Abstract
Bacterial endophthalmitis is a blinding infectious disease typically acquired during ocular surgery. We previously reported significant alterations in retinal metabolism during Staphylococcus (S) aureus endophthalmitis. However, the changes in retinal lipid composition during endophthalmitis are unknown. Here, using a mouse model of S. aureus endophthalmitis and an untargeted lipidomic approach, we comprehensively analyzed temporal alterations in total lipids and oxylipin in retina. Our data showed a time-dependent increase in the levels of lipid classes, sphingolipids, glycerolipids, sterols, and non-esterified fatty acids, whereas levels of phospholipids decreased. Among lipid subclasses, phosphatidylcholine decreased over time. The oxylipin analysis revealed increased prostaglandin-E2, hydroxyeicosatetraenoic acids, docosahexaenoic acid, eicosapentaenoic acid, and α-linolenic acid. In-vitro studies using mouse bone marrow-derived macrophages showed increased lipid droplets and lipid-peroxide formation in response to S. aureus infection. Collectively, these findings suggest that S. aureus-infection alters the retinal lipid profile, which may contribute to the pathogenesis of bacterial endophthalmitis.
Keywords: Endophthalmitis, S. aureus, Lipidomic, Oxylipins, Inflammation, retina
Introduction
Bacterial endophthalmitis is an infectious eye disease that occurs due to the entry of bacterial pathogens into the eye following surgical or accidental trauma [1, 2]. Endophthalmitis is considered a medical emergency, as it can lead to permanent vision loss, if left untreated. As the aging population increases and continues to undergo eye surgeries for cataracts, diabetic retinopathy, glaucoma, and age-related macular degeneration (AMD), the incidences of postsurgical bacterial endophthalmitis are likely to rise [3–6]. While the etiology of endophthalmitis varies depending on postsurgical or posttraumatic complications and geographic location, in general, Gram-positive bacteria remain the leading cause of approximately ~80% of bacterial endophthalmitis. Over the last decade, our lab has been using an established mouse model of Staphylococcus aureus endophthalmitis to mimic postsurgical bacterial endophthalmitis [7, 8]. S. aureus is a commensal bacteria that predominantly colonizes the skin and nares of up to 30% of all people [9] and is a major causes of severe endophthalmitis [4]. Patients with endophthalmitis experience red eye, pain, photophobia, and blurred or lost vision [10, 11]. The current treatment for endophthalmitis involves intravitreal injection of broad-spectrum antibiotics, vancomycin and ceftazidime which target Gram-positive bacteria, and Gram-negative bacteria, respectively. [12, 13]. However, the emergence of antibiotic resistance among ocular pathogens poses a significant threat to the management of ocular infections [14, 15]. Moreover, there is consensus on use of anti-inflammatory therapeutic to reduce intraocular inflammation during endophthalmitis. Therefore, the discovery of novel antimicrobials and immunomodulatory therapies are warranted to overcome these challenges.
Our previous study established the contribution of different S. aureus virulence factors to the pathogenesis of endophthalmitis. [16]. In addition to bacterial virulence factors, uncontrolled inflammation also causes irreversible retinal tissue damage during endophthalmitis [17, 18]. Therefore, it is important to understand the underlying mechanisms regarding intraocular inflammation and tissue damage in endophthalmitis to properly develop new therapeutic modalities.
Given the complexity of host-pathogen interactions, we utilized high throughput technologies such as transcriptomics, [19] and metabolomics, [20] to identify altered signaling pathways and their potential roles in the pathogenesis of bacterial endophthalmitis. Our integrated omics and systems biology approach showed a dysregulation of energy metabolism during S. aureus endophthalmitis [20, 21] and found that S. aureus infection increases glycolytic response, which fuels inflammatory response, during endophthalmitis [22]. Furthermore, the intraocular administration of the glycolytic inhibitor, 2-deoxy-glucose (2DG), effectively reduced inflammatory mediators [23]. Similarly, our metabolomic analysis uncovered the role of a TCA cycle metabolite, itaconate in regulating innate immune responses during endophthalmitis [20]. Moreover, the untargeted metabolomics also indicated altered lipid metabolism. This prompted us to perform lipidomic analysis in the bacterial endophthalmitis mouse model.
Lipids are a diverse group of organic molecules that serve critical functions in various physiological processes within the retina [24]. They are essential components of the cell membrane and contribute to the structural integrity and stability of retinal cells. Dysregulation of lipid metabolism in the retina can lead to a variety of pathological conditions, including inflammation [25], cell death [26], and retinal damage [27]. One of the mechanisms of cell death is by enhanced lipid peroxidation [28]. Since the retina is enriched in polyunsaturated fatty acids (PUFAs), retinal cells are more prone to lipid peroxidation-mediated cell death. Lipid peroxidation is triggered by a variety of free radicals, including reactive oxygen species, who levels are increased during endophthalmitis [29]. Similarly, dysregulation of sphingolipids is associated with the development of various retinal diseases, such as glaucoma, AMD [30], and retinitis pigmentosa [31, 32]. In animal models of these disorders, ceramide (Cer) has been discovered to be a typical mediator of inflammation and cell death [33]. Although the production of inflammatory lipids has been shown to cause retinal tissue damage [34], their role during intraocular infections, such as bacterial endophthalmitis, remains elusive.
The recent advancement in lipidomic analysis in various biological processes and diseases [35–38] and its ability to accurately analyze a large number of lipid classes and sub-classes [39, 40], led us to utilize the high throughput liquid chromatography-mass spectrometry (LC-MS) approach for bacterial endophthalmitis. Here, we report temporal changes in total lipids and oxylipins in S. aureus-infected mouse retina and cultured mouse bone marrow-derived macrophages (BMDMs). Our study provides evidence of lipid dysregulation and enhanced lipid peroxide formation during bacterial infection.
Materials and methods:
Mice
Both male and female C57BL/6 mice aged 8 to 12 weeks were procured from the Jackson Laboratory (Bar Harbor, Maine, USA). The mice were housed in a restricted-access Division of Laboratory Animal Resources (DLAR) facility at Kresge Eye Institute (Detroit, MI, USA), maintained in a 12:12 light/dark cycle at 22 °C temperature, and fed on Lab Diet rodent chow (Pico Laboratory, St. Louis, MO, USA) and ad libitum of water. The Institutional Animal Care and Use Committee (IACUC) at Wayne State University approved all procedures (protocol # IACUC-22-04-4557) under the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research.
Mouse model of bacterial endophthalmitis
Endophthalmitis was induced in B6 mice by intravitreal injection of S. aureus strain RN6390, as reported in prior studies [41, 42]. Briefly, the overnight bacterial culture in TSB broth was centrifuged, resuspended, and rinsed twice with sterile PBS. The bacterial inoculum was subsequently adjusted to a concentration of 5000 CFU in 2 μL of sterile PBS. Mice were anesthetized using a ketamine-xylazine and eyes were injected either with PBS (control) or bacterial suspension using a 34-gauge needle under a microscope [20]. Disease progression was monitored using a slit lamp exam and photomicrographs. At the specified time post-S. aureus infection, mice were humanely euthanized utilizing isoflurane, followed by cervical dislocation to ensure death. Subsequently, eyes were enucleated, and the retinal tissues were harvested. The excised tissues were immediately snap-frozen in liquid nitrogen for the lipidomic analysis. All experimental procedures were conducted in strict adherence to the Association for Research in Vision and Ophthalmology (ARVO) guidelines for animal research and approval from Institutional Animal Care and Use Committee (IACUC-22-04-4557) of Wayne State University.
Isolation of mouse bone marrow-derived macrophages (BMDMs)
Mouse BMDMs were obtained in the manner previously described [20, 23]. In short, mice were euthanized and, their tibia and femur bone marrow were harvested using (Roswell Park Memorial Institute) RPMI-1640 medium containing 10% (Fetal Bovine Serum) FBS and 0.2 mM (Ethylenediaminetetraacetic acid) EDTA. To pellet the cells, centrifugation was performed at 400 × g for 5 minutes at 4°C. Red blood cells (RBCs) were removed via the addition of 0.2% (Sodium chloride) NaCl solution for 20–30 seconds, followed by 1.6% NaCl, and then centrifuged. The cell pellets were then washed with RPMI-1640 media after RBC lysis. For macrophage differentiation, cells were resuspended and seeded in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/mL streptomycin, and 10 ng/m Macrophage Colony-Stimulating Factor (M-CSF). The cells were incubated at 37°C in 5% CO2. Six days after differentiation, 4 × 106 BMDMs/mL were cultured in 60 mm Petri dishes. To challenge the BMDMs, S. aureus was added at a multiplicity of infection (MOI) of 10:1 and incubated for 8 h. Finally, the cells were harvested and snap-frozen in liquid nitrogen for lipidomic analysis.
Lipid droplet and lipid peroxidation fluorescence microscopy
BMDMs were infected with S. aureus for six hours in four-well chamber slide (Fisher Scientific, Rochester, NY, USA). Afterward, cells were either stained with the lipid droplet detection dye (488 green, Sigma Millipore, catalog number SCT144) or lipid peroxidation detection dye (Invitrogen, catalog number C10445) in accordance with the manufacturer’s instructions. After 30 minutes of staining, cells were rinsed three times in PBS and observed under a fluorescent microscope (Keyence microscope, Itasca, IL).
Lipid extraction for LC-MS
An internal standard and calibration mixture of 100 μM each of di-myristoyl phospholipids (PG, PE, PS, PA), 100 μM PC (46:0), 100 μM SM (30:1), and 25 μM TG were added along with retinal or BMDM samples on dry ice in 10 μl amounts (14:1). 300 μl of −20C chilled 75% methanol with 1mM BHT (butylated hydroxytoluene) an antioxidant 0.5 mm zirconium oxide (ZO) beads were added to each sample. Samples were homogenized in a Bullet Blender tissue homogenizer and placed on ice. Each sample received 60 μl of methanol and 1 mL of MTBE (Methyl tert-butyl ether) before being vortexed for 60 minutes at room temperature. The samples were vortexed for an additional 15-minutes with the addition of 170 μl of water, followed by 15 minutes of centrifugation. The precipitated lipids were once again isolated from the supernatants and transferred to new test tubes. A speed-vaccum was used to dry the combined extracts overnight. They were then resuspended in 400 μl of isopropanol containing 0.01% BHT.
Mass-spectrometry analysis and Instrument parameters
In positive and negative ionization modes, a Thermo Scientific LTQ-Orbitrap Velos mass spectrometer was used to record the 100,000-resolution MS spectra at m/z 400 m/z 200–1200. Using an electrospray ionization source, 5 μL of the sample was flow-injected at 5 μL/min. Using a 20 mM ammonium format, the sample to injection solvent were 2:1 (v:v) isopropanol : methanol. A spray voltage of 4.0 kV was used, with an ion transfer tube temperature of 275 °C, the S-lens value was 50%, with an ion trap fill time of 300 ms. Before injecting the next sample, the LC tubing, autosampler, and ESI source were flushed with 1 mL isopropanol after two minutes of MS signal averaging. Pooled QC samples from all research samples were evaluated in random order, interspersed with solvent and extraction blank injections. After MS data evaluation, we “Recalibrate Offline” option in Thermo Fishers scientific Xcalibur™ Software was used to recalibrate the masses of the internal calibration standards and numerous common endogenous mammalian lipid species. Database-identified lipid species were confirmed and examined using higher-energy collisional dissociation (HCD) MS/MS at 60,000 resolution with a normalized collision energy of 25 for positive ions and 60 for negative ions. MS/MS scans were prompted by distinct inclusion lists for positive and negative ionization modalities.
Statistical analysis
We used the R Project for Statistical Computing (version 4.2) for all data processing and statistical analyses. The proportional composition of lipids in each sample was calculated, and a generalized linear model was fitted to test the differentially expressed lipids using the edgeR package (2–4). A Wilcoxon rank sum test was also performed for each lipid, and p-values were adjusted using the Benjamini-Hochberg method. A principal component analysis (PCA) was performed to visualize the similarity of the lipid profiles within and between groups. Heatmaps and volcano plots were generated to visualize the levels and significant alterations in the lipid profiles at different time points. The data are presented as the mean along with the standard deviation. To assess the statistical significance between the experimental groups, one-way ANOVA (with Dunnett’s multiple comparisons) and non-parametric t-tests were employed. GraphPad Prism 9.2 (GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses. A 95% confidence interval was utilized for all statistical tests, and a p-value of <0.05 was considered statistically significant.
Results
S. aureus infection alters retinal lipidome during endophthalmitis
We recently reported metabolic perturbations and their therapeutic potential during S. aureus endophthalmitis [20]. To determine global changes in lipids, we performed untargeted lipidomics using retinal tissue from S. aureus-infected or uninfected (mock) mouse eyes, as shown in the schematics (Fig. 1A). Principal component analysis (PCA) revealed clear clustering of different experimental groups, indicating time-dependent changes in retinal lipid profiles (Fig. 1B). S. aureus infection and time course (12, 24, 48 h) seem to be the primary drivers of lipid alterations during endophthalmitis. To identify differential production of lipids across a set time course, we analyzed our data based on lipid classes and subclasses (Fig. 1C). Among the major classes of lipids, the data showed a significant reduction in total phospholipid levels, with a 13.5% decline at 24 h and 21.9% at 48 h post-infection. In contrast, levels of sphingolipids, glycerolipids, sterol, and non-esterified fatty acids were increased in a time-dependent manner with a significant change at 48 h time point (Fig. 1D). Overall, these results show that S. aureus infection induces significant alterations in retinal lipid metabolism.
Figure 1. Untargeted retinal lipidomic analysis in bacterial endophthalmitis.
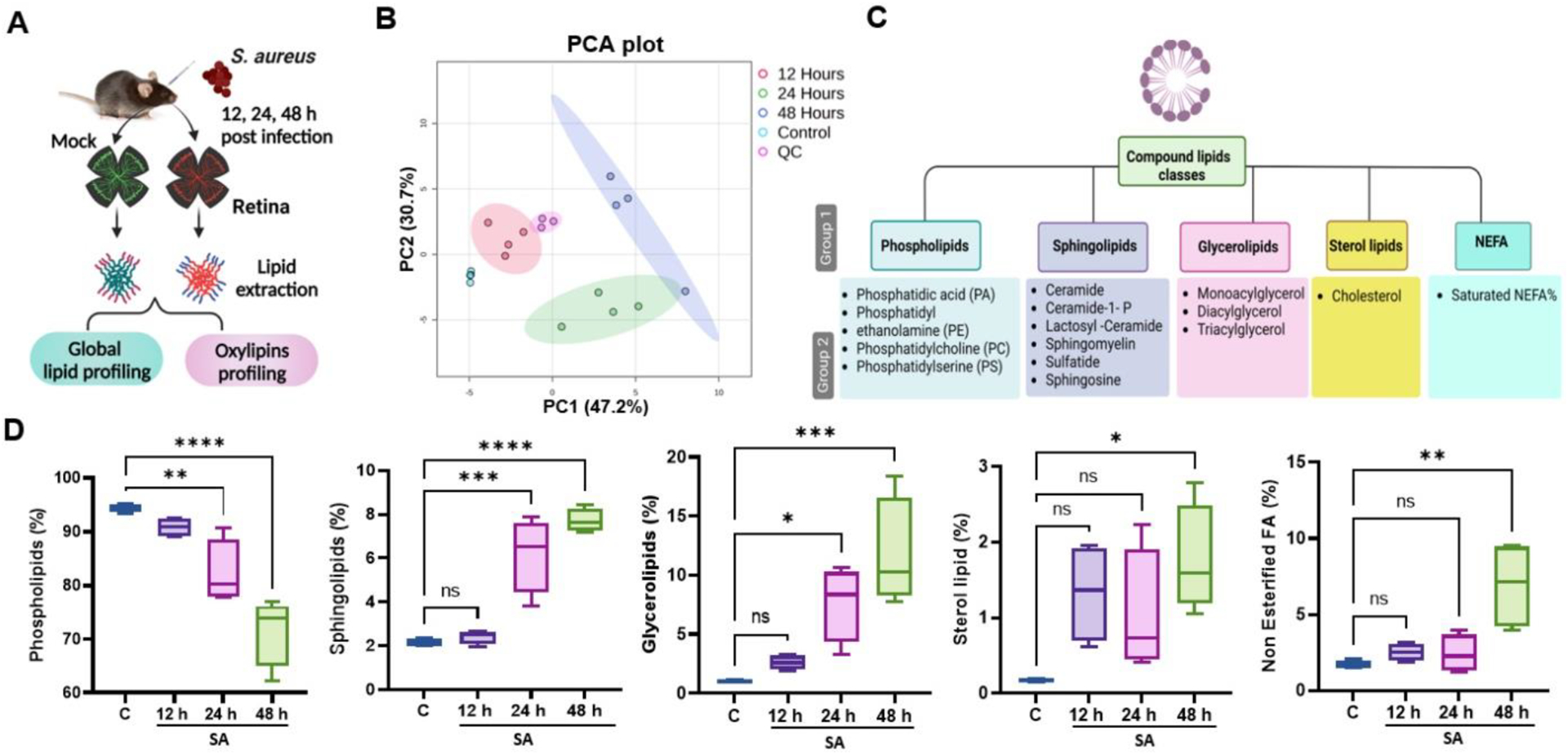
(A) Schematic of experimental design for lipidomic analysis in bacterial endophthalmitis. Retinal tissue from uninfected (mock) or S. aureus (SA)-infected eyes (n=4) at three different time points (12, 24 or 48 h) were used for lipidomic analysis. (B) Principal component analysis (PCA) of untargeted lipidomic data shows distinct clustering of various experimental groups at different time intervals with QC (Quality control). (C) Schematic of the lipid classification used in this study indicating five major lipid classes and their subclasses and species. (D) Lipidomic analysis showing temporal percentage changes in the mock (c) versus SA-infected retinal tissue. Statistical analysis was performed using one-way ANOVA, with significance levels of (*) p<0.05, (**) p<0.01, (***) p<0.001, (****) p<0.0001, ns indicating non-significance. Comparisons were made between uninfected control (C) vs. SA-infected retina.
Differential changes in lipid subclasses during S. aureus endophthalmitis
Next, we examined the temporal alterations in lipid subclasses in response to S. aureus infection. To visualize these alterations, a heatmap was generated, representing the different lipid subclasses at various time points post-infection (Fig. 2A). Our analysis revealed a time-dependent decrease in the phospholipid subclass, phosphatidylcholine (PC) whereas the relative abundance of phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI), exhibits the biphasic expression pattern, increased post 24 h infection, then declined at 48 h time point. Among the glycerolipids, sphingolipids and sterol subclasses, levels of triacylglycerol (TAG), diacylglycerol (DAG), monoacylglycerol (MAG), sphingomyelin (SM), and ceramide (Cer) exhibited a time-dependent increase (Fig. 2B).
Figure 2. Alterations in lipid subclasses during S. aureus endophthalmitis.
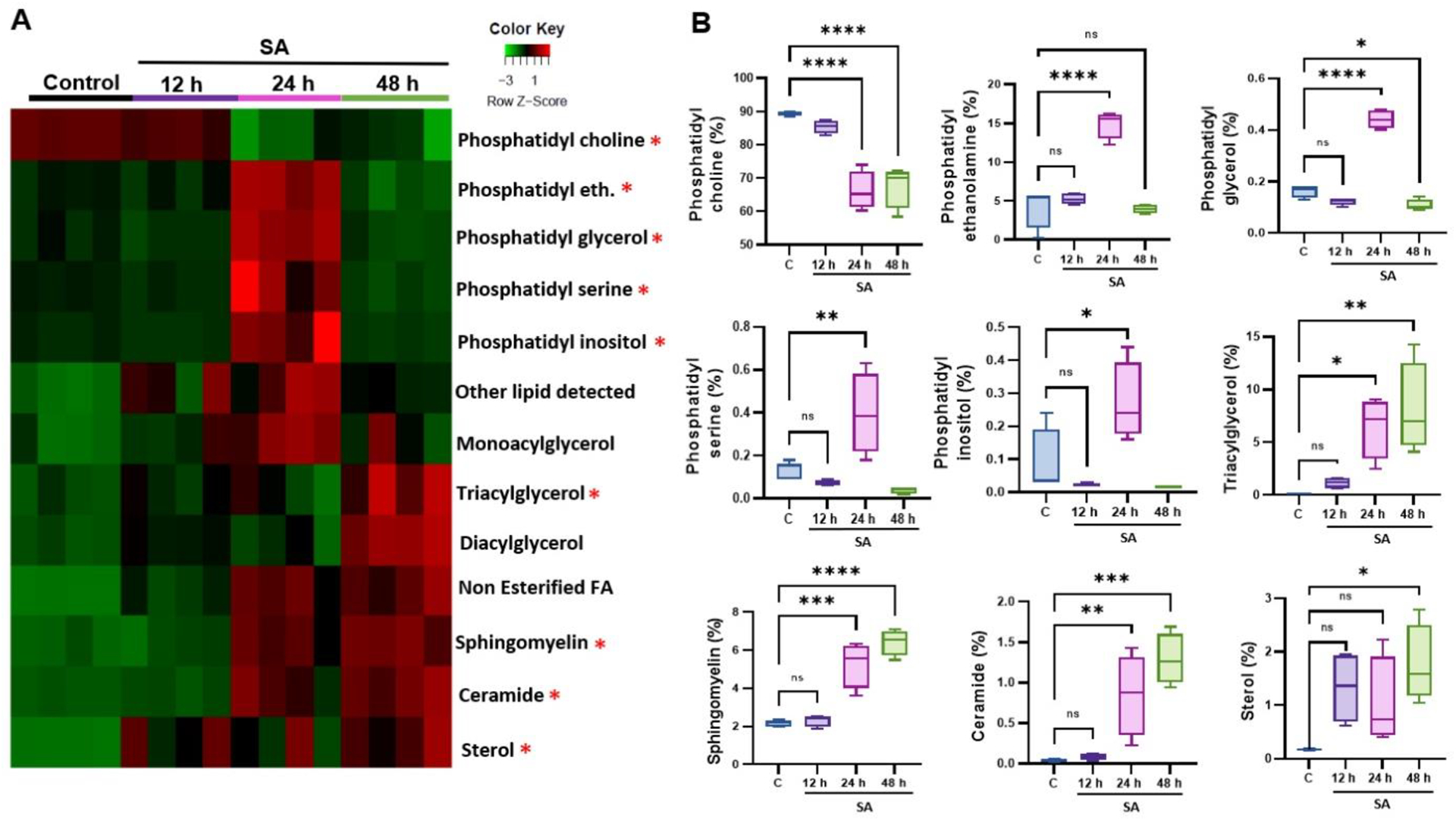
(A) Heatmap of lipid subclasses showing significant alterations at indicated time points (12, 24, and 48 h) post-infection. (B) Bar diagram showing temporal changes in some representative lipid subclasses in mock (c) versus S. aureus (SA)- infected mouse retinal tissue. Statistical analysis was performed using one-way ANOVA, with significance levels of (*) p<0.05, (**) p<0.01, (***) p<0.001, (****) p<0.0001, ns indicating non-significance. Comparisons were made between the uninfected control (C) vs. SA-infected retina.
Since lipids can be metabolized into various species, we generated a heatmap of the top 21 lipid subspecies whose production were significantly altered (Fig. 3A). Our analysis revealed that diacyl-PC levels showed a significant reduction of 27.5% at 24 h and 47.3% at 48 h over time, and diacyl-PE showed an increase at 24 h followed by a sharp decline at the 48h time point. As shown in the heatmap, relative abundance of sterol, ceramide (Cer), and sphingomyelin (SM) lipid species increased at later time points (24 and 48 h). The levels of non-esterified fatty acid (NEFA) lipid species, such as polyunsaturated NEFA, monounsaturated NEFA, and saturated NEFA, were also higher at the 48h time point. Among sterol lipids, the percentage of free cholesterol and cholesteryl esters showed an increase at 24 and 48 h. Additionally, we found that the glycerolipid species, monoacylglycerol (MAG), diacylglycerol (DAG), and triacylglycerol (TAG), were significantly increased at both 24 and 48 h time points, while with acylcarnitine’s and hydroxy-acylcarnitine’s lipid species peaked at 24 h time point. Interestingly, VLC-SM species showed an increase at the 24 h time point (Fig. 3B). These results indicate altered lipid metabolism during endophthalmitis, and the relative abundance of lipid subclasses might play a role in the disease pathobiology.
Figure 3. Alterations in lipid species during S. aureus endophthalmitis.
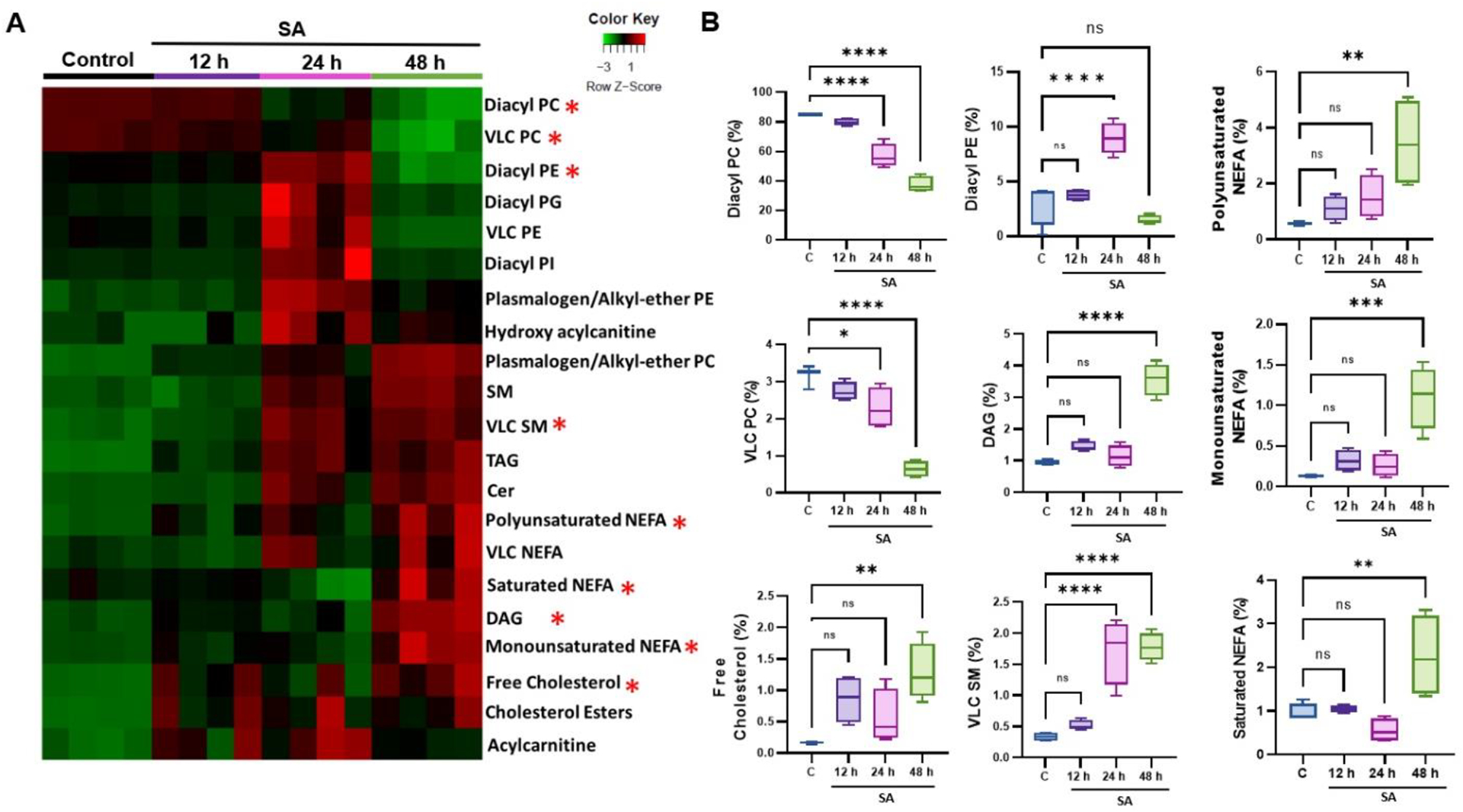
(A) Heatmap of lipid species showing significant alterations at indicated time points (12, 24, and 48h) post-infection. (B) Bar diagram showing temporal changes in some representative lipid species in mock (c) versus S. aureus (SA)-infected mouse retinal tissue. Statistical analysis was performed using one-way ANOVA, with significance levels of (*) p<0.05, (**) p<0.01, (***) p<0.001, (****) p<0.0001, ns indicating non-significance. Comparisons were made between uninfected control (C) vs. SA- infected retina.
Alterations in retinal oxylipins during S. aureus endophthalmitis
Our untargeted lipidomic analysis showed significant alterations in various lipid classes and subclasses. Next, we assessed the production of oxylipins, which are bioactive lipid metabolites derived from polyunsaturated fatty acids (PUFAs) via cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) pathways (Fig. 4A) and play a key role in regulating the inflammatory response [43]. We used the targeted oxylipins and fatty acids/fatty acid metabolites, which consist of >90 lipid subclasses (Supplementary Table 1). Principal component analysis (PCA) showed distinct clusters indicating alterations in oxylipin composition at various time points (12, 24, 48 h) post-infection (Fig. 4B). We then generated a heatmap to visualize the differential production of oxylipins at the early (12 h), mid (24 h), and later (48 h) stages of endophthalmitis (Fig. 5A). The oxylipin data analysis revealed a time-dependent increase in levels of eicosapentaenoic acid (EPA; C20: 5n3), docosahexaenoic acid (DHA; C22: 6n3), α-linolenic acid (ALA; C18:3n3) and 19/20-Di-hydroxydocosapentaenoic acid (19/20 DIHDPA) derived from ω-3 fatty acid and 5-hydroxyeicosatetraenoic acid (5-HETEs), prostaglandin E2 (PGE2), arachidonic acid (AA; C20: 4n6), adrenic acid (AdA; C22: 4n6), and linolenic acid (LA; C18: 2n6) derived from the ώ-6 fatty acid (Fig. 5B). Overall, ω-3 and ω-6 fatty acid derivatives were identified as the dominant class of oxylipins present in the infected mouse retinas. The volcano plot also identified significant differences in oxylipins in control vs. SA-infected tissues at 12, 24, and 48 h time points (Fig. 5C–E). Collectively, these results show differential production of oxylipins during endophthalmitis.
Figure 4. Targeted retinal oxylipin analysis in bacterial endophthalmitis.
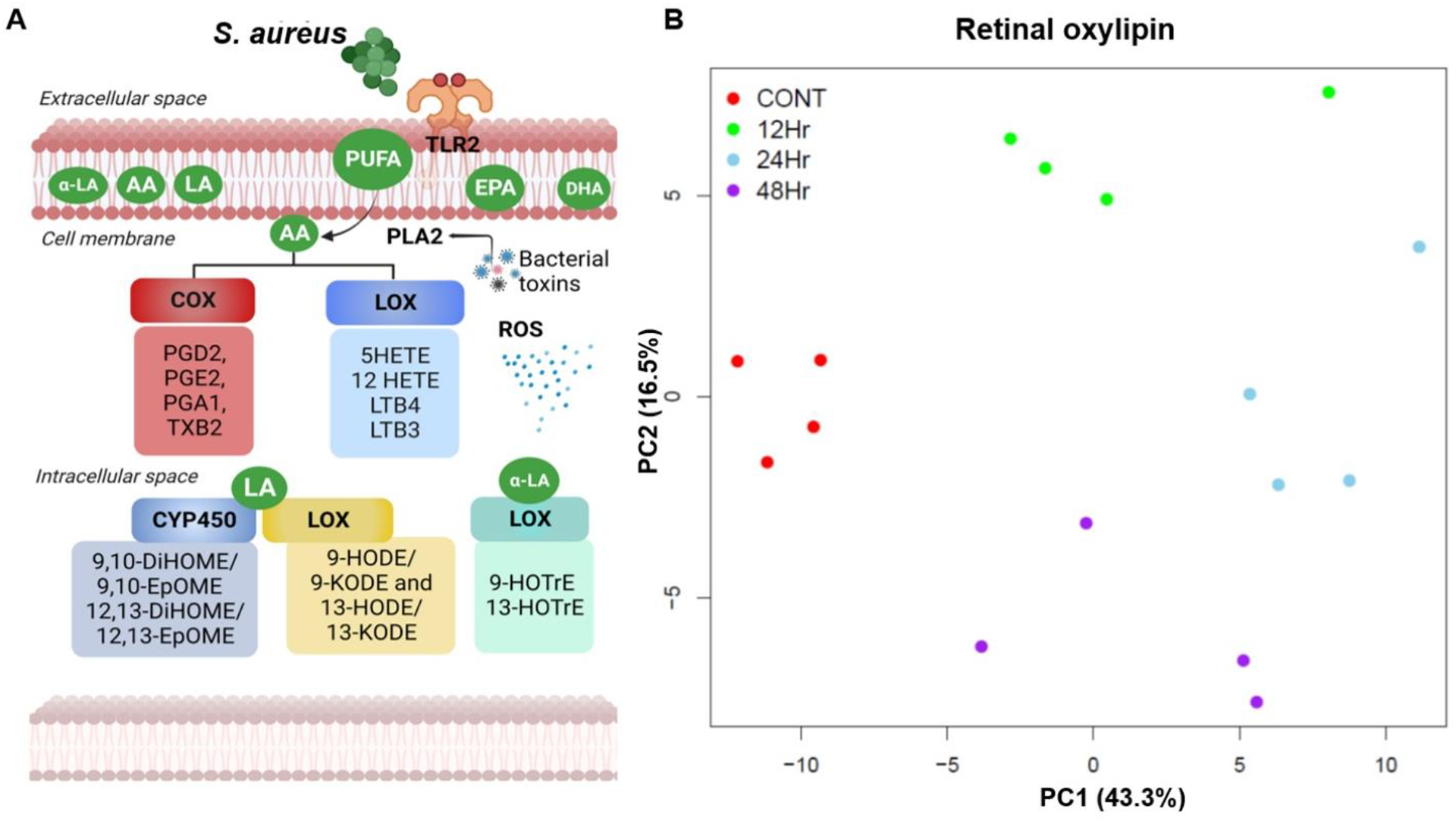
(A) Schematic representation of generation of oxylipins based on their sources and metabolic pathways regulated by three key enzymes, COX, LOX, and CYP450. (B) Principal component analysis (PCA) of retinal oxylipins from mock-infected and S. aureus (SA) infected mouse retinas (n=4) at 12, 24, and 48 h post-infection.
Figure 5. Alterations in retinal oxylipin production during S. aureus endophthalmitis.
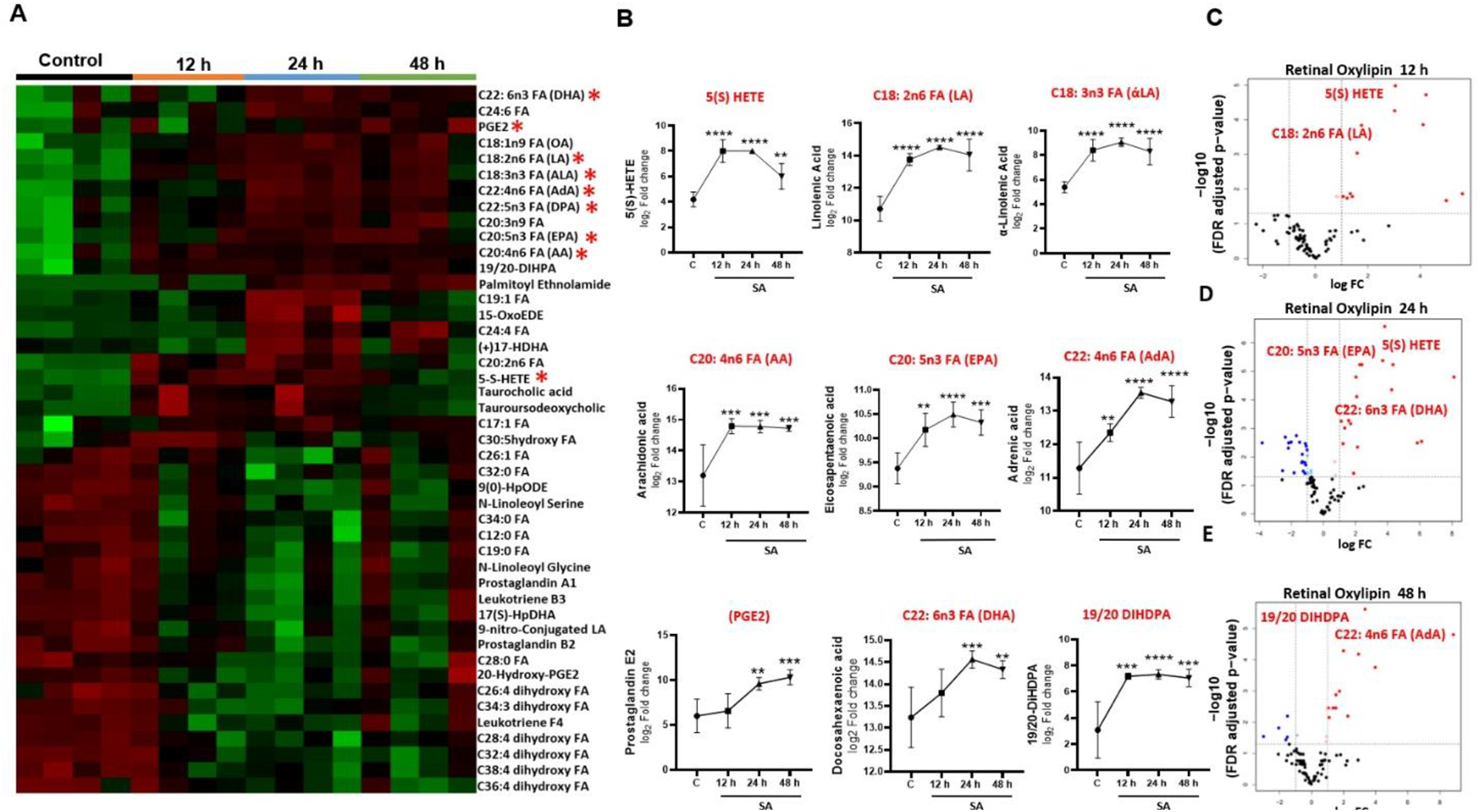
(A) Heat map showing temporal changes in the relative abundance of retinal oxylipins during endophthalmitis. (B) Line graph showing fold changes in oxylipins with significant alterations at indicated time post infection. (C) Volcano plot showing the differentially produced oxylipins with red dots indicating increased and blue dots showing reduced levels of oxylipins from the centerline (y-axis) and above a certain significance threshold. Statistical analysis was performed using one-way ANOVA, with significance levels of (*) p<0.05, (**) p<0.01, (***) p<0.001, (****) p<0.0001, ns indicating non-significance. Comparisons were made between uninfected control (C) vs. SA- infected retina.
S. aureus infection induces lipid peroxidation and alters oxylipin in BMDMs
Our in vivo data showed that S. aureus infection alters both total lipids and oxylipin during endophthalmitis. Next, we sought to determine the effects of bacterial infection on macrophages, one of the innate immune cells that infiltrate the eye during endophthalmitis and regulate the inflammatory response [44–46]. First, we assessed the rate of lipid droplet formation in response to S. aureus infection. Our data showed that within 6 h post-infection, BMDMs exhibited increased formation of lipid droplets (Fig. 6A&B), suggesting potential modulation in the lipid metabolism. However, during microbial infection or tissue injury, lipids are commonly oxidized via lipid peroxidation, which is perceived as a threat by the innate immune system [47]. Thus, we measured lipid peroxidation using a fluorescent dye (Lipid Peroxidation, live cell dye) and observed a significant increase in its levels in S. aureus infected BMDMs (Fig. 6 C&D). These findings imply that the enhanced generation of lipid ROS might play an active role in the pathogenesis of bacterial endophthalmitis.
Figure 6. S. aureus infection induced lipid droplets and lipid peroxidation in BMDMs.
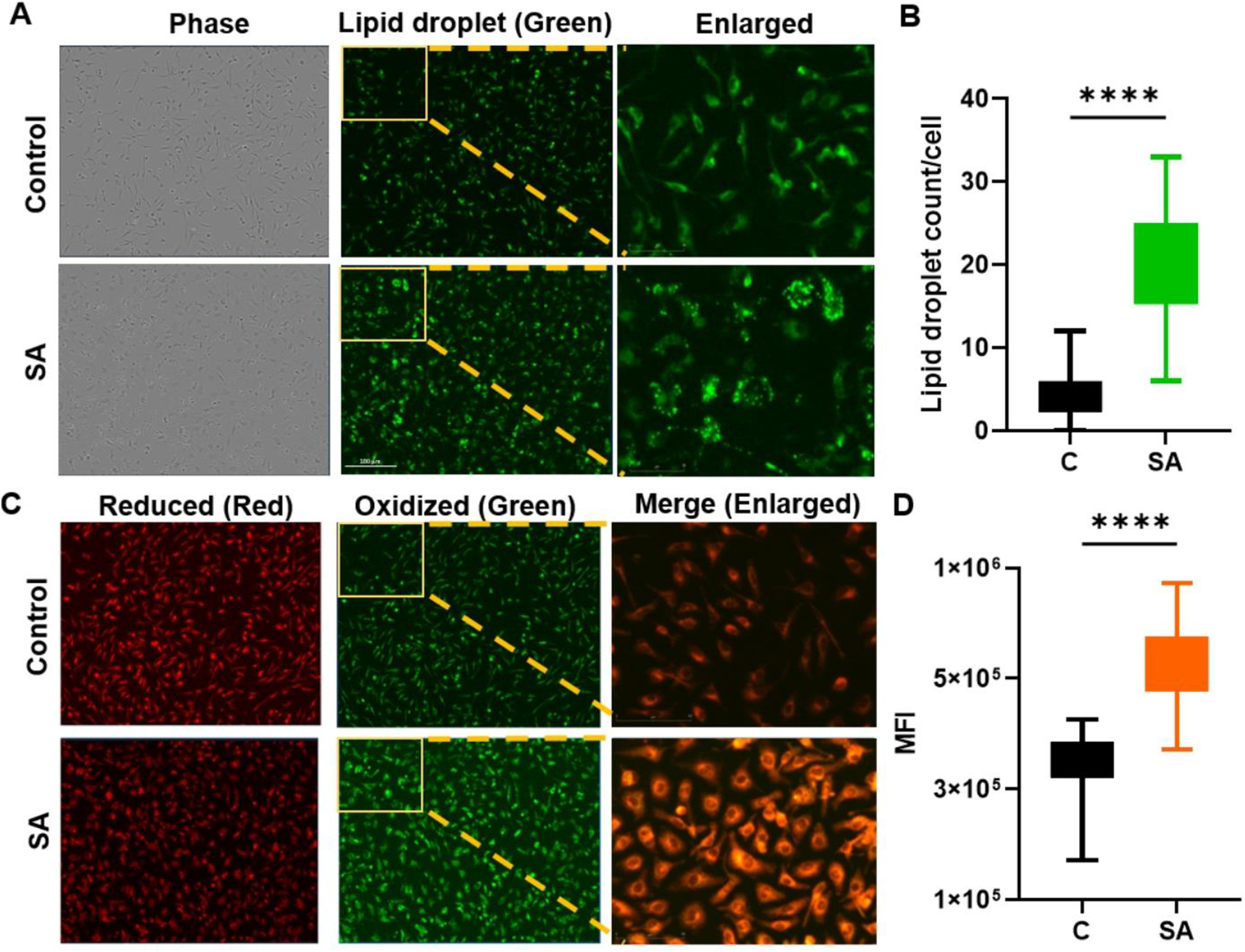
BMDMs were isolated from the bones of B6 mice and cells were infected with S. aureus (SA) for 6 h. (A) Cells were stained with specific dye to visualize lipid droplets (green). (B) Semi-quantitative analysis was performed by counting lipid droplets ~50 cells/image. (C) The formation of lipid peroxidation (LPO) in ratiometric dye which gives shift from red (reduced lipids) to green (oxidized lipids) indicating lipid peroxidation, Scale bar; 100μm. Images are representative of two independent experiments. (D) Quantitative analysis was of lipid peroxidation was performed by measuring mean fluorescence intensity (MFI). Statistical analysis was performed using an unpaired, t-test, with significance levels of (****) p<0.0001. Comparisons were made between uninfected control (C) vs. SA- infected BMDM.
Next, we assessed the production of oxylipins in BMDMs and observed significant differences in control versus S. aureus infected cells. The heatmap analysis depicted increased levels of oxylipins in BMDMs in response to S. aureus-infection (Fig. 7A). Furthermore, PCA analysis, provides compelling evidence by the separation of experimental groups (Fig. 7B) as well as in the volcano plot (Fig 7C). Notably, we observed increased levels of 20-HETEs, along with other unsaturated fatty acids including DTA (C22; 6n4), ETA (C22; 3n9), OA (C18; 1n9), α-LA (C18; 3n3) and LA (C18; 2n6) (Fig. 7D). Collectively, these results indicate that S. aureus alters lipid metabolism with increased lipid ROS and 20-HETEs formation in innate immune cells.
Figure 7. Oxylipin formation in S. aureus infected BMDMs.
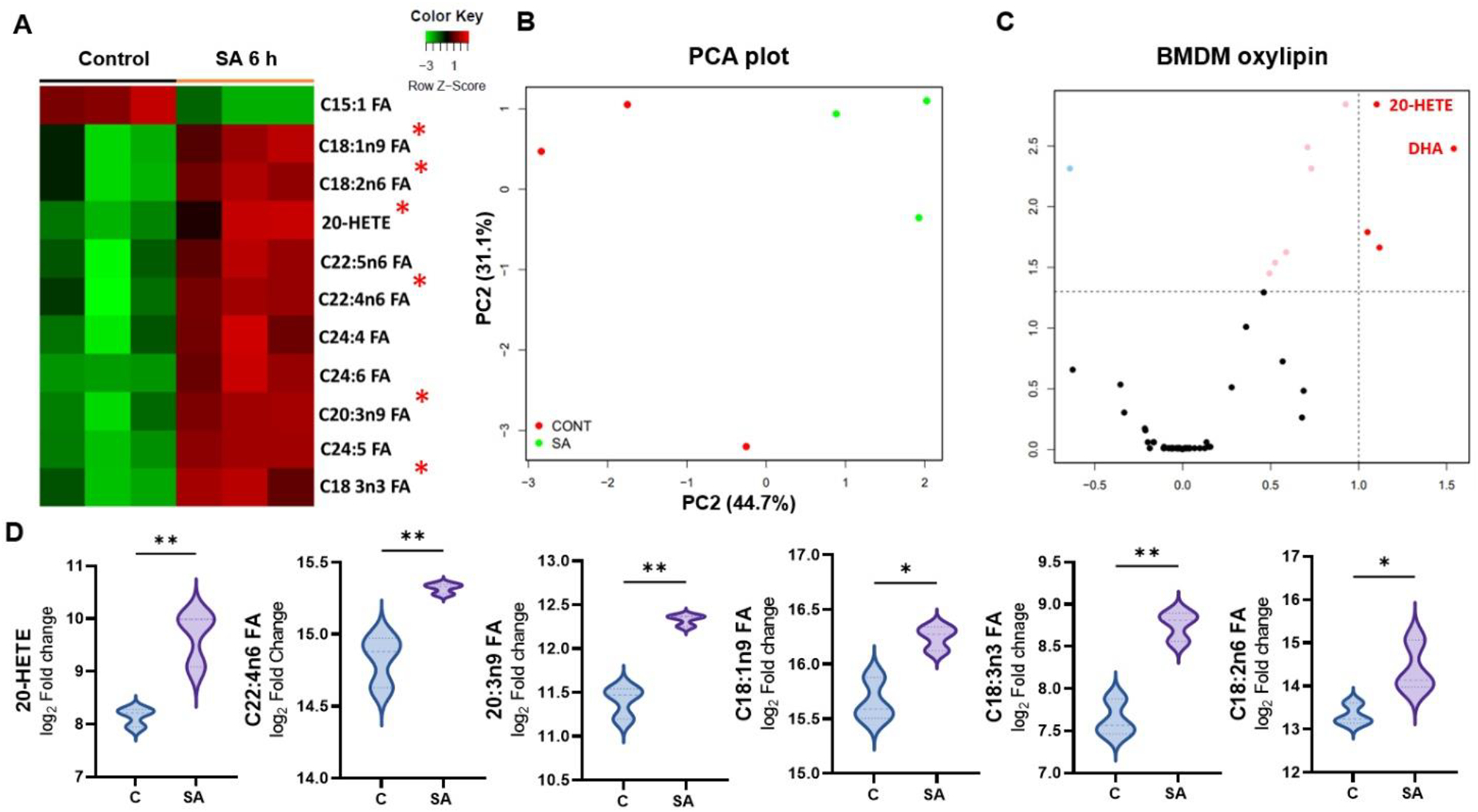
(A) Heatmap showing the changes in production of various oxylipins in BMDMs (n=3) in response to S. aureus (SA) infection for 6h. (B) Principal Component Analysis (PCA) of oxylipins, showing the distinct clustering of control and SA-infected BMDMs. (C) Volcano plot showing differentially expressed lipids represented by dots located above the significance threshold and far from the centerline (y-axis). The upregulated oxylipins (red) located on the right-hand side of the plot. (D) Violin plot showing the most significantly upregulated oxylipins, plotted based on the heatmap analysis. Statistical analysis was performed using an unpaired, t-test, with significance levels of (*) p<0.05, (**) p<0.01. Comparisons were made between uninfected control (C) vs. SA- infected BMDM.
Discussion
Lipids are involved in various physiological processes, including energy storage, membrane formation, and cell signaling [48]. Understanding lipid metabolism and changes in lipid profiles during microbial infection can provide valuable insights into the disease pathogenesis. The role of lipid mediators, such as prostaglandins, leukotrienes, and platelet-activating factors, in modulating immune responses is already well established [49, 50], in different diseases, including ocular surface diseases [51]. However, alterations in lipid metabolism and their role in bacterial endophthalmitis are unknown. Thus, using the lipidomic approach, our study defines temporal changes in retinal lipid composition in response to bacterial infection and provides insight into the pathogenesis of bacterial endophthalmitis.
Our experimental model of endophthalmitis involves direct inoculation of microbial pathogens, S. aureus, in the vitreous cavity of B6 mice [16, 41, 44]. This results in the activation and production of inflammatory mediators by resident retinal cells such as microglia [52], Müller glia [53, 54], and retinal pigment epithelium (RPE) [54]. However, as the infection progresses, infiltrating innate immune cells, mainly neutrophils, contribute to intraocular inflammation during endophthalmitis [55]. Although, essential to restrain an infection, an uncontrolled inflammatory response causes retinal tissue damage, resulting in vision loss during endophthalmitis [56]. Hence, we used mouse retinal tissues for multiomics studies to identify genes and pathways altered during disease progression [19, 20]. Using a similar approach for lipidomic analysis, our study shows significant changes in retinal lipid classes and subclasses during bacterial endophthalmitis. Our first observation was that among the six major classes of lipids, the relative abundance of sphingolipids, glycerolipids, and sterols increased in a time-dependent manner, whereas phospholipid levels were decreased.
Sphingolipids are the major class of retinal lipids that play a key role in multiple physiological processes and are essential for retinal function; thus, dysregulation of sphingolipids has been implicated in various retinal diseases [31]. Our data showed the levels of ceramide, one of the bioactive sphingolipids, were increased at 24 and 48 h post-infection, which corroborated with increased levels of inflammatory mediators in endophthalmitis [7, 41]. In the retina, increased ceramides have been shown to promote inflammation and cell death, including apoptosis [57, 58] and parthanatosis [59]. Additionally, increased ceramide levels have been reported in aqueous humor and in the retina of experimental uveitis [60]. Therefore, increased ceramide during bacterial endophthalmitis indicates its role in the activation of an innate immune response, thereby facilitating immune cell recruitment into the eye. Further, ceramide accumulation can enhance retinal cell death observed in our model, including those triggered by mitochondrial dysfunction [54, 56]. Sphingomyelin, another subgroup of sphingolipids found higher in our analysis, is hydrolyzed to generate ceramide [61]. Literature suggest that, increased ceramide levels due to sphingomyelin hydrolysis have been associated with retinal cell death in conditions such as diabetic retinopathy [62] and AMD [63]. Importantly, one of the potential mechanisms by which ceramide causes cell death and inflammation is by inducing endoplasmic reticulum (ER) stress [64, 65]. We also reported an essential form of ER stress in TLR2-mediated innate response in bacterial endophthalmitis [66]. However, further studies are needed to test whether the observed ER stress in endophthalmitis is due to ceramide accumulation. Moreover, inhibition of ceramide biosynthesis has been shown to protect the retina [57, 67]. Therefore, targeting ceramide metabolism could ameliorate retinal tissue damage during bacterial endophthalmitis.
Glycerolipids, also known as glycerides, are a class of lipids consisting of a glycerol backbone bonded with one or more fatty acid chains. They are important components of cell membranes and serve as a source of energy in the form of triglycerides [68]. Our lipidomic analysis showed an increase in the sub-classes of glycerolipids such as triacylglycerol (TAG) in the bacterial-infected retina. The breakdown of triglycerides can lead to the production of free fatty acids, some of which can serve as signaling molecules that modulate the immune response. On the contrary, increased retinal TAG levels could promote the survival and proliferation of S. aureus during endophthalmitis due to the utilization of TAG as a nutrient source [69]. The excessive breakdown of TAGs, which exceeds the cell’s capacity to process the resulting free fatty acids, can lead to lipotoxicity [70] and impair autophagy in the retina [71]. Increased lipotoxicity in the retina can trigger mitochondrial damage in endothelial cells, resulting in the development of diabetic retinopathy [72]. Therefore, regulated retinal TAG metabolism during endophthalmitis could limit the availability of free fatty acids for bacterial growth.
Our lipidomic analysis also revealed increased levels of sterols, an essential class of lipids inherent to eukaryotic cell membranes necessary to maintain their structural and functional integrity. Within the sterols, we observed that the relative abundance of free cholesterol did not change at the early stages of infection but increased at the 48h time point. The perturbations in cholesterol homeostasis, a central aspect of sterol regulation, have been associated with an array of retinal pathologies [73]. Increased retinal cholesterol levels have been shown to contribute to the pathogenesis of AMD and diabetic retinopathy [74, 75], along with the formation of hyperreflective crystalline structures, which are likely cholesterol crystals, have been reported in diabetic retinas [76]. Interestingly, a recent study showed bacteria, including S. aureus, can adhere to cholesterol crystals and feeds on cholesterol, and cause endocarditis [77].Further studies are warranted to better understand the role of cholesterol metabolism during endophthalmitis and test whether cholesterol-lowering drugs could be used as adjunct therapeutics.
Our temporal analysis showed significant increase in levels of non-esterified fatty acids (NEFAs), including monounsaturated, saturated, and polyunsaturated, especially at the later time points i.e., 48h post infection. NEFAs play key role in maintaining cell membrane fluidity that is necessary for effective signal transduction [78]. However, increased levels of NEFAs may promote inflammation, thereby increasing inflammation-mediated tissue damage [79]. The high plasma levels of polyunsaturated NEFA were found to correlate with the severity of COVID 19, indicating its role as a potential biomarker [80]. Thus, understanding the proinflammatory effect of NEFAs in the retina, might provide new therapeutic target to resolve intraocular inflammation in bacterial endophthalmitis.
Another lipid class with substantial alterations observed in our study were phospholipids, with a significant reduction in levels of phosphatidylcholine (PC) which is the largest subclass of retinal phospholipids that make the cell membrane [81]. However, the levels of other phospholipids such as phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), and phosphatidylglycerol (PG), did not change during the course of the S. aureus infection, with the exception at 24 h time point showing higher levels. These results corroborate the previous finding showing that the retina is one of the tissues with the highest content of polyunsaturated fatty acids (PUFAs) [82]. Being the essential constituents of cell membranes, these fatty acids, when released by specific phospholipases, act as substrates to generate bioactive lipids in turn regulate intracellular signaling, including inflammation. The major class of these bioactive lipids is referred to as oxylipins, which are derived from PUFAs through enzymatic or nonenzymatic oxidation processes. The key enzymes involved in oxylipin synthesis are cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) which primarily act on arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) in animals, and linoleic acid (LA) and α-linolenic acid (ALA) in plants. However, depending on the availability of these substrates and the activity of specific oxygenase enzymes, oxylipins can exert either pro- or anti-inflammatory effects via the action of lipid mediators in modulating the inflammatory milieu [83]. Our analysis shows differential levels of oxylipins and their substrates during bacterial endophthalmitis. We observed a time-dependent increase in levels of EPA, DHA, and AA in the S. aureus-infected retina. The lipid mediators derived from the COX and LOX pathways originating from arachidonic acid (AA) are often linked to a pro-inflammatory response. On the other hand, the mediators originating from EPA and DHA are generally characterized as either having lower inflammatory potential or being anti-inflammatory mediators [84, 85]. Additionally, EPA and DHA have been shown to have direct antimicrobial effects against a variety of bacteria, including S. aureus, the most common cause of bacterial endophthalmitis. These fatty acids have been shown to disrupt bacterial cell membranes and interfere with bacterial metabolism, ultimately leading to bacterial death [86]. These results indicate that EPA and DHA supplementation might be protective during endophthalmitis via production of anti-inflammatory lipid mediators and inhibiting bacterial growth.
In the context of inflammatory lipid mediators, our data showed increased levels of 5-hydroxy-eicosatetraenoic acids (5-HETEs), which are produced by the metabolism of AA, and have been implicated in the pathobiology of various retinal diseases [87–89]. Notably, previous studies have shown that both 12-HETEs and 5-HETEs contribute to retinal damage in diabetic retinopathy [90, 91]. Furthermore, in a murine sepsis model, specific inhibition of 20-HETE synthase significantly reduced mortality. This effect was attributed to reduction in 20-HETE induced ferroptosis in peripheral blood mononuclear cells [92]. In the retina, 5-HETEs increases oxidative stress and inflammation, which culminate into enhanced retinal cell death [93, 94]. 5-HETEs also cause the breach of blood-retinal barrier, leading to fluid leakage and edema, a hallmark of proliferative diabetic retinopathy [95].
Our oxylipin analysis also revealed increased levels of prostaglandin E2 (PGE2), which are produced from AA by COX enzymes. These lipid mediators are known to play important roles in the regulation of inflammatory eye diseases such as dry eye and pathological retinal angiogenesis [96–98]. Thus, PGE2 could be a promising therapeutic target to promote inflammation resolution in endophthalmitis. Our in vitro analysis of BMDMs further revealed significant changes in oxylipins, including increased production of HETEs, in response to S. aureus infection. One of the mechanisms by which 5-HETEs cause tissue damage is via production of lipid ROS [99] triggering ferroptosis cell death [100]. In support, our data showed that S. aureus induced the accumulation of lipid droplets and oxidized lipids in BMDMs. However, future studies are needed to understand the lipid ROS and ferroptosis axis in bacterial endophthalmitis.
Limitation of the study:
While we comprehensively analyzed lipid dysregulation in bacterial endophthalmitis, there are several limitations of the study. 1) Lipids are complex biomolecules composed of > 50, 000 species [101]. It is important to acknowledge that our untargeted lipidomic analysis might have not detected low abundant, highly volatile, temporally regulated, lipids such as specialized pro-resolving lipid mediators (SPMs). only showed significant alterations in major lipid classes and subclasses. 2) Among the gram-positive bacteria, coagulase-negative staphylococci (CoNS) are the major (>80%) cause of bacterial endophthalmitis. It would be insightful to compare lipid changes during CoNS endophthalmitis using our mouse model of S. epidermidis endophthalmitis [55]. 3) S. aureus produces several lipases as a virulence factor to modify host lipids [102]. Although our bacterial preparation is unlikely to have lipase activity from the spent media, mutant S. aureus strains lacking specific lipases can be utilized to induce endophthalmitis to determine their contributions in retinal lipid composition [103].
In summary, our study offers valuable insights into the changes in retinal lipids in bacterial endophthalmitis. However, it is important to acknowledge that the biological implications of these lipid alterations are intricate and cannot be predicted solely based on lipidomic data. Consequently, this study primarily serves as a foundation for generating hypotheses that warrant further investigation through targeted studies, thus bringing new therapeutic developments for ocular bacterial infections.
Supplementary Material
Highlights.
Retinal lipidome is altered during bacterial (S. aureus) endophthalmitis.
The levels of sphingolipids, glycerolipids, sterols, and non-esterified fatty acids increases, while phospholipid levels decline.
Oxylipin analysis revealed higher levels of PGE2, 5HETEs, DHA, and AA in response to S. aureus infection.
S. aureus infection enhances lipid droplets and lipid-peroxide formation.
Acknowledgments
This study was supported by National Institute of Health (NIH) Grants R21AI135583, R01EY026964, and R01 EY027381 awarded to A.K. We would like to acknowledge the Research to Prevent Blindness (RPB) for their unrestricted grant to the Kresge Eye Institute/Department of Ophthalmology, Visual, and Anatomical Sciences. The immunology core is supported by an NEI vision center grant P30EY004068. The funders had no role in the design of the study, data collection, data analysis, interpretation of the results, or in the decision to submit the work for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare no conflict of interest.
Data availability:
All data are contained within the article. Any or additional data are available from the corresponding authors upon reasonable request.
References
- [1].Sheu SJ, Endophthalmitis, Korean J Ophthalmol 31(4) (2017) 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Durand ML, Endophthalmitis, Clin Microbiol Infect 19(3) (2013) 227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu AM, Wu CM, Tseng VL, Greenberg PB, Giaconi JA, Yu F, Lum F, Coleman AL, Characteristics Associated With Receiving Cataract Surgery in the US Medicare and Veterans Health Administration Populations, JAMA Ophthalmol 136(7) (2018) 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Callegan MC, Engelbert M, Parke DW 2nd, Jett BD, Gilmore MS, Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions, Clin Microbiol Rev 15(1) (2002) 111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, Ah Tong BAM, Arunga S, Bachani D, Bascaran C, Bastawrous A, Blanchet K, Braithwaite T, Buchan JC, Cairns J, Cama A, Chagunda M, Chuluunkhuu C, Cooper A, Crofts-Lawrence J, Dean WH, Denniston AK, Ehrlich JR, Emerson PM, Evans JR, Frick KD, Friedman DS, Furtado JM, Gichangi MM, Gichuhi S, Gilbert SS, Gurung R, Habtamu E, Holland P, Jonas JB, Keane PA, Keay L, Khanna RC, Khaw PT, Kuper H, Kyari F, Lansingh VC, Mactaggart I, Mafwiri MM, Mathenge W, McCormick I, Morjaria P, Mowatt L, Muirhead D, Murthy GVS, Mwangi N, Patel DB, Peto T, Qureshi BM, Salomao SR, Sarah V, Shilio BR, Solomon AW, Swenor BK, Taylor HR, Wang N, Webson A, West SK, Wong TY, Wormald R, Yasmin S, Yusufu M, Silva JC, Resnikoff S, Ravilla T, Gilbert CE, Foster A, Faal HB, The Lancet Global Health Commission on Global Eye Health: vision beyond 2020, Lancet Glob Health 9(4) (2021) e489–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD, The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001, Ophthalmology 112(8) (2005) 1388–94. [DOI] [PubMed] [Google Scholar]
- [7].Talreja D, Singh PK, Kumar A, In Vivo Role of TLR2 and MyD88 Signaling in Eliciting Innate Immune Responses in Staphylococcal Endophthalmitis, Invest Ophthalmol Vis Sci 56(3) (2015) 1719–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Singh PK, Donovan DM, Kumar A, Intravitreal Injection of the Chimeric Phage Endolysin Ply187 Protects Mice from Staphylococcus aureus Endophthalmitis, Antimicrobial Agents and Chemotherapy 58(8) (2014) 4621–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sakr A, Brégeon F, Mège JL, Rolain JM, Blin O, Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections, Front Microbiol 9 (2018) 2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thompson KN, Alshaikhsalama AM, Wang AL, Evaluation of the Clinical Course of Endogenous Endophthalmitis, J Vitreoretin Dis 7(5) (2023) 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu LJ, Chen X, Adelman RA, Clinical Etiologies, Microbial Spectrum, Antibiotic Susceptibilities, and Visual Acuity Outcomes of Acute Endophthalmitis, J Ocul Pharmacol Ther 36(7) (2020) 534–539. [DOI] [PubMed] [Google Scholar]
- [12].Novosad BD, Callegan MC, Severe bacterial endophthalmitis: towards improving clinical outcomes, Expert Rev Ophthalmol 5(5) (2010) 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Benz MS, Scott IU, Flynn HW Jr., Unonius N, Miller D, Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases, Am J Ophthalmol 137(1) (2004) 38–42. [DOI] [PubMed] [Google Scholar]
- [14].Lakhundi S, Zhang K, Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology, Clin Microbiol Rev 31(4) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bertino JS Jr., Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics, Clin Ophthalmol 3 (2009) 507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kumar A, Kumar A, Role of Staphylococcus aureus Virulence Factors in Inducing Inflammation and Vascular Permeability in a Mouse Model of Bacterial Endophthalmitis, PLOS ONE 10(6) (2015) e0128423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miller FC, Coburn PS, Huzzatul MM, LaGrow AL, Livingston E, Callegan MC, Targets of immunomodulation in bacterial endophthalmitis, Prog Retin Eye Res 73 (2019) 100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singh PK, Kumar A, Mitochondria mediates caspase-dependent and independent retinal cell death in Staphylococcus aureus endophthalmitis, Cell Death Discovery 2(1) (2016) 16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rajamani D, Singh PK, Rottmann BG, Singh N, Bhasin MK, Kumar A, Temporal retinal transcriptome and systems biology analysis identifies key pathways and hub genes in Staphylococcus aureus endophthalmitis, Sci Rep 6 (2016) 21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh S, Singh PK, Jha A, Naik P, Joseph J, Giri S, Kumar A, Integrative metabolomics and transcriptomics identifies itaconate as an adjunct therapy to treat ocular bacterial infection, Cell Rep Med 2(5) (2021) 100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, Hu B, Yip CC, Tsang JO, Huang X, Chai Y, Yang D, Hou Y, Chik KK, Zhang X, Fung AY, Tsoi HW, Cai JP, Chan WM, Ip JD, Chu AW, Zhou J, Lung DC, Kok KH, To KK, Tsang OT, Chan KH, Yuen KY, Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study, Lancet Microbe 1(1) (2020) e14–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar A, Giri S, Kumar A, 5-Aminoimidazole-4-carboxamide ribonucleoside-mediated adenosine monophosphate-activated protein kinase activation induces protective innate responses in bacterial endophthalmitis, Cell Microbiol 18(12) (2016) 1815–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Francis R, Singh PK, Singh S, Giri S, Kumar A, Glycolytic inhibitor 2-deoxyglucose suppresses inflammatory response in innate immune cells and experimental staphylococcal endophthalmitis, Exp Eye Res 197 (2020) 108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fliesler SJ, Introduction to the Thematic Review Series: Seeing 2020: lipids and lipid-soluble molecules in the eye, J Lipid Res 62 (2021) 100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hsueh YJ, Chen YN, Tsao YT, Cheng CM, Wu WC, Chen HC, The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases, Int J Mol Sci 23(3) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, Zhang Z, Wang X, Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1, Mol Cell 81(2) (2021) 355–369 e10. [DOI] [PubMed] [Google Scholar]
- [27].Fu Z, Chen CT, Cagnone G, Heckel E, Sun Y, Cakir B, Tomita Y, Huang S, Li Q, Britton W, Cho SS, Kern TS, Hellstrom A, Joyal JS, Smith LE, Dyslipidemia in retinal metabolic disorders, EMBO Mol Med 11(10) (2019) e10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Danielli M, Perne L, Jarc Jovicic E, Petan T, Lipid droplets and polyunsaturated fatty acid trafficking: Balancing life and death, Front Cell Dev Biol 11 (2023) 1104725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gaschler MM, Stockwell BR, Lipid peroxidation in cell death, Biochem Biophys Res Commun 482(3) (2017) 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shiwani HA, Elfaki MY, Memon D, Ali S, Aziz A, Egom EE, Updates on sphingolipids: Spotlight on retinopathy, Biomed Pharmacother 143 (2021) 112197. [DOI] [PubMed] [Google Scholar]
- [31].Simon MV, Basu SK, Qaladize B, Grambergs R, Rotstein NP, Mandal N, Sphingolipids as critical players in retinal physiology and pathology, J Lipid Res 62 (2021) 100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mondal K, Mandal N, Role of Bioactive Sphingolipids in Inflammation and Eye Diseases, Adv Exp Med Biol 1161 (2019) 149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen H, Tran JT, Brush RS, Saadi A, Rahman AK, Yu M, Yasumura D, Matthes MT, Ahern K, Yang H, LaVail MM, Mandal MN, Ceramide signaling in retinal degeneration, Adv Exp Med Biol 723 (2012) 553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaur G, Singh NK, Inflammation and retinal degenerative diseases, Neural Regen Res 18(3) (2023) 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang T, Chen H, Du X, Li N, Chen Y, Min H, Differences in aqueous humor protein profiles in patients with proliferative diabetic retinopathy before and after conbercept treatment, J Proteomics 276 (2023) 104838. [DOI] [PubMed] [Google Scholar]
- [36].Achten R, Thijs J, van der Wal M, van Luijk C, van Luin M, El Amrani M, Knol E, Delemarre E, Jager CDH, de Graaf M, Bakker D, de Boer J, van Wijk F, de Bruin-Weller M, High dupilumab levels in tear fluid of atopic dermatitis patients with moderate-to-severe ocular surface disease, Clin Transl Allergy 13(1) (2023) e12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nattinen J, Aapola U, Nukareddy P, Uusitalo H, Clinical Tear Fluid Proteomics-A Novel Tool in Glaucoma Research, Int J Mol Sci 23(15) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Menta V, Agarwal S, Das US, Moksha L, Srividya G, Anandan AM, Srinivasan B, Iyer G, Velpandian T, Angayarkanni N, Ocular surface sphingolipids associate with the refractory nature of vernal keratoconjunctivitis: newer insights in VKC pathogenesis, Br J Ophthalmol 107(4) (2023) 461–469. [DOI] [PubMed] [Google Scholar]
- [39].Azbukina NV, Chistyakov DV, Goriainov SV, Kotelin VI, Fedoseeva EV, Petrov SY, Sergeeva MG, Iomdina EN, Zernii EY, Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma, Biology (Basel) 10(7) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Han X, Gross RW, The foundations and development of lipidomics, J Lipid Res 63(2) (2022) 100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Singh PK, Singh S, Wright RE 3rd, Rattan R, Kumar A, Aging, But Not Sex and Genetic Diversity, Impacts the Pathobiology of Bacterial Endophthalmitis, Invest Ophthalmol Vis Sci 61(14) (2020) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kumar A, Singh PK, Zhang K, Kumar A, Toll-like receptor 2 (TLR2) engages endoplasmic reticulum stress sensor IRE1alpha to regulate retinal innate responses in Staphylococcus aureus endophthalmitis, FASEB J 34(10) (2020) 13826–13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Serhan CN, Chiang N, Van Dyke TE, Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators, Nat Rev Immunol 8(5) (2008) 349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kumar A, Singh PK, Ahmed Z, Singh S, Kumar A, Essential Role of NLRP3 Inflammasome in Mediating IL-1β Production and the Pathobiology of Staphylococcus aureus Endophthalmitis, Infect Immun 90(5) (2022) e0010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kumar A, Singh PK, Zhang K, Kumar A, Toll-like receptor 2 (TLR2) engages endoplasmic reticulum stress sensor IRE1α to regulate retinal innate responses in Staphylococcus aureus endophthalmitis, The FASEB Journal 34(10) (2020) 13826–13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Singh S, Singh PK, Kumar A, Butyrate Ameliorates Intraocular Bacterial Infection by Promoting Autophagy and Attenuating the Inflammatory Response, Infect Immun 91(1) (2023) e0025222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhivaki D, Kagan JC, Innate immune detection of lipid oxidation as a threat assessment strategy, Nature Reviews Immunology 22(5) (2022) 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Horn A, Jaiswal JK, Structural and signaling role of lipids in plasma membrane repair, Curr Top Membr 84 (2019) 67–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Christie PE, Henderson WR Jr., Lipid inflammatory mediators: leukotrienes, prostaglandins, platelet-activating factor, Clin Allergy Immunol 16 (2002) 233–54. [PubMed] [Google Scholar]
- [50].Bennett M, Gilroy DW, Lipid Mediators in Inflammation, Microbiology Spectrum 4(6) (2016) 4.6.06. [DOI] [PubMed] [Google Scholar]
- [51].Flitter BA, Fang X, Matthay MA, Gronert K, The potential of lipid mediator networks as ocular surface therapeutics and biomarkers, Ocul Surf 19 (2021) 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kochan T, Singla A, Tosi J, Kumar A, Toll-Like Receptor 2 Ligand Pretreatment Attenuates Retinal Microglial Inflammatory Response but Enhances Phagocytic Activity toward Staphylococcus aureus, Infect Immun 80(6) (2012) 2076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shamsuddin N, Blair J, Kumar A, Toll Like Receptor 2 Mediates The Innate Immune Response Of Retinal Muller Glia To Staphylococcus Aureus, Investigative Ophthalmology & Visual Science 52(14) (2011) 2959–2959. [Google Scholar]
- [54].Das S, Singh S, Satpathy S, Bhasin M, Kumar A, Transcriptomics and systems biology identify nonantibiotic drugs for the treatment of ocular bacterial infection, iScience 25(9) (2022) 104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Das S, Singh S, Kumar A, Bacterial Burden Declines But Neutrophil Infiltration and Ocular Tissue Damage Persist in Experimental Staphylococcus epidermidis Endophthalmitis, Front Cell Infect Microbiol 11 (2021) 780648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Singh PK, Kumar A, Mitochondria mediates caspase-dependent and independent retinal cell death in Staphylococcus aureus endophthalmitis, Cell Death Discov 2 (2016) 16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen H, Tran J-TA, Eckerd A, Huynh T-P, Elliott MH, Brush RS, Mandal NA, Inhibition of de novo ceramide biosynthesis by FTY720 protects rat retina from light-induced degeneration, Journal of Lipid Research 54(6) (2013) 1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ranty M-L, Carpentier S, Cournot M, Rico-Lattes I, Malecaze F, Levade T, Delisle M-B, Quintyn J-C, Ceramide production associated with retinal apoptosis after retinal detachment, Graefe’s Archive for Clinical and Experimental Ophthalmology 247(2) (2009) 215–224. [DOI] [PubMed] [Google Scholar]
- [59].Prado Spalm FH, Vera MS, Dibo MJ, Simón MV, Politi LE, Rotstein NP, Ceramide Induces the Death of Retina Photoreceptors Through Activation of Parthanatos, Mol Neurobiol 56(7) (2019) 4760–4777. [DOI] [PubMed] [Google Scholar]
- [60].Wang HY, Wang Y, Zhang Y, Wang J, Xiong SY, Sun Q, Crosslink between lipids and acute uveitis: a lipidomic analysis, Int J Ophthalmol 11(5) (2018) 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gault CR, Obeid LM, Hannun YA, An overview of sphingolipid metabolism: from synthesis to breakdown, Adv Exp Med Biol 688 (2010) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fox TE, Han X, Kelly S, Merrill AH 2nd, Martin RE, Anderson RE, Gardner TW, Kester M, Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy, Diabetes 55(12) (2006) 3573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Landowski M, Bowes Rickman C, Targeting Lipid Metabolism for the Treatment of Age-Related Macular Degeneration: Insights from Preclinical Mouse Models, J Ocul Pharmacol Ther 38(1) (2022) 3–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liu Z, Xia Y, Li B, Xu H, Wang C, Liu Y, Li Y, Li C, Gao N, Li L, Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca2+ homeostasis in human adenoid cystic carcinoma cells, Cell & Bioscience 4(1) (2014) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Park W-J, Park J-W, The role of sphingolipids in endoplasmic reticulum stress, FEBS Letters 594(22) (2020) 3632–3651. [DOI] [PubMed] [Google Scholar]
- [66].Kumar A, Singh PK, Zhang K, Kumar A, Toll-like receptor 2 (TLR2) engages endoplasmic reticulum stress sensor IRE1α to regulate retinal innate responses in Staphylococcus aureus endophthalmitis, Faseb j 34(10) (2020) 13826–13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P, Ghidoni R, Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa, Proc Natl Acad Sci U S A 107(43) (2010) 18706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alves-Bezerra M, Cohen DE, Triglyceride Metabolism in the Liver, Compr Physiol 8(1) (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Delekta PC, Shook JC, Lydic TA, Mulks MH, Hammer ND, Staphylococcus aureus Utilizes Host-Derived Lipoprotein Particles as Sources of Fatty Acids, J Bacteriol 200(11) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lipke K, Kubis-Kubiak A, Piwowar A, Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge, Cells 11(5) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Heckel E, Cagnone G, Agnihotri T, Cakir B, Das A, Kim JS, Kim N, Lavoie G, Situ A, Pundir S, Sun Y, Wünnemann F, Pierce KA, Dennis C, Mitchell GA, Chemtob S, Rezende FA, Andelfinger G, Clish CB, Roux PP, Sapieha P, Smith LE, Joyal JS, Triglyceride-derived fatty acids reduce autophagy in a model of retinal angiomatous proliferation, JCI Insight 7(6) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kumar B, Kowluru A, Kowluru RA, Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy, Invest Ophthalmol Vis Sci 56(5) (2015) 2985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ramachandra Rao S, Fliesler SJ, Cholesterol homeostasis in the vertebrate retina: biology and pathobiology, J Lipid Res 62 (2021) 100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pikuleva IA, Curcio CA, Cholesterol in the retina: the best is yet to come, Prog Retin Eye Res 41 (2014) 64–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Busik JV, Lipid metabolism dysregulation in diabetic retinopathy, J Lipid Res 62 (2021) 100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jenkins AJ, Grant MB, Busik JV, Lipids, hyperreflective crystalline deposits and diabetic retinopathy: potential systemic and retinal-specific effect of lipid-lowering therapies, Diabetologia 65(4) (2022) 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boumegouas M, Raju M, Gardiner J, Hammer N, Saleh Y, Al-Abcha A, Kalra A, Abela GS, Interaction between bacteria and cholesterol crystals: Implications for endocarditis and atherosclerosis, PLOS ONE 17(2) (2022) e0263847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Keen H, Chlouverakis C, Metabolism of Isolated Rat Retina. The Role of Non-Esterified Fatty Acid, Biochem J 94(2) (1965) 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wood LG, Scott HA, Garg ML, Gibson PG, Innate immune mechanisms linking non-esterified fatty acids and respiratory disease, Progress in lipid research 48(1) (2009) 27–43. [DOI] [PubMed] [Google Scholar]
- [80].Nguyen M, Bourredjem A, Piroth L, Bouhemad B, Jalil A, Pallot G, Le Guern N, Thomas C, Pilot T, Bergas V, Choubley H, Quenot JP, Charles PE, Lagrost L, Deckert V, de Barros JP, Guinot PG, Masson D, Binquet C, Gautier T, Blot M, g. Lymphonie study, High plasma concentration of non-esterified polyunsaturated fatty acids is a specific feature of severe COVID-19 pneumonia, Sci Rep 11(1) (2021) 10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Acar N, Berdeaux O, Grégoire S, Cabaret S, Martine L, Gain P, Thuret G, Creuzot-Garcher CP, Bron AM, Bretillon L, Lipid Composition of the Human Eye: Are Red Blood Cells a Good Mirror of Retinal and Optic Nerve Fatty Acids?, PLOS ONE 7(4) (2012) e35102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Fliesler AJ, Anderson RE, Chemistry and metabolism of lipids in the vertebrate retina, Progress in Lipid Research 22(2) (1983) 79–131. [DOI] [PubMed] [Google Scholar]
- [83].Kahnt AS, Schebb NH, Steinhilber D, Formation of lipoxins and resolvins in human leukocytes, Prostaglandins Other Lipid Mediat 166 (2023) 106726. [DOI] [PubMed] [Google Scholar]
- [84].Lundström SL, Levänen B, Nording M, Klepczynska-Nyström A, Sköld M, Haeggström JZ, Grunewald J, Svartengren M, Hammock BD, Larsson BM, Eklund A, Wheelock Å M, Wheelock CE, Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure, PLoS One 6(8) (2011) e23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Li C, Wu X, Liu S, Shen D, Zhu J, Liu K, Role of Resolvins in the Inflammatory Resolution of Neurological Diseases, Front Pharmacol 11 (2020) 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Coraca-Huber DC, Steixner S, Wurm A, Nogler M, Antibacterial and Anti-Biofilm Activity of Omega-3 Polyunsaturated Fatty Acids against Periprosthetic Joint Infections-Isolated Multi-Drug Resistant Strains, Biomedicines 9(4) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Birkle DL, Bazan NG, Lipoxygenase- and cyclooxygenase-reaction products and incorporation into glycerolipids or radiolabeled arachidonic acid in the bovine retina, Prostaglandins 27(2) (1984) 203–16. [DOI] [PubMed] [Google Scholar]
- [88].Leung HH, Galano JM, Crauste C, Durand T, Lee JC, Combination of Lutein and Zeaxanthin, and DHA Regulated Polyunsaturated Fatty Acid Oxidation in H(2)O(2)-Stressed Retinal Cells, Neurochem Res 45(5) (2020) 1007–1019. [DOI] [PubMed] [Google Scholar]
- [89].Zhao T, Wang Y, Guo X, Li H, Jiang W, Xiao Y, Deng B, Sun Y, Altered oxylipin levels in human vitreous indicate imbalance in pro-/anti-inflammatory homeostasis in proliferative diabetic retinopathy, Exp Eye Res 214 (2022) 108799. [DOI] [PubMed] [Google Scholar]
- [90].Elmasry K, Ibrahim AS, Saleh H, Elsherbiny N, Elshafey S, Hussein KA, Al-Shabrawey M, Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy, Diabetologia 61(5) (2018) 1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tang J, Kern TS, Inflammation in diabetic retinopathy, Prog Retin Eye Res 30(5) (2011) 343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wu J, Liu Q, Zhang X, Tan M, Li X, Liu P, Wu L, Jiao F, Lin Z, Wu X, Wang X, Zhao Y, Ren J, The interaction between STING and NCOA4 exacerbates lethal sepsis by orchestrating ferroptosis and inflammatory responses in macrophages, Cell Death Dis 13(7) (2022) 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY, Gurel Z, Sheibani N, Maddipati KR, Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization, Diabetes 60(2) (2011) 614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dong L, Wang H, Chen K, Li Y, Roles of hydroxyeicosatetraenoic acids in diabetes (HETEs and diabetes), Biomed Pharmacother 156 (2022) 113981. [DOI] [PubMed] [Google Scholar]
- [95].Wang MH, Hsiao G, Al-Shabrawey M, Eicosanoids and Oxidative Stress in Diabetic Retinopathy, Antioxidants (Basel, Switzerland) 9(6) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Xie T, Zhang Z, Cui Y, Shu Y, Liu Y, Zou J, Wang M, Wang Y, Yang Q, Pan X, Cai J, Sun X, Yao Y, Wang X, Prostaglandin E2 promotes pathological retinal neovascularisation via EP4R-EGFR-Gab1-AKT signaling pathway, Experimental Eye Research 205 (2021) 108507. [DOI] [PubMed] [Google Scholar]
- [97].Deng C, Chen S, Li X, Luo H, Zhang Q, Hu P, Wang F, Xiong C, Sun T, Zhang X, Role of the PGE2 receptor in ischemia-reperfusion injury of the rat retina, Mol Vis 26 (2020) 36–47. [PMC free article] [PubMed] [Google Scholar]
- [98].Lekhanont K, Sathianvichitr K, Pisitpayat P, Anothaisintawee T, Soontrapa K, Udomsubpayakul U, Association between the levels of prostaglandin E2 in tears and severity of dry eye, Int J Ophthalmol 12(7) (2019) 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gaschler MM, Stockwell BR, Lipid peroxidation in cell death, Biochemical and Biophysical Research Communications 482(3) (2017) 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Li J, Cao F, Yin H.-l., Huang Z.-j., Lin Z.-t., Mao N, Sun B, Wang G, Ferroptosis: past, present and future, Cell Death & Disease 11(2) (2020) 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Swinnen JV, Dehairs J, A beginner’s guide to lipidomics, The Biochemist 44(1) (2022) 20–24. [Google Scholar]
- [102].Cadieux B, Vijayakumaran V, Bernards MA, McGavin MJ, Heinrichs DE, Role of lipase from community-associated methicillin-resistant Staphylococcus aureus strain USA300 in hydrolyzing triglycerides into growth-inhibitory free fatty acids, Journal of bacteriology 196(23) (2014) 4044–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kumar NG, Contaifer D Jr., Wijesinghe DS, Jefferson KK, Staphylococcus aureus Lipase 3 (SAL3) is a surface-associated lipase that hydrolyzes short chain fatty acids, PLOS ONE 16(10) (2021) e0258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article. Any or additional data are available from the corresponding authors upon reasonable request.


