Abstract
The Aryl Hydrocarbon Receptor (AhR) is a ligand-activated transcriptional factor pivotal in responding to environmental stress and maintaining cellular homeostasis. Exposure to specific xenobiotics or industrial compounds in the environment activates AhR and its subsequent signaling, inducing oxidative stress and related toxicity. Past research has also identified and characterized several classes of endogenous ligands, particularly some tryptophan (Trp) metabolic/catabolic products, that act as AhR agonists, influencing a variety of physiological and pathological states, including the modulation of immune responses and cell death. Heavy metals, being non-essential elements in the human body, are generally perceived as toxic and hazardous, originating either naturally or from industrial activities. Emerging evidence indicates that heavy metals significantly influence AhR activation and its downstream signaling. This review consolidates current knowledge on the modulation of the AhR signaling pathway by heavy metals, explores the consequences of co-exposure to AhR ligands and heavy metals, and investigates the interplay between oxidative stress and AhR activation, focusing on the regulation of immune responses and ferroptosis.
Keywords: AhR, Oxidative Stress, Heavy Metals, Immune Checkpoint, Ferroptosis
Introduction
The aryl hydrocarbon receptor (AhR) is a conserved, ligand-activated transcription factor belonging to the basic helix-loop-helix (bHLH)/Per-ARNT-Sim (PAS) family of proteins 1. It features distinct domains that regulate its functions: the N-terminal bHLH domain is responsible for recognizing DNA binding sites, PAS domain A stabilizes heterodimerization with ARNT, and PAS domain B governs the binding pocket for ligands 2. Early studies of AhR primarily centered on its toxicological aspects, as it was known to be activated by environmental pollutants such as dioxins 3. Upon AhR activation induced by these environmental xenobiotics, the metabolism of these substances results in the production of reactive oxygen species (ROS), which can disrupt cellular homeostasis over an extended period 4. Extensive research over the past two decades has investigated the regulatory relationship between AhR activation and oxidative stress 5,6. As commonly known, heavy metal ions are strongly associated with the generation of reactive oxygen species (ROS) and subsequent oxidative toxicity upon cellular exposure 7. Therefore, the heavy metals-induced oxidative stress is highly implied to interact with AhR activation and its downstream target genes’ transcription 8. This concise review aims to provide a summary of the mechanisms through which various heavy metals activate the AhR signaling pathway and how heavy metals-induced oxidative stress can influence the regulation of AhR-related homeostasis.
AhR signaling and Its Ligands
In the canonical AhR signaling axis, AhR is released from a protein complex consisting of Src (SRC proto-oncogene, non-receptor tyrosine kinase), hsp90 (heat shock protein 90), p23 (PTGES3), and AhR-interacting protein (AIP) upon binding with an AhR ligand. This process exposes the nuclear localization signal of AhR, resulting in its nuclear translocation. In the nucleus, AhR dimerizes with aryl hydrocarbon receptor nuclear translocator (ARNT) and binds to xenobiotic response elements (XREs), initiating transactivation of targeting genes 9–11. Among AhR targeted genes are Phase I metabolic enzymes including the cytochrome P450 superfamily proteins (CYPs), which introduce reactive and polar groups to xenobiotic molecules 12. However, AhR also induces expression of target genes that function far beyond metabolism of xenobiotics 13. In fact, AhR plays a crucial role in development, metabolic homeostasis, and immune responses 14–16, and it regulates expression of immunomodulatory proteins including programmed cell death 1 (PD-1), programmed death-ligand 1 (PD-L1), and indoleamine 2,3-dioxygenase 1 (IDO1) 11, 17.
AhR activity is regulated by a wide range of small molecular compounds from the environment, diets, and metabolized by the host and its commensal microorganisms 10,11,18,19. AhR was first identified and characterized through its activation by a diverse range of environmental ligands, including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) 20. TCDD is a byproduct originating from the use of herbicides such as Agent Orange 21. It has been observed to persistently contaminate the soil and riverbed sediments over extended periods 22. PAHs are a group of organic compounds that are formed during incomplete combustion of organic materials such as fossil fuels, wood, and tobacco. Notably, benzo[a]pyrene (BaP) and dioxins are also known as pre-carcinogens or carcinogens. PCBs are a class of synthetic organic chemicals that are widely used in electrical equipment, hydraulic fluids, and other industrial applications, and PCBs can persist in the environment due to their long half-lives 23. These environmental pollutant compounds are regarded as hazardous, toxic, and carcinogenic due to their ability to persist within the human body following exposure 24,25. They are resistant to metabolic degradation, resulting in prolonged AhR activation 26,27. This sustained activation disrupts the regulatory program of downstream genes, elevates oxidative stress levels, and disturbs overall homeostasis 26.
Among the endogenous ligands for AhR are a series of compounds derived from tryptophan (Trp) metabolism/catabolism 28–31, including 6-formylindolo[3,2-b] carbazole (FICZ), kynurenine (Kyn), indirubin, oxindole and indole 3-pyruvic acid (I3P). FICZ is converted from Trp either under UVB irradiation as a photoproduct or after exposure to H2O2 for 14 days at room temperature 32,33; Kyn is derived from Trp by the enzyme metabolism of TDO (Tryptophan 2,3-dioxygenase)/IDO1 and arylformamidase (AFMID) 30; Indirubin is a catabolic product of Trp that is metabolized by indole hydroxylation within the gut microbiota 34. Oxindole, one of indole products, is primarily derived from Trp metabolism of gut microbiota, and its formation has been identified under cellular oxidation in our previous study 35,36. I3P is a metabolic product of Trp catalyzed by IL4I1, an amino acid dioxygenase 37.
Hence, an increasing number of studies have started to recognize Trp metabolites as indispensable factors in investigating the influence of AhR on cell survival, immune responses, and overall homeostasis.
AhR and Immune Checkpoint Genes
Immuno-modulators, or checkpoint proteins, are molecules that regulate the immune response, ensuring a balance between activation and suppression to maintain immune homeostasis 38. IDO1 is a heme enzyme, which plays a critical role in regulating the tryptophan metabolism in the body, with its activity leading to the degradation of tryptophan and the production of kynurenine 39. IDO-1 has been regarded as a third arm of the immune checkpoint 40,41. Numerous prior studies have demonstrated that high expression of IDO1 is associated with an immunosuppressive effect, resulting in poor prognosis and outcomes in various solid tumors and hematologic malignancies 42,43. The primary mechanism underlying the upregulation of IDO1 that contributes to immune tolerance is the inhibition of T cell activity in a tryptophan-depleting environment. The high expression of IDO1 in the tumor microenvironment depletes tryptophan, hindering T cells from utilizing it for self-activation 30,44. Additionally, IDO1 induces the general control nonderepressible 2 (GCN2) kinase and suppresses the mammalian target of rapamycin 1 (mTOR1) pathway in T cells, thereby inhibiting their tryptophan-withdrawal functions 45,46. The production of kynurenine, an AhR ligand, continually stimulates AhR activation, leading to increased IDO1 transcription and the formation of a positive feedback loop 47. Moreover, AhR activation facilitates the trans differentiation of Th17 cells into regulatory T cells, further downregulating T cell activity 48.
PD-1/PD-L1 are considered as major components of the immune checkpoint that negatively regulate T-cell functions 49,50. PD-L1, also referred to as B7-H1 and CD274, is an immune checkpoint inhibitor that binds to its receptor PD-1 expressed by T cells and other immune cells 51. The activation of PD-1/PD-L1 pathway serves to suppress autoimmunity and promote immune tolerance under inflammatory conditions 52. However, elevated expression of PD-L1 on tumors triggers an “adaptive immune mechanism” to evade anti-tumor responses and is strongly associated with advanced disease state and unfavorable prognosis in various cancers 53. In earlier studies, AhR has been shown to regulate the transactivation of programmed cell death protein 1 (PD-1) and its ligand PD-L1, which are critical genes involved in immune suppression 17,54,55. It has been reported that tobacco smoking carcinogens induced PD-L1 expression both in vivo and in vitro, which is highly dependent on AhR activation 56. In a murine orthotopic oral cancer (MOC) model, AhR expression up-regulates T cell exhaustion in the tumor-infiltrating T cells and increases the expression of PD-1/PD-L1, while AhR-deficient model shows a lower PD-1/PD-L1 expression level and a long-lasting antitumor immune response 54. Furthermore, the results obtained from both RT-PCR and NanoString PanCancer Immune Profiling Panel clearly demonstrated that AhR knockout significantly down-regulated the mRNA level of PD-L1 54. In T cells, Kyn induces expression of PD-1 through the AhR signaling axis, thus suppressing their cytotoxicity 17. In patients with gastrointestinal cancer, the immunomodulatory roles of AhR and its regulation of IDO1, PD-1/PD-L1 have also been identified. Strong evidence suggests that high expression of IDO1 and PD-L1 is associated with an early clinical stage and absence of lymphatic, which is AhR activation dependent 57. Tryptophan 2,3-dioxygenase (TDO2), which has a similar function to IDO1, induces AhR-mediated PD-L1 transactivation to disrupt immune response 58. Additionally, AhR/TDO2/kynurenine positive feedback loop facilitates liver metastasis of colon cancer via PD-L1-mediated immune evasion 58. Given the crucial role of the immune checkpoint in cancer development, newly implemented immunotherapies targeting the blockage of IDO1 and/or PD-1/PD-L1 have better health outcomes of patients with various malignancies including melanoma, gastrointestinal cancer, breast cancer, and lung cancer. Although a phase III clinical trial failed to show an improved anti-tumor response with IDO1 inhibitor epacadostat 59, it is possible that pro-survival activities of Trp-derivatives in the tumor microenvironment may greatly reduce its efficacy. Therefore, it’s worth considering the supplementary blockade of AhR activation as a viable strategy to enhance the anti-tumor efficacy of IDO1 inhibitors.
AhR and Oxidative Stress
The term “oxidative stress” refers to an elevation in the oxidation state within cells. This shift in cellular redox homeostasis typically occurs due to an increase in the production of reactive oxygen species and a failure of cellular antioxidant defenses 60. Elevated ROS resulting from exposure to environmental toxins or disrupted metabolic processes can have detrimental effects on organisms 61. For example, tobacco smoke is a well-known ROS producer by generating hydrogen peroxide to disrupt antioxidant system 62. Benzo(a)pyrene, a well-known tobacco smoke byproduct, has been found to elevate ROS level in cells 5. Moreover, benzo(a)pyrene as an AhR activator is largely generated and consistently induce the activation of cytochrome P450 enzymes during smoking 63.
Extensive research in the past has demonstrated that AhR activation plays a major role in the maintenance of oxidative homeostasis, which is accomplished through the regulation of xenobiotic metabolism, detoxification, and the modulation of antioxidant defenses 64. Thus, dysregulated AhR activation can lead to oxidative stress and associated pathologies. Some researchers believe that when activated by TCDD, one of the most common environmental dioxins and an exogenous ligand of AhR, AhR may act as an inducer of oxidative stress 65. The cytochrome P450 enzymes induced by TCDD are known to incompletely reduce O2, resulting in the generation of superoxide and hydrogen peroxide due to poor coupling of electron flow 66. TCDD has been implicated in the formation of the superoxide anion in rat brain with resultant generation of lipid peroxides, and this effect is controlled in part by the AhR complex 4. Additionally, TCDD has been shown to induce xanthine oxidase and xanthine dehydrogenase (XO/XDH) activities in the liver of mice 67. This induction leads to the production of reactive oxygen species (ROS), which is dependent on AhR activation. An increase in ROS parameters is observed in liver when AhR downstream targets including cytochrome P450 (CYP) enzymes are activated to metabolize chemical compounds during the detoxification process 68. One major rationale for AhR as an oncogene is that ROS accumulation due to increased CYP activities, leading to severe oxidative stress and increased DNA damage, favoring malignant transformation 68,69. Mitochondria, as the major site of reactive oxygen production, also serves as a regulator of TCDD-induced oxidative stress. By treating mice with TCDD, succinate stimulated mitochondrial H2O2 production is elevated in the early weeks and remains significantly elevated after eight weeks. Meanwhile, the level of mitochondrial H2O2 production in AhR knock-out mice was one-fifth that found in wild-type mice 70,71. Thus, a proposed mechanism by which TCDD-induced AhR activation contributes to oxidative stress is inhibition of electron transport at complex III, producing a persistent accumulation of succinate-dependent superoxide and H2O2 production in mitochondria 66. Moreover, it has been reported that exposure to TCDD induced a degradation of mitochondria AhR (mitoAhR), which is localized to the inter-membrane space (IMS) of the organelle. The TCDD-induced degradation of mitoAhR significantly disrupted the cellular respiration and expression level of proteins within the mitochondrial proteome, resulting in mitochondrial dysfunction and TCDD-induced toxicity 72. Therefore, these experiments limit the relationship between AHR and ROS to the relationship where AHR is an inducer of ROS.
However, several lines of recent evidence suggest that ROS plays an essential role in activating AhR, thus enhancing expression of its downstream targets. In breast cancer cells, ROS accumulation is strongly correlated with increasing AhR expression, triggering AhR nuclear translocation, and promoting its transcriptional activity 73. In addition, UVB irradiation or hydrogen peroxide treatment causes the accumulation of FICZ, which is mediated by inhibitory effect on CYP enzymes, thereby activating AhR 6. Furthermore, it has been demonstrated that H2O2 gradually transforms tryptophan into FICZ at room temperature 33. This gradual conversion process produces nanomolar concentrations of FICZ, which are sufficient to induce AhR activation. In addition to the conversion of tryptophan to FICZ, kynurenine, as a typical AhR endogenous ligand, has also been identified as one of the degradation products resulting from tryptophan oxidation under ROS exposure 74. In our previous finding, we identified 2-oxindole as a new compound generated in vitro during oxidative stress. 2-oxindole, also a Trp-derivative, was capable of activating AhR, leading to upregulation of CYP1A2, PD-L1, and IDO1 36. These lines of evidence strongly suggest that H2O2 and ROS can induce or accelerate the generation of AhR ligands in vivo.
Furthermore, recent research has uncovered a regulatory association between AhR and lipid peroxidation. While AhR’s metabolism induced by exogenous ligands can result in lipid peroxides, AhR plays a vital role in reducing levels of lipid peroxidation and providing resistance against ferroptosis. 75. Ferroptosis is an iron-dependent cell death process, which is distinct from other forms of programmed cell death such as apoptosis, necrosis, and autophagy 76. Ferroptotic cell death is marked by the presence of lipid species derived from peroxidation. Extensive research in the past has identified and characterized important gene products involved in regulating ferroptosis including those involved in iron metabolism (e.g., HO-1), anti-lipid peroxidation and anti-oxidation (e.g., GPX4), glutathione synthesis rate (e.g., SLC7A11, SLC3A2), and transcription factors 77–81. Several lines of studies have implicated the involvement of AhR in modulating the sensitivity to ferroptosis. (I) AhR activation may regulate ferroptosis by modulating the expression of genes involved in ferroptotic responses. For example, AhR is capable of upregulating Nrf2 and its target gene, heme oxygenase-1 (HO-1) 82,83. Both Nrf2 and HO-1 are known to exert antioxidant and cytoprotective effects, thus inhibiting ferroptosis. In addition, Kyn and I3P have been shown to suppress ferroptosis 84,85. Since both Kyn and I3P are AhR agonists, it is conceivable that AhR and its signaling may potentiate ferroptotic responses. Furthermore, AhR may directly regulate expression of SLC7A11 to inhibit ferroptosis. Our studies revealed that the promoter region of SLC7A11 contains at least one XRE, a consensus binding motif of AhR, and AhR-deficiency suppresses expression of SLC7A11 after treatment with erastin (unpublished results).
Heavy Metal Toxicity and AhR
Heavy metal toxicity.
Heavy metals toxicity refers to the process by which certain metals or metalloids can elicit cellular and/or genetic alterations, leading to the dysfunction of physiological system, organ damage, and development of cancer 86. One common feature associated with toxicity and/or carcinogenicity of metals/metalloids listed above is the perturbation of the oxidative state in the cells 87. In fact, many toxic metals/metalloids induce the production of reactive oxygen species (ROS) through Fenton- and Haber-Weiss-type reactions, which are thought to be the main culprit in their carcinogenesis 87.
Arsenic is a toxic metalloid that is abundant in the environment. It is generally thought that the resulting oxidative damage to cellular components is attributed to arsenic’s toxic effects and its role in carcinogenesis 88. Arsenic exposure induces oxidative stress by promoting the generation of ROS and impairing cellular antioxidant defenses 89. Furthermore, arsenic triggers significant morphological disruptions in mitochondrial integrity, accompanied by a decrease in mitochondrial membrane potential. These mitochondrial modifications are recognized as key loci where unregulated generation of superoxide anion radicals occurs 90.
Chromium is a metallic element that exists in different oxidation states, with hexavalent chromium [Cr(VI)] being the most toxic and carcinogenic form 91. Acute exposure to Cr(VI) causes cytotoxicity and genotoxicity on cells, including the generation of ROS, perturbing cell signaling, induction of DNA double-strand breaks, and cell cycle arrest 92,93, 94,95. Cr(VI)-generated ROS are important in causing various adverse health effects including mutagenesis and tumorigenesis. Under aerobic conditions, microsomal enzymes reduce Cr(VI) to Cr(V), which is accompanied by the generation of hydroxyl radical (HO•) and superoxide (O2•) 96. Superoxide (O2•) is converted to hydrogen peroxide (H2O2) by superoxide dismutase 97. Therefore, Cr(VI) carcinogenesis is initiated or promoted by metabolic reduction of Cr(VI), producing ROS that can induce genotoxic effects, elicit inflammatory responses, and alter survival signaling pathways.
Cadmium is a heavy metal commonly found in the environment and it is classified as a human carcinogen, primarily associated with the development of lung cancer and prostate cancer 98. Cadmium is known to promote oxidative stress, disrupting cellular signaling pathways, and perturbing cellular genetics 99. Evidence showed that cadmium had a capacity to replace copper and iron from cytoplasmic and membrane proteins, including ferritin and apoferritin 100. This cadmium-induced replacement results in an accumulation of unbound or inadequately chelated copper and iron ions, triggering oxidative stress through Fenton reactions 87.
Lead, as a soft metal, has been wildly applied in the production of lead-acid batteries, oxides for paint, glass, and pigments 101. The primary organ system most susceptible to lead-induced toxicity is the central nervous system (CNS) 102. Additionally, both acute and chronic lead exposure can adversely affect the proper functioning of the kidney and reproductive organs 102. The toxicity of lead hinges on its disruption of the equilibrium between the production of ROS and the antioxidant defense mechanisms 103. Lead shares electrons with sulfhydryl groups of antioxidant enzymes to establish covalent bonds, which inhibits their enzyme activities, leading to a depletion of glutathione 86. Moreover, studies found that the mechanism of toxicity of lead also includes raising free radical levels and inducing lipid peroxidation 104.
AhR activities impacted by heavy metals.
The connection between heavy metals and AhR activation has been the subject of thorough investigation in previous studies. Various hypotheses have been proposed to elucidate the mechanism underlying heavy metals induced AhR activation. However, despite differences in the effects of various heavy metals on AhR activation, a universally accepted explanation for the mechanism remains elusive.
Arsenic (As3+), presenting in elevated levels in drinking water and recognized as a potential human carcinogen, has demonstrated with the ability to induce AhR nuclear translocation and accumulation in Hepa-1 cells with a comparable efficacy to that of TCDD 105. In addition, it has been found that As3+ exposure increase XRE-dependent luciferase activity and elevate CYP1A1 protein levels 106 Meanwhile, As3+ have synergistic effect with BaP on phase1 enzyme CYP1A1, phase 2 enzyme NQO1 and potentiates AhR ligands-mediated stability of CYP1A1 mRNA 107. However, the precise mechanisms underlying the ability of As3+ to induce CYP1A1 mRNA transcription via AhR activation remain unclear. One possible explanation is that As3+ increases HO-1 expression at the time, which facilitate heme degradation. This process generates biliverdin and subsequently bilirubin, which have been demonstrated to act as AhR ligands 108. Nevertheless, despite the observed elevation of pulmonary CYP1A1 expression and an accompanying increase in total plasma bilirubin concentrations, administering bilirubin directly to the lungs through intra-tracheal injection failed to increase AhR activation and CYP1A1 mRNA expression 109. The oxidative stress triggered by arsenic has been observed to liberate arachidonic acid from glycerophospholipids, potentially resulting in AhR activation 110. This could represent another plausible mechanism through which arsenic modulates the AhR signaling pathway.
As a ROS producer, Cr(VI) undergoes rapid reduction to chromium (V) and chromium (III) upon entering cells, which is accompanied by the generation of superoxide and H2O2 111,112. In our previous research, we discovered that Cr(VI)-induced ROS generation stimulates AhR activation 36. The production of oxindole, a potent AhR ligand, is driven by the H2O2 generated because of Cr(VI) exposure 36. The induction of AhR downstream genes by Cr(VI) exposure was inhibited by both AhR inhibitors and AhR genomic knockdown, which confirmed the regulation of AhR by Cr(VI) exposure 36. Furthermore, we found that Cr(VI) exhibited synergy with I3P, an AhR ligand, in inducing the expression of CYP1A2 (unpublished data). Thus, we conclude that Cr(VI) regulates AhR activation by ROS generation.
Cadmium (Cd2+) is well known for its hepatic and renal toxicity 113,114. Earlier studies have shown that the accumulation of Cd2+ affected total hepatic CYP450 enzymes activities in both in vitro/in vivo models 115,116. In studies conducted in El-Kadi’s laboratory, Cd2+ was observed to enhance AhR nuclear translocation and elevate XRE-dependent luciferase activity, indicating that the induction of the CYP1A1 gene’s transcription by Cd2+ is dependent on the AhR signaling pathway 8. Moreover, Cd2+ was found to exhibit a synergistic effect on AhR activation whe n combined with AhR ligands or the pro-oxidant buthionine sulfoximine 7, suggesting that Cd2+ induces AhR activation by augmenting oxidative stress.
Lead (Pb2+) exposure has been shown to induce strong oxidative stress and antioxidant deficiency, leading to organs impairment and potential carcinogenesis 117. Initial investigations noted a substantial elevation in AhR levels upon exposure to Pb2+, accompanied with induction of the downstream gene CYP1A1 118. In addition, Pb2+ enhanced the TCDD-induced increase in CYP1A1 mRNA levels in a manner dependent on both time and dosage 119. In research conducted in Korashy’s laboratory, lung toxicity has been associated with AhR activation and CYP1A1 induction induced by Pb2+ exposure 118. The initiation of inflammation and apoptosis in rat lung tissue by Pb2+ was asserted to rely on both oxidative stress and AhR activation 120. Moreover, an elevation in the expression of CYP1A1 mRNA and protein levels was observed in Pb2+ induced cardiotoxicity, indicating a regulation role of AhR in cardiotoxicity 121. Co-exposure to BSO and Pb2+ enhanced CYP1A1 mRNA level, suggesting that Pb2+ regulate AhR signaling pathway through a mechanism dependent on oxidative stress 122.
Immuno-modulation upon Exposure to Heavy Metals
Early studies have provided a complex interaction between aberrant immune homeostasis and exposure to heavy metals while the mechanisms remain elusive. Previous studies have demonstrated that exposure to heavy metals can have diverse immune-related effects, encompassing the induction of proinflammatory cytokine dysregulation, hypersensitivity reactions, autoimmunity, immunosuppression, and disturbances in T-cell function 123. For example, arsenic was found to regulate the activity of both innate and adaptive immunity by various cellular mechanisms. Chronic arsenic exposure markedly disrupted the differentiation process of peripheral blood monocytes into fully matured macrophages and inhibited the activity of transcriptional factors (such as NF-κB-related survival pathways and the liver X receptor), ultimately leading to a reduction in the expression of its target genes and disruption of cholesterol efflux 124–126. Moreover, arsenic has been observed to significantly impair the activation and functionality of T cells, disrupting their differentiation and proliferation processes 127. Long-term arsenic exposure induced reductions in lymphoproliferation, cytokine secretion, and an imbalance in Th1/Th2 and Th17/Treg differentiation, contributing to sever immunosuppression and immunotoxicity 128,129.
Lead-induced immunotoxicity has been observed in both animal model experiments and occupational research studies 130,131. Previous studies have reported that lead release elevated levels of tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-6, and IL-12 in macrophages 132. In addition, a cohort study observed that lead-exposed workers exhibit higher plasma IL-10 levels and a tendency towards higher plasma TNF-alpha levels compared to non-exposed workers 133. Lead has also been observed to reduce the percentage of CD4 cells and the expression of the CD4 surface antigen 134,135. Furthermore, exposure to lead was reported to increase drug hypersensitivity syndrome (DHS) and sensitize food allergy by direct inhibition of antigen-specific T cell activation 136,137.
Cadmium has been linked to numerous inflammatory manifestations and autoimmune responses across various tissues 124. Cadmium-induced extensive ROS generation leads to the upregulation of several proinflammatory cytokines (such as IL-6, IL-8, TNF-α, and IL-1β) and inflammatory transcription factors like NF-κB, which facilitate additional leukocyte accumulation and contribute to a secondary injury 138,139. In addition, evidence has been shown that AhR is involved in the lung leukocyte proinflammatory cytokine (IL-6) response to cadmium exposure 140, suggesting an interaction between AhR and heavy metals modulated immune response.
It has been known that AhR plays an important role in both innate and adaptive immunity, partly through mediating expression of immune checkpoint proteins including IDO1, PD-1 and PD-L1 54,56,141. Our previous studies have shown that H2O2 and ROS generated AhR ligand oxindole significantly induced IDO1 and PD-L1 expression in both concentration- and time-dependent fashion 36. Given the fact that heavy metals regulate AhR by ROS generation, we propose a novel hypothesis to elucidate the mechanism linking heavy metals and immune regulation. This hypothesis posits that heavy metals regulate immune checkpoint genes through the generation of ROS, thereby inducing the production of endogenous AhR ligands, particularly oxindole. This, in turn, leads to an increase in the expression of immune checkpoint genes such as IDO1 and PD-L1 through an AhR activation-dependent mechanism. Given this hypothesis, we demonstrated that Cr(VI) induced expression of PD-L1 and IDO1 in HCT116 cells in a concentration- and time-dependent manner (unpublished data). Combined with the above findings strongly suggest that heavy metals potentially function as modulator of immune checkpoint genes in vivo and that the heavy metals induced oxidative stress may significantly compromise immune responses via deregulating AhR signaling.
Ferroptotic Responses impacted by Heavy Metals
As mentioned earlier, heavy metals can stimulate the generation of ROS, which react with membrane polyunsaturated fatty acids (PUFAs) to generate lipid peroxides, ultimately triggering lipid peroxidation 142,143. Pioneering research has recently provided compelling evidence linking heavy metal-induced lipid peroxidation to ferroptosis, a process closely associated with metal toxicity 144. Chronic As3+ exposure has been found to trigger ferroptosis in a mice model, with mechanisms involving iron accumulation, disruption of mitochondrial function, endoplasmic reticulum stress and significant reductions in anti-ferroptosis protein expressions (such as SLC7A11, GPX4), ultimately resulting in cerebral cortex neuronal loss and cell death within the testicular tissue 145,146. Additional evidence has revealed that arsenic exposure induce mitochondrial reactive oxygen species (MtROS)-dependent autophagy in pancreatic cells, leading to ferroptosis by modulating iron homeostasis 147. Furthermore, arsenic exposure has been revealed to upregulate ACSL4 (acyl-CoA synthetase long-chain family member 4) under ferroptosis 148. The inhibition of ACSL4 has been shown to significantly suppress ferroptosis, inflammation, and lipid accumulation triggered by arsenic exposure, highlighting ACSL4 as a promising new target for anti-ferroptotic strategies 148.
Recent studies have observed that Cd induced liver damage by inducing ferroptosis 149. One mechanism by which Cd induces ferroptosis is through the augmentation of endoplasmic reticulum (ER) stress, accompanied by the activation of the PERK-eIF2α-ATF4-CHOP signaling pathway 149. Moreover, exposure to Cd led to an elevation in cellular iron content and oxidative stress, resulting in the upregulation of iron death-related genes (such as ASCL4, PTGS2, and NOX1) expression, while concurrently repressing the expression of anti-ferroptosis genes (like GPX4 and FTH1) 150. Intriguingly, contrary to its role as a ferroptosis inducer, chronic exposure to Cd was discovered to inhibit erastin-induced ferroptosis by reducing cellular iron content, elevating GSH levels, and upregulating SLC7A11 expression in prostate cancer cells 151,152. The anti-ferroptosis function of Cd increases cancer cell viability via a possible modulation of miR-128-3p/SLC7A11 signaling 151. This finding suggests a dual-edged effect of heavy metals in ferroptosis, which depends on both the duration and dosage of exposure.
Compelling studies have revealed a connection between lead-induced toxicity in the central nervous system (CNS) and ferroptosis. Utilizing the ferroptosis database for bioinformatics analysis, it was discovered that 16 ferroptosis-related genes exhibited differential expression in choroid plexus epithelial cells after exposure to lead 153. Furthermore, Pb2+ significantly inhibited GPX4 and SLC7A11 expression level and decreased cell viability, leading to massive ferroptosis and a potential blood-cerebrospinal fluid barrier dysfunction 153. Ferroptosis-induced lead neurotoxicity has been observed in HT22 cells. Acute exposure to lead resulted in the accumulation of iron, a reduction in glutathione (GSH), and a significant downregulation of the expression of SLC7A11 154. These studies imply that SLC7A11 plays as an important regulator of lead induced ferroptosis.
Even though Cr(VI) is known to induce oxidative stress and high dose Cr(VI) exposure directly contribute to cell death, we observed that at low concentrations, Cr(VI) suppressed erastin-induced ferroptosis and cell death (unpublished data). Given our observations that Cr(VI) activates AhR signaling 36, we hypothesized that Cr(VI) suppress ferroptosis induced by erastin by inducing AhR activation, with mechanism involving a positive feedback loop between AhR, its transcriptional targets (e.g., IDO1), and metabolic products (e.g., Kyn) catalyzed by these enzymes. In addition, we observed that AhR-null cells, as originally described 155, contained a higher basal level of HO-1 than wild-type cells and erastin treatment further increased expression of HO-1(unpublished data). This observation suggests that AhR may be directly involved in the negative regulation of HO-1 during ferroptosis. It is known that heme degradation by HO-1 leads to the release of free iron, which can participate in the Fenton reaction, resulting in highly reactive hydroxyl radicals 156. The increased level of these radicals is expected to contribute to lipid peroxidation, a key feature of ferroptosis. Thus, this line of observation introduces a novel perspective to elucidate how heavy metals modulate ferroptosis through an AhR-dependent pathway.
Summary
In this review, we discuss the intricate relationship between oxidative stress and the AhR signaling pathway, particularly under the influence of heavy metal exposure, such as [Cr(VI). Recent findings underscore that AhR not only modulates oxidative stress but also experiences reciprocal regulation through stress-induced endogenous ligands. Specifically, oxidative stress can trigger the formation of metabolites from cellular components, like amino acids and lipids, that serve as AhR agonists.
Our proposed model, illustrated in Figure 1, outlines the activation of AhR signaling by oxidative stress, which is a critical response to environmental metal toxicants, including cadmium, arsenic, lead, and chromium. These metals/metalloids induce the production of ROS, leading to the generation of amino acid-derived compounds such as 2-oxindole, Kyn, and I3P. These substances act in turn as AhR ligands, causing the receptor to translocate to the nucleus and bind to XRE to regulate the transcription of target genes. Upon activation, AhR transactivates expression of genes related to ferroptosis, such as SLC7A11 and heme oxygenase 1 (HO-1), and immuno-modulatory responses, such as IDO1 and PD-L1.
Figure 1:
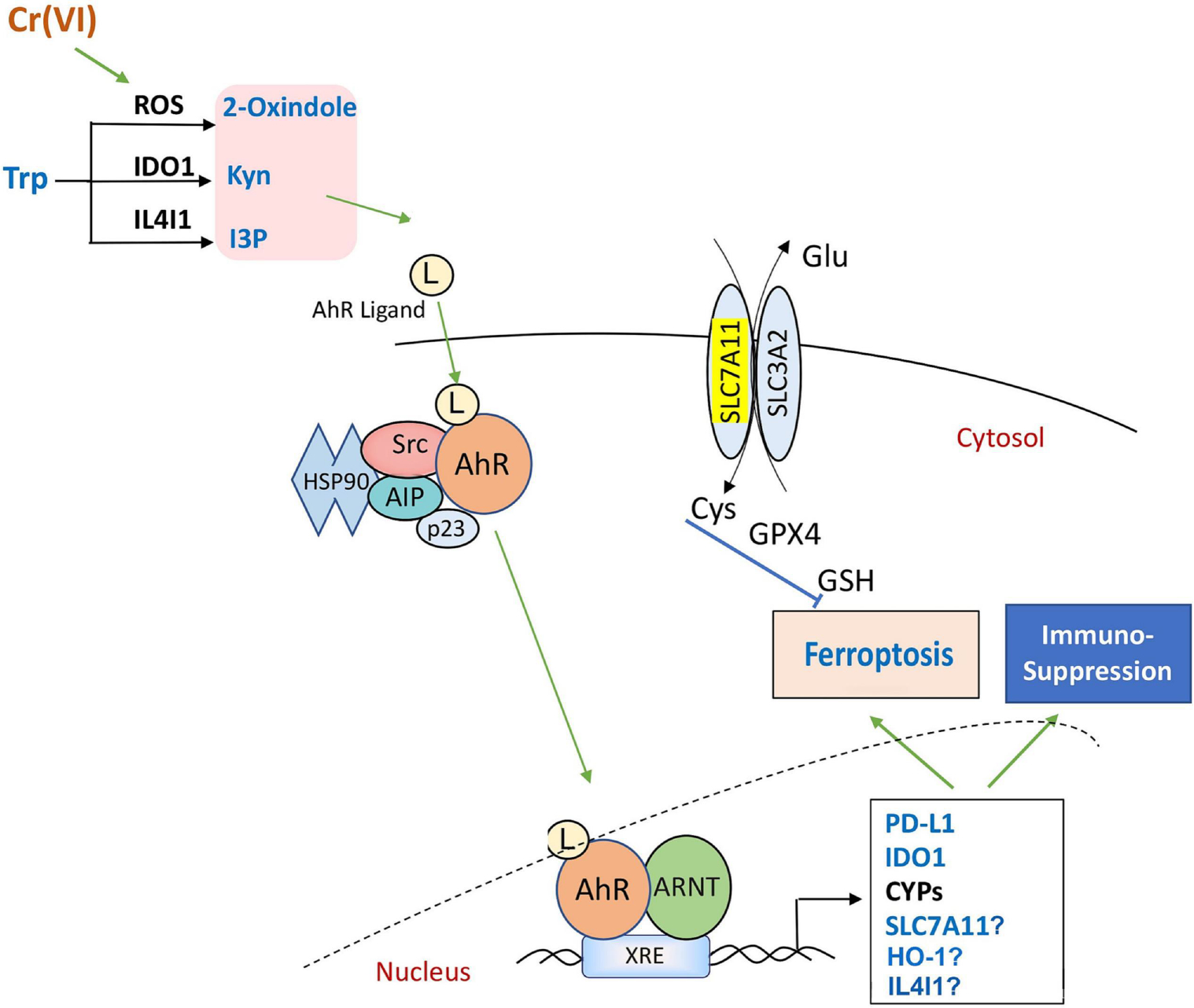
AhR activation under oxidative stress: This model illustrates the process of AhR activation in response to oxidative stress, involving the generation of Trp-derivatives. Upon activation, AhR plays a crucial role in the transcriptional regulation of target genes. This activation has significant biological consequences, including the suppression of immune responses and ferroptosis, providing insights into the complex interplay between AhR signaling and cellular stress responses.
In this comprehensive review, we explore the complex interaction between various heavy metals and the AhR signaling pathway. We place particular emphasis on highlighting oxidative stress as a key mechanism underlying heavy metal-induced AhR activation. Additionally, we provide an extensive overview of the regulatory interaction between AhR and heavy metals and their impact on immune responses and ferroptosis. Furthermore, we propose a novel hypothesis suggesting that the regulation of immune responses and ferroptosis by heavy metals may be dependent on AhR activation. This novel perspective may contribute to unraveling the previously unknown mechanisms by which heavy metals may exert impact on immune responses and ferroptosis via the AhR signaling axis.
Highlights:
Oxidative stress as a key mechanism underlying heavy metal-induced AhR activation.
AhR activation is intricately linked to oxidative stress, immune regulation, and the induction of ferroptosis.
ACKNOWLEDGEMENT
We would like to thank the lab members, especially Dr. Byeong Choi and Ms. Eunji Lee, for various assistances and discussions. This work was supported in part by National Institutes of Health (NIH) grants R01CA213159 and R01CA216987 to W.D.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT author statement
Ziyue Kou: Conceptualization, Writing - Original Draft, Writing - Review & Editing, Resources, Validation
Franklin Tran: Writing - Review & Editing
Wei Dai: Writing - Review & Editing, Supervision, Funding acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nebert DW Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res 67, 38–57, doi: 10.1016/j.plipres.2017.06.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu D & Rastinejad F Structural characterization of mammalian bHLH-PAS transcription factors. Curr Opin Struct Biol 43, 1–9, doi: 10.1016/j.sbi.2016.09.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii-Kuriyama Y & Kawajiri K Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc Jpn Acad Ser B Phys Biol Sci 86, 40–53, doi: 10.2183/pjab.86.40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsharif NZ, Lawson T & Stohs SJ Oxidative stress induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin is mediated by the aryl hydrocarbon (Ah) receptor complex. Toxicology 92, 39–51, doi: 10.1016/0300-483x(94)90166-x (1994). [DOI] [PubMed] [Google Scholar]
- 5.Tsuji G et al. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-smediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J Dermatol Sci 62, 42–49, doi: 10.1016/j.jdermsci.2010.10.017 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Wincent E et al. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A 109, 4479–4484, doi: 10.1073/pnas.1118467109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anwar-Mohamed A, Elbekai RH & El-Kadi AO Regulation of CYP1A1 by heavy metals and consequences for drug metabolism. Expert Opin Drug Metab Toxicol 5, 501–521, doi: 10.1517/17425250902918302 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Elbekai RH & El-Kadi AO The role of oxidative stress in the modulation of aryl hydrocarbon receptor-regulated genes by As3+, Cd2+, and Cr6+. Free Radic Biol Med 39, 1499–1511, doi: 10.1016/j.freeradbiomed.2005.07.012 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Hahn ME Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact 141, 131–160, doi: 10.1016/s0009-2797(02)00070-4 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Kou Z & Dai W Aryl hydrocarbon receptor: Its roles in physiology. Biochem Pharmacol 185, 114428, doi: 10.1016/j.bcp.2021.114428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothhammer V & Quintana FJ The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 19, 184–197, doi: 10.1038/s41577-019-0125-8 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Nebert DW & Dalton TP The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nature Reviews Cancer 6, 947–960, doi: 10.1038/nrc2015 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Sutter TR & Greenlee WF Classification of Members of the Ah Gene Battery. Chemosphere 25, 223–226, doi:Doi 10.1016/0045-6535(92)90519-W (1992). [DOI] [Google Scholar]
- 14.Veldhoen M, Hirota K, Christensen J, O’Garra A & Stockinger B Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. Journal of Experimental Medicine 206, 43–49, doi: 10.1084/jem.20081438 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robles R et al. The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology 141, 450–453, doi:Doi 10.1210/En.141.1.450 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Lahvis GP et al. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Molecular Pharmacology 67, 714–720, doi: 10.1124/mol.104.008888 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Liu Y et al. Tumor-Repopulating Cells Induce PD-1 Expression in CD8(+) T Cells by Transferring Kynurenine and AhR Activation. Cancer Cell 33, 480–494 e487, doi: 10.1016/j.ccell.2018.02.005 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Che X & Dai W Aryl Hydrocarbon Receptor: Its Regulation and Roles in Transformation and Tumorigenesis. Curr Drug Targets 20, 625–634, doi: 10.2174/1389450120666181109092225 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Jaglin M et al. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front Neurosci 12, 216, doi: 10.3389/fnins.2018.00216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julliard W, Fechner JH & Mezrich JD The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front Immunol 5, 458, doi: 10.3389/fimmu.2014.00458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.in Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam (1994). [PubMed]
- 22.Olson KR & Morton LW Long-term fate of Agent Orange and dioxin TCDD contaminated soils and sediments in Vietnam hotspots. Open Journal of Soil Science 9, 1 (2019). [Google Scholar]
- 23.Wirgin I et al. Mechanistic basis of resistance to PCBs in Atlantic tomcod from the Hudson River. Science 331, 1322–1325, doi:science.1197296 [pii] 10.1126/science.1197296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boffetta P, Mundt KA, Adami HO, Cole P & Mandel JS TCDD and cancer: a critical review of epidemiologic studies. Crit Rev Toxicol 41, 622–636, doi: 10.3109/10408444.2011.560141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perera F Carcinogenicity of airborne fine particulate benzo(a)pyrene: an appraisal of the evidence and the need for control. Environ Health Perspect 42, 163–185, doi: 10.1289/ehp.8142163 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Wang Y, Fu Y, Yin Y & Xu K Modulating AHR function offers exciting therapeutic potential in gut immunity and inflammation. Cell Biosci 13, 85, doi: 10.1186/s13578-023-01046-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens EA, Mezrich JD & Bradfield CA The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127, 299–311, doi: 10.1111/j.1365-2567.2009.03054.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ala M Tryptophan metabolites modulate inflammatory bowel disease and colorectal cancer by affecting immune system. Int Rev Immunol, 1–20, doi: 10.1080/08830185.2021.1954638 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Perez-Castro L, Garcia R, Venkateswaran N, Barnes S & Conacci-Sorrell M Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J, doi: 10.1111/febs.16245 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platten M, Wick W & Van den Eynde BJ Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res 72, 5435–5440, doi: 10.1158/0008-5472.CAN-12-0569 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Wei GZ et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2021091118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed DN & Mukhtar H FICZ: A Messenger of Light in Human Skin. J Invest Dermatol 135, 1478–1481, doi: 10.1038/jid.2015.52 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Smirnova A et al. Evidence for New Light-Independent Pathways for Generation of the Endogenous Aryl Hydrocarbon Receptor Agonist FICZ. Chem Res Toxicol 29, 75–86, doi: 10.1021/acs.chemrestox.5b00416 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Roager HM & Licht TR Microbial tryptophan catabolites in health and disease. Nat Commun 9, 3294, doi: 10.1038/s41467-018-05470-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong F et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes 12, 1–24, doi: 10.1080/19490976.2020.1788899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kou Z et al. Oxidative stress modulates expression of immune checkpoint genes via activation of AhR signaling. Toxicol Appl Pharmacol 457, 116314, doi: 10.1016/j.taap.2022.116314 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadik A et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 182, 1252–1270 e1234, doi: 10.1016/j.cell.2020.07.038 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Jalalvand M, Darbeheshti F & Rezaei N Immune checkpoint inhibitors: review of the existing evidence and challenges in breast cancer. Immunotherapy 13, 587–603, doi: 10.2217/imt-2020-0283 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Mellor AL & Munn DH IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4, 762–774, doi: 10.1038/nri1457 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD & Allison JP Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210, 1389–1402, doi: 10.1084/jem.20130066 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, Zhang R, Li S & Liu J IDO1: An important immunotherapy target in cancer treatment. Int Immunopharmacol 47, 70–77, doi: 10.1016/j.intimp.2017.03.024 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Mangaonkar A et al. A novel immunohistochemical score to predict early mortality in acute myeloid leukemia patients based on indoleamine 2,3 dioxygenase expression. Sci Rep 7, 12892, doi: 10.1038/s41598-017-12940-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wainwright DA et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18, 6110–6121, doi: 10.1158/1078-0432.CCR-12-2130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mellor AL & Munn DH Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today 20, 469–473, doi: 10.1016/s0167-5699(99)01520-0 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Munn DH et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642, doi: 10.1016/j.immuni.2005.03.013 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Fallarino F et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 176, 6752–6761, doi: 10.4049/jimmunol.176.11.6752 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Routy JP, Routy B, Graziani GM & Mehraj V The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int J Tryptophan Res 9, 67–77, doi: 10.4137/IJTR.S38355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gagliani N et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225, doi: 10.1038/nature14452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai J, Wang D, Zhang G & Guo X The Role Of PD-1/PD-L1 Axis In Treg Development And Function: Implications For Cancer Immunotherapy. Onco Targets Ther 12, 8437–8445, doi: 10.2147/OTT.S221340 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willsmore ZN et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: treatment of melanoma and immune mechanisms of action. Eur J Immunol, doi: 10.1002/eji.202048747 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Freeman GJ et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192, 1027–1034, doi: 10.1084/jem.192.7.1027 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharpe AH, Wherry EJ, Ahmed R & Freeman GJ The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8, 239–245, doi: 10.1038/ni1443 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Hudson K, Cross N, Jordan-Mahy N & Leyland R The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front Immunol 11, 568931, doi: 10.3389/fimmu.2020.568931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kenison JE et al. The aryl hydrocarbon receptor suppresses immunity to oral squamous cell carcinoma through immune checkpoint regulation. Proc. Natl. Acad. Sci. U. S. A. 118, doi: 10.1073/pnas.2012692118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen G et al. Icariside I - A novel inhibitor of the kynurenine-AhR pathway with potential for cancer therapy by blocking tumor immune escape. Biomed Pharmacother 153, 113387, doi: 10.1016/j.biopha.2022.113387 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Wang GZ et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun 10, 1125, doi: 10.1038/s41467-019-08887-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrot-Applanat M et al. Differential Expression of Genes Involved in Metabolism and Immune Response in Diffuse and Intestinal Gastric Cancers, a Pilot Ptudy. Biomedicines 10, doi: 10.3390/biomedicines10020240 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyazaki T et al. Stemness and immune evasion conferred by the TDO2-AHR pathway are associated with liver metastasis of colon cancer. Cancer Sci 113, 170–181, doi: 10.1111/cas.15182 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao Y et al. What is the prospect of indoleamine 2,3-dioxygenase 1 inhibition in cancer? Extrapolation from the past. J Exp Clin Cancer Res 40, 60, doi: 10.1186/s13046-021-01847-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pizzino G et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017, 8416763, doi: 10.1155/2017/8416763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panich U, Sittithumcharee G, Rathviboon N & Jirawatnotai S Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int 2016, 7370642, doi: 10.1155/2016/7370642 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valavanidis A, Vlachogianni T & Fiotakis K Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 6, 445–462, doi: 10.3390/ijerph6020445 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iqbal J et al. Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc Natl Acad Sci U S A 110, 11115–11120, doi: 10.1073/pnas.1220919110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grishanova AY & Perepechaeva ML Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int J Mol Sci 23, doi: 10.3390/ijms23126719 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogel CFA, Van Winkle LS, Esser C & Haarmann-Stemmann T The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol 34, 101530, doi: 10.1016/j.redox.2020.101530 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reichard JF, Dalton TP, Shertzer HG & Puga A Induction of oxidative stress responses by dioxin and other ligands of the aryl hydrocarbon receptor. Dose Response 3, 306–331, doi: 10.2203/dose-response.003.03.003 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugihara K et al. Aryl hydrocarbon receptor (AhR)-mediated induction of xanthine oxidase/xanthine dehydrogenase activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Biophys Res Commun 281, 1093–1099, doi: 10.1006/bbrc.2001.4464 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Dostalek M et al. Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J Biol Chem 283, 17147–17157, doi: 10.1074/jbc.M802447200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou D, Shao L & Spitz DR Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res 122, 1–67, doi: 10.1016/B978-0-12-420117-0.00001-3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senft AP et al. Dioxin increases reactive oxygen production in mouse liver mitochondria. Toxicol Appl Pharmacol 178, 15–21, doi: 10.1006/taap.2001.9314 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Senft AP et al. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med 33, 1268–1278, doi: 10.1016/s0891-5849(02)01014-6 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Hwang HJ et al. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome. Toxicol Appl Pharmacol 304, 121–132, doi: 10.1016/j.taap.2016.04.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kubli SP et al. AhR controls redox homeostasis and shapes the tumor microenvironment in BRCA1-associated breast cancer. Proc Natl Acad Sci U S A 116, 3604–3613, doi: 10.1073/pnas.1815126116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnett GV et al. Probing the Tryptophan Environment in Therapeutic Proteins: Implications for Higher Order Structure on Tryptophan Oxidation. J Pharm Sci 108, 1944–1952, doi: 10.1016/j.xphs.2018.12.027 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Peng Y et al. AhR Promotes the Development of Non-small cell lung cancer by Inducing SLC7A11-dependent Antioxidant Function. J Cancer 14, 821–834, doi: 10.7150/jca.82066 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirschhorn T & Stockwell BR The development of the concept of ferroptosis. Free Radic Biol Med 133, 130–143, doi: 10.1016/j.freeradbiomed.2018.09.043 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y & Gu W p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ 29, 895–910, doi: 10.1038/s41418-022-00943-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Fan Z, Yang Y & Gu C Iron metabolism and its contribution to cancer (Review). Int J Oncol 54, 1143–1154, doi: 10.3892/ijo.2019.4720 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Hu W et al. Ferroptosis and Its Role in Chronic Diseases. Cells 11, doi: 10.3390/cells11132040 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu R, Li X & Zhao G Beclin1-mediated ferroptosis activation is associated with isoflurane-induced toxicity in SH-SY5Y neuroblastoma cells. Acta Biochim Biophys Sin (Shanghai) 51, 1134–1141, doi: 10.1093/abbs/gmz104 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Song X et al. AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System Xc(−) Activity. Curr Biol 28, 2388–2399 e2385, doi: 10.1016/j.cub.2018.05.094 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miao W, Hu L, Scrivens PJ & Batist G Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem 280, 20340–20348, doi: 10.1074/jbc.M412081200 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Gao S et al. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem Toxicol 59, 739–747, doi: 10.1016/j.fct.2013.07.032 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Zeitler L et al. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. Elife 10, doi: 10.7554/eLife.64806 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fiore A et al. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell 82, 920–932 e927, doi: 10.1016/j.molcel.2022.02.007 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaishankar M, Tseten T, Anbalagan N, Mathew BB & Beeregowda KN Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7, 60–72, doi: 10.2478/intox-2014-0009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koedrith P & Seo YR Advances in carcinogenic metal toxicity and potential molecular markers. Int J Mol Sci 12, 9576–9595, doi: 10.3390/ijms12129576 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi H, Shi X & Liu KJ Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255, 67–78, doi: 10.1023/b:mcbi.0000007262.26044.e8 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Flora SJ, Bhadauria S, Kannan GM & Singh N Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol 28, 333–347 (2007). [PubMed] [Google Scholar]
- 90.Jomova K et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31, 95–107, doi: 10.1002/jat.1649 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Salnikow K & Zhitkovich A Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol 21, 28–44, doi: 10.1021/tx700198a (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ovesen JL et al. Long-term exposure to low-concentrations of Cr(VI) induce DNA damage and disrupt the transcriptional response to benzo[a]pyrene. Toxicology 316, 14–24, doi:S0300-483X(13)00326-0 [pii] 10.1016/j.tox.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez-Martin FJ et al. Long-term Coexposure to Hexavalent Chromium and B[a]P Causes Tissue-Specific Differential Biological Effects in Liver and Gastrointestinal Tract of Mice. Toxicol Sci 146, 52–64, doi:kfv070 [pii] 10.1093/toxsci/kfv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen L, Ovesen JL, Puga A & Xia Y Distinct contributions of JNK and p38 to chromium cytotoxicity and inhibition of murine embryonic stem cell differentiation. Environ Health Perspect 117, 1124–1130, doi: 10.1289/ehp.0800157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan Y, Ovesen JL & Puga A Long-term exposure to hexavalent chromium inhibits expression of tumor suppressor genes in cultured cells and in mice. J Trace Elem Med Biol 26, 188–191, doi:S0946-672X(12)00063-6 [pii] 10.1016/j.jtemb.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borthiry GR, Antholine WE, Kalyanaraman B, Myers JM & Myers CR Reduction of hexavalent chromium by human cytochrome b5: generation of hydroxyl radical and superoxide. Free Radic Biol Med 42, 738–755; discussion 735–737, doi: 10.1016/j.freeradbiomed.2006.10.055 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Afonso V, Champy R, Mitrovic D, Collin P & Lomri A Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 74, 324–329, doi: 10.1016/j.jbspin.2007.02.002 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Wang Z & Yang C Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis. Semin Cancer Biol 57, 95–104, doi: 10.1016/j.semcancer.2019.01.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beyersmann D & Hartwig A Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 82, 493–512, doi: 10.1007/s00204-008-0313-y (2008). [DOI] [PubMed] [Google Scholar]
- 100.Price DJ & Joshi JG Ferritin. Binding of beryllium and other divalent metal ions. J Biol Chem 258, 10873–10880 (1983). [PubMed] [Google Scholar]
- 101.Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN & Tsatsakis AM Lead toxicity update. A brief review. Med Sci Monit 11, RA329–336 (2005). [PubMed] [Google Scholar]
- 102.Abdel-Warith AA, Younis EMI, Al-Asgah NA, Rady AM & Allam HY Bioaccumulation of lead nitrate in tissues and its effects on hematological and biochemical parameters of Clarias gariepinus. Saudi J Biol Sci 27, 840–845, doi: 10.1016/j.sjbs.2020.01.015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Timbrell JA Principles of biochemical toxicology. (CRC press, 2008). [Google Scholar]
- 104.Dasharathy S et al. Mutagenic, Carcinogenic, and Teratogenic Effect of Heavy Metals. Evid Based Complement Alternat Med 2022, 8011953, doi: 10.1155/2022/8011953 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Ghiaty MA, Alqahtani MA & El-Kadi AOS Arsenic trioxide (ATO) up-regulates cytochrome P450 1A (CYP1A) enzymes in murine hepatoma Hepa-1c1c7 cell line. Environ Toxicol Pharmacol 101, 104214, doi: 10.1016/j.etap.2023.104214 (2023). [DOI] [PubMed] [Google Scholar]
- 106.Elbekai RH & El-Kadi AO Modulation of aryl hydrocarbon receptor-regulated gene expression by arsenite, cadmium, and chromium. Toxicology 202, 249–269, doi: 10.1016/j.tox.2004.05.009 (2004). [DOI] [PubMed] [Google Scholar]
- 107.Kann S et al. Arsenite-induced aryl hydrocarbon receptor nuclear translocation results in additive induction of phase I genes and synergistic induction of phase II genes. Mol Pharmacol 68, 336–346, doi: 10.1124/mol.105.011841 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Albores A et al. Sodium arsenite induced alterations in bilirubin excretion and heme metabolism. J Biochem Toxicol 4, 73–78, doi: 10.1002/jbt.2570040202 (1989). [DOI] [PubMed] [Google Scholar]
- 109.Seubert JM, Sinal CJ & Bend JR Acute sodium arsenite administration induces pulmonary CYP1A1 mRNA, protein and activity in the rat. J Biochem Mol Toxicol 16, 84–95, doi: 10.1002/jbt.10022 (2002). [DOI] [PubMed] [Google Scholar]
- 110.Schaldach CM, Riby J & Bjeldanes LF Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry 38, 7594–7600, doi: 10.1021/bi982861e (1999). [DOI] [PubMed] [Google Scholar]
- 111.Wise JP Jr., Young JL, Cai J & Cai L Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ Int 158, 106877, doi: 10.1016/j.envint.2021.106877 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Branicky R, Noe A & Hekimi S Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217, 1915–1928, doi: 10.1083/jcb.201708007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niture S et al. Role of Autophagy in Cadmium-Induced Hepatotoxicity and Liver Diseases. J Toxicol 2021, 9564297, doi: 10.1155/2021/9564297 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seif MM, Madboli AN, Marrez DA & Aboulthana WMK Hepato-Renal protective Effects of Egyptian Purslane Extract against Experimental Cadmium Toxicity in Rats with Special Emphasis on the Functional and Histopathological Changes. Toxicol Rep 6, 625–631, doi: 10.1016/j.toxrep.2019.06.013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alexidis AN, Rekka EA & Kourounakis PN Influence of mercury and cadmium intoxication on hepatic microsomal CYP2E and CYP3A subfamilies. Res Commun Mol Pathol Pharmacol 85, 67–72 (1994). [PubMed] [Google Scholar]
- 116.Anjum F, Raman A, Shakoori AR & Gorrod JW An assessment of cadmium toxicity on cytochrome P-450 and flavin monooxygenase-mediated metabolic pathways of dimethylaniline in male rabbits. J Environ Pathol Toxicol Oncol 11, 191–195 (1992). [PubMed] [Google Scholar]
- 117.Virgolini MB & Aschner M Molecular Mechanisms of Lead Neurotoxicity. Adv Neurotoxicol 5, 159–213, doi: 10.1016/bs.ant.2020.11.002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Attafi IM, Bakheet SA & Korashy HM The role of NF-kappaB and AhR transcription factors in lead-induced lung toxicity in human lung cancer A549 cells. Toxicol Mech Methods 30, 197–207, doi: 10.1080/15376516.2019.1687629 (2020). [DOI] [PubMed] [Google Scholar]
- 119.Korashy HM & El-Kadi AO Regulatory mechanisms modulating the expression of cytochrome P450 1A1 gene by heavy metals. Toxicol Sci 88, 39–51, doi: 10.1093/toxsci/kfi282 (2005). [DOI] [PubMed] [Google Scholar]
- 120.Attafi IM et al. Lead Nitrate Induces Inflammation and Apoptosis in Rat Lungs Through the Activation of NF-kappaB and AhR Signaling Pathways. Environ Sci Pollut Res Int 29, 64959–64970, doi: 10.1007/s11356-022-19980-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ansari MA, Maayah ZH, Bakheet SA, El-Kadi AO & Korashy HM The role of aryl hydrocarbon receptor signaling pathway in cardiotoxicity of acute lead intoxication in vivo and in vitro rat model. Toxicology 306, 40–49, doi: 10.1016/j.tox.2013.01.024 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Korashy HM & El-Kadi AO The role of redox-sensitive transcription factors NF-kappaB and AP-1 in the modulation of the Cyp1a1 gene by mercury, lead, and copper. Free Radic Biol Med 44, 795–806, doi: 10.1016/j.freeradbiomed.2007.11.003 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Hultman P & Michael Pollard K in Handbook on the Toxicology of Metals (Fourth Edition) (eds Nordberg Gunnar F., Fowler Bruce A., & Nordberg Monica) 379–398 (Academic Press, 2015). [Google Scholar]
- 124.Popov Aleksandrov A et al. Immunomodulation by heavy metals as a contributing factor to inflammatory diseases and autoimmune reactions: Cadmium as an example. Immunol Lett 240, 106–122, doi: 10.1016/j.imlet.2021.10.003 (2021). [DOI] [PubMed] [Google Scholar]
- 125.Lemarie A et al. Arsenic trioxide induces apoptosis of human monocytes during macrophagic differentiation through nuclear factor-kappaB-related survival pathway down-regulation. J Pharmacol Exp Ther 316, 304–314, doi: 10.1124/jpet.105.092874 (2006). [DOI] [PubMed] [Google Scholar]
- 126.Bellamri N, Morzadec C, Fardel O & Vernhet L Arsenic and the immune system. Current Opinion in Toxicology 10, 60–68, doi: 10.1016/j.cotox.2018.01.003 (2018). [DOI] [Google Scholar]
- 127.Dangleben NL, Skibola CF & Smith MT Arsenic immunotoxicity: a review. Environ Health 12, 73, doi: 10.1186/1476-069X-12-73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Biswas R et al. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum Exp Toxicol 27, 381–386, doi: 10.1177/0960327108094607 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Li J et al. Imbalanced immune responses involving inflammatory molecules and immune-related pathways in the lung of acute and subchronic arsenic-exposed mice. Environ Res 159, 381–393, doi: 10.1016/j.envres.2017.08.036 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Yucesoy B, Turhan A, Ure M, Imir T & Karakaya A Effects of occupational lead and cadmium exposure on some immunoregulatory cytokine levels in man. Toxicology 123, 143–147, doi: 10.1016/s0300-483x(97)00107-8 (1997). [DOI] [PubMed] [Google Scholar]
- 131.Iavicoli I, Carelli G, Stanek EJ 3rd, Castellino N & Calabrese EJ Below background levels of blood lead impact cytokine levels in male and female mice. Toxicol Appl Pharmacol 210, 94–99, doi: 10.1016/j.taap.2005.09.016 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Flohe SB, Bruggemann J, Herder C, Goebel C & Kolb H Enhanced proinflammatory response to endotoxin after priming of macrophages with lead ions. J Leukoc Biol 71, 417–424 (2002). [PubMed] [Google Scholar]
- 133.Valentino M et al. Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum Exp Toxicol 26, 551–556, doi: 10.1177/0960327107073817 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Fischbein A et al. Phenotypic aberrations of CD3+ and CD4+ cells and functional impairments of lymphocytes at low-level occupational exposure to lead. Clin Immunol Immunopathol 66, 163–168, doi: 10.1006/clin.1993.1020 (1993). [DOI] [PubMed] [Google Scholar]
- 135.Mishra KP, Rani R, Yadav VS & Naik S Effect of lead exposure on lymphocyte subsets and activation markers. Immunopharmacol Immunotoxicol 32, 446–449, doi: 10.3109/08923970903503668 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Mener DJ et al. Lead exposure and increased food allergic sensitization in U.S. children and adults. Int Forum Allergy Rhinol 5, 214–220, doi: 10.1002/alr.21460 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Mishra KP Lead exposure and its impact on immune system: a review. Toxicol In Vitro 23, 969–972, doi: 10.1016/j.tiv.2009.06.014 (2009). [DOI] [PubMed] [Google Scholar]
- 138.Phuagkhaopong S et al. Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-kappaB pathways. Neurotoxicology 60, 82–91, doi: 10.1016/j.neuro.2017.03.001 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Olszowski T, Baranowska-Bosiacka I, Gutowska I & Chlubek D Pro-inflammatory properties of cadmium. Acta Biochim Pol 59, 475–482 (2012). [PubMed] [Google Scholar]
- 140.Kulas J et al. Aryl hydrocarbon receptor is involved in the proinflammatory cytokine response to cadmium. Biomedical and Environmental Sciences 34, 192–202 (2021). [DOI] [PubMed] [Google Scholar]
- 141.Wirthgen E & Hoeflich A Endotoxin-Induced Tryptophan Degradation along the Kynurenine Pathway: The Role of Indolamine 2,3-Dioxygenase and Aryl Hydrocarbon Receptor-Mediated Immunosuppressive Effects in Endotoxin Tolerance and Cancer and Its Implications for Immunoparalysis. J Amino Acids 2015, 973548, doi: 10.1155/2015/973548 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yin H, Xu L & Porter NA Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 111, 5944–5972, doi: 10.1021/cr200084z (2011). [DOI] [PubMed] [Google Scholar]
- 143.Higdon A, Diers AR, Oh JY, Landar A & Darley-Usmar VM Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J 442, 453–464, doi: 10.1042/BJ20111752 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aschner M et al. Ferroptosis as a mechanism of non-ferrous metal toxicity. Arch Toxicol 96, 2391–2417, doi: 10.1007/s00204-022-03317-y (2022). [DOI] [PubMed] [Google Scholar]
- 145.Meng P et al. Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol Environ Saf 194, 110360, doi: 10.1016/j.ecoenv.2020.110360 (2020). [DOI] [PubMed] [Google Scholar]
- 146.Tang Q et al. Ferroptosis is newly characterized form of neuronal cell death in response to arsenite exposure. Neurotoxicology 67, 27–36, doi: 10.1016/j.neuro.2018.04.012 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Wei S et al. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. Journal of Hazardous Materials 384, 121390, doi: 10.1016/j.jhazmat.2019.121390 (2020). [DOI] [PubMed] [Google Scholar]
- 148.Wei S et al. Ferroptosis mediated by the interaction between Mfn2 and IREalpha promotes arsenic-induced nonalcoholic steatohepatitis. Environ Res 188, 109824, doi: 10.1016/j.envres.2020.109824 (2020). [DOI] [PubMed] [Google Scholar]
- 149.He Z et al. Cadmium induces liver dysfunction and ferroptosis through the endoplasmic stress-ferritinophagy axis. Ecotoxicology and Environmental Safety 245, 114123, doi: 10.1016/j.ecoenv.2022.114123 (2022). [DOI] [PubMed] [Google Scholar]
- 150.Hao R et al. Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ Toxicol 37, 41–51, doi: 10.1002/tox.23376 (2022). [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y et al. LncRNA OIP5-AS1 inhibits ferroptosis in prostate cancer with long-term cadmium exposure through miR-128-3p/SLC7A11 signaling. Ecotoxicol Environ Saf 220, 112376, doi: 10.1016/j.ecoenv.2021.112376 (2021). [DOI] [PubMed] [Google Scholar]
- 152.Wang Y, Ma Y & Jiang K The role of ferroptosis in prostate cancer: a novel therapeutic strategy. Prostate Cancer Prostatic Dis 26, 25–29, doi: 10.1038/s41391-022-00583-w (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shi F et al. Pb induces ferroptosis in choroid plexus epithelial cells via Fe metabolism. Neurotoxicology 95, 107–116, doi: 10.1016/j.neuro.2023.01.005 (2023). [DOI] [PubMed] [Google Scholar]
- 154.Wang W et al. MiR-378a-3p/SLC7A11 regulate ferroptosis in nerve injury induced by lead exposure. Ecotoxicol Environ Saf 239, 113639, doi: 10.1016/j.ecoenv.2022.113639 (2022). [DOI] [PubMed] [Google Scholar]
- 155.Fu Y et al. Disruption of the tumor suppressor-like activity of aryl hydrocarbon receptor by arsenic in epithelial cells and human lung cancer. Int J Biol Sci 19, 1983–2001, doi: 10.7150/ijbs.81423 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tyrrell R Redox regulation and oxidant activation of heme oxygenase-1. Free Radic Res 31, 335–340, doi: 10.1080/10715769900300901 (1999). [DOI] [PubMed] [Google Scholar]


