Abstract

Polymer membranes employed in gas separation play a pivotal role in advancing environmental sustainability, energy production, and gas purification technologies. Despite their significance, the current design and manufacturing of these membranes lack cradle-to-cradle approaches, contributing to plastic waste pollution. This study explores emerging solutions, including the use of biodegradable biopolymers such as polyhydroxybutyrate (PHB) and membrane recycling, with a focus on the specific impact of mechanical recycling on the performance of biodegradable gas separation membranes. This research represents the first systematic exploration of recycling biodegradable membranes for gas separation. Demonstrating that PHB membranes can be recycled and remanufactured without solvents using hot-melt extrusion and 3D printing, the research highlights PHB’s promising performance in developing more sustainable CO2 separations, despite an increase in gas permeability with successive recycling steps due to reduced polymer molecular weight. The study emphasizes the excellent thermal, chemical, and mechanical stability of PHB membranes, albeit with a marginal reduction in gas selectivity upon recycling. However, limitations in PHB’s molecular weight affecting extrudability and processability restrict the recycling to three cycles. Anticipating that this study will serve as a foundational exploration, we foresee more sophisticated recycling studies for gas separation membranes, paving the way for a circular economy in future membrane technologies.
Keywords: polymer membranes, gas separation, 3D printing, polymer recycling, cradle-to-cradle manufacturing, solvent-free fabrication, biopolymer
1. Introduction
Polymer membranes provide a cost-effective and environmentally friendly means to selectively separate gas mixtures by exploiting variations in gas solubilities, and/or diffusivities.1−3 In contrast to conventional separation methods like absorption, adsorption, and cryogenic distillation, polymer membranes offer advantages such as lower energy consumption, diminished environmental impact, and more compact equipment design.2,3 These distinctive features make polymer membranes indispensable for sustainable processes, encompassing carbon capture, biogas processing, and air and hydrogen purification.4−7
Historically, membrane development and research have prioritized functional efficiency, often neglecting environmental sustainability. Furthermore, given the typical replacement cycle of industrial membrane modules every three to five years,8 polymer membranes have contributed to substantial plastic waste, with nonbiodegradable materials frequently ending up in landfills or natural ecosystems.9 Consequently, there is a compelling need to design membranes with a cradle-to-cradle perspective, a sustainable design approach focusing on a product full lifecycle from production to reuse, ensuring that all materials can be repurposed or returned safely to the environment and considering their features throughout their life cycle.
Innovative approaches to establish a circular economy for polymer membranes involve the utilization of biodegradable polymers sourced from biological origins.10 Notably, cellulose acetate has undergone extensive exploration and application in gas membranes due to its distinctive attributes such as ease of processing, versatility, and eco-friendliness.11−17 Similarly, chitosan (CS) membranes have demonstrated effectiveness in both water treatment and gas separation.18−21 The molecular structure of CS, abundant in amino and hydroxyl groups, has proven to enhance CO2 absorption, especially in water-swollen states or when incorporated into mixed matrix membranes.22−25 Another illustration is provided by polylactic acid membranes, exhibiting effectiveness in biogas separation with a CO2 permeability of 70 Barrer and a selectivity of approximately 285 for CO2/CH4 and 26 for H2/CO2.26,27
In addition to the utilization of biodegradable polymers, recent research has concentrated on the recycling of end-of-life (EoL) polymeric membranes, particularly those employed in water treatment. According to Tian et al.,28 the recycling strategy should be tailored to the membrane condition. Severely damaged membranes undergo dissolution in organic solvents, followed by refabrication. For example, Patel et al. extracted polyvinylidene fluoride (PVDF) polymer using dimethylformamide (DMF).29 Wang et al.30 utilized N-methyl-2-pyrrolidone and DMF to dissolve EoL hollow fiber and flat sheet PVDF microfiltration/ultrafiltration (MF/UF) membranes. Subsequently, these membranes were reprocessed through the phase inversion method to produce recycled PVDF MF or UF membranes, demonstrating their potential as substrates for nanofiltration/reverse osmosis (NF/RO) membrane production.
For less damaged membranes, recycling occurs through regeneration, upcycling, or downcycling. Tian et al. employed PolarClean, an eco-friendly solvent, to recycle membranes used in municipal wastewater treatment.31,32 Some authors have embraced upcycling technology, incorporating interfacial polymerization (IP)—a polycondensation reaction—to manufacture polyamide NF/RO membranes on EoL MF/UF membranes. Recycled NF membranes have also been produced from EoL MF membranes using various techniques, including three-dimensional (3D) printing.33−36
The objective of this research is to design biodegradable gas separation membranes and evaluate their recycling potential using solventless mechanical methods. In this investigation, poly(3-hydroxybutyrate) (PHB) is chosen as the membrane precursor due to its status as a bioderived and biodegradable plastic.37,38 Moreover, it is the most common type of polyhydroxyalkanoate (PHA) variations,39 thus serving as a model compound for others. PHB stands out as a compelling alternative to petroleum-based polymers across a range of applications. Additionally, PHB’s compatibility with the extrusion 3D printing process allows for precise control over the membrane’s structure, which is crucial for achieving the desired gas separation performance. This selection is further justified by PHB biosynthesis from bacterial sources and its current investigation at a pilot scale for production as a byproduct of activated sludge in wastewater treatment plants,40 underscoring its bioderived nature and highlighting its potential integration into existing infrastructural systems for sustainable applications. Furthermore, only limited studies have explored PHB for gas separation. Follain et al.41 utilized homopolymer PHB and copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) or PHBV, achieving selectivity ranges of 1.5 to 6.7 for CO2/O2 and 4.8 to 19 for CO2/N2, respectively. In a separate study, Siracusa et al.42 observed selectivity values ranging from 1.7 to 2.5 for CO2/O2 and 2.2 to 3.4 for CO2/N2 in PHB films. Nevertheless, PHB warrants further exploration due to its environmental characteristics.
This research employs mechanical recycling through hot-melt extrusion and manufacturing via 3D printing to eliminate the use of solvents. Membrane fabrication and recycling often rely on solvents, posing risks to sustainability, operational safety, environmental impact, and human health.43 Additionally, 3D printing, a layer-by-layer membrane fabrication technique, offers benefits such as design flexibility, rapid prototyping, cost-effectiveness for small batches, and reduced waste. Using an advanced form of 3D printing, the membrane’s structural attributes can be precisely controlled, including thickness, surface area, and overall geometry.44 This technique expands the boundaries of traditional membrane fabrication, increasing the surface area by adjusting the geometry and controlling the membrane thickness.
This study marks the first systematic exploration of recycling biodegradable gas separation membranes and introduces the development of 3D-printed filaments using pristine PHB. Furthermore, it investigates the recycling potential of the polymer and its impact on the physicochemical properties. This research represents a significant step toward the development of circular membranes with improved environmental, economic, and social characteristics.
2. Methodology
2.1. Materials
PHB used in this investigation had a molecular weight of 550 kg/mol and a granule size of 5 mm. It was purchased from Goodfellow Cambridge Ltd.—UK. The chemical structure of the PHB polymer is presented in Figure 1. To assess the barrier properties of the membranes, we used oxygen (O2), nitrogen (N2), methane (CH4), and carbon dioxide (CO2) from Sigma-Aldrich with purities of ≥99.5%.
Figure 1.

Chemical structure of PHB.
2.2. Membrane Preparation
The membrane fabrication process was initiated with the drying of the as-received PHB components in a vacuum oven at 60 °C for 2 h to remove any residual moisture. Subsequently, PHB polymer granules were fed into a Noztek Touch single-screw extruder, running at 10 rpm and 165 °C, to create pure PHB filaments. It is important to note that all filaments were generated without additives. These filaments were fed to an Ender 3 Pro 3D printer equipped with a 0.4 mm nozzle. The printer generated membranes with a thickness of 0.3 and 4.7 mm in diameter. The printing process was specified to maintain nozzle and bed temperatures of 220 and 50 °C, respectively, and print at a speed of 40 mm/s. Membranes were then thoroughly characterized to understand their physicochemical properties and gas permeation properties. Finally, the membranes were cut into smaller pieces and passed through the extruder to generate new filaments. Note that membranes were not grinded as this process had a significant effect on the degradation of the polymer as described in Section 3.2.
Figure 2 illustrates the iterative “polymer extrusion–membrane printing–membrane characterization” process employed to evaluate the recyclability of biodegradable membranes. Notably, consistent filament development in each recycling loop necessitated modifications to the extruder parameters, as detailed in Section 3.
Figure 2.
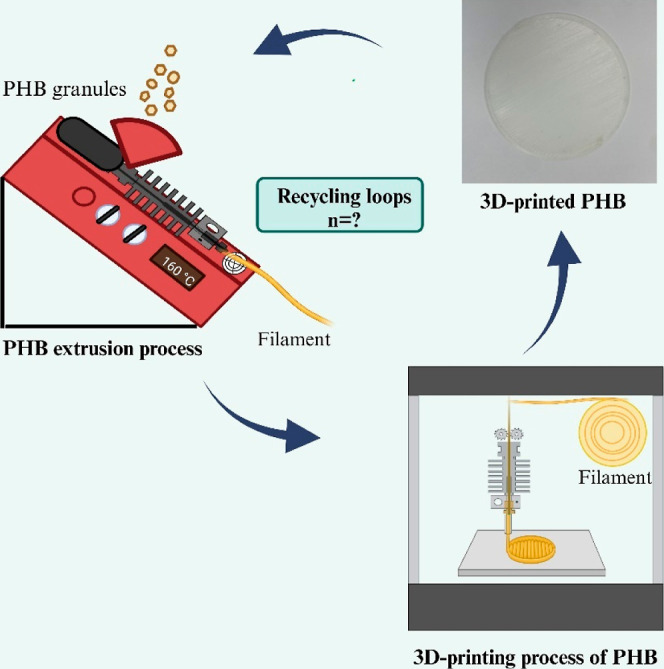
Schematic procedure of the recycling and 3D-printing cycle of PHB membranes, highlighting the closed loop from PHB granules to the printed membrane.
2.3. Membrane Characterization
2.3.1. Structural Characterization
The supramolecular structure of the membrane was analyzed by powder X-ray diffraction (PXRD). The PXRD scanning was performed on a STOE STADI P double setup operated at 40 kV and 40 mA using pure CuKα1 radiation with wavelength (λ) = 1.54059 Å. The scans ranged from 5 to 50° 2θ, with a step-size of 0.02° and a scan speed of 0.2 s per step. Likewise, the morphological characteristics of the fabricated membrane surface and cross sections were analyzed using a variable pressure scanning electron microscopy (SEM, SU3900, Hitachi, Japan).
2.3.2. Chemical Characterization
Fourier transform infrared spectroscopy (FTIR, Spectrum 100 PerkinElmer USA), configured with a total reflectance cell ranging from 4000 to 650 cm–1 was utilized for the chemical structure analysis. The FTIR analysis was aimed at ascertaining the functional group features of the respective membranes. As a standard procedure, prior to the analysis of the actual samples, a baseline scan was conducted in transmission mode with a spectral resolution of 4 cm–1, recording spectra across the entire reflectance cell range.
2.3.3. Thermal Characterization
The thermal characteristics (stability and degradation) of the membranes were assessed by both thermogravimetric analysis (TGA) and a differential scanning calorimeter (DSC). As a precautionary measure, prior to the thermal analysis by both TGA and DSC, the respective membrane samples were vacuum-dried overnight at 80 °C. The TGA was conducted using a thermogravimetric analyzer (TGA, TA Instruments Q-500). Approximately 20 mg of the respective membrane was fed into an alumina crucible and heated at the rate of 10 °C min–1, under an argon flow rate of 60 mL/min, from a temperature of 20 to 600 °C. Then, the thermal degradation, described in terms of weight loss as a function of temperature, was determined at the first derivative peak of the TGA curve, using the Setsoft 2000 thermal analysis software. The DSC analysis was done utilizing a DSC (TA Instruments Q2000), equipped with an intercooler refrigeration system. The respective membrane samples were subjected to a cycle of heating–cooling–heating at temperature ranging from −50 to 200 °C at a heating rate of 10 °C min–1 and nitrogen flow rate of 20 mL/min. Baseline curvature was minimized in this analysis by using an empty holder of aluminum as a reference in the alternative sample holder of the DSC. The degree of crystallinity (Xc) was determined using the following eq 1.
| 1 |
where ΔHm is the melting enthalpy per unit of weight of the PHB sample and ΔH100% denotes the enthalpy per unit weight of the 100% crystalline PHB, which is assumed to be 146 J/g.
2.3.4. Gel Permeation Chromatography
The Agilent gel permeation chromatography (GPC) and size exclusion chromatography (SEC) analysis was performed using an Agilent Infinity II MDS instrument configured with a differential refractive index, viscometry, dual-angle light scatters (LS), and variable wavelength UV detectors. The configuration of the equipment consisted of 2 × PLgel Mixed D columns (300 × 7.5 mm) and a PLgel 5 μm guard column. CHCl3 was used as the eluent, and ethanol was added as a flow rate marker. Prior to the actual sample analysis, the system was calibrated between 535 and 1,591,000 g mol–1 using poly(methyl methacrylate), and polystyrene standards (Agilent EasiVials). The respective samples to be analyzed were solubilized overnight, then filtered through a 0.22 μm pore size nylon membrane prior to injection. The samples were analyzed at a flow rate and temperature of 1 mL min–1 and 30 °C, respectively. Critical parameters of the PHB polymer, including dispersity (PD), molecular weight mass average (Mw), and number-average (Mn) were determined before and after membrane fabrication by using Agilent GPC/SEC software.
2.3.5. Mechanical Property Analysis
The mechanical properties of the respective PHB membranes were analyzed according to the ASTMD 882 standard method using an INSTRON (3369, England) instrument at room temperature. The following fabricated membranes PHB-1, PHB-2, and PHB-3 were investigated for tensile stress, tensile strain at break, and tensile modulus. The tensile strength is equal to the ratio of the maximum load to the cross-sectional area. The elongation of the polymer films was evaluated as the percentage of elongation. This was done by taking the ratio of the change in length and the original length of the films, expressed as a percentage. A total of three samples from each cycle were subjected to testing. In order to minimize the inaccuracy resulting from material overlap during the process of 3D printing, tension was intentionally applied in alignment with the printing direction (Figure S5).
2.3.6. Gas Sorption Analysis
The BET surface area and pore size distribution of the PHB, PHB-1, PHB-2, and PHB-3 were analyzed to assess the formation of any micropores. This was done through nitrogen adsorption and desorption measurements at 77 K, using a Micromeritics 3Flex 3500 volumetric gas sorption analyzer. The specific surface area was calculated by employing the Brunauer–Emmett–Teller (BET) method. This involved regression analysis of relative pressure data ranging from 0 to 1.0, in accordance with the guidelines provided by the manufacturer.
2.3.7. Gas Permeation Test
Single gas (CO2, O2, CH4, and N2) permeation tests of the membranes were measured by means of a constant volume/variable pressure time-lag apparatus. The gases were fed at both fixed pressure and variable pressure values at 25 °C, and permeability was evaluated from the steady-state rate of pressure increase at a fixed downstream volume. A detailed description of the procedure and experimental setup is presented.45
3. Results and Discussion
3.1. PHB Filament Manufacturing for 3D Printing
This study centered on producing PHB membranes via 3D printing, involving the creation of PHB filaments from both fresh granules and recycled PHB 3D-printed membranes. Table 1 outlines the temperature and motor speed utilized during each extrusion cycle. The initial membrane, PHB-1, was crafted from fresh PHB granules at 165 °C and 10 rpm, yielding high-quality, consistent filaments. After PHB-1 characterization, the membranes were re-extruded, using the same extruder process parameters, to produce similar quality filaments. These filaments were used to produce PHB-2, and the recycling continued with the temperature reduced to 155 °C for optimal 3D printing in PHB-3. However, due to changes in the mechanical properties of PHB on re-extrusion, the polymer melt was not sufficiently viscous to be processed following a third extrusion at 145 °C.
Table 1. Parameters of Extruded PHB Polymer Filaments.
| PHB polymer membrane | extrusion temperature (°C) | extruder motor speed (rpm) |
|---|---|---|
| PHB granule | 165 | 10 |
| first cycle of the printed membrane, PHB-1 | 165 | 10 |
| second cycle of the printed membrane, PHB-2 | 155 | 10 |
| third cycle of the printed membrane, PHB-3 | 145 | 10 (unsuccessful) |
Each recycling step exhibited a decreased viscosity, necessitating lower extrusion temperatures. Despite this, all fabricated membranes, including those from recycled PHB, displayed ease of handling, high flexibility, and flawless surfaces, as depicted in Figure 3. Notably, no distinguishable differences were observed in the surfaces or coloration between membranes produced from original PHB granules and the recycled PHB polymer.
Figure 3.
3D-printed PHB polymeric membranes: (a) illustration of PHB-1 flexibility; (b) PHB-1; (c) PHB-2; (d) PHB-3.
3.2. GPC: Molecular Weight Changes During Recycling
GPC was used to characterize PHB and determine the molecular weight distributions at different recycling loops. Table 2 shows the values for Mn, Mw, and the polydispersity index (PDI) (calculated as Mw/Mn) of the different recycled membranes. Mn represents the statistical average molecular weight of all polymer chains in a sample, which can be estimated through polymerization mechanisms or ascertained by techniques that assess the quantity of molecules in a specific weight sample, like colligative methods, including end-group assay. In contrast to Mn, Mw incorporates the molecular weight of each chain when calculating the average molecular weight, meaning that heavier chains have a more significant impact on Mw. Mw is measured using methods focusing on molecular size instead of just the number of molecules, such as light scattering techniques.46
Table 2. Summary of Polymer Properties, Mn, Mw, and the PDI of Neat PHB Granules and Ground PHB and PHB Membranes.
| membrane samples | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|
| PHB granule | 550,000 | ||
| PHB grinded | 60,975 | 142,712 | 2.3 |
| PHB-1 | 86,121 | 224,699 | 2.6 |
| PHB-2 | 68,358 | 200,559 | 2.9 |
| PHB-3 | 63,657 | 186,303 | 2.9 |
The results show that the raw PHB granules have the highest Mw. Upon thermal recycling, the polymer chains were shortened, reducing the Mw. This observation is consistent with those reported across the literature.47,48 The observed reduction in Mw and Mn is attributed to polymer chain scission, as depicted in Figure 4, which shows a shortening of the polymer chains. Chain scission results in fewer entanglements and a decrease in the viscosity across successive recycling cycles. Concurrently, the PDI value increased after each cycle, indicating that chain scission led to a more varied distribution of chain lengths within the polymer.
Figure 4.
Schematic of the thermal degradation of PHB by random chain scission.
Although all experiments to produce the membranes in this work were subject to melt extrusion; as a control experiment, raw PHB granules were subject to a grinding process to assess the effect of mechanical action on the reduction of the molecular weight. The results showed a significant reduction in Mw due to chain scission caused by the grinding process. In contrast, as shown in Table 2, recycled membranes revealed that the 3D printing process resulted in no significant reduction in Mw, even after multiple recycling cycles, when compared to grinding. Although PHB undergoes a dual-extrusion process during its application in 3D printing; initially, PHB is extruded to create the filament, which is subsequently used in the 3D printing process and then extruded again during printing. This repeated exposure to high temperatures may adversely affect the material’s thermal stability. This implies that the mechanical force from the grinding process impacted the polymer more than did the thermal effect. This assertion is supported by the conclusions reported in previously published studies.48−50
3.3. Structural Characterization
The XRD spectra of the neat PHB granule, and the respective cycles of recycled PHB are presented in Figure S1. The diffractograms show similar crystalline characteristics depicting orthorhombic crystalline planes,51,52 with three characteristic crystalline peaks at 2θ 13.5, 16.9, and 25.5° assigned to the (020), (110), and (130) planes of the orthorhombic unit cell. This observation is consistent with the findings acknowledged by the Joint Committee on Powder Diffraction Standards (JCPDS).53 In addition, several other weaker reflections were observed in the spectra. For instance, the peak at a 2θ of 21.02°, which is assigned to reflection of the (021) plane shows that the respective 3D-printed PHB membrane spectra have some amount of orthorhombic β-form crystal with zigzag conformation. The manifestation of the β-form crystal could contribute to the appearance of a shoulder prior to the melting peak on the DSC, which is similar to the observation reported in the referenced study.52 The XRD images show that the crystallinity of the 3D-printed membrane was not significantly affected by the recycling process. This finding is supported by the appearance of characteristic crystalline peaks for PHB, PHB-1, PHB-2, and PHB-3, all displaying the same 2θ values.
The morphological structure of each PHB membrane was examined via SEM to assess any potential changes in the membranes. Figures S2 and 5 display the surface and cross-section SEM images, respectively, of the PHB-1, PHB-2, and PHB-3 membranes. The PHB-1 membrane, with a higher molecular weight, displayed a defect-free, smooth surface with clear layering lines characteristic of 3D printing (Figure S2a1). Layering lines on the membrane surface lead to an increase in surface area compared to conventional flat membranes.54 3D printing can therefore enable membrane design optimization for superior gas separation performance. PHB-1 membrane’s cross-section (Figure 5a) showed a dense structure, suggesting tightly packed polymer chains typical of high molecular weight polymers. This density is attributed to chain entanglements limiting mobility and resulting in a compact formation.55−57Figure S2b1 shows a defect-free surface for the PHB-2 membrane. However, voids or pore structures were observed in the cross-section (Figure 5b), indicating a less dense polymeric structure compared to the PHB-1 membrane. This could be due to a lower molecular weight, reducing chain entanglements and consequently increasing the number of chain ends. The GPC analysis in Table 2 revealed that membrane PHB-2 has lower Mn and Mw compared to PHB-1, which implies more chain ends that are more mobile and less tightly packed than the middle parts of the chain. As a result, areas near the chain ends tend to have more free volume and allow for a more open structure.56,58,59 This trend was also observed on PHB-3 membranes, which displayed the lowest molecular weight values (Figure 5c).
Figure 5.

SEM images for (a) PHB-1, (b) PHB-2, and (c) PHB-3 cross section.
To confirm the formation of pores, BET surface areas of PHB-1, PHB-2, and PHB-3 were determined through low-pressure nitrogen gas adsorption at −195 °C. The resulting BET surface areas were found to be 1.05 m2/g for PHB-1, 1.18 m2/g for PHB-2, and 1.28 m2/g for PHB-3. With each recycling cycle, the observed gradual rise in surface area may indicate the formation of additional voids (Supporting Information, S1).
3.4. Chemical Characterization
FTIR analysis was performed on the membranes to assess any potential changes in the chemical structure of the polymer. Figure S3 displays the FTIR spectra of the PHB granule and different membranes. The scanning range for FT-IR was 500–4000 cm–1, revealing several key features. A prominent absorption band at 1720 cm–1 was observed, representing the ester carbonyl group, aligned with the C=O stretching bond within the molecular chain. Between 1163 and 1210 cm–1, a series of absorption bands indicated ester group C–O bond stretching. The bending vibrations seen at 2969 and 2932 cm–1 were indicative of the presence of the methyl group, and the peak at 1378 cm–1 was associated with the methyl group symmetric bending. The band at 1454 cm–1 suggested the asymmetric bending of the −CH2 and −CH3 groups. Moreover, a moderately intense band at 3435 cm–1 was identified as belonging to the hydroxyl group, which agrees with a typical FTIR spectrum of PHB reported in literature51,60 The findings imply that recycling the polymer did not alter its chemical properties nor introduce any new chemical components.61
3.5. Thermal Characterization
The evaluation of the thermal properties of the fabricated PHB membranes was conducted by using TGA and DSC, as illustrated in Figure 6. The TGA shows no major differences between samples with an initial thermal degradation phase between 235 and 293 °C, resulting in an 85% reduction in weight. Further degradation was observed between 293 and 400 °C, resulting in an additional 11% weight loss. PHB exhibited a significant thermal degradation at 400 °C, corresponding to a weight loss of 96%. Also, a minor shift is observed at the beginning of the curve after each cycle, as depicted in Figure 6a. Notably, the degradation curves of the respective PHB membrane display comparable patterns as those previously published in literature and their characteristic temperatures remain consistent.41
Figure 6.
Thermal analysis of membranes: (a) TGA; (b) DSC curves for the 2nd heating cycle.
Figure 6b shows the DSC curves for the second heating cycle run performed during heating up to 180 °C for the 3D-printed PHB granule, PHB-1, PHB-2, and PHB-3, respectively. The thermal behavior of these membranes followed a similar trend. The main melting peaks were observed at Tm = 161 °C for the PHB granule and at Tm ranging from 156 to 158 °C for the respective recycled membranes (PHB-1, PHB-2, and PHB-3). It can be noted that after each recycling process, there was a gradual reduction of the melting temperature. This decrease in Tm can be attributed to the high temperatures and shear stresses encountered during 3D printing and the extrusion process, which lead to chain scission or the breaking of molecular chains in the polymer (Figure 4). This phenomenon is evident from the data in GPC result Table 2. Indeed, polymers with a lower molecular weight typically exhibit lower melting temperatures as they have fewer molecular entanglements and reduced intermolecular forces, thereby facilitating easier movement of the chains relative to each other and enabling a smoother transition to a liquid state.62,63Table 3 shows the values of the thermal properties (Tcc—cold crystallization; Tm—melting point for the first and second heating cycle; ΔHm—Enthalpy for the first and second heating cycle, and Xc %—degree of crystallinity). It was observed that the Tcc and ΔHm2 is stably maintained for all the membranes and occurred at approximately Tcc = 109 °C and ΔHm2 = 70 J/g. The Tcc is primarily influenced by the ability of polymer chains to arrange into a crystalline structure. These findings support the observation previously stated that the recycling of the PHB polymer did not alter the regularity of the polymer chains and, as such, did not impact the crystallization rate.
Table 3. Summarized Crystallinity of Membranes.
| membrane | Tm1 (°C) | Tm2 (°C) | ΔHm1 (J/g) | ΔHm2 (J/g) | Tcc (°C) | Xc % |
|---|---|---|---|---|---|---|
| PHB | 163 | 161.5 | 71.2 | 76.1 | 109.3 | 52.1 |
| PHB-1 | 158 | 157.7 | 70.7 | 75.6 | 109.1 | 51.7 |
| PHB-2 | 157 | 156.5 | 68.6 | 75.5 | 108.9 | 51.7 |
| PHB-3 | 157 | 156.1 | 70.9 | 75.2 | 109.7 | 51.5 |
3.6. Membrane Mechanical Properties
Table 4 presents a comprehensive summary of the mechanical properties of recycled PHB membranes, displaying Young’s modulus, tensile strength at break, and elongation at break. The initial data reveal that PHB-1 possesses a Young modulus of 3052 MPa, which exhibits a gradual decrease in subsequent membranes PHB-2 and PHB-3. Young’s modulus is a critical measure, reflecting the material resistance to deformation under stress, essentially quantifying the ratio of stress to strain. The observed reduction in Young’s modulus can be attributed to the polymer chain scission occurring with each recycling cycle. Longer polymer chains, which are more effective in stress transfer, give way to shorter chains, resulting in decreased stiffness and a lower Young modulus. Additionally, the repeated heating process during recycling may lead to the degradation of key structural elements of the polymer.
Table 4. Mechanical Properties of Fabricated PHB Membranes.
| membranes | Young’s modulus (MPa) | tensile strength at break (MPa) | elongation at break (%) |
|---|---|---|---|
| PHB-1 | 3052 | 18.8 | 3.1 |
| PHB-2 | 2963 | 17.7 | 2.7 |
| PHB-3 | 2954 | 16.3 | 2.5 |
Moreover, the observed data indicate a minor reduction in both tensile strength and elongation at break. This reduction corresponds to a decrease in softness and flexibility when subjected to mechanical stress, as depicted in Figure 7. This decline in mechanical properties can be attributed to repeated recycling, which is expected to shorten polymer chains, resulting in a less compact structure with fewer entanglements and a reduced molecular weight, as explained by refs (47,48, and 64) These changes, primarily resulting from polymer chain scission and structural degradation during recycling, highlight the challenges in maintaining the mechanical integrity of recycled polymers. This understanding is crucial for optimizing recycling processes and enhancing the performance of recycled materials in practical applications.
Figure 7.

Tensile stress vs strain curve of PHB membranes.
3.7. Gas Permeation Tests
Figure 8a displays the outcomes of single gas permeation tests (N2, CH4, O2, and CO2) conducted at 25 °C with a constant 3 bar feed pressure. This test evaluated the impact of recycling cycles on the separation performance of 3D-printed membranes created using the PHB polymer recycled at different cycles. PHB-1 displayed a CO2 permeability of 6 Barrer (0.02 GPU) and a selectivity of 13, 6, and 3 for CO2/N2, CO2/CH4, and O2/N2, respectively. PHB-2 revealed a modest enhancement in CO2 permeability to 7 Barrer (0.023 GPU). However, this was coupled with a discernible decrease in selectivity, yielding values of 12, 5.2, and 2.8 for CO2/N2, CO2/CH4, and O2/N2, respectively. Lastly, PHB-3 demonstrated the highest CO2 permeability among the tested membranes at 8.2 Barrer (0.027 GPU). Nonetheless, this was concomitant with a further reduction in selectivity.
Figure 8.
Pure gas permeability and ideal gas selectivity.
The physicochemical properties of a polymer, such as mechanical, thermal, viscosity, permeability, and others, are dictated by two elements: (a) chemical composition and (b) supramolecular structure.65,66 By demonstrating that the polymer did not undergo any chemical change (Figure S3), we can conclude that any change in permeability is due to changes in the supramolecular structure. Supramolecular structure is affected by polymer chain lengths, polymer crystallinity, and solvent/additive effects.67−69 As crystallinity remained the same (Figure S1) and no additional additives or solvents were used in the process, the data suggest that the cause for permeability changes is due to changes in molecular weight.
Gas transport through dense polymeric membranes is governed by the solution-diffusion mechanism, wherein a gas molecule initially adsorbs at the interface between the feed and the membrane, then diffuses through the membrane, and finally desorbs on the permeate side. Consequently, permeance is influenced by two key factors: solubility and diffusivity, both of which are a function of the membrane materials properties.70,71 Gas solubility is influenced by factors such as the condensability of the gas, indicated by its critical temperature (Tc), and the interaction between the gas and polymer chains. Similarly, gas diffusivity is impacted by several elements, including the size of the gas molecule as measured by its kinetic diameter (DK), the membrane fractional free volume, the interaction between gas and polymer chains, and the flexibility of the polymer chains.72 The results indicated that the gas permeability of the membrane progressively increased as the number of PHB polymer recycling cycles increase, following the sequence PHB-3 > PHB-2 > PHB-1. As the number of recycling cycles increases, the polymer chains are shortened, as indicated by a reduction in the molecular weight (Table 2); this, in turn, increases the free volume at the molecular level, allowing for quicker diffusion of small gas molecules.73,74 The permeability differences for N2, CH4, O2, and CO2 between the PHB-1 and PHB-3 membranes were 46, 47, 30, and 37%, respectively. Additionally, the results showed that the membranes exhibited size-selective behavior, with permeability decreasing as the kinetic diameter of the gas increased (CO2 > O2 > CH4 > N2). This observation aligns with other research findings and comparable with polymers of a similar type and structure, like other PHAs, as reported in various sources.41,42,49 Notably, CO2 permeability remained significantly high across all recycled membranes, largely due to the predominant positive effect of solubility,49 smaller kinetic diameter, and higher critical temperature (Tc = 31.05 °C and DK = 0.33 nm). Owing to these factors, CO2 gave a better solubility and diffusivity than CH4 (Tc = −82.45 °C and DK = 0.38 nm), O2 (Tc = −118.55 °C and DK = 0.346 nm), and N2 (Tc = −147.05 °C and DK = 0.364 nm), thereby leading to promising selectivity values for CO2/N2 and CO2/CH4.75 Given that the gas permeability values improved with the increase in PHB recycling cycles for the three recycled PHB membranes, it is concluded that membrane gas permeability is not adversely affected by polymer recycling.
In contrast, the selectivity of the membrane pair gases (CO2/N2, CO2/CH4, and O2/N2) gradually decreased with an increase in the number of PHB polymer recycling cycles, following the order PHB-3 < PHB-2 < PHB-1, as shown in Figure 8b. The decrease in CO2/N2, CO2/CH4, and O2/N2 selectivity between the PHB-1 and PHB-3 membranes was 9, 9, and 12%, respectively. As previously mentioned, the recycling cycles lead to a rearrangement of polymer chains, creating more free volume, and some defects and voids begin to emerge, reducing the gas selectivity of the membrane. This is due to morphological changes, as demonstrated in the cross-sectional SEM images of PHB-2 and PHB-3 (Figure 5). Nevertheless, it can be inferred from the observed increase in gas permeability and the slight reduction in ideal selectivity that polymer recycling can effectively be utilized in creating polymer membranes without significantly compromising their general properties. Moreover, as illustrated in the Robeson upper bound plot (Figure S7), the membrane’s performance aligns with the permeability and selectivity demonstrated by numerous biodegradable polymers.41,49,76 Given these characteristics, the PHB membranes explored in this study emerge as eco-friendly and solvent-free alternatives in comparison to their counterparts. This performance positions them as promising candidates for sustainable CO2 separations.
3.8. Physical Aging and Stability Tests
In this study, PHB-3 membranes were exposed to a single feed of CO2 gas at 3 bar pressure for 360 h continuously to evaluate the membrane stability. The results revealed a slight reduction in CO2 permeability after 360 h, amounting to only 3%. This suggests that the sample was stable throughout the test. This finding indicates that recycled PHB membranes are likely to retain their effectiveness without significant aging under similar continuous CO2 feed conditions. The resistance of the membrane against physical aging may be attributed to the constant occupation of free volume within the polymer matrix by CO2 molecules, which restricted the movement of the polymer chains, as suggested in the literature.77
Another membrane stability test was conducted for the PHB-3 membrane, and the gas permeability of all species (CO2, O2, CH4, and N2) was tested periodically for 730 h. In between tests, the membrane was stored in dry air. Figure 9 shows that the PHB-3 membrane displayed a slight decrease in the relative permeability for all gases. Notably, the CO2 permeability diminished by 4% within the first 340 h, with a further 1% decrease observed between 340 and 730 h. Conversely, N2, O2, and CH4 showed a more pronounced reduction in permeability compared to CO2, therefore, enhancing the membrane selectivity for CO2/CH4, CO2/N2, and O2/N2.
Figure 9.

Relative permeability and relative ideal selectivity over time of the PHB-3 membrane.
The reduction in permeance is attributed to an increase in polymer crystallinity over time as PHB undergoes significant cold-crystallization and structural rearrangement due to its glass transition temperature being below room temperature (Tg ∼ 2 °C).78,79 When PHB is stored at or above its Tg, the polymer chains acquire enough mobility to gradually form crystallites. However, over time, at temperatures above Tg, PHB can undergo annealing, allowing the chains to reorient and evenly distribute across the material. Even so, we recommend long-term stability tests to thoroughly understand the impact of physical aging and degradation over time on the gas permeability through PHB.
4. Conclusions
This study focuses on creating 3D-printed gas separation membranes using PHB, an environmentally friendly and biodegradable polymer, while investigating their recycling potential. The recycling method involves extrusion to recycle PHB membranes, followed by their reconstruction via 3D printing with each recycling cycle undergoing thorough characterization at every phase.
Our findings indicate that successive recycling rounds of PHB membranes led to decreased polymer chain length, directly impacting their thermal, mechanical, and gas permeation traits. Recycled membranes displayed heightened gas permeability but suffered a decline in gas selectivity. Nonetheless, these membranes exhibited stability during extended testing (over 300 h). This study underscores several key discoveries pertinent to the engineering of circular and sustainable gas separation membranes:
-
(1)
PHB, a biodegradable and sustainable polymer, can create 3D-printed membranes without the need for additives.
-
(2)
PHB membranes demonstrate promising performance for more sustainable CO2 separations.
-
(3)
3D printing proves to be a reliable method for manufacturing consistent PHB membranes.
-
(4)
The mechanical recycling of PHB membranes to produce new ones results in reduced polymer chain length with subsequent effects on physicochemical properties.
-
(5)
The gas permeation and selectivity of recycled PHB membranes suffer slight changes, deemed minor for gas separation applications.
Acknowledgments
The authors would like to express their gratitude to the Kuwait Institute for Scientific Research (KISR) for providing financial support for Sharifah Alkandari’s PhD studies. We would like to acknowledge the GPC experimentation and data collection, performed by WASC (polymer characterization facility) operated by Warwick University, EPSRC grant code EP/V007688/1. Moreover, the hot-melt extrusion work and 3D printing manufacturing was funded by BioMed 4.0, EPSRC grant code EP/V051083/1.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsaenm.4c00060.
Characterization of the 3D-printed PHB membranes including XRD patterns, FT-IR spectra, SEM, DSC, and nitrogen adsorption–desorption isotherms for 3D-printed PHB granules and recycled PHB membranes (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bernardo P.; Drioli E.; Golemme G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48 (10), 4638–4663. 10.1021/ie8019032. [DOI] [Google Scholar]
- Pandey P.; Chauhan R. S. Membranes for Gas Separation. Prog. Polym. Sci. 2001, 26 (6), 853–893. 10.1016/S0079-6700(01)00009-0. [DOI] [Google Scholar]
- Sidhikku Kandath Valappil R.; Ghasem N.; Al-Marzouqi M. Current and Future Trends in Polymer Membrane-Based Gas Separation Technology: A Comprehensive Review. J. Ind. Eng. Chem. 2021, 98, 103–129. 10.1016/j.jiec.2021.03.030. [DOI] [Google Scholar]
- Siagian U. W. R.; Raksajati A.; Himma N. F.; Khoiruddin K.; Wenten I. G. Membrane-Based Carbon Capture Technologies: Membrane Gas Separation vs. Membrane Contactor. J. Nat. Gas Sci. Eng. 2019, 67, 172–195. 10.1016/j.jngse.2019.04.008. [DOI] [Google Scholar]
- Scholes C. A.; Stevens G. W.; Kentish S. E. Membrane Gas Separation Applications in Natural Gas Processing. Fuel 2012, 96, 15–28. 10.1016/j.fuel.2011.12.074. [DOI] [Google Scholar]
- Chen X. Y.; Vinh-Thang H.; Ramirez A. A.; Rodrigue D.; Kaliaguine S. Membrane Gas Separation Technologies for Biogas Upgrading. RSC Adv. 2015, 5 (31), 24399–24448. 10.1039/C5RA00666J. [DOI] [Google Scholar]
- Mohshim D. F.; Mukhtar H. b.; Man Z.; Nasir R. Latest Development on Membrane Fabrication for Natural Gas Purification: A Review. J. Eng. 2013, 2013, 101746. 10.1155/2013/101746. [DOI] [Google Scholar]
- Ignatyev I. A.; Thielemans W.; Vander Beke B. Recycling of Polymers: A Review. ChemSusChem 2014, 7 (6), 1579–1593. 10.1002/cssc.201300898. [DOI] [PubMed] [Google Scholar]
- Baker R. W.Membrane Technology and Applications; Richard W., Ed.; John Wiley & Sons, 2012. [Google Scholar]
- Othman S. H. Bio-Nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-Sized Filler. Agric. Agric. Sci. Procedia. 2014, 2, 296–303. 10.1016/j.aaspro.2014.11.042. [DOI] [Google Scholar]
- Nikolaeva D.; Azcune I.; Tanczyk M.; Warmuzinski K.; Jaschik M.; Sandru M.; Dahl P. I.; Genua A.; Loïs S.; Sheridan E.; Fuoco A.; Vankelecom I. F. J. The Performance of Affordable and Stable Cellulose-Based Poly-Ionic Membranes in CO2/N2 and CO2/CH4 Gas Separation. J. Membr. Sci. 2018, 564, 552–561. 10.1016/j.memsci.2018.07.057. [DOI] [Google Scholar]
- Wu J.; Yuan Q. Gas Permeability of a Novel Cellulose Membrane. J. Membr. Sci. 2002, 204 (1–2), 185–194. 10.1016/S0376-7388(02)00037-6. [DOI] [Google Scholar]
- Gopi S.; Pius A.; Kargl R.; Kleinschek K. S.; Thomas S. Fabrication of Cellulose Acetate/Chitosan Blend Films as Efficient Adsorbent for Anionic Water Pollutants. Polym. Bull. 2019, 76 (3), 1557–1571. 10.1007/s00289-018-2467-y. [DOI] [Google Scholar]
- Wsoo M. A.; Shahir S.; Mohd Bohari S. P.; Nayan N. H. M.; Razak S. I. A. A Review on the Properties of Electrospun Cellulose Acetate and Its Application in Drug Delivery Systems: A New Perspective. Carbohydr. Res. 2020, 491, 107978. 10.1016/j.carres.2020.107978. [DOI] [PubMed] [Google Scholar]
- Raza A.; Farrukh S.; Hussain A.; Khan I.; Othman M. H. D.; Ahsan M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes 2021, 11 (4), 245. 10.3390/membranes11040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z.; Ottesen V.; Deng J.; Helberg R. M. L.; Deng L. A Brief Review of Nanocellulose Based Hybrid Membranes for CO2 Separation. Fibers 2019, 7 (5), 40. 10.3390/fib7050040. [DOI] [Google Scholar]
- Alkandari S. H.; Castro-Dominguez B. Electro-Casting for Superior Gas Separation Membrane Performance and Manufacturing. ACS Appl. Mater. Interfaces 2023, 15 (48), 56600–56611. 10.1021/acsami.3c14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar V.; Asghari M.; Dashti A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2 (3), 204–226. 10.1002/cben.201400025. [DOI] [Google Scholar]
- Xu D.; Hein S.; Wang K. Chitosan Membrane in Separation Applications. Mater. Sci. Technol. 2008, 24 (9), 1076–1087. 10.1179/174328408X341762. [DOI] [Google Scholar]
- Yang R.; Li H.; Huang M.; Yang H.; Li A. A Review on Chitosan-Based Flocculants and Their Applications in Water Treatment. Water Res. 2016, 95, 59–89. 10.1016/j.watres.2016.02.068. [DOI] [PubMed] [Google Scholar]
- Beppu M. M.; Vieira R. S.; Aimoli C. G.; Santana C. C. Crosslinking of Chitosan Membranes Using Glutaraldehyde: Effect on Ion Permeability and Water Absorption. J. Membr. Sci. 2007, 301 (1–2), 126–130. 10.1016/j.memsci.2007.06.015. [DOI] [Google Scholar]
- Xiao S.; Feng X.; Huang R. Y. M. Trimesoyl Chloride Crosslinked Chitosan Membranes for CO2/N2 Separation and Pervaporation Dehydration of Isopropanol. J. Membr. Sci. 2007, 306 (1–2), 36–46. 10.1016/j.memsci.2007.08.021. [DOI] [Google Scholar]
- Ito A.; Sato M.; Anma T. Permeability of CO2 through Chitosan Membrane Swollen by Water Vapor in Feed Gas. Die angewandte Makromolekulare Chemie 1997, 248, 85–94. 10.1002/apmc.1997.052480105. [DOI] [Google Scholar]
- Abdul Khalil A. K.; Saurabh C. K.; Adnan A.; Nurul Fazita M. R.; Syakir M. I.; Davoudpour Y.; Rafatullah M.; Abdullah C. K. M.; Haafiz M. K. M.; Dungani R. A Review on Chitosan-Cellulose Blends and Nanocellulose Reinforced Chitosan Biocomposites: Properties and Their Applications. Carbohydr. Polym. 2016, 150, 216–226. 10.1016/j.carbpol.2016.05.028. [DOI] [PubMed] [Google Scholar]
- Li N.; Wang Z.; Wang J. Water-Swollen Carboxymethyl Chitosan (CMC)/Polyamide (PA) Membranes with Octopus-Branched Nanostructures for CO2 Capture. J. Membr. Sci. 2022, 642, 119946. 10.1016/j.memsci.2021.119946. [DOI] [Google Scholar]
- Iulianelli A.; Russo F.; Galiano F.; Desiderio G.; Basile A.; Figoli A. PLA Easy Fil - White-Based Membranes for CO2 Separation. Greenhouse Gases: Sci. Technol. 2019, 9 (2), 360–369. 10.1002/ghg.1853. [DOI] [Google Scholar]
- Iulianelli A.; Algieri C.; Donato L.; Garofalo A.; Galiano F.; Bagnato G.; Basile A.; Figoli A. New PEEK-WC and PLA Membranes for H2 Separation. Int. J. Hydrogen Energy 2017, 42 (34), 22138–22148. 10.1016/j.ijhydene.2017.04.060. [DOI] [Google Scholar]
- Tian C.; Chen J.; Bai Z.; Wang X.; Dai R.; Wang Z. Recycling of End-of-Life Polymeric Membranes for Water Treatment: Closing the Loop. J. Membr. Sci. Lett. 2023, 3 (2), 100063. 10.1016/j.memlet.2023.100063. [DOI] [Google Scholar]
- Patel R. V.; Raj G. B.; Chaubey S.; Yadav A. Investigation on the Feasibility of Recycled Polyvinylidene Difluoride Polymer from Used Membranes for Removal of Methylene Blue: Experimental and DFT Studies. Water Sci. Technol. 2022, 86 (1), 194–210. 10.2166/wst.2022.193. [DOI] [PubMed] [Google Scholar]
- Wang X.; Xiao C.; Pan J.; Hu X.; Huan G. Recycled Performance of Waste Polyvinylidene Fluoride Hollow Fiber Membrane. Gaofenzi Cailiao Kexue Yu Gongcheng/Polymeric Materials Science and Engineering 2017, 33, 107–113. 10.16865/j.cnki.1000-7555.2017.04.019. [DOI] [Google Scholar]
- Tian C.; Wang T.; Han H.; Dai R.; Wang Z. Green Solvent Cleaning Removes Irrecoverable Foulants from End-of-Life Membranes in Membrane Bioreactors: Efficacy and Mechanisms. Environ. Sci. Technol. 2022, 56 (17), 12563–12572. 10.1021/acs.est.2c02321. [DOI] [PubMed] [Google Scholar]
- Tian C.; Chen J.; Li X.; Dai R.; Wang Z. Chemical Cleaning-solvent Treatment-hydrophilic Modification Strategy for Regenerating End-of-Life PVDF Membrane. J. Membr. Sci. 2023, 669, 121325. 10.1016/j.memsci.2022.121325. [DOI] [Google Scholar]
- Chowdhury M. R.; Steffes J.; Huey B. D.; McCutcheon J. R. 3D Printed Polyamide Membranes for Desalination. Science 2018, 361 (6403), 682–686. 10.1126/science.aar2122. [DOI] [PubMed] [Google Scholar]
- Dai R.; Zhou H.; Wang T.; Qiu Z.; Long L.; Lin S.; Tang C. Y.; Wang Z. Nanovehicle-Assisted Monomer Shuttling Enables Highly Permeable and Selective Nanofiltration Membranes for Water Purification. Nat. Water 2023, 1 (3), 281–290. 10.1038/s44221-022-00010-3. [DOI] [Google Scholar]
- Bai Y.; Liu B.; Li J.; Li M.; Yao Z.; Dong L.; Rao D.; Zhang P.; Cao X.; Villalobos L. F.; Zhang C.; An Q.-F.; Elimelech M. Microstructure Optimization of Bioderived Polyester Nanofilms for Antibiotic Desalination via Nanofiltration. Sci. Adv. 2023, 9 (18), eadg6134 10.1126/sciadv.adg6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B.; Ulbricht M.; Hu C.; Fan H.; Wang X.; Pan Y.-R.; Hosseini S. S.; Panglisch S.; Van der Bruggen B.; Wang Z. Membrane Life Cycle Management: An Exciting Opportunity for Advancing the Sustainability Features of Membrane Separations. Environ. Sci. Technol. 2023, 57 (8), 3013–3020. 10.1021/acs.est.2c09257. [DOI] [PubMed] [Google Scholar]
- McAdam B.; Brennan Fournet M.; McDonald P.; Mojicevic M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12 (12), 2908. 10.3390/polym12122908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomietto P.; Russo F.; Galiano F.; Loulergue P.; Salerno S.; Paugam L.; Audic J.-L.; De Bartolo L.; Figoli A. Sustainable Fabrication and Pervaporation Application of Bio-Based Membranes: Combining a Polyhydroxyalkanoate (PHA) as Biopolymer and CyreneTM as Green Solvent. J. Membr. Sci. 2022, 643, 120061. 10.1016/j.memsci.2021.120061. [DOI] [Google Scholar]
- Usurelu C. D.; Badila S.; Frone A. N.; Panaitescu D. M. Poly(3-Hydroxybutyrate) Nanocomposites with Cellulose Nanocrystals. Polymers 2022, 14 (10), 1974. 10.3390/polym14101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Sagastume F.; Hjort M.; Cirne D.; Gérardin F.; Lacroix S.; Gaval G.; Karabegovic L.; Alexandersson T.; Johansson P.; Karlsson A.; Bengtsson S.; Arcos-Hernández M.; Magnusson P.; Werker A. Integrated Production of Polyhydroxyalkanoates (PHAs) with Municipal Wastewater and Sludge Treatment at Pilot Scale. Bioresour. Technol. 2015, 181, 78–89. 10.1016/j.biortech.2015.01.046. [DOI] [PubMed] [Google Scholar]
- Follain N.; Chappey C.; Dargent E.; Chivrac F.; Cretois R.; Marais S. Structure and Barrier Properties of Biodegradable Polyhydroxyalkanoate Films. J. Phys. Chem. C 2014, 118, 6165–6177. 10.1021/jp408150k. [DOI] [Google Scholar]
- Siracusa V.; Ingrao C.; Karpova S. G.; Olkhov A. A.; Iordanskii A. L. Gas Transport and Characterization of Poly(3 Hydroxybutyrate) Films. Eur. Polym. J. 2017, 91, 149–161. 10.1016/j.eurpolymj.2017.03.047. [DOI] [Google Scholar]
- Clark J. H.; Tavener S. J. Alternative Solvents: Shades of Green. Org. Process Res. Dev. 2007, 11 (1), 149–155. 10.1021/op060160g. [DOI] [Google Scholar]
- Roy Barman S.; Gavit P.; Chowdhury S.; Chatterjee K.; Nain A. 3D-Printed Materials for Wastewater Treatment. JACS Au 2023, 3 (11), 2930–2947. 10.1021/jacsau.3c00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkandari S. H.; Lightfoot J.; Castro-Dominguez B. Asymmetric Membranes for Gas Separation: Interfacial Insights and Manufacturing. RSC Adv. 2023, 13 (21), 14198–14209. 10.1039/D3RA00995E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agilent T.Polymer Molecular Weight Distribution and Definitions of MW Averages Technical Overview. 2015, USA. www.agilent.com/chem/gpc-sec (accessed Dec 14, 2023).
- Lee D.; Lee Y.; Kim I.; Hwang K.; Kim N. Thermal and Mechanical Degradation of Recycled Polylactic Acid Filaments for Three-Dimensional Printing Applications. Polymers 2022, 14, 5385. 10.3390/polym14245385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso A. G.; Rosa D. Mechanical, Thermal and Morphological Characterization of Recycled LDPE/Corn Starch Blends. Carbohydr. Polym. 2005, 59, 1–9. 10.1016/j.carbpol.2004.08.018. [DOI] [Google Scholar]
- Papchenko K.; Degli Esposti M.; Minelli M.; Fabbri P.; Morselli D.; De Angelis M. G. New Sustainable Routes for Gas Separation Membranes: The Properties of Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Cast from Green Solvents. J. Membr. Sci. 2022, 660, 120847. 10.1016/j.memsci.2022.120847. [DOI] [Google Scholar]
- Oliveira R. R. d.; Oliveira T. A. d.; Silva L. R. C. d.; Barbosa R.; Alves T. S.; Carvalho L. H.; Rodrigues D. T. Effect of Reprocessing Cycles on the Morphology and Mechanical Properties of a Poly(Propylene)/Poly(Hydroxybutyrate) Blend and Its Nanocomposite. Mater. Res. 2021, 24 (4), e20200372 10.1590/1980-5373-mr-2020-0372. [DOI] [Google Scholar]
- Zainuddin M. Z.; Abu Bakar A. A.; Adam A. N.; Abdullah S. M.; Tamchek N.; Alauddin M. S.; Mahat M. M.; Wiwatcharagoses N.; Alforidi A.; Ghazali M. I. M. Mechanical and Structural Properties of Polyhydroxybutyrate as Additive in Blend Material in Additive Manufacturing for Medical Applications. Polymers 2023, 15 (8), 1849. 10.3390/polym15081849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frone A. N.; Nicolae C. A.; Eremia M. C.; Tofan V.; Ghiurea M.; Chiulan I.; Radu E.; Damian C. M.; Panaitescu D. M. Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly (3-Hydroxybutyrate) Films. Polymers 2020, 12 (11), 2446. 10.3390/polym12112446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quispe M. M.; Lopez O. V.; Boina D. A.; Stumbé J. F.; Villar M. A. Glycerol-Based Additives of Poly (3-Hydroxybutyrate) Films. Polym. Test. 2021, 93, 107005. 10.1016/j.polymertesting.2020.107005. [DOI] [Google Scholar]
- Al-Shimmery A.; Mazinani S.; Ji J.; Chew Y. J.; Mattia D. 3D Printed Composite Membranes with Enhanced Anti-Fouling Behaviour. J. Membr. Sci. 2019, 574, 76–85. 10.1016/j.memsci.2018.12.058. [DOI] [Google Scholar]
- Shirvani H.; Sadeghi M.; Taheri Afarani H.; Bagheri R. Polyurethane/Poly(Vinyl Alcohol) Blend Membranes for Gas Separation. Fibers Polym. 2018, 19 (5), 1119–1127. 10.1007/s12221-018-1023-6. [DOI] [Google Scholar]
- Tomé L. C.; Guerreiro D. C.; Teodoro R. M.; Alves V. D.; Marrucho I. M. Effect of Polymer Molecular Weight on the Physical Properties and CO2/N2 Separation of Pyrrolidinium-Based Poly(Ionic Liquid) Membranes. J. Membr. Sci. 2018, 549, 267–274. 10.1016/j.memsci.2017.12.019. [DOI] [Google Scholar]
- Taheri P.; Raisi A.; Salehi Maleh M. CO 2-Selective Poly (Ether-Block-Amide)/Polyethylene Glycol Composite Blend Membrane for CO 2 Separation from Gas Mixtures. Environ. Sci. Pollut. Res. 2021, 28, 38274–38291. 10.1007/s11356-021-13447-y/Published. [DOI] [PubMed] [Google Scholar]
- Hiemenz P. C.; Lodge T.. Polymer Chemistry; CRC Press, 2007. [Google Scholar]
- Ravve A.Principles of Polymer Chemistry; Springer: US, 2000. [Google Scholar]
- Trakunjae C.; Boondaeng A.; Apiwatanapiwat W.; Kosugi A.; Arai T.; Sudesh K.; Vaithanomsat P. Enhanced Polyhydroxybutyrate (PHB) Production by Newly Isolated Rare Actinomycetes Rhodococcus Sp. Strain BSRT1–1 Using Response Surface Methodology. Sci. Rep. 2021, 11 (1), 1896. 10.1038/s41598-021-81386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Plackett D.; Chen L. X. L. Kinetics and Mechanism of the Monomeric Products from Abiotic Hydrolysis of Poly[(R)-3-Hydroxybutyrate] under Acidic and Alkaline Conditions. Polym. Degrad. Stab. 2005, 89 (2), 289–299. 10.1016/j.polymdegradstab.2004.12.026. [DOI] [Google Scholar]
- Rivas L. F.; Casarin S. A.; Nepomuceno N. C.; Alencar M. I.; Agnelli J. A. M.; Medeiros E. S. d.; Wanderley Neto A. d. O.; Oliveira M. P. d.; Medeiros A. M. d.; Santos A. S. F. e. Reprocessability of PHB in Extrusion: ATR-FTIR, Tensile Tests and Thermal Studies. Polimeros 2017, 27 (2), 122–128. 10.1590/0104-1428.2406. [DOI] [Google Scholar]
- Foster L. J. R.; Tighe B. J. Centrifugally Spun Polyhydroxybutyrate Fibres: Accelerated Hydrolytic Degradation Studies. Polym. Degrad. Stab. 2005, 87 (1), 1–10. 10.1016/j.polymdegradstab.2003.11.012. [DOI] [Google Scholar]
- El-Khair A.; Ali A. A. The Mechanical Behaviour of Recycled High Density Polyethylene. International Journal of Modern Engineering Research (IJMER) 2013, 3 (2), 798–808. [Google Scholar]
- Kloos J.; Jansen N.; Houben M.; Nijmeijer K.; Schenning A. P. H. J.; Borneman Z. Molecular Order Determines Gas Transport through Smectic Liquid Crystalline Polymer Membranes with Different Chemical Compositions. ACS Appl. Polym. Mater. 2022, 4 (10), 7426–7436. 10.1021/acsapm.2c01154. [DOI] [Google Scholar]
- Mehra N.; Jeske M.; Yang X.; Gu J.; Kashfipour M. A.; Li Y.; Baughman J. A.; Zhu J. Hydrogen-Bond Driven Self-Assembly of Two-Dimensional Supramolecular Melamine-Cyanuric Acid Crystals and Its Self-Alignment in Polymer Composites for Enhanced Thermal Conduction. ACS Appl. Polym. Mater. 2019, 1 (6), 1291–1300. 10.1021/acsapm.9b00111. [DOI] [Google Scholar]
- Ma W.; Yuan H.; Wang X. The Effect of Chain Structures on the Crystallization Behavior and Membrane Formation of Poly(Vinylidene Fluoride) Copolymers. Membranes 2014, 4 (2), 243–256. 10.3390/membranes4020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L.; Xi F.; Wang X.; Xuan J.; Jiao K. Effects of Side Chain Length on the Structure, Oxygen Transport and Thermal Conductivity for Perfluorosulfonic Acid Membrane: Molecular Dynamics Simulation. J. Electrochem. Soc. 2019, 166 (8), F511–F518. 10.1149/2.0791908jes. [DOI] [Google Scholar]
- Satake A. The Solvent Effect on Weak Interactions in Supramolecular Polymers: Differences between Small Molecular Probes and Supramolecular Polymers. ChemPlusChem 2020, 85 (7), 1542–1548. 10.1002/cplu.202000400. [DOI] [PubMed] [Google Scholar]
- Prasetya N.; Himma N. F.; Sutrisna P. D.; Wenten I. G.; Ladewig B. P. A Review on Emerging Organic-Containing Microporous Material Membranes for Carbon Capture and Separation. Chem. Eng. J. 2020, 391, 123575. 10.1016/j.cej.2019.123575. [DOI] [Google Scholar]
- Wang M.; Wang Z.; Zhao S.; Wang J.; Wang S. Recent Advances on Mixed Matrix Membranes for CO2 Separation. Chin. J. Chem. Eng. 2017, 25, 1581–1597. 10.1016/j.cjche.2017.07.006. [DOI] [Google Scholar]
- Salehi Maleh M.; Raisi A. Comparison of Porous and Nonporous Filler Effect on Performance of Poly (Ether-Block-Amide) Mixed Matrix Membranes for Gas Separation Applications. Chem. Eng. Res. Des. 2019, 147, 545–560. 10.1016/j.cherd.2019.05.038. [DOI] [Google Scholar]
- Soleimani M.; Khan S.; Mendenhall D.; Lau W.; Winnik M. A. Effect of Molecular Weight Distribution on Polymer Diffusion during Film Formation of Two-Component High-/Low-Molecular Weight Latex Particles. Polymer 2012, 53 (13), 2652–2663. 10.1016/j.polymer.2011.12.012. [DOI] [Google Scholar]
- Jiun Y. L.; Tze C. T.; Moosa U.; Tawawneh M. A. A. Effects of Recycling Cycle on Used Thermoplastic Polymer and Thermoplastic Elastomer Polymer. Polym. Polym. Compos. 2016, 24 (9), 735–740. 10.1177/096739111602400909. [DOI] [Google Scholar]
- Xu S.; Zhou H.; Jia H.; Xu J.; Liu D.; Zhang M.; Qu Y.; Ma W.; Jiang P.; Zhao W.; Wang X. Preparation and High CO2/CH4 Selectivity of ZSM-5/Ethyl Cellulose Mixed Matrix Membranes. Mater. Res. Express 2021, 8 (2), 026403. 10.1088/2053-1591/abe321. [DOI] [Google Scholar]
- Lehermeier H. J.; Dorgan J. R.; Way J. D. Gas Permeation Properties of Poly(Lactic Acid). J. Membr. Sci. 2001, 190 (2), 243–251. 10.1016/S0376-7388(01)00446-X. [DOI] [Google Scholar]
- Ma C.; Koros W. J. Physical Aging of Ester-Cross-Linked Hollow Fiber Membranes for Natural Gas Separations and Mitigation Thereof. J. Membr. Sci. 2018, 551, 214–221. 10.1016/j.memsci.2018.01.049. [DOI] [Google Scholar]
- Crétois R.; Chenal J. M.; Sheibat-Othman N.; Monnier A.; Martin C.; Astruz O.; Kurusu R.; Demarquette N. R. Physical Explanations about the Improvement of PolyHydroxyButyrate Ductility: Hidden Effect of Plasticizer on Physical Ageing. Polymer 2016, 102, 176–182. 10.1016/j.polymer.2016.09.017. [DOI] [Google Scholar]
- Anbukarasu P.; Sauvageau D.; Elias A. The Effects of Solvent Casting Temperature and Physical Aging on polyhydroxybutyrate-graphene Nanoplatelet Composites. Polym. Compos. 2021, 42 (3), 1451–1461. 10.1002/pc.25915. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






