ABSTRACT
The microbial ecology of raw milk cheeses is determined by bacteria originating from milk and milk-producing animals. Recently, it has been shown that members of the Bifidobacterium mongoliense species may become transmitted along the Parmigiano Reggiano cheese production chain and ultimately may colonize the consumer intestine. However, there is a lack of knowledge regarding the molecular mechanisms that mediate the interaction between B. mongoliense and the human gut. Based on 128 raw milk cheeses collected from different Italian regions, we isolated and characterized 10 B. mongoliense strains. Comparative genomics allowed us to unveil the presence of enzymes required for the degradation of sialylated host-glycans in B. mongoliense, corroborating the appreciable growth on de Man-Rogosa-Sharpe (MRS) medium supplemented with 3’-sialyllactose (3′-SL) or 6’-sialyllactose (6′-SL). The B. mongoliense BMONG18 was chosen, due to its superior ability to utilize 3′-SL and mucin as representative strain, to investigate its behavior when co-inoculated with other bifidobacterial species. Conversely, members of other bifidobacterial species did not appear to benefit from the presence of BMONG18, highlighting a competitive scenario for nutrient acquisition. Transcriptomic data of BMONG18 reveal no significant differences in gene expression when cultivated in a gut simulating medium (GSM), regardless of whether cheese was included or not. Furthermore, BMONG18 was shown to exhibit high adhesion capabilities to HT29-MTX human cells, in line with its colonization ability of a human host.
IMPORTANCE
Fermented foods are nourishments produced through controlled microbial growth that play an essential role in worldwide human nutrition. Research interest in fermented foods has increased since the 80s, driven by growing awareness of their potential health benefits beyond mere nutritional content. Bifidobacterium mongoliense, previously identified throughout the production process of Parmigiano Reggiano cheese, was found to be capable of establishing itself in the intestines of its consumers. Our study underscores molecular mechanisms through which this bifidobacterial species, derived from food, interacts with the host and other gut microbiota members.
KEYWORDS: bifidobacteria, microbiota, microbiome, dairy food microorganisms, microbial genomics, microbe–host interactions
INTRODUCTION
It is universally recognized that foods, besides being considered a source of nutrients for humans and their associated intestinal microbiota, also represent a potential transfer vehicle of microbes to the human gut, where they may influence host health through modulation of the intestinal microbiota composition (1). In this context, a crucial role is played by the microbiota of fermented foods, whose beneficial effects are conferred by microorganisms and their metabolites (2). Fermented foods encompass a variety of foods and beverages, ranging from, to name but a few, cheese, yogurt, kefir, and sauerkraut. The fermentative transformation of carbohydrate substrates through controlled microbial growth and biochemical conversions has been investigated extensively in order to understand nutritional function and assess organoleptic properties mediated by microorganisms (3). Dairy products, including yogurt and raw milk cheeses, are among the most commonly consumed fermented foods in Western culture (4). Raw milk cheeses are made from milk that is subjected to a temperature not exceeding 40°C, allowing the preservation of viable milk-derived microorganisms that possess metabolic potential to process the food matrix (5). Raw milk represents an optimal environment for the colonization and proliferation of numerous microorganisms. Its chemical-physical features render raw milk-based cheese a highly nutritional substrate, enriched with compounds made accessible through the metabolic activities of different microbial populations (6). Hence, its microbial ecology depends on the milk microbiota itself and on microbes acquired from the environment where the cheese is manufactured (7). These microbial characteristics, as well as agricultural, processing, and ripening techniques, influence, modify, and ultimately govern the particular raw milk microbiota composition (8).
The microbiota of raw milk cheeses is characterized by different microbial taxa belonging predominantly to Streptococcus, Staphylococcus, Lactobacillus, and Lactococcus genera, responsible for the production of aromatic molecules that endow the final product with various organoleptic and physical characteristics, in particular its aroma and texture (9). Among the bacteria that have been identified as part of the raw milk cheese microbiota, certain members of the genus Bifidobacterium deserve to be mentioned (10). In particular, members of the taxa Bifidobacterium crudilactis and Bifidobacterium mongoliense are relevant in the context of raw milk (8, 11, 12). Interestingly, it has been reported that certain bifidobacterial species grow and survive in milk and dairy products due to genes that are dedicated to the metabolism of substrates (e.g., carbohydrates and peptides) typically present in dairy matrices (13). In this context, it has recently been shown that B. mongoliense occurring in the microbiota of Parmigiano Reggiano cheese is horizontally transferred along the entire cheese production chain and, subsequently, into the intestine of individuals who daily consume this food (12).
The main aim of the present study was to isolate and characterize 10 novel B. mongoliense strains associated with raw milk cheeses, and to investigate their genomic content and phylogenetic relationships. Comparative genomic analyses were performed to investigate the glycobiome of the B. mongoliense species, followed by in vitro validation through growth assays employing different carbon sources. Then, because in silico analyses of the B. mongoliense taxon revealed a close evolutionary distance between strains, we selected a representative strain, i.e., BMONG18, to delve deeper into the specific traits and behaviors of the taxon. Thus, we investigated the potential functional impact of B. mongoliense BMONG18 strain with multiple in vitro experiments involving other bifidobacterial species, HT29-MTX human cell lines, and dedicated growth media mimicking intestinal and cheese microenvironments.
RESULTS AND DISCUSSION
Genetic characterization of novel B. mongoliense strains
A previous investigation of the resident microbiome of 128 Italian raw milk cheeses revealed that B. mongoliense is the most prevalent bifidobacterial species (8). Nevertheless, to date, the genetic information of the B. mongoliense taxon is based on a rather limited number of genotypes (14, 15). Consequently, to further investigate the genetic variability of this species, a cultivation-dependent strategy was employed, allowing the isolation of 10 novel B. mongoliense strains from different raw milk cheeses and cheesemaking sites (Table 1). Subsequently, genetic characterization of these strains was carried out by decoding their genome sequences through a next-generation sequencing (NGS) method, resulting in 19 to 29 contigs with sequence fold coverage ranging from 164 to 393 (Table 1). Assembled contigs of each B. mongoliense genome allowed the prediction of their genome length, which ranged from 1,991 to 2,150 kb, as well as of the number of open reading frames (ORFs), which ranged from 1,605 to 1,790 ORFs (Table 1). Unlike previously described bifidobacteria, such as Bifidobacterium dentium and Bifidobacterium adolescentis (16, 17), members of the B. mongoliense species displayed relatively little variation in their genome length and ORF number, suggesting a conserved genome structure, whose genetic composition was further investigated through in silico comparative genomic techniques.
TABLE 1.
Summary table of assembly data of B. mongoliense genomes
| Strain | 16S rRNA identitya (%) | ANIa (%) | Completeness (%) | Contamination (%) | Avg coverage | No. of contigs | Genome length | No. of genes | Isolation source | Accession no. |
|---|---|---|---|---|---|---|---|---|---|---|
| BMONG18 | 99.93 | 98.95 | 98.82 | 0.01 | 201.86 | 25 | 2,132,753 | 1,764 | Parmesan cheese DOP | QRAJ01 |
| 2099B | 100 | 98.89 | 98.91 | 0.01 | 171.47 | 26 | 2,111,539 | 1,709 | Parmesan cheese DOP | JBBMLP000000000 |
| 2254B | 99.87 | 99.09 | 98.91 | 0.01 | 190.19 | 23 | 2,137,522 | 1,718 | Formai de mut DOP | JBBMLO000000000 |
| 2255B | 99.47 | 99.06 | 98.91 | 0.01 | 234.91 | 19 | 2,149,534 | 1,761 | Puzzone di Moena DOP | JBBMLN000000000 |
| 2256B | 100 | 98.99 | 98.91 | 0.01 | 173.59 | 19 | 1,991,151 | 1,605 | Strachitunt DOP | JBBMLM000000000 |
| 2257B | 100 | 98.7 | 98.91 | 0.01 | 164.29 | 30 | 2,152,313 | 1,790 | Castelmagno DOP | JBBMLL000000000 |
| 2258B | 100 | 98.88 | 98.91 | 0.01 | 221.7 | 28 | 2,109,495 | 1,720 | Asiago DOP | JBBMLK000000000 |
| 2259B | 100 | 98.84 | 98.91 | 0.01 | 226.23 | 19 | 2,136,905 | 1,734 | Bitto DOP | JBBMLJ000000000 |
| 2260B | 100 | 98.95 | 98.91 | 0.01 | 292.57 | 27 | 2,067,515 | 1,665 | Pecorino DOP | JBBMLI000000000 |
| 2261B | 100 | 98.93 | 98.91 | 0.01 | 281.14 | 22 | 2,154,803 | 1,765 | Puzzone di Moena DOP | JBBMLH000000000 |
| 2262B | 100 | 98.75 | 98.91 | 0.01 | 185.15 | 29 | 2,154,453 | 1,790 | Castelmagno DOP | JBBMLG000000000 |
Comparison based on the type strain DSM 21395 sequences.
Pangenome analysis of the B. mongoliense species
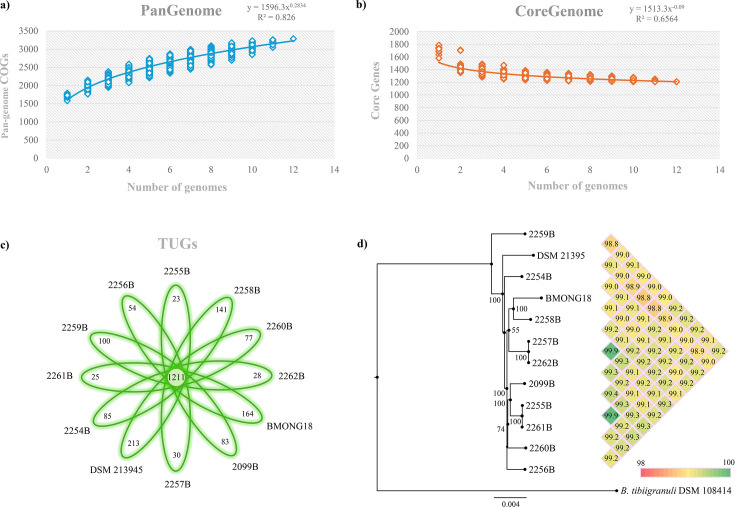
A comparative genomic analysis was performed employing the predicted proteome of the 10 isolated strains belonging to the B. mongoliense species, together with the proteome sequences of the publicly available type strain and strain BMONG18 (Table 1). In this context, a pangenome analysis was performed using a previously described method based on cluster of orthologous groups (COGs) (18, 19), allowing the identification of 3,285 COGs. The graphical representation of the pangenome revealed that the power trend line had not reached a plateau, indicating that the genetic diversity of the B. mongoliense taxon had not been fully covered by analyzing these 12 genomes only (Fig. 1a). Thus, a larger number of genomes will have to be incorporated in this comparative analysis to fully encompass the B. mongoliense pangenome. Our comparative analysis revealed 1,211 COGs that are shared by all 12 B. mongoliense genotypes, thus representing 70% of the whole pangenome (Fig. 1b). The 12 B. mongoliense genomes were also valuable in disclosing the genetic variability of the taxon. In this context, identification of truly unique genes (TUGs), i.e., genes that are present in just one genome of the analyzed strains, was performed revealing significant variability among strains, ranging from 23 to 213 TUGs in 2255B and DSM 21395, respectively (Fig. 1c). Strains with the lower number of TUGs (ranging from 23 to 30) were retrieved from different cheesemakers, highlighting a genomic relatedness of cheese-related bifidobacteria covering different Italian regions. In contrast, the higher number of TUGs identified in the genome of B. mongoliense type strain DSM 21395, which was not sequenced as part of this study, reflected its origin, i.e., the traditional Mongolian beverage Airag produced from fermented mare milk (Fig. 1c).
Fig 1.
B. mongoliense pangenome, core genome, and phylogenetic tree. Panel (a) illustrates the pangenome size based on the sequential insertion of the 12 B. mongoliense genomes. Panel (b) shows the core-genome size via the sequential addition of the genomes, whereas panel (c) reports the number of TUGs per each B. mongoliense genome. Panel (d) represents the phylogenetic tree of the B. mongoliense species (left) and a color scheme incorporating the ANI values between each analyzed strain (right).
Evaluation of intraspecies genomic variability of the B. mongoliense species
The genomes of the isolated B. mongoliense strains were analyzed through the average nucleotide identity (ANI) approach to further explore the genomic variability of this taxon (Fig. 1d; Table S1). This analysis revealed high genome identity levels among members of the B. mongoliense taxon, with associated ANI values ranging from 98.8% to 99.9% (Fig. 1d; Table S1). Of note, the analyzed B. mongoliense strains exhibit an average ANI value of 99.2%, indicative of very limited genetic variability of this bifidobacterial taxon compared to other bifidobacterial species, for which an ANI value exceeding 98.2% has been considered to represent limited genome variability (17). These findings suggest that the isolated members of this bifidobacterial species are adapted to the ecological niche represented by dairy foods. Interestingly, within the taxon, the type strain of B. mongoliense showed the lowest ANI values compared to other analyzed genomes, ranging from 98.8% to 99.1%, which, as reported above, is consistent with its diverse origin.
An investigation into the phylogeny of the B. mongoliense taxon was performed starting with the proteomes deduced from the genome sequences of the 12 B. mongoliense strains (20, 21). The type strain of Bifidobacterium tibiigranuli (22, 23) was also employed due to its phylogenetic relatedness to the B. mongoliense taxon (24). The obtained phylogenetic tree, as suggested by ANI analysis and TUG distribution, showed that the B. mongoliense type strain as well as 2259B was markedly separated through evolutionary distance from other B. mongoliense strains (Fig. 1d). Thus, our phylogenetic analysis corroborates that from an evolutionary perspective, strains 2259B and DSM 21395 seemed to have followed a specific evolutionary route associated with the kind of cheese and geography. Nonetheless, the obtained phylogenetic tree highlights a close evolutionary distance between B. mongoliense strains, indicating high conservation of the genetic heritage and reflecting the characteristics of the biological matrix of isolation (Fig. 1d).
The carbohydrate-active enzyme arsenal among members of the B. mongoliense species
To uncover the carbohydrate-active enzyme repertoire of the B. mongoliense species, the genomes of the 10 newly sequenced B. mongoliense strains coupled with the two publicly available genomes were screened to predict genes encoding glycosyl hydrolases (GHs), glycosyltransferases (GTs), and polysaccharide lyases (PLs) using the carbohydrate-active enzyme (CAZy) database (25). The genomic analyses revealed the presence of 726 genes encoding various glycosyl-related enzymatic functions across the 12 B. mongoliense strains (Table S2). Predicted carbohydrate-active enzymes encompassing 14 GH families were shared by all B. mongoliense strains (Fig. 2) due to the high level of genomic synteny identified for B. mongoliense. To unveil the unique feature of B. mongoliense, its glycobiome was then compared with that of various bifidobacterial species isolated from dairy-based foods that share a similar ecological niche, such as Bifidobacterium aquikefiri, B. crudilactis, Bifidobacterium psychraerophilum, and B. tibiigranuli (20) (Table S3). Remarkably, GH33, which is predicted to be involved in the degradation of sialyloligosaccharides (Fig. 2), was absent in all other species, representing an apparently unique feature of the B. mongoliense species. Predicted β-N-acetylhexosaminidase (GH20) was identified in the genomes of six B. mongoliense strains, whereas the predicted exo-α-sialidase (GH33) appeared to be only missing in chromosome of strain 2256B (Table S2), representing enzymes involved in human milk oligosaccharide (HMO) or bovine milk oligosaccharide (BMO) degradation. The presence of a secretion signal sequence was evaluated for each enzyme, revealing that together with other GHs, members of the GH33 were predicted to represent extracellular enzymes (Table S2).
Fig 2.
Carbohydrate-active enzymes of B. mongoliense and other bifidobacterial species harboring fermented foods. The graphical representation shows a map of GHs, GTs, and PL, with the percentage within each genome representing the enzyme’s corresponding gene frequency in the chromosome sequences of the species analyzed. The final column displays the cumulative prevalence of GH, GT, and PL in families with all strains.
To corroborate in silico genome-based analyses, growth experiments encompassing all 12 B. mongoliense strains on de Man-Rogosa-Sharpe (MRS) medium containing monosaccharides such as glucose, galactose, ribose, and sialic acid, as well as disaccharides, i.e., lactose, melibiose, and lactulose, as the only carbon source, were performed (Fig. 3). B. mongoliense strains showed appreciable growth on 3’-sialyllactose (3′-SL) as well as on 6’-sialyllactose (6′-SL), which are sialylated trisaccharides present in human and bovine milk (26). Interestingly, strains that showed the highest growth yield on sialylated trisaccharides were also predicted to translocate their GH33 to the extracellular environment, i.e., strains 2254B, 2255B, 2259B, 2261B, 2099B, and DSM 21395 (Table S2; Fig. 3). Overall, the ability of B. mongoliense strains to grow on sialylated saccharides is consistent with their ability to utilize sialic acid as the sole carbon source (Fig. 3) and with the presence of GH33 in their genome (Fig. 2). A genomic investigation revealed the presence of a gene cluster encoding the complete pathway for sialic acid degradation and transport, i.e., the nan operon consisting of nanA, nanB, nanC, nanD, nanH, nanK, and nanT, as well as the nagA and nagB homologues, which are presumed to be responsible for the conversion of N-acetylglucosamine-6-phosphate to fructose-6-phosphate. The absence of the nan operon in strain 2256B and the absence of the gene encoding for the GH33 in its genome corroborate that this strain is the only B. mongoliense strain shown to be unable to grow on sialic acid as the sole carbon source. As previously characterized in Bifidobacterium breve (27), the ability to metabolize sialylated saccharides can improve bifidobacterial colonization and persistence in the gut of lactating animals during the first stages of life as well as in those individuals frequently taking bovine milk as part of their daily diet.
Fig 3.
Growth abilities of the B. mongoliense strains on different carbon sources. Growth performances of the B. mongoliense strains were measured by assessing optical density (OD) values after 96 h of incubation. For OD values between 0 and 0.3, the strain was assumed to be unable to grow in the sugar, whereas for OD values between 0.3 and 1, the strain was determined to be able to sustain growth on the particular carbohydrate. LNT, lacto-N-tetraose; FOS, fructooligosaccharides.
Co-cultivation assay between B. mongoliense and other bifidobacterial strains
To expand our knowledge on the ability and behavior of B. mongoliense to colonize the human gut, we assayed if and how this bacterial species engages in trophic interactions with other key members of the human gut microbiota (28, 29). Due to its superior (compared to the other tested B. mongoliense strains) ability to utilize 3′-SL and mucin, B. mongoliense BMONG18 was selected as the representative strain to investigate its behavior when co-cultivated in MRS medium supplemented with HMOs, starch, or mucin as sole carbon sources with Bifidobacterium bifidum PRL2010, Bifidobacterium longum PRL2022, and/or B. adolescentis PRL2023. We then compared these results to the growth profiles obtained when these strains were co-cultivated in pairs (bi-associations) on identical substrates. Co-cultivation experiments with HMOs were performed in MRS supplemented with 3’-sialyllactose (3′-SL) and 2’-fucosyllactose (2′-FL). These carbohydrates were selected to represent carbon sources that are expected to be present in the human gut from infancy to adulthood, as they are derived from human/bovine milk (30, 31). Overall, qPCR evaluation of B. mongoliense BMONG18 revealed that this taxon is able to utilize 3′-SL and mucin-based medium for growth (Fig. 4). In this context, it has already been demonstrated that B. mongoliense is able to metabolize most oligosaccharides present in bovine milk, including 3′-SL, due to an extracellular α-sialidase (14). Among all possible combinations with bifidobacterial strains, the quantity of BMONG18 cells was shown to increase up to threefold in those bi-associations that include B. longum PRL2022 or B. adolescentis PRL2023 cells when cultivated on MRS supplemented with all different carbohydrates compared to their corresponding mono-associations (Fig. 4). Hence, these findings suggest that the observed B. mongoliense growth expansion is linked to its ability to better metabolize the available substrates, competing with the co-inoculated microbe for resources and benefitting from its presence, thus establishing a competitive scenario for nutritional resources. In contrast, each bacterial partner, i.e., PRL2010, PRL2022, and PRL2023, did not appear to benefit from the presence of BMONG18 (Fig. 4). In fact, the bacterial load of PRL2010 decreased almost twofold when co-cultivated with BMONG18 in MRS medium supplemented with mucin when compared to its mono-association (Fig. 4). Furthermore, B. mongoliense appears to influence PRL2010 growth, leading to a competitive interaction for HMOs, i.e., 3′-SL and 2′-FL, when these strains were cultured in bi-association (Fig. 4). Another interesting finding was the onefold decrease of the cell load of PRL2022 when co-cultivated with B. mongoliense BMONG18 on MRS supplemented with mucin (Fig. 4). These findings imply that B. mongoliense actively engages in metabolic competition with other microorganisms that are usually members of the human gut microbiota, potentially influencing the overall microbial balance. Such trophic interactions may, therefore, play an important role in shaping the composition and functionality of the gut microbiota.
Fig 4.
B. mongoliense BMONG18 growth observed through co-cultivation with other bifidobacterial species. The four panels show the cell number quantification of B. mongoliense BMONG18, B. bifidum PRL2010, B. longum PRL2022, and B. adolescentis PRL2023 strains in mono- and bi-association on the MRS medium supplemented with HMOs, starch, and mucin, indicated above each panel by qPCR. Results of the qPCR experiments are expressed by bars indicating fold change (FC) of cell number quantification after 24 h of growth on MRS medium compared with the respective inoculum (T0). The y-axis indicates the FC of genome copy number per milliliter of bacterial culture, whereas the x-axis displays the name of the strains involved in mono- and bi-associations. The dotted bars represent the amount of each bifidobacterial strain present at T0.
Functional investigation of B. mongoliense in a gut- or cheese-simulated environment
Given the distribution of B. mongoliense in fermented and dairy products and its potential interaction with the human intestinal microbiota, B. mongoliense strain BMONG18 was used to perform three different growth assays in cheese-based medium, gut simulating growth medium (GSM), and GSM plus sterilized cheese (see Materials and Methods), in order to assess associated transcriptomic profiles when present in cheese or in the intestinal environment. For this purpose, RNA was extracted from BMONG18 cells cultivated under the aforementioned conditions and subjected to RNA sequencing, generating a total of 16,517,495 quality-filtered reads with an average of 1,835,277 reads per sample. Only genes exhibiting transcriptional variations with a log fold change (log FC) of ≥1 in combination with a value of P ≤ 0.05 calculated through correction for multiple comparisons using the false discovery rate (FDR) procedure were considered as significantly differentially transcribed between the three conditions assessed. Transcriptomic data did not reveal remarkable differences in the obtained gene expression patterns of B. mongoliense strain BMONG18 when cultivated in GSM with or without the inclusion of cheese. Conversely, a change in the transcription profile occurred when BMONG18 strain was cultivated in cheese broth compared to GSM assays. Under such cultivation conditions, and among the 179 genes that were upregulated, genes encoding a predicted argininosuccinate synthase (2016B_1510), acetylglutamate kinase (2016B_1506), and N-acetyl-gamma-glutamyl-phosphate reductase (2016B_1504) were detected, which are predicted to be involved in arginine biosynthesis, along with several other enzymes implicated in the urea cycle such as argininosuccinate lyase (2016B_1511) and ornithine carbamoyltransferase (2016B_1508) (Fig. 5a) (Table S4 to S6). Contextually, milk and cheese are valuable sources of arginine, an amino acid that plays important roles in various physiological processes of the human host (32, 33). These include nitric oxide synthesis to promote circulation, polyamine synthesis to promote proliferation, protein synthesis, growth hormone production, as well as supporting immune response and accelerating healing processes (34, 35). Moreover, we observed an upregulation of different ABC-type transporter-encoding genes, as well as genes for ABC-type carbohydrate-specific permeases, ATP-binding proteins, and transporters belonging to the major facilitator superfamily (MFS), possibly involved in the uptake of sugars or other compounds when strain BMONG18 is cultivated on a substrate simulating cheese environment. Other transcriptionally enhanced genes of B. mongoliense BMONG18 cells encompassed those encoding glucose-6-phosphate isomerase (2016B_0244), galactose-1-phosphate uridyltransferase (2016B_0336), and glycoside hydrolase family 3 protein (2016B_1436), whose functions are related to the interconversion of sugars in monosaccharides channeled into the “bifid-shunt” metabolic pathway for energy generation (Fig. 5b) (Table S4 to S6). RNA sequencing data were validated by RT-qPCR experiments, which showed a not significant upregulation of the abovementioned genes in BMONG18 when grown in the cheese-based medium compared to GSM and GSM supplemented with cheese (Fig. S2). Remarkably, we also observed an increase in the transcription of genes encoding the diverse subunits of the ATPase system of the BMONG18 strain, likely triggered by medium acidification due to enhanced metabolic activity. Furthermore, the upregulation of lipoteichoic acid synthase family protein (2016B_1474) detected in the BMONG18 strain cultivated in cheese broth, as opposed to gut environment-simulating medium conditions, supports the concept of cheese being a favorable niche for B. mongoliense (Table S4 to S6).
Fig 5.
Transcriptional modulation of B. mongoliense BMONG18 genes when grown under three different broth conditions. Panels (a–b) summarize the number of genes per functional category. The image reports transcriptional modulation of genes, expressed as the average of the normalized count reads obtained from each independent biological triplicate.
Ability of B. mongoliense to interact with the human host as evaluated through in vitro assays
A recent study demonstrated that B. mongoliense exhibits the ability to be transferred through the cheese production chain, to persist in the final product, and to potentially colonize the human intestine (12). In this context, the ability of B. mongoliense to adhere to human intestinal cells may support the notion that this bacterium is able to colonize the human gut. In addition, such an adhesion ability may support its potential interaction with other members of the gut microbiota as well as with the human host (36, 37). We, therefore, investigated the adhesive abilities of B. mongoliense cells to human intestinal cells represented by HT29-MTX monolayers following a previously published protocol (37, 38). HT29-MTX monolayers are a widely used mucus-producing cell model mimicking the mucosal surface of the human gut epithelium (38, 39). Adhesion experiments were performed on a subset of four representative B. mongoliense strains, i.e., strains 2256B, BMONG18, 2258B, and the type strain DSM 21395, chosen through a hierarchical clustering analysis in order to cover the biodiversity of the species (Fig. S1). The number of microbial cells attached to HT29-MTX monolayers was assessed to calculate the adhesion index, which represents the number of bacterial cells adhering to 100 human intestinal cells (37). The adhesion capabilities of the selected strains were determined and shown to be higher than those of the reference bacterium, i.e., B. bifidum strain PRL2010, which clearly displayed an adhesive phenotype to human mucosa (adhesion index of 714,666 ± 49,026) (Fig. 6a and b) (38, 40). Furthermore, B. mongoliense 2256B and BMONG18 strains exhibited significantly higher adhesion abilities compared to the type strain (adhesion indexes of 1,226,666 ± 139,535 and 1,118,666 ± 190,447, compared to 369,333 ± 118,793, respectively) (Kruskal-Wallis test P < 0.05) and the negative control Bifidobacterium animalis subsp. lactis Bb-12 (adhesion index of 18,666 ± 7,542) (Kruskal-Wallis test P < 0.01) (Fig. 6a and b) (38, 41). In addition, an adhesion assay on mucin was performed, which showed that all tested B. mongoliense strains show a larger adhesion ability to mucin when compared to the negative control Bb-12 (45.74%) (Fig. 6c). In particular, 2256B and BMONG18 strains were shown to exhibit the highest adhesion capabilities with a percentage of 92.44% and 88.47%, respectively. Moreover, there was a significant difference in the adhesion ability of 2256B and BMONG18 strains compared to that of the type strain DSM 21395 (82.11%) (Kruskal-Wallis test P < 0.05) (Fig. 6c). These results highlight the strong adhesive properties of B. mongoliense cells to enteric epithelial cells, indicating their potential for long-term colonization in the human gut. This adhesive capability suggests that B. mongoliense strains can establish a stable presence within the gut environment, potentially impacting the microbiota composition, its associated metabolic and, consequently, gut health.
Fig 6.
Adhesion of B. mongoliense cells to HT29-MTX cell monolayers and mucin. Panel (a) depicts the quantification of the adhesion performances of different B. mongoliense strains to HT29-MTX cell monolayers, expressed as the adhesion index. The vertical bars indicate standard deviations, and the asterisks indicate Kruskal-Wallis test P values. Panel (b) shows light microscopic images of HT29-MTX monolayer cells as observed with Giemsa staining of B. mongoliense strains. The bifidobacterial strains shown in each image are (i) B. mongoliense 2256B, (ii) B. mongoliense BMONG18, (iii) B. mongoliense 2258B, (iv) B. mongoliense DSM 21395, (v) B. bifidum PRL2010, and (vi) B. animalis subsp. lactis Bb-12. Panel (c) represents the percent adhesion values of B. mongoliense strains to mucin. The percentage of relative adhesion for each strain is determined by calculating the CFU values before and after the adhesion assay. The error bars represent the standard deviation of all assays, and the asterisks indicate Kruskal-Wallis test P values < 0.05.
Conclusions
Given the wide interest in fermented foods as a vehicle of nutrients and metabolites, as well as a carrier of microbial cells that can influence consumers’ health, detailed understanding of these aspects has been an obvious scientific pursuit. The microbiota of cheese, especially of raw milk cheese, has been extensively studied, although further investigation into the ecology of the bacterial populations inhabiting dairy products is warranted. This is particularly desirable for bacterial species with specific properties that can positively influence gut microbiota composition as well as overall host health. In this study, B. mongoliense strains, isolated from different raw milk cheeses, were characterized at genomic and phenotypic levels to assess their genetic heritage and nutritional requirements. Furthermore, microbe–host interactions were explored to better understand colonization and adhesion capabilities to mucin and intestinal epithelial cells. Remarkably, the glycobiome of B. mongoliense, when compared with those of many bifidobacterial species isolated from dairy-based foods, was shown to be uniquely characterized by the presence of a GH33 family member, which is predicted to be involved in the removal of sialic acid moieties from host-glycans such as BMOs and HMOs. Similarly to members of GH20, these enzymes may mediate the interaction between B. mongoliense and mammals, as previously shown for human-related bifidobacterial species such as B. bifidum, B. breve, and B. longum. In this context, it has been demonstrated that host-glycans are driving factors in shaping the gut microbiota. Because B. mongoliense is able to utilize 3′-SL and mucin-based medium for growth, this ability may provide a competitive advantage in interacting with the human gut. Furthermore, co-culture experiments demonstrated that B. mongoliense utilizes nutritional resources by effectively competing with other species of intestinal bifidobacteria, a trait that is expected to be advantageous for intestinal colonization.
MATERIALS AND METHODS
Bacterial strain isolation
One gram of cheese sample was mixed with 9 mL of phosphate-buffered saline (PBS), pH = 6.5. Serial dilutions and subsequent plating were performed using MRS agar medium (Scharlau Chemie, Barcelona, Spain) supplemented with 0.05% (wt/col) L-cysteine hydrochloride, 50 µg/mL mupirocin (Delchimica, Italy), and 1% lactose (Merck, Germany). Agar plates were incubated in an anaerobic atmosphere (2.99% H2, 17.01% CO2, and 80% N2) in a chamber (Concept 400; Ruskin) at 37°C for 96 h. Colonies, chosen based on different sizes and morphologies, were otherwise randomly picked, and the identification of putative newly isolated B. mongoliense strains was achieved through the Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) Biotyper Sirius (Bruker) using the manufacturer’s software FlexControl and the MALDI-Biotyper software (MBT). In detail, a single bacterial colony grown on MRS agar was transferred onto a spot of the MSP 96 target polished steel BC MALDI target plate (Bruker). Subsequently, the bacterial sample was overlaid with 1 µL of matrix solution containing 10 mg/mL a-cyano-4-hydroxycinnamic acid (HCCA, Sigma-Aldrich) resolved in 50% acetonitrile (Carlo Erba) and 2.5% trifluoro-acetic acid (TFA, Carlo Erba) and air dried (42, 43). The MALDI target plate was then introduced into the spectrophotometer for automated measurement and data interpretation. The obtained mass spectra were processed with the MALDI Biotyper 3.0 software package (Bruker) containing reference spectra, including different microbial species. According to the criteria recommended by the manufacturer, a score of ≥2.000 indicates a significant similarity between the obtained spectrum and the database entry. Each sample was analyzed in duplicate (two spots for each sample).
The default parameter settings used were as follows: positive linear mode, laser frequency 200 Hz, ion source 1 = 19.84 kV, ion source 2 = 18.07 kV, Bruker’s MBT_FC and MBT_AutoX methods, and a mass range between 2,000 and 20,000 Da. Moreover, before analysis, calibration was performed with a bacterial test standard (Bruker) containing an extract of Escherichia coli DH5a.
Growth conditions of bifidobacterial strains and DNA isolation
Bifidobacterial species, i.e., B. mongoliense, B. bifidum PRL2010, B. longum PRL2022, B. adolescentis PRL2023, and B. animalis subsp. lactis Bb-12, were grown at 37°C under anaerobic conditions (2.99% H2, 17.01% CO2, and 80% N2) in a chamber (Concept 400; Ruskin) in MRS broth (Scharlau Chemie, Barcelona, Spain) supplemented with 0.05% (wt/vol) L-cysteine hydrochloride.
To perform chromosomal DNA extraction, the 10 B. mongoliense strains previously isolated were cultivated in MRS broth supplemented with 0.05% (wt/vol) L-cysteine hydrochloride in an anaerobic atmosphere at 37°C for 96 h. Subsequently, cells from 10 mL of the culture were harvested by centrifugation at 6,000 rpm for 8 min, and the obtained cell pellet was used for DNA extraction using the GenElute bacterial genomic DNA kit (Sigma-Aldrich) following the manufacturer’s guide.
Genome sequencing, assembly, and annotation
The DNA extracted from the 10 B. mongoliense strains was subjected to whole-genome sequencing using MiSeq (Illumina) at GenProbio srl, Parma, Italy (www.genprobio.com) according to the supplier’s protocol (Illumina). Individual genome libraries were generated using the Nextera XT preparation kit and were loaded into a 600-cycle (250-bp paired ends) flow cell version 3 (Illumina). Raw DNA sequence reads (fastq files) obtained from genome sequencing were assembled using the MEGAnnotator2 pipeline (44, 45). Briefly, SPAdes software was used for the de novo assembly of the genome sequences with the pipeline option “‐‐carefull” and a list of “21,33,55,77,99,127” k-mer sizes (46), whereas protein-encoding genes were predicted for contigs greater than 1,000 bp using Prodigal (47). Functional annotation of the predicted genes was achieved through DIAMOND (using the --sensitive option in search of query coverage >50 and e-value <1 × 10−8) (48) against the RefSeq database of NBCI resized with a CD-HIT sequence identity threshold of 70% (49), and further investigated by InterProScan (50). Moreover, tRNA genes were determined using tRNAscan-SE 2.0 (51), and rRNA loci were identified with barrnap (https://github.com/tseemann/barrnap).
The MEGAnnotator2 pipeline (44) also assessed genome quality using the checkM software (52), whereas fastANI (53) was employed for analyzing the ANI between strains of B. mongoliense. The rigorous quality assessment of reconstructed genome sequences revealed predicted levels of completeness >98% and levels of contamination <1% (Table 1). The complete genome sequence of B. mongoliense DSM 21395 was used as a reference to determine the orientation of each assembled contig.
Pangenome analyses and phylogenomic tree reconstruction
All pangenome calculations were performed using PanGenomes Analysis Pipeline (PGAP [54]) as described previously (19, 55). In detail, orthologous protein sequences were identified in genome sequences using BLAST analysis (cutoff E-value = 1 × 10−5; 50% identity over at least 80% of sequence coverage) and then organized into functional COGs through the MCL algorithm (graph-based Markov clustering algorithm) using the gene family (GF) method. Pangenome profiles were produced through an optimized procedure integrated into the PGAP software based on a presence/absence matrix including all COGs identified in the given genomes. The concatenated protein sequences of core genes were aligned using Mafft v7.453 (56) and then employed to build correspondent phylogenomic trees through the neighbor-joining method in ClustalW version 2.1 (57). Visual core genome-based phylogenomic trees were developed using FigTree software (http://tree.bio.ed.ac.uk/software/figtree/).
Glycobiome profiling
The proteome of B. mongoliense strains was screened for genes predicted to encode carbohydrate-active enzymes based on sequence similarity to genes classified in the CAZy database (58). Thus, each gene sequence was screened for orthologues through the dbCAN3 pipeline (59) using HMMER v3.3.2 (cutoff e-value of 1*10−15 and coverage >0.35) against the dbCAN and dbCAN-sub databases and DIAMOND (cutoff e-value <1*10−102) against the CAZy database. Positive results on all three databases were used to predict the glycobiome of each strain. Furthermore, the presence and location of signal peptide cleavage sites in amino acid sequences were identified using the SignalP 4.1 software (60).
Carbohydrate growth assay
In vitro growth assays with different carbon sources were performed on all B. mongoliense strains isolated, including the type strain DSM 21395 and BMONG18. In detail, the strains were cultivated overnight on a semisynthetic MRS medium supplemented with 0.05% (wt/vol) L-cysteine hydrochloride at 37°C under anaerobic conditions (61, 62). Subsequently, cells were diluted in MRS without glucose to obtain an OD600nm = 1, and 15 µL of the diluted cells was inoculated in 135 µL of MRS without glucose supplemented with 1% (wt/vol) of a particular sugar in a 96-well microtiter plate and incubated in an anaerobic cabinet. Specifically, each carbohydrate was dissolved in sterilized MRS without glucose and made sterile using a 0.2-µm filter. Cell growth was evaluated by monitoring the OD at 600 nm using a plate reader (Biotek). After 96 h of growth, each plate was read in discontinuous mode in triplicate. Each reading was ahead of 30 s of shaking at medium speed. Cultures were performed in triplicates for each strain, and the resulting growth data sets were expressed as the average OD600nm of these independent biological replicates. Carbohydrates tested in this study were purchased from Merck and Fisher Scientific, ACROS Organics. The MRS medium without any added carbohydrate was used as the control medium.
Adhesion to HT29-MTX cells
Bifidobacterial adhesion to HT29-MTX cells was assessed following the protocol described by Guglielmetti et al. and Serafini et al. (37, 38). Briefly, human colorectal adenocarcinoma HT29-MTX cells (kindly provided by Prof. A. Baldi, University of Milan) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 µg/mL streptomycin, and 100 U/mL penicillin, and were maintained in standard culture conditions. For the experiments, the HT29-MTX cells were seeded on microscopy cover glasses previously settled into 10 cm2 Petri dishes. Confluent cells were carefully washed twice with PBS before bacterial cells were added. B. mongoliense strains, B. bifidum PRL2010, and B. animalis subsp. lactis Bb-12 were grown as previously described, until a concentration of 5 × 107 CFU mL−1 was reached. The strains were then centrifuged at 3,000 rpm for 8 min, resuspended in PBS (pH 7.3), and incubated with monolayers of the HT29-MTX cells. After a 1-h incubation at 37°C, cultures were washed twice with 2 mL of PBS to remove unbound bacteria. The cells were then fixed with 1 mL of methanol and were incubated for 8 min at room temperature. The cells were then stained with 1.5 mL of Giemsa stain solution (1:20) (Sigma-Aldrich, Milan, Italy) and were left in the dark for 30 min at room temperature. After two washes with 2 mL of PBS, the cover glasses were removed from the Petri plate, mounted on a glass slide, and examined using a phase-contrast microscope Zeiss Axiovert 200 (objective, 100×/1.4 oil). Adherent bacteria in 20 randomly selected microscopic fields were counted and averaged. The proportion of bacterial cells that remained attached to the HT29-MTX monolayer was determined to reflect the extent of specific host–microbe interaction. The adhesion index represents the average number of bacterial cells attached to 100 HT29-MTX cells (37, 40, 41). A nonparametric Kruskal-Wallis test was applied for the detection of statistically significant differences. All assays were performed at least in triplicate.
Mucin adhesion assay
The effect of bifidobacterial adhesion on mucin was assessed by adapting the protocol described by Valeriano et al. (63). Briefly, 100 µL of a 1 mg mL−1 sterile mucin dissolved in PBS (pH 7.4) was aliquoted into a 96-well microtiter plate (Sarstedt, Germany) and was incubated overnight at 4°C. Subsequently, each well was washed with 200 µL of PBS, rinsed, filled with 100 µL of a 20 mg mL−1 sterile bovine serum albumin solution, and incubated at 4°C for 2 h. B. mongoliense strains were grown at 37°C under anaerobic conditions (2.99% H2, 17.01% CO2, and 80% N2) (Concept 400; Ruskin) in MRS broth (Scharlau Chemie, Barcelona, Spain) supplemented with 0.05% (wt/vol) L-cysteine HCl. Bifidobacterial growth was monitored until a concentration of 108 CFU mL−1 was reached. One hundred microliters of a corresponding bacterial suspension, previously washed and resuspended in PBS, was added in each well and incubated under anaerobic conditions at 37°C for 1 h. After incubation, each well was washed three times with 200 µL of PBS to remove unbound bacteria. Then, 200 µL of 0.5% (vol/vol) Triton X-100 was added and incubated at room temperature for 2 h, with gentle agitation to detach the adherent bacteria. The viable cell count expressed as CFU mL−1 was determined in all cases by plating on MRS medium. Each assay was performed in triplicate. Percentage adhesion was calculated as follows:
Quantification of bifidobacterial strains in co-cultivation trials
Cells of each of the following strains, B. mongoliense BMONG18, B. bifidum PRL2010, B. longum PRL2022, B. adolescentis PRL2023, or these strains in bi-association, were inoculated in 6 mL of MRS (without any carbohydrate; Scharlau Chemie, Barcelona, Spain) supplemented with 1% of either HMOs, i.e., 3’-sialyllactose (3′-SL), 2’-fucosyllactose (2′-FL), starch, or mucin (Sigma-Aldrich) as the sole carbon source in triplicates. Cell suspensions were mixed and incubated at 37°C for 24 h under anaerobic conditions. Bacterial cell cultivations were performed in triplicate (biological replicates). Bacterial strain enumerations during the experiments were determined by quantitative real-time PCR (qPCR) and were performed at the beginning (Fig. 4) and at the end of the growth experiments. qPCR experiments were based on a specific primer pair targeting a gene present in a single copy within the genome of B. mongoliense BMONG18, B. bifidum PRL2010, B. longum PRL2022, and B. adolescentis PRL2023 (Table S7). These experiments were performed following the protocol previously described by Turroni et al. (28).
BMONG18 cultivation in a gut- and cheese-simulated environment
B. mongoliense BMONG18 was inoculated in 30 mL of human gut simulating growth medium (GSM), which had previously been described (64), and in the same GSM plus the addition of autoclaved cheese in 2% (wt/vol) proportion. Additional growth assays were performed in a cheese-simulated medium prepared by mixing shaved Parmesan cheese with sodium citrate pH 7.5. The suspension was incubated at 42°C for 50 min, followed by centrifugation at 8,000 rpm for 20 min and filtration through a sterile dressing to remove fats. Subsequently, the medium was autoclaved at 121°C for 20 min. These growth experiments were performed in triplicate. Then, all performed growth assays were incubated under anaerobic conditions at 37°C for 6 h. Finally, cultures were centrifuged at 7,000 rpm for 5 min, and the obtained pellets were then used for RNA extraction. Both pellets and supernatants were stored at −80°C until they were processed.
RNA extraction and sequencing
Total RNA of each considered condition (fecal medium, fecal medium plus autoclaved cheese and cheese broth, in triplicate) was extracted as follows. Briefly, bacterial cell pellets were resuspended in 1 mL of QIAzol lysis reagent (Qiagen, Germany) in a sterile tube containing glass beads (Merk, Germany). Cells were lysed by alternating 2 min of stirring the mix on a Precellys 24 homogenizer (Bertin Instruments, France) with 2 min of static cooling on ice. These steps were repeated three times. The lysed cells were centrifuged at 12,000 rpm for 15 min, and the upper phase was recovered. The RNA samples were then purified using the RNeasy mini kit (Qiagen, Germany) following the manufacturer’s instructions. RNA concentration and purity were evaluated using a spectrophotometer (Eppendorf, Germany). For RNA sequencing, total RNA (from 100 ng to 1 µg) was treated to remove rRNA by using the QIAseq FastSelect following the manufacturer’s instructions (Qiagen, Germany). The rRNA depletion yield was checked using a 2200 TapeStation (Agilent Technologies, USA). Then, a whole transcriptome library was constructed using the TruSeq Stranded mRNA Sample preparation kit (Illumina, San Diego, USA). Samples were loaded into a NextSeq high-output v2 kit (150 cycles) (Illumina) as the technical support guide indicated. The obtained RNA-seq reads were filtered to remove low-quality reads (minimum mean quality 20, minimum length 150 bp) as well as any remaining ribosomal loci using the METAnnotatorX2 pipeline (65). Then, the filtered RNA-seq data were aligned to the BMONG18 genome sequence with BWA (66) software. To quantify gene expression, the number of reads mapping each BMONG18 gene (read count) was computed utilizing HTSeq software (67), operating in union mode.
Statistical analyses
Differences in bacterial abundance at the species level in qPCR experiments, as well as variations in adhesion to mucin and HT29 cells, were evaluated by nonparametric independent-samples Kruskal-Wallis test analysis using IBM SPSS Statistics for Windows. Comparison of count-based expression data across bacterial growth conditions was performed through edgeR package (68). Specifically, after using the counts per million (CPM) function of edgeR to transform raw counts into CPM and log2-counts per million (log-CPM) values, genes with low counts (CPM < 1) in all conditions were excluded. Following that, the trimmed mean of M-values (TMM) method was implemented to normalize the reads count for varying library size (sample-specific effect). In differential gene expression analysis, the log2 ratio was used to describe the average log fold change in gene expression between the reference sample and each test sample. FDR procedure was employed in multiple hypothesis testing to correct for multiple comparisons.
ACKNOWLEDGMENTS
We thank GenProbio Srl for the financial support from the Laboratory of Probiogenomics. Part of this research was conducted at the high-performance computing (HPC) facility of the University of Parma. We also thank Prof. Antonietta Baldi of the University of Milan for kindly providing HT29-MTX human colon carcinoma-derived cells.
G.L., F.F., and M.V. are funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 – Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Award Number: Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods - Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods.” D.V.S/ is a member of The APC Microbiome Institute funded by the Science Foundation Ireland (SFI) through the Irish Government’s National Development Plan (Grant numbers SFI/12/RC/2273a and SFI/12/RC/2273b). The cost of the equipment MALDI‐TOF MS Biotyper Sirius used for this experimental investigation was partly supported by the University of Parma through the Scientific Instrumentation Upgrade Programme 2020.
G.L. performed all experiments and wrote the manuscript; G.A.L. supervised all bioinformatics analyses and wrote the manuscript; C.T. performed bioinformatics analyses related to RNAseq and phylogenetics; F.F. performed bioinformatics analyses related to the pangenome and comparative genomics; E.C. isolated the microbial strains. M.G.B. performed the experiments with human cell lines; O.B. supervised the project for the experiments with human cell lines; D.V.S., F.T., and M.V. designed the study, supervised the project, and edited the manuscript.
Contributor Information
Gabriele Andrea Lugli, Email: gabrieleandrea.lugli@unipr.it.
Edward G. Dudley, The Pennsylvania State University, University Park, Pennsylvania, USA
DATA AVAILABILITY
The genome sequence of B. mongoliense strains was deposited in the GenBank database under the BioProject assigned code PRJNA1092624. Individual accession numbers are reported in Table 1. Raw sequences of transcriptomics experiments are accessible through the SRA study BioProject PRJNA1111329.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.01244-24.
Fig. S1 and S2.
Legends for Fig. S1 and S2.
Tables S1 to S7.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, Gänzle M, Kort R, Pasin G, Pihlanto A, Smid EJ, Hutkins R. 2017. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol 44:94–102. doi: 10.1016/j.copbio.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 2. Marco ML, Sanders ME, Gänzle M, Arrieta MC, Cotter PD, De Vuyst L, Hill C, Holzapfel W, Lebeer S, Merenstein D, Reid G, Wolfe BE, Hutkins R. 2021. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol 18:196–208. doi: 10.1038/s41575-020-00390-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leeuwendaal NK, Stanton C, O’Toole PW, Beresford TP. 2022. Fermented foods, health and the gut microbiome. Nutrients 14:1527. doi: 10.3390/nu14071527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hess JM, Stephensen CB, Kratz M, Bolling BW. 2021. Exploring the links between diet and inflammation: dairy foods as case studies. Adv Nutr 12:1S–13S. doi: 10.1093/advances/nmab108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Klerk JN, Robinson PA. 2022. Drivers and hazards of consumption of unpasteurised bovine milk and milk products in high-income countries. PeerJ 10:e13426. doi: 10.7717/peerj.13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ivey M, Massel M, Phister TG. 2013. Microbial interactions in food fermentations. Annu Rev Food Sci Technol 4:141–162. doi: 10.1146/annurev-food-022811-101219 [DOI] [PubMed] [Google Scholar]
- 7. Mayo B, Rodríguez J, Vázquez L, Flórez AB. 2021. Microbial interactions within the cheese ecosystem and their application to improve quality and safety. Foods 10:602. doi: 10.3390/foods10030602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fontana F, Longhi G, Alessandri G, Lugli GA, Mancabelli L, Tarracchini C, Viappiani A, Anzalone R, Ventura M, Turroni F, Milani C. 2023. Multifactorial microvariability of the Italian raw milk cheese microbiota and implication for current regulatory scheme. mSystems 8:e0106822. doi: 10.1128/msystems.01068-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siebert A, Hofmann K, Staib L, Doll EV, Scherer S, Wenning M. 2021. Amplicon-sequencing of raw milk microbiota: impact of DNA extraction and library-PCR. Appl Microbiol Biotechnol 105:4761–4773. doi: 10.1007/s00253-021-11353-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milani C, Alessandri G, Mancabelli L, Lugli GA, Longhi G, Anzalone R, Viappiani A, Duranti S, Turroni F, Ossiprandi MC, van Sinderen D, Ventura M. 2019. Bifidobacterial distribution across Italian cheeses produced from raw milk. Microorganisms 7:599. doi: 10.3390/microorganisms7120599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delcenserie V, Gavini F, Beerens H, Tresse O, Franssen C, Daube G. 2007. Description of a new species, bifidobacterium crudilactis sp. nov., isolated from raw milk and raw milk cheeses. Syst Appl Microbiol 30:381–389. doi: 10.1016/j.syapm.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 12. Milani C, Duranti S, Napoli S, Alessandri G, Mancabelli L, Anzalone R, Longhi G, Viappiani A, Mangifesta M, Lugli GA, Bernasconi S, Ossiprandi MC, van Sinderen D, Ventura M, Turroni F. 2019. Colonization of the human gut by bovine bacteria present in parmesan cheese. Nat Commun 10:1286. doi: 10.1038/s41467-019-09303-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milani C, Turroni F, Duranti S, Lugli GA, Mancabelli L, Ferrario C, van Sinderen D, Ventura M. 2016. Genomics of the genus bifidobacterium reveals species-specific adaptation to the glycan-rich gut environment. Appl Environ Microbiol 82:980–991. doi: 10.1128/AEM.03500-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bondue P, Milani C, Arnould E, Ventura M, Daube G, LaPointe G, Delcenserie V. 2020. Bifidobacterium mongoliense genome seems particularly adapted to milk oligosaccharide digestion leading to production of antivirulent metabolites. BMC Microbiol 20:111. doi: 10.1186/s12866-020-01804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delcenserie V, Taminiau B, Gavini F, de Schaetzen M-A, Cleenwerck I, Theves M, Mahieu M, Daube G. 2013. Detection and characterization of bifidobacterium crudilactis and B. mongoliense able to grow during the manufacturing process of French raw milk cheeses. BMC Microbiol 13:239. doi: 10.1186/1471-2180-13-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duranti S, Milani C, Lugli GA, Mancabelli L, Turroni F, Ferrario C, Mangifesta M, Viappiani A, Sánchez B, Margolles A, van Sinderen D, Ventura M. 2016. Evaluation of genetic diversity among strains of the human gut commensal Bifidobacterium adolescentis. Sci Rep 6:23971. doi: 10.1038/srep23971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lugli GA, Tarracchini C, Alessandri G, Milani C, Mancabelli L, Turroni F, Neuzil-Bunesova V, Ruiz L, Margolles A, Ventura M. 2020. Decoding the genomic variability among members of the Bifidobacterium dentium species. Microorganisms 8:1720. doi: 10.3390/microorganisms8111720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milani C, Lugli GA, Duranti S, Turroni F, Bottacini F, Mangifesta M, Sanchez B, Viappiani A, Mancabelli L, Taminiau B, Delcenserie V, Barrangou R, Margolles A, van Sinderen D, Ventura M. 2014. Genomic encyclopedia of type srains of the genus Bifidobacterium. Appl Environ Microbiol 80:6290–6302. doi: 10.1128/AEM.02308-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lugli GA, Duranti S, Albert K, Mancabelli L, Napoli S, Viappiani A, Anzalone R, Longhi G, Milani C, Turroni F, Alessandri G, Sela DA, van Sinderen D, Ventura M. 2019. Unveiling genomic diversity among members of the species Bifidobacterium pseudolongum, a widely distributed gut commensal of the animal kingdom. Appl Environ Microbiol 85:e03065-18. doi: 10.1128/AEM.03065-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lugli GA, Milani C, Turroni F, Duranti S, Mancabelli L, Mangifesta M, Ferrario C, Modesto M, Mattarelli P, Jiří K, van Sinderen D, Ventura M. 2017. Comparative genomic and phylogenomic analyses of the bifidobacteriaceae family. BMC Genomics 18:568. doi: 10.1186/s12864-017-3955-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lugli GA, Milani C, Duranti S, Mancabelli L, Mangifesta M, Turroni F, Viappiani A, van Sinderen D, Ventura M. 2018. Tracking the taxonomy of the genus Bifidobacterium based on a phylogenomic approach. Appl Environ Microbiol 84:e02249-17. doi: 10.1128/AEM.02249-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eckel VPL, Vogel RF. 2020. Genomic and physiological insights into the lifestyle of Bifidobacterium species from water kefir. Arch Microbiol 202:1627–1637. doi: 10.1007/s00203-020-01870-7 [DOI] [PubMed] [Google Scholar]
- 23. Eckel VPL, Ziegler LM, Vogel RF, Ehrmann M. 2020. Bifidobacterium tibiigranuli sp. nov. isolated from homemade water kefir. Int J Syst Evol Microbiol 70:1562–1570. doi: 10.1099/ijsem.0.003936 [DOI] [PubMed] [Google Scholar]
- 24. Lugli GA, Alessandri G, Milani C, Viappiani A, Fontana F, Tarracchini C, Mancabelli L, Argentini C, Ruiz L, Margolles A, van Sinderen D, Turroni F, Ventura M. 2021. Genetic insights into the dark matter of the mammalian gut microbiota through targeted genome reconstruction. Environ Microbiol 23:3294–3305. doi: 10.1111/1462-2920.15559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. 2009. The carbohydrate-active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–8. doi: 10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang L, Lin Q, Zhang J, Shi Y, Pan L, Hou Y, Peng X, Li W, Wang J, Zhou P. 2023. Qualitative and quantitative changes of oligosaccharides in human and animal milk over lactation. J Agric Food Chem 71:15553–15568. doi: 10.1021/acs.jafc.3c03181 [DOI] [PubMed] [Google Scholar]
- 27. Egan M, O’Connell Motherway M, Ventura M, van Sinderen D. 2014. Metabolism of sialic acid by Bifidobacterium breve UCC2003. Appl Environ Microbiol 80:4414–4426. doi: 10.1128/AEM.01114-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turroni F, Özcan E, Milani C, Mancabelli L, Viappiani A, van Sinderen D, Sela DA, Ventura M. 2015. Glycan cross-feeding activities between bifidobacteria under in vitro conditions. Front Microbiol 6:1030. doi: 10.3389/fmicb.2015.01030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duranti S, Lugli GA, Milani C, James K, Mancabelli L, Turroni F, Alessandri G, Mangifesta M, Mancino W, Ossiprandi MC, Iori A, Rota C, Gargano G, Bernasconi S, Di Pierro F, van Sinderen D, Ventura M. 2019. Bifidobacterium bifidum and the infant gut microbiota: an intriguing case of microbe-host co-evolution. Environ Microbiol 21:3683–3695. doi: 10.1111/1462-2920.14705 [DOI] [PubMed] [Google Scholar]
- 30. Chassard C, Lacroix C. 2013. Carbohydrates and the human gut microbiota. Curr Opin Clin Nutr Metab Care 16:453–460. doi: 10.1097/MCO.0b013e3283619e63 [DOI] [PubMed] [Google Scholar]
- 31. Díaz R, Garrido D. 2024. Screening competition and cross-feeding interactions during utilization of human milk oligosaccharides by gut microbes. Microbiome Res Rep 3:12. doi: 10.20517/mrr.2023.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Górska-Warsewicz H, Rejman K, Laskowski W, Czeczotko M. 2019. Milk and dairy products and their nutritional contribution to the average polish diet. Nutrients 11:1771. doi: 10.3390/nu11081771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auestad N, Layman DK. 2021. Dairy bioactive proteins and peptides: a narrative review. Nutr Rev 79:36–47. doi: 10.1093/nutrit/nuab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martí i Líndez A-A, Reith W. 2021. Arginine-dependent immune responses. Cell Mol Life Sci 78:5303–5324. doi: 10.1007/s00018-021-03828-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. 2009. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168. doi: 10.1007/s00726-008-0210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rautava S, Walker WA. 2007. Commensal bacteria and epithelial cross talk in the developing intestine. Curr Gastroenterol Rep 9:385–392. doi: 10.1007/s11894-007-0047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guglielmetti S, Tamagnini I, Mora D, Minuzzo M, Scarafoni A, Arioli S, Hellman J, Karp M, Parini C. 2008. Implication of an outer surface lipoprotein in adhesion of Bifidobacterium bifidum to Caco-2 cells. Appl Environ Microbiol 74:4695–4702. doi: 10.1128/AEM.00124-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Serafini F, Strati F, Ruas-Madiedo P, Turroni F, Foroni E, Duranti S, Milano F, Perotti A, Viappiani A, Guglielmetti S, Buschini A, Margolles A, van Sinderen D, Ventura M. 2013. Evaluation of adhesion properties and antibacterial activities of the infant gut commensal Bifidobacterium bifidum PRL2010. Anaerobe 21:9–17. doi: 10.1016/j.anaerobe.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 39. Vazquez-Gutierrez P, de Wouters T, Werder J, Chassard C, Lacroix C. 2016. High iron-sequestrating bifidobacteria inhibit enteropathogen growth and sdhesion to intestinal epithelial cells In vitro Front Microbiol 7:1480. doi: 10.3389/fmicb.2016.01480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rizzo SM, Alessandri G, Lugli GA, Fontana F, Tarracchini C, Mancabelli L, Viappiani A, Bianchi MG, Bussolati O, van Sinderen D, Ventura M, Turroni F. 2023. Exploring molecular interactions between human Milk hormone insulin and bifidobacteria. Microbiol Spectr 11:e0066523. doi: 10.1128/spectrum.00665-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turroni F, Serafini F, Foroni E, Duranti S, O’Connell Motherway M, Taverniti V, Mangifesta M, Milani C, Viappiani A, Roversi T, Sánchez B, Santoni A, Gioiosa L, Ferrarini A, Delledonne M, Margolles A, Piazza L, Palanza P, Bolchi A, Guglielmetti S, van Sinderen D, Ventura M. 2013. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium–host interactions. Proc Natl Acad Sci U S A 110:11151–11156. doi: 10.1073/pnas.1303897110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulthess B, Bloemberg GV, Zbinden R, Böttger EC, Hombach M. 2014. Evaluation of the bruker MALDI biotyper for identification of gram-positive rods: development of a diagnostic algorithm for the clinical laboratory. J Clin Microbiol 52:1089–1097. doi: 10.1128/JCM.02399-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Werner G, Fleige C, Fessler AT, Timke M, Kostrzewa M, Zischka M, Peters T, Kaspar H, Schwarz S. 2012. Improved identification including MALDI-TOF mass spectrometry analysis of group D streptococci from bovine mastitis and subsequent molecular characterization of corresponding enterococcus faecalis and enterococcus faecium isolates. Vet Microbiol 160:162–169. doi: 10.1016/j.vetmic.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 44. Lugli GA, Fontana F, Tarracchini C, Milani C, Mancabelli L, Turroni F, Ventura M. 2023. MEGAnnotator2: a pipeline for the assembly and annotation of microbial genomes. Microbiome Res Rep 2:15. doi: 10.20517/mrr.2022.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lugli GA, Milani C, Mancabelli L, van Sinderen D, Ventura M. 2016. MEGAnnotator: a user-friendly pipeline for microbial genomes assembly and annotation. FEMS Microbiol Lett 363:fnw049. doi: 10.1093/femsle/fnw049 [DOI] [PubMed] [Google Scholar]
- 46. Prjibelski A, Antipov D, Meleshko D, Lapidus A, Korobeynikov A. 2020. Using SPAdes De Novo assembler. Curr Protoc Bioinformatics 70:e102. doi: 10.1002/cpbi.102 [DOI] [PubMed] [Google Scholar]
- 47. Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buchfink B, Reuter K, Drost HG. 2021. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat Methods 18:366–368. doi: 10.1038/s41592-021-01101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fu L, Niu B, Zhu Z, Wu S, Li W. 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28:3150–3152. doi: 10.1093/bioinformatics/bts565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong SY, Lopez R, Hunter S. 2014. InterProScan 5: genome-scale protein function classification. Bioinformatics 30:1236–1240. doi: 10.1093/bioinformatics/btu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chan PP, Lin BY, Mak AJ, Lowe TM. 2021. tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res 49:9077–9096. doi: 10.1093/nar/gkab688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. doi: 10.1038/s41467-018-07641-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao Y, Wu J, Yang J, Sun S, Xiao J, Yu J. 2012. PGAP: pan-genomes analysis pipeline. Bioinformatics 28:416–418. doi: 10.1093/bioinformatics/btr655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tarracchini C, Lugli GA, Mancabelli L, Milani C, Turroni F, Ventura M. 2020. Assessing the genomic variability of gardnerella vaginalis through comparative genomic analyses: evolutionary and ecological implications. Appl Environ Microbiol 87:e02188-20. doi: 10.1128/AEM.02188-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. doi: 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 58. Drula E, Garron ML, Dogan S, Lombard V, Henrissat B, Terrapon N. 2022. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res 50:D571–D577. doi: 10.1093/nar/gkab1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng J, Ge Q, Yan Y, Zhang X, Huang L, Yin Y. 2023. dbCAN3: automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res 51:W115–W121. doi: 10.1093/nar/gkad328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 61. Argentini C, Lugli GA, Tarracchini C, Fontana F, Mancabelli L, Viappiani A, Anzalone R, Angelini L, Alessandri G, Longhi G, Bianchi MG, Taurino G, Bussolati O, Milani C, van Sinderen D, Turroni F, Ventura M. 2024. Genomic and ecological approaches to identify the Bifidobacterium breve prototype of the healthy human gut microbiota. Front Microbiol 15:1349391. doi: 10.3389/fmicb.2024.1349391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Argentini C, Lugli GA, Tarracchini C, Fontana F, Mancabelli L, Viappiani A, Anzalone R, Angelini L, Alessandri G, Bianchi MG, Taurino G, Bussolati O, Milani C, van Sinderen D, Turroni F, Ventura M. 2024. Ecology- and genome-based identification of the Bifidobacterium adolescentis prototype of the healthy human gut microbiota. Appl Environ Microbiol 90:e0201423. doi: 10.1128/aem.02014-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Valeriano VD, Parungao-Balolong MM, Kang DK. 2014. In vitro evaluation of the mucin-adhesion ability and probiotic potential of lactobacillus mucosae LM1. J Appl Microbiol 117:485–497. doi: 10.1111/jam.12539 [DOI] [PubMed] [Google Scholar]
- 64. Macfarlane GT, Macfarlane S, Gibson GR. 1998. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb Ecol 35:180–187. doi: 10.1007/s002489900072 [DOI] [PubMed] [Google Scholar]
- 65. Milani C, Lugli GA, Fontana F, Mancabelli L, Alessandri G, Longhi G, Anzalone R, Viappiani A, Turroni F, Sinderen D, Ventura M. 2021. METAnnotatorX2: a comprehensive tool for deep and shallow metagenomic data set analyses. mSystems. doi: 10.1128/mSystems.00583-21:e0058321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li H, Durbin R. 2009. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anders S, Pyl PT, Huber W. 2015. HTSeq--a python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2.
Legends for Fig. S1 and S2.
Tables S1 to S7.
Data Availability Statement
The genome sequence of B. mongoliense strains was deposited in the GenBank database under the BioProject assigned code PRJNA1092624. Individual accession numbers are reported in Table 1. Raw sequences of transcriptomics experiments are accessible through the SRA study BioProject PRJNA1111329.