Abstract
Background
Chemotherapy is an important approach for lung cancer patients. The study was designed to evaluate the feasibility of the compound probiotic supplements in improving the quality of life for lung cancer patients undergoing chemotherapy.
Methods
This randomized, double‐blind, placebo‐controlled trial enrolled chemotherapy‐naive patients with lung cancer who were scheduled to receive platinum‐based doublet chemotherapy. All eligible patients were randomly administered (1:1) compound probiotic supplements (group BP‐1) or placebo (group C) for two chemotherapy cycles. The EORTC QLQ C30 questionnaire scores were evaluated before the first, second, and third cycles of chemotherapy. The primary endpoint was the difference in the EROTC QLQ C30 questionnaire score between the two groups after two cycles of chemotherapy.
Results
A total of 110 patients were recruited from March 2021 to January 2022. After undergoing two cycles of chemotherapy, group BP‐1 were significantly better in various dimensions of the overall quality of life, role function, nausea and vomiting, appetite loss, constipation, and diarrhea relative to group C (76.90 ± 18.31 vs. 58.89 ± 17.17; 93.33 ± 11.58 vs. 85.93 ± 15.06; 0.00 ± 0.00 vs. 27.04 ± 29.15; 6.67 ± 13.53 vs. 22.22 ± 18.80; 0.95 ± 5.63 vs. 28.15 ± 22.42; 2.86 ± 9.47 vs. 15.56 ± 16.82; p < 0.05, respectively). The incidence of nausea and vomiting, appetite loss, constipation, and diarrhea in group BP‐1 was significantly lower than in group C (0% vs. 71.43%, 16.67% vs. 57.14%, 2.38% vs. 63.27%, and 7.14% vs. 42.86%, respectively, p < 0.001).
Conclusions
Compound probiotic supplements can improve the quality of life and relieve chemotherapy‐related gastrointestinal side effects for lung cancer patients receiving platinum‐based doublet chemotherapy. (Chinese Clinical Trial Registry: ChiCTR1800019269).
Keywords: chemotherapy, gut microbiota, lung cancer, probiotics, quality of life
Our study demonstrated that oral compound probiotics supplements can improve the quality of life and relieve chemotherapy‐related gastrointestinal adverse events for lung cancer patients receiving platinum‐based chemotherapy.
INTRODUCTION
Despite significant improvements in the realm of anticancer strategies, whatever chemotherapy is administered as a monotherapy or in combination with other treatment paradigms encompassing surgery, radiotherapy, immune checkpoint inhibitors (ICIs), antiangiogenesis agents, targeted therapies, and beyond, it remains an important treatment strategy for almost all cancers, especially lung cancer, the most prevalent and fatal malignant tumor in the population. 1 , 2
Platinum‐based doublet chemotherapy occupies an important role in the management of lung cancer; however, there are treatment‐associated side effects which include nausea and vomiting, fatigue, appetite loss, pain, constipation, diarrhea, and many other debilitating symptoms that may deteriorate the quality of life for patients undergoing chemotherapy, and even necessitate dose reduction or discontinuation. 3 , 4
Chemotherapy can also damage the gastrointestinal epithelial cells and result in a disorder of the gut microbiome. 5 , 6 Gut microbiota has been proven to protect the intestinal mucosa, prevent intestinal inflammation, and build the immune ecology of the whole body. 7 , 8 Previous studies showed that certain cytotoxic drugs such as cyclophosphamide, fluorouracil, and etoposide appeared to have antibacterial properties in plasma, and medications such as irinotecan, fluorouracil would affect the diversity of the gut microbiome, 9 , 10 which may result in gastrointestinal mucositis, exacerbating the mucositis caused by chemotherapeutic drugs, thereby leading to severe gastrointestinal complications in patients receiving chemotherapy. 11 , 12 , 13 , 14
Many strategies for controlling chemotherapy‐related adverse events have been applied in clinical practice during the past decades, but the management situation is not optimistic in the real world. 3 , 15 , 16 Emerging evidence favors the strategy of gut microbiota regulation for ameliorating chemotherapy‐related adverse events. 17 In particular, the feasibility of probiotic supplementation to ameliorate chemotherapy‐related adverse effects has been demonstrated in preclinical and clinical studies. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Nevertheless, whether administering compound probiotics can relieve chemotherapy‐related adverse events for lung patients undergoing platinum‐based doublet chemotherapy is rarely reported. This study attempted to determine whether oral compound probiotic supplements can reduce chemotherapy‐related adverse effects and improve lung cancer patients quality of life during chemotherapy.
METHODS
This prospective, randomized, placebo‐controlled, multicenter clinical study was conducted at three major cancer centers in Sichuan province, China: Thoracic Oncology Ward, West China Hospital, Sichuan University; Cancer Center, No.7 People's Hospital of Chengdu; Cancer Center, People's Hospital of Sichuan Province. The study was approved by the Ethics Committee of the West China Hospital of Sichuan University and conformed to the Declaration of Helsinki. The study was registered in the Chinese Clinical Trial Registry (registration no.: ChiCTR1800019269). All eligible patients provided written informed consent.
Patients
This study intended to screen and enroll chemotherapy‐naive patients with lung cancer who were scheduled to receive platinum‐based doublet chemotherapy. The quality of life score of lung cancer patients during chemotherapy was around 60 (Figure 1). It was expected that it could be improved to 70 by using a compound probiotic preparation, taking the test level α as 0.05, assuming that the number of cases in both groups was equal, the minimum sample size to be included in each group was n1 = n2 = 49, N = 98, considering 10% of patients were excluded, the final number of cases would be 110.
FIGURE 1.
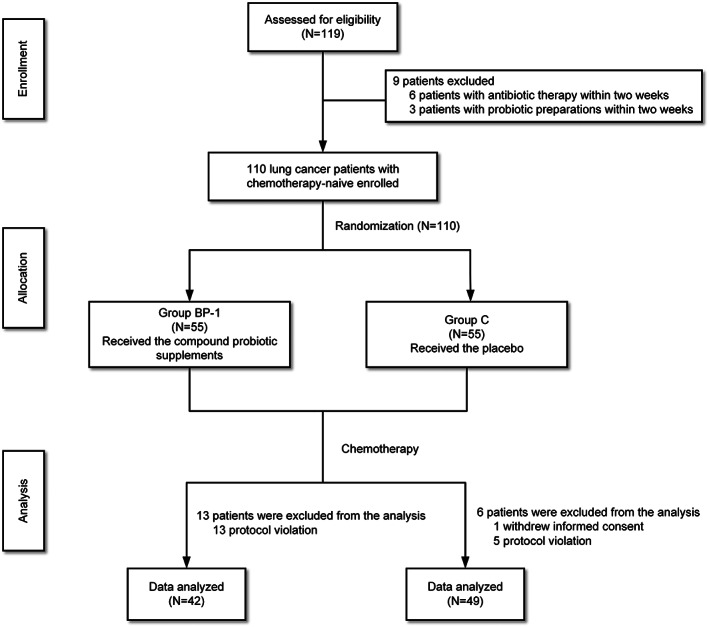
The flow chart of patient disposition.
Patients who met all of the following criteria were included: (1) pathologically confirmed with lung cancer (including non‐small cell lung cancer and small cell lung cancer); (2) chemotherapy‐naive; (3) aged between 18 and 75 years old; (4) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; (5) receiving chemotherapy for the first time with a regimen of cisplatin/carboplatin (AUC ≥5) in combination with one of these following agents: paclitaxel, docetaxel, gemcitabine, vinorebine, pemetrexed, etoposide, and who were supposed to receive two or more cycles of chemotherapy.
The main exclusion criteria were as follows: (1) Patients who received recent (within 2 weeks before chemotherapy) antibiotic therapy or proton pump inhibitor therapy, (2) patients who had recently (within 2 weeks before chemotherapy) taken some probiotic preparations, (3) patients with chronic gastrointestinal diseases or gastrointestinal metastatic tumor, (4) patients with severe systemic metabolic diseases or immune system diseases.
Based on the computer‐generated program, enrolled patients were randomly assigned (1:1) to either the compound probiotic supplements group (group BP‐1) or the placebo group (group C). A total of 110 random identification numbers were created by the computer before patients enrolled. Each enrolled patient was given an identification number based on the enrolment order and assigned to the corresponding group based on the identification number. The process mentioned above was performed by the Cancer Psychology and Health Management Committee of the Sichuan Cancer Society and was double‐blind for both subjects and researchers.
Treatment
The enrolled patients were administered the platinum‐based doublet chemotherapy regimen recommended by NCCN/CSCO guidelines. 26
The oral compound probiotic supplements (Hua Wei Yi probiotic solid drink, Yiga Bio‐technology Chengdu Co., LTD, Chengdu, China) contained oligofructose (added at >93.69%), Bifidobacterium lactis Bi‐07, Lactobacillus acidophilus NCFM, Lactobacillus rhamnosus HN001, Bifidobacterium lactis HN019. It was packaged in an aluminum‐plastic film bag and a maltodextrin‐based placebo with the same appearance and taste. All compound probiotics/placebos in this study were received from Yiga Bio‐technology Chengdu Co., Ltd (via the Cancer Psychology and Health Management Committee of the Sichuan Cancer Society. The corresponding compound probiotic supplement/placebo with an identification number was assigned when a patient was enrolled. The Cancer Psychology and Health Management Committee of the Sichuan Cancer Society kept the assignment information list and was responsible for compound probiotic supplement/placebo distribution. The enrolled patients were given compound probiotic supplements/placebos at the beginning of the first cycle of chemotherapy, one sachet (2 g) dissolved in cold water or indoor temperature twice a day until the start of the third chemotherapy cycle.
Follow‐up and data collection
All enrolled patients were evaluated for two chemotherapy cycles. The EORTC QLQ C30 questionnaire was completed independently by the enrolled patients prior to the first, second, and third cycles of chemotherapy. The scale consisted of 30 items, including five functional dimensions (physical function, role function, cognitive function, emotional function, and social function), nine symptom dimensions (fatigue, pain, nausea and vomiting, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties), and one dimension of overall quality of life. 27 For the functional and overall quality of life dimensions, higher scores indicated better functional status and quality of life. In contrast, for the symptom dimensions, higher scores indicated more severe symptoms or problems.
The National Cancer Institute Common Toxicity Criteria (version 4.0) was used for the evaluation of adverse events.
Statistical analysis
The primary endpoint was the difference in the EROTC QLQ C30 questionnaire score between the two groups after two cycles of chemotherapy. For qualitative data, we used the chi‐square test to determine whether there was a difference between groups BP‐1 and C. The Mann–Whitney U or independent‐sample t‐test were used to compare the differences for quantitative data. The above statistical analysis was performed with SPSS version 27 software. Two‐sided p‐values < 0.05 were determined to be statistically significant.
RESULTS
Patient characteristics
From March 2021 to January 2022, 110 patients were enrolled, of whom one patient withdrew their informed consent and 18 patients were excluded due to protocol violation. A total of 91 patients were included in the final statistics. No significant differences were observed between the two groups at baseline (Table 1).
TABLE 1.
Baseline demographic characteristics of 91 lung cancer patients.
| Characteristic | Group C (N = 49) | Group BP‐1 (N = 42) | p‐value |
|---|---|---|---|
| Age (year), mean (SD) | 60.06 (7.67) | 58.95 (8.51) | 0.515 |
| Gender, n (%) | |||
| Male | 33 (67.35) | 30 (71.43) | 0.674 |
| Female | 16 (32.65) | 12 (28.57) | |
| Ethnicity, n (%) | |||
| Han | 48 (97.96) | 41 (97.62) | 1.000 |
| Other | 1 (2.04) | 1 (2.38) | |
| History of allergy, n (%) | |||
| No | 49 (100.00) | 42 (100.00) | ‐ |
| History of significant past, n (%) | |||
| No | 49 (100.00) | 42 (100.00) | ‐ |
| Stage, n (%) | 0.249 | ||
| I | 12 (24.49) | 6 (14.29) | |
| II | 8 (16.33) | 14 (33.33) | |
| III | 19 (38.78) | 15 (35.71) | |
| IV | 10 (20.41) | 7 (16.67) | |
| History of surgery, n (%) | |||
| Yes | 27 (55.10) | 25 (59.52) | 0.671 |
| No | 22 (44.90) | 17 (40.48) | |
| Performance status, n (%) | |||
| 0 | 40 (81.63) | 32 (76.19) | 0.524 |
| 1 | 9 (18.37) | 10 (23.81) | |
| Chemotherapy regimen | |||
| Pemetrexed + carboplatin | 19 (38.78) | 16 (38.10) | 0.680 |
| Pemetrexed + cisplatinum | 6 (12.24) | 7 (16.67) | |
| Etoposide + cisplatinum | 5 (10.20) | 7 (16.67) | |
| Gemcitabine + cisplatinum | 2 (4.08) | 1 (2.38) | |
| Paclitaxel + carboplatin | 7 (14.29) | 7 (16.67) | |
| Paclitaxel + cisplatinum | 10 (20.41) | 4 (9.52) | |
Abbreviations: SD, standard deviation.
EORTC QLQ C30 questionnaire analysis
No significant difference was observed in the scores of the EORTC QLQ C30 questionnaire at baseline between the two groups (Table 2).
TABLE 2.
Quality of life before and after receiving interventions and chemotherapy treatment in different groups.
| Dimensions Mean (SD) | Group C (N = 49) | Group BP‐1 (N = 42) | p‐value |
|---|---|---|---|
| Overall quality of life | |||
| Before the first cycle of chemotherapy | 67.21 (23.92) | 73.20 (19.56) | 0.304 |
| Before the second cycle of chemotherapy | 61.55 (19.12) | 75.45 (15.46) | 0.001 |
| Before the third cycle of chemotherapy | 58.89 (17.17) | 76.90 (18.31) | <0.001 |
| Functional dimensions | |||
| Physical function | |||
| Before the first cycle of chemotherapy | 87.83 (13.36) | 85.23 (10.67) | 0.085 |
| Before the second cycle of chemotherapy | 89.24 (8.41) | 88.11 (10.08) | 0.666 |
| Before the third cycle of chemotherapy | 90.96 (8.18) | 89.62 (9.66) | 0.595 |
| Role function | |||
| Before the first cycle of chemotherapy | 88.41 (16.80) | 84.23 (17.54) | 0.230 |
| Before the second cycle of chemotherapy | 91.29 (12.70) | 91.89 (12.80) | 0.770 |
| Before the third cycle of chemotherapy | 85.93 (15.06) | 93.33 (11.58) | 0.023 |
| Cognitive function | |||
| Before the first cycle of chemotherapy | 87.68 (16.27) | 90.09 (12.08) | 0.712 |
| Before the second cycle of chemotherapy | 91.29 (12.18) | 92.34 (14.48) | 0.392 |
| Before the third cycle of chemotherapy | 88.52 (13.68) | 93.33 (11.58) | 0.093 |
| Emotional function | |||
| Before the first cycle of chemotherapy | 87.32 (13.69) | 80.86 (17.88) | 0.086 |
| Before the second cycle of chemotherapy | 88.07 (14.86) | 88.96 (15.72) | 0.837 |
| Before the third cycle of chemotherapy | 87.04 (13.36) | 90.95 (11.32) | 0.244 |
| Social function | |||
| Before the first cycle of chemotherapy | 76.45 (24.24) | 77.93 (15.74) | 0.942 |
| Before the second cycle of chemotherapy | 83.33 (17.24) | 84.23 (21.50) | 0.518 |
| Before the third cycle of chemotherapy | 86.67 (17.26) | 87.62 (16.83) | 0.839 |
| Symptom dimensions | |||
| Fatigue | |||
| Before the first cycle of chemotherapy | 19.32 (14.52) | 23.72 (13.65) | 0.175 |
| Before the second cycle of chemotherapy | 21.26 (21.95) | 15.77 (13.19) | 0.443 |
| Before the third cycle of chemotherapy | 16.30 (13.10) | 11.90 (12.93) | 0.118 |
| Pain | |||
| Before the first cycle of chemotherapy | 15.94 (20.17) | 10.81 (17.22) | 0.213 |
| Before the second cycle of chemotherapy | 17.75 (25.68) | 6.76 (13.87) | 0.021 |
| Before the third cycle of chemotherapy | 10.00 (13.94) | 8.10 (10.97) | 0.648 |
| Nausea and vomiting | |||
| Before the first cycle of chemotherapy | 5.07 (10.46) | 4.05 (9.13) | 0.715 |
| Before the second cycle of chemotherapy | 22.46 (24.14) | 4.05 (9.13) | <0.001 |
| Before the third cycle of chemotherapy | 27.04 (29.15) | 0.00 (0.00) | <0.001 |
| Dyspnea | |||
| Before the first cycle of chemotherapy | 21.74 (20.14) | 27.03 (17.28) | 0.169 |
| Before the second cycle of chemotherapy | 19.57 (24.92) | 14.41 (16.74) | 0.510 |
| Before the third cycle of chemotherapy | 17.78 (18.26) | 13.33 (16.57) | 0.291 |
| Insomnia | |||
| Before the first cycle of chemotherapy | 26.81 (22.90) | 22.52 (20.87) | 0.412 |
| Before the second cycle of chemotherapy | 20.30 (26.74) | 10.81 (15.82) | 0.130 |
| Before the third cycle of chemotherapy | 20.00 (22.92) | 14.29 (16.74) | 0.351 |
| Appetite loss | |||
| Before the first cycle of chemotherapy | 10.87 (17.29) | 8.11 (16.49) | 0.390 |
| Before the second cycle of chemotherapy | 22.46 (24.40) | 9.91 (15.45) | 0.010 |
| Before the third cycle of chemotherapy | 22.22 (18.80) | 6.67 (13.53) | <0.001 |
| Constipation | |||
| Before the first cycle of chemotherapy | 8.70 (16.38) | 6.31 (13.24) | 0.556 |
| Before the second cycle of chemotherapy | 23.91 (24.00) | 6.31 (13.24) | <0.001 |
| Before the third cycle of chemotherapy | 28.15 (22.42) | 0.95 (5.63) | <0.001 |
| Diarrhea | |||
| Before the first cycle of chemotherapy | 7.97 (14.38) | 5.41 (14.73) | 0.266 |
| Before the second cycle of chemotherapy | 9.42 (22.95) | 1.80 (7.64) | 0.056 |
| Before the third cycle of chemotherapy | 15.56 (16.82) | 2.86 (9.47) | <0.001 |
| Financial difficulties | |||
| Before the first cycle of chemotherapy | 30.43 (32.07) | 26.13 (32.52) | 0.455 |
| Before the second cycle of chemotherapy | 23.91 (31.94) | 19.82 (30.89) | 0.443 |
| Before the third cycle of chemotherapy | 20.00 (26.01) | 18.10 (28.40) | 0.491 |
Abbreviations: SD, standard deviation.
After one cycle of chemotherapy and compound probiotic supplement/placebo treatment, the scores of the two groups (group BP‐1 vs. group C) showed a statistically significant difference in the following dimensions: overall quality of life (75.45 ± 15.46 vs. 61.55 ± 19.12, p = 0.001), pain (6.76 ± 13.87 vs. 17.75 ± 25.68, p = 0.021), nausea and vomiting (4.05 ± 9.13 vs. 22.46 ± 24.14, p < 0.001), appetite loss (9.91 ± 15.45 vs. 22.46 ± 24.40, p = 0.010), and constipation (6.31 ± 13.24 vs. 23.91 ± 24.00, p < 0.001). The incidence of nausea and vomiting, appetite loss, constipation, and diarrhea in group BP‐1 was 16.67%, 26.19%, 16.67%, and 4.76%, respectively, significantly lower than that of the group C: 63.27%, 53.06%, 57.14%, and 18.37%, respectively (p < 0.05) (Table 2, Figure 2a).
FIGURE 2.
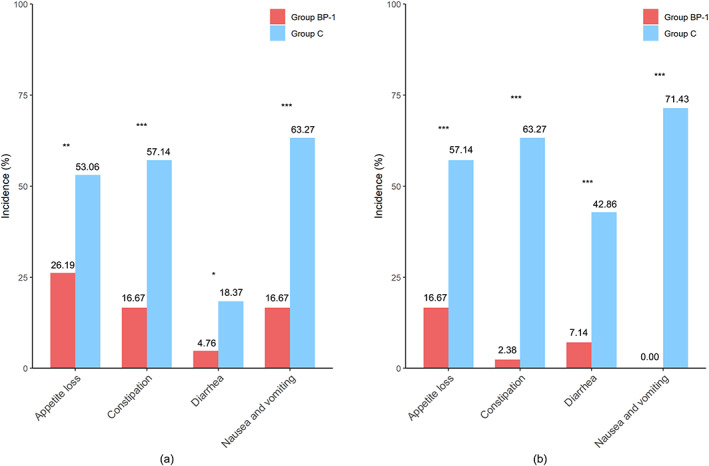
The prevalence of chemotherapy‐related gastrointestinal reactions between group BP‐1 and group C. (a) The incidence of chemotherapy‐related gastrointestinal reactions during the first cycle of chemotherapy. (b) The incidence of chemotherapy‐related gastrointestinal reactions during the second cycle of chemotherapy; *p < 0.05; **p < 0.01; ***p < 0.001.
After 2 cycles of the study treatment, there was a significant difference in the dimensions, including overall quality of life (76.90 ± 18.31 vs. 58.89 ± 17.17, p < 0.001), role function (93.33 ± 11.58 vs. 85.93 ± 15.06, p = 0.023), nausea and vomiting (0.00 ± 0.00 vs. 27.04 ± 29.15, p < 0.001), appetite loss (6.67 ± 13.53 vs. 22.22 ± 18.80, p < 0.001), constipation (0.95 ± 5.63 vs. 28.15 ± 22.42, p < 0.001) and diarrhea (2.86 ± 9.47 vs. 15.56 ± 16.82, p < 0.001) between the group BP‐1 and group C. The incidence of nausea and vomiting, appetite loss, constipation, and diarrhea in group BP‐1 was significantly lower than in group C (0% vs. 71.43%, 16.67% vs. 57.14%, 2.38% vs. 63.27%, and 7.14% vs. 42.86%, respectively, p < 0.001) (Table 2, Figure 2b).
Adverse events
No grade>3 adverse events were observed. There were no differences in adverse reactions between the two groups, except for the incidence of gastrointestinal reactions. In particular, the incidence of nausea and vomiting, constipation, anorexia, and diarrhea were lower in group BP‐1 than in group C (Table 3).
TABLE 3.
Adverse events.
| Toxicity | Grade 1 | Grade 2 | Grade 3 | p‐value | |||
|---|---|---|---|---|---|---|---|
| Group BP‐1 N (%) | Group C N (%) | Group BP‐1 N (%) | Group C N (%) | Group BP‐1 N (%) | Group C N (%) | ||
| Neutropenia | 10 (23.81) | 14 (28.57) | 6 (14.29) | 9 (18.37) | 1 (2.38) | 2 (4.08) | 0.778 |
| Anemia | 8 (19.05) | 15 (30.61) | 8 (19.05) | 4 (8.16) | 0 (0.00) | 1 (2.04) | 0.209 |
| Thrombocytopenia | 9 (21.43) | 7 (14.29) | 1 (2.38) | 2 (4.08) | ‐ | ‐ | 0.624 |
| Sensory neuropathy | 5 (11.90) | 6 (12.24) | 1 (2.38) | 3 (6.12) | 1 (2.38) | 0 (0.00) | 0.509 |
| Nausea and vomiting | 5 (11.90) | 17 (34.69) | 2 (4.76) | 16 (32.65) | 0 (0.00) | 2 (4.08) | <0.001 |
| Anorexia | 10 (23.81) | 22 (44.90) | 1 (2.38) | 6 (12.24) | ‐ | ‐ | 0.007 |
| Constipation | 7 (16.67) | 26 (53.06) | 0 (0.00) | 5 (10.20) | ‐ | ‐ | <0.001 |
| Diarrhea | 3 (7.14) | 13 (26.53) | 0 (0.00) | 7 (14.29) | 0 (0.00) | 1 (2.04) | <0.001 |
| Fatigue | 19 (45.24) | 21 (42.86) | 7 (16.67) | 10 (20.41) | 0 (0.00) | 2 (4.08) | 0.413 |
| Pain | 3 (7.14) | 7 (14.29) | 2 (4.76) | 6 (12.24) | ‐ | ‐ | 0.201 |
| Dyspnea | 3 (7.14) | 6 (12.24) | 0 (0.00) | 3 (6.12) | ‐ | ‐ | 0.098 |
| Insomnia | 5 (11.90) | 5 (10.20) | 3 (7.14) | 5 (10.20) | ‐ | ‐ | 0.857 |
| Dizziness | 3 (7.14) | 5 (10.20) | 1 (2.38) | 0 (0.00) | ‐ | ‐ | 0.408 |
| Alopecia | 4 (9.52) | 3 (6.12) | 2 (4.76) | 2 (4.08) | ‐ | ‐ | 0.816 |
DISCUSSION
Our study demonstrated that compound probiotic supplements can improve the quality of life and relieve platinum‐based doublet chemotherapy‐induced gastrointestinal adverse reactions for lung cancer patients undergoing chemotherapy. Previous clinical studies have also indicated that probiotics may ameliorate chemotherapy‐induced adverse effects. Jiang et al. found that a probiotic combination (Bifidobacterium longum, Lactobacillus lactis, and Enterococcus faecium) can ameliorate the severity of oral mucositis via gut microbiota modulation for nasopharyngeal carcinoma patients who were undergoing concurrent radiochemotherapy. 28 Probiotic combinations containing Bifidobacterium infants, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus have also been shown to be effective in attenuating chemotherapy‐related gastrointestinal complications, especially diarrhea for colorectal cancer patients who were undergoing postoperative chemotherapy. 22 As for lung cancer patients receiving platinum‐based doublet chemotherapy, Clostridium butyricum can relieve chemotherapy‐related diarrhea. 25 However, clinical studies exploring the usage of probiotics to mitigate chemotherapy‐related adverse effects have predominantly concentrated on colorectal cancer and head and neck carcinoma, with fewer studies targeting lung cancer patients. In addition, there has been limited exploration regarding whether combination probiotic preparations containing Lactobacillus and Bifidobacterium can improve chemotherapy‐related adverse effects for lung cancer patients. Our study provides preliminary evidence favoring the potential benefits of compound probiotic supplements to ameliorate chemotherapy‐related adverse effects and the possibility of compound probiotic clinical application in managing chemotherapy‐related complications among lung cancer patients.
It is our inaugural endeavor to improve the quality of life for lung cancer patients who are undergoing chemotherapy through compound probiotic supplements. In this report, after two cycles of compound probiotic supplement/placebo treatment along with platinum‐based doublet chemotherapy, a significant difference in some questionnaire dimensions was shown between the two groups. Most of all, the overall quality of life score in group BP‐1 was significantly better than that in group C, and so was the score of role function. In other words, patients in group BP‐1 maintained a relatively good quality of life during the chemotherapy course, which was aggravated in group C. Moreover, the prevalence of nausea and vomiting, appetite loss, constipation, and diarrhea in group BP‐1 was significantly lower than in group C. The above results imply that the adverse effects caused by chemotherapy may worsen the quality of life. A randomized controlled trial reported a similar situation: the quality of life analysis of KEYNOTE‐024 showed significantly higher scores in the QLQ‐30 questionnaire for nausea and vomiting, constipation, and diarrhea in the chemotherapy group. 29 The symptom control and quality of life investigation conducted in the LUX‐Lung 3 trial performed the QLQ‐30 questionnaire, revealing that 63% of patients in the chemotherapy group experienced nausea and vomiting, and 24% of patients experienced diarrhea following pemetrexed plus cisplatin treatment. 30 Our study observed that the prevalence of nausea and vomiting in group C was 63.27% and 71.43% before the second and third cycle of chemotherapy, respectively; the incidence of diarrhea was 18.37% and 42.86%, respectively, which were close to the findings from the LUX‐Lung 3 trial. Meanwhile, the incidence and the QLQ‐30 questionnaire scores of diarrhea and vomiting in the BP‐1 group were lower than those reported in the LUX‐Lung 3 trial. These findings indicate that the compound probiotics supplements can relieve gastrointestinal side effects; for example, diarrhea and vomiting, thereby maintaining the patient's quality of life during chemotherapy.
How do the probiotics work on improving the quality of life in patients receiving chemotherapy?
Chemotherapeutic agents can disturb the composition and diversity of the gut microbiota, correlated with adverse effects such as diarrhea, appetite loss, etc. 5 , 6 , 31 Considering the close connection between the composition of gut microbiota and short‐chain fatty acids (SCFAs) production, 32 it is plausible to hypothesize that chemotherapy‐related gastrointestinal reactions may be attributed to a decline in SCFA levels resulting from an imbalance in gut microbiota post‐chemotherapy. SCFAs have been demonstrated to attenuate chemotherapy‐related toxicities due to their anti‐inflammatory, antioxidant, and protective characteristics. 32 SCFAs can also be against chemotherapy‐induced intestinal injury via immunoregulation, promoting crypt cell proliferation and maintaining epithelial integrity. 32 , 33 In addition, postoperative chemotherapy may lead to a decline in gut phylum Firmicutes levels for colorectal cancer patients, 22 which are known to be an important source of SCFAs. 34 Bifidobacterium and Lactobacillus are SCFA‐producing microbiota; supplementation with compound probiotics can potentially reduce chemotherapy‐related gastrointestinal adverse events for lung cancer patients by restoring SCFA levels and thus improving quality of life. Our aim is to validate this in future studies.
It is a pity that the sample size of this study was limited after 19 patients were excluded from the final statistical analysis. Further exploration of the variation of gut microbiota and SCFA levels are needed to elucidate the potential mechanisms of compound probiotic supplements to ameliorate chemotherapy‐related adverse effects. In addition, many patients with early‐stage lung cancer were included in our study, and our future studies will be focused on patients with advanced lung cancer. Our study observed that compound probiotic supplements could effectively alleviate gastrointestinal adverse events. We still need to explore whether compound probiotic supplements can improve other chemotherapy‐related adverse reactions and improve the quality of life of cancer patients in future studies. Probiotic agents have been reported to provide a survival benefit for lung cancer patients treated with ICIs. 35 Our study focused mainly on the management of chemotherapy‐related adverse effects and neglected the observation of treatment efficacy, which will be further explored in a subsequent study. However, this study is the first step in evaluating compound probiotic supplement intervention in improving the quality of life and relieving the symptoms of patients suffering from the adverse effects of chemotherapy. We have found a positive trend from the current study. We also plan further clinical trials to provide more robust evidence to confirm the advantages of compound probiotic supplements to lung cancer patients undergoing chemotherapy.
In conclusion, oral compound probiotic supplements can improve the quality of life and relieve chemotherapy‐related gastrointestinal adverse events for lung cancer patients receiving platinum‐based chemotherapy.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, Yu Sun and Jiang Zhu; Data curation, Jialong Han, Ping Chen, and Ke Xie; Formal analysis, Hao Wei, Zhiying Yue, and Jialong Han; Funding acquisition, Yu Sun and Jiang Zhu; Investigation, Jialong Han, Ping Chen, and Ke Xie; Methodology, Yu Sun and Jiang Zhu; Project administration, Yu Sun and Jiang Zhu; Resources, Jialong Han, Ping Chen, and Ke Xie; Software, Hao Wei and Zhiying Yue; Supervision, Yu Sun and Jiang Zhu; Validation, Yu Sun and Jiang Zhu; Visualization, Hao Wei; Writing – original draft preparation, Hao Wei, Zhiying Yue, and Jialong Han; Writing – Review and editing, Jiang Zhu.
CONFLICT OF INTEREST STATEMENT
The Authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank all those who participated in this study. The Cancer Psychology and Health Management Committee of the Sichuan Cancer Society supported this trial.
Wei H, Yue Z, Han J, Chen P, Xie K, Sun Y, et al. Oral compound probiotic supplements can improve the quality of life for patients with lung cancer during chemotherapy: A randomized placebo‐controlled study. Thorac Cancer. 2024;15(2):182–191. 10.1111/1759-7714.15177
Hao Wei, Zhiying Yue, Jialong Han contributed equally to this work and share first authorship.
Contributor Information
Yu Sun, Email: 55880810@qq.com.
Jiang Zhu, Email: zhujiang@wchscu.cn.
REFERENCES
- 1. Kalemkerian GP, Schneider BJ. Advances in small cell lung cancer. Hematol Oncol Clin North Am. 2017;31(1):143–156. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 3. Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, et al. On the receiving end—patient perception of the side‐effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19(2):203–208. [DOI] [PubMed] [Google Scholar]
- 4. Roviello G, Iannone LF, Bersanelli M, Mini E, Catalano M. The gut microbiome and efficacy of cancer immunotherapy. Pharmacol Ther. 2022;231:107973. [DOI] [PubMed] [Google Scholar]
- 5. Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier‐Colame V, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide‐induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–943. [DOI] [PubMed] [Google Scholar]
- 6. Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rakoff‐Nahoum S, Foster KR, Comstock LE. The evolution of cooperation within the gut microbiota. Nature. 2016;533(7602):255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ivanov II, Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr Opin Microbiol. 2011;14(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pouncey AL, Scott AJ, Alexander JL, Marchesi J, Kinross J. Gut microbiota, chemotherapy and the host: the influence of the gut microbiota on cancer treatment. Ecancermedicalscience. 2018;12:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibson RJ. Gut microbiome and intestinal mucositis: a new challenge for researchers. Cancer Biol Ther. 2009;8(6):512–513. [DOI] [PubMed] [Google Scholar]
- 11. Gibson RJ, Keefe DM. Cancer chemotherapy‐induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer. 2006;14(9):890–900. [DOI] [PubMed] [Google Scholar]
- 12. Valdagni R, Vavassori V, Rancati T, Fellin G, Baccolini M, Bianchi C, et al. Increasing the risk of late rectal bleeding after high‐dose radiotherapy for prostate cancer: the case of previous abdominal surgery. Results from a prospective trial. Radiother Oncol. 2012;103(2):252–255. [DOI] [PubMed] [Google Scholar]
- 13. Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011;71(3):661–665. [DOI] [PubMed] [Google Scholar]
- 14. Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–344. [DOI] [PubMed] [Google Scholar]
- 15. Aapro M, Molassiotis A, Dicato M, Peláez I, Rodríguez‐Lescure Á, Pastorelli D, et al. The effect of guideline‐consistent antiemetic therapy on chemotherapy‐induced nausea and vomiting (CINV): the pan European emesis registry (PEER). Ann Oncol. 2012;23(8):1986–1992. [DOI] [PubMed] [Google Scholar]
- 16. Sun Y, Zheng Y, Yang X, Xie K, Du C, He L, et al. Incidence of chemotherapy‐induced nausea and vomiting among cancer patients receiving moderately to highly emetogenic chemotherapy in cancer centers in Sichuan. China J Cancer Res Clin Oncol. 2021;147(9):2701–2708. [DOI] [PubMed] [Google Scholar]
- 17. Xu Y, Du H, Chen Y, Ma C, Zhang Q, Li H, et al. Targeting the gut microbiota to alleviate chemotherapy‐induced toxicity in cancer. Crit Rev Microbiol. 2023;1‐17:1–17. [DOI] [PubMed] [Google Scholar]
- 18. Lee TH, Park D, Kim YJ, Lee I, Kim S, Oh CT, et al. Lactobacillus salivarius BP121 prevents cisplatin‐induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p‐cresol sulfate via alleviating dysbiosis. Int J Mol Med. 2020;45(4):1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsiao YP, Chen HL, Tsai JN, Lin MY, Liao JW, Wei MS, et al. Administration of Lactobacillus reuteri combined with clostridium butyricum attenuates cisplatin‐induced renal damage by gut microbiota reconstitution, increasing butyric acid production, and suppressing renal inflammation. Nutrients. 2021;13(8):2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osterlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97(8):1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reyna‐Figueroa J, Barrón‐Calvillo E, García‐Parra C, Galindo‐Delgado P, Contreras‐Ochoa C, Lagunas‐Martínez A, et al. Probiotic supplementation decreases chemotherapy‐induced gastrointestinal side effects in patients with acute leukemia. J Pediatr Hematol Oncol. 2019;41(6):468–472. [DOI] [PubMed] [Google Scholar]
- 22. Huang F, Li S, Chen W, Han Y, Yao Y, Yang L, et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients. 2023;15(2):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma A, Tilak T, Bakhshi S, Raina V, Kumar L, Chaudhary S, et al. Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem cell transplantation. ESMO Open. 2016;1(6):e000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation‐ and chemotherapy‐induced mucositis in patients with head and neck cancer: a randomized double‐blind placebo‐controlled study. Eur J Cancer. 2012;48(6):875–881. [DOI] [PubMed] [Google Scholar]
- 25. Tian Y, Li M, Song W, Jiang R, Li YQ. Effects of probiotics on chemotherapy in patients with lung cancer. Oncol Lett. 2019;17(3):2836–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–v243. [DOI] [PubMed] [Google Scholar]
- 27. Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM, et al. General population normative data for the EORTC QLQ‐C30 health‐related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153–163. [DOI] [PubMed] [Google Scholar]
- 28. Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double‐blind, placebo‐controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019;125(7):1081–1090. [DOI] [PubMed] [Google Scholar]
- 29. Brahmer JR, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Health‐related quality‐of‐life results for pembrolizumab versus chemotherapy in advanced, PD‐L1‐positive NSCLC (KEYNOTE‐024): a multicentre, international, randomised, open‐label phase 3 trial. Lancet Oncol. 2017;18(12):1600–1609. [DOI] [PubMed] [Google Scholar]
- 30. Yang JC, Hirsh V, Schuler M, Yamamoto N, O'Byrne KJ, Mok TS, et al. Symptom control and quality of life in LUX‐lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3342–3350. [DOI] [PubMed] [Google Scholar]
- 31. Prisciandaro LD, Geier MS, Butler RN, Cummins AG, Howarth GS. Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy‐induced intestinal mucositis. Crit Rev Food Sci Nutr. 2011;51(3):239–247. [DOI] [PubMed] [Google Scholar]
- 32. Al‐Qadami GH, Secombe KR, Subramaniam CB, Wardill HR, Bowen JM. Gut microbiota‐derived short‐chain fatty acids: impact on cancer treatment response and toxicities. Microorganisms. 2022;10(10):2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y, Zhang Y, Wei K, He J, Ding N, Hua J, et al. Review: effect of gut microbiota and its metabolite SCFAs on radiation‐induced intestinal injury. Front Cell Infect Microbiol. 2021;11:577236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2):133–139. [DOI] [PubMed] [Google Scholar]
- 35. Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, et al. Association of Probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res. 2020;8(10):1236–1242. [DOI] [PubMed] [Google Scholar]


