Key Points
Question
What percentage of US adults consume at least 1 of 6 potentially hepatotoxic botanical products?
Findings
In this survey study analyzing nationally representative data from 9685 adults, 4.7% of US adults reported exposure to 6 potentially hepatotoxic botanicals: turmeric was most frequently reported, followed in order by green tea, ashwagandha, Garcinia cambogia, red yeast rice, and black cohosh products. Botanical product users were significantly older, more educated, and more likely to have arthritis compared with nonusers.
Meaning
The results of this study suggest that clinicians should be aware of possible adverse events from consumption of these largely unregulated products.
Abstract
Importance
Use of herbal and dietary supplements (HDSs) accounts for an increasing proportion of drug hepatotoxicity cases. Turmeric or curcumin, green tea extract, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha are the most frequently reported hepatoxic botanicals, but their prevalence and reasons for use in the general population are unknown.
Objective
To assess the prevalence and clinical characteristics of adult consumers of 6 potentially hepatoxic botanicals.
Design, Setting, and Participants
This survey study analyzed nationally representative data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative, cross-sectional survey of the general US population. Prescription drug and HDS exposure data in the past 30 days were analyzed, and 2020 US Census data were used for population estimates. Data were analyzed July 1, 2023, to February 1, 2024.
Exposures
Adult NHANES participants enrolled between January 2017 and March 2020.
Main Outcomes and Measures
Baseline weighted characteristics of HDS users and users of 6 potentially hepatotoxic botanical products were compared with non–HDS users. Multivariable analysis was undertaken to identify factors associated with HDS use or at-risk botanical use.
Results
Among 9685 adults enrolled in this NHANES cohort, the mean (SE) age was 47.5 (0.5) years, and 51.8% (95% CI, 50.2%-53.4%) were female. The overall prevalence of HDS product use was 57.6% (95% CI, 55.9%-59.4%), while the prevalence of using the 6 botanicals of interest was 4.7% (95% CI, 3.9%-5.7%). Turmeric-containing botanicals were most commonly used (n = 236), followed by products containing green tea (n = 92), ashwagandha (n = 28), Garcinia cambogia (n = 20), red yeast rice (n = 20), and black cohosh (n = 19). Consumers of these 6 botanicals were significantly older (adjusted odds ratio [AOR], 2.36 [95% CI, 1.06-5.25]; P = .04 for 40-59 years of age and AOR, 3.96 [95% CI, 1.93-8.11]; P = .001 for ≥60 years of age), had a higher educational level (AOR, 4.78 [95% CI, 2.62-8.75]; P < .001), and were more likely to have arthritis (AOR, 2.27 [95% CI, 1.62-3.29]; P < .001) compared with non–HDS users. An estimated 15 584 599 (95% CI, 13 047 571-18 648 801) US adults used at least 1 of the 6 botanical products within the past 30 days, which was similar to the estimated number of patients prescribed potentially hepatotoxic drugs, including simvastatin (14 036 024 [95% CI, 11 202 460-17 594 452]) and nonsteroidal anti-inflammatory drugs (14 793 837 [95% CI, 13 014 623-16 671 897]). The most common reason for consuming turmeric and green tea was to improve or maintain health.
Conclusions and Relevance
In this survey study, an estimated 15.6 million US adults consumed at least 1 botanical product with liver liability within the past 30 days, comparable with the number of people who consumed nonsteroidal anti-inflammatory drugs and a commonly prescribed hypolipidemic drug. Given a lack of regulatory oversight on the manufacturing and testing of botanical products, clinicians should be aware of possible adverse events from consumption of these largely unregulated products.
This survey study assesses the prevalence of use and clinical characteristics of consumers of the 6 most frequently reported hepatoxic botanicals, including turmeric or curcumin, green tea extract, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha, among US adults.
Introduction
Herbal and dietary supplements (HDSs) include a multitude of products consumed by millions of people every day to improve their general health and to treat minor ailments. Over 80 000 HDS products can be purchased without a prescription at various unregulated retail outlets or via the internet.1 The largest group of HDS products used include multivitamins, minerals, vitamin D, omega-3 fatty acid, and calcium with well-defined ingredients on the label. However, an estimated 5% to 12% of HDS products are plant-derived, complex multi-ingredient botanicals.2,3 Chemical analyses of HDS products associated with confirmed liver toxic effects show frequent discrepancies between product labels and detected ingredients.3 The safety and efficacy of HDSs are not well established due to the lack of regulatory requirements by the US Food and Drug Administration for human pharmacokinetic or prospective clinical trials prior to marketing.4
The Drug Induced Liver Injury Network (DILIN), a multicenter US observational program that collects and analyzes data from patients with hepatotoxic effects attributed to various drugs and HDS products, found that the proportion of DILI cases from HDSs nearly tripled from 7% in 2004 to 2005 to 20% in 2013 to 2014.5,6 The most commonly implicated botanical products in the DILIN include turmeric, kratom, green tea extract, and Garcinia cambogia, with potentially severe and even fatal liver injury.7,8,9,10,11,12,13 Furthermore, the multicenter Acute Liver Failure Study Group has also demonstrated that an increasing proportion of DILI-related acute liver failure cases were caused by HDSs, increasing from 12.4% in 1998 to 2007, to 21.1% in 2007 to 2015.14
The National Health and Nutrition Examination Survey (NHANES) is a periodic, population-based study of the general US population that includes comprehensive data regarding HDS use.15 In the current study, the proportion of NHANES patients who reported exposure to 6 potentially hepatotoxic botanicals—turmeric or curcumin, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha—were identified.7,8,9,10,11,12,13 The clinical features and baseline demographics of these individuals along with their self-reported reasons for taking these products are reviewed herein and compared with non–HDS users. To determine population level estimates of exposure to these products, US census data were used.
Methods
Study Population
This survey study used data from NHANES, a cross-sectional, nationally representative survey designed to monitor the health and nutrition of the civilian noninstitutionalized resident US population that has been conducted in 2-year cycles since 1999.16 NHANES was approved by the research ethics review board of the US Centers for Disease Control and Prevention National Center for Health Statistics, with written informed consent obtained from all adult participants. NHANES collects data from interviews, standardized physical examinations, and analyses of obtained blood and other biological specimens. Due to the COVID-19 pandemic, data collection for the NHANES 2019-2020 cycle was interrupted. Therefore, for the present analysis, data collected from January 2019 to March 2020 among adults older than 18 years of age were combined with data from the NHANES 2017 to 2018 cycle to form a nationally representative sample of NHANES 2017 to March 2020 prepandemic data.17 The crude response rates during 2017 to March 2020 were 51.4% for children and adolescents aged 2 to 19 years of age and 43.9% for adults aged 20 years or older.17 All data used in this analysis were extracted from publicly available datasets.18 This study followed the American Association for Public Opinion Research (AAPOR) reporting guideline for survey studies.
NHANES Data Collection
Information on participant age, sex, race and ethnicity, marital status, educational level, family income to poverty level index, and medical history were collected through questionnaires. Race and ethnicity data were collected because of a potential difference in the prevalence of HDS use. Race and ethnicity were based on self-report and were categorized as Mexican and non-Mexican Hispanic, non-Hispanic Asian (persons having origins in any of the original peoples of East Asia, Southeast Asia, or Indian subcontinent), non-Hispanic Black or African American, non-Hispanic White, and other (eg, American Indian, Alaska Native, Native Hawaiian, Pacific Islander, >1 race, or any other race). HDS and prescription drug use data were collected through personal interviews for the 30-day period prior to the survey date. The use of HDSs reported in NHANES 2017 to 2018 and 2019 to 2020 is detailed in the NHANES Dietary Supplement Database 1999-2020.19 An HDS ingredient was classified as a botanical if it is part of plant, tree, shrub, or herb. We targeted our analysis to investigate national levels of exposure to the 6 most frequently implicated causes of HDS-DILI cases in the DILIN.7,8,9,10,11,12,13 The 6 potentially hepatotoxic botanicals of interest in our study, including turmeric or curcumin, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha, were identified by their ingredient and supplement identification numbers (eTable 1 in Supplement 1). However, the daily dose of an HDS product consumed by an individual patient was not recorded or available for analysis. Furthermore, confirmation of the ingredients listed on HDS product labels via analytical chemistry methods was also not available.
Approximately 95% of adults 18 years or older provided blood samples at the mobile examination centers. The blood samples were tested at central laboratories using standard protocols to determine routine laboratory parameters (eg, complete blood count, comprehensive panel) as well as fasting glycated hemoglobin, cholesterol, and triglyceride levels.
Echosens North America Vibration-Controlled Transient Elastography was performed at the mobile examination centers by NHANES technicians. Controlled attenuation parameter was used to quantify the presence and severity of hepatic steatosis. Similarly, the liver stiffness measurement score in kilopascals was used to estimate the severity of hepatic fibrosis.20
Self-reported chronic medical conditions that were specifically captured were current or prior history of hypertension, diabetes, coronary heart disease, stroke, arthritis, chronic obstructive pulmonary disease, thyroid disorder, cancer, and liver condition. Liver conditions included viral, autoimmune, genetic liver disease, drug- or medication-induced liver disease, alcoholic liver disease, metabolic dysfunction–associated steatotic liver disease (formerly nonalcoholic fatty liver disease), liver cyst, liver abscess, and cirrhosis. Smokers were defined as individuals who smoked 100 or more cigarettes. Regular alcohol consumption was defined as mean alcohol consumption in the past 12 months of 1 or more alcoholic beverages per day for women and 2 or more alcoholic beverages per day for men.
Population Size Estimates
Data from the 2020 US Census were used to estimate the size with associated 95% CIs of the resident population 18 years of age or older.21 The prevalence of use of the 6 at risk botanicals was compared with the prevalence of widely prescribed potentially hepatotoxic medications with a LiverTox likelihood score of A or B, which included nonsteroidal anti-inflammatory drugs (NSAIDs), sertraline (antidepressant drug), and simvastatin (hypolipidemic drug).22,23 The NSAID prescriptions included ibuprofen, naproxen, meloxicam, celecoxib, indomethacin, ketorolac, piroxicam, and sulindac. The LiverTox likelihood score is a 5-point scale (A to E) that estimates whether a medication is a cause of liver injury: A indicates well-known cause, with more than 50 published cases; B, highly likely cause, with 12 to 49 published cases; C, probable cause, with 4 to 11 published cases; D, possible cause, with 1 to 3 published cases; E, unlikely cause; Eb, suspected but unproven cause; and X, unknown.23
Statistical Analysis
The complex survey design factors in the NHANES, including sample weights, clustering, and stratification, were accounted for as specified in the NHANES statistical analysis guideline.17 Baseline weighted characteristics were compared and summarized as estimated percentages or means, with a margin of error, following the AAPOR reporting guidance for survey studies.24 Categorical variables were compared using Fisher exact tests or χ2 tests if more than 2 categories existed, and continuous variables were compared using the t test. Multivariable analysis of factors that had a value of P < .10 with univariate analysis was performed to evaluate for factors associated with any HDS use as well as botanical products of interest exposure, adjusted for age group, sex, race and ethnicity, marital status, alcohol use, smoking, poverty index, and educational level. Each chronic medical condition was analyzed after adjusting for age, sex, race and ethnicity, marital status, alcohol use, smoking, income, and educational level. Odds ratios (ORs) and 95% CIs are reported. Median numbers of HDS products and prescription drugs were compared using Mann-Whitney tests. All statistical analyses were conducted from July 1, 2023, to February 1, 2024, using STATA/SE version 16.1 (StataCorp LLC). A 2-sided P < .05 was considered statistically significant.
Results
Overall HDS Use in the NHANES 2017-2020 Cohort
Among 9685 adults enrolled in this NHANES cohort, the mean (SE) age was 47.5 (0.5) years, 4971 (51.8% [95% CI, 50.2-53.4]) were female, 4714 (48.2% [95% CI, 46.6-49.8]) were male, 2121 (16.3% [95% CI, 13.5%-19.6%]) were Mexican or non-Mexican Hispanic, 1169 (5.9% [95% CI, 4.3%-8.2%]) were non-Hispanic Asian, 2552 (11.5% [95% CI, 8.8%-14.7%]) were non-Hispanic Black, 3369 (62.2% [95% CI, 57.1%-67.0%]) were non-Hispanic White, and 474 (4.1% [95% CI, 3.4%-4.8%]) were other race or ethnicity (Table 1). Overall, 5271 adults (57.6% [95% CI, 55.9%-59.4%]) reported using at least 1 HDS product within the past 30 days. HDS users were significantly older (mean [SE] age, 51.9 [0.7] vs 41.5 [0.4] years; P < .001) and more likely to be female (57.7% [95% CI, 55.2%-60.1%] vs 43.7% [95% CI, 42.5%-45.0]; P < .001), non-Hispanic White (67.6% [95% CI, 62.5%-72.4%] vs 54.8% [95% CI, 49.1%-60.3%]; P < .001), married (63.5% [95% CI, 59.7%-67.0%] vs 59.0% [95% CI, 57.0%-60.9%]; P < .001), and have a higher level of education (68.6% [95% CI, 66.0%-72.0%] vs 52.6% [95% CI, 50.9%-59.2%]; P < .001) compared with non–HDS users. HDS users were also less likely to smoke (39.6% [95% CI, 36.8%-42.4%] vs 44.1% [95% CI, 40.7%-47.5%]; P = .03) and less likely to be below the poverty line (9.5% [95% CI, 7.6%-11.8%] vs 18.3% [95% CI, 16.0%-20.7%]; P < .001), indicative of a higher socioeconomic status. Body mass index (calculated as weight in kilograms divided by height in meters squared; mean [SE], 29.7 [0.2] vs 29.9 [0.2]; P = .37) and history of alcohol use (87.2% [95% CI, 85.0%-89.0%] vs 86.3% (95% CI, 83.4%-88.8%; P = .63) were similar in the 2 groups.
Table 1. Clinical Characteristics of Adults With vs Without HDS Use in NHANES 2017-2020a.
| Characteristic | Adults, No./% (95% CI) | P value | ||
|---|---|---|---|---|
| Overall (n = 9685) | With HDS use (n = 5271) | Without HDS use (n = 4414) | ||
| Age, mean (SE), y | 47.5 (0.5) | 51.9 (0.7) | 41.5 (0.4) | <.001 |
| Sex | ||||
| Female | 4971/51.8 (50.2–53.4) | 3026/57.7 (55.2-60.1) | 1945/43.7 (42.5-45.0 | <.001 |
| Male | 4714/48.2 (46.6-49.8) | 2245/42.3 (39.9-44.8) | 2469/56.3 (55.0-57.5) | |
| Race and ethnicity | <.001 | |||
| Mexican or Other non-Mexican Hispanic | 2121/16.3 (13.5-19.6) | 1013/13.2 (10.6-16.3) | 1108/20.5 (16.8-24.8) | |
| Non-Hispanic Asian | 1169/5.9 (4.3-8.2) | 642/5.7 (4.0-8.0) | 527/6.3 (4.6-8.7) | |
| Non-Hispanic Black | 2552/11.5 (8.8-14.7) | 1258/9.5 (7.2-12.5) | 1294/14.1 (11-18) | |
| Non-Hispanic White | 3369/62.2 (57.1-67.0) | 2098/67.6 (62.5-72.4) | 1271/54.8 (49.1-60.3) | |
| Otherb | 474/4.1 (3.4-4.8) | 260/3.9 (3.1-5) | 214/4.3 (3.5-5.2) | |
| Married | 5275/61.6 (59-64.1) | 3034/63.5 (59.7-67.0) | 2241/59.0 (57.0-60.9) | <.001 |
| BMI, mean (SE) | 29.8 (0.2) | 29.7 (0.2) | 29.9 (0.2) | .37 |
| No. of HDS products used, median (IQR) | 3 (1-5) | 1 (1-3) | NA | NA |
| ≥1 Prescribed drug used | 5663/58.3 (55.8-60.7) | 3725/70.1 (67.7-72.4) | 1938/42.2 (39.5-44.9) | <.001 |
| No. of prescribed drugs used, median (IQR) | 3 (1-5) | 3 (1-6) | 2 (1-4) | .27c |
| Smoked >100 cigarettes | 3886/41.5 (39.1-43.8) | 2070/39.6 (36.8-42.4) | 1816/44.1 (40.7-47.5) | .03 |
| Regular alcohol used | 4983/86.8 (85-88.3) | 2664/87.2 (85.0-89.0) | 2319/86.3 (83.4-88.8) | .63 |
| Income: poverty ratio <1, No. (%) | 1640/13.1 (11.4-15.1) | 667/9.5 (7.6-11.8) | 973/18.3 (16.0-20.7) | <.001 |
| Some college or higher | 5228/61.9 (59.8-66.4) | 3269/68.6 (66.0-72.0) | 1959/52.6 (50.9-59.2) | <.001 |
| Chronic medical disorder | ||||
| Hypertension | 3575/31.7 (29.4-34.1) | 2300/37.1 (34.4-40) | 1275/24.4 (21.9-26.9) | <.001 |
| Diabetes | 1687/13.7 (13-14.6) | 1109/16.8 (15.7-18) | 578/9.6 (8.4-10.9) | <.001 |
| Coronary heart disease | 421/4.2 (3.2-5.5) | 294/5.1 (3.8-6.7) | 127/3.03 (2.2-4.2) | .001 |
| Stroke | 486/3.8 (3.3-4.4) | 312/4.5 (3.7-5.4) | 174/2.8 (2.2-3.7) | .006 |
| Arthritis | 2812/27.9 (25.3-30.6) | 1930/33.8 (30.4-37.5) | 882/19.5 (17.7-21.4) | <.001 |
| COPD | 846/8.6 (7.6-9.8) | 519/9 (7.6-10.7) | 327/8.1 (6.7-9.8) | .43 |
| Thyroid disorder | 1079/12.3 (11.4-13.2) | 785/16.2 (15-17.5) | 294/6.8 (5.6-8.1) | <.001 |
| Cancer | 1004/11.6 (10.6-12.7) | 732/15 (13.7-16.4) | 272/6.8 (5.6-8.3) | <.001 |
| Liver disorder | 462/4.6 (3.9-5.3) | 297/5.3 (4.5-6.4) | 165/3.5 (2.8-4.4) | .002 |
| Glycated hemoglobin, mean (SE), % | 5.6 (0.0) | 5.7 (0.0) | 5.6 (0.0) | .007 |
| Total cholesterol, mean (SE), mg/dL | 186.9 (1.2) | 188.6 (1.4) | 184.4 (1.4) | .009 |
| Triglyceride, mean (SE), mg/dL | 138.9 (2.5) | 135.9 (2.6) | 143 (2.9) | .003 |
| ALT, mean (SE), U/L | 22.6 (0.3) | 22.1 (0.3) | 23.4 (0.5) | .015 |
| AST, mean (SE), U/L | 21.8 (0.2) | 21.8 (0.3) | 21.8 (0.2) | .98 |
| ALP, mean (SE), U/L | 75.7 (0.5) | 74.5 (0.6) | 77.4 (0.6) | .001 |
| CAP score, mean (SE), dB/m | 263.3 (1.4) | 262.9 (1.8) | 263.9 (1.5) | .54 |
| LSM, mean (SE), kPa | 5.9 (0.12) | 5.8 (0.15) | 5.9 (0.11) | .37 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAP, controlled attenuation parameter measured in decibels per meter; COPD, chronic obstructive pulmonary disorder; HDS, herbal dietary supplement; LSM, liver stiffness measurement, in kilopascal; NA, not applicable; NHANES, National Health and Nutrition Examination Survey.
SI conversion factor: To convert HbA1c percentage to proportion of total, multiply by 0.01; total cholesterol level to millimoles per liter, by 0.0259; triglyceride level to millimoles per liter, by 0.0113; ALT, AST, and ALP levels to microkatals per liter, by 0.0167.
Data are weighted characteristics and are reported as means with SEs for continuous variables and percentages with 95% CIs for categorical variables.
Other race and ethnicity included American Indian, Alaska Native, Native Hawaiian, Pacific Islander, more than 1 race, or any other race.
Mann-Whitney test.
Average alcohol consumption in the past 12 months 1 or more drinks per day for women and 2 or more drinks per day for men.
Consistent with their increased age, HDS users were significantly more likely to have hypertension, diabetes, coronary heart disease, stroke, arthritis, thyroid disorder, cancer, and liver conditions compared with non–HDS users. The median (range) number of HDS products used within 30 days was 1 (1-22). HDS users were also significantly more likely than non–HDS users to be taking a concomitant prescription medication (70.1% [95% CI, 67.7%-72.4%] vs 42.2% [95% CI, 39.5%-44.9%]; P < .001).
Consistent with their higher prevalence of diabetes, HDS users had significantly higher hemoglobin A1C and total cholesterol levels, but they had lower triglyceride levels. Furthermore, HDS users tended to have lower serum alanine aminotransferase and alkaline phosphatase levels compared with non–HDS users (Table 1). However, there was no significant difference for controlled attenuation parameter or liver stiffness measurement scores between the 2 groups.
Use of 6 Potentially Hepatotoxic Botanicals in NHANES 2017 to 2020
In total, 731 of 9685 US adults assessed (7.5%) used a botanical-containing HDS product within the last 30 days, and 350 participants (4.7% [95% CI, 3.9%-5.7%]) used at least 1 of the 6 botanical products of interest within the past 30 days (eFigure in Supplement 1). The most commonly used potentially hepatotoxic botanical products were turmeric or curcumin (n = 236) and green tea (n = 92), followed by ashwagandha (n = 28), Garcinia cambogia (n = 20), red yeast rice (n = 20), and black cohosh (n = 19). The number of unique products was 118 for turmeric, 26 for ashwagandha, 66 for green tea, 13 for Garcinia cambogia, 11 for black cohosh, 10 for red yeast rice, and 275 for other botanicals (eTable 2 in Supplement 1). Among 350 patients, 291 had exposure to only 1 of the 6 botanicals, 51 had exposure to 2 botanicals, and 8 had exposure to 3 or more.
Characteristics of the 6 potentially hepatotoxic botanical users (n = 350) were compared with those with no HDS use (n = 4414) (Table 2). At-risk botanical users were significantly older (mean [SE] age, 51.7 [2.0] vs 41.5 [0.4] years; P < .001) and more likely to be female (56.9% [95% CI, 47.7%-65.5%] vs 43.7% [95% CI, 42.5%-45.0%]; P = .005), non-Hispanic White (75.2% [95% CI, 65.4%-82.9%] vs 54.8% [95% CI, 49.1%-60.3%]; P < .001), married (66% [95% CI, 58.5%-72.7%] vs 59.0% [95% CI, 57.0%-60.9%]; P = .001), and have some college degree or higher (82.8% [95% CI, 76.7%-87.5%] vs 52.6% [95% CI, 50.9%-59.2%]; P < .001), and were less likely to be below the poverty line (5.1% [95% CI, 3.0%-8.8%] vs 18.3% [95% CI, 16.0%-20.7%]; P < .001). Among at-risk botanical users, the median number of HDS products used was 4 (range, 2-7) and was highest among those who consumed red yeast rice and ashwagandha (median [IQR], 7 [4-11]). Individuals who used at least 1 the 6 botanicals of interest were also more likely to be taking a prescription medication compared with non–HDS users (66.0% [95% CI, 58.9%-71.8%] vs 42.0% [95% CI, 39.5%-44.9%]; P < .001). Furthermore, the botanical users were more likely to have arthritis (40.0% [95% CI, 32.4%-48.1%] vs 19.5% [95% CI, 17.7%-21.4%]; P < .001), thyroid disorder (15.8% [95% CI, 11.0%-22.1%] vs 6.8% [95% CI, 5.6%-8.1%]; P = .004), and cancer (14.0% [95% CI, 9.7%-19.6%] vs 6.8% [95% CI, 5.6%-8.3%]; P = .006) compared with non–HDS users.
Table 2. Clinical Characteristics of Adults With vs Without Use of 6 Indexed Botanical Productsa.
| Characteristic | Adults, No./% (95% CI) | P value for 6-indexed HDS vs no HDS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Turmeric (n = 236) | Green tea (n = 92) | Garcinia cambogia (n = 20) | Black cohosh (n = 19) | Red yeast rice (n = 20) | Ashwagandha (n = 28) | 6-Indexed HDS (n = 350) | No HDS (n = 4414) | ||
| Age, mean (SE), y | 52.2 (2.8)b | 53.2 (2.2)b | 44.3 (2.4) | 53.2 (4.2)b | 67.2 (2.2)b | 51.7 (4.2)b | 51.7 (2.0)b | 41.5 (0.4) | <.001 |
| Sex | |||||||||
| Female | 132/51.2 (41.6-60.7) | 54/68.3 (54.5-79.5)b | 16/70 (36.6-90.4) | 18/87.5 (42.9-98.5)b | 12/66.8 (43.2-84.2) | 14/61.9 (33.9-83.7) | 202/56.9 (47.7-65.5)b | 1945/43.7 (42.5-45.0) | .005 |
| Male | 104/48.8 (39.3-58.4) | 37/31.7 (20.5-45.5) | 4/29.9 (9.6-63.4) | 1/12.5 (1.5-57.1) | 8/33.2 (15.8-56.8) | 14/38.1 (16.3-66.1) | 145/43.1 (34.5-52.3) | 2473/56.3 (55.0-57.5) | |
| Race and ethnicity | <.001 | ||||||||
| Mexican or other non-Mexican Hispanic | 41/9.6 (5.6-16) | 19/14.8 (7.5-27.2) | 6/25.3 (7.9-57.3) | 2/3 (0.6-14.4) | 4/14.5 (4.6-37) | 4/7 (2-21.9) | 65/11.2 (6.7-18.1) | 1108/20.5 (16.8-24.8) | |
| Non-Hispanic Asian | 16/2 (1.2-3.4) | 9/3.9 (1.2-12.2) | 0 | 1/0.93 (0.1-6.4) | 1/4.3 (0.4-32.4) | 2/3.6 (0.7-16.2) | 26/2.5 (1.6-4) | 527/6.3 (4.6-8.7) | |
| Non-Hispanic Black | 44/5.7 (3.5-9.2) | 25/10.5 (5.9-17.8) | 5/11.3 (18.9-45.6) | 3/4.4 (1.3-13.8) | 4/6.3 (2.1-17.5) | 3/2.4 (0.6-8.3) | 67/6 (3.8-9) | 1294/14.1 (11-18) | |
| Non-Hispanic White | 119/77.8 (68.2-85.1)b | 36/69.8 (60-80.1) | 6/40.7 (14-74) | 12/90.8 (82.4-95.4)b | 11/74.9 (48.8-90.3) | 17/84.4 (67.4-93.4)b | 168/75.2 (65.4-82.9)b | 1271/54.8 (49.1-60.3) | |
| Otherc | 16/5 (2.1-11.1) | 2/1.1 (0.2-5.7) | 3/22.8 (6-57.5) | 1/0.91 (0.1-7.3) | 0 | 2/2.6 (1.5-4.5) | 21/5.1 (2.5-10.4) | 214/4.3 (3.5-5.2) | |
| Married | 145/68.7 (60-76.3)b | 56/65.1 (46.6-80) | 10/53.4 (29.4-76) | 8/53.3 (25.4-79.4) | 13/67 (37.9-87.1) | 16/71.9 (47.7-87.8) | 208/66 (58.5-72.7)b | 2241/59 (57-60.9) | .001 |
| BMI, mean (SD) | 28.9 (0.7) | 29 (1.0) | 36.2 (2.5b) | 27.1 (2.2) | 27 (0.7b) | 26.5 (1.9) | 28.9 (0.6) | 29.9 (0.22) | .12 |
| No. of HDS products used, median (IQR) | 4 (2-7) | 4 (2-6) | 2 (2-5) | 4 (2-7) | 7 (5-12) | 7 (4-11) | 4 (2-7) | NA | NA |
| ≥1 Prescribed drug used | 165/66 (57.2-74.2) | 65/76 (61.2-86.8) | 11/48 (23.7-73.6) | 15/69 (37.3-89.5) | 15/86 (62.8-95.4) | 15/73 (49-88.5) | 234/66.0(58.9-71.8) | 1938/42.0 (39.5-44.9) | <.001 |
| No. of prescribed drugs used, median (IQR) | 3 (1-5) | 2 (1-5) | 2 (1-5) | 3 (2-7) | 4 (1-5) | 2 (1-5) | 3 (1-5) | 2 (1-4) | <.001d |
| Smoked >100 cigarettes | 84/36.3 (27.6-46) | 29/32.9 (23.3-44.2) | 10/57.9 (29.9-81.6) | 9/45.2 (19.9-73.3) | 6/30.1 (13.5-54.4) | 12/39.5 (20.4-64.5) | 123/36.4 (28.6-45.2) | 1819/44.1 (40.7-47.5) | .23 |
| Alcohol usee | 144/91.5 (83.5-95.8) | 58/89.5 (71.3-96.7) | 14/83.4 (36.6-97.8) | 11/83.6 (33.3-98.1) | 8/96.4 (72.1-99.6) | 13/91.4 66.3-98.3) | 210/91.4 (85.7-95) | 2319/86.3 (83.4-88.8) | .06 |
| Income: poverty ratio <1 | 12/4.1 (2-8.3)b | 6/2.5(1-6.5)b | 4/17.6 (6.8-38.6) | 3/6.2 (1.1-28.7) | 0b | 3/6.8 (3.2-13.4)b | 25/5.1 (3.0-8.8)b | 973/18.3 (16.0-20.7) | <.001 |
| Some college or higher | 187/85.8 (78.4-91)b | 70/85.6 (73.3-92.8)b | 14/62.9 (31.7-86.1) | 15/89.5 (67.5-97.2)b | 16/73.5 (41.9-91.5) | 24/91.4 (66.5-98.3)b | 265/82.8 (76.7-87.5)b | 1959/52.6 (50.9-59.2) | <.001 |
| Chronic medical condition | |||||||||
| Hypertension | 96/29.2 (22-37.6) | 33/32.2 (20.3-47) | 8/54.6 (24.9-81.4) | 5/14.4 (4-40.6) | 12/69.5 (40.8-88.3)b | 9/32.4 (14.5-57.4) | 132/30 (23.8-37.1) | 1275/24.4 (21.9-26.9) | .06 |
| Diabetes | 42/12.1 (7.1-19.8) | 20/8.3 (4.7-14.2) | 5/34.2 (13.7-63.1)b | 4/6.3 (1.7-20.9) | 6/16.8 (5.8-40) | 3/3.5 (1.9-6.2)b | 67/12.7 (8.6-18.3) | 578/9.6 (8.4-10.9) | .23 |
| Coronary heart disease | 8/1.9 (0.6-6.3) | 3/1.6 (0.6-4.1) | 0b | 0b | 0b | 0b | 10/1.6 (0.5-4.5) | 127,3.03 (2.2-4.2) | .15 |
| Stroke | 9/3.7 (1.5-8.9) | 3/1.7 (0.4-7.1) | 0b | 1/3.6 (0.3-29.1) | 0b | 2/3.5 (0.4-25.3) | 14/3.5 (1.7-6.7) | 174/2.8 (2.2-3.7) | .61 |
| Arthritis | 106/40.9 (31.7-50.7)b | 29/33.4 (21.3-48.3)b | 9/47.1 (23.7-71.8)b | 9/45.8 (20.2-73.8)b | 10/62 (38.7-80.8)b | 8/27.6 (10-56.6) | 146/40.0 (32.4-48.1)b | 882/19.5 (17.7-21.4) | <.001 |
| COPD | 18/4.7 (2.1-10.2) | 4/1.8 (0.5-6.4)b | 1/0.67 (0.1-5.3)b | 1/2.9 (3.7-19.8) | 2/6 (0.9-32.3) | 1/0.4 (0.1-3)b | 22/4 (2-7.8)b | 327/8.1 (6.7-9.8) | .02 |
| Thyroid disorder | 34/15.1 (9.6-23)b | 11/20.1 (10.4-35.3)b | 2/7.8 (1.5-32.8) | 7/34.8 (13.2-65.3)b | 6/30 (13.4-53.5)b | 5/28 (9.8-58.1) | 53/15.8 (11.0-22.1)b | 294/6.8 (5.6-8.1) | .004 |
| Cancer | 30/13.6 (8.4-21.1)b | 9/16.2 (7.1-32.9)b | 2/13.6 (3.4-41.3)b | 1/5.8 (0.7-34.6) | 3/6 (1.7-18.8) | 4/14.5 (3.7-42.7)b | 44/14.0 (9.7-19.6)b | 272/6.8 (5.6-8.3) | .006 |
| Liver disorder | 11/2.7 (1.3-5.7) | 2/4.2 (0.6-23.6) | 0 | 1/10.4 (1.3-49.8) | 0 | 1/1.6 (0.2-11.9) | 12/2.8 (1.3-6) | 165/3.5 (2.8-4.4) | .51 |
| Glycated hemoglobin, mean (SE), % | 5.6 (0.1) | 5.8 (0.1) | 6.3 (0.5) | 5.8 (0.2) | 5.8 (0.2) | 5.4 (0.1)b | 5.6 (0.1) | 5.6 (0.0) | .89 |
| Total cholesterol, mean (SE), mg/dL | 188.1 (5.3) | 205 (4.9)b | 179.4 (9.5) | 197.8 (9.7) | 217.7 (7.9)b | 196.1 (4.8)b | 190.7 (4.2) | 184.4 (1.37) | .13 |
| Triglyceride, mean (SE), mg/dL | 122 (6.5)b | 126.9 (10.5) | 186.9 (49) | 130.7 (12.6) | 111.5 (12.8b | 104.3 (17.7)b | 126.8 (5.7)b | 143 (2.9) | .001 |
| ALT, mean (SE), U/L | 22.7 (0.8 | 21.3 (0.9) | 25.7 (2.2 | 20.5 (2) | 17.3 (1.5)b | 25.2 (3) | 22.3 (0.7) | 23.4 (0.5) | .20 |
| AST, mean (SE), U/L | 22 (0.6) | 21.6 (0.8) | 23.1 (1.6) | 21 (1.8) | 19 (0.6)b | 23.3 (1.8) | 21.9 (0.5) | 21.8 (0.2) | .95 |
| ALP, mean (SE), U/L | 71.8 (1.3)b | 75 (3.6) | 65.5 (5.6) | 69.1 (6) | 66.8 (3.6)b | 71 (4.3) | 70.7 (1.2)b | 77.4 (0.6) | <.001 |
| CAP score, mean (SE), dB/m | 255.3 (7.8) | 257.6 (9.6) | 283 (15.5) | 235 (10.7)b | 249.9 (11.8) | 262 (12.9) | 254.6 (5.9) | 263.9 (1.5) | .12 |
| LSM, mean (SE), kPa | 5.2 (0.15) | 4.9 (0.26)b | 10.5 (3.8) | 5.2 (0.7) | 4.7 (0.2)b | 5 (0.4)b | 5.5 (0.25) | 5.9 (0.11) | .17 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAP, controlled attenuation parameter measured in decibels per meter; COPD, chronic obstructive pulmonary disease; HDS, herbal dietary supplement; LSM, liver stiffness measurement, in kilopascal; NA, not applicable.
SI conversion factor: To convert HbA1c percentage to proportion of total, multiply by 0.01; total cholesterol level to millimoles per liter, by 0.0259; triglyceride level to millimoles per liter, by 0.0113; ALT, AST, and ALP levels to microkatals per liter, by 0.0167.
Data are weighted characteristics and are reported as means with SEs for continuous variables and percentages with 95% CIs for categorical variables.
P value significant compared with no HDS.
Other race and ethnicity included American Indian, Alaska Native, Native Hawaiian, Pacific Islander, more than 1 race, or any other race.
Mann-Whitney test.
Mean alcohol consumption in the past 12 months 1 or more drinks per day for women and 2 or more drinks per day for men.
Laboratory parameters were generally similar, but at-risk botanical users had significantly lower triglyceride and alkaline phosphatase levels (Table 2). However, there was no significant difference for glycated hemoglobin, serum aminotransferase levels or liver elastography parameters.
Multivariable Regression Analysis
Independent factors associated with HDS use included older age (adjusted OR [AOR], 1.77 [95% CI, 1.36-2.32]; P < .001 for 40-59 years; AOR, 3.97 [95% CI, 2.99-5.28]; P < .001 for ≥60 years,), female sex (AOR, 1.76 [95% CI, 1.42-2.18]; P < .001), non-Hispanic White race and ethnicity (AOR, 1.25 [95% CI, 1.04-1.49]; P = .02), poverty ratio higher than 1 (AOR, 1.41 [95% CI, 1.11-1.77]; P = .006), and some college education (AOR, 2.02 [95% CI, 1.61-2.52]; P < .001) (Table 3). In addition, the presence of hypertension (AOR, 1.37 [95% CI, 1.11-1.70]; P < .001), diabetes (AOR, 1.55 [95% CI, 1.21-1.97]; P < .001), arthritis (AOR, 1.31 [95% CI, 1.13-1.52]; P = .001) and thyroid disorder (AOR, 1.60 [95% CI, 1.13-2.25]; P = .01) remained significantly associated with HDS use after adjusting for age, sex, race and ethnicity, marital status, smoking, income, and education level. Features associated with at-risk botanical users included older age (AOR, 2.36 [95% CI, 1.06-5.25]; P = .04 for 40-59 years of age; AOR, 3.96 [95% CI, 1.93-8.11]; P = .001 for age ≥60 years), some college education (AOR, 4.78 [95% CI, 2.62-8.75]; P < .001), and the presence of arthritis (AOR, 2.27 [95% CI, 1.62-3.29]; P < .001) after adjusting for other covariates (Table 3).
Table 3. Multivariable Analysis for Factors Associated With HDS Use or With 6 Indexed Botanical Products vs No HDS Usea.
| Variable | HDS vs no HDS, AOR (95% CI) | P value | 6 Indexed HDS vs no HDS, AOR (95% CI) | P value |
|---|---|---|---|---|
| Age, y | ||||
| 40-59 | 1.77 (1.36-2.32) | <.001 | 2.36 (1.06-5.25) | .04 |
| ≥60 | 3.97 (2.99-5.28) | <.001 | 3.96 (1.93-8.11) | .001 |
| Female | 1.76 (1.42-2.18) | <.001 | 1.61 (0.99-2.61) | .06 |
| Non-Hispanic White | 1.25 (1.04-1.49) | .02 | 1.38 (0.80-2.35) | .23 |
| Married | 0.93 (0.77-1.12) | .45 | 0.94 (0.63-1.41) | .77 |
| Alcohol useb | 0.99 (0.72-1.38) | .98 | 1.82 (0.90-3.68) | .09 |
| Smokerc | 1.17 (0.94-1.47) | .16 | 0.94 (0.51-1.72) | .83 |
| Poverty index ratio >1 | 1.41 (1.11-1.77) | .006 | 2.15 (0.90-5.16) | .08 |
| Some college or above | 2.02 (1.61-2.52) | <.001 | 4.78 (2.62-8.75) | <.001 |
| Hypertension | 1.37 (1.11-1.70) | <.001 | 1.18 (0.73-1.91) | .50 |
| Diabetes | 1.55 (1.21-1.97) | .001 | 1.43 (0.81-2.52) | .20 |
| Arthritis | 1.31 (1.13-1.52) | .001 | 2.27 (1.62-3.29) | <.001 |
| Coronary heart disease | 1.55 (0.91-2.66) | .10 | 0.45 (0.09-2.34) | .33 |
| Stroke | 1.22 (0.83-1.78) | .30 | 0.97 (0.32-2.97) | .96 |
| COPD | 0.87 (0.59-1.29) | .48 | 0.51 (0.20-1.30) | .15 |
| Thyroid disorder | 1.60 (1.13-2.25) | .01 | 1.32 (0.84-2.06) | .21 |
| Cancer | 1.18 (0.80-1.74) | .39 | 1.10 (0.55-2.20) | .79 |
| Liver condition | 1.58 (0.76-3.27) | .21 | 0.65 (0.22-1.90) | .42 |
Abbreviations: AOR, adjusted odds ratio; COPD, chronic obstructive pulmonary disorder; HDS, herbal and dietary supplements.
Medical condition was adjusted for age, sex, race and ethnicity, marital status, income, educational level, and smoking for HDS vs no HDS, and adjusted for age, sex, race and ethnicity, marital status, income, and educational level for 6 indexed botanical products vs no HDS.
Mean alcohol consumption in the past 12 months 1 or more drinks per day for women and 2 or more drinks per day for men.
Smoked more than 100 cigarettes.
Stated Reasons for Using the 6 Potentially Hepatotoxic Botanicals
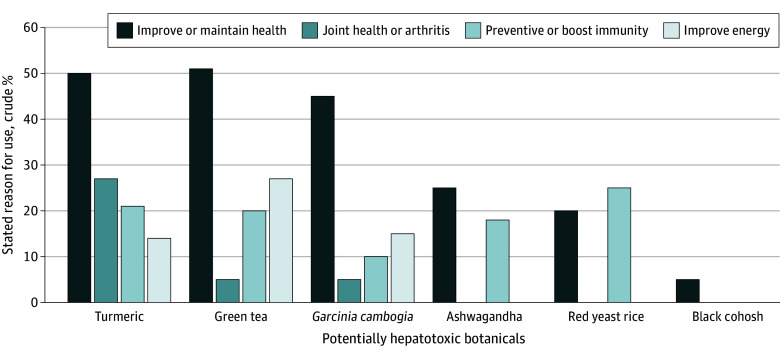
The vast majority of at-risk botanical users were doing so of their own accord, and use of these products was not recommended by their health care providers (87.6% [95% CI, 82.8%-91.9%] for turmeric; 80.4% [95% CI, 63.8%-92.8%] for green tea; 100% for Garcinia cambogia; 63.2% [ 95% CI, 23.5%-82.5%] for black cohosh; 40% [95% CI, 39.9%-92.2%] for red yeast rice; and 96.4% [95% CI, 75.8%-99.7%] for ashwagandha). The most common reasons for using the botanical were to improve or maintain health or to prevent health problems or boost immunity (Figure 1). In addition, 64 turmeric users (26.8%) consumed those products for joint health or arthritis, and 25 green tea users (27.2%) were trying to improve their energy level. In total, 14 Garcinia cambogia users (70.0%) were trying to lose weight and had the highest median body mass index and proportion with diabetes (34.2% [95% CI, 13.7%-63.1%]) (Table 2). Similarly, 84.2% of black cohosh users were taking these products to treat hot flashes, and the vast majority of these patients were women (87.5% [95% CI, 42.9%-98.5%]). The main stated reason to consume red yeast rice was for heart health (90.0%), and these individuals tended to be older and had the second highest incidence of diabetes.
Figure 1. Self-Reported Reasons for Consuming 6 Potentially Hepatotoxic Botanical Products.
US Population Estimates of Exposure to Botanicals of Interest
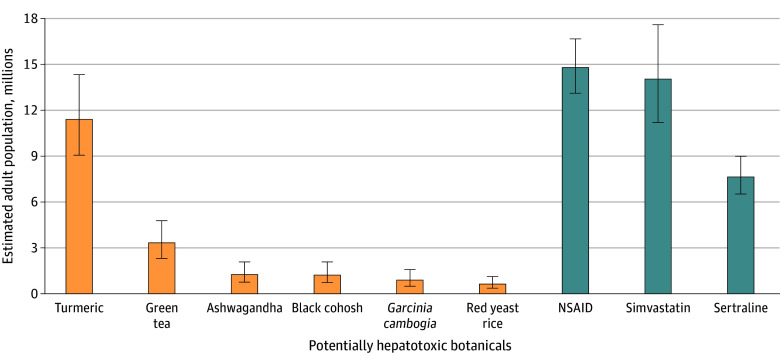
Extrapolating from the NHANES data, we observed approximately 4.7% (95% CI, 4.0%-5.7%), or an estimated 15 584 599 (95% CI, 13 047 571-18 648 801), of US adults used at least 1 of the 6 potentially hepatotoxic botanical products within the past 30 days. The most common products used were turmeric or curcumin, estimated at 11 400 151 (95% CI, 906 813-14 332 559) adults, and green tea, estimated at 3 327 790 (95% CI, 2 306 389-4 777 520) adults. An estimated 1 252 040 (95% CI, 757 813-2 075 750) adults used ashwagandha, 1 219 091 (95% CI, 724 865-32 075 750) adults used black cohosh, 889 607 (95% CI, 494 226-1 581 524) adults used Garcinia cambogia, and 626 020 (95% CI, 362 433-1 120 246) adults used red yeast rice.
The prevalence of using the 6 potentially hepatotoxic botanical products was compared with the prevalence of the use of known hepatotoxic prescription medications (LiverTox class A or B) that are used for similar indications. Approximately 14 793 837 (95% CI, 13 014 623-16 671 897) US adults used prescription nonsteroidal anti-inflammatory drugs, which are typically used for indications similar to those for turmeric (ie, pain or arthritis). Simvastatin, a hypolipidemic drug used to treat and prevent cardiovascular disease similar to the use for red yeast rice, was consumed by 14 036 024 (95% CI, 11 202 460-17 594 452) individuals. The prevalence of sertraline use was 7 676 980 (95% CI, 6 523 786-8 994 917) individuals. The comparison of the botanical products of interest with commonly prescribed prescription medications is shown in Figure 2 (based on the 2020 US Census estimated total resident population 18 years of age or older of 329 484 123, July 1, 202021).
Figure 2. United States Population Estimates for Use of 6 Potentially Hepatotoxic Botanical Products Compared With 3 Commonly Prescribed Medications.
Data are based on 2020 US Census estimated total resident population 18 years of age or older of 329 484 123 July 1, 2020.21 Whiskers represent 95% CI. NSAIDs indicates nonsteroidal anti-inflammatory drugs.
Discussion
This survey study assessed the prevalence and clinical characteristics of consumers of the 6 most frequently reported hepatoxic botanicals, including turmeric or curcumin, green tea extract, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha, in a representative sample of US adults. We estimated that at least 15.6 million US adults used at least 1 of 6 potentially hepatotoxic botanical products within the past 30 days, which was similar to estimated number of US adults prescribed an NSAID or simvastatin.
The Dietary Supplement Health and Education Act (DSHEA) of 1994 defined an HDS as a product that contains a “dietary ingredient,” such as vitamins, minerals, herbs or botanicals, amino acids, and dietary substances that are intended to supplement the diet.25 HDS use has dramatically increased over time in the United States. from 32.9% in the NHANES I cohort (1971-1974) to 52% in the NHANES 2011-2012 cohort and 57.6% in the NHANES 2017-2018 cohort.2,26 The economic impact of the HDS products industry in the US is profound, with over $150 billion in marketplace sales in 2023, and rivals that of all prescription drugs combined.27 The most common HDSs used are multivitamins or minerals, calcium, fish oil, botanical supplements, and vitamin C.28,29 In the US, a variety of adverse events related to HDS use have been described, with an estimated 23 000 annual emergency department visits and 2154 hospitalizations in 2014.30 The incidence of HDS-DILI is also increasing over time and accounts for over 20% of cases of liver injury recorded in the DILIN prospective registry.7 The HDS-DILI can be not only severe, leading to hepatocellular injury with jaundice, but also fatal, leading to death or liver transplantation.31,32,33,34,35,36,37 Kesar et al35 reported the number of liver transplants due to HDS-DILI in 2010 through 2020 increased over 70% when compared with 1994 through 2009.
The prevalence of the use of potentially hepatotoxic botanical products rather than use of benign, non-hepatotoxic HDSs, such as vitamins and minerals, has not been systematically studied nor reported. The present study found that between January 2017 and March 2020, approximately 15 million US adults consumed at least 1 potentially hepatotoxic botanical product within the past 30 days, which was comparable to the number of people taking potentially hepatotoxic prescription drugs, such as simvastatin, NSAIDs, and sertraline (Figure 2).
The clinical characteristics of the users of the 6 botanical products of interest were similar to overall HDS users, with older age, more women and non-Hispanic White individuals, higher income, and higher level of education among HDS users compared with non–HDS users.38,39,40 Use of HDSs has also been shown to be more prevalent among individuals with chronic medical conditions, including cardiovascular disorders, cancer, and obesity.26,40,41,42,43,44,45,46,47 In our study, we found diabetes, arthritis, and thyroid disorder independently associated with HDS use, but not cardiovascular disorder or cancer. Arthritis was independently associated with the use of the 6 potentially hepatotoxic botanical products and with overall HDS use (Table 3).
The reasons for botanical use varied substantially by the specific products as well as the age, gender, and demographic features of the individual product users (Table 2 and Figure 1). For example, turmeric-containing products were most commonly used for joint health or arthritis due to the widespread belief that turmeric may have antioxidant and anti-inflammatory properties as touted in ayurvedic medicine.48 However, multiple randomized clinical trials have failed to demonstrate any efficacy of turmeric-containing products in osteoarthritis.48,49 Green tea–containing products were mostly used as energy supplements. However, multiple studies have failed to demonstrate any objective evidence of weight loss and sustained improvement in mood or energy levels with products that contain high levels of catechins or polyphenols found in green tea extract.50,51,52 Garcinia cambogia was commonly used for weight loss (70%), as it has been touted that hydroxycitric acid promotes weight loss.53 Black cohosh was used for hot flashes, and ashwagandha was used as muscle builder.
In the United States, HDSs are regulated under the general umbrella of foods and are not intended to be taken for disease treatment or prevention.54,55 Assuming that HDS products are generally safe similar to foods, the FDA does not require manufacturers to verify the ingredients in a given product or lot. But recent studies by DILIN have shown substantial discrepancies between product labels and the results of mass spectroscopy of the actual products.3 In addition, human bioavailability and safety studies are not required prior to the marketing of an HDS product unless the formulation contains a novel chemical entity that was not known prior to 1994. The active ingredients and components in botanical products are even more challenging to standardize due to the impact of changes in soil, local environment, and batch to batch variation in plant or cultivar production. The majority of the at-risk botanical users in this study consumed these products without clinician recommendations presumably due to the touted benefits of the products being marketed. The number of HDS products marketed in the US increased from 4000 in 1993 to 55 000 in 2012, and approximately 80 000 products were available by 2022.1,56
Limitations
Our study has several important limitations. First, the survey response rate for the January 2017 to March 2020 prepandemic cohort was low, at 43.9% for adults aged 20 years or older. Since NHANES is a cross-sectional study, there was no opportunity to determine associations with clinical outcomes, such as episodes of idiosyncratic hepatotoxic effects. In addition, this survey sample size was not adequate to detect hepatotoxic effects from botanicals or other adverse events since these arise in less than 1% of exposed individuals. Thus, our study was not designed to identify any causal relationship between consumption of the 6 botanicals of interest and the development of liver injury over time. Lastly, use of HDS products and medications was obtained by self-report in NHANES and not independently verified by source documents. The ingredients data used in this study may be limited in accuracy due to poor governmental regulation and confirmation of the ingredients listed on the product label, given that previous analysis has shown discrepancies between product labels and detected ingredients.3 However, NHANES is the largest available nationally representative database with detailed information regarding dietary supplement product usage in the United States.
Conclusions
This survey study found that in the NHANES 2017 to March 2020 study, over 7% of US adults used a botanical-containing HDS product within the last 30 days and that the 6 products most commonly implicated in liver injury in the US are popular among US adults and used as frequently as common hypolipidemic drugs, NSAIDs and antidepressants. In light of the lack of regulatory oversight on the manufacturing and testing of botanical products, it is recommended that clinicians obtain a full medication and HDS use history when evaluating patients with unexplained symptoms or liver test abnormalities. Considering widespread and growing popularity of botanical products, we urge government authorities to consider increasing the regulatory oversight on how botanicals are produced, marketed, tested, and monitored in the general population.
eTable 1. Ingredient Identification Number and Supplement Identification Number Identified the 6 Potential Hepatotoxic Botanical Products
eTable 2. Botanical Products by Name (Alphabetical)
eFigure. Herbal and Dietary Supplement Use Among U.S. Adults Enrolled in NHANES 2017-2020
Data Sharing Statement
References
- 1.American Medical Association . Dietary supplements: underregulated, unknown, and maybe unsafe. Accessed January 19, 2024. https://www.ama-assn.org/delivering-care/public-health/dietary-supplements-underregulated-unknown-and-maybe-unsafe
- 2.Mishra S, Stierman B, Gahche JJ, Potischman N. Dietary Supplement Use Among Adults: United States, 2017–2018. NCHS Data Brief, No. 399. National Center for Health Statistics; 2021. [PubMed] [Google Scholar]
- 3.Navarro V, Avula B, Khan I, et al. The contents of herbal and dietary supplements implicated in liver injury in the United States are frequently mislabeled. Hepatol Commun. 2019;3(6):792-794. doi: 10.1002/hep4.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avigan MI, Mozersky RP, Seeff LB. Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci. 2016;17(3):331. doi: 10.3390/ijms17030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana RJ, Watkins PB, Bonkovsky HL, et al. ; DILIN Study Group . Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55-68. doi: 10.2165/00002018-200932010-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363-373. doi: 10.1002/hep.28813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierard S, Coche JC, Lanthier P, et al. Severe hepatitis associated with the use of black cohosh: a report of two cases and an advice for caution. Eur J Gastroenterol Hepatol. 2009;21(8):941-945. doi: 10.1097/MEG.0b013e3283155451 [DOI] [PubMed] [Google Scholar]
- 8.Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. 2013;58(9):2682-2690. doi: 10.1007/s10620-013-2687-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng EX, Rossi S, Fontana RJ, et al. Risk of liver injury associated with green tea extract in SLIMQUICK weight loss products: results from the DILIN prospective study. Drug Saf. 2016;39(8):749-754. doi: 10.1007/s40264-016-0428-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoofnagle JH, Bonkovsky HL, Phillips EJ, et al. ; Drug-Induced Liver Injury Network . HLA-Bb35:01 and green tea-induced liver injury. Hepatology. 2021;73(6):2484-2493. doi: 10.1002/hep.31538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuppalanchi R, Bonkovsky HL, Ahmad J, et al. ; Drug-Induced Liver Injury Network . Garcinia cambogia, either alone or in combination with green tea, causes moderate to severe liver injury. Clin Gastroenterol Hepatol. 2022;20(6):e1416-e1425. doi: 10.1016/j.cgh.2021.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philips CA, Valsan A, Theruvath AH, et al. ; Liver Research Club India . Ashwagandha-induced liver injury: a case series from India and literature review. Hepatol Commun. 2023;7(10):e0270. doi: 10.1097/HC9.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halegoua-DeMarzio D, Navarro V, Ahmad J, et al. Liver injury associated with turmeric-a growing problem: ten cases from the Drug-Induced Liver Injury Network [DILIN]. Am J Med. 2023;136(2):200-206. doi: 10.1016/j.amjmed.2022.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman L, Gottfried M, Whitsett M, et al. Clinical features and outcomes of complementary and alternative medicine induced acute liver failure and injury. Am J Gastroenterol. 2016;111(7):958-965. doi: 10.1038/ajg.2016.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health Office of Dietary Supplements . Dietary Supplement Label Database (DSLD). Accessed July 1, 2023. https://dsld.od.nih.gov/
- 16.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999-2010. Vital Health Stat 1. 2013;(56):1-37. [PubMed] [Google Scholar]
- 17.Stierman B, Afful J, Carroll MD, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files—Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports; No. 158. National Center for Health Statistics; 2021. [Google Scholar]
- 18.Centers for Disease Control and Prevention . NHANES 2017-March 2020 pre-pandemic. Accessed July 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-2020
- 19.Centers for Disease Control and Prevention . NHANES 1999-2020 data documentation, codebook, and frequencies. Accessed July 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/1999-2000/DSII.htm#Analytic_Notes
- 20.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960-974. doi: 10.1053/j.gastro.2008.01.034 [DOI] [PubMed] [Google Scholar]
- 21.United States Census Bureau . 2020 U.S. Census results. Accessed July 1, 2023. https://www.census.gov/programs-surveys/decennial-census/decade/2020/2020-census-results.html
- 22.ClinCalc.com. The top 300 of 2021 provided by the ClinCalc Drugstats Database. Accessed December 1, 2023. https://clincalc.com/DrugStats/Top300Drugs.aspx
- 23.National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury, 2012-. Updated June 20, 2024. Accessed January 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK547852/ [PubMed]
- 24.American Association for Public Opinion Research . Best practices for survey research. Accessed May 22, 2024. https://aapor.org/standards-and-ethics/best-practices
- 25.US Food and Drug Administration . Dietary Supplement Health and Education Act of 1994; Public Law 103–417. US Food and Drug Administration; 1994. [Google Scholar]
- 26.Gahche JJ, Bailey RL, Potischman N, et al. Federal monitoring of dietary supplement use in the resident, civilian, noninstitutionalized US population, National Health and Nutrition Examination Survey. J Nutr. 2018;148(suppl 2):1436S-1444S. doi: 10.1093/jn/nxy093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Council for Responsible Nutrition. The science behind the supplements: economic impact of the dietary supplement industry. Accessed January 31, 2024. https://www.crnusa.org/resources/economic-impact-dietary-supplement-industry#:~:text=In%20the%20United%20States%2C%20the,for%20health%20and%20wellness%20products
- 28.Farina EK, Austin KG, Lieberman HR. Concomitant dietary supplement and prescription medication use is prevalent among US adults with doctor-informed medical conditions. J Acad Nutr Diet. 2014;114(11):1784-90.e2. doi: 10.1016/j.jand.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 29.Du M, Luo H, Blumberg JB, et al. Dietary supplement use among adult cancer survivors in the United States. J Nutr. 2020;150(6):1499-1508. doi: 10.1093/jn/nxaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geller AI, Shehab N, Weidle NJ, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med. 2015;373(16):1531-1540. doi: 10.1056/NEJMsa1504267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estes JD, Stolpman D, Olyaei A, et al. High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch Surg. 2003;138(8):852-858. doi: 10.1001/archsurg.138.8.852 [DOI] [PubMed] [Google Scholar]
- 32.Chalasani N, Fontana RJ, Bonkovsky HL, et al. ; Drug Induced Liver Injury Network (DILIN) . Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934, 1934.e1-1934.e4. doi: 10.1053/j.gastro.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García-Cortés M, Borraz Y, Lucena MI, et al. Liver injury induced by “natural remedies”: an analysis of cases submitted to the Spanish Liver Toxicity Registry [in Spanish]. Rev Esp Enferm Dig. 2008;100(11):688-695. doi: 10.4321/s1130-01082008001100004 [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) . Notes from the field: acute hepatitis and liver failure following the use of a dietary supplement intended for weight loss or muscle building—May-October 2013. MMWR Morb Mortal Wkly Rep. 2013;62(40):817-819. [PMC free article] [PubMed] [Google Scholar]
- 35.Bonkovsky HL, Kleiner DE, Gu J, et al. ; U.S. Drug Induced Liver Injury Network Investigators . Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65(4):1267-1277. doi: 10.1002/hep.28967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenig G, Callipari C, Smereck JA. Acute liver injury after long-term herbal “liver cleansing” and “sleep aid” supplement use. J Emerg Med. 2021;60(5):610-614. doi: 10.1016/j.jemermed.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 37.Kesar V, Channen L, Masood U, et al. Liver transplantation for acute liver injury in Asians is more likely due to herbal and dietary supplements. Liver Transpl. 2022;28(2):188-199. doi: 10.1002/lt.26260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satia-Abouta J, Kristal AR, Patterson RE, Littman AJ, Stratton KL, White E. Dietary supplement use and medical conditions: the VITAL study. Am J Prev Med. 2003;24(1):43-51. doi: 10.1016/S0749-3797(02)00571-8 [DOI] [PubMed] [Google Scholar]
- 39.Mah E, Chen O, Liska DJ, Blumberg JB. Dietary supplements for weight management: a narrative review of safety and metabolic health benefits. Nutrients. 2022;14(9):1787. doi: 10.3390/nu14091787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor EA, Evans CV, Ivlev I, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327(23):2334-2347. doi: 10.1001/jama.2021.15650 [DOI] [PubMed] [Google Scholar]
- 41.Mangione CM, Barry MJ, Nicholson WK, et al. ; US Preventive Services Task Force . Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(23):2326-2333. doi: 10.1001/jama.2022.8970 [DOI] [PubMed] [Google Scholar]
- 42.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol. 2004;160(4):339-349. doi: 10.1093/aje/kwh207 [DOI] [PubMed] [Google Scholar]
- 43.Rock CL. Multivitamin-multimineral supplements: who uses them? Am J Clin Nutr. 2007;85(1):277S-279S. doi: 10.1093/ajcn/85.1.277S [DOI] [PubMed] [Google Scholar]
- 44.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103(3):323-328. [DOI] [PubMed] [Google Scholar]
- 45.Miller MF, Bellizzi KM, Sufian M, Ambs AH, Goldstein MS, Ballard-Barbash R. Dietary supplement use in individuals living with cancer and other chronic conditions: a population-based study. J Am Diet Assoc. 2008;108(3):483-494. doi: 10.1016/j.jada.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Rahman O. Dietary supplements use among adults with cancer in the United States: a population-based study. Nutr Cancer. 2021;73(10):1856-1863. doi: 10.1080/01635581.2020.1820050 [DOI] [PubMed] [Google Scholar]
- 47.Barrett LA, Xing A, Sheffler J, et al. Assessing the use of prescription drugs and dietary supplements in obese respondents in the National Health and Nutrition Examination Survey. PLoS One. 2022;17(6):e0269241. doi: 10.1371/journal.pone.0269241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods. 2017;6(10):92. doi: 10.3390/foods6100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng L, Yang T, Yang K, et al. Efficacy and safety of curcumin and Curcuma longa extract in the treatment of arthritis: a systematic review and meta-analysis of randomized controlled trial. Front Immunol. 2022;13:891822. doi: 10.3389/fimmu.2022.891822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hursel R, Viechtbauer W, Dulloo AG, et al. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev. 2011;12(7):e573-e581. doi: 10.1111/j.1467-789X.2011.00862.x [DOI] [PubMed] [Google Scholar]
- 51.Rondanelli M, Riva A, Petrangolini G, et al. Effect of acute and chronic dietary supplementation with green tea catechins on resting metabolic rate, energy expenditure and respiratory quotient: a systematic review. Nutrients. 2021;13(2):644. doi: 10.3390/nu13020644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akbarialiabad H, Dahroud MD, Khazaei MM, Razmeh S, Zarshenas MM. Green tea, a medicinal food with promising neurological benefits. Curr Neuropharmacol. 2021;19(3):349-359. doi: 10.2174/1570159X18666200529152625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mena-García A, Bellaizac-Riascos AJ, Rada-Mendoza M, Chito-Trujillo DM, Ruiz-Matute AI, Sanz ML. Quality Evaluation of dietary supplements for weight loss based on Garcinia cambogia. Nutrients. 2022;14(15):3077. doi: 10.3390/nu14153077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.US Food and Drug Administration. Dietary supplements. Accessed January 1, 2024. https://www.fda.gov/food/dietary-supplements
- 55.US Food and Drug Administration. FDA’s regulation of dietary supplements with Dr. Cara Welch: Q&A with FDA podcast/transcript. Accessed January 1, 2024. https://www.fda.gov/drugs/news-events-human-drugs/fdas-regulation-dietary-supplements-dr-cara-welch
- 56.Nahin RL, Pecha M, Welmerink DB, Sink K, DeKosky ST, Fitzpatrick AL; Ginkgo Evaluation of Memory Study Investigators . Concomitant use of prescription drugs and dietary supplements in ambulatory elderly people. J Am Geriatr Soc. 2009;57(7):1197-1205. doi: 10.1111/j.1532-5415.2009.02329.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Ingredient Identification Number and Supplement Identification Number Identified the 6 Potential Hepatotoxic Botanical Products
eTable 2. Botanical Products by Name (Alphabetical)
eFigure. Herbal and Dietary Supplement Use Among U.S. Adults Enrolled in NHANES 2017-2020
Data Sharing Statement