Abstract
PURPOSE:
The purpose of this study was to compare early visual outcomes, epithelial healing, and stromal haze between transepithelial photorefractive keratectomy (Trans-PRK) using smart pulse technology (SPT) with traditional Trans-PRK.
METHODOLOGY:
This study is a retrospective, comparative study conducted at a private eye center in “Riyadh, Saudi Arabia,” investigating myopic patients who underwent either Trans-PRK with SPT (study group) or traditional Trans-PRK (control group). The patients were assessed preoperatively and followed up at 1 week and 2 months postoperatively. The main outcomes included uncorrected distance visual acuity (UDVA), corneal haze, and corneal epithelial defect.
RESULTS:
This study included 501 eyes, of them, 222 eyes (44.3%) underwent Trans-PRK with SPT. The UDVA in the study group was significantly better 1 week postoperatively (P < 0.05). For the 2-month follow-up visits, there was no significant difference between the groups. Epithelium healing and stromal haze were comparable in the two groups without significant differences between them.
CONCLUSION:
Transepithelial photorefractive keratectomy with SPT yielded better short-term visual outcomes than traditional Transepithelial photorefractive keratectomy.
Keywords: Myopia, refractive surgery, smart pulse technology, transepithelial photorefractive keratectomy
Introduction
Throughout the world, the main cause of visual impairment is uncorrected refractive errors.[1] When it comes to surgical methods in correcting these refractive errors, excimer laser photorefractive keratectomy (PRK) remains a very commonly used method and a dominant option.
Although PRK has been proven to be effective, predictable, and safe in the treatment of some refractive errors,[2] it has several disadvantages, including postoperative pain, corneal haze, and occasional myopic regression.[3] With the continuous evolution of the excimer laser procedures to overcome these disadvantages and to improve effectiveness, predictability, and accuracy, a new one-step technique was introduced, which is transepithelial PRK (Trans-PRK). The excimer laser ablation in this modality takes place by simultaneously ablating the epithelium and stroma in precise and uniform manner,[4] instead of the classic uniform PRK epithelial removal, which required the need for alcohol epithelial debridement or mechanical removal of the epithelium.[5,6] Trans-PRK requires only one-step removal of the epithelium and stroma, without the need for instruments to come in contact with the cornea, thus takes significantly less surgical time, resulting in less postoperative pain, faster wound healing, and faster visual recovery than conventional PRK.[7] This technique became available on the SCHWIND AMARIS platform, in which surface ablation is performed by applying varying amounts of laser energy from the center to the periphery to resemble the curvature of the cornea.[8] To further improve the Amaris laser platform, SCHWIND introduced a new optional software feature named smart pulse technology (SPT) that ameliorates the stromal bed contour and diminishes surface irregularities immediately after the laser treatment and, as a result, would offer better visual recovery, especially during the early days of postoperative period. Moreover, thanks to the smoother corneal stroma bed, faster re-epithelization along with a lower incidence of postoperative haze is also expected. In addition, when comparing traditional Trans-PRK to Trans-PRK with SPT, it was shown that traditional Trans-PRK has slower visual acuity recovery and instant postoperative discomfort as major drawbacks.[9,10] Furthermore, excimer laser PRK is associated with corneal haze in 2%–4% or more. Corneal haze level is related to the level of stromal surface irregularities that remain after undergoing surface ablation.[11] The aim of this retrospective, cohort study is to compare early visual outcomes, epithelial healing, and stromal haze between Trans-PRK using SPT with traditional Trans-PRK in treating myopic patients.
Methodology
This retrospective comparative study was conducted at a private eye center in “Riyadh, Saudi Arabia” with the approval of the hospital’s Institutional Ethics Review Board. Data were collected from April 2022 to June 2022. The sample consisted of 501 eye participants who underwent either Trans-PRK with SPT (study group) or traditional Trans-PRK (control group) for the treatment of myopia with or without astigmatism and were done by one surgeon (TK). Patients who underwent previous refractive surgeries or who had other ocular pathologies were excluded. All patients were consulted and informed about the risks and benefits of the surgery.
Patients assessment
All patients had a full preoperative ophthalmologic examination, including best-corrected visual acuity, uncorrected distance visual acuity (UDVA), slit-lamp biomicroscopy, Goldmann tonometry, dilated funduscopy, and corneal topography. The data sheet was set for the evaluation of the patients’ demographic data and their clinical outcomes, which included the measurement of UDVA, corneal haze, and corneal epithelial healing at 1 week and 2 months after the surgery.
Surgical technique
For the control group, drops of topical anesthesia were applied in the eye. Povidone-iodine scrub was applied on the lids and lashes. Periorbital skin scrubbing was performed with povidone-iodine, the surgical field was isolated using a sterile drape, and a lid speculum was placed. Laser ablation of the corneal surface was performed using a single-step Trans-PRK with the targeted refractive correction that was calculated preoperatively. Mitomycin C (MMC) 0.02% was applied on the corneal surface. The duration of MMC application was 30 seconds, except in eyes with moderate-to-high myopia (more than −4.00 D), in which the MMC was applied for 1–2 min, according to the spherical equivalent (SE). The ocular surface was then washed copiously with the balanced salt solution. At the end, one drop of each topical corticosteroid and topical antibiotic was applied on the cornea, and a bandage contact lens was placed. The postoperative regimen included topical antibiotic eye drops four times a day for 10–14 days (until the complete healing of the corneal epithelium), corticosteroid eye drops four times a day (tapered over 4 weeks), nonsteroidal anti-inflammatory drops for pain relief four times a day for 3 days, and preservative-free artificial tears as needed. The bandage contact lens was left in place for approximately 1 week until complete healing of the corneal epithelium. The exact same steps were performed in the study group, with the addition of applying SPT during the corneal ablation.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 26.0 (IBM-SPSS, Armonk, New York, USA). Counts and percentages were used to summarize categorical variables, and the mean ± standard deviation (SD) was used for continuous variables. The Chi-square test was used to assess the association between categorical variables. Hypothesis testing was performed at 5% level of significance.
Results
Table 1 shows the preoperative characteristics of the patients. The mean preoperative logMAR UDVA −1.03 ± 0.87 in the TPRK group and −0.98 ± 0.86 in the smart pulse TPRK group (P = 0.073). No statistically significant difference was observed between the groups in the mean (SD) of SE, UDVA, age, or gender.
Table 1.
Preoperative data (results section)
| TPRK | Pulse TPRK-smart | P | |
|---|---|---|---|
| Eyes, n (%) | 281 (50.7) | 273 (49.3) | |
| Age (years), mean±SD (range) | 6.6±27.04 (56–18) | 6.8±27.74 (50–18) | 0.220 |
| Gender (%) | |||
| Male | 30.2 | 29.7 | 0.882 |
| Female | 69.8 | 70.3 | |
| UDVA (logMAR), mean±SD (range) | 0.9±1.03 (1.3–0.05) | 0.9±0.98 (1.3–0.05) | 0.073 |
| Spherical equivalent (D), mean±SD (range) | 1.4±−3.02 (−0.13–−6.88) | 1.1±−2.77 (−0.75–−5.63) | 0.067 |
UDVA: Uncorrected distance visual acuity, Trans-PRK: Transepithelial photorefractive keratectomy, SD: Standard deviation
The mean SE of eyes measured preoperatively and at all the follow-up visits are given in Figure 1. The mean SE of the eyes decreased in all groups after the procedure at 1 week and this normalization continued throughout the subsequent follow-up visits. There were no statistically significant differences between the groups in the mean SE at the follow-up period.
Figure 1.
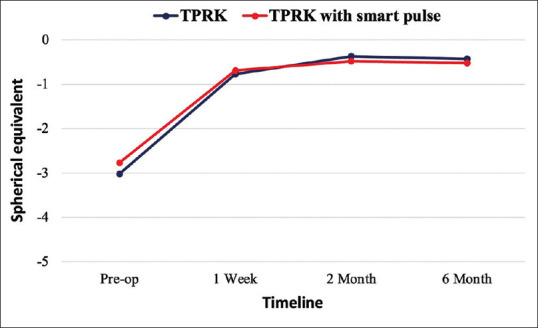
The mean spherical equivalents at all visits. Trans-PRK = Transepithelial photorefractive keratectomy
Figure 2 shows the postoperative logMAR UDVA in the last visit. The Snellen UDVA ranged from 20/20 to 20/40 in both groups. The percentage of eyes with Snellen UDVA of 20/25 or better was significantly higher in the smart pulse TPRK group (89.4%) compared to the TPRK group (82.2%) (P = 0.016).
Figure 2.
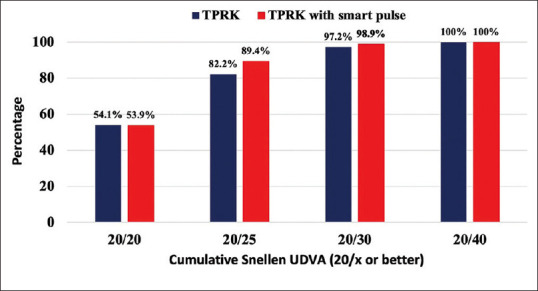
Cumulative percentage of eyes achieving Snellen uncorrected distance visual acuity at final visit. UDVA = Uncorrected distance visual acuity, Trans-PRK = Transepithelial photorefractive keratectomy
Figure 3 shows the percentage of eyes that were within different levels of the attempted SE correction. At the last follow-up visit, 40.6% of eyes in the TPRK group and 52% of eyes in the smart pulse TPRK group were within ±0.50 D of the attempted SE correction (P = 0.007). No statistically significant differences were reported in the percentages of eyes, achieving a final SE within ±0.25 D, ±1.00 D, and ±2.00 (P = 0.16, P = 0.12, and P = 0.12, respectively).
Figure 3.
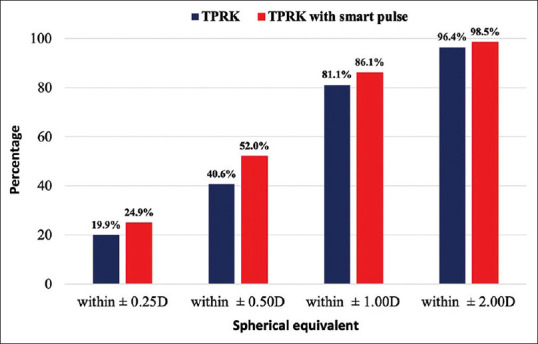
Postoperative spherical equivalent refractive predictability. Trans-PRK = Transepithelial photorefractive keratectomy
Table 2 shows the postoperative logMAR UDVA and SE results. The logMAR UDVA at 1 week ranged from 0 to 0.90 in the TPRK group and from 0 to 0.70 in the smart pulse TPRK group with a mean of 0.13 ± 0.1 and 0.10 ± 0.08, respectively (P = 0.012). At 2 months, the mean SE was −0.37 ± 0.7 in the TPRK group and −0.48 ± 0.6 in the smart pulse TPRK group (P = 0.173). The mean logMAR UDVA and SE was significantly better in both groups in all follow-up visits compared to the preoperative data. There was no statistically significant difference between the groups, regarding SE at 1-week, 2-month, and 6-month follow-up visits (P = 0.256, P = 0.173, P = 0.517, respectively).
Table 2.
Postoperative data (results section)
| TPRK | Pulse TPRK-smart | P † | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean±SD | Range | P* | Mean±SD | Range | P* | ||
| UDVA (logMAR) | |||||||
| 1 week | 0.1±0.13 | 0.90–0 | <0.001 | 0.08±0.10 | 0.70–0 | <0.001 | 0.012 |
| 2 months | 0.05±0.1 | 0.90–0 | <0.001 | 0.09±0.03 | 0.90–0 | <0.001 | 0.049 |
| 6 months | 0.2±0.05 | 1.00–0 | <0.001 | 0.08±0.02 | 0.70–0 | <0.001 | 0.137 |
| SE (D) | |||||||
| 1 week | 0.8±−0.77 | 1.63–−4.63 | <0.001 | 0.6±−0.69 | 1.13–−3.75 | <0.001 | 0.256 |
| 2 months | 0.7±−0.37 | 1.50–−3.38 | <0.001 | 0.6±−0.48 | 1.38–−2.50 | <0.001 | 0.173 |
| 6 months | 0.9±−0.43 | 1.50–−3.38 | <0.001 | 0.6±−0.52 | 1.00–−3.38 | <0.001 | 0.517 |
*Difference between pre- and post-operative data, †Difference between the 2 groups. UDVA: Uncorrected distance visual acuity, Trans-PRK: Transepithelial photorefractive keratectomy, SD: Standard deviation
Discussion
Our results obtained from 501 eyes showed a significant improvement of UDVA after 1 week in favor to Trans-PRK surgery with SPT over the traditional Trans-PRK surgery. However, this significant difference faded after 2 months of follow-up. No significant difference was noted between the two groups, regarding epithelial healing and stromal haze.
PRK provided a superior effect regarding the efficacy and safety of corneal refractive surgeries to treat errors of refraction. New techniques have been introduced to accelerate visual recovery and reduce corneal haze and postoperative pain, including femtosecond assisted and small incision lenticule extraction (SMILE).[7] Trans-PRK is considered to remove epithelium and stroma in one step without mechanical removal of the epithelium or cornea contact with the surgical instruments.[5]
The SPT has been introduced to provide a characteristic spot geometry ablation that avoids unwanted extra thermal load and pulse energy. Furthermore, the excimer laser coupled with SPT could improve UDVA post 6 months compared to standard techniques by reducing irregularity in the superficial stromal.[11,12] The application of SPT in laser ablation surgeries was associated with rapid UDVA recovery and epithelium healing.[12] Even though Trans-PRK with or without SPT was reported to be superior to alcohol-assisted PRK, when regarding better visual acuity.[13] On the contrary, another cohort study on 792 eyes reported that clinical outcomes of femtosecond assisted laser in situ keratomileusis (FS-LASIK) were slightly better than those of Trans-PRK for high-grade myopia with efficacy index values of 0.92 in Trans-PRK group and 0.95 in FS-LASIK group, however, this could be explained that the preoperative SE of the Trans-PRK group was higher.[14] The use of mitomycin is suggested to reduce corneal haze, additionally, in a recent study, Gab-Alla et al. investigated the safety and efficacy of Trans-PRK using SPT with MMC for correction of post-SMILE myopic residual refractive errors with 100% correction 12 months postoperative.[15]
In terms of UDVA, Lin et al. reported a rapid recovery and correction of monocular and binocular UDVA in 50% and 72% of examined 2239 myopic eyes, respectively, treated by Trans-PRK with SPT. Further, monocular UDVA of 20/25 or better was detected in 94% 3-month postoperative.[16] This comes consistent with our results that show a UDVA of 20/30 or better in 98.9% of examined eyes. Similarly, a significant rapid improvement in visual rehabilitation 1 day and 1 week after Trans-PRK with SPT surgery had been reported (P < 0.05) that faded after 1 month postoperatively.[12] In a retrospective case series, Lin et al. evaluated postoperative corneal asphericity in low, moderate, and high myopic 106 eyes, SmartSurf (ACE) technique showed an advantage in improving the vision quality and the onset of presbyopia symptoms after undergoing laser refractive correction.[17] In another trial to investigate the role of SPT in aberration-free all-surface laser ablation surgery, the group with SPT achieved better visual acuity.[18]
Other studies have demonstrated that an increase in root mean square (RMS) and higher order aberrations (HOAs) of the anterior cornea are correlated with the preoperative SE and the central corneal ablation depth, although PRK-induced increased HOAs by 2.4-fold with respect to the anterior corneal surface when the ablation depth is <77 μm.[19,20] However, Du et al. reported a significant increase in RMS HOAs up to three times in both groups treated with and without SPT, the result was against the effect of SPT to reduce epithelium roughness and the postoperative spherical aberration.[18]
Regarding other postoperative complications, Aslanides and Kymionis reported a lower pain score in Trans-PRK with SPT surgery group whereas corneal haze was similar in both groups. This also comes consistent with our results.[12] No significant difference in epithelial healing time was detected in aberration-free all-surface laser ablation surgery with and without SPT.[18] However, there is a clear association between the residual corneal stromal roughness surface and how smooth the corneal epithelial cells regenerate and migrate as well as the rate of fibroblast proliferation.[21] The development of haze after PRK is directly associated with increased wound-healing keratocytes.[22] Hence, the smoother plane may improve UDVA and reduce corneal opacity that could be achieved by SPT. On the other hand, there is a linear association between the deeper the ablation and the hyperplasia of the epithelium that led to refractive regression.[23]
The current study had some limitations, including the retrospective design of the study and a short-term follow-up of 2 months. Furthermore, lack of assessment of the mean re-epithelialization time and pain sensation. In addition, we did not have enough representative samples of the elderly, as our study included only two patients above the age of 50.
Conclusion
Transepithelial photorefractive keratectomy with SPT yielded better short-term visual outcomes than traditional Transepithelial photorefractive keratectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Xi L, Zhang C, He Y. Clinical outcomes of transepithelial photorefractive keratectomy to treat low to moderate myopic astigmatism. BMC Ophthalmol. 2018;18:115. doi: 10.1186/s12886-018-0775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spadea L, Giovannetti F. Main complications of photorefractive keratectomy and their management. Clin Ophthalmol. 2019;13:2305–15. doi: 10.2147/OPTH.S233125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu YL, Tseng CC, Lin CP. Visual performance after excimer laser photorefractive keratectomy for high myopia. Taiwan J Ophthalmol. 2017;7:82–8. doi: 10.4103/tjo.tjo_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adib-Moghaddam S, Soleyman-Jahi S, Salmanian B, Omidvari AH, Adili-Aghdam F, Noorizadeh F, et al. Single-step transepithelial photorefractive keratectomy in myopia and astigmatism:18-month follow-up. J Cataract Refract Surg. 2016;42:1570–8. doi: 10.1016/j.jcrs.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Wang DM, Du Y, Chen GS, Tang LS, He JF. Transepithelial photorefractive keratectomy mode using SCHWIND-ESIRIS excimer laser: Initial clinical results. Int J Ophthalmol. 2012;5:334–7. doi: 10.3980/j.issn.2222-3959.2012.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luger MH, Ewering T, Arba-Mosquera S. Consecutive myopia correction with transepithelial versus alcohol-assisted photorefractive keratectomy in contralateral eyes: One-year results. J Cataract Refract Surg. 2012;38:1414–23. doi: 10.1016/j.jcrs.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Naderi M, Jadidi K, Mosavi SA, Daneshi SA. Transepithelial photorefractive keratectomy for low to moderate myopia in comparison with conventional photorefractive keratectomy. J Ophthalmic Vis Res. 2016;11:358–62. doi: 10.4103/2008-322X.194070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadlallah A, Fahed D, Khalil K, Dunia I, Menassa J, El Rami H, et al. Transepithelial photorefractive keratectomy: Clinical results. J Cataract Refract Surg. 2011;37:1852–7. doi: 10.1016/j.jcrs.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Randleman JB, Loft ES, Banning CS, Lynn MJ, Stulting RD. Outcomes of wavefront-optimized surface ablation. Ophthalmology. 2007;114:983–8. doi: 10.1016/j.ophtha.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 10.Manche EE, Haw WW. Wavefront-guided laser in situ keratomileusis (Lasik) versus wavefront-guided photorefractive keratectomy (Prk): A prospective randomized eye-to-eye comparison (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2011;109:201–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Vinciguerra P, Camesasca FI, Vinciguerra R, Arba-Mosquera S, Torres I, Morenghi E, et al. Advanced surface ablation with a new software for the reduction of ablation irregularities. J Refract Surg. 2017;33:89–95. doi: 10.3928/1081597X-20161122-01. [DOI] [PubMed] [Google Scholar]
- 12.Aslanides IM, Kymionis GD. Trans advanced surface laser ablation (TransPRK) outcomes using SmartPulse technology. Cont Lens Anterior Eye. 2017;40:42–6. doi: 10.1016/j.clae.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 13.D’Oria F, Fernández-Buenaga R, Casanova L, García-Corral MJ, Vega A, Alio JL. Surface ablation outcomes in high myopia with different epithelium removal techniques. J Cataract Refract Surg. 2021;47:1175–82. doi: 10.1097/j.jcrs.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 14.Gershoni A, Mimouni M, Livny E, Bahar I. Z-LASIK and trans-PRK for correction of high-grade myopia: Safety, efficacy, predictability and clinical outcomes. Int Ophthalmol. 2019;39:753–63. doi: 10.1007/s10792-018-0868-4. [DOI] [PubMed] [Google Scholar]
- 15.Gab-Alla A. SmartSurf ACE transepithelial photorefractive keratectomy with mitomycin C enhancement after small incision lenticule extraction. Eye and Vision. 2021;8:1–10. doi: 10.1186/s40662-021-00254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin DT, Holland SP, Mosquera SA, Covello A. Immediate visual recovery after photorefractive keratectomy with new laser beam profile. Invest Ophthalmol Vis Sci. 2019;60:5072. [Google Scholar]
- 17.Lin DT, Holland SP, Verma S, Hogden J, Arba-Mosquera S. Postoperative corneal asphericity in low, moderate, and high myopic eyes after transepithelial PRK using a new pulse allocation. J Refract Surg. 2017;33:820–6. doi: 10.3928/1081597X-20170920-02. [DOI] [PubMed] [Google Scholar]
- 18.Du X, Zhang J, Su M, Cao W, Zeng S, Wang Q, et al. Clinical outcomes of aberration-free all surface laser ablation (ASLA) versus aberration-free ASLA assisted by smart pulse technology in high myopia: A one-year follow-up study. J Ophthalmol. 2021;2021:2588765. doi: 10.1155/2021/2588765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juhasz E, Kranitz K, Sandor GL, Gyenes A, Toth G, Nagy ZZ. Wavefront properties of the anterior and posterior corneal surface after photorefractive keratectomy. Cornea. 2014;33:172–6. doi: 10.1097/ICO.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 20.Serrao S, Lombardo G, Ducoli P, Lombardo M. Long-term corneal wavefront aberration variations after photorefractive keratectomy for myopia and myopic astigmatism. J Cataract Refract Surg. 2011;37:1655–66. doi: 10.1016/j.jcrs.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier CA, Epstein D, Holden BA, Tengroth B, Fagerholm P, Hamberg-Nyström H, et al. Epithelial alterations following photorefractive keratectomy for myopia. J Refract Surg. 1995;11:113–8. doi: 10.3928/1081-597X-19950301-11. [DOI] [PubMed] [Google Scholar]
- 22.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–45. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 23.Erie JC. Corneal wound healing after photorefractive keratectomy: A 3-year confocal microscopy study. Trans Am Ophthalmol Soc. 2003;101:293–333. [PMC free article] [PubMed] [Google Scholar]


