Abstract
This study was aimed to create a bioactive hydrogel form with PVA/PVP (polyvinyl alcohol/poly(N-vinylpyrrolidone) polymer using acetone and ethanol extractions of Jania rubens red algae and investigate some pharmaceutical properties. The anti-candidal activity and some inhibition performance of J. rubens/PVA/PVP hydrogel were investigated on Candida tropicalis which is one of the important causes of bloodstream infections. The physicochemical properties of J. rubens/PVA/PVP hydrogel were revealed using FTIR and swelling-absorption tests. The volatile compounds of J. rubens extracts were examined by GCMS. By mixing the extracts in equal proportions, PVA/PVP-based hydrogel was prepared. According to the results, Cumulative Drug Release was stable at 25 °C for the first 5 h. The IZ (inhibition zone) and MIC (minimum inhibitory concentration) of J. rubens/PVA/PVP hydrogel were 9.01 mm and 80.20 mg/mL, respectively. It was found that logarithmic reduction and percent reduction were seen as 1.5 CFU/mL and 97.5%, respectively, on C. tropicalis exposed to J. rubens/PVA/PVP hydrogel in the first 5 min of the incubation. After exposure of C. tropicalis to J. rubens/PVA/PVP, the number of viable cells transferred from the gel to water was between 76.1 and 73.1% in high glucose medium, while it was between 92.2 and 80.8% for the PVA/PVP hydrogel under the same conditions. As a result, PVA/PVP hydrogel was made bioactive with J. rubens extracts for the first time in this study, and its potential for use as a functional anticandidal hydrogel on C. tropicalis has been demonstrated.
Keywords: Jania rubens, Candida tropicalis, Hydrogel, Anti-candidal activity
Introduction
Jania rubens is a member of the Corallinaceae family of red algae (Rhodophyta). It spreads in the Mediterranean-Black Sea, North-East Atlantic, Indian Ocean, and China Sea [1]. J. rubens are distinguished from other algae by the absence of flagella in their sexual reproduction. The thalli are multicellular, and the basis of the thallus structure is filamentous. The wall of thallus cells is made of cellulose and various pectic compounds. The inner layer is cellulose, and the outer layer is mucilage pectin. Many studies have shown that J. rubens is used in treating some diseases and is responsible for many biological activities due to its active ingredients of fatty acids, alkanes, sterols, vitamins, trace elements, halides, mannitol, and some proteins. In ancient times, it was used to treat intestinal ulceration and radioisotope poisoning, and it stated that it benefited from its hypoglycemic, fibrinolytic, and lipolytic activities [2–4]. Another biological activity of the algae is its antimicrobial potential due to secondary metabolites in its content. In previous studies, Ismail-Ben Ali et al. reported that dichloromethane and dichloromethane/methanol extracts of J. rubens were active on Staphylococcus aureus, and Micrococcus sp. [5]. It was found that J. rubens had the highest antibacterial activity against S. aureus [6]. It has been reported that methanol, dichloromethane, hexane, chloroform, and essential oil extracts of J. rubens are highly effective against Streptococcus faecalis, Bacillus subtilis, S. aureus, S. epidermidis, Escherichia coli, Salmonella typhimurium, and Candida albicans microorganisms [1].
C. tropicalis is a member of the kingdom Fungi, of the division Ascomycota, and genus Candida. The frequency of spread varies depending on the geography where the invasive disease occurs and causes 3–66% of candidemia [7]. Studies have reported that after infecting the patient, C. tropicalis causes rapid spread and high mortality rate, especially in patients with weakened immune systems [8, 9]. In recent years, Candida tropicalis has been the leading non-albicans Candida species causing nosocomial fungal bloodstream infections [10]. This has led to the idea that C. tropicalis is responsible for 50% of Candida infections. Geographical structure and epidemiological changes have shown that Candida glabrata replaced C. tropicalis in some cases [11]. In a recent study, an increase in the incidence of bloodstream infections caused by Candida parapsilosis and Candida tropicalis was observed during the Coronavirus disease 2019 epidemic [12]. Candida tropicalis is one of the most known Candida species in terms of its virulence and drug resistance. One of the most important reasons for this is the ability of yeast to adhere to surfaces and form biofilms [13]. It is often resistant to the immune system cells of the host it infects and likely to serve as a reservoir for persistent sources of infection [14]. Compared to other Candida species, the strong biofilm-forming ability of C. tropicalis provides resistance to the varying pH and osmolarity range in the environment [15]. Therefore, they are highly resistant to antifungal treatments and cause dangerous infections with species such as C. albicans and C. glabrata [16, 17]. Treatment with C. tropicalis has been reported to produce strains increasingly resistant to fluconazole and other antifungal agents. This highlights the need to investigate new alternative therapeutics with antifungal effects [18, 19].
Hydrogels are semi-fluid, soft, or slightly rigid structures of cross-linked hydrophilic polymeric networks. Various hydrogels can be formed depending on the crosslinking points and mechanical strength of three-dimensional mesh. They can be divided into several classes based on their source, method of preparation, electrical charge, configuration, crosslinking, and function [20]. Some of these include physical hydrogels containing hydrogen bonds, van der Waals and hydrophobic interactions, and chemical hydrogels with covalent bonds [21]. Hydrogels developed by many chemical and physical methods have found application in tissue engineering, pharmaceutical, and biomedical fields [22]. Especially, hydrogels have significant advantages in wound care and other pharmaceutical treatments. These include features such as keeping humidity constant and reducing pain due to the cooling effect in the microenvironment [23]. Polyvinyl alcohol (PVA) and poly(N-vinylpyrrolidone) (PVP), which are widely used in hydrogel synthesis, are a group of polymers that are biocompatible and have extremely low cytotoxicity [24, 25]. PVA/PVP-based hydrogels developed for areas such as wound treatments and drug delivery devices can be processed using different methods such as freeze–thaw and γ-irradiation techniques [26, 27].
Plant-based hydrogels can be used as hydrogel sources because they are natural, harmless, and renewable [27]. Among them, cellulose-based hydrogels [28] are the most widely known. Hydrogels based on starch [29], pectin [30], or soy protein-polysaccharide [31] have biomedical and food applications. Functional hydrogels with different properties are developed using algal resources. The primary material of algae-based hydrogels was mostly polysaccharides (alginate, carrageenan, starch, agarose, porphyrin, and cellulose) [32]. Apart from these, we rarely come across hydrogels containing extracts prepared to reveal bioactive components of algae such as phenolic and volatile components. For example, Chlorella vulgaris green algae were extracted in dry form in TBS buffer (Tris–HCl-saline, pH 7.5), and this extract, which contains various bioactive compounds, was hydrogelized with the carbopol agent [33]. In another study, the extraction of Cladophora glomerata using acetone, methanol, and ethanol solvents was investigated, and various glomerata hydrogels were developed using GCMS analysis and subsequent extracts. The study emphasized that this hydrogel could potentially be developed as a skin care product [34]. In another study, polyamidoamine-based hydrogels containing photoactive chlorophyll a obtained from Spirulina seaweed extract were developed for potential applications in photodynamic therapy [35]. Red algae, which contain various photoprotective and antiphotoaging compounds, are organic sources that will enrich hydrogels with bioactive molecules [36]. Two anthocyanins (malonylchisonine and 4′-demalonylsalvianin), found in J. rubens extract and belonging to the flavonoid class, are important components known to provide anti-aging and protection against UV [37]. In another study, the volatile components n-docosane, n-eicosane, and n-tetratriacontane (5.58%) were detected [1]. However, there are no studies in which these compounds are integrated into hydrogel structures.
In this study, anti-candidal activity and the time-dependent death dynamic against Candida tropicalis of J. rubens/PVA/PVP hydrogel were reported for the first time. The characteristic features of this hydrogel and the volatile content of the mixed extract were revealed using GCMS and FTIR, respectively.
Experimental Sections
Preparation of J. rubens Ethanol and Acetone Extractions
J. rubens was collected from Mersin Akkum beach (38.193003, 26.770186) in Turkey (2020). J. rubens samples were brought to Mersin University, Faculty of Fisheries laboratory with the help of a cold chain. Identification of J. rubens was made by Prof. Dr. Deniz Ayas. The extraction method was studied by modifying traditional methods to reveal bioactive components of J. rubens [38, 39]. The sand on the J. rubens surface was cleaned by several washes, left to dry, and ground in the robot. In preparation for the extraction, approximately 4 g of J. rubens sample was soaked in 40 mL of ethanol and acetone solvents separately for 1 day and then stirred on a magnetic stirrer for 2 h. Then, the sample (0.1 g/mL) was filtered, and the filtrate was passed through a sterile 0.45 µm filter and stored at 4 °C to be used in the experiments.
Analysis of Total Phenol in J. rubens Extracts
The total phenol content of J. rubens extractions was investigated using the Folin–Ciocalteu method applied by Erdoğan Eliuz [40]. Each extraction was performed in 3 parallels. First, 0.5 mL of Folin–Ciocalteu reagent and 0.5 mL of sample solutions were placed in the tubes and mixed. It was kept in the dark for 5 min, and then, 2 mL of Na2CO3 (200 g L−1) solution and 3 mL of distilled water were added, remixed, and kept in the dark for 30 min. Absorbance measurements were made at 700 nm using a UV spectrophotometer. The gallic acid standard was used to calculate the concentrations of the extracts. The data obtained are given as milligrams of gallic acid (mg GAE/100 g dw) [40].
Analysis of Volatile Compounds in J. rubens Extracts by GC–MS
GC–MS (Gas Chromatography-Mass Spectrometry) analysis of J. rubens extracts were performed using a 7890A GC-5975C MSD (Agilent) instrument and HP-5MS column (30 m × 250 µm × 0.25 µm). In the analysis, helium with a flow rate of 1 mL/min was used as the carrier gas, and the furnace temperature was started at 50 °C, kept at this temperature for 3 min, and increased to 300 °C with 10 °C/min increments per minute and kept at this temperature for 6 min. The injection volume for each sample is 1 μL, and the ionization voltage is 70 eV. Separated components were evaluated by comparing them with NIST 2008 (National Institute of Standards and Technology) and National Standards Institute data [41].
Preparation of J. rubens-Based PVA-PVP Hydrogel
Freeze–thaw method was used to prepare the J. rubens-based PVA-PVP hydrogel. PVA (20 wt%) and PVP (12 wt%) were dissolved in doubly distilled water and heated in a water bath at 90 °C for 5 and 15 min, respectively. After the temperature of each solution reached approximately 90 °C, PVA/PVP in different compositions were mixed. Then, 5%, 10%, or 20% of J. rubens extracts (J. rubens ethanol and acetone extracts in v/v ratio) were added, separately. The polymer solutions were stirred under magnetic stirring for 2 h at room temperature. The mixture was poured into Petri plates and kept directly frozen for 16 h at − 18 °C. The frozen hydrogels were then thawed at room temperature for 8 h. This freezing/thawing process to crosslink the polymer was repeated three times [22]. Among the prepared hydrogels, analyses were continued with hydrogels that are effective against C. tropicalis in liquid form (by testing disc diffusion test method). In this study, PVA/PVP hydrogel formed with a 20% extract (100 mg/mL) mixture was studied.
Swelling Measurements of J. rubens/PVA/PVP Hydrogel
J. rubens hydrogel, completely dried in the lyophilizer, was weighed on a precision balance and then completely immersed in 200 mL of distilled water. It was left at room temperature for 3 h and hung for 10 min to remove excess water. Equilibrium swelling (ES) was calculated according to Eq. 1.
| 1 |
W1 and W2 are the weights of dry and swollen gel, respectively.
FTIR Analysis of J. rubens/PVA/PVP Hydrogel
The FT-IR (Fourier Transform Infrared Spectrophotometer) spectra of the J. rubens hydrogel were analyzed using FT-IR (Brand: Jasco FT/IR-6700) in ATR mode, with a spectral measurement range of 4000–500 cm−1. In the hydrogel, bioactive compounds based on aromatic, phenyl, and acyl groups were determined according to their FT-IR spectra [42].
Determination of IZ and MIC of J. rubens/PVA-PVP Hydrogel on C. tropicalis
Before testing, the yeast was inoculated on SDA (Sabouraud dextrose agar) solid medium and incubated at 37 °C for 18–24 h. At the end of 1-day incubation, colonies were taken directly from the single fallen colonies on the agar plate with the help of a loop, and the McFarland (~ 10 CFU/mL) was adjusted with saline. Fluconazole was used for yeasts as a positive control antibiotic.
According to the disc diffusion method, a certain amount of microorganism solution adjusted according to McFarland 0.5 was spread on the Petri dish with MHA agar, and 6 mm diameter discs were added in the middle of the Petri plate. Each disc was filled with 20 µL of liquid form of J. rubens/PVA/PVP and incubated at 37 °C for 24 h. When evaluating the results, the diameters of the (IZ) were measured in millimeters using the Images program. To calculate the MIC, the double dilution concentrations of the hydrogel were studied as in the mentioned experiment [40]. A graph of the extract concentration (%) and inhibition zone was plotted with the results. The % inhibition was calculated using the following formula in Eq. 2. Anti-candidal activity of J. rubens/PVA/PVP was compared with PVA/PVP hydrogel and J. rubens extracts as a negative control, and all tests were repeated thrice.
| 2 |
In Vitro Cumulative Drug Release
0.1 g of J. rubens-loaded hydrogel was weighed and added to 100 mL of PBS (7.4) as a release medium. Triplicate samples were shaken in a shaking incubator at 80 rpm for 8 h. Samples taken at certain time intervals were filtered through a 0.45 µm membrane filter and replaced with fresh medium. The results were measured by the spectrophotometric (280 nm) method as a function of time. The averages of the experiments were taken, and a graph was created according to the formula in Eq. 3 [43].
| 3 |
Mt is the amount of J. rubens mix-extract released from the hydrogel at time t and M∞ is the estimated amount of J. rubens mix-extract loaded into the hydrogel.
Time-Related Dynamics of Mortality of C. tropicalis on Exposure to J. rubens/PVA/PVP Hydrogel
Five microliters of C. tropicalis inoculum (McFarland 0.5) was transferred into the tubes previously added with J. rubens/PVA/PVP hydrogel (50 µL) and vortexed for 5 min. The same procedure was also prepared for negative control with PVA/PVP hydrogel. All samples were incubated at 37 °C at certain time intervals (5, 15, 25 min) in a shaking incubator. Afterward, the samples were taken out of the incubator and taken into sterile glass tubes containing 1 mL of saline and shaken for 5 min in a shaking incubator to allow the yeasts to pass into the water. After 10−2 serial dilutions of the samples, 5 µL of sample from the tube was inoculated into MHA plates and incubated at 37 °C for 24 h. After 1 day, colonies were counted visually, and logarithmic reduction and percent inhibition (Eqs. 4 and 5). Petri dishes in which more than 300 colonies were counted were not taken into account in the experiments, and experiments were performed three times for each time interval [44].
| 4 |
| 5 |
Testing of J. rubens/PVA/PVP as an Adsorbent of C. tropicalis
The TTC-DRA method is a method used to determine the viability of microbial cells after treatment with antimicrobial agents. The dehydrogenase enzyme found in living cells produces insoluble red 2,3,5-triphenyl formazan (TF) and is reduced by the hydrogen acceptor TTC dye molecule in microbial culture. Therefore, to determine TTC-DRA in cells, the absorbance value of the TF molecule is measured at 485 nm. In this study, the absorption of C. tropicalis cells by J. rubens/PVA/PVP hydrogel was evaluated. Hydrogel cut to approximately 1 cm2 in size was combined with C. tropicalis (McFarland 0.5) culture (1 mL) under aseptic conditions and vortexed. After waiting for 30 min, the gel was removed from the yeast solution and transferred to a 1 mL water medium containing TTC dye (0.01 mg/mL) and glucose (0.01 or 3 g/L to mimic a clean and dirty organic environment condition). All samples were kept in the incubator for 8 h to stain live cells. Then, all tubes were vortexed for 5 min to move the attached cells into the water and then kept at 37 °C for 15 min. This process was repeated 3 times. Live yeast cells transferred to water were measured spectrophotometrically. PVA/PVP hydrogel (non-glucose) was used for control. The DRA (dehydrogenase activity) is formulated as follows (Eq. 6):
| 6 |
where ODx and ODc represent the absorbance of the treated and control samples, respectively.
Statistical Analysis
MIC, IZ, and Log red. analyses and significance were tested using One-way ANOVA with post-hoc Tukey HSD Test (p-value < 0.05).
Results and Discussions
Total Phenol Content and GCMS Analysis of J. rubens Extracts
The total phenolic contents of the extracts were determined as 0.002 mgGAE/100 for J. rubens ethanol and 0.004 mgGAE/100 for acetone on a dry weight basis, respectively. In general, it is stated that there are phenolic compounds in the range of 1.5–4.1 mg GAE/g in crude methanolic extracts of red seaweed [3]. Phenolic compounds are commonly found in plants, including seaweeds, and have been reported to exhibit a wide variety of biological activities, including antimicrobial properties. Reports revealed that phenolic compounds are the most effective compounds in red algae. These compounds are known to be critical metabolites responsible for antimicrobial activity [45].
GC-MS Analysis of J. rubens Extracts
Extraction of secondary metabolites found in plants is difficult due to their insoluble nature. In the literature, solvents such as methanol, ethanol, acetone, n-hexane, isooctane, and ethyl acetate are used effectively in secondary metabolite extractions. It has been reported that ethanol and acetone are effective in dissolving tannins, polyphenols, flavonols, terpenoids, and alkaloids [46, 47]. In our study, we used ethanol and acetone for the detection of volatile compounds of J. rubens. According to the GC-MS analysis, while the main compounds of J. rubens EE were phenol, 2,4-bis(1,1-dimethylethyl) (25.64%), 4.alpha.,7,8a.beta.-Trimethyl-3,4, 4a in ethanol extract. as beta.,5,6,8a-hexahydronaphthale n-1(2H)-one (3.99%), hexadecanoic acid, methyl ester (14.59%), ethyl palmitate (5.14%), oleic acid, methyl ester (6.43%), the major compounds of J. rubens AE were diacetone alcohol (85.57%), ethyl palmitate (3.11%), octadecanoic acid, and ethyl ester (6.04%) (Table 1).
Table 1.
GCMS analysis of J. rubens AE and EE extracts
| Rt | Compound | J. rubens EE | J. rubens AE | ||
|---|---|---|---|---|---|
| % | Q | % | Q | ||
| 4.197 | Diacetone alcohol | - | - | 85.57 | 83 |
| 14.483 | Phenol, 2,4-bis(1,1-dimethylethyl) | ||||
| 25.64 | 96 | - | - | ||
| 24.490 | 4.alpha.,7,8a.beta.-trimethyl-3,4,4a.beta.,5,6,8a-hexahydronaphthale n-1(2H)-one | 3.99 | 78 | - | - |
| 26.804 | Hexadecanoic acid, methyl ester | ||||
| 14.59 | 99 | - | - | ||
| 27.825 | Ethyl palmitate | ||||
| 5.14 | 99 | 3.11 | 98 | ||
| 29.226 | Oleic acid, methyl ester | ||||
| 6.43 | 93 | - | - | ||
| 30.276 | Octadecanoic acid, ethyl ester | ||||
| - | - | 6.04 | 99 | ||
RT retention time
In the literature, Karabay-Yavasoglu et al. identified 40 compounds as a result of GC–MS analysis of volatile components of J. rubens in their study [1]. The main volatile components of J. rubens were n-docosane (6.35%), n-eicosane (5.77%), and n-tetratriacontane (5.58%). In addition, this extract did not affect Candida albicans growth, and the IZ was found as zero. In another study, The GC–MS analysis of the crude extract of J. rubens revealed that the main chemical constituent was 1-( +)-ascorbic acid 2,6-dihexadecanoate (35.48%) followed by icosapent (6.91%), trans-13 octadecenoic acid (5.04%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (4.66%), heptadecane (4.01%), and 1,2-benzenedicarboxylic acid (3.10%) [3]. Phenol, 2,4-bis(1,1-dimethylethyl) was found in fermented seaweeds and Kappaphycus spp. red algae enhanced with endophytic mushroom fermentation [48, 49]. In this study, phenol, 2,4-bis(1,1-dimethylethyl) substance in J. rubens EE (25.64%) could limit the antimicrobial activity of microorganisms other than C. tropicalis. Because, it has been reported that the related compound acidified the environment and caused the reduction of reactive oxygen species (ROS), which causes the inhibition of pathogens [50]. Methyl palmitate, which is found in significant amounts in J. rubens EE, has been previously detected in Ulva australis [51]. Contrary to other studies, compounds such as 2,4-bis(1,1-dimethylethyl), hexadecanoic acid, methyl ester (14.59%), and ethyl palmitate detected in this study may have affected Candida tropicalis.
Characteristic Properties of J. rubens/PVA/PVP Hydrogel
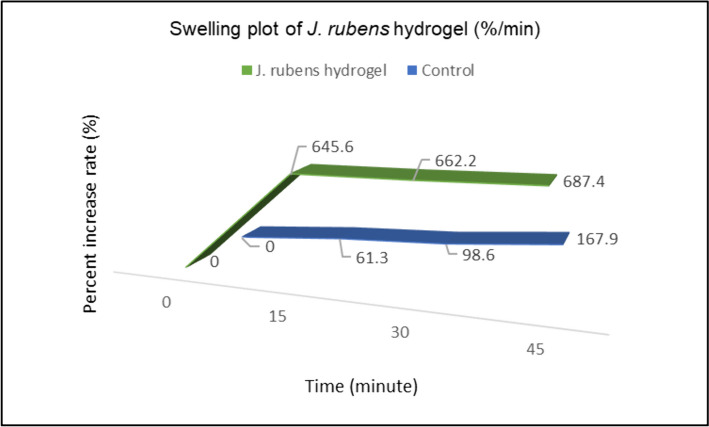
The time-dependent swelling property of J. rubens/PVA/PVP hydrogel is shown in the graph (Fig. 1). When this graph was examined, it was observed that the swelling behavior of J. rubens/PVA/PVP reached its maximum level in the first 15 min with a 645.6% increase rate. Afterward, the swelling rate slowed down and remained stable for 45 min. The initial weight of the control hydrogel (PVA/PVP) was 0.0428 g, while the initial weight of J. rubens/PVA/PVP hydrogel was 0.0949 g. This difference comes from the algae extract added from the algae in the J. rubens/PVA/PVP gel.
Fig. 1.
Swelling rate (%) on J. rubens/PVA/PVP hydrogel and control (PVA/PVP hydrogel)
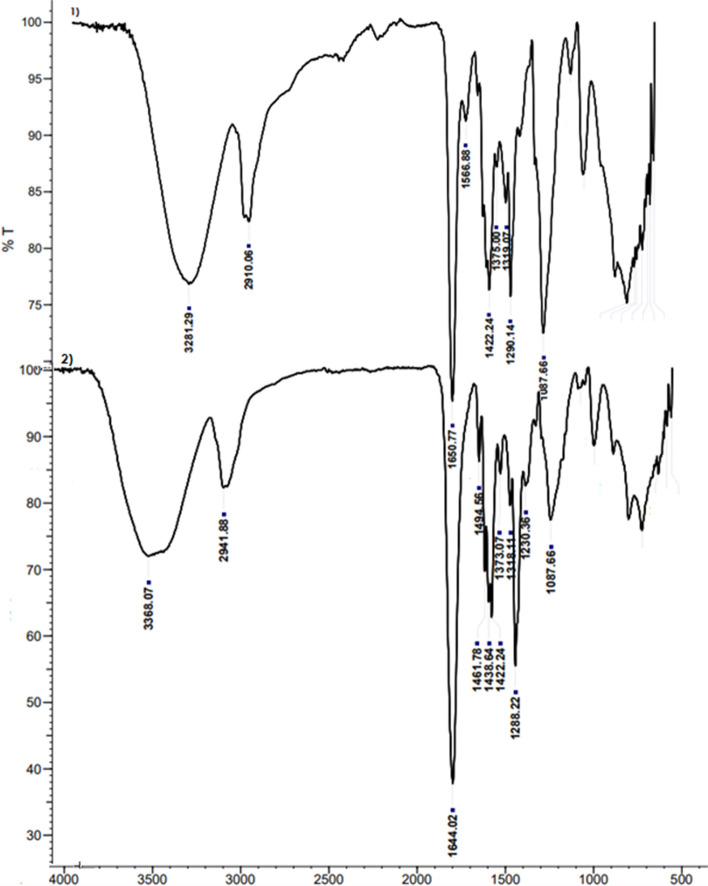
The IR spectra of the PVA/PVP (Fig. 2-1) and J. rubens/PVA/PVP hydrogel (Fig. 2-2) were shown in the wavenumber range of 4000 to 400 cm−1. In the FT-IR of pure hydrogel alone, 3281.29 cm−1 (O–H groups; stretch modes in water and hydroxyl), 2910.06 cm−1 (alkyl groups), 1650.77 cm−1 (C = O), 1566.88 cm−1, 1422.24 cm−1 (C-H vibration), 1375.00 cm−1 and 1319.07 cm−1 (CH3 vibration), 1290.14 cm−1, and 1087.66 cm−1 (C-O stretching) peaks were detected. Fusing PVP with PVA is 3281.27 cm−1, where the O–H stretch of PVA is high. C = O stretching of PVP was seen from 1660 cm−1 to 1655–1651 cm−1. These results demonstrate the intermolecular hydrogen bonding between the hydroxyl groups of PVA and the carbonyl groups of PVP. These results are consistent with previous reports [52]. All major peaks related to hydroxyl and acetate groups were observed in the FTIR spectra of PVA. The large bands observed between 3550 and 3200 cm−1 are due to O–H extending from intermolecular and intermolecular hydrogen bonds. The vibration band followed between 2840 and 3000 cm−1 is due to alkyl groups. Mixture gel of PVA and PVP shows that hydrogen-bonded single bond OH groups (at about 3200–3500 cm−1) and C = O peak shifts (about 1650–1680 cm−1) and dominant intermolecular interactions in PVA occur [52]. In the FT-IR of J. rubens hydrogel, bands of 3368.07 cm−1 (phenol), 2941.88 cm−1 (lipid acyl chain), 1644.02 cm−1 (aromatic C–C stretch), 1494, 56 cm−1 (phenyl groups), 1461.78 cm−1 (C-H vibration), 1438.64 (C-H vibration), 1422.24 (C-H vibration), 1373.07 cm−1 and 1318.11 cm−1 (CH3 vibration), 1288.22 cm−1 (C-O stretching vibrations), 1230.36 (C-N vibrations), and 1087.66 cm−1 (C-O; C–C and C-H) were found. Bands at and around 1400 cm−1 of the hydrogel indicate that the gel contains many residual organic groups.
Fig. 2.
IR spectra of PVA/PVP (1) and J. rubens/PVA/PVP hydrogel (2). T (%): transmittance
Cumulative Drug Release
As shown in Fig. 3, the release of J. rubens from PVA/PVP hydrogels increased with increasing temperature. This shows that the spreading of J. rubens/PVA/PVP will accelerate with temperature. At 37 °C, the maximum release was observed from the 1st hour to the 5th hour, and the order is as follows: 0%, 13.5%, 19.8%, 37.8%, and 50% respectively (p-value ≤ 0.05). There is a significant oscillation after the 3rd hour at 25 °C. These are 8.7%, 12.3%, and 19.6% respectively. At 10 °C, no significant oscillation was observed in the first 5 h (p-value ≤ 0.05).
Fig. 3.
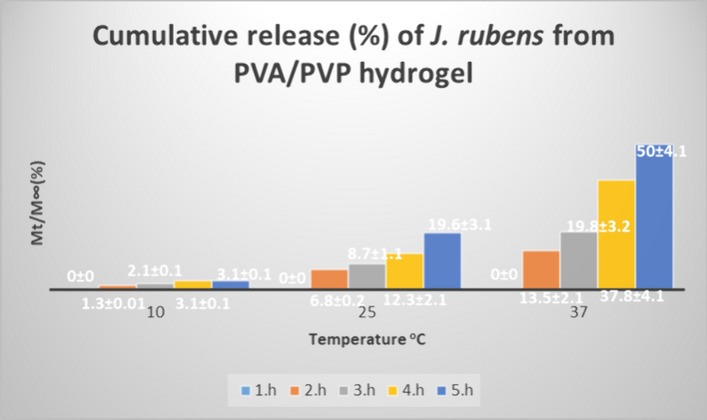
Cumulative release of J. rubens from PVA/PVP hydrogel at 10 °C, 25 °C, and 37 °C in buffer solutions of pH 7.4
Anti-candidal Activities of J. rubens/PVA/PVP Hydrogel
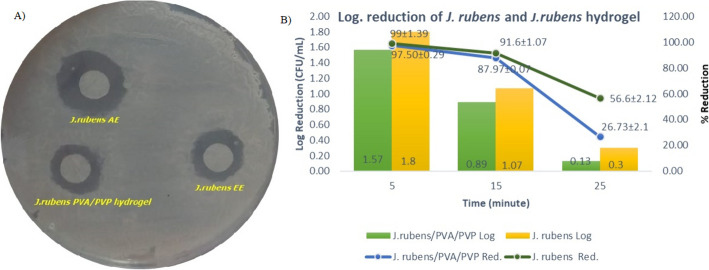
Antimicrobial activities of J. rubens/PVA/PVP hydrogel against C. tropicalis were tested (p-value < 0.05). The results obtained are given in Table 2. The IZ and MIC of J. rubens/PVA/PVP hydrogel were 9.01 mm and 80.20 mg/mL, respectively. The ethanol and acetone extractions of J. rubens showed inhibition zones of 10.14 mm and 12.37 mm against C. tropicalis (p-value < 0.05) (Fig. 4A). The MIC values of J. rubens ethanol and acetone extracts were determined as 91.5 mg/mL and 99.25 mg/mL, respectively (p-value ≤ 0.05).
Table 2.
IZ diameters (mm) and MIC values (mg/mL) of J. rubens/PVA/PVP and J. rubens EE and AE against C. tropicalis
| J. rubens/PVA/PVP | PVA/PVP | J. rubens EE | J. rubens AE | Fluconazole (120 µg/mL) | |
|---|---|---|---|---|---|
| IZ (mm) | 9.01a ± 0.1 | 0 | 10.14a ± 0.1 | 12.37a ± 0.1 | 30.0 ± 0.02 µg/mL |
| MIC (mg/mL) | 80.20b ± 2.1 | 0 | 91.5b ± 3.7 | 99.25b ± 3.7 | 25 ± 0.12 µg/mL |
Statistical differences are indicated by a different letter in each line (p-value < 0.05)
Fig. 4.
Inhibition zones of J. rubens EE, J. rubens AA, and J. rubens/PVA/PVP (A); time-dependent death dynamics of C. tropicalis exposed to J. rubens/PVA/PVP hydrogel (B)
Antifungal activities of J. rubens have been reported in several previous publications [1, 5, 53]. However, there are rare publications about the antifungal activities of J. rubens on Candida tropicalis. The extracts of methanol, diethyl ether, ethyl acetate, petroleum ether, ethanol, and acetone of J. rubens highly affected C. tropicalis between 10 and 30 mm [54, 55]. Polyhydroxysteroids such as cholesta-8-en-3β,5α,6α,25-tetrol and cholesta-8(14)-en-3β,5α,6α-25-tetrol extracted from another algae species (Lamellodysidea herbacea) were inhibited to C. tropicalis with 13 and 11 mmat 10 mg/disc [56]. These results were similar to our data (≥ 10 mm).
Dynamics of Time-Related Death of C. tropicalis When Exposed to J. rubens/PVA/PVP
In this study, the microbicidal effect of J. rubens/PVA/PVP hydrogel was determined dynamically over time (Fig. 4B). In the first 5 min, of incubation of C. tropicalis fungus exposed to the hydrogel, logarithmic reduction and reduction were 1.5 ± 0.12 CFU/mL and 97.5% ± 0.7, respectively. It was calculated as 0.89 ± 0.15 CFU/mL and 87.97 ± 3.6% at the 15th minutes and 0.13 ± 0.12 and 26.73% ± 23.11% at the 25th minutes. In the study, it can be concluded that J. rubens/PVA/PVP hydrogel affected C. tropicalis, significantly, in the first 5 min (p ≤ 0.5).
Testing of J. rubens/PVA/PVP as an Adsorbent of C. tropicalis
The following study was conducted to understand the viability of C. tropicalis cells exposed to J. rubens hydrogel surface compared to the control. Penetration and inhibition of Candida on the J. rubens hydrogel surface were expected when Candida cells were compared to the bioactive substances in the hydrogel. Cells that survived and migrated into water were measured spectrophotometrically. The density of living cells adhering to J. rubens/PVA/PVP hydrogel and then passing into water was found to be higher than PVA/PVP hydrogel alone. The rate of live cells of C. tropicalis absorbed to J. rubens/PVA/PVP was 75.2%, 66.2%, 60.2%, 60.1%, and 60% in low glucose environment, respectively, while they were 76.1%, 75.1%, 75.1%, 74%, and 73.1% in high glucose environment within 5, 20, 40, 80, and 120 min, respectively. While PVA/PVP hydrogel absorbed C. tropicalis cells at the level of 91.2%, 81.5%, 80.2%, 80%, and 80% in low glucose environment, they were 92.2%, 87.5%, 82.2%, 80.9%, and 80.8% in high glucose environment within 5, 20, 40, 80, and 120 min, respectively. In this case, we can say that the fungal absorption capacity of phenolic gel is higher (Table 3).
Table 3.
TTC-DRA (%) of C. tropicalis after J. rubens/PVA/PVP and PVA/PVP hydrogels treatment in low-glucose (0.01 g/mL) and high-glucose (3 g/L) environmental conditions
| Time (min) | DRA (%) | |||
|---|---|---|---|---|
| J. rubens/PVA/PVP | PVA/PVP | |||
| Low-glucose | High-glucose | Low-glucose | High-glucose | |
| 5 | 75.2a ± 0.1 | 76.1a ± 0.1 | 91.2a ± 0.1 | 92.2a ± 0.1 |
| 20 | 66.2a ± 0.1 | 75.1a ± 0.1 | 81.5a ± 0.1 | 87.5a ± 0.1 |
| 40 | 60.2a ± 0.1 | 75.1a ± 0.1 | 80.2a ± 0.1 | 82.2a ± 0.1 |
| 80 | 60.1a ± 0.1 | 74a ± 0.1 | 80a ± 0.1 | 80.9a ± 0.1 |
| 120 | 60a ± 0.1 | 73.1a ± 0.1 | 80a ± 0.1 | 80.8a ± 0.1 |
Statistical differences are indicated by a different letter in each column (p-value < 0.05)
When hydrogels are cross-linked with their parent material, they form an insoluble three-dimensional structure that increases water absorption capacity [57]. The ability to rapidly absorb water supports biocompatibility, which is an advantage in pharmaceutical, cell delivery systems, or antimicrobial research. Hydrogel formulations containing methylcellulose [58], Ginja cherry extract [59], clove bud (Syzygium aromaticum), olive leaf extract [60], and thyme (Origanum vulgare) [61] essential oils have been synthesized due to their ability to absorb water and antimicrobial activity. Among these, methylcellulose has been recommended for use in oral mucosa and/or vaginal applications due to its ability to adhere to mucosal surfaces [62, 63]. In this study, it appears that J. rubens-based hydrogel rapidly absorbs the microorganism together with water in the water environment where Candida is present (Fig. 5). The presence of fewer Candida cells in the J. rubens/PVA/PVP hydrogel compared to the control surface (PVA/PVP) may be associated with the inhibition of C. tropicalis by the phenolic extract. In vivo studies are needed to make hydrogel forms of bioactive substances from J. rubens ethanol or acetone extracts applicable. In particular, acetone is mentioned to be limited in use due to toxicity. However, a few studies have shown that acetone can also be used in in vivo applications at certain concentrations (< 20 mmol/kg) [64, 65].
Fig. 5.
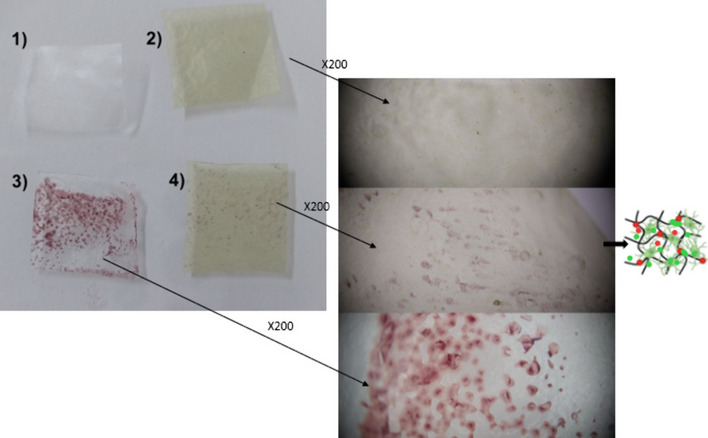
Absorbance of C. tropicalis by the gels. PVA/PVP free gel (1-control), J. rubens/PVA/PVP free gel (2), cell loaded PVA/PVP, and J. rubens/PVA/PVP (3 and 4, respectively)
Conclusion
Candida tropicalis has been inhibited by many algae extracts in the literature including J. rubens. This study tested the effectiveness of J. rubens secondary metabolites in hydrogel form for the first time. The results showed that the hydrogel was highly active against C. tropicalis. In a time-dependent death study, it was found that J. rubens PVA/PVP hydrogel stopped the development of C. tropicalis in the first 5 min. At the same time, J. rubens hydrogel has physicochemical properties that can be used actively in many areas, such as pathogen absorbent, drug coating, wound treatment, and functional food. J. rubens-based PVA/PVP hydrogel can be used in wound healing, development of cosmetic products, design of antimicrobial materials, and development of biomedical sanitary pads and other medical products. In the next study, it is planned to conduct in vitro biocompatibility analysis of the bioactive hydrogel, in vitro healing assay in C. tropicalis-infected wound (scratch migration assay), and in vivo healing assay in C. tropicalis-infected wound. Future in vivo studies are planned to investigate multi-infection caused by symbiotic relationships with other Candida members other than C. tropicalis.
Acknowledgements
The authors would like to thank the Advanced Technology Education Research and Application Center for GCMS and the Çukurova University Central Research Center for FTIR.
Author Contribution
Elif Erdoğan Eliuz: writing and editing the original draft and revising, writing, and editing the discussion. It helped shape and provide critical feedback on the results. Meltem Boran: writing the original article, planning the experiments, discussing the results, and writing the discussion. Deniz Ayas: writing and editing the original draft and evaluating, revising, and editing the discussion. He evaluated the data and provided critical feedback. All authors have read and accepted the final version of the manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This academic work was funded by the Mersin University Research Fund (Project No: 2022–1-TP2-4647).
Data Availability
All experimental data and analyses of the study are included in this published article.
Declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Karabay-Yavasoglu, N. U., Sukatar, A., Ozdemir, G., et al. (2007). Antimicrobial activity of volatile components and various extracts of the red alga Janiarubens. Phytotherapy Research,21, 153–156. 10.1002/ptr.2045 [DOI] [PubMed] [Google Scholar]
- 2.Awad, N. E. (2004). Bioactive brominated diterpenes from the marine red alga Janiarubens (L.) Lamx. Phytotherapy Research,18, 275–279. 10.1002/ptr.1273 [DOI] [PubMed] [Google Scholar]
- 3.El-Din, S. M. M., & El-Ahwany, A. M. (2016). Bioactivity and phytochemical constituents of marine red seaweeds (Janiarubens, Corallinamediterranea, and Pterocladiacapillacea). Journal Taibah University for Science,10, 471–484. 10.1016/j.jtusci.2015.06.004 [Google Scholar]
- 4.Aydin, B. (2022). Antioxidant properties of some macroalgae harvested from the Iskenderun Bay Turkey. Fresenius Environ Bulletin and Advances in Food Sciences,31, 2145–2152. [Google Scholar]
- 5.Ismail-Ben Ali, A., El Bour, M., Ktari, L., Bolhuis, H., Ahmed, M., Boudabbous, A., & Stal, L. J. (2012). Jania rubens-associated bacteria: Molecular identification and antimicrobial activity. Journal of applied phycology,24, 525–534. [Google Scholar]
- 6.Sujatha, R., Siva, D., & Nawas, P. M. A. (2016). Screening of phytochemical profile and antibacterial activity of various solvent extracts of marine algae Sargassumswartzii. World Scientific News,115, 27–40. [Google Scholar]
- 7.Ann Chai, L. Y., Denning, D. W., & Warn, P. (2010). Candida tropicalis in human disease. Critical reviews in microbiology,36(4), 282–298. [DOI] [PubMed] [Google Scholar]
- 8.Costa, S. F., Marinho, I., Araujo, E. A. P., Manrique, A. E. I., Medeiros, E. A. S., & Levin, A. S. (2000). Nosocomial fungaemia: A 2-year prospective study. Journal of Hospital Infection,45(1), 69–72. [DOI] [PubMed] [Google Scholar]
- 9.Sipsas, N. V., Lewis, R. E., Tarrand, J., Hachem, R., Rolston, K. V., Raad, I. I., & Kontoyiannis, D. P. (2009). Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007) stable incidence but changing epidemiology of a still frequently lethal infection. Cancer,115(20), 4745–4752. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., Diekema, D. J., Andes, D., Arendrup, M. C., Brown, S. D., Lockhart, S. R., ... & Perlin, D. S. (2011). Clinical breakpoints for the echinocandins and Candida revisited: Integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resistance Updates, 14(3), 164–176. [DOI] [PubMed]
- 11.Yang, Y. L., Lin, C. C., Chang, T. P., Lauderdale, T. L., Chen, H. T., Lee, C. F., ... & Lo, H. J. (2012). Comparison of human and soil Candida tropicalis isolates with reduced susceptibility to fluconazole. PloS one, 7(4), e34609. [DOI] [PMC free article] [PubMed]
- 12.Pienthong, T., Visuttichaikit, S., Apisarnthanarak, P., Jantarathaneewat, K., Weber, D. J., & Apisarnthanarak, A. (2023). Increase in the incidence of Candida parapsilosis and Candida tropicalis bloodstream infections during the coronavirus disease 2019 (COVID-19) pandemic. Antimicrobial Stewardship & Healthcare Epidemiology,3(1), e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuza-Alves, D. L., Silva-Rocha, W. P., & Chaves, G. M. (2017). An update on Candida tropicalis based on basic and clinical approaches. Frontiers in microbiology,8, 281220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalheiro, M., & Teixeira, M. C. (2018). Candida biofilms: Threats, challenges, and promising strategies. Frontiers in medicine,5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng, Y. K., Chen, Y. C., Hou, C. J., Deng, F. S., Liang, S. H., Hoo, S. Y., Hsu, C. C., Ke, C. L., & Lin, C. H. (2020). Evaluation of biofilm formation in Candida tropicalis using a silicone-based platform with synthetic urine medium. Microorganisms,8(5), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva, S., Negri, M., Henriques, M., Oliveira, R., Williams, D. W., & Azeredo, J. (2012). Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS microbiology reviews,36(2), 288–305. [DOI] [PubMed] [Google Scholar]
- 17.de Souza, C. M., Dos Santos, M. M., Furlaneto-Maia, L., & Furlaneto, M. C. (2023). Adhesion and biofilm formation by the opportunistic pathogen Candida tropicalis: What do we know? Canadian journal of microbiology,69(6), 207–218. [DOI] [PubMed] [Google Scholar]
- 18.Meccatti, V. M., Oliveira, J. R. D., Figueira, L. W., LagareiroNetto, A. A., Zamarioli, L. S., Marcucci, M. C., Camargo, S. E. A., Carvalho, C. A. T., & Oliveira, L. D. D. (2021). Rosmarinus officinalis L (rosemary) extract has antibiofilm effect similar to the antifungal nystatin on Candida samples. Anais da Academia Brasileira de Ciencias,93, e20190366. [DOI] [PubMed] [Google Scholar]
- 19.Meccatti, V. M., Santos, L. F., de Carvalho, L. S., Souza, C. B., Carvalho, C. A. T., Marcucci, M. C., Hasna, A. A., & de Oliveira, L. D. (2023). Antifungal action of herbal plants’ glycolic extracts against Candida species. Molecules,28(6), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed, E. M. (2015). Hydrogel: Preparation, characterization, and applications: A review. Journal of advanced research,6, 105–121. 10.1016/j.jare.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadinejad, R., Maleki, H., Larraneta, E., Fajardo, A. R., Nik, A. B., Shavandi, A., & Thakur, V. K. (2019). Status and future scope of plant-based green hydrogels in biomedical engineering. Applied Materials Today,16, 213–246. 10.1016/j.apmt.2019.04.010 [Google Scholar]
- 22.Husain, M. S. B., Gupta, A., Alashwal, B. Y., & Sharma, S. (2018). Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources, Part A433A: Recovery, Utilization, and Environmental Effects+A465,40, 2388–2393. 10.1080/15567036.2018.1495786 [Google Scholar]
- 23.Frehner, E., & Watts, R. (2016). Evidence summary: Wound management-hydrogel dressings without additional therapeutic additives. Wound Practice & Research: Journal of Australian Wound Management Association,24, 59–60. [Google Scholar]
- 24.Kamoun, E. A., Chen, X., Eldin, M. S. M., & El-Refaie, K. S. (2015). Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arabian Journal of Chemistry,8, 1–14. 10.1016/j.arabjc.2014.07.005 [Google Scholar]
- 25.Kokabi, M., Sirousazar, M., & Hassan, Z. M. (2007). PVA–clay nanocomposite hydrogels for wound dressing. European polymer journal,43, 773–781. 10.1016/j.eurpolymj.2006.11.030 [Google Scholar]
- 26.El-Mohdy, H. L. A., & Ghanem, S. (2009). Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. Journal of Polymer Research,16, 1–10. 10.1007/s10965-008-9196-0 [Google Scholar]
- 27.Chang, C., & Zhang, L. (2011). Cellulose-based hydrogels: Present status and application prospects. Carbohydrate polymer,84, 40–53. 10.1016/j.carbpol.2010.12.023 [Google Scholar]
- 28.Sannino, A., Demitri, C., & Madaghiele, M. (2009). Biodegradable cellulose-based hydrogels: Design and applications. Materials,2, 353–373. 10.3390/ma2020353 [Google Scholar]
- 29.Jacob, J., Haponiuk, J. T., Thomas, S., & Gopi, S. (2018). Biopolymer based nanomaterials in drug delivery systems: A review. Materials today chemistry,9, 43–55. [Google Scholar]
- 30.Mehrali, M., Thakur, A., Kadumudi, F. B., Pierchala, M. K., Cordova, J. A. V., Shahbazi, M. A., Mehrali, M., Pennisi, C. P., Orive, G., & Gaharwar, A. K. (2019). Pectin methacrylate (PEMA) and gelatin-based hydrogels for cell-delivery: Converting waste-materials into biomaterials. ACS Applied Materials Interfaces,11, 12283–12297. 10.1021/acsami.9b00154 [DOI] [PubMed] [Google Scholar]
- 31.Birch, N. P., Barney, L. E., Pandres, E., Peyton, S. R., & Schiffman, J. D. (2015). Thermal-responsive behavior of a cell compatible chitosan/pectin hydrogel. Biomacromolecules,16, 1837–1843. 10.1021/acs.biomac.5b00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaumont, M., Tran, R., Vera, G., Niedrist, D., Rousset, A., Pierre, R., & Forget, A. (2021). Hydrogel-forming algae polysaccharides: From seaweed to biomedical applications. Biomacromolecules,22, 1027–1052. 10.1021/acs.biomac.0c01406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Melo, R. G., de Andrade, A. F., Bezerra, R. P., Viana Marques, D. D. A., da Silva, V. A., Paz, S. T., de Lima Filho, J. L., & Porto, A. L. F. (2019). Hydrogel-based Chlorella vulgaris extracts: A new topical formulation for wound healing treatment. Journal of applied phycology,31, 3653–3663. [Google Scholar]
- 34.Petchsomrit, A., Chanthathamrongsiri, N., Jiangseubchatveera, N., Manmuan, S., Leelakanok, N., Plianwong, S., Siranonthana, N., & Sirirak, T. (2023). Extraction, antioxidant activity, and hydrogel formulation of marine Cladophoraglomerata. Algal Research,71, 103011. [Google Scholar]
- 35.Rizzi, V., Gubitosa, J., Fini, P., Fraix, A., Sortino, S., Agostiano, A., & Cosma, P. (2021). Development of Spirulina sea-weed raw extract/polyamidoamine hydrogel system as novel platform in photodynamic therapy: Photostability and photoactivity of chlorophyll a. Materials Science and Engineering: C,119, 111593. [DOI] [PubMed] [Google Scholar]
- 36.Kasanah, N., Ulfah, M., Imania, O., Hanifah, A. N., & Marjan, M. I. D. (2022). Rhodophyta as potential sources of photoprotectants, antiphotoaging compounds, and hydrogels for cosmeceutical application. Molecules,27(22), 7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixit, D., & Reddy, C. R. K. (2017). Non-targeted secondary metabolite profile study for deciphering the cosmeceutical potential of red marine macro alga Janiarubens—An LCMS-based approach. Cosmetics,4(4), 45. [Google Scholar]
- 38.Horincar, V. B., Parfene, G., & Bahrim, G. (2011). Evaluation of bioactive compounds in extracts obtained from three Romanian marine algae species. Romanian Biotechnological Letters,16(6), 71–78. [Google Scholar]
- 39.Eliuz, E., Börekçi, N. S., & Ayas, D. (2019). The antimicrobial activity of Enteromorpha sp. methanolic extract and gelatin film solution against on some pathogens. Marine Science and Technology Bulletin,8(2), 58–63. [Google Scholar]
- 40.ErdoğanEliuz, E. A. (2022). Antibacterial activity and antibacterial mechanism of ethanol extracts of Lentinula edodes (Shiitake) and Agaricusbisporus (button mushroom). International Journal of Environmental Health Research,32, 1828–1841. 10.1080/09603123.2021.1919292 [DOI] [PubMed] [Google Scholar]
- 41.Yabalak, E., Ibrahim, F., Eliuz, E. A. E., Everest, A., & Gizir, A. M. (2022). Evaluation of chemical composition, trace element content, antioxidant and antimicrobial activities of Verbascumpseudoholotrichum. Plant Biosystems,2022(156), 313–322. 10.1080/11263504.2020.1852332 [Google Scholar]
- 42.Yabalak, E., & Eliuz, E. A. E. (2023). Hydrochar synthesis of from waste human hair, incorporation with phenolic extract of Morus alba and evaluation as a natural anti-Staphylococcus aureus agent. The Journal of Supercritical Fluids,192, 105804. 10.1016/j.supflu.2022.105804 [Google Scholar]
- 43.Wang, Q., Li, S., Wang, Z., Liu, H., & Li, C. (2009). Preparation and characterization of a positive thermoresponsive hydrogel for drug loading and release. Journal of Applied Polymer Sciences,111, 1417–1425. 10.1002/app.29026 [Google Scholar]
- 44.Singh, N., Singh, R. K., Bhunia, A. K., & Stroshine, R. L. (2002). Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157: H7 on lettuce and baby carrots. LWT Food Science and Technology,35, 720–729. 10.1006/fstl.2002.0933 [Google Scholar]
- 45.Anand, U., Jacobo-Herrera, N., Altemimi, A., & Lakhssassi, N. A. (2019). Comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites,9, 1–13. 10.3390/metabo9110258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altıok, E., Bayçın, D., Bayraktar, O., & Ülkü, S. (2008). Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Separation and Purification Technology,62(2), 342–348. [Google Scholar]
- 47.Jha, A. K., & Sit, N. (2022). Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends in Food Science & Technology,119, 579–591. [Google Scholar]
- 48.Harikrishnan M, Prakash P, Jayabaskaran C, Bhat, S. (2021). Multi-functional bioactive secondary metabolites derived from endophytic fungi of marine algal origin. Current Research Microbial Sciences, 2, (100037). 10.1016/j.crmicr.2021.100037. [DOI] [PMC free article] [PubMed]
- 49.Norakma, M. N., Zaibunnisa, A. H., & Razarinah, W. W. (2022). The changes of phenolics profiles, amino acids and volatile compounds of fermented seaweed extracts obtained through microbial fermentation. Materials Today,48, 815–821. 10.1016/j.matpr.2021.02.366 [Google Scholar]
- 50.Romero-Correa, M. T., Villa-Gómez, R., Castro-Mercado, E., & Garcie-Pineda, E. (2014). The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiological Molecular Plant Pathology,87, 32–41. [Google Scholar]
- 51.Trentin, R., Custódio, L., Rodrigues, M. J., Moschin, E., Sciuto, K., da Silva, J. P., & Moro, I. (2020). Exploring Ulva australis Areschoug for possible biotechnological applications: In vitro antioxidant and enzymatic inhibitory properties, and fatty acids contents. Algal Research,50, 101980. [Google Scholar]
- 52.Jaipakdee, N., Pongjanyakul, T., Limpongsa, E. (2018). Preparation and characterization of poly (vinyl alcohol)-poly (vinyl pyrrolidone) mucoadhesive buccal patches for delivery of lidocaine HCL. International Journal of Applied Pharmaceutics, 10, 115–123. 10.22159/ijap.2018v10i1.23208.
- 53.Soliman, F. M., El-Tohamy, S. F., Fathy, M. M., Ramdan, A., Afify, N. A., & Sanad, O. A. (1994). Phytochemical and pharmacological investigation of the red alga Jania rubens (L.) Lamx. Carbo-hydrates, mannitol and pharmacological screening. Journal of Drug Research of Egypt,21, 165–180. [Google Scholar]
- 54.Ismail-Ben, A., El Bour, M., Ktari, L., Bolhuis, H., Ahmed, M., Boudabbous, A., & Stal, L. J. (2012). Janiarubens-associated bacteria: Molecular identification and antimicrobial activity. Journal of Applied Phycology,2012(24), 525–534. 10.1007/s10811-011-9758-0 [Google Scholar]
- 55.Sheikh, H., El-Naggar, A., & Al-Sobahi, D. (2018). Evaluation of antimycotic activity of extracts of marine algae collected from Red Sea Coast, Jeddah, Saudi Arabia. Journal of Biosciences and Medicines,6, 51–68. 10.4236/jbm.2018.64004 [Google Scholar]
- 56.Sauleau, P., Retailleau, P., Vacelet, J., & Bourguet-Kondracki, M. L. (2005). New polychlorinated pyrrolidinones from the Red Sea marine sponge Lamellodysideaherbacea. Tetrahedron,61, 955–963. 10.1016/j.tet.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 57.Slaughter, B. V., Khurshid, S. S., Fisher, O. Z., Khademhosseini, A., & Peppas, N. A. (2009). Hydrogels in regenerative medicine. Advanced Materials,21, 3307–3329. 10.1002/adma.200802106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serra, E., Saubade, F., Ligorio, C., Whitehead, K., Sloan, A., Williams, D. W., Hidalgo-Bastida, A., Verran, J., & Malic, S. (2020). Methylcellulose hydrogel with Melissa officinalis essential oil as a potential treatment for oral candidiasis. Microorganisms,8, 1–13. 10.3390/microorganisms8020215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campos, D., Piccirillo, C., Pullar, R. C., Castro, M. L., & Pintado, M. M. E. (2014). Characterization and antimicrobial properties of food packaging methylcellulose films containing stem extract of Ginja cherry. Journal of the Science of Food and Agriculture,94, 2097–2103. 10.1002/jsfa [DOI] [PubMed] [Google Scholar]
- 60.Ayana, B. B., & Turhan, K. N. (2009). Use of antimicrobial methylcellulose films to control Staphylococcus aureus during storage of Kasar cheese and science. Packaging Technology and Science,22, 461–469. 10.1002/pts.870 [Google Scholar]
- 61.Otoni, C. G., Pontes, S. F. O., Medeiros, E. A. A., & Soares, N. F. (2014). Edible films from methylcellulose and nanoemulsions of clove bud (Syzygiumaromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. Journal of Agricultural and Food Chemistry,62, 5214–5219. 10.1021/jf501055f [DOI] [PubMed] [Google Scholar]
- 62.Klouda, L. (2015). Thermoresponsive hydrogels in biomedical applications: A seven-year update. European Journal of Pharmaceutics and Biopharmaceutics,97, 338–349. 10.1016/j.ejpb.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 63.Gafitanu, C. A., Filip, D., Cernatescu, C., Rusu, D., Tuchilus, C. G., Macocinschi, D., & Zaltariov, M. (2017). Design preparation and evaluation of HPMC-based PAA or SA freeze-dried scaffolds for vaginal delivery of fluconazole. Pharmaceutical Research,34, 2185–2196. 10.1007/s11095-017-2226-z [DOI] [PubMed] [Google Scholar]
- 64.Rho, J. M., Anderson, G. D., Donevan, S. D., & White, H. S. (2002). Acetoacetate, acetone, and dibenzylamine (a contaminant in L-(+)-β-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia,43(4), 358–361. [DOI] [PubMed] [Google Scholar]
- 65.Jayesh, K., Helen, L. R., Vysakh, A., Binil, E., & Latha, M. S. (2017). In vivo toxicity evaluation of aqueous acetone extract of Terminalia bellirica (Gaertn.) Roxb. fruit. Regulatory Toxicology and Pharmacology,86, 349–355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All experimental data and analyses of the study are included in this published article.