Abstract
The purpose of the present study was to assess the nutritional value of yeast culture (YC) and to explore the effect of YC on growth performance and health of piglets fed low-protein diets. In Exp. 1, 12 growing barrows were allocated into control diet and YC diet treatments to determine the available energy of YC. Results showed that the digestible energy and metabolizable energy of YC are 12.12 and 11.66 MJ/kg dry matter (DM), respectively. In Exp. 2, 12 growing barrows were surgically equipped with a T-cannula near the distal ileum and were assigned to 2 dietary treatments (nitrogen-free diet and YC diet), and the amino acid digestibility of YC was determined. In Exp. 3, a total of 96 weaned piglets were randomly divided into 4 treatments, including low-protein basal diet (Basal), Basal + 0.5% YC (0.5%YC), Basal + 1.0% YC (1.0%YC), and Basal + 1.5% YC (1.5%YC). The results were as follows: YC supplementation linearly improved the weight gain and feed intake ratio (P < 0.001), linearly increased the activity of glutathione peroxidase on d 14 (P = 0.032) and linearly decreased the concentration of malondialdehyde on d 14 (P = 0.008) and d 32 (P = 0.004) in serum, and linearly decreased the concentration of total short-chain fatty acid on d 14 in feces (P = 0.045). Compared with other treatments, 1.5%YC group showed a greater abundance of various probiotics, such as Prevotellaceae, Prevotella and Turicibacter. In Exp. 4, twelve growing barrows with an ileal T-cannula were randomly assigned to Control and 1.5%YC treatments to clarify the impact of YC supplementation on nitrogen balance and nutrient digestibility. Results showed that YC had no significant effect on nitrogen efficiency and nutrient digestibility, except for trend of reducing the total tract digestibility of organic matter (P = 0.067). In conclusion, the present study assessed the digestible and metabolizable energy values (12.12 and 11.66 MJ/kg DM, respectively) and standardized ileal digestibility of amino acid (from 43.93% to 82.65%) of YC in pig feed and demonstrated that moderate supplementation of YC (1.5% of diet) can effectively improve feed conversion efficiency, enhance antioxidant capacity, and promote a balanced gut microbiota in piglets.
Keywords: Yeast culture, Available energy, Digestible amino acids, Low-protein diet, Pig
1. Introduction
Antibiotics are effective in promoting animal growth, preventing disease spread, and are a conventional solution for post-weaning diarrhea in piglets (Becattini et al., 2016; Wang et al., 2023b; Faccin et al., 2020). However, research has indicated a significant correlation between antibiotic use, pathogenicity in organisms, and antibiotic resistance (Darby et al., 2023; Liu et al., 2018; Roselli et al., 2005). After the prohibition of antibiotic growth promoters (AGP) used in feed, piglets experienced a more pronounced reduction in nutrient digestion and absorption efficiency under weaning conditions, leading to more serious nutritional diarrhea and even death (Barton, 2000). Therefore, the development of alternative technologies to replace antibiotics in piglet production is of significant importance with many prospects (Li et al., 2018; Lillehoj et al., 2018; Low et al., 2021; Omonijo et al., 2018).
Under normal production conditions, low-protein diets can accurately satisfy the nutritional requirements of pigs, and reduce feed costs and pollutant emissions without affecting growth performance (Wang et al., 2018). Recent studies showed that low-protein diets can improve intestinal villi development and promote nutrient digestion and absorption, making it a potentially viable strategy as an alternative to AGP in alleviating weaning stress in piglets (Opapeju et al., 2009; Zhou et al., 2022a). Nevertheless, recent investigations have indicated that after removing AGP, piglets fed with low-protein diets, which were formulated with conventional feedstuffs but not highly digestible protein sources, may display decreased digestive enzyme activity and weakened nutrient digestion capacity (Yu et al., 2019, 2017). Therefore, in the absence of AGP, conventional low-protein diets may not be adequate to satisfy the varied nutritional requirements of piglets, including essential amino acids (AA) and other essential nutrients (Zhou et al., 2020). Facilitating the digestion and absorption of nutrients in piglets fed low-protein diets has become an urgent problem.
Yeast culture (YC) is mainly composed of yeast cell metabolites, fermented variant culture media, and a small amount of inactivated yeast cells (Pang et al., 2022; Shurson, 2018). YC has the potential to promote intestinal health and exhibit immunomodulatory abilities (He et al., 2021; Xu et al., 2018). Otherwise, in both swine and ruminant animals, it has been investigated that supplementation with YC can enhance growth performance, boost milk production, and stimulate the immune system (Nocek et al., 2011). In addition, supplementation with YC in pig feed has been demonstrated to elevate the digestibility of dry matter, gross energy, and crude protein, indicating that YC could improve dietary utilization (Shen et al., 2009). Moreover, the structure and metabolic activity of the gut microbiota profoundly impacts host health, and numerous studies have suggested that YC promotes animal health through this mechanism (Dias et al., 2018; Wang et al., 2023a; Waititu et al., 2017). Therefore, the combination of YC with a low-protein diet is considered a potential nutritional strategy for post-weaning piglets. Since 2013, YC has been officially classified by the catalogue of feed materials as a feed ingredient rather than a feed additive. However, due to the highly effective growth-promoting and health-enhancing properties of YC, it is typically used in relatively small amounts in feed. Currently, deficiency of reference information about the nutritional composition of YC in pig production has led to difficulties in accurately applying its nutritional value when used in feed formulation.
The aims of the current study are: 1) to determine the chemical composition, available energy, and standardized ileal AA digestibility of YC as a feedstuff; 2) to assess the impact of YC supplementation in a low-protein diet on the growth performance, serum parameters, and intestinal health of piglets, and determine the optimal inclusion level of YC; and 3) to investigate the influence of YC on the nutrient digestibility and nitrogen (N) balance in pigs. Ultimately, the goal of this study is to contribute scientific evidence for the practical application of YC under antibiotic-free, low-protein conditions.
2. Materials and methods
2.1. Animal ethic statement
This research adhered to the guidelines for experimental protocols and animal welfare in China. All animal experimentation procedures and uses were approved by the Animal Care Committee of Beijing University of Agriculture (protocol no. BUA2023027, Beijing, China).
2.2. Experimental materials
Four experiments were performed at the Metabolism Laboratory of Dabeinong (Yutian) Swine Science Experiment Center located in Yutian, Hebei, China. The YC used in the present study was produced by fermenting high-quality feed ingredients such as corn and soybean meal with Saccharomyces cerevisiae, and was obtained from Beijing China-Agri Hong Ke Bio-Technology Co, Ltd., China. The analyzed chemical composition of the YC are shown in Table 1.
Table 1.
Composition of the yeast culture (as-fed basis).1
| Item | Content |
|---|---|
| Conventional nutrition, % | |
| Dry matter | 92.96 |
| Gross energy, MJ/kg | 17.12 |
| Crude protein | 23.03 |
| Starch | 8.70 |
| Ether extract | 3.98 |
| Neutral detergent fiber | 49.76 |
| Acid detergent fiber | 18.64 |
| Crude fiber | 11.92 |
| Ash | 8.74 |
| Calcium | 0.92 |
| Amino acid, % | |
| Phosphorus | 0.99 |
| Arginine | 1.11 |
| Histidine | 0.51 |
| Leucine | 1.07 |
| Isoleucine | 0.60 |
| Lysine | 0.75 |
| Methionine | 0.23 |
| Phenylalanine | 0.69 |
| Threonine | 0.58 |
| Tryptophan | 0.20 |
| Valine | 0.81 |
| Alanine | 0.95 |
| Asparagine | 1.37 |
| Cysteine | 0.34 |
| Glutamine | 3.11 |
| Glycine | 0.91 |
| Proline | 0.98 |
| Serine | 0.72 |
| Tyrosine | 0.38 |
| Organic acid, μg/g | |
| Lactate | 53,900 |
| Acetate | 5100 |
| Propionate | 340 |
| Butyrate | 60 |
| Lactic acid | 2.20 |
| Antitrophic factor, mg/kg | |
| Aflatoxin B1 | 0 |
| Zearalenone | 0.04 |
| Deoxynivalenol | 0 |
| Antigenic proteins, % | |
| Mannan | 9.04 |
| β-Glucan | 20.38 |
| Polypeptide | 9.26 |
Yeast culture were purchased from Beijing China-Agri Hong Ke Bio-Technology Co, Ltd., China.
2.3. Exp. 1: Available energy value
This experiment was performed to assess the energy utilization efficiency, digestible energy (DE) and metabolizable energy (ME) of YC in pig diets. Twelve crossbred growing barrows (Duroc × Yorkshire × Landrace; initial body weight [BW] 29.0 ± 6.5 kg) were randomly allocated into 2 treatments (Control diet and YC diet) equally, and the DE and ME of the YC were determined. The nutritional level of the Control diet exceeded the requirements for a growing pig (NRC, 2012). The content of YC in the YC diet was 24.30%. The composition and nutrient levels of diets are shown in Table 2.
Table 2.
Composition and nutrient levels of diets in Exp. 1 (as-fed basis, %).
| Item | Control diet | YC diet |
|---|---|---|
| Ingredients | ||
| Corn | 74.80 | 56.10 |
| Soybean meal | 22.20 | 16.65 |
| Yeast culture1 | 24.30 | |
| Dicalcium phosphate | 1.20 | 1.20 |
| L-Lysine·HCl | 0.20 | 0.15 |
| Limestone | 0.80 | 0.80 |
| NaCl | 0.30 | 0.30 |
| Vitamin and trace element premix2 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Nutrient levels3 | ||
| Dry matter | 91.27 | 91.08 |
| Gross energy, MJ/kg | 16.30 | 16.45 |
| Crude protein | 18.34 | 17.48 |
| Ether extract | 1.92 | 3.82 |
| Neutral detergent fiber | 11.53 | 16.91 |
| Acid detergent fiber | 6.63 | 9.67 |
| Crude fiber | 2.94 | 5.75 |
| Ash | 4.59 | 6.16 |
| Calcium | 0.65 | 0.88 |
| Phosphorus | 0.52 | 0.70 |
YC = yeast culture.
Yeast culture were purchased from Beijing China-Agri Hong Ke Bio-Technology Co, Ltd., China.
Vitamin and trace element premix provided the following per kilogram of diets: vitamin A 5512 IU, vitamin D3 2200 IU, vitamin E 30 IU, vitamin K3 2.2 mg, vitamin B12 27.6 μg, riboflavin 4.0 mg, pantothenic acid 14.0 mg, niacin 30 mg, choline chloride 400 mg, folic acid 0.7 mg, oryzanin 1.5 mg, pyridoxine 3 mg, biotin 44 μg, Mn 40 mg (MnSO4), Fe 75 mg (FeSO4∙H2O), Zn 75 mg (ZnSO4), Cu 100 mg (CuSO4∙5H2O), I 0.3 mg (KI), Se 0.3 mg (Na2SeO3).
Nutrient levels were all analyzed levels.
The pigs were individually housed in stainless-steel metabolic cages (1.4 m × 0.6 m × 0.6 m), where the environment was maintained at 22 ± 2 °C, humidity varied from 50% to 60% during the experiment, and water was freely available at all times. The feed supply was equal to 4% of BW and was divided equally into 2 meals and fed to each pig at 08:00 and 17:00 every day. The experiment lasted 19-d divided into the first 7 d for adaptation to the stainless-steel metabolic cages, the next 7 d for adaptation to the diets, and the last 5 d for feces and urine collection. During the 19-d experiment period, the feed intake of each pig was recorded at every feeding time. The feces were collected into plastic bags (one pig per bag) quickly when they appeared in the metabolic crates and stored at −20 °C. The bucket contained HCl (10 mL; 6 mol/L) to limit microorganism multiplication and reduce the loss of ammonia. Upon excretion by the pig, urine flows down the deflector of the metabolic cage and into the container. The urine volume was recorded every day and the urine samples (20% of the total volume) were collected and stored at −20 °C after being filtered through gauze.
2.4. Exp. 2: Digestible AA content
This experiment was conducted to determine the apparent ileal digestibility (AID) and standardized ileal digestibility (SID) of crude protein and AA of YC fed to pigs. Twelve crossbred growing barrows (initial BW: 27.5 ± 1.3 kg; Duroc × Yorkshire × Landrace) were surgically equipped with a T-cannula near the distal ileum and were randomly assigned to 2 dietary treatments (N-free diet and YC diet) equally (Stein et al., 1998). The N-free diet was made to detect the endogenous AA losses, and 40% of YC as the sole AA source was contained in the YC diet (Table 3).
Table 3.
Composition and nutrient levels of diets in Exp. 2 (as-fed basis, %).
| Item | Nitrogen -free diet | YC diet |
|---|---|---|
| Ingredients | ||
| Yeast culture1 | 40.00 | |
| Cornstarch | 69.00 | 33.40 |
| Sucrose | 20.00 | 20.00 |
| Soybean oil | 3.00 | 3.00 |
| Dicalcium phosphate | 1.60 | 1.60 |
| Cellulose acetate2 | 4.00 | |
| Potassium carbonate | 0.30 | |
| Magnesium oxide | 0.10 | |
| Chromic oxide2 | 0.30 | 0.30 |
| Limestone | 0.90 | 0.90 |
| Salt | 0.30 | 0.30 |
| Mineral and vitamin premix3 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Nutrient levels4 | ||
| Dry matter | 89.12 | 90.78 |
| Crude protein | 0.23 | 9.12 |
| Arginine | 0.42 | |
| Histidine | 0.19 | |
| Leucine | 0.42 | |
| Isoleucine | 0.21 | |
| Lysine | 0.27 | |
| Methionine | 0.09 | |
| Phenylalanine | 0.02 | 0.28 |
| Threonine | 0.22 | |
| Tryptophan | 0.09 | |
| Valine | 0.31 | |
| Alanine | 0.39 | |
| Asparagine | 0.03 | 0.54 |
| Cysteine | 0.05 | 0.15 |
| Glutamine | 1.21 | |
| Glycine | 0.38 | |
| Proline | 0.42 | |
| Serine | 0.28 | |
| Tyrosine | 0.22 | |
YC = yeast culture.
Yeast culture were purchased from Beijing China-Agri Hong Ke Bio-Technology Co, Ltd., China.
Made by Chemical Reagents Company, Beijing, China.
Mineral and vitamin premix provided the following per kilogram of diets: vitamin A 5512 IU, vitamin D3 2200 IU, vitamin E 30 IU, vitamin K3 2.2 mg, vitamin B12 27.6 μg, riboflavin 4.0 mg, pantothenic acid 14.0 mg, niacin 30 mg, choline chloride 400 mg, folic acid 0.7 mg, oryzanin 1.5 mg, pyridoxine 3 mg, biotin 44 μg, Mn 40 mg (MnSO4), Fe 75 mg (FeSO4∙H2O), Zn 75 mg (ZnSO4), Cu 100 mg (CuSO4∙5H2O), I 0.3 mg (KI), Se 0.3 mg (Na2SeO3).
Nutrient levels were all analyzed levels.
The stainless-steel metabolic cages, environment, and feeding method were the same as those in Exp. 1. The whole experiment lasted 21 d within which the first 14 d were for recovery, then 5 d for adaptation to the diets, and the last 2 d for ileal digesta collection. The feed supply was equal to 4% of BW and was divided equally into 2 meals and fed to each pig at 08:00 and 17:00 every day. Plastic bags were used to collect the ileal digesta continuously from 08:00 to 17:00. Whenever the ileal digesta filled the plastic bags, or at least every 30 min, the bags were removed and replaced and the samples were stored in the −20 °C refrigerator. Further processing of the digesta samples followed the description by Pan et al. (2017) throughout the manuscript.
2.5. Exp. 3: Growth performance, antioxidant capacity and microbiota
This experiment was conducted to investigate the effects of YC supplementation in low-protein diets on growth performance, serum parameters, and fecal microbiota of weaned piglets. A total of ninety-six crossbred piglets (initial BW: 7.4 ± 1.2 kg; Duroc × Yorkshire × Landrace) were allocated to 4 dietary treatments, with 6 pens per group and 4 pigs per pen (2 barrows and 2 gilts), as follows: 1) low-protein basal diet (Basal); 2) Basal + 0.5% YC (0.5%YC); 3) Basal + 1.0% YC (1.0%YC); 4) Basal + 1.5% YC (1.5%YC). As the available energy values and digestible AA content of YC had already been determined in the preceding two experiments, the diet for the present experiment was formulated following NRC (2012) based on these results (Table 4).
Table 4.
Composition and nutrient levels of diets in Exp. 3 (as-fed basis, %).
| Item | Basal diet | 0.5%YC | 1.0%YC | 1.5%YC |
|---|---|---|---|---|
| Ingredients | ||||
| Corn | 33.72 | 33.22 | 32.72 | 32.22 |
| Extruded corn | 15.00 | 15.00 | 15.00 | 15.00 |
| Cassava meal | 4.00 | 4.00 | 4.00 | 4.00 |
| Soybean meal | 14.10 | 14.10 | 14.10 | 14.10 |
| Extruded soybean | 2.00 | 2.00 | 2.00 | 2.00 |
| Wheat bran | 2.50 | 2.50 | 2.50 | 2.50 |
| Fish meal | 5.00 | 5.00 | 5.00 | 5.00 |
| Sucrose | 2.00 | 2.00 | 2.00 | 2.00 |
| Glucose | 1.00 | 1.00 | 1.00 | 1.00 |
| Low protein whey powder | 5.00 | 5.00 | 5.00 | 5.00 |
| Broken rice | 10.00 | 10.00 | 10.00 | 10.00 |
| Soy protein concentrate | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean oil | 0.30 | 0.30 | 0.30 | 0.30 |
| Monocalcium phosphate | 0.42 | 0.42 | 0.42 | 0.42 |
| Limestone | 0.98 | 0.98 | 0.98 | 0.98 |
| NaCl | 0.30 | 0.30 | 0.30 | 0.30 |
| L-Lysine HCl | 0.48 | 0.48 | 0.48 | 0.48 |
| DL-Methionine | 0.17 | 0.17 | 0.17 | 0.17 |
| L-Threonine | 0.19 | 0.19 | 0.19 | 0.19 |
| L-Tryptophan | 0.06 | 0.06 | 0.06 | 0.06 |
| L-Valine | 0.11 | 0.11 | 0.11 | 0.11 |
| L-Isoleucine | 0.07 | 0.07 | 0.07 | 0.07 |
| L-Leucine | 0.02 | 0.02 | 0.02 | 0.02 |
| Choline chloride1 | 0.08 | 0.08 | 0.08 | 0.08 |
| Vitamin and mineral premix2 | 0.50 | 0.50 | 0.50 | 0.50 |
| Yeast culture3 | 0.50 | 1.00 | 1.50 | |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutritional value4 | ||||
| Net energy, kcal/kg | 2520 | 2518 | 2516 | 2514 |
| Crude protein | 17.50 | 17.52 | 17.54 | 17.56 |
| Calcium | 0.74 | 0.74 | 0.74 | 0.74 |
| Total phosphorus | 0.66 | 0.66 | 0.66 | 0.66 |
| Crude fiber | 2.45 | 2.45 | 2.45 | 2.45 |
| Neutral detergent fiber | 8.53 | 8.53 | 8.53 | 8.53 |
| Acid detergent fiber | 2.61 | 2.61 | 2.61 | 2.61 |
| SID Lys | 1.22 | 1.22 | 1.22 | 1.22 |
| SID SAA | 0.67 | 0.67 | 0.67 | 0.67 |
| SID Thr | 0.72 | 0.72 | 0.72 | 0.72 |
| SID Trp | 0.21 | 0.21 | 0.21 | 0.21 |
| SID Val | 0.77 | 0.77 | 0.77 | 0.77 |
| SID Leu | 1.22 | 1.22 | 1.22 | 1.22 |
| SID Ile | 0.63 | 0.63 | 0.63 | 0.63 |
| Nutrient levels5 | ||||
| Dry matter | 90.65 | 90.21 | 89.98 | 90.13 |
| Gross energy, MJ/kg | 17.83 | 17.77 | 17.81 | 17.79 |
| Crude protein | 17.45 | 17.51 | 17.51 | 17.49 |
| Crude fiber | 2.75 | 2.65 | 2.77 | 2.79 |
| Neutral detergent fiber | 9.17 | 9.23 | 9.17 | 9.35 |
| Acid detergent fiber | 3.06 | 3.24 | 3.17 | 3.19 |
| Ash | 3.66 | 3.64 | 3.85 | 3.79 |
YC = yeast culture; SAA = sulfur-containing amino acid; SID = standardized ileal digestible.
Made by Chemical Reagents Company, Beijing, China.
Vitamin and mineral premix provided the following per kilogram of diets: vitamin A 5512 IU, vitamin D3 2200 IU, vitamin E 30 IU, vitamin K3 2.2 mg, vitamin B12 27.6 μg, riboflavin 4.0 mg, pantothenic acid 14.0 mg, niacin 30 mg, choline chloride 400 mg, folic acid 0.7 mg, oryzanin 1.5 mg, pyridoxine 3 mg, biotin 44 μg, Mn 40 mg (MnSO4), Fe, 75 mg (FeSO4∙H2O) Zn, 75 mg (ZnSO4), Cu 100 mg (CuSO4∙5H2O), I 0.3 mg (KI), Se 0.3 mg (Na2SeO3).
Yeast culture were purchased from Beijing China-Agri Hong Ke Bio-Technology Co, Ltd., China.
Nutritional value was calculated according to NRC (NRC, 2012).
Nutrient levels were all analyzed levels.
All the pigs were housed in a nursery with controlled environmental conditions, featuring pens with sturdy concrete floors, and were provided unrestricted access to both feed and water. The whole experiment lasted 32 d and pigs were fed their respective diets freely. Body weight and feed consumption of piglets were recorded on d 1, 14, and 32, respectively. The experiment involved recording the feed intake of each pen throughout the study, and subsequently calculating the average daily gain, average daily feed intake, and weight gain and feed intake ratio.
At 06:00 on d 14 and 32, blood samples were collected aseptically through the anterior vena cava from a pig whose weight was close to the average weight per pen. To obtain the serum sample, we centrifuged the blood sample at 3000 × g for 10 min at 4 °C and immediately stored it at −80 °C until analysis. On d 14 and 32 of the experiment, two pigs from each pen were selected, and fresh fecal samples (about 20 g) were collected by manual stimulation of rectum. Then, the fecal samples of two pigs from the same pen were mixed and transferred into a 10-mL centrifuge tube and were stored at −80 °C for the determination of microbial composition and short-chain fatty acid content.
2.6. Exp. 4: Nutrient digestibility
This experiment was conducted to investigate the effects of YC supplementation on total tract and ileal nutrient digestibility of pigs fed low-protein diets. Twelve crossbred growing barrows (initial BW: 23.5 ± 2.0 kg; Duroc × Yorkshire × Landrace) were surgically equipped with a T-cannula near the distal ileum and were randomly assigned to 2 dietary treatments. There were 6 replicates in each diet group and 1 pig per replicate. The experimental diets included a basal low-protein diet (Control diet) and a Control diet supplemented with 1.5% YC (1.5%YC). Diets were supplemented with 0.3% chromium oxide as an exogenous indicator for the calculation of SID and AID, AA and crude protein digestibility. The composition and nutrient levels of diets in the Exp. 4 are presented in Table 5.
Table 5.
Composition and nutrient levels of diets in Exp. 4 (as-fed basis, %).
| Item | Control diet | YC diet |
|---|---|---|
| Ingredients | ||
| Corn | 75.59 | 74.09 |
| Soybean meal | 10.96 | 10.96 |
| Wheat bran | 8.88 | 8.88 |
| Limestone | 0.76 | 0.76 |
| Dicalcium phosphate | 1.30 | 1.30 |
| Salt | 0.40 | 0.40 |
| Vitamin and mineral premix1 | 0.50 | 0.50 |
| L-Lysine HCl | 0.69 | 0.69 |
| DL-Methionine | 0.14 | 0.14 |
| L-Threonine | 0.23 | 0.23 |
| L-Tryptophan | 0.06 | 0.06 |
| L-Valine | 0.19 | 0.19 |
| Chromic oxide2 | 0.30 | 0.30 |
| Yeast culture3 | 1.50 | |
| Total | 100.00 | 100.00 |
| Calculated nutrient levels4 | ||
| Net energy, MJ/kg | 10.28 | 10.28 |
| Crude protein | 13.50 | 13.50 |
| SID Lys | 0.98 | 0.98 |
| SID Met + Cys | 0.55 | 0.55 |
| SID Thr | 0.59 | 0.59 |
| SID Trp | 0.17 | 0.17 |
| SID Val | 0.64 | 0.64 |
| Nutrient levels5 | ||
| Dry matter | 90.65 | 90.75 |
| Gross energy, MJ/kg | 16.55 | 16.72 |
| Crude protein | 13.91 | 14.20 |
| Crude fiber | 3.75 | 3.88 |
| Neutral detergent fiber | 13.11 | 14.60 |
| Acid detergent fiber | 4.13 | 4.06 |
| Ash | 4.92 | 5.26 |
| Arginine | 0.58 | 0.58 |
| Histidine | 0.43 | 0.43 |
| Leucine | 1.08 | 1.11 |
| Isoleucine | 0.41 | 0.42 |
| Lysine | 0.84 | 0.90 |
| Methionine | 0.27 | 0.24 |
| Phenylalanine | 0.52 | 0.54 |
| Threonine | 0.51 | 0.52 |
| Tryptophan | 0.12 | 0.13 |
| Valine | 0.69 | 0.72 |
| Alanine | 0.67 | 0.70 |
| Asparagine | 0.90 | 0.92 |
| Cysteine | 0.20 | 0.20 |
| Glutamine | 1.95 | 2.02 |
| Glycine | 0.42 | 0.44 |
| Proline | 0.75 | 0.77 |
| Serine | 0.52 | 0.54 |
| Tyrosine | 0.34 | 0.32 |
YC = yeast culture; SID = standardized ileal digestible.
Vitamin and mineral provided the following per kilogram of diets: vitamin A 5512 IU, vitamin D3 2200 IU, vitamin E 30 IU, vitamin K3 2.2 mg, vitamin B12 27.6 μg, riboflavin 4.0 mg, pantothenic acid 14.0 mg, niacin 30 mg, choline chloride 400 mg, folic acid 0.7 mg, oryzanin 1.5 mg, pyridoxine 3 mg, biotin 44 μg, Mn 40 mg (MnSO4), Fe 75 mg (FeSO4·H2O), Zn 75 mg (ZnSO4), Cu 100 mg (CuSO4·5H2O), I 0.3 mg (KI), Se 0.3 mg (Na2SeO3).
Made by Chemical Reagents Company, Beijing, China.
Yeast culture were purchased from Beijing China-Agri Hong Ke Bio-Technology Co, Ltd., China.
Nutritional value was calculated according to NRC (NRC, 2012).
Nutrient levels were all analyzed levels.
The whole experiment lasted 28 d of which the first 14 d were for recovery, the next 7 d for adaptation to the diets, the next 5 d for feces and urine collection, and the last 2 d for ileal digesta collection. The stainless-steel metabolic cages, environment and feeding method were the same as those in Exp. 1. Fecal, urine, and ileal digesta samples were collected and treated in the same method as in Exp. 1 and Exp. 2, respectively.
2.7. Sample preparation and chemical analyses
In Exp. 1 and Exp. 4, the total feces and urine were mixed respectively for each experimental pig, and then 250 g subsamples were collected and dried for 72 h at 65 °C and were ground through a 1-mm screen. The urine sample (20% of the total volume) was collected and filtered through gauze. Feces and urine samples were stored at 4 and −20 °C individually for analysis. In Exp. 2 and Exp. 4, samples of digesta from each pig were thawed, mixed, subsampled, and then freeze-dried (Tofflon Freezing Drying systems, China). The ileal digesta samples were stored at −20 °C until analysis.
Chemical analysis was performed twice for each sample of feed, feces, urine, and ileal digesta. According to the Association of Official Analytical Chemists procedures (AOAC, 2000), samples of YC, feces, urine, digesta and diets were measured for dry matter (DM) (method 930.15), ether extract (method 920.39) and crude protein (method 954.01). The crude fiber (method 978.10), calcium (method 984.04), ash (method 942.05) and phosphorus (method 965.17), and YC material were analyzed according to the AOAC (2006). Otherwise, we used a fiber analyzer to determine acid detergent (method 973.18) and neutral detergent fiber (method 993.21) in YC and feed ingredients (AOAC, 2012). The gross energy (GE) of feed, feces, urine and YC was measured by an automatic isoperibol oxygen bomb calorimeter (Parr 6300 calorimeter; Parr Instrument Company, USA). Samples of each diet were analyzed for the specific array and concentration of amino acids. Initially, samples were hydrolyzed with 6 mol/L HCl (at 110 °C for 24 h) before analysis of 15 amino acids with the Amino Acid Analyzer (L-8900, Hitachi, Japanese). Williams et al. (1962) described the analysis process of chromium in detail. In addition, serum biochemical, antioxidant and immunological indicators and dietary starch were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, China). Organic acid and antitrophic factor were determined according to previous studies (Marconi et al., 2007; Wacoo et al., 2014). Antigenic proteins, such as mannan, β-Glucan and polypeptide were determined using ELISA kit (QiYi Bioengineering Institute, China).
In Exp. 3, the detection method for short-chain fatty acids in fecal samples is as follows: approximately 1.5 g of thawed fecal samples were placed into a centrifuge tube, mixed with sterile water (1.5 mL) and centrifuged at 15,000 × g for 10 min at 4 °C to obtain the supernatant. Afterward, the supernatant was drained into a gas chromatograph vial by a sterile syringe and mixed with 1 mL of 25% metaphosphoric acid. According to the procedure mentioned by Yu et al. (2023), the short-chain fatty acid content in the fecal samples was analyzed using a gas chromatograph (GC-1890B, Agilent Technologies, USA).
The method for assessing the microbial community structure in fecal samples of Exp. 3 is according to the previous studies (Zhou et al., 2022a, 2022b). In simple terms, based on the instructions of the manufacturer, a stool mini kit (TianGen Bioengineering Institute, China) was used for extracting DNA from fecal samples. The DNA concentration was determined by 1% agarose gel (Thermo Scientific, USA). The 16S rRNA gene of the bacterial V3–V4 hypervariable region was amplified by a thermocycling polymerase chain reaction system (GeneAmp 9700, ABI, USA). Miseq platform (Allwegene, China) was used to purify, pool, and deep sequence the resulting amplicons. Within Uparse (version 7.0.1090 http://drive5.com/uparse/), all qualified sequences were clustered into operational taxonomic units (OTU) with a 97% similarity. Sparse curves, alpha and beta diversity were generated and calculated as previously described (Zhou et al., 2020).
2.8. Calculation
In Exp. 1, the DE and ME of YC was calculated according to Adeola (2001) as follows:
| DEd = (GEi − GEf)/Fi, |
| DEy = [DEd − (1 − X) × DEd/0.97]/X, |
| MEd = (GEi − GEf − GEu)/Fi, |
| MEy = [MEd − (1 − X) × MEd/0.97]/X, |
where DEd and DEy indicate DE values in diet and YC, respectively. MEd and MEy indicate ME values in diet and YC, respectively. The GEi, GEf, GEu, and Fi represent the total GE intake, the total GE content in feces and urine, and total feed intake for 5 d, respectively. X represents the percentage of energy replaced by YC in the basal diet.
In Exp. 2, according to the method described by Stein et al. (2007), the AA digestibility of YC samples was calculated. Because the YC was the only ingredient in the diet that provided AA, dietary values also represent the digestibility for YC. The AID of AA in YC diet was calculated as follows:
| AID = [1 − (AAdigesta/AAdiet) × (Crdiet/Crdigesta)] × 100, |
where AID represents the apparent ileal digestibility of an AA (%), the subscripts “digesta” and “diet” represent the AA concentrations in the ileal digesta and diets (g/kg of DM), and Crdiet and Crdigesta are the chromium concentration in the diets and ileal digesta (g/kg of DM). The AID of CP was calculated following the same equation above.
The endogenous loss of AA for each pig fed the N-free diet was calculated using the following equation:
| IAAend = [AAdigesta × (Crdiet/Crdigesta)], |
where AAdigesta represented the concentrations (g/kg of DM) of the substance in ileal digesta of pigs fed a N-free diet. The endogenous loss of CP was calculated using the same method.
Furthermore, the SID of AA was calculated as follows:
| SID = AID + (IAAend/AAdiet) × 100, |
where the SID is the standardized ileal digestibility of an AA (%).
2.9. Statistical analysis
The PROC MIXED procedure of SAS 9.4 (SAS Inc., USA) was used to perform data analysis. All data were checked for normal distribution and homogeneous variance using the UNIVARIATE procedure. In Exp. 1, 2 and 4, data were obtained with each pig as one experimental unit. In Exp. 3, data were obtained with each pen as one experimental unit. GLM models of SAS 9.4 and Tukey's tests were used to analyze the experimental data. Data obtained by ANOVA are shown as the means ± standard error of mean (SEM). Differences at a P-value ≤0.05 were considered significant, and differences at a 0.05 < P-value ≤ 0.10 were considered a tendency.
The α diversity of the fecal bacterial community was analyzed using the Mann–Whitney U test and Kruskal–Wallis test. The statistical significance of the principal coordinate analysis (PCoA) of microbial compositions between the treatments was performed using the QIIME software package (version 2) and was based on Bray–Curtis distance metrics. Linear discriminant analysis effect size was used to compare differences in taxonomic levels, including phylum, class, order, family, and genus.
3. Results
3.1. Available energy of YC
After substituting 24.3% of the energy component in the diet with YC, there was no significant impact on the gross energy of the diet, but a significant reduction in the DE (P < 0.001) and ME (P < 0.001) of the diet was observed, with no change in the efficiency of converting DE to ME. Analysis of the effective energy values for YC, as shown in Table 6 revealed DE and ME values of 11.04 and 10.62 MJ/kg (as-fed basis) and 12.12 and 11.66 MJ/kg (as-DM basis), respectively.
Table 6.
The digestible and metabolizable energy values of diets and yeast culture in Exp. 1 (as-fed basis).1
| Item | Control diet | YC diet | SEM | P-value | YC |
|
|---|---|---|---|---|---|---|
| as-fed basis | as-DM basis | |||||
| Gross energy, MJ/kg | 16.30 | 16.45 | 0.072 | 0.212 | 17.12 | 18.41 |
| Digestible energy, MJ/kg | 15.21a | 14.17b | 0.263 | <0.001 | 11.04 | 12.12 |
| Metabolizable energy, MJ/kg | 14.80a | 13.76b | 0.268 | <0.001 | 10.62 | 11.66 |
| ME/DE, % | 97.32 | 97.10 | 0.012 | 0.338 | 96.21 | 96.20 |
| Predicted net energy, MJ/kg2 | 7.36 | 6.70 | ||||
YC = yeast culture; ME/DE = metabolizable energy/digestible energy; DM = dry matter; SEM = standard error of mean.
a,bValues in a row with no common superscripts differ significantly (P < 0.05).
Control diet = basal diet without YC; YC diet = basal diet supplemented with 24.3% YC.
The prediction equation is based on the model from the China Agricultural University: NE = 0.25 + 0.62 × DE + 0.03 × starch content.
3.2. AA ileal digestibility of YC
The digestibility of AA is presented in Table 7. The results indicated that the SID of YC was significantly higher than the AID (P < 0.05; except for tyrosine). The only negative value was observed in the AID of proline (−27.99%), with an SID of 48.29%. Additionally, among the essential AA, methionine exhibits the lowest digestibility, with an AID of 38.81% and a SID of 43.93%.
Table 7.
The apparent ileal digestibility, apparently ileal digestible content, standardized ileal digestibility and standardized ileal digestible content of amino acid in yeast culture in Exp. 2.
| Item | AID, % | AIDAA, % | SID, % | SIDAA, % | SEM | P-value |
|---|---|---|---|---|---|---|
| Crude protein | 58.19b | 13.40B | 65.31a | 15.04A | 1.483 | <0.001 |
| Essential amino acid | ||||||
| Arginine | 61.13b | 0.68B | 69.22a | 0.77A | 2.647 | <0.001 |
| Histidine | 68.45b | 0.35B | 75.52a | 0.39A | 2.880 | <0.001 |
| Leucine | 62.17b | 0.67B | 67.26a | 0.72A | 1.711 | <0.001 |
| Isoleucine | 55.61b | 0.33B | 60.72a | 0.36A | 1.303 | <0.001 |
| Lysine | 47.32b | 0.35B | 53.43a | 0.40A | 2.206 | <0.001 |
| Methionine | 38.81b | 0.09B | 43.93a | 0.10A | 2.103 | <0.001 |
| Phenylalanine | 62.00b | 0.43B | 70.07a | 0.48A | 3.807 | <0.001 |
| Threonine | 52.53b | 0.30B | 57.72a | 0.33A | 1.520 | <0.001 |
| Tryptophan | 47.77b | 0.10B | 52.94a | 0.11A | 0.791 | <0.001 |
| Valine | 58.46b | 0.47B | 61.56a | 0.50A | 1.188 | <0.001 |
| Non-essential amino acid | ||||||
| Alanine | 62.86b | 0.60B | 72.97a | 0.69A | 2.555 | <0.001 |
| Asparagine | 56.08b | 0.77B | 66.18a | 0.91A | 3.404 | <0.001 |
| Glutamine | 70.99b | 2.21B | 81.05a | 2.52A | 1.786 | <0.001 |
| Glycine | 36.20b | 0.33B | 66.52a | 0.61A | 5.273 | <0.001 |
| Proline | −27.99b | −0.27B | 48.29a | 0.47A | 10.332 | <0.001 |
| Serine | 64.75b | 0.47B | 74.89a | 0.54A | 2.409 | <0.001 |
| Tyrosine | 84.63 | 0.32 | 82.65 | 0.31 | 0.990 | 0.313 |
AID = apparent ileal digestibility; AIDAA = apparently ileal digestible amino acid content; SID = standardized ileal digestibility; SIDAA = standardized ileal digestible amino acid content; SEM = standard error of mean.
a,bValues in a row with no common superscripts differ significantly between SID and AID (P < 0.05).
A,BValues in a row with no common superscripts differ significantly between SIDAA and AIDAA (P < 0.05).
3.3. Modulation of piglet growth performance by YC
The supplementation of a small amount of YC to the low-protein diet had no significant impact on the average daily gain and average daily feed intake of weaned growing pigs (Table 8). However, it is worth noting that the moderate supplementation of YC (1.5%) showed linearly a trend of increasing average daily gain in 15 to 32 d (P = 0.078) and 1 to 32 d (P = 0.092). Furthermore, the addition of 1.0% or 1.5% YC linearly improved the weight gain and feed intake ratio of piglets, whether during the first 14 d, the subsequent 18 d, or the entire 32-d trial period (P < 0.05).
Table 8.
Effect of yeast culture applied to a low-protein diet on the growth performance of growing pigs in Exp. 3.
| Item | Dietary treatments1 |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| Basal diet | 0.5%YC | 1.0%YC | 1.5%YC | Treatment | Linear | Quadratic | ||
| Body weight, kg | ||||||||
| 1 to 14 d | 7.06 | 7.07 | 7.08 | 7.09 | 0.183 | 0.996 | 0.843 | 0.982 |
| 15 to 32 d | 9.82 | 9.86 | 9.72 | 9.98 | 0.186 | 0.734 | 0.561 | 0.406 |
| 1 to 32 d | 17.96 | 18.30 | 18.46 | 19.18 | 0.687 | 0.341 | 0.084 | 0.680 |
| ADG, kg/d | ||||||||
| 1 to 14 d | 0.20 | 0.20 | 0.19 | 0.21 | 0.013 | 0.114 | 0.496 | 0.135 |
| 15 to 32 d | 0.45c | 0.47b | 0.49b | 0.51a | 0.035 | 0.024 | 0.078 | 0.754 |
| 1 to 32 d | 0.34c | 0.35bc | 0.36b | 0.38a | 0.021 | 0.033 | 0.092 | 0.778 |
| ADFI, kg/d | ||||||||
| 1 to 14 d | 0.42 | 0.40 | 0.38 | 0.40 | 0.026 | 0.312 | 0.182 | 0.217 |
| 15 to 32 d | 1.44 | 1.45 | 1.48 | 1.50 | 0.103 | 0.782 | 0.547 | 0.943 |
| 1 to 32 d | 0.81 | 0.81 | 0.81 | 0.83 | 0.051 | 0.213 | 0.780 | 0.785 |
| G:F | ||||||||
| 1 to 14 d | 0.47c | 0.50b | 0.49b | 0.52a | 0.002 | 0.006 | <0.001 | 0.602 |
| 15 to 32 d | 0.31c | 0.32bc | 0.33b | 0.34a | 0.004 | 0.028 | <0.001 | 0.696 |
| 1 to 32 d | 0.42c | 0.43b | 0.44b | 0.46a | 0.004 | <0.001 | <0.001 | 0.744 |
YC = yeast culture; ADG = average daily gain; ADFI = average daily feed intake; G:F = weight gain and feed intake ratio; SEM = standard error of mean.
a–cValues in a row with no common superscripts differ significantly (P < 0.05).
Basal diet, 0.5%YC, 1.0%YC, 1.5%YC referred to supplementing with 0%, 0.5%, 1.0%, 1.5% YC in a low-protein diet.
3.4. Serum parameters
On d 14 of the experiment, the addition of YC quadratically modulated the serum levels of catalase (CAT) (P < 0.001) and total antioxidant capacity (T-AOC) (P = 0.003) in growing pigs, and linearly regulated the concentrations of malondialdehyde (MDA) (P = 0.008) and glutathione peroxidases (GSH-Px) (P = 0.032) in growing pig serum (Table 9). On d 32 of the experiment, the dietary supplementation of YC quadratically regulated CAT (P = 0.024) in growing pig serum, and linearly regulated albumin (ALB) (P < 0.001), glucose (GLU) (P = 0.011), high-density lipoprotein cholesterol (HDL-C) (P < 0.001), light-density lipoprotein cholesterol (LDL-C) (P = 0.019), MDA (P = 0.004), GSH-Px (P = 0.017), and T-AOC (P = 0.016).
Table 9.
Effect of yeast culture applied to a low-protein diet on serum parameters of growing pigs in Exp. 3.
| Item | Dietary treatments1 |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal diet | 0.5%YC | 1.0%YC | 1.5%YC | Treatment | Linear | Quadratic | |||
| ALB, g/L | 14 d | 16.09 | 20.06 | 18.60 | 20.84 | 2.506 | 0.891 | 0.127 | 0.608 |
| 32 d | 24.70c | 27.09b | 33.34a | 29.49b | 2.127 | 0.044 | <0.001 | 0.050 | |
| TP, g/L | 14 d | 45.36 | 49.06 | 51.64 | 53.13 | 4.951 | 0.324 | 0.114 | 0.764 |
| 32 d | 54.15 | 62.09 | 58.32 | 61.69 | 4.225 | 0.481 | 0.175 | 0.458 | |
| GLU, mmol/L | 14 d | 2.60c | 3.95a | 3.36b | 3.31b | 0.462 | 0.039 | 0.291 | 0.055 |
| 32 d | 4.41c | 5.36b | 5.57b | 6.03a | 0.588 | 0.048 | 0.011 | 0.564 | |
| UN, mmol/L | 14 d | 2.93 | 3.84 | 3.46 | 2.67 | 0.742 | 0.329 | 0.623 | 0.121 |
| 32 d | 3.58 | 4.40 | 3.69 | 4.34 | 0.353 | 0.228 | 0.176 | 0.731 | |
| TG, mmol/L | 14 d | 0.28 | 0.32 | 0.34 | 0.32 | 0.058 | 0.124 | 0.397 | 0.399 |
| 32 d | 0.41 | 0.49 | 0.49 | 0.46 | 0.065 | 0.171 | 0.703 | 0.188 | |
| TC, mmol/L | 14 d | 1.63 | 1.99 | 1.81 | 1.81 | 0.241 | 0.276 | 0.653 | 0.314 |
| 32 d | 2.09 | 1.73 | 2.21 | 2.11 | 0.281 | 0.633 | 0.576 | 0.513 | |
| HDL-C, mmol/L | 14 d | 0.87 | 0.95 | 0.77 | 0.78 | 0.103 | 0.414 | 0.214 | 0.636 |
| 32 d | 0.45c | 0.57b | 0.83a | 0.74a | 0.106 | 0.038 | <0.001 | 0.134 | |
| LDL-C, mmol/L | 14 d | 0.52 | 0.35 | 0.48 | 0.35 | 0.093 | 0.117 | 0.221 | 0.693 |
| 32 d | 0.42b | 0.37c | 0.47b | 0.64a | 0.094 | 0.024 | 0.019 | 0.088 | |
| ALT, U/L | 14 d | 55.77 | 60.78 | 60.06 | 69.10 | 11.266 | 0.426 | 0.284 | 0.808 |
| 32 d | 62.98 | 59.50 | 87.56 | 54.61 | 13.322 | 0.639 | 0.957 | 0.134 | |
| AST, U/L | 14 d | 41.76 | 40.82 | 42.96 | 44.23 | 1.363 | 0.275 | 0.476 | 0.327 |
| 32 d | 43.56 | 42.81 | 46.32 | 45.13 | 0.857 | 0.118 | 0.351 | 0.431 | |
| CAT, U/mL | 14 d | 7.12ab | 6.78b | 5.53c | 7.69a | 0.548 | 0.043 | 0.563 | <0.001 |
| 32 d | 2.40b | 1.99c | 2.02c | 3.01a | 0.398 | 0.029 | 0.153 | 0.024 | |
| SOD, U/mL | 14 d | 77.65 | 77.99 | 75.70 | 80.86 | 2.706 | 0.404 | 0.406 | 0.221 |
| 32 d | 91.29a | 88.50b | 88.72b | 91.68a | 2.243 | 0.041 | 0.846 | 0.088 | |
| MDA, mmol/L | 14 d | 7.13 | 6.30 | 4.66 | 4.13 | 0.752 | 0.092 | 0.008 | 0.234 |
| 32 d | 8.23a | 7.03b | 5.66c | 5.11c | 0.854 | 0.011 | 0.004 | 0.271 | |
| GSH-Px, U/mL | 14 d | 405.60 | 424.80 | 437.82 | 482.70 | 13.218 | 0.071 | 0.032 | 0.198 |
| 32 d | 334.40c | 387.20a | 384.00a | 348.80b | 0.509 | 0.042 | 0.017 | 0.028 | |
| T-AOC, U/mL | 14 d | 0.20 | 0.22 | 0.24 | 0.25 | 0.018 | 0.191 | 0.125 | 0.003 |
| 32 d | 0.19 | 0.21 | 0.24 | 0.24 | 0.057 | 0.226 | 0.016 | 0.784 | |
ALB = albumin; TP = total protein; GLU = glucose; UN = urea nitrogen; TG = total triglycerides; TC = total cholesterol; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; ALT = alanine aminotransferase; AST = aspartate transaminase; CAT = catalase; SOD = superoxide dismutase; MDA = malondialdehyde; GSH-Px = glutathione peroxidases; T-AOC = total antioxidant capacity; YC = yeast culture; SME = standard error of mean.
a–cValues in a row with no common superscripts differ significantly (P < 0.05).
Basal diet, 0.5%YC, 1.0%YC, 1.5%YC referred to supplementing with 0%, 0.5%, 1.0%, 1.5% YC in a low-protein diet.
3.5. Fecal short-chain fatty acids
On the 14th day of the experiment, the supplementation of YC linearly reduced the concentration of total short-chain fatty acids in growing pig feces (P = 0.045; Table 10). It showed a trend of decreasing concentrations of acetate (P = 0.077) and isobutyrate (P = 0.068), while showing a trend of quadratic changes in isovaleric acid concentration with YC supplementation (P = 0.071). On d 32 of the experiment, the addition of YC to the diet exhibited a linear trend of reducing the concentration of total short-chain fatty acids in growing pig feces (P = 0.096).
Table 10.
Effect of yeast culture applied to a low-protein diet on fecal short-chain fatty acids concentration (μg/g) of growing pigs in Exp. 3.
| Item | Dietary treatments1 |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal diet | 0.5%YC | 1.0%YC | 1.5%YC | Treatment | Linear | Quadratic | |||
| Acetate | 14 d | 160.38a | 158.94a | 158.52a | 140.30b | 10.016 | 0.037 | 0.077 | 0.256 |
| 32 d | 127.67 | 101.39 | 113.38 | 97.77 | 5.786 | 0.688 | 0.387 | 0.791 | |
| Propionate | 14 d | 70.96 | 60.88 | 67.91 | 67.90 | 7.772 | 0.247 | 0.933 | 0.372 |
| 32 d | 55.19 | 45.80 | 39.32 | 43.06 | 9.173 | 0.366 | 0.162 | 0.323 | |
| Isobutyrate | 14 d | 7.45a | 6.99b | 7.11b | 5.37c | 0.968 | 0.044 | 0.068 | 0.366 |
| 32 d | 5.07 | 4.16 | 3.61 | 4.26 | 1.168 | 0.732 | 0.437 | 0.353 | |
| Butyrate | 14 d | 50.76 | 53.51 | 42.58 | 49.94 | 7.561 | 0.688 | 0.584 | 0.677 |
| 32 d | 37.48 | 36.01 | 32.04 | 33.32 | 6.722 | 0.712 | 0.458 | 0.788 | |
| Isovalerate | 14 d | 11.48b | 13.31a | 11.23b | 10.24c | 1.053 | 0.031 | 0.102 | 0.071 |
| 32 d | 6.45 | 5.91 | 5.03 | 6.51 | 0.926 | 0.711 | 0.812 | 0.146 | |
| Valerate | 14 d | 11.47 | 10.48 | 10.88 | 8.58 | 1.938 | 0.287 | 0.195 | 0.647 |
| 32 d | 5.84 | 6.59 | 4.30 | 5.98 | 1.143 | 0.342 | 0.618 | 0.571 | |
| Total short-chain fatty acid | 14 d | 312.50a | 304.11b | 298.23b | 282.33c | 9.643 | 0.037 | 0.045 | 0.684 |
| 32 d | 237.70a | 199.86b | 197.68b | 190.90b | 8.231 | 0.046 | 0.096 | 0.619 | |
YC = yeast culture; SEM = standard error of mean.
a–cValues in a row with no common superscripts differ significantly (P < 0.05).
Basal diet, 0.5%YC, 1.0%YC, 1.5%YC referred to supplementing with 0%, 0.5%, 1.0%, 1.5% YC in a low-protein diet.
3.6. Modulation of fecal bacterial community by YC
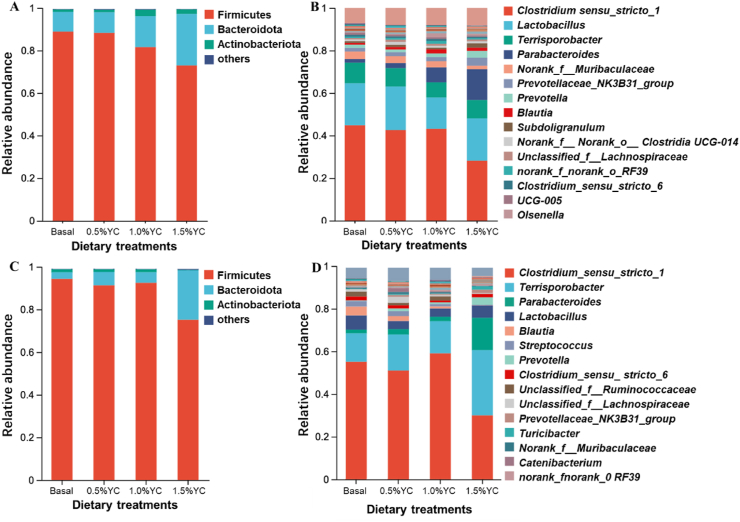
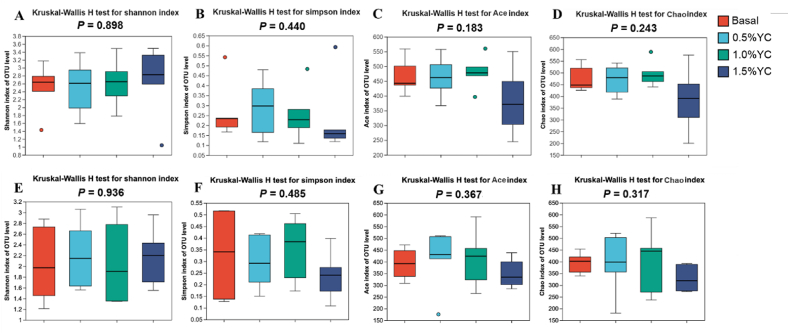
An analysis at the phylum level consistently showed that Firmicutes (83.43% and 89.28% of the sequences, respectively at d 14 and 32) and Bacteroidetes (14.50% and 9.50% of the sequences, respectively at d 14 and 32) dominated the gut microbiota composition of piglets (Fig. 1). At the genus level, Clostridium_sensu_stricto 1, Lactobacillus, Terrisporobacter and Parabacteroides were the dominant bacteria. The Ace, Chao, and Sobs indexes, as well as the Shannon index, revealed that bacterial community richness and diversity showed no significant distinctions among the different treatment groups (Fig. 2).
Fig. 1.
Fecal bacterial community at the phylum and genus levels of pigs fed a low-protein diet with different content of yeast culture (YC). Microbial community bar plot of phyla with an abundance of 0.015% or greater at 14 d (A), and at 32 d (C); microbial community bar plot of genus with a proportion of 0.015% or higher at 14 d (B) and at 32 d (D). Basal diet, 0.5%YC, 1.0%YC, 1.5%YC referred to supplementing with 0%, 0.5%, 1.0%, 1.5% YC in a low-protein diet.
Fig. 2.
The α diversity of the fecal bacterial community of pigs fed a low-protein diet with different content of yeast culture (YC). Shannon index (A), Simpson index (B), ACE index (C), and Chao index (D) of the fecal bacterial community at 14 d; Shannon index (E), Simpson index (F), ACE index (G), and Chao index (H) of the fecal bacterial community at 32 d. Basal diet, 0.5%YC, 1.0%YC, 1.5%YC referred to supplementing with 0%, 0.5%, 1.0%, 1.5% YC in a low-protein diet. OTU = operational taxonomic units.
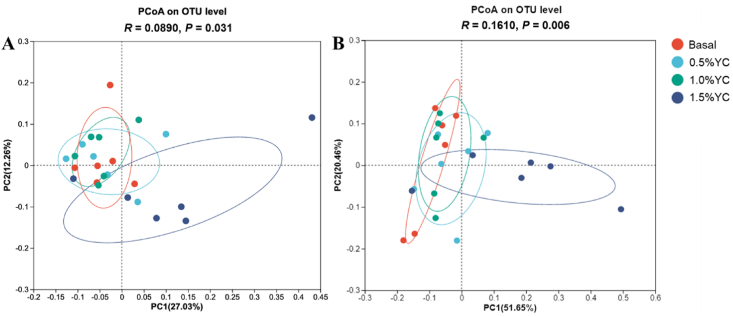
To examine microbial communities, a PCoA was conducted on treatment groups using unweighted_unifrac analyses of bacterial communities at the operational taxonomic unit level. The PCoA demonstrated that the addition of YC in low-protein diets led to alterations in both taxonomic and functional structures of microbial communities on d 14 and 32, respectively (Fig. 3).
Fig. 3.
Principal coordinate analysis (PCoA) of the microbiota from feces of pigs fed a low-protein diet with different content of yeast culture (YC). Principal coordinate analysis of treatment groups based on unweighted_unifrac analyses of bacterial communities at the operational taxonomic unit level. The distances between the symbols on the ordination plot reflect the relative dissimilarities in the community structures. Basal diet, 0.5%YC, 1.0%YC, 1.5%YC referred to supplementing with 0%, 0.5%, 1.0%, 1.5% YC in a low-protein diet. OTU = operational taxonomic units.
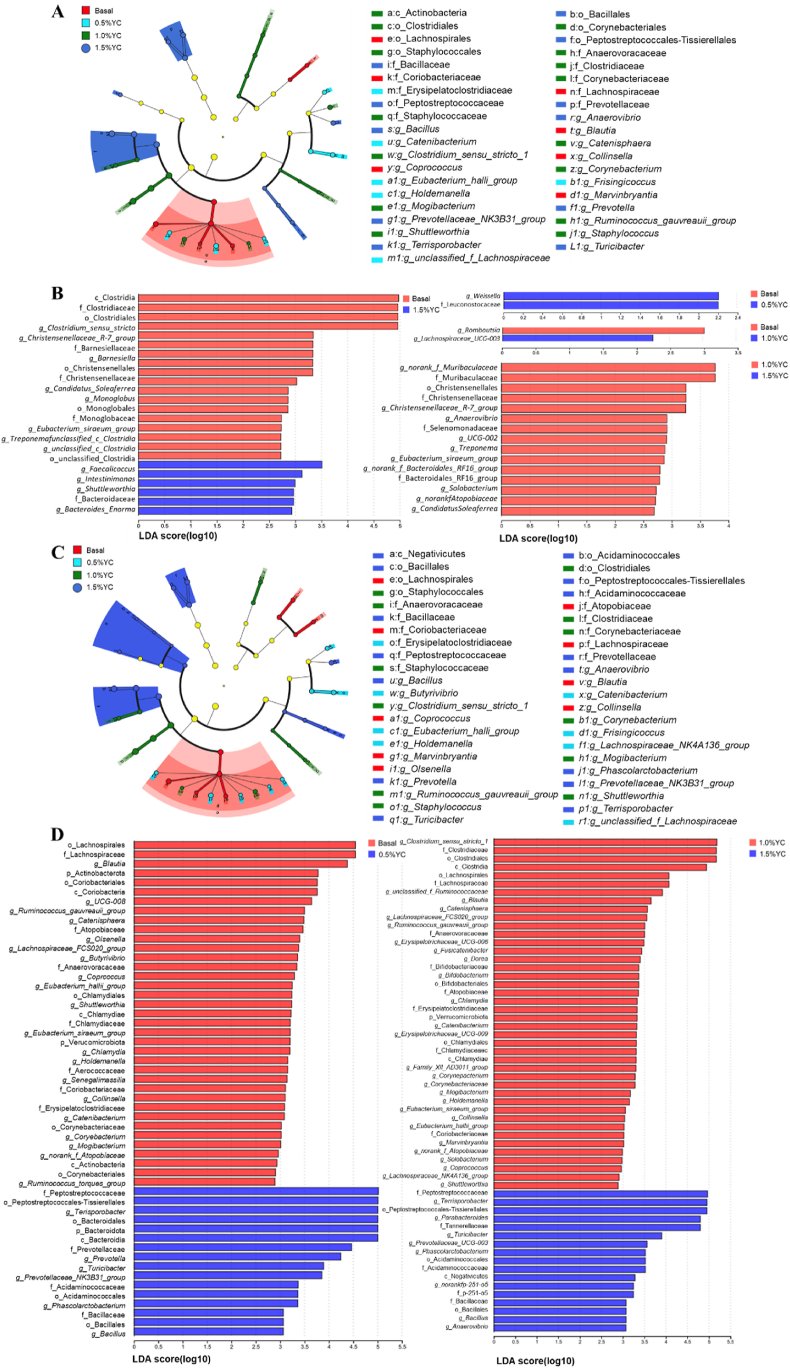
Figure 4 illustrates notable distinctions in the microbial community among the various treatment groups. Many bacteria such as Prevotellaceae, Prevotella and Turicibacter were in greater abundance in the 1.5%YC treatment than the Basal diet, the 0.5%YC or the 1.0%YC treatments. Compared with other treatments, the Basal treatment had an increased abundance of Lachnospirales, Blautia, Collinsella and Coprococcus.
Fig. 4.
LEfSe effect size results of the microbiota in fecal samples of pigs fed a low-protein diet with different content of yeast culture (YC). Histogram of the linear discriminant analysis scores computed for the differentially abundant features in the fecal bacteria between Basal, 0.5%YC, 1.0%YC and 1.5%YC treatments at d 14 (A) and at d 32 (C); the results of the comparison between each two treatments at d 14 (B) and at d 32 (D). The linear discriminant analysis bars indicate the microbial groups within treatments with linear discriminant analysis (LDA) scores higher than 2.0. The differentially abundant clades in each treatment are represented by colors in the cladograms, and the linear discriminant analysis scores of these clades indicate the degrees of statistical and biological differences. Basal diet, 0.5%YC, 1.0%YC, 1.5%YC referred to supplementing with 0%, 0.5%, 1.0%, 1.5% YC in a low-protein diet.
3.7. YC exhibits a minor impact on nutrient digestibility
As shown in Table 11, the addition of YC has no significant impact on the N balance in growing pigs. Additionally, supplementing the diet with 1.5% YC had no significant effect on the total tract digestibility of neutral detergent fiber, acid detergent fiber, and CP of pigs, but had a tendency of reducing the digestibility of organic matter (P = 0.067; Table 12). Moreover, the additional supplementation of YC has no significant impact on the apparent ileal digestibility and apparent total tract digestibility of various AA (Table 13).
Table 11.
Effect of yeast culture applied to a low-protein diet on the nitrogen balance of pigs in Exp. 4.1
| Item | Control diet | YC diet | SEM | P-value |
|---|---|---|---|---|
| Average daily feed intake, g/d | 806.00 | 826.50 | 146.497 | 0.893 |
| Dietary N intake, g/d | 17.94 | 18.78 | 3.303 | 0.801 |
| Urinary N excretion, g/d | 1.79 | 1.87 | 0.061 | 0.837 |
| Fecal N excretion, g/d | 3.82 | 3.93 | 0.557 | 0.488 |
| Total N excretion, g/d | 5.61 | 5.80 | 0.573 | 0.502 |
| Retention of N, g/d2 | 12.33 | 12.98 | 1.841 | 0.886 |
| Total N excretion/intake of N, % | 31.27 | 30.88 | 1.009 | 0.414 |
| Urinary N excretion/N excretion, % | 31.92 | 32.24 | 1.683 | 0.628 |
| Fecal N excretion/N excretion, % | 68.08 | 67.76 | 1.687 | 0.622 |
| Apparent biological value, %3 | 87.32 | 87.41 | 0.487 | 0.825 |
| Protein efficiency of utilization, %4 | 68.73 | 69.12 | 2.004 | 0.413 |
N = nitrogen; YC = yeast culture; SEM = standard error of mean.
Control diet: a basal low-protein diet; YC diet: a Control diet supplemented with 1.5% YC.
Retention of nitrogen (%) = [(intake of nitrogen − fecal nitrogen-urinary nitrogen)/intake of nitrogen] × 100.
Apparent biological value (%) = [(intake of nitrogen − fecal nitrogen-urinary nitrogen)/(intake of nitrogen − fecal nitrogen)] × 100.
Protein efficiency of utilization (%) = [(intake of nitrogen − fecal nitrogen-urinary nitrogen)/intake of nitrogen] × 100.
Table 12.
Effect of yeast culture applied to a low-protein diet on the total tract digestibility of nutrients in Exp. 4 (as-fed basis, %).1
| Item | Control diet | YC diet | SEM | P-value |
|---|---|---|---|---|
| Crude protein | 84.99 | 82.15 | 1.977 | 0.182 |
| Neutral detergent fiber | 48.71 | 46.56 | 2.144 | 0.641 |
| Acid detergent fiber | 51.54 | 48.11 | 5.216 | 0.537 |
| Organic matter | 90.54 | 88.90 | 0.681 | 0.067 |
YC = yeast culture; SEM = standard error of mean.
Control diet: a basal low-protein diet; YC diet: a Control diet supplemented with 1.5% YC.
Table 13.
The apparent ileal digestibility (%) and apparent total tract digestibility (%) of crude protein and amino acid in diets with yeast culture in Exp. 4.1
| Item | Apparent ileal digestibility |
Apparent total tract digestibility |
||||||
|---|---|---|---|---|---|---|---|---|
| Control diet | YC diet | SEM | P-value | Control diet | YC diet | SEM | P-value | |
| Crude protein | 85.13 | 82.87 | 1.601 | 0.232 | 95.14 | 93.53 | 2.041 | 0.664 |
| Essential amino acid | ||||||||
| Arginine | 85.93 | 83.35 | 2.584 | 0.313 | 95.90 | 94.25 | 1.854 | 0.981 |
| Histidine | 85.72 | 83.73 | 1.991 | 0.351 | 94.44 | 92.69 | 2.468 | 0.407 |
| Leucine | 76.01 | 74.26 | 1.758 | 0.647 | 95.56 | 94.01 | 1.883 | 0.804 |
| Isoleucine | 84.56 | 82.36 | 2.209 | 0.264 | 93.16 | 90.95 | 2.821 | 0.687 |
| Lysine | 86.40 | 84.68 | 1.723 | 0.727 | 95.65 | 94.37 | 1.747 | 0.609 |
| Methionine | 78.21 | 77.13 | 1.087 | 0.448 | 96.18 | 94.62 | 1.735 | 0.171 |
| Phenylalanine | 84.26 | 82.72 | 1.545 | 0.354 | 94.39 | 92.49 | 2.343 | 0.890 |
| Threonine | 83.59 | 80.50 | 3.083 | 0.339 | 93.94 | 91.62 | 2.497 | 0.874 |
| Tryptophan | 85.24 | 82.57 | 2.661 | 0.510 | 94.35 | 93.30 | 2.321 | 0.310 |
| Valine | 85.22 | 83.16 | 2.054 | 0.462 | 94.86 | 93.04 | 2.091 | 0.741 |
| Non-essential amino acid | ||||||||
| Alanine | 84.57 | 82.47 | 2.103 | 0.423 | 94.50 | 92.73 | 2.188 | 0.548 |
| Asparagine | 83.85 | 80.90 | 2.956 | 0.321 | 93.87 | 91.45 | 2.659 | 0.704 |
| Cysteine | 83.99 | 80.92 | 3.074 | 0.457 | 95.71 | 94.15 | 1.829 | 0.711 |
| Glutamine | 86.10 | 83.81 | 2.304 | 0.513 | 96.46 | 95.20 | 1.410 | 0.798 |
| Glycine | 88.65 | 81.53 | 7.127 | 0.347 | 92.40 | 89.68 | 3.144 | 0.766 |
| Proline | 75.69 | 73.63 | 2.061 | 0.335 | 96.74 | 95.34 | 1.441 | 0.811 |
| Serine | 84.05 | 81.22 | 2.848 | 0.420 | 94.41 | 92.02 | 2.308 | 0.947 |
| Tyrosine | 83.69 | 81.34 | 2.358 | 0.320 | 93.77 | 90.66 | 2.836 | 0.773 |
YC = yeast culture; SEM = standard error of mean.
Control diet: a basal low-protein diet; YC diet: a Control diet supplemented with 1.5% YC.
4. Discussion
Low-protein diets have become an alternative approach towards optimizing the intestinal health of pigs, and their combination with YC may represent a potential nutritional strategy for post-weaning piglets. From the perspective of satisfying nutritional requirements, the current research assessed available energy values and digestible AA content of YC. Moreover, the current study explored the performance of YC supplementation in antibiotic-free, low-protein piglet diets and evaluated the impact of adding YC on the foregut digestibility of nutrients in pigs, providing a foundation in data for the rational use of YC in low-protein diets.
The present study indicates that the DE and ME of YC are 12.12 and 11.66 MJ/kg DM, respectively, which are close to the energy values of conventional feed ingredients like corn, sorghum, and barley. The crude protein content is 23.03%, which is lower than that of protein-rich feed ingredients like soybean meal (He et al., 2022; Li et al., 2015). Factors affecting feedstuff energy values in pig feed have been summarized by Noblet and Goff (2001). They found that the DE and ME values in feed ingredients are negatively correlated with the content of acid detergent fiber and neutral detergent fiber, while positively correlated with the protein content in feed ingredients. The relatively high content of neutral detergent fiber and acid detergent fiber, along with a relatively low content of crude protein in the YC, contributes to its relatively low nutritional value. The current study evaluates the nutritional value of YC as a feed ingredient for pig diets for the first time. Despite the relatively low inclusion rate of YC in pig feed, accurately evaluating its value has the benefit of precisely meeting the nutritional needs of pigs, which in turn can contribute to savings in feed costs. Additionally, this research detected various beneficial functional components in YC, including mannooligosaccharides, β-Glucans, and peptides, among others. A previous study showed that β-Glucans from yeast cell walls can markedly increase the daily weight gain of weaned piglets, and the high lactate content in YC can lower stomach pH, inhibit the proliferation of harmful gut microbes, and promote intestinal health (Eicher et al., 2006). Therefore, we have supplemented the text with relevant content: in the present study, the various components contained in the yeast culture acted holistically to exert a probiotic effect.
During weaning, piglets transition from easily digestible sow milk to solid pellet feed. The underdeveloped intestines of piglets struggle to adapt, leading to reduced feed intake and low nutrient conversion efficiency, ultimately impairing their growth performance. YC contains alcohols, aromatic compounds, and other flavor-enhancing substances that improve feed palatability and increase feed intake in animals (Waititu et al., 2017). Research has shown that adding 1 g/kg of YC to weaned piglets significantly improves their growth performance (Mathew et al., 1998; Shen et al., 2009). In our study, we found that adding 1.5% YC numerically increased the growth rate of piglets and significantly improved weight gain and feed intake ratio. We speculate that this may be because YC reduces the impact of stress on weaned piglets. In intensive farming environments, the control group piglets might have had difficulties adapting, leading to ineffective stress-induced energy consumption, while the piglets in the 1.5%YC group showed less impact from stress (Ji et al., 2021; Blavi et al., 2021). However, it should be further noted that when YC is utilized in large quantities as a feed ingredient, its primary function is to provide nutrients. Conversely, when added in small amounts, its effectiveness may not rely on the AA and energy it provides.
Serum biochemical indicators can reflect the health and metabolism status, as well as changes in tissue and organ functions. The total protein content in piglet serum can reflect the protein metabolism and immune function. Elevated levels of ALB in the serum typically indicate enhanced metabolism and immune function in piglets (Liu et al., 2022). Blood GLU is direct source of energy for life activities. GLU content in the blood can reflect the state of health (Montenegro and Dori-Hacohen, 2020). Increased serum GLU levels within the normal range can enhance immunity and promote animal growth (Gopinath and Etherton, 1989; Kvidera et al., 2017). In the current experiment, YC supplementation significantly increased the serum ALB and GLU content at d 32, which may be related to enhanced stress resistance and improved growth performance.
In pig farming, numerous stressors contribute to the generation of a substantial amount of reactive oxygen species within pigs. The antioxidant system plays a crucial role in health, and antioxidant capacity is considered a vital indicator for evaluating animal health (Chen et al., 2021). The GSH-Px, SOD, and T-AOC are key indicators representing the ability to eliminate free radicals (Lee and Park, 2021). MDA is the predominant breakdown product resulting from lipid peroxidation, potentially causing cellular damage, including aging and apoptosis. Elevated levels of MDA indicate oxidative stress (Han et al., 2018). The results of the present study indicate that supplemented 1.5% YC enhances the activities of T-AOC, SOD, CAT, and GSH-Px in the serum of pigs while significantly reducing the level of MDA. This effect may be attributed to functional components in YC, such as high levels of β-Glucans, mannans, peptides, and organic acids. Research showed that β-Glucans effectively reduce free radical concentrations, elevate serum T-AOC and SOD activities, and enhance the antioxidant capacity (Kogan et al., 2005). Furthermore, β-Glucans and mannans can increase intracellular GSH-Px activity, reducing intracellular reactive oxygen species and MDA content (Guo et al., 2019).
Protein is a primary component of pork products, and N balance experiments are an authoritative method for evaluating protein deposition efficiency in feed. The results of Exp. 4 showed that YC supplementation does not impact N utilization efficiency. In fact, due to the high fiber content in YC, it might potentially lead to a decrease in nutrient utilization efficiency (Zhang et al., 2013). However, in the present trial, YC only compromised the total tract digestibility of organic matter, with no significant effect on fiber, crude protein or AA digestibility. Moreover, it significantly improved feed conversion efficiency in piglets. The intake of digestible nutrients is used more efficiently in the YC group than the controls. This represents a post-absorptive effect, enabling the metabolic energy to be more effectively transformed into net gain energy.
Due to remarkable impact of intestinal microbiota and its metabolites on host nutrient utilization (Munyaka et al., 2015), we investigated the influence of varying levels of YC supplementation under low-protein conditions on pig fecal short-chain fatty acids and microbial community structures. The feces from the posterior end of the pig colon can be obtained by the continuous rectal stimulation, and it reflects the bacterial structure of the posterior intestinal fermentation. The results indicate that YC supplementation markedly altered the fecal microbial community, and the differences in microbial structure increased with the duration of treatment. On both d 14 and 32 of the experiment, the 1.5%YC group exhibited a significant increase in the abundance of Prevotellaceae and Prevotella in piglet feces compared to the other treatment groups. The majority of Prevotellaceae and Prevotella members have the capability to break down a diverse array of complex oligosaccharides and polysaccharides (Dou et al., 2017) and benefit host starch metabolism. Additionally, Prevotellaceae and Prevotella play crucial roles in the modulation of inflammation and caspase-8-mediated maturation of interleukin 1β (Lukens et al., 2014), and are linked to the degradation of mucin, leading to a decrease in the intestinal mucin layer (Brinkman et al., 2011). Furthermore, Turicibacter also showed enrichment in the 1.5%YC treatment group on both the d 14 and 32 of the experiment. Research indicates that a decrease in Turicibacter abundance may result in intestinal dysbiosis, causing disruption of the epithelial barrier, ultimately resulting in elevated serum interleukin 2 levels (Gao et al., 2019). Additionally, decreased Turicibacter abundance has been observed in a canine model of idiopathic inflammatory bowel disease. Turicibacter has been demonstrated to alleviate kidney damage in mouse models, attributed to its anti-inflammatory properties (Jiang et al., 2018). The series of findings above indicate that the moderate YC supplementation is beneficial for improving the gut microbiota structure, thereby promoting feed efficiency and intestinal health. Unexpectedly, in the present experiment, the addition of YC resulted in a decrease in the total short-chain fatty acid content, and this phenomenon remained consistent on both d 14 and 32 of the experiment. This contrasts with previous research findings, and the specific reasons for this observation warrant further investigation.
5. Conclusion
The DE and ME of YC in pig feed are 12.12 and 11.66 MJ/kg DM, respectively. Additionally, the present study also assessed the AID (from −27.99% to 84.63%) and SID (from 43.93% to 82.65%) of AA and SID crude protein (15.04%) in YC. Appropriate supplementation of YC, at a dietary concentration of 1.5%, can effectively improve feed conversion efficiency, enhance antioxidant capacity, and promote a balanced gut microbiota in piglets fed low protein diets. The current study provides a data foundation and a theoretical basis for the rational use of YC in formulating pig feed.
Credit author statement
Baocheng Hu: Conceptualization, Methodology, Project administration, Formal analysis, Writing - Original draft. Tairan Liu: Investigation. Bing Xia: Investigation, Formal analysis. Yanjun Dong: Investigation, Project administration. Ming Liu: Funding acquisition, Methodology, Project administration. Ming Liu: Validation, Resources. Junyan Zhou: Conceptualization, Supervision, Project administration, Writing - Review & Editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2021YFD1301003), Science and Technology innovation support program of Beijing University of Agriculture (HHXD2022011), National Natural Science Foundation of China (32302797) and Research Fund for Academic Degree & Graduate Education of Beijing University of Agriculture (2023YJS035). We thank Beijing China-Agri Hong Ke Bio-Technology Co, Ltd. for providing YC used in the present study.
Footnotes
Peer review under the responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adeola O. In: Swine nutrition. Lewis J., Southern L.L., editors. CRC Press; Washington, DC: 2001. Digestion and balance techniques in pigs; pp. 903–916. [Google Scholar]
- AOAC . Association of Official Analytical Chemists; Arlington: 2000. Official methods of analysis. 17th revision. [Google Scholar]
- AOAC . Official methods of analysis. 19th revision. Association of Official Analytical Chemists; Arlington: 2012. [Google Scholar]
- AOAC . Association of Official Analytical Chemists; Arlington: 2006. Official methods of analysis. 17th revision. [Google Scholar]
- Barton M.D. Antibiotic use in animal feed and its impact on human healt. Nutr Res Rev. 2000;13:279–299. doi: 10.1079/095442200108729106. [DOI] [PubMed] [Google Scholar]
- Becattini S., Taur Y., Pamer E.G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blavi L., Solà-Oriol D., Llonch P., López-Vergé S., Martín-Orúe S.M., Pérez J.F. Management and feeding strategies in early life to increase piglet performance and welfare around weaning: a review. Animals. 2021;11(2):302. doi: 10.3390/ani11020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman B.M., Hildebrand F., Kubica M., Goosens D., Del F.J., Declercq W., et al. Caspase deficiency alters the murine gut microbiome. Cell Death Dis. 2011;2:e220. doi: 10.1038/cddis.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xie Y., Zhong R., Han H., Liu L., Chen L., et al. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets. J Anim Sci. 2021;99 doi: 10.1093/jas/skab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby E.M., Trampari E., Siasat P., Gaya M.S., Alav I., Webber M.A., et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol. 2023;21:280–295. doi: 10.1038/s41579-022-00820-y. [DOI] [PubMed] [Google Scholar]
- Dias A.L.G., Freitas J.A., Micai B., Azevedo R.A., Greco L.F., Santos J.E.P. Effect of supplemental yeast culture and dietary starch content on rumen fermentation and digestion in dairy cows. J Dairy Sci. 2018;101(1):201–221. doi: 10.3168/jds.2017-13241. [DOI] [PubMed] [Google Scholar]
- Dou S., Gadonna-Widehem P., Rome V., Hamoudi D., Rhazi L., Lakhal L., et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher S.D., McKee C.A., Carroll J.A., Pajor E.A. Supplemental vitamin C and yeast cell wall β-glucan as growth enhancers in newborn pigs and as immunomodulators after an endotoxin challenge after weaning. J Anim Sci. 2006;84:2352–2360. doi: 10.2527/jas.2005-770. [DOI] [PubMed] [Google Scholar]
- Faccin J.E.G., Tokach M.D., Allerson M.W., Woodworth J.C., DeRouchey J.M., Dritz S.S., et al. Relationship between weaning age and antibiotic usage on pig growth performance and mortality. J Anim Sci. 2020;98:skaa363. doi: 10.1093/jas/skaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Jiang Q., Ji H., Ning J., Li C., Zheng H. Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. Biochim Biophys Acta Mol Basis Dis. 2019;1865 doi: 10.1016/j.bbadis.2019.165541. [DOI] [PubMed] [Google Scholar]
- Gopinath R., Etherton T.D. Effects of porcine growth hormone on glucose metabolism of pigs: I. Acute and chronic effects on plasma glucose and insulin status. J Anim Sci. 1989;67(3):682–688. doi: 10.2527/jas1989.673682x. [DOI] [PubMed] [Google Scholar]
- Guo W., Gu X., Tong Y., Wang X., Wu J., Chang C. Protective effects of mannan/β-glucans from yeast cell wall on the deoxyniyalenol-induced oxidative stress and autophagy in IPEC-J2 cells. Int J Biol Macromol. 2019;135:619–629. doi: 10.1016/j.ijbiomac.2019.05.180. [DOI] [PubMed] [Google Scholar]
- Han Y., Tang C., Zhao Q., Zhan T., Zhang K., Han Y., et al. Effects of dietary supplementation with combinations of organic and medium chain fatty acids as replacements for chlortetracycline on growth performance, serum immunity, and fecal microbiota of weaned piglets. Livest Sci. 2018;216:210–218. [Google Scholar]
- He T., Zheng Y., Piao X., Long S. Determination of the available energy, standardized ileal digestibility of amino acid of fermented corn germ meal replacing soybean meal in growing pig diets. Anim Nutr. 2022;9:259–268. doi: 10.1016/j.aninu.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Gao Y., Guo Z., Yang Z., Wang X., Liu H., et al. Effects of fermented wheat bran and yeast culture on growth performance, immunity, and intestinal microflora in growing-finishing pigs. J Anim Sci. 2021;99 doi: 10.1093/jas/skab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Bi Y., Cheng Z., Liu R., Zhang X., Shu Y., Li X., Bao J., Liu H. Impact of early socialization environment on social behavior, physiology and growth performance of weaned piglets. Appl Anim Behav Sci. 2021;238 [Google Scholar]
- Jiang Q., He X., Zou Y., Ding Y., Li H., Chen H. Altered gut microbiome promotes proteinuria in mice induced by adriamycin. AMB Express. 2018;8:31. doi: 10.1186/s13568-018-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan G., Staško A., Bauerová K., Polovka M., Šoltés L., Brezová V., et al. Antioxidant properties of yeast (1→3)-beta-D-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritis. Carbohydrate Polymers. 2005;61:18–28. [Google Scholar]
- Kvidera S.K., Horst E.A., Mayorga E.J., Sanz-Fernández M.V., Abuajamieh M., Baumgard L.H. Estimating glucose requirements of an activated immune system in growing pigs. J Anim Sci. 2017;95(11):5020–5029. doi: 10.2527/jas2017.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Park Y.S. The emerging roles of antioxidant enzymes by dietary phytochemicals in vascular diseases. Life. 2021;11:199. doi: 10.3390/life11030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang F., Wu F., Wang J., Liu L., Lai C., et al. Chemical composition,energy and amino acid digestibility in double-low rapeseed meal fed to growing pigs. J Anim Sci Biotechnol. 2015;6:37. doi: 10.1186/s40104-015-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zheng J., Deng K., Chen L., Zhao X., Jiang X., et al. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. J Anim Sci. 2018;96:3302–3318. doi: 10.1093/jas/sky197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M.E., Chi F., Cravens R.L., et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 2018;49:76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Ma X., Jiang X. Effects of immobilized antimicrobial peptides on growth performance, serum biochemical index, inflammatory factors, intestinal morphology, and microbial community in weaning pigs. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.872990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Espinosa C.D., Abelilla J.J., Casas G.A., Lagos L.V., Lee S.A., et al. Non-antibiotic feed additives in diets for pigs: a review. Anim Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C.X., Tan L., Ab M.N., Pusparajah P., Goh B.H., Chan K.G., et al. Unveiling the impact of antibiotics and alternative methods for animal husbandry: a review. Antibiotics. 2021;10:578. doi: 10.3390/antibiotics10050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens J.R., Gurung P., Vogel P., Johnson J.R., Carter R.A., Mcgoldrick D.J., et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516:246–249. doi: 10.1038/nature13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A.G., Chattin S.E., Robbins C.M., Golden D.A. Effects of a direct-fed yeast culture on enteric microbial populations, fermentation acids, and performance of weanling pigs. J Anim Sci. 1998;76:2138–2145. doi: 10.2527/1998.7682138x. [DOI] [PubMed] [Google Scholar]
- Munyaka P.M., Nandha N.K., Kiarie E., Nyachoti C.M., Khafipour E. Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poult Sci. 2015;95:528–540. doi: 10.3382/ps/pev333. [DOI] [PubMed] [Google Scholar]
- Marconi O., Floridi S., Montanari L. Organic acids profile in tomato juice by HPLC with UV detection. J Food Quality. 2007;30:253–266. [Google Scholar]
- Montenegro R.E., Dori-Hacohen G. Morality in sugar talk: presenting blood glucose levels in routine diabetes medical visits. Soc Sci Med. 2020;253 doi: 10.1016/j.socscimed.2020.112925. [DOI] [PubMed] [Google Scholar]
- NRC . 11th revised ed. National Academy Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Noblet J., Goff G.L. Effect of dietary fibre on the energy value of feeds for pigs. Anim Feed Sci Tech. 2001;90:35–52. [Google Scholar]
- Nocek J.E., Holt M.G., Oppy J. Effects of supplementation with yeast culture and enzymatically hydrolyzed yeast on performance of early lactation dairy cattle. J Dairy Sci. 2011;94:4046–4056. doi: 10.3168/jds.2011-4277. [DOI] [PubMed] [Google Scholar]
- Omonijo F.A., Ni L., Gong J., Wang Q., Lahaye L., Yang C. Essential oils as alternatives to antibiotics in swine production. Anim Nutr. 2018;4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opapeju F.O., Krause D.O., Payne R.L., Rademacher M., Nyachoti C.M. Effect of dietary protein level on growth performance, indicators of enteric health, and gastrointestinal microbial ecology of weaned pigs induced with postweaning colibacillosis. J Anim Sci. 2009;87:2635–2643. doi: 10.2527/jas.2008-1310. [DOI] [PubMed] [Google Scholar]
- Pan L., Shang Q.H., Wu Y., Ma X.K., Long S.F., Liu L., et al. Concentration of digestible and metabolizable energy, standardized ileal digestibility, and growth performance of pigs fed diets containing sorghum produced in the United States or corn produced in China. J Anim Sci. 2017;95:4880–4892. doi: 10.2527/jas2017.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Zhang H., Wen H., Wan H., Wu H., Chen Y., et al. Yeast probiotic and yeast products in enhancing livestock feeds utilization and performance: an overview. J Fungi. 2022;8:1191. doi: 10.3390/jof8111191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli M., Finamore A., Britti M.S., Bosi P., Oswald I., Mengheri E. Alternatives to in-feed antibiotics in pigs: evaluation of probiotics, zinc or organic acids as protective agents for the intestinal mucosa. A comparison of in vitro and in vivo results. Anim Res. 2005;54:203–218. [Google Scholar]
- Shen Y., Piao X., Kim S.W., Wang L., Liu P., Yoon I., et al. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci. 2009;87:2614–2624. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- Shurson G.C. Yeast and yeast derivatives in feed additives and ingredients: sources, characteristics, animal responses, and quantification methods. Anim Feed Sci Technol. 2018;235:60–76. [Google Scholar]
- Stein H.H., Sève B., Fuller M.F., Moughan P.J., De Lange C.F.M. Invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J Anim Sci. 2007;85:172–180. doi: 10.2527/jas.2005-742. [DOI] [PubMed] [Google Scholar]
- Stein H.H., Shipley C.F., Easter R.A. Technical Note: A technique for inserting a T-cannula into the distal ileum of pregnant sows. J Anim Sci. 1998;76:1433–1436. doi: 10.2527/1998.7651433x. [DOI] [PubMed] [Google Scholar]
- Wacoo A.P., Wendiro D., Vuzi P.C., Hawumba J.F. Methods for detection of aflatoxins in agricultural food crops. J Appl Chem. 2014;2014 [Google Scholar]
- Waititu S.M., Yin F., Patterson R., Yitbarek A., Rodriguez-Lecompte J.C., Nyachoti C.M. Dietary supplementation with a nucleotide-rich yeast extract modulates gut immune response and microflora in weaned pigs in response to a sanitary challenge. Animal. 2017;11:2156–2164. doi: 10.1017/S1751731117001276. [DOI] [PubMed] [Google Scholar]
- Wang X., Li F., Zhang N., Ungerfeld E., Guo L., Zhang X., Wang M., Ma Z. Effects of supplementing a yeast culture in a pelleted total mixed ration on fiber degradation, fermentation parameters, and the bacterial community in the rumen of sheep. Anim Feed Sci Technol. 2023;296 [Google Scholar]
- Wang C., Wang M., Chu Y., Sun R., Li J., Li A., et al. Antibiotic resistance patterns and molecular characterization of streptococcus suis isolates from swine and humans in China. Microbiol Spectr. 2023;11 doi: 10.1128/spectrum.00309-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhou J., Wang G., Cai S., Zeng X., Qiao S. Advances in low-protein diets for swine. J Anim Sci Biotechnol. 2018;9:1–14. doi: 10.1186/s40104-018-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.H., David D.J., Iismaa O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J Agric Sci. 1962;59:381–385. [Google Scholar]
- Xu J., Li Y., Yang Z., Li C., Liang H., Wu Z., et al. Yeast probiotics shape the gut microbiome and improve the health of early-weaned piglets. Front Microbiol. 2018;9:2011. doi: 10.3389/fmicb.2018.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Zhu W., Hang S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch Anim Nutr. 2019;73:287–305. doi: 10.1080/1745039X.2019.1614849. [DOI] [PubMed] [Google Scholar]
- Yu M., Zhang C., Yang Y., Mu C., Su Y., Yu K., et al. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. J Anim Sci Biotechnol. 2017;8:60. doi: 10.1186/s40104-017-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Li L., Zhao H., Tu Y., Liu M., Jiang L., et al. Characterization of the dynamic changes of ruminal microbiota colonizing citrus pomace waste during rumen incubation for volatile fatty acid production. Microbiol Spectr. 2023;11 doi: 10.1128/spectrum.03517-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Li D., Liu L., Zang J., Duan Q., Yang W., Zhang L. The effects of dietary fiber level on nutrient digestibility in growing pigs. J Anim Sci Biotechnol. 2013;4:1–7. doi: 10.1186/2049-1891-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wang Y., Zeng X., Zhang T., Li P., Yao B., et al. Effect of antibiotic-free, low-protein diets with specific amino acid compositions on growth and intestinal flora in weaned pigs. Food Funct. 2020;11:493–507. doi: 10.1039/c9fo02724f. [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang L., Yang L., Yang G., Zeng X., Qiao S. Different dietary starch patterns in low-protein diets: effect on nitrogen efficiency, nutrient metabolism, and intestinal flora in growing pigs. J Anim Sci Biotechnol. 2022;13:78. doi: 10.1186/s40104-022-00704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wang Y., Wang L., Tu J., Yang L., Yang G., et al. Compromised hindgut microbial digestion, rather than chemical digestion in the foregut, leads to decreased nutrient digestibility in pigs fed low-protein diets. Nutrients. 2022;14:2793. doi: 10.3390/nu14142793. [DOI] [PMC free article] [PubMed] [Google Scholar]