Abstract
Background:
Several case reports and a few studies have reported that hypothyroid patients have elevated serum potassium levels. However, hypothyroidism has not been widely accepted as a cause of hyperkalemia.
Objectives:
This study aims to evaluate the incidence of hyperkalemia and factors influencing serum potassium levels in thyroid cancer patients with hypothyroidism during thyroid hormone withdrawal before radioactive iodine (RAI) treatment.
Methods:
We conducted a retrospective review of electronic medical records from January 2017 to June 2021, involving 956 thyroid cancer patients post-thyroidectomy and undergoing RAI. Laboratory parameters, including serum potassium levels, were collected in both euthyroid (<1 year prior to RAI) and hypothyroid states.
Results:
Among 508 patients (mean age 52 years, 79.3% female), hyperkalemia (potassium ⩾ 5.0 mEq/L) occurred in 2.8%, without severe hyperkalemia (potassium ⩾ 6.5 mEq/L). The hypothyroid state exhibited significantly higher serum potassium than the euthyroid state [4.16 (IQR, 3.94-4.41) vs 4.10 (IQR, 3.90-4.35) mEq/L, P < .01]. The mean change in potassium levels between the euthyroid and hypothyroid state was 0.05 ± 0.17 mEq/L. Pre-thyroid hormone withdrawal (euthyroid state) factors associated with serum potassium levels in the hypothyroid state included age, use of angiotensin-converting enzyme inhibitors, diabetes mellitus, serum BUN/creatinine, serum potassium levels, hemoglobin A1c (positive correlation); and thiazide use and eGFR (negative correlation). In the hypothyroid state, hyperkalemia was more likely in patients with serum potassium ⩾4.2 mEq/L (OR 9.36, P < .01) or free T4 ⩾1.38 ng/dL (OR 7.05, P < .01) during the euthyroid state.
Conclusions:
The incidence of hyperkalemia was low in our hypothyroid cohorts. However, physicians should remain vigilant for cases with risk factors for developing hyperkalemia.
Keywords: Electrolyte, iodine radioisotopes, hyperkalemia, hypothyroidism, thyroid neoplasms, water-electrolyte imbalance
Plain Language Summary:
Serum Potassium in Hypothyroid Patients
A retrospective cohort study of thyroid cancer patients undergoing radioactive iodine therapy found a low incidence of hyperkalemia (2.8%) during hypothyroidism. Patients exhibited higher serum potassium levels, influenced by factors included age, medication use (ACEI), diabetes mellitus, and initial potassium levels. Elevated initial potassium and thyroid hormone levels were associated with higher risk of hypothyroid-related hyperkalemia. Physicians should remain vigilant for hyperkalemia in these patients, particularly those with predisposing factors, warranting further mechanistic studies and broader validations.
Introduction
Thyroid hormones play a pivotal role in renal development and physiology, affecting kidney structure, renal hemodynamics, glomerular filtration rate (GFR), tubular function, renal water and electrolyte handling, as well as the renin-angiotensin-aldosterone system (RAAS). 1 Hyponatremia is a common electrolyte disturbance in clinically hypothyroid patients. In acute hypothyroidism, observed in patients with differentiated thyroid cancer undergoing thyroid hormone withdrawal for radioactive iodine (RAI) therapy, it is primarily attributed to reduced GFR, resulting in diminished water delivery to the distal tubular segments. In chronic hypothyroidism, the mechanism involves decreased free water excretion due to elevated antidiuretic hormone (ADH) levels. 2 On the contrary, patients with thyrotoxicosis have reported experiencing hypomagnesemia and hypercalcemia.3,4 Thyrotoxic hypokalemic periodic paralysis is an unusual complication of thyrotoxicosis due to a massive intracellular shift of potassium. 5
Hypothyroidism has not been recognized as a contributing factor to hyperkalemia. 6 However, emerging evidence highlight the association between hypothyroidism and hyperkalemia. Elevated serum potassium levels have been reported in chronic hypothyroidism in dogs 7 and in patients undergoing thyroid hormone withdrawal.8 -11 A previous retrospective study reported that patients undergoing thyroid hormone withdrawal exhibited elevated serum potassium levels, with some experiencing severe hyperkalemia and abnormal electrocardiogram findings necessitating potassium-lowering therapy. 9
Because of the limited available data regarding the connection between hypothyroidism and hyperkalemia, this study aims to determine the incidence of hyperkalemia and identify the factors affecting serum potassium levels in thyroid cancer patients experiencing hypothyroidism due to thyroid hormone withdrawal before undergoing RAI treatment.
Material and Methods
Study population
In this retrospective cohort study, we recruited individuals aged 18 years and older diagnosed with thyroid cancer who had undergone near-total or total thyroidectomy and underwent thyroid hormone withdrawal as part of the preparation for RAI therapy between January 2017 and June 2021 at Ramathibodi Hospital. Elevating endogenous thyroid-stimulating hormone (TSH) levels involves a 3 to 4 week withdrawal of levothyroxine to reach an optimal TSH level (⩾30 mIU/L), enhancing the efficacy of RAI treatment.
Exclusions comprised patients with chronic kidney disease stages 4 to 5, diabetes mellitus with a hemoglobin A1c exceeding 9%, those facing terminal illness, and individuals lacking complete laboratory data on serum potassium and TSH levels. These exclusion were made because the prevalence of hyperkalemia is known to increase in patients with CKD, particularly in stages 4 to 5,12,13 and serum potassium is more likely to increase in individuals with poorly controlled diabetes mellitus.14,15
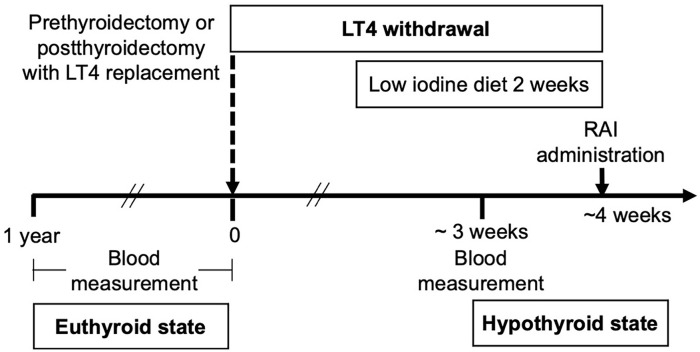
By reviewing electronic medical records, we collected demographic data, medical history, and laboratory parameters in both euthyroid state and hypothyroid states. Patients undergoing RAI therapy at our hospital followed the local standard protocol illustrated in Figure 1. Blood samples were first collected during the euthyroid state (within 1 year before RAI) and subsequently during the hypothyroid state after thyroid hormone withdrawal. None of the blood samples exhibited hemolysis. This study was approved by the Human Research Ethics Committee of the Faculty of Medicine Ramathibodi Hospital, Mahidol University (MURA2021/549). The requirement for written informed consent was waived by the ethics committee due to the retrospective nature of the study.
Figure 1.
The standard protocol of thyroid hormone withdrawal for thyroid cancer patients who have undergone radioactive iodine therapy at our hospital.
Abbreviations: LT4, levothyroxine; RAI, radioactive iodine.
Outcomes
The primary outcome assessed the incidence of hyperkalemia, defined as a serum potassium level equal to or exceeding 5.0 mEq/L, in thyroid cancer patients experiencing hypothyroidism due to thyroid hormone withdrawal before receiving RAI treatment. Severe hyperkalemia was defined as serum potassium ⩾6.5 mEq/L.
The secondary outcomes included factors influencing serum potassium levels in a hypothyroid state, including renal function, other electrolytes, and liver function tests in both hypothyroid and euthyroid states. Estimated GFR (eGFR) was calculated using the CKD-EPI equation. The factors affecting hyperkalemia and the increase in serum potassium levels by 0.5 mEq/L or more in patients in a hypothyroid state compared with a euthyroid state (Delta K+) were also investigated.
Laboratory measurement
Reference ranges of laboratory parameters according to the manufacturers are followed: blood urea nitrogen (BUN), 9 to 20 mg/dL (male), 7 to 18 mg/dL (female); creatinine, 0.73 to 1.18 mg/dL (male), 0.55 to 1.02 mg/dL (female); sodium, 136 to 145 mEq/L; potassium, 3.5 to 5.1 mEq/L; TSH, 0.35 to 4.94 mIU/L; free triiodothyronine (T3), 1.58 to 3.91 pg/mL; free thyroxine (T4), 0.70 to 1.48 ng/dL; aspartate aminotransferase (AST), 5 to 34 U/L; alanine aminotransferase (ALT), 0 to 55 U/L; alkaline phosphatase, 40 to 150 U/L; gamma-glutamyl transferase, <38 U/L.
Statistical analyses
We determined the sample size based on a previous study, 9 where 4.6% of thyroidectomized patients with hypothyroidism from thyroid hormone withdrawal before RAI therapy experienced hyperkalemia. To achieve 80% power, this study required a minimum of 117 participants, with a significance level set at P-value < .05. Consequently, our goal is to collect data from at least 900 patients, considering the potential for incomplete data due to the retrospective nature of the study and the annual count of approximately 200 thyroid cancer patients undergoing RAI therapy at our hospital.
The incidence of hyperkalemia was expressed as a percentage. Normally distributed continuous data were presented as mean ± SD and analyzed using a paired t-test. Non-normally distributed continuous data were presented as medians with interquartile ranges (IQRs) and analyzed using a Wilcoxon signed-rank test. Correlations between continuous variables were determined through a mixed-effects model and Spearman’s rank correlation. Logistic regression analysis was performed to evaluate risk factors for odds ratio calculation, with 95% confidence intervals (CI). All statistical analyses were performed using Stata version 17 (College Station, TX: StataCorp LLC).
Results
During the study period, 956 thyroid cancer patients who had undergone thyroidectomy received RAI therapy. We excluded 448 patients, as shown in Figure 2. The main reason for incomplete data (428 patients) was the absence of laboratory records in a euthyroid state, as many patients were referred to our hospital for RAI therapy already developing hypothyroidism, with no available prior laboratory data.
Figure 2.
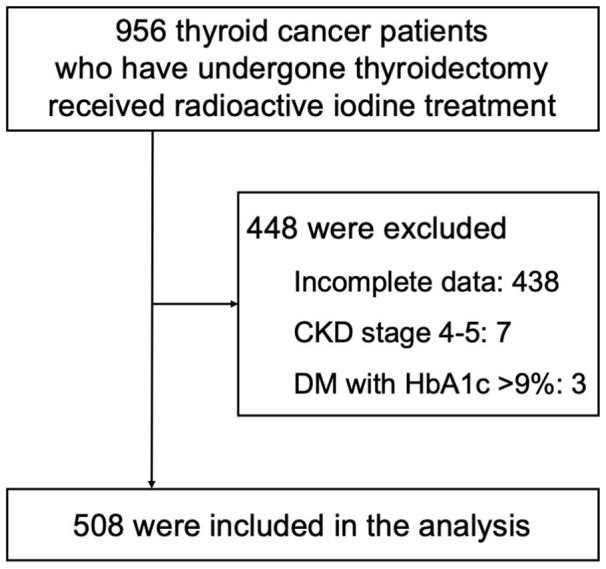
Flow of study participants.
Abbreviations: CKD, chronic kidney disease; DM, diabetes mellitus; HbA1c, hemoglobin A1c.
The analysis included a cohort of 508 patients, with a mean age 52.0 ± 14.6 years. Among them, 79.3% were female, 35.4% had hypertension, 17.3% had diabetes mellitus, and 17.1% were taking RAAS inhibitors including angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). Additional characteristics are detailed in Table 1.
Table 1.
Baseline characteristics.
| Baseline characteristics | N = 508 |
|---|---|
| Age, years | 52.0 ± 14.6 |
| Female, no. (%) | 403 (79.3%) |
| Comorbidities, no. (%) | |
| Cerebrovascular disease | 12 (2.4%) |
| Diabetes mellitus | 88 (17.3%) |
| Hypertension | 180 (35.4%) |
| Ischemic heart disease | 10 (2.0%) |
| eGFR 45-59 mL/min/1.73 m 2 | 16 (3.2%) |
| Concomitant medications, no. (%) | |
| Insulin | 5 (1.0%) |
| ACEI | 19 (3.7%) |
| ARB | 68 (13.4%) |
| Thiazide | 16 (3.2%) |
| Potassium-sparing diuretic | 3 (0.6%) |
| Loop diuretic | 3 (0.6%) |
| Non-selective beta-blocker | 4 (0.8%) |
| Beta-1 selective blocker | 41 (8.0%) |
| Beta-blocker with alpha-blocking activity | 5 (1.0%) |
Abbreviations: ACEIs, angiotension-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate.
Primary outcome: Incidence of hyperkalemia
Fourteen patients (2.8% of 508 patients) developed hyperkalemia, and none of them developed severe hyperkalemia. Among these 14 patients, the serum potassium level in a hypothyroid state was significantly higher than in the euthyroid state (5.18 ± 0.15 vs 4.43 ± 0.32 mEq/L, P < .001), with a difference in serum potassium levels between the hypothyroid and euthyroid states (Delta K+) of 0.74 ± 0.32 mEq/L. Details for these 14 patients are provided in Table 2. No precipitating factors contributing to hyperkalemia were identified. After reviewing the data following the resumption of thyroid hormone usage in these patients, only 5 individuals had available laboratory data. It was observed that all patients returned to normokalemia, and their renal function also returned to baseline, as shown in Table 2.
Table 2.
Details of 14 patients who developed hyperkalemia (serum potassium ⩾ 5.0 mEq/L).
| No. | Age | Sex | Euthyroid State (Pre RAI) | Delta K+ | Hypothyroid State (TH Withdrawal) | Euthyroid State (Post RAI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Cr | eGFR | K+ | Cr | eGFR | TSH | K+ | Cr | eGFR | Drugs | ||||
| 1 | 65 | F | 4.35 | 0.61 | 96.5 | 0.65 | 5.0 | 0.88 | 70.9 | 45.4 | - | - | - | MFM, DPP4i |
| 2 | 37 | F | 4.98 | 0.63 | 115.4 | 0.04 | 5.02 | 0.79 | 96.3 | 12.9 | - | - | - | - |
| 3 | 67 | F | 4.24 | 0.57 | 96.6 | 0.78 | 5.02 | 0.91 | 65.7 | 17.9 | - | - | - | - |
| 4 | 45 | M | 4.52 | 0.86 | 105.5 | 0.52 | 5.04 | 1.14 | 77.8 | 64.0 | - | - | - | MFM, SU |
| 5 | 56 | F | 4.41 | 0.69 | 98.7 | 0.66 | 5.07 | 1.04 | 60.4 | 26.5 | 4.31 | 0.68 | 97.8 | - |
| 6 | 66 | F | 3.84 | 0.81 | 76.2 | 1.26 | 5.1 | 1.03 | 57 | 61.1 | 4.63 | 0.79 | 78 | ARBs |
| 7 | 29 | F | 4.65 | 0.6 | 123.2 | 0.49 | 5.14 | 0.92 | 84.1 | 98.9 | - | - | - | - |
| 8 | 86 | F | 4.29 | 1.16 | 42.6 | 0.87 | 5.16 | 1.31 | 36.8 | 100 | - | - | - | - |
| 9 | 69 | M | 4.28 | 0.93 | 83.5 | 0.93 | 5.21 | 1.44 | 49.2 | 77.9 | 4.66 | 0.88 | 87.6 | - |
| 10 | 38 | F | 3.94 | 0.59 | 117.1 | 1.28 | 5.22 | 0.81 | 92.1 | 61.2 | - | - | - | - |
| 11 | 60 | F | 4.48 | 0.64 | 97 | 0.78 | 5.26 | 0.73 | 89.5 | 100 | 4.96 | 0.62 | 96.6 | - |
| 12 | 50 | F | 4.46 | 0.54 | 114 | 0.90 | 5.36 | 0.69 | 104.4 | 78.7 | - | - | - | MFM, SU, ARB, CCB |
| 13 | 72 | M | 4.75 | 0.98 | 77.3 | 0.66 | 5.41 | 1.44 | 48.5 | 42.4 | 4.95 | 1.05 | 70.6 | CCB |
| 14 | 58 | F | 4.89 | 0.67 | 97.6 | 0.56 | 5.45 | 0.82 | 78.9 | 52.2 | - | - | - | - |
| Summary | 57 ± 16 | M:F = 3:11 | 4.43 ± 0.32 | 0.73 ± 0.19 | 95.8 ± 21.0 | 0.74 ± 0.32 | 5.18 ± 0.15 | 1.00 ± 0.25 | 72.3 ± 20.2 | 59.9 ± 29.2 | ||||
Abbreviations: ARBs, angiotensin receptor blocker; CCB, calcium channel blocker; Cr, creatinine; DPP4i, dipeptidyl peptidase-4 inhibitor; eGFR, estimated glomerular filtration rate; F, female; K+, potassium; M, male; MFM, metformin; No., number; RAI, radioactive iodine; SU, sulfonylurea; TH, thyroid hormone; TSH, thyroid stimulating hormone.
Units for each parameter: age, years; Cr, mg/dL; eGFR, mL/min/1.73 m 2 ; K+, mEq/L; TSH, mIU/L.
Delta K+ is a difference in serum potassium levels between the hypothyroid and euthyroid states.
Secondary outcomes
Laboratory parameters: Euthyroid state versus hypothyroid state
When comparing the laboratory parameters of all patients in the euthyroid and hypothyroid states, we observed that the serum potassium levels were significantly higher in the hypothyroid state compared to the euthyroid state: 4.16 (IQR, 3.94-4.41) vs 4.10 (IQR, 3.90-4.35) mEq/L; P < .01. The mean Delta K+, was 0.047 ± 0.173 mEq/L. Furthermore, we found that the eGFR, serum sodium, and serum alkaline phosphatase were all significantly lower in the hypothyroid state than in the euthyroid state. In contrast, in a hypothyroid state, serum levels of creatinine, AST, and ALT were all significantly higher (Table 3).
Table 3.
Comparison of laboratory parameters of all patients in the euthyroid state and the hypothyroid state (N = 508).
| Laboratory Parameters | Euthyroid | Hypothyroid | P-value |
|---|---|---|---|
| BUN (mg/dL) | 11 (IQR 9-14) | 11 (IQR 9-13) | <.001 |
| Creatinine (mg/dL) | 0.68 (IQR 0.6-0.81) | 0.90 (IQR 0.79-1.09) | <.001 |
| eGFR (mL/min/1.73m2) | 98.7 (IQR 86.8-111.7) | 76.7 (IQR 63.2-90) | <.001 |
| Sodium (mEq/L) | 140.3 (IQR 139-142) | 139.5 (IQR 138-141) | <.001 |
| Potassium (mEq/L) | 4.10 (IQR 3.9-4.35) | 4.16 (IQR 3.94-4.405) | <.01 |
| TSH (mIU/L) | 1.21 (IQR 0.39-2.00) | 75.1 (IQR 53.617-100) | <.001 |
| Free T3 (pg/mL) | 2.47 (IQR 2.17-2.77) | N/A | N/A |
| Free T4 (ng/mL) | 1.04 (IQR 0.94-1.18) | N/A | N/A |
| Hemoglobin A1c (%) | 5.83 (IQR 5.5-6.54) | 5.95 (IQR 5.655-6.55) | .4863 |
| Fasting plasma glucose (mg/dL) | 96 (IQR 90-105) | 101 (IQR 95-111) | .9498 |
| Aspartate aminotransferase (U/L) | 23 (IQR 20-28) | 38 (IQR 28-52) | <.001 |
| Alanine aminotransferase (U/L) | 21 (IQR 15-30) | 32 (IQR 21-53) | <.001 |
| Alkaline phosphatase (U/L) | 69.5 (IQR 56-82) | 65.0 (IQR 52-78) | <.001 |
| Gamma-glutamyl transferase (U/L) | 21 (IQR 15-36) | 23 (IQR 16-39) | <.001 |
Abbreviations: BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine.
Correlations between serum potassium levels and other parameters in the hypothyroid state
In a hypothyroid state, serum potassium levels showed significant correlations with age (r = .174; P < .01), particularly in individuals aged 60 years or older (r = .128; P < .01). Additionally, positive correlations were observed with, hemoglobin A1c (r = .361, P < .01), diabetes mellitus (r = .130, P < .01), use of ACEIs (r = .123, P < .01), serum creatinine (r = .118, P < .01), metformin use (r = .114, P < .01), and serum BUN (r = .015, P < .01). Conversely, there were negative correlations with the use of thiazide (r = −.122, P < .01) and eGFR (r = −.161, P < .01).
Pre-thyroid hormone withdrawal factors predicting serum potassium levels during the hypothyroid state
After we analyzed data by a mixed-effects model, we observed the relationship between serum potassium levels during the hypothyroid state and pre-thyroid hormone withdrawal factors. The coefficient estimates for serum potassium were 0.047 (95%CI, 0.013-0.081; P < .005), serum creatinine 0.175 (95%CI, 0.055-0.300; P < .005), serum BUN 0.012 (95%CI, 0.006-0.018; P < .001), hemoglobin A1c 0.133 (95%CI, 0.065-0.201; P < .001), age 0.003 (95%CI, 0.001-0.005; P < .005), use of ACEIs 0.162 (95%CI, 0.017-0.308; P < .05), and use of thiazide −0.340 (95%CI, −0.496 to −0.184; P < .001).
Factors influencing Delta K+ ⩾0.5 mEq/L
The serum potassium levels in the euthyroid state (OR 0.13, P < .001) and patient’s age (OR 1.02, P < .05) were identified as independent factors associated with Delta K+ 0.5 mEq/L.
Pre-thyroid hormone withdrawal factors influencing hyperkalemia
We analyzed the clinical parameters of patients who developed hyperkalemia and found that serum potassium levels in a euthyroid state (OR 7.90, P < .01) and serum free T4 levels in a euthyroid state (OR 4.43, P < .05) were independent factors associated with the development of hyperkalemia during hypothyroidism.
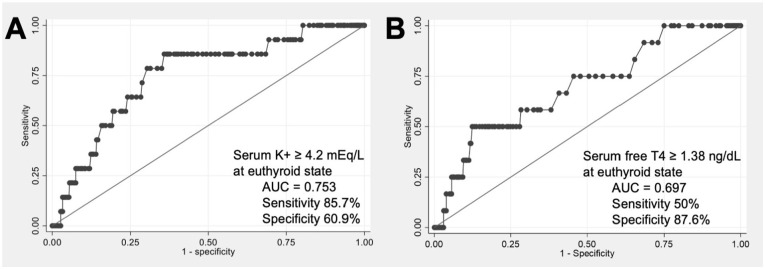
Using receiver operating characteristic (ROC) curve analysis demonstrated in Figure 3, we identified that a serum potassium level in the euthyroid state ⩾4.2 mEq/L exhibits 85.7% sensitivity and 60.9% specificity in predicting hyperkalemia (AUC = 0.753). Additionally, serum free T4 levels in the euthyroid state ⩾1.38 ng/dL demonstrated 50% sensitivity and 87.6% specificity for predicting hyperkalemia (AUC = 0.697).
Figure 3.
ROC of serum potassium (A) and free T4 levels (B) predicting the development of hyperkalemia during hypothyroidism.
Discussion
In this study, despite observing significantly higher serum potassium levels in the hypothyroid state compared to the euthyroid state, we found a very low incidence of hyperkalemia (2.8%) in thyroid cancer patients with hypothyroidism resulting from thyroid hormone withdrawal before RAI treatment, and severe hyperkalemia did not occur. There was a robust correlation between hyperkalemia and both serum potassium (⩾4.2 mEq/L) and free T4 levels (⩾1.38 ng/dL) in a euthyroid state. Furthermore, various factors were associated with the serum potassium levels in the hypothyroid state, including positive correlations with serum potassium levels during the euthyroid state, serum BUN/creatinine, hemoglobin A1c in both euthyroid and hypothyroid states, diabetes mellitus, metformin use, use of ACEIs, and age. Additionally, there was a negative correlation with the use of thiazide and eGFR.
The incidence of hyperkalemia in our study (2.8%) was lower than in previous studies (4.3%-12%).9 -11 This discrepancy can be attributed to the higher eGFR in our cohorts, which averaged 98.8 mL/min/1.73 m 2 . Hyperkalemia may result from impaired renal function, as the eGFR has been demonstrated to have a nearly linear relationship with hyperkalemia. 16 The observed variability in the incidence of hyperkalemia is associated with eGFR levels, as evidenced by rates of 4.3% at a mean eGFR of 75.0, 10 4.6% at a mean eGFR of 77.6, 9 and 12% at a mean eGFR of 56.5 mL/min/1.73 m 2 . 11
Hypothyroidism reduces GFR and renal plasma flow.17,18 Elevated serum creatinine levels can appear within as little as 2 weeks of significant hypothyroidism, with a rapid normalization typically observed following levothyroxine administration in acute hypothyroidism cases. 19 These GFR alterations were validated using exogenous GFR markers, specifically 51Cr-EDTA clearance.20,21 Another mechanism of hypothyroidism-induced hyperkalemia involves the RAAS. Reports have suggested a decrease in RAAS associated with hypothyroidism,10,22 -26 although there is currently no evidence of a change in aldosterone levels. Since RAAS stimulates the function of Na+-K+-ATPase pump function, reduced RAAS activity may decrease pump activity, potentially causing hyperkalemia. Studies in both animals and humans have reported reduced concentrations of Na+-K+-ATPase pumps during hypothyroidism.7,27 -29
To caution against hyperkalemia during the hypothyroid state, factors to be considered align with traditional contributors to hyperkalemia, including serum potassium, renal function, old age, diabetes mellitus, and the use of ACEIs/ARBs.6,30 High serum potassium levels in the euthyroid phase appear appears straightforwardly predisposing to hyperkalemia during the hypothyroid phase. However, we identified interesting factors, such as metformin use and high normal levels of serum free T4 in the euthyroid state. Since metformin has not been directly associated with hyperkalemia, it may act as a confounding factor in patients with diabetes mellitus. The causative role of the rapid decline in thyroid hormone levels after thyroid hormone withdrawal in inducing higher serum potassium remains uncertain. The author hypothesizes that thyroid hormone stimulates Na+-K+-ATPase pump activity, promoting potassium influx into cells.29,31 A rapid transition from euthyroid to hypothyroid states may unmask hyperkalemia due to reduced Na+-K+-ATPase activity, potentially explaining why patients with higher free T4 levels during the euthyroid state may experience more hyperkalemia in the hypothyroid phase.
Consistent with previous findings,32,33 our study revealed hyponatremia following thyroid hormone withdrawal, with a mean reduction in serum sodium of 0.91 mEq/L. None of the participants developed severe hyponatremia (serum sodium <125 mEq/L). Additionally, significant elevations in liver enzymes were observed during the hypothyroid state, consistent with findings from earlier studies.34 -36
The strength of this study is the inclusion of the largest number of participants to date focusing on serum potassium levels in the hypothyroid state compared with the euthyroid state. Our study has several limitations. It was a retrospective study, leading to inevitable missing data and uncontrolled confounding factors. The exclusion of a significant number of patients due to missing euthyroid state data potentially biases the results. The study population consisted only of thyroid cancer patients, with a predominance of female participants. In some patients, TSH levels did not reach the target of >30 mIU/L. In addition, the blood sampling times in the euthyroid state varied for each patient. Future prospective research in the general population could offer more insights into the association between hypothyroidism and hyperkalemia. Additionally, further studies on mechanisms are needed for comprehensive understanding.
Conclusion
The incidence of hyperkalemia was low in hypothyroid patients in our cohorts. However, physician need to be cautious regarding the potential occurrence of hyperkalemia during the hypothyroid state, especially in patients with risk factors for developing hyperkalemia. During the preparation of RAI treatment, it is necessary to assess these risk factors including high serum potassium levels, impaired renal function, old age, diabetes mellitus, and the use of ACEIs/ARBs. Future research should focus on mechanistic studies or broader population studies to validate these findings.
Acknowledgments
The authors thank Dr. Kunlawat Thadanipon and Nattacha Chumsunthorn for their outstanding support in statistical analysis. The abstract was presented at the 45th Annual Meeting of the European Thyroid Association, in Milan, Italy.
Footnotes
ORCID iDs: Wichana Chamroonrat  https://orcid.org/0000-0002-8781-1265
https://orcid.org/0000-0002-8781-1265
Chutintorn Sriphrapradang  https://orcid.org/0000-0001-8294-8601
https://orcid.org/0000-0001-8294-8601
Declarations
Ethics Approval and Consent to Participate: This study was approved by the Human Research Ethics Committee of the Faculty of Medicine Ramathibodi Hospital, Mahidol University (MURA2021/549). The requirement for written informed consent was waived by the ethics committee due to the retrospective nature of the study. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent for Publication: Not applicable
Author contributions: P.T. and C.S. designed the study. All authors collected and analyzed the data. The first draft of manuscript was written by P.T. All authors read and approved the final manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials: Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Narasaki Y, Sohn P, Rhee CM. The interplay between thyroid dysfunction and kidney disease. Semin Nephrol. 2021;41:133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liamis G, Filippatos TD, Liontos A, Elisaf MS. Management of endocrine disease: hypothyroidism-associated hyponatremia: mechanisms, implications and treatment. Eur J Endocrinol. 2017;176:R15-R20. [DOI] [PubMed] [Google Scholar]
- 3. Benker G, Breuer N, Windeck R, Reinwein D. Calcium metabolism in thyroid disease. J Endocrinol Invest. 1988;11:61-69. [DOI] [PubMed] [Google Scholar]
- 4. Wuttke H, Kessler FJ. [Clinical significance of serum magnesium concentration in thyrotoxicosis (author's transl)]. Med Klin. 1976;71:235-238. [PubMed] [Google Scholar]
- 5. Salih M, van Kinschot CMJ, Peeters RP, et al. Thyrotoxic periodic paralysis: an unusual presentation of hyperthyroidism. Neth J Med. 2017;75:315-320. [PubMed] [Google Scholar]
- 6. Kim MJ, Valerio C, Knobloch GK. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2023;107:59-70. [PubMed] [Google Scholar]
- 7. Schaafsma IA, van Emst MG, Kooistra HS, et al. Exercise-induced hyperkalemia in hypothyroid dogs. Domest Anim Endocrinol. 2002;22:113-125. [DOI] [PubMed] [Google Scholar]
- 8. Shakir MK, Krook LS, Schraml FV, Hays JH, Clyde PW. Symptomatic hyponatremia in association with a low-iodine diet and levothyroxine withdrawal prior to I131 in patients with metastatic thyroid carcinoma. Thyroid. 2008;18:787-792. [DOI] [PubMed] [Google Scholar]
- 9. Horie I, Ando T, Imaizumi M, Usa T, Kawakami A. Hyperkalemia develops in some thyroidectomized patients undergoing thyroid hormone withdrawal in preparation for radioactive iodine ablation for thyroid carcinoma. Endocr Pract. 2015;21:488-494. [DOI] [PubMed] [Google Scholar]
- 10. Niri T, Horie I, Ando T, et al. Renal function and plasma renin activity as potential factors causing hyperkalemia in patients with thyroid carcinoma undergoing thyroid hormone withdrawal for radioactive iodine therapy. Endocr Pract. 2020;26:197-206. [DOI] [PubMed] [Google Scholar]
- 11. Takata N, Miyagawa M, Okada T, et al. Effect of preparation method for radioactive iodine therapy on serum electrolytes. Jpn J Radiol. 2023;41:1247-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mu F, Betts KA, Woolley JM, et al. Prevalence and economic burden of hyperkalemia in the United States Medicare population. Curr Med Res Opin. 2020;36:1333-1341. [DOI] [PubMed] [Google Scholar]
- 14. McNair P, Madsbad S, Christiansen C, Christensen MS, Transbøl I. Hyponatremia and hyperkalemia in relation to hyperglycemia in insulin-treated diabetic out-patients. Clin Chim Acta. 1982;120:243-250. [DOI] [PubMed] [Google Scholar]
- 15. Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract. 1989;7:163-167. [DOI] [PubMed] [Google Scholar]
- 16. Kovesdy CP, Matsushita K, Sang Y, et al.; CKD Prognosis Consortium. Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis consortium meta-analysis. Eur Heart J. 2018;39:1535-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160:503-515. [DOI] [PubMed] [Google Scholar]
- 18. Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23:22-26. [DOI] [PubMed] [Google Scholar]
- 19. Kreisman SH, Hennessey JV. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch Intern Med. 1999;159:79-82. [DOI] [PubMed] [Google Scholar]
- 20. Villabona C, Sahun M, Roca M, et al. Blood volumes and renal function in overt and subclinical primary hypothyroidism. Am J Med Sci. 1999;318:277-280. [DOI] [PubMed] [Google Scholar]
- 21. Karanikas G, Schütz M, Szabo M, et al. Isotopic renal function studies in severe hypothyroidism and after thyroid hormone replacement therapy. Am J Nephrol. 2004;24:41-45. [DOI] [PubMed] [Google Scholar]
- 22. Barreto-Chaves ML, Carrillo-Sepúlveda MA, Carneiro-Ramos MS, Gomes DA, Diniz GP. The crosstalk between thyroid hormones and the renin-angiotensin system. Vasc Pharmacol. 2010;52:166-170. [DOI] [PubMed] [Google Scholar]
- 23. van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol. 2009;160:205-215. [DOI] [PubMed] [Google Scholar]
- 24. Zdrojewicz Z, Plamieniak Z. [Plasma renin activity in women with hypothyroidism]. Pol Tyg Lek. 1993;48:595-NaN6, 598. [PubMed] [Google Scholar]
- 25. Elias AN, Kyaw T, Valenta LJ, Meshkinpour H. The renin-angiotensin system in hypothyroidism of short duration. Horm Metab Res. 1986;18:349-351. [DOI] [PubMed] [Google Scholar]
- 26. Marchant C, Brown L, Sernia C. Renin-angiotensin system in thyroid dysfunction in rats. J Cardiovasc Pharmacol. 1993;22:449-455. [DOI] [PubMed] [Google Scholar]
- 27. Ismail-Beigi F. Thyroid hormone regulation of Na,K-ATPase expression. Trends Endocrinol Metab. 1993;4:152-155. [DOI] [PubMed] [Google Scholar]
- 28. Lei J, Nowbar S, Mariash CN, Ingbar DH. Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L762-L772. [DOI] [PubMed] [Google Scholar]
- 29. Phakdeekitcharoen B, Phudhichareonrat S, Pookarnjanamorakot C, et al. Thyroid hormone increases mRNA and protein expression of Na+-K+-ATPase alpha2 and beta1 subunits in human skeletal muscles. J Clin Endocrinol Metab. 2007;92:353-358. [DOI] [PubMed] [Google Scholar]
- 30. Goia-Nishide K, Coregliano-Ring L, Rangel ÉB. Hyperkalemia in diabetes mellitus setting. Diseases. 2022;10:20. doi: 10.3390/diseases10020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin SH, Huang CL. Mechanism of thyrotoxic periodic paralysis. J Am Soc Nephrol. 2012;23:985-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vannucci L, Parenti G, Simontacchi G, et al. Hypothyroidism and hyponatremia: data from a series of patients with iatrogenic acute hypothyroidism undergoing radioactive iodine therapy after total thyroidectomy for thyroid cancer. J Endocrinol Invest. 2017;40:49-54. [DOI] [PubMed] [Google Scholar]
- 33. Cao JJ, Yun CH, Xiao J, et al. Analysis of the incidence and influencing factors of hyponatremia before (131)I treatment of differentiated thyroid carcinoma. World J Clin Cases. 2021;9:11173-11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Targher G, Montagnana M, Salvagno G, et al. Association between serum TSH, free T4 and serum liver enzyme activities in a large cohort of unselected outpatients. Clin Endocrinol. 2008;68:481-484. [DOI] [PubMed] [Google Scholar]
- 35. Piantanida E, Ippolito S, Gallo D, et al. The interplay between thyroid and liver: implications for clinical practice. J Endocrinol Invest. 2020;43:885-899. [DOI] [PubMed] [Google Scholar]
- 36. Al-Janabi G, Hassan HN, Al-Fahham A. Biochemical changes in patients during hypothyroid phase after thyroidectomy. J Med Life. 2022;15:104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]