Abstract
Diversion colitis (DC) is characterized by mucosal inflammation in the defunctioned segment of the colon following a colostomy or ileostomy. The major causes of DC are an increase in the number of aerobic bacteria, a lack of short-chain fatty acids (SCFAs), and immune disorders in the diverted colon. However, its exact pathogenesis remains unknown. Various treatment strategies for DC have been explored, although none have been definitively established. Treatment approaches such as SCFAs, 5-aminosalicylic acid enemas, steroid enemas, and irrigation with fibers have been attempted, yielding various degrees of efficacies in mitigating mucosal inflammation. However, only individual case reports demonstrating the limited effect of the following therapies have been published: leukocytapheresis, dextrose (hypertonic glucose) spray, infliximab, an elemental diet, and coconut oil. The usefulness of probiotics for treating DC has recently been reported. Furthermore, fecal microbiota transplantation (FMT) has emerged as a promising treatment for DC. This review provides an update on the treatment strategies of DC, with a particular focus on FMT and its relationship with the intestinal microbiota. FMT may become the first choice of treatment for some patients in the future because of its low medical costs, ease of use, and minimal side effects. Furthermore, FMT can also be used for postoperative DC prophylaxis.
Keywords: diversion colitis, pouchitis, ileitis, inflammatory bowel disease, treatment, fecal microbiota transplantation, microbiota
INTRODUCTION
Diversion colitis (DC) was first described by Morson and Dawson [1] in 1974 as a nonspecific inflammation in the diverted colon. Glotzer et al. [2] labeled this inflammation as “diversion colitis” in 1981. A prospective study reported that almost all patients had colitis post-colostomy, as evidenced by endoscopic analyses at 3–36 months of follow-up [3]. Symptomatic cases comprise approximately 30% of all DC cases diagnosed via endoscopic studies, and the precise pathogenesis of this condition remains elusive. Although various symptoms are reportedly associated with the disease, including abdominal discomfort, tenesmus, anorectal pain, mucous discharge, and rectal bleeding [4, 5], no definitive treatment strategies for DC have been established. Diversion pouchitis is characterized by inflammation of the ileal pouch due to fecal stream exclusion and a subsequent lack of nutrients from luminal bacteria, which is similar to DC. Therefore, the key difference between pouchitis and diversion pouchitis is whether the lesion is exposed to the fecal stream. Patients generally present with varying symptoms, such as tenesmus, bloody or mucus-like discharge, and abdominal pain [6]. Although the incidence of diversion pouchitis is unknown, it is more common in patients with an underlying inflammatory bowel disease (IBD). Nonsurgical approaches for treating diversion pouchitis include the use of short-chain fatty acids (SCFAs), topical 5-aminosalicylic acid (5-ASA), and topical glucocorticoids. Unfortunately, efficacy study outcomes are conflicting, and the only curative approach is surgical reanastomosis with the reestablishment of gut continuity [6,7,8].
Since our previous review publication on the treatments for DC and pouchitis [9], numerous new treatments have emerged, with increasing reports on fecal microbiota transplantation (FMT). However, an unmet need to summarize these therapeutic options exists. Therefore, this review provides an update on the treatment strategies of DC, particularly focusing on FMT and its relationship with the intestinal microbiota. We believe that the information compiled in this review will help physicians in treating DC cases. By increasing the number of treated cases, we hope to support the establishment of novel criteria for disease assessment and therapeutic decision trees.
Literature analysis
A literature search was conducted using the PubMed and Ovid databases, and the search terms “diversion colitis” or “diversion pouchitis” were used to extract studies published over the last 45 years. All appropriate English-language publications from relevant journals were selected. We have summarized the available information on the pathogenesis, treatment, and clinical course of DC.
CLINICAL CHARACTERISTICS
Pathogenesis
The basic mechanisms underlying DC remain unclear. Glotzer et al. [2] hypothesized that the condition might originate from bacterial overgrowth, the presence of harmful bacterial species, nutritional deficiencies, toxins, or disturbances in the symbiotic relationship between luminal bacteria and the mucosal layer. Studies have reported a reduction in the concentrations of carbohydrate-fermenting anaerobic and pathogenic bacteria in defunctioning colons [10,11,12,13], indicating that the overgrowth of anaerobic or pathogenic bacteria is unlikely to be an important etiological factor. Conversely, an increase in nitrate-reducing bacteria has been observed in patients with DC [14]. These bacteria produce nitric oxide (NO), which is protective at low concentrations but toxic to the colonic tissue at higher levels [15]. Therefore, an increase in nitrate-reducing bacteria may result in toxic levels of NO, leading to DC.
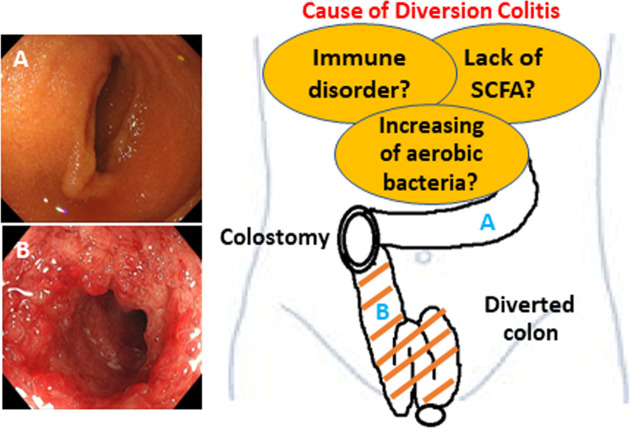
Recently, ischemia has been proposed as a potential cause of DC [16]. This theory is rooted in the changes to the luminal flora due to fecal stream interruption. Normal luminal bacteria produce SCFAs, such as butyric acid. Butyrate is the principal oxidative substrate of colonocytes [17], and patients with DC may experience improvements following topical treatment with SCFAs, particularly butyrate enemas [10, 18]. This hypothesis is supported by evidence suggesting that SCFAs relax vascular smooth muscle and that butyrate deficiency may induce increased tone in the pelvic arteries, leading to relative ischemia of the colorectal mucosa and intestinal wall [10]. Therefore, additional basic research is necessary to discern disease mechanisms. Figure 1 summarizes the pathogenesis of this disease.
Fig. 1.
Schematic presentation of diversion colitis and pouchitis.
An increase in the number of aerobic bacteria, a lack of short-chain fatty acids (SCFAs), and immune disorders in the diverted colon are the major causes of diversion colitis.
A. normal afferent ileal loop; B. diversion colitis.
TREATMENT STRATEGIES
The goal of treatment is to reduce or eliminate symptoms. Patients who desire stoma closure and have acceptable risks are deemed suitable to undergo surgery to reestablish intestinal continuity. In a prospective study, Son et al. reported that DC severity is correlated with diarrhea occurrence after ileostomy reversal and may adversely affect a patient’s quality of life [19]. Pharmacological treatments are required for symptomatic patients with permanent stomas and those who cannot undergo stoma closure due to technical difficulties, poor anal sphincter function, or persistent perianal sepsis. However, no established standard therapy for DC and pouchitis exists, given the unknown etiology of these conditions. A total of 61 articles matched our definitions of DC and pouchitis. Tables 1and 2 present the methods, advantages, and disadvantages of each therapy in the order in which they were first reported.
Table 1. Summary of pharmacologic treatments for diversion colitis and pouchitis 1.
| Treatment | Ref | First reported in the year | Procedure/Standard dosage | Efficacy |
|---|---|---|---|---|
| Surgical anastomosis | [2, 4, 3, 21, 22] | 1981 | Mobilization of both ends of the bowel with either sutured or stapled anastomosis. | This is the most effective method of alleviating the signs and symptoms. |
| Corticosteroids | [23, 24] | 1987 | Hydrocortisone (100 mg per 60 mL bottle) enema is administered once daily for up to 3 weeks. | Response to treatment is generally seen in 3 to 5 days. |
| Occasional treatment may be given for 2 to 3 months depending on clinical response. | ||||
| Short-chain-fatty acids (SCFAs) | [10, 26, 28,29,30] | 1989 | SCFA enema rectally twice a day for 2 weeks, and then tapered according to response over 2 to 4 weeks. | Varying effect. |
| 5-aminosalicylic acid (5ASA) enemas | [31,32,33] | 1991 | 4 g mesalamine in 60 ml suspension, administered rectally once-daily for 4 to 5 weeks. | Varying effect. |
| Irrigation with Fibers | [35, 36] | 2004 | Solution containing 5% Fibers (10 g/day) for 7 days. | The endoscopic score, used to quantify the intensity of the imflammation of the mucosa at the diverted colon, diminished after treatment. |
| Leukocytapheresis | [37] | 2014 | Leukocytapheresis, at a flow rate of 40 mL/min for 60 min, once weekly for 5 weeks; following low dose of metronidazole and ciprofloxacin, another set of weekly leukocytapheresis was added. | Signficant improvement in pouchitis disease activity index (PDAI) from 14 to 1. |
| Autologous fecal transplantation | [55,56,57,58,59] | 2015 | Feces were collected from the colostomy bag, diluted with 600 mL of sterile saline (0.9 %), stirred, and filtered three times using an ordinary coffee filter, and irrigation was done endoscopically. | All symptoms improved dramatically within 5 days after the first treatment. |
| Colonoscopy performed at 28 days after the first treatment showed no major signs of inflammation in the colonic stump. | ||||
| This procedure was repeated 3 times within 4 weeks (on Days 0, 10, and 28). | ||||
| Dextrose (hypertonic glucose) spray | [7] | 2017 | Endoscopically sprayed with 150 mL of 50% dextrose via a catheter. | Follow-up pouchoscopy 2 weeks after the dextrose spray showed normal pouch mucosa with no evidenceof bleeding or mucosal friability. |
| Infliximab | [42] | 2021 | The infliximab dose was 5 mg/kg, repeated at 2 and 6 weeks after the initial dose. | This therapy dramatically improved the colonic inflammation and alleviated the patient’s symptoms. |
| Total colonoscopy performed at 4 weeks after initiating this protocol showed almost complete resolution of the inflammation. | ||||
| Elemental diet | [45] | 2021 | An exclusive elemental diet and the low-fibre, fat-limited exclusion (LOFFLEX) diet. | Significant improvement in symptoms with a decrease in bowel motions, rectal discharge, and pain few weeks after employing an elemental diet. |
| Probiotics | [50, 51] | 2021 | Slow infusion of a solution of 4.5 mg of probiotics diluted in 250 mL of 0.9% physiological saline for 20–30 min. | A significant decrease in endoscopic pathological findings (mucosal friability, mucous erosions, polyps, edema, erythema and stenosis) and in histological findings (follicular hyperplasia, eosinophils, cryptic abscesses, lymphocyte infiltration, plasma cell infiltration and architecture distortion) was observed in a group stimulated with probiotics (p<0.001). |
| Coconut oil | [52] | 2022 | Daily local administration of 100 mL prewarmed coconut oil as a rectal enema. | One week after initiation of daily coconut oil administrations, the patient reported reduced abdominal pain and mucus secretions. |
| After another 6 weeks of continuous therapy, hematochezia and mucus secretion completely stopped. | ||||
| In a sigmoidoscopy performed after 8 weeks of daily therapy, clear improvement of endoscopic and histologic signs of inflammation were observed. |
Table 2. Summary of pharmacologic treatments for diversion colitis and pouchitis 2.
| Treatment | Ref | Complications/Main side effects | Special points at issue |
|---|---|---|---|
| Surgical anastomosis | [2, 3, 4, 21, 22] | Bleeding, infection, anastomotic leak, anastomotic stricture, anesthetic risks. | Because a differential diagnosis can be difficult, IBD may be reactivated, requiring futher treatment. |
| Corticosteroids | [23, 24] | Local pain and burning, occasionnally rectal bleeding.Prolonged treatment may result in systemic absorption, causing systemic side effects. | One of the first-line agents for symptomatic diversion colitis, with varying effectivenes. |
| Short-chain-fatty acids (SCFAs) | [10, 26, 28,29,30] | None. | One of the expensive pharmacologic treatment options. |
| 5-aminosalicylic acid (5ASA) enemas | [31,32,33] | Occasionally produces acute intolerance manifested by cramping, acute abdominal pain, bloody diarrhea, fever, headche, and rash. | For mild to moderrate proctitis, proctosigmoiditis, or distal colitis. |
| Irrigation with Fibers | [35, 36] | Probably none. | Only one study showed role of treatment in the preoperative management of colostomy. |
| Leukocytapheresis | [37] | The common side effects are nausea, vomiting, fever, chills, and nasal obstruction. | Only a case report, few serious side effects but expensive, good treatment for elderly patients. |
| Autologous fecal transplantation | [55,56,57,58,59] | None, patient’s tolerance is required. | Safe and inexpensive, patient’s motivation needed. |
| Dextrose (hypertonic glucose) spray | [7] | It has a very low chance of causing transient hyperglycemia because there is no direct injection of the hypertonic solution into blood vessels. | Only a case report, safe and inexpensive, this treatment has a potential for the side effect of bleeding. |
| Infliximab | [42] | Infliximab may cause not only common side effects but also serious side effects including infections. | Only a case report, the results in this case should be interpreted with caution because intravenous prednisolone was used with infliximab, and it is difficult to estimate the effect of therapy with infliximab alone. |
| Elemental diet | [45] | None. | Onset of effects was relatively slow. |
| Probiotics | [50, 51] | None. | A prospective, randomized, double-blind, controlled study, safe and effective. |
| Coconut oil | [52] | No adverse events. | Only a case report, safe and inexpensive. |
IBD: inflammatory bowel disease.
Surgery
The primary therapeutic objective for DC is to restore bowel continuity, thereby reinstating luminal flow. This approach alleviates symptoms and assists the bowel in returning to its normal state. Several studies have proven that reanastomosis is consistently effective in halting DC symptoms [2, 20,21,22,23]. Reanastomosis of diverted segments in patients with pre-existing IBD is a challenging decision to make because inflammation in the diverted segment could be indicative of IBD or DC, each of which dictates a different course of action [3, 4, 23]. Typically, resection is not required. The indications for resection include uncontrolled perianal sepsis, perianal fistulous disease, anal incontinence, and uncontrolled symptoms related to DC.
Corticosteroids
In 1984, Glotzer et al. [2] reported on several patients with DC who were treated with steroid enemas. In 1999 and 2000, respectively, Jowett and Cobden, and Lim et al. reported on the efficacy of steroid enemas [23, 24]. Corticosteroids are the first-line agents for symptomatic DC with varying effectiveness.
Short-chain fatty acids
Short-chain-fatty acids (SCFAs), primarily butyrate, are the major fuel sources for the epithelium. The absence of the diverted tract may lead to mucosal atrophy and inflammation. Bacteria produce SCFAs as by-products of carbohydrate fermentation in the colonic lumen, and SCFAs provide the primary energy source for colonic mucosal cells [25]. In human neutrophils, SCFAs have been shown to reduce the production of reactive oxygen species, which are the agents of oxidative tissue damage [26]. DC treatment with SCFAs or butyrate has shown inconsistent results. In 1989, Harig et al. [10] reported on the efficacy of SCFAs. Komorowski [20] reported similar results in four patients with DC treated with SCFA irrigation. However, Fazio et al. and Haque and West failed to demonstrate histological or endoscopic improvements [6, 27]. The differences in response may be partly attributed to disease grouping. Recently, several studies have reported the usefulness of SCFAs, including butyrate [28, 29]. Luceri et al. [30] proposed that butyrate enemas may prevent atrophy of the diverted colon/rectum, thereby improving the recovery of tissue integrity.
5-aminosalicylic acid
The utility of 5-ASA enemas in DC was first reported by Triantafillidis et al. in 1991 [31]. Similar results were also reported by Tripodi et al. [32]. Caltabiano et al. [33] reported that a 5-ASA enema reduced oxidative DNA damage in the colonic mucosa and mucosal damage in rats in a DC model. Mucosal disorders may be improved by the protective action against oxidative DNA damage and the anti-inflammatory action of 5-ASA [34].
Irrigation with fibers
DC resolution, as evidenced by endoscopic and histological examinations, has been reported after irrigation of the diverted segment of the colon with fibers [35, 36]. De Oliveira-Neto and de Aguilar-Nascimento [36] investigated the effect of irrigating the colorectal mucosa with a solution of fibers in patients who had undergone a colostomy. In 11 patients with loop colostomies, the diverted colorectal segment was irrigated with a solution containing 5% fibers (10 g/d) for 7 days. This fiber irrigation improved inflammation within the defunctionalized colon, suggesting that this therapy plays a role in the preoperative management of colostomies [36], potentially decreasing the high incidence of diarrhea after intestinal transit reestablishment.
Leukocytapheresis
Watanabe et al. [37] reported successful treatment using leukocytapheresis in a patient with chronic antibiotic-refractory diversion pouchitis following ileal pouch-anal anastomosis for ulcerative colitis (UC) with diverting ileostomy. The diverted pouch mucosa is less exposed to the fecal stream and pathogens. Therefore, altered immunity likely plays a major role in diversion pouchitis progression. Leukocytapheresis, which addresses altered immunity, appears to be a reasonable approach for antibiotic-refractory pouchitis following ileal J-pouch anal anastomosis for UC with diverting ileostomy; its effectiveness, in this case, suggests that altered immunity is a key contributing factor compared with dysbiosis, bacterial pathogens, and ischemia.
Dextrose spray
Nyabanga and Shen [7] reported on a patient with UC with severe hematochezia and diffuse mucosal bleeding in a diverted ileal pouch who was successfully treated with an endoscopic spray of hypertonic glucose (50% dextrose). Hypertonic glucose may function through osmotic dehydration and sclerosant effects, inducing long-term mural necrosis and fibrotic obliteration of the mucosal vessels. Glucose spraying is safe, inexpensive, and has a low risk of complications. Therefore, this approach can reduce recurrent bleeding and the need for surgical intervention.
Infliximab
Clinically, DC tends to occur in patients with pre-existing IBD, and the clinicopathological findings of both conditions can mimic each other [21, 38]. The effect of infliximab in an experimental animal model of DC was recently reported, and Buanaim et al. suggested that it had a positive effect against DC based on their experimental model [39,40,41]. Kido et al. [42] reported a DC case in which infliximab suppressed acute inflammation refractory to standard medical management and successfully prevented the need for total colonic resection.
Elemental diet
An exclusive elemental diet has been shown to induce and maintain remission in patients with Crohn’s disease [43, 44]. However, evidence is lacking regarding the use of an elemental diet and low-fiber, fat-limited exclusion (LOFFLEX) diet in managing DC [9]. An exclusively elemental diet provides patients with essential and nonessential amino acids, fats, carbohydrates, and vitamins in a form that is more effectively absorbed and less allergenic in patients with gastrointestinal disorders [43]. The LOFFLEX diet is designed to help reintroduce foods after enteral feeding and comprises foods reported to cause the least symptoms in patients with IBD [45]. Lane et al. [46] reported on a patient with a flare of DC who was treated with diet alone. The use of an elemental diet that decreases the antigen load in the gut may help regulate autoimmunity, which can cause expanded DC [47].
Probiotics
Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts [48]. They interact with the intestinal mucosa, thereby reducing pro-inflammatory substance production [49]. Rodríguez-Padilla et al. [50, 51] designed a prospective, randomized, double-blind, controlled study and reported that preoperatively stimulating the efferent loop with probiotics through the dysfunctional bowel to allow slow infusion can reduce the endoscopic and histopathological alterations of DC. Probiotic stimulation of the efferent loop is a safe and effective method for reducing macroscopic and microscopic colitis and symptoms in the short term after reconstructive surgery.
Coconut oil
Zundler et al. [52] reported on a patient with fistulizing Crohn’s disease complicated by DC who was successfully treated with topical coconut oil application. Daily local administration of 100 mL prewarmed coconut oil as a rectal enema was initiated based on the rationale that coconut oil contains fatty acids with comparatively short chain lengths [53]. Although they are not identical to the SCFAs established in DC therapy, they are commensally produced and reduced in the inflamed mucosa [54], suggesting a positive impact on epithelial metabolism, as postulated for SCFAs [10].
Autologous fecal microbiota transplantation
Tables 3, 4, 5, 6 present detailed data from case reports of autologous FMT for DC and diversion pouchitis. In 2015, Gundling et al. [55] reported that autologous FMT might be an effective and safe option for relapsing DC after standard therapies fail, and Kalla et al. [56] published a similar report in 2019. In 2019, we reported for the first time the usefulness of autologous FMT for DC and diversion pouchitis and intestinal microbiota evaluation pre- and post-treatment [57]. Subsequently, Donahue et al. [58] reported the treatment of a DC patient with FMT as the first choice, which also improved lumen narrowing. In 2022, we performed FMT in five patients with mild DC and observed improvements in clinical symptoms and endoscopic findings. We also evaluated the gut microbiota pre- and post-treatment [59].
Table 3. Clinical characteristics of the reviewed cases of diversion colitis and pouchitis 1.
| Case | Authors | Ref | Reporting | Country | Age | Sex | Primary illness | Type of diversion |
| (No) | (No) | year | (years) | (Male/Female) | (reason for diversion) | (surgical procedure) | ||
| 1 | Gundling et al. | [55] | 2015 | Germany | 75 | F | Chronic constipation | Permanent end-colostomy |
| 2 | Kalla et al. | [56] | 2019 | UK | 47 | F | Severe constipation | Subtotal colectomy and ileorectal anastomosis |
| 3 | Tominaga et al. | [57] | 2019 | Japan | 43 | F | Ulcerative colitis pancolitis-type | Total proctocolectomy with ileal pouch–anal anastomosis → ileostomy |
| 4 | Donahue et al. | [58] | 2022 | USA | 20 | M | Gunshot wound | Repair of two sigmoid colonic injuries and diverting colostomy |
| 5 | Tominaga et al. | [59] | 2022 | Japan | 49 | F | Rectal cancer | Low anterior resection + colostomy |
| 6 | Tominaga et al. | [59] | 2022 | Japan | 66 | F | Rectal cancer | Low anterior resection + colostomy |
| 7 | Tominaga et al. | [59] | 2022 | Japan | 67 | F | Retroperitoneal abscess | Left hemicolectomy + transverse colostomy |
| 8 | Tominaga et al. | [59] | 2022 | Japan | 74 | F | Ovarian cancer | Transverse colostomy |
| 9 | Tominaga et al. | [59] | 2022 | Japan | 67 | F | Rectovaginal septum cancer | Transverse colostomy |
Table 4. Clinical characteristics of the reviewed cases of diversion colitis and pouchitis 2.
| Case | Authors | Ref | Period of up to diagnosisfrom operation | Symptoms | Endoscopy findings | Diagnosis |
|---|---|---|---|---|---|---|
| (No) | (No) | |||||
| 1 | Gundling et al. | [55] | N/A | Tenesmus and severe rectal pain | Severe inflammation on colonic stump | Diversion colitis |
| 2 | Kalla et al. | [56] | 23 years | Recurrent bloody rectal discharge and anorectal pain | Severe active inflammation with evidence of contact bleeding | Diversion colitis |
| 3 | Tominaga et al. | [57] | 13 months | Tenesmus and perianal pain | Severe ileitis and pouchitis | Diversion ileitis and pouchitis |
| 4 | Donahue et al. | [58] | 8 months | None | Severe disuse colitis with the small caliber of the colon | Diversion colitis |
| 5 | Tominaga et al. | [59] | 6 months | None | Ulcerative Colitis Endoscopic Index of Severity (UCEIS) 2 | Diversion colitis |
| 6 | Tominaga et al. | [59] | 6 months | None | UCEIS 2 | Diversion colitis |
| 7 | Tominaga et al. | [59] | 16 months | Mucous stool | UCEIS 3 | Diversion colitis |
| 8 | Tominaga et al. | [59] | 18 months | Mucous stool | UCEIS 3 | Diversion colitis |
| 9 | Tominaga et al. | [59] | 40 months | Bloody stool and tenesmus | UCEIS 3 | Diversion colitis |
Table 5. Summary of treatments for the reviewed cases of diversion colitis and pouchitis.
| Case | Authors | Ref | Ineffective treatment | Effective treatment |
|---|---|---|---|---|
| (No) | (No) | |||
| 1 | Gundling et al. | [55] | Enemas containing 5-aminosalicylic acid and steroids plus antibiotic therapy | A total of 3 autologous FMTs over 4 weeks (Days 0, 10, and 28). |
| 2 | Kalla et al. | [56] | Steroid enemas, 5ASAs, SCFA enemas, and antibiotic therapies | A total of 3 autologous FMTs over 4 weeks (Days 0, 10, and 29). |
| 3 | Tominaga et al. | [57] | Antibiotics, corticosteroids and immunosuppressive agents (azathioprine) | Inject faeces from the stoma every day for a month and 3 times per week thereafter. |
| 4 | Donahue et al. | [58] | None | Inject fecal material into the mucous fstula 1–2 times a day for 1 year. |
| 5 | Tominaga et al. | [59] | None | Inject autologous feces from the stoma once every 3 days for 4 weeks. |
| 6 | Tominaga et al. | [59] | None | Inject autologous feces from the stoma once every 3 days for 4 weeks. |
| 7 | Tominaga et al. | [59] | None | Inject autologous feces from the stoma once every 3 days for 4 weeks. |
| 8 | Tominaga et al. | [59] | None | Inject autologous feces from the stoma once every 3 days for 4 weeks. |
| 9 | Tominaga et al. | [59] | None | Inject autologous feces from the stoma once every 3 days for 4 weeks. |
ASAs: amino salicyclic acids; SCFA: short chain fatty acids; FMT: fecal microbiota transplantation.
Table 6. Summary of the prognoses of the reviewed cases of diversion colitis and pouchitis.
| Case | Authors | Ref | Prognosis |
|---|---|---|---|
| (No) | (No) | ||
| 1 | Gundling et al. | [55] | All symptoms improved dramatically within 5 days. Colonoscopy performed at 28 days after the first treatment showed no major signs of inflammation in the colonic stump. |
| 2 | Kalla et al. | [56] | Endoscopic and histological evidence of remission. |
| 3 | Tominaga et al. | [57] | Patient’s condition improved markedly. |
| 4 | Donahue et al. | [58] | Sigmoid colon appeared healthy and large enough to perform a colocolonic anastomosis, healthy appearing mucosa with a small amount of stool in the rectum. |
| 5 | Tominaga et al. | [59] | Symptomatic improvement within 2 weeks of starting FMT, UCEIS score 0 at 1 month after the treatment. |
| 6 | Tominaga et al. | [59] | Symptomatic improvement within 2 weeks of starting FMT, UCEIS score 0 at 1 month after the treatment. |
| 7 | Tominaga et al. | [59] | Symptomatic improvement within 2 weeks of starting FMT, UCEIS score 1 at 1 month after the treatment. |
| 8 | Tominaga et al. | [59] | Symptomatic improvement within 2 weeks of starting FMT, UCEIS score 0 at 1 month after the treatment. |
| 9 | Tominaga et al. | [59] | Symptomatic improvement within 2 weeks of starting FMT, UCEIS score 1 at 1 month after the treatment. |
FMT: fecal microbiota transplantation; UCEIS: Ulcerative Colitis Endoscopic Index of Severity.
The mean age of the nine patients (one male and eight females) reported to have been treated with FMT to date is 56.4 (20–75) years. The proportions of patients according to their various primary conditions (reasons for diversion) are as follows: constipation, n=2; rectal cancer, n=2; ulcerative colitis, n=1; retroperitoneal abscess, n=1; ovarian cancer, n=1; rectovaginal septum cancer, n=1; and gunshot wound, n=1. Additionally, the time from diagnosis to operation ranged from 6 months to 23 years (Table 3). All nine patients showed endoscopic inflammation; however, three were asymptomatic (Table 4). Six of the nine patients underwent FMT as the first choice, and three were refractory to various treatments, including 5-ASA, SCFAs, and prednisolone (Table 5). In all the reports, the onset of efficacy was rapid, with improvement in clinical symptoms within a few days to 2 weeks; improvement was observed endoscopically 1 month later in most cases. No major adverse effects were observed in any of these studies (Table 6). Therefore, FMT decreases inflammation in the defunctionalized colon, suggesting that this therapy plays a role in preoperatively managing colostomies. Furthermore, FMT may become the first treatment option for some patients in the future because of its low medical cost, ease of use, and minimal side effects.
Summary of pharmacological treatments
Pharmacological treatment is generally indicated for the temporary control of symptoms during preparation for surgery. It is occasionally used in patients who are not considered surgical candidates because of severe medical comorbidities, poor sphincter function, or technical difficulties. In our review, SCFAs, 5-ASA enemas, steroid enemas, and irrigation with fibers were explored, and they have been reported to demonstrate various efficacies for mucosal inflammation. However, for some therapies, such as leukocytapheresis, dextrose (hypertonic glucose) spray, infliximab, an elemental diet, and coconut oil, only case reports have been published, limiting the generalizability of the findings from one case study to other settings. Recently, the usefulness of autologous FMT for DC has been reported, and it may become the first treatment choice for some patients in the future because of its low medical cost, ease of use, and minimal side effects. However, for autologous FMT to be successful in DC treatment, patients must be reliable; that is, they should be self-motivated to perform fecal transplantation independently. Therefore, given the limited number of cases, further investigations should determine the optimal frequency and duration of treatment before reestablishing intestinal continuity.
CONCLUSION
Although most patients with a diversion remain asymptomatic, DC occurs in almost all such patients. It generally resolves after the closure of the colostomy. However, patients with significant symptoms or histories of colitis or diarrhea should undergo complete proximal and distal colonic evaluations before stoma closure, and certain treatments should not be delayed in these patients. Furthermore, patients with a permanent diversion should undergo periodic pharmacological treatment; however, the efficacy of these treatments has not been clearly confirmed. Therefore, this review of the various therapies for DC will hopefully be useful for determining future treatments.
AUTHOR CONTRIBUTIONS
K Tominaga and K Kamimura wrote the manuscript. Y Kojima, Y Kawata, K Takahashi, H Sato, A Tsuchiya, and S Terai collected the data. All authors read and approved the final version of the manuscript.
FUNDING
The authors disclose the receipt of the following financial support for the research and authorship of this article. This work was supported by a Grant-in-Aid for Early-Career Scientists (19K17393) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. K Tominaga also received a research incentive award from The Intestinal Microbiology Society.
AVAILABILITY OF DATA AND MATERIALS
All the data supporting this review are available within the article.
CONFLICT OF INTEREST
The authors have no conflict of interest.
REFERENCES
- 1.Morson BC, Dawson IMP. 1972. Gastrointestinal pathology, 1st ed. Blackwellfic Publications, London. [Google Scholar]
- 2.Glotzer DJ, Glick ME, Goldman H. 1981. Proctitis and colitis following diversion of the fecal stream. Gastroenterology 80: 438–441. [PubMed] [Google Scholar]
- 3.Korelitz BI, Cheskin LJ, Sohn N, Sommers SC. 1984. Proctitis after fecal diversion in Crohn’s disease and its elimination with reanastomosis: implications for surgical management. Report of four cases. Gastroenterology 87: 710–713. [PubMed] [Google Scholar]
- 4.Szczepkowski M, Kobus A, Borycka K. 2008. How to treat diversion colitis?—Current state of medical knowledge, own research and experience. Acta Chir Iugosl 55: 77–81. [DOI] [PubMed] [Google Scholar]
- 5.Ma CK, Gottlieb C, Haas PA. 1990. Diversion colitis: a clinicopathologic study of 21 cases. Hum Pathol 21: 429–436. [DOI] [PubMed] [Google Scholar]
- 6.Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, Schroeder TK. 1995. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg 222: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyabanga CT, Shen B. 2017. Endoscopic treatment of bleeding diversion pouchitis with high-concentration dextrose spray. ACG Case Rep J 4: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorgun E, Remzi FH. 2004. Complications of ileoanal pouches. Clin Colon Rectal Surg 17: 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tominaga K, Kamimura K, Takahashi K, Yokoyama J, Yamagiwa S, Terai S. 2018. Diversion colitis and pouchitis: a mini-review. World J Gastroenterol 24: 1734–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harig JM, Soergel KH, Komorowski RA, Wood CM. 1989. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med 320: 23–28. [DOI] [PubMed] [Google Scholar]
- 11.Baek SJ, Kim SH, Lee CK, Roh KH, Keum B, Kim CH, Kim J. 2014. Relationship between the severity of diversion colitis and the composition of colonic bacteria: a prospective study. Gut Liver 8: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neut C, Colombel JF, Guillemot F, Cortot A, Gower P, Quandalle P, Ribet M, Romond C, Paris JC. 1989. Impaired bacterial flora in human excluded colon. Gut 30: 1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tominaga K, Tsuchiya A, Mizusawa T, Matsumoto A, Minemura A, Oka K, Takahashi M, Yosida T, Kawata Y, Takahashi K, et al. 2020. Evaluation of intestinal microbiota, short-chain fatty acids, and immunoglobulin a in diversion colitis. Biochem Biophys Rep 25: 100892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neut C, Guillemot F, Colombel JF. 1997. Nitrate-reducing bacteria in diversion colitis: a clue to inflammation? Dig Dis Sci 42: 2577–2580. [DOI] [PubMed] [Google Scholar]
- 15.McCafferty DM, Mudgett JS, Swain MG, Kubes P. 1997. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology 112: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 16.Villanacci V, Talbot IC, Rossi E, Bassotti G. 2007. Ischaemia: a pathogenetic clue in diversion colitis? Colorectal Dis 9: 601–605. [DOI] [PubMed] [Google Scholar]
- 17.Velázquez OC, Lederer HM, Rombeau JL. 1997. Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol 427: 123–134. [PubMed] [Google Scholar]
- 18.Kiely EM, Ajayi NA, Wheeler RA, Malone M. 2001. Diversion procto-colitis: response to treatment with short-chain fatty acids. J Pediatr Surg 36: 1514–1517. [DOI] [PubMed] [Google Scholar]
- 19.Son DN, Choi DJ, Woo SU, Kim J, Keom BR, Kim CH, Baek SJ, Kim SH. 2013. Relationship between diversion colitis and quality of life in rectal cancer. World J Gastroenterol 19: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komorowski RA. 1990. Histologic spectrum of diversion colitis. Am J Surg Pathol 14: 548–554. [DOI] [PubMed] [Google Scholar]
- 21.Orsay CP, Kim DO, Pearl RK, Abcarian H. 1993. Diversion colitis in patients scheduled for colostomy closure. Dis Colon Rectum 36: 366–367. [DOI] [PubMed] [Google Scholar]
- 22.Lusk LB, Reichen J, Levine JS. 1984. Aphthous ulceration in diversion colitis. Clinical implications. Gastroenterology 87: 1171–1173. [PubMed] [Google Scholar]
- 23.Jowett SL, Cobden I. 2000. Diversion colitis as a trigger for ulcerative colitis. Gut 46: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim AG, Langmead FL, Feakins RM, Rampton DS. 1999. Diversion colitis: a trigger for ulcerative colitis in the in-stream colon? Gut 44: 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray FE, O’Brien MJ, Birkett DH, Kennedy SM, LaMont JT. 1987. Diversion colitis. Pathologic findings in a resected sigmoid colon and rectum. Gastroenterology 93: 1404–1408. [PubMed] [Google Scholar]
- 26.Liu Q, Shimoyama T, Suzuki K, Umeda T, Nakaji S, Sugawara K. 2001. Effect of sodium butyrate on reactive oxygen species generation by human neutrophils. Scand J Gastroenterol 36: 744–750. [DOI] [PubMed] [Google Scholar]
- 27.Haque S, West AB. 1992. Diversion colitis—20 years a-growing. J Clin Gastroenterol 15: 281–283. [PubMed] [Google Scholar]
- 28.Pal K, Tinalal S, Al Buainain H, Singh VP. 2015. Diversion proctocolitis and response to treatment with short-chain fatty acids—a clinicopathological study in children. Indian J Gastroenterol 34: 292–299. [DOI] [PubMed] [Google Scholar]
- 29.Schauber J, Bark T, Jaramillo E, Katouli M, Sandstedt B, Svenberg T. 2000. Local short-chain fatty acids supplementation without beneficial effect on inflammation in excluded rectum. Scand J Gastroenterol 35: 184–189. [DOI] [PubMed] [Google Scholar]
- 30.Luceri C, Femia AP, Fazi M, Di Martino C, Zolfanelli F, Dolara P, Tonelli F. 2016. Effect of butyrate enemas on gene expression profiles and endoscopic/histopathological scores of diverted colorectal mucosa: a randomized trial. Dig Liver Dis 48: 27–33. [DOI] [PubMed] [Google Scholar]
- 31.Triantafillidis JK, Nicolakis D, Mountaneas G, Pomonis E. 1991. Treatment of diversion colitis with 5-aminosalicylic acid enemas: comparison with betamethasone enemas. Am J Gastroenterol 86: 1552–1553. [PubMed] [Google Scholar]
- 32.Tripodi J, Gorcey S, Burakoff R. 1992. A case of diversion colitis treated with 5-aminosalicylic acid enemas. Am J Gastroenterol 87: 645–647. [PubMed] [Google Scholar]
- 33.Caltabiano C, Máximo FR, Spadari APP, da Conceição Miranda DD, Serra MM, Ribeiro ML, Martinez CA. 2011. 5-aminosalicylic acid (5-ASA) can reduce levels of oxidative DNA damage in cells of colonic mucosa with and without fecal stream. Dig Dis Sci 56: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 34.Grisham MB, Granger DN. 1988. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci 33 Suppl: 6S–15S. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal VP, Schimmel EM. 1989. Diversion colitis: a nutritional deficiency syndrome? Nutr Rev 47: 257–261. [DOI] [PubMed] [Google Scholar]
- 36.de Oliveira-Neto JP, de Aguilar-Nascimento JE. 2004. Intraluminal irrigation with fibers improves mucosal inflammation and atrophy in diversion colitis. Nutrition 20: 197–199. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe C, Hokari R, Miura S. 2014. Chronic antibiotic-refractory diversion pouchitis successfully treated with leukocyteapheresis. Ther Apher Dial 18: 644–645. [DOI] [PubMed] [Google Scholar]
- 38.Geraghty JM, Talbot IC. 1991. Diversion colitis: histological features in the colon and rectum after defunctioning colostomy. Gut 32: 1020–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buanaim RP, Pereira JA, Campos FG, Kotze PG, Goto EFK, Mendonça RLS, Kanno DT, Martinez CAR. 2019. Effects of anti-TNF-α in experimental diversion colitis. Acta Cir Bras 34: e201901004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves Junior AJT, Pereira JA, Ávila MG, Domingues FA, Sato DT, Martinez CAR. 2021. Tissue content of metalloproteinase-9 and collagen in the colon with and without fecal stream after intervention with infliximab in rats subjected to Hartmann’s surgery. Acta Cir Bras 36: e360401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delben AG, Martinez CAR, Sato DT, Pereira JA, Dos Santos Mendonça RL, Kanno DT, de Lourdes Setsuko Ayrizono M. 2021. Evaluation of the effects of biological therapy with infliximab in an experimental model of diversion colitis. J Gastrointest Surg 25: 2681–2683. [DOI] [PubMed] [Google Scholar]
- 42.Kido M, Tamura R, Yasui Y, Okajima H. 2021. Novel application of infliximab for diversion colitis. BMJ Case Rep 14: e243284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rostami K, Al Dulaimi D. 2015. Elemental diets role in treatment of high ileostomy output and other gastrointestinal disorders. Gastroenterol Hepatol Bed Bench 8: 71–76. [PMC free article] [PubMed] [Google Scholar]
- 44.González-Huix F, de León R, Fernández-Bañares F, Esteve M, Cabré E, Acero D, Abad-Lacruz A, Figa M, Guilera M, Planas R, et al. 1993. Polymeric enteral diets as primary treatment of active Crohn’s disease: a prospective steroid controlled trial. Gut 34: 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woolner J, Parker T, Kirby G, Hunter J. 1998. The development and evaluation of a diet for maintaining remission in Crohn’s disease. J Hum Nutr Diet 11: 1–11. [Google Scholar]
- 46.Lane A, Dalkie N, Henderson L, Irwin J, Rostami K. 2021. An elemental diet is effective in the management of diversion colitis. Gastroenterol Hepatol Bed Bench 14: 81–84. [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. 2005. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis 11: 580–588. [DOI] [PubMed] [Google Scholar]
- 48.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. 2014. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11: 506–514. [DOI] [PubMed] [Google Scholar]
- 49.Biagioli M, Capobianco D, Carino A, Marchianò S, Fiorucci C, Ricci P, Distrutti E, Fiorucci S. 2019. Divergent effectiveness of multispecies probiotic preparations on intestinal microbiota structure depends on metabolic properties. Nutrients 11: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodríguez-Padilla Á, Morales-Martín G, Pérez-Quintero R, Gómez-Salgado J, Rada-Morgades R, Ruiz-Frutos C. 2021. Diversion colitis: macro and microscopic findings after probiotics stimulation. Biology (Basel) 10: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-Padilla Á, Morales-Martín G, Pérez-Quintero R, Rada-Morgades R, Gómez-Salgado J, Ruiz-Frutos C. 2021. Diversion colitis and probiotic stimulation: effects of bowel stimulation prior to ileostomy closure. Front Med (Lausanne) 8: 654573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zundler S, Dietz L, Matzel KE, Geppert CI, Becker E, Rath T, Neurath MF, Atreya R. 2018. Successful long-term treatment of diversion colitis with topical coconut oil application. Am J Gastroenterol 113: 1908–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orsavova J, Misurcova L, Ambrozova JV, Vicha R, Mlcek J. 2015. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci 16: 12871–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Preter V, Machiels K, Joossens M, Arijs I, Matthys C, Vermeire S, Rutgeerts P, Verbeke K. 2015. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut 64: 447–458. [DOI] [PubMed] [Google Scholar]
- 55.Gundling F, Tiller M, Agha A, Schepp W, Iesalnieks I. 2015. Successful autologous fecal transplantation for chronic diversion colitis. Tech Coloproctol 19: 51–52. [DOI] [PubMed] [Google Scholar]
- 56.Kalla R, Pitt M, Sharma A. 2019. The role of autologous fecal microbiota transplantation in diversion colitis: a case report. Inflamm Bowel Dis 25: e29–e30. [DOI] [PubMed] [Google Scholar]
- 57.Tominaga K, Tsuchiya A, Yokoyama J, Terai S. 2019. How do you treat this diversion ileitis and pouchitis? Gut 68: 593–758. [DOI] [PubMed] [Google Scholar]
- 58.Donahue CA, Chaudhry V, Mantilla N. 2022. Autologous fecal transplant for the treatment of microcolon due to diversion colitis. Tech Coloproctol 26: 79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tominaga K, Tsuchiya A, Mizusawa T, Matsumoto A, Minemura A, Oka K, Takahashi M, Yoshida T, Kojima Y, Ogawa K, et al. 2021. Utility of autologous fecal microbiota transplantation and elucidation of microbiota in diversion colitis. DEN Open 2: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting this review are available within the article.