Abstract
The ability of mitochondria to transform the energy we obtain from food into cell phosphorylation potential has long been appreciated. However, recent decades have seen an evolution in our understanding of mitochondria, highlighting their significance as key signal-transducing organelles with essential roles in immunity that extend beyond their bioenergetic function. Importantly, mitochondria retain bacterial motifs as a remnant of their endosymbiotic origin that are recognised by innate immune cells to trigger inflammation and participate in anti-microbial defence. This review aims to explore how mitochondrial physiology, spanning from oxidative phosphorylation (OxPhos) to signalling of mitochondrial nucleic acids, metabolites, and lipids, influences the effector functions of phagocytes. These myriad effector functions include macrophage polarisation, efferocytosis, anti-bactericidal activity, antigen presentation, immune signalling, and cytokine regulation. Strict regulation of these processes is critical for organismal homeostasis that when disrupted may cause injury or contribute to disease. Thus, the expanding body of literature, which continues to highlight the central role of mitochondria in the innate immune system, may provide insights for the development of the next generation of therapies for inflammatory diseases.
Subject terms: Mitochondria, Energy metabolism
Introduction
Mitochondria are double-membraned organelles found in the cytoplasm of virtually all eukaryotic organisms. They contain their own genetic material, a circular chromosome termed mitochondrial DNA (mtDNA)1, differentiating them from most other eukaryotic subcellular structures with the exception of chloroplasts. It is proposed that mitochondria originated from an endosymbiotic event between an α-proteobacterial ancestor and an archaeal host of the Lokiarchaeota phylum over 2.5 billion years ago2, which acted as a primary driving force in eukaryotic evolution3. These dynamic and morphologically diverse organelles have captivated scientists for decades, inspiring several conceptual and theoretical advances across scientific disciplines, from evolution to metabolism and medicine4. Perhaps the most pervasive analogy for mitochondria is as the ‘powerhouse of the cell’, an analogy derived from the chemiosmotic theory of oxidative phosphorylation (OxPhos) introduced by the paradigm-shifting work of Peter Mitchell and Jennifer Moyle in the 1960s5. Structurally, mitochondria possess an outer mitochondrial membrane (OMM) that encloses the organelle and an inner mitochondrial membrane (IMM) that forms numerous folds called cristae, which increase the surface area available for ATP synthesis by chemiosmotic coupling. In fact, mitochondrial bioenergetics, the ability of energy-transducing pathways in mitochondria to maintain cell phosphorylation potential, is a leading theory of how endosymbiosis triggered the explosion, diversification, and multi-cellularity associated with the eukaryotic domain of life3,6. Equally fascinating is the idea that a break in mitochondrial endosymbiosis may even be a basis for inflammatory diseases in the modern age7.
The use of the terms mitochondrial function and dysfunction in the scientific literature often directly relates to mitochondrial OxPhos8. Although it has been proposed that this terminology is misleading and should be avoided8, it is also argued that these terms represent appropriate umbrella terms to describe overall mitochondrial health9. Despite these debates on terminology, it is clear that the powerhouse analogy only tells one part of a larger story. In the modern era, mitochondria are now known to act as central organising hubs coordinating biosynthetic and signalling modalities with the ability to influence fate and function decision-making across cell and tissue types4,10. This inherent complexity in mitochondrial biology has led to the proposition of mitochondria as processors of the cell and it has been suggested we refer to it as the mitochondrial information processing system (MIPs)4. While only time will tell if this newly suggested terminology persists, mitochondrial signal transduction is emerging as a critically important regulator of cellular and systemic physiology. This concept is perfectly illustrated in cells of our innate immune system, a universal and evolutionarily ancient form of host defence against infection and tissue damage11. Key components of the innate immune system include physical barriers like the skin and mucous membranes, as well as cellular and chemical defences such as phagocytes (e.g., dendritic cells (DCs), neutrophils, and macrophages), natural killer cells, and antimicrobial proteins like complement and interferons. These components work together to recognise and eliminate pathogens, initiate inflammation to recruit immune cells, and activate the adaptive immune response if needed. Our objective in this review article is to underscore the importance of mitochondrial signal transduction during the innate immune response using clear examples and a focus on phagocytes, rather than providing an exhaustive list of all studies and signals in this growing field. The integrated nature of mitochondrial physiology for the generation of these important signals will also be highlighted.
Mitochondrial bioenergetics
OxPhos
The electron transport chain (ETC) is a crucial component of aerobic respiration, occurring within the IMM of eukaryotic cells. The ETC consists of a series of protein complexes (I, II, III, IV) and electron carriers, including flavoproteins, cytochromes, and ubiquinone. These complexes work together to transfer electrons derived from the oxidation of redox equivalents, NADH and FADH2, down a series of reactions, ultimately to molecular oxygen (O2), the terminal electron acceptor. As electrons move along the ETC, they release energy that is utilised to pump protons (H+) across the IMM, establishing an electrochemical gradient known as the proton motive force (Δp). The electrochemical gradient then drives the synthesis of ATP via F0F1-ATP synthase (also known as Complex V) in a process referred to as chemiosmosis5. This method of maintaining cell phosphorylation potential is far superior to the other major alternative energy-transducing metabolic pathway, glycolysis12. The oxidation of glucose to pyruvate yields a net gain of 2 molecules of ATP per molecule of glucose, whereas the complete oxidation of glucose by OxPhos yields ~32 molecules of ATP12. If pyruvate is reduced to lactate in the presence of O2, this is commonly referred to as the Warburg effect or aerobic glycolysis, first observed in carcinoma cells13,14. However, it is now apparent that modulation of both OxPhos and aerobic glycolysis is a critical feature of metabolic remodelling in stimulated innate immune cells, such as macrophages and DCs.
Macrophages are phenotypically plastic phagocytic cells widely distributed throughout the body and can adopt a variety of polarisation states depending on their environment15. DCs on the other hand are primarily found in tissues that interface with the external environment, such as the skin, respiratory tract, and gastrointestinal tract16. Here, they act as important sentinels for the capture and processing of antigens to initiate adaptive immune responses. Classically activated macrophages, defined experimentally by stimulation with lipopolysaccharide (LPS) with or without interferon-gamma (IFN-γ) but can also include other microbial products, are inflammatory in nature and required to counteract pathogenic microorganisms15. On the other hand, anti-inflammatory macrophages, often generated experimentally using IL-4, IL-13, or IL-10 stimulation, are associated with the resolution of inflammation, wound healing, and type II immune responses15.
Classical activation of macrophages and stimulation of DCs by Toll-like receptor (TLR) ligands (also known as pathogen-associated molecular patterns (PAMPs)), results in the suppression of mitochondrial respiration and an increase in aerobic glycolysis (Fig. 1A)17–20. Mechanistically, respiratory impairment has been linked to the inducible nitric oxide synthase (iNOS), also known as NOS2, and increased nitric oxide (NO) production18,20. NO is a free radical that has long been known to inhibit ETC complexes in macrophages21–24. More recently, NO has been shown to reduce the protein levels of complexes I, II, III, and IV and impair the activity of complexes I, II, and IV in macrophages co-stimulated with LPS and IFN-γ25,26. In contrast, IL-4-stimulated macrophages exhibit increased OxPhos, a process dependent on PPARγ-coactivator-1β (PGC1β)-mediated mitochondrial biogenesis, CD36-dependent lysosomal lipolysis, and fatty acid oxidation (FAO) (Fig. 1B)27,28. As such, FAO-driven mitochondrial respiration is required for effective type II immune responses against parasitic helminth infections28. Intriguingly, IL-4-stimulated macrophages readily repolarise into classical inflammatory macrophages20. However, NO-mediated inhibition of OxPhos prevents the repolarisation of inflammatory macrophages highlighting the importance of mitochondrial bioenergetics for macrophage plasticity20. Similarly, the anti-inflammatory cytokine IL-10 antagonises classical macrophage polarisation by suppressing aerobic glycolysis and increasing OxPhos29. This positive impact of IL-10 on mitochondrial respiratory function is linked to the restriction of iNOS expression, increased arginase 2 levels, reduced NO production, and suppression of mammalian targets of rapamycin (mTOR)29,30.
Fig. 1. Mitochondrial bioenergetics in innate immunity.
A NO produced by inducible nitric oxide synthase (iNOS) inhibits OxPhos and increases mtROS following prolonged LPS stimulation. Increased mtROS has bactericidal activity. Impaired OxPhos promotes tolerance and prevents alternative activation with subsequent IL-4 challenge. IL-10 antagonises NO by increasing mitochondrial Arg2. B IL-4/IL-13 increases OxPhos by enhancing glutamine anaplerosis and FAO. Increased OxPhos following IL-4/IL-13 training facilitates a hyperinflammatory response with subsequent LPS challenge and exhibits improved anti-mycobacterial responses. C NLRP3 inflammasome activation depends on mitochondrial PCr that is converted to ATP in the cytosol by CKB.
In inflammatory macrophages or DCs, the precise reason behind the shift away from mitochondrial respiration and toward aerobic glycolysis is still unclear. This acute inflammatory response is generally short-lived when compared to the more prolonged activities of alternatively activated macrophages. Indeed, evidence suggests that inhibiting glycolysis limits the activation and survival of DCs and impairs cytokine production in macrophages, particularly the pro-inflammatory cytokine IL-1β18,19,31. Specifically, the rapid glycolytic burst downstream of TLR signalling in DCs facilitates anabolic processes, such as de novo fatty acid synthesis, enabling the expansion of membranes for protein secretion32. This suggests that the observed metabolic switch is essential for function, perhaps by allowing for the rapid synthesis of cytoplasmic ATP and reducing equivalents for these energetic processes. However, one alternative hypothesis is that by suppressing mitochondrial respiration this acts as an “off switch” for an acute inflammatory response, thereby promoting tolerance. This notion is supported by kinetic analyses of metabolic reprogramming and cytokine levels in classically activated macrophages with links to the mitohormetic impact of mitochondrial-derived reactive oxygen species (mtROS) and reactive electrophilic species (mtRES)33,34. In line with this concept, Garaude et al.35 demonstrated a transient decrease in complex I-containing super complexes and a switch to complex II-mediated mitochondrial respiration early after E. coli infection, which was required for anti-bacterial immunity35. However, at later timepoints, complex II activity had decreased relative to the uninfected controls35. Additional support comes from IL-4/IL-13 training of macrophages, which enhances anti-mycobacterial killing and pro-inflammatory cytokine production in a manner dependent on OxPhos36. Nevertheless, it can also lead to a hyperinflammatory response following subsequent LPS exposure that can potentially drive pathology37. Therefore, this hyperinflammatory phenotype in the absence of respiratory chain suppression may provide insights into the role of this metabolic remodelling process.
More recently, the mitochondrial ETC has also been shown to be essential for activation of the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome in macrophages38. NLRP3 serves as an intracellular sensor capable of detecting a wide array of microbial motifs, endogenous danger signals, and environmental irritants, leading to the formation and activation of the inflammasome complex39. This complex comprises a sensor component (NLRP3), an adaptor (ASC, also known as PYCARD), and an effector (caspase 1)39. Structurally, NLRP3 is a tripartite protein containing an amino-terminal pyrin domain (PYD), a central NACHT domain, and a carboxy-terminal leucine-rich repeat domain (LRR domain), with the NACHT domain exhibiting ATPase activity crucial for NLRP3 self-association and function39. Upon activation, the effector caspase 1 cleaves pro-IL-1β, pro-IL-18 and gasdermin D (GSDMD) to their mature forms, triggering pyroptosis and pro-inflammatory cytokine release39. While the mechanism by which NLRP3 senses such diverse stimuli has been extensively investigated and a direct link with mitochondria has long been established, the precise signalling involved remains unclear40. Billingham and colleagues utilised pharmacological inhibitors targeting complex I, II, III, and V to investigate this link and confirmed impairments in NLRP3 inflammasome activation upon inhibition of OxPhos38. This effect was reversed following functional complementation of complex I and complex III utilising ectopic expression of Saccharomyces cerevisiae NADH dehydrogenase (NDI1) or Ciona intestinalis alternative oxidase (AOX)38. Mechanistically, the authors found that mitochondrial ATP synthesis via phosphocreatine (PCr) and cytosolic creatine kinase B (CKB), which generates cytosolic ATP from PCr, was necessary for NLRP3 activation (Fig. 1C)38. These findings underscore the intricate interplay between OxPhos and innate immune responses.
While this study firmly connects mitochondrial bioenergetics to NLRP3, it’s important to note some conflicting reports. NLRP3 inflammasome activation can occur in a K+ efflux-dependent and K+ efflux-independent manner41,42. K+ efflux-dependent activation is reportedly unrelated to mitochondrial bioenergetics41, while Imiquimod and CL097 trigger K+ efflux-independent NLRP3 activation by inhibiting mitochondrial complex I41. However, complex I inhibition or PCr depletion prior to CL097 treatment still impaired IL-1β release, suggesting additional mechanisms are involved during CL097 signalling38. Furthermore, treatment of macrophages with the complex II inhibitor malonate increased intracellular succinate levels but had a modest impact on IL-1β release43. However, malonate is a negatively charged dicarboxylate with poor membrane permeability44. It is unclear how malonate enters the cell at neutral pH, what intracellular concentrations of malonate were achieved, or if it led to a significant impairment in mitochondrial respiration. As such, the use of multiple ETC inhibitors alongside measures of respiration by Billingham et al.38 provides solid evidence for the involvement of mitochondrial OxPhos. Despite this, important questions remain regarding why ATP produced by glycolysis or direct ATP export to the cytosol via the adenine nucleotide transporter (ANT) are insufficient to support NLRP3 activity. The data also suggests that there is no role for mtROS in NLRP3 activation in contrast to previous reports40,45–49. This highlights the importance of conducting further research into this complex process in order to clarify underlying mechanisms and to aid any potential therapeutic targeting in the future.
mtROS
ROS are chemically reactive molecules containing oxygen, traditionally thought of as agents of cellular damage. Indeed, cytosolic ROS produced by NADPH oxidase 2 (NOX2) in innate immune cells are known to directly damage pathogens through the oxidation of lipids and DNA50–53. Beyond these NOX enzymes, which evolved as anti-microbicidal tools of phagocytes54, a consequence of the use of mitochondrial OxPhos for energy transduction is the generation of mtROS55,56. mtROS, notably superoxide (O2•−) and, following dismutation, hydrogen peroxide (H2O2), are predominantly formed at complex I or complex III of the ETC55. The significant contribution of mtROS to inflammatory redox signalling in innate immune cells, as well as anti-microbial immunity, has become increasingly prominent over the years57–59.
For instance, stimulation of Toll-like receptors (TLR) 1,2, and 4 on the surface of innate immune cells and within their phagosome initiates various signalling pathways within the cell60. Among others, it causes mitochondrial migration towards the phagolysosome through the activation of the serine-threonine kinases Mst1 and Mst257,61–63. Simultaneously, West et al.57 observed mtROS production in macrophages following cell-surface TLR stimulation (Fig. 1)57. Interestingly, the production of mtROS that was induced by TLR binding is specific to antimicrobial defence, as it was not observed after stimulation of endosomal TLRs that function primarily in antiviral defence57. In response to a methicillin-resistant Staphylococcus aureus infection, mtROS production is also stimulated, leading to the formation of mitochondria-derived vesicles (MDVs)64. These MDVs delivered mitochondrial matrix enzyme manganese superoxide dismutase (SOD2) to bacteria-filled phagosomes, enhancing bacterial clearance.
Beyond their direct antimicrobial effects, infection-induced mtROS can trigger the production of pro-inflammatory cytokines. Herb et al.65 demonstrated that Listeria monocytogenes infected murine macrophages generate mtROS, which enter the cytosol and induce secretion of pro-inflammatory cytokines65. Likewise, complex I-derived mtROS are implicated in the stabilisation of hypoxia-inducible factor 1 alpha (HIF-1α) and expression of IL-1β downstream of prolonged TLR4 activation59,66. As previously mentioned, mtROS have been repeatedly implicated in the activation of the NLRP3 inflammasome and the subsequent maturation of IL-1β and IL-18, through an indirect mechanism that will be discussed further on40,45,46,48,49,67,68. Interestingly, a gain-of-function mutation in leucine-rich repeat kinase 2 (Lrrk2G2019S), which is associated with familial Parkinson’s disease, leads to increased mtROS and a functional switching of cell death pathways in macrophages69. Specifically, mtROS redirects GSDMD to mitochondrial membranes triggering a switch to necroptosis and a hyperinflammatory response to Mycobacterium tuberculosis infection69. In agreement, ROS-mediated oxidation of cysteine 192 in GSDMD has also been shown to promote GSDMD oligomerisation and pyroptotic cell death70. The idea that mtROS are pro-inflammatory in nature is further supported by the anti-inflammatory action of mitophagy. Mitophagy serves as a protective mechanism against excessive mtROS by selectively degrading damaged mitochondria, as observed with IL-10 antagonism of LPS triggered inflammation30, while the absence of autophagy also results in ROS-dependent amplification of retinoic acid-inducible gene I (RIG-I)-like signalling71–73.
Despite the emerging importance of mtROS signalling, the relative contribution of complex I versus complex III to mtROS generation in inflammatory macrophages is currently a topic of debate56,59,74. The role of complex I in OxPhos is to harvest electrons from NADH and transfer them to the ubiquinone (CoQ) pool while pumping protons across the IMM. This forward electron transfer (FET) will occur if the difference in reduction potential between the NAD+/NADH and the CoQ/CoQH2 couples (ΔEh) is sufficient to pump protons against Δp, which is composed of the mitochondrial membrane potential (ΔΨm) and pH gradient (i.e. 2ΔEh > 4Δp)56. When 4Δp > 2ΔEh, electrons can also be transferred in the reverse direction, known as reverse electron transport (RET), from the CoQ pool through complex I to flavin mononucleotide (FMN), and subsequently passed to O2 to generate O2•−56. Indeed, current evidence favours this model of activation downstream of TLR4 activation, albeit from indirect measurements58,59,66. On the other hand, complex III transfers electrons from CoQH2 to cytochrome c (cyt c) via the Q-cycle and can generate O2•− at the Qo site56. However, the physiological relevance of O2•− production at complex I is thought to be higher than that of complex III55,56. Despite this, complex III-derived mtROS is reported to drive oxidative DNA damage in macrophages enforcing reliance on NAD+ salvage pathways to sustain aerobic glycolysis and pro-inflammatory cytokine production74. The evidence for complex III mtROS derives primarily from the use of Qo site inhibitor myxothiazol, while showing no impact of rotenone74. However, since RET is dependent on 4Δp > 2ΔEh, which will be impacted by complex III inhibition, the use of this compound cannot exclude mtROS production at complex I56.
Mitochondria in neutrophils, historically undervalued due to their preference for glycolysis, have recently gained recognition for their involvement in neutrophil extracellular traps (NETs), motility, degranulation, and respiratory burst75. The production of ROS by neutrophils during the respiratory burst is a key mechanism for regulating infection and inflammation75. While mtROS do not directly contribute to intracellular ROS stores, it is implicated in the oxidative burst caused by NOX2 activation and degranulation76. Notably, production of mtROS regulates neutrophil motility in vivo, as demonstrated by Zhou et al. using a zebrafish model77.
NETs are complex networks comprised of modified chromatin and bactericidal proteins, which were initially associated with cell death in a process termed NETosis. It is now recognised that NETosis exists in two forms: the prolonged ‘suicidal’ NETosis and the rapid ‘vital’ NETosis that leaves neutrophils alive78–80. Classically, NETosis was believed to be dependent on ROS produced by cytosolic NOX281. However, Douda et al.82 and Reithofer et al.83 elucidated the mechanisms behind a second NOX-independent NETosis type, demonstrating that calcium (Ca2+)-dependent NETosis requires Ca2+ influx from lysosomes or the extracellular space. Mitochondria sense these elevated Ca2+ levels, generating mtROS. Both cytoplasmic Ca2+ and mtROS generated at complex III of the ETC are required for activation of peptidyl arginine deiminase 4 (PAD4), crucial for chromatin decondensation and NETosis82–84. However, the evidence for complex III-derived O2•− was determined from the use of the Qi site inhibitor antimycin A56. There was no decrease observed with myxothiazol or the complex III-specific O2•− suppressor, S3QEL84,85. As such, the source of mtROS in neutrophils remains to be definitively determined. Finally, NETs containing mtDNA oxidised by mtROS induce high levels of type I interferon (IFN) signalling and are reported to contribute to systemic lupus erythematosus (SLE)86,87.
This emerging role for mtROS in inflammation and anti-microbial activity highlights a critical repurposing of mitochondrial function away from OxPhos toward redox signalling. However, many open questions remain about how such signals propagate from mitochondria in the presence of abundant anti-oxidants to engage their reported targets in different cellular compartments. One hypothesis posits that mtROS signalling to the cytosol is achieved by localised redox relays involving peroxiredoxins and glutathione peroxidases88,89, which remains to be explored in the context of innate immune signalling. Alternatively, a second proposal is the floodgate model, which involves the inactivation of scavenging enzymes, enabling the oxidation of target proteins by H2O289. While redox signalling may be important for cellular and organismal homeostasis, it can also contribute to disease pathology under certain circumstances59,90 and so identifying the source of mtROS will be a critical question to address in the future. To elucidate the source of mtROS in innate immune cells, genetic models will likely be required. One model, the ND6 G14600A mtDNA mutation, which leads to a proline to leucine substitution at position 25 in the ND6 subunit of complex I (ND6-P25L), may be used in the future56,91. Importantly, the mutant complex I is fully active for NADH oxidation and has little impact on FET, but cannot generate ROS by RET91. It also protects the heart from ischaemia-reperfusion (I/R) injury, a process driven by succinate oxidation and O2•− production by RET90–92.
Mitochondrial membrane potential (ΔΨm) and Ca2+
In addition to its role in maintaining cell phosphorylation potential, ΔΨm is indispensable for multiple aspects of mitochondrial physiology, including mtROS production and the transport of many proteins, metabolites, and ions56,93. Mills et al. have highlighted that LPS stimulation augments ΔΨm in macrophages, which together with the enhanced oxidation of succinate by complex II, results in accumulation of mtROS and elevated Il1b gene expression59. Conversely, alternatively activated IL-4-stimulated macrophages exhibit a dissipated ΔΨm when treated with the lipid immunomodulator prostaglandin E2 (PGE2)94. Mechanistically, PGE2-induced dissipation of ΔΨm was related to the malate-aspartate shuttle and led to voltage-dependent changes in gene expression, partly regulated by the transcription factor ETS variant 1 (ETV1)94. These studies are noteworthy as they provide evidence that external stimuli, in this case LPS and PGE2, can alter ΔΨm, thereby inducing mitochondria-to-nucleus retrograde communication and fine-tuning macrophage polarisation states.
Moreover, other roles for ΔΨm have recently emerged in different innate immune subsets. Efferocytosis, the successful clearance of apoptotic cells by phagocytes, effectively doubles the content of the engulfing cell, thereby introducing many more metabolites95. Park and colleagues illustrated that the mitochondrial membrane protein uncoupling protein 2 (UCP2), which lowers ΔΨm, is essential for the functional clearance of apoptotic target cells but not for the clearance of synthetic targets96. Similarly, aged DCs that exhibited lower ΔΨm and coupling efficiency were less efficient at endocytosing irradiated cells and cross-presenting antigens to T cells than their younger counterparts97. This effect of reduced ΔΨm on antigen processing and presentation has also been observed as a result of physiological carbon monoxide production98. Furthermore, inducing mitochondrial dysfunction in younger DCs diminished their phagocytic and cross-presenting capacity, whereas mtROS specifically affected cross-presentation. This aligns with the work of Oberkampf and colleagues, who demonstrated that mtROS regulate cross-presentation to cytotoxic T cells by plasmacytoid DCs (pDCs)99.
Another important aspect of the ΔΨm is its role as the driving force behind the uptake of Ca2+ into the mitochondrial matrix. Cytosolic Ca2+ serves as a pivotal intracellular signalling messenger, implicated in processes such as exocytosis, cell motility, and apoptosis100. Regulation of cytosolic Ca2+ primarily occurs through Ca2+ uptake from the extracellular space and release from organelles, such as the endoplasmic reticulum. Elevated levels of cytosolic Ca2+ trigger Ca2+ influx into the mitochondrial matrix through the mitochondrial calcium uniporter complex (MCU), thereby buffering cytosolic Ca2+ and regulating mitochondrial respiration93. This complex consists of the channel-forming subunit MCU and its regulators MICU1, MICU, MCUb, EMRE, MCUR1 and miR-25101.
The MCU has been the subject of many studies investigating its role in macrophage function. For instance, the MCU functions as a regulator of phagocytosis-dependent NLRP3 inflammasome activation in response to bacterial challenges102–105. Mechanistically, mitochondrial Ca2+ uptake inhibits endosomal sorting complex required for transport (ESCRT)-mediated phagolysosomal membrane repair, which enables NLRP3 activation105. Additionally, expression of MCU and MICU1 inversely correlate with age, resulting in reduced mitochondrial Ca2+ uptake in aging macrophages106. This leads to an amplification of cytosolic Ca2+ oscillations, a major driver of nuclear factor kappa B (NF-κB) activation and inflammation. Interestingly, the abundance of the dominant-negative subunit MCUb is associated with macrophage polarisation during skeletal muscle regeneration, indicating that the composition of the MCU complex influences macrophage phenotypes107. This was underscored by Lu et al.108, who investigated the role of the MCU in atherosclerosis-mediated efferocytosis dysfunction. Using an MCU-specific inhibitor, they were able to attenuate the upregulation of MCU and MCUR1 and the downregulation of MCUb induced by oxidised low-density lipoprotein, which coincided with reduced production of ROS and pro-inflammatory cytokine and improved efferocytosis108. In DCs, circadian changes in mitochondrial Ca2+ have also been found to regulate antigen processing and T cell activation109. These rhythmic changes in mitochondrial Ca2+ were driven by the circadian control of key regulators of the mitochondrial calcium uniporter complex, including MCUb and EMRE.
Finally, recent work by Monteith et al.110 demonstrated that the MCU, and the resulting Ca2+ flux, steers neutrophils away from primary degranulation and towards suicidal NETosis110. Murine neutrophils deficient in MICU1 exhibited increased bactericidal activity, particularly in the presence of macrophages or during systemic S. aureus infection110. Moreover, activation of the MCU and mitochondrial Ca2+ uptake promotes neutrophil polarisation and chemotaxis, further emphasizing the critical importance of mitochondrial Ca2+ dynamics in innate immune cells111. All of these studies on ΔΨm and Ca2+ together illustrate how virtually all key effector functions of innate immune cells are governed by mitochondrial physiology and strongly illustrate the concept of mitochondria as an information processing system.
Mitochondrial nucleic acid signalling
mtDNA
Mitochondrial nucleic acids encompass the entire genetic material found within mitochondria, which includes mtDNA and mitochondrial RNA (mtRNA). The primary component, mtDNA, exists in multiple copies within each mitochondrion, with the number varying depending on the cell type and energy demand1. In humans, mtDNA consists of a circular, double-stranded molecule containing approximately 16,500 base pairs1. Unlike nuclear DNA, mtDNA is only inherited matrilineally, reflecting its unique evolutionary history and mode of transmission1. Within mtDNA, there are 37 regions encoding essential genes critical for mitochondrial function, including 13 subunits of the ETC involved in OxPhos, as well as transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs) necessary for mitochondrial translation1. However, this only represents a minor component of the mitochondrial proteome, with the remaining 99% encoded by the nuclear genome112. The similarities between eukaryotic mtDNA and bacterial DNA is a key piece of evidence for the endosymbiotic origin of mitochondria. However, these properties also allow mitochondrial signals to act as endogenous danger-associated molecular patterns (DAMPs) to drive inflammation113–115.
mtDNA, akin to bacterial DNA, possesses a significant proportion of hypomethylated CpG dinucleotides, which are motifs recognised by TLR9 to trigger an innate immune response116. Tissue injury resulting from trauma can induce a systemic inflammatory response syndrome (SIRS), which shares clinical similarities with sepsis115. In SIRS, the release of mitochondrial DAMPs, including N-formyl peptides and mtDNA, activate polymorphonuclear neutrophils (PMNs)115. This activation leads to degranulation and organ injury following TLR9 sensing of mtDNA (Fig. 2)115. Additionally, mtDNA and TLR9 activation drive NET formation and lung injury during primary graft dysfunction after lung transplantation117. Furthermore, previous research by Oka et al.118 demonstrated that mtDNA escape from autophagy in cardiomyocytes contributes to TLR9-mediated inflammation and subsequent heart failure118. Collectively, these studies suggest that TLR9 sensing of mtDNA is essential for driving pathological sterile inflammation following injury.
Fig. 2. Mitochondrial nucleic acid signalling in innate immunity.
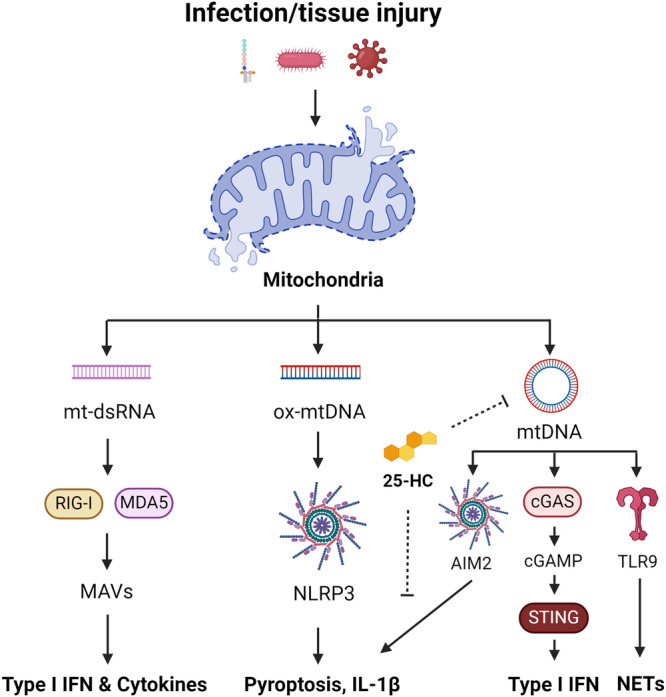
Infection with bacteria or viruses, as well as tissue injury, can lead to mitochondrial damage and the release of mitochondrial nucleic acids, including mtDNA and mt-dsRNA. mt-dsRNA can be sensed by RIG-I and MDA5, which signal via MAVS, to promote the expression of type I IFN and pro-inflammatory cytokines. Ox-mtDNA is a reported ligand for the NLRP3 inflammasome triggering pyroptosis and IL-1β maturation. mtDNA also activates the AIM2 inflammasome, cGAS-STING pathway, and TLR9 to drive type I IFN and NETosis. 25-HC can inhibit mtDNA release and AIM2 activation arising from elevated cholesterol.
In addition to TLR9, mtDNA can also be detected by other intracellular sensors such as the absence in melanoma 2 (AIM2) inflammasome119, the NLRP3 inflammasome46–49, and the cyclic GMP–AMP synthase (cGAS)-stimulator of interferon response cGAMP interactor 1 (STING) pathway (Fig. 2)120. AIM2, a cytosolic DNA sensor, triggers the maturation of IL-1β and pyroptosis in response to mtDNA release119,121. Research by Dang et al.119 highlights the role of 25-hydroxycholesterol (25-HC) in limiting cholesterol-dependent mtDNA release following bacterial infection or LPS stimulation in macrophages119. This suggests that macrophages employ mechanisms to preserve mitochondrial integrity and prevent excessive AIM2-mediated inflammation.
While AIM2 can sense mtDNA, newly synthesized and oxidised mtDNA (ox-mtDNA) fragments are reported to activate the NLRP3 inflammasome, driving the processing of IL-1β38. Recent in vitro studies propose that the pyrin domain of NLRP3 shares a protein fold with DNA glycosylases, potentially enabling recognition of ox-mtDNA122. However, an unidentified mediator may also be involved. Zhu and colleagues identified an orphan receptor, Nur77, which binds both intracellular LPS and mtDNA, leading to non-canonical NLRP3 activation123. This mechanism was found to be independent of canonical activation and unlikely to serve as an ox-mtDNA receptor for canonical NLRP3 signalling. Recent findings have also cast doubt on the role of mtROS production in canonical NLRP3 inflammasome activation38. As such, further research is required to understand the precise role of mtROS and ox-mtDNA in this signalling axis.
cGAS functions as both a nuclear and cytosolic protein, responding to cytosolic double-stranded DNA by catalysing the formation of cGAMP, a second messenger that initiates an inflammatory response via STING120,124. The activation of STING by cGAMP promotes type I IFN production via the transcription factor interferon-regulatory factor 3 (IRF3), initiating an antiviral immune response. The cGAS-STING pathway plays a crucial role in sensing intracellular pathogens, including M. tuberculosis125, herpesvirus120, dengue virus126, norovirus127, influenza A virus128, encephalomyocarditis virus128 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV2)129, which all promote the release of mtDNA to enhance detection and antiviral signalling. Moreover, cGAS is also involved in detecting extracellular bacteria including Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus130. These findings indicate that the surveillance of mitochondrial integrity cooperates with viral and bacterial sensing mechanisms to fully engage the host innate immune response. However, in the case of SARS-CoV2 and coronavirus disease (COVID19), this can lead to severe lung inflammation and pathology, primarily driven by macrophages129.
mtRNA
The process of mtDNA transcription and translation occurs primarily within the mitochondria, facilitated by the mtRNA machinery. mtRNA includes precursor transcripts that undergo processing to produce mature mRNAs, tRNAs, and rRNAs, which are essential for mitochondrial protein synthesis. A consequence of the bacterial origin of circular mtDNA is that it is subject to bidirectional transcription, which generates overlapping transcripts capable of forming long double-stranded RNA (dsRNA) structures131–133.
Similar to mtDNA, mitochondrial dsRNA (mt-dsRNA) has been found to trigger a type I IFN response mediated by the cytosolic viral RNA sensors, RIG-I134 or melanoma differentiation-associated protein 5 (MDA5) (Fig. 2)133. Deletion of the autophagy protein IRGM1 in macrophages has been shown to impair mitophagy and drive inflammation via TLR7 signalling135. TLR7, an endosomal TLR, senses viral and bacterial single-stranded RNA (ssRNA)136,137, suggesting it may also sense mtRNA following mitochondrial damage. Supporting this notion, inhibition of the TCA cycle enzyme fumarate hydratase (FH) in LPS-stimulated macrophages, which impairs mitochondrial respiration, is reported to drive IFNβ release via the combined action of RIG-I, MDA5 and TLR7138. However, further work is required to determine if this is the case. Suppression of inflammatory mitochondrial RNA species also appears crucial to prevent autoimmunity. Defects in RNA editing by ADAR1139, essential to prevent dsRNA/MDA5-mediated inflammation, and TLR7 gain-of-function mutations140, are previously underappreciated mechanisms of common inflammatory diseases, such as SLE.
Together, these studies provide compelling evidence that mitochondrial nucleic acid signalling regulates host innate immune responses to resolve the infection. However, these signalling events must be tightly controlled in order to prevent immunopathology. This dual role reinforces the concept of mitochondria as sequestered processors within the cell, highlighting the importance of maintaining this endosymbiotic relationship7.
Mitochondrial metabolite and lipid signalling
TCA cycle remodelling, signalling and anti-microbial action
The tricarboxylic acid cycle (TCA cycle), also known as the citric acid cycle or the Krebs cycle, is a fundamental aspect of cellular metabolism141. Consisting of a series of enzymatic reactions, the TCA cycle plays a crucial role in extracting energy from carbohydrates, fats, and proteins to produce NADH and FADH2, which then fuel the ETC for ATP synthesis141–143. Besides its energy-generating function, the TCA cycle contributes to biosynthetic processes by providing precursors for the synthesis of amino acids, nucleotides, and other essential biomolecules143. Recent studies have also revealed intricate connections between TCA cycle remodelling and innate immunity, unveiling a novel dimension of immune regulation19,33,142,144–146. Importantly, metabolic intermediates generated from the TCA cycle, or TCA cycle metabolites themselves, serve as signalling molecules that modulate immune responses beyond their roles in bioenergetics or biosynthetic pathways, which have been extensively reviewed elsewhere142,147–150. Here, we will highlight several key findings in this area of research focusing on the mechanisms and kinetics of TCA cycle remodelling, and the anti-microbial and/or signalling roles of α-ketoglutarate, succinate, fumarate, and itaconate.
As discussed earlier, inflammatory macrophages and DCs suppress OxPhos in a manner dependent on NO production18,20. However, TCA cycle remodelling downstream of TLR4 activation occurs in stages eventually leading to the initial accumulation of succinate and itaconate, followed by their decrease after prolonged stimulation (Fig. 3A)19,138,145,151. This process is reported to occur in two stages33, but a case for three stages could also be made152. In the first stage, LPS stimulation transiently increases mitochondrial respiration152. This stage is dependent on the mitochondrial glycerol 3-phosphate dehydrogenase (GPD2), a component of the glycerol phosphate shuttle, which enhances glucose oxidation to fuel acetyl-CoA-mediated histone acetylation of key inflammatory genes152. Importantly, acetyl-CoA is synthesized by the ATP-citrate lyase in the cytosol from mitochondrial-derived citrate152–154. After this first stage, two different breakpoints of the TCA cycle have been proposed. The first metabolic breakpoint occurs at isocitrate dehydrogenase (IDH), while the second break point occurs at complex II, also known as succinate dehydrogenase (SDH) (early)33,144. The breakpoint at IDH has been attributed to the decreased expression and activity downstream of autocrine type I IFN signalling155. Conversely, the breakpoint at complex II has been attributed to immunoresponsive gene 1 (IRG1), also known as cis-aconitate decarboxylase (CAD), mediated itaconate synthesis. Itaconate acts as a weak competitive inhibitor of complex II145,146,151 and is also reported to inhibit IDH2156, linking itaconate to both TCA cycle breakpoints. The third stage of TCA cycle reprogramming (late) is largely driven by the inhibition of pyruvate dehydrogenase complex (PDHC) and the oxoglutarate dehydrogenase complex (OGDC)33. Mechanistically, this is controlled by dynamic changes in the lipoylation state of both PDHC and OGDC E2 subunits and phosphorylation of the PDHC E1 subunit33. Additionally, this may be linked to NO production, which inhibits the TCA cycle enzyme aconitase 2 (ACO2) and PDHC25,157. These two stages of metabolic reprogramming are crucial mechanisms to support acute phase inflammation and restrict a hyperinflammatory response.
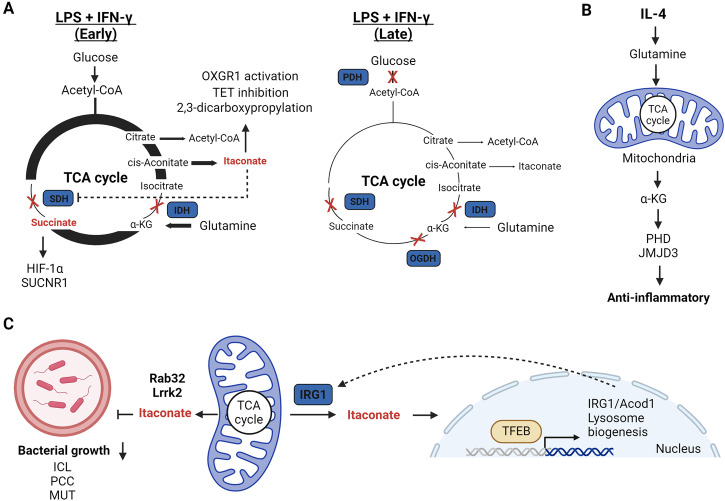
Fig. 3. Mitochondrial metabolite signalling and anti-bacterial activity in innate immunity.
A TCA cycle remodelling during early and later phases of stimulation post LPS and IFN-γ. Early- to mid-phase changes lead to increased itaconate and succinate levels that can signal through a variety of mechanisms before decreasing at a later stage. B IL-4-mediated increases in glutamine anaplerosis leads to high levels of α-KG that promotes anti-inflammatory gene expression via increased PHD and JMJD3 activity. C Mitochondrial-derived itaconate is trafficked into phagolysosomes in a TFEB, Rab32, Lrrk2-dependent manner where it is directly anti-bactericidal via inhibition of ICL, PCC, or MUT. Itaconate also activates TFEB to promote lysosome biogenesis.
The accumulation and release of succinate has emerged as a crucial signal influencing innate immune responses in both normal and pathological states. In macrophages, elevated levels of succinate are reportedly exported from mitochondria to the cytosol during TCA cycle rewiring, stabilising HIF-1α and thereby promoting the synthesis of pro-inflammatory cytokines such as IL-1β19,33. HIF-1α stability is regulated by prolyl hydroxylases (PHDs), which target it for degradation by the proteasome, requiring α-KG as a substrate. Notably, α-KG has demonstrated the ability to dampen the activation of pro-inflammatory macrophages, supporting endotoxin tolerance post-activation (Fig. 3B)158,159. Mechanistically, α-KG suppresses IKKβ and NF-κB in a PHD-dependent manner and impedes the stabilisation of HIF-1α158,159. Thus, a reduced α-KG:succinate ratio is associated with the pro-inflammatory phenotype. In contrast, glutamine-derived α-KG is also required for alternative macrophage activation driving FAO and Jmjd3-dependent epigenetic reprogramming of IL-4 target genes158. Succinate oxidation at complex II within mitochondria is also proposed to drive mtROS from complex I by RET, thereby stabilising HIF-1α92. Given the reports of complex II inhibition by itaconate and dimethyl malonate (DMM), which increase succinate levels, reduce HIF-1α, and limit IL-1β43,59,145, succinate oxidation is likely a stronger driving force for HIF-1α stabilisation than succinate accumulation per se and requires further investigation. In DCs, succinate is associated with the mobilisation of intracellular Ca2+, leading to migratory responses and acting synergistically with TLR ligand stimulation to produce pro-inflammatory cytokines160. In this instance, succinate drives this response via binding to its G-protein coupled receptor succinate receptor 1 (SUCNR1), also known as GPR91, on the cell surface. Strikingly, extracellular liver succinate can drive inflammation and non-alcoholic fatty liver disease (NAFLD), which indicates succinate can act as a mitochondrial DAMP161. However, succinate-SUCNR1 can also promote hyperpolarisation of anti-inflammatory macrophages162 and decrease inflammatory markers in adipose tissue163, suggesting that succinate signalling is context specific.
Itaconate has emerged as a potent immunoregulatory metabolite primarily synthesized by cells of the myeloid lineage150. In macrophages, itaconate plays dual roles as a potent anti-bactericidal metabolite and an immunomodulator that restricts the production of pro-inflammatory cytokines (Fig. 3C)145,150,151,164. Previously, the anti-bacterial properties of itaconate were attributed to its interference with bacterial growth through the inhibition of enzymes such as isocitrate lyase (ICL) in the glyoxylate cycle151,165 or propionyl-CoA carboxylase (PCC) in the citramalate cycle166. More recently, itaconate was reported to undergo conversion into the CoA derivative itaconyl-CoA167, which limits M. tuberculosis growth by inhibiting B12-depencent methylmalonyl-CoA mutase (MUT)168. As such, itaconate can target multiple enzymes of pathogen propionate metabolism to enforce nutrient stress. To combat intracellular bacteria such as Legionella169 and Salmonella170, mitochondrial-derived itaconate is delivered to phagolysosomes. In the case of Salmonella infection, this host defence mechanism relies on a scaffolding complex involving mitochondria, IRG1/CAD, the GTPase Rab32, Lrrk2 and Salmonella-containing vacuoles (SCVs)170,171. This crosstalk between mitochondria and phagolysosomes is dependent on the lysosomal biogenesis factor transcription factor EB (TFEB)172. Itaconate, in turn, induces lysosome formation by disrupting mTOR/14-3-3-mediated cytosolic retention of TFEB173. Therefore, itaconate is both a direct anti-bactericidal agent and co-ordinator of cellular lysosomal signalling. However, many pathogens have evolved intricate mechanisms in an attempt to evade the anti-bacterial action of itaconate. For instance, Yersinia pestis and Pseudomonas aeruginosa encode the enzymes itaconate CoA transferase, itaconyl-CoA hydratase, and (S)-citramalyl-CoA lyase that metabolise itaconate to pyruvate and acetyl-CoA and promote their survival in macrophages174. Conversely, M. tuberculosis encode the bifunctional enzyme β-hydroxyacyl-CoA lyase required for itaconate and leucine catabolism175. This nicely highlights the evolutionary arms races that occur between primary pathogens and host immune responses.
Beyond its anti-bacterial role, itaconate exhibits immunomodulatory properties via several mechanisms, for an in-depth analysis this has been nicely reviewed elsewhere150,176,177. Initially recognised as an anti-inflammatory metabolite for its ability to inhibit complex II145, itaconate has since been identified as a mildly electrophilic compound capable of alkylating protein cysteine thiols164, a process termed 2,3-dicarboxypropylation, and glutathione178. In addition, itaconate has also been identified as a competitive inhibitor of the TET family of α-KG-dependent DNA dioxygenases179 and a ligand of the α-KG receptor OXGR1180. The mild electrophilic nature of itaconate enables derivatives, such as dimethyl itaconate (DMI) or 4-octyl itaconate (4-OI), to modify various metabolic enzymes, redox regulators, and immune proteins150,176. Target modification by itaconate derivatives leads to activation of the anti-oxidant and stress-responsive transcription factors nuclear factor erythroid 2-related factor 2 (NRF2) and activating transcription factor 3 (ATF3), which in turn can limit pro-inflammatory cytokines such as IL-1β and IL-6164,178. NRF2 stabilisation is also decreased in IRG1-deficient macrophages and Kupffer cells under certain contexts suggesting a role for endogenous itaconate in NRF2 stabilisation178,181,182. However, treatment with underivatised itaconate has mixed results with regard to NRF2, increasing stability in some instances183, but not in others43 for unclear reasons. Furthermore, itaconate derivatives also alkylate key enzymes of glycolysis, including fructose-bisphosphate aldolase A (ALDOA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), thereby curbing aerobic glycolysis associated with pro-inflammatory macrophage activation184,185. The list of targets modified by itaconate derivatives continues to grow and current data indicates they may represent a novel class of anti-inflammatory agents with clinical potential during infection and inflammatory disease176,177. Itaconate accumulation, in combination with NO, is also an important mediator of innate immune tolerance limiting NLRP3 inflammasome activation and pyroptosis through mechanisms dependent on complex II inhibition or it’s electrophilic properties186–188. Furthermore, myeloid-derived IRG1 dampens neutrophil-mediated lung inflammation following M. tuberculosis infection, underscoring the importance of itaconate in vivo189. However, following trauma, itaconate-producing neutrophils play an important role in tissue inflammation and the wound healing process following tendon injury190. In contrast, itaconate production in DCs impairs anti-parasitic immune responses by promoting mtDNA-dependent PD-L1 expression following Plasmodium chabaudi infection, which limits CD8+ T cells191. This suggests that the beneficial effects of itaconate synthesis may vary depending on the pathogen involved. In summary, these studies highlight the importance of the mitochondrial IRG1-itaconate axis in regulating the innate immune response to pathogens, and for the most part, in restricting hyperinflammatory responses. However, the relative importance of endogenous itaconate cysteine reactivity versus metabolic perturbations during an immune response remains to be determined and requires further investigation.
Similarly to itaconate, fumarate is a mildly electrophilic metabolite that can modify protein cysteine thiols and glutathione, a process termed succination138. Fumarate levels increase following inflammatory macrophage activation in a mechanism dependent on glutamine anaplerosis and induction of aspartate-arginosuccinate shunt138,144. Inhibiting this shunt limits arginine synthesis and leads to a reduction in pro-inflammatory mediators, including NO and IL-6144. Fumarate accumulation can also enhance TNF-α production by inhibiting autocrine IL-10 signalling in macrophages138 and inhibiting lysine demethylase 5 (KDM5) histone demethylases in monocytes192. Furthermore, fumarate accumulation has also been implicated in anti-bacterial defence owing to its cysteine reactivity35, which can intoxicate pathogens including Mycobacterium tuberculosis193. The intricate interactions between the TCA cycle and innate immunity underscore the significance of metabolic reprogramming in shaping immune responses. Understanding the regulatory roles of TCA cycle intermediates, such as succinate and itaconate, opens new avenues for therapeutic interventions using immunomodulatory metabolite derivatives.
Cardiolipin signalling
Cardiolipin is a unique phospholipid found predominantly in the IMM of eukaryotic cells and can be found in most bacterial species194. Structurally, it consists of two phosphatidyl groups linked by a glycerol backbone, resulting in a dimeric structure. The presence of four acyl chains contributes to its distinctive conical shape, which promotes curvature of the membrane and cristae morphology194. Approximately 10-15% of all mitochondrial phospholipid content is cardiolipin194. Cardiolipin stabilises the respiratory chain complexes to support mitochondrial bioenergetics, whilst also being implicated in protein import, mitophagy, apoptosis and mitochondrial dynamics194. In addition to these identified functions, cardiolipin is emerging as a regulator of innate immune signalling and inflammatory cell death.
Cardiolipin found in human serum has been observed to have an interesting role in immune regulation195. It has been reported to promote the surface expression of the non-polymorphic major histocompatibility complex (MHC) class I-like molecule CD1d in DCs, a process that relies on peroxisome proliferator-activated receptor (PPAR) nuclear hormone receptors195. Furthermore, CD1d is capable of binding to bacterial and eukaryotic cardiolipin and when presented by DCs, can activate splenic and hepatic γδ T cells in vivo196. These findings suggest that DCs play a crucial role in antigen presentation of bacterial cardiolipin following infection or mitochondrial cardiolipin following tissue injury, which may represent a key immunosurveillance mechanism.
In macrophages, cardiolipin has been implicated in supporting NLRP3 inflammasome activation (Fig. 4)197. Research suggests that cardiolipin interacts with NLRP3 after translocation to OMM, indicating that the outer membrane is a critical site for co-ordinating NLRP3 signalling197. Notably, NLRP3 activation was hindered when cardiolipin synthase (CSL) was genetically silenced197. Recent findings also indicate that GSDMD causes mitochondrial damage by permeabilising both the IMM and OMM69,198,199. Mechanistically, impairing cardiolipin biosynthesis or the transfer of cardiolipin to the OMM by the scramblase PLSCR3 prevented GSDMD recruitment and subsequent pyroptosis198. However, high-resolution structures of NLRP3-activated ASC complexes using cryo-electron tomography do not show co-localisation with mitochondria, despite supporting GSDMD-mediated mitochondrial pore formation199. This data suggests that NLRP3 signalling at the OMM may not occur as previously suggested. However, it’s important to note that an earlier interaction between NLRP3 and the OMM, which may not have been captured in the structure, cannot be conclusively ruled out.
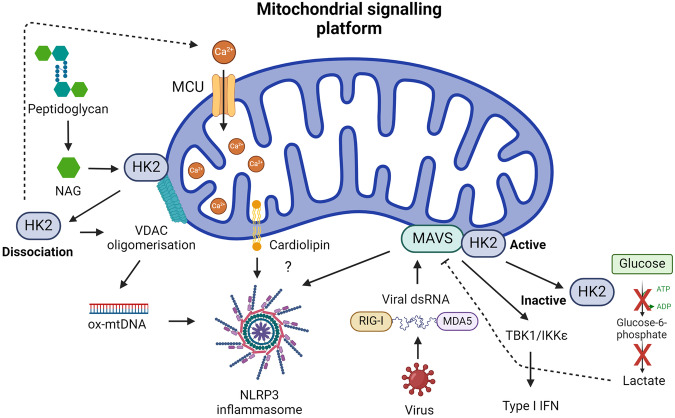
Fig. 4. Mitochondrial signalling platform in innate immunity.
MAVS localized to the OMM integrates the sensing of viral RNA by RIG-I or MDA5 to trigger a type I IFN response and recruit NLRP3 to mitochondria, an event also attributed to cardiolipin. HK2 association with MAVS and mitochondria promotes its enzymatic activity, which can lead to increased lactate and MAVS inhibition. An inhibition that’s relieved when HK2 dissociates from mitochondria. HK2 can also associate with VDAC and sense NAG, a breakdown product of bacterial peptidoglycan. HK2 dissociation from VDAC promotes mitochondrial Ca2+ uptake, VDAC oligomerisation, and mtDNA release to activate the NLRP3 inflammasome.
Furthermore, Reynolds et al. (2023) reported that loss of cardiolipin biosynthesis, achieved by silencing CSL in macrophages, also impaired Il1b expression via a complex II-dependent mechanism26. This indicates that a loss of cardiolipin could more broadly impact the pro-inflammatory response and limit NLRP3-mediated IL-β release by reducing pro-IL-1β levels. Indeed, the importance of cardiolipin in mitochondrial respiration and cristae architecture presents a challenge in distinguishing its role in bioenergetics from its involvement in OMM signalling when its biosynthesis is disrupted. Mitochondrial respiration relies on the proper functioning of respiratory chain complexes embedded within the IMM, where cardiolipin plays a crucial role in stabilising and optimising their activity. Disruption of cardiolipin biosynthesis can impair mitochondrial respiration, affecting cellular ATP production and potentially influencing NLRP3 inflammasome activation, as bioenergetics status is a known regulator of this process38. As such, the precise role of cardiolipin is unclear and will require sophisticated experimental approaches to disentangle.
Mitochondria as a signalling platform
A key facet of mitochondrial signal transduction is found in the organelles ability to function as a scaffold, thereby facilitating cellular signalling cascades. Central to this paradigm is mitochondrial antiviral signalling protein (MAVS), also known as IFNβ promoter stimulator 1 (IPS1), CARD adaptor inducing IFNβ (CARDIF) and virus-induced signalling adaptor (VISA), a key mediator that interfaces with RIG-I-like receptors (RLRs)200. As such, MAVS serves as a critical nexus for the integration of intracellular antiviral signalling (Fig. 4)201. Structurally, MAVS is a 540-amino acid protein comprising three distinct functional domains: an N-terminal CARD domain, a proline-rich region, and a C-terminal transmembrane domain. The N-terminal CARD domain of MAVS facilitates interaction with the CARD domains of RLRs, including RNA helicases RIG-I and MDA5202. This interaction is pivotal for initiating signalling events leading to the release of type I IFN and the activation of the NF-κB and IRF pathways203–205. The subcellular localisation of MAVS adds an additional layer of complexity to its function. MAVS dynamically associates with the OMM, endoplasmic reticulum, and peroxisomes, suggesting a versatile role in coordinating antiviral responses across distinct cellular compartments206. Upon viral infection, peroxisomal MAVS induces the rapid IFN-independent expression of defence factors that provide short-term protection, whereas mitochondrial MAVS activates a delayed IFN-dependent signalling pathway, which amplifies and stabilises the antiviral response206.
Upon activation, MAVS undergoes oligomerisation, thereby forming fibrils that induce membrane remodelling and signalling complex assembly207,208. Independent of RLR sensing of RNA, mtROS can promote MAVS oligomerisation and type I IFN production in SLE patients207, which suggest MAVS may act as a mitochondrial redox sensor. Like cardiolipin, MAVS is also reported to recruit the NLRP3 inflammasome to mitochondria, thereby triggering its activation209,210. This appears to occur when using standard NLRP3 stimuli209 and in response to Sendai Virus, also known as murine respirovirus, infection210. Notably, while a structural study failed to capture an interaction between NLRP3 and mitochondria using cryo-electron ET199, this study was not conducted in the context of viral infection. Therefore, it remains possible that NLRP3 interacts with mitochondria following viral sensing and MAVS oligomerisation, an aspect that warrants further investigation.
The localisation of MAVS to the mitochondrial membrane suggests a potential interplay with mitochondrial dynamics within macrophages. Recent studies propose that MAVS may influence mitochondrial morphology and function, thereby modulating the metabolic profile of macrophages during the course of an antiviral response. Specifically, the fusion mechanisms of the OMM are rigorously governed by Mitofusin 1 (Mfn1) and Mitofusin 2 (Mfn2), exerting regulatory control over MAVS activity211. Surprisingly, while Mfn1 positively regulates MAVS-mediated antiviral responses, its close homolog Mfn2 directly inhibits MAVS, possibly unrelated to its function in mitochondrial dynamics212,213. Thus, although Mfn1 and Mfn2 share the function of inducing mitochondrial fusion, they play opposing roles in viral innate immunity. Mitochondrial dynamics, especially mitochondrial fusion, appears crucial for the innate immune response. Conversely, promoting mitochondrial fission, via dynamin-related protein 1 (DRP1), inhibits MAVS activity during viral infection214,215.
Hexokinase 2 (HK2), a key enzyme in glucose metabolism has recently been identified as a novel interactor with MAVS (Fig. 4)216,217. When MAVS is inactive, it forms a complex with HK2, inducing its localisation to the mitochondria, where it associates with the OMM through its interaction with the voltage-dependent anion channel (VDAC), and maintaining its enzymatic activity216. RLR signalling disrupts glucose metabolism, leading to the downregulation of glycolysis. Mechanistically, MAVS, in its active state, binds to RIG-I, releasing HK2 into the cytoplasm, impairing its activity and subsequent glucose metabolism. HK2 inactivation leads to the decrease of intracellular lactate levels, which can inhibit RLR/MAVS signalling216. This intricate regulation suggests a role for the MAVS-HK2 axis in connecting the innate immune response with cellular bioenergetics during viral challenges. In macrophages, HK2 is also reported to associate with VDAC on mitochondria to act as an innate immune sensor for bacterial peptidoglycan218. Phagosomal processing of peptidoglycan leads to the release of N-acetylglucosamine (NAG) that inhibits HK2 triggering its dissociation from the OMM and activates NLRP3218. Mechanistically, HK2 dissociation from the OMM promotes mitochondrial Ca2+ uptake, VDAC oligomerisation and the release of mtDNA219. Finally, in DCs, TLR activation promotes HK2 association with mitochondria to facilitate the rapid induction of glycolysis, which was essential for DC activation32.
In summary, the mitochondrial signalling platform, often centred around MAVS, HK2 and VDAC, serve as a crucial nexus orchestrating innate immune responses against bacterial and viral infections. The convergence of mitochondrial dynamics and antiviral signalling pathways underscores the intricate cellular mechanisms deployed to counteract pathogenic threats. Future research endeavours focused on unravelling the complexities of RLR recruitment to mitochondria are poised to enhance our comprehension of this vital axis in innate immunity. Collectively, the studies on NLRP3 also highlight how all facets of mitochondrial physiology are intertwined and work together to drive activation of this complicated signalling complex.
Future outlook and concluding remarks
Much of the research conducted thus far has involved extensive in vitro stimulations of bone marrow- or monocyte-derived macrophages, DCs, and neutrophils to model in vivo cell populations. While these model systems are valuable for studying innate immune cell biology, they do not precisely replicate tissue-resident or infiltrating in vivo cell populations. The latter are often shaped by a complex and dynamic microenvironment that is difficult to reproduce in vitro220. However, there are now expanding toolkits emerging that will facilitate the measurement of metabolic genes and metabolism in immune cells in vitro and in vivo. Experimental changes to the medium composition and cell culture geometry can now more closely reproduce in vivo conditions without over complicating experimental methodologies221–223. Significant progress has been made in single-cell techniques, including single-cell RNA sequencing (scRNA-seq), which has been used to identify OxPhos as a distinguishing feature of tissue-resident macrophages across different organs under steady state and obesogenic conditions224. High-dimensional spectral flow cytometry has also identified tissue-resident macrophage metabolic heterogeneity during helminth infection225. Other emerging techniques, such as single-cell energetic metabolism by profiling translation inhibition (SCENITH), allow the study of energy metabolism using flow cytometry and have been applied to in vitro and ex vivo human and murine myeloid populations226,227. Finally, progress is also being made in mass spectrometry imaging (MSI), which has been applied for joint protein-metabolite profiling of single immune and cancer cells228. These expanding metabolic toolkits will enable greater investigations of mitochondrial metabolism and signalling in innate immune cell populations and beyond.
Since the designation of mitochondria as the ‘powerhouse of the cell’, further research has revealed, as discussed here, their role as centrally positioned signalling hubs essential for innate immune signalling. However, while the importance of mitochondria cannot be overstated, many aspects of how they influence innate immune function remain unclear. There remain many outstanding questions to be addressed in future work to better understand the role of mitochondria in innate immunity. This is exemplified by the NLRP3 inflammasome, which is evidently regulated by mitochondrial function (Table 1). What is the precise role of specific mitochondrial signals such as mtROS, cardiolipin, and ATP synthesis for the activation of this inflammatory signalling complex during bacterial and viral infection? And are these involved in NLRP3 activation in vivo? What is the source of mtROS and is it dependent on RET?56 The answer to these outstanding questions may aid with therapeutic targeting of this process during infection or inflammatory disease. Finally, given the importance of mitochondria to innate immune cell biology, to what extent are mitochondrial diseases a manifestation of innate immune cell dysfunction?229 Or what proportion of more common autoimmune disorders are driven by a break in mitochondrial endosymbiosis?7 We hope this review will inspire research into these and many other questions that remain to be explored and will promote a clearer comprehension of the extensive role of mitochondria in innate immunity.
Table 1.
Mitochondrial signal transduction and NLRP3 activation.
| Mitochondrial signal | Signalling | Outcome | Cell type |
|---|---|---|---|
| ATP and PCr synthesis | Cytosolic ATP synthesis by CKB | NLRP3 activation, IL-1β | Macrophage |
| mtROS (Complex I-derived) | HIF-1α stabilisation and Il1b transcription | NLRP3 activation, IL-1β | Macrophage |
| mtDNA, mtROS | Release of ox-mtDNA fragments | NLRP3 activation, IL-1β | Macrophage |
| Itaconate | Complex II inhibition, electrophilic properties | Inhibition of NLRP3 and pyroptosis | Macrophage |
| Cardiolipin | Biosynthesis and translocation to OMM | NLRP3 recruitment and activation, IL-1β, pyroptosis | Macrophage |
| MAVS | Oligomerisation on OMM | NLRP3 recruitment and activation, IL-1β | Macrophage |
| Mfn1 | MAVS activation | NLRP3 activation, IL-1β | Macrophage |
| Mfn2 | MAVS inhibition | NLRP3 inhibition | Macrophage |
| DRP1 | MAVS inhibition | NLRP3 inhibition | Macrophage |
| HK2 dissociation from OMM | Mitochondrial Ca2+ uptake increase, VDAC oligomerisation | mtDNA release, NLRP3 activation, IL-1β | Macrophage |
Acknowledgements
We would like to thank Dr. Alva Casey for her helpful comments and the Medical Research Council (MRC) (MC_UU_00028). Figures 1 to 4 were created using Biorender.com.
Author contributions
E.M., R.K., and D.G.R. wrote and edited the manuscript. E.M. and R.K. made the table. D.G.R. conceptualised the topic and made the figures with input from E.M. and R.K.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stewart, J. B. & Chinnery, P. F. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat. Rev. Genet.16, 530–542 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Spang, A. et al. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature521, 173–179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane, N. & Martin, W. The energetics of genome complexity. Nature467, 929–934 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Picard, M. & Shirihai, O. S. Mitochondrial signal transduction. Cell Metab34, 1620–1653 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature191, 144–148 (1961). [DOI] [PubMed] [Google Scholar]
- 6.Ryan, D. G., Frezza, C. & O’Neill, L. A. J. TCA cycle signalling and the evolution of eukaryotes. Curr. Opin. Biotechnol.68, 72–88 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy, M. P. & O’Neill, L. A. J. A break in mitochondrial endosymbiosis as a basis for inflammatory diseases. Nature626, 271–279 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Monzel, A. S., Enríquez, J. A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab.5, 546–562 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mailloux, R. J., Treberg, J., Grayson, C., Agellon, L. B. & Sies, H. Mitochondrial function and phenotype are defined by bioenergetics. Nat. Metab.5, 1641–1641 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Chandel, N. S. Mitochondria as signaling organelles. BMC Biol12, 34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janeway, C. A. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol.20, 197–216 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Pfeiffer, T., Schuster, S. & Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science292, 504–507 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Wang, Y. & Patti, G. J. The Warburg effect: a signature of mitochondrial overload. Trends Cell Biol33, 1014–1020 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warburg, O. The metabolism of carcinoma cells1. J. Cancer Res.9, 148–163 (1925). [Google Scholar]
- 15.Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity41, 14–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabeza-Cabrerizo, M., Cardoso, A., Minutti, C. M., Pereira da Costa, M. & Reis e Sousa, C. Dendritic cells revisited. Annu. Rev. Immunol.39, 131–166 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood115, 4742–4749 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everts, B. et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood120, 1422–1431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature496, 238–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Bossche, J. et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep.17, 684–696 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Cleeter, M. W., Cooper, J. M., Darley-Usmar, V. M., Moncada, S. & Schapira, A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett345, 50–54 (1994). [DOI] [PubMed] [Google Scholar]
- 22.Drapier, J. C. & Hibbs, J. B. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J. Immunol. Baltim. Md 1950140, 2829–2838 (1988). [PubMed] [Google Scholar]
- 23.Granger, D. L. & Lehninger, A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J. Cell Biol.95, 527–535 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltrán, B., Mathur, A., Duchen, M. R., Erusalimsky, J. D. & Moncada, S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc. Natl. Acad. Sci. USA97, 14602–14607 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmieri, E. M. et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun.11, 698 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds, M. B. et al. Cardiolipin coordinates inflammatory metabolic reprogramming through regulation of Complex II disassembly and degradation. Sci. Adv.9, eade8701 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vats, D. et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab4, 13–24 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, S. C.-C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol.15, 846–855 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowling, J. K. et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat. Commun.12, 1460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S. & Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science356, 513–519 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, F. et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab28, 463–475.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everts, B. et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat. Immunol.15, 323–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seim, G. L. et al. Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation. Nat. Metab.1, 731–742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timblin, G. A. et al. Mitohormesis reprogrammes macrophage metabolism to enforce tolerance. Nat. Metab.3, 618–635 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garaude, J. et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol.17, 1037–1045 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundahl, M. L. et al. Macrophage innate training induced by IL-4 and IL-13 activation enhances OXPHOS driven anti-mycobacterial responses. eLife11, e74690 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czimmerer, Z. et al. The epigenetic state of IL-4-polarized macrophages enables inflammatory cistromic expansion and extended synergistic response to TLR ligands. Immunity55, 2006–2026.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billingham, L. K. et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol.23, 692–704 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swanson, K. V., Deng, M. & Ting, J. P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol.19, 477–489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature469, 221–225 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Groß, C. J. et al. K+ Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity45, 761–773 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity38, 1142–1153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swain, A. et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat. Metab.2, 594–602 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prag, H. et al. Ischemia-selective protection against cardiac ischemia/reperfusion injury by malonate. J. Mol. Cell. Cardiol.173, S5 (2022). [Google Scholar]
- 45.Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science320, 674–677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol.12, 222–230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 Inflammasome during Apoptosis. Immunity36, 401–414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature560, 198–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xian, H. et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity55, 1370–1385.e8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Praticò, D. In vivo measurement of the redox state. Lipids36, S45–S47 (2001). [DOI] [PubMed] [Google Scholar]
- 51.O’Rourke, E. J. et al. Pathogen DNA as target for host-generated oxidative stress: Role for repair of bacterial DNA damage in Helicobacter pylori colonization. Proc. Natl. Acad. Sci. USA100, 2789–2794 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu, G. Y. et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med.202, 209–215 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staudinger, B. J., Oberdoerster, M. A., Lewis, P. J. & Rosen, H. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J. Clin. Invest.110, 1151–1163 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambeth, J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol.4, 181–189 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J.417, 1–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casey, A. M. & Murphy, M. P. Uncovering the source of mitochondrial superoxide in pro-inflammatory macrophages: Insights from immunometabolism. Biochim. Biophys. Acta BBA - Mol. Basis Dis.1868, 166481 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature472, 476–480 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carneiro, F. R. G., Lepelley, A., Seeley, J. J., Hayden, M. S. & Ghosh, S. An essential role for ECSIT in Mitochondrial Complex I Assembly and mitophagy in macrophages. Cell Rep22, 2654–2666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills, E. L. et al. Succinate Dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell167, 457–470.e13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlee, M. & Hartmann, G. Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol.16, 566–580 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chong, A., Lima Celia, A., Allan David, S., Nasrallah Gheyath, K. & Garduño Rafael, A. The purified and recombinant Legionella pneumophila Chaperonin alters mitochondrial trafficking and microfilament organization. Infect. Immun.77, 4724–4739 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geng, J. et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat. Immunol.16, 1142–1152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horwitz, M. A. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med158, 1319–1331 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abuaita, B. H., Schultz, T. L. & O’Riordan, M. X. Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control Phagosome-localized Staphylococcus aureus. Cell Host Microbe24, 625–636.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herb, M. et al. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci. Signal.12, eaar5926 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Kelly, B., Tannahill, G. M., Murphy, M. P. & O’Neill, L. A. J. Metformin inhibits the production of reactive oxygen species from NADH:Ubiquinone Oxidoreductase to limit induction of Interleukin-1β (IL-1β) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages. J. Biol. Chem.290, 20348–20359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruz, C. M. et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem.282, 2871–2879 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pétrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ.14, 1583–1589 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Weindel, C. G. et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell185, 3214–3231.e23 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Devant, P. et al. Gasdermin D pore-forming activity is redox-sensitive. Cell Rep.42, 112008 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee, H.-T. et al. The pathogenesis of systemic lupus erythematosus - From the viewpoint of oxidative stress and mitochondrial dysfunction. Mitochondrion30, 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Sun, K. et al. Autophagy-deficient Kupffer cells promote tumorigenesis by enhancing mtROS-NF-κB-IL1α/β-dependent inflammation and fibrosis during the preneoplastic stage of hepatocarcinogenesis. Cancer Lett.388, 198–207 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Tal, M. C. et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci.106, 2770–2775 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cameron, A. M. et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol.20, 420–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeon, J. H., Hong, C. W., Kim, E. Y. & Lee, J. M. Current understanding on the metabolism of neutrophils. Immune Netw20, e46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vorobjeva, N. et al. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol.96, 254–265 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Zhou, W. et al. Neutrophil-specific knockout demonstrates a role for mitochondria in regulating neutrophil motility in zebrafish. Dis. Model. Mech.11, dmm033027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med.13, 463–469 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Pilsczek, F. H. et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol.185, 7413–7425 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Yipp, B. G. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med18, 1386–1393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuchs, T. A. et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol.176, 231–241 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Douda, D. N., Khan, M. A., Grasemann, H. & Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci.112, 2817–2822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reithofer, M. et al. Alum triggers infiltration of human neutrophils ex vivo and causes lysosomal destabilization and mitochondrial membrane potential-dependent NET-formation. Faseb J34, 14024–14041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunham-Snary, K. J. et al. Mitochondria in human neutrophils mediate killing of Staphylococcus aureus. Redox Biol.49, 102225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orr, A. L. et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Biol.11, 834–836 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med.22, 146–153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang, H., Li, T., Chen, S., Gu, Y. & Ye, S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of Metformin. Arthritis Rheumatol67, 3190–3200 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Travasso, R. D. M., Sampaio Dos Aidos, F., Bayani, A., Abranches, P. & Salvador, A. Localized redox relays as a privileged mode of cytoplasmic hydrogen peroxide signaling. Redox Biol12, 233–245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reczek, C. R. & Chandel, N. S. ROS-dependent signal transduction. Curr. Opin. Cell Biol.33, 8–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature515, 431–435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yin, Z. et al. Structural basis for a complex I mutation that blocks pathological ROS production. Nat. Commun.12, 707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin, J. L. et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab.1, 966–974 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zorova, L. D. et al. Mitochondrial membrane potential. Anal. Biochem.552, 50–59 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanin, D. E. et al. Mitochondrial membrane potential regulates nuclear gene expression in macrophages exposed to Prostaglandin E2. Immunity49, 1021–1033.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doran, A. C., Yurdagul, A. & Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol.20, 254–267 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park, D. et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature477, 220–224 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chougnet, C. A. et al. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J. Immunol.195, 2624–2632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Riquelme, S. A., Pogu, J., Anegon, I., Bueno, S. M. & Kalergis, A. M. Carbon monoxide impairs mitochondria-dependent endosomal maturation and antigen presentation in dendritic cells. Eur. J. Immunol.45, 3269–3288 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Oberkampf, M. et al. Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat. Commun.9, 2241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol.1, 11–21 (2000). [DOI] [PubMed] [Google Scholar]
- 101.Marchi, S. & Pinton, P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J. Physiol.592, 829–839 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li, T. et al. Listeria monocytogenes upregulates mitochondrial calcium signalling to inhibit LC3-associated phagocytosis as a survival strategy. Nat. Microbiol.6, 366–379 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Rimessi, A. et al. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun.6, 6201 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Rimessi, A. et al. Pharmacological modulation of mitochondrial calcium uniporter controls lung inflammation in cystic fibrosis. Sci. Adv.6, eaax9093 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dong, H. et al. Mitochondrial calcium uniporter promotes phagocytosis-dependent activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA119, e2123247119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seegren, P. V. et al. Reduced mitochondrial calcium uptake in macrophages is a major driver of inflammaging. Nat. Aging3, 796–812 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feno, S. et al. The dominant-negative mitochondrial calcium uniporter subunit MCUb drives macrophage polarization during skeletal muscle regeneration. Sci. Signal.14, eabf3838 (2021). [DOI] [PubMed] [Google Scholar]
- 108.Lu, N. et al. Modulation of oxidized low-density lipoprotein-affected macrophage efferocytosis by mitochondrial calcium uniporter in a murine model. Immunol. Lett.263, 14–24 (2023). [DOI] [PubMed] [Google Scholar]
- 109.Cervantes-Silva, M. P. et al. The circadian clock influences T cell responses to vaccination by regulating dendritic cell antigen processing. Nat. Commun.13, 7217 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monteith, A. J. et al. Mitochondrial Calcium Uniporter affects neutrophil bactericidal activity during Staphylococcus aureus infection. Infect. Immun.90, e0055121 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng, X. et al. Phosphorylation of dynamin-related protein 1 at Ser616 regulates mitochondrial fission and is involved in mitochondrial calcium uniporter-mediated neutrophil polarization and chemotaxis. Mol. Immunol.87, 23–32 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res.49, D1541–D1547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mills, E. L., Kelly, B. & O’Neill, L. A. J. Mitochondria are the powerhouses of immunity. Nat. Immunol.18, 488–498 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Yoon, J., Kim, S., Lee, M. & Kim, Y. Mitochondrial nucleic acids in innate immunity and beyond. Exp. Mol. Med.55, 2508–2518 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature464, 104–107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol.5, 190–198 (2004). [DOI] [PubMed] [Google Scholar]
- 117.Mallavia, B. et al. Mitochondrial DNA stimulates TLR9-dependent neutrophil extracellular trap formation in primary graft dysfunction. Am. J. Respir. Cell Mol. Biol.62, 364–372 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature485, 251–255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dang, E. V., McDonald, J. G., Russell, D. W. & Cyster, J. G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell171, 1057–1071.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature520, 553–557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernandes-Alnemri, T., Yu, J.-W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature458, 509–513 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cabral, A. et al. Differential Binding of NLRP3 to non-oxidized and Ox-mtDNA mediates NLRP3 Inflammasome Activation. Commun. Biol.6, 1–12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu, F. et al. The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome. Immunity56, 753–767.e8 (2023). [DOI] [PubMed] [Google Scholar]
- 124.Marchi, S., Guilbaud, E., Tait, S. W. G., Yamazaki, T. & Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol.23, 159–173 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wiens, K. E. & Ernst, J. D. The Mechanism for Type I Interferon Induction by Mycobacterium tuberculosis is Bacterial Strain-Dependent. PLoS Pathog12, e1005809 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun, B. et al. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci. Rep.7, 3594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jahun, A. S. et al. Leaked genomic and mitochondrial DNA contribute to the host response to noroviruses in a STING-dependent manner. Cell Rep.42, 112179 (2023). [DOI] [PubMed] [Google Scholar]
- 128.Moriyama, M., Koshiba, T. & Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun.10, 4624 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Domizio, J. D. et al. The cGAS–STING pathway drives type I IFN immunopathology in COVID-19. Nature603, 145–151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou, C.-M. et al. Identification of cGAS as an innate immune sensor of extracellular bacterium Pseudomonas aeruginosa. iScience24, 101928 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aloni, Y. & Attardi, G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc. Natl. Acad. Sci. USA68, 1757–1761 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Young, P. G. & Attardi, G. Characterization of double-stranded RNA from HeLa cell mitochondria. Biochem. Biophys. Res. Commun.65, 1201–1207 (1975). [DOI] [PubMed] [Google Scholar]
- 133.Dhir, A. et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature560, 238–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tigano, M., Vargas, D. C., Tremblay-Belzile, S., Fu, Y. & Sfeir, A. Nuclear sensing of breaks in mitochondrial DNA enhances immune surveillance. Nature591, 477–481 (2021). [DOI] [PubMed] [Google Scholar]
- 135.Rai, P. et al. IRGM1 links mitochondrial quality control to autoimmunity. Nat. Immunol.22, 312–321 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science303, 1526–1529 (2004). [DOI] [PubMed] [Google Scholar]
- 137.Mancuso, G. et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol.10, 587–594 (2009). [DOI] [PubMed] [Google Scholar]
- 138.Hooftman, A. et al. Macrophage fumarate hydratase restrains mtRNA-mediated interferon production. Nature615, 490–498 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li, Q. et al. RNA editing underlies genetic risk of common inflammatory diseases. Nature608, 569–577 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature605, 349–356 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krebs, H. A. & Johnson, W. A. The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett117, K2–K10 (1980). [DOI] [PubMed] [Google Scholar]
- 142.Ryan, D. G. & O’Neill, L. A. J. Krebs cycle reborn in macrophage immunometabolism. Annu. Rev. Immunol.38, 289–313 (2020). [DOI] [PubMed] [Google Scholar]
- 143.Inigo, M., Deja, S. & Burgess, S. C. Ins and outs of the TCA Cycle: The central role of Anaplerosis. Annu. Rev. Nutr.41, 19–47 (2021). [DOI] [PubMed] [Google Scholar]
- 144.Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity42, 419–430 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Lampropoulou, V. et al. Itaconate links inhibition of Succinate Dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab24, 158–166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cordes, T. et al. Immunoresponsive Gene 1 and Itaconate inhibit Succinate Dehydrogenase to modulate intracellular succinate levels. J. Biol. Chem.291, 14274–14284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ryan, D. G. et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab.1, 16–33 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martínez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun.11, 102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ryan, D. G., Peace, C. G. & Hooftman, A. Basic mechanisms of Immunometabolites in shaping the immune response. J. Innate Immun.15, 925–943 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Neill, L. A. J. & Artyomov, M. N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol.19, 273–281 (2019). [DOI] [PubMed] [Google Scholar]
- 151.Michelucci, A. et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA110, 7820–7825 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Langston, P. K. et al. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat. Immunol.20, 1186–1195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Infantino, V. et al. The mitochondrial citrate carrier: a new player in inflammation. Biochem. J.438, 433–436 (2011). [DOI] [PubMed] [Google Scholar]
- 154.Infantino, V., Iacobazzi, V., Palmieri, F. & Menga, A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem. Biophys. Res. Commun.440, 105–111 (2013). [DOI] [PubMed] [Google Scholar]
- 155.Souza, D. P. D. et al. Autocrine IFN-I inhibits isocitrate dehydrogenase in the TCA cycle of LPS-stimulated macrophages. J. Clin. Invest.129, 4239–4244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Heinz, A. et al. Itaconate controls its own synthesis via feedback-inhibition of reverse TCA cycle activity at IDH2. Biochim. Biophys. Acta Mol. Basis Dis.1868, 166530 (2022). [DOI] [PubMed] [Google Scholar]
- 157.Palmieri, E. M. et al. Pyruvate dehydrogenase operates as an intramolecular nitroxyl generator during macrophage metabolic reprogramming. Nat. Commun.14, 5114 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu, P.-S. et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol.18, 985–994 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Abla, H., Sollazzo, M., Gasparre, G., Iommarini, L. & Porcelli, A. M. The multifaceted contribution of α-ketoglutarate to tumor progression: An opportunity to exploit? Semin. Cell Dev. Biol.98, 26–33 (2020). [DOI] [PubMed] [Google Scholar]
- 160.Rubic, T. et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat. Immunol.9, 1261–1269 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Mills, E. L. et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab.3, 604–617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Trauelsen, M. et al. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Rep.35, 109246 (2021). [DOI] [PubMed] [Google Scholar]
- 163.Keiran, N. et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol.20, 581–592 (2019). [DOI] [PubMed] [Google Scholar]
- 164.Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature556, 113–117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rao, G. R. & McFadden, B. A. Isocitrate lyase from Pseudomonas indigofera: IV. Specificity and inhibition. Arch. Biochem. Biophys.112, 294–303 (1965). [DOI] [PubMed] [Google Scholar]
- 166.Berg, I. A., Filatova, L. V. & Ivanovsky, R. N. Inhibition of acetate and propionate assimilation by itaconate via propionyl-CoA carboxylase in isocitrate lyase-negative purple bacterium Rhodospirillum rubrum. FEMS Microbiol. Lett.216, 49–54 (2002). [DOI] [PubMed] [Google Scholar]
- 167.Shen, H. et al. The human knockout gene CLYBL connects Itaconate to Vitamin B12. Cell171, 771–782.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ruetz, M. et al. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science366, 589–593 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Naujoks, J. et al. IFNs modify the Proteome of Legionella-Containing vacuoles and restrict infection via IRG1-derived Itaconic acid. PLoS Pathog12, e1005408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen, M. et al. Itaconate is an effector of a Rab GTPase cell-autonomous host defense pathway against Salmonella. Science369, 450–455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lian, H. et al. Parkinson’s disease kinase LRRK2 coordinates a cell-intrinsic itaconate-dependent defence pathway against intracellular Salmonella. Nat. Microbiol.8, 1880–1895 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schuster, E.-M. et al. TFEB induces mitochondrial itaconate synthesis to suppress bacterial growth in macrophages. Nat. Metab.4, 856–866 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang, Z. et al. Itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Mol. Cell82, 2844–2857.e10 (2022). [DOI] [PubMed] [Google Scholar]
- 174.Sasikaran, J., Ziemski, M., Zadora, P. K., Fleig, A. & Berg, I. A. Bacterial itaconate degradation promotes pathogenicity. Nat. Chem. Biol.10, 371–377 (2014). [DOI] [PubMed] [Google Scholar]
- 175.Wang, H. et al. An essential bifunctional enzyme in Mycobacterium tuberculosis for itaconate dissimilation and leucine catabolism. Proc. Natl. Acad. Sci. USA116, 15907–15913 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peace, C. G. & O'Neill, L. A. J. The role of itaconate in host defense and inflammation. J. Clin. Invest.132, e148548 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McGettrick, A. F. & O’Neill, L. A. Two for the price of one: itaconate and its derivatives as an anti-infective and anti-inflammatory immunometabolite. Curr. Opin. Immunol.80, 102268 (2023). [DOI] [PubMed] [Google Scholar]
- 178.Bambouskova, M. et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature556, 501–504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen, L.-L. et al. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat. Cell Biol.24, 353–363 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeng, Y.-R. et al. The immunometabolite itaconate stimulates OXGR1 to promote mucociliary clearance during the pulmonary innate immune response. J. Clin. Invest.133, e160463 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang, H. et al. Pre-operative exercise therapy triggers anti-inflammatory trained immunity of Kupffer cells through metabolic reprogramming. Nat. Metab.3, 843–858 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song, J. et al. Itaconate suppresses atherosclerosis by activating a Nrf2-dependent antiinflammatory response in macrophages in mice. J. Clin. Invest.134, e173034 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen, F. et al. Citraconate inhibits ACOD1 (IRG1) catalysis, reduces interferon responses and oxidative stress, and modulates inflammation and cell metabolism. Nat. Metab.4, 534–546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qin, W. et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat. Chem. Biol.15, 983–991 (2019). [DOI] [PubMed] [Google Scholar]
- 185.Liao, S.-T. et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat. Commun.10, 5091 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hooftman, A. et al. The immunomodulatory metabolite Itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab.32, 468–478.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bambouskova, M. et al. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep.34, 108756 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Domínguez-Andrés, J. et al. The Itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab.29, 211–220.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 189.Nair, S. et al. Irg1 expression in myeloid cells prevents immunopathology during M. tuberculosis infection. J. Exp. Med.215, 1035–1045 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Crossley, J. L. et al. Itaconate-producing neutrophils regulate local and systemic inflammation following trauma. JCI Insight8, e169208 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ramalho, T. et al. Itaconate impairs immune control of Plasmodium by enhancing mtDNA-mediated PD-L1 expression in monocyte-derived dendritic cells. Cell Metab. S1550-4131(24)00008–1 10.1016/j.cmet.2024.01.008. (2024) [DOI] [PMC free article] [PubMed]
- 192.Arts, R. J. W. et al. Glutaminolysis and Fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab.24, 807–819 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ruecker, N. et al. Fumarase deficiency causes protein and metabolite succination and intoxicates Mycobacterium tuberculosis. Cell Chem. Biol.24, 306–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Falabella, M., Vernon, H. J., Hanna, M. G., Claypool, S. M. & Pitceathly, R. D. S. Cardiolipin, mitochondria, and neurological disease. Trends Endocrinol. Metab.32, 224–237 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Leslie, D. S. et al. Serum lipids regulate dendritic cell CD1 expression and function. Immunology125, 289–301 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dieudé, M. et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells in the normal murine Repertoire. J. Immunol. Baltim. Md 1950186, 4771–4781 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity39, 311–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miao, R. et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity56, 2523–2541.e8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu, Y. et al. Cryo-electron tomography of NLRP3-activated ASC complexes reveals organelle co-localization. Nat. Commun.14, 7246 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature437, 1167–1172 (2005). [DOI] [PubMed] [Google Scholar]
- 201.Seth, R. B., Sun, L., Ea, C.-K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell122, 669–682 (2005). [DOI] [PubMed] [Google Scholar]
- 202.Wang, X. et al. MAVS Expression in Alveolar macrophages is essential for host resistance against Aspergillus fumigatus. J. Immunol. Baltim. Md 1950209, 346–353 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sun, Q. et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity24, 633–642 (2006). [DOI] [PubMed] [Google Scholar]
- 204.Xu, L.-G. et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell19, 727–740 (2005). [DOI] [PubMed] [Google Scholar]
- 205.Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol.6, 981–988 (2005). [DOI] [PubMed] [Google Scholar]
- 206.Dixit, E. et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell141, 668–681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Buskiewicz, I. A. et al. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci. Signal.9, ra115–ra115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hwang, M.-S. et al. MAVS polymers smaller than 80 nm induce mitochondrial membrane remodeling and interferon signaling. FEBS J286, 1543–1560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Subramanian, N., Natarajan, K., Clatworthy, M. R., Wang, Z. & Germain, R. N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell153, 348–361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park, S. et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J. Immunol. Baltim. Md 1950191, 4358–4366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Castanier, C., Garcin, D., Vazquez, A. & Arnoult, D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep.11, 133–138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Onoguchi, K. et al. Virus-Infection or 5′ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLOS Pathog6, e1001012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yasukawa, K. et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci. Signal.2, ra47 (2009). [DOI] [PubMed] [Google Scholar]
- 214.Hanada, Y. et al. MAVS is energized by Mff which senses mitochondrial metabolism via AMPK for acute antiviral immunity. Nat. Commun.11, 5711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen, S. et al. TBK1-mediated DRP1 targeting confers nucleic acid sensing to reprogram mitochondrial dynamics and physiology. Mol. Cell80, 810–827.e7 (2020). [DOI] [PubMed] [Google Scholar]
- 216.Zhang, W. et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell178, 176–189.e15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chao, C.-C. et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell179, 1483–1498.e22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial Peptidoglycan. Cell166, 624–636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Baik, S. H. et al. Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation. Sci. Immunol.8, eade7652 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mass, E., Nimmerjahn, F., Kierdorf, K. & Schlitzer, A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat. Rev. Immunol.23, 563–579 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell169, 258–272.e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vande Voorde, J. et al. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv.5, eaau7314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sapudom, J. et al. 3D in vitro M2 macrophage model to mimic modulation of tissue repair. Npj Regen. Med.6, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wculek, S. K. et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity56, 516–530.e9 (2023). [DOI] [PubMed] [Google Scholar]
- 225.Heieis, G. A. et al. Metabolic heterogeneity of tissue-resident macrophages in homeostasis and during helminth infection. Nat. Commun.14, 5627 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Argüello, R. J. et al. SCENITH: A flow cytometry based method to functionally profile energy metabolism with single cell resolution. Cell Metab.32, 1063–1075.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Verberk, S. G. S. et al. An integrated toolbox to profile macrophage immunometabolism. Cell Rep. Methods2, 100192 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hu, T. et al. Single-cell spatial metabolomics with cell-type specific protein profiling for tissue systems biology. Nat. Commun.14, 8260 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hanaford, A. & Johnson, S. C. The immune system as a driver of mitochondrial disease pathogenesis: a review of evidence. Orphanet J. Rare Dis.17, 335 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]