Abstract
Purpose
The effect of soy products on prostate cancer (PCA) remains a topic of debate. This study aimed to investigate the association between soy products consumption and the incidence of PCA.
Materials and Methods
A search was conducted in the PubMed, EBSCO, Web of Science, and Cochrane Library databases up to December 2023. The adjusted odds ratio (OR) and corresponding 95% confidence interval (CI) were used to assess the association.
Results
A total of 22 articles, comprising 1,409,213 participants, were included for this meta-analysis. The results indicate that soy products reduce the risk of PCA (OR=0.94, 95% CI=0.91–0.97, p<0.001), especially in cases of localized or low-grade PCA (OR=0.94, 95% CI=0.90–0.97, p<0.001), but exhibit no effect on non-localized or high-grade PCA (OR=0.96, 95% CI=0.91–1.01, p=0.085). Furthermore, increased consumption frequency was negatively associated with PCA risk. Specifically, soy products can reduce the risk of PCA in African Americans (OR=0.89, p=0.006) and Latinos (OR=0.93, p=0.036), but have no impact on Japanese (OR=0.99, p=0.655), Chinese (OR=0.90, p=0.155), and Whites (OR=0.96, p=0.133). Non-fermented soy products were associated with a reduction in the incidence rate of PCA (OR=0.93, 95% CI=0.90–0.96, p<0.001), while fermented soy products had no effect on the incidence rate of PCA (OR=1.10, 95% CI=0.98–1.22, p=0.096).
Conclusions
The consumption of soy products can reduce the overall risk of PCA among men. Various factors, including soy products-related factors (e.g., consumption, frequency), population-related factors (e.g., race), and PCA-related factors (e.g., PCA stage) collectively influence the effect of soy products on PCA.
Keywords: Diet, Meta-analysis, Prostatic neoplasms, Risk assessment, Soy foods
Graphical Abstract
INTRODUCTION
Prostate cancer (PCA) is currently the second most prevalent cancer among men globally and the sixth leading cause of cancer-related mortality as of 2020 [1]. The burden of PCA is anticipated to escalate, with projections indicating 499,000 deaths and 1.7 million new cases by 2030 [2]. Both endogenous (e.g., family history [3], race [4], genetic syndrome [5]) and exogenous (e.g., smoking [6], obesity [7]) factors can affect the onset of PCA.
Nutrition and lifestyle habits, being readily modifiable, are considered as potential effective preventive strategies against cancer [8]. On the one hand, lifestyle habits such as increased physical activity [9], reduced sedentary behavior [10], and non-smoking [6] are considered to reduce the risk of PCA. On the other hand, dietary choices exert a notable influence on PCA incidence, with different dietary patterns and food types having varying impacts [11]. For instance, the plant-based diets [12], low-fat diets [13], and Mediterranean diet [14] are inversely associated with the risk of PCA. In addition, numerous studies have explored the impact of dairy products [15], soy products [16], fruits [17], vegetables [18], and dietary fiber [19] on PCA risk.
Among existing studies, divergent findings persist regarding the effect of soy products on PCA risk. Notably, there is no unified conclusion on whether soy products impact the occurrence of PCA. For instance, Greenlee et al. [20] posited that a high intake of soy products promotes the development of PCA, while Kirsh et al. [21] reported inconclusive findings regarding the association between soy products and PCA risk. Moreover, subgroup analyses across various studies, considering PCA stage and racial demographics, exhibit substantial heterogeneity. For example, Kurahashi et al. [22] proposed an increased risk of metastatic PCA associated with soy products, whereas Park et al. [23] asserted a risk reduction in metastatic PCA with soy product consumption. Furthermore, published meta-analyses on this topic have failed to reach a consistent conclusion. Zhang et al. [24] contended that soy products do not mitigate the risk of PCA, while Hwang et al. [25] believed otherwise. These discrepancies highlight the ongoing debate and the need for further investigation.
This meta-analysis is necessary because previous analyses have not comprehensively addressed the potential sources of heterogeneity, such as genetic differences, dietary patterns, and lifestyle factors across different populations. By conducting a systematic review and meta-analysis of relevant observational studies, this study aims to provide a more nuanced understanding of the relationship between soy products and PCA, accounting for these factors. This will offer preventive insights for the male population and potentially guide dietary recommendations.
MATERIALS AND METHODS
1. Search strategy
A comprehensive search of studies investigating the association between soy products and PCA risk was conducted across electronic databases of PubMed, Web of Science, EBSCO, and Cochrane Library until December 2023. The search strategy utilized the following keywords: “prostate cancer” and “soy products”. The complete search formula used to identify relevant studies included the following terms: (“prostate cancer” OR “prostate neoplasm” OR “PCA” OR “prostatic carcinoma”) AND (“soy” OR “soy products” OR “bean product” OR “soymilk” OR “soybean milk” OR “bean” OR “soy milk” OR “isoflavone” OR “bean curd” OR “beancurd” OR “tofu” OR “soy protein”). Supplementary Table 1 presents the specific retrieval strategies. Additionally, the retrieved studies and the reference lists of recent reviews were also examined for potentially relevant studies. In case of duplicate literature, the original article was included if the study was published both as an abstract and as a full article. Also, for studies that were periodically updated and reported, only the most recent or comprehensive articles were included. This meta-analysis adhered to the MOOSE (meta-analysis of observational studies in epidemiology) guidelines [26]. The PICOS (population, intervention/exposure, comparison, outcome, and setting) criteria were used to describe the research question. This meta-analysis’s PROSPERO registration number was CRD42024493149.
2. Selection criteria
Eligible criteria had been formulated. The specific criteria were as follows:
Inclusion criteria: (1) all included studies must be observational studies. (2) All studies included available data that reported the relationship between soy products and PCA. (3) The main exposure of the study was soy products and the outcome was a risk of PCA. Exclusion criteria: (1) the study lacked a reference group or control group. (2) The study was conducted on PCA population and utilized mortality or recovery rate as the outcomes. (3) The study did not contain full-text articles. (4) The study was not published in English. (5) The study was published in duplicate.
Two researchers independently applied a search strategy to select studies from the database and independently reviewed the titles and abstracts of these articles for eligibility for inclusion. In cases of uncertainty, the full text will be searched for further selection. When necessary, authors are contacted for more information about their research. In case of disagreement, discussions were held with a third researcher. When consensus could not be reached, the study was excluded.
3. Data extraction
A standardized data collection form, mutually agreed upon, was used for the systematic extraction of all pertinent information. Information was extracted as follows: the author’s name, year of publication, study type, age, exposure assessment, number of participants, number of PCA cases, variables adjusted in the statistical analyses, and outcomes. To ensure the objectivity and accuracy of the data, two researchers independently extracted data from each study, with disagreements resolved by a third researcher.
4. Bias risk and quality assessment
The quality assessment of each included study was conducted using the Newcastle–Ottawa Quality Assessment Scale (NOS) checklist, a validated tool designed for the evaluation of non-randomized studies. The NOS checklist comprises eight items classified into three aspects including selection, comparability, and outcome. The maximum score on this checklist is nine and scores between seven and nine were identified as high study quality.
5. Objectives and endpoints
The primary objective was to investigate the relationship between soy product consumption and the incidence of PCA. The secondary objectives were to explore the relationship between PCA incidence and various soy product subgroups (e.g., consumption levels, frequency of consumption, types of soy products), soy products and PCA across different demographics (e.g., racial groups), and the association between soy products and different PCA subtypes (e.g., advanced PCA, localized PCA). The results obtained after adjusting for relevant confounding factors were uniformly adopted for data processing from the included articles.
6. Statistical analysis
The Stata software version 12 (StataCorp) was used to analyze the data. A 95% confidence interval (CI) was utilized to evaluate the relationship between soy products and PCA risk. p-values less than 0.05 considered statistically significant. Heterogeneity among the included studies was assessed quantitatively using the Q statistic and I2 statistic. For statistical results, values of p<0.10 and I2>50% were considered representative of statistically significant heterogeneity. A random-effects model was employed to incorporate data due to the variety of soy products so as to increase the credibility of the results. When more than ten studies were included [27,28], sensitivity analysis and publication bias test were performed to evaluate the stability and reliability of the results. Publication bias was evaluated by the Begg’s test.
RESULTS
1. Literature search
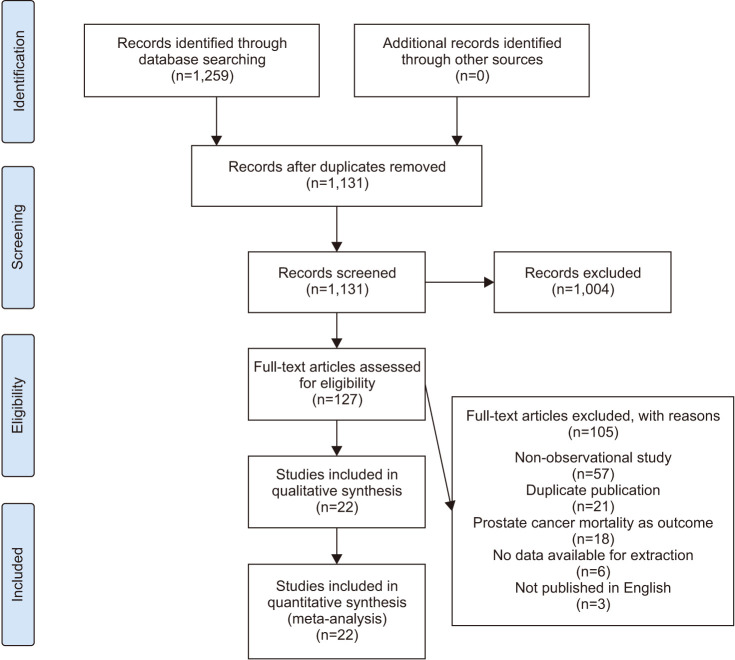
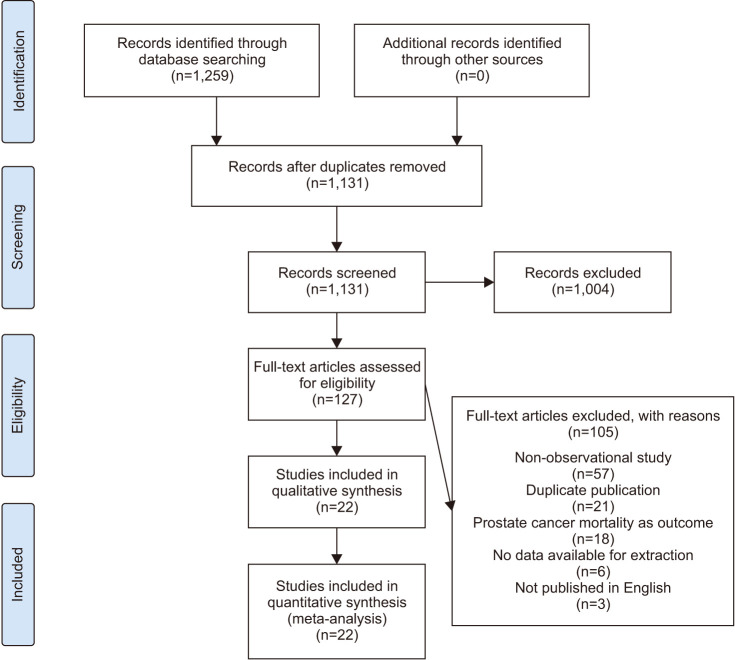
A total of 1,259 pertinent articles were identified in the PubMed, EBSCO, Web of Science, and Cochrane Library databases. No additional records were identified through other sources. After removing 128 duplicate articles, 1,004 articles were excluded based on their titles or abstracts. The remaining 127 articles underwent full-text scrutiny. Among these, 105 articles were eliminated due to reasons such as non-observational study design (n=57), duplicate publication (n=21), not exploring the risk of PCA (n=18), no data available for extraction (n=6), and not published in English (n=3). Ultimately, 22 articles [20,21,22,23,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], encompassing a total of 1,409,213 participants, were selected for this meta-analysis. Fig. 1 shows the flow diagram about the selection of articles.
Fig. 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2009 flow diagram. A schematic flow for the selection of articles included in this meta-analysis.
2. Characteristic of studies
Among the 22 included studies, 10 were cohort studies (1,402,720 participants and 61,740 PCA cases) and 12 were case-control studies (6,493 controls and 6,260 PCA cases). The publication years of these studies ranged from 1988 to 2022, with follow-up durations spanning from 4.2 to 21.0 years. Geographically, 12 studies were conducted in Asia, 8 in America, and 2 in Europe. Regarding age at recruitment, 3 studies did not specify an upper age limit, and 1 study did not set a lower age limit, and 1 study had no accessible data. The median age for analysis ranged from 51.0 to 72.2 years, but data was lacking in 8 studies. Data collection methods and exposure assessment varied, 15 studies employing participants completed questionnaires, 2 studies conducting interviews, and 5 studies utilizing a combination of questionnaires and interviews. In addition, the adjustment of potential confounding factors varied among studies. Most of the adjustments included parameters were age, geographic area, physical activity, body mass index, family history of PCA, total energy intake, smoking, and alcohol consumption. Table 1 and Supplementary Table 2 contained detailed characteristics of the included studies.
Table 1. Characteristics of included observational studies in the meta-analysis.
| Study | Country | Time of experiment (y) | Age at recruitment (y) | Median age (y) | Median follow-up time (y) | No. of PCA cases | No. of participants |
|---|---|---|---|---|---|---|---|
| Severson et al. [43] (1989) | America | 1965–1968 | 46–68 | 54.0 | 21.0 | 174 | 7,999 |
| Jacobsen et al. [32] (1998) | America | 1976–1992 | ≥25 | NA | NA | 225 | 12,395 |
| Greenlee et al. [20] (2004) | America | 2000–2002 | 50–76 | NA | NA | 1,891 | 35,441 |
| Allen et al. [29] (2004) | Japan | 1963–1981 | 18–99 | 51.0 | 16.9 | 196 | 18,115 |
| Nomura et al. [39] (2004) | Japan | 1971–1975 | 30–75 | NA | 19.4 | 304 | 5,826 |
| Kurahashi et al. [22] (2007) | Japan | 1995–2004 | 45–74 | NA | 5.0 | 307 | 325,371 |
| Kirsh et al. [21] (2007) | America | 1993–2001 | 55–74 | 63.3 | 4.2 | 1,338 | 29,361 |
| Park et al. [23] (2008) | America | 1993–1996 | 45–75 | NA | 8.0 | 4,404 | 82,483 |
| Petimar et al. [41] (2017) | America | 1985–2008 | 18–75 | NA | 18.0 | 52,680 | 842,149 |
| Sawada et al. [42] (2020) | Japan | 1995–2016 | 45–74 | 57.6 | 16.9 | 221 | 43,580 |
| Oishi et al. [40] (1988) | Japan | 1981–1984 | 50–79 | 70.6 | 6.4 | 117 | 227 |
| Sung et al. [45] (1999) | China | 1995–1996 | 40–85 | NA | NA | 90 | 270 |
| Villeneuve et al. [46] (1999) | Canada | 1994–1997 | 50–74 | NA | NA | 1,623 | 3,246 |
| Kolonel et al. [34] (2000) | China | 1987–1991 | ≤84 | 70.5 | 9.5 | 1,619 | 3,237 |
| Lee et al. [35] (2003) | China | 1989–1992 | 50–89 | 60.6 | NA | 133 | 398 |
| Sonoda et al. [44] (2004) | Japan | 1996–2002 | 59–73 | 65.2 | 5.2 | 140 | 280 |
| Jian et al. [33] (2004) | China | 2001–2002 | ≥45 | 72.2 | 4.3 | 130 | 404 |
| McCann et al. [37] (2005) | America | 1986–1991 | 30–75 | 65.0 | NA | 433 | 971 |
| Hedelin et al. [31] (2006) | Sweden | 2001–2002 | 35–79 | 66.8 | 6.7 | 1,314 | 2,096 |
| Heald et al. [30] (2007) | Scotland | 1998–2001 | 50–74 | 67.2 | NA | 433 | 916 |
| Nagata et al. [38] (2007) | Japan | 1996–2003 | 59–73 | 60.3 | 10.2 | 200 | 400 |
| Li et al. [36] (2008) | China | 1998–2000 | ≥50 | 71.4 | NA | 28 | 308 |
PCA, prostate cancer; NA, not available.
3. Soy products overall effect
Twenty studies recorded data (1,379,632 participants) on the risk of PCA associated with total soy product consumption, with 65,909 participants newly diagnosed with PCA during the follow-up period. The analysis indicated that men who consumed soy products exhibited a significantly lower risk of PCA than those who never or rarely consumed soy products (odds ratio [OR]=0.94, 95% CI=0.91–0.97, p<0.001), with moderate heterogeneity observed (I2=40.3%). In essence, the intake of soy products demonstrated a protective effect against PCA risk. Furthermore, both cohort studies (OR=0.98, 95% CI=0.95–1.00) (I2=19.0%) and case-control studies (OR=0.94, 95% CI=0.94–0.97) (I2=40.3%) consistently concluded that soy products consumption reduces the risk of PCA in men. Detailed data is contained in Table 2.
Table 2. Effects of soy products on PCA incidence.
| Subgroup analysis | No. of studies | OR | 95% CI | p-value | Heterogeneity (I2) (%) | ||
|---|---|---|---|---|---|---|---|
| Soy products | 20 | 0.94 | 0.91–0.97 | <0.001 | 40.3 | ||
| Cohort study | 9 | 0.98 | 0.95–1.00 | 0.056 | 19.0 | ||
| Case-control study | 11 | 0.94 | 0.94–0.97 | <0.001 | 40.3 | ||
| Soy products consumption | |||||||
| 0–50 g/day | 11 | 0.99 | 0.97–1.00 | 0.143 | 0.0 | ||
| 50–100 g/day | 12 | 0.98 | 0.93–1.04 | 0.586 | 41.7 | ||
| 100–150 g/day | 11 | 0.93 | 0.87–0.99 | 0.018 | 35.5 | ||
| >150 g/day | 6 | 0.91 | 0.82–1.01 | 0.078 | 64.1 | ||
| Soy products consumption frequency | |||||||
| <1 time/day | 3 | 0.94 | 0.84–1.05 | 0.254 | 0.0 | ||
| ≥1 time/day | 6 | 0.80 | 0.65–0.99 | 0.038 | 30.5 | ||
| Racial demographics | |||||||
| African Americans | 2 | 0.89 | 0.81–0.97 | 0.006 | 55.5 | ||
| Japanese | 2 | 0.99 | 0.93–1.05 | 0.655 | 0.0 | ||
| Whites | 2 | 0.96 | 0.90–1.01 | 0.133 | 0.0 | ||
| Latinos | 2 | 0.93 | 0.87–1.00 | 0.036 | 8.9 | ||
| Chinese | 2 | 0.90 | 0.79–1.04 | 0.155 | 0.0 | ||
| Prostate cancer stage | |||||||
| Non-localized or high-grade | 5 | 0.96 | 0.91–1.01 | 0.085 | 54.0 | ||
| Localized or low grade | 3 | 0.94 | 0.90–0.97 | <0.001 | 19.9 | ||
| Soy products subtypes | |||||||
| Fermented soy products | 7 | 1.10 | 0.98–1.22 | 0.096 | 10.7 | ||
| Natto | 2 | 0.98 | 0.77–1.24 | 0.837 | 15.8 | ||
| Miso | 5 | 1.15 | 1.00–1.33 | 0.047 | 16.9 | ||
| Non-fermented soy products | 18a | 0.93 | 0.90–0.96 | <0.001 | 44.3 | ||
| Tofu | 10 | 0.93 | 0.85–1.01 | 0.099 | 28.8 | ||
| Legumes | 8 | 0.95 | 0.92–0.98 | 0.001 | 45.5 | ||
| Soy milk | 10 | 0.84 | 0.75–0.93 | 0.001 | 47.4 | ||
PCA, prostate cancer; OR, odds ratio; CI, confidence interval.
a:Some studies have also explored the effects of soy milk, tofu, and soybeans on prostate cancer. There are overlapping groups between the studies, so the total number is less than the sum of the studies in the following three subgroups.
4. Soy products consumption
The study employed grams per day (g/day) as the unit of measurement for soy product consumption. Twelve studies (involving 1,336,156 participants) investigated the impact of varying levels of soy product consumption on PCA risk. A total of 63,023 participants were newly diagnosed with PCA during the study period. According to the grouping criteria delineated in the included studies, the data were categorized into ‘0–50 g/day group’, ‘50–100 g/day group’, ‘100–150 g/day group’, and ‘>150 g/day group’. The results showed that men’s intake of soy products within the range of 0–50 g/day (OR=0.99, p=0.143) (I2=0.0%), 50–100 g/day (OR=0.98, p=0.586) (I2=41.7%), 100–150 g/day (OR=0.93, p=0.018) (I2=35.5%), and >150 g/day (OR=0.91, p=0.078) (I2=64.1%) demonstrated no significant impact on PCA incidence. With the increase in soy products intake, the incidence of PCA tended to gradually decrease, although statistically significance was not attained. The detailed data is contained in Table 2.
5. Soy products consumption frequency
In this study, the frequency of soy product consumption was quantified as times per day (time/day). The data were grouped based on a frequency of 1 time/day. Six studies explored the effect of soy products on PCA at different consumption frequencies. Among the 42,290 participants, a total of 2,363 were newly diagnosed with PCA. Subgroup analysis showed that when the frequency was <1 time/day, there was no significant association between soy products consumption and PCA incidence (OR=0.94, 95% CI=0.84–1.05, p=0.254). However, when the frequency was ≥1 time/day, soy product consumption was associated with a reduced incidence of PCA (OR=0.80, 95% CI=0.65–0.99, p=0.038), with statistically significant differences observed. The detailed data are presented in Table 2.
6. Racial demographics
The correlation between soy products consumption and PCA risk is affected by racial demographics. Two studies provided relevant data, categorizing the population into African Americans, Latinos, Whites, Japanese, and Chinese. Among the 85,720 participants, a total of 6,023 were newly diagnosed with PCA. The results revealed that soy products exhibited a protective effect on the prostate among African Americans (OR=0.89, p=0.006) and Latinos (OR=0.93, p=0.036), thereby reducing the risk of PCA in male population. However, no significant effect was observed among Japanese (OR=0.99, p=0.655), Chinese (OR=0.90, p=0.155), and Whites (OR=0.96, p=0.133). Detailed data are presented in Table 2.
7. PCA stage
Regarding the relationship between soy products and different stages of PCA, 5 studies (1,211,029 participants) provided data on localized or low-grade PCA, and 3 studies (1,299,338 participants) contained data about non-localized or high-grade PCA. The analysis indicated that soy products were associated with a reduced risk of localized or low-grade PCA (OR=0.94, 95% CI=0.90–0.97, p<0.001) with low heterogeneity (I2=19.9%). However, no significant effect was observed for non-localized or high-grade PCA (OR=0.96, 95% CI=0.91–1.01, p=0.085) with moderate heterogeneity (I2=54.0%). Detailed data are presented in Table 2.
8. Soy products subtypes
Various subtypes of soy products have different effects on PCA risk. Eighteen studies (involving 1,379,001 participants) provided data on non-fermented soy products and 7 studies (involving 395,976 participants) provided data on fermented soy products. The analysis showed that non-fermented soy products had a protective effect on PCA (OR=0.93, 95% CI=0.90–0.96, p<0.001). Conversely, fermented soy products had no significant effect on PCA (OR=1.10, 95% CI=0.98–1.22, p=0.096). Among non-fermented soy products, legumes (OR=0.95, p=0.001) and soy milk (OR=0.84, p=0.001) significantly reduced the risk of PCA in the male population, but tofu (OR=0.93, p=0.099) exhibited no significant effect on PCA. Among fermented soy products, natto (OR=0.98, p=0.837) showed no association with the incidence of PCA, while miso (OR=1.15, p=0.047) was identified as a potential risk factor for PCA in men, leading to a slight increase in incidence with minor heterogeneity observed. Detailed data are presented contained in Table 2.
9. Study quality
The NOS checklist was adopted to rigorously assess the quality of the included observational studies in this meta-study. Regarding cohort studies, 90% were of high quality (NOS score ≥7). For case-control studies, 91.67% were of high quality (NOS score ≥7). Supplementary Tables 3 and 4 explicitly recorded the assessment of risk of bias.
10. Publication bias and sensitivity analysis
Begg’s test was used to evaluate publication bias. The results of Begg’s test indicated no significant publication bias among the included articles (p>0.05). Sensitivity analysis was conducted to assess whether any individual studies affected the overall results. The results suggested that the analysis was relatively stable.
DISCUSSION
Through rigorous data analysis, this study has elucidated that soy products have a protective effect within the male population, effectively lowering the incidence rate of PCA, especially localized or low-grade PCA, but having no significant effect on non-localized or high-grade PCA. Both cohort and case-control studies showed consistent results. Consumption frequency and race were associated with PCA risk. Although statistical significance was not consistently achieved, a trend was observed suggesting that higher consumption of soy products may associated with a gradual reduction in PCA incidence. Specifically, a consumption frequency of ≥1 time/day was linked to a diminished risk of PCA, while frequencies of <1 time/day showed no significant effect. Soy products can reduce the risk of PCA in African Americans and Latinos, whereas Japanese, Chinese, and White populations did not exhibit a significant association. Subtype analysis revealed that non-fermented soy products, including legumes and soy milk, demonstrated a noteworthy reduction in PCA incidence, whereas tofu exhibited no discernible effect. Conversely, fermented soy products had no effect on PCA incidence, with natto showing no effect and miso potentially increasing the incidence rate of PCA in subtype analysis.
Given the intricate composition of soy products, there is no unified mechanism to explain their impact on the incidence rate of PCA. However, prevailing theories suggest that high level of isoflavones (including genistein, daidzein, etc.) present in soy products inhibit PCA epithelial cell growth, thereby conferring a protective effect on the prostate [30,47,48]. Research has demonstrated that isoflavones can effectively impede the growth of LAPC-4 and PC-3 PCA cells in a dose-dependent manner by inhibiting angiogenesis during PCA growth and preventing cancer cell adhesion to blood vessel surfaces [49,50]. Meanwhile, isoflavones possess antioxidant properties [51], which can inhibit tyrosine kinase activity and regulate signaling pathways such as AKT [52], MAPK [53], and others [54], thereby influencing the expression of genes that control cell survival [55], the cell cycle [56], and apoptosis [57]. Additionally, they enhance the DNA repair system to jointly inhibit tumor occurrence [58]. The effects of isoflavones can be cumulative over time, with prolonged and intensified exposure to soy products enhancing their efficacy. These mechanistic insights corroborate the findings of the present study, affirming the biological validity of the observed negative correlation between PCA risk and soy product consumption frequency among the male population.
A recent emerging perspective suggests that the protective effect of soy products on PCA depends on their androgen-like and anti-androgenic effects. Previous research underscores the pivotal role of androgen levels in male prostate health, highlighting prolonged androgen exposure or heightened cellular androgen responsiveness as significant PCA risk factors [16]. Exogenous estrogen supplementation has been shown to suppress hormone release from the anterior pituitary gland, reduce luteinizing hormone, and decrease the production of androgen by the testes, thereby exerting anti-androgenic effects. On the one hand, the relatively high level of isoflavones and 17-β-estradiol, a type of phytoestrogen with strong estrogenic activity [59], has a similar structure. Isoflavones can bind to a large number of estrogen receptors β in prostate tissue and play an anti-androgenic role [60,61]. Moreover, isoflavones have been observed to downregulate levels of androgen receptor (AR) and prostate-specific antigen, collectively mitigating androgen-mediated prostatic stimulation [16]. On the other hand, soy products contain natural androgens, and exogenous supplementation of phytoestrogens can induce hormone metabolism to convert into androgens, leading to an increase in circulating androgens and an increased risk of PCA [62].
Presently, there is a prevailing inclination among researchers to attribute greater significance to the anti-androgenic properties of soy products and the inherent anticancer effects of isoflavones compared to the deleterious impact of androgens on the prostate [63]. Moreover, it is recognized that anti-androgenic effects may lead to a reactive increase in the number of ARs and an increase in sensitivity to androgen in prostate cells, leading to the occurrence of hormone-sensitive tumors [64]. Studies have delineated that as PCA progresses, mutations or epigenetic silencing of DNA repair genes within cancer cells may compromise their responsiveness to hormone therapy, thereby facilitating the transition from hormone-dependent to hormone-independent tumor growth. This phenomenon assumes particular significance in the management of advanced-stage PCA patients [65]. This may explain why the soy product consumption is associated with a protective effect on localized or low-grade PCA, while the effect on non-localized or high-grade PCA is not significant. In addition, according to epidemiological and biological data, the potential etiology of PCA is not only influenced by genes but also by the interaction between exposure and genetic environment [66]. The differential impact observed across racial and geographical demographics may be attributed to background factors modulating PCA susceptibility via population-specific gene-environment interactions [67,68]. This, to some extent, supports the results found in this study that soy product intake can reduce the incidence of PCA in African American and Latino populations but has no effect on the incidence of PCA in Japanese, White, and Chinese populations.
Regarding the conclusions drawn from our investigation of non-fermented and fermented soy products, our research aligns with previous studies [25,69], demonstrating that non-fermented soy products can reduce the incidence rate of PCA, while fermented soy products show no discernible impact on PCA incidence. However, our research presents differing conclusions regarding the impact of a specific soy products on PCA. For example, Hwang et al. [25] believe that tofu can significantly reduce the incidence rate of PCA, whereas our research finds that tofu has no impact on PCA incidence. Conversely, they [25] suggest that soy milk has no effect on PCA, but our research indicates that soy milk can reduce the risk of PCA. Given the complexity of human diet and the different confounding factors addressed in various studies, we should exercise caution when drawing conclusions about the impact of individual soy products on PCA, as any bias may alter the results. Interestingly, our study identified an unexpected increase in PCA incidence associated with miso consumption, which contrasts with previous findings [25]. We hypothesize that the high sodium content in miso may lead to hyperosmotic dehydration and cellular necrosis, potentially promoting PCA initiation, proliferation, and metastasis. However, further rigorous investigation is warranted to validate this conjecture.
Reviewing the same type of studies, Yan and Spitznagel’s study [70] reached a similar conclusion to the present study, showing that soy products reduce the risk of PCA in men (OR=0.70, 95% CI=0.59–0.83). However, they did not conduct subgroup analyses on soy product consumption and race, thus limiting their ability to provide comprehensive insights into the male population from various perspectives. The study by Applegate et al. [69] conducted a subgroup analysis by region, but there was only one study in the European group, which may have led to an overestimation of their findings and a lack of reliability. Furthermore, while our study analyzed isoflavones, it is essential to note the varying definitions of isoflavones across different studies. For example, Reger et al. [48] defined isoflavones as including genistein, daidzein, formononetin, biochanin A and coumestrol, while Heald et al. [30] defined isoflavones as including genistein, daidzein, and equol. Given the limited number of studies analyzed and the heterogeneity observed among them, caution must be exercised in drawing definitive conclusions.
Although this meta-analysis yielded comprehensive and objective conclusions, several potential limitations warrant consideration. Firstly, variations in study design, populations, sample sizes, risk assessment methodologies, and adjustment for confounding factors varied among the included studies may introduce bias and diminish confidence in the conclusions. To address this variability, a random-effects model was employed to assess the impact of soy products on PCA. Secondly, most studies use food frequency questionnaires to evaluate diet, while a few studies use an interview format. This inevitably leads to evaluation or measurement errors in the dietary evaluation process, which may result in biased results. Additionally, considerable heterogeneity existed in the lifestyle habits and geographic locations of the study populations, further complicating the analysis. To mitigate these issues, relevant data were meticulously selected for statistical analysis, and adjustments were made for a maximal number of potential confounding factors to enhance result accuracy. Thirdly, the unavailability of subgroup data, such as PCA type subgroup data, race status subgroup data, etc., in all trials posed challenges for conducting certain subgroup analyses in this study. Thus, large-scale observational studies are still needed to further validate the relevant conclusions.
In addition to limitations, this meta-analysis boasts several strengths. Foremost among them is the inclusion of a substantial number of observational studies, encompassing over 1.4 million participants in Asia, Europe, and America. The extensive observational population enhances the reliability and credibility of the study’s conclusions. Additionally, this study grouped the extracted data (by PCA stage, race or soy products subtype) and performed subgroup analyses to comprehensively explore the effect of soy products on different populations and PCA types. In sum, this meta-analysis yielded meaningful insights that may offer novel perspectives for PCA prevention strategies among male populations.
CONCLUSIONS
This meta-analysis found that soy products reduce the risk of PCA, especially localized or low-grade PCA. The frequency of consumption was negatively associated with PCA risk. Soy products can reduce the risk of PCA in African Americans and Latinos but have no impact in Japanese, Chinese and White populations. Non-fermented soy products demonstrate a reduction in PCA incidence, whereas fermented soy products do not show a significant effect. Considering the relevant limitations, large-scale prospective cohort studies are still needed to further confirm the conclusions of this study.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: This research was supported by grants from the National College Students Innovation and Entrepreneurship Training Program (grant number: 202310343074S).
- Research conception and design: Yiping Huang and Wenyan Wang.
- Data acquisition: Yiping Huang and Jianxiang Jin.
- Statistical analysis: Yiping Huang and Wenyan Wang.
- Data analysis and interpretation: Yiping Huang and Jianxiang Jin.
- Drafting of the manuscript: Wenyan Wang and Jianxiang Jin.
- Critical revision of the manuscript: Wenyan Wang and Jianxiang Jin.
- Obtaining funding: Yiping Huang and Jianxiang Jin.
- Administrative, technical, or material support: Wenyan Wang and Jianxiang Jin.
- Supervision: Yiping Huang and Jianxiang Jin.
- Approval of the final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4111/icu.20240186.
The search strategy in PubMed, EBSCO, Web of Science, and Cochrane Library database
Characteristics of included observational studies in the meta-analysis
Quality assessment of cohort studies included
Quality assessment of case control studies included
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77:38–52. doi: 10.1016/j.eururo.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Barber L, Gerke T, Markt SC, Peisch SF, Wilson KM, Ahearn T, et al. Family history of breast or prostate cancer and prostate cancer risk. Clin Cancer Res. 2018;24:5910–5917. doi: 10.1158/1078-0432.CCR-18-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity - United States, 2001-2017. MMWR Morb Mortal Wkly Rep. 2020;69:1473–1480. doi: 10.15585/mmwr.mm6941a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhen JT, Syed J, Nguyen KA, Leapman MS, Agarwal N, Brierley K, et al. Genetic testing for hereditary prostate cancer: current status and limitations. Cancer. 2018;124:3105–3117. doi: 10.1002/cncr.31316. [DOI] [PubMed] [Google Scholar]
- 6.Jochems SHJ, Fritz J, Häggström C, Järvholm B, Stattin P, Stocks T. Smoking and risk of prostate cancer and prostate cancer death: a pooled study. Eur Urol. 2023;83:422–431. doi: 10.1016/j.eururo.2022.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Saha A, Kolonin MG, DiGiovanni J. Obesity and prostate cancer - microenvironmental roles of adipose tissue. Nat Rev Urol. 2023;20:579–596. doi: 10.1038/s41585-023-00764-9. [DOI] [PubMed] [Google Scholar]
- 8.Ballon-Landa E, Parsons JK. Nutrition, physical activity, and lifestyle factors in prostate cancer prevention. Curr Opin Urol. 2018;28:55–61. doi: 10.1097/MOU.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 9.Shephard RJ. Physical activity and prostate cancer: an updated review. Sports Med. 2017;47:1055–1073. doi: 10.1007/s40279-016-0648-0. [DOI] [PubMed] [Google Scholar]
- 10.Hermelink R, Leitzmann MF, Markozannes G, Tsilidis K, Pukrop T, Berger F, et al. Sedentary behavior and cancer-an umbrella review and meta-analysis. Eur J Epidemiol. 2022;37:447–460. doi: 10.1007/s10654-022-00873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser GE, Jaceldo-Siegl K, Orlich M, Mashchak A, Sirirat R, Knutsen S. Dairy, soy, and risk of breast cancer: those confounded milks. Int J Epidemiol. 2020;49:1526–1537. doi: 10.1093/ije/dyaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb S, Fu BC, Bauer SR, Pernar CH, Chan JM, Van Blarigan EL, et al. Association of plant-based diet index with prostate cancer risk. Am J Clin Nutr. 2022;115:662–670. doi: 10.1093/ajcn/nqab365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oczkowski M, Dziendzikowska K, Pasternak-Winiarska A, Włodarek D, Gromadzka-Ostrowska J. Dietary factors and prostate cancer development, progression, and reduction. Nutrients. 2021;13:496. doi: 10.3390/nu13020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baguley BJ, Skinner TL, Jenkins DG, Wright ORL. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: a pilot randomised control trial. Clin Nutr. 2021;40:245–254. doi: 10.1016/j.clnu.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Mikami K, Ozasa K, Miki T, Watanabe Y, Mori M, Kubo T, et al. JACC Study Group. Dairy products and the risk of developing prostate cancer: a large-scale cohort study (JACC Study) in Japan. Cancer Med. 2021;10:7298–7307. doi: 10.1002/cam4.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–132. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taborelli M, Polesel J, Parpinel M, Stocco C, Birri S, Serraino D, et al. Fruit and vegetables consumption is directly associated to survival after prostate cancer. Mol Nutr Food Res. 2017;61:1600816. doi: 10.1002/mnfr.201600816. [DOI] [PubMed] [Google Scholar]
- 18.Kerley CP. Vegetable consumption and progression of prostate cancer. JAMA. 2020;323:2528–2529. doi: 10.1001/jama.2020.6726. [DOI] [PubMed] [Google Scholar]
- 19.Deschasaux M, Pouchieu C, His M, Hercberg S, Latino-Martel P, Touvier M. Dietary total and insoluble fiber intakes are inversely associated with prostate cancer risk. J Nutr. 2014;144:504–510. doi: 10.3945/jn.113.189670. [DOI] [PubMed] [Google Scholar]
- 20.Greenlee H, White E, Patterson RE, Kristal AR Vitamins and Lifestyle (VITAL) Study Cohort. Supplement use among cancer survivors in the Vitamins and Lifestyle (VITAL) study cohort. J Altern Complement Med. 2004;10:660–666. doi: 10.1089/acm.2004.10.660. [DOI] [PubMed] [Google Scholar]
- 21.Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, et al. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. 2007;99:1200–1209. doi: 10.1093/jnci/djm065. [DOI] [PubMed] [Google Scholar]
- 22.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S Japan Public Health Center-Based Prospective Study Group. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;16:538–545. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN Multiethnic Cohort Study. Legume and isoflavone intake and prostate cancer risk: the Multiethnic Cohort Study. Int J Cancer. 2008;123:927–932. doi: 10.1002/ijc.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Feng H, Qluwakemi B, Wang J, Yao S, Cheng G, et al. Phytoestrogens and risk of prostate cancer: an updated meta-analysis of epidemiologic studies. Int J Food Sci Nutr. 2017;68:28–42. doi: 10.1080/09637486.2016.1216525. [DOI] [PubMed] [Google Scholar]
- 25.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Gu L, Huang X, Li S, Mao D, Shen Z, Khadaroo PA, et al. A meta-analysis of the medium- and long-term effects of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass. BMC Surg. 2020;20:30. doi: 10.1186/s12893-020-00695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Gu L, Shen Z, Mao D, Khadaroo PA, Su H. A meta-analysis of comparison of proximal gastrectomy with double-tract reconstruction and total gastrectomy for proximal early gastric cancer. BMC Surg. 2019;19:117. doi: 10.1186/s12893-019-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen NE, Sauvaget C, Roddam AW, Appleby P, Nagano J, Suzuki G, et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Control. 2004;15:911–920. doi: 10.1007/s10552-004-1683-y. [DOI] [PubMed] [Google Scholar]
- 30.Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br J Nutr. 2007;98:388–396. doi: 10.1017/S0007114507700703. [DOI] [PubMed] [Google Scholar]
- 31.Hedelin M, Bälter KA, Chang ET, Bellocco R, Klint A, Johansson JE, et al. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate. 2006;66:1512–1520. doi: 10.1002/pros.20487. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States) Cancer Causes Control. 1998;9:553–557. doi: 10.1023/a:1008819500080. [DOI] [PubMed] [Google Scholar]
- 33.Jian L, Zhang DH, Lee AH, Binns CW. Do preserved foods increase prostate cancer risk? Br J Cancer. 2004;90:1792–1795. doi: 10.1038/sj.bjc.6601755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 35.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12:665–668. [PubMed] [Google Scholar]
- 36.Li XM, Li J, Tsuji I, Nakaya N, Nishino Y, Zhao XJ. Mass screening-based case-control study of diet and prostate cancer in Changchun, China. Asian J Androl. 2008;10:551–560. doi: 10.1111/j.1745-7262.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 37.McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, et al. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer. 2005;53:33–41. doi: 10.1207/s15327914nc5301_4. [DOI] [PubMed] [Google Scholar]
- 38.Nagata Y, Sonoda T, Mori M, Miyanaga N, Okumura K, Goto K, et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr. 2007;137:1974–1979. doi: 10.1093/jn/137.8.1974. [DOI] [PubMed] [Google Scholar]
- 39.Nomura AM, Hankin JH, Lee J, Stemmermann GN. Cohort study of tofu intake and prostate cancer: no apparent association. Cancer Epidemiol Biomarkers Prev. 2004;13:2277–2279. [PubMed] [Google Scholar]
- 40.Oishi K, Okada K, Yoshida O, Yamabe H, Ohno Y, Hayes RB, et al. A case-control study of prostatic cancer with reference to dietary habits. Prostate. 1988;12:179–190. doi: 10.1002/pros.2990120208. [DOI] [PubMed] [Google Scholar]
- 41.Petimar J, Wilson KM, Wu K, Wang M, Albanes D, van den Brandt PA, et al. A pooled analysis of 15 prospective cohort studies on the association between fruit, vegetable, and mature bean consumption and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:1276–1287. doi: 10.1158/1055-9965.EPI-16-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawada N, Iwasaki M, Yamaji T, Shimazu T, Inoue M, Tsugane S Japan Public Health Center-based Prospective Study Group. Soy and isoflavone consumption and subsequent risk of prostate cancer mortality: the Japan Public Health Center-based Prospective Study. Int J Epidemiol. 2020;49:1553–1561. doi: 10.1093/ije/dyaa177. [DOI] [PubMed] [Google Scholar]
- 43.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49:1857–1860. [PubMed] [Google Scholar]
- 44.Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, Okumura K, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95:238–242. doi: 10.1111/j.1349-7006.2004.tb02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sung JF, Lin RS, Pu YS, Chen YC, Chang HC, Lai MK. Risk factors for prostate carcinoma in Taiwan: a case-control study in a Chinese population. Cancer. 1999;86:484–491. [PubMed] [Google Scholar]
- 46.Villeneuve PJ, Johnson KC, Kreiger N, Mao Y The Canadian Cancer Registries Epidemiology Research Group. Risk factors for prostate cancer: results from the Canadian National Enhanced Cancer Surveillance System. Cancer Causes Control. 1999;10:355–367. doi: 10.1023/a:1008958103865. [DOI] [PubMed] [Google Scholar]
- 47.Křížová L, Dadáková K, Kašparovská J, Kašparovský T. Isoflavones. Molecules. 2019;24:1076. doi: 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reger MK, Zollinger TW, Liu Z, Jones JF, Zhang J. Dietary intake of isoflavones and coumestrol and the risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2018;142:719–728. doi: 10.1002/ijc.31095. [DOI] [PubMed] [Google Scholar]
- 49.Ajdžanovic V, Filipovic B, Miljic D, Mijatovic S, Maksimovic-Ivanic D, Miler M, et al. Prostate cancer metastasis and soy isoflavones: a dogfight over a bone. EXCLI J. 2019;18:106–126. [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmoud AM, Zhu T, Parray A, Siddique HR, Yang W, Saleem M, et al. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS One. 2013;8:e78479. doi: 10.1371/journal.pone.0078479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57:1–10. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R, et al. Deregulation of PI3K/Akt/mTOR signaling pathways by isoflavones and its implication in cancer treatment. Anticancer Agents Med Chem. 2013;13:1014–1024. doi: 10.2174/18715206113139990117. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- 54.Touny LH, Banerjee PP. Identification of both Myt-1 and Wee-1 as necessary mediators of the p21-independent inactivation of the cdc-2/cyclin B1 complex and growth inhibition of TRAMP cancer cells by genistein. Prostate. 2006;66:1542–1555. doi: 10.1002/pros.20495. [DOI] [PubMed] [Google Scholar]
- 55.Mace TA, Ware MB, King SA, Loftus S, Farren MR, McMichael E, et al. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci Rep. 2019;9:5068. doi: 10.1038/s41598-019-41687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ying C, Hsu JT, Hung HC, Lin DH, Chen LF, Wang LK. Growth and cell cycle regulation by isoflavones in human breast carcinoma cells. Reprod Nutr Dev. 2002;42:55–64. doi: 10.1051/rnd:2002006. [DOI] [PubMed] [Google Scholar]
- 57.Pang D, Yang C, Luo Q, Li C, Liu W, Li L, et al. Soy isoflavones improve the oxidative stress induced hypothalamic inflammation and apoptosis in high fat diet-induced obese male mice through PGC1-alpha pathway. Aging (Albany NY) 2020;12:8710–8727. doi: 10.18632/aging.103197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitagishi Y, Kobayashi M, Matsuda S. Defective DNA repair systems and the development of breast and prostate cancer (review) Int J Oncol. 2013;42:29–34. doi: 10.3892/ijo.2012.1696. [DOI] [PubMed] [Google Scholar]
- 59.Rizzo J, Min M, Adnan S, Afzal N, Maloh J, Chambers CJ, et al. Soy protein containing isoflavones improves facial signs of photoaging and skin hydration in postmenopausal women: results of a prospective randomized double-blind controlled trial. Nutrients. 2023;15:4113. doi: 10.3390/nu15194113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canivenc-Lavier MC, Bennetau-Pelissero C. Phytoestrogens and health effects. Nutrients. 2023;15:317. doi: 10.3390/nu15020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38:15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- 62.Ramírez-de-Arellano A, Pereira-Suárez AL, Rico-Fuentes C, López-Pulido EI, Villegas-Pineda JC, Sierra-Diaz E. Distribution and effects of estrogen receptors in prostate cancer: associated molecular mechanisms. Front Endocrinol (Lausanne) 2022;12:811578. doi: 10.3389/fendo.2021.811578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakai S, Fujita M, Kamei Y. Health promotion effects of soy isoflavones. J Nutr Sci Vitaminol (Tokyo) 2020;66:502–507. doi: 10.3177/jnsv.66.502. [DOI] [PubMed] [Google Scholar]
- 64.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 65.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119–1134. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8:754. doi: 10.3390/nu8120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rebbeck TR. Prostate cancer genetics: variation by race, ethnicity, and geography. Semin Radiat Oncol. 2017;27:3–10. doi: 10.1016/j.semradonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Applegate CC, Rowles JL, Ranard KM, Jeon S, Erdman JW. Soy consumption and the risk of prostate cancer: an updated systematic review and meta-analysis. Nutrients. 2018;10:40. doi: 10.3390/nu10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer. 2005;117:667–669. doi: 10.1002/ijc.21266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The search strategy in PubMed, EBSCO, Web of Science, and Cochrane Library database
Characteristics of included observational studies in the meta-analysis
Quality assessment of cohort studies included
Quality assessment of case control studies included