Abstract
Drug addiction and the circuitry for learning and memory are intimately intertwined. Drugs of abuse create strong, inappropriate, and lasting memories that contribute to many of their destructive properties, such as continued use despite negative consequences and exceptionally high rates of relapse. Studies in Drosophila melanogaster are helping us understand how drugs of abuse, especially alcohol, create memories at the level of individual neurons and in the circuits where they function. Drosophila is a premier organism for identifying the mechanisms of learning and memory. Drosophila also respond to drugs of abuse in ways that remarkably parallel humans and rodent models. An emerging consensus is that, for alcohol, the mushroom bodies participate in the circuits that control acute drug sensitivity, not explicitly associative forms of plasticity such as tolerance, and classical associative memories of their rewarding and aversive properties. Moreover, it is becoming clear that drugs of abuse use the mushroom body circuitry differently from other behaviors, potentially providing a basis for their addictive properties.
Drug addiction is intimately interconnected with the mechanisms of learning and memory (Berke and Hyman 2000; Kelley 2004; Hyman et al. 2006). It has been long recognized that inappropriate conditioning and valence assignment underlie substance use disorders (Wikler 1984). The remarkably strong associations that drugs of abuse create with external cues and internal states are reflected in many experimental paradigms used to model aspects of addiction. For example, animals readily associate spatial cues with drug administration, they quickly learn to perform tasks to receive drugs, and they remember drug/cue associations for extraordinarily long times. Behavioral changes resulting from drug experiences without explicit cue pairing—including preference, sensitization, and tolerance—also reflect pharmacologically induced changes in brain function that are tied to the risk for developing a substance use disorder (Schuckit 2000; Nestler 2002). Memories of experiences with drugs of abuse are atypically persistent, contributing to the high risk for relapse that is characteristic of drugs of abuse (Dong et al. 2017).
The molecular and circuit mechanisms for learning and memory overlap with those for drugs of abuse. Fundamental processes including synaptic plasticity, regulation of the expression of specific genes, and chromatin structural changes are shared (Berke and Hyman 2000; Hegde and Smith 2019). Although the circuitry where plasticity occurs for drugs of abuse and learning and memory in the mammalian brain at least partially overlap, it is likely that more precise and detailed understanding will reveal important distinctions (Nestler 2002). In most cases, the individual neurons in specific brain regions in mammals that are responsible for the actions of drugs of abuse are not yet identified. Moreover, subtle and not so subtle differences in the temporal and molecular ways the neurons are used—individually and in circuits and networks—are beginning to emerge. There are numerous clues as to how the potent and lasting memories of addictive drugs differ from more benign forms of memory, but work is needed to identify clear mechanistic distinctions that may reveal why drugs are so different.
The fruit fly (or vinegar fly) Drosophila melanogaster is useful for studying the actions of drugs of abuse. Because of their ecology, they are mostly used to model aspects of alcohol use disorder, and the actions of ethanol are the main focus of this review. D. melanogaster is attracted to sites of fermentation that serve as a source of food, a place to find mates, a conducive environment for the development of the young, and protection of the young from certain forms of predation (Devineni and Heberlein 2013; Kacsoh et al. 2013). Allelic variation in the gene that encodes alcohol dehydrogenase and contributes to ethanol metabolism is linked to adaptations to environments with different levels of fermentation (Parsons 1973). Genetic factors that influence processes other than metabolism also contribute to ethanol adaptation, as first discovered in populations in a winery cellar (Parsons 1973; McKenzie and Parsons 1974). Early studies further found that female flies prefer to lay their eggs on ethanol-containing substrates, a demonstration of a form of drug preference (McKenzie and Parsons 1972). In addition to ethanol, Drosophila is used to study the molecular and cellular mechanisms of cocaine, nicotine, amphetamines, and caffeine (Cummins-Beebee et al. 2023). For example, cocaine, by binding to the plasma membrane transporters for the biogenic amines in flies, drives drug sensitivity and sensitization (Corey et al. 1994; Demchyshyn et al. 1994; McClung and Hirsh 1998; Bainton et al. 2000). Flies given volatilized (crack) cocaine develop stereotypies, as do humans, suggesting perhaps surprisingly deep parallels in drug action on the brains of highly divergent species.
How D. melanogaster, humans, and certain vertebrate models respond to ethanol is similar. Naturally occurring variation in sensitivity to ethanol inebriation is due in part to genetic differences that include overlapping sets of genes between species (Morozova et al. 2009; Parker et al. 2020). In D. melanogaster, repeated ethanol exposure causes tolerance to its negative effects and sensitization to its stimulant effects (Scholz et al. 2000; Lee et al. 2008). An initial aversion to ethanol intake converts to preference with alcohol pre-exposure or continuous access (Ja et al. 2007; Devineni and Heberlein 2009; Peru et al. 2013). Flies will assign valence to neutral cues that were paired with ethanol (Kaun et al. 2011). Moreover, flies will reinstate at high levels of drinking after a period of abstinence, and repeated exposures can result in aversion-resistant alcohol seeking (Devineni and Heberlein 2009; Kaun et al. 2011; Shohat-Ophir et al. 2012). Neuromodulators and neurotransmitters central to drug action appear to have conserved function; most prominently, dopamine is critical for acute drug responses and for experience-dependent plasticity from flies to humans (Bainton et al. 2000; Kong et al. 2010b; Kaun et al. 2011; Kanno et al. 2021). In contrast, there exist closely related species for vertebrates and for invertebrates in which the relationship of the neurochemical and behavioral effects appears to be decoupled. For example, Drosophila simulans that is closely related to D. melanogaster does not prefer ethanol (McKenzie and McKechnie 1979). The shared neurochemical and behavioral effects of ethanol in D. melanogaster, Mus musculus, and humans may be examples of convergent evolution for the actions of drugs of abuse. However, the surprising depth of similarity suggests that certain core operations of the brain are more alike than different through evolutionary time. Thus, although our current knowledge in limited species supports conservation of drug action at the molecular and circuit level, studies in additional species may reveal important distinctions, in drug interactions with evolutionarily conserved circuitry, drug disposition, or evolving circuits.
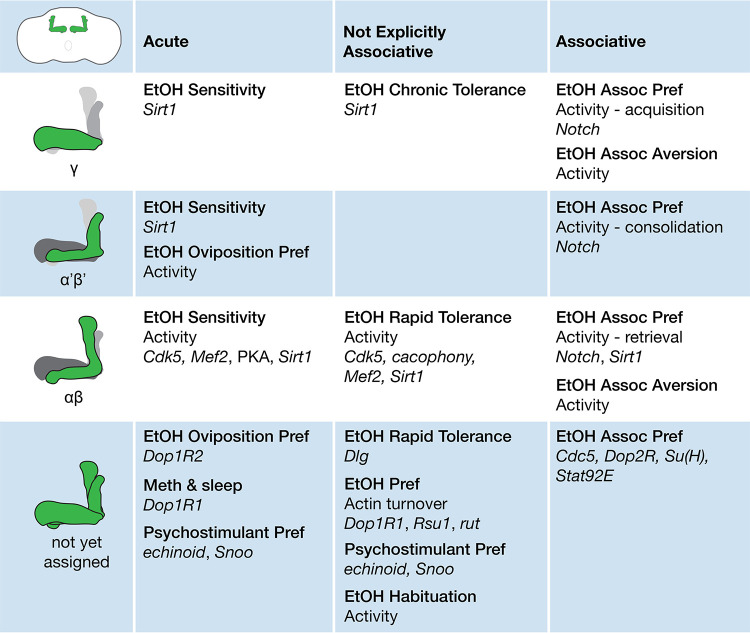
Here, we review the roles of the mushroom bodies in the actions of drugs of abuse in adult Drosophila. The mushroom bodies are the major site of learning and memory, and the circuitry and its function are some of the best understood in any organism. The mushroom bodies are comprised of the intrinsic Kenyon cell neurons that are organized into highly parallel fiber tracts arranged into lobes. The lobes are named α/β, α′/β′, and γ. The Kenyon cells receive sensory inputs, and transmit information to a small number of mushroom body output neurons (MBONs) that encode approach and avoidance behaviors. The MBONs tile across the Kenyon cell lobes to create multiple discrete zones in each lobe. The zones are also innervated by the DAN dopamine neurons that convey internal states and unconditioned stimuli to modify sensory information and select MBONs. The Kenyon cells are broadly innervated by two large interneurons, the APL and the DPM, that appear to play important roles in learning and memory.
As in humans and rodents, drugs of abuse in flies engage learning and memory mechanisms. The emerging picture is that drugs of abuse likely use the mushroom bodies differently from other conditioning events. Some aspects of drug learning and memory may occur outside of the mushroom body circuitry, suggesting that addiction-like circuitry in flies is distributed, as in mammals (Knabbe et al. 2022; Larnerd et al. 2023). An exciting prospect is that defining the genes and circuits for drugs of abuse can reveal the deeper functional analogies between the brains of flies and vertebrates.
This review is organized according to three types of drug experiences: their acute actions in drug-naive animals, repeated or prolonged drug exposures designed to measure plasticity but lacking explicit associative cues, and associative pairing. The mushroom bodies function in all stages of drug experience, which appears consistent with how the mushroom bodies function in other behaviors (Fig. 1). Because addiction is a progressive disease, how the shifting functions of the mushroom bodies relate to one another may help reveal some of the complex encoding mechanisms unique to addictive substances.
Figure 1.
Circuit and genetic functions of the mushroom body Kenyon cell–intrinsic neurons for the actions of drugs of abuse. The functions of mushroom body inputs and outputs (dopamine neurons, APL, DPM, and MBON output neurons) are detailed in the text of this review.
Acute actions of drugs of abuse and the Drosophila mushroom bodies
The acute effects of drugs of abuse can be measured as sensitivity or resistance to their pharmacological and behavioral actions, and as attraction or aversion to the drug. All drugs of abuse acutely activate dopaminergic pathways that are tied to reward at doses that cause euphoric highs or inebriation. Higher or prolonged acute drug doses engage additional neural mechanisms, including aversive neural stress and immune pathways. These and other well-characterized neural pathways that are involved in the response to drugs of abuse appear to be conserved at least in part from flies to humans (Scaplen and Kaun 2016; Chvilicek et al. 2020). Moreover, acute drug exposure initiates multiple forms of plasticity to drug behavioral, cellular, and molecular actions. Decreased sensitivity to the negative effects of ethanol and increased sensitivity to its positive effects correlates with increased risk for alcohol use disorder in humans, suggesting that the acute actions of the drug are relevant to addiction mechanisms (Schuckit 1994; Morean and Corbin 2010). This relationship holds true in rodents (Parker et al. 2020).
Drosophila react to their first ethanol encounter in a highly stereotyped manner. At low to moderate ethanol doses, flies increase locomotor activity. Flies are attracted to lower ethanol concentrations and are repelled by higher concentrations. Female flies prefer to lay their eggs on ethanol-containing substrates. With increasing ethanol doses flies become uncoordinated and then sedated, and they recover after clearing the ethanol using conserved metabolic pathways. Ethanol blood/internal ethanol concentrations are similar between flies and mammals for analogous behavioral responses (Wolf et al. 2002).
Drug sensitivity
Ethanol sensitivity in flies is typically measured by locomotor stimulation at low and moderate drug doses and sedation at higher doses. Approaches to understanding ethanol sensitivity in Drosophila include gene mutants, functional assessment of genes regulated by ethanol, anatomical mapping of the sites of gene function, and anatomical mapping of neurons whose activity regulates ethanol responses.
Hr38 is an immediate early response gene transcription factor and the sole Drosophila ortholog of the Nr4a1–3 family in mammals. Hr38 mRNA is rapidly and transiently induced by binge-like ethanol exposure and by neuronal activity (Fujita et al. 2013; Adhikari et al. 2019). Hr38 inducibility is limited to the first binge-like experience, suggesting that it functions to mark naive responding (Larnerd et al. 2023). Expressed sparsely in the mushroom body Kenyon cells constitutively, Hr38 induction includes the addition of newly Hr38-expressing Kenyon cells. Flies heterozygous for a null Hr38 mutation have decreased ethanol sensitivity. Hr38 induction by ethanol is dependent on the Mef2 transcriptional activator. Blocking Mef2 activity in α/β Kenyon cells with dominant negative Mef2 decreases ethanol sensitivity. The role of the α′/β′ and the γ lobes was not tested. Thus the mushroom body α/β lobes can support ethanol sensitivity through a Mef2-dependent signaling event that may include Hr38 induction.
Sirt1 is a nuclear histone/protein deacetylase that regulates the behavioral responses to drugs of abuse in rodents and in flies (Ferguson et al. 2013). Sirt1 functions in the mushroom bodies to promote ethanol sensitivity, and Sirt1 regulation of ethanol sensitivity is an adult function of the gene (Engel et al. 2016). Sirt1 is required in each of the three mushroom body lobes, implicating a broader role of the mushroom bodies in the regulation of ethanol sensitivity (Larnerd et al. 2023). Moreover, Sirt1 is critical for the termination of the induction of Hr38 gene expression by ethanol, and it more broadly facilitates ethanol-dependent gene expression regulation in flies (Engel et al. 2016; Adhikari et al. 2019). However, it seems unlikely that ethanol regulation of gene expression is a mechanism to determine ethanol sensitivity given the short duration of the assay. Sirt1 may be required in steady state to maintain chromatin in a particular conformation in the mushroom bodies; when the steady state is transiently disrupted, the functional properties of the mushroom bodies may be altered, causing a decrease in ethanol sensitivity.
The function of two additional genes was tested in the α/β lobes of the mushroom bodies for their role in ethanol sensitivity. Inhibition of protein kinase A (PKA) in the α/β lobes decreases ethanol sensitivity, as does RNAi-directed reduction in Cdk5 expression (Rodan et al. 2002; Lange and Wolf 2023). PKA activity is central to cAMP signaling that is critical for both ethanol sensitivity and learning and memory across species (Newton and Messing 2006; Kandel 2012; Davis 2023). Likewise, Cdk5 is a pleiotropic kinase that plays multiple roles in synaptic and neuronal physiology and that is implicated in responses to drugs of abuse and in memory (Fischer et al. 2005; Benavides et al. 2007; Smith-Trunova et al. 2015; Pao and Tsai 2021).
The role of the mushroom bodies in ethanol sensitivity does not appear to include a requirement for neuronal activity. Blocking Kenyon cell–evoked transmission with tetanus toxin, hyperpolarizing the Kenyon cells with the Kir2.1 inwardly rectifying potassium channel, and decreasing Kenyon cell expression of the Cav2.1 voltage-gated calcium channel had no effect on ethanol sensitivity (Engel et al. 2016; Lange and Wolf 2023; Larnerd et al. 2023). Indeed, selective chemical ablation of the mushroom bodies during development had no effect on ethanol sensitivity in adults (Rodan et al. 2002). This is in contrast to the adult-specific ethanol sensitivity role of Sirt1. Notably, blocking evoked transmission with tetanus toxin in the Kenyon cells in flies also mutant for Sirt1 results in increased ethanol sensitivity, the opposite behavioral response compared to Sirt1 mutants alone. Thus, Sirt1 may normally block the mushroom bodies from releasing neurotransmitters or neuromodulators in response to ethanol and participating in circuits that respond to an acute inebriating ethanol exposure. Expression of Sirt1 and other regulators of chromatin structure are readily altered by a variety of contexts and prior experiences, providing a potential mechanism that can tune how the first drug experience is perceived and perhaps remembered (Campbell and Wood 2019).
In summary, ethanol sensitivity can be controlled by genetic pathways in each of the three mushroom body lobes, and acute ethanol exposure causes changes in mushroom body gene expression. The relationships of the molecular pathways implicated by the mushroom body ethanol sensitivity genes remain to be explored.
Drug attraction or aversion in drug-naive Drosophila
Smell, taste, and ingestion of ethanol can trigger approach or avoidance behavior in drug-naive flies. The response of the flies can depend on drug context, concentration, and the length of exposure. Attraction and aversion represent the inherent volitional nature of ethanol for Drosophila.
Innate olfactory attraction to ethanol
Olfactory sensory pathways provide a large fraction of the input to the mushroom bodies. Olfactory attraction to ethanol is studied by motion tracking and with olfactory traps, the latter of which captures flies that venture near an odor source such as ethanol (Schneider et al. 2012; van Breugel et al. 2018). Neurons expressing serotonin and the norepinephrine-like octopamine regulate ethanol odor attraction, and neurons expressing these amines innervate the mushroom body circuitry (Sinakevitch and Strausfeld 2006; Lee et al. 2011; Xu et al. 2016; Claßen and Scholz 2018). The octopamine and serotonin neurons controlling innate olfactory preference may function in olfactory processing pathways upstream of the mushroom bodies, in lateral horn circuitry that can code for odor valence and identity, or in other pathways that influence mushroom body function (Das Chakraborty et al. 2022).
Innate oviposition preference for ethanol
Ethanol can reach concentrations that inebriate flies in fermenting plants. In the laboratory, mated Drosophila females, given a choice, lay more eggs on ethanol/sucrose medium than on sucrose alone medium. The choice of substrate is typically made quickly as internal ethanol concentrations are likely increasing. A combination of olfactory and taste cues guide oviposition substrate choice (Azanchi et al. 2013). Blocking presynaptic release from mushroom body α′/β′ neurons, but not other mushroom body lobe neurons, decreases oviposition preference for ethanol. Dopamine neurons of positive and negative valence that innervate the mushroom bodies promote and inhibit ethanol oviposition choice, respectively. The dopamine D1-like receptor Dop1R2 in the mushroom bodies is critical for oviposition preference. Thus, female preference for laying eggs on ethanol substrates likely involves a dopaminergic signal biased toward choosing ethanol that signals onto D1-like receptors, influencing the α′/β′ mushroom body Kenyon cell neuron promotion of ethanol substrate choice.
Early developing drug intake preference
Ethanol-naive Drosophila tend to avoid ethanol intake (presented at 5%–15% concentrations), but they develop ethanol preference over the course of days (see the next section for a discussion for acquired preference) (Ja et al. 2007; Devineni and Heberlein 2009). Flies can develop ethanol preference early with behavioral and genetic manipulation, and this may reflect innate preference. The CAFE assay is most widely used to measure preference. Flies are presented capillaries that contain a caloric liquid (sucrose that can have added yeast for protein), either with or without ethanol, a setup that is similar to two-bottle choice paradigms used in rodent studies of ethanol. The flies drink over a 12- to 16-h period and the ratio of the volumes consumed gives a preference index. A second preference assay, the FRAPPE, presents the choices arrayed in microwell plates with access given to the flies for 15–30 min (Peru et al. 2013). Despite the possibility for learning and memory in the CAFE given its longer duration, outcomes are indistinguishable in the cases in which both CAFE and FRAPPE were performed, suggesting that the CAFE can, in some cases, assess innate preference. An ethanol avoidance pathway in ethanol-naive flies appears to suppress an underlying ethanol preference pathway (Ojelade et al. 2015; Engel et al. 2016). Anatomical mapping for the ethanol avoidance pathway is still crude, but the current evidence points to a nonmushroom body origin. Genetic evidence in Drosophila and mice implicates actin dynamics in initial ethanol responses as well as in memory formation (Offenhäuser et al. 2006; Rothenfluh and Cowan 2013; Lamprecht 2016). Indeed, increasing actin turnover in all mushroom body neurons in adults causes faster acquisition of ethanol preference (Butts et al. 2019). This may reflect faster learning or altered innate behavioral dynamics.
Cocaine and morphine consumption are aversive to Drosophila, whereas methamphetamine consumption is mildly appetitive (Kanno et al. 2021). The role of the mushroom body in the preference for cocaine and methamphetamine intake was tested by reducing the expression of genes that were identified in a genome-wide association study of preference in a panel of inbred wild-derived Drosophila strains (Highfill et al. 2019). Changes in psychostimulant preference occurred with mushroom body knockdown of two genes: echinoid, which encodes a cell adhesion molecule, and the Sno (Snoo) oncogene that regulates the Smad signaling pathway. Moreover, DAN-specific knockdown of echinoid, Snoo, and the musashi RNA-binding protein all changed the preference for cocaine. Thus the early evidence points to a role for the mushroom bodies in regulating the preference for multiple drugs of abuse (Highfill et al. 2019). Mushroom body gene expression is markedly altered in response to cocaine consumption (Baker et al. 2021). Genes affected by cocaine consumption in the mushroom bodies overlap with known regulators of drug responses, including the insulin receptor, the Slo potassium channel, the Mef2 transcription factor, and the Rutabaga type I adenylyl cyclase. Acute methamphetamine intake also disturbs sleep in Drosophila, as it does in vertebrates (Andretic et al. 2005). Methamphetamine exerts its effects at least in part by increasing dopamine neurotransmission. In flies the effect on sleep is mediated by the dopamine D1 receptor Dop1R1 in the mushroom bodies (Andretic et al. 2008).
In summary, initial responses to the acute administration of multiple drugs of abuse share sites of action in the mushroom body circuitry. Drug sensitivity mechanisms appear to engage genetic pathways rather than neurotransmission events in Kenyon cells. In contrast, mechanisms for innate preference to oviposit on or consume drug-containing substrates appear to use both changes in gene expression and synaptic signaling.
Not explicitly associative actions of drugs of abuse and the Drosophila mushroom bodies
Drug exposure that is repeated one or more times causes drug-induced behavioral and molecular changes. Sensitization, habituation, tolerance, and acquired preference are all types of behavioral plasticity induced upon repeat drug use without explicit pairing with cues. These forms of plasticity may be distinct from associative forms with explicit cue pairing. Sensitization is an important measure of the actions of drugs of abuse in rodents, and it is observed from humans to invertebrates (Lister 1987; Pierce and Kalivas 1997). In Drosophila, repeated exposure to methamphetamine, cocaine, and ethanol results in escalating locomotor and other behavioral activity (McClung and Hirsh 1998; Lee et al. 2008; Rigo et al. 2021). Sensitization is yet to be studied in relation to the mushroom bodies.
Drug habituation
Habituation to drugs of abuse in the nervous system is defined as a rapid reduction in response intensity to repeated sensory exposures. Habituation may also occur due to coincident stimuli, such as the internal events associated with using the drug (e.g., taste) and the external cues around the drug (e.g., environment). The mushroom bodies are critical for olfactory sensory processing; therefore, habituation may attenuate the strength of olfactory cues associated with a drug. Indeed, the mushroom bodies have a demonstrated role for the habituation of a locomotor startle response to novel odors, including ethanol. The ethanol odor–induced startle response fails to habituate when (1) the mushroom bodies are ablated, (2) the evoked activity of the mushroom bodies is blocked with tetanus toxin, or (3) the Rutabaga adenylyl cyclase, which functions in the mushroom bodies for learning and memory, is mutated (Cho et al. 2004). As a fly interacts with a drug over time, the process of habituation can decrease the salience of environmental cues associated with drug use. Hence the landscape of sensory information during drug use becomes less impactful to the organism. Concurrently, the drug is chemically altering brain function, potentially changing how the brain processes sensory information. Therefore, sensory habituation to the drug might result in a dual effect: Not only can it change how environmental information is perceived, but it can also interact with the drug-induced changes in brain function, ultimately influencing the overall impact of the drug on the brain.
Drug tolerance
Tolerance is a difference in sensitivity to a drug, after exposure to that drug. Therefore, tolerance is an acquired effect of drug experience that may manifest as resistance to the negative aspects of drug use or sensitization to the positive effects. For ethanol, adult flies do not develop metabolic/pharmacokinetic tolerance: They do not alter the rate of drug clearance (Geer et al. 1988; Scholz et al. 2000). Hence, the observed tolerance arises from a functional/pharmacodynamic change. Different forms of ethanol tolerance can be induced by the type of exposure, and there is growing evidence that these forms are anatomically, molecularly, and functionally distinct, including their engagement with the mushroom bodies. Tolerance is classically categorized as acute (within session), rapid (after the first exposure is fully metabolized), and chronic (Fadda and Rossetti 1998). Although all forms of ethanol tolerance exist in flies, acute tolerance is less well-studied. Similarly understudied is repeated tolerance, which has distinct behavioral outcomes compared to chronic tolerance in flies (Ranson et al. 2020; Larnerd et al. 2023). Like acute tolerance, it is not yet known if repeated tolerance engages mushroom body circuitry. Finally, we note that tolerance classification in flies differs from that in rodents and humans. In flies chronic ethanol tolerance results from a prolonged low-dose ethanol exposure, whereas in mammals it also includes tolerance resulting from repeated ethanol exposures.
Rapid and chronic ethanol tolerance have been characterized in relation to known functions of the mushroom bodies, such as olfactory memory. For example, previously identified long-term memory mutants were screened in rapid and chronic tolerance assays (Dubnau et al. 2003; Berger et al. 2008). Several memory mutants harbored deficits in acquiring rapid and/or chronic tolerance too. The mushroom bodies are a well-documented site for olfactory conditioning that creates long-term memory, which is distinct from earlier types of memory in part by its requirement for new protein synthesis. Rapid tolerance develops normally in flies fed the translational inhibitor cycloheximide, but chronic tolerance does not, suggesting that chronic tolerance depends on new protein synthesis (Berger et al. 2004). Olfactory conditioning that creates long-term memory also depends on mushroom body usage of the immediate early gene transcription factor Kayak, related to Fos in mammals, and the cAMP response element-binding protein (CREB) (Miyashita et al. 2018). Chronic ethanol-induced kayak expression and CREB signaling are exciting prospects for further characterization of chronic tolerance. Chronic tolerance requires CREB signaling, based on the expression of a dominant negative form of CREB in the adult brain. However, mushroom body–specific testing revealed a dispensable role for CREB (Larnerd et al. 2023). Thus, chronic ethanol exposure appears to create a long-term memory-like state. Studies like these demonstrate the overlap and distinctions between genetic mechanisms that support memory and those that create tolerance to prior ethanol doses.
Drug tolerance: rapid ethanol tolerance
Rapid ethanol tolerance is measured after the complete metabolism of the initiating exposure, a binge-like dose of ethanol that is strongly inebriating to sedating. Rapid tolerance is formed and maintained in part by chromatin state and possibly gene expression programs (Berkel and Pandey 2017). Mef2, a transcription factor that is highly expressed in the mushroom bodies, is required for rapid tolerance. A dominant negative form of Mef2 revealed that its signaling is required in the mushroom body α/β lobes. Mef2 induces the immediate early gene transcription factor Hr38, which itself promotes rapid tolerance in a dose-dependent manner. The histone/protein deacetylase Sirt1 terminates Hr38 induction to allow rapid tolerance expression, a role that has been localized to the mushroom body α/β lobes (Adhikari et al. 2019). The roles for Mef2 and Sirt1 in supporting rapid tolerance have yet to be tested in other Kenyon cells or the extrinsic neurons of the mushroom bodies.
The anatomical structure and synaptic function of the mushroom bodies have also been explored for rapid tolerance. Drosophila mutants with mushroom body abnormalities, such as flies mutant for the Ras GTPase–activating protein Vap, do not develop rapid tolerance (De Belle and Heisenberg 1996; Scholz et al. 2000). NMDA receptor–mediated synaptic plasticity is a target of ethanol, with acute ethanol antagonizing NMDAR function (Nagy 2008). NMDAR mutants show reduced rapid tolerance (Maiya et al. 2012). NMDAR clustering on the postsynaptic membrane is aided by scaffold proteins PSD-95 and SAP97, whose Drosophila homolog is Dlg1. Dlg1 mutants show a rapid tolerance deficit that is restored when the DlgS97 isoform, homolog of mammalian SAP97, is expressed in mushroom body neurons (Maiya et al. 2012). Last, the mushroom body α/β lobes require neuronal activity to support rapid tolerance (Engel et al. 2016; Lange and Wolf 2023). Thus, the synaptic physiology of the mushroom body supports rapid ethanol tolerance. Manipulations of small molecule neurotransmitter signaling provide additional, indirect evidence that the mushroom bodies are implicated for rapid tolerance. Kenyon cells receive input from extrinsic mushroom body neurons, including GABAergic signals from both the DPMs and APLs. Kenyon cell GABA reception occurs via ionotropic GABAA and/or metabotropic GABAB, both of which have been implicated in ethanol tolerance. Pharmacological agonism of GABAB augments rapid tolerance in Drosophila (Dzitoyeva et al. 2003). The Kenyon cells additionally receive octopaminergic signals from the APLs. Octopamine is synthesized from tyramine via the enzyme tyramine β-hydroxylase (TBH). The octopamine-deficient mutant TbhM18 shows a deficit in rapid tolerance (Scholz et al. 2000; Berger et al. 2004). Taken together, the mushroom bodies encompass many candidate sites of altered signaling for encoding tolerance to acute ethanol, but direct tests of all circuitry have yet to be performed.
Finally, rapid tolerance is composed of two memory-like states, an anesthesia-sensitive and an anesthesia-resistant state (Larnerd et al. 2023). Administration of a brief cold shock to flies mutant for the GTPase Radish almost completely erases rapid tolerance. Cold shock treatment is used to ablate anesthesia-sensitive memory (ASM) formed by odor-shock aversive training, and mutation of Radish selectively eliminates anesthesia-resistant memory (ARM) that is coformed along with ASM. Moreover, the temporal decay of rapid tolerance, over 24–36 h, is similar to that for odor-shock aversive training (Berger et al. 2004; Margulies et al. 2005). Aversive odor-shock ASM and ARM are encoded by the APL, DPM, and Kenyon cell mushroom body circuitry (Pitman et al. 2011; Bourouliti and Skoulakis 2022; Davis 2023). Thus, current evidence suggests that rapid tolerance is protein synthesis independent and consists of labile and consolidated encoding mechanisms that may localize to the mushroom body circuitry.
Drug tolerance: chronic ethanol tolerance
Chronic ethanol tolerance occurs after the metabolism of the initiating exposure, a long-term low dose of ethanol. Like rapid tolerance, chronic tolerance is encoded into the genome via changes in chromatin state (Berkel and Pandey 2017). Unlike rapid tolerance, the immediate early response gene Hr38 is dispensable for chronic tolerance. Hr38 is not inducible following chronic ethanol conditions, likely because of chromatin compaction by Class I/II histone deacetylases (HDACs) (Larnerd et al. 2023). Feeding flies nicotinamide, which blocks the Sirtuin class of NAD-dependent HDACs, after the initiating dose causes a reduction in chronic tolerance, suggesting that Sirtuins help maintain the adaptations to chronic ethanol. However, one NAD-dependent Sirtuin, Sirt1, plays an opposite role and suppresses chronic tolerance. Sirt1 exerts its tolerance-suppressing effects in adults and specifically in the mushroom body γ lobe, but not the other lobes (Larnerd et al. 2023). Mechanisms for these chronic tolerance-encoding chromatin states are currently incomplete, especially how they function in specific mushroom body cell types. The synaptic function of the mushroom bodies has also been tested for chronic ethanol tolerance. Evoked activity from the mushroom bodies blocked with tetanus toxin disrupts chronic tolerance, even in the Sirt1 mutant background, which develops high chronic tolerance on its own. Hence, mushroom body neurotransmission is critical and may additionally regulate the Sirt1-dependent suppression of chronic tolerance. Consistent with this, hyperpolarizing all Kenyon cells in adults using the Kir2.1 inwardly rectifying potassium channel reduces chronic tolerance development (Larnerd et al. 2023). Ultimately, a yet-identified subpopulation of Kenyon cells likely needs to actively promote tolerance to chronic ethanol. Importantly, chronic ethanol tolerance differs from classical long-term memory in that the CREB pathway acts outside the Kenyon cells.
In summary, different forms of ethanol tolerance are encoded into distinct circuits in the mushroom bodies. Namely, a Sirt1-dependent mechanism exists in the α/β lobes to promote rapid tolerance and in the γ lobe to inhibit chronic tolerance. There is the requisite neuronal activity of broad and specific Kenyon cell populations for rapid and chronic ethanol tolerance behaviors.
Relationship between ethanol sensitivity and ethanol tolerance
A correlation exists between ethanol sensitivity and tolerance phenotypes in flies mutant for a variety of genes: Reduced ethanol sensitivity correlates with reduced rapid tolerance (Berger et al. 2008; Kong et al. 2010a; Chvilicek et al. 2024). In some cases in which the spatial or temporal actions of a gene were mapped, its role in sensitivity and tolerance can be separated. For example, the cacophony calcium channel subunit-encoding gene promotes rapid tolerance in the mushroom body α/β neurons, whereas its role in ethanol sensitivity maps to other neurons (Lange and Wolf 2023). Sirt1 provides a different example, in which its role in promoting ethanol sensitivity maps to all three mushroom body lobes, and its role in promoting rapid tolerance maps specifically to the α/β lobes (Larnerd et al. 2023). Thus, ethanol sensitivity and rapid tolerance likely share genetic pathways that can be used in the same or different cells.
Drug preference
Drug preference is the innate or acquired consumption or attraction to the drug itself. Attraction to drug-related cues is also possible, as exemplified in conditioned place preference schemes; however, these behaviors are explicitly associative. Here, we describe current findings on the mushroom body regulation of learned but nonassociative drug preference.
Drosophila exhibit a learned attraction to or increase in consumption of drugs under repeated or prolonged conditions. This can be evaluated across days using the two-choice CAFE assay, in which flies can self-administer sucrose solutions with or without low levels of ethanol. Acquired ethanol preference requires the activity of DANs—namely, the PAM cluster—and it requires dopamine reception in the Kenyon cells they target via Dop1R1. Consistent with this, Dop1R1 protein levels increase in the medial lobes of the mushroom bodies with ethanol (Kanno et al. 2021). The gene Ras suppressor 1 (Rsu1) is required in the mushroom bodies in adults for acquired ethanol preference, further demonstrating the importance of actin turnover in Kenyon cells (Ojelade et al. 2015; Butts et al. 2019). Also, the rut type I adenylyl cyclase is broadly required in the mushroom bodies for learned preference (Xu et al. 2012). Taken together, dopaminergic and cAMP signaling mechanisms are paramount for the mushroom body's response to prolonged ethanol consummatory behavior.
Different priming doses of ethanol can also shape acquired preference to ethanol, which is measured after the priming dose by the CAFE assay. An acute inebriating or sedating dose of ethanol causes preference for ethanol that is typically measured at least 16 hours later (Peru et al. 2013; Engel et al. 2016; Larnerd et al. 2023). A chronic, nonsedating dose also causes acquired preference. However, an inebriating dose of ethanol repeated daily causes learned aversion (Larnerd et al. 2023). Preference from primed ethanol pre-exposure, similar to the preference formed during prolonged ethanol exposure, maps at least in part to the mushroom bodies (Butts et al. 2019).
The mushroom bodies also regulate acquired preference to drugs other than ethanol. Flies exposed to the psychostimulants cocaine and methamphetamine develop acquired preference. Candidate genes identified in a genome-wide association study of preference were directly tested in mushroom body neurons and DANs for regulating such drug intake behaviors over time (Highfill et al. 2019). The Dop1R1 dopamine receptor and Snoo, a regulator of Smad signaling, are each required in DANs for methamphetamine acquired preference. Moreover, the mushroom bodies require Snoo for cocaine acquired preference, and the echinoid cell adhesion molecule for methamphetamine acquired preference, respectively (Highfill et al. 2019). Therefore, the mushroom body intrinsic and extrinsic neurons regulate psychostimulant intake behaviors.
In summary, behavioral plasticity that is not explicitly associative, such as sensitization, habituation, tolerance, and acquired preference, all represent the longer term and likely stable neural adaptations that build from multiple encounters with drugs of abuse. There exists direct and indirect evidence that the mushroom bodies regulate almost all of these drug behaviors through the following means: (1) changes in chromatin state, (2) molecular mechanisms for memory encoding, and (3) cellular signaling events. It is evident that the mushroom bodies play a critical role in shaping the motivational behaviors that follow prolonged use of several drugs of abuse, even when their pharmacological actions differ.
Explicitly associative actions of drugs of abuse and the Drosophila mushroom bodies
The ability to form an association between a stimulus and an event—to store it as a memory and to retrieve it upon exposure to the affiliated stimulus—for anticipating the reoccurrence of the event has an evolutionary advantage. However, learning and memory mechanisms are vulnerable to drugs of abuse to manifest maladaptive behaviors like aversion-resistant cue-seeking and long persistence. Drug-induced associations can reveal how reward and aversion are encoded in the brain. Addiction-related associative memories occur in Drosophila and enormous strides are being made to tease apart the involved circuitry and to understand the nature of these associative memories, predominantly using ethanol as a drug.
Behavioral paradigms to study the associative actions of alcohol
When ethanol is presented along with an odor, flies learn to form an association between the odor and ethanol's valuation. To quantitatively study the associative action of ethanol, typically flies are sequentially presented with two odors, with one of them simultaneously paired with inebriating doses of ethanol vapor. These training events can be spaced akin to standard long-term memory paradigms or massed akin to ARM paradigms. To assess learning post-training, flies are tested in a Y maze with the paired and unpaired odors presented as a choice. Spaced training with inebriating amounts of ethanol causes short-term aversion to the odor that was previously paired with ethanol and a longer-term attraction toward the paired odor that lasts for at least 7 days. Thus, ethanol supports alcohol-associative preference (also termed cue-induced ethanol seeking) in Drosophila (Kaun et al. 2011; Petruccelli et al. 2018). The association formed is between the cue and the pharmacological or intoxicating effect of ethanol rather than its odor (Nunez et al. 2018). Importantly, the long-lasting attraction is aversion resistant: Flies will endure electric shock to pursue odor cues predictive of ethanol. This aversion resistance is markedly stronger than that created for sugar reward cues after appetitive training (Kaun et al. 2011).
Ethanol dose and duration of exposure play an important role in shaping the valence, strength, and perdurance of cue-induced ethanol memories, as does the frequency of exposure and intertrial interval length (Nunez et al. 2018). In contrast to spaced training, massed training results in binge-like ethanol exposures and generates short-lived reward memories (Nunez et al. 2018). Larvae also develop an alcohol-associative preference, with similar parametric requirements, potentially providing a model to understand why drug use during late developmental periods is high risk for developing substance use disorders (Berger et al. 2023).
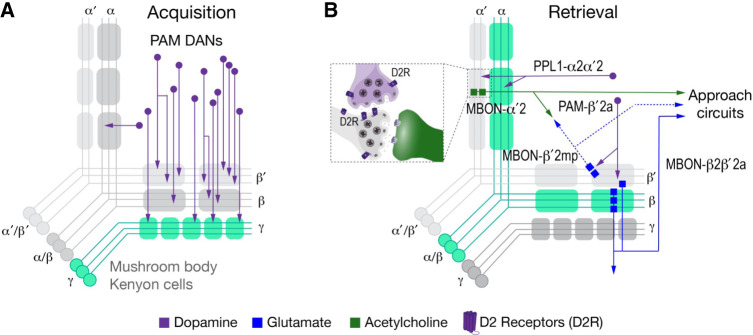
Mushroom body circuitry involved in alcohol-associative preference
Alcohol-associative preference and alcohol-associative aversion are extensively encoded into the mushroom body circuitry. Alcohol memories can be divided into three sequential phases: the learning phase for acquisition, the consolidation phase when the memory is written into the circuitry, and the retrieval phase that demarcates the expression of memory. The sequential phases of acquisition, consolidation, and retrieval of alcohol-associative preference, respectively, require neuronal activity in the γ, α′/β′, and α/β mushroom body lobes (Fig. 2; Kaun et al. 2011). Dopaminergic input also shows phase-specific roles. Drosophila dopamine neurons can be separated into the reward-encoding PAM cluster that primarily innervates the mushroom bodies and a smaller group of all other dopamine neurons that include aversion-encoding neurons that innervate the mushroom bodies. Neuronal activity is required in the PAM dopamine neurons during acquisition and retrieval for alcohol-associative preference (Fig. 2A; Scaplen et al. 2020). The other dopamine neurons are required specifically during retrieval (Kaun et al. 2011). Dopaminergic neuron activity is dispensable for the short-term alcohol-associative aversion, as are the amines dopamine and serotonin. Conditioned aversion is encoded by neuronal activity in the γ and α/β or just α/β Kenyon cells (Kaun et al. 2011). Thus, spaced training for ethanol creates separable appetitive and aversive memory traces that engage the mushroom body circuitry differently.
Figure 2.
Mushroom body circuitry for alcohol-associative preference. (A) Diagram of the mushroom bodies, depicting the vertical (α′, α) and horizontal (β′, β, γ) lobe Kenyon cells, overlayed with the compartments that are defined by dopaminergic input neurons and mushroom body output neurons (MBONs). The PAM dopaminergic input neurons are required for the acquisition of alcohol-associative preference and innervate the horizontal lobes in a precise pattern. (B) PAM dopaminergic inputs and MBON outputs are important for the retrieval of alcohol-associative preference memories. Inset depicts a possible site of action for the dopamine D2 receptor D2R. Highlighted in green are the Kenyon cells that support acquisition and retrieval. The α′/β′ neurons support consolidation. The diagrams are adapted, with permission, from Scaplen et al. (2020).
Refinement of the dopamine circuit identified specific mushroom body input–output pathways for alcohol-associative preference (Fig. 2B). Activity of PAM dopamine neurons that innervate a specific zone of the β′ lobe, the β′2a neurons, and their paired output neurons, the β2β′2a MBONs, promote retrieval of ethanol reward memories (Scaplen et al. 2020). The activity of a second MBON that receives input from the β′2 zone, the β′2mp neurons, inhibits ethanol reward memory formation during consolidation. Because the α′/β′ Kenyon cells promote ethanol memory consolidation, a current model is that dopamine input to the β′2 zone inhibits the ethanol memory consolidation, and that inhibition of consolidation facilitates later ethanol memory retrieval. The β′2 compartment is also implicated in sugar and water rewards (Lin et al. 2014; May et al. 2020). However, the β′2 region encodes the appetitive properties of sugar, a different aspect of reward than ethanol. Thus, ethanol may act through a natural reward pathway but use the circuit differently, providing a high-resolution view of ethanol circuit co-option.
A second circuit for alcohol-associated preference requires the activity of distinct dopamine neurons and MBONs that innervate the α′2 zone of the mushroom bodies (Scaplen et al. 2020). This circuit promotes retrieval of the ethanol reward memory. There exist parallel circuits for retrieval of long-term water and food reward memories, but they use different dopamine neurons and MBONs that mostly innervate other zones of the mushroom body (Plaçais et al. 2013; Ichinose et al. 2015; Lee et al. 2020). The activity of additional MBONs—γ4 > γ1γ2, β′2mp or γ5β′2a, α′2, γ2α′1, or α′3 (m & ap)—support either acquisition or retrieval of ethanol reward memories (Aso et al. 2014).
Molecular mechanisms for alcohol-associative preference and aversion in the mushroom bodies
The highly conserved Notch intercellular signaling pathway plays a critical physiological role in alcohol-associative preference. Scabrous (Sca) is a secreted glycoprotein that acts as a dimer with the Notch ligand Delta to activate the Su(H)-dependent Notch signaling pathway. Sca mutants specifically disrupt alcohol-associative preference, and adult knockdown of Notch in any of the three mushroom body lobes had the same effect (Kaun et al. 2011; Petruccelli et al. 2018). Importantly, the 3× ethanol:odor spaced training progressively activates the Notch signaling pathway. Notch activation leads to changes in gene expression through the Su(H) transcription factor. Ethanol changes Su(H) binding at the dopamine D2 receptor gene Dop2R, resulting in a change in the expression of Dop2R splice isoforms. Dop2R functions in the mushroom bodies to regulate alcohol-associative preference. Hence spaced training with ethanol causes a switch in Dop2R isoforms in the mushroom bodies to promote ethanol reward–associative memories.
Other targets of the Notch/Su(H) pathway showed shifts in gene isoform expression with ethanol spaced training, including the Stat92E gene that encodes a component of the JAK/STAT signaling pathway and that functions in the mushroom bodies for alcohol-associative preference (Petruccelli et al. 2018, 2020; Wilson et al. 2023). The Stat92E isoform switching induced by ethanol spaced training was still detectable at 24 hours, when ethanol reward memories are typically tested. Hence, alternative RNA transcript splicing might contribute to encoding distinct experiences in the mushroom bodies.
The histone/protein deacetylase Sirt1 functions in both alcohol-associative aversion and alcohol-associative preference, in addition to its roles in ethanol sensitivity, ethanol preference, rapid ethanol tolerance, and chronic ethanol tolerance. Whereas Sirt1 null mutants are defective for both the short-term ethanol aversion memory and the longer-term ethanol reward memory, selective knockdown of Sirt1 in mushroom body α/β neurons specifically blocked ethanol reward memory (Engel et al. 2016). Thus, alcohol-associative aversion and preference pathways occur in distinct regions of the mushroom body. Why Sirt1 plays such a broad role in ethanol behavioral responses remains to be determined.
In summary, distinct subsets of mushroom body neurons harbor unique cellular and molecular mechanisms to alter neuron function, which ultimately encodes sequential aspects of the drug:cue pairing process. The associations that are created and maintained in the mushroom bodies with drugs of abuse, especially ethanol, contribute to long-lasting maladaptive behavior. The high-resolution circuit discovery of how drug and natural reward pathways differ presages similar advances in other drug plasticity pathways.
Perspective
Behaviors associated with drugs of abuse may interact with each other and with nondrug forms of learning and memory to strengthen the risk for addiction. These behaviors can theoretically interact over space (i.e., in shared neuronal populations that regulate multiple behaviors) and interact over time (i.e., during coincident internal states caused by separate behaviors or with traces of past experience). The mushroom bodies are also a candidate site for drug-related behaviors to influence each other spatiotemporally. Indeed, some genes and mushroom body circuits regulate both ethanol sensitivity and tolerance. For example, a Mef2–Sirt1 transcriptional program regulates ethanol sensitivity and rapid tolerance in the mushroom body α/β lobes, where Sirt1 also promotes ethanol reward. Early behaviors associated with drugs of abuse may also compound across a lifetime of use: Mushroom body responses may change over time to accumulate and/or set future responses to drug experiences. Finally, drugs of abuse have broad access to the brain and can simultaneously impact the function of not only the mushroom bodies, but also mushroom body afferent (e.g., olfactory) and efferent (e.g., approach and avoidance) pathways, providing additional layers for behavioral action and plasticity (Keesey and Hansson 2022). Of particular note is that ethanol is a strongly valenced olfactory cue and that olfactory pathways are directly connected to the mushroom bodies. Future studies might aim to (1) sort out the drug versus nondrug influences on mushroom body circuitry, (2) categorize the altered responses of the mushroom bodies as deriving from anatomical/structural plasticity versus activity/signaling events, and (3) test mushroom body circuits known to regulate drug responses for controlling multiple drug-related behaviors. How different drugs of abuse use the mushroom body circuitry is a promising avenue of future research, as it may help us hone in on why drugs of abuse differ from other reinforcers in their potency and perdurance. The level of circuit detail that is now possible to achieve in Drosophila is another reason that flies continue to be important for understanding drugs of abuse, especially as the accuracy and depth of natural behavior encoding mechanisms advance alongside.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053815.123.
Freely available online through the Learning & Memory Open Access option.
References
- Adhikari P, Orozco D, Randhawa H, Wolf FW. 2019. Mef2 induction of the immediate early gene Hr38/Nr4a is terminated by Sirt1 to promote ethanol tolerance. Genes Brain Behav 18: e12486. 10.1111/gbb.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Kim YC, Jones FS, Han KA, Greenspan RJ. 2008. Drosophila D1 dopamine receptor mediates caffeine-induced arousal. Proc Natl Acad Sci 105: 20392–20397. 10.1073/pnas.0806776105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, van Swinderen B, Greenspan RJ. 2005. Dopaminergic modulation of arousal in Drosophila. Curr Biol 15: 1165–1175. 10.1016/j.cub.2005.05.025 [DOI] [PubMed] [Google Scholar]
- Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guérin G, Plaçais P-Y, Robie AA, Yamagata N, Schnaitmann C, et al. 2014. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife 3: e04580. 10.7554/eLife.04580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azanchi R, Kaun KR, Heberlein U. 2013. Competing dopamine neurons drive oviposition choice for ethanol in Drosophila. Proc Natl Acad Sci 110: 21153–21158. 10.1073/pnas.1320208110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT-Y, Singh CM, Moore MS, Neckameyer WS, Heberlein U. 2000. Dopamine modulates acute responses to cocaine, nicotine, and ethanol in Drosophila. Curr Biol 10: 187–194. 10.1016/S0960-9822(00)00336-5 [DOI] [PubMed] [Google Scholar]
- Baker BM, Mokashi SS, Shankar V, Hatfield JS, Hannah RC, Mackay TFC, Anholt RRH. 2021. The Drosophila brain on cocaine at single-cell resolution. Genome Res 31: 1927–1937. 10.1101/gr.268037.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, Olausson P, Yan Z, Taylor JR, Bibb JA. 2007. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci 27: 12967–12976. 10.1523/JNEUROSCI.4061-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Heberlein U, Moore MS. 2004. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin Exp Res 28: 1469–1480. 10.1097/01.ALC.0000141817.15993.98 [DOI] [PubMed] [Google Scholar]
- Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. 2008. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res 32: 895–908. 10.1111/j.1530-0277.2008.00659.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Yapıcı B, Scholz H. 2023. The function of ethanol in olfactory associative behaviors in Drosophila melanogaster larvae. PLoS ONE 18: e0276714. 10.1371/journal.pone.0276714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. 2000. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515–532. 10.1016/S0896-6273(00)81056-9 [DOI] [PubMed] [Google Scholar]
- Berkel TDM, Pandey SC. 2017. Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res 41: 666–680. 10.1111/acer.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouliti A, Skoulakis EMC. 2022. Anesthesia resistant memories in Drosophila, a working perspective. Int J Mol Sci 23: 8527. 10.3390/ijms23158527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts AR, Ojelade SA, Pronovost ED, Seguin A, Merrill CB, Rodan AR, Rothenfluh A. 2019. Altered actin filament dynamics in the Drosophila mushroom bodies lead to fast acquisition of alcohol consumption preference. J Neurosci 39: 8877–8884. 10.1523/JNEUROSCI.0973-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RR, Wood MA. 2019. How the epigenome integrates information and reshapes the synapse. Nat Rev Neurosci 20: 133–147. 10.1038/s41583-019-0121-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Heberlein U, Wolf FW. 2004. Habituation of an odorant-induced startle response in Drosophila. Genes Brain Behav 3: 127–137. 10.1111/j.1601-183x.2004.00061.x [DOI] [PubMed] [Google Scholar]
- Chvilicek MM, Titos I, Rothenfluh A. 2020. The neurotransmitters involved in Drosophila alcohol-induced behaviors. Front Behav Neurosci 14: 607700. 10.3389/fnbeh.2020.607700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvilicek MM, Seguin A, Lathen DR, Titos I, Cummins-Beebe PN, Pabon MA, Miscevic M, Nickel E, Merrill CB, Rodan AR, et al. 2024. Large analysis of genetic manipulations reveals an inverse correlation between initial alcohol resistance and rapid tolerance phenotypes. Genes Brain Behav 23: e12884. doi:10.1111/gbb.12884
- Claßen G, Scholz H. 2018. Octopamine shifts the behavioral response from indecision to approach or aversion in Drosophila melanogaster. Front Behav Neurosci 12: 131. 10.3389/fnbeh.2018.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey JL, Quick MW, Davidson N, Lester HA, Guastella J. 1994. A cocaine-sensitive Drosophila serotonin transporter: cloning, expression, and electrophysiological characterization. Proc Natl Acad Sci 91: 1188–1192. 10.1073/pnas.91.3.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins-Beebee PN, Chvilicek MM, Rothenfluh A. 2023. The stage-based model of addiction—using Drosophila to investigate alcohol and psychostimulant responses. Int J Mol Sci 24: 10909. 10.3390/ijms241310909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Chakraborty S, Chang H, Hansson BS, Sachse S. 2022. Higher-order olfactory neurons in the lateral horn support odor valence and odor identity coding in Drosophila. eLife 11: e74637. 10.7554/eLife.74637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. 2023. Learning and memory using Drosophila melanogaster: a focus on advances made in the fifth decade of research. Genetics 224: iyad085. 10.1093/genetics/iyad085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belle JS, Heisenberg M. 1996. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: a case study of the mushroom body miniature gene (mbm). Proc Natl Acad Sci 93: 9875–9880. 10.1073/pnas.93.18.9875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchyshyn LL, Pristupa ZB, Sugamori KS, Barker EL, Blakely RD, Wolfgang WJ, Forte MA, Niznik HB. 1994. Cloning, expression, and localization of a chloride-facilitated, cocaine-sensitive serotonin transporter from Drosophila melanogaster. Proc Natl Acad Sci 91: 5158–5162. 10.1073/pnas.91.11.5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. 2009. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol 19: 2126–2132. 10.1016/j.cub.2009.10.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. 2013. The evolution of Drosophila melanogaster as a model for alcohol research. Annu Rev Neurosci 36: 121–138. 10.1146/annurev-neuro-062012-170256 [DOI] [PubMed] [Google Scholar]
- Dong Y, Taylor JR, Wolf ME, Shaham Y. 2017. Circuit and synaptic plasticity mechanisms of drug relapse. J Neurosci 37: 10867–10876. 10.1523/JNEUROSCI.1821-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Chiang A-S, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al. 2003. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol 13: 286–296. 10.1016/S0960-9822(03)00064-2 [DOI] [PubMed] [Google Scholar]
- Dzitoyeva S, Dimitrijevic N, Manev H. 2003. γ-Aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila : adult RNA interference and pharmacological evidence. Proc Natl Acad Sci 100: 5485–5490. 10.1073/pnas.0830111100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel GL, Marella S, Kaun KR, Wu J, Adhikari P, Kong EC, Wolf FW. 2016. Sir2/Sirt1 links acute inebriation to presynaptic changes and the development of alcohol tolerance, preference, and reward. J Neurosci 36: 5241–5251. 10.1523/JNEUROSCI.0499-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. 1998. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol 56: 385–431. 10.1016/S0301-0082(98)00032-X [DOI] [PubMed] [Google Scholar]
- Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, Renthal W, Neve R, Liu X, Shao N, et al. 2013. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci 33: 16088–16098. 10.1523/JNEUROSCI.1284-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai L-H. 2005. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48: 825–838. 10.1016/j.neuron.2005.10.033 [DOI] [PubMed] [Google Scholar]
- Fujita N, Nagata Y, Nishiuchi T, Sato M, Iwami M, Kiya T. 2013. Visualization of neural activity in insect brains using a conserved immediate early gene, Hr38. Curr Biol 23: 2063–2070. 10.1016/j.cub.2013.08.051 [DOI] [PubMed] [Google Scholar]
- Geer BW, McKechnie SW, Bentley MM, Oakeshott JG, Quinn EM, Langevin ML. 1988. Induction of alcohol dehydrogenase by ethanol in Drosophila melanogaster. J Nutr 118: 398–407. 10.1093/jn/118.3.398 [DOI] [PubMed] [Google Scholar]
- Hegde AN, Smith SG. 2019. Recent developments in transcriptional and translational regulation underlying long-term synaptic plasticity and memory. Learn Mem 26: 307–317. 10.1101/lm.048769.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfill CA, Baker BM, Stevens SD, Anholt RRH, Mackay TFC. 2019. Genetics of cocaine and methamphetamine consumption and preference in Drosophila melanogaster. PLoS Genet 15: e1007834. 10.1371/journal.pgen.1007834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598. 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Ichinose T, Aso Y, Yamagata N, Abe A, Rubin GM, Tanimoto H. 2015. Reward signal in a recurrent circuit drives appetitive long-term memory formation. eLife 4: e10719. 10.7554/eLife.10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. 2007. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci 104: 8253–8256. 10.1073/pnas.0702726104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacsoh BZ, Lynch ZR, Mortimer NT, Schlenke TA. 2013. Fruit flies medicate offspring after seeing parasites. Science 339: 947–950. 10.1126/science.1229625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. 2012. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 5: 14. 10.1186/1756-6606-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno M, Hiramatsu S, Kondo S, Tanimoto H, Ichinose T. 2021. Voluntary intake of psychoactive substances is regulated by the dopamine receptor Dop1R1 in Drosophila. Sci Rep 11: 3432. 10.1038/s41598-021-82813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. 2011. A Drosophila model for alcohol reward. Nat Neurosci 14: 612–619. 10.1038/nn.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesey IW, Hansson BS. 2022. Neuroecology of alcohol preference in Drosophila. Annu Rev Entomol 67: 261–279. 10.1146/annurev-ento-070721-091828 [DOI] [PubMed] [Google Scholar]
- Kelley AE. 2004. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44: 161–179. 10.1016/j.neuron.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Knabbe J, Protzmann J, Schneider N, Berger M, Dannehl D, Wei S, Strahle C, Tegtmeier M, Jaiswal A, Zheng H, et al. 2022. Single-dose ethanol intoxication causes acute and lasting neuronal changes in the brain. Proc Natl Acad Sci 119: e2122477119. 10.1073/pnas.2122477119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW. 2010a. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res 34: 302–316. 10.1111/j.1530-0277.2009.01093.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW. 2010b. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE 5: e9954. 10.1371/journal.pone.0009954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R. 2016. The role of actin cytoskeleton in memory formation in amygdala. Front Mol Neurosci 9: 23. 10.3389/fnmol.2016.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AP, Wolf FW. 2023. Alcohol sensitivity and tolerance encoding in sleep regulatory circadian neurons in Drosophila. Addict Biol 28: e13304. 10.1111/adb.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larnerd C, Adhikari P, Valdez A, Del Toro A, Wolf FW. 2023. Rapid and chronic ethanol tolerance are composed of distinct memory-like states in Drosophila. J Neurosci 43: 2210–2220. 10.1523/JNEUROSCI.1348-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-G, Kim Y-C, Dunning JS, Han K-A. 2008. Recurring ethanol exposure induces disinhibited courtship in Drosophila. PLoS ONE 3: e1391. 10.1371/journal.pone.0001391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P-T, Lin H-W, Chang Y-H, Fu T-F, Dubnau J, Hirsh J, Lee T, Chiang A-S. 2011. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci 108: 13794–13799. 10.1073/pnas.1019483108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W-P, Chiang M-H, Chang L-Y, Lee J-Y, Tsai Y-L, Chiu T-H, Chiang H-C, Fu T-F, Wu T, Wu C-L. 2020. Mushroom body subsets encode CREB2-dependent water-reward long-term memory in Drosophila. PLoS Genet 16: e1008963. 10.1371/journal.pgen.1008963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Owald D, Chandra V, Talbot C, Huetteroth W, Waddell S. 2014. Neural correlates of water reward in thirsty Drosophila. Nat Neurosci 17: 1536–1542. 10.1038/nn.3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. 1987. The effects of repeated doses of ethanol on exploration and its habituation. Psychopharmacology (Berl) 92: 78–83. 10.1007/BF00215483 [DOI] [PubMed] [Google Scholar]
- Maiya R, Lee S, Berger KH, Kong EC, Slawson JB, Griffith LC, Takamiya K, Huganir RL, Margolis B, Heberlein U. 2012. DlgS97/SAP97, a neuronal isoform of discs large, regulates ethanol tolerance. PLoS ONE 7: e48967. 10.1371/journal.pone.0048967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies C, Tully T, Dubnau J. 2005. Deconstructing memory in Drosophila. Curr Biol 15: R700–R713. 10.1016/j.cub.2005.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CE, Rosander J, Gottfried J, Dennis E, Dus M. 2020. Dietary sugar inhibits satiation by decreasing the central processing of sweet taste. eLife 9: e54530. 10.7554/eLife.54530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C, Hirsh J. 1998. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol 8: 109–112. 10.1016/S0960-9822(98)70041-7 [DOI] [PubMed] [Google Scholar]
- McKenzie JA, McKechnie SW. 1979. A comparative study of resource utilization in natural populations of Drosophila melanogaster and D. simulans. Oecologia 40: 299–309. 10.1007/BF00345326 [DOI] [PubMed] [Google Scholar]
- McKenzie JA, Parsons PA. 1972. Alcohol tolerance: an ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia 10: 373–388. 10.1007/BF00345738 [DOI] [PubMed] [Google Scholar]
- McKenzie JA, Parsons PA. 1974. Microdifferentiation in a natural population of Drosophila melanogaster to alcohol in the environment. Genetics 77: 385–394. 10.1093/genetics/77.2.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kikuchi E, Horiuchi J, Saitoe M. 2018. Long-term memory engram cells are established by c-Fos/CREB transcriptional cycling. Cell Rep 25: 2716–2728.e3. 10.1016/j.celrep.2018.11.022 [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. 2010. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res 34: 385–395. 10.1111/j.1530-0277.2009.01103.x [DOI] [PubMed] [Google Scholar]
- Morozova TV, Ayroles JF, Jordan KW, Duncan LH, Carbone MA, Lyman RF, Stone EA, Govindaraju DR, Ellison RC, Mackay TFC, et al. 2009. Alcohol sensitivity in Drosophila: translational potential of systems genetics. Genetics 183: 733–745, 1SI–12SI. 10.1534/genetics.109.107490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. 2008. Alcohol related changes in regulation of NMDA receptor functions. Curr Neuropharmacol 6: 39–54. 10.2174/157015908783769662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. 2002. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem 78: 637–647. 10.1006/nlme.2002.4084 [DOI] [PubMed] [Google Scholar]
- Newton PM, Messing RO. 2006. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther 109: 227–237. 10.1016/j.pharmthera.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Nunez KM, Azanchi R, Kaun KR. 2018. Cue-induced ethanol seeking in Drosophila melanogaster is dose-dependent. Front Physiol 9: 438. 10.3389/fphys.2018.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhäuser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D'Angelo E, Frassoni C, Amadeo A, Tocchetti A, et al. 2006. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell 127: 213–226. 10.1016/j.cell.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Ojelade SA, Jia T, Rodan AR, Chenyang T, Kadrmas JL, Cattrell A, Ruggeri B, Charoen P, Lemaitre H, Banaschewski T, et al. 2015. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci 112: E4085–E4093. 10.1073/pnas.1417222112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao P-C, Tsai L-H. 2021. Three decades of Cdk5. J Biomed Sci 28: 79. 10.1186/s12929-021-00774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CC, Lusk R, Saba LM. 2020. Alcohol sensitivity as an endophenotype of alcohol use disorder: exploring its translational utility between rodents and humans. Brain Sci 10: 725. 10.3390/brainsci10100725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons PA. 1973. Genetics of resistance to environmental stresses in Drosophila populations. Annu Rev Genet 7: 239–265. 10.1146/annurev.ge.07.120173.001323 [DOI] [PubMed] [Google Scholar]
- Peru YdP, Ojelade SA, Penninti PS, Dove RJ, Nye MJ, Acevedo SF, Lopez A, Rodan AR, Rothenfluh A. 2013. Long-lasting, experience-dependent alcohol preference in Drosophila. Addict Biol 19: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli E, Feyder M, Ledru N, Jaques Y, Anderson E, Kaun KR. 2018. Alcohol activates scabrous-notch to influence associated memories. Neuron 100: 1209–1223.e4. 10.1016/j.neuron.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli E, Brown T, Waterman A, Ledru N, Kaun KR. 2020. Alcohol causes lasting differential transcription in Drosophila mushroom body neurons. Genetics 215: 103–116. 10.1534/genetics.120.303101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. 1997. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev 25: 192–216. 10.1016/S0165-0173(97)00021-0 [DOI] [PubMed] [Google Scholar]
- Pitman JL, Huetteroth W, Burke CJ, Krashes MJ, Lai SL, Lee T, Waddell S. 2011. A pair of inhibitory neurons are required to sustain labile memory in the Drosophila mushroom body. Curr Biol 21: 855–861. 10.1016/j.cub.2011.03.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaçais PY, Trannoy S, Friedrich AB, Tanimoto H, Preat T. 2013. Two pairs of mushroom body efferent neurons are required for appetitive long-term memory retrieval in Drosophila. Cell Rep 5: 769–780. 10.1016/j.celrep.2013.09.032 [DOI] [PubMed] [Google Scholar]
- Ranson DC, Ayoub SS, Corcoran O, Casalotti SO. 2020. Pharmacological targeting of the GABAB receptor alters Drosophila’s behavioural responses to alcohol. Addict Biol 25: e12725. 10.1111/adb.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo F, Filošević A, Petrović M, Jović K, Andretić Waldowski R. 2021. Locomotor sensitization modulates voluntary self-administration of methamphetamine in Drosophila melanogaster. Addict Biol 26: e12963. 10.1111/adb.12963 [DOI] [PubMed] [Google Scholar]
- Rodan AR, Kiger JA, Heberlein U. 2002. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci 22: 9490–9501. 10.1523/JNEUROSCI.22-21-09490.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Cowan CW. 2013. Emerging roles of actin cytoskeleton regulating enzymes in drug addiction: actin or reactin’? Curr Opin Neurobiol 23: 507–512. 10.1016/j.conb.2013.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaplen KM, Kaun KR. 2016. Reward from bugs to bipeds: a comparative approach to understanding how reward circuits function. J Neurogenet 30: 133–148. 10.1080/01677063.2016.1180385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaplen KM, Talay M, Nunez KM, Salamon S, Waterman AG, Gang S, Song SL, Barnea G, Kaun KR. 2020. Circuits that encode and guide alcohol-associated preference. eLife 9: e48730. 10.7554/eLife.48730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Ruppert M, Hendrich O, Giang T, Ogueta M, Hampel S, Vollbach M, Büschges A, Scholz H. 2012. Neuronal basis of innate olfactory attraction to ethanol in Drosophila. PLoS ONE 7: e52007. 10.1371/journal.pone.0052007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Ramond J, Singh CM, Heberlein U. 2000. Functional ethanol tolerance in Drosophila. Neuron 28: 261–271. 10.1016/S0896-6273(00)00101-X [DOI] [PubMed] [Google Scholar]
- Schuckit MA. 1994. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151: 184–189. 10.1176/ajp.151.2.184 [DOI] [PubMed] [Google Scholar]
- Schuckit MA. 2000. Genetics of the risk for alcoholism. Am J Addict 9: 103–112. 10.1080/10550490050173172 [DOI] [PubMed] [Google Scholar]
- Shohat-Ophir G, Kaun KR, Azanchi R, Heberlein U. 2012. Sexual deprivation increases ethanol intake in Drosophila. Science 335: 1351–1355. 10.1126/science.1215932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinakevitch I, Strausfeld NJ. 2006. Comparison of octopamine-like immunoreactivity in the brains of the fruit fly and blow fly. J Comp Neurol 494: 460–475. 10.1002/cne.20799 [DOI] [PubMed] [Google Scholar]
- Smith-Trunova S, Prithviraj R, Spurrier J, Kuzina I, Gu Q, Giniger E. 2015. Cdk5 regulates developmental remodeling of mushroom body neurons in Drosophila. Dev Dyn 244: 1550–1563. 10.1002/dvdy.24350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breugel F, Huda A, Dickinson MH. 2018. Distinct activity-gated pathways mediate attraction and aversion to CO2 in Drosophila. Nature 564: 420–424. 10.1038/s41586-018-0732-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. 1984. Conditioning factors in opiate addiction and relapse. J Subst Abuse Treat 1: 279–285. 10.1016/0740-5472(84)90008-4 [DOI] [PubMed] [Google Scholar]
- Wilson A, Periandri EM, Sievers M, Petruccelli E. 2023. Drosophila Stat92E signaling following pre-exposure to ethanol. Neurosci Insights 18: 26331055221146755. 10.1177/26331055221146755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT-Y, Heberlein U. 2002. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci 22: 11035–11044. 10.1523/JNEUROSCI.22-24-11035.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chan T, Shah V, Zhang S, Pletcher SD, Roman G. 2012. The propensity for consuming ethanol in Drosophila requires rutabaga adenylyl cyclase expression within mushroom body neurons. Genes Brain Behav 11: 727–739. 10.1111/j.1601-183X.2012.00810.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, He J, Kaiser A, Gräber N, Schläger L, Ritze Y, Scholz H. 2016. A single pair of serotonergic neurons counteracts serotonergic inhibition of ethanol attraction in Drosophila. PLoS ONE 11: e0167518. 10.1371/journal.pone.0167518 [DOI] [PMC free article] [PubMed] [Google Scholar]