Abstract
Nanomaterial advancements have driven progress in central and peripheral nervous system applications such as tissue regeneration and brain-machine interfacing. Ideally, neural interfaces with native tissue should seamlessly integrate, a process which is often mediated by the interfacial material properties. Surface topography and material chemistry are significant extracellular stimuli that can influence neural cell behavior to facilitate tissue integration and augment therapeutic outcomes. This review characterizes topographical modifications, including micropillars, microchannels, surface roughness, and porosity, implemented on regenerative scaffolding and brain-machine interfaces. Their impact on neural cell response is summarized through neurogenic outcome and mechanistic analysis. We also review the effects of surface chemistry on neural cell signaling with common interfacing compounds like carbon-based nanomaterials, conductive polymers, and biologically inspired matrices. Finally, the impact of these extracellular mediated neural cues on intracellular signaling cascades is discussed to provide perspective on the manipulation of neuron and neuroglia cell microenvironments to drive therapeutic outcomes.
Keywords: Surface topography, Substrate chemistry, Cell signaling, Neurogenesis, Neural Interfaces
Graphical Abstract

Surface topography and material chemistry are significant extracellular stimuli that influence neural cell behavior to facilitate tissue integration and augment therapeutic outcomes. This review characterizes topographical modifications and surface chemistry on neural cell signaling to improve neural interface outcomes. The impact of extracellular mediated cues on intracellular signaling cascades provides perspective on the manipulation of neural microenvironments to drive therapeutic success.
1.0. Introduction:
The central and peripheral nervous systems (CNS and PNS, respectively) are complex networks having electrophysiological and biochemical signals that dictate sensory, motor, and visceral processes. Studying the functional relationships of CNS neurons and neuroglia is essential to understanding region-specific neural behavior and how it changes across normal and abnormal physiological conditions. Initially, neural interface technologies were utilized as tools for research to decode spatiotemporal function of CNS tissue [1–5]. As mapping experiments became more complex and region-specific activity was uncovered, numerous neural interface applications arose including an increased attention to chronic interfacing therapies and neural regeneration efforts [6–8]. Since then, neural interface technologies have grown considerably, focusing on the seamless reintegration of neural tissue into implanted substrates to facilitate therapeutic outcomes that include nervous tissue regeneration and the restoration of sensory and motor function. Although the relationship between neurons and neuroglia allows for remarkable tissue plasticity and function, innate neuroprotective mechanisms can frustrate therapeutic modalities such as tissue regeneration and brain-machine interfaces (BMI) [9–11]. The success of these therapies relies on the proliferation, maturation, and integration of neurons/neuroglia onto engineered constructs implanted within the native tissue. In recent years, complex nanomaterial development and surface engineering techniques have been the focus of neural interface engineering to improve upon conventional materials that lack bioactivity and chronic interfacing properties. Substrate topography and chemistry can significantly influence the behavior of interfacing neural cells by manipulating the tissue microenvironment. Consequently, the development of interfacing materials capable of optimizing biocompatibility and the therapeutic outcome has increased dramatically. Although neural regenerative engineering and BMI interfacing may differ in application, their interface requirements are not mutually exclusive and oftentimes benefit from similar surface engineering techniques that encourage neural cell recruitment and healthy tissue reintegration.
For tissue regeneration applications, the regeneration and reintegration of neural tissue rely on a scaffold’s biochemical, bioelectrical, and biomechanical characteristics which should mimic the native extracellular matrix (ECM) (Figure 1). Scaffolds interact with the cellular microenvironment to proliferate, differentiate, and reintegrate cells as functional members of the surrounding native tissue. Several design considerations can minimize cell death, decrease inflammation, and maximize cell penetration. Substrate nanotopography is a powerful tool to manipulate and guide cell signaling through extracellular cues and guidance. The surface properties and modifications of scaffolding materials, such as surface roughness, porosity, and micropillar/microgroove formation, have all been shown to manipulate cell behavior to achieve enhanced therapeutic outcomes [17–21]. In addition to topographical alterations, interface chemistry impacts stability and biocompatibility due to the direct molecular-level interactions at the membrane/ECM surface. Although less characterized, the chemical structure of interfacing surfaces elicits varying levels of surface receptor expression which impacts membrane protein compositions, ion concentration gradients, and potential intracellular cascade activation [22–24].
Figure 1.
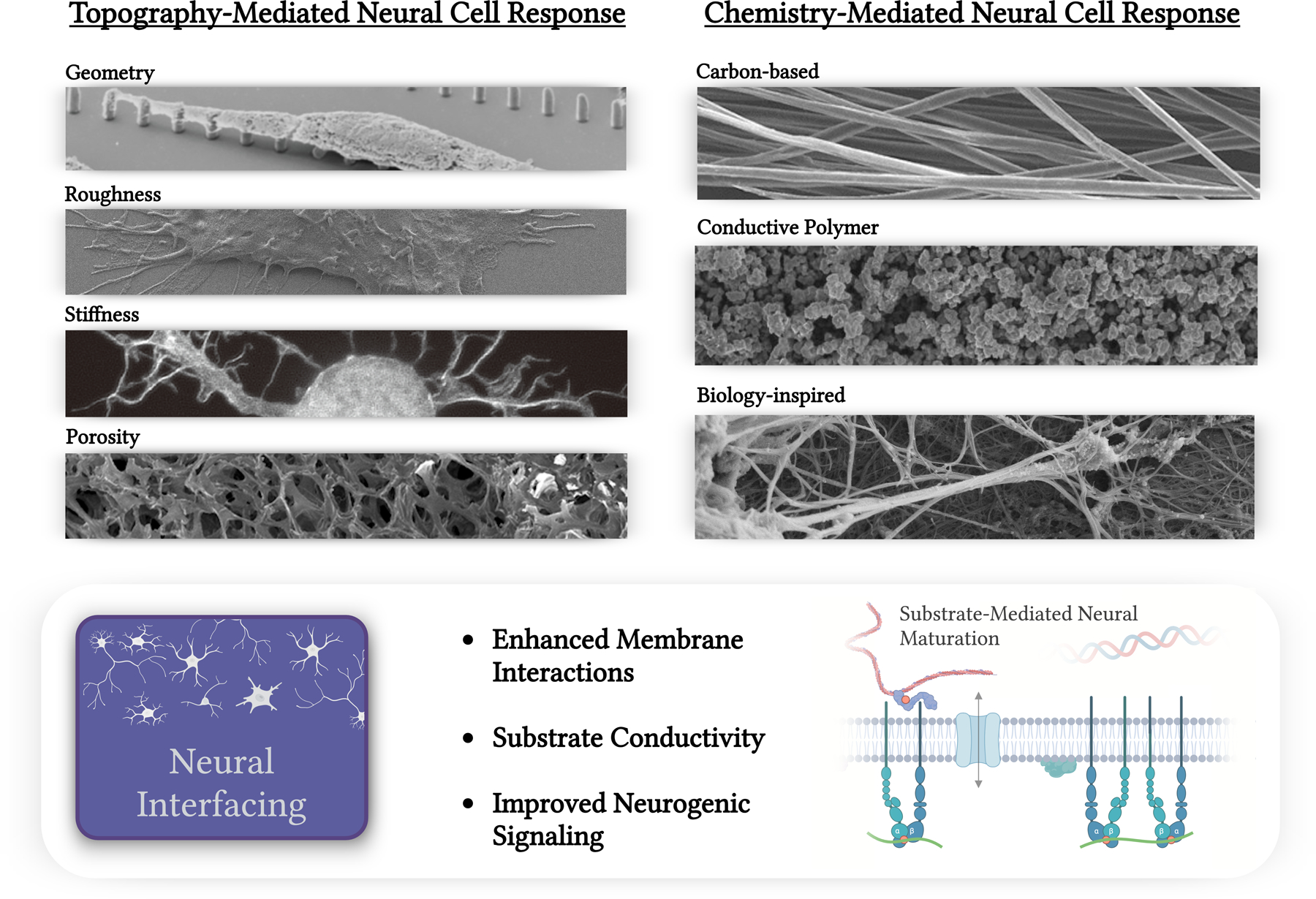
Summary of physical modifications to neural interfaces including porosity [12], geometry [13], stiffness [14], and roughness. All cited categories are from works adapted to this figure under the Creative Commons license (CC BY 3.0 or 4.0). Additional chemical substrate modifications are categorized into carbon-based [15], conductive polymer [16], or biologically-inspired chemistries. All cited categories are from works adapted to this figure under the Creative Commons license (CC BY 3.0 or 4.0). Neural interface applications discussed within include brain machine interfacing and CNS/PNS regenerative scaffolding.
Implanted BMI electrodes for stimulating and recording nearby neurons aim to achieve neural reintegration while simultaneously minimizing innate immune reactions. Electrophysiological recordings rely on detecting spatiotemporal variations in ionic concentrations generated by neurogenic cells. A healthy device-tissue interface will enhance the signal-to-noise (SNR) ratio for recorded signals, reduce power consumption for stimulation, and increase the functional longevity of the device. For many invasive BMI applications, the foreign body response (FBR) continues to frustrate chronic phase interfacing through reactive tissue encapsulation. Neuroglia are driven to the interface of BMI devices through the compounded impact of insertion trauma, microenvironmental inflammatory signaling, mechanical mismatch, and poor device interface properties. The accumulation of reactive neuroglia physically and electrically isolates the device, negatively impacting its stability and signal quality [25, 26]. Surface topography and chemistry can increase adhesion and enhance electrogenic cell behavior while reducing the recruitment of reactive neuroglia, enabling healthy interfaces. By modulating protruding nanostructures and promoting focal adhesion site formation, the cleft between the device and cell membrane is strengthened and the seal resistance of the surrounding tissue is enhanced [27]. In addition, chemical modifications such as incorporating carbon nanotubes (CNTs) and conductive polymers (CPs) can regulate the response of neurons/neuroglia through receptor-specific molecular interactions and electrochemical reactions during signal transduction [28]. An increasing number of in vivo electrophysiology studies are incorporating surface engineering and material chemistry into probe design, targeting both the functional (electrode sites) and non-functional areas, to minimize the immune response and enhance neural proliferation at the implant site. However, to the best of the authors’ knowledge, these studies have primarily been in animals s. Further research and development are needed to advance surface topography and chemistry modifications into human studies and evaluate their ability to enhance the chronic performance of BMIs.
Since surface topography and chemistry can have a significant influence on neural cell behavior, this review article summarizes the progress in developing surface-mediated neurogenic cues for regenerative and BMI applications. We also summarize mechanistic studies involving high-resolution transcriptomic analysis that characterize neural cell response through signaling cascades that impact neurogenesis and electrogenic function.
2.0. Etched and Patterned Nano/Micro-Topography:
Manipulating substrate geometry is an effective way to influence cell response as the brain’s microenvironment is sensitive to external forces and stimulation. Strategies to reduce neuroinflammation are especially important as inflammation can prevent the desired neural integration and regeneration, including neural proliferation and differentiation. Well-studied surface modifications include roughness, micropillars, microgrooves, pores, and stiffness variability (Figure 2). The impact these topographies have on cellular behavior can be evaluated through neural viability, maturation, and inflammation. In addition, mechanistic studies are conducted to elucidate the specific cellular mechanisms and pathways responsible for the changes in neural behavior.
Figure 2.
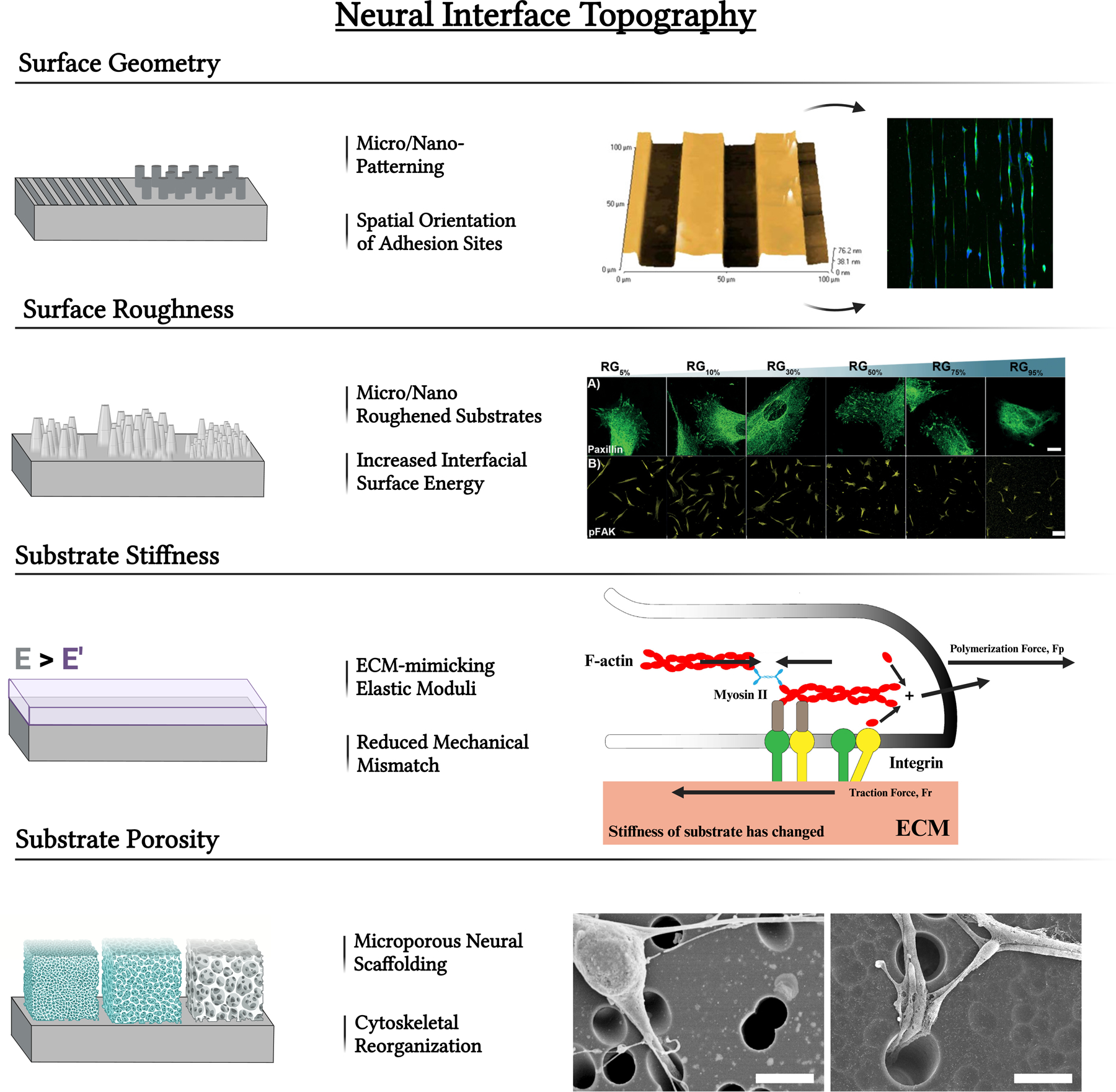
Neural interface modifications including the alteration of substrate geometry using micro/nano-patterning techniques. These can result in microgrooves, microchannels, micropillars, or other modifications that impact the spatial orientation of adhesion sites. Figure adapted with permission from Pardo-Figuerez et al. [29] under the Creative Commons license (CC BY 3.0 or 4.0). Alteration of interfacial energy by altering surface roughness also impacts neural cell response. Figure adapted from [30] under the Creative Commons license (CC BY 3.0 or 4.0). Neural cell interactions may also be impacted by altering surface porosity for network growth. Figure adapted from [31] under the Creative Commons license (CC BY 3.0 or 4.0). Substrate stiffness similar to native tissue ECM can facilitate a superior device-tissue integration. Figure adapted from [32] under the Creative Commons license (CC BY 3.0 or 4.0).
2.1. Surface Geometry:
2.1.1. Micropillars:
Micropillar topography features structures having high aspect ratio are capable of directing change in stem cell and neural cell behavior through contact and nano compressive characteristics. Arrays of micropillars interact with cell networks to induce and regulate cell adhesion through mechanotransduction via the formation of focal adhesion complexes [33]. Depending on the array design, these adhesions can deform/elongate the nuclear envelope, promote radial migration, and enhance cellular organization based on the control of spacing in the array. Functionalization of micropillar topographies can direct differentiation of stem cells into cell lineage termination and has achieved regulation of proliferation and cell fate through the Ca2+ ion-dependent Wnt signaling [34].
Several studies investigated the mechanism of micropillar topography interaction with the cell interface, permitting operational control of adhesion and subsequent signaling of the cellular network. Doolin et al. studied the interaction of mesenchymal stem cells (MSCs) through micropillar assays, and the effect of spacing and height on mechanotransductive responses through the cell-surface interface [35]. Over three weeks, MSCs within micropillar arrays displayed mechanical deformation as the adhesion to the substrate directed cell mobility, cytoskeletal deformation forces, and the forces acting on the nucleus for elongation. Narrowing of array spacing resulted in robust MSC infiltration from 2D culture to the array; actin expression in the formation of focal adhesions was increased. Adhesion to micropillar arrays have also demonstrated a significant contribution to nuclear deformation which has been implicated in enhanced differentiation, proliferation, and migration of cells [36, 37] (Figure 3A). Micropillar arrays facilitate a high rate of binding to fibronectin in the ECM, giving rise to greater cell mobility mechanisms. Cellular mobility plays a significant role in developing cell signaling networks and tissue formation. In the context of neural networks, characterized by their plastic structure, cellular mobility emerges as a dominant factor and exerts a profound influence in the formation of intracellular signaling pathways. Activation of mechanosensing pathways to cell stretching, like that observed in the stretch-activated ion channel PIEZO1, has a significant influence on matrix mechanical signal transduction and also impacts neural stem cell or neuroglia differentiation lineages [38].
Figure 3.
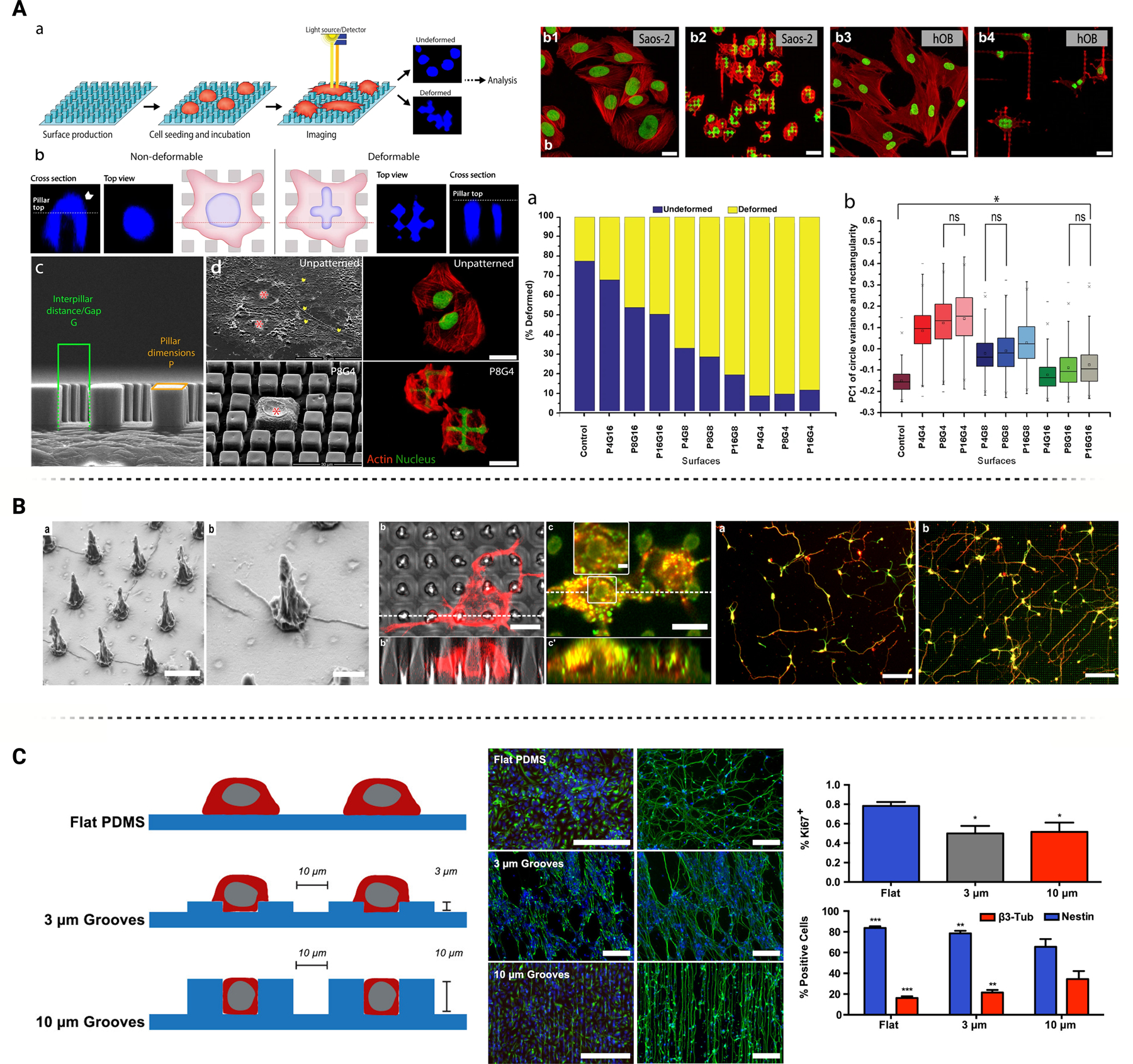
Modifications to neural interface surface geometries including micropillars and etched microgroove/microchannels demonstrate key influence on neural cell behavior. (A) Micropillar induced nuclear deformations over patterned surfaces demonstrate heightened nuclear elasticity and actin activity over Saos-2 cells compared to unpatterned topographies. Figure adopted with permission from Ermis et al. [37] under the Creative Commons license (CC BY 3.0 or 4.0). (B) PH3T micropillar geometries combined with light excitation demonstrate critical upregulation of key neuronal markers MAP2 and TUJ1. Figure adopted with permission from Milos et al. [40] under the Creative Commons license (CC BY 3.0 or 4.0). (C) PDMS microchannels modulate neuronal-linked epigenetic factors at different key depths and channel widths, as well as significant impact on NOTCH pathway capabilities. Surface topographies promote neural lineage and maturation amongst neural stem cell cultures. Figures adopted with permission from Milos et.al. [47]
Micropillar interaction with neural cells can also facilitate intracellular signaling pathway activity. Cutarelli et al. observed the impact of micropillar silicon substrates in inducing adhesion and differentiation of pluripotent stem cells to promote cortical cell maturation [39]. These substrates produced radial migration distinctive of cortical progenitor maturation in vivo. Expression of SOX2 and Nestin were unaffected by micropillar culture, while neuronal marker beta III tubulin was downregulated which indicated sustained stem cell multipotency. Differentiation experiments demonstrated culture upregulation of MAP2 and CUX1 mature neuron markers and increased regulation of beta III tubulin promoting differentiation signaling from stem cells to cortical progenitors. Milos et al. investigated how neuronal cell growth could be modulated through an interface with micropillar substrate topography [40]. Micropillar arrays fabricated of semiconducting polymer, P3HT (Figure 3B), coupled with light excitation resulted in an accumulation of charge (Ca2+ ions) along the cell membrane interface. The resulting interfacial electric field can affect downstream pathways for neuronal functions in growth and differentiation. Local charge density resulted in increased intracellular calcium levels and influenced calmodulin interactions and protein kinase C phosphorylation. Dependent on the material utilized, the geometry of a nanopillar substrate may also influence downstream differentiation of stem cells including the ability to upregulate specific gene markers in Tuj1 and MAP2 pathways for neural-typical differentiation as well as the inhibition of astrocyte activation.
Liang et al. performed a comprehensive study in which a piezoelectric poly (vinylidene fluoride) (PVDF) nanopillar array was fabricated through hot pressing, creating an array with hexagonal bases that would taper up to a round column. Fourteen days of rat bone marrow stem cell culture on nanopillar arrays induced neurite outgrowth and natural elongation, indicative of neural differentiation and maturation. Although the focus of this study was ultrasound stimulation, noteworthy findings emerged in the absence of ultrasound. Under 0 W of ultrasound stimulation, quantitative polymerase chain reaction (qPCR) results at 21 days of incubation revealed a differential expression of the Tuj1 gene, indicating potential early-stage differentiation. This suggests that the unique geometry of the nanopillar, leading to the inherent narrowing of cell morphology albeit higher pseudopod count in adhesion to the substrate, facilitated material deformation, subsequently elucidating a piezoelectric discharge. This local electric field discharge likely activated calcium-dependent voltage channels, thereby regulating the NFkB protein complex [41]. NFkB protein complex activation can play a significant role in the functionalization of neural-like cells and is an influencing factor of neurite outgrowth within ganglion neurons [42].
Astrocyte and neuroglial cell formation have been the subject of several studies investigating the regulatory role of micro/nanopillar substrate geometry. Nanopillar PVDF arrays lead to a downregulation of GFAP astrocyte markers compared to tissue culture plates and natural films [43]. The decrease in GFAP protein expression indicates a reduction in astrocyte formation from seeded stem cells. To elucidate the mechanism behind the downregulation of neuroglial formation, researchers explored the activation of Wnt-mediated cellular pathway, which is calcium-dependent and strongly associated with neural differentiation through electrical stimulation. Activation of Wnt pathway may subsequently trigger Notch pathway activation as a downstream mechanism [44].
In addition, astrocyte and neuroglia respond to the mechanical tension exerted by the pillar geometry upon seeding. The surface tension generated by the pillar geometry restricts cell motility and narrows cell morphologies. This unique characteristic enables the influx of ions through dependent channels, as well as the activation of underlying pathways including Hippo, integrin signaling, and the transcription factor Yes-associated protein (YAP)/TAZ. Therefore, the interaction between glial cell adhesion complexes and nanopillar substrates may regulate the formulation of glial cells through mechanosensing activation of YAP/TAZ [45, 46].
2.1.2. Microgroove/Microchannels:
Microchannel surface modification entails etching longitudinal grooves aligned through the substrate; the resulting topography can influence cell behavior due to defined regions of peaks and valleys. These surfaces provide a degree of alignment that may regulate the drive for neural differentiation from stem cell pluripotency, and enhance proliferation through surface interactions [48]. Moreover, microchannels have been used as nerve guidance conduits (NGCs) to promote nerve growth and guide axonal extension after peripheral nerve injuries. NGCs offer an alternative to invasive surgical procedures like autografts, as they can mimic in vivo nerve situations [49]. These beneficial interface interactions are valuable for neural-based cell therapies and can facilitate a microenvironment for neural system functionalization.
Numerous studies have effectively highlighted the cellular mechanisms involved in microchannel interfaces to explore adherence mechanisms and how this topography influences differentiation, proliferation, and various life cycle functions. Microchannel interfaces may influence neural cell behavior, including neurite alignment, cell maturation, differentiation of human mesenchymal stem cells (hMSCs) into neural stem cells (NSCs), mechanotransduction on cell shape, and modulation of epigenetic markers [50]. Hsu. et al. observed NSC interactions with microchannel textured substrates of polydimethylsiloxane (PDMS) and the mechanisms through which resulting surface impacted differentiation capacity and functional cell behavior (Figure 3C). Enhanced alignment, particularly with channels having 10 μm ridge/grove width, was achieved. As channel depth was increased, the expression of epigenetic factors AcH3, AcH4, and H3K9me3 was upregulated, often associated with the expression of neurogenic markers NeuroD and BDNF. Significant expression of these markers is associated with nuclear envelope elongation through mechanotransduction following the adhesion of NSCs to the substrate interface, which plays a significant role in ordering intracellular signals among neural networks due to the complex interactions with cell ECM. Finally, a knockdown assay isolated the Notch pathway activation independent of cell-cell contact, and microchannel surface topography was observed to downregulate the Notch pathway [47]. Microgroove topography affects the differentiation potential of neural stem cells, as evidenced by its influence on the Notch signaling pathway through geometric sensing. Notch signaling target genes were altered through the activation of histone deacetylase (HDAC). Histone deacetylation signaling in parallel with topographic-induced epigenetic modulation enhanced the potential for stem cell neuronal differentiation. Additionally, proliferation could be affected by the epigenetic factor Ki67+, demonstrating similar results with the downregulation of proliferation over larger microchannel surface interactions [51].
Microgroove topographies have also demonstrated control of the signaling and function of neuroglia. Singh et al. explored the morphological and biochemical responses of astrocytes interacting with micro-grooved topographies. Modified microgroove surfaces having varying depths resulted in decreased proliferation compared to smooth surfaces which may result from nuclear elongation and subsequent chromatin condensation. Additionally, in exploring the signaling mechanism of topographically induced astrocyte behavior, this study quantified a differential expression in calcium ion concentration. Neuroglia signal transduction has been demonstrated to occur through calcium ion channel activity. Therefore, heightened activity of calcium signaling pathways may influence astrocyte cell behavior, including cellular function especially in contributing to complex neural networks. However, further studies are required to understand this effect [52, 53].
Geometrical alignment of multiple microchannels has allowed observation of interfacial interactions of multiple neural populations with high reliability and measurement via state-of-art sensing techniques. Novel microfabrication procedures can integrate microgroove architectures in microfluidic systems with multielectrode arrays (MEAs), field-effect transistors (FETs), and chemical sensors, enabling the simultaneous stimulation and recording of neural populations to monitor cellular interactions [54], and information flow via synapses [55].
Microgroove channels have been demonstrated as a powerful tool for neural regenerative engineering, particularly through nerve interfacing and repair. Musick et al. used a soft multichannel neural electrode interface between sciatic nerve cells within rats over three months and demonstrated regeneration and electrical measurement potential in mice spinal cord injury (SCI) models. At 2, 8, and 12 weeks post-implantation, histological imaging demonstrated regeneration of the sciatic nerve showing both myelinated and unmyelinated axons, Schwann cells, and connective tissue surrounding the implant indicative of neural regeneration capacity through the surface interface. Researchers observed the formation of an artificial fascicular structure between the regenerating neurons and the microchannel array, as well as heavy collagen and connective tissue along the channel walls indicative of the potential for neural cell adhesion. Chronic recording was studied through the sciatic nerve by observing the conductance of electrical signaling through the microchannel array up to 3 months following the operation. Electrophysiological recording revealed action potential firing was synchronous to the gait cycle of the animal. Increased spike rate over time was attributed to neuronal maturation through the miniature nerve fascicle development and axon myelination. A parallel microchannel (110 × 120 μm2 with a wall thickness of 50 μm and length of 50 mm) allowed directional maturation of regenerating neurons to allow for nerve repair and the quality electrical signal conduction through the sciatic nerve to conduct a gait cycle [56]. Similar observations have been repeated through several different in vitro studies including microchannel arrays cultured with dorsal root ganglion cells [57] and cortical cells [58]. In vivo studies with sciatica nerve deformities [59] demonstrated ideal adhesion of neural cells as well as increased ordered neurite growth and extension. The parallel alignment of microchannels allows for ordering neural cell outgrowth and maturation into potential regenerative repair. Additionally, high throughput electrical stimulation to cultured neural cells [58] or electrical action potential observation in vivo would further quantify the maturation of nerve repair [59] with microchannel electrode arrays demonstrating high-quality resolution in recording electrical activity through nerve fascicles across the sciatic nerve.
Aligned microchannels have also emerged as a promising approach for developing artificial NGCs to address peripheral nerve injuries. NGCs can incorporate micro- or nanopatterns with repeated ridges and grooves that are embedded in biocompatible flexible materials such as PDMS and SU-8 following microelectromechanical systems (MEMS) fabrication techniques [60]. These structures are rolled to form 3D designs with longitudinal channels that mimic the microarchitecture of the endoneurium tube, thereby facilitating axonal growth, nerve guidance, and subsequent regeneration. Park et al. presented a biodegradable NGC with tunable microchannel sizes with the ability to align the regrowth of nerve fibers and recruited host stem cells for enhanced functional regeneration [61]. In their study, micropatterned poly(L-lactide-co-ε-caprolactone) (PLCL) sheets were utilized in conjunction with stem-cell recruitment factors (substance P, SP) to create a conducive environment for nerve regeneration and proliferation. The multichannel NGC device was evaluated both in vitro, by assessing the spatial guidance of PC12 cells, and in vivo, by examining the regrowth of injured rat sciatic nerve. The results demonstrated a significant improvement in regeneration outcomes. Shelly et al. explored the control of neuronal cell polarity through micropatterned strips of semaphorin 3A placed in a microchannel substrate design. Undifferentiated neurons exposed to Sema3A in a microchannel design exhibited polarization of neural cells into dendrites through the suppression of axonal development. Elevation of the cGMP/PKG signaling pathway was observed through FRET signaling and was hypothesized to downregulate the activity of cAMP and PKA signaling pathways which play a significant role in the axonal development via LKB1 and GSK3-B phosphorylation. Additionally, in vivo assays in cortical neurons seeded with Sema3A downregulation in microchannels resulted in polarization defects and reduced growth length of neural cells [62]. Microfluidic technology introduces unique capabilities for exploring neural cell signaling by providing greater control of cell fate and functionalization through the cell-substrate interaction and tunable functionalization. This nuanced approach holds great promise in advancing our understand of neural signaling and opens new avenues for precise interventions in cellular behavior and functional outcomes.
2.2. Surface Roughness:
Surface roughness describes the quantifiable deviations in normal vectors across a microtopography. The roughness indicates the measure of micro (and nano)-irregularities across the material interface [63]. Rough surface topography typically encourages cell adhesion through the entrapment of fibrin on the contact interface, leading to a greater interface energy-driven proliferative effect among adhered cells. Topographical roughness along a surface creates a region of high interfacial energy with increased wettability and can lead to improvements in cellular adhesion [64, 65]. In the interaction between substrate topography and cellular environment, the adsorption of proteins has been studied as a potential adhesion region for the cell-substrate interface (Figure 4A). Interfacial energy drives protein adhesion and is proportional to the contact area with the substrate [66]. Driven by a change in interfacial adhesive energy and interface protein adsorption, surface roughness modification can augment neural cell proliferation, differentiation, and innate function. Characterization of this interface and the signaling mechanisms between cells and roughened surface should demonstrate the associated cell-substrate interactions and the mechanism through which this interface interacts and drives cellular function [67].
Figure 4.
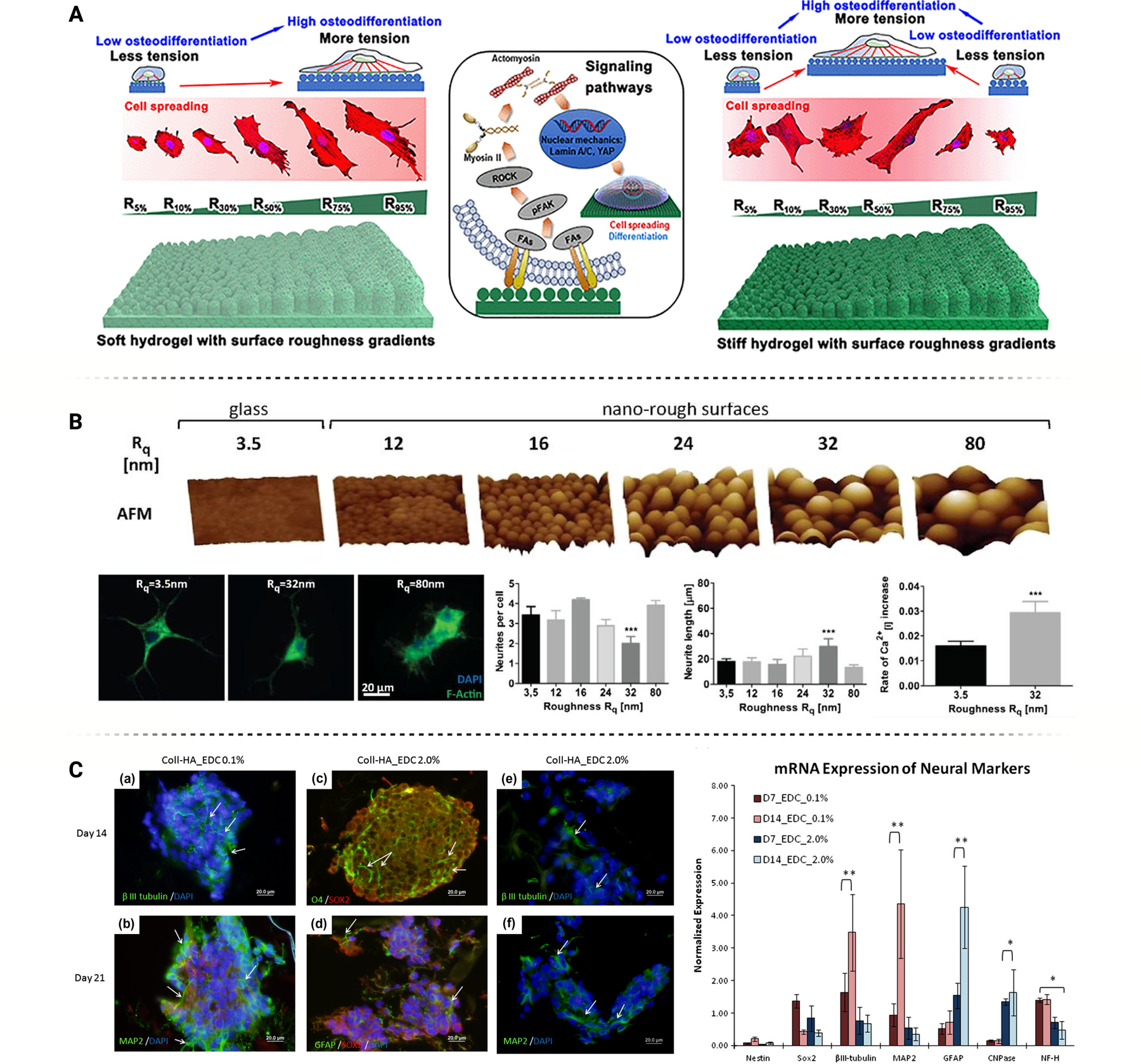
(A) Soft and stiff hydrogels, each with the same surface roughness gradients, differentially impact cellular behavior. Figure adapted from Hou et al. [55] with permission. (B) Surfaces with an optimal stochastic nanoroughness, Rq = ~ 23 nm, induce increased neuronal differentiation and longer neurite outgrowth, as compared to smooth surfaces, Rq = ~ 3.5 nm. Figure adapted from Blumenthal et al. [65] under Creative Commons License (CC BY 3.0 or 4.0). (C) Hydrogel stiffness, controlled through concentration of crosslinking agent (EDC), influences stem cell differentiation towards different neural lineages. Figure adapted from Her et al. [83] with permission.
Focal adhesion kinase (FAK) is a crucial component of cellular adhesion and ECM interactions when interfacing with the topography of a surface. Several research groups have demonstrated the capability of cellular components to sense and facilitate adhesion complexes based on varying nano-scaled roughness among different cell types. Schwartz et al. demonstrated the impact of FAK complex formation of osteoblasts on differentiated micro-rough titanium substrates, inducing the growth factor TGF-B1 for differentiation modulation [68]. Similarly, Deligianni et al. reported comparable adhesion profile results through the attachment of human bone marrow cells onto hydroxyapatite substrates with varying roughness gradients [69]. Although using different cell types, the findings offer a practical explanation for modulation of the adhesion profile through surface roughness, with both studies observing greater cell viability and proliferation rates through an increased adherence at higher roughness values in substrates such as silicon. Researchers hypothesize that the correlation between FAK phosphorylation and favorable adhesion to a nano-roughened substrate is due to surface contact angle and wettability and thus the interfacial energy of a substrate surface. Fan et al. supported this hypothesis with primary substantia nigra neurons cultured on silicon substrates etched to variable roughness gradients [70]. Through tyrosine hydroxylase (TH) immunostaining and SEM imaging analysis, surfaces with average roughness ranges of 20–30 nm yielded greater cell viability and improved adherence behavior; neuronal cells were able to recognize and migrate based on the magnitude of surface roughness. Cell membrane activity tended towards maximizing interfacial contact area and contact strain between the surface and the cell. In addition to viability control, surface roughness can influence various cellular functions such as inducing neural differentiation pathways and intracellular signaling for functional management.
Several studies investigated the impact of surface roughness on cell-substrate interactions and the mechanism through which stem cells can be induced to differentiate into neural cells. As reported in Pan et al., gene upregulation of ISL1, NeuroD1, and NeuroG1 were observed in evaluating induced pluripotent stem cells seeded onto nano-rough PDMS substrates with RT-PCR, indicating neuronal differentiation after eight days in culture [71]. Increased interfacial energy and adherence profiles allowed neural-induced differentiation by aligning focal adhesion complexes. By providing efficiently aligned anchor points, cytoskeleton and nuclei elongation provided the necessary mechanical tension to alter gene expression through nuclear deformation [36]. In the rearranging of cell nuclear structure, increased expression of the nuclear matrix protein lamin A/C, especially in early differentiation stages, showed a correlation between cellular mechanosensing of nanotopographical cues and epigenetic changes that occur as a cell undergoes differentiation.
Interestingly, the findings presented by Brunetti et al. contradict the prevailing trend of augmented neurogenesis on nano-roughened substrates. Despite this discrepancy, the study highlighted neural cell sensitivity to nanoscale roughness and its importance on FAK activation. Immunohistochemical analysis of Vinculin and Golgi complex of SH-SY5Y cells cultured on nano-roughened gold substrates provided valuable insights into the potential for focal adhesion and cellular viability, respectively. Notably, cellular adhesion and nanoscale roughness were inversely correlated, and roughened substrates demonstrated decreased neuronal polarization and Golgi apparatus functionality through randomized unordered focal adhesion profiles [72]. One plausible explanation for this observation is the adverse impact of nonspecific protein adsorption due to increased substrate wettability. Dysregulated laminin and fibronectin adsorption could result in poor ECM layer formation, impairing neuron adhesion complex functionalization. Hence, careful consideration should be given to the material composition of nano-roughened substrates, as deviations from the general trend of neurogenesis may be due to other factors that alter substrate binding affinity.
Topographical modifications have demonstrated the ability to alter the immune response through microglia and astrocyte formation, which play a vital role in the foundation of immunomodulatory effects of neural immune cells and the development of healthy neural networks. Unlike smooth surfaces, nano-roughened surfaces supporting microglia in vivo and in vitro have exhibited higher induced M2 phagocytic activity indicative of healing mechanism activation [73]. Additionally, higher antioxidant activities, decreased inflammatory markers, and increased anti-inflammatory effects were observed in rough surfaces compared to smoother surface conditions. The inflammatory polarization of primary microglia was observed on smooth substrates compared to roughened groups with cellular adhesion molecule (L1) conjugated to the surface. These modified surfaces activated anti-inflammatory markers such as CD206, CD209, CD163 and the upregulation of Arg-1, Il-10, and TGF B-1 (M2 polarization). Limiting focal adhesion capabilities on a rough surface would demonstrate decreased microglial coverage mediating attachment and spreading microglial cells [74]. Astrocyte interactions determined by topographical roughness are also determined through the mechanical responsiveness of cells concerning topographical roughness. At root mean square roughness (Rq) values of 32 nm, a notable shift in astrocyte behavior towards migratory patterns was observed, accompanied by the reduction in the astrocyte forming factor, evident through discernible morphological changes within cell culture (Figure 4B). Knockdown of mechanosensing pathways that allow for neuron-astrocyte interactions such as Piezo 1, TRPC, and TRPC 6 negated the impact of nanotopography, highlighting the significance of mechanoresponsive receptor expression in neuroglia [75].
Surface roughness also plays an important role when designing neural implants. Traditionally, metals such as gold (Au), platinum (Pt), platinum-iridium (Pt-Ir), and titanium (Ti) are used to fabricate microelectrode arrays for recording electrophysical activity due to their high biocompatibility [76, 77]. Pt, in particular, is regarded as the preferred metal for neural implants owing to its excellent electrochemical stability, impermeability, and corrosion resistance [78]. However, metallic electrodes exhibit certain limitations. The substantial mismatch in Young’s modulus (E) between Pt (154–172 GPa [79]) and neural tissue (0.6–15.2 kPa for human brain [80, 81]) is believed to contribute to tissue inflammation at the implant site, resulting from shear between the metal and surrounding tissue during motion [82]. While the substrate material is commonly identified as the problem, efforts have been directed towards modifying the electrode material to improve neural attachment through increased surface roughness, hence establishing a more favorable interface. Another limitation arises as electrodes are scaled down to match the size of individual neurons, aiming to achieve higher spatial resolution and density. This reduction in size leads to an increase in the charge per unit area that must pass through the electrode for stimulation, and an increase in impedance which negatively impacts recordings [83, 84].
To address both limitations, surface roughening through coatings or modification of the metallic layer has shown promise in enhancing tissue integration and reducing impedance by increasing the electrochemically active area [85–87]. While inorganic coatings like platinum black (Pt-black) [84, 88], titanium nitride (TiN) [89, 90], and iridium oxide (IrOx) [91, 92] have demonstrated improved charge transfer capability and impedance levels, no reports are found on their positive effect on cell attachment or proliferation. In contrast, organic coatings such as CNTs and conductive polymers (e.g., PEDOT) have shown enhanced electrochemical performance and improved neural interfaces, as they can be functionalized to influence biological responses. A comprehensive discussion on organic coatings will be provided in the subsequent sections of this review.
2.3. Substrate Stiffness:
The stiffness of a material surface has considerable influence over intracellular chemical signal production. Neural cells are particularly sensitive to changes in these microenvironmental cues considering their native ECM is comprised of a soft matrix of glycosaminoglycans, proteoglycans, glycoproteins, and low levels of fibrous proteins [93]. The stiffness of brain ECM is 1–3.5kPa, which is substantially softer by several orders of magnitude than most materials used to construct nervous system interfaces [78]. This mechanical discrepancy has been a significant research focus, spurring the development of materials that reduce this mechanical mismatch. Additional work has characterized the cellular mechanisms most impacted by changes in substrate stiffness (Figure 4C). These mechanisms can dictate neurite outgrowth, adhesion quality, synapse formation, and neuroglia behavior at the surface interface which are essential for neuro-regenerative and interfacing applications [94].
The recognition of substrate stiffness plays a pivotal role in modulating essential intracellular signals through the formation of adhesion complexes and activation of FAK. Nonreceptor tyrosine kinase assumes a critical role in mechanosensing, orchestrating integrin and talin interactions between a cell and its microenvironment [95]. FAK complexes have been established as vital mediators in interfacing between cells and the ECM environment, and can respond to changes in microenvironment characteristics including the nano-stiffness of a material. By altering cell adhesion capabilities, several downstream signaling pathways may in turn be activated, subsequently inducing characteristic changes in cell behavior, such as alterations in proliferation rates and differentiation capabilities [96]. Numerous studies have demonstrated the capability of variable substrate stiffness modulating FAK activation in neural cells. Ozgun et al. illustrated this phenomenon in the differentiation of neuroblastomas (SH-SY5Y) across polyacrylamide gels of different stiffness (0.1, 1, and 50 kPa) [97]. The markers p-FAK and Tuj1 indicated neural-like development, which was increased on softer substrates closely resembling natural ECM stiffness. The abundance of p-FAK markers, especially in neuroblastoma culture on soft hydrogels, signifies strengthened focal adhesion and integrin binding interactions, creating strong binding interactions that stimulate neural maturation. FAK activation may also contribute to further downstream pathway signaling, thereby exerting diverse effects on neural cell functionalization. Zhang et al. further elucidated the modulation of FAK activation in PC12 cells over PDMS substrates with varying stiffness to observe cytoskeletal structure, viability, and the influence of matrix stiffness on drug delivery related effects [98]. PDMS stiffness was controlled by altering the ratio of base and curing agent (46.7, 5.3, and 0.1 kPa), creating diverse ECM environments equivalent to collagenous bone, mammary tumor, and adult brain parenchyma, respectively. PC12 cells, when interacting with substrates of varying stiffness, exhibited substantial alterations in neural-like phenotypes including adhesion profile, viability, and cytoskeletal structure. The number and size of actin stress fibers and focal adhesions complexes underwent significant modifications with decreasing stiffness, with the 0.1 kPa substrate promoting the highest state of these adhesion profiles among the cytoskeleton.
The mechanistic effect of neural cell activation on substrate stiffness was explored through RNA transcriptome analysis activation of FAK and related downstream pathways. Through the use of Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) term analysis, researchers observed differentially expressed genes in related categories of cellular and biological processes contributing to the structural realignment of neural cells on softer substrates. Genes such as EGFR, KRAS, SYT6, and PRKCA exhibited differential expression across varying substrate rigidity, indicating signal transducer activity and the activation of significant neural processes. Downstream activation of the EGFR/P13K/AKT pathway was found to be differentially expressed across variable stiffness environments. This signaling pathway, known for its role in regulating neural cell phenotype and cytoskeletal rearrangement following mechanosensing, provides additional insight into the mechanism behind neural cell behavior given substrate topographical cues. Similarly, Saha et al. demonstrated the capability of substrate rigidity to modulate the signaling of neural stem cells and the adhesion capability of focal interfaces as well as the interactions between bsp-RGD - αvβ3 integrin through the interactions of hydrogel substrates [99]. Neural stem cells cultured on moduli most similar to the physiological stiffness of brain tissue (500 Pa) exhibited a heightened level of neuronal marker beta III tubulin, indicating neural differentiation when compared to hydrogels of varying stiffness profiles (100 – 10,000 Pa).
YAP/TAZ is a master regulator for mechanotransduction and serves as a downstream body in the neural signal propagation from several membrane bound protein signaling pathways including FAK and RhoA, acting to provide nuclear response to substantial stimuli [100]. Regulation of several cell functions can be modulated through this body including cellular homeostasis, proliferation, and cellular differentiation [101, 102]. In response to microenvironment stiffness, YAP/TAZ can be modulated to influence transcriptional machinery which is done through Hippo signaling pathway including the RhoA and LATS1/2 signaling bodies [103]. A mechanical stimulus from a substrate interface may then phosphorylate YAP/TAZ, leading to transduction into the nucleus for phenotypic modification. Particularly in softer substrates, increased focal adhesion may increase upstream RAP2 signaling which in turn influences activity of RhoA, MST1/2, and LATS1/2 to modulate YAP/TAZ. In addition to being regulated primarily through RhoA, YAP/TAZ signaling may also be influenced by various other bodies responding to substrate interfacing [104]. Regulation of this transcriptional effector has demonstrated key control in modulating stem cell fate and influencing neural lineages through substrate interfacial stimuli. Musah et al. reported on the regulation of YAP over hydrogel substrates of variable stiffnesses 0.7–10 kPa, highlighting its impact on stem cell pluripotency and neural-like differentiation [105]. Notably, the formation of focal adhesions along stiff hydrogels promoted long-term stem cell renewal over a 2-week period. Conversely, on compliant soft surfaces, increased f-actin adhesion activity and subsequent depletion of YAP increased transcriptional activity, pushing greater differentiation into neural-like lineages. Inhibiting YAP activity, expedited and enhanced differentiation into neural-like lineages more efficiently than conventional differentiation methods. Differentiation was characterized through differential gene expression of NEUROG2, NEUROD1, SLC17A6, and analysis of electrophysiological functional attributes, revealing traces of spontaneous postsynaptic currents along differentiated cells. Similarly, Engler et al. observed the positive influence of collagen-coated substrates with modulus mimicking brain tissue (Ebrain ∼ 0.1–1 kPa) on the ability of MSCs to differentiate towards neural lineages [106]. Activation of adhesion complexes drove YAP signaling modulation, allowing for the regulation of neural-like development through substrate-cell interfacing. The capability to promote neural cell activity based on YAP/TAZ signaling activity and cell stiffness has allowed for several innovative approaches in neural tissue engineering. Biomedical applications could benefit from the control over YAP/TAZ signaling, particularly for neural systems where the response of cells to mechanical stimuli, such as stiffness, can have significant control over this signaling system and offer a consideration towards substrate design in influencing neural cell behavior.
Neuroglial cells are significantly impacted by substrate stiffness. A study conducted by Blaschke et al. evaluated the functional effects of microglia cultured on surfaces of varying stiffness [107]. Primary rat microglia were grown on PDMS substrates with stiffnesses similar to physiological (0.6 – 1.0 kPa) and supraphysiological (1.2 MPa) values. Soft substrates led to increased proliferative capacity of microglia and M2 polarization, as seen by increased BrdU uptake and increased CD206 expression, respectively. To elucidate response mechanisms to substrate stiffness, microglia were treated with DID to block stretch-dependent chloride channels, which have been shown to control microglial activation. CD206 expression was completely inhibited, indicating that stretch-dependent chloride channels are a primary mechanism in which soft substrates trigger microglia’s anti-inflammatory state. Furthermore, a study conducted by Hu et al. investigated the impact of matrix stiffness on astrocyte phenotype, specifically in regards to scar formation [108]. Notably, astrocytes in the soft matrix had upregulated GFAP and IL-1β expression, suggesting that matrix stiffness mediates astrocyte phenotype and activation. More specifically, matrix softening activated astrocytes into their reactive phenotype, which leads to the formation of glial scars. On the other hand, matrix stiffening reverts astrogliosis, triggering the astrocytes to revert back to their native phenotype. Mechanistic studies revealed that these phenotype shifts in response to matrix stiffness are mediated by YAP activity. Specifically, a decrease in YAP activity is associated with reactive astrogliosis along with an upregulation of GFAP. As illustrated by these studies, substrate stiffness should be a significant consideration when designing interfacing biomaterials due to the complex influence this characteristic has on glial cell binding, neural proliferation, and synaptic strength.
For penetrating neural interfaces that disrupt healthy tissue during implantation to the target region, an ongoing debate exists on probe material stiffness. The implanted probe, or foreign body, elicits an immune response cascade and formation of a surrounding glial scar sheath [109]. This scar increases the distance between the electrode sites and the neuronal processes, degrading achievable SNR for electrophysiological recordings. Traditional rigid penetrating materials such as silicon, glass, or carbon fiber have been shown to exacerbate the immune response at the targeted site [110]. Recent reports suggest that the use of ‘soft’ and flexible probe materials with reduced Young’s modulus (< 10 GPa) can mitigate the severity and size of glial encapsulation post-surgery, enabling long-term interface stability [111–113]. The mechanical trauma and large difference in mechanical stiffness between the implant and tissue can greatly impact the ability to record from neurons near the device-tissue interface over time [9, 114]. With advancements in thin film fabrication techniques such as MEMS, flexible polymeric probes with integrated MEAs have been developed, commonly utilizing polyimide and poly(p-xylylene) (Parylene C) as backbone materials.
Parylene C, a widely used bioinert thermoplastic polymer, has found extensive applications as a tissue-engineering scaffold and as an electrically insulating coating and substrate for neural stimulation and recording applications, including an investigational device used in clinical trials [115–117]. While Parylene can be used as a coating on solid and rigid electrodes, flexible microfabricated Parylene C-based MEAs have gained popularity for invasive stimulation and recording applications in the nervous system. This popularity stems from its apparent reduction of neural immune cell binding and favorable adhesion properties for neuron binding in vitro [118]. In vivo, the performance of the material becomes more complex as invasive devices penetrate through healthy tissue, triggering immune response. Lo et al. suggested that long term gliosis resulting from mechanical trauma and stiffness differences could be mitigated by smaller, more flexible Parylene C-based MEAs, although their study involved only sham devices without electrodes [119]. Other studies have demonstrated acute and chronic implantation of functional Parylene C MEAs. One study demonstrated a decrease in neural loss in targeted implantation area from 40% to 12–17% when comparing rigid silicon probes with soft Parylene C probes [26]. Similar to the findings of Lo et al., safe implantation of mechanically compliant probes often requires the use of stiff introducer or biodegradable polymer coating [111, 113, 120]. The ability to apply polymeric coatings on top of Parylene substrates opens avenues for further modification, such as a drug delivery vector or composite for more nuanced interfacing dynamics [121, 122].
Poly(ethylene glycol) (PEG) derivatives have been explored extensively beyond tissue interfacing applications. Standalone PEG systems are stiffer than neural tissue (~2 MPa) and have not demonstrated significant promise in vivo as a viable solution to maximizing tissue integration and decreasing adverse neuroglial reactions [123, 124]. PEG-based hydrogels have been developed to improve mechanical properties of PEG. PEGDA (PEG-diacrylate) is formed when the PEG chain ends are functionalized with acrylate groups and crosslinked to create a covalently linked hydrogel network. PEGDA stiffness down to ~300 Pa can be achieved by the soluble implementation of alloc-presenting monomers; through the alteration of the allyl-to-acrylate molar ratio, the reduced stiffness PEGDA falls within the range of neural tissue to significantly improve neural cell growth and neurite extension [125].
Hydrogels consist of a 3D network of hydrophilic polymer chains and can be derived from both synthetic and natural materials. They have tunable structural, chemical, and mechanical properties, and strong biocompatibility depending on the specific material [126–128]. This versatility allows hydrogels to be modified, and as a result, they are commonly used to interface with the CNS. Hydrogel polymerization is oftentimes leveraged as a step to incorporate electrochemical elements without compromising the mechanical characteristics of the scaffold. The blending of hydrogel polymers around electroactive elements creates interpenetrating polymer networks (IPNs) that provide enhancements to hydrogel electrochemical properties while typically preserving interface biocompatibility [129, 130]. Unique instances of polymer blends that include crosslinked and linear chains may entangle to form semi-IPNs which, in the case of conductive polymers, leads to the synthesis of conductive polymer hydrogels (CPHs) [131, 132]. In other cases, electrochemical composite hydrogels may be synthesized by physically suspending conductive elements such as nanoparticles, nanotubes, etc. in a percolation network to enhance electrical conductivity [133–135]. This methodology can be applied across a wide variety of conductive fillers while preserving the primary advantages of hydrogel-based interfaces including the reduced reactive tissue recruitment caused by interface mechanical mismatch.
Matrigel scaffolds have also been utilized as a cell binding membrane for neural interfacing applications including regenerative scaffolds and microelectrode coatings. Impressive neuro-proliferative effects have been observed using Matrigel as a basement membrane for growth in vitro [136]. Shen et al. also demonstrated that Matrigel-based composite microelectrode coatings could serve as a viable interfacing material for stimulation and recording while diminishing glial cell response. Matrigel-COL1 composite coatings were able to decrease adjacent GFAP, Tau1, NF, and CS56 staining while increasing localized NeuN+ signal [137]. While the mechanical and neurogenic properties of Matrigel lend themselves towards promising scaffold systems in the CNS, its chemical composition warrants further consideration. Since Matrigel is an ECM excretion from Englebreth-Holm-Swarm tumors in mice, the reconstituted basement membrane will have innate composition variability [138]. Although most proteins within the Matrigel ECM are structurally relevant (laminin, COL IV, enactin), there are detectable levels of intracellular proteins that will inevitably interact with interfacing cells in vitro or in vivo depending on the experimental application. Therefore, the experimental results gathered from Matrigel-based interfacing studies should be approached with caution due to the variability guaranteed by its composition heterogeneity [139].
Despite significant progress in developing various designs for ‘soft’ penetrating probes, several challenges still need to be addressed to establish long-term, stable BMI. For a more comprehensive discussion on flexible penetrating neural interfaces and the challenges associated with their fabrication and implantation, the reader is referred to the reviews by Weltman et al. and Thielen et al., respectively [109, 110].
2.4. Porous Substrates:
The porosity of a substrate also impacts the differentiation and proliferation of cells. Overall porosity and individual pore size can be controlled and their impact on various neural cell types, including neurons, oligodendrocytes, and astrocytes have been studied [140, 141] (Figure 5A). Porous substrates offer a three-dimensional (3D) microenvironment that mimics the ECM, thereby providing structural support, facilitating nutrient transport, and promoting cell-to-cell interactions. While hydrogels have primarily been the focus of porosity related research due to their inherent porosity, porous scaffolds and microporous membranes fabricated from polymers, silks, glass, and metals have also shown great promise. We refer readers to the reviews on material selection and fabrication for porous interfaces from Maksoud et al. and Wen et al. [142, 143]. In addition, comprehensive gene analysis has been applied to determine the specific porosity-dependent pathways that impact cellular behavior. These endeavors enable researchers to design materials with a modified porosity to enhance neural cell interactions with the substrate and desired outcomes.
Figure 5.
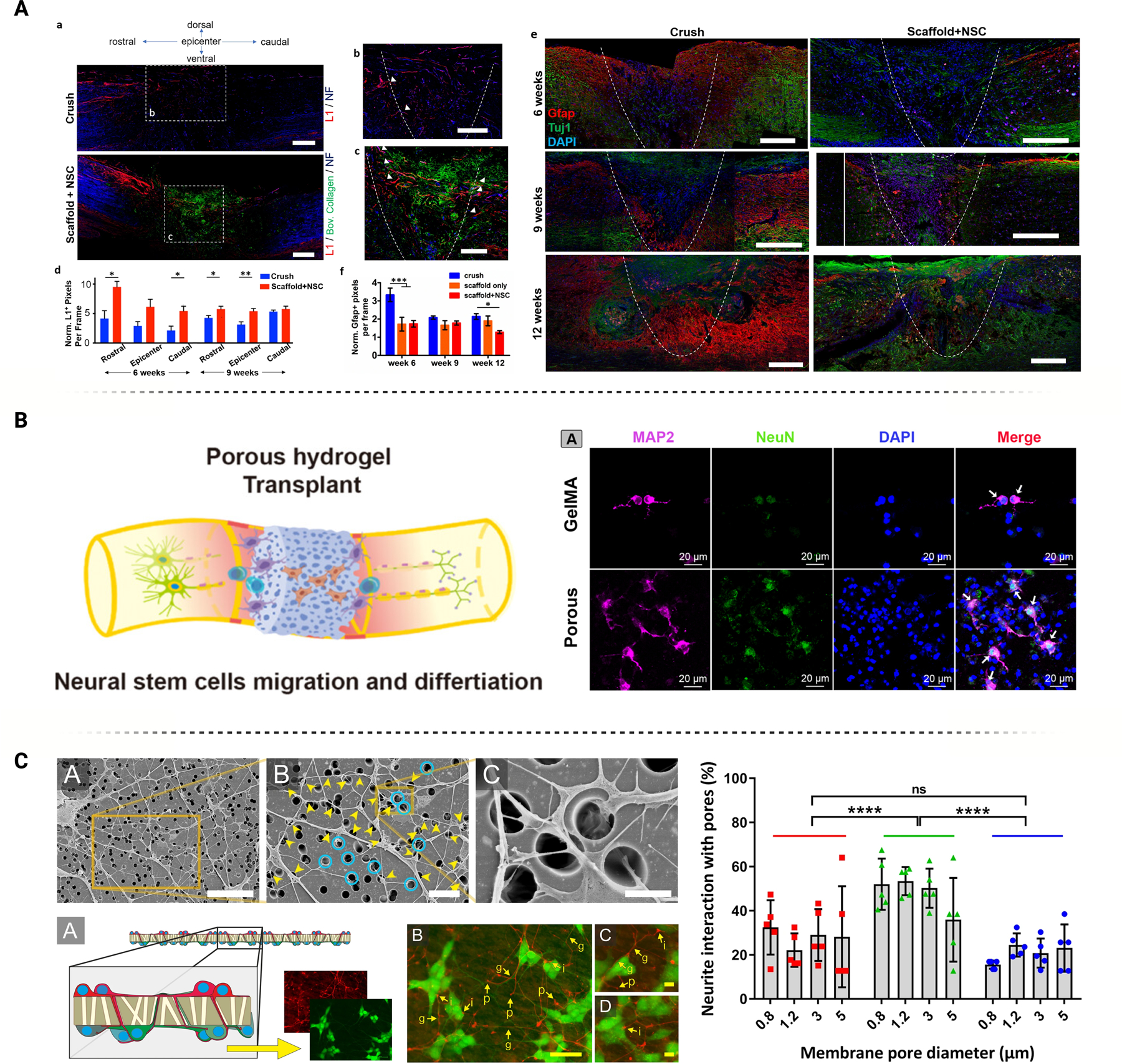
Porosity, including individual pore size and overall material porosity, can impact neural behavior in a variety of different ways. (A) Porous collagen scaffolds deliver neural stem cells to lesion sites of spinal cord injuries, improving axonal elongation and reducing astrogliosis. Figure adapted from Kourgiantaki et al. [121] under Creative Commons License (CC BY 3.0 or 4.0). (B) GelMA hydrogel with inner connective pores enables cell infiltration, in turn promoting NSC differentiation. Figure adapted from Shi et al. [58] with permission. (C) Neurons can interact with the pores in many ways, including entering the pore itself, crossing over it, or skirting around the edge. Pore diameter can impact these interactions. Figure adapted from George et al. [21] under Creative Commons License (CC BY 3.0 or 4.0).
Many studies investigated the effect of hydrogel porosity on neural cell differentiation and maturation. Shi et al. compared the behavior of NSCs encapsulated in porous and non-porous GelMA hydrogels. In vitro testing revealed that the NSCs in the porous hydrogel migrated further, indicating better cellular infiltration, and exhibited higher viability than in the non-porous counterpart. The porous hydrogel also promoted better differentiation of the NSCs into mature neurons, as seen by the upregulated levels of NeuN and MAP2 (Figure 5B). A deeper analysis revealed that these NSCs specifically differentiated into mature motor neurons given the upregulation of motor neuron progenitor genes, ISLET1, MAP2, and HB9. In vivo implantation of porous hydrogels into rat SCI models demonstrated better functional recovery, diminished inflammatory response, and reduced systematic apoptosis. The porous hydrogel also improved endogenous NSC activation and proliferation while enhancing neurogenesis and neuronal differentiation. Notably, the porous hydrogel had a faster degradation rate than the non-porous version. This degradation may result from a enahnced material exchange rate across the pores or the loss of the agent responsible for inner pore foaming. While faster degradation may be considered a disadvantage for specific applications, improved neuronal differentiation and maturation can be achieved. In fact, enhanced formation of cell lineage and faster hydrogel degradation may signify an advantage to promote the complete reintegration of new tissue [68].
In addition to regenerative applications, porous hydrogels have been used to study the intricacies of neural circuits. Yan et al. developed highly porous and biocompatible hydrogels that effectively modeled highly complex three-dimensional neural networks. A polyacrylic acid/polyvinyl alcohol/polyethylene glycol (PAA/PVA/PEG) hydrogel was designed to serve as an in vitro model that would mimic brain tissue and support neuronal growth. Interestingly, low expressions of GFAP revealed that the hydrogel was astrocyte-resistant, likely due to the highly hydrophilic nature of the hydrogel. However, Tuj1 staining and SEM imaging demonstrated that the porous hydrogel enabled adhesion and differentiation of the neurons and promoted neurite outgrowth and the formation of interactive and complex 3D networks. The biological validity of these neuronal networks was confirmed through recording of spontaneous action potentials and optogenetic stimulation, resulting in frequency-dependent electrical spikes. These results suggest that this porous hydrogel can be used as a model for functional 3D neural network activity, however the lack of neuroglia recruitment limits in vivo translatability [144].
Pore size is a critical factor that can significantly influence cellular behavior. Li et al. conducted a thorough study to determine how different pore sizes affected the differentiation of neural cells, including neurons, oligodendrocytes, and astrocytes, using methacrylamide chitosan (MAC) mixed with porogen D-mannitol. To evaluate neural progenitor stem cell (NPSC) differentiation, the hydrogels were cultured in cell-specific differentiation media. Beta III tubulin, RIP, and GFAP IHC confirmed lineage-specific differentiation. Interestingly, while the porous groups produced significantly higher counts of desired cell types than control groups, there was no significant difference in population proportion for different pore sizes (Figure 5C). Additionally, the total cell count decreased with increased porosity, which may indicate that the pores limit NPSC proliferation while promoting differentiation [145].
To elucidate the mechanisms behind enhanced differentiation, Li et al. investigated oxygen diffusion rates in hydrogels of different pore sizes. The results demonstrated that higher porosity was associated with faster oxygen diffusion, creating a more favorable microenvironment that increased cell survival and differentiation. Overall, this study further demonstrated that porous networks improve not only neurogenesis, but also oligodendrogenesis and astrogenesis. However, there was no significant correlation between pore size and neural differentiation [145]. The limited range of pore sizes used (4060 ± 160 to 7600 ± 1550 µm2) may explain this observation. A wider range of pore sizes is likely necessary to observe significant differences in cellular behavior. When using hydrogels for nerve tissue engineering, porosity and pore size can impact cell migration. Specifically, pores with diameters less than 2 µm can inhibit migration. Smaller pores also restrict diffusion, limiting cell survival and differentiation [145, 146]. Another study by Nguyen et al. investigated the anti-inflammatory effects of porous hydrogels on murine BV2 microglia. Specifically, they created hydrogels composed of fucoidan, sodium alginate, and gelatin (SaGFu) with pore diameters varying from 60 – 100 μm and observed that larger pore size better supported microglial growth. Additionally, the anti-inflammatory effect of these hydrogels was determined by measuring the release of several bioactive substances, namely NO, PGE2, and ROS, following microglial activation by LPS stimulation. Results demonstrated that the SaGFu hydrogels could effectively inhibit the production of these inflammatory substances, with the most porous hydrogels having the most significant inhibitory effect. Mechanistic analysis revealed inhibition of NF-κB p65 activation and translocation into the nucleus, which plays a vital role in inflammation and in many neural diseases and disorders [147].
Hydrogel substrates and coatings also have unique drug delivery capabilities, avoiding the complexity of incorporating engineered micro/nanofluidic channels. Porous materials, such as hydrogels, offer an advantage when designing BMI applications and devices, as anti-inflammatory agents can be embedded into the matrix to enhance the tissue-neural interface by reducing the tissue immune response at the implant site. For instance, Huang et al. proposed a novel aerosol jet printing technique to accurately deploy anti-inflammatory nanogels on a flexible polyimide neural probe, constituting a 3D nanocarrier-based interface [148]. The coating, composed of amphiphilic silicone-modified chitosan and natural antioxidant OPC agent, mimicked the physical properties of brain, which alleviated tissue edema at acute phase. Furthermore, this 3D nanocarrier-based membrane reduced tissue trauma in the chronic stages, which decreased the population of activated glia and astrocytes at the implant site and prolonged neuronal survival by 28 days.
Aside from hydrogels, other scaffolds can be modified to control neural differentiation and proliferation, including synthetic sources like poly-l-lactic acid (PLLA) or natural materials such as collagen to mimic the composition of native ECM [149, 150]. Yuan et al. designed double-layer collagen membranes to treat spinal cord injuries. Unequal pore sizes were incorporated to maximize cell adhesion area within the inner compartment (100 μm) and minimize scar tissue formation on the outer contact layer (10 μm). NSC-seeded scaffolds implanted into rat SCI models displayed significantly improved functional recovery four weeks post-op [151]. Furthermore, Ganguly et al. studied how a nanoporous substrate can attenuate the astrocytic response after brain electrode implantation, which often results in a glial scar. Specifically, they cultured rat cortical neurons on anodic aluminum oxide (AAO) surfaces with varying pore sizes (nonporous control, small pore surface with an average pore size of 21.1 ± 2.3 nm, and a large pore surface with an average pore size of 90.3 ± 3.5 nm). Viability assays revealed that the small pore surfaces promoted adhesion and survival of the astrocytes, while the large pore surfaces negatively impacted viability. Focal adhesion number and distribution were evaluated using EGFP fluorescence, suggesting that surfaces with small and no pores (control) had similar numbers of focal adhesions, whereas surfaces with larger pores exhibited significantly more focal adhesions. Additionally, the small pore case had more peripheral focal adhesions than central adhesions, which suggests that peripheral adhesions are responsible for increased cell adhesion. Conversely, the large pore case had more central focal adhesions and the nonporous control had an equal distribution of peripheral and central focal adhesions. These results suggest that astrocytes prefer surfaces with smaller pores rather than larger pores as seen by their improved viability and adhesion [152]. This contrasts with neurons and microglia, which have been observed to exhibit improved behavior on surfaces with larger pores [146, 147]. Given that neural networks rely on the interconnected behavior between these neural cells, the pore size of any therapeutic interface must be carefully evaluated to determine a size that will effectively modulate neural behavior overall, rather than focusing on one specific cell type.
Substantial progress has been made to identify the cellular pathways and mechanisms enhanced by porous substrates. Jin et al. investigated the differences between porous and permeable membranes for human embryonic stem cell (hESC) growth and differentiation. Through a global gene expression profiling analysis, the study revealed that hESCs grown on porous membranes exhibited upregulation of several ECM genes (collagen type XI ɑ1, laminin ɑ3 and ɣ1, and catenin ɑ1, δ1, and β1), integrin, and collagen genes (integrin β1, β5, and ɑV, and collagen type XII ɑ1, type XVI ɑ1, and type XI ɑ1). The heightened expression of cadherin-1 and connective tissue growth factors suggested an upregulation of cell–cell interactions. Furthermore, matrix metallopeptidase (MMP) family proteins along with CD44 were also increased, suggesting enhanced cell–cell and cell–ECM interactions. The upregulation of catenin, collagen, and integrin genes indicated the initiation of Wnt signaling pathway by chemical and topographical properties of the porous membrane substrates. This was corroborated by the detection of translocation of β-catenin into the nucleus along with the upregulation of MMP proteins, which are directly activated by Wnt/β-catenin signaling [153].
Porous substrates were combined with micropillars to create surface morphologies resembling ECM. Wei et al. introduced a nanocomposite structure consisting of biocompatible copolymer poly-lactic-co-glycolic acid (PLGA) nanofibers on PDMS micropillars. The authors employed conventional microfabrication to produce PDMS slabs followed by electrospinning of PLGA nanofibers. The nanocomposite surface was visually evaluated after a 48-h culture of human glioblastoma cells, revealing improved cell morphology compared to the control group cultured on PDMS pillars alone. Moreover, astrocyte proliferation on the nanocomposite exhibited cell morphologies similar to those observed in vivo, as opposed to the control group cultured on flat PDMS. The results were further evaluated through calcium imaging, which demonstrated higher signal amplitudes in primary hippocampal neurons cultured on the nanocomposite substrate (0.038 ± 0.004 DF/F) compared to those on the PDMS control (0.016 ± 0.001 DF/F) [154].
3.0. Synthetic and Biological-Based Substrate Chemistry:
Apart from surface geometry and profile, surface chemistry of topographically modified substrates can play an important role in influencing cell behavior and fate. Cell-to-substrate interactions can drive the success or failure of the intended function of the interfacing material. For instance, a regenerative tissue approach may require an interfacing material with cell-recognizable surface chemistries to enhance cellular proliferation while discouraging inflammatory behavior. In brain stimulation and recording, more complex surface chemistries must be implemented to maximize the functional efficacy of the device interfacing with biological tissue. The significant range of applications that involves direct contact between neural tissue and the foreign body increases the opportunity for novel interface nanomaterial development.
Typically, neural tissue health must be maintained regardless of other specifications required of the interfacing materials. Optimal tissue health may be difficult to achieve based on the types of materials chosen for neural cell interfacing. With a growing interest in nanomaterial coatings and composite-based scaffoldings, it is important to classify and organize the families of materials being studied and their effects on downstream signaling in native neuron populations. Due to the invasive nature of BMI applications, it is also crucial to consider neuroglia recruitment towards these interfaces and how their behavior influences overall tissue health. High-resolution transcriptomic analysis in recent years has driven deeper understanding of these downstream effects resulting from substrate interfacing dynamics. These substrates and scaffolds include carbon-based, polymer-based, and biologically-inspired materials that all uniquely influence neural cell interactions at the tissue interface (Figure 6). The transcriptomic analysis of these interactions has been beneficial in elucidating the cellular mechanisms activated within the scope of individual studies. However, a complete review of these findings may provide a comprehensive insight into the effects of interface material chemistry and neural cell behavior.
Figure 6.
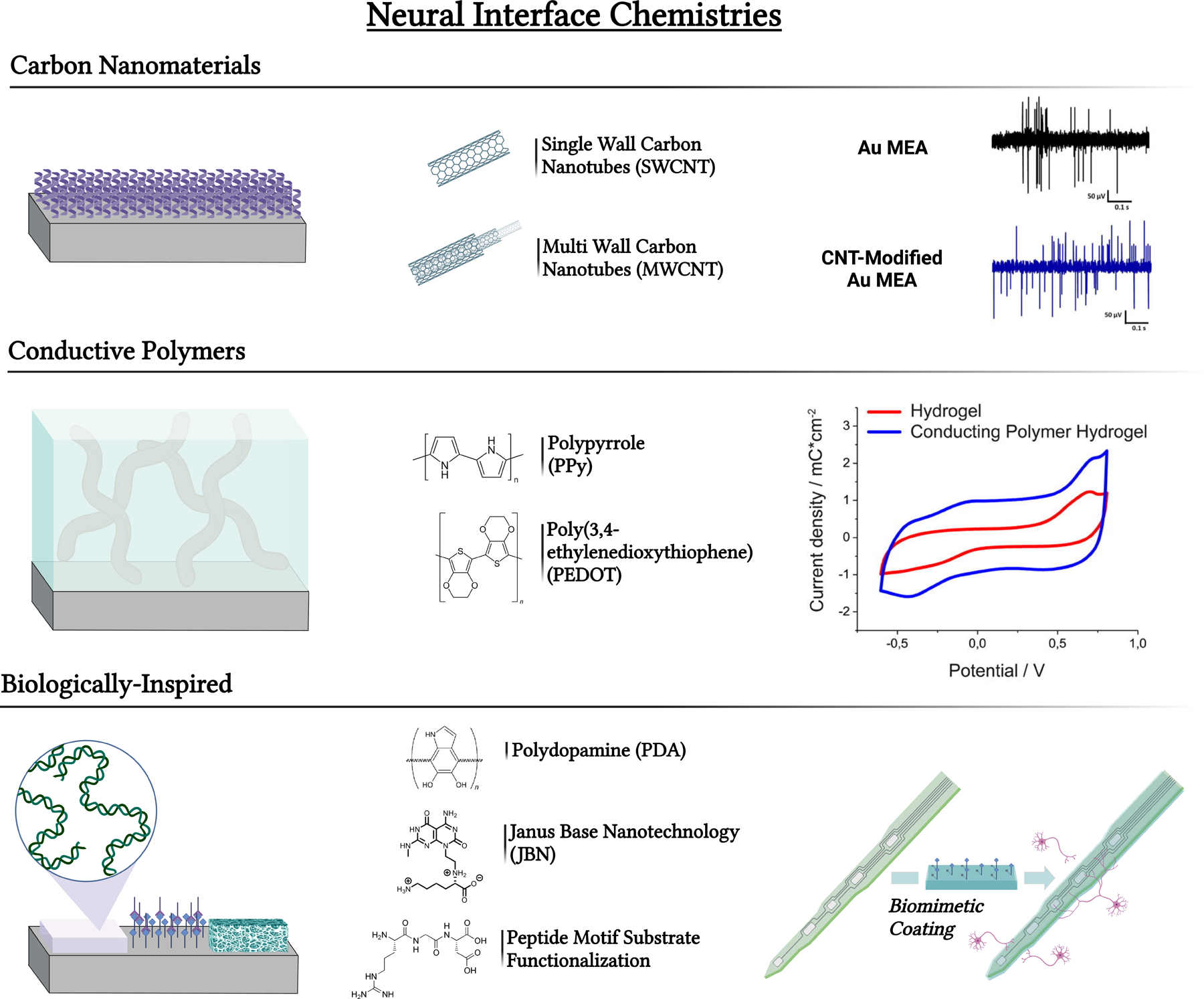
Common neural interface chemistries can impact therapeutic outcomes depending on conductivity, bioactivity, toxicity, etc. Chemical composition alone can influence cell behavior which can further impact the therapeutic success of the interface. Common interface chemistries include carbon nanomaterials, conductive polymers, and biologically inspired materials. Figures adapted with permission from Vafaiee et al. and Kleber et al., respectively [132, 155].
3.1. Carbon Nanomaterials/Coatings:
Carbon nanomaterials, particularly carbon nanotubes (CNTs), have gained significant attention for their favorable properties, including high surface aspect ratio, robust strength, and high electrical conductivity. In addition, their tunable surface chemistry allows for functional modifications at tissue interfaces. However, recent work has documented the disparity in experimental results regarding the cytotoxicity of carbon nanomaterials, including impurities and long-term oxidative stress. Therefore, it is essential to consider the acute and chronic implications of carbon nanomaterial interactions at bio-interfaces, including the inevitable internalization of these materials through natural processes such as endocytosis or pinocytosis. Especially for chronic-term interfacing, the integration or internalization of carbon nanomaterials require additional consideration due to their lack of degradability, leading to the formation of reactive oxygen species (ROS) and natural inflammatory responses [156, 157]. Therefore, the beneficial signaling interactions between carbon nanomaterial interfaces and neural cells should be closely characterized and the cautions associated with acute and chronic phase interfacing be elucidated.
A few studies investigated the cellular mechanisms during neural cell interfacing with CNT surfaces due to their positive influence on proliferation and differentiation. Shao et al. performed RNAseq on NSCs grown on CNT nanocomposites composed of multi-walled CNTs (MWCNTs) and poly(dimethyldiallylammonium chloride) (PDDA). In vitro IHC and electrochemical analysis demonstrated significant neurite outgrowth and electrophysiological maturation (Figure 7A). Differentially expressed gene analysis was performed on enriched biological processes, cellular components, and molecular functions to elucidate the mechanisms governing this response. Adhesion-mediated processes were significantly upregulated including cell-substrate, cell-matrix, and cell-cell interactions. Differential gene expression indicated that integrin-mediated adhesion was a primary actuator for increased binding activity. Increased binding affinity to modified substrates typically increased membrane stress caused by cell-substrate mediated junctions [158]. The implication of integrin involvement is unsurprising, as multiple studies have discussed the importance of integrin-mediated adhesion on cellular development and maturation, particularly with nanoscale-modified substrates. With respect to non-bonding interactions, Mondal et al. recently characterized interfacing mechanics involved in CNT binding with adhesion proteins secreted from canine-induced pluripotent stem cells, revealing multiple non-bonding interactions such as hydrogen bonding, salt-bridge, π-cation, and π-π stacking interactions [159]. Cytoskeletal reorganization was observed following CNT binding which manifested actin-filament-based movement and cell contraction. This filament-based reorganization was also responsible for biological processes induced by CNT composite binding, including the myelination processes during NSC differentiation. Neuron ensheathment, myelination, and myelin maintenance were differentiation expressed in gene ontology (GO) analysis. Gap junction activity was also significantly regulated compared to the control, demonstrating the enhanced cell-to-cell activity encouraged by CNT binding. Apart from the specialized binding sites encouraged by CNTs, upregulated matrix secretion activity was also apparent based on GO term analysis. These results agree with previous studies that found significant upregulation of cell-secreted adhesion proteins induced by carbon nanomaterial binding [160]. These results demonstrate a synergistic benefit of synthetic scaffolding that encourages the natural secretion of adhesion-mediating proteins such as laminin, fibronectin, and collagen [160].
Figure 7.
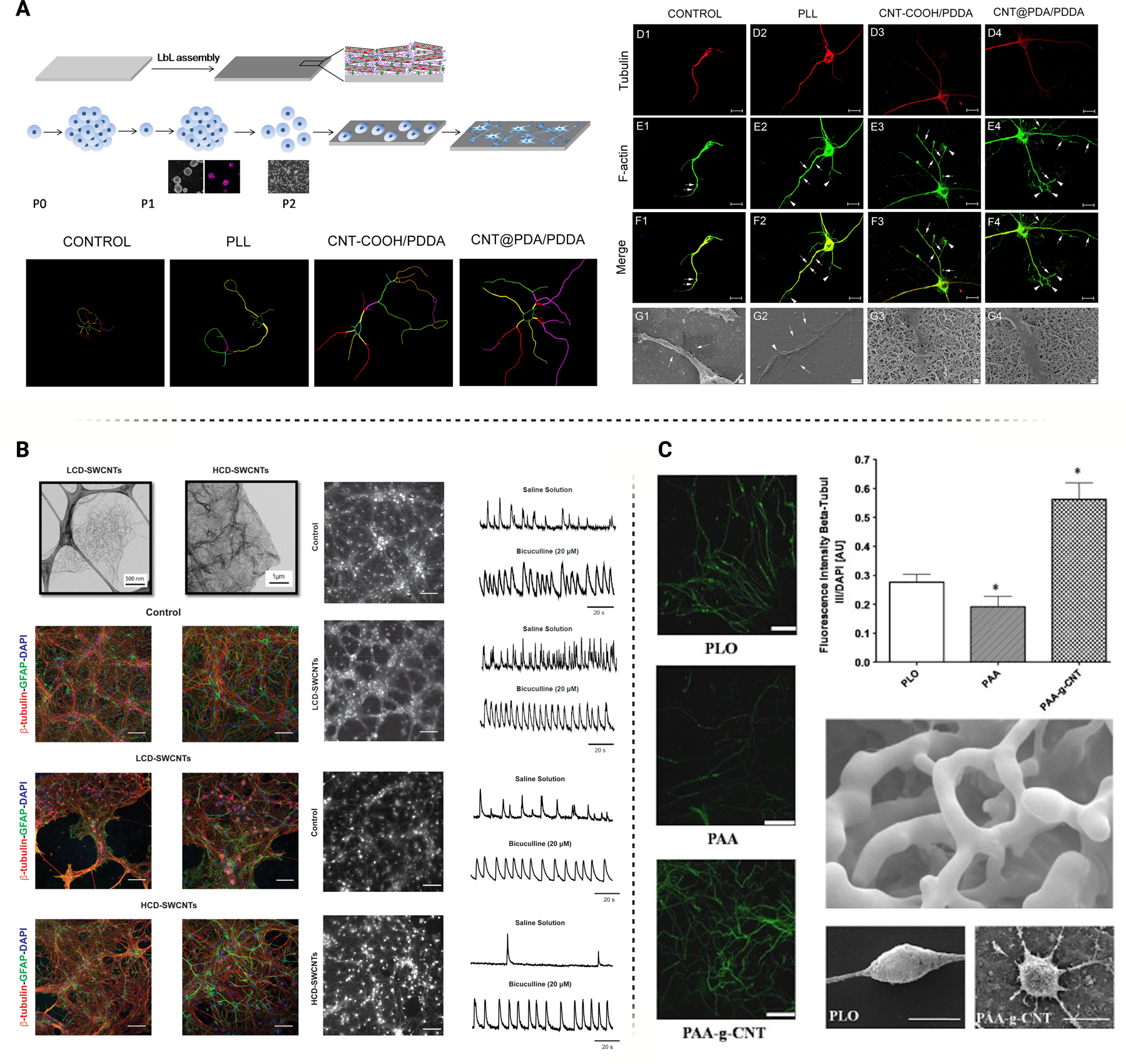
A) Neural stem cell behavior may be actuated through interfacing with CNT composite scaffolds, improving adhesion, viability, neurite outgrowth, and differentiation. Figure adapted with permission from Shao et al. [158] B) Standalone CNT films can impact the development of neural networks and augment signal transduction. The degree of crosslinking between CNTs also impact the strength of the neural circuitry. Figure adapted from Barrejòn et al. with permission [164] C) Poly(acrylic acid) modified with CNTs demonstrate similar improvements in neural viability and neurite outgrowth. Figure adapted with permission from Chao et al. [165]
KEGG analysis can highlight crucial signaling regulatory processes that mediate the communication between binding dynamics and cellular response, like proliferation, differentiation, and cell fate. Recent studies investigated the involvement of FAK as a significant regulatory gateway for downstream signaling during neural cell development. FAK is a non-receptor tyrosine kinase highly expressed in mammalian cells and its innate activity regulates neurite outgrowth and synaptic plasticity. FAK activation also leads to Src binding, which results from integrin-induced signal transduction [161]. Since the proliferative effects of CNT substrates are mainly classified as an integrin-dependent process, FAK activation becomes a significant contributor to the downstream activation of cytoskeletal rearrangement and differentiation pathways like MAPK/ERK, PI3K/AKT, and WNT. The crosstalk between these downstream signaling pathways and FAK activation is well documented, and these differentiation pathways can induce neurite outgrowth, neural excitability, synaptic plasticity, and neural maturation [162–165] (Figure 7B and 7C). FAK is also a known regulator of the Rho GTPase family, a group of guanine nucleotide-binding proteins functioning as binary molecular switches that regulate cell growth cycle, cytokinesis, and cell-cell/cell-matrix interactions. The Rho GTPase family, particularly RhoA, Rac1, and Cdc42, can augment many cellular processes, particularly neural cell development and maturation [166, 167]. CNT’s ability to increase binding affinity with neural cells encourages innate bioactivity partially due to the stimulation of FAK through integrin-mediated adhesion and the natural secretion of adhesion proteins.
The chemistry of carbon nanomaterials must be distinguished from the effects of topographically modified substrates. Chen et al. demonstrated that carboxylated MWCNTs alone could direct neural differentiation in human bone marrow mesenchymal stem cells (hBMMSCs) without exogenous neurogenic factor stimulation. The proposed underlying mechanism driven by MWCNTs involved increased synaptophysin expression as early as eight days after MWCNT interfacing, further promoting endogenous neural protein expression. Synaptophysin is an integral membrane-bound glycoprotein in presynaptic vesicles and is crucial for neural network formation during the early stages of differentiation [168]. Early synaptophysin expression will facilitate adjacent neuron connectivity and increase cell-to-cell signaling during network maturation. Further gene expression analysis found a significant upregulation of BDNF, IGF1, NGF, PAX6, SOX9, and MASH1. The simultaneous upregulation of SOX2 and downregulation of VEGF, FGFR, and FGF2 suggested an inhibitory mechanism for restricting chondrocyte or osteocyte differentiation. This inhibitory mechanism was also seen in hMSC behavior following CNT interfacing. Studies have shown that the SMAD1-dependent BMP signaling pathway is suppressed after interfacing with carboxylated SWCNTs, which may further explain the signaling mechanisms at play during CNT-driven neural differentiation [169]. Absorption testing has also found that neurogenic factors like BDNF, IGF1, NGF, and GDNF were all bound to MWCNT following in vitro culture [168, 170]. This suggests the involvement of a positive feedback mechanism in which endogenous production of neurogenic growth factors is easily trapped to MWCNT after differentiation is induced, which further enhances receptor tyrosine kinase pathways such as RAS or PI3K/AKT. Additional literature confirms that CNTs possess the physicochemical properties to allow for protein absorption and the large surface area of CNT scaffolds can act as an efficient substrate for endogenous protein binding [171].
While CNTs increase function-specific adhesion, such as induced by synaptophysin expression, the electrochemical properties of carbon nanomaterials significantly affect electrical signaling in mature and immature neurons [172]. This affinity for enhancing neural signaling provides a perspective into complex direct and indirect mechanisms deployed during CNT integration and proliferation. Fabbro et al. investigated the passive electrochemical function of rat spinal neurons interfaced with MWCNTs, observing a 37% membrane capacitance decrease corresponding to a 12% decrease in soma diameter after eight days of in vitro culture. Voltage clamps examined the expression of voltage-gated ion channels at eight days, which showed an 87% increase in MWCNT-interfacing neurons [173]. Additional RNA sequencing analysis revealed significantly upregulated gene ontology groups such as cell chemotaxis, proliferation, and differentiation. NEDD4, which facilitates the degradation of proteins including Na+ voltage-gated ion channels, was downregulated after interfacing with MWCNTs. Cellot et al. provided a more in-depth perspective of signal propagation behavior on hippocampal neurons interfacing with single-wall or multi-wall CNTs. Through single-cell current-clamp recording techniques, neurons interfacing with CNTs were found to elicit a higher frequency of current backpropagation into distal dendrites during standard signal propagation down the length of the axon. Increased signal backpropagation led to a significantly higher intracellular concentration of Ca2+ through calcium electrogenesis [174]. The observed after-depolarization potential (ADP) was confirmed using Ca2+ blockers that abolished dendritic signal backpropagation. Cellot et al. reproduced these studies on indium tin oxide (ITO) and RADA16 substrates to emulate other factors that may elicit this unique electrochemical response, like material conductivity and nano roughness. This signal backpropagation behavior was not seen, which suggests that this behavior is CNT-specific. It is proposed that a direct resistive/capacitive coupling between neurons and fast repetitive voltage signals generated by neurons is enhanced at areas of discontinuous CNT-membrane contacts, reinforcing signaling especially at distal dendrites [174]. Further work by Cellot et al. demonstrated how hippocampal neurons might interpret their substrate and alter synaptic activity once bound to CNT scaffolding. Interfacing with CNT scaffolding significantly increased the colocalization of VGAT and g2 at synapse interaction, indicating functional GABAA innervation throughout the developing neural network in vitro. Interestingly, typical GABAA receptor phenomena, such as short-term synaptic depression, was nearly eliminated in CNT-bound neurons and was reversed in the presence of tetrodotoxin (TTX) incubation [175].
The functional impact of CNTs on neuron electrophysiology and maturation is especially interesting when considering the implications of significant Ca2+ influx during the early stages of neural network formation. Ca2+ signaling cascades and their impact on neural proliferation and differentiation are well documented. Calcineurin-NFAT signaling is critical for neurotrophin-mediated axonal growth and guidance while CAMP and Ca2+/calmodulin (CAM)-dependent protein kinases (CaMKs) directly impact differentiation regulators like CREB. Induction of NeuroD in immature neural cells has also been noted as a direct result of intracellular Ca2+ increase [176]. Other pathways like PKA, RAS/MEK/ERK, and CaMKK can be activated downstream due to Ca2+ influx and a synergistic promotion of growth factor receptor activation is likely. Through their unique electrochemical and physical properties, CNTs profoundly impact neuron physiology through traditional FAK-driven cascades and less common Ca2+ mediated signaling due to their impact on synaptic activity and voltage-gated ion channel expression.
CNTs also influence intracellular calcium signaling in neuroglia such as astrocytes which secrete gliotransmitters to regulate neural homeostasis. Intracellular calcium is essential for astrocytes to function as a counterpart to neuronal membrane change [177]. Astrocytes modulate excitation/inhibition balance and neurotransmitter clearance, making them a key component of healthy neural tissue. Lee et al. investigated the regulation of gliotransmission, glutamate uptake ability, and resting intracellular calcium concentration while interfacing with MWCNT substrates. Adhesion and proliferation of astrocytes were increased following MWCNT interfacing and intracellular calcium concentration was significantly higher in astrocytes bound to MWCNT compared to the control. Pathview gene analysis identified TRPV1 as the mechanosensitive Ca2+ channel responsible for increased intracellular Ca2+, leading to increased gliotransmission [178].
Graphene has gained interest for neuroscience applications requiring transparent electrically conductive interfaces. Its single-layer hexagonal arrangement of carbon atoms offers excellent carrier mobility, chemical stability, and optical transparency, making it a promising candidate for interfacing electrical-excitable tissues [179]. The biocompatibility of graphene has been extensively characterized, and its influence on neural cell behavior has been well-documented for in vitro applications [180, 181]. Li et al. conducted a study on graphene substrates for neurite growth and culture, demonstrating successful growth of mouse hippocampal cells and enhanced neurite sprouting and proliferation when compared with tissue culture polystyrene (TCPS) substrates [182]. Western blot analysis revealed that growth-associated protein (GAP-43) expression was greatly enhanced in the graphene coatings compared to the TCPS control group. Additional evidence highlights the potential of graphene scaffolds to accelerate the adhesion, proliferation, and differentiation of MSCs. Interestingly, the ability of graphene to act as a preconcentration substrate for osteogenetic (dexamethasone and β-glycerolphosphate) inducers demonstrated to promote stem cell lineage specification [183]. Furthermore, single layer graphene was found to modulate neuronal communication by positively influencing the extracellular ion distribution at the interface with hippocampal neurons, a key regulator of neuronal excitability [184]. This ability of graphene to influence ion channel conductance may have implications for synaptic activity and the development of future neuroprosthetics.
Graphene probes have been employed in vivo. The exceptional flexibility of monolayer graphene allows it to be transferred and conform to any substrate, such as flexible polymer films for neural implants, facilitating easy integration into the fabrication process of soft MEAs or FETs [185, 186]. The Garrido group developed graphene-based probe implants capable of recording neural signals and stimulating specific brain regions with high reliability. Furthermore, graphene’s high optical transparency overcomes limitations associated with conventional opaque electrode materials [187]. Thunemann et al. developed a graphene-based MEA integrated with 2-photon imaging and artifact-free optogenetic stimulation, enabling the visualization of neural activity and dynamics in deeper brain regions with high spatial resolution [188]. Integrating in vivo optical imaging and stimulation methods provide an avenue for multi-modal BMI.
Although carbon-based nanomaterials continue to show significant promise, particularly at the interface between neural tissue and foreign body, the contradictory reports surrounding toxicity remain a topic of debate. One of the primary factors contributing to these inconsistent findings is the metal impurities and contaminants generated during the fabrication and functionalization process of graphene and CNTs. Transitional metals like Fe, Y, Ni, Mo, and Co may all be introduced during the fabrication and purification process of these carbon materials [189]. Furthermore, the large-scale production required for industry applications may limit the use of CNTs and graphene. Although graphene and one-dimensional CNT have similar crystalline structure and chemical composition, the flat sheets of graphene strongly differ from the rolled tube configuration of CNTs. This variation in shape leads to different cytotoxicity levels, making it a vital parameter to consider when designing long-term studies [190]. Besides chemical impurities and shape configuration, additional attention must be paid to the eventual degradation or bio-integration at the interface between carbon nanomaterials and neural tissue. Although carbon nanomaterials are not biodegradable, long-term integration and uptake with neural tissue can pose potential problems, particularly with respect to the functional applications of CNTs and graphene. Release and/or uptake of carbon nanomaterials into multiple cell types have activated microglia to produce reactive oxygen species (ROS) driven inflammatory processes [190]. Increased intracellular ROS leads to macromolecular disruption which disturbs cellular homeostasis and leads to neuroinflammation and apoptosis. Disrupting the delicate interface between brain tissue and device through inflammatory-driven processes may lead to neuroprosthetic isolation and failure [191, 192]. These factors must be considered when discussing the prospect of carbon nanomaterials at the CNS/PNS interface.
3.2. Conductive Polymer-Based Nanomaterial/Coating:
Conductive polymers have high electrical conductivity and wide range of modifications that can aid in regeneration and signal conduction. Polymers such as polypyrrole (PPy) and poly(3,4-ethylene dioxythiophene) (PEDOT) have been extensively characterized and their compatibility and impact on neural cells are well documented [193, 194]. Conductive polymers possess a series of alternating single and double bonds with overlapping pi-bonds, allowing the free movement of electrons between atoms. Once a dopant is introduced, these polymers may be oxidized and their backbones are disrupted, which allows the passage of electrons under the application of electrical potential [195]. Interest in these materials stems from their ability to act as an electrically conductive scaffold to enhance neural regeneration or as a conductive coating for intraneural stimulation and recording. Due to their acceptable biocompatibility, work focusing on the relationship between chemical structure interactions and neural cell behavior is relatively limited. Current literature focuses on exploring the effect of electrical stimulation on neural cell behavior using a wide range of conductive polymeric materials. The complementary role of polymeric materials as a signal generator and a mediator of direct cell membrane interaction is often overlooked with a significant focus on the effect of electrical stimulation alone. The effects on stimulated neural cells are attributed to the polymeric chemistry of the substrate driving direct membrane interactions. The relationship between CNS tissue and conductive polymer scaffolds or coatings is nuanced, particularly when the goal is regenerating and integrating neurons and neuroglia in a normal physiological state. It is therefore essential to explore the molecular interactions between the material chemistry of these conductive polymers and the cellular components existing at the CNS interface. This will allow us to understand the short- and long-term effects of conductive polymer interfacing with and without electrical stimulation.
The main precursor of PPy, heterocyclic pyrrole, is a compound found in many pharmacologically active substances including antidepressants, anti-tumor, and neuroprotective therapeutics [196]. Due to the anti-inflammatory and neurogenic effects demonstrated by PPy derivatives, the chemical structure of PPy likely influences intracellular signaling pathways. Apart from the conductivity of PPy, membrane interactions with heterocyclic pyrrole may provide an added benefit of neural tissue interfacing due to its intrinsic neurogenic properties. Skopalová et al. investigated the effect of PPy, PPy oligomers, low molecular-weight byproducts, and residual impurities present in PPy after its synthesis on the neurogenesis of stem cells [197]. Embryoid body (EB) encapsulations of embryonic stem cells (ESC) were cultured with 1, 5, and 25% PPy extract for five days and allowed to culture for an additional 11 days with serum-free medium after exposure. EBs exposed to PPy extract exhibited increased neurite growth, with 5% concentration providing the most extensive neurogenesis. Early neurogenesis markers PAX6, SOX1, and MASH1 were all significantly upregulated in EBs exposed to 5% PPy extract, and western blot analysis showed an increase in N-cadherin, N-CAM, beta III tubulin, and doublecortin protein expression. 10% PPy extract treatment and subsequent analysis found a significant decrease in Akt phosphorylation while ERK phosphorylation was slightly increased. Due to the unmodified phosphorylation of upstream kinase GSK3, it was theorized that PPy extract mediates neurogenic differentiation through the modulation of the Akt signaling pathway.
Conductive polymers are typically insoluble, and their native hydrophobicity tends to limit cell adhesion and proliferation desired for CNS interfacing applications. For this reason, direct membrane interactions with PPy substrates are limited but can be promoted via surface modification with ligands and anionic dopants. When synthesizing PPy scaffolds or coatings for neurological applications, different dopants can be introduced to augment the physical and electrochemical properties of the conductive polymer and encourage interactions with neural ECM and membranes. Dopant selection can also increase the surface’s hydrophilicity, decreasing the probability of nonspecific protein adsorption while enhancing the rate of neural cell adhesion [201, 202]. With this tunability in mind, current research focuses on optimizing surface chemistry characteristics to encourage neural cell binding and differentiation. Thompson et al. investigated the use of dopants including para-toluene sulfonate (pTS), dodecyl benzene sulfonate (DBS), poly(4-styrene sulfonate) (PSS), hyaluronic acid (HA), chondroitin sulfate (CS), and poly(2-methoxy aniline-5-sulfonic acid (PMAS) [201]. Spinal ganglion neuron (SGN) explants were cultured on PPy films doped with these anions. Following cytotoxicity and neurotrophic drug release testing in vitro, PPy/pTS was the only doped PPy film to perform favorably in both criteria. Interestingly, in cell adhesion and neurite outgrowth studies without stimulated drug release, PPy/PMAS adherent SGNs did not exhibit neurite extension or non-neural cell bed proliferation. Adjacent to the PPy/PMAS film, however, both neurite and non-neural cell bed growth was observed which highlighted the importance of neural cell adhesion molecules and their interactions with these modified PPy films. Lundin et al. demonstrated similar variability when comparing the use of DBS, tosylate (TsO), perchlorate (ClO4), and chloride (Cl). Interestingly, DBS was the only group able to sustain NSC viability while epithelial cell adhesion was comparable amongst PPy/Cl, PPy/ClO4, and PPy/DBS [203].
Biologically-inspired dopants may also facilitate a more chemically recognizable structure between the substrate and neural cells. Stauffer et al. considered two peptide sequences derived from laminin (p31 and p20) and used them as dopants in the electro-polymerization of PPy. Electrochemical performance on recording electrodes was performed and subsequent fluorescence staining of PPy/p31-p20, PPy/p31, and PPy/PSS demonstrated significantly greater neurite outgrowth in PPy doped with p31 and p20. Interestingly, PPy/p31 trended towards a higher binding affinity to astrocytes compared to p20 which highlights the sensitivity in neural cell binding dependent on the counterion [202]. PPy coatings on neural probes have also been reported for in vivo studies with minimal tissue response [204]. For instance, PPy/ CDPGYIGSR coated gold electrodes showed promising results in lengthening the life of neural recording devices. Electrophysiological recordings were stable for a 2-week period and immunocytochemical studies indicated a significantly improved interaction of neuronal tissue to the coated sites when compared to uncoated Au electrodes [205].
PEDOT is a promising conductive polymer owing to its thermoelectric properties, electrical conductivity, stretchability, transparency, and solution processability [206, 207], that can impact the neural proliferation and differentiation of NSCs and other cell types. Similar to PPy, dopants are a critical consideration when designing PEDOT constructs to enhance cell interactions and improve electrochemical performance. Cellot et al. investigated PEDOT doped with polystyrene sulfonate (PSS) and its impact on the development of neural synaptic networks [198]. Like PPy, the dopant used impacts the physical and electrochemical characteristics of the substrate, which in turn influences the membrane/ECM interactions that develop during neural tissue integration (Figure 8A). PEDOT/PSS, doped with varying levels of ethylene glycol (EG) demonstrated significant wettability which naturally increased the binding affinity of rat hippocampal neurons. A mixed population of postsynaptic currents confirmed healthy single-cell membrane passive activity, indicating neurons connected by functionally active synapses after binding to PEDOT/PSS substrates. Interestingly, GFAP expression density was reduced after interfacing with PEDOT/PSS compared to poly-ornithine substrates acting as a control, indicating a potential underlying mechanism that decreases neuroglial binding. These findings were consistent when compared to Forcelli et al. in which similar decreases in astrocyte binding to conductive polymers such as poly(3-methyl-thiophene) (P3MT) and Poly(3,4-ethylenedioxypyrole) (PEDOP) was observed [193]. Without electrical stimulation, Ostrakhovitch et al. investigated the relationship between innate substrate conductivity and NSC and P19 pluripotent embryonal (P19 EC) carcinoma cell differentiation [199]. ITO (2.5 S cm-2), P3HT (8.77 x 10-5 S cm-2), PBT (2.27 x 10-5 S cm-2), PEDOT:PEG (2.63 x 10-3 S cm-2), and electrodeposited PEDOT (1.89 x 10-2 S cm-2) were used as substrates for neural differentiation and a correlation between high conductivity and increased differentiation was found. Without retinoic acid (RA) stimulation, P19 aggregates and NSC grown on electrodeposited PEDOT and ITO differentiated into neurons comparable to RA-induced groups. The percentage of cell differentiation was directly related to substrate conductance as polythiophene groups with lower conductance (PBT and P3HT) did not support the differentiation of NSCs and P19s (Figure 8B). Additionally, the PI3/AKT pathway was found to be inhibited upon PEDOT binding similar to the effects of RA-induced differentiation. It is hypothesized that these results stem from redox reactions at the interface of electroactive substrates and cell monolayer via changes in intracellular distribution of redox couples and therefore the intracellular redox potential [199].
Figure 8.
A) Poly-ornithine substrates modified with PEDOT demonstrated reduced neuroglial binding indicated by a decrease in GFAP expression in neural cultures. Single cell electrophysiology confirmed robust signal transfer on PEDOT-modified films in vitro. Figure adapted with permission from Cellot et al. [198] B) A decrease in substrate conductivity led to a decrease in neural cell differentiation. Similar to RA-induced differentiation, proliferative pathways were inhibited on PEDOT films leading to enhanced neurite outgrowth. Figure adapted with permission from Ostrakhovitch et al. [199] C) PEDOT vs. PEDOT/CNT composite interfaces demonstrate an enhanced neural response on PEDOT/CNT substrates, further linking substrate conductivity and differentiation potential. Figure adapted with permission from Dominguez-Alfaro [200].
Pisciotta et al. investigated the practices of spin coating vs. electro polymerization of PEDOT (SC-PEDOT vs. ED-PEDOT, respectively) and their impact on human dental pulp stem cells (hDPSCs). Cells grown on SC-PEDOT presented fibroblast-like morphology while ED-PEDOT directed more spindle-like morphology which closely relates to their neural crest derivation. The stemness of these cells was maintained which was confirmed through Nestin and SOX10 staining. ED-PEDOT also showed superior beta III tubulin and MAP2 expression and displayed a morphological shift towards neuron-like cells. Interestingly, ED-PEDOT film also maintained the expression of FasL, a member of the Fas/FasL pathway responsible for maintaining a cell’s ability to modulate the local immune response. ED-PEDOT surfaces resulted in induction of neurogenic commitment and maintenance of immunomodulatory properties necessary for complex neuroregenerative therapeutic strategies. Electro polymerization of PEDOT also increases surface roughness with a negligible increase in hydrophobicity which further supports the increase in neural cell binding and proliferation on ED-PEDOT [208]. Notably, studies have shown composition dependent differences in neural differentiation on different conductive polymer and graphene substrate composites. Dominguez-Alfaro et al. demonstrated a significant increase in neural maturation using SH-SY5Y neuroblastoma cells on PEDOT/CNT vs. PEDOT substrates. PEDOT/CNT produced a significantly higher expression of beta III tubulin and MAP2 with a shift towards mature neural morphology. Since CNTs have a higher natural conductivity relative to conductive polymers like PEDOT, the relationship between neural differentiation and substrate conductivity seems closely related even without applying an electrical field [200].
3.3. Biologically-based Nanomaterials/Coatings:
Biomimicry has become a popular strategy for tissue regeneration and interfacing applications. Biologically recognizable materials that emulate native ECM components allow for the development of biocompatible microenvironments that increase tissue integration and manipulate cellular microenvironments to optimize adhesion, proliferation, and differentiation. In addition to the incorporation of bioactive molecules, biologically-inspired nanomaterials typically hold the advantage of minimal to no cytotoxicity at therapeutic concentrations. Biodegradability without releasing toxic byproducts may also be viewed as an advantage in applications requiring seamless integration within the CNS or PNS. Biologically-inspired materials may also be used with CNTs or CPs to maximize the benefit of electrophysiological stimulation while ensuring biocompatibility at the interface. Regardless of the application, materials derived from naturally expressed origins have proven to be an effective way of programming cell response by minimizing immune cell activity and increasing healthy proliferation/maturation.
Bioactive material coatings are often inspired by components in neural ECM including glycosaminoglycans (GAGs) and laminin. These materials include amino acids that facilitate the adhesion, migration, and maturation of neural cells through membrane recognition of natural binding motifs. Various signaling responses have been observed depending on the binding motif included in the scaffolding. For instance, significant increases in adhesion, proliferation, and differentiation of neural cells and stem cells resulted when incorporating RGD (fibronectin mimetic) and IKVAV (laminin mimetic) into scaffolding [209]. Ruan et al. incorporated IKVAV peptide amphiphiles into three-dimensional scaffolding to differentiate BMSCs into neural cells without media stimulation [210]. Farrukh et al. functionalized a polylysine (PL) hydrogel with IKVAV to promote neurogenesis in cortical progenitor cells at varying substrate stiffnesses. IKVAV/PL promoted ITB1 expression, leading to positive downstream effects on adhesion-based signaling and overall neurogenesis (Figure 9A). A significant synergy was identified between IKVAV and PL functional groups that increased adhesion and displayed well-developed fast voltage-gated inward currents typical of Na+ channel activation [211]. Interestingly, IKVAV and PL alone showed no significant difference in focal adhesion expression through ITB1 IHC, reflected by poor voltage-gated current activity. Schwann cell activity on electrospun polycaprolactone (PCL) scaffolding functionalized with DOPA and IKVAV provided extremely well-controlled directional growth and myelination for regenerative scaffolding applications. The incorporation of IKVAV into aligned and unaligned PCL substrates was analyzed through RNAseq and RT-qPCR to differentiate between topographically and biochemically influenced Schwann cell activity. The data suggests TGF-B pathway activity as a response to biochemical cues from IKVAV interactions [212]. Compared to other peptide motifs like RGD, IKVAV has a substantially different membrane interaction dependent on cell type (Figure 9B). For example, RGD incorporated into polyimide substrates demonstrates significant recruitment of T98-G glial cells and 3T3 fibroblast cells with low PC12 neural adhesion. IKVAV presented the inverse binding profile with significant neuron binding and minimal immune cell activity [213]. Additional evidence shows that in a neuron-astrocyte-microglia co-culture, RGD motif substrates can increase the neural spheroid formation and microglial adhesion and proliferation. Although microglia are necessary for neural network development, inflammatory cytokine concentrations were upregulated, suggesting additional effects beyond enhanced co-culture crosstalk [214, 215]. This must be an essential factor when designing a microenvironment to discourage the innate foreign body reaction upon implantation for devices like BMI probes if RGD functionalization is considered.
Figure 9.
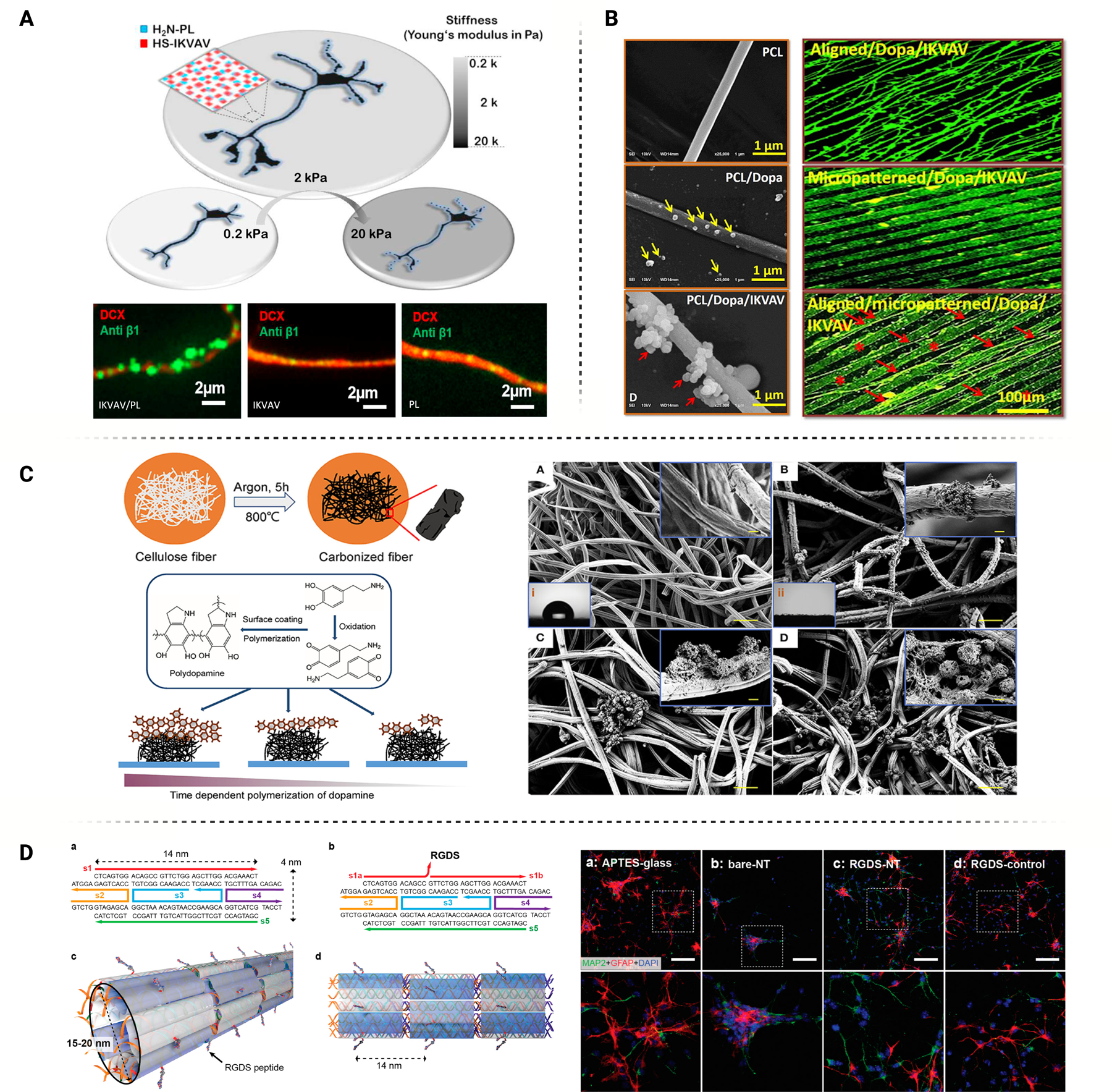
A) Standalone benefits of IKVAV and PL were insignificant, however synergistic benefits of IKVAV and PL functional groups for neural binding were identified through integrin IHC. Figure adapted with permission from Farrukh et al. [211] B) PCL electrospun fibers functionalized with DOPA and IKVAV provided extremely well controlled directional growth and myelination of Schwann cells. Figure adapted with permission from Li et al. [212] C) Polydopamine-modified carbonized microfibers increase neural stem cell adhesion, organization, and intracellular coupling. Figure adapted with permission from Yang et al. [219] D) DNA nanotubes covalently functionalized with RGDS peptides for cellular recognition and binding. DNA nanotube substrates preferentially differentiated neural stem cells into neurons rather than astrocytes. Figure adapted with permission from Stephanopoulos et al. [222]
Recently, mussel-inspired adhesive techniques have garnered attention as a viable coating option for conductive substrates like CNTs and CPs [216]. Polydopamine and other materials are oxidants or reductants due to their oxidizing quinolinyl and reductive catechol. Biomolecules can react with carbon on the benzene rings while primary amino groups in proteins react with PDA in the quinone form. PDA coatings can increase the hydrophilicity, which increases the binding affinity of the coated conductive polymer. Various studies have found that postsynaptic density protein-95 (PSD-95), beta III tubulin, and MAP2 significantly increased due to PDA neural stem cell binding to PDA-modified conductive polymers [217]. The promotion of neuritogenesis, differentiation, and proliferation have been confirmed through stimulated and non-stimulated binding experiments [218, 219] (Figure 9C). In vivo performance of PDA is favorable depending on the secondary component involved in the PDA composite. PDA/PSB (a zwitterionic particle fixed with catechol-mediated binding) demonstrated neuronal cell binding to implanted microelectrodes while minimizing glial cell attraction. This is due to PSB’s non-specific binding characteristics, which minimized microglia and reactive astrocyte recruitment while utilizing the adhesive benefits of PDA as a base coating [220]. Regarding mechanistic molecular analysis, Yang et al. found that although carbon/PDA microfibrous scaffolds did not increase FAK or ITB1 expression, vinculin was significantly upregulated, suggesting protein-specific involvement of PDA-bound neural cells. Although the secondary signaling affected by these interactions between NSCs and PDA is still unknown, the adhesive benefits of PDA are well characterized, and future mechanistic studies may help elucidate pathway involvement.
The development and popularity of biologically-inspired coatings has also influenced the designing of BMIs to enhance their long-term implantation. For instance, Lee et al. reported an improved performance of lubricant coated brain-penetrating flexible electrodes during in vivo measurements. The anti-biofouling thin lubricant exhibited near-frictionless properties and strong repellency to both nonpolar and polar liquids, which lead to a two-fold increase in the recording channel count and a drastic improvement in SNR by minimizing the insertion friction, and an 8-to-16 week extension in the recordings by reducing the immune response at the implant site [221].
DNA-based architecture is quickly emerging as a promising modality for therapeutic strategies like drug delivery and biological tissue interfacing. Precise control over DNA sequences to create two- and three-dimensional structures generate opportunities for complex nanomaterial composites that are biocompatible while manipulating the microenvironment of interfacing tissues. Integrin and FAK expression analysis has been performed on other cell types interfacing with DNA-based ECM with favorable regulation of cytoskeletal remodeling and proliferation [223]. Differentiation of NPCs into mature neuron phenotypes has been achieved by conjugating binding peptides to DNA-based nanotubes that act as nanofiber scaffolding—briefly, five strands of DNA self-assemble into a two-dimensional tile consisting of two parallel DNA helices. The edges of these tiles contain short single-stranded DNA segments that direct two-dimensional lattice assembly. These tiles then curve to form a closed nanotube structure (Figure 9D). RGD conjugation to DNA nanotubes increased MAP2 expression in NSC cultures while limiting GFAP expression, indicating a restriction in astrocyte development [222]. Janus base nanotubes (JBNts) are another biomimetic DNA-based system for therapeutic interfacing. JBNts are self-assembled from guanine and cytosine (GĈ) or thymine and adenine (ÂT) units, which mimic DNA base pairs via hydrogen bonds and the base stacking effect [224, 225]. The self-assembled DNA-mimicking structure of JBNts has demonstrated excellent bio-integration in terms of improved cell adhesion, proliferation and long-term functions with various types of tissue cells [226, 227]. JBNts utilize the assembly of DNA base units into a sp2 hybridized aromatic-ring system (rosette). Long-distance delocalization of electrons is achieved following π-π base stacking of the rosettes similar to the electron motility mechanisms of a CNT. Based on the biomolecule-based materials self-assembled through non-covalent bonding, JBNts hold significant potential as a neural interfacing material. Favorable neural cell adhesion and maturation results have been achieved in vitro along with successful acute-phase in vivo studies, pointing towards ins potential for neural interfacing applications [228].
4.0. Future Directions and Applications:
Substrate nanotopography can be altered in many ways, with each alteration having a different impact on neural cell behavior. As such, modifying a substrate’s surface architecture can facilitate controllable and predictable cell behavior with relevance to a wide range of biomedical applications. The desired cell responses may be achieved, including growth, differentiation, and proliferation, solely through substrate modifications, potentially minimizing the burden on BMI design and manufacturing.
Mechanistic studies seek to uncover the internal cellular mechanisms and pathways activated in response to external stimuli from the substrate. Such understanding can drive new strategies to alter a substrate’s topography to trigger the expression of a specific pathway, allowing for precise control of cell behavior. However, despite the numerous improvements in both RNA transcriptome analysis, as well as new novel imaging modalities to provide high-resolution imaging of interfaces at cellular and sub-cellular scales, comprehensive mechanistic studies that inform strategies to precisely target intracellular pathways via topographical interactions are still required.
Single-cell sequencing offers additional avenues for mechanistic exploration into the modulation of cellular functions, establishing differentially upregulated target genes related to both adhesion and specific cell processes that could allow for more in-depth pathway tracing and understanding of how geometry influences specific signaling components. The potential to manipulate cell behavior by controlling pore size or to use a range of different pore sizes to influence the behavior of multiple cell types simultaneously offer intriguing possibilities that have yet to be realized. More advanced topographical geometries such as micropillars and microchannels offer greater control over surface topography with impact on cellular functions like proliferation of neural cell lineages and the differentiation of stem cells to neuronal lineages. However, additional studies on signaling modulation and the mechanistic impact of cell-substrate interfacing are needed to drive best practice recommendations. Advanced interfacial interactions may influence both long-range signaling across cell-cell contacts, as well as traditional mechanosensing adhesion complexes. The improvements in RNA transcriptome analysis will allow for further in-depth explorations into how advanced geometrical conformations influence neural signaling systems, as well as the various modulative effects a cell interfacing with a unique topography may have. Similarly, in regard to nano grooved topographies, it is well known that focal adhesion complexes help to elucidate a mechanoresponse. This process involves physiological changes to cell structures and the activation of adhesion-related protein complexes, allowing for the modulation of cell processes, including proliferation, differentiation, and cell fate. Exploring the mechanisms within downstream FAK activation and probing how mechanosensing may influence other adhesion contacts and cell-cell contacts can further improve mechanistic understanding of cellular capabilities to sense nanoroughness topography and the appropriate modulation to functionalization that may come from it.
Interface material chemistry is also an important consideration, particularly for sensitive applications that require intimate and sustained integration with the host tissue. The development of electrically conductive materials to augment nervous tissue interactions has directed attention toward the stimulation and recording capacity of these biomaterial coatings. Oftentimes, the impressive electrochemical capabilities of these materials can overshadow their potential impact as a standalone interface due to their chemical makeup. For instance, many of these materials have innate neurogenic properties without the introduction of exogenous stimuli. This can increase their utility as a scaffolding or coating material and provide additional insight into the impact of surface chemistry on cell membrane to ECM interactions. Materials like carbon nanotubes with high lateral π-π electron mobilization can impact the likelihood and efficiency of spontaneous signal transduction, which has a profound effect on gated ion channel expression and downstream signaling effects. ECM-mediated control over ion transport and electrochemical gradients is especially important in neural networks that rely on optimal contribution from neurons and neuroglia to maintain homeostasis.
In developing neural networks for regeneration approaches, spontaneous signal propagation can play a pivotal role with respect to stem cell differentiation and glial cell control. Substrates with innate electrical conductivity such as CNTs, PPy, and JBNt can indirectly impact cell maturation through their positive influence on gated ion transport expression. Numerous investigations into these materials have highlighted their impact on intracellular calcium transport which influences the activity of several neurogenic pathways. Additional work has provided substantial insight into neuroglial cell activity on electroactive substrates which should be considered when designing a scaffold or coating material. The body of work elucidating the impact of substrate chemical structure on neural cell behavior is substantially less when compared to topography-based studies. There is an opportunity to leverage recent advances in sequencing technologies to address the impact of substrate chemical structure on tissue microenvironment control. Transcriptome analysis can provide speculative insight into the ways these biomaterial scaffolds/coatings are manipulating cell behavior through membrane interactions. Further understanding of these interactions can help inform the development of more effective composite materials and even push the development of novel interfaces that increase electrochemical function and biocompatibility. Finally, the incorporation of biologically-inspired materials as a standalone or composite interface with neural tissue continues to gain momentum due to their innate biocompatibility and recognizable surface chemistry. PDA and peptide-based composites may be advantageous for applications that require high surface binding affinity and low cytotoxicity. Their negative impact on electrical conductivity limits their use as an intermediate for stimulation and recording purposes, however novel composites may mitigate this issue and promote healthy neural integration.
These emerging surface modification techniques and new chemistries have garnered significant interest for their potential to positively enhance device-tissue interactions at the cellular and microenvironment scale. Future advancements in these techniques are likely to improve therapeutic outcomes of applications such as scaffold-based regenerative engineering and BMI stimulation/recording. Novel nanomaterial development continues to help balance interface bioactivity and long-term functional maintenance while the increasing accessibility of transcriptomic analysis provides researchers with additional resources to manipulate and control tissue microenvironments. Current limitations for these modification techniques include practical implementation into regenerative therapies and brain machine interfaces which may require additional development to adapt prototypes convenient for early in vitro and in vivo studies as well as considerations for the process scalability for applying a particular surface modification or material.
Figure 10.
The potential applications for neural interface engineering will improve chronic stability, balance bioactivity and conductivity, and provide easier-to-implement practices in biomedical research. These improvements will help to accelerate the development and success of therapeutic modalities that rely on complex and intimate interfacing with nervous tissue.
Acknowledgements:
We would like to acknowledge partial support from U01NS126046, U24NS113647, NIH 7R01AR072027, NIH 1R21AR079153-01A1, NSF Career Award 1905785, NSF 2025362, NSF 2234570, NASA 80JSC022CA006, DOD W81XWH2110274 and the University of Connecticut.
References:
- [1].Strumwasser F, “Long-term recording from single neurons in brain of unrestrained mammals,” Science, vol. 127, no. 3296, pp. 469–470, 1958. [DOI] [PubMed] [Google Scholar]
- [2].Dow B, Vautin R, and Bauer R, “The mapping of visual space onto foveal striate cortex in the macaque monkey,” Journal of Neuroscience, vol. 5, no. 4, pp. 890–902, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dario P et al. , “Neural interfaces for regenerated nerve stimulation and recording,” IEEE Transactions on Rehabilitation Engineering, vol. 6, no. 4, pp. 353–363, 1998, doi: 10.1109/86.736149. [DOI] [PubMed] [Google Scholar]
- [4].Kipke DR et al. , “Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities,” Journal of Neuroscience, vol. 28, no. 46, pp. 11830–11838, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kozai TDY, “The History and Horizons of Microscale Neural Interfaces,” (in eng), Micromachines (Basel), vol. 9, no. 9, Sep 6 2018, doi: 10.3390/mi9090445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adewole DO et al. , “The Evolution of Neuroprosthetic Interfaces,” (in eng), Crit Rev Biomed Eng, vol. 44, no. 1–2, pp. 123–52, 2016, doi: 10.1615/CritRevBiomedEng.2016017198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Teleanu RI, Gherasim O, Gherasim TG, Grumezescu V, Grumezescu AM, and Teleanu DM, “Nanomaterial-Based Approaches for Neural Regeneration,” (in eng), Pharmaceutics, vol. 11, no. 6, Jun 8 2019, doi: 10.3390/pharmaceutics11060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Takagi Y, “History of Neural Stem Cell Research and Its Clinical Application,” (in eng), Neurol Med Chir (Tokyo), vol. 56, no. 3, pp. 110–24, 2016, doi: 10.2176/nmc.ra.2015-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Polikov VS, Tresco PA, and Reichert WM, “Response of brain tissue to chronically implanted neural electrodes,” Journal of neuroscience methods, vol. 148, no. 1, pp. 1–18, 2005. [DOI] [PubMed] [Google Scholar]
- [10].Grill WM, Norman SE, and Bellamkonda RV, “Implanted neural interfaces: biochallenges and engineered solutions,” Annual review of biomedical engineering, vol. 11, pp. 1–24, 2009. [DOI] [PubMed] [Google Scholar]
- [11].Ereifej ES et al. , “Implantation of Neural Probes in the Brain Elicits Oxidative Stress,” (in eng), Front Bioeng Biotechnol, vol. 6, p. 9, 2018, doi: 10.3389/fbioe.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rasoulianboroujeni M et al. , “Dual Porosity Protein-based Scaffolds with Enhanced Cell Infiltration and Proliferation,” Scientific Reports, vol. 8, no. 1, p. 14889, 2018/October/05 2018, doi: 10.1038/s41598-018-33245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fan S et al. , “Guiding the Patterned Growth of Neuronal Axons and Dendrites Using Anisotropic Micropillar Scaffolds,” (in eng), Adv Healthc Mater, vol. 10, no. 12, p. e2100094, Jun 2021, doi: 10.1002/adhm.202100094. [DOI] [PubMed] [Google Scholar]
- [14].Tanaka A, Fujii Y, Kasai N, Okajima T, and Nakashima H, “Regulation of neuritogenesis in hippocampal neurons using stiffness of extracellular microenvironment,” (in eng), PLoS One, vol. 13, no. 2, p. e0191928, 2018, doi: 10.1371/journal.pone.0191928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kunisaki A et al. , “Carbon-nanotube yarns induce axonal regeneration in peripheral nerve defect,” Scientific Reports, vol. 11, no. 1, p. 19562, 2021/October/01 2021, doi: 10.1038/s41598-021-98603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang MZ, Dai CL, and Lu DH, “Polypyrrole porous micro humidity sensor integrated with a ring oscillator circuit on chip,” (in eng), Sensors (Basel), vol. 10, no. 11, pp. 10095–104, 2010, doi: 10.3390/s101110095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jain D, Mattiassi S, Goh EL, and Yim EKF, “Extracellular matrix and biomimetic engineering microenvironment for neuronal differentiation,” (in eng), Neural Regen Res, vol. 15, no. 4, pp. 573–585, Apr 2020, doi: 10.4103/1673-5374.266907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Onesto V et al. , “Nano-topography Enhances Communication in Neural Cells Networks,” Scientific Reports, vol. 7, no. 1, p. 9841, 2017/August/29 2017, doi: 10.1038/s41598-017-09741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tonazzini I, Meucci S, Faraci P, Beltram F, and Cecchini M, “Neuronal differentiation on anisotropic substrates and the influence of nanotopographical noise on neurite contact guidance,” Biomaterials, vol. 34, no. 25, pp. 6027–6036, 2013/August/01/ 2013, doi: 10.1016/j.biomaterials.2013.04.039. [DOI] [PubMed] [Google Scholar]
- [20].Chen W et al. , “Nanotopography regulates motor neuron differentiation of human pluripotent stem cells,” (in eng), Nanoscale, vol. 10, no. 7, pp. 3556–3565, Feb 15 2018, doi: 10.1039/c7nr05430k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang K et al. , “Nanotopographical Manipulation of Focal Adhesion Formation for Enhanced Differentiation of Human Neural Stem Cells,” ACS Applied Materials & Interfaces, vol. 5, no. 21, pp. 10529–10540, 2013/November/13 2013, doi: 10.1021/am402156f. [DOI] [PubMed] [Google Scholar]
- [22].Joseph G, Orme RP, Kyriacou T, Fricker RA, and Roach P, “Effects of Surface Chemistry Interaction on Primary Neural Stem Cell Neurosphere Responses,” (in eng), ACS Omega, vol. 6, no. 30, pp. 19901–19910, Aug 3 2021, doi: 10.1021/acsomega.1c02796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stukel JM and Willits RK, “Mechanotransduction of Neural Cells Through Cell-Substrate Interactions,” (in eng), Tissue Eng Part B Rev, vol. 22, no. 3, pp. 173–82, Jun 2016, doi: 10.1089/ten.TEB.2015.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keselowsky BG, Collard DM, and García AJ, “Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation,” Proceedings of the National Academy of Sciences, vol. 102, no. 17, pp. 5953–5957, 2005, doi: doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Szarowski DH et al. , “Brain responses to micro-machined silicon devices,” Brain Research, vol. 983, no. 1, pp. 23–35, 2003/September/05/ 2003, doi: 10.1016/S0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- [26].Seymour JP and Kipke DR, “Neural probe design for reduced tissue encapsulation in CNS,” (in eng), Biomaterials, vol. 28, no. 25, pp. 3594–607, Sep 2007, doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- [27].Spira ME and Hai A, “Multi-electrode array technologies for neuroscience and cardiology,” Nature Nanotechnology, vol. 8, no. 2, pp. 83–94, 2013/February/01 2013, doi: 10.1038/nnano.2012.265. [DOI] [PubMed] [Google Scholar]
- [28].Redondo-Gómez C, Leandro-Mora R, Blanch-Bermúdez D, Espinoza-Araya C, Hidalgo-Barrantes D, and Vega-Baudrit J, “Recent Advances in Carbon Nanotubes for Nervous Tissue Regeneration,” Advances in Polymer Technology, vol. 2020, p. 6861205, 2020/February/11 2020, doi: 10.1155/2020/6861205. [DOI] [Google Scholar]
- [29].Pardo-Figuerez M et al. , “Controlled Arrangement of Neuronal Cells on Surfaces Functionalized with Micropatterned Polymer Brushes,” ACS Omega, vol. 3, no. 10, pp. 12383–12391, 2018/October/31 2018, doi: 10.1021/acsomega.8b01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hou Y et al. , “Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells,” Small, 10.1002/smll.201905422 vol. 16, no. 10, p. 1905422, 2020/March/01 2020, doi: 10.1002/smll.201905422. [DOI] [PubMed] [Google Scholar]
- [31].George JH et al. , “A closer look at neuron interaction with track-etched microporous membranes,” Scientific Reports, vol. 8, no. 1, p. 15552, 2018/October/19 2018, doi: 10.1038/s41598-018-33710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Si W, Gong J, and Yang X, “Substrate stiffness in nerve cells,” Brain Science Advances, vol. 9, no. 1, pp. 24–34, 2023, doi: 10.26599/bsa.2023.9050002. [DOI] [Google Scholar]
- [33].Szabó Á, Liliom H, Fekete Z, Schlett K, and Pongrácz A, “SU-8 microstructures alter the attachment and growth of glial cells in vitro,” Materials Today Communications, vol. 27, p. 102336, 2021/June/01/ 2021, doi: 10.1016/j.mtcomm.2021.102336. [DOI] [Google Scholar]
- [34].Mei F et al. , “Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis,” Nature Medicine, vol. 20, no. 8, pp. 954–960, 2014/August/01 2014, doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Doolin MT and Stroka KM, “Integration of Mesenchymal Stem Cells into a Novel Micropillar Confinement Assay,” Tissue Eng Part C Methods, vol. 25, no. 11, pp. 662–676, 2019/September/11 2019, doi: 10.1089/ten.TEC.2019.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ankam S, Teo BKK, Pohan G, Ho SWL, Lim CK, and Yim EKF, “Temporal Changes in Nucleus Morphology, Lamin A/C and Histone Methylation During Nanotopography-Induced Neuronal Differentiation of Stem Cells,” Frontiers in Bioengineering and Biotechnology, Original Research vol. 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ermis M, Akkaynak D, Chen P, Demirci U, and Hasirci V, “A high throughput approach for analysis of cell nuclear deformability at single cell level,” Scientific Reports, vol. 6, no. 1, p. 36917, 2016/November/14 2016, doi: 10.1038/srep36917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pathak MM et al. , “Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells,” Proceedings of the National Academy of Sciences, vol. 111, no. 45, pp. 16148–16153, 2014/November/11 2014, doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cutarelli A et al. , “Vertically-Aligned Functionalized Silicon Micropillars for 3D Culture of Human Pluripotent Stem Cell-Derived Cortical Progenitors,” Cells, vol. 9, no. 1, doi: 10.3390/cells9010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Milos F et al. , “High Aspect Ratio and Light-Sensitive Micropillars Based on a Semiconducting Polymer Optically Regulate Neuronal Growth,” ACS Applied Materials & Interfaces, vol. 13, no. 20, pp. 23438–23451, 2021/May/26 2021, doi: 10.1021/acsami.1c03537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kong Y et al. , “Wireless Localized Electrical Stimulation Generated by an Ultrasound-Driven Piezoelectric Discharge Regulates Proinflammatory Macrophage Polarization,” Advanced Science, 10.1002/advs.202100962 vol. 8, no. 13, p. 2100962, 2021/July/01 2021, doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gutierrez H and Davies AM, “Regulation of neural process growth, elaboration and structural plasticity by NF-κB,” (in eng), Trends Neurosci, vol. 34, no. 6, pp. 316–25, Jun 2011, doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liang L et al. , “Piezotronic effect determined neuron-like differentiation of adult stem cells driven by ultrasound,” Nano Energy, vol. 90, p. 106634, 2021/December/01/ 2021, doi: 10.1016/j.nanoen.2021.106634. [DOI] [Google Scholar]
- [44].Kasai M, Satoh K, and Akiyama T, “Wnt signaling regulates the sequential onset of neurogenesis and gliogenesis via induction of BMPs,” Genes Cells, vol. 10, no. 8, pp. 777–783, 2005/August 2005, doi: 10.1111/j.1365-2443.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- [45].Marinval N and Chew SY, “Mechanotransduction assays for neural regeneration strategies: A focus on glial cells,” APL Bioengineering, vol. 5, no. 2, p. 021505, 2021/June/01 2021, doi: 10.1063/5.0037814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lavado A, Gangwar R, Paré J, Wan S, Fan Y, and Cao X, “YAP/TAZ maintain the proliferative capacity and structural organization of radial glial cells during brain development,” Developmental Biology, vol. 480, pp. 39–49, 2021/December/01/ 2021, doi: 10.1016/j.ydbio.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hsu C-C et al. , “Biophysical Regulations of Epigenetic State and Notch Signaling in Neural Development Using Microgroove Substrates,” ACS Applied Materials & Interfaces, vol. 14, no. 29, pp. 32773–32787, 2022/July/27 2022, doi: 10.1021/acsami.2c01996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Otomo A et al. , “Efficient differentiation and polarization of primary cultured neurons on poly(lactic acid) scaffolds with microgrooved structures,” Scientific Reports, vol. 10, no. 1, p. 6716, 2020/April/21 2020, doi: 10.1038/s41598-020-63537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sarker M, Naghieh S, McInnes AD, Schreyer DJ, and Chen X, “Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration,” Biotechnology Journal, vol. 13, no. 7, p. 1700635, 2018, doi: 10.1002/biot.201700635. [DOI] [PubMed] [Google Scholar]
- [50].Sato K, Egami A, Odake T, Tokeshi M, Aihara M, and Kitamori T, “Monitoring of intercellular messengers released from neuron networks cultured in a microchip,” (in eng), J Chromatogr A, vol. 1111, no. 2, pp. 228–32, Apr 14 2006, doi: 10.1016/j.chroma.2005.06.099. [DOI] [PubMed] [Google Scholar]
- [51].Eftekhari BS, Eskandari M, Janmey PA, Samadikuchaksaraei A, and Gholipourmalekabadi M, “Surface Topography and Electrical Signaling: Single and Synergistic Effects on Neural Differentiation of Stem Cells,” Advanced Functional Materials, 10.1002/adfm.201907792 vol. 30, no. 25, p. 1907792, 2020/June/01 2020, doi: 10.1002/adfm.201907792. [DOI] [Google Scholar]
- [52].Singh AV et al. , “Astrocytes Increase ATP Exocytosis Mediated Calcium Signaling in Response to Microgroove Structures,” Scientific Reports, vol. 5, no. 1, p. 7847, 2015/January/19 2015, doi: 10.1038/srep07847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bazargani N and Attwell D, “Astrocyte calcium signaling: the third wave,” Nature Neuroscience, vol. 19, no. 2, pp. 182–189, 2016/February/01 2016, doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- [54].Shi M et al. , “Glia co-culture with neurons in microfluidic platforms promotes the formation and stabilization of synaptic contacts,” Lab on a Chip, 10.1039/C3LC50249J vol. 13, no. 15, pp. 3008–3021, 2013, doi: 10.1039/C3LC50249J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang R et al. , “Ultrasensitive Graphene Optoelectronic Probes for Recording Electrical Activities of Individual Synapses,” Nano Letters, vol. 18, no. 9, pp. 5702–5708, 2018/September/12 2018, doi: 10.1021/acs.nanolett.8b02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Musick KM et al. , “Chronic multichannel neural recordings from soft regenerative microchannel electrodes during gait,” Scientific Reports, vol. 5, no. 1, p. 14363, 2015/September/24 2015, doi: 10.1038/srep14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Srinivasan A, Guo L, and Bellamkonda RV, “Regenerative microchannel electrode array for peripheral nerve interfacing,” in 2011 5th International IEEE/EMBS Conference on Neural Engineering, 27 April-1 May 2011 2011, pp. 253–256, doi: 10.1109/NER.2011.5910535. [DOI] [Google Scholar]
- [58].Wieringa P, Wiertz R, Feber J, and Rutten W, Neural Growth Into A Microchannel Network: Towards A Regenerative Neural Interface. 2009, pp. 51–55. [Google Scholar]
- [59].Lacour SP, Fitzgerald JJ, Lago N, Tarte E, McMahon S, and Fawcett J, “Long Micro-Channel Electrode Arrays: A Novel Type of Regenerative Peripheral Nerve Interface,” IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 17, no. 5, pp. 454–460, 2009, doi: 10.1109/TNSRE.2009.2031241. [DOI] [PubMed] [Google Scholar]
- [60].Srinivasan A et al. , “Microchannel-based regenerative scaffold for chronic peripheral nerve interfacing in amputees,” (in eng), Biomaterials, vol. 41, pp. 151–65, Feb 2015, doi: 10.1016/j.biomaterials.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Park D et al. , “Micropattern-based nerve guidance conduit with hundreds of microchannels and stem cell recruitment for nerve regeneration,” npj Regenerative Medicine, vol. 7, no. 1, p. 62, 2022/October/20 2022, doi: 10.1038/s41536-022-00257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shelly M et al. , “Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth,” Neuron, vol. 71, no. 3, pp. 433–446, 2011/August/11 2011, doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cai S, Wu C, Yang W, Liang W, Yu H, and Liu L, “Recent advance in surface modification for regulating cell adhesion and behaviors,” Nanotechnology Reviews, vol. 9, no. 1, pp. 971–989, 2020, doi: doi: 10.1515/ntrev-2020-0076. [DOI] [Google Scholar]
- [64].Majhy B, Priyadarshini P, and Sen AK, “Effect of surface energy and roughness on cell adhesion and growth - facile surface modification for enhanced cell culture,” (in eng), RSC Adv, vol. 11, no. 25, pp. 15467–15476, Apr 21 2021, doi: 10.1039/d1ra02402g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hou Y et al. , “Surface Roughness and Substrate Stiffness Synergize To Drive Cellular Mechanoresponse,” Nano Letters, vol. 20, no. 1, pp. 748–757, 2020/January/08 2020, doi: 10.1021/acs.nanolett.9b04761. [DOI] [PubMed] [Google Scholar]
- [66].Khan SP, Auner GG, and Newaz GM, “Influence of nanoscale surface roughness on neural cell attachment on silicon,” Nanomedicine: Nanotechnology, Biology and Medicine, vol. 1, no. 2, pp. 125–129, 2005/June/01/ 2005, doi: 10.1016/j.nano.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [67].Xia J, Yuan Y, Wu H, Huang Y, and Weitz DA, “Decoupling the effects of nanopore size and surface roughness on the attachment, spreading and differentiation of bone marrow-derived stem cells,” Biomaterials, vol. 248, p. 120014, 2020/July/01/ 2020, doi: 10.1016/j.biomaterials.2020.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shi M et al. , “Cell Infiltrative Inner Connected Porous Hydrogel Improves Neural Stem Cell Migration and Differentiation for Functional Repair of Spinal Cord Injury,” ACS Biomaterials Science & Engineering, vol. 8, no. 12, pp. 5307–5318, 2022/December/12 2022, doi: 10.1021/acsbiomaterials.2c01127. [DOI] [PubMed] [Google Scholar]
- [69].Deligianni DD, Katsala ND, Koutsoukos PG, and Missirlis YF, “Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength,” Biomaterials, vol. 22, no. 1, pp. 87–96, 2001/January 2001, doi: 10.1016/s0142-9612(00)00174-5. [DOI] [PubMed] [Google Scholar]
- [70].Fan YW, Cui FZ, Hou SP, Xu QY, Chen LN, and Lee IS, “Culture of neural cells on silicon wafers with nano-scale surface topograph,” Journal of Neuroscience Methods, vol. 120, no. 1, pp. 17–23, 2002/October/15/ 2002, doi: 10.1016/S0165-0270(02)00181-4. [DOI] [PubMed] [Google Scholar]
- [71].Pan F et al. , “Topographic effect on human induced pluripotent stem cells differentiation towards neuronal lineage,” Biomaterials, vol. 34, no. 33, pp. 8131–8139, 2013/November/01/ 2013, doi: 10.1016/j.biomaterials.2013.07.025. [DOI] [PubMed] [Google Scholar]
- [72].Brunetti V et al. , “Neurons sense nanoscale roughness with nanometer sensitivity,” Proceedings of the National Academy of Sciences, vol. 107, no. 14, pp. 6264–6269, 2010/April/06 2010, doi: 10.1073/pnas.0914456107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim Y et al. , “Nano-Architectural Approaches for Improved Intracortical Interface Technologies,” Frontiers in Neuroscience, Review vol. 12, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kushwah N, Woeppel K, Dhawan V, Shi D, and Cui XT, “Effects of neuronal cell adhesion molecule L1 and nanoparticle surface modification on microglia,” Acta Biomaterialia, vol. 149, pp. 273–286, 2022/September/01/ 2022, doi: 10.1016/j.actbio.2022.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Blumenthal NR, Hermanson O, Heimrich B, and Shastri VP, “Stochastic nanoroughness modulates neuron–astrocyte interactions and function via mechanosensing cation channels,” Proceedings of the National Academy of Sciences, vol. 111, no. 45, pp. 16124–16129, 2014/November/11 2014, doi: 10.1073/pnas.1412740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Stensaas SS and Stensaas LJ, “Histopathological evaluation of materials implanted in the cerebral cortex,” Acta Neuropathologica, vol. 41, no. 2, pp. 145–155, 1978/January/01 1978, doi: 10.1007/BF00689766. [DOI] [PubMed] [Google Scholar]
- [77].Merrill DR, Bikson M, and Jefferys JG, “Electrical stimulation of excitable tissue: design of efficacious and safe protocols,” (in eng), J Neurosci Methods, vol. 141, no. 2, pp. 171–98, Feb 15 2005, doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- [78].Scholten K and Meng E, “Materials for microfabricated implantable devices: a review,” Lab on a Chip, 10.1039/C5LC00809C vol. 15, no. 22, pp. 4256–4272, 2015, doi: 10.1039/C5LC00809C. [DOI] [PubMed] [Google Scholar]
- [79].“Platinum - Properties and Applications.” https://www.azom.com/article.aspx?ArticleID=601 (accessed November 8th 2023, 2023).
- [80].Green MA, Bilston LE, and Sinkus R, “In vivo brain viscoelastic properties measured by magnetic resonance elastography,” (in eng), NMR Biomed, vol. 21, no. 7, pp. 755–64, Aug 2008, doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- [81].Taylor Z and Miller K, “Reassessment of brain elasticity for analysis of biomechanisms of hydrocephalus,” (in eng), J Biomech, vol. 37, no. 8, pp. 1263–9, Aug 2004, doi: 10.1016/j.jbiomech.2003.11.027. [DOI] [PubMed] [Google Scholar]
- [82].Lind G, Linsmeier CE, and Schouenborg J, “The density difference between tissue and neural probes is a key factor for glial scarring,” Scientific Reports, vol. 3, no. 1, p. 2942, 2013/October/15 2013, doi: 10.1038/srep02942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mierzejewski M, Steins H, Kshirsagar P, and Jones PD, “The noise and impedance of microelectrodes,” (in eng), J Neural Eng, vol. 17, no. 5, p. 052001, Oct 15 2020, doi: 10.1088/1741-2552/abb3b4. [DOI] [PubMed] [Google Scholar]
- [84].Desai SA, Rolston JD, Guo L, and Potter SM, “Improving impedance of implantable microwire multi-electrode arrays by ultrasonic electroplating of durable platinum black,” (in eng), Front Neuroeng, vol. 3, p. 5, 2010, doi: 10.3389/fneng.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Aregueta-Robles UA, Woolley AJ, Poole-Warren LA, Lovell NH, and Green RA, “Organic electrode coatings for next-generation neural interfaces,” (in eng), Front Neuroeng, vol. 7, p. 15, 2014, doi: 10.3389/fneng.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cogan SF, Troyk PR, Ehrlich J, and Plante TD, “In vitro comparison of the charge-injection limits of activated iridium oxide (AIROF) and platinum-iridium microelectrodes,” IEEE Transactions on Biomedical Engineering, vol. 52, no. 9, pp. 1612–1614, 2005, doi: 10.1109/TBME.2005.851503. [DOI] [PubMed] [Google Scholar]
- [87].Hallab NJ, Bundy KJ, O’Connor K, Moses RL, and Jacobs JJ, “Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion,” (in eng), Tissue Eng, vol. 7, no. 1, pp. 55–71, Feb 2001, doi: 10.1089/107632700300003297. [DOI] [PubMed] [Google Scholar]
- [88].Tang R et al. , “Fabrication of strongly adherent platinum black coatings on microelectrodes array,” Science China Information Sciences, vol. 57, no. 4, pp. 1–10, 2014/April/01 2014, doi: 10.1007/s11432-013-4825-6. [DOI] [Google Scholar]
- [89].Rodrigues F et al. , “Fabrication and characterization of polyimide-based ‘smooth’ titanium nitride microelectrode arrays for neural stimulation and recording,” (in eng), J Neural Eng, vol. 17, no. 1, p. 016010, Dec 13 2019, doi: 10.1088/1741-2552/ab4dbb. [DOI] [PubMed] [Google Scholar]
- [90].Janders M, Egert U, Stelzle M, and Nisch W, “Novel thin film titanium nitride micro-electrodes with excellent charge transfer capability for cell stimulation and sensing applications,” in Proceedings of 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 31 Oct.-3 Nov. 1996 1996, vol. 1, pp. 245–247 vol.1, doi: 10.1109/IEMBS.1996.656936. [DOI] [Google Scholar]
- [91].Negi S, Bhandari R, Rieth L, and Solzbacher F, “In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays,” (in eng), Biomed Mater, vol. 5, no. 1, p. 15007, Feb 2010, doi: 10.1088/1748-6041/5/1/015007. [DOI] [PubMed] [Google Scholar]
- [92].Lu Y, Wang T, Cai Z, Cao Y, Yang H, and Duan YY, “Anodically electrodeposited iridium oxide films microelectrodes for neural microstimulation and recording,” Sensors and Actuators B: Chemical, vol. 137, no. 1, pp. 334–339, 2009/March/28/ 2009, doi: 10.1016/j.snb.2008.11.036. [DOI] [Google Scholar]
- [93].Lam D et al. , “Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array,” Scientific Reports, vol. 9, no. 1, p. 4159, 2019/March/11 2019, doi: 10.1038/s41598-019-40128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Her GJ, Wu H-C, Chen M-H, Chen M-Y, Chang S-C, and Wang T-W, “Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages,” Acta Biomaterialia, vol. 9, no. 2, pp. 5170–5180, 2013/February/01/ 2013, doi: 10.1016/j.actbio.2012.10.012. [DOI] [PubMed] [Google Scholar]
- [95].Michael KE, Dumbauld DW, Burns KL, Hanks SK, and García AJ, “Focal Adhesion Kinase Modulates Cell Adhesion Strengthening via Integrin Activation,” Molecular Biology of the Cell, vol. 20, no. 9, pp. 2508–2519, 2009, doi: 10.1091/mbc.e08-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schober M et al. , “Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics,” Journal of Cell Biology, vol. 176, no. 5, pp. 667–680, 2007, doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ozgun A, Erkoc-Biradlı FZ, Bulut O, and Garipcan B, “Substrate stiffness effects on SH-SY5Y: The dichotomy of morphology and neuronal behavior,” Journal of Biomedical Materials Research Part B: Applied Biomaterials, vol. 109, no. 1, pp. 92–101, 2021, doi: 10.1002/jbm.b.34684. [DOI] [PubMed] [Google Scholar]
- [98].Zhang C et al. , “Exploration of the Effects of Substrate Stiffness on Biological Responses of Neural Cells and Their Mechanisms,” ACS Omega, vol. 5, no. 48, pp. 31115–31125, 2020/December/08 2020, doi: 10.1021/acsomega.0c04279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Saha K et al. , “Substrate modulus directs neural stem cell behavior,” (in eng), Biophys J, vol. 95, no. 9, pp. 4426–38, Nov 1 2008, doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cai X, Wang K-C, and Meng Z, “Mechanoregulation of YAP and TAZ in Cellular Homeostasis and Disease Progression,” (in English), Frontiers in Cell and Developmental Biology, Review vol. 9, 2021-May-24 2021, doi: 10.3389/fcell.2021.673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Virdi JK and Pethe P, “Biomaterials Regulate Mechanosensors YAP/TAZ in Stem Cell Growth and Differentiation,” Tissue Engineering and Regenerative Medicine, vol. 18, no. 2, pp. 199–215, 2021/April/01 2021, doi: 10.1007/s13770-020-00301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Scott KE, Fraley SI, and Rangamani P, “A spatial model of YAP/TAZ signaling reveals how stiffness, dimensionality, and shape contribute to emergent outcomes,” Proceedings of the National Academy of Sciences, vol. 118, no. 20, p. e2021571118, 2021, doi: doi: 10.1073/pnas.2021571118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Li Y, Wang J, and Zhong W, “Regulation and mechanism of YAP/TAZ in the mechanical microenvironment of stem cells (Review) Erratum in / 10.3892/mmr.2021.12265,” Mol Med Rep, vol. 24, no. 1, p. 506, 2021/July/01 2021, doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nukuda A et al. , “Stiff substrates increase YAP-signaling-mediated matrix metalloproteinase-7 expression,” Oncogenesis, vol. 4, no. 9, pp. e165–e165, 2015/September/01 2015, doi: 10.1038/oncsis.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Musah S et al. , “Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification,” Proceedings of the National Academy of Sciences, vol. 111, no. 38, pp. 13805–13810, 2014, doi: doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Engler AJ, Sen S, Sweeney HL, and Discher DE, “Matrix Elasticity Directs Stem Cell Lineage Specification,” Cell, vol. 126, no. 4, pp. 677–689, 2006, doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [107].Blaschke SJ et al. , “Substrate Elasticity Exerts Functional Effects on Primary Microglia,” (in English), Frontiers in Cellular Neuroscience, Original Research vol. 14, 2020-November-05 2020, doi: 10.3389/fncel.2020.590500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hu Y et al. , “Matrix stiffness changes affect astrocyte phenotype in an in vitro injury model,” NPG Asia Materials, vol. 13, no. 1, p. 35, 2021/April/16 2021, doi: 10.1038/s41427-021-00304-0. [DOI] [Google Scholar]
- [109].Thielen B and Meng E, “A comparison of insertion methods for surgical placement of penetrating neural interfaces,” (in eng), J Neural Eng, vol. 18, no. 4, Apr 26 2021, doi: 10.1088/1741-2552/abf6f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Weltman A, Yoo J, and Meng E, “Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Microfabrication,” (in eng), Micromachines (Basel), vol. 7, no. 10, Oct 4 2016, doi: 10.3390/mi7100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang X et al. , “A Parylene Neural Probe Array for Multi-Region Deep Brain Recordings,” (in eng), J Microelectromech Syst, vol. 29, no. 4, pp. 499–513, Aug 2020, doi: 10.1109/jmems.2020.3000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Luan L et al. , “Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration,” (in eng), Sci Adv, vol. 3, no. 2, p. e1601966, Feb 2017, doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hara SA, Kim BJ, Kuo JT, Lee CD, Meng E, and Pikov V, “Long-term stability of intracortical recordings using perforated and arrayed Parylene sheath electrodes,” (in eng), J Neural Eng, vol. 13, no. 6, p. 066020, Dec 2016, doi: 10.1088/1741-2560/13/6/066020. [DOI] [PubMed] [Google Scholar]
- [114].Bettinger CJ, Ecker M, Kozai TDY, Malliaras GG, Meng E, and Voit W, “Recent advances in neural interfaces—Materials chemistry to clinical translation,” MRS Bulletin, vol. 45, no. 8, pp. 655–668, 2020/August/01 2020, doi: 10.1557/mrs.2020.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chang TY et al. , “Cell and Protein Compatibility of Parylene-C Surfaces,” Langmuir, vol. 23, no. 23, pp. 11718–11725, 2007/November/01 2007, doi: 10.1021/la7017049. [DOI] [PubMed] [Google Scholar]
- [116].Kim BJ and Meng E, “Micromachining of Parylene C for bioMEMS,” Polymers for Advanced Technologies, vol. 27, no. 5, pp. 564–576, 2016, doi: 10.1002/pat.3729. [DOI] [Google Scholar]
- [117].Lin CY et al. , “Bio-Compatibility and Bio-Insulation of Implantable Electrode Prosthesis Ameliorated by A-174 Silane Primed Parylene-C Deposited Embedment,” (in eng), Micromachines (Basel), vol. 11, no. 12, Nov 30 2020, doi: 10.3390/mi11121064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Leung BK, Biran R, Underwood CJ, and Tresco PA, “Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry,” Biomaterials, vol. 29, no. 23, pp. 3289–3297, 2008/August/01/ 2008, doi: 10.1016/j.biomaterials.2008.03.045. [DOI] [PubMed] [Google Scholar]
- [119].Lo MC et al. , “Evaluating the in vivo glial response to miniaturized parylene cortical probes coated with an ultra-fast degrading polymer to aid insertion,” (in eng), J Neural Eng, vol. 15, no. 3, p. 036002, Jun 2018, doi: 10.1088/1741-2552/aa9fad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sohal HS et al. , “The sinusoidal probe: a new approach to improve electrode longevity,” (in English), Frontiers in Neuroengineering, Original Research vol. 7, 2014-April-29 2014, doi: 10.3389/fneng.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Golda-Cepa M et al. , “Multifunctional PLGA/Parylene C Coating for Implant Materials: An Integral Approach for Biointerface Optimization,” ACS Applied Materials & Interfaces, vol. 8, no. 34, pp. 22093–22105, 2016/August/31 2016, doi: 10.1021/acsami.6b08025. [DOI] [PubMed] [Google Scholar]
- [122].Lee CD et al. , “Matrigel coatings for Parylene sheath neural probes,” Journal of Biomedical Materials Research Part B: Applied Biomaterials, vol. 104, no. 2, pp. 357–368, 2016, doi: 10.1002/jbm.b.33390. [DOI] [PubMed] [Google Scholar]
- [123].Lee HC, Gaire J, Currlin SW, McDermott MD, Park K, and Otto KJ, “Foreign Body Response to Intracortical Microelectrodes Is Not Altered with Dip-Coating of Polyethylene Glycol (PEG),” (in eng), Front Neurosci, vol. 11, p. 513, 2017, doi: 10.3389/fnins.2017.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhao Z and Zharnikov M, “Elastic Properties of Poly(ethylene glycol) Nanomembranes and Respective Implications,” (in eng), Membranes (Basel), vol. 12, no. 5, May 10 2022, doi: 10.3390/membranes12050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chapla R, Alhaj Abed M, and West J, “Modulating Functionalized Poly(ethylene glycol) Diacrylate Hydrogel Mechanical Properties through Competitive Crosslinking Mechanics for Soft Tissue Applications,” (in eng), Polymers (Basel), vol. 12, no. 12, Dec 16 2020, doi: 10.3390/polym12123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Naahidi S et al. , “Biocompatibility of hydrogel-based scaffolds for tissue engineering applications,” Biotechnology Advances, vol. 35, no. 5, pp. 530–544, 2017/September/01/ 2017, doi: 10.1016/j.biotechadv.2017.05.006. [DOI] [PubMed] [Google Scholar]
- [127].Madhusudanan P, Raju G, and Shankarappa S, “Hydrogel systems and their role in neural tissue engineering,” Journal of The Royal Society Interface, vol. 17, no. 162, p. 20190505, 2020, doi: doi: 10.1098/rsif.2019.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ziai Y et al. , “Conducting polymer-based nanostructured materials for brain-machine interfaces,” (in eng), Wiley Interdiscip Rev Nanomed Nanobiotechnol, vol. 15, no. 5, p. e1895, Sep-Oct 2023, doi: 10.1002/wnan.1895. [DOI] [PubMed] [Google Scholar]
- [129].Lu Y et al. , “Poly(vinyl alcohol)/poly(acrylic acid) hydrogel coatings for improving electrode–neural tissue interface,” Biomaterials, vol. 30, no. 25, pp. 4143–4151, 2009/September/01/ 2009, doi: 10.1016/j.biomaterials.2009.04.030. [DOI] [PubMed] [Google Scholar]
- [130].Thomas DA and Sperling LH, “Chapter 11 - Interpenetrating Polymer Networks,” in Polymer Blends, Paul DR and Newman S Eds.: Academic Press, 1978, pp. 1–33. [Google Scholar]
- [131].Rinoldi C et al. , “Three-Dimensional Printable Conductive Semi-Interpenetrating Polymer Network Hydrogel for Neural Tissue Applications,” Biomacromolecules, vol. 22, no. 7, pp. 3084–3098, 2021/July/12 2021, doi: 10.1021/acs.biomac.1c00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kleber C, Bruns M, Lienkamp K, Rühe J, and Asplund M, “An interpenetrating, microstructurable and covalently attached conducting polymer hydrogel for neural interfaces,” Acta Biomaterialia, vol. 58, pp. 365–375, 2017/August/01/ 2017, doi: 10.1016/j.actbio.2017.05.056. [DOI] [PubMed] [Google Scholar]
- [133].Ye L et al. , “Carbon Nanotube–Hydrogel Composites Facilitate Neuronal Differentiation While Maintaining Homeostasis of Network Activity,” Advanced Materials, vol. 33, no. 41, p. 2102981, 2021, doi: 10.1002/adma.202102981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rinoldi C et al. , “In Vivo Chronic Brain Cortex Signal Recording Based on a Soft Conductive Hydrogel Biointerface,” ACS Applied Materials & Interfaces, vol. 15, no. 5, pp. 6283–6296, 2023/February/08 2023, doi: 10.1021/acsami.2c17025. [DOI] [PubMed] [Google Scholar]
- [135].Chong J et al. , “Highly conductive tissue-like hydrogel interface through template-directed assembly,” Nature Communications, vol. 14, no. 1, p. 2206, 2023/April/18 2023, doi: 10.1038/s41467-023-37948-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Novikova LN, Mosahebi A, Wiberg M, Terenghi G, Kellerth JO, and Novikov LN, “Alginate hydrogel and matrigel as potential cell carriers for neurotransplantation,” (in eng), J Biomed Mater Res A, vol. 77, no. 2, pp. 242–52, May 2006, doi: 10.1002/jbm.a.30603. [DOI] [PubMed] [Google Scholar]
- [137].Shen W et al. , “Extracellular matrix-based intracortical microelectrodes: Toward a microfabricated neural interface based on natural materials,” Microsystems & Nanoengineering, vol. 1, no. 1, p. 15010, 2015/June/29 2015, doi: 10.1038/micronano.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Passaniti A, Kleinman HK, and Martin GR, “Matrigel: history/background, uses, and future applications,” (in eng), J Cell Commun Signal, vol. 16, no. 4, pp. 621–626, Dec 2022, doi: 10.1007/s12079-021-00643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hughes CS, Postovit LM, and Lajoie GA, “Matrigel: a complex protein mixture required for optimal growth of cell culture,” (in eng), Proteomics, vol. 10, no. 9, pp. 1886–90, May 2010, doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- [140].Kourgiantaki A et al. , “Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury,” npj Regenerative Medicine, vol. 5, no. 1, p. 12, 2020/June/15 2020, doi: 10.1038/s41536-020-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Farrukh A, Zhao S, and del Campo A, “Microenvironments Designed to Support Growth and Function of Neuronal Cells,” (in English), Frontiers in Materials, Review vol. 5, 2018-November-02 2018, doi: 10.3389/fmats.2018.00062. [DOI] [Google Scholar]
- [142].Maksoud FJ et al. , “Porous biomaterials for tissue engineering: a review,” Journal of Materials Chemistry B, 10.1039/D1TB02628C vol. 10, no. 40, pp. 8111–8165, 2022, doi: 10.1039/D1TB02628C. [DOI] [PubMed] [Google Scholar]
- [143].Wen Y et al. , “3D printed porous ceramic scaffolds for bone tissue engineering: a review,” Biomaterials Science, 10.1039/C7BM00315C vol. 5, no. 9, pp. 1690–1698, 2017, doi: 10.1039/C7BM00315C. [DOI] [PubMed] [Google Scholar]
- [144].Yan M, Wang L, Wu Y, Wang L, and Lu Y, “Three-dimensional highly porous hydrogel scaffold for neural circuit dissection and modulation,” Acta Biomaterialia, vol. 157, pp. 252–262, 2023/February/01/ 2023, doi: 10.1016/j.actbio.2022.12.011. [DOI] [PubMed] [Google Scholar]
- [145].Li H, Wijekoon A, and Leipzig ND, “3D Differentiation of Neural Stem Cells in Macroporous Photopolymerizable Hydrogel Scaffolds,” PLOS ONE, vol. 7, no. 11, p. e48824, 2012, doi: 10.1371/journal.pone.0048824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].George J, Hsu C-C, Nguyen LTB, Ye H, and Cui Z, “Neural tissue engineering with structured hydrogels in CNS models and therapies,” Biotechnology Advances, vol. 42, p. 107370, 2020/September/01/ 2020, doi: 10.1016/j.biotechadv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- [147].Nguyen V-T et al. , “Anti-inflammatory effects of sodium alginate/gelatine porous scaffolds merged with fucoidan in murine microglial BV2 cells,” International Journal of Biological Macromolecules, vol. 93, pp. 1620–1632, 2016/December/01/ 2016, doi: 10.1016/j.ijbiomac.2016.05.078. [DOI] [PubMed] [Google Scholar]
- [148].Huang W-C et al. , “Multifunctional 3D Patternable Drug-Embedded Nanocarrier-Based Interfaces to Enhance Signal Recording and Reduce Neuron Degeneration in Neural Implantation,” Advanced Materials, vol. 27, no. 28, pp. 4186–4193, 2015, doi: 10.1002/adma.201500136. [DOI] [PubMed] [Google Scholar]
- [149].Yang F, Xu CY, Kotaki M, Wang S, and Ramakrishna S, “Characterization of neural stem cells on electrospun poly(L-lactic acid) nanofibrous scaffold,” Journal of Biomaterials Science, Polymer Edition, vol. 15, no. 12, pp. 1483–1497, 2004/January/01 2004, doi: 10.1163/1568562042459733. [DOI] [PubMed] [Google Scholar]
- [150].Amini S, Salehi H, Setayeshmehr M, and Ghorbani M, “Natural and synthetic polymeric scaffolds used in peripheral nerve tissue engineering: Advantages and disadvantages,” Polymers for Advanced Technologies, vol. 32, no. 6, pp. 2267–2289, 2021, doi: 10.1002/pat.5263. [DOI] [Google Scholar]
- [151].Yuan N, Tian W, Sun L, Yuan R, Tao J, and Chen D, “Neural stem cell transplantation in a double-layer collagen membrane with unequal pore sizes for spinal cord injury repair,” (in eng), Neural Regen Res, vol. 9, no. 10, pp. 1014–9, May 15 2014, doi: 10.4103/1673-5374.133160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ganguly D, Johnson CDL, Gottipati MK, Rende D, Borca-Tasciuc DA, and Gilbert RJ, “Specific Nanoporous Geometries on Anodized Alumina Surfaces Influence Astrocyte Adhesion and Glial Fibrillary Acidic Protein Immunoreactivity Levels,” ACS Biomaterials Science & Engineering, vol. 4, no. 1, pp. 128–141, 2018/January/08 2018, doi: 10.1021/acsbiomaterials.7b00760. [DOI] [PubMed] [Google Scholar]
- [153].Jin S, Yao H, Krisanarungson P, Haukas A, and Ye K, “Porous membrane substrates offer better niches to enhance the Wnt signaling and promote human embryonic stem cell growth and differentiation,” (in eng), Tissue Eng Part A, vol. 18, no. 13–14, pp. 1419–30, Jul 2012, doi: 10.1089/ten.TEA.2011.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wei J, Pozzi D, Ulloa Severino FP, Torre V, and Chen Y, “Fabrication of PLGA nanofibers on PDMS micropillars for neuron culture studies,” Microelectronic Engineering, vol. 175, pp. 67–72, 2017/May/05/ 2017, doi: 10.1016/j.mee.2017.01.015. [DOI] [Google Scholar]
- [155].Vafaiee M, Mohammadpour R, Vossoughi M, Asadian E, Janahmadi M, and Sasanpour P, “Carbon Nanotube Modified Microelectrode Array for Neural Interface,” (in eng), Front Bioeng Biotechnol, vol. 8, p. 582713, 2020, doi: 10.3389/fbioe.2020.582713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Mohanta D, Patnaik S, Sood S, and Das N, “Carbon nanotubes: Evaluation of toxicity at biointerfaces,” (in eng), J Pharm Anal, vol. 9, no. 5, pp. 293–300, Oct 2019, doi: 10.1016/j.jpha.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Pulskamp K, Diabaté S, and Krug HF, “Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants,” Toxicology Letters, vol. 168, no. 1, pp. 58–74, 2007/January/10/ 2007, doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [158].Shao H et al. , “Carbon nanotube multilayered nanocomposites as multifunctional substrates for actuating neuronal differentiation and functions of neural stem cells,” (in eng), Biomaterials, vol. 175, pp. 93–109, Aug 2018, doi: 10.1016/j.biomaterials.2018.05.028. [DOI] [PubMed] [Google Scholar]
- [159].Mondal T et al. , “Thin films of functionalized carbon nanotubes support long-term maintenance and cardio-neuronal differentiation of canine induced pluripotent stem cells,” Nanomedicine: Nanotechnology, Biology and Medicine, vol. 40, p. 102487, 2022/February/01/ 2022, doi: 10.1016/j.nano.2021.102487. [DOI] [PubMed] [Google Scholar]
- [160].Imaninezhad M, Schober J, Griggs D, Ruminski P, Kuljanishvili I, and Zustiak SP, “Cell Attachment and Spreading on Carbon Nanotubes Is Facilitated by Integrin Binding,” (in eng), Front Bioeng Biotechnol, vol. 6, p. 129, 2018, doi: 10.3389/fbioe.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Li W et al. , “Activation of FAK and Src are receptor-proximal events required for netrin signaling,” Nature Neuroscience, vol. 7, no. 11, pp. 1213–1221, 2004/November/01 2004, doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Dinsmore CJ and Soriano P, “MAPK and PI3K signaling: At the crossroads of neural crest development,” (in eng), Dev Biol, vol. 444 Suppl 1, no. Suppl 1, pp. S79–s97, Dec 1 2018, doi: 10.1016/j.ydbio.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yu JSL and Cui W, “Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination,” Development, vol. 143, no. 17, pp. 3050–3060, 2016, doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- [164].Barrejón M, Rauti R, Ballerini L, and Prato M, “Chemically Cross-Linked Carbon Nanotube Films Engineered to Control Neuronal Signaling,” ACS Nano, vol. 13, no. 8, pp. 8879–8889, 2019/August/27 2019, doi: 10.1021/acsnano.9b02429. [DOI] [PubMed] [Google Scholar]
- [165].Chao T-I et al. , “Carbon nanotubes promote neuron differentiation from human embryonic stem cells,” Biochemical and Biophysical Research Communications, vol. 384, no. 4, pp. 426–430, 2009/July/10/ 2009, doi: 10.1016/j.bbrc.2009.04.157. [DOI] [PubMed] [Google Scholar]
- [166].Govek EE, Newey SE, and Van Aelst L, “The role of the Rho GTPases in neuronal development,” (in eng), Genes Dev, vol. 19, no. 1, pp. 1–49, Jan 1 2005, doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- [167].Stankiewicz TR and Linseman DA, “Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration,” (in eng), Front Cell Neurosci, vol. 8, p. 314, 2014, doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Chen Y-S and Hsiue G-H, “Directing neural differentiation of mesenchymal stem cells by carboxylated multiwalled carbon nanotubes,” Biomaterials, vol. 34, no. 21, pp. 4936–4944, 2013/July/01/ 2013, doi: 10.1016/j.biomaterials.2013.03.063. [DOI] [PubMed] [Google Scholar]
- [169].Liu D, Yi C, Zhang D, Zhang J, and Yang M, “Inhibition of Proliferation and Differentiation of Mesenchymal Stem Cells by Carboxylated Carbon Nanotubes,” ACS Nano, vol. 4, no. 4, pp. 2185–2195, 2010/April/27 2010, doi: 10.1021/nn901479w. [DOI] [PubMed] [Google Scholar]
- [170].Zhang T, Tang M, Yao Y, Ma Y, and Pu Y, “MWCNT interactions with protein: surface-induced changes in protein adsorption and the impact of protein corona on cellular uptake and cytotoxicity,” (in eng), Int J Nanomedicine, vol. 14, pp. 993–1009, 2019, doi: 10.2147/ijn.S191689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Tonelli FM et al. , “Carbon nanotube interaction with extracellular matrix proteins producing scaffolds for tissue engineering,” (in eng), Int J Nanomedicine, vol. 7, pp. 4511–29, 2012, doi: 10.2147/ijn.S33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Malarkey EB and Parpura V, “Carbon nanotubes in neuroscience,” (in eng), Acta Neurochir Suppl, vol. 106, pp. 337–41, 2010, doi: 10.1007/978-3-211-98811-4_62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Fabbro A et al. , “Adhesion to carbon nanotube conductive scaffolds forces action-potential appearance in immature rat spinal neurons,” (in eng), PLoS One, vol. 8, no. 8, p. e73621, 2013, doi: 10.1371/journal.pone.0073621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Cellot G et al. , “Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts,” Nature Nanotechnology, vol. 4, no. 2, pp. 126–133, 2009/February/01 2009, doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- [175].Cellot G et al. , “Carbon nanotube scaffolds tune synaptic strength in cultured neural circuits: novel frontiers in nanomaterial-tissue interactions,” (in eng), J Neurosci, vol. 31, no. 36, pp. 12945–53, Sep 7 2011, doi: 10.1523/jneurosci.1332-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].West AE et al. , “Calcium regulation of neuronal gene expression,” Proceedings of the National Academy of Sciences, vol. 98, no. 20, pp. 11024–11031, 2001, doi: doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Min JO and Yoon BE, “Glia and gliotransmitters on carbon nanotubes,” (in eng), Nano Rev Exp, vol. 8, no. 1, p. 1323853, 2017, doi: 10.1080/20022727.2017.1323853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Lee W-S et al. , “Improved gliotransmission by increasing intracellular Ca2+ via TRPV1 on multi-walled carbon nanotube platforms,” Journal of Nanobiotechnology, vol. 20, no. 1, p. 367, 2022/August/11 2022, doi: 10.1186/s12951-022-01551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Schmidt C, “Bioelectronics: The bionic material,” Nature, vol. 483, no. 7389, pp. S37–S37, 2012/March/01 2012, doi: 10.1038/483S37a. [DOI] [PubMed] [Google Scholar]
- [180].Bramini M et al. , “Interfacing Graphene-Based Materials With Neural Cells,” (in eng), Front Syst Neurosci, vol. 12, p. 12, 2018, doi: 10.3389/fnsys.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Zhang Y, Wang S, and Yang P, “Effects of Graphene-Based Materials on the Behavior of Neural Stem Cells,” Journal of Nanomaterials, vol. 2020, p. 2519105, 2020/July/03 2020, doi: 10.1155/2020/2519105. [DOI] [Google Scholar]
- [182].Li N et al. , “The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates,” (in eng), Biomaterials, vol. 32, no. 35, pp. 9374–82, Dec 2011, doi: 10.1016/j.biomaterials.2011.08.065. [DOI] [PubMed] [Google Scholar]
- [183].Lee WC et al. , “Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide,” (in eng), ACS Nano, vol. 5, no. 9, pp. 7334–41, Sep 27 2011, doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- [184].Pampaloni NP et al. , “Single-layer graphene modulates neuronal communication and augments membrane ion currents,” (in eng), Nat Nanotechnol, vol. 13, no. 8, pp. 755–764, Aug 2018, doi: 10.1038/s41565-018-0163-6. [DOI] [PubMed] [Google Scholar]
- [185].Kostarelos K, Vincent M, Hebert C, and Garrido JA, “Graphene in the Design and Engineering of Next-Generation Neural Interfaces,” (in eng), Adv Mater, vol. 29, no. 42, Nov 2017, doi: 10.1002/adma.201700909. [DOI] [PubMed] [Google Scholar]
- [186].Masvidal-Codina E et al. , “High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors,” (in eng), Nat Mater, vol. 18, no. 3, pp. 280–288, Mar 2019, doi: 10.1038/s41563-018-0249-4. [DOI] [PubMed] [Google Scholar]
- [187].Esteban-Linares A et al. , “Graphene-based microfluidic perforated microelectrode arrays for retinal electrophysiological studies,” Lab on a Chip, 10.1039/D3LC00064H vol. 23, no. 9, pp. 2193–2205, 2023, doi: 10.1039/D3LC00064H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Thunemann M et al. , “Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays,” Nature Communications, vol. 9, no. 1, p. 2035, 2018/May/23 2018, doi: 10.1038/s41467-018-04457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Liu Y, Zhao Y, Sun B, and Chen C, “Understanding the Toxicity of Carbon Nanotubes,” Accounts of Chemical Research, vol. 46, no. 3, pp. 702–713, 2013/March/19 2013, doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- [190].Zhang Y et al. , “Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells,” (in eng), ACS Nano, vol. 4, no. 6, pp. 3181–6, Jun 22 2010, doi: 10.1021/nn1007176. [DOI] [PubMed] [Google Scholar]
- [191].Lotti F, Ranieri F, Vadalà G, Zollo L, and Di Pino G, “Invasive Intraneural Interfaces: Foreign Body Reaction Issues,” (in English), Frontiers in Neuroscience, Review vol. 11, 2017-September-06 2017, doi: 10.3389/fnins.2017.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Carnicer-Lombarte A, Chen S-T, Malliaras GG, and Barone DG, “Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics,” (in English), Frontiers in Bioengineering and Biotechnology, Systematic Review vol. 9, 2021-April-15 2021, doi: 10.3389/fbioe.2021.622524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Forcelli PA et al. , “Histocompatibility and in vivo signal throughput for PEDOT, PEDOP, P3MT, and polycarbazole electrodes,” (in eng), J Biomed Mater Res A, vol. 100, no. 12, pp. 3455–62, Dec 2012, doi: 10.1002/jbm.a.34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Chatterjee K, “In vitro Growth and Differentiation of Neuronal Synaptic Network on PEDOT: Tosylate Substrate,” 2017. [Google Scholar]
- [195].Skorupa M, Więcławska D, Czerwińska-Główka D, Skonieczna M, and Krukiewicz K, “Dopant-Dependent Electrical and Biological Functionality of PEDOT in Bioelectronics,” (in eng), Polymers (Basel), vol. 13, no. 12, Jun 11 2021, doi: 10.3390/polym13121948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ahmad S, Alam O, Naim MJ, Shaquiquzzaman M, Alam MM, and Iqbal M, “Pyrrole: An insight into recent pharmacological advances with structure activity relationship,” European journal of medicinal chemistry, vol. 157, pp. 527–561, 2018. [DOI] [PubMed] [Google Scholar]
- [197].Skopalová K et al. , “Modulation of Differentiation of Embryonic Stem Cells by Polypyrrole: The Impact on Neurogenesis,” (in eng), Int J Mol Sci, vol. 22, no. 2, Jan 6 2021, doi: 10.3390/ijms22020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Cellot G et al. , “PEDOT:PSS Interfaces Support the Development of Neuronal Synaptic Networks with Reduced Neuroglia Response In vitro,” (in eng), Front Neurosci, vol. 9, p. 521, 2015, doi: 10.3389/fnins.2015.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Ostrakhovitch EA, Byers JC, O’Neil KD, and Semenikhin OA, “Directed differentiation of embryonic P19 cells and neural stem cells into neural lineage on conducting PEDOT-PEG and ITO glass substrates,” (in eng), Arch Biochem Biophys, vol. 528, no. 1, pp. 21–31, Dec 1 2012, doi: 10.1016/j.abb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- [200].Dominguez-Alfaro A, Alegret N, Arnaiz B, Salsamendi M, Mecerreyes D, and Prato M, “Toward Spontaneous Neuronal Differentiation of SH-SY5Y Cells Using Novel Three-Dimensional Electropolymerized Conductive Scaffolds,” (in eng), ACS Appl Mater Interfaces, vol. 12, no. 51, pp. 57330–57342, Dec 23 2020, doi: 10.1021/acsami.0c16645. [DOI] [PubMed] [Google Scholar]
- [201].Thompson BC, Moulton SE, Richardson RT, and Wallace GG, “Effect of the dopant anion in polypyrrole on nerve growth and release of a neurotrophic protein,” Biomaterials, vol. 32, no. 15, pp. 3822–3831, 2011/May/01/ 2011, doi: 10.1016/j.biomaterials.2011.01.053. [DOI] [PubMed] [Google Scholar]
- [202].Stauffer WR and Cui XT, “Polypyrrole doped with 2 peptide sequences from laminin,” Biomaterials, vol. 27, no. 11, pp. 2405–2413, 2006/April/01/ 2006, doi: 10.1016/j.biomaterials.2005.10.024. [DOI] [PubMed] [Google Scholar]
- [203].Lundin V, Herland A, Berggren M, Jager EW, and Teixeira AI, “Control of neural stem cell survival by electroactive polymer substrates,” (in eng), PLoS One, vol. 6, no. 4, p. e18624, Apr 11 2011, doi: 10.1371/journal.pone.0018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Wang X et al. , “Evaluation of biocompatibility of polypyrrole in vitro and in vivo,” Journal of Biomedical Materials Research Part A, vol. 68A, no. 3, pp. 411–422, 2004, doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- [205].Cui X, Wiler J, Dzaman M, Altschuler RA, and Martin DC, “In vivo studies of polypyrrole/peptide coated neural probes,” Biomaterials, vol. 24, no. 5, pp. 777–787, 2003/February/01/ 2003, doi: 10.1016/S0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- [206].Gueye MN, Carella A, Faure-Vincent J, Demadrille R, and Simonato J-P, “Progress in understanding structure and transport properties of PEDOT-based materials: A critical review,” Progress in Materials Science, vol. 108, p. 100616, 2020/February/01/ 2020, doi: 10.1016/j.pmatsci.2019.100616. [DOI] [Google Scholar]
- [207].Kayser LV and Lipomi DJ, “Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS,” (in eng), Adv Mater, vol. 31, no. 10, p. e1806133, Mar 2019, doi: 10.1002/adma.201806133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Pisciotta A et al. , “PEDOT: PSS promotes neurogenic commitment of neural crest-derived stem cells,” (in eng), Front Physiol, vol. 13, p. 930804, 2022, doi: 10.3389/fphys.2022.930804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Patel R et al. , “Ile-Lys-Val-ala-Val (IKVAV) peptide for neuronal tissue engineering,” Polymers for Advanced Technologies, vol. 30, no. 1, pp. 4–12, 2019, doi: 10.1002/pat.4442. [DOI] [Google Scholar]
- [210].Ruan H et al. , “Biofunctionalized self-assembly of peptide amphiphile induces the differentiation of bone marrow mesenchymal stem cells into neural cells,” Molecular and Cellular Biochemistry, vol. 450, no. 1, pp. 199–207, 2019/January/01 2019, doi: 10.1007/s11010-018-3386-9. [DOI] [PubMed] [Google Scholar]
- [211].Farrukh A et al. , “Bifunctional Hydrogels Containing the Laminin Motif IKVAV Promote Neurogenesis,” (in eng), Stem Cell Reports, vol. 9, no. 5, pp. 1432–1440, Nov 14 2017, doi: 10.1016/j.stemcr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Li G et al. , “Bionic microenvironment-inspired synergistic effect of anisotropic micro-nanocomposite topology and biology cues on peripheral nerve regeneration,” Science Advances, vol. 7, no. 28, p. eabi5812, 2021, doi: doi: 10.1126/sciadv.abi5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Massia SP, Holecko MM, and Ehteshami GR, “In vitro assessment of bioactive coatings for neural implant applications,” Journal of Biomedical Materials Research Part A, vol. 68A, no. 1, pp. 177–186, 2004, doi: 10.1002/jbm.a.20009. [DOI] [PubMed] [Google Scholar]
- [214].Balion Z et al. , “Cerebellar Cells Self-Assemble into Functional Organoids on Synthetic, Chemically Crosslinked ECM-Mimicking Peptide Hydrogels,” (in eng), Biomolecules, vol. 10, no. 5, May 12 2020, doi: 10.3390/biom10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Revkova VA et al. , “Spidroin Silk Fibers with Bioactive Motifs of Extracellular Proteins for Neural Tissue Engineering,” ACS Omega, vol. 6, no. 23, pp. 15264–15273, 2021/June/15 2021, doi: 10.1021/acsomega.1c01576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Lee H, Dellatore SM, Miller WM, and Messersmith PB, “Mussel-inspired surface chemistry for multifunctional coatings,” (in eng), Science, vol. 318, no. 5849, pp. 426–30, Oct 19 2007, doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Yan J, Wu R, Liao S, Jiang M, and Qian Y, “Applications of Polydopamine-Modified Scaffolds in the Peripheral Nerve Tissue Engineering,” (in eng), Front Bioeng Biotechnol, vol. 8, p. 590998, 2020, doi: 10.3389/fbioe.2020.590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Kim S, Jang LK, Jang M, Lee S, Hardy JG, and Lee JY, “Electrically Conductive Polydopamine-Polypyrrole as High Performance Biomaterials for Cell Stimulation in Vitro and Electrical Signal Recording in Vivo,” (in eng), ACS Appl Mater Interfaces, vol. 10, no. 39, pp. 33032–33042, Oct 3 2018, doi: 10.1021/acsami.8b11546. [DOI] [PubMed] [Google Scholar]
- [219].Yang Y, Zhang Y, Chai R, and Gu Z, “A Polydopamine-Functionalized Carbon Microfibrous Scaffold Accelerates the Development of Neural Stem Cells,” (in English), Frontiers in Bioengineering and Biotechnology, Original Research vol. 8, 2020-June-23 2020, doi: 10.3389/fbioe.2020.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Golabchi A, Wu B, Cao B, Bettinger CJ, and Cui XT, “Zwitterionic polymer/polydopamine coating reduce acute inflammatory tissue responses to neural implants,” (in eng), Biomaterials, vol. 225, p. 119519, Dec 2019, doi: 10.1016/j.biomaterials.2019.119519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Lee Y, Shin H, Lee D, Choi S, Cho I-J, and Seo J, “A Lubricated Nonimmunogenic Neural Probe for Acute Insertion Trauma Minimization and Long-Term Signal Recording,” Advanced Science, vol. 8, no. 15, p. 2100231, 2021, doi: 10.1002/advs.202100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Stephanopoulos N et al. , “Bioactive DNA-Peptide Nanotubes Enhance the Differentiation of Neural Stem Cells Into Neurons,” Nano Letters, vol. 15, no. 1, pp. 603–609, 2015/January/14 2015, doi: 10.1021/nl504079q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Aldaye FA, Senapedis WT, Silver PA, and Way JC, “A structurally tunable DNA-based extracellular matrix,” (in eng), J Am Chem Soc, vol. 132, no. 42, pp. 14727–9, Oct 27 2010, doi: 10.1021/ja105431h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Fenniri H et al. , “Helical rosette nanotubes: design, self-assembly, and characterization,” (in eng), J Am Chem Soc, vol. 123, no. 16, pp. 3854–5, Apr 25 2001, doi: 10.1021/ja005886l. [DOI] [PubMed] [Google Scholar]
- [225].Zhou L, Yau A, Zhang W, and Chen Y, “Fabrication of a Biomimetic Nano-Matrix with Janus Base Nanotubes and Fibronectin for Stem Cell Adhesion,” (in eng), J Vis Exp, no. 159, May 10 2020, doi: 10.3791/61317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lee J, Sands I, Zhang W, Zhou L, and Chen Y, “DNA-inspired nanomaterials for enhanced endosomal escape,” Proceedings of the National Academy of Sciences, vol. 118, no. 19, p. e2104511118, 2021, doi: 10.1073/pnas.2104511118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Zhou L, Zhang W, Lee J, Kuhn L, and Chen Y, “Controlled Self-Assembly of DNA-Mimicking Nanotubes to Form a Layer-by-Layer Scaffold for Homeostatic Tissue Constructs,” ACS Applied Materials & Interfaces, vol. 13, no. 43, pp. 51321–51332, 2021/November/03 2021, doi: 10.1021/acsami.1c13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Sands I et al. , “Electrically Conductive DNA-Inspired Coating for Intracortical Neural Microelectrodes,” bioRxiv, p. 2023.06.19.545632, 2023, doi: 10.1101/2023.06.19.545632. [DOI] [Google Scholar]


