Abstract
Background
Diabetic wounds, characterized by their chronic nature, represent a critical challenge for patients with diabetes, often leading to amputation and mortality. Although stem cells show great promise, their use is limited by challenges related to stability and tumorigenicity. The secretome of stem cells, comprising molecules released by these cells, offers a potential alternative to the challenges associated with stem cell therapy and provides a promising solution for diabetic wound healing.
Objective
We conducted a systematic review and meta-analysis of relevant preclinical studies to evaluate the effectiveness of stem cell secretomes in treating diabetic wounds.
Methods
The protocol registration for this systematic review and meta-analysis was recorded in the PROSPERO database (CRD42023473726). Databases were searched from their inception until November 20, 2023. The quality assessment of the included studies was performed utilizing the CAMARADES 10-item Quality Checklist. Statistical analyses were conducted using a random-effects model to calculate standardized mean differences (SMD) and 95% confidence intervals (CI), with heterogeneity assessed via the I² statistic. The primary outcome evaluated was the wound closure rate, while secondary outcomes included parameters such as the number of fibroblasts, neutrophils, and macrophages.
Results
Twenty studies were included, comprising 382 animal subjects, and five of which were eligible for quantitative evaluation in a meta-analysis. The stem cell secretome significantly improved the wound closure rate (SMD = 9.63; 95% CI = 2.01 −17.25; P = 0.01, I2 = 76%) and reduced the number of neutrophils (SMD = −8.47; 95% CI = −13.05 to −3.90; P = 0.0003) and macrophages (SMD = −5.32; 95% CI = −9.09 to −1.55; P = 0.006).
Conclusion
This review suggests that stem cell secretomes have potential as a novel therapeutic strategy for diabetic wound healing, enhancing wound closure rates and reducing inflammation. These findings support the use of stem cell secretomes as a safer and more stable alternative to direct stem cell therapy, but further clinical studies are needed to confirm these results in human patients.
Key words: Diabetic wound, Secretome, Stem cell, Wound healing
Graphical Abstract
Introduction
Diabetic wounds, also referred to as diabetic ulcers, occur in individuals with diabetes due to impaired self-healing capabilities.1 The primary characteristic is a slow healing process, attributed to diabetes-induced damage to blood vessels and nerves.2 Patients with diabetes often experience diminished tissue regeneration, complicating the wound healing process.3 This reduction is linked to hyperglycemia, which causes stiffened blood vessels, reduced circulation, and microvascular dysfunction, leading to decreased tissue oxygenation.4 Altered blood vessels in patients with diabetes also hinder leukocyte migration into wounds, increasing susceptibility to infections.5 The high prevalence of diabetic wounds poses a significant risk of complications, leading to amputation (14-24%) or death (40%).6,7 Besides stringent blood sugar control and infection prevention, meticulous wound care is crucial to minimizing severe complications.
Current treatments for diabetic wounds include antibiotics, antimicrobial agents, and specialized wound care therapies.8,9 However, their effectiveness in expediting optimal healing processes remains limited. Stem cells offer a potentially revolutionary solution for this challenge. A meta-analysis by Sun et al. revealed that stem cells are more beneficial in improving wound closure rates for diabetic patients (odds ratio (OR) = 8.20, CI = 5.33–12.62).10 Notably, this meta-analysis included studies utilizing various stem cell types, such as bone marrow-derived mesenchymal stem cells (BM-MSCs), peripheral blood mesenchymal stem cells (PB-MSCs), and human umbilical cord mesenchymal stem cells (HUC-MSCs), highlighting the diversity of stem cell sources studied for diabetic wound healing. Their remarkable regenerative capabilities make them a promising choice for accelerating the healing process in individuals with diabetes. However, a limitation of that meta-analysis is that the included studies did not distinguish stem cells from other types of somatic cells, potentially leading to confounded results. Moreover, it is essential to acknowledge that, like any medical breakthrough, the use of stem cells is not without drawbacks. Some studies have suggested potential carcinogenicity, posing risks to individuals with diabetes.11, 12, 13, 14 Additionally, concerns regarding the stability of stem cells limit their long-term effectiveness in stimulating tissue regeneration.15,16
To address these challenges, the stem cell secretome—the conditioned medium produced by stem cells—has emerged as a promising alternative. The secretome contains a complex mixture of bioactive molecules, including growth factors, cytokines, extracellular vesicles, and exosomes, which are key players in the regenerative process. Unlike whole stem cells, the secretome is cell-free, alleviating concerns about uncontrolled cell proliferation and potential tumorigenicity. Furthermore, the secretome is more stable, easier to standardize, and less immunogenic, making it a safer and more practical option for clinical applications.17,18 Preclinical in vivo studies using secretomes derived from various mesenchymal stem cell sources—human bone marrow-derived mesenchymal stem cells (hBM-MSCs), human adipose tissue-derived mesenchymal stem cells (hAD-MSCs), and human umbilical cord-derived mesenchymal stem cells (hUC-MSCs)—have demonstrated their efficacy in promoting tissue repair and wound healing.19, 20, 21, 22
However, it is important to note that existing preclinical studies on the effectiveness of the stem cell secretome for diabetic wound healing often have small sample sizes and considerable variation in quality, resulting in a low power of evidence. Therefore, this comprehensive systematic review and meta-analysis was conducted to consolidate findings from previous animal studies on the effects of stem cell secretome in repairing diabetic wounds. The primary goal of this analysis was to provide a comprehensive evaluation of the wound healing efficacy of the stem cell secretome in in vivo diabetic animal models. Since the secretome can be obtained from various types of stem cells, this study also aimed to explore the differential impact of these sources to provide greater clarity on their therapeutic potential.
Materials and Methods
Protocol and registration
The systematic review adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Table S1).23 Initial steps involved a preliminary search and piloting of the study selection process. The review protocol was registered on the Prospectively Registered Systematic Reviews (PROSPERO) platform with the registration number CRD42023473726.
Search strategy and selection criteria
The exploration of literature involved searching through four electronic databases: PubMed, Scopus, ScienceDirect, and EBSCOhost. Relevant studies were retrieved using keywords such as ``(secretome OR 'conditioned medium')'' AND ``(ulcer OR wound)'' AND ``(diabetes OR diabetic)'' AND ``('in vivo' OR 'preclinical'),'' which were adjusted as needed for compatibility with other databases. Searches were conducted until November 20, 2023, without restrictions on publication dates.
Study selection
Two reviewers (CS and NW), possessing comparable experience and skills, independently assessed the titles and abstracts of the articles. In instances where the information was unclear based on the title and abstract, the entire document was retrieved for a thorough examination. Any discrepancies in opinion were deliberated and resolved through consensus with the third and fourth reviewers (GW and AFAM). The study selection process adhered to the predefined inclusion and exclusion criteria, which are outlined in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Publication Type | Research studies published in English version | Reviews, conference abstracts, editorial letters |
| Animals/Population | Animals models established using different methods, regardless of species, age, weight, or gender. | Nondiabetic animal model |
| Intervention/Exposure | Experimental groups received stem cell secretome as monotherapy | Experimental groups received nonstem cell secretome or stem cell secretome combined with other medication/treatment |
| Comparator/Control | The corresponding control groups were treated with a blank treatment or received a placebo | Without control group and/or compared with other medicine rather than placebo or blank |
| Study design | Observational studies with separate treatment groups | Cross sectional studies |
| Outcome measure | Outcome related to diabetic wound healing, such as ulcer size, ulcer depth, wound closure rate, and histopathological changes | Lack of outcome indicator |
The research included in the appendices of this systematic review and meta-analysis was chosen because it aligns with the PICO approach as follows:
-
•
Populations (P): Diabetic animal models
-
•
Interventions (I): Treatment with stem cell secretome
-
•
Control (C): Groups receiving only the vehicle composition or a placebo
-
•
Outcomes (O): Primary outcome: wound closure rate; secondary outcomes: histology profile, encompassing the quantity of fibroblasts, neutrophils, and macrophages.
As shown in Table 1, these criteria were used to guide the inclusion of studies in the analysis.
Assessment of the quality of the included studies
Two independent reviewers (CS and NW) assessed the risk of bias of the in vivo studies included in this systematic review. The risk of potential bias for each study was evaluated using the 10-item quality checklist from CAMARADES (Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies).24 The CAMARADES risk of bias assessment tool is a specialized tool to evaluate bias in systematic reviews of animal experiments. It consists of 10 criteria: publication in a peer-reviewed journal, control of temperature, random allocation to treatment or control groups, blinded induction of the experimental model, blinded assessment of outcomes, use of an anesthetic agent that does not have significant protective or toxic effects, appropriate selection of animal models, calculation of sample size, compliance with animal welfare regulations, and disclosure of potential conflicts of interest. Adjustments were made to the checklist as needed, particularly for criterion C9 (compliance with animal welfare regulations). In some cases, studies did not explicitly report all components of animal welfare compliance (e.g., environmental temperature and postoperative analgesia); however, studies meeting at least three out of the seven components outlined in the guidelines were included. Additionally, no new criteria were added, but we clarified C6 regarding the use of anesthetic agents to exclude those with significant protective or toxic effects on the skin. Any discrepancies between the two reviewers in terms of both methods were resolved through discussion and consensus with the third and fourth reviewers (GW and AFAM).
Data extraction
Data extraction was independently conducted by two authors (CS and NW), and any discrepancies were verified by the third and fourth investigators (GW and AFAM). A spreadsheet was employed to gather information from the included studies, encompassing details such as the author's name(s), publication date, animal species, sample size, method or agent used to induce diabetes, type of stem cell serving as the source of secretome, characteristics of the control group regimen, route of administration, duration of analysis, and primary and secondary outcomes. For continuous quantitative outcomes, mean values, standard deviations (SD), and sample sizes were extracted. In cases where a study involved multiple intervention groups, data extraction was focused solely on the group receiving the stem cell secretome and the control group. The final results were discussed among all investigators to ensure consensus.
Data synthesis and statistical analysis
Data analyses were performed using RevMan 5.3 (Cochrane Collaboration, Oxford, United Kingdom) and SPSS version 29 software (IBM Corp., New York, United States). The Mantel-Haenszel technique was employed to estimate the standard mean difference (SMD) with a 95% confidence interval (CI) for continuous outcomes. Statistical significance was determined using a threshold P-value of ≤0.05.25 Higgins and Thompson's I2 statistic was used to assess heterogeneity, indicating the extent to which variation among trials was attributable to differences rather than sampling error. I2 values were categorized as follows to determine the level of heterogeneity: 0-25% for low, 26-75% for moderate, and above 75% for high levels of heterogeneity.26 A random-effects model was applied for all analyses due to the limited number of studies in each meta-analysis, which precluded sensitivity analyses to investigate heterogeneity. Additionally, Egger's test was conducted to explore the potential impact of publication bias.24 The pooled estimate was calculated as a standard mean difference (SMD) with a 95% confidence interval (CI) for continuous outcomes.
Results
Search of studies
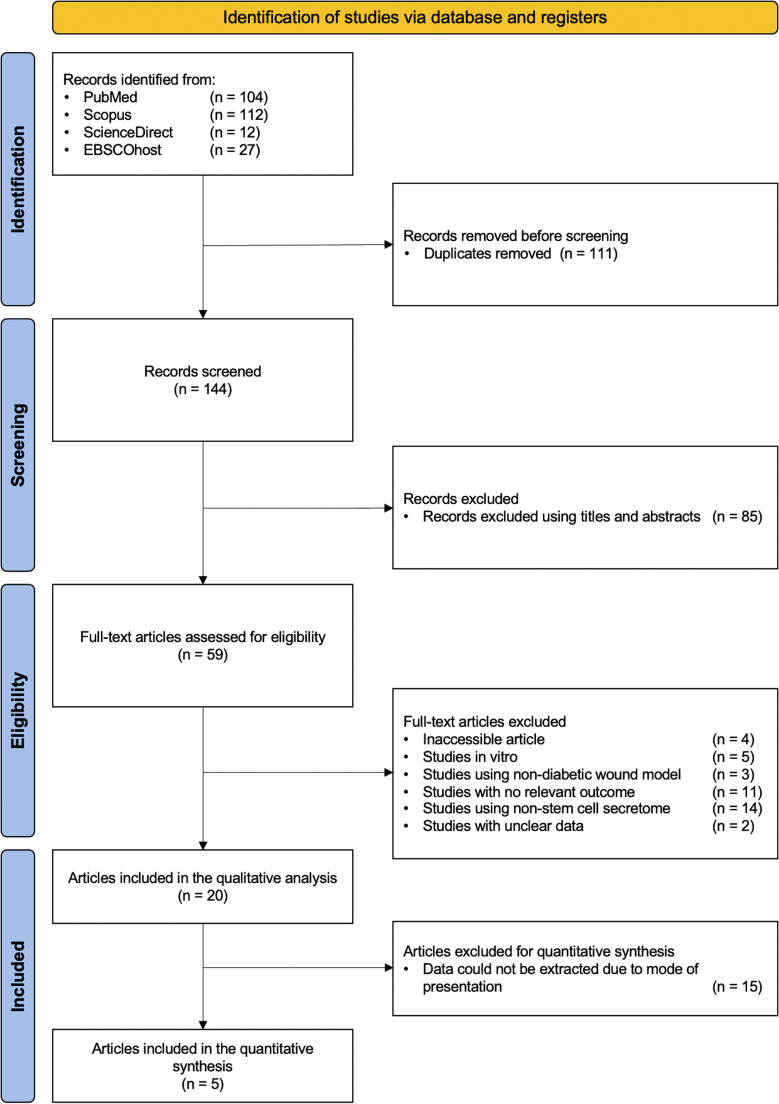
A total of 255 pertinent articles were identified in the four databases (Table S2). After removing duplicates, 144 studies were retained for title and abstract review. After a detailed examination, 85 studies that were not relevant to this investigation were excluded. After reviewing the full text of the remaining 59 studies, 33 articles were excluded for not meeting at least one exclusion criterion. Moreover, 4 articles were excluded due to inaccessibility. Despite attempts to retrieve these studies via institutional access and correspondence with the authors, we were unable to obtain their full texts. Additionally, 2 articles were excluded because the data presented were unclear and insufficient for extraction and analysis. Attempts to contact the authors for clarification were unsuccessful. Ultimately, 20 studies were selected for qualitative analysis,18,20, 21, 22,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 and among them, 5 qualified for further evaluation in the meta-analysis.28,30,34,36,41 The detailed flow chart illustrating the literature identification and selection process is depicted in Figure 1.
Figure 1.
Flowchart of the study selection process.
Characteristics of included studies
The characteristics of the 20 studies are summarized in Table 2. All studies, conducted in English between 2013 and 2023, involved 382 subjects, with 191 in the experimental group and 191 in the control group. Sprague–Dawley rats (SD), Wistar rats, C57BL/6 mice, and transgenic mice were the four different species utilized in 3 studies,18,22,36 7 studies,21,28,29,34,35,39,40 3 studies,27,30,38 and 7 studies, respectively.20,31, 32, 33,37,41,42 The animal sample size ranged from 3 to 18, with a median sample size of 18 rats. Diabetic models were induced using streptozotocin or alloxan, a high-fat diet, or genetically modified animal models. Regarding the type of stem cell used as a source of secretome, eight types were identified: human adipose tissue-derived mesenchymal stem cells (hAD-MSCs),20,28,42 human bone marrow-derived mesenchymal stem cells (hBM-MSCs),21,29,34,35,37,39,40 human menstrual blood‐derived mesenchymal stem cells (MenSCs),30 human umbilical cord Wharton's jelly stem cells (hUC-WJSCs),31, 32, 33 human umbilical cord-derived mesenchymal stem cells (hUC-MSCs),22,27,36,41 human endothelial cell-differentiated mesenchymal stem cells (hEC-MSCs),38 human fetal mesenchymal stem cells (hF-MSCs),18 and human foreskin-derived dermal stem cells (hFDSCs).42 Control groups in the included studies received either a placebo, phosphate buffer saline (PBS), unconditioned medium, or no treatment. Treatment administration methods included intraperitoneal (i.p.), intravenous (i.v.), subcutaneous (s.c.), intradermal, and topical routes. Follow-up durations varied in each study, ranging from 9 to 28 days.
Table 2.
Study characteristics.
| Study | Animal Species | Sample size (Treatment group/ Control group) |
Diabetic inducing agent or method | Type of stem cell | Control | Route of administration | Follow Up (days) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Asadi et al.28 | Wistar adult virgin male rats | 6/6 | Streptozotocin | Human adipose tissue-derived mesenchymal stem cells (hAD-MSCs) | Placebo | Intraperitoneal (i.p.) | 16 | ↓Colony forming unit (CFU), ↑wound closure rate, ↑binding stiffness, ↑stress high load, ↓neutrophils, ↓macrophages, ↓total inflammatory cells, ↑fibroblasts, ↑new blood vessels, ↑volume of new epidermis and dermis |
| Bagheri et al.29 | Adult male Wistar rats | 18/18 | Streptozotocin | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | Placebo | Intraperitoneal (i.p.) | 15 | ↑Bending stiffness, ↑high stress load, ↓total number of mast cells |
| Dalirfardouei et al.30 | Inbred Male C57BL/6 mice | 18/18 | Streptozotocin | Human menstrual blood‐derived mesenchymal stem cells (MenSCs) | Phosphate buffer saline (PBS) | Intradermal | 14 | ↑wound closure rate, ↑re-epithelialization, ↑VEGF expression, ↑new blood vessels, ↓type I collagen expression, ↑type III collagen expression |
| Fong et al.31 | Diabetic mice (Strain BKS.Cg-Dock7m +/+Leprdb/J) | 9/9 | N/A | Human umbilical cord Wharton's jelly stem cells (hUC-WJSCs) | Unconditioned medium (UCM) | Intradermal | 14 | ↑Wound closure rate, ↑VEGF expression, ↓TIMP-1 expression, ↓ICAM-1 expression |
| Fridoni et al.34 | Wistar male adult rats | 18/18 | Streptozotocin | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | Placebo | Intraperitoneal (i.p.) | 15 | ↑Angiogenesis, ↓neutrophils, ↓macrophages, ↑fibroblasts, ↑bFGF expression, ↑HIF‐1α expression, SDF‐1α expression |
| Gregorio et al.20 | Transgenic mice (BKS.Cg-m+/+Leprdb/J) | 6/6 | N/A | Human adipose tissue-derived mesenchymal stem cells (hAD-MSCs) | Placebo | Intravenous (i.v.) | 14 | ↑Wound closure rate, ↑wound epidermal area, ↓collagen deposition, ↓type I collagen, ↑angiogenesis, ↑IGF-1 expression, ↑ANG-1 expression, ↑PDGF expression |
| Hendrawan et al.36 | Adult male Sprague–Dawley (SD) rats | 3/3 | Streptozotocin | Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) | Without any medication | Intradermal | 14 | ↑Wound closure rate, ↑re-epithelialization, ↑length of epithelium, ↑collagen-deposited area |
| Kouhkheil et al.35 | Wistar male adult rats | 6/6 | Streptozotocin | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | Placebo | Intraperitoneal (i.p.) | 15 | ↓CFU, ↑bending stiffness, ↑stress high load |
| Kouhkheil et al.21 | Wistar male adult rats | 18/18 | Streptozotocin | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | Placebo | Intraperitoneal (i.p.) | 15 | ↓Total number of mast cells, ↓CFU |
| Liu et al.22 | Sprague Dawley rats | 8/8 | Streptozotocin | Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) | Placebo | Intravenous (i.v.) | 28 | ↓VCAM-1 expression, ↓ICAM-1 expression, ↓endothelial thickness, ↓average optical density (AOD) |
| Ma et al.37 | Diabetic mice (Strain BKS.Cg-Dock7m +/+Leprdb/J) | N/A | N/A | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | Placebo | Injection | 24 | ↑Wound closure rate, ↑epidermal thickness |
| Ormazabal et al.38 | C57Bl/6 J mice | N/A | High fat diet (HFD) for 120 days | Human endothelial cell-differentiated mesenchymal stem cells (hEC-MSCs) | Placebo | Injection | 9 | ↑Wound closure rate |
| Pouriran et al.39 | Wistar male adult rats | 7/7 | Streptozotocin | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | Placebo | Intraperitoneal (i.p.) | 15 | ↑Bending stiffness, ↑maximum force, ↑stress high load, ↑energy absorption |
| Raj et al.33 | Transgenic mice (BKS.Cg-m+/+Leprdb/J) | 9/9 | N/A | Human umbilical cord Wharton's jelly stem cells (hUC-WJSCs) | Placebo | Topical | 28 | ↑Epidermis thickness, ↑dermis thickness, ↑average collagen area, ↑ECM regulation, ↑collagen biosynthesis, ↑chemoattraction of immune cells, ↑angiogenesis, ↑scarless wound healing |
| Saheli et al.40 | Wistar male adult rats | 18/18 | Alloxan monohydrate | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | Placebo | Intraperitoneal (i.p.) | 15 | ↑Percentage of healed wounds, ↓inflammation score, ↑collagen density, ↑microvessels, ↔lymphocytes, ↓neutrophils, ↓macrophages, ↑fibroblasts, ↑EGF expression, ↑bFGF expression |
| Shrestha et al.41 | Transgenic mice (BKS.Cg-m+/+Leprdb/J) | 6/6 | N/A | Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) | Placebo | Subcutaneous (s.c.) | 14 | ↑Wound closure rate, ↑relative granulation area, ↑wound score, ↑capillary density, ↑VEGF expression, ↑PDGF expression, ↑KGF expression |
| Tam et al.32 | Transgenic mice (BKS.Cg-m+/+Leprdb/J) | 12/12 | N/A | Human umbilical cord Wharton's jelly stem cells (hUC-WJSCs) | Placebo | Topical | 28 | ↑Wound closure rate, ↑VEGF expression, ↓TIMP-1 expression, ↓ICAM-1 expression |
| Wang et al.18 | Sprague Dawley rats | 12/12 | Streptozotocin | Human fetal mesenchymal stem cells (hF-MSCs) | Placebo | Topical | 28 | ↓Defect area, ↓index of re-epithelialization, ↓granulation area, ↑alpha-SMA expression, ↑CD31 expression, ↓NIMP-R14 expression, ↓IL-1β expression |
| Xin et al.42 | C57BLKS/J db/db diabetic mice | 5/5 | N/A | Human foreskin-derived dermal stem cell (hFDSCs) | Placebo | Topical | 14 | ↓Percentage of the wound area, ↓wound healing time, ↑CD31 expression, ↓CD68 expression, ↓CD86 expression, ↑CD206 expression, ↑collagen volume |
| Human adipose tissue-derived mesenchymal stem cells (hAD-MSCs) | ||||||||
| Zhang et al.27 | C57BL/6 J female mice | 12/12 | Streptozotocin | Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) | Placebo | Subcutaneous (s.c.) | 14 | ↑Wound closure rate, ↑new blood vessels, ↓IL-1β expression, ↔TGF-β expression, ↓TNF-α expression, ↓IL-6 expression, ↑IL-10 expression, ↑VEGF expression |
Notes: N/A = not applicable; “↑” indicates an increase in parameters evaluated; “↔” indicates no change in parameters evaluated; “↓” indicates a decrease in parameters evaluated.
The primary outcome, wound closure rate, was assessed in 12 studies.20,27,28,30, 31, 32,36, 37, 38,40, 41, 42 The secondary outcomes determined in this study were colony forming unit (CFU), binding stiffness, high stress load, maximum force, energy absorption, neutrophils, macrophages, mast cells, lymphocytes, total inflammatory cells, fibroblasts, new blood vessels, volume of new epidermis and dermis, re-epithelialization, type I and type III collagen, angiogenesis, endothelial thickness, length of epithelium, granulation area, average optical density (AOD), and the expression of several growth factors.
Quality assessment of included studies
Table 3 illustrates the reported risk of bias for each publication incorporated in this meta-analysis. The risk of bias for each study was assessed using the CAMARADES 10-item quality checklist. Criteria met ranged from 4/10 to 8/10, with an average score of 6.3/10. All included studies were peer-reviewed publications, utilized an appropriate animal model (diabetic animal model), employed an anaesthetic agent without significant protective or toxic effects on the skin, and disclosed potential conflicts of interest. However, 12 of them did not mention the control of temperature,18,21,27, 28, 29,33, 34, 35,37,38,40,41 and seven did not provide an explanation for the randomization process.18,29,31,33,34,38,40 None of studies reported the methods used for blinding the assessment of the outcome. Only one study in this meta-analysis provided a specific description of sample-size calculations.21 Two studies did not declare compliance with animal welfare regulations.34,40
Table 3.
Assessment of risk of bias using CAMARADES 10-items quality checklist.
| Study | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asadi et al.28 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Bagheri et al.29 | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Dalirfardouei et al.30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Fong et al.31 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Fridoni et al.34 | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Gregorio et al.20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Hendrawan et al.36 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Kouhkheil et al.35 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Kouhkheil et al.21 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Liu et al.22 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Ma et al.37 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Ormazabal et al.38 | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Pouriran et al.39 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Raj et al.33 | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Saheli et al.40 | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Shrestha et al.41 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Tam et al.32 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Wang et al.18 | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Xin et al.42 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||||
| Zhang et al.27 |
Notes: Studies fulfilling the criteria of the following: C1: peer-reviewed publication; C2: control of temperature; C3: random allocation to treatment or control; C4: blinded induction of model (group randomly after modeling); C5: blinded assessment of outcome; C6: use of anesthetic agent without significant protective and toxic effects on skin; C7: appropriate animal model (diabetic model); C8: sample size calculation; C9: compliance with animal welfare regulations (including three or more of the following points: preoperative anesthesia, postoperative analgesia, nutrition, disinfection, environment temperature, environment humidity, circadian rhythm, and euthanasia); C10: statement of potential conflict of interests.
Despite variability in quality scores, studies with lower scores (4/10) were retained due to their potential contribution to the evidence base. The inclusion of these studies was justified because they still met key methodological criteria that ensured relevance to the research question. Specifically, studies with lower scores were retained as they employed appropriate animal models (diabetic models), used an anesthetic agent without significant adverse effects, and were peer-reviewed publications. Additionally, the use of studies with lower quality scores in the meta-analysis allows for a broader and more comprehensive evaluation of the effects of the stem cell secretome in diabetic wound healing. However, acknowledging the potential biases introduced by including this study is essential. Mitigation was addressed through adherence to strict inclusion criteria and comprehensive quality assessment using the CAMARADES checklist.
Meta-analysis results
Wound closure rate
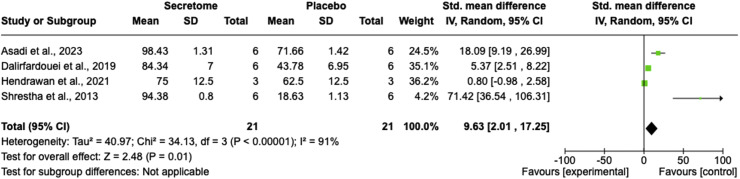
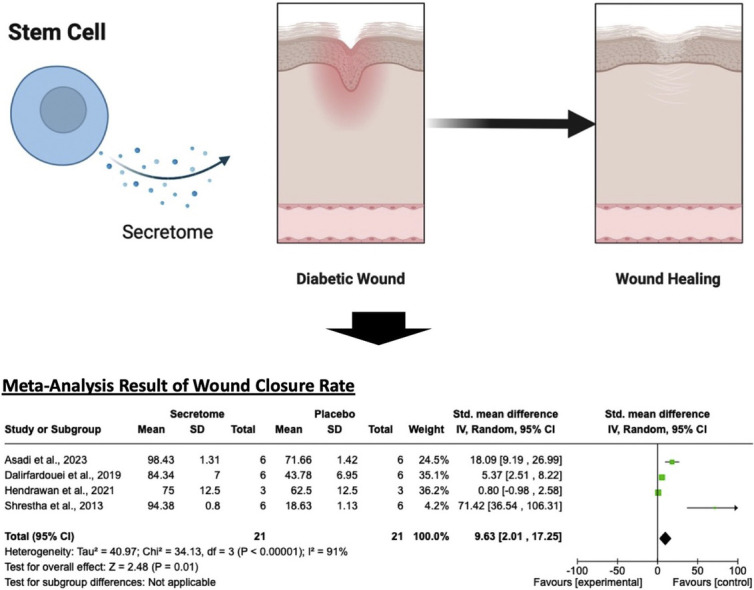
As a primary outcome, four studies examined the wound closure rate parameter,28,30,36,41 revealing that the stem cell secretome significantly enhanced the progressivity of wound healing compared with the control group (SMD = 9.63; 95% CI = 2.01–17.25; P = 0.01, I2 = 91%; Figure 2). The random-effect model was employed due to the significant heterogeneity among the included studies.
Figure 2.
Wound closure rate comparison between the stem cell secretome and control groups using a forest plot.
Number of fibroblasts
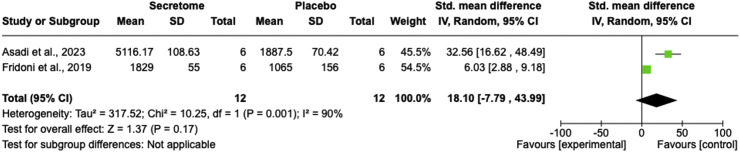
Two studies compared the stem cell secretome and control groups regarding fibroblast numbers.28,34 The pooled results indicated that the stem cell secretome failed to significantly increase the number of fibroblasts compared to the control group (SMD = 18.10; 95% CI = −7.79 to 43.99; P = 0.17; I2 = 90%; Figure 3).
Figure 3.
Number of fibroblasts comparison between the stem cell secretome and control groups using a forest plot.
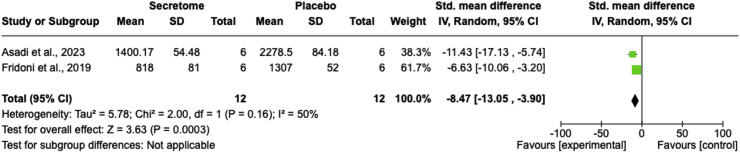
Number of neutrophils
In terms of the number of neutrophils, two studies demonstrated a significant reduction in the number of neutrophils after treatment with stem cell secretome compared with the control group (SMD = −8.47; 95% CI = −13.05 to −3.90; P = 0.0003; I2 = 50%; Figure 4).28,34
Figure 4.
Number of neutrophils comparison between the stem cell secretome and control groups using a forest plot.
Number of macrophages
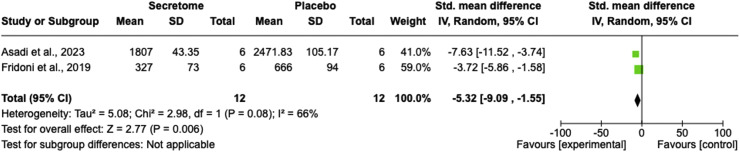
Two studies compared the stem cell secretome with the control group based on the number of macrophages.28,34 The pooled results indicated that the stem cell secretome significantly decreased the number of macrophages compared to the control group (SMD = −5.32; 95% CI = −9.09 to −1.55; P = 0.006; I2 = 66%; Figure 5).
Figure 5.
Number of macrophages comparison between the stem cell secretome and control groups using a forest plot.
Publication bias
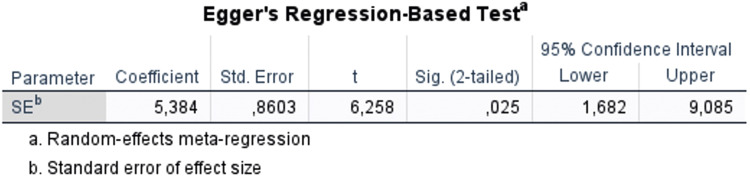
Egger's test assessed publication bias in this meta-analysis and identified several potential biases. Owing to the constrained inclusion of studies in the meta-analysis, the number of studies included for each parameter was limited. Only the wound closure rate parameter met the criteria for analyzing publication bias. The results suggested that the examined wound closure rate displayed potential publication bias (P = 0.025 (< 0.05); t = 6.258, Figure 6). Notably, only one lower-quality study was included in the meta-analysis,34 and the parameters it addressed involved only two studies, precluding sensitivity analysis or publication bias assessment for these parameters. Despite these constraints, including lower-quality studies enhances the comprehensiveness of the analysis by expanding the evidence base.
Figure 6.
Publication bias with regard to wound closure rate interpretated by Egger's regression test.
Discussion
Summary of evidence
To our knowledge, this is the first preclinical systematic review and meta-analysis assessing the effectiveness of the stem cell secretome in healing wounds in diabetic animal models. The analysis incorporated 20 high-quality studies involving 382 rats with diabetic wound animal models. The results revealed that the stem cell secretome significantly enhanced the wound closure rate, concurrently reducing the number of neutrophils and macrophages in diabetic wound animal models. These findings suggest that the stem cell secretome holds promise as a potential treatment for wounds in individuals with diabetes. However, we observed high heterogeneity in the outcomes of wound closure rate and the number of fibroblasts in this meta-analysis.
The observed heterogeneity in wound closure rates and other outcomes could be partially attributed to the diverse sources of secretome and stem cells used. For example, secretomes derived from hBM-MSCs, hAD-MSCs, and hUC-MSCs vary in their composition of growth factors and cytokines, potentially influencing their efficacy.43,44 Previous studies have shown that hBM-MSCs secretome contains higher concentrations of vascular endothelial growth factor (VEGF), while hAD-MSCs produce more anti-inflammatory cytokines, such as interleukin-10 (IL-10), and hUC-MSCs exhibit higher levels of epidermal growth factor (EGF).45, 46, 47 These variations may explain differences in wound healing efficacy observed in preclinical studies and highlight the importance of tailoring secretome sources to specific therapeutic needs. However, the limited number of studies prevented us from analyzing the primary cause of this high heterogeneity through subgroup analysis. Factors such as follow-up duration, stem cell types, routes of administration, modeling methods, and sample sizes may all contribute to these variations. Therefore, future high-quality studies with larger sample sizes are crucial to validate our findings and enable more comprehensive subgroup analyses to better understand these differences.
Strengths
To the best of our knowledge, this is the first systematic review and meta-analysis documenting the efficacy of the stem cell secretome for managing diabetic wounds in animal models. All the studies included were observational, comprising 20 trials involving 382 animals, with 5 meeting the criteria for further investigation in the meta-analysis. The studies encompassed in the qualitative analysis (systematic review) were notably diverse, and their collective outcomes consistently endorse the potential effectiveness of stem cell secretome in the healing of diabetic wounds.
Limitations
Nevertheless, this study is subject to several limitations. First, despite conducting searches across four databases without imposing restrictions on the publication date, it remains possible that pertinent studies were overlooked. Second, inherent selection bias may exist, as negative outcomes may not have been consistently reported or published. Third, the limited availability of studies with identical quantitative parameters limited the pool of studies available for inclusion in the meta-analysis. This contributes to high heterogeneity, the causes of which are challenging to identify owing to the inability to conduct subgroup or sensitivity analyses. Fourth, considerable variations in animal species, sample sizes, diabetic modeling methods, specific types of stem cells used as sources of secretome, approaches to the route of administration, and follow-up durations were observed among the included studies.
Implications of research
The use of animal models has become indispensable for optimizing and streamlining clinical trials. Various methods can be employed to model diabetic wounds in animal models. Chemical substances, such as streptozotocin and alloxan, can induce diabetes in animals. Upon uptake into β cells, streptozotocin undergoes cleavage into glucose and methylnitrosourea components. The alkylating properties of methylnitrosourea fragment DNA, leading to the destruction of β cells and resulting in insulin-dependent diabetes.48 Alloxan, on the other hand, induces the generation of reactive oxygen species (ROS) with rapid uptake by pancreatic β cells. Additionally, a high-fat diet leading to obesity can indirectly induce diabetes.49 Consuming a diet high in saturated fat reduces the number of broad islets in the pancreas, impacting the insulin response and predisposing individuals to glucose sensitivity and diabetes.50 Transgenic animal models modified to develop diabetes are widely used because of their ease of use.51 However, each method has distinct capabilities, including variations in mechanisms, inductive power, and effects on the physiology of other body systems.52 Although not a primary factor, variations in modeling methods can contribute to high heterogeneity in the results of meta-analyses.
The assessment of wound healing efficacy often revolves around the wound closure rate as the primary outcome. Meta-analysis results revealed that the stem cell secretome significantly improved the diabetic wound closure rate (P = 0.01). This improvement is attributed to the secretome's composition of growth factors, including epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and other regenerative substances.53 However, quantitatively identifying the most significant growth factor is challenging owing to the lack of data for each factor. Qualitatively, the stem cell secretome has been shown to enhance various growth factors, including VEGF, bFGF, EGF, platelet-derived growth factor (PDGF), and keratinocyte growth factor (KGF).20,27,30, 31, 32,34,40,41 The insignificant improvement in the number of fibroblasts after stem cell secretome administration (P = 0.17) suggests that VEGF, a fibroblast production factor, is not the primary substance supporting the regenerative ability of the stem cell secretome.
Beyond regenerative aspects, the inflammatory system is crucial for supporting wound healing. Anti-inflammatory agents play pivotal roles in the final phase of inflammation during wound healing.54 The findings of this study indicated that the stem cell secretome has notable anti-inflammatory properties, as indicated by the decrease in neutrophils and macrophages (P = 0.0003 and P = 0.006, respectively). Neutrophils and macrophages, which function as pro-inflammatory cells, contribute to the prevention of infections throughout the wound healing process. After the completion of their activities, it is crucial to suppress the proliferation and migration of these cells to initiate the proliferative phase.55
Suggestions for future studies
Future studies should address several key areas to further validate and expand the findings of this review. First, larger, multicenter studies involving diverse animal models are needed to better understand the impact of various types of stem cell secretome. These studies should also explore optimal delivery methods and dosages. Second, long-term follow-up studies are essential to assess the stability and durability of the regenerative effects of the stem cell secretome. Third, given the heterogeneity observed in this meta-analysis, subgroup analyses based on factors such as stem cell source, administration routes, and diabetic models would provide further insights into the specific conditions under which the stem cell secretome is the most effective. Finally, future studies should standardized reporting of outcome measures, including consistent wound healing metrics and detailed histological analyses, to enhance the comparability and robustness of future research.
Conclusion
This preclinical systematic review and meta-analysis offers novel insights into the potential of the stem cell secretome as a therapeutic approach for diabetic wound healing. By synthesizing data from animal studies, this review highlights the significant effects of the stem cell secretome in improving wound closure rates, the primary outcome, and reducing neutrophil and macrophage counts, thereby supporting its anti-inflammatory properties. This review contributes significantly to preclinical research by emphasizing the promising therapeutic benefits of the stem cell secretome in diabetic wound treatment. However, it is crucial to note that these results were derived from preclinical animal models, and caution is needed when extrapolating these findings to human clinical applications. This review underscores the importance of considering the stem cell source and secretome composition when evaluating their therapeutic potential. Identifying the most effective secretome source could optimized treatments for diabetic wounds and pave the way for personalized therapeutic strategies in clinical settings. Further clinical studies are needed to validate the efficacy and safety of the stem cell secretome in human patients.
Authors’ Contribution
CS and NW: conceptualization; CS: methodology, software, and visualization; GW, KME, and NW: resources; CS, GW, KME, ARD, SG, AA, and NW: data curation; GW and KME: validation; CS, GW, KME, and NW: formal analysis; CS, GW, and NW: investigation; GW and NW: supervision; CS, ARD, SG, and AA: writing—original draft preparation; GW, KME, and NW: review and editing; CS and NW: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors thank the Rector of Universitas Padjadjaran for funding the APC.
Funding
This study was supported by grants from Beasiswa Unggulan Pascasarjana Padjadjaran (BUPP) of Universitas Padjadjaran with grant numbers 791/UN6.WR3/TU.00/2022.
Availability of Data and Material
All of relatable data can be requested upon reasonable request from the corresponding author.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2025.100778.
Appendix. Supplementary materials
References
- 1.Wang X., Yuan C.X., Xu B., Yu Z. Diabetic foot ulcers: classification, risk factors and management. World J Diabetes. 2022;13(12):1049–1065. doi: 10.4239/wjd.v13.i12.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raja J.M., Maturana M.A., Kayali S., Khouzam A., Efeovbokhan N. Diabetic foot ulcer: a comprehensive review of pathophysiology and management modalities. World J Clin Cases. 2023;11(8):1684–1693. doi: 10.12998/wjcc.v11.i8.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mieczkowski M., Mrozikiewicz-Rakowska B., Kowara M., Kleibert M., Czupryniak L. The problem of wound healing in diabetes—from molecular pathways to the design of an animal model. Int J Mol Sci. 2022;23(14):7930. doi: 10.3390/ijms23147930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horton W.B., Barrett E.J. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev. 2021;42(1):29–55. doi: 10.1210/endrev/bnaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pezhman L., Tahrani A., Chimen M. Dysregulation of leukocyte trafficking in type 2 diabetes: mechanisms and potential therapeutic avenues. Front Cell Dev Biol. 2021;9:624184. doi: 10.3389/fcell.2021.624184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeyaraman K., Berhane T., Hamilton M., Chandra A.P., Falhammar H. Mortality in patients with diabetic foot ulcer: a retrospective study of 513 cases from a single centre in the Northern Territory of Australia. BMC Endocr Disord. 2019;19(1):1. doi: 10.1186/s12902-018-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao X., Li S.H., El Akkawi M.M., Fu X.B., Liu H.W., Huang Y.S. Surgical amputation for patients with diabetic foot ulcers: a Chinese expert panel consensus treatment guide. Front Surg. 2022;9:1003339. doi: 10.3389/fsurg.2022.1003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavitha K.V. Choice of wound care in diabetic foot ulcer: a practical approach. World J Diabetes. 2014;5(4):546–556. doi: 10.4239/wjd.v5.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez-Acuña J.M., Cardenas-Cadena S.A., Marquez-Salas P.A., et al. Diabetic foot ulcers: current advances in antimicrobial therapies and emerging treatments. Antibiotics. 2019;8(4):193. doi: 10.3390/antibiotics8040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y., Zhao J., Zhang L., Li Z., Lei S. Effectiveness and safety of stem cell therapy for diabetic foot: a meta-analysis update. Stem Cell Res Ther. 2022;13(1):416. doi: 10.1186/s13287-022-03110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao Y., Agboola O.S., Hu X., Wu Y., Lei L. Tumorigenic and immunogenic properties of induced pluripotent stem cells: a promising cancer vaccine. Stem Cell Rev Rep. 2020;16(6):1049–1061. doi: 10.1007/s12015-020-10042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lezmi E., Benvenisty N. The tumorigenic potential of Human pluripotent stem cells. Stem Cells Transl Med. 2022;11(8):791–796. doi: 10.1093/stcltm/szac039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z. Assessing tumorigenicity in stem cell-derived therapeutic products: a critical step in safeguarding regenerative medicine. Bioengineering. 2023;10(7):857. doi: 10.3390/bioengineering10070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacey G.N., Andrews P.W., Barbaric I., et al. Stem cell culture conditions and stability: a joint workshop of the PluriMes consortium and pluripotent stem cell platform. Regenerative Med. 2019;14(3):243–255. doi: 10.2217/rme-2019-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhalter M.D., Rudolph K.L., Sperka T. Genome instability of ageing stem cells-induction and defence mechanisms. Ageing Res Rev. 2015;23:29–36. doi: 10.1016/j.arr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhandi C., Mohammed A.F.A., Wilar G., El-Rayyes A., Wathoni N. Effectiveness of mesenchymal stem cell secretome on wound healing: a systematic review and meta-analysis. Tissue Eng Regen Med. 2023;20:1053–1062. doi: 10.1007/s13770-023-00570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B., Pang M., Song Y., et al. Human fetal mesenchymal stem cells secretome promotes scarless diabetic wound healing through heat-shock protein family. Bioeng Transl Med. 2023;8(1):e10354. doi: 10.1002/btm2.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bormann D., Gugerell A., Ankersmit H.J., Mildner M. Therapeutic application of cell secretomes in cutaneous wound healing. Journal of Investigative Dermatology. 2023;143(6):893–912. doi: 10.1016/j.jid.2023.02.019. [DOI] [PubMed] [Google Scholar]
- 20.De Gregorio C., Contador D., Diáz D., et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11(1):168. doi: 10.1186/s13287-020-01680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouhkheil R., Fridoni M., Abdollhifar M.A., et al. Impact of photobiomodulation and condition medium on mast cell counts, degranulation, and wound strength in infected skin wound healing of diabetic rats. Photobiomodul Photomed Laser Surg. 2019;37(11):706–714. doi: 10.1089/photob.2019.4691. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Chen J., Liang H., et al. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res Ther. 2022;13(1):258. doi: 10.1186/s13287-022-02927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Revista Espanola de Nutricion Humana y Dietetica. 2016;20(2):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lian Y., Zhu H., Guo X., et al. Antiosteoporosis effect and possible mechanisms of the ingredients of Radix Achyranthis Bidentatae in animal models of osteoporosis: systematic review and meta-analysis of in vivo studies. J Orthop Surg Res. 2023;18(1):531. doi: 10.1186/s13018-023-04031-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2. John Wiley & Sons; Chichester (UK): 2019. pp. 413–444. [Google Scholar]
- 26.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S., Chen L., Zhang G., Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther. 2020;11(1):39. doi: 10.1186/s13287-020-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadi R., Mostafavinia A., Amini A., et al. Acceleration of a delayed healing wound repair model in diabetic rats by additive impacts of photobiomodulation plus conditioned medium of adipose-derived stem cells. J Diabetes Metab Disord. 2023;22:1551–1560. doi: 10.1007/s40200-023-01285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagheri M., Amini A., Abdollahifar M.A., et al. Effects of photobiomodulation on degranulation and number of mast cells and wound strength in skin wound healing of streptozotocin-induced diabetic rats. Photomed Laser Surg. 2018;36(8):415–423. doi: 10.1089/pho.2018.4453. [DOI] [PubMed] [Google Scholar]
- 30.Dalirfardouei R., Jamialahmadi K., Jafarian A.H., Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–568. doi: 10.1002/term.2799. [DOI] [PubMed] [Google Scholar]
- 31.Fong C.Y., Tam K., Cheyyatraivendran S., et al. Human Wharton's jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J Cell Biochem. 2014;115(2):290–302. doi: 10.1002/jcb.24661. [DOI] [PubMed] [Google Scholar]
- 32.Tam K., Cheyyatraviendran S., Venugopal J., et al. A nanoscaffold impregnated with human Wharton's jelly stem cells or its secretions improves healing of wounds. J Cell Biochem. 2014;115(4):794–803. doi: 10.1002/jcb.24723. [DOI] [PubMed] [Google Scholar]
- 33.Raj V., Claudine S., Subramanian A., et al. Histological, immunohistochemical, and genomic evaluation of excisional and diabetic wounds treated with human Wharton's jelly stem cells with and without a nanocarrier. J Cell Biochem. 2019;120(7):11222–11240. doi: 10.1002/jcb.28398. [DOI] [PubMed] [Google Scholar]
- 34.Fridoni M., Kouhkheil R., Abdollhifar M.A., et al. Improvement in infected wound healing in type 1 diabetic rat by the synergistic effect of photobiomodulation therapy and conditioned medium. J Cell Biochem. 2019;120(6):9906–9916. doi: 10.1002/jcb.28273. [DOI] [PubMed] [Google Scholar]
- 35.Kouhkheil R., Fridoni M., Piryaei A., et al. The effect of combined pulsed wave low-level laser therapy and mesenchymal stem cell-conditioned medium on the healing of an infected wound with methicillin-resistant staphylococcal aureus in diabetic rats. J Cell Biochem. 2018;119(7):5788–5797. doi: 10.1002/jcb.26759. [DOI] [PubMed] [Google Scholar]
- 36.Hendrawan S., Kusnadi Y., Lagonda C.A., et al. Wound healing potential of human umbilical cord mesenchymal stem cell conditioned medium: an in vitro and in vivo study in diabetes-induced rats. Vet World. 2021;14(8):2109–2117. doi: 10.14202/vetworld.2021.2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma D., Kua J.E.H., Lim W.K., Lee S.T., Chua A.W.C. In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy. 2015;17(8):1036–1051. doi: 10.1016/j.jcyt.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Ormazabal V., Nova-Lampeti E., Rojas D., et al. Secretome from Human mesenchymal stem cells-derived endothelial cells promotes wound healing in a type-2 diabetes mouse model. Int J Mol Sci. 2022;23(2):941. doi: 10.3390/ijms23020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pouriran R., Piryaei A., Mostafavinia A., et al. The effect of combined pulsed wave low-level laser therapy and Human bone marrow mesenchymal stem cell-conditioned medium on open skin wound healing in diabetic rats. Photomed Laser Surg. 2016;34(8):345–354. doi: 10.1089/pho.2015.4020. [DOI] [PubMed] [Google Scholar]
- 40.Saheli M., Bayat M., Ganji R., et al. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch Dermatol Res. 2020;312(5):325–336. doi: 10.1007/s00403-019-02016-6. [DOI] [PubMed] [Google Scholar]
- 41.Shrestha C., Zhao L., Chen K., He H., Mo Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int J Endocrinol. 2013;2013:592454. doi: 10.1155/2013/592454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin Y., Xu P., Wang X., Chen Y., Zhang Z., Zhang Y. Human foreskin-derived dermal stem/progenitor cell-conditioned medium combined with hyaluronic acid promotes extracellular matrix regeneration in diabetic wounds. Stem Cell Res Ther. 2021;12(1):49. doi: 10.1186/s13287-020-02116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trigo C.M., Rodrigues J.S., Camões S.P., Solá S., Miranda J.P. Mesenchymal stem cell secretome for regenerative medicine: where do we stand? J Adv Res. 2024 doi: 10.1016/j.jare.2024.05.004. in press. [DOI] [PubMed] [Google Scholar]
- 44.Kaokaen P., Pangjantuk A., Kunhorm P., Promjantuek W., Chaicharoenaudomrung N., Noisa P. Conditioned medium of human umbilical cord-mesenchymal stem cells cultivated with human cord blood serum enhances stem cell stemness and secretome profiles. Toxicology in Vitro. 2024;103:105973. doi: 10.1016/j.tiv.2024.105973. [DOI] [PubMed] [Google Scholar]
- 45.Vilaça-Faria H., Marote A., Lages I., et al. Fractionating stem cells secretome for Parkinson's disease modeling: is it the whole better than the sum of its parts? Biochimie. 2021;189:87–98. doi: 10.1016/j.biochi.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Kuang P.P., Liu X.Q., Li C.G., et al. Mesenchymal stem cells overexpressing interleukin-10 prevent allergic airway inflammation. Stem Cell Res Ther. 2023;14(1):369. doi: 10.1186/s13287-023-03602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Z.Y., Sun X., Xu X.L., Zhao Q., Peng J., Wang Y. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res. 2015;10(4):651–658. doi: 10.4103/1673-5374.155442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 49.Fajarwati I., Solihin D.D., Wresdiyati T., Batubara I. Self-recovery in diabetic Sprague Dawley rats induced by intraperitoneal alloxan and streptozotocin. Heliyon. 2023;9(5):e15533. doi: 10.1016/j.heliyon.2023.e15533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasad M., Rajagopal P., Devarajan N., et al. A comprehensive review on high -fat diet-induced diabetes mellitus: an epigenetic view. Journal of Nutritional Biochemistry. 2022;107:109037. doi: 10.1016/j.jnutbio.2022.109037. [DOI] [PubMed] [Google Scholar]
- 51.Kottaisamy C.P.D., Raj D.S., Prasanth Kumar V., Sankaran U. Experimental animal models for diabetes and its related complications—A review. Lab Anim Res. 2021;37(1):23. doi: 10.1186/s42826-021-00101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King A.J.F. The use of animal models in diabetes research. Br J Pharmacol. 2012;166(3):877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park S.R., Kim J.W., Jun H.S., Roh J.Y., Lee H.Y., Hong I.S. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Molecular Therapy. 2018;26(2):606–617. doi: 10.1016/j.ymthe.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macleod A.S., Kwock J.T. Inflammation in wound repair: role and function of Inflammation in wound repair. Wound Healing: Stem Cells Repair and Restorations, Basic and Clinical Aspects. 2018:177–194. doi: 10.1002/9781119282518.ch14. [DOI] [Google Scholar]
- 55.Wu Y.S., Chen S.N. Apoptotic cell: linkage of inflammation and wound healing. Front Pharmacol. 2014;5:1. doi: 10.3389/fphar.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of relatable data can be requested upon reasonable request from the corresponding author.