Abstract
Background
Funders and scientific journals use peer review to decide which projects to fund or articles to publish. Reviewer training is an intervention to improve the quality of peer review. However, studies on the effects of such training yield inconsistent results, and there are no up‐to‐date systematic reviews addressing this question.
Objectives
To evaluate the effect of peer reviewer training on the quality of grant and journal peer review.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 27 April 2022.
Selection criteria
We included randomized controlled trials (RCTs; including cluster‐RCTs) that evaluated peer review with training interventions versus usual processes, no training interventions, or other interventions to improve the quality of peer review.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. completeness of reporting and 2. peer review detection of errors. Our secondary outcomes were 1. bibliometric scores, 2. stakeholders' assessment of peer review quality, 3. inter‐reviewer agreement, 4. process‐centred outcomes, 5. peer reviewer satisfaction, and 6. completion rate and speed of funded projects. We used the first version of the Cochrane risk of bias tool to assess the risk of bias, and we used GRADE to assess the certainty of evidence.
Main results
We included 10 RCTs with a total of 1213 units of analysis. The unit of analysis was the individual reviewer in seven studies (722 reviewers in total), and the reviewed manuscript in three studies (491 manuscripts in total).
In eight RCTs, participants were journal peer reviewers. In two studies, the participants were grant peer reviewers. The training interventions can be broadly divided into dialogue‐based interventions (interactive workshop, face‐to‐face training, mentoring) and one‐way communication (written information, video course, checklist, written feedback). Most studies were small.
We found moderate‐certainty evidence that emails reminding peer reviewers to check items of reporting checklists, compared with standard journal practice, have little or no effect on the completeness of reporting, measured as the proportion of items (from 0.00 to 1.00) that were adequately reported (mean difference (MD) 0.02, 95% confidence interval (CI) −0.02 to 0.06; 2 RCTs, 421 manuscripts).
There was low‐certainty evidence that reviewer training, compared with standard journal practice, slightly improves peer reviewer ability to detect errors (MD 0.55, 95% CI 0.20 to 0.90; 1 RCT, 418 reviewers).
We found low‐certainty evidence that reviewer training, compared with standard journal practice, has little or no effect on stakeholders' assessment of review quality in journal peer review (standardized mean difference (SMD) 0.13 standard deviations (SDs), 95% CI −0.07 to 0.33; 1 RCT, 418 reviewers), or change in stakeholders' assessment of review quality in journal peer review (SMD −0.15 SDs, 95% CI −0.39 to 0.10; 5 RCTs, 258 reviewers).
We found very low‐certainty evidence that a video course, compared with no video course, has little or no effect on inter‐reviewer agreement in grant peer review (MD 0.14 points, 95% CI −0.07 to 0.35; 1 RCT, 75 reviewers).
There was low‐certainty evidence that structured individual feedback on scoring, compared with general information on scoring, has little or no effect on the change in inter‐reviewer agreement in grant peer review (MD 0.18 points, 95% CI −0.14 to 0.50; 1 RCT, 41 reviewers, low‐certainty evidence).
Authors' conclusions
Evidence from 10 RCTs suggests that training peer reviewers may lead to little or no improvement in the quality of peer review. There is a need for studies with more participants and a broader spectrum of valid and reliable outcome measures. Studies evaluating stakeholders' assessments of the quality of peer review should ensure that these instruments have sufficient levels of validity and reliability.
Keywords: Humans; Bias; Checklist; Peer Review; Peer Review, Research; Publishing; Reproducibility of Results
Plain language summary
What are the benefits of training peer reviewers?
Key messages
• Training peer reviewers may have little or no effect on the quality of peer review. • Larger, well‐designed studies are needed to give better estimates of the effect.
What is a peer reviewer?
A peer reviewer is a person who evaluates the research work done by another person. The peer reviewer is usually a researcher with skills similar to those needed to conduct the research they evaluate.
What is peer review used for?
Both funders and publishers of research can be uncertain if a research project or report is of good quality. Many use peer reviewers to evaluate the quality of a project or report.
How could peer review quality be improved by training reviewers?
Training peer reviewers might make them better at identifying strengths and weaknesses in the research they assess.
What did we want to find out?
We wanted to find out if training peer reviewers increased the quality of their work.
What did we do?
We searched for studies that looked at training for peer reviewers compared with no training, different types of training, or standard journal or funder practice. We extracted information and summarized the results of all the relevant studies. We rated our confidence in the evidence based on factors such as study methods and sizes.
What did we find?
We found 10 studies that involved a total of 1213 units of analysis (722 reviewers and 491 manuscripts). Eight studies included only journal peer reviewers. The remaining two studies included grant peer reviewers.
Main results
Emails reminding peer reviewers to check items of reporting checklists, compared with standard journal practice, probably have little or no effect on the completeness of reporting (evidence from 2 studies with 421 manuscripts).
Reviewer training, compared with standard journal practice, may slightly improve peer reviewer ability to detect errors (evidence from 1 study with 418 reviewers). The reviewers who received training identified 3.25 out of 9 errors on average, whereas the reviewers who received no training identified 2.7 out of 9 errors on average.
Reviewer training, compared with standard journal practice, may have little or no effect on stakeholders' assessment of review quality (evidence from 6 studies with 616 reviewers and 60 manuscripts).
We are unsure about the effect of a video course, compared with no video course, on agreement between reviewers (evidence from 1 study with 75 reviewers).
Structured individual feedback on scoring, compared with general information on scoring, may have little or no effect on the change in agreement between reviewers (evidence from 1 study with 41 reviewers).
What are the limitations of the evidence?
We have little confidence in most of the evidence because most studies lacked important information and included too few reviewers. Additionally, it is unclear whether the studies measured peer review quality in a valid and reliable way.
How up to date is this evidence?
The evidence is up to date to April 2022.
Summary of findings
Summary of findings 1. Summary of findings. Peer reviewer training versus standard journal practice or no training.
| Population: peer reviewers Settings: journal or grant peer review Intervention: training Comparison: standard journal practice or no training | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | SMD† (95% CI) | No of reviewers/ manuscripts (studies) | Certainty of evidence (GRADE) | Comments | |
| No training | Training | |||||
|
Completeness of reporting Proportion of items adequately reported, from 0.00 to 1.00, higher number indicates more complete reporting. |
The mean proportion of adequately reported items was 0.58. | The MD was 0.02 more (0.02 less to 0.06 more). | — | 421 manuscripts (2 RCTs) | ⊕⊕⊕⊝ Moderatea |
Reminding peer reviewers to check items of reporting checklists, compared with standard journal practice, probably has little or no effect on completeness of reporting. |
|
Peer reviewer detection of errors Number of major errors identified, assessed by 2 researchers independently, from 0 to 9, higher number indicates better detection. |
The mean number of errors identified was 2.7. | The MD was 0.55 errors more (0.20 more to 0.90 more). | — | 418 reviewers (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
Peer reviewer training, compared with standard journal practice, may slightly improve numbers of major errors identified. |
|
Stakeholders' assessment of review quality (final score) Assessed by 2 editors independently using the RQI from 1 to 5 (average of the 2 ratings), higher scores indicate higher quality. |
The mean final review quality score was 2.74. | — | SMD 0.13 SDs (−0.07 to 0.33) | 418 reviewers (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
Peer reviewer training, compared with standard journal practice, may have little or no effect on stakeholders' assessment of review quality. |
|
Stakeholders' assessment of review quality (change score) Assessed using either: A) a 5‐point scale, change scores range from −4 to 4 (3 studies); B) a 5‐point scale, expressed by "slope of quality score change"(1 study); or C) the MQAI, from −144 to 144 (1 study). In all cases, higher scores indicate increased review quality. |
The mean change in review quality was: A) 0.09 points. B) −0.23 points. C) 4.87 points. |
— | SMD −0.15 SDs (−0.39 to 0.10) | 258 reviewers (5 RCTs) | ⊕⊕⊝⊝ Lowa,c |
Peer reviewer training, compared with standard journal practice, may have little or no effect on the change in stakeholders' assessment of review quality. |
|
Inter‐reviewer agreement (final score) Assessed using the average deviation between reviewers, from −4 to 0, higher scores indicate higher agreement. |
The mean average deviation was −1.03 points. | The MD was 0.14 points higher (0.07 lower to 0.35 higher). | — | 75 reviewers (1 RCT) | ⊕⊝⊝⊝ Very lowd,e |
We are uncertain about the effect of a video course, compared with no video course, on agreement between reviewers, assessed using the average deviation between reviewers. |
|
Inter‐reviewer agreement (change score) Assessed using the average score difference between reviewers, possible changes scores range from −9 to 9, higher scores indicate increased agreement. |
The mean change in average score difference was 0.16 points. | The MD was 0.18 points higher (0.14 lower to 0.50 higher). | — | 41 reviewers (1 RCT) | ⊕⊕⊝⊝ Lowd |
Structured individual feedback on scoring, compared with general information on scoring, may have little or no effect on the change in agreement between reviewers. |
| Bibliometric scores | — | — | — | — | — | No studies reported this outcome. |
| * The effect in the intervention group (and its 95% CI) is based on the assumed effect in the comparison group and the relative effect of the intervention (and its 95% CI).
† SMD is used as a summary statistic in meta‐analysis when the studies all assess the same outcome but measure it in a variety of ways. For interpretation of the SMD, 0.2 is considered a small effect, 0.5 a moderate effect, and 0.8 a large effect. CI: confidence interval; MD: Mean difference; MQAI: Manuscript Quality Assessment Instrument; RQI: Review Quality Instrument; SD: standard deviation; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for imprecision: wide CI exceeds (or likely exceeds) threshold for both no effect and an important effect. b Downgraded one level for risk of bias: high risk of bias due to lack of blinding of participants and personnel, incomplete outcome data, and unclear allocation concealment. c Downgraded one level for risk of bias: unclear risk of bias due to selective outcome reporting in all five studies, unclear or high risk of bias due to incomplete outcome data in three studies, unclear allocation concealment in three studies, and unclear blinding in two studies. d Downgraded two levels for imprecision: limited number of participants, and wide CI exceeds (or likely exceeds) threshold for both no effect and an important effect. e Downgraded one level for indirectness: participants were not only reviewers, and the intervention was limited to interpreting the review scale; and one level for risk of bias: unclear allocation concealment, sequence generation, blinding, completeness of outcome data, and selective outcome reporting.
Background
Both research funders and scientific journals use peer review to decide which projects to fund or articles to publish, and to review methods and improve reporting of journal articles. Most funders and journals have a set of criteria and specific instructions for reviewing project proposals or articles. Some funders and journals train their reviewers to increase compliance with the defined review criteria. Training can involve various interventions and methods, including (but not limited to) training sessions, courses, handbooks, written instructions, feedback, and guidance. The primary goal of training is to improve the quality of peer review.
There are different outcome measures for evaluating the quality of peer review. These include how well the research project or article performs in terms of different bibliometrics, stakeholders' evaluation of peer reviews, agreement between reviewers, and authors' adherence to guidelines.
Several studies have shown that both grant and journal peer reviewers perform suboptimally on several important outcome measures (Bornmann 2011; Guthrie 2017). Reviewer training is aimed at improving these outcomes, but studies on the effects of training have yielded inconsistent results (Bruce 2016).
Description of the methods being investigated
According to Elsevier, one of the world's largest scholarly publishers, "Reviewers evaluate article submissions to journals based on the requirements of that journal, predefined criteria, and the quality, completeness and accuracy of the research presented. They provide feedback on the paper, suggest improvements and make a recommendation to the editor about whether to accept, reject or request changes to the article" (Elsevier 2023).
Both funders and journals usually have a set of formal documents describing the aims and the scope of their work (e.g. a mission statement), the specific criteria that funding applications and articles should meet (e.g. guidelines, call for proposals), and details of the scales used for scoring and how the criteria should be scored (e.g. peer reviewer scoring instructions). Peer reviewer training should address the aims of the funder or journal, the peer review criteria, and the scoring instructions, with the goal of improving adherence to these measures and thus peer review quality.
Surveys of peer reviewers show that they receive little formal training in peer review, that they would like more guidance, and that they think it would improve the quality of the peer review (Mulligan 2013; Sense About Science 2009; Warne 2016). Training can be delivered in various ways. Some funders and journals use passive training strategies, such as guidelines, written instructions, handbooks, or videos; while others use more active strategies, such as live training sessions, online courses, or mentoring. Most strategies are implemented before the peer review, but there are also post‐review training strategies, such as feedback or evaluations to train peer reviewers for their next peer review session.
How these methods might work
Peer reviewer training might improve the peer review process through several mechanisms.
Research has shown that peer reviewers' interpretation and weighting of criteria can vary (Abdoul 2012), and the tasks considered critical by peer reviewers (e.g. evaluating risk of bias) are often incongruent with the tasks requested by the funding programme officers and journal editors (e.g. providing recommendations for publication; Chauvin 2015). Training could help to reduce these discrepancies, which otherwise might lower the inter‐rater and intra‐rater reliability of peer review.
Human judgement and decision‐making processes are susceptible to cognitive biases, and research suggests this might also be the case in grant and journal peer review (Bornmann 2006; Gallo 2018; Langfeldt 2006; Lee 2013; Walker 2015). Studies have found that the following factors influence reviews or similar assessments.
Social influence: the influence of one reviewer's assessment on another (Lane 2020; Teplitskiy 2019)
Matthew effect or status bias: the tendency for signals of status in a manuscript or grant proposal (such as prestigious affiliations or well‐known author names) to affect the overall assessment of the manuscript or proposal (Bol 2018; Huber 2022)
Similarity bias or cognitive distance: when the degree of similarity between authors and reviewers affects the outcome of the review (Wang 2015)
Anchor effects: the tendency for assessments to be influenced too heavily by the first piece of information presented, such as the review score that happens to be presented at the start of a group discussion (Roumbanis 2017)
Sequence effects: the influence of the order in which items are reviewed on different assessment settings (Bhargava 2014; Danziger 2011; Olbrecht 2010; Sattler 2015).
Educating peer reviewers about the risk of biases and how to avoid them might reduce these effects.
Furthermore, in some instances, peer review is conducted in groups, and social psychological phenomena such as groupthink, group polarization, or bandwagon effects might influence decisions, particularly in panel discussions and applicant interviews associated with funding decisions (Olbrecht 2010). Showing peer reviewers how to avoid these pitfalls might increase the reliability and validity of peer review.
Why it is important to do this review
Scholarly peer review is an undertaking of massive proportions. A total of 1,714,780 new articles, originating from 5282 journal titles, were indexed in 2022 in MEDLINE alone (National Library of Medicine 2022). One 2015 publication estimated that there were about 34,550 scholarly peer‐reviewed journals in the world, with a combined annual production of approximately 2.5 million articles (Ware 2015). The editorial decision to publish or reject manuscripts submitted to these journals relies heavily on peer review and has significant consequences for both researchers and research output. Researchers are likely to be evaluated on how often and in which journals they publish when they apply for jobs or grants. Additionally, research output is affected not only by the peer reviewers' recommendations regarding publication, but also their suggestions regarding revisions to the manuscript, including the methodology of the study.
Worldwide, large funds are distributed through grant application processes in which experts and peers consider whether a project is worthy of support. According to one review, "peer review decisions award an estimated >95% of academic medical research funding" (Guthrie 2017). In the USA, the National Institutes of Health alone invests around USD 45 billion a year in medical research, and more than 84% of this is "awarded for extramural research, largely through almost 50,000 competitive grants" (National Institutes of Health 2022). In the EU, the Horizon Europe programme will grant nearly EUR 96 billion, funding thousands of projects and researchers, and involving thousands of peer reviewers (European Commission 2021). More than 20,000 peer reviewers were involved in the first three years of Horizon Europe's predecessor, Horizon 2020 (European Commission 2018). As with journal peer review, the consequences are significant. Not only does peer review affect the distribution of research funds, but success in grant applications may also affect researchers' future chances of success (Bol 2018).
The literature is inconclusive on the effect of peer reviewer training on the quality of peer review. No systematic reviews have studied the effects of training in grant peer review (Guthrie 2017; Sattler 2015), and the most recent review of studies on reviewer training in journal peer review is current to June 2015 (Bruce 2016). One realist synthesis on what works for peer review in research funding concluded that "Evidence of the broader impact of [...] interventions on the research ecosystem is still needed, and future research should aim to identify processes that consistently work to improve peer review across funders and research contexts" (Recio‐Saucedo 2022).
Grant and journal peer review are very similar processes, often using overlapping criteria (such as methodological quality, impact, and originality). We decided to include both types of peer review in this systematic review to increase power. One Cochrane Methodology Review addresses journal peer review (Jefferson 2007), and another addresses grant peer review (Demicheli 2007). Both focus on peer review as a measure for improving the quality of funded research and study reports, and they both highlight important challenges. As our review focuses on training as a measure to address some of these challenges, we believe it will complement the two existing reviews.
Objectives
To evaluate the effect of peer reviewer training on the quality of grant and journal peer review.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs; including cluster‐RCTs) that evaluated training interventions for peer reviewers versus usual processes, no training interventions, or other interventions to improve the quality of peer review.
Types of data
We included quantitative data on estimated intervention effects, using both continuous and dichotomous variables.
Types of methods
Any intervention aimed at training peer reviewers was eligible. Based on surveys of peer reviewers and previous research on the effects of training, we expected the following interventions to be the most prevalent.
Guidelines, instructions, checklists, and templates
Guidance or mentoring
Feedback and evaluation
Workshops, seminars, and webinars
Self‐administered online/video courses
Interventions could be specific to journals or funders, or could be aimed at any type of peer review. Workshops, seminars, and webinars are usually held in groups. Some of the interventions are more time‐consuming than others. Feedback and evaluation are typically given after or during the peer review process.
Types of outcome measures
Our main outcome was the quality of the review, however measured. Based mainly on one systematic review of interventions to improve journal peer review, we expected to find the outcome measures listed below (Bruce 2016). Reporting of our prespecified outcome measures was not an inclusion criterion of this review. We expected to find both objective and subjective outcome reporting, and we analysed both.
Primary outcomes
Completeness of reporting in articles, based on relevant guidelines such as the CONsolidated Standards for Reporting Trials (CONSORT; Schulz 2010), the Template for Intervention Description and Replication (TIDieR; Hoffmann 2014), and Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT; Chan 2013)
Peer reviewer detection of errors: identification of flaws in manuscripts and proposals
Secondary outcomes
Bibliometric scores, such as citation rates, Altmetrics score, and save rate
Stakeholders' assessment of peer review quality: evaluations of peer reviewers or their reviews by stakeholders, such as grant administration or editors (e.g. scored with instruments like the Review Quality Instrument (RQI; van Rooyen 1999) or the Manuscript Quality Assessment Instrument (MQAI; Goodman 1994))
Inter‐reviewer agreement: degree of agreement between peer reviewers (changes in inter‐rater reliability measures like weighted kappa and intraclass correlation)
Process‐centred outcomes, such as speed or cost of reviewing
Peer reviewer satisfaction with the review process
Completion rate and speed of funded projects (applicable only to grant peer review)
Search methods for identification of studies
We searched for all published and unpublished studies of the effect of peer reviewer training on the quality of peer review, without restrictions on language or publication status. One Information Specialist (HS) developed the search strategy, and another Information Specialist peer reviewed it.
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (Wiley; searched 27 April 2022)
MEDLINE (Ovid; 1946 to 26 April 2022)
Embase (Ovid; 1947 to 26 April 2022)
PsycINFO (Ovid; 1806 to April Week 3 2022)
ERIC (Ovid; 1965 to March 2022)
ProQuest Dissertations & Theses Global (ProQuest; searched 27 April 2022)
CINAHL (EBSCO; 1982 to 7 February 2021)
Web of Science Indexes (SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI; Clarivate; searched 27 April 2022)
OpenGrey (searched 7 February 2021)
We performed all searches on 27 April 2022, except OpenGrey and CINAHL, which we searched in February 2021. Appendix 1 shows the full search strategies.
We also searched the following trials registries for planned and ongoing trials.
U.S. National Institutes of Health Ongoing Trials Registry ClinicalTrials.gov (clinicaltrials.gov/)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; trialsearch.who.int/)
Open Science Framework (OSF; osf.io/search)
Searching other resources
We checked the reference lists of included studies and any relevant systematic reviews for references to relevant trials (Horsley 2011).
We contacted major research funding agencies and researchers who were known or expected to have conducted relevant research to ask them for information on relevant trials.
Data collection and analysis
Selection of studies
An Information Specialist (HS) conducted the searches and removed duplicates using EndNote X9. Three review authors (JOH, IS, TD) were involved in assessing the studies in three steps. First, two review authors independently screened the titles and abstracts of the records and eliminated those that were clearly irrelevant. Second, two review authors read the full‐text articles and study protocols of all potentially eligible records to decide which trials to include. Third, we compared the results from the two independent screenings; in case of disagreements, another review author (AF) assessed the studies. The three authors involved in the discussion made the final decision of whether to include or exclude a study through electronic communication. We recorded the selection process in sufficient detail to create a PRISMA flow diagram (Page 2021).
Two of the review authors (JOH, IS) are authors of one of the included studies (Hesselberg 2021). In this case, two other review authors (AF, TD) screened the article for inclusion.
We used Covidence for the screening process (Covidence 2021).
Data extraction and management
One review author (JOH) extracted general information from the studies. Two of three review authors (JOH, TD, IS) independently extracted the remaining data from each study using the template provided in Covidence (Covidence 2021). Two review authors (JOH, TD) piloted the data extraction form on two studies. In case of disagreements, a review author who was blinded to the details of the disagreement (AF) assessed the studies. The three authors involved in the discussion made the final decision through electronic communication.
We extracted the following study characteristics.
General information: author details, year and language of publication
Methods: study design, study setting, withdrawals, total duration of the study, date of study
Participants: numbers and proportions in each group, peer reviewer experience, field of expertise, gender, language, country of residence
Interventions: detailed description of the intervention, comparison, how the intervention was designed and by whom, delivery format, temporal length of intervention, who delivered the intervention
Outcomes: prespecified primary and secondary outcomes and any other relevant outcomes, time points reported
Notes: study funding, conflicts of interest
For studies that required translation, we would have asked for help from colleagues or from Cochrane Crowd. If this did not resolve the issue, we would have contacted the authors and asked for help to retrieve the relevant information. If all else failed, we would have hired professional translators.
Two of the review authors (JOH, IS) are authors of one of the included studies (Hesselberg 2021). In this case, two other review authors (AF, TD) performed data extraction.
We used the TIDieR checklist to describe the components of the intervention (Hoffmann 2014).
Assessment of risk of bias in included studies
Two of three review authors (JOH, IS, TD) independently assessed the risk of bias of each study using the original Cochrane risk of bias tool (RoB 1; Higgins 2011). We performed the risk of bias assessments in Covidence and transferred the results to Review Manager Web (RevMan Web 2022). When the published report provided insufficient information, we contacted the study authors for clarification. In case of disagreements, a third review author (AF) assessed the risk of bias. The three authors involved in the discussion made the final decision through electronic communication.
Two review authors (JOH, IS) are authors of one of the included studies (Hesselberg 2021). For this study, another two review authors (AF, TD) assessed risk of bias.
We assessed the risk of bias across the following domains.
Random sequence generation and allocation concealment (selection bias)
Blinding of participants and personnel (performance bias) and outcome assessors (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias
We judged each study to be at high, unclear, or low risk of bias in each domain.
Measures of the effect of the methods
We collected all outcomes reported in the studies along with how they were measured (self‐report, chart‐abstraction, other objective primary or secondary outcomes). For included outcomes, we extracted the intervention effect estimate reported by the study authors, along with its confidence interval (CI) and the method of statistical analysis used to calculate it.
When a study reported more than one measure for the same outcome, we used the primary outcome as defined by the study authors. If the primary outcome was not clearly defined, we would have chosen the outcome most similar to approaches used across included trials.
We requested additional information from the study authors if reports did not contain sufficient data (Young 2011).
Continuous outcomes
For continuous outcomes, we analysed data based on the mean, standard deviation (SD), and the number of people assessed in both the intervention and comparison groups to calculate the mean difference (MD) and 95% CI. When different studies reported the same outcome using different scales, we used the standardized mean difference (SMD) with its 95% CI.
Dichotomous outcomes
For dichotomous outcomes, we planned to analyse data based on the number of events and the number of people assessed in the intervention and comparison groups. We would have used these to calculate the risk ratio (RR) and 95% CI.
Unit of analysis issues
Cluster‐randomized trials
We planned to analyse cluster‐randomized trials using the average cluster size and the value of the intraclass correlation coefficient (ICC). We would have followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the trials had not reported ICC estimates, we would have requested them from the trial authors, and if these attempts were unsuccessful, we would have used an estimate from a similar trial. We planned to perform a sensitivity analysis, omitting trials that had not accounted for clustering.
Studies with multiple intervention groups
For studies that reported multiple intervention groups, we included only the groups that were relevant to our review. We excluded or combined arms when conducting meta‐analyses with pairwise comparisons.
Dealing with missing data
We assessed each study for missing data and attrition, and we contacted study authors to request missing data and provide reasons for and characteristics of dropouts (Young 2011).
Assessment of heterogeneity
We assessed statistical heterogeneity by inspecting the forest plot visually for outliers and CI overlap. We considered the presence of outliers and lack of CI overlap as indicators of possible heterogeneity. We used the Chi2 test to assess statistical heterogeneity, with the significance level set at P < 0.10 (Deeks 2017). We also used the I2 statistic and would have interpreted results above 30% as possible heterogeneity (Higgins 2003). We also planned to use subgroup and sensitivity analyses to assess heterogeneity.
Assessment of reporting biases
If any of the meta‐analyses had included 10 or more studies, we would have investigated publication (or other small‐study) bias by visually inspecting funnel plots for skewness. We would have further investigated skewness/asymmetry using Egger's test for continuous outcomes and Harbord's test for dichotomous outcomes (Egger 1997; Harbord 2006). If we detected asymmetry, we would have discussed possible explanations and considered performing sensitivity analyses.
Data synthesis
We meta‐analysed data to provide an overall effect estimate. To the best of our knowledge, there was no evidence to suggest that different training interventions would have markedly different effects. We therefore presumed similar effects for all training interventions, across both grant and journal peer review, and meta‐analysed the data collectively. We used Review Manager Web for data analysis (RevMan Web 2022).
Based on the expected variability in samples and interventions, we used a random‐effects model to incorporate this heterogeneity. For continuous variables, we used the inverse variance method. For dichotomous variables, we used the Mantel‐Haenszel method (Mantel 1959).
When several studies measured the same outcomes but used different tools, we calculated the SMD and 95% CI using the inverse variance method in Review Manager Web (RevMan Web 2022).
GRADE and summary of findings table
We created a summary of findings table for following outcomes.
Completeness of reporting
Peer reviewer detection of errors
Bibliometrics scores
Stakeholders' assessment of review quality
Inter‐reviewer agreement
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Guyatt 2008). We used methods and recommendations described in section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We justified all decisions to downgrade the certainty of the evidence in footnotes to the summary of findings table.
Subgroup analysis and investigation of heterogeneity
If heterogeneity had been above the thresholds described in Assessment of heterogeneity, we may have conducted the following subgroup analyses to investigate possible sources of heterogeneity.
Type of peer review: although several peer review criteria overlap between grants and journals (e.g. methodological quality, originality, and impact), there are also important differences. For example, in grant peer review, the expected feasibility of the study and the merits of the research environment for the proposed research are essential criteria that are not a part of journal peer review. This might result in increased heterogeneity.
Degree of interaction: training interventions that demand more interaction from the peer reviewers are expected to yield better outcomes than those that demand little input. Ideally, time spent in training should be the basis of a subgroup analysis. However, we were doubtful that a sufficient number of studies would have precise measures of this. As an alternative, we would have analysed types of training that are likely to demand different degrees of interaction from the peer reviewers. Training interventions expected to demand a higher degree of interaction are personal mentoring/guidance/feedback/evaluation and seminars/workshops/webinars, whereas training interventions expected to demand a lower degree of interaction are guidelines/written or verbal instructions and checklists/template.
Sensitivity analysis
We would have conducted sensitivity analyses under the following circumstances.
If cluster‐randomized trials had not adjusted for clustering (i.e. we would have excluded data from non‐adjusted cluster‐randomized trials).
If we detected significant heterogeneity (we would have inspected forest plots to determine possible sources).
If some studies were at high risk of selection bias (we would have excluded the studies deemed high risk).
Results
Description of studies
Results of the search
The searches yielded 27,750 records, all identified through searches via databases and registries and none through other sources. After removing 14,422 duplicates, we were left with 13,328 unique records.
After screening titles and abstracts, we excluded 13,305 records and assessed 23 full‐text reports for eligibility. We excluded 14 full‐text reports. Two of the remaining reports each described two studies (Callaham 2002a Study 1; Callaham 2002a Study 2, Callaham 2002b); only one of the studies described in Callaham 2002b was eligible for inclusion in this review. The total number of included studies was 10. Figure 1 illustrates the study selection process in a PRISMA flow chart.
1.
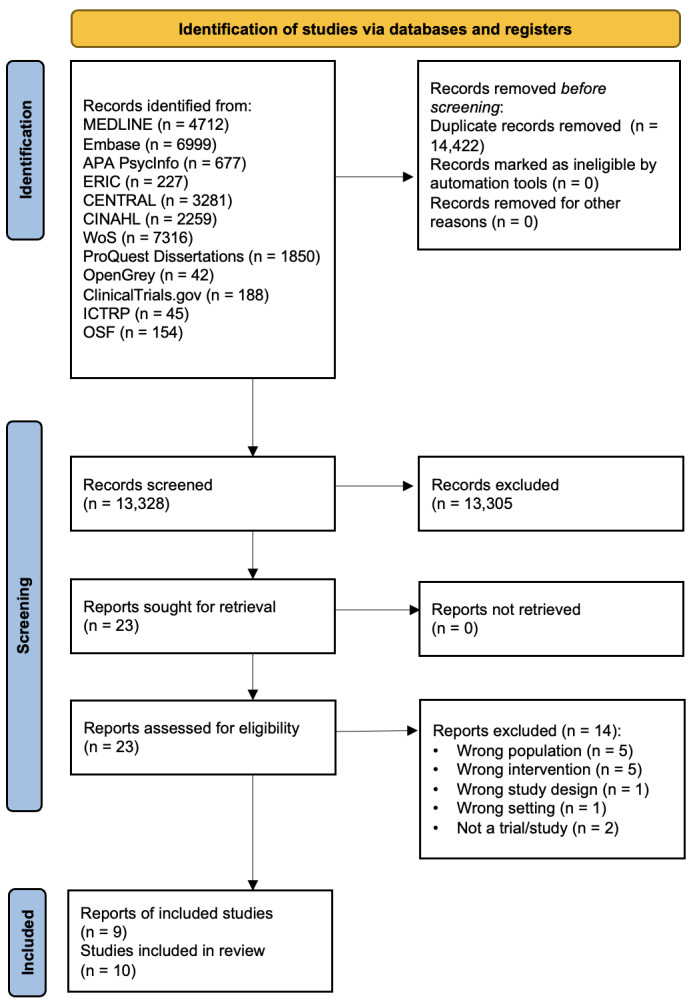
Included studies
The Characteristics of included studies table provides the details of each study.
We included 10 studies (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Hesselberg 2021; Houry 2012; Sattler 2015; Schroter 2004; Speich 2023 Study 1; Speich 2023 Study 2). All the studies measured the effects of peer reviewer training compared to no training or standard journal or funder practice. Eight included studies published results between 2002 and 2022, and an author of the two remaining studies provided results through personal communication in 2023 (Speich 2023 Study 1; Speich 2023 Study 2).
Design
All studies were parallel‐group RCTs with individual randomization. The number of randomized participants varied from 22 (Callaham 2002b) to 418 (Schroter 2004). The unit of analysis was the individual reviewer in seven studies (722 reviewers in total; Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Hesselberg 2021; Houry 2012; Sattler 2015; Schroter 2004), and the reviewed manuscript in three studies (491 manuscripts in total; Cobo 2007; Speich 2023 Study 1; Speich 2023 Study 2). The total number of units randomized was 1213, with 660 allocated to a training intervention and 553 to a control group.
Participants
In eight studies, the participants were journal peer reviewers (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Houry 2012; Schroter 2004; Speich 2023 Study 1; Speich 2023 Study 2). Most participants (1097 of 1213) were included in these studies. In three of them, the unit of randomization was submitted manuscripts (Cobo 2007; Speich 2023 Study 1; Speich 2023 Study 2).
In two studies, the participants were grant peer reviewers (Hesselberg 2021; Sattler 2015).
One study was conducted at five different journals based in the USA or the UK (Speich 2023 Study 2). Four studies were conducted at journals based in the USA (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Houry 2012), two studies at journals based in the UK (Schroter 2004; Speich 2023 Study 1), and one study at a journal based in Spain (Cobo 2007). One study was conducted at a funder in Norway (Hesselberg 2021), and one was conducted at a university in the USA (Sattler 2015).
These countries most likely do not align with the participants' countries of residence. However, in the two studies that provided this information, the participants' country of residence and the country where the study was conducted did coincide (Hesselberg 2021; Sattler 2015).
Interventions
The interventions can be broadly divided into dialogue‐based interventions and one‐way communication. One study had two intervention arms, with interventions from both categories: face‐to‐face training and self‐teaching (Schroter 2004).
Two studies used only dialogue‐based interventions: an interactive workshop in Callaham 2002b and mentoring in Houry 2012.
Seven studies used interventions based on one‐way communication: an email reminder to check items of guidelines (Speich 2023 Study 1; Speich 2023 Study 2), written feedback or written information on how to do reviews (Callaham 2002a Study 1; Callaham 2002a Study 2; Hesselberg 2021), a checklist (Cobo 2007), and an 11‐minute video course (Sattler 2015). See Table 2 for a summary of study interventions.
1. Summary of study interventions.
| Study | Setting | Intervention | Control |
| Callaham 2002a Study 1 | Journal peer review | Summary of goals for quality review + editor's numerical rating | Standard journal practice |
| Callaham 2002a Study 2 | Journal peer review | Study 1 + copy of excellent review | Standard journal practice |
| Callaham 2002b | Journal peer review | 4‐hour, highly interactive workshop | Standard journal practice |
| Cobo 2007 | Journal peer review | Checklist (clinical reviewer + checklist) | Standard review + a standard letter |
| Hesselberg 2021 | Grant peer review | 1‐page, structured individual feedback report on scoring | 1‐page, non‐specific, general information on scoring |
| Houry 2012 | Journal peer review | Mentoring | Standard journal practice |
| Sattler 2015 | Grant peer review | 11‐minute video course | Written information only |
| Schroter 2004 | Journal peer review | Face‐to‐face training or self‐teaching | Standard journal practice |
| Speich 2023 Study 1 | Journal peer review | Email reminder to check items of SPIRIT guidelines | Standard journal practice |
| Speich 2023 Study 2 | Journal peer review | Email reminder to check items of CONSORT guidelines | Standard journal practice |
CONSORT: Consolidated Standards of Reporting Trials; SPIRIT: Standard Protocol Items: Recommendations for Interventional Trials.
Outcome measures
Three studies evaluated one of our predefined primary outcomes (completeness of reporting or peer reviewer detection of errors; Schroter 2004; Speich 2023 Study 1; Speich 2023 Study 2). Two studies measured completeness of reporting as the portion of adequately reported items from the SPIRIT guidelines (Speich 2023 Study 1) or the CONSORT guidelines (Speich 2023 Study 2). Schroter 2004 measured peer reviewer detection of errors by registering the number of major errors detected in a manuscript with deliberately inserted flaws.
Eight studies reported one of our secondary outcome measures. Six journal peer review studies evaluated stakeholders' assessment of review quality or change in stakeholders' assessment of review quality as the main outcome, using a variety of instruments and measures (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Houry 2012; Schroter 2004). Schroter 2004 used the average of two editors' assessments of review quality based on the RQI (van Rooyen 1999). Callaham 2002a Study 1, Callaham 2002a Study 2, and Callaham 2002b reported change in review quality assessed by editors on a five‐point scale used by the Annals of Emergency Medicine (Callaham 1998). Houry 2012 reported the slope of change in quality assessed independently by two researchers on the five‐point scale used by the Annals of Emergency Medicine, and Cobo 2007 reported the change in the average of two editors' assessment of review quality using the MQAI (Goodman 1994).
Both grant peer review studies used inter‐reviewer agreement as the main outcome (Hesselberg 2021, Sattler 2015). Sattler 2015 used intraclass correlation to measure agreement between participants. For ease of analysis and interpretation, we accessed the original data and used the participants' average deviation from the mean as the outcome. Greater deviation represents greater disagreement.
Excluded studies
See the Characteristics of excluded studies table.
We excluded 14 studies at full text review stage for the following reasons.
Ineligible population (not reviewers): Blanco 2020; Ghannad 2021; MacAuley 1998; Strowd 2014; Wong 2017
Ineligible intervention (not training): NCT03751878; Cobo 2011; Green 1989; Jones 2019; Pitman 2019
Ineligible study design (not an RCT): Chauvin 2017
Ineligible setting (not journal or grant peer review): Crowe 2011
Not a trial/study: DiDomenico 2017; Gardner 1986
Ongoing studies
At the time of the search, two records were protocols of ongoing studies (Speich 2023 Study 1; Speich 2023 Study 2). Results from these studies were presented at a conference in September 2022. On 11 March 2023, we contacted the lead author, who sent us a manuscript draft with all the details we needed to include the two studies.
Risk of bias in included studies
We contacted the authors of all studies for clarification on unclear risk of bias items; authors of five studies replied (Hesselberg 2021; Sattler 2015; Schroter 2004; Speich 2023 Study 1; Speich 2023 Study 2).
Figure 2 shows the review authors' judgements about each risk of bias item for each study, and Figure 3 shows the review authors judgements about each risk of bias item as percentages across all studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias; graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
Allocation
Random sequence generation
Nine studies randomized participants using computer‐generated random sequences, so were at low risk of bias in this domain (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Hesselberg 2021; Houry 2012; Schroter 2004; Speich 2023 Study 1; Speich 2023 Study 2). Sattler 2015 provided no information on the method of randomization (unclear risk of bias).
Allocation concealment
We rated five studies at low risk of bias for allocation concealment (Callaham 2002b; Hesselberg 2021; Houry 2012; Speich 2023 Study 1; Speich 2023 Study 2). The remaining five studies provided insufficient details on allocation concealment so were at unclear risk (Callaham 2002a Study 1; Callaham 2002a Study 2; Cobo 2007; Sattler 2015; Schroter 2004).
Blinding
Blinding of participants and personnel
Six studies reported adequate blinding of participants and personnel (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Hesselberg 2021; Speich 2023 Study 1; Speich 2023 Study 2).
In Cobo 2007, it was unclear if both the participants and the editorial team engaging with them were blinded. In two studies, the personnel were blinded, but it was unclear if the participants were blinded (Houry 2012; Sattler 2015). We considered Schroter 2004 at high risk of performance bias, as no participants in either group were blinded.
Blinding of outcome assessors
Outcome assessors were blinded in seven studies (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Hesselberg 2021; Schroter 2004; Speich 2023 Study 1; Speich 2023 Study 2). In Cobo 2007, the outcome assessors were able to identify the manuscripts that had been reviewed by a reviewer using a checklist. However, the report states that "after stratifying by response to [the] blinding questions, the overall conclusion on the main effects remain the same". Based on this clarification, we considered the risk of detection bias to be unclear. The two remaining studies provided no information on blinding of assessors so were also at unclear risk (Houry 2012; Sattler 2015).
Incomplete outcome data
We judged five studies at low risk of attrition bias (Cobo 2007; Hesselberg 2021; Houry 2012; Speich 2023 Study 1; Speich 2023 Study 2). Two studies reported very low levels of attrition (Cobo 2007; Hesselberg 2021), and one study reported an intention‐to‐treat analysis that yielded similar results to the main analysis (Houry 2012). Two studies reported high rates of exclusions, but the exclusions were similar in the intervention and control groups (Speich 2023 Study 1; Speich 2023 Study 2).
We judged three studies at unclear risk of attrition bias: they reported low attrition rates but did not provide reasons for missing data (Callaham 2002a Study 1; Callaham 2002a Study 2; Sattler 2015).
We judged two studies at high risk of attrition bias: they reported high rates of attrition with no description of the difference between responders and non‐responders (Callaham 2002b; Schroter 2004).
Selective reporting
Three studies were preregistered, so we judged them at low risk of reporting bias (Hesselberg 2021; Speich 2023 Study 1; Speich 2023 Study 2). We contacted the authors of the remaining studies to request information on any preplanned analyses (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Houry 2012; Sattler 2015; Schroter 2004). We received no such information, so considered these studies at unclear risk of reporting bias.
Other potential sources of bias
We identified no other potential sources of bias.
Effect of methods
Primary outcomes
Three studies reported one of our two predefined primary outcomes (Schroter 2004; Speich 2023 Study 1; Speich 2023 Study 2).
Completeness of reporting
Two studies measured the portion of adequately reported items from the SPIRIT checklist (Speich 2023 Study 1) or the CONSORT checklist (Speich 2023 Study 2). These studies included 421 manuscripts randomized to journal peer reviewers receiving either an email reminder to check items of SPIRIT or CONSORT guidelines or standard journal practice. Reminding peer reviewers to use reporting guidelines probably had little or no effect on completeness of reporting compared to standard journal practice (MD 0.02, 95% CI −0.02 to 0.06; moderate‐certainty evidence; Analysis 1.1). We downgraded the certainty of the evidence by one level for imprecision.
1.1. Analysis.

Comparison 1: Training versus no training or standard journal practice, Outcome 1: Completeness of reporting (proportion of items adequately reported, from 0.00 to 1.00)
Peer reviewer detection of errors
Schroter 2004 reported the number of major errors identified in a manuscript with deliberately inserted flaws. This study included 418 journal peer reviewers. Training compared with standard journal practice may slightly improve peer reviewer detection of errors (MD 0.55, 95% CI 0.20 to 0.90; low‐certainty evidence; Analysis 1.2). We downgraded the certainty of the evidence by two levels for risk of bias and imprecision.
1.2. Analysis.

Comparison 1: Training versus no training or standard journal practice, Outcome 2: Peer reviewer detection of errors (number of errors identified, from 0 to 9)
Secondary outcomes
Eight studies evaluated one of our predefined secondary outcomes (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Hesselberg 2021; Houry 2012; Sattler 2015; Schroter 2004).
Bibliometric scores
No studies reported bibliometric scores.
Stakeholders' assessment of review quality
Six studies, including a total of 676 participants, reported stakeholder's assessment of review quality (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Houry 2012; Schroter 2004). All the studies indicated little or no effect of the training interventions.
One study, including 418 reviewers, reported review quality as a final score (Schroter 2004). Reviewer training, compared with standard journal practice, may have little or no effect on stakeholders' assessment of review quality (SMD 0.13, 95% CI −0.07 to 0.33; low‐certainty evidence; Analysis 1.3). We downgraded the certainty of the evidence by two levels for risk of bias and imprecision.
1.3. Analysis.

Comparison 1: Training versus no training or standard journal practice, Outcome 3: Stakeholders' assessment of review quality
The five remaining studies, including 258 reviewers, reported change in review quality (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Cobo 2007; Houry 2012). Reviewer training compared with standard journal practice may have little or no effect on change in stakeholders' assessment of review quality (SMD −0.15, 95% CI −0.39 to 0.10; low‐certainty evidence; Analysis 1.3). We downgraded the certainty of the evidence by two levels for risk of bias and imprecision.
Inter‐reviewer agreement
Two studies reported levels of agreement between reviewers (Hesselberg 2021; Sattler 2015).
Sattler 2015, which included 75 reviewers, reported inter‐reviewer agreement as a final score. We are unsure about the effect of a peer reviewer training video, compared with no video, on inter‐reviewer agreement (MD 0.14, 95% CI −0.07 to 0.35; very low‐certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence by three levels for risk of bias, imprecision, and indirectness.
1.4. Analysis.

Comparison 1: Training versus no training or standard journal practice, Outcome 4: Inter‐reviewer agreement
Hesselberg 2021, which included 41 reviewers, reported change in agreement. Structured individual feedback on scoring, compared with general information on scoring, may have little or no effect on change in inter‐reviewer agreement (MD 0.18, 95% CI −0.14 to 0.50; low‐certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence by two levels for imprecision.
Process‐centred outcomes
No studies reported process‐centred outcomes.
Peer reviewer satisfaction
No studies reported peer reviewer satisfaction.
Completion rate and speed of funded projects
No studies reported completion rate and speed of funded projects.
Discussion
Summary of main results
We found moderate‐certainty evidence from two RCTs that reminding peer reviewers of important reporting guideline items has little or no effect on completeness of reporting. We found low‐certainty evidence from one RCT that peer reviewer training slightly increases peer reviewer detection of errors, but the improvement was not meaningful. There was low‐certainty evidence that different types of peer reviewer training have little or no effect on stakeholders' assessment of review quality (final score and change score) and change in inter‐reviewer agreement. Finally, there was very low‐certainty evidence that a video course has little or no effect on inter‐reviewer agreement (final score).
In summary, the evidence from 10 RCTs suggests that training peer reviewers may lead to little or no improvement in the quality of peer review. New research may change our conclusions.
Overall completeness and applicability of evidence
In general, the included studies had relevant settings, interventions, participants, and comparators. One exception is Sattler 2015, where the participants included people other than peer reviewers, and the outcome measure was agreement in the interpretation of descriptions of different review scores, not agreement in the review of research proposals.
When this review was submitted (5 April 2023), the mean number of years since publication of the included reviews was 13.1 (median 16.0 years, range 0.6 to 20.9). However, we do not believe the relevance of the interventions or the typical review setting changed significantly over these years
There are some concerns regarding the methods of measuring the outcomes, particularly stakeholder's assessment of peer review quality. One systematic review of the tools used to assess the quality of peer review concluded that the "development and validation process [of these tools] is questionable and the concepts evaluated by these tools vary widely" (Superchi 2019). In addition, inter‐rater reliability for the tools used in the included studies is generally low, no studies assessed criterion validity, and no studies provided a definition of overall quality. Hence, it is debatable whether stakeholders can assess peer review quality in a meaningful way and whether the tools used to guide the assessments are valid and reliable.
We considered grant and journal peer review as equal processes because they share many important traits. However, there are some differences that might limit the generalizability of results from one to the other. For example, it is reasonable to assume that grant peer reviewers are older and more experienced, are more often paid, and discuss more with other peer reviewers. However, we are unaware of any studies comparing grant to journal peer review, and we suspect that the variance is greater within each process rather than between the two.
We have a similar concern related to the selection of interventions. Although our assessments did not reveal problematic levels of heterogeneity, it could be argued that the interventions are not sufficiently similar. For example, a workshop is likely to have both more impact and higher compliance rates than an email reminder to check items of reporting guidelines, and it may have been better not to mix the different interventions in one analysis.
Furthermore, we consider the homogeneity of the outcome measures to be a disadvantage. The included studies reported only four of our prespecified outcomes: completeness of reporting, peer reviewer detection of errors, stakeholders' assessment of peer review quality, and inter‐reviewer agreement. While these are important measures, they are far from the only outcomes of interest. Editors, funders, researchers, and other end‐users like policymakers and patients are not primarily interested in whether reviewers detect errors, whether they agree, or how the editors assess the review quality; they are likely more interested in whether training peer reviews can improve the scientific output, resulting in a greater impact on end‐users' lives. Impact‐related outcomes such as new patents or products, contributions to policy change, or changes in treatment guidelines seem more relevant, albeit difficult to measure.
Quality of the evidence
Seven of the 10 included studies had unclear or high risk of bias in two or more of the seven domains. Several studies lacked information on allocation concealment and outcome data. Seven studies did not preregister any hypotheses or outcome measures. However, as no studies reported any important effect of the intervention, we consider it unlikely that the hypotheses or outcomes were altered in a way that would have affected the overall results.
The main reasons for downgrading the certainty of the evidence were risk of bias and imprecision (wide CIs exceeding or likely exceeding the threshold for both no effect and an important effect, and limited number of participants).
We downgraded one outcome (inter‐reviewer agreement; final score) for indirectness due to the questionable relevance of methods of measuring peer review quality (see Overall completeness and applicability of evidence).
Potential biases in the review process
Two review authors independently extracted all data and assessed the risk of bias. We resolved any disagreements by discussion or by involving a third review author. The use of RoB 1 is a possible weakness, as there is now a second version of the risk of bias tool (RoB 2).
In the search for unpublished trials, we contacted a selection of journals, funders, and researchers. Our choice of whom to contact was based on prior knowledge, scanning references, and superficial web searches; we could have made a more systematic effort to determine the most relevant sources of information.
Two study authors (IS and JOH) were involved in one of the included studies (Hesselberg 2021). For that study, two other review authors (AF and TD) performed data extraction and risk of bias assessment. We believe this was the best way of overcoming any potential biases. However, as the authors of the included study (IS and JOH) and the review authors assessing the study for inclusion (AF and TD) are all members of the same Cochrane author group, this might still be considered a limitation. In future updates, this could be subject to alteration if Cochrane's conflict of interest guidelines change.
The results of our review may be affected by publication bias. Although we conducted a thorough search and contacted large journals, and funders, we identified no unpublished studies. However, there is a vast number of journals and funders, and peer review processes are well suited to controlled experiments due to easy access to participants and data. Additionally, many funders and journals are familiar with the benefits of doing controlled research. For these reasons, we believe there may be unpublished research, but we consider this concern insufficient to downgrade the certainty of the evidence.
Agreements and disagreements with other studies or reviews
We are aware of two previous reviews that have addressed reviewer training. They both found similar results to ours, based on one or more of the same studies included in our review. The most recent review analysed several interventions, including reviewer training, for improving journal peer review (Bruce 2016). The meta‐analysis of the training interventions included data from five studies that were also included in this review (Callaham 2002a Study 1; Callaham 2002a Study 2; Callaham 2002b; Houry 2012; Schroter 2004). Bruce 2016 concluded that "Training had no impact on the quality of the peer review report".
A Cochrane Methodology Review of editorial peer review concluded that "There is no evidence that referees' training has any effect on the quality of the outcome" (Jefferson 2007). However, the searches for that review were performed in 2004, and the results are likely outdated.
Authors' conclusions
Implication for systematic reviews and evaluations of healthcare.
Evidence from 10 RCTs suggests that training peer reviewers may lead to little or no improvement in the quality of peer review. There is a need for studies with more participants and a broader spectrum of valid and reliable outcome measures. Future studies should pay particular attention to the possible heterogeneity between interventions aimed at grant reviewers versus journal reviewers, and between different types of training.
We believe journals and funders are in an ideal position to initiate such research. Their staff often have research experience, they organize huge quantities of reviews, and data on the review process are often collected systematically through editorial and grant manager systems. Settings like these lend themselves to testing interventions in randomized controlled trials.
Implication for methodological research.
The small number of included studies, the low number of participants in most studies, and the questionable validity and reliability of measures of peer review quality highlight the need for additional randomized trials. These trials should evaluate outcome measures of higher relevance and quality.
To increase relevance and generalizability, future studies should ideally involve multiple journals or funders, as the review settings and standard practice can vary greatly between them.
Studies that evaluate stakeholders' assessments of the quality of peer review should clearly define what constitutes good quality in that specific setting, and ensure that the instruments have sufficient validity and reliability.
History
Protocol first published: Issue 11, 2020
Acknowledgements
We wish to thank information specialist Marit Johansen at the Norwegian Institute of Public Health for reviewing the search strategy and offering important suggestions.
We also wish to thank secretary‐general Hans Christian Lillehagen at the Norwegian Foundation Dam for providing feedback on and supporting the project in its initial stages. His feedback was of particular importance to the selection of criteria for considering studies for the review.
Editorial contributions
Cochrane Methodology Review Group supported the authors in the development of this systematic review. The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Mike Clarke, Co‐ordinating Editor of the Cochrane Methodology review Group, Queen's University Belfast, UK.
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Marwah Anas El‐Wegoud, Cochrane Central Editorial Service.
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service.
Copy Editor (copy editing and production): Julia Turner, Cochrane Central Production Service.
Peer reviewers (provided comments and recommended an editorial decision): Camilla Hansen Nejstgaard, Centre for Evidence‐Based Medicine Odense (CEBMO) & Cochrane Denmark; Dawid Pieper, Brandenburg Medical School, Germany; Professor Philippa Middleton, SAHMRI, University of Adelaide; Valerie Wells Research Associate, Information Scientist MRC/CSO Social and Public Health Sciences Unit School of Health and Wellbeing University of Glasgow (search review).
Appendices
Appendix 1. Search strategy
Ovid MEDLINE(R)
ALL <1946 to April 26, 2022>
Date searched: 27 April 2022
| 1 | Peer Review/ or Peer Review, Research/ | 15162 |
| 2 | peer review*.tw,kf. | 43906 |
| 3 | 1 or 2 | 53557 |
| 4 | Education, Professional/ or exp Inservice Training/ or Mentors/ or Mentoring/ | 46344 |
| 5 | (train* or workshop* or school* or feedback or mentor* or coach* or teach* or taught or educat* or exercis* or guid* or instruct* or practice or handbook* or manual* or course* or seminar* or webinar* or procedure* or program* or assessment* or evaluation* or checklist* or check‐list* or correspond* or template*).tw,kf. | 7824904 |
| 6 | 4 or 5 | 7837640 |
| 7 | 3 and 6 | 30592 |
| 8 | ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (exp animals/ not humans.sh.) | 1326893 |
| 9 | 7 and 8 | 4712 |
Embase Classic+Embase
<1947 to 2022 April 26>
Date searched: 27 April 2022
| 1 | "peer review"/ | 35193 |
| 2 | peer review*.tw,kw. | 50891 |
| 3 | 1 or 2 | 72623 |
| 4 | education/ or continuing education/ or course content/ or education program/ or in service training/ or mentoring/ or mentor/ or postgraduate education/ or refresher course/ or teaching/ or workshop/ | 685025 |
| 5 | (train* or workshop* or school* or feedback or mentor* or coach* or teach* or taught or educat* or exercis* or guid* or instruct* or practice or handbook* or manual* or course* or seminar* or webinar* or procedure* or program* or assessment* or evaluation* or checklist* or check‐list* or correspond* or template*).tw,kw. | 10636114 |
| 6 | 4 or 5 | 10800658 |
| 7 | 3 and 6 | 38563 |
| 8 | (randomized controlled trial/ or randomization/ or (random*.tw,kw. or trial.ti.)) not (Animal experiment/ not (human experiment/ or human/)) | 1927927 |
| 9 | 7 and 8 | 6999 |
APA PsycInfo
<1806 to April Week 3 2022>
Date searched: 27 April 2022
| 1 | Peer Evaluation/ | 3232 |
| 2 | peer review*.tw. | 10570 |
| 3 | 1 or 2 | 12616 |
| 4 | exp Teaching/ or Training/ or Personnel Training/ or Inservice Training/ or Education/ or Continuing Education/ or Professional Development/ or Mentor/ | 219319 |
| 5 | (train* or workshop* or school* or feedback or mentor* or coach* or teach* or taught or educat* or exercis* or guid* or instruct* or practice or handbook* or manual* or course* or seminar* or webinar* or procedure* or program* or assessment* or evaluation* or checklist* or check‐list* or correspond* or template*).tw. | 2427361 |
| 6 | 4 or 5 | 2437073 |
| 7 | 3 and 6 | 8817 |
| 8 | (Randomized Controlled Trials/ or (random* or double‐blind).tw. or trial.ti.) not exp animals/ | 232886 |
| 9 | 7 and 8 | 677 |
ERIC
<1965 to March 2022>
Date searched: 27 April 2022
| 1 | Peer Evaluation/ | 5619 |
| 2 | peer review*.tw. | 3870 |
| 3 | 1 or 2 | 8064 |
| 4 | Training/ or Professional Training/ or Research Training/ or Education/ or Inservice Education/ or Professional Development/ or Workshops/ or Mentors/ or Facilitators/ or Teaching Guides/ | 85439 |
| 5 | (train* or workshop* or school* or feedback or mentor* or coach* or teach* or taught or educat* or exercis* or guid* or instruct* or practice or handbook* or manual* or course* or procedure* or program* or assessment* or evaluation* or checklist* or check‐list* or correspond* or template*).tw. | 1683834 |
| 6 | 4 or 5 | 1684097 |
| 7 | 3 and 6 | 7938 |
| 8 | Randomized Controlled Trials/ or (random* or double‐blind).tw. or trial.ti. | 40068 |
| 9 | 7 and 8 | 227 |
Cochrane Central Register of Controlled Trials
Date searched: 27 April 2022
| #1 | ([mh ^"Peer Review"] OR [mh ^"Peer Review, Research"]) | 83 |
| #2 | peer NEXT review*:ti,ab,kw | 3875 |
| #3 | #1 OR #2 | 3875 |
| #4 | ([mh ^"Education, Professional"] OR [mh "Inservice Training"] OR [mh ^Mentors] OR [mh ^Mentoring]) | 1330 |
| #5 | (train* OR workshop* OR school* OR feedback OR mentor* OR coach* OR teach* OR taught OR educat* OR exercis* OR guid* OR instruct* OR practice OR handbook* OR manual* OR course* OR seminar* OR webinar* OR procedure* OR program* OR assessment* OR evaluation* OR checklist* OR (check NEXT list*) OR correspond* OR template*):ti,ab,kw | 926815 |
| #6 | #4 OR #5 | 926815 |
| #7 | #3 AND #6 | 3348 |
| #8 | #3 AND #6 in Trials | 3281 |
Web of Science
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years
Date searched: 27 April 2022
| #1 | TS="peer review*" | 93,585 |
| #2 | TS=(train* OR workshop* OR school* OR feedback OR mentor* OR coach* OR teach* OR taught OR educat* OR exercis* OR guid* OR instruct* OR practice OR handbook* OR manual* OR course* OR seminar* OR webinar* OR procedure* OR program* OR assessment* OR evaluation* OR checklist* OR check‐list* OR correspond* OR template*) | 15,736,076 |
| #3 | TS=random* OR TI=trial | 2,415,547 |
| #4 | #1 AND #2 AND #3 | 7,316 |
CINAHL (EBSCO)
Date searched: 7 February 2021
| S1 | MH Peer Review | 7,948 |
| S2 | "peer review*" | 24,035 |
| S3 | S1 OR S2 | 24,035 |
| S4 | (MH Education OR MH Teaching OR MH Teaching Materials+ OR MH Staff Development OR MH Seminars and Workshops OR MH Mentorship) | 188,444 |
| S5 | (train* OR workshop* OR school* OR feedback OR mentor* OR coach* OR teach* OR taught OR educat* OR exercis* OR guid* OR instruct* OR practice OR handbook* OR manual* OR course* OR seminar* OR webinar* OR procedure* OR program* OR assessment* OR evaluation* OR checklist* OR check‐list* OR correspond* OR template*) | 3,517,085 |
| S6 | S4 OR S5 | 3,560,721 |
| S7 | S3 AND S6 | 15,425 |
| S8 | (((MH randomized controlled trials OR MH double‐blind studies OR MH single‐blind studies OR MH triple‐blind Studies OR MH random assignment) OR (random* OR double‐blind) OR TI trial) NOT (MH animals+ NOT (MH animals+ AND MH humans))) | 531,957 |
| S9 | S7 AND S8 | 2,259 |
OpenGrey
Date searched: 7 February 2021 (database not updated since)
"peer review*" AND (train* OR workshop* OR school* OR feedback OR mentor* OR coach* OR teach* OR taught OR educat* OR exercis* OR guid* OR instruct* OR practice OR handbook* OR manual* OR course* OR procedure* OR program* OR assessment* OR evaluation* OR checklist* OR "check‐list*" OR correspond* OR template*)
42 hits
ProQuest Dissertations & Theses Global
Date searched: 27 April 2022
[STRICT] noft("peer review*") AND noft((train* OR workshop* OR school* OR feedback OR mentor* OR coach* OR teach* OR taught OR educat* OR exercis* OR guid* OR instruct* OR practice OR handbook* OR manual* OR course* OR seminar* OR webinar* OR procedure* OR program* OR assessment* OR evaluation* OR checklist* OR check‐list* OR correspond* OR template*)) AND (ft(trial OR random* OR double‐blind))
1850 hits
Data and analyses
Comparison 1. Training versus no training or standard journal practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Completeness of reporting (proportion of items adequately reported, from 0.00 to 1.00) | 2 | 421 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 1.2 Peer reviewer detection of errors (number of errors identified, from 0 to 9) | 1 | 418 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.20, 0.90] |
| 1.3 Stakeholders' assessment of review quality | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Final score | 1 | 418 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.07, 0.33] |
| 1.3.2 Change score | 5 | 258 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.39, 0.10] |
| 1.4 Inter‐reviewer agreement | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Final score | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.07, 0.35] |
| 1.4.2 Change score | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.14, 0.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Callaham 2002a Study 1.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: September 1998–September 2000 Setting: reviewers and reviews at the Annals of Emergency Medicine Withdrawals: 16 (10 control subjects and 6 intervention subjects) of 51 |
|
| Data |
Inclusion criteria: reviewers were selected from the entire pool according to their performance in the previous 2 years. Reviewers were eligible for analysis only if they completed ≥ 2 rated reviews during the study. The study targeted low‐volume, low‐quality reviewers. Exclusion criteria: reviewers who completed < 2 rated reviews during the study. Number of participants: 35 (20 in intervention group, 15 in control group) Group differences: minor differences at baseline in number of reviews past 2 years. However, no SDs or CIs are provided for most outcomes. Country of residence: not stated Field of expertise: not stated but likely emergency medicine Gender: not specified Language: not stated Peer reviewer experience: the average number of manuscripts reviewed in the previous 2 years was 7.3 in the intervention group and 7.5 in the control group. |
|
| Comparisons | Standard journal practice | |
| Outcomes |
Stakeholders' assessment of review quality (change score), assessed by editors using a 5‐point scale
|
|
| Identification |
Sponsorship source: not stated Country: USA Setting: University/Annals of Emergency Medicine Authors: Michael L Callaham, Robert K Knopp, E John Gallagher Institution: Department of Emergency Medicine, University of California, San Francisco, CA Email: mlc@medicine.ucsf.edu Address: Michael L Callaham, MD, Department of Emergency Medicine, University of California, Box 0208, San Francisco, CA Conflicts of interest: Callaham is the Editor‐in‐Chief of Annals of Emergency Medicine (3 June 2021). Unclear if he held this position when conducting the study. |
|
| Interventions | Summary of goals for quality review and editor's numerical rating. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Statview 5.0 was used for randomizations, but additional information is sparse. |
| Allocation concealment | Unclear | It is unclear who allocated reviewers and how allocation was organized. However, the intervention was delivered after the review, and it is unlikely that the process until the written feedback was affected by the allocation. |
| Blinding of participants and personnel All outcomes | Yes | Quote: "(reviewers were blinded to author identity and the editor's rating of their review, and authors were blinded to reviewer identity)." Comment: reviewers blinded to author identity and editor's rating, but not to the intervention. The editor (evaluator) was blinded to reviewer identity, reviewer participation, enrolment, and study purpose. |
| Blinding of outcome assessors All outcomes | Yes | Main outcome measure was the editor's routine quality rating (1–5) of all reviews, and the editor was blinded to enrolment, reviewer ID, and study purpose. |
| Incomplete outcome data All outcomes | Unclear | 51 reviewers eligible for entry, 16 (10 control subjects and 6 intervention subjects) completed insufficient rated reviews during the study period, leaving 35 with sufficient data. More reviewers in the control group were lost to follow‐up. It is unclear if any eligible reviewers were denied participation in the study, and it is unclear whether lost to follow‐up means "missing at random". |
| Selective outcome reporting | Unclear | No preregistration available to check whether planned outcomes and analyses were left out or if the main outcome was switched. We asked the authors for information on preplanned analyses, but they did not respond. It is possible, but not likely, that there is some selective reporting: possible because there is no protocol, unlikely because the reported outcome measure seems the most reasonable one. The study authors also state explicitly that they opted for that outcome measure rather than a more comprehensive one (under Methods). In addition, the results were disappointing, which suggests the study authors did not withhold other findings. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Callaham 2002a Study 2.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: April 2000–January 2002 Setting: reviewers and reviews at the Annals of Emergency Medicine Withdrawals: 32 (15 control subjects and 17 intervention subjects) of 127 |
|
| Data |
Inclusion criteria: reviewers were selected from the entire pool according to their performance in the previous 2 years. Reviewers were eligible for analysis only if they completed ≥ 2 rated reviews during the study. The study targeted low‐volume, average‐quality reviewers. Exclusion criteria: reviewers who completed < 2 rated reviews during the study Number of participants: 95 (49 in intervention group, 46 in control group) Group differences: minor differences at baseline in number of reviews in past 2 years. However, no SDs or CIs are provided for most outcomes. Country of residence: not stated Field of expertise: not stated but likely emergency medicine Gender: not specified Language: not stated Peer reviewer experience: the average number of manuscripts reviewed in the previous 2 years was 7.7 in the intervention group and 7.1 in the control group. |
|
| Comparisons | Standard journal practice | |
| Outcomes |
Editors' assessment of review quality (change score), assessed by editors using a 5‐point scale
|
|
| Identification |
Sponsorship source: not stated Country: USA Setting: University/Annals of Emergency Medicine Authors: Michael L Callaham, Robert K Knopp, E John Gallagher Institution: Department of Emergency Medicine, University of California, San Francisco, CA Email: mlc@medicine.ucsf.edu Address: Michael L Callaham, MD, Department of Emergency Medicine, University of California, Box 0208, San Francisco, CA Conflicts of interest: Callaham is the Editor‐in‐Chief of Annals of Emergency Medicine (3 June 2021). Unclear if he held this position when conducting the study. |
|
| Interventions | Summary of goals for quality review, editor's numerical rating and a copy of excellent review | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Statview 5.0 was used for randomizations, but additional information is sparse. |
| Allocation concealment | Unclear | It is unclear who allocated reviewers and how allocation was organized. However, the intervention was delivered after the review, and it is unlikely that the process until the written feedback was affected by the allocation. |
| Blinding of participants and personnel All outcomes | Yes | Quote: "(reviewers were blinded to author identity and the editor's rating of their review, and authors were blinded to reviewer identity)." Comment: reviewers blinded to author identity and editor's rating, but not to the intervention. The editor (evaluator) was blinded to reviewer identity, reviewer participation, enrolment, and study purpose. |
| Blinding of outcome assessors All outcomes | Yes | Main outcome measure was the editor's routine quality rating (1–5) of all reviews, and the editor was blinded to enrolment, reviewer ID, and study purpose. |
| Incomplete outcome data All outcomes | Unclear | 127 reviewers were eligible; 32 (15 control subjects and 17 intervention subjects) did not complete sufficient reviews, leaving 95 with sufficient data for analysis. Analyses by the ITT principle. Numbers of reviewers with too few reviews to be included are well described. Distribution was fairly equal between groups. It is unclear if any eligible reviewers were denied participation in the study. |
| Selective outcome reporting | Unclear | No preregistration available to check whether planned outcomes and analyses were left out or if the main outcome was switched. We asked the authors for information on preplanned analyses, but they did not respond. It is possible, but not likely, that there is some selective reporting: possible because there is no protocol, unlikely because the reported outcome measure seems the most reasonable one. The study authors also state explicitly that they opted for that outcome measure rather than a more comprehensive one (under Methods). In addition, the results were disappointing, which suggests the study authors did not withhold other findings. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Callaham 2002b.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: 1999 (workshop) to 2001/2002 (follow‐up) Setting: reviews at Annals of Emergency Medicine Withdrawals: 63/75 in the intervention group (of 75 reviewers randomized to the intervention, 29 agreed to attend, and 12 attended. 1 had insufficient data). No information is specified for the control group, but "Those not invited served as the group from which control reviewers matched for previous review quality and volume were selected". 150 reviewers were invited in total, and only 11 were left in the analysis in both groups. |
|
| Data |
Inclusion criteria: reviewers with average quality scores during the previous 2 years Exclusion criteria: attendance at a prior workshop, guest reviewer status, membership on the journal's editorial board Number of participants: 22 analysed (11 in intervention group, 11 in control group) Group differences: not stated. The control reviewers were selected from invited reviewers who did not attend the workshop (nonattendees) and matched for previous review quality and volume. Country of residence: not stated Field of expertise: not stated but likely emergency medicine Gender: not specified Language: not stated. Most likely well versed in English Peer reviewer experience: average number of reviews in the past 2 years was 5.36 in the control group and 4.82 in the intervention group. Unclear if there was a minimum requirement for number of reviews in past 2 years. |
|
| Comparisons | Standard journal practice | |
| Outcomes |
Stakeholders' assessment of review quality (change score), assessed by editors using a 5‐point scale
|
|
| Identification |
Sponsorship source: not stated Country: USA Setting: University/Annals of Emergency Medicine Authors: Michael L Callaham, David L Schriger Institution: Department of Emergency Medicine, University of California‐San Francisco, San Francisco, CA Email: mlc@medicine.ucsf.edu Address: Michael L Callaham, MD, Department of Emergency Medicine, University of California, Box 0208, San Francisco, CA Conflicts of interest: Callaham is the Editor‐in‐Chief of Annals of Emergency Medicine (3 June 2021). Unclear if he held this position when conducting the study. |
|
| Interventions | A formal, 4‐hour, highly interactive workshop on peer review held simultaneously with a major meeting in the specialty and run by 2 senior editors of the journal (including a formally trained methodology and statistics editor) | |
| Notes | This article described 2 studies; only study 2 was randomized. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Quote: "In study 2, 150 reviewers with average quality scores during the previous 2 years were randomized (StatView 5.0, SAS, Carey, NC) to be invited to the previously described workshop in October 1999 or not to be invited." Comment: computer‐generated randomization |
| Allocation concealment | Yes | Quote: "If invited reviewers did not respond to letters and e‐mails, follow‐up telephone calls were made first by journal staff and, ultimately, by the first author. Efforts at contact were continued until a specific response was obtained from those invited." Comment: insufficient information regarding how allocation concealment was handled. Those involved in enrolment and assignment were not blinded, but this is unlikely to have had any effect on the outcome (unless they wanted the intervention to fail). |
| Blinding of participants and personnel All outcomes | Yes | Clearly the personnel involved in the workshop were not blinded, but in this case, they should be considered an integral part of the intervention. Editors were blinded to the identity of study participants, whether they attended the workshop, and the purpose of the study. Reviewers were blinded to the purpose of the study. |
| Blinding of outcome assessors All outcomes | Yes | Quote: "Editors were blinded as to the identity of study participants, whether they attended the workshop and the purpose of both studies." Comment: Editors' quality reviews served as the outcome. |
| Incomplete outcome data All outcomes | No | Of 150 invited subjects, only 22 were included in the analyses. It is not clear if only 11 subjects in the intervention group actually agreed to participate in the workshop, or whether some participated but were left out of the analyses. The 11 subjects in the control group were matched to the 11 subjects in the intervention group, and it is unclear if they were "representative" of those randomized to the control group as a whole. |
| Selective outcome reporting | Unclear | The study was not preregistered. We asked the study authors for information on preplanned analyses, but they did not respond. Selective outcome reporting is not considered likely (as the outcome is the editors' score), but cannot be ruled out. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Cobo 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: May 2004–March 2005 Setting: journal peer review (Medicina Clinica, a peer‐reviewed weekly biomedical journal) Withdrawals: loss to follow‐up (before intervention): 5 in control group, 4 in intervention group; rejected manuscripts (after intervention): 5 in control group; 5 in intervention group |
|
| Data |
Inclusion criteria: manuscripts sent to the "original" and "brief original" sections (original primary research, including statistical analysis) of the journal between May 2004 and March 2005 Exclusion criteria: manuscripts with "other" study design, (i.e. qualitative research reports, case series, editorials, non‐systematic reviews, and letters to the editor). Articles not fitting the journal's editorial policy were excluded. Number of participants: 60 manuscripts (28 in intervention group, 32 in control group) Group differences: there were some differences in baseline overall quality score between the groups, but according to the report, the were not significant. Unclear what that assumption is based on; it seems there was no analysis to test these differences. All information is based on manuscripts characteristics. The report provides no information on the clinical reviewers. Country of residence: no information on the reviewers as the study population was defined as the submitted manuscripts. Field of expertise: no information on the reviewers as the study population was defined as the submitted manuscripts. Gender: no information on the reviewers as the study population was defined as the submitted manuscripts. Language: no information on the reviewers as the study population was defined as the submitted manuscripts. Peer reviewer experience: no information on the reviewers as the study population was defined as the submitted manuscripts. |
|
| Comparisons | Normal procedure | |
| Outcomes | Stakeholders' assessment of review quality (change score), assessed by 2 researchers independently using the MQAI
|
|
| Identification |
Sponsorship source: Grant PI041945 from the Carlos III Institute of Health (Spain) Country: Spain Setting: journal peer review in Medicina Clinica (a peer‐reviewed weekly Spanish biomedical journal) Authors: Erik Cobo, Albert Selva‐O'Callagham, Josep‐Maria Ribera, Francesc Cardellach, Ruth Dominguez, Miquel Vilardell Institution: Department of Statistics and Operations Research, Technological University of Catalonia, Barcelona, Spain, Medicina Clínica, Barcelona, Spain Email: erik.cobo@upc.edu Address: Department of Statistics and Operations Research, Technological University of Catalonia, Barcelona, Spain, Medicina Clínica, Barcelona, Spain Conflicts of interest: 5 of the authors are external partial‐time employees (around 5%–10% of total income) of Elsevier, publisher of Medicina Clinica. |
|
| Interventions | Checklist | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Quote: "We randomly allocated the manuscripts accepted for review into four groups defined by the interventions"; "Then, manuscripts were randomly allocated (by AS) using a computer program that first stratifies by study type, and second allocates to intervention groups while minimizing differences in initial quality. Then, the manuscripts followed the proper editorial procedure, according to their assigned group." |
| Allocation concealment | Unclear | Insufficient reporting of the allocation procedure. |
| Blinding of participants and personnel All outcomes | Unclear | Unclear if the editorial team was blinded. Apart from half of them receiving the intervention, the clinical reviewers seem to be unaware of the study objective and randomization. Unclear whether it is possible for a reviewer to be in multiple intervention groups, based on randomization of manuscripts and the possibility for receiving several manuscripts over the trial period. |
| Blinding of outcome assessors All outcomes | Unclear | Quote: "Two evaluators (EC, RD) independently rated the reporting quality of manuscripts at initial submission and following peer review and revision, according to the MQAI. Both knew the initial and final status but were blinded to the intervention group."; "On the use of a checklist, the evaluators were able to guess the intervention group in 65.3% (95% CI from 53.5 to 76.0%) of the 75 over 99 (75.8%) cases analyzed." According to the authors the overall conclusion the main effects remained the same after stratifying by response to both of the blinding questions. However, the data are not provided. |
| Incomplete outcome data All outcomes | Yes | 5 manuscripts in the control group and 4 in the intervention group were lost to follow‐up. However, this was before the intervention was delivered and was due to the authors not responding within the deadline. It is unlikely this would have affected the outcome in any meaningful way. After the reviews, 5 manuscripts were rejected in each group. It is unclear if the editors rejecting were blinded or not. However, it is unlikely that this would have had an effect on the result as the rejected manuscripts were of low quality. |
| Selective outcome reporting | Unclear | The study was not preregistered. We asked the study authors for information on preplanned analyses, but they did not respond. Selective outcome reporting is not considered likely (as the results are disappointing), but cannot be ruled out. |
| Other sources of bias | Yes | No other sources of bias identified. |
Hesselberg 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: 2018 (spring and autumn) Setting: grant review committees Withdrawals: 1 member of a review committee withdrew due to acute illness. |
|
| Data |
Inclusion criteria: all reviewers who served in the funding programme Health ("Helse") at the Norwegian Foundation Dam in 2018 Exclusion criteria: reviewers who had not served on the corresponding review committee the previous year (the individual feedback report was based on reviews conducted the previous year) Number of participants: 41 (19 in intervention group, 22 in control group) Group differences: some potentially key differences, as shown in Table 1 (especially age) Country of residence: Norway Field of expertise: not stated Gender: 56% women Language: not stated Peer reviewer experience: average years of experience as reviewer for the foundation was 2.5 years (SD 3.08), and there was no difference between the groups. |
|
| Comparisons | 1‐page, nonspecific, general information on scoring | |
| Outcomes | Disagreement (change score), assessed using the average score difference between reviewers
|
|
| Identification |
Sponsorship source: Foundation Dam (Norway) Country: Norway Setting: grant review committee Authors: Jan‐Ole Hesselberg, Knut Inge Fostervold, Pål Ulleberg, Ida Svege Institution: Foundation Dam (Norway) Email: janohes@student.sv.uio.no Address: Foundation Dam Conflicts of interest: JOH is the chief programme officer and IS is the head of development at the funder of this study, the Norwegian Foundation Dam (Stiftelsen Dam). |
|
| Interventions | 1‐page, structured individual feedback report on scoring | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Quote: "The participants were randomized in two rounds, one for each committee. The randomization was done by JOH in Microsoft® Excel for Mac version 16.10 by using the rand.between function and drawing a random number between 1 and 10,000 for each participant. The half with the lowest numbers were assigned to the general feedback group, the half with the highest numbers were assigned to the individual feedback group." |
| Allocation concealment | Yes | Quote: "The participants were randomized in two rounds, one for each committee. The randomization was done by JOH in Microsoft® Excel for Mac version 16.10 by using the rand.between function and drawing a random number between 1 and 10,000 for each participant. The half with the lowest numbers were assigned to the general feedback group, the half with the highest numbers were assigned to the individual feedback group." |
| Blinding of participants and personnel All outcomes | Yes | Double‐blind. |
| Blinding of outcome assessors All outcomes | Yes | The study author performing the statistical analysis (IS) was blinded. |
| Incomplete outcome data All outcomes | Yes | No missing data (except for 1 participant, which is described well, and data not imputed). |
| Selective outcome reporting | Yes | Protocol available at osf.io/n4fq3, no indication of selective reporting. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Houry 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Setting: USA, Emory University, California, Annals of Emergency Medicine Withdrawals: 4 subjects randomized to the mentorship group never contacted their mentors so did not receive the intervention. The study used a per‐protocol analysis, excluding the 4 subjects. The report states "we also performed a corresponding intention‐to‐treat analysis that yielded essentially identical results." Duration and date: total duration of the study 4.5 years, dates of study April 2006–October 2010 |
|
| Data |
Inclusion criteria: individuals newly added to the journal's reviewer ranks during the study period. Each candidate had to have published at ≥ 2 first‐author peer‐reviewed publications. Exclusion criteria: none stated Number of participants: 35 (20 in intervention group, 15 in control group) Group differences: none Country of residence: not stated Field of expertise: no information. However, these were reviewers for the Annals of Emergency Medicine Gender: not stated Language: no information. However, the manuscripts were in English, so the reviewers should be competent in English. Peer reviewer experience: 21/46 (46%) had no previous reviewer experience. 12/46 (26%) had reviewed for ≥ 3 journals. |
|
| Comparisons | Standard journal practice | |
| Outcomes | Stakeholders' assessment of review quality (change score), assessed by editors using a 5‐point scale, expressed by "slope of quality score change"
|
|
| Identification |
Sponsorship source: not stated Country: USA Setting: university Authors: Debra Houry, Steven Green, Michael Callaham Institution: Department of Emergency Medicine, University of California Email: Michael.Callaham@ucsf.edu Address: Department of Emergency Medicine, University of California, San Francisco, CA, USA Conflicts of interest: the authors declare that they have no competing interests. |
|
| Interventions | Mentoring | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Quote: "We used computer‐generated randomization to assign new reviewers to either the intervention or control group." |
| Allocation concealment | Yes | Computer‐generated randomization and only a senior editor was aware of who was to receive the intervention. |
| Blinding of participants and personnel All outcomes | Unclear | Quote: "Over the course of the study editors invited reviewers in their standard fashion, without knowledge of which new reviewers were assigned to the mentorship or control groups." Comment: personnel were blinded but unclear if participants were blinded. |
| Blinding of participants and personnel All outcomes | Unclear | Quote: "Over the course of the study editors invited reviewers in their standard fashion, without knowledge of which new reviewers were assigned to the mentorship or control groups." Comment: personnel were blinded but unclear if participants were blinded. |
| Blinding of outcome assessors All outcomes | Unclear | Quote: "All reviews at Annals are routinely rated by editors for quality on a previously reported 5‐point scale that has demonstrated moderate reliability and is comparable to the scale validated by van Rooyen." Comment: unclear if the editors were aware of allocation when assessing the reviews. |
| Incomplete outcome data All outcomes | Yes | Quote: "per‐protocol analysis excluding these four subjects; however we also performed a corresponding intention‐to‐treat analysis that yielded essentially identical results (data not shown)." Comment: 4 subjects in the intervention group did not receive the intervention and were excluded from the analysis. The researchers state that an ITT analysis "yielded essentially identical results" but the analyses are not included in the paper. |
| Selective outcome reporting | Unclear | No mention of preregistered outcomes or if some planned analyses were changed or not. We contacted the study authors for clarification but received no response. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Sattler 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: not stated in the article, but in the data set first data were collected 1 September 2011 and the last were collected 26 January 2012 Setting: university Withdrawals: not stated |
|
| Data |
Inclusion criteria: "We identified 180 individuals by performing a web‐based search for Public Health programs and sent an e‐mail soliciting participation. If a response was not received within 1 1/2 weeks, we sent an e‐mail or called. They were offered $50 in exchange for their participation." Exclusion criteria: none stated Number of participants: 46 (24 in intervention group, 22 in control group) Group differences: group data are not reported, only data for total sample Country of residence: USA Field of expertise: reviewers highest academic titles were PhD (80%), MD (11%), ScD (4%), DPharmacy (2.5%), and both a PhD and MD (2.5%). Gender: 47% women (35/75) Language: English (US) Peer reviewer experience: "Participants also were asked whether they had any extramural grant review experience. Those with no extramural review experience (all Assistant Professors; N = 29) were coded as "novices," and those with experience (Assistant, Associate, and Full Professors; N = 46) were coded as "experienced" reviewers. Experienced reviewers had served on an average of 19 extramural review panels (SD = 27) whereas novice reviewers had served on no review panels (M = 0, SD = 0)." |
|
| Comparisons | Written information regarding how to use the scoring scale | |
| Outcomes | In the publication, the authors report Rating Scale Knowledge, Inter‐Rater Reliability (intraclass correlation)
For the analyses of the review, we obtained the raw scores and calculated the average deviation between reviewers.
|
|
| Identification |
Sponsorship source: U.S. Department of Energy and ORAU Country: USA Setting: university Authors name: David N Sattler, Patrick E McKnight, Linda Naney, Randy Mathis Institution: Department of Psychology, Western Washington University Email: pmckmigh@gmu.edu(PM); David.Sattler@wwu.edu(DS) Address: Department of Psychology, Western Washington University, Bellingham, WA Conflicts of interest: the authors declared that no competing interests. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. |
|
| Interventions | 11‐minute video course on how to use the scoring scale | |
| Notes | Unclear exactly how reviewers were recruited and how many agreed to participate. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Unclear | No information about how randomization was performed. |
| Allocation concealment | Unclear | No information about how randomization was performed. |
| Blinding of participants and personnel All outcomes | Unclear | Participants in both groups (training and control) were sent to a secure website with information. Only the training group got to see the training programme video online. It is unclear if lack of blinding the participants would cause bias. There were no personnel involved in this. |
| Blinding of outcome assessors All outcomes | Unclear | No information about blinding the outcome assessors. |
| Incomplete outcome data All outcomes | Unclear | 180 subjects were contacted, 75 completed the interventions. It is unclear what happened to the 105 subjects who were contacted but did not complete the courses. |
| Selective outcome reporting | Unclear | The data set includes several other outcome measures that are not mentioned in the paper. Unclear if the study was preregistered. We contacted the study authors for clarification but received no response. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Schroter 2004.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: no information Setting: reviews and reviewers at BMJ. However, in the Declarations section the authors state that "Because members of BMJ staff were involved in the conduct of this research and writing the paper, assessment and peer review have been carried out entirely by external advisers. No member of BMJ staff has been involved in making the decision on the paper." Withdrawals: 87 of 609 randomized reviewers did not complete review 1, 82 of 522 did not complete review 2, and 22 of 440 did not complete review 3. |
|
| Data |
Inclusion criteria: consenting reviewers Exclusion criteria: 647 reviewers were excluded, but there is no mention of exclusion criteria. Number of participants: 418 (262 in intervention group, 256 in control group) Group differences: the investigators "used a stratified permuted blocks randomisation method to ensure that the groups were similar in terms of factors known to influence the quality of reviews (age, current investigators in medical research projects, postgraduate training in epidemiology, postgraduate training in statistics, and editorial board members of a scientific or medical journal)." Country of residence: not stated Field of expertise: epidemiology, statistics, editorial board member Gender: 72.6% male (379/522 in review 1) Language: not stated Peer reviewer experience: no information. However, of 522 reviewers completing review 1, 253 had experience as editorial board members of a scientific or medical journal. |
|
| Comparisons | Standard journal practice | |
| Outcomes |
Number of major errors identified
Stakeholders' assessment of review quality (final score), assessed by 2 editors independently using the RQI
|
|
| Identification |
Sponsorship source: the study was funded by the NHS London Regional Office Research and Development Directorate.
Country: UK Setting: reviews and reviewers at the BMJ Authors: Sara Schroter Institution: BMJ Editorial Office Email: sschroter@bmj.com Address: BMJ Editorial Office, BMA House, Tavistock Square, London WC1H 9JR, UK Conflicts of interest: RS is the editor of the BMJ. SS, NB, and SE review for the BMJ. |
|
| Interventions | Face‐to‐face training or self‐teaching | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Quote: "We randomised consenting reviewers into three groups: two intervention groups and a control group. We used a stratified permuted blocks randomisation method to ensure that the groups were similar in terms of factors known to influence the quality of reviews (age, current investigators in medical research projects, postgraduate training in epidemiology, postgraduate training in statistics, and editorial board members of a scientific or medical journal)." |
| Allocation concealment | Unclear | No information about concealment. |
| Blinding of participants and personnel All outcomes | No | No information about blinding in article In email correspondence with Schroter (7 July 2021): "Participants were not blinded. They were told which group they were randomised to when sent the first paper to review. They also knew if they were in a training group as they either received a package in the post or came in for training. Those in the control group were told when they were sent the second paper to review that if we found the training materials effective that we would send these to them at the end of the trial." |
| Blinding of outcome assessors All outcomes | Yes | Quote: "Because members of BMJ staff were involved in the conduct of this research and writing the paper, assessment and peer review have been carried out entirely by external advisers. No member of BMJ staff has been involved in making the decision on the paper."; "Two researchers blind to the identity and study group of the reviewer independently assessed the number of major errors reported in each review. We used the total number of major errors identified averaged across the two raters. Inclusion of the minor errors in the total error score made no difference to the results." |
| Incomplete outcome data All outcomes | No | Considerable attrition rate in self‐taught group between review 1 and 2; more than in face‐to‐face and control groups (which are quite similar). |
| Selective outcome reporting | Unclear | No available prespecified analysis plan. Not mentioned in the publication, but confirmed by the author by email. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Speich 2023 Study 1.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: June 2020–May 2021 Setting: peer review in the journal BMJ Open Withdrawals: 2193 manuscripts were screened, 245 randomized, and 178 included in the analysis. |
|
| Data |
Inclusion criteria: eligible manuscripts needed to report an RCT protocol. Manuscripts flagged as eligible were randomized if they were sent out for peer review (randomization after the first peer reviewer accepted the invitation). Exclusion criteria: none specified Number of participants: 245 manuscripts randomized and 178 included in the analysis (90 in the intervention group, 88 in the control group) Group differences: the unit of randomization was submitted manuscripts. No information on the reviewers. Country of residence: the unit of randomization was submitted manuscripts. No information on the reviewers. Field of expertise: the unit of randomization was submitted manuscripts. No information on the reviewers. Gender: the unit of randomization was submitted manuscripts. No information on the reviewers. Language: the unit of randomization was submitted manuscripts. No information on the reviewers. Peer reviewer experience: the unit of randomization was submitted manuscripts. No information on the reviewers. |
|
| Comparisons | Standard journal practice | |
| Outcomes | The primary outcome was the difference in the mean proportion of adequately reported items in published articles between the 2 groups.
|
|
| Identification |
Sponsorship source: "Benjamin Speich was supported by an Advanced Postdoc. Mobility (P300PB_177933) and a Return Postdoc.Mobility (P4P4PM_194496) grant from the Swiss National Science Foundation, as well as a research grant from the Research Foundation of the University of Basel. Szimonetta Lohner was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00498/17/5) and a research grant from the National Research, Development and Innovation Office of Hungary (NKFIH_FK_143633)." The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Country: UK Setting: peer review in the journal BMJ Open Authors name: Benjamin Speich Institution: Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK Email: benjamin.speich@usb.ch Address: CLEAR Methods Center, Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland, Totengässlein 3 4051 Basel, Switzerland Conflicts of interest: BS and MB received unrestricted grants from Moderna (2021/22) for studies unrelated to the presented work. SS is employed by the BMJ. EM, and AC are employed by the Public Library of Science; AC is a PLOS ONE editor. During the design and initial implementation IP was an employee by the Public Library of Science. DM, SH, and IB are members of the CONSORT executive and authors of the CONSORT 2010 statement. AWC and DM are authors of the SPIRIT 2013 Statement. DM, MMS, PD, and PR are members of the EQUATOR network. All study authors declared no other competing interests exist. |
|
| Interventions | Email reminder to check items of SPIRIT guidelines | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Quote: "Eligible manuscripts were randomized as soon as the first peer reviewer accepted the invitation to peer review the article. Randomization was conducted by the corresponding author (BS) through the Study‐Randomizer system20 using a 1:1 allocation (random block sizes between 2 and 8) [...]." |
| Allocation concealment | Yes | Quote: "Eligible manuscripts were randomized as soon as the first peer reviewer accepted the invitation to peer review the article. Randomization was conducted by the corresponding author (BS) through the Study‐Randomizer system20 using a 1:1 allocation (random block sizes between 2 and 8) [...]." |
| Blinding of participants and personnel All outcomes | Yes | Quote: "Authors and peer reviewers were not informed about the study. Editors were not actively informed about the randomization." |
| Blinding of outcome assessors All outcomes | Yes | Quote: "Outcomes assessors, independently assessing in duplicate the adequacy of reporting in the published version of the articles, were blinded." |
| Incomplete outcome data All outcomes | Yes | Quote: "For SPIRIT‐PR 2, 193 manuscripts were screened, 245 randomized and 178 included in the analysis." Comment: similar exclusion in both groups after randomization. |
| Selective outcome reporting | Yes | Quote: "The primary outcome will be the mean proportion of the 10 items that are adequately reported in the published articles." Comment: primary outcome was preregistered. |
| Other sources of bias | Yes | No indication of other sources of bias. |
Speich 2023 Study 2.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group Duration and date: July 2019–July 2021) Setting: peer review at 7 journals: BMJ Open, The BMJ, British Journal of Sports Medicine, British Journal of Ophthalmology, Heart, PLOS Medicine, PLOS ONE Withdrawals: 30,067 manuscripts were screened, 510 randomized, and 243 included in the analysis. |
|
| Data |
Inclusion criteria: eligible manuscripts needed to report an RCT protocol. Manuscripts flagged as eligible were randomized if they were sent out for peer review (randomization after the first peer reviewer accepted the invitation). Exclusion criteria: none specified Number of participants: 510 manuscripts randomized and 243 included in the analysis (122 in the intervention group, 121 in the control group) Group differences: the unit of randomization was submitted manuscripts. No information on the reviewers. Country of residence: the unit of randomization was submitted manuscripts. No information on the reviewers. Field of expertise: the unit of randomization was submitted manuscripts. No information on the reviewers. Gender: the unit of randomization was submitted manuscripts. No information on the reviewers. Language: the unit of randomization was submitted manuscripts. No information on the reviewers. Peer reviewer experience: the unit of randomization was submitted manuscripts. No information on the reviewers. |
|
| Comparisons | Standard journal practice | |
| Outcomes | The primary outcome of this study was the difference in the mean proportion of adequately reported C‐short items in published articles between the 2 groups.
|
|
| Identification |
Sponsorship source: "Benjamin Speich was supported by an Advanced Postdoc. Mobility (P300PB_177933) and a Return Postdoc.Mobility (P4P4PM_194496) grant from the Swiss National Science Foundation, as well as a research grant from the Research Foundation of the University of Basel. Szimonetta Lohner was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00498/17/5) and a research grant from the National Research, Development and Innovation Office of Hungary (NKFIH_FK_143633)." The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Country: UK Setting: peer review at 7 journals: BMJ Open, The BMJ, British Journal of Sports Medicine, British Journal of Ophthalmology, Heart, PLOS Medicine, PLOS ONE Authors name: Benjamin Speich Institution: Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK Email: benjamin.speich@usb.ch Address: CLEAR Methods Center, Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland, Totengässlein 3 4051 Basel, Switzerland Conflicts of interest: BS and MB received unrestricted grants from Moderna (2021/22) for studies unrelated to the presented work. SS is employed by the BMJ. EM, and AC are employed by the Public Library of Science; AC is a PLOS ONE editor. During the design and initial implementation IP was an employee by the Public Library of Science. DM, SH, and IB are members of the CONSORT executive and authors of the CONSORT 2010 statement. AWC and DM are authors of the SPIRIT 2013 Statement. DM, MMS, PD, and PR are members of the EQUATOR network. All study authors declared no other competing interests exist. |
|
| Interventions | Email reminder to check items of CONSORT guidelines | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Random sequence generation | Yes | Quote: "Eligible manuscripts were randomized as soon as the first peer reviewer accepted the invitation to peer review the article. Randomization was conducted by the corresponding author (BS) through the Study‐Randomizer system20 using a 1:1 allocation (random block sizes between 2 and 8) and stratification by journal (for CONSORT‐PR)." |
| Allocation concealment | Yes | Quote: "Eligible manuscripts were randomized as soon as the first peer reviewer accepted the invitation to peer review the article. Randomization was conducted by the corresponding author (BS) through the Study‐Randomizer system20 using a 1:1 allocation (random block sizes between 2 and 8) and stratification by journal (for CONSORT‐PR)." |
| Blinding of participants and personnel All outcomes | Yes | Quote: "Authors and peer reviewers were not informed about the study. Editors were not actively informed about the randomization." |
| Blinding of outcome assessors All outcomes | Yes | Quote: "Outcomes assessors, independently assessing in duplicate the adequacy of reporting in the published version of the articles, were blinded." |
| Incomplete outcome data All outcomes | Yes | Quote: "For CONSORT‐PR a total of 34,067 manuscripts were assessed for eligibility by BS between July 2019 and July 2021 based on title, abstracts, and full texts if necessary. Out of those, 510 eligible manuscripts were randomized, and 243 were published and included in the analysis." Comment: similar exclusion in both groups after randomization. |
| Selective outcome reporting | Yes | Quote: "The primary outcome will be the mean proportion of the 10 items that are adequately reported in the published articles." Comment: primary outcome was preregistered. |
| Other sources of bias | Yes | No indication of other sources of bias. |
CI: confidence interval; CONSORT: CONsolidated Standards for Reporting Trials; EQUATOR: Enhancing the Quality and Transparency of Research; ITT: intention‐to‐treat; MQAI: Manuscript Quality Assessment Instrument; RCT: randomized controlled trial; RQI: Review Quality Instrument; SD: standard deviation; SPIRIT: Standard Protocol Items: Recommendations for Interventional Trials.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blanco 2020 | Ineligible population. |
| Chauvin 2017 | Ineligible study design. |
| Cobo 2011 | Ineligible intervention. |
| Crowe 2011 | Ineligible setting. |
| DiDomenico 2017 | Not a trial/study. |
| Gardner 1986 | Not a trial/study. |
| Ghannad 2021 | Ineligible population. |
| Green 1989 | Ineligible intervention. |
| Jones 2019 | Ineligible intervention. |
| MacAuley 1998 | Ineligible population. |
| NCT03751878 | Ineligible intervention. |
| Pitman 2019 | Ineligible intervention. |
| Strowd 2014 | Ineligible population. |
| Wong 2017 | Ineligible population. |
Differences between protocol and review
See Hesselberg 2020 (protocol).
Methods > Criteria for considering studies for this review > Types of outcome measures
We changed the phrasing of the primary outcome Peer reviewer detection of errors, as it was always the intention to include any study that used error detection as an outcome, regardless of the origin of the errors. We changed the wording from "Identification of deliberate, inserted flaws in manuscripts and proposals" to "Identification of flaws in manuscripts and proposals".
Methods > Search methods for identification of studies > Electronic searches
In the Electronic searches section and in Appendix 1, we provided a more detailed description of the search strategy in the review compared to the protocol. However, we did not change the search strategy itself.
Methods > Data collection and analysis > Data extraction and management
There are some differences regarding which of the review authors were involved in the different stages of data collection and analysis. The protocol states that IS and JOH would screen the studies for inclusion, while JOH and TD would extract data and assess the risk of bias. However, IS, JOH, and TD were involved in all these steps. In addition, IS and JOH were authors of one of the included studies. For this study, AF and TD performed screening, data extraction, and risk of bias assessment.
Additionally, we added information on what we would have done if we had encountered studies that required translation.
Methods > Data collection and analysis > Measures of the effect of the methods To bring our methods more in line with what we would have done and what we believe we will do in updated versions of this review, we modified the following paragraph.
Protocol: "If more than one adherence outcome is reported within the same study, we will use the primary outcome as defined by the study authors. If a primary outcome is not clearly defined, we will calculate and use the median value from all relevant outcomes."
Review: "When a study reported more than one measure for the same outcome, we used the primary outcome as defined by the study authors. If the primary outcome was not clearly defined, we would have chosen the outcome most similar to approaches used across included trials."
Methods > Data collection and analysis > Assessment of reporting biases To make it clear that we would only have used funnel plots if any meta‐analysis had included 10 or more studies, we modified the following sentence.
Protocol: "If our review had included ten or more included studies, we would have investigated publication (or other small‐study) bias by visually inspecting funnel plots for skewness."
Review: "If any of the meta‐analyses had included 10 or more studies, we would have investigated publication (or other small‐study) bias by visually inspecting funnel plots for skewness."
Contributions of authors
JOH, HS, TD, IS, and AF developed the protocol. HS searched for the studies and obtained copies of them. JOH, TD, and IS selected studies to include, with AF serving as the arbitrator. JOH, TD, and IS extracted data from the studies. JOH entered data into RevMan. JOH and TD carried out and interpreted the analysis. JOH drafted the final review. All authors contributed to the final version of the review
Sources of support
Internal sources
-
University of Oslo, Norway
The University of Oslo is the affiliation of the lead author (JOH). No monetary support, but access to subscription‐based journals was provided.
External sources
-
Stiftelsen Dam, Norway
This systematic review is a part of a PhD that is funded by The Norwegian Foundation Dam (Stiftelsen Dam). Jan‐Ole Hesselberg is the chief programme officer and Ida C. Svege is head of programme development at the foundation.
Declarations of interest
JOH: together with IS, JOH is an author of one of the studies included in this review (Hesselberg 2021). For this study, AF and TD performed screening, data extraction, and risk of bias assessment. JOH is the chief programme officer at The Norwegian Foundation Dam (Stiftelsen Dam), a research funder that also funded the PhD which this systematic review is a part of. IS: together with JOH, is an author of one of the studies included in this review (Hesselberg 2021). For this study, AF and TD performed screening, data extraction, and risk of bias assessment. IS is the head of programme development at The Norwegian Foundation Dam (Stiftelsen Dam). TD: none known. HS: none known. AF: none known.
New
References
References to studies included in this review
Callaham 2002a Study 1 {published data only}
- Callaham ML, Knopp RK, Gallagher EJ. Effect of written feedback by editors on quality of reviews: two randomized trials. Journal of the American Medical Association 2002;287(21):2781-3. [DOI: 10.1001/jama.287.21.2781] [DOI] [PubMed] [Google Scholar]
Callaham 2002a Study 2 {published data only}
- Callaham ML, Knopp RK, Gallagher EJ. Effect of written feedback by editors on quality of reviews: two randomized trials. Journal of the American Medical Association 2002;287(21):2781-3. [DOI: 10.1001/jama.287.21.2781] [DOI] [PubMed] [Google Scholar]
Callaham 2002b {published data only}
- Callaham ML, Schriger DL. Effect of structured workshop training on subsequent performance of journal peer reviewers. Annals of Emergency Medicine 2002;40(3):323-8. [DOI] [PubMed] [Google Scholar]
Cobo 2007 {published data only}
- Cobo E, Selva-O'Callagham A, Ribera JM, Cardellach F, Dominguez R, Vilardell M. Statistical reviewers improve reporting in biomedical articles: a randomized trial. PLOS ONE 2007;2(3):e332. [DOI: 10.1371/journal.pone.0000332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesselberg 2021 {published data only}
- Hesselberg J-O, Fostervold KI, Ulleberg P, Svege I. Individual versus general structured feedback to improve agreement in grant peer review: a randomized controlled trial. Research Integrity and Peer Review 2021;6:12. [DOI: 10.21203/rs.3.rs-461626/v1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houry 2012 {published data only}
- Houry D, Green S, Callaham M. Does mentoring new peer reviewers improve review quality? A randomized trial. BMC Medical Education 2012;12:83. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sattler 2015 {published data only}
- Sattler DN, McKnight PE, Naney L, Mathis R. Grant peer review: improving inter-rater reliability with training. PLOS ONE 2015;10(6):e0130450. [DOI: 10.1371/journal.pone.0130450] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroter 2004 {published data only}
- Schroter S, Black N, Evans S, Carpenter J, Godlee F, Smith R. Effects of training on quality of peer review: randomised controlled trial. BMJ 2004;328(7441):673. [DOI: 10.1136/bmj.38023.700775.AE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Speich 2023 Study 1 {unpublished data only}
- Speich B, Mann E, Schönenberger CM, Mellor K, Griessbach AN, Dhiman P, et al. Reminding peer reviewers of the most important reporting guideline items to improve completeness in published articles: primary results of two randomized controlled trials. Personal communication with author 2023. [DOI] [PMC free article] [PubMed]
- Speich B. Impact of a short form of the SPIRIT checklist for peer reviewers to improve the reporting of protocols for randomised controlled trials published in biomedical journals: a randomised controlled trial. Open Science Framework 2020. [DOI: 10.17605/OSF.IO/Z2HM9] [DOI] [PMC free article] [PubMed]
Speich 2023 Study 2 {published data only}
- Speich B, Mann E, Schönenberger CM, Mellor K, Griessbach AN, Dhiman P, et al. Reminding peer reviewers of the most important reporting guideline items to improve completeness in published articles: primary results of two randomized controlled trials. Personal communication with author 2023. [DOI] [PMC free article] [PubMed]
- Speich B, Schroter S, Briel M, Moher D, Puebla I, Clark A, et al. Impact of a short version of the CONSORT checklist for peer reviewers to improve the reporting of randomised controlled trials published in biomedical journals: study protocol for a randomised controlled trial. BMJ Open 2020;10(3):e035114. [DOI: 10.1136/bmjopen-2019-035114] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Blanco 2020 {published data only}
- Blanco D, Schroter S, Aldcroft A, Moher D, Boutron I, Kirkham JJ, et al. Effect of an editorial intervention to improve the completeness of reporting of randomised trials: a randomised controlled trial. BMJ Open 2020;10(5):e036799. [DOI: 10.1136/bmjopen-2020-036799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chauvin 2017 {published data only}
- Chauvin A, Moher D, Altman D, Schriger DL, Alam S, Hopewell S, et al. A protocol of a cross-sectional study evaluating an online tool for early career peer reviewers assessing reports of randomised controlled trials. BMJ Open 2017;7(9):1-10. [DOI: 10.1136/bmjopen-2017-017462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cobo 2011 {published data only}
- Cobo E, Cortes J, Ribera JM, Cardellach F, Selva-O'Callaghan A, Kostov B, et al. Effect of using reporting guidelines during peer review on quality of final manuscripts submitted to a biomedical journal: masked randomised trial. BMJ 2011;343:d6783. [DOI: 10.1136/bmj.d6783] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowe 2011 {published data only}
- Crowe M, Sheppard L, Campbell A. Comparison of the effects of using the Crowe Critical Appraisal Tool versus informal appraisal in assessing health research: a randomised trial. International Journal of Evidence-Based Healthcare 2011;9(4):444-9. [DOI: 10.1111/j.1744-1609.2011.00237.x] [DOI] [PubMed] [Google Scholar]
DiDomenico 2017 {published data only}
- DiDomenico RJ, Baker WL, Haines AT. Improving peer review: what reviewers can do. American Journal of Health-System Pharmacy 2017;74(24):2080-4. [DOI: 10.2146/ajhp170190] [DOI] [PubMed] [Google Scholar]
Gardner 1986 {published data only}
- Gardner MJ, Machin D, Campbell MJ. Use of check lists in assessing the statistical content of medical studies. British Medical Journal (Clinical Research Edition) 1986;292(6523):810-2. [DOI: 10.1136/bmj.292.6523.810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghannad 2021 {published data only}
- Ghannad M, Yang B, Leeflang M, Aldcroft A, Bossuyt PM, Schroter S, et al. A randomized trial of an editorial intervention to reduce spin in the abstract's conclusion of manuscripts showed no significant effect. Journal of Clinical Epidemiology 2021;130:69-77. [DOI: 10.1016/j.jclinepi.2020.10.014] [DOI] [PubMed] [Google Scholar]
Green 1989 {published data only}
- Green JG, Calhoun F, Nierzwicki L, Brackett J, Meier P. Rating intervals: an experiment in peer review. FASEB Journal 1989;3(8):1987-92. [DOI: 10.1096/fasebj.3.8.2721858] [DOI] [PubMed] [Google Scholar]
Jones 2019 {published data only}
- Jones CW, Adams A, Weaver MA, Schroter S, Misemer BS, Schriger D, et al. Peer reviewed evaluation of registered end-points of randomised trials (the PRE-REPORT study): protocol for a stepped-wedge, cluster-randomised trial. BMJ Open 2019;12:e066624. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacAuley 1998 {published data only}
- MacAuley D, McCrum E, Brown C. Randomised controlled trial of the READER method of critical appraisal in general practice. BMJ 1998;316(7138):1134-7. [DOI: 10.1136/bmj.316.7138.1134] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03751878 {published data only}
- NCT03751878. Evaluating the impact of assessing during peer review the CONSORT checklist submitted by authors. clinicaltrials.gov/study/NCT03751878?tab=history (first received 21 November 2018).
Pitman 2019 {published data only}
- Pitman A, Underwood R, Hamilton A, Tyrer P, Yang M. Enhanced peer-review for optimising publication of biomedical papers submitted from low- and middle-income countries: feasibility study for a randomised controlled trial. BJPsych Open 2019;5(2):e20. [DOI: 10.1192/bjo.2018.89] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strowd 2014 {published data only}
- Strowd R, Wong V, Aragon-Garcia R, Moon Y, Ford B, Haut S, et al. Use of mentored peer review of standardized manuscripts as an educational tool for neurology residents (P1.335). Neurology 2014;82(10 Supplement):P1.335. [Google Scholar]
Wong 2017 {published data only}
- Wong VS, Strowd RE, Aragon-Garcia R, Moon YO, Ford B, Haut SR, et al. Mentored peer review of standardized manuscripts as a teaching tool for residents: a pilot randomized controlled multi-center study. Research Integrity & Peer Review 2017;2:6. [DOI: 10.1186/s41073-017-0032-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdoul 2012
- Abdoul H, Perrey C, Amiel P, Tubach F, Gottot S, Durand-Zaleski I, et al. Peer review of grant applications: criteria used and qualitative study of reviewer practices. PLOS One 2012;7(9):e46054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhargava 2014
- Bhargava S, Fisman R. Contrast effects in sequential decisions: evidence from speed dating. Review of Economics and Statistics 2014;96(3):444-57. [Google Scholar]
Bol 2018
- Bol T, Vaan M, de Rijt A. The Matthew effect in science funding. Proceedings of the National Academy of Sciences of the United States of America 2018;115(19):4887-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bornmann 2006
- Bornmann L, Daniel H-D. Potential sources of bias in research fellowship assessments: effects of university prestige and field of study. Research Evaluation 2006;15(3):209-19. [Google Scholar]
Bornmann 2011
- Bornmann L. Scientific peer review. Annual Review of Information Science and Technology 2011;45(1):197-245. [Google Scholar]
Bruce 2016
- Bruce R, Chauvin A, Trinquart L, Ravaud P, Boutron I. Impact of interventions to improve the quality of peer review of biomedical journals: a systematic review and meta-analysis. BMC Medicine 2016;14(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Callaham 1998
- Callaham ML, Baxt WG, Waeckerle JF, Wears RL. Reliability of editors’ subjective quality ratings of peer reviews of manuscripts. Journal of the American Medical Association 1998;280(3):229-31. [DOI: 10.1001/jama.280.3.229] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chauvin 2015
- Chauvin A, Ravaud P, Baron G, Barnes C, Boutron I. The most important tasks for peer reviewers evaluating a randomized controlled trial are not congruent with the tasks most often requested by journal editors. BMC Medicine 2015;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2021 [Computer program]
- Covidence. Version accessed 29 May 2019. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org.
Danziger 2011
- Danziger S, Levav J, Avnaim-Pesso L. Extraneous factors in judicial decisions. Proceedings of the National Academy of Sciences of the United States of America 2011;108(17):6889-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Demicheli 2007
- Demicheli V, Di Pietrantonj C. Peer review for improving the quality of grant applications. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: MR000003. [DOI: 10.1002/14651858.MR000003.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsevier 2023
- Elsevier. Role of a reviewer. www.elsevier.com/reviewers/role (accessed 11 Mar 2023).
European Commission 2018
- European Commission. HORIZON 2020 in full swing. Three years on. Key facts and figures 2014-2016. European Commission, 2018. Available from: www.kowi.de/Portaldata/2/Resources/horizon2020/H2020-2014-2016-Key-Facts-and-Figures.pdf.
European Commission 2021
- Directorate-General for Research and Innovation. Horizon Europe, budget: Horizon Europe - the most ambitious EU research & innovation programme ever. Publications Office of the European Union 2021. [DOI: 10.2777/202859] [DOI]
Gallo 2018
- Gallo S, Thompson L, Schmaling K, Glisson S. Risk evaluation in peer review of grant applications. Environment Systems and Decisions 2018;38(2):216-29. [Google Scholar]
Goodman 1994
- Goodman SN. Manuscript quality before and after peer review and editing at Annals of Internal Medicine. Annals of Internal Medicine 1994;121(1):11-21. [DOI: 10.7326/0003-4819-121-1-199407010-00003] [DOI] [PubMed] [Google Scholar]
Guthrie 2017
- Guthrie S, Ghiga I, Wooding S. What do we know about grant peer review in the health sciences? F1000Research 2017;6:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ (Clinical Research Ed.) 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Horsley 2011
- Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: MR000026. [DOI: 10.1002/14651858.MR000026.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huber 2022
- Huber J, Sabiou A, Kerschbamer, Smith VL. Nobel and novice: author prominence affects peer review. Proceedings of the National Academy of Sciences of the United States of America 2022;119(41):e2205779119. [DOI: 10.1073/pnas.2205779119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2007
- Jefferson T, Rudin M, Brodney Folse S, Davidoff F. Editorial peer review for improving the quality of reports of biomedical studies. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: MR000016. [DOI: 10.1002/14651858.MR000016.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lane 2020
- Lane JN, Teplitskiy M, Gray G, Ranu H, Menietti M, Guinan E, et al. Conservatism Gets Funded? A Field Experiment on the Role of Negative Information in Novel Project Evaluation. Harvard Business School Technology & Operations Mgt. Unit Working Paper No. 21-007, 2020. Available from: papers.ssrn.com/sol3/papers.cfm?abstract_id=3656495. [WEBSITE: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3656495]
Langfeldt 2006
- Langfeldt L. The policy challenges of peer review: managing bias, conflict of interests and interdisciplinary assessments. Research Evaluation 2006;15(1):31-41. [Google Scholar]
Lee 2013
- Lee CJ, Sugimoto CR, Zhang G, Cronin B. Bias in peer review. Journal of the American Society for Information Science and Technology 2013;64(1):2-17. [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. JNCI: Journal of the National Cancer Institute 1959;22(4):719-48. [PubMed] [Google Scholar]
Mulligan 2013
- Mulligan A, Hall L, Raphael E. Peer review in a changing world: an international study measuring the attitudes of researchers. Journal of the American Society for Information Science and Technology 2013;64(1):132-61. [Google Scholar]
National Institutes of Health 2022
- National Institutes of Health. Budget. www.nih.gov/about-nih/what-we-do/budget (accessed 11 March 2023). [WEBSITE: https://www.nih.gov/about-nih/what-we-do/budget]
National Library of Medicine 2022
- National Library of Medicine. MEDLINE PubMed Production Statistics. National Library of Medicine 2022. [WEBSITE: https://www.nlm.nih.gov/bsd/medline_pubmed_production_stats.html]
Olbrecht 2010
- Olbrecht M, Bornmann L. Panel peer review of grant applications: what do we know from research in social psychology on judgment and decision-making in groups? Research Evaluation 2010;19(4):293-304. [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;371:n71. [DOI: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Recio‐Saucedo 2022
- Recio-Saucedo A, Crane K, Meadmore K, Fackrell K, Church H, Fraser S, et al. What works for peer review and decision-making in research funding: a realist synthesis. Research Integrity and Peer Review 2022;7(2):1-28. [DOI: 10.1186/s41073-022-00120-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Roumbanis 2017
- Roumbanis L. Academic judgments under uncertainty: A study of collective anchoring effects in Swedish Research Council panel groups. Social Studies of Science 2017;47(1):95-116. [DOI: 10.1177/0306312716659789] [DOI] [PubMed] [Google Scholar]
Sattler 2015
- Sattler DN, McKnight PE, Naney L, Mathis R. Grant peer review: improving inter-rater reliability with training. PLOS One 2015;10(6):e0130450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. [DOI: 10.1186/1741-7015-8-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sense About Science 2009
- Sense About Science. Peer Review Survey 2009: Full Report. senseaboutscience.org/activities/peer-review-survey-2009/ (accessed 30 July 2020).
Superchi 2019
- Superchi C, González JA, Solà I, Cobo E, Hren D, Boutron I. Tools used to assess the quality of peer review reports: a methodological systematic review. BMC Medical Research Methodology 2019;19(1):48. [DOI: 10.1186/s12874-019-0688-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teplitskiy 2019
- Teplitskiy M, Gray G, Guinan E, Ranu H, Menietti M, Lakhani KR. Do Experts Listen to Other Experts? Field Experimental Evidence from Scientific Peer Review. Harvard Business School, 2019.. Avaialble from www.hbs.edu/ris/Publication%20Files/19-107_06115731-d0ae-4a11-ab1d-ecaec2118921.pdf.
van Rooyen 1999
- Rooyen S, Black N, Godlee F. Development of the review quality instrument (RQI) for assessing peer reviews of manuscripts. Journal of Clinical Epidemiology 1999;52(7):625-9. [DOI] [PubMed] [Google Scholar]
Walker 2015
- Walker R, Barros B, Conejo R, Neumann K, Telefont M. Personal attributes of authors and reviewers, social bias and the outcomes of peer review: a case study. F1000Research 2015;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015
- Wang Q, Sandström U. Defining the role of cognitive distance in the peer review process with an explorative study of a grant scheme in infection biology. Research Evaluation 2015;24(3):271-81. [Google Scholar]
Ware 2015
- Ware M, Mabe M. The STM Report - an overview of scientific and scholarly journal publishing. Available from www.stm-assoc.org/2015_02_20_STM_Report_2015.pdf 2015.
Warne 2016
- Warne V. Rewarding reviewers – sense or sensibility? A Wiley study explained. Learned Publishing 2016;29(1):41-50. [Google Scholar]
Young 2011
- Young T, Hopewell S. Methods for obtaining unpublished data. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: MR000027. [DOI: 10.1002/14651858.MR000027.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hesselberg 2020
- Hesselberg J-O, Dalsbø TK, Stromme H, Svege I, Fretheim A. Reviewer training for improving grant and journal peer review. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: MR000056. [DOI: 10.1002/14651858.MR000056] [DOI] [PMC free article] [PubMed] [Google Scholar]


