Abstract
Background and Aim
Functional bowel disorders (FBDs), including irritable bowel syndrome (IBS) and others, are conditions without a physically identifiable etiology that, as a result, are difficult to treat. Alternatives to traditional medical interventions are needed because IBS patients require more of physician time and higher healthcare spending. The goal of this study was to determine the efficacy of alternative lifestyle interventions for patients with FBDs seen in an integrative medicine (IM) clinic at an academic medical center.
Methods
We performed a retrospective chart review to determine whether patients with FBDs had improvement in symptoms following predominantly nutrition‐based IM interventions that included recommendations for dietary supplements and elimination diets. We measured symptoms before and after intervention (average time between measurements 8.75 months) using a medical symptoms questionnaire (MSQ) commonly used to quantify symptom change in IM clinics.
Results
Digestive tract symptoms, as measured by the MSQ, improved significantly in patients (n = 57) with FBDs following IM intervention. The MSQ Digestive Tract subtotal for FBD patients decreased from 10.2 (SD, 5.4) to 7.2 (SD, 5.2) (P < 0.001) after IM intervention.
Conclusions
Patients in an IM clinic had improved digestive tract symptoms scores following IM intervention. Because nutrition‐based interventions were the primary intervention recommended by IM providers, primary care physicians and gastroenterologists may wish to consider referring FBD patients to registered dietitian‐nutritionists (RDNs) skilled in implementing elimination diets.
Keywords: functional bowel disorders; functional gastrointestinal disorders; gluten‐free diet; integrative medicine; irritable bowel syndrome; low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet
Functional bowel disorders (FBDs), including irritable bowel syndrome (IBS), are conditions without a physically identifiable etiology that, as a result, are difficult to treat. We performed a retrospective chart review to determine whether patients with FBDs had improvement in symptoms following nutrition‐based Integrative Medicine interventions. Digestive tract symptoms measured by the Medical Symptoms Questionnaire improved significantly in patients (n = 57) with FBDs following intervention.
Introduction
Functional bowel disorders (FBDs) include irritable bowel syndrome (IBS), functional bloating (FB), diarrhea (FD), functional constipation (FC), and unspecified functional bowel disorders (FBD‐U). 1 FBDs are a subset of a larger classification of functional gastrointestinal disorders (FGIDs), which are more recently referred to as disorders of gut–brain interaction (DGBI). FGIDs are diagnosed based on patient‐reported gastrointestinal (GI) symptoms, despite a lack of identifiable structural or biochemical abnormalities by routine investigation. FGIDs are not life‐threatening conditions, but they negatively impact patients' quality of life. 2 IBS is one of the most common FGIDs. 3 Patients with IBS report pain related to defecation and altered bowel habits (change in stool frequency and/or consistency). IBS symptoms are characterized by constipation‐dominance (IBS‐C), diarrhea‐dominance (IBS‐D), mixed symptomatology (IBS‐M), or are unclassified (IBS‐U). 4 IBS has both complex pathophysiology and treatment, with much to still be understood about the condition. 5 Patients with FB, FD, and FC experience similar changes in bowel habits (constipation, diarrhea) or bloating without pain.
Internal medicine and family physicians refer about one‐third of their IBS patients to gastroenterology specialists. 6 Gastroenterologists report that though these patients are less ill than other GI patients, they require more of the specialists' time, 6 possibly reflecting some of the psychosocial effects of IBS. Because IBS patients pose a burden on physicians with limited time, alternative forms of care that can improve patient symptoms are needed.
Integrative medicine (IM) combines therapies from conventional medicine and complementary and alternative medicine in a patient‐centered manner and addresses the full range of physical, emotional, mental, social, spiritual, and environmental influences that affect a person's health. 7 , 8 , 9 The therapeutic relationship between the practitioner and the patient that the latter need 10 is difficult to develop within the limited time conventional medical providers have with patients. In contrast, IM practitioners have significant time with patients to do clinical assessment and rapport‐building, and IM healthcare providers can suggest and implement lifestyle interventions for FGID patients. 11 , 12
Because many IM interventions are based on lifestyle changes (e.g. diet, exercise, sleep), we chose an IM clinic at the University of Kansas to study the impact of dietary interventions on persons with FBDs. The aim of this study was to determine whether interventions for FBD patients at an IM clinic were effective, based on symptom report and measurement. Secondary outcomes for this study include effectiveness of IM interventions on types of FBD. Because IM is by definition individualized per patient, 6 the interventions tested here are a group of therapies that vary from patient to patient with some overlapping recommendations.
Methods
Patients
This study was a retrospective chart review to describe interventions for patients with FGIDs at an IM clinic at an academic medical center (KU IM). This study was approved by the Institutional Review Board of the University of Kansas Medical Center. A total of 547 randomly selected charts were screened for fit‐to‐study criteria. Patient charts were identified for inclusion in the study if the patient was diagnosed with any FGID (e.g. IBS, functional bowel abnormality, FC). We identified 85 patients with FGID who were seen in the IM clinic (charts screened May 2016). Of those, 57 met the following inclusion criteria: age 21–89 years; attended at least three clinic appointments during which they saw at least one IM practitioner such as a medical doctor (MD), a nurse practitioner (APRN), or a physician assistant (PA) and/or a registered dietitian‐nutritionist (RDN); and had completed the medical symptom questionnaire (MSQ) initially and during follow‐up. Patients were excluded from the study if their only consultation was with a registered dietitian (likely no medical diagnoses in the chart), or if the patient attended fewer than three appointments in the IM clinic. Charts did not include a verification of intervention completion; therefore, patients were included without confirmation of intervention completion. Organic GI diseases were not ruled out. Based on initial diagnosis by IM practitioners, 10 different diagnoses were listed in the included charts: IBS, IBS‐D, IBS‐C, IBS‐M, FGID, functional disorder of intestine, FC‐C, IBS, functional bowel abnormality, and FD. A Board‐certified gastroenterologist reviewed all records and confirmed the final diagnosis based on the Rome IV diagnostic criteria, physician documentation, and patient‐reported symptoms to rule out the presence of alarm features 13 of organic GI diseases (family history of colorectal cancer, unexplained rectal bleeding, etc.).The criteria for diagnosis were the presence or absence of pain and the presence or absence of constipation, diarrhea, or bloating. The six final diagnoses included IBS‐C, IBS‐D, IBS‐M, FD, FC, and FB. In general, IM patients find the clinic by a variety of means: practitioner referral, word of mouth, or their own discovery of the clinic.
Integrative medicine interventions
Once a patient was selected for study inclusion, his or her chart was reviewed to characterize the interventions recommended to the patient. The “intervention appointment” was defined as the appointment that followed the initial or “baseline” appointment when the practitioner reviews lab results with the patient and makes a treatment plan or “intervention.” The intervention might include recommendations from a diagnosing healthcare provider (provider) such as an MD, APRN, or a PA and/or an RDN. Both the provider and RDN interventions were included if the appointments were within 6 months of each other.
Initially, 38 total interventions were identified, which subsequently collapsed into nine intervention categories. The nine categories were (i) elimination diet (e.g. recommend that a patient eliminate gluten, casein, and/or eggs for a certain number of weeks to determine if symptoms resolve or improve), (ii) vitamin or mineral supplementation, (iii) magnesium supplementation, (iv) GI‐related supplement (including probiotic, digestive enzyme, betaine hydrochloric acid), (v) fermented foods, (vi) water (hydration), (vii) non‐diet lifestyle modification (physical activity, stress management), (viii) referral (not including referral to RDN, since RDN interventions were included in the data), and (ix) GI‐related medication prescription.
After the nine IM intervention categories were defined, each patient's intervention appointment(s) were tallied for the intervention(s) he or she received. Once all charts were reviewed, totals for each of the nine categories of integrative intervention were compiled to determine which interventions were most often and least often recommended to IM patients. The recommended elimination diets were based on laboratory testing results (serum IgG, fecal IgA, and/or genetic testing for celiac or gluten‐sensitivity genetics).
Symptom measurement
Patients at KU IM completed an MSQ at each clinic visit to document their symptoms. The MSQ is a clinical and research tool, 14 , 15 , 16 , 17 organized by body system (e.g. head, nose, digestive tract, energy, emotions, etc.) with a rating scale of 0–4 for each symptom. A patient reporting a 0 never or almost never has the symptom, while a 4 reflects frequent symptoms and the reported effect is severe. Digestive‐tract‐specific MSQ symptoms include nausea/vomiting, diarrhea, constipation, bloated feeling, belching/passing gas, heartburn, and intestinal/stomach pain. The average time between baseline MSQ and follow‐up (post‐intervention) MSQ was 8.75 months (about 35 weeks). We defined “interventions” to include both recommendations from the diagnosing provider (MD, PA, APRN) and the RDN.
Statistical analysis
We used SPSS statistical software to perform all analyses. We compared the change in baseline MSQ digestive tract scores to those post intervention. Power calculation was based on an assumed moderate effect size of 0.45, because no comparable previous data were available. We determined that at least 40 chart reviews were required to achieve 80% power at an α = 0.05 to detect a moderate effect size of 0.45 (Cohen's d). No adjustment for multiple testing was considered for secondary outcomes (IBS) or subgroup analyses; therefore, these results should be considered exploratory. For outcomes with the normality assumption satisfied (overall FGID and IBS patient data), a paired t‐test was performed to determine whether the difference between the baseline and post‐intervention MSQ digestive tract scores were significantly different. For outcomes with the normality assumption violated (FB, FC, FD patients), a Wilcoxon signed‐rank test was used to determine whether the means for the pre‐ and post‐intervention MSQ digestive scores were different. All tests were considered significant at P < 0.05.
Results
Fifty‐seven patients fit study criteria with an average age of 49.8 ± 13.7 years (range 22–80 years), and 84.2% (n = 48) were women. Of the 57 patients, 43 (75.4%) improved their digestive tract symptoms scores from baseline to post intervention, whereas symptoms worsened in 9 (15.8%) and were unchanged in 5 (8.8%).
The most common intervention recommended by IM providers was to follow an elimination diet. The recommendation was made to patients at 103 (78.6%) of the 131 total patient intervention appointments. The second most common recommendation was vitamin or mineral supplements, recommended at 83 of 131 patient intervention appointments (63.4%). Providers or RDNs recommended that patients take GI‐related supplements (digestive enzymes, probiotics, and/or betaine HCl) 48.9% of the time. A provider or RDN suggested magnesium supplementation at 45.0% of intervention appointments. The next most common interventions for FBD patients were non‐diet lifestyle interventions (e.g. increase exercise, manage stress) (32.1% of appointments), increase water consumption (29.0%), non‐dietary referral (any referral that was not to a registered dietician) (25.2%), and consumption of fermented foods (16.0%). GI‐related medications were prescribed at 32.9% of intervention appointments (24 out of 73 appointments with providers because RDNs cannot prescribe medications). Practitioners may have recommended more than one intervention. The average time between baseline MSQ and follow‐up MSQ was 8.75 months (about 35 weeks).
The mean baseline digestive tract score for all FBD patients was 10.2 (SD, 5.4) and the mean post‐intervention score was 7.2 (SD, 5.2) (P < 0.0001, Table 1, Fig. 1).
Table 1.
GI MSQ score symptom change by diagnosis and symptom type of functional bowel disorder
| Diagnosis | Pre‐intervention mean GI MSQ | Post‐intervention mean GI MSQ | P‐value |
|---|---|---|---|
| All IBS (n = 45) | 11.4 ± 5.2 | 7.8 ± 5.1 | <0.001 |
| IBS‐C (n = 18) | 11.4 ± 4.7 | 8.3 ± 3.5 | 0.005 |
| IBS‐D (n = 16) | 10.7 ± 5.8 | 6.0 ± 5.9 | <0.001 |
| IBS‐M (n = 11) | 12.4 ± 5.4 | 9.5 ± 5.9 | 0.02 |
| FB (n = 3) | 7.7 ± 5.5 | 7 ± 9.5 | 0.75 |
| FC (n = 7) | 5 ± 2.8 | 4.1 ± 2.4 | 0.33 |
| FD (n = 2) | 6 ± 7.1 | 5 ± 7.1 | 0.50 |
| FBD (n = 57) | 10.2 ± 5.4 | 7.2 ± 5.2 | <0.001 |
FB, functional bloating, FC, functional constipation; FD, functional diarrhea; IBS, irritable bowel syndrome; IBS‐C, irritable bowel syndrome‐constipation dominant; IBS‐D, irritable bowel syndrome‐diarrhea dominant; IBS‐M, irritable bowel syndrome‐mixed bowel habits.
Figure 1.
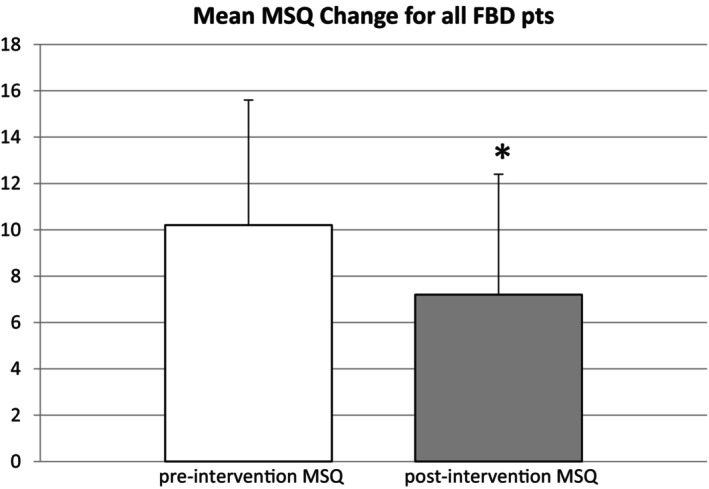
Mean pre‐ and post‐intervention MSQ scores for all FBD patients with error bars. Mean change in MSQ score was −3.0 and the change in MSQ scores for all FBD patients before and after intervention was statistically significant (P < 0.001). FBD, Functional bowel disorder; GI, gastrointestinal; MSQ, medical symptoms questionnaire.
For all patients with IBS (n = 45), the mean MSQ score decreased from 11.4 (SD, 5.2) to 7.8 (SD, 5.1) (P < 0.001, Fig. 2). Patients within IBS subcategories also showed significant symptom improvement (BS‐C [P = 0.005], IBS‐D [P < 0.001], and IBS‐M [P = 0.02]), while the symptoms did not change significantly for the other FBDs (Table 1).
Figure 2.
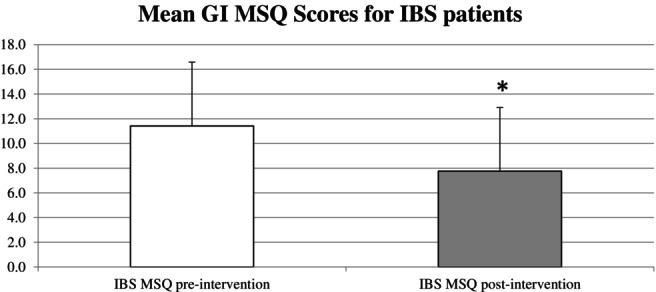
Mean pre‐ and post‐intervention MSQ GI scores for IBS patients with error bars. Mean change in MSQ score was −3.66 (SD, 4.0), and the change in MSQ scores for all IBS patients before and after intervention was statistically significant (P < 0.001). GI, gastrointestinal; IBS, irritable bowel syndrome; MSQ, medical symptoms questionnaire; Pts, patients.
Discussion
Our study suggests that IM interventions for patients with IBS are effective. One of the most common interventions was an elimination diet, and the most common foods eliminated were (in descending order) gluten and/or grains, gluten (alone), dairy or casein, and “other foods” (e.g. beef, pork, caffeine, and others). Interestingly, none of the providers specifically recommended the low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet, which is one of the more cited dietary interventions for IBS patients. The low FODMAPs diet and a traditional IBS diet are both effective at alleviating IBS symptoms. 18 , 19 Our data suggest that a less strict elimination diet may effectively alleviate symptoms in patients with IBS. Future prospective studies should compare use of less strict elimination diets to the low FODMAP diet, which may be easier for patients to follow.
Our results suggest that integrative medical interventions for FBDs appear to be most effective at lowering GI‐related symptoms for patients with IBS‐D and general diarrhea‐dominant symptoms (including both IBS‐D and FD); however, no statistical adjustment was made for subgroup analyses and these results should be considered exploratory. Our results confirm findings from the literature. A clinical review from the Journal of the American Medical Association 10 suggests that holistic lifestyle interventions such as dietary changes are appropriate for patients with IBS. Thus, studying the effectiveness of dietary interventions among a population of patients in an IM clinic is appropriate.
The effectiveness of interventions at KU IM may have been enhanced by defining “interventions” to include both recommendations from the diagnosing provider (MD, PA, APRN) and the RDN. Most patients with IBS report stronger confidence that lifestyle recommendations would help them, but patients adhere more to medication recommendations. 20 Patients in an IM setting may be more willing to make lifestyle changes because these patients seek this additional health care and often pay out of pocket for their care.
Not all patients benefited from IM interventions. Some possible explanations for patients with refractory symptoms include (i) patients not following recommended therapies, (ii) expense of carrying out the recommended intervention(s) (although this explanation may be less likely since the patients in this clinic pay out of pocket for this care), and (iii) complications of IBS not understood such as psychosocial‐related symptoms (e.g. early life trauma) and “rectal perceptual thresholds.” 21 For future research, a validated questionnaire such as the IBS Satisfaction with Care (IBS‐SAT) 22 questionnaire could be used to determine whether patient satisfaction differs between conventional and integrative IBS care.
In addition to dietary interventions, other IM interventions can effectively treat IBS symptoms, including peppermint oil, Chinese herb preparations (specifically, preparations made in the United States), soluble fiber, probiotics, mind–body therapies (cognitive behavioral therapy [CBT] and hypnosis), and acupuncture. 23 , 24
There are several limitations to this study. First, this is a retrospective chart review with a small sample of randomly selected charts from a single clinic. Second, we cannot pinpoint the specific interventions suggested to patients as the reason for their improvement because benefit may have come from the holistic approach in our IM clinic. The heterogeneity in IM treatments suggested prevents direct association between treatment and benefit as does the lack of a comparison arm. Third, patients were self‐paying and may have been more motivated to comply with interventions. Fourth, we did not determine which patients became Rome IV criteria‐negative after the intervention. In addition, we were unable to ensure that all patients completed both an initial and follow‐up MSQ. We also had a few patients with non‐IBS FBDs (i.e. FB, FD, FC), so we cannot draw conclusions for patients who did not have IBS. Another limitation is that the MSQ is a general‐symptoms questionnaire and is not specific to IBS patients; however, it has been used in other research to measure change in patient‐reported symptoms over time. 15 , 16 , 17 For future studies, the IBS quality of life (IBS‐QOL) 25 could be used to more accurately assess the impact on the quality of life by these patients' conditions. A placebo effect cannot be ruled out as a possible explanation for improvement in most patients; however, Spiller 26 suggests that the ideal length of any IBS clinical trial should be longer than 12 weeks because the placebo effect diminishes at 12 weeks. The average time between baseline MSQ and follow‐up MSQ was 8.75 months (about 35 weeks), which is well beyond that period. 26 Finally, since organic GI diseases were not ruled out, patients could have undiagnosed conditions such as celiac disease, inflammatory bowel disease, or colon cancer.
Our study adds to the evidence that dietary interventions that are less stringent than the low FODMAP diet may effectively reduce IBS symptoms in most patients. Because the study was retrospective in nature, we were able to assess the way that KU IM treats FBDs without influencing methods or the choice of interventions used during the study. KU IM used mostly laboratory‐test‐based interventions to determine the specific elimination diet (instead of a broad spectrum of foods, like FODMAP foods). Using a low FODMAP diet appears to be similarly efficacious but may be more difficult for patients to adhere to. 27
IM interventions for patients with FBDs may be effective, especially for those with IBS. Primary care physicians and gastroenterologists should consider dietary interventions as a first‐line therapy for patients with IBS. These healthcare providers could refer IBS patients to RDNs to implement dietary interventions for efficacy and to save physician time. 6 An interdisciplinary approach implementing several interventions over time may be worthwhile to help patients to (i) understand and follow the interventions and (ii) address the chronic nature of the condition over time.
Declaration of conflict of interest: The authors report no personal or financial disclosures or conflicts of interest.
References
- 1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 2. Wilson A, Longstreth G, Knight K et al. Quality of life in managed care patients with irritable bowel syndrome. Manag. Care Interface. 2004; 17: 24–28, 34. [PubMed] [Google Scholar]
- 3. Sperber AD, Bangdiwala SI, Drossman DA et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology. 2021; 160: 99–114 e3. [DOI] [PubMed] [Google Scholar]
- 4. Lacy BE, Mearin F, Chang L et al. Bowel disorders. Gastroenterology. 2016; 150: 1393–1407 e5. [DOI] [PubMed] [Google Scholar]
- 5. Brandt LJ, Chey WD, Foxx‐Orenstein AE et al. An evidence‐based systematic review on the management of irritable bowel syndrome. Am. J. Gastroenterol. 2009; 104: S8–S35. [DOI] [PubMed] [Google Scholar]
- 6. Lacy BE, Rosemore J, Robertson D, Corbin DA, Grau M, Crowell MD. Physicians' attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand. J. Gastroenterol. 2006; 41: 892–902. [DOI] [PubMed] [Google Scholar]
- 7. Horrigan B, Lewis S, Abrams DI, Pechura C. Integrative medicine in America—how integrative medicine is being practiced in clinical centers across the United States. Glob. Adv. Health Med. 2012; 1: 18–94. [Google Scholar]
- 8. Hu X‐Y, Lorenc A, Kemper K, Liu J‐P, Adams J, Robinson N. Defining integrative medicine in narrative and systematic reviews: a suggested checklist for reporting. Eur. J. Integr. Med. 2015; 7: 76–84. [Google Scholar]
- 9. Academic Consortium for Integrative Medicine and Health . Definition of Integrative Medicine and Health Academic Consortium for Integrative Medicine and Health . 2016. Available from URL: https://www.imconsortium.org/about/about-us.cfm
- 10. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 11. Maizes V, Rakel D, Niemiec C. Integrative medicine and patient‐centered care. Explore J. Sci. Heal. 2009; 5: 277–289. [DOI] [PubMed] [Google Scholar]
- 12. Dossett ML, Cohen EM, Cohen J. Integrative medicine for gastrointestinal disease. Prim. Care. 2017; 44: 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel P, Bercik P, Morgan DG et al. Prevalence of organic disease at colonoscopy in patients with symptoms compatible with irritable bowel syndrome: cross‐sectional survey. Scand. J. Gastroenterol. 2015; 50: 816–823. [DOI] [PubMed] [Google Scholar]
- 14. Hernandez MB, Blavo C, Hardigan PC, Perez AM, Hage K. Differences in perceived stress, depression, and medical symptoms among medical, nursing, and physician assistant students: a latent class analysis. Ann. Behav. Sci. Med. Educ. 2010; 16: 35–39. [Google Scholar]
- 15. Lerman RH. Nutritional approach for relief of joint discomfort: a 12‐week, open‐case series and illustrative case report. Integr. Med. 2015; 14: 52–61. [PMC free article] [PubMed] [Google Scholar]
- 16. Mallar M. The efficacy of complementary and alternative medicine approaches for the treatment of depression in an integrative healthcare setting . 2008.
- 17. Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi‐disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019; 11: e4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Böhn L, Störsrud S, Liljebo T et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015; 149: 1399–1407.e2. [DOI] [PubMed] [Google Scholar]
- 19. Manning LP, Yao CK, Biesiekierski JR. Therapy of IBS: Is a low FODMAP diet the answer? Front Psychiatry. 2020; 11: 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whitehead WE, Levy RL, Von Korff M et al. The usual medical care for irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004; 20: 1305–1315. [DOI] [PubMed] [Google Scholar]
- 21. Guthrie E, Creed F, Fernandes L et al. Cluster analysis of symptoms and health seeking behaviour differentiates subgroups of patients with severe irritable bowel syndrome. Gut. 2003; 52: 1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dorn SD, Morris CB, Schneck SE et al. Development and validation of the irritable bowel syndrome satisfaction with care scale. Clin. Gastroenterol. Hepatol. 2011; 9: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 23. Michelfelder AJ, Lee KC, Bading EM. Integrative medicine and gastrointestinal disease. Prim. Care. 2010; 37: 255–267. [DOI] [PubMed] [Google Scholar]
- 24. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta‐analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018; 48: 1044–1060. [DOI] [PubMed] [Google Scholar]
- 25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig. Dis. Sci. 1998; 43: 400–411. [DOI] [PubMed] [Google Scholar]
- 26. Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am. J. Med. 1999; 107: 91–97. [DOI] [PubMed] [Google Scholar]
- 27. Nanayakkara WS, Skidmore PM, O'Brien L, Wilkinson TJ, Gearry RB. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clin. Exp. Gastroenterol. 2016; 9: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]


