Keywords: brain hyperoxia, central vasodilation, hyperthermia, hypothermia, peripheral vasoconstriction
Abstract
Fentanyl is the leading contributor to drug overdose deaths in the United States. Its potency, rapid onset of action, and lack of effective reversal treatment make the drug much more lethal than other opioids. Although it is understood that fentanyl is dangerous at higher doses, the literature surrounding fentanyl’s physiological effects remains contradictory at lower doses. To explore this discrepancy, we designed a study incorporating electrochemical assessment of oxygen in the brain (nucleus accumbens) and subcutaneous space, multisite thermorecording (brain, skin, muscle), and locomotor activity at varying doses of fentanyl (1.0, 3.0, 10, 30, and 90 µg/kg) in rats. In the nucleus accumbens, lower doses of fentanyl (3.0 and 10 µg/kg) led to an increase in oxygen levels while higher doses (30 and 90 µg/kg) led to a biphasic pattern, with an initial dose-dependent decrease followed by an increase. In the subcutaneous space, oxygen decreases started to appear at relatively lower doses (>3 µg/kg), had shorter onset latencies, and were stronger and prolonged. In the temperature experiment, lower doses of fentanyl (1.0, 3.0, and 10 µg/kg) led to an increase in brain, skin, and muscle temperatures, while higher doses (30 and 90 µg/kg) resulted in a dose-dependent biphasic temperature change, with an increase followed by a prolonged decrease. We also compared oxygen and temperature responses induced by fentanyl over six consecutive days and found no evidence of tolerance in both parameters. In conclusion, we report that fentanyl’s effects are highly dose-dependent, drawing attention to the importance of better characterization to adequately respond in emergent cases of illicit fentanyl misuse.
NEW & NOTEWORTHY By using electrochemical oxygen sensors in freely moving rats, we show that intravenous fentanyl induces opposite changes in brain oxygen at varying doses, increasing at lower doses (<10 µg/kg) and inducing a biphasic response, decrease followed by increase, at higher doses (>10–90 µg/kg). In contrast, fentanyl-induced dose-dependent oxygen decreases in the subcutaneous space. We consider the mechanisms underlying distinct oxygen responses in the brain and periphery and discuss naloxone’s role in alleviating fentanyl-induced brain hypoxia.
INTRODUCTION
Fentanyl is a major contributor to death in the current opioid epidemic (1–4). Three factors: high drug potency, rapidity of its action, and low efficiency of naloxone as an antidote, make the drug especially dangerous compared to other weaker opioids like morphine and heroin. While fentanyl is widely used clinically to induce analgesia and anesthesia, it may lead to dangerous physiological effects in an uncontrolled environment, inducing deep sedation, bradycardia, muscle rigidity, and respiratory depression (2, 5, 6). At high doses, respiratory depression appears to be the primary factor leading to brain hypoxia, coma, and death (7–9).
Clinical and preclinical studies have investigated the effects of fentanyl at variable doses and through different routes of administration in both humans and experimental animals. However, much of these data remain either contradictory or nebulous. For example, values of fentanyl toxicity vary within an unusually wide range. While human doses used for general anesthesia are within 0.5 to 10 μg/kg with bolus intravenous injection, life-threatening respiratory complications that require immediate medical attention may also occur within this dose range. Additionally, the estimated human lethal dose is ∼2 mg or ∼29 μg/kg (7). Estimates of the lethal dose 50 (LD50) of intravenous fentanyl in rats vary from 1 to 3 mg/kg (fentanyl safety lists from Advanz Pharma; Covetrus North America) and 2.91 mg/kg (10) to >90 μg/kg (7) and 25 μg/kg (11, 12). However, some of these estimates were obtained in anesthetized rats, which represents a different factor that may affect the response to differing doses of fentanyl. Since the physiological effects of drugs depend on the dose and route of drug delivery, the issue of the dose dependency of fentanyl’s physiological and behavioral effects of intravenous fentanyl requires careful investigation in freely moving rats. This was the primary goal of this study, in which we used oxygen sensors coupled with high-speed amperometry to examine fentanyl-induced changes in oxygen dynamics in the brain and periphery.
Pharmacokinetic data obtained in human patients and rats reveal that intravenous-delivered fentanyl rapidly enters the brain and peaks in concentration within the first few minutes and then is followed by a similarly rapid descent toward the baseline (13). While the effects of intravenous fentanyl in humans are exceptionally rapid, plethysmography data assessing respiratory depression by monitoring breathing activity show slower dynamics, with delayed peaks and more prolonged duration (7, 14–17). This slow dynamic differs from data obtained with electrochemical measurements of brain oxygen levels, which provide second-scale time resolution (18, 19). In this case, brain oxygen levels drop very rapidly, reach the nadir within several minutes, and show a posthypoxic oxygen increase. While our previous data were obtained with one moderate dose of fentanyl (20 or 40 μg/kg), it is unknown how oxygen levels will change at different doses within the range of no effect, nonfatal and fatal overdose, and what mechanisms underlie these changes. This again underscores the need for dose-response studies assessing oxygen dynamics in the brain and periphery under physiologically relevant conditions.
Repeated exposure to fentanyl or “drug experience” is another factor that may determine changes in brain oxygen induced by fentanyl. Tolerance, i.e., the weakening of the physiological effects resulting from repeated drug dosing, is a known feature of opioid drugs and is often mentioned with respect to fentanyl-induced respiratory depression (14, 20). To verify tolerance of fentanyl-induced oxygen responses at different doses, they were examined following repeated treatment sessions.
Our electrochemical recordings were supplemented by monitoring of brain and body temperatures and locomotor activity. While it is often believed that sedative drugs have hypothermic effects, intravenous heroin at relatively low doses induces powerful sedation but increases brain and body temperature through retention of body heat via peripheral vasoconstriction (21,22). Robust increases in brain temperature, within 38–39°C, also occur during heroin self-administration maintained by low drug doses (23). As it is still unknown whether this holds true for fentanyl along its wide range of doses, we used multisite temperature recordings with determination of temperature differentials between the brain, temporal muscle, and subcutaneous space to assess the effects of the drug on metabolic brain activity and the tone of peripheral blood vessels (24, 25). Since oxygen fluctuations and temperature parameters are both clinically relevant and potentially interrelated, we also assessed their relationships.
MATERIALS AND METHODS
Subjects
Fifteen adult male Long-Evans rats (Charles River Laboratories) weighing 450–520 g (mean: 482 ± 7 g) at the time of surgery were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12–12 light-dark cycle (7 AM light on) with food and water available ad libitum. All procedures were approved by the National Institute on Drug Abuse–Intramural Research Program Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH Publication 865-23) and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Maximal care was taken to minimize the number of experimental animals and any possible discomfort or suffering at all stages of the study.
Overview of the Study
This study describes the results of two experiments conducted in freely moving rats. In the first experiment, we examined changes in oxygen levels in the nucleus accumbens (NAc) and subcutaneous space induced by fentanyl at different doses. In the second experiment, we used the same treatment protocol but examined drug-induced temperature changes in the NAc and two peripheral locations.
Surgical Preparations
Surgical procedures for the electrochemical assessment of oxygen have been described in detail in a prior study (22). Under general anesthesia (80 mg/kg ketamine HCl and 8 mg/kg xylazine HCl, respectively, with additional boosting at 30% of initial doses as necessary), rats were chronically implanted with Pt-Ir oxygen sensors (model 7002-02; Pinnacle Technology, Inc., Lawrence, KS) in two locations. One sensor was implanted in the medial segment of the NAc [anterior-posterior (AP) at +1.2 mm in front of bregma, medial-lateral (ML) at ±0.8 mm, and dorsal-ventral (DV) at +7.2–7.6 mm from the skull surface], according to coordinates from the rat brain atlas (26), while the other sensor was implanted in the subcutaneous space in the frontal area of the rat’s head. This area is densely vascularized, and the sensor implanted in this area does not move during behavioral activation, allowing it to provide artifact-free electrochemical recording. Both sensors were secured with dental acrylic to three stainless steel screws threaded into the skull. During the same surgery, rats were implanted with a chronic jugular catheter, which ran subcutaneously to the head mount. Rats were allowed a minimum of 5 days of postoperative recovery and at least three daily habituation sessions (∼6 h each) in the recording environment. Jugular catheters were flushed daily with 0.2 mL heparinized saline to maintain patency.
Surgical procedures for thermorecording experiments have also been described in detail in a prior study (27). Each rat was implanted with three copper-constantan thermocouple electrodes in the NAc shell, temporal muscle, and subcutaneously along the nasal ridge with the tip ∼15 mm anterior to bregma. Target coordinates of the recordings in the right NAc shell were AP at +1.2 mm in front of bregma, ML at ±0.8 mm, and DV at +7.6 mm from the skull surface, according to coordinates of the rat brain atlas (26). The probes were secured with dental acrylic to the three stainless steel screws threaded into the skull. Postoperative procedures were identical to those in the first experiment.
Electrochemical Detection of Oxygen
For in vivo oxygen detection, we used Pinnacle oxygen sensors coupled with high-speed amperometry. The principles of electrochemical oxygen detection, construction of oxygen sensors, and their calibration have been described in detail in prior studies (18, 28).
Experimental Procedures
This article describes the results of two experiments conducted in two groups of rats, which underwent the same drug treatments during repeated daily sessions. At the onset of each recording session, rats were briefly anesthetized (<2 min) with isoflurane and their electrochemical (experiment 1) or temperature (experiment 2) sensors were connected to the recording instruments via an electrically shielded flexible cable and a multichannel electrical swivel. A catheter extension mounted on the cable was used to allow stress- and cue-free drug delivery from outside the cage. After stabilization of baselines, rats received five repeated injections of fentanyl at increasing doses (1, 3, 10, 30, and 90 μg/kg) delivered with increasing postinjection time intervals (30, 40, 60, 90, and 90 min, respectively). Since fentanyl was used at different doses, it was delivered via two catheter extensions containing different drug concentrations. While the speed of fentanyl injection was about the same for each dose, injection duration differed for each dose from 0.1 to 0.9 mL. Upon completion of treatments, rats were lightly anesthetized by isoflurane and disconnected from the recording instruments. Rats were allowed to recover from anesthesia, and their jugular catheters were flushed with heparinized saline before being returned to the animal colony. The same protocols were maintained during the repeated daily sessions (n = 6). In a few sessions, we were unable to maintain this protocol due to technical issues with the recording quality of electrochemical or temperature sensors and catheter patency.
In both experiments, fentanyl (50 μg/mL fentanyl citrate injection; Hospira Inc., Lake Forest, IL) was delivered via slow (from 10 to 40 s for lower and higher doses) intravenous injections. In both clinical and preclinical studies, fentanyl is used at different doses and routes of administration. Our treatment protocol covered a wide range of doses, known to induce noticeable physiological changes in both humans and rats. The 1 μg/kg dose was selected as the minimal, presumably ineffective dose, and the 90 μg/kg was selected as the maximal dose at which fentanyl induces robust twitching, extreme muscle rigidity, full tail erection, and seizure-like convulsions. This dose range extends beyond the generally accepted human lethal dose (2 mg or 29 µg/kg; Ref. 7) but remains within the range of lethal dose 50 (LD50) for intravenous administration in rats, which varies widely across different studies (0.025–2.91 mg/kg; Refs. 10, 11). The two lowest doses (1 and 3 μg/kg) are within the range used for clinical analgesia (50–100 µg/70 kg or 0.7–1.4 μg/kg) and for maintenance of self-administration behavior in rats (3 μg/kg; Ref. 29).
Histological Verification of Electrode Placements
After experiments were complete, rats were deeply anesthetized with isoflurane and decapitated, and their brains were extracted and stored in a 10% formalin solution. Then, the brains were cut and analyzed for verification of the locations of cerebral implants and possible tissue damage in the area of electrochemical recording.
Data Analysis
Electrochemical data were analyzed in a slow (1-min) and a rapid (4-s) time resolution. Because each individual sensor differed in substrate sensitivity, currents were first converted into concentrations (μM) based on sensitivity calibrations provided by the manufacturer and then into percent (%) changes in oxygen concentration. Values from 1 min before the drug injection were averaged and set as the 100% baseline for slow and rapid time-course analyses. One-way repeated measure ANOVA (followed by Fisher’s least significance difference post hoc tests) was used to evaluate the statistical significance of drug-induced oxygen responses. To assess the relationships between oxygen changes in the NAc and subcutaneous space, we used time-dependent correlation analyses, which demonstrate how the change in oxygen in one location (brain) is related to changes in oxygen in another location (periphery). For text clarity, the quantitative results of most statistical evaluations are shown in Supplemental Tables S1–S3.
Temperature data were sampled at 2-s time intervals and analyzed with 1-min time bins and presented as both absolute and relative changes with respect to the moment of drug administration. We also calculated NAc minus muscle and skin minus muscle temperature gradients that were used to determine the effects of fentanyl on brain metabolic activity and skin vessel tone. Locomotor data were assessed with 1-min time resolution. One-way repeated measure ANOVA (followed by Fisher’s least significance difference post hoc tests) was used to evaluate the statistical significance of drug-induced changes in temperature. Two-way repeated-measure ANOVA was used to analyze between-group differences in the effects of fentanyl in the brain and peripheral location. We also used time-dependent correlation analyses to determine relationships between changes in oxygen and brain-muscle and skin-muscle differentials. Quantitative results of multiple statistical evaluations are shown in Supplemental Table S4.
RESULTS
Dose Dependency of Fentanyl’s Effects on Oxygen Levels in the NAc and Subcutaneous Space
The effects of fentanyl on oxygen levels in the NAc and subcutaneous space were examined in 10 rats during 34 daily sessions. These data were analyzed at a slow, 1-min time resolution for the entire duration of postinjection time interval and with a fast, 4-s resolution for the first 10 min postinjection.
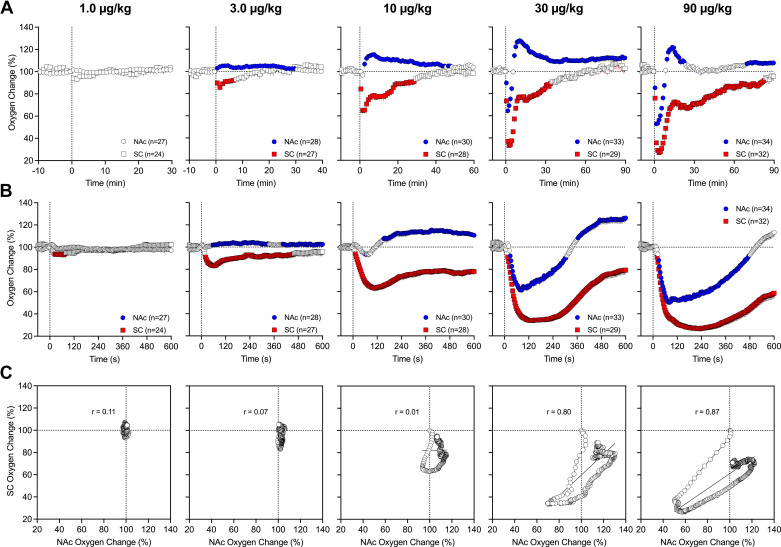
Fentanyl at a 1 μg/kg dose has no significant effects on oxygen levels in both the NAc and subcutaneous space analyzed with slow temporal resolution (Fig. 1A). At a 3 μg/kg dose, fentanyl induced opposite changes in oxygen with a significant increase in the brain and a significant decrease in the subcutaneous space. The effect in the subcutaneous space was stronger but less prolonged than in the NAc. A similar, but stronger, increase in the NAc and decrease in the subcutaneous space was seen at a 10 μg/kg dose. The effects in both locations were also more prolonged, with a ∼30-min increase in the brain and an ∼10-min decrease in the subcutaneous space. At 10 µg/kg, a transient and weak postinjection brain oxygen decrease was seen in the brain, resulting in a longer latency to subsequent larger and more prolonged increase. When the fentanyl dose was tripled (30 μg/kg), the effect in the brain was biphasic, with a strong but transient decrease (nadir at 60% of baseline and duration ∼6 min) followed by a strong and more prolonged increase (peak at 125% of baseline). In contrast, the effect in the subcutaneous space was monophasic, stronger, and more prolonged than in the brain (nadir at ∼30% and duration at ∼50 min). In this case, the decrease in the subcutaneous space was more rapid (onset at ∼16 s) than in the brain, reaching significance during the first minute after injection. The effect of fentanyl at the largest, 90 μg/kg dose was like that at the 30 μg/kg dose, but the decreases in both locations were stronger and more prolonged (NAc nadir at 50%, duration ∼8 min; subcutaneous space nadir at ∼25%, and duration at ∼85 min). Similarly, the effect was biphasic in the brain and monophasic in the subcutaneous space. Oxygen changes in both locations had a relatively low variability and small standard errors, which were mostly within the size of symbols shown in Fig. 1.
Figure 1.
Mean ± SE changes in oxygen levels in the nucleus accumbens (NAc) and subcutaneous (SC) space induced by intravenous fentanyl: dose dependence. A: slow (1-min bins) changes in oxygen levels calculated in percent vs. the preinjection baseline (100%). B: rapid (4-s bins) changes vs. the preinjection baseline (100%). Filled symbols show values significantly different from the preinjection baseline; n, number of averaged oxygen responses. C: correlative relationships in oxygen dynamics between the 2 recording locations. Each graph depicts a regression line and a coefficient of correlation between NAc and subcutaneous space oxygen change. Vertical dashed lines in A and B show moments of drug injections.
A more precise picture of fentanyl-induced oxygen fluctuations was obtained when the data were analyzed with high temporal resolution (Fig. 1B). In this case, a weak decrease in skin oxygen levels became significant at a 1 mg/kg dose. This analysis revealed that oxygen decreases in the subcutaneous space were more rapid than changes in the brain, being significant at 12, 16, and 20 s from the injection onset for 10, 30, and 90 μg/kg doses, respectively. Decreases in brain oxygen occurred with longer latencies, reaching significance at 64, 28, and 28 s after the injection onset. Oxygen decreases in the subcutaneous space induced by the largest dose of fentanyl were larger in both amplitude (mean: 28%, SD: 12.4%, and range: 9%–58%) and duration (mean: 77.3 min, SD: 22.7 min, and range: 12–90 min) than similar changes seen in the NAc (amplitude: mean: 53%, SD: 8.0%, and range: 34–74%; duration: 10 min, SD: 4–41 min, and range: 5–23 min).
To examine the relationship between oxygen changes in the brain and periphery, we used time-dependent correlation analyses, which show how changes in one parameter relate to changes in a second parameter (Fig. 1C). This analysis confirmed no effect of fentanyl in both the brain and periphery at the lowest dose and a weak decrease in the subcutaneous space at a 3 μg/kg dose. At 10 μg/kg, fentanyl induced a different pattern of changes in both locations. Initially, oxygen rapidly and powerfully decreased in the subcutaneous space but only transiently decreased in the NAc. Then, subcutaneous space oxygen began to increase from its nadir and NAc oxygen tonically increased. In this case, the decrease is more rapid than in the brain. At 90 μg/kg, a rapid, strong, and correlative decrease was seen in both locations. Then, from 90 s in the brain and 210 s in subcutaneous space, concentration curves began to increase toward the preinjection baseline. While the decrease was ∼8 min in the brain, it continued for almost 90 min in the subcutaneous space.
Behaviorally, the effects of fentanyl were characterized by hypoactivity and muscle rigidity (visualized as stiff flexion of the limb and tail erection) evident at 10-, 30-, and 90-μg doses. While not quantified in this experiment, hypoactivity, muscle rigidity, and decreases in spontaneous respiration were stronger with higher fentanyl doses. Rats injected with the 90 μg/kg dose also occasionally presented with convulsive movements that mimicked tonic-clonic seizures within the first minute following injection onset.
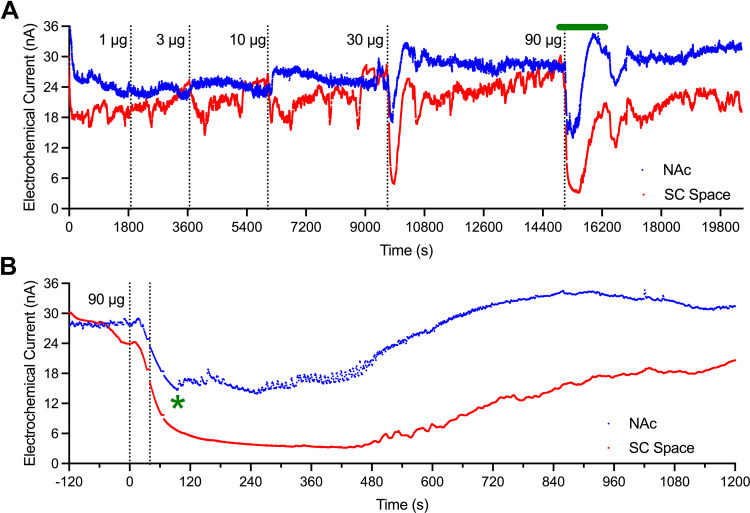
Figure 2 depicts a representative 1-day session of oxygen monitoring during repeated fentanyl injections. Electrochemical currents were recorded with 1-s time resolution during entire session (Fig. 2A), and currents induced by 90 μg/kg fentanyl are better visualized in the enlarged time frame (Fig. 2B). Oxygen levels in the subcutaneous space began to decrease from ∼15 s after the onset of a 40-s injection until the cessation of apnea and appearance of breathing at ∼90 s (green asterisk). Oxygen levels in the NAc began to decrease from ∼20 s postinjection and reached the lowest point before rebounding when breathing resumed. The initial breathing was rare as shown in fluctuations of NAc electrochemical signal. At ∼8–10 min from the injection, fluctuations disappeared suggesting a return to more frequent and deep breathing activity. At this time, brain oxygen levels were near to baseline or began to increase. While changes in oxygen level in the subcutaneous space were larger and more prolonged than in the brain, breathing-related fluctuations were absent in this location. Another original example of fentanyl-induced changes in reduction current is shown in Supplemental Fig. S1.
Figure 2.
Representative single-session record of changes in reduction currents recorded from the nucleus accumbens (NAc; blue) and subcutaneous (SC) space (red) with repeated injections of fentanyl at different doses (A). Original data (1-s bins) were transported from the recording software into the Prism program, then inverted. The bolded green line above A represents the time interval that is magnified and shown in B. Vertical dashed lines show the timing of drug injections. Green asterisk in B shows the time of the first breath after apnea. Subsequent fluctuations in NAc current were associated with individual breaths. These fluctuations were absent in currents recorded from the subcutaneous space.
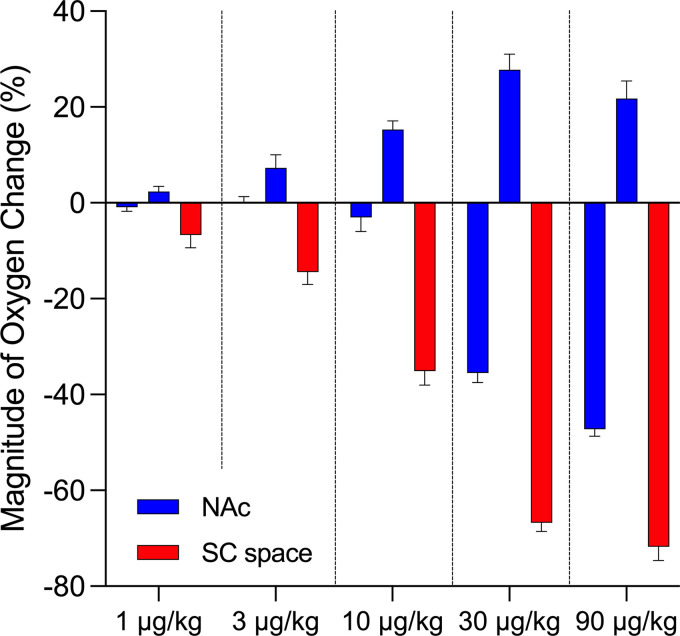
The amplitudes of oxygen decrease and increase in both NAc and subcutaneous space elicited by fentanyl at different doses are shown schematically in Fig. 3. In the NAc, fentanyl induces weak oxygen increases at low doses (1 and 3 µg/kg), but biphasic responses, a postinjection decrease followed by a weaker, more-prolonged increase, at higher doses (10, 30, and 90 µg/kg). Among the biphasic responses, the postinjection oxygen decrease occurred at the smallest amplitude at 10 μg/kg and at exponentially larger amplitudes at higher doses. At the highest dose, an oxygen drop in NAc occurred at ∼50% of baseline (mean: 53%; range: 34%–74%). In contrast, fentanyl-induced monophasic oxygen decreases in the subcutaneous space that increased in amplitude with increases in drug doses. An oxygen decrease in the subcutaneous space was seen to a minimal extent at 1 μg/kg but became significant at 3 μg/kg. Notably, the robust decrease in subcutaneous space oxygen fell to ∼30% of baseline (mean: 28%; range: 9%–58%) after injection of 90 µg/kg fentanyl.
Figure 3.
Fentanyl-induced changes in oxygen responses in the brain and peripheral tissue: dose dependance. Bar graphs show mean ± SE changes in amplitude of oxygen responses induced by fentanyl at 1, 3, 10, 30, and 90 μg/kg doses. Red graphs show changes in subcutaneous (SC) space and blue graphs show mean amplitude for both the initial decrease and subsequent increase in nucleus accumbens (NAc). Mean amplitudes of oxygen decreases and increases were calculated as an average of maximal increases and decreases in individual tests. Vertical dashed lines separate effects at different doses.
Dependency of Fentanyl’s Effects on Oxygen Levels in the NAc and subcutaneous Space Depending on the Age and Weight of Rats
Although the rats used in this study were similar in age (mean: 134 days; range: 113–157 days) and weight (mean: 484 g; range: 445–525 g), we examined relationships between brain oxygen decrease and both the age and weight of rats. Analysis with data from the 90 µg/kg fentanyl group, which induced the strongest changes in brain oxygen levels, showed that oxygen responses assessed by their maximal amplitude are independent of both age (n = 32; r = −0.27) and weight (n = 32; r = −0.21).
Dose Dependency of Fentanyl’s Effects on Temperature Parameters and Locomotor Activity
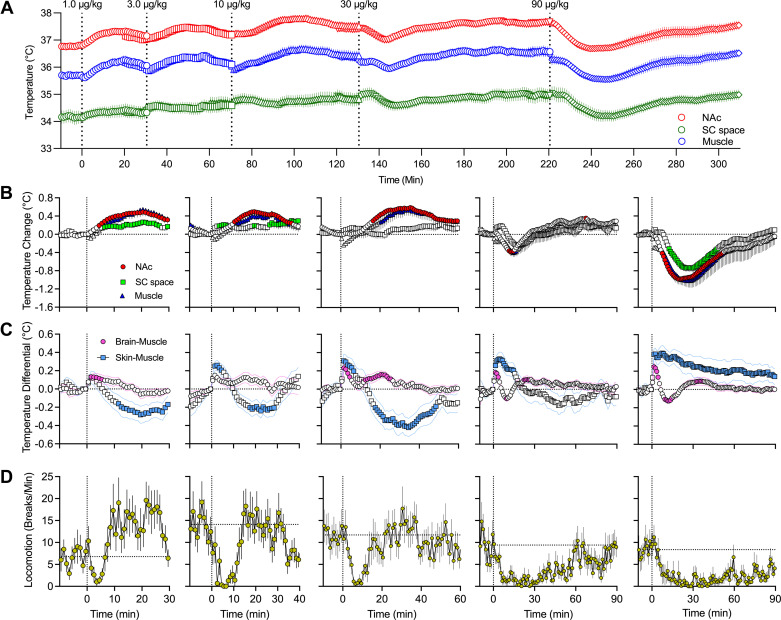
Fentanyl-induced temperature changes in the NAc, temporal muscle, and subcutaneous space were examined in a separate group of rats (n = 5; 31 daily sessions) using a treatment protocol identical to that in our oxygen experiment. We analyzed absolute temperature changes in each recording location within the entire session (Fig. 4A), relative changes for each drug dose (Fig. 4B), and changes in brain-muscle and skin-muscle temperature differentials (Fig. 4C). In this experiment, we also assessed the behavioral effects of fentanyl using conventional recording of locomotor activity (Fig. 4D).
Figure 4.
Mean ± SE changes of different temperature parameters induced by intravenous fentanyl: dose dependence. A: slow absolute changes in temperature in the nucleus accumbens (NAc), temporal muscle, and subcutaneous (SC) space. B: relative temperature responses for each dose of fentanyl. C: changes in NAc-muscle and subcutaneous space-muscle temperature differentials. D: changes in locomotion for each fentanyl dose. Filled symbols in B and C show values significantly different from preinjection baseline. Vertical dashed lines in all graphs show the timings of fentanyl injections. Horizonal dashed lines in A–C show basal levels (level of no change = 0°C) and in D show mean basal locomotion for 10 min before fentanyl injections. Locomotor activity was measured using four photobeams located at the chamber’s walls and quantified in photobeam breaks per minute.
Consistent with our previous studies (23, 25), basal temperature values differed in each location, with highest values in the NAc (36.81 ± 0.10°C), lower values in temporal muscle (35.70 ± 0.12°C), and lowest values in the subcutaneous space (34.15 ± 0.23°C). As shown in Fig. 4, fentanyl induced significant changes in temperatures evident in most locations and at most doses. At the lowest 1 μg/kg dose, temperature in all locations slightly increased, with a transient increase in brain-muscle differential and delayed decrease in skin-muscle differential. Delayed temperature increases were also evident at larger doses (3 and 10 µg/kg), but the effect becomes biphasic (rapid increase followed by decrease) at a 30 μg/kg dose, with only strong and prolonged brain temperature decrease at the maximal tested dose (90 µg/kg). A transient increase in brain-muscle differential was evident in each fentanyl dose (∼7–8 min), but changes in skin-muscle differential were more complex. With a modest dose (3 or 10 µg/kg), skin-muscle differential phasically increased but then tonically decreased below baseline. At a larger dose (30 µg/kg), we observe only a transient increase, which becomes strong and very prolonged at the largest dose (90 µg/kg). In addition to the more phasic changes following fentanyl injections, the basal temperature before each injection gradually and significantly increased during the session. This tonic increase was similar in amplitude (0.77–0.85°C) at each location.
Fentanyl at each dose decreased locomotor activity; hypoactivity was dose dependent, with a transient decrease at the lowest dose and progressively stronger and more prolonged decreases at higher doses (Fig. 4D).
Relationships between Fentanyl-Induced Changes in Temperature and Brain Oxygen
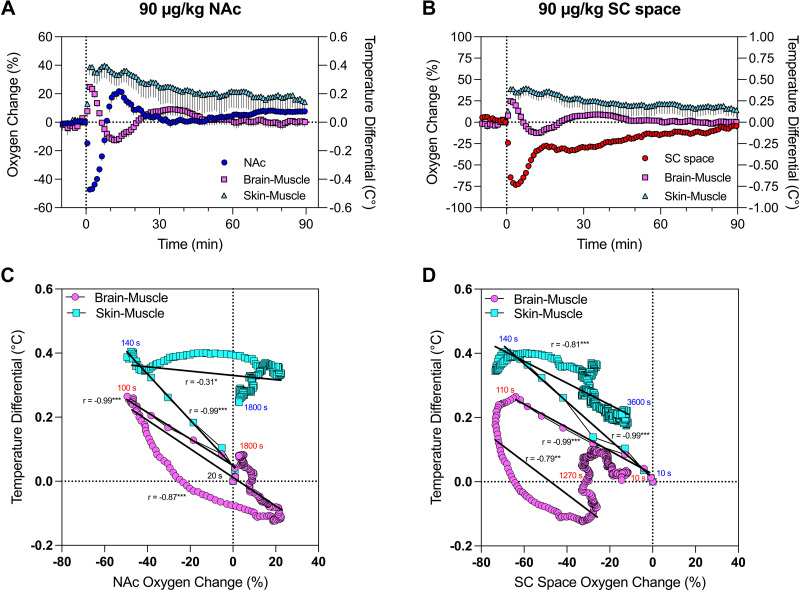
Next, we examined whether and how fentanyl-induced changes in brain and peripheral oxygen levels relate to the two temperature differentials, which reflect changes in metabolic brain activity (brain-muscle) and peripheral vascular tone (skin-muscle) (24, 25, 30). This analysis was conducted only for the maximal 90 μg/kg fentanyl dose. In this case, data were shown with high temporal resolution (10 s) for better representation of their phasic changes.
As shown in Fig. 5, A and C, brain oxygen change was tightly negatively correlated with brain-muscle differentials during the descending phase of oxygen response (r = −0.99; P < 0.001). After ∼20 s from the injection onset, both parameters changed rapidly, reached the lowest point at ∼100 s, and slowly returned toward baseline, showing a much weaker nonlinear correlation (r = 0.31; P < 0.05). Brain oxygen and skin-muscle differential also showed equally rapid but opposite changes (r = −0.99; P < 0.001), peaking at ∼140 s after injection onset. Then, both parameters slowly returned to baseline, showing a weaker and less linear correlation (r = 0.87; P < 0.001). While the hypoxic phase of brain oxygen response was transient, the fentanyl-induced change in skin-muscle differential was robust and prolonged, reaching preinjection levels at ∼1,800 s.
Figure 5.
Correlative relationships between fentanyl-induced changes in brain and peripheral oxygen and temperature parameters. A and B: mean ± SE oxygen changes in nucleus accumbens (NAc; A) and subcutaneous (SC) space (B) assessed in relation to the 2 temperature differentials induced by intravenous fentanyl at a 90 μg/kg dose. C and D: correlative relationships between changes in NAc oxygen (C) and subcutaneous space (D) and the 2 temperature differentials. Each curve has coefficient of correlation (r) and regression lines (*P < 0.05; **P < 0.01; ***P < 0.001). Note that data shown in A and C have different y-axis scales.
The relationship between oxygen changes in the subcutaneous space and temperature differentials had both similarities and differences from those found for changes in NAc oxygen (Fig. 5, B and D). Here, skin oxygen decreases were strongly negatively correlated with increases in the brain-muscle differential (r = −0.99; P < 0.001) from ∼20 s to 110 s, when skin oxygen decrease reached its minimal values. Then, both parameters slowly correlatively returned toward baseline (r = −0.79; P < 0.001). A similarly strong linear correlation was evident between skin oxygen decrease and skin-muscle differential (r = −0.99; P < 0.001) during the time interval from 20 s to 140 s when the decrease in subcutaneous oxygen reached its lowest point. Then, the direction of changes in both parameters was inverted (110–120 s) and slowly descended toward baseline but did not reach it at 60 min postinjection.
Fentanyl’s Effects on NAc Oxygen Levels: Drug Experience
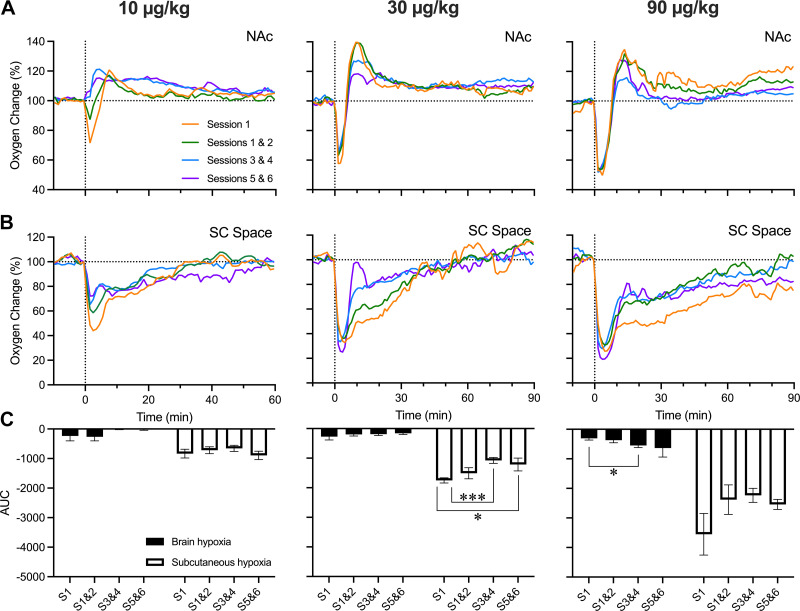
Rats in our experiments received the same fentanyl treatment during six repeated sessions. Although we obtained mean oxygen responses over all sessions, changes may have been affected by tolerance. To clarify this issue, we sorted data into four groups based on the session number (1, 1–2, 3–4, and 5–6; i.e., 1 represents the first day of recording) and examined whether fentanyl-induced oxygen response depends on prior exposure. This analysis was conducted for 10, 30, and 90 μg/kg doses, and the areas under the curve for oxygen decreases were compared for brain and subcutaneous oxygen responses (Fig. 6).
Figure 6.
Changes in oxygen responses in the nucleus accumbens (NAc; A) and subcutaneous (SC) space (B) across grouped daily sessions. Data are shown for 3 doses of fentanyl: 10, 30, and 90 μg/kg. C: mean changes in fentanyl-induced oxygen decreases (area under the curve) in the brain and subcutaneous space for 3 doses of fentanyl. Vertical dashed lines in all graphs show the timings of fentanyl injections and horizonal dashed lines show basal levels (level of no change = 0°C). AUC, area under the curve; S, session. *P < 0.05; ***P < 0.001, statistical significance of between-session oxygen responses.
As shown in Fig. 6, the patterns of oxygen responses elicited by fentanyl did not differ significantly across treatment sessions. Although not significant, decreases in NAc oxygen induced by fentanyl at a 10 μg/kg dose were seen during the first two treatment sessions but disappeared on later days (Fig. 6A). No changes in subcutaneous oxygen responses were found in different treatment days, but the initial decrease was the largest in magnitude in the first treatment session across all three doses (Fig. 6B). Experience-dependent changes in oxygen responses in the NAc were not found with larger drug doses, but the biphasic pattern was evident in each treatment day, and the response durations were similar (Fig. 6A). However, the sample sizes for each treatment day were much lower (n = 4–6) than for the whole sample, resulting in greater variability and weaker probability of revealing possible between-session differences.
An area under the curve analysis was performed to quantify the degree of hypoxia caused by fentanyl injection at different doses (Fig. 6C). At the lowest dose, fentanyl induced minimal decreases in brain oxygen, which disappeared in subsequent treatment days. Changes in oxygen in the subcutaneous space were stronger but similar in each treatment day. At a moderate dose, oxygen decreases became weaker, with a significant effect in the subcutaneous space. Student’s t tests were then conducted to test for significance between sessions within each fentanyl dose. Decreases in oxygen levels in the subcutaneous space induced by fentanyl at the largest dose also became weaker with subsequent treatment days, but brain oxygen decreases showed the opposite effect, becoming stronger on repeated treatment days.
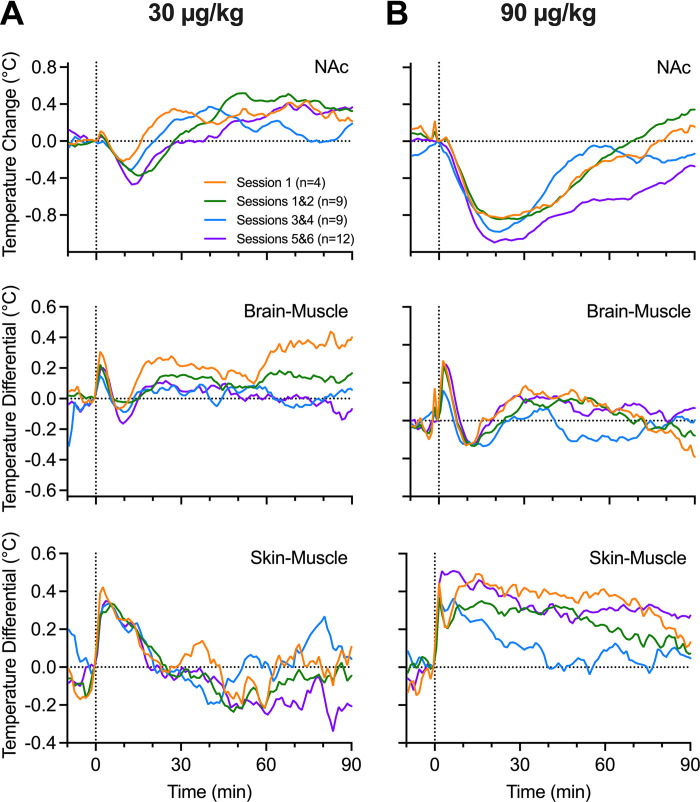
Fentanyl’s Effects on Temperature Parameters: Drug Experience
The potential for tolerance of fentanyl’s temperature effects was also evaluated in a similar manner (Fig. 7). In this case, all analyzed temperature parameters show similar changes in both the brain and periphery with both 30 and 90 μg/kg doses. As shown in Fig. 7, oxygen curves for NAc temperature and both temperature differentials showed similar changes without any significant between-group differences.
Figure 7.
Mean changes in nucleus accumbens (NAc) oxygen, brain-muscle differentials, and skin-muscle differentials across grouped treatment sessions for fentanyl at 30 μg/kg (A) and 90 μg/kg (B). Vertical dashed lines in all graphs show the timing of fentanyl injections and horizonal dashed lines show basal levels (level of no change = 0°C). S, session.
DISCUSSION
Our study revealed that intravenous fentanyl has distinct, dose-dependent effects on brain oxygenation, modestly increasing oxygen levels at lower doses (1.0–10 μg/kg) and inducing biphasic, decrease followed by increase, effects at higher doses (>10 μg/kg). This biphasic pattern contrasted monophasic dose-dependent oxygen decreases in the subcutaneous space, which were stronger than decreases seen in the brain and appeared with shorter onset latencies (10–20 s). Therefore, the hypoxic effects of fentanyl in the brain occur at doses that are higher than those for maintaining self-administration (1.25–5.0 μg/kg; Ref. 29) and decreasing oxygen levels in the peripheral locations (1–3 μg/kg). Short onset latencies, rapid changes, and brief durations of fentanyl-induced brain hypoxia also agree with pharmacokinetic data obtained in humans and rats. Fentanyl concentration in arterial blood is known to peak at ∼15 s after intravenous bolus injection at a clinically relevant dose (100 μg/70 kg) and then rapidly drops to 10% of the peak at ∼180 s (13). Fentanyl levels in brain tissue increased at a slower rate, with ∼35 s to reach 50% of peak brain concentration and 360 s to reach a peak. Similarly, rapid pharmacokinetics of intravenous fentanyl have been shown in rats (31).
Potential Mechanisms Underlying the Effects of Fentanyl on Brain Oxygenation
Bidirectional pattern of fentanyl-induced brain oxygen responses in this study has several important differences from the results of most plethysmography studies, as they report monophasic and prolonged inhibitory effects of fentanyl on respiratory activity (7, 17, 32, 33). Although cessation or inhibition of respiration is the primary factor determining oxygen decreases in the brain and peripheral tissues, oxygen levels in the brain’s extracellular space also depend on metabolic activity and the tone of cerebral vessels, which affect cerebral blood flow and oxygen entry from arterial blood to the brain’s extracellular space. Oxygen decreases in the subcutaneous space also depend on the tone of blood vessels, which appear to be constricted by fentanyl as suggested by our thermorecording experiments. By constricting peripheral blood vessels, blood is redistributed from the peripheral domain to the central domain, with subsequent cerebral vasodilation and increased cerebral blood flow. Thus, decreasing blood flow in the periphery may allow more arterial blood to enter brain tissue (functional hyperemia), supplying more oxygen and counteracting oxygen decreases due to respiratory depression. This is an important adaptive mechanism elicited by multiple arousing stimuli and is responsible for both physiological increases in cerebral blood flow and functional brain hyperthermia (30). This mechanism may be responsible for modest increases in brain oxygen levels induced by intravenous heroin and oxycodone at low behaviorally active doses (50 μg/kg and 0.15–0.3 mg/kg; Refs. 18, 28, respectively). In contrast to heroin, which induces strong peripheral vasoconstriction and larger increases in brain temperature (21), fentanyl induced a weaker effect, evident only at low drug doses (1–10 μg/kg), preceded by rapid but transient vasodilation, and disappearing at the highest dose used in this study. Furthermore, the impact of peripheral vasoconstriction becomes weaker with increases in drug dose, and at the largest dose fentanyl induces skin vasodilation, which is responsible for increased heat dissipation and decreased brain and body temperature.
There is another important factor that may contribute to fentanyl-induced oxygen increases that follow rapid oxygen decreases. Since respiratory depression results in intra-cerebral accumulation of CO2, a powerful vasodilator (34–36), cerebral vasodilation and increased global cerebral blood flow may contribute to the posthypoxic hyperoxia, as another adaptive mechanism opposing oxygen decreases due to respiratory depression.
The rapid oxygen dynamics induced by intravenous fentanyl suggest the possible involvement of peripheral opioid receptors in triggering brain oxygen responses. Opioid receptors are expressed on both central and peripheral locations, including the terminals of sensory nerves that densely innervate blood vessels. Activation of these receptors by fentanyl circulating in the cardiovascular system may trigger an excitatory signal that rapidly reaches the central nervous system and induces an efferent response. We proposed this mechanism in a prior study (37), which showed that naloxone methiodide (2.0 mg/kg), which has very limited permeability via the blood-brain barrier (BBB), has similar attenuating effects on heroin-induced hypoxia with naloxone HCl (0.2 mg/kg), which easily crosses the BBB. While it is known that fentanyl easily crosses the BBB, time is needed for intravenously delivered drugs to reach its centrally located receptor sites and directly interact with them.
Despite rapidity and strength, decreases in brain oxygen induced by intravenous fentanyl are relatively brief. At the largest fentanyl dose (90 μg/kg), when oxygen levels decreased to ∼50% of preinjection baseline, the duration of decrease lasted ∼10 min postinjection. In the subcutaneous space, when the larger oxygen decrease fell to ∼25% of baseline, its duration was much longer (mean ∼23 min). Therefore, hypoxia in the periphery is much stronger and more prolonged than in the brain due to brain-specific adaptive mechanisms that oppose oxygen decreases due to drug-induced respiratory depression. Therefore, the periphery may be more susceptible to decreases in oxygen compared to the central nervous system.
We found that both oxygen and temperature responses induced by intravenous fentanyl are surprisingly similar at different treatment days with no evidence of tolerance. Tolerance is a known feature of opioid drugs (5), which is often mentioned in clinical studies with respect to fentanyl-induced respiratory depression (14). Humans who used opioid drugs chronically (38) or rats exposed to continuous fentanyl delivery via chronically implanted minipumps (17) showed weaker respiratory responses to intravenous fentanyl than in drug-naive individuals. However, repeated fentanyl injections did not show evident tolerance to respiratory effects (17). In contrast, other studies showed clear tolerance of respiratory effects following repeated daily injections of fentanyl (14). These differences may be related to differences in monitored parameters, the small number of tests, and the dosage or timing parameters of drug treatments in our study.
Although our current recordings were conducted in the NAc as a representative brain structure, we previously found that the pattern of oxygen responses induced by heroin in the NAc and amygdala was quite similar, suggesting that drug-induced oxygen responses tend to be more global and largely independent of structure (39). Minor structural differences were also found with respect to changes in brain glucose (40) and brain temperature (24, 25). This may be true for hypoxic responses, which depend primarily on vascular density, which appears to be similar in different grey structures. While not proven experimentally, fentanyl-induced posthypoxic oxygen increases could be also similar since they appear to result primarily from accumulation of CO2 and other vasodilators. This issue is complex and clearly requires further investigation.
Functional Implications: Relevance in Understanding and Attenuating the Hazardous Effects of Illicit Fentanyl
Constant oxygen delivery is essential for any tissue, but the brain is exceptionally sensitive to the lack of oxygen, with brain cells starting to die within 5 min after cessation of oxygen supply (41–43). Living organisms may better tolerate robust decreases in oxygen levels if they are transient, but the harmful effects of hypoxia progressively increase as it becomes more prolonged (44, 45). Despite strong rigidity, appearance of convulsions, and robust hypoxia, the rats in our study did not perish after fentanyl administration at a 90 μg/kg dose (n = 34), and most rats showed unchanged responses following repeated daily tests. Thus this dose with intravenous delivery did not appear to be lethal in awake rats. While objective data collected from human drug users are limited, fentanyl at much lower doses may induce serious health complications, including death.
However, fentanyl use by drug users differs significantly from well-controlled studies conducted in animals. In cases of drug abuse, the dose of injected fentanyl remains unknown and may often be large, producing strong, toxic effects. Based on measurements of peak concentration of fentanyl in blood after its intravenous delivery (46) and autopsy data in overdosed individuals, the possible injected dose at the time of death was within 10–100 µg/kg (31, 47); fentanyl at this dose is highly toxic, and its intravenous delivery may result in death. Despite evident differences between rats and humans, recent studies with modeling of medium and high fentanyl overdose (1.63 mg and 2.97 mg or 23 and 43 µg/kg with intravenous bolus administration, respectively) revealed very rapid decreases in oxygen partial pressure, with a maximal drop at 2–3 min postinjection (48, 49). At a higher dose, the maximal drop in ventilation occurred in less than 1 min postinjection, while the maximal increase in carbon dioxide occurred ∼5 min postinjection. Interestingly, the change in blood flow to the brain was biphasic, initially increasing and peaking at 1.5 min and then decreasing to the lowest point at ∼7.5 min.
Fentanyl use by drug users also differs from rat models in the content and purity of the injected drug, which is often contaminated with an unknown amount of other neuroactive drugs, including cocaine, benzodiazepines, xylazine, or other drugs (3). Nearly half of fentanyl overdose deaths involve multiple drugs, and these drug additions could significantly increase the toxicity of fentanyl.
Recently, we showed that the addition of xylazine, an alpha2-adrenergic agonist and a veterinary tranquilizer used for general analgesia, strongly potentiates fentanyl-induced brain hypoxia by eliminating the posthypoxic oxygen increase (50). Since the harmful effects of brain hypoxia exponentially increase when hypoxia becomes more prolonged, potentiation of fentanyl-induced brain hypoxia by the addition of xylazine may explain the higher lethality of these mixtures, which have become more common in the illicit drug market in the United States (1, 51, 52). In contrast to the full blockade of fentanyl’s hypoxic effects by naloxone (0.2 mg/kg), this drug was much less effective in attenuating oxygen decreases induced by the fentanyl-xylazine mixture (53). Thus increased lethality of fentanyl-xylazine mixtures may result from both the potentiation of fentanyl-induced hypoxia and the lower effectiveness of naloxone in attenuating hypoxia.
Brain hypoxia induced by intravenous fentanyl is relatively brief (5–6 min for a 30 µg/kg and 8–10 min for 90 µg/kg dose), placing essential constraints on therapeutic interventions to block or attenuate cerebral hypoxia. To be effective, a therapeutic drug must be delivered by the most rapid route within a relatively short time window; the effectiveness of this treatment declines within minutes after fentanyl intravenous delivery.
Naloxone is the gold standard for the treatment of opioid overdose, but its effectiveness is much lower for fentanyl compared to other slower-acting opioids (4, 33, 54–56). An increase in naloxone dose (to 4 and 8 mg/70 kg) has been proposed as a possible solution, but its value in increasing survival has been questioned (57–60). Furthermore, naloxone used in heavy opioid users at high doses has life-threatening side effects, including acute withdrawal, tachycardia, pulmonary edema, cardiac arrhythmia, etc. (4).
The brief duration of cerebral hypoxia may explain the low therapeutic efficiency of naloxone. When naloxone at a relatively low dose (0.2 mg/kg) is delivered intravenously to freely moving rats as a pretreatment, it fully blocks oxygen responses induced by intravenous fentanyl (20 µg/kg) in both the brain and subcutaneous space (19). However, when naloxone is injected 10 min after fentanyl, after brain hypoxia has already ceased, it induces rapid hyperlocomotion and a transient rise in brain oxygen but has minimal effects on subsequent oxygen levels. Therefore, the therapeutic window for naloxone to attenuate rapid brain hypoxia in rats is less than 10 min, consistent with human observations of rapid lethality within 10 min postfentanyl. An even shorter therapeutic window of naloxone (1 min) has been suggested by a study monitoring vocal cord closure, another possible life-threatening effect of intravenous fentanyl (11) contributing to the development of cerebral hypoxia. However, the latter study was conducted in anesthetized rats when fentanyl is much more toxic (25–50 µg/kg lethality) than in nonanesthetized rats. While quick naloxone treatment is possible with adequate preparation (i.e., in hospital settings with readily available naloxone), in real-world conditions, naloxone could be delivered at much longer postfentanyl time, after the initial brain hypoxic effects had already decreased or entirely ceased, diminishing its therapeutic efficiency.
DATA AVAILABILITY
Raw data and the results of their primary analyses are available on request from Dr. E. A. Kiyatkin (National Institute on Drug Abuse–Intramural Research Program; ekiyatki@intra.nida.nih.gov).
SUPPLEMENTAL MATERIAL
Supplemental Fig. S1 and Supplemental Tables S1–S4: https://doi.org/10.6084/m9.figshare.25898848.
GRANTS
The study was supported by the National Institute on Drug Abuse–Intramural Research Program (NIH Grant 1ZIADA000566-12; to E. A. Kiyatkin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C. and E.A.K. conceived and designed research; S.C., M.R.N., and E.A.K. performed experiments; S.C., M.R.N., and E.A.K. analyzed data; S.C. and E.A.K. interpreted results of experiments; S.C., M.R.N., and E.A.K. prepared figures; E.A.K. drafted manuscript; S.C., M.R.N., and E.A.K. edited and revised manuscript; S.C., M.R.N., and E.A.K. approved final version of manuscript.
REFERENCES
- 1. Friedman J, Montero F, Bourgois P, Wahbi R, Dye D, Goodman-Meza D, Shover C. Xylazine spreads across the US: a growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend 233: 109380, 2022. doi: 10.1016/j.drugalcdep.2022.109380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han Y, Yan W, Zheng Y, Khan Z, Yan K, Lu L. The rising crisis of elicit fentanyl use, overdose, and potential therapeutic strategies. Transl Psychiatry 9: 282, 2019. doi: 10.1038/s41398-019-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Compton VM, Valentino RJ, DuPont RL. Polysubstance use in the U.S. opioid crisis. Mol Psychiatry 26: 41–50, 2021. doi: 10.1038/s41380-020-00949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skolnick P. Treatment of overdose in the synthetic opioid era. Pharmacol Ther 233: 108019, 2022. doi: 10.1016/j.pharmthera.2021.108019. [DOI] [PubMed] [Google Scholar]
- 5. Jaffe JH, Knapp CM, Ciraulo DA. Opiates: clinical aspects. In: Substance Abuse (3rd ed.), edited by Lowinson JH, Ruiz P, Millman RB, Langrod JG. Baltimore, MD: Williams & Wilkins, 2005, p. 158–166. [Google Scholar]
- 6. Baud FJ. Mechanisms of opioid-induced overdose: experimental approach to clinical concerns. Ann Pharm Fr 67: 353–359, 2009. doi: 10.1016/j.pharma.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 7. Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94: 825–834, 2005. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 8. Yeadon M, Kitchen I. Opioids and respiration. Prog Neurobiol 33: 1–16, 1989. doi: 10.1016/0301-0082(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 9. Pattinson KT. Opioids and the control of respiration. Br J Anaesth 100: 747–758, 2008. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 10. Van Bever WF, Niemegeers CJ, Janssen PA. Synthetic analgesics. Synthesis and pharmacology of the diastereoisomers of N-(3-methyl-1-(2-phenylethyl)-4-piperidyl)-N-phenylpropanamide and N-(3-methyl-1-(1-methyl-2-phenylethyl)-4-piperidyl)-N-phenylpropanamide. J Med Chem 17: 1047–1051, 1974. doi: 10.1021/jm00256a003. [DOI] [PubMed] [Google Scholar]
- 11. Miner NB, Schutzer WE, Zarnegarnia Y, Janowsky A, Torralva R. Fentanyl causes naloxone-resistant vocal cord closure: a platform for testing overdose treatments. Drug Alcohol Depend 227: 108974, 2021. doi: 10.1016/j.drugalcdep.2021.108974. [DOI] [PubMed] [Google Scholar]
- 12. Lui PW, Lee TY, Chan SH. Fentanyl-induced muscle rigidity in unanesthetized and ketamine- or thiopental-anesthetized rats. Anesthesiology 70: 984–990, 1989. doi: 10.1097/00000542-198906000-00017. [DOI] [PubMed] [Google Scholar]
- 13. Metz C, Göbel L, Gruber M, Hoerauf KH, Taeger K. Pharmacokinetics of human cerebral opioid extraction: a comparative study on sufentanil, fentanyl, and alfentanil in a patient after severe head injury. Anesthesiology 92: 1559–1567, 2000. doi: 10.1097/00000542-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 14. Laferrière A, Colin-Durand J, Moss IR. Ontogeny of respiratory sensitivity and tolerance to the mu-opioid agonist fentanyl in rat. Brain Res Dev Brain Res 156: 210–217, 2005. doi: 10.1016/j.devbrainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 15. Kuo A, Wyse BD, Meutermans W, Smith MT. In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile. Br J Pharmacol 172: 532–548, 2015. doi: 10.1111/bph.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yadav SK, Kumar D, Kumar P, Gupta PK, Bhattacharya R. Biochemical, oxidative, and physiological changes caused by acute exposure of fentanyl and Its 3 analogs in rodents. Int J Toxicol 37: 28–37, 2018. doi: 10.1177/1091581817750560. [DOI] [PubMed] [Google Scholar]
- 17. Marchette RCN, Carlson ER, Frye EV, Hastings LE, Vendruscolo JCM, Mejias-Torres G, Lewis SJ, Hampson A, Volkow ND, Vendruscolo LF, Koob GF. Heroin- and fentanyl-induced respiratory depression in a rat plethysmography model: potency, tolerance, and sex differences. J Pharmacol Exp Ther 385: 117–134, 2023. doi: 10.1124/jpet.122.001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solis E, Jr,Cameron-Burr KT, Shaham Y, Kiyatkin EA. Fentanyl-induced brain hypoxia triggers brain hyperglycemia and biphasic changes in brain temperature. Neuropsychopharmacology 43: 810–819, 2018. doi: 10.1038/npp.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curay CM, Irwin MR, Kiyatkin EA. The pattern of brain oxygen response induced by intravenous fentanyl limits the time window of therapeutic efficacy of naloxone. Neuropharmacology 231: 109507, 2023. doi: 10.1016/j.neuropharm.2023.109507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ling GS, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J Pharmacol Exp Ther 232: 149–155, 1985. [PubMed] [Google Scholar]
- 21. Bola RA, Kiyatkin EA. Brain temperature effects of intravenous heroin: state dependency, environmental modulation, and the effects of dose. Neuropharmacology 126: 271–280, 2017. doi: 10.1016/j.neuropharm.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA. Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro 4: ENEURO.0151-17.2017, 2017. doi: 10.1523/ENEURO.0151-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kiyatkin EA, Wise RA. Brain and body hyperthermia associated with heroin self-administration in rats. J Neurosci 22: 1072–1080, 2002. doi: 10.1523/JNEUROSCI.22-03-01072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci (Landmark Ed) 15: 73–92, 2010. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiyatkin EA. Brain temperature and its role in physiology and pathophysiology: lessons from 20 years of thermorecording. Temperature (Austin) 6: 271–333, 2019. doi: 10.1080/23328940.2019.1691896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998. [Google Scholar]
- 27. Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci 22: 930–938, 2005. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- 28. Kiyatkin EA. Physiological and drug-induced fluctuations in brain oxygen and glucose assessed by substrate-sensitive sensors coupled with high-speed amperometry. In: Compendium of In Vivo Monitoring in Real-Time Molecular Neuroscience, edited by Wilson GS, Michael AC. Singapore: World Scientific, 2019, p. 219–250, [Google Scholar]
- 29. Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40: 421–428, 2015. doi: 10.1038/npp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiyatkin EA. Functional role of peripheral vasoconstriction: not only thermoregulation but much more. J Integr Neurosci 20: 755–764, 2021. doi: 10.31083/j.jin2003080. [DOI] [PubMed] [Google Scholar]
- 31. Hug CC Jr, Murphy MR. Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology 55: 369–375, 1981. doi: 10.1097/00000542-198110000-00006. [DOI] [PubMed] [Google Scholar]
- 32. Hill R, Santhakumar R, Dewey W, Kelly E, Henderson G. Fentanyl depression of respiration: comparison with heroin and morphine. Br J Pharmacol 177: 254–266, 2020. doi: 10.1111/bph.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dahan A, Franko TS, Carroll JW, Craig DS, Crow C, Galinkin JL, Garrity JC, Peterson J, Rausch DB. Fact vs. fiction: naloxone in the treatment of opioid-induced respiratory depression in the current era of synthetic opioids. Front Public Health 12: 1346109, 2024. doi: 10.3389/fpubh.2024.1346109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt CF, Kety SS. Recent studies of cerebral blood flow and cerebral metabolism in man. Trans Assoc Am Physicians 60: 52–58, 1947. [PubMed] [Google Scholar]
- 35. Battisti-Charbonney A, Fisher JA, Duffin J. Respiratory, cerebrovascular and cardiovascular responses to isocapnic hypoxia. Respir Physiol Neurobiol 179: 259–268, 2011. doi: 10.1016/j.resp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 36. Kontos HA. Regulation of the cerebral circulation. Annu Rev Physiol 43: 397–407, 1981. doi: 10.1146/annurev.ph.43.030181.002145. [DOI] [PubMed] [Google Scholar]
- 37. Perekopskiy D, Afzal A, Jackson SN, Muller L, Woods AS, Kiyatkin EA. The role of peripheral opioid receptors in triggering heroin-induced brain hypoxia. Sci Rep 10: 833, 2020. doi: 10.1038/s41598-020-57768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Algera MH, Olofsen E, Moss L, Dobbins RL, Niesters M, van Velzen M, Groeneveld GJ, Heuberger J, Laffont CM, Dahan A. Tolerance to opioid-induced respiratory depression in chronic high-dose opioid users: a model-based comparison with opioid-naive individuals. Clin Pharmacol Ther 109: 637–645, 2021. doi: 10.1002/cpt.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomas SA, Curay CM, Kiyatkin EA. Relationships between oxygen changes in the brain and periphery following physiological activation and the actions of heroin and cocaine. Sci Rep 11: 6355, 2021. doi: 10.1038/s41598-021-85798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solis E Jr, Bola RA, Fasulo BJ, Kiyatkin EA. Brain hyperglycemia induced by heroin: association with metabolic neural activation. ACS Chem Neurosci 8: 265–271, 2017. doi: 10.1021/acschemneuro.6b00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin RL, Lloyd HG, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci 17: 251–257, 1994. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 42. Rainaldi MA, Perlman JM. Pathophysiology of birth asphyxia. Clin Perinatol 43: 409–422, 2016. doi: 10.1016/j.clp.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 43. Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation 26: 5–13, 2010. doi: 10.3233/NRE-2010-0531. [DOI] [PubMed] [Google Scholar]
- 44. Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest 106: 723–731, 2000. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bailey DM. Oxygen and brain death; back from the brink. Exp Physiol 104: 1769–1779, 2019. doi: 10.1113/EP088005. [DOI] [PubMed] [Google Scholar]
- 46. Schleimer R, Benjamini E, Eisele J, Henderson G. Pharmacokinetics of fentanyl as determined by radioimmunoassay. Clin Pharmacol Ther 23: 188–194, 1978. doi: 10.1002/cpt1978232188. [DOI] [PubMed] [Google Scholar]
- 47. Palmer RB. Fentanyl in postmortem forensic toxicology. Clin Toxicol (Phila) 48: 771–784, 2010. doi: 10.3109/15563650.2010.525514. [DOI] [PubMed] [Google Scholar]
- 48. Mann J, Samieegohar M, Chaturbedi A, Zirkle J, Han X, Ahmadi SF, Eshleman A, Janowsky A, Wolfrum K, Swanson T, Bloom S, Dahan A, Olofsen E, Florian J, Strauss DG, Li Z. Development of a translational model to assess the impact of opioid overdose and naloxone dosing on respiratory depression and cardiac arrest. Clin Pharmacol Ther 112: 1020–1032, 2022. doi: 10.1002/cpt.2696. [DOI] [PubMed] [Google Scholar]
- 49. Strauss DG, Li Z, Chaturbedi A, Chakravartula S, Samieegohar M, Mann J, Nallani SC, Prentice K, Shah A, Burkhart K, Boston J, Fu Y-HA, Dahan A, Zineh I, Florian JA. Intranasal naloxone repeat dosing strategies and fentanyl overdose: a simulation-based randomized clinical trial. JAMA Netw Open 7: e2351839, 2024. doi: 10.1001/jamanetworkopen.2023.51839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Choi S, Irwin MR, Kiyatkin EA. Xylazine effects on opioid-induced brain hypoxia. Psychopharmacology (Berl) 240: 1561–1571, 2023. doi: 10.1007/s00213-023-06390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ayub S, Parnia S, Poddar K, Bachu AK, Sullivan A, Khan AM, Ahmed S, Jain L. Xylazine in the opioid epidemic: a systematic review of case reports and clinical implications. Cureus 15: e36864, 2023. doi: 10.7759/cureus.36864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruiz-Colón K, Chavez-Arias C, Díaz-Alcalá JE, Martínez MA. Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: a comprehensive review of the literature. Forensic Sci Int 240: 1–8, 2014. doi: 10.1016/j.forsciint.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 53. Choi S, Irwin MR, Noya MR, Shaham Y, Kiyatkin EA. Combined treatment with naloxone and the alpha2 adrenoceptor antagonist atipamezole reversed brain hypoxia induced by a fentanyl-xylazine mixture in a rat model. Neuropsychopharmacology 49: 1104–1112, 2023. doi: 10.1038/s41386-023-01782-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Skolnick P. On the front lines of the opioid epidemic: rescue by naloxone. Eur J Pharmacol 835: 147–153, 2018. doi: 10.1016/j.ejphar.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 55. Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf 9: 63–88, 2018. doi: 10.1177/2042098617744161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Dorp E, Yassen A, Dahan A. Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf 6: 125–132, 2007. doi: 10.1517/14740338.6.2.125. [DOI] [PubMed] [Google Scholar]
- 57. Moss RB, Carlo DJ. Higher doses of naloxone are needed in the synthetic opioid era. Subst Abuse Treat Prev Policy 14: 6, 2019. doi: 10.1186/s13011-019-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdelal R, Banerjee AR, Carlberg-Racich S, Darwaza N, Ito D, Epstein J. The need for multiple naloxone administrations for opioid overdose reversals: a review of the literature. Subst Abus 43: 774–784, 2022. doi: 10.1080/08897077.2021.2010252. [DOI] [PubMed] [Google Scholar]
- 59. Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl) 239: 2063–2081, 2022. doi: 10.1007/s00213-022-06125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yugar B, McManus K, Ramdin C, Nelson LS, Parris MA. Systematic review of naloxone dosing and adverse events in the emergency department. J Emerg Med 65: e188–e198, 2023. doi: 10.1016/j.jemermed.2023.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1 and Supplemental Tables S1–S4: https://doi.org/10.6084/m9.figshare.25898848.
Data Availability Statement
Raw data and the results of their primary analyses are available on request from Dr. E. A. Kiyatkin (National Institute on Drug Abuse–Intramural Research Program; ekiyatki@intra.nida.nih.gov).