Abstract
Apelin, a (neuro) vasoactive peptide, plays a prominent role in controlling water balance and cardiovascular functions. Apelin and its receptor co-localize with vasopressin in magnocellular vasopressinergic neurons. Apelin receptors (Apelin-Rs) are also expressed in the collecting ducts of the kidney, where vasopressin type 2 receptors are also present. Apelin and vasopressin interact at the brain and renal levels to maintain body fluid homeostasis by regulating diuresis in opposite directions. Apelin and angiotensin II have opposite effects on the regulation of blood pressure (BP). Angiotensin II, by binding to AT1 receptors present in VSMCs, induces intracellular calcium mobilization and vasoconstriction, while apelin, by binding to Apelin-R present on vascular endothelium, increases nitric oxide production and induces vasodilation. Apelin also plays a crucial role in the regulation of cardiac function. Apelin-deficient and Apelin-R-deficient mice develop progressive myocardial dysfunction with ageing and are susceptible to heart failure in response to pressure overload. Since the half-life of apelin is very short in vivo (in the minute range), several metabolically stable apelin analogs and non-peptidic Apelin-R agonists have been developed, with potential applications in diverse diseases. In this review, we highlight the interaction between apelin and vasopressin in the regulation of water balance and that between apelin and angiotensin II in the regulation of BP. Additionally, we underline the protective effects of apelin in cardiac function. Lastly, we discuss the beneficial effects of Apelin-R activation in different pathological states such as hyponatremia, hypertension, and heart failure.
Keywords: Aqueous diuresis, Blood pressure, Cardiac function;, G Protein-Coupled Receptor, Apelin receptor
Introduction
In 1993, the APJ receptor cDNA was cloned from a human genomic library [1]. This sequence codes for an orphan seven-transmembrane-domain G-protein-coupled receptor composed of 380 amino acids. This receptor was first named APJ for ‘putative angiotensin II (Ang II) type 1 receptor (AT1-R)-related protein’. APJ has also been cloned in rodents [2–4], where it shares 31% protein sequence identity with the murine AT1 type 1a receptor. Cyclic AMP (cAMP) production experiments, carried out on eukaryotic cells stably expressing the rat APJ receptor, showed that this receptor did not bind Ang II, Ang III, or Ang IV [3] and remained an orphan receptor. In 1998, Tatemoto et al. isolated the endogenous ligand of the human APJ receptor from bovine stomach extracts [5]. The isolated peptide was named ‘apelin’ for the endogenous ligand of the APJ receptor, and the APJ receptor was renamed the apelin receptor (Apelin-R).
Apelin and its precursor, preproapelin
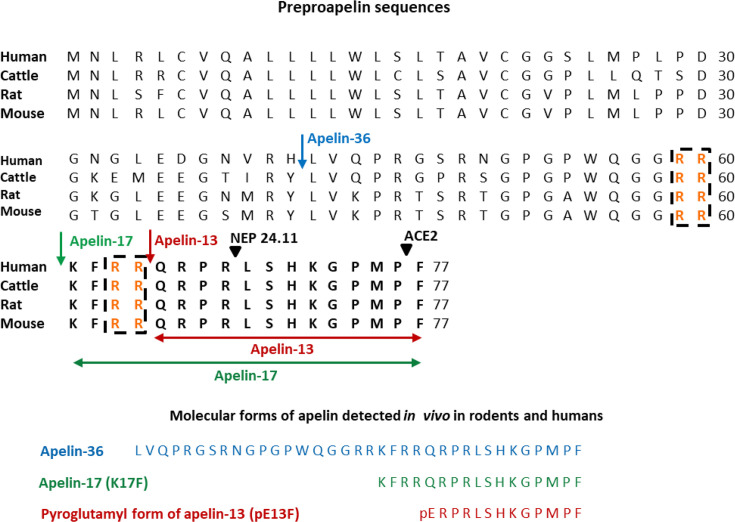
Apelin is derived from a large precursor, preproapelin, composed of 77 amino acids [5]. The human apelin gene is located on the X chromosome, and the open reading phase is localized in two exons (1 and 2) separated by an intron of approximately 6 kb. The 3' non-coding part is also found in two exons (2 and 3). These data may explain the presence of two transcripts of different sizes, 3.0 and 3.5 kb, in varying proportions depending on the tissue studied [6]. Preproapelin has been isolated from several species [5–7], and sequence alignment between mice, rats, cattle, and humans revealed strict conservation of the last 17 amino acids at the C-terminal part of the precursor, corresponding to apelin-17 or K17F (Figure 1). In vivo, preproapelin gives rise to several molecular forms of apelin, which differ in length, corresponding to the last 36, 17, or 13 amino acids at the C-terminal part of the precursor (Figure 1). Apelin-13 is spontaneously pyroglutamylated in vivo at its N-terminus, giving rise to the pyroglutamylated form of apelin-13 or pE13F [5] (Figure 1). In rat brain and plasma, the major molecular forms are pE13F and, in smaller quantities, K17F [9]. In the testis and uterus, apelin-36 predominates, while in the mammary glands, both apelin-36 and pE13F are found [10]. In human plasma, K17F, pE13F, and, to a lesser extent, apelin-36 are predominantly detected [11].
Figure 1. Amino-acid sequences of the apelin precursor, preproapelin, in humans, cattle, rats, and mice, and the molecular forms of apelin detected in vivo.
The blue arrow indicates the beginning of the sequence of apelin-36, the green one that of the sequence of apelin-17 (K17F), which is strictly conserved in mammals, and the red one that of the apelin-13 sequence. The dibasic doublets (in orange) are framed by black dashed boxes. The black arrows show the cleavage sites by neutral endopeptidase 24.11 (NEP 24.11, EC 3.4.24.11) and angiotensin-converting enzyme 2 (ACE2, EC 3.4.17.23). The various molecular forms of apelin detected in vivo in humans and rodents: apelin-36, apelin-17, and the pyroglutamyl form of apelin-13 (pE13F). Figure adapted from [8] with permission from the copyright holders.
Apelin receptor signaling pathways
The different molecular forms of apelin (apelin-36, K17F, and pE13F) have a subnanomolar affinity for the Apelin-R [12,13]. The acidic residues of Apelin-R located on the surface of the receptor, Glu172, Asp282, and Asp92, are involved in apelin binding via their interactions with basic residues present in the endogenous peptide, apelin (in pE13F: Arg2, Arg4, and Lys8). Several laboratories have shown that activation of murine and human Apelin-Rs by the different apelin isoforms induces the inhibition of adenylate cyclase through its Gαi coupling [3,7,14–19].
Apelin-36, K17F, and pE13F also increase intracellular calcium mobilization ([Ca2+]i) in Ntera2 human teratocarcinoma cells (NT2N), basophil-derived cells (RBL-2H3), or HEK-293 cells stably expressing the human Apelin-R [12,20–22]. Furthermore, in 2008, Hus-Citharel et al. showed that K17F decreased Ang II-induced [Ca2+]i mobilization in microdissected glomerular arterioles, and stimulation of Apelin-R by K17F or pE13F modulates vascular tone via nitric oxide (NO) production [23–25].
Apelin-R stimulation can also induce the activation of kinase-related signaling pathways. In human umbilical vein endothelial cells (HUVECs) and Chinese Hamster Ovary cells (CHO) expressing the mouse Apelin-R, Apelin-R stimulation by apelin induces the activation of phosphatidylinositol 3-kinase (PI3K)/Akt and the MAP kinase (mitogen-activated protein kinase) pathway, stimulating the phosphorylation of ribosomal protein kinase S6 (p70S6K) [15,19,26]. Furthermore, in 2009, D'Aniello et al. showed that apelin induces the phosphorylation of p70S6 kinase in murine embryonic stem cells via a pathway dependent on the ERK1 and ERK2 (extracellular signal-regulated kinases, ERK1/2) enzymes [27]. ERK1/2 is phosphorylated in a Gαi- and protein kinase C-dependent manner in CHO cells stably expressing the mouse Apelin-R [15,18].
As with most GPCRs, apelin, upon binding to its receptor, induces the phosphorylation of Apelin-R by GPCR kinases (GRKs), followed by the recruitment of β-arrestin1/2 and the internalization of Apelin-R via a clathrin-dependent mechanism (12–13,15,20,21,27). K17F leads to more extensive internalization than pE13F. The C-terminal phenylalanine of pE13F or K17F is inserted at the bottom of the Apelin-R binding site, into a hydrophobic cavity composed of tryptophans at positions 152 (Trp152) in transmembrane domain IV (TM IV) and 259 (Trp259) in TM VI, and phenylalanine at position 255 (Phe255) in TM VI [13]. The residues Phe255 and Trp259, by interacting with the C-terminal phenylalanine of pE13F or K17F, were crucial for the internalization of Apelin-R but played no role in apelin binding to its receptor or in the coupling of Apelin-R to Gαi. Deletion of the C-terminal phenylalanine of K17F (leading to K16P) or its substitution by alanine (giving rise to K17A) strongly diminishes the peptide’s ability to trigger Apelin-R internalization, without affecting its affinity for the receptor or its ability to activate Gαi coupling [13,16,28]. All these data point to a functional dissociation between Apelin-R coupling to Gαi and receptor internalization. This suggests that Apelin-R may exist in different conformations depending on the ligand binding, triggering the activation of distinct signaling pathways and varying biological effects [13]. Thus, Apelin-R may exhibit functional selectivity or biased signaling—on one hand through its coupling with Gαi and on the other through recruitment of β-arrestin 1/2. This hypothesis was confirmed by Ceraudo et al. in 2014, who showed that K17F activates ERK1/2 phosphorylation in a β-arrestin- and Gαi-protein-dependent manner, whereas K16P activates ERK1/2 phosphorylation only through Gαi [29].
Another endogenous ligand for the apelin receptor: elabela/apela
In 2013, another endogenous ligand for the Apelin-R, apela (Apelin-R early endogenous ligand, also known as Elabela/ Toddler; encoded by a gene on chromosome 4) was discovered [30,31]. The sequence identity between apelin and apela is weak, but both originate from two different precursors that are processed to generate several isoforms [32]. The gene for apela encoded a 54-amino-acid precursor. The 22 aa signal peptide is removed to generate apela-32, which is secreted and bioactive [33,34]. The cleavage of apela-32 by furin was predicted to produce two fragments, apela-22 and apela-11, composed of 21 and 11 amino acids, respectively [30,31]. Apela-11 is fully conserved between species. Apela-32 and apela-22 exhibit subnanomolar affinity for the Apelin-R, whereas apela-11 is less potent [35]. Apela is broadly expressed during development, whereas in adults, high apela mRNA expression is found in the prostate and kidney [36]. Elabela/apela increases urine output and water intake in adult rats, suggesting a role for elabela/apela in the regulation of body fluid homeostasis [37,38]. For a review on apela, see [32].
Distribution of apelin and its receptor in the brain
Reverse transcription and quantitative gene amplification [10,12], in situ hybridization and Northern blot analysis [4,6] have shown that mRNAs encoding preproapelin or Apelin-R are heterogeneously distributed in various brain structures in rats and humans. With regard to apelin, the development of a high-affinity and selective polyclonal antibody against K17F has enabled to visualize the presence of apelinergic neurons in the rat brain (Figure 2) [9,14,39]. The cell bodies of these neurons are particularly abundant in the hypothalamus and medulla oblongata, structures involved in neuroendocrine control, drinking and feeding behaviors, and BP regulation. In particular, immunolabeling was observed in the supraoptic nucleus (SON) and the magnocellular part of the paraventricular nucleus (PVN) of the hypothalamus, the arcuate nucleus, the lateral reticular nucleus, and the nucleus ambiguous (Figure 2) [39]. The fibers and terminals of the apelinergic neurons are more widely distributed, and are particularly dense in the inner layer of the median eminence and in the posterior pituitary (Figure 2) [39,40], suggesting that PVN and SON apelinergic neurons project, like vasopressinergic and oxytocinergic magnocellular neurons, in the posterior pituitary gland. Immunofluorescence double-labeling studies confirmed this hypothesis, showing that apelin co-localized with arginine-vasopressin (AVP; antidiuretic hormone) [9,41] and oxytocin [40] in hypothalamic magnocellular neurons. Numerous apelinergic fibers have also been visualized in the region along the third ventricle, in the lamina terminalis (composed of the subfornical organ, the vascular organ of the lamina terminalis, and the median preoptic nucleus) (Figure 2), involved in the regulation of drinking behavior [42]. Like apelin, its receptor is widely distributed in the rat brain [3,4,6]. Apelin-R mRNA expression was observed by in situ hybridization in the piriform and entorhinal cortex, hippocampus, and structures containing monoaminergic neurons (dopamine, serotonin, and noradrenaline), such as the substantia nigra pars compacta, the dorsal raphe nucleus, and the locus coeruleus. Very strong Apelin-R expression is detected in apelin-rich hypothalamic nuclei, including the PVN, SON, and arcuate nucleus, as well as in the pineal gland and the anterior and intermediate pituitary lobes. In addition, double labeling using immunohistochemistry and in situ hybridization has demonstrated that in the PVN and SON, mRNA encoding Apelin-R [14,43] is co-expressed with AVP and apelin in magnocellular vasopressinergic neurons known to express AVP type 1 a (V1a) and 1b (V1b) receptors [44].
Figure 2. Distribution of apelineric cell bodies and nerve fibers in the adult rat brain.
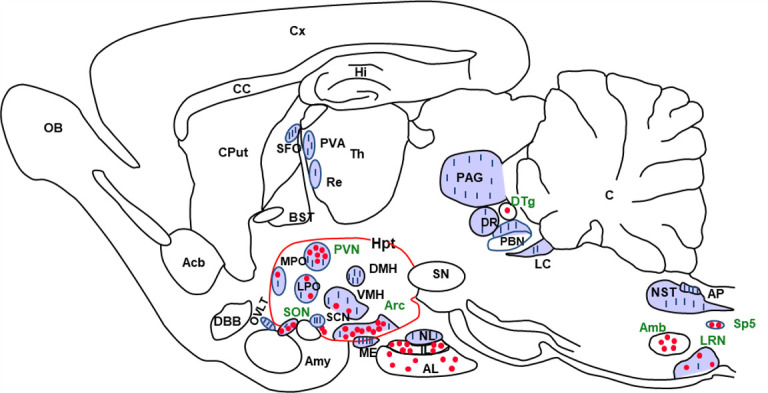
Neuroanatomical distribution of apelin immunoreactive cell bodies and nerve fibers in a sagittal section of rat brain. Cell bodies are shown as dots and nerve fibers are shown as lines. Acb, nucleus accumbens; Amb, nucleus ambiguus; Amy, amygdala; AL, anterior lobe of the pituitary gland; AP, area postrema; Arc, arcuate nucleus; BST, nucleus of the layer of the stria terminalis; C, cerebellum; CC, corpus callosum; Cput, caudate putamen; Cx, cerebral cortex; DBB, diagonal band of Broca; DMH, dorsomedial nucleus of the hypothalamus; DR, dorsal raphe nucleus; DTg, dorsal tegmental nucleus; HI, hippocampus; Hpt, hypothalamus; IL, intermediate pituitary lobe; LC, locus coerulus; LPO, lateral preoptic area; LRN, lateral reticular nucleus; ME, median eminence; MPO, median preoptic nucleus; NL, neural lobe of the pituitary gland; NST, nucleus of the solitary tract; OB, olfactory bulb; OVLT, vascular organ of the lamina terminalis; PAG, periaqueductal grey matter; PBN, parabrachial nucleus; PVA, paraventicular nucleus of the thalamus; PVN, paraventicular nucleus of the hypothalamus; Re, reuniens nucleus of the thalamus; SCN, suprachiasmatic nucleus; SFO, subfornical organ; SN, substantia nigra; SON, supraotpic nucleus; Sp5, spinal nucleus of the trigeminal nerve; Th, thalamus; VMH, ventromedian nucleus of the hypothalamus. Figure adapted from [39] with permission from the copyright holders.
Distribution of apelin and its receptor in the kidney
mRNAs encoding preproapelin, as well as Apelin-R, are expressed in the kidney of rats and humans [4,12,17]. Apelin immunoreactivity is also detected in endothelial cells (ECs) of small intrarenal vessels in humans [45]. In rats, apelin expression has been detected in tubular epithelial cells, glomeruli, and vascular epithelial cells [46], but other authors have reported that apelin expression remains mainly restricted to cells in the medulla [47]. Finally, apelin immunoreactivity has also been visualized in human collecting ducts [48] and in rat medullary collecting ducts, where it co-localizes with aquaporin 2 (AQP2) expression [49]. Apelin-R mRNA expression is detected in ECs and smooth muscle cells of rat glomerular arterioles [24]. In glomeruli, Apelin-R mRNA expression is approximately eight times higher than in the nephron segments, where it is nevertheless present in all segments [4,24]. Finally, the Apelin-R transcript is also localized in the collecting ducts where AVP type 2 receptors (V2R) are also expressed [50]. Apelin-R mRNA expression is lower in the cortical collecting ducts compared with the collecting ducts of the outer medulla and higher in the collecting ducts of the inner medulla [24,47].
Distribution of apelin and its receptor in vessels and heart
Apelin and its receptor are present in the cardiovascular system. High levels of preproapelin and Apelin-R mRNAs, as well as apelin immunoreactivity, have been detected in the rat heart [4,12,17]. Preproapelin mRNA has also been identified in the human heart, though at lower levels [12]. Using radioimmunoassay and immunohistochemistry, apelin has been shown to be highly expressed in ECs and vascular smooth muscle cells (VSMCs) of human cardiac vessels, and in the endocardial EC lining the right atrium [45,51,52]. Apelin and its receptor are abundantly expressed in rat and human arteries and veins [10,45,51,53].
Involvement of apelin in the regulation of body fluid homeostasis via central and renal effects
Central action of apelin on the activity of magnocellular vasopressinergic neurons, systemic AVP release, and diuresis
It has long been established that magnocellular neurosecretory neurons projecting into the posterior pituitary gland release AVP, an antidiuretic and vasoconstrictive peptide, into the bloodstream in response to variations in plasma osmolality and blood volume [54,55] under the action of various neurohormones, including Ang II, Ang III, and natriuretic peptides [56,57]. The co-localization of AVP/apelin in hypothalamic magnocellular neurons and the close association of receptors for both peptides with these neurons suggested the existence of an interaction between AVP and apelin and the possibility of an apelinergic response to osmotic and volemic stimuli. Thus, independently of the direct autocrine feedback regulation exerted by AVP, which increases its own release via its type 1 receptors (V1-R) located on vasopressinergic neurons [57,58], apelin could also modulate the activity of these neurons, and consequently the release of AVP into the bloodstream, by acting on its receptors expressed by vasopressinergic neurons. This hypothesis was checked in the lactating rat model, in which magnocellular vasopressinergic neuron hyperactivity was observed [59] in order to conserve as much water of the body for optimal milk production for newborns [60]. In this model, K17F, injected into the third ventricle, decreases the phasic electrical activity of vasopressinergic neurons, reduces AVP release from the posterior pituitary into the bloodstream, and induces aqueous diuresis [9] (Figure 3). Similarly, a significant reduction in AVP release into the bloodstream was observed after the ICV administration of K17F or pE13F in mice dehydrated during 24 h [14], a condition that also leads to vasopressinergic neuron hyperactivity. These results suggest that apelin, like AVP, may be released by vasopressinergic cell bodies, at the somatodendritic level, to inhibit both the activity of these neurons and systemic AVP release by means of a direct action on apelin autoreceptors located on these neurons. In this way, apelin acts as a natural inhibitor of the antidiuretic effect of AVP and is involved in osmoregulation.
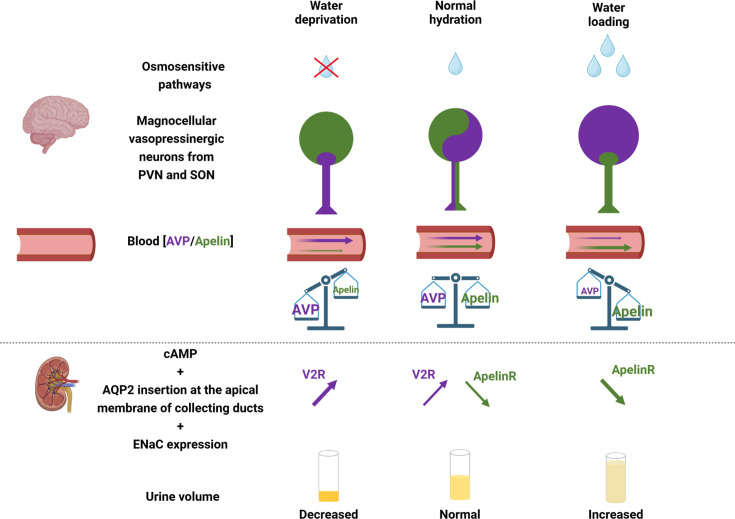
Figure 3. Apelin and vasopressin (AVP), the Yin and the Yang of water balance.
Under physiological conditions, apelin (green) and AVP (purple) are released by magnocellular vasopressinergic neurons at levels appropriate to plasma osmolality. In the renal collecting duct, AVP acts on V2R to increase cAMP production and the insertion of aquaporin 2 (AQP2) at the apical membrane, leading to water reabsorption and urine output decrease. Conversely, apelin, through its action on Apelin-R, has an opposing effect. Under physiological conditions, water reabsorption is adequate and plasma sodium concentrations are normal. Following dehydration, vasopressinergic neurons are strongly activated and AVP is released into the bloodstream more rapidly than it is synthesized, leading to a decrease in neuronal AVP content, while apelin accumulates in the neurons rather than being released. In contrast, under water loading, vasopressinergic neurons are inhibited, which stops AVP release in the blood circulation and results in neuronal AVP accumulation. Conversely, the release of apelin into the bloodstream increases rapidly, leading to a depletion of neuronal apelin content. Thereby, neuronal and plasma apelin levels are regulated by osmotic stimuli in an opposite direction to AVP. Figure adapted from [61] with permission from the copyright holders and made with Biorender©.
Renal action of apelin in the regulation of diuresis
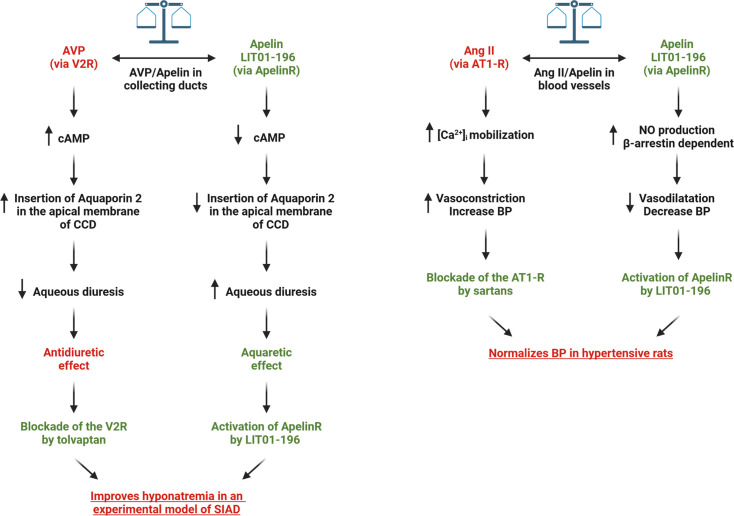
The collecting duct is known to be the site of the antidiuretic action of AVP [62,63]. In this segment, AVP, by stimulating V2R, induces an increase in cAMP production and activates the protein kinase A that phosphorylates the water channel, AQP2. This results in the insertion of phosphorylated AQP2 into the apical membrane of the main cells of the collecting duct [64,65], leading to water reabsorption, decreased diuresis, and increased urinary osmolality. The presence of Apelin-R mRNA along the collecting duct suggests that apelin may act as an aquaretic peptide not only through a central action but also through a direct action on the collecting duct. In line with this hypothesis, application of K17F to the collecting duct inhibits cAMP production induced by (deamino-Cys1, D-Arg8)-vasopressin (dDAVP), a specific and selective V2R agonist (Figures 3 and 4) [67]. Furthermore, in lactating rats, IV administration of K17F induced a significant decrease in AQP2 immunostaining at the apical membrane of the collecting duct along a cortico-medullary gradient (Figures 3 and 4) [67]. Finally, K17F injected IV in lactating rats [24] or apelin-13 IV administered in increasing doses [37] or infused IV for 24 h in adult male rats [38] significantly increases diuresis in a dose-dependent manner (Figures 3 and 4). Simultaneously, there is a significant decrease in urinary osmolality with no change in Na+ and K+ excretion. These data were recently reinforced by the work of Boulkeroua et al. [68]. These authors reported that, in a highly differentiated mouse cortical collecting duct cell line (mpkCCD) expressing V2R and Apelin-R, application of apelin-13 decreased dDAVP-induced phosphorylation and apical membrane expression of AQP2 after 30–60 min of treatment, as well as dDAVP-induced expression of AQP2 mRNA after 8–24 h of treatment. More recently, another study showed that the diuretic action of pE13F would involve the cAMP/protein kinase A/soluble prorenin receptor pathway in the collecting duct [49].
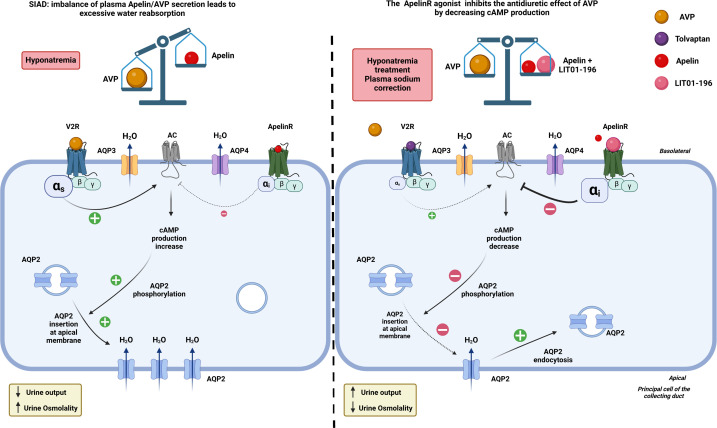
Figure 4. In an experimental model of SIAD, AVP secretion into the bloodstream is abnormally high in relation to plasma osmolality.
By binding to V2R in the collecting duct, AVP increases cAMP production, mobilizes aquaporin 2 (AQP2) to the apical membrane, and enhances water reabsorption, leading to hyponatremia. Administration of LIT01-196, a metabolically stable analogue of apelin-17 (K17F), stimulates Apelin-R located in the collecting ducts. This inhibits AVP-induced cAMP production, reduces the mobilization of AQP2 to the apical membrane, and decreases water reabsorption, thereby promoting diuresis. Moreover, Apelin-R activation reduces transepithelial amiloride-sensitive sodium current and decreases αENaC subunit expression, which in turn reduces sodium reabsorption. This leads to a progressive improvement in hyponatremia. Figure adapted from [66] with permission from the copyright holders and made with Biorender©.
Moreover, apelin gene expression in the brain has also been reported to be hydration-sensitive (88). Thus, the aquaretic effect of apelin is due in part to its direct action in the kidney, which inhibits the antidiuretic effect of AVP, occurring via V2R in the collecting duct. The opposing effects of apelin and AVP in this structure help to control plasma osmolality by regulating water reabsorption by the kidney (Figure 3).
It is also worth noting that the function of AQP2 is tightly coordinated with that of the Epithelial Sodium Channel (ENaC), a channel that constitutes the rate-limiting step for transepithelial sodium transport in the collecting duct. Interestingly, Ayari and Chraibi [69] have recently reported that in mpkCCD cells in the continuous presence of aldosterone, apelin-13 reduces transepithelial amiloride-sensitive sodium current and decreases αENaC subunit expression. AVP at a high concentration increases ENaC activity and reduces sodium excretion [70]. Sodium and water conservation or excretion are often associated. This suggests that apelin and AVP are also involved in the regulation of water balance by conversely regulating the ability of the kidney to excrete sodium because of their opposite effects on ENaC activity and expression.
Apelin-R mRNA expression has also been detected in ECs and VSMCs of glomerular arterioles [24], and the application of apelin on these arterioles pre-contracted by Ang II induces vasorelaxation. This vasodilatory effect of apelin is NO-dependent and directly linked to the inhibition of Ang II-induced increase in [Ca2+]i [24]. Apelin-R mRNA expression in glomeruli and glomerular arterioles and the apelin-dependent vasorelaxation in muscular efferent arterioles (which originate from the juxtamedullary cortex and give rise to the vasa recta), strongly suggest that apelin, by binding to its receptors located in these structures, causes an increase in renal blood flow, which also participates in the increase in diuresis induced by this peptide [24].
Taken together, these data underline the crucial role of apelin in the maintenance of body fluid homeostasis through its action not only at the brain level, where it inhibits AVP release from the posterior pituitary into the bloodstream, but also directly at the renal level, where it blocks the antidiuretic effect of AVP in the collecting duct and increases renal blood flow (Figure 3).
Opposite regulation of apelin and vasopressin in the maintenance of body fluid homeostasis
The co-localization of AVP and apelin within the same neurons and their opposing actions on diuresis led to an investigation of the regulation of these peptides in the maintenance of body fluid homeostasis. Although both peptides are co-localized in the same neurons, in rats dehydrated for 24 h, the hyperactivity of vasopressinergic neurons and the concomitant increase in systemic AVP secretion are accompanied by a decrease in plasma apelin levels (Figure 3) [9]. Parallel quantitative immunohistochemical studies in water-deprived rats revealed an increase in apelin neuronal content in vasopressinergic magnocellular neurons of the PVN and SON, while the neuronal content of AVP fell sharply (Figure 3) [41]. This suggests that, in water-deprived conditions, apelin accumulates in vasopressinergic neurons rather than being released. Furthermore, the increase in neuronal apelin content observed in dehydrated animals was inhibited by ICV administration of a selective V1 receptor antagonist and mimicked by ICV administration of AVP [9,41]. This suggests that this increase is due to increased somatodendritic release of AVP secondary to dehydration. This interpretation implies that apelin and AVP are released differentially by vasopressinergic magnocellular neurons in which they are co-localized. In line with this hypothesis, double labeling studies using confocal microscopy have shown that the two peptides are largely found in neurosecretory granules of distinct size and distribution in magnocellular vasopressinergic neurons [9,41].
Taken together, these data show that during dehydration, apelin and AVP play a complementary role in maintaining body fluid homeostasis. While increased somatodendritic release of AVP enhances the phasic electrical activity of vasopressinergic neurons [57,58], thereby facilitating the release of AVP into the bloodstream, apelin accumulates in these neurons instead of being released into the bloodstream, reducing the inhibition it exerts on vasopressinergic neurons [9], and thereby amplifying the phasic electrical activity of vasopressinergic neurons (Figure 3).
This cross-regulation of apelin and AVP following osmotic stimulation has also been demonstrated in humans [11]. In that study, ten healthy male volunteers were subjected either to a 12 h water restriction followed by an infusion of hypertonic saline in order to increase plasma osmolality or to oral water loading in order to decrease plasma osmolality. An increase in plasma osmolality is accompanied by a fall in plasma apelin levels, while plasma AVP levels increase linearly. Conversely, the decrease in plasma osmolality produced a reduction in plasma AVP concentration and a rapid rise in plasma apelin concentration (Figure 3).
These results show, in humans, that apelin and AVP secretions are conversely regulated by osmotic stimuli, suggesting that both apelin and AVP could play an important role in maintaining body fluid homeostasis in both humans and rodents (Figure 3).
Involvement of the apelin/AVP balance in hyponatremia
Hyponatremia
Hyponatremia, defined as a plasma sodium concentration of less than 135 mmol/L, is the most common electrolyte disorder in hospitalized patients. Hyponatremia has been associated with various pathologies, including the syndrome of inappropriate antidiuresis (SIAD), chronic heart failure, chronic renal failure, and cirrhosis [71]. Hyponatremia is important to be detected as it is associated with high mortality rates [72–74] and may be a marker of underlying disease.
Syndrome of inappropriate antidiuresis
SIAD, previously known as syndrome of inappropriate antidiuretic hormone secretion (SIADH), is the most common cause of hyponatremia. Many clinical conditions can cause SIAD, including tumors, which can ectopically secrete AVP, central nervous system disorders, and lung disease. A number of drugs can also induce a SIAD, by increasing AVP secretion, such as tricyclic antidepressants, serotonin reuptake inhibitors, and opiates, and/or by potentiating its effect, such as carbamazepine, chlorpropamide, and anti-inflammatory drugs [75]. In SIAD, plasma AVP levels are abnormally high with respect to plasma osmolality [71]. By acting on the V-2 receptors present in the collecting ducts, the high concentration of AVP increased cAMP production, leading to greater insertion of AQP2 into the apical membrane, resulting in higher water reabsorption and reduced urine output. This results in an increased blood volume and plasma sodium dilution, responsible for hyponatremia (Figure 4) [76]. Severe symptoms, such as coma, convulsions, and respiratory arrest, are usually associated with acute, life-threatening hyponatremia. Less severe symptoms such as headache, irritability, nausea/vomiting, mental slowing, confusion, and disorientation are observed in chronic hyponatremia [77]. Given the opposite regulation of plasma apelin and AVP levels by osmotic stimuli in healthy subjects, the response of apelin to the osmoregulatory defect of AVP in SIAD was investigated [78]. In SIAD patients, age- and sex-adjusted plasma levels of apelin and copeptin (a 39-amino acid peptide derived, like AVP, from pre-pro-vasopressin, but more stable than AVP and released in equimolar quantities with AVP into the bloodstream, shown to be a good marker of AVP secretion) are increased by 26% and 75%, respectively, compared with those measured in healthy subjects [78]. Apelin/copeptin ratios as a function of natremia were outside the 95% predicted physiological limits for 86% of SIAD patients that may contribute to the corresponding water metabolism defect [78]. It was therefore hypothesized that the activation of the Apelin-R by an agonist of this receptor could counteract the increased water reabsorption induced by increased AVP levels and correct hyponatremia.
Effects of a metabolically stable apelin analog in an experimental model of hyponatremia
Development and pharmacological characterization of LIT01-196
In mouse plasma, K17F and pE13F have a half-life (ex vivo) of 4.6 and 7.2 min, respectively [25], and pE13F has a half-life of 14 min in rat plasma [79]. After perfusion of apelin-36 in healthy human subjects, its half-life is less than 5 min [80]. The in vivo half-life of K17F in the bloodstream after IV administration is 44 and 50 s in mice and rats, respectively [66]. These short half-lives are due to the rapid metabolism of apelin by enzymes such as angiotensin-converting enzyme 2 (ACE2, EC 3.4.17.23) or neutral endopeptidase 24.11 (NEP, neprilysin, EC 3.4.24.11) [28,81]. The rapid disappearance of apelin in vivo has encouraged the development of metabolically stable apelin analogs for potential therapeutic applications.
Numerous approaches, such as PEGylation [82–84], chemical modifications within the apelin RPRL motif [20], palmitoylation, and the use of unnatural amino acids [85–87] or main chain modifications (cyclization) [88–90], have been used to increase the half-life of apelin. Most studies aimed at developing metabolically stable analogs of apelin have focused on pE13F [25,86,88–91] and apelin-36 [82,83]. However, K17F has a tenfold higher affinity than pE13F for the Apelin-R, and is more potent at inducing ß-arrestin mobilization (20-fold) and rat Apelin-R internalization (10- to 30-fold) than pE13F [25] and is also more active in reducing BP than pE13F [16].
Taking into account these data, metabolically stable K17F analogs have been developed using the attachment of an N-terminal C16 fatty acid chain (PALMitoylation) or polyethylene glycol chain (PEGylation) on K17F. The N-terminally PEGylated apelin-17 (A2) is a highly protease-resistant analog (t1/2 = 18 h in plasma), with high affinity for the Apelin-R (subnanomolar) and a pronounced blood pressure (BP) lowering effect [87]. Another original strategy, based on the introduction of a fluorocarbon chain directly to the N-terminal part of K17F was also used, generating LIT01-196 [25]. LIT01-196 has a high affinity for the Apelin-R (Ki = 0.08 nM) and is much more stable in plasma (half-life > 24 h) than K17F (4.6 min). LIT01-196 showed remarkable resistance to plasma degradation enzymes, with >90% of the peptide remaining intact after 24 h incubation in mouse plasma at 37°C. LIT01-196 exhibits a complete agonist profile for Apelin-R, whether for cAMP production, ERK1/2 phosphorylation, β-arrestin recruitment, or Apelin-R internalization.
Effects of LIT01-196 on AVP release and on the antidiuretic effect of AVP in control animals
Intracerebroventricular (ICV) administration of LIT01-196 in increasing doses in mice significantly decreases systemic AVP release induced by dehydration, in a dose-dependent manner, and is 160-fold more effective than K17F [25]. These data suggest that centrally administered LIT01-196, like K17F, rapidly reaches hypothalamic structures, the PVN and SON, and then acts on Apelin-Rs expressed by magnocellular vasopressinergic neurons to inhibit AVP release into the blood circulation and increase aqueous diuresis. Furthermore, in the kidney, at the level of the collecting duct, LIT01-196 decreases dDAVP-induced cAMP production and the insertion of phosphorylated AQP2 at the apical surface, leading to decreased water reabsorption and consequently increased aqueous diuresis [25,66] (Figures 4 and 5). LIT01-196 and tolvaptan, a V2 receptor antagonist, administered at an equimolar dose of 900 nmol/kg by the subcutaneous route to normonatremic rats, similarly increase 24 h urine output by 79% and 77%, respectively, and decrease urinary osmolality by 52% and 40%, respectively (Figure 4) [66]. The urine output increase was accompanied by a significant increase in water intake in rats treated with tolvaptan (+ 37%) and a slight non-significant increase for those treated with LIT01-196 (+ 11%). The urinary sodium excretion fraction was not modified by treatment with LIT01-196 or tolvaptan [66]. On the other hand, LIT01-196, like K17F, induces vasorelaxation of rat juxtamedullary arterioles that give rise to the vasa recta [25], suggesting that LIT01-196, like K17F, may also increase urine output by increasing renal blood flow [24].
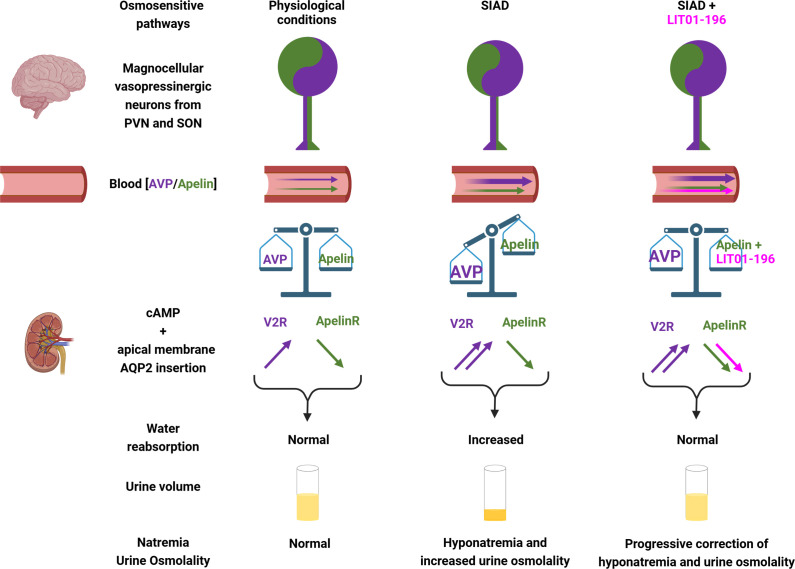
Figure 5. Apelin and vasopressin regulation in SIAD and effects of LIT01-196 in this pathology.
In normal conditions of hydration, apelin and AVP, co-localized in magnocellular vasopressinergic neurons, are released in the blood circulation, and the balance between apelin and AVP allows both peptides by activating at the kidney level the V2R and the Apelin-R to induce normal water reabsorption and urine volume and ensure normal natremia. In SIAD, there is a high amount of AVP released in the blood circulation relative to osmolality, whereas plasma apelin levels are slightly modified, and the AVP/apelin balance is not reached, increasing water reabsorption, decreasing aqueous diuresis, and leading to hyponatremia. In SIAD, treatment with LIT01-196 allows to re-establish the balance between AVP and apelin by the supplementary activation of the Apelin-Rby LIT01-196. This counteracts vasopressin-induced water reabsorption and correct hyponatremia. AVP, arginine-vasopressin; ENaC, epithelial sodium channel; PVN, paraventicular nucleus of the hypothalamus; SON, supraotpic nucleus; V2R, AVP type 2 receptor. Figure adapted from [66] with permission from the copyright holders and made with Biorender©.
Taken together, these data indicate that LIT01-196, through its central and renal effects, appears to be an effective aquaretic and could be particularly useful for the treatment of water retention and hyponatremia.
Effects of LIT01-196 on the antidiuretic effect of AVP in an experimental model of hyponatremia
Subcutaneous infusion of AVP (sc, 30 ng/h) to rats for 4 days, combined with a semiliquid diet, results in a decrease in urine output, an increase in urinary osmolality, and a decrease in plasma sodium levels, stable at around 100 mmol/L for 2–4 days after the start of infusion. In these conditions, the balance between apelin and AVP is lost. Administration of LIT01-196 (900 nmol/kg = 3 mg/kg, sc) in this hyponatremia model for 2 days (to supplement the low rate of apelin compared with AVP) significantly inhibits the effects of AVP on urine output and urinary osmolality and induces a progressive correction of plasma sodium levels (Figures 4 and 5) [66]. As previously published [92], tolvaptan, at an equimolar dose, similarly inhibits the antidiuretic effects of AVP. However, if the dose of tolvaptan is increased, an even greater increase in diuresis occurs, in contrast with what is observed when the dose of LIT01-196 is increased. These data suggest that LIT01-196, by re-establishing the apelin/AVP balance in the collecting duct, inhibits the increased production of cAMP induced by continuous infusion of AVP, thereby inhibiting the massive insertion of AQP2 at the apical membrane of the collecting duct, leading to inhibition of water reabsorption by the kidney and an increase in aqueous diuresis, resulting in the progressive correction of hyponatremia (Figures 4 and 5).
Consequently, in SIAD, the activation of the Apelin-R by LIT01-196 in the collecting duct (Figure 5), compared with blocking the effects of AVP on the V2R by tolvaptan, would result in less severe polyuria and may be better tolerated than when treated with V2 receptor antagonists.
The various physiological effects associated with Apelin-R activation result from the activation of different signaling pathways. Consequently, the development of metabolically stable biased K17F analogs, which could target only the Gi signaling pathway involved in the aquaretic effect of apelin, represents an interesting strategy to improve their specificity of action on water metabolism.
Moreover, treatment with metabolically stable apelin analogs may also be useful for the treatment of autosomal dominant polycystic kidney disease [93], a disorder linked to excessive AVP secretion, although chronic treatment with tolvaptan is effective but associated with intense thirst, polyuria (24 h diuresis around 6 L/day), nocturia [94], and a rapid plasma sodium concentration correction [95,96] or for the treatment of nephrogenic syndrome of inappropriate antidiuresis (NSIAD). NISAD was due to gain-of-function mutations of the V2R. Patients with NSIAD have reduced free water excretion and concentrated urine despite hyponatremia and low or undetectable circulating AVP levels [97]. Nevertheless, mutated V2R displayed constitutive cAMP production and a high rate of ß-arrestin-dependent constitutive internalization [98,99]. Tolvaptan and satavaptan are inefficient in vitro as well as in vivo in reducing constitutive cAMP production induced by these variants [100,101]. Thereby, the use of a metabolically stable apelin analog for the treatment of NSIAD could be of potential therapeutic interest, since, by activating the Apelin-R, it will reduce cAMP production, decreasing the insertion of AQP-2 at the apical membrane of collecting duct cells, and thereby increasing urine output. This remains to be assessed in further experimental preclinical and clinical studies.
Cardiovascular effects of apelin
Effects of apelin and apelin analogs in the regulation of blood pressure
Apelin, by acting on the endothelium of human mammary artery, human splanchnic arteries, or rat glomerular arterioles, induces vasodilation by increasing NO [24,102,103]. In contrast, apelin induces vasoconstriction of VSMCs in endothelium-denuded arteries [53]. Several in vivo studies have reported that apelin administered in normotensive rodents induces a rapid dose-dependent and NO-dependent BP decrease [23,102,104,105]. This vasodilatory effect has also been observed in humans, where infusions of apelin-13 and apelin-36 result in a dose-dependent and NO-dependent arteriolar vasodilation in the forearm [88,106]. In healthy volunteers, apelin-13 induces a BP decrease, reduces peripheral vascular resistance, and induces a slight increase in heart rate, which was probably a compensatory effect to the decrease in BP [80].
The apelin system has opposite effects to the renin-angiotensin system in the control of BP (Figure 6) [107,108]. Both systems may also reciprocally regulate each other. Apelin decreases Ang II-induced [Ca2+]i mobilization in glomerular arterioles, inducing a reversion of the Ang II-induced vasoconstriction (Figure 6) [24], and Apelin-R-deficient mice exhibited a higher BP increase induced by Ang II compared with that measured in wild type mice in the same conditions. Moreover, the baseline BP of double mutant mice homozygous for both Apelin-R and AT1-R was significantly elevated compared with that of AT1-R-deficient mice [102]. These results demonstrate that apelin, by activating the Apelin-R, plays a counterregulatory role against the pressor action of Ang II via the production of NO. Interestingly, in the blood circulation, ACE2, a zinc metalloprotease, anchored in ECs, with its active site in the bloodstream may also be involved in this regulation [109]. ACE2 converts Ang II (vasoconstrictor) into another peptide angiotensin 1–7 (vasorelaxant) by removing the C-terminal phenylalanine of Ang II [110]. Likewise, by removing the C-terminal phenylalanine of K17F, ACE2 converts K17F (vasorelaxant) into K16P, the capacity of which to decrease BP and increase cardiac contractility is highly reduced [16,28].
Figure 6. Potential therapeutic effects of LIT01-196 in SIAD and hypertension.
By activating ApelinR in the collecting duct, LIT01-196 would counteract the overactivation of V2R by decreasing cAMP production, leading to a decrease in the insertion of aquaporin 2 in the apical membrane, resulting in the increase in aqueous diuresis. Together with tolvaptan that inhibits V2R, LIT01-196 would improve hyponatremia in an experimental model of SIADH. In the other hand, by activating ApelinR in the endothelial cells of blood vessel, LIT01-196 would counteract the overactivation of AT1-R by increasing NO production in a β-arrestin-dependent manner, leading to a vasodilatation and a decrease in blood pressure. Together with sartans that block AT1-R, LIT01-196 would normalizes blood pressure in hypertensive rats. The figure was made with Biorender©. Ang II, angiotensin II; AT1-R, angiotensin II type 1 receptor; AVP, arginine-vasopressin; BP, blood pressure; CCD, cortical collecting duct; NO, nitric oxide; SIAD, syndrome of inappropriate antidiuresis; SIADH, syndrome of inappropriate antidiuretic hormone; V2R, AVP type 2 receptor.
Flahault et al. [105] have studied in hypertensive DOCA-salt rats (an experimental model of hypertension, resistant to treatment with blockers of the renin-angiotensin system exhibiting low plasma renin levels and high plasma AVP levels) the effects of K17F and the metabolically stable apelin analog, LIT01-196: Acute IV LIT01-196 administration, in increasing doses, dose-dependently decreases arterial BP with ED50 values of 9.8 and 3.1 nmol/kg in normotensive rats and DOCA-salt rats, respectively. This effect occurs for both via a NO-dependent mechanism (Figure 6). Moreover, acute subcutaneous LIT01-196 administration (90 nmol/kg) normalizes arterial BP in conscious hypertensive DOCA-salt rats for more than 7 h. The LIT01-196-induced BP decrease remains unchanged after four consecutive daily subcutaneous administrations of 90 nmol/kg, and does not induce any alteration of plasma sodium and potassium levels and kidney function. Thereby, activating the Apelin-R may constitute a novel pharmacological approach for the treatment of hypertension (Figure 6).
In addition, different studies have shown that the administration of apelin 13 in specific brain nuclei, the paraventricular nucleus, the nucleus tractus solitarius, or the rostral ventrolateral medulla in normotensive or spontaneously hypertensive rats (SHR) increased BP [111,112]. Moreover, microinjection of [Pyr1]apelin-13 into any of the three circumventricular organs (subfornical organ, organum vasculosum of the lamina terminalis, and area postrema) decreased BP, and this was greater in hypertensive rats than in normotensive rats [113]. On the other hand, central administration of apelin 13 was shown to decrease the pressor response induced by sudden stress in SHR but not in normotensive rats. It also abolished the difference in stress-induced BP increases between normotensive rats and SHR [114]. Further studies are required to deepen the role of brain apelin in the regulation of BP under various physiopathological conditions.
Effects of apelin and apelin analogs in the regulation of cardiac function
Apelin is one of the most potent endogenous positive inotropic peptides [115,116]. Apelin increases conduction velocity in cardiomyocytes and induces a shortening of action potential in atrial myocytes [117,118]. Apelin potently inhibited Ang II-induced atrial fibrosis and the subsequent vulnerability to atrial fibrillation induction [119]. Apelin administration increases cardiac output in vivo in rodents [116,120]. Subcutaneous infusion of apelin-13 in rats over 3 weeks was shown to decrease Ang II-induced cardiac fibrosis and plasminogen activator inhibitor type-1 (PAI-1) gene expression, through which Ang II causes cardiovascular remodeling [121].
Apelin-deficient mice exhibit progressive cardiac contractility impairment together with systolic dysfunction in the absence of histological abnormalities. Moreover, heart failure produced by pressure overload in apelin-deficient mice is also more severe than in wild-type mice [122]. Apelin infusion for 2 weeks in apelin-deficient mice reestablished the impaired cardiac function at the same level measured in wild-type mice [123].
Administration of apelin in rodents after myocardial infarction improved functional recovery and reduced infarct size, most likely due to increased NO production and angiogenesis [124,125]. Administration of apelin for 2 weeks after aortic banding prevented cardiac remodeling by inhibiting myocyte hypertrophy, cardiac fibrosis, and ventricular dysfunction [126]. In heart failure patients, acute administration of apelin, by intravenous route, increases cardiac output and left ventricular (LV) ejection fraction while reducing BP and vascular resistance [80,127].
In renal hypertensive rats that exhibit some of the common phenotypes of human hypertension-induced heart failure, including cardiac hypertrophy and fibrosis, diastolic dysfunction, and increased brain natriuretic peptide, as well as decreased stroke volume (SV) and cardiac output (CO), chronic treatment with BMS-986224, a non-peptidic orally active Apelin-R agonist, elicits cardiac improvements in SV and CO. There are no significant differences in LV mass, cardiac fibrosis, LV end-diastolic volume, or LV ejection fraction [128]. This is in contrast with enalapril, an angiotensin I converting enzyme inhibitor, which prevented cardiac hypertrophy, cardiac fibrosis, and diastolic dysfunction.
In an experimental model of heart failure after myocardial infarction, chronic treatment with another non-peptidic orally active Apelin-R agonist, AM-8123 [129], resulted in sustained improvement in systolic function, reflected by a significant increase in LV ejection fraction and a decrease in both end-diastolic volume and end-systolic volume. Additionally, chronic AM-8123 administration improved diastolic function (isovolumic relaxation time and the E/E′ ratio), but LV end-diastolic pressure and tau (the relaxation constant) are not modified in contrast with treatment with an AT1-R antagonist, losartan. Chronic administration of AMG-8123 also attenuated fibrosis but has no effect on cardiac hypertrophy. Together these data suggest that the activation of the Apelin-R by an ApelinR agonist could constitute an additional approach for the treatment of heart failure.
Moreover, during sepsis, the heart is one of the organs that are vulnerable to damage. The delivery of apelin in septic rodents was effective in attenuating myocardial injury and improving cardiac function [130,131]. In a rat model of sepsis, apelin-13 showed superior performance compared with dobutamine (inotrope commonly used in sepsis) to significantly improve LV function and allow higher survival rate [132]. Apelin was also shown to decrease cardiomyocyte apoptosis in septic rats, leading to an improvement of cardiac function [132,133]. Altogether, this suggests that targeting the Apelin/APJ system may be a promising strategy to treat cardiac dysfunction in sepsis.
Conclusions
The experimental data obtained to date demonstrate that apelin exerts an aquaretic action through central and renal effects: through its inhibitory role on the phasic electrical activity of vasopressinergic neurons and the secretion of AVP into the bloodstream and through its direct action at the kidney level on renal microcirculation and tubular function via inhibition of the antidiuretic effect of AVP mediated by V2 receptors in the collecting duct. In addition, following water deprivation or water loading, in both humans and rodents, AVP and apelin are conversely regulated to optimize the systemic release of AVP in the bloodstream and thus avoid additional water loss from the kidneys during dehydration, or to facilitate the release of apelin to increase water elimination in order to restore water balance during water loading. These data show that the balance between apelin and AVP plays a crucial role in maintaining body fluid homeostasis in humans and rodents. Clinically, in SIAD patients, the apelin/AVP balance is altered, which contributes to the defect in water metabolism. Activation of Apelin-R by a metabolically stable apelin-17 analog, LIT01-196, by inhibiting the antidiuretic effect of AVP, increasing aqueous diuresis and moderately increasing water intake, and progressively correcting hyponatremia, may represent an additional therapeutic approach for the treatment of hyponatremia (Figure 6).
The apelin system is also a promising therapeutic target for a range of cardiovascular diseases. There is evidence for the benefit of Apelin-R agonists in experimental models of hypertension. Ang II and apelin have opposite effects on the regulation of BP, whereas Ang II, by binding to AT1 receptors present in VSMCs, induces intracellular calcium mobilization and vasoconstriction, apelin, by binding to Apelin-Rs present on vascular endothelium, increases NO production, and induces vasodilation. Moreover, after myocardial infarction, whereas high Ang II is deleterious, apelin is protective. Treatment with an angiotensin I-converting enzyme inhibitor, by blocking the formation of Ang II, is the treatment of reference to prevent the development of heart failure, treatment with an Apelin-R agonist may be useful to preserve cardiac function and avoid hypertrophy and fibrosis (Figure 6).
Abbreviations
- ACE2
angiotensin converting enzyme 2
- AT1-R
angiotensin II type 1 receptor
- AVP
arginine-vasopressin
- Ang II
angiotensin II
- Apelin-R
apelin receptor
- BP
blood pressure
- CCD
cortical collecting duct
- CO
cardiac output
- EC
endothelial cell
- ENaC
epithelial sodium channel
- HUVEC
human umbilical vein endothelial cell
- ICV
intracerebroventricular
- LV
left ventricular
- NEP
neutral endopeptidase
- NO
nitric oxide
- NSIAD
nephrogenic syndrome of inappropriate antidiuresis
- PAI-1
plasminogen activator inhibitor type 1
- PVN
paraventricular nucleus
- SHR
spontaneously hypertensive rat
- SIAD
syndrome of inappropriate antidiuresis
- SIADH
syndrome of inappropriate antidiuretic hormone secretion
- SON
supraoptic nucleus
- SV
stroke volume
- TM IV
transmembrane domain IV
- V2-R
AVP type 2 receptor
- VSMC
vascular smooth muscle cell
- mpkCCD
mouse cortical collecting duct cell line
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the French National Institute of Health and Medical Research, the Collège de France, the French Federation for Cardiology (R24097FF) and the University Paris-Saclay (HeaDS Core 2024)
CRediT Author Contribution
P.C. & C.L-C. wrote the original draft and reviewed manuscript. All authors approved the final version of the manuscript.
References
- 1.O’Dowd B.F., Heiber M., Chan A., Heng H.H., Tsui L.C., Kennedy J.L., et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 2.Devic E., Rizzoti K., Bodin S., Knibiehler B., Audigier Y. Amino acid sequence and embryonic expression of msr/APJ, the mouse homolog of Xenopus X-msr and human APJ. Mech. Dev. 1999;84:199–203. doi: 10.1016/s0925-4773(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 3.De Mota N., Lenkei Z., Llorens-Cortès C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology. 2000;72:400–407. doi: 10.1159/000054609. [DOI] [PubMed] [Google Scholar]
- 4.O’Carroll A.M., Selby T.L., Palkovits M., Lolait S.J. Distribution of mRNA encoding B78/APJ, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim. Biophys. Acta. 2000;1492:72–80. doi: 10.1016/s0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 5.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X., et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 6.Lee D.K., Cheng R., Nguyen T., Fan T., Kariyawasam A.P., Liu Y., et al. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 7.Habata Y., Fujii R., Hosoya M., Fukusumi S., Kawamata Y., Hinuma S., et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta. 1999;1452:25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 8.Galanth C., Hus-Citharel A., Li B., Llorens-Cortès C. Apelin in the control of body fluid homeostasis and cardiovascular functions. Curr. Pharm. Des. 2012;18:789–798. doi: 10.2174/138161212799277770. [DOI] [PubMed] [Google Scholar]
- 9.De Mota N., Reaux-Le Goazigo A., El Messari S., Chartrel N., Roesch D., Dujardin C., et al. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamata Y., Habata Y., Fukusumi S., Hosoya M., Fujii R., Hinuma S., et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim. Biophys. Acta. 2001;1538:162–171. doi: 10.1016/s0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 11.Azizi M., Iturrioz X., Blanchard A., Peyrard S., De Mota N., Chartrel N., et al. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J. Am. Soc. Nephrol. 2008;19:1015–1024. doi: 10.1681/ASN.2007070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medhurst A.D., Jennings C.A., Robbins M.J., Davis R.P., Ellis C., Winborn K.Y., et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003;84:1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 13.Iturrioz X., Gerbier R., Leroux V., Alvear-Perez R., Maigret B., Llorens-Cortes C. By interacting with the C-terminal Phe of apelin, Phe255 and Trp259 in helix VI of the apelin receptor are critical for internalization. J. Biol. Chem. 2010;285:32627–32637. doi: 10.1074/jbc.M110.127167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reaux A., De Mota N., Skultetyova I., Lenkei Z., El Messari S., Gallatz K., et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J. Neurochem. 2001;77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 15.Masri B., Lahlou H., Mazarguil H., Knibiehler B., Audigier Y. Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem. Biophys. Res. Commun. 2002;290:539–545. doi: 10.1006/bbrc.2001.6230. [DOI] [PubMed] [Google Scholar]
- 16.El Messari S., Iturrioz X., Fassot C., De Mota N., Roesch D., Llorens-Cortes C. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J. Neurochem. 2004;90:1290–1301. doi: 10.1111/j.1471-4159.2004.02591.x. [DOI] [PubMed] [Google Scholar]
- 17.Hosoya M., Kawamata Y., Fukusumi S., Fujii R., Habata Y., Hinuma S., et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem. 2000;275:21061–21067. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- 18.Masri B., Morin N., Pedebernade L., Knibiehler B., Audigier Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 2006;281:18317–18326. doi: 10.1074/jbc.M600606200. [DOI] [PubMed] [Google Scholar]
- 19.Bai B., Tang J., Liu H., Chen J., Li Y., Song W. Apelin-13 induces ERK1/2 but not p38 MAPK activation through coupling of the human apelin receptor to the Gi2 pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2008;40:311–318. doi: 10.1111/j.1745-7270.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 20.Choe W., Albright A., Sulcove J., Jaffer S., Hesselgesser J., Lavi E., et al. Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J. Neurovirol. 2000;6:S61–9. doi: 10.3109/13550280009006383. [DOI] [PubMed] [Google Scholar]
- 21.Zhou N., Fan X., Mukhtar M., Fang J., Patel C.A., DuBois G.C., et al. Cell–cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology (Auckl) 2003;307:22–36. doi: 10.1016/S0042-6822(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhou N., Zhang X., Fan X., Argyris E., Fang J., Acheampong E., et al. The N-terminal domain of APJ, a CNS-based coreceptor for HIV-1, is essential for its receptor function and coreceptor activity. Virol. (Auckl.) 2003;317:84–94. doi: 10.1016/j.virol.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Tatemoto K., Takayama K., Zou M.X., Kumaki I., Zhang W., Kumano K., et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 24.Hus-Citharel A., Bouby N., Frugière A., Bodineau L., Gasc J.-M., Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 2008;74:486–494. doi: 10.1038/ki.2008.199. [DOI] [PubMed] [Google Scholar]
- 25.Gerbier R., Alvear-Perez R., Margathe J.-F., Flahault A., Couvineau P., Gao J., et al. Development of original metabolically stable apelin-17 analogs with diuretic and cardiovascular effects. FASEB J. 2017;31:687–700. doi: 10.1096/fj.201600784R. [DOI] [PubMed] [Google Scholar]
- 26.Eyries M., Siegfried G., Ciumas M., Montagne K., Agrapart M., Lebrin F., et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ. Res. 2008;103:432–440. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 27.D’Aniello C., Lonardo E., Iaconis S., Guardiola O., Liguoro A.M., Liguori G.L., et al. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circ. Res. 2009;105:231–238. doi: 10.1161/CIRCRESAHA.109.201186. [DOI] [PubMed] [Google Scholar]
- 28.Wang W., McKinnie S.M.K., Farhan M., Paul M., McDonald T., McLean B., et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension. 2016;68:365–377. doi: 10.1161/HYPERTENSIONAHA.115.06892. [DOI] [PubMed] [Google Scholar]
- 29.Ceraudo E., Galanth C., Carpentier E., Banegas-Font I., Schonegge A.M., Alvear-Perez R., et al. Biased signaling favoring gi over β-arrestin promoted by an apelin fragment lacking the C-terminal phenylalanine. J. Biol. Chem. 2014;289:24599–24610. doi: 10.1074/jbc.M113.541698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chng S.C., Ho L., Tian J., Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Pauli A., Norris M.L., Valen E., Chew G.-L., Gagnon J.A., Zimmerman S., et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read C., Nyimanu D., Williams T.L., Huggins D.J., Sulentic P., Macrae R.G.C., et al. International union of basic and clinical pharmacology. CVII. structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler Is a scond endogenous peptide ligand. Pharmacol. Rev. 2019;71:467–502. doi: 10.1124/pr.119.017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soulet F., Bodineau C., Hooks K.B., Descarpentrie J., Alves I., Dubreuil M, et al. ELA/APELA precursor cleaved by furin displays tumor suppressor function in renal cell carcinoma through mTORC1 activation. JCI Insight. 2020;5:129070. doi: 10.1172/jci.insight.129070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang P., Read C., Kuc R.E., Buonincontri G., Southwood M., Torella R., et al. Elabela/Toddler is an Endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation. 2017;135:1160–1173. doi: 10.1161/CIRCULATIONAHA.116.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couvineau P., Llorens-Cortes C., Iturrioz X. Elabela/Toddler and apelin bind differently to the apelin receptor. FASEB J. 2020;34:7989–8000. doi: 10.1096/fj.201903029R. [DOI] [PubMed] [Google Scholar]
- 36.Chen H., Wang L., Wang W., Cheng C., Zhang Y., Zhou Y., et al. ELABELA and an ELABELA fragment protect against AKI. J. Am. Soc. Nephrol. 2017;28:2694–2707. doi: 10.1681/ASN.2016111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng C., Chen H., Yang N., Feng Y., Hsueh A.J.W. Apela regulates fluid homeostasis by binding to the APJ receptor to activate Gi signaling. J. Biol. Chem. 2015;290:18261–18268. doi: 10.1074/jbc.M115.648238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murza A., Sainsily X., Coquerel D., Côté J., Marx P., Besserer-Offroy É., et al. Discovery and structure-activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J. Med. Chem. 2016;59:2962–2972. doi: 10.1021/acs.jmedchem.5b01549. [DOI] [PubMed] [Google Scholar]
- 39.Reaux A., Gallatz K., Palkovits M., Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002;113:653–662. doi: 10.1016/s0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 40.Brailoiu G.C., Dun S.L., Yang J., Ohsawa M., Chang J.K., Dun N.J. Apelin-immunoreactivity in the rat hypothalamus and pituitary. Neurosci. Lett. 2002;327:193–197. doi: 10.1016/s0304-3940(02)00411-1. [DOI] [PubMed] [Google Scholar]
- 41.Reaux-Le Goazigo A., Morinville A., Burlet A., Llorens-Cortes C., Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 2004;145:4392–4400. doi: 10.1210/en.2004-0384. [DOI] [PubMed] [Google Scholar]
- 42.Johnson A.K., Cunningham J.T., Thunhorst R.L. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin. Exp. Pharmacol. Physiol. 1996;23:183–191. doi: 10.1111/j.1440-1681.1996.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 43.O’Carroll A.M., Lolait S.J. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraopric nuclei by osmotic stimuli. J. Neuroendocrinol. 2003;15:661–666. doi: 10.1046/j.1365-2826.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- 44.Hurbin A., Boissin-Agasse L., Orcel H., Rabié A., Joux N., Desarménien M.G., et al. The V1a and V1b, but not V2, vasopressin receptor genes are expressed in the supraoptic nucleus of the rat hypothalamus, and the transcripts are essentially colocalized in the vasopressinergic magnocellular neurons. Endocrinology. 1998;139:4701–4707. doi: 10.1210/endo.139.11.6320. [DOI] [PubMed] [Google Scholar]
- 45.Kleinz M.J., Skepper J.N., Davenport A.P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 2005;126:233–240. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Ipoll E., Pluvinet R., Torras J., Olivar R., Franquesa M, et al. In vivo therapeutic efficacy of intra-renal CD40 silencing in a model of humoral acute rejection. Gene Ther. 2011:945–952. doi: 10.1038/gt.2011.39. [DOI] [PubMed] [Google Scholar]
- 47.O’Carroll A.-M., Salih S., Griffiths P.R., Bijabhai A., Knepper M.A., Lolait S.J. Expression and functional implications of the renal apelinergic system in rodents. PLoS One. 2017;12:e0183094. doi: 10.1371/journal.pone.0183094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Falco M., De Luca L., Onori N., Cavallotti I., Artigiano F., Esposito V., et al. Apelin expression in normal human tissues. In Vivo. 2002;16:333–336. https://www.researchgate.net/publication/10979539_Apelin_expression_in_normal_human_tissues [PubMed] [Google Scholar]
- 49.Chen Y., Xu C., Hu J., Deng M., Qiu Q., Mo S., et al. Diuretic action of apelin-13 mediated by inhibiting cAMP/PKA/sPRR pathway. Front. Physiol. 2021;12:642274. doi: 10.3389/fphys.2021.642274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostrowski N.L., Lolait S.J., Bradley D.J., O’Carroll A.M., Brownstein M.J., Young W.S., 3rd Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology. 1992;131:533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- 51.Kleinz M.J., Davenport A.P. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul. Pept. 2004;118:119–125. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Chen M.M., Ashley E.A., Deng D.X.F., Tsalenko A., Deng A., Tabibiazar R., et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–1439. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 53.Katugampola S.D., Maguire J.J., Matthewson S.R., Davenport A.P. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br. J. Pharmacol. 2001;132:1255–1260. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manning M., Lowbridge J., Haldar J., Sawyer W.H. Design of neurohypophyseal peptides that exhibit selective agonistic and antagonistic properties. Fed. Proc. 1977;36:1848–1852. [PubMed] [Google Scholar]
- 55.Brownstein M.J., Russell J.T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- 56.Zini S., Fournie-Zaluski M.C., Chauvel E., Roques B.P., Corvol P., Llorens-Cortes C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11968–11973. doi: 10.1073/pnas.93.21.11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouzènes L., Desarménien M.G., Hussy N., Richard P., Moos F.C. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J. Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ludwig M. Dendritic release of vasopressin and oxytocin. J. Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 59.Poulain D.A., Wakerley J.B., Dyball R.E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc. R. Soc. Lond., B, Biol. Sci. 1977;196:367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- 60.Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 61.Llorens-Cortes C., Moos F. Apelin and vasopressin: two work better than one. J. Neuroendocrinol. 2012;24:1085–1086. doi: 10.1111/j.1365-2826.2012.02316.x. [DOI] [PubMed] [Google Scholar]
- 62.Terada Y., Tomita K., Nonoguchi H., Yang T., Marumo F. Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J. Clin. Invest. 1993;92:2339–2345. doi: 10.1172/JCI116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morello J.P., Bichet D.G. Nephrogenic diabetes insipidus. Annu. Rev. Physiol. 2001;63:607–630. doi: 10.1146/annurev.physiol.63.1.607. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen S., Chou C.L., Marples D., Christensen E.I., Kishore B.K., Knepper M.A. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sands J.M., Naruse M., Baum M., Jo I., Hebert S.C., Brown E.M., et al. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J. Clin. Invest. 1997;99:1399–1405. doi: 10.1172/JCI119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flahault A., Girault-Sotias P.-E., Keck M., Alvear-Perez R., De Mota N., Estéoulle L., et al. A metabolically stable apelin-17 analog decreases AVP-induced antidiuresis and improves hyponatremia. Nat. Commun. 2021;12:305. doi: 10.1038/s41467-020-20560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hus-Citharel A., Bodineau L., Frugière A., Joubert F., Bouby N., Llorens-Cortes C. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology. 2014;155:4483–4493. doi: 10.1210/en.2014-1257. [DOI] [PubMed] [Google Scholar]
- 68.Boulkeroua C., Ayari H., Khalfaoui T., Lafrance M., Besserer-Offroy É., Ekindi N., et al. Apelin-13 regulates vasopressin-induced aquaporin-2 expression and trafficking in kidney collecting duct cells. Cell. Physiol. Biochem. 2019;53:687–700. doi: 10.33594/000000165. [DOI] [PubMed] [Google Scholar]
- 69.Ayari H., Chraibi A. Apelin-13 decreases epithelial sodium channel (ENaC) expression and activity in kidney collecting duct cells. Cell. Physiol. Biochem. 2022;56:1–12. doi: 10.33594/000000488. [DOI] [PubMed] [Google Scholar]
- 70.Bankir L., Bichet D.G., Bouby N. Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am. J. Physiol. Renal Physiol. 2010;299:F917–28. doi: 10.1152/ajprenal.00413.2010. [DOI] [PubMed] [Google Scholar]
- 71.Ellison D.H., Berl T. Clinical practice. the syndrome of inappropriate antidiuresis. N. Engl. J. Med. 2007;356:2064–2072. doi: 10.1056/NEJMcp066837. [DOI] [PubMed] [Google Scholar]
- 72.Kim W.R., Biggins S.W., Kremers W.K., Wiesner R.H., Kamath P.S., Benson J.T., et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N. Engl. J. Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waikar S.S., Mount D.B., Curhan G.C. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am. J. Med. 2009;122:857–865. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovesdy C.P., Lott E.H., Lu J.L., Malakauskas S.M., Ma J.Z., Molnar M.Z., et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peri A. Management of hyponatremia: causes, clinical aspects, differential diagnosis and treatment. Expert Rev. Endocrinol. Metab. 2019;14:13–21. doi: 10.1080/17446651.2019.1556095. [DOI] [PubMed] [Google Scholar]
- 76.Verbalis J.G., Goldsmith S.R., Greenberg A., Korzelius C., Schrier R.W., Sterns R.H, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am. J. Med. 2013;126:S1–42. doi: 10.1016/j.amjmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Verbalis J.G. Euvolemic hyponatremia secondary to the syndrome of inappropriate antidiuresis. Front. Horm. Res. 2019;52:61–79. doi: 10.1159/000493238. [DOI] [PubMed] [Google Scholar]
- 78.Blanchard A., Steichen O., De Mota N., Curis E., Gauci C., Frank M., et al. An abnormal apelin/vasopressin balance may contribute to water retention in patients with the syndrome of inappropriate antidiuretic hormone (SIADH) and heart failure. J. Clin. Endocrinol. Metab. 2013;98:2084–2089. doi: 10.1210/jc.2012-3794. [DOI] [PubMed] [Google Scholar]
- 79.Murza A., Belleville K., Longpré J.-M., Sarret P., Marsault É. Stability and degradation patterns of chemically modified analogs of apelin-13 in plasma and cerebrospinal fluid. Biopolymers. 2014;102:297–303. doi: 10.1002/bip.22498. [DOI] [PubMed] [Google Scholar]
- 80.Japp A.G., Cruden N.L., Barnes G., van Gemeren N., Mathews J., Adamson J., et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121:1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 81.McKinnie S.M.K., Fischer C., Tran K.M.H., Wang W., Mosquera F., Oudit G.Y., et al. The Metalloprotease neprilysin degrades and inactivates apelin peptides. Chembiochem. 2016;17:1495–1498. doi: 10.1002/cbic.201600244. [DOI] [PubMed] [Google Scholar]
- 82.Jia Z.Q., Hou L., Leger A., Wu I., Kudej A.B., Stefano J, et al. Cardiovascular effects of a PEGylated apelin. Peptides. 2012;38:181–188. doi: 10.1016/j.peptides.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Galon-Tilleman H., Yang H., Bednarek M.A., Spurlock S.M., Paavola K.J., Ko B, et al. Apelin-36 modulates blood glucose and body weight independently of canonical APJ receptor signaling. J. Biol. Chem. 2017;292:1925–1933. doi: 10.1074/jbc.M116.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer C., Lamer T., Fernandez K., Gheblawi M., Wang W., Pascoe C., et al. Optimizing PEG-extended apelin analogues as cardioprotective drug leads: importance of the KFRR motif and aromatic head group for improved physiological activity. J. Med. Chem. 2020;63:12073–12082. doi: 10.1021/acs.jmedchem.0c01395. [DOI] [PubMed] [Google Scholar]
- 85.Paul O.F., Mary F.P.R. Apelin analogues. WO 2015/165936 Al 2015
- 86.Juhl C., Els-Heindl S., Schönauer R., Redlich G., Haaf E., Wunder F., et al. Development of potent and metabolically stable APJ ligands with high therapeutic potential. ChemMedChem. 2016;11:2378–2384. doi: 10.1002/cmdc.201600307. [DOI] [PubMed] [Google Scholar]
- 87.Fischer C., Lamer T., Wang W., McKinnie S.M.K., Iturrioz X., Llorens-Cortes C, et al. Plasma kallikrein cleaves and inactivates apelin-17: Palmitoyl- and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents. Eur. J. Med. Chem. 2019;166:119–124. doi: 10.1016/j.ejmech.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 88.Brame A.L., Maguire J.J., Yang P., Dyson A., Torella R., Cheriyan J., et al. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension. 2015;65:834–840. doi: 10.1161/HYPERTENSIONAHA.114.05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murza A., Sainsily X., Côté J., Bruneau-Cossette L., Besserer-Offroy É., Longpré J.-M., et al. Structure-activity relationship of novel macrocyclic biased apelin receptor agonists. Org. Biomol. Chem. 2017;15:449–458. doi: 10.1039/c6ob02247b. [DOI] [PubMed] [Google Scholar]
- 90.Trân K., Murza A., Sainsily X., Coquerel D., Côté J., Belleville K., et al. A systematic exploration of macrocyclization in apelin-13: impact on binding, signaling, stability, and cardiovascular effects. J. Med. Chem. 2018;61:2266–2277. doi: 10.1021/acs.jmedchem.7b01353. [DOI] [PubMed] [Google Scholar]
- 91.Murza A., Besserer-Offroy É., Côté J., Bérubé P., Longpré J.-M., Dumaine R., et al. C-Terminal modifications of apelin-13 significantly change ligand binding, receptor signaling, and hypotensive action. J. Med. Chem. 2015;58:2431–2440. doi: 10.1021/jm501916k. [DOI] [PubMed] [Google Scholar]
- 92.Miyazaki T., Yamamura Y., Onogawa T., Nakamura S., Kinoshita S., Nakayama S., et al. Therapeutic effects of tolvaptan, a potent, selective nonpeptide vasopressin V2 receptor antagonist, in rats with acute and chronic severe hyponatremia. Endocrinology. 2005;146:3037–3043. doi: 10.1210/en.2004-1590. [DOI] [PubMed] [Google Scholar]
- 93.Torres V.E., Chapman A.B., Devuyst O., Gansevoort R.T., Grantham J.J., Higashihara E., et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boertien W.E., Meijer E., Renken R.J, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am. J. Kidney Dis. 2015;65:833–841. doi: 10.1053/j.ajkd.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 95.Verbalis J.G., Adler S., Schrier R.W., Berl T., Zhao Q., Czerwiec F.S., et al. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur. J. Endocrinol. 2011;164:725–732. doi: 10.1530/EJE-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tzoulis P., Waung J.A., Bagkeris E., Carr H., Khoo B., Cohen M., et al. Real-life experience of tolvaptan use in the treatment of severe hyponatraemia due to syndrome of inappropriate antidiuretic hormone secretion. Clin. Endocrinol. (Oxf) 2016;84:620–626. doi: 10.1111/cen.12943. [DOI] [PubMed] [Google Scholar]
- 97.Feldman B.J., Rosenthal S.M., Vargas G.A., Fenwick R.G., Huang E.A., Matsuda-Abedini M., et al. Nephrogenic syndrome of inappropriate antidiuresis. N. Engl. J. Med. 2005;352:1884–1890. doi: 10.1056/NEJMoa042743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rochdi M.D., Vargas G.A., Carpentier E., Oligny-Longpré G., Chen S., Kovoor A., et al. Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis: implications for treatments. Mol. Pharmacol. 2010;77:836–845. doi: 10.1124/mol.109.061804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tiulpakov A., White C.W., Abhayawardana R.S., See H.B., Chan A.S., Seeber R.M., et al. Mutations of vasopressin receptor 2 including novel L312S have differential effects on trafficking. Mol. Endocrinol. 2016;30:889–904. doi: 10.1210/me.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Erdélyi L.S., Mann W.A., Morris-Rosendahl D.J., Groß U., Nagel M., Várnai P., et al. Mutation in the V2 vasopressin receptor gene, AVPR2, causes nephrogenic syndrome of inappropriate diuresis. Kidney Int. 2015;88:1070–1078. doi: 10.1038/ki.2015.181. [DOI] [PubMed] [Google Scholar]
- 101.Decaux G., Vandergheynst F., Bouko Y., Parma J., Vassart G., Vilain C. Nephrogenic syndrome of inappropriate antidiuresis in adults: high phenotypic variability in men and women from a large pedigree. J. Am. Soc. Nephrol. 2007;18:606–612. doi: 10.1681/ASN.2006090987. [DOI] [PubMed] [Google Scholar]
- 102.Ishida J., Hashimoto T., Hashimoto Y., Nishiwaki S., Iguchi T., Harada S., et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J. Biol. Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 103.Salcedo A., Garijo J., Monge L., Fernández N., Luis García-Villalón A., Sánchez Turrión V, et al. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul Pept. Abbreviated form: Role of nitric oxide and prostanoids. Regul Pept. 2007;144:50–55. doi: 10.1016/j.regpep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 104.Lee D.K., Saldivia V.R., Nguyen T., Cheng R., George S.R., O’Dowd B.F. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–236. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 105.Flahault A., Keck M., Girault-Sotias P.-E., Esteoulle L., De Mota N., Bonnet D., et al. LIT01-196, a metabolically stable Apelin-17 analog, normalizes blood pressure in hypertensive DOCA-Salt rats via a NO synthase-dependent mechanism. Front. Pharmacol. 2021;12:715095. doi: 10.3389/fphar.2021.715095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Japp A.G., Cruden N.L., Amer D.A.B., Li V.K.Y., Goudie E.B., Johnston N.R., et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008;52:908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 107.Ashley E., Chun H.J., Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J. Mol. Cell. Cardiol. 2006;41:778–781. doi: 10.1016/j.yjmcc.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 108.Chapman F.A., Maguire J.J., Newby D.E., Davenport A.P., Dhaun N. Targeting the apelin system for the treatment of cardiovascular diseases. Cardiovasc. Res. 2023;119:2683–2696. doi: 10.1093/cvr/cvad171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arendse L.B., Danser A.H.J., Poglitsch M., Touyz R.M., Burnett J.C., Jr, Llorens-Cortes C., et al. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019;71:539–570. doi: 10.1124/pr.118.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 111.Seyedabadi M., Goodchild A.K., Pilowsky P.M. Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton. Neurosci. 2002;101:32–38. doi: 10.1016/s1566-0702(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 112.Zhang F., Sun H.-J., Xiong X.-Q., Chen Q., Li Y.-H., Kang Y.-M., et al. Apelin-13 and APJ in paraventricular nucleus contribute to hypertension via sympathetic activation and vasopressin release in spontaneously hypertensive rats. Acta Physiol. (Oxf). 2014;212:17–27. doi: 10.1111/apha.12342. [DOI] [PubMed] [Google Scholar]
- 113.Griffiths P.R., Lolait S.J., Paton J.F.R., O’Carroll A.-M. Circumventricular organ apelin receptor knockdown decreases blood pressure and sympathetic drive responses in the spontaneously hypertensive rat. Front. Physiol. 2021;12:711041. doi: 10.3389/fphys.2021.711041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gomolka R.S., Cudnoch-Jedrzejewska A., Czarzasta K., Szczepanska-Sadowska E. Reduction of pressor response to stress by centrally acting apelin in spontaneously hypertensive rats. J. Basic Clin. Physiol. Pharmacol. 2015;26:233–236. doi: 10.1515/jbcpp-2014-0066. [DOI] [PubMed] [Google Scholar]
- 115.Szokodi I., Tavi P., Földes G., Voutilainen-Myllylä S., Ilves M., Tokola H., et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 2002;91:434–440. doi: 10.1161/01.res.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 116.Berry M.F., Pirolli T.J., Jayasankar V., Burdick J., Morine K.J., Gardner T.J., et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation. 2004;110:II187–93. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 117.Farkasfalvi K., Stagg M.A., Coppen S.R., Siedlecka U., Lee J., Soppa G.K., et al. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem. Biophys. Res. Commun. 2007;357:889–895. doi: 10.1016/j.bbrc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 118.Cheng C.-C., Weerateerangkul P., Lu Y.-Y., Chen Y.-C., Lin Y.-K., Chen S.-A., et al. Apelin regulates the electrophysiological characteristics of atrial myocytes. Eur. J. Clin. Invest. 2013;43:34–40. doi: 10.1111/eci.12012. [DOI] [PubMed] [Google Scholar]
- 119.Lv W., Zhang L., Cheng X., Wang H., Qin W., Zhou X., et al. Apelin inhibits angiotensin II-Induced atrial fibrosis and atrial fibrillation via TGF-β1/Smad2/α-SMA pathway. Front. Physiol. 2020;11:583570. doi: 10.3389/fphys.2020.583570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ashley E.A., Powers J., Chen M., Kundu R., Finsterbach T., Caffarelli A., et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc. Res. 2005;65:73–82. doi: 10.1016/j.cardiores.2004.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siddiquee K., Hampton J., McAnally D., May L., Smith L. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. Br. J. Pharmacol. 2013;168:1104–1117. doi: 10.1111/j.1476-5381.2012.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuba K., Zhang L., Imai Y., Arab S., Chen M., Maekawa Y., et al. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 2007;101:e32–42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 123.Ceylan-Isik A.F., Kandadi M.R., Xu X., Hua Y., Chicco A.J., Ren J, et al. Apelin administration ameliorates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell. Cardiol. 2013;63:4–13. doi: 10.1016/j.yjmcc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Wang W., McKinnie S.M.K., Patel V.B., Haddad G., Wang Z., Zhabyeyev P., et al. Loss of apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic apelin analogues. J. Am. Heart Assoc. 2013;2:e000249. doi: 10.1161/JAHA.113.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Azizi Y., Faghihi M., Imani A., Roghani M., Nazari A. Post-infarct treatment with [pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides. 2013;46:76–82. doi: 10.1016/j.peptides.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 126.Pchejetski D., Foussal C., Alfarano C., Lairez O., Calise D., Guilbeau-Frugier C., et al. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur. Heart J. 2012;33:2360–2369. doi: 10.1093/eurheartj/ehr389. [DOI] [PubMed] [Google Scholar]
- 127.Barnes G.D., Alam S., Carter G., Pedersen C.M., Lee K.M., Hubbard T.J., et al. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circ. Heart Fail. 2013;6:482–491. doi: 10.1161/CIRCHEARTFAILURE.111.000077. [DOI] [PubMed] [Google Scholar]
- 128.Gargalovic P., Wong P., Onorato J., Finlay H., Wang T., Yan M, et al. In Vitro and In Vivo evaluation of a small-molecule APJ (Apelin Receptor) agonist, BMS-986224, as a potential treatment for heart failure. Circ. Heart Fail. 2021;14:e007351. doi: 10.1161/CIRCHEARTFAILURE.120.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ason B., Chen Y., Guo Q., Hoagland K.M., Chui R.W., Fielden M, et al. Cardiovascular response to small-molecule APJ activation. JCI Insight. 2020;5:e132898. doi: 10.1172/jci.insight.132898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coquerel D., Lamoureux J., Chagnon F., Trân K., Sage M., Fortin-Pellerin E., et al. Apelin-13 in septic shock: effective in supporting hemodynamics in sheep but compromised by enzymatic breakdown in patients. Sci. Rep. 2021;11:22770. doi: 10.1038/s41598-021-02087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song Q., Wang X., Cao Z., Xin C., Zhang J., Li S. The apelin/apj system: a potential therapeutic target for sepsis. J. Inflamm. Res. 2024;17:313–330. doi: 10.2147/JIR.S436169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chagnon F., Coquerel D., Salvail D., Marsault E., Dumaine R., Auger-Messier M., et al. Apelin compared with dobutamine exerts cardioprotection and extends survival in a rat model of endotoxin-induced myocardial dysfunction. Crit. Care Med. 2017;45:e391–e398. doi: 10.1097/CCM.0000000000002097. [DOI] [PubMed] [Google Scholar]
- 133.Luo Q., Liu G., Chen G., Guo D., Xu L., Hang M., et al. Apelin protects against sepsis‑induced cardiomyopathy by inhibiting the TLR4 and NLRP3 signaling pathways. Int. J. Mol. Med. 2018;42:1161–1167. doi: 10.3892/ijmm.2018.3665. [DOI] [PubMed] [Google Scholar]