Abstract
Background:
Seminal plasma exosomes are now recognized to play a complex role in the regulation of the female reproductive system infertility. The objective of this study was to assess the effect of exosomes derived from the sperm of men with oligoasthenoteratozoospermia on endometrial implantation-related genes.
Methods:
To isolate the exosomes, we employed an ultracentrifugation method on samples derived from 10 fertile men with normal sperm parameters and 10 men with oligoasthenoteratozoospermia. The size distribution and ultrastructure of the exosomes were then characterized using transmission electron microscopy and dynamic light scattering. We detected an exosome marker using western blot analysis and confirmed the cytoplasmic localization of the exosomes by incubating them with DiI dye and visualizing them using fluorescence microscopy. After 6 hours of in vitro treatment of endometrial epithelial cells with 100 µg/ml seminal exosome, the endometrial receptivity genes were examined using qRT-PCR. To perform data analysis and quantification, we utilized Image J and Prism software. P< 0.05 were considered statistically significant.
Results:
After 6 hours of treatment, the mRNA levels of MUC1, LIF, G-CSF, CX3CL1, and VEGF were significantly downregulated in the endometrial epithelial cells treated with oligoasthenoteratozoospermia exosomes compared to the normal group. Although changes were observed in the mean mRNA levels of IL8 and TGF-β genes in the oligoasthenoteratozoospermia group compared to the normal group, these differences did not reach statistical significance (p > 0.05).
Conclusions
Oligoasthenoteratozoospermia exosomes have a distinct effect on endometrial receptivity compared to normal exosomes, leading to reduced expression of implantation-related genes.
Key Words: Embryo implantation, Endometrium, Exosome, Semen, Infertility
Introduction
Infertility is still common among couples around the world and is associated with numerous economic, psychological, and social problems. Male infertility plays an important role in 20–50% of cases of infertility. Despite the improvement of new technologies and diagnostic procedures, many of these men remain infertile (1). Oligoasthenoteratozoospermia (OAT) infertility in men is not a rare condition, but it is uncommon. Most of these men are generally healthy, and the precise cause of the impaired spermatogenesis is often unknown (2). Nowadays, intracytoplasmic sperm injection (ICSI) technology gives even these men an opportunity to become fathers (3). Assisted reproductive technology (ART) is a widely used approach for treating infertility. Nonetheless, a significant challenge with ART is the relatively low success rates in terms of implantation, pregnancy, and live birth (4).
The low implantation rate in ART is thought to be primarily due to dysregulated endometrial function and improper priming resulting from non-physiological stimulation with gonadotropins, as well as the lack of seminal plasma (5). In response to the significant demands for actions that improve implantation and subsequent live birth rates, a number of systemic treatments have been developed (6).
Seminal plasma has a critical role in completing several processes of fertilization, many of which are not completely understood (7). Seminal plasma may be used therapeutically to improve maternal immunological tolerance, endometrial receptivity, and the competence and rate of implantation of developing embryos during ART cycles (7). The most likely strategy appears to be to use seminal plasma as a physiological stimulator of the female reproductive system and for its function in priming the endometrium and maternal immune system to improve implantation and live birth rates in ART cycles. According to experimental findings and tests on many human and animal endometriums, seminal plasma may play a significant role in embryo implantation process (8).
Exosomes are tiny membrane vesicles secreted into the body fluids by many cell types (9). Compared to other fluids, semen contains a greater concentration of them. Exosomes carry a payload of lipid molecules, phospholipids, proteins, cholesterol, mRNAs, and miRNAs. Seminal exosomes play a major role in the female reproductive tract for effective fertilization (10). In this study, our goal was to determine whether using exosomes from patients with OAT would affect the expression of endometrial receptivity genes in vivo compared to a normal group.
Materials and Methods
Participants selection
This study was performed at the Shahid Akbar Abadi Hospital In vitro fertilization (IVF) Centre, Iran University of Medical Sciences, Tehran, Iran, from April 2022 to February 2023.
Totally 10 men with OAT undergoing infertility treatment and 10 men with proven fertility as a normal group were selected. The average age of men in the OAT and normal groups was 33.80±3.134 and 33.24±3.218, respectively. Male participants in both groups were excluded if they had abnormal endocrine testing, varicocele, excessive alcohol or drug use, or erectile dysfunction.
Endometrial biopsies were collected from five healthy young women in the proliferative phase of a natural menstrual cycle who had no hormonal therapy or an atypical uterus. The average age of women in participant in this study was 27.5±2.
Semen Analysis
The semen samples were collected through masturbation and put in non-toxic, sterile containers during 2-4 days of abstinence from sexual activity. After liquefaction at room temperature for 20 minutes, sperm count, motility, and morphology were analyzed according to the World Health Organization (WHO) guidelines. The percentage of immotile sperm, the total concentration of sperm, and sperm motility, including progressive and non-progressive motility, were all measured using a Makler chamber, under 400x magnification. The morphology of the sperms was examined using a Diff-Quick staining kit (Avicenna, Iran), and the resulting slides were assessed under a light microscope (Olympus, Japan) at 100× magnification (11).
Exosome Isolation
Semen samples were centrifuged at 1,000 g for 10 min at 25 °C to remove cell debris. Then, the supernatant was further centrifuged at 10,000 g for 20 min at 4 °C, followed by centrifugation of the resulting upper layer at 18,000 g for 45 min at 4 °C. Finally, the remaining seminal plasma was subjected to ultracentrifugation (Beckman, Germany) at 100,000 g for 120 min. After undergoing another round of ultracentrifugation, the pellets were washed with PBS. Pellets containing exosomes were resuspended in PBS and stored at -80 °C. The concentration of whole exosome protein was determined using the Bradford method (12).
Measurement of Exosome Size
After the isolation procedure, exosomes were mixed with PBS and their sizes were assessed using Dynamic Light Scattering (DLS) (Nano-flex 180°, Germany) (13).
Transmission Electron Microscopy (TEM)
The exosomes were placed on Formvar/carbon-coated Electron Microscopy (EM) grids and dried with filter paper. Subsequently, they were negatively stained with 2% uranyl acetate (Sigma-Aldrich, USA) for two minutes at room temperature. Images of the exosomes were captured using an LEO 906 Transmission Electron Microscope (Zeiss, Germany) operating at an acceleration voltage of 100 kV.
Western Blotting
From each sample, 50 µg, was loaded on SDS-polyacrylamide gel and then transferred onto polyvinylidene fluoride (PVDF) membranes (Sigma-Aldrich, Germany). The membranes were blocked using Tris-buffered saline with 0.1% Tween® 20 detergent (TBST) (Sigma-Aldrich, Germany), and then incubated with primary anti-CD9 antibody (ID: 928) (1:500) overnight at 4 °C. After washing with TBST, the blots were incubated for 2 hours at room temperature with a horseradish peroxidase(HRP)-conjugated secondary antibody (1:10,000; Abcam Company, UK). Immunodetection was carried out following another round of TBST washing. The chemiluminescent peroxidase substrate (ECL; Pars Tous, Iran) and blots (Fujifilm, REF 47410) were examined using X-ray films and scanned with a densitometer (GS-800; Bio-Rad, USA) (14).
Endometrial Tissue Staining
Tissue structure was assessed using H&E staining. Endometrial tissue was collected, fixed with paraformaldehyde, and processed as follows: it was dehydrated, embedded in paraffin, sectioned into 5 µm slices, deparaffinized in xylene, rehydrated in graded ethanol, stained with H&E, and imaged using a light microscope (Olympus, Japan) for histological analysis (15, 16).
Endometrial Cells culture
With the aid of a scalpel, the endometrial drop was initially placed in 1-3 ml vials of phosphate-buffered saline (PBS). After a rinse in PBS, it was incubated for 2-3 hours with a mixture of 6.4 mg/ml collagenase, 125 U/ml hyaluronidase, and 0.1 nmol/L gentamicin (Gibco, USA), until epithelial sheets emerged. The filtrate was then backwashed and cultured in a medium consisting of Dulbecco's modified Eagle's medium (DMEM) and 25% MCDB-105, supplemented with 10% charcoal-stripped fetal bovine serum and 5 mg/ml insulin. Stromal cells were allowed to attach to the bottom of the flask, while epithelial cells adhered to the flask's surface in keratinocyte serum-free medium (KSFM; Gibco). The cells were cultured in 6-well plates until they reached confluence, which typically took 10-14 days (17).
Flow cytometry
The cell suspension was mixed with an antibody conjugated to the cytokeratin-18 marker to identify epithelial cells, and the collected cells were confirmed using cytokeratin-18 FITC markers (Abcam company, UK). Utilizing the Flow Jo program, data analysis was done (17).
Exosome Internalization into endometrial epithelial cells (EECs) with DiI Labelling
Five µg Purified exosomes were incubated by 15 µM 2 M 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI dye, Sigma-Aldrich, USA) for 30 minutes at room temperature. Cells were rinsed with PBS and centrifuged at 100,000 g for two hours to eliminate residual DiI. The small exosome pellets have been suspended after being washed three times with PBS. In a 12-well plate, 100×103 EECs were incubated with Dil-labelled exosomes (Dil-Exos) for 6 h. The EECs were then rinsed in PBS, fixed in 4% paraformaldehyde, and stained for 15 minutes with 40,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, USA). A fluorescent microscope (Olympus, Japan) was applied to follow the cells' uptake of Dil-Exos (18).
In further experiments, endometrial epithelial cells were co-incubated with exosomes from two groups for 6 hours. The first group consisted of exosomes from fertile men with normal semen parameters, while the second group consisted of exosomes from men with infertility due to OAT. A control group was included, which consisted of endometrial cells grown in the same medium as the case group but without any exosomes. Each experiment was repeated three times.
The extraction and synthesis of RNA and cDNA
The expression levels of Vascular endothelial growth factor (VEGF) (ID: 7422), Transforming Growth Factor-β (TGF-β) (ID: 7040), chemokine (C-X3-C motif) ligand1 (CX3Cl1) (ID: 6376), Interleukin- 6 (IL-6) (ID: 3569), Mucin 1 (MUC1) (ID: 4582), Leukemia Inhibitory Factor (LIF) (ID: 3976), Granulocyte colony-stimulating factor (G-CSF) (ID: 12985), Interleukin-8 (IL-8) (ID: 3576), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ID: 2597) genes were determined using reverse transcription-polymerase chain reaction (RT-PCR) on each specimen. Total RNA was extracted from the semen samples (sperm) of patients with recurrent ICSI failure and the control group using the RNX-plus method according to the manufacturer's instructions (Gene All; Seoul, Korea). The integrity of the extracted RNA and its concentration were assessed by agarose electrophoresis and measuring the absorbance ratio between 260 and 280 nm. Complementary DNA (cDNA) synthesis was carried out using the Revert Aid First Strand cDNA Synthesis Kit and 1μg of total RNA (Bonbiotech, Iran).
Quantitative real-time PCR analysis (qRT-PCR)
For each reaction, the Polymerase chain reaction (PCR) mixture contained 1 μL of each primer (3 pmol/μL), 7.5 μL of SYBR premix Ex Taq II (Ampliqon, Denmark), and 12.5 ng of cDNA made up to a final volume of 20 μL with dH2O. Triplicate tests were run on each reaction. Specific primer pairs were designed using Gene Runner 6 (Table 1). The qRT-PCR procedure involved an initial denaturation step of 15 minutes at 95 °C followed by 40 repeated cycles of 10 seconds at 95 °C and 35 seconds at 60 °C. The expression levels of VEGF, TGF-β, CX3Cl1, IL-6, MUC1, LIF, G-CSF, IL-8, and GAPDH mRNAs were normalized to the expression of the housekeeping gene, GAPDH, for each sample. The relative expression was calculated using the CT method (19, 20).
Table 1.
Primer sequences used in Real-time PCR.
| Genes | Forward sequences | Reverse sequences | PCR product size (bp) |
|---|---|---|---|
| h-VEGF | CGGCGAAGAGAAGAGACACA | GGAGGAAGGTCAACCACTCA | 196 |
| h-CX3CL1 | CATGGCTCCGTTATCTCTGTC | TGCCTGGTTCTGTTGATAGTG | 169 |
| h-G-CSF | GAGAAGCTGGTGAGTGAGGCA | CGCCATTCCCAGTTCTTCCAT | 186 |
| h-IL-8 | ACTCCAAACCTTTCCACCC | TTCTCAGCCCTCTTCAAAAACT | 175 |
| h-IL-6 | CCCACACAGACAGCCACTCA | TGCCAGTGCCTCTTTGCTGC | 136 |
| h-LIF | AGGTCTTGGTGGTAGGAGTTGTG | TGATAGGGGTGATGGGGAGGG | 87 |
| h-TGFβ | CTGAGATGCTGGGACTCTGATAA | GTCTTCTTCACTATCCCCCACT | 119 |
| h-MUC1 | CAGCACCGACTACTACCAAGA | CAGATCCTGGCCTGAACTTAAT | 113 |
| h-GAPDH | CTTTGGTATCGTGGAAGGAC | GCAGGGATGATGTTCTGG | 126 |
Statistical Analysis
Statistical analysis was performed using both the T-test and one-way ANOVA. The results were computed using version 8 of PRISM. P< 0.05 were considered statistically significant, and the results were presented as mean ± standard deviation (SD).
Results
Collection and Analysis of Samples
To isolate and characterize semen exosomes, seminal plasma samples were collected from both normal and OAT patients with semen parameters evaluated according to the WHO criteria. The results of the analysis showed significant differences between the two groups in terms of sperm count, motility, and morphology (Fig. 1).
Fig. 1.

The patients' sperm characteristics were evaluated, and significant differences were found in sperm count, motility, and morphology between the two populations. The results are presented as mean ± SD (n = 10), and **** indicates a statistically significant difference with a P< 0.0001.
Isolation of Exosomes
According to TEM examination, a population of spherical, tiny, electron-dense vesicles with double membranes was found. The DLS analysis showed that the SEs from the two groups exhibited remarkably similar size profiles, with mean SD diameters of 101±12.32 nm for the normal group and 95.97±8.58 nm for the OAT group. The particles in both groups ranged in size from 50 to 130 nm. A Western blot analysis revealed the presence of the CD9 marker in the exosome samples from both the ED and OAT groups. One sample was chosen at random from each group for Western blotting.
Fig. 2.

Images captured by transmission electron microscopy show exosomes. a) from men in the normal group. b) men in the OAT group. C) Western blots of exosomes from normal men (1) and exosomes from OAT men (2) show the presence of the CD9 exosome surface marker. Scale bars in a) and b) represent 100 nm.
Fig. 3.

DLS validation of exosome size. a) Exosomes from men in Normal group. b) from OAT group. Mean SD is utilized to represent the results. c) Exosome average size is used for each group (n = 10) P> 0.05. Each group's exosome size is represented by a picture.
Histological Dating and Flow Cytometry
The H&E staining of the endometrial sample (Fig. 4b) indicated that the endometrium was in the proliferative phase. Quantitative flow cytometric analysis revealed that 84.6% of the endometrial cells expressed cytokeratin-18 (Fig. 4c).
Fig. 4.
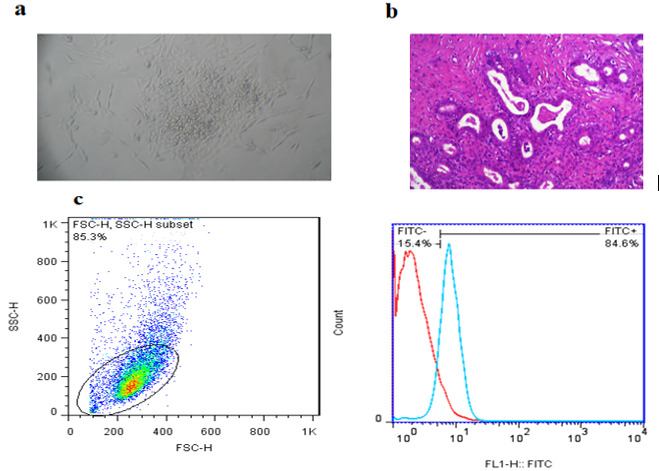
Verification of EECs: a) Primary cell culture of EECs. b) Image of endometrium in the proliferative phase taken under a light microscope; scale bars: 100 micrometers. c) Results from flow cytometry demonstrate that EECs expressed cytokeratin-18.
Internalization of exosomes
A fluorescent microscope (Olympus Co., Tokyo, Japan) was used to identify the cytoplasmic location of exosomes following the incubation of DiI-labelled SEs with EECs. Exosome uptake after 6 h is depicted in (Fig. 5). This finding suggests that EECs can internalize exosomes, which are necessary for subsequent cell functions.
Fig. 5.

Exosomes internalization within EECs. After 6h, EECs internalized DiI-labeled exosomes (red). DAPI (blue) is used to mark the nuclei of EECs. 20 nm scale bars.
Genes expression after exosomes treatment
As shown in (Fig. 6), the mean mRNA levels of TGF-β, IL-8, LIF, IL-6, VEGF, G-CSF, CX3CL1, and MUC1 increased significantly in EECs treated with the normal group exosomes compared with the control and OAT groups after 6h (P< 0.05). Our result show that there were no significant differences between IL8 and TGF-β expressions in EECs treated with normal exosomes after 6h compared with EECs treated with OAT exosomes.
Fig. 6.

Gene’s expression in EECs following normal and OAT exosome treatments for 6 h. The assessment of mRNA levels was done using qRT-PCR. GAPDH was used as an internal control (n = 10) and results were also reported as mean SD. *P< 0.05. two experimental groups are compared to the control groups. The *, **, ***, and **** indicate P< 0.05, P< 0.01, P< 0.001, and P< 0.0001, respectively.
Discussion
In this study, we assessed the mRNA expression levels of implantation-related genes in EECs treated with exosomes from individuals with OAT and those with normal semen parameters. Exosomes from both groups were found to have a size of almost 100 nm, as determined by TEM and DLS studies, and western blot results confirmed the presence of the CD9 exosome marker. Our findings suggest that the expression of genes related to endometrial receptivity is significantly reduced when EECs are treated with exosomes from individuals with OAT compared to those from fertile men with normal semen parameters. In situations where only a few sperm are detectable in the ejaculate, such as in cases of OAT, intracytoplasmic sperm injection (ICSI) has made it possible to generate embryos and achieve pregnancy (21). During ART cycles, one or two embryos are transferred into the womb using a small amount of fluid. Despite this, failed embryo implantation remains the biggest barrier to achieving a successful pregnancy. Low implantation rates in transfers of 'good quality' embryos may be due to insufficient endometrial receptivity."(22). Poor endometrial receptivity has been reported to be responsible for two-thirds of implantation failures. To increase implantation rates following ART procedures, a variety of therapeutic techniques have been employed (23). Recent research suggests that the female reproductive tract's response to seminal fluid plays a fascinating and likely significant role in determining the receptivity of the endometrium to embryo implantation and subsequent pregnancy development (24).
In contrast to regular sexual activity, where the male partner ejaculates in the vagina, exposing his partner to seminal fluid, during ART procedures, the female reproductive system does not encounter seminal plasma. Bellinge found that depositing semen in the upper vaginal region increases the likelihood of successful embryo implantation during IVF cycles (25).
It is possible that delivering a significant volume of seminal plasma directly into the uterus may have an impact on the implantation procedure (24).
Previous studies have shown that exosomes derived from seminal plasma possess immunosuppressive properties and can function as natural killer cells in the female reproductive tract. Furthermore, the presence of small RNAs in seminal exosomes can influence target cells and potentially modulate the immune response (26). Numerous studies have demonstrated that the miRNA of exosomes can regulate intracellular interactions under cell culture conditions (27). These miRNAs have the potential to inhibit immune responses, viral infections, and cancer cell proliferation. Additionally, seminal components trigger the release of embryotrophic cytokines that directly contribute to the successful development of the early embryo (28). Abu-Halima investigated the profile expression of microRNAs in fertile men and OAT infertile individuals (29). Given the role of exosomes in the female reproductive tract and the variations in their composition, it is plausible that seminal plasma exosomes from fertile and infertile men can exert distinct effects on endometrial receptivity and signaling pathways, leading to either an increase or decrease in endometrial receptivity. The decrease in endometrial receptivity, which can result in lower implantation rates, is thought to be due to the disruption of various molecular processes, including growth factor, cytokine, and hormone signaling (30). LIF, MUC1, VEGF, G-CSF, CX3CL1, IL-6, IL-8, and TGF-β play crucial roles in angiogenesis, proliferation, and tissue remodeling in the endometrium. Downregulation or disruption in the expression of these genes can lead to recurrent implantation failure (RIF) and recurrent pregnancy loss (31-33). Previous studies show that in women who experience recurrent miscarriage of unclear cause, seminal plasma pessaries have been used effectively to increase implantation rates (28).
It is possible that exosomes derived from infertile OAT men may decrease the expression of crucial genes that are essential for implantation due to differences in their composition and miRNA content when compared to exosomes from healthy fertile men. Our findings indicate that the use of exosomes or seminal plasma from these infertile men does not aid in improving endometrial receptivity or embryo implantation. However, studying seminal plasma exosomes and miRNA may provide insights into the molecular mechanisms underlying infertility and implantation failure. The authors suggest that further research is necessary to explore the differences in the composition of seminal exosomes between fertile and infertile men and their impact on the implantation process.
The use of seminal plasma exosomes from OAT patients is not beneficial in improving endometrial receptivity or clinical outcomes for patients undergoing IVF. On the other hand, exosomes derived from semen of healthy men have been shown to upregulate endometrial receptivity genes and may potentially serve as a natural adjunct to IVF in the future.
Ethical Consideration
This study was supported by the Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.293).
Funding information
This work was supported by the Iran University of Medical Sciences under grant number 98-4-4-15581.
Declaration of competing interests
The authors declare no conflicts of interest.
Acknowledgements
All experiments were performed in the laboratories of the Department of Anatomy and the Centre for Cellular and Molecular Research (CMRC), IUMS, Tehran, Iran. The present study was supported by Iran University of Medical Sciences.
References
- 1.Barratt CLR, Wang C, Baldi E, Toskin I, Kiarie J, Lamb DJ. What advances may the future bring to the diagnosis, treatment, and care of male sexual and reproductive health? Fertil Steril. 2022;117(2):258–267. doi: 10.1016/j.fertnstert.2021.12.013. other Editorial Board Members of the WHO Laboratory Manual for the Examination and Processing of Human Semen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed Khosroshahi L, Parhizkar F, Kachalaki S, Aghebati-Maleki A, Aghebati-Maleki L. Immune checkpoints and reproductive immunology: Pioneers in the future therapy of infertility related Disorders? Int Immunopharmacol. 2021;99:107935. doi: 10.1016/j.intimp.2021.107935. [DOI] [PubMed] [Google Scholar]
- 5.Schjenken JE, Glynn DJ, Sharkey DJ, Robertson SA. TLR4 Signaling Is a Major Mediator of the Female Tract Response to Seminal Fluid in Mice. Biol Reprod. 2015;93(3):68. doi: 10.1095/biolreprod.114.125740. [DOI] [PubMed] [Google Scholar]
- 6.Luchetti CG, Mikó E, Szekeres-Bartho J, Paz DA, Motta AB. Dehydroepiandrosterone and metformin modulate progesterone-induced blocking factor (PIBF), cyclooxygenase 2 (COX2) and cytokines in early pregnant mice. J Steroid Biochem Mol Biol. 2008;111(3-5):200–7. doi: 10.1016/j.jsbmb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi H, Csabai T, Gorgey E, Rashidiani S, Parhizkar F, Aghebati-Maleki L. Composition and effects of seminal plasma in the female reproductive tracts on implantation of human embryos. Biomed Pharmacother. 2022;151:113065. doi: 10.1016/j.biopha.2022.113065. [DOI] [PubMed] [Google Scholar]
- 8.Fabian D, Juhás S, Il'ková G, Koppel J. Dose- and time-dependent effects of TNFalpha and actinomycin D on cell death incidence and embryo growth in mouse blastocysts. Zygote. 2007;15(3):241–9. doi: 10.1017/S0967199407004200. [DOI] [PubMed] [Google Scholar]
- 9.Vickram AS, Srikumar PS, Srinivasan S, Jeyanthi P, Anbarasu K, Thanigaivel S, et al. Seminal exosomes - An important biological marker for various disorders and syndrome in human reproduction. Saudi J Biol Sci. 2021;28(6):3607–3615. doi: 10.1016/j.sjbs.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samanta L, Parida R, Dias TR, Agarwal A. The enigmatic seminal plasma: a proteomics insight from ejaculation to fertilization. Reprod Biol Endocrinol. 2018;16(1):41. doi: 10.1186/s12958-018-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torki A, Amirmozafari N, Talebi M, Talebi A. Using the PCR and Blood Agar in Diagnosis of Semen Bacterial Contamination of Fertile and Infertile Men. Rep Biochem Mol Biol. 2021;10(3):402–411. doi: 10.52547/rbmb.10.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdica V, Giacomini E, Alteri A, Bartolacci A, Cermisoni GC, Zarovni N, et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil Steril. 2019;111(5):897–908.e2. doi: 10.1016/j.fertnstert.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Paktinat S, Hashemi SM, Ghaffari Novin M, Mohammadi-Yeganeh S, Salehpour S, Karamian A, Nazarian H. Seminal exosomes induce interleukin-6 and interleukin-8 secretion by human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2019;235:71–76. doi: 10.1016/j.ejogrb.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Amjadi F, Salehi E, Zandieh Z, Rashidi M, Taleahmad S, Javedani Masrour M, et al. Comparative evaluation of NOTCH signaling molecules in the endometrium of women with various gynecological diseases during the window of implantation. Iran J Basic Med Sci. 2019;22(4):426–431. doi: 10.22038/ijbms.2019.32961.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shams LM, Minaei-Tehrani D, Gholipour H, Nohehkhan M. Effects of quinazolinones on Balb/C mice embryonic livers. Indian J Exp Biol. 2011;49(3):183–90. [PubMed] [Google Scholar]
- 16.Gholipour H, Lahijani MS. Teratogenic Effects of Two New Derivatives of Quinazolinones on Balb/C Mice Embryos and Newborns: A Literature Review. Curr Res J Biol. 2017;9(2):23–31. [Google Scholar]
- 17.Ajdary M, Ashrafi M, Aflatoonian R, Mehdizadeh M. The role of sperm in inducing genomic changes in the implantation: An experimental study. Andrologia. 2021;53(7):e14077. doi: 10.1111/and.14077. [DOI] [PubMed] [Google Scholar]
- 18.Taghdidri Nooshabadi V, Verdi J, Ebrahimi-Barough S, Mowla J, Atlasi MA, Mazoochi T, et al. Endometrial mesenchymal stem cell-derived exosome promote endothelial cell angiogenesis in a dose dependent manner: a new perspective on regenerative medicine and cell-free therapy. Arch Neurosci. 2019;6(4):e94041. [Google Scholar]
- 19.Govahi A, Amjadi F, Nasr-Esfahani MH, Raoufi E, Mehdizadeh M. Accompaniment of Time-Lapse Parameters and Cumulus Cell RNA-Sequencing in Embryo Evaluation. Reprod Sci. 2022;29(2):395–409. doi: 10.1007/s43032-021-00748-3. [DOI] [PubMed] [Google Scholar]
- 20.Zare R, Anvari K, Mohajertehran F, Farshbaf A, Pakfetrat A, et al. Association between Tissue Expression of Toll-Like Receptor and Some Clinicopathological Indices in Oral Squamous Cell Carcinoma. Rep Biochem Mol Biol. 2022;11(2):200–208. doi: 10.52547/rbmb.11.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordhoff V, Fricke RK, Schüring AN, Zitzmann M, Kliesch S. Treatment strategies for severe oligoasthenoteratozoospermia (OAT) (<0.1 million/mL) patients. Andrology. 2015;3(5):856–63. doi: 10.1111/andr.12077. [DOI] [PubMed] [Google Scholar]
- 22.Timeva T, Shterev A, Kyurkchiev S. Recurrent implantation failure: the role of the endometrium. J Reprod Infertil. 2014;15(4):173–83. [PMC free article] [PubMed] [Google Scholar]
- 23.Moharrami T, Ai J, Ebrahimi-Barough S, Nouri M, Ziadi M, Pashaiefar H, et al. Influence of Follicular Fluid and Seminal Plasma on The Expression of Endometrial Receptivity Genes in Endometrial Cells. Cell J. 2021;22(4):457–466. doi: 10.22074/cellj.2021.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford G, Ray A, Gudi A, Shah A, Homburg R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta-analysis. Hum Reprod Update. 2015;21(2):275–84. doi: 10.1093/humupd/dmu052. [DOI] [PubMed] [Google Scholar]
- 25.Bellinge BS, Copeland CM, Thomas TD, Mazzucchelli RE, O'Neil G, Cohen MJ. The influence of patient insemination on the implantation rate in an in vitro fertilization and embryo transfer program. Fertil Steril. 1986;46(2):252–6. doi: 10.1016/s0015-0282(16)49521-x. [DOI] [PubMed] [Google Scholar]
- 26.Vickram AS, Samad HA, Latheef SK, Chakraborty S, Dhama K, Sridharan TB, et al. Human prostasomes an extracellular vesicle - Biomarkers for male infertility and prostate cancer: The journey from identification to current knowledge. Int J Biol Macromol. 2020;146:946–958. doi: 10.1016/j.ijbiomac.2019.09.218. [DOI] [PubMed] [Google Scholar]
- 27.Lyu Y, Kaddour H, Kopcho S, Panzner TD, Shouman N, Kim EY. Human Immunodeficiency Virus (HIV) Infection and Use of Illicit Substances Promote Secretion of Semen Exosomes that Enhance Monocyte Adhesion and Induce Actin Reorganization and Chemotactic Migration. Cells. 2019;8(9):1027. doi: 10.3390/cells8091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aflatoonian A, Ghandi S, Tabibnejad N. The effect of intercourse around embryo transfer on pregnancy rate in assisted reproductive technology cycles. Int J Fertil Steril. 2009;2(4):169–72. [Google Scholar]
- 29.Abu-Halima M, Ludwig N, Hart M, Leidinger P, Backes C, Keller A, et al. Altered micro-ribonucleic acid expression profiles of extracellular microvesicles in the seminal plasma of patients with oligoasthenozoospermia. Fertil Steril. 2016;106(5):1061–1069.e3. doi: 10.1016/j.fertnstert.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Koot YE, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochim Biophys Acta. 2012;1822(12):1943–50. doi: 10.1016/j.bbadis.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Wu F, Chen X, Liu Y, Liang B, Xu H, Li TC, Wang CC. Decreased MUC1 in endometrium is an independent receptivity marker in recurrent implantation failure during implantation window. Reprod Biol Endocrinol. 2018;16(1):60. doi: 10.1186/s12958-018-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81(3):1118–22. doi: 10.1210/jcem.81.3.8772585. [DOI] [PubMed] [Google Scholar]
- 33.Bollwein H, Sowade C, Stolla R. The effect of semen extender, seminal plasma and raw semen on uterine and ovarian blood flow in mares. Theriogenology. 2003;60(4):607–16. doi: 10.1016/s0093-691x(03)00084-0. [DOI] [PubMed] [Google Scholar]


