Abstract
Treating cognitive impairment is a holy grail of modern clinical neuroscience. In the past few years, non-invasive brain stimulation is increasingly emerging as a therapeutic approach to ameliorate performance in patients with cognitive impairment and as an augmentation approach in persons whose cognitive performance is within normal limits. In patients with Alzheimer’s disease, better understanding of brain connectivity and function has allowed for the development of different non-invasive brain stimulation protocols. Recent studies have shown that transcranial stimulation methods enhancing brain plasticity with several modalities have beneficial effects on cognitive functions. Amelioration has been shown in preclinical studies on behaviour of transgenic mouse models for Alzheimer’s pathology and in clinical studies with variable severity of cognitive impairment. While the field is still grappling with issues related to the standardization of target population, frequency, intensity, treatment duration and stimulated region, positive outcomes have been reported on cognitive functions and on markers of brain pathology. Here we review the most encouraging protocols based on repetitive transcranial magnetic stimulation, transcranial direct current stimulation, transcranial alternating current stimulation, visual-auditory stimulation, photobiomodulation and transcranial focused ultrasound, which have demonstrated efficacy to enhance cognitive functions or slow cognitive decline in patients with Alzheimer’s disease. Beneficial non-invasive brain stimulation effects on cognitive functions are associated with the modulation of specific brain networks. The most promising results have been obtained targeting key hubs of higher-level cognitive networks, such as the frontal-parietal network and the default mode network. The personalization of stimulation parameters according to individual brain features sheds new light on optimizing non-invasive brain stimulation protocols for future applications.
Keywords: transcranial magnetic stimulation, transcranial direct current stimulation, transcranial alternating current stimulation, photobiomodulation, transcranial focused ultrasound, Alzheimer’s disease
Treating cognitive impairment is a holy grail of modern clinical neuroscience. Koch et al. review new approaches using non-invasive brain stimulation to try to enhance cognitive function or slow cognitive decline in patients with Alzheimer’s disease.
Introduction
The estimated global number of persons suffering from dementia currently exceeds 57 million people, a number that is expected to increase by nearly 300% to 152 million by 2050.1 The global socioeconomic costs of dementia are projected to increase from USD 1.3 trillion to USD 9.1 trillion in 2050. Treating dementia is climbing up as a priority in the agenda of governmental agencies and international organizations, and increasing numbers of initiatives are aiming to offer early interventions.2 Alzheimer’s disease (AD) is the first cause of dementia, accounting for 60%–80% of dementia cases. Thanks to an in-depth understanding of AD pathophysiology and technological advancements, it is now possible to detect AD pathology before the onset of dementia and to estimate the individual’s risk of developing dementia in the following years.3 Therefore, there is an increasing interest in early treatment of individuals with mild cognitive impairment (MCI) or cognitively unimpaired with dementia risk factors, such as subjective cognitive complaints, APOE ε4 genotype, positivity to AD biomarkers, cardiovascular comorbidity and lifestyle-related risk factors.4
Non-invasive brain stimulation (NIBS) is attracting increasing interest as a possible approach to treat cognitive and functional impairment in patients with neurodegenerative disorders. The field is rapidly evolving with novel techniques and protocols that offer the possibility to develop tailored interventions for AD.5 The most popular NIBS methods include repetitive transcranial magnetic stimulation (rTMS) and transcranial electrical stimulation (tES) based on either direct (tDCS) or alternating (tACS) current. More recently, sensory (visual and/or auditory) stimulation in the gamma (γ) frequency (typically 30–100 Hz), photobiomodulation (PBM) and transcranial focused ultrasound (tFUS) have also drawn the attention of the scientific community. RTMS is currently approved and reimbursed in several countries (such as the USA, the Netherlands and Australia) for the treatment of resistant depression, obsessive compulsive disorder and migraine, based on level A evidence of efficacy.6 Current literature on clinical efficacy of NIBS in patients with overt AD dementia is promising yet conflicting, reaching level B evidence for rTMS and level C evidence for some tES protocols.7-9 While no NIBS technique has currently been approved by the FDA with cognitive indications, one personalized rTMS system and one device for γ-sensory stimulation have recently received the FDA’s breakthrough device designation for digital therapeutics in AD.
Historically, a topographical approach (i.e. the notion of a one-to-one mapping between regions and symptoms) has inspired the field of clinical and cognitive neuroscience including NIBS interventions for AD. However, recent evidence suggests that a network-based approach (i.e. the notion that cognitive functions are regulated by interactions of interconnected hubs over large and distributed areas) more accurately fits clinical and experimental observations. Indeed, NIBS techniques commonly stimulate specific brain areas, but their effects may spread to structurally or functionally connected brain areas, leading to widespread functional effects.10 Currently, knowledge about brain network organization is increasingly informing NIBS interventions, a trend that is expected to expand in the future thanks to precision medicine approaches leveraging on sophisticated approaches combining NIBS with neuroimaging/electrophysiology.5 Such interventions have initially focused on circuits supporting cognitive functions, such as the frontoparietal network involved in working memory and executive functions, and the dorsal and ventral networks for goal and stimulus-driven attentional processes.5 More recently, NIBS has been applied to the default mode network (DMN), a circuit including the precuneus, the hippocampus and the retrosplenial cortex with a key role in autobiographical and episodic memory.11 Functional MRI (fMRI) and high-density EEG, also in combination with TMS, can be used to personalize stimulation parameters, by identifying specific networks with high spatial and temporal precision, allowing accurate targeting for NIBS and defining the best parameters for stimulation at an individual level. fMRI and EEG measures of connectivity can also be used not only to personalize stimulation parameters but also to monitor the effects of a certain therapy as biomarkers of stimulation efficacy.5,12
The aim of this review is to summarize the current evidence on NIBS techniques applied to patients with cognitive impairment within the entire spectrum of clinical presentation ranging from initial memory impairment to overt dementia. We therefore incorporated studies exploring the potential of different NIBS techniques in patients with overt dementia, patients with MCI or prodromal AD and individuals with subjective cognitive decline (SCD).
Stimulation techniques
It is important to emphasize that the different NIBS have different mechanisms of action. For instance, rTMS exerts strong and durable changes in neural plasticity and can modulate long-range connectivity.10 tDCS mainly alters membrane potentials leading to changes in neuronal excitability, while tACS and sensory stimulation mainly entrain neuronal oscillations.7-9
Transcranial magnetic stimulation
TMS is based on the electromagnetic induction of an electric field through a coil connected to a capacitor-discharge stimulator. A time-varying magnetic field penetrates the skull and induces a weak and highly localized electric current directly in the region of the cortex beneath the coil, generating polarization of specific neuronal populations. When a focal coil (i.e. figure-of-eight coil) is used, stimulation is strongest in the region beneath the centre of the coil, independently from the electrical conduction properties of the skull. Despite the focal nature of the stimulation, the effects of TMS can extend beyond the immediate site of stimulation by propagating trans-synaptically in interconnected areas along neural networks.5,13,14
When TMS is applied in repetitive trains of pulses with specific stimulation patterns, i.e. rTMS, it can modulate cortical activity in the long-term depending on the stimulation frequency. High frequency rTMS (>5 Hz) induces excitatory effects whereas low frequency rTMS (<1 Hz) induces inhibitory effects, resulting in long-lasting synaptic plasticity-related changes. Although the precise mechanisms are still debated, it is commonly assumed that rTMS protocols, including theta-burst stimulation (TBS), induce long-lasting changes in cortical excitability by modulating the neuronal cells’ synaptic activity, similar to what occurs during long-term potentiation (LTP) or depression (LTD) in animal models.13
The impairment of LTP-like cortical plasticity has recently been identified as one of the key neurophysiological features in AD.15 From this perspective, rTMS might be an ideal tool to restore altered LTP and promote functional rearrangements of synaptic activity. Experimental studies in animal models of AD have shown that rTMS restores key neurophysiological mechanisms related to synaptic functions, such as LTP and ion channel activity. RTMS-rescued deficits in LTP and spatial memory of rats with amyloid-β (Aβ) injection, indicating that rTMS non-invasively and effectively increases hippocampal neurotrophins and NMDA-receptor contents in Aβ1-42-induced toxicity model rats.16 Moreover, rTMS counteracts the reduction in neuronal excitability and ion channel activity in dentate gyrus granule neurons, as demonstrated by patch clamp recordings.17
Other studies confirmed that rTMS decreases Aβ and phosphorylated tau deposits, increases neurogenic proteins, such as brain-derived neurotrophic factor, and reduces pro-inflammatory cytokines such as IL-6 and TNF-α.18,19 In animal models of AD, rTMS treatment inhibits the expression of BACE1 and elevates the level of IDE, suggesting that the reduction of Aβ load could be attributed to the inhibition of Aβ production and facilitation of Aβ degradation.20 Importantly, rTMS treatment significantly increased the drainage efficiency of brain clearance pathways, including the glymphatic system in brain parenchyma and the meningeal lymphatics, in the 5xFAD mouse model.21
Transcranial electrical stimulation
TES techniques, such as tDCS and tACS, modulate brain excitability through a weak electric current (typically at 1–2 mA of intensity) between two or more electrode patches placed on the scalp (Table 1). tDCS is based on a direct current flowing through two (or more) electrodes, i.e. anode and cathode, that can modulate membrane potential, and therefore enhancing (anodal tDCS) or decreasing (cathodal tDCS) cortical excitability, involving changes in GABA and glutamate receptors.22 TACS is based on an alternating (sinusoidal) current to entrain brain oscillations depending on the frequency of the stimulation.23 TACS can drive brain oscillations by entraining target oscillatory generators with stimulation bursts at specific frequencies.24 An increasing body of evidence suggests that γ brain oscillations play a crucial, even though not fully understood, role in normal cognitive performance and AD.24,25 Gamma oscillations typically emerge from the coordinated interaction of brain excitation and inhibition, are prominent across multiple brain regions, including the hippocampus, and are involved in various higher brain functions including long-term memory, working memory, attention, sensory processing, neural communication and synaptic plasticity.26 Abnormal low frequency oscillations and dysregulation of γ-oscillations have been observed in AD patients,24,27 and might contribute to the cognitive deficits and synaptic dysfunctions commonly observed in the disease. Indeed, synaptic dysfunction, along with amyloid and tau deposition, is an early event in AD pathophysiology, preceding neuronal loss.15,28-30 The disruption of γ-oscillations in AD is proportional to disease severity, possibly resulting from the loss of GABAergic inhibitory interneurons.24 In light of this, γ-tACS provides a unique opportunity to modulate relevant brain oscillations in a frequency-specific manner and to assess their functional impact on distinct cognitive functions. However, it has to be noticed that the pathophysiology of gamma-band alteration represents a common denominator for different neuropsychiatric disorders, not being specific for AD.31 While experimental evidence in animal models of AD is lacking, PET imaging research indicates that the effect of γ-tACS in patients with AD may occur through a decrease of tau burden in the medial temporal cortex, while Aβ and microglial activation are not significantly influenced by stimulation.32 Moreover, a novel technology based on electrical stimulation, transcranial temporal interference electrical stimulation, now allows us to target deep brain structures, that are involved in cognitive processing, in humans, such as the hippocampus or the striatum.33-36 This method could further expand the armamentarium of NIBS in AD with the unique ability to target deep structures focally in non-invasive and safe ways.
Table 1.
Features of non-invasive brain stimulation techniques
| NIBS technique | Physical phenomenon | Frequency | Intensity | Duration | Required personnel | Setting requirements | Estimated device cost |
|---|---|---|---|---|---|---|---|
| Repetitive transcranial magnetic stimulation (rTMS) | Repetitive electromagnetic field | 5–50 Hz | 0.8–1.5 T | 2–24 weeks 3–5 sessions/week 800–3000 pulses/session |
Health professional | Hospital/clinic | €60–120k (€40–60k)a |
| Transcranial direct current stimulation (tDCS) | Continuous electrical current | NA | 1–2 mA | 2–5 weeks 2–5 sessions/week 15–45 min/session |
Health professional Trained caregiver |
Hospital/clinic Home–based |
€5–20k |
| Transcranial alternating current stimulation (tACS) | Alternating electrical current | 40 Hz | 1–2 mA | 4–6 weeks 2–5 sessions/week 30–60 min/session |
Health professional Trained caregiver |
Hospital/clinic Home–based |
€5–20k |
| Sensory stimulation | Repetitive time-varying sensory stimuli (flashes, sounds, vibrations) | 40 Hz | NA | 12–24 weeks 5 sessions/week 60 min/session |
Health professional Trained caregiver |
Hospital/clinic Home–based |
€1–5k |
| Photobiomodulation (PBM) | Light emitted from a laser or light-emitting diode | Red to near infrared spectrum (600 nm–810 nm) | <60 J/cm² | 8 weeks 3–5 sessions/week 10–25 min/session |
Health professional | Hospital/clinic Home–based |
€2–10k |
| Transcranial focused ultrasound (tFUS) | Focused ultrasound pulses | <700 kHz; brief trains applied at 1–5 Hz (for TPS) | 0.2–0.3 mJ/mm2 | 2–4 weeks 5 sessions/week 1000–6000 pulses/session |
Health professional | Hospital/clinic | €40–80k |
Intensity, frequency, duration ranges are based on the values most reported in the reviewed studies. NA = not applicable; TPS = transcranial pulse stimulation.
aCost of neuronavigation system, which is needed for precise targeting.
Sensory stimulation
Sensory stimulation may be used to entrain brain oscillations based on the frequency of the time-varying sensory stimuli, given the evidence that exogenous stimuli flickering at a given frequency can induce activity at the same frequency in the brain (Table 1).37 Sensory stimulation commonly consists of lights (visual stimulation) and sounds (auditory stimulation), and more recently also tactile vibrations (vibrotactile stimulation), alone or combined in a multisensory approach, time-varying at a specific frequency. The entrainment of γ-oscillations with sensory stimulation in AD might be a viable approach to improve cognition by restoring brain oscillations and reducing AD pathology (see the ‘Transcranial electrical stimulation’ for more information on the relevance of γ-oscillations for cognition in AD).38 In mouse models of AD, γ-sensory stimulation was reported to reduce the levels of amyloid and phosphorylated tau, affect microglia, preserve neuronal and synaptic density across multiple brain areas, and modify cognitive performance.39 However, the exact mechanisms underlying γ-sensory stimulation remain to be elucidated as a recent study failed to replicate effects on amyloid levels and microglia.40
Photobiomodulation
PBM, also known as low-level laser therapy, involves the use of light in the red to near infrared spectrum from a laser or light-emitting diode.41 PBM targets mainly the mitochondrial cytochrome c oxidase and leads to the activation of several signalling molecules, including cyclic AMP.41 PBM in experimental models of AD was followed by a dose-dependent reduction in the size and number of Aβ plaques in the neocortex and hippocampus,42 increased adenosine triphosphate levels, mitochondrial function, c-fos expression43 and reduced hyperphosphorylated tau, neurofibrillary tangles and oxidative stress markers.42
Transcranial focused ultrasound
Focused ultrasound (FUS) enables deep brain areas to be reached with high spatial specificity compared to other NIBS approaches, offering the unique ability to stimulate key AD regions, such as the hippocampus and medial temporal lobe. When applied at low acoustic intensities, tFUS has the capacity to either enhance or diminish the excitability of specific brain areas.44 The neuromodulatory effects of tFUS can persist beyond the duration of sonication, influencing short-term brain excitability and connectivity, inducing long-term plasticity and modulating behaviour. Typically, tFUS operates at frequencies <700 kHz, markedly lower than those employed in diagnostic ultrasound. In patients with AD, tFUS has demonstrated the ability to transiently alter the permeability of the blood–brain barrier (BBB).45
Safety of NIBS techniques
The safety profiles of NIBS techniques are of paramount importance as their application broadens in both research and clinical domains. TMS is generally well tolerated, with the most common side effects being mild headaches or scalp discomfort following sessions.46 Although TMS carries a rare risk of inducing seizures, adherence to established guidelines and screening patients for potential complications, such as a history of seizures or the presence of metal implants in the head, ensures that this risk remains minimal. Both tDCS and tACS might lead to mild skin irritation or a burning sensation at the electrode sites.46 To mitigate these skin-related effects, proper skin preparation is crucial and it is essential that electrodes do not dry out during sessions. Adherence to recommended current densities and durations is also important for these techniques. Sensory stimulation is generally considered low risk, though some individuals may experience discomfort or sensitivity to specific stimuli, necessitating continuous monitoring and potential adjustments. PBM and tFUS are considered safe, yet their long-term safety profiles require further investigation.
Clinical and biological effects of NIBS
Efficacy of NIBS in patients with Alzheimer’s disease dementia
In patients with dementia due to AD, rTMS is the most investigated NIBS technique followed by tDCS, while fewer than five studies are available for tACS, sensory stimulation, PBM and tFUS.
Repetitive transcranial magnetic stimulation
Recent meta-analyses have reported significant favourable effects of rTMS on several cognitive functions, including global cognition and memory in patients with dementia due to AD. The dorsolateral prefrontal cortex (DLPFC), precuneus and temporal lobe were the most common target regions.7-9,47-52 Most studies used standard rTMS protocols at high frequency (between 10 and 20 Hz), with a minority using TBS protocols. These works reported improvement in clinical scales of global cognition such as the Mini-Mental State Examination (MMSE) and Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) as well as measurements of autonomies of daily living, such as the Alzheimer’s Disease Cooperative Study—Activities of Daily Living Scale (ADCS-ADL). Moreover, it was investigated which parameters are the most influential in the efficacy of the stimulation, focusing on whether a personalized approach, that uses participant-specific neuroimaging scans to select target regions, is more effective than a standardized one-size-fits-all approach (i.e. a predefined region for all participants).7 Stimulations based on either personalized or standardized approaches resulted in similar clinical outcomes. Possible explanations for this unexpected finding may be the insufficient number of studies on personalized approaches, the use of structural MRI to identify the target region (while using the patient’s functional or structural connectivity profile may lead to a greater degree of personalization), and the evidence that NIBS has a widespread effect on the brain extending beyond the target region. A direct correlation between the effect size and the total number of rTMS pulses delivered during the intervention was also reported, while other rTMS parameters did not affect efficacy.7 Besides improving cognition, rTMS also has a positive effect on behavioural and psychological symptoms in patients with AD dementia.48-51 Other approaches used high frequency rTMS in conjunction with concurrent cognitive training, with rTMS being delivered during a few weeks at six different cortical sites, with patients receiving cognitive training overlapping with TMS application. This approach showed some promising results, although it is not clear how cognitive training itself may have produced some beneficial effects.53 A large multisite double-blind randomized trial tested the short and long-term efficacy of rTMS applied to patients with AD at mild-to-moderate stages, in doses of either 2 or 4 weeks of treatment (5 days/week), whilst compared with 4 weeks of sham rTMS. The overall results show significant cognitive improvement after treatment up to 2 months post-treatment with either sham or active coils.54
More recently, the personalization of rTMS parameters has been implemented using TMS in combination with EEG to identify—in each patient—the most effective location and intensity of stimulation able to directly activate neural responses above the stimulated area, the precuneus, within the DMN.12 Taking advantage of combined TMS stimulation and EEG recordings, the study showed that a rTMS intervention of 6 months, consisting of an intensive 2-week phase (five sessions/week) followed by a maintenance 22-week phase (one session/week), applied over the precuneus, proved beneficial for cognitive and functional independence measurements with positive effects on the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), ADAS-Cog, MMSE and ADCS-ADL, compared to sham. Moreover, rTMS enhanced gamma oscillations and stabilized cortical excitability within the DMN after 24 weeks of treatment.12 Another study using fMRI-guided rTMS, reported increased dynamic functional connectivity in the DMN, which positively correlated with changes in global cognition, after 2 weeks of high frequency parietal stimulation, but not at a 12-week follow-up. The change in connectivity at 2 weeks was associated with improvement in global cognition and was specific to the DMN.55
Personalization with fMRI is also showing promising effects in terms of clinical outcomes by hippocampal network–targeted stimulation. In a study involving 41 adults with early AD and amyloid biomarker evidence, participants were randomly assigned to either personalized rTMS or sham stimulation, with 20 sessions over 4 weeks and assessments at 4 and 8 weeks. The rTMS group showed significant improvement in the ADAS-Cog, especially in the memory domain, at 8 weeks. Additionally, the rTMS group had greater improvement on the CDR-SB scale at 4 weeks and 8 weeks, and on the Seoul Instrumental Activities of Daily Living scale at 8 weeks. Functional MRI analysis indicated that rTMS increased connectivity between the hippocampus and precuneus, correlating with improvements in the ADAS-Cog.56
Transcranial direct current stimulation
Several meta-analyses have reported that tDCS improves global cognition and memory in patients with AD dementia.49,57 The most frequently targeted regions were the DLPFC and temporal lobe.57 A recent meta-analysis suggested that tDCS showed greater effectiveness when applied to temporal regions, as opposed to frontal regions.58 The potential benefit of focusing on temporal areas rather than frontal areas in AD could be explained by the alterations in the functional connectivity observed in the medial temporal lobe during the early stages of the AD continuum.59 Overall, the effect of tDCS on cognition is weaker than the effect of rTMS,39 or even not statistically different from sham tDCS,50 possibly due to the limited number of studies and high heterogeneity of data.49,57 This discrepancy might be explained by differences between both techniques, such as higher spatial resolution and neurophysiological specificity in rTMS.60 For instance, a study comparing the neurophysiological effects of both techniques showed that 10 Hz rTMS increased the cortical excitability in the stimulation region in comparison with tDCS.61 However, the comparative effect of these two techniques on brain functioning in clinical populations is still not clear, necessitating additional investigation in future studies.
Transcranial alternating current stimulation
Few studies to date have examined the clinical effects of tACS in patients with AD and the evidence is to be considered more preliminary compared to tDCS. A recent non-randomized study in AD patients showed that γ-tACS applied over the left DLPFC can improve memory performance when combined with cognitive training.62 Comparison of the post-intervention and 1-month follow-up assessments indicated that the tACS group showed a trend towards a better improvement than the non-tACS group.62 γ-TACS targeting temporal regions can increase blood perfusion63 and—in a small case series—reduce tau burden32 in these regions, without inducing clear beneficial effects on cognition. Despite the small evidence available, there are several ongoing randomized controlled trials using home-based treatment as a novel, interesting approach.64
Sensory stimulation
Only a few studies have investigated the clinical efficacy of sensory stimulation in patients with AD. Preliminary evidence suggests that γ-sensory (visual, auditory or combined) stimulation in AD patients may improve global cognition,65 memory,66 sleep and daily living activities,67 increase functional connectivity in the DMN and visual network, and reduce hippocampal and ventricular atrophy,66 but not amyloid burden.68
Photobiomodulation
The clinical use of photobiostimulation in patients with AD has not been tested in large samples of patients to date. A recent pilot study showed an improvement in memory and executive functions in mild-to-moderate AD patients.69 This encouraging observation provides preliminary support for the design of larger trials in AD patients.
Transcranial focused ultrasound
Preclinical studies have suggested that low intensity tFUS may have therapeutic potential for AD by opening the BBB, reducing amyloid pathology, and improving cognition. A recent small study tested image-guided tFUS to the right hippocampus in eight patients with AD. No evidence of transient BBB opening was found on T1 dynamic contrast-enhanced MRI. However, immediate and recognition memory were significantly improved on the verbal learning test. PET image analysis demonstrated increased metabolic activity in the right hippocampus.70 Further larger sham-controlled trials with longer follow-up are warranted to evaluate the efficacy and safety of tFUS in patients with AD.
A different approach uses a novel clinical sonication technique, based on single ultrashort ultrasound pulses (transcranial pulse stimulation, TPS) which markedly differs from existing FUS techniques. Recently, an uncontrolled study in 35 AD patients provided preliminary evidence that TPS can target the memory network, improve its functional connectivity and global cognition over a period of 3 months.71
Efficacy of NIBS in patients with mild cognitive impairment
In patients with MCI, tDCS is the technique for which the most data are available, followed by rTMS and tACS, while only one observational study is currently available for each of sensory stimulation, PBM and tFUS.
Repetitive transcranial magnetic stimulation
Several randomized controlled studies reported clinical results on rTMS in patients with MCI.11,72-77 These studies implemented rTMS protocols with relatively heterogeneous parameters (frequency: 5–20 Hz, intensity: 80–120% of resting motor threshold, duration: 10–30 sessions, number of pulses: 1500–3000 per session) and target regions (left DLPFC in three studies; bilateral DLPFC in two studies; precuneus and right DLPFC in one study, respectively). Overall, these studies found a positive, yet heterogeneous, effect on cognition, with improved memory being the most commonly reported positive outcome,11,72,73 followed by improved global cognition,74,75 visuo-spatial performances,76,77 attention75 and language.76 Improvement in apathy has also been reported.75
From a biological perspective, rTMS increased connectivity dynamics within the ventral attention and the left fronto-parietal networks after stimulation of the bilateral DLPFC76 and decreased connectivity dynamics within the DMN after stimulation of the right DLPFC.72 Another study reported increased neural activity in the precuneus accompanied by a modification of effective connectivity dynamics between the precuneus and medial frontal areas after stimulation of the precuneus.11 Enhancement of beta brain oscillations after stimulation of the precuneus has also been reported.11
A total of 14 patients with SCD and 16 patients with MCI were enrolled in a recent study and received 10 Hz rTMS intervention on the bilateral precuneus for 2 weeks. Neurocognitive scales, structural and fMRI were collected at enrolment and after the rTMS intervention. The precuneus rTMS not only enhanced episodic memory in SCD, but also improved multiple cognitive domains in MCI. Post-rTMS intervention, more brain regions showed decreased functional connectivity in SCD and MCI patients, suggesting that precuneus rTMS may protect cerebral cortical plasticity by reducing excessive functional compensation and thus improve cognitive function.78
Recent advances highlighted a new accelerated intermittent theta burst stimulation (aiTBS) protocol, consisting of multiple sessions per day and higher overall pulse doses, in brain modulation. This approach has been developed to treat depression to reduce the duration of rTMS treatment by applying several stimulations sessions daily for multiple days.79
Twenty-four older adults with amnestic MCI (aMCI) due to possible AD were enrolled in a recent phase I trial of open-label accelerated iTBS to the left DLPFC (eight stimulation sessions of 600 pulses of iTBS/day for 3 days).80 Accelerated iTBS induced a significant, large effect size (d = 0.98) improvement in fluid cognition using the NIH Toolbox Cognition Battery from pretreatment to post-treatment in MCI patients.80 Another similar study enrolled 45 patients in early clinical stages of AD and they were randomly assigned to either receive real or sham accelerated iTBS over the left DLPFC. Real stimulation showed markedly better performances in the group average of auditory verbal learning test scores compared to baseline. TMS-EEG revealed that iTBS reinforced this memory-related cortical mechanism by increasing cortical excitability and β-oscillatory activity underlying the TMS target. These novel findings implicated that aiTBS targeting the left DLPFC is rapid-acting, safe and tolerable in AD patients.81
Transcranial direct current stimulation
TDCS has been tested in several randomized controlled studies reporting results on single or multiple sessions of tDCS in patients with MCI. Compared to rTMS protocols, tDCS studies present greater homogeneity of stimulation parameters as all applied excitatory anodal stimulation. Among these, 14 targeted the left prefrontal area, three the right temporo-parietal area, and two the left temporal area. A strong agreement is also observable in the intensity of stimulation, with almost 80% (15/19) of the published studies applying anodal tDCS at 2 mA of intensity, whether only two studies used 1.5 mA and two used 1 mA. The duration of the intervention was more heterogeneous (number of sessions: 1–36, session duration: 15–30 min). Overall, these studies report a positive effect of tDCS on global cognition82-85 and over specific cognitive domains such as memory,84-93 attention87,93 and executive functions.87,90,91 It is also important to note that some studies used tDCS during cognitive training, reporting larger and more specific effects on cognitive abilities than sham tDCS or cognitive training alone.82,89,94 A similar combined approach has been used with a 12-week protocol in which tDCS was used during a Tai-Chi training in 20 older adults with MCI.95 The authors showed that anodal tDCS combined with Tai-Chi training led to greater improvement in dual-task gait performance.95
From a biological perspective, anodal tDCS of the left DLPFC resulted in changes in key nodes of the DMN, ventral attention network and frontal-parietal network, such as the precuneus and insular cortex,96 in increased temporal pole functional amplitude in MCI APOE ε4 carriers but not in non-carriers,97 and in increased regional cerebral metabolism in the dorsolateral, ventrolateral and medial prefrontal cortices, the dorsal anterior cingulate, the anterior and posterior insular regions, and the hippocampal and parahippocampal regions.98 In contrast, tDCS of the right posterior parietal cortex increased the segregation levels of the DMN and the dorsal attention network, which were restored to a normal range of values99 and resulted in the enhancement of GABA levels along with a reduction of glutamate/GABA ratio.100
Moreover, a recent randomized trial showed that cognitive training with concurrent anodal tDCS of the left DLPFC, compared to cognitive training with sham stimulation, did not lead to superior performance enhancements in patients with MCI or SCD. Together, these findings do not support the immediate benefit of the combined intervention on the trained function. Future research needs to explore whether individualized protocols for both training and stimulation parameters might further enhance treatment gains.101
Transcranial alternating current stimulation
TACS is emerging as a promising approach to treat MCI patients, with randomized controlled studies reporting positive results on tACS in patients with MCI.102-104 However, these studies used single session γ-tACS (intensity: 2–3 mA, duration: 30–60 min) targeting the DLPFC or precuneus. Overall, γ-tACS positively affected cognition, with improved executive functions (but not attention) after stimulation of the DLPFC102 and improved episodic and associative memory after stimulation of the precuneus.103,104 From a biological perspective, γ-tACS resulted in the enhancement of beta brain oscillations after stimulation of the DLPFC102 and enhancement of beta and gamma and reduction of theta oscillations after stimulation of the precuneus.104 Moreover, γ-tACS of the precuneus also led to an improvement of cholinergic transmission evaluated using a TMS protocol assessing short-latency afferent inhibition, an indirect measure of cholinergic neurotransmission.103,104
Sensory stimulation
Evidence for the use of sensory stimulation in MCI is still preliminary. An uncontrolled feasibility study in 10 patients with MCI due to AD reported increased functional connectivity within the DMN (i.e. between the posterior cingulate cortex and precuneus) and downregulation of immune factors, but no change in amyloid and tau CSF levels after 8 weeks of gamma visual and auditory flicker stimulation.105
Photobiomodulation
PBM has recently been tested as a potential treatment for patients with MCI. In a single session study, MCI patients who received PBM demonstrated significant improvement in visual memory and reduced haemodynamic response during the task, suggesting a more efficient neural recruitment following treatment.106 A randomized, double-blind, placebo-controlled study in MCI and early AD participants is currently ongoing to test the efficacy, safety and impact of 24 sessions of transcranial PBM delivered over 8 weeks on cognition.107
Transcranial focused ultrasound
The use of tFUS in MCI has not been investigated in depth. A recent open-label study recruited 19 patients with MCI to receive neuro-navigated TPS intervention for 2 weeks, with three sessions per week, reaching statistically significant effects on global cognition and executive functions after the intervention.108
Efficacy of NIBS in individuals with subjective cognitive decline
Repetitive transcranial magnetic stimulation
In elderly subjects with SCD, a study suggested a possible effect of rTMS in transiently improving associative memory.109 It was a single-session study targeting the prefrontal cortex, with fMRI before and after stimulation. The improvement was accompanied by increased activation of the right prefrontal and bilateral posterior cortex during a face-name association task at fMRI post-stimulation.109 A randomized sham-controlled study on rTMS in SCD reported positive effects of multiple sessions of precuneus stimulation on episodic memory.110 The study showed improvement of episodic memory associated with a decreased connectivity between some hippocampal subregions and other brain regions (i.e. between the middle cognitive hippocampal subregion and the left parahippocampal gyrus, and between the posterior perceptual hippocampal subregion and the left middle temporal gyrus), but no significant effects on depression levels.110 However, a limitation of this study was the small sample size (eight subjects in the rTMS group versus five subjects in the sham group). While no evidence is available for other risk factors, a study on rTMS in cognitively unimpaired APOE ɛ4 allele carriers is currently ongoing.111 In a recent study, after undergoing 2 weeks of rTMS treatment, notable changes were observed in the effective connectivity patterns of the DMN and Central Executive Network in patients with SCD, including improvements in episodic memory. Specifically, the effective connectivity of the angular gyrus to the precuneus, anterior cingulate gyrus, medial frontal gyrus and inferior occipital gyrus in the DMN increased.112
Transcranial direct current stimulation
Few studies have investigated the use of tDCS in SCD patients, all targeting the left prefrontal cortex.113-115 The first two studies showed that single-session tDCS (1.5 mA, 15 min) improved or maintained episodic memory.113,114 Greater memory improvement was associated with higher DMN functional connectivity and temporal lobe thickness in one study.114 The third study showed that 12 tDCS sessions (2 mA, 20 min) associated with cognitive training was more effective than multi-session cognitive training alone in alleviating concerns regarding memory impairment in elderly SCD individuals.115
Transcranial alternating current stimulation
A recent study assessed tACS at theta frequency over the medial prefrontal cortex in SCD, showing an improvement in episodic memory, associated with a change in EEG current source density.116 In healthy individuals, recent evidence suggests that tACS applied at theta and gamma frequencies may improve short- and long-term memory.117 Recently, a 4-day treatment of tACS showed long-lasting memory improvement lasting up to a month in the elderly, putting the basis for novel clinical applications of tACS in individuals at high risk of dementia.118
Interpretation
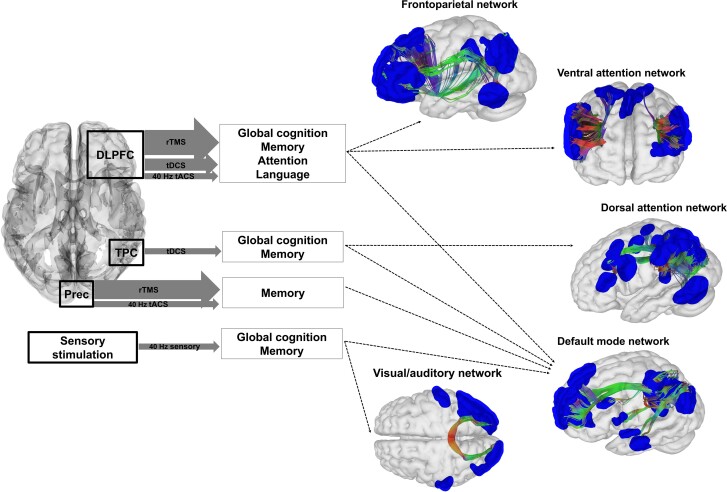
Increasing amounts of evidence suggest that NIBS might be a promising approach to enhance cognitive functions or slow cognitive decline in individuals with AD at different stages of severity. The most promising results with NIBS have been obtained targeting key hubs of higher cognitive networks, such as the frontal-parietal network and the DMN. Specifically, the strongest effect has been obtained with rTMS at high frequency (10–20 Hz) targeting the (i) DLPFC, leading to improvements in global cognition and specific cognitive domains (e.g. memory, attention and language); and (ii) the precuneus, resulting in improvements in memory. A weaker effect on these cognitive functions has been reported for anodal tDCS, mostly targeting the DLPFC and temporal regions, while evidence for γ-tACS applied over the precuneus and DLPFC, as well as for γ sensory stimulation, PBM and tFUS, is preliminary (Fig. 1).
Figure 1.
Effect of non-invasive brain stimulation on cognition in persons at high risk of Alzheimer’s disease dementia or with Alzheimer’s disease dementia. The image summarizes the degree of evidence that non-invasive brain stimulation (NIBS) techniques can improve cognitive functions in individuals at high risk of dementia or with Alzheimer’s disease (AD) dementia. NIBS techniques based on magnetic or electrical stimulation (e.g. rTMS, tDCS, 40 Hz tACS) commonly target the dorsolateral prefrontal cortex (DLPFC), the precuneus (Prec), or the temporoparietal cortex (TPC), while NIBS techniques based on sensory or electrical stimulation (e.g. auditory/visual, tACS) can target specific rhythms (e.g. 40 Hz). NIBS protocols variably affect cognitive functions and these effects are associated with the modulation of specific brain networks. Arrow size denotes the degree of evidence for each NIBS technique (thicker = higher). Functional brain networks are shown in blue and axonal connections with red/green-blue colour coding. Network hubs and axons were derived from resting state functional MRI and diffusion weighted images of a volunteer scanned on 7 T MRI. rTMS = repetitive transcranial magnetic stimulation; tDCS = transcranial direct current stimulation; 40 Hz tACS = transcranial alternate current stimulation in the gamma frequency; 40 Hz sensory = visual and/or auditory sensory stimulation in the gamma frequency; L = left; R = right.
A crucial aspect that warrants further exploration is the sustainability of these benefits in the mid to long-term. While the immediate effects of NIBS are promising, the clinical relevance of these interventions hinges on their lasting impact. Prolonged neuromodulatory treatments, often spanning from two to several weeks, necessitate significant allocation of human resources and place demands on patients for consistent compliance. Available literature on the mid and long-term effects of NIBS is still emerging with preliminary findings, suggesting that while some benefits might persist, others may wane over time, possibly benefitting from booster sessions or continued treatment. The feasibility of such extended treatments, both in terms of resource allocation and patient adherence, is a pivotal consideration. One possible alternative is the development of home-based protocols, allowing patients to receive treatment in the comfort and familiarity of their own home, reducing distress and costs associated with institutional-based care, and increasing patients’ compliance. This aspect is crucial, especially in the case of interventions, such as tACS or tDCS, that do not require advanced technological solutions, such as rTMS.
Despite the promising results mentioned above for patients with AD, research on the clinical efficacy of NIBS in MCI and especially individuals with SCD is still in its infancy. NIBS has been used in large samples of patients with mild-to-moderate dementia with sham-controlled design and for longer periods of time reaching level B evidence for rTMS and level C evidence for some tDCS studies. On the other hand, the literature in patients with MCI and SCD still lacks this level of evidence.
Apart from the beneficial effects reported on cognition and independence of daily living, preliminary evidence is showing that NIBS techniques, such as rTMS and tDCS, can potentially alleviate behavioural disturbances, such as apathy in individuals with cognitive impairment.75,119,120 In patients with MCI, high frequency rTMS over the left DLPFC significantly improved apathy, global cognition and executive function compared to sham.75 In patients with AD, simultaneous application of rTMS and tDCS (rTMS-tDCS) over the bilateral angular gyrus resulted in greater improvement in apathy than sham.121 This new application of NIBS is of great interest as there are no effective pharmacological interventions for apathy in AD or MCI and apathy can have detrimental effects on patient quality of life, dementia severity, disease progression and caregiver burden.119
The potential of NIBS techniques to interact with key mechanisms of brain physiology, such as plasticity, connectivity, and excitability is yet to be fully exploited. The current evidence indicates that the effect of NIBS on cognitive functions is associated with the modulation of specific brain networks, e.g. changes in episodic memory generally involve the DMN, while improvements in global cognition are linked to the frontoparietal network. The personalization of stimulation parameters according to individual brain features sheds new light on optimizing NIBS protocols. Indeed, the preliminary assessment of individual neurophysiological traits, for instance by combining TMS with EEG or fMRI, opens the possibility of developing personalized stimulation protocols with a potentially significant clinical impact. In this scenario, the possibility of creating novel protocols characterized by more robust and long-lasting beneficial effects on cognition, is a realistic expectation. In the future, personalized NIBS protocols may be developed further to reduce the inter-individual variability that currently limit the use of common protocols, such as theta burst stimulation,122 and to increase the overall beneficial effects on cognitive functions. For instance, the dual application of transcranial magnetic and electrical stimulation holds promise in enhancing brain plasticity and connectivity.123
It is also important to consider that some techniques can be used safely at home, tremendously extending the scalability of these interventions. In pursuing more effective treatments for cognitive decline, an emerging avenue of research involves the integration of NIBS with pharmacological interventions that act on neurotransmission or with cognitive training. NIBS could prime the brain to be more receptive to the effects of the drugs, while the drugs could augment the neural plasticity induced by NIBS. This synergic approach could potentiate the benefits of each treatment modality, yielding more robust and sustained cognitive improvements. Current evidence seems to suggest that NIBS could have a relevant impact in treating cognition, behaviour and functional symptoms of patients with AD. Hence the broad clinical impact of NIBS could easily be added on top of pharmacological treatments targeting Aβ deposition, that are showing promise in reducing cognitive decline.124,125 Moreover, NIBS methods could be used to enhance local delivery of drugs acting on the CNS. MRI-guided low-intensity FUS has been shown to reversibly open the BBB, with the potential to non-invasively deliver therapeutic agents to target brain regions in patients with AD and other neurodegenerative conditions.45
It is unclear how the combination of NIBS with cognitive training may lead to a substantial improvement compared to the application of NIBS alone. Some studies directly tested the combination of NIBS with different platforms for cognitive exercise reporting favourable effects on memory functions.62 However, there are no studies that systematically compared the additional value of cognitive training in addition to NIBS.
Adopting a one-size-fits-all approach treating cognitive decline is unrealistic. Future studies should consider the heterogeneity of the risk factors for progression, whether lifestyle-related or genetic/biological, and devise personalized approaches.2 Moreover, no evidence exists to support NIBS efficacy regarding risk factors, such as APOE ε4 genotype, positivity to AD biomarkers, cardiovascular comorbidity, or lifestyle-related risk factors. Importantly, studies on patients with AD dementia provide valuable hints about future research avenues, such as the use of personalized approaches to select target regions and the combination of NIBS and other techniques (e.g. EEG, fMRI), as well as more evidence on the biological outcomes and on sensory stimulation.
Memory clinics are faced with increasing numbers of individuals complaining of cognitive decline who show no or very mild cognitive impairment on neurocognitive testing seeking interventions to ameliorate their cognitive performance.4 Currently, clinicians have little to offer in addition to the traditional symptomatic pharmacological interventions. While the available evidence on NIBS has not yet reached the threshold to change clinical practice significantly, it is strong enough to challenge the notion that cognition cannot be modulated. These promising non-pharmacological treatments could provide a path towards better treatment and care for patients throughout their late life.
Contributor Information
Giacomo Koch, Experimental Neuropsychophysiology Lab, Santa Lucia Foundation IRCCS, 00179 Rome, Italy; Department of Neuroscience and Rehabilitation, University of Ferrara and Center for Translational Neurophysiology of Speech and Communication, Italian Institute of Technology (IIT), 44121 Ferrara, Italy.
Daniele Altomare, Department of Clinical and Experimental Sciences, University of Brescia, 25123 Brescia, Italy.
Alberto Benussi, Neurology Unit, Department of Medical, Surgical and Health Sciences, University of Trieste, 34127 Trieste, Italy.
Lucie Bréchet, Department of Basic Neurosciences, Faculty of Medicine, University of Geneva, 1205 Geneva, Switzerland.
Elias P Casula, Experimental Neuropsychophysiology Lab, Santa Lucia Foundation IRCCS, 00179 Rome, Italy; Department of System Medicine, University of Tor Vergata, 00133 Rome, Italy.
Alessandra Dodich, Center for Mind/Brain Sciences (CIMeC), University of Trento, 38068 Rovereto, Italy.
Michela Pievani, Laboratory Alzheimer’s Neuroimaging and Epidemiology, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, 25123 Brescia, Italy.
Emiliano Santarnecchi, Precision Neuroscience and Neuromodulation Program, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, 02114 Boston, USA.
Giovanni B Frisoni, Laboratory of Neuroimaging of Aging (LANVIE), University of Geneva, 1205 Geneva, Switzerland; Geneva Memory Center, Geneva University Hospitals, 1205 Geneva, Switzerland.
Funding
G.K. has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101017716 (Neurotwin) and from the Italian Ministry of University No. PE00000006 ‘A multiscale integrated approach to the study of the nervous system in health and disease’ MNESYS.
Competing interests
G.K. has received funding (competitive grants) not related to the current manuscript from the Alzheimer Drug Discovery Foundation (ADDF), European Commission Horizon 2020, Italian Ministry of Health, Italian Ministry of Education (MIUR), Brightfocus Foundation. G.K. also received funding from PIAM farmaceutici Spa and Epitech Group. G.K. is scientific co-founder and holds stocks of Sinaptica Therapeutics. G.K. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from: Epitech, Roche, Novo Nordisk. G.K. has the following patent issued: Combination drug formulations including rotigotine and an acetylcholinesterase inhibitor for the treatment of neurodegenerative diseases (n. 20230381512); Systems and methods for providing personalized targeted non-invasive stimulation to a brain network (n. 11998740). D.A. has received funding not related to the current manuscript through Swiss National Science Foundation and received a prize from Fondation Recherche Alzheimer. A.B. has received funding not related to the current manuscript through Fondation Recherche Alzheimer—SCOR, Fondazione Cariplo Airalzh, served as DSMB Chairman for the project Non-invasive Brain Stimulation for Gamma-induction and Cognitive Enhancement in FTD (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA). Patents issued: Method of generating a diagnostic index for Alzheimer’s disease, electronic apparatus for implementing the method and system (n. 102016000110051); Nontherapeutic method of stimulation with gamma-tACS (n. 102021000000776). L.B. has received funding not related to the current manuscript through Swiss National Science Foundation. E.P.C. has received funding from the Italian Ministry of University and Research under the National Recovery and Resilience Plan (Fondi DM 502/2022—PNRR MC42 Bando Giovani Ricercatori). E.S. has received funding not related to the current manuscript from National Institutes of Health (NIH), Alzheimer Drug Discovery Foundation (ADDF). E.S. is scientific co-founder and holds stocks of Sinaptica Therapeutics. E.S. has a patent issued on Systems and methods for providing personalized targeted non-invasive stimulation to a brain network (n. 11998740). G.B.F. has received funding not related to the current manuscript through the Private Foundation of Geneva University Hospitals from: A.P.R.A.—Association Suisse pour la Recherche sur la Maladie d’Alzheimer, Genève; Fondation Segré, Genève; Ivan Pictet, Genève; Race Against Dementia Foundation, London, UK; Fondation Child Care, Genève; Fondation Edmond J. Safra, Genève; Fondation Minkoff, Genève; Fondazione Agusta, Lugano; McCall Macbain Foundation, Canada; Nicole et René Keller, Genève; Fondation AETAS, Genève. He has received funding through the University of Geneva or Geneva University Hospitals: for IISSs from ROCHE Pharmaceuticals OM Pharma EISAI Pharmaceuticals Biogen Pharmaceuticals and Novo Nordisk; for competitive research projects from: H2020, Innovative Medicines Initiative (IMI), IMI2, Swiss National Science Foundation, and VELUX Foundation. G.B.F. has received consulting fees from: Biogen, Diadem, Roche. All to his institution. G.B.F. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from: Biogen, Roche, Novo Nordisk, GE HealthCare. All to his institution.
References
- 1. GBD 2019 Dementia Forecasting Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frisoni GB, Altomare D, Ribaldi F, et al. Dementia prevention in memory clinics: Recommendations from the European task force for brain health services. Lancet Reg Health Eur. 2023;26:100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ranson JM, Rittman T, Hayat S, et al. Modifiable risk factors for dementia and dementia risk profiling. A user manual for Brain Health Services—Part 2 of 6. Alzheimers Res Ther. 2021;13:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frisoni GB, Molinuevo JL, Altomare D, et al. Precision prevention of Alzheimer’s and other dementias: Anticipating future needs in the control of risk factors and implementation of disease-modifying therapies. Alzheimers Dement. 2020;16:1457–1468. [DOI] [PubMed] [Google Scholar]
- 5. Menardi A, Rossi S, Koch G, et al. Toward noninvasive brain stimulation 2.0 in Alzheimer’s disease. Ageing Res Rev. 2022;75:101555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen SL, Bikson M, Badran BW, George MS. A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices. Brain Stimul. 2022;15:73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menardi A, Dotti L, Ambrosini E, Vallesi A. Transcranial magnetic stimulation treatment in Alzheimer’s disease: A meta-analysis of its efficacy as a function of protocol characteristics and degree of personalization. J Neurol. 2022;269:5283–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Šimko P, Kent JA, Rektorova I. Is non-invasive brain stimulation effective for cognitive enhancement in Alzheimer’s disease? An updated meta-analysis. Clin Neurophysiol. 2022;144:23–40. [DOI] [PubMed] [Google Scholar]
- 9. Gu L, Xu H, Qian F. Effects of non-invasive brain stimulation on Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:410–424. [DOI] [PubMed] [Google Scholar]
- 10. Pini L, Manenti R, Cotelli M, Pizzini FB, Frisoni GB, Pievani M. Non-invasive brain stimulation in dementia: A complex network story. Neurodegener Dis. 2019;18:281–301. [DOI] [PubMed] [Google Scholar]
- 11. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. NeuroImage. 2018;169:302–311. [DOI] [PubMed] [Google Scholar]
- 12. Koch G, Casula EP, Bonnì S, et al. Precuneus magnetic stimulation for Alzheimer’s disease: A randomized, sham-controlled trial. Brain. 2022;145:3776–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siebner HR, Funke K, Aberra AS, et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin Neurophysiol. 2022;140:59–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage. 2012;62:2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Lorenzo F, Ponzo V, Bonnì S, et al. Long-term potentiation–like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann Neurol. 2016;80:202–210. [DOI] [PubMed] [Google Scholar]
- 16. Tan T, Xie J, Liu T, et al. Low-frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) reverses Aβ1–42-mediated memory deficits in rats. Exp Gerontol. 2013;48:786–794. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Z, Ding C, Fu R, Wang J, Zhao J, Zhu H. Low-frequency rTMS modulated the excitability and high-frequency firing in hippocampal neurons of the Alzheimer’s disease mouse model. Brain Res. 2024;1831:148822. [DOI] [PubMed] [Google Scholar]
- 18. Li K, Wang X, Jiang Y, et al. Early intervention attenuates synaptic plasticity impairment and neuroinflammation in 5xFAD mice. J Psychiatr Res. 2021;136:204–216. [DOI] [PubMed] [Google Scholar]
- 19. McNerney MW, Heath A, Narayanan SK, Yesavage J. Repetitive transcranial magnetic stimulation improves brain-derived neurotrophic factor and cholinergic signaling in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 2022;86:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang H, Zhu Y, Liao L, et al. The long-term effects of intermittent theta burst stimulation on Alzheimer’s disease-type pathologies in APP/PS1 mice. Brain Res Bull. 2023;202:110735. [DOI] [PubMed] [Google Scholar]
- 21. Lin Y, Jin J, Lv R, et al. Repetitive transcranial magnetic stimulation increases the brain’s drainage efficiency in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paulus W. Chapter 26 transcranial direct current stimulation (tDCS). In: Paulus W, Tergau F, Nitsche MA, Rothwell JG, Ziemann U, Hallett M, eds. Supplements to clinical neurophysiology. Transcranial magnetic stimulation and transcranial direct current stimulation; vol 56. Elsevier; 2003:249–254. doi: 10.1016/S1567-424X(09)70229-6 [DOI] [PubMed] [Google Scholar]
- 23. Antal A, Paulus W. Transcranial alternating current stimulation (tACS). Front Hum Neurosci. 2013;7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Traikapi A, Konstantinou N. Gamma oscillations in Alzheimer’s disease and their potential therapeutic role. Front Syst Neurosci. 2021;15:782399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17:777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Casula EP, Pellicciari MC, Bonnì S, et al. Decreased frontal gamma activity in Alzheimer disease patients. Ann Neurol. 2022;92:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. [DOI] [PubMed] [Google Scholar]
- 29. Francesco DL, Koch G. Synaptic impairment: The new battlefield of Alzheimer’s disease. Alzheimers Dement. 2021;17:314–315. [DOI] [PubMed] [Google Scholar]
- 30. Mecca AP, Chen MK, O’Dell RS, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 2020;16:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manippa V, Palmisano A, Nitsche MA, et al. Cognitive and neuropathophysiological outcomes of gamma-tACS in dementia: A systematic review. Neuropsychol Rev. 2024;34:338–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dhaynaut M, Sprugnoli G, Cappon D, et al. Impact of 40 Hz transcranial alternating current stimulation on cerebral tau burden in patients with Alzheimer’s disease: A case series. J Alzheimers Dis. 2022;85:1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Violante IR, Alania K, Cassarà AM, et al. Non-invasive temporal interference electrical stimulation of the human hippocampus. Nat Neurosci. 2023;26:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Popa T, Beanato E, Wessel MJ, et al. Effects of hippocampal noninvasive theta-burst stimulation on consolidation of associative memory in healthy older adults. bioRxiv. [Preprint] doi: 10.1101/2023.10.11.554933 [DOI]
- 35. Wessel MJ, Beanato E, Popa T, et al. Noninvasive theta-burst stimulation of the human striatum enhances striatal activity and motor skill learning. Nat Neurosci. 2023;26:2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vassiliadis P, Beanato E, Popa T, et al. Non-invasive stimulation of the human striatum disrupts reinforcement learning of motor skills. Nat Hum Behav. 2024;8:1581–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24:333–339. [DOI] [PubMed] [Google Scholar]
- 38. Iaccarino HF, Singer AC, Martorell AJ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martorell AJ, Paulson AL, Suk HJ, et al. Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell. 2019;177:256–271.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soula M, Martín-Ávila A, Zhang Y, et al. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat Neurosci. 2023;26:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abijo A, Lee CY, Huang CY, Ho PC, Tsai KJ. The beneficial role of photobiomodulation in neurodegenerative diseases. Biomedicines. 2023;11:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex—Evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Enengl J, Hamblin MR, Dungel P. Photobiomodulation for Alzheimer’s disease: Translating basic research to clinical application. J Alzheimers Dis. 2020;75:1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarica C, Nankoo JF, Fomenko A, et al. Human studies of transcranial ultrasound neuromodulation: A systematic review of effectiveness and safety. Brain Stimul. 2022;15:737–746. [DOI] [PubMed] [Google Scholar]
- 45. Meng Y, Goubran M, Rabin JS, et al. Blood–brain barrier opening of the default mode network in Alzheimer’s disease with magnetic resonance-guided focused ultrasound. Brain. 2023;146:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rossi S, Antal A, Bestmann S, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol. 2021;132:269–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin Y, Jiang WJ, Shan PY, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: A systematic review and meta-analysis. J Neurol Sci. 2019;398:184–191. [DOI] [PubMed] [Google Scholar]
- 48. Teselink J, Bawa KK, Koo GK, et al. Efficacy of non-invasive brain stimulation on global cognition and neuropsychiatric symptoms in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res Rev. 2021;72:101499. [DOI] [PubMed] [Google Scholar]
- 49. Chu CS, Li CT, Brunoni AR, et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: A component network meta-analysis. J Neurol Neurosurg Psychiatry. 2021;92:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X, Mao Z, Yu X. The role of noninvasive brain stimulation for behavioral and psychological symptoms of dementia: A systematic review and meta-analysis. Neurol Sci. 2020;41:1063–1074. [DOI] [PubMed] [Google Scholar]
- 51. Vacas SM, Stella F, Loureiro JC, Simões do Couto F, Oliveira-Maia AJ, Forlenza OV. Noninvasive brain stimulation for behavioural and psychological symptoms of dementia: A systematic review and meta-analysis. Int J Geriatr Psychiatry. 2019;34:1336–1345. [DOI] [PubMed] [Google Scholar]
- 52. Chou YH, Ton That V, Sundman M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2020;86:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sabbagh M, Sadowsky C, Tousi B, et al. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimers Dement. 2019;16:641–650. [DOI] [PubMed] [Google Scholar]
- 54. Moussavi Z, Uehara M, Rutherford G, et al. Repetitive transcranial magnetic stimulation as a treatment for Alzheimer’s disease: A randomized placebo-controlled double-blind clinical trial. Neurotherapeutics. 2024;21:e00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei L, Zhang Y, Wang J, et al. Parietal-hippocampal rTMS improves cognitive function in Alzheimer’s disease and increases dynamic functional connectivity of default mode network. Psychiatry Res. 2022;315:114721. [DOI] [PubMed] [Google Scholar]
- 56. Jung YH, Jang H, Park S, et al. Effectiveness of personalized hippocampal network–targeted stimulation in Alzheimer disease: A randomized clinical trial. JAMA Netw Open. 2024;7:e249220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Majdi A, van Boekholdt L, Sadigh-Eteghad S, Mc Laughlin M. A systematic review and meta-analysis of transcranial direct-current stimulation effects on cognitive function in patients with Alzheimer’s disease. Mol Psychiatry. 2022;27:2000–2009. [DOI] [PubMed] [Google Scholar]
- 58. Fernandes SM, Mendes AJ, Rodrigues PFS, Conde A, Rocha M, Leite J. Efficacy and safety of repetitive Transcranial Magnetic Stimulation and transcranial Direct Current Stimulation in memory deficits in patients with Alzheimer’s disease: Meta-analysis and systematic review. Int J Clin Health Psychol. 2024;24:100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berron D, van Westen D, Ossenkoppele R, Strandberg O, Hansson O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain. 2020;143:1233–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2:241–245. [DOI] [PubMed] [Google Scholar]
- 61. Simis M, Adeyemo BO, Medeiros LF, Miraval F, Gagliardi RJ, Fregni F. Motor cortex-induced plasticity by noninvasive brain stimulation: A comparison between transcranial direct current stimulation and transcranial magnetic stimulation. Neuroreport. 2013;24:973. [DOI] [PubMed] [Google Scholar]
- 62. Moussavi Z, Kimura K, Kehler L, de Oliveira Francisco C, Lithgow B. A novel program to improve cognitive function in individuals with dementia using transcranial alternating current stimulation (tACS) and tutored cognitive exercises. Front Aging. 2021;2:632545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sprugnoli G, Munsch F, Cappon D, et al. Impact of multisession 40 Hz tACS on hippocampal perfusion in patients with Alzheimer’s disease. Alzheimers Res Ther. 2021;13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Altomare D, Libri I, Alberici A, et al. Plasma biomarkers increase diagnostic confidence in patients with Alzheimer’s disease or frontotemporal lobar degeneration. Alzheimers Res Ther. 2024;16:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clements-Cortes A, Ahonen H, Evans M, Freedman M, Bartel L. Short-term effects of rhythmic sensory stimulation in Alzheimer’s disease: An exploratory pilot study. J Alzheimers Dis. 2016;52:651–660. [DOI] [PubMed] [Google Scholar]
- 66. Chan D, Suk HJ, Jackson BL, et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: Results of feasibility and pilot studies. PLoS One. 2022;17:e0278412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cimenser A, Hempel E, Travers T, et al. Sensory-evoked 40-Hz gamma oscillation improves sleep and daily living activities in Alzheimer’s disease patients. Front Syst Neurosci. 2021;15:746859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ismail R, Hansen AK, Parbo P, et al. The effect of 40-Hz light therapy on amyloid load in patients with prodromal and clinical Alzheimer’s disease. Int J Alzheimer’s Dis. 2018;2018:e6852303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blivet G, Relano-Gines A, Wachtel M, Touchon J. A randomized, double-blind, and sham-controlled trial of an innovative brain-gut photobiomodulation therapy: Safety and patient compliance. J Alzheimers Dis. 2022;90:811–822. [DOI] [PubMed] [Google Scholar]
- 70. Jeong YJ, Yoon HJ, Kang DY, Park KW. Quantitative comparative analysis of amyloid PET images using three radiopharmaceuticals. Ann Nucl Med. 2023;37:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beisteiner R, Matt E, Fan C, et al. Transcranial pulse stimulation with ultrasound in Alzheimer’s disease—A new navigated focal brain therapy. Adv Sci. 2020;7:1902583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cui H, Ren R, Lin G, et al. Repetitive transcranial magnetic stimulation induced hypoconnectivity within the default mode network yields cognitive improvements in amnestic mild cognitive impairment: A randomized controlled study. J Alzheimers Dis. 2019;69:1137–1151. [DOI] [PubMed] [Google Scholar]
- 73. Drumond Marra HL, Myczkowski ML, Maia Memória C, et al. Transcranial magnetic stimulation to address mild cognitive impairment in the elderly: A randomized controlled study. Behav Neurol. 2015;2015:e287843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gy RR, Jv RL, J RG, et al. Effect of transcranial magnetic stimulation as an enhancer of cognitive stimulation sessions on mild cognitive impairment: Preliminary results. Psychiatry Res. 2021;304:114151. [DOI] [PubMed] [Google Scholar]
- 75. Padala PR, Padala KP, Lensing SY, et al. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 2018;261:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Esposito S, Trojsi F, Cirillo G, et al. Repetitive transcranial magnetic stimulation (rTMS) of dorsolateral prefrontal cortex may influence semantic fluency and functional connectivity in fronto-parietal network in mild cognitive impairment (MCI). Biomedicines. 2022;10:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cirillo G, Pepe R, Siciliano M, et al. Long-Term neuromodulatory effects of repetitive transcranial magnetic stimulation (rTMS) on plasmatic matrix metalloproteinases (MMPs) levels and visuospatial abilities in mild cognitive impairment (MCI). Int J Mol Sci. 2023;24:3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ge H, Chen S, Che Z, et al. rTMS regulates homotopic functional connectivity in the SCD and MCI patients. Front Neurosci. 2023;17:1301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van Rooij SJH, Arulpragasam AR, McDonald WM, Philip NS. Accelerated TMS—Moving quickly into the future of depression treatment. Neuropsychopharmacology. 2024;49:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aghamoosa S, Lopez J, Rbeiz K, et al. A phase I trial of accelerated intermittent theta burst rTMS for amnestic MCI. J Neurol Neurosurg Psychiatry. 2024;95:1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lin H, Liang J, Wang Q, et al. Effects of accelerated intermittent theta-burst stimulation in modulating brain of Alzheimer’s disease. Cereb Cortex. 2024;34:bhae106. [DOI] [PubMed] [Google Scholar]
- 82. Gonzalez PC, Fong KNK, Brown T. Transcranial direct current stimulation as an adjunct to cognitive training for older adults with mild cognitive impairment: A randomized controlled trial. Ann Phys Rehabil Med. 2021;64:101536. [DOI] [PubMed] [Google Scholar]
- 83. Lu H, Chan SSM, Chan WC, Lin C, Cheng CPW, Linda Chiu Wa L. Randomized controlled trial of TDCS on cognition in 201 seniors with mild neurocognitive disorder. Ann Clin Transl Neurol. 2019;6:1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fileccia E, Di Stasi V, Poda R, et al. Effects on cognition of 20-day anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex in patients affected by mild cognitive impairment: A case-control study. Neurol Sci. 2019;40:1865–1872. [DOI] [PubMed] [Google Scholar]
- 85. Satorres E, Escudero Torrella J, Real E, Pitarque A, Delhom I, Melendez JC. Home-based transcranial direct current stimulation in mild neurocognitive disorder due to possible Alzheimer’s disease. A randomised, single-blind, controlled-placebo study. Front Psychol. 2023;13:1071737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gu J, Li D, Li Z, et al. The effect and mechanism of transcranial direct current stimulation on episodic memory in patients with mild cognitive impairment. Front Neurosci. 2022;16:811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stonsaovapak C, Hemrungroj S, Terachinda P, Piravej K. Effect of anodal transcranial direct current stimulation at the right dorsolateral prefrontal cortex on the cognitive function in patients with mild cognitive impairment: A randomized double-blind controlled trial. Arch Phys Med Rehabil. 2020;101:1279–1287. [DOI] [PubMed] [Google Scholar]
- 88. Manenti R, Sandrini M, Gobbi E, Binetti G, Cotelli M. Effects of transcranial direct current stimulation on episodic memory in amnestic mild cognitive impairment: A pilot study. J Gerontol B Psychol Sci Soc Sci. 2020;75:1403–1413. [DOI] [PubMed] [Google Scholar]
- 89. Martin DM, Mohan A, Alonzo A, et al. A pilot double-blind randomized controlled trial of cognitive training combined with transcranial direct current stimulation for amnestic mild cognitive impairment. J Alzheimers Dis. 2019;71:503–512. [DOI] [PubMed] [Google Scholar]
- 90. Gomes MA, Akiba HT, Gomes JS, Trevizol AP, de Lacerda ALT, Dias ÁM. Transcranial direct current stimulation (tDCS) in elderly with mild cognitive impairment: A pilot study. Dement Neuropsychol. 2019;13:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Das N, Spence JS, Aslan S, et al. Cognitive training and transcranial direct current stimulation in mild cognitive impairment: A randomized pilot trial. Front Neurosci. 2019;13:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Murugaraja V, Shivakumar V, Sivakumar PT, Sinha P, Venkatasubramanian G. Clinical utility and tolerability of transcranial direct current stimulation in mild cognitive impairment. Asian J Psychiatr. 2017;30:135–140. [DOI] [PubMed] [Google Scholar]
- 93. Rodella C, Bernini S, Panzarasa S, et al. A double-blind randomized controlled trial combining cognitive training (CoRe) and neurostimulation (tDCS) in the early stages of cognitive impairment. Aging Clin Exp Res. 2022;34:73–83. [DOI] [PubMed] [Google Scholar]
- 94. de Sousa AVC, Grittner U, Rujescu D, Külzow N, Flöel A. Impact of 3-day combined anodal transcranial direct current stimulation-visuospatial training on object-location memory in healthy older adults and patients with mild cognitive impairment. J Alzheimers Dis. 2020;75:223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liao YY, Liu MN, Wang HC, Walsh V, Lau CI. Combining transcranial direct current stimulation with tai chi to improve dual-task gait performance in older adults with mild cognitive impairment: A randomized controlled trial. Front Aging Neurosci. 2021;13:766649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. He F, Li Y, Li C, Fan L, Liu T, Wang J. Repeated anodal high-definition transcranial direct current stimulation over the left dorsolateral prefrontal cortex in mild cognitive impairment patients increased regional homogeneity in multiple brain regions. PLoS One. 2021;16:e0256100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kang DW, Wang SM, Kim TY, et al. Impact of transcranial direct current stimulation on cognitive function, brain functional segregation, and integration in patients with mild cognitive impairment according to amyloid-Beta deposition and APOE ε4-allele: A pilot study. Brain Sci. 2021;11:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yun K, Song IU, Chung YA. Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimers Res Ther. 2016;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Iordan AD, Ryan S, Tyszkowski T, Peltier SJ, Rahman-Filipiak A, Hampstead BM. High-definition transcranial direct current stimulation enhances network segregation during spatial navigation in mild cognitive impairment. Cereb Cortex. 2022;32:5230–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lengu K, Ryan S, Peltier SJ, et al. Effects of high definition-transcranial direct current stimulation on local GABA and glutamate levels among older adults with and without mild cognitive impairment: An exploratory study. J Alzheimers Dis. 2021;84:1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Antonenko D, Fromm AE, Thams F, et al. Cognitive training and brain stimulation in patients with cognitive impairment: A randomized controlled trial. Alzheimers Res Ther. 2024;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kim J, Kim H, Jeong H, Roh D, Kim DH. tACS as a promising therapeutic option for improving cognitive function in mild cognitive impairment: A direct comparison between tACS and tDCS. J Psychiatr Res. 2021;141:248–256. [DOI] [PubMed] [Google Scholar]
- 103. Benussi A, Cantoni V, Cotelli MS, et al. Exposure to gamma tACS in Alzheimer’s disease: A randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul. 2021;14:531–540. [DOI] [PubMed] [Google Scholar]
- 104. Benussi A, Cantoni V, Grassi M, et al. Increasing brain gamma activity improves episodic memory and restores cholinergic dysfunction in Alzheimer’s disease. Ann Neurol. 2022;92:322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. He Q, Colon-Motas KM, Pybus AF, et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimers Dement (N Y). 2021;7:e12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chan AS, Lee TL, Hamblin MR, Cheung MC. Photobiomodulation enhances memory processing in older adults with mild cognitive impairment: A functional near-infrared spectroscopy study. J Alzheimers Dis. 2021;83:1471–1480. [DOI] [PubMed] [Google Scholar]
- 107. Iosifescu DV, Song X, Gersten MB, et al. Protocol report on the transcranial photobiomodulation for Alzheimer’s disease (TRAP-AD) study. Healthcare (Basel). 2023;11:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fong TKH, Cheung T, Ngan STJ, et al. Transcranial pulse stimulation in the treatment of mild neurocognitive disorders. Ann Clin Transl Neurol. 2023;10:1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Solé-Padullés C, Bartrés-Faz D, Junqué C, et al. Repetitive transcranial magnetic stimulation effects on brain function and cognition among elders with memory dysfunction. A randomized sham-controlled study. Cereb Cortex. 2006;16:1487–1493. [DOI] [PubMed] [Google Scholar]
- 110. Chen J, Ma N, Hu G, et al. rTMS modulates precuneus-hippocampal subregion circuit in patients with subjective cognitive decline. Aging (Albany NY). 2020;13:1314–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pievani M, Mega A, Quattrini G, et al. Targeting default mode network dysfunction in persons at risk of Alzheimer’s disease with transcranial magnetic stimulation (NEST4AD): Rationale and study design. J Alzheimers Dis. 2021;83:1877–1889. [DOI] [PubMed] [Google Scholar]
- 112. Liang S, Deng W, Li X, et al. Biotypes of major depressive disorder: Neuroimaging evidence from resting-state default mode network patterns. NeuroImage Clin. 2020;28:102514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Manenti R, Sandrini M, Gobbi E, et al. Strengthening of existing episodic memories through non-invasive stimulation of prefrontal cortex in older adults with subjective memory complaints. Front Aging Neurosci. 2017;9:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vaqué-Alcázar L, Mulet-Pons L, Abellaneda-Pérez K, et al. tDCS-induced memory reconsolidation effects and its associations with structural and functional MRI substrates in subjective cognitive decline. Front Aging Neurosci. 2021;13:695232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Stoynova N, Laske C, Plewnia C. Combining electrical stimulation and cognitive control training to reduce concerns about subjective cognitive decline. Brain Stimul. 2019;12:1083–1085. [DOI] [PubMed] [Google Scholar]
- 116. Varastegan S, Kazemi R, Rostami R, Khomami S, Zandbagleh A, Hadipour AL. Remember NIBS? tACS improves memory performance in elders with subjective memory complaints. Geroscience. 2023;45:851–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Reinhart RMG, Nguyen JA. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci. 2019;22:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Grover S, Wen W, Viswanathan V, Gill CT, Reinhart RMG. Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation. Nat Neurosci. 2022;25:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jin Y, Li J, Xiao B. Efficacy and safety of neuromodulation for apathy in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 2024;171:17–24. [DOI] [PubMed] [Google Scholar]
- 120. Padala PR, Boozer EM, Lensing SY, et al. Neuromodulation for apathy in Alzheimer’s disease: A double-blind, randomized, sham-controlled pilot study. J Alzheimers Dis. 2020;77:1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hu Y, Jia Y, Sun Y, et al. Efficacy and safety of simultaneous rTMS–tDCS over bilateral angular gyrus on neuropsychiatric symptoms in patients with moderate Alzheimer’s disease: A prospective, randomized, sham-controlled pilot study. Brain Stimul. 2022;15:1530–1537. [DOI] [PubMed] [Google Scholar]
- 122. Corp DT, Bereznicki HGK, Clark GM, et al. Large-scale analysis of interindividual variability in theta-burst stimulation data: Results from the ‘Big TMS Data Collaboration’. Brain Stimul. 2020;13:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Maiella M, Casula EP, Borghi I, et al. Simultaneous transcranial electrical and magnetic stimulation boost gamma oscillations in the dorsolateral prefrontal cortex. Sci Rep. 2022;12:19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330:512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. [DOI] [PubMed] [Google Scholar]