Abstract
Objective
This study aimed to explore the relationship between adverse childhood experiences (ACEs), sleep, and lower extremity function in older adults using a nationally representative cohort.
Methods
This study included 4,439 participants aged 60 years or older (mean age: 67.2 ± 5.7 years) from the China Health and Retirement Longitudinal Study (CHARLS) 2015 national survey and the 2014 Life History Survey. ACEs, sleep duration, and sleep quality were assessed through self-report, and lower extremity function was measured using the Short physical performance battery (SPPB). The relationships between ACEs, sleep, and lower extremity function were analyzed using multivariate linear regression model and restricted cubic splines.
Results
After adjusting for covariates, older adults with four or more ACEs exhibited worse lower extremity function compared to those with no ACEs (β: -0.175). 6–8 h of sleep was associated with improved lower extremity function (β: 0.119), while good sleep quality was also associated with higher lower extremity function scores (β: 0.177). Age-related differences revealed that the association between four or more ACEs and reduced lower extremity function (β: -0.431) was significant only in individuals aged 70 years and older. In the 60–69 years age group, the sleep duration of 6–8 h was significantly related to better lower extremity function (β: 0.150), however, in those aged 70 years and older, more than 8 h of sleep was associated with poorer function (β: -0.378). Furthermore, good sleep quality was associated with better lower extremity function in individuals aged 70 years and older (β: 0.246).
Conclusion
ACEs, particularly household mental illness and parental disability, are associated with poorer lower extremity function in older adults. Normal sleep duration and good sleep quality are linked to better lower extremity function and may mitigate the negative effects of ACEs. However, these associations vary by age.
Keywords: Adverse childhood experiences, Sleep quality, Sleep duration, Lower extremity function
Introduction
Lower extremity function is a key determinant of quality of life and independent living in older adults [1, 2]. Research indicates that impaired lower extremity function significantly increases the risk of falls [3], and approximately one-third of community-dwelling older adults experience falls annually, which are a leading cause of both death and disability in this demographic [4, 5]. In China, falls are the primary direct cause of death among older adults [6], with estimated direct medical costs of RMB 5 billion and social costs ranging from RMB 60 billion to 80 billion [7].Moreover, age-related decline in lower extremity function is associated with various adverse health outcomes, including hospitalization [8] and disability [9]. Therefore, identifying risk factors associated with lower extremity function is essential for promoting healthy aging.
Adverse childhood experiences (ACEs) encompass a range of traumatic or stressful events occurring before the age of 18, including abuse, neglect, and family dysfunction [10]. The prevalence of ACEs among diverse populations in China varies from 50.5 to 75.0% [11–13]. Study have shown that ACEs, such as maltreatment, can negatively impact physical health and function later in life [14]. Specifically, ACEs are associated with cognitive decline [15] and reduced physical functioning in later life [16]. However, one study suggested that older adults with ACEs are more prone to lower extremity dysfunction [17]. Conversely, another study found that ACEs do not directly cause increased physical limitations in adulthood [18]. A significant gap persists in the literature, with a shortage of nationally representative, high-quality studies examining the relationship between ACEs and lower extremity function in older Chinese adults.
Studies have shown that physiological changes linked to aging cause widespread sleep disturbances in the elderly [19]. Poor sleep quality or abnormal sleep duration not only disrupts the hypothalamic-pituitary-adrenal axis, resulting in hormonal imbalances [20], but also leads to elevated levels of inflammation [21, 22]. Furthermore, substantial evidence shows that short sleep duration is linked to reduced mobility [23] and increased disability [24], whereas long sleep duration is associated with decreased walking speed [23]. Poor sleep quality is linked to diminished physical performance [23] and increased disability [25]. However, some studies suggest that both insufficient and excessive sleep are linked to decreased lower extremity function [26], whereas other research shows that the increased incidence of lower extremity dysfunction is not associated with sleep duration [27]. A longitudinal study has found that poor sleep quality is associated with a decline in lower extremity function in the elderly [28], whereas a cross-sectional study found that poor sleep quality is linked to poor lower extremity function in the elderly men, but the relationship is reversed in women [29]. This indicates gender heterogeneity in the relationship between sleep quality and lower extremity function, and age-related heterogeneity requires further exploration. Therefore, further investigation into the relationship between sleep and lower extremity function is warranted. Evidence indicates that both ACEs and sleep influence muscle function [30, 31], but it remains unclear whether sleep modulates the relationship between ACEs and lower extremity function in older adults.
Based on this, this study aims to investigate the relationship between ACEs and sleep patterns with lower extremity limb function in older adults, using a nationally representative sample from the China Health and Retirement Longitudinal Study (CHARLS). Additionally, physical performance changes in Asian older adults exhibit significant age-related heterogeneity, as the study will analyze age-related heterogeneity in this relationship.
Methods
Study participants
Data for this study were obtained from the 2015 survey and the 2014 Life History Survey of the China Health and Retirement Longitudinal Study (CHARLS). CHARLS is a national longitudinal study conducted from 2008 to 2020, involving residents aged 45 years or older across 28 Chinese provinces. The study utilized a multi-stage, stratified, probability-proportional-to-size sampling methodology. It includes data on demographic background, health status and function, social and economic status, and retirement information [32]. The 2014 Life History Survey provided detailed information on respondents’ family history, health history, education history, wealth history, and work history. CHARLS received ethical approval from the Ethical Committee of Peking University (IRB00001052-11015), and all participants voluntarily provided informed consent.
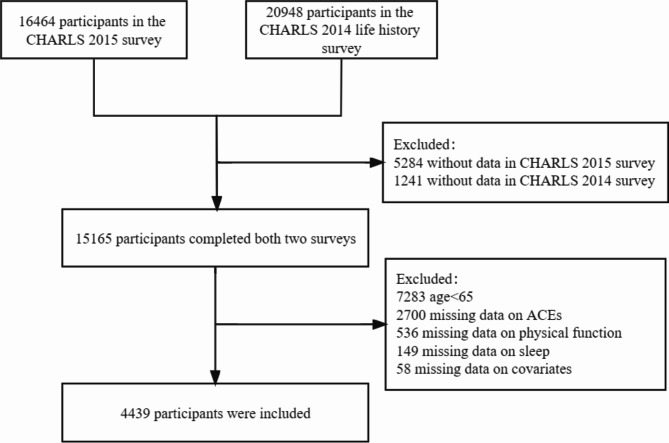
In this study, data from 16,406 respondents in the 2015 survey were matched with data from 20,948 participants in the 2014 Life history Survey. Participants were excluded if they met any of the following criteria: (1) age below 60 years, (2) missing data on adverse childhood experiences, sleep, and physical performance, or (3) missing information on covariates. Ultimately, 4,439 older participants (mean age: 67.2 ± 5.7 years) were included in the final analysis. Figure 1 illustrates the participant selection process.
Fig. 1.
Flowchart of study participant selection
Lower extremity function
Lower extremity function was assessed using the Short Physical Performance Battery (SPPB). All participants completed the SPPB Chinese test following standardized instructions provided by a trained assessor. The SPPB consists of three components: walking speed test, repeated chair stand test, and balance test. Each component is scored from 0 to 4, leading to a total score that ranges from 0 to 12; higher scores indicate better lower extremity function [2].
1) Balance Test: The balance test includes semi-tandem, full-tandem, and side-by-side stands. Participants initially perform a semi-tandem stand, placing one heel beside the other foot’s toes. Those unable to maintain this position for 10 s proceed to side-by-side stands, with feet placed adjacent to each other. Those maintaining the semi-tandem stand for 10 s move to the full-tandem stand, with one heel placed directly in front of the other foot’s toes. Scoring reflects the duration of each stance: side-by-side for 10 s and semi-tandem for less than 10 s (1 point); semi-tandem for 10 s and full-tandem for 0–2 s (2 points); full-tandem for 3–9 s (3 points); full-tandem for 10 s or longer (4 points); or inability to perform the test (0 points) [33].
2) Walking Speed: Participants were instructed to walk along a 2.5-meter line twice at their usual walking pace, with the option to use canes or assistive devices as needed. The faster time from the two attempts was used for statistical analysis. Scoring criteria were as follows: ≤0.43 m/s (1 point), 0.44 to 0.60 m/s (2 points), 0.61 to 0.77 m/s (3 points), ≥ 0.78 m/s (4 points), and failure to complete the test (0 points) [33].
3) Repeated Chair Stand Test: Participants were instructed to sit in a standard chair (47 cm seat height) and cross their arms over their chest. They were required to stand up and sit down as quickly as possible, repeating the cycle five times consecutively without pausing or using their arms for support. The total time to complete the five repetitions was recorded. Scoring criteria were as follows: ≥16.7 s (1 point), 13.7 to 16.6 s (2 points), 11.2 to 13.6 s (3 points), ≤ 11.1 s (4 points), and failure to complete the test (0 points) [33].
Adverse childhood experiences
Based on previous research [34, 35], we identified 12 ACEs indicators from the CHARLS dataset: physical abuse, emotional neglect, domestic violence, peer bullying, unsafe neighborhoods, parental death, parental disability, sibling death, household mental illness, substance abuse, parental separation or divorce, and incarcerated family members. Participants were categorized into three groups according to the cumulative number of ACEs: 0, 1–3, and 4 or more.
Sleep duration and quality
Participants were asked, “In the past month, how many hours of actual sleep did you get each night on average?” (According to previous reports, the recommended sleep duration for middle-aged and elderly individuals in China is 6–8 h per day [36]). Based on their responses, sleep duration was categorized into three groups: short (< 6 h), recommended (6–8 h), and long (> 8 h). Additionally, participants’ sleep quality was assessed by asking about the number of days they experienced poor sleep, with responses classified as follows: none (< 1 day), some (1–2 days), occasional (3–4 days), and most (5–7 days). Sleep quality was further categorized as good (< 1 day), fair (1–2 or 3–4 days), and poor (5–7 days).
Covariates
Based on previous studies [37, 38], the covariates in this study include: (1) demographics: age, gender, place of residence (urban and rural-urban or other), and employment status (employed or unemployed); (2) lifestyle: including current smoking status (yes or no) and current drinking status (yes or no); and (3) comorbidities: hypertension, diabetes mellitus, stroke, heart disease, chronic lung disease, and cancer (yes with one of these, no otherwise).
Statistical analysis
Descriptive statistics were performed to summarize all socio-demographic characteristics, behavioral and lifestyle factors, and chronic disease data. Continuous variables were described using means and standard deviations, while categorical data were summarized with frequencies and percentages. Associations between individual ACEs, cumulative ACEs scores, and outcomes including sleep quality, sleep duration, and lower extremity function were examined using multivariate linear regression model. Three models were developed: Model 1, which excluded covariates; Model 2, adjusted for gender and age; and Model 3, adjusted for all covariates, including age, gender, residence, job status, smoking status, drinking status, and chronic diseases. Beta coefficients and 95% confidence intervals (95% CI) were calculated to report the strength of these associations. Additionally, stratification and interaction analyses were performed based on age groups (60–69 years vs. ≥70 years). Nonlinearity was explored using restricted cubic splines (RCS). Fully adjusted RCS linear analyses were conducted to assess the relationships between the number of ACEs and sleep outcomes, as well as their interactions with lower extremity function scores, stratified by age group. Statistical analyses were performed using STATA 17.0 (Stata Corp., College Station, TX, USA) and R version 4.3.3.
Results
Participant characteristics
Among the 4,439 participants included in this study, 2,292 (51.63%) were male and 2,147 (48.37%) were female, with a mean age of 67.2 ± 5.7 years. A total of 3,265 participants (73.6%) reported experiencing at least one ACE, and 245 participants (5.5%) reported experienced four or more ACEs. The majority of participants were from rural areas and reported no history of smoking or alcohol consumption. Participants who experienced four or more ACEs were more likely to be female and from rural areas than those who did not experience ACEs. (Table 1)
Table 1.
Characteristics of participants by number of ACEs
| Characteristics | Overall | No. of ACEs | ||
|---|---|---|---|---|
| 0 (n = 1174) | 1–3 (n = 3020) | ≥ 4 (n = 245) | ||
| Age, mean (SD) | 67.2(5.7) | 66.7(5.4) | 67.3(5.8) | 67.6(6.5) |
| Gender | ||||
| Men | 2292(51.63) | 571(48.64) | 1606(53.18) | 115(46.94) |
| Women | 2147(48.37) | 603(51.36) | 1414(46.82) | 130(53.06) |
| Residence | ||||
| Urban and urban-rural | 1101(24.80) | 307(26.15) | 741(24.54) | 53(21.63) |
| Rural and other | 3338(75.20) | 867(73.85) | 2279(75.46) | 192(78.37) |
| Job status | ||||
| Employed | 2676(60.28) | 703(59.88) | 1824(60.40) | 149(60.82) |
| Unemployed | 1763(39.72) | 471(40.12) | 1196(39.60) | 96(39.18) |
| Smoking status | ||||
| Yes | 1301(29.31) | 331(28.19) | 903(29.90) | 67(27.35) |
| No | 3138(70.69) | 843(71.81) | 2117(70.10) | 178(72.65) |
| Drinking status | ||||
| Yes | 1534(34.56) | 380(32.37) | 1068(35.36) | 86(35.10) |
| No | 2905(65.44) | 794(67.63) | 1,952(64.64) | 159(64.90) |
| Chronic diseases | ||||
| Yes | 2050(46.18) | 533(45.40) | 1401(46.39) | 116(47.35) |
| No | 2389(53.82) | 641(54.60) | 1619(53.61) | 129(52.65) |
Relationship of adverse childhood experiences and sleep and their combinations to lower extremity functioning
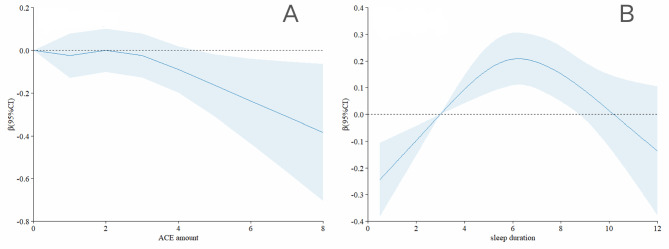
Table 2 presents the results of the multiple linear regression analyses examining the associations between ACEs and sleep and lower extremity function. In model 3, participants who experienced four or more ACEs had a significantly lower lower extremity function score by 0.175 (β: -0.175, 95% CI: -0.345 to -0.005) compared to those who did not experience ACEs. Findings for specific ACEs indicated that lower extremity function scores decreased by 0.111 and 0.122 for participants experiencing household mental illness and parental disability, respectively (household mental illness β: -0.111, 95% CI: -0.214 to -0.008; parental disability β: -0.122, 95% CI: -0.212 to -0.033). According to the restricted cubic spline model, lower extremity function scores decreased as the number of ACEs increased (Fig. 2A). Regarding sleep, lower extremity function scores were 0.119 points higher with 6–8 h of sleep compared to less than 6 h of sleep (β: 0.119, 95% CI: 0.039 to 0.198). Furthermore, participants with good sleep quality had higher lower extremity function scores by 0.177 points (β: 0.177, 95% CI: 0.082 to 0.272) compared to those with poor sleep quality. The restricted cubic spline model revealed an inverted U-shaped relationship between sleep duration and lower extremity function scores, indicating improvement with sleep durations up to 6 h and decline thereafter (Fig. 2B).
Table 2.
Association between the ACEs and sleep with lower extremity physical performance
| Variables | N | β (95% CI) | ||
|---|---|---|---|---|
| Model1 | Model2 | Model3 | ||
| No. of ACEs | ||||
| 0 | 1174 | Ref | Ref | Ref |
| 1–3 | 3020 | 0.005(-0.083 to 0.094) | 0.013(-0.072 to 0.097) | 0.013(-0.070 to 0.097) |
| ≥ 4 | 245 | -0.218(-0.398 to -0.037)* | -0.179(-0.351 to -0.006)* | -0.175(-0.345 to -0.005)* |
| ACE indicator | ||||
| physical abuse | 1173 | 0.074(-0.014 to 0.162) | -0.006(-0.090 to 0.078) | -0.011(-0.093 to 0.072) |
| emotional neglect | 1447 | 0.009(-0.073 to 0.092) | 0.035(-0.044 to 0.114) | 0.028(-0.050 to 0.106) |
| household substance abuse | 319 | -0.033(-0.183 to 0.117) | -0.039(-0.182 to 0.104) | -0.066(-0.207 to 0.075) |
| household mental illness | 652 | -0.135(-0.244 to -0.026)* | -0.119(-0.223 to -0.015)* | -0.111(-0.214 to -0.008)* |
| domestic violence | 357 | -0.112(-0.254 to 0.031) | -0.097(-0.232 to 0.039) | -0.083(-0.217 to 0.051) |
| incarcerated household member | 22 | 0.298(-0.252 to 0.849) | 0.235(-0.289 to 0.760) | 0.231(-0.287 to 0.749) |
| parental separation or divorce | 31 | -0.184(-0.648 to 0.280) | -0.174(-0.616 to 0.269) | -0.167(-0.604 to 0.269) |
| unsafe neighborhood | 340 | -0.243(-0.388 to -0.098)* | -0.158(-0.296 to -0.019)* | -0.135(-0.272 to 0.002) |
| bullying | 577 | -0.021(-0.136 to 0.094) | -0.055(-0.165 to 0.054) | -0.060(-0.168 to 0.048) |
| parental death | 1138 | -0.068(-0.156 to 0.021) | -0.010(-0.095 to 0.074) | -0.005(-0.088 to 0.079) |
| sibling death | 945 | 0.051(-0.044 to 0.145) | 0.066(-0.024 to 0.156) | 0.060(-0.029 to 0.149) |
| parental disability | 924 | -0.113(-0.208 to -0.018)* | -0.130(-0.221 to -0.039)* | -0.122(-0.212 to -0.033)* |
| Sleep duration | ||||
| < 6 h | 1507 | Ref | Ref | Ref |
| 6–8 h | 2478 | 0.235(0.151 to 0.318)* | 0.139(0.058 to 0.219)* | 0.119(0.039 to 0.198)* |
| > 8 h | 454 | -0.111(-0.248 to 0.026) | -0.106(-0.237 to 0.025) | -0.091(-0.221 to 0.039) |
| Sleep quality | ||||
| Bad | 943 | Ref | Ref | Ref |
| Fair | 1193 | 0.118(0.006 to 0.229)* | 0.100(-0.007 to 0.206) | 0.083(-0.022 to 0.189) |
| Good | 2303 | 0.291(0.192 to 0.390)* | 0.207(0.111 to 0.303)* | 0.177(0.082 to 0.272)* |
* Significant at the 5% level
Fig. 2.
Association Between the Number of ACEs and sleep with lower extremity physical performance in restricted cubic spline model. The models were adjusted for age, gender, residence, job status, smoking status, drinking status, and chronic diseases
Figure 2 Association Between the Number of ACEs and sleep with lower extremity physical performance in restricted cubic spline model. The models were adjusted for age, gender, residence, job status, smoking status, drinking status, and chronic diseases.
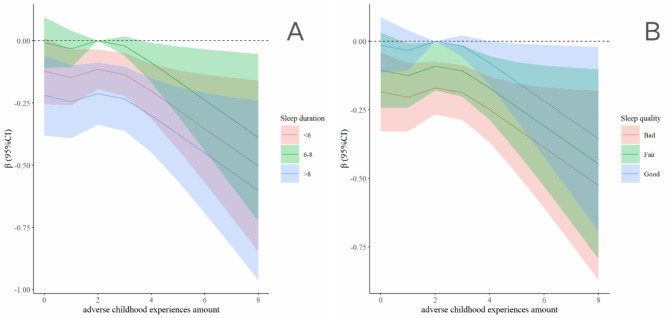
Figure 3 depicts the restricted cubic spline illustrating the relationship between sleep duration, sleep quality, and ACEs with respect to lower extremity function. Among all participants, those sleeping between 6 and 8 h exhibited lower extremity function least impacted by ACEs, compared to those sleeping less than 6 or more than 8 h (Fig. 3A). Additionally, improvements in sleep quality were associated with a progressive reduction in the impact of ACEs on lower extremity function (Fig. 3B).
Fig. 3.
Association Between the combination of the ACEs and sleep with lower extremity physical performance in restricted cubic spline model. The models were adjusted for age, gender, residence, job status, smoking status, drinking status, and chronic diseases
Age differences in the relationship between adverse childhood experiences and sleep and their combinations with lower extremity function
Table 3 presents the results of a multiple linear regression analysis examining the relationship between the number of ACEs, sleep, and lower extremity function across different age groups. The analysis indicated that age significantly modulated the association between ACEs, sleep, and lower extremity function (P for interaction = 0.000). Specifically, having four or more ACEs was associated with decreased lower extremity function (β: -0.431, 95% CI: -0.811 to -0.050) exclusively in individuals aged 70 and older, in comparison to those without ACEs (Table 3). Additionally, a sleep duration of 6 to 8 h was associated with better lower extremity function in the 60–69 age group (β: 0.150, 95% CI: 0.066 to 0.234). In contrast, within the 70 + age group, sleeping more than 8 h was associated with poorer lower extremity function (β: -0.378, 95% CI: -0.645 to -0.111) (Table 3). Additionally, good sleep quality was associated with better lower extremity function in individuals over 70, while sleep quality had no significantly effect on lower extremity function in the 60–69 age group (Table 3).
Table 3.
Association between the number of ACEs and sleep with lower extremity physical performance grouped by age
| Variables |
N
(age 60–69/≥70) |
Age group | P for interaction | |
|---|---|---|---|---|
| 60–69 | 70 and above | |||
| No. of ACEs | 0.000 | |||
| 0 | 859/315 | Ref | Ref | |
| 1–3 | 2092/928 | 0.004(-0.083 to 0.090) | 0.050(-0.144 to 0.244) | |
| ≥ 4 | 169/76 | -0.060(-0.240 to 0.119) | -0.431(-0.811 to -0.050)* | |
| Sleep duration | 0.000 | |||
| < 6 h | 1021/486 | Ref | Ref | |
| 6–8 h | 1813/665 | 0.150(0.066 to 0.234)* | 0.052(-0.127 to 0.230) | |
| > 8 h | 286/168 | 0.072(-0.071 to 0.214) | -0.378(-0.645 to -0.111)* | |
| Sleep quality | 0.000 | |||
| Bad | 671/272 | Ref | Ref | |
| Fair | 838/355 | 0.156(0.045 to 0.266)* | -0.068(-0.308 to 0.173) | |
| Good | 1611/692 | 0.246(0.146 to 0.345)* | 0.025(-0.192 to 0.241) | |
* Significant at the 5% level
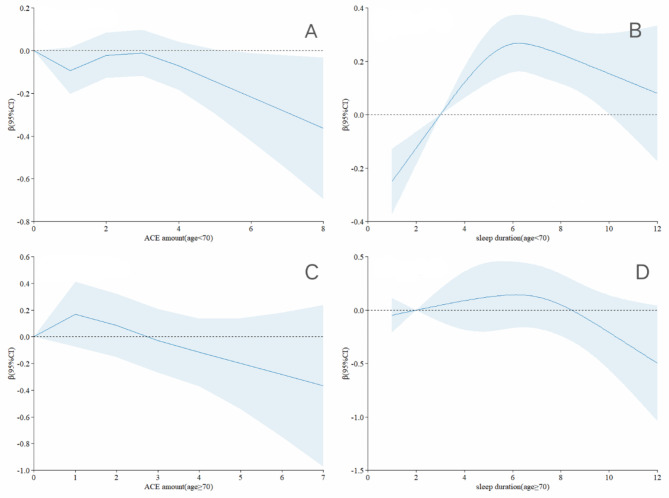
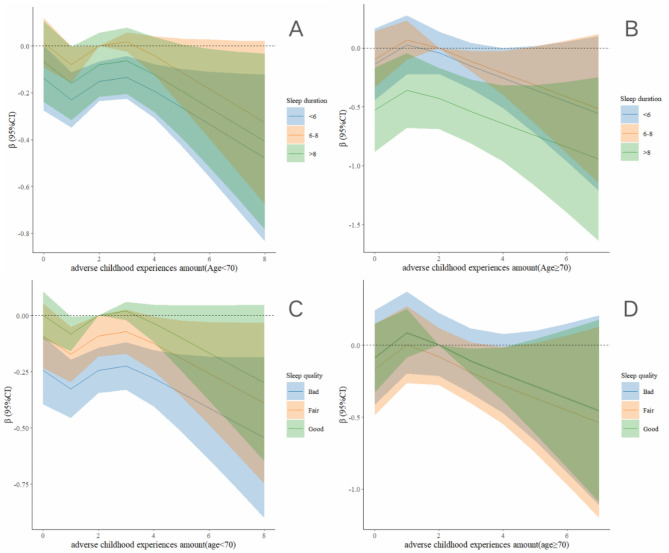
Figure 4 presents the results of the restricted cubic spline analysis regarding the relationship between the number of ACEs and both sleep duration and quality, as well as lower extremity function across different age groups. The analysis shown a positive correlation between having 0–3 ACEs and lower extremity function among participants aged 70 years or older, compared to those younger than 70 years (Fig. 4A C). Additionally, the relationship between sleep duration and lower extremity function exhibited an inverted U-shaped shape in both age groups. However, individuals younger than 70 years and those 70 years or older were more significantly affected by short sleep and prolonged sleep, respectively (Fig. 4B D).
Fig. 4.
Association Between the Number of ACEs and sleep duration with lower extremity physical performance by age. The models were adjusted for gender, residence, job status, smoking status, drinking status, and chronic diseases
Figure 5 presents the results restricted cubic spline analysis, examining the relationship between ACEs, sleep duration, and lower extremity function across different age groups. In both age subgroups, participants who reported sleeping 6–8 h per night exhibited better lower extremity function relative to the number of ACEs, compared to those who slept less than 6 h or more than 8 h (Fig. 5A B). Additionally, among participants aged 60–69 years, those with high-quality sleep demonstrated the optimal lower extremity function relative to ACEs. Conversely, among participants aged 70 years or older, lower extremity function was similarly influenced by ACEs, regardless of sleep quality (Fig. 5C D).
Fig. 5.
Association Between the combination of the ACEs and sleep with lower extremity physical performance in restricted cubic spline model grouped by age. The models were adjusted for gender, residence, job status, smoking status, drinking status, and chronic diseases
Discussion
The results of this study suggest that a higher number of ACEs (≥ 4) is associated with poorer lower extremity function in older adults (≥ 70 years), particularly in those with a history of household mental illness and parental disability. Moreover, the study found that a normal sleep duration (6–8 h per night) correlates with better lower extremity function, while both shorter and longer sleep durations adversely affect it. Additionally, older adults aged 60–69 years are more susceptible to the adverse effects of short sleep periods, while those aged 70 years and older are more vulnerable to prolonged sleep periods. Furthermore, good sleep quality is associated with better lower extremity function in older adults aged 60–69 years. The findings suggest that maintaining a normal sleep duration may mitigate the negative effects of ACEs on lower extremity function in older adults, while good sleep quality helps to counteract the decline in lower extremity function associated with ACEs in the 60–69 years age group.
The study found that a higher number of ACEs is associated with decreased lower extremity function in adults aged 70 years and older. This finding is consistent with previous research [17], which indicated that older Americans over the age of 50 who experienced ACEs are more likely to suffer from lower limb dysfunction. Studies suggest that ACEs may indirectly influence lower extremity function by fostering unhealthy behaviors, including smoking, overeating, and physical inactivity [39, 40], as well as by affecting brain structures such as the hippocampus [41–44]. Moreover, physical injuries sustained from childhood physical abuse could act as a risk factor for the decline in lower extremity function during later life [14]. Additionally, household mental illness and parental disability also contribute to the decline in lower extremity function. Studies have shown that parental mental health problems increase the risk of mental disorders in offspring, either due to genetic inheritance or disruption of the parent-child relationship [45], and these mental disorders are associated with a decline in physical function [46]. Parental disabilities not only pass on to their children [47]but also lead to inadequate responses to emergencies, posing a threat to the children’s safety and increasing the risk of accidents [48].
Our findings reveal an inverted U-shaped relationship between sleep duration and lower extremity function in older adults, indicating that 6–8 h of sleep per night is associated with optimal lower extremity function, consistent with previous research [26]. Existing research indicates that individuals that sleeping more than 9 h may experience a 15% reduction in weekly physical activity [49], negatively impacting lower extremity function [50]. Both short sleep duration and poor sleep quality impact muscle mass and strength via hormonal pathways related to muscle metabolism [51], are also associated with cognitive decline [52], further affecting lower extremity function [53, 54]. Notably, we find that younger older adults were more susceptible to short sleep durations, whereas older adults are more prone to the impacts of prolonged sleep. This age-related difference may be attributed to increased sleep disturbances with advancing age, which older adults often mitigate by extending their sleep duration [55, 56]. Additionally, better sleep quality was associated with better lower extremity function in individuals aged 60–69 years, but this association was not found in those over 70 years. This discrepancy may be attributed to older adults’ tendency to underreport sleep issues [30]. Consequently, it is recommended that older adults enhance sleep quality through modifications in physical activity to help prevent declines in lower extremity function [31].
We have found that normal sleep duration and good sleep quality are associated with a reduced negative impact of ACE on lower limb function, which we hypothesize may be related to muscle function. We have found for the first time that normal sleep duration and good sleep quality are associated with a reduced negative impact of ACE on lower limb function. ACEs may cause skeletal muscle damage and reduced muscle strength through increased inflammation and immune dysfunction [38]. Adequate sleep duration and good quality sleep are associated with improved muscle strength and mass by optimizing hormone-related muscle proteolysis, energy metabolism and maintaining physical activity levels [49–51]. However, the protective effect of sleep quality was not observed in adults over the age of 70, suggesting that age-related changes in sleep quality may affect its ability to protect individuals from early life adversity. Age-related changes in the neuroendocrine system that regulates sleep and stress responses may reduce the protective role of sleep against early life trauma [57]. Therefore, older adults who have experienced ACEs are recommended to aim for 6–8 h of quality sleep per night to mitigate the effects on lower extremity function.
This study has several strengths. First, we not only considered the total number of ACEs experienced by participants but also identified associations between specific types of ACEs and lower extremity function. Second, we investigated whether age-related factors influenced the associations between ACEs, sleep, and lower extremity function. This study also has several limitations. First, the data on ACEs were collected through participant recall, which may introduce bias. Second, certain ACEs, such as sexual assault, were not included in the study. Third, due to the cross-sectional nature of our study, it is not possible to determine the temporal relationship between the variables. Fourth, since the sample is only from China, caution is required when extrapolating the results to other cultural contexts or countries. Fifth, failing to stratify the analysis according to sleep characteristics has limited the depth and richness of the article.
Conclusion
ACEs is associated with poorer lower extremity function in older adults, especially household mental illness and parental disability. Normal sleep duration and good sleep quality are associated with better lower extremity function in the elderly. There was a positive association between normal sleep duration and good sleep quality and the negative effects of ACEs on lower extremity function in older adults. However, these associations had different age heterogeneity. It is recommended that policymakers prioritize addressing the long-term effects of Adverse Childhood Experiences (ACEs) on lower extremity function in older adults by implementing strategies to improve sleep quality and duration, which could help alleviate the negative impact of ACEs on physical health.
Acknowledgements
We thank the participants of the China Health and Retirement Longitudinal Study for their participation in these large-scale efforts; their support made it possible to collect data that could be used for this study.
Author contributions
Conception and design of study: X.D.;Collating data: J.X.;Analysis and/or interpretation of data: X. D., J.X.;Drafting the manuscript: J.X.;Revising the manuscript critically: T.K., M.D.;Approval of the version of the manuscript to be published: J.X., X.D., M.D., T.K.
Funding
This research was supported by the Natural Science Foundation of Shandong Province (ZR2024QG047), the China Postdoctoral Science Foundation (2023M742059), and Postdoctoral Innovation Project of Shandong Province (SDCX-RS-202400011).
Data availability
The data for this article comes from the China Health and Retirement Longitudinal Study. Available from https://charls.pku.edu.cn/en/.
Declarations
Ethics approval and consent to participate
The Ethics Committee of Peking University granted approval for CHARLS (IRB00001052-11015). All participants provided written informed consent. This research followed the guidance of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaqiang Xiao and Xiaosheng Dong contributed equally to this work and are listed as co-first authors.
References
- 1.Shinkai S, et al. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing. 2000;29(5):441–6. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka S, et al. -Hospital fall risk prediction by Objective Measurement of Lower Extremity Function in a High-Risk Population. J Am Med Dir Assoc. 2023;24(12):1861–e18672. [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18(2):141–58. [DOI] [PubMed] [Google Scholar]
- 5.James SL, et al. The global burden of falls: global, regional and national estimates of morbidity and mortality from the global burden of Disease Study 2017. Inj Prev. 2020;26(Supp 1):i3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jue H, Aihui Y. Research progress on falls of the elderly in domestic nursing institutions. Medical Journal of Chinese People’s Health; 2018.
- 7.Wang L, et al. The Perceived knowledge of fall Prevention in nurses Working in Acute Care hospitals in China and the United States. J Patient Saf. 2022;18(2):e580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hoski S, et al. Physical function and Frailty for Predicting adverse outcomes in older primary care patients. Arch Phys Med Rehabil. 2020;101(4):592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 10.Gilgoff R, et al. Adverse childhood experiences, outcomes, and interventions. Pediatr Clin North Am. 2020;67(2):259–73. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Q, et al. Parental alcoholism, adverse childhood experiences, and later risk of personal alcohol abuse among Chinese medical students. Biomed Environ Sci. 2008;21(5):411–9. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, et al. Adverse childhood experiences and interaction with methamphetamine use frequency in the risk of methamphetamine-associated psychosis. Drug Alcohol Depend. 2014;142:295–300. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Mersky JP, Topitzes J. Adverse childhood experiences and psychological well-being in a rural sample of Chinese young adults. Child Abuse Negl. 2020;108:104658. [DOI] [PubMed] [Google Scholar]
- 14.Irving SM, Ferraro KF. Reports of abusive experiences during childhood and adult health ratings: personal control as a pathway? J Aging Health. 2006;18(3):458–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L, et al. Association of Adverse Childhood Experiences and social isolation with later-life cognitive function among adults in China. JAMA Netw Open. 2022;5(11):e2241714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Said SMS, Adly NN, Abdul-Rahman SA. Executive function and physical function among Community-Dwelling Egyptian older adults. J Alzheimers Dis. 2021;80(4):1583–9. [DOI] [PubMed] [Google Scholar]
- 17.Lee VM, et al. Adverse childhood experiences and Aging-Associated Functional Impairment in a National Sample of Older Community-Dwelling adults. J Gen Intern Med. 2023;38(15):3362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottenhoff JSE, et al. Adverse childhood experiences are not Associated with patient-reported outcome measures in patients with Musculoskeletal illness. Clin Orthop Relat Res. 2019;477(1):219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miner B, Kryger MH. Sleep in the Aging Population. Sleep Med Clin. 2017;12(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9. [DOI] [PubMed] [Google Scholar]
- 21.Mullington JM, et al. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24(5):775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrie JE, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II study. Am J Epidemiol. 2013;178(6):956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenholm S, et al. Sleep-related factors and mobility in older men and women. J Gerontol Biol Sci Med Sci. 2010;65(6):649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz RA, et al. The relationship between sleep and physical function in community-dwelling adults: a pilot study. Fam Community Health. 2014;37(4):298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park M, et al. Sleep complaints and incident disability in a community-based cohort study of older persons. Am J Geriatr Psychiatry. 2014;22(7):718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman SE, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30(10):1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arias-Fernández L, et al. Sleep patterns and physical function in older adults attending primary health care. Fam Pract. 2021;38(2):147–53. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira RL, et al. Longitudinal association of sleep quality with physical performance measures: SABE cohort study, Brazil. Public Health. 2024;235:56–62. [DOI] [PubMed] [Google Scholar]
- 29.Denison HJ, et al. Poor sleep quality and physical performance in older adults. Sleep Health. 2021;7(2):205–11. [DOI] [PubMed] [Google Scholar]
- 30.Gu D, et al. Sociodemographic and health correlates of sleep quality and duration among very old Chinese. Sleep. 2010;33(5):601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanderlinden J, Boen F, van Uffelen JGZ. Effects of physical activity programs on sleep outcomes in older adults: a systematic review. Int J Behav Nutr Phys Act. 2020;17(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, et al. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guralnik JM, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol Biol Sci Med Sci. 2000;55(4):M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L, et al. Adverse childhood experiences and subsequent chronic diseases among Middle-aged or older adults in China and associations with demographic and socioeconomic characteristics. JAMA Netw Open. 2021;4(10):e2130143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, et al. Adverse childhood experiences and their impacts on subsequent depression and cognitive impairment in Chinese adults: a nationwide multi-center study. J Affect Disord. 2023;323:884–92. [DOI] [PubMed] [Google Scholar]
- 36.Chen WC, Wang XY. Longitudinal associations between sleep duration and cognitive impairment in Chinese elderly. Front Aging Neurosci. 2022;14:1037650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, et al. Depressive symptoms mediate associations of adverse childhood experiences and chronic lung diseases: a mediation effect analysis. J Affect Disord. 2024;345:342–8. [DOI] [PubMed] [Google Scholar]
- 38.Lin L, et al. Adverse childhood experiences and handgrip strength among middle-aged and older adults: a cross-sectional study in China. BMC Geriatr. 2022;22(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felitti VJ, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 40.Winger JG, et al. Diet and exercise intervention adherence and health-related outcomes among older long-term breast, prostate, and colorectal cancer survivors. Ann Behav Med. 2014;48(2):235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ezzati A, et al. Transcranial doppler and lower extremity function in older adults: Einstein Aging Study. J Am Geriatr Soc. 2017;65(12):2659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moon SY, et al. Prospective associations between white matter hyperintensities and lower extremity function. Neurology. 2018;90(15):e1291–7. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y-K, Ham B-J, Han K-M. Interactive effects of genetic polymorphisms and childhood adversity on brain morphologic changes in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:4–13. [DOI] [PubMed] [Google Scholar]
- 44.Dempster KS, et al. Linking the hemodynamic consequences of adverse childhood experiences to an altered HPA axis and acute stress response. Brain Behav Immun. 2021;93:254–63. [DOI] [PubMed] [Google Scholar]
- 45.Mattejat F, Remschmidt H. The children of mentally ill parents. Deutsches Ärzteblatt International. 2008;105(23):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chafetz L, et al. Predictors of physical functioning among adults with severe mental illness. Psychiatric Serv. 2006;57(2):225–31. [DOI] [PubMed] [Google Scholar]
- 47.Saxton M. Why members of the disability community oppose prenatal diagnosis and selective abortion. Prenatal Testing & Disability Rights Ed Erik Parens & Adrienne Asch, 2000. 1(16): pp. 608–610.
- 48.Pituch E, et al. Parenting with a physical disability and cognitive impairments: a scoping review of the needs expressed by parents. Disabil Rehabil. 2022;44(13):3285–300. [DOI] [PubMed] [Google Scholar]
- 49.Ayas NT, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–4. [DOI] [PubMed] [Google Scholar]
- 50.Botoseneanu A, et al. Metabolic syndrome and the benefit of a physical activity intervention on lower-extremity function: results from a randomized clinical trial. Exp Gerontol. 2021;150:111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piovezan RD et al. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res Rev, 2015. 23(Pt B): pp. 210– 20. [DOI] [PubMed]
- 52.Li M, Wang N, Dupre ME. Association between the self-reported duration and quality of sleep and cognitive function among middle-aged and older adults in China. J Affect Disord. 2022;304:20–7. [DOI] [PubMed] [Google Scholar]
- 53.Beaudart C, et al. Assessment of muscle function and physical performance in Daily Clinical Practice: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal diseases (ESCEO). Calcif Tissue Int. 2019;105(1):1–14. [DOI] [PubMed] [Google Scholar]
- 54.Givan A, et al. Cognitive impairment and low physical function among older Mexican americans: findings from a 20-year follow-up(☆). Ann Epidemiol. 2022;70:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu HF, et al. Sleep problems in Chinese elderly in Hong Kong. Sleep. 1999;22(6):717–26. [DOI] [PubMed] [Google Scholar]
- 56.Chen HC, Hsu NW, Chou P. The Association between Sleep Duration and hand grip strength in Community-Dwelling older adults: the Yilan Study, Taiwan. Sleep, 2017. 40(4). [DOI] [PubMed]
- 57.Mcewen B, Morrison J. The brain on stress: vulnerability and plasticity of the Prefrontal Cortex over the Life Course. Neuron, 2013(1): p. 79. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this article comes from the China Health and Retirement Longitudinal Study. Available from https://charls.pku.edu.cn/en/.