Abstract
This guideline is the first Iranian guideline developed for the diagnosis, management, and treatment of hyperlipidemia in adults. The members of the guideline developing group (GDG) selected 9 relevant clinical questions and provided recommendations or suggestions to answer them based on the latest scientific evidence. Recommendations include the low-density lipoprotein cholesterol (LDL-C) threshold for starting drug treatment in adults lacking comorbidities was determined to be over 190 mg/dL and the triglyceride (TG) threshold had to be >500 mg/dl. In addition to perform fasting lipid profile tests at the beginning and continuation of treatment, while it was suggested to perform cardiovascular diseases (CVDs) risk assessment using valid Iranian models. Some recommendations were also provided on lifestyle modification as the first therapeutic intervention. Statins were recommended as the first line of drug treatment to reduce LDL-C, and if its level was high despite the maximum allowed or maximum tolerated drug treatment, combined treatment with ezetimibe, proprotein convertase subtilisin/kexin type 9 inhibitors, or bile acid sequestrants was suggested. In adults with hypertriglyceridemia, pharmacotherapy with statin or fibrate was recommended. The target of drug therapy in adults with increased LDL-C without comorbidities and risk factors was considered an LDL-C level of <130 mg/dl, and in adults with increased TG without comorbidities and risk factors, TG levels of <200 mg/dl. In this guideline, specific recommendations and suggestions were provided for the subgroups of the general population, such as those with CVD, stroke, diabetes, chronic kidney disease, elderly, and women.
Keywords: Adult, clinical practice guideline, hyperlipidemia, Iran
INTRODUCTION
Hyperlipidemia is a common metabolic disorder and one of the risk factors for chronic diseases, including cardiovascular diseases (CVDs), caused by eating disorders, obesity, genetic diseases (e.g., familial hypercholesterolemia [FH]), or other diseases (e.g., diabetes).[1] According to the World Health Organization (WHO), hyperlipidemia is significantly associated with more than half of the global cases of ischemic heart disease.[2] Although hyperlipidemia encompasses a wide range of lipid abnormalities, the increase in total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) has attracted the most attention due to its important role in the development of atherosclerosis.[3,4]
The level of plasma lipids in normal people in different societies is different due to genetic and lifestyle differences, including eating habits and physical activities. The average level of TC has been reported to be 202 mg/dL in Western men and 165 mg/dL in Chinese men.[3] The findings of a systematic review that examined lipid components indicated that the prevalence of hyperlipidemia in Iran was significant. The overall prevalence rates of TC ≥200 mg/dL and TC ≥240 mg/dL in adults over 20 years old were 42% and 17%, respectively.[5] The prevention and control of CVD risk factors, including hyperlipidemia, is considered a preliminary measure in primary or secondary prevention and should be a priority for the health-care system to reduce the burden of CVDs.[4] The results of studies show that the management of hyperlipidemia reduces mortality and CVD occurrences by 30%.[3,4,5,6,7,8] The value of reducing TC and LDL-C in the primary and secondary prevention of CVD is evident based on the results of several epidemiological studies.[3]
Hyperlipidemia is often a lifelong but manageable disease. However, if hyperlipidemia is not treated, since it is progressive, it often leads to severe underlying vascular disease processes that can be fatal. Chronic exposure to high levels of serum lipids in early adulthood increases the risk of coronary heart disease (CHD) in a dose-dependent manner.[9]
The incidence of CVD in individuals with LDL-C ≥190 mg/dL, compared to those with LDL-C <130 mg/dL, occurs 10–20 years earlier in men and 20–30 years earlier in women. It has also been found that the risk of atherosclerotic CVD (ASCVD) is more than four times higher in these people (hazard ratio = 4.1, 95% confidence interval).[10]
Considering the difference in the determinants of diseases in different societies, to solve some health problems, it is necessary to adopt multidisciplinary national policies and do local and national planning and policy-making according to the local plans.[11] The management of hyperlipidemia based on accurate and up-to-date clinical guidelines is important and possible. Considering that one of the known obstacles in the management of some diseases in society is the lack of knowledge and awareness of doctors or their failure to follow the instructions for the control and care of this disease,[12] it is necessary to provide them with a set of clinical solutions based on the latest scientific evidence of the world (which naturally causes the least harm and damage to the patients and is based on the health-care structure of the country).
In different countries, different clinical guidelines have been developed for the prevention, control, and treatment of hyperlipidemia[6,13,14,15] that are different from each other in terms of focus or priority area (prevention, diagnosis, and treatment), subject area (all lipid or cholesterol disorders), guideline users, and the target group (children, adults, or special groups).
Due to the lack of Iranian guidelines in many field including hyperlipidemia, most physicians follow the guidelines of other countries or regions. However, due to the differences in the structure and environment of providing health services as well as in the social and economic situation between countries and considering a request by the Ministry of Health of Iran in this regard, the first Iranian guideline to the management, diagnosis, and treatment of hyperlipidemia in adults was developed.
DEFINITIONS
Hyperlipidemia is defined as an increase in the plasma concentration of lipids (triglycerides [TG], TC, and their transporter lipoproteins in the blood; cholesterol, LDL-C, and very LDL-C).[6] In this guideline, due to the importance of LDL-C in the occurrence of atherosclerosis diseases and the high prevalence of TG in Iran, the main focus was on LDL-C and TG.
Severe FH is defined as LDL-C >400 mg/dL (10 mmol/L), LDL-C higher than 310 mg/dL (8 mmol/L) with a risk factor, or LDL-C over 190 mg/dL (5 mmol/L) along with two high-risk factors.[14]
STUDY DESIGN
Participating groups in the developing of guideline
Four groups participated in the development of the Iranian hyperlipidemia guidelines:
Steering committee
This group consisted of the executive, main collaborators, and related officials in the Ministry of Health. It was responsible for selecting the members of other groups and coordinating and supervising the implementation of the process of guideline preparation.
Guideline development group
It consist of 38 experts from universities of medical sciences and related scientific societies in the fields of cardiology, internal medicine, endocrinology, nephrology, neurology, nutrition, epidemiology, pharmacology, health economics, as well as physicians, nurses, and representatives of patients. The members were selected based on their specialty, experience and interest from different parts at the national level. Two methodologists facilitated the guideline developing group (GDG) group sessions.
Systematic review group
This independent group was responsible for developing the evidence following an extensive search in the literature and performing systematic review.
External review group
It consisted of a number of experts in various fields related to the subject, as well as influential people in policy-making in this field, who they extensively evaluated the guideline.
Declaration of interest
To identify the types of conflicts of interest (e.g., finance, work, research, and consultancy), the standard conflict of interest approved by the Ministry of Health of Iran was signed by all members in the groups developing guidelines including steering committee (SC), GDG, systematic review group (SRG), and external review group (ERG) in two stages (at the beginning of phase 1 and phase 3). There were no cases of conflict of interest in the completed forms; however, according to prior planning, it was decided that if any conflict of interest was identified, while maintaining the confidentiality of information, the cases would be managed by the SC and then possible measures, such as exclusion from participation in the group, restriction of participation in discussions, or restriction to vote on the relevant recommendations, would be adopted.
The scope of the guideline
The scope of the guideline defined the main axes of the clinical questions, included the functional area, the target group, and the outcome of the guideline as well as the questions that were supposed to be answered (Population, Intervention, Comparison, and Outcomes [PICOs]). The functional scope of this guideline, according to a consensus through voting by GDG members, was diagnosis, management, and treatment; therefore, this guideline did not deal with prevention and screening.
The target group of the guideline was male and female adults (18 years and older) with hyperlipidemia (we considered high LDL-C or high TG in this guideline). Moreover, subgroups consist of people suffering from type 1 and type 2 diabetes, CVD, chronic kidney disease (CKD), ischemic stroke, FH, fatty liver, as well as the elderly and women (intended to be pregnant, pregnant, and lactating) were considered in the developing of recommendations. To determine and rank the outcomes, the members of the SC prepared an initial list of outcomes related to the diagnosis, management, and treatment of hyperlipidemia suggested by the GDG members and sent it to them through an electronic form to rank each outcome according to its relative importance from one to nine. Afterward, the average score for each outcome, which indicated the relative importance of that outcome, was determined so that the average of 7–9 indicated that the outcome was critical, 4–7 was important, and 3-1 was not important. Based on this, the relative importance of the outcomes was determined and the critical outcomes for the design of PICO questions were identified.
At the next stage, the members of the GDG were asked to present their initial questions regarding the diagnosis, management, and treatment of hyperlipidemia. These questions were reviewed by the SC and a small working group of GDG. After removing irrelevant and repetitive items and merging similar questions, 9 questions remained which were transformed to PICOs. The working group members then created an analytical framework that showed the impact of interventions on intermediate and final outcomes and specified the order of the 9 PICO questions to better visualize them and integrate them along the patient management pathway [Figure 1]. Due to the COVID-19 pandemic and the geographical dispersion of the members, the SC organized online meetings with the members of the GDG. These meetings, which accounted for 9, were held with the purpose of determining the scope of the guidelines and designing the PICO questions. Furthermore, to identify the values and preferences of patients, a session of focus group discussion was held with the participation of 6 patients having various blood lipid disorders. During this session, the participants’ preferences for diagnosis, medicinal and nonmedicinal therapy (i.e., lifestyle), and required training were extracted and considered in the design of PICO questions and preparation of recommendations and suggestions.
Figure 1.
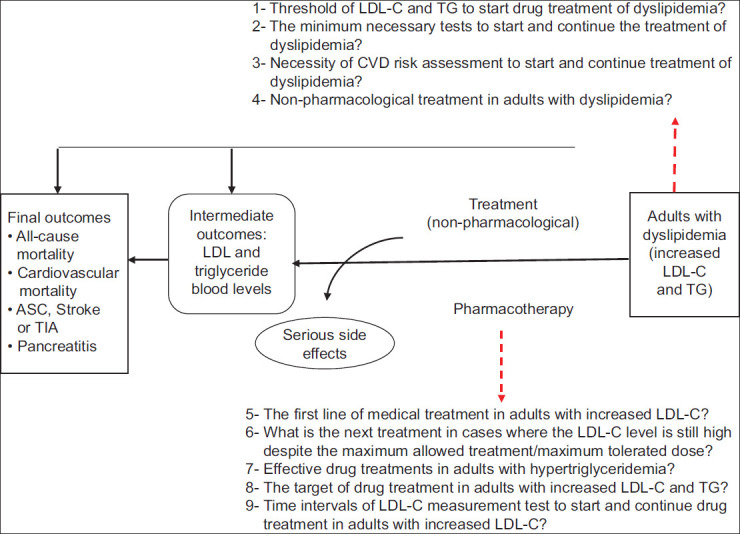
Analytical framework for the management of dyslipidemia in adults. LDL-C = Low-density lipoprotein cholesterol; CVDs = Cardiovascular diseases; TG = Triglyceride
Search for evidence
All PICO questions were submitted to the SRG. The SRG performed a systematic search for each PICO question in PubMed, Embase, and Cochrane Library databases without a time limit. At first, an Umbrella Systematic Review was conducted on all previous systematic reviews. In this way, the latest evidence related to new systematic reviews was found, and since there was no new systematic review for one of the PICO questions, a new one was conducted.
The evidence used in developing the recent hyperlipidemia guidelines was also reviewed. The systematic reviews found were evaluated in terms of their up-to-date, the degree of compliance with the PICO questions, the assessment of their methodology by the AMSTAR tool, the provision of sufficient information to assess the certainty of the evidence, and reporting evidence in the subgroups of the guideline; subsequently, the most appropriate ones were selected. A total of 167 studies, including 97 systematic reviews and meta-analyses, 2 network meta-analyses, 41 clinical trials, were examined.
Turning evidence into decision (evidence-to-decision)
After searching for scientific evidence through a systematic review, certainty in and quality of evidence were rated by the standard GRADE method. In this process, the risk of bias, imprecision, indirectness, and inconsistency were investigated. In addition, another overview was conducted by the SC to examine other decision-making criteria in the framework of the Evidence to Decision tables, and the evidence related to the criteria of patients’ values and preferences, health benefits and harms, resources, costs, acceptability, the feasibility of implementation, and health equity indicators were searched.
Compilation of recommendations and suggestions
To compile the recommendations, 9 two-h online meetings were held with the members of the GDG. In each meeting, a PICO question was introduced and then the evidence found for it was presented in the form of a GRADE evidence profile by the expert who performed the SR and sometimes with the presence of the head of the SRG. Subsequently, the members of the GDG, with the guidance of methodologists, completed the evidence-to-decision tables by reviewing the evidence found regarding the criteria of patients’ values and preferences, the overall certainty of the evidence, health benefits and harms, resources, costs, acceptability by key stakeholders involved, the feasibility of implementing the recommendations according to the different levels of providing health services in Iran, and health equity indicators in the recommendations as well as the agreement. Based on this table, recommendations and suggestions were compiled.
The strength of a recommendation indicated the extent to which the GDG was confident that the desirable effects of the recommendations (e.g., beneficial health outcomes) outweigh the potential undesirable effects (e.g., side effects). The recommendations of this guideline were divided into two groups based on the strength of their supporting evidence.
Recommendation: A recommendation that the GDG was confident that the favorable effects of adhering to it outweigh its adverse effects.
Suggestion: It is a recommendation for which there was greater uncertainty regarding the quality of evidence, the balance of benefits and harms, values and preferences, and the use of resources; however, the GDG concluded that the positive effects of adhering to it probably outweighed the negative effects.
In this guideline, 31 recommendations and 17 suggestions were developed for adults with hyperlipidemia.
External evaluation
The initial report of the guideline was presented to the ERG to evaluate based on the AGREE Reporting Checklist as a guideline evaluation tool to evaluate the draft guideline in terms of validity, reliability, clarity, clinical applicability, clinical flexibility, and documentation. It is worth mentioning that according to the standard procedure, none of the members of the ERG was a member of the GDG. Then, based on their opinions and comments, the guideline was re-examined and some parts were modified.
Update time
The update time for the developed guideline was set for 3 years by the GDG.
Evidence review and recommendations
Low-density lipoprotein cholesterol and triglyceride thresholds for starting drug therapy
| It is recommended that the LDL-C threshold be determined at LDL-C ≥190 mg/dL for the onset of drug treatment in adults with increased LDL-C (who lack comorbidities or risk factors) It is recommended to start pharmacotherapy in patients with CVD, ischemic stroke, diabetes (over 40 years old), chronic kidney failure (eGFR <60 mL/min/1.73 m2), FH, regardless of LDL-C level It is recommended that in people with increased TG, the TG threshold be considered TG ≥500 mg/dL to start drug treatment It is suggested that the threshold of TG be determined at TG ≥200 mg to start drug treatment in the subgroups (diabetes, CVDs, ischemic stroke, FH, and nonalcoholic fatty liver disease) |
LDL-C=Low-density lipoprotein cholesterol; CVD=Cardiovascular disease; TG=Triglyceride; FH=Familial hypercholesterolemia; eGFR=Estimated glomerular filtration rate
Based on evidence, there is a causal relationship between the LDL-C level and the risk of ASCVD development.[16,17,18] Moreover, an increase in plasma TG level has been identified as an independent predictor of CVD risk even in patients who have reached the target of LDL-C treatment with statin therapy. Regarding the adverse effects of lipid-lowering drugs, the evidence showed that the frequency of side effects did not differ between various treatments based on different thresholds [Box 1].[19]
In a systematic review that included 8 clinical trial studies, 30,048 patients were evaluated at the baseline and after a 1-year follow-up in terms of the reduction in LDL-C levels and the incidence of CVDs following statin use. The results showed that following statin use, at LDL-C levels of 125 to <150, there was a 36% reduction in the risk of major cardiovascular events, a 33% decrease in the occurrence of major coronary events, and a 42% decline in the outcome of major cerebrovascular events. In addition, at the 150 to <175 level of LDL-C, a 29% reduction in the risk of major cardiovascular events, a 22% decrease in the occurrence of coronary events, and a 57% decline in the outcome of major cerebrovascular events were observed.[20]
In general, the expected benefits were large and significant without any significant disadvantages. The overall certainty of the evidence was high and the clinical trial studies were of high quality.
Evidence-to-decision considerations
Patients, experts, members of the medical team, and policy-makers all emphasized the importance of starting pharmacological treatment.
From the point of view of patients, blood lipid control is crucial to preventing the complications of hyperlipidemia, such as CVDs. From the perspective of experts and medical team members, although pharmacotherapy is essential to prevent the complications of hyperlipidemia, the patient’s acceptance to start drug therapy would differ based on the patient’s age, knowledge, education, health literacy level, living conditions, socioeconomic status, and level of trust in the doctor.[21] By lowering the threshold to 5 mg in the general population, more people will be exposed to treatment, and therefore, more resources will be needed; however, as the threshold is lowered, the complications are reduced as well. A systematic review on the cost-effectiveness of hyperlipidemia treatment in low- and middle-income countries showed that treating high cholesterol at a threshold of 5.7 mmol/L comes at a higher cost per disability-adjusted life-years averted than at a threshold of 6.2 mmol/L.[5]
Minimum tests required to initiate and continue treatment
| It is recommended for all adults (over 20 years old) with increased LDL-C and TG (in whom the causes of secondary hyperlipidemia have been ruled out) lipid profile tests (LDL-C, TG, TC, HDL-C, AST, and ALT) should be done on fasting at the beginning of treatment and for follow-up It is suggested that if a person’s lipid profile tests (LDL-C, TG, TC, and HDL-C) have been done in the last 6 weeks, they should be considered a baseline test and not repeated It is recommended that for adults with increased LDL-C and TG that are taking statins for the first time, elderly people, or those who have a history of pain or muscle weakness to start and follow up treatment, and for patients who experience pain or muscle weakness during treatment, CK test should be done to follow the treatment |
LDL-C=Low-density lipoprotein cholesterol; TG=Triglyceride; HDL-C=High-density lipoprotein cholesterol; CK=Creatine kinase; AST=Aspartate aminotransferase; ALT=Alanine transaminase; TC=Total cholesterol
In most of the evidence, it is mentioned to measure the serum lipid profile in fasting or nonfasting form, which includes TC, LDL-C, high-density lipoprotein cholesterol (HDL-C), non-HDL-C, and TG; if TG is higher than 400 mg/dL, it is recommended to measure LDL-C directly and on fasting [Box 2].[6,13,22,23]
About 3% of patients under treatment with statins show an elevated level of liver enzymes, including alanine aminotransferase (ALT) and/or aspartate aminotransferase, in the 1st year of medication.[24] This increase is usually mild (<3 times the normal limit) and does not indicate liver damage clinically or histologically in the absence of increased bilirubin or synthetic liver dysfunction.[25,26] Elevated transaminases often return to baseline with statin continuation or adjusting the dose of statin therapy.[27]
Statin-related muscle events are reported as the most common side effects in statin users. Clinical manifestations vary from mild symptoms to muscle damage manifested by marked elevation of creatine kinase (CK) and/or rhabdomyolysis. These events are reversible with prompt discontinuation of statins or dose titration.[28,29,30] To check the adverse effects of statin, it is necessary to perform ALT and CK tests before starting the treatment so that they can be used as a baseline value to start the treatment and follow-up.[27,31] Measuring CK before starting treatment can help to diagnose and determine the severity of muscle damage, and it is reasonable to perform it for people at risk, such as patients using multiple drugs (polypharmacy), patients with proven muscle diseases, or people with high-intensity physical activity.[27] On the other hand, several studies have concluded that an increase in aminotransferase levels caused by statins is rare, and statin users may not be significantly different from normal people.[27,32,33,34]
Evidence-to-decision considerations
Most patients are aware of the importance of conducting tests to start and follow-up treatment; nevertheless, the cost of some tests and the need to repeat the tests to follow up the treatment make it difficult for some people to visit regularly. To reduce these barriers, the panel members suggested that lipid tests performed during 6 weeks before be considered as the first baseline test.
Tests intervals for measuring low-density lipoprotein cholesterol
| It is recommended that to continue drug treatment in adults with increased LDL-C, lipid profile should be measured at intervals of 6–12 weeks until the therapeutic target is reached, and then repeated at intervals of 6–12 months It is recommended that to continue drug treatment in adults with increased LDL-C, liver enzymes should be repeated 3 months after the start of treatment and then 12 months in case of a change in the type or dose of statin or in case of liver symptoms |
LDL-C=Low-density lipoprotein cholesterol
Evidence and rationale
The review of guidelines, systematic review studies, and clinical trials has shown that the results of tests at different time intervals are an important indicator of the success of dyslipidemia treatment.[6,7,8,9,10,35,36,37] Frequent lipid monitoring allows clinicians to assess patient response to therapy and promote adherence, which provides the opportunity for further titration of therapy based on objective criteria. Several cycles of drug titration and reassessment may be necessary to achieve an optimal lipid profile in patients with high hyperlipidemia or in those who respond less well to treatment. Considering the significant variability in the response to treatment with lipid-lowering drugs and the adverse effect of reduced adherence to treatment, repeated lipid testing is necessary to increase the provision of guideline-based medical care.[10,38]
Clinical evidence suggests that different time intervals, including 4–12 weeks after starting statin therapy to determine patient adherence and then every 3–12 months to follow up lipoprotein tests, are clinically effective.[39,40,41,42,43,44,45] Similarly, based on the guidelines of the Task Force of the European Society of Cardiology and the European Atherosclerosis Society for the management of dyslipidemias, it is recommended to repeat the tests every 8 (±4) weeks after adjusting the treatment until the treatment target is reached and then perform them annually.[46] On the other hand, others have argued that performing repeated tests to achieve the treatment target is clinically inefficient, and routine annual tests increase health-care costs, laboratory burden, and false-positive results.[47,48]
Evidence-to-decision considerations
The results of a qualitative study conducted on patients suffering from different types of dyslipidemia showed that although the patients acknowledged the necessity of repeating the tests at certain time intervals to follow the treatment process, they faced some obstacles, such as the high cost of the tests, which affected their adherence.[49]
The required resources (e.g., expert manpower, equipment, and laboratories) to repeat the tests in the mentioned time intervals were evaluated as moderate by the members of the guideline development group.
For primary prevention, annual follow-up tests, which can reduce the risk of CVD by 20%, are more cost-efficient and effective than repeated follow-up tests. For secondary prevention, annual surveillance using a TC threshold of 155 mg/dL or an LDL-C threshold of 77 mg/dL is less expensive and more effective than frequent surveillance, and biannual surveillance is even more cost-effective than annual surveillance.[8] The recommended intervals for repeating the tests are likely to be accepted by the key stakeholders, and it is possible to implement them considering the existing infrastructure of the country.
Cardiovascular disease risk assessment
| It is suggested that to start and continue drug treatment in adults with increased LDL-C and TG, if possible, a CVD risk assessment should be performed It is suggested to use Iranian risk assessment models in case that CVD risk assessment is performed |
LDL-C=Low-density lipoprotein cholesterol; TG=Triglyceride; CVD=Cardiovascular disease
Well-known CVD risk assessment models and charts have been developed and updated over the past five decades, including the Framingham Risk Score,[50] Pooled Cohort Equations recommended by the American College of Cardiology (2013), and the American Heart Association guidelines for the risk assessment of CVDs,[51] as well as the risk prediction models of SCORE,[52] ASSIGN,[53] Q-Risk,[54] PROCAM,[55] and Globorisk.[56]
A collection of evidence shows that risk assessment leads to improved risk management.[57] However, drawing a risk diagram is based on the general outline of risk factors that are distinct in different populations. Consequently, each risk assessment diagram is specific to that population and is unlikely be valid across populations.[32] Iranian models for determining the risk of ASCVDs in 10 years, which were designed based on laboratory and nonlaboratory findings of cohort studies conducted in Iran, i.e. the Persian ASCVD Risk Stratification and simplified Persian ASCVD Risk Stratification,[58,59] are simple and accessible and can be used for risk assessment.
In general, the overall confidence of the evidence found for risk assessment was assessed as moderate. Moreover, no evidence was found regarding the adverse effects of performing a risk assessment to start and continue pharmacotherapy.
Evidence-to-decision considerations
No direct evidence was found on the cost-effectiveness of starting and continuing treatment with or without risk assessment; however, the members of the GDG assessed the cost of conducting risk assessment (e.g. costs related to screening tests, obtaining information on risk factors, capacity building in health system employees to conduct a risk assessment, and care costs) as moderate. According to the WHO, the costs related to performing screening tests and delay in starting treatment due to CVD risk assessment are significant in countries with limited resources. Some evidence suggests that in low-resource countries, adding an additional step before initiating treatment may increase disparities because patients with limited access to health-care services may experience delays in treatment or even fail to receive treatment.[60]
Although conducting a risk assessment is acceptable to key beneficiaries, the possibility of its implementation is limited due to the overcrowding of patients in private offices and government clinics, doctors’ lack of enough time, and the need for more human resources. Time limitation, lack of perceived usefulness, insufficient knowledge, and inconsistency in the published recommendations have been stated as common reasons for physicians not using global risk assessment tools for CVDs.[61] Simplifying these models and empowering educated nursing staff (who are often the first people referred to) to perform risk assessment under the supervision of a doctor can be effective in remedying the current deficiencies in treatment.[62]
Nonpharmacological treatment
| It is recommended that in adults with elevated LDL-C or TG or both (who lack comorbidities or risk factors), lifestyle modification should be considered the first therapeutic intervention, which includes the following It is recommended that they keep their weight in a healthy range and have proper nutrition as follows Eliminate the consumption of trans fatty acids and consume less saturated fatty acids and replace them with unsaturated oils, preferably canola and olive oil Reduce the consumption of red meat and replace it with low-fat white meat, marine sources of protein (e.g., fish), low-fat dairy products, and vegetable proteins (e.g., soy and beans) Reduce the consumption of refined grains (e.g., rice, bread, and white flour) and replace them with whole grains (3 units or more per day) Consume fruits and vegetables (5 units or more) per day Eat nuts at least 5 times a week Reduce the consumption of sweets and products containing sugar and simple sugar It is recommended that adults do at least 150–300 min of moderate-intensity aerobic physical activity, at least 75–150 min of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate-intensity and vigorous-intensity activity during the week Adults are recommended to do 2 or more days of moderate-to-high-intensity muscle-strengthening activities that involve all major muscle groups It is recommended to avoid any type of tobacco (e.g., cigarettes and hookah) and alcohol in any amount, and in case of previous consumption, to quit it It is suggested to start drug treatment if LDL-C and TG levels do not reach the treatment target after at least 6 months from the beginning of lifestyle modification |
LDL-C=Low-density lipoprotein cholesterol; TG=Triglyceride
Changing lifestyles, especially in terms of diet and physical activity, have been mentioned in the evidence to reduce blood lipids.[63,64,65] Evidence shows that high saturated fat intake increases LDL-C concentration. LDL-C is an established risk factor for the development of CVD. A meta-analysis of 26 trials reported that every 1 mmol/L reduction in plasma LDL-C level was associated with a 20% reduction in the risk of CHD-related mortality.[66] There is strong evidence indicating the existence of an association between protective factors, including the consumption of vegetables, nuts, and a high-quality “Mediterranean” diet, and reduced CVD, as well as a relationship between harmful factors, including the consumption of trans fatty acids and high-glycemic index foods, and an increased incidence of CVD. There is moderate-quality evidence regarding the relationship between fish consumption, marine fatty acids, folate, whole grains, dietary Vitamins E and C, beta-carotene, alcohol, fruits, and fiber with decreased incidence of CVD.[63] The results of a network meta-analysis study revealed that all vegetable oils were more effective in reducing cholesterol and LDL-C compared to butter.[67]
Weight loss also affects TC and LDL-C levels, however, minimally. In obese individuals, a decrease of 0.2 mmol/L (8 mg/dL) in LDL-C concentration is observed for every 10 kg of weight loss.[68,69] The reduction in LDL-C levels because of regular physical exercise is even less. The benefits of weight loss and physical exercise are likely to influence other CVD risk factors, particularly hypertension and diabetes. In the case of hypertriglyceridemia, weight loss increases insulin sensitivity and decreases TG levels. Regular physical exercise reduces the level of plasma TG more than the effect of weight loss.[70,71] The consumption of foods rich in carbohydrates and simple sugars has harmful effects on TG levels. On the other hand, a diet rich in fiber and low glycemic in people with hypertriglyceridemia,[72] especially in the case of people with diabetes, plays an important role in improving their condition.[72,73]
Moderate alcohol consumption (≤10 g/day [1 unit] for men and women) is acceptable for those who drink alcohol if TG levels are not elevated. Alcohol consumption has a harmful effect on TG levels, especially in people with hypertriglyceridemia.[73] Quitting smoking has clear benefits on overall CV risk and especially on HDL-C levels.[71]
Evidence-to-decision considerations
The importance of treating dyslipidemia is well-accepted by most patients, healthcare providers, professional societies, and public and private organizations. The results of a qualitative survey regarding the values and preferences of patients showed that they preferred changing their lifestyle over taking medicine and they were willing to receive education regarding nutrition and herbal medicines to control their blood lipids. They emphasized the practicality of the recommendations provided (e.g. for lifestyle) and considered them a guarantee for the implementation of the recommendations[49]
The overall certainty of the evidence found for lifestyle change as the first treatment intervention was high and numerous positive effects were attributed to it.[63,64,65,66] The findings of a network meta-analysis showed that moderate-dose statin and high-intensity exercise were effective in improving arterial stiffness, and that high-intensity exercise interventions could be considered a suitable alternative to moderate-dose statin therapy.[4] Regarding the unfavorable lifestyle and the consumption of alcohol and smoking, the results of studies also reported numerous adverse effects, including an increased risk of mortality due to all causes, specifically CVDs and cancers[74,75]
The GDG felt that health disparities were likely to be reduced with this treatment approach. Considering the nature of this intervention, GDG members underestimated the required costs and resources.
From the point of view of the GDG members, this intervention was acceptable to the key stakeholders and was implementable.
First line pharmacological treatment
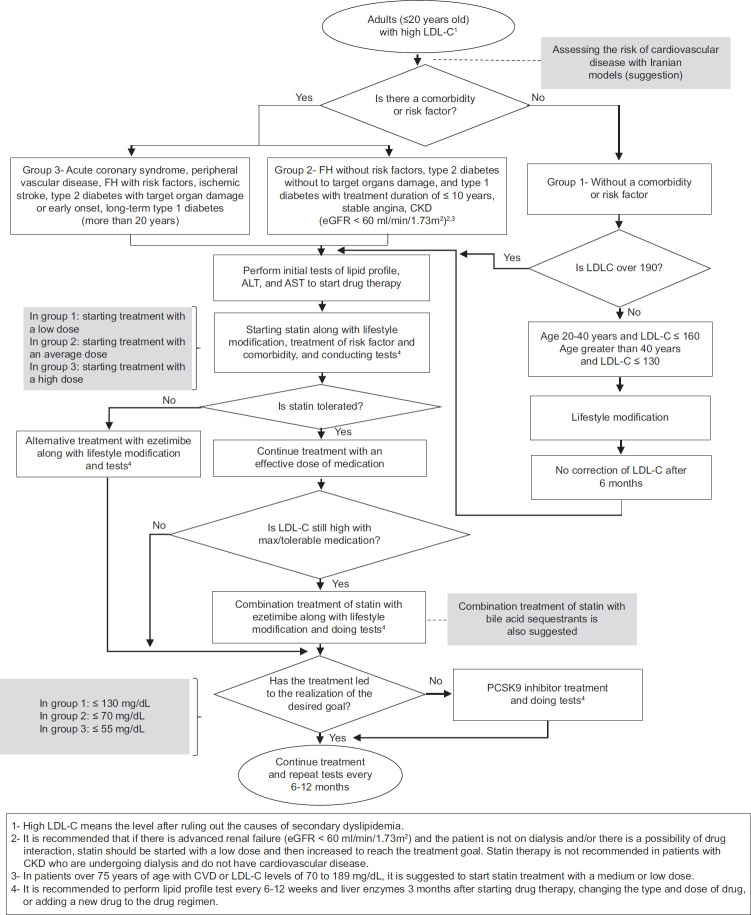
| It is recommended that statins be used as the first line of medical treatment in adults with elevated LDL-C It is recommended to continue following the lifestyle modification recommendations along with drug therapy It is suggested to consider increasing the dose of statin before introducing a combination therapy In women Statins are recommended in women with the same approach and targets as men Statins are not recommended during pregnancy or breastfeeding or when planning for pregnancy During pregnancy or breastfeeding or when planning for pregnancy, it is recommended to use bile acid sequestrants in patients with FH or those who have a severe increase in LDL-C Elderly It is suggested to start statin treatment with a medium or low dose in patients over 75 years of age with CVDs or with LDL-C levels of 70–189 mg/dL It is suggested to stop statin treatment in adults 75 years or older with multiple diseases, reduced physical cognitive function, and decreased life expectancy CVD It is recommended to start or continue high-dose statin therapy as soon as possible in all patients with the ACS or ischemic stroke, regardless of the initial LDL-C level It is recommended to start or continue moderate-dose statin therapy in patients with CVD, in whom high-dose statin therapy is contraindicated or who have experienced statin-related side effects, or who have heart failure due to ischemic heart disease Diabetes Statins with moderate doses are recommended for adults over 40 years of age with type 1 or 2 diabetes In adults with type 2 diabetes who have several major risk factors for CVDs, high-dose statin therapy is recommended It is recommended to use statin with a medium dose in people aged 20–40 years who have long-term diabetes (10 years of type 2 diabetes, 20 years of type 1 diabetes), albuminuria (at least 30 μg albumin/mg creatinine), glomerular filtration rate (eGFR <60 mL/min/1.73 m2), retinopathy, neuropathy, or peripheral vascular disease Severe FH It is recommended to start high-dose statin in people with severe FH Kidney failure It is recommended that patient with an advanced renal failure (eGFR <60 mL/min/1.73 m2) and are not on dialysis or there is a possibility of drug interaction, statin therapy should be started with a low dose and then increased to reach the treatment target Statin therapy is not recommended in patients with CKD who are under or must undergo dialysis |
LDL-C=Low-density lipoprotein cholesterol; CVDs=Cardiovascular diseases; CKD=Chronic kidney disease; FH=Familial hypercholesterolemia; eGFR=Estimated glomerular filtration rate; ACS=Acute coronary syndrome
Drug therapy along with lifestyle modification is necessary to manage hyperlipidemia. Statin should be used as the first line of drug treatment to reduce lipoprotein. Available evidence recommends the use of moderate to high doses of statins.[38,41] The results of a large number of meta-analysis studies on the effects of statins show that by reducing LDL-C levels, the risk of CVDs decreases.[39,40,41,76,77] In a meta-analysis of 26 prospective clinical trial studies with more than 170,000 participants, the results revealed that every 38 mg/dL reduction in LDL-C level decreased major vascular events by 23%, death from the peripheral arterial disease by 20%, stroke by 17%, and overall mortality by 10% during 5 years.[38]
The results of a meta-analysis study (including 27 clinical trials) indicated that neither the relative decline in major vascular events nor coronary artery reconstruction for LDL-C reduction was significantly different between genders.[41] However, statins are contraindicated in pregnant women. In a meta-analysis of 6 studies on pregnant women exposed to statins, no increased risk of birth defects was observed compared to controls; however, the risk of miscarriage was increased.[43] In comparison with healthy women, those with FH are not at increased risk for preterm birth, having low birth weight infants, or congenital malformations; nevertheless, unknown biases cannot be ruled out; therefore, women with homozygous FH should consult an experienced lipid expert so that bile acid sequestrants can be prescribed for them if needed.[44]
In terms of the effectiveness of statin therapy on the elderly, the results of a meta-analysis of 28 clinical trial studies indicated that overall, statin treatment or a more intense statin regimen resulted in a 21% reduction in major vascular events, a 24% decrease in coronary events, a 25% decline in coronary artery reconstruction processes for per 1 mmol/L reduction in LDL-C. Since the elderly may be at higher risk of side effects from statin use and may have less adherence to high-dose therapy, moderate-intensity statin therapy may be preferable.[78]
Most adults over 40 years of age with diabetes are at moderate-to-severe risk of CVDs.[79,80,81] Based on several clinical trial studies, in patients with diabetes, statin treatment significantly reduces the incidence of cardiovascular events.[82,83,84,85] In addition, the results of a meta-analysis study of clinical trials showed that statin treatment with a medium dose in diabetic patients led to a 25% reduction in the risk of CVDs, and this decrease was not different in type 1 and type 2 diabetes patients. Although the results of studies reveal that moderate-dose statin therapy has significant benefits in diabetic patients, the residual risk of statin therapy is still high in these patients (8.5% of cardiovascular events in 3.8 years).[86] Strong evidence shows that the benefits of statin therapy are related to the degree of risk and intensity of treatment.[38]
People with familial or severe hypercholesterolemia (LDL-C equal to or above 190 mg/dL) are at high risk of CVDs[87,88] and early and frequent coronary events.[89] The results of a clinical trial study on 6595 people showed that following the daily intake of 40 mg of pravastatin, the incidence of heart attacks and death due to CVDs decreased in these patients.[90] Moreover, the results of a retrospective cohort study indicated that statin treatment reduced the risk of heart attack, CHD, and death from other causes in patients with FH.[91] Considering that a higher dose of statin causes a greater reduction in the risk of CVDs, in people with familial or severe hypercholesterolemia, statin therapy with the maximum tolerated dose is recommended.[38]
Statin therapy is effective in preventing CHD and stroke in patients with mild to moderate CKD; nonetheless, its effects are unclear in people with more advanced diseases, particularly those on dialysis. The results of a meta-analysis study on 28 clinical trials with 183,419 patients having renal disorders showed that statin treatment reduced the risk of the first major vascular event in these patients by 21% per mmol/L reduction in LDL-C. By reducing eGFR, smaller relative effects on major vascular events were observed.[92]
Regarding the adverse effects of statins, the findings of studies indicate that the use of statins is not associated with an increase in the risk of serious adverse effects, myalgia, or serious liver damage; the risk of diabetes; and cancer.[93,94]
Evidence-to-decision considerations
In a study by Brodney et al., when asked about patients’ preferences for statin therapy after reviewing information related to benefits and personal risks, 45% of participants reported that they would definitely or probably choose statin therapy. With a 10-year increase in the risk of CVD, the percentage of people choosing statin therapy increased from 31% for those with a risk of < 5% to 82.6% for those with a risk of more than 50%. Willingness to use statin treatment was related to increased health literacy and knowledge scores of the participants.[21]
The members of the GDG evaluated the resources and costs required for statin therapy as moderate to low. The results of studies show that statin treatment for patients who are at risk of ASCVD and have an LDL-C ≥ 160 mg/dL can have lifelong health benefits and can save health care costs.[93] It has also been determined that statins are cost-effective in the primary and secondary prevention of ASCVD events in individuals at moderate to high risk or in individuals with LDL ≥ 190 mg/dL.[95]
Since statin drugs in Iran are covered by insurance and are offered at a lower cost in both the public and charitable sectors, it seems that the prescription of statins does not lead to an increase in health inequalities.
Other researchers have found that relatively affluent patients receive more secondary prevention drugs. Patients with higher socioeconomic status and better education may have higher expectations about their health care and are therefore more likely to demand preventive medications. User fees for primary care visits and for prescription drugs also reduce access for poorer people.[96,97,98]
Obstacles mentioned by doctors to prescribe statins in studies include the lack of support resources (impossibility of following up with patients who show resistance to statins), the problem of classifying patients based on risk assessment, inconsistency of dyslipidemia management guidelines for specialists and family doctors, uncertain (doubtful) benefits of statin treatment in certain groups of patients, limited visit time for patients, weak doctor–patient relationship, and the lack of regular patient visits.[99] However, from the point of view of the panel members, statin therapy is accepted by the key stakeholders and it can be implemented considering the country’s infrastructure.
Treatment of elevated low-density lipoprotein cholesterol despite the maximum allowed/tolerated drug treatment
| In adults with increased LDL-C, in whom LDL-C level is still high despite using a maximum allowed treatment or maximum tolerated drug treatment, a combination treatment of statin with ezetimibe is recommended It is recommended to continue following the lifestyle modification recommendations along with drug therapy Treatment in patients with diabetes, FH, ACS (after 4 to 6 weeks of treatment), peripheral arterial disease and ischemic stroke, who have not reached the therapeutic target with the maximum tolerated dose of statin and ezetimibe, a combination with a PCSK9 inhibitor is recommended It is suggested that in adults with increased LDL-C who take statins, if the target is not achieved, statin in combination with bile acid sequestrants should be considered It is suggested that in adults with increased LDL-C, if statin is not tolerated at any dose, ezetimibe should be prescribed alone, and if there is no response or if the target is not reached, a PCSK9 inhibitor should also be added to ezetimibe |
LDL-C=Low-density lipoprotein cholesterol; FH=Familial hypercholesterolemia; ACS=Acute coronary syndrome; PCSK9=Proprotein convertase subtilisin/kexin type 9
Intolerance of statin or failure of patients to reach the treatment target even with the use of a high dose of statin limits the use of high-dose statin in some patients. Due to these limitations, some patients need to add other drug groups to achieve the treatment target. The addition of ezetimibe to a high-dose statin to further reduce LDL-C has been mentioned in some evidence. The results of a meta-analysis study showed that statin and ezetimibe combination therapy resulted in a mean LDL-C reduction of 14% compared to high-dose statin monotherapy,[100] 21%–27% compared to placebo, 11%–15% compared to statin monotherapy, and 13%–20% compared to doubling the dose of statin.[101] The addition of ezetimibe to a statin does not seem to increase the incidence of elevated CK levels more than that with statin therapy alone. Life-threatening liver failure with ezetimibe alone or in combination with statins is very rare.[102]
The results of the IMPROVE-IT clinical trial study, which included 18,144 patients with acute coronary syndrome (ACS), revealed that when the LDL-C level reached 53 mg/dL using the combination of a moderate-dose statin and ezetimibe, a significant reduction was observed in major adverse cardiac events, compared to the LDL-C level of 70 mg/dL achieved with statin alone. The greatest benefits of combination therapy were observed in patients with diabetes and the elderly over 75 years old.[103] The results of another study (2021) on 17,999 patients with ACS showed that the addition of ezetimibe to a statin consistently reduced the risk of cardiovascular events in post-ACS patients regardless of baseline LDL-C values; so that for per 1 mmoL decrease in LDL-C level, 21% relative risk reduction was observed in the baseline LDL-C level of 50–70 mg/dL, 16% in the baseline LDL-C level of 70–100 mg/dL, and 13% in the baseline LDL-C level of 100–125 mg/dL.[104]
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are promising drugs for the treatment of FH.[16,105,106] Two clinical trial studies were conducted to investigate the efficacy and safety of PCSK9 inhibitors in patients with heterozygous FH, who were 18 years of age and were receiving the maximum tolerated dose of statins. The results of these studies showed a favorable safety profile and a 50% greater reduction in LDL-C.[106,107]
The strategy of adding ezetimibe to statins before adding PCSK9 inhibitors is recommended because ezetimibe is a widely-available generic drug, its safety and tolerability have already been proven, and it has been used for decades, while PCSK9 inhibitors have been introduced, approved, and entered the market recently.[103]
A clinical trial study on the clinical outcomes of evolocumab use in 27,564 ASCVD patients having LDL-C levels >70 mg/dL and receiving statins indicated that the use of Evolocumab in combination with statins reduced LDL-C levels by an average of 30 mg/dL, as a result of which the risk of cardiovascular events decreased.[108]
The results of a meta-analysis study showed that the combination of statin and bile acid sequestrants led to a 16.2% decrease in LDL-C compared to statin alone.[109]
Evidence-to-decision considerations
In regard to combination therapy, most patients prefer to take fewer medications. Multi-drug regimens and complex drug regimens reduce patient adherence to treatment. Therefore, in prescribing multi-drug regimens, to increase patient adherence, it is important to pay attention to the patient’s age and socioeconomic status, presence of comorbidities, frequency of drug use, drug side effects, and drug cost and accessibility, and whether the drug is covered by insurance.[110] The results of a systematic review on the cost-effectiveness of hyperlipidemia treatment in low- and middle-income countries by comparing cross-sectional studies showed that treatment with polypills was generally more cost-effective than treatment with statins alone.[111]
The cost-effectiveness of evolocumab varies from country to country, which is mainly due to different population characteristics, CVD risk factors, efficacy assumptions, and drug prices.[112] The results of a study investigating the cost-effectiveness of evolocumab in the treatment of dyslipidemia in Saudi Arabia showed that evolocumab was a cost-effective treatment option for patients with clinically evident ASCVD and heterozygous FH whose LDL-C levels were not controlled by conventional drugs.[113] Figure 2 shows the algorithm for the diagnosis, management, and treatment of adult with high LDL-C.
Figure 2.
The algorithm for the diagnosis, management, and treatment of adult with high low-density lipoprotein cholesterol. LDL-C = Low-density lipoprotein cholesterol
Pharmacological treatments in hypertriglyceridemia
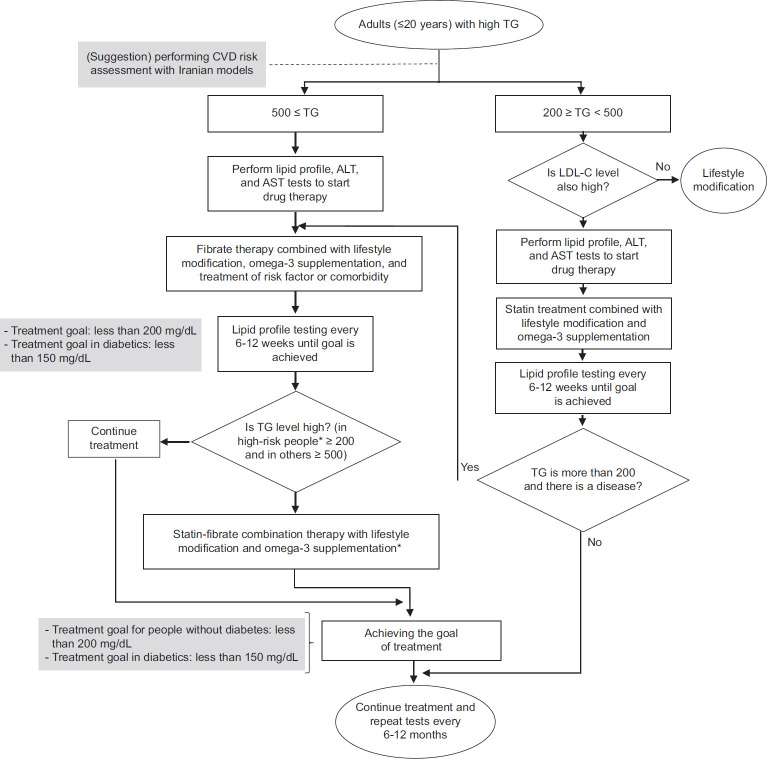
| It is recommended that in adults with hypertriglyceridemia who have high LDL-C, statin therapy be started with a TG level of 200–499 mg It is recommended to start drug therapy with fibrates if the TG level is ≥500 mg/dL along with normal LDL-C It is recommended to continue following the lifestyle modification recommendations along with drug therapy It is suggested that if the TG level remains >200 mg/dL with statin use, in high-risk people (including CVDs, ischemic stroke, diabetes, nonalcoholic fatty liver disease, and pancreatitis), TG-lowering drugs, such as fibrate, should be used It is suggested to add omega-3 acid supplements in patients who have hypertriglyceridemia and are under medical treatment |
LDL-C=Low-density lipoprotein cholesterol; CVDs=Cardiovascular diseases; TG=Triglyceride
Few studies have been conducted on the effect of TG-lowering drugs on patients with hypertriglyceridemia. Although the risk of CVD increases when fasting TG is more than 150 mg/dL, the use of such drugs as statins, fibrates, PCSK9 inhibitors, and n-3 PUFAs, especially Icosapent ethyl, to lower TG levels in high-risk patients may be considered only when TG are more than 200 mg.[114] To prevent acute pancreatitis, it is reasonable to lower TG whenever its level exceeds 500 mg/dL. By eliminating underlying factors, consuming a very low-fat diet, and adding fibrates or omega-3 fatty acids to the diet of patients with severe hypertriglyceridemia, TG can be continuously reduced.[115] In a meta-analysis on the effect of statins on TG amount, the average reduction of TG by statins was 15.1%–31.3%, and rosuvastatin led to TG reduction more than atorvastatin and simvastatin at the same dose (P < 0.05). In patients with hypertriglyceridemia, who receive statins, the reduction of TG was numerically less than LDL-C, and the significant reduction of LDL-C was dependent on the choice and dose of statin.[19]
In the face of secondary causes, statins alone cannot prevent the increase of TG levels and the development of acute pancreatitis caused by hypertriglyceridemia. Therefore, in patients with dyslipidemia, especially atherogenic dyslipidemia, fibrates are a useful treatment option alone or in combination with statins because they are generally well tolerated, alone or in combination with statins.[114]
Fibrates lower TG levels by an average of 20%–50% and also increase HDL-C levels by about 10%–20%. Fibrates have variable effects on LDL-C, including that in patients with hypertriglyceridemia they may increase plasma LDL-C levels by 10%–15%.[116,117]
Evidence also supports a dose-dependent beneficial effect of fish oil on serum TG, particularly among individuals with elevated TG. Consumption of fish oil moderately improves HDL-C as well.[118] In a meta-analysis including data from 13 trials, it was concluded that marine omega-3 supplements reduce the risk of myocardial infarction, CHD incidence and death, and CVD incidence and death, and that the risk reduction appears to be linearly related to the dose of marine omega-3.[119]
Evidence-to-decision considerations
The overall certainty of the evidence found for drug treatments that were effective against hypertriglyceridemia was moderate, and numerous beneficial effects have been reported for these treatments, including reduced risk of CVD and pancreatitis; reduction in clinical events in the general population and in patients with CKD, use of health care resources, and costs; and an effective decrease of TG, as well as an increase in HDL-C level and improvement of lipid, glucose metabolism, and liver function.[120,121,122,123,124] Although there have been some reports regarding treatment with fibrates as adverse effects in the form of mild side effects (e.g., gastrointestinal disorders, skin reactions, and decreased libido) and long-term complications (e.g. liver damage, muscle damage, gallstone formation, venous thromboembolism, increased homocysteine levels, and increased creatinine level,[124,125] they can definitely be controlled by selecting the appropriate target population and monitoring the patients under treatment.[124] Figure 3 displays the algorithm for the diagnosis, management, and treatment of hypertriglyceridemia.
Figure 3.
The algorithm for the diagnosis, management, and treatment of adult with high triglyceride. *In this guideline, diabetes (type 1 and 2), cardiovascular diseases, ischemic stroke, familial hypercholesterolemia, and non-alcoholic fatty liver have been considered. CVDs = Cardiovascular diseases; TG = Triglyceride; ALT = Alanine transaminase
Treatment targets for low-density lipoprotein cholesterol and triglyceride
| It is recommended that in adults with increased LDL-C who lack comorbidities and risk factors, the target of medical treatment should be <130 mg/dL It is recommended to target the drug therapy at reducing the level of LDL-C to <70 mg/dL and >50% reduction from the initial level of LDL-C in patients with FH without risk factors, type 2 diabetes without target organ damage, type 1 diabetes with treatment duration ≤10 years, stable angina, and CKD (eGFR <60 mL/min/1.73 m2) It is recommended to target the drug therapy at reducing the LDL-C level to <55 mg/dL and >50% reduction from the initial LDL-C level in patients with FH along with a disease or risk factor, type 2 diabetes with target organ damage or early onset long-term type 1 diabetes (>20 years), ACS, peripheral vascular disease, and ischemic stroke It is recommended that in adults with increased TG who lack comorbidities or risk factors, the target of drug treatment should be <200 mg/dL It is suggested that in patients with high TG and diabetes, the target of drug treatment should be <150 mg/dL |
LDL-C=Low-density lipoprotein cholesterol; TG=Triglyceride; FH=Familial hypercholesterolemia; ACS=Acute coronary syndrome; CKD=Chronic kidney disease; eGFR=Estimated glomerular filtration rate
In the clinical management of hyperlipidemia, a reasonable target is to attempt to bring lipid levels within the normal range. However, for people at risk, more aggressive targets should be set.[126] The results of meta-analysis studies show that the greater the absolute reduction of LDL-C, the greater the decrease in CVD risk.[16,41,76,127]
Defining the treatment target will lead to a more specific treatment process. The target-based treatment approach can also help doctor–patient communication and increase treatment adherence.
Choosing the target of reducing LDL-C by 50% from baseline or decreasing LDL-C to a lower level than a certain target is still debated; however, in some cases, these two targets appear to be complementary. For patients with untreated baseline LDL-C levels close to the target, a target of a 50% reduction in LDL-C levels may be more helpful than selecting a specific target for LDL-C levels. On the other hand, other studies have shown that the inter individual variability in the percent response of LDL-C to a given dose of statin is largely dependent on the pretreatment level, which may not always be known in each patient; therefore, an absolute target of LDL-C would be more appropriate.[13]
A clinical trial study of 10,000 patients with stable angina having LDL-C levels <130 mg/dL showed that lowering LDL-C levels to 70 mg/dL, using high-dose statins, could reduce the risk of cardiovascular events by 22%.[128] Another clinical trial study on 4700 patients with a history of ischemic or transient stroke in the past 6 months showed that at an LDL-C level of <73 mg in the group receiving statins, the risks of stroke and CVD decreased by 16% and 20%, respectively. These results indicate that bringing the level of LDL-C to nearly 70 mg through statin administration is beneficial in patients with ischemic stroke.[129]
Evidence shows that targeted reduction of LDL-C levels to below-target levels, through using combination therapy in very high-risk patients, is beneficial for preventing major cardiac events.[17,130]
Evidence-to-decision considerations
Treatment of patients with hyperlipidemia should be focused on bringing LDL-C levels as close as possible to the target values; regarding this, the treatment should be based on a step-by-step approach and through a joint decision-making process between the doctor and the patient. This approach is similar to clinical practice, where treatment escalation is considered based on anticipated benefits, side effects, and most importantly patient preferences.[114]
The results of a systematic review on the cost-effectiveness of hyperlipidemia treatment in low- and middle-income countries that included a review of 22 studies showed that most drug treatment strategies for hyperlipidemia were either cost-effective or very cost-effective.[5]
CONCLUSION
The members of the GDG, who were experienced people from various educational groups and representatives of most universities of medical sciences and related national associations in the country, tried to answer the most important clinical questions regarding the diagnosis, management, and treatment of hyperlipidemia. Although guidelines cannot answer all the challenges associated with the management of the disease and reflect the expected changes in related research, the authors believe that this guideline presents significant recommendations and suggestions that will be extremely helpful for patients and service providers in managing the disease. This is because this guideline was prepared using the standard method in the development of the national guidelines of hyperlipidemia, with the active and interactive participation of both specialists in related fields and general practitioners, nurses, and patients, along with methodologists and was based on the local context of Iran, using the latest available scientific evidence, the presence of several active committees, and obtaining the opinion of the external evaluation group.
It should be explained that in many guidelines, the recommendations are based on a 10-year risk assessment of CVDs. Although CVD risk assessment was acceptable to beneficiaries, members of the current guideline development group concluded that providing population-based recommendations on the basis of CVD risk assessment had limitations, especially when implemented in the office setting. Finally, the recommendations related to lifestyle modification and related behavioral interventions were emphasized as a way to manage the disease alone or along with drug therapy to increase treatment adherence and achieve the target of treatment and reduction of atherosclerosis diseases. As a result, this guideline was developed to the target groups as an important source of information in accordance with the latest scientific documents accepted by important international scientific assemblies in this field.
Financial support and sponsorship
This study was funded by Vice-Chancellor for Research of Iran’s MOH.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This project (with number 51329) was prepared based on the request of the Vice-Chancellor for Research of Iran’s MOH. We appreciate the specialist, faculty members and researchers who contributed to the various stages of project. We also appreciate the patients for participating in the meetings and expressing their values and preferences, and the support team members and IT experts who cooperated with us in holding the meetings.
REFERENCES
- 1.Hill MF, Bordoni B. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. Hyperlipidemia. [Google Scholar]
- 2.Grundy SM, Arai H, Barter P, Bersot TP, Betteridge DJ, Carmena R, et al. An international atherosclerosis society position paper: Global recommendations for the management of dyslipidemia: Executive summary. Atherosclerosis. 2014;232:410–3. doi: 10.1016/j.atherosclerosis.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Tabatabaei-Malazy O, Qorbani M, Samavat T, Sharifi F, Larijani B, Fakhrzadeh H. Prevalence of dyslipidemia in Iran: A systematic review and meta-analysis study. Int J Prev Med. 2014;5:373–93. [PMC free article] [PubMed] [Google Scholar]
- 4.Hovsepian S, Kelishadi R, Djalalinia S, Farzadfar F, Naderimagham S, Qorbani M. Prevalence of dyslipidemia in Iranian children and adolescents: A systematic review. J Res Med Sci. 2015;20:503–21. doi: 10.4103/1735-1995.163979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husain MJ, Spencer G, Nugent R, Kostova D, Richter P. The cost-effectiveness of hyperlipidemia medication in low- and middle-income countries: A review. Glob Heart. 2022;17:18. doi: 10.5334/gh.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Dansinger ML, Williams PT, Superko HR, Schaefer EJ. The importance of cholesterol follow-up testing under current statin treatment guidelines. Prev Med. 2019;121:150–7. doi: 10.1016/j.ypmed.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Perera R, McFadden E, McLellan J, Lung T, Clarke P, Pérez T, et al. Optimal strategies for monitoring lipid levels in patients at risk or with cardiovascular disease: A systematic review with statistical and cost-effectiveness modelling. Health Technol Assess. 2015;19:1–401. doi: 10.3310/hta191000. vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia X, Al Rifai M, Ramsey DJ, Ahmed ST, Akeroyd JM, Nambi V, et al. Association between lipid testing and statin adherence in the veterans affairs health system. Am J Med. 2019;132:e693–700. doi: 10.1016/j.amjmed.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Jia X, Ramsey DJ, Rifai MA, Ahmed ST, Akeroyd JM, Dixon DL, et al. Impact of lipid monitoring on treatment intensification of cholesterol lowering therapies (from the Veterans affairs healthcare system) Am J Cardiol. 2020;125:874–9. doi: 10.1016/j.amjcard.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Motlagh ME, Kelishadi R, Amirkhani MA, Ziaoddini H, Dashti M, Aminaee T, et al. Double burden of nutritional disorders in young Iranian children: Findings of a nationwide screening survey. Public Health Nutr. 2011;14:605–10. doi: 10.1017/S1368980010002399. [DOI] [PubMed] [Google Scholar]
- 12.Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens (Greenwich) 2008;10:644–6. doi: 10.1111/j.1751-7176.2008.08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74:1376–414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, et al. American Heart Association guide for improving cardiovascular health at the community level, 2013 update: A scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation. 2013;127:1730–53. doi: 10.1161/CIR.0b013e31828f8a94. [DOI] [PubMed] [Google Scholar]
- 16.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA. 2016;316:1289–97. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 17.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 18.HPS3/TIMI55-REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–27. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 19.Karlson BW, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. A VOYAGER meta-analysis of the impact of statin therapy on low-density lipoprotein cholesterol and triglyceride levels in patients with hypertriglyceridemia. Am J Cardiol. 2016;117:1444–8. doi: 10.1016/j.amjcard.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: A meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–94. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodney S, Valentine KD, Sepucha K, Fowler FJ, Jr., Barry MJ. Patient preference distribution for use of statin therapy. JAMA Netw Open. 2021;4:e210661. doi: 10.1001/jamanetworkopen.2021.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–50. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Berberich AJ, Hegele RA. A modern approach to dyslipidemia. Endocr Rev. 2022;43:611–53. doi: 10.1210/endrev/bnab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. Spectrum of statin hepatotoxicity: Experience of the drug-induced liver injury network. Hepatology. 2014;60:679–86. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrick C, Bahrainy S, Gill EA. Statins and the liver. Cardiol Clin. 2015;33:257–65. doi: 10.1016/j.ccl.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Bays H, Cohen DE, Chalasani N, Harrison SA The National Lipid Association’s Statin Safety Task Force. An assessment by the statin liver safety task force: 2014 update. J Clin Lipidol. 2014;8:S47–57. doi: 10.1016/j.jacl.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Chien SC, Chen PS, Huang YH, Tang SC, Li YH, Yeh HI. 2019 Taiwan Society of Lipids and Atherosclerosis expert consensus statement on statin intolerance. J Formos Med Assoc. 2019;118:1385–92. doi: 10.1016/j.jfma.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society consensus panel statement on assessment, aetiology and management. Eur Heart J. 2015;36:1012–22. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian consensus working group update (2016) Can J Cardiol. 2016;32:S35–65. doi: 10.1016/j.cjca.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA The National Lipid Association’s Muscle Safety Expert Panel. An assessment by the statin muscle safety task force: 2014 update. J Clin Lipidol. 2014;8:S58–71. doi: 10.1016/j.jacl.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 31.McKenney JM, Davidson MH, Jacobson TA, Guyton JR National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the national lipid association statin safety assessment task force. Am J Cardiol. 2006;97:89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Liu J, Xie W, Qi Y. Cardiovascular risk assessment: A global perspective. Nat Rev Cardiol. 2015;12:301–11. doi: 10.1038/nrcardio.2015.28. [DOI] [PubMed] [Google Scholar]
- 33.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: Population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118:618–24. doi: 10.1016/j.amjmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Schiele F, Quignot N, Khachatryan A, Gusto G, Villa G, Kahangire D, et al. Clinical impact and room for improvement of intensity and adherence to lipid lowering therapy: Five years of clinical follow-up from 164,565 post-myocardial infarction patients. Int J Cardiol. 2021;332:22–8. doi: 10.1016/j.ijcard.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Tran C, Vo V, Taylor P, Koehn DA, Virani SS, Dixon DL. Adherence to lipid monitoring and its impact on treat intensification of LDL-C lowering therapies at an urban academic medical center. J Clin Lipidol. 2022;16:491–7. doi: 10.1016/j.jacl.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 37.May HT, Knowlton KU, Anderson JL, Lappé DL, Bair TL, Muhlestein JB. High-statin adherence over 5 years of follow-up is associated with improved cardiovascular outcomes in patients with atherosclerotic cardiovascular disease: Results from the IMPRES study. Eur Heart J Qual Care Clin Outcomes. 2022;8:352–60. doi: 10.1093/ehjqcco/qcab024. [DOI] [PubMed] [Google Scholar]
- 38.Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genser B, März W. Low density lipoprotein cholesterol, statins and cardiovascular events: A meta-analysis. Clin Res Cardiol. 2006;95:393–404. doi: 10.1007/s00392-006-0403-x. [DOI] [PubMed] [Google Scholar]
- 40.Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: A network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–24. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 41.Cholesterol Treatment Trialists’ (CTT) Collaboration. Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 42.Cholesterol Treatment Trialists’ (CTT) Collaborators. Kearney PM, Blackwell L, Collins R, Keech A, Simes J, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 43.Vahedian-Azimi A, Bianconi V, Makvandi S, Banach M, Mohammadi SM, Pirro M, et al. A systematic review and meta-analysis on the effects of statins on pregnancy outcomes. Atherosclerosis. 2021;336:1–11. doi: 10.1016/j.atherosclerosis.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Graham DF, Raal FJ. Management of familial hypercholesterolemia in pregnancy. Curr Opin Lipidol. 2021;32:370–7. doi: 10.1097/MOL.0000000000000790. [DOI] [PubMed] [Google Scholar]
- 45.Tikkanen MJ, Holme I, Cater NB, Szarek M, Faergeman O, Kastelein JJ, et al. Comparison of efficacy and safety of atorvastatin (80 mg) to simvastatin (20 to 40 mg) in patients aged<65 versus>or=65 years with coronary heart disease (from the Incremental DEcrease through Aggressive Lipid Lowering [IDEAL] study) Am J Cardiol. 2009;103:577–82. doi: 10.1016/j.amjcard.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi O, Glasziou PP, Perera R, Shimbo T, Suwa J, Hiramatsu S, et al. Lipid re-screening: What is the best measure and interval? Heart. 2010;96:448–52. doi: 10.1136/hrt.2009.172619. [DOI] [PubMed] [Google Scholar]
- 48.Glasziou PP, Irwig L, Heritier S, Simes RJ, Tonkin A. LIPID Study Investigators. Monitoring cholesterol levels: Measurement error or true change? Ann Intern Med. 2008;148:656–61. doi: 10.7326/0003-4819-148-9-200805060-00005. [DOI] [PubMed] [Google Scholar]
- 49.Development and Adapting Guidelines Department. Qualitative Evaluation Report of Values and Preferences of Dyslipidemia Patients. 2021 [Google Scholar]
- 50.D’Agostino RB, Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 51.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canoy D, Nazarzadeh M, Copland E, Bidel Z, Rao S, Li Y, et al. How much lowering of blood pressure is required to prevent cardiovascular disease in patients with and without previous cardiovascular disease? Curr Cardiol Rep. 2022;24:851–60. doi: 10.1007/s11886-022-01706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodward M, Brindle P, Tunstall-Pedoe H SIGN Group on Risk Estimation. Adding social deprivation and family history to cardiovascular risk assessment: The ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93:172–6. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: Prospective open cohort study. BMJ. 2007;335:136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310–5. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 56.Hajifathalian K, Ueda P, Lu Y, Woodward M, Ahmadvand A, Aguilar-Salinas CA, et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): A pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015;3:339–55. doi: 10.1016/S2213-8587(15)00081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet. 2005;365:434–41. doi: 10.1016/S0140-6736(05)17833-7. [DOI] [PubMed] [Google Scholar]
- 58.Hassannejad R, Mansourian M, Marateb H, Mohebian MR, Gaziano TA, Jackson RT, et al. Developing non-laboratory cardiovascular risk assessment charts and validating laboratory and non-laboratory-based models. Glob Heart. 2021;16:58. doi: 10.5334/gh.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarrafzadegan N, Hassannejad R, Marateb HR, Talaei M, Sadeghi M, Roohafza HR, et al. PARS risk charts: A 10-year study of risk assessment for cardiovascular diseases in Eastern Mediterranean Region. PLoS One. 2017;12:e0189389. doi: 10.1371/journal.pone.0189389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. Guideline for the Pharmacological Treatment of Hypertension in Adults: Web Annex A: Summary of Evidence. World Health Organization. 2021 [PubMed] [Google Scholar]
- 61.Dallongeville J, Banegas JR, Tubach F, Guallar E, Borghi C, De Backer G, et al. Survey of physicians’ practices in the control of cardiovascular risk factors: The EURIKA study. Eur J Prev Cardiol. 2012;19:541–50. doi: 10.1177/1741826711407705. [DOI] [PubMed] [Google Scholar]
- 62.Becker J. Cardiovascular risk assessment and hyperlipidemia. Crit Care Nurs Clin North Am. 2008;20:277–85. doi: 10.1016/j.ccell.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–69. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Global status report on noncommunicable diseases 2014. World Health Organization. 2014 (No. WHO/NMH/NVI/15.1) [Google Scholar]
- 65.Cavero-Redondo I, Deeks JJ, Alvarez-Bueno C, Jolly K, Saz-Lara A, Price M, et al. Comparative effect of physical exercise versus statins on improving arterial stiffness in patients with high cardiometabolic risk: A network meta-analysis. PLoS Med. 2021;18:e1003543. doi: 10.1371/journal.pmed.1003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, et al. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health. 2018;3:e419–28. doi: 10.1016/S2468-2667(18)30135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwingshackl L, Bogensberger B, Benčič A, Knüppel S, Boeing H, Hoffmann G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J Lipid Res. 2018;59:1771–82. doi: 10.1194/jlr.P085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr., Brehm BJ, et al. Effects of low-carbohydrate versus low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 69.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: A meta-analysis. Am J Clin Nutr. 1992;56:320–8. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 70.Huffman KM, Hawk VH, Henes ST, Ocampo CI, Orenduff MC, Slentz CA, et al. Exercise effects on lipids in persons with varying dietary patterns-does diet matter if they exercise? Responses in studies of a targeted risk reduction intervention through defined exercise I. Am Heart J. 2012;164:117–24. doi: 10.1016/j.ahj.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Natale C, Annuzzi G, Bozzetto L, Mazzarella R, Costabile G, Ciano O, et al. Effects of a plant-based high-carbohydrate/high-fiber diet versus high-monounsaturated fat/low-carbohydrate diet on postprandial lipids in type 2 diabetic patients. Diabetes Care. 2009;32:2168–73. doi: 10.2337/dc09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yasin YJ, Banoub JAM GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2018;392:1015–35. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster HM, Celis-Morales CA, Nicholl BI, Petermann-Rocha F, Pell JP, Gill JM, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: A prospective analysis of the UK Biobank cohort. Lancet Public Health. 2018;3:e576–85. doi: 10.1016/S2468-2667(18)30200-7. [DOI] [PubMed] [Google Scholar]
- 75.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2018;392:1015–35. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cholesterol Treatment Trialists’ (CTT) Collaborators. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 78.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–15. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karmali KN, Goff DC, Jr., Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–68. doi: 10.1016/j.jacc.2014.06.1186. [DOI] [PubMed] [Google Scholar]
- 80.Muntner P, Colantonio LD, Cushman M, Goff DC, Jr., Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–15. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong ND, Glovaci D, Wong K, Malik S, Franklin SS, Wygant G, et al. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res. 2012;9:146–52. doi: 10.1177/1479164112436403. [DOI] [PubMed] [Google Scholar]
- 82.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 83.Collins R, Armitage J, Parish S, Sleigh P, Peto R Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 84.Knopp RH, d’Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: The atorvastatin study for prevention of coronary heart disease endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–85. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 85.Sever PS, Poulter NR, Dahlöf B, Wedel H, Collins R, Beevers G, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial – Lipid-lowering arm (ASCOT-LLA) Diabetes Care. 2005;28:1151–7. doi: 10.2337/diacare.28.5.1151. [DOI] [PubMed] [Google Scholar]
- 86.de Vries FM, Denig P, Pouwels KB, Postma MJ, Hak E. Primary prevention of major cardiovascular and cerebrovascular events with statins in diabetic patients: A meta-analysis. Drugs. 2012;72:2365–73. doi: 10.2165/11638240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Lamiquiz-Moneo I, Civeira F, Mateo-Gallego R, Laclaustra M, Moreno-Franco B, Tejedor MT, et al. Diagnostic yield of sequencing familial hypercholesterolemia genes in individuals with primary hypercholesterolemia. Rev Esp Cardiol (Engl Ed) 2021;74:664–73. doi: 10.1016/j.rec.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9–19. doi: 10.1161/CIRCULATIONAHA.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nanchen D, Gencer B, Muller O, Auer R, Aghlmandi S, Heg D, et al. Prognosis of patients with familial hypercholesterolemia after acute coronary syndromes. Circulation. 2016;134:698–709. doi: 10.1161/CIRCULATIONAHA.116.023007. [DOI] [PubMed] [Google Scholar]
- 90.Vallejo-Vaz AJ, Robertson M, Catapano AL, Watts GF, Kastelein JJ, Packard CJ, et al. Low-Density lipoprotein cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of low-density lipoprotein cholesterol levels of 190 mg/dL or above: Analyses from the WOSCOPS (west of Scotland coronary prevention study) 5-year randomized trial and 20-year observational follow-up. Circulation. 2017;136:1878–91. doi: 10.1161/CIRCULATIONAHA.117.027966. [DOI] [PubMed] [Google Scholar]
- 91.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, et al. Efficacy of statins in familial hypercholesterolaemia: A long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cholesterol Treatment Trialists’ (CTT) Collaboration. Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: A meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–39. doi: 10.1016/S2213-8587(16)30156-5. [DOI] [PubMed] [Google Scholar]
- 93.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: Evidence report and systematic review for the US preventive services task force. JAMA. 2016;316:2008–24. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 94.Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: A study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390–9. doi: 10.1161/CIRCOUTCOMES.111.000071. [DOI] [PubMed] [Google Scholar]
- 95.Ohsfeldt RL, Gandhi SK, Fox KM, McKenney JM. Statin cost-effectiveness comparisons using real-world effectiveness data: Formulary implications. Value Health. 2008;11:1061–9. doi: 10.1111/j.1524-4733.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- 96.Odden MC, Pletcher MJ, Coxson PG, Thekkethala D, Guzman D, Heller D, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med. 2015;162:533–41. doi: 10.7326/M14-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stocks NP, Ryan P, McElroy H, Allan J. Statin prescribing in Australia: Socioeconomic and sex differences. A cross-sectional study. Med J Aust. 2004;180:229–31. [PubMed] [Google Scholar]
- 98.Thomsen RW, Johnsen SP, Olesen AV, Mortensen JT, Bøggild H, Olsen J, et al. Socioeconomic gradient in use of statins among Danish patients: Population-based cross-sectional study. Br J Clin Pharmacol. 2005;60:534–42. doi: 10.1111/j.1365-2125.2005.02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butalia S, Lee-Krueger RC, McBrien KA, Leung AA, Anderson TJ, Quan H, et al. Barriers and facilitators to using statins: A qualitative study with patients and family physicians. CJC Open. 2020;2:530–8. doi: 10.1016/j.cjco.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J, Egolum U, Parihar H, Cooley M, Ling H. Effect of ezetimibe added to high-intensity statin therapy on low-density lipoprotein cholesterol levels: A meta-analysis. Cardiol Res. 2021;12:98–108. doi: 10.14740/cr1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morrone D, Weintraub WS, Toth PP, Hanson ME, Lowe RS, Lin J, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: A pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012;223:251–61. doi: 10.1016/j.atherosclerosis.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 102.Phan BA, Dayspring TD, Toth PP. Ezetimibe therapy: Mechanism of action and clinical update. Vasc Health Risk Manag. 2012;8:415–27. doi: 10.2147/VHRM.S33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 104.Oyama K, Giugliano RP, Blazing MA, Park JG, Tershakovec AM, Sabatine MS, et al. Baseline low-density lipoprotein cholesterol and clinical outcomes of combining ezetimibe with statin therapy in IMPROVE-IT. J Am Coll Cardiol. 2021;78:1499–507. doi: 10.1016/j.jacc.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 105.Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. doi: 10.1093/eurheartj/ehv370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 107.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 108.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: Insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk) Circulation. 2018;137:338–50. doi: 10.1161/CIRCULATIONAHA.117.032235. [DOI] [PubMed] [Google Scholar]
- 109.Alder M, Bavishi A, Zumpf K, Peterson J, Stone NJ. A meta-analysis assessing additional LDL-C reduction from addition of a bile acid sequestrant to statin therapy. Am J Med. 2020;133:1322–7. doi: 10.1016/j.amjmed.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 110.Aslam F, Haque A, Lee V, Foody J. Patient adherence and preference considerations in managing cardiovascular risk: Focus on single pill and amlodipine/atorvastatin fixed combination. Patient Prefer Adherence. 2009;3:61–6. doi: 10.2147/ppa.s4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baumgartner A, Drame K, Geutjens S, Airaksinen M. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12:190. doi: 10.3390/pharmaceutics12020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Azari S, Rezapour A, Omidi N, Alipour V, Behzadifar M, Safari H, et al. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: A systematic review. Heart Fail Rev. 2020;25:1077–88. doi: 10.1007/s10741-019-09874-2. [DOI] [PubMed] [Google Scholar]
- 113.Alghamdi A, Balkhi B, Altowaijri A, Al-Shehri N, Ralph L, Marriott ER, et al. Cost-effectiveness analysis of evolocumab for the treatment of dyslipidemia in the Kingdom of Saudi Arabia. Pharmacoecon Open. 2022;6:277–91. doi: 10.1007/s41669-021-00300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 115.Wiggins BS, Saseen JJ, Page RL, 2nd, Reed BN, Sneed K, Kostis JB, et al. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2016;134:e468–95. doi: 10.1161/CIR.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 116.Li YH, Ueng KC, Jeng JS, Charng MJ, Lin TH, Chien KL, et al. 2017 Taiwan lipid guidelines for high risk patients. J Formos Med Assoc. 2017;116:217–48. doi: 10.1016/j.jfma.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 117.McKeage K, Keating GM. Fenofibrate: A review of its use in dyslipidaemia. Drugs. 2011;71:1917–46. doi: 10.2165/11208090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 118.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Hu Y, Hu FB, Manson JE. Marine Omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8:e013543. doi: 10.1161/JAHA.119.013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kayıkçıoğlu M, Shahbazova S, İbrahimov F, Can LH. Cumulative non-HDL-cholesterol burden in patients with hypertriglyceridemia receiving long-term fibrate therapy: Real life data from a lipid clinic cohort. Turk Kardiyol Dern Ars. 2020;48:359–67. doi: 10.5543/tkda.2019.25169. [DOI] [PubMed] [Google Scholar]
- 121.Tenenbaum A, Fisman EZ. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 2012;11:125. doi: 10.1186/1475-2840-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yokote K, Yamashita S, Arai H, Araki E, Matsushita M, Nojima T, et al. Effects of pemafibrate on glucose metabolism markers and liver function tests in patients with hypertriglyceridemia: A pooled analysis of six phase 2 and phase 3 randomized double-blind placebo-controlled clinical trials. Cardiovasc Diabetol. 2021;20:96. doi: 10.1186/s12933-021-01291-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hadjivasilis A, Kouis P, Kousios A, Panayiotou A. The Effect of fibrates on kidney function and chronic kidney disease progression: A systematic review and meta-analysis of randomised studies. J Clin Med. 2022;11:768. doi: 10.3390/jcm11030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Okopień B, Bułdak Ł, Bołdys A. Benefits and risks of the treatment with fibrates – A comprehensive summary. Expert Rev Clin Pharmacol. 2018;11:1099–112. doi: 10.1080/17512433.2018.1537780. [DOI] [PubMed] [Google Scholar]
- 125.Nurmohamed NS, Dallinga-Thie GM, Stroes ES. Targeting apoC-III and ANGPTL3 in the treatment of hypertriglyceridemia. Expert Rev Cardiovasc Ther. 2020;18:355–61. doi: 10.1080/14779072.2020.1768848. [DOI] [PubMed] [Google Scholar]
- 126.Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 127.Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: A systematic review and meta-analysis. JAMA. 2018;319:1566–79. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 129.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 130.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]