Abstract
Background
Persistent mortality in adults hospitalized due to acute COVID-19 justifies pursuit of disease mechanisms and potential therapies. The aim was to evaluate which virus and host response factors were associated with mortality risk among participants in Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3) trials.
Methods
A secondary analysis of 2625 adults hospitalized for acute SARS-CoV-2 infection randomized to 1 of 5 antiviral products or matched placebo in 114 centers on 4 continents. Uniform, site-level collection of participant baseline clinical variables was performed. Research laboratories assayed baseline upper respiratory swabs for SARS-CoV-2 viral RNA and plasma for anti–SARS-CoV-2 antibodies, SARS-CoV-2 nucleocapsid antigen (viral Ag), and interleukin-6 (IL-6). Associations between factors and time to mortality by 90 days were assessed using univariate and multivariable Cox proportional hazards models.
Results
Viral Ag ≥4500 ng/L (vs <200 ng/L; adjusted hazard ratio [aHR], 2.07; 1.29–3.34), viral RNA (<35 000 copies/mL [aHR, 2.42; 1.09–5.34], ≥35 000 copies/mL [aHR, 2.84; 1.29–6.28], vs below detection), respiratory support (<4 L O2 [aHR, 1.84; 1.06–3.22]; ≥4 L O2 [aHR, 4.41; 2.63–7.39], or noninvasive ventilation/high-flow nasal cannula [aHR, 11.30; 6.46–19.75] vs no oxygen), renal impairment (aHR, 1.77; 1.29–2.42), and IL-6 >5.8 ng/L (aHR, 2.54 [1.74–3.70] vs ≤5.8 ng/L) were significantly associated with mortality risk in final adjusted analyses. Viral Ag, viral RNA, and IL-6 were not measured in real-time.
Conclusions
Baseline virus-specific, clinical, and biological variables are strongly associated with mortality risk within 90 days, revealing potential pathogen and host-response therapeutic targets for acute COVID-19 disease.
Keywords: acute COVID-19, viral factors, host response
COVID-19 mortality risk of virus, host factors.
Graphical Abstract
Graphical Abstract.
(See the Editorial Commentary by Trøseid on pages 1504–5.)
Mortality in adults hospitalized with coronavirus disease 2019 (COVID-19) is unacceptably high, ranging from 5% to 15% during Delta, early Omicron, and later Omicron variant periods [1], justifying continued assessment of clinical variable and biomarker associations with relevant outcomes. By leveraging clinical trials that enroll patients with standardized data and biospecimen collection, secondary analyses of trial data provide a resource to better understand predictors of clinical outcomes, and may also identify higher-risk subpopulations defined by biological variables for whom mechanism-based interventions may be considered in future studies.
COVID-19 risk-prediction models have utilized patient demographic variables, including older age, and male sex, and comorbid conditions, including diabetes mellitus, hypertension, chronic lung disease, and cardiovascular disease, that are associated with worse COVID-19 outcomes [2–4]. Integration of electronic health record–based clinical and laboratory data [5, 6] and blood-based host biomarkers that reveal inflammatory status improves model prediction [7–9], and can prospectively identify COVID-19 trial eligibility [10, 11]. However, biomarkers of host tissue injury or pathogen burden that require measurement outside of clinical laboratories have not been broadly reported for COVID-19 mortality risk prediction.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral burden quantified by viral RNA or antigen levels in the upper respiratory tract has shown an inconsistent association with clinical outcomes and little relation to systemic markers [12–14]. In contrast, serum SARS-CoV-2 RNA levels are generally associated with markers of clinical COVID-19 disease progression, including mortality [15]. More recent work has also demonstrated the importance of plasma viral antigen as an independent predictor of in-hospital outcomes [16, 17].
Adding plasma-based viral markers plus inflammatory and tissue damage biomarkers to clinical data may reveal pathogen-host COVID-19 disease mechanisms and help identify precision-based therapies appropriate for higher-risk disease populations [18, 19]. The aim of this work was to evaluate the association of baseline variables with the risk of mortality by integrating virus-specific, host-related, and clinical factors among participants enrolled in Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3) randomized clinical trials.
METHODS
Participants
We report secondary analyses of 2625 adults aged 18 years and older hospitalized for acute COVID-19 who were enrolled in the Institutional Review Board (IRB)–approved TICO platform trial (NCT04501978) from August 2020 to November 2021 across 114 sites on 4 continents [20–25]. Participants were required to have an attributable symptom onset 12 days or less prior to enrollment, with additional trial-specific criteria included in the Supplementary Methods (pp. 2–5). For the modified intention-to-treat (mITT) population (n = 2625), participants were randomly assigned to receive 1 of 5 antiviral products (bamlanivimab, sotrovimab, amubarvimab–romlusevimab, tixagevimab–cilgavimab, and ensovibep) or matched placebo and received all or part of the assigned study product. Following informed consent, baseline clinical data and biospecimens were collected 0–24 hours prior to randomization and evaluated in this study. Pulmonary status was re-assessed immediately prior to randomization.
Central Laboratory Measurements
Baseline plasma samples were used to measure interleukin-6 (IL-6) via electrochemiluminescence (Meso Scale Discovery), anti-spike (-S) neutralizing antibody (Ab) using a surrogate viral neutralization test (GenScript cPass) (Supplementary Methods, p. 6), anti-nucleocapsid (-N)–binding Abs (Bio-Rad Platelia SARS-CoV-2 Total Antibody Test) (Supplementary Methods, p. 6), and quantitative plasma SARS-CoV-2 nucleocapsid antigen (viral Ag) by microbead-based immunoassay (Quanterix) [17]. SARS-CoV-2 viral RNA levels were measured from a midturbinate nasal (upper respiratory) swab collected concurrent with the plasma sample [16, 26], quantified and assessed for the Delta variant using reverse transcriptase–polymerase chain reaction (RT-PCR) assay (Supplementary Methods, pp. 6–7). Subsequent sequencing analyses revealed 99.6% concordance with the RT-PCR assay for the Delta variant.
Clinical Data
Common case-report forms collected baseline data on each TICO trial participant, including demographic characteristics, geographic location, infection time period, pre–COVID-19 comorbidities, SARS-CoV-2 vaccination status, days since COVID-19 symptom onset, concomitant medications, COVID-19 clinical severity including pulmonary status, modified Borg dyspnea scale, National Early Warning Score (NEWS) [27], and clinical laboratory measurements (a full list of all variables under each category is available in the Supplementary Methods, pp. 7–8).
Statistical Analysis
Baseline factors associated with mortality through day 90 were identified using univariate and multivariable Cox proportional hazards models. All multivariable models presented included adjustment for age, sex, race/ethnicity, residence, geographical region, infection time period, active or placebo treatment group, and baseline pulmonary status. Informed by prior COVID-19 studies, 3 models were constructed to test for associations with time to mortality: 1 model examining pre-disease participant characteristics (demographics, body mass index [BMI], and comorbid conditions) [2–4], 1 model examining disease incident characteristics, and 1 model combining the significant results of the other 2 models (excluding comorbid conditions observed in <5% of participants). The multivariable model for disease incident characteristics included symptom duration, vaccination, viral RNA, viral Ag, anti-S Ab, anti-N Ab, measures of clinical severity, C-reactive protein (CRP), absolute lymphocyte count (ALC), estimated glomerular filtration rate (eGFR), and IL-6 [7–11]. Adjusted hazard ratios (aHRs; 95% confidence interval [CI]) with significance (P < .05) are reported; an aHR greater than 1 signified worse mortality.
Participants were followed until day 90, death, or loss to follow-up, and for the primary analysis were censored at the date last known to be alive. Some baseline values of IL-6 and viral Ag were missing (5.7% and 3.1%, respectively), so multiple imputation was used to assess the sensitivity of the complete case analysis to missingness of these variables (Supplementary Methods, pp. 9–10).
The presence of interactions between viral Ag and other baseline variables was assessed by including an interaction term in the adjusted Cox proportional hazards model. For the final adjusted model, we tested the proportional hazards assumption and subsequently conducted an analysis stratified by geographical region. The relationship between viral Ag as a continuous variable and mortality HR was plotted using a restricted cubic spline. While the significance level has been controlled at the conventional level of 5%, due to the large number of hypothesis tests presented here the results should be interpreted carefully.
Two additional sensitivity analyses were performed. The first limited the cohort to only participants randomized to placebo to exclude any treatment effect. A second cohort excluded participants randomized to the tixagevimab–cilgavimab trial that enrolled the most participants and a greater proportion of higher respiratory support, helping assess the validity with lower respiratory illness severity [23]. All analyses were conducted using SAS, version 9.4 (SAS Institute), or R, version 4.0 (R Foundation for Statistical Computing). Further details on statistical methods are provided in the Supplementary Methods (pp. 2–10).
RESULTS
Baseline Clinical and Virus-Specific Cohort Characteristics
Between August 2020 and November 2021, 2694 adults hospitalized for acute SARS-CoV-2 infection were enrolled in 5 TICO randomized controlled trials. We report on baseline variables as risk factors for mortality for 2625 participants in the mITT population (Table 1). The median age was 57 years (interquartile range [IQR], 46–68 years), 58% were male, 42% Black or Hispanic, and 78% of participants were enrolled in the United States.
Table 1.
Baseline Characteristics
| Did Not Die (n = 2364) | Died (n = 261) | Percentage in Subgroupa (n = 2625) | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Demographics | |||||
| Age, median (IQR), y | 56 (45–67) | 67 (57–76) | |||
| 18–39 y | 347 | 97.2 | 10 | 2.8 | 13.6 |
| 40–49 y | 457 | 94.8 | 25 | 5.2 | 18.4 |
| 50–59 y | 585 | 93.3 | 42 | 6.7 | 23.9 |
| 60–69 y | 502 | 87.3 | 73 | 12.7 | 21.9 |
| 70–79 y | 344 | 84.7 | 62 | 15.3 | 15.5 |
| ≥80 y | 129 | 72.5 | 49 | 27.5 | 6.8 |
| Sex | |||||
| Male | 1355 | 89.5 | 159 | 10.5 | 57.7 |
| Female | 1009 | 90.8 | 102 | 9.2 | 42.3 |
| Race/ethnicity | |||||
| Asian | 112 | 92.6 | 9 | 7.4 | 4.6 |
| Black | 558 | 88.9 | 70 | 11.1 | 23.9 |
| Hispanic | 430 | 89.0 | 53 | 11.0 | 18.4 |
| White | 1178 | 90.4 | 125 | 9.6 | 49.6 |
| Other | 86 | 95.6 | 4 | 4.4 | 3.4 |
| Region | |||||
| Africa | 100 | 76.3 | 31 | 23.7 | 5.0 |
| Asia | 38 | 90.5 | 4 | 9.5 | 1.6 |
| Europe | 378 | 96.2 | 15 | 3.8 | 15.0 |
| United States | 1848 | 89.8 | 211 | 10.2 | 78.4 |
| Residence | |||||
| Independent, without assistance | 2233 | 90.4 | 237 | 9.6 | 94.1 |
| Other | 131 | 84.5 | 24 | 15.5 | 5.9 |
| Infection time period | |||||
| Pre-2021 | 377 | 93.3 | 27 | 6.7 | 15.4 |
| January–June 2021 | 983 | 92.8 | 76 | 7.2 | 40.3 |
| July–November 2021 | 1004 | 86.4 | 158 | 13.6 | 44.3 |
| Treatment group | |||||
| Active | 1345 | 91.0 | 133 | 9.0 | 56.3 |
| Placebo | 1147 | 88.8 | 128 | 11.2 | 43.7 |
| COVID-19 characteristics | |||||
| Symptom duration, median (IQR) d | 8 (6–10) | 8 (5–9) | |||
| <5 d | 359 | 90.2 | 39 | 9.8 | 15.2 |
| 5–7 d | 686 | 89.7 | 79 | 10.3 | 29.1 |
| 8–10 d | 985 | 90.4 | 104 | 9.6 | 41.5 |
| >10 d | 334 | 89.5 | 39 | 10.5 | 14.2 |
| No. of vaccine doses | |||||
| 0 | 1949 | 90.7 | 201 | 9.3 | 82.6 |
| 1 | 166 | 90.2 | 18 | 9.8 | 7.1 |
| 2 | 228 | 85.1 | 40 | 14.9 | 10.3 |
| Plasma viral Ag, median (IQR), ng/L | 1296 (199–4152) | 4775 (1038–11702) | |||
| 1000+ | 1270 | 87.3 | 185 | 12.7 | 57.2 |
| <1000 | 1030 | 94.7 | 58 | 5.3 | 42.8 |
| Upper respiratory viral RNA | |||||
| Negative | 325 | 97.3 | 9 | 2.7 | 13.3 |
| <35 000 copies/mL | 1007 | 92.6 | 80 | 7.4 | 43.3 |
| 35 000+ copies/mL | 930 | 85.2 | 161 | 14.8 | 43.4 |
| Anti-S Ab | |||||
| Positive | 1200 | 91.5 | 112 | 8.5 | 51.6 |
| Negative | 1100 | 89.4 | 131 | 10.6 | 48.4 |
| Anti-N Ab | |||||
| Positive | 1442 | 90.9 | 144 | 9.1 | 62.3 |
| Negative | 859 | 89.7 | 99 | 10.3 | 37.7 |
| Variant | |||||
| Delta | 729 | 84.1 | 138 | 15.9 | 48.6 |
| Not Delta | 844 | 92.0 | 73 | 8.0 | 51.4 |
| Comorbid conditions | |||||
| Diabetes | |||||
| Yes | 651 | 88.0 | 89 | 12.0 | 28.2 |
| No | 1713 | 90.9 | 172 | 9.1 | 71.8 |
| Hypertension | |||||
| Yes | 1057 | 88.0 | 144 | 12.0 | 45.8 |
| No | 1307 | 91.8 | 117 | 8.2 | 54.2 |
| Renal impairment | |||||
| Yes | 217 | 83.5 | 43 | 16.5 | 9.9 |
| No | 2147 | 90.8 | 218 | 9.2 | 90.1 |
| BMI, median (IQR), kg/m2 | 31 (26–36) | 28 (25–35) | |||
| <18.5 (underweight) | 39 | 81.3 | 9 | 18.8 | 1.8 |
| 18.5–24.9 (healthy) | 382 | 88.6 | 49 | 11.4 | 16.5 |
| 25–29.9 (overweight) | 671 | 88.2 | 90 | 11.8 | 29.1 |
| 30–39.9 (obese) | 914 | 92.0 | 80 | 8.0 | 38.0 |
| ≥40 (morbidly obese) | 350 | 91.6 | 32 | 8.4 | 14.6 |
| Concomitant medications prior to randomization | |||||
| Immunomodulators | |||||
| Yes | 134 | 79.3 | 35 | 20.7 | 6.4 |
| No | 2230 | 90.8 | 226 | 9.2 | 93.6 |
| Corticosteroids | |||||
| Yes | 1584 | 88.7 | 202 | 11.3 | 68.0 |
| No | 780 | 93.0 | 59 | 7.0 | 32.0 |
| Remdesivir | |||||
| Yes | 1457 | 90.5 | 153 | 9.5 | 61.3 |
| No | 907 | 89.4 | 108 | 10.6 | 38.7 |
| COVID-19 severity | |||||
| Pulmonary status | |||||
| No O2 | 636 | 96.5 | 23 | 3.5 | 25.1 |
| O2 <4 L/min | 906 | 95.3 | 45 | 4.7 | 36.2 |
| O2 ≥4 L/min | 623 | 85.5 | 106 | 14.5 | 27.8 |
| Noninvasive ventilation/HFNC | 199 | 69.6 | 87 | 30.4 | 10.9 |
| Borg dyspnea scale | |||||
| 0–2 (nothing to slight) | 1071 | 92.8 | 83 | 7.2 | 47.8 |
| 3–4 (moderate–somewhat severe) | 706 | 89.8 | 80 | 10.2 | 32.5 |
| 5–10 (severe–maximal) | 406 | 85.5 | 69 | 14.5 | 19.7 |
| NEWS | |||||
| <2 | 306 | 96.8 | 10 | 3.2 | 12.1 |
| 2–3 | 796 | 93.6 | 54 | 6.4 | 32.5 |
| 4–5 | 755 | 90.5 | 79 | 9.5 | 31.9 |
| ≥6 | 495 | 80.9 | 117 | 19.1 | 23.4 |
| Lymphocytes, median (IQR) ×109/L | 0.85 (0.59–1.24) | 0.61 (0.37–0.90) | |||
| <0.9 | 1235 | 86.6 | 191 | 13.4 | 55.3 |
| 0.9–1.5 | 750 | 94.2 | 46 | 5.8 | 30.9 |
| >1.5 | 337 | 94.4 | 20 | 5.6 | 13.8 |
| Serum creatinine, median (IQR), µmol/L (mg/dL) | 74.3 (61.9–96.4) 0.84 (0.70–1.09) | 88.4 (66.3–125.6) 1.00 (0.75–1.42) | |||
| <97.3 (1.1) | 1783 | 92.3 | 149 | 7.7 | 73.7 |
| 97.3–132.6 (1.1–1.5) | 356 | 86.6 | 55 | 13.4 | 15.7 |
| >132.6 (1.5) | 220 | 79.4 | 57 | 20.6 | 10.6 |
| eGFR, median (IQR), mL/min/1.73m2 | 92 (71–108) | 73 (46–97) | |||
| <60 | 406 | 80.2 | 100 | 19.8 | 19.3 |
| ≥60 | 1953 | 92.4 | 161 | 7.6 | 80.7 |
| CRP, median (IQR), mg/L | 60 (26–112) | 93 (51–161) | |||
| <50 | 995 | 94.0 | 63 | 6.0 | 40.9 |
| 50–75 | 378 | 91.7 | 34 | 8.3 | 15.9 |
| >75 | 956 | 85.8 | 158 | 14.2 | 43.1 |
| IL-6, median (IQR), ng/L | 5 (2–13) | 15 (8–35) | |||
| ≤5.8 | 1189 | 96.4 | 44 | 3.6 | 49.8 |
| >5.8 | 1047 | 84.3 | 195 | 15.7 | 50.2 |
Abbreviations: Ab, antibody; Ag, antigen; Anti-N, anti-nucleocaspid; Anti-S, anti-spike; BMI, body mass index; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HFNC, high-flow nasal canula; IL-6, interleukin 6; IQR, interquartile range; O2, supplemental oxygen; NEWS, National Early Warning Score.
aColumn percentages.
The median (IQR) time from symptom onset to randomization was 8 days [6, 10], and 10% of participants had previously received 2 vaccine doses. The overall median (IQR) baseline plasma viral Ag was 1445 ng/L (234–4731 ng/L), and 57% of participants had a viral Ag of 1000 ng/L or greater [16]. Forty-three percent had viral RNA levels of 35 000 or more copies/mL, 52% were positive for anti-S and 62% were positive for anti-N SARS-CoV-2 antibodies. Baseline COVID-19 therapies included the following: corticosteroids (68%), remdesivir (61%; first-dose median, 1 day [IQR, 1–2 days] prior to randomization). A full set of baseline variables with associated percentages of survival and death are presented in Supplementary Table 1. Timing from symptom onset, most recent positive test, and hospitalization to randomization are described by TICO trial (Supplementary Table 2).
Clinical variables to assess COVID-19 severity are also reported in Table 1. At baseline, most participants required no or low levels of oxygen support. Twenty percent reported “severe-maximal” dyspnea (score, 5–10) on the Borg dyspnea scale. Other clinical biomarkers of inflammation or organ damage were also frequently abnormal, including an ALC of less than 0.9 × 109/L (55%) and CRP greater than 75 mg/L (43%).
Association of Participant Clinical and Virus-Specific Variables With Mortality Risk
We assessed demographic variables and pre-existing comorbidities for association with time to mortality by day 90 (Table 2). A Kaplan–Meier curve for mortality was constructed (Supplementary Figure 1), with 84 (3.2%) participants censored prior to day 90. Compared with the United States, participants enrolled in Europe had lower mortality (aHR, .33; .20–.57), and participants enrolled in Africa had higher mortality (aHR, 3.88; 2.34–6.43).
Table 2.
Predictors of 90-Day Mortality: Demographics and Comorbidities
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| No. of Pts | HR (95% CI) | HR (95% CI) | P | |
| Demographics | ||||
| Age (per 10 y older) | 2625 | 1.55 (1.42–1.69) | 1.70 (1.55–1.87) | <.001 |
| 18–39 y | 357 | (ref) | (ref) | |
| 40–49 y | 482 | 1.85 (.89–3.86) | 2.05 (.98–4.29) | .055 |
| 50–59 y | 627 | 2.39 (1.20–4.76) | 2.72 (1.36–5.45) | .005 |
| 60–69 y | 575 | 4.72 (2.44–9.14) | 5.29 (2.71–10.33) | <.001 |
| 70–79 y | 406 | 5.76 (2.95–11.23) | 7.83 (3.97–15.45) | <.001 |
| ≥80 y | 178 | 11.29 (5.72–22.29) | 16.81 (8.37–33.78) | <.001 |
| Race/ethnicity | ||||
| Asian | 121 | .77 (.39–1.52) | .87 (.36–2.13) | .77 |
| Black | 628 | 1.19 (.89–1.59) | 0.77 (.53–1.10) | .153 |
| Hispanic | 483 | 1.14 (.82–1.57) | 1.23 (.88–1.72) | .23 |
| White | 1303 | (ref) | (ref) | |
| Other | 90 | .46 (.17–1.23) | .48 (.18–1.30) | .147 |
| Sex | ||||
| Male | 1514 | (ref) | (ref) | |
| Female | 1111 | .87 (.68–1.11) | .89 (.69–1.14) | .34 |
| Residence | ||||
| Independent without assistance | 2470 | .60 (.39–.91) | .84 (.54–1.30) | .43 |
| All other | 155 | (ref) | (ref) | |
| Region | ||||
| Africa | 131 | 2.63 (1.81–3.84) | 3.88 (2.34–6.43) | <.001 |
| Asia | 42 | .90 (.33–2.41) | .98 (.26–3.70) | .98 |
| Europe | 393 | .36 (.21–.61) | .33 (.20–.57) | <.001 |
| United States | 2059 | (ref) | (ref) | |
| Infection time period | ||||
| Pre-2021 | 404 | .47 (.31–.70) | .54 (.35–.82) | .004 |
| January–June 2021 | 1059 | .51 (.39–.67) | 1.05 (.76–1.44) | .77 |
| July–November 2021 | 1162 | (ref) | (ref) | |
| Treatment group | ||||
| Active | 1478 | .79 (.62–1.01) | .81 (.64–1.04) | .095 |
| Placebo | 1147 | (ref) | (ref) | |
| Comorbid conditions | ||||
| BMI (kg/m2) | ||||
| <18.5 (underweight) | 48 | 1.67 (.82–3.40) | .93 (.45–1.93) | .85 |
| 18.5–24.9 (healthy) | 431 | (ref) | (ref) | |
| 25–29.9 (overweight) | 761 | 1.03 (.73–1.45) | 1.02 (.71–1.47) | .90 |
| 30–39.9 (obese) | 994 | .69 (.48–.98) | .80 (.55–1.17) | .25 |
| ≥40 (morbidly obese) | 382 | .73 (.47–1.14) | 1.13 (.70–1.83) | .62 |
| Asthma | ||||
| Yes | 260 | .83 (.54–1.29) | 1.01 (.65–1.57) | .97 |
| No | 2365 | |||
| COPD | ||||
| Yes | 167 | 1.09 (.67–1.75) | .81 (.49–1.32) | .39 |
| No | 2458 | |||
| Diabetes mellitus | ||||
| Yes | 740 | 1.33 (1.03–1.72) | 1.09 (.84–1.42) | .50 |
| No | 1885 | |||
| Heart failure | ||||
| Yes | 116 | 1.77 (1.11–2.82) | 1.28 (.78–2.09) | .33 |
| No | 2509 | |||
| Hypertension | ||||
| Yes | 1201 | 1.49 (1.17–1.90) | 1.14 (.88–1.48) | .31 |
| No | 1424 | |||
| Renal impairment | ||||
| Yes | 260 | 1.89 (1.36–2.62) | 1.49 (1.06–2.10) | .022 |
| No | 2365 | |||
| HIV | ||||
| Yes | 42 | 1.52 (.68–3.42) | 1.61 (.71–3.65) | .25 |
| No | 2583 | |||
| Other immune suppression | ||||
| Yes | 82 | 2.00 (1.19–3.36) | 1.97 (1.16–3.36) | .012 |
| No | 2543 | |||
| Malignancy | ||||
| Yes | 106 | 2.79 (1.86–4.18) | 2.30 (1.51–3.50) | <.001 |
| No | 2519 | |||
| Number of comorbiditiesb | ||||
| 0 | 952 | (ref) | (ref) | |
| 1 | 790 | 1.45 (1.04–2.01) | 1.13 (.80–1.58) | .49 |
| 2+ | 573 | 1.68 (1.19–2.37) | 1.28 (.89–1.85) | .182 |
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; HR, hazard ratio; Pts, patients; ref, reference group.
aAdjusted for age, sex, race/ethnicity, residence, geographical region, infection time period, treatment group, and baseline pulmonary status.
bIncluding asthma, COPD, diabetes, heart failure, hypertension, renal impairment, HIV, other immune suppression, and malignancy.
We also assessed medications administered prior to randomization for their association with mortality (Supplementary Table 3), including remdesivir (aHR, .65; .48–.86), corticosteroids (aHR, .94; .68–1.29), prophylactic-dose heparin (aHR, 1.14; .86–1.51), and intermediate/therapeutic-dose heparin (aHR, 1.47; .97–2.24).
Among COVID-19 characteristics (Table 3), symptom duration and vaccination status were not significantly associated with time to mortality in adjusted models. Higher plasma antigenemia was significantly associated with increased mortality as a binary factor (aHR, 2.24; 95% CI: 1.66–3.02; viral Ag ≥1000 ng/L), or by quartiles (aHR, 2.99; 95% CI: 2.02–4.43, for viral Ag ≥4500 ng/L vs Ag <200 ng/L). Viral RNA values less than 35 000 (aHR, 2.50; 95% CI: 1.25–4.99) and 35 000 or higher (aHR, 3.94; 95% CI: 2.00–7.76) copies/mL were associated with increased mortality (vs viral RNA below the limit of detection). The presence of either anti–SARS-CoV-2 Ab was associated with reduced mortality. To better characterize the association of effective vaccination with mortality risk, we evaluated the impact of having received 2 vaccine doses with a positive anti-S Ab (n = 181), a group that had a comparative mortality risk aHR of .66 (95% CI: .41–1.07; P = .09).
Table 3.
Predictors of 90-Day Mortality: COVID-19 Characteristics and Clinical Severity
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| No. of Pts | HR (95% CI) | HR (95% CI) | P | |
| COVID-19 characteristics | ||||
| Symptom duration | ||||
| <5 d | 398 | (ref) | (ref) | |
| 5–7 d | 765 | 1.06 (.73–1.56) | 1.01 (.68–1.50) | .95 |
| 8–10 d | 1089 | .97 (.67–1.41) | .99 (.67–1.45) | .96 |
| >10 d | 373 | 1.07 (.69–1.67) | 1.05 (.65–1.68) | .85 |
| Number of vaccine doses | ||||
| 0 | 2150 | (ref) | (ref) | |
| 1 | 184 | 1.05 (.65–1.70) | .90 (.55–1.47) | .67 |
| 2 | 268 | 1.64 (1.17–2.31) | .98 (.67–1.44) | .92 |
| Anti-S Ab status | ||||
| Positive | 1312 | .80 (.62–1.03) | .59 (.46–.77) | <.001 |
| Negative | 1231 | (ref) | (ref) | |
| Anti-N Ab status | ||||
| Positive | 1586 | .88 (.68–1.14) | .74 (.57–.97) | .031 |
| Negative | 958 | (ref) | (ref) | |
| Anti-S × anti-N Ab | ||||
| Anti-S+, anti-N+ | 1058 | .77 (.57–1.04) | .54 (.39–.73) | <.001 |
| Anti-S+, anti-N– | 254 | .57 (.38–.96) | .37 (.22–.64) | <.001 |
| Anti-S–, anti-N+ | 527 | .80 (.56–1.14) | .69 (.48–.99) | .043 |
| Anti-S–, anti-N– | 704 | (ref) | (ref) | |
| Plasma viral Ag, ng/L | ||||
| <200 | 609 | (ref) | (ref) | |
| 200–1499 | 680 | 1.06 (.67–1.68) | .97 (.61–1.54) | .89 |
| 1500–4499 | 595 | 1.45 (.93–2.27) | 1.38 (.88–2.16) | .162 |
| ≥4500 | 659 | 3.78 (2.58–5.55) | 2.99 (2.02–4.43) | <.001 |
| 1000+ | 1455 | 2.49 (1.85–3.34) | 2.24 (1.66–3.02) | <.001 |
| <1000 | 1088 | (ref) | (ref) | |
| Upper respiratory viral RNA | ||||
| Negative | 334 | (ref) | (ref) | |
| <35 000 copies/mL | 1087 | 2.79 (1.40–5.55) | 2.50 (1.25–4.99) | .009 |
| 35 000+ copies/mL | 1091 | 5.79 (2.96–11.32) | 3.94 (2.00–7.76) | <.001 |
| Clinical severity | ||||
| Pulmonary status | ||||
| No O2 | 659 | (ref) | (ref) | |
| O2 <4 L/min | 951 | 1.36 (.82–2.25) | 1.75 (1.05–2.90) | .032 |
| O2 ≥4 L/min | 729 | 4.44 (2.83–6.97) | 5.16 (3.27–8.16) | <.001 |
| Noninvasive ventilation/HFNC | 286 | 10.12 (6.39–16.02) | 15.37 (9.29–25.44) | <.001 |
| Borg dyspnea scale | ||||
| 0–2 (nothing to slight) | 1154 | (ref) | (ref) | |
| 3–4 (moderate–somewhat severe) | 786 | 1.44 (1.06–1.96) | 1.09 (.79–1.50) | .60 |
| 5–10 (severe–maximal) | 475 | 2.07 (1.50–2.85) | 1.46 (1.04–2.05) | .029 |
| NEWS | ||||
| <2 | 316 | (ref) | (ref) | |
| 2–3 | 850 | 2.05 (1.04–4.03) | .98 (.44–2.17) | .96 |
| 4–5 | 834 | 3.11 (1.61–6.00) | 1.00 (.44–2.26) | 1.00 |
| ≥6 | 612 | 6.66 (3.49–12.70) | 1.60 (.70–3.63) | .26 |
| Lymphocytes, × 109/L | ||||
| <0.9 | 1426 | (ref) | (ref) | |
| 0.9–1.5 | 796 | .42 (.30–.57) | .50 (.36–.70) | <.001 |
| >1.5 | 357 | .41 (.26–.65) | .50 (.31–.81) | .005 |
| Serum creatinine, µmol/L (mg/dL) | ||||
| <1.1 | 1932 | (ref) | (ref) | |
| 1.1–1.5 | 411 | 1.80 (1.32–2.45) | 1.67 (1.21–2.32) | .002 |
| >1.5 | 277 | 2.92 (2.15–3.96) | 2.77 (1.98–3.87) | <.001 |
| eGFR, mL/min/1.73 m2 | ||||
| <60 | 506 | 2.82 (2.20–3.62) | 2.29 (1.74–3.03) | <.001 |
| ≥60 | 2114 | (ref) | (ref) | |
| CRP, mg/L | ||||
| <50 | 1058 | (ref) | (ref) | |
| 50–75 | 412 | 1.40 (.92–2.12) | 1.21 (.80–1.84) | .37 |
| >75 | 1114 | 2.46 (1.84–3.30) | 1.71 (1.27–2.31) | <.001 |
| IL-6, ng/L | ||||
| ≤5.8 | 1233 | (ref) | (ref) | |
| >5.8 | 1242 | 4.74 (3.42–6.58) | 3.20 (2.28–4.47) | <.001 |
Abbreviations: Ab, antibody; Ag, antigen; Anti-N, anti-nucleocaspid; Anti-S, anti-spike; CI, confidence interval; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HFNC, high-flow nasal cannula; HR, hazard ratio; IL-6, interleukin 6; NEWS, National Early Warning Score; O2, supplemental oxygen; Pts, patients; ref, reference group.
aAdjusted for age, sex, race/ethnicity, residence, geographical region, infection time period, treatment group, and baseline pulmonary status.
For clinical measures of COVID-19 severity (Table 3), compared with participants requiring no oxygen, we observed significant and increasing mortality risk for higher baseline respiratory support requirements. A severe to maximal Borg scale score versus a score of nothing to slight was associated with increased mortality risk (aHR, 1.46; 1.04–2.05), even after adjusting for level of respiratory support. In addition, clinical and laboratory variables that are markers for systemic inflammation (CRP, IL-6) or organ-level damage (ALC, serum creatinine, eGFR) were also individually significantly associated with mortality in adjusted analyses.
Association Between Participant Variables and Impact on Mortality Risk
A model that further examined the impact of plasma viral Ag by also adjusting for anti-S by anti-N Ab status demonstrated that mortality risk was primarily derived from viral Ag level (Supplementary Table 4), as the highest aHR was only partially reduced in the presence of 1 or both Abs (Supplementary Figure 2). Models that examined the impact of either anti–SARS-CoV-2 Ab in conjunction with log10 viral Ag revealed that neither Ab was a significant predictor of mortality given the effect of viral Ag and other covariates (Supplementary Table 4). Additionally, when we assessed viral antigen adjusted for demographic variables, treatment arm, and respiratory severity, the aHR for mortality appeared to increase for increasing viral Ag levels (P < .001) (Supplementary Figure 3).
We also evaluated the relationship between plasma viral Ag and upper respiratory viral RNA and their association with mortality. Most participants with viral Ag greater than 200 ng/L had quantifiable viral RNA (1720/1876, 91.7%), and in participants with quantifiable viral RNA, the aHR for mortality was significantly increased when viral Ag was 4500 ng/L or greater (Supplementary Table 5). Participants without quantifiable viral RNA and viral Ag less than 200 ng/L had a significantly lower mortality risk than those with quantifiable viral RNA and viral Ag less than 200 ng/L, with cumulative data suggesting these factors were additive (Supplementary Figure 4).
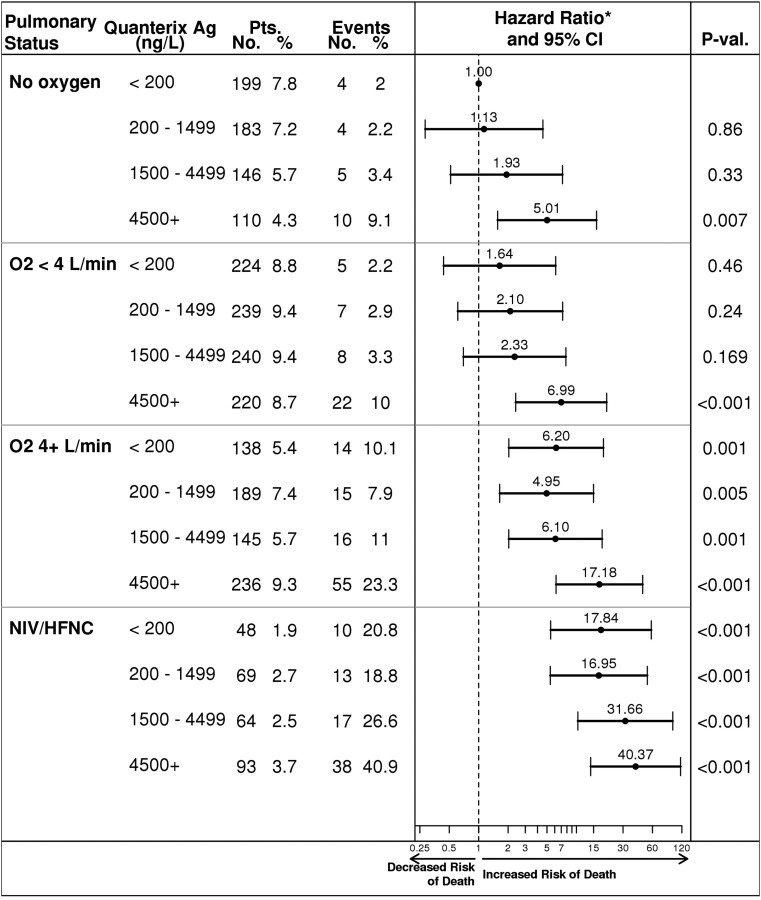
To better understand the relationship between viral Ag level and specific clinical variables and their association with mortality, we first evaluated viral Ag level and baseline pulmonary status (Figure 1, Supplementary Table 6). There was an additive impact of higher viral Ag levels on the hazard for mortality among participants requiring noninvasive ventilation (NIV) or high-flow nasal cannula (HFNC), maximally with viral Ag greater than 4500 ng/L (aHR, 40.4; 14.0–116.4). The impact of viral Ag and other clinical variables, including Borg dyspnea scale, NEWS score, CRP, IL-6, ALC, and eGFR, on mortality was assessed (Supplementary Figures 5–10). Mortality risk appeared to be additive among participants with elevated inflammatory markers CRP (>75 mg/L) (Supplementary Figure 7) or IL-6 (>5.8 ng/L) (Supplementary Figure 8) or reduced ALC (<0.9 × 109/L) (Supplementary Figure 9) in the presence of higher viral Ag levels.
Figure 1.
Day 90 mortality by baseline pulmonary and plasma viral antigen status—adjusted for age, sex, race/ethnicity, residence, geographical region, infection period, and treatment group. Abbreviations: Ag, antigen; CI, confidence interval; HFNC, high-flow nasal cannula; L, Liters; min, minute; NIV, noninvasive ventilation; No, number; O2, oxygen; Pts., patients; P-val., P value. *Adjusted for age, gender, race/ethnicity, residence, geographical region, date of infection, and treatment group.
We also assessed the relationship of CRP and IL-6, systemic markers of inflammation, with viral Ag. CRP levels greater than 75 mg/L (aHR, 1.40 [1.03–1.91] vs CRP <50 mg/L) or IL-6 greater than 5.8 ng/L (aHR, 2.59 [1.83–3.66] vs IL-6 ≤5.8 ng/L) were significantly associated with increased mortality risk even after adjusting for log10 viral Ag (Supplementary Tables 7 and 8); viral Ag remained significantly associated with mortality risk after adjusting for CRP (aHR, 3.03; 2.02–4.53) or IL-6 (aHR, 2.31; 1.53–3.48) for Ag levels of 4500 or greater vs less than 200 ng/L, with minimal impact due to collinearity between CRP and IL-6 (Supplementary Table 9).
A multivariable model that included key viral and clinical factors demonstrated significant associations with mortality risk for viral Ag, viral RNA, pulmonary status, IL-6, and eGFR, along with demographic variables age, region, and infection period (Table 4). Visualization of adjusted survival curves by plasma viral Ag (Supplementary Figure 11), upper respiratory viral RNA (Supplementary Figure 12), or IL-6 (Supplementary Figure 13) supports the model results in Table 4. For this model, the proportional hazards assumption was violated (P = .004), with region a significant variable when tested individually (P < .001). Because the effect of region could differ over time during the COVID-19 pandemic, we re-ran the model with region as a stratification variable, and observed similar results for other covariates (Supplementary Table 10) with a valid proportional hazards assumption (P = .26). An association between missingness of IL-6 and viral Ag and mortality was observed, and the association between viral Ag and mortality was not accounted for by other relevant predictors (Supplementary Table 11). To account for variable missingness, the final model fit using multiple imputation revealed consistent results (Supplementary Table 12).
Table 4.
Predictors of Mortality: Multivariable Model Adjusted for Relevant Viral and Clinical Factors
| No. of Ptsa | HR (95% CI)b | P | |
|---|---|---|---|
| Age | |||
| 18–39 y | 323 | (ref) | |
| 40–49 y | 436 | 1.94 (.90–4.20) | .093 |
| 50–59 y | 559 | 1.91 (.91–4.01) | .088 |
| 60–69 y | 497 | 3.55 (1.73–7.29) | <.001 |
| 70–79 y | 359 | 4.29 (2.07–8.89) | <.001 |
| ≥80 y | 156 | 8.00 (3.72–17.22) | <.001 |
| Race/ethnicity | |||
| Asian | 118 | .97 (.39–2.42) | .95 |
| Black | 559 | .66 (.44–1.00) | .052 |
| Hispanic | 426 | 1.27 (.87–1.84) | .21 |
| White | 1143 | (ref) | |
| Other | 84 | .39 (.12–1.25) | .113 |
| Sex | |||
| Male | 1347 | (ref) | |
| Female | 983 | .88 (.66–1.16) | .36 |
| Residence | |||
| Independent without assistance | 2194 | .72 (.44–1.18) | .19 |
| All other | 136 | (ref) | |
| Region | |||
| Africa | 129 | 5.45 (3.10–9.58) | <.001 |
| Asia | 42 | .88 (.23–3.34) | .85 |
| Europe | 369 | .34 (.19–.60) | <.001 |
| United States | 1790 | (ref) | |
| Infection time period | |||
| Pre-2021 | 374 | .56 (.36–.89) | .013 |
| January–June 2021 | 923 | .97 (.68–1.37) | .86 |
| July–November 2021 | 1033 | (ref) | |
| Treatment group | |||
| Active | 1318 | .84 (.64–1.10) | .21 |
| Placebo | 1012 | (ref) | |
| Pulmonary status | |||
| No O2 | 584 | (ref) | |
| O2 < 4 L/min | 852 | 1.84 (1.06–3.22) | .032 |
| O2 ≥ 4 L/min | 650 | 4.41 (2.63–7.39) | <.001 |
| Noninvasive ventilation/HFNC | 244 | 11.30 (6.46–19.75) | <.001 |
| Plasma viral Ag, ng/L | |||
| <200 | 549 | (ref) | |
| 200–1499 | 616 | 1.03 (.61–1.75) | .90 |
| 1500–4499 | 553 | 1.24 (.74–2.09) | .41 |
| ≥4500 | 612 | 2.07 (1.29–3.34) | .003 |
| Upper respiratory viral RNA | |||
| Negative | 305 | (ref) | |
| <35 000 copies/mL | 1024 | 2.42 (1.09–5.34) | .029 |
| 35 000+ copies/mL | 1001 | 2.84 (1.29–6.28) | .010 |
| Lymphocytes, × 109/L | |||
| ≤1.5 | 2007 | (ref) | |
| >1.5 | 323 | .77 (.45–1.31) | .34 |
| eGFR, mL/min/1.73 m2 | |||
| <60 | 439 | 1.77 (1.29–2.42) | <.001 |
| ≥60 | 1891 | (ref) | |
| CRP,c mg/L | |||
| <50 | 958 | (ref) | |
| 50–75 | 368 | .96 (.60–1.52) | .85 |
| >75 | 1004 | 1.10 (.78–1.55) | .58 |
| IL-6, ng/L | |||
| ≤5.8 | 1141 | (ref) | |
| >5.8 | 1189 | 2.54 (1.74–3.70) | <.001 |
Abbreviations: Ag, antigen; CI, confidence interval; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HFNC, high-flow nasal cannula; HR, hazard ratio; IL-6, interleukin 6; O2, supplemental oxygen; Pts, patients; ref, reference group.
aNumber of participants in subgroup with all variables in multivariate model available (n = 2330).
bAdjusted for all variables in the table.
cAlso adjusted as a binary variable (≤75, >75 mg/L), with an adjusted HR of .89 (.67, 1.20); P = .45 (not shown).
In an analysis limited to participants randomized to placebo, significant associations with mortality risk for viral Ag, higher levels of respiratory support, and clinical laboratory markers remained (Supplementary Table 13). When excluding participants from the tixagevimab–cilgavimab TICO trial, viral Ag, O2 levels of 4 L/min or greater or NIV/HFNC respiratory support levels, as well as several clinical laboratory markers retained significance with similar point estimates as the primary model (Supplementary Table 14).
DISCUSSION
In a secondary analysis of data from 2625 participants enrolled in 5 placebo-controlled clinical trials conducted under the TICO master protocol, we observed that several virus-specific and host biomarkers had a strong association with mortality risk by day 90. Importantly, biomarkers assayed in research laboratories, including plasma viral Ag, upper respiratory viral RNA, and IL-6, were among the most strongly associated with mortality risk, adding potential biological insights to the robust literature on COVID-19 prognosis and prediction factors that have typically relied on clinically obtained data [28–30]. Our results support emerging data for plasma viral antigen as an important predictor of clinical outcomes following SARS-CoV-2 infection [16, 17], but also reveal the importance of upper respiratory viral RNA. These results also suggest additive mortality risk for individuals with higher viral Ag or viral RNA levels and abnormal biomarker values that reflect systemic inflammation or organ damage. Our findings support continued assessment of therapies that reduce plasma viral replication or target pathways of tissue injury to attempt to limit organ damage and related clinical outcomes.
When we assessed the relationship of virus-specific variables with each other and to mortality, SARS-CoV-2 plasma viral antigen and upper respiratory viral RNA, as opposed to anti–SARS-CoV-2 Abs, emerged as key risk factors, yet the impact of antiviral therapy on these viral measures and subsequent clinical outcomes is not known. Notably, because vaccination rates were low during our study, it may be important to evaluate novel antiviral therapies in the current state of increased vaccination rates and bivalent SARS-CoV-2 vaccine availability, yet reports of waning primary series vaccine effectiveness against Omicron [31].
Prior COVID-19 trials demonstrated steroid or anti–IL-6/IL-6 receptor therapeutic benefit [10, 32, 33], as well as steroid-induced CRP reduction [34]. However, our final adjusted model suggests that IL-6 but not CRP is associated with mortality; thus, it may be important to further evaluate anti-inflammatory therapies in combination with antiviral therapies that effectively reduce plasma viral Ag and viral RNA levels. The absence of real-time assays for viral antigen, RNA, or IL-6 currently limits deploying this type of adaptive, precision-based therapeutic approach.
In addition to confirming prior COVID-19 studies that identify the degree of baseline respiratory support as a strong predictor of mortality risk [33], our analyses show additive risk for mortality among participants with higher levels of virus who require NIV or HFNC levels of respiratory support. Interestingly, greater respiratory distress quantified on the Borg dyspnea scale was also associated with increased mortality risk, even after adjusting for baseline respiratory support requirements, and may suggest a potential relationship between increased dyspnea and higher viral antigen burden or other clinical markers of inflammation and multiorgan injury.
Limitations
There are a few noteworthy limitations in our findings. One, our cohort was predominantly not vaccinated (∼80%); therefore, some results may be harder to interpret as we consider current COVID-19 interventions. Two, we observed noteworthy regional differences in aHR for mortality. The median time from symptom onset to randomization was similar across regions, and the number of trial participants on room air at baseline per region was not proportional to observed morality HRs), suggesting that illness duration or severity at baseline may not be major contributors to regional differences in mortality risk. Variability in trial accrual by site and region, along with uncertainty around directive COVID-19–related care and post-hospitalization resources, precludes full evaluation of the validity of apparent regional differences in mortality risk. Three, COVID-19 therapies, including vaccines, remdesivir, or corticosteroids for COVID-19, may be subject to confounding by indication and impact the clinical trajectory in unmeasured ways not accounted for in adjusted analyses using only baseline data.
Conclusions
In a large, diverse cohort comprising TICO clinical trial participants, several virus-related and clinical variables measured early during hospitalization for COVID-19 were significantly associated with the risk of mortality within 90 days. With their levels strongly associated with increased mortality, high viral antigen and viral RNA may represent ongoing viral replication and possible systemic spread that could warrant augmentation of antiviral therapy. Furthermore, host biomarkers measured at early time points, including eGFR and IL-6, were strongly associated with mortality. As such, it is critical to study how abnormal biomarkers reflect the host viral response at the organ and tissue levels and to identify and prioritize potential precision-based therapeutic targets, particularly as additional real-time biomarker assays become available.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Neil R Aggarwal, Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Jacquie Nordwall, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
Dominique L Braun, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Lucy Chung, CAMRIS International (under contract no. 75N93019D00025 with National Institute of Allergy and Infectious Diseases, Department of Health and Human Services), National Institute of Health, Bethesda, Maryland, USA.
Jordan Coslet, Velocity Clinical Research, Chula Vista, California, USA.
Tatyana Der, Department of General Internal Medicine, Duke University School of Medicine, Durham, North Carolina, USA.
Nnakelu Eriobu, Institute of Human Virology Nigeria, Abuja, Nigeria.
Adit A Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Awori J Hayanga, Department of Cardiovascular Thoracic Surgery, West Virginia University School of Medicine, Morgantown, West Virginia, USA.
Helene Highbarger, Virus Isolation and Serology Laboratory, Frederick National Laboratory, National Cancer Institute, Frederick, Maryland, USA.
Mark Holodniy, Veterans Affairs Palo Alto Health Care System, Division of Infectious Diseases and Geographic Medicine, Stanford University, Palo Alto, California, USA.
Juan P Horcajada, Department of Infectious Diseases, Hospital del Mar Research Insititute, UPF, Barcelona, Spain; CIBERINFEC, Instituto de Salud Carlos III, Madrid, Spain.
Mamta K Jain, Division of Infectious Diseases and Geotropical Medicine, UT Southwestern Medical Center and Parkland Health and Hospital System, Dallas, Texas, USA.
Kami Kim, Division of Infectious Disease and International Medicine, Morsani College of Medicine, University of South Florida and Global Emerging Diseases Institute, Tampa General Hospital, Tampa, Florida, USA.
Sylvain Laverdure, Laboratory of Human Retrovirology and Immunoinformatics, Frederick National Laboratory, National Cancer Institute, Frederick, Maryland, USA.
Jens Lundgren, CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Righospitalet, University of Copenhagen, Copenhagen, Denmark.
Ven Natarajan, Laboratory of Molecular Cell Biology, Frederick National Laboratory, National Cancer Institute, Frederick, Maryland, USA.
Hien H Nguyen, Division of Infectious Diseases, Veterans Affairs Northern California, University of California, Davis, Sacramento, California, USA.
Sarah L Pett, The Medical Research Council Clinical Trials Unit at UCL, Institute of Clinical Trials and Methodology, University College London, London, United Kingdom; Institute for Global Health, University College London, London, United Kingdom.
Andrew Phillips, Institute for Global Health, University College London, London, United Kingdom.
Garyphallia Poulakou, Third Department of Medicine and Laboratory National and Kapodistrian University of Athens Medical School, Athens, Greece.
David A Price, Newcastle Upon Tyne NHUS Hospitals Foundation Trust, Newcastle Upon Tyne, United Kingdom.
Philip Robinson, Infection Prevention and Hospital Epidemiology, Hoag Memorial Hospital Presbyterian, Newport Beach, California, USA.
Angela J Rogers, Division of Pulmonary, Allergy, and Critical Care Medicine, Stanford University, Palo Alto, California, USA.
Uriel Sandkovsky, Division of Infectious Diseases, Baylor University Medical Center, Dallas, Texas, USA.
Katy Shaw-Saliba, National Institute of Allergy and Infectious Diseases/National Institutes of Health, Bethesda, Maryland, USA.
Jeffrey M Sturek, Division of Pulmonary and Critical Care Medicine, Department of Medicine, UVA Health, Charlottesville, Virginia, USA.
Barbara W Trautner, Michael E. DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, Houston, Texas, USA.
Michael Waters, Velocity Clinical Research, Chula Vista, California, USA.
Cavan Reilly, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
for the ACTIV-3/TICO Study Group:
David Sahner, John Tierney, Susan E Vogel, Betsey R Herpin, Mary C Smolskis, Laura A McKay, Kelly Cahill, Page Crew, Ratna Sardana, Sharon Segal Raim, Lisa Hensely, Johsua Lorenzo, Rebecca Mock, Judith Zuckerman, Negin Atri, Mark Miller, David Vallee, Lucy Chung, Nayon Kang, Kevin Barrett, Stacey J Adam, Sarah Read, Ruxandra Draghia-Akli, Judy Currier, Eric Hughes, Rachel H Harrigan, Laura Amos, Amy Carlsen, Anita Carter, Gary Collins, Bionca Davis, Eileen Denning, Alain DuChene, Kate Eckroth, Nicole Engen, Alex Frase, Greg Gandits, Birgit Grund, Merrie Harrison, Nancy Hurlbut, Payton Kaiser, Joseph Koopmeiners, Gregg Larson, Sue Meger, Shweta Sharma Mistry, Thomas Murray, Ray Nelson, Kien Quan, Siu Fun Quan, Cavan Reilly, Lianne Siegel, Greg Thompson, David Vock, Jamie Walski, Annetine C Gelijns, Alan J Moskowitz, Emilia Bagiella, Ellen Moquete, Karen O'Sullivan, Mary E Marks, Evan Accardi, Emily Kinzel, Sarah Burris, Gabriela Bedoya, Lola Gupta, Jessica R Overbey, Milerva Santos, Marc A Gillinov, Marissa A Miller, Wendy C Taddei-Peters, Kathleen Fenton, Uriel Sandkovsky, Robert L Gottlieb, Michael Mack, Mezgebe Berhe, Clinton Haley, Emma Dishner, Christopher Bettacchi, Kevin Golden, Erin Duhaime, Madison Ryan, Sarah Burris, Catherine Tallmadge, Lorie Estrada, Felecia Jones, Samatha Villa, Samatha Wang, Raven Robert, Tanquinisha Coleman, Laura Clariday, Rebecca Baker, Mariana Hurutado-Rodriguez, Nazia Iram, Michelle Fresnedo, Allyson Davis, Kiara Leonard, Noelia Ramierez, Jon Thammavong, Krizia Duque, Emma Turner, Fisher Tammy, Dianna Robinson, Desirae Ransom, Nicholas Maldonado, Erica Lusk, Aaron Killian, Palacious Adriana, Edilia Solis, Janet Jerrow, Matthew Watts, Heather Whitacre, Elizabeth Cothran, Peter K Smith, Christina E Barkauskas, Andrew M Vekstein, Emily R Ko, Grace R Dreyer, Neil Stafford, Megan Brooks, Tatyana Der, Marie Witte, Ruwan Gamarallage, John Franzone, Noel Ivey, Rebecca H Lumsden, Nilima Mosaly, Ahmaad Mourad, Thomas L Holland, Mary Motta, Kathleen Lane, Lauren M McGowan, Jennifer Stout, Heather Aloor, Kennesha M Bragg, Barvina Toledo, Beth McLendon-Arvik, Barbara Bussadori, Beth A Hollister, Michelle Griffin, Dana M Giangiacomo, Vicente Rodriguez, Gordon Bokhart, Sharon M Eichman, Patrick E Parrino, Stephen Spindel, Aditya Bansal, Katherine Baumgarten, Johnathan Hand, Derek Vonderhaar, Bobby Nossaman, Sylvia Laudun, DeAnna Ames, Shane Broussard, Nilmo Hernandez, Geralyn Isaac, Huan Dinh, Yiling Zheng, Sonny Tran, Hunter McDaniel, Nicolle Crovetto, Emerson Perin, Briana Costello, Prasad Manian, M Rizwan Sohail, Alexander Postalian, Punit Hinsu, Carolyn Watson, James Chen, Melyssa Fink, Lydia Sturgis, Kim Walker, Kim Mahon, Jennifer Parenti, Casey Kappenman, Aryn Knight, Jeffrey M Sturek, Andrew Barros, Kyle B Enfield, Alexandra Kadl, China J Green, Rachel M Simon, Ashley Fox, Kara Thornton, Amy Adams, Vinay Badhwar, Sunil Sharma, Briana Peppers, Paul McCarthy, Troy Krupica, Arif Sarwari, Rebecca Reece, Lisa Fornaresico, Chad Glaze, Raquel Evans, Fang Di, Shawn Carlson, Tanja Aucremanne, Connie Tennant, Sutton Lisa Giblin, Sabrina Buterbaugh, Roger Williams, Robin Bunner, Jay H Traverse, Frank Rhame, Joshua Huelster, Rajesh Kethireddy, Irena Davies, Julianne Salamanca, Christine Majeski, Paige Skelton, Maria Zarambo, Andrea Sarafolean, Michael E Bowdish, Zea Borok, Noah Wald-Dickler, Douglass Hutcheon, Amytis Towfighi, Mary Lee, Meghan R Lewis, Brad Spellberg, Linda Sher, Aniket Sharma, Anna P Olds, Chris Justino, Edward Loxano, Chris Romero, Janet Leong, Valentina Rodina, Christine Quesada, Luke Hamilton, Jose Escobar, Brad Leshnower, William Bender, Milad Sharifpour, Jeffrey Miller, Woodrow Farrington, Kim T Baio, Mary McBride, Michele Fielding, Sonya Mathewson, Kristina Porte, Missy Maton, Chari Ponder, Elisabeth Haley, Christine Spainhour, Susan Rogers, Derrick Tyler, Ronson J Madathil, Joseph Rabin, Andrea Levine, Kapil Saharia, Ali Tabatabai, Christine Lau, James S Gammie, Maya-Loren Peguero, Kimberly McKernan, Mathew Audette, Emily Fleischmann, Kreshta Akbari, Myounghee Lee, Andrew Chi, Hanna Salehi, Alan Pariser, Phuong Tran Nyguyen, Jessica Moore, Adrienne Gee, Shelika Vincent, Richard A Zuckerman, Alexander Iribarne, Sara Metzler, Samantha Shipman, Haley Johnson, Crystallee Newton, Doug Parr, Leslie Miller, Beth Schelle, Sherry McLean, Howard R Rothbaum, Michael S Alvarez, Shivam P Kalan, Heather H Germann, Jennifer Hendershot, Karen Moroney, Karen Herring, Sharri Cook, Pam Paul, Rebecca Walker-Ignasiak, Crystal North, Cathryn Oldmixon, Nancy Ringwood, Ariela Muzikansky, Richard Morse, Laura Fitzgerald, Haley D Morin, Roy G Brower, Lora A Reineck, Karen Bienstock, Jay H Steingrub, Peter K Hou, Jay S Steingrub, Mark A Tidswell, Lori-Ann Kozikowski, Cynthia Kardos, Leslie DeSouza, Sarah Romain, Sherell Thornton-Thompson, Daniel Talmor, Nathan Shapiro, Konstantinos Andromidas, Valerie Banner-Goodspeed, Michael Bolstad, Katherine L Boyle, Payton Cabrera, Arnaldo deVilla, Joshua C Ellis, Ana Grafals, Sharon Hayes, Conor Higgins, Lisa Kurt, Nicholas Kurtzman, Kimberly Redman, Elinita Rosseto, Douglas Scaffidi, Nathan Shapiro, Michael R Filbin, Kathryn A Hibbert, Blair Parry, Justin Margolin, Brooklynn Hillis, Rhonda Hamer, Kelsey Brait, Caroline Beakes, Brenna McKaig, Eleonore Kugener, Alan E Jones, James Galbraith, Utsav Nandi, Rebekah Peacock, Gregory Hendey, Kirsten Kangelaris, Kimia Ashktorab, Rachel Gropper, Anika Agrawal, Kimberley J Yee, Alejandra E Jauregui, Hanjing Zhuo, Eyad Almasri, Mohamed Fayed, Kinsley A Hubel, Alyssa R Hughes, Rebekah L Garcia, George W Lim, Steven Y Chang, Gregory Hendey, Michael Y Lin, Julia Vargas, Hena Sihota, Rebecca Beutler, Trisha Agarwal, Jennifer G Wilson, Rosemary Vojnik, Cynthia Perez, Jordan H McDowell, Jonasel Roque, Henry Wang, Ryan M Huebinger, Bela Patel, Elizabeth Vidales, Timothy Albertson, Erin Hardy, Richart Harper, Marc A Moss, Amiran Baduashvili, Lakshmi Chauhan, David J Douin, Flora Martinez, Lani L Finck, Jill Bastman, Michelle Howell, Carrie Higgins, Jeffrey McKeehan, Jay Finigan, Peter Stubenrauch, William J Janssen, Christine Griesmer, Olivia VerBurg, Robert C Hyzy, Pauline K Park, Kristine Nelson, Jake I McSparron, Ivan N Co, Bonnie R Wang, Jose Jimenez, Norman Olbrich, Kelli McDonough, Shijing Jia, Sinan Hanna, Michelle N Gong, Lynne D Richardson, Rahul Nair, Brenda Lopez, Omowunmi Amosu, Obiageli Offor, Hiwet Tzehaie, William Nkemdirim, Sabah Boujid, Jarrod M Mosier, Cameron Hypes, Elizabeth Salvagio Campbell, Billie Bixby, Boris Gilson, Anitza Lopez, Christian Bime, Sairam Parthasarathy, Ariana M Cano, R Duncan Hite, Thomas E Terndrup, Herbert P Wiedemann, Kristin Hudock, Hammad Tanzeem, Harshada More, Jamie Martinkovic, Susan Sellers, Judy Houston, Mary Burns, Simra Kiran, Tammy Roads, Sarah Kennedy, Abhijit Duggal, Nirosshan Thiruchelvam, Kiran Ashok, Alexander H King, Omar Mehkri, Siddharth Dugar, Debasis Sahoo, Donald M Yealy, Derek C Angus, Alexandra J Weissman, Tina M Vita, Emily Berryman, Catherine L Hough, Akram Khan, Olivia F Krol, Emmanuel Mills, Mistry Kinjal, Genesis Briceno, Raju Reddy, Kinsley Hubel, Milad K Jouzestani, Madeline McDougal, Rupali Deshmukh, Nicholas J Johnston, Bryce H Robinson, Staphanie J Gundel, Sarah C Katsandres, Peter Chen, Sam S Torbati, Tanyalak Parimon, Antonina Caudill, Brittany Mattison, Susan E Jackman, Po-En Chen, Emad Bayoumi, Cristabelle Ojukwu, Devin Fine, Gwendolyn Weissberg, Katherine Isip, Yunhee Choi-Kuaea, Shaunt Mehdikhani, Tahir B Dar, Nsole Biteghe Fleury Augustin, Dana Tran, Jennifer Emilow Dukov, Yuri Matusov, June Choe, Niree A Hindoyan, Timothy Wynter, Ethan Pascual, Gregg J Clapham, Lisa Herrera, Antonia Caudill, D Shane O'Mahony, Sonam T Nyatsatsang, David M Wilson, Julie A Wallick, Alexandria M Duven, Dakota D Fletcher, Chadwick Miller, D Clark Files, Kevin W Gibbs, Lori S Flores, Mary E LaRose, Leigha D Landreth, D Rafael Palacios, Lisa Parks, Madeline Hicks, Andrew J Goodwin, Edward F Kilb, Caitlan T Lematty, Kerilyn Patti, Abigail Grady, April Rasberry, Peter E Morris, Jamie L Sturgill, Evan P Cassity, Sanjay Dhar, Ashley A Montgomery-Yates, Sarah N Pasha, Kirby P Mayer, Brittany Bissel, Terren Trott, Shahnaz Rehman, Wit Marjolein de, Jessica Mason, Joseph Bledsoe, Kirk U Knowlton, Samuel Brown, Michael Lanspa, Lindsey Leither, Ithan Pelton, Brent P Armbruster, Quinn Montgomery, Naresh Kumar, Melissa Fergus, Karah Imel, Ghazal Palmer, Brandon Webb, Carolyn Klippel, Hannah Jensen, Sarah Duckworth, Andrew Gray, Tyler Burke, Dan Knox, Jenna Lumpkin, Valerie T Aston, Darrin Applegate, Erna Serezlic, Katie Brown, Mardee Merril, Estelle S Harris, Elizabeth A Middleton, Macy A G Barrios, Jorden Greer, Amber D Schmidt, Melissa K Webb, Roert Paine, Sean J Callahan, Lindsey J Waddoups, Misty B Yamane, Wesley H Self, Todd W Rice, Jonathan D Casey, Jakea Johnson, Christopher Gray, Margaret Hays, Megan Roth, Vidya Menon, Moiz Kasubhai, Anjana Pillai, Jean Daniel, Daniel Sittler, Balavenkatesh Kanna, Nargis Jilani, Francisco Amaro, Jessica Santana, Aleksandr Lyakovestsky, Issa Madhoun, Louis Marie Desroches, Nicole Amadon, Alaa Bahr, Imaan Ezzat, Maryanne Guerrero, Joane Padilla, Jessie Fullmer, Inderpreet Singh, Syed Hamad Ali Shah, Rajeev Narang, Polly Mock, Melissa Shadle, Brenda Hernandez, Kevin Welch, Andrea Payne, Gabriela Ertl, Daniel Canario, Isabel Barrientos, Danielle Goss, Mattie DeVries, Ibidolapo Folowosele, Dorothy Garner, Mariana Gomez, Justin Price, Ekta Bansal, Jim Wong, Jason Faulhaber, Tasaduq Fazili, Brian Yeary, Ruth Ndolo, Christina Bryant, Bridgette Smigeil, Philip Robinson, Rana Najjar, Patrice Jones, Julie Nguyen, Christina Chin, Hassan Taha, Salah Najm, Christopher Smith, Jason Moore, Talal Nassar, Nick Gallinger, Amy Christian, D'Amber Mauer, Ashley Phipps, Michael Waters, Karla Zepeda, Jordan Coslet, Rosalynn Landazuri, Jacob Pineda, Nicole Uribe, Jose Ruiz Garcia, Cecilia Barbabosa, Kaitlyn Sandler, J Scott Overcash, Adrienna Marquez, Hanh Chu, Kia Lee, Kimberly Quillin, Andrea Garcia, Pauline Lew, Ralph Rogers, Fadi Shehadeh, Evangelia K Mylona, Matthew Kaczynski, Quynh-Lam Tran, Gregorio Benitez, Biswajit Mishra, Lewis Oscar Felix, Maria Tsikala Vafea, Eleftheria Atalla, Robin Davies, Salma Hedili, Maria Andrea Monkeberg, Sandra Tabler, Britt Harrington, Meegada Sreenath, Venkata Sandeep Koripalli, Prithvi Muddana, Lakshay Jain, Chaitanya Undavalli, Parasa Kavya, Mofoluwaso Ibiwoye, Hameed Akilo, Bryce D Lovette, Jamie-Crystal Wylie, Diana M Smith, Kenneth Poon, Paula Eckardt, Rubio-Gomez Heysu, Nithya Sundararaman, Doris Alaby, Candice Sareli, Adriana Sánchez, Laura Popielski, Amy Kambo, Kimberley Viens, Melissa Turner, Michael J Vjecha, Amy Weintrob, Indira Brar, Norman Markowitz, Erika Pastor, Roweena Corpuz, George Alangaden, John McKinnon, Mayur Ramesh, Erica Herc, Nicholas Yared, Lanfranco Odaliz Abreu, Emanuel Rivers, Jennifer Swiderek, Gupta Ariella Hodari, Pardeep Pabla, Sonia Eliya, Jehan Jazrawi, Jeremy Delor, Mona Desai, Aaron Cook, Jaehne Anja Kathrina, Gill Jasreen Kaur, Sheri Renaud, Siva Sarveswaran, Edward Gardner, James Scott, Monica Bianchini, Casey Melvin, Gina Kim, David Wyles, Kevin Kamis, Rachel Miller, Ivor Douglas, Jason Haukoos, Carrie Hicks, Susana Lazarte, Rubria Marines-Price, Alice Osuji, Barbine Tchamba Agbor Agbor, Tianna Petersen, Dena Kamel, Laura Hansen, Angie Garcia, Christine Cha, Azadeh Mozaffari, Rosa Hernandez, James Cutrell, Barbine Tchamba Agbor Agbor, Mina Kim, Natalie DellaValle, Sonia Gonzales, Charurut Somboonwit, Asa Oxner, Lucy Guerra, Michael Hayes, Thi Nguyen, Thanh Tran, Avenette Pinto, Timothy Hatlen, Betty Anderson, Ana Zepeda-Gutierrez, Dannae Martin, Cindi Temblador, Avon Cuenca, Roxanne Tanoviceanu, Martha Prieto, Mario Guerrero, Dannae Martin, Eric Daar, Ramiro Correa, Gabe Hartnell, Glenn Wortmann, Saumil Doshi, Theresa Moriarty, Melissa Gonzales, Kristin Garman, Jason V Baker, Anne Frosch, Rachael Goldsmith, Brian Driver, Christine Frank, Tzivia Leviton, Matthew Prekker, Hodan Jibrell, Melanie Lo, Jonathan Klaphake, Shari Mackedanz, Linh Ngo, Kelly Garcia-Myers, Ken M Kunisaki, Chris Wendt, Anne Melzer, Erin Wetherbee, Dimitri Drekonja, Alexa Pragman, Aimee Hamel, Abbie Thielen, Ken M Kunisaki, Miranda Hassler, Mary Walquist, Michael Augenbraun, Jensen George, Demeo Lynette, Motria Mishko, Lorraine Thomas, Luis Tatem, Jack Dehovitz, Mahsa Abassi, Anne-Marie Leuck, Via Rao, Matthew Pullen, Darlette Luke, Derek LaBar, Theresa Christiansen, Diondra Howard, Kousick Biswas, Cristin Harrington, Amanda Garcia, Tammy Bremer, Tara Burke, Brittany Koker, Anne Davis-Karim, David Pittman, Shikha S Vasudeva, Jaylynn R Johnstone, Kate Agnetti, Ruby Davis, Barbara Trautner, Casey Hines-Munson, John Van, Laura Dillon, Yiqun Wang, Stephanie Nagy-Agren, Shikha Vasudeva, Tracy Ochalek, Erin Caldwell, Edward Humerickhouse, David Boone, William McGraw, David J Looney, Sanjay R Mehta, Scott Thompson Johns, John Melissa St, Jacqueline Raceles, Emily Sear, Stephen Funk, Rosa Cesarini, Michelle Fang, Keith Nicalo, Wonder Drake, Beatrice Jones, Teresa Holtman, Hien H Nguyen, Archana Maniar, Eric A Johnson, Lam Nguyen, Michelle T Tran, Thomas W Barrett, Tera Johnston, John T Huggins, Tatsiana Y Beiko, Heather Y Hughes, William C McManigle, Nichole T Tanner, Ronald G Washburn, Magdalena Ardelt, Patricia A Tuohy, Jennifer L Mixson, Charles G Hinton, Nicola Thornley, Heather Allen, Shannon Elam, Barry Boatman, Brittany J Baber, Rudell Ryant, Brentin Roller, Chinh Nguyen, Amani Morgan Mikail, Marivic Hansen, Paola Lichtenberger, Gio Baracco, Carol Ramos, Lauren Bjork, Melyssa Sueiro, Phyllis Tien, Heather Freasier, Theresa Buck, Hafida Nekach, Mark Holodniy, Aarthi Chary, Kan Lu, Theresa Peters, Jessica Lopez, Susanna Yu Tan, Robert H Lee, Aliya Asghar, Tasadduq Karim Karyn Isip, Katherine Le, Thao Nguyen, Shinn Wong, Dorthe Raben, Daniel D Murray, Tomas O Jensen, Lars Peters, Bitten Aagaard, Charlotte B Nielsen, Katharina Krapp, Bente Rosdahl Nykjær, Christina Olsson, Katja Lisa Kanne, Anne Louise Grevsen, Zillah Maria Joensen, Tina Bruun, Ane Bojesen, Frederik Woldbye, Nick E Normand, Frederik V L Esman, Thomas Benfield, Clara Lundetoft Clausen, Nichlas Hovmand, Simone Bastrup Israelsen, Katrine Iversen, Caecilie Leding, Karen Brorup Pedersen, Louise Thorlacius-Ussing, Michaela Tinggaard, Sandra Tingsgard, Louise Krohn-Dehli, Dorthe Pedersen, Signe Villadsen, Jens-Ulrik Staehr Jensen, Rikke Overgaard, Ema Rastoder, Christian Heerfordt, Caroline Hedsund, Christian Phillip Ronn, Peter Thobias Kamstrup, Dorthe Sandbaek Hogsberg, Christina Bergsoe, Christian Søborg, Nuria M S Hissabu, Bodil C Arp, Lars Ostergaard, Nina Breinholt Staerke, Yordanos Yehdego, Ane Sondergaard, Isik S Johansen, Pedersen Andreas Arnholdt, Fredrikke C Knudtzen, Lykke Larsen, Mathias A Hertz, Thilde Fabricius, Inge K Holden, Susan O Lindvig, Marie Helleberg, Jan Gerstoft, Ole Kirk, Tina Bruun, Tomas Ostergaard Jensen, Birgitte Lindegaard Madsen, Thomas Ingemann Pedersen, Zitta Barrella Harboe, Birgit Thorup Roge, Thomas Michael Hansen, Matilde Kanstrup Glesner, Sandra Valborg Lofberg, Ariella Denize Nielsen, von Huth Sebastian Leicht, Henrik Nielsen, Rikke Krog Thisted, Kristine Toft Petersen, Maria Ruwald Juhl, Daria Podlekareva, Stine Johnsen, Helle Frost Andreassen, Lars Pedersen, Cecilia Ebba Clara Ellinor Lindnér, Lothar Wiese, Lene Surland Knudsen, Nikolaj Julian Skrøder Nytofte, Signe Ravn Havmøller, Maria Expósito, José Badillo, Ana Martínez, Elena Abad, Ana Chamorro, Ariadna Figuerola, Lourdes Mateu, Sergio España, Maria Constanza Lucero, José Ramón Santos, Gemma Lladós, Cristina Lopez, Lydia Carabias, Daniel Molina-Morant, Cora Loste, Carmen Bracke, Adrian Siles, Eduardo Fernández-Cruz, Marisa Di Natale, Sergiu Padure, Jimena Gomez, Cristina Ausin, Eva Cervilla, Héctor Balastegui, Carmen Rodríguez Sainz, Paco Lopez, Javier Carbone, Mariam Escobar, Leire Balerdi, Almudena Legarda, Montserrat Roldan, Laura Letona, José Muñoz, Daniel Camprubí, Jose R Arribas, Rocio Montejano Sánchez, Beatriz Díaz-Pollán, Stefan Mark Stewart, Irene Garcia, Alberto Borobia, Marta Mora-Rillo, Vicente Estrada, Noemi Cabello, M J Nuñez-Orantos, I Sagastagoitia, J R Homen, E Orviz, Adrián Sánchez Montalvá, Juan Espinosa-Pereiro, Pau Bosch-Nicolau, Fernando Salvador, Joaquin Burgos, Jose Luis Morales-Rull, Anna Maria Moreno Pena, Cristina Acosta, Cristina Solé-Felip, Juan P Horcajada, Elena Sendra, Silvia Castañeda, Inmaculada López-Montesinos, Joan Gómez-Junyent, Carlota Gudiol Gonzáles, Guilermo Cuervo, Miquel Pujol, Jordi Carratalà, Sebastià Videla, Huldrych Günthard, Dominique L Braun, Emily West, Khadija M'Rabeth-Bensalah, Mareile L Eichinger, Manuela Grüttner-Durmaz, Christina Grube, Veronika Zink, Josefine Goes, Gerd Fätkenheuer, Jakob J Malin, Tengiz Tsertsvadze, Akaki Abutidze, Nikoloz Chkhartishvili, Revaz Metchurtchlishvili, Marina Endeladze, Marcin Paciorek, Dominik Bursa, Dominika Krogulec, Piotr Pulik, Anna Ignatowska, Andrzej Horban, Elzbieta Bakowska, Justyna Kowaska, Agnieszka Bednarska, Natalia Jurek, Agata Skrzat-Klapaczynska, Carlo Bienkowski, Malgorzata Hackiewicz, Michal Makowiecki, Antoni Platowski, Roman Fishchuk, Olena Kobrynska, Khrystyna Levandovska, Ivanna Kirieieva, Mykhailo Kuziuk, Pontus Naucler, Emma Perlhamre, Lotta Mazouch, Anthony Kelleher, Mark Polizzotto, Catherine Carey, Christina C Chang, Sally Hough, Sophie Virachit, Sarah Davidson, Daniel J Bice, Katherine Ognenovska, Gesalit Cabrera, Ruth Flynn, Barnaby E Young, Po Ying Chia, Tau Hong Lee, Ray J Lin, David C Lye, Sean W X Ong, Ser Hon Puah, Tsin Wen Yeo, Shiau Hui Diong, Juwinda Ongko, He Ping Yeo, Nnakelu Eriobu, Vivian Kwaghe, Habib Zaiyad, Godwin Idoko, Blessing Uche, Poongulali Selvamuthu, Nagalingeswaran Kumarasamy, Faith Ester Beulah, Narayan Govindarajan, Kowsalya Mariyappan, Marcelo H Losso, Cecilia Abela, Renzo Moretto, Carlos G Belloc, Jael Ludueña, Josefina Amar, Marcelo H Losso, Javier Toibaro, Laura Moreno Macias, Lucia Fernandez, Pablo S Frare, Sebastian R Chaio, Valeria Pachioli, Stella M Timpano, Marisa del Lujan Sanchez, Mariana de Paz Sierra, Vanina Stanek, Waldo Belloso, Flavia L Cilenti, Ricardo N Valentini, Martin E Stryjewski, Nicolas Locatelli, Riera Maria C Soler, Clara Salgado, Ines M Baeck, Castelnuovo Valentina Di, Stella M Zarza, Fleur Hudson, Mahesh K B Parmar, Anna L Goodman, Jonathan Badrock, Adam Gregory, Katharine Goodall, Nicola Harris, James Wyncoll, S Bhagani, A Rodger, A Luntiel, C Patterson, J Morales, E Witele, A-M Preston, A Nandani, D A Price, Aiden Hanrath, Jeremy Nell, Bijal Patel, Carole Hays, Geraldine Jones, Jade Davidson, Anna L Goodman, T Bawa, M Mathews, A Mazzella, K Bisnauthsing, L Aguilar-Jimenez, F Borchini, S Hammett, Giota Touloumi, Nikos Pantazis, Vicky Gioukari, Tania Souliou, A Antoniadou, K Protopapas, D Kavatha, S Grigoropoulou, R-N Tziolos, C Oikonomopoulo, C Moschopoulos, N G Koulouris, K Tzimopoulos, A Koromilias, K Argyraki, P Lourida, P Bakakos, I Kalomenidis, V Vlachakos, Z Barmparessou, E Balis, S Zakynthinos, I Sigala, N Gianniou, E Dima, S Magkouta, E Synolaki, S Konstanta, M Vlachou, P Stathopoulou, P Panagopoulos, V Petrakis, D Papazoglou, E Tompaidou, E Isaakidou, G Poulakou, V Rapti, K Leontis, T Nitsotolis, K Athanasiou, K Syrigos, K Argyraki, M-D Myrodia, K Kyriakoulis, I Trontzas, M Arfara-Melanini, V Kolonis, Cissy Kityo, Henry Mugerwa, Francis Kiweewa, Ivan Kimuli, Joseph Lukaakome, Christoher Nsereko, Gloria Lubega, Moses Kibirige, William Nakahima, Deus Wangi, Evelyne Aguti, Lilian Generous, Rosemary Massa, Margaret Nalaki, Felix Magala, Phiona Kaweesi Nabaggala, Robert Kidega, Cissy Kityo, Henry Mugerwa, Oryem Daizy Faith, Apio Florence, Ocung Emmanuel, Mugoonyi Paul Beacham, Amone Geoffrey, Dridah Nakiboneka, Paska Apiyo, Francis Kiweewa, Bruce Kirenga, Ivan Kimuli, Angella Atukunda, Winters Muttamba, Kyeyume Remmy, Ivan Segawa, Nsubuga Pheona, David Kigere, Queen Lailah Mbabazi, Ledra Boersalino, Grace Nyakoolo, Francis Kiweewa, Aniongo Fred, Alice Alupo, Doryn Ebong, Edson Monday, Ritah Norah Nalubwama, Milton Kainja, Munu Ambrose, Vanon Kwehayo, Mary Grace Nalubega, Augustine Ongoli, Stephen Obbo, Nicholus Sebudde, Jeniffer Alaba, Geoffrey Magombe, Harriet Tino, E E Emmanuel Obonya, Joseph Lutaakome, Jonathan Kitonsa, Martin Onyango, Tukamwesiga Naboth, Hadijah Naluyinda, Regina Nanyunja, Muttiibwa Irene, Biira Jane, Kyobejja Wimfred, Ssemazzi Leonard, Tkiinomuhisha Deus, Namasaba Babra, Paul Taire, Joseph Lutaakome, Evelyn Nabankema, Joseph Ogavu, Oscar Mugerwa, Ivan Okoth, Raymond Mwebaze, Timothy Mugabi, Anthony Makhoba, Phiona Arikiriza, Nabuuma Theresa, Hope Nakayima, Kisuule Frank, Patrícia Ramgi, Kássia Pereira, Anu Osinusi, Huyen Cao, Paul Klekotka, Karen Price, Ajay Nirula, Suzette Osei, Craig Tipple, Angela Wills, Amanda Peppercorn, Helen Watson, Rajesh Gupta, Elizabeth Alexander, Erik Mogalian, Leo Lin, Xiao Ding, David Margolis, Li Yan, Jean-Luc Girardet, Ji Ma, Zhi Hong, Quing Zhu, Seth Seegobin, Michael Gibbs, Mickel Latchman, Katarzyna Hasior, Jerome Bouquet, Jianxin Wei, Katie Streicher, Albert Schmelzer, Dennis Brooks, Jonny Butcher, Dimitar Tonev, Douglas Arbetter, Philippe Damstetter, Philippe Legenne, Michael Stumpp, Susana Goncalves, Krishnan Ramanathan, Richa Chandra, Beth Baseler, Marc Teitelbaum, Adam Schechner, H Preston Holley, Shirley Jankelevich, Amy Adams, Nancy Becker, Suzanne Dolney, Debbie Hissey, Shelly Simpson, Mi Ha Kim, Joy Beeler, Liam Harmon, Mabel Asomah, Yvonne Jato, April Stottlemyer, Olivia Tang, Sharon Vanderpuye, Lindsey Yeon, Molly Buehn, Vanessa Eccard-Koons, Sadie Frary, Leah MacDonald, Jennifer Cash, Lisa Hoopengardner, Jessica Linton, Marylu Schaffhauser, Michaela Nelson, Mary Spinelli-Nadzam, Calvin Proffitt, Christopher Lee, Theresa Engel, Laura Fontaine, C K Osborne, Matt Hohn, Michael Galcik, DeeDee Thompson, Stacey Kopka, Denise M Shelley, Gregg Mendez, Shawn Brown, Sara Albert, Abby Balde, Michelle Baracz, Mona Bielica, Shere Billouin-Frazier, Jay Choudary, Mary Dixon, Carolyn Eyler, Leanne Frye, Jensen Gertz, Lisa Giebeig, Neelam Gulati, Liz Hankinson, Debi Hogarty, Lynda Huber, Gary Krauss, Eileen Lake, Meryan Manandhar, Erin Rudzinski, Jen Sandrus, Connie Suders, Ven Natarajan, Adam W Rupert, Michael Baseler, Danielle Lynam, Tom Imamichi, Sylvain Laverdure, Ashley McCormack, Sharada Paudel, Kyndal Cook, Kendra Haupt, Ayub Khan, Allison Hazen, Yunden Badralmaa, Kenneth Smith, Bhakti Patel, Amanda Kubernac, Robert Kubernac, Marie L Hoover, Courtney Solomon, Marium Rashid, Joseph Murphy, Craig Brown, Nadine DuChateau, Sadie Ellis, Adam Flosi, Lisa Fox, Les Johnson, Rich Nelson, Jelena Stojanovic, Amy Treagus, Christine Wenner, and Richard Williams
Notes
Acknowledgments. On behalf of the TICO study group, the authors’ writing group thanks all the TICO trial participants for their participation and engagement, as this work would not have been possible without them.
Disclaimer. Investigators from the National Institutes of Health, the funder, were directly involved in all aspects of this study, including study design, data collection, analysis, and interpretation, as well as report writing. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the National Institutes of Health (NIH) or the US government.
Financial support. The trial was sponsored and primarily funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, Maryland. This trial was funded in part with federal funds from NIAID, including grant number U01-AI136780, and the National Cancer Institute, NIH, under contract number 75N91019D00024, task order number 75N91020F00039, and NIH agreement 1OT2HL156812 through the National Heart, Lung, and Blood Institute (NHLBI) CONNECTS program. No support was provided for medical writing.
References
- 1. Adjei S, Hong K, Molinari NM, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the Omicron and Delta variant pandemic periods—United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim L, Garg S, O'Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021; 72:e206–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ram-Mohan N, Kim D, Zudock EJ, et al. SARS-CoV-2 RNAemia predicts clinical deterioration and extrapulmonary complications from COVID-19. Clin Infect Dis 2022; 74:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kowsar R, Rahimi AM, Sroka M, et al. Risk of mortality in COVID-19 patients: a meta- and network analysis. Sci Rep 2023; 13:2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Espejo-Paeres C, Espliguero RA, Uribarri A, et al. Predictors of poor prognosis in healthy, young, individuals with SARS-CoV-2 infections. Clin Microbiol Infect 2022; 28:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C mortality score. BMJ 2020; 370:m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abers MS, Delmonte OM, Ricotta EE, et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 2021; 6:e144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calvet J, Berenguer-Llergo A, Gay M, et al. Biomarker candidates for progression and clinical management of COVID-19 associated pneumonia at time of admission. Sci Rep 2022; 12:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146:128–36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Recovery Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021; 27:1752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyapati A, Wipperman MF, Ehmann PJ, et al. Baseline severe acute respiratory syndrome viral load is associated with coronavirus disease 2019 severity and clinical outcomes: post hoc analyses of a phase 2/3 trial. J Infect Dis 2021; 224:1830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magleby R, Westblade LF, Trzebucki A, et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2021; 73:e4197–e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salto-Alejandre S, Berastegui-Cabrera J, Camacho-Martinez P, et al. SARS-CoV-2 viral load in nasopharyngeal swabs is not an independent predictor of unfavorable outcome. Sci Rep 2021; 11:12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodríguez-Serrano DA, Roy-Vallejo E, Zurita Cruz ND, et al. Detection of SARS-CoV-2 RNA in serum is associated with increased mortality risk in hospitalized COVID-19 patients. Sci Rep 2021; 11:13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ACTIV-3/TICO Study Group; Rogers AJ, Wentworth D, et al. The association of baseline plasma SARS-CoV-2 nucleocapsid antigen level and outcomes in patients hospitalized with COVID-19. Ann Intern Med 2022; 175:1401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wick KD, Leligdowicz A, Willmore A, et al. Plasma SARS-CoV-2 nucleocapsid antigen levels are associated with progression to severe disease in hospitalized COVID-19. Crit Care 2022; 26:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beitler JR, Thompson BT, Baron RM, et al. Advancing precision medicine for acute respiratory distress syndrome. Lancet Respir Med 2022; 10:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Q, Pennini ME, Bergmann JN, et al. Applying lessons learned from COVID-19 therapeutic trials to improve future ALI/ARDS trials. Open Forum Infect Dis 2022; 9:ofac381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Douin DJ, Siegel L, Grandits G, et al. Evaluating primary endpoints for COVID-19 therapeutic trials to assess recovery. Am J Respir Crit Care Med 2022; 206:730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ACTIV-3/TICO LY-CoV555 Study Group; Lundgren JD, Grund B, et al. A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med 2021; 384:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ACTIV-3/TICO Study Group; Barkauskas C, Mylonakis E, et al. Efficacy and safety of ensovibep for adults hospitalized with COVID-19 : a randomized controlled trial. Ann Intern Med 2022; 175:1266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group . Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial. Lancet Respir Med 2022; 10:972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray DD, Babiker AG, Baker JV, et al. Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: therapeutics for inpatients with COVID-19 (TICO/ACTIV-3). Clin Trials 2022; 19:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group . Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis 2022; 22:622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ACTIV-3/TICO Bamlanivimab Study Group; Lundgren JD, Grund B, et al. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels : a randomized controlled trial. Ann Intern Med 2022; 175:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abbott TEF, Cron N, Vaid N, Ip D, Torrance HDT, Emmanuel J. Pre-hospital National Early Warning Score (NEWS) is associated with in-hospital mortality and critical care unit admission: a cohort study. Ann Med Surg (Lond) 2018; 27:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of COVID-19: systematic review and critical appraisal. BMJ 2020; 369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reyes LF, Murthy S, Garcia-Gallo E, et al. Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the international severe acute respiratory and emerging infection consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Res 2022; 8:00552-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams K, Rhoads JP, Surie D, et al. Vaccine effectiveness of primary series and booster doses against COVID-19 associated hospital admissions in the United States: living test negative design study. BMJ 2022; 379:e072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. REMAP-CAP Investigators; Gordon AC, Mouncey PR, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med 2021; 384:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RECOVERY Collaborative Group; Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: an open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr 2021; 133(7–8):303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.