Abstract
Background
Guidelines suggest that adults with diabetes and kidney disease receive treatment with angiotensin‐converting‐enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB). This is an update of a Cochrane review published in 2006.
Objectives
We compared the efficacy and safety of ACEi and ARB therapy (either as monotherapy or in combination) on cardiovascular and kidney outcomes in adults with diabetes and kidney disease.
Search methods
We searched the Cochrane Kidney and Transplants Register of Studies to 17 March 2024 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
We included studies evaluating ACEi or ARB alone or in combination, compared to each other, placebo or no treatment in people with diabetes and kidney disease.
Data collection and analysis
Two authors independently assessed the risk of bias and extracted data. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) or standardised mean difference (SMD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
One hundred and nine studies (28,341 randomised participants) were eligible for inclusion. Overall, the risk of bias was high.
Compared to placebo or no treatment, ACEi may make little or no difference to all‐cause death (24 studies, 7413 participants: RR 0.91, 95% CI 0.73 to 1.15; I2 = 23%; low certainty) and with similar withdrawals from treatment (7 studies, 5306 participants: RR 1.03, 95% CI 0.90 to 1.19; I2 = 0%; low certainty). ACEi may prevent kidney failure (8 studies, 6643 participants: RR 0.61, 95% CI 0.39 to 0.94; I2 = 0%; low certainty).
Compared to placebo or no treatment, ARB may make little or no difference to all‐cause death (11 studies, 4260 participants: RR 0.99, 95% CI 0.85 to 1.16; I2 = 0%; low certainty). ARB have uncertain effects on withdrawal from treatment (3 studies, 721 participants: RR 0.85, 95% CI 0.58 to 1.26; I2 = 2%; low certainty) and cardiovascular death (6 studies, 878 participants: RR 3.36, 95% CI 0.93 to 12.07; low certainty). ARB may prevent kidney failure (3 studies, 3227 participants: RR 0.82, 95% CI 0.72 to 0.94; I2 = 0%; low certainty), doubling of serum creatinine (SCr) (4 studies, 3280 participants: RR 0.84, 95% CI 0.72 to 0.97; I2 = 32%; low certainty), and the progression from microalbuminuria to macroalbuminuria (5 studies, 815 participants: RR 0.44, 95% CI 0.23 to 0.85; I2 = 74%; low certainty).
Compared to ACEi, ARB had uncertain effects on all‐cause death (15 studies, 1739 participants: RR 1.13, 95% CI 0.68 to 1.88; I2 = 0%; low certainty), withdrawal from treatment (6 studies, 612 participants: RR 0.91, 95% CI 0.65 to 1.28; I2 = 0%; low certainty), cardiovascular death (13 studies, 1606 participants: RR 1.15, 95% CI 0.45 to 2.98; I2 = 0%; low certainty), kidney failure (3 studies, 837 participants: RR 0.56, 95% CI 0.29 to 1.07; I2 = 0%; low certainty), and doubling of SCr (2 studies, 767 participants: RR 0.88, 95% CI 0.52 to 1.48; I2 = 0%; low certainty).
Compared to ACEi plus ARB, ACEi alone has uncertain effects on all‐cause death (6 studies, 1166 participants: RR 1.08, 95% CI 0.49 to 2.40; I2 = 20%; low certainty), withdrawal from treatment (2 studies, 172 participants: RR 0.78, 95% CI 0.33 to 1.86; I2 = 0%; low certainty), cardiovascular death (4 studies, 994 participants: RR 3.02, 95% CI 0.61 to 14.85; low certainty), kidney failure (3 studies, 880 participants: RR 1.36, 95% CI 0.79 to 2.32; I2 = 0%; low certainty), and doubling of SCr (2 studies, 813 participants: RR 1.14, 95% CI 0.70 to 1.85; I2 = 0%; low certainty).
Compared to ACEi plus ARB, ARB alone has uncertain effects on all‐cause death (7 studies, 2607 participants: RR 1.02, 95% CI 0.76 to 1.37; I2 = 0%; low certainty), withdrawn from treatment (3 studies, 1615 participants: RR 0.81, 95% CI 0.53 to 1.24; I2 = 0%; low certainty), cardiovascular death (4 studies, 992 participants: RR 3.03, 95% CI 0.62 to 14.93; low certainty), kidney failure (4 studies, 2321 participants: RR 1.15, 95% CI 0.67 to 1.95; I2 = 29%; low certainty), and doubling of SCr (3 studies, 2252 participants: RR 1.18, 95% CI 0.85 to 1.64; I2 = 0%; low certainty).
Comparative effects of different ACEi or ARB and low‐dose versus high‐dose ARB were rarely evaluated. No study compared different doses of ACEi.
Adverse events of ACEi and ARB were rarely reported.
Authors' conclusions
ACEi or ARB may make little or no difference to all‐cause and cardiovascular death compared to placebo or no treatment in people with diabetes and kidney disease but may prevent kidney failure. ARB may prevent the doubling of SCr and the progression from microalbuminuria to macroalbuminuria compared with a placebo or no treatment. Despite the international guidelines suggesting not combining ACEi and ARB treatment, the effects of ACEi or ARB monotherapy compared to dual therapy have not been adequately assessed. The limited data availability and the low quality of the included studies prevented the assessment of the benefits and harms of ACEi or ARB in people with diabetes and kidney disease. Low and very low certainty evidence indicates that it is possible that further studies might provide different results.
Keywords: Humans; Angiotensin Receptor Antagonists; Angiotensin Receptor Antagonists/therapeutic use; Angiotensin-Converting Enzyme Inhibitors; Angiotensin-Converting Enzyme Inhibitors/therapeutic use; Bias; Cause of Death; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Diabetes Mellitus, Type 2/drug therapy; Diabetic Nephropathies; Diabetic Nephropathies/prevention & control; Disease Progression; Drug Therapy, Combination; Randomized Controlled Trials as Topic
Plain language summary
Angiotensin‐converting‐enzyme inhibitors and angiotensin receptor blockers for preventing the progression of diabetic kidney disease
Key messages
‐ Angiotensin‐converting‐enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) may reduce kidney failure in people with diabetes and kidney disease.
‐ We are not sure whether ACEi, ARB or various combinations or doses prevent death or heart disease in people with diabetes and kidney disease.
Why treat people with diabetes who have chronic kidney disease?
Kidney disease is experienced by about one‐quarter to one‐half of people who have diabetes, usually 20 to 25 years after the onset of diabetes. Approximately one‐third of those who have diabetes with kidney disease will progress to kidney failure and require treatment with dialysis or kidney transplantation. Blood pressure‐lowering treatments prevent heart disease and enable patients to avoid or delay the need for dialysis or kidney transplantation. Two drug classes ‐ ACEi and ARB ‐ have been considered particularly effective at improving the health and well‐being of people with diabetes. We examine whether these drugs prevent kidney failure, death and heart complications in people who have diabetes and kidney disease.
What did we want to find out?
We wanted to find out if ACEi, ARB or combinations of treatment prevented the progression of kidney disease in adults with diabetes and kidney disease.
What did we do?
We searched for all trials that assessed the benefits and harms of randomly allocated ACEi, ARB, or various combinations for people with diabetes and chronic kidney disease. We compared and summarised the trials' results and rated our confidence in the information based on factors such as trial methods and sizes.
What did we find?
We found 109 studies involving 28,341 adults. ACEi and ARB may prevent kidney failure in people with diabetes and kidney disease. ACEi, ARB, or various combinations had uncertain effects on death or heart disease in people with diabetes and kidney disease.
What are the limitations of the evidence?
The small number of studies (per comparison) and the small size of the studies were limitations in this review. Not all the studies provided data about the outcomes we were interested in. We are unsure about the results.
How up‐to‐date is the evidence?
The evidence is up‐to‐date as of March 2024.
Summary of findings
Summary of findings 1. Angiotensin‐converting‐enzyme inhibitors versus placebo or no treatment for people with diabetes and kidney disease.
| ACEi versus placebo or no treatment for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: multinational (Australia, Austria, Canada, Chile, Denmark, France, Japan, India, Israel, Italy, Sweden, The Netherlands, UK, USA and other countries not specified, including Europe and North Africa) Intervention: ACEi Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | ACEi | |||||
|
All‐cause death Median follow‐up 2 years |
125 per 1000 | 11 fewer per 1000 (34 fewer to 19 more) |
RR 0.91 (0.73 to 1.15) |
7413 (24) | ⊕⊕⊝⊝ low1,2 | ACEi may make little or no difference to all cause death compared to placebo or no treatment in people with diabetes and kidney disease |
|
Cardiovascular death Median follow‐up 2 years |
49 per 1000 | 16 fewer per 1000 (43 fewer to 126 more) |
RR 0.67 (0.13 to 3.57) |
5625 (17) | ⊕⊝⊝⊝ very low1,2,3 | It is very uncertain whether ACEi makes any difference to cardiovascular death compared to placebo or no treatment people with diabetes and kidney disease |
|
Doubling of SCr Median follow‐up 3.8 years |
43 per 1000 | 13 fewer per 1000 (23 fewer to 0) |
RR 0.69 (0.47 to 1.01) |
6702 (8) | ⊕⊝⊝⊝ very low1,2,3 | It is very uncertain whether ACEi makes any difference to doubling of SCr compared to placebo or no treatment people with diabetes and kidney disease |
|
Fatal or nonfatal stroke Median follow‐up 7 years |
47 per 1000 | 1 more per 1000 (9 fewer to 15 more) |
RR 1.02 (0.80 to 1.31) |
4944 (2) | ⊕⊕⊝⊝ low1,2 | ACEi may make little or no difference to fatal or nonfatal stroke compared to placebo or no treatment people with diabetes and kidney disease |
|
Fatal or nonfatal myocardial infarction Median follow‐up 3 years |
31 per 1000 | 6 fewer per 1000 (13 fewer to 3 more) |
RR 0.79 (0.57 to 1.09) |
5100 (3) | ⊕⊕⊝⊝ low2,4 | ACEi may make little or no difference to fatal or nonfatal myocardial infarction compared to placebo or no treatment people with diabetes and kidney disease |
|
Micro‐ to macroalbuminuria Median follow‐up 2 years |
225 per 1000 |
128 fewer per 1000 (162 fewer to 81 fewer) |
RR 0.43 (0.28 to 0.64) |
2282 (19) | ⊕⊝⊝⊝ very low2,3,5 | It is very uncertain whether ACEi makes any difference to progression from micro‐ to macroalbuminuria compared to placebo or no treatment people with diabetes and kidney disease |
|
Micro‐ to normoalbuminuria Median follow‐up 2 years |
104 per 1000 |
209 more per 1000 (89 more to 404 more) |
RR 3.01 (1.86 to 4.88) |
1959 (17) | ⊕⊝⊝⊝ very low2,3,5 | It is very uncertain whether ACEi makes any difference to regression from micro‐ to normoalbuminuria compared to placebo or no treatment people with diabetes and kidney disease |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: Angiotensin‐converting‐enzyme inhibitors; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. Some studies had unclear risks for sequence generation, all had unclear allocation concealment, and the majority of them were not blinded (participant/investigator and/or outcomes assessor)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to moderate statistical heterogeneity
4 Evidence certainty was downgraded by one level due to study limitations. All studies had unclear risks for allocation concealment, and the majority of them were not blinded (outcomes assessor)
5 Evidence certainty was downgraded by one level due to study limitations. Some studies had unclear risks for sequence generation and/or allocation concealment, and the majority of them were not blinded (participant/investigator and/or outcomes assessor)
Summary of findings 2. Angiotensin receptor blockers versus placebo or no treatment for people with diabetes and kidney disease.
| ARB versus placebo or no treatment for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: multinational (Canada, China, Hong Kong, Japan, Thailand, USA and other countries unspecified from Asia and Europe) Intervention: ARB Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | ARB | |||||
|
All‐cause death Median follow‐up 1 year |
136 per 1000 | 1 fewer per 1000 (20 fewer to 22 more) |
RR 0.99 (0.85 to 1.16) |
4260 (11) | ⊕⊕⊝⊝ low1,2 | ARB may make little or no difference to all‐cause death compared to placebo or no treatment in people with diabetes and kidney disease |
|
Cardiovascular death Median follow‐up 1 year |
7 per 1000 | 17 more per 1000 (0 to 78 more) |
RR 3.36 (0.93 to 12.07) |
878 (6) | ⊕⊕⊝⊝ low1,2 | ARB have uncertain effects on cardiovascular death compared to placebo or no treatment in people with diabetes and kidney disease |
|
Doubling of SCr Median follow‐up 3 years |
282 per 1000 | 45 fewer per 1000 (79 fewer to 8 fewer) |
RR 0.84 (0.72 to 0.97) |
3280 (4) | ⊕⊕⊝⊝ low3,4 | ARB may prevent doubling of SCr compared to placebo or no treatment in people with diabetes and kidney disease |
|
Fatal or nonfatal stroke Median follow‐up 2.8 years |
39 per 1000 | 9 fewer per 1000 (26 fewer to 30 more) |
RR 0.76 (0.32 to 1.77) |
619 (2) | ⊕⊕⊝⊝ low2,3 | ARB have uncertain effects on fatal or nonfatal stroke compared to placebo or no treatment in people with diabetes and kidney disease |
|
Fatal or nonfatal myocardial infarction Median follow‐up 2.8 years |
23 per 1000 | 13 fewer per 1000 (20 fewer to 15 more) |
RR 0.43 (0.11 to 1.65) |
619 (2) | ⊕⊕⊝⊝ low2,3 | ARB have uncertain effects on fatal or nonfatal myocardial infarction compared to placebo or no treatment in people with diabetes and kidney disease |
|
Micro‐ to macroalbuminuria Median follow‐up 1.8 years |
425 per 1000 | 238 fewer per 1000 (327 fewer to 64 fewer) |
RR 0.44 (0.23 to 0.85) |
815 (5) | ⊕⊕⊝⊝ low1,5 | ARB may prevent progression from micro‐ to macroalbuminuria compared to placebo or no treatment in people with diabetes and kidney disease |
|
Micro‐ to normoalbuminuria Median follow‐up 2 years |
41 per 1000 | 188 more per 1000 (6 fewer to 1468 more) |
RR 5.58 (0.85 to 36.81) |
671 (4) | ⊕⊝⊝⊝ very low1,2,5 | It is very uncertain whether ARB makes any difference to regression from micro‐ to normoalbuminuria compared to placebo or no treatment in people with diabetes and kidney disease |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARB: Angiotensin receptor blockers; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. Some studies had unclear risks for sequence generation, all had unclear allocation concealment and the majority or all of them were not blinded (participant/investigator and/or outcomes assessor)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to study limitations. All studies had unclear allocation concealment and some of them were not blinded (outcomes assessor)
4 Evidence certainty was downgraded by one level due to low statistical heterogeneity
5 Evidence certainty was downgraded by one level due to substantial statistical heterogeneity
Summary of findings 3. Angiotensin receptor blockers versus angiotensin‐converting‐enzyme inhibitors for people with diabetes and kidney disease.
| ARB versus ACEi for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: multinational (northern Europe, Canada, Hong Kong, Japan, India, Italy, Slovenia, Spain, Thailand, The Netherlands, Turkey) Intervention: ARB Comparison: ACEi | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| ACEi | ARB | |||||
|
All‐cause death Median follow‐up 1 year |
31 per 1000 | 4 more per 1000 (10 fewer to 28 more) |
RR 1.13 (0.68 to 1.88) |
1739 (15) | ⊕⊕⊝⊝ low1,2 | ARB have uncertain effects on all‐cause death compared to ACEi in people with diabetes and kidney disease |
|
Cardiovascular death Median follow‐up 1 year |
10 per 1000 | 2 more per 1000 (6 fewer to 20 more) |
RR 1.15 (0.45 to 2.98) |
1606 (13) | ⊕⊕⊝⊝ low1,2 | ARB have uncertain effects on cardiovascular death compared to ACEi in people with diabetes and kidney disease |
|
Doubling of SCr Median follow‐up 2.7 years |
72 per 1000 | 9 fewer per 1000 (35 fewer to 35 more) |
RR 0.88 (0.52 to 1.48) |
767 (2) | ⊕⊕⊝⊝ low2,3 | ARB have uncertain effects on doubling of SCr compared to ACEi in people with diabetes and kidney disease |
|
Fatal or nonfatal stroke Median follow‐up 2.8 years |
19 per 1000 | 1 fewer per 1000 (12 fewer to 24 more) |
RR 0.92 (0.38 to 2.24) |
1146 (5) | ⊕⊕⊝⊝ low1,2 | ARB have uncertain effects on fatal or nonfatal stroke compared to ACEi in people with diabetes and kidney disease |
|
Fatal or nonfatal myocardial infarction Median follow‐up 2.7 years |
23 per 1000 | 6 more per 1000 (9 fewer to 35 more) |
RR 1.25 (0.62 to 2.50) |
1209 (6) | ⊕⊕⊝⊝ low1,2 | ARB have uncertain effects on fatal or nonfatal myocardial infarction compared to ACEi in people with diabetes and kidney disease |
|
Micro‐ to macroalbuminuria Median follow‐up 1 year |
103 per 1000 | 3 fewer per 1000 (34 fewer to 43 more) |
RR 0.97 (0.67 to 1.42) |
965 (4 | ⊕⊕⊝⊝ low1,2 | ARB have uncertain effects on progression from micro‐ to microalbuminuria compared to ACEi in people with diabetes and kidney disease |
|
Micro‐ to normoalbuminuria Median follow‐up 1 year |
695 per 1000 | 28 fewer per 1000 (139 fewer to 111 more) |
RR 0.96 (0.62 to 1.48) |
216 (4) | ⊕⊕⊝⊝ low 2,4 | ARB have uncertain effects on regression from micro‐ to normoalbuminuria compared to ACEi in people with diabetes and kidney disease |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: Angiotensin‐converting‐enzyme inhibitors; ARB: Angiotensin receptor blockers; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. Some studies had unclear risks for sequence generation and/or allocation concealment, and the majority of them were not blinded (participant/investigator and/or outcomes assessor)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to study limitations. Some studies were not blinded (participant/investigator and/or outcomes assessor)
4 Evidence certainty was downgraded by one level due to study limitations. All studies had unclear risks for allocation concealment and were not blinded (participant/investigator and outcomes assessor)
Summary of findings 4. Dual therapy (angiotensin‐converting‐enzyme inhibitors plus angiotensin receptor blockers) versus placebo or no treatment for people with diabetes and kidney disease.
| Duel therapy (ACEi plus ARB) versus placebo or no treatment for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: Japan Intervention: duel therapy (ACEi plus ARB) Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No, of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | ACEi + ARB | |||||
|
All‐cause death1 Follow‐up 1.8 years |
No events | No events | ‐‐ | 90 (1) | ⊕⊕⊝⊝ low2,3 | Studies were not designed to measure effects of dual therapy compared to placebo to no treatment on all‐cause death in people with diabetes and kidney disease |
|
Cardiovascular death4 Follow‐up 1.8 years |
No events | No events | ‐‐ | 90 (1) | ⊕⊕⊝⊝ low2,3 | Studies were not designed to measure effects of dual therapy compared to placebo to no treatment on cardiovascular death in people with diabetes and kidney disease |
| Doubling of SCr | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Fatal or nonfatal stroke | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Fatal or nonfatal myocardial infarction | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
|
Micro‐ to macroalbuminuria5 Follow‐up 1.8 years |
800 per 1000 | 720 fewer per 1000 (760 fewer to 608 fewer) |
RR0.10 (0.05 to 0.24) |
82 (1) | ⊕⊕⊝⊝ low2,3 | Studies were not designed to measure effects of dual therapy compared to placebo or no treatment on progression from micro‐ to macroalbuminuria in people with diabetes and kidney disease |
|
Micro‐ to normoalbuminuria6 Follow‐up 1.8 years |
No events | 17/72** |
RR 5.27 (0.34 to 81.57) |
82 (1) | ⊕⊕⊝⊝ verylow2,7 | Studies were not designed to measure effects of dual therapy compared to placebo or no treatment on regression from micro‐ to normoalbuminuria in people with diabetes and kidney disease |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Events rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group. ACEi: Angiotensin‐converting‐enzyme inhibitors; ARB: Angiotensin receptor blockers; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All‐cause death was reported in a single study
2 Evidence certainty was downgraded by one level due to study limitations. The study had unclear risks for allocation concealment and was not blinded (participant/investigator and outcomes assessor)
3 Evidence certainty was downgraded by one level due to imprecision
4 Cardiovascular death was reported in a single study
5 Micro‐ to macroalbuminuria was reported in a single study
6 Micro‐ to normoalbuminuria was reported in a single study
7 Evidence certainty was downgraded by two levels due to imprecision (including optimal information size not met)
Summary of findings 5. Angiotensin‐converting‐enzyme inhibitors (ACEi) alone versus ACEi plus angiotensin receptor blockers for people with diabetes and kidney disease.
| ACEi alone versus ACEi plus ARB for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: Italy, Japan, Slovenia, Spain, Turkey Intervention: ACEi alone Comparison: ACEi plus ARB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| ACEi + ARB | ACEi | |||||
|
All‐cause death Median follow‐up 1.8 years |
34 per 1000 | 3 more per 1000 (17 fewer to 48 more) |
RR 1.08 (0.49 to 2.40) |
1166 (6) | ⊕⊕⊝⊝ low1,2 | ACEi alone has uncertain effects on all‐cause death compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Cardiovascular death Median follow‐up 1 year |
4 per 1000 | 8 more per 1000 (2 fewer to 55 more) |
RR 3.02 (0.61 to 14.85) |
994 (4) | ⊕⊕⊝⊝ low1,2 | ACEi alone has uncertain effects on cardiovascular death compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Doubling of SCr Median follow‐up 2.7 years |
75 per 1000 | 11 more per 1000 (22 fewer to 64 more) |
RR 1.14 (0.70 to 1.85) |
813 (2) | ⊕⊕⊝⊝ low2,3 | ACEi alone has uncertain effects on doubling of SCr compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Fatal or nonfatal stroke Median follow‐up 4.5 years |
18 per 1000 | 5 fewer per 1000 (14 fewer to 22 more) |
RR 0.72 (0.23 to 2.24) |
775 (2) | ⊕⊕⊝⊝ low2,3 | ACEi alone has uncertain effects on fatal or nonfatal stroke compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Fatal or nonfatal myocardial infarction Median follow‐up 3.6 years |
33 per 1000 | 18 fewer per 1000 (27 fewer to 4 more) |
RR 0.44 (0.17 to 1.12) |
880 (3) | ⊕⊕⊝⊝ low2,3 | ACEi alone has uncertain effects on fatal or nonfatal myocardial infarction compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Micro‐ to macroalbuminuria Median follow‐up 1.2 years |
88 per 1000 | 22 more per 1000 (29 fewer to 116 more) |
RR 1.25 (0.67 to 2.32) |
958 (3) | ⊕⊝⊝⊝ very low1,2,4 | It is very uncertain whether ACEi alone makes any difference to progression from micro‐ to macroalbuminuria compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Micro‐ to normoalbuminuria Median follow‐up 1 year |
333 per 1000 | 27 more per 1000 (87 fewer to190 more) |
RR 1.08 (0.74 to 1.57) |
145 (3) | ⊕⊕⊝⊝ low1,5 | ACEi alone has uncertain effects on regression from micro‐ to normoalbuminuria compared to ACEi plus ARB in people with diabetes and kidney disease |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: Angiotensin‐converting‐enzyme inhibitors; ARB: Angiotensin receptor blockers; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. Some studies had unclear risks for allocation concealment, and the majority of them were not blinded (participant/investigator and/or outcomes assessor)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to study limitations. The studies were not blinded (participant/investigator and/or outcomes assessor)
4 Evidence certainty was downgraded by one level due to moderate statistical heterogeneity
5 Evidence certainty was downgraded by one level due to study limitations. All studies had unclear risks for allocation concealment, and they were not blinded (participant/investigator and/or outcomes assessor)
Summary of findings 6. Angiotensin receptor blockers (ARB) alone versus angiotensin‐converting‐enzyme inhibitors plus ARB for people with diabetes and kidney disease.
| ARB alone versus ACEi plus ARB for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: Italy, Japan, Slovenia, Spain, Turkey, USA Intervention: ARB alone Comparison: ACEi plus ARB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| ACEi + ARB | ARB | |||||
|
All‐cause death Median follow‐up 2 years |
62 per 1000 | 1 more per 1000 (15 fewer to 23 more) |
RR 1.02 (0.76 to 1.37) |
2607 (7) | ⊕⊕⊝⊝ low1,2 | ARB alone has uncertain effects on all‐cause death compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Cardiovascular death Median follow‐up 1 year |
4 per 1000 | 8 more per 1000 (1 fewer to 56 more) |
RR 3.03 (0.62 to 14.93) |
992 (4) | ⊕⊕⊝⊝ low1,2 | ARB alone has uncertain effects on cardiovascular death compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Doubling of SCr Median follow‐up 2.5 years |
59 per 1000 | 11 more per 1000 (9 fewer to 38 more) |
RR 1.18 (0.85 to 1.64) |
2252 (3) | ⊕⊕⊝⊝ low2,3 | ARB alone has uncertain effects on doubling of SCr compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Fatal or nonfatal stroke Median follow‐up 3.4 years |
23 per 1000 | 5 fewer per 1000 (15 fewer to 15 more) |
RR 0.76 (0.35 to 1.65) |
2223 (3) | ⊕⊕⊝⊝ low2,3 | ARB alone has uncertain effects on fatal or nonfatal stroke compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Fatal or nonfatal myocardial infarction Median follow‐up 2.7 years |
57 per 1000 | 17 fewer per 1000 (29 fewer to 1 more) |
RR 0.70 (0.49 to 1.01) |
2321 (4) | ⊕⊕⊝⊝ low2,3 | ARB alone has uncertain effects on fatal or nonfatal myocardial infarction compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Micro‐ to macroalbuminuria Median follow‐up 1.8 years |
51 per 1000 | 4 more per 1000 (12 fewer to 25 more) |
RR 1.07 (0.77 to 1.49) |
2410 (4) | ⊕⊕⊝⊝ low1,2 | ARB alone has uncertain effects on progression from micro‐ to macroalbuminuria compared to ACEi plus ARB in people with diabetes and kidney disease |
|
Micro‐ to normoalbuminuria Median follow‐up 1 year |
333 per 1000 | 13 more per 1000 (87 fewer to 150 more) |
RR 1.04 (0.74 to 1.45) |
151 (3) | ⊕⊕⊝⊝ low1,2 | ARB alone has uncertain effects on regression from micro‐ to normoalbuminuria compared to ACEi plus ARB in people with diabetes and kidney disease |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: Angiotensin‐converting‐enzyme inhibitors; ARB: Angiotensin receptor blockers; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. Some studies had unclear risks for allocation concealment, and the majority of them were not blinded (participant/investigator and/or outcomes assessor)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to study limitations. The studies were not blinded (participant/investigator and/or outcomes assessor)
Summary of findings 7. Angiotensin‐converting‐enzyme inhibitors (ACEi) versus another ACEi for people with diabetes and kidney disease.
| ACEi versus another ACEi for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: Hong‐Kong, Italy, Taiwan Intervention: ACEi Comparison: another ACEi | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| Another ACEi | ACEi | |||||
|
All‐cause death1 Median follow‐up 0.5 years |
No events | No events | ‐‐ | 269 (3) | ⊕⊕⊝⊝ low2,3 | Studies were not designed to measure effects of ACEi compared to another ACEi on all‐cause death in people with diabetes and kidney disease |
|
Cardiovascular death4 Median follow‐up 0.5 years |
No events | No events | ‐‐ | 269 (3) | ⊕⊕⊝⊝ low2,3 | Studies were not designed to measure effects of ACEi compared to another ACEi on cardiovascular death in people with diabetes and kidney disease |
| Doubling of SCr | Not reported |
Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
|
Fatal or nonfatal stroke |
Not reported |
Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
|
Fatal or nonfatal myocardial infarction |
Not reported |
Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Micro‐ to macroalbuminuria | Not reported |
Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Micro‐ to normoalbuminuria | Not reported |
Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: Angiotensin‐converting‐enzyme inhibitors; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All‐cause death was reported in a single study
2 Evidence certainty was downgraded by one level due to study limitations. All studies had unclear risks for allocation concealment, and the majority of them were not blinded (participant/investigator and/or outcomes assessor)
3 Evidence certainty was downgraded by one level due to imprecision
4 Cardiovascular death was reported in a single study
Summary of findings 8. Angiotensin receptor blocker (ARB) versus another ARB for people with diabetes and kidney disease.
| ARB versus another ARB for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: multinational (Argentina, Australia, Brazil, Canada, Mexico, New Zealand, South Korea, Taiwan, Thailand, USA, Japan, Europe, Asia, and South Africa) Intervention: ARB Comparison: another ARB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| Another ARB | ARB | |||||
|
All‐cause death Median follow‐up 1 year |
21 per 1000 | 9 fewer per 1000 (20 fewer to 125 more) |
RR 0.58 (0.05 to 7.08) |
2041 (5) | ⊕⊝⊝⊝ very low1,2,3 | It is very uncertain whether ARB makes any difference to all‐cause death compared to another ARB in people with diabetes and kidney disease |
|
Cardiovascular death Median follow‐up 1 year |
9 per 1000 | 3 more per 1000 (5 fewer to 25 more) |
RR 1.34 (0.47 to 3.82) |
1360 (4) | ⊕⊕⊝⊝ low1,2 | ARB have uncertain effects on cardiovascular death compared to another ARB in people with diabetes and kidney disease |
|
Doubling of SCr4 Follow‐up 1 year |
7 per 1000 | 0 per 1000 (6 fewer to 28 more) |
RR 1.00 (0.20 to 4.94) |
857 (1) | ⊕⊕⊝⊝ low2,5 | Studies were not designed to measure effects of ARB compared to another ARB on doubling of serum creatinine in people with diabetes and kidney disease |
|
Fatal or nonfatal stroke6 Follow‐up 1 year |
12 per 1000 | 15 more per 1000 (3 fewer to 64 more) |
RR 2.21 (0.77 to 6.29) |
857 (1) | ⊕⊕⊝⊝ low2,5 | Studies were not designed to measure effects of ARB compared to another ARB on fatal or nonfatal stroke in people with diabetes and kidney disease |
|
Fatal or nonfatal myocardial infarction7 Follow‐up 1 year |
26 per 1000 | 17 fewer per 1000 (23 fewer to 4 more) |
RR 0.36 (0.12 to 1.14) |
857 (1) | ⊕⊕⊝⊝ low2,5 | Studies were not designed to measure effects of ARB compared to another ARB on fatal or nonfatal myocardial infarction in people with diabetes and kidney disease |
|
Micro‐ to macroalbuminuria 8 Follow‐up 1 year |
90 per 1000 | 31 more per 1000 (12 fewer to 96 more) |
RR 1.34 (0.87 to 2.07) |
716 (1) | ⊕⊕⊝⊝ low 2,5 | Studies were not designed to measure effects of ARB compared to another ARB on progression from micro‐ to macroalbuminuria in people with diabetes and kidney disease |
|
Micro‐ to normoalbuminuria9 Follow‐up 1 year |
11 per 1000 | 0 per 1000 (8 fewer to 32 more) |
RR 0.98 (0.25 to 3.88) |
716 (1) | ⊕⊕⊝⊝ low2,5 | Studies were not designed to measure effects of ARB compared to another ARB on regression from micro‐ to normoalbuminuria in people with diabetes and kidney disease |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARB: Angiotensin receptor blockers; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. All studies had unclear risks for allocation concealment, and the majority of them were not blinded (participant/investigator and/or outcomes assessor)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to important statistical heterogeneity
4 Doubling of SCr was reported in a single study
5 Evidence certainty was downgraded by one level due to study limitations. The studies had unclear risks for sequence generation and were not blinded (outcomes assessor)
6 Fatal or nonfatal stroke was reported in a single study
7 Fatal or nonfatal myocardial infarction was reported in a single study
8 Micro‐ to macroalbuminuria was reported in a single study
9 Micro‐ to normoalbuminuria was reported in a single study
Summary of findings 9. Low‐dose angiotensin receptor blockers (ARB) versus high‐dose ARB for people with diabetes and kidney disease.
| Low‐dose ARB versus high‐dose ARB for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: Canada Intervention: low‐dose ARB Comparison: high‐dose ARB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| High‐dose ARB | Low‐dose ARB | |||||
| All‐cause death1 | No events | No events | ‐‐ | 156 (2) | ⊕⊕⊝⊝ low2,3 | Studies were not designed to measure effects of low‐dose ARB compared to high‐dose ARB on all‐cause death in people with diabetes and kidney disease |
|
Cardiovascular death4 Follow‐up 0.6 years |
No events | No events | ‐‐ | 156 (2) | ⊕⊕⊝⊝ low2,3 | Studies were not designed to measure effects of low‐dose ARB compared to high‐dose ARB on cardiovascular death in in people with diabetes and kidney disease |
| Doubling of SCr | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Fatal or nonfatal stroke | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Fatal or nonfatal myocardial infarction | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Micro‐ to macroalbuminuria | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Micro‐ to normoalbuminuria | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARB: Angiotensin receptor blockers; CI: Confidence interval; RR: Risk ratio; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All‐cause death was reported in a single study
2 Evidence certainty was downgraded by one level due to study limitations. The studies had unclear risks for sequence generation and were not blinded (outcomes assessor)
3 Evidence certainty was downgraded by one level due to imprecision
4 Cardiovascular death was reported in a single study
Summary of findings 10. Low‐dose angiotensin receptor blockers (ARB) plus spironolactone versus high‐dose ARB plus spironolactone for people with diabetes and kidney disease.
| Low‐dose ARB plus spironolactone versus high‐dose ARB plus spironolactone for people with diabetes and kidney disease | ||||||
|
Patient or population: people with diabetes and kidney disease Settings: single centre Intervention: low‐dose ARB plus spironolactone Comparison: high‐dose ARB plus spironolactone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| High‐dose ARB + spironolactone | Low‐dose ARB + spironolactone | |||||
| All‐cause death | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Cardiovascular death | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Doubling of SCr | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Fatal or nonfatal stroke | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Fatal or nonfatal myocardial infarction | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Micro‐ to macroalbuminuria | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Micro‐ to normoalbuminuria | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARB: Angiotensin receptor blockers; CI: Confidence interval; SCr: Serum creatinine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Diabetes‐related kidney disease, defined as the presence of albuminuria in people with type 1 and type 2 diabetes with or without reduced kidney function, occurs in 25% to 40% of people with diabetes within 20 to 25 years of diagnosis (Bonino 2019; Ritz 1999). People who have diabetes and kidney disease experience both microvascular complications (causing nephropathy, retinopathy and neuropathy) and damage to large blood vessels (leading to heart disease, stroke, peripheral vascular disease and related death). People with diabetes progress through a pathway of compensatory glomerular hypertrophy, then progressive loss of kidney structure and function due to glomerulosclerosis, showing clinically as microalbuminuria (urine albumin excretion (UAE) 30 to 300 mg/d), then macroalbuminuria (> 300 mg/d). Progressive kidney failure requires kidney replacement therapy (KRT) (dialysis or kidney transplantation) (Mogensen 1995; Mogensen 1999; Tabriziani 2018; Vaidya 2022) and markedly increases the risk of death, cardiovascular events and hospitalisation (Go 2004; Stringer 2013). About one‐third of patients who have diabetes and kidney disease will eventually progress to kidney failure (Ritz 1999; Yuan 2017).
Description of the intervention
Drugs to delay the progression of diabetes‐related kidney disease include angiotensin‐converting‐enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB). ACEi and ARB block the activity of angiotensin II (Messerli 2018; Ritter 2011), a central component of blood pressure (BP) regulation. Blockade of angiotensin II leads to generalised arterial vasodilatation, which lowers BP and glomerular arteriolar vasodilatation (Arendshorst 1999). Dilatation of glomerular arterioles (particularly the efferent arteriole) lowers glomerular pressure and directly protects kidney structure and function, leading to reduced proteinuria and slowing progressive kidney failure. In addition, interruption of angiotensin II activity ameliorates kidney tissue fibrosis.
Recently, newer glucose‐lowering interventions have been developed for preventing the progression of kidney disease in people with diabetes and kidney disease, including sodium‐glucose‐linked co‐transport‐2 (SGLT‐2) inhibitors (CREDENCE 2019; DAPA‐CKD 2021). SGLT‐2 inhibitors block the SGLT‐2 protein located in the proximal convoluted tubule of the nephron, leading to the reabsorption of glucose in the kidney and, therefore, lower plasma glucose concentrations (Joshi 2021).
How the intervention might work
BP reductions have been shown in meta‐analyses to lower the risk of kidney failure on average by 10% and slow the progression of albuminuria (a predictive marker of kidney failure and death) to a similar extent in populations at risk of cardiovascular disease (Cheng 2014; Lv 2012). For people who have diabetes without kidney disease, ACEi reduces death and the progression of kidney disease, whereas treatment benefits for ARB are less certain, and combination therapy may be more effective than ACEi alone (Lv 2012a) for reducing albuminuria. Since large‐scale randomised controlled trials (RCTs) have shown that ACEi (HOPE 1996) and ARB (IDNT 2001; IRMA‐2 2001; RENAAL 2001) slow the deterioration of kidney function and reduce proteinuria, these have become the most broadly used agents in people with diabetes and kidney disease. Global guidelines recommend ACEi and ARB in people with diabetes and kidney disease (JNC 8 2014; KDIGO Blood Pressure Guidelines 2021; NKF KDOQI Guidelines 2004).
Why it is important to do this review
ACEi and ARB are considered first‐line therapies in relation to emerging therapies that reduce glomerular filtration pressure to prevent the progression of kidney disease in people with diabetes and kidney disease. Due to the large number of new studies, the original published Cochrane review (Strippoli 2006) has been updated.
Objectives
We compared the efficacy and safety of ACEi and ARB therapy (either as monotherapy or in combination) on cardiovascular and kidney outcomes in adults with diabetes and kidney disease.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs of at least six months' duration that evaluated ACEi or ARB therapy (monotherapy or in combination) in treating diabetes‐related kidney disease. We did not include quasi‐RCTs in which treatment assignment is decided through methods such as date of birth or day of the week.
Types of participants
RCTs enrolling adults with diabetes and kidney disease were included regardless of the severity of albuminuria. We included participants who had either microalbuminuria (equivalence of a UAE of 30 to 300 mg/d) or macroalbuminuria (UAE > 300 mg/d). Patients were included either if they reported microalbuminuria or macroalbuminuria regardless of the estimated glomerular filtration rate (eGFR) or with eGFR < 90 mL/min/1.73 m2, regardless of albuminuria. We did not include participants who had an eGFR < 15 mL/min/1.73 m2 treated with dialysis, kidney transplantation or supportive care.
Types of interventions
Interventions of direct interest
We included studies that evaluated one or more of the following interventions: ACEi or ARB alone, in combination, directly with each other, placebo or no treatment.
Types of outcome measures
This review did not exclude studies that did not report our outcomes of interest.
Primary outcomes
Primary efficacy outcome
All‐cause death
Primary safety outcome
Withdrawal from treatment for any cause
Secondary outcomes
Secondary efficacy outcomes
Cardiovascular death
Kidney failure
Fatal or nonfatal myocardial infarction (MI)
Fatal or nonfatal stroke
Doubling of serum creatinine (SCr) or 50% reduction in GFR
Progression from micro‐ to macroalbuminuria
Regression from micro‐ to normoalbuminuria
Secondary safety outcomes
Hyperkalaemia
Cough
Headache
Peripheral oedema
Impotence
Presyncope/dizziness
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 17 March 2024 through contact with the Information Specialist using search terms relevant to this review without language restriction.
The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. The Specialised Register section of information about Cochrane Kidney and Transplant provides details of search strategies and a list of handsearched journals, conference proceedings, and current awareness alerts.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies
We searched grey literature sources (e.g. abstracts, dissertations and theses) in addition to those already included in the Cochrane Kidney and Transplant Specialised Register.
Data collection and analysis
Selection of studies
In this updated review, the search strategies described in Appendix 1 were used to obtain titles and abstracts of studies that might be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable based on the inclusion criteria for this review. However, studies and reviews that might include relevant data or information on studies were retained initially, and their full‐text versions were analysed. Any discrepancy was solved by discussion with a third author.
Data extraction and management
Data extraction was carried out independently by the same two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together, and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancies between published versions were highlighted. When necessary, the authors of the included studies were contacted for further information.
Outcome data
We extracted from each included study the following (as defined by study investigators): number of patients experiencing one or more events (all‐cause death, withdrawal from the study for any cause, cardiovascular death, kidney failure, fatal or nonfatal MI, fatal or nonfatal stroke, doubling of SCr, reduction in GFR, change in GFR, GFR at the end of the treatment, progression from microalbuminuria to macroalbuminuria, regression from microalbuminuria to normoalbuminuria, regression from macroalbuminuria to microalbuminuria, regression from macroalbuminuria to normoalbuminuria, hyperkalaemia, cough, headache, peripheral oedema, impotence and dizziness), the duration of the intervention, the number of participants, the drop‐out rates, the trial investigators' definition of response and the interventions being compared.
Data on potential effect modifiers
We extracted from each study data that might act as effect modifiers.
Duration of follow‐up
Type 1 and 2 diabetes
Presence of hypertension at baseline
Stage of kidney disease (microalbuminuria, macroalbuminuria, both microalbuminuria and macroalbuminuria)
Sponsorship (for‐profit, not‐for‐profit, unclear)
The mean age of the study participants
Allocation concealment (low risk versus unclear or high risk)
Blinding of outcome assessment (low risk versus unclear or high risk).
Other data
We extracted the following additional information from each included study.
Proportion men
Baseline eGFR
Non‐randomised antihypertensive co‐interventions
Specific randomised intervention (drug type within each class).
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2022) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Two independent authors assessed the risk of bias in selected studies. Any disagreement was resolved through discussion and consultation with a third author. When necessary, the study authors were contacted for further information. When a description of what was reported to have happened was provided, a judgment on the risk of bias was made for each domain based on the following three categories.
High risk of bias
Low risk of bias
Unclear risk of bias.
Measures of treatment effect
For dichotomous outcomes (e.g. all‐cause death, withdrawn from the trial for any cause, cardiovascular death, kidney function), results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. change in GFR or GFR reported at the end of the treatment), the mean difference (MD) and its 95% CI were used. The final results are presented in International System (SI) units. When investigators did not report crude event data, available reported risk estimates and their 95% CIs were included in the meta‐analyses.
Cluster‐randomised studies
We anticipated that studies using clustered randomisation were controlled for clustering effects. In case of doubt, we planned to contact the authors to ask for individual participant data to calculate an estimate of the intra‐cluster correlation coefficient (ICC). If this was not possible, we obtained external estimates of the ICC from a similar study or a study of a similar population as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). If ICCs from other sources were used, we reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. No cluster‐randomised studies were identified.
Cross‐over studies
Cross‐over studies were to be analysed using data from the first study period before cross‐over. No cross‐over studies were included.
Dealing with missing data
Any further information or clarification required from the authors was requested by written or electronic correspondence, and relevant information obtained in this manner was included in the review. Missing dichotomous outcome data were managed according to the intention‐to‐treat principle (ITT). It was assumed that participants in the full analysis set who dropped out after randomisation had a negative outcome. Sensitivity analyses were also undertaken to examine the effect of the decision to use imputed data.
To evaluate the presence of clinical and methodological heterogeneity, we generated descriptive statistics for trial and study population characteristics across all eligible trials that compare each pair of interventions. We assessed the presence of clinical heterogeneity within each pairwise comparison by comparing these characteristics.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plots. We then quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). The following guide to interpreting I² values was used.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test or a CI for I²) (Higgins 2022).
Assessment of reporting biases
Funnel plots were used to assess the potential existence of small study bias (Higgins 2022).
Data synthesis
Data were pooled using the random‐effects model, but the fixed‐effect model was also used to ensure the robustness of the chosen model and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis to explore possible sources of heterogeneity considering.
Type of diabetes (type I versus type II)
Presence of hypertension
Microalbuminuria versus macroalbuminuria.
Sensitivity analysis
We limited analyses to studies in which allocation concealment and attrition were adjudicated low risk of bias. Sensitivity analyses excluding small studies were not planned.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in summary of findings tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2022a). The summary of findings tables also include an overall grading of the evidence related to each main outcome using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2022b). We presented the following outcomes in the summary of findings tables.
All‐cause death
Cardiovascular death
Doubling of SCr
Fatal or nonfatal stroke
Fatal or nonfatal MI
Micro‐ to macroalbuminuria
Micro‐ to normoalbuminuria
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
For this 2024 update, we searched the Cochrane Kidney and Transplant Register of Studies (March 2024) and identified 563 new reports. Sixty new studies (207 reports) were included, 84 studies (327 reports) were excluded, and three ongoing studies were identified. Four new studies are awaiting classification (recently completed; no data available/abstract‐only publication). We also identified 195 new reports of existing included studies.
We reassessed and deleted one study awaiting classification (wrong population) and excluded two included studies (one study used the wrong comparator (Mathiesen 1991), and one was a report of an existing included study (Parving 1989).
A total of 109 studies were included (471 reports, 28,341 randomised participants), 84 were excluded, four are awaiting classification, and three are ongoing (Figure 1).
1.
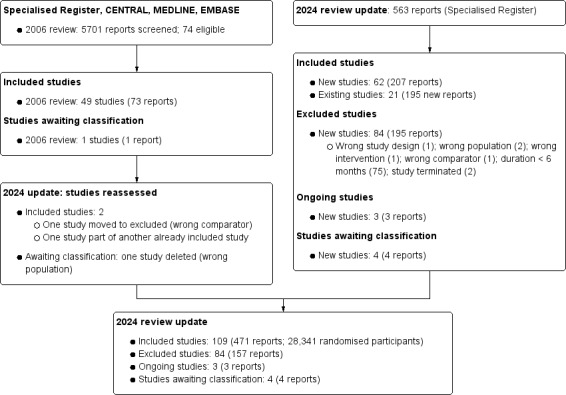
Flow diagram show study selection
Included studies
ide The characteristics of the participants and the interventions in the included studies are detailed in the Characteristics of included studies tables.
Study design, setting and characteristics
Study duration varied from six months to 9.5 years, with a median of 1.5 years. No studies had a cross‐over or cluster design. Studies were conducted from 1987 to 2021 in Australia (four studies), Austria (one study), Brazil (one study), Canada (three studies), Chile (two studies), China (five studies), Columbia (one study), Denmark (seven studies), France (two studies), Hong‐Kong (four studies), India (five studies), Israel (one study), Italy (eight studies), Italy and Slovenia (one study), Italy and the UK (one study), Japan (13 studies), Japan and Hong‐Kong (one study), the Netherlands (three studies), Serbia (one study), Spain (four studies), Sweden (one study), Taiwan (one study), Thailand (one study), Turkey (five studies), the UK (four studies), the USA (15 studies), multinational (13 studies), and one study did not provide any specific details about the country. Forty‐five studies received at least some funding from pharmaceutical companies.
Study participants
The sample size varied from nine to 4,937 participants (median of 58 participants). The mean study age ranged from 22 years to 74 years (median 55 years).
Twenty‐nine studies were performed in patients with type I diabetes, 56 studies in patients with type II diabetes, seven studies included patients with both type I and type II diabetes, and 16 studies did not specify the type of diabetes.
Only 35 studies reported information regarding the eGFR or stage of chronic kidney disease (CKD).
Fifty‐three studies were performed in people with microalbuminuria, 22 in people with microalbuminuria, 14 studies in people with both microalbuminuria and microalbuminuria, and one study included people with normoalbuminuria, microalbuminuria or macroalbuminuria with kidney impairment. Twelve studies reported albuminuria in general, and five studies did not provide information about the presence of albuminuria but were performed in patients with kidney impairment.
Thirty‐five studies enrolled hypertensive patients, 42 studies enrolled patients without hypertension, 17 studies enrolled patients with and without hypertension, and 15 studies did not report information about hypertension.
Interventions
A broad range of interventions have been reported in the included studies. The interventions are listed below.
ACEi versus placebo or no treatment
Fifty‐seven studies compared ACEi to placebo or no treatment.
Benazapril: two studies (AIPRI 1996; Niu 2008)
Captopril: 16 studies (Bilo 1993; Capek 1994; CAPTOPRIL 1992; Chase 1993; Cocchi 1989; Hansen 1994; Hommel 1995; JAPAN‐IDDM 2002; Laffel 1995; Larsen 1990; Lee 1990; Muirhead 1999; Parving 1989; Romero 1993; Viberti 1994; Yoldi 1995)
Cilazapril: one study (Phillips 1993)
Enalapril: 14 studies (ABCD 1996; Ahmad 1997; Ahmad 2003; Bauer 1992; Durruty 1990; Durruty 1996; ESPRIT 1992; Lebovitz 1994; Marre 1987; Ravid 1993; Sano 1994; SOLVD (Treatment) 1990; Stornello 1988; Stornello 1989)
Fosinopril: three studies (Carella 1999; PREVEND IT 2000; Tong 2006)
Lisinopril: five studies (Bakris 1994; Crepaldi 1998; EUCLID 1997; O'Donnell 1993; Poulsen 2001)
Perindopril: eight studies (MDNSG 1993; Cordonnier 1999; Jerums 2001; Jerums 2004; Nankervis 1998; White 2001; Xia 1996; Yao 2001a)
Ramipril: six studies (ATLANTIS 2000; Bojestig 2001; DIABHYCAR 1996; Garg 1998; HOPE 1996; Trevisan 1995)
Trandolapril: one study (Nakamura 2002a)
Temocapril: one study (Ogawa 2007).
ARB versus placebo or no treatment
Sixteen studies compared ARB to placebo or no treatment.
Candesartan: two studies (Nakamura 2002a; Ogawa 2007)
Irbesartan: two studies (IDNT 2001; IRMA‐2 2001)
Losartan: five studies (Mehdi 2009; RENAAL 2001; Sawaki 2008; Tan 2002; Weil 2012)
Olmesartan: one study (ORIENT 2006)
Telmisartan: three studies (INNOVATION 2005; Krairittichai 2009; TRANSCEND 2009)
Valsartan: three studies (ABCD‐2V 2006; Dragovic 2003; Muirhead 1999).
ARB versus ACEi
Twenty‐four studies compared ARB to ACEi.
Candesartan versus trandolapril: one study (Nakamura 2002a)
Candesartan versus temocapril: one study (Ogawa 2007)
Candesartan versus lisinopril: two studies (CALM 2000; Schram 2005)
Candesartan versus enalapril: one study (Rizzoni 2005)
Candesartan versus enalapril or trandolapril: one study (Sato 2003)
Irbesartan versus lisinopril: one study (PRONEDI 2013)
Losartan versus enalapril: four studies (Castelao 1999; Deyneli 2006; Lacourciere 2000; Tutuncu 2001)
Losartan versus fosinopril: one study (Kavgaci 2002)
Losartan versus lisinopril: two studies (Atmaca 2006; Bansal 2004)
Losartan plus ACEi (enalapril, lisinopril, temocapril or imidapril) versus ACEi plus ACEi (enalapril, imidapril or delapril): one study (Abe 2007a)
Telmisartan versus enalapril: two studies (Arpitha 2020; DETAIL 2002)
Telmisartan versus lisinopril: one study (Sengul 2006)
Telmisartan versus ramipril: two studies (ONTARGET 2004; Raj 2021)
Valsartan versus enalapril: one study (Ko 2005)
Valsartan versus captopril: one study (Muirhead 1999)
Valsartan versus benazepril: one study (Ruggenenti 2019)
Any available ACEi versus any available ARB: one study (LIRICO 2007)
ACEi plus ARB versus placebo or no treatment
Two studies compared ACEi plus ARB to placebo or no treatment.
Temocapril plus candesartan versus no treatment: one study (Ogawa 2007)
Trandolapril plus candesartan versus placebo: one study (Nakamura 2002a)
ACEi or ARB alone versus ACEi plus ARB
ACEi versus ACEi plus ARB
Twelve studies compared ACEi to ACEi plus ARB.
Benazepril versus benazepril plus valsartan: one study (Ruggenenti 2019)
Enalapril versus enalapril plus losartan: two studies (Titan 2011; Tutuncu 2001)
Lisinopril versus lisinopril plus losartan: two studies (Atmaca 2006; Bansal 2004)
Lisinopril versus lisinopril plus candesartan: one study (CALM II 2003)
Lisinopril versus lisinopril plus irbesartan: one study (PRONEDI 2013)
Lisinopril versus lisinopril plus telmisartan: one study (Sengul 2006)
Ramipril versus ramipril plus telmisartan: one study (ONTARGET 2004)
Tandolapril versus tandolapril plus candesartan: one study (Nakamura 2002a)
Temocapril versus temocapril plus candesartan: one study (Ogawa 2007)
Any available ACEi versus any available ACEi plus any available ARB: one study (LIRICO 2007)
ARB versus ACEi plus ARB
Twelve studies compared ARB to ACEi plus ARB.
Candesartan versus tandolapril plus candesartan: two studies (CAT 2008: Nakamura 2002a)
Candesartan versus temocapril plus candesartan: one study (Ogawa 2007)
Irbesartan versus lisinopril plus irbesartan: one study (PRONEDI 2013)
Losartan versus lisinopril plus losartan: three studies (Atmaca 2006; Bansal 2004; VA NEPHRON‐D 2009)
Losartan versus enalapril plus losartan: one study (Tutuncu 2001)
Telmisartan versus lisinopril plus telmisartan: one study (Sengul 2006)
Telmisartan versus ramipril plus telmisartan: one study (ONTARGET 2004)
Valsartan versus benazepril plus valsartan: one study (Ruggenenti 2019)
Any available ARB versus any available ACEi plus any available ARB: one study (LIRICO 2007)
ACEi versus a combination of two different ACEi
One study compared ACEi to a combination of two different ACEi.
Ramipril versus captopril plus ramipril: one study (Lewis 1995)
ACEi versus another ACEi
Four studies compared ACEi versus another ACEi.
Captopril versus enalapril: two studies (Cheng 1990; Lin 1995)
Captopril versus imidapril: one study (JAPAN‐IDDM 2002)
Imidapril versus ramipril: one study (Fogari 2013a)
ARB versus another ARB
Six studies compared ARB versus another ARB.
Candesartan versus valsartan: one study (Yoneda 2007)
Fimasartan versus losartan: one study (FANTASTIC 2017)
Telmisartan versus losartan: one study (AMADEO 2008)
Telmisartan versus valsartan: one study (VIVALDI 2005)
Telmisartan versus candesartan versus valsartan versus losartan: one study (Arai 2008)
Telmisartan versus candesartan versus olmesartan versus losartan: one study (Nakamura 2010c)
Low‐dose versus high‐dose ARB
Five studies compared low‐dose versus high‐dose ARB.
Low‐dose candesartan versus high‐dose candesartan: two studies (CAT 2008; SMART 2009)
Low‐dose irbesartan versus high‐dose irbesartan: one study (Chen 2018b)
Low‐dose irbesartan versus high‐dose irbesartan with spironolactone in both arms: one study (Chen 2018b)
Low‐dose telmisartan versus high‐dose telmisartan: one study (Kitamura 2020)
Low‐dose valsartan versus high‐dose valsartan: one study (DROP 2006)
Excluded studies
In this 2024 update, 84 studies (157 records) were excluded. The reasons for exclusion were wrong study design (one study), wrong population (two studies), wrong intervention (three studies), wrong comparator (one study), treatment duration of less than six months (75 studies), or the study was terminated (two studies).
Ongoing studies
Our search identified three studies that have yet to be completed.
ACEi plus ARB (Losartan plus enalapril) with ARB (losaratan) (CTRI/2023/05/052593)
ARB (azilsartan) versus ARB (losartan) (NCT05753696)
ACEi (enalapril) versus ARB (azilsartan) (TCTR20220426002).
Studies awaiting classification
Our search identified four studies: two published more than 10 years ago but never fully reported (Andrysiak‐Mamos 1997; Vijay 2000), and two recently completed studies that will be assessed in a future update of this review (Limonte 2024; Vineela 2023).
Risk of bias in included studies
Figure 2 summarises the risks of bias for studies overall, and Figure 3 reports the risks of bias in each individual study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Methods for generating the random sequence were deemed to be at low risk of bias in 76 studies. In 33 studies, the methods were unclear as they stated they were randomised but provided no further details on how this was undertaken.
Allocation concealment
In 10 studies, allocation concealment was judged to have a low risk of bias, while the risk of bias for allocation concealment was unclear in 99 studies.
Blinding
Performance bias
Fifty‐four studies were blinded and considered to be at low risk of bias for performance bias. Fifty‐five studies were not blinded and were considered at high risk of performance bias.
Detection bias
Forty‐nine studies were based on laboratory assessment and were considered at low risk of bias, and 58 studies were considered at high risk of bias for blinding of outcome assessment since they reported patient‐centred outcomes, including adverse events. The risk of detection bias was unclear in two studies.
Incomplete outcome data
Thirty‐seven studies met the criteria for low risk of attrition bias. Thirty‐seven studies were considered at high risk of attrition bias as there was a differential loss to follow‐up between treatment groups, or high attrition rates in the treatment groups. Loss to follow‐up was commonly due to withdrawal from the study or adverse events. The risk of attrition bias was unclear in 35 studies.
Selective reporting
Eleven studies reported expected and clinically relevant outcomes and were deemed to be at low risk of bias. Ninety‐eight studies did not report patient‐centred outcomes of death or adverse events.
Other potential sources of bias
Thirty‐eight studies appeared to be free from other sources of bias. Twenty studies were considered to be at high risk of bias due to the potential role of funding, and for 51 studies, the assessment of other sources of bias was unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10.
ACEi versus placebo or no treatment
Forty studies (Ahmad 1997; Ahmad 2003; AIPRI 1996; ATLANTIS 2000; Bakris 1994; Bauer 1992; Bilo 1993; Bojestig 2001; Capek 1994; CAPTOPRIL 1992; Chase 1993; Crepaldi 1998; DIABHYCAR 1996; Durruty 1996; ESPRIT 1992; EUCLID 1997; Garg 1998; Hansen 1994; Hommel 1995; HOPE 1996; JAPAN‐IDDM 2002; Jerums 2001; Jerums 2004; Laffel 1995; Larsen 1990; Marre 1987; Muirhead 1999; Nankervis 1998; O'Donnell 1993; Ogawa 2007; Parving 1989; Phillips 1993; Poulsen 2001; Ravid 1993; Romero 1993; Sano 1994; Stornello 1988; Tong 2006; Trevisan 1995; Viberti 1994) comparing ACEi versus placebo or no treatment in patients with diabetes and kidney disease, during a median follow‐up of two years, could be meta‐analysed. The certainty of the evidence was low or very low for all outcomes (Table 1).
Primary outcomes
Compared to placebo or no treatment, ACEi may make little or no difference to all‐cause death (Analysis 1.1 (24 studies, 7413 participants): RR 0.91, 95% CI 0.73 to 1.15; I2 = 23%; low certainty evidence).
1.1. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 1: Death: any cause
Compared to placebo or no treatment, ACEi may result in similar withdrawals from treatment due to any cause (Analysis 1.2 (7 studies, 5306 participants): RR 1.03, 95% CI 0.90 to 1.19; I2 = 0%; low certainty evidence).
1.2. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 2: Withdrawn
Secondary outcomes
It is uncertain whether ACEi made any difference in cardiovascular death (Analysis 1.3 (17 studies, 5625 participants): RR 0.67, 95% CI 0.13 to 3.57; I2 = 46%; very low certainty evidence) compared with placebo to no treatment.
1.3. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 3: Cardiovascular death
ACEi may prevent kidney failure (Analysis 1.4 (8 studies, 6643 participants): RR 0.61, 95% CI 0.39 to 0.94; I2 = 0%; low certainty evidence), but may make little or no difference to fatal or nonfatal MI (Analysis 1.5 (3 studies, 5100 participants): RR 0.79, 95% CI 0.57 to 1.09; I2 = 0%; low certainty evidence) or fatal or nonfatal stroke (Analysis 1.6 (2 studies, 4944 participants): RR 1.02, 95% CI 0.80 to 1.31; I2 = 0%; low certainty evidence)
1.4. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 4: Kidney failure
1.5. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 5: Fatal or nonfatal myocardial infarction
1.6. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 6: Fatal or nonfatal stroke
The effects on doubling of SCr (Analysis 1.7 (8 studies, 6702 participants): RR 0.69, 95% CI 0.47 to 1.01; I2 = 37%; very low certainty evidence) were uncertain, compared with placebo or no treatment. There was moderate heterogeneity in treatment effects observed among studies.
1.7. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 7: Doubling of serum creatinine
ACEi had uncertain effects on GFR (Analysis 1.8 (3 studies, 116 participants): RR 0.91, 95% CI 0.55 to 1.51; I2 = 0%; low certainty evidence) and change in GFR (Analysis 1.9 (3 studies, 88 participants): MD 0.20 mL/min/1.73 m2, 95% CI ‐0.50 to 0.90; I2 = 53%; low certainty evidence) compared with placebo or no treatment. There was moderate heterogeneity in treatment effects observed among studies.
1.8. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 8: Reduction in GFR
1.9. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 9: Change in GFR [mL/min/1.73 m²]
It is uncertain whether ACEi made any difference to GFR at the end of treatment (Analysis 1.10 (7 studies, 188 participants): MD ‐1.41 mL/min/1.73 m2, 95% CI ‐8.79 to 5.97; I2 = 72%; very low certainty evidence) compared with placebo or no treatment. There was moderate heterogeneity in treatment effects observed among studies.
1.10. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 10: GFR (at end of treatment) [mL/min/1.73 m²]
It is uncertain whether ACEi made any difference in the progression from microalbuminuria to macroalbuminuria (Analysis 1.11 (19 studies, 2282 participants): RR 0.43, 95% CI 0.28 to 0.64; I2 = 54%; very low certainty evidence) or the regression from microalbuminuria to normoalbuminuria (Analysis 1.12 (17 studies, 1959 participants): RR 3.01, 95% CI 1.86 to 4.88; I2 = 39%; very low certainty evidence) compared with placebo or no treatment. There was moderate heterogeneity in treatment effects observed among studies.
1.11. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 11: Micro‐ to macroalbuminuria
1.12. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 12: Micro‐ to normoalbuminuria
ACEi had uncertain effects on hyperkalaemia (Analysis 1.13 (4 studies, 1289 participants): RR 0.82, 95% CI 0.48 to 1.40; I2 = 0%; low certainty evidence), but may increase cough (Analysis 1.14 (10 studies, 6615 participants): RR 3.26, 95% CI 2.30 to 4.60; I2 = 0%; low certainty evidence) compared with placebo or no treatment.
1.13. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 13: Hyperkalaemia
1.14. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 14: Cough
ACEi had uncertain effects on headache (Analysis 1.15 (5 studies, 6244 participants): RR 0.84, 95% CI 0.34 to 2.12; I2 = 0%; low certainty evidence) and impotence (Analysis 1.16 (5 studies, 1528 participants): RR 1.02, 95% CI 0.26 to 3.91; I2 = 0%; low certainty evidence) compared with placebo or no treatment.
1.15. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 15: Headache
1.16. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 16: Impotence
Muirhead 1999 reported that ACEi did not differ from placebo in the number of participants reporting dizziness (Analysis 1.17 (58 participants): RR 3.00, 95% CI 0.33 to 27.18; very low certainty evidence).
1.17. Analysis.

Comparison 1: ACEi versus control (placebo or no treatment), Outcome 17: Dizziness
ARB versus placebo or no treatment
Fifteen studies (ABCD‐2V 2006; Dragovic 2003; IDNT 2001; INNOVATION 2005; IRMA‐2 2001; Krairittichai 2009; Lacourciere 2000; Mehdi 2009; Muirhead 1999; Ogawa 2007; ORIENT 2006; RENAAL 2001; Sawaki 2008; Tan 2002; Weil 2012) comparing ARB versus placebo or no treatment in patients with diabetes and kidney disease, during a median follow‐up of 1.8 years, could be meta‐analysed. The certainty of the evidence was low or very low for all outcomes (Table 2).
Primary outcomes
Compared to placebo or no treatment, ARB may make little or no difference to all‐cause death (Analysis 2.1 (11 studies, 4260 participants): RR 0.99, 95% CI 0.85 to 1.16; I2 = 0%; low certainty evidence).
2.1. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 1: Death: any cause
Compared to placebo or no treatment, ARB had uncertain effects on withdrawal from treatment due to any cause (Analysis 2.2 (3 studies, 721 participants): RR 0.85, 95% CI 0.58 to 1.26; I2 = 2%; low certainty evidence).
2.2. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 2: Withdrawn
Secondary outcomes
Compared to placebo or no treatment, ARB had uncertain effects on cardiovascular death (Analysis 2.3 (6 studies, 878 participants): RR 3.36, 95% CI 0.93 to 12.07; low certainty evidence).
2.3. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 3: Cardiovascular death
ARB may prevent kidney failure (Analysis 2.4 (3 studies, 3227 participants): RR 0.82, 95% CI 0.72 to 0.94; I2 = 0%; low certainty evidence) compared to placebo or no treatment,
2.4. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 4: Kidney failure
ARB had uncertain effects on fatal or nonfatal MI (Analysis 2.5 (2 studies, 619 participants): RR 0.43, 95% CI 0.11 to 1.65; low certainty evidence) and fatal or nonfatal stroke (Analysis 2.6 (2 studies, 619 participants): RR 0.76, 95% CI 0.32 to 1.77; I2 = 0%; low certainty evidence) compared with placebo or no treatment.
2.5. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 5: Fatal and nonfatal myocardial infarction
2.6. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 6: Fatal or nonfatal stroke
ARB may prevent doubling of SCr (Analysis 2.7 (4 studies, 3280 participants): RR 0.84, 95% CI 0.72 to 0.97; I2 = 32%; low certainty evidence).
2.7. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 7: Doubling of serum creatinine
ARB had uncertain effects on GFR reduction (Analysis 2.8 (2 studies, 171 participants): RR 0.25, 95% CI 0.03 to 2.14; low certainty evidence) compared with placebo or no treatment. Krairittichai 2009 reported that ARB may reduce GFR at the end of treatment (Analysis 2.9 (80 participants): MD ‐11.57 mL/min/1.73 m2, 95% CI ‐21.73 to ‐1.41; low certainty evidence) compared with no treatment.
2.8. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 8: Reduction in GFR
2.9. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 9: GFR (at end of treatment) [mL/min/1.73 m²]
ARB may prevent the progression from microalbuminuria to macroalbuminuria (Analysis 2.10 (5 studies, 815 participants): RR 0.44, 95% CI 0.23 to 0.85; I2 = 74%; low certainty evidence) compared with placebo or no treatment. There was moderate heterogeneity in treatment effects observed among studies. However, it was uncertain whether ARB made any difference in the regression from microalbuminuria to normoalbuminuria (Analysis 2.11 (4 studies, 671 participants): RR 5.58, 95% CI 0.85 to 36.81; I2 = 86%; very low certainty evidence) compared with placebo or no treatment. There was substantial heterogeneity in treatment effects observed among studies.
2.10. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 10: Micro‐ to macroalbuminuria
2.11. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 11: Micro‐ to normoalbuminuria
ARB may increase hyperkalaemia (Analysis 2.12 (4 studies, 1299 participants): RR 5.29, 95% CI 1.89 to 14.85; I2 = 0%; low certainty evidence), and may increase cough (Analysis 2.13 (3 studies, 784 participants): RR 2.81, 95% CI 2.13 to 3.71; I2 = 0%; low certainty evidence) compared with placebo or no treatment.
2.12. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 12: Hyperkalaemia
2.13. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 13: Cough
Muirhead 1999 reported that compared with placebo, ARB made no difference to headache (Analysis 2.14 (91 participants): RR 0.47, 95% CI 0.03 to 7.22) or dizziness (Analysis 2.15 (91 participants): RR 0.16, 95% CI 0.01 to 3.78; very low certainty evidence).
2.14. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 14: Headache
2.15. Analysis.

Comparison 2: ARB versus control (placebo or no treatment), Outcome 15: Dizziness
Data were not available for other secondary outcomes.
ARB versus ACEi
Nineteen studies (Abe 2007a; Arpitha 2020; Atmaca 2006; Bansal 2004; DETAIL 2002; Deyneli 2006; Kavgaci 2002; Ko 2005; Krairittichai 2009; Lacourciere 2000; LIRICO 2007; Muirhead 1999; Ogawa 2007; PRONEDI 2013; Ruggenenti 2019; Sato 2003; Schram 2005; Sengul 2006; Tutuncu 2001) comparing ARB versus ACEi in patients with diabetes and kidney disease, during a median follow‐up of one year, could be meta‐analysed. The certainty of the evidence was low or very low for all outcomes (Table 3).
Primary outcomes
Compared to ACEi, ARB had uncertain effects on all‐cause death (Analysis 3.1 (15 studies, 1739 participants): RR 1.13, 95% CI 0.68 to 1.88; I2 = 0%; low certainty evidence) and withdrawal from treatment due to any cause (Analysis 3.2 (6 studies, 612 participants): RR 0.91, 95% CI 0.65 to 1.28; I2 = 0%; low certainty evidence).
3.1. Analysis.

Comparison 3: ARB versus ACEi, Outcome 1: Death: any cause
3.2. Analysis.

Comparison 3: ARB versus ACEi, Outcome 2: Withdrawn: any cause
Secondary outcomes
ARB had uncertain effects on cardiovascular death (Analysis 3.3 (13 studies, 1606 participants): RR 1.15, 95% CI 0.45 to 2.98; I2 = 0%; low certainty evidence), kidney failure (Analysis 3.4 (3 studies, 837 participants): RR 0.56, 95% CI 0.29 to 1.07; I2 = 0%; low certainty evidence), fatal or nonfatal MI (Analysis 3.5 (6 studies, 1209 participants): RR 1.25, 95% CI 0.62 to 2.50; I2 = 0%; low certainty evidence), fatal or nonfatal stroke (Analysis 3.6 (5 studies, 1146 participants): RR 0.92, 95% CI 0.38 to 2.24; I2 = 0%; low certainty evidence) and doubling of SCr (Analysis 3.7 (2 studies, 767 participants): RR 0.88, 95% CI 0.52 to 1.48; I2 = 0%; low certainty evidence) compared with ACEi.
3.3. Analysis.

Comparison 3: ARB versus ACEi, Outcome 3: Cardiovascular death
3.4. Analysis.

Comparison 3: ARB versus ACEi, Outcome 4: Kidney failure
3.5. Analysis.

Comparison 3: ARB versus ACEi, Outcome 5: Fatal or nonfatal myocardial infarction
3.6. Analysis.

Comparison 3: ARB versus ACEi, Outcome 6: Fatal or nonfatal stroke
3.7. Analysis.

Comparison 3: ARB versus ACEi, Outcome 7: Doubling of serum creatinine
The effect of ARB on GFR reduction (Analysis 3.8: 91 participants) was not estimable, as no events were reported in the eligible study.
3.8. Analysis.

Comparison 3: ARB versus ACEi, Outcome 8: Reduction in GFR
ARB had uncertain effects on the progression from microalbuminuria to macroalbuminuria (Analysis 3.9 (4 studies, 965 participants): RR 0.97, 95% CI 0.67 to 1.42; I2 = 0%; low certainty evidence) and regression from microalbuminuria to normoalbuminuria (Analysis 3.10 (4 studies, 216 participants): RR 0.80, 95% CI 0.62 to 1.16; I2 = 27%; low certainty evidence) compared with ACEi. There was moderate heterogeneity in treatment effects observed among studies.
3.9. Analysis.

Comparison 3: ARB versus ACEi, Outcome 9: Micro‐ to macroalbuminuria
3.10. Analysis.

Comparison 3: ARB versus ACEi, Outcome 10: Micro‐ to normoalbuminuria
ARB had uncertain effects on hyperkalaemia (Analysis 3.11 (5 studies, 891 participants): RR 1.08, 95% CI 0.71 to 1.64; I2 = 4%; low certainty evidence), but may have less cough (Analysis 3.12 (4 studies, 868 participants): RR 0.21, 95% CI 0.08 to 0.54; I2 = 0%; low certainty evidence) compared with ACEi.
3.11. Analysis.

Comparison 3: ARB versus ACEi, Outcome 11: Hyperkalaemia
3.12. Analysis.

Comparison 3: ARB versus ACEi, Outcome 12: Cough
Muirhead 1999 reported no difference in headache (Analysis 3.13 (91 participants): RR 1.43, 95% CI 0.06 to 34.04; very low certainty evidence) between ARB and ACEi.
3.13. Analysis.

Comparison 3: ARB versus ACEi, Outcome 13: Headache
Ruggenenti 2019 reported that the effect of ARB compared with ACEi on impotence (Analysis 3.14: 70 participants) was not estimable, as no events were reported.
3.14. Analysis.

Comparison 3: ARB versus ACEi, Outcome 14: Impotence
ARB had uncertain effects on dizziness (Analysis 3.15 (2 studies, 115 participants): RR 0.07, 95% CI 0.00 to 1.28; low certainty evidence) compared with ACEi.
3.15. Analysis.

Comparison 3: ARB versus ACEi, Outcome 15: Dizziness
Data were not available for other secondary outcomes.
ACEi plus ARB (dual therapy) versus placebo or no treatment
Ogawa 2007 (90 participants) compared ACEi plus ARB versus no treatment during a follow‐up of 1.8 years. The study was not designed to evaluate the majority of the outcomes specified in this systematic review. The certainty of the evidence was low or very low for all outcomes (Table 4).
Primary outcomes
Ogawa 2007 reported that the effect of ACEi plus ARB dual therapy compared to no treatment on all‐cause death (Analysis 4.1: 90 participants) was not estimable, as no events were reported. Data were not available for withdrawn from treatment due to any cause.
4.1. Analysis.

Comparison 4: Dual therapy (ACEi+ARB) versus control (placebo or no treatment), Outcome 1: Death: any cause
Secondary outcomes
Ogawa 2007 reported that the effect of ACEi plus ARB compared to no treatment on cardiovascular death (Analysis 4.2: 90 participants) was not estimable, as no events were reported.
4.2. Analysis.

Comparison 4: Dual therapy (ACEi+ARB) versus control (placebo or no treatment), Outcome 2: Cardiovascular death
Ogawa 2007 reported ACEi plus ARB may prevent the progression from microalbuminuria to macroalbuminuria (Analysis 4.3 (82 participants): RR 0.10, 95% CI 0.05 to 0.24; low certainty evidence), but there was no difference in regression from microalbuminuria to normoalbuminuria (Analysis 4.4 (82 participants): RR 5.27, 95% CI 0.34 to 81.57; very low certainty evidence) compared with no treatment.
4.3. Analysis.

Comparison 4: Dual therapy (ACEi+ARB) versus control (placebo or no treatment), Outcome 3: Micro‐ to macroalbuminuria
4.4. Analysis.

Comparison 4: Dual therapy (ACEi+ARB) versus control (placebo or no treatment), Outcome 4: Micro‐ to normoalbuminuria
Data were not available for other secondary outcomes.
ACEi or ARB (monotherapy) versus ACEi plus ARB (dual therapy)
ACEI alone versus ACEi plus ARB
Eight studies (Atmaca 2006; Bansal 2004; LIRICO 2007; Ogawa 2007; PRONEDI 2013; Ruggenenti 2019; Sengul 2006; Tutuncu 2001) comparing ACEi versus ACEi plus ARB in patients with diabetes and kidney disease, during a median follow‐up of one year, could be meta‐analysed. The certainty of the evidence was low or very low for all outcomes (Table 5).
Primary outcomes
Compared to ACEi plus ARB, ACEi alone had uncertain effects on all‐cause death (Analysis 5.1.1 (6 studies, 1166 participants): RR 1.08, 95% CI 0.49 to 2.40; I2 = 20%; low certainty evidence) and withdrawal from treatment due to any cause (Analysis 5.2.1 (2 studies, 172 participants): RR 0.78, 95% CI 0.33 to 1.86; I2 = 0%; low certainty evidence).
5.1. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 1: Death: any cause
5.2. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 2: Withdrawn: any cause
Secondary outcomes
ACEi alone had uncertain effects on cardiovascular death (Analysis 5.3.1 (4 studies, 994 participants): RR 3.02, 95% CI 0.61 to 14.85; low certainty evidence), kidney failure (Analysis 5.4.1 (3 studies, 880 participants): RR 1.36, 95% CI 0.79 to 2.32; I2 = 0%; low certainty evidence), fatal or nonfatal MI (Analysis 5.5.1 (3 studies, 880 participants): RR 0.44, 95% CI 0.17 to 1.12; I2 = 0%; low certainty evidence), fatal or nonfatal stroke (Analysis 5.6.1 (2 studies, 775 participants): RR 0.72, 95% CI 0.23 to 2.24; I2 = 0%; low certainty evidence), and doubling of SCr (Analysis 5.7.1 (2 studies, 813 participants): RR 1.14, 95% CI 0.70 to 1.85; I2 = 0%; low certainty evidence) compared with ACEi plus ARB. Reduction in GFR was not reported.
5.3. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 3: Cardiovascular death
5.4. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 4: Kidney failure
5.5. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 5: Fatal or nonfatal myocardial infarction
5.6. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 6: Fatal or nonfatal stroke
5.7. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 7: Doubling of serum creatinine
It was very uncertain whether ACEi alone made any difference in the progression from microalbuminuria to macroalbuminuria (Analysis 5.9.1 (3 studies, 958 participants): RR 1.25. 95% CI 0.67 to 2.32; I2 = 36%; very low certainty evidence) compared with ACEi plus ARB. There was moderate heterogeneity in treatment effects observed among studies.
5.9. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 9: Micro‐ to macroalbuminuria
ACEi alone had uncertain effects on regression from microalbuminuria to normoalbuminuria (Analysis 5.10.1 (3 studies, 145 participants): RR 1.08, 95% CI 0.74 to 1.57; I2 = 25%; low certainty evidence), hyperkalaemia (Analysis 5.11.1 (4 studies, 900 participants): RR 1.01, 95% CI 0.71 to 1.44; I2 = 0%; low certainty evidence) and cough (Analysis 5.12.1 (2 studies, 730 participants): RR 2.15, 95% CI 0.89 to 5.22; low certainty evidence) compared with ACEi plus ARB.
5.10. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 10: Micro‐ to normoalbuminuria
5.11. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 11: Hyperkalaemia
5.12. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 12: Cough
Ruggenenti 2019 reported that ACEi alone made no difference to impotence (Analysis 5.13.1 (67 participants): RR 0.32, 95% CI 0.01 to 7.68; very low certainty evidence) compared with ACEi plus ARB.
5.13. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 13: Impotence
Tutuncu 2001 reported that the effect of ACEi alone compared with ACEi plus ARB on dizziness (Analysis 5.14.1: 22 participants) was not estimable, as no events were reported.
5.14. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 14: Dizziness
Data were not available for other secondary outcomes.
ARB alone versus ACEi plus ARB
Nine studies (Atmaca 2006; Bansal 2004; LIRICO 2007; Ogawa 2007; PRONEDI 2013; Ruggenenti 2019; Sengul 2006; Tutuncu 2001; VA NEPHRON‐D 2009) comparing ARB alone versus ACEi plus ARB in patients with diabetes and kidney disease, during a median follow‐up of 1.4 years, could be meta‐analysed. The certainty of the evidence was low or very low for all outcomes (Table 6).
Primary outcomes
ARB alone had uncertain effects on all‐cause death (Analysis 5.1.2 (7 studies, 2607 participants): RR 1.02, 95% CI 0.76 to 1.37; I2 = 0%; low certainty evidence) and withdrawal from treatment due to any cause (Analysis 5.2.2 (3 studies, 1615 participants): RR 0.81, 95% CI 0.53 to 1.24; I2 = 0%; low certainty evidence) compared with ACEi plus ARB.
Secondary outcomes
ARB alone had uncertain effects on cardiovascular death (Analysis 5.3.2 (4 studies, 992 participants): RR 3.03, 95% CI 0.62 to 14.93; low certainty evidence), kidney failure (Analysis 5.4.2 (4 studies, 2321 participants): RR 1.15, 95% CI 0.67 to 1.95; I2 = 29%; low certainty evidence), fatal or nonfatal MI (Analysis 5.5.2 (4 studies, 2321 participants): RR 0.70, 95% CI 0.49 to 1.01; I2 = 0%; low certainty evidence), fatal or nonfatal stroke (Analysis 5.6.2 (3 studies, 2223 participants): RR 0.76, 95% CI 0.35 to 1.65; I2 = 12%; low certainty evidence), and doubling of SCr (Analysis 5.7.2 (3 studies, 2252 participants): RR 1.18, 95% CI 0.85 to 1.64; I2 = 0%; low certainty evidence) compared with ACEi plus ARB.
VA NEPHRON‐D 2009 reported that ARB alone made no difference in the reduction in GFR (Analysis 5.8.1 (1448 participants): RR 1.32, 95% CI 0.96 to 1.82; very low certainty evidence) compared with ACEi plus ARB.
5.8. Analysis.

Comparison 5: Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB), Outcome 8: Reduction in GFR
ARB alone had uncertain effects on the progression from microalbuminuria to macroalbuminuria (Analysis 5.9.2 (4 studies, 2410 participants): RR 1.07, 95% CI 0.77 to 1.49; I2 = 0%; low certainty evidence) and the regression from microalbuminuria to normoalbuminuria (Analysis 5.10.2 (3 studies, 151 participants): RR 1.04, 95% CI 0.74 to 1.45; I2 = 0%; low certainty evidence) compared with ACEi plus ARB.
It was uncertain whether ARB alone made any difference to hyperkalaemia (Analysis 5.11.2 (5 studies, 2341 participants): RR 0.79, 95% CI 0.46 to 1.37; I2 = 67%; very low certainty evidence) compared with ACEi plus ARB. There was moderate heterogeneity in treatment effects observed among the studies. ARB alone had uncertain effects on cough (Analysis 5.12.2 (2 studies, 728 participants): RR 0.14, 95% CI 0.02 to 1.17; low certainty evidence) compared with ACEi plus ARB.
Ruggenenti 2019 reported that ARB alone made no difference to impotence (Analysis 5.13.2 (1 study, 69 participants, RR 0.31, 95% CI 0.01 to 7.27; very low certainty evidence) compared with ACEi plus ARB, whilst the effect on dizziness (Analysis 5.14.2: 22 participants) was not estimable, as no events were reported.
Data were not available for other secondary outcomes.
ACEi versus another ACEi
Three studies (Chen 2018b; Fogari 2013a; Lin 1995) comparing an ACEi (captopril or imidapril) versus another ACEi (enalapril or ramipril) in patients with diabetes and kidney disease during a median follow‐up of one year could be meta‐analysed. Studies were not designed to evaluate the majority of the outcomes specified in this systematic review. The certainty of the evidence was low or very low for all outcomes (Table 7).
Primary outcomes
The effect of ACEi versus another ACEi on all‐cause death (Analysis 6.1: 3 studies, 269 participants) was not estimable since zero events were reported in eligible studies.
6.1. Analysis.

Comparison 6: ACEi versus another ACEi, Outcome 1: Death: any cause
ACEi have uncertain effects on withdrawal from treatment due to any cause (Analysis 6.2 (2 studies, 194 participants): RR 0.87, 95% CI 0.28 to 2.76; I2 = 0%; low certainty evidence) compared with another ACEi.
6.2. Analysis.

Comparison 6: ACEi versus another ACEi, Outcome 2: Withdrawn
Secondary outcomes
The effect of ACEi versus another ACEi on cardiovascular death (Analysis 6.3: 3 studies, 269 participants) was not estimable, as no events were reported in any of the eligible studies.
6.3. Analysis.

Comparison 6: ACEi versus another ACEi, Outcome 3: Cardiovascular death
Chen 2018b reported that captopril had no difference to hyperkalaemia (Analysis 6.4 (16 participants): RR 3.75, 95% CI 0.18 to 80.19; very low certainty evidence) compared with enalopril.
6.4. Analysis.

Comparison 6: ACEi versus another ACEi, Outcome 4: Hypekalaemia
Fogari 2013a reported imidapril may reduce cough (Analysis 6.5 (176 participants): RR 0.21, 95% CI 0.07 to 0.59; low certainty evidence) but made no difference to headache (Analysis 6.6 (176 participants): RR 1.00, 95% CI 0.21 to 4.82; very low certainty evidence) compared with ramipril.
6.5. Analysis.

Comparison 6: ACEi versus another ACEi, Outcome 5: Cough
6.6. Analysis.

Comparison 6: ACEi versus another ACEi, Outcome 6: Headache
Data were not available for other secondary outcomes.
ARB versus another ARB
Five studies (AMADEO 2008; Arai 2008; FANTASTIC 2017; Nakamura 2010c; VIVALDI 2005) comparing an ARB (fimasartan, telmisartan) versus another ARB (candasartan, losartan, olmesartan, valsartan) in patients with diabetes and kidney disease during a median follow‐up of one year could be meta‐analysed. The certainty of the evidence was low or very low for all outcomes (Table 8).
Primary outcomes
It was uncertain whether one ARB made any difference to all‐cause death (Analysis 7.1 (5 studies, 2041 participants): RR 0.58, 95% CI 0.05 to 7.08; I2 = 88%; very low certainty evidence) compared with another ARB. Substantial heterogeneity was observed among studies. Data were not available for patients who were withdrawn from treatment due to any cause.
7.1. Analysis.

Comparison 7: ARB versus another ARB, Outcome 1: Death: any cause
Secondary outcomes
ARB had uncertain effects on cardiovascular death (Analysis 7.2 (4 studies, 1360 participants): RR 1.34, 95% CI 0.47 to 3.82; low certainty evidence) compared with another ARB.
7.2. Analysis.

Comparison 7: ARB versus another ARB, Outcome 2: Cardiovascular death
VIVALDI 2005 reported that compared to valsartan, telisartan made no difference to kidney failure (Analysis 7.3 (857 participants): RR 0.88, 95% CI 0.32 to 2.40; very low certainty evidence), fatal or nonfatal MI (Analysis 7.4 (857 participants): RR 0.36, 95% CI 0.12 to 1.14; very low certainty evidence), fatal or nonfatal stroke (Analysis 7.5 (857 participants): RR 2.21, 95% CI 0.77 to 6.29; very low certainty evidence), doubling of SCr (Analysis 7.6 (857 participants): RR 1.00, 95% CI 0.20 to 4.94; very low certainty evidence), GFR at the end of treatment (Analysis 7.7 (716 participants): MD ‐0.70 mL/min/1.73 m2, 95% CI ‐4.00 to 2.60; very low certainty evidence), regression from macroalbuminuria to microalbuminuria (Analysis 7.8 (716 participants): RR 1.34, 95% CI 0.87 to 2.07; very low certainty evidence), and regression from macroalbuminuria to normoalbuminuria (Analysis 7.9 (716 participants): RR 0.98, 95% CI 0.25 to 3.88; very low certainty evidence).
7.3. Analysis.

Comparison 7: ARB versus another ARB, Outcome 3: Kidney failure
7.4. Analysis.

Comparison 7: ARB versus another ARB, Outcome 4: Fatal or nonfatal myocardial infarction
7.5. Analysis.

Comparison 7: ARB versus another ARB, Outcome 5: Fatal or nonfatal stroke
7.6. Analysis.

Comparison 7: ARB versus another ARB, Outcome 6: Doubling of serum creatinine
7.7. Analysis.

Comparison 7: ARB versus another ARB, Outcome 7: GFR (at end of treatment) [mL/min/1.7 3m²]
7.8. Analysis.

Comparison 7: ARB versus another ARB, Outcome 8: Macro‐to microalbuminuria
7.9. Analysis.

Comparison 7: ARB versus another ARB, Outcome 9: Macro‐to normoalbuminuria
ARB had uncertain effects on hyperkalaemia (Analysis 7.10 (2 studies, 1065 participants): RR 1.10, 95% CI 0.27 to 4.50; I2 = 24%; low certainty evidence) and dizziness (Analysis 7.11 (1 study, 349 participants): RR 10.81, 95% CI 0.60 to 194.08; very low certainty evidence) compared with another ARB.
7.10. Analysis.

Comparison 7: ARB versus another ARB, Outcome 10: Hyperkalaemia
7.11. Analysis.

Comparison 7: ARB versus another ARB, Outcome 11: Dizziness
Data were not available for other secondary outcomes.
Low‐dose versus high‐dose ARB
Four studies (Chen 2018b; DROP 2006; Kitamura 2020; SMART 2009) comparing low‐dose ARB versus high‐dose ARB in patients with diabetes and kidney disease during a median follow‐up of one year could be meta‐analysed. Studies were not designed to evaluate the majority of the outcomes specified in this systematic review. The certainty of the evidence was low or very low for all outcomes (Table 9).
Primary outcomes
The effect of low‐dose compared with high‐dose ARB on all‐cause death (Analysis 8.1: 2 studies, 156 participants) was not estimable, as no events were reported.
8.1. Analysis.

Comparison 8: Low versus high‐dose ARB, Outcome 1: Death: any cause
Kitamura 2020 reported that low‐dose ARB had uncertain effects on withdrawal from treatment due to any cause (Analysis 8.2 (11 participants): RR 0.45, 95% CI 0.03 to 7.39; very low certainty evidence).
8.2. Analysis.

Comparison 8: Low versus high‐dose ARB, Outcome 2: Withdrawn: any cause
Secondary outcomes
The effect of low‐dose compared with high‐dose ARB on cardiovascular death (Analysis 8.3: 2 studies, 156 participants) was not estimable, as no events were reported.
8.3. Analysis.

Comparison 8: Low versus high‐dose ARB, Outcome 3: Cardiovascular death
Chen 2018b reported no difference to change in GFR with low‐dose ARB (Analysis 8.4 (101 participants): MD 0.30 mL/min/1.73 m2, 95% CI ‐1.76 to 2.36; very low certainty evidence) compared with high‐dose ARB.
8.4. Analysis.

Comparison 8: Low versus high‐dose ARB, Outcome 4: Change in GFR [mL/min/1.73 m²]
Low‐dose ARB had uncertain effects on hyperkalaemia (Analysis 8.5 (3 studies, 422 participants): RR 1.11, 95% CI 0.18 to 6.69; I2 = 0%; low certainty evidence) compared with high‐dose ARB.
8.5. Analysis.

Comparison 8: Low versus high‐dose ARB, Outcome 5: Hyperkalaemia
DROP 2006 reported no difference in headaches (Analysis 8.6 (306 participants): RR 0.58, 95% CI 0.27 to 1.25; very low certainty evidence) and dizziness (Analysis 8.7 (306 participants): RR 0.44, 95% CI 0.17 to 1.10; very low certainty evidence) with low‐dose ARB compared with high‐dose ARB.
8.6. Analysis.

Comparison 8: Low versus high‐dose ARB, Outcome 6: Headache
8.7. Analysis.

Comparison 8: Low versus high‐dose ARB, Outcome 7: Dizziness
Data were not available for other secondary outcomes.
Low‐dose ARB plus spironolactone versus high‐dose ARB plus spironolactone
Chen 2018b compared low‐dose ARB plus spironolactone versus high‐dose ARB plus spironolactone during a follow‐up of 1.4 years. The study was not designed to evaluate the outcomes specified in this systematic review. The certainty of the evidence was very low for all outcomes (Table 10).
Primary outcomes
All‐cause death and withdrawal from treatment due to any cause were not reported.
Secondary outcomes
Chen 2018b reported low‐dose ARB plus spironolactone made no difference to change in GFR (Analysis 9.1 (101 participants): MD 2.30 mL/min/1.73 m2, 95% CI ‐0.00 to 4.60; very low certainty evidence) or hyperkalaemia (Analysis 9.2 (101 participants): RR 0.16, 95% CI 0.02 to 1.26; very low certainty evidence) compared with high‐dose ARB plus spironolactone.
9.1. Analysis.

Comparison 9: Low‐dose ARB + spironolactone versus high‐dose ARB + spironolactone, Outcome 1: Change in GFR [mL/min/1.73 m²]
9.2. Analysis.

Comparison 9: Low‐dose ARB + spironolactone versus high‐dose ARB + spironolactone, Outcome 2: Hyperkalaemia
Data were not available for other secondary outcomes.
Sensitivity and subgroup analyses
ACEi versus placebo or no treatment
Subgroup analysis for cardiovascular death: stratified by type of diabetes
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.17), suggesting that different types of diabetes do not modify the effect of ACEi on the risk of cardiovascular death (Analysis 10.1). However, a smaller number of participants contributed data to the type I diabetes than to the type II diabetes subgroup, meaning that the analysis may not be able to detect subgroup differences.
10.1. Analysis.

Comparison 10: ACEi versus control: cardiovascular death (subgroup analyses), Outcome 1: Type of diabetes
Subgroup analysis for cardiovascular death: stratified by the presence of hypertension
The test for subgroup differences, stratified by the presence of hypertension, was not estimable since zero events were reported in both arms in all but one study (Analysis 10.2). It was not possible to detect the effect of ACEi on the risk of cardiovascular death between the subgroups.
10.2. Analysis.

Comparison 10: ACEi versus control: cardiovascular death (subgroup analyses), Outcome 2: Presence of hypertension
Subgroup analysis for cardiovascular death: stratified by microalbuminuria versus macroalbuminuria
The test for subgroup differences, stratified by the presence of microalbuminuria or macroalbuminuria, was not estimable since zero events were reported in both arms in all studies (Analysis 10.3). It was not possible to detect the effect of ACEi on the risk of cardiovascular death between the subgroups.
10.3. Analysis.

Comparison 10: ACEi versus control: cardiovascular death (subgroup analyses), Outcome 3: Microalbuminuria versus macroalbuminuria
Subgroup analysis for progression from microalbuminuria to microalbuminuria: stratified by type of diabetes
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.59), suggesting that different types of diabetes do not modify the effect of ACEi on the risk of progression from microalbuminuria to macroalbuminuria (Analysis 11.1). However, a smaller number of participants contributed data to the type I diabetes than to the type II diabetes subgroup, meaning that the analysis may not be able to detect subgroup differences.
11.1. Analysis.

Comparison 11: ACEi versus control: microalbuminuria to macroalbuminuria (subgroup analyses), Outcome 1: Type of diabetes
Subgroup analysis for progression from microalbuminuria to microalbuminuria: stratified by the presence of hypertension
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.17), suggesting that the presence of hypertension does not modify the effect of ACEi on the risk of progression from microalbuminuria to macroalbuminuria (Analysis 11.2). However, a smaller number of studies and participants contributed data to patients with hypertension than to patients without hypertension subgroups, meaning that the analysis may not be able to detect subgroup differences.
11.2. Analysis.

Comparison 11: ACEi versus control: microalbuminuria to macroalbuminuria (subgroup analyses), Outcome 2: Presence of hypertension
Subgroup analysis for progression from microalbuminuria to microalbuminuria: stratified by microalbuminuria versus macroalbuminuria
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.65), suggesting that the presence of microalbuminuria or macroalbuminuria does not modify the effect of ACEi on the risk of progression from microalbuminuria to macroalbuminuria (Analysis 11.3). However, a smaller number of studies and participants contributed data to the macroalbuminuria than to the microalbuminuria subgroup, meaning that the analysis may not be able to detect subgroup differences.
11.3. Analysis.

Comparison 11: ACEi versus control: microalbuminuria to macroalbuminuria (subgroup analyses), Outcome 3: Microalbuminuria versus macroalbuminuria
Subgroup analysis for regression from microalbuminuria to normoalbuminuria: stratified by type of diabetes
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.33), suggesting that different types of diabetes do not modify the effect of ACEi on regression from microalbuminuria to normoalbuminuria (Analysis 12.1). However, a smaller number of participants contributed data to the type I diabetes than to the type II diabetes subgroup, meaning that the analysis may not be able to detect subgroup differences.
12.1. Analysis.

Comparison 12: ACEi versus control: microalbuminuria to normoalbuminuria (subgroup analyses), Outcome 1: Type of diabetes
Subgroup analysis for regression from microalbuminuria to normoalbuminuria: stratified by the presence of hypertension
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.74), suggesting that the presence of hypertension does not modify the effect of ACEi on regression from microalbuminuria to normoalbuminuria (Analysis 12.2). However, a smaller number of studies and participants contributed data to patients with hypertension than to patients without hypertension subgroups, meaning that the analysis may not be able to detect subgroup differences.
12.2. Analysis.

Comparison 12: ACEi versus control: microalbuminuria to normoalbuminuria (subgroup analyses), Outcome 2: Presence of hypertension
Subgroup analysis for regression from microalbuminuria to normoalbuminuria: stratified by microalbuminuria versus macroalbuminuria
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.59), suggesting that the presence of microalbuminuria or macroalbuminuria does not modify the effect of ACEi on regression from microalbuminuria to normoalbuminuria (Analysis 12.3). However, a smaller number of studies and participants contributed data to the macroalbuminuria than to the microalbuminuria subgroup, meaning that the analysis may not be able to detect subgroup differences.
12.3. Analysis.

Comparison 12: ACEi versus control: microalbuminuria to normoalbuminuria (subgroup analyses), Outcome 3: Microalbuminuria versus macroalbuminuria
Sensitivity analysis for adequate allocation concealment: cardiovascular death
Sensitivity analysis for cardiovascular death was not possible because there were no studies with adequate allocation concealment.
Sensitivity analysis for adequate allocation concealment: progression from microalbuminuria to macroalbuminuria
Considering only studies with adequate allocation concealment, ACEi may prevent the progression from microalbuminuria to macroalbuminuria (Analysis 13.1 (3 studies, 232 participants): RR 0.47, 95% CI 0.21 to 1.05; I2 = 0%; low certainty evidence) compared to placebo or no treatment.
13.1. Analysis.

Comparison 13: ACEi versus control: sensitivity analysis (adequate allocation concealment), Outcome 1: Micro‐ to macroalbuminuria
Sensitivity analysis for adequate allocation concealment: regression from microalbuminuria to normoalbuminuria
Considering only studies with adequate allocation concealment, ACEi probably increases the regression from microalbuminuria to normoalbuminuria (Analysis 13.2 (3 studies, 232 participants): RR 4.21, 95% CI 1.94 to 9.14; I2 = 0%; moderate certainty evidence) compared to placebo or no treatment.
13.2. Analysis.

Comparison 13: ACEi versus control: sensitivity analysis (adequate allocation concealment), Outcome 2: Micro‐ to normoalbuminuria
Sensitivity analysis for low risk of attrition: cardiovascular death
Considering only studies with a low risk of attrition bias, the effects of ACEi compared to placebo or no treatment on cardiovascular death (Analysis 14.1: 2 studies, 35 participants) were not estimable since zero events were reported.
14.1. Analysis.

Comparison 14: ACEi versus control: sensitivity analysis (low risk of attrition), Outcome 1: Cardiovascular death
Sensitivity analysis for low risk of attrition: progression from microalbuminuria to macroalbuminuria
Considering only studies with a low risk of attrition bias, it was very uncertain whether ACEi made any difference in the progression from microalbuminuria to macroalbuminuria (Analysis 14.2 (3 studies, 261 participants): RR 0.87, 95% CI 0.20 to 3.92; I2 = 58%; very low certainty evidence) compared to placebo or no treatment. There was moderate heterogeneity in treatment effects observed among the studies.
14.2. Analysis.

Comparison 14: ACEi versus control: sensitivity analysis (low risk of attrition), Outcome 2: Micro‐ to macroalbuminuria
Sensitivity analysis for low risk of attrition: regression from microalbuminuria to normoalbuminuria
Considering only studies with a low risk of attrition bias, it was very uncertain whether ACEi made any difference in the regression from microalbuminuria to normoalbuminuria (Analysis 14.3 (2 studies, 36 participants): RR 6.04, 95% CI 0.77 to 47.71; I2 = 0%; very low certainty evidence) compared to placebo or no treatment.
14.3. Analysis.

Comparison 14: ACEi versus control: sensitivity analysis (low risk of attrition), Outcome 3: Micro‐ to normoalbuminuria
Other sensitivity and subgroup analyses
Sensitivity and subgroup analyses were not possible when comparing ARB versus placebo or no treatment, ARB versus ACEi, ACEi plus ARB dual therapy versus placebo or no treatment, ACEi or ARB monotherapy versus ACEi plus ARB dual therapy, ACEi versus another ACEi, ARB versus another ARB, or low‐dose versus high‐dose ARB with or without spironolactone due to the insufficient number of available studies.
Discussion
Summary of main results
ACEi or ARB may make little or no difference to the risks of all‐cause and cardiovascular death, fatal and non‐fatal stroke or MI compared with placebo or no treatment in patients with diabetes and kidney disease. ACEi or ARB may prevent kidney failure compared to placebo or no treatment. ARB may prevent the doubling of SCr and progression from microalbuminuria to macroalbuminuria compared to placebo or no treatment.
To date, there is insufficient evidence to define the role of ACEi or ARB on regression from microalbuminuria to normoalbuminuria and withdrawal from treatment for any cause. The benefits on survival, cardiovascular and kidney outcomes of ACEi compared to ARB are uncertain in this population because direct comparisons were based on small studies with a limited number of events. The efficacy of ACEi or ARB monotherapy on death, withdrawal from treatment due to any cause, cardiovascular events, and kidney outcomes is uncertain compared to ACEi plus ARB dual therapy. The effects of two different ACEi or ARBs, low‐dose and high‐dose ARB, were rarely reported. No study compared different doses of ACEi. Adverse events were rarely reported.
Overall completeness and applicability of evidence
In this updated review, we included 62 new studies. Despite the larger number of studies, there was limited certainty about the effects of treatments on death, withdrawal from treatment due to any cause, cardiovascular events (fatal or nonfatal MI, fatal or nonfatal stroke) and kidney outcomes (kidney failure, doubling of SCr, progression from microalbuminuria to macroalbuminuria and regression from microalbuminuria to normoalbuminuria) between treatment groups. Potential adverse events were rarely and inconsistently reported. KDIGO Blood Pressure Guidelines 2021 and NKF KDOQI Guidelines 2004 recommend ACEi and ARB for preventing CKD in people with diabetes to reduce the risk of cardiovascular outcomes and death. Despite these international guidelines suggesting not combining ACEi and ARB treatment, the effects of ACEi or ARB monotherapy compared to dual therapy have not been adequately assessed. The external validity of the review may be limited as most of the studies were conducted in high‐income countries, and approximately 50% of the included studies had a short follow‐up (from six months to 1.5 years). Standardisation of outcome reporting in future studies as prioritised by the Standardised Outcomes in Nephrology (SONG) by patients, caregivers and health professionals may improve the existing evidence and our certainty in the findings.
Quality of the evidence
While the analyses included data from comprehensive search and unpublished data, selective reporting of outcomes may reduce the certainty of our conclusions, especially for cardiovascular and kidney outcomes. We used the Cochrane risk of bias domains and the GRADE methodology (GRADE 2008) to evaluate the certainty of the evidence. The treatment estimates were low to very low certainty evidence for all outcomes. The majority of studies did not report adequate allocation concealment or attrition, although sensitivity analyses did not find differences in treatment effects when analyses were restricted to studies at a lower risk of bias. Sources of heterogeneity (including type of diabetes, presence of hypertension, microalbuminuria and macroalbuminuria) in the included studies remained unexplained through subgroup analyses, as few studies with a limited number of events and participants contributed data.
Low and very low certainty evidence indicates that it is possible that further studies might provide different results.
Potential biases in the review process
This review was conducted using standard Cochrane methods, with rigid inclusion criteria for RCTs only. A highly sensitive search of the Cochrane Kidney Transplant specialised register was undertaken without language restriction up to March 2024. The registry contains hand‐searched literature and conference proceedings, maximising the inclusion of grey literature in this review. We directly contacted the authors to request additional data. Two independent investigators performed data extraction, data analysis, and risk of bias assessments, and all authors checked consistency. However, there were some potential sources of bias due to data availability in the individual studies.
Selective outcomes reporting was a limitation across the included studies.
Some treatment estimates included evidence of moderate to substantial heterogeneity between treatment interventions, but due to the limited number of participants and events in the included studies, fully accounting for the sources of bias could not be estimated.
According to international guidelines, most studies were at high risk of bias and/or underpowered, preventing the evaluation of the effects of dual therapy.
Differentiation between interventions in patients with type I and type II diabetes was not addressed.
Ethnicity, a key determinant in calculating GFR, was not adequately explored.
We were not able to investigate albuminuria despite the fact that albumin:creatinine ratio was not normally distributed among studies.
Sensitivity analyses excluding small studies were not planned or possible due to the limited number of included studies.
It is possible that some studies were missed because they were set out as non‐inferiority studies and terminated early due to poor recruitment.
eGFR categories or CKD stages were rarely reported, preventing a clear evaluation of the clinical context to assess key cardiovascular outcomes and the progression of kidney disease.
Adverse events were infrequently and inconsistently reported.
Formal assessment for publication bias through visualisation of asymmetry in funnel plots was precluded for many treatments and outcomes because of few studies.
Agreements and disagreements with other studies or reviews
This updated Cochrane review is consistent with the findings reported in a previous systematic review comparing ACEi or ARB with placebo, where treatments may make little or no difference to risks of all‐cause and cardiovascular death, had uncertain effects on regression from microalbuminuria to normoalbuminuria, but may prevent kidney failure (Coleman 2019). Our review confirmed that ARB may prevent the doubling of SCr and progression from microalbuminuria to macroalbuminuria, while our review did not find any improvement using ACEi for these outcomes. The different inclusion criteria might explain the differences between these two reviews. Coleman 2019 included studies with ≥ 100 participants with type II diabetes and micro‐ or macroalbuminuria with or without background antihypertensive during a follow‐up of ≥ 12 months.
Our findings were consistent with Wang 2018, which reported that both ACEi and ARB did not reduce death. In contrast to the findings of this review, Wang 2018 showed that only ARB prevented kidney failure, and both ACEi and ARB prevented the doubling of SCr. Wang 2018 used a Jadad score for the quality assessment of the included studies and did not use GRADE for the evaluation of the evidence.
A previous network meta‐analysis by Zhang 2020 included 44 RCTs with 42,319 patients with non‐dialysis CKD and compared ACEi, ARB or their combination to other antihypertensive treatments (e.g. calcium channel blockers, beta‐blockers, diuretics), placebo or control. Our findings are similar and showed that ACEi plus ARB did not reduce cardiovascular events compared to ACEi or ARB monotherapy, ARB did not reduce hypokalaemia compared to ACEi, and ACEi increased the risk of cough compared to placebo. Conversely, Zhang 2020 reported that ACEi, ARB or their combination decreased all‐cause and cardiovascular death compared with placebo. However, these discrepancies derived from the exclusion criteria reported in Zhang 2020, which did not include patients with GFR > 60 mL/min/1.73 m2 or studies without pre‐specified cardiovascular and kidney outcomes, and for these reasons, the study population may not be comparable to our population.
Authors' conclusions
Implications for practice.
Overall evidence ratings and recommendations for ACEi or ARB to prevent cardiovascular and kidney outcomes in people with diabetes and kidney disease are summarised using the GRADE system for grading evidence (GRADE 2011).
The current guidelines (KDIGO Blood Pressure Guidelines 2021; NKF KDOQI Guidelines 2004) recommend ACEi and ARB for preventing CKD in people with diabetes to reduce the risk of cardiovascular outcomes and death. Low certainty evidence suggested that ACEi or ARB may make little or no difference to risks of death and uncertain effects on cardiovascular events but may prevent kidney failure in people with diabetes and kidney disease.
ARB may prevent the doubling of SCr and the progression from microalbuminuria to macroalbuminuria compared with placebo or no treatment.
The absolute effects of ACEi or ARB on reducing the risk of death are summarised quantitatively based on risk estimates from this updated systematic review (Table 1; Table 2).
The relative benefits of ACEi or ARB monotherapy versus ACEi plus ARB dual therapy are insufficient to inform clinical practice due to the paucity and the low quality of the included studies, although the KDIGO Blood Pressure Guidelines 2021 and the NKF KDOQI Guidelines 2004 discourage the use dual therapy.
The effects of ACEi plus ARB dual therapy on hyperkalaemia are uncertain compared to ACEi or ARB monotherapy. However, ACEi plus ARB dual therapy in people with diabetes and kidney disease is often discontinued due to hyperkalaemia. Patiromer and sodium zirconium cyclosilicate are newer potassium binders that have been shown to reduce serum potassium levels in people receiving dual renin‐angiotensin‐aldosterone therapy and might be promising treatments to prevent hyperkalaemia in this setting (Georgianos 2018).
The effects of direct comparison between two different ACEi or ARB, or low‐dose versus high‐dose of the same antihypertensive agent, are uncertain.
Implications for research.
There is currently little data to assess the benefits and harms of ACEi or ARB in people with diabetes and kidney disease, although ACEi and ARB may prevent kidney failure compared with placebo or no treatment.
Future well powered, high quality and well‐conducted RCTs should investigate key cardiovascular outcomes and identify the appropriate dose of ACEi and ARB therapy in people with diabetes and kidney disease.
Additional studies that evaluate ACEi or ARB monotherapy compared to ACEi plus ARB dual therapy in people with diabetes and kidney disease would inform clinical practice.
Studies could use standardised cardiovascular and kidney outcomes to increase trial relevance to stakeholders, including patients and health professionals.
What's new
| Date | Event | Description |
|---|---|---|
| 20 May 2024 | Amended | Minor correction to abstract |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 24 April 2024 | New search has been performed | Risk of bias, GRADE assessment incorporated |
| 24 April 2024 | New citation required but conclusions have not changed | 82 new studies included, no change to conclusions |
| 23 February 2014 | Amended | Protocol updated for review update and inclusion of network meta‐analysis. Changed terminology of angiotensin II receptor antagonists (AIIRA) to angiotensin receptor blockers (ARB) |
| 23 February 2014 | Amended | Added author (Suetonia Palmer) |
| 26 November 2013 | Amended | Search strategies updated |
| 18 March 2010 | Amended | Contact details updated. |
| 13 May 2009 | Amended | Contact details updated. |
| 26 August 2008 | Amended | Converted to new review format. |
Acknowledgements
Cochrane 2024 update
We are indebted to Narelle Willis for editorial support and Gail Higgins for providing updated search strategies for this review. Suetonia Palmer is supported by a grant from the Canterbury Medical Research Foundation (grant number 13/01) and is funded by a Rutherford Discovery Fellowship from the Royal Society of New Zealand.
We wish to thank Janice Pogue and the HOPE triallists, Drs M Ravid, PJ Phillips, HH Parving, R Romero, S Katayama, EM Mathiesen, BR Brenner, Carmen Bonifati, Maria Craig and KC Tan who provided data of their studies upon request.
The authors are grateful to the following peer reviewers for their time and comments: Tahseen A. Chowdhury (Consultant in Diabetes, The Royal London Hospital, London UK); Vishnu Vardhan Garla; Dr Rebecca Suckling (Epsom and St Helier University Hospital); Dr Daniel van Raalte (Internist‐Endocrinologist Amsterdam UMC)."
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. ACEi versus control (placebo or no treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death: any cause | 24 | 7413 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.73, 1.15] |
| 1.2 Withdrawn | 7 | 5306 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.19] |
| 1.3 Cardiovascular death | 17 | 5625 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.13, 3.57] |
| 1.4 Kidney failure | 8 | 6643 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.94] |
| 1.5 Fatal or nonfatal myocardial infarction | 3 | 5100 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.09] |
| 1.6 Fatal or nonfatal stroke | 2 | 4944 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.80, 1.31] |
| 1.7 Doubling of serum creatinine | 8 | 6702 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.01] |
| 1.8 Reduction in GFR | 3 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.55, 1.51] |
| 1.9 Change in GFR [mL/min/1.73 m²] | 3 | 88 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.50, 0.90] |
| 1.10 GFR (at end of treatment) [mL/min/1.73 m²] | 7 | 188 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐8.79, 5.97] |
| 1.11 Micro‐ to macroalbuminuria | 19 | 2282 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.64] |
| 1.12 Micro‐ to normoalbuminuria | 17 | 1959 | Risk Ratio (M‐H, Random, 95% CI) | 3.01 [1.86, 4.88] |
| 1.13 Hyperkalaemia | 4 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.48, 1.40] |
| 1.14 Cough | 10 | 6615 | Risk Ratio (M‐H, Random, 95% CI) | 3.26 [2.30, 4.60] |
| 1.15 Headache | 5 | 6244 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.34, 2.12] |
| 1.16 Impotence | 5 | 1528 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.91] |
| 1.17 Dizziness | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.33, 27.18] |
Comparison 2. ARB versus control (placebo or no treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Death: any cause | 11 | 4260 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.85, 1.16] |
| 2.2 Withdrawn | 3 | 721 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.58, 1.26] |
| 2.3 Cardiovascular death | 6 | 878 | Risk Ratio (M‐H, Random, 95% CI) | 3.36 [0.93, 12.07] |
| 2.4 Kidney failure | 3 | 3227 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.72, 0.94] |
| 2.5 Fatal and nonfatal myocardial infarction | 2 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.11, 1.65] |
| 2.6 Fatal or nonfatal stroke | 2 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.77] |
| 2.7 Doubling of serum creatinine | 4 | 3280 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.97] |
| 2.8 Reduction in GFR | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.14] |
| 2.9 GFR (at end of treatment) [mL/min/1.73 m²] | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐11.57 [‐21.73, ‐1.41] |
| 2.10 Micro‐ to macroalbuminuria | 5 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.23, 0.85] |
| 2.11 Micro‐ to normoalbuminuria | 4 | 671 | Risk Ratio (M‐H, Random, 95% CI) | 5.58 [0.85, 36.81] |
| 2.12 Hyperkalaemia | 4 | 1299 | Risk Ratio (M‐H, Random, 95% CI) | 5.29 [1.89, 14.85] |
| 2.13 Cough | 3 | 784 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [2.13, 3.71] |
| 2.14 Headache | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.03, 7.22] |
| 2.15 Dizziness | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.78] |
Comparison 3. ARB versus ACEi.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Death: any cause | 15 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.68, 1.88] |
| 3.2 Withdrawn: any cause | 6 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.28] |
| 3.3 Cardiovascular death | 13 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.45, 2.98] |
| 3.4 Kidney failure | 3 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.29, 1.07] |
| 3.5 Fatal or nonfatal myocardial infarction | 6 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.62, 2.50] |
| 3.6 Fatal or nonfatal stroke | 5 | 1146 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.38, 2.24] |
| 3.7 Doubling of serum creatinine | 2 | 767 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.52, 1.48] |
| 3.8 Reduction in GFR | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.9 Micro‐ to macroalbuminuria | 4 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.67, 1.42] |
| 3.10 Micro‐ to normoalbuminuria | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.16] |
| 3.11 Hyperkalaemia | 5 | 891 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.71, 1.64] |
| 3.12 Cough | 4 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.54] |
| 3.13 Headache | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.06, 34.04] |
| 3.14 Impotence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.15 Dizziness | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.28] |
Comparison 4. Dual therapy (ACEi+ARB) versus control (placebo or no treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Death: any cause | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3 Micro‐ to macroalbuminuria | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.05, 0.24] |
| 4.4 Micro‐ to normoalbuminuria | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 5.27 [0.34, 81.57] |
Comparison 5. Single therapy (ACEi or ARB) versus dual therapy (ACEi+ARB).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Death: any cause | 7 | 3773 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.33] |
| 5.1.1 ACEi | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.49, 2.40] |
| 5.1.2 ARB | 7 | 2607 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.76, 1.37] |
| 5.2 Withdrawn: any cause | 3 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.18] |
| 5.2.1 ACEi | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.33, 1.86] |
| 5.2.2 ARB | 3 | 1615 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.53, 1.24] |
| 5.3 Cardiovascular death | 4 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 3.03 [0.98, 9.34] |
| 5.3.1 ACEi | 4 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 3.02 [0.61, 14.85] |
| 5.3.2 ARB | 4 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 3.03 [0.62, 14.93] |
| 5.4 Kidney failure | 4 | 3201 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.95, 1.77] |
| 5.4.1 ACEi | 3 | 880 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.79, 2.32] |
| 5.4.2 ARB | 4 | 2321 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.67, 1.95] |
| 5.5 Fatal or nonfatal myocardial infarction | 4 | 3201 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.47, 0.93] |
| 5.5.1 ACEi | 3 | 880 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.17, 1.12] |
| 5.5.2 ARB | 4 | 2321 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.49, 1.01] |
| 5.6 Fatal or nonfatal stroke | 3 | 2998 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.48, 1.38] |
| 5.6.1 ACEi | 2 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.23, 2.24] |
| 5.6.2 ARB | 3 | 2223 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.65] |
| 5.7 Doubling of serum creatinine | 3 | 3065 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.53] |
| 5.7.1 ACEi | 2 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.70, 1.85] |
| 5.7.2 ARB | 3 | 2252 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.85, 1.64] |
| 5.8 Reduction in GFR | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.8.1 ARB | 1 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.96, 1.82] |
| 5.9 Micro‐ to macroalbuminuria | 4 | 3368 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.85, 1.41] |
| 5.9.1 ACEi | 3 | 958 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.67, 2.32] |
| 5.9.2 ARB | 4 | 2410 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.77, 1.49] |
| 5.10 Micro‐ to normoalbuminuria | 3 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 5.10.1 ACEi | 3 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.57] |
| 5.10.2 ARB | 3 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.45] |
| 5.11 Hyperkalaemia | 5 | 3241 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.20] |
| 5.11.1 ACEi | 4 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.71, 1.44] |
| 5.11.2 ARB | 5 | 2341 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.46, 1.37] |
| 5.12 Cough | 2 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.04, 9.72] |
| 5.12.1 ACEi | 2 | 730 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.89, 5.22] |
| 5.12.2 ARB | 2 | 728 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.17] |
| 5.13 Impotence | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 2.95] |
| 5.13.1 ACEi | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.68] |
| 5.13.2 ARB | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.27] |
| 5.14 Dizziness | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.14.1 ACEi | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.14.2 ARB | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 6. ACEi versus another ACEi.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Death: any cause | 3 | 269 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.2 Withdrawn | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.28, 2.76] |
| 6.3 Cardiovascular death | 3 | 269 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.4 Hypekalaemia | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 3.75 [0.18, 80.19] |
| 6.5 Cough | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.59] |
| 6.6 Headache | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.21, 4.82] |
Comparison 7. ARB versus another ARB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Death: any cause | 5 | 2041 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.05, 7.08] |
| 7.2 Cardiovascular death | 4 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.47, 3.82] |
| 7.3 Kidney failure | 1 | 857 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.32, 2.40] |
| 7.4 Fatal or nonfatal myocardial infarction | 1 | 857 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.14] |
| 7.5 Fatal or nonfatal stroke | 1 | 857 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [0.77, 6.29] |
| 7.6 Doubling of serum creatinine | 1 | 857 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.20, 4.94] |
| 7.7 GFR (at end of treatment) [mL/min/1.7 3m²] | 1 | 716 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐4.00, 2.60] |
| 7.8 Macro‐to microalbuminuria | 1 | 716 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.87, 2.07] |
| 7.9 Macro‐to normoalbuminuria | 1 | 716 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.25, 3.88] |
| 7.10 Hyperkalaemia | 2 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.27, 4.50] |
| 7.11 Dizziness | 1 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 10.81 [0.60, 194.08] |
Comparison 8. Low versus high‐dose ARB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Death: any cause | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.2 Withdrawn: any cause | 1 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.03, 7.39] |
| 8.3 Cardiovascular death | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.4 Change in GFR [mL/min/1.73 m²] | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐1.76, 2.36] |
| 8.5 Hyperkalaemia | 3 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.18, 6.69] |
| 8.6 Headache | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.25] |
| 8.7 Dizziness | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.17, 1.10] |
Comparison 9. Low‐dose ARB + spironolactone versus high‐dose ARB + spironolactone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Change in GFR [mL/min/1.73 m²] | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐0.00, 4.60] |
| 9.2 Hyperkalaemia | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.26] |
Comparison 10. ACEi versus control: cardiovascular death (subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Type of diabetes | 12 | 5427 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.13, 3.57] |
| 10.1.1 Type I | 7 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.60] |
| 10.1.2 Type II | 5 | 5131 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 10.2 Presence of hypertension | 12 | 5361 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 10.2.1 Patients with hypertension | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.2.2 Patients without hypertension | 11 | 5311 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 10.3 Microalbuminuria versus macroalbuminuria | 15 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.60] |
| 10.3.1 Microalbuminuria | 14 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.60] |
| 10.3.2 Macroalbuminuria | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 11. ACEi versus control: microalbuminuria to macroalbuminuria (subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Type of diabetes | 16 | 2219 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.26, 0.61] |
| 11.1.1 Type I | 10 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.28, 0.64] |
| 11.1.2 Type II | 6 | 1466 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.75] |
| 11.2 Presence of hypertension | 17 | 1079 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.49] |
| 11.2.1 Patients with hypertension | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.12, 0.45] |
| 11.2.2 Patients without hypertension | 15 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.27, 0.59] |
| 11.3 Microalbuminuria versus macroalbuminuria | 18 | 2207 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.66] |
| 11.3.1 Microalbuminuria | 16 | 1918 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.26, 0.72] |
| 11.3.2 Macroalbuminuria | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.19, 0.68] |
Comparison 12. ACEi versus control: microalbuminuria to normoalbuminuria (subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Type of diabetes | 15 | 1912 | Risk Ratio (M‐H, Random, 95% CI) | 2.99 [1.75, 5.11] |
| 12.1.1 Type I | 11 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 3.66 [2.27, 5.90] |
| 12.1.2 Type II | 4 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.52, 6.64] |
| 12.2 Presence of hypertension | 15 | 756 | Risk Ratio (M‐H, Random, 95% CI) | 3.44 [2.26, 5.23] |
| 12.2.1 Patients with hypertension | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 4.79 [0.63, 36.44] |
| 12.2.2 Patients without hypertension | 13 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 3.38 [2.20, 5.20] |
| 12.3 Microalbuminuria versus macroalbuminuria | 16 | 1884 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.74, 4.82] |
| 12.3.1 Microalbuminuria | 15 | 1820 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [1.68, 4.78] |
| 12.3.2 Macroalbuminuria | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 6.13 [0.39, 96.97] |
Comparison 13. ACEi versus control: sensitivity analysis (adequate allocation concealment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Micro‐ to macroalbuminuria | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.05] |
| 13.2 Micro‐ to normoalbuminuria | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 4.21 [1.94, 9.14] |
Comparison 14. ACEi versus control: sensitivity analysis (low risk of attrition).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Cardiovascular death | 2 | 35 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 14.2 Micro‐ to macroalbuminuria | 3 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.20, 3.92] |
| 14.3 Micro‐ to normoalbuminuria | 2 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 6.04 [0.77, 47.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ABCD 1996.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | A single‐blind study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An Endpoint Committee blinded to randomisation assignment will ascertain the diagnosis of myocardial infarction, cerebrovascular accident, congestive heart failure and sudden death based on all available pertinent information." Comment: outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. For subjective outcomes the endpoint committee was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to permit adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participant baseline characteristics and cointerventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
ABCD‐2V 2006.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The original randomisation list was secured within the Data Coordinating Center of the CPC." Comment: Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants baseline characteristics and cointerventions were not reported only for albuminuric participants (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | A single‐blind study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent Endpoint Committee, which was blinded to the study intervention arms, reviewed all cardiovascular events. The Endpoint Committee reviewed all hospital admissions that appeared to be related to cardiovascular events." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. For subjective outcomes the endpoint committee was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The participant was nevertheless included in the intention to treat analyses." |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants' baseline characteristics and co‐interventions were not reported only for albuminuric participants. It was not clear if the funder could influence data analysis and study reporting or interpretation. The study was terminated early |
Abe 2007a.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan + ACEi group
ACEi + ACEi group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Quote: "We carried out randomisation using envelopes." Comment: Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Ahmad 1997.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | A single‐blind study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 103/120 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Ahmad 2003.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 73/85 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
AIPRI 1996.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Benazepril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participant baseline characteristics and co‐interventions were not reported (only for 21 participants of our interest). It was not clear if funder could influence data analysis and study reporting or interpretation |
AMADEO 2008.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group
Losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eighty percent of the total cohort completed the trial, 345 and 342 patients in the telmisartan and losartan groups, respectively." Comment: 687/860 participants completed the study |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Arai 2008.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group
Valsartan group
Candesartan group
Losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Arpitha 2020.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Telmisartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study is considered at high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. There were no source of funding. No other apparent sources of bias |
ATLANTIS 2000.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Ramipril group 1
Ramipril group 2
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients baseline characteristics |
| Allocation concealment (selection bias) | Low risk | Quote from Wang 2018: "Assignments were placed in sealed, sequenced, opaque envelopes, which study coordinators opened at time of enrolment to assign participants to ACTH treatment or no relapse‐preventing treatment." Comment: Allocation concealment is considered as adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 134/140 participants were reported in the analysis |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Atmaca 2006.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Losartan group
Lisinopril + Losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26/34 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics and co‐interventions. Funder was not reported. No other apparent sources of bias |
Bakris 1994.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/22 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Bansal 2004.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Losartan group
Lisinopril + losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30/46 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. Funder was not reported |
Bauer 1992.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24/33 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Bilo 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18/24 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Bojestig 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Ramipril group
Ramipril group 2
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 51/55 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
CALM 2000.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Candesartan group
Lisinopril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants co‐interventions were not reported |
CALM II 2003.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Candesartan + lisinopril
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported only for albuminuric participants (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants' baseline characteristics and co‐interventions were not reported only for albuminuric participants. It was not clear if funder could influence data analysis and study reporting or interpretation |
Capek 1994.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/20 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
CAPTOPRIL 1992.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 301/409 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participant baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Carella 1999.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Fosinopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/10 participants completed the study (per study group) |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Castelao 1999.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In the abstract was not clearly stated if the study was a randomised controlled trial. Participants baseline characteristics and cointerventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and cointerventions were not reported. Funder was not reported |
CAT 2008.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Candesartan + trandolapril group
Candesartan group 1 (high dose)
Candesartan group 2 (moderate dose)
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and cointerventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported. Numbers per group were not reported |
| Other bias | Unclear risk | Participants' baseline characteristics and cointerventions were not reported. Funder was not reported |
Chase 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Chen 2018b.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Irbesartan group (low dose)
Irbesartan group (high dose)
Irbesartan group (low dose) + spironolactone
Irbesartan group (high dose) + spironolactone
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by the block randomisation method using a random number table generated by Statistics Analysis System (SAS)." |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 206/218 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if the pharmaceutical company influenced the data (authors declared that they bought the drugs from them) |
Cheng 1990.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Enalapril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16/18 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Cocchi 1989.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. Funder was not reported |
Cordonnier 1999.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/22 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. Funder was unlikely to influence data analysis and study reporting or interpretation |
Crepaldi 1998.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 58/96 completed the study |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported (mortality, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro to macroalbuminuria) |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
DETAIL 2002.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Telmisartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on permuted blocks, with a block size of four." Comment: Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Actual five‐year values were available for 62 subjects in the telmisartan group and 74 in the enalapril group, and analyses of values based on the last observation carried forward were performed for all 103 and 113 subjects." Comment: 168/250 participants completed the 5‐year follow‐up. However, it is unclear the number of participants included in the analysus |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Deyneli 2006.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two treatment groups using Arcus QuickStat software." Comment: A computer‐generation model is considered as a low risk of bias. There was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered opaque, sealed envelopes were prepared according to the list generated by the program and used for the randomisation." Comment: Allocation concealment is considered as adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24/26 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
DIABHYCAR 1996.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Ramipril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additonal information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The trial ended prematurely for 838 (17%) participants: 678 (14%) withdrew and refused to be followed up subsequently (344 (14%) taking ramipril and 334 (14%) taking placebo); and 160 (3.2%) were lost to follow up (62 (2.5%) taking ramipril and 98 (4.0%) taking placebo), and their primary end point status is unknown." [...] "4074 (82.9%) participants were followed to 31 March 2001, died, or presented with a non‐fatal primary end point." |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro to macroalbuminuria) |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Dragovic 2003.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Valsartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funding was not reported. No other apparent sources of bias |
DROP 2006.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Valsartan group 1
Valsartan group 2
Valsartan group 3
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 306/391 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Durruty 1990.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to permit adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. Funding was not reported |
Durruty 1996.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/21 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported (only for 21 participants of our interest). Funding was not reported |
ESPRIT 1992.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was prepared electronically. [...] Patients allocation number were linked to treatment in a block size of three." |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, subjective measures were included in outcomes and were possibly influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33/36 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
EUCLID 1997.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was stratified by centre and albuminuric status. [...] Patients were randomly assigned to lisinopril or placebo with a block size of four. Separate schemes were created for each stratum (microalbuminuric and normoalbuminuric), with a FORTRAN computer program validated against the SAS RANUNI random‐number generator." Comment: A computer‐generation model is considered as a low risk of bias. There was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes were supplied to each centre and the coordinating centre so that the code could be broken in an emergency." Comment: Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to permit adjudication only in patients with micro‐ and macroalbuminuria |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants baseline characteristics were not reported only for patients with micro‐ or macroalbuminuria. Funder was likely to influence data analysis and study reporting or interpretation |
FANTASTIC 2017.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants |
Baseline characteristics
|
|
| Interventions | 1a: Fimasartan group at standard BP control group (SBP < 140 mm Hg)
1b: Fimasartan group at strict BP control group (SBP < 130 mm Hg)
2a: Losartan group at standard BP control group (SBP < 140 mm Hg)
2b: Losartan group (at strict BP control group (SBP < 130 mm Hg)
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported sufficiently to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 326/351 subjects completed the study. Safety analyses were reported based on 349 participants. However, ITT was used |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Fogari 2013a.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Imidapril group
Ramipril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "At each visit, adverse events (AEs) spontaneously reported by patients or elicited by the investigators were recorded." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 176/211 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Garg 1998.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Ramipril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Addtional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Hansen 1994.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug) Not reported |
|
| Outcomes | Outcomes reported
|
|
| Notes | Addditonal information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Quite similar participants' baseline characteristics (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Quite similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Hommel 1995.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants baseline characteristics and co‐interventions were not reported. Funder was not reported |
HOPE 1996.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants |
Baseline characteristics
|
|
| Interventions | Ramipril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two‐by‐two factorial design study." Comment: Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All primary and secondary outcomes were documented on separate forms and centrally assessed by the event committee (who were unaware of the participants’ assigned treatments) according to standard definitions." Comment: Outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform adjudication in only people with diabetes |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported. |
| Other bias | High risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation. The study was stopped 6 months early |
IDNT 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Irbesartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned by a central office to one of three treatment regimens." |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1139/1148 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Similar participants baseline characteristics and co‐interventions. Funder was likely to influence data analysis and study reporting or interpretation |
INNOVATION 2005.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group 1
Telmisartan group 2
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional infromation
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 514/527 participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants' baseline characteristics were not reported. It was not clear if funder could influence data analysis and study reporting or interpretation |
IRMA‐2 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Irbesartan group 1
Irbesartan group 2
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent, blinded end‐point committee adjudicated all major cardiovascular events." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 611 patients were included in the ITT analysis |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Similar participants baseline characteristics and co‐interventions. Funder was likely to influence data analysis and study reporting or interpretation |
JAPAN‐IDDM 2002.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Imidapril group
Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eighty‐one eligible cases were randomised centrally." |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All parameters were determined centrally at SRL TeijinBio (Hachiouji, Tokyo)." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 59/81 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants' baseline characteristics and co‐interventions. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Jerums 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by a pharmacist who had no information about participants." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Jerums 2004.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Blinded endpoint." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation. Not reported in sufficient information to assess it as low risk, so we decided to assess it as high risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 45/77 participants completed the study (7 participants were lost to follow‐up) |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | The study was terminated prematurely. Similar participants' baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Kavgaci 2002.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan group
Fosinopril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Kitamura 2020.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group 1
Telmisartan group 2
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study is considered at high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/3 participants in the intervention group 1 and 2/8 participants in the intervention group 2 discontinued. However, ITT principle was used. |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar baseline characteristics were reported in participants with CKD and diabetes. There were no source of funding |
Ko 2005.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Valsartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. There were some differences between participants' baseline characteristics (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation process involved the use of sealed envelopes." Comment: Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform adjudication only in people with micro and macroalbuminuria (one patient dropped in the valsartan group but there was not reported if they had micro/macroalbuminuria) |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported (mortality, cardiovascular events and reduction in GFR or doubling of serum creatinine or progression from micro to macroalbuminuria) |
| Other bias | Unclear risk | There were some differences between participants baseline characteristics, co‐interventions were similar between groups. Funder was not reported |
Krairittichai 2009.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. There were some differences between participants' baseline characteristics (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | There were some differences between participants' baseline characteristics, but co‐interventions were similar between groups. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Lacourciere 2000.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study design
Baseline characteristics
|
|
| Interventions | Losartan group
Enalapril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92/103 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Laffel 1995.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participnats completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participant baseline characteristics and co‐interventions. It was not clear if the funder could influence data analysis and study reporting or interpretation |
Larsen 1990.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/20 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics and co‐interventions. Funder was not reported. No other apparent sources of bias |
Lebovitz 1994.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Lee 1990.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Addtional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation was not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/22 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants baseline characteristics and co‐interventions were not reported. Funder was not reported |
Lewis 1995.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril + ramipril group
Placebo + ramipril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "An external advisory board reviewed all medical, ethical, and statistical considerations." Comment: Although an external advisory board reviewed the endpoint, it was not reported if they were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 96/129 participants completed the two years of follow‐up |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants baseline characteristics and co‐interventions were not reported. It was not clear if funder could influence data analysis and study reporting or interpretation |
Lin 1995.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Captopril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Addtional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
LIRICO 2007.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | ACEi group
ARB group
ACEi + ARB group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized using an electronically generated random list created by the study statistician stratified by centre and in randomly permuted blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated to study treatment by investigators via telephone contact with staff at a central study office. The allocation sequence was concealed to central office staff until after a participant was irreversibly allocated to a treatment group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants and physicians were not blinded to study allocation post‐randomisation." Comment: An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"...blinded endpoint (PROBE) design." "...outcome assessment for the primary composite outcome was carried out by an independent committee that was unaware of treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | Low risk | Protocol was published. Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro to macroalbuminuria) |
| Other bias | Low risk | Similar participants' baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Marre 1987.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation schedule was kept in the hospital pharmacy." Comment: Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the investigator nor the patients were aware of the type of treatment each patients was receiving in this double blind study." Comment: A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
MDNSG 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation was not reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes reported were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred; however, , publications were only abstracts |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were included in the analysis; however, publications were only abstracts |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. Funder was not reported |
Mehdi 2009.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
* 1 patient withdrew before 1st dose |
|
| Interventions | Losartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 39/54 participants completed the 52 weeks period (42/54 completed the 48 weeks period) |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression to micro‐ to macroalbuminuria) |
| Other bias | Low risk | Similar participants baseline characteristics and cointerventions. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Muirhead 1999.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Valsartan group
Valsartan group 2
Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 103/122 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Nakamura 2002a.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Trandolapril group
Candesartan cilexetil group
Trandolapril + Candesartan cilexetil group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Nakamura 2010c.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan group
Candesartan group
Olmesartan group
Telmisartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was little difference in patients' baseline characteristics that could deter an imbalance in randomisation |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients were randomly assigned in a blinded manner." Comment: Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Quote: "Before treatment, age, sex, SBP, DBP, body mass index, serum creatinine, HbA1c, T‐chol and TG, 24‐hour Ccr, urinary LFABP, 8‐OHdG, UAE, and co‐administered drugs differed little between the 4 treatment groups." Comment: Little differences in participants' baseline characteristics were reported. Funder was not reported |
Nankervis 1998.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31/40 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Niu 2008.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Benazepril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additonal information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. Funding was not reported |
O'Donnell 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27/32 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participant baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Ogawa 2007.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Temocapril group
Candesartan group
Temocapril + candesartan group 1
Candesartan + temocapril group 2
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | A single‐blind study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 156/170 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
ONTARGET 2004.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Ramipril group
Telmisartan group
Ramipril + telmisartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned by a central automated system between November 2001 and May 2004 and were followed until March 2008. [...] Randomization was stratified by hospital or clinic." |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomly assigned by telephone through a central automated system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
ORIENT 2006.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Olmesartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After written informed consent was obtained and following the run‐in period, eligible patients were randomised into olmesartan group or placebo group by the registration centre in Japan (EPS, Tokyo, Japan) through fax contact. The centre assigned each patient by the dynamic allocation method, depending on whether or not they were using ACEIs, further stratified by UACR and serum creatinine." |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, caregivers, the people carrying out examinations and people assessing the outcomes were blinded to group assignment. [...] All persons involved in the study were unaware of the drug assignments, except for the person in charge of drug assignment who was not involved in the study." Comment: Investigator and participants were unaware of the treatment allocation groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The IDMC examined the data in a blinded fashion, except for serious adverse events for which a causal relationship with the study drug cannot be ruled out." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 566/577 participants completed the study (< 5% lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro to macroalbuminuria) |
| Other bias | High risk | The study was terminated earlier. Similar participant baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Parving 1989.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32/33 participants completed the study with differences between groups |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events, reduction in GFR or doubling of SCr, or progression from micro to macroalbuminuria) |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Phillips 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Cilaxapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics were not reported (possible imbalance in randomisation was not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. It was not clear if funder could influence data analysis and study reporting or interpretation |
Poulsen 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participants baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
PREVEND IT 2000.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Fosinopril group
Control group 1a
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The independent end point committee of the active trial period reviewed all end points, and the members had no knowledge of subject's treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported for diabetic patients. Funder was not reported |
PRONEDI 2013.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Irbesartan group
Lisinopril + irbesartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was created using SPSS statistical software (IBM‐SPSS Statistics, www.ibm.com/SPSS_Statistics) and stratified by centre using random block sizes of 8 patients within strata with a 1:1:2 allocation." Comment: Computer‐generated is considered as a low risk of bias. There was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence, generated by an investigator with no clinical involvement in the trial, was concealed in opaque sealed envelopes from the researcher enrolling and assessing participants." Comment: It was not clear if the envelopes were numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro to macroalbuminuria) |
| Other bias | Low risk | Quote: "The trial was sponsored, designed, run, and analysed by the investigators of Hospital Fundación de Alcorcón." Comment: Similar participant baseline characteristics and co‐interventions. Although one of the sponsor included a pharmaceutical company, funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Raj 2021.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group
Ramipril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated random numbers was considered at low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open label study is considered at high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91/100 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participant baseline characteristics and co‐interventions were not adequately reported. There was no source of funding |
Ravid 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 94/108 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics and co‐interventions. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
RENAAL 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent end‐points committee whose members were unaware of the patients’ treatment assignments reviewed the data to determine which patients had reached the end points." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included into the analysis |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro to macroalbuminuria) |
| Other bias | High risk | The study was discontinued before the planned follow‐up. Similar participant baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Rizzoni 2005.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Candesartan group
Co‐interventions (non‐randomised antihypertensive drugs
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Addtional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics and co‐interventions. Funder was not reported. No other apparent sources of bias |
Romero 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics and co‐interventions. Funder was not reported. No other apparent sources of bias |
Ruggenenti 2019.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Valsartan group
Benazepril group
Valsartan + benazepril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible participants were randomly assigned (1:1:1) to treatment with benazepril, valsartan or a combination of both at the centralized treatment assignment secretariat by an independent investigator according to a web‐based, computer‐generated randomisation list created using SAS." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible participants were randomly assigned (1:1:1) to treatment with benazepril, valsartan or a combination of both at the centralized treatment assignment secretariat by an independent investigator according to a web‐based, computer‐generated randomisation list created using SAS." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither participants nor care‐providers were masked to group assignment." Comment: An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blind‐endpoint." "An adjudicating group, whose members were not aware of the treatment assignments, reviewed the data to determine which patients had reached study endpoints and to evaluate safety." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics and co‐interventions. Funder did not influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Sano 1994.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 48/62 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics and co‐interventions. Funder was not reported. No other apparent sources of bias |
Sato 2003.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | ACEi group
Candesartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics and co‐interventions. Funder was not reported. No other apparent sources of bias |
Sawaki 2008.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform adjudication in patients with microalbuminuria |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Schram 2005.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Candesartan group
Lisinopril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform adjudication in patients with microalbuminuria |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics. Although a pharmaceutical company supported the study, funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Sengul 2006.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Lisinopril group
Telmisartan group
Lisinopril + telmisartan group
Telmisartan + lisinopril group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study for the 28 weeks period |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics and co‐interventions. Funder was not reported. No other apparent sources of bias |
SMART 2009.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Candesartan group 1
Candesartan group 2
Candesartan group 3
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients’ allocation was blocked with a block size of 3 in a 1:1:1 ratio; patients were stratified according to degree of proteinuria as > 3 g/d or between 1 and 3 g/d." Comment: Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported for diabetic patients (possible imbalance in randomisation non clearly reported) |
| Allocation concealment (selection bias) | Low risk | Quote: "Interactive voice response system" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participant baseline characteristics and co‐interventions were not reported (only for diabetic participants ). Funder influenced the data analysis and study reporting or interpretation |
SOLVD (Treatment) 1990.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Similar participants' baseline characteristics were not reported for only diabetic patients with proteinuria (possible imbalance in randomisation was not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from McCallum 2019: "Cause of each patient’s death was determined by the principal investigator at each centre on the basis of blinded review." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. Death was assessed by the principal investigator, who was blind to the treatment assigned. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform an adjudication in people with diabetes |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participant baseline characteristics were not reported for only diabetic patients with proteinuria. Funder was unlikely to influence data and analysis |
Stornello 1988.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Stornello 1989.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Control group
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
Tan 2002.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Titan 2011.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril + placebo group
Enalapril + losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participant baseline characteristics. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Tong 2006.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Fosinopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Quite similar participants' baseline characteristics and co‐interventions (possible imbalance in randomisation was not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "At each visit, patients were asked to report any spontaneous adverse events. No specific questionnaire was used." Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, subjective measures were included in outcomes, and were possibly influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21/38 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Quite similar participant baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
TRANSCEND 2009.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported only for diabetic patients with kidney disease (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from Tobe 2011: "Investigators, participants, and all trial collaborators were blinded to treatment allocation." Comment: Investigator and participants were unaware of the treatment allocation groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Tobe 2011: "Investigators, participants, and all trial collaborators were blinded to treatment allocation except for a trial‐independent statistician and the members of the independent data safety and monitoring board. [...] All primary outcomes were adjudicated by an independent committee." Comment: The independent committee was not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participant baseline characteristics and co‐interventions were not reported only for diabetic patients with kidney disease. Funder was unlikely to influence data analysis and study reporting or interpretation |
Trevisan 1995.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Ramipril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 108/122 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar participant baseline characteristics. It was not clear if funder could influence data analysis and study reporting or interpretation |
Tutuncu 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Enalapril group
Losartan group
Enalapril + Losartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34/37 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Low risk | Similar participants baseline characteristics. Funder was not reported. No other apparent sources of bias |
VA NEPHRON‐D 2009.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan + placebo group
Losartan + lisinopril group
Cointerventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation program |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was completed by the site coordinator at each study site using a web based randomisation program provided by the CSP Clinical Research Pharmacy Coordinating centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participant, study personnel at treatment office blinded to treatment assignment." Comment: A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adjudication of endpoints (e.g. ESKD) or complications was conducted by individuals blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included into the analysis |
| Selective reporting (reporting bias) | Low risk | Protocol was published. Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro‐ to macroalbuminuria) |
| Other bias | High risk | In October 2012, the data and safety monitoring committee recommended to the sponsor that the study treatment be stopped, primarily on account of safety concerns due to increased rates of serious adverse events, hyperkalaemia, and AKI in the combination‐therapy group as compared with the monotherapy group, along with low conditional power (< 5% for the observed trend) to detect a treatment effect on the primary endpoint. Funder was unlikely to influence data analysis and study reporting or interpretation |
Viberti 1994.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 225/235 participants completed the study |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participant baseline characteristics and co‐interventions were not reported. Funder was unlikely to influence data analysis and study reporting or interpretation |
VIVALDI 2005.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Telmisartan group
Valsartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned in a 1:1 ratio to once‐daily treatment with telmisartan 40 mg or valsartan 80 mg." Comment: Methods for generating the random sequence were not reported in sufficient detail to assess risk. However, there was no imbalance in patients' baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 716/885 participants completed the study |
| Selective reporting (reporting bias) | Low risk | Protocol was published. Clinically relevant outcomes that would be expected for this type of intervention were reported (death, cardiovascular events and reduction in GFR or doubling of SCr or progression from micro to macroalbuminuria) |
| Other bias | Unclear risk | Similar participant baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
Weil 2012.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Losartan group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment group was assigned by computer‐generated random blocks of <10 subjects stratified by albumin category." Comment: A computer‐generated is considered as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, some outcomes (adverse events) could be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | High risk | Protocol was published. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Quite similar participant baseline characteristics and co‐interventions. It was not clear if funder could influence data analysis and study reporting or interpretation |
White 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
*Sufficient tissue to provide material for detailed electron microscopic examination |
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 5/9 and 6/10 participants had sufficient tissue to provide material for detailed electron microscopic examination |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participant baseline characteristics and co‐interventions were not reported. Funder was unlikely to influence data analysis and study reporting or interpretation |
Xia 1996.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Participants baseline characteristics and co‐interventions were not reported. Funder was not reported |
Yao 2001a.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Perindopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participant baseline characteristics and co‐interventions were not reported. Funding was not reported |
Yoldi 1995.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Captopril group
Control group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating the random sequence were not reported in sufficient detail to assess risk. Participants' baseline characteristics and co‐interventions were not reported (possible imbalance in randomisation not clearly reported) |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participants baseline characteristics and co‐interventions were not reported. Funder was not reported |
Yoneda 2007.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Candesartan group
Valsartan group
Co‐interventions (non‐randomised antihypertensive drug)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned by computer to either candesartan (8 mg daily) or valsartan (80 mg daily)." |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Participant baseline characteristics and co‐interventions were not reported. Founder was not reported |
ACEi: angiotensin‐converting‐enzyme inhibitor; AER: albumin excretion rate; ARB: angiotensin receptor blocker; BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; CAB: coronary artery bypass; CCB: calcium channel blocker; CHF: congestive heart failure; CKD: chronic kidney disease; CrCl: creatinine clearance; CRP: C‐reactive protein; CVA: cerebrovascular accident; CVD: cerebrovascular disease; CVE: cardiovascular event; DBP: diastolic blood pressure; DM: diabetes mellitus; ESKD: end‐stage kidney disease; FBG: fasting blood glucose; GFR: glomerular filtration rate; HD: haemodialysis; HDL: high‐density lipoprotein; IBW: ideal body weight; IDDM: insulin‐dependent diabetes mellitus; KRT: kideny replacement therapy; LDL: low‐density lipoprotein; LVEF: left ventricular ejection fraction; MAP: mean arterial pressure; MI: myocardial infarction; NSAIDs: nonsteroidal anti‐inflammatory drugs; NYHA: New York Heart Association; PDG: postprandial blood glucose; PD: peritoneal dialysis; PVD: peripheral vascular disease; RAAS: renin‐angiotensin‐aldosterone system; RCT: randomised contrlled trial; SBP: systolic blood pressure; SC: subcutaneous; SCr: serum creatinine; TIA: transient ischaemic attack; UAE: urinary albumin excretion; UACR: urinary albumin:creatinine ratio; ULN: upper limit of normal; UPCR: urinary protein:creatinine ratio; UPE: urinary protein excretion; UTI: urinary tract infection; WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acbay 2001 | Intervention < 6 months |
| Agarwal 2001 | Intervention < 6 months |
| Alam 2001 | Intervention < 6 months |
| Andersen 1999 | Intervention < 6 months |
| Andersen 2000 | Intervention < 6 months |
| Bakris 2000 | Intervention < 6 months |
| Cetinkaya 2002 | Intervention < 6 months |
| Cetinkaya 2004 | Intervention < 6 months (ACEi and ARB for 12 weeks and then dual therapy was performed for 12 weeks) |
| Chan 1997 | Intervention < 6 months |
| Chonko 1993 | Intervention < 6 months |
| Christensen 2001a | Intervention < 6 months |
| Daikuhara 2012 | Not RCT (patients were randomised 12 weeks before with ARB in both arms, but at T0, they received CCBs in a non‐randomised fashion) |
| Dhillon 2003 | Intervention < 6 months |
| DIRECT Studies 2002 | Wrong population: patients did not have albuminuria or kidney impairment |
| DREAM 2004 | Wrong population: patients did not have albuminuria or kidney impairment |
| Dreyling 1990 | Intervention < 6 months |
| Drummond 1989 | Intervention < 6 months |
| Esnault 2005 | Intervention < 6 months |
| EUCTR2021‐001661‐21‐DK | Wrong intervention: dapaglifozin versus empagliflozin |
| Fliser 2005 | Intervention < 6 months |
| Garg 2005 | Intervention < 6 months |
| Haneda 2004 | Intervention < 6 months |
| Hommel 1986 | Intervention < 6 months |
| Hoque 2009 | Intervention < 6 months |
| Hou 2017a | Intervention < 6 months |
| Houlihan Losartan 2002 | Intervention < 6 months |
| Igarashi 2006 | Intervention < 6 months |
| Inigo 2000 | Intervention < 6 months |
| Insua 1988 | Intervention < 6 months |
| Islam 2005 | Intervention < 6 months |
| Jacobsen 2002 | Intervention < 6 months |
| Jacobsen 2003 | Intervention < 6 months |
| Jacobsen 2003a | Intervention < 6 months |
| Jiao 2022 | Intervention < 6 months |
| Keilani 1993 | Intervention < 6 months |
| Kincaid‐Smith 2002 | Intervention < 6 months |
| Lim 2007 | Intervention < 6 months |
| Masuda 2009 | Intervention < 6 months |
| Mathiesen 1991 | Wrong intervention: captopril + bendrofluazide versus control |
| Matos 2005 | Intervention < 6 months |
| Meier 2011 | Intervention < 6 months (first phase 2 months) |
| Mimran 1988 | Intervention < 6 months |
| Mårup 2022 | Wrong intervention: ACEi, ARB and spironolactone with or without patiromer |
| NCT00171600 | Intervention < 6 months; study was discontinued |
| NCT00208221 | Intervention < 6 months; study was terminated due to recruitment issues |
| NCT04238702 | Intervention < 6 months |
| NCT05189015 | Wrong comparator: olmesartan versus amlodipine |
| NCT05593575 | Intervention < 6 months |
| New 1998 | Intervention < 6 months |
| Okura 2012 | Intervention < 6 months |
| Parvanova 2001 | Intervention < 6 months |
| Pedersen 1992 | Intervention < 6 months |
| Pruijm 2013 | Intervention < 6 months |
| Rasi Hashemi 2012 | Intervention < 6 months |
| Romanelli 1989 | Intervention < 6 months |
| Rossing 2002 | Intervention < 6 months |
| Rossing 2003 | Intervention < 6 months |
| Rossing 2003a | Intervention < 6 months |
| Rossing 2005a | Intervention < 6 months |
| Rossing 2005b | Intervention < 6 months |
| Saka 2004 | Intervention < 6 months |
| Salem 2021 | Intervention < 6 months |
| Sasso 2002 | Intervention < 6 months |
| Schjoedt 2005a | Intervention < 6 months |
| Schjoedt 2009a | Intervention < 6 months |
| Schmieder 2005 | Intervention < 6 months |
| Seher 2017 | Intervention < 6 months |
| Shand 2002 | Intervention < 6 months; cross‐over study |
| SHIELD 2003 | Intervention < 6 months |
| Shu 1997 | Intervention < 6 months |
| Song 2003 | Intervention < 6 months |
| Song 2004a | Intervention < 6 months |
| Song 2006 | Intervention < 6 months |
| Suehiro 2021 | Intervention < 6 months |
| Suzuki 2011 | Intervention < 6 months |
| Tan 2010 | Intervention < 6 months |
| TRENDY 2007 | Intervention < 6 months |
| Turab 2008 | Intervention < 6 months |
| van Nieuwenhoven 2004 | Intervention < 6 months |
| Vongterapak 1998 | Intervention < 6 months |
| Wiegmann 1992 | Intervention < 6 months |
| Wouda 2021 | Intervention < 6 months |
| Zandbergen 2003 | Intervention < 6 months |
| Zhang 2019c | Intervention < 6 months |
ACEi: angiotensin‐converting‐enzyme inhibitor; ARB: angiotensin receptor blocker
Characteristics of studies awaiting classification [ordered by study ID]
Andrysiak‐Mamos 1997.
| Methods | Study design
|
| Participants | Study characteristics
Baseline characteristics
|
| Interventions | Enalapril group
Lisinopril group
Co‐interventions (non‐randomised antihypertensive drug)
|
| Outcomes | Outcomes reported
|
| Notes | Additional information
|
Limonte 2024.
| Methods | Awaiting assessment |
| Participants | Awaiting assessment |
| Interventions | Awaiting assessment |
| Outcomes | Awaiting assessment |
| Notes |
Vijay 2000.
| Methods | Study design
|
| Participants | Study characteristics
Baseline characteristics
|
| Interventions | Treatment group not clearly stated
Comparison group not clearly stated
Co‐interventions (non‐randomised antihypertensive drug)
|
| Outcomes | Outcomes reported
|
| Notes | Additional information
|
Vineela 2023.
| Methods | Awaiting assessment |
| Participants | Awaiting assessment |
| Interventions | Awaiting assessment |
| Outcomes | Awaiting assessment |
| Notes | Awaiting assessment |
BP: blood pressure; M/F: male/female; RCT: randomised controlled trial; SD: standard deviation; UAE: urinary albumin excretion
Characteristics of ongoing studies [ordered by study ID]
CTRI/2023/05/052593.
| Study name | Dual RAAS blockage in primary glomerular diseases |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Losartan + enalapril group
Losartan group
|
| Outcomes | Planned outcomes
|
| Starting date | Approval date: 1 March 2023 Registration date: 12 May 2023 |
| Contact information | Dr Tukaram Jamale Email: tukaramjamale@yahoo.co.in |
| Notes | Funding: Department of Nephrology KEM Hospital Parel Mumba |
NCT05753696.
| Study name | Azilsartan in patients with diabetic kidney disease and hypertension |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Azilsartan group
Losartan group
|
| Outcomes | Planned outcomes
|
| Starting date | Estimated start date: 1 April 2023 |
| Contact information | Chen Zhida Email: 715264276@qq.com |
| Notes | Additional information
|
TCTR20220426002.
| Study name | Comparison of efficacy in renoprotection between azilsartan medoxomil and enalapril: a randomized, open‐labeled controlled trial |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Azilsartan group
Enalapril group
Co‐interventions (non‐randomised antihypertensive drug)
|
| Outcomes | Planned outcomes
|
| Starting date | Date of first enrolment: 18 April 2018 Registration date: 26 April 2022 |
| Contact information | Dr. Ployrawee Thanaprirax ployraweeth@gmail.com |
| Notes | Additional information
|
ACEi: angiotensin‐converting enzyme inhibitor; AKI: acute kidney injury; ARB: angiotensin receptor blocker; BP: blood pressure; BUN: blood urea nitrogen; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; FSGS: focal segmental glomerulosclerosis; HbA1c: glycosylated haemoglobin; IgAN: Ig A nephropathy; MCD: minimal change disease; MN: menbranous nephropathy; MPGN: membranoproliferative glomerulonephritis; RAAS: renin‐angiotensin‐aldosterone system; RCT: randomised controlled trial; SBP: sydtolic blood pressure; UACR: urinary albumin‐creatinine ratio
Differences between protocol and review
Risk of bias assessment tool has replaced Quality assessment checklist. We have added the following outcomes: change in GFR, GFR at end of treatment, regression from microalbuminuria to normoalbuminuria and regression from macroalbuminuria to either microalbuminuria or normoalbuminuria.
GRADE assessment has been used.
Contributions of authors
Cochrane 2024 update
Patrizia Natale: Data extraction, data entry, data analysis, data interpretation, writing the review
Suetonia Palmer: Design, conduct, data analysis, data interpretation, writing the review
Sankar Navaneethan: Data interpretation, writing of review
Jonathan Craig: Design, conduct, data interpretation, writing the review
Giovanni Strippoli: Design, conduct, data interpretation, writing the review
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Patrizia Natale: No relevant interests were disclosed
Suetonia Palmer: No relevant interests were disclosed
Sakar Navaneethan: Baylor College of Medicine (Employment), AstraZeneca (Independent Contractor ‐ Data And Safety Monitoring), Vifor Pharma (Independent Contractor ‐ Consultant), Eli Lilly and Company (Independent Contractor ‐ Consultant), Boehringer Ingelheim (Independent Contractor ‐ Consultant), Keryx Biopharmaceuticals, Inc. (Money paid to institution: Grant / Contract), Bayer (Independent Contractor ‐ End Point Review Committee), U.S. Department of Veterans Affairs (Employment)
Jonathan Craig: No relevant interests were disclosed
Giovanni Strippoli: No relevant interests were disclosed
Edited (no change to conclusions)
References
References to studies included in this review
ABCD 1996 {published data only}
- Estacio RO, Jeffers B, Biggerstaff S, Schrier RW. Effects of a calcium channel antagonist versus an ACE inhibitor on diabetic nephropathy [abstract no: S1064]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):114A. [CENTRAL: CN-00445260] [Google Scholar]
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23 Suppl 2:B54-64. [MEDLINE: ] [PubMed] [Google Scholar]
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Estacio RO, Mehler P, Esler A, Schrier RW. Aggressive lowering of blood pressure in normotensive type 2 diabetic patients: beneficial effects on stroke, progression of retinopathy and nephropathy [abstract no: A0763]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):146A. [CENTRAL: CN-00445261] [Google Scholar]
- Estacio RO, Savage S, Nagel NJ, Schrier RW. Baseline characteristics of participants in the Appropriate Blood pressure Control in Diabetes trial. Controlled Clinical Trials 1996;17(3):242-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Estacio RO, Schrier RW. Antihypertensive therapy in type 2 diabetes: implications of the appropriate blood pressure control in diabetes (ABCD) trial. American Journal of Cardiology 1998;82(9B):9-14R. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation 2003;107(5):753-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Savage S, Estacio RO, Jeffers B, Schrier RW. Urinary albumin excretion as a predictor of diabetic retinopathy, neuropathy, and cardiovascular disease in NIDDM. Diabetes Care 1996;19(11):1243-48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Savage S, Johnson Nagel N, Estacio RO, Feig PU, MacCarthy EP, et al. The ABCD (Appropriate Blood Pressure Control in Diabetes) trial. Rationale and design of a trial of hypertension control (moderate or intensive) in type II diabetes. Online Journal of Current Clinical Trials 1993;Doc No 104. [PMID: ] [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney International 2002;61(2):1086-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Jeffers B. Appropriate Blood pressure Control in NIDDM (ABCD) trial. Diabetologia 1996;39(12):1646-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schrier RW, Savage S. Appropriate Blood Pressure Control in type II diabetes (ABCD Trial): implications for complications. American Journal of Kidney Diseases 1992;20(6):653-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Stabler SP, Estacio R, Jeffers BW, Cohen JA, Allen RH, Schrier RW. Total homocysteine is associated with nephropathy in non-insulin-dependent diabetes mellitus. Metabolism: Clinical & Experimental 1999;48(9):1096-101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ABCD‐2V 2006 {published data only}
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. American Journal of Hypertension 2006;19(12):1241-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Estacio RO, Esler A, Lundgren R, Schrier RW. Aggressive blood pressure control with valsartan in normotensive type 2 diabetic patients results in regression of albuminuria [abstract no: SU-PO165]. Journal of the American Society of Nephrology 2004;15(Oct):568A. [CENTRAL: CN-00644364] [Google Scholar]
Abe 2007a {published data only}
- Abe H, Minatoguchi S, Ohashi H, Murata I, Minagawa T, Okuma T, et al. Renoprotective effect of the addition of losartan to ongoing treatment with an angiotensin converting enzyme inhibitor in type-2 diabetic patients with nephropathy. Hypertension Research - Clinical & Experimental 2007;30(10):929-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmad 1997 {published data only}
- Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care 1997;20(10):1576-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmad 2003 {published data only}
- Ahmad J, Shafique S, Abidi SM, Parwez I. Effect of 5-year enalapril therapy on progression of microalbuminuria and glomerular structural changes in type 1 diabetic subjects. Diabetes Research & Clinical Practice 2003;60(2):131-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
AIPRI 1996 {published data only}
- Apperloo AJ, Rensma PL. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curren CG. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):597. [MEDLINE: ] [PubMed] [Google Scholar]
- Hogan TJ, Elliott WJ, Seto AH, Bakris GL. Antihypertensive treatment with and without benazepril in patients with chronic renal insufficiency: a US economic evaluation. Pharmacoeconomics 2002;20(1):37-47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Carbarns IR, Maschio G, Mann JF, Ponticelli C, Ritz E, et al. Long-term progression of chronic renal insufficiency in the AIPRI Extension Study. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Kidney International - Supplement 1997;63:S63-6. [MEDLINE: ] [PubMed] [Google Scholar]
- Locatelli F, Del Vecchio L, Andrulli S, Marai P, Tentori F. The role of underlying nephropathy in the progression of renal disease. Kidney International - Supplement 2000;75:S49-55. [MEDLINE: ] [PubMed] [Google Scholar]
- Mann JF, Maschio G, Alberti D, AIPRI investigators. Perspectives of ACE inhibition in progressive renal failure [abstract no: 104]. Nephrology 1997;3(Suppl 1):S39. [CENTRAL: CN-00461248] [Google Scholar]
- Maschio G, Alberti D, Janin G, Locatelli F, Mann J, Motolese M, et al. The use of benazepril in the treatment of progressive renal disease [abstract]. In: 13th International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:65. [CENTRAL: CN-00550723]
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. New England Journal of Medicine 1996;334(15):939-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maschio G, Alberti D, Locatelli F, Mann JF, Motolese M, Ponticelli C, et al. Angiotensin-converting enzyme inhibitors and kidney protection: the AIPRI trial. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study Group. Journal of Cardiovascular Pharmacology 1999;33 Suppl 1:S16-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maschio G, Alberti D, Locatelli F. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):597. [CENTRAL: CN-00550722] [PubMed] [Google Scholar]
- Pedersen EB. Reduced progression, increased mortality in chronic renal disease after treatment with the angiotensin-converting enzyme inhibitor benazepril [Nedsat progressionshastighed ved kronisk nyresygdom efter behandling med den angiotensinkonverterende enzymhaemmer benazepril, men ogsa oget mortalite]. Ugeskrift for Laeger 1996;158(41):5798-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Hout BA, Simeon GP, McDonnell J, Mann JF. Economic evaluation of benazepril in chronic renal insufficiency. Kidney International - Supplement 1997;63:S159-62. [MEDLINE: ] [PubMed] [Google Scholar]
AMADEO 2008 {published data only}
- AMADEO: A trial to compare telmisartan 40mg titrated to 80mg versus losartan 100mg in hypertensive type-2 diabetic patients with overt nephropathy. The Protection Trial Programme - Micardis Protection 2005. [CENTRAL: CN-00583661]
- Bakris G, Burgess E, Weir M, Davidai G, Koval S. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney International 2008;74(3):364-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bakris G, Burgess E, Weir M, Koval S. Influence of glycaemic control on proteinuria in patients with type 2 diabetes and overt nephropathy and hypertension: results of the AMADEO trial [abstract no: 0601-P]. Diabetes 2007;56(Suppl 1):A159. [CENTRAL: CN-00765394] [Google Scholar]
- Moranne O, Bakris G, Fafin C, Favre G, Pradier C, Esnault VL. Determinants and changes associated with aldosterone breakthrough after angiotensin II receptor blockade in patients with type 2 diabetes with overt nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(10):1694-701. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranne OP, Bakris GL, Esnault VL. Predictors of aldosterone breakthrough with ARB treatment in diabetic nephropathy [abstract no: F-FC315]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):73A. [Google Scholar]
Arai 2008 {published data only}
- Arai T, Ohashi H. Angiotensin receptor blockers and microalbuminuria in hypertensive patients with early (microalbuminuric) stage diabetic nephropathy. Molecular Medicine Reports 2008;1(3):391-3. [PMID: ] [PubMed] [Google Scholar]
Arpitha 2020 {published data only}
- Arpitha KS, Lakshminarayana K. A comparative study of efficacy of enalapril versus telmisartan in patients with diabetic nephropathy. National Journal of Physiology, Pharmacy & Pharmacology 2020;10(10):894‐9. [EMBASE: 2005173747] [Google Scholar]
ATLANTIS 2000 {published data only}
- O'Hare P, Bilbous R, Mitchell T, O'Callaghan CJ, Viberti GC. Low-dose ramipril reduces microalbuminuria in type 1 diabetic patients without hypertension: results of a randomized controlled trial. Diabetes Care 2000;23(12):1823-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Atmaca 2006 {published data only}
- Atmaca A, Gedik O. Effects of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and their combination on microalbuminuria in normotensive patients with type 2 diabetes. Advances in Therapy 2006;23(4):615-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakris 1994 {published data only}
- Bakris GL, Slataper R, Vicknair N, Sadler R. ACE inhibitor mediated reductions in renal size and microalbuminuria in normotensive, diabetic subjects. Journal of Diabetes & its Complications 1994;8(1):2-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bansal 2004 {published data only}
- Bansal R, Agarwal SK, Tiwari SC. Short term effects of maximum tolerable doses of ACE-inhibitor (lisinopril), angiotensin receptor blocker (losartan) and add-on losartan on proteinuria and glomerular filtration rate [abstract]. Indian Journal of Nephrology 2004;14(3):110-1. [CENTRAL: CN-01658567] [Google Scholar]
- Bansal R, Agarwal SK. Study of maximum tolerable doses of ACE-I (lisinopril), ARB (losartan) and add-on ARB on proteinuria and progression of renal disease in patients of type 2 diabetes with early nephropathy: results of one year follow up [abstract no: CNO-9]. In: 36th Annual Conference of the Indian Society of Nephrology (ISNCON 2005); 2005 Dec 1-3; Cochin, India. 2005:34. [CENTRAL: CN-01658566]
Bauer 1992 {published data only}
- Bauer JH, Reams GP, Hewett J, Klachko D, Lau A, Messina C, et al. A randomized, double-blind, placebo-controlled trial to evaluate the effect of enalapril in patients with clinical diabetic nephropathy. American Journal of Kidney Diseases 1992;20(5):443-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bilo 1993 {published data only}
- Bilo H, Kluitman E, Ballegooie E, Potter van Loon BJ, Bakker K, Michels B, et al. Long term use of captopril or nifedipine in normotensive microalbuminuric patients with insulin-dependent diabetes mellitus. Diabetes Research 1993;23(3):115-22. [MEDLINE: ] [PubMed] [Google Scholar]
- Bilo HJ, Ballegooie E, Potter van Loon BJ, Gans RO, Donker AJ. Captopril or nifedipine in normotensive microalbuminuric IDDM patients [abstract no: 85P]. Journal of the American Society of Nephrology 1992;3(3):331. [CENTRAL: CN-00583442] [Google Scholar]
Bojestig 2001 {published data only}
- Bojestig M, Karlberg BE, Lindstrom T, Nystrom FH. Reduction of ACE activity is insufficient to decrease microalbuminuria in normotensive patients with type 1 diabetes. Diabetes Care 2001;24(5):919-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CALM 2000 {published data only}
- Cooper ME, Mogensen CE, CALM Study Group. Role of candesartan, lisinopril and their combination on blood pressure and albuminuria in hypertensive microalbuminuric diabetic subjects [abstract no: 84]. Nephrology 2000;5(3):A87. [CENTRAL: CN-00509141] [Google Scholar]
- Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000;321(7274):1440-4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CALM II 2003 {published data only}
- Andersen NH, Knudsen ST, Poulsen PL, Poulsen SH, Helleberg K, Eiskjaer H, et al. Dual blockade with candesartan cilexetil and lisinopril in hypertensive patients with diabetes mellitus: rationale and design. Journal of the Renin-Angiotensin-Aldosterone System 2003;4(2):96-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Andersen NH, Poulsen PL, Knudsen ST, Poulsen SH, Eiskjaer H, Hansen KW, et al. Long-term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes: the CALM II study. Diabetes Care 2005;28(2):273-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Knudsen ST, Andersen NH, Poulsen SH, Eiskjaer H, Hansen KW, Helleberg K, et al. Pulse pressure lowering effect of dual blockade with candesartan and lisinopril vs. high-dose ACE inhibition in hypertensive type 2 diabetic subjects: a CALM II study post-hoc analysis. American Journal of Hypertension 2008;21(2):172-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Capek 1994 {published data only}
- Capek M, Schnack C, Ludvik B, Kautzky-Willer A, Banyai M, Prager R. Effects of captopril treatment versus placebo on renal function in type 2 diabetic patients with microalbuminuria: a long-term study. Clinical Investigator 1994;72(12):961-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CAPTOPRIL 1992 {published data only}
- Bain R, Rohde R, Hunsicker LG, McGill J, Kobrin S, Lewis EJ. A controlled clinical trial of angiotensin-converting enzyme inhibition in type I diabetic nephropathy: study design and patient characteristics. The Collaborative Study Group. Journal of the American Society of Nephrology 1992;3(4 Suppl):S97-103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Breyer J, McGill J, Nahman S, Lewis E, Bain R, Cooper M, et al. Predictors of the rate of progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy [abstract no: 38P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):301. [CENTRAL: CN-00483327] [Google Scholar]
- Breyer JA, Bain RP, Evans JK, Nahman NS Jr, Lewis EJ, Cooper M, et al. Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. The Collaborative Study Group. Kidney International 1996;50(5):1651-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Breyer JA, Hunsicker LG, Bain RP, Lewis EJ. Angiotensin converting enzyme inhibition in diabetic nephropathy. The Collaborative Study Group. Kidney International - Supplement 1994;45:S156-60. [MEDLINE: ] [PubMed] [Google Scholar]
- Hebert LA, Bain RP, Verme D, Cattran D, Whittier FC, Tolchin N, et al. Remission of nephrotic range proteinuria in type I diabetes. Collaborative Study Group. Kidney International 1994;46(6):1688-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Bain R, Hunsicker LG, Collaborative Study Group. A controlled clinical trial of angiotensin-converting enzyme inhibition in type 1 diabetic nephropathy [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:424. [CENTRAL: CN-00626026]
- Lewis EJ, Bain RP, Rohde RD, Hunsicker LG, Collaborative Study Group. A controlled clinical trial of angiotensin-converting enzyme (ACE) inhibition in type 1 diabetic nephropathy [abstract no: 39P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):305. [CENTRAL: CN-00484813] [Google Scholar]
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. New England Journal of Medicine 1993;329(20):1456-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis EJ. The captopril trial [abstract]. In: 13th International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:66. [CENTRAL: CN-00550439]
- Rodby R, Firth L, Lewis E. An economic analysis of captopril in the treatment of diabetic nephropathy [abstract no: 16P]. Journal of the American Society of Nephrology 1994;5(3):382. [Google Scholar]
- Rodby RA, Rohde RD, Bain RP, Sharon Z, Pohl MA, Lewis EJ, et al. The urine protein to creatinine ratio (p/c) as a predictor of 24 hour urine protein excretion (24up) in type 1 diabetics with nephropathy [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):308. [CENTRAL: CN-00485628] [DOI] [PubMed] [Google Scholar]
- Rodby RA, Rohde RD, Sharon Z, Pohl MA, Bain RP, Lewis EJ. The urine protein to creatinine ratio as a predictor of 24-hour urine protein excretion in type 1 diabetic patients with nephropathy. The Collaborative Study Group. American Journal of Kidney Diseases 1995;26(6):904-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weiss MF, Rodby RA, Justice AC, Hricik DE. Free pentosidine and neopterin as markers of progression rate in diabetic nephropathy. Collaborative Study Group. Kidney International 1998;54(1):193-202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carella 1999 {published data only}
- Carella MJ, Gossain VV, Jones J. The effects of a low-dose regimen of fosinopril on elevated urinary albumin excretion in normotensive type 1 diabetic patients. Journal of Medicine 1999;30(5-6):305-20. [MEDLINE: ] [PubMed] [Google Scholar]
Castelao 1999 {published data only}
- Castelao AM, Ramos R, Sanchez-Zamorano MA, Alsina J. Comparison of losartan vs enalapril in type 2 diabetes mellitus patients with diabetic nephropathy [abstract no: A0652]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):127A. [CENTRAL: CN-00550750] [Google Scholar]
CAT 2008 {published data only}
- Nakao N, Fujimori A, Hasegawa A, Matsushima H. A combination treatment of an angiotensin-converting enzyme inhibitor and angiotensin-receptor blocker can inhibit plasma renin-angiotensin system more completely and persistently as compared with high or moderate dose angiotensin-receptor blocker treatments in type 2 diabetic nephropathy: nested cohort study of candesartan-trandolapril trial in diabetic CKD (CAT Trial) [abstract no: MP156]. NDT Plus 2008;1(Suppl 1):ii285. [Google Scholar]
Chase 1993 {published data only}
- Chase HP, Garg SK, Harris S, Hoops S, Jackson WE, Holmes DL. Angiotensin-converting enzyme inhibitor treatment for young normotensive diabetic subjects: a two-year trial. Annals of Ophthalmology 1993;25(8):284-9. [MEDLINE: ] [PubMed] [Google Scholar]
Chen 2018b {published data only}
- Chen Y, Liu P, Chen X, Li Y, Zhang F, Wang Y. Effects of different doses of irbesartan combined with spironolactone on urinary albumin excretion rate in elderly patients with early type 2 diabetic nephropathy. American Journal of the Medical Sciences 2018;355(5):418-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheng 1990 {published data only}
- Cheng IK, Ma JT, Yeh GR, Chan MK. Comparison of captopril and enalapril in the treatment of hypertension in patients with non-insulin dependent diabetes mellitus and nephropathy. International Urology & Nephrology 1990;22(3):295-303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cocchi 1989 {published data only}
- Cocchi R, Degli EE, et al. Does captopril reduce proteinuria in normotensive, non-azotaemic diabetic patients? [abstract]. Nephrology Dialysis Transplantation 1989;4(5):433. [CENTRAL: CN-00260423] [Google Scholar]
Cordonnier 1999 {published data only}
- Cordonnier DJ, Pinel N, Barro C, Maynard M, Zaoui P, Halimi S, et al. Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. The Diabiopsies Group. Journal of the American Society of Nephrology 1999;10(6):1253-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cordonnier DJ, Pinel N, Barro C, Zaoui P. Renal interstital expansion is modified by perindopril in true diabetic glomerulosclerosis (DG) in type 2 (NIDDM) diabetic patients. a 2 years sequential biopsy study [abstract no: A0522]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):110A. [CENTRAL: CN-00444912] [Google Scholar]
- Cordonnier DJ, Pinel N. Renal interstitial expansion is modified by perindopril in true diabetic glomerulosclerosis (DG) in type 2 (NIDDM) diabetic patients. A 2 years sequential biopsy study [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A89. [CENTRAL: CN-00261382] [Google Scholar]
- Langham R, Gow RM, Zhang Y, Kelly DJ, Cordonnier D, Pinel N, et al. Blockade of the renin-angiotensin system attenuates renal TGF-b gene expression and activity in human diabetic nephropathy [abstract no: M-PO20058]. Nephrology 2005;10(Suppl):A30. [Google Scholar]
- Langham RG, Kelly DJ, Cox AJ, Thomson NM, Holthofer H, Zaoui P, et al. Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition [abstract no: F-PO529]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):167A. [DOI] [PubMed] [Google Scholar]
- Langham RG, Kelly DJ, Gow RM, Zhang Y, Cordonnier DJ, Pinel N, et al. Transforming growth factor-beta in human diabetic nephropathy: effects of ACE inhibition. Diabetes Care 2006;29(12):2670-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crepaldi 1998 {published data only}
- Crepaldi G, Carta Q, Deferrari G, Mangili R, Navalesi R, Santeusanio F, et al. Effects of lisinopril and nifedipine on the progression to overt albuminuria in IDDM patients with incipient nephropathy and normal blood pressure. The Italian Microalbuminuria Study Group in IDDM. Diabetes Care 1998;21(1):104-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DETAIL 2002 {published data only}
- Barnett A. Preventing renal complications in type 2 diabetes: results of the diabetics exposed to telmisartan and enalapril trial. Journal of the American Society of Nephrology 2006;17(4 Suppl 2):S132-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. New England Journal of Medicine 2004;351(19):1952-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- DETAIL: Diabetics exposed to telmisartan and enalapril [brochure]. The Protection Trial Programme - Micardis Protection 2005:20.
- Rippin J, Bain SC, Barnett AH, DETAIL S. Rationale and design of diabetics exposed to telmisartan and enalapril (DETAIL) study [Erratum in: N Engl J Med. 2005 Apr 21;352(16)1731]. Journal of Diabetes & its Complications 2002;16(3):195-200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deyneli 2006 {published data only}
- Deyneli O, Yavuz D, Velioglu A, Cacina H, Aksoy N, Haklar G, et al. Effects of ACE inhibition and angiotension II receptor blockade on glomerular basement membrane protein excretion and change selectivity in type 2 diabetic patients. JRAAS - Journal of the Renin-Angiotensin-Aldosterone System 2006;7(2):98-103. [EMBASE: 44383504] [DOI] [PubMed] [Google Scholar]
DIABHYCAR 1996 {published data only}
- Azizi M, Menard J, Peyrard S, Lievre M, Marre M, Chatellier G. Assessment of patients' and physicians' compliance to an ACE inhibitor treatment based on urinary N-acetyl Ser-Asp-Lys-Pro determination in the Noninsulin-Dependent Diabetes, Hypertension, Microalbuminuria, Proteinuria, Cardiovascular Events, and Ramipril (DIABHYCAR) study. Diabetes Care 2006;29(6):1331-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lievre M, Marre M, Chatellier G, Plouin P, Reglier J, Richardson L, et al. The non-insulin-dependent diabetes, hypertension, microalbuminuria or proteinuria, cardiovascular events, and ramipril (DIABHYCAR) study: design, organization, and patient recruitment. DIABHYCAR Study Group. Controlled Clinical Trials 2000;21(4):383-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marre M, Lievre M, Chatellier G, Mann J, Passa P, Menard J. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ 2004;328(7438):495-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre M, Lievre M, Chatellier G, Vasmant D, Mann J, Passa P, et al. Low-dose ramipril (1.25 mg/day) does not decrease cardiovascular events in type 2 diabetes patients with microalbuminuria/proteinuria: the DIABHYCAR (type 2 DIABetes, HYpertension, CArdiovascular events and Ramipril) study [abstract no: 185]. In: 38th Annual Meeting of the European Association for the Study of Diabetes (EASD); 2002 Sept 1-5; Budapest, Hungary. 2002.
- Marre M, Lievre M, Vasmant D, Gallois Y, Hadjadj S, Reglier JC, et al. Determinants of elevated urinary albumin in the 4,937 type 2 diabetic subjects recruited for the DIABHYCAR Study in Western Europe and North Africa. Diabetes Care 2000;23 Suppl 2:B40-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Passa P, Chatellier G. The DIAB-HYCAR Study. Diabetologia 1996;39(12):1662-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vaur L, Gueret P, Lievre M, Chabaud S, Passa P. Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: observations from the DIABHYCAR (type 2 DIABetes, Hypertension, CArdiovascular Events and Ramipril) study. Diabetes Care 2003;26(3):855-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Velho G, Potier L, Mohammedi K, Fumeron F, Hadjadj S, Marre M, et al. AVP gene variants, plasma copeptin and nephropathy in type 2 diabetes [abstract no: 183]. Diabetologia 2018;61(Suppl 1):S93-4. [EMBASE: 624032797] [Google Scholar]
Dragovic 2003 {published data only}
- Dragovic T, Hrvacevic R, Ajdinovic B, Vujanic S. Efficacy of valsartan in the treatment of persistent microalbuminuria in normotensive patients with type 1 diabetes [Efikasnost valsartana u terapiji perzistentne mikroalbuminurije kod normotenzivnih bolesnika sa tipom 1 secerne bolesti]. Vojnosanitetski Pregled 2003;60(5):555-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dragovic T, Mijuskovic Z, Karajovic J, Andelkovic Z. Effect of AT1 receptor blockers on plasma lipid profiles in type 1 diabetic patients with incipient diabetic nephropathy [Efekat primene blokatora AT1 receptora na lipidni profil kod bolesnika sa secernom bolesti tip 1 I pocetnom dijabetesnom nefropatijom]. Jugoslovenska Medicinska Biohemija 2005;24(1):21-6. [EMBASE: 40188044] [Google Scholar]
DROP 2006 {published data only}
- Hollenberg NK, Parving H, Viberti G, Remuzzi G. The Diovan Reduction Of Proteinutria (DROP) study: albuminuria response to high-doses of valsartan in type 2 diabetes mellitus [abstract no: TH-PO244]. Journal of the American Society of Nephrology 2006;17(Abstracts):158A. [CENTRAL: CN-00602042] [Google Scholar]
- Hollenberg NK, Parving HH, Viberti G, Remuzzi G, Ritter S, Zelenkofske S, et al. Albuminuria response to very high-dose valsartan in type 2 diabetes mellitus. Journal of Hypertension 2007;25(9):1921-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weir MR, Hollenberg NK, Remuzzi G, Zappe DH, Meng X, Parving HH. Varying patterns of the antihypertensive and antialbuminuric response to higher doses of renin-angiotensin-aldosterone system blockade in albuminuric hypertensive type 2 diabetes mellitus patients. Journal of Hypertension 2011;29(10):2031-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weir MR, Hollenberg NK, Zappe DH, Meng X, Parving HH, Viberti G, et al. Antihypertensive effects of double the maximum dose of valsartan in African-American patients with type 2 diabetes mellitus and albuminuria. Journal of Hypertension 2010;28(1):186-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Durruty 1990 {published data only}
- Durruty P, Krause P, Perez F, Garcia de los RM, Lopez G, Durruty G. Microalbuminuria in insulin-dependent diabetics: the prevention of diabetic nephropathy [Microalbuminuria en diabeticos insulinodependientes: prevencion de la nefropatia diabetica]. Revista Medica de Chile 1990;118(12):1319-25. [MEDLINE: ] [PubMed] [Google Scholar]
Durruty 1996 {published data only}
- Durruty P, Tapia JC, Ugarte C, Perez E, Krause P, Soto N, et al. Urinary albumin excretion in non-insulin-dependent diabetic patients. Effects of an angiotensin-converting enzyme inhibitor [Excrecion urinaria de albumina en diabeticos no insulino-dependientes. Efecto de un inhibidor de la enzima convertidora de angiotensina]. Revista Medica de Chile 1996;124(9):1036-44. [MEDLINE: ] [PubMed] [Google Scholar]
ESPRIT 1992 {published data only}
- Effect of 3 years of antihypertensive therapy on renal structure in type 1 diabetic patients with albuminuria: the European Study for the Prevention of Renal Disease in Type 1 Diabetes (ESPRIT). Diabetes 2001;50(4):843-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Viberti GC, Bilous RW, El Nahas AM, Hersh A, Remuzzi G. A pilot, multicentre, randomized, double-blind, parallel study evaluating the efficacy and tolerability of enalapril, nifedipine retard and placebo on the evolution of diabetic nephropathy in normotensive, insulin-dependent diabetic patients with increased urinary albumin excretion. Journal of Nephrology 1992;5:99-109. [Google Scholar]
- White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 2002;51(10):3083-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EUCLID 1997 {published data only}
- Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet 1998;351(9095):28-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fuller JH, EUCLID Study Group, Anzalone D. Renal effect of lisinopril in insulin dependent diabetes mellitus (IDDM) without hypertension [abstract no: A0560]. Journal of the American Society of Nephrology 1996;7(9):1357. [CENTRAL: CN-00583621] [Google Scholar]
- Penno G, Chaturvedi N, Talmud PJ, Cotroneo P, Manto A, Nannipieri M, et al. Effect of angiotensin-converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: findings from the EUCLID randomized controlled trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes 1998;47(9):1507-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997;349(9068):1787-92. [MEDLINE: ] [PubMed] [Google Scholar]
- Schalkwijk CG, Chaturvedi N, Twaafhoven H, Hinsbergh VW, Stehouwer CD. Amadori-albumin correlates with microvascular complications and precedes nephropathy in type 1 diabetic patients. European Journal of Clinical Investigation 2002;32(7):500-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, Chaturvedi N, Hinsbergh VW, Stehouwer CDA. Amadori-albumin correlates with microvascular complications and precedes nephropathy in type 1 diabetic patients [abstract no: A4431]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):847A. [Google Scholar]
- Sjolie AK, Chaturvedi N, Fuller J. Effect of lisinopril on progression of retinopathy and microalbuminuria in normotensive subjects with insulin-dependent diabetes mellitus [Effekt af lisinopril pa progression af retinopati og mikroalbuminuri hos normotensive personer med insulinafhaengig diabetes mellitus. The EUCLID Study Group]. Ugeskrift for Laeger 1999;161(7):949-52. [MEDLINE: ] [PubMed] [Google Scholar]
FANTASTIC 2017 {published data only}
- Kim JY, Son JW, Park S, Yoo TH, Kim YJ, Ryu DR, et al. FimAsartaN proTeinuriA SusTaIned reduCtion in comparison with losartan in diabetic chronic kidney disease (FANTASTIC): study protocol for randomized controlled trial. Trials [Electronic Resource] 2017;18(1):632. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo TH, Hong SJ, Kim S, Shin S, Kim DK, Lee JP, et al. The FimAsartaN proTeinuriA SusTaIned reduCtion in comparison with losartan in diabetic chronic kidney disease (FANTASTIC) trial. Hypertension Research - Clinical & Experimental 2022;45(12):2008‐17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fogari 2013a {published data only}
- Fogari R, Mugellini A, Zoppi A, Gualtierotti R, Lazzari P, Derosa G, et al. Effect of imidapril versus ramipril on urinary albumin excretion in hypertensive patients with type 2 diabetes and microalbuminuria. Expert Opinion on Pharmacotherapy 2013;14(18):2463-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garg 1998 {published data only}
- Garg SK, Chase HP, Jackson WE, Harris S, Carmain JA, Hansen MH, et al. Renal and retinal changes after treatment with ramipril and pentoxifyline in subjects with IDDM. Annals of Ophthalmology - Glaucoma 1998;30(1):33-7. [EMBASE: 28128821] [Google Scholar]
Hansen 1994 {published data only}
- Captopril reduces the risk of nephropathy in IDDM patients with microalbuminuria. The Microalbuminuria Captopril Study Group. Diabetologia 1996;39(5):587-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansen KW, Klein F, Christensen PD, Sorensen K, Andersen PH, Moller J, et al. Effects of captopril on ambulatory blood pressure, renal and cardiac function in microalbuminuric type 1 diabetic patients. Diabete et Metabolisme 1994;20(5):485-93. [MEDLINE: ] [PubMed] [Google Scholar]
- Mogensen CE, Cooper M, European Microalbuminuria Captopril Study Group. Captopril (C) delays progression to overt renal disease in insulin-dependent diabetes mellitus (IDDM) patients with microalbuminuria [abstract]. Journal of the American Society of Nephrology 1992;3(3):336. [CENTRAL: CN-00461342] [Google Scholar]
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994;271(4):275-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Viberti G, European Microalbuminuria Captopril Study Group (EMCSG). The effect of captopril on the progression of microalbuminuria in insulin-dependent diabetes [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:424. [CENTRAL: CN-00740489]
- Viberti GC, Laffel L, Gans DJ, Microalbuminuria CSG. Secondary prevention of diabetic nephropathy by captopril in patients with insulin-dependent diabetes mellitus (IDDM) and microalbuminuria [abstract]. Journal of the American Society of Nephrology 1994;5(3):385. [Google Scholar]
Hommel 1995 {published data only}
- Hommel E, Jensen B, Parving H. Long-term effect of captopril on kidney function in normotensive insulin dependent diabetic patients (IDDM) with diabetic nephropathy [abstract no: 444]. Journal of the American Society of Nephrology 1995;6(3):450. [CENTRAL: CN-00484393] [Google Scholar]
HOPE 1996 {published data only}
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators [Erratum appears in Lancet 2000 Sep 2;356(9232):860]. Lancet 2000;355(9200):253-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Gerstein HC, Bosch J, Pogue J, Taylor DW, Zinman B, Yusuf S. Rationale and design of a large study to evaluate the renal and cardiovascular effects of an ACE inhibitor and vitamin E in high-risk patients with diabetes. The MICRO-HOPE Study. Microalbuminuria, cardiovascular, and renal outcomes. Heart Outcomes Prevention Evaluation. Diabetes Care 1996;19(11):1225-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Pogue J, Mann JF, Lonn E, Dagenais GR, McQueen M, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 2005;48(9):1749-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerstein HC. Diabetes and the HOPE study: implications for macrovascular and microvascular disease. International Journal of Clinical Practice. Supplement 2001;(117):8-12. [MEDLINE: ] [PubMed] [Google Scholar]
- Hoogwerf BJ, Young JB. The HOPE study. Ramipril lowered cardiovascular risk, but vitamin E did not. Cleveland Clinic Journal of Medicine 2000;67(4):287-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lamy A, Yusuf S, Pogue J, Gafni A. Cost implications of the use of ramipril in high-risk patients based on the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 2003;107(7):960-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Gerstein HC, Pogue J, Yi Q, Hoogwerf BJ, Bosch J, et al. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk diabetic patients [abstract no:1051-29]. Journal of the American College of Cardiology 2002;39(Suppl 2):291A. [Google Scholar]
- Lonn E, Mathew J, Pogue J, Johnstone D, Danisa K, Bosch J, et al. Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high-risk patients. European Journal of Cardiovascular Prevention & Rehabilitation 2003;10(6):420-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Roccaforte R, Yi Q, Bosch J, Probstfield J, Sleight P, et al. Ramipril prevents major cardiovascular events in high-risk women: results of the HOPE trial [abstract no: 1051-28]. Journal of the American College of Cardiology 2002;39(Suppl 2):290A. [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, et al. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care 2002;25(11):1919-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency (RI) as predicator of cardiovascular (CV) outcomes and impact of ramipril: the HOPE study [abstract no: A0836]. Journal of the American Society of Nephrology 2000;11(Sept):156A. [CENTRAL: CN-00671781] [Google Scholar]
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Annals of Internal Medicine 2001;134(8):629-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Yi QL, Franke J, Lonn EM, Hoogwerf BJ, et al. Progression of renal insufficiency in type 2 diabetes with and without microalbuminuria: results of the Heart Outcomes and Prevention Evaluation (HOPE) randomized study. American Journal of Kidney Diseases 2003;42(5):936-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Yi QL, Lonn EM, Hoogwerf BJ, Rashkow A, et al. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. Journal of the American Society of Nephrology 2003;14(3):641-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, et al. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Kidney International 2004;65(4):1375-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Yi QL, Sleight P, Dagenais GR, Gerstein HC, Lonn EM, et al. Serum potassium, cardiovascular risk, and effects of an ACE inhibitor: results of the HOPE study. Clinical Nephrology 2005;63(3):181-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001;104(14):1615-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McQueen MJ, Lonn E, Gerstein HC, Bosch J, Yusuf S. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scandinavian Journal of Clinical & Laboratory Investigation Supplement 2005;240:143-56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sleight P, Yusuf S, Pogue J, Tsuyuki R, Diaz R, Probstfield J, et al. Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet 2001;358(9299):2130-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smieja M, Gnarpe J, Lonn E, Gnarpe H, Olsson G, Yi Q, et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2003;107(2):251-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The HOPE (Heart Outcomes Prevention Evaluation) Study: the design of a large, simple randomized trial of an angiotensin-converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. The HOPE study investigators. Canadian Journal of Cardiology 1996;12(2):127-37. [MEDLINE: ] [PubMed] [Google Scholar]
- Veres A, Fust G, Smieja M, McQueen M, Horvath A, Yi Q, et al. Relationship of anti-60 kDa heat shock protein and anti-cholesterol antibodies to cardiovascular events. Circulation 2002;106(22):2775-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 2000;342(3):154-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients [Erratum in: 2000 May 4;342(18):1376; Erratum in: N Engl J Med 2000 Mar 9;342(10):748]. New England Journal of Medicine 2000;342(3):145-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IDNT 2001 {published data only}
- Aguilar D, Goldhaber SZ, Gans DJ, Levey AS, Porush JG, Lewis JB, et al. Clinically unrecognized Q-wave myocardial infarction in patients with diabetes mellitus, systemic hypertension, and nephropathy. American Journal of Cardiology 2004;94(3):337-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. American Journal of Kidney Diseases 2005;45(2):281-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atkins RC, Briganti EM, Wiegmann TB. Effect of baseline proteinuria and change in proteinuria with treatment on the risk of renal endpoints in the Irbesartan Diabetic Nephropathy Trial (IDNT) [abstract no: F-FC033]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):7A. [CENTRAL: CN-00444265] [Google Scholar]
- Atkins RC, Jerums G, Gilbert RE, Lewis EJ, Hunsicker LG. A clinical trial in patients with overt type 2 diabetic nephropathy [abstract no: 11]. Nephrology 2002;7(Suppl 3):A3. [CENTRAL: CN-00790194] [Google Scholar]
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Annals of Internal Medicine 2003;138(7):542-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. Journal of the American Society of Nephrology 2005;16(7):2170-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berl T, Hunsicker LG, Pfeffer MA, Pohl MA. Impact of baseline and achieved blood pressure on cardiovascular events (CV) in type 2 diabetic nephropathy [abstract no: F-FC023]. Journal of the American Society of Nephrology 2003;14(Nov):5-6A. [CENTRAL: CN-00550409] [DOI] [PubMed] [Google Scholar]
- Busch M, Franke S, Wolf G, Brandstadt A, Ott U, Gerth J, et al. The advanced glycation end product N(epsilon)-carboxymethyllysine is not a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertension. American Journal of Kidney Diseases 2006;48(4):571-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Busch M, Franke S, Wolf G, Rohde RD, Stein G. Serum levels of the advanced glycation end products Nepsilon-carboxymethyllysine and pentosidine are not influenced by treatment with the angiotensin receptor II type 1 blocker irbesartan in patients with type 2 diabetic nephropathy and hypertension. Nephron 2008;108(4):c291-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coyle D, Rodby R, Soroka S, Levin A, Muirhead N, Cotret PR, et al. Cost-effectiveness of irbesartan 300 mg given early versus late in patients with hypertension and a history of type 2 diabetes and renal disease: a Canadian perspective. Clinical Therapeutics 2007;29(7):1508-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Evans M, Bain S, Beckham C, Hogan S, Bilous R. Irbesartan delays progression of nephropathy as measured by estimated glomerular filtration rate: Post-hoc analysis of the Irbesartan Diabetic Nephropathy Trial [abstract no: P405]. Diabetic Medicine 2009;26(Suppl 1):158-9. [EMBASE: 70342532] [DOI] [PubMed] [Google Scholar]
- Evans M, Bain SC, Hogan S, Bilous RW. Irbesartan delays progression of nephropathy as measured by estimated glomerular filtration rate: post hoc analysis of the Irbesartan Diabetic Nephropathy Trial. Nephrology Dialysis Transplantation 2012;27(6):2255-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Friedman AN, Hunsicker LG, Selhub J, Bostom AG. C-reactive protein as a predictor of total arteriosclerotic outcomes in type 2 diabetic nephropathy. Kidney International 2005;68(2):773-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Friedman AN, Hunsicker LG, Selhub J, Bostom AG. Total plasma homocysteine and arteriosclerotic outcomes in type 2 diabetes with nephropathy. Journal of the American Society of Nephrology 2005;16(11):3397-402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney International 2012;82(3):330-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lambers Heerspink HJ, Laverman GD, Lewis J, Parving HH, Zeeuw D. Both hypokalemia and hyperkalemia predict cardiovascular risk during blood pressure lowering therapy in patients with diabetes and nephropathy [abstract no: SA-PO2407]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):663A. [Google Scholar]
- Lambers Heerspink HJ, Weldegiorgis M, Inker LA, Gansevoort R, Parving HH, Dwyer JP, et al. Estimated GFR decline as a surrogate end point for kidney failure: A post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) Study and Irbesartan Diabetic Nephropathy Trial (IDNT). American Journal of Kidney Diseases 2014;63(2):244-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis E, Ritz E. Blood pressure-independent nephroprotection by irbesartan - results of the IDNT study [abstract no: V3-1]. Deutsche Medizinische Wochenschrift 2001;126(Suppl 3):S158. [CENTRAL: CN-00383472] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine 2001;345(12):851-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Collaborative SG. A clinical trial in patients with overt type 2 diabetic nephropathy [abstract no: A1138]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):220A. [CENTRAL: CN-00444266] [Google Scholar]
- Lewis EJ, Hunsicker LG, Rodby RA. A clinical trial in type 2 diabetic nephropathy. American Journal of Kidney Diseases 2001;38(4 Suppl 1):S191-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis EJ. The role of angiotensin II receptor blockers in preventing the progression of renal disease in patients with type 2 diabetes. American Journal of Hypertension 2002;15(10 (Pt 2)):123-8S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Annemans L, Roze S, Lamotte M, Lapuerta P, Gabriel S, et al. Irbesartan is projected to be cost and life saving in the USA for treatment of patients with type 2 diabetes, hypertension, and microalbuminuria [abstract no F-PO603]. Journal of the American Society of Nephrology 2003;14(Nov):193A. [Google Scholar]
- Palmer AJ, Annemans L, Roze S, Lamotte M, Rodby RA, Bilous RW. An economic evaluation of the Irbesartan in Diabetic Nephropathy Trial (IDNT) in a UK setting. Journal of Human Hypertension 2004;18(10):733-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Annemans L, Roze S, Lamotte M, Rodby RA, Cordonnier DJ. An economic evaluation of irbesartan in the treatment of patients with type 2 diabetes, hypertension and nephropathy: cost-effectiveness of Irbesartan in Diabetic Nephropathy Trial (IDNT) in the Belgian and French settings. Nephrology Dialysis Transplantation 2003;18(10):2059-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pohl M, Rodby R, Clark W, Hunsicker L, Lewis E, Anzalone D, et al. Irbesartan diabetic nephropathy trial (IDNT), a multicenter collaborative trial of angiotensin II receptor antagonism on renal function, mortality, and morbidity in hypertensive type 2 diabetic patients with nephropathy [abstract no: A0910]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):178A. [Google Scholar]
- Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. Journal of the American Society of Nephrology 2005;16(10):3027-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rodby RA, Chiou CF, Borenstein J, Smitten A, Sengupta N, Palmer AJ, et al. The cost-effectiveness of irbesartan in the treatment of hypertensive patients with type 2 diabetic nephropathy. Clinical Therapeutics 2003;25(7):2102-19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rodby RA, Rohde RD, Clarke WR, Hunsicker LG, Anzalone DA, Atkins RC, et al. The Irbesartan type II diabetic nephropathy trial: study design and baseline patient characteristics. For the Collaborative Study Group. Nephrology Dialysis Transplantation 2000;15(4):487-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schutte E, Lambers Heerspink HJ, Lutgers HL, Bakker SJ, Vart P, Wolffenbuttel BH, et al. Serum bicarbonate and kidney disease progression and cardiovascular outcome in patients with diabetic nephropathy: a post hoc analysis of the RENAAL (Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) Study and IDNT (Irbesartan Diabetic Nephropathy Trial). American Journal of Kidney Diseases 2015;66(3):450-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smink PA, Bakker SJ, Laverman GD, Berl T, Cooper ME, Zeeuw D, et al. An initial reduction in serum uric acid during angiotensin receptor blocker treatment is associated with cardiovascular protection: a post-hoc analysis of the RENAAL and IDNT trials. Journal of Hypertension 2012;30(5):1022-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stoycheff N, Stevens LA, Collaborative Study Group, Levey AS. The relationship between total proteinuria, albuminuria, and the signs and symptoms of nephrotic syndrome in diabetic kidney disease [abstract no: SU-PO832]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):769A. [CENTRAL: CN-01912555] [Google Scholar]
INNOVATION 2005 {published data only}
- INNOVATION: Incipient to overt: angiotensin II receptor blocker, telmisartan, investigation on type II diabetic nephropathy. The Protection Trial Programme - Micardis Protection 2005.
- Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iawamoto Y, et al. Incipient to overt: angiotensin II blocker, telmisartan, investigation on type 2 diabetic nephropathy (INNOVATION) study [abstract no: TH-PO243]. Journal of the American Society of Nephrology 2006;17(Abstracts):158A. [CENTRAL: CN-00601997] [Google Scholar]
- Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, et al. Microalbuminuria reduction with telmisartan in normotensive and hypertensive Japanese patients with type 2 diabetes: a post-hoc analysis of the Incipient to Overt: Angiotensin II Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) study. Hypertension Research - Clinical & Experimental 2008;31(4):657-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 2007;30(6):1577-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, et al. The telmisartan renoprotective study from incipient nephropathy to overt nephropathy--rationale, study design, treatment plan and baseline characteristics of the incipient to overt: angiotensin II receptor blocker, telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) Study. Journal of International Medical Research 2005;33(6):677-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ogawa D, Makino H. INNOVATION study. Nippon Rinsho - Japanese Journal of Clinical Medicine 2010;68 Suppl 9:451-4. [MEDLINE: ] [PubMed] [Google Scholar]
IRMA‐2 2001 {published data only}
- Andersen S, Brochner-Mortensen J, Parving HH. Kidney function during and after withdrawal of long-term irbesartan treatment in patients with type 2 diabetes and microalbuminuria [abstract no: F-FC025]. Journal of the American Society of Nephrology 2003;14(Nov):6A. [DOI] [PubMed] [Google Scholar]
- Andersen S, Brochner-Mortensen J, Parving HH. Kidney function during and after withdrawal of long-term irbesartan treatment in patients with type 2 diabetes and microalbuminuria. Diabetes Care 2003;26(12):3296-302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Andersen S, Mischak H, Zurbig P, Parving HH, Rossing P. Urinary proteome analysis enables assessment of renoprotective treatment in type 2 diabetic patients with microalbuminuria. BMC Nephrology 2010;11:29. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broedbaek K, Henriksen T, Weimann A, Petersen M, Andersen JT, Afzal S, et al. Long-term effects of Irbesartan treatment and smoking on nucleic acid oxidation in patients with type 2 diabetes and microalbuminuria: an Irbesartan in patients with type 2 diabetes and Microalbuminuria (IRMA 2) substudy. Diabetes Care 2011;34(5):1192-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle D, Rodby R, Soroka S, Levin A, Muirhead N, Cotret PR, et al. Cost-effectiveness of irbesartan 300 mg given early versus late in patients with hypertension and a history of type 2 diabetes and renal disease: a Canadian perspective. Clinical Therapeutics 2007;29(7):1508-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Engelen L, Persson F, Ferreira I, Rossing P, Hovind P, Teerlink T, et al. Irbesartan treatment does not influence plasma levels of the advanced glycation end products N(epsilon)(1-carboxymethyl)lysine and N(epsilon)(1-carboxyethyl)lysine in patients with type 2 diabetes and microalbuminuria. A randomized controlled trial. Nephrology Dialysis Transplantation 2011;26(11):3573-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hellemons ME, Persson F, Bakker SJ, Rossing P, Parving HH, Zeeuw D, et al. Initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria: a post hoc analysis of the IRMA-2 trial. Diabetes Care 2011;34(9):2078-83. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert H, Parving HH, Brochner-Mortensen J, Gomis R, Arner P. Irbesartan in patients with type 2 diabetes mellitus and microalbuminura (IRMA 2) [abstract]. Deutsche Medizinische Wochenschrift 2001;126(Suppl 3):S158. [CENTRAL: CN-00383453] [Google Scholar]
- Llewelyn DE, Garcia-Puig J. How different urinary albumin excretion rates can predict progression to nephropathy and the effect of treatment in hypertensive diabetics. Journal of the Renin-Angiotensin-Aldosterone System 2004;5(3):141-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Annemans L, Roze S, Lamotte M, Lapuerta P, Gabriel S, et al. Irbesartan is projected to be cost and life saving in the USA for treatment of patients with Type 2 diabetes, hypertension, and microalbuminuria [abstract no: F-PO603]. Journal of the American Society of Nephrology 2003;14(Nov):193A. [Google Scholar]
- Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. Effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. Ugeskrift for Laeger 2001;163(40):5519-24. [MEDLINE: ] [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. New England Journal of Medicine 2001;345(12):870-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Persson F, Rossing P, Hovind P, Parving H. Impact of arterial blood pressure on development of diabetic nephropathy at different systolic blood pressure levels in patients with type 2 diabetes and persistent microalbuminuria: an IRMA 2 substudy [abstract no: TH-PO263]. Journal of the American Society of Nephrology 2006;17(Abstracts):162A. [CENTRAL: CN-00601996] [Google Scholar]
- Persson F, Rossing P, Hovind P, Stehouwer C, Schalkwijk C, Tarnow L, et al. Impact of new biomarkers on development of diabetic nephropathy in the irbesartan in patients with type 2 diabetes and microalbuminuria (IRMA 2) study [abstract no: SA-PO356]. Journal of the American Society of Nephrology 2005;16(Abstracts):635A. [Google Scholar]
- Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk C, Tarnow L, et al. Irbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: an IRMA 2 substudy. Diabetes 2006;55(12):3550-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk C, Tarnow L, et al. The effect of irbesartan treatment on vascular biomarkers in patients with type 2 diabetes and microalbuminuria. An irbesartan in patients with type 2 diabetes and microalbuminuria (IRMA 2) substudy [abstract no: SA-PO357]. Journal of the American Society of Nephrology 2005;16(Abstracts):635A. [PMID: ] [Google Scholar]
- Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk CG, Tarnow L, et al. Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scandinavian Journal of Clinical & Laboratory Investigation 2008;68(8):731-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Persson F, Rossing P, Schalkwijk C, Stehouwer CD, Parving H. N-terminal pro brain natriuretic peptide (NTproBNP) in the irbesartan in patients with type 2 diabetes and microalbuminuria study. An IRMA 2 substudy [abstract no: TH-PO1006]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):338A. [Google Scholar]
- Piazza M, Hanssen NM, Persson F, Scheijen JL, de Waarenburg MP, Greevenbroek MM, et al. Irbesartan treatment does not influence plasma levels of the dicarbonyls methylglyoxal, glyoxal and 3-deoxyglucosone in participants with type 2 diabetes and microalbuminuria: an IRMA2 sub-study. Diabetic Medicine 2021;38(9):e14405. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
JAPAN‐IDDM 2002 {published data only}
- Katayama S, Kikkawa R, Isogai S, Sasaki N, Matsuura N, Tajima N, et al. Effect of captopril or imidapril on the progression of diabetic nephropathy in Japanese with type 1 diabetes mellitus: a randomized controlled study (JAPAN-IDDM). Diabetes Research & Clinical Practice 2002;55(2):113-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jerums 2001 {published data only}
- Jerums G, Allen TJ, Campbell DJ, Cooper ME, Gilbert RE, Hammond JJ, et al. Long-term comparison between perindopril and nifedipine in normotensive patients with type 1 diabetes and microalbuminuria. American Journal of Kidney Diseases 2001;37(5):890-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thomas MC, Jerums G, Tsalamandris C, Macisaac R, Panagiotopoulos S, Cooper ME, et al. Increased tubular organic ion clearance following chronic ACE inhibition in patients with type 1 diabetes. Kidney International 2005;67(6):2494-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jerums 2004 {published data only}
- Jerums G, Allen TJ, Campbell DJ, Cooper ME, Gilbert RE, Hammond JJ, et al. Long-term renoprotection by perindopril or nifedipine in non-hypertensive patients with type 2 diabetes and microalbuminuria. Diabetic Medicine 2004;21(11):1192-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kavgaci 2002 {published data only}
- Kavgaci H, Sahin A, Onder Ersoz H, Erem C, Ozdemir F. The effects of losartan and fosinopril in hypertensive type 2 diabetic patients. Diabetes Research & Clinical Practice 2002;58(1):19-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kitamura 2020 {published data only}
- Kitamura M, Arai H, Abe S, Ota Y, Muta K, Furusu A, et al. Renal outcomes of treatment with telmisartan in patients with stage 3–4 chronic kidney disease: a prospective, randomized, controlled trial (JINNAGA). SAGE Open Medicine 2020;8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2005 {published data only}
- Ko GT, Tsang CC, Chan HC. Stabilization and regression of albuminuria in Chinese patients with type 2 diabetes: a one-year randomized study of valsartan versus enalapril. Advances in Therapy 2005;22(2):155-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krairittichai 2009 {published data only}
- Krairittichai U, Chaisuvannarat V. Effects of dual blockade of renin-angiotensin system in type 2 diabetes mellitus patients with diabetic nephropathy. Journal of the Medical Association of Thailand 2009;92(5):611-7. [MEDLINE: ] [PubMed] [Google Scholar]
Lacourciere 2000 {published data only}
- Lacourciere Y, Belanger A, Godin C, Halle JP, Ross S, Wright N, et al. Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney International 2000;58(2):762-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laffel 1995 {published data only}
- Captopril reduces the risk of nephropathy in IDDM patients with microalbuminuria. The Microalbuminuria Captopril Study Group. Diabetologia 1996;39(5):587-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Laffel L, Gans DJ, McGill JB, Captopril Nephropathy Study Group. Captopril decreases the rate of progression of renal disease in normotensive, insulin-dependent diabetes mellitus (IDDM) patients with microalbuminuria [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):304. [CENTRAL: CN-00484730] [Google Scholar]
- Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. American Journal of Medicine 1995;99(5):497-504. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Larsen 1990 {published data only}
- Larsen M, Hommel E, Parving HH, Lund-Andersen H. Protective effect of captopril on the blood-retina barrier in normotensive insulin-dependent diabetic patients with nephropathy and background retinopathy. Graefes Archive for Clinical & Experimental Ophthalmology 1990;228(6):505-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lebovitz 1994 {published data only}
- Lebovitz H, Cnaan A, Wiegmann T, Broadstone V, Schwartz S, Sica D, et al. Enalapril slows the progressioin of renal disease in non-insulin dependent diabetes mellitus (NIDDM): results of a 3-yr multicenter, randomized, prospective, double-blind study [abstract]. Journal of the American Society of Nephrology 1992;3(3):335. [CENTRAL: CN-0046114] [Google Scholar]
- Lebovitz HE, Wiegmann TB, Cnaan A, Shahinfar S, Sica DA, Broadstone V, et al. Renal protective effects of enalapril in hypertensive NIDDM: role of baseline albuminuria. Kidney International - Supplement 1994;45:S150-5. [MEDLINE: ] [PubMed] [Google Scholar]
Lee 1990 {published data only}
- Lee S, Kim TY, Lim JS, Kim HK, Lee JW. Prospective study of effect of converting enzyme inhibitor, captopril, on renal function and proteinuria in diabetic nephropathy [abstract]. In: 11th International Congress of Nephrology. 1990:160A.
Lewis 1995 {published data only}
- Lewis EJ, Rohde R, Raymond B, Collaborative Study Group. A follow-up study of the course of nephropathy in type I diabetes mellitus [abstract no: P1222]. Nephrology 1997;3(Suppl 1):S379. [CENTRAL: CN-00461163] [Google Scholar]
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. American Journal of Kidney Diseases 1999;34(5):809-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rodby R, Rohde R, Evans J, Bain R, Mulcahy W, Lewis E. Design and baseline characteristics of the study of the effect of intensity of blood pressure (BP) management on the progression of type 1 diabetic nephropathy [abstract]. Journal of the American Society of Nephrology 1994;5(3):382. [CENTRAL: CN-00644275] [DOI] [PubMed] [Google Scholar]
- Rodby RA, Rohde R, Evans J, Bain RP, Mulcahy WS, Lewis EJ. The study of the effect of intensity of blood pressure management on the progression of type 1 diabetic nephropathy: study design and baseline patient characteristics. Collaborative Study Group. Journal of the American Society of Nephrology 1995;5(10):1775-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 1995 {published data only}
- Lin M, Yang Y, Chiang H, Lee D, Wang S, Chang M, et al. Beneficial effects of angiotensin-converting enzyme inhibitors on cardiovascular and renal functions in patients with hypertension and diabetes. Acta Cardiologica Sinica 1995;11(1):30-8. [EMBASE: 25120726] [Google Scholar]
LIRICO 2007 {published data only}
- Maione A, Nicolucci A, Craig JC, Tognoni G, Moschetta A, Palasciano G, et al. Angiotensin converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in microalbuminuric patients with one or more cardiovascular risk factors. Protocol of the Long-term Impact of RAS Inhibition on Cardiorenal Outcomes randomized trial (LIRICO) [ACE-inibizione, antagonismo recettoriale e terapia combinata in soggetti microalbuminurici con fattori di rischio cardiovascolare. Protocollo dello studio randomizzato multicentrico ''Long-term impact of RAS inhibition on cardiorenal outcomes-LIRICO]. Giornale Italiano di Nefrologia 2007;24(5):446-56. [MEDLINE: ] [PubMed] [Google Scholar]
- Maione A, Nicolucci A, Craig JC, Tognoni G, Moschetta A, Palasciano G, et al. Protocol of the long-term impact of RAS inhibition on cardiorenal outcomes (LIRICO) randomized trial. Journal of Nephrology 2007;20(6):646-55. [MEDLINE: ] [PubMed] [Google Scholar]
- Saglimbene V, Palmer SC, Ruospo M, Natale P, Maione A, Nicolucci A, et al. The Long-Term Impact of Renin-Angiotensin System (RAS) Inhibition on Cardiorenal Outcomes (LIRICO): a randomized, controlled trial. Journal of the American Society of Nephrology 2018;29(12):2890-99. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strippoli GF, Palmer S, Saglimbene VM, Ruospo M, Hegbrant JB, Craig JC. ACE inhibitor, ARB, or combined therapy in patients with diabetes and microalbuminuria: the long-term impact of RAS inhibition on cardiorenal outcomes (LIRICO) randomized clinical trial [abstract no: SA-OR115]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):103. [EMBASE: 633700766] [Google Scholar]
Marre 1987 {published data only}
- Marre M, Chatellier G, Leblanc H, Guyene TT, Menard J, Passa P. Prevention of diabetic nephropathy with enalapril in normotensive diabetics with microalbuminuria. BMJ 1988;297(6656):1092-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre M, Leblanc H, Suarez L, Guyenne TT, Menard J, Passa P. Converting enzyme inhibition and kidney function in normotensive diabetic patients with persistent microalbuminuria. British Medical Journal Clinical Research Ed 1987;294(6585):1448-52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre M. Microalbuminuria and ACE inhibition in non-hypertensive diabetics. Journal of Diabetic Complications 1990;4(2):84-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marre M. Recent advance in the treatment of diabetic nephropathy: angiotensin I converting enzyme (ACE) inhibitors. Journal of Diabetic Complications 1991;5(2-3):91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MDNSG 1993 {published data only}
- Gilbert RE, Jerums G, Allen TJ, Hammond J, Cooper ME, Melbourne Diabetic Nephropathy Study Group. Effect of different antihypertensive agents in normotensive microalbuminuric patients with IDDM and NIDDM [abstract no: 16P]. Journal of the American Society of Nephrology 1994;5(3):377. [Google Scholar]
- Melbourne Diabetic Nephropathy Study Group, Allen T, Cooper ME, Jerums G, De Luise M, Alford F, et al. Effects of different antihypertensive agents in normotensive microalbuminuric type I and type II diabetic patients [abstract]. Kidney International 1994;45:1270. [Google Scholar]
- Melbourne Diabetic Nephropathy Study Group, Cooper ME, Allen T, Jerums G, DeLuise M, Alford F, et al. Effects of different antihypertensive agents in normotensive microalbuminuric type I and type II diabetic patients [abstract]. In: 12th International Congress of Nephrology. 1993:424.
Mehdi 2009 {published data only}
- Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. Journal of the American Society of Nephrology 2009;20(12):2641-50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Effect of an angiotensin receptor blocker (ARB) or a mineralocorticoid antagonist (MRA) added-on to maximally-dosed angiotensin converting enzyme inhibitor (ACEi) regimen in diabetic nephropathy (DN) [abstract no: TH-FC128]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):28A. [CENTRAL: CN-00756304] [Google Scholar]
- Mehdi UF, Adams-Huet B, Toto RD. Mechanism of hyperkalemia during combined renin angiotensin aldosterone system (RAAS) blockade in diabetic nephropathy [abstract no: SA-PO2246]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):623A. [Google Scholar]
- Mulder S, Lambers Heerspink HJ, Perco P, Toto RD, Pena M. Validation of a systems biology derived urinary metabolite panel for prediction of albuminuria response to spironolactone therapy in type 2 diabetes [abstract no: FR-PO461]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):539-40. [EMBASE: 633734135] [Google Scholar]
- Srivastava A, Adams-Huet B, Vega GL, Toto RD. Effect of losartan and spironolactone on lipoprotein metabolism in diabetic nephropathy [abstract no: 266]. American Journal of Kidney Diseases 2012;59(4):A81. [EMBASE: 71074879] [Google Scholar]
- Srivastava A, Adams-Huet B, Vega GL, Toto RD. Effect of losartan and spironolactone on triglyceride-rich lipoproteins in diabetic nephropathy. Journal of Investigative Medicine 2016;64(6):1102-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto R, Mortazavi K, Jones K, Roman-Latorre J, Dominguez M, Blackstone S, et al. Combined blockade with the renin-angiotensin-aldosterone system to improve outcomes in diabetics with nephropathy (DN) [abstract no: PUB582]. Journal of the American Society of Nephrology 2005;16:908A. [Google Scholar]
- Van Buren PN, Adams-Huet B, Nguyen M, Molina C, Toto RD. Potassium handling with dual renin-angiotensin system inhibition in diabetic nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(2):295-301. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren PN, Adams-Huet B, Toto R. Achieving blood pressure goals in high risk patients with diabetic nephropathy [abstract no: 272]. Journal of Investigative Medicine 2010;58(2):432. [EMBASE: 70981115] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren PN, Adams-Huet B, Toto RD. Effective antihypertensive strategies for high-risk patients with diabetic nephropathy. Journal of Investigative Medicine 2010;58(8):950-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muirhead 1999 {published data only}
- Cheung R, Lewanczuk RZ, Rodger NW, Huff MW, Oddou-Stock P, Botteri F, et al. The effect of valsartan and captopril on lipid parameters in patients with type II diabetes mellitus and nephropathy. International Journal of Clinical Practice 1999;53(8):584-92. [MEDLINE: ] [PubMed] [Google Scholar]
- Muirhead N, Feagan BF, Mahon J, Lewanczuk RZ, Rodger NW, Botteri F, et al. The effects of valsartan and captopril on reducing microalbuminuria in patients with type 2 diabetes mellitus: a placebo-controlled trial. Current Therapeutic Research, Clinical & Experimental 1999;60(12):650-60. [EMBASE: 30036150] [Google Scholar]
Nakamura 2002a {published data only}
- Nakamura T, Ushiyama C, Osada S, Takahashi Y, Shimada N, Ebihara I, et al. Combination therapy of trandolapril and candesartan cilexetil reduces microalbuminuria and urinary endothelin-1 excretion in patients with type 2 diabetes. Clinical & Experimental Nephrology 2002;6(3):135-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ushiyama C, Suzuki S, Shimada N, Sekizuka K, Ebihara I, et al. Comparison between the angiotensin II receptor antagonist candesartan cilexetil and the angiotensin-converting enzyme inhibitor trandolapril in microalbuminuria of patients with early diabetic nephropathy. Nephron 2000;86(2):247. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2010c {published data only}
- Nakamura T, Fujiwara N, Sato E, Ueda Y, Sugaya T, Koide H. Renoprotective effects of various angiotensin II receptor blockers in patients with early-stage diabetic nephropathy. Kidney & Blood Pressure Research 2010;33(3):213-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nankervis 1998 {published data only}
- Nankervis A, Nicholls K, Kilmartin G, Allen P, Ratnaike S, Martin FI. Effects of perindopril on renal histomorphometry in diabetic subjects with microalbuminuria: a 3-year placebo-controlled biopsy study. Metabolism: Clinical & Experimental 1998;47(12 Suppl 1):12-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niu 2008 {published data only}
- Niu W, Zhao S. The effect of benazepril in treatment of the patients with early diabetic nephropathy with normotensive and microalbuminuria. China Tropical Medicine 2008;8(2):229-30. [CENTRAL: CN-00796267] [Google Scholar]
O'Donnell 1993 {published data only}
- O'Donnell MJ, Rowe BR, Lawson N, Horton A, Gyde OH, Barnett AH. Placebo-controlled trial of lisinopril in normotensive diabetic patients with incipient nephropathy. Journal of Human Hypertension 1993;7(4):327-32. [MEDLINE: ] [PubMed] [Google Scholar]
Ogawa 2007 {published data only}
- Ogawa S, Takeuchi K, Mori T, Nako K, Tsubono Y, Ito S. Effects of monotherapy of temocapril or candesartan with dose increments or combination therapy with both drugs on the suppression of diabetic nephropathy. Hypertension Research - Clinical & Experimental 2007;30(4):325-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ONTARGET 2004 {published data only}
- Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurology 2011;10(1):43-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, Clase CM, Mente A, Mann JF, Sleight P, et al. Albuminuria and rapid loss of GFR and risk of new hip and pelvic fractures. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(2):233-40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, O'Donnell M, Mann JF, Anderson C, Fagard R, et al. Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Archives of Internal Medicine 2011;171(2):142-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, Ryden L, Schumacher H, Probstfield J, Commerford P, et al. Effects of telmisartan on glucose levels in people at high risk for cardiovascular disease but free from diabetes: the TRANSCEND study. Diabetes Care 2011;34(9):1902-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm M, Baumhakel M, Teo K, Sleight P, Probstfield J, Gao P, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation 2010;121(12):1439-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bohm M, Schumacher H, Laufs U, Sleight P, Schmieder R, Unger T, et al. Effects of nonpersistence with medication on outcomes in high-risk patients with cardiovascular disease. American Heart Journal 2013;166(2):306-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Clase CM, Barzilay J, Gao P, Smyth A, Schmieder RE, Tobe S, et al. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney International 2017;91(3):683-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cukierman-Yaffe T, Gerstein HC, Anderson C, Zhao F, Sleight P, Hilbrich L, et al. Glucose intolerance and diabetes as risk factors for cognitive impairment in people at high cardiovascular risk: results from the ONTARGET/TRANSCEND research programme. Diabetes Research & Clinical Practice 2009;83(3):387-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dans AL, Teo K, Gao P, Chen JH, Jae-Hyung K, Yusoff K, et al. In a subgroup of high-risk Asians, telmisartan was non-inferior to ramipril and better tolerated in the prevention of cardiovascular events. PLoS ONE [Electronic Resource] 2010;5(12):e13694. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan M, Mente A, Teo KK, Gao P, Sleight P, Dagenais G, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 2012;126(23):2705-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dunkler D, Dehghan M, Teo KK, Heinze G, Gao P, Kohl M, et al. Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Internal Medicine 2013;173(18):1682-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallieni M, Cozzolino M, Brancaccio D, Giovannini M. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2019. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heerspink HJ, Gao P, Zeeuw D, Clase C, Dagenais GR, Sleight P, et al. The effect of ramipril and telmisartan on serum potassium and its association with cardiovascular and renal events: results from the ONTARGET trial. European Journal of Preventive Cardiology 2014;21(3):299-309. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kappert K, Bohm M, Schmieder R, Schumacher H, Teo K, Yusuf S, et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 2012;126(8):934-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kappert K, Bohm M, Schmieder R, Schumacher H, Teo K, Yusuf S, et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 2012;126(8):934-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clinical Research in Cardiology 2014;103(2):149-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, Bilo G, Gao P, Fagard R, Redon J, et al. Ambulatory blood pressure values in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). Hypertension 2012;60(6):1400-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Schumacher H, Bohm M, Mann JF, Redon J, Facchetti R, et al. Visit-to-visit blood pressure variability and renal outcomes: results from ONTARGET and TRANSCEND trials. Journal of Hypertension 2020;38(10):2050-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Schumacher H, Redon J, Verdecchia P, Schmieder R, Jennings G, et al. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET). Circulation 2011;124(16):1727-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Ryden L, et al. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. Journal of Hypertension 2013;31(2):414-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372(9638):547-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen MM, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both in people at high vascular risk: results from the ONTARGET trial [abstract no: F-FC209]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):48A. [CENTRAL: CN-00747294] [Google Scholar]
- Narayanan RM, Chi LS, Choo LL, Ying YL, Fang SC. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2020. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nonoguchi H, Nanami M, Hasuike Y, Kuragano T, Nakanishi T. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2019-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Redon J, Mancia G, Sleight P, Schumacher H, Gao P, Pogue J, et al. Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). Journal of the American College of Cardiology 2012;59(1):74-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruilope LM, Redon J, Schmieder R. Cardiovascular risk reduction by reversing endothelial dysfunction: ARBs, ACE inhibitors, or both? Expectations from the ONTARGET Trial Programme. Vascular Health & Risk Management 2007;3(1):1-9. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Schmieder RE, Mann JFE, Dyal L, Pogue J, Copland I, Teo KK, et al. ONTARGET: cardiovascular outcome in patients at high cardiovascular risk and with chronic kidney disease (CKD) [abstract no: TH-PO886]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):309A. [CENTRAL: CN-00747295] [Google Scholar]
- Shiffman D, Pare G, Louie JZ, Rowland CM, Devlin JJ, McQueen M. A gene variant in the CERS2 gene is associated with worsening albuminuria in diabetic patients of ON TARGET and TRANSCEND [abstract no: SA-PO351]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):707A. [Google Scholar]
- Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. Journal of Hypertension 2009;27(7):1360-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sleight P. Clinical evidence from ONTARGET: the value of an angiotensin II receptor blocker and an angiotensin-converting enzyme inhibitor. Journal of Hypertension - Supplement 2009;27(5):S23-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. American Heart Journal 2004;148(1):52-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, et al. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011;123(10):1098-107. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Dagenais G, Healey J, Gao P, Dans AL, Chazova I, et al. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. Journal of Hypertension 2012;30(5):1004-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Sleight P, Mancia G, Fagard R, Trimarco B, Schmieder RE, et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation 2009;120(14):1380-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wetzels JF. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2020. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yu LT, Zhu J, Tan HQ, Wang GG, Teo KK, Liu LS. Telmisartan, ramipril, or both in high-risk Chinese patients: analysis of ONTARGET China data. Chinese Medical Journal Jun;124(12):1763-8. [MEDLINE: ] [PubMed] [Google Scholar]
ORIENT 2006 {published data only}
- Imai E, Chan J, Ito S, Haneda M, Makino H. Impact of olmesartan with or without ace inhibitor on renal and cardiovascular protection in type 2 diabetes with overt proteinuria [abstract no: Su322]. NDT Plus 2010;3(Suppl 3):iii416-7. [EMBASE: 70484542] [Google Scholar]
- Imai E, Chan J, Ito S, Haneda M, Makino H. Olmesartan reduces incidence of end stage diabetic nephropathy trial: ORIENT study [abstract no: F-FC313]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):72A. [Google Scholar]
- Imai E, Chan JC, Ito S, Yamasaki T, Kobayashi F, Haneda M, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 2011;54(12):2978-86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai E, Chan JCN, Ito S, Haneda M, Mankino H. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetic patients with overt nephropathy [abstract no: SA764]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Imai E, Haneda M, Chan JC, Yamasaki T, Kobayashi F, Ito S, et al. Reduction and residual proteinuria are therapeutic targets in type 2 diabetes with overt nephropathy: a post hoc analysis (ORIENT-proteinuria). Nephrology Dialysis Transplantation 2013;28(10):2526-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Imai E, Haneda M, Ito S, Kobayashi F, Yamasaki T, Chan J, et al. Proteinuria is a therapeutic target for renoprotection in patients with type 2 diabetes with overt nephropathy: A sub-analysis of ORIENT study [abstract no: FO027]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii13. [EMBASE: 70766565] [Google Scholar]
- Imai E, Haneda M, Yamasaki T, Kobayashi F, Harada A, Ito S, et al. Effects of dual blockade of the renin-angiotensin system on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy and hypertension in the ORIENT: a post-hoc analysis (ORIENT-Hypertension). Hypertension Research - Clinical & Experimental 2013;36(12):1051-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai E, Ito S, Haneda M, Chan JC, Makino H. Olmesartan reducing incidence of endstage renal disease in diabetic nephropathy trial (ORIENT): rationale and study design. Hypertension Research - Clinical & Experimental 2006;29(9):703-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Imai E, Ito S, Haneda M, Harada A, Kobayashi F, Yamasaki T, et al. Effects of blood pressure on renal and cardiovascular outcomes in Asian patients with type 2 diabetes and overt nephropathy: a post hoc analysis (ORIENT-blood pressure). Nephrology Dialysis Transplantation 2016;31(3):447-54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda E, Imai E, Kobayashi F, Kashihara N, Nangaku M. Importance of eGFR change as a surrogate end point of ESRD in a randomized controlled trial [abstract no: FR-PO490]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):527. [EMBASE: 633703127] [Google Scholar]
Parving 1989 {published data only}
- Parving HH, Hommel E, Damkjaer Nielsen M, Giese J. Effect of captopril on blood pressure and kidney function in normotensive insulin dependent diabetics with nephropathy. BMJ 1989;299(6698):533-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parving HH, Hommel E, Jensen BR, Hansen HP. Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney International 2001;60(1):228-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phillips 1993 {published data only}
- Phillips PJ, Phillipou G, Bowen KM, Lowe J, Yue DK, Wischusen J, et al. Diabetic microalbuminuria and cilazapril. American Journal of Medicine 1993;94(4A):58-60S. [MEDLINE: ] [PubMed] [Google Scholar]
Poulsen 2001 {published data only}
- Poulsen PL, Ebbehoj E, Mogensen CE. Lisinopril reduces albuminuria during exercise in low grade microalbuminuric type 1 diabetic patients: a double blind randomized study. Journal of Internal Medicine 2001;249(5):433-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Poulsen PL, Ebbehoj E, Nosadini R, Fioretto P, Deferrari G, Crepaldi G, et al. Early ACE-i intervention in microalbuminuric patients with type 1 diabetes: effects on albumin excretion, 24 h ambulatory blood pressure, and renal function. Diabetes & Metabolism 2001;27(2 Pt 1):123-8. [MEDLINE: ] [PubMed] [Google Scholar]
PREVEND IT 2000 {published data only}
- Asselbergs FW, Diercks GF, Hillege HL, Boven AJ, Janssen WM, Voors AA, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 2004;110(18):2809-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Asselbergs FW, Roon AM, Hillege HL, Jong PE, Gans RO, Smit AJ, et al. Effects of fosinopril and pravastatin on carotid intima-media thickness in subjects with increased albuminuria. Stroke 2005;36(3):649-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Asselbergs FW, van der Harst P, Roon AM, Hillege HL, Jong PE, Gans RO, et al. Long-term effects of pravastatin and fosinopril on peripheral endothelial function in albuminuric subjects. Atherosclerosis 2008;196(1):349-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atthobari J, Asselbergs FW, Boersma C, Vries R, Hillege HL, Gilst WH, et al. Cost-effectiveness of screening for albuminuria and subsequent treatment with an ACE-inhibitor; a pharmaco-economic analysis [abstract no: TH-FC169]. Journal of the American Society of Nephrology 2005;16:36A. [CENTRAL: CN-00583782] [Google Scholar]
- Atthobari J, Asselbergs FW, Boersma C, Vries R, Hillege HL, Gilst WH, et al. Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the prevention of renal and vascular endstage disease (PREVEND) study and the prevention of renal and vascular endstage disease intervention trial (PREVEND IT). Clinical Therapeutics 2006;28(3):432-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atthobari J, Brantsma AH, Gansevoort RT, Visser ST, Asselbergs FW, Gilst WH, et al. The effect of statins on urinary albumin excretion (UAE) and glomerular filtration rate (GFR): a randomized controlled trial (RCT) and observational cohort study [abstract no: F-FC151]. Journal of the American Society of Nephrology 2005;16:71A. [CENTRAL: CN-00583784] [Google Scholar]
- Atthobari J, Brantsma AH, Gansevoort RT, Visser ST, Asselbergs FW, Gilst WH, et al. The effect of statins on urinary albumin excretion and glomerular filtration rate: results from both a randomized clinical trial and an observational cohort study. Nephrology Dialysis Transplantation 2006;21(11):3106-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bello AK, Zeeuw D, El Nahas M, Brantsma AH, Bakker SJ, Jong PE, et al. Impact of weight change on albuminuria in the general population. Nephrology Dialysis Transplantation 2007;22(6):1619-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brouwers FP, Asselbergs FW, Hillege HL, Boer RA, Gansevoort RT, Veldhuisen DJ, et al. Long-term effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria:Ten years of follow-up of Prevention of Renal and Vascular End-stage Disease Intervention Trial (PREVEND IT). American Heart Journal 2011;161(6):1171-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- De Jong MA, Eisenga MF, Ballegooijen AJ, Beulens JWJ, Vervloet MG, Navis G, et al. Fibroblast growth factor 23 and new-onset chronic kidney disease in the general population: the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Nephrology Dialysis Transplantation 2021;36(1):121-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diercks GF, Janssen WM, Boven AJ, Bak AA, Jong PE, Crijns HJ, et al. Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular ENdstage Disease Intervention Trial [PREVEND IT]). American Journal of Cardiology 2000;86(6):635-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Geluk CA, Asselbergs FW, Hillege HL, Bakker SJ, Jong PE, Zijlstra F, et al. Impact of statins in microalbuminuric subjects with the metabolic syndrome: a substudy of the PREVEND Intervention Trial. European Heart Journal 2005;26(13):1314-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pouwels KB, Visser ST, Hak E. Effect of pravastatin and fosinopril on recurrent urinary tract infections. Journal of Antimicrobial Chemotherapy 2013;68(3):708-14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wal RM, Gansevoort RT, Harst P, Boomsma F, Thijs Plokker HW, Veldhuisen DJ, et al. Predictors of angiotensin-converting enzyme inhibitor-induced reduction of urinary albumin excretion in nondiabetic patients. Hypertension 2006;48(5):870-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- de Wal RM, van der Harst P, Gerritsen WB, van der Horst F, Plokker TH, Gansevoort RT, et al. Plasma matrix metalloproteinase-9 and ACE-inhibitor-induced improvement of urinary albumin excretion in non-diabetic, microalbuminuric subjects. Journal of the Renin-Angiotensin-Aldosterone System 2007;8(4):177-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Harst P, Asselbergs FW, Hillege HL, Voors AA, Veldhuisen DJ, Gilst WH. Effect of fosinopril treatment on serum C-reactive protein levels in patients with microalbuminuria. American Journal of Cardiology 2008;102(2):223-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PRONEDI 2013 {published data only}
- Fernandez Juarez G, Luno J, Barrio V, Vinuesa SG, Praga M, Goicoechea M, et al. Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy: a randomized trial. American Journal of Kidney Diseases 2013;61(2):211-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fernandez-Juarez G, Luno J, Barrio V, Vinuesa SG, Praga M, Goicoechea M, et al. 25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(11):1870-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Juarez G, Villacorta PJ, Luno Fernandez JL, Martinez-Martinez E, Cachofeiro V, Barrio Lucia V, et al. High levels of circulating TNFR1 increase the risk of all-cause mortality and progression of renal disease in type 2 diabetic nephropathy. Nephrology 2017;22(5):354-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fernandez-Juarez GM, Perez JV, Martinez EM, Cachofeiro V, Tato A, Ocana J, et al. Circulating TNF receptor levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin angiotensin system [abstract no: SP300]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii478-9. [EMBASE: 72207626] [Google Scholar]
- Luno J, Gerna Maria F, Vinuesa SG, Goicoechea M, Dominguez JO, Losada LC, et al. Vitamin D levels and renal progression in type 2 diabetic patients with blockade of the renin angiotensin system [abstract no: TH-PO438]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):201A. [Google Scholar]
- Luno J, Gerna Maria F, Vinuesa SG, Praga M. Effect of dual blockade of renin angiotensin system on the progression of type 2 diabetic nephropathy [abstract no: TH-OR051]. Journal of the American Society of Nephrology 2011;22(Abstracts):12A. [Google Scholar]
Raj 2021 {published data only}
- Raj A, Verma N, Pal A, Chattopadhyay R. Comparative study of the renal effect of telmisartan and ramipril in patients with hypertension and type 2 diabetes mellitus. Journal of Cardiovascular Disease Research 2021;12(6):1056‐62. [EMBASE: 2017637667] [Google Scholar]
Ravid 1993 {published data only}
- Ravid M, Clutter WE. Long-term effect of enalapril on normotensive type II diabetes mellitus. Annals of Internal Medicine 1993;119(Suppl 1):6. [EMBASE: 23289262] [Google Scholar]
- Ravid M, Lang R, Rachmani R, Lishner M. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Archives of Internal Medicine 1996;156(3):286-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Ravid M, Neumann L, Lishner M. Plasma lipids and the progression of nephropathy in diabetes mellitus type II: effect of ACE inhibitors. Kidney International 1995;47(3):907-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Annals of Internal Medicine 1993;118(8):577-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ravid M, Savin H, Jutrin I, Bental T, Lang R, Lishner M. Long-term effect of ACE inhibition on development of nephropathy in diabetes mellitus type II. Kidney International - Supplement 1994;45:S161-4. [MEDLINE: ] [PubMed] [Google Scholar]
RENAAL 2001 {published data only}
- Appel GB, Radhakrishnan J, Avram MM, DeFronzo RA, Escobar-Jimenez F, Campos MM, et al. Analysis of metabolic parameters as predictors of risk in the RENAAL study. Diabetes Care 2003;26(5):1402-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, Dijk DJ, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Archives of Internal Medicine 2003;163(13):1555-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boner G, Cooper ME, McCarroll K, Brenner BM, Zeeuw D, Kowey PR, et al. Adverse effects of left ventricular hypertrophy in the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) study. Diabetologia 2005;48(10):1980-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, Zeeuw D, Grunfeld JP, Keane WF, Kurokawa K, et al. The losartan renal protection study--rationale, study design and baseline characteristics of RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan). Journal of the Renin-Angiotensin-Aldosterone System 2000;1(4):328-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 2001;345(12):861-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Burgess ED, Carides GW, Gerth WC, Marentette MA, Chabot I. Losartan reduces the costs associated with nephropathy and end-stage renal disease from type 2 diabetes: economic evaluation of the RENAAL study from a Canadian perspective. Canadian Journal of Cardiology 2004;20(6):613-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Carides GW, Shahinfar S, Dasbach EJ, Keane WF, Gerth WC, Alexander CM, et al. The impact of losartan on the lifetime incidence of end-stage renal disease and costs in patients with type 2 diabetes and nephropathy. Pharmacoeconomics 2006;24(6):549-58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Carr AA, Kowey PR, Devereux RB, Brenner BM, Dahlof B, Ibsen H, et al. Hospitalizations for new heart failure among subjects with diabetes mellitus in the RENAAL and LIFE studies. American Journal of Cardiology 2005;96(11):1530-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan JC, Wat NM, So WY, Lam KS, Chua CT, Wong KS, et al. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes: an Asian perspective from the RENAAL study. Diabetes Care 2004;27(4):874-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Citarella A, Mantovani LG, Cammarota S, Menditto E, Riegler S, De Portu S. Pharmacoeconomic consequences of losartan therapy in patiens undergoing diabetic end-stage renal disease [abstract]. Value in Health 2009;12(7):A406-7. [EMBASE: 70002899] [Google Scholar]
- Dasbach EJ, Shahinfar S, Santanello NC, Simpson RL, Haffner SM, RENAAL Investigators. Quality of life in persons with NIDDM and nephropathy at baseline: the losartan renal protection study (RENAAL) [abstract no: A0823]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):160A. [CENTRAL: CN-00550370] [Google Scholar]
- Eijkelkamp WB, Zhang Z, Brenner BM, Cooper ME, Devereux RB, Dahlof B, et al. Renal function and risk for cardiovascular events in type 2 diabetic patients with hypertension: the RENAAL and LIFE studies. Journal of Hypertension 2007;25(4):871-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Journal of the American Society of Nephrology 2007;18(5):1540-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fisman EZ, Tenenbaum A, Motro M. Losartan and diabetic nephrology: commentaries on the RENAAL study. Cardiovascular Diabetology 2002;1:2. [CENTRAL: CN-00498713] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth WC, Remuzzi G, Viberti G, Hannedouche T, Martinez-Castelao A, Shahinfar S, et al. Losartan reduces the burden and cost of ESRD: public health implications from the RENAAL study for the European Union. Kidney International - Supplement 2002;82:S68-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerth WC, Ribeiro AB, Ferder LF, Amato DJ, Carides GW. Losartan reduced the burden of ESRD: public health implications from the RENAAL study for Latin America [abstract no: PUB143]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):699a. [CENTRAL: CN-00445462] [Google Scholar]
- Herman WH, Shahinfar S, Carides GW, Dasbach EJ, Brenner BM, RENAAL Investigators. Losartan reduces the costs associated with ESRD: the RENAAL study economic evaluation [abstract no: A1078]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):208A. [CENTRAL: CN-00445701] [Google Scholar]
- Herman WH, Shahinfar S, Carides GW, Dasbach EJ, Gerth WC, Alexander CM, et al. Losartan reduces the costs associated with diabetic end-stage renal disease: the RENAAL study economic evaluation. Diabetes Care 2003;26(3):683-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holtkamp FA, Zeeuw D, Thomas MC, Cooper ME, Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney International 2011;80(3):282-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keane WF, Brenner BM, Zeeuw D, Grunfeld JP, Lash J, McGill J, et al. The RENAAL ESRD risk score: a risk score system for predicting end stage renal disease in patients with type 2 diabetes and nephropathy [abstract no: F-PO925]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):249a. [CENTRAL: CN-00446044] [Google Scholar]
- Keane WF, Brenner BM, Zeeuw D, Grunfeld JP, McGill J, Mitch WE, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney International 2003;63(4):1499-507. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keane WF, Carides GW, Shahinfar S, Dasbach EJ, Gerth WC, Alexander CM, et al. The impact of losartan on the lifetime incidence of end-stage renal disease and associated costs: lifetime projections based on the RENAAL study [abstract no: F-P1000]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):264a. [CENTRAL: CN-00446045] [Google Scholar]
- Keane WF, Zhang Z, Lyle PA, Cooper ME, Zeeuw D, Grunfeld JP, et al. Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL Study. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(4):761-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Chan JC, Cooper ME, Keane WF, Shahinfar S, Zhang Z. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes: a subanalysis of Japanese patients from the RENAAL study. Clinical & Experimental Nephrology 2006;10(3):193-200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kvetny J, Gregersen G, Pedersen RS. Randomized placebo-controlled trial of perindopril in normotensive, normoalbuminuric patients with type 1 diabetes mellitus. QJM 2001;94(2):89-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney International 2012;82(3):330-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lambers Heerspink HJ, Laverman GD, Lewis J, Parving HH, Zeeuw D. Both hypokalemia and hyperkalemia predict cardiovascular risk during blood pressure lowering therapy in patients with diabetes and nephropathy [abstract no: SA-PO2407]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):663A. [Google Scholar]
- Lambers Heerspink HJ, Weldegiorgis M, Inker LA, Gansevoort R, Parving HH, Dwyer JP, et al. Estimated GFR decline as a surrogate end point for kidney failure: A post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) Study and Irbesartan Diabetic Nephropathy Trial (IDNT). American Journal of Kidney Diseases 2014;63(2):244-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leung WY, So WY, Tong PC, Lo MK, Lee KF, Ko GT, et al. The renoprotective effects of structured care in a clinical trial setting in type 2 diabetic patients with nephropathy. Nephrology Dialysis Transplantation 2004;19(10):2519-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Losartan and the kidney protection. The RENAAL study [Il losartan e la nefroprotezione. Lo studio RENAAL]. Recenti Progressi in Medicina 2001;92(12):788. [MEDLINE: ] [PubMed] [Google Scholar]
- McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. American Journal of Kidney Diseases 2014;64(5):714-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miao Y, Dobre D, Lambers Heerspink HJ, Brenner BM, Cooper ME, Parving HH, et al. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial [Erratum appears in Diabetologia. 2011 Aug;54(8):2209]. Diabetologia 2011;54(1):44-50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Dobre D, Lambers Heerspink HJ, Shahnaz S, Brenner BM, Cooper M, et al. Serum potassium and renal outcomes in the RENAAL trial [abstract no: SA-PO2742]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):738A. [Google Scholar]
- Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension 2011;58(1):2-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mohanram A, Zhang J, Shahinfar S, Keane WF, Brenner BM, Toto RD. Baseline hemoglobin predicts decline in renal function in patients with Type 2 diabetes and nephropathy [abstract no: SA-PO468]. Journal of the American Society of Nephrology 2003;14(Nov):399A. [CENTRAL: CN-00791121] [Google Scholar]
- Mohanram A, Zhang J, Shahinfar S, Keane WF, Brenner BM, Toto RD. Distribution of hemoglobin concentration in patients with Type 2 diabetes and nephropathy [abstract no: SA-PO721]. Journal of the American Society of Nephrology 2003;14(Nov):455-6A. [CENTRAL: CN-00792633] [Google Scholar]
- Mohanram A, Zhang J, Shahinfar S, Keane WF, Toto RD. Anemia is an independent risk factor for progression to end-stage renal disease (ESRD) in type 2 diabetics with nephropathy: results from the reduction in endpoints in non-insulin dependent diabetes mellitus with the angiotensin II antagonist losartan (RENAAL) trial [abstract no: F-FC034]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):8A. [CENTRAL: CN-00446798] [Google Scholar]
- Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney International 2004;66(3):1131-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mohanram A, Zhang Z, Shahinfar S, Lyle PA, Toto RD. The effect of losartan on hemoglobin concentration and renal outcome in diabetic nephropathy of type 2 diabetes. Kidney International 2008;73(5):630-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mohanram A, Zhang Z, Shahinfar S, Toto RD. The renoprotective effect of angiotensin II receptor blockade in Type 2 diabetics with nephropathy and impact on anemia [abstract no: F-PO993]. Journal of the American Society of Nephrology 2005;16:553A. [CENTRAL: CN-00796616] [Google Scholar]
- Ottenbros SA, Lambers Heerspink HJ, Laverman GD, Bakker SJL, Shahinfar S, Parving H, et al. Association between changes in serum uric acid and renoprotection in the RENAAL trial [abstract no: F-PO1927]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):552A. [Google Scholar]
- Parving HH, Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE et al. Effect of losartan on renal and cardiovascular complications of patients with type 2 diabetes and nephropathy [Effekt af losartan pa nyre- og kardiovaskulaere komplikationer hos patienter med type 2-diabetes og nefropati]. Ugeskrift for Laeger 2001;163(40):5514-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Parving HH, Zeeuw D, Cooper ME, Remuzzi G, Liu N, Lunceford J, et al. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. Journal of the American Society of Nephrology 2008;19(4):771-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi G, Ruggenenti P, Perna A, Dimitrov BD, Zeeuw D, Hille DA, et al. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. Journal of the American Society of Nephrology 2004;15(12):3117-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Risler T. New aspects in renal protection by angiotensin receptor antagonists: the RENAAL study [abstract no: V3-3]. Deutsche Medizinische Wochenschrift 2001;126(Suppl 3):S158. [CENTRAL: CN-00384083] [Google Scholar]
- Schutte E, Lambers Heerspink HJ, Lutgers HL, Bakker SJ, Vart P, Wolffenbuttel BH, et al. Serum bicarbonate and kidney disease progression and cardiovascular outcome in patients with diabetic nephropathy: a post hoc analysis of the RENAAL (Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) Study and IDNT (Irbesartan Diabetic Nephropathy Trial). American Journal of Kidney Diseases 2015;66(3):450-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Seng WK, Hwang SJ, Han DC, Teong CC, Chan J, Burke TA, et al. Losartan reduces the costs of diabetic end-stage renal disease: an Asian perspective. Nephrology 2005;10(5):520-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shahinfar S, Brenner BM, Cooper M, Dahlof B, Devereux RB, Edelman JM, et al. Serum creatinine and risk for cardiovascular events in patients with diabetes: RENAAL and LIFE [abstract no: SU-PO314]. Journal of the American Society of Nephrology 2003;14(Nov):603A. [CENTRAL: CN-00644168] [Google Scholar]
- Shahinfar S, Brenner BM, Mogensen CE, Ramjit D, Gleim G, Zeeuw D, et al. Losartan treatment effect on renal outcomes in type 2 diabetic patients with nephropathy after adjusting for an imbalance in baseline proteinuria [abstract no: M336]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):105. [CENTRAL: CN-00447688] [Google Scholar]
- Shahinfar S, Dickson TZ, Ahmed T, Zhang Z, Ramjit D, Smith RD, et al. Losartan in patients with type 2 diabetes and proteinuria: observations from the RENAAL Study. Kidney International - Supplement 2002;82:S64-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shahinfar S, Mogensen CE, Ramjit D, Gleim G, Zeeuw D, Keane WF, et al. The importance of baseline distribution of proteinuria in renal outcomes trials: lessons from RENAAL [abstract no: SU-PO674]. Journal of the American Society of Nephrology 2003;14(Nov):682A. [CENTRAL: CN-00550564] [DOI] [PubMed] [Google Scholar]
- Shahinfar S, Zhang Z, Ramjit D, Gleim G, Snapinn S, Keane WF, et al. The albumin/creatine ratio and 24 hour proteinuria excretion have similar predictive power for renal endpoints in diabetic nephropathy [abstract no: SU-PO675]. Journal of the American Society of Nephrology 2003;14(Nov):682A. [CENTRAL: CN-00796078] [Google Scholar]
- Shahinfar S, Zeeuw D, Dickson TZ, Zhang Z, Gleim G, Ramjit D, et al. The impact of blood pressure on the anti-proteinuric effect of losartan in Type II diabetes [abstract no: SU-PO308]. Journal of the American Society of Nephrology 2003;14(Nov):601A. [CENTRAL: CN-00796480] [Google Scholar]
- Smink PA, Bakker SJ, Laverman GD, Berl T, Cooper ME, Zeeuw D, et al. An initial reduction in serum uric acid during angiotensin receptor blocker treatment is associated with cardiovascular protection: a post-hoc analysis of the RENAAL and IDNT trials. Journal of Hypertension 2012;30(5):1022-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Souchet T, Durand Zaleski I, Hannedouche T, Rodier M, Gaugris S, Passa P. An economic evaluation of losartan therapy in type 2 diabetic patients with nephropathy: an analysis of the RENAAL study adapted to France. Diabetes & Metabolism 2003;29(1):29-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stanton R. Blood pressure and diabetic nephropathy. Current Diabetes Reports 2003;3(6):483-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Szucs TD, Sandoz MS, Keusch GW. The cost-effectiveness of losartan in type 2 diabetics with nephropathy in Switzerland--an analysis of the RENAAL study. Swiss Medical Weekly 2004;134(31-32):440-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tershakovec A, Keane WF, Zhang Z, Appel GB, Lyle PA, McGill J, et al. Effect of LDL-cholesterol and treatment with losartan on end-stage renal disease in the RENAAL study [abstract no: SA-PO956]. Journal of the American Society of Nephrology 2005;16:766A. [CENTRAL: CN-00793052] [DOI] [PubMed] [Google Scholar]
- Tershakovec AM, Keane WF, Zhang Z, Lyle PA, Appel GB, McGill JB, et al. Effect of LDL cholesterol and treatment with losartan on end-stage renal disease in the RENAAL study. Diabetes Care 2008;31(3):445-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Winkelmayer WC, Zhang Z, Shahinfar S, Cooper ME, Avorn J, Brenner BM. Efficacy and safety of angiotensin II receptor blockade in elderly patients with diabetes. Diabetes Care 2006;29(10):2210-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wong KS, Gerth WC, Yong KM, Carides GW, Choong HL, Woo KT. Losartan reduced the costs associated with ESRD: implications from the RENAAL study for Singapore General Hospital [abstract no: SAP0802]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):429A. [CENTRAL: CN-00448408] [Google Scholar]
- Zhang Z, Shahinfar S, Keane WF, Ramjit D, Dickson TZ, Gleim GW, et al. Importance of baseline distribution of proteinuria in renal outcomes trials: lessons from the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) study. Journal of the American Society of Nephrology 2005;16(6):1775-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Portu S, Citarella A, Cammarota S, Menditto E, Mantovani LG. Pharmaco-economic consequences of losartan therapy in patients undergoing diabetic end stage renal disease in EU and USA. Clinical & Experimental Hypertension (New York) 2011;33(3):174-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004;110(8):921-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney International 2004;65(6):2309-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rizzoni 2005 {published data only}
- Rizzoni D, Porteri E, De Ciuceis C, Sleiman I, Rodella L, Rezzani R, et al. Effect of treatment with candesartan or enalapril on subcutaneous small artery structure in hypertensive patients with noninsulin-dependent diabetes mellitus. Hypertension 2005;45(4):659-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Romero 1993 {published data only}
- Romero R, Salinas I, Lucas A, Abad E, Reverter JL, Johnston S, et al. Renal function changes in microalbuminuric normotensive type II diabetic patients treated with angiotensin-converting enzyme inhibitors. Diabetes Care 1993;16(4):597-600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruggenenti 2019 {published data only}
- Ruggenenti P, Cortinovis M, Parvanova A, Trillini M, Iliev IP, Bossi AC, et al. Preventing microalbuminuria with benazepril, valsartan, and benazepril-valsartan combination therapy in diabetic patients with high-normal albuminuria: a prospective, randomized, open-label, blinded endpoint (PROBE) study. PLoS Medicine 2021;18(7):e1003691. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P, Trillini M, Barlovic DP, Cortinovis M, Pisani A, Parvanova A, et al. Effects of valsartan, benazepril and their combination in overt nephropathy of type 2 diabetes: A prospective, randomized, controlled trial. Diabetes, Obesity & Metabolism 2019;21(5):1177-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sano 1994 {published data only}
- Sano T, Hotta N, Kawamura T, Matsumae H, Chaya S, Sasaki H, et al. Effects of long-term enalapril treatment on persistent microalbuminuria in normotensive type 2 diabetic patients: results of a 4-year, prospective, randomized study. Diabetic Medicine 1996;13(2):120-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sano T, Kawamura T, Matsumae H, Sasaki H, Nakayama M, Hara T, et al. Effects of long-term enalapril treatment on persistent micro-albuminuria in well-controlled hypertensive and normotensive NIDDM patients. Diabetes Care 1994;17(5):420-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sato 2003 {published data only}
- Sato A, Tabata M, Hayashi K, Saruta T. Effects of the angiotensin II type 1 receptor antagonist candesartan, compared with angiotensin-converting enzyme inhibitors, on the urinary excretion of albumin and type IV collagen in patients with diabetic nephropathy. Clinical & Experimental Nephrology 2003;7(3):215-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sawaki 2008 {published data only}
- Sawaki H, Terasaki J, Fujita A, Nakagawa S, Kanatsuna N, Sadahiro K, et al. A renoprotective effect of low dose losartan in patients with type 2 diabetes. Diabetes Research & Clinical Practice 2008;79(1):86-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schram 2005 {published data only}
- Schram MT, Ittersum FJ, Spoelstra-de Man A, Dijk RA, Schalkwijk CG, Ijzerman RG, et al. Aggressive antihypertensive therapy based on hydrochlorothiazide, candesartan or lisinopril as initial choice in hypertensive type II diabetic individuals: effects on albumin excretion, endothelial function and inflammation in a double-blind, randomized clinical trial. Journal of Human Hypertension 2005;19(6):429-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sengul 2006 {published data only}
- Sengul AM, Altuntas Y, Kurklu A, Aydin L. Beneficial effect of lisinopril plus telmisartan in patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Research & Clinical Practice 2006;71(2):210-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SMART 2009 {published data only}
- Burgess E, Muirhead N, Rene de Cotret P, Chiu A, Pichette V, Tobe S, et al. Supramaximal dose of candesartan in proteinuric renal disease. Journal of the American Society of Nephrology 2009;20(4):893-900. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess ED, Muirhead N, Cotret PR. A double-blind randomized controlled trial of high dose candesartan cilexetil in proteinuric renal disease - results from SMART (Supra Maximal Atacand Renal Trial) [abstract no: SA-FC104]. Journal of the American Society of Nephrology 2007;18(Abstracts):57A. [Google Scholar]
- Muirhead N, Burgess E, Rene de Cotret P, SMART Investigators. A randomised controlled trial of high dose candesartan in the treatment of proteinuric renal disease: design and baseline characteristics [abstract no: SA-PO261]. Journal of the American Society of Nephrology 2004;15(Oct):357-8A. [CENTRAL: CN-00550711] [Google Scholar]
SOLVD (Treatment) 1990 {published data only}
- Al-Ahmad A, Levey A, Rand W, Majunath G, Salem D, Gregory D, et al. Anemia and renal insufficiency as risk factors for mortality in patients with left ventricular dysfunction [abstract no: A0739]. Journal of the American Society of Nephrology 2000;11(Sept):137A. [CENTRAL: CN-00550523] [Google Scholar]
- Al-Ahmad AM, Sarnak MJ, Rand W, Gregory D, Konstam MA, Salem DN, et al. Level of renal function as an independent predictor of mortality in patients with left ventricular dysfunction [abstract no: A0783]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):152A. [CENTRAL: CN-00550524] [Google Scholar]
- Alsheikh-Ali AA, Trikalinos TA, Ruthazer R, Terrin N, Wong JB, Sarnak MJ, et al. Risk of arrhythmic and nonarrhythmic death in patients with heart failure and chronic kidney disease. American Heart Journal 2011;161(1):204-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bansal N, Tighiouart H, Weiner D, Griffith J, Vlagopoulos P, Salem D, et al. Anemia as a risk factor for kidney function decline in heart failure [abstract no: TH-PO928]. Journal of the American Society of Nephrology 2005;16:322A. [CENTRAL: CN-01658120] [Google Scholar]
- Bansal N, Tighiouart H, Weiner D, Griffith J, Vlagopoulos P, Salem D, et al. Anemia as a risk factor for kidney function decline in individuals with heart failure. American Journal of Cardiology 2007;99(8):1137-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bowling CB, Sanders PW, Allman RM, Rogers WJ, Patel K, Aban IB, et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD Treatment trial. International Journal of Cardiology 2013;167(1):151-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capes SE, Gerstein HC, Negassa A, Yusuf S. Enalapril prevents clinical proteinuria in diabetic patients with low ejection fraction. Diabetes Care 2000;23(3):377-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gregory DD, Sarnak MJ, Konstam MA, Pereira B, Salem D. Impact of chronic kidney disease and anemia on hospitalization expense in patients with left ventricular dysfunction. American Journal of Cardiology 2003;92(11):1300-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Knight EL, Glynn RJ, McIntyre KM, Mogun H, Avorn J. Predictors of decreased renal function in patients with heart failure during angiotensin-converting enzyme inhibitor therapy: results from the studies of left ventricular dysfunction (SOLVD). American Heart Journal 1999;138(5 (Pt 1)):849-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McCallum W, Tighiouart H, Ku E, Salem D, Sarnak MJ. Acute declines in estimated glomerular filtration rate on enalapril and mortality and cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Kidney International 2019;96(5):1185-94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum WI, Tighiouart H, Ku E, Salem DN, Sarnak MJ. Kidney function outcomes following RAAS inhibition in patients with heart failure [abstract no: FR-PO175]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):464. [EMBASE: 633734726] [Google Scholar]
- McCallum WI, Tighiouart H, Ku E, Salem DN, Sarnak MJ. Mortality outcomes related to acute declines in eGFR following RAAS inhibition in patients with heart failure [abstract no: FR-OR016]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):43. [EMBASE: 633736772] [Google Scholar]
- Riedinger MS, Dracup KA, Brecht ML. Quality of life in women with heart failure, normative groups, and patients with other chronic conditions. American Journal of Critical Care 2002;11(3):211-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Studies of left ventricular dysfunction (SOLVD)--rationale, design and methods: two trials that evaluate the effect of enalapril in patients with reduced ejection fraction [Erratum in: Am J Cardiol 1990 Oct 15;66(12):1026]. American Journal of Cardiology 1990;66(3):315-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circulation: Heart Failure 2011;4(6):685-91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions [Erratum in: N Engl J Med 1992 Dec 10;327(24):1768]. New England Journal of Medicine 1992;327(10):685-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New England Journal of Medicine 1991;325(5):293-302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stornello 1988 {published data only}
- Stornello M, Valvo EV, Puglia N, Scapellato L. Angiotensin converting enzyme inhibition with a low dose of enalapril in normotensive diabetics with persistent proteinuria. Journal of Hypertension - Supplement 1988;6(4):S464-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stornello 1989 {published data only}
- Stornello M, Valvo EV, Scapellato L. Angiotensin converting enzyme inhibition in normotensive type II diabetics with persistent mild proteinuria. Journal of Hypertension - Supplement 1989;7(6):S314-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan KC, Chow WS, Ai VH, Lam KS. Effects of angiotensin II receptor antagonist on endothelial vasomotor function and urinary albumin excretion in type 2 diabetic patients with microalbuminuria. Diabetes/Metabolism Research Reviews 2002;18(1):71-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Titan 2011 {published data only}
- Tavares G, Venturini G, Padilha K, Zatz R, Pereira AC, Thadhani RI, et al. 1,5-Anhydroglucitol predicts CKD progression in macroalbuminuric diabetic kidney disease: results from non-targeted metabolomics. Metabolomics 2018;14(4):39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Titan S, Vieira JM Jr, Barros R, Zatz R. Urinary biomarkers and prediction of proteinuria progression in diabetic nephropathy [abstract no: M327]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Titan SM, Vieira JM Jr, Barros RT, Zatz R. The effect of enalapril and losartan association therapy on proteinuria progression in advanced diabetic nephropathy: a double-blind randomized clinical trial [abstract no: TH-FC129]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):28A. [Google Scholar]
- Titan SM, Vieira JM Jr, Dominguez WV, Barros RT, Zatz R. ACEI and ARB combination therapy in patients with macroalbuminuric diabetic nephropathy and low socioeconomic level: a double-blind randomized clinical trial. Clinical Nephrology 2011;76(4):273-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Titan SM, Vieira JM Jr, Dominguez WV, Moreira SR, Pereira AB, Barros RT, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. Journal of Diabetes & its Complications 2012;26(6):546-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(2):241-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tong 2006 {published data only}
- Tong PC, Ko GT, Chan WB, Ma RC, So WY, Lo MK, et al. The efficacy and tolerability of fosinopril in Chinese type 2 diabetic patients with moderate renal insufficiency. Diabetes, Obesity & Metabolism 2006;8(3):342-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TRANSCEND 2009 {published data only}
- Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurology 2011;10(1):43-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, Clase CM, Mente A, Mann JF, Sleight P, et al. Albuminuria and rapid loss of GFR and risk of new hip and pelvic fractures. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(2):233-40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, O'Donnell M, Mann JF, Anderson C, Fagard R, et al. Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Archives of Internal Medicine 2011;171(2):142-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, Ryden L, Schumacher H, Probstfield J, Commerford P, et al. Effects of telmisartan on glucose levels in people at high risk for cardiovascular disease but free from diabetes: the TRANSCEND study. Diabetes Care 2011;34(9):1902-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm M, Baumhakel M, Teo K, Sleight P, Probstfield J, Gao P, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation 2010;121(12):1439-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Clase CM, Barzilay J, Gao P, Smyth A, Schmieder RE, Tobe S, et al. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney International 2017;91(3):683-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cukierman-Yaffe T, Gerstein HC, Anderson C, Zhao F, Sleight P, Hilbrich L, et al. Glucose intolerance and diabetes as risk factors for cognitive impairment in people at high cardiovascular risk: results from the ONTARGET/TRANSCEND research programme. Diabetes Research & Clinical Practice 2009;83(3):387-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dans AL, Teo K, Gao P, Chen JH, Jae-Hyung K, Yusoff K, et al. In a subgroup of high-risk Asians, telmisartan was non-inferior to ramipril and better tolerated in the prevention of cardiovascular events. PLoS ONE [Electronic Resource] 2010;5(12):e13694. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan M, Mente A, Teo KK, Gao P, Sleight P, Dagenais G, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 2012;126(23):2705-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation 2007;115(11):1371-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kappert K, Bohm M, Schmieder R, Schumacher H, Teo K, Yusuf S, et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 2012;126(8):934-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clinical Research in Cardiology 2014;103(2):149-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Schumacher H, Bohm M, Mann JF, Redon J, Facchetti R, et al. Visit-to-visit blood pressure variability and renal outcomes: results from ONTARGET and TRANSCEND trials. Journal of Hypertension 2020;38(10):2050-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann J, Schmieder R, Dyal L, Schumacher H, Dagenais G, Teo K, et al. Renal outcomes with telmisartan in 5926 people at high vascular risk, results of the TRANSCEND study, a randomised placebo controlled trial [abstract no: SA777]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Mann JF, Schmieder RE, Dyal L, McQueen MJ, Schumacher H, Pogue J, et al. Effect of telmisartan on renal outcomes: a randomized trial. Annals of Internal Medicine 2009;151(1):1-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shiffman D, Pare G, Louie JZ, Rowland CM, Devlin JJ, McQueen M. A gene variant in the CERS2 gene is associated with worsening albuminuria in diabetic patients of ON TARGET and TRANSCEND [abstract no: SA-PO351]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):707A. [Google Scholar]
- Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. American Heart Journal 2004;148(1):52-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, et al. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011;123(10):1098-107. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Dagenais G, Healey J, Gao P, Dans AL, Chazova I, et al. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. Journal of Hypertension 2012;30(5):1004-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Sleight P, Mancia G, Fagard R, Trimarco B, Schmieder RE, et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation 2009;120(14):1380-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial [Erratum in: Lancet. 2008 Oct 18;372(9647):1384]. Lancet 2008;372(9644):1174-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. New England Journal of Medicine 2008;358(15):1547-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trevisan 1995 {published data only}
- Trevisan R, Tiengo A. Effect of low-dose ramipril on microalbuminuria in normotensive or mild hypertensive non-insulin-dependent diabetic patients. North-East Italy Microalbuminuria Study Group. American Journal of Hypertension 1995;8(9):876-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tutuncu 2001 {published data only}
- Tutuncu NB, Gurlek A, Gedik O. Efficacy of ACE inhibitors and ATII receptor blockers in patients with microalbuminuria: a prospective study. Acta Diabetologica 2001;38(4):157-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VA NEPHRON‐D 2009 {published data only}
- Fried LF, Duckworth W, Zhang JH, O'Connor T, Brophy M, Emanuele N, et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D). Clinical Journal of the American Society of Nephrology: CJASN 2009;4(2):361-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LF, Emanuele N, Zhang JH, Brophy M, Conner T, Duckworth W, et al. Combined angiotensin inhibition for treatment of diabetic nephropathy: VA nephron D [abstract no: HI-OR03]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):1B. [Google Scholar]
- Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. New England Journal of Medicine 2013;369(20):1892-903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fried LF, Huang Y, Zhang JH, Palevsky PM, Johnson GR, Seliger SL, et al. ESRD and mortality after VA NEPHRON-D [abstract no: TH-PO717]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):284. [EMBASE: 633704957] [Google Scholar]
- Huang Y, Johnson G, Kyriakides T, Zhang J. Ascertaining study participant safety using centralized electronic medical records in a clinical trial setting - lessons learned from the veteran affairs NEPHRON-D trial [abstract no: P27]. Trials 2017;18(Suppl 1):11. [EMBASE: 620925249] [Google Scholar]
- Leehey DJ, Zhang JH, Emanuele N, Whaley-Connell A, Palevsky PM, Reilly RF, et al. Blood pressure and renal outcomes in diabetic kidney disease: results from the VA NEPHRON-D trial [abstract no: TH-OR037]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):11A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehey DJ, Zhang JH, Emanuele NV, Whaley-Connell A, Palevsky PM, Reilly RF, et al. BP and renal outcomes in diabetic kidney disease: The Veterans Affairs Nephropathy in Diabetes Trial. Clinical Journal of The American Society of Nephrology: CJASN 2015;10(12):2159-69. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevsky PM, Zhang JH, Seliger SL, Emanuele N, Fried LF. Incidence, severity, and outcomes of AKI associated with dual renin-angiotensin system blockade. Clinical Journal of the American Society of Nephrology: CJASN 2016;11(11):1944-53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seliger SL, Zhang JH, Emanuele N, Palevsky PM, Fried LF. Risk and severity of hyperkalemia with combined angiotensin antagonism in diabetic nephropathy- the VA-NEPHRON-D study [abstract no: SA-PO1089]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):5B. [Google Scholar]
- Zhang JH, O'Connor T, Fried L. Titration management of study drugs in the VA NEPHRON-D trial [abstract no: P34]. Clinical Trials 2010;7(4):466. [EMBASE: 70462836] [Google Scholar]
Viberti 1994 {published data only}
- Viberti GC, Laffel L, Gans DJ, Microalbuminuria Captopril Study Group. Secondary prevention of diabetic nephropathy by captopril in patients with insulin-dependent diabetes mellitus (IDDM) and microalbuminuria [abstract no: 119P]. Journal of the American Society of Nephrology 1994;5(3):385. [Google Scholar]
- Viverti GC, Microalbuminuria Captopril Study Group. Treatment of normotensive microalbuminuric IDDM patients [abstract]. In: 13th International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:66. [CENTRAL: CN-00550656]
VIVALDI 2005 {published data only}
- Boger RH, Schwedhelm E, Maas R, Quispe-Bravo S, Skamira C. ADMA and oxidative stress may relate to the progression of renal disease: rationale and design of the VIVALDI study. Vascular Medicine 2005;10 Suppl 1:S97-102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Galle J, Schwedhelm E, Pinnetti S, Boger RH, Wanner C. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathy. Nephrology Dialysis Transplantation 2008;23(10):3174-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- VIVALDI: A trial to investigate the efficacy of telmisartan 80mg versus valsartan 160mg in hypertensive type-2 diabetic patients with overt nephropathy. The Protection Trial Programme - Micardis Protection 2005.
Weil 2012 {published data only}
- Afshinnia F, Nair V, Lin J, Rajendiran TM, Soni T, Byun J, et al. Increased lipogenesis and impaired beta-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI insight 2019;4(21):e130317. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker HC, Mauer M, Saulnier PJ, Harder JL, Nair V, Boustany-Kari CM, et al. Changes in albuminuria but not GFR are associated with early changes in kidney structure in type 2 diabetes. Journal of the American Society of Nephrology 2019;30(6):1049-59. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanamas SK, Saulnier PJ, Fufaa GD, Wheelock K, Hanson RL, Knowler WC, et al. Long-term effect of losartan on kidney disease in American Indians with type 2 diabetes [abstract no: 536-P]. Diabetes 2016;65(Suppl 1):A140. [EMBASE: 620236877] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanamas SK, Saulnier PJ, Fufaa GD, Wheelock KM, Weil EJ, Hanson RL, et al. Long-term effect of losartan on kidney disease in American Indians with type 2 diabetes: a follow-up analysis of a randomized clinical trial. Diabetes Care 2016;39(11):2004-10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, et al. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 2013;62(9):3224-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney International 2012;82(9):1010-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2001 {published data only}
- White KE, Pinel N, Bilous RW. Interstitial volume changes, but not glomerulopathy, are limited by angiotensin converting enzyme inhibition after 2 years of therapy in type 2 diabetic patients with nephropathy [abstract no: A0668]. Journal of the American Society of Nephrology 2000;11(Sept):124A. [Google Scholar]
- White KE, Pinel N, Cordonnier DJ, Bilous RW. Does ACE inhibition slow progression of glomerulopathy in patients with Type 2 diabetes mellitus? Diabetic Medicine 2001;18(11):933-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xia 1996 {published data only}
- Xia H, He R, Chen J. Protective effect of angiotensin converting enzyme inhibitor on renal function in normotensive non-insulin dependent diabetes mellitus patients with early diabetic nephropathy and microalbuminuria. Chung-Hua Nei Ko Tsa Chih [Chinese Journal of Internal Medicine] 1996;35(8):533-6. [MEDLINE: ] [PubMed] [Google Scholar]
Yao 2001a {published data only}
- Yao B, Hu G, Li Y, Liao Z, Zhang Y. The effect of perindopril in treatment of early diabetic nephropathy with normal blood pressure and microalbuminuria. Chung-Hua Nei Ko Tsa Chih [Chinese Journal of Internal Medicine] 2001;40(12):826-8. [MEDLINE: ] [PubMed] [Google Scholar]
Yoldi 1995 {published data only}
- Yoldi A, Llorente I, Monreal M, Diez J, Salvador J. Is the suppression of growth hormone involved in the kidney-protecting effects of captopril in patients with diabetic nephropathy? [abstract]. In: ISN XIII International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:198. [CENTRAL: CN-00509572]
Yoneda 2007 {published data only}
- Yoneda T, Takeda Y, Usukura M, Oda N, Takata H, Yamamoto Y, et al. Aldosterone breakthrough during angiotensin II receptor blockade in hypertensive patients with diabetes mellitus. American Journal of Hypertension 2007;20(12):1329-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acbay 2001 {published data only}
- Acbay O. Effects of low-dose losartan treatment on persistent microalbuminuria in normotensive type 1 diabetic subjects. Journal of Endocrinological Investigation 2001;24(8):608-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Agarwal 2001 {published data only}
- Agarwal R, Siva S, Dunn SR, Sharma K. Add-on angiotensin II receptor blockade lowers urinary transforming growth factor-beta levels. American Journal of Kidney Diseases 2002;39(3):486-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Agarwal R. Add-on angiotensin receptor blockade with maximized ACE inhibition. Kidney International 2001;59(6):2282-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Agarwal R. Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. American Journal of Physiology - Renal Physiology 2003;284(4):F863-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alam 2001 {published data only}
- Alam SM, Abbas A, Vishnu R, Sharma BN. Study of ramipril, losartan and benazapril efficacy on microalbuminuria [abstract no: CNP 17]. Indian Journal of Nephrology 2001;11(3):118. [CENTRAL: CN-00460261] [Google Scholar]
Andersen 1999 {published data only}
- Andersen S, Parving H, Blouch K, Deckert M, Myers BD. Angiotensin II receptor blockade and barrier function in diabetic nephropathy [abstract no: A0642]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):125A. [CENTRAL: CN-00550555] [Google Scholar]
Andersen 2000 {published data only}
- Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney International 2000;57(2):601-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parving HH, Andersen S, Rossing P, Hansen BV, Tarnow L. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy [abstract no: A0679]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):132A. [DOI] [PubMed] [Google Scholar]
Bakris 2000 {published data only}
- Bakris GL, Siomos M, Bolton WK, Hebert l, Agerwall R, Catanzaro D, et al. Differential effects of valsartan (V) and lisinopril (L) on potassium [K+] homeostasis in hypertensive patients with nephropathy [abstract no: A0349]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):68A. [CENTRAL: CN-00550414] [Google Scholar]
- Bakris GL, Siomos M, Richardson D, Janssen I, Bolton WK, Hebert L, et al. ACE inhibition or angiotensin receptor blockade: impact on potassium in renal failure. VAL-K Study Group. Kidney International 2000;58(5):2084-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cetinkaya 2002 {published data only}
- Cetinkaya R, Odabas AR, Selcuk NY. Combination of enalapril and losartan therapy in diabetic nephropathy [abstract no: T129]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):225. [CENTRAL: CN-00509126] [Google Scholar]
Cetinkaya 2004 {published data only}
- Cetinkaya R, Odabas AR, Selcuk Y. Anti-proteinuric effects of combination therapy with enalapril and losartan in patients with nephropathy due to type 2 diabetes. International Journal of Clinical Practice 2004;58(5):432-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 1997 {published data only}
- Chan JC, Critchley JA, Tomlinson B, Chan TY, Cockram CS. Antihypertensive and anti-albuminuric effects of losartan potassium and felodipine in Chinese elderly hypertensive patients with or without non-insulin-dependent diabetes mellitus. American Journal of Nephrology 1997;17(1):72-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chonko 1993 {published data only}
- Chonko A, Donaldson D, Moore W, MacDougall M, Wiegmann T. Ramipril (R) has antiproteinuric effect and maintains renal function in patients with type 1 diabetes mellitus (DM): a prospective double-blind dose finding trials (PRT) [abstract no: 39P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):302. [CENTRAL: CN-00483510] [Google Scholar]
Christensen 2001a {published data only}
- Christensen PK, Lund S, Parving H. Maintained autoregulation of glomerular filtration rate during candesartan treatment in hypertensive type 2 diabetic patients [abstract no: A4356]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):832-3A. [CENTRAL: CN-00550725] [Google Scholar]
- Christensen PK, Lund S, Parving HH. Autoregulated glomerular filtration rate during candesartan treatment in hypertensive type 2 diabetic patients. Kidney International 2001;60(4):1435-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Daikuhara 2012 {published data only}
- Daikuhara H, Kikuchi F, Ishida T. The combination of OLmesartan and a CAlcium channel blocker (azelnidipine) or candesartan and a calcium channel blocker (amlodipine) in type 2 diabetic hypertensive patients: the OLCA study. Diabetes & Vascular Disease Research 2012;9(4):280-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dhillon 2003 {published data only}
- Dhillon KK, Mason E, Nanovic L, Laplace L, McCune TR. Combining ACE inhibitors and angiotensin II receptor blockers to decrease proteinuria in patients with diabetic nephropathy [abstract no: SU-PO310]. Journal of the American Society of Nephrology 2003;14(Nov):602A. [Google Scholar]
DIRECT Studies 2002 {published data only}
- Bilous R, Chaturvedi N, Sjolie AK, Fuller J, Klein R, Orchard T, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Annals of Internal Medicine 2009;151(1):11-20, W3-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bilous RW, Parving H, Nishi C. DIRECT-Renal: The effect of the angiotensive type 1 receptor blocker candesartan on the development of microalbuminuria in type 1 and type 2 diabetes [abstract no: LB-003]. In: American Society of Nephrology (ASN) Renal Week; 2008 Nov 4-9; Philadelphia, PA. 2008. [CENTRAL: CN-00724910]
- Bilous RW, Parving H, Orchard TJ, Klein R, Porta M, Fuller J, et al. Renin angiotensin system blockade is effective in preventing microalbuminuria in hypertensive but not normotensive people with type 2 diabetes; further analysis of the DIRECT Programme [abstract no: 223]. Diabetologia 2010;53(Suppl 1):S99. [EMBASE: 70262263] [Google Scholar]
- Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 2008;372(9647):1394-402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Sjoelie AK, Svensson A. The DIabetic Retinopathy Candesartan Trials (DIRECT) Programme, rationale and study design. Journal of the Renin-Angiotensin-Aldosterone System 2002;3(4):255-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eickhoff MK, Frimodt-Moller M, Lindhardt M, Dakna M, Mischak H, Rossing P. Is urinary proteomics useful to predict retinopathy in type 2 diabetic patients in the DIRECT 2 study [abstract no: FR-PO607]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):499A. [CENTRAL: 641103335] [Google Scholar]
- Lindhardt M, Persson F, Zurbig P, Stalmach A, Mischak H, Zeeuw D, et al. Urinary proteomics predict onset of microalbuminuria in normoalbuminuric type 2 diabetic patients, a sub-study of the DIRECT-Protect 2 study. Nephrology Dialysis Transplantation 2017;32(11):1866-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lindhardt M, Persson FI, Zurbig P, Stalmach A, Mischak H, Zeeuw D, et al. Urinary proteomics predict onset of microalbuminuria in a cohort of normoalbuminuric type 1 diabetic patients in the DIRECT 1 study [abstract no: FR-OR048]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):41A. [Google Scholar]
- Lindhardt M, Persson FI, Zurbig P, Stalmach A, Mischak H, Zeeuw D, et al. Urinary proteomics predict onset of microalbuminuria in normoalbuminuric type 2 diabetic patients in the DIRECT 2 study [abstract no: TH-PO507]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):213A. [Google Scholar]
- Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet 2008;372(9647):1385-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sjolie AK, Porta M, Parving HH, Bilous R, Klein R. The DIabetic REtinopathy Candesartan Trials (DIRECT) Programme: baseline characteristics. Journal of the Renin-Angiotensin-Aldosterone System 2005;6(1):25-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tillin T, Chaturvedi N, Fuller J, Orchard TJ. Candesartan reduces vascular complications in type 2 diabetic patients without cardiovascular disease or microalbuminuria [abstract]. Diabetes 2009;58(Suppl 1A). [EMBASE: 70136499] [Google Scholar]
DREAM 2004 {published data only}
- Bangalore S, Messerli FH. Effect of ramipril on the incidence of diabetes. New England Journal of Medicine 2007;356(5):522-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, et al. Effect of ramipril on the incidence of diabetes. New England Journal of Medicine 2006;355(15):1551-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dagenais GR, Gerstein HC, Holman R, Budaj A, Escalante A, Hedner T, et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care 2008;31(5):1007-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Epstein BJ, Cooper-DeHoff RM. Effect of ramipril on the incidence of diabetes. New England Journal of Medicine 2007;356(5):523-4. [MEDLINE: ] [PubMed] [Google Scholar]
- Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial [Erratum in: Lancet. 2006 Nov 18;368(9549):1770]. Lancet 2006;368(9541):1096-105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Yusuf S, Holman R, Bosch J, Pogue J. Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trial. Diabetologia 2004;47(9):1519-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Potter BJ, LeLorier J. Effect of ramipril on the incidence of diabetes. New England Journal of Medicine 2007 Feb 1;356(5):522-3. [MEDLINE: ] [PubMed] [Google Scholar]
Dreyling 1990 {published data only}
- Dreyling KW, Grotz W, Keller E, Schollmeyer P. Angiotensin converting enzyme (ACE) - inhibitors induce blood count changes in patients with diabetic nephropathy [abstract]. In: 11th International Congress of Nephrology. 1990:160A.
Drummond 1989 {published data only}
- Drummond K, Levy-Marchal C, Laborde K, Kindermans C, Wright C, Dechaux M, et al. Enalapril (E) in normotensive Type 1 diabetes (DM-1): a double blind cross-over study in patients with elevated glomerular filtration rate (GFR) and renal plasma flow (RPF) [abstract]. Kidney International 1988;33(1):189. [CENTRAL: CN-00583510] [Google Scholar]
- Drummond K, Levy-Marchal C, Laborde K, Kindermans C, Wright C, Dechaux M, et al. Enalapril does not alter renal function in normotensive, normoalbuminuric, hyperfiltering type 1 (insulin-dependent) diabetic children. Diabetologia 1989;32(4):255-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esnault 2005 {published data only}
- Ekhlas Eid A, Nguyen J, Delcroix C, Moutel M, Esnault VL. Effect of diuretics after dual blockade of renin-angiotensin-aldosterone system on severe proteinuria [abstract no: TO22]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:434-5. [CENTRAL: CN-00509172]
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. Journal of the American Society of Nephrology 2005;16(2):474-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Esnault VL, Ekhlas A, Nguyen JM, Delcroix C, Moutel MG. Angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB) and diuretics for refractory proteinuria [abstract no: SU-PO1030]. Journal of the American Society of Nephrology 2003;14(Nov):762A. [CENTRAL: CN-00583888] [Google Scholar]
EUCTR2021‐001661‐21‐DK {published data only}
- EUCTR2021-001661-21-DK. Feasibility of aggressively lowering urine albumin in individuals with kidney biopsy-proven diabetic kidney disease - a pilot study. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-001661-21-DK (date accessed: 29 February 2024).
Fliser 2005 {published data only}
- Fliser D, Wagner K, Loos A, Tsikas D, Haller H. Chronic angiotensin II receptor blockade reduces intra(renal) vascular resistance in patients with type 2 diabetes mellitus [abstract no: MP121]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v240. [CENTRAL: CN-00740561] [DOI] [PubMed] [Google Scholar]
- Fliser D, Wagner KK, Loos A, Tsikas D, Haller H. Chronic angiotensin II receptor blockade reduces (intra)renal vascular resistance in patients with type 2 diabetes. Journal of the American Society of Nephrology 2005;16(4):1135-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garg 2005 {published data only}
- Garg JP, Ellis R, Elliott WJ, Hasabou N, Chua D, Chertow GM, et al. Angiotensin receptor blockade and arterial compliance in chronic kidney disease: a pilot study. American Journal of Nephrology 2005;25(4):393-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haneda 2004 {published data only}
- Haneda M, Kikkawa R, Sakai H, Kawamori R. Antiproteinuric effect of candesartan cilexetil in Japanese subjects with type 2 diabetes and nephropathy. Diabetes Research & Clinical Practice 2004;66(1):87-95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hommel 1986 {published data only}
- Hommel E, Parving HH, Mathiesen E, Edsberg B, Damkjaer NM, Nielsen M, et al. Effect of captopril on kidney function in insulin-dependent diabetic patients with nephropathy. British Medical Journal Clinical Research Ed 1986;293(6545):467-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoque 2009 {published data only}
- Hoque R, Rahman MS, Iqbal M. Effect of enalapril and losartan on proteinuria in type 2 diabetic nephropathy patients. Bangladesh Medical Research Council Bulletin 2009;35(2):44-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hou 2017a {published data only}
- Hou Y, Zhang F, Liu Z, Su S, Wu X, Wang Z. Effect of telmisartan and enalapril on ventricular remodeling and kidney prognosis of patients with coronary artery disease complicated with diabetic nephropathy. Experimental & Therapeutic Medicine 2017;13(1):131-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houlihan Losartan 2002 {published data only}
- Houlihan C, Allen T, Hovey A, Jenkins M, Cooper M, Jerums G. A low salt diet in patients with type II diabetes significantly amplifies the effects of angiotensin II receptor blockade with losartan [abstract no: 17]. Nephrology 2000;5(3):A71. [CENTRAL: CN-00509238] [Google Scholar]
- Houlihan CA, Akdeniz A, Tsalamandris C, Cooper ME, Jerums G, Gilbert RE. Urinary transforming growth factor-beta excretion in patients with hypertension, type 2 diabetes, and elevated albumin excretion rate: effects of angiotensin receptor blockade and sodium restriction. Diabetes Care 2002;25(6):1072-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Houlihan CA, Allen T, Hovey A, Jenkins M, Cooper M, Jerums G. Comparison of regular versus low sodium diet on the effects of losartan in hypertensive subjects with type II diabetes [abstract no: A0624]. Journal of the American Society of Nephrology 2000;11(Sept):116A. [CENTRAL: CN-00615874] [Google Scholar]
- Houlihan CA, Allen TJ, Baxter AL, Panangiotopoulos S, Casley DJ, Cooper ME, et al. A low-sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care 2002;25(4):663-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Igarashi 2006 {published data only}
- Igarashi M, Hirata A, Kadomoto Y, Tominaga M. Dual blockade of angiotensin II with enalapril and losartan reduces proteinuria in hypertensive patients with type 2 diabetes. Endocrine Journal 2006;53(4):493-501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Inigo 2000 {published data only}
- Inigo PJ, Esmatjes E, Lario S, Flores L, Ruilope LM, Campistol JM. Losartan decreases TGF-b1 plasma levels and urinary albumin excretion in patients with diabetes type-II and microalbuminuria [abstract no: A0630]. Journal of the American Society of Nephrology 2000;11(Sept):117A. [Google Scholar]
Insua 1988 {published data only}
- Insua A, Ribstein J, Mimran A. Comparative effect of captopril and nifedipine in normotensive patients with incipient diabetic nephropathy. Postgraduate Medical Journal 1988;64 Suppl 2:59-62. [MEDLINE: ] [PubMed] [Google Scholar]
Islam 2005 {published data only}
- Iqbal MM, Islam MN, Rahman MH, Banerjee SK, Hassan MS, Hossain RM, et al. Effect of renin angiotensin system inhibitors (ACEI) and blockers (ATRB) on proteinuria and blood pressure reduction in patients with diabetic nephropathy [abstract no: M-PO20059]. Nephrology 2005;10(Suppl):A30-1. [Google Scholar]
- Iqbal MM, Islam MN, Rahman MH, Banerjee SK, Hossain RM, Islam MS, et al. Tolerance of high dose enalapril and losartan for proteinuria and blood pressure reduction in patients with chronic kidney disease [abstract no: SO008]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv5. [Google Scholar]
- Islam MN, Mansur MA, Ahmed Z, Iqbal MM, Rahman MH, Ahmed T, et al. Alterations in serum K+ level at different doses of enalapril and losartan for treating type 2 diabetic CKD patients [abstract no: FP219]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi92. [CENTRAL: CN-00755274] [Google Scholar]
Jacobsen 2002 {published data only}
- Jacobsen P, Andersen S, Hansen BV, Parving HH. Dual blockade of the renin-angiotensin system in type 1 diabetic patients with diabetic nephropathy [abstract no: A0777]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):148A. [CENTRAL: CN-01912811] [Google Scholar]
- Jacobsen P, Andersen S, Rossing K, Hansen BV, Parving HH. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrology Dialysis Transplantation 2002;17(6):1019-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jacobsen PK, Rossing P, Parving HH. Impact of renin angiotensin system blockade on night to day blood pressure ratio in diabetic nephropathy. Nephrology Dialysis Transplantation 2006;21(7):2030-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jacobsen 2003 {published data only}
- Jacobsen P, Andersen S, Jensen BR, Parving HH. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. Journal of the American Society of Nephrology 2003;14(4):992-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jacobsen PK, Rossing P, Parving HH. Impact of renin angiotensin system blockade on night to day blood pressure ratio in diabetic nephropathy. Nephrology Dialysis Transplantation 2006;21(7):2030-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jacobsen 2003a {published data only}
- Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney International 2003;63(5):1874-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jacobsen PK, Rossing P, Parving HH. Impact of renin angiotensin system blockade on night to day blood pressure ratio in diabetic nephropathy. Nephrology Dialysis Transplantation 2006;21(7):2030-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jiao 2022 {published data only}
- Jiao S, Dong Y, Chang X, Wu Y, Li H. Effects of alpha lipoic acid combined with olmesartan medoxomil on blood glucose and oxidation indicators in patients with diabetic nephropathy: a protocol for a parallel, randomized, double-blind, controlled clinical trial. Medicine 2022;101(17):e29080. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keilani 1993 {published data only}
- Keilani T, Schlueter WA, Levin ML, Batlle DC. Improvement of lipid abnormalities associated with proteinuria using fosinopril, an angiotensin-converting enzyme inhibitor. Annals of Internal Medicine 1993;118(4):246-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schlueter W, Keilani T, Batlle DC. Metabolic effects of converting enzyme inhibitors: focus on the reduction of cholesterol and lipoprotein(a) by fosinopril. American Journal of Cardiology 1993;72(20):37-44H. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kincaid‐Smith 2002 {published data only}
- Kincaid-Smith P, Fairley K, Packham D. Comparison of the effect of 50% increase in angiotensin converting enzyme inhibitor (ACEI) with a combination of candesartan and ACEI on proteinuria and blood pressure in chronic renal disease [abstract no: A0392]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):75A. [CENTRAL: CN-00446096] [Google Scholar]
- Kincaid-Smith P, Fairley K, Packham D. Comparison of the effect of 50% increase in angiotensin converting enzyme inhibitor (ACEI) with a combination of candesartan and ACEI on proteinuria and blood pressure in chronic renal disease [abstract no: P99]. Nephrology 2002;7(Suppl 3):A25. [CENTRAL: CN-00792336] [Google Scholar]
- Kincaid-Smith P, Fairley K, Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrology Dialysis Transplantation 2002;17(4):597-601. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kincaid-Smith P, Fairley K. A fall in urine protein with combined angiotensin converting enzyme inhibitor (ACEI) and aII receptor antagonist (AIIRA) treatment predicts a good outcome at three years in patients with chronic renal disease with continuing reduction in urine protein levels. Diabetic and obese patients with a poor response to combined ACEI and AIIRA treatment have a poor outcome [abstract no: M419]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):131. [CENTRAL: CN-00446094] [Google Scholar]
- Kincaid-Smith PS, Fairley KF, Packham D. Randomised controlled crossover study of the effect on proteinuria of adding an angiotensin 11-receptor antagonist (ARA) to an angiotensin converting enzyme inhibitor (ACEI) [abstract no: A1825]. Journal of the American Society of Nephrology 2000;11(Sept):349A. [CENTRAL: CN-00626048] [Google Scholar]
- Kincaid-Smith PS, Fairley KF. A fall in urine protein with combined angiotensin converting enzyme inhibitor (ACEI) and AII receptor antagonist (AIIRA) treatment predicts a good outcome at three years in patients with chronic renal disease (mean serum creatinine level of 0.18 mmols/litre mean urinary protein level of 2.3 grams/24 hours). Diabetic and obese patients with poor response to combined ACEI and AIIRA treatment have a poor outcome [abstract no: PUB059]. Journal of the American Society of Nephrology 2003;14(Nov):786A. [Google Scholar]
Lim 2007 {published data only}
- Lim SC, Koh AF, Goh SK, Chua CL, Heng BL, Subramaniam T, et al. Angiotensin receptor antagonist vs. angiotensin-converting enzyme inhibitor in Asian subjects with type 2 diabetes and albuminuria - a randomized crossover study. Diabetes, Obesity & Metabolism 2007;9(4):477-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lim SC, Liu JJ, Subramaniam T, Sum CF. Elevated circulating alpha-klotho by angiotensin II receptor blocker losartan is associated with reduction of albuminuria in type 2 diabetic patients. Journal of the Renin-Angiotensin-Aldosterone System 2014;15(4):487-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mårup 2022 {published data only}
- Mårup FH, Peters CD, Christensen JH, Birn H. Can patiromer allow for intensified renin-angiotensin-aldosterone system blockade with losartan and spironolactone leading to decreased albuminuria in patients with chronic kidney disease, albuminuria and hyperkalaemia? An open-label randomised controlled trial: morphCKD. BMJ open 2022;12(2):e057503. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masuda 2009 {published data only}
- Masuda S, Tamura K, Wakui H, Kanaoka T, Ohsawa M, Maeda A, et al. Effects of angiotensin II type 1 receptor blocker on ambulatory blood pressure variability in hypertensive patients with overt diabetic nephropathy. Hypertension Research - Clinical & Experimental 2009;32(11):950-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tamura K, Wakui H, Masuda SI, Kanaoka T, Ohsawa M, Maeda A, et al. Effects of angiotensin II type 1 receptor blocker on ambulatory blood pressure profile in hypertensive patients with overt diabetic nephropathy [abstract no: P295]. Hypertension 2009;54(4):e89. [EMBASE: 70024188] [DOI] [PubMed] [Google Scholar]
Mathiesen 1991 {published data only}
- Hansen PM, Mathiesen ER, Kofoed-Enevoldsen A, Deckert T. Possible effect of angiotensin-converting enzyme inhibition on glomerular charge selectivity. Journal of Diabetes & its Complications 1995;9(3):158-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansen PM, Mathiesen ER, Kofoed-Enevoldsen A. Possible effect of angiotensin converting enzyme (ACE) inhibition on glomerular charge selectivity [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:425. [CENTRAL: CN-00583297]
- Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ 1991;303(6794):81-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen ER, Hommel E, Hansen HP, Parving HH. Preservation of normal GFR with long term captopril treatment in normotensive IDDM patients with microalbuminuria [abstract no: A0546]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):115A. [CENTRAL: CN-00446655] [Google Scholar]
- Mathiesen ER, Hommel E, Hansen HP, Smidt UM, Parving HH. Randomised controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ 1999;319(7201):24-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen ER, Hommel E, Smidt UM, Parving H. Efficacy of captopril in normotensive diabetic patients with microalbuminuria - 8 years follow-up [abstract no: 670]. Journal of the American Society of Nephrology 1995;6(3):452. [CENTRAL: CN-00583618] [Google Scholar]
Matos 2005 {published data only}
- Matos JP, Lourdes Rodrigues M, Ismerim VL, Boasquevisque EM, Genelhu V, Francischetti EA. Effects of dual blockade of the renin angiotensin system in hypertensive type 2 diabetic patients with nephropathy. Clinical Nephrology 2005;64(3):180-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Matos JP, Lourdes Rodrigues M, Valenca D, Ismerim V, Boasquevisque E, Barroso S, et al. Dual blockade of the renin-angiotensin system reduces plasma aldosterone, proteinuria and urinary tgb-b1 in type 2 diabetic patients with nephropathy [abstract no: MP135]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:275. [CENTRAL: CN-00636139]
Meier 2011 {published data only}
- Meier P, Maillard M, Meier R, Burnier M. Antiproteinuric effect of losartan: is a higher dose as effective as an association with an ACE inhibitor? Results of a randomised triple-crossover study in proteinuric patients [abstract no: F-PO1920]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):543A. [Google Scholar]
- Meier P, Maillard M, Tremblay S, Meier R, Burnier M. Comparative 24h blood pressure effects of increasing doses of losartan and the association of losartan and an ACE inhibitor in proteinuric patients [abstract no: SA-PO2249]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):617A. [Google Scholar]
- Meier P, Maillard MP, Meier JR, Tremblay S, Gauthier T, Burnier M. Combining blockers of the renin-angiotensin system or increasing the dose of an angiotensin II receptor antagonist in proteinuric patients: a randomized triple-crossover study. Journal of Hypertension 2011;29(6):1228-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mimran 1988 {published data only}
- Mimran A, Insua A, Ribstein J, Monnier L, Bringer J, Mirouze J. Contrasting effects of captopril and nifedipine in normotensive patients with incipient diabetic nephropathy. Journal of Hypertension 1988;6(11):919-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00171600 {published data only}
- Antialbuminuric effects of valsartan and lisinopril [Comparative, open multicenter trial assessing the effect on albumin excretion rate of 320mg valsartan (with or without HCTZ) Vs 40mg lisinopril (with or without HCTZ) on hypertensive patients with diabetic and non-diabetic nephropathy and albuminuria]. www.clinicaltrials.gov/ct/show/NCT00171600 (first received 15 September 2005).
NCT00208221 {published data only}
- Higher dose of ramipril versus addition of telmisartan-ramipril in hypertension and diabetes [Comparison of a higher dose of ramipril to the addition of telmisartan 80 mg+ramipril 10 mg in patients with hypertension and diabetes]. www.clinicaltrials.gov/ct2/show/NCT00208221 (first received 21 September 2005).
NCT04238702 {published data only}
- Renohemodynamic Effects of Combined empagliflOzin and LosARtan (RECOLAR) [A randomized, comparator-controlled, cross-over intervention study to assess renal hemodynamics of mono- and combination therapy with SGLT-2 inhibitor empagliflozin and RAS-inhibitor losartan in patients with type 2 diabetes mellitus]. www.clinicaltrials.gov/show/NCT04238702 (first received 23 October 2021).
NCT05189015 {published data only}
- Effect of olmesartan on angiotensin(1-7) levels and vascular functions in diabetes and hypertension (Ang(1-7)) [Effect of olmesartan or amlodipine on serum angiotensin(1-7) levels and vascular functions in patients with type 2 diabetes and hypertension]. www.clinicaltrials.gov/show/NCT05189015 (first received 12 January 2022).
NCT05593575 {published data only}
- Efficacy and safety of SPH3127 tablets on treating the diabetic kidney disease [A multicenter, randomized, double-blind, active-controlled, parallel, dose-finding phase 2 clinical study to evaluate the efficacy and safety of SPH3127 tablets in the treatment of diabetic kidney disease]. www.clinicaltrials.gov/show/NCT05593575 (first received 25 October 2022).
New 1998 {published data only}
- New JP, Marshall SM, Bilous RW. Renal autoregulation is normal in newly diagnosed, normotensive, NIDDM patients. Diabetologia 1998;41(2):206-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okura 2012 {published data only}
- Okura T, Kojima M, Machida H, Sugiyama M, Kato T, Komada T, et al. Effects of up-titration of candesartan versus candesartan plus amlodipine on kidney function in type 2 diabetic patients with albuminuria. Journal of Human Hypertension 2012;26(4):214-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parvanova 2001 {published data only}
- Parvanova P, Pisoni R, Dimitrov BD, Iliev I, Perna A, Ruggenenti P, et al. Relative renoprotective effect of ACE inhibitors (ACEi), angiotensin II antagonists (ATA), ACEi and ATA combination and dihydrophyridine calcium channel blockers (dCCBs) in overt nephropathy of type 2 diabetes [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):153A. [CENTRAL: CN-00447108] [Google Scholar]
Pedersen 1992 {published data only}
- Pedersen MM, Hansen KW, Schmitz A, Sorensen K, Christensen CK, Mogensen CE. Effects of ACE inhibition supplementary to beta blockers and diuretics in early diabetic nephropathy. Kidney International 1992;41(4):883-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pruijm 2013 {published data only}
- Pruijm M, Hofmann L, Zanchi A, Maillard M, Forni V, Muller ME, et al. Blockade of the renin-angiotensin system and renal tissue oxygenation as measured with BOLD-MRI in patients with type 2 diabetes. Diabetes Research & Clinical Practice 2013;99(2):136-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rasi Hashemi 2012 {published data only}
- Rasi Hashemi S, Noshad H, Tabrizi A, Mobasseri M, Tayebi Khosroshahi H, Heydarnejad M, et al. Angiotensin receptor blocker and N-acetyl cysteine for reduction of proteinuria in patients with type 2 diabetes mellitus. Iranian Journal of Kidney Diseases 2012;6(1):39-43. [MEDLINE: ] [PubMed] [Google Scholar]
Romanelli 1989 {published data only}
- Romanelli G, Giustina A, Cimino A, Valentini U, Agabiti-Rosei E, Muiesan G, et al. Short term effect of captopril on microalbuminuria induced by exercise in normotensive diabetics. BMJ 1989;298(6669):284-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossing 2002 {published data only}
- Rossing K, Christensen PK, Jensen BR, Parving HH. Dual blockade of the renin-angiotensin system in diabetic nephropathy: a randomized double-blind crossover study. Diabetes Care 2002;25(1):95-100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rossing K, Christensen PK, Jensen BR, Parving HH. Dual blockade of the renin-angiotensin system in type 2 diabetic patients with diabetic nephropathy [abstract no: A0813]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):155A. [CENTRAL: CN-00444816] [Google Scholar]
Rossing 2003 {published data only}
- Rossing K, Christensen PK, Hansen BV, Carstensen B, Parving HH. Optimal dose of candesartan for renoprotection in type 2 diabetic patients with nephropathy: a double-blind randomized cross-over study. Diabetes Care 2003;26(1):150-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossing 2003a {published data only}
- Rossing K, Jacobsen P, Pietraszek L, Parving HH. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care 2003;26(8):2268-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rossing K, Jacobsen PK, Pietraszek L, Parving H. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE-inhibitor in diabetic nephropathy [abstract no: SU-PO307]. Journal of the American Society of Nephrology 2003;14(Nov):601A. [CENTRAL: CN-00644353] [DOI] [PubMed] [Google Scholar]
Rossing 2005a {published data only}
- Rossing K, Mischak H, Parving HH, Christensen PK, Walden M, Hillmann M, et al. Impact of diabetic nephropathy and angiotensin II receptor blockade on urinary polypeptide patterns. Kidney International 2005;68(1):193-205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossing 2005b {published data only}
- Nielsen SE, Rossing K, Hess G, Zdunek D, Jensen BR, Parving HH, et al. The effect of RAAS blockade on markers of renal tubular damage in diabetic nephropathy: u-NGAL, u-KIM1 and u-LFABP. Scandinavian Journal of Clinical & Laboratory Investigation 2012;72(2):137-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Rossing K, Jensen BR, Parving HH, Rossing P. Tubular damage in type 2 diabetic nephropathy: the effect of ultrahigh doses of irbesartan [abstract no: 1225]. Diabetologia 2010;53(Suppl 1):S484. [EMBASE: 70263264] [Google Scholar]
- Rabbani N, Adaikalakoteswari A, Rossing K, Rossing P, Tarnow L, Parving HH, et al. Effect of irbesartan treatment on plasma and urinary markers of protein damage in patients with type 2 diabetes and microalbuminuria. Amino Acids 2012;42(5):1627-39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rossing K, Schjoedt KJ, Jensen BR, Boomsma F, Parving HH. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney International 2005;68(3):1190-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rossing K, Schjoedt KJ, Parving H. Reduction of plasma N-terminal pro-brain natriuretic peptide by angiotensin II receptor blockade in type 2 diabetic patients with microalbuminuria [abstract no: SA-PO043]. Journal of the American Society of Nephrology 2006;17(Abstracts):585A. [Google Scholar]
- Rossing K, Schjoedt KJ, Schalkwijk CG, Stehouwer CDA, Parving H, Rossing P. Effects of recommended and ultra high doses of irbesartan on biomarkers of inflammation and endothelial dysfunction in type 2 diabetic patients with microalbuminuria [abstract no: SA-PO1040]. Journal of the American Society of Nephrology 2007;18(Abstracts):572A. [Google Scholar]
Saka 2004 {published data only}
- Saka S, Yildiz N, Yildiz M, Orhon A, Isgüven P, Bakirci N, et al. Short-term comparison on antiproteinuric effects of losartan and enalapril in children with insulin-dependent diabetes mellitus and nephropathy [abstract no: OPFC47]. Pediatric Nephrology 2004;19(9):C86. [Google Scholar]
Salem 2021 {published data only}
- Salem M, Sallam AM, Abdel-Aleem E, El-Mesallamy HO. Effect of lisinopril and verapamil on angiopoietin 2 and endostatin in hypertensive diabetic patients with nephropathy: a randomized trial. Hormone & Metabolic Research 2021;53(7):470‐7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sasso 2002 {published data only}
- Sasso FC, Carbonara O, Persico M, Iafusco D, Salvatore T, D'Ambrosio R, et al. Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension: a randomized double-blind placebo-controlled crossover study. Diabetes Care 2002;25(11):1909-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schjoedt 2005a {published data only}
- Schjoedt KJ, Jacobsen P, Rossing K, Boomsma F, Parving HH. Dual blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy: the role of aldosterone. Hormone & Metabolic Research 2005;37 Suppl 1:4-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schjoedt 2009a {published data only}
- Nielsen SE, Schjoedt KJ, Astrup AS, Tarnow L, Lajer M, Hansen PR, et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabetic Medicine 2010;27(10):1144-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schjoedt KJ, Astrup AS, Persson F, Frandsen E, Boomsma F, Rossing K, et al. Optimal dose of lisinopril for renoprotection in type 1 diabetic patients with diabetic nephropathy: a randomised crossover trial. Diabetologia 2009;52(1):46-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schjoedt KJ, Astrup AS, Persson F, Parving H, Rossing P. Optimal dose of lisinopril for renoprotection in type 1 diabetic patients with diabetic nephropathy [abstract no: SA-PO1041]. Journal of the American Society of Nephrology 2007;18(Abstracts):572A. [Google Scholar]
Schmieder 2005 {published data only}
- Fleischmann EH, Klingbeil AU, Schneider MP, Delles C, Schmieder RE. The effect of treatment with ultra high dose candesartan (64mg) on proteinuria: Interim results of a double-blind, randomized, multi-center study [abstract no: M427]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):133-4. [CENTRAL: CN-00445352] [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Delles C, Veelken R. Additive effect of ultra high dose (64 mg) candesartan on proteinuria: a double blind randomized study [abstract no: W-PO20003]. Nephrology 2005;10(Suppl 1):A244. [CENTRAL: CN-00602110] [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Delles C, Veelken R. Dose-response effect of angiotensin receptor blocker candesartan on proteinuria: a double blind, randomized study [abstract no: SP149]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v69. [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Veelken R, Delles C. Additional antiproteinuric effect of ultra high dose (64mg) candesartan: a double blind, randomized study [abstract no: F-P0243]. Journal of the American Society of Nephrology 2004;15(Oct):119A. [CENTRAL: CN-00602112] [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Veelken R, Delles C. Additional antiproteinuric effect of ultrahigh dose candesartan: a double-blind, randomized, prospective study. Journal of the American Society of Nephrology 2005;16(10):3038-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Seher 2017 {published data only}
- Seher S, Iqbal A, Irum T. Comparing the effectiveness of lisinopril and losartan potassium in treatment of microalbuminurea in newly diagnosed type II diabetes mellitus. Medical Forum Monthly 2017;28(8):63-6. [EMBASE: 618953685] [Google Scholar]
Shand 2002 {published data only}
- Shand B. Comparative study of the effects of enalapril and losartan on glomerular size selectivity in patients with proteinuria with and without diabetes [abstract]. Australian & New Zealand Journal of Medicine 1999;29:596. [CENTRAL: CN-00319649] [Google Scholar]
- Shand BI, Lewis JG, Elder PA, Agarwal M. Antihypertensive treatment and glomerular size selectivity in hypertensive patients with renal parenchymal disease and proteinuria. Clinical Nephrology 2002;58(4):321-4. [MEDLINE: ] [PubMed] [Google Scholar]
- Shand BI, McGregor DO, Agarwal M, Lynn KL, Lewis J, Elder PA. Comparative study of the effects of enalapril and losartan on glomerular size selectivity in patients with proteinuria [abstract no: 22]. Nephrology 2000;5(1-2 Suppl):A8. [CENTRAL: CN-00319649] [Google Scholar]
SHIELD 2003 {published data only}
- Bakris GL, Weir MR. Achieving goal blood pressure in patients with type 2 diabetes: conventional versus fixed-dose combination approaches. Journal of Clinical Hypertension 2003;5(3):202-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MR, Bakris MD, SHIELD (Study of Hypertension and the Efficacy of Lotrel in Diabetes). Benefits of BP control using ACE/CCB in type 2 diabetics [abstract no: 91]. American Journal of Kidney Diseases 2002;39(4):A33. [CENTRAL: CN-00403065] [Google Scholar]
Shu 1997 {published data only}
- Shu G, Yin D, et al. Clinical observation on benazepril treatment of non-insulin-dependent diabetes mellitus patients with diabetic nephropathy [abstract no: P1220]. Nephrology 1997;3(Suppl 1):S378. [CENTRAL: CN-0191223] [Google Scholar]
Song 2003 {published data only}
- Kim M, Song JH, Lee SW, Lee SY, Park GH. Renoprotective effect of dual blockade of renin-angiotensin system by the combination of ACE inhibitor and ATII receptor antagonist in hypertensive IgA nephropathy [abstract no: SU-FC071]. Journal of the American Society of Nephrology 2004;15(Oct):59A. [CENTRAL: CN-00626045] [Google Scholar]
- Song JH, Lee SW, Suh JH, Kim ES, Hong SB, Kim KA, et al. The effects of dual blockade of the renin-angiotensin system on urinary protein and transforming growth factor-beta excretion in 2 groups of patients with IgA and diabetic nephropathy. Clinical Nephrology 2003;60(5):318-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2004a {published data only}
- Song JH, Lee SY, Park GH, Lee SW, Kim M. Effects of combination therapy of ACE inhibitor and ATII receptor blocker on proteinuria and renal TGF-b production in Type II overt diabetic nephropathy: a comparison with doubling dose of single therapies [abstract no: SU-PO154]. Journal of the American Society of Nephrology 2004;15(Oct):566A. [Google Scholar]
Song 2006 {published data only}
- Song JH, Cha SH, Lee HJ, Lee SW, Park GH, Lee SW, et al. Effect of low-dose dual blockade of renin-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney disease. Nephrology Dialysis Transplantation 2006;21(3):683-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Song JH, Lee SW, Kim M, Park GH, Lee SW. Renoprotective benefits of low-dose combination of ACE inhibitor and ATII receptor blocker in advanced stage of overt diabetic nephropathy [abstract no: M-PO20056]. Nephrology 2005;10(Suppl):A30. [Google Scholar]
Suehiro 2021 {published data only}
- Suehiro T, Tsuruya K, Yoshida H, Tsujikawa H, Yamada S, Tanaka S, et al. Stronger effect of azilsartan on reduction of proteinuria compared to candesartan in patients with CKD: a randomized crossover trial. Kidney & Blood Pressure Research 2021;46(2):173‐84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Suzuki 2011 {published data only}
- Suzuki K, Aizawa Y. Evaluation of dosing time-related anti-hypertensive efficacy of valsartan in patients with type 2 diabetes. Clinical & Experimental Hypertension (New York) 2011;33(1):56-62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2010 {published data only}
- Tan F, Mukherjee JJ, Lee KO, Lim P, Liew CF. Dual blockade of the renin-angiotensin-aldosterone system is safe and effective in reducing albuminuria in Asian type 2 diabetic patients with nephropathy. Singapore Medical Journal 2010;51(2):151-6. [MEDLINE: ] [PubMed] [Google Scholar]
TRENDY 2007 {published data only}
- Schmieder RE, Delles C, Mimran A, Fauvel JP, Ruilope L. Effects of telmisartan versus ramipril on endothelium function of the renal vasculature in Type 2 - diabetes [abstract no: M-PO20027]. Nephrology 2005;10(Suppl 1):A22-3. [CENTRAL: CN-00740460] [Google Scholar]
- Schmieder RE, Delles C, Mimran A, Fauvel JP, Ruilope LM. Impact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetes. Diabetes Care 2007;30(6):1351-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Delles CC, Mimran A, Fauvel JP, Ruilope L. Effects of telmisartan versus ramipril on endothelium function of the renal vasculature in type 2 diabetes [abstract no: MP127]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v242-3. [CENTRAL: CN-00740460] [Google Scholar]
- Schmieder RE, Schlaich MP, Mimran A, Fauvel JP, Ruilope L. Pathogenetic factors of atherosclerosis are disparately influenced by telmisartan and ramipril in Type 2 diabetes [abstract no: MO005]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv289. [CENTRAL: CN-00740458] [Google Scholar]
- Schmieder RE, Schlaich MP, Mimran A, Fauvel JP, Ruilope LM. Pathogenetic factors of atherosclerosis are disparately influenced by telmisartan and ramipril in type 2 diabetes [abstract no: TH-FC151]. Journal of the American Society of Nephrology 2006;17(Abstracts):33A. [CENTRAL: CN-00740458] [Google Scholar]
- TRENDY: Telmisartan versus ramipril in renal endothelial dysfunction. The Protection Trial Programme - Micardis Protection 2005.
Turab 2008 {published data only}
- Turab SM, Mashori GR, Naqvi A, Rabbi SF. Proteinuria a target for reno-protection in hypertensive patients with type 2 diabetic nephropathy. Medical Forum Monthly 2008;19(9):4-8. [EMBASE: 354488777] [Google Scholar]
van Nieuwenhoven 2004 {published data only}
- Nieuwenhoven FA, Rossing K, Andersen S, Oliver N, Goldschmeding R. Beneficial effect of dual blockade of the renin-angiotensin system (RAS) on urinary connective tissue growth factor (CTGF) in Type 2 diabetic patients with nephropathy [abstract no: SU-PO180]. Journal of the American Society of Nephrology 2004;15(Oct):571A. [CENTRAL: CN-00583815] [Google Scholar]
Vongterapak 1998 {published data only}
- Vongterapak S, Dahlan W, Nakasatien S, Anuntakulnatee T, Suppanich M, Kittipoom W, et al. Impediment of the progressions of microalbuminuria and hyperlipidemia in normotensive type 2 diabetes by low-dose ramipril. Journal of the Medical Association of Thailand 1998 Sep;81(9):671-81. [MEDLINE: ] [PubMed] [Google Scholar]
Wiegmann 1992 {published data only}
- Wiegmann TB, Herron KG, Chonko AM, MacDougall ML, Moore WV. Effect of angiotensin-converting enzyme inhibition on renal function and albuminuria in normotensive type I diabetic patients. Diabetes 1992;41(1):62-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wouda 2021 {published data only}
- Wouda RD, Waanders F, Zeeuw D, Navis G, Vogt L. Diminished antiproteinuric effect of the angiotensin receptor blocker losartan during high potassium intake in patients with CKD. Clinical Kidney Journal 2021;14(10):2170‐6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zandbergen 2003 {published data only}
- Zandbergen AA, Baggen MG, Lamberts SW, Bootsma AH, Zeeuw D, Ouwendijk RJ. Effect of losartan on microalbuminuria in normotensive patients with type 2 diabetes mellitus. A randomized clinical trial. Annals of Internal Medicine 2003;139(2):90-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zandbergen AA, Lamberts SW, Baggen MG, Janssen JA, Boersma E, Bootsma AH. The IGF-I system and the renal and haemodynamic effects of losartan in normotensive patients with type 2 diabetes mellitus: a randomized clinical trial. Clinical Endocrinology 2006;64(2):203-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2019c {published data only}
- Zhang X, Yan Y, Shi J, Ma T, Hu C. Clinical observation of ACEI combined with ARB for treatment of diabetic nephropathy in elderly patients. International Journal of Clinical & Experimental Medicine 2019;12(6):7630-6. [EMBASE: 2002255351] [Google Scholar]
References to studies awaiting assessment
Andrysiak‐Mamos 1997 {published data only}
- Andrysiak-Mamos E, Majkowska L, Pynka S, Czekalski S. The reduction in 24 h blood pressure and diminution of microalbuminuria in incipient diabetic nephropathy treated with ACEI during 2 years [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A90. [CENTRAL: CN-00509146] [Google Scholar]
Limonte 2024 {published data only}
- Limonte CP, Prince DK, Hoofnagle AN, Galecki A, Hirsch IB, Tian F, et al. Associations of biomarkers of tubular injury and inflammation with biopsy features in type 1 diabetes. Clinical Journal of the American Society of Nephrology: CJASN 2024;19(1):44-55. [CENTRAL: CN-00509146] [DOI: 10.2215/CJN.0000000000000333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vijay 2000 {published data only}
- Vijay V, Jayaraman M, Snehalatha C, Shina K, Ramachandran A. Progression of persistent microalbuminuria in hypertensive versus normotensive type 2 diabetic patients: a follow-up study [abstract no: CNO18]. Indian Journal of Nephrology 2000;10:108. [Google Scholar]
Vineela 2023 {published data only}
- Vineela KN, Kodudula S, Sindu P, Srisangeetha G. A comparative study on the pleiotropic effects of Olmesartan and Telmisartan in hypertensive patients with type 2 diabetes mellitus: a randomized, prospective, open labelled, hospital based study. Journal of Krishna Institute of Medical Sciences University 2023;12(3):113‐21. [EMBASE: 2026550529] [Google Scholar]
References to ongoing studies
CTRI/2023/05/052593 {published data only}
- Dual RAAS blockage in primary glomerular diseases. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/05/052593 (registration date: 12 May 2023). [Google Scholar]
NCT05753696 {published data only}
- Azilsartan in patients with diabetic kidney disease and hypertension [The effect of azilsartan in patients with diabetic kidney disease and hypertension]. https://clinicaltrials.gov/show/NCT05753696 (date first posted: 3 March 2023).
TCTR20220426002 {published data only}
- Comparison of efficacy in renoprotection between Azilsartan medoxomil and Enalapril: a randomized, open-labeled controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20220426002 (registration date: 26 April 2022).
Additional references
Arendshorst 1999
- Arendshorst WJ, Brannstrom K, Ruan X. Actions of angiotensin II on the renal microvasculature. Journal of the American Society of Nephrology : JASN 1999;10 Suppl 11:S149-61. [PMID: ] [PubMed] [Google Scholar]
Bonino 2019
- Bonino B, Leoncini G, De Cosmo S, Greco E, Russo GT, Giandalia A, et al. Antihypertensive treatment in diabetic kidney disease: the need for a patient-centered approach. Medicina (Kaunas, Lithuania) 2019;55(7):382. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2014
- Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Internal Medicine 2014;174(5):773-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coleman 2019
- Coleman CI, Weeda ER, Kharat A, Bookhart B, Baker WL. Impact of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on renal and mortality outcomes in people with Type 2 diabetes and proteinuria. Diabetic Medicine 2019;37(1):44-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
CREDENCE 2019
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine 2019;380(24):2295-306. [PMID: ] [DOI] [PubMed] [Google Scholar]
DAPA‐CKD 2021
- Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes & Endocrinology 2021;9(1):22-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Georgianos 2018
- Georgianos PI, Agarwal R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney International 2018;93(2):325-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Go 2004
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine 2004;351(13):1296-305. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviewsof Interventions version 6.3 (updated February 2022), Cochrane, 2022. Available from www.training.cochrane.org/handbook.
JNC 8 2014
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) [Erratum in: JAMA. 2014 May 7;311(17):1809]. JAMA 2014;311(5):507-20. [DOI: 10.1001/jama.2013.284427] [PMID: ] [DOI] [PubMed] [Google Scholar]
Joshi 2021
- Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart 2021;107(13):1032-8. [EMBASE: 634351712] [DOI] [PMC free article] [PubMed] [Google Scholar]
KDIGO Blood Pressure Guidelines 2021
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney International Supplement 2021;99(1):S1-87. [DOI: 10.1016/j.kint.2020.11.003] [DOI] [Google Scholar]
Lv 2012
- Lv J, Neal B, Ehteshami P, Ninomiya T, Woodward M, Rodgers A, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS Medicine 2012;9(8):e1001293. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lv 2012a
- Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, Strippoli GF. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD004136. [DOI: 10.1002/14651858.CD004136.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Messerli 2018
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? Journal of the American College of Cardiology 2018;71(13):1474-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mogensen 1995
- Mogensen CE. Microalbuminuria in prediction and prevention of diabetic nephropathy in insulin-dependent diabetes mellitus patients. Journal of Diabetes & its Complications 1995;9(4):337-49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mogensen 1999
- Mogensen CE. Drug treatment for hypertensive patients in special situations: diabetes and hypertension. Clinical & Experimental Hypertension (New York) 1999;21(5-6):895-906. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NKF KDOQI Guidelines 2004
- K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. Guideline 11: use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in CKD. https://kidneyfoundation.cachefly.net/professionals/KDOQI/guidelines_bp/guide_11.htm (date accessed: 4 March 2024).
Ritter 2011
- Ritter JM. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers in hypertension. BMJ 2011;342:d1673. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ritz 1999
- Ritz E, Ortho SR. Nephropathy in patients with type 2 diabetes mellitus. New England Journal of Medicine 1999;341(15):1127-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available fromwww.training.cochrane.org/handbook.
SONG
- Standardised Outcomes in Nephrology. https://songinitiative.org/ (accessed: 4 March 2024).
Stringer 2013
- Stringer S, Sharma P, Dutton M, Jesky M, Ng K, Kaur O, et al. The natural history of, and risk factors for, progressive chronic kidney disease (CKD): the renal impairment in secondary care (RIISC) study; rationale and protocol. BMC Nephrology 2013;14:95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabriziani 2018
- Tabriziani H, Lipkowitz MS, Vuong NR. Chronic kidney disease, kidney transplantation and oxidative stress: a new look to successful kidney transplantation. Clinical Kidney Journal 2018;11(1):130–5. [EMBASE: 621480394] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaidya 2022
- Vaidya SR, Aeddula NR. Chronic kidney disease. StatPearls 2022. [https://www.ncbi.nlm.nih.gov/books/NBK535404/] [PubMed] [Google Scholar]
Wang 2018
- Wang K, Hu J, Luo T, Wang Y, Yang S, Qing H, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney & Blood Pressure Research 2018;43(3):768-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yuan 2017
- Yuan CM, Nee R, Ceckowski KA, Knight KR, Abbott KC. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clinical Kidney Journal 2017;10(2):257–62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2020
- Zhang Y, He D, Zhang W, Xing Y, Guo Y, Wang F, et al. ACE inhibitor benefit to kidney and cardiovascular outcomes for patients with non‑dialysis chronic kidney disease stages 3–5: a network meta‑analysis of randomised clinical trial. Drugs 2020;80(8):797–811. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Strippoli 2004
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004;329(7470):828-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strippoli 2006
- Strippoli GFM, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD006257. [DOI: 10.1002/14651858.CD006257] [DOI] [PMC free article] [PubMed] [Google Scholar]


