Abstract
Background and Purpose
This study was an open-label, dose-escalation, phase 1 clinical trial to determine the safety and dose of EN001 for patients with Duchenne muscular dystrophy (DMD). EN001, developed by ENCell, are allogeneic early-passage Wharton’s jelly-derived mesenchymal stem cells that originate at the umbilical cord, with preclinical studies demonstrating their high therapeutic efficacy for DMD.
Methods
This phase 1 clinical trial explored the safety and tolerability of EN001 as a potential treatment option for patients with DMD. Six pediatric participants with DMD were divided into two subgroups of equal size: low-dose EN001 (5.0×105 cells/kg) and high-dose EN001 (2.5×106 cells/kg). All participants were monitored for 12 weeks after EN001 administration to assess its safety. Dose-limiting toxicity (DLT) was evaluated across 2 weeks post administration. Exploratory efficacy was evaluated by measuring serum creatine kinase levels, and functional evaluations—including spirometry, myometry, the North Star Ambulatory Assessment, and the 6-minute walk test—were conducted at week 12 and compared with the baseline values.
Results
No participants experienced serious adverse events related to EN001 injection during the 12-week follow-up period. Mild adverse events included injection-related local erythema, edema, parosmia, and headache, but DLT was not observed. Functional evaluations at week 12 revealed no significant changes from baseline.
Conclusions
These results demonstrated that EN001 are safe and well tolerated for patients with DMD, and did not cause serious adverse events. The efficacy of EN001 could be confirmed through larger-scale future studies that incorporate repeated dosing and have a randomized controlled trial design.
Keywords: mesenchymal stem cells, Duchenne muscular dystrophy, phase 1 clinical trial, cell therapy
Graphical Abstract
INTRODUCTION
Duchenne muscular dystrophy (DMD) affects 1 in 3,500 live male births and is a common neuromuscular X-linked recessive disorder caused by a mutation in DMD that weakens normal dystrophin production.1 Patients with DMD experience progressive loss of muscle mass and eventually lose the ability to walk independently in their teens. Moreover, dysfunction of the dystrophin-glycoprotein complex leads to cardiac dysfunction, respiratory insufficiency, and gradual and irreversible DMD disease progression.2
Various therapeutic targets based on DMD pathogenesis3 have been explored in both preclinical and clinical studies to determine if they alter its natural progression.4 Few therapies have significantly altered the clinical course of DMD despite the global efforts in conducting clinical trials. Corticosteroids such as prednisone and deflazacort remain the gold-standard treatment for improving muscle strength and reducing the inflammation associated with DMD pathogenesis.5 However, long-term corticosteroid use is associated with weight gain, short stature, and osteoporosis.6 As an emerging and promising treatment option, gene therapies that target gene replacement or alteration of the mRNA transcript in DMD are currently being developed and evaluated in clinical trials, with one therapy (delandistrogene moxeparvovec, Sarepta Therapeutics, MA, USA) receiving US Food and Drug Administration (FDA) approval in June 2024.7 Several drugs are also under development that target the pathogenic mechanisms of DMD including anti-inflammation, antifibrosis, and muscle regeneration. Furthermore, cell therapies have been developed for patients with DMD.8
Mesenchymal stem cells (MSCs) are considered promising candidates for treating degenerative disorders such as DMD. Numerous recent clinical trials have found that MSCs are safe and beneficial treatments for various pathologies.9,10 MSCs are mesoderm-tissue-derived cells that can be obtained from autologous or allogeneic sources.11,12 They can differentiate into adipogenic, chondrogenic, and osteogenic lineages, and even undergo myogenic differentiation when engrafted in muscle tissues.13,14,15 Various proteins secreted by MSCs generally exhibit therapeutic effects via paracrine action. These effects include inflammation regulation and immune response inhibition at lesion sites.16,17
Intravenous administration of EN001 was found in previous studies to improve biochemical, pathohistological, and behavioral abnormalities in a DMD mouse model using mdx mice.18,19,20,21 Due to the potential of EN001 in treating DMD, we conducted a phase 1 clinical trial to evaluate the safety and dose-limiting toxicity (DLT) of a single intravenous administration of EN001.
METHODS
Study design
An open-label, dose-escalating phase 1 clinical trial was conducted to determine the safety and dose of EN001 in patients with DMD. The protocol and consent form were reviewed and approved by the Ministry of Food and Drug Safety of the Republic of Korea (approval no. 33735) and the Institutional Review Board of Samsung Medical Center (IRB No. 2021-09-075).
The sample size was determined using the traditional 3+3 cohort design method. The dose of EN001 was increased in a stepwise manner, starting at a low dose that was presumed safe based on preclinical experiments. Subjects in each group were monitored for 12 weeks after EN001 administration to assess safety. DLT was evaluated for 2 weeks after administration, and was defined as adverse events at grade 3 or higher on version 5.0 of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE).22 The Safety Review Committee reviewed all safety data to determine the dose escalation once the DLT of all subjects in the low-dose group was evaluated.
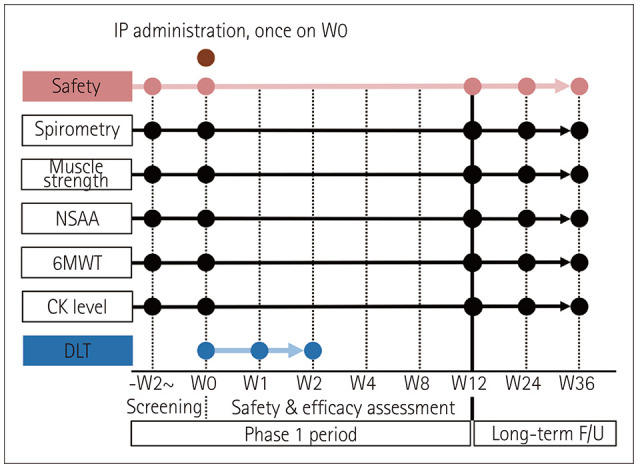
The serum creatine kinase (CK) level was measured at each visit to evaluate exploratory efficacy. Moreover, functional evaluations (spirometry, myometry, North Star Ambulatory Assessment [NSAA], and the 6-minute walk test [6 MWT]) were performed at week 12 and compared with baseline. The phase 1 clinical trial was conducted for 12 weeks (Fig. 1). Long-term follow-up studies will be conducted for up to 5 years after EN001 administration. The interim data at 24 and 36 weeks compared with baseline data are presented as Supplementary Figs. 1, 2, 3, 4 (in the online-only Data Supplement).
Fig. 1. Study design. 6MWT, six-minute walk test; CK, creatine kinase; DLT, dose-limiting toxicity; F/U, follow-up; IP, investigational product; NSAA, North Star Ambulatory Assessment; W, week.
Participants
All subjects were registered from January 18, 2022 to October 8, 2022. The inclusion criteria were as follows: 2–18 years old, male, carrying DMD mutations, phenotypic evidence of DMD (proximal muscle weakness, waddling gait, and Gowers’ sign), and receiving a stable dose of systemic corticosteroids that was expected to remain constant throughout the study. The exclusion criteria were as follows: left ventricular ejection fraction (LVEF) <50% on echocardiography, requiring respiratory assistance, receiving invasive mechanical ventilation through tracheostomy, exposure to gene therapy or ataluren within 24 weeks of screening, or clinically significant laboratory results obtained during screening.
Cell preparation
The procedure for manufacturing the Wharton’s jelly-derived MSCs (WJ-MSCs; EN001) was reviewed and approved by the Institutional Review Board of Samsung Medical Center (Seoul, Republic of Korea; approval number: 2016-07-102). The umbilical cords were donated by pregnant mothers who gave informed consent. A batch of WJ-MSCs was extracted from a single umbilical cord and cultured according to the standard operating procedures of the Good Manufacturing Practice facility of ENCell (Seoul, Republic of Korea).
In brief, WJ-MSCs were expanded by triple sub-passaging to obtain a sufficient number of stem cells, which took an average of 20 days. The composition of the conditioned medium was Minimum Essential Media alpha (MEMα; cat. no. 12571, Gibco, Carlsbad, CA, USA) supplemented by 10% fetal bovine serum (cat. no. 16000, Gibco), and 0.25 µg/mL gentamicin (cat. no. 15710, Gibco). The culture vessels were incubated at 37℃ with 5% CO2. WJ-MSCs at passage 3 were prepared at 5.0×106 cells/10 mL/cryobag and suspended using a cryopreservation solution developed by ENCell (patent application number 10-2023-0025103, Republic of Korea). The cryobags containing EN001 were stored at −196℃ in liquid nitrogen until they were used. Various specifications were evaluated to confirm that EN001 met the predefined quality attributes, including appearance, sterility, mycoplasma testing, endotoxin, total number of cells, cell viability, and flow cytometry (identity: CD44, CD73, CD90, CD105, and CD166; purity: CD11b, CD14, CD19, CD34, CD45, and HLA-DR). This trial used a batch of EN001 that matched the predefined criteria.
Administration procedures
EN001 were supplied in a frozen state. One bag of EN001 (10 mL) was intravenously administered within 10 min after being thawed in a bath of 37-℃ water. Each subject received hydrocortisone (1–2 mg/kg), lorazepam (0.1 mg/kg; maximum 2 mg), ondansetron (5 mg/m2), and chlorpheniramine (1 mg for 2- to 6-year-olds and 2 mg for 6- to 12-year-olds) as premedication at the discretion of the investigator.
The EN001 dose to be applied in the clinical trial was determined based on previous preclinical tests.21 An effective dose of 5×104 cells/20 g (2.5×106 cells/kg) was set as the high dose, and a one-fifth dose of 1×104 cells/20 g (5.0×105 cells/kg) was set as the low dose. The vital signs, physical state, and electrocardiograms of each subject were monitored throughout EN001 administration to closely observe any infusion-related adverse events such as fever, hypertension, hypotension, tachycardia, hyperventilation, rash, and shock. The monitored parameters included blood pressure, body temperature, pulse rate, respiration rate, and oxygen saturation.
Outcomes
Safety was evaluated as the primary endpoint according to the occurrence of any adverse events, serious adverse events related to EN001 administration, DLT, and clinically significant abnormalities. Subjects were assessed at each visit to identify adverse events and serious adverse events related to EN001 administration. All adverse events and serious adverse events related to EN001 administration were graded from 1 to 5 according to NCI-CTCAE (version 5.0). A physical examination, vital sign monitoring, blood cell count, urinalysis, and tests of renal function, blood chemistry, and coagulation were conducted at each visit.
The exploratory efficacy outcomes included serum CK, spirometry, myometry, NSAA, and 6 MWT.
Serum CK measurements
Serum CK was measured in a blood test at each visit, and all other variables were measured at week 12.
Myometry measurements
The muscle strengths of the left and right knee extensors and elbow flexors were measured using myometry with a portable hand-held dynamometer (MicroFET2, Hoggan Scientific, Salt Lake City, UT, USA) according to a standardized procedure to assess upper and lower limb strength. Myometry was measured at baseline and week 12.
North Star Ambulatory Assessment
The NSAA is a validated clinical scale comprising 17 items with 3 response categories (2, 1, and 0) that measure motor function and disease progression. This assessment was conducted at baseline and week 12.
Six-minute walk test
The 6 MWT was performed according to a standardized procedure, which involved walking at a normal pace across 30 m for 6 minutes. The test was conducted indoors on a flat, no slippery surface. The 6 MWT was measured at baseline and week 12.
Statistical analyses
Continuous variables were presented as mean±standard deviation values, and categorical variables were presented as frequencies and proportions in each group.
Safety analysis and drug tolerance assessment
All adverse events among all collected reports were analyzed. Adverse events were summarized according to the Medical Dictionary for Regulatory Activities (version 25.1).23 The numbers and proportions of subjects who experienced adverse events and their occurrence were presented for each group.
Exploratory efficacy
Within-group (low- and high-dose groups) comparisons of the exploratory efficacy variables were analyzed using the Wilcoxon signed-rank test. Between-group comparisons of the change in the results for each functional test were analyzed at each visit using the exact Wilcoxon rank-sum test. Any increase in the number of type I errors due to multiple comparisons was corrected using the Bonferroni method where necessary. Statistical Analysis System software (version 9.4, SAS Institute, Cary, NC, USA) was used for all statistical analyses.
RESULTS
Participants
Seven subjects who met the inclusion criteria were screened, and six were enrolled in this clinical trial (Supplementary Fig. 5 in the online-only Data Supplement). All of the clinical trial procedures were conducted with the written informed consent of the subjects or their legally acceptable representatives. Seven visits were conducted, which included screening visits. The three subjects in each of the low- and high-dose groups received EN001 at 5.0×105 cells/kg and 2.5×106 cells/kg, respectively. All subjects who received EN001 were included in the analysis group.
Baseline data
The enrolled subjects were aged 10.0–15.8 years: 11.9±2.4 years and 12.7±2.8 years in the low- and high-dose groups, respectively (Table 1). The time since DMD diagnosis ranged from 2.4 to 13.9 years. All subjects were ambulatory and received a stable dose of glucocorticoid throughout the clinical trial. Echocardiography indicated that LVEF was ≥50% before EN001 administration in all subjects. No other functional or structural abnormalities were observed. Subjects were administered EN001 according to the clinical trial protocol based on their body weight.
Table 1. Baseline demographic information of all enrolled subjects.
| Patient | Low-dose group | L1 | L2 | L3 | High-dose group | H1 | H2 | H3 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | 11.9±2.4 | 11.0 | 10.0 | 14.6 | 12.7±2.8 | 15.8 | 11.8 | 10.5 | ||
| Sex | - | Male | Male | Male | - | Male | Male | Male | ||
| Height (cm) | 132.9±1.2 | 132.7 | 131.9 | 134.2 | 130.0±4.1 | 132.6 | 132.1 | 125.2 | ||
| Weight (kg) | 36.7±4.1 | 32.8 | 41.0 | 36.4 | 35.4±6.5 | 37.9 | 28.0 | 40.2 | ||
| Dystrophin gene mutation | - | Exon 51 deletion | Exons 43–79 deletion c.6254G>A, p.W2085* | Exons 34–51 deletion | - | Exon 43 duplication | Exon 2 duplication | Exon 18 c.1901C>G p.S634X | ||
| Duration of disease (yr) | 7.3±5.9 | 5.6 | 2.4 | 13.9 | 8.5±3.2 | 11.8 | 5.4 | 8.2 | ||
| Clinical manifestations | - | Calf muscle pseudo-hypertrophy, Gowers’ sign, waddling gait | Calf muscle pseudo-hypertrophy, Gowers’ sign, waddling gait | Calf muscle pseudo-hypertrophy, Gowers’ sign, waddling gait | - | Calf muscle pseudo-hypertrophy, Gowers’ sign, waddling gait | Calf muscle pseudo-hypertrophy, Gowers’ sign, waddling gait | Calf muscle pseudo-hypertrophy, Gowers’ sign, waddling gait | ||
| Glucocorticoid used | - | Deflazacort at 24 mg/day | Prednisolone at 20 mg/day | Deflazacort at 18 mg/day | - | Deflazacort at 24 mg/day | Deflazacort at 18 mg/day | Deflazacort at 24 mg/day | ||
| Baseline characteristics | ||||||||||
| Serum CK (IU/L) | 6,095±1,689 | 8,042 | 5,215 | 5,028 | 8,244±5,740 | 3,477 | 14,615 | 6,639 | ||
| Total NSAA score (points) | 20.3±4.0 | 18 | 25 | 18 | 16.7±10.7 | 8 | 28 | 14 | ||
| 6 MWT (m) | 299.4±59.6 | 329.5 | 338.0 | 230.7 | 295.9±43.1 | 274.2 | 345.6 | 268.0 | ||
| Muscle strength (lbs) | ||||||||||
| Knee extensor | 9.3±1.6 | 8.4 | 11.2 | 8.4 | 7.8±3.4 | 4.7 | 11.4 | 7.2 | ||
| Elbow flexor | 5.1±2.7 | 3.0 | 8.1 | 4.2 | 3.8±2.1 | 1.9 | 3.3 | 6.1 | ||
| Spirometer | ||||||||||
| FVC% (%) | 83.7±17.5 | 66 | 101 | 84 | 51.3±7.2 | 99 | 87 | 100 | ||
| MIP (cm H2O) | 50.3±8.4 | 46 | 60 | 45 | 51.3±12.1 | 47 | 42 | 65 | ||
| MEP (cm H2O) | 45.7±10.8 | 41 | 58 | 38 | 45.0±6.1 | 41 | 52 | 42 | ||
| PEFR (L/min) | 198.1±26.9 | 226.2 | 195.5 | 172.6 | 221.4±15.5 | 205.9 | 236.9 | 221.3 | ||
| IP administration information | ||||||||||
| IP infusion volume (5.0×105 cells/mL) | 36.7±4.1 | 32.8 | 41.0 | 36.4 | 176.3±32.1 | 187.8 | 140.0 | 201.0 | ||
| Infusion-related adverse events | - | None | None | None | - | None | None | None | ||
Values are presented as mean±standard deviation or numbers only.
6 MWT, six-minute walk test; CK, creatine kinase; FVC%, percentage of predicted forced vital capacity; H, high-dose group; IP, investigational product; L, low-dose group; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; NSAA, North Star Ambulatory Assessment; PEFR, peak expiratory flow rate.
Adverse events
There were 18 adverse events, and all subjects experienced at least 1 after EN001 administration: 6 and 12 in the low- and high-dose groups, respectively (Table 2). No serious adverse events or unexpected serious adverse events were observed. Injection-related local erythema, edema, and pain were reported in two subjects, both of which subsided in 1 day. Three patients complained of headaches, and one reported parosmia (discomfort related to the smell of the investigational product [IP]). No subjects experienced DLT or an infusion-related serious adverse event such as fever, hypertension, hypotension, tachycardia, hyperventilation, rash, or shock.
Table 2. Summary of adverse events during the study period.
| Adverse event (according to NCI-CTCAE version 5.0) | Low-dose group (n=3) | High-dose group (n=3) | |||||
|---|---|---|---|---|---|---|---|
| No. of patients | No. of adverse events | Grade | No. of patients | No. of adverse events | Grade | ||
| Gastrointestinal disorders | 1 | 1 (5.6) | 1 | - | - | - | |
| Dyspepsia | 1 | 1 (5.6) | 1 | - | - | - | |
| General disorders and administration-site conditions | 1 | 2 (11.1) | 1 | 2 | 6 (33.3) | 1 | |
| Chest pain | 1 | 1 (5.6) | 1 | - | - | - | |
| Infusion-site erythema | - | - | - | 2 | 2 (11.1) | 1 | |
| Infusion-site pain | - | - | - | 2 | 2 (11.1) | 1 | |
| Infusion-site swelling | - | - | - | 2 | 2 (11.1) | 1 | |
| Tenderness | 1 | 1 (5.6) | 1 | - | - | - | |
| Infections and infestations | 2 | 2 (11.1) | 1 | 2 | 2 (11.1) | 1 | |
| SARS-CoV-2 | 2 | 2 (11.1) | 1 | - | - | ||
| Nasopharyngitis | - | - | - | 2 | 2 (11.1) | 1 | |
| Musculoskeletal and connective-tissue disorders | - | - | - | 1 | 1 (5.6) | 1 | |
| Bone pain | - | - | - | 1 | 1 (5.6) | 1 | |
| Nervous system disorders | 1 | 1 (5.6) | 1 | 2 | 3 (16.7) | 1 | |
| Headache | 1 | 1 (5.6) | 1 | 2 | 2 (11.1) | 1 | |
| Parosmia* | - | - | - | 1 | 1 (5.6) | 1 | |
Values are presented as n (%) or numbers only.
*Reported adverse event term: discomfort related to the IP smell.
-, no data; IP, investigational product; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
An additional 3 adverse events were reported (increasing the total to 21) in follow-up data up to 36 weeks, all of which were grade 1. No delayed adverse events related to EN001 were reported (Supplementary Table 1 in the online-only Data Supplement).
Outcomes
Spirometry, myometry, NSAA, 6 MWT, and the serum CK level were the exploratory efficacy outcomes.
Spirometry
Spirometry tests were conducted on all six participants at baseline. All subjects completed the test at week 12, except for subject H2 because of bone pain. In terms of the forced vital capacity percentage (FVC%), the low-dose group presented a 6.67%±6.81% increase from baseline at week 12, and the high-dose group presented a 1.00%±1.41% increase with no significant difference (Fig. 2). Four of the remaining five participants presented slight improvement to or maintenance of FVC%, which was not significant. Some data from other spirometry tests (maximal inspiratory pressure [MIP], maximal expiratory pressure [MEP], and peak expiratory flow rate) exhibited small increases relative to baseline, but none were attributable to the intervention (Supplementary Fig. 1 in the online-only Data Supplement).
Fig. 2. Changes in spirometer parameters following the administration of WJ-MSCs. Subject H2 was unable to perform a spirometry test because of bone pain at week 12 and was excluded from the mean and SD calculations. Measurements at a specific time point are marked with a circle, triangle, or diamond. The group average is presented as a bar graph indicating mean and SD values. No statistical difference was observed. A: FVC%. B: MIP. C: MEP. D: PEFR. FVC%, percentage of predicted forced vital capacity; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; PEFR, peak expiratory flow rate; SD, standard deviation; WJ-MSCs, Wharton’s jelly-derived mesenchymal stem cells.
Myometry
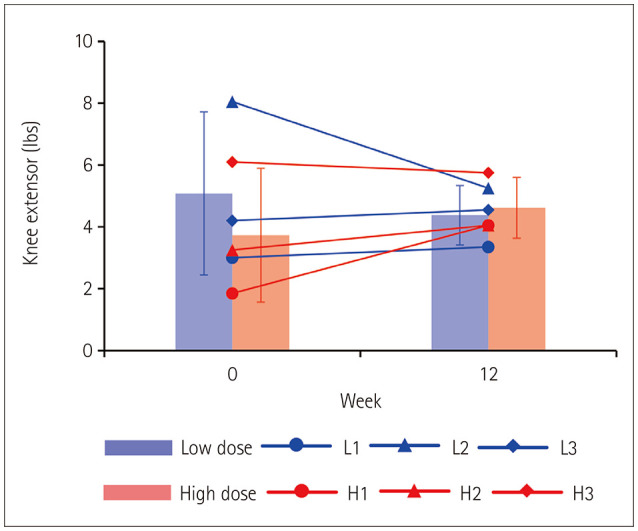
There were various changes between the dose groups and muscle regions as follows: knee extensor muscle strength in the low-dose group decreased by 0.70±1.82 lbs after 12 weeks, while that in the high-dose group increased by 0.88±1.28 lbs (Fig. 3); specifically, four of six test subjects presented increased knee extensor muscle strength. The knee extensor muscle strength in the high-dose group had even increased at 24 and 36 weeks of follow-ups (Supplementary Fig. 2A in the online-only Data Supplement). However, the elbow flexor muscle strength slightly decreased up to the 36-week follow-up (Supplementary Fig. 2B in the online-only Data Supplement).
Fig. 3. Changes in muscle strength following WJ-MSC administration. Muscle strength was measured for the knee extensor at the quadriceps and for the knee extensors of both right and left knees, and their values were averaged. Measurements at a specific time point are marked with a circle, triangle, or diamond. The group average is presented as a bar graph indicating mean and SD values. No statistical difference was observed. SD, standard deviation; WJ-MSC, Wharton’s jelly-derived mesenchymal stem cell.
North Star Ambulatory Assessment
The NSAA yielded exploratory efficacy outcomes for overall motor function. The score for those who received low-dose EN001 decreased by 3.33±4.51 points from baseline to 12 weeks, and that for the high-dose groups decreased by 3.00±3.00 points (Table 3). Regarding individual subject changes, subject L3 improved from 18 to 19 points, and subject H3 remained constant at 14 points. Two of the six test subjects showed increased or sustained scores. The trend line of NSAA scores appeared mostly consistent across the 36 weeks of follow-up, except for the increase in subject H1 (Supplementary Fig. 3 in the online-only Data Supplement).
Table 3. Changes in NSAA following the administration of WJ-MSCs.
| Test | Low-dose group | High-dose group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 (11.0)* | L2 (10.0)* | L3 (14.6)* | H1 (15.8)* | H2 (11.8)* | H3 (10.5)* | |||||||
| W0 | W12 | W0 | W12 | W0 | W12 | W0 | W12 | W0 | W12 | W0 | W12 | |
| 1. Stand | 1 | 1† | 2 | 1 | 1 | 1† | 1 | 1† | 2 | 1 | 1 | 1† |
| 2. Walk | 1 | 1† | 1 | 1† | 1 | 1† | 1 | 1† | 1 | 1† | 1 | 1† |
| 3. Stand up from chair | 1 | 0 | 1 | 1† | 1 | 1† | 0 | 0 | 1 | 1† | 1 | 1† |
| 4. Stand on one leg – right | 2 | 2† | 1 | 1† | 2 | 2† | 1 | 1† | 2 | 2† | 1 | 0 |
| 5. Stand on one leg – left | 2 | 2† | 2 | 2† | 2 | 2† | 1 | 0 | 2 | 2† | 2 | 1 |
| 6. Climb box step – right | 1 | 1† | 2 | 1 | 1 | 1† | 0 | 0 | 2 | 1 | 0 | 1‡ |
| 7. Descend box step – right | 1 | 1† | 2 | 2† | 1 | 1† | 0 | 0 | 1 | 1 | 1 | 1† |
| 8. Climb box step – left | 1 | 1† | 2 | 1 | 1 | 1† | 0 | 0 | 2 | 1 | 1 | 1† |
| 9. Descend box step – left | 1 | 1† | 2 | 1 | 1 | 1† | 0 | 0 | 1 | 1† | 0 | 1‡ |
| 10. Lift head from supine | 2 | 2† | 1 | 0 | 2 | 2† | 2 | 1 | 2 | 2† | 2 | 2† |
| 11. Sit down | 1 | 1† | 2 | 1 | 1 | 2‡ | 1 | 1† | 2 | 2† | 1 | 1† |
| 12. Rise from floor | 1 | 1† | 1 | 1† | 1 | 1† | 0 | 0 | 1 | 1† | 1 | 1† |
| 13. Stand on heels | 1 | 0 | 1 | 1† | 0 | 0 | 0 | 0 | 1 | 1† | 0 | 0 |
| 14. Jump | 1 | 0 | 2 | 2† | 2 | 2† | 0 | 0 | 2 | 1 | 1 | 1† |
| 15. Hop on right leg | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| 16. Hop on left leg | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2† | 0 | 0 |
| 17. Run 10 m | 1 | 1† | 1 | 1† | 1 | 1† | 1 | 0 | 1 | 1† | 1 | 1† |
| Total score | 18 | 15 | 25 | 17 | 18 | 19‡ | 8 | 5 | 28 | 22 | 14 | 14† |
| Average (difference from baseline) | 3.33±4.51 | 3.00±3.00 | ||||||||||
*Subject number (age at baseline, years); †Items that remained constant at the baseline score, unless the baseline score was 0, even if the score remained the same; ‡Items that improved from baseline.
NSAA, North Star Ambulatory Assessment; W, week; WJ-MSC, Wharton’s jelly-derived mesenchymal stem cell.
Six-minute walk test
The low- and high-dose groups presented decreases in 6 MWT scores of −13.77±18.02 m and −47.37±36.92 m from 12 weeks to baseline, respectively. One of the test subjects increased their 6 MWT score (Supplementary Fig. 4 in the online-only Data Supplement).
CK level
CK levels were reduced compared with baseline in most visits after EN001 administration (Fig. 4); these reductions were observed until 12 weeks. However, some subjects presented a rapid increase in CK level during the SARS-CoV-2 infection period. CK levels were reduced or maintained in both groups according to the follow-up data up to 36 weeks, except for the high-dose group at week 36 (Supplementary Table 2 in the online-only Data Supplement).
Fig. 4. Changes in serum CK level following WJ-MSC administration. Serum CK levels were measured from baseline to week 12 post-WJ-MSC administration. Although the CK levels increased rapidly at the time of SARS-CoV-2 infection in some subjects, all data were included in the analysis. Measurements at a specific time point are marked with a circle, triangle, or diamond. The group average is presented as a bar graph. No statistical difference was observed. CK, creatine kinase; WJ-MSC, Wharton’s jelly-derived mesenchymal stem cell.

Cardiac function as assessed using echocardiography
Two patients in each group underwent follow-up examinations at 6–8 months after EN001 administration, the results of which revealed good left ventricular function and trivial tricuspid regurgitation. Follow-up examinations were conducted after 1 year of treatment on four patients, including the above-mentioned two patients, and all results were normal.
DISCUSSION
Six pediatric patients with DMD were administered a single EN001 dose in this phase 1 clinical trial, demonstrating its safety and tolerability. No patients in this study experienced serious adverse events related to EN001 administration during the 12-week follow-up. Only mild adverse events were observed, such as skin reactions at the injection site, headache, and parosmia. The extended follow-up data revealed no functional deterioration of DMD. These results validated the safety and tolerability of single-dose EN001, which lays the groundwork for forthcoming clinical trials to validate the efficacy and safety of multiple dosages of EN001.
The mechanisms underlying EN001 are anti-apoptosis and anti-fibrosis. WJ-MSCs were injected into the tail vein of mdx mouse models in previous preclinical studies. Intravenously administered WJ-MSCs secreted various trophic factors that can address the many pathological symptoms of DMD, instead of direct differentiation into skeletal muscle. More specifically, WJ-MSCs secrete lymphotactin, also known as XCL1. This protein protects against the apoptosis of skeletal muscle cells. MMP-1 secreted by WJ-MSC reduces skeletal muscle fibrosis by upregulating microRNA-499.18,21,24
In comparison with existing commercially available MSC therapies, EN001 as early-passage MSC therapy exhibit several advantages due to their therapeutic efficacy and safety. In general, a subcultivation process must be conducted to produce a sufficient number of MSCs for supplying clinical trials or commercial markets. The total cultivation time increases with the number of subcultivations, accelerating cellular senescence and ultimately reducing the therapeutic potential of stem cells.25,26,27 Recent studies have demonstrated that the therapeutic efficacy of MSCs decreases following serial subcultivation. Specifically, the progress of subcultivation changes the morphology and decreases the differentiation potential of MSCs, resulting in a reduced ability to migrate to lesion sites.26 Minimizing the total cultivation time and number of subcultivations is therefore vital to prevent senescence in MSCs. This was supported by comparing preclinical experiments that the number of MSCs administered at an early passage was approximately ten times lower—with better therapeutic effect—than those administered at a late passage.21,28,29,30 Therefore, EN001 (WJ-MSCs at passage 3) were expected to produce a higher therapeutic efficacy in patients with DMD, accompanied by a reduced dose of EN001 compared with other MSC therapies.
The most significant advantage of utilizing early-passage MSC therapy is its safety profile. Early-passage MSCs may serve to minimize the risk of infusion-related and unexpected adverse events due to reduced doses and a lower immunogenicity profile.27 Serious adverse events therefore did not occur during the follow-up period from EN001 administration. Instead, only transient and mild adverse events were reported, including infusion-site pain, swelling, and erythema, all of which improved within several hours of the infusion. Specifically, one patient complained that IP smelled strange for approximately 1 day. The other reported adverse events were mild dyspepsia and headache, which disappeared within several hours after administration ceased. Two published studies on MSC therapy in DMD found similarly to our trial that no any adverse events occurred during 2–3 years of follow-up.10,31 Most clinical studies of MSC administration not aimed at DMD also found MSCs to be safe and feasible, with only minor side effects.32,33 MSC therapy is therefore considered safe when compared with other modalities such as gene and pluripotent cell therapies.
Further regarding safety, the impact of the formulation (or diluent) within the body is as significant as that of the therapeutics used. The most common side effect in cell therapy is transient fever, due to a reaction to the diluent of residual phosphate-buffered saline (PBS).33,34 The present study overcame this by applying an advanced EN001 formulation that comprised xeno-free, injectable, and compatible substances with WJ-MSCs (patent application number 10-2023-0025103, Republic of Korea). Compared with general diluents such as normal saline, PBS, or MEMα, this improved stability and reduced the formation of WJ-MSC aggregates (Supplementary Fig. 6 in the online-only Data Supplement). No adverse effects were observed when the diluent was administered into the tail vein of athymic mice. The advanced EN001 formulation was therefore determined to be safe with no incidence of fever, and did not affect how the phase 1 clinical trial results were interpreted.
This study evaluated the efficacies of EN001 as the exploratory efficacy outcomes, and the endpoints were determined according to preclinical experiments.18,21 We assessed changes in muscle strength using several functional tests. Although we observed increased grip strength in the mdx mouse model after WJ-MSC administration, it was difficult to confirm improved extremity muscle strength using myometry in the short-term evaluation period (12 weeks) of this phase 1 clinical trial. The NSAA and 6 MWT also presented similar results to myometry, with no differences observed between the low- and high-dose groups. Although the score for each item of the NSAA increased, total NSAA scores remained the same or reduced below the baseline values. Such a decreased NSAA score was also found by Muntoni et al.35 These results were therefore insufficient to demonstrate the exploratory efficacy of a single EN001 administration in patients with DMD. Evaluating efficacy outcomes necessitates long-term follow-up due to the slowly progressive nature of DMD, with most clinical studies adopting a 1- or 2-year period. The present phase 1 clinical trial focused on proving the safety and tolerability of EN001, which required a shorter study period. The efficacy outcomes will need to be assessed in subsequent long-term controlled clinical trials.
Serum CK levels showed a decreasing trend from EN001 administration to week 12 (Fig. 4) but without significance. Although serum CK levels have a low clinical significance as a marker of disease activity, the decreasing trend observed from baseline to week 12 may indicate a temporary suppression of skeletal muscle cell destruction and inflammation. This suppression is potentially linked to the anti-apoptotic, anti-inflammatory, and anti-fibrotic effects of WJ-MSCs. Serum CK levels returned to their baseline level after 12 weeks in all patients. It could not be determined in this study whether the decreasing trend of serum CK level was a transient fluctuation or a therapy-induced effect. Our future studies will investigate whether repeated EN001 administrations can achieve sustained suppression of muscle cell destruction and inflammation.
In the pulmonary function test, lung capacity (quantified as FVC%) presented an increasing trend at week 12 in the high- and low-dose groups (Fig. 2), and at week 36 in the low-dose group (Supplementary Fig. 1 in the online-only Data Supplement), but without significance. Indicators of respiratory muscle strength (MIP and MEP) also did not differ significantly between baseline and the 12-week follow-up. MIP and MEP are used to assess the inspiratory and expiratory abilities in patients with muscular dystrophy and serve as clinical criteria for weaning off ventilatory support. This study found no improvement in respiratory muscle strength during either the short- or long-term follow-up period of single-dose EN001. Again, repeated EN001 administration may yield more evidence for improved muscle function, including proximal muscle power and pulmonary function.
Akat and Karaöz8 identified 12 systematic reviews of cell therapies for DMD reported on in public databases. Among them, four cases received MSC therapy and two received cardiosphere-derived cell (CDC) therapy. However, only two reports on CDC therapies for DMD were accessible on clinicaltrials.gov: HOPE-Duchenne36 and HOPE-2.37 CAP-1002, which is one of the leading cell therapies for DMD, is a CDC therapy that achieves skeletal and cardiac muscle growth and protection. According to the recent report of the HOPE-2 phase 2 trial (clinicaltrials.gov ID NCT03406780), four CAP-1002 administrations at 3-month intervals were safe and well tolerated in eight patients with DMD. Moreover, McDonald et al.37 demonstrated that CAP-1002 slows DMD progression at 12 months post administration, with significant changes in mid-level elbow performance according to the upper limb score (performance of upper limb [PUL] 1.2, the primary efficacy endpoint) and cardiac magnetic resonance imaging (MRI) scoring (exploratory endpoint) compared with a placebo.
The two investigational drugs (EN001 and CAP-1002) are similar in that both are allogeneic cell therapies and require pretreatment to inhibit allergic responses (Table 4). Both are allogeneic cell therapeutics but with different sources and cell types: EN001 are MSCs obtained from Wharton’s jelly, which is part of the umbilical cord, whereas CAP-1002 are CDC obtained from myocardial tissues. The expected mechanism of action is the same as for paracrine effects, where several proteins, exosomes, or miRNAs are secreted and indirectly promote immune modulation, anti-fibrosis, and muscle regeneration. However, the efficacy endpoints are different: the outcome measures for EN001 include serum CK level, ambulatory assessment, and respiratory function, while those for CAP-1002 are PUL and cardiac MRI evaluations. The intended therapeutic effects of EN001 therefore focused on lower-limb skeletal muscle and respiratory muscle and those of CAP-1002 focused on upper-limb skeletal muscle and cardiac muscle. These two types of studies are complementary, and there remains a need to confirm the efficacy of EN001 in nonambulatory patients.
Table 4. Comparison of EN001 and CAP-1002.
| Category | EN001 | CAP-1002 |
|---|---|---|
| Trial design | Open-label, dose-escalation, phase 1 trial | Multicenter, randomized, double-blind, placebo-controlled, phase 2 trial |
| Cell type | Allogeneic, mesenchymal stem cells | Allogeneic, cardiosphere-derived cells |
| Dosage | Low-dose group: 5.0×105 cells/kg High-dose group: 2.5×106 cells/kg Intravenous, single infusion |
Experimental: 1.5×108 cells Placebo Intravenously every 3 months for a total of four infusions |
| Pretreatment | H1 blocker, glucocorticoid, benzodiazepines, ondansetron, and acetaminophen 30 minutes before infusion | H1 and H2 blockers at 1 h before infusion. Glucocorticoids at 12–14 h, and again 2–3 h after infusion |
| Primary endpoint | Safety at 3 months | PUL 1.2 at 12 months, and safety at 12 months* |
| Secondary endpoint | Serum CK level and functional test (NSAA, 6 MWT, myometry, and lung capacity) at 3 months | PUL 2.0 at 3, 6, and 9 months; cardiac MRI assessment at 6 and 12 months |
| Expected mechanism of action | Paracrine action | Paracrine action |
*OLE HOPE-2 open-label extension study.
6 MWT, six-minute walk test; CK, creatine kinase; MRI, magnetic resonance imaging; NSAA, North Star Ambulatory Assessment; OLE, open-label extension; PUL, performance of upper limb.
It was difficult to directly compare these two newly developed drugs due to differences in study design, dosage, and efficacy endpoints. However, Weil et al.38 investigated the comparative efficacies of allogeneic MSC and CDCs in swine. In that study, both MSCs and CDCs exhibited similar therapeutic effects on myocardial regeneration in a large animal model of the hibernating myocardium. It can therefore be expected that EN001 and CAP-1002 will exert similar therapeutic effects on muscle regeneration in patients with DMD, despite our study not focusing on improvements in cardiac function.
Only a few treatment options currently exist that slow disease progression and improve motor function in DMD. Despite their limitations, oral corticosteroids are the current gold-standard treatment for DMD, as described in the Introduction. However, corticosteroids can help retain the ability to walk independently for an additional ≥5 years as well as preserve lung function and prevent scoliosis in patients.39,40 Regarding gene-modifying therapies, ataluren helps to produce full-length dystrophin protein via ribosomal readthrough of mRNA having a premature stop codon from nonsense mutations.41 Antisense oligonucleotides therapies are currently approved by the US FDA and used in clinical patients with the mutation corresponding to each drug, which includes eteplirsen and drisapersen that target deletions including exon 51, golodirsen, viltolarsen, and casimersen, which follow a similar mechanism by targeting the deletion of exons 53 and 45.42 However, these treatments are restricted to patients with specific genetic mutations, and their effectiveness has yet to be established.2,3,7
Furthermore, delandistrogene moxeparvovec has recently been approved by the US FDA for treating both ambulatory pediatric patients with DMD aged 4–5 years who have a confirmed DMD mutation and nonambulatory patients aged ≥4 years.43 The approval process was accelerated because of the expression of therapy-driven micro-dystrophin observed in patients treated using this novel therapy,44 but completing the approval process may depend on confirmatory trials. These gene therapies involve inserting part of the dystrophin gene into the patient via an adeno-associated virus with a restricted containing load, which results in a phenotype similar to Becker muscular dystrophy in which the muscle weakness progresses gradually. Crucially, no therapeutics are currently available for muscular dystrophy aside from those for DMD. WJ-MSCs such as EN001 are therefore valuable alternative treatments that can be used in addition to current gene-modifying treatments. Furthermore, these treatments have the potential for expansion to other forms of muscular dystrophy for which no cure currently exists.
This study was the first phase 1 clinical trial applying MSCs to treat patients with DMD. However, its limitations as a single dosing study involving a small sample and the presence of SARS-CoV-2 in some patients during the 12-week follow-up period made it difficult to confirm the efficacy of EN001. Similar to this study, a phase 1 clinical trial of gene therapy that used a chimeric adeno-associated virus capsid variant (AAV 2.5) to deliver the dystrophin gene was conducted on six patients with DMD.45 Another study recruited eight patients with DMD and demonstrated the safety of exon-45-skipping gene therapy.46 This trend toward small-scale phase 1 trials seems to be consistent when the investigational drug is a cell and gene therapy. The first-in-human trial should be seriously considered because the history of cell and gene therapies is shorter than that of conventional drugs such as small molecules and antibodies, meaning that unexpected adverse events may occur. We plan to continuously assess the safety and efficacy of EN001 through long-term follow-up studies and additional clinical trials to overcome this limitation, including by performing a multicenter, randomized, double-blind, placebo-controlled, phase 1/2 trial in patients with DMD (clinicaltrials.gov ID: NCT06328725).
In conclusion, WJ-MSCs (EN001) were safely administered to pediatric patients with DMD without serious adverse events or DLT. This research has important implications for the clinical application of EN001 as a therapeutic strategy for DMD. Further evaluations of the efficacy of stem-cell therapies and the development of standardized protocols are required through large-scale studies to confirm its therapeutic utility.
Acknowledgments
The authors are grateful to the patients and their families for their participation in this study.
Footnotes
- Conceptualization: Jeehun Lee.
- Data curation: Jiwon Lee.
- Formal analysis: Jiwon Lee, Jeong-Yi Kwon.
- Funding acquisition: Jeehun Lee, Jong Wook Chang, Hong Bae Jeon.
- Investigation: Jeehun Lee, Jiwon Lee.
- Methodology: Jeehun Lee, Mira Kim, Jeong-Yi Kwon.
- Project administration: Jeehun Lee.
- Resources: Jeehun Lee, Jong Wook Chang.
- Software: Jiwon Lee.
- Supervision: Jeehun Lee.
- Validation: Jiwon Lee, Sang Eon Park, Hyeongseop Kim.
- Visualization: Sang Eon Park, Hyeongseop Kim.
- Writing—original draft: Jeehun Lee, Jiwon Lee, Sang Eon Park, Mira Kim.
- Writing—review & editing: Jeehun Lee, Jiwon Lee, Mira Kim, Hyeongseop Kim, Hong Bae Jeon.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Human Wharton’s jelly-derived mesenchymal stem cells were supplied by ENCell Co, Ltd.; the sponsor was not involved in the interpretation of the data, writing of the report, or the decision to submit the manuscript for publication.
Funding Statement: This study was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant: HR22C1363 and HI14C3484). This study was also supported by Future Medicine 2030 Project of the Samsung Medical Center (SMO1240041), and Korean Fund for Regenerative Medicine funded by Ministry of Science and ICT, and Ministry of Health and Welfare (RS-2023-00223069, Republic of Korea).
Availability of Data and Material
All data are incorporated into the article and its online supplementary material.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2024.0299.
Summary of adverse events, including long-term follow-up data
Serum CK level for each subject, including long-term follow-up data
Changes in spirometer parameters including the interim follow-up data collected up to week 36. Data are mean and SD values. Subject H2 was unable to perform a spirometry test because of bone pain at week 12 and was excluded from the calculation of the mean and SD at week 12. The baseline for each group is indicated by a dotted line. The mean for each group at a specific time point is marked with a blue or red circle along with the SD. No statistical difference was observed. A: FVC%. B: MIP. C: MEP. D: PEFR. FVC%, percentage of predicted forced vital capacity; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; PEFR, peak expiratory flow rate; SD, standard deviation.
Changes in muscle strength including the interim follow-up data collected up to week 36. Data are mean and SD values. The muscle strengths of the knee extensor (A) and elbow flexor (B) were measured using the quadriceps and biceps, respectively. Right and left muscles were measured, and their values were averaged. The baseline for each group is indicated by a dotted line. The mean for each group at a specific time point is marked with a blue or red circle along with the SD. No statistical difference was observed. SD, standard deviation.
Changes in the NSAA score for each subject including the interim follow-up data collected up to week 36. Trend line for each subject is indicated. Red and blue lines indicate the low-dose and high-dose groups, respectively. The black dotted line represents the natural history of NSAA score reduction in patients with Duchenne muscular dystrophy. NSAA, North Star Ambulatory Assessment.
Changes in scores for the 6MWT including the interim follow-up data collected up to week 36. Data are mean and SD values. The baseline for each group is indicated by a dotted line. The mean for each group at a specific time point is marked with a blue or red circle along with the SD. No statistical difference was observed. 6MWT, six-minute walk test; SD, standard deviation.
Overview of the study scheme.
Stability test of the EN001 formulation. A: The viability of MSCs was measured using trypan blue staining (magnification: ×4). CS5 and CS10 are commercial products. The conventional cryopreservative solution comprised fetal bovine serum and dimethyl sulfoxide. B: After thawing, the ratio of cell clumping was measured. MSCs were diluted by the EN001 formulation or MEMα. C: Viability of MSCs as measured using trypan blue staining. MSCs were diluted by EN001 formulation or MEMα. Data are mean and SD values. DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; MEMα, Minimum Essential Media alpha; MSCs, mesenchymal stem cells; SD, standard deviation.
References
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Chang M, Cai Y, Gao Z, Chen X, Liu B, Zhang C, et al. Duchenne muscular dystrophy: pathogenesis and promising therapies. J Neurol. 2023;270:3733–3749. doi: 10.1007/s00415-023-11796-x. [DOI] [PubMed] [Google Scholar]
- 3.Bez Batti Angulski A, Hosny N, Cohen H, Martin AA, Hahn D, Bauer J, et al. Duchenne muscular dystrophy: disease mechanism and therapeutic strategies. Front Physiol. 2023;14:1183101. doi: 10.3389/fphys.2023.1183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts TC, Wood MJA, Davies KE. Therapeutic approaches for Duchenne muscular dystrophy. Nat Rev Drug Discov. 2023;22:917–934. doi: 10.1038/s41573-023-00775-6. [DOI] [PubMed] [Google Scholar]
- 5.McDonald CM, Sajeev G, Yao Z, McDonnell E, Elfring G, Souza M, et al. Deflazacort vs prednisone treatment for Duchenne muscular dystrophy: a meta-analysis of disease progression rates in recent multicenter clinical trials. Muscle Nerve. 2020;61:26–35. doi: 10.1002/mus.26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heydemann A, Siemionow M. A brief review of Duchenne muscular dystrophy treatment options, with an emphasis on two novel strategies. Biomedicines. 2023;11:830. doi: 10.3390/biomedicines11030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson G, Conner H, Groneman M, Blavo C, Parmar MS. Duchenne muscular dystrophy: current treatment and emerging exon skipping and gene therapy approach. Eur J Pharmacol. 2023;947:175675. doi: 10.1016/j.ejphar.2023.175675. [DOI] [PubMed] [Google Scholar]
- 8.Akat A, Karaöz E. Cell therapy strategies on Duchenne muscular dystrophy: a systematic review of clinical applications. Stem Cell Rev Rep. 2024;20:138–158. doi: 10.1007/s12015-023-10653-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:545. doi: 10.1186/s13287-021-02609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai A, Baspinar O, Yeşilyurt A, Sun E, Aydemir Çİ, Öztel ON, et al. Efficacy of stem cell therapy in ambulatory and nonambulatory children with Duchenne muscular dystrophy–phase I–II. Degener Neurol Neuromuscul Dis. 2018;8:63–77. doi: 10.2147/DNND.S170087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand N, Zubair AC. Autologous versus allogeneic mesenchymal stem cell therapy: the pros and cons. Surgery. 2022;171:1440–1442. doi: 10.1016/j.surg.2021.10.057. [DOI] [PubMed] [Google Scholar]
- 13.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 14.Otto A, Collins-Hooper H, Patel K. The origin, molecular regulation and therapeutic potential of myogenic stem cell populations. J Anat. 2009;215:477–497. doi: 10.1111/j.1469-7580.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Choi Y, Jung N, Yu Y, Ryu KH, Kim HS, et al. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int J Mol Med. 2016;37:1209–1220. doi: 10.3892/ijmm.2016.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhao MM, Cui JZ, Cui Y, Li R, Tian YX, Song SX, et al. Therapeutic effect of exogenous bone marrow derived mesenchymal stem cell transplantation on silicosis via paracrine mechanisms in rats. Mol Med Rep. 2013;8:741–746. doi: 10.3892/mmr.2013.1580. [DOI] [PubMed] [Google Scholar]
- 18.Choi A, Park SE, Jeong JB, Choi SJ, Oh SY, Ryu GH, et al. Anti-fibrotic effect of human Wharton’s Jelly-derived mesenchymal stem cells on skeletal muscle cells, mediated by secretion of MMP-1. Int J Mol Sci. 2020;21:6269. doi: 10.3390/ijms21176269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Park SE, Jeong JB, Oh SJ, Choi A, Kim YH, et al. Wharton’s Jelly-derived mesenchymal stem cells with high aurora kinase A expression show improved proliferation, migration, and therapeutic potential. Stem Cells Int. 2022;2022:4711499. doi: 10.1155/2022/4711499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EJ, Kim SJ, Jeon SY, Chung S, Park SE, Kim JS, et al. Glutaminase-1 inhibition alleviates senescence of Wharton’s Jelly-derived mesenchymal stem cells via senolysis. Stem Cells Transl Med. 2024;13:873–885. doi: 10.1093/stcltm/szae053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SE, Jeong JB, Oh SJ, Kim SJ, Kim H, Choi A, et al. Wharton’s Jelly-derived mesenchymal stem cells reduce fibrosis in a mouse model of Duchenne muscular dystrophy by upregulating microRNA 499. Biomedicines. 2021;9:1089. doi: 10.3390/biomedicines9091089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 5.0; 2017 [Internet] Washington, DC: United States Department of Health and Human Services; [cited 2024 May 22]. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. [Google Scholar]
- 23.International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Introductory guide MedDRA Version 25.1; 2022 [Internet] Geneva: ICH; [cited 2024 May 22]. Available from: https://admin.meddra.org/sites/default/files/guidance/file/intguide_25_1_English.pdf. [Google Scholar]
- 24.Kwon S, Ki SM, Park SE, Kim MJ, Hyung B, Lee NK, et al. Anti-apoptotic effects of human Wharton’s Jelly-derived mesenchymal stem cells on skeletal muscle cells mediated via secretion of XCL1. Mol Ther. 2016;24:1550–1560. doi: 10.1038/mt.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9:131. doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SH, Lee MH, Koo MA, Seon GM, Park YJ, Kim D, et al. Stem cell passage affects directional migration of stem cells in electrotaxis. Stem Cell Res. 2019;38:101475. doi: 10.1016/j.scr.2019.101475. [DOI] [PubMed] [Google Scholar]
- 27.Sareen N, Sequiera GL, Chaudhary R, Abu-El-Rub E, Chowdhury SR, Sharma V, et al. Early passaging of mesenchymal stem cells does not instigate significant modifications in their immunological behavior. Stem Cell Res Ther. 2018;9:121. doi: 10.1186/s13287-018-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyano K, Ikehata M, Ohshima K, Yoshida Y, Nose Y, Yoshihara SI, et al. Intravenous administration of human mesenchymal stem cells derived from adipose tissue and umbilical cord improves neuropathic pain via suppression of neuronal damage and anti-inflammatory actions in rats. PLoS One. 2022;17:e0262892. doi: 10.1371/journal.pone.0262892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neves AF, Camargo C, Premer C, Hare JM, Baumel BS, Pinto M. Intravenous administration of mesenchymal stem cells reduces Tau phosphorylation and inflammation in the 3xTg-AD mouse model of Alzheimer’s disease. Exp Neurol. 2021;341:113706. doi: 10.1016/j.expneurol.2021.113706. [DOI] [PubMed] [Google Scholar]
- 30.Ramalho BDS, Almeida FM, Sales CM, de Lima S, Martinez AMB. Injection of bone marrow mesenchymal stem cells by intravenous or intraperitoneal routes is a viable alternative to spinal cord injury treatment in mice. Neural Regen Res. 2018;13:1046–1053. doi: 10.4103/1673-5374.233448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajput BS, Chakrabarti SK, Dongare VS, Ramirez CM, Deb KD. Human umbilical cord mesenchymal stem cells in the treatment of Duch-enne muscular dystrophy: safety and feasibility study in India. J Stem Cells. 2015;10:141–156. [PubMed] [Google Scholar]
- 32.Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13:366. doi: 10.1186/s13287-022-03054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baranovskii DS, Klabukov ID, Arguchinskaya NV, Yakimova AO, Kisel AA, Yatsenko EM, et al. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022;9:7. doi: 10.21037/sci-2022-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66:209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 35.Muntoni F, Domingos J, Manzur AY, Mayhew A, Guglieri M, et al. UK NorthStar Network. Categorising trajectories and individual item changes of the North Star Ambulatory Assessment in patients with Duchenne muscular dystrophy. PLoS One. 2019;14:e0221097. doi: 10.1371/journal.pone.0221097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor M, Jefferies J, Byrne B, Lima J, Ambale-Venkatesh B, Ostovaneh MR, et al. Cardiac and skeletal muscle effects in the randomized HOPE-Duchenne trial. Neurology. 2019;92:e866–e878. doi: 10.1212/WNL.0000000000006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald CM, Marbán E, Hendrix S, Hogan N, Ruckdeschel Smith R, Eagle M, et al. Repeated intravenous cardiosphere-derived cell therapy in late-stage Duchenne muscular dystrophy (HOPE-2): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2022;399:1049–1058. doi: 10.1016/S0140-6736(22)00012-5. [DOI] [PubMed] [Google Scholar]
- 38.Weil BR, Suzuki G, Leiker MM, Fallavollita JA, Canty JM., Jr Comparative efficacy of intracoronary allogeneic mesenchymal stem cells and cardiosphere-derived cells in swine with hibernating myocardium. Circ Res. 2015;117:634–644. doi: 10.1161/CIRCRESAHA.115.306850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landfeldt E, Ferizović N, Buesch K. Timing of clinical interventions in patients with Duchenne muscular dystrophy: a systematic review and grading of evidence. J Neuromuscul Dis. 2022;9:353–364. doi: 10.3233/JND-220804. [DOI] [PubMed] [Google Scholar]
- 40.Guglieri M, Bushby K, McDermott MP, Hart KA, Tawil R, Martens WB, et al. Developing standardized corticosteroid treatment for Duchenne muscular dystrophy. Contemp Clin Trials. 2017;58:34–39. doi: 10.1016/j.cct.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercuri E, Osorio AN, Muntoni F, Buccella F, Desguerre I, Kirschner J, et al. Safety and effectiveness of ataluren in patients with nonsense mutation DMD in the STRIDE registry compared with the CINRG Duchenne Natural History Study (2015–2022): 2022 interim analysis. J Neurol. 2023;270:3896–3913. doi: 10.1007/s00415-023-11687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parent Project Muscular Dystrophy. Duchenne drug development pipeline [Internet] Washington, DC: Parent Project Muscular Dystrophy; [cited 2024 Sep 4]. Available from: https://www.parentprojectmd.org/Duchenne-drug-development-pipeline. [Google Scholar]
- 43.U.S. FDA. FDA expands approval of gene therapy for patients with Duchenne muscular dystrophy [Internet] Silver Spring: U.S. FDA; [cited 2024 Sep 4]. Available from: https://www.fda.gov/news-events/press-announcements/fda-expands-approval-gene-therapy-patients-duchenne-muscular-dystrophy. [Google Scholar]
- 44.Hoy SM. Delandistrogene moxeparvovec: first approval. Drugs. 2023;83:1323–1329. doi: 10.1007/s40265-023-01929-x. [DOI] [PubMed] [Google Scholar]
- 45.Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daiichi Sankyo. Study of DS-5141b in patients with Duchenne muscular dystrophy [Internet] Tokyo: Daiichi Sankyo; [cited 2024 Sep 4]. Available from: https://clinicaltrials.gov/study/NCT02667483. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of adverse events, including long-term follow-up data
Serum CK level for each subject, including long-term follow-up data
Changes in spirometer parameters including the interim follow-up data collected up to week 36. Data are mean and SD values. Subject H2 was unable to perform a spirometry test because of bone pain at week 12 and was excluded from the calculation of the mean and SD at week 12. The baseline for each group is indicated by a dotted line. The mean for each group at a specific time point is marked with a blue or red circle along with the SD. No statistical difference was observed. A: FVC%. B: MIP. C: MEP. D: PEFR. FVC%, percentage of predicted forced vital capacity; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; PEFR, peak expiratory flow rate; SD, standard deviation.
Changes in muscle strength including the interim follow-up data collected up to week 36. Data are mean and SD values. The muscle strengths of the knee extensor (A) and elbow flexor (B) were measured using the quadriceps and biceps, respectively. Right and left muscles were measured, and their values were averaged. The baseline for each group is indicated by a dotted line. The mean for each group at a specific time point is marked with a blue or red circle along with the SD. No statistical difference was observed. SD, standard deviation.
Changes in the NSAA score for each subject including the interim follow-up data collected up to week 36. Trend line for each subject is indicated. Red and blue lines indicate the low-dose and high-dose groups, respectively. The black dotted line represents the natural history of NSAA score reduction in patients with Duchenne muscular dystrophy. NSAA, North Star Ambulatory Assessment.
Changes in scores for the 6MWT including the interim follow-up data collected up to week 36. Data are mean and SD values. The baseline for each group is indicated by a dotted line. The mean for each group at a specific time point is marked with a blue or red circle along with the SD. No statistical difference was observed. 6MWT, six-minute walk test; SD, standard deviation.
Overview of the study scheme.
Stability test of the EN001 formulation. A: The viability of MSCs was measured using trypan blue staining (magnification: ×4). CS5 and CS10 are commercial products. The conventional cryopreservative solution comprised fetal bovine serum and dimethyl sulfoxide. B: After thawing, the ratio of cell clumping was measured. MSCs were diluted by the EN001 formulation or MEMα. C: Viability of MSCs as measured using trypan blue staining. MSCs were diluted by EN001 formulation or MEMα. Data are mean and SD values. DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; MEMα, Minimum Essential Media alpha; MSCs, mesenchymal stem cells; SD, standard deviation.
Data Availability Statement
All data are incorporated into the article and its online supplementary material.




