Abstract
Background
The ovary is a central organ in the reproductive system that produces oocytes and synthesizes and secretes steroid hormones. Healthy development and regular cyclical change in the ovary is crucial for regulating reproductive processes. However, the key genes and metabolites that regulate ovarian development and pregnancy have not been fully elucidated. This study conducted high-throughput RNA sequencing and untargeted metabolite profiling of the ovarian tissues from Chenghua pigs at four stages, including postnatal day 3 (D3), puberty at the age of about 125 days (Pub), sexual maturity at the age of about 365 days (Y1), and 105 days after pregnancy at the age of about 360 days (Pre).
Results
A total of 9,264 and 1,593 differentially expressed genes (DEGs) were identified during ovarian development and pregnancy. Several key genes involved in ovarian development, including SQLE, HMGCS1, MSMO1, SCARB1, CYP11A1, HSD3B1, HSD17B1, and SERPINE1 were identified. Similarly, LUM, FN1, PLAUR, SELP, SDC1, and VCAN were considered to be associated with pregnancy maintenance. Overexpression of HSD17B1 in granulosa cells significantly upregulated estrogen synthesis-related genes (HSD3B1, CYP11A1, and STAR); meanwhile, overexpression of PLAUR promotes granulosa cell proliferation. Furthermore, 66, 24, 77, and 7 differentially expressed miRNAs (DEMis) were found, leading to the selection of key miRNAs such as ssc-miR-206, ssc-miR-107, ssc-miR-429, ssc-miR-210, and ssc-miR-133a-3p by differential miRNA-targeted mRNA interaction network; meanwhile, ssc-miR-133a-3p was validated to have a targeting relationship with KCNA1 by dual-luciferase reporter systems assay. At the metabolic levels, androstenedione, 17a-hydroxyprogesterone, dehydroepiandrosterone, and progesterone were identified, with their synthesis regulated by these DEGs in the ovarian steroidogenesis pathway. Furthermore, treatment of cells with androstenedione upregulated the expression of HSD3B1, CYP11A1, and STAR.
Conclusions
This study revealed the dynamic changes in the transcriptome and metabolome of pig ovaries across developmental stages and gestation, indicating that it may provide new theoretical insights for improving sow fertility.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-11122-3.
Keywords: Pig, Ovary, Development, Gestation, MRNA, MiRNA, Metabolome
Background
The ovary is the foundation of the sexual reproduction process and is responsible for producing oocytes and secreting hormones, thus regulating reproductive functions and endocrine balance to maintain fertility and health. The quality and quantity of oocytes directly determine the fertilization rate and embryo count, affecting reproductive efficiency. Moreover, sex hormones play a crucial role in inducing oestrus and supporting embryonic development, which is essential for mating and conception. Incomplete development or dysfunction of the ovary can lead to blocked ovulation and abnormal hormone levels and subsequently affect the regulation of the reproductive cycle, embryo implantation, fetal development, and maintenance of pregnancy. Abnormalities during the development of the ovaries can lead to ovarian dysfunction, which in turn can trigger a range of diseases, such as premature ovarian failure (POF) [1], polycystic ovary syndrome (PCOS) [2], and even ovarian cancer [3]. Therefore, studying ovarian development holds significant reference values for research on female reproductive health and assisted reproductive technologies.
Follicles, as the fundamental functional units of the ovary, drive the development of the ovaries through the process of follicular development [4]. Follicle development begins in the embryonic or neonatal period [5]. Primordial follicles are composed of oocytes and pregranulosa cells differentiated from somatic cells surrounding the oocytes, which then enter a dormant state [6]. During puberty, some dormant primordial follicles become activated, with oocytes growing rapidly and flattened pregranulosa cells becoming cuboidal [7]. Multiple signaling pathways regulate primordial follicle activation, with the mammalian target of rapamycin complex 1 (mTORC1)-kit ligand (KITL)-oocyte KIT-PI3K signaling pathway in pregranulosa cells being crucial for the physiological activation of primordial follicles [8]. Activated primordial follicles become growing follicles, which progress through the primary and secondary follicle stages to become antral follicles [9]. The development of growing follicles is characterized by rapid accumulation of RNA and proteins and an increase in mitochondria, with granulosa cells proliferating and differentiating into multiple layers, oocytes growing rapidly, and their volume increasing more than 100-fold [10]. The outermost granulosa cells consist of theca cells, have secretory capabilities, and synthesize estrogen in conjunction with granulosa cells [11, 12]. This process is cyclical recruitment regulated by follicle-stimulating hormone (FSH) and involves interactions among various molecules and signaling pathways between oocytes, granulosa cells, and theca cells, as detailed in the reviews by McGee, Edson, and Rimon-Dahari [9, 13, 14].
Before developing into antral follicles, growing follicles are mainly subjected to autocrine and paracrine regulation mediated by oocyte-secreted factors (OSFs) [9]. Several members of the TGF-β superfamily, including anti-Müllerian hormone (AMH), glial cell-derived neurotrophic factor (GDNF), activin/inhibin, growth and differentiation factor (GDF), bone morphogenetic protein (BMP) and TGF-β, have been reported to be involved in the regulation of the follicular developmental process and cellular communication within the follicle [15]. The hypothalamic-pituitary–gonadal (HPG) axis plays a crucial role in the development of the antral follicles to ovulation [16, 17]. The hypothalamus releases gonadotropin-releasing hormone (GnRH), which prompts the anterior pituitary gland to secrete luteinizing hormone (LH) and FSH [18, 19]. These two hormones are collectively known as gonadotropins. FSH and LH circulate in the bloodstream to the ovaries, producing progesterone, estrogen, and testosterone [20]. Mature follicles secrete large amounts of estradiol, further stimulating the pituitary gland to release more gonadotropins, primarily LH and some FSH. Elevated levels of LH lead to rupture of the follicle and ovulation of the mature follicle [21]. Folliculogenesis is accompanied by the formation and degeneration of the corpus luteum [10]. The corpus luteum secretes progesterone in preparation for pregnancy. If fertilization and implantation do not occur, the corpus luteum degenerates and hormone levels decrease, initiating a new cycle of follicular development [10]. Notably, only a few growing follicles can undergo cyclical recruitment under the regulation of FSH after puberty, growing and maturing until ovulation, while most growing follicles undergo atresia and degeneration [22–24]. Follicular atresia is primarily characterized by granulosa cell apoptosis, which can be mitigated by autophagy activation. Autophagy activation can promote granulosa cell proliferation, delay atresia [25], and ensure ovarian function by maintaining homeostasis [26]. Conversely, inhibiting autophagy in oocytes of primordial follicles leads to infertility in female mice [27].
In recent years, with the development of high-throughput sequencing technology, multi-omics analysis has become a powerful tool for studying ovarian development and function. RNA-seq technology can be used to identify new mRNA transcripts [28], screen related candidate genes [29, 30], analyze the regulatory network of related genes, and compare transcriptome heterogeneity across samples [31]. For instance, Varik et al. analyzed the effects of the plasticizer di(2-ethylhexyl) phthalate (DEHP) on cholesterol biosynthesis, steroidogenesis pathways, and follicular growth [32]. Dhori et al. unveiled novel gene signatures and signaling pathways associated with granulosa cells (GCs) function and oocyte maturation through transcriptome datasets integration [33]. In addition, the global transcriptome spectrum reveals the transcriptional characteristics of human oocytes at multiple stages (GV, MI, and MII) before ovulation, as well as the interactions between oocytes and cumulus cells [34, 35]. Follicular development and ovulation are regulated not only by genes but also by small RNAs, which are involved in these processes. Some studies have shown that miR-224 plays an important role in the development of mouse follicles by regulating the proliferation of cumulus and perifollicular granulosa cells, as well as the expression levels of enzymes related to steroid hormone production [36]. In addition, certain miRNAs, such as miR-125a-5p [37], miR-26b [38], and miR-21 [39], are involved in the regulation of granulosa cell apoptosis during follicle development, and miR-376a can regulate the apoptotic process of oocytes [40]. Other studies have shown that miRNAs such as miR-133b [41], miR-125a-3p [42], and miR-125b [43] are associated with the release of oocytes during ovulation. These miRNAs can regulate the maturation and release of oocytes, thereby affecting the ovulation rhythm. Additionally, plasma-derived exosome miR-10a-5p promotes the development of premature ovarian failure (POF) [44]. The miR-34c from follicular fluid-derived extracellular vesicles regulates the acquisition of oocyte competence and has an impact on embryonic development [45]. Multiple researchers have employed single-cell RNA sequencing (scRNA-seq) technology to meticulously characterize a variety of cell types and their subpopulations in the ovaries of different species [46–50]. By constructing a comprehensive cellular atlas of the ovary, researchers have successfully identified a range of new marker genes [49, 51–55]. These advancements have not only broadened our understanding of ovarian cell growth and interactions but also deepened our knowledge of ovarian physiology at the molecular level. The reports on the use of metabolomics to study ovarian tissue mainly include reports of ovarian physiological characteristics and related diseases [56, 57].
As a high-quality local pig breed with good reproductive performance, the Chenghua pig not only has significant economic value in farming but also serves as an important biomedical research model and a valuable reference for human reproductive health. Although many studies have focused on the molecular mechanisms of ovarian development in mice and humans, relatively few systematic studies have been conducted on ovarian development in agricultural animals, especially in Chenghua pigs. In particular, ovarian changes during different developmental stages and physiological states (e.g., birth, puberty, maturation, and gestation) have not been fully investigated. The key genes and metabolites that regulate ovarian development and pregnancy have not been fully elucidated. This study aimed to reveal the key genes and metabolic pathways affecting ovarian development and function by systematically analyzing the transcriptome and metabolome changes in the ovaries of Chenghua pigs at different developmental stages and physiological states. The specific objectives of this study were (1) to construct a transcriptome and metabolome database of the ovaries during early childhood, puberty, maturity, and gestation; (2) to identify differentially expressed genes and significantly altered metabolites at different developmental stages and in different physiological states; and (3) to analyze the role of key genes and metabolic pathways in the regulation of ovarian development and function. This study not only helps to improve the reproductive performance of Chenghua pigs but also provides new perspectives and methods for the study of mammalian ovarian development and may also have a wide range of potential applications in agricultural production and reproductive medicine.
Materials and methods
Experimental animals and sample preparation
In this study, ovarian tissues were collected at four-time points, including postnatal day 3 (D3), puberty at the age of about 125 days (Pub), sexual maturity at the age of about 365 days (Y1), and 105 days after pregnancy at the age of about 360 days (Pre). The onset of puberty is determined by observing the sow's vulva and oestrus behavior. These sows from the same boar family were obtained from the Chengdu Livestock and Poultry Genetic Resources Protection Center (Sichuan, China). All the Chenghua sows were in good health and had no history of reproductive diseases. They were kept under the same environmental conditions with a comparable level of nutrition. Three healthy sows with similar ages were selected from each age group (Table S1), transported for 1 h to the laboratory and fasted for 12 h, and then their serum and ovaries were collected for this study (n = 3). Prior to animal euthanasia, blood was collected into anticoagulant tubes via jugular venipuncture, followed by centrifugation to separate the serum, which was then aliquoted and stored at −80°C. Afterward, the animals were electrically stunned (90 V, 10 s, 50 Hz) to induce immediate unconsciousness and exsanguinated as necessary to minimize suffering. The ovary tissue was collected, washed with 1X PBS, cut into 3–5 mm2 fragments, and immediately snap-frozen in liquid nitrogen for total RNA extraction.
Hematoxylin and eosin (HE) staining
Following the manufacturer's instructions, the collected ovarian tissues were fixed in 10% paraformaldehyde (Beyotime, CHN) for 24 h, dehydrated using an ethanol gradient of varying concentrations (Chron, CHN), placed in xylene to make it transparent, and embedded in paraffin until solidified. The tissues were then sectioned into 3–4 µm thick slices, stained with hematoxylin and eosin (Servicebio, CHN), dehydrated, sealed, and examined under a microscope (Leica LAS X, GER).
Enzyme-linked immunosorbent assay (ELISA)
The ELISA kits were purchased from Quanzhou Ruixin Biotechnology Co., Ltd. The concentrations of FSH, LH, estradiol (E2), and progesterone (PROG) in the serum were quantified according to the manufacturer's instructions. LH and FSH were detected by sandwich ELISA, while E2 and PROG were detected by competitive ELISA with sensitivity and detection range detailed in Table S2. A four-parameter logistic (4-PL) curve fitting was applied to generate a standard equation based on the concentration and the corresponding optical density (OD) value of the standard. Then, the sample concentration was calculated based on their OD values.
RNA extraction and quality control
According to the instruction manual, total RNA was extracted from ovarian tissues using TRIzol Reagent (Invitrogen, USA). RNA integrity number (RIN) was evaluated using the Agilent RNA 6000 Nano Reagents Part 1 Assay Kit on the Agilent 2100 Bioanalyzer system (Agilent Technologies, USA) based on the 28S and 18S rRNA peak ratio and background noise. The Bioanalyzer system generated an electropherogram and calculated RNA concentration from the fluorescence signal intensity.
cDNA library and miRNA library construction and sequencing
The cDNA library was constructed after a quality test. Briefly, messenger ribonucleic acids (mRNAs) were enriched from total RNA using magnetic beads linked with oligo (dT). Then fragmented mRNAs in lysis buffer. First-strand cDNA was synthesized using interrupted mRNA as a template, and second-strand cDNA was synthesized using dUTP as a substrate. The next step involved the end-repair of double-stranded cDNA fragments, followed by the addition of a single 'A' nucleotide to the 3' ends of the blunt fragments. Finally, the cDNAs were amplified by Polymerase Chain Reaction (PCR) to establish a strand-specific transcriptome library. The methods used for miRNA library preparation differ slightly from those used for cDNA library preparation. A certain number of qualified RNA samples were collected, and the 3’ and 5’ adapters were connected to the samples in sequence. Afterward, reverse transcription-PCR was performed, and the target band of the PCR products was purified via PAGE and dissolved in EB solution. The cDNA and miRNA PCR products were denatured and circularized to obtain a single-stranded cyclized product. Single-stranded circular DNA molecules are subjected to rolling-cycle amplification for replication, generating DNA nanoballs (DNBs) containing multiple DNA copies. The obtained DNBs are then loaded into reticulated vias on the chip through high-density DNA nano-chip technology and sequenced using combinatorial probe-anchor synthesis (cPAS) based on the DNBSEQ high-throughput platform.
RNA-Seq data analysis
The sequencing data were filtered using SOAPnuke (v1.5.6) by (1) eliminating reads containing sequencing adapters, (2) discarding reads with a low-quality base ratio exceeding 20% (base quality ≤ 15), and (3) removing reads whose unknown base ('N' base) ratio exceeded 5%. Afterward, clean reads were obtained and saved in FASTQ format. Further analysis and data mining were conducted using the Dr. Tom Multi-omics Data mining system [58]. Clean reads were aligned to the reference genome and the reference gene set using HISAT2 (v2.1.0) [59] and Bowtie2 (v2.3.4.3) [60] software, respectively. The gene expression levels were quantified by RSEM (v1.3.1) [61]. Heatmaps were generated by pheatmap (v1.0.8) [62] to visualize the differential gene expression across different samples. Differential expression analysis was conducted using the DESeq2(v1.4.5) [63] with a significance threshold of Q value ≤ 0.05. To gain insight into the phenotypic changes, enrichment analysis of the annotated differentially expressed genes was conducted using GO and KEGG with the Phyper tool based on the hypergeometric test. The significant levels of terms and pathways were adjusted by the Q value with a stringent threshold of Q value ≤ 0.05.
Small RNA analysis
The raw sequencing data are referred to as raw tags. We obtained clean tags by using SOAPnuke to filter tags in the following situations: tags with low quality; tags containing 5' primer contaminants; tags lacking 3' primer; tags lacking insertion; and tags containing poly-A. Following filtration, the clean tags were aligned to the reference genome and other small RNA databases, such as siRNA, miRbase, snoRNA, and piRNA, using Bowtie2 (v2.2.5) [60]. In particular, cmsearch was performed for Rfam mapping [64]. The miRDeep2 [65] package was used to identify potentially novel miRNAs. RNAhybrid [66] and miRanda [67] were used to predict the target genes of the miRNAs. The expression levels of small RNAs were quantified by enumerating absolute numbers of molecules using unique molecular identifiers (UMIs) [68]. Data analysis, mapping, and mining were further performed utilizing Dr. Tom's Multi-omics Data Mining System.
Quantitative real-time polymerase chain reaction (qRT-PCR)
QRT-PCR validated RNA-Seq results for three randomly selected differential genes and miRNAs, using GAPDH and U6 snRNA as reference genes. Primers were designed via NCBI and synthesized by Tsingke (Table S3), with reverse primers for miRNAs and U6 provided in the Mir-X miRNA First Strand Synthesis Kit (Takara, JPN). SYBR Green II dye (Vazyme, CHN) was used. Each sample was analyzed in triplicate. The relative expression levels of the tested genes were calculated using the 2−ΔΔCT method.
Cell culture and transfection
Porcine granulosa cells (GCs) obtained from Meisen CTCC (Zhejiang, China) were cultured in DMEM supplemented with 10% FBS at 37℃ with 5% CO2 until reaching 70–80% confluency. The cells were then seeded into 6 or 96-well plates, divided into five groups, including blank control, overexpression (pEGFP-N1-HSD17B1 and pEGFP-N1-PLAUR), empty plasmids (pEGFP-N1-NC), siRNA interference (si-HSD17B1 or PLAUR), and siRNA negative control group (si-NC) for overexpression and knockdown analysis. The recombinant plasmids and siRNAs were synthesized by Tsingke Biotechnology Co., Ltd (Table S4). Each group was tested in triplicate. Lipid complexes were prepared using Lipofectamine™ 3000 according to the manufacturer's instructions (Thermo Fisher Scientific, USA) and added to the culture medium for 4—6 h. Then, the medium was replaced with a complete culture medium and incubated for 24—72 h to achieve gene overexpression and knockdown. Take bright and dark-field fluorescence photos of cells under a Leica LAS X microscope (40x). After the designated incubation time, total RNA was extracted using an RNA isolation kit for evaluation of target gene levels by qPCR (Table S3).
Cell proliferation assay
Cell proliferation was assessed using cell counting kit-8 (CCK-8) (Coolaber, CHN) at 0, 24, 48, and 72 h. Briefly, cells are seeded into 96-well plates at a density of 1*103 per well and transfected with 100 ng of overexpression plasmid, siRNA, and negative control, respectively. After incubation for 24, 48, and 72 h, add 10 µL of CCK-8 reagent to each well and incubate for 1 h. Measure the absorbance at 450 nm using a MultiSKAN GO microplate reader (Thermo, USA).
Dual-luciferase reporter systems assay
Wild-type (WT) and mutant (MUT) sequences of KCNA1 were designed and synthesized using the PCR-based Accurate Synthesis (PAS) method (Table S4). These sequences were cloned into the pSicheck2.0 vector to create recombinant plasmids (KCNA1-WT and KCNA1-MUT). Simultaneously, ssc-miR-133a-3p mimics were synthesized in vitro (Table S4). The components were co-transfected into 293 T cells using Lipo3000 (Thermo Fisher Scientific, USA). 293 T cells were plated in a 6-well plate and incubated at 37℃ with 5% CO2 for 24 h. One hour prior to transfection, the culture medium was replaced with 10% FBS lacking antibiotics. Six hours post-transfection, the medium was refreshed with 10% FBS. After 48 h, firefly and sea kidney luciferase activity were measured to assess the targeting effects of ssc-miR-133a-3p on KCNA1 using the Duo-LiteTM Luciferase Assay System (Vazyme, CHN).
Clustering of time-series expression profiles.
Using short time series expression miner (STEM1.3.7), the gene expression patterns were first preset and then classified based on the correlation coefficient between each gene and the preset trend. The gene expression data of the four-time point samples were clustered and analyzed for their expression patterns.
Metabolite extraction and UPLC-MS analysis
A total of 25 mg of the sample with 2 small magnetic beads was added to the centrifuge tube, and 10 µL of the prepared internal standard 1 was added. Methanol, acetonitrile, and water were used to prepare the precooled extraction reagent at a ratio of 2:2:1 by volume, and after 800 µL was added to each sample, the mixture was ground at 50 Hz for 5 min. The ground samples were left to precipitate at −20°C for 2 h and then centrifuged for 15 min at 25000 g *4°C. After centrifugation, 600 µL of each was added to a new EP tube and freeze-dried. Then, 600 µL of 50% methanol was added to the tube and shaken until completely dissolved. 25000 g *4℃ centrifugation was performed for 15 min. The supernatant was placed in a new EP tube for UPLC-MS analysis, and 10 µL of each sample was mixed to form a QC sample. Waters 2777C UPLC (Waters, USA) in series with Q Exactive HF high-resolution mass spectrometer (Thermo Fisher Scientific, USA) was used for metabolite separation and detection.
Androstenedione treatment
Androstenedione (4-androstene-3,17-dione, C19H26O2) from Beijing Solarbio Science & Technology Co., Ltd. was dissolved in DMSO to prepare a 1*10–2 mol/L stock solution and diluted with DMEM to final concentrations of 100 μM (Group I), 10 μM (Group II), and 1 μM (Group III). Granulosa cells were treated with these media for 24 h (n = 3). Total RNA was then extracted for qPCR analysis of estrogen synthesis-related genes (Table S3).
Statistical analysis
All experimental data were obtained from at least three independent experiments. Values are shown as the mean ± standard error of the mean. GraphPad Prism (v9.0.0) statistics software was used for statistical analysis. The differences between groups were calculated using Student's t-test. A significance level of < 0.05 was considered to be statistically significant; * p < 0.05; ** p < 0.01; *** p < 0.001.
Results
Characteristics of ovarian histomorphology and levels of, plasma hormone
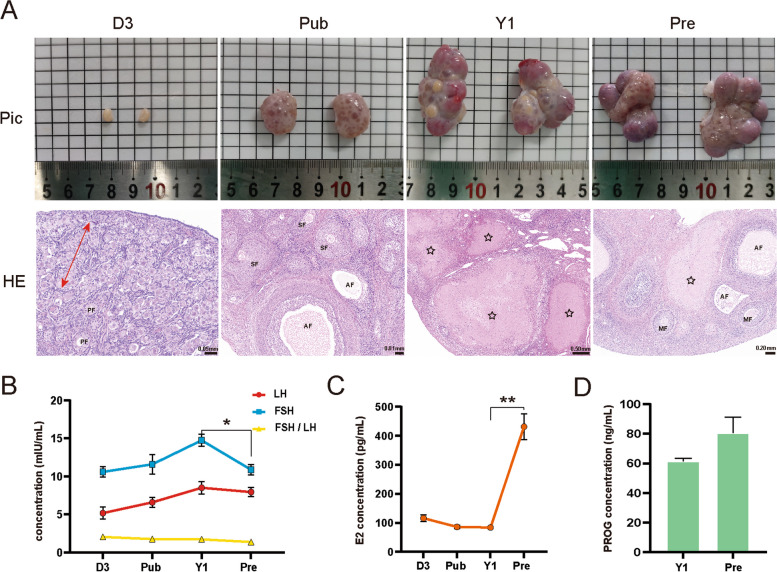
The ovaries underwent significant morphological changes during developmental and physiological stages (Fig. 1A). At birth (D3), the ovaries had a smooth renal shape, and no follicles were visible. When entering puberty (Pub), many follicles begin to develop, and numerous follicles filled with follicular fluid can be seen, giving the ovary a mulberry-like appearance. As the developmental cycle progressed, the ovarian surface was covered with follicles of varying diameters, and corpora lutea formed after ovulation, resembling a cluster of grapes (Y1). With age, the ovarian volume gradually increased, and the follicles became more numerous. During pregnancy, the ovaries were covered by a large amount of corpus luteum formed after ovulation and some growing follicles (Pre).
Fig. 1.
Histomorphological characteristics of ovarian tissue and serum hormone levels. A Ovarian photos and HE stained sections in D3, Pub, Y1, and Pre. Red arrowhead area means primordial follicles groups; PF: primary follicles; SF: secondary follicles; AF: antral follicles; ☆: corpus luteum. MF: mature follicles. B Concentration of luteinizing hormone (LH) and follicle stimulating hormone (FSH) (mIU/mL), and the ratio of FSH and LH. C Concentration of estradiol (E2) in serum, pg/mL. D Progesterone (PROG) concentration in serum of Y1 and Pre, ng/mL. D3: postnatal day 3; Pub: puberty, Y1: sexual maturity at the age of about one year after birth (Y1), Pre: pregnancy; the same below. Mean ± SD. n = 3. *: p < 0.05. **: p < 0.01
Histologically, HE staining revealed a significant difference in these ovaries (Fig. 1A, Fig. S1). The ovarian cortex displayed many proliferating oogonia and a few primordial follicles at birth (D3). More secondary, antral, and mature follicles were observed in the ovaries during puberty. Many corpus rubrum, corpus luteum, and corpus albicans were observed at the stage of sexual maturity (Y1), indicating recent ovulation and the current luteal phase. During pregnancy, more corpus luteum and antral follicles were filled with follicular fluid in the ovaries, indicating a robust reproductive reserve capacity.
During the development and pregnancy of the ovaries, the ELISA results showed that the levels of FSH and LH gradually increased, then peaked at sexual maturity (Y1), whereas FSH significantly decreased during pregnancy (p < 0.05); meanwhile, FSH/LH was less than 2 and gradually decreased, indicating that the basic reserve function and periodic hormone regulation of the ovary were in a normal state (Fig. 1B). Estradiol (E2) levels remained almost unchanged during the first three stages but significantly increased during pregnancy (p < 0.01), averaging 430.94 pg/mL (Fig. 1C). Similarly, progesterone (PROG) levels were greater during pregnancy compared to Y1, contributing to the maintenance of pregnancy (Fig. 1D).
Differentially expressed mRNAs (DEGs) and functional annotation
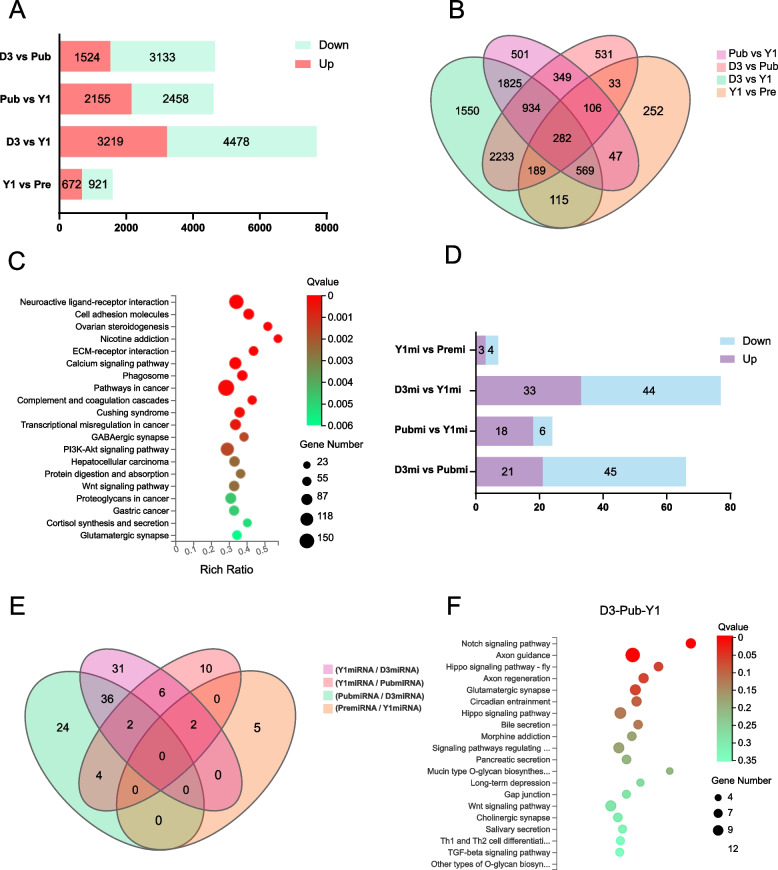
RNA sequencing analysis showed that a total of 18,753 genes were expressed in the four groups (acc GSE271331), and the genes in each group were sorted by their expression levels (according to average TPM). The top 20 genes with high expression levels were showed in Table 1, most of which are ribosomal protein genes involved in genetic information processing. Compared with those in D3 group, there were 4,657 DEGs in Pub group (|log2FC|≥ 1 and Q value ≤ 0.05). Similarly, 4613, 7697, and 1593 DEGs were identified in the comparison groups of Pub vs Y1, D3 vs Y1, and Y1 vs Pre, respectively (Fig. 2A). Among them, 282 genes were differentially expressed in all four comparison groups (Fig. 2B). The 20 DEGs with the greatest differences for each comparison group were shown in Table 2. Microglobulin-β (MSMB) was highly expressed in Y1 and significantly upregulated compared to D3, indicating that it may be a key gene in ovarian development. The expression levels of phosphoenolpyruvate carboxy kinase 1 (PCK1), steroidogenic acute regulatory protein (STAR), ornithine decarboxylase-like (LOC100520618), and L-amino-acid oxidase-like (LOC100525099) tended to increase overall during ovarian development.
Table 1.
The top 20 genes with high expression levels
| D3 | Pub | Y1 | Pre | ||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Average TPM | Gene Symbol | Average TPM | Gene Symbol | Average TPM | Gene Symbol | Average TPM |
| EEF1A1 | 13,368.41 | EEF1A1 | 13,656.76 | MSMB | 22,335.18 | RLN2 | 16,455.09 |
| RPS28 | 11,009.23 | RPS28 | 13,174.50 | TIMP1 | 15,204.46 | RPS28 | 13,516.83 |
| RPL37 | 8309.54 | RPS27 | 10,340.94 | EEF1A1 | 10,342.35 | EEF1A1 | 12,870.50 |
| RPS27 | 7872.32 | RPL37 | 9240.17 | RPS28 | 10,105.74 | RPL37 | 11,378.66 |
| RPL36 | 7423.08 | TPT1 | 8137.20 | RPL37 | 7183.80 | RPS27 | 10,862.95 |
| TPT1 | 6676.00 | RPS12 | 7583.72 | RPLP1 | 6984.63 | TMSB10 | 9054.25 |
| RPLP1 | 6510.59 | RPLP1 | 7052.59 | FTH1 | 6970.65 | TPT1 | 8664.55 |
| RPS12 | 6407.98 | TMSB10 | 6946.34 | RPS12 | 6823.27 | RPLP1 | 8448.99 |
| RPS20 | 6155.57 | RPS20 | 6937.13 | TMSB10 | 6739.87 | RPS12 | 8253.95 |
| RPL35 | 5813.64 | RPL36 | 6917.44 | TPT1 | 6704.20 | RPL36 | 7847.32 |
| RPS19 | 5570.46 | FTH1 | 6240.50 | HSD3B1 | 6663.94 | RPS20 | 7508.51 |
| RPS27A | 5565.55 | RPS19 | 6143.58 | RPS27 | 6474.54 | RPS19 | 7426.73 |
| RPS8 | 5268.80 | RPL35 | 6027.37 | RPL36 | 5977.07 | TMSB4X | 6978.26 |
| RPS17 | 5218.73 | RPS26 | 5978.14 | RPS26 | 5673.65 | RPS8 | 6808.99 |
| RPS11 | 5192.52 | RPS8 | 5607.61 | RPL35 | 4939.22 | RPL35 | 6796.93 |
| RPL34 | 5129.18 | RPS11 | 5484.86 | RPL31 | 4931.09 | RPS27A | 6693.47 |
| RPL31 | 5119.11 | RPL26 | 5311.26 | RPS19 | 4832.14 | RPS26 | 6693.30 |
| RPS26 | 5092.48 | RPS27A | 5235.59 | RPLP2 | 4509.73 | FTH1 | 6450.09 |
| RPL26 | 5019.77 | RPL31 | 5231.59 | RPS20 | 4503.08 | RPS11 | 6356.58 |
| RPL10 | 4959.68 | RPS29 | 5191.16 | RPL10 | 4362.40 | RPL34 | 6009.44 |
Fig. 2.
Identification and annotation of differentially expressed genes (DEGs) and miRNAs (DEMis). A The number of DEGs and their up-regulation and down-regulation relationships. B Veen plots of DEGs in four comparative groups. C Top 20 KEGG pathways enriched by DEGs in the D3 VS Pub comparison group. D The number of DEMis and their up-regulation and down-regulation relationships. E Veen plots of DEMis in four comparative groups. F Top 20 KEGG pathways rich in target genes of DEMis in the D3, Pub, and Y1 comparison groups
Table 2.
The top 20 DEGs with fold changes among the different comparison groups
| D3 vs Pub | Pub vs Y1 | D3 vs Y1 | Y1 vs Pre |
|---|---|---|---|
| LOC100624086↑ | LOC100520618↑ | STAR↑ | CXCL11↓ |
| LOC110261743↑ | LOC100621844↑ | LOC100520618↑ | COCH↑ |
| CYP19A3↑ | LOC100525099↑ | LOC100525099↑ | PKD1L2↓ |
| BSP1↑ | CPS1↑ | PCK1↑ | LOC100522201↓ |
| SFTPC↑ | LOC100623188↑ | MSMB↑ | SOHLH1↓ |
| IHH↑ | UNC93A↑ | LOC110261743↑ | LOC102162205↓ |
| PCK1↑ | MSMB↑ | LOC100621844↑ | LOC110256334↓ |
| STAR↑ | LOC110256334↑ | CYP11A1↑ | LOC100525099↓ |
| CYP17A1↑ | LOC100523909↑ | LOC110260994↑ | SERPINB2↓ |
| LOC100519511↓ | GPR45↓ | TMEM163↓ | RPP14↓ |
| SPTA1↑ | AVPR1A↑ | TMPRSS9↓ | BSP1↓ |
| ATP13A4↓ | LOC110256817↓ | SERPINB2↑ | CPS1↓ |
| LOC100736962↓ | TMEM215↑ | DNAH14↓ | VSNL1↓ |
| SSTR3↓ | DAO↓ | LOC100156977↓ | UNC93A↓ |
| VGF↑ | CLIC6↑ | TKTL1↓ | MRO↓ |
| MOBP↑ | DIO1↑ | TRIML2↓ | LOC100156325↑ |
| CFAP65↓ | DUOX2↑ | LOC106509328↓ | SERPINA3-2↑ |
| FAM92B↓ | OPCML↓ | LGR6↓ | LOC110261162↓ |
| DMBT1↓ | SPTA1↓ | FMR1NB↓ | LOC100623188↓ |
| LOC110255823↑ | SLC39A2↓ | CRSP3↓ | LOC100623351↓ |
To better discover the molecular mechanisms of the DEGs in regulating ovarian development and late pregnancy maintenance, a KEGG functional enrichment analysis was conducted on them. The top 20 significantly enriched KEGG pathway terms of each comparison group were shown in Figs. 2C and S2A-C. The DEGs of D3 and Pub were significantly enriched in pathways related to cell adhesion, proliferation, differentiation, and apoptosis, such as neuroactive ligand-receptor interaction, cell adhesion molecules, and ovarian steroidogenesis, etc. (Fig. 2C). The DEGs of Pub vs Y1 were mainly enriched in metabolic pathways such as carbon metabolism, fatty acid metabolism, biosynthesis of unsaturated fatty acids, biosynthesis of amino acids, and reproductive-related pathways such as steroid biosynthesis and ovarian steroidogenesis, as well as energy metabolism pathways such as the calcium signaling pathway, oxidative phosphorylation, and the citrate cycle (TCA cycle) (Fig. S2A). The DEGs of D3 vs Y1, excluding pathways significantly enriched in diseases, were mainly enriched in cellular processes such as phagosome, focal adhesion, and lysosome, as well as metabolic processes such as carbon metabolism and oxidative phosphorylation, as well as signal transduction pathways such as calcium signaling, cAMP signaling, and TNF signaling pathway (Fig. S2B). The DEGs in the Y1 vs Pre comparison were significantly enriched in the oxidative phosphorylation, carbon metabolism, fatty acid metabolism, ECM-receptor interaction, steroid biosynthesis, biosynthesis of amino acids, citrate cycle (TCA cycle), and PPAR signaling pathways (Fig. S2C).
Then, the DEGs of the D3 vs Pub, Pub vs Y1, and D3 vs Y1 comparison groups were merged, and the annotated GO terms were classified (Fig. S2D). In biological processes, these DEGs participate in multicellular organismal process, localization, developmental process, cell proliferation, and biological adhesion related to ovarian development. The candidate DEGs in the cellular component category were mainly related to cell organelles and membrane parts. In the molecular functional category, candidate genes mainly have functions such as binding and biological activities of participating in various substances involved in the transcription and translation process of genetic material.
Differentially expressed miRNAs (DEMis) and functional annotation
Small RNA sequencing analysis discovered that a total of 66, 24, 77, and 7 differentially expressed miRNAs were screened in the comparison groups of D3 vs Pub, Pub vs Y1, D3 vs Y1, and Y1 vs Pre, respectively (|log2FC|≥ 1 and Q value ≤ 0.05), and the up-and downregulation results were shown on Fig. 2D. There were no differentially expressed miRNAs in the intersection of the four comparison groups. However, two miRNAs, novel-ssc-miR845-3p and novel-ssc-miR845-5p, which were differentially expressed among the three comparison groups of D3, Pub, and Y1, may be key miRNAs involved in ovarian development (Fig. 2E). After merging the 115 differentially expressed miRNAs from D3 vs Pub, Pub vs Y1, and D3 vs Y1, target gene prediction was performed using RNAhybrid and miRanda software. There may be interactions between them and 619 mRNA types, as well as between them and 1971 other types of RNA (Table S5). Two hundred eighty-one target genes were predicted among the 7 differentially expressed miRNAs in the Y1 vs Pre comparison group (Table S6).
KEGG pathway enrichment analysis of these target genes revealed that they were significantly enriched in the notch signaling pathway, axon guidance pathway, circadian entrainment pathway, Ras signaling pathway, and glutamatergic synapse pathway (Figs. 2F, S2E). Similarly, we classified the target gene-annotated GO entries predicted by all the DEMis of the D3 vs Pub, Pub vs Y1, and D3 vs Y1 comparison groups (Fig. S2F), as well as the target genes predicted by the DEMis of the Y1 vs Pre comparison group (Fig. S2G).
Validation of RNA expression by qRT-PCR
QRT-PCR was conducted to validate the RNA sequencing results. From all the identified DEGs and DEMis, three randomly selected DEGs (NPC2, SCARB1, ANGPTL4) and DEMis (ssc-miRNA-21-5p, ssc-miRNA-451, ssc-miRNA-146a-5p) were analyzed. The expression patterns obtained from qRT-PCR and RNA-seq were highly consistent (Fig. S3), indicating the high reproducibility and reliability of the gene expression profiling from RNA-seq results.
Screening of key genes regulating ovarian development and pregnancy
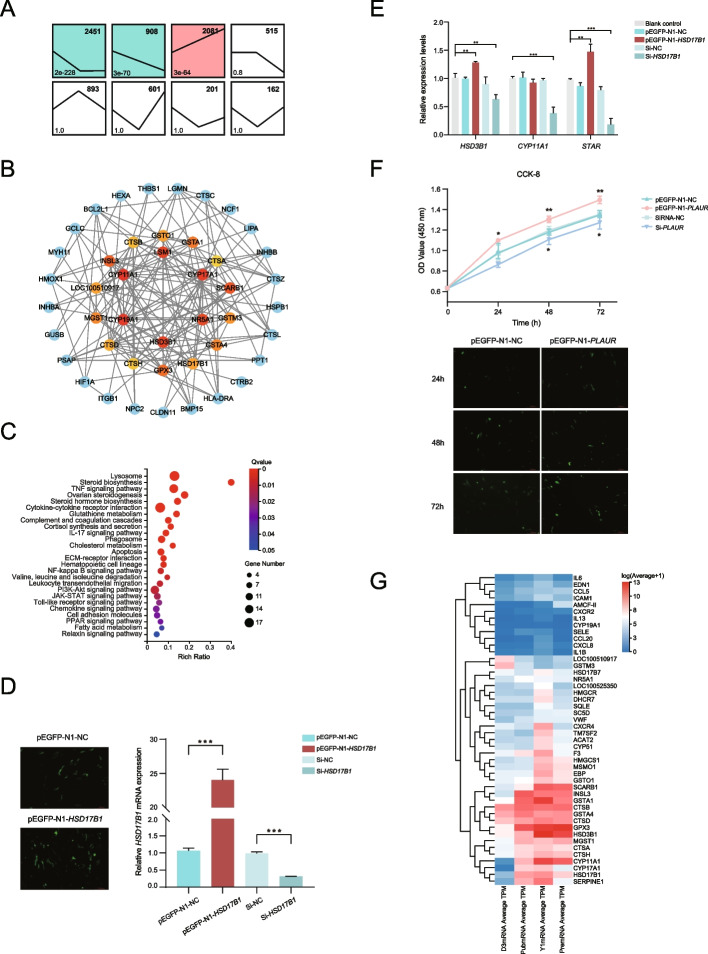
Short time series expression miner (STEM) was used to perform expression pattern clustering analysis on DEGs between the D3 vs Pub and Pub vs Y1 comparison groups to screen for key genes regulating ovarian development. These genes had eight expression patterns (Fig. 3A), of which 2451 genes were downregulated first and then remained unchanged, 908 gene expression levels continued to decrease, and 2081 genes were continuously upregulated at three time points. The remaining expression patterns were not significant. Continuously upregulated genes may positively promote ovarian development, whereas previously downregulated genes may only function in the early stages of development. These enriched genes may be key genes involved in gene regulation.
Fig. 3.
Key genes regulating ovarian development. A Clusters of expression patterns of DEGs. The top right corner represents the number of genes, and the bottom left corner represents the significance p-value. B The protein–protein interaction (PPI) relationship between DEGs of D3 vs Pub. The redder the color, the higher the score for Cytohubba. C KEGG enrichment analysis of hub genes in D3, Pub, and Y1 comparison groups. D Evaluation of HSD17B1 overexpression and knockdown effects. Fluorescence imaging of overexpression plasmid transfection, scale: 500 μm (Left). Relative expression level of HSD17B1 (Right). E Relative expression levels of key genes. F OD value changes of granulosa cells at different time points under various treatments (Up). Fluorescence imaging of overexpression plasmid transfection of PLAUR, scale: 500 μm (down). G Heatmap of hub genes for comparison groups D3, Pub, and Y1
To further explore the interactions between proteins encoded by differentially expressed genes, we selected genes with high expression levels (FPKM > 50) and high fold change values among the four comparison groups, and protein–protein interaction (PPI) analysis was performed via String and Cytoscape, respectively. Cytohubba software was used to identify the top 20 genes according to the score (Figs. 3B, S4A-C). Among them, CTSB, CTSH, CTSD, and CTSA are enriched in the lysosome (map04142) and apoptosis (map04210); SQLE, HSD17B7, EBP, TM7SF2, SC5D, DHCR7, and MSMO1 mainly participate in steroid biosynthesis (map00100); and SCARB1. LOC100525350, CYP11A1, CYP17A1, CYP19A1, HSD3B1, HSD17B1, and HSD17B7 are mainly involved in ovarian steroidogenesis (map04913) and steroid hormone synthesis (map00140), cortisol synthesis and secretion (map04927); CCL5, ICAM1, AMCF-II, END1, IL1B, SELE, IL6, IL13, CXCR2, CXCR4, CXCL8, and CCL20 are significantly enriched in the TNF signaling pathway (map04668), IL-17 signaling pathway (map04657) and cytokine‒cytokine receptor interactions (map04060); GSTA4, GSTM3, GPX3, GSTO1, MGST1, GSTA1, and LOC100510917 are significantly enriched in glutathione metabolism (map00480); VWF is involved in ECM-receptor interaction (map04512); HMGCS1 involved in PPAR signaling pathway (map03320), INSL3 is involved in the relaxin signaling pathway (map04926) (Fig. 3C).
The protein synthesized by HSD17B1 is a critical isoenzyme in ovarian granulosa cells, responsible for regulating estradiol synthesis [69]. In this study, the gene was successfully overexpressed or knocked down in granulosa cells (Fig. 3D), and the expression levels of upstream genes closely associated with estrogen synthesis were evaluated. The results indicated that overexpression of HSD17B1 for 48 h significantly increased HSD3B1 and STAR expression, while knockdown reduced HSD3B1, CYP11A1, and STAR levels (Fig. 3E). The interaction between PLAU and PLAUR has been reported to promote bovine granulosa cell proliferation via the cAMP-ERK1/2 signaling pathway [70] but its role in pigs remains unexplored. We conducted PLAUR overexpression and knockdown to investigate its impact on cell proliferation. Similarly, overexpression of PLAUR promoted granulosa cell proliferation, whereas knockdown inhibited (Fig. 3F). These results underscore the functions of HSD17B1 in steroidogenesis and PLAUR in granulosa cell proliferation, further supporting our hypothesis.
In summary, combined with gene expression levels (Fig. 3G), the following key candidate genes involved in ovarian development were screened, including SQLE, HMGCS1, MSMO1, SCARB1, CYP11A1, HSD3B1, HSD17B1, and SERPINE1. Similarly, some key genes involved in pregnancy regulation, such as LUM, FN1, PLAUR, SELP, SDC1, and VCAN, were screened according to the above methods (Fig. S4C-E).
Integrated analysis of differentially expressed mRNAs and miRNAs
MiRNAs can complement the 3'UTR of target mRNAs and negatively regulate gene expression at the posttranscriptional level, leading to mRNA degradation or translation inhibition. Therefore, this study predicted target genes for various comparison groups of DEMis, screened for genes that overlapped with the RNA-seq results, and conducted subsequent correlation analysis, which revealed upregulated mRNAs with downregulated miRNAs and downregulated mRNAs with upregulated miRNA, respectively. A network diagram was constructed to illustrate the associations between key miRNAs and their target genes. Among the upregulated miRNAs, the novel ssc-miR650-3p was associated with five genes. The degree scores of novel ssc-miR1219-3p, novel ssc-miR1432-5p, novel ssc-miR406-3p, and ssc-miR-382 were all 4, indicating that they were associated with four genes (Fig. S5A). Similarly, among the downregulated miRNAs, 6 had a degree score of 4, indicating a possible association with the four DEGs (Fig. S5B).
Among the miRNAs upregulated in puberty, novel-ssc-miR1432-5p targets PREP and is enriched in the renin-angiotensin system pathway. Ssc-miR-206 targets the ADCY10 gene and is enriched for circadian entrainment, growth hormone synthesis, secretion and action, and the apelin signaling pathway. Ssc-miR-497, ssc-miR-107, and novel-ssc-miR1248-5p target the ROR1 gene and are enriched in the Wnt signaling pathway (Fig. 4A). Among the downregulated miRNAs expressed during puberty, the ssc-miR-429, ssc-miR-210, novel-ssc-miR843-3p, novel-ssc-miR876-5p, novel-ssc-miR140-3p, and ssc-miR-10383 target genes involved in cytokine‒cytokine receptor interaction, insulin secretion, growth hormone synthesis, secretion and action, as well as the p53, AMPK, MAPK, PI3K-Akt, notch, HIF-1, TNF, FoxO, and mTOR signaling pathways, were significantly associated with follicular development (Fig. 4B). At sexual maturity, novel-ssc-miR870-5p, novel-ssc-miR359-5p, novel-ssc-miR573-5p, novel-ssc-miR1158-3p, and novel-ssc-miR429-5p were upregulated and expressed, and they targeted GRIN2A, MAML3, TLE6, IRS1, WWC1, and GRIN2A (Fig. 4C).
Fig. 4.
Key miRNAs and their target genes. A Upregulated miRNAs during puberty and their target genes. B Downregulated miRNAs during puberty and their target genes. C Upregulated miRNAs at the Y1 stage and their target genes. The circle represents mRNA, the red polygon represents upregulated miRNA, and the green polygon represents downregulated miRNA. D Relative luciferase activity between KCNA1 and ssc-miR-133a-3p. n = 3
To verify the targeting relationship between the DEMis and their predicted target genes, we performed dual-luciferase reporter assays. Among them, ssc-miR-133a-3p (Fig S5A) was significantly upregulated during pregnancy and its target gene, KCNA1, encoding a voltage-dependent potassium channel protein that primarily functions in the nervous system, potentially influencing the brain development of embryos [71]. The results showed that ssc-miR-133a-3p significantly inhibited the luciferase activity of the wild-type KCNA1 recombinant plasmid (p < 0.001) (Fig. 4E), establishing its target relationship with KCNA1.
The overall analysis of ovarian metabolites
After peak extraction and identification of the offline data detected by LC–MS/MS, the base peak chromatograms (BPC), PCA, and coefficient of variation (CV) analysis of the QC samples were plotted to determine the quality of the metabolomic data. The results showed that the system error was small, the experimental results had high repeatability, and the data quality was good, which can be used for further analysis (Fig. S6A-C).
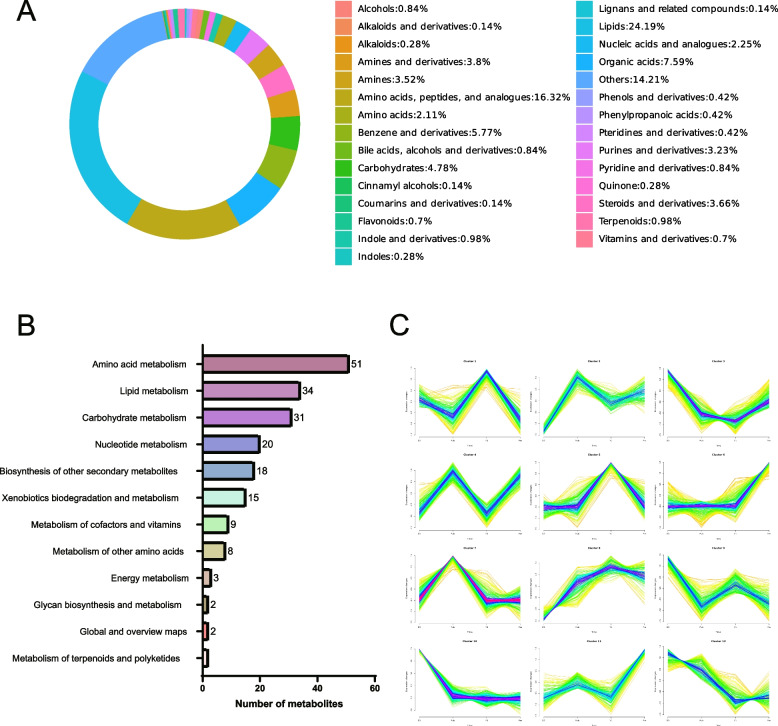
A total of 2,453 metabolites were detected in the ovarian tissues of the 4 groups and were mainly divided into 29 categories. According to the classification results of the metabolites, lipids ranked first, accounting for 24.19%; amino acids, peptides, and analogues accounted for 16.32%; and organic acids accounted for 7.59%. We also observed that steroids and their derivatives accounted for 3.66%, ranking seventh (Fig. 5A). The metabolites of these ovarian tissues mainly participate in amino acid, lipid, carbohydrate, and nucleotide metabolism; the biosynthesis of other secondary metabolites; xenobiotic biodegradation; metabolism; etc. (Fig. 5B).
Fig. 5.
Overall analysis of ovarian metabolites. A Metabolite classification donut chart. B Kegg pathway bar chart of metabolites. C Metabolite expression trend chart. The X-axis represents each time point, and the Y-axis represents the normalized expression value
According to the expression information, metabolites can be grouped into related clusters, and metabolites with the same expression pattern can be clustered into the same cluster. The Mfuzz software package was used to cluster metabolite expression levels from samples at different time points. The figure below shows the various metabolite clusters (Fig. 5C). These metabolites were clustered into 12 clusters. The expression levels of metabolites in clusters 2, 4, and 7 peaked in Pub, and these metabolites may be key metabolites involved in regulating the initial oestrus phase. In addition, the expression levels of metabolites in clusters 6 and 11, which may be key metabolites involved in regulating pregnancy, reached their highest levels during pregnancy.
Screening and analysis of differential abundant metabolites (DAMs)
A total of 249 DAMs were found in the D3 vs Pub group, of which 110 were upregulated and 139 were downregulated. The Pub vs Y1 group had a total of 76 DAMs, of which 29 were upregulated and 47 were downregulated. The D3 vs Y1 group had a total of 167 DAMs, of which 72 were upregulated and 96 were downregulated. There were a total of 21 DAMs in the Y1 vs Pre group, of which 10 were upregulated and 11 were downregulated (Fig. 6A). In summary, the most differentially abundant metabolites were detected between the ovaries of 3-day-old and pubescent pigs, while the fewest differentially abundant metabolites were detected between the ovarian tissues of pregnant and 1-year-old pigs. The PCA results also showed high reproducibility among the three biological replicates in each group, with significant differences between D3 and the other 3 groups. Some similarities were observed between the Y1 and Pre groups (Fig. 6B).
Fig. 6.
Screening and analysis of differential metabolites (DMs). A Bar chart of the number of differential metabolites in four comparison groups. B PCA analysis for all samples. PC1 represents the first principal component and PC2 represents the second principal component. C-D Metabolic pathway enrichment analysis of differential metabolites from D3 vs Pub comparison group. C is bubble diagram, (D) is network diagram. E Cluster analysis of the expression levels of the key differential metabolites from D3 vs Pub comparison group
Metabolic pathway enrichment analysis of DAMs based on the KEGG database revealed significantly altered metabolic pathways, thereby contributing to the interpretation of biological phenotypes. In this report, the metabolic pathways with p values < 0.05 were defined as the metabolic pathways with significant enrichment of DAMs, and the top 20 metabolic pathways (less than 20, use all data) with the smallest p values were drawn as bubble charts (Figs. 6C, S7A). Simultaneously, a network diagram of the top 5 metabolic pathways with the smallest p values and their associated metabolites and enzymes was drawn (Figs. 6D, S7B). The amino acid metabolism pathway was significantly enriched in all four comparison groups. Bile secretion, protein digestion and absorption, ABC transporters, ovarian steroidogenesis, and steroid hormone biosynthesis were enriched in multiple comparison groups. To further investigate the significant metabolite changes during ovarian development, we plotted metabolite heatmaps showing substantial differences in metabolic pathways significantly enriched in the four comparison groups (Figs. 6E, S7C). The expression levels of dehydroepiandrosterone (DHEA), androstenedione, 17alpha-hydroxyprogesterone, (2R)−2,3-dihydroxypropanoic acid, and 3-dehydrosphinganine in Pub were significantly greater than those in D3. The expression levels of L-acetylcarnitine, shikimic acid, alpha-D-mannose 1-phosphate, and DL-carnitine in Y1 were significantly greater than those in Pub. The expression levels of progesterone, 17alpha-hydroxyprogesterone, 5alpha-pregenan-3,20-dione, and DHEA in Y1 were significantly greater than those in D3. The expression level of carnosine in Pre was significantly greater than that in Y1, while DHEA was significantly lower than in Y1.
Based on the FC values and KEGG enrichment analysis results, key metabolites involved in ovarian development were identified, including androstenedione, 17alpha-hydroxyprogesterone, dehydroepiandrosterone (DHEA), progesterone, and carnosine. These metabolites are mainly involved in ovarian steroid metabolism.
Combined analysis of the transcriptome and metabolome
Ovarian development mainly relies on the regulation of hormones (cortisol, FSH, LH) and signaling pathways. The metabolic pathways of the DEGs and significantly DAMs highly correlated with ovarian steroid production in the four comparison groups were visualized (Fig. 7A). In the ovarian steroidogenesis pathway, the expression levels of DEGs such as CYP11A1, HSD3B1, CYP17A1, and HSD17B1 increase with the growth cycle. Similarly, the levels of DAMs, such as 17α-hydroxyprogesterone, dehydroepiandrosterone, and androstenedione also increased, consistent with changes in gene expression. Interestingly, changes in metabolite concentrations can feed back and regulate gene expression. Granulosa cells cultured in vitro were treated with varying concentrations of androstenedione solution for 24 h (Fig. 7B). The results demonstrated that the androstenedione treatment significantly upregulated the expression levels of steroidogenesis-related genes STAR, HSD17B1, and HSD3B1, with the highest expression observed at a concentration of 1 × 10⁻⁶ M (Fig. 7C).
Fig. 7.
Genes and metabolites involved in the ovarian steroidogenesis pathway. The rectangle represents the coding gene, and the circle represents the metabolite product. B Granulosa cell status after 24 h androstenedione treatment at varying concentrations. Scale: 500 μm. NC: Negative control, I: 100 μM, II: 10 μM, III 1 μM. C Relative expression levels of genes related to estrogen synthesis
Discussion
Ovarian development is a dynamic process that involves multiple stages, including the activation of primordial follicles during initial recruitment, the growth of follicles, or follicular atresia, and cyclic recruitment [5]. Studying ovarian development and its regulatory mechanisms will help to gain a deeper understanding of the ovarian state at various developmental stages. In this study, we conducted a comprehensive analysis of the transcriptomics and metabolomics of porcine ovarian tissues at different developmental stages (infancy, puberty, sexual maturity, and pregnancy). Key genes, miRNAs, and metabolites related to ovarian development and pregnancy regulation were identified. From the gene expression analysis, we identified important genes associated with ovarian development, including HMGCS1, MSMO1, SCARB1, SERPINE1, HSD3B1, CYP11A1, and HSD17B1. Similarly, genes such as LUM, FN1, PLAUR, SELP, SDC1, and VCAN were identified as crucial for pregnancy regulation. Furthermore, integrating miRNA data allowed us to identify key miRNAs, such as ssc-miR-206, ssc-miR-107, ssc-miR-429, ssc-miR-210, and novel-ssc-miR140-3p. Key metabolites, including androstenedione, 17a-hydroxyprogesterone, dehydroepiandrosterone (DHEA), and progesterone, were identified through variance analysis and KEGG enrichment analysis. These metabolites were involved in ovarian steroid metabolism and regulated by the products of related genes.
Among the key genes associated with ovarian development, several are related to cholesterol synthesis and absorption. SQLE participates in the initial step of the cholesterol synthesis pathway. HMGCS1 is involved in catalyzing the formation of mevalonate, a crucial intermediate in cholesterol synthesis. MSMO1 is a key enzyme in the steroid biosynthesis pathway and participates in the final stages of cholesterol synthesis by converting 24-dehydrocholesterol to cholesterol [72]. Notably, SQLE expression is downregulated in individuals with diminished ovarian reserve (DOR) [73]. SCARB1, expressed as scavenger receptor class B member 1 (SR-BI) in ovarian stromal cells, theca cells, and the corpus luteum, is involved in cholesterol uptake [74]. Plasma progesterone levels are significantly reduced in SCARB1 knockout mice, indicating that SCARB1 expression is crucial for maintaining ovarian cholesterol homeostasis and luteal steroidogenesis [75]. Our results reveal a strong correlation among SQLE, MSMO1, and HMGCS1, with their expression rising during ovarian development, indicating increased cholesterol synthesis for sex hormone production and cell membrane formation. Elevated SCARB1 expression suggests enhanced exogenous cholesterol uptake. Cholesterol is a precursor molecule for sex hormones, including estrogens and progestogens, which are critical for the ovarian cycle, follicular development, ovulation, and overall reproductive health. Therefore, the activity and expression levels of SQLE, HMGCS1, MSMO1, and SCARB1 may indirectly influence the production of sex hormones in the ovaries, impacting ovarian development and function.. Additionally, SERPINE1 (plasminogen activator inhibitor 1), a member of the serine protease inhibitor (SERPIN) superfamily, shares high nucleotide (78%) and amino acid (86%) identities with its human counterpart [76, 77]. SERPINE1 has been demonstrated to be expressed in the uterus of both humans and mice and serves as a crucial regulatory protein involved in extracellular matrix remodeling and cell adhesion, promoting cell survival [78–82]. Qu et al. showed that SERPINE1 promoted granulosa cell proliferation via the Erk1/2 pathway in vitro [83]. SERPINE1 was highly expressed during puberty and sexual maturity, linked to apelin and HIF-1 pathways. Apelin supports angiogenesis for follicular development and luteal function, while HIF-1 is essential for ovulation. These findings suggest that SERPINE1 plays a significant role in ovulation and corpus luteum formation, potentially playing a crucial role in ovarian tissue remodeling and hormone regulation.
Members of the cytochrome P450 family are key genes involved in ovarian steroidogenesis. The first step in steroidogenesis is the conversion of cholesterol to pregnenolone, and the rate-limiting enzyme catalyzing this step is encoded by CYP11A1. This step serves as the common starting point for the synthesis of all steroid hormones. The expression level of CYP11A1 determines a cell’s capacity for steroid production. Prolonged stimulation of cells can increase the amount of P450scc, thereby enhancing the basal level of steroid production and the response to steroidogenic signals [84]. In vitro experiments indicate that upregulating the transcriptional activity of StAR and CYP11A1 can enhance progesterone production [85]. CYP17A1 encodes an enzyme response for catalyzing 17α-hydroxylation and 17,20-lyase reactions, which are particularly crucial in the adrenal glands and gonads. The activity of this enzyme is a pivotal branching point in sex hormone synthesis [86]. Experiments in vitro revealed that changes in CYP17A1 mRNA and protein expression led to alterations in androstenedione, testosterone, and estrone levels in the corpus luteum [87]. Within the 3β-hydroxysteroid dehydrogenase (3β-HSD) family, the type 1 enzyme (HSD3B1) primarily catalyzes the conversion of pregnenolone to progesterone in the early stages of steroid biosynthesis. Previous research has indicated that HSD3B1 acts as a rate-limiting enzyme in steroid transformation, making it a critical enzyme in steroidogenesis [88], reducing mRNA expression of HSD3B1 in the corpus luteum impaired the synthesis of progesterone [89]. The expression and activity of hydroxysteroid 17β dehydrogenase 1, encoded by the HSD17B1 gene, increase upon LH stimulation. It efficiently catalyzes the reversible conversion of the weak estrogen precursor estrone (E1) to the potent estrogen estradiol (E2), which is essential for normal ovarian development [90, 91]. HSD17B1 is the main isoenzyme in ovarian granulosa cells and plays a central role in regulating circulating estradiol concentrations [91], local estrogen production, and the local promotion of follicular development, differentiation, and maturation [92]. Inhibiting HSD17B1 impairs the synthesis of 17β-estradiol and diminishes its effects, thereby directly hindering ovarian follicle development. It was discovered that enhanced HSD17B1 expression increased the estrogen response [93]. Sequencing results showed that the expression levels of HSD17B1, CYP17A1, HSD3B1, and CYP11A1 increased with ovarian development. Optimal levels of them support normal ovarian development, follicle maturation, and hormone production necessary for reproductive health. Our experiment also demonstrated that overexpression of HSD17B1 can promote the expression of STAR, HSD3B1, and CYP11A1 that related to the estrogen synthesis pathway.
Among the key genes related to pregnancy regulation, LUM plays an important role in the cardiovascular system and is associated with angiogenesis, making it a potential marker for placental function [94, 95]. This study hypothesizes that LUM is related to fetal cardiovascular development. FN1 encodes fibronectin 1, which is involved in focal adhesion and interactions with the extracellular matrix (ECM) receptor [96]. Research by Mingju Sun et al. indicated that FN1 can improve the maturation of oocyte nuclei and cytoplasm by promoting the activation of the PI3K signaling pathway [97]. Integrin β5 (ITGB5) is a member of the integrin β subfamily and typically pairs with integrin αV (ITGAV) to form the αVβ5 integrin. This integrin is involved in cell-ECM interactions, influencing cell adhesion, migration, and proliferation. During pregnancy, integrins are crucial for placental formation and function and regulate placental cell adhesion, migration, and angiogenesis, thereby ensuring that the fetus receives adequate nutrients and oxygen [98]. PLAUR encodes the urokinase-type plasminogen activator receptor (UPAR), which is particularly important for cellular proliferation and tissue remodeling during embryo implantation [99]. Our study found that PLAUR promoted granulosa cell proliferation in vitro, underscoring its crucial role in cellular proliferation. Selectins, a family of calcium-dependent type I transmembrane glycoproteins, along with their ligands, are involved in various physiological processes, such as leukocyte homing, as well as pathological processes, including cancer and human implantation [100, 101]. L-selectin and its ligands play important roles in adhesion between the blastocyst and the mother during embryo implantation. Moreover, P-selectin and E-selectin participate in the recognition of the maternal immune system of implanted embryos, promoting the migration of trophoblast cells within the spiral artery of the decidua [101]. The SDC1 (syndecan-1) gene is involved in the differentiation of trophoblast cells into multinucleated syncytiotrophoblasts (STB). SDC1 is one of the key genes promoted by uterine epithelial cells during the blastocyst implantation process, facilitating the differentiation of trophoblasts into invasive STBs [102].
miRNAs are small noncoding RNAs that play important roles in regulating the expression of numerous genes related to follicular development, including key processes such as occlusion and ovulation [103]. The functions of various miRNAs have been studied. miR-206 acts as a tumor suppressor by blocking estrogen signaling pathways, particularly those mediated by the G protein-coupled estrogen receptor (GPER). miR-206 can inhibit the migration of EOC cells induced by 17β-estradiol (E2) and the selective GPER agonist G1 by reducing the protein levels of PFKFB3 [104]. This study predicted that ssc-miR-206 targets ADCY10, which encodes soluble adenylyl cyclase (sAC). sAC can regulate estrogen production and granulosa cell proliferation by amplifying the FSH-stimulated cAMP/PKA pathway [105]. ADCY10 expression decreased during puberty, potentially due to the upregulation of ssc-miR-206. Research has shown that miR-497 can inhibit the migration and invasion of ovarian cancer cells by directly targeting and suppressing the expression of SMURF1 [106]. M1 macrophages promote the activation of primordial follicles by secreting extracellular vesicles containing miR-107, consistent with the stimulatory role of M1 macrophages in ovarian function. miR-107 activates the PI3K/mTOR signaling pathway by targeting PTEN [107]. This pathway plays a critical role in the transition of follicles from a dormant state to a growing state. The predicted target genes of ssc-miR-497 and ssc-miR-107 are both ROP1, which are crucial for embryonic development, but may lead to tumorigenesis when overexpressed [108]. Maintaining low expression levels of ROR1 helps prevent tumor occurrence. MiR-429 may regulate the expression of the luteinizing hormone beta subunit (LHβ) by targeting the 3'-UTR of ZEB1 mRNA. The loss or dysfunction of miR-429 could lead to reduced fertility in females [109]. RIMS2 targeted by ssc-miR- 429 plays a role in the presynaptic membrane of nerve terminals, regulating the release of neurotransmitters [110]. This may be related to the release of intracellular steroid hormones. In zebrafish ovarian cells, miR-210 has been shown to promote immune responses and inhibit genes related to oocyte meiosis through simulation experiments, indicating its key role in coordinating transcriptomic changes in fish ovaries [111], but there is limited research on this topic in mammals. Ssc-miR-210 targets the IRS1 (insulin receptor substrate 1), which is involved in the IRS1/AKT pathway and can affect liver metabolism [112]. This could be a new target for the treatment of polycystic ovary syndrome. Studies have shown elevated levels of miR-140-3p in natural killer (NK) cells from ovarian cancer patients, while MAPK1 expression is decreased. After IL-2 treatment, miR-140-3p levels decrease, and MAPK1 expression increases, suggesting that miR-140-3p may be related to the activation state of NK cells [113]. ssc-miR-140-3p is predicted to target FAS. The FAS-mediated death receptor signaling pathway is associated with apoptosis of ovarian granulosa cells, and equine chorionic gonadotropin can promote follicle development by reducing the expression of pro-apoptotic factors Fas/FasL [114]. Other novel miRNAs, such as novel-ssc-miR843-3p, novel-ssc-miR573-5p, novel-ssc-miR429-5p, and novel-ssc-miR1432-5p, require further investigation.
In our metabolomic analysis, we identified significantly differentially expressed metabolites involved in the steroidogenesis pathway, including androstenedione, 17α-hydroxyprogesterone, DHEA, and progesterone, and conducted a combined analysis of the differentially expressed genes. Progesterone is synthesized into 17-hydroxyprogesterone in theca interstitial cells under the catalysis of CYP17A1, which is further converted into androstenedione. Androstenedione can be used as an oral supplement to increase testosterone levels. Female rats given high oral doses of androstenedione can induce some liver cytochromes P450 [115]. Androstenedione treatment significantly upregulated STAR, HSD17B1, and HSD3B1 in granulosa cells in vitro, highlighting its role in promoting steroidogenesis as a key precursor in the ovarian steroidogenesis pathway. DHEA can also be converted into androstenedione via 3β-HSD catalysis. These metabolites are closely interconnected and are regulated by multiple genes in a coordinated manner. At the transcriptome level, HSD17B1 and CYP17A1 are highly expressed in Pub and Y1, while HSD3B1 and CYP11A1 are highly expressed in Y1 and Pre. Progesterone levels peak during puberty, while the other three metabolites show lower expression levels during the D3 and Pub stages, with increased levels during sexual maturity and pregnancy, consistent with the expression levels of the genes responsible for regulating these metabolites.
Our study contributes to the field by providing a transcriptomic and metabolomic atlas of porcine ovarian development. This integrative approach reveals the dynamic regulatory network of ovarian development and pregnancy maintenance, highlighting new key genes and metabolic pathways that can be used to improve reproductive efficiency in pigs and potentially other mammals. This study identified new candidate genes and potential pathways for further understanding the regulatory mechanisms of follicular development and ovarian function. This study has several limitations. While we performed overexpression and knockdown experiments on two key DEGs (HSD17B1 and PLAUR) and confirmed the targeting relationship between ssc-miR-133a-3p and KCAN1, as well as validated the role of androstenedione in granulosa cells in vitro, further functional studies are necessary to make the conclusions more convincing. Specifically, the regulatory relationships (e.g., upregulation/downregulation effects) of the validated miRNA-mRNA remain unexplored. And functional validation of the identified genes and metabolites through in vivo studies is necessary to confirm their roles and mechanisms.
Conclusion
In this study, we examined the expression of mRNAs, miRNAs, and metabolites in the ovaries at different developmental stages and during pregnancy. Several genes related to ovarian development were identified, including SQLE, HMGCS1, MSMO1, SCARB1, CYP11A1, HSD3B1, HSD17B1, and SERPINE1. Key genes crucial for maintaining pregnancy, such as LUM, FN1, PLAUR, SELP, SDC1, and VCAN, were also identified. KEGG analysis indicated that these DEGs were primarily enriched in the steroid biosynthesis pathway and various signaling pathways. Additionally, several key miRNAs that regulate mRNA expression, such as ssc-miR-206, ssc-miR-497, ssc-miR-107, ssc-miR-133a-3p and ssc-miR-429, were identified. Correspondingly, we constructed a miRNA—mRNA interaction network. Through untargeted metabolomics analysis, key metabolites were identified, including androstenedione, 17alpha-hydroxyprogesterone, dehydroepiandrosterone (DHEA), progesterone, and carnosine. This study provides a reference for further research on ovarian development and pregnancy maintenance in pigs.
Supplementary Information
Acknowledgements
The authors would like to thank the Chengdu Livestock and Poultry Genetic Resources Protection Center for providing the experimental animals. RNA sequencing was performed at BGI Genomics, BGI SHENZHEN, China.
Authors’ contributions
BP and JC performed the experiments, analyzed and interpreted the data, prepared figures and/or tables, authored and reviewed drafts of the article. BP, JC and KF performed the experiments and analyzed the data. TZ conceived and designed the experiments, performed the experiments. YJ, conceived and designed the experiments, reviewed drafts, confirmed the accuracy of data, graphs, tables, and materials, and approved the final draft.
Funding
This work is supported by the Sichuan Science and Technology Program (2021ZDZX0008) and the State Key Laboratory of Swine and Poultry Breeding Industry.
Data availability
The datasets generated and/or analysed during the current study are available in the [Gene Expression Omnibus] repository with the primary GEO accession GSE271331. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE271331.
Declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations provided by the Regulations of the Administration of Affairs Concerning Experimental Animals (China, 2017) for animal experiments. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (Approval No.20240469). All the efforts were made to minimize the suffering of the pig.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Binyun Pan, Jin Chai and Kaixin Fei contributed equally to this work.
References
- 1.Qin X, Zhao Y, Zhang T, Yin C, Qiao J, Guo W, Lu B. TrkB agonist antibody ameliorates fertility deficits in aged and cyclophosphamide-induced premature ovarian failure model mice. Nat Commun. 2022;13(1):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31. [DOI] [PubMed] [Google Scholar]
- 3.Kroeger PT Jr, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol. 2017;29(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J, Ma X, Li F, Liu J. Exposure to multiple pyrethroid insecticides affects ovarian follicular development via modifying microRNA expression. Sci Total Environ. 2022;828:154384. [DOI] [PubMed] [Google Scholar]
- 5.Dai Y, Bo Y, Wang P, Xu X, Singh M, Jia L, Zhang S, Niu S, Cheng K, Liang J. Asynchronous embryonic germ cell development leads to a heterogeneity of postnatal ovarian follicle activation and may influence the timing of puberty onset in mice. BMC Biol. 2022;20(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res. 1961;24:495–507. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015;21(6):779–86. [DOI] [PubMed] [Google Scholar]
- 8.John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction (Cambridge, England). 2007;133(5):855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svoboda P, Franke V, Schultz RM. Sculpting the transcriptome during the oocyte-to-embryo transition in mouse. Curr Top Dev Biol. 2015;113:305–49. [DOI] [PubMed] [Google Scholar]
- 11.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–80. [DOI] [PubMed] [Google Scholar]
- 12.Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37(7):1344–9. [DOI] [PubMed] [Google Scholar]
- 13.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–14. [DOI] [PubMed] [Google Scholar]
- 14.Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. Mol Mechan Cell Diff Gonad Dev. 2016;58:167–90. [DOI] [PubMed] [Google Scholar]
- 15.Clarke HJ. Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdiscip Rev Dev Biol. 2018;7(1):e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikhael S, Punjala-Patel A, Gavrilova-Jordan L. Hypothalamic-pituitary-ovarian axis disorders impacting female fertility. Biomedicines. 2019;7(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao S, Lopez-Tello J, Sferruzzi-Perri AN. Developmental programming of the female reproductive system—a review. Biol Reprod. 2021;104(4):745–70. [DOI] [PubMed] [Google Scholar]
- 18.Gougeon AL. Dynamics for human growth: morphologic, dynamic, and functional aspects. The ovary. 2003;2:25–43. [Google Scholar]
- 19.Messinis IE. From menarche to regular menstruation: endocrinological background. Ann N Y Acad Sci. 2006;1092(1):49–56. [DOI] [PubMed] [Google Scholar]
- 20.Bousfield GR, Dias JA. Synthesis and secretion of gonadotropins including structure-function correlates. Rev Endocr Metab Disord. 2011;12(4):289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: Parallels With Inflammatory Processes. Endocr Rev. 2019;40(2):369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight PG, Satchell L, Glister C. Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol. 2012;359(1):53–65. [DOI] [PubMed] [Google Scholar]
- 23.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30(5):438–64. [DOI] [PubMed] [Google Scholar]
- 24.Gosden R, Laing S, Felicio L, Nelson J, Finch C. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28(2):255–60. [DOI] [PubMed] [Google Scholar]
- 25.Xi H, Wang Z, Li M, Duan X, Li Y. Paeoniflorin promotes ovarian development in mice by activating mitophagy and preventing oxidative stress. Int J Mol Sci. 2024;25(15):8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Hu M, Ying R, Zou J, Du Z, Lin L, Lan T, Wang H, Hou Y, Cheng H, et al. RAB37-mediated autophagy guards ovarian homeostasis and function. Autophagy. 2024;20(12):2738–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong MZ, Ouyang YC, Gao SC, Gu LJ, Guo JN, Sun SM, Wang ZB, Sun QY. Protein phosphatase 4 maintains the survival of primordial follicles by regulating autophagy in oocytes. Cell Death Dis. 2024;15(9):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du ZQ, Eisley CJ, Onteru SK, Madsen O, Groenen MA, Ross JW, Rothschild MF. Identification of species-specific novel transcripts in pig reproductive tissues using RNA-seq. Anim Genet. 2014;45(2):198–204. [DOI] [PubMed] [Google Scholar]
- 29.Kwon SG, Hwang JH, Park DH, Kim TW, Kang DG, Kang KH, Kim IS, Park HC, Na CS, Ha J, et al. Identification of differentially expressed genes associated with litter size in Berkshire pig placenta. PLoS ONE. 2016;11(4):e0153311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song H, Zhu L, Li Y, Ma C, Guan K, Xia X, Li F. Exploiting RNA-sequencing data from the porcine testes to identify the key genes involved in spermatogenesis in Large White pigs. Gene. 2015;573(2):303–9. [DOI] [PubMed] [Google Scholar]
- 31.Rooda I, Hassan J, Hao J, Wagner M, Moussaud-Lamodière E, Jääger K, Otala M, Knuus K, Lindskog C, Papaikonomou K, et al. In-depth analysis of transcriptomes in ovarian cortical follicles from children and adults reveals interfollicular heterogeneity. Nat Commun. 2024;15(1):6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varik I, Zou R, Bellavia A, Rosenberg K, Sjunnesson Y, Hallberg I, Holte J, Lenters V, Van Duursen M, Pedersen M, et al. Reduced ovarian cholesterol and steroid biosynthesis along with increased inflammation are associated with high DEHP metabolite levels in human ovarian follicular fluids. Environ Int. 2024;191:108960. [DOI] [PubMed] [Google Scholar]
- 33.Dhori X, Gioiosa S, Gonfloni S. An integrated analysis of multiple datasets reveals novel gene signatures in human granulosa cells. Scientific data. 2024;11(1):972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y, Zhang R, Zhang S, Ji Y, Zhou Q, Leng L, Meng F, Gong F, Lu G, Lin G, et al. Transcriptomic profiles reveal the characteristics of oocytes and cumulus cells at GV, MI, and MII in follicles before ovulation. Journal of ovarian research. 2023;16(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ducreux B, Ferreux L, Patrat C, Fauque P. Overview of Gene Expression Dynamics during Human Oogenesis/Folliculogenesis. Int J Mol Sci. 2023;25(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao G, Yin M, Lian J, Tian H, Liu L, Li X, Sun F. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Molecular endocrinology (Baltimore, Md). 2010;24(3):540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, Li D, Zhang S, Xing Y, Gao Y, Wu J. MicroRNA-125a-5p induces mouse granulosa cell apoptosis by targeting signal transducer and activator of transcription 3. Menopause (New York, NY). 2016;23(1):100–7. [DOI] [PubMed] [Google Scholar]
- 38.Lin F, Li R, Pan ZX, Zhou B, Yu DB, Wang XG, Ma XS, Han J, Shen M, Liu HL. miR-26b promotes granulosa cell apoptosis by targeting ATM during follicular atresia in porcine ovary. PLoS ONE. 2012;7(6):e38640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010;83(2):286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Jiang X, Zhang Y, Xu B, Hua J, Ma T, Zheng W, Sun R, Shen W, Cooke HJ, et al. microRNA 376a regulates follicle assembly by targeting Pcna in fetal and neonatal mouse ovaries. Reproduction (Cambridge, England). 2014;148(1):43–54. [DOI] [PubMed] [Google Scholar]
- 41.Dai A, Sun H, Fang T, Zhang Q, Wu S, Jiang Y, Ding L, Yan G, Hu Y. MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Lett. 2013;587(15):2474–82. [DOI] [PubMed] [Google Scholar]
- 42.Grossman H, Chuderland D, Ninio-Many L, Hasky N, Kaplan-Kraicer R, Shalgi R. A novel regulatory pathway in granulosa cells, the LH/human chorionic gonadotropin-microRNA-125a-3p-Fyn pathway, is required for ovulation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(8):3206–16. [DOI] [PubMed] [Google Scholar]
- 43.Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci USA. 2014;111(8):3008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee YZ, Cheng SH, Lin YF, Wu CC, Tsai YC. The Beneficial Effects of Lacticaseibacillus paracasei subsp. paracasei DSM 27449 in a Letrozole-Induced Polycystic Ovary Syndrome Rat Model. Int J Mol Sci. 2024;25(16):8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benedetti C, Pavani KC, Gansemans Y, Azari-Dolatabad N, Pascottini OB, Peelman L, Six R, Fan Y, Guan X, Deserranno K, et al. From follicle to blastocyst: microRNA-34c from follicular fluid-derived extracellular vesicles modulates blastocyst quality. Journal of animal science and biotechnology. 2024;15(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JJ, Ge W, Zhai QY, Liu JC, Sun XW, Liu WX, Li L, Lei CZ, Dyce PW, De Felici M, et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice. PLoS Biol. 2020;18(12):e3001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang JJ, Tian Y, Li MH, Feng YQ, Kong L, Zhang FL, Shen W. Single-cell transcriptome dissection of the toxic impact of Di (2-ethylhexyl) phthalate on primordial follicle assembly. Theranostics. 2021;11(10):4992–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian Y, Zhang MY, Zhao AH, Kong L, Wang JJ, Shen W, Li L. Single-cell transcriptomic profiling provides insights into the toxic effects of Zearalenone exposure on primordial follicle assembly. Theranostics. 2021;11(11):5197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Dong J, Yan L, Yong J, Liu X, Hu Y, Fan X, Wu X, Guo H, Wang X, et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell. 2017;20(6):858-873.e854. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, Liu Z, Min Z, Hu H, Jing Y, et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell. 2020;180(3):585-600.e519. [DOI] [PubMed] [Google Scholar]
- 51.Zhao ZH, Ma JY, Meng TG, Wang ZB, Yue W, Zhou Q, Li S, Feng X, Hou Y, Schatten H, et al. Single-cell RNA sequencing reveals the landscape of early female germ cell development. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2020;34(9):12634–45. [DOI] [PubMed] [Google Scholar]
- 52.Niu W, Spradling AC. Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc Natl Acad Sci USA. 2020;117(33):20015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S, et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Yan Z, Qin Q, Nisenblat V, Chang HM, Yu Y, Wang T, Lu C, Yang M, Yang S, et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Mol Cell. 2018;72(6):1021-1034.e1024. [DOI] [PubMed] [Google Scholar]
- 55.Fan X, Moustakas I, Bialecka M, Del Valle JS, Overeem AW, Louwe LA, Pilgram GSK, van der Westerlaken LAJ, Mei H, de Sousa Lopes Chuva SM. Single-cell transcriptomics analysis of human small antral follicles. Int J Mol Sci. 2021;22(21):11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Zhu S, Shu W, Guo Y, Guan Y, Zeng J, Wang H, Han L, Zhang J, Liu X, et al. Characterization of Metabolic Patterns in Mouse Oocytes during Meiotic Maturation. Mol Cell. 2020;80(3):525-540.e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai J, Cai J, Zhang T, Pang M, Xu X, Bai J, Liu Y, Qin Y. Transcriptome and metabolome analyses reveal the mechanism of corpus luteum cyst formation in pigs. Genes. 2023;14(10):1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics (Oxford, England). 2008;24(5):713–4. [DOI] [PubMed] [Google Scholar]
- 59.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolde R, Kolde MR. Package ‘pheatmap.’ R package. 2015;1(7):790. [Google Scholar]
- 63.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics (Oxford, England). 2013;29(22):2933–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedländer MR, Chen W, Adamidi C, Maaskola J, Einspanier R, Knespel S, Rajewsky N. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol. 2008;26(4):407–15. [DOI] [PubMed] [Google Scholar]
- 66.Krüger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34(Web Server issue):W451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2(11):e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kivioja T, Vähärautio A, Karlsson K, Bonke M, Enge M, Linnarsson S, Taipale J. Counting absolute numbers of molecules using unique molecular identifiers. Nat Methods. 2012;9(1):72–4. [DOI] [PubMed] [Google Scholar]
- 69.Vaskivuo TE, Mäentausta M, Törn S, Oduwole O, Lönnberg A, Herva R, Isomaa V, Tapanainen JS. Estrogen receptors and estrogen-metabolizing enzymes in human ovaries during fetal development. J Clin Endocrinol Metab. 2005;90(6):3752–6. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, Yu B, Liu X, Hu J, Yang Y, Namei E, Yang B, Bai Y, Qian Y, Li H. The cAMP-ERK1/2 signaling pathway regulates urokinase-type plasminogen activator-induced bovine granulosa cell proliferation. Reproduction (Cambridge, England). 2020;160(6):853–62. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z, Chen Z, Ye S, He Y, Huang S, Yuan X, Chen Z, Zhang H, Li J. Genome-wide association study for reproductive traits in a Duroc pig population. Animals. 2019;9(10):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Li C, Li Q, Li G, Li W, Li H, Kang X, Tian Y. Novel regulatory factors in the hypothalamic-pituitary-ovarian axis of hens at four developmental stages. Front Genet. 2020;11:591672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He R, Zhao Z, Yang Y, Liang X. Using bioinformatics and metabolomics to identify altered granulosa cells in patients with diminished ovarian reserve. PeerJ. 2020;8:e9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang XL, Liu L, Wang N, Chen ZJ, Zhang C. The function of high-density lipoprotein and low-density lipoprotein in the maintenance of mouse ovarian steroid balance. Biol Reprod. 2017;97(6):862–72. [DOI] [PubMed] [Google Scholar]
- 75.Jiménez LM, Binelli M, Bertolin K, Pelletier RM, Murphy BD. Scavenger receptor-B1 and luteal function in mice. J Lipid Res. 2010;51(8):2362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weisz F, Bartenschlager H, Knoll A, Mileham A, Deeb N, Geldermann H, Čepica S. Association analyses of porcine SERPINE1 reveal sex-specific effects on muscling, growth, fat accretion and meat quality. Anim Genet. 2012;43(5):614–9. [DOI] [PubMed] [Google Scholar]
- 77.Bijnens AP, Knockaert I, Cousin E, Kruithof EK, Declerck PJ. Expression and characterization of recombinant porcine plasminogen activator inhibitor-1. Thromb Haemost. 1997;77(2):350–6. [PubMed] [Google Scholar]
- 78.Christensen L, Simonsen ACW, Heegaard CW, Moestrup SK, Andersen JA, Andreasen PA. Immunohistochemical localization of urokinase-type plasminogen activator, type-1 plasminogen-activator inhibitor, urokinase receptor and α2-macroglobulin receptor in human breast carcinomas. Int J Cancer. 1996;66(4):441–52. [DOI] [PubMed] [Google Scholar]
- 79.Teesalu T, Blasi F, Talarico D. Embryo implantation in mouse: fetomaternal coordination in the pattern of expression of uPA, uPAR, PAI-1 and α2MRLRP genes. Mech Dev. 1996;56(1–2):103–16. [DOI] [PubMed] [Google Scholar]
- 80.Azimi I, Petersen RM, Thompson EW, Roberts-Thomson SJ, Monteith GR. Hypoxia-induced reactive oxygen species mediate N-cadherin and SERPINE1 expression, EGFR signalling and motility in MDA-MB-468 breast cancer cells. Sci Rep. 2017;7(1):15140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andreasen PA. PAI-1-a potential therapeutic target in cancer. Curr Drug Targets. 2007;8(9):1030–41. [DOI] [PubMed] [Google Scholar]
- 82.Kasyapa CS, Kunapuli P, Hawthorn L, Cowell JK. Induction of the plasminogen activator inhibitor-2 in cells expressing the ZNF198/FGFR1 fusion kinase that is involved in atypical myeloproliferative disease. Blood. 2006;107(9):3693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qu X, Guo S, Yan L, Zhu H, Li H, Shi Z. TNFα-Erk1/2 signaling pathway-regulated SerpinE1 and SerpinB2 are involved in lipopolysaccharide-induced porcine granulosa cell proliferation. Cell Signal. 2020;73:109702. [DOI] [PubMed] [Google Scholar]
- 84.LaVoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med. 2009;234(8):880–907. [DOI] [PubMed] [Google Scholar]
- 85.Dou YD, Zhao H, Huang T, Zhao SG, Liu XM, Yu XC, Ma ZX, Zhang YC, Liu T, Gao X, et al. STMN1 Promotes Progesterone Production Via StAR Up-regulation in Mouse Granulosa Cells. Sci Rep. 2016;6:26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nimz M, Spitschak M, Fürbass R, Vanselow J. The pre-ovulatory luteinizing hormone surge is followed by down-regulation of CYP19A1, HSD3B1, and CYP17A1 and chromatin condensation of the corresponding promoters in bovine follicles. Mol Reprod Dev. 2010;77(12):1040–8. [DOI] [PubMed] [Google Scholar]
- 87.Grzesiak M, Knapczyk-Stwora K, Ciereszko RE, Wieciech I, Slomczynska M. Alterations in luteal production of androstenedione, testosterone, and estrone, but not estradiol, during mid- and late pregnancy in pigs: effects of androgen deficiency. Theriogenology. 2014;82(5):720–33. [DOI] [PubMed] [Google Scholar]
- 88.Sánchez P, Torres JM, Olmo A, O’Valle F, Ortega E. Effects of environmental stress on mRNA and protein expression levels of steroid 5alpha-Reductase isozymes in adult rat brain. Horm Behav. 2009;56(3):348–53. [DOI] [PubMed] [Google Scholar]
- 89.Blitek A, Szymanska M, Pieczywek M, Morawska-Pucinska E. Luteal P4 synthesis in early pregnant gilts after induction of estrus with PMSG/hCG. Anim Reprod Sci. 2016;166:28–35. [DOI] [PubMed] [Google Scholar]
- 90.Labrie F, Luu-The V, Lin S-X, Claude L, Simard J, Breton R, Bélanger A. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62(1):148–58. [DOI] [PubMed] [Google Scholar]
- 91.Piprek RP: Molecular mechanisms of cell differentiation in gonad development, vol. 58: Springer; 2016.
- 92.Poutanen M, Isomaa V, Peltoketo H, Vihko R. Role of 17β-hydroxysteroid dehydrogenase type 1 in endocrine and intracrine estradiol biosynthesis. J Steroid Biochem Mol Biol. 1995;55(5–6):525–32. [DOI] [PubMed] [Google Scholar]
- 93.Järvensivu P, Saloniemi-Heinonen T, Awosanya M, Koskimies P, Saarinen N, Poutanen M. HSD17B1 expression enhances estrogen signaling stimulated by the low active estrone, evidenced by an estrogen responsive element-driven reporter gene in vivo. Chem Biol Interact. 2015;234:126–34. [DOI] [PubMed] [Google Scholar]
- 94.Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican-and fibromodulin-deficient mice. J Biol Chem. 2002;277(38):35532–40. [DOI] [PubMed] [Google Scholar]
- 95.Gao Q, Pan H-T, Lin X-H, Zhang J-Y, Jiang Y, Tian S, Chen L-T, Liu M-E, Xiong Y-M, Huang H-F. Altered protein expression profiles in umbilical veins: insights into vascular dysfunctions of the children born after in vitro fertilization. Biology of Reproduction. 2014;91(3):71. [DOI] [PubMed] [Google Scholar]
- 96.Ottone T, Silvestrini G, Piazza R, Travaglini S, Gurnari C, Marchesi F, Nardozza AM, Fabiani E, Attardi E, Guarnera L, et al. Expression profiling of extramedullary acute myeloid leukemia suggests involvement of epithelial-mesenchymal transition pathways. Leukemia. 2023;37(12):2383–94. [DOI] [PubMed] [Google Scholar]
- 97.Sun M, Wang X, Bi F, Xiang H, Wang N, Gao W, Liu Y, Lv Z, Li Y, Huan Y. Fibronectin 1 supports oocyte in vitro maturation in pigs. Int J Biol Macromol. 2024;264:130590. [DOI] [PubMed] [Google Scholar]
- 98.Velez C, Williamson D, Riesco O, Martin P, Garcia M, Yaful G, Koncurat M. Estrogens, progesterone and integrin-fibronectin interactionduring porcine placentation. Placenta. 2015;36(4):500–500. [Google Scholar]
- 99.Asano Y, Iwaki T, Umemura K, Kanayama N, Itoh H. Fibrin-mediated growth restriction of early-stage human trophoblasts is switched to growth promotion through fibrinolysis. Human reproduction (Oxford, England). 2021;36(12):3108–21. [DOI] [PubMed] [Google Scholar]
- 100.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9(6):263–8. [DOI] [PubMed] [Google Scholar]
- 101.Feng Y, Ma X, Deng L, Yao B, Xiong Y, Wu Y, Wang L, Ma Q, Ma F. Role of selectins and their ligands in human implantation stage. Glycobiology. 2017;27(5):385–91. [DOI] [PubMed] [Google Scholar]
- 102.Ruane PT, Garner T, Parsons L, Babbington PA, Wangsaputra I, Kimber SJ, Stevens A, Westwood M, Brison DR, Aplin JD. Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation. Human reproduction (Oxford, England). 2022;37(4):777–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tesfaye D, Gebremedhn S, Salilew-Wondim D, Hailay T, Hoelker M, Grosse-Brinkhaus C, Schellander K. MicroRNAs: tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction (Cambridge, England). 2018;155(3):R121-r135. [DOI] [PubMed] [Google Scholar]
- 104.Boscaro C, Ramaschi GE, Trevisi L, Cignarella A, Bolego C. MiR-206 inhibits estrogen signaling and ovarian cancer cell migration without affecting GPER. Life Sci. 2023;333:122135. [DOI] [PubMed] [Google Scholar]
- 105.Chen H, Chan HC. Amplification of FSH signalling by CFTR and nuclear soluble adenylyl cyclase in the ovary. Clin Exp Pharmacol Physiol. 2017;44(Suppl 1):78–85. [DOI] [PubMed] [Google Scholar]
- 106.Wang W, Ren F, Wu Q, Jiang D, Li H, Peng Z, Wang J, Shi H. MicroRNA-497 inhibition of ovarian cancer cell migration and invasion through targeting of SMAD specific E3 ubiquitin protein ligase 1. Biochem Biophys Res Commun. 2014;449(4):432–7. [DOI] [PubMed] [Google Scholar]
- 107.Xiao Y, Peng X, Peng Y, Zhang C, Liu W, Yang W, Dou X, Jiang Y, Wang Y, Yang S, et al. Macrophage-derived extracellular vesicles regulate follicular activation and improve ovarian function in old mice by modulating local environment. Clin Transl Med. 2022;12(10):e1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y, Zhang D, Guo Y, Lu B, Zhao ZJ, Xu X, Chen Y. Tyrosine Kinase ROR1 as a Target for Anti-Cancer Therapies. Front Oncol. 2021;11:680834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hasuwa H, Ueda J, Ikawa M, Okabe M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science. 2013;341(6141):71–3. [DOI] [PubMed] [Google Scholar]
- 110.Mechaussier S, Almoallem B, Zeitz C, Van Schil K, Jeddawi L, Van Dorpe J, Dueñas Rey A, Condroyer C, Pelle O, Polak M, et al. Loss of Function of RIMS2 Causes a Syndromic Congenital Cone-Rod Synaptic Disease with Neurodevelopmental and Pancreatic Involvement. Am J Hum Genet. 2020;106(6):859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Gelderen TA, Ribas L. miR-210 promotes immune- and suppresses oocyte meiosis-related genes in the zebrafish ovarian cells. Genomics. 2024;116(2):110820. [DOI] [PubMed] [Google Scholar]
- 112.Cao M, Zhao Y, Chen T, Zhao Z, Zhang B, Yuan C, Wang X, Chen L, Wang N, Li C, et al. Adipose mesenchymal stem cell-derived exosomal microRNAs ameliorate polycystic ovary syndrome by protecting against metabolic disturbances. Biomaterials. 2022;288:121739. [DOI] [PubMed] [Google Scholar]
- 113.Wang J, Zhu M, Zhou X, Wang T, Xi Y, Jing Z, Xi W. MiR-140–3p inhibits natural killer cytotoxicity to human ovarian cancer via targeting MAPK1. J Biosci. 2020;45:66. [PubMed] [Google Scholar]
- 114.Guan S, Guo L, Zhang T, Zhu B, Wang X, Zhang C. Effects of gonadotropin on Fas and/or FasL expression and proliferation in rat ovary. Theriogenology. 2015;83(1):21–9. [DOI] [PubMed] [Google Scholar]
- 115.Badawy MT, Sobeh M, Xiao J, Farag MA. Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences. Molecules (Basel, Switzerland). 2021;26(20):6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the [Gene Expression Omnibus] repository with the primary GEO accession GSE271331. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE271331.