Abstract
This study aimed to evaluate the efficacy of using ground herb-based phytobiotics and essential oil-based phytobiotics in pig diets on intestinal health and growth performance (GP) of nursery pigs challenged with F18+Escherichia coli. Forty nursery pigs (6.4 ± 0.1 kg) at 21 d of age were individually housed and assigned to 4 dietary treatments in a randomized complete block design, with body weight and sex as blocking factors. Basal diets were fed to pigs for 28 d in 3 phases. Treatments were negative control (NC): basal diet, non-challenged; positive control (PC): basal diet, challenged with F18+E. coli; HP: PC + 1% ground herb-based phytobiotics (Salcochek Pro, Ayurvet Limited, Kaushambi, India); EP: PC + 1% essential oil-based phytobiotics (Liq-biotic, Ayurvet Limited). The GP was recorded for each phase and fecal score (FS) was measured daily. On day 7 postweaning, the challenged groups were orally inoculated with F18+E. coli (2.0 × 1010 CFU), the NC treatment received a sterile saline solution. On day 28, pigs were euthanized to collect jejunal samples to evaluate intestinal health and relative abundance (RA) of jejunal mucosa-associated microbiota. Data were analyzed using the MIXED procedure on SAS 9.4. The PC increased (P < 0.05) the RA of Prevotellaceae, Lachnospiraceae, and Ruminococcaceae when compared to NC. The HP reduced (P < 0.05) the RA of Veillonellaceae, Prevotellaceae, and Lachnospiraceae when compared to PC. The EP tended to reduce the RA of Streptococcaceae (P = 0.073) and Corynebacteriaceae (P = 0.074) when compared to PC. The PC increased (P < 0.05) occludin and tended to increase (P = 0.096) toll-like receptor-4 (TLR4) when compared to NC. The PC decreased (P < 0.05) average daily gain and average daily feed intake when compared to NC in days 7 to 28. The PC increased FS (P < 0.05) compared to the HP and EP days 7 to 11. The HP and EP decreased (P < 0.05) FS when compared to PC during days 7 to 11 and days 7 to 18. In conclusion, F18+E. coli challenge disrupted the jejunal mucosa-associated microbiota, increased TLR4 expression and FS, and consequently reduced GP. Both HP and EP phytobiotics supported intestinal morphology during the challenge to F18+E. coli by supporting enterocyte maturation. The HP and EP treatments exhibited antimicrobial-like effects by altering the jejunal mucosa-associated microbiota and reduced FS during the first 2 weeks post-challenge. The HP treatment showed potential antioxidant effects.
Keywords: F18+Escherichia coli, intestinal health, phytobiotics, oxidative stress, nursery pigs
Ground herb-based and essential oil-based phytobiotics supported intestinal morphology in nursery pigs challenged with F18+E. coli. Both phytobiotics exhibited antimicrobial properties and reduced fecal scores, offering promising insights into swine nutrition and disease management.
Introduction
Postweaning diarrhea (PWD) frequently occurs due to the stress generated during the weaning period, predisposing pigs to enterotoxigenic Escherichia coli infection. Enterotoxigenic E. coli expressing F18 fimbriae and producing heat-stable toxin A (Sta) and heat-stable toxin B (STb) are the primary causes of diarrhea in nursery pigs (Liu et al., 2014; Castro et al., 2022). Generally, PWD occurs between the first and second weeks postweaning, causing watery diarrhea with potential mortality rates between 1.5% and 25% (Duarte et al., 2023). The repercussions of such infections extend beyond the gastrointestinal tract, including a diminished growth rate, and substantial economic losses within pig production systems (Coddens et al., 2017).
The optimal performance of pigs is highly linked to maintaining optimal intestinal conditions. The small intestine is not only a critical site for nutrient digestion and absorption but also the largest component of the animal’s immune system (Chassaing et al., 2014). Among the nutritional strategies explored to promote intestinal health, phytobiotics, plant-derived additives with bioactive compounds, have gained attention for their potential to enhance growth performance (GP) and intestinal health (Kommera et al., 2006; Kikusato, 2021).
Phytobiotics involve various plant-based sources, including ground herbs and essential oils, which provide unique combinations of bioactive compounds with functional properties. Ground herbs, such as Aegle marmelos, Berberis aristata, Holarrhena antidysenterica, and Punica granatum, are rich in coumarins, flavonoids, saponins, steroid alkaloids, and tannins. These compounds exhibit antidiarrheal (Gilani et al., 2010; Rahman and Parvin, 2014; Sharma et al., 2015), antimicrobial (Sinha et al., 2013; Mujeeb et al., 2014; Jahan et al., 2022), anti-inflammatory (Raj et al., 2020), and antioxidant (Lamichhane et al., 2014; Bassiri-Jahromi, 2018) properties. Similarly, essential oils from plants like Eucalyptus globulus, Mentha piperita, Ocimum sanctum, and Trachyspermum ammi are composed of eucalyptol, menthol, thymol, and other phenolic compounds with antimicrobial and antioxidant activities (Bairwa et al., 2012; Yamani et al., 2016; Čmiková et al., 2023).
Evaluating the effects of ground herb-based phytobiotics and essential oil-based phytobiotics could provide new insights directly into the modulation of jejunal mucosa-associated microbiota to improve intestinal health and in nursery pigs challenged with F18+E. coli. In the present study, it was hypothesized that the dietary inclusion of ground herb-based phytobiotics could reduce oxidative stress, support intestinal barrier integrity, and improve fecal score (FS), thereby regulating inflammatory responses. Additionally, essential oil-based phytobiotics will increase beneficial bacteria in the jejunal mucosa reducing jejunal oxidative stress, collectively mitigating the detrimental effects of F18+E. coli in the jejunum of nursery pigs. The objective of this study was to evaluate the efficacy of dietary inclusion of ground herb-based phytobiotics and essential oil-based phytobiotics on the diversity and relative abundance (RA) of jejunal mucosa-associated microbiota and mucosal immune response in the jejunum of nursery pigs challenged with F18+E. coli.
Materials and Methods
The experimental protocol was approved by the Institutional Animal Care and Use Committee of North Carolina State University. Two different phytobiotics were used in this study (Table 1) 1) ground herb-based phytobiotics (Salcochek Pro, Ayurvet Limited, Kaushambi, India) mainly composed of A. marmelos (14.4%), Berberis aristate (14.4%), H. antidysenterica (14.4%), P. granatum (14.4%), and Woodfordia fruticose (14.4%); and 2) essential oil-based phytobiotics (Liq-biotic, Ayurvet Limited) mainly composed of moisture (98.3 %), M. piperita (0.75%), E. globulus (0.65%), O. sanctum (0.05), and Trychyspermum ammi (0.05%).
Table 1.
Composition of ground herb-based phytobiotics and essential oil-based phytobiotics
| Item | Component | Content, % |
|---|---|---|
| Ground herb-based phytobiotics1 | Holarrhena antidysenterica | 14.4 |
| Berberis aristate | 14.4 | |
| Punica granatum | 14.4 | |
| Aegle marmelos | 14.4 | |
| Woodfordia fruticose | 14.4 | |
| Others | 28.0 | |
| Essential oil-based phytobiotics2 | Moisture | 96.3 |
| Ocimum sanctum | 0.05 | |
| Mentha piperita | 0.75 | |
| Eucalyptus globulus | 0.65 | |
| Trychyspermum ammi | 0.05 | |
| Others | 2.20 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
Animals, design, and diets
The experiment was conducted at the Metabolism Education Unit at North Carolina State University (Raleigh, NC). Forty pigs (PIC 337 × Camborough 22) at 21 d of age (20 barrows and 20 gilts) with initial body weight (BW) of 6.4 ± 0.1 kg were allotted to 4 dietary treatments based on a randomized complete block design with sex and BW as a block. The basal diets were composed of a corn and soybean meal-based formulation. The dietary treatments consisted of a negative control (NC): basal diet, non-challenged pigs; positive control (PC): basal diet, challenged with F18+E. coli on day 7; test 1 diet (HP): basal diet + 1% ground herb-based phytobiotics (Salcochek Pro, Ayurvet Limited), incorporated into the feed during the mixing process, and challenged with F18+E. coli on day 7; and test 2 diet (EP): basal diet + 1% essential oil-based phytobiotics (Liq-biotic, Ayurvet Limited), incorporated into the feed during the mixing process, and challenged with F18+E. coli on day 7 postweaning. The inclusion level (1%) of both phytobiotics was decided to provide 5 g phytobiotics per day based on preliminary titration studies (Patilba et al., 2017). The phytobiotics were supplemented replacing corn in the basal diet and diets were formulated meeting the nutrient requirements of NRC (2012), as shown in Table 2. Pigs were housed individually with free access to feed and water during 28 d feeding period (4 wk) based on 3 phase feeding program: phase 1 (days 0 to 11), phase 2 (days 11 to 21), and phase 3 (days 21 to 28).
Table 2.
Composition of basal diets (as-fed basis)
| Item | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| Feedstuff, % | |||
| Corn, yellow | 41.96 | 49.52 | 60.57 |
| Whey permeate, 80% lactose | 19.00 | 13.00 | 6.00 |
| Soybean meal, 48% CP | 18.50 | 23.50 | 28.50 |
| Poultry meal | 9.00 | 5.00 | 0.00 |
| Fish meal | 5.00 | 3.00 | 0.00 |
| Enzyme treated SBM1 | 3.00 | 1.50 | 0.00 |
| Poultry fat | 1.10 | 1.70 | 1.42 |
| l-Lys HCl | 0.58 | 0.47 | 0.46 |
| l-Met | 0.27 | 0.19 | 0.16 |
| l-Thr | 0.20 | 0.14 | 0.14 |
| l-Trp | 0.03 | 0.01 | 0.00 |
| l-Val | 0.02 | 0.01 | 0.03 |
| Dicalcium phosphate | 0.00 | 0.38 | 0.95 |
| Limestone | 0.44 | 0.68 | 0.87 |
| Salt | 0.22 | 0.22 | 0.22 |
| Vitamin premix2 | 0.03 | 0.03 | 0.03 |
| Mineral premix3 | 0.15 | 0.15 | 0.15 |
| Calculated composition | |||
| Dry matter, % | 90.8 | 90.3 | 89.5 |
| ME, kcal/kg | 3,401 | 3,400 | 3,351 |
| SID4 Lys, % | 1.50 | 1.35 | 1.23 |
| SID Met + Cys, % | 0.82 | 0.74 | 0.68 |
| SID Trp, % | 0.25 | 0.22 | 0.20 |
| SID Thr, % | 0.88 | 0.79 | 0.73 |
| SID Val, % | 0.95 | 0.87 | 0.78 |
| Ca, % | 0.85 | 0.80 | 0.70 |
| STTD P5, % | 0.45 | 0.40 | 0.33 |
| Total P, % | 0.70 | 0.64 | 0.58 |
1HP300 (Hamlet Protein, Findlay, OH, USA).
2The vitamin premix provided the following per kilogram of complete diet: 6,613.8 IU of vitamin A as vitamin A acetate, 992.0 IU of vitamin D3, 19.8 IU of vitamin E, 2.64 mg of vitamin K as menadione sodium bisulfate, 0.03 mg of vitamin B12, 4.63 mg of riboflavin, 18.52 mg of d-pantothenic acid as calcium pantothenate, 24.96 mg of niacin, and 0.07 mg of biotin.
3The trace mineral premix provided the following per kilogram of complete diet: 4.0 mg of Mn as manganous oxide, 165 mg of Fe as ferrous sulfate, 165 mg of Zn as zinc sulfate, 16.5 mg of Cu as copper sulfate, 0.30 mg of I as ethylenediamine di-hydroiodide, and 0.30 mg of Se as sodium selenite.
4Standardized ileal digestible.
5Standardized total tract digestible phosphorus.
On d 7 postweaning, 30 pigs were orally inoculated with F18+E. coli (challenged groups: PC, HP, and EP) in 4 separate doses (1 mL each) over 2 d, with the following dosage concentrations: dose 1 (4.5 × 109 CFU/pig), dose 2 (6.5 × 109 CFU/pig), dose 3 (3.75 × 109 CFU/pig), and dose 4 (4.75 × 109 CFU/pig), for a final concentration of 2.0 × 1010 CFU/pig. The F18+E. coli of strain 2144 (O147: non-motile) was originally isolated from piglets with PWD and produced STa and STb (Sun et al., 2021). Pigs in the unchallenged group received a sterile saline solution. The cultures of the F18+E. coli strains were prepared following the standard laboratory protocol as previously reported (Duarte et al., 2020; Xu et al., 2022; Jang et al., 2023). Piglets and sows were not vaccinated against E. coli prior to this study. To minimize the possibility of cross-contamination, non-challenged pigs were housed in the same room separated by 2.10 m from challenged pigs. Solid barriers were also used to separate pens, and biosecurity protocols, including changing gloves and footwear, were followed between treatment areas. All daily procedures were conducted first with the non-challenged groups before interacting with the challenge group.
GP and FS
Pigs and the feed disappearance were individually weighed at the end of each phase to determine GP parameters, including BW, average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratio (G:F). FS was recorded every day during the entire period based on a 1 to 5 scale, 1) very firm stool, 2) normal firm stool, 3) moderately loose stool, 4) loose, watery stool, and 5) very watery stool (Duarte et al., 2020).
Sample collection
After day 28 of the study, all pigs were euthanized by exsanguination after a penetrating captive bolt to the head, to collect jejunal tissues (3 m away from the pyloric duodenal junction), rinsed with a saline solution (0.9%) to remove digesta content. The mid-jejunum was scraped to obtain intestinal mucosa placed in Eppendorf tubes (2 mL) and stored at −80 °C (after snap-freezing in liquid nitrogen, immediately after collection). A segment from the mid-jejunum was removed and placed in a 5 mL tube, immediately placed into liquid nitrogen, and then stored at −80 °C. Another segment of mid-jejunum tissue was removed, rinsed with a 0.9% saline solution, and stored in a 50 mL Falcon tube containing 10% buffered formaldehyde.
Diversity and RA of jejunal mucosa-associated microbiota
Mucosa samples collected from the mid-jejunum were to determine the diversity and RA of jejunal mucosa-associated microbiota. The samples were sent to a commercial laboratory (Zymo Research, Irvine, CA) for 16S rRNA microbiome sequencing analysis. Utilizing the ZymoBIOMICS-96 MagBead DNA Kit (Zymo Research). The generation of the alpha diversity rare fraction plot and the amplicon sequence variant (ASV) table was executed using Qiime (version 1.9.1), with a depth of sequencing of 20,000 × sample preparation. The DADA2 pipeline was used to infer unique amplicon sequences that were inferred from the raw reads. Zymo Research Database, an internally curated 16S database, was utilized as a reference to assign taxonomy, using Uclust from QUIIME v.1.9.1. Alpha and beta diversity were evaluated using the website program, MicrobiomeAnalyst (QC, CA). The ASV data were transformed to RA. At each level, ASV information characterized by an RA of less than 0.5% and sequences that were not assigned were combined and collectively labeled as “Others” as previously described by Kim et al. (2019). Beta diversity was assessed using the Bray-Curtis distance method, as described by Deng et al. (2023).
Relative mRNA expression of genes in jejunal tissue
Tissue samples obtained from the mid-jejunum were weighed (50 to 100 mg) and subjected to homogenization in 1 mL of TRIzol reagent (15-596-026, Invitrogen, Waltham, MA) utilizing the Bead Mill 24 homogenizer (Thermo Fisher Scientific Inc.). The homogenization process involved 2 cycles at 4.5 m/s for 30 s each, with a 20 s interval on ice between cycles, as previously described by Jang et al. (2023). Post-homogenization, the samples were centrifugated for 10 min at 12,000 × g at 4 °C. Following centrifugation, the resulting supernatant was transferred to a 1.5 mL centrifuge tube containing 200 µL of chloroform (Thermo Fisher Scientific Inc.) and gently vortexed for 1 min. The combined tubes were incubated at room temperature for 10 min, followed by another centrifugation for 15 min at 12,000 × g at 4 °C. The resulting supernatant was cautiously removed, leaving the pellet and dried within a fume hood for approximately 20 min until complete evaporation of the supernatant. The obtained RNA underwent quality and quantity assessment using spectrometry, as detailed by Jang et al. (2023). Subsequently, the extracted RNA was reverse transcribed into cDNA using a commercial kit (RevertAid First Strand cDNA Synthesis, Thermo Fisher Scientific Inc.), following the manufacturer’s instructions. For quantitative real-time polymerase chain reaction (qPCR), the CFX Connect Real-Time PCR Detection System (BioRad, Hercules, CA, USA) was employed, using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific Inc.) and oligonucleotide primers synthesized by Millipore Sigma (Burlington, MA). The genes evaluated were nod-like receptor 1 (NOD1); nod-like receptor 2 (NOD2); toll-like receptor 2 (TLR2); toll-like receptor 4 (TLR4); claudin (CLD); occludin (OCC); zonula occludens-1 (ZO-1). The primers are listed in Table 3. The relative expression of each gene was normalized using delta–delta–Ct method as described previously (Jang et al., 2023).
Table 3.
Sequence of primers for microbial sensing and intestinal integrity in the jejunum of nursery pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Gene3 | Primer sequences (5′–3′)4 | Accession number | Size |
|---|---|---|---|
| NOD1 | F: AACACCGATCCAGTGAGCAG R: AAATGGTCTCGCCCTCCTTG |
NM_001114277.1 | 230 |
| NOD2 | F: GTGCCTCCCCTCTAGACTCA R: ACGAACCAGGAAGCCAAGAG |
NM_001105295.1 | 191 |
| TLR2 | F: GGGCTGCGTTCATTCATCAG R: CTGCAGAGGATGGATGGCAA |
XM_005653576.3 | 132 |
| TLR4 | F: CGTGCAGGTGGTTCCTAACA R: GGTTTGTCTCAACGGCAACC |
NM_001113039.2 | 326 |
| CLD | F: AAACCGTGTGGGAACAACCA R: CACATGAAAATGGCTTCCCTC |
NM_001244539.1 | 196 |
| OCC | F: TCAGGTGCACCCTCCAGATT R: AGGAGGTGGACTTTCAAGAGG |
XP_005672579.1 | 169 |
| ZO-1 | F: CAGAGACCAAGAGCCGTCC R: TGCTTCAAGACATGGTTGGC |
XM_003480423.4 | 105 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NOD1, nod-like receptor 1; NOD2, nod-like receptor 2; TLR2, toll-like receptor 2; TLR4, toll-like receptor 4; CLD, claudin; OCC, occludin; ZO-1, zonula occludens-1.
4F, forward; R, reverse.
Inflammatory cytokines, humoral immune status, and oxidative stress parameters in the jejunum
Mucosa samples collected from the mid-jejunum were weighed (1 g) and placed in 1 mL of phosphate-buffered saline (PBS) on ice. Subsequently, the samples were homogenized using a tissue homogenizer (Tissuemiser; Thermo Fisher Scientific Inc.) the samples underwent centrifugation at 14,000 × g for 15 min, following the methodology described by Holanda and Kim (2021). Upon centrifugation, the supernatant was carefully extracted, divided into 5 aliquots, and stored at −80 °C for subsequent analyses.
The concentrations of total protein, malondialdehyde (MDA), protein carbonyl, immunoglobulin G (IgG), immunoglobulin A (IgA), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8), were determined using commercially available kits as per the provided instructions. Optical density (OD) values were measured using a plate reader (Synergy HT, BioTek Instruments, Winooski, VT) and analyzed with corresponding software (Gen5 Data Analysis Software, BioTek Instruments). Concentrations of each analyte were calculated by comparing the resulting OD values to the absorbance of standard curves following the guidelines provided in the manual.
To proceed with the laboratory analysis, the supernatant derived from the earlier homogenization of mucosal samples underwent a dilution step (1:60) in PBS to achieve a suitable concentration range (20 to 2,000 μg/mL). The determination of total protein concentration was carried out utilizing the Pierce BCA Protein Assay Kit (#23225, Thermo Fisher Scientific Inc.), following the methodology outlined by Holanda et al. (2020). Absorbance readings were taken at 562 nm, and the resulting total protein concentrations for each sample were employed for normalization in subsequent colorimetric assays. The measurement of MDA concentration in the mucosa was conducted using the OxiSelect TBARS MDA Quantitation Assay Kit (#STA-330, Cell Biolabs, Inc., San Diego, CA), following the protocol detailed by Moita et al. (2021). The working range for the standard is between 0.98 and 125 µM/L, with absorbance readings conducted at a wavelength of 532 nm. The MDA concentration was calculated using the standard and expressed as nmol/mg protein. For the quantification of protein carbonyl, the OxiSelect Protein Carbonyl ELISA Kit (#STA-310, Cell Biolabs, Inc.) was utilized in accordance with the methodology outlined by Moita et al. (2021). Supernatants were appropriately diluted with PBS to obtain a protein concentration of 10 µg/mL before measurement. The standard was prepared within the working range of 0.375 to 7.5 nmol/mg protein. All steps were performed in accordance with the provided manual, and absorbance readings were taken at a wavelength of 450 nm, with concentrations expressed as nmol/mg protein. The concentrations of IgG and IgA were determined using ELISA kits (E101-104 and E101-102, Bethyl Laboratories, Inc., Montgomery, TX) following the protocol outlined by Holanda et al. (2020). Mucosal sample supernatants were appropriately diluted with PBS to achieve dilution factors of 1:1,600 and 1:400 for IgG and IgA, respectively, ensuring the working range for measurement. Absorbance readings were taken at a wavelength of 450 nm, and concentrations were reported as µg/mg of protein. The TNF-α concentration was quantified using the Porcine TNF-α Immunoassay Kit (#PTA00, R&D Systems, Minneapolis, MN, USA), as detailed in previous work (Cheng et al., 2021). Absorbance readings at 450 nm, corrected at 570 nm, were used to determine the final TNF-α concentration, expressed as pg/mg protein. The concentration of IL-6 was determined by employing the Porcine IL-6 DuoSet ELISA kit (#DY686, R&D Systems) in accordance with the method outlined by Deng et al. (2023). Similarly, for the measurement of IL-8 concentration, the Porcine IL-8/CXCL8 DuoSet ELISA kit (#DY535, R&D Systems) was used following the procedure described in previous work (Jang and Kim, 2019). Prior to analysis, samples were diluted with reagent diluent at a 1:5 ratio. Absorbance readings were taken at 450 nm, with correction at 570 nm. All steps were executed following the manufacturer’s protocol.
Intestinal morphology and cell proliferation (Ki-67+) in the crypt of the jejunum
Jejunal tissues were used for morphological evaluation. Following sampling, sections extracted from the mid-jejunum of each pig were fixed in a 10% formalin solution for a duration of 3 d. Subsequently, the samples were transversely cut into 2 sections, placed in a cassette, and immersed in a 70% ethanol solution. These prepared sample sections were then shipped to the University of North Carolina School of Medicine Lineberger Comprehensive Cancer Center (Chapel Hill, NC) for further processing. Formalin-fixed tissues were processed using a Leica ASP 6025s tissue processor, embedded in paraffin (Leica Paraplast), and sectioned at a thickness of 5 µm. Chromogenic Immunohistochemistry was performed on tissue slides using the Ventana Discovery automated staining platform (Roche). Antigen retrieval was carried out using Ventana’s CC1 solution (pH 8.5), followed by blocking, and incubation with a Ki-67 primary antibody (12202S, Cell Signaling Technology) diluted at 1:400. A secondary antibody (Discovery OmniMap anti-Rabbit HRP, Roche) was applied, and Ki-67+ cells were visualized with Discovery Chromo Maps DAB (Roche), followed by nuclear counterstaining with Hematoxylin II (Roche). Stained slides were dehydrated and the cover slipped with Cytoseal 60 (Epredia). A PC was included in the staining procedure. The evaluation of samples involved the use of an Olympus CX31 microscope (Lumenera Corporation, Ottawa, Canada) and the Infinity 2-2 digital CCD software. For each sample, images capturing 10 intact villi, and their associated crypts were obtained and measured, as previously detailed (Jang et al., 2020; Cheng et al., 2021). The measurements included villus height (VH) from the top to the junction of the villus and crypt, and crypt depth (CD) from the junction of the villus and crypt to the crypt’s bottom. The villus height to crypt depth (VH:CD) ratio was determined by dividing the measured VH by CD. In the Teledyne Lumenera INFINITY ANALYZE 7 software, the 10 images for each sample were imported, and the number of Ki-67+ cells in the crypt was counted. A single person conducted image collection and analysis of the intestinal morphology samples, and the averaged results of 10 measurements per pig were reported as a singular value per pig.
Statistical analysis
Data was analyzed using Proc MIXED SAS 9.4 software (SAS Inc., Cary, NC, USA). Initial BW and sex were considered as blocks. The statistical model included dietary treatments as fixed effects and blocks as random effects. Experimental unit was the individually housed pig. A power test was conducted to determine the appropriate number of replications needed for the study to determine statistical significance for an expected mean difference of 11% to 12% at P < 0.05. Based on previous studies conducted with pigs with a similar genetic background in the same research facility (Duarte et al., 2020; Duarte and Kim, 2022; Jang et al., 2023), a coefficient of variation of 7.5% was utilized. The power of test (1—beta) at 95% the power analysis indicated an 80%, the minimum number of replications for each treatment was 10 (Aaron and Hays, 2004). A pre-determined contrast was utilized to determine the differences of least squares means between NC vs. PC treatments, to test the effect of challenge with F18+E. coli, and between PC vs. HP, and PC vs. EP treatments. The data related to diarrhea incidence were analyzed using the Proc Freq of SAS. The analysis of similarities (ANOSIM) was conducted to assess the beta diversity of mucosa-associated microbiota, with the results visualized using principal coordinate analysis (PCoA) based on Bray-Curtis distance. Statistical differences were considered significant with P < 0.05 and tendency with 0.05 ≤ P < 0.10.
Results
Diversity and RA of jejunal mucosa-associated microbiota
There were no differences between PC and NC treatment for alpha diversity of jejunal mucosa-associated microbiota (Table 4). The HP treatment decreased (P < 0.05) alpha diversity of Chao1, Shannon, and Simpson when compared to PC treatment. The microbial community was visualized using PCoA based on Bray-Curtis distance, which confirmed that the PC changed (P < 0.05) microbiota composition in the jejunal mucosa of nursery pigs compared to the NC group (Figure 1). There were no significant differences in beta diversity of the jejunal mucosa-associated microbiota for HP and EP compared to PC treatment.
Table 4.
Alpha diversity of jejunal mucosa-associated microbiota at species level in pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Treatment3 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | HP | EP | SEM | NC vs. PC | PC vs. HP | PC vs. EP |
| Chao1 | 37 | 36 | 28 | 32 | 2.0 | 0.921 | 0.013 | 0.240 |
| Shannon | 1.5 | 1.7 | 1.1 | 1.2 | 0.2 | 0.557 | 0.021 | 0.581 |
| Simpson | 0.7 | 0.7 | 0.4 | 0.6 | 0.1 | 0.824 | 0.018 | 0.487 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
Figure 1.
PCoA plot in the jejunal mucosa-associated microbiota at the species level in nursery pigs fed diets supplemented with ground herb-based phytobiotics and essential oil-based phytobiotics. NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics (Salcochek Pro, Ayurvet Limited), challenged with F18+E. coli; EP: basal diet + 1% essential oil-based phytobiotics (Liq-biotic, Ayurvet Limited), challenged with F18+E. coli. The X-axis and Y-axis represent the principal component axes, with the percentages indicating the proportion of variation explained by each component. Points of different colors correspond to samples from different treatments (NC, PC, HP, and EP), and the closer the 2 points are, the more similar their species composition. The P value for Bray-Curtis for NC vs. PC: (P = 0.025); PC vs. HP (P = 0.458); PC vs. EP (P = 0.744).
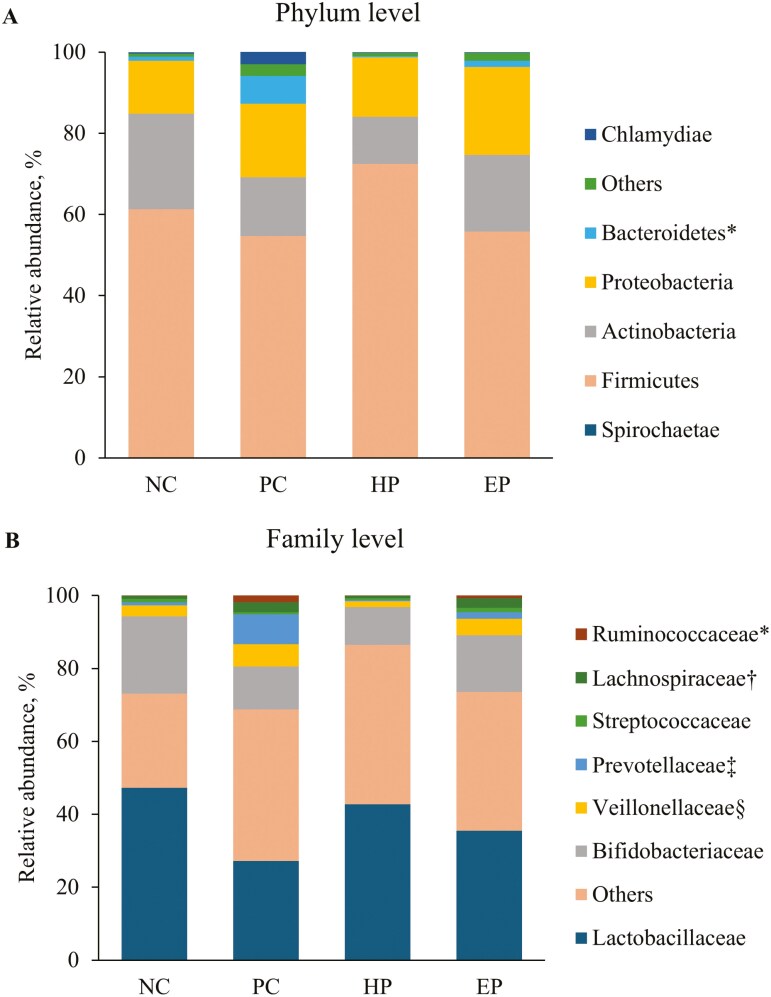
At phylum level (Table 5), the PC treatment increased (P < 0.05) the RA of Bacteroidetes when compared to NC treatment (fig. 2A). The PC treatment tended to decrease (P = 0.085) the RA of Actinobacteria, whereas PC treatment tended to increase (P = 0.074) Spirochaetae when compared to NC treatment. The HP treatment decreased (P < 0.05) Bacteriodetes when compared to PC treatment, whereas EP treatment tended to reduce (P = 0.057) Bacteriodetes when compared to PC treatment.
Table 5.
RA of jejunal mucosa-associated microbiota at the phylum level in pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Treatment3 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | HP | EP | SEM | NC vs. PC | PC vs. HP | PC vs. EP |
| Firmicutes | 61.29 | 54.56 | 72.47 | 55.66 | 8.15 | 0.534 | 0.125 | 0.927 |
| Actinobacteria | 23.52 | 14.46 | 11.55 | 18.91 | 3.87 | 0.085 | 0.593 | 0.433 |
| Proteobacteria | 13.06 | 18.11 | 14.63 | 21.61 | 8.94 | 0.669 | 0.781 | 0.788 |
| Bacteroidetes | 1.00 | 6.84 | 0.40 | 1.55 | 1.95 | 0.022 | 0.018 | 0.057 |
| Chlamydiae | 0.39 | 3.05 | 0.18 | 0.18 | 1.58 | 0.172 | 0.165 | 0.180 |
| Spirochaetae | < 0.01 | 0.15 | 0.02 | 0.12 | 0.07 | 0.074 | 0.145 | 0.758 |
| Others | 0.74 | 2.83 | 0.75 | 1.97 | 0.70 | 0.029 | 0.040 | 0.401 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
Figure 2.
RA of jejunal mucosa-associated microbiota at (A) phylum and (B) genus level in pigs fed diets supplemented with ground herb-based phytobiotics and essential oil-based phytobiotics. Each pattern represents a particular bacterial phylum. Phylum sequences that did not achieve 0.5% within each phylum were combined as “Others”. NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics (Salcochek Pro, Ayurvet Limited), challenged with F18+E. coli; EP: basal diet + 1% essential oil-based phytobiotics (Liq-biotic, Ayurvet Limited), challenged with F18+E. coli. (A). * Bacteroidetes, NC vs. PC: (P < 0.05), PC vs. HP: (P < 0.05), PC vs. EP (P = 0.057). (B) *Ruminococcaceae NC vs. PC: (P < 0.05), PC vs. HP: (P = 0.062), PC vs. EP (P = 0.238); †Lachnospiraceae NC vs. PC: (P < 0.05), PC vs. HP: (P < 0.05), PC vs. EP (P = 0.941); ‡Prevotellaceae NC vs. PC: (P < 0.05), PC vs. HP: (P < 0.05), PC vs. EP (P = 0.111); §Veillonellaceae NC vs. PC: (P = 0.133), PC vs. HP: (P < 0.05), PC vs. EP (P = 0.480).
At the family level (Table 6), the PC treatment increased (P < 0.05) Prevotellaceae, Lachnospiraceae, and Ruminococcaceae when compared to NC treatment (Figure 2B) and tended to decrease the RA of Lactobacillaceae (P = 0.092), and Bifidobacteriaceae (P = 0.050) when compared to NC treatment. The HP treatment decreased (P < 0.05) the RA of Veillonellaceae, Prevotellaceae, and Lachnospiraceae when compared to NC treatment, and tended to reduce (P = 0.062) RA of Ruminococcaceae when compared to PC treatment, whereas EP treatment tended to increase the RA of Streptococcaceae (P = 0.073) and Corynebacteriaceae (P = 0.074) when compared to PC treatment.
Table 6.
RA of jejunal mucosa-associated microbiota at family level in pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Treatment3 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | HP | EP | SEM | NC vs. PC | PC vs. HP | PC vs. EP |
| Lactobacillaceae | 47.26 | 27.17 | 42.77 | 36.49 | 8.79 | 0.092 | 0.212 | 0.569 |
| Bifidobacteriaceae | 21.14 | 12.78 | 10.38 | 15.84 | 3.50 | 0.050 | 0.780 | 0.486 |
| Staphylococcaceae | 6.94 | 14.14 | 25.26 | 5.96 | 6.86 | 0.644 | 0.149 | 0.591 |
| Helicobacteraceae | 11.71 | 18.64 | 14.13 | 20.95 | 8.72 | 0.734 | 0.902 | 0.676 |
| Veillonellaceae | 2.99 | 6.10 | 1.59 | 4.54 | 1.54 | 0.133 | 0.043 | 0.480 |
| Prevotellaceae | 0.92 | 4.18 | 0.37 | 1.83 | 1.17 | 0.045 | 0.028 | 0.111 |
| Coriobacteriaceae | 1.15 | 2.24 | 0.93 | 2.11 | 0.61 | 0.179 | 0.132 | 0.884 |
| Lachnospiraceae | 0.82 | 2.75 | 0.61 | 2.67 | 0.69 | 0.041 | 0.033 | 0.941 |
| Erysipelotrichaceae | 0.68 | 0.70 | 1.02 | 1.44 | 0.37 | 0.970 | 0.527 | 0.170 |
| Leuconostocaceae | 0.94 | 0.95 | 0.19 | 0.91 | 0.63 | 0.999 | 0.386 | 0.966 |
| Chlamydiaceae | 0.39 | 0.47 | 0.18 | 0.18 | 0.21 | 0.765 | 0.332 | 0.263 |
| Streptococcaceae | 0.90 | 0.62 | 0.52 | 1.19 | 0.21 | 0.328 | 0.726 | 0.073 |
| Corynebacteriaceae | 0.86 | 0.24 | 0.12 | 1.15 | 0.34 | 0.180 | 0.803 | 0.074 |
| Ruminococcaceae | 0.09 | 1.84 | 0.09 | 0.71 | 0.72 | 0.049 | 0.062 | 0.238 |
| Others | 3.21 | 7.18 | 1.84 | 4.03 | 1.44 | 0.048 | 0.014 | 0.149 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
At the genus level (Table 7), the PC treatment tended to decrease the RA of Lactobacillus (P = 0.092) and Bifidobacterium (P = 0.050) when compared to NC treatment. The HP treatment tended to reduce the RA of Megasphera (P = 0.074) and Dialister (P = 0.052) when compared to PC treatment. The EP treatment increased (P < 0.05) Syntrophococcus when compared to PC treatment, whereas EP tended to increase Streptococcus (P = 0.076) and Corynebacterium (P = 0.098) when compared to PC treatment.
Table 7.
RA of jejunal mucosa-associated microbiota at genus level in pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Treatment3 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | HP | EP | SEM | NC vs. PC | PC vs. HP | PC vs. EP |
| Lactobacillus | 47.24 | 27.12 | 42.76 | 34.25 | 8.80 | 0.092 | 0.211 | 0.581 |
| Bifidobacterium | 21.13 | 12.77 | 10.38 | 15.36 | 3.50 | 0.050 | 0.777 | 0.486 |
| Staphylococcus | 6.92 | 12.08 | 25.24 | 5.76 | 6.86 | 0.647 | 0.148 | 0.560 |
| Helicobacter | 11.71 | 15.84 | 14.13 | 20.95 | 8.72 | 0.734 | 0.902 | 0.676 |
| Megasphaera | 1.10 | 2.67 | 0.74 | 1.38 | 0.63 | 0.134 | 0.074 | 0.285 |
| Olsenella | 0.98 | 2.45 | 0.82 | 1.88 | 0.58 | 0.146 | 0.116 | 0.777 |
| Weissella | 0.94 | 0.95 | 0.19 | 4.15 | 1.41 | 0.999 | 0.703 | 0.131 |
| Mitsuokella | 1.11 | 1.10 | 0.53 | 1.04 | 0.39 | 0.976 | 0.187 | 0.899 |
| Chlamydia | 0.39 | 3.05 | 0.18 | 0.18 | 1.58 | 0.172 | 0.165 | 0.180 |
| Streptococcus | 0.90 | 0.62 | 0.52 | 1.18 | 0.21 | 0.324 | 0.728 | 0.076 |
| Syntrophococcus | 0.53 | 0.64 | 0.40 | 1.70 | 0.31 | 0.777 | 0.571 | 0.019 |
| Sharpea | 0.29 | 0.23 | 0.84 | 0.97 | 0.31 | 0.887 | 0.168 | 0.109 |
| Corynebacterium | 0.66 | 0.17 | 0.09 | 0.80 | 0.25 | 0.157 | 0.824 | 0.098 |
| Dialister | 0.54 | 0.64 | 0.10 | 0.44 | 0.19 | 0.701 | 0.052 | 0.483 |
| Selenomonas | 0.09 | 0.28 | 0.03 | 0.35 | 0.14 | 0.280 | 0.189 | 0.773 |
| Prevotella | 0.08 | 0.45 | 0.06 | 0.11 | 0.17 | 0.102 | 0.111 | 0.179 |
| Others | 5.39 | 18.94 | 2.99 | 9.50 | 4.73 | 0.020 | 0.011 | 0.135 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
At the species level (Table 8), the PC treatment decreased (P < 0.05) the RA of Lactobacillus delbrueckii, Bifidobacterium dentium, Lactobacillus salivarius, and Bifidobacterium boum when compared to NC treatment. The HP treatment increased (P < 0.05) the RA of Staphylococcus saprophyticus and Staphylococcus cohnii-nepalensis when compared to PC treatment and tended to decrease (P = 0.054) the RA of Dialister succinatiphilus. The EP treatment increased (P < 0.05) the RA of Syntrophococcus sp. when compared to PC treatment.
Table 8.
RA of mucosa-associated microbiota at species level of pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Treatment3 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | HP | EP | SEM | NC vs. PC | PC vs. HP | PC vs. EP |
| Helicobacter rappini | 11.68 | 13.08 | 14.03 | 20.13 | 8.51 | 0.897 | 0.934 | 0.575 |
| Bifidobacterium thermacidophilum-thermophilum | 7.07 | 7.98 | 6.36 | 13.04 | 2.97 | 0.816 | 0.698 | 0.248 |
| Lactobacillus delbrueckii | 20.58 | 9.00 | 18.20 | 12.97 | 4.32 | 0.045 | 0.130 | 0.530 |
| Lactobacillus mucosae | 9.02 | 6.21 | 8.89 | 8.46 | 2.26 | 0.351 | 0.401 | 0.515 |
| Staphylococcus kloosii | 2.04 | 6.61 | 6.55 | 1.08 | 3.53 | 0.287 | 0.989 | 0.243 |
| Staphylococcus saprophyticus-xylosus | 1.67 | 1.98 | 4.11 | 2.95 | 1.35 | 0.863 | 0.266 | 0.694 |
| Bifidobacterium dentium | 10.06 | 2.11 | 2.51 | 0.04 | 1.84 | 0.001 | 0.853 | 0.339 |
| Staphylococcus saprophyticus | 1.35 | 0.93 | 4.24 | 1.31 | 0.99 | 0.748 | 0.023 | 0.794 |
| Staphylococcus cohnii-nepalensis | 0.73 | 1.27 | 9.81 | 0.18 | 2.86 | 0.887 | 0.040 | 0.794 |
| Bifidobacterium boum | 4.08 | 1.55 | 1.51 | 2.25 | 0.86 | 0.026 | 0.971 | 0.558 |
| Megashpaera sp. | 1.07 | 2.33 | 0.74 | 1.34 | 0.63 | 0.136 | 0.079 | 0.281 |
| Chlamydia muridarum | 0.39 | 3.05 | 0.18 | 0.18 | 1.58 | 0.172 | 0.165 | 0.180 |
| Lactobacillus salivarius | 1.79 | 0.33 | 0.79 | 0.71 | 0.60 | 0.004 | 0.530 | 0.611 |
| Olsenella profusa | 0.81 | 1.12 | 0.61 | 0.79 | 0.41 | 0.567 | 0.378 | 0.579 |
| Syntrophococcus sp. | 0.46 | 0.63 | 0.38 | 1.66 | 0.31 | 0.664 | 0.540 | 0.021 |
| Weissellat hailandensis | 0.65 | 0.86 | 0.17 | 2.28 | 0.73 | 0.838 | 0.538 | 0.223 |
| Sharpea azabuensis | 0.29 | 0.23 | 0.82 | 0.97 | 0.29 | 0.887 | 0.168 | 0.109 |
| Helicobacter equorum | 0.02 | 1.90 | 0.02 | 0.84 | 0.58 | 0.014 | 0.018 | 0.189 |
| Mitsuokella multacida | 0.34 | 0.62 | 0.44 | 0.32 | 0.28 | 0.276 | 0.511 | 0.291 |
| Dialister succinatiphilus | 0.53 | 0.63 | 0.10 | 0.44 | 0.19 | 0.702 | 0.054 | 0.495 |
| Lactobacillus sp. | 11.95 | 8.02 | 4.72 | 2.95 | 2.72 | 0.279 | 0.391 | 0.207 |
| Others | 13.08 | 28.94 | 14.38 | 24.80 | 6.08 | 0.056 | 0.095 | 0.639 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
Relative mRNA expression of genes in jejunal tissue
The F18+E. coli challenge did not alter the expression of NOD1 and NOD2 between treatments (Table 9). Although no differences were observed in NOD1, NOD2, TLR2, CLD, and ZO-1 expression, the PC treatment tended to increase (P = 0.096) TLR4 expression when compared to NC treatment and increased (P < 0.05) OCC expression when compared to NC treatment.
Table 9.
Relative gene expression of mid-jejunum markers of pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Item3 | Treatment4 | SEM | P value | |||||
|---|---|---|---|---|---|---|---|---|
| NC | PC | HP | EP | NC vs. PC | PC vs. HP | PC vs. EP | ||
| NOD1 | 1.104 | 1.058 | 1.322 | 0.947 | 0.196 | 0.870 | 0.349 | 0.691 |
| NOD2 | 1.034 | 1.036 | 1.276 | 0.945 | 0.149 | 0.993 | 0.263 | 0.672 |
| TLR2 | 1.047 | 0.845 | 0.788 | 0.684 | 0.133 | 0.289 | 0.764 | 0.399 |
| TLR4 | 1.064 | 2.990 | 2.075 | 2.353 | 0.796 | 0.096 | 0.422 | 0.575 |
| CLD | 1.027 | 1.685 | 1.243 | 2.450 | 0.518 | 0.287 | 0.472 | 0.189 |
| OCC | 0.997 | 1.408 | 1.568 | 1.370 | 0.113 | 0.014 | 0.324 | 0.809 |
| ZO-1 | 1.003 | 1.091 | 1.169 | 1.000 | 0.092 | 0.509 | 0.551 | 0.492 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NOD1, Nucleotide-binding oligomerization domain 1; NOD2, Nucleotide-binding oligomerization domain; TLR2, Toll-like receptor 2; TLR4, Toll-like receptor 2; CLD, claudin; OCC, occludin; ZO-1, Zonula occludens-1.
4NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
Oxidative stress and immune parameters in the jejunal mucosa
The PC treatment showed no difference in inflammatory markers (TNF-α, IL-6, and IL-8), immune response markers (IgA and IgG), or oxidative markers (MDA and protein carbonyl) in mucosa samples from the mid-jejunum at 28 d post-challenge when compared to NC treatment (Table 10). However, the HP treatment tended to decrease (P = 0.053) protein carbonyl in mucosa samples from the mid-jejunum compared to the PC treatment at 28 d post-challenge.
Table 10.
Oxidative damage products and immune response from mid-jejunal mucosa of pigs fed with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Item3 | Treatment4 | SEM | P value | |||||
|---|---|---|---|---|---|---|---|---|
| NC | PC | HP | EP | NC vs. PC | PC vs. HP | PC vs. EP | ||
| Unit/mg protein | ||||||||
| IL-6, pg | 29.7 | 27.0 | 36.2 | 23.9 | 4.7 | 0.642 | 0.153 | 0.652 |
| IL-8, pg | 1.45 | 1.42 | 1.21 | 1.55 | 0.22 | 0.931 | 0.494 | 0.693 |
| TNFα, pg | 3.67 | 2.85 | 3.97 | 3.72 | 0.57 | 0.334 | 0.159 | 0.260 |
| IgA, μg | 2.89 | 2.70 | 2.43 | 2.64 | 0.37 | 0.703 | 0.625 | 0.915 |
| IgG, μg | 2.14 | 1.88 | 2.47 | 2.17 | 0.45 | 0.686 | 0.353 | 0.648 |
| MDA, μmol | 0.42 | 0.41 | 0.45 | 0.40 | 0.09 | 0.911 | 0.716 | 0.970 |
| Protein carbonyl, nmol | 1.34 | 1.71 | 0.95 | 1.48 | 0.27 | 0.332 | 0.053 | 0.567 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3IL-6, interleukin-6, IL-8, interleukin-8; TNFα, tumor necrosis factor-alpha; IgA, immunoglobulin A; IgG, immunoglobulin G; MDA, malondialdehyde.
4NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
Intestinal morphology and cell proliferation (Ki-67+) in the crypt of the jejunum
The PC treatment decreased (P < 0.05) VH when compared to NC treatment (Table 11), whereas HP and EP were not different in VH when compared to PC treatment. There were no differences in CD between PC and NC treatments, but HP and EP decreased (P < 0.05) CD when compared to PC treatment. The VH:CD ratio was similar between NC and PC treatment, whereas HP and EP increased (P < 0.05) VH:CD ratio when compared to PC treatment. The PC treatment decreased (P < 0.05) cell proliferation number Ki-67+ when compared to NC treatment. The HP and EP decreased (P < 0.05) cell proliferation number Ki-67+ when compared to PC treatment.
Table 11.
Intestinal morphology and cell proliferation in nursery pigs fed diets ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Item4 | Treatments3 | SEM | P value | |||||
|---|---|---|---|---|---|---|---|---|
| NC | PC | HP | EP | NC vs. PC | PC vs. HP | PC vs. EP | ||
| VH, µm | 486 | 422 | 467 | 440 | 22.0 | 0.034 | 0.115 | 0.486 |
| CD, µm | 177 | 164 | 132 | 130 | 8.0 | 0.206 | 0.004 | 0.002 |
| VH:CD | 2.79 | 2.62 | 3.54 | 3.38 | 0.16 | 0.432 | 0.001 | 0.002 |
| Ki-67+5, unit | 53.9 | 67.4 | 48.3 | 53.4 | 3.2 | 0.005 | 0.001 | 0.004 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NC, basal diet, non-challenged pigs; PC basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
4VH, villus height; CD, crypt depth; VH:CD, Villus height to Crypt depth ratio.
5Ki-67+, cell proliferation rate.
Fecal score
The incidence of diarrhea in the PC treatment was 30% higher compared to the HP and EP treatments during the period days 7 to 11 (Figure 3). The HP and EP treatment decreased (P < 0.05) FS when compared to PC treatment during the period days 7 to 11 and days 7 to 18.
Figure 3.
Incidence of diarrhea of pigs fed diets supplemented with ground herb-based phytobiotics and essential oil-based phytobiotics. NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics (Salcochek Pro, Ayurvet Limited), challenged with F18+E. coli; EP: basal diet + 1% essential oil-based phytobiotics (Liq-biotic, Ayurvet Limited), challenged with F18+E. coli.
Growth performance
The PC treatment tended to have decreased BW at day 18 (P = 0.056) and day 21 (P = 0.059) when compared to NC treatment (Table 12). The PC treatment showed a reduced (P < 0.05) ADG in the last 2 phases of the study and reduced (P < 0.05) ADFI during the 3 phases of the study when compared to NC treatment. However, the PC treatment had no difference in G:F ratio when compared to NC treatment. Overall, ADG was still reduced by 25% and the final BW was still decreased by 2.4 kg in PC treatment when compared to NC treatment. As regards phytobiotics, results show that during the last periods post-challenge, there were no differences for HP and EP treatments for BW, ADG, and, consequently, G:F ratio, when compared to PC treatment.
Table 12.
GP of pigs fed diets supplemented with ground herb-based phytobiotics1 and essential oil-based phytobiotics2
| Treatment3 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | HP | EP | SEM | NC vs. PC | PC vs. HP | PC vs. EP |
| BW, kg | ||||||||
| Day 0 | 6.42 | 6.41 | 6.43 | 6.45 | 0.13 | 0.947 | 0.876 | 0.824 |
| Day 7 | 6.37 | 6.31 | 6.27 | 6.37 | 0.18 | 0.805 | 0.855 | 0.824 |
| Day 11 | 7.27 | 6.72 | 6.67 | 6.79 | 0.29 | 0.196 | 0.893 | 0.863 |
| Day 18 | 10.58 | 9.18 | 9.10 | 9.36 | 0.50 | 0.056 | 0.906 | 0.807 |
| Day 21 | 12.22 | 10.66 | 10.10 | 10.82 | 0.56 | 0.059 | 0.494 | 0.836 |
| Day 28 | 16.07 | 13.67 | 12.72 | 13.87 | 0.72 | 0.024 | 0.354 | 0.851 |
| ADG, g/d | ||||||||
| Days 0 to 7 (pre challenge) | -6 | -15 | -24 | -11 | 17 | 0.732 | 0.719 | 0.881 |
| Days 7 to 28 | 462 | 351 | 307 | 357 | 28 | 0.009 | 0.287 | 0.872 |
| Days 0 to 11 (phase1) | 77 | 28 | 21 | 31 | 23 | 0.143 | 0.815 | 0.929 |
| Days 11 to 21 (phase 2) | 495 | 393 | 344 | 403 | 33 | 0.034 | 0.290 | 0.840 |
| Days 21 to 28 (phase 3) | 551 | 431 | 374 | 435 | 40 | 0.044 | 0.322 | 0.946 |
| Days 0 to 28 (overall) | 345 | 260 | 225 | 265 | 24 | 0.017 | 0.308 | 0.874 |
| ADFI, g/d | ||||||||
| Days 0 to 7 (pre challenge) | 69 | 55 | 45 | 66 | 13 | 0.475 | 0.589 | 0.562 |
| Days 7 to 28 | 730 | 539 | 477 | 522 | 38 | 0.001 | 0.254 | 0.743 |
| Days 0 to 11 (phase1) | 129 | 87 | 82 | 96 | 17 | 0.095 | 0.838 | 0.699 |
| Days 11 to 21 (phase 2) | 648 | 499 | 456 | 479 | 41 | 0.015 | 0.463 | 0.737 |
| Days 21 to 28 (phase 3) | 1,038 | 781 | 668 | 780 | 58 | 0.004 | 0.176 | 0.989 |
| Days 0 to 28 (overall) | 565 | 418 | 364 | 408 | 30 | 0.002 | 0.218 | 0.807 |
| G:F | ||||||||
| Days 0 to 7 (pre challenge) | -0.21 | -2.70 | -2.39 | -1.77 | 2.17 | 0.422 | 0.105 | 0.764 |
| Days 7 to 28 | 0.63 | 0.64 | 0.65 | 0.68 | 0.03 | 0.827 | 0.846 | 0.299 |
| Days 0 to 11 (phase1) | 0.57 | 0.32 | 0.17 | 0.38 | 0.75 | 0.118 | 0.245 | 0.190 |
| Days 11 to 21 (phase 2) | 0.76 | 0.79 | 0.76 | 0.86 | 0.03 | 0.542 | 0.498 | 0.114 |
| Days 21 to 28 (phase 3) | 0.53 | 0.55 | 0.57 | 0.56 | 0.05 | 0.739 | 0.807 | 0.885 |
| Days 0 to 28 (overall) | 0.61 | 0.61 | 0.62 | 0.64 | 0.03 | 0.869 | 0.738 | 0.428 |
1Salcochek Pro (Ayurvet Limited).
2Liq-biotic (Ayurvet Limited).
3NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics, challenged with F18+E. coli; EP, basal diet + 1% essential oil-based phytobiotics, challenged with F18+E. coli.
Discussion
This study provides insights into how ground herb-based phytobiotics and essential oil-based phytobiotics differently influence intestinal health in nursery pigs challenged with F18+E. coli. Specifically, the findings demonstrate how these phytobiotics mitigate disruption to the jejunal mucosa-associated microbiota and support intestinal morphology, offering practical applications for improving intestinal health and performance in pig production.
Intestinal health is crucial as it is the primary site of digestion, nutrient absorption, and defense against pathogens, playing a key role in stress response in nursery pigs (Tang et al., 2022). In the present study, the F18+E. coli challenge induced jejunal mucosal-associated microbiota disruption and compromised intestinal morphology, evidenced by altered VH, CD, and tight junction protein expression (OCC) consistent with previous reports (Duarte et al., 2023; Jang et al., 2023). These changes were accompanied by disruption to the jejunal mucosa-associated microbiota, including reduced RA of Actinobacteria, known producers of antimicrobial agents (Barka et al., 2016), and increased susceptibility to intestinal inflammation and epithelial damage (Xu et al., 2023). Additionally, it was observed that F18+E. coli tended to reduce RA of Lactobacillus, which inhibits pathogenic bacteria through the production of lactic acid, antimicrobial peptides, and various metabolites. These actions contribute to improved GP and influence microbiota composition as well as immune indices in pigs (Pringsulaka et al., 2015; Zhang et al., 2023). Elevated TLR4 levels in challenged pigs reflected an active inflammatory response to the pathogen, consistent with its role in recognizing bacterial endotoxins like lipopolysaccharides from Gram-negative bacteria and activating innate immune signaling pathways (Sabroe et al., 2008; Hu et al., 2014). This aligns with studies showing that bacterial recognition by TLR4 can upregulate inflammatory responses (Chen et al., 2013).
Both phytobiotics mitigated these adverse effects, but through different mechanisms. The HP reduced microbial diversity, as observed by lower Chao1, Shannon, and Simpson indices, potentially limiting the proliferation of pathogenic bacteria (Xia and Sun, 2023). The bioactive compounds in HP, such as alkaloids and phenolics, can bind to bacterial DNA and RNA, altering their structure and disrupting replication, transcription, and protein synthesis of harmful bacteria (Jin et al., 2010). Additionally, alkaloids can interfere with bacterial metabolic pathways, leading to cell death of harmful bacteria (Du et al., 2020). Interestingly, HP treatment tended to reduce the RA of Megasphaera and Dialister, which are known to produce short-chain fatty acids or to modulate the host’s immune system and produce propionate (Yoshikawa et al., 2018; Sakamoto et al., 2022), this reduction of Megasphaera could suggest that HP has the ability to selectively modulate jejunal mucosal-associated microbiota population. Furthermore, the impact of oxidative stress on intestinal health was also considered in the present study, using oxidative damage markers such as MDA and protein carbonyl. These markers serve as immediate indicators of oxidative stress, reflecting tissue damage and impaired function (Dalle-Donne et al., 2003; Cordiano et al., 2023). The HP demonstrated the potential to reduce oxidative stress, as reflected by a trend toward decreased protein carbonyl levels. This likely contributed to preserving tight junction integrity by reducing proteins and DNA damage (Dalle-Donne et al., 2003; Mateos and Bravo, 2007). The antioxidant and anti-inflammatory properties of flavonoids and tannins in HP could have further protected against epithelial damage and promoted intestinal recovery (Chandra et al., 2016; Tong et al., 2022). In contrast, the EP phytobiotics positively modulated jejunal mucosa-associated microbiota, by exerting jejunal conditions to shift the RA of beneficial bacteria such as Syntrophococcus and Corynebacterium, whereas reducing pathogenic populations such as Bacteroidetes, which is related to a lower capacity of the host to obtain energy from ingested feed and a lower capacity to store fat (Guo et al., 2008). Although the study did not directly assess the antimicrobial properties of the phytobiotics, changes in microbiota composition suggest mechanisms involving bacterial membrane disruption and inhibition of biomolecule synthesis (Jin et al., 2010; Valenzuela-Grijalva et al., 2017; Chen et al., 2020). The bioactive compounds in EP, such as thymol and eugenol, are related to disrupting the lipid fraction of bacterial membranes, altering membrane permeability, and causing leakage of intracellular materials (Trombetta et al., 2005; Guimarães et al., 2019). Similarly, although EP also contains antioxidant compounds like thymol and eugenol (Nagoor Meeran et al., 2017; da Silva et al., 2018), its lipid-focused antioxidant mechanism may be less effective in reducing overall oxidative stress compared to the broader activity of tannins and flavonoids in HP (Nagababu et al., 2010; Archana et al., 2011).
Despite differences in the mechanisms of action, the morphological changes observed in this study further highlight the protective effects of phytobiotics. The F18+E. coli challenge caused villus atrophy and crypt hyperplasia, as evidenced by reduced VH and increased CD in challenged pigs. However, supplementation with both types of phytobiotics improved intestinal morphology, as indicated by higher VH:CD ratios and reduced CD (Du et al., 2016; Chen et al., 2019). These results are supported by reduced Ki-67+ cells in phytobiotic-treated pigs, suggesting decreased cellular turnover and the presence of mature enterocytes (Paiva et al., 2014; Miller et al., 2018). The bioactive compounds in ground herb-based phytobiotics, such as alkaloids, flavonoids, and tannins, may promote epithelial regeneration and support intestinal structure (Peng et al., 2019; Pei et al., 2020; Toschi et al., 2022), while thymol and eugenol in essential oil-based phytobiotics also likely contribute to maintaining intestinal integrity (Rocha-Caldas et al., 2015; Hui et al., 2020). These improvements in intestinal morphology were accompanied by better FS, indicating reduced diarrhea severity (Figure 4). Whereas HP demonstrated prolonged reductions in diarrhea, likely due to their bioactive compounds’ ability to regulate gastrointestinal motility and electrolyte balance (Teke et al., 2010; Rahman et al., 2013; Damissie et al., 2023). The EP was more effective in the early post-challenge period. Though GP was not a primary focus of this study, the F18+E. coli challenge negatively impacted feed intake and growth during the initial postweaning period. This aligns with previous findings that suggest feed consumption is reduced under pathogenic stress (Li et al., 2019).
Figure 4.
The FS of pigs fed diets supplemented with ground herb-based phytobiotics and essential oil-based phytobiotics. NC, basal diet, non-challenged pigs; PC, basal diet, challenged with F18+E. coli; HP, basal diet + 1% ground herb-based phytobiotics (Salcochek Pro, Ayurvet Limited), challenged with F18+E. coli; EP: basal diet + 1% essential oil-based phytobiotics (Liq-biotic, Ayurvet Limited), challenged with F18+E. coli. * days 3 to 7, NC vs. PC: (P = 0.259), PC vs. HP: (P = 0.007), PC vs. EP: (P = 0.527); day 7, NC vs. PC: (P = 0.238), PC vs. HP: (P = 0.045), PC vs. EP: (P = 0.080); day 8, NC vs. PC: (P = 0.061), PC vs. HP: (P = 0.018), PC vs. EP: (P = 0.527); day 10, NC vs. PC: (P = 0.029), PC vs. HP: (P = 0.751), PC vs. EP: (P = 0.362); day 27, NC vs. PC: (P = 1.000), PC vs. HP: (P = 0.385), PC vs. EP: (P = 0.035).
In conclusion, this study highlights the negative impacts of F18+E. coli on the jejunal mucosa-associated microbiota, immune responses, and intestinal morphology in nursery pigs. Both ground herb-based phytobiotics and essential oil-based phytobiotics influenced the composition of jejunal mucosa-associated microbiota by decreasing harmful bacteria, enhancing VH to crypt ratio, and promoting mucosal recovery through increased cell renewal and reducing FS in the first 2 wk post-challenge. However, the composition of ground herb-based phytobiotics, containing alkaloids, coumarins, flavonoids, saponins, and tannins, reduced oxidative stress, as shown by the tendency to decreased protein carbonyl levels in the jejunal mucosa. The distinct properties of herb-based and essential oil-based phytobiotics underscore their potential as natural interventions for improving intestinal health during E. coli challenges, and understanding their impacts is key to optimizing strategies for pig well-being.
Acknowledgments
Financial supports for this research from North Carolina Agricultural Foundation (#660101 and #665610, Raleigh, NC, USA), USDA-NIFA Hatch (#02893, Washington DC, USA), and Ayurvet Limited (Kaushambi, India). Technical support was provided by members of the Kim Lab for animal handling, sampling, and laboratory analysis.
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- BW
body weight
- G:F
gain to feed ratio
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IL-6
interleukin-6
- IL-8
interleukin-8
- MDA
malondialdehyde
- NOD1
nucleotide-binding oligomerization domain containing 1
- NOD2
nucleotide-binding oligomerization domain containing 2
- OCC
occludin
- PWD
postweaning diarrhea
- Sta
heat-stable toxin A
- Stb
heat-stable toxin B
- TLR2
toll-like receptor 2
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor-alpha
- VH
villus height
- VH:CD
villus height to crypt depth ratio
- ZO-1
zonula occludens-1
Contributor Information
Yesid Garavito-Duarte, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Marcos Elias Duarte, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Sung Woo Kim, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Conflict of interest statement
Yesid Garavito-Duarte, Marcos Duarte, and Sung Woo Kim declare that they have no conflict of interest.
Author contributions
Yesid Garavito-Duarte (Data curation, Formal analysis, Investigation, Writing—original draft, Writing—review & editing), Marcos Duarte (Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing), and Sung Woo Kim (Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing—original draft, Writing—review & editing).
Literature Cited
- Aaron, D. K., and Hays V. W... 2004. How many pigs? Statistical power considerations in swine nutrition experiments. J. Anim. Sci. 82:45–54. doi: https://doi.org/ 10.2527/2004.8213_supplE245x [DOI] [PubMed] [Google Scholar]
- Archana, P., Rao B. N., and Rao B. S. S... 2011. In vivo radioprotective potential of thymol, a monoterpene phenol derivative of cymene. Mutat. Res. 726:136–145. doi: https://doi.org/ 10.1016/j.mrgentox.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Bairwa, R., Sodha R. S., and Rajawat B. S... 2012. Trachyspermum ammi. Pharmacogn. Rev. 6:56–60. doi: https://doi.org/ 10.4103/0973-7847.95871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka, E. A., Vatsa P., Sanchez L., Gaveau-Vaillant N., Jacquard C., Klenk H. -P., Clément C., Ouhdouch Y., and Wezel G. P... 2016. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 80:1–43. doi: https://doi.org/ 10.1128/mmbr.00019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiri-Jahromi, S. 2018. Punica granatum (pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 12:345. doi: https://doi.org/ 10.4081/oncol.2018.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, J., Barros M. M., Araújo D., Campos A. M., Oliveira R., Silva S., and Almeida C... 2022. Swine enteric colibacillosis: current treatment avenues and future directions. Front. Vet. Sci. 9:981207. doi: https://doi.org/ 10.3389/fvets.2022.981207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, S. R., Diwan A. D., and Panche A. N... 2016. Flavonoids: an overview. J. Nutr. Sci. 5:e47. doi: https://doi.org/ 10.1017/jns.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing, B., Kumar M., Baker M. T., Singh V., and Vijay-Kumar M... 2014. Mammalian gut immunity. Biomed. J. 37:246–258. doi: https://doi.org/ 10.4103/2319-4170.130922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F., Liu Y., Zhu H., Hong Y., Wu Z., Hou Y., Li Q., Ding B., Yi D., and Chen H... 2013. Fish oil attenuates liver injury caused by LPS in nursery pigs associated with inhibition of TLR4 and nucleotide-binding oligomerization domain protein signaling pathways. Innate Immun. 19:504–515. doi: https://doi.org/ 10.1177/1753425912472003 [DOI] [PubMed] [Google Scholar]
- Chen, J., Kang B., Yao K., Fu C., and Zhao Y... 2019. Effects of dietary Macleaya cordata extract on growth performance, immune responses, antioxidant capacity, and intestinal development in weaned piglets. J. Appl. Anim. Res. 47:349–356. doi: https://doi.org/ 10.1080/09712119.2019.1636800 [DOI] [Google Scholar]
- Chen, J., Tang C., Zhang R., Ye S., Zhao Z., Huang Y., Xu X., Lan W., and Yang D... 2020. Metabolomics analysis to evaluate the antibacterial activity of the essential oil from the leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol. 253:112652. doi: https://doi.org/ 10.1016/j.jep.2020.112652 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. C., Duarte M. E., and Kim S. W... 2021. Nutritional and functional values of lysed Corynebacterium glutamicum cell mass for intestinal health and growth of nursery pigs. J. Anim. Sci. 99:skab331. doi: https://doi.org/ 10.1093/jas/skab331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čmiková, N., Galovičová L., Schwarzová M., Vukic M. D., Vukovic N. L., Kowalczewski P., Bakay L., Kluz M. I., Puchalski C., and Kačániová M... 2023. Chemical composition and biological activities of Eucalyptus globulus essential oil. Plants. 12:1076. doi: https://doi.org/ 10.3390/plants12051076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddens, A., Loos M., Vanrompay D., Remon J. P., and Cox E... 2017. Cranberry extract inhibits in vitro adhesion of F4 and F18(+) Escherichia coli to pig intestinal epithelium and reduces in vivo excretion of pigs orally challenged with F18(+) verotoxigenic E. coli. Vet. Microbiol. 202:64–71. doi: https://doi.org/ 10.1016/j.vetmic.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Cordiano, R., Di Gioacchino M., Mangifesta R., Panzera C., Gangemi S., and Minciullo P. L... 2023. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: an update. Molecules. 28:5979. doi: https://doi.org/ 10.3390/molecules28165979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne, I., Rossi R., Giustarini D., Milzani A., and Colombo R... 2003. Protein carbonyl treatments as biomarkers of oxidative stress. Clin. Chim. Acta. 329:23–38. doi: https://doi.org/ 10.1016/s0009-8981(03)00003-2 [DOI] [PubMed] [Google Scholar]
- Damissie, G., Tolossa K., Hymete A., and Tadesse S... 2023. Antidiarrheal coumarins from Psydrax schimperianus (A. Rich.) Bridson roots. J. Ethnopharmacol. 316:116705. doi: https://doi.org/ 10.1016/j.jep.2023.116705 [DOI] [PubMed] [Google Scholar]
- da Silva, F. F. M., Monte F. J. Q., de Lemos T. L. G., do Nascimento P. G. G., de Medeiros Costa A. K., and de Paiva L. M. M... 2018. Eugenol derivatives: synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 12:34. doi: https://doi.org/ 10.1186/s13065-018-0407-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Z., Duarte M. E., and Kim S. W... 2023. Efficacy of soy protein concentrate replacing animal protein supplements in mucosa-associated microbiota, intestinal health, and growth performance of nursery pigs. Anim. Nutr. 14:235–248. doi: https://doi.org/ 10.1016/j.aninu.2023.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, E., Wang W., Gan L., Li Z., Guo S., and Guo Y... 2016. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 7:19. doi: https://doi.org/ 10.1186/s40104-016-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, G. -F., Le Y. -J., Sun X., Yang X. -Y., and He Q. -Y... 2020. Proteomic investigation into the action mechanism of berberine against Streptococcus pyogenes. J. Proteomics. 215:103666. doi: https://doi.org/ 10.1016/j.jprot.2020.103666 [DOI] [PubMed] [Google Scholar]
- Duarte, M. E., and Kim S. W... 2022. Significance of mucosa-associated microbiota and its impacts on intestinal health of pigs challenged with F18+E. coli. Pathogens. 11:589. doi: https://doi.org/ 10.3390/pathogens11050589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., Tyus J., and Kim S. W... 2020. Synbiotic effects of enzyme and probiotics on intestinal health and growth of newly nursery pigs challenged with enterotoxigenic F18+Escherichia coli. Front. Vet. Sci. 7:573. doi: https://doi.org/ 10.3389/fvets.2020.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., Garavito-Duarte Y., and Kim S. W... 2023. Impacts of F18+Escherichia coli on intestinal health of nursery pigs and dietary interventions. Animals. 13:2791. doi: https://doi.org/ 10.3390/ani13172791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani, A. H., Khan A., Khan A. -u, Bashir S., Rehman N. -u, and Mandukhail S. -u-R... 2010. Pharmacological basis for the medicinal use of Holarrhena antidysenterica in gut motility disorders. Pharm. Biol. 48:1240–1246. doi: https://doi.org/ 10.3109/13880201003727960 [DOI] [PubMed] [Google Scholar]
- Guimarães, A. C., Meireles L. M., Lemos M. F., Guimarães M. C. C., Endringer D. C., Fronza M., and Scherer R... 2019. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 24:2471. doi: https://doi.org/ 10.3390/molecules24132471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X., Xia X., Tang R., Zhou J., Zhao H., and Wang K... 2008. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47:367–373. doi: https://doi.org/ 10.1111/j.1472-765X.2008.02408.x [DOI] [PubMed] [Google Scholar]
- Holanda, D. M., and Kim S. W... 2021. Investigation of the efficacy of mycotoxin-detoxifying additive on health and growth of newly-nursery pigs under deoxynivalenol challenges. Anim. Biosci. 34:405–416. doi: https://doi.org/ 10.5713/ajas.20.0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda, D. M., Yiannikouris A., and Kim S. W... 2020. Investigation of the efficacy of a postbiotic yeast cell wall-based blend on newly-nursery pigs under a dietary challenge of multiple mycotoxins with emphasis on deoxynivalenol. Toxins. 12:504. doi: https://doi.org/ 10.3390/toxins12080504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C. H., Song Z. H., Xiao K., Song J., Jiao L. F., and Ke Y. L... 2014. Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs. Innate Immun. 20:478–486. doi: https://doi.org/ 10.1177/1753425913499947 [DOI] [PubMed] [Google Scholar]
- Hui, Q., Ammeter E., Liu S., Yang R., Lu P., Lahaye L., and Yang C... 2020. Eugenol attenuates inflammatory response and enhances barrier function during lipopolysaccharide-induced inflammation in the porcine intestinal epithelial cells. J. Anim. Sci. 98:skaa245. doi: https://doi.org/ 10.1093/jas/skaa245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan, F., Alvi S. S., and Islam M. H... 2022. Berberis aristata and its secondary metabolites: insights into nutraceutical and therapeutical applications. Pharmacol. Res. - Modern Chin. Med. 5:100184. doi: https://doi.org/ 10.1016/j.prmcm.2022.100184 [DOI] [Google Scholar]
- Jang, K. B., and Kim S. W... 2019. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly nursery pigs. J. Anim. Sci. 97:4875–4882. doi: https://doi.org/ 10.1093/jas/skz334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B., Purvis J. M., and Kim S. W... 2020. Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health, and gut-associated microbiome of offspring. J. Anim. Sci. 98:skaa227. doi: https://doi.org/ 10.1093/jas/skaa227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B., Moita V. H. C., Martinez N., Sokale A., and Kim S. W... 2023. Efficacy of zinc glycinate reducing zinc oxide on intestinal health and growth of nursery pigs challenged with F18+Escherichia coli. J. Anim. Sci. 101:skad035. doi: https://doi.org/ 10.1093/jas/skad035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, J., Hua G., Meng Z., and Gao P... 2010. Antibacterial mechanisms of berberine and reasons for little resistance of bacteria. Chin. Herb. Med. 3:27–35. doi: https://doi.org/ 10.3969/j.issn.1674-6384.2011.01.007 [DOI] [Google Scholar]
- Kikusato, M. 2021. Phytobiotics to improve health and production of broiler chickens: functions beyond the antioxidant activity. Anim. Biosci. 34:345–353. doi: https://doi.org/ 10.5713/ab.20.0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. W., Holanda D. M., Gao X., Park I., and Yiannikouris A... 2019. Efficacy of a yeast cell wall extract to mitigate the effect of naturally co-occurring mycotoxins contaminating feed ingredients fed to young pigs: Impact on gut health, microbiome, and growth. Toxins. 11:633. doi: https://doi.org/ 10.3390/toxins11110633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommera, S. K., Mateo R. D., Neher F. J., and Kim S. W... 2006. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian-Australas. J. Anim. Sci. 19:1784–1789. doi: https://doi.org/ 10.5713/ajas.2006.1784 [DOI] [Google Scholar]
- Lamichhane, B., Adhikari S., Shrestha P., and Shrestha B. G... 2014. Study of phytochemical, antioxidant, antimicrobial and anticancer activity of Berberis aristata. J. Trop. Life Sci. 4:01–07. doi: https://doi.org/ 10.11594/jtls.04.01.01 [DOI] [Google Scholar]
- Li, Q., Burrough E. R., Gabler N. K., Loving C. L., Sahin O., Gould S. A., and Patience J. F... 2019. A soluble and highly fermentable dietary fiber with carbohydrases improved gut barrier integrity markers and growth performance in F18 ETEC challenged pigs. J. Anim. Sci. 97:2139–2153. doi: https://doi.org/ 10.1093/jas/skz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., Yuan C., Meng X., Du Y., Gao R., Tang J., and Shi D... 2014. Frequency of virulence factors in Escherichia coli isolated from suckling pigs with diarrhea in China. Vet. J. 199:286–289. doi: https://doi.org/ 10.1016/j.tvjl.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Mateos, R., and Bravo L... 2007. Chromatographic and electrophoretic methods for the analysis of biomarkers of oxidative damage to macromolecules (DNA, lipids, and proteins). J. Sep. Sci. 30:175–191. doi: https://doi.org/ 10.1002/jssc.200600314 [DOI] [PubMed] [Google Scholar]
- Miller, I., Min M., Yang C., Tian C., Gookin S., Carter D., and Spencer S. L... 2018. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 24:1105–1112.e5. doi: https://doi.org/ 10.1016/j.celrep.2018.06.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita, V. H. C., Duarte M. E., da Silva S. N., and Kim S. W... 2021. Supplemental effects of functional oils on the modulation of mucosa-associated microbiota, intestinal health, and growth performance of nursery pigs. Animals (Basel). 11:1591. doi: https://doi.org/ 10.3390/ani11061591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujeeb, F., Bajpai P., and Pathak N... 2014. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed. Res. Int. 2014:97606. doi: https://doi.org/ 10.1155/2014/497606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagababu, E., Rifkind J. M., Boindala S., and Nakka L... 2010. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol. Biol. 610:165–180. doi: https://doi.org/ 10.1007/978-1-60327-029-8_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoor Meeran, M. F., Javed H., Al Taee H., Azimullah S., and Ojha S. K... 2017. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 8:380. doi: https://doi.org/ 10.3389/fphar.2017.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient Requirements of Swine: Eleventh Revised Edition. Washington, DC: The National Academies Press. [Google Scholar]
- Paiva, D., Walk C., and McElroy A... 2014. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult. Sci. 93:2752–2762. doi: https://doi.org/ 10.3382/ps.2014-04148 [DOI] [PubMed] [Google Scholar]
- Patilba, M. P., Borthakur A., Yadav V., and Ravikanth K... 2017. Efficacy of polyherbal formulation in the treatment of diarrhea in broiler birds. Global J. Res. Anal. 6:484–485. [Google Scholar]
- Pei, R., Liu X., and Bolling B... 2020. Flavonoids and gut health. Curr. Opin. Biotechnol. 61:153–159. doi: https://doi.org/ 10.1016/j.copbio.2019.12.018 [DOI] [PubMed] [Google Scholar]
- Peng, J., Zheng T. T., Li X., Liang Y., Wang L. J., Huang Y. C., and Xiao H. T... 2019. Plant-derived alkaloids: the promising disease-modifying agents for inflammatory bowel disease. Front. Pharmacol. 10:351. doi: https://doi.org/ 10.3389/fphar.2019.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsulaka, O., Rueangyotchanthana K., Suwannasai N., Watanapokasin R., Amnueysit P., Sunthornthummas S., Sukkhum S., Sarawaneeyaruk S., and Rangsiruji A... 2015. In vitro screening of lactic acid bacteria for multi-strain probiotics. Livest. Sci. 174:66–73. doi: https://doi.org/ 10.1016/j.livsci.2015.01.016 [DOI] [Google Scholar]
- Rahman, S., and Parvin R... 2014. Therapeutic potential of Aegle marmelos (L.)—An overview. Asian Pac. J. Trop. Dis. 4:71–77. doi: https://doi.org/ 10.1016/s2222-1808(14)60318-2 [DOI] [Google Scholar]
- Rahman, M. K., Barua S., Islam M. F., Islam M. R., Sayeed M. A., Parvin M. S., and Islam M. E... 2013. Studies on the anti-diarrheal properties of leaf extract of Desmodium puchellum. Asian. Pac. J. Trop. Biomed. 3:639–43; discussion 642. doi: https://doi.org/ 10.1016/S2221-1691(13)60129-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Caldas, G. F., Oliveira A. R., Araújo A. V., Lafayette S. S., Albuquerque G. S., Silva-Neto Jda C., Costa-Silva J. H., Ferreira F., Costa J. G., and Wanderley A. G... 2015. Gastroprotective mechanisms of the monoterpene 1,8-Cineole (Eucalyptol). PLoS One. 10:e0134558. doi: https://doi.org/ 10.1371/journal.pone.0134558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabroe, I., Parker L., Dower S., and Whyte M... 2008. The role of TLR activation in inflammation. J. Pathol. 214:126–135. doi: https://doi.org/ 10.1002/path.2264 [DOI] [PubMed] [Google Scholar]
- Sakamoto, M., Ikeyama N., Iino T., and Ohkuma M... 2022. Growth of succinate consumer Dialister hominis is supported by Bacteroides thetaiotaomicron. Anaerobe. 77:102642. doi: https://doi.org/ 10.1016/j.anaerobe.2022.102642 [DOI] [PubMed] [Google Scholar]
- Sharma, D. K., Gupta V. K., Kumar S., Joshi V., Mandal R. S., Prakash A. G., and Singh M... 2015. Evaluation of antidiarrheal activity of ethanolic extract of Holarrhena antidysenterica seeds in rats. Vet. World. 8:1392–1395. doi: https://doi.org/ 10.14202/vetworld.2015.1392-1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, S., Sharma A., Reddy P. H., Rathi B., Prasad N. V. S. R. K., and Vashishtha A... 2013. Evaluation of phytochemical and pharmacological aspects of Holarrhena antidysenterica. J. Pharm. Res. 6:488–492. doi: https://doi.org/ 10.1016/j.jopr.2013.04.004 [DOI] [Google Scholar]
- Sun, Y., Duarte M. E., and Kim S. W... 2021. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim. Nutr. 7:326–333. doi: https://doi.org/ 10.1016/j.aninu.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., Xiong K., Fang R., and Li M... 2022. Weaning stress and intestinal health of piglets: a review. Front. Immunol. 13:1042778. doi: https://doi.org/ 10.3389/fimmu.2022.1042778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teke, G. N., Kuiate J. R., Kueté V., Teponno R. B., Tapondjou L. A., and Vilarem G... 2010. Antidiarrheal activity of extracts and compounds from Trilepisium madagascariense stem bark. Indian J. Pharmacol. 42:157–163. doi: https://doi.org/ 10.4103/0253-7613.66839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, Z., He W., Fan X., and Guo A... 2022. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 8:803657. doi: https://doi.org/ 10.3389/fvets.2021.803657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi, A., Piva A., and Grilli E... 2022. Phenol-rich botanicals modulate oxidative stress and epithelial integrity in intestinal epithelial cells. Animals (Basel). 12:2188. doi: https://doi.org/ 10.3390/ani12172188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta, D., Castelli F., Sarpietro M. G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., and Bisignano G... 2005. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 49:2474–2478. doi: https://doi.org/ 10.1128/AAC.49.6.2474-2478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Grijalva, N. V., Pinelli-Saavedra A., Muhlia-Almazan A., Domínguez-Díaz D., and González-Ríos H... 2017. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 59:8. doi: https://doi.org/ 10.1186/s40781-017-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Y., and Sun J... 2023. Alpha diversity. In: Xia, Y., and Sun J., editors, Bioinformatic and statistical analysis of microbiome data: from raw sequences to advanced modeling with QIIME 2 and R. Cham: Springer International Publishing; p. 289–333. [Google Scholar]
- Xu, X., Duarte M. E., and Kim S. W... 2022. Postbiotic effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+Escherichia coli. J. Anim. Sci. 100:skac210. doi: https://doi.org/ 10.1093/jas/skac210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Jia Z., Xiao S., Long C., and Wang L... 2023. Effects of Enterotoxigenic Escherichia coli challenge on jejunal morphology and microbial community profiles in weaned crossbred piglets. Microorganisms. 11:2646. doi: https://doi.org/ 10.3390/microorganisms11112646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamani, H. A., Pang E. C., Mantri N., and Deighton M. A... 2016. Antimicrobial activity of Tulsi (Ocimum tenuiflorum) essential oil and their major constituents against three species of bacteria. Front. Microbiol. 7:681. doi: https://doi.org/ 10.3389/fmicb.2016.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa, S., Araoka R., Kajihara Y., Ito T., Miyamoto H., and Kodama H... 2018. Valerate production by Megasphaera elsdenii isolated from pig feces. J. Biosci. Bioeng. 125:519–524. doi: https://doi.org/ 10.1016/j.jbiosc.2017.12.016 [DOI] [PubMed] [Google Scholar]
- Zhang, D., Wang S., Li G., Chen M., Liu H., and Ji H... 2023. Two doses of Lactobacillus induced different microbiota profiles and serum immune indices in pigs. J. Funct. Foods. 102:105405. doi: https://doi.org/ 10.1016/j.jff.2023.105405 [DOI] [Google Scholar]
- Raj, H., Gupta A., Upmanyu N.. 2020. Anti-inflammatory effect of Woodfordia fructicosa leaves ethanolic extract on adjuvant- and carrageenan-treated rats.. Antiinflamm. Antiallergy Agents Med. Chem. 19:103–112. doi: https://doi.org/ 10.2174/1871523018666190222120127. [DOI] [PMC free article] [PubMed] [Google Scholar]