Abstract
Antimicrobial peptides (AMPs) are molecules found in most organisms, playing a vital role in innate immune defense against pathogens. Their mechanism of action involves the disruption of bacterial cell membranes, causing leakage of cellular contents and ultimately leading to cell death. While AMPs typically lack a defined structure in solution, they often assume a defined conformation when interacting with bacterial membranes. Given this structural flexibility, we investigated whether intrinsically disordered regions (IDRs) with AMP-like properties could exhibit antimicrobial activity. We tested 14 peptides from different IDRs predicted to have antimicrobial activity and found that nearly all of them did not display the anticipated effects. These peptides failed to adopt a defined secondary structure and had compromised membrane interactions, resulting in a lack of antimicrobial activity. We hypothesize that evolutionary constraints may prevent IDRs from folding, even in membrane-like environments, limiting their antimicrobial potential. Moreover, our research reveals that current antimicrobial predictors fail to accurately capture the structural features of peptides when dealing with intrinsically unstructured sequences. Hence, the results presented here may have far-reaching implications for designing and improving antimicrobial strategies and therapies against infectious diseases.
Keywords: Antimicrobial peptide, Disordered proteins, Peptide design, Prediction
1. Introduction
Antimicrobial peptides (AMPs) are naturally occurring molecules found in all forms of life, from bacteria to humans, and hold promise as an alternative to conventional antibiotics [1], [2]. AMPs may be present either as secreted peptides, such as defensins, or as hidden segments within larger proteins, which are activated under certain conditions [3], [4]. Hemocidin, for example, is released by the proteolytic cleavage of the α-chain of human hemoglobin [5], [6]. Unlike traditional antibiotics, which often target specific bacterial functions or metabolic pathways, AMPs disrupt essential physical structures within bacterial cells, including the cell membrane and cell wall, thereby reducing the likelihood of resistance development [7], [8]. This unique mode of action positions AMPs as promising agents for treating infections caused by antibiotic-resistant bacterial strains.
AMPs are characterized by their small size, typically ranging from 12 to 50 amino acids, their cationic nature, and amphipathic properties in their mature form [2], [9]. These features enable them to selectively interact with microbial membranes. Cationic AMPs are attracted to negatively charged components in microbial membranes, such as phosphatidylglycerol and lipopolysaccharides, while the membranes of mammalian cells are typically more neutral, containing a higher proportion of zwitterionic phospholipids like phosphatidylcholine [8], [10]. This charge difference contributes to the specific targeting of microbial cells, which is essential for therapeutic applications. The amphipathic structure of AMPs, featuring both hydrophobic and hydrophilic regions, further facilitates their interaction with the lipid bilayer [11], [12]. This dual nature allows AMPs to align themselves at the membrane interface, with hydrophobic residues facing the lipid core and hydrophilic residues interacting with the aqueous environment [11], [13]. This orientation is crucial for subsequent membrane insertion and disruption.
Remarkably, most AMPs are unstructured in aqueous solutions, existing as random coils or extended conformations [14], [15]. However, upon interaction with the hydrophobic environment of lipid bilayers, they undergo significant conformational changes [14]. Membrane binding often leads to the adoption of specific secondary structures, primarily α-helices [14], [16]. The adoption of helical conformations within the membrane allows AMPs to penetrate more deeply, disrupt lipid packing, and ultimately compromise membrane integrity [14], [17]. This structural flexibility enables AMPs to adapt to various target membranes and exert their antimicrobial action through multiple mechanisms [11], [18], [19].
IDRs lack stable secondary structures [20], [21], [22]. However, under specific conditions, such as interactions with other proteins or the presence of certain ligands, these regions can adopt distinct structures through a process called conditional folding [23], [24]. Given this inherent structural flexibility, it is reasonable to ask whether intrinsically disordered regions (IDRs) in proteins can function like encrypted AMPs under suitable conditions. Should these unstructured regions that contain cationic and amphipathic sequences may form helical structures in hydrophobic environments, then they might function as encrypted AMPs upon binding to bacterial membranes. For instance, recently have been reported some IDRs that have cationic intrinsically disordered antimicrobial peptides (CIDAMPs) [25] and also specific regions that directly regulate the protein's anti-infective action [26], [27].
In this study, we investigated IDRs that have AMP-compatible amino acid compositions and found that most of them did not demonstrate the expected activity. We reasoned that evolutionary constraints may prevent these regions from adopting a defined structure, even in membrane-like environments. Although our findings do not rule out the possibility that some disordered regions have evolved for specific antimicrobial purposes, such cases may be rare. Our results also reveal that existing antimicrobial peptide prediction tools often fail to accurately assess IDRs, leading to increased false positive rates.
2. Results and discussion
2.1. Searching for antimicrobial peptides in intrinsically disordered regions
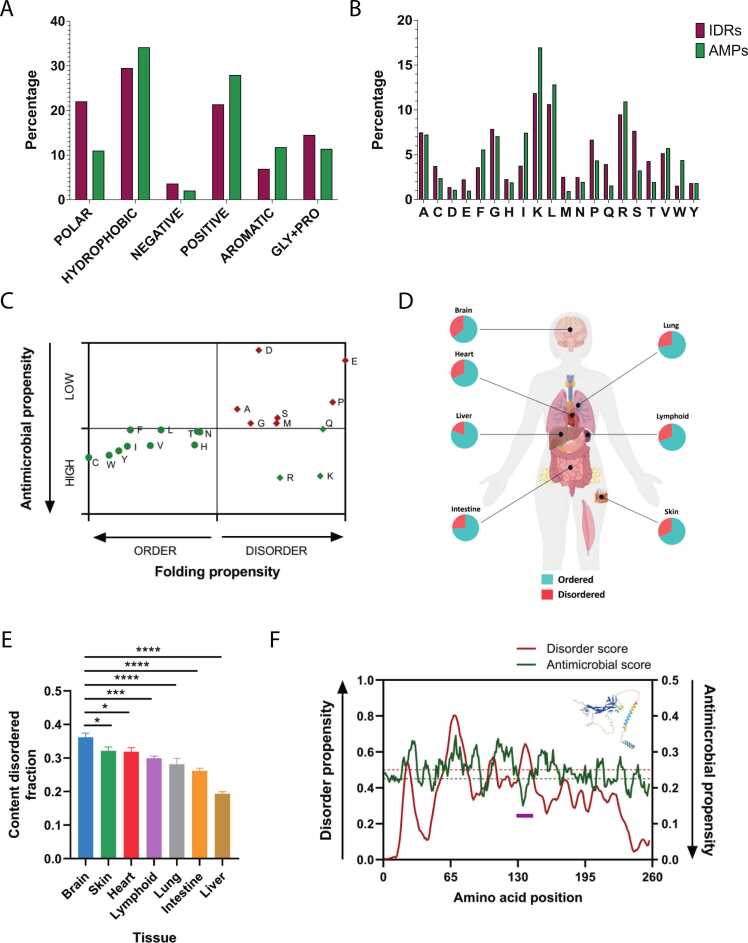
To explore the possible correlation between the structural characteristics of IDRs and the antimicrobial activity of peptides, we examined how these align with AMP properties. We first identified segments in the human proteome that were unstructured as by AlphaFold predictions, cationic, and 10–25 residues long, aligning with conventional AMP properties. Out of 42987 IDRs, 10879 met the average AMP length criteria, with 4469 being cationic. Using multiple computational prediction tools, 1592 of these cationic sequences were identified as potential AMPs by at least three out of five algorithms (Supplementary File 1). This suggests that approximately 3.7% of human proteome IDRs might function as potential encrypted AMPs. Also, we compared the amino acid composition of these encrypted AMPs with canonical AMPs (Fig. 1A, B). We found that IDR-derived AMPs contained, on average, more polar residues, while canonical AMPs had a higher content of hydrophobic and aromatic residues. Specifically, canonical AMPs were enriched in Leu, Ile, Phe, and Trp, while IDR-derived AMPs had a higher presence of Ser, Thr, Gln, and Pro. These differences can be attributed to the different propensities of these amino acids for disorder [28], [29] and antimicrobial activity [30] (Fig. 1C). For instance, Ser and Pro tend to be disordered but have limited antimicrobial action, while hydrophobic residues like Trp and Ile have strong antimicrobial properties but tend to be more ordered. Both groups were characterized by the presence of Lys and Arg and a minimal presence of Glu and Asp, reflecting the imposed positive charge characteristic of AMPs.
Fig. 1.
Analysis of disordered proteins and amino acid propensities. (A) Proportional comparison of amino acid types in canonical AMPs versus IDR-encrypted AMPs, (B) Difference in amino acid percentages in canonical AMPs compared to IDR-encrypted AMPs, (C) Correlation between amino acids' antimicrobial and folding propensities. Amino acid propensities for order and disorder, based on the DisProt scale [28], are depicted as circles and diamonds, respectively. Amino acids with predicted antimicrobial propensities, as determined by M. Torrent et al. [30], are highlighted in red (low propensity) and green (high propensity), (D) Representation of ordered and disordered regions in various tissues through pie charts, (E) Tissue-specific disordered fraction predictions using the espN tool [31]. Error bars correspond to the standard error of the mean. The ANOVA test was used to evaluate significant differences between the brain and other tissues (denoted as * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.001), (F) Diagram showing neurotrophin-3's disorder (red) and antimicrobial (green) predictions with threshold markers (0.5 and 0.225, respectively) in dashed lines. The selected peptide of neurotrophin-3 (purple segment) is from the segment with highest antimicrobial propensity.
Additionally, we assessed the tissue-specific distributions of these encrypted AMPs in IDRs (Fig. 1D). We selected key genes that were uniquely expressed in different tissues, with evidence at the protein level, including the brain, skin, heart, lymphoid tissue, lung, intestine, and liver (Supplementary File 2). By cross-referencing this information with the MobiDB protein disorder database [31], we observed a higher prevalence of IDRs in brain proteins compared to other tissues (Fig. 1E). However, we could not find any significant differences in the proportion of IDRs with AMP-like properties between the different tissues (Supplementary File 2).
In summary, despite compositional differences, many IDRs consistently exhibited potential antimicrobial properties, warranting further investigation. Hence, we focused our subsequent research on brain proteins due to the prevalent occurrence of structurally disordered proteins in this organ.
2.2. Evaluation of antimicrobial activity
We analyzed secreted brain proteins with the AMPA software [32] to identify potential antimicrobial regions that overlapped with IDRs (Supplementary File 3). An example of this approach is depicted for neurotrophin-3 (Fig. 1F). From these results, we selected 14 peptides (labeled 1–14) derived from intrinsically disordered regions, three control peptides (H1-H3) from alpha-helical segments, and the standard AMP control LL-37. We evaluated the peptides using five prediction tools (CAMPR4, AI4AMP, AmpGram, HydrAMP, and sAMPpred) and assessed the consensus among these predictors (Table 1). Additionally, we tested the peptides against four bacterial strains, Escherichia coli, Pseudomona aeruginosa, Staphylococcus aureus and Micrococcus luteus, to determine their antimicrobial activity in vitro (Table 2).
Table 1.
Prediction scores of peptides derived from intrinsically disordered regions and control peptides.
| ID | UniProt code | Sequence | CAMPR4 | AI4AMP | HydrAMP | AmpGram | sAMPpred | Consensus |
|---|---|---|---|---|---|---|---|---|
| 1 | Q9NQ76 | GRKYHYVPHRQNNSTR | + | - | + | - | + | + ++ |
| 2 | P11487 | VNGKGRPRRGFKTRRTQKSSLFLP | + | + | + | - | + | + ++ + |
| 3 | Q8IUH2 | VRRGRRPARPGTR | + | + | - | + | + | + ++ + |
| 4 | O15018 | EKRRGGKKRKTHQGPVLD | + | + | - | + | + | + ++ + |
| 5 | Q3ZCN5 | IVRKLNLMIPWSIF | + | - | + | - | + | + ++ |
| 6 | P51884 | KGRVFSKLKQLKKLHINHN | + | + | + | + | + | + ++ ++ |
| 7 | O15018 | KKGKRTRKFGVISR | + | + | - | + | + | + ++ + |
| 8 | P43235 | KKTHRKQYNNKV | + | + | - | - | + | + ++ |
| 9 | O15018 | LIMLRRFKHKAHSTYN | + | + | + | + | + | + ++ ++ |
| 10 | P20783 | NRTSRRKRYAEHKSH | + | + | - | - | + | + ++ |
| 11 | Q17RW2 | RAHRTRRGKVSPTAKTKSL | + | + | + | + | + | + ++ ++ |
| 12 | O00622 | SSLKKGKKSSKTKKSP | + | + | + | + | + | + ++ ++ |
| 13 | Q14623 | VGSRRRPPRKLVPLAY | + | + | + | + | + | + ++ ++ |
| 14 | Q9UBX7 | QRLRWLRDWKSS | + | + | - | - | + | + ++ |
| H1 | P01562 | KKYFRRITLYLTE | + | + | - | - | + | + ++ |
| H2 | O00634 | RDAWTRRLRRLQRRERRGRSS | + | + | + | - | + | + ++ + |
| H3 | P02776 | DLQAPLYKKIIKKLL | + | + | + | + | + | + ++ ++ |
| LL-37 | P49913 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | + | + | + | + | + | + ++ ++ |
Table 2.
Antimicrobial activity of IDR-derived AMPs and control peptides.
| ID |
Minimal Inhibitory Concentration (µM) |
|||
|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | M. luteus | |
| 1 | > 100 | > 100 | > 100 | > 100 |
| 2 | > 100 | > 100 | > 100 | > 100 |
| 3 | > 100 | > 100 | > 100 | > 100 |
| 4 | > 100 | > 100 | > 100 | > 100 |
| 5 | 50 | 50 | 100 | 25 |
| 6 | 50 | 50 | 100 | 25 |
| 7 | > 100 | > 100 | > 100 | > 100 |
| 8 | > 100 | > 100 | > 100 | > 100 |
| 9 | > 100 | > 100 | > 100 | > 100 |
| 10 | > 100 | > 100 | > 100 | > 100 |
| 11 | > 100 | > 100 | > 100 | > 100 |
| 12 | > 100 | > 100 | > 100 | > 100 |
| 13 | > 100 | > 100 | > 100 | > 100 |
| 14 | > 100 | > 100 | > 100 | > 100 |
| H1 | 6.3 | 12.5 | 12.5 | 12.5 |
| H2 | 25 | 25 | 50 | 50 |
| H3 | 12.5 | 12.5 | 25 | 25 |
| LL-37 | 1.6 | 1.6 | 25 | 1.6 |
IDR-derived peptides showed varying results across different AMP predictors. However, despite the inherent variability, most of them were predicted to be antimicrobial by at least three out of five tools (Table 1). The consensus estimate, indicating the overall agreement among predictors, suggested strong antimicrobial activity for the selected candidates. However, experimental results revealed a different scenario. Most of these peptides (peptides 1–4, 7–14) did not exhibit any antimicrobial activity against the bacterial strains tested, even at concentrations up to 100 µM (Table 2). However, we cannot exclude that some of these peptides may display activity on other strains, as some AMPs have been proven effective against other pathogens. Two peptides (5 and 6) showed only minimal activity, with effective concentrations of 50 µM against E. coli and P. aeruginosa, and 25–100 µM against M. luteus and S. aureus, respectively (Table 2). These findings suggest that despite positive predictions, IDRs may not readily adopt functional antimicrobial conformations, highlighting the complexity of translating sequence-based predictions into functional antimicrobial peptides.
Control peptides H1-H3, derived from alpha-helical segments, were included to validate our approach. The prediction pattern for these peptides was similar to that of peptides 1–14, suggesting no bias in the behavior of the predictors between these sets of peptides (Table 1). However, unlike IDR-derived peptides, control peptides demonstrated substantial antimicrobial activity. Peptide H1 was particularly effective, with concentrations of 6.3 µM and 12.5 µM required to inhibit bacterial cell growth (Table 2). Peptides H2 and H3 also exhibited considerable activity, with concentrations ranging from 12.5 to 50 µM. These values are consistent with the control peptide LL-37, which displayed minimal inhibitory concentrations of 1.6 and 25 µM [33], [34].
The difference between the IDR-derived peptides and the control peptides underlines the importance of considering structural context when predicting antimicrobial activity. In this context, IDR-derived peptides, despite possessing compositions compatible with canonical AMPs, may not be suitable as antimicrobials. These findings collectively indicate that structural properties, which are not explicitly considered in the predictions, play a crucial role in antimicrobial activity.
2.3. Structural characterization of peptides
Building on the assessment of antimicrobial activity, we further explored the structural characteristics of the selected peptides. The structure was evaluated using three approaches: by comparison with the original structure in the parental protein, by predicting the structure with PepFold [35], [36] and AlphaFold [37], [38], [39], and experimentally, by using circular dichroism [40], [41].
Most IDR-derived peptides were characterized as random coils by both predictive tools, PepFold and AlphaFold (Table 3), with experimental data largely validating these predictions (Supplementary Figure 1). While SDS micelles typically induce folding in AMPs, most IDR-derived peptides remained in a random coil conformation (Table 3). Only a few peptides (6, 10, and 14), exhibited a substantial folding by adopting an alpha-helix conformation in SDS micelles (Table 3). Yet, despite this induced folding in a membrane-like environment, these peptides lacked significant antimicrobial activity, except for 6, which showed some residual activity (Table 2). Conversely, control peptides H1-H3, derived from alpha-helical segments in the original proteins, consistently showed high helical content in SDS micelles and high antimicrobial activity. These control peptides, including LL-37, underline the correlation between helical structure and functionality [42], [43], [44], [45].
Table 3.
Predicted and experimentally determined secondary structures of peptides.
| ID | Original structure | PepFold | AlphaFold | Helix (%) | Beta (%) | Coil (%) |
|---|---|---|---|---|---|---|
| 1 | Random coil | Random coil | Random coil | 6.4 | 8.8 | 84.8 |
| 2 | Random coil | Helix | Random coil | 40.6 | 0.6 | 58.8 |
| 3 | Random coil | Random coil | Random coil | 0.70 | 29.8 | 69.5 |
| 4 | Random coil | Random coil | Random coil | 20.1 | 14.6 | 65.3 |
| 5 | Random coil | Random coil | Random coil | 43.3 | 6.3 | 50.4 |
| 6 | Random coil | Helix | Random coil | 99.6 | 0.4 | 0.0 |
| 7 | Random coil | Random coil | Random coil | 18.1 | 16.0 | 65.9 |
| 8 | Random coil | Helix | Random coil | 8.4 | 27.7 | 63.9 |
| 9 | Random coil | Helix | Helix | 19.6 | 36.4 | 44.0 |
| 10 | Random coil | Helix | Helix | 61.5 | 3.9 | 34.6 |
| 11 | Random coil | Random coil | Random coil | 24.2 | 8.4 | 67.4 |
| 12 | Random coil | Helix | Random coil | 16.8 | 7.7 | 75.5 |
| 13 | Random coil | Random coil | Random coil | 37.3 | 5.0 | 57.7 |
| 14 | Random coil | Helix | Helix | 59.3 | 4.7 | 36.0 |
| H1 | Helix | Helix | Helix | 43.9 | 12.4 | 43.7 |
| H2 | Helix | Helix | Helix | 97.7 | 0 | 2.3 |
| H3 | Helix | Helix | Helix | 98.9 | 1.1 | 0 |
| LL-37 | Helix | Helix | Helix | 85.4 | 0.5 | 14.1 |
These results illustrate a complex relationship between structural characteristics and antimicrobial activity. While helical content is required for antimicrobial function, this relationship is not always straightforward, as suggested by peptides 6, 10, and 14. Hence, the propensity to form helices does not always translate into antimicrobial action, pointing to the multifactorial nature of peptide activity.
2.4. Binding affinity and organization to membranes
To gain further insights into the structural and functional behavior of the selected peptides, we calculated the Gibbs free energy (ΔG) of binding for three different types of membranes using the FMAP thermodynamic model: SDS micelles, DPC micelles, and Gram-negative bacterial membranes [46], [47].
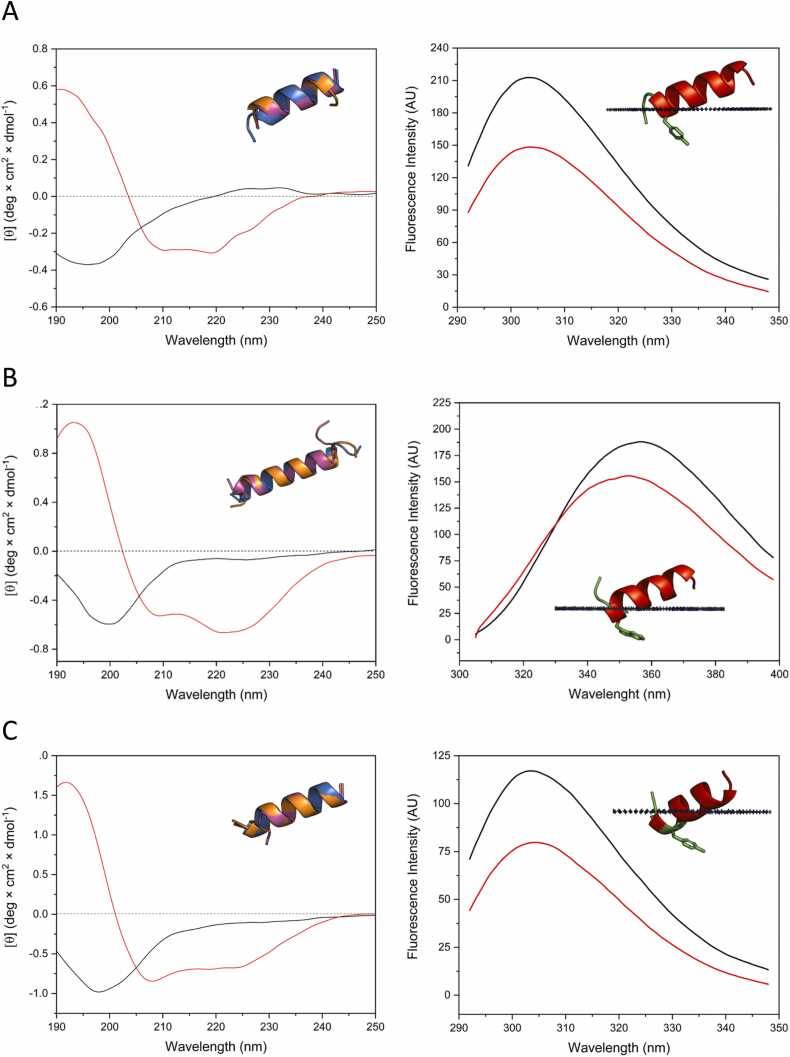
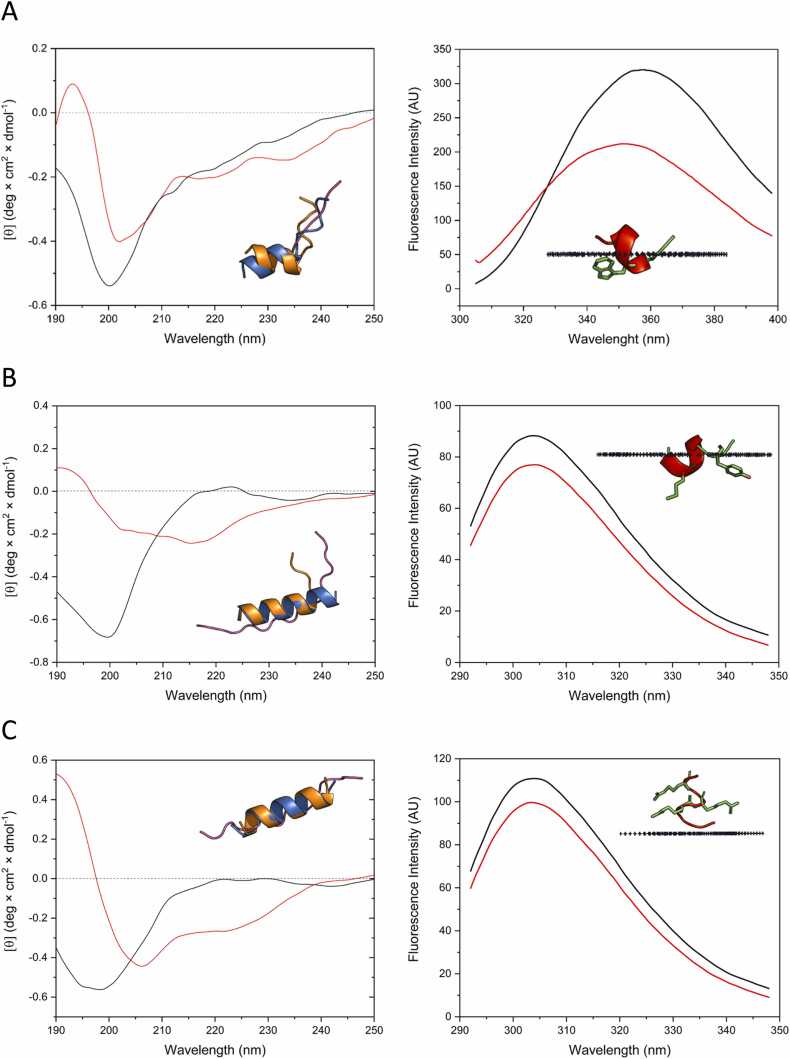
Control peptides H1-H3, characterized as helices, demonstrated substantial negative ΔG values for all membrane types, ranging from − 3.4 to − 5.0 kcal/mol (Table 4, Fig. 2). These values indicate strong and favorable binding, consistent with their previously observed antimicrobial activity (Table 2), and similar to other AMPs [48], [49]. The binding to membranes is also supported by a decrease in the intrinsic fluorescence of the aromatic residues upon binding to membranes [50], [51] (Fig. 2). Among the IDR-derived peptides, most displayed small ΔG values across all types of membranes, suggesting weak or negligible binding (Table 4), which correlates with the absence of antibacterial activity (Table 2) [52]. Notably, peptide 6, which previously exhibited a high helical content, also displayed strong binding to SDS and DPC micelles (−4.5 kcal/mol) but weaker binding to Gram-negative membranes (−2.9 kcal/mol). Similarly, peptides 5 and 9 showed significant binding energies, particularly to Gram-negative membranes, with values of − 4.3 and − 5.0 kcal/mol, respectively. In this sense, the binding energies correlate to some extent with antimicrobial activity (Table 2), as peptides 5 and 6, which demonstrated residual activity against bacterial strains, also exhibited favorable binding to Gram-negative membranes (Table 2, Fig. 3).
Table 4.
Calculated Gibbs free energy of binding (ΔG) for different membrane environments. Helix tilt and depth measurements were calculated for bacterial membranes.
| ID | Peptide Structure determined by CD | ΔG SDS (kcal/mol) | ΔG DPC (kcal/mol) | ΔG bacterial membrane (kcal/mol) | Tilt (º)a | Depth (Å)a |
|---|---|---|---|---|---|---|
| 1 | Random coil | -0.7 | -0.7 | -0.7 | — | — |
| 2 | Random coil | -3.4 | -3.3 | -3.6 | 78 | 2.5 |
| 3 | Random coil | -0.1 | -0.1 | -0.1 | — | — |
| 4 | Random coil | -0.6 | -0.6 | -0.6 | 53 | 0.5 |
| 5 | Random coil | -3.2 | -3.3 | -4.3 | 72 | 1.8 |
| 6 | Helix | -4.5 | -4.5 | -2.9 | 65 | 2.1 |
| 7 | Random coil | -1.8 | -1.8 | -1.8 | 55 | 2.4 |
| 8 | Random coil | -0.4 | -0.4 | -0.4 | — | — |
| 9 | Random coil | -5.8 | -5.6 | -5.0 | 86 | 6.8 |
| 10 | Helix | -0.3 | -0.3 | -0.3 | 82 | 0.0 |
| 11 | Random coil | -0.7 | -0.7 | -0.8 | 73 | 0.0 |
| 12 | Random coil | -0.5 | -0.5 | -0.5 | 89 | 1.9 |
| 13 | Random coil | -1.5 | -1.5 | -1.7 | 76 | 1.2 |
| 14 | Helix | -3.8 | -3.8 | -3.8 | 87 | 2.7 |
| H1 | Helix | -4.6 | -4.9 | -3.4 | 78 | 6.2 |
| H2 | Helix | -4.5 | -4.3 | -3.8 | 68 | 5.0 |
| H3 | Helix | -5.0 | -4.8 | -4.4 | 71 | 4.2 |
| LL-37 | Helix | -6.2 | -6.3 | -6.2 | 85 | 4.6 |
Tilt angles and depth insertion were calculated only in bacterial membranes. Peptides 1, 3, and 8 could not form any helices according to the FMAP model. The full LL-37 is too long for FMAP analysis, and the results correspond to the peptide core (FKRIVQRIKDFLR).
Fig. 2.
Structural characteristics of control peptides. The figure presents the structural properties of control peptides H1 (A), H2 (B), and H3 (C), showing various aspects across different panels. The left panel shows the CD profile in water (black) and SDS micelles (red), alongside the structure in the original protein (purple), PepFold prediction (orange), and AlphaFold prediction (blue). In the right panel, membrane interactions are measured through the intrinsic fluorescence of tyrosine (H1 and H3) and tryptophan (H2), showing a marked decrease in fluorescence upon membrane binding. Additionally, the helical peptide regions in contact with or embedded in the membrane are displayed, as predicted by the FMAP model.
Fig. 3.
Structural Characteristics of Peptides 5, 9 and, 10. The figure presents the structural properties of control peptides 5 (A), 9 (B), and 10 (C), showing various aspects across different panels. The left panel shows the CD profile in water (black) and SDS micelles (red), alongside the structure in the original protein (purple), PepFold prediction (orange), and AlphaFold prediction (blue). In the right panel, membrane interactions are measured through the intrinsic fluorescence of tyrosine (5) and tryptophan (9 and 10), showing the decrease in fluorescence upon membrane binding. Additionally, the helical peptide regions in contact with or embedded in the membrane are displayed, as predicted by the FMAP model.
3. Conclusions
Our findings provide a general understanding of cationic IDR-derived AMPs. First, our results stress the importance of considering multiple factors, including amino acid composition, membrane binding, and structure. Overall, to exhibit antimicrobial properties, peptides must not only have a defined amino acid composition but a strong affinity for membranes and they need to adopt a specific structure upon binding. Our observations are supported by other studies on cationic IDR-derived AMPs, such as histatin-5 [26] and rEchAMP [27]. Experimental studies by NMR show that histatin-5 has an alpha-helix structure in dimyristoyl-phosphatidylcholine lipid vesicles [53], and the CD spectra for protein rEchAMP show an alpha-helix conformation in SDS micelles [27]. These observations support the idea that a structural rearrangement in membrane-like environments is required for AMP activity. However, peptides derived from IDRs, despite possessing compositions compatible with AMPs, appear to be largely unable to meet these criteria. Hence, it is reasonable to propose that IDRs in proteins may be evolutionarily constrained to remain disordered, and these constraints may prevent the peptides from satisfying the necessary conditions to function as AMPs. The higher prevalence of polar residues and the depletion in aromatic residues in IDRs may be a key factor to restrict antimicrobial activity. Additionally, our results indicate that AMP predictors should consider these factors. Even the most successful predictor, HydrAMP, failed to accurately predict 50% of the IDR-derived peptides, highlighting an existing bias in AMP predictors when dealing with disordered regions. This underscores the need for a more nuanced approach in AMP prediction, accounting for the unique characteristics of IDRs and their impact on antimicrobial activity. Considering properties like structural propensity, particularly the formation of α-helices, and the Gibbs free energy of binding (ΔG) in membrane models could be beneficial. Hence, employing tools, such as FMAP, that are capable of predicting the helical behavior or structural folding of peptides in various environments, could prove useful. Also, conducting a structural analysis of peptides using advanced computational methods like AlphaFold or PepFold could significantly enhance prediction accuracy prior to experimental validation. It is important to note that our results may not be applicable to anionic AMPs, and discrepancies could emerge, particularly when peptides fail to adopt alpha-helical conformations in membrane-like environments.
4. Experimental procedures
4.1. Materials
Peptides were synthesized by ProteoGenix (Schiltigheim, France). Escherichia coli was sourced from the Coli Genetic Stock Center (BW25113) and Pseudomonas aeruginosa ATCC 15692, Staphylococcus aureus ATCC 12600 and Micrococcus luteus ATCC 15307 was acquired from the Spanish Type Culture Collection (CECT, Valencia, Spain). The Escherichia coli Total Lipid Extract was purchased from Avanti Polar Lipids (Alabaster, AL).
4.2. Data preprocessing and antimicrobial activity prediction
To define the disordered regions in the human proteome, we used AlphaFold (AF) accessibility score, as reported by Norman Davey in https://github.com/normandavey/ProcessedAlphafold. Sequences matching the AMP criterion (length 10–25 residues, positive net charge) underwent further analysis and confirmation using peptide predictors, including CAMPR4 (http://camp.bicnirrh.res.in/) [54], AI4AMP (https://axp.iis.sinica.edu.tw/AI4AMP/) [55], AmpGram (http://biongram.biotech.uni.wroc.pl/AmpGram/) [56], HydrAMP (https://hydramp.mimuw.edu.pl/) [57], and sAMPpred (http://bliulab.net/sAMPpred-GAT/) [58]. The sequences selected for specific tissue analysis, were selected from the Protein Atlas (https://www.proteinatlas.org/) from those that are tissue-specific and show evidence both at the protein and transcript levels. Disordered regions in these sequences were evaluated using the MobiDB server (https://mobidb.bio.unipd.it/). Antimicrobial segments were identified using the antimicrobial peptide predictor AMPA (https://tcoffee.crg.eu/apps/ampa/do). The main differences between AMP predictors arise from three main aspects: the composition of the training databases, the algorithms used for classification, and the specific physicochemical and structural features used by the prediction algorithm. The training databases vary significantly in the number and diversity of peptides classified as active (AMPs) and inactive (non-AMPs), which is critical for model training and validation. The lack of sequences unequivocally identified as non-AMPs requires to resort to generated negative datasets through the use of random peptide generation tools. Regarding the algorithms used for classification, there is a broad spectrum that includes, but is not limited to, support vector machines, deep learning models, and graph neural networks. Each of these methodologies brings its own strengths and limitations, influenced by how they process data and identify patterns within the datasets. The selection of physicochemical properties and structural features considered for prediction also varies among different predictors. Feature selection is important since it directly influences the ability of the model to accurately distinguish between AMPs and non-AMPs based on their molecular characteristics. The cumulative effect of these differences has a notable impact on the prediction accuracy of the models. Evidence suggests that leveraging a combination of different predictors to establish a consensus score may enhance the reliability of AMP predictions [59]. This approach can mitigate the individual shortcomings of each, thereby providing a more robust framework for the identification of AMPs. A summary of the principal features of AMP predictors is included in Table 5.
Table 5.
Principal features of AMP predictor tools.
| Predictor | Training Datasets | Number of AMPs/non-AMPs | Algorithma | Features | Mode of generate negative dataset |
|---|---|---|---|---|---|
| CAMPR4 | InverPep, YADAMP, LAMP, C-PamP | 24.243/24.243 | RF (Best accuracy), SVM, ANN | Sequence-based composition and physicochemical properties of AMPs | 25 non-AMPs experimentally validated, non-secreted and random coil proteins from UniProt database and random peptides generation |
| AI4AMP | APD3, CAMPR3, DRAMP, LAMP | 6.623/6.623 | PC6 Deep learning | Physicochemical properties of AMPs | Preprocessed UniProt data and random peptides generation |
| HydrAMP | dbAMP, AMP Scanner, DRAMP | 11.131/11.131 | GNN | Sequence-based composition, predicted structural information and physicochemical properties of AMPs | Preprocessed UniProt data |
| AmpGram | APD3, CAMPR3, ADAM, PhytAMP, AMPer, AntiBP2, BACTIBASE, LAMP | 2.463/2.463 | RF | Sequence-based composition | Preprocessed UniProt data |
| sAMPpred-GAT | SATPdb, ADAM, AMPfun, APD3, CAMPR3, LAMP, DRAMP, dbAMP | 5.536/5.536 | GNN | Sequence-based composition, predicted structural information and physicochemical properties of AMPs | Preprocessed UniProt data |
RF: Random Forest, SVM: Support Vector Machine, ANN: Artificial Neural Networks and, GNN: Graph Neural Networks
The 14 synthesized peptides were selected according to the following criterion: peptides with the higher antibacterial propensity according to AMPA and validated by AMP predictors with the consensus metric. Original proteins belong to secreted proteins in the brain tissue with evidence at protein level, according to UniProt (Supplementary File 3).
4.3. Liposome preparation
The Escherichia coli Total Lipid Extract, at a concentration of 25 mg/mL, was diluted in chloroform to produce a 10 mg/mL solution. 200 μL of the lipid suspension were then placed in a temperature-controlled bath at 37 °C until complete solvent evaporation. The resulting lipid film was hydrated using 2 mL of phosphate-buffered saline (PBS: NaCl 136.8 mM, KH2PO4 2.7 mM, Na2HPO4 7.7 mM, pH 7.4), at 60 °C with continuous agitation for 1 h, achieving a final vesicle concentration of 1 mg/mL. The resulting vesicles were frozen and thawed repeatedly and homogenized through 11 extrusion cycles at 60 °C using polycarbonate membranes with a 0.4 µm pore size. This process yielded large unilamellar vesicles (LUVs) of a 0.4 µm defined size.
4.4. Minimum inhibitory concentration (MIC)
The antimicrobial activity was determined using the microdilution method endorsed by the National Committee of Laboratory Safety and Standards (NCLSS), adapted for AMPs [60]. In brief, overnight bacterial cell cultures were grown to an optical density (OD) of 0.5 at 600 nm in Mueller Hinton Broth (MHB; Condalab, Torrejón de Ardoz, Spain). Sequential 1:2 peptide dilutions were dispensed into sterile polypropylene 96-well plates (Greiner, Frickenhausen, Germany) with MHB medium that contained 0.2% (w/v) bovine serum albumin (BSA) and 0.02% glacial acetic acid, resulting in a final peptide concentration range of 0.1–100 µM. Then, the bacterial culture was added to each well to achieve a final concentration of 5 × 105 CFU/mL and the plates were incubated at 37 °C with orbital shaking at 250 rpm overnight. The minimum inhibitory concentration (MIC) was identified as the lowest peptide concentration that inhibited bacterial growth. Results represent the average of three independent experiments.
4.5. Circular dichroism
Circular dichroism (CD) experiments were conducted at room temperature using a J-815 CD spectropolarimeter (Jasco, Tokyo, Japan) with 1.0 nm bandwidth and a 1.0 mm optical path-length quartz cuvette. Peptides were dissolved in ultra-pure water and in 10 mM SDS solution, to simulate a membrane microenvironment [61], with a final peptide concentration of 50 μM. Spectra were recorded in the 190–250 nm range with 15 spectra accumulation to enhance the signal-to-noise ratio. Baselines of samples without peptides, either in water or micelle solutions, were subtracted to obtain the peptide contribution. Data was subsequently processed in OriginPro 2022, using the Savitzky–Golay method for smoothing. The molar mean ellipticity [θ] was calculated using:
Where L denotes the path length (cm) and [M] the peptide concentration (mol/L). The secondary structure estimation was performed by CDPro (https://sites.google.com/view/sreerama) using the CONTINLL algorithm with the reference protein sets 7 [62].
4.6. Fluorescence measurements
Experiments were performed on a Varian Cary Eclipse fluorescence spectrophotometer (Agilent, Santa Clara, California). Emission spectra for tyrosine (Tyr) and tryptophan (Trp) fluorescence were recorded at excitation wavelengths of 275 nm and 295 nm, respectively. The 295 nm excitation wavelength was chosen to capture Trp emission data without interference from the Tyr signal. Slit settings were 2 nm for excitation and between 5–10 nm for emission, depending on the sample. Emission spectra were collected from 295–345 nm and 305–395 nm for peptides containing Tyr and Trp, respectively, with a scan rate of 60 nm/min in a 10 × 10 mm cuvette with immediate stirring post-sample mixing. Intrinsic peptide fluorescence was measured both in the absence and presence of LUVs in PBS at 25 °C, with a final peptide to LUV ratio of 1:200. Spectra acquired in the presence of liposomes were corrected for light scattering by subtracting the LUV background. Data was processed using OriginPro 2022.
CRediT authorship contribution statement
Roberto Bello-Madruga: Data curation, Methodology, Validation, Formal analysis, Software, Investigation, Writing – original draft, Visualization, Writing – review & editing. Marc Torrent Burgas: Conceptualization, Supervision, Writing - original draft, Visualization, Writing – review & editing, Funding acquisition, Project administration.
Conflict of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Acknowledgments
This study was funded by a Research Grant 2022 of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the Spanish Ministerio de Ciencia e Innovación (PDC2021–121544-I00 reference MCIN/AEI/10.13039/501100011033 and European Union Next GenerationEU/ PRTR, and project PID2020–114627RB-I00 reference MCIN/AEI /10.13039/501100011033), all to MT. This work has been co-financed by the Spanish Ministry of Science and Innovation with funds from the European Union NextGenerationEU, from the Recovery, Transformation and Resilience Plan (PRTR-C17. I1) and from the Autonomous Community of Catalonia within the framework of the Biotechnology Plan Applied to Health. RB-M is a recipient of a FPI fellowship (PRE2021–097678).
Author contributions
MTB designed, directed, obtained funding for, and coordinated the study. RB-M performed the experiments. RB-M wrote an initial version of the manuscript, subsequently edited by MTB. All authors contributed to editing and revising the final version of the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.csbj.2024.02.008.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Wang L., et al. Therapeutic peptides: current applications and future directions. Signal Transduct Target Ther. 2022;7(1):48. doi: 10.1038/s41392-022-00904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayab S., et al. A review of antimicrobial peptides: its function, mode of action and therapeutic potential. Int J Pept Res Ther. 2022;28(1):46. [Google Scholar]
- 3.Mookherjee N., et al. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19(5):311–332. doi: 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A., et al. Antimicrobial peptides: a new hope in biomedical and pharmaceutical fields. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.668632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liepke C., et al. Human hemoglobin-derived peptides exhibit antimicrobial activity: a class of host defense peptides. J Chromatogr B Anal Technol Biomed Life Sci. 2003;791(1-2):345–356. doi: 10.1016/s1570-0232(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 6.Parish C.A., et al. Broad-spectrum antimicrobial activity of hemoglobin. Bioorg Med Chem. 2001;9(2):377–382. doi: 10.1016/s0968-0896(00)00263-7. [DOI] [PubMed] [Google Scholar]
- 7.Huang X., Li G. Antimicrobial peptides and cell-penetrating peptides: non-antibiotic membrane-targeting strategies against bacterial infections. Infect Drug Resist. 2023;16:1203–1219. doi: 10.2147/IDR.S396566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong H., et al. How do antimicrobial peptides disrupt the lipopolysaccharide membrane leaflet of Gram-negative bacteria? J Colloid Interface Sci. 2023;637:182–192. doi: 10.1016/j.jcis.2023.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Mahlapuu M., et al. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6 doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savini F., et al. Binding of an antimicrobial peptide to bacterial cells: Interaction with different species, strains and cellular components. Biochim Et Biophys Acta (BBA) - Biomembr. 2020;1862(8) doi: 10.1016/j.bbamem.2020.183291. [DOI] [PubMed] [Google Scholar]
- 11.Benfield A.H., Henriques S.T. Mode-of-action of antimicrobial peptides: membrane disruption vs. intracellular mechanisms. Front Med Technol. 2020;2 doi: 10.3389/fmedt.2020.610997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parchebafi A., et al. The dual interaction of antimicrobial peptides on bacteria and cancer cells; mechanism of action and therapeutic strategies of nanostructures. Microb Cell Factor. 2022;21(1):118. doi: 10.1186/s12934-022-01848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S., et al. The structure-mechanism relationship and mode of actions of antimicrobial peptides: a review. Trends Food Sci Technol. 2021;109:103–115. [Google Scholar]
- 14.Skvortsova P., et al. Spectroscopic study of antimicrobial peptides: structure and functional activity. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2022;264 doi: 10.1016/j.saa.2021.120273. [DOI] [PubMed] [Google Scholar]
- 15.Kang S.J., Nam S.H., Lee B.J. Engineering approaches for the development of antimicrobial peptide-based antibiotics. Antibiot (Basel) 2022;11(10) doi: 10.3390/antibiotics11101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabelka I., Vácha R. Advances in molecular understanding of α-helical membrane-active peptides. Acc Chem Res. 2021;54(9):2196–2204. doi: 10.1021/acs.accounts.1c00047. [DOI] [PubMed] [Google Scholar]
- 17.Lin L., et al. Membrane-disruptive peptides/peptidomimetics-based therapeutics: promising systems to combat bacteria and cancer in the drug-resistant era. Acta Pharm Sin B. 2021 doi: 10.1016/j.apsb.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei J., et al. The antimicrobial peptides and their potential clinical applications. Am J Transl Res. 2019;11(7):3919–3931. [PMC free article] [PubMed] [Google Scholar]
- 19.Huan Y., et al. Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ando T. In: Intrinsically Disordered Proteins (IDPs), in High-Speed Atomic Force Microscopy in Biology: Directly Watching Dynamics of Biomolecules. Action T.Ando., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2022. pp. 201–225. [Google Scholar]
- 21.Uversky V.N., Oldfield C.J., Dunker A.K. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37:215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 22.Rani P., Baruah A., Biswas P. Does lack of secondary structure imply intrinsic disorder in proteins? A sequence analysis. Biochim Biophys Acta. 2014;1844(10):1827–1834. doi: 10.1016/j.bbapap.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Piovesan D., Monzon A.M., Tosatto S.C.E. Intrinsic protein disorder and conditional folding in AlphaFoldDB. Protein Sci. 2022;31(11) doi: 10.1002/pro.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alderson T.R., et al. Systematic identification of conditionally folded intrinsically disordered regions by AlphaFold2. Proc Natl Acad Sci. 2023;120(44) doi: 10.1073/pnas.2304302120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latendorf T., et al. Cationic intrinsically disordered antimicrobial peptides (CIDAMPs) represent a new paradigm of innate defense with a potential for novel anti-infectives. Sci Rep. 2019;9(1):3331. doi: 10.1038/s41598-019-39219-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCaslin T.G., et al. Specific metallo-protein interactions and antimicrobial activity in Histatin-5, an intrinsically disordered salivary peptide. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-52676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A., et al. Structural and mechanistic insights into EchAMP: a antimicrobial protein from the Echidna milk. Biochim Et Biophys Acta (BBA) - Biomembr. 2019;1861(6):1260–1274. doi: 10.1016/j.bbamem.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Theillet F.X., et al. The alphabet of intrinsic disorder: I. Act like a Pro: on the abundance and roles of proline residues in intrinsically disordered proteins. Intrinsically Disord Proteins. 2013;1(1) doi: 10.4161/idp.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quaglia F., et al. DisProt in 2022: improved quality and accessibility of protein intrinsic disorder annotation. Nucleic Acids Res. 2022;50(D1):D480–d487. doi: 10.1093/nar/gkab1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrent M., Nogués V.M., Boix E. A theoretical approach to spot active regions in antimicrobial proteins. BMC Bioinformatics. 2009;10:373. doi: 10.1186/1471-2105-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piovesan D., et al. MobiDB: 10 years of intrinsically disordered proteins. Nucleic Acids Res. 2023;51(D1):D438–d444. doi: 10.1093/nar/gkac1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrent M., et al. AMPA: an automated web server for prediction of protein antimicrobial regions. Bioinformatics. 2011;28(1):130–131. doi: 10.1093/bioinformatics/btr604. [DOI] [PubMed] [Google Scholar]
- 33.Bello-Madruga R., et al. The C-terminus of panusin, a lobster β-defensin, is crucial for optimal antimicrobial activity and serum stability. Pharmaceutics. 2023;15(6) doi: 10.3390/pharmaceutics15061777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leszczyńska K., et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J Antimicrob Chemother. 2012;68(3):610–618. doi: 10.1093/jac/dks434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thévenet P., et al. PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012;40(W1):W288–W293. doi: 10.1093/nar/gks419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamiable A., et al. PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016;44(W1):W449–W454. doi: 10.1093/nar/gkw329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald E.F., et al. Benchmarking AlphaFold2 on peptide structure prediction. Structure. 2023;31(1):111–119.e2. doi: 10.1016/j.str.2022.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jumper J., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirdita M., et al. ColabFold: making protein folding accessible to all. Nat Methods. 2022;19(6):679–682. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenfield N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1(6):2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micsonai A., et al. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc Natl Acad Sci. 2015;112(24):E3095–E3103. doi: 10.1073/pnas.1500851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavelka A., et al. Recombinant production of human antimicrobial peptide LL- 37 and its secondary structure. Biologia. 2023 [Google Scholar]
- 43.Oliveira N.G.J., et al. Physicochemical-guided design of cathelicidin-derived peptides generates membrane active variants with therapeutic potential. Sci Rep. 2020;10(1):9127. doi: 10.1038/s41598-020-66164-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y., Huang J., Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell. 2010;1(2):143–152. doi: 10.1007/s13238-010-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S.-K., et al. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci Rep. 2016;6(1) doi: 10.1038/srep27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lomize A.L., Hage J.M., Pogozheva I.D. Membranome 2.0: database for proteome-wide profiling of bitopic proteins and their dimers. Bioinformatics. 2018;34(6):1061–1062. doi: 10.1093/bioinformatics/btx720. [DOI] [PubMed] [Google Scholar]
- 47.Lomize A.L., et al. Membranome: a database for proteome-wide analysis of single-pass membrane proteins. Nucleic Acids Res. 2017;45(D1):D250–d255. doi: 10.1093/nar/gkw712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayyed-Ahmad A., Khandelia H., Kaznessis Y.N. Relative free energy of binding between antimicrobial peptides and SDS or DPC micelles. Mol Simul. 2009;35(10-11):986–997. doi: 10.1080/08927020902902742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrushchenko V.V., et al. Thermodynamics of the interactions of tryptophan-rich cathelicidin antimicrobial peptides with model and natural membranes. Biochim Et Biophys Acta (BBA) - Biomembr. 2008;1778(4):1004–1014. doi: 10.1016/j.bbamem.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Torrent M., et al. Topography studies on the membrane interaction mechanism of the eosinophil cationic protein. Biochemistry. 2007;46(3):720–733. doi: 10.1021/bi061190e. [DOI] [PubMed] [Google Scholar]
- 51.Ambrosio R.L., et al. The antimicrobial peptide 1018-K6 interacts distinctly with eukaryotic and bacterial membranes, the basis of its specificity and bactericidal activity. Int J Mol Sci. 2022;23(20) doi: 10.3390/ijms232012392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anselmo S., et al. Peptide–membrane interactions monitored by fluorescence lifetime imaging: a study case of transportan 10. Langmuir. 2021;37(44):13148–13159. doi: 10.1021/acs.langmuir.1c02392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raj P.A., Marcus E., Sukumaran D.K. Structure of human salivary histatin 5 in aqueous and nonaqueous solutions. Biopolymers. 1998;45(1):51–67. doi: 10.1002/(SICI)1097-0282(199801)45:1<51::AID-BIP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 54.Gawde U., et al. CAMPR4: a database of natural and synthetic antimicrobial peptides. Nucleic Acids Res. 2022;51(D1):D377–D383. doi: 10.1093/nar/gkac933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin T.T., et al. AI4AMP: an antimicrobial peptide predictor using physicochemical property-based encoding method and deep learning. mSystems. 2021;6(6) doi: 10.1128/mSystems.00299-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burdukiewicz M., et al. Proteomic screening for prediction and design of antimicrobial peptides with AmpGram. Int J Mol Sci. 2020;21(12):4310. doi: 10.3390/ijms21124310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szymczak P., et al. Discovering highly potent antimicrobial peptides with deep generative model HydrAMP. Nat Commun. 2023;14(1):1453. doi: 10.1038/s41467-023-36994-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan K., et al. sAMPpred-GAT: prediction of antimicrobial peptide by graph attention network and predicted peptide structure. Bioinformatics. 2022;39(1) doi: 10.1093/bioinformatics/btac715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y., et al. Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat Biotechnol. 2022;40(6):921–931. doi: 10.1038/s41587-022-01226-0. [DOI] [PubMed] [Google Scholar]
- 60.Sandín D., et al. Rationally modified antimicrobial peptides from the N-terminal domain of human RNase 3 show exceptional serum stability. J Med Chem. 2021;64(15):11472–11482. doi: 10.1021/acs.jmedchem.1c00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perinelli D.R., et al. Surfactant self-assembling and critical micelle concentration: one approach fits all? Langmuir. 2020;36(21):5745–5753. doi: 10.1021/acs.langmuir.0c00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sreerama N., Woody R.W. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000;287(2):252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material