Key Points
Question
Is patient participation in cancer drug trials associated with a survival benefit?
Findings
In this systematic review and meta-analysis of 39 studies (85 comparisons), participation in trials by patients with cancer was associated with greater survival benefit compared with routine care (hazard ratio [HR], 0.76). However, survival benefit was not significantly greater when only high-quality studies were pooled (HR, 0.9) or when the sample was adjusted for possible publication bias (HR, 0.94).
Meaning
After accounting for biases and confounders, cancer clinical trial participation was not associated with a survival benefit.
Abstract
Importance
Many cancer clinical investigators view clinical trials as offering better care for patients than routine clinical care. However, definitive evidence of clinical benefit from trial participation (hereafter referred to as the participation effect) has yet to emerge.
Objective
To conduct a systematic review and meta-analysis of the evidence examining whether patient participation in cancer trials was associated with greater survival benefit compared with routine care.
Data Sources
Studies were found through PubMed and Embase (January 1, 2000, until August 31, 2022), as well as backward and forward citation searching.
Study Selection
Studies were included that compared overall survival of trial participants and routine care patients.
Data Extraction and Synthesis
Data extraction and methodological quality assessment were completed by 2 independent coders using Covidence software. Data were pooled using a random-effects model and analyzed based on the quality of the comparison between trial participants and routine care patients (ie, extent to which studies controlled for bias and confounders).
Main Outcomes and Measures
The hazard ratio (HR) for overall survival of trial participants vs routine care patients.
Results
Thirty-nine publications were included, comprising 85 comparisons of trial participants and routine care patients. The meta-analysis revealed a statistically significant overall survival benefit for trial participants (HR, 0.76 [95% CI, 0.69-0.82]) when all studies were pooled, regardless of design or quality. However, survival benefits diminished in study subsets that matched trial participants and routine care patients for eligibility criteria (HR, 0.85 [95% CI, 0.75-0.97]) and disappeared when only high-quality studies were pooled (HR, 0.91 [95% CI, 0.80-1.05]). They also disappeared when estimates were adjusted for potential publication bias (HR, 0.94 [95% CI, 0.86-1.03]).
Conclusions and Relevance
Many studies suggest a survival benefit for cancer trial participants. However, these benefits were not detected in studies using designs addressing important sources of bias and confounding. Pooled results of high-quality studies are not consistent with a beneficial effect of trial participation on its own.
This systematic review and meta-analysis examines whether patient participation in cancer trials is associated with greater survival benefit compared with routine care.
Introduction
Many people believe that patients achieve better clinical outcomes because of participation in clinical trials.1,2,3,4,5,6,7,8 This phenomenon is commonly called the trial effect, which is often attributed to closer monitoring and access to new treatments in trials. However, the assertion that trials confer medical benefits for participants rests on observational methodologies that are notoriously prone to bias and confounding.1,2,3,4,9,10,11 These observational methodologies are precisely the forms of evidence that motivate the conduct of rigorous trials.
Two reviews of cancer clinical trials based mostly on studies published in the 1990s found inconclusive evidence that participation in trials confers survival benefits.1,5 Two other major systematic reviews in other disease areas have been published,12,13 and neither showed evidence that patients in trials have better outcomes than patients outside of trials.
Numerous reports supporting a trial effect have been published since these reviews.14,15,16,17,18,19,20 Changes in trial practices since the 1990s, such as improved patient monitoring or greater inclusivity, might influence survival benefit for trial participation. Recent studies also vary in the extent to which they control for factors that might produce a spurious suggestion of survival benefit. For example, survival benefits associated with trial participation may reflect the confounding influence of trials selecting for patients with better prognoses, rather than the benefits of closer monitoring.
The primary aim of this study was to conduct a systematic review and meta-analysis of the evidence examining whether patient participation in cancer trials (hereafter referred to as trial participants) was associated with greater survival benefit when compared with patients who did not participate in trials (hereafter referred to as routine care patients), taking into consideration various sources of bias and confounding. The study assessed pooled overall survival based on subgroups related to quality of the comparison between trial participants and routine care patients (eg, accounting for trial eligibility, treatment effect, prognostic confounders) to explore design features associated with detection of survival benefits in trial participants.
Methods
Theoretical Framework
We defined treatment effect as the effects of trial participation on outcomes that are mediated by assignment to the experimental intervention in the trial. We defined participation effect as the effects of trial participation that are not mediated by assignment to the experimental intervention in the trial. We refer to the combination of these 2 as the trial effect, which includes all outcome differences between trial participants and routine care patients that are attributable to trial participation (eTable 1 and eTable 2 in Supplement 1). The present study is primarily aimed at isolating the participation effect, which we defined as outcome differences (eg, in overall survival time) attributed to trial participation that are unrelated to receiving an investigational intervention and not the result of confounding or measurement errors. Participation effects in trials might arise because of better management of medication adverse effects or improved medication adherence.
Search Strategy and Selection Criteria
We performed searches of PubMed and Embase (for articles published until August 31, 2022) for studies comparing survival outcomes for trial participants and routine care patients in cancer, limiting our search to studies published on or after January 1, 2000. We limited our search to 2000 and onward because previous systematic reviews have included older publications.5,13 Also, older studies are not representative of the current landscape of clinical trials and often lack the detailed information needed for the present systematic review. Reference lists of found articles were also searched for eligible articles. The following keywords and variants were used to identify relevant articles: cancer, oncology, clinical trial, retrospective cohort, trial participation, trial effect, participation bias, nonparticipant, and nontrial (full list provided in eTable 3 in Supplement 1). Because the literature on trial effect uses different terminologies, we also conducted backward citation searches (searching publications cited in publications we found) and forward citation searches (searching publications that cited publications we found).
Publications were first screened based on titles and abstracts and then full texts were screened by 2 authors (R.I. and H.M.) for studies meeting the following inclusion criteria: (1) use of hazard ratios (HRs) to compare overall survival in a group of trial participants to a group of routine care patients (regardless of whether trial participant groups derived from a randomized trial), (2) treatment includes a drug/biologic, and (3) studies were conducted in patients with cancer. We excluded publications that were (1) focused on trials examining surgical procedures or indirect interventions (eg, programs); (2)commentaries, editorials, letters, or other nonresearch articles; and (3) non–English-language studies (eTable 4 in Supplement 1). Screening was completed using Covidence software.21 Disagreements were resolved through consensus.
Data Extraction
We extracted information from all studies for the following domains: patient demographics, treatment characteristics, and various quality items deemed important for adjustment when measuring participation effects (see below). We also extracted overall survival HRs for trial participants and routine care patients (receiving the same treatment, if available), regardless of whether the former derived from a treatment or control arm. When multiple HRs were available for a comparison, we extracted the HR that reflected the most adjustments for quality factors. Following extraction, study authors were contacted for missing information. All studies were extracted by 2 independent coders with disagreements reconciled by discussion.
Quality scoring in meta-analyses of observational studies using standard methods (eg, ROBINS-I) involves conceptual and practical difficulties22,23,24 and is not customized to discern factors that specifically bear on estimating trial and participation effects. Also, some items in such tools involve many different components that are likely to be at play with routine care patient groups (eg, bias in selection of participants into studies might reflect differences in eligibility, prognostic factors, or consent in the trial participant and routine care patient groups). Instead, we began our study by creating a directed acyclic graph (eFigure 1 in Supplement 1) to identify factors that could cause a participation effect as well as factors that might confound or bias estimates thereof. Sixteen factors associated with confounding or bias were identified. From this, we created a 16-point scoring scale, assigning 1 quality point for each factor addressed in the primary study (eTable 5 in Supplement 1). Of note, some primary studies were not focused on measuring participation effects; our scale reflected quality with respect to estimating participation effects, not the primary objectives of original studies. A leave-one-out meta-analysis of the quality factors is included in eTable 6 in Supplement 1. Quality factors included differences between trial participants and routine care patients in cancer treatment, eligibility criteria, timeframe, demographics (eg, age, sex, race and ethnicity), and medical history (comorbidities, cancer stage, histology, performance status, and line of treatment). To examine the impact of quality scores on estimates of participation effects, we categorized primary studies into 3 similarly sized subgroups of low (≤6 points in our score), medium (7 points), and high (≥8 points) quality levels. Additional post hoc analyses include grouping primary studies into 2 and 5 quality score subgroups and are reported in eFigures 2 and 3 in Supplement 1.
Statistical Analysis
Because studies in our sample involved different indications and drugs, a standard meta-analysis would not be possible. Instead, meta-analytic methods were used to explore the impacts of quality factors (eg, accounting for treatment and eligibility) and general study characteristics (eg, sponsorship, study location) on pooled effects and heterogeneity.25 For this, a DerSimonian and Laird random-effects model was used to pool survival hazard ratios of trial participants vs routine care patients.26 This model is appropriate when pooling estimates from heterogeneous studies employing multiple designs and cancer types. All statistical analyses were completed using R version 4.3.0 (R Foundation).27 We used funnel plots, Begg28 and Egger29 tests, and the trim-and-fill method30 to explore potential publication biases.
This systematic review followed the PRISMA reporting guidelines (eTable 7 in Supplement 1).31 A protocol was preregistered on Open Science Framework (https://osf.io/b7h9w).32 Other information, including the dataset and codebook, are available on Open Science Framework.
Results
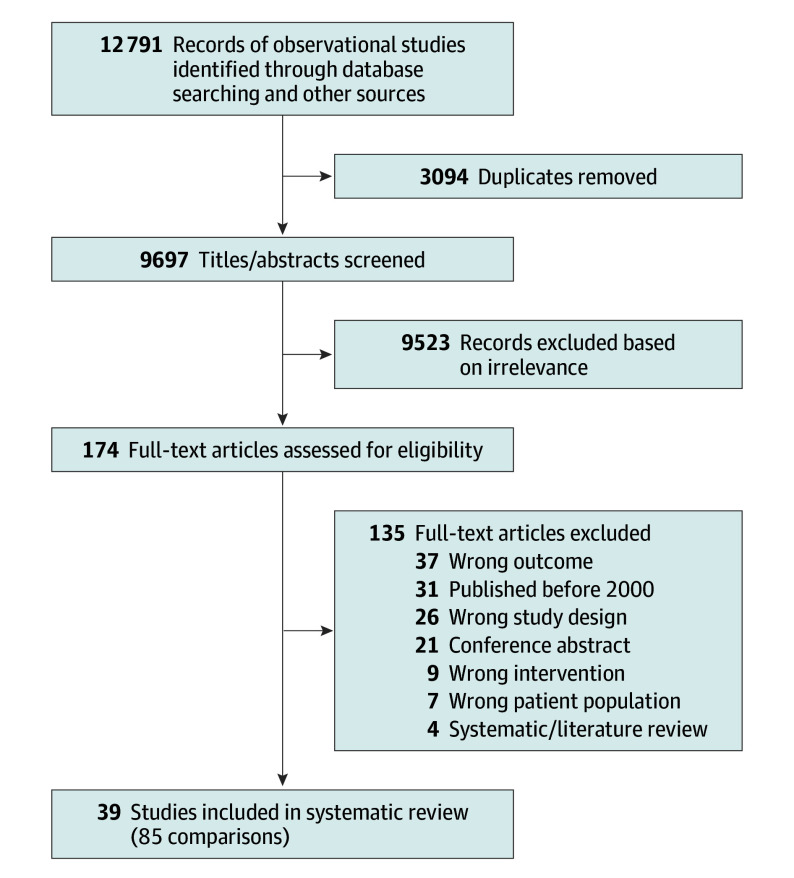
Following screening, 39 studies (85 total comparisons) were eligible for inclusion in the meta-analysis (Figure 1). Of these studies, 32 comparisons comparing trial participants and routine care patients aimed to measure the trial effect. The median sample sizes for the trial participant and routine care patient groups were 209 and 409 patients, respectively (Table 1). Characteristics of individual publications are available in eTable 8 and quality scores of individual studies are available in eTable 9 in Supplement 1. Examples of studies included in the sample are provided in the Box,33,34,35,36,37 along with an explanation of their quality.
Figure 1. Selection of Studies in the Systematic Review and Meta-Analysis.
Thirty-nine studies were included, which involved 85 comparisons.
Table 1. Study Characteristics of 85 Included Comparisons.
| Study characteristic | No. (%)a |
|---|---|
| Year of publication | |
| 2000-2005 | 2 (2) |
| 2006-2011 | 7 (8) |
| 2012-2017 | 33 (39) |
| 2018-2022 | 43 (51) |
| Study location | |
| North America | 64 (75) |
| Asia | 9 (11) |
| Europe | 9 (11) |
| Australia | 3 (4) |
| Cancer | |
| Hematologic | 18 (21) |
| Breast | 14 (16) |
| Lung | 12 (14) |
| CNS | 6 (7) |
| Prostate | 6 (7) |
| Melanoma | 5 (6) |
| Pancreatic | 4 (5) |
| Otherb | 20 (24) |
| Advanced/metastatic patients | 28 (33) |
| Patient populationc | |
| Adult | 75 (89) |
| Pediatric | 5 (6) |
| Mixed | 4 (5) |
| Routine care group institution source | |
| Single institution | 21 (25) |
| Multiple institutions | 64 (75) |
| Routine care group data source | |
| Registry | 67 (79) |
| Medical records | 18 (21) |
| Sample size, median (IQR) | |
| Trial participants | 209 (96-397) |
| Routine care patients | 409 (173-1523) |
| Age, median (IQR), yc,d | |
| Trial participants | 59 (53.3-62.0) |
| Routine care patients | 62 (57-67) |
| Male sex, median (IQR), %c,e | |
| Trial participants | 57 (0-70.5) |
| Routine care patients | 54.5 (0-67.5) |
| Female sex, median (IQR), %c,e | |
| Trial participants | 42 (28.5-99.0) |
| Routine care patients | 44.5 (32.5-100.0) |
Abbreviation: CNS, central nervous system.
Percentages may not add to 100 due to rounding.
Other cancer types included bladder, cervical, colorectal, esophageal, gastric, head and neck, kidney, ovarian, and mixed solid tumors.
Excludes studies with missing data.
Unweighted medians of reported means or medians.
Unweighted medians of reported percentages.
Box. Examples of Included Studies and Results.
Abdel-Rahman (2019)37 compared survival of patients with localized prostate cancer treated in clinical trials and patients registered in the Surveillance, Epidemiology, and End Results Program database. This study was deemed high quality: it accounted for age, sex, race and ethnicity, comorbidities, stage, performance status, line of treatment, treatment, eligibility, and timeframe. The study did not show evidence of a survival difference between patients treated in a clinical trial (n = 397) vs routine care (n = 1718) (hazard ratio [HR], 0.79 [95% CI, 0.45-1.39]; quality score = 12 points).
Tanai et al (2011)36 compared survival of patients with unresectable or recurrent gastric cancer treated with chemotherapy in trials and patients who were offered trial participation using medical records. This study was deemed high quality: it accounted for age, sex, race and ethnicity, stage, histology, performance status, line of treatment, treatment, eligibility, timeframe, and data source for patient groups. They found no evidence of a survival difference in patients treated in a clinical trial (n = 190) vs patients treated in routine care who refused trial participation (n = 96) (HR, 0.83 [95% CI, 0.62-1.10]; quality score = 12 points).
Le Du et al (2016)35 was scored as medium quality. It compared survival of patients with breast cancer in and out of clinical trials using medical records, and it accounted for age, sex, race and ethnicity, comorbidities, line of treatment, eligibility, timeframe, data source for patient groups, but not performance status, histology, stage, or treatment. They found no evidence of a survival difference in patients treated in a clinical trial (n = 285) vs patients treated in routine care (n = 367) (HR, 0.89 [95% CI, 0.72-1.10]; quality score = 7 points).
Elumalai et al (2022)34 compared survival of patients treated with docetaxel for metastatic castration-resistant prostate cancer in and out of clinical trials using medical records. This study was low quality with respect to estimation of participation effects. It accounted for sex, performance status, treatment, and timeframe, but not for factors including age, race and ethnicity, comorbidities, stage, histology, line of treatment, eligibility, or data source for patient groups. They found a survival benefit for trial participants (n = 2070) compared with routine care patients (n = 178) (HR, 0.57 [95% CI, 0.48-0.68]; quality score = 3 points).
Mayers et al (2001)33 compared survival of patients with breast carcinoma who participated in a clinical trial with those who did not using medical records. This study was low quality with respect to estimation of participation effects. It accounted for sex, stage, treatment, timeframe, data source for patient groups, but not for factors including age, race and ethnicity, comorbidities, histology, performance status, line of treatment, or eligibility. They reported a survival benefit for trial participants (n = 160) compared with routine care patients (n = 519), but results were not statistically significant (HR, 0.77 [95% CI, 0.57-1.05]; quality score = 3 points).
The original pooled HR for all included studies without regard to quality subgroups was 0.76 (95% CI, 0.69-0.82), suggesting a statistically significant survival benefit for trial participants in the highly heterogeneous sample (I2 = 88%; Figure 2). When studies were grouped according to their overall quality score based on susceptibility to bias or confounding, the lowest scoring group reported the largest survival benefit for trial participants (HR, 0.64 [95% CI, 0.58-0.72]) and high heterogeneity (I2 = 83%). The intermediate group reported results between the low and high groups (HR, 0.85 [95% CI, 0.73-0.98]; I2 = 53%), and the highest scoring group reported no significant survival benefit (HR, 0.91 [95% CI, 0.80-1.05]) and showed high heterogeneity (I2 = 89%) (Figure 2). Other groupings by quality produced consistent trends toward null estimates with greater quality (eFigures 2 and 3 in Supplement 1).
Figure 2. Pooled Overall Survival by Quality Adjustment Scores.
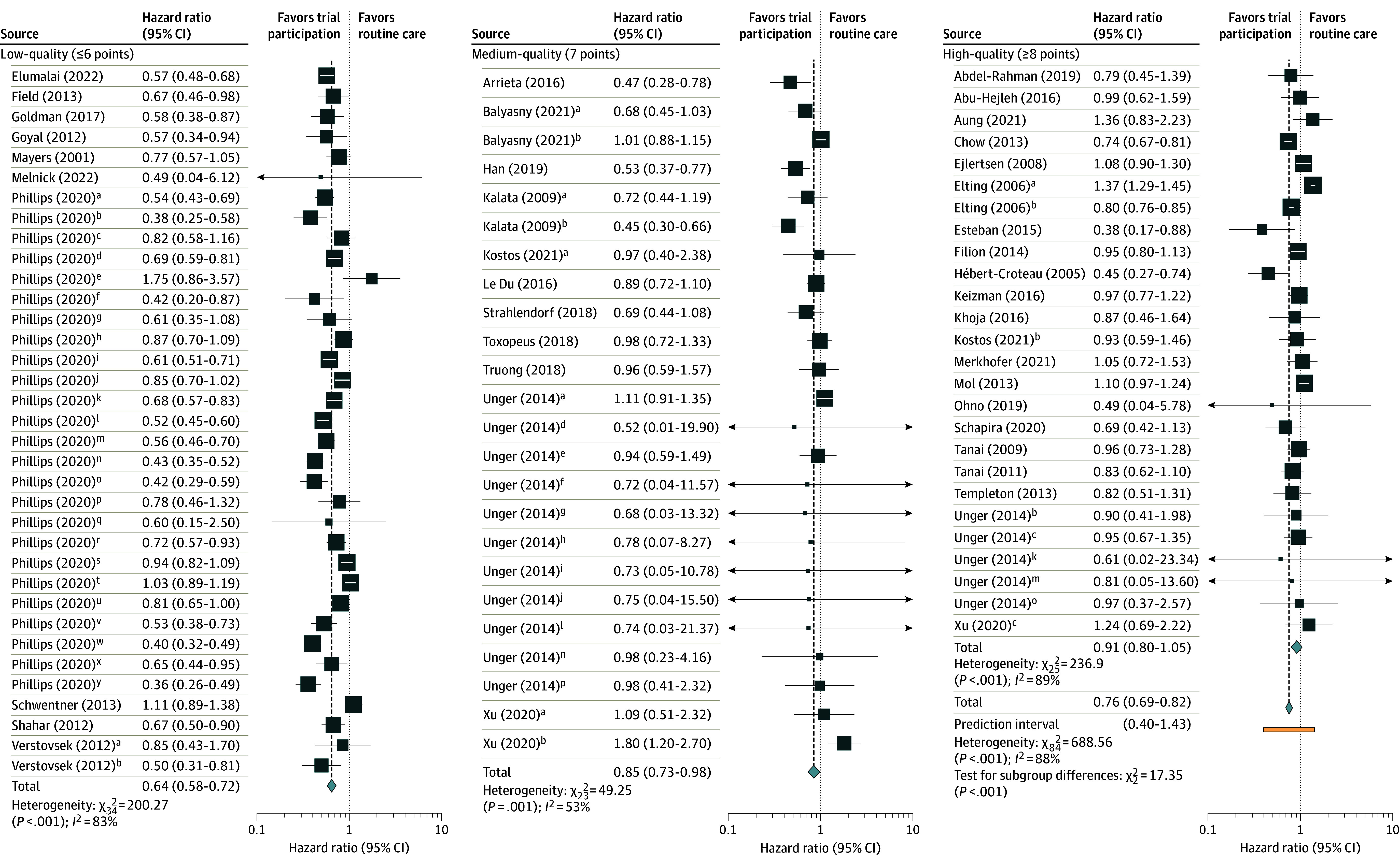
All studies are listed in the References list at the end of this article. Quality was assessed on a 16-point scale with higher scores indicating less potential for confounding or bias. Studies were classified as low-quality (≤6 points), medium-quality (7 points), and high-quality (≥8 points). See eTable 5 in Supplement 1 for factors used in scoring and eTable 9 in Supplement 1 for quality scores of individual studies. Some studies had multiple comparisons and, to differentiate them, superscript letters are noted by each comparison. The distinctions can be found in eTable 8 in Supplement 1.
Significant evidence of publication bias was found using 2 of the 3 methods deployed. A funnel plot (eFigure 4 in Supplement 1) and Egger test (P = .007) suggested possible publication bias against studies failing to show a participation effect. The Begg test did not show publication bias (P = .48). The trim-and-fill method added 30 comparisons to the 85 (n = 115) with a random effects pooled HR regressing to 0.94 (95% CI, 0.86-1.03).
All studies matched trial participants and routine care patients by cancer type, but varied in the extent to which they addressed other confounders and biases. This might explain the observed survival benefit for trial participants in the overall pooled estimate (Table 2). Overall, high heterogeneity is a possible indication of studies that were lower-quality for estimating participation effects. For instance, studies that lacked information on whether trial participants and routine care patients were matched on trial eligibility were the most heterogeneous and had the strongest effects (HR, 0.76 [95% CI, 0.63-0.92]; Q = 266.5), whereas studies known to have matched on trial eligibility had less heterogeneity and a higher HR (0.85 [95% CI, 0.75-0.97]; Q = 37.0). Similarly, studies that did not report whether they included individuals who chose not to participate in the trial were included in the routine care group (Q = 653.9) were also highly heterogeneous. In all cases but 1 (line of treatment), studies that accounted for prognostic confounders (another set of quality factors) produced smaller estimates of survival benefit for trial participants.
Table 2. Results of Subgroup Analyses of Comparisons by Various Characteristics That Indicate Quality of Comparison in Publications.
| Characteristics | No. of comparisons | Pooled HR random model | Heterogeneity | Significance P valuea | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | Q | Q P value | |||
| All comparisons | 85 | 0.76 (0.69-0.82) | <.001 | 688.6 | <.001 | NA |
| Patient characteristics | ||||||
| Accounts for age | 53 | 0.85 (0.77-0.93) | <.001 | 301.7 | <.001 | .002 |
| Does not account for age | 32 | 0.66 (0.59-0.75) | <.001 | 204.2 | <.001 | |
| Accounts for sex | 57 | 0.85 (0.77-0.93) | <.001 | 348.0 | <.001 | <.001 |
| Does not account for sex | 28 | 0.64 (0.56-0.72) | <.001 | 173.7 | <.001 | |
| Accounts for race/ethnicity | 42 | 0.89 (0.79-1.00) | .056 | 276.4 | <.001 | <.001 |
| Does not account for race and ethnicity | 43 | 0.68 (0.61-0.75) | <.001 | 268.0 | <.001 | |
| Accounts for comorbidities | 14 | 0.81 (0.66-1.00) | .05 | 214.7 | <.001 | .40 |
| Does not account for comorbidities | 71 | 0.74 (0.68-0.81) | <.001 | 349.9 | <.001 | |
| Accounts for stage | 47 | 0.87 (0.78-0.97) | .01 | 291.1 | <.001 | <.001 |
| Does not account for stage | 38 | 0.67 (0.60-0.74) | <.001 | 217.9 | <.001 | |
| Accounts for histology | 34 | 0.94 (0.82-1.07) | .33 | 246.5 | <.001 | <.001 |
| Does not account for histology | 51 | 0.68 (0.62-0.75) | <.001 | 286.2 | <.001 | |
| Accounts for performance status | 23 | 0.80 (0.68-0.94) | .01 | 256.1 | <.001 | .39 |
| Does not account for performance status | 62 | 0.74 (0.67-0.81) | <.001 | 320.2 | <.001 | |
| Accounts for line of treatment | 43 | 0.72 (0.64-0.82) | <.001 | 575.0 | <.001 | .15 |
| Does not account for line of treatment | 42 | 0.81 (0.73-0.90) | <.001 | 112.0 | <.001 | |
| Accounts for trial eligibility | 17 | 0.85 (0.75-0.97) | .01 | 37.0 | .002 | .17 |
| Does not account for trial eligibility | 52 | 0.73 (0.66-0.81) | <.001 | 244.1 | <.001 | |
| Missing data on trial eligibility | 16 | 0.76 (0.63-0.92) | .004 | 266.5 | <.001 | |
| Routine care group does not include trial refusers | 2 | 0.96 (0.73-1.26) | .76 | 0.1 | .74 | .004 |
| Routine care group includes trial refusers | 14 | 0.93 (0.83-1.04) | .18 | 19.2 | .12 | |
| Missing data on the inclusion of trial refusers | 69 | 0.73 (0.66-0.81) | <.001 | 653.9 | <.001 | |
| Treatment characteristics | ||||||
| Accounts for treatment effect (same treatments) | 45 | 0.69 (0.62-0.77) | <.001 | 291.8 | <.001 | .01 |
| Does not account for treatment effect (different treatments) | 30 | 0.87 (0.76-0.99) | .04 | 58.3 | <.001 | |
| Missing data on treatment effect | 10 | 0.88 (0.69-1.11) | .28 | 191.8 | <.001 | |
| Setting characteristics | ||||||
| Similar time period for comparison groups | 59 | 0.80 (0.72-0.88) | <.001 | 460.1 | <.001 | .08 |
| Different time period for comparison groups | 26 | 0.69 (0.60-0.78) | <.001 | 149.8 | <.001 | |
| Compares routine care group to a single trialb | 54 | 0.73 (0.65-0.82) | <.001 | 301.4 | <.001 | .32 |
| Compares routine care group to multiple trialsb | 31 | 0.79 (0.70-0.90) | <.001 | 322.4 | <.001 | |
| Other characteristics | ||||||
| Routine care group data source is registries | 67 | 0.75 (0.67-0.82) | <.001 | 632.8 | <.001 | .42 |
| Routine care group data source is medical records | 18 | 0.80 (0.70-0.91) | .001 | 53.7 | <.001 | |
| Accounts for the same trial and routine care group sourcesc | 29 | 0.82 (0.74-0.90) | <.001 | 70.8 | <.001 | .18 |
| Does not account for the same trial and routine care group sourcesc | 56 | 0.74 (0.65-0.83) | <.001 | 617.3 | <.001 | |
| Trial sample size of ≥200 | 48 | 0.75 (0.67-0.84) | <.001 | 613.1 | <.001 | .73 |
| Trial sample size of <200 | 37 | 0.77 (0.69-0.85) | <.001 | 69.1 | <.001 | |
Shows whether there is a significant difference between items in each subgroup (eg, in the first category, the P value of .002 shows that there is a statistically significant difference in survival estimates for comparisons that did vs did not account for age.
Studies that compared routine care patient groups to the same trial only included 1 trial in the trial group, whereas comparing to multiple trials included more than 1 trial in the trial participant group.
Studies accounting for the same trial and routine care sources indicated that both groups were from the same source. For example, the Surveillance, Epidemiology, and End Results (SEER) Program covers all the US, so it is not the same location, but if both trial and routine care groups are from the SEER database, then they accounted for similar group sources.
Subgroup analyses based on different study characteristics were also conducted, without regard for quality indicators. Comparisons from studies in the US (n = 31) showed an HR of 0.87 (95% CI, 0.75-1.02), while those conducted in other countries (n = 54) had an HR of 0.72 (95% CI, 0.65-0.79) (eTable 10 in Supplement 1).
Discussion
In this systematic review and meta-analysis of 39 studies comparing outcomes among patients with cancer participating in clinical trials vs those receiving routine care, we found that typical pooled analyses suggest that trial participation is associated with greater survival benefit. However, survival benefits for participation diminish or disappear in studies that account for various sources of bias and confounding. Accounting for factors such as eligibility and some prognostic confounders reduced the magnitude of overall participation effects. For example, no statistically significant trial effect was observed for studies that accounted for comorbidities, histology, or race and ethnicity. When a subgroup analysis was conducted based on quality score to isolate participation effects, there was a greater apparent survival benefit in low-quality studies than high-quality studies. The high-quality studies made more efforts to reduce heterogeneity between trial participants and routine care patients.
Because preregistries are not widely used for observational studies, there are no direct ways to test for publication bias. However, indirect statistical tests suggest a disproportionate number of small studies indicating large survival benefits for trial participants, which is consistent with publication bias. Statistically correcting for potential publication bias produced an HR that was inconsistent with a significant survival benefit for trial participants.
Previous systematic reviews, including those focused on cancer, have not detected clear evidence that patients in randomized trials have better outcomes than patients outside them.1,5,9,12,13 Despite this, many factors may contribute to the perception that patients have better outcomes in trials. One is that participants in trials often experience better care processes, including more frequent imaging. Another is the notorious efficacy-effectiveness gap. Trials often show survival outcomes that exceed those in clinical care.38,39 Indeed, the literature on efficacy-effectiveness gaps complements our findings: prognostic variables such as performance status that appear to explain better outcomes in trials, when adjusted for in comparisons of trial participants and routine care patients, diminish apparent participation effects. A third factor is the regular publication of studies, similar to many in the current sample, that suggest a participation effect but do not account for relevant confounders. Similar to prior studies,1,5 the current study suggests that methodological rigor and quality continues to present a challenge for some publications asserting estimates of the participation effect.
This study leaves many questions about participation effects unresolved. Some studies suggest that patients have improved quality of life, greater cost savings, or benefit from incidental findings when participating in trials.40,41,42 Other studies have suggested that patients have worse quality of life outcomes.43,44 The current protocol set out to measure such benefits. However, outcomes such as quality of life or anxiety are often not measured in trials, much less in routine care patients. It was not possible to identify a critical mass of studies on patient-reported outcomes to include in the meta-analysis. The analysis also does not address whether participation effects might occur with interventions involving greater skill with administration (eg, surgical procedures).
Limitations
This meta-analysis has limitations. First, many studies in the review were missing data or provided unclear information leading to exclusions and/or difficulties in interpreting studies. Authors were contacted to mitigate these difficulties. Due to incomplete descriptions on how trial participants and routine care patients were matched, it was also difficult to determine how well quality factors were implemented. For example, treatments were often very poorly described and therefore treatment similarity was not always clear for studies claiming to have matched treatments between groups. Second, the scale used for measuring study quality was created expressly for this study. Although the scoring scale was systematic and reflected sources of bias for trial effect studies, scales with different sets of factors, factor prioritization with weighted scores, or score cut-offs might produce findings that differ from those reported above. However, the main purpose of stratifying by quality was to explore relationships between low adjustment and detection of participation effects.
Third, as previously noted,1 the absence of standard terminologies makes it extremely difficult to conduct literature searches for primary reports of the participation effect. It cannot be ruled out that some reports were not captured in the search. Fourth, the study provides a picture of participation benefit associated with current research practices. The possibility that other trial approaches might produce different results cannot be excluded. For example, trials often select for patients that have fewer comorbidities or better performance status. Participation effects might be more manifest where trials relax such criteria. Fifth, any attempt to study the benefits of trial participation is subject to the fact that there is no random assignment among trial participants and routine care patients. Even ideally designed studies may be confounded, and such confounders could either mask or amplify participation effect estimates. The best that can be concluded is that participation effect estimates grow narrower the more analyses account for various confounders. It cannot be ruled out that benefits of participation might be revealed were it possible to conduct a randomized trial testing the benefits of participating in a randomized trial.
Conclusions
In this meta-analysis, evidence that cancer trial participation results in survival benefits was mostly driven by studies that do not account for factors that could bias or confound such estimates. When analysis was restricted to studies that account for such factors (eg, eligibility, prognostic confounders), effects regress toward a null effect. These findings may strike some trial advocates as discouraging, given how hard they work to improve patient outcomes within trials. However, a more reassuring interpretation is that there is no evidence that excluding patients from trials due to geography, nonavailability of trials in their condition, or ineligibility deprives them of survival opportunities.
Educational Objective: To identify the key insights or developments described in this article.
-
The authors refer to a phenomenon called the “trial effect.” What is the trial effect?
An increase in adverse events beyond what might otherwise be anticipated, likely on account of regular assessment throughout the trial period.
Improved outcomes for both placebo and treatment groups due to systematic exclusion of sicker patients who may not be able to complete therapy.
The belief that participation in clinical trials yields better outcomes, potentially on account of closer monitoring and access to new treatments.
-
What were the results of this meta-analysis of 85 comparisons across 39 studies?
High-quality studies demonstrated a statistically meaningful survival benefit (hazard ratio, 0.64), while low-quality studies did not (hazard ratio, 0.91).
The original pooled hazard ratio for all included studies was 0.76, suggesting a statistically significant survival benefit for those participating in a clinical trial.
Trial participants experienced more adverse events, including incidental mortality, than patients undergoing routine care.
-
How do the authors interpret their findings?
Evidence for a trial effect is mostly driven by studies that did not adequately account for factors that could bias or confound results.
That trial participation resulted in survival benefit suggests the need to augment routine care processes to more closely resemble the care provided as part of clinical trials.
While there was evidence of publication bias, statistical correction for publication bias continued to yield a meaningful survival benefit for trial participants.
eResults
Data sharing statement
References
- 1.Braunholtz DA, Edwards SJL, Lilford RJ. Are randomized clinical trials good for us (in the short term)? evidence for a “trial effect”. J Clin Epidemiol. 2001;54(3):217-224. doi: 10.1016/S0895-4356(00)00305-X [DOI] [PubMed] [Google Scholar]
- 2.Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. Published online September 4, 2015. doi: 10.1136/bmj.h4672 [DOI] [PubMed] [Google Scholar]
- 3.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267-277. doi: 10.1016/j.jclinepi.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menezes P. Trial effect: the road from efficacy to effectiveness. Clin Investig (Lond). 2012;2(5):443-445. doi: 10.4155/cli.12.34 [DOI] [Google Scholar]
- 5.Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363(9405):263-270. doi: 10.1016/S0140-6736(03)15383-4 [DOI] [PubMed] [Google Scholar]
- 6.Engelbak Nielsen Z, Eriksson S, Schram Harsløf LB, et al. Are cancer patients better off if they participate in clinical trials? a mixed methods study. BMC Cancer. 2020;20(1):401. doi: 10.1186/s12885-020-06916-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godskesen TE, Petri S, Eriksson S, Halkoaho A, Mangset M, Nielsen ZE. The culture of hope and ethical challenges in clinical trials: a qualitative study of oncologists and haematologists’ views. Clin Ethics. 2020;15(1):29-38. doi: 10.1177/1477750919897379 [DOI] [Google Scholar]
- 8.Godskesen TE, Petri S, Eriksson S, et al. When nursing care and clinical trials coincide: a qualitative study of the views of nordic oncology and hematology nurses on ethical work challenges. J Empir Res Hum Res Ethics. 2018;13(5):475-485. doi: 10.1177/1556264618783555 [DOI] [PubMed] [Google Scholar]
- 9.Bouzalmate-Hajjaj A, Massó Guijarro P, Khan KS, Bueno-Cavanillas A, Cano-Ibáñez N. Benefits of participation in clinical trials: an umbrella review. Int J Environ Res Public Health. 2022;19(22):15368. doi: 10.3390/ijerph192215368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lantos JD. The “inclusion benefit” in clinical trials. J Pediatr. 1999;134(2):130-131. doi: 10.1016/S0022-3476(99)70400-2 [DOI] [PubMed] [Google Scholar]
- 11.Joffe S. Framing the benefits of cancer clinical trials. Arch Pediatr Adolesc Med. 2010;164(3):293-294. doi: 10.1001/archpediatrics.2009.293 [DOI] [PubMed] [Google Scholar]
- 12.Fernandes N, Bryant D, Griffith L, et al. Outcomes for patients with the same disease treated inside and outside of randomized trials: a systematic review and meta-analysis. CMAJ. 2014;186(16):E596-E609. doi: 10.1503/cmaj.131693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Published online July 16, 2008. Cochrane Database of Syst Rev. doi: 10.1002/14651858.MR000009.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrieta O, Carmona A, Ramírez-Tirado LA, et al. Survival of patients with advanced non-small cell lung cancer enrolled in clinical trials. Oncology. 2016;91(4):185-193. doi: 10.1159/000447404 [DOI] [PubMed] [Google Scholar]
- 15.Esteban D, Tovar N, Jiménez R, et al. Patients with relapsed/refractory chronic lymphocytic leukaemia may benefit from inclusion in clinical trials irrespective of the therapy received: a case-control retrospective nalysis. Blood Cancer J. 2015;5(10):e356-e356. doi: 10.1038/bcj.2015.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman C, Tchack J, Robinson EM, et al. Outcomes in melanoma patients treated with BRAF/MEK-directed therapy or immune checkpoint inhibition stratified by clinical trial versus standard of care. Oncology. 2017;93(3):164-176. doi: 10.1159/000475715 [DOI] [PubMed] [Google Scholar]
- 17.Han JJ, Kim JW, Suh KJ, et al. Clinical characteristics and outcomes of patients enrolled in clinical trials compared with those of patients outside clinical trials in advanced gastric cancer. Asia Pac J Clin Oncol. 2019;15(3):158-165. doi: 10.1111/ajco.13145 [DOI] [PubMed] [Google Scholar]
- 18.Melnick KF, Miller P, Carmichael E, et al. The trial effect in patients with glioblastoma: effect of clinical trial enrollment on overall survival. J Neurooncol. 2022;159(2):479-484. doi: 10.1007/s11060-022-04083-8 [DOI] [PubMed] [Google Scholar]
- 19.Ohno S, Mukai H, Narui K, et al. Participants in a randomized controlled trial had longer overall survival than non-participants: a prospective cohort study. Breast Cancer Res Treat. 2019;176(3):631-635. doi: 10.1007/s10549-019-05276-y [DOI] [PubMed] [Google Scholar]
- 20.Strahlendorf C, Pole JD, Barber R, et al. Enrolling children with acute lymphoblastic leukaemia on a clinical trial improves event-free survival: a population-based study. Br J Cancer. 2018;118(5):744-749. doi: 10.1038/bjc.2017.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covidence systematic review software. Accessed May 1, 2024. http://www.covidence.org
- 22.West S, King V, Carey T, et al. Systems to rate the strength of scientific evidence. In: AHRQ Evidence Report Summaries. Vol 47. Agency for Healthcare Research and Quality; 2002:1-11. [PMC free article] [PubMed] [Google Scholar]
- 23.Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054-1060. doi: 10.1001/jama.282.11.1054 [DOI] [PubMed] [Google Scholar]
- 24.Igelström E, Campbell M, Craig P, Katikireddi SV. Cochrane’s risk of bias tool for non-randomized studies (ROBINS-I) is frequently misapplied: a methodological systematic review. J Clin Epidemiol. 2021;140:22-32. doi: 10.1016/j.jclinepi.2021.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao G, Lopez-Jimenez F, Boyd J, et al. ; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council . Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American Heart Association. Circulation. 2017;136(10):e172-e194. doi: 10.1161/CIR.0000000000000523 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT; Cochrane Collaboration , ed. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Wiley-Blackwell; 2020. [Google Scholar]
- 27.R Core Team . R: A Language and Environment for Statistical Computing. Published online 2023. https://www.R-project.org/
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Published online March 29, 2021. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.OSF Home. Measuring participation effects on survival in cancer clinical trials: a systematic review and meta-analysis. Updated February 1, 2024. Accessed May 1, 2024. http://OSF.IO/KEUBC
- 33.Mayers C, Panzarella T, Tannock IF. Analysis of the prognostic effects of inclusion in a clinical trial and of myelosuppression on survival after adjuvant chemotherapy for breast carcinoma. Cancer. 2001;91(12):2246-2257. [PubMed] [Google Scholar]
- 34.Elumalai T, Barker C, Elliott T, et al. Translation of Prognostic and pharmacodynamic biomarkers from trial to non-trial patients with metastatic castration-resistant prostate cancer treated with docetaxel. Clin Oncol. 2022;34(7):e291-e297. doi: 10.1016/j.clon.2022.01.040 [DOI] [PubMed] [Google Scholar]
- 35.Le Du F, Fujii T, Park M, et al. Impact of clinical trial on survival outcomes. Breast Cancer Res Treat. 2016;159(2):273-281. doi: 10.1007/s10549-016-3942-5 [DOI] [PubMed] [Google Scholar]
- 36.Tanai C, Nakajima TE, Nagashima K, et al. Characteristics and outcomes of patients with advanced gastric cancer who declined to participate in a randomized clinical chemotherapy trial. J Oncol Pract. 2011;7(3):148-153. doi: 10.1200/JOP.2010.000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Rahman O. Comparison of outcomes of radiotherapy-treated localized prostate cancer patients within a clinical trial setting versus real-life setting. Future Oncol. 2019;15(11):1269-1277. doi: 10.2217/fon-2018-0539 [DOI] [PubMed] [Google Scholar]
- 38.Boyle JM, Hegarty G, Frampton C, et al. Real-world outcomes associated with new cancer medicines approved by the Food and Drug Administration and European Medicines Agency: A retrospective cohort study. Eur J Cancer. 2021;155:136-144. doi: 10.1016/j.ejca.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Templeton AJ, Booth CM, Tannock IF. Informing Patients About Expected Outcomes: The Efficacy-Effectiveness Gap. J Clin Oncol. 2020;38(15):1651-1654. doi: 10.1200/JCO.19.02035 [DOI] [PubMed] [Google Scholar]
- 40.McGrath-Lone L, Ward H, Schoenborn C, Day S. The effects of cancer research participation on patient experience: a mixed-methods analysis. Eur J Cancer Care (Engl). 2016;25(6):1056-1064. doi: 10.1111/ecc.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandelblatt JS, Makgoeng SB, Luta G, et al. A planned, prospective comparison of short-term quality of life outcomes among older patients with breast cancer treated with standard chemotherapy in a randomized clinical trial vs. an observational study: CALGB #49907 and #369901. J Geriatr Oncol. 2013;4(4):353-361. doi: 10.1016/j.jgo.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Julian-Reynier C, Genève J, Dalenc F, et al. ; Patients’ Committee for Clinical Trials of the Ligue Nationale Contre le Cancer . Assessment of care by breast cancer patients participating or not participating in a randomized controlled trial: a report with the Patients’ Committee for Clinical Trials of the Ligue Nationale Contre le Cancer. J Clin Oncol. 2007;25(21):3038-3044. doi: 10.1200/JCO.2006.08.9367 [DOI] [PubMed] [Google Scholar]
- 43.Thompson CA, Hugo SE, Swetz KM, et al. End-of-life care in a population-based cohort of cancer patients: clinical trial participation versus standard of care. BMJ Support Palliat Care. 2013;3(2):181-187. doi: 10.1136/bmjspcare-2012-000295 [DOI] [PubMed] [Google Scholar]
- 44.Enzinger AC, Zhang B, Weeks JC, Prigerson HG. Clinical trial participation as part of end-of-life cancer care: associations with medical care and quality of life near death. J Pain Symptom Manage. 2014;47(6):1078-1090. doi: 10.1016/j.jpainsymman.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eResults
Data sharing statement