Abstract
Introduction
The objective of this study was to examine the association between retinal thickness (RT) fluctuations and best corrected visual acuity (BCVA) in eyes with neovascular AMD, macular edema secondary to RVO, and DME treated with anti-VEGF therapy.
Methods
A systematic search of Ovid MEDLINE, EMBASE, and the Cochrane Library was performed from January 2006 to March 2024. Studies comparing visual or anatomic outcomes of patients treated with anti-VEGF therapy, stratified by magnitudes of RT fluctuation, were included. ROBINS-I and Cochrane RoB 2 tools were used to assess risk of bias, and certainty of evidence was evaluated with GRADE criteria. Meta-analysis was performed with a random-effects model. Primary outcomes were final BCVA and change in BCVA relative to baseline.
Results
15,725 articles were screened; 15 studies were identified in the systematic review and 5 studies were included in the meta-analysis. Final ETDRS VA was significantly worse in eyes with the highest level of RT fluctuation (weighted mean difference [WMD] = 7.86 letters; 95% CI, 4.97, 10.74; p < 0.00001; I2 = 81%; 3,136 eyes). RT at last observation was significantly greater in eyes with high RT fluctuations (WMD = −27.35 μm; 95% CI, −0.04, 54.75; p = 0.05; I2 = 88%; 962 eyes).
Conclusions
Final visual outcome is associated with magnitude of RT fluctuation over the course of therapy. It is unclear whether minimizing RT fluctuations would help optimize visual outcomes in patients treated with anti-VEGF therapy. These findings are limited by a small set of studies, risk of bias, and considerable heterogeneity.
Keywords: Retinal thickness fluctuation, Visual acuity, Anti-vascular endothelial growth factor, Age-related macular degeneration, Retinal vein occlusion, Diabetic macular edema
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) therapy is indicated in several conditions, including neovascular age-related macular degeneration (nAMD), macular edema (ME) secondary to retinal vein occlusion (RVO), and diabetic macular edema (DME) secondary to diabetic retinopathy (DR) [1]. Adherence to therapy is crucially important as significant visual compromise may result due to inadequate treatment [2, 3].
Optical coherence tomography (OCT) imaging is crucial for monitoring disease activity and morphologic changes in the retina, and the presence of certain OCT patterns may be helpful in prognosticating visual outcomes [4]. Retinal thickness (RT) is a closely scrutinized metric that is followed in eyes with ME. Indeed, in the setting of DME, studies have demonstrated a correlation between elevated RT and worse visual outcomes [5]. In nAMD, elevated RT secondary to intraretinal fluid (IRF) is associated with worse best corrected visual acuity (BCVA) compared to elevated RT secondary to subretinal fluid (SRF) [6]. A growing body of literature has examined the association between RT fluctuations and VA in nAMD, RVO, and DME. For example, Evans and colleagues [7] conducted a post hoc analysis of the IVAN and CATT trials and found that greater variability in RT over the course of treatment was associated with poorer final VA and increased likelihood of developing macular atrophy and fibrosis. Similarly, Chakravarty et al. [8] observed worse visual outcomes in highly fluctuating nAMD eyes. Thus, large fluctuations in RT may reflect a suboptimal anti-VEGF treatment regimen and may be indicative of poor visual prognosis. Therefore, a rigorous analysis of the potential impact of RT fluctuations on visual outcomes is needed to further guide anti-VEGF treatment decisions. In this systematic review and meta-analysis, we consolidated findings from studies that have reported on the association between RT fluctuations and VA outcomes in patients treated with anti-VEGF therapy for nAMD, RVO, and DME.
Methods
Search Strategy and Eligibility Criteria
We performed a systematic literature search on Ovid MEDLINE, EMBASE, and Cochrane Library from January 2006 to April 2024. Our search strategy for Ovid MEDLINE can be found in Supplementary Table 1 (for all online suppl. material, see https://doi.org/10.1159/000539648). We included peer-reviewed studies comparing functional and anatomical outcomes of patients treated with anti-VEGF agents for retinal vascular diseases, stratified by categories of standard deviation (SD) of RT to quantify RT fluctuations. We included patient populations from the following settings: nAMD, DME, and ME secondary to RVO. We excluded (I) studies that did not use RT SD as a measure of RT fluctuations, (II) studies that did not present outcomes stratified by RT SD categories (e.g., tertiles or quartiles), (III) studies in which patients received intravitreal corticosteroids or laser therapy as part of their treatment regimen, and (IV) studies that did not provide the sample size, mean, or SD for at least one of our considered outcomes. Studies that stratified patients by categories of RT SD but did not provide sufficient data to perform meta-analysis (e.g., due to missing data) were included as part of the systematic review but were excluded from the meta-analysis. We also excluded studies with no published results, no English full-text, literature reviews, systematic reviews, meta-analyses, conference proceedings, letters to editors, and theses. Our study adhered to the tenets of the Declaration of Helsinki, and we did not require institutional ethics approval. The protocol for our study was registered in the International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42022382400).
Study Selection and Data Collection
At least two independent authors (B.P., A.M., A.H., and J.G.) performed title and abstract screening for all articles followed by full-text screening on Covidence (Veritas Health Innovation, Melbourne, VIC, Australia). Two independent authors collected data (B.P. and A.M.) on Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). When necessary, an impartial author (M.M.P.) was consulted for conflict resolution. The following baseline characteristics were collected: study name, country, year of publication, retinal vascular disease, number of males, total eyes, right eyes, phakic eyes, pseudophakic/aphakic eyes, anti-VEGF agent, treatment regimen, BCVA, RT, type of RT reported, and the SD of RT fluctuation in study groups. In studies stratifying patients by quartiles of fluctuation, quartiles 1 (Q1) and 4 (Q4) represented the least and greatest RT fluctuation, respectively.
Our primary outcomes were final BCVA and change in BCVA from baseline. Our secondary outcomes were final RT and incidence of IRF and SRF per quartile of RT fluctuation. A subgroup analysis was also conducted to evaluate primary and secondary endpoints in eyes with nAMD.
Risk of Bias and Certainty of Evidence Assessment
The risk of bias (ROB) of included studies was assessed by two independent authors (A.H. and J.G.). The certainty of evidence associated with each study outcome was also evaluated by two independent authors (A.M. and B.P.). We used the risk of bias tool 2 (ROB2) for RCTs [9] and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies [10]. We evaluated the certainty of evidence of each outcome in our meta-analysis with the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) tool [11].
Statistical Analysis
We performed our meta-analysis using a random-effects model on Review Manager 5.4 (RevMan 5.4; The Nordic Cochrane Centre, Cochrane, Copenhagen, Denmark) and weighted every outcome by number of eyes. Weighted mean difference (WMD) and SD was reported for continuous outcomes. The inverse variance method was applied to continuous outcomes. Outcomes with I2 >50% were associated with substantial heterogeneity and >75% were associated with considerable heterogeneity [12]. We used methods outlined in Beck et al. [13] (2003) to calculate Snellen fractions and methods outlined in Khoshnood et al. [14] (2010) to convert logMAR values to ETDRS letters. To evaluate the robustness of our results, a subgroup analysis was restricted to nAMD patients only, and a leave-one-out sensitivity analysis was performed for all included studies. All p values were two-sided, and we considered p values ≤0.05 as statistically significant. For the primary analysis, Q1/Q2 and Q3/Q4 eyes were pooled, and for the sensitivity analysis, Q1 eyes were compared against Q4.
Results
Study Screening and Baseline Demographics
Title and abstract screening was performed on 15,725 articles. Overall, 61 articles underwent full-text screening, and 15 studies met inclusion criteria (Fig. 1) [7, 8, 15–27]. There were 10 observational studies [8, 15–17, 19, 21, 22, 25–27] and 5 post hoc analyses of RCTs (Table 1) [7, 18, 20, 23, 24]. Various measures of RT were employed, including central subfield thickness (CST) [8, 15, 16, 18, 19, 21, 23–25], central retinal thickness (CRT) [17, 27], central foveal thickness [20], foveal thickness [22, 26], and foveal center point thickness [7]. A variety of treatment regimens were employed across studies, including pro re nata (PRN), treat-and-extend, and fixed-dosing intervals (Table 1). Moreover, 11 studies included treatment-naïve patients only; 1 study included both treatment-naïve and pretreated patients; 1 study included patients who were treatment-naïve for at least 12 months; and 2 studies did not report on the treatment status of patients (Table 1). The patient examination frequency at which OCT scans were taken was also varied across studies; however, 6 of 12 studies (50%) examined patients at least every 3 months (Table 1). Of all included studies, 5 were included in the meta-analysis as these were the only studies that provided BCVA data stratified by categories of RT SD [7, 8, 22, 24, 26]. Overall, 7,563 eyes were included at baseline [7, 8, 15–27]. Moreover, 40.0% of patients were male, and the mean patient age was 75.1 ± 10.7 years. The mean number of anti-VEGF injections was 8.83 ± 5.0 (N = 1,933 eyes) [8, 15–17, 21, 22, 25–27], and the mean follow-up period was 24.2 months. A summary of baseline characteristics is provided in Table 2.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchartoutlining study exclusion criteria.
Table 1.
General study information, including country, study design, condition, and anti-VEGF agent used
| Reference | Country | Study design | Condition | Anti-VEGF agent | Baseline treatment status | Treatment regimen | Examination frequency |
|---|---|---|---|---|---|---|---|
| Chakravarthy et al. [8] (2021) | UK | Retrospective observational | nAMD | Any licensed agent | N/A | N/A | Minimum of 2 OCT scans during maintenance phase |
| Chen et al. [15] (2022) | USA | Retrospective observational | nAMD | BEV/RAN/AFL | Treatment-naïve | N/A | Every 3 months |
| Cheong et al. [16] (2021) | Singapore | Prospective observational | nAMD | BEV/RAN/AFL | Treatment-naïve | PRN or treat-and-extend | N/A |
| Ciucci et al. [17] (2022) | Italy | Retrospective observational | nAMD | AFL | N/A | Loading phase followed by bimonthly; PRN after 1 year | N/A |
| Dugel et al. [18] (2022) (Hawk and Harrier) | USA | Post hoc RCT | nAMD | AFL/BRO | Treatment-Naïve | Loading phase followed by BRO every 12 weeks and then 8 weeks, and AFL every 8 weeks | Every 4 weeks |
| Evans et al. [7] (2020) (IVAN and CATT) | USA | Post hoc RCT | nAMD | BEV/RAN | Treatment-naïve | Monthly or PRN | Every 3 months |
| Guo et al. [27] (2023) | China | Retrospective observational | nAMD | RAN | Treatment-naïve | Loading dose for 3 months followed by PRN or treat-and-extend | N/A |
| Lai and Lai, [19] (2021) | Hong Kong | Retrospective observational | nAMD | BEV/RAN/AFL | Treatment-naïve | 3 loading doses followed by PRN | Minimum of 3 OCT scans in 24-month follow-up period |
| Sheth et al. [20] (2022) (Harbor) | USA | Post hoc RCT | nAMD | RAN | Treatment-naïve | Monthly or PRN | Every month |
| Chen et al. [21] (2020) | USA | Retrospective observational | CRVO/BRVO | BEV/RAN/AFL | Treatment-naïve | N/A | Every 3 months |
| Nagasato et al. [22] (2022) | Japan | Retrospective observational | BRVO | AFL/RAN | Treatment-naïve | PRN | Every 3 months for 12 months followed by every 12 months |
| Nagasato et al. [26] (2024) | Japan | Retrospective observational | CRVO | AFL/RAN | Treatment-naïve | PRN | Every 3 months or earlier |
| Scott et al. [23] (2022) (SCORE2) | USA | Post hoc RCT | CRVO/HRVO | BEV/AFL | Treatment-naïve and pretreated | Monthly or treat-and-extend | Every 4 weeks for 6 months |
| Starr et al. [24] (2021) (Protocol T) | USA | Post hoc RCT | DME | BEV/RAN/AFL | Treatment-naïve within last 12 months | Modified PRN | Every 1 month for 12 months followed by every 2 months and then every 4 months |
| Wang et al. [25] (2022) | USA | Retrospective observational | DME | BEV/RAN/AFL | Treatment-naïve | PRN | Every 3 months |
BEV, bevacizumab; RAN, ranibizumab; AFL, aflibercept; BRO, brolucizumab; PRN, pro re nata; OCT, optical coherence tomography.
Table 2.
Baseline data by study
| Reference | Injections, mean (SD) | Eyes (initial), N | Eyes (final), N | Follow-up duration | Male, n (%) | Age, mean (SD), years | Eye laterality (right), n (%) |
|---|---|---|---|---|---|---|---|
| Chakravarthy et al. [8] (2021) | 8.9 (4.0) | 403 | 403 | 24 months | 141 (37) | 77.8 (6.8) | N/A |
| Chen et al. [15] (2022) | 14.1 (4.9) | 422 | 422 | 24 months | 154 (36.5) | 83.6 (8.3) | 232 (55) |
| Cheong et al. [16] (2021) | 7.6 (3.4) | 141 | 141 | 12 months | 56 (39.7) | 70.5 (9.2) | N/A |
| Ciucci et al. [17] (2022) | 9.07 (2.6) | 41 | 41 | 24.93 months | 15 (36.6) | 78.56 (7.51) | N/A |
| Dugel et al. [18] (2022) (Harrier) | N/A | 739 | 739 | 22.09 months | 317 (42.9) | 75.1 (8.24) | N/A |
| Dugel et al. [18] (2022) (Hawk) | N/A | 1,078 | 1,078 | 22.09 months | 469 (43.5) | 76.5 (8.68) | N/A |
| Evans et al. [7] (2020) | N/A | 1,731 | 1,731 | 24 months | 673 (38.9) | 78.6 (7.4) | N/A |
| Guo et al. [27] (2023) | 4.5 (2.5) | 76 | 76 | 120 months | 19 (25.0) | N/A | N/A |
| Lai and Lai, [19] (2021) | Median: 7 (IQR 4–10) | 64 | 64 | 24 months | 38 (61.3) | 75.3 (9.4) | N/A |
| Sheth et al. [20] (2022) | N/A | 1,097 | 1,097 | 24 months | 446 (40.7) | 78.7 (8.4) | N/A |
| Chen et al. [21] (2020) | 7.4 (2.2) | 134 | 134 | 12 months | 58 (43.3) | 73.4 (10.7) | 69 (51.5) |
| Nagasato et al. [22] (2022) | 5.8 (4.6) | 309 | 309 | 50.6 months | 118 (38.2) | 67.5 (10) | N/A |
| Nagasato et al. [26] (2024) | 5.6 (3.6) | 141 | 141 | 24 months | 88 (62.4) | 69.3 (12.4) | N/A |
| Scott et al. [23] (2022) | 6 | 362 | 362 | 12 months | N/A | N/A | N/A |
| Starr et al. [24] (2021) | N/A | 559 | 559 | 24 months | 289 (51.7) | 59.9 (10.4) | N/A |
| Wang et al. [25] (2022) | 8.2 (2.4) | 266 | 266 | 12 months | 141 (53.0) | 61.0 (11.3) | 141 (53.0) |
| Overall | 8.83 (4.96) (N = 1,933) | 7,563 (n = 15) | 7,563 (n = 15) | 24.16 months | 3,022 (40.0) | 75.11 (10.67) (n = 13) | 442 (53.8) (n = 3) |
n, number of studies; N, number of eyes; SD, standard deviation.
Visual Acuity
Mean initial BCVA across all studies was 59.8 ± 15.1 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters (N = 7,125) [7, 8, 15–22, 24–26], and mean final BCVA was 67.6 ± 18.7 letters (N = 4,141) [7, 8, 15–17, 21, 22, 24–26]. The overall change in BCVA was 6.3 ± 16.7 letters (N = 3,515) (Table 3) [7, 8, 15, 21, 24, 25]. Patients stratified in quartile 1 (i.e., least RT fluctuation) had a mean initial BCVA of 66.3 ± 11.3 letters (N = 1,112) [7, 8, 18, 24] and a mean final BCVA of 74.6 ± 15.0 letters (N = 785) [7, 8, 22, 24, 26], resulting in a mean improvement of 7.0 ± 13.4 letters (N = 671) (Table 4) [7, 8, 24]. Patients stratified in quartile 4 (i.e., greatest RT fluctuation) had a mean initial BCVA of 55.5 ± 14.2 letters (N = 1,111) [7, 8, 18, 24] and a mean final BCVA of 61.5 ± 21.2 letters (N = 784) [7, 8, 22, 24, 26], leading to a mean change of 5.6 ± 19.5 letters (N = 672) (Fig. 2) [7, 8, 24].
Table 3.
Visual acuity data by study
| Reference | Eyes (baseline), N | Eyes (final), N | Initial BCVA, mean (SD), ETDRS | BCVA at 3 months, mean (SD), ETDRS | BCVA at 12 months, mean (SD), ETDRS | BCVA at 24 months, mean (SD), ETDRS | Final BCVA, mean (SD), ETDRS | Change in BCVA, mean (SD), ETDRS |
|---|---|---|---|---|---|---|---|---|
| Chakravarty et al. [8] (2021) | 403 | 403 | 58.2 (10.4) | 63.3 (12.5) | N/A | 61.6 (15.8) | 61.6 (15.8) | 3.4 (14.5) |
| Chen et al. [15] (2022) | 422 | 422 | 58.8 (19.2) | N/A | 64.3 (17.8) | 62.4 (20.6) | 62.4 (20.6) | 3.7 (20.8) |
| Cheong et al. [16] (2021) | 141 | 141 | 49.9 (17.8) | N/A | N/A | N/A | 56 (16.1) | 5.6 |
| Ciucci et al. [17] (2021) | 41 | 41 | 66.09 (32.6) | N/A | N/A | N/A | 65.85 (29.5) | −0.24 |
| Dugel et al. [18] (2022) (Harrier) | 739 | 739 | 61.2 (12.76) | N/A | N/A | N/A | N/A | N/A |
| Dugel et al. [18] (2022) (Hawk) | 1,078 | 1,078 | 60.6 (13.71) | N/A | N/A | N/A | N/A | N/A |
| Evans et al. [7] (2020) | 1,731 | 1,731 | 61.0 (14.0) | N/A | N/A | N/A | 66.5 (18.5) (n = 1,725) | 5.5 (16.0) |
| Guo et al. [27] (2023) | 76 | 76 | Median: 60.5 (50.0, 70.0) | N/A | N/A | N/A | N/A | N/A |
| Lai and Lai, [19] (2021) | 64 | 64 | 55.5 (20) | N/A | N/A | N/A | N/A | N/A |
| Sheth et al. [20] (2022) | 1,097 | 1,097 | 53.9 (12.7) | N/A | N/A | N/A | N/A | N/A |
| Chen et al. [21] (2020) | 134 | 134 | 52.8 (20.9) | N/A | 65.9 (17.3) | N/A | 65.9 (17.3) | 13.1 (20.3) |
| Nagasato et al. [22] (2022) | 309 | 309 | 70 (15) | N/A | N/A | N/A | 80.5 (14) | 10.5 |
| Nagasato et al. [26] (2024) | 141 | 141 | 52.5 (26) | N/A | N/A | 59 (28.5) | 59 (28.5) | 6.5 |
| Scott et al. [23] (2022) | 362 | 362 | N/A | N/A | N/A | N/A | N/A | N/A |
| Starr et al. [24] (2021) | 559 | 559 | 65 (11) | N/A | 77 (10) | 77 (12) | 77 (12) | 12 (14) |
| Wang et al. [25] (2022) | 266 | 266 | 63.5 (15.7) | N/A | 69.0 (13.8) | N/A | 69.0 (13.8) | 5.1 (16.1) |
| Overall | 7,563 (n = 15) | 7,563 (n = 15) | 59.81 (15.13) (N = 7,125) | N/A | 70.5 (15.3) (N = 1,381) | 67.23 (19.15) (N = 1,525) | 67.59 (18.68) (N = 4,141) | 6.34 (16.65) (N = 3,515) |
n, number of studies; N, number of eyes; SD, standard deviation; BCVA, best corrected visual acuity; ETDRS, Early Treatment of Diabetic Retinopathy Study.
Table 4.
Visual acuity and RT data stratified by quartiles
| Quartile | Eyes, N | Age, mean (SD), years | Male (%) | Initial BCVA, mean (SD), ETDRS | Final BCVA, mean (SD), ETDRS | Overall change in BCVA, mean (SD), ETDRS | Initial RT, mean (SD), µm | Final RT, mean (SD), µm | Overall change in RT, mean (SD), µm |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1,453 (n = 7) | 74.44 (10.60) (n = 3) | 238/572 (41.6; n = 2) | 66.32 (11.30) (N = 1,112) | 74.55 (15.04) (N = 785) | 6.95 (13.37) (N = 671) | 323.05 (64.53) (N = 1,089) | 264.12 (68.22) (N = 241) | N/A |
| 2 | 1,420 (n = 7) | 74.67 (10.46) (n = 3) | 253/573 (44.2; n = 2) | 62.97 (11.77) (N = 1,111) | 70.42 (16.85) (N = 784) | 6.35 (14.40) (N = 672) | 387.32 (74.60) (N = 1,103) | 271.25 (68.99) (N = 240) | N/A |
| 3 | 1,435 (n = 7) | 74.90 (10.77) (n = 3) | 247/573 (43.1; n = 2) | 59.94 (13.11) (N = 1,111) | 68.69 (17.88) (N = 783) | 7.20 (14.84) (N = 671) | 454.62 (105.79) (N = 1,100) | 283.88 (84.62) (N = 240) | N/A |
| 4 | 1,435 (n = 7) | 74.41 (11.74) (n = 3) | 234/572 (40.9; n = 2) | 55.53 (14.22) (N = 1,111) | 61.45 (21.20) (N = 784) | 5.56 (19.45) (N = 672) | 605.07 (187.88) (N = 1,108) | 301.03 (117.34) (N = 241) | N/A |
n, number of studies; N, number of eyes; SD, standard deviation; BCVA, best corrected visual acuity; ETDRS, Early Treatment of Diabetic Retinopathy Study; RT, retinal thickness.
Fig. 2.
Bar plot comparison of initial and final best corrected visual acuity (BCVA) by quartiles. The final BCVA trends downwards with increasing quartiles of RT fluctuation. Bars represent mean BCVA, and error bars represent SD. ETDRS, Early Treatment of Diabetic Retinopathy Study. *p ≤ 0.05.
Retinal Thickness
Mean initial RT across all studies was 440.8 ± 163.8 μm (N = 7,201) [7, 8, 15–22, 24–27], and mean final RT was 290.3 ± 106.0 μm (N = 2,492) [8, 15–17, 21, 22, 24–27], resulting in a mean change of −109.3 ± 144.2 μm (N = 1,598) (Table 5) [15, 16, 21, 24, 25, 27]. In quartile 1, the mean initial and final RTs were 323.1 ± 64.5 μm (N = 1,089) [7, 8, 18, 24] and 264.1 ± 68.2 μm (N = 241), respectively (Table 2) [8, 24]. In quartile 4, the mean initial and final RTs were 605.1 ± 187.9 μm (N = 1,108) [7, 8, 18, 24] and 301.0 ± 117.3 μm (N = 241), respectively (Fig. 3) [8, 24].
Table 5.
Retinal thickness data by study
| Reference | Eyes (baseline), N | Eyes (final), N | Initial RT, mean (SD), μm | RT at 3 months, mean (SD), μm | RT at 12 months, mean (SD), μm | RT at 24 months, mean (SD), μm | Final RT, mean (SD), μm | Change in RT, mean (SD), μm |
|---|---|---|---|---|---|---|---|---|
| Chakravarty et al. [8] (2021) | 403 | 403 | 350.1 (109.7) | 248.0 (66.7) | N/A | 239.2 (64.9) | 239.2 (64.9) | N/A |
| Chen et al. [15] (2022) | 422 | 422 | 331.2 (97.6) | N/A | 259.9 (53.7) | 253.4 (53.6) | 253.4 (53.6) | −77.8 (104.7) |
| Cheong et al. [16] (2021) | 141 | 141 | 495.2 (223.3) | N/A | 343 (162.9) | N/A | 343 (162.9) | −150.7 (176) |
| Ciucci et al. [17] (2021) | 41 | 41 | 348.5 (164.4) | N/A | N/A | N/A | 235.6 (163.8) | −112.9 |
| Dugel et al. [18] (2022) (Harrier) | 739 | 739 | 469.5 (161.57) | N/A | N/A | N/A | N/A | N/A |
| Dugel et al. [18] (2022) (Hawk) | 1,078 | 1,078 | 462.5 (160.31) | N/A | N/A | N/A | N/A | N/A |
| Evans et al. [7] (2020) | 1,731 | 1,731 | 440.1 (167.4) | N/A | N/A | N/A | N/A | N/A |
| Guo et al. [27] (2023) | 76 | 76 | 293.6 (89.1) | N/A | 252.5 (68.6) | N/A | 252.5 (68.6) | −41.3 (104.1) |
| Lai and Lai, [19] (2021) | 64 | 64 | 364 (113) | N/A | N/A | N/A | N/A | N/A |
| Sheth et al. [20] (2022) | 1,097 | 1,097 | 440.7 (151.2) | N/A | N/A | N/A | N/A | N/A |
| Chen et al. [21] (2020) | 134 | 134 | 488.6 (165) | N/A | 334.3 (131.9) | N/A | 334.3 (131.9) | −154.3 (210.2) |
| Nagasato et al. [22] (2022) | 309 | 309 | 503.1 (162.4) | N/A | N/A | N/A | 287.1 (103.3) | −216 |
| Nagasato et al. [26] (2024) | 141 | 141 | 661.1 (257.4) | N/A | N/A | 328.9 (157.8) | 328.9 (157.8) | −332.2 |
| Scott et al. [23] (2022) | 362 | 362 | N/A | N/A | N/A | N/A | N/A | N/A |
| Starr et al. [24] (2021) | 559 | 559 | 453 (127) | N/A | 318 (96) | 310 (91) | 310 (91) | −145 (143) |
| Wang et al. [25] (2022) | 266 | 266 | 396.9 (109.7) | N/A | 337.7 (100.7) | N/A | 337.7 (100.7) | −59.2 (114.8) |
| Overall | 7,563 (n = 15) | 7,563 (n = 15) | 440.82 (163.82) (N = 7,201) | N/A | 306.39 (103.74) (N = 1,598) | 277.38 (91.68) (N = 1,525) | 290.35 (105.98) (N = 2,492) | −109.32 (144.20) (N = 1,598) |
n, number of studies; N, number of eyes; SD, standard deviation; RT, retinal thickness.
Fig. 3.
Bar plot comparison of initial and final retinal thickness (RT) by quartiles. There is a modest increase in final RT with increasing quartiles of fluctuation. Bars represent mean BCVA, and error bars represent SD. *p ≤ 0.05.
GRADE and Risk of Bias Assessment
Of three outcomes in the primary analysis, our GRADE assessment found that BCVA at last study observation was associated with a moderate certainty of evidence. The change in BCVA from baseline and RT at last study observation were associated with a low certainty of evidence (online suppl. Table 2).
The Cochrane risk of bias tool 2 was used to conduct ROB assessment for five post hoc analyses of RCTs. A low ROB across the five domains of bias was generally observed. There were some concerns around author conflict(s) of interest and industry sponsorship. Our ROB assessment of 10 observational studies via the ROBINS-I tool found that 52 (74.3%) domains had a low ROB, and 18 (25.7%) domains had a moderate risk of bias. The following represents the proportion of observational studies with a low ROB across ROBINS-I domains: bias in classification of interventions (100%), bias due to deviations from intended interventions (90.0%), bias in measurement of outcomes (90.0%), bias due to confounding (70.0%), bias in selection of participants into study (60.0%), bias due to missing data (60.0%), and bias due to selection of the reported results (50.0%) (online suppl. Tables 3, 4).
Meta-Analysis
Comparison of Final VA, VA Change, and Final RT by Quartile
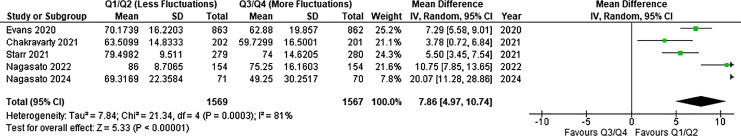
Two post hoc analyses [7, 24] and 3 retrospective studies [8, 22, 26] reported final BCVA data in patients stratified by quartiles of RT fluctuations. Overall, the final visual outcome in Q1/Q2 eyes (N = 1,569) was significantly better at final follow-up compared to Q3/Q4 eyes (N = 1,567) (WMD = 7.86 letters; 95% CI, 4.97, 10.74; p < 0.00001; I2 = 81%; GRADE = moderate; Fig. 4) [7, 8, 22, 24, 26]. No significant differences were observed with respect to the change in BCVA between Q1/Q2 (N = 1,343) and Q3/Q4 (N = 1,343) (WMD = 0.12 letters; 95% CI, −1.87, 2.12; p = 0.90; I2 = 60%; GRADE = low; Fig. 5) [7, 8, 24]. The RT was significantly lower by final follow-up in Q1/Q2 eyes (N = 481) relative to Q3/Q4 eyes (N = 481) (WMD = −27.35 μm; 95% CI, −0.04, 54.75; p = 0.05; I2 = 88%; GRADE = low; Fig. 6) [8, 24].
Fig. 4.
Forest plot depicting final VA comparison between Q1/Q2 and Q3/Q4 eyes, demonstrating favorable BCVA outcomes in Q1/Q2 eyes with less RT fluctuations. Blocks represent point estimates; horizontal lines, 95% confidence intervals; diamond, overall effect estimate of meta-analysis. SD, standard deviation.
Fig. 5.
Forest plot comparing best corrected visual acuity (BCVA) change relative to baseline in Q1/Q2 versus Q3/Q4 eyes, demonstrating no significant differences in mean BCVA changes. Blocks represent point estimates; horizontal lines, 95% confidence intervals; diamond, overall effect estimate of meta-analysis. SD, standard deviation.
Fig. 6.
Forest plot comparing retinal thickness (RT) at final follow-up between Q1/Q2 and Q3/Q4 eyes, demonstrating lower mean final RT in Q1/Q2 (less RT fluctuations). Blocks represent point estimates; horizontal lines, 95% confidence intervals; diamond, overall effect estimate of meta-analysis. SD, standard deviation.
Sensitivity Analysis
Leave-one-out sensitivity analysis was performed for the following outcomes: BCVA at last study observation and change in BCVA from baseline (online suppl. Fig. 1, 2). The results from the leave-one-out analysis were consistent with the findings from the overall analysis of both outcomes.
Analysis of Q1 versus Q4 eyes was significant for final BCVA (p < 0.00001) and final RT (p = 0.02) (online suppl. Fig. 3). This was consistent with the results from the pooled analysis of Q1/Q2 versus Q3/Q4.
Subgroup Analysis – nAMD
Subgroup analysis of two nAMD studies [7, 8] revealed significantly better final visual outcomes in Q1/Q2 eyes (N = 1,065) when compared to Q3/Q4 eyes (N = 1,063), with considerable heterogeneity (WMD = 5.57 letters; 95% CI, 2.13, 9.01; p = 0.002; I2 = 62%; online suppl. Fig. 4).
Subgroup Analysis – RVO
A subgroup analysis of two RVO studies [22, 26] demonstrated significantly better visual outcomes in Q1/Q2 eyes (N = 225) when compared to Q3/Q4 eyes (N = 224), with considerable heterogeneity (WMD = 14.45 letters; 95% CI, 5.51, 23.38; p = 0.002; I2 = 74%; online suppl. Fig. 5).
Incidence of IRF and SRF
A meta-analysis of this outcome could not be performed due to insufficient data.
Systematic Review
Neovascular Age-Related Macular Degeneration Studies
This systematic review identified 10 studies that examined the association between RT fluctuations and visual outcomes in eyes with nAMD treated with anti-VEGF therapy. The majority of studies stratified patients by quartiles of fluctuation; however, one study stratified patients by tertiles [16]. In general, the visual outcome was significantly worse in eyes with high RT fluctuations compared to eyes with low fluctuations. Indeed, Ciucci et al. [17] found a significantly inverse association between CRT/SD and final BCVA (Pearson’s r = 0.43; p = 0.005), indicating that greater CRT fluctuation was associated with poorer visual outcome. Similarly, Lai and colleagues [19] observed a significant correlation between final BCVA and CST fluctuation (p < 0.0001; Spearman’s ρ = 0.54). Extent of BCVA improvement was also significantly greater in eyes with lower RT fluctuations [16, 18, 19]. Evans et al. [7] found that the likelihood of developing geographic atrophy and fibrosis was greater in eyes with higher RT fluctuations. Chakravarthy et al. [8] demonstrated that IRF fluctuations played a more important role than SRF with respect to final visual outcome. Moreover, Sheth et al. [20] found that SRF fluctuations did not impact final visual outcome. Eyes with lower RT fluctuations had a larger proportion of patient visits who presented with SRF only compared to eyes with moderate and high RT fluctuation [16]. Guo and colleagues [27] demonstrated that increased RT fluctuations over the course of 1 year of anti-VEGF therapy were associated with a greater risk of legal blindness (≤35 ETDRS).
Retinal Vein Occlusion Studies
Three studies investigated the association between RT fluctuations and visual outcome in eyes with ME secondary to RVO [21–23]. Two studies examined patients with BRVO [21, 22] and three studies examined patients with CRVO [21, 23, 26]. One study examined patients with HRVO [23]. The final visual outcome was significantly better in eyes with smaller RT fluctuations [21–23]. Chen et al. [21] observed a difference of 10 letters between quartiles 1 and 4 (p < 0.001), reflecting the worse visual prognosis in highly fluctuating eyes. Nagasato et al. [22] observed longer defects in the foveal ellipsoid zone (EZ) in BRVO eyes with the largest RT fluctuations. A similar study by Nagasato et al. [26] also demonstrated longer EZ defects in eyes with CRVO with larger fluctuations. One study found significant differences in RT fluctuations depending on the anti-VEGF agent used. Specifically, larger RT fluctuations were observed in eyes receiving bevacizumab (BEV) injections relative to aflibercept (AFL) injections (p < 0.0001) [23].
Diabetic Macular Edema Studies
Two studies examined RT fluctuations in eyes treated with anti-VEGF therapy for DME [24, 25]. Patients were stratified into quartiles of RT fluctuation. Overall, significantly worse visual outcomes were observed in eyes with high RT fluctuations. For example, Wang et al. [25] observed a difference of 9.7 ETDRS letters between quartiles 1 and 4 at final follow-up (p < 0.001).
Discussion
This systematic review and meta-analysis investigated the association between RT fluctuations and BCVA in patients treated with anti-VEGF therapy in the setting of nAMD, RVO, and DME. Overall, our systematic review and meta-analysis demonstrated that eyes with greater RT fluctuations experienced significantly poorer visual outcomes at final follow-up in all three eye diseases. Additionally, the RT at final follow-up was significantly lower in eyes with lower fluctuations over the course of treatment. The change in BCVA was not significantly different between Q1/Q2 and Q3/Q4 eyes when results were pooled in a meta-analysis. These results are limited by the presence of considerable heterogeneity and the relatively low number of studies included in the meta-analysis.
Although studies have demonstrated an association between greater RT fluctuations and poorer visual outcomes, the exact mechanism has not been determined. Cheong and colleagues [16] hypothesize that photoreceptor viability is perturbed due to repeated shrinking and stretching of photoreceptors, and the directional sensitivity of photoreceptors is impaired due to perturbations in cone structure and alignment. Further evidence of photoreceptor damage secondary to RT fluctuations was demonstrated by Nagasato et al. [22] and Nagasato et al. [26], where greater foveal thickness fluctuations in eyes with BRVO and CRVO, respectively, were associated with longer EZ defects, reflecting photoreceptor damage. Evans et al. [7] showed that highly fluctuating eyes had a greater risk of developing GA and fibrosis, both of which have been associated with poorer visual outcomes in the setting of nAMD [7, 28]. Ultimately, further studies are needed to elucidate which of these mechanisms resulted in poorer visual prognosis in highly fluctuating eyes.
Several anti-VEGF treatment regimens may be used, including PRN, treat-and-extend, and fixed-dosing intervals [29]. In a PRN regimen, injections are administered in a reactive manner in the setting of macular fluid recurrence [3]. In a treat-and-extend regimen, the eye is injected at each visit until there is resolution of disease activity, followed by subsequent injections with increasing time intervals in between [3, 29]. The goal of the proactive treat-and-extend regimen is to prevent fluid recurrence and provide anti-VEGF therapy in the absence of fluid [3, 7, 29]. Notably, one study found that patients undergoing a PRN regimen had larger RT fluctuations relative to patients on a monthly treatment regimen [20]. The extent of RT fluctuations therefore becomes important to consider when weighing the risks and benefits of the PRN treatment regimen. In principle, a treat-and-extend regimen may lead to relatively lower fluctuations in RT; however, this regimen may not always be appropriate based on patient and physician preferences [3]. In practice, clinicians should consider the potential for poor visual outcomes in patients with high RT fluctuations when adopting an anti-VEGF treatment regimen.
There is conflicting evidence with regards to the impact of the number of injections on the likelihood of greater RT fluctuation. Evans et al. [7] found that an increased number of injections was associated with higher RT fluctuations. Conversely, Lai et al. [19] observed no significant differences in injection burden between quartiles of fluctuation. Nevertheless, anti-VEGF undertreatment has been implicated as a risk factor for poorer visual outcomes in the setting of nAMD, BRVO, and DME, and it is possible that higher RT fluctuations may mediate this relationship [30, 31]. Patients with the highest RT fluctuations had the lowest BCVA and highest RT at baseline in this study, suggesting that these patients present with more advanced disease, resulting in greater treatment demand and higher RT fluctuations. The impact of specific anti-VEGF agents on RT fluctuations is also yet to be elucidated; however, Scott et al. [23] interestingly observed significantly greater CST fluctuations in eyes receiving BEV injections compared to AFL injections. Further studies are needed to assess the different impact of various anti-VEGF agents on the magnitude of RT fluctuations.
This meta-analysis has important limitations to consider. Only five studies met eligibility criteria for the meta-analysis, resulting in a relatively low sample size. The results are further limited by considerable heterogeneity, which may limit generalizability. Given a paucity of data, eyes with AMD, DME, and RVO were combined in the overall meta-analysis, introducing heterogeneity. Variable study design may have contributed to increased heterogeneity. Missing and/or incomplete patient data in individual studies may give rise to sampling bias. Additionally, the measures of RT varied between studies, and this introduces further heterogeneity and may lead to under- or over-estimation of treatment effects on disease progression. Evans et al. [7] reported thickness at the foveal center point, whereas two studies measured CST [8, 24]. Two studies reported FT as a measure of RT [22, 26]. We did not restrict studies based on follow-up duration due to a paucity of evidence in this setting, but the studies included in the meta-analysis had long-term follow-up for our outcomes of interest (range: 24 months–50.6 months). Another limitation of this meta-analysis is the potential for anti-VEGF undertreatment as an explanation for the association between RT fluctuations and VA outcome, given that the mean number of anti-VEGF injections was greater in eyes with high RT fluctuations [7, 8, 22]. It is possible that eyes with more severe disease at baseline are predisposed to greater RT fluctuations, suggesting that RT fluctuations may not cause poor visual outcomes but are rather a surrogate for disease severity. The potential for diurnal variation in RT thickness serves as another limitation for this study as the measured RT may be impacted by the time of day. Indeed, previous studies have demonstrated variations in ME over the course of the day in patients with DME [32] and CRVO [33]. None of the studies included in this review commented on the potential impact of diurnal variation. Given the potential prognostic value of assessing RT fluctuations, future studies may benefit from adopting a standardized method of measuring RT fluctuations, assessing the impact of duration of RT fluctuations on final VA, and accounting for anti-VEGF undertreatment.
This systematic review of sixteen studies and meta-analysis of five studies found that VA outcomes were significantly worse in highly fluctuating eyes in patients treated with anti-VEGF therapy. Further studies are needed to quantify the impact of RT fluctuations on VA outcomes and whether different anti-VEGF agents mediate differences in RT fluctuations.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature. This study was conducted ethically and in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
M.M.P.: financial support (to institution) – PSI Foundation, Fighting Blindness Canada.
D.T.W.: consultant – AbbVie, Alcon, Apellis, Bayer, Bausch Health, Biogen, Boehringer Ingelheim, Novartis, Ripple Therapeutics, Roche, Topcon, and Zeiss. Research grants from Novartis, Roche.
Funding Sources
No funding was provided for this study.
Author Contributions
Bhadra U. Pandya, Andrew Mihalache, Amin Hatamnejad, Justin Grad, Marko M. Popovic, and David T. Wong: conception and design, acquisition of data, analysis and interpretation of data, preparation of the manuscript, and final approval of the completed manuscript.
Funding Statement
No funding was provided for this study.
Data Availability Statement
All available data are available in this article and online as supplementary information. Further inquiries may be directed to the corresponding author.
Supplementary Material.
Supplementary Material.
References
- 1. Cornel S, Adriana ID, Mihaela TC, Speranta S, Algerino DS, Mehdi B, et al. Anti-vascular endothelial growth factor indications in ocular disease. Rom J Ophthalmol. 2015;59(4):235–42. [PMC free article] [PubMed] [Google Scholar]
- 2. Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freund KB, Korobelnik J-F, Devenyi R, Framme C, Galic J, Herbert E, et al. Treat-and-extend regimens with anti-vegf agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35(8):1489–506. [DOI] [PubMed] [Google Scholar]
- 4. Hwang HS, Chae JB, Kim JY, Kim DY. Association between hyperreflective dots on spectral-domain optical coherence tomography in macular edema and response to treatment. Invest Ophthalmol Vis Sci. 2017;58(13):5958–67. [DOI] [PubMed] [Google Scholar]
- 5. Ou WC, Brown DM, Payne JF, Wykoff CC. Relationship between visual acuity and retinal thickness during anti-vascular endothelial growth factor therapy for retinal diseases. Am J Ophthalmol. 2017;180:8–17. [DOI] [PubMed] [Google Scholar]
- 6. Patil NS, Mihalache A, Dhoot AS, Popovic MM, Muni RH, Kertes PJ. Association between visual acuity and residual retinal fluid following intravitreal anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration: a systematic review and meta-analysis. JAMA Ophthalmol. 2022;140(6):611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans RN, Reeves BC, Maguire MG, Martin DF, Muldrew A, Peto T, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti-vascular endothelial growth factor agents. JAMA Ophthalmol. 2020;138(10):1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chakravarthy U, Havilio M, Syntosi A, Pillai N, Wilkes E, Benyamini G, et al. Impact of macular fluid volume fluctuations on visual acuity during anti-VEGF therapy in eyes with nAMD. Eye. 2021;35(11):2983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 10. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deeks J, Higgins J, Altman D. Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley and Sons; 2008. [Google Scholar]
- 13. Beck RW, Moke PS, Turpin AH, Ferris FL 3rd, SanGiovanni JP, Johnson CA, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. [DOI] [PubMed] [Google Scholar]
- 14. Khoshnood B, Mesbah M, Jeanbat V, Lafuma A, Berdeaux G. Transforming scales of measurement of visual acuity at the group level. Ophthalmic Physiol Opt. 2010;30(6):816–23. [DOI] [PubMed] [Google Scholar]
- 15. Chen ER, Chen AX, Greenlee TE, Conti TF, Briskin IN, Urbano CA, et al. Macular thickness fluctuation in neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor. Can J Ophthalmol. 2022;57(5):350–6. [DOI] [PubMed] [Google Scholar]
- 16. Cheong KX, Teo AWJ, Cheung CMG, Too IHK, Chakravarthy U, Teo KYC. Association between retinal thickness variation and visual acuity change in neovascular age-related macular degeneration. Clin Exp Ophthalmol. 2021;49(5):430–8. [DOI] [PubMed] [Google Scholar]
- 17. Ciucci F, Ioele G, Bardocci A, Lofoco G, Antonelli B, Gaetano CD, et al. Central retinal thickness fluctuations in patients treated with anti-VEGF for neovascular age related macular degeneration. Eur J Ophthalmol. 2022;32(4):2388–94. [DOI] [PubMed] [Google Scholar]
- 18. Dugel PU, Jhaveri CD, Chakravarthy U, Wykoff CC, Singh RP, Hamilton R, et al. Effect of retinal thickness variability on visual outcomes and fluid persistence in neovascular age-related macular degeneration: a post hoc analysis of the HAWK and HARRIER studies. Retina. 2022;42(3):511–8. [DOI] [PubMed] [Google Scholar]
- 19. Lai TYY, Lai RYK. Association between retinal thickness variability and visual acuity outcome during maintenance therapy using intravitreal anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. J Pers Med. 2021;11(10):1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheth V, D’Rozario M, Gune S, Blotner S. Fluctuations in central foveal thickness and association with vision outcomes with anti-VEGF therapy for nAMD: HARBOR post hoc analysis. BMJ Open Ophthalmol. 2022;7(1):e000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen AX, Greenlee TE, Conti TF, Briskin IN, Singh RP. Fluctuations in macular thickness in patients with retinal vein occlusion treated with anti-vascular endothelial growth factor agents. Ophthalmol Retina. 2020;4(12):1158–69. [DOI] [PubMed] [Google Scholar]
- 22. Nagasato D, Muraoka Y, Tanabe M, Nishigori N, Osaka R, Mitamura Y, et al. Foveal thickness fluctuation in anti-VEGF treatment for branch retinal vein occlusion: a long-term study. Ophthalmol Retina. 2022;6(7):567–74. [DOI] [PubMed] [Google Scholar]
- 23. Scott IU, Oden NL, VanVeldhuisen PC, Ip MS, Blodi BA. SCORE2 Investigator Group. SCORE2 Report 17: macular thickness fluctuations in anti-VEGF-treated patients with central or hemiretinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2022;2605:1491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Starr MR, Salabati M, Mahmoudzadeh R, Patel LG, Ammar MJ, Hsu J, et al. Fluctuations in central subfield thickness associated with worse visual outcomes in patients with diabetic macular edema in clinical trial setting. Am J Ophthalmol. 2021;232:90–7. [DOI] [PubMed] [Google Scholar]
- 25. Wang VY, Kuo BL, Chen AX, Wang K, Greenlee TE, Conti TF, et al. Fluctuations in macular thickness in patients with diabetic macular oedema treated with anti-vascular endothelial growth factor agents. Eye. 2022;36:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagasato D, Muraoka Y, Tanabe M, Nishigori N, Osaka R, Mitamura Y, et al. Foveal thickness fluctuations in anti-VEGF treatment for central retinal vein occlusion. Ophthalmol Sci. 2024;4(2):100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo Y, Wu J, Zheng X, Yin C, Wu Z. The first-year variation in central retinal thickness predicts legal blindness in patients with neovascular age-related macular degeneration. Ophthalmic Res. 2023;66(1):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casalino G, Stevenson MR, Bandello F, Chakravarthy U. Tomographic biomarkers predicting progression to fibrosis in treated neovascular age-related macular degeneration: a multimodal imaging study. Ophthalmol Retina. 2018;2(5):451–61. [DOI] [PubMed] [Google Scholar]
- 29. Mantel I. Optimizing the anti-vegf treatment strategy for neovascular age-related macular degeneration: from clinical trials to real-life requirements. Transl Vis Sci Technol. 2015;4(3):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monés J, Singh RP, Bandello F, Souied E, Liu X, Gale R. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: the need for a change of mindset. Ophthalmologica. 2020;243:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Yuksel B, Karti O, Celik O, Kerci SG, Kusbeci T. Low frequency ranibizumab versus dexamethasone implant for macular oedema secondary to branch retinal vein occlusion. Clin Exp Optom. 2018;101(1):116–22. [DOI] [PubMed] [Google Scholar]
- 32. Frank RN, Schulz L, Abe K, Iezzi R. Temporal variation in diabetic macular edema measured by optical coherence tomography. Ophthalmology. 2004;111(2):211–7. [DOI] [PubMed] [Google Scholar]
- 33. Gupta B, Grewal J, Adewoyin T, Pelosini L, Williamson TH. Diurnal variation of macular oedema in CRVO: prospective study. Graefes Arch Clin Exp Ophthalmol. 2009;247(5):593–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data are available in this article and online as supplementary information. Further inquiries may be directed to the corresponding author.