Abstract
Background
Bacterial pathogens frequently encounter host-derived metabolites during their colonization and invasion processes, which can serve as nutrients, antimicrobial agents, or signaling molecules for the pathogens. The essential nutrient choline (Cho) is widely known to be utilized by a diverse range of bacteria and may undergo conversion into the disease-associated metabolite trimethylamine (TMA). However, the impact of choline metabolism on bacterial physiology and virulence remains largely unexplored.
Methods
Here, we employed an in vitro infection model to investigate the role of Cho in intracellular survival and virulence of Pseudomonas aeruginosa (P. aeruginosa). Additionally, a comprehensive RNA-seq based transcriptomic analysis and various phenotypic assays were performed to elucidate the impacts of Cho on P. aeruginosa.
Results
We observed that the Cho metabolite glycine betaine (GB) effectively reduced intracellular levels of cyclic-di-GMP (c-di-GMP). Supplementation of Cho or GB in P. aeruginosa had thus affected c-di-GMP regulated phenotypes, such as pyoverdine production, biofilm formation, and mobility. Depletion of Cho metabolism through knockout of the betAB operon resulted in compromised intracellular survival of P. aeruginosa. Notably, the P. aeruginosa betAB mutant elicited a more robust protective inflammatory response compared to the wild-type strain.
Conclusion
Our study showed that P. aeruginosa Cho metabolism not only interferes host nutritional immunity, but also directly affect multiple virulence phenotypes through modulation of c-di-GMP signaling.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10375-3.
Keywords: Pseudomonas aeruginosa, Choline metabolism, Cyclic-di-GMP, Virulence, Biofilm
Background
The metabolism of host-derived molecules represents a crucial strategy for orchestrating the interactions between eukaryotic hosts and their associated bacterial pathogens, symbionts, and commensals [1]. Bacterial pathogens utilize various mechanisms, including the production of virulence factors and engagement in collective behaviors (e.g., quorum sensing and biofilm formation), to exploit host-derived resources for enhanced survival within the host [1, 2]. A notable host-derived molecule that serves as a source of carbon, nitrogen, and precursors for numerous biomolecules across diverse pathogens is quaternary amine alcohol Cho [3, 4]. In infection sites such as the lungs, urinary tract, skin, and eyes, bacterial pathogens synthesize several enzymes to acquire or metabolize Cho from phosphatidylcholine, acetylcholine, and phosphorylcholine [5–7]. Nevertheless, relatively few studies have investigated how Cho metabolism influences the physiology and virulence of these pathogens.
Bacterial pathogens have evolved numerous metabolic pathways for the acquisition and utilization of specific molecules within their hosts [1, 8]. Previous studies have demonstrated that host-derived aromatic amino acids in the lungs of individuals with cystic fibrosis (CF) can act as signals to modulate quorum sensing (QS) in P. aeruginosa [9, 10], which coordinates population-dependent gene expression and regulates bacterial group behaviors such as biofilm formation and swarming motility [11]. Additionally, host-derived cues including iron, bile, bicarbonate, arginine, and norspermidine have also been implicated in the regulation of quorum sensing and c-di-GMP signaling across various bacterial species [12–15]. C-di-GMP is a ubiquitous second messenger known to regulate virulence, motility, and biofilm formation in multiple bacterial pathogens. It is synthesized by diguanylate cyclases (DGCs) and degraded by c-di-GMP phosphodiesterases (PDEs), functioning through binding to diverse protein or riboswitch effectors [16, 17].
P. aeruginosa is a multidrug resistant and opportunistic human pathogen that is the leading cause of CF infections and nosocomial infections such as ventilator-associated pneumonia (VAP). It causes a high rate of persistent infections and a high mortality rate despite adequate antibiotic treatment in P. aeruginosa infection [18, 19]. P. aeruginosa has evolved multiple pathways to utilize Cho and is an experimentally model organism to study the regulatory pathways controlling Cho and GB homeostasis in Cho-catabolizing bacteria [20]. After entering cells, Cho can be further catabolized into glycine betaine (GB) through betA and betB genes followed by sequential demethylation to dimethylglycine by demethylase genes (gbcA-B) and other nutrients [5, 21]. Furthermore, transcriptomic analysis of P. aeruginosa growing in sputum of CF patients showed a strong induction of genes involved in Cho catabolism including betA and betB [22–24], which suggests a relationship between Cho metabolism and virulence in P. aeruginosa infection. More importantly, deletion of the betAB genes decreased the survival of P. aeruginosa in the mouse lung infection model. However, deletion of the GB demethylase genes (gbcA-B), required for catabolism of GB, did not affect P. aeruginosa survival in the mouse lungs [25]. Even though these studies suggest that the conversion of Cho to GB contributes to the survival of P. aeruginosa during infection, the underlying mechanisms involved are not fully understood [23, 26].
In the present study, we utilized an in vitro macrophage infection model to elucidate the mechanisms by which Cho metabolism impacts virulence and its regulatory pathways in P. aeruginosa. Initially, we conducted a proteomic analysis that demonstrated active expression of Cho metabolism-related proteins in P. aeruginosa following macrophage engulfment. Subsequently, we found that betAB deficiency significantly diminished the intracellular survival and virulence of P. aeruginosa. Furthermore, the addition of glyceryl-betaine (GB) to P. aeruginosa markedly suppressed the expression of c-di-GMP-related genes and their associated phenotypes, including pyoverdine synthesis, biofilm formation, and motility. Importantly, the betAB mutant of P. aeruginosa elicited a stronger protective inflammatory response compared to the wild-type strain. Our findings suggest that Cho metabolism not only interferes with c-di-GMP signaling but also plays a role in modulating host immune responses during P. aeruginosa infections.
Methods
Strains and growth conditions
The P. aeruginosa PAO1 and E. coli strains were cultured in Luria Bertani (LB) broth or on agar plates supplemented with 1.5% Bacto agar, at a temperature of 37 °C unless otherwise specified. Appropriate antibiotics were added to the media, with concentrations (µg mL− 1) as follows: ampicillin (Amp) at 100 µg mL-1 for E. coli strains; gentamycin (Gm) at either 100 µg mL− 1 or 200 µg mL− 1, and carbenicillin (Cb) at 150 µg mL-1 for P. aeruginosa.
Construction of P. aeruginosa mutant strains
The P. aeruginosa clean gene knockout mutant strains were obtained as previously described [27]. Briefly, two pairs of the corresponding primers were used to amplify the upstream and downstream of the betA, betB, gbcA and gbcB genes followed by ligated to the suicide vector PK18 using Gibson Assembly master mix (New England Biolabs [NEB]). The ligated PK18 plasmid (keep in E. coli Top10) was transferred to PAO1 by conjugal mating with the help of E. coli harbor pRK600 vector after verifying the sequence. Gentamicin was used for selection of the first homologous recombinants. The selected colonies were then streaked onto LB agar with 20% sucrose to select the second homologous recombinants. Finally, the betA, betB and betAB double mutant, gbcA, gbcB and gbcAB double mutants were verified by PCR. All the strains and plasmids were listed in Table 1.
Table 1.
Strains and plasmid list
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| P. aeruginosa Strains | ||
| PAO1 | P. aeruginosa WT | Lab collection |
| ∆gbcAB | In-frame gbcA and gbcB deletions in PAO1 | [23] |
| ∆betAB | In-frame betA and betB deletions in PAO1 | [23] |
| ∆PA0285 | In-frame PA0285 deletions in PAO1 | [26] |
| ∆PA0285 + pUCP22::cdrA-gfp | ∆PA0285 with plasmid pUCP22::cdrA-gfp | [26] |
| E. coli | ||
| Top 10 | F2 mcrA D(mrr-hsd RMS-mcrBC) f 80lacZ DM15 DlacX74 recA1 araD139D(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Tiangen |
| Plasmids | ||
| pK18 | Small mobilizable vector, Gmr, sucrose sensitive (sacB) | [27] |
| RK600 | Cmr ColE1 oriV RK2 mob1 tra1; helper plasmid in triparental matings | [29] |
| pUCP22::cdrA-gfp | pCdrA::gfps pUCP22Not-PcdrA-RBS-CDS-RNase III-gfp(Mut3)-T0-T1, Ampr Gmr | [26] |
Gmr, gentamicin resistance; Cmr, chloramphenicol resistance
Intracellular survival and virulence assay
The intracellular survival and virulence assays were conducted as previously described with minor modifications [28]. The murine macrophage cell line Raw264.7 was seeded at a density of 1 × 105 cells per well in 24-well culture plates containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. Subsequently, the cells were infected with mid-log phase PAO1 cultured in agrobacterium minimal medium ABTG, either supplemented with or without GB, at various multiplicities of infection for one hour. Following bacterial internalization, the cells were washed twice with pre-warmed phosphate-buffered saline (PBS) to remove extracellular bacteria and maintained in DMEM containing gentamicin (Gm) at a concentration of 100 µg mL− 1 for an additional 3 h. Supernatants were collected for lactate dehydrogenase (LDH) detection according to the manufacturer’s instructions (YEASEN, 40209ES76), after which the cells were treated with gentamicin at a concentration of 200 µg mL− 1 to eliminate any remaining extracellular organisms for another hour following the initial incubation period. Cells were then rinsed once with PBS and lysed using 0.1% Triton X-100 before being plated onto LB agar plates for colony-forming unit (CFU) counting.
RNA sequencing and analysis
The RNA extraction and sequencing analysis were carried out as previously described [28]. Briefly, PAO1 control group and 0.1 mM GB treatment group were cultured to mid-log phase with 3 biological replicates. Then, the total RNAs were extracted using RNApure Bacteria Kit (CWBIO, CW0539S). The integrity of RNA was examined by TapeStation RNA ScreenTape with RNA size distributions. Illumina NovaSeq 6000 sequencing platform was used for RNA sequencing to generate paired-end reads of 100 nt. which were trimmed and mapped to PAO1 genome (NC_002516.2) by CLC Genomics Workbench 9.0 (Qiagen). The differentially expressed genes was analyzed by DESeq 2 with statistical significance (fold change > 1, adjusted P < 0.05) based on the transcript count table. The reason why we choose the cut-off of fold change > 1 is that the fold changes of differentially expressed genes are not very high. GO and KEGG enrichment analysis were performed with DAVID Bioinformatics Resources. The volcano charts and pathway enrichment analysis map were obtained by R studio (version 4.0.2).
Real-time qPCR verification
Total RNA from the 0.1 mM glyceryl-betaine (GB) treatment group and the control group was extracted using an RNeasy Minikit (Qiagen, Germany), followed by DNase treatment (Qiagen, Germany) and reverse transcription to cDNA (TaKaRa). RT-qPCR assays were conducted with Hieff qPCR SYBR Green Master Mix (Yeasen) and a qPCR system (Roche), adhering to the manufacturers’ instructions with three technical replicates for each sample. Data analysis was performed using LightCycler 96 software (Roche). The relative expression levels of target genes were calculated by normalizing against the expression of the housekeeping gene rpsL.
Fluorescence reporter assays
The plasmid containing gfp fusions to the c-di-GMP related gene (cdrA) was transformed into PAO1 or ∆betAB mutant. Subsequently, overnight cultures of P. aeruginosa carrying pcdrA-gfp were diluted to an optical density at 600 nm (OD600) of 0.01. Each well of a 96-well microtiter plate was then inoculated with 200 µL of the bacterial mixture supplemented with or without Cho and GB, in triplicate replicates. Finally, the plate was incubated in a Tecan Infinite 200 PRO microplate reader at 37 °C, where the program was set to read every 15 min for a total of 96 cycles at a wavelength of 590 nm. After normalizing the fluorescence data with OD600 values, the time point corresponding to the peak of Gaussian distribution (10 h) was selected for statistical analysis [28].
Biofilm quantification
The PAO1 strain was cultured in 96-well microplates containing ABTG supplemented with either 0.1 mM GB, 0.1 mM Cho, or no additional supplements for a duration of 24 h at a temperature of 37 °C. After the incubation period, the cultures were discarded, and the microplates were rinsed with tap water. Subsequently, the resulting biofilm was stained with a solution of 0.1% crystal violet for a duration of 15 min, solubilized using a solution consisting of 30% acetic acid, and quantified by measuring absorbance at a wavelength of 550 nm.
Pyoverdine production
The pyoverdine assay was conducted as previously reported [28]. PAO1 and the betAB mutant, grown overnight at 37 °C, were suspended in ABTG medium to an optical density (OD600) of 0.01, supplemented with either 0.1 mM GB, 0.1 mM Cho, or no additional supplements. The OD600 and fluorescence emission for each well were measured using a Tecan Infinite 200 PRO microplate reader over a period of 16 h, with an excitation wavelength of 400 nm and an emission wavelength of 460 nm. Poverdine production was calculated by normalizing the fluorescence value (arbitrary units, AU) with cell density (OD600).
Swimming assay
Swimming motility was assayed as previously described on BM2 agar plates (0.4% [wt/vol] glucose, 62 mM potassium phosphate buffer [pH = 7], 0.3% [wt/vol] Difco agar, 2 mM MgSO4, 10 M FeSO4, 0.1% [wt/vol] Casamino Acids) with 0.1 mM GB, 0.1 mM Cho or not [29]. All plates were inoculated at the center with 2 µL of overnight bacterial culture at 37 °C for 18 h. Subsequently, the colony parameter was subsequently quantified using ImageJ software, and the statistical analysis was performed using SPSS. The results were visualized using GraphPad Prism.
IL-1β and IL-18 detection
Overnight cultures of PAO1 and the betAB mutant were re-inoculated in LB medium at a dilution ratio of 1:100 for 3 h. Subsequently, Raw264.7 macrophages were infected with PAO1 and the betAB mutant at a multiplicity of infection (MOI) of 100 for 6 h. The levels of IL-1β and IL-18 in the cell supernatants were measured using ELISA kits (CUSABIO, CSB-E04609m, CSB-E08054m) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SPSS (PASW Statistics 18, one-way ANOVA). Quantitative data were visualized using GraphPad Prism. All experiments in the manuscript were independently conducted at least three times. Data are presented as mean ± standard deviation (S.D.). Significance was determined at a threshold of p < 0.05.
Results
Deletion of choline metabolism gene betAB leads to decreasedP. aeruginosa survival and virulence
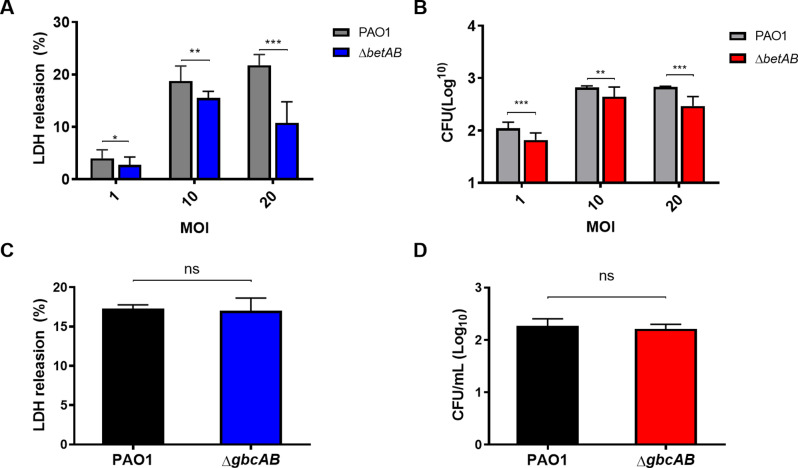
Given that Cho catabolism is implicated in the pathogenesis of P. aeruginosa in mouse lung infection models [25], and a strong induction of betA and betB genes has been associated with P. aeruginosa infections in cystic fibrosis (CF) patients as well as acute murine pneumonia [22–24], we aim to investigate the role of Cho metabolism in the virulence and survival of P. aeruginosa using an in vitro macrophage infection model. As illustrated in Fig. 1, Raw264.7 macrophages infected with P. aeruginosa at various multiplicities of infection (MOIs) demonstrated that betAB deletion mutants exhibited significantly reduced intracellular virulence (Fig. 1A) and survival (Fig. 1B) compared to the PAO1 wild-type strain. Importantly, no defects in intracellular viability or virulence were observed for gbcA and gbcB mutants (Fig. 1C-D). These findings are consistent with previous studies indicating that the virulence deficiency observed in betAB mutants is not attributable to GB catabolism or its downstream metabolites [25]. However, potential mechanisms underlying the attenuation of intracellular viability and virulence in P. aeruginosa betAB deletion mutants remain unclear.
Fig. 1.
Impact of Cho metabolism on P. aeruginosa survival and virulence. Effect of betAB deletion on the virulence (lactate dehydrogenase release, LDH) and intracellular survival (colony forming units, CFU) on P. aeruginosa (A-B). Effect of gbcAB deletion on the virulence (LDH) and intracellular survival (CFU) on P. aeruginosa (C-D). Statistical analysis was done using One way-ANOVA. Significance * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001. n.s. = not significant. The data represent the mean ± SD of four biological replicates
Exogenous GB increases the intracellular survival and virulence of P. aeruginosa.
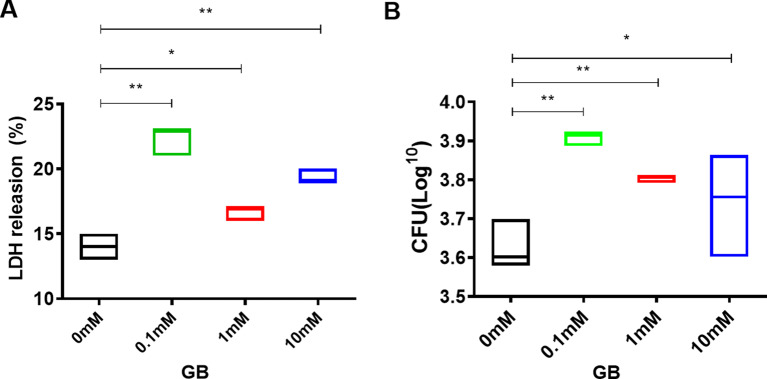
We are interested in elucidating the underlying mechanisms by which Cho or GB contributes to the survival of P. aeruginosa within macrophage cells. We firstly examined the intracellular viability and virulence of PAO1 cultured with exogenous GB in ABTG minimal medium. The addition of exogenous GB significantly enhanced the virulence (Fig. 2A) and survival rate of P. aeruginosa within macrophages at various concentrations, particularly at a concentration of 0.1 mM GB compared to the control group (Fig. 2B). Typically, GB serves as an osmoprotectant or undergoes metabolic processes to acquire carbon and/or nitrogen resources during P. aeruginosa infection [20]. Therefore, we subsequently employed transcriptomic analysis to evaluate its global impact on P. aeruginosa.
Fig. 2.
Effects of GB on the intracellular survival and virulence of P. aeruginosa. Raw264.7 cells were infected with mid-log phase of PAO1 that cultured with multiple concentrations of GB for 3 h. The virulence and intracellular survival rate were evaluated by LDH release (A) and CFU (B) respectively. Statistical analysis was performed by One-way ANOVA. Significance * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001.The data represent the mean ± SD of three biological replicates
GB negatively regulates the expression of c-di-GMP related genes in P. aeruginosa.
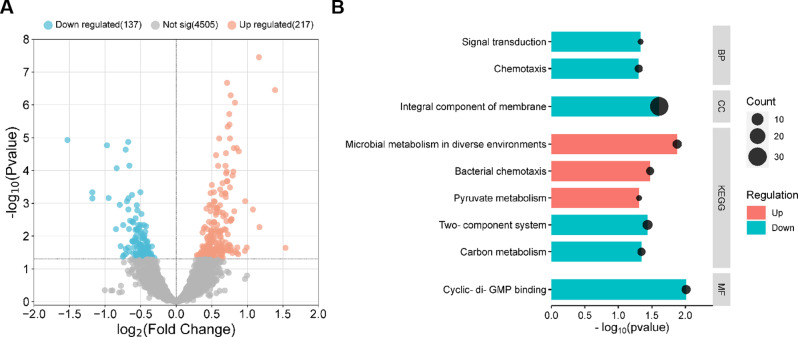
In order to thoroughly decode the regulatory effects of GB on P. aeruginosa, we performed a RNA-seq based transcriptomic analysis to compare the 0.1 mM GB treated P. aeruginosa cells and the control cells. The results showed that 354 genes were differentially expressed when P. aeruginosa cells were treated by 0.1 mM GB compared with untreated control (Fig. 3A and supplementary Table 1). Further investigation revealed that these differentially expressed genes (DEGs) was enriched in several signaling pathways including the c-di-GMP (c-di-GMP synthetases (DGCs) and the PliZ receptor) signaling pathway (Fig. 3B; Table 2). C-di-GMP is recognized as a second messenger that exerts its regulatory function by binding with various effectors, including PilZ domain proteins, transcription factors, kinases or phosphorylases, degenerate DGCs, or PDEs [17, 30–32]. In addition, other pathways including flagellum organization and swarming motility were also enriched. C-di-GMP signaling has been widely reported to coordinate multiple essential pathways in P. aeruginosa including biofilm formation, pyoverdine production, motility of organism and regulation of virulence [33].
Fig. 3.
Transcriptomic analysis for the impact of GB on P. aeruginosa. Volcano chart of changed genes between 0.1mM GB treated group and blank control (A). Gene ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the significantly changed genes between 0.1mM GB treated group and blank control (B)
Table 2.
Differentially expressed genes involved in c-di-GMP pathway in P. aeruginosa after 0.1 mM GB treatment
| Locus tag | Name | Domain signiture | Activity | Log2Fold change | Function | References |
|---|---|---|---|---|---|---|
| DGCs | ||||||
| PA1107 | roeA | GGEEF | DGC | -0.39 | EPS production | [34, 35] |
| PA0575 | rmcA | GGEEF, EAL | ND | -0.40 | Biofilm development, motility | [36] |
| PA4929 | nicD | GGDEF | DGC | -0.36 | Nutrient-induced biofilm dispersal | [37] |
| PA4843 | acbA | GGEEF | DGC | -0.56 | Biofilm development | [38] |
|
Receptors/ effectors |
||||||
| PA2799 | PilZ | -0.78 | [39] | |||
| PA4608 | mapZ | PilZ | -0.56 | [39, 40] |
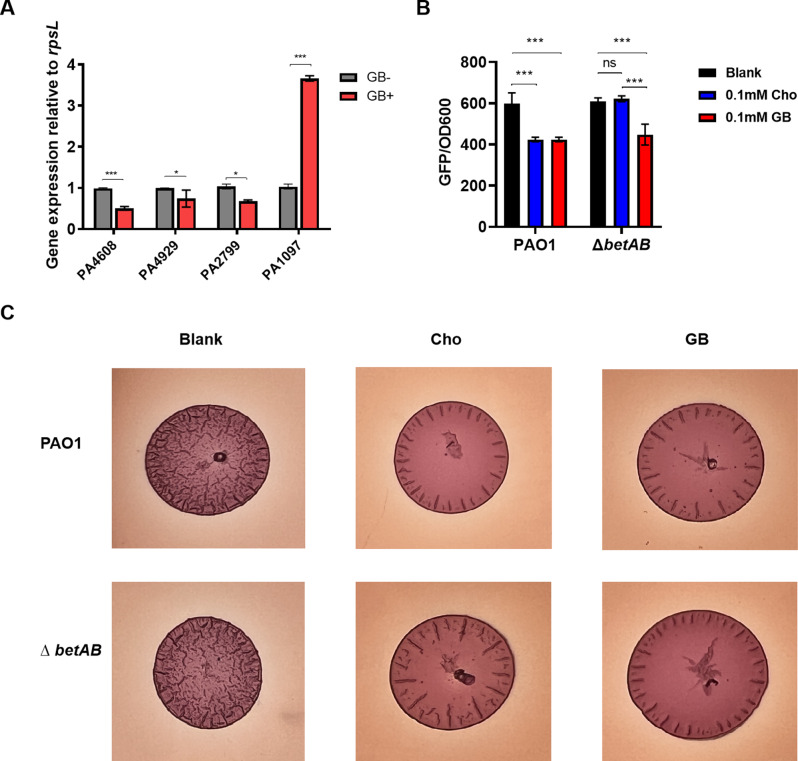
The reduction in the expression of key genes involved in DGCs and c-di-GMP receptors, including the fleQ (PA1077) receptor, which is expected to be highly expressed in a low c-di-GMP environment but was not detected in the RNA-seq, was further confirmed by quantitative real-time reverse transcription-PCR (qRT-PCR) analysis (Fig. 4A). Furthermore, we used a fluorescent reporter strain carrying the pcdrA-gfp fusion plasmid to quantify intracellular c-di-GMP levels for validatting the transcriptomic data, as CdrA is positively regulated by intracellular c-di-GMP molecules [41]. As shown in Fig. 4B, 0.1 mM Cho and GB effectively inhibited cdrA expression in PAO1. The deletion of betAB genes abolished the inhibitory effect of Cho on cdrA expression but did not affect the inhibitory effect of GB. This confirms that GB is the critical metabolite to suppress c-di-GMP signaling.
Fig. 4.
Cho and GB exhibited down-regulatory effects on c-di-GMP related phenotypes. RT-PCR varify the effects of GB on the expression of c-di-GMP-related gene relative to rpsL (A). The impact of Cho and GB on the expression of the c-di-GMP reporter gene PcdrA-gfp in both PAO1 and betAB mutant strains (B). Congo red colony morphology assay revealed the inhibitory effect of 0.1 mM Cho and 0.1 mM GB on c-di-GMP production in both PAO1 and betAB mutant (C). Statistical analysis was conducted by One-way ANOVA. * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001.The data represent the mean ± SD of three biological replicates
Considering the relatively modest fold changes observed in differentially expressed DGCs and c-di-GMP effectors, we postulated that the reduction of c-di-GMP levels in P. aeruginosa is a cumulative consequence of GB activity. Consequently, we proceeded to investigate whether GB treatment influences the colony phenotype regulated by c-di-GMP using the Congo Red assay, which has been widely employed as an indirect measure of c-di-GMP levels across various bacterial species due to its ability to assess exopolysaccharide production [42]. As expected, the control plate exhibited pronounced wrinkle formation and Congo red adsorption for both PAO1 and the betAB mutant. However, on 0.1 mM GB and 0.1 mM GB Cho plates in PAO1, a smooth colony with only a thin wrinkle along the periphery was observed. In accordance with the PcdrA-gfp assay, the betAB deletion mutant only formed a smooth colony on the GB plates, while still formed partially wrinkled colony on Cho plates (Fig. 4C). These results suggest that Cho and its metabolite GB could negatively affect the c-di-GMP signaling pathway. Given the ability of high intracellular c-di-GMP levels to attenuate virulence production in various bacterial species [43, 44], we anticipated that deletion of betAB would result in impaired intracellular survival and diminished virulence during infections.
Cho and GB inhibited c-di-GMP regulated phenotypes in P. aeruginosa
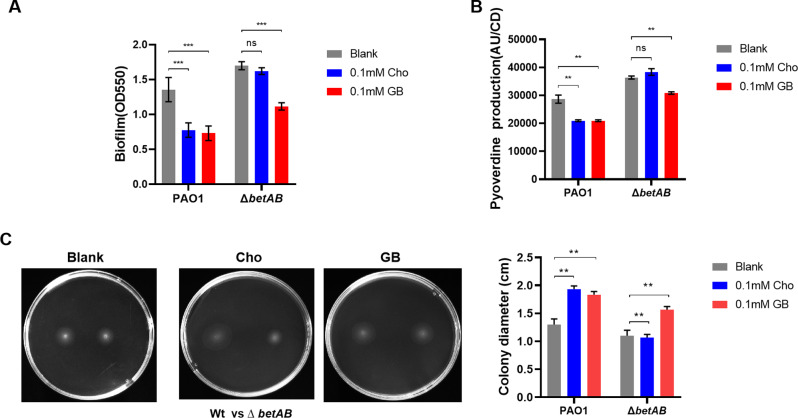
Increasing evidence suggests that elevated intracellular levels of c-di-GMP promote pyoverdine synthesis and biofilm formation in P. aeruginosa [27], whereas reduced intracellular c-di-GMP content is associated with increased virulence [33, 45]. We hypothesized that the betAB-mediated Cho metabolism to GB would impact c-di-GMP-mediated virulence phenotypes in P. aeruginosa. As anticipated, the addition of both 0.1 mM GB and choline significantly decreased biofilm formation (Fig. 5A) and pyoverdine synthesis (Fig. 5B), while enhancing swimming motility in PAO1 (Fig. 5C). In contrast, only the addition of GB, but not Cho, led to a reduction in biofilm formation (Fig. 5A) and pyoverdine synthesis (Fig. 5B), as well as an increase in swimming motility in the betAB deletion mutant strain (Fig. 5C). Collectively, our study suggested that the diffierence in c-di-GMP due to the failure conversion of Cho to GB may contributed to the survival defects of betAB mutant.
Fig. 5.
Cho and GB interfere c-di-GMP regulated phenotypes in P. aeruginosa. Biofilm formation of PAO1 and betAB mutant after cultured overnight with 0.1mM Cho or GB in ABTG medium (A). Poverdine production of PAO1 and betAB mutant after cultured overnight with 0.1mM Cho or GB in ABTG medium (B). Swarming motility of PAO1 and betAB mutant on BM2 plates supplemented with 0.1 mM Cho or GB, significance evaluation was listed on the right (C). Data was presented as mean ± SD of four biological replicates. Statistical analysis was conducted by One Way-ANOVA, ** Indicates p < 0.01, *** Indicates p < 0.001
Cho metabolism regulates host immune response to P. aeruginosa
Numerous studies have implicated Cho metabolism in modulating macrophage immune responses towards infections, including the secretion of IL-1β and IL-18 [46, 47]. Therefore, we hypothesized that the diminished intracellular survival capacity in the P. aeruginosa betAB mutant is not solely attributed to the loss of choline conversion, which ultimately triggers c-di-GMP regulated virulence. Additionally, it is plausible that bacterial Cho metabolism also contributes to host immune defenses. To test our hypothesis, we investigated the impact of betAB gene knockout on IL-1β and IL-18 production following P. aeruginosa infection. Consistent with our expectations, the deletion of betAB genes in P. aeruginosa resulted in a significantly elevated level of cytokine production compared to the wild-type strain (Fig. 6). This is consistent with the reduced intracellular survival rate and virulence in the betAB mutant, as IL-1β and IL-18 production constitutes a protective inflammatory response that aids in eradicating pathogens under physiological conditions [48–50]. Our findings strongly support the notion that choline facilitates P. aeruginosa’s evasion of host immune defenses. Overall, our data demonstrate that failure to convert Cho leads to reduced c-di-GMP related virulence and an enhanced host immune response against P. aeruginosa, contributing to the intracellular survival defect observed in the betAB mutant.
Fig. 6.
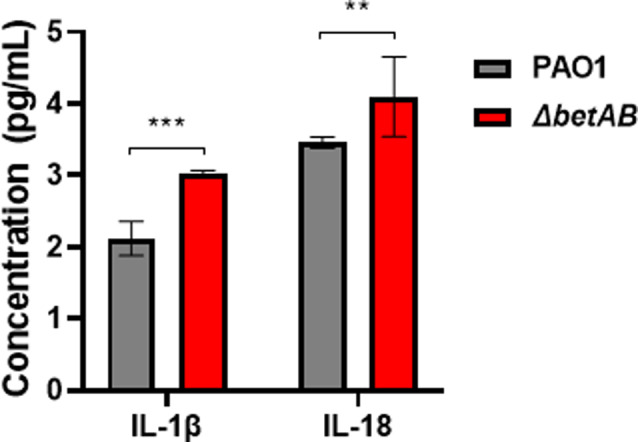
Deletion of betAB gene in P. aeruginosa enhances the production of IL-1β and IL-18 during macrophage infection. Raw264.7 macrophages were infected with log-phase PAO1 or betAB mutant for 6 h. Subsequently, the supernatant was collected and analyzed for IL-1β and IL-18 levels. The data shown are from one representative of three independent experiments. ** Indicates p < 0.01, *** Indicates p < 0.001
Discussion
P. aeruginosa is the cause of chronic lung infection in cystic fibrosis patients and a major contributor to hospital-acquired infections. Like many bacterial pathogens, P. aeruginosa employs various mechanisms to acquire host-derived nutrients such as iron and amino acids for successful establishment of infections. Previous studies have demonstrated significant upregulation of genes involved in Cho transportation and catabolism in the transcriptomes of P. aeruginosa growing in sputum from both CF patients and healthy individuals [26, 51]. Furthermore, Cho acquisition and glycerophosphocholine oxidation were found to be crucial for the survival of P. aeruginosa in mouse infection models; however, the underlying mechanism remains unclear [25]. In this study, we investigated the impact of Cho metabolism on P. aeruginosa physiology during macrophage infections (Fig. 7).
Fig. 7.
Model for Cho regulating the virulence of P. aeruginosa
Cho is an essential nutrient for eukaryotic cells which is required for synthesis of phospholipid and phosphatidylcholine. Recent study showed that Cho uptake is boosted in macrophages after LPS activation, and the increase of Cho transporter and uptake is important for full capacity of macrophage immune responsiveness and cytokine secretion [46]. Another report showed that impaired Cho uptake by macrophages could alter the mitochondrial lipid profile, attenuate its ATP synthesis and arrest NLRP3 inflammasome activation and cytokine production [47]. Thus our study here showed that P. aeruginosa is actively uptake Cho and its metabolite GB, which is an example of nutrient competition of pathogens and host cells that might attenuate the host immune response. Recent studies from other group as well as our own group showed that extracellular pathogens such as Staphylococcus aureus and P. aeruginosa are able to survive inside host phagocytes as non-growing persistent cells. These intracellular persistent cells still have active gene expression profiles even without dividing, which might drain the Cho from inside of macrophage cells and affect the macrophage immunometabolism [52]. Further studies should explore the impact of Cho uptake by intracellular pathogens to host immune cells.
It is well known that bacterial pathogens have distinct gene expression profiles and physiology at the infection sites when compared to laboratory cultivation conditions. For example, iron limitation stress is encountered by most of the bacteria growing at the infection sites, which can induce production of siderophore and other virulence mechanisms [53]. Besides, intracellular P. aeruginosa could induce QS-regulated cellular innate immune suppression [54]. C-di-GMP signaling is widely used mechanism by bacterial pathogens to modulate their physiology to adapt to the complex host environments, which can sense environmental cues surrounding bacterial cells and rapidly adjust the intracellular c-di-GMP pool via turning on the activity of DGCs or PDEs [27]. Our group has previously showed that host derived ROS cause extensive oxidative stress to P. aeruginosa and might serve as the driven force for selecting the hyperbiofilm forming wspF mutant with high intracellular c-di-GMP levels [28]. The host derived NO is one of the most studied factors to activate PDEs in multiple bacterial species and lower their intracellular c-di-GMP and cause biofilm inhibition [55]. Previous study has implicated haemolytic phospholipase C (PlcH) in P. aeruginosa virulence and deletion of betAB results in decreased survival compared to knock out of the plcH receptor (plcHR), suggesting that regulation of plcH expression is not the only role for GB during infection [56]. In this study, we provided evidence that GB metabolism serves as a host-derived factor capable of activating PDEs and decreasing intracellular c-di-GMP levels in P. aeruginosa. Despite the fact that GB treatment only moderately reduces the expression of several DGCs and does not show strong activity in suppressing c-di-GMP levels, multiple phenotypic assays consistently demonstrate that GB is able to modulate c-di-GMP-dependent virulence traits, such as exopolysaccharide production, pyoverdine synthesis, biofilm formation, bacterial motility, and intracellular survival. We hypothesize that the reduction of c-di-GMP levels in P. aeruginosa could be attributed to the cumulative effect of GB inhibiting the formation of multiple DGCs. Interestingly, even though we found a reduction of the c-di-GMP in P. aeruginosa after Cho addition, we noticed a strong upregulation of the fleQ gene. FleQ is known to be a c-di-GMP responsive transcription factor that increases the formation of biofilm in a lower c-di-GMP environment and allows to exopolysaccharide (EPS) production for biofilm formation at high c-di-GMP levels [57]. Further efforts will be engaged to identify the molecular mechanism about Cho metabolite regulation on c-di-GMP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- TMA Disease
associated metabolite trimethylamine
- Cho
Choline
- GB
Glycine betaine
- C-di-GMP
Cyclic-di-GMP
- DGCs
Diguanylate cyclases
- PDEs
Phosphodiesterases
- VAP
Ventilator-associated pneumonia
- MOI
Multiplicity of infection
- LDH
Lactate dehydrogenase release
Author contributions
Yachun Zhou and Yu Zhang collected, processed, analyzed and draft the manuscript. Xiangke Duan participated in the design the study. Tian Zhou partly contributed to the experiment. Yingyue Deng reviewed this manuscript. Lei Liu participated in design this study. Anmin Ren helped in materal preparation. Lin Zhong provide some resource. Liang Yang and Dongjing Liu contributed to study design, guide and manuscript verifcation. All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the Shenzhen Science and Technology Program (KQTD20200909113758004), National Natural Science Foundation of China (91951204, 32270196, 32200155, 32400154 and 32200053), Guangdong High-level Personnel of Special Support Program (2019QN01Y163), HaiYa Young Scientist Foundation of Shenzhen University General Hospital (2024-HY013), Guangdong Basic and Applied Basic Research Foundation (2019A1515110640, 2020A1515010316 and 2023A1515110104), Guangdong Yiyang Healthcare Charity Foundation (JZ2023015).
Data availability
RNA-seq data have been deposited into the Sequence Read Archive database (https://www.ncbi.nlm.nih.gov/sra) with the bioProject accession number: PRJNA1027243.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yachun Zhou and Yu Zhang contributed equaly.
Contributor Information
Dongjing Liu, Email: dangban-ldj@szsy.sustech.edu.cn.
Liang Yang, Email: yangl@sustech.edu.cn.
References
- 1.Brown SA, Palmer KL, Whiteley M. Revisiting the host as a growth medium. Nat Rev Microbiol. 2008;6:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteley M. The host as a growth medium: targeting bacterial nutrient uptake in New Treatment Strategies. Pediatr Pulmonol. 2013;48:154–55. [Google Scholar]
- 3.Chittim CL, del Campo AM, Balskus EP. Gut bacterial phospholipase ds support disease-associated metabolism by generating choline. Nat Microbiol. 2019;4:155–63. [DOI] [PubMed] [Google Scholar]
- 4.Kortstee GJ. The aerobic decomposition of choline by microorganisms. I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as the sole carbon and nitrogen source. Archives Microbiol. 1970;71:235–44. [PubMed] [Google Scholar]
- 5.Lisa TA, Garrido MN, Domenech CE. Induction of acid phosphatase and cholinesterase activities in ps. Aeruginosa and their in-vitro control by choline, acetylcholine and betaine. Mol Cell Biochem. 1983;50:149–55. [DOI] [PubMed] [Google Scholar]
- 6.Wright JR, Clements JA. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987;136:426–44. [DOI] [PubMed] [Google Scholar]
- 7.Salvano MA, Lisa TA, Domenech CE. Choline transport in Pseudomonas aeruginosa. Mol Cell Biochem. 1989;85:81–89. [DOI] [PubMed]
- 8.Garber ED. The host as a growth medium. Annals New York Acad Sciences-Series. 1960;88:1187–194. [DOI] [PubMed]
- 9.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189:8079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer KL, Mashburn LM, Singh PK, Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol. 2005;187:5267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno-Gamez S, Hochberg ME, van Doorn GS. Quorum sensing as a mechanism to harness the wisdom of the crowds. Nat Commun. 2023;14. [DOI] [PMC free article] [PubMed]
- 12.Koestler BJ, Waters CM. Bile acids and bicarbonate inversely regulate Intracellular Cyclic di-GMP in Vibrio cholerae. Infect Immun. 2014;82:3002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koestler BJ, Waters CM. Intestinal GPS: bile and bicarbonate control cyclic di-GMP to provide Vibrio cholerae spatial cues within the small intestine. Gut Microbes. 2014;5:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karatan E, Duncan TR, Watnick PI. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J Bacteriol. 2005;187:7434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernier SP, Ha DG, Khan W, Merritt JH, O’Toole GA. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res Microbiol. 2011;162:680–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a Universal Bacterial Second Messenger. Microbiol Mol Biol Rev. 2013;77:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenal U, Reinders A, Lori C. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol. 2017;15:271–84. [DOI] [PubMed] [Google Scholar]
- 18.Trouillet JL, Vuagnat A, Combes A, Kassis N, Chastre J, Gibert C. Pseudomonas aeruginosa ventilator-associated pneumonia: comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin Infect Dissases. 2002;34:1047–54. [DOI] [PubMed] [Google Scholar]
- 19.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wargo MJ. Homeostasis and catabolism of choline and glycine betaine: lessons from Pseudomonas aeruginosa. Appl Environ Microbiol. 2013;79:2112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bremer E. Liberate and grab it, ingest and digest it. The GbdR regulon of the pathogen Pseudomonas aeruginosa. J Bacteriol. 2014;196:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi E, Falcone M, Molin S, Johansen HK. High-resolution in situ transcriptomics of Pseudomonas aeruginosa unveils genotype independent patho-phenotypes in cystic fibrosis lungs. Nat Commun. 2018;9:3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son MS, Matthews WJ Jr., Kang Y, Nguyen DT, Hoang TT. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun. 2007;75:5313–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damron FH, Oglesby-Sherrouse AG, Wilks A, Barbier M. Dual-seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia. Sci Rep. 2016;6:39172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wargo MJ. Choline Catabolism to Glycine Betaine contributes to Pseudomonas aeruginosa Survival during murine lung infection. PLoS ONE. 2013; 8. [DOI] [PMC free article] [PubMed]
- 26.Cattoir V, Narasimhan G, Skurnik D, Aschard H, Roux D, Ramphal R, et al. Transcriptional response of mucoid Pseudomonas aeruginosa to human respiratory mucus. mBio. 2013;3:e00410–00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YC, Yuan MJ, Mohanty A, Yam JKH, Liu Y, Chua SL, et al. Multiple diguanylate cyclase-coordinated regulation of pyoverdine synthesis in Pseudomonas aeruginosa. Environ Microbiol Rep. 2015;7:498–507. [DOI] [PubMed] [Google Scholar]
- 28.Chua SL, Ding YC, Liu Y, Cai Z, Zhou JN, Swarup S et al. Reactive oxygen species drive evolution of pro-biofilm variants in pathogens by modulating cyclic-di-GMP levels. Open Biology 2016; 6. [DOI] [PMC free article] [PubMed]
- 29.Coleman SR, Bains M, Smith ML, Victor Spicer Y, Lao PK, Taylor N, et al. The small RNAs PA2952.1 and PrrH as regulators of virulence, motility, and iron metabolism in Pseudomonas aeruginosa. Appl Environ Microbiol. 2021;87:e02182–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol. 2005;187:1792–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt AJ, Ryjenkov DA, Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol. 2005;187:4774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasteva PV, Sondermann H. Versatile modes of cellular regulation via cyclic dinucleotides. Nat Chem Biol. 2017;13:350–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua SL, Liu Y, Yam JK, Chen Y, Vejborg RM, Tan BG, et al. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat Commun. 2014;5:4462. [DOI] [PubMed] [Google Scholar]
- 34.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–73. [DOI] [PubMed] [Google Scholar]
- 35.Bernier SP, Ha DG, Khan W, Merritt JH, O’Toole GA. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res Microbioly. 2011;162:680–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Y, Xi N, Hai E, Zhang X, Guo J, Lin Z, et al. PA0575 (RmcA) interacts with other c-di-GMP metabolizing proteins in Pseudomonas aeruginosa PAO1. J Gen Appl Microbiol. 2023;68:232–41. [DOI] [PubMed] [Google Scholar]
- 37.Kulasekara HD, Ventre I, Kulasekara BR, Lazdunski A, Filloux A, Lory S. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol Microbiol. 2005;55:368–80. [DOI] [PubMed] [Google Scholar]
- 38.Marvig RL, Sommer LM, Molin S, Johansen HK. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet. 2015;47:57–64. [DOI] [PubMed] [Google Scholar]
- 39.Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. The second messenger bis-(3’-5’)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol Microbiol. 2007;65:876–95. [DOI] [PubMed] [Google Scholar]
- 40.Liao J, Schurr MJ, Sauer K. The MerR-like regulator BrlR confers biofilm tolerance by activating multidrug efflux pumps in Pseudomonas aeruginosa biofilms. J Bacteriol. 2013;195:3352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, et al. Fluorescence-based reporter for gauging cyclic Di-GMP levels in Pseudomonas aeruginosa. Appl Environ Microbiol. 2012;78:5060–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones CJ, Wozniak DJ. Congo Red Stain identifies Matrix overproduction and is an indirect measurement for c-di-GMP in many species of Bacteria. Methods Mol Biol. 2017;1657:147–56. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava D, Waters CM. A tangled web: regulatory connections between quorum sensing and cyclic Di-GMP. J Bacteriol. 2012;194:4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall CL, Lee VT. Cyclic-di-GMP regulation of virulence in bacterial pathogens. Wiley Interdisciplinary Reviews-RNA. 2018;9. [DOI] [PMC free article] [PubMed]
- 45.Chua SL, Liu Y, Li YY, Ting HJ, Kohli GS, Cai Z et al. Reduced intracellular c-di-GMP Content increases expression of Quorum sensing-regulated genes in Pseudomonas aeruginosa. Front Cell Infect Microbiol. 2017; 7. [DOI] [PMC free article] [PubMed]
- 46.Snider SA, Margison KD, Ghorbani P, LeBlond ND, O’Dwyer C, Nunes JRC, et al. Choline transport links macrophage phospholipid metabolism and inflammation. J Biol Chem. 2018;293:11600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Lopez E, Zhong ZY, Stubelius A, Sweeney SR, Booshehri LM, Antonucci L, et al. Choline uptake and metabolism modulate macrophage IL-1β and IL-18 production. Cell Metabol. 2019;29:1350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2007;292:L312–322. [DOI] [PubMed] [Google Scholar]
- 49.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol. 2004;172:3377–81. [DOI] [PubMed] [Google Scholar]
- 50.McHugh BJ, Wang R, Li HN, Beaumont PE, Kells R, Stevens H, et al. Cathelicidin is a fire alarm, generating protective NLRP3-dependent airway epithelial cell inflammatory responses during infection with Pseudomonas aeruginosa. PLoS Pathogen. 2019;15:e1007694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Son MS, Matthews WJ, Kang Y, Nguyen DT, Hoang TT. Vivo evidence of pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun. 2007;75:5313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell DG, Huang L, VanderVen BC. Immunometabolism at the interface between macrophages and pathogens. Nat Rev Immunol. 2019;19:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonciarz RL, Renslo AR. Emerging role of ferrous iron in bacterial growth and host-pathogen interaction: new tools for chemical (micro)biology and antibacterial therapy. Curr Opin Chem Biol. 2021;61:170–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan XK, Boo ZZ, Chua SL, Chong KHC, Long ZQ, Yang Rl, Zhou YC, Janela B, Chotirmall SH, Ginhoux F, Hu QH, Wu B, Yang L. A bacterial quorum sensing regulated protease inhibits host immune responses by cleaving death domains of innate immune adaptors. Adv Sci, 2023; 2304891. [DOI] [PMC free article] [PubMed]
- 55.Chua SL, Liu Y, Yam JKH, Chen YC, Vejborg RM, Tan BGC et al. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat Commun. 2014;5:4462. [DOI] [PubMed]
- 56.Wargo MJ, Ho TC, Gross MJ, Whittaker LA, Hogan DA. GbdR regulates Pseudomonas aeruginosa plcH and pchP transcription in response to Choline catabolites. Infect Immun. 2009;77:1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickman JW, Harwood CS. Identification of FleQ from as a c-di-GMP-responsive transcription factor. Mol Microbiol. 2008;69:376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited into the Sequence Read Archive database (https://www.ncbi.nlm.nih.gov/sra) with the bioProject accession number: PRJNA1027243.