Abstract
Background
Congenital uterine anomalies (CUA) can be associated with impairments of early and late pregnancy events.
Objective
To assess the impact of CUA on reproductive outcomes in pregnancies conceived spontaneously or after assisted reproduction.
Material and Methods
Systematic review and meta-analysis of cohort studies comparing patients with CUA versus women with normal uterus. A structured literature search was performed in leading scientific databases to identify prospective and retrospective studies. The Newcastle-Ottawa scale, adapted to AHRQ standards, was used to assess the risk of bias. Pooled odds ratios (OR) were calculated. Publication bias and statistical heterogeneity were assessed, and meta-regression was used to analyse the heterogeneity.
Main outcome measures
Miscarriage, ectopic pregnancy, placental abruption, term, and premature rupture of membranes (PROM), malpresentation at delivery, preterm delivery prior to 37, 34 and 32 weeks, caesarean delivery, intrauterine growth restriction/small for gestational age, foetal mortality and perinatal mortality.
Results
32 studies were included. CUAs increased significantly the risk of first/second trimester miscarriage (OR:1.54;95%CI:1.14-2.07), placental abruption (OR:5.04;3.60-7.04), PROM (OR:1.71;1.34-2.18), foetal malpresentation at delivery (OR:21.04;10.95-40.44), preterm birth (adjusted OR:4.34;3.59-5.21), a caesarean delivery (adjusted OR:7.69;4.17-14.29), intrauterine growth restriction/small for gestational age (adjusted OR:50;6.11-424), foetal mortality (OR:2.07;1.56-2.73) and perinatal mortality (OR:3.28;2.01-5.36).
Conclusions
CUA increases the risk of complications during pregnancy, delivery, and postpartum. Complications most frequent in CUA patients were preterm delivery, foetal malpresentation, and caesarean delivery.
What is new?
Bicornuate uterus was associated with the highest number of adverse outcomes, followed by didelphys, subseptate and septate uterus.
Keywords: Congenital uterine anomalies, Müllerian anomalies, pregnancy outcome, obstetric complications, labour complications, neonatal outcome
Introduction
Congenital uterine anomalies (CUA) are uncommon entities caused by abnormal development, fusion, or resorption of Müllerian ducts during organogenesis, which results in defects in canalisation, unification, or conformation of Müllerian-derived structures. Their prevalence is difficult to assess, due to the lack of a universally accepted classification and differences in diagnostic methods or the population profiles reported in the available studies. A systematic review published by Chan et al. (2011b) estimated that CUA are present in 5.5% of infertile patients, in 13.3% of women with previous miscarriage and in 24.5% of women affected by both conditions.
CUA comprises a broad spectrum of congenital defects, characterised by different degrees of distortion of the uterine anatomy, which could generate different levels of perinatal risk. The association with reproductive outcomes has been extensively analysed. Until now, four systematic reviews on the association between CUA and obstetrical and perinatal risks have been published (Chan et al., 2011a; Grimbizis et al., 2001; Kim et al., 2021; Venetis et al., 2014). However, they include observational studies which are affected considerably by the risk of bias. Recently, several studies looking at the correlation between CUA and adverse pregnancy outcomes have been published.
The objective of this systematic review with meta-analysis is to evaluate the association between CUA with adverse pregnancy outcomes. Eligibility of studies will follow the actual criteria for risk of bias assessment of observational studies that complement score-based scales with domain-based evaluation, particularly concerning comparability between exposed and non-exposed patients.
Materials and methods
Protocol registration
The systematic review and meta-analysis protocol were defined according to MOOSE guidelines and registered at PROSPERO (CRD42023380794).
Study selection
For eligibility, the following inclusion criteria were applied: a) Prospective and retrospective cohort studies analysing the effects of CUA on obstetrical and perinatal outcomes in spontaneous or ART pregnancies in patients affected by infertility, recurrent pregnancy loss, or general population; b) Fair or high quality studies with an adequate level of comparability between exposed and non-exposed patients; c) Peer-reviewed articles published in English, French, German or Spanish between January 1980 and April 2022.
As exclusion criteria we considered case-control design, insufficient information on the population considered or on diagnostic techniques used, lack of adjustment for potential confounders, studies considering comparisons other than those of interest, and non-comparative studies.
Information sources and searches
A systematic review of primary studies was carried out by investigators in well-recognised scientific databases (MEDLINE, EMBASE, Current Contents, Web of Science, and Cochrane Database Register for Clinical Trials, ClinicalTrials.gov and Google Scholar). Search terms and limits are provided in Appendix 1 and were adapted to specific syntax in the different databases. Cross references were hand-searched.
Studies selection and individual risk of bias assessment
Identified studies were initially classified according to title and abstract by two different authors. Studies concordantly selected were full text evaluated, and discrepancies were solved by consensus with a third evaluator.
Eligibility criteria were applied to the studies selected for full-text evaluation. Risk of bias was assessed using Newcastle-Ottawa Scale (NOS) for cohort studies, and qualified according to the standards of United States Agency for Healthcare Research and Quality (AHRQ) (Wells et al., 2021). Only studies qualified as good or fair quality (granted with 1 or 2 stars in the comparability domain), were included, considering age and parity as main potential confounders for all outcomes analysis. Reasons for exclusion were discussed and summarised. The studies selection process was in accordance with MOOSE Statement recommendations for systematic reviews (Stroup et al., 2000).
Data collection, outcomes, and summary measures
Data extraction from selected studies was performed by one of the authors and verified by a co-author, using a pre-designed form. Definitions and classification categories for CUA used by authors were specifically checked.
We considered as outcomes first and second trimester miscarriage, ectopic pregnancy, placental abruption, term, and preterm premature rupture of membranes (PROM/PPROM) foetal malposition or abnormal presentation at delivery, preterm delivery, preterm delivery prior to 34 and prior to 32 weeks, caesarean delivery, intrauterine growth restriction or small for gestational age (IUGR/ SGA), foetal mortality and perinatal mortality. Odds ratios of these outcomes were considered as summary measures.
Statistical analysis
Articles favourable for quantitative synthesis were meta-analysed applying a random-effects model (DerSimonian and Kacker, 2007). Pooled odds ratios (OR) and its 95% confidence interval were used as pooled effect measures.
Statistic heterogeneity was estimated by Cochran’s Q and I2 statistics. Q statistics with p values <0.05 was considered statistically significant. I2 values were evaluated considering critical thresholds previously defined (Borenstein et al., 2021; Higgins and Thompson, 2002).
Publication bias was assessed through funnel plots for each outcome. In case of obtaining non- conclusive plots, we applied Begg’s and Egger’s tests. Meta-regression based on random-effects models (Borenstein et al., 2021) was applied to adjust the effect on overall estimates affected by high statistical heterogeneity (I2 >50%), considering as covariates the type of cohort-study design (classical versus matched controls cohort studies), studied population (general population, infertile patients and previous pregnancy loss history) and type of pregnancy (singleton or multiple).
Review Manager 5.4.1 was used to calculate pooled estimated effects and heterogeneity and Stata software 17 was used for publication-bias and meta-regression analysis.
Results
Systematic review
The structured searches identified 12794 reports. From these, 6461 were screened by title and 328 by title and abstract. A final subset of 78 studies were full-text evaluated (Figure 1), of which 32 (Ban-Frangez et al., 2009; Ben-Rafael et al., 1991; Cahen-Peretz et al., 2017; Cai et al., 2021; Cooney et al., 1998; Crane et al., 2012; Chen et al., 2018; Chen et al., 2019; Erez et al., 2007; Hiersch et al., 2016; Hua et al., 2011; Jayaprakasan et al., 2011; Kong et al., 2021; Leible et al., 1998; Li et al., 2017; Lu et al., 2021; Marianna et al., 2022; Mastrolia et al., 2017; Mastrolia et al., 2018; Ouyang et al., 2020; Ozgur et al., 2017; Pleş et al., 2018; Prior et al., 2018; Qiu et al., 2022; Saravelos et al., 2010; Sugiura-Ogasawara et al., 2010; Surrey et al., 2018; Takami et al., 2014; Tomaževič et al., 2010; Zambrotta et al., 2021; Zlopasa et al., 2007) fulfilled inclusion criteria (Table SI). Scores granted by Newcastle-Ottawa Score and quality assessment using AHRQ standards are presented in Appendix 2. Reasons for exclusion of non-included studies (Acién, 1993; Acién et al., 2014; Airoldi et al., 2005; Akar et al., 2005; Alonso Pacheco et al., 2019; Ben-Rafael et al., 1990; Colacurci et al., 1996; Chen et al., 2013; Elsokkary et al., 2018; Fedele and Bianchi, 1995; Fox et al., 2019; Fox et al., 2014; Gabbai et al., 2018; Ghi et al., 2012; Grimbizis et al., 2001; Hynes et al., 2021; Jaslow and Kutteh, 2013; Lavergne et al., 1996; Liang and Hu, 2010; Ludwin, 2018; Maneschi et al., 1995; Neal et al., 2019; Portuondo et al., 1986; Raga et al., 1997; Ravasia et al., 1999; Ridout et al., 2019; Rogers and Needham, 1985; Salim et al., 2003; Sendag et al., 2010; Shuiqing et al., 2002; Sorensen and Trauelsen, 1987; Sugiura-Ogasawara et al., 2015; Tofoski and Antovska, 2014; Tomazevic et al., 2007; Tonguc et al., 2011; Woelfer et al., 2001; Zhang et al., 2010; Zupi et al., 1996) are described in Table SII.
Figure 1.
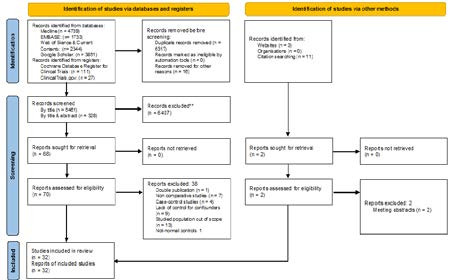
PRISMA flowchart diagram.
Meta-analysis
Miscarriage
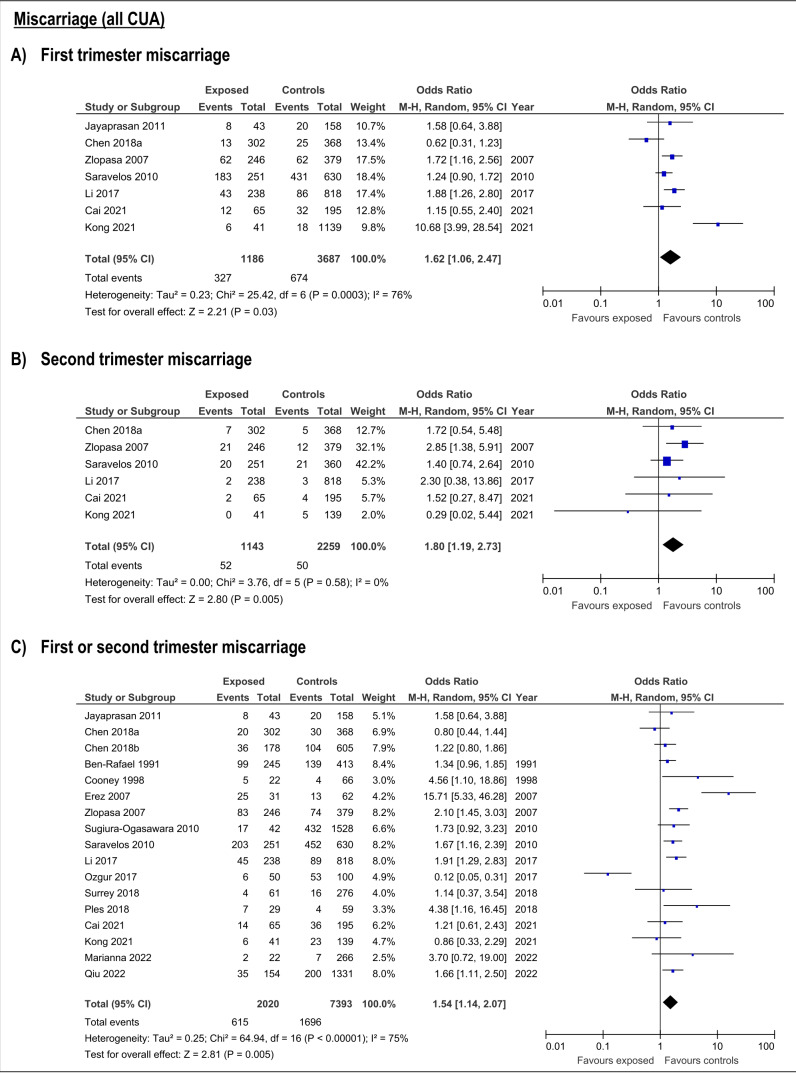
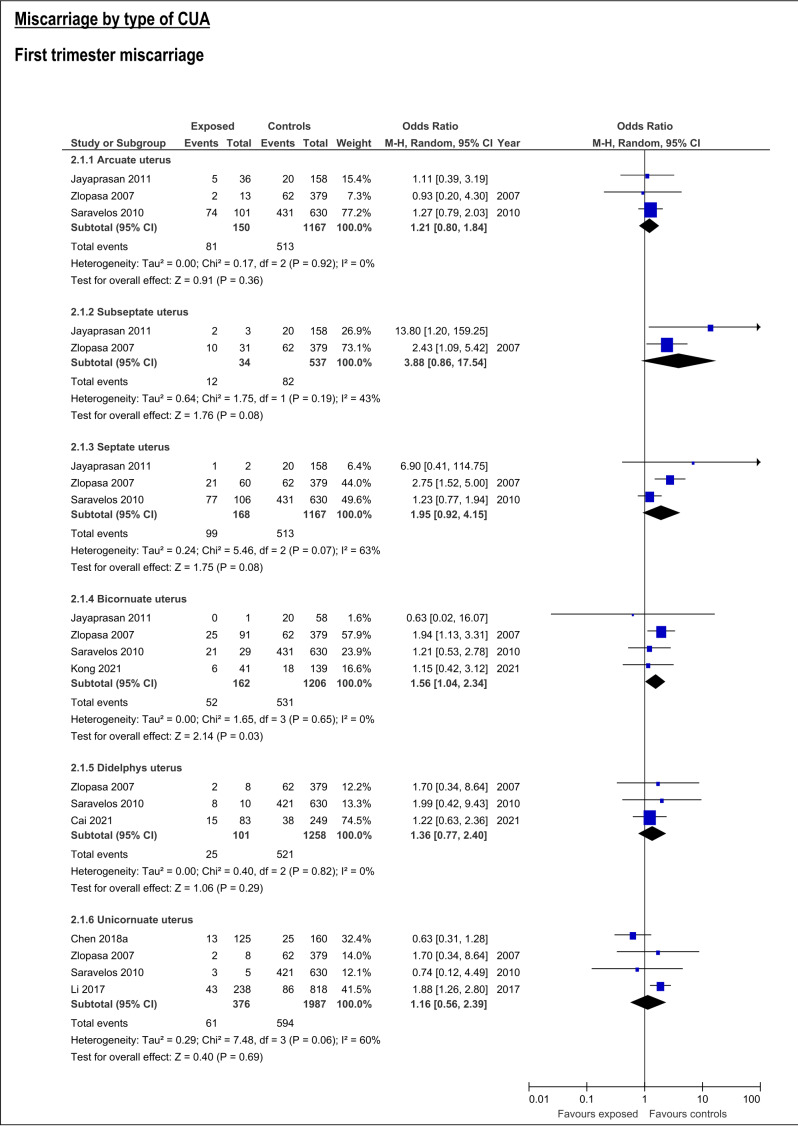
CUA increased the risk of first trimester miscarriage (OR:1.62, 95%CI:1.06-2.47; 7 studies; I2:76%) (Table SIII; Figure 2. This risk was only detectable for bicornuate uterus (OR:1.56; 95%CI:1.04-2.34, 4 studies; I2:0%), and resulted not significant for arcuate, septate, subseptate, didelphys and unicornuate uterus (Table SIII; Figure 3).
Figure 2.
Forest plots of individual and pooled effects of CUA (combined) on first (A), second trimester (B) and any trimester (C) miscarriage risk.
Figure 3.
Forest plots of individual and pooled effects on first trimester miscarriage by type of CUA.
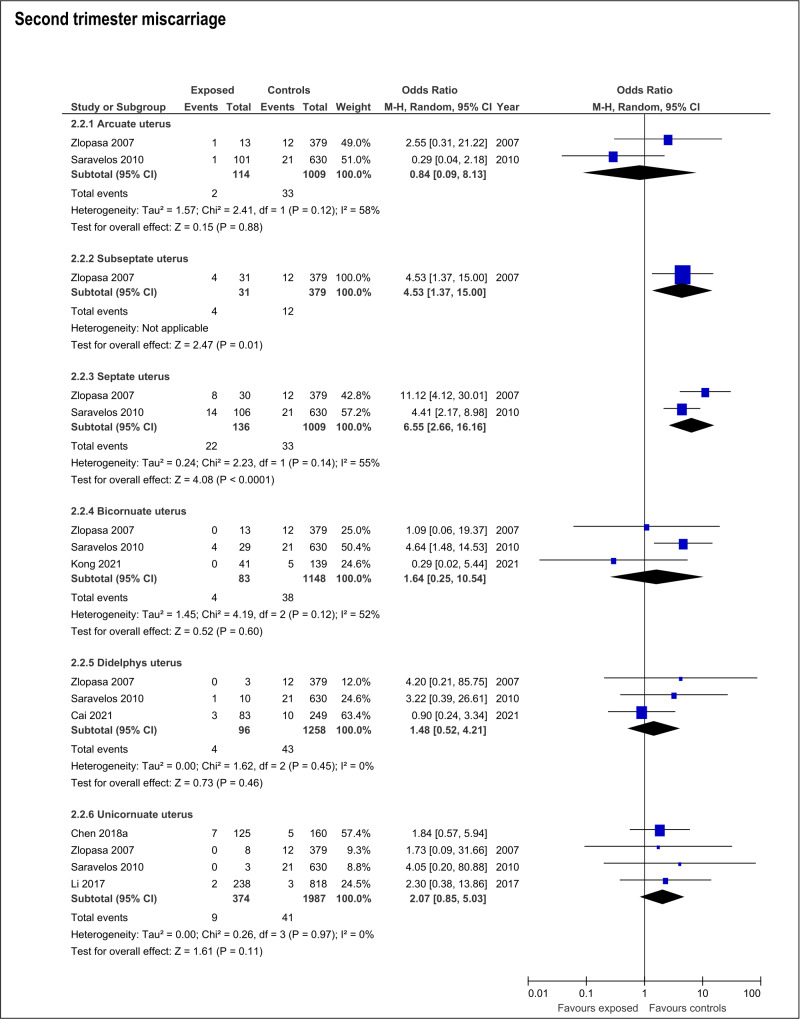
The presence of any CUA increased risk of second trimester miscarriage (OR:1.8; 95%CI:1.19- 2.73; 6 studies; I2:0%) (Table SIII; Figure 2). This risk was only present with septate uteri (OR:6.65; 95%CI:2.66-16.16; 2 studies; I2:55%). The estimated effect of the subseptate uterus on this outcome derived from a single study (OR:4.53; 95%CI:1.37-15.0) (Zlopasa et al., 2007). The rest of the evaluated anomalies (arcuate, didelphys, bicornuate and unicornuate uterus) showed no association with risk of second trimester miscarriage (Table SIII; Figure 4).
Figure 4.
Forest plots of individual and pooled effects on second trimester miscarriage by type of CUA.
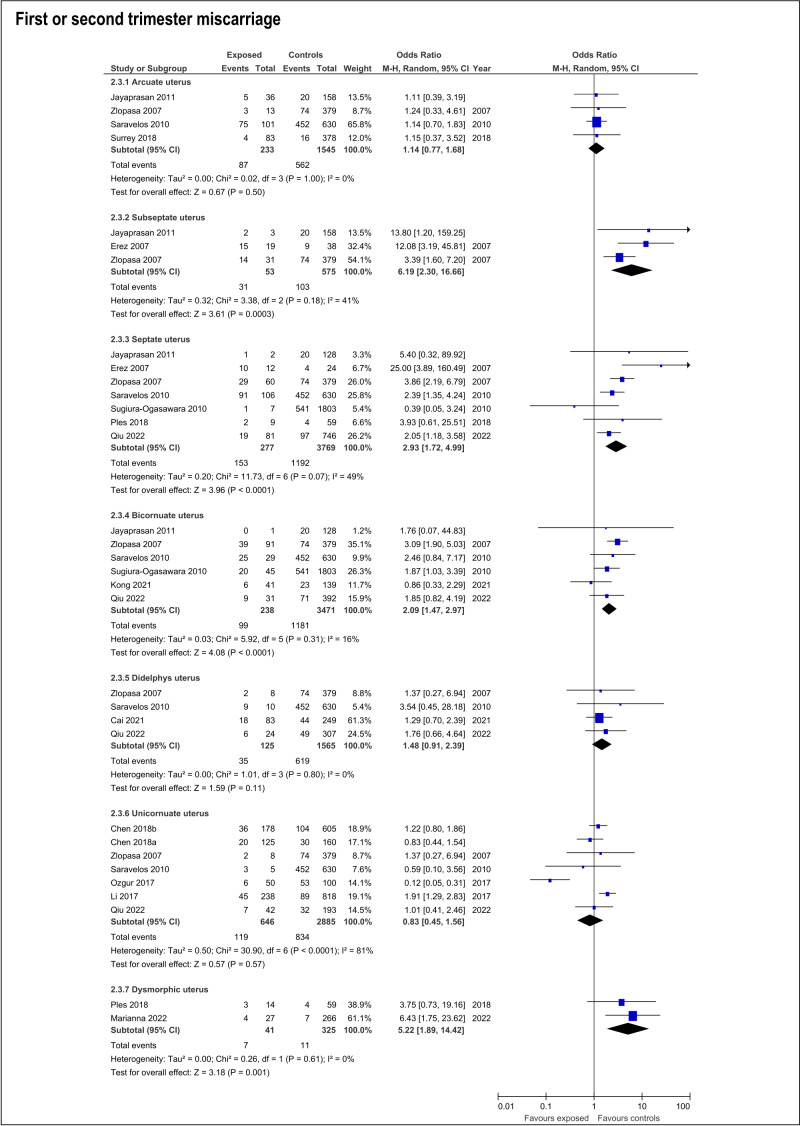
The risk of miscarriage in any trimester increases in the presence of any CUA (OR:1.54; 95%CI:1.14- 2.07; 17 studies; I2: 75%) (Table SIII; ). The specific anomalies that have increased risk of miscarriage in the first or second trimester were subseptate uterus (OR:6.19; 95%CI:2.3-16.66; 3 studies; I2:41%), septate (OR:2.93; 95%CI:1.72-4.99; 7 studies; I2:49%), bicornuate (OR:2.09; 95%CI:1.47-2.97; 6 studies; I2:16%) and T-shaped uterus (OR:5.22; 95%CI:1.89-14.42). On the contrary, arcuate, didelphys and unicornuate uterus did not increase the risk of miscarriage (Table SIII; Figure 5).
Figure 5.
Forest plots of individual and pooled effects on any trimester miscarriage by type of CUA.
Ectopic pregnancy
Ectopic pregnancy is not increased in patients with CUA (OR:1.30; 95%CI:0.82-2.05; 6 studies; I2:0%) (Table SIII; Figure S1). When analysed by type of anomaly, only septate uteri had a significant increased risk of ectopic pregnancy (OR:2.04; 95%CI:2.03-4.04, 2 studies; I2:49%) (Qiu et al., 2022; Saravelos et al., 2010). Effects of arcuate, and T-shaped uterus were estimated each one from a single study (Marianna et al., 2022; Saravelos et al., 2010), whereas effects of didelphys, bicornuate and unicornuate uterus derived from the data of two (Qiu et al., 2022; Saravelos et al., 2010), three (Kong et al., 2021; Qiu et al., 2022; Saravelos et al., 2010) and four studies (Chen X. et al., 2018; Li et al., 2017; Qiu et al., 2022; Saravelos et al., 2010) respectively (Table SIII; Figure S2).
Placental abruption
Presence of any CUA increased risk of placental abruption (OR:5.04; 95%CI:3.66-7.04; 6 studies; I2:40%) (Table SIII; Figure S3). This event is more frequent in patients with subseptate (OR:17.45; 95%CI:5.05-60.22; 1 study) and bicornuate uterus (OR:12.11; 95%CI:3.14-46.74; 2 studies; I2:81%). No effects of septate, didelphys and unicornuate uterus were found analysing data from a single study (Takami et al., 2014) (Table SIII; Figure S4).
PROM/PPROM
Combined PROM-PPROM risk was increased in patients with CUA (OR:1.71; 95%CI:1.34-2.18; 9 studies; I2:65%) (Table SIII; Figure S5), specifically with bicornuate uterus (OR:1.79; 95%CI: 1.37- 2.33; 2 studies; I2: 0%). No effect was detected for unicornuate uterus (OR:0.46; 95%CI:0.18-1.21, 1 study) (Lu et al., 2021) (Table SIII; Figure S6).
Fetal Malpresentation at delivery
Fetal malpresentation at the time of delivery was consistently increased in women with CUA (OR:21.04; 95%CI:10.95-40.44; 7 studies; I2:97%) (Table SIII; Figure S7). Arcuate uterus (OR:11.38; 95%CI:1.49-87.07; 2 studies; I2:41%), subseptate uterus (OR:25.62; 95%CI:10.69-60.85; 2 studies; I2:25%), septate uterus (OR:45.48; 95%CI:16.97- 121.89; 2 studies; I2:0%), uterus didelphys (OR:19.15; 95%CI:15.16-24.18; 3 studies; I2:0%), unicornuate (OR:32.74; 95%CI:6.21-172.67; 3 studies; I2:53%) and bicornuate uterus (OR:17.96; 95%CI:12.19-26.47; 3 studies; I2:27%) (Table SIII; Figure S8).
Preterm delivery
The risk of preterm delivery is higher in patients with CUA, both in the global analysis (adjusted OR:4.34; 95%CI:3.59-5.21; 19 studies; I2:56%) and for most anomalies. Estimated OR of preterm birth were 8.91 (95%CI:3.1-25.63) for arcuate uterus (2 studies; I2: 0%), 5.24 (95%CI:1.87- 14.67) for subseptate uterus (2 studies; I2:58%), 4.62 (95%CI:2.43-8.8) for didelphys uterus (7 studies; I2:74%) and 4.45 (95%CI:1.29-15.5) for T-shaped uterus (1 study). Significant Adjusted OR were found for bicornuate (OR:4.9; 95%CI:3.93- 6.11; 7 studies; I2:8%) and unicornuate uterus (OR:3.85; 95%CI:1.84- 8.16; 8 studies; I2:0%). After adjustment, the effect of septate on the risk of preterm delivery became not significant (adjusted OR:1.04 (95%CI: 0.51-2.01; 5 studies; I2:0%) (Table SIII; Figure S9 and Figure S10).
Preterm birth before 34 weeks of gestational age was also more frequent in patients with CUA (OR:5.36; 95%CI:4.29-6.7; 6 studies; I2:12%). The risk of prematurity prior to 34 weeks was increased in presence of didelphys uterus (OR:53.78; 95%CI:5.43-532.94; 1 study) and bicornuate uterus (OR:11.34; 95%CI:1.14-112.75, 1 study) (Crane et al., 2012). No significant effect of septate and unicornuate uterus were detected, and no estimates were available for arcuate, subseptate and T-shaped uterus (Table SIII; Figure S9 and Figure S11).
The rate of preterm delivery before 32 weeks was not affected by CUA (adjusted OR:1.64; 95%CI:0.91-2.97; 6 studies; I2:0%). Septate, didelphys, bicornuate and unicornuate and uterus did not show association with this outcome (Table SIII; Figure S9 and Figure S12). Data to estimate effects of arcuate, subseptate and T-shaped uterus were not available.
Caesarean delivery
Compared to women with normal uterus, the caesarean delivery rate was higher in patients with any type of CUA (adjusted OR:7.69; 95%CI:4.17- 14.29; 16 studies; I2:96%) (Table SIII; Figure S13). Caesarean rate was also increased in patients with subseptate uterus (OR:11.27; 95%CI:3.01-42.23; 2 studies; I2:58%), uterus didelphys (adjusted OR:29.9; 95%CI:8.24-126.4; 6 studies; I2:75%), bicornuate (adjusted OR:23.8; 95%CI:10.17-55.7; 6 studies; I2: 46%) and unicornuate uterus (adjusted OR:12.1; 95%CI:5.64-26.5; 6 studies; I2: 0%). Arcuate and septate uterus showed no association with caesarean delivery rate (Table SIII; Figure S14). No estimations for T-shaped effect were available.
IUGR/SGA
The risk of IUGR or SGA, considered as a combined outcome, was higher in patients affected by CUA (adjusted OR:50.0; 95%CI:6.11-424; 9 studies; I2:83%) (Table SIII; Figure S15). The rates of IUGR/ SGA were increased in pregnancies of women with subseptate (OR:2.54; 95%CI:1.10-5.89; 2 studies; I2:0%), didelphys (OR:3.82; 95%CI:1.93-7.56; 3 studies; I2:36%), and bicornuate uteri (OR:2.75; CI95%: 1.96-3.86; 4 studies; I2:0%). Arcuate, septate and unicornuate uteri were not associated with this outcome (Table SIII; Figure S16).
Foetal and perinatal mortality
Risk of foetal mortality was increased in patients with CUA (OR:2.07; 95%CI:1.56-2.73; 9 studies; I2:10%) (Table SIII; Figure S17). Foetal demise was also more frequent in the presence of uterus didelphys (OR:2.67; 95%CI:1.29-5.51; 3 studies; I2:0%), bicornuate (OR:3.46; 95%CI:2.0-5.99; 3 studies; I2:0%) and unicornuate (OR:2.36; 95%CI:1.23-4.54; 3 studies; I2:0%). Arcuate, subseptate and septate uteri were not associated with increased risk of foetal demise (Table SIII; Figure S18).
Perinatal mortality was higher in women diagnosed with any CUA (OR:3.28; 95%CI:2.01- 5.36; 6 studies; I2:56%) (Table SIII; Figure S19). Specifically, the didelphys (OR:6.69; 95%CI:1.59- 28.15; 2 studies; I2:25%), bicornuate (OR:4.25; 95%CI:1.56-11.6; 2 studies; I2:0%) and unicornuate uterus (OR:3.05; 95%CI:1.75-5.31; 3 studies; I2:0%). No association was found between arcuate, septate, subseptate uterus and increased perinatal mortality (Table SIII; Figure S20).
Analyses of funnel-plot and results of Begg’s and Egger’s tests, performed, when necessary, did not reveal relevant risk of reporting bias.
Discussion
Summary of main results
This meta-analytic review supports the association between CUA and adverse obstetrical and perinatal outcomes. Considering the different types of CUA individually, most frequent defects can be classified as U1 (T-shaped), U2 (septate or subseptate), U3 (bicorporate) and U4 (categories of ESGE classification were associated to relevant adverse outcomes. Both septate and subseptate uterus, as well as bicornuate and didelphys uterus, increased risks of miscarriage, preterm birth, foetal malpresentation at delivery, IUGR and need for caesarean delivery. By contrast, arcuate and unicornuate uterus were associated with a significantly lower number of adverse outcomes.
Comparison with previous studies
Our meta-analysis included 32 studies, which is more than those selected by Chan et al. (2011a) and Venetis et al. (2014), although less than those included in the 2021 Kim’s meta-analysis. Twelve of the studies selected by Kim et al. (2021) were not included in our meta-analysis: one was published out of time limits (Forde et al., 1978) while the other eleven did not achieve the minimum score on the AHRQ-adapted NOS scale (Acién, 1993; Fox et al., 2014; Liang and Hu, 2010; Maneschi et al., 1995; Neal et al., 2019; Ravasia et al., 1999; Shuiqing et al., 2002; Sorensen and Trauelsen, 1987; Woelfer et al., 2001; Zhang et al., 2010; Zupi et al., 1996). Our meta-analysis includes seven studies not considered by Kim et al. (2021) (Cai et al., 2021; Crane et al., 2012; Chen et al., 2018; Lu et al., 2021; Marianna et al., 2022; Qiu et al., 2022; Surrey et al., 2018).
We have detected a significantly increased risk in all the outcomes analysed, with the exception of ectopic pregnancy. We have identified a higher rate of caesarean delivery and intrauterine foetal death, which is consistent with Kim et al. (2021). Our results also support an increased risk of IUGR/ SGA, in line with Kim et al. (2021) but in contrast to Venetis et al. (2014). In our analysis, the pooled effects for several outcomes affected by high levels of statistical heterogeneity, which were not reduced by meta-regression, and that are similar to those estimated in Kim’s in his meta-analysis (Kim et al., 2021).
Canalisation anomalies has been consistently associated with increased risk of miscarriage, as concluded in our meta-analysis and those by Chan et al. (2011a), Venetis et al. (2014), and Kim et al. (2021). According to our results, septate uterus was associated with second trimester but not with first trimester miscarriage, in contrast with the estimation of previous meta-analysis. Our estimation on association of septate uterus on first trimester miscarriage risk is based in pooled results of three good quality studies, which totalled 612 events and 1335 patients. On the contrary, previous meta-analysis included studies performed on small samples and/or excluded from our criteria by low quality scoring. For subseptate uterus, we identified only an increased risk of second trimester miscarriage, contrary to Kim et al. (2021).
Bicornuate uterus is the unification defect most commonly associated with risk of miscarriage, as our study and those of Chan et al. (2011a), Kim et al. (2021), and Venetis et al. (2014) reported. With regard to the pathogenic implication of each type of defect in gestational loss, it should be recalled that bicornuate uterus and the septate/ subseptate uterus share to some extent certain similar characteristics that may explain their causal association with pregnancy loss, such as reduced volume and distensibility of the uterine cavity or abnormal vascularization (Venetis et al., 2014). None of the meta-analyses have found a correlation between uterus didelphys and the risk of miscarriage. Unicornuate uterus seems related to miscarriage according to Venetis et al. (2014), and with first trimester miscarriage as described Chan et al. (2011a).
We found no association between arcuate uterus and risks of miscarriage, which differs from what was previously reported by Chan et al. (2011a), Kim et al. (2021), Venetis et al. (2014). However, this result could be biased by the difficulty in discriminating arcuate uterus from normal or subseptate uterus, due to changes in diagnostic criteria and to differences in clinical imaging accuracy. Therefore, it must be assumed that a proportion of uterus classified as arcuate in the included studies would be considered normal according to current diagnostic criteria. Finally, in our analysis T-shaped uterus markedly increased the risk of first or second trimester miscarriage.
Ectopic pregnancy was more frequent in patients with septate uterus (derived from a single study), which is different from Kim et al. (2021) review, which includes 11 studies on this item (10 of them excluded from our meta-analysis).
Kim et al. (2021) identified an increased risk of placental abruption for all types of CUA, and Venetis et al. (2014) only for arcuate and septate uterus. We found an increased risk of placental abruption only for subseptate and bicornuate uterus. However, as it was obtained from a single study (Takami et al., 2014), it should be considered with caution.
Risk for combined outcome PROM/PPROM from our data revealed a significant association with bicornuate uterus. This result is comparable with that obtained by Venetis et al. (2014), who found increased risk of PROM for arcuate and septate uterus, and by Kim et al. (2021), in whose analysis PPROM rate was increased for all types of CUA.
We found that the preterm delivery rate is increased in most CUA. Previous meta-analyses identified an increased risk of prematurity for subseptate, unicornuate, bicornuate and didelphys uterus (Chan et al., 2011a; Kim et al., 2021; Venetis et al., 2014). Arcuate uterus was not associated with preterm delivery risk according to Chan, Venetis and Kim studies (Kim et al., 2021; Venetis et al., 2014; Chan et al.,2011a), but showed a strong association in our study (OR:8.91; 95%CI:3.10- 25.63). The estimate of Kim et al. (2021) is based on ten studies, eight of which were not included in our synthesis. Septate uterus increases the risk of preterm delivery according to the three previous meta-analyses. This risk was not significant when adjusted by meta-regression, as it depended on the design of the selected studies. Risk of preterm delivery <34 weeks was significantly associated with unification defects (bicornuate and didelphys uterus). Only Venetis et al. (2014) has estimated this correlation, and found an association with most CUA, but not with arcuate uterus. Although never analysed in the past, we did not find correlation between CUA and prematurity <32 weeks with septate uterus or unification defects.
Regarding IUGR/SGA, the three previous meta- analyses (Chan et al., 2011a; Kim et al., 2021; Venetis et al., 2014) found an association with unification defects, especially with didelphys and bicornuate uteri, which is consistent with our results. Canalization defects are less consistently associated with this outcome. Subseptate uterus increases the risk of IUGR/SGA both according to Kim’s study and ours, although Venetis estimated an increased risk of IUGR only for septate uterus (Venetis et al., 2014).
Concerning delivery and postnatal events, malpresentation at delivery is the most frequently reported adverse outcomes for all types of CUA. Caesarean delivery is more frequent in patients carrying canalization defects, as concluded meta- analysis from Kim et al. (2021) and our study. Unification defects also increase the risk of caesarean section, as also by Kim et al. (2021). Chan et al. (2011a) and Venetis et al. (2014) did not consider this outcome. Finally, our results support an increase of risk of foetal mortality in patients with CUA due to unification disorders, while Kim et al. (2021) identified this increased risk only in patients with unicornuate uterus. Perinatal mortality is also associated with unification defects. In our study all the unification defects had increased perinatal mortality, whereas Kim’s meta-analysis detected this increase for unicornuate, bicornuate and septate uterus (Kim et al., 2021).
Strengths and limitations
Strengths of our study derive from the strict selection criteria. This meta-analysis is the first to estimate pooled effects of T-shaped uterus on pregnancy outcomes obtaining estimates on relevant outcomes (miscarriage, ectopic pregnancy, and prematurity). Our study also provides the first estimation of CUA effects on preterm delivery <32 weeks. Additionally, meta-regression models were applied as an effort to analyse and control the observed heterogeneity.
Retrospective design of most included studies and differences in population of interest, sample sizes, procedures applied for the diagnosis –such as hysterosalpingography or 2D ultrasound–, characteristics of non-exposed patients and design of included studies should be considered as limitations. In addition, classification categories used in includes studies do not correspond with more recent and widely accepted classification schemes of CUA. We have not performed the re-classification of the exposure categories into actual classifications, to avoid the risk of bias potentially associated. Certain outcomes, such as placental abruption and preterm delivery, have been analysed from single studies. Furthermore, several of the ‘effect’ estimates present high levels of statistical heterogeneity, which have not been substantially reduced by meta-regression. Specifically, 30 of the 71 estimates of global effects were affected by high statistical heterogeneity (I2>50%), despite the 10 adjustments.
Implications for clinical practice
The accurate estimation of risks associated with a specific CUA requires a precise diagnosis and an appropriate standardised classification. In clinical practice, 3-D ultrasound constitutes actually the first- choice image assessment of CUA. In certain cases, complementary tests such as MRI or hysteroscopy may be necessary. There is increasing evidence about the usefulness of hysteroscopic metroplasty in reducing the risk of miscarriage by correction of septate and subseptate uterus (Carrera et al., 2022; Jiang et al., 2023), as well as dysmorphic uterus (Garzon et al., 2020). The surgical treatment of unification defects is more complex with no evidence of improving perinatal prognosis.
Implications for research
Most studies diagnose and classify CUA according to the first version of the AFS classification. It may be of interest to re-analyse the reproductive risks associated with CUA using the ESHRE/ ESGE or ASRM revised classification, which may help to better estimate the risks associated with these characterised anomalies. The development of prospective studies that apply the most recent classifications are needed.
Conclusions
CUA are associated with an increased risk of complications in early and late pregnancy. Complications associated with most of the CUA were preterm delivery, malpresentation at delivery, and caesarean delivery. Moreover, our results do not clearly define a profile of preferential association between type of Müllerian defects and category of complications.
Supplementary material
Included studies.
Not included studies and reasons for exclusion.
Summary of estimated effects of CUA on pregnancy and neonatal outcomes.
Forest plot of individual and pooled effects on ectopic pregnancy of all CUA
Forest plots of individual and pooled effects on ectopic pregnancy by type of CUA.
Forest plot of individual and pooled effects on placental abruption of all CUA (combined).
Forest plots of individual and pooled effects on placental abruption by type of CUA.
Forest plot of individual and pooled effects on PROM/PPROM of all CUA (combined).
Forest plots of individual and pooled effects on PROM/PPROM by type of CUA.
Forest plot of individual and pooled effects on fetal malpresentation at delivery of all CUA (combined).
Forest plots of individual and pooled effects on fetal malpresentation at delivery by type of CUA.
Forest plots of individual and pooled effects on preterm delivery (A) preterm delivery < 34 wweks (B) and preterm delivery < 32 weeks of all CUA (combined).
Forest plots of individual and pooled effects on preterm delivery by type of CUA.
Forest plots of individual and pooled effects on preterm delivery < 34 weeks by type of CUA.
Forest plots of individual and pooled effects on preterm delivery < 32 weeks by type of CUA.
Forest plot of individual and pooled effects on cesarean delivery of all CUA (combined).
Forest plots of individual and pooled effects on cesarean delivery by type of CUA.
Forest plot of individual and pooled effects on IUGR/SGA of all CUA (combined).
Forest plots of individual and pooled effects on IUGR/SGA by type of CUA.
Forest plot of individual and pooled effects on fetal mortality of all CUA (combined).
Forest plots of individual and pooled effects on fetal mortality by type of CUA.
Forest plot of individual and pooled effects on perinatal mortality of all CUA (combined).
Forest plots of individual and pooled effects on perinatal mortality by type of CUA.
Appendix 1: NLM (PubMed) structured search
Appendix 2: included studies: newcastle-Ottawa scale scores
Footnotes
Acknowledgements: The authors thank the Spanish Fertility Society for the logistical support received for the preparation of this article.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Acién P. Reproductive performance of women with uterine malformations. Hum Reprod. 1993;8:122–126. doi: 10.1093/oxfordjournals.humrep.a137860. [DOI] [PubMed] [Google Scholar]
- 2.Acién P, Acién M, Mazaira N, et al. Reproductive outcome in uterine malformations with or without an associated unilateral renal agenesis. J Reprod Med. 2014;59:69–75. [PubMed] [Google Scholar]
- 3.Airoldi J, Berghella V, Sehdev H, et al. Transvaginal ultrasonography of the cervix to predict preterm birth in women with uterine anomalies. Obstet Gynecol. 2005;106:553–556. doi: 10.1097/01.AOG.0000173987.59595.e2. [DOI] [PubMed] [Google Scholar]
- 4.Akar ME, Bayar D, Yildiz S, et al. Reproductive outcome of women with unicornuate uterus. Aust N Z J Obstet Gynaecol. 2005;45:148–150. doi: 10.1111/j.1479-828X.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 5.Alonso Pacheco L, Laganà AS, Garzon S, et al. Hysteroscopic outpatient metroplasty for t-shaped uterus in women with reproductive failure: Results from a large prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;243:173–178. doi: 10.1016/j.ejogrb.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Ban-Frangez H, Tomazevic T, Virant-Klun I, et al. The outcome of singleton pregnancies after IVF/ICSI in women before and after hysteroscopic resection of a uterine septum compared to normal controls. Eur J Obstet Gynecol Reprod Biol. 2009;146:184–187. doi: 10.1016/j.ejogrb.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Rafael Z, Seidman DS, Recabi K, et al. Uterine anomalies. A retrospective, matched-control study. J Reprod Med. 1991;36:723–727. [PubMed] [Google Scholar]
- 8.Ben-Rafael Z, Seidman DS, Recabi K, et al. The association of pregnancy-induced hypertension and uterine malformations. Gynecol Obstet Invest. 1990;30:101–104. doi: 10.1159/000293227. [DOI] [PubMed] [Google Scholar]
- 9. Borenstein M, Hedges LV, Higgins JPT, et al. Heterogeneity. Meta-regression. In Introduction to meta-analysis (2nd ed) John Wiley & Sons. 2021; 197-212. [Google Scholar]
- 10.Cahen-Peretz A, Sheiner E, Friger M, et al. The association between Müllerian anomalies and perinatal outcome. J Matern Fetal Neonatal Med. 2017;32:51–57. doi: 10.1080/14767058.2017.1370703. [DOI] [PubMed] [Google Scholar]
- 11.Cai P, Ouyang Y, Lin G, et al. Pregnancy outcomes of women with congenital uterus didelphys after in-vitro fertilization- embryo transfer. Ultrasound Obstet Gynecol. 2021;54:543–549. doi: 10.1002/uog.24750. [DOI] [PubMed] [Google Scholar]
- 12.Carrera M, Perez Millan F, Alcazar JL, et al. Effect of hysteroscopic metroplasty on reproductive outcomes in women with septate uterus: Systematic review and meta- analysis. J Minim Invasive Gynecol. 2022;29:465–475. doi: 10.1016/j.jmig.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Colacurci N, De Placido G, Mollo A, et al. Reproductive outcome after hysteroscopic metroplasty. Eur J Obstet Gynecol Reprod Biol. 1996;66:147–150. doi: 10.1016/0301-2115(96)02417-7. [DOI] [PubMed] [Google Scholar]
- 14.Cooney MJ, Benson CB, Doubilet PM. Outcome of pregnancies in women with uterine duplication anomalies. J Clin Ultrasound. 1998;26:3–6. doi: 10.1002/(sici)1097-0096(199801)26:1<3::aid-jcu2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Crane J, Scott H, Stewart A, et al. Transvaginal ultrasonography to predict preterm birth in women with bicornuate or didelphus uterus. J Matern Fetal Neonatal Med. 2012;25:1960–1964. doi: 10.3109/14767058.2012.675372. [DOI] [PubMed] [Google Scholar]
- 16.Chan YY, Jayaprakasan K, Tan A, et al. Reproductive outcomes in women with congenital uterine anomalies: A systematic review. Ultrasound Obstet Gynecol. 2011a;38:371–382. doi: 10.1002/uog.10056. [DOI] [PubMed] [Google Scholar]
- 17.Chan YY, Jayaprakasan K, Zamora J, et al. The prevalence of congenital uterine anomalies in unselected and high-risk populations: A systematic review. Hum Reprod Update. 2011b;17:761–771. doi: 10.1093/humupd/dmr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SQ, Deng N, Jiang HY, et al. Management and reproductive outcome of complete septate uterus with duplicated cervix and vaginal septum: Review of 21 cases. Arch Gynecol Obstet. 2013;287:709–714. doi: 10.1007/s00404-012-2622-x. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Liu P, Sheng Y, et al. The impact of unicornuate uterus on perinatal outcomes after IVF/ICSI cycles: A matched retrospective cohort study. J Matern Fetal Neonatal Med. 2019;32:2469–2474. doi: 10.1080/14767058.2018.1438403. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Nisenblat V, Yang P, et al. Reproductive outcomes in women with unicornuate uterus undergoing in vitro fertilization: A nested case-control retrospective study. Reprod Biol Endocrinol. 2018;16:64. doi: 10.1186/s12958-018-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Kacker R. Random-effects model for meta- analysis of clinical trials: An update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Elsokkary M, Elshourbagy M, Labib K, et al. Assessment of hysteroscopic role in management of women with recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2018;31:1494–1504. doi: 10.1080/14767058.2017.1319925. [DOI] [PubMed] [Google Scholar]
- 23.Erez O, Dukler D, Novack L, et al. Trial of labor and vaginal birth after cesarean section in patients with uterine mullerian anomalies: A population based study. Am J Obstet Gynecol. 2007;196:537.:e1-11. doi: 10.1016/j.ajog.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Fedele L, Bianchi S. Hysteroscopic metroplasty for septate uterus. Obstet Gynecol Clin North Am. 1995;22:473–489. [PubMed] [Google Scholar]
- 25.Forde P, O’Driscoll D, Murphy H. Pregnancy associated with uterine abnormality. Ir Med J. 1978;71:164–165. [PubMed] [Google Scholar]
- 26.Fox NS, Connolly CT, Hill MB, et al. Pregnancy outcomes in viable pregnancies with a septate uterus compared with viable pregnancies after hysteroscopic uterine septum resection. Am J Obstet Gynecol MFM. 2019;1:136–143. doi: 10.1016/j.ajogmf.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Fox NS, Roman AS, Stern EM, et al. Type of congenital uterine anomaly and adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2014;27:949–953. doi: 10.3109/14767058.2013.847082. [DOI] [PubMed] [Google Scholar]
- 28.Gabbai D, Harlev A, Friger M, et al. Pregnancy outcomes among patients with recurrent pregnancy loss and uterine anatomic abnormalities. J Perinat Med. 2018;46:728–734. doi: 10.1515/jpm-2016-0411. [DOI] [PubMed] [Google Scholar]
- 29.Garzon S, Laganà AS, Di Spiezio Sardo A, et al. Hysteroscopic metroplasty for t-shaped uterus: A systematic review and meta-analysis of reproductive outcomes. Obstet Gynecol Surv. 2020;75:431–444. doi: 10.1097/OGX.0000000000000807. [DOI] [PubMed] [Google Scholar]
- 30.Ghi T, De Musso F, Maroni E, et al. The pregnancy outcome in women with incidental diagnosis of septate uterus at first trimester scan. Hum Reprod. 2012;27:2671–2675. doi: 10.1093/humrep/des215. [DOI] [PubMed] [Google Scholar]
- 31.Grimbizis GF, Camus M, Tarlatzis BC, et al. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update. 2001;7:161–174. doi: 10.1093/humupd/7.2.161. [DOI] [PubMed] [Google Scholar]
- 32.Hiersch L, Yeoshoua E, Miremberg H, et al. The association between mullerian anomalies and short-term pregnancy outcome. J Matern Fetal Neonatal Med. 2016;29:2573–2578. doi: 10.3109/14767058.2015.1098613. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 34.Hua M, Odibo AO, Longman RE, et al. Congenital uterine anomalies and adverse pregnancy outcomes. Am J Obstet Gynecol. 2011;205:558.:e-5. doi: 10.1016/j.ajog.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Hynes JS, Schwartz AR, Wheeler SM, et al. Rates of preterm birth in multiparous women with congenital uterine anomalies. Am J Obstet Gynecol MFM. 2021;3:100392. doi: 10.1016/j.ajogmf.2021.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaslow CR, Kutteh WH. Effect of prior birth and miscarriage frequency on the prevalence of acquired and congenital uterine anomalies in women with recurrent miscarriage: A cross-sectional study. Fertil Steril. 2013;99:1916–1922.:e1. doi: 10.1016/j.fertnstert.2013.01.152. [DOI] [PubMed] [Google Scholar]
- 37.Jayaprakasan K, Chan YY, Sur S, et al. Prevalence of uterine anomalies and their impact on early pregnancy in women conceiving after assisted reproduction treatment. Ultrasound Obstet Gynecol. 2011;37:727–732. doi: 10.1002/uog.8968. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Wang L, Wang B, et al. Reproductive outcomes of natural pregnancy after hysteroscopic septum resection in patients with a septate uterus: A systematic review and meta- analysis. Am J Obstet Gynecol MFM. 2023;5:100762. doi: 10.1016/j.ajogmf.2022.100762. [DOI] [PubMed] [Google Scholar]
- 39.Kim MA, Kim HS, Kim YH. Reproductive, obstetric and neonatal outcomes in women with congenital uterine anomalies: A systematic review and meta-analysis. J Clin Med. 2021;10:4797. doi: 10.3390/jcm10214797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong WY, Zhao SR, Deng K, et al. Effects of bicornuate uterus on pregnancy and obstetric outcomes of in vitro fertilization/ intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2021;258:132–138. doi: 10.1016/j.ejogrb.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 41.Lavergne N, Aristizabal J, Zarka V, et al. Eur J Obstet Gynecol Reprod Biol. Uterine anomalies and in vitro fertilization: What are the results. 1996;68:29–34. doi: 10.1016/0301-2115(96)02459-1. [DOI] [PubMed] [Google Scholar]
- 42.Leible S, Muñoz H, Walton R, et al. Uterine artery blood flow velocity waveforms in pregnant women with müllerian duct anomaly: A biologic model for uteroplacental insufficiency. Am J Obstet Gynecol. 1998;178:1048–1053. doi: 10.1016/s0002-9378(98)70546-0. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Ouyang Y, Yi Y, et al. Pregnancy outcomes of women with a congenital unicornuate uterus after ivf-embryo transfer. Reprod Biomed Online. 2017;35:583–591. doi: 10.1016/j.rbmo.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Liang F, Hu W. Pregnancy complications and obstetric outcomes among women with congenital uterine malformations. Int J Gynecol Obstet. 2010;109:159–160. doi: 10.1016/j.ijgo.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y, Ji M, Deng S, et al. Association of a unicornuate uterus with adverse obstetric outcomes: A retrospective cohort study. Journal of Obstetrics and Gynaecology Research. 2021 doi: 10.1111/jog.14911. [DOI] [PubMed] [Google Scholar]
- 46. Ludwin A. Re: Outcome of assisted reproduction in women with congenital uterine anomalies: a prospective observational study. Ultrasound Obstet Gynecol. 2018;51:110–117. doi: 10.1002/uog.18935. M. Prior, A. Richardson, S. Asif, L. Polanski, M. Parris-Larkin, J. Chandler, L. Fogg, P. Jassal, J. G. Thornton, N. J. Raine-Fenning. [DOI] [PubMed] [Google Scholar]
- 47.Maneschi F, Zupi E, Marconi D, et al. Hysteroscopically detected asymptomatic Müllerian anomalies. Prevalence and reproductive implications. J Reprod Med. 1995;40:684–688. [PubMed] [Google Scholar]
- 48.Marianna A, Karine T, Armine C, et al. The impact of t-shaped uterine cavity anomaly on IVF outcomes: More questions than answers. J Gynecol Obstet Hum Reprod. 2022;51:102293. doi: 10.1016/j.jogoh.2021.102293. [DOI] [PubMed] [Google Scholar]
- 49.Mastrolia SA, Baumfeld Y, Hershkovitz R, et al. Bicornuate uterus is an independent risk factor for cervical os insufficiency: A retrospective population based cohort study. J Matern Fetal Neonatal Med. 2017;30:2705–2710. doi: 10.1080/14767058.2016.1261396. [DOI] [PubMed] [Google Scholar]
- 50.Mastrolia SA, Baumfeld Y, Hershkovitz R, et al. Independent association between uterine malformations and cervical insufficiency: A retrospective population-based cohort study. Arch Gynecol Obstet. 2018;297:919–926. doi: 10.1007/s00404-018-4663-2. [DOI] [PubMed] [Google Scholar]
- 51.Neal SA, Morin SJ, Werner MD, et al. Three-dimensional ultrasound diagnosis of t-shaped uterus is associated with adverse pregnancy outcomes after embryo transfer. Reprod Biomed Online. 2019;39:777–783. doi: 10.1016/j.rbmo.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang Y, Cai P, Gong F, et al. The risk of twin pregnancies should be minimized in patients with a unicornuate uterus undergoing IVF-ET. Sci Rep. 2020;10:5571. doi: 10.1038/s41598-020-62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozgur K, Bulut H, Berkkanoglu M, et al. Reproductive outcomes of IVF patients with unicornuate uteri. Reprod Biomed Online. 2017;34:312–318. doi: 10.1016/j.rbmo.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 54.Pleş L, Alexandrescu C, Ionescu CA, et al. Three-dimensional scan of the uterine cavity of infertile women before assisted reproductive technology use. Medicine (Baltimore) 2018;97:e12764. doi: 10.1097/MD.0000000000012764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portuondo JA, Camara MM, Echanojauregui AD, et al. Müllerian abnormalities in fertile women and recurrent aborters. J Reprod Med. 1986;31:616–619. [PubMed] [Google Scholar]
- 56.Prior M, Richardson A, Asif S, et al. Outcome of assisted reproduction in women with congenital uterine anomalies: A prospective observational study. Ultrasound Obstet Gynecol. 2018;51:110–117. doi: 10.1002/uog.18935. [DOI] [PubMed] [Google Scholar]
- 57.Qiu J, Du T, Chen C, et al. Impact of uterine malformations on pregnancy and neonatal outcomes of IVF/ICSI-frozen embryo transfer. Hum Reprod. 2022;37:428–446. doi: 10.1093/humrep/deac003. [DOI] [PubMed] [Google Scholar]
- 58.Raga F, Bauset C, Remohi J, et al. Reproductive impact of congenital Müllerian anomalies. Hum Reprod. 1997;12:2277–2281. doi: 10.1093/humrep/12.10.2277. [DOI] [PubMed] [Google Scholar]
- 59.Ravasia DJ, Brain PH, Pollard JK. Incidence of uterine rupture among women with mullerian duct anomalies who attempt vaginal birth after cesarean delivery. Am J Obstet Gynecol. 1999;181:877–881. doi: 10.1016/s0002-9378(99)70318-2. [DOI] [PubMed] [Google Scholar]
- 60.Ridout AE, Ibeto LA, Ross GN, et al. Cervical length and quantitative fetal fibronectin in the prediction of spontaneous preterm birth in asymptomatic women with congenital uterine anomaly. Am J Obstet Gynecol. 2019;221:341.:e1-9. doi: 10.1016/j.ajog.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 61.Rogers MS, Needham PG. Unicornuate uterus and reproductive performance. Aust N Z J Obstet Gynaecol. 1985;25:144–145. doi: 10.1111/j.1479-828x.1985.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 62.Salim R, Regan L, Woelfer B, et al. A comparative study of the morphology of congenital uterine anomalies in women with and without a history of recurrent first trimester miscarriage. Hum Reprod. 2003;18:162–166. doi: 10.1093/humrep/deg030. [DOI] [PubMed] [Google Scholar]
- 63.Saravelos SH, Cocksedge KA, Li TC. The pattern of pregnancy loss in women with congenital uterine anomalies and recurrent miscarriage. Reprod Biomed Online. 2010;20:416–422. doi: 10.1016/j.rbmo.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Sendag F, Mermer T, Yucebilgin S, et al. Reproductive outcomes after hysteroscopic metroplasty for uterine septum. Clin Exp Obstet Gynecol. 2010;37:287–289. [PubMed] [Google Scholar]
- 65.Shuiqing M, Xuming B, Jinghe L. Pregnancy and its outcome in women with malformed uterus. Chin Med Sci J. 2002;17:242–245. [PubMed] [Google Scholar]
- 66.Sorensen SS, Trauelsen AGH. Obstetric implications of minor mullerian anomalies in oligomenorrheic women. Am J Obstet Gynecol. 1987;156:1112–1118. doi: 10.1016/0002-9378(87)90121-9. [DOI] [PubMed] [Google Scholar]
- 67.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 68.Sugiura-Ogasawara M, Lin BL, Aoki K, et al. J Obstet Gynaecol. Does surgery improve live birth rates in patients with recurrent miscarriage caused by uterine anomalies. 2015;35:155–158. doi: 10.3109/01443615.2014.936839. [DOI] [PubMed] [Google Scholar]
- 69.Sugiura-Ogasawara M, Ozaki Y, Kitaori T, et al. Midline uterine defect size is correlated with miscarriage of euploid embryos in recurrent cases. Fertil Steril. 2010;93:1983–1988. doi: 10.1016/j.fertnstert.2008.12.097. [DOI] [PubMed] [Google Scholar]
- 70.Surrey ES, Katz-Jaffe M, Surrey RL, et al. Fertil Steril. Arcuate uterus: Is there an impact on in vitro fertilization outcomes after euploid embryo transfer. 2018;109:638–643. doi: 10.1016/j.fertnstert.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Takami M, Aoki S, Kurasawa K, et al. A classification of congenital uterine anomalies predicting pregnancy outcomes. Acta Obstet Gynecol Scand. 2014;93:691–697. doi: 10.1111/aogs.12400. [DOI] [PubMed] [Google Scholar]
- 72.Tofoski G, Antovska V. Influence of hysteroscopic metroplasty on reproductive outcome in patients with infertility and recurrent pregnancy loss. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2014;35:95–103. doi: 10.2478/prilozi-2014-0012. [DOI] [PubMed] [Google Scholar]
- 73.Tomazevic T, Ban-Frangez H, Ribic-Pucelj M, et al. Small uterine septum is an important risk variable for preterm birth. Eur J Obstet Gynecol Reprod Biol. 2007;135:154–157. doi: 10.1016/j.ejogrb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Tomaževič T, Ban-Frangež H, Virant-Klun I, et al. Septate, subseptate and arcuate uterus decrease pregnancy and live birth rates in IVF/ICSI. Reprod Biomed Online. 2010;21:700–705. doi: 10.1016/j.rbmo.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 75.Tonguc EA, Var T, Batioglu S. Hysteroscopic metroplasty in patients with a uterine septum and otherwise unexplained infertility. Int J Gynecol Obstet. 2011;113:128–130. doi: 10.1016/j.ijgo.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 76.Venetis CA, Papadopoulos SP, Campo R, et al. Clinical implications of congenital uterine anomalies: A meta-analysis of comparative studies. Reprod Biomed Online. 2014;29:665–683. doi: 10.1016/j.rbmo.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Retrieved 6th June 2021 from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 78.Woelfer B, Salim R, Banerjee S, et al. Reproductive outcomes in women with congenital uterine anomalies detected by three-dimensional ultrasound screening. Obstet Gynecol. 2001;98:1099–1103. doi: 10.1016/s0029-7844(01)01599-x. [DOI] [PubMed] [Google Scholar]
- 79.Zambrotta E, Di Gregorio LM, Di Guardo F, et al. Congenital uterine anomalies and perinatal outcomes: A retrospective single-center cohort study. Clin Exp Obstet Gynecol. 2021;48:160–163. [Google Scholar]
- 80.Zhang Y, Zhao YY, Qiao J. Obstetric outcome of women with uterine anomalies in China. Chin Med J (Engl) 2010;123:418–422. [PubMed] [Google Scholar]
- 81.Zlopasa G, Skrablin S, Katafatic D, et al. Uterine anomalies and pregnancy outcome following resectoscope metroplasty. Int J Gynecol Obstet. 2007;98:129–133. doi: 10.1016/j.ijgo.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Zupi E, Solima E, Marconi D, et al. Uterine anomalies prevalence and reproductive outcome in women undergoing diagnostic hysteroscopy. Gynaecol Endosc. 1996;5:147–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Included studies.
Not included studies and reasons for exclusion.
Summary of estimated effects of CUA on pregnancy and neonatal outcomes.
Forest plot of individual and pooled effects on ectopic pregnancy of all CUA
Forest plots of individual and pooled effects on ectopic pregnancy by type of CUA.
Forest plot of individual and pooled effects on placental abruption of all CUA (combined).
Forest plots of individual and pooled effects on placental abruption by type of CUA.
Forest plot of individual and pooled effects on PROM/PPROM of all CUA (combined).
Forest plots of individual and pooled effects on PROM/PPROM by type of CUA.
Forest plot of individual and pooled effects on fetal malpresentation at delivery of all CUA (combined).
Forest plots of individual and pooled effects on fetal malpresentation at delivery by type of CUA.
Forest plots of individual and pooled effects on preterm delivery (A) preterm delivery < 34 wweks (B) and preterm delivery < 32 weeks of all CUA (combined).
Forest plots of individual and pooled effects on preterm delivery by type of CUA.
Forest plots of individual and pooled effects on preterm delivery < 34 weeks by type of CUA.
Forest plots of individual and pooled effects on preterm delivery < 32 weeks by type of CUA.
Forest plot of individual and pooled effects on cesarean delivery of all CUA (combined).
Forest plots of individual and pooled effects on cesarean delivery by type of CUA.
Forest plot of individual and pooled effects on IUGR/SGA of all CUA (combined).
Forest plots of individual and pooled effects on IUGR/SGA by type of CUA.
Forest plot of individual and pooled effects on fetal mortality of all CUA (combined).
Forest plots of individual and pooled effects on fetal mortality by type of CUA.
Forest plot of individual and pooled effects on perinatal mortality of all CUA (combined).
Forest plots of individual and pooled effects on perinatal mortality by type of CUA.