Abstract
Although low-dose lactulose has shown a good theoretical foundation for the treatment of ulcerative colitis (UC) in previous studies, the exact effects and mechanism remain unclear. The rats were randomly distributed into 5 groups, i.e., normal drinking water was provided for an initial 14 days in blank control group, 4% dextran sulfate sodium was used for modeling in the remaining 4 groups. During the 15-24th day, rats in the blank control group were administered with 0.9% saline (0.5 ml/d) by gavage. In the rest 4 groups, rats were administered 0.9% saline (0.5 ml/d, UC model), mesalazine (400 mg/kg/d), lactulose (1000 mg/kg/d), and lactulose + mesalazine (two-drug combination) by gavage. In addition to symptoms and pathological changes, serum IL-6, TNF-α, and High-sensitivity C-reactive protein(Hs-CRP) by ELISA analysis, mRNA and protein expression levels of TLR-2, TLR-4, Nuclear factor-κB(NF-κB), IL-6, and TNF-α in colon tissues by RT-qPCR and WB analyses respectively. Meanwhile, short-chain fatty acid(SCFAs) and intestinal flora were analyzed. Low-dose lactulose improved symptoms (diarrhea, blood in stool, weight loss) and pathological inflammation. In addition to serum IL-6, TNF-α, and Hs-CRP, the mRNA and protein expression levels of TLR-2, TLR-4, NF-κB, IL-6 and TNF-α in the colon were down-regulated with the intervention of lactulose.Meanwhile, lactulose decreased the ileocecal PH, increased SCFAs and altered the intestinal flora. Low-dose lactulose may be beneficial to UC by regulating TLRs/NF-κB pathway, reducing ileocecal PH, increasing SCFAs, regulating intestinal flora and improving the intestinal mucosal barrier. Meanwhile, low-dose lactulose and mesalazine may have additive effects upon combination.
Keywords: Lactulose, Ulcerative colitis, Short-chain fatty acids, Intestinal flora
Subject terms: Ulcerative colitis, Ulcerative colitis, Gastrointestinal models
Introduction
Ulcerative colitis (UC) is one of the chronic inflammatory bowel diseases with recurrent episodes. Considering the unclarified pathogenesis, the clinical treatment efficacy remains unsatisfactory, seriously affecting the quality of life of the patients and imposing a significant economic burden1. In recent times, extensive research on exploring mechanistic views suggested that the precise reasons for UC could be related to various immune, genetic, and environmental factors2. Moreover, intestinal mucosal barrier damage could be a key factor in the pathogenesis of UC. In several instances, patients with UC experienced intestinal dysbiosis3, which could be closely related to intestinal microbial metabolites and intestinal microenvironment.
Prebiotics can interact with the intestinal flora and are fermented in the intestine to short-chain fatty acids. These metabolites of prebiotics can regulate immunity and anti-inflammation, maintaining a normal intestinal mucosal barrier and improving the diversity of the intestinal flora to retain physiological functions4. Thus, it is feasible to substantially alter the intestinal microecology of UC patients through the intervention of prebiotics in treating UC.
Among various prebiotics, lactulose has emerged as one of the important prebiotics, elevating intestinal luminal osmolality, promoting intestinal excretion of toxins, acidification, and regulating intestinal flora. These important actions can be beneficial in the UC treatment5, which remains unexplored comprehensively. Notably, lactulose has been found to produce different therapeutic effects at altered doses6. In an instance, a low dose of ≤ 10 g/day for adults mainly produces a prebiotic effect, causing no adverse events of diarrhea or bloating symptoms. In another instance, it has been suggested that the temporary laxative threshold for lactulose was estimated to be 0.26 g/kg body weight, a dose of approximately 13 g/day for a 50 kg adult7. Along this line, several reports demonstrated that low-dose lactulose typically improved the quality of life in patients with UC8. In animal-based UC models, lactulose at a low dose could exert anti-inflammatory effects by modulating the Nuclear factor-κB(NF-κB) pathway, indicating its therapeutic role9,10. Nevertheless, the precise mechanism remains to be investigated.
Motivated by these considerations, this study aimed to use low-dose lactulose to treat dextran sodium sulfate (DSS) induced UC model of rats. Further, the effects of low-dose lactulose were observed on the symptoms of UC, intestinal tissue inflammation, inflammatory pathways, inflammatory factors, intestinal mucosal mechanical barriers, short-chain fatty acids and intestinal bacterial flora, exploring the mechanisms involved.
Materials and methods
Subjects
Sprague Dawley (SD) rats (Male, 235–340 g) were provided by the ANIMAL EXPERIMENTS CENTER OF XINJIANG MEDICAL UNIVERSITY (Urumqi,China; Production license batch number:SCXK 2018–0002; Using license number:SYXK 2018–0003) .SD rats housed in the specific pathogen-free (SPF) environment of the Animal Experimentation Center of Xinjiang Medical University. Notably, the environmental conditions of the SPF were maintained at a temperature of 20–26 °C, relative humidity of 40- 70%, and the diurnal altered time of 12 h/12 h. SD rats were adaptively fed for 1 week before the experiment. Finally, the experiment was terminated by subjecting the animals to 2% pentobarbital sodium to anesthetize them .The rats were euthanized by inhalation of CO2.
All the experimental protocols were approved by the Experimental Animal Ethics Committee of Xinjiang Medical University (Xinjiang, China;IACUC-20210805–07). All experiments were performed in accordance with relevant guidelines and regulations. All experiments were performed in accordance with ARRIVE guidelines.
UC model establishment
In this study, a 4% DSS solution was used for UC modeling. To establish the UC model, rats were allowed to drink water containing 4% DSS (M.W 40,000) freely for 14 days, the DSS solution was dissolved and replaced daily. Further, the successful establishment of the UC model was determined based on the criteria of symptoms and pathological manifestations. On the one hand, the symptoms included rats showing weight loss, loss of appetite, reduced activity, mental deterioration, diarrhea, positive occult blood in stools in the pre-modeling period, and bloody stools with the naked eye with the prolongation of time. On the other hand, the pathological manifestations of hematoxylin and eosin (H&E) staining of colonic tissues included the incomplete epithelium of the colonic mucosa, with ulcers and erosions. In addition, the mucosal layer and/or submucosal layer were infiltrated with lymphocytes and neutrophils. Moreover, several crypts could be seen as crypt inflammation and crypt abscesses.
Animal grouping
SD rats (n = 40) were randomly divided into 5 experimental groups, including blank control, UC model, lactulose, mesalazine, and two-drug combination treatment groups, with 8 rats in each group. For the first 14 days, the blank control group was administered drinking water without DSS, and the remaining 4 groups were given drinking water containing 4% DSS (M.W 40,000) freely. After 14 days of modeling, the blank control and the UC model groups were administered with 0.5 ml of 0.9% saline by gavage, along with 1000 mg/kg of lactulose once a day in the lactulose treatment group, and 400 mg/kg of mesalazine by gavage once a day group in the mesalazine treatment group. To explore their combinatorial effect, the two-drug combination group of rats was administered 1000 mg/kg lactulose + 400 mg/kg mesalazine by gavage once a day for 10 consecutive days. It should be noted that the rats in the treatment groups were free to eat and drink.
The dose of lactulose was selected according to the literature and preliminary experiment. In the preliminary experiment, we found low-dose lactulose (500 mg/kg/d and 1000 mg/kg/d) improved diarrhea and hematochezia in SD rats, and the literature indicates that lactulose is more effective in the treatment of colitis with increasing doses at low doses9, we selected 1000 mg/kg/d as the intervention dose of lactulose. The dose of mesalamine was selected according to the literature, and a mesalamine dose of 400 mg/kg/d in SD rats was equivalent to a therapeutic dose in humans with UC.
Disease activity index score
During the experiment, the changes in the body weight, blood in stool, and fecal character were recorded daily. The disease activity index(DAI) score was calculated based on Cooper’s scoring criteria11.
Morphological assessments
To demonstrate various morphological assessments, the colonic length was measured along with the colonic mucosa damage index (CMDI) score and colonic tissue damage index score. After terminating the treatment, the rats were anesthetized with sodium pentobarbital, and the length of the separated whole colon (ileum to rectum) was measured. Further, the colon was dissected by extending the longitudinal axis. After rinsing the intestinal tube with saline, the CMDI score was performed using Wallace’s scoring criteria12. The descending colon tissue was retained in the upper 1 cm of the rectum, fixed in 4% paraformaldehyde solution, and stained with HE to observe the histopathological changes. Notably, two pathologists in the direction of digestion were asked to perform the colonic tissue damage index score. The score was determined based on the sum of five scores, including inflammation, lesion depth, crypt destruction, lesion extent, and regeneration under a light microscope, using a blind method.
The colon microstructure was determined using electron microscopic observations. The transmission electron microscope (TEM, JEOL JEM F200) was applied to observe colon epithelial cells, microvilli, intercellular junctions, and mitochondrial morphology.
ELISA
The ELISA analysis was performed to determine the expression levels of various serum inflammatory markers, including IL-6, TNF-α, and High-sensitivity C-reactive protein(Hs-CRP) levels, according to the manufacturer’s instructions (Jianglai, China). Further, the absorbance values at 450 nm were detected on an enzyme labeling instrument (Thermo Fisher Scientific, Waltham, USA) and calculated using the standard curve method.
RT-qPCR analysis
The RT-qPCR analysis was performed to determine the expression levels of various inflammatory indicators. Initially, the total RNA from the dissected colon tissues was extracted using a grinder (KZ-111 grinder, Wuhan Sevier Biotechnology Co., Ltd., Wuhan, China). Further, the quality of RNA OD260/OD280 was determined to be at 1.95–2.0, and cDNA was synthesized using a cDNA synthesis kit (Takara, Japan). Then, the primers for TLR-2, TLR-4, NF-κB, IL-6, TNF-α, and β-actin were designed based on the literature. The sequences of the primers are shown in Table S1. Further, the amplification was carried out on a real-time fluorescence quantitative PCR instrument (QuantStudioTM 1, Plus) with reference to the fluorescence quantitative PCR kit (Kjeldahl Biotechnologie GmbH, Germany). The reaction conditions are shown in Table S2. The relative expression levels of TLR-2, TLR-4, NF-κB, IL-6, and TNF-α compared with the β-actin as an internal reference gene were calculated using the 2-ΔΔCt value.
Western blotting
Initially, the protein was extracted from the clean, rinsed colon tissues of rats. Further, the complete protein was quantified using the Bio-Rad assay kit (Bio-Rad, Hercules, USA). Further, the required quantity of extracted protein was separated through sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Then, the gels were blotted onto a polyvinylidene difluoride (PVDF) membrane, which was incubated with the primary antibodies (IL-6 1:1000, Affinity China, DF6087; TNF-α 1:2000, Abcam British, ab205587; NF-κB 1:500, Bioss China,bs-2695R; TLR-2 1:2000, Abcam British, ab209217; TLR-4 1:1000, Bioss China, bs-20595R) overnight at 4 ℃. Further, the membranes were incubated with the secondary antibody (bs-0295G-HRP, Goat anti Rabbit, Bioss China, protected from light). Finally, the protein expression bands were detected using the Bio-Rad Western blot imager. The gray-scale value was analyzed using ImageJ software.
Analysis of intestinal flora and metabolites
PH-Test: Moreover, the pH values of the contents of the ileocecal, transverse colon, and sigmoid colon were determined using a pH meter.
Detection of short-chain fatty acids (SCFAs): Gas chromatography-mass spectrometry (GC–MS) was used to determine the amount of SCFAs in ileocecal contents (8 cases per group) against the 2-methyl valeric acid as the internal standard. Initially, the samples were pretreated and detected using a Shimadzu GC2030-QP2020 NX GC–MS instrument equipped with an Agilent HP-FFAP capillary (30 m × 250 μm × 0.25 μm, Agilent Technologies, USA). After injection in split mode with 1 μL of analyte, helium was used as the carrier gas at a column flow rate of 1 ml/min and a spacer purge flow rate of 3 ml/min. After a 3.5 min solvent delay, mass spectrometry data were collected in full-scan mode with a m/z range of 33–150.
16S rRNA gene sequencing: Initially, DNA of colonic contents were extracted using a fecal genomic DNA extraction kit (Tiangen Biotech Co., Ltd., Beijing, China). Further, the DNA sequencing was performed using the PacBio Sequel platform from Aqi Biotech Co., Ltd. (Shanghai, China). This platform was designed for sequencing the 16S rRNA gene by using primer pairs. The primer sequences were 27F (5'-AGRGTTTGATYNTGGCTCAG-3'), 1492R (5'-TASGGHTACCTTGTTASGACTT-3'), and the length of the sequencing was 1500 bp. It should be noted that the primer sequences were identified and removed using Cutadapt. Then, these primer sequences were spliced, and chimeric reads were removed using USEARCH to obtain high-quality sequences. These sequences were further clustered with 97% similarity to obtain the operational taxonomic unit (OTU) table. The amplicon sequence variants (ASVs) were obtained by denoising the quality-control data using the DADA2 method in QIIME213. The NCBI database was used to annotate the ASVs of 16S ribosomal RNA (rRNA) genes, thus obtaining the taxonomic information of each ASV and generating a table of relative abundance at each taxonomic level.
Statistical analysis
Typically, SPSS 26.0 and GraphPad Prism 9.0 software were used for analysis and statistical charting. The data that did not meet the requirements of ANOVA were expressed as the median (lower quartile, upper quartile) . The two groups that did not meet the requirements of ANOVA were compared using the Mann–Whitney U test. The data that met ANOVA were compared using the two independent samples t-test for two groups and one-way ANOVA for more than 3 groups. The ACE and Shannon–Wiener diversity indices were used to analyze the alpha diversity of the intestinal flora. The Principal Coordinates Analysis (PCoA) was used to analyze the beta diversity. Linear discriminant analysis Effect Size(LEfSe) analysis was used to analyze the significance of the differences between the groups, considering a value of P < 0.05 statistically significant.
Results
Low-dose lactulose improves symptoms and reduces inflammatory changes in colonic histopathology in UC rats
As depicted in Fig. 1A, on days 1–14, rats in the blank control group showed a smooth increase in their body weight. Contrarily, rats in the remaining four modeling groups (UC model, lactulose, mesalazine, and two-drug combination treatment groups) showed a slower increase in body weight. Notably, the sluggish growth in the body weight of the DSS modeling rats was not obvious on days 8–14. Moreover, the body weight of rats from day 14 to 24 in the blank control group increased steadily. The results showed an insignificant increase in the body weight of rats in the UC model group. In contrast, the rats in the three treatment groups (lactulose treatment group, mesalazine treatment group, and two-drug combination treatment group) presented a gradual increase in their body weights.
Fig. 1.
Symptoms of rats in 5 different treatment groups. (A) The data show changes in the body weight during 24 days in 5 treatment groups. (B) The image shows the DAI score during 24 days of treatment in 5 treatment groups of rats.
Regarding symptoms, the DAI scores of the rats in the blank control group remained at 0 (Fig. 1B). After 14 days of modeling, the rats in the remaining four groups showed blood in the stools and loose stools. Moreover, it should be noted that the DAI scores gradually increased until day 14. At the end of modeling, the UC model group, after 10 days of treatment, still showed blood and occult blood-positive stools, dilute stools, and relatively high DAI scores. Contrarily, the rats in the other three treatment groups showed no blood in the stools, even on days 20–24. The stools turned normal or soft, and their DAI scores gradually decreased to 0. Although the 2 groups of rats given lactulose treatment showed no aggravated diarrhea, an ameliorating effect on blood in stool, loose stool, and weight loss was observed.
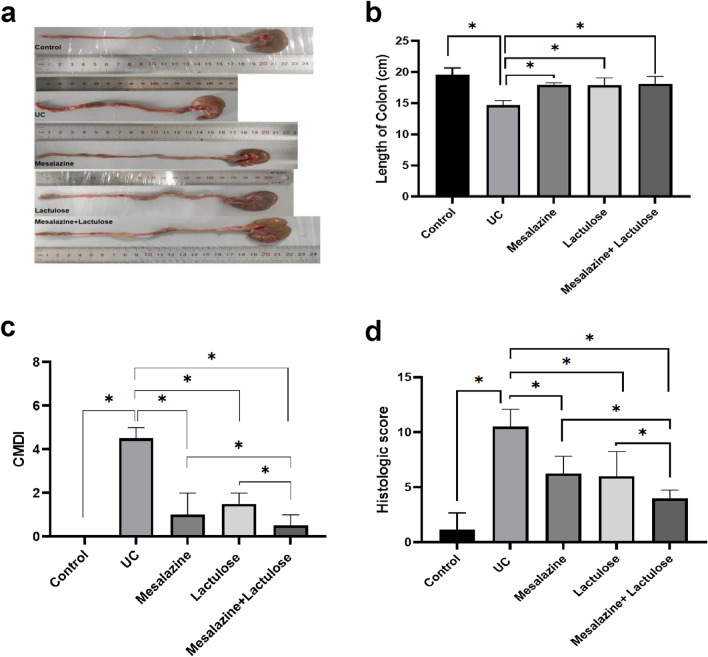
The visual morphological attributes were observed by dissecting the colons from the sacrificed rats. As shown in Fig. 2a and b, the colon length of rats in the UC model group was significantly shorter than that of the blank control group (P < 0.05). Contrarily, the length of the colon in the rest of the three treatment groups (Lactulose, Mesalazine, and Combinatorial) was significantly longer than that in the UC model group (P < 0.05).
Fig. 2.
Colonic histopathological observations in treatment 5 groups of rats. (a) The images show the length of the colon. (b) The data show the comparison of 5 groups concerning the length of the colon. (c) The data present the colon mucosa damage index in 5 different treatment groups. (d) The data show histologic scores in 5 different treatment groups. Data are presented as mean ± SD, and *indicates P < 0.05.
Further, CMDI scores were performed according to colonic mucosal inflammation, ulceration and intestinal adhesion of rats. The colonic tissue damage index was used to evaluate the inflammation of the colonic tissue stained by HE. As shown in Fig. 2c and d, the CMDI and colonic tissue damage index of the UC model treatment group were significantly increased compared with those of the blank control treatment group (P < 0.05). The CMDI and colonic tissue damage index of the three treatment groups were significantly decreased compared with those of the UC model group (P < 0.05). Moreover, the CMDI and colonic tissue damage index of the two-drug combination treatment group were lower than those of the two single-drug treatment groups (P < 0.05).
The colonic tissue sections were observed using HE staining (Fig. 3). Accordingly, the blank control group showed intact colonic epithelial structure, regular crypt morphology, and no inflammatory cell infiltration. The UC model treatment group could evidently present colonic ulcers, incomplete epithelium, lymphocyte, and neutrophil infiltration in the mucosa and submucosal layer. It should be noted that the crypts appeared to have crypt inflammation, crypt abscess, crypt branching, and other alterations, as well as a significant reduction in cup-shaped cells. The lactulose treatment group presented scattered lymphocyte and neutrophil infiltration in colon sections. Moreover, the submucosal lymphoid follicles were observed in some tissues, with relatively more intact intestinal epithelium. Nevertheless, no ulcerative changes were observed, but with significantly increased cup cells compared with the UC model group.
Fig. 3.
Representative images show HE-stained colon tissues in 5 different treatment groups (Low magnification: × 50, Medium magnification: × 100).
Low-dose lactulose reduces systemic and localized inflammation levels
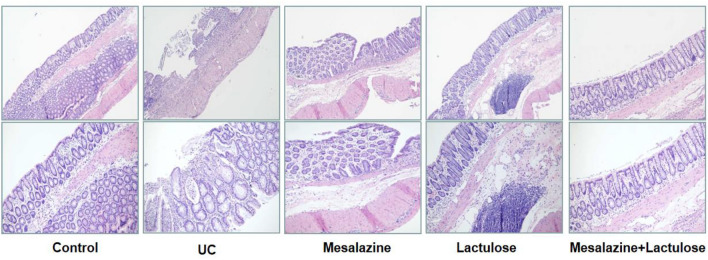
Further, the expression levels of IL-6, TNF-α, and Hs-CRP in rat serum were measured by ELISA assay. It was observed that the expression levels of IL-6, TNF-α, and Hs-CRP in the UC model group were significantly higher than those in the blank control group. Contrarily, the above inflammatory markers could be down-regulated in the three treatment groups (P < 0.05, Fig. 4).
Fig. 4.
The data present the expression levels of serum inflammatory factors of (a) IL-6, (b) TNF-α, and (c) Hs-CRP. Data are presented as mean ± SD, and * indicates p < 0.05.
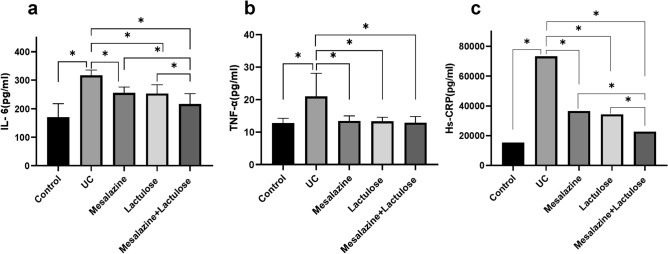
Further, the mRNA expression levels of IL-6, TNF-α, TLR-2, TLR-4, and NF-κB in colon tissues were detected using RT-qPCR analysis. As shown in Fig. 5, the mRNA expression levels of IL-6, TNF-α, TLR-2, TLR-4, and NF-κB in the UC model group were significantly higher than those in the blank control group. Contrarily, the RT-qPCR analysis of tissues from all three treatment groups showed down-regulated expression levels of IL-6, TNF-α, TLR-2, TLR-4, and NF-κB mRNAs (P < 0.05). Notably, the down-regulation expression of the notified mRNAs was more pronounced in the combination treatment group compared to the monotherapy (P < 0.05, Fig. 5).
Fig. 5.
RT-qPCR analysis determines the gene expression levels. The images show mRNA levels of (a) IL-6, (b) TNF-α, (c) NF-κB, (d) TLR-2, and (e) TLR-4. * indicates P < 0.05.
The expression levels of IL-6, TNF-α, TLR-2, TLR-4, and NF-κB proteins were detected by WB analysis. Similar to the RT-qPCR analysis, it was observed that the expression levels of IL-6, TNF-α, TLR-2, TLR-4, and NF-κB proteins were found to be significantly higher in the UC model group compared to that of the blank control group. As anticipated, the three treatment groups showed down-regulated expression levels of the notified proteins (P < 0.05, Fig. 6).
Fig. 6.
(a) Representative images show the WB bands of different proteins, including IL-6, TNF-α,NF-κB, TLR-2, and TLR-4, as well as β-actin as the internal control. Western blot analysis showed the protein expression comparison of (b) IL-6, (c) TNF-α, (d) NF-κB, (e) TLR-2 and (f) TLR-4.
Low-dose lactulose improves the mechanical barrier of colonic mucosa and repairs mitochondrial structure
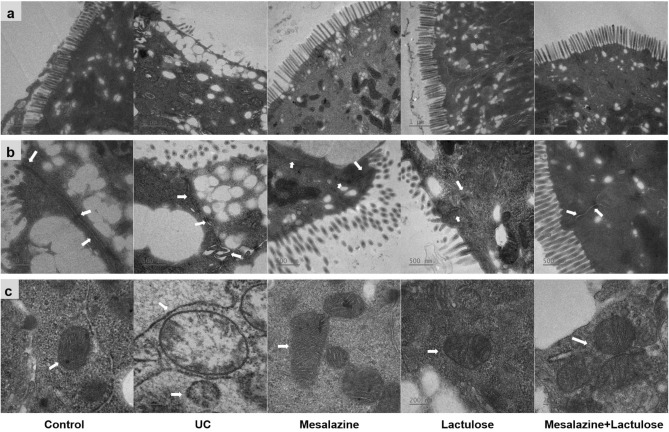
As depicted in the TEM observations, the colonic epithelial cells in the blank control group were structurally intact, with uniformly arranged microvilli (Fig. 7). Contrarily, the UC model group showed broken colonic epithelial cells with chaotically structured and partially missing microvilli. The colonic microvilli of the colons of the rats in the rest of the treatment groups were neatly arranged. The microvilli lengths of the two treatment groups with lactulose were increased compared to those in the remaining three groups. In the normal control treatment group, the tight junctions, intermediate junctions, and bridging grains were intact with narrow gaps. In the UC model group, the intercellular gaps were significantly widened, discontinuous, and partially broken. On the contrary, the intercellular junctions were relatively tight without any break in the rest of the three treatment groups. The mitochondria in the cells of the normal group of rats were structurally intact. In contrast, the mitochondria of the UC model group showed lysis and cristae disruption, with significantly reduced granules. Contrarily, the mitochondria of the rest of the three treatment groups showed increased volume and slightly loose cristae structure, which further improved compared with that of the UC model group.
Fig. 7.
The TEM observations show the ultrastructure of colonic mucosa in 5 different groups, regarding (a) microvilli (× 5000), (b) tight junction, intermediate junction, and bridging grains (× 10,000), and (c) morphology of mitochondria (× 20,000).
Low-dose lactulose increases SCFAs and decreases pH in the ileocecal region
As shown in Fig. 8, the pH values of the ileocecal region, transverse colon, and sigmoid colon of rats in different treatment groups were measured. It was observed that the pH values of the ileocecal contents of the two treatment groups containing lactulose were significantly decreased, while all of them were maintained > 6. It should be noted that the above changes were not obvious in the transverse colon and sigmoid colon. The SCFAs analysis of the ileocecal contents showed that acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, and hexanoic acid in the UC model treatment group were lower than those in the blank control group (P < 0.05). Contrarily, the SCFAs content increased after the administration of mesalazine, lactulose, and a combination of medications. Among them, an increase in acetic acid, propionic acid, and butyric acid levels was more pronounced in the lactulose-treated group (Table S3).
Fig. 8.
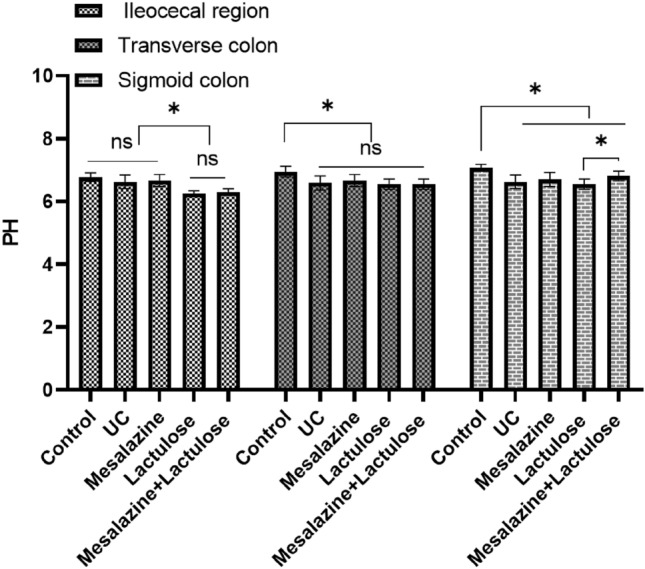
The pH levels in the ileocecal region, transverse colon, and sigmoid colon of 5 treatment groups. Data are presented as mean ± SD; * indicates P < 0.05; “ns “ represents no significant difference.
Low-dose lactulose affects intestinal flora
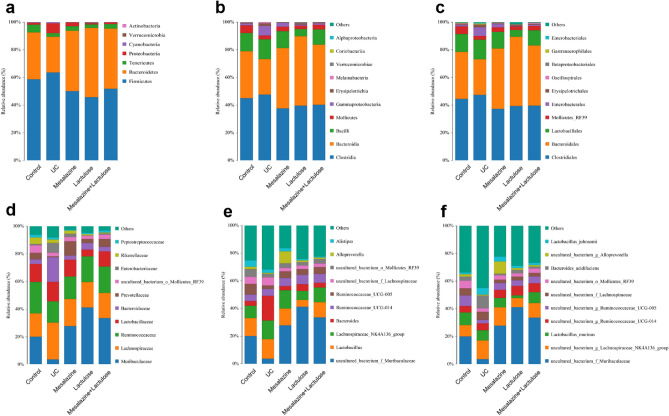
Alpha diversity analysis: From the ACE and Shannon’s indices, it was observed that the abundance and diversity of the flora decreased in the UC model group compared to the blank control group. Contrarily, the abundance and diversity of the three treatment groups increased compared to that of the UC model group (Fig. 9a and b).
Fig. 9.
Alpha diversity and beta diversity analyses of gut microbiota in 5 different treatment groups. The data show (a) the abundance of microbiota (ACE index) and (b) the diversity of microbiota (Shannon index). (c) The data present the PcoA approach of microbiota in 5 different treatment groups of rats.
Beta diversity analysis: The PcoA approach indicated that the differences within each group were insignificant. Moreover, the composition of the blank control group and the UC model group were more different, while was similar to the three treatment groups. Accordingly, the composition of these three groups was similar to that of the blank control group (Fig. 9c).
As depicted in Figs. 10 and 11, the abundance of Bacteroidetes, Ruminococcaceae_UCG_005, Muribaculaceae, and their genus-level and species-level bacteria, as well as Prevotellaceae in the UC model group was significantly decreased compared to that of the blank control group. Moreover, various bacteria, such as Proteobacteria, Firmicutes, Gammaproteobacteria, Clostridiales, Enterobacterales, Enterobacteriaceae, Bacteroides, and Baceroides_acidifaciens were significantly more abundant in the UC model group than the control group of rats. The trend of the distribution of the bacterial flora in the three treatment groups was observed to be contrary to that of the UC model group, which was similar to that of the blank control group. On the one end, in the mesalazine treatment group, the abundance of Prevotellaceae and Alloprevotella and their species-level bacteria was significantly increased. On the other hand, in the lactulose-treated group, the increase in abundance of Muribaculaceae and its genus-level and species-level bacteria was more pronounced than in the other treatment groups.
Fig. 10.
The microbiota composition in 5 different treatment groups. The data show microbiota composition and comparison of microbiota in rats on the phylum (a), class (b), order (c), family (d), genus (e), and species levels (f).
Fig. 11.
Characteristics of intestinal microbiota composition by LEfSe analysis in 5 groups. (a) Distribution histogram of LDA value, LDA > 4. (b) The cladogram based on LEfSe.
Discussion
UC is one of the chronic inflammatory bowel diseases with recurrent episodes. Notably, the clinical treatment remains unsatisfactory due to the unclarified precise mechanistic views in terms of pathogenesis. Previous studies indicated that prebiotics were fermented into SCFAs in the intestinal tract, which could regulate immunity and anti-inflammatory effect, maintaining a normal intestinal mucosal barrier and improving the diversity of the intestinal flora to maintain intestinal physiological functions14. As an important prebiotic, lactulose can elevate intestinal luminal osmolality and promote intestinal endotoxin excretion, acidification, and regulation of intestinal flora15. Considering these advantageous characteristics, lactulose is considered beneficial for UC treatment. Accordingly, previous studies on animal experiments and population studies indicated the positive effects of low-dose lactulose on UC. Nevertheless, the precise mechanism of lactulose against UC remains unclear.
In this study, lactulose at a low dose of 1000 mg/kg/d was applied to intervene in a UC rat model to observe its therapeutic effect and explore the mechanism. Considering the clinical symptoms, it was observed that lactulose at a low dose of 1000 mg/kg/d showed no aggravated diarrhea in UC rats. On the contrary, it showed a certain therapeutic effect on diarrhea and hematochezia. The low dose of 1000 mg/kg/d in this study was equivalent to a human use dose of about 10 g/d. The resultant adverse events in this study were consistent with the results of many reported studies based on human trials, showing that low-dose lactulose (1–10 g/d) exhibited a prebiotic effect16with almost no gastrointestinal symptoms or other adverse effects. Moreover, some previous reports indicated that its laxative threshold was estimated to be 0.26 g/kg/d7. Nevertheless, dose dependence was observed in terms of gastrointestinal symptoms in clinical settings. The present study suggested that low-dose lactulose treatment improved blood in stool and diarrhea and increased body weight in UC rats. Moreover, it was observed that low-dose lactulose reduced the inflammatory changes in colonic histopathology by CMDI and Colonic Damage Index scores. Interestingly, the combination of lactulose and mesalazine exhibited an additive therapeutic effect in reducing the inflammation of colonic histopathology.
Notably, the etiology of UC is multifactorial and complex, in which abnormal activation of the immune system and overproduction of inflammatory factors play important roles in UC17. It was observed from the experimental results that a low-dose lactulose treatment significantly reduced the expression levels of IL-6, TNF-α, and Hs-CRP in serum. Moreover, lactulose downregulated the mRNA and protein expression levels of IL-6, TNF-α, TLR-2, TLR-4, and NF-κB in colonic tissues. Previous studies indicated the importance of TLRs in the intestinal mucosal immune barrier as important transmembrane receptors and signaling receptors for innate immunity18. As an important receptor for lipoteichoic acid (LTA) in Gram-positive bacteria19, the TLR-2 ligands were more widespread than those of TLR-4. Moreover, the ligands for TLR-4 are the Gram-negative bacterial lipopolysaccharide (LPS)20. Accordingly, the TLRs/NF-κB pathway plays a key role as an inflammatory signaling pathway in the pathogenesis and progression of UC diseases, regulating pro-inflammatory cytokines, autophagy function, reactive oxygen mediator production, and apoptosis in the inflammatory cascade [119]. The TLRs recognize pathogen-associated molecular patterns (PAMPs), transmitting signals to the intracytoplasm through the TIR region. These ultimately activate NF-κB and mitogen-associated protein kinase (IRAK) P38 via MYD88 and IL-1 receptor-associated protein kinase (IRAK), thereby releasing cytokines such as IL-6, TNF-α, and nitric oxide synthase, and generating various inflammatory responses [ 21]. In the present study, it was observed that low-dose lactulose could reduce systemic and localized colonic inflammation by modulating the TLRs/NF-κB signaling pathway. Accordingly, lactulose and mesalazine showed an additive therapeutic effects in modulating the TLRs/NF-κB inflammatory pathway.
In an instance, it was demonstrated that the impaired intestinal mucosal barrier function was one of the causative factors of UC22. Under the electron microscope, it was observed that the UC group in this study possessed defective colonic epithelial cells, disorganized, partially missing microvillus structure, and significantly widened and discontinuous intercellular space. In addition, several predominant reasons included the direct exposure of the intestinal mucosal epithelial cells and lamina propria single-nucleated cells, as well as the high upregulation of the intestinal mucosal TLR-2 and TLR-4 in a large number of intestinal flora. Moreover, the binding of TLR-2 to the LTA of the Gram-positive bacteria and the binding of TLR-4 to the LPS of the Gram-negative bacteria could cause excessive activation of NF-κB, thus triggering an inflammatory response. After treatment with lactulose, lactulose significantly improved the intestinal mucosal barrier, improved the structure, tight junctions, intermediate junctions, and the integrity of bridging particles of the colonic microvilli in UC rats. These consequences could result in a significant improvement of the mechanical barrier, reducing the direct exposure of epithelial cells and lamina propria single nuclei to a large number of intestinal flora, in turn lowering the TLR-2 and TLR-4 expressions, resulting in the alleviation of inflammatory response. Simultaneously, in conjunction with colonic pathologic manifestations, it was observed that the UC model group showed reduced cup cells, while lactulose treatment increased cup cells, thus mucus secretion, which could improve the chemical barrier in UC.
In addition, electron microscopy observations indicated that microvilli length was increased in the lactulose-treated groups, which might be related to the fructose content of lactulose. In an instance, Samuel et al. demonstrated that consumption of fructose in a mouse model improved intestinal cell survival and increased the length of the intestinal villi, thus enlarging the intestinal surface area and increasing the absorption of nutrients23. In addition, it was demonstrated that the growth-promoting effect of fructose in breastfed infants might be related to this mechanism24. In contrast, microvillus length was determined by the balance between the rates of proliferation and death of epithelial cells, correlating with stem cell division in the crypts25, which could be closely related to mitochondrial function.
In this study, it was observed that the mitochondria of intestinal epithelial cells in the UC model rats showed lysis and cristae damage. The predominant reason for direct damage of mitochondria in intestinal epithelial cells could be due to the excessive generation of inflammatory factors and reactive oxygen species (ROS) released by immune cells, as well as gut microbial metabolites, leading to mitochondrial DNA damage, dysfunction, and disruption of the structural integrity of mitochondria26. The mitochondrial damage could affect epithelial cells, epithelial tight junctions and secretion barriers, increase intestinal permeability, and increase ROS levels, exacerbate the inflammatory responses27. Moreover, mitochondrial dysfunction could lead to necrosis or apoptosis of intestinal epithelial cells, releasing damage-associated molecular patterns that could activate TLRs and further activate the inflammatory response28. Notably, these findings in this study were reportedly consistent with the absence of intestinal mucosal epithelium, incomplete tight junctions, and increased inflammatory factors by activation of TLRs/NF-κB signaling pathway in UC rats. After lactulose treatment, the mitochondrial regeneration and repair were significantly improved with the relative structural integrity, corresponding to restoration of intestinal epithelial structure, lengthening of microvilli repair, improving mucosal barrier function, and reducing inflammatory responses.
Typically, the prebiotic effect of low-dose lactulose provides excellent theoretical evidence for the UC treatment. In the present study, it was verified that low-dose lactulose reduced the pH value of the ileocecal region of UC rats. The mechanism might be related to lactic acid, short-chain fatty acids, and hydrogen decomposed by lactulose29. However, the pH value of > 6 in the ileocecal region showed no substantial effect on the pH-dependent efficacy of mesalazine. The levels of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, and hexanoic acid were higher after low-dose lactulose treatment, with a more pronounced increase in the acetic acid, propionic acid, and butyric acid compared to other treatment groups. Previous studies indicated that acetic acid could be one of the main SCFAs produced by fermentation of carbohydrates30, contributing to immune regulation and simultaneously enhancing intestinal absorption of minerals, such as calcium and magnesium31. Although butyrate could not be directly produced during carbohydrate fermentation, the intestinal bacterial flora could interact with each other, thus converting it to produce butyrate. Notably, butyrate could play an important role in maintaining the integrity of the intestinal mucosal barrier, lowering inflammation, and regulating immunity. Bothe and colleagues demonstrated the important role of butyrate in maintaining the integrity of the intestinal mucosal barrier, as well as reducing inflammation and regulating immunity. In addition, the authors found that low-dose lactulose could dose-dependently increase the level of SCFAs16, which could not be obvious in high-dose lactulose. In the present study, the experimental results on inflammatory factors, inflammatory pathways, and intestinal mucosal barrier indicated that lactulose could improve intestinal function and reduce inflammation levels in UC by increasing SCFAs in UC.
Regarding the intestinal flora, the UC model group showed a significant increase in the abundance of Proteobacteria, Firmicutes, Clostridium, Enterobacteriaceae, and Bacteroides,which were in agreement with the findings of He and coworkers3. It should be noted that an increased abundance of bacteria in the order Enterobacteriaceae, family Enterobacteriaceae,could trigger a strong inflammatory response through a high expression of lipopolysaccharides by binding to TLR-432. Moreover, it has been shown that Bacteroidesspp. could affect amino acid transport metabolism and energy production33. In addition, it could stimulate the cleavage of the intercellular adhesion protein E-cadherin in colonic epithelial cells through the secretion of metalloproteinase toxins both in vivo and in vitro, leading to an increase in the permeability of the colonic mucosa and the activation of the NF-κB pathway thereby exacerbating inflammatory responses34. Bacteroides acidifaciens, a bacterium associated with colonic inflammation, belongs to the family Bacteroidetes, which can be elevated during inflammation but decreases rapidly after reducing colitis. In an instance, Zhou and colleagues demonstrated that Bacteroides acidifacienswas significantly elevated in a DSS-induced animal model of UC, which was in agreement with our results35. Li and Peng et al. demonstrated that its increase could be associated with intestinal inflammation and could cause diarrhea symptoms36. Moreover, the excess Bacteroides acidifacienscould produce mucus-degrading enzymes, leading to a thinning of the mucus layer, resulting in damage to the intestinal epithelium by harmful bacteria or antigens in the gut37,38. Clostridium is a group of Gram-positive anaerobic bacteria, including the well-known pathogenic clostridia, such as Clostridium botulinum, Clostridium difficile, Clostridium perfringens, and Clostridium tetani, as well as beneficial Clostridium butyricum, among others. An increase in intestinal Clostridium difficileusually results in the development of dehydration, abdominal cramps, watery diarrhea, loss of appetite and weight loss, and, in severe cases, rectal bleeding39, which can exacerbate UC. Several infections based on Clostridium innocuummay lead to poor clinical remission in UC40. Moreover, Clostridium butyricumcan cause colonic microorganisms to produce SCFAs via the butyric acid kinase pathway, which may modulate intestinal immune homeostasis, improve gastrointestinal barrier function, and reduce inflammation41, which may be beneficial to host health. In the present study. Clostridium showed changes in the UC model group, which might be related to an increase in the number of deleterious genera of this species.
Meanwhile, it was observed that the abundance of SCFAs-producing bacteria, i.e., Muribaculaceae, Prevotellaceae, and Ruminococcaceae_UCG_005, was significantly reduced in the UC model group. Currently, several reports indicated that Muribaculaceae inhibited CD8+T cell activation to tolerate immune stimulation, negatively correlating with an inflammatory state42. Moreover, the production of large amounts of acetate and propionate could play an important role in maintaining the integrity of the intestinal barrier function and anti-inflammatory properties43. To this end, Ruminococcaceae_UCG_005 was suggested to be a protective butyrate-producing gut bacterium44,45, which could induce beneficial metabolism by enhancing mitochondrial activity, improving energy metabolism, and activating intestinal gluconeogenesis46. In addition, Prevotellaceaeis a bacterium that produces SCFAs, possessing anti-inflammatory properties in immune cells and inhibiting the growth of invasive pathogens47. The production of Prevotellaceaecan be increased with the use of probiotics48. In a case, Dadi and colleagues demonstrated that Prevotellaceaewas negatively correlated with transcription factor P3 which can encode a pro-inflammatory factor in the colonic lymph nodes49, had a therapeutic effect on diarrhea. The reduced abundance of SCFAs-producing bacteria in the UC model group also suggested that a reduction in SCFAs could play an important role in UC pathogenesis.
The low-dose lactulose treatment significantly improved the diversity of intestinal flora in the UC model group of rats. Contrarily, the distribution of the flora mentioned above after lactulose intervention was opposite to that in UC model group. Among them, the abundance of bacteria at Muribaculaceae and its genus and species levels was significantly higher than that of other treatment groups. Combined with the physiological functions of these florae, lactulose treatment increased the abundance of SCFAs-producing bacteria (e.g., Prevotellaceae, Muribaculaceae, and Ruminococcaceae_UCG_005). Moreover, it showed a decrease in pro-inflammatory-associated bacteria (e.g., Clostridium, Enterobacterales, Enterobacteriaceae, Bacteroides, and Bacteroides acidifaciens) and opportunistic pathogenic bacteria (e.g., Proteobacteria), thereby reducing UC intestinal inflammation and improving the intestinal mucosal biological barrier.
Conclusion
In summary, the intervention of UC in rats using lactulose at a low dose of 1000 mg/kg/d showed no aggravation of the diarrhea symptoms and produced some prebiotic effects, which could significantly improve the symptoms of UC rats. The lactulose treatment could reduce systemic and localized colonic inflammation through TLRs/NF-κB pathway, repair colonic epithelial structure, improve intercellular junctions, repair mitochondrial structure, reduce ileocecal pH, increase the content of SCFAs, regulate the intestinal flora (mainly increase the SCFAs-producing bacteria, and reduce pro-inflammatory bacteria and opportunistic pathogens), contributing to treat UC. Together, lactulose and mesalazine showed additive therapeutic effects in modulating the TLRs/NF-κB inflammatory pathway and reducing pathological inflammation in colonic tissues.However, the overall distribution of intestinal flora in rat UC model is different from that in human, further studies of low-dose lactulose on UC patients are needed.
Supplementary Information
Acknowledgements
The authors thank the platform support of the The First Affiliated Hospital of Xinjiang Medical University. Supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (No.2021D01C334) and National Natural Science Foundation of China (No. 82460110).
Author contributions
M C wrote the main manuscript text ; M C and WM Y completed the experiment; P Y completed project management and thesis verification. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available in the NCBI repository at https://www.ncbi.nlm.nih.gov/sra/PRJNA1177842. The accession number: PRJNA1177842.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Acknowledgments section in the original version of this Article was incomplete. The Acknowledgments section now reads: “The authors thank the platform support of the The First Affiliated Hospital of Xinjiang Medical University.” now reads: “The authors thank the platform support of the The First Affiliated Hospital of Xinjiang Medical University. Supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (No.2021D01C334) and National Natural Science Foundation of China (No. 82460110).”
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/13/2025
A Correction to this paper has been published: 10.1038/s41598-025-93098-y
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86823-0.
References
- 1.Collaborators, G. I. B. D. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol5(1), 17–30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos, G. P. & Papadakis, K. A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc94(1), 155–165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He, X. et al. Bacterial O-GlcNAcase genes abundance decreases in ulcerative colitis patients and its administration ameliorates colitis in mice. Gut70(10), 1872–1883 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu, P. et al. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res165, 105420 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Talley N J, Abreu M T, Achkar J P, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol, 2011, 106 Suppl 1: S2–25; quiz S6. [DOI] [PubMed]
- 6.Ruszkowski, J. & Witkowski, J. M. Lactulose: Patient- and dose-dependent prebiotic properties in humans. Anaerobe59, 100–106 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Oku, T. & Okazaki, M. Transitory laxative threshold of trehalose and lactulose in healthy women. J Nutr Sci Vitaminol (Tokyo)44(6), 787–798 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Hafer, A. et al. Effect of oral lactulose on clinical and immunohistochemical parameters in patients with inflammatory bowel disease: a pilot study. BMC Gastroenterology7(1), 36 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rumi, G. et al. Protective effect of lactulose on dextran sulfate sodium-induced colonic inflammation in rats. Dig Dis Sci49(9), 1466–1472 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Hu, G. et al. Prevention of dextran sulfate sodium (DSS)-induced ulcerative colitis in mice by a synbiotic approach using probiotic and lactulose. Lett Appl Microbiol76(5), 36 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Cooper, H. S. et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest69(2), 238–249 (1993). [PubMed] [Google Scholar]
- 12.Wallace, J. L. et al. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology102(1), 18–27 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol37(8), 852–857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalile, B. et al. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol16(8), 461–478 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Odenwald, M. A. et al. Bifidobacteria metabolize lactulose to optimize gut metabolites and prevent systemic infection in patients with liver disease. Nat Microbiol8(11), 2033–2049 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bothe, M. et al. Dose-Dependent Prebiotic Effect of Lactulose in a Computer-Controlled In Vitro Model of the Human Large Intestine. Nutrients9(7), 767 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgart, D. C. & Carding, S. R. Inflammatory bowel disease: cause and immunobiology. Lancet369(9573), 1627–1640 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Korneev, K. V. et al. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine89, 127–135 (2017). [DOI] [PubMed] [Google Scholar]
- 19.de Paiva N M, Ayrizono M L, Milanski M, et al. Differential expression of TLR2, TLR4 and JNK in mucosa of ileal pouches for ulcerative colitis. Is there a role for bacterial antigen pathway in asymptomatic patients?. Int J Clin Exp Med, 2011, 4(3): 179–86. [PMC free article] [PubMed]
- 20.Hornef, M. W. et al. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med195(5), 559–570 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp, R. et al. P2X7 Interactions and Signaling - Making Head or Tail of It. Front Mol Neurosci12, 183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merga, Y., Campbell, B. J. & Rhodes, J. M. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis32(4), 475–483 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Taylor, S. R. et al. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature597(7875), 263–267 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goran, M. I. et al. Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients9(2), 146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall, P. A. et al. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci107(Pt 12), 3569–3577 (1994). [DOI] [PubMed] [Google Scholar]
- 26.Ghezzi, D. & Zeviani, M. Assembly factors of human mitochondrial respiratory chain complexes: physiology and pathophysiology. Adv Exp Med Biol748, 65–106 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Jackson, D. N. et al. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut69(11), 1928–1938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature560(7717), 198–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson, G. R., McCartney, A. L. & Rastall, R. A. Prebiotics and resistance to gastrointestinal infections. British Journal of Nutrition93(S1), S31–S34 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Chambers, E. S. et al. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep7(4), 198–206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakan, T., Tuohy, K. M. & Janssen-van, S. G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Frontiers in Nutrition8, 672925 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey, H. et al. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest Res22(1), 15–43 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun, X. et al. L-Arginine and N-carbamoylglutamic acid supplementation enhance young rabbit growth and immunity by regulating intestinal microbial community. Asian-Australas J Anim Sci33(1), 166–176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.EC W, CL S. Bacteroides spp. and diarrhea. Current opinion in infectious diseases, 2010, 23(5): 470–4. [DOI] [PMC free article] [PubMed]
- 35.Zhou, R. et al. Coptis chinensis and Berberine Ameliorate Chronic Ulcerative Colitis: An Integrated Microbiome-Metabolomics Study. Am J Chin Med51(8), 2195–2220 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Li, X. et al. Gut-Kidney Impairment Process of Adenine Combined with Folium sennae-Induced Diarrhea: Association with Interactions between Lactobacillus intestinalis, Bacteroides acidifaciens and Acetic Acid, Inflammation, and Kidney Function. Cells11(20), 3261 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan S, Waliullah S, Godfrey V, et al. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med, 2020, 12(567): eaay6218 [DOI] [PubMed]
- 38.Zhu, W. et al. 1,25(OH)(2)D(3) deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model. Gut Pathog11, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alhobayb, T. & Ciorba, M. A. Clostridium difficile in inflammatory bowel disease. Curr Opin Gastroenterol39(4), 257–262 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le, P. H. et al. Clostridium innocuum infection in hospitalised patients with inflammatory bowel disease. J Infect84(3), 337–342 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Stoeva, M. K. et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes13(1), 1–28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shang, L. et al. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics (Basel)10(6), 643 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagkouvardos, I. et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome7(1), 28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, Z. et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw Open4(7), e2118811 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, H. J. et al. Growth performance, digestibility, blood metabolites, ruminal fermentation, and bacterial communities in response to the inclusion of gallic acid in the starter feed of preweaning dairy calves. J Dairy Sci105(4), 3078–3089 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Hartstra, A. V. et al. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care38(1), 159–165 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Zhou, X. et al. Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct12(12), 5637–5649 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Wu, W. Y. et al. Silicon-containing water intake confers antioxidant effect, gastrointestinal protection, and gut microbiota modulation in the rodents. PLoS One16(3), e0248508 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dadi T H, Vahjen W, Zentek J, et al. Lythrum salicaria L. herb and gut microbiota of healthy post-weaning piglets. Focus on prebiotic properties and formation of postbiotic metabolites in ex vivo cultures. J Ethnopharmacol, 2020, 261: 113073. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the NCBI repository at https://www.ncbi.nlm.nih.gov/sra/PRJNA1177842. The accession number: PRJNA1177842.