Summary
Cancer progression and therapeutic resistance are closely linked to a stemness phenotype. Here, we introduce a protein-expression-based stemness index (PROTsi) to evaluate oncogenic dedifferentiation in relation to histopathology, molecular features, and clinical outcomes. Utilizing datasets from the Clinical Proteomic Tumor Analysis Consortium across 11 tumor types, we validate PROTsi’s effectiveness in accurately quantifying stem-like features. Through integration of PROTsi with multi-omics, including protein post-translational modifications, we identify molecular features associated with stemness and proteins that act as active nodes within transcriptional networks, driving tumor aggressiveness. Proteins highly correlated with stemness were identified as potential drug targets, both shared and tumor specific. These stemness-associated proteins demonstrate predictive value for clinical outcomes, as confirmed by immunohistochemistry in multiple samples. The findings emphasize PROTsi’s efficacy as a valuable tool for selecting predictive protein targets, a crucial step in customizing anti-cancer therapy and advancing the clinical development of cures for cancer patients.
Keywords: stemness, cancer, proteomics, multiomics, drug targets, biomarkers, mass spectrometry, machine learning, kinase activity, tumor plasticity
Graphical abstract

Highlights
-
•
Proteomic-based stemness index reveals aggressive tumor subtypes
-
•
Drug targets for anti-cancer therapy can be predicted by the proteomic stemness phenotype
-
•
Post-translational modifications associated with stemness are shared across tumors
-
•
Stemness-associated proteins can predict clinical outcomes and serve as biomarkers
Kołodziejczak-Guglas et al. introduce PROTsi, a protein-expression-based stemness index that measures tumor aggressiveness across multiple cancer types. Using machine learning on Clinical Proteomic Tumor Analysis Consortium multi-omics data, including post-translational modifications, they identify actionable stemness-associated protein targets, paving the way for refined precision oncology and accelerated cancer therapy research.
Introduction
Malignant cells evade differentiation and unlock naturally restricted phenotypic plasticity to avoid terminal differentiation and continue proliferation.1 This phenotypic plasticity reflects the capability to switch between distinct cellular states, such as cancer stem cells (CSCs) and non-CSCs, differentiated and progenitor-like cells, quiescent and proliferative states, and drug-resistant and drug-sensitive states. Compelling evidence indicates that cellular plasticity can contribute to tumor progression, metastasis, and therapeutic resistance.1,2,3 Moreover, stem cell features, often referred to as “stemness,” have been associated with poor outcomes in a variety of cancers.4,5,6 Several studies, including our previous work, dedicated significant efforts and described distinct approaches aimed at measuring stemness in tumors utilizing transcriptomic and epigenomic features.4,7,8
Contemporary research on molecular features and mechanisms of cancer is increasingly oriented toward proteomics.9,10 Proteomics provides functional information, including protein activities, modifications, and interactions, allowing deeper mechanistic insights into biological processes both physiological and pathophysiological.11,12 Proteomics enables the identification of protein biomarkers for disease diagnosis and prognosis and for monitoring treatment response13 and allows the identification and validation of targets for drugs, the study of drug-protein interactions, and the assessment of drug efficacy in preclinical and clinical trials.14,15 Additionally, proteomics reveals mechanisms of drug resistance, supporting the development of personalized therapies.16,17,18
With a particular emphasis on proteomic analyses, the Clinical Proteomic Tumor Analysis Consortium (CPTAC) has enhanced our understanding of fundamental molecular mechanisms underlying cancer by consolidating data across the proteogenomic spectrum, integrating proteomics and phosphoproteomics with whole-exome and whole-genome sequencing, DNA methylation, and RNA sequencing.19,20
Here, we provide a new protein-expression-based stemness index (PROTsi) for assessing the degree of oncogenic dedifferentiation in tumor samples. Proteogenomic datasets obtained from tumor samples analyzed by CPTAC were indexed by PROTsi, confirming the efficacy of PROTsi to quantify stem-like features. In turn, this allows for a deeper understanding of cancer biology and mechanisms responsible for tumor resistance, along with the identification of candidate protein therapeutic targets and biomarkers of cancer progression.
Results
Novel stemness index classifier PROTsi measures oncogenic dedifferentiation and allows for stratification of tumor and normal adjacent tissue samples
We used a machine-learning algorithm based on one-class logistic regression (OCLR) trained on the proteomic data from 207 genetically diverse non-transformed induced pluripotent stem cells (iPSCs) to extract stemness features (Table S1C). To ensure comparability with the CPTAC dataset, we identified common proteins between the Human Induced Pluripotent Stem Cell Initiative and CPTAC proteomic datasets.
The created model was then used to predict stemness in tumors and generate a novel PROTsi, which ranges from low (0) to high (1) stemness (Figures 1A–1C; Table S1A). We validated PROTsi in independent datasets of cancer and non-CSCs (Figure S1A). The validation results show that PROTsi performs consistently across various datasets, clearly distinguishing between stem cells (embryonic stem cellss, iPSCs) and differentiated cells (ICC_N, LUAD_N, hFF), with different tumors stratifying in intermediate positions (Figure S1A; Table S1D).
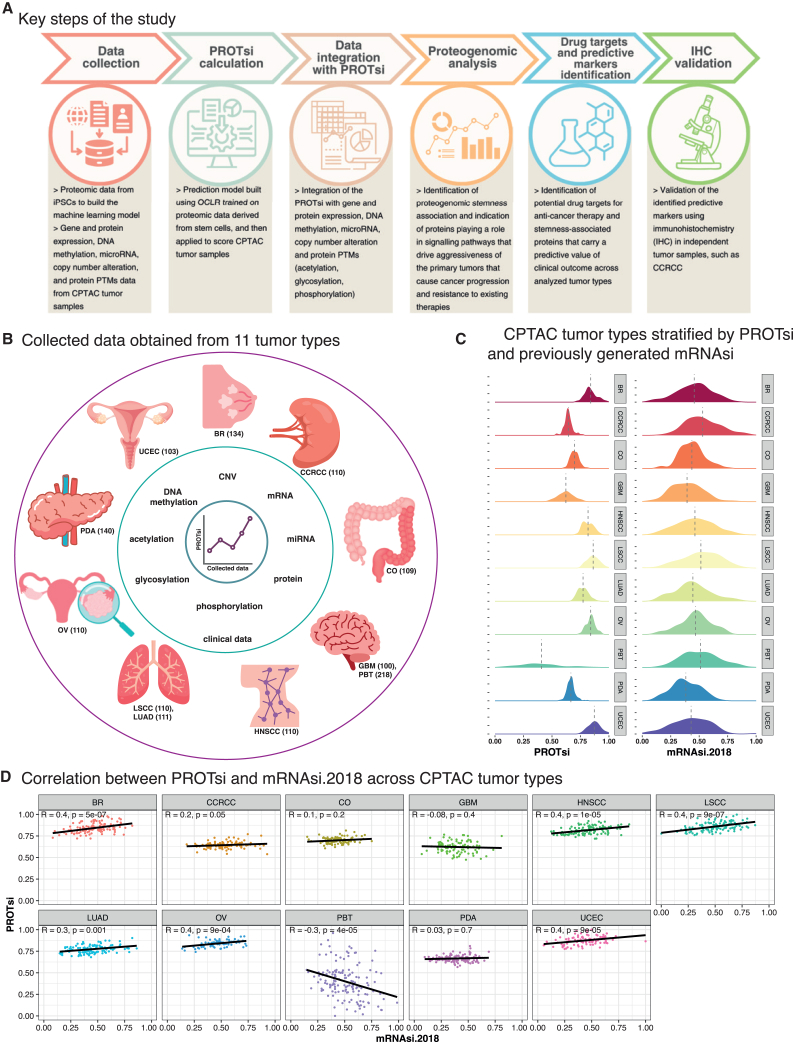
Figure 1.
Stratification of CPTAC tumor types with newly developed protein-expression-based stemness index (PROTsi)
(A) Key steps of the study: data collection, PROTsi calculation, data integration with PROTsi, proteogenomic analysis, drug targets and predictive markers identification, and IHC validation.
(B) Collected data obtained from 11 tumor types. The outer circle layer includes 11 CPTAC tumor types (BR, breast cancer; CCRCC, clear cell renal cell carcinoma; CO, colon cancer; GBM, glioblastoma; PBT, pediatric brain tumors; HNSCC, head and neck squamous cell carcinoma; LSCC, lung squamous cell carcinoma; LUAD, lung adenocarcinoma; OV, ovarian cancer; PDA, pancreatic ductal adenocarcinoma; and UCEC, uterine corpus endometrial carcinoma), with the total number of samples that served for proteogenomic and clinical data collection used for downstream analysis.
(C) Tumor types stratified by PROTsi (right) and previously generated mRNAsi.2018 (left).
(D) Correlation between mRNAsi.2018 and PROTsi across tumor types. p < 0.05 is considered statistically significant.
See also Figure S1.
We applied PROTsi to over 1,300 CPTAC samples obtained from 11 types of primary tumors of the breast (BR), ovary (OV), lung (squamous cell [LSCC] and adenocarcinoma [LUAD]), kidney (CCRCC), uterus (UCEC), brain (pediatric [PBT] and adult [GBM]), head and neck (HNSCC), colon (CO), and pancreas (pancreatic ductal adenocarcinoma [PDA]) and found an association of PROTsi with molecular and histopathologic features and with clinical outcomes (Tables S1A and S1E). Most tumors had higher stemness scores than non-tumor samples, and PROTsi efficiently segregated tumor and non-tumor samples for all the tumor types, except PDA (Figure S1B), likely due to the low purity of PDA samples, which include abundant admixed cancer-associated fibroblasts together with nests of malignant cells. The highest PROTsi was observed in basal BR (Figure S1C), which exhibits an aggressive phenotype associated with a dedifferentiated state.21 Likewise, when stratifying the histological types of PBTs, the highest PROTsi was observed for World Health Organization grade IV atypical teratoid/rhabdoid tumor (ATRT) (Figure S1C), which originates from embryonic cells and is one of the most aggressive brain malignancies.22 Similarly, PROTsi usually increased with malignancy grade and pathologic stage (Figures S1D and S1E). PROTsi is also predictive of clinical outcomes in HNSCC and UCEC (progression-free survival [PFS]) (Figure S1F) and CCRCC (overall survival [OS]) (Figure S1G).
PROTsi positively correlates with published transcriptomic-based stemness scores, including our previous stemness model based on transcriptomic data (mRNAsi.2018; Figure 1D).4 Compared to mRNAsi.2018, PROTsi was more effective in distinguishing between tumor and non-tumor samples. Because PROTsi showed less variation than mRNAsi in tumors, there was cleaner separation from non-neoplastic tumors, which was most apparent in CCRCC, where mRNAsi.2018 failed to distinguish tumor from non-tumor, whereas PROTsi succeeded (Figure S1B). Additionally, PROTsi better differentiated between higher grades of LUAD, UCEC, PDA, and PBT (Figure S1C), while mRNAsi.2018 only performed better in stratification of grade 4 CCRCC (Figure S1C).
Interestingly, PROTsi did not correlate with mRNAsi.2018 in adult brain tumors (GBM) and inversely correlated in PBT (Figure 1D). All GBM samples in this study are grade 4, while PBT includes seven different histologies, from low-grade to high-grade tumors (Table S1A). PROTsi was lowest in GBM and PBT, but it also showed the greatest variation among all tumors when comparing molecular stratification by PROTsi and mRNAsi.2018 (Figure S1C). This likely reflects not only the influence of tumor-specific factors among GBM and PBT but also the contributions of the cancer-associated non-neoplastic stroma in brain cancer and the highly heterogeneous nature of brain tumors. PROTsi effectively stratified PBT by aggressiveness, whereas gene expression data cannot (Figures S1C and S1D). For example, pediatric high-grade gliomas showed higher PROTsi levels compared to low-grade gliomas, while mRNAsi.2018 failed to distinguish between these markedly different tumor types (Figure S1C). Therefore, PROTsi and mRNAsi complement each other, with PROTsi excelling in protein-level analysis and providing a more accurate assessment of tumor aggressiveness.
Identification of stemness-associated proteins and their correlation with proteogenomic and clinical data
Integration of PROTsi with multi-omics data, including copy-number variation (CNV), DNA methylation, mRNA, microRNA (miRNA), and protein expression, was performed to elucidate the intricate relationships between different molecular layers. The correlation between PROTsi and protein expression identified a subset of proteins strongly correlated with stemness in each individual cancer (Table S2), capturing cancer-specific details in stemness-associated protein patterns, acknowledging the heterogeneity across cancers.
For each tumor, we selected 12 proteins most positively correlated with stemness (i.e., those with high expression linked to high PROTsi scores in tumor samples) and 12 proteins most negatively correlated (i.e., those with low expression associated with high PROTsi) as representative stemness-related proteins. The proteogenomic features of these 24 proteins were then explored in each cancer type (Figure 2). An expanded representation of the stemness-associated multi-omics data is provided in Figures S2A–S2H.
Figure 2.
PROTsi integration with data on copy-number variation, mRNA, miRNA and protein expression, and clinical outcomes across tumors
Shown are 12 proteins positively and 12 proteins negatively correlated with stemness, along with their molecular mechanisms in cancer stemness across tumors. The top bar represents PROTsi values from low (left) to high (right), with samples as vertical bars. Left columns detail copy-number variation (CNV) and miRNA and PROTsi correlation, while right columns show protein functional annotation, including therapeutic use, tier classification,23 and functional family. Overall survival and progression-free survival (PFS) columns indicate predictive value with hazard ratio (HR) and p value, adjusted for age at diagnosis. Statistical significance (∗p < 0.05) is marked by asterisks.
See also Figures S2 and S3.
In general, for proteins positively correlated with PROTsi, mRNA expression and CNV also showed positive correlation with PROTsi, while miRNA targeting the corresponding gene was negatively correlated (Figures 2 and S2; Table S3A). Taking as an example the relationship between PROTsi and proteogenomic data in HNSCC, we demonstrate high PROTsi values correlated with high ATP-dependent RNA helicase A (DHX9) protein expression, high mRNA expression, positive correlation with CNV and, simultaneously, negative correlation with miRNA expression and no association with DNA methylation (Figures 2 and S2C). In contrast, high PROTsi values correlated with low Optineurin (OPTN) levels, low mRNA expression, high miRNA expression, and no changes in CNV and DNA methylation (Figures 2 and S2C). A few stemness-related proteins among our selection showed epigenetic regulation such as DNA topoisomerase 1 (TOP1) in UCEC, where high protein and mRNA expression, copy number, and promoter hypomethylation correlated with high PROTsi (Figures 2 and S2G).
Although PROTsi only showed prognostic associations with OS and PFS in limited milieus after adjustment for age, sex, and tumor purity (Figures S1F and S1G), we queried whether individual stemness-related proteins might be more informative when interrogated separately (Figures 2 and S2). Several stemness-associated proteins exhibited prognostic significance, with higher levels linked to increased risk and lower levels associated with reduced risk. Notably, for a significant subset of these proteins (Table S3B), the expression of the corresponding genes does not show prognostic value, highlighting the advantage of using protein biomarkers.
To assess the robustness and accuracy of PROTsi, we performed external validation using independent datasets for PDA and LUAD. Stemness-related proteins identified in CPTAC samples also displayed positive correlations (correlation > 0.5, p < 0.05) in the validation cohorts for both PDA and LUAD (Figures S3A and S3C; Table S4). For PDA, which also had survival data available, proteins associated with poor prognosis in the CPTAC dataset—such as KPNA2, MCM2, MCM3, MCM4, and NUDCD1—exhibited consistent OS hazard ratios in the PDA validation dataset (Figure S3B). These findings validate PROTsi’s reliability and generalizability in identifying biologically relevant correlations, reinforcing its utility for predicting protein biomarkers of cancer progression.
The identified stemness-associated proteins represent a rich assortment of functional categories, and their association with clinical outcomes underscores their relevance in cancer progression. The vast majority of these proteins currently lack targeting by existing cancer therapies (Figures 2 and S2), positioning them as potentially untapped resources for future drug development. However, a significant number have already been recognized as drug targets or potential candidates according to the five-tier classification in Target LinkedOmics, offering opportunities for drug repurposing or novel therapeutic strategies.23
PROTsi correlates with specific protein post-translational modifications and allows the selection of functionally active protein targets for anti-cancer therapy
Many protein post-translational modifications (PTMs) that modify protein activity, structure, locations, functions, and protein-protein interactions in tumor cells have been reported to correlate with tumor progression, growth, and survival.24,25 We investigated the relationship between stemness and PTMs such as acetylation, glycosylation, and phosphorylation in the CPTAC tumors (Figures 3A–3C; Table S5). Various stemness-associated modified sites, including acetylation, N-linked glycans, and phosphorylation, are shared across high stemness tumors (Figures 3A–3C; Table S5).
Figure 3.
Protein PTMs associated with cancer stemness
Top: correlation of stemness and PTMs at site level. Shared acetylation (A), glycosylation (B), and phosphorylation (C) sites across tumors. UpSet plots display positively (red) and negatively (blue) correlated sites for each modification type. Bottom: correlation of stemness and PTMs at protein level. Protein acetylation, glycosylation, and phosphorylation correlated with stemness in shared tumors. Chord diagrams display the top 20 modified proteins with positive (D) and negative (E) correlations.
Using BR, GBM, LSCC, LUAD, and UCEC acetylation data, we found that these tumors shared 71 acetylation sites positively correlated with stemness and 21 negatively correlated (Figure 3A). Most of the common stemness-associated acetylation sites are involved in cell-cycle and transcription regulation. Among 71 shared acetylation sites, the identification of poly (ADP-ribose) polymerase 1 (PARP1) acetylation at K105 and K108 in all tumors suggests a functional link between PARP1 acetylation and cancer stemness (Table S5). These modifications are highly correlated with stemness, indicating that acetylated PARP1 may promote CSCs’ survival and therapy resistance. Given the role of PARP1 in DNA repair,26 its acetylation likely enhances the CSCs’ DNA repair capabilities, contributing to tumor resilience. This may explain high-stemness tumor resistance and suggest that PARP1 acetylation aids survival. Conversely, the exploration of acetylation sites negatively correlated with stemness revealed that in low stemness tumors, SEPTIN11 is being acetylated at multiple sites: K180, K367, K282, K371, K362, K205, K200, K258, and K311 (Table S5). Septins act as scaffolds for protein-protein interactions and contribute to carcinogenesis.27 Although the specific acetylation of SEPTIN11 at these sites has never been documented as contributing to cancer, we indicate a potential link between these events and the stemness phenotype. BR and LSCC showed the most similar patterns of positively correlated acetylation, with 206 positively associated acetylation sites in common, while the most similar patterns of negative association were found in GBM and LSCC, presenting 153 acetylation sites in common (Figure 3A). The identification of shared acetylation sites correlated with PROTsi in different cancers suggests a significant role of acetylation in maintaining a stem cell-like state in cancer.
Tumors shared 214 positively stemness-correlated phosphorylation sites among 7 cancer types (Figure 3C). Negative correlation was less frequent, with 104 shared sites between 5 cancer types (PDA, HNSCC, UCEC, LUAD, and LSCC) and only 7 sites shared across all cancer types. The presence of unique acetylation sites (e.g., FBN1_K1027 in LSCC), N-linked glycans (e.g., PYCARD_N9H10F1S2G0 in HNSCC), and phosphorylation sites (e.g., HYOU1_S742 in CCRCC) in particular tumors (Table S5) suggests modification-specific stemness regulation in those cancers.
Analysis of glycosylation correlated with stemness revealed only a few sites, either positively or negatively correlated with stemness, shared among the seven cancers (Figure 3B). Of these, negative correlation was more frequent than positive correlation.
We also investigated stemness-associated PTMs across tumors by aggregating each protein site. Figures 3D and 3E show the top 20 proteins undergoing PTMs that are associated with high (Figure 3D) and low (Figure 3E) stemness and that are shared across tumors (Table S5). The protein nucleolin (NCL), for example, is acetylated at 13 common sites (K102, K109, K116, K124, K132, K16, K294, K377, K398, K572, K79, K87, and K95) in high stemness tumors (Figure 3D). NCL is a multifunctional protein, involved in ribosome biogenesis and proliferation,28 and alterations in its acetylation status may have functional consequences for ribosome biogenesis, which is often dysregulated in cancer cells.29 No data currently link these modifications to ribosome biogenesis in these cancers, emphasizing the need to explore their functional impact. Likewise, the serine/arginine repetitive matrix protein 2 (SRRM2) presented numerous hyperphosphorylated sites in high stemness tumors (Figure 3D) (e.g., SRRM2_T1003 and SRRM2_S1083 [CCRCC, GBM, HNSCC, LSCC, LUAD, and UCEC] and SRRM2_T1043 [GBM, LSCC, LUAD, PDA, and UCEC]), while neuroblast differentiation-associated protein (AHNAK) indicated hypophosphorylated sites in high stemness tumors (Figure 3E) (e.g., AHNAK_S572 [CCRCC, GBM, HNSCC, LSCC, LUAD, PDA, and UCEC] and AHNAK_S5857 [CCRCC, GBM, HNSCC, LSCC, LUAD, and UCEC]). Similar PTM patterns are observed for other proteins and shared across tumors (Figures 3D and 3E; Table S5). Of note, there are modified proteins that, although shared across distinct tumors, are enriched in specific tumor types, such as fibronectin (FN1) and low-density lipoprotein receptor-related protein 1 (LRP1), which show glycosylation of distinct sites highly associated with CCRCCs with high stemness (Figure 3D), i.e., DQCIVDDITYNVNDTFHKR-N5H5F0S1G0 (FN1 peptide sequence with glycosylation at the fifth asparagine, consisting of 5 hexoses and 1 N-acetylhexosamine) and DQCIVDDITYNVNDTFHKR-N4H5F1S0G0 (FN1 peptide sequence with glycosylation at the fourth asparagine, consisting of 5 hexoses, 1 fucose, and no N-acetylhexosamine), and other LRP1 glycopeptides representing variations in glycosylation at different asparagine residues, with different glycan components combinations. Our findings clearly link PTMs and cancer stemness. Key PTMs, particularly acetylation, drive stemness by enhancing CSC survival and existing resistance to therapies.
Kinase activity score reveals stemness-associated protein kinases
We integrated kinase activity scores obtained from phosphoproteomic data with PROTsi to discover the most activated and inactive stemness-associated protein kinases (Figures 4 and S4; Table S6). Positive association with stemness was observed for kinases from the CMGC, CK1, and Atypical families, for most kinases from the Other family, and for some kinases belonging to the CAMK family. Kinases in this cluster show higher activity in high stemness tumors across several cancers. Cyclin-dependent kinases from the CMGC family such as CDK1, CDK2, CDK7, and others regulate cell-cycle progression, which may explain their high association with stemness as CSCs exhibit enhanced proliferative potential.30 The high kinase activity across multiple cancers suggests a potentially conserved role in maintaining stem cell-like properties among cancer cells. Some kinases show increased activity in particular cancers. For example, the only kinase from the AGC family in the positive cluster, microtubule-associated serine/threonine-protein kinase-like (MASTL), shows strong positive correlation with stemness, particularly in HNSCC.
Figure 4.
Activity score of kinases associated with stemness
Activity distributions of kinases, with synchronized association with PROTsi stratified by tumor types. Kinases of consistent correlation direction in all tumor types and of significance in at least eight tumor types were selected. Kinases with positive correlations or negative correlations were grouped separately in the plot, with the kinase group identity annotated on the left. Samples of each tumor type were ordered by the PROTsi scores with lowest to the left.
See also Figure S4.
Conversely, kinases from the TKL, CAMK, AGC, TK, and STE families show negative correlations with stemness overall, suggesting that their reduced activity might play an important role in tumorigenesis and aggressive features acquisition. Several TK family kinases, such as epidermal growth factor receptor, hematopoietic cell kinase, and erb-b2 receptor tyrosine kinase 4, which are well-known drivers of tumorigenesis, are highlighted, and their deactivation in high stemness tumors might point to chemoresistance or non-genetic mechanisms of drug tolerance by reducing their kinase activities and activating compensatory mechanisms of growth.31
Several of the highly stemness-associated kinases have been already tested as targeted therapies, offering clinical relevance for our findings. For example, CHEK1, a member of the CAMK kinase group, was found to be highly activated in high stemness tumors (Figures 4 and S4), while prexasertib, a CHEK1 inhibitor, is a promising drug for highly aggressive tumors.32 Haspin, a serine/threonine-protein kinase, also demonstrates increased activity in high stemness tumors, particularly in BR, UCEC, and OV (Figure 4), suggesting a role in supporting CSC-like features. Haspin regulates histone H3 via phosphorylation at threonine 3, which is essential for the configuration of chromatin during mitosis.33 Increased Haspin activity in these high stemness samples may therefore promote uncontrolled proliferation and contribute to genomic instability.1,34 The Haspin inhibitor CHR-6494, previously studied in highly aggressive BR,35,36,37 could be also widely tested following our analysis. The therapeutic potential of targeting Haspin is further underscored by its synergistic action when combined with other mitotic regulators, such as Aurora B kinase inhibitors.38,39 These findings could guide new therapies and deepen the understanding of kinases in cancer.
Identification of protein-protein interactions and biological pathways associated with stemness unravels new functional networks underpinning tumor pathobiology
The identification of protein-protein interaction networks and biological pathways provides understanding of the molecular relationships and potential functional associations among the identified stemness-related proteins in analyzed tumors, and it may offer insights into the intricate cancer stemness regulatory mechanisms.
Protein-protein interaction analysis was conducted using Cytoscape40 and the STRING database41 on the top 700 proteins most correlated with stemness (100 per tumor) (Figure 5A; Table S3A). These proteins were categorized as pan-cancer proteins, proteins common for multiple tumors, and specific proteins shared across two tumors only (Figures S5A and S5B), and specific for the given tumor type (Figures S5C and S5D). Among the proteins, both the upregulated and downregulated protein sets exhibited a number of protein-protein interactions, forming a densely interconnected “main” network. A few downregulated proteins disconnected from the main network, indicating potential functional differences or unique regulatory roles in cancer stemness. Notably, certain downregulated proteins also play a crucial role in the network—for example, interferon regulatory factor 6 (IRF6) is downregulated in CCRCC, yet it interacts with the main network via SMARCA4, an upregulated protein (Figure 5A). This interplay between upregulated and downregulated proteins suggests a complex regulatory landscape. IRF6, a transcription factor involved in epithelial development,42 and SMARCA4, a core component of the chromatin-remodeling SWI/SNF complex,43 collectively impact gene expression and chromatin structure. IRF6 dysregulation has been associated with aberrant epithelial-to-mesenchymal transition (EMT),44 while SMARCA4 mutations are linked to ovarian cancer and hepatocellular carcinoma.45 Both proteins contribute to maintaining genomic stability and cellular identity, and their disruption may contribute to cancer initiation and CCRCC progression.
Figure 5.
Protein-protein interactions and key biological pathways associated with stemness identified in tumors
(A) Protein-protein interactions among 100 stemness-associated proteins, with red indicating upregulation and blue indicating downregulation.
(B) Top 10 enriched Reactome pathways associated with stemness, ranked by statistical significance (p < 0.05). Red dots represent p values, and maroon dots indicate false discovery rate values.
(C) Stemness-associated pathways shared across tumor types. Maroon rectangles highlight significant involvement of stemness-related proteins in Reactome pathways present in at least two tumor types (p < 0.05).
See also Figure S5.
We next conducted pathway analysis to identify key biological pathways associated with cancer stemness enriched in each tumor (Figure 5B; Table S7A). The top 10 enriched Reactome pathways for each tumor encompass numerous cellular processes, including cell-cycle regulation, DNA repair, and immune response pathways, which are crucial in tumor development and progression.
Notably, pathways consistently enriched across multiple tumors (Figure 5C), including mRNA splicing and processing, support the importance of post-transcriptional regulation of stemness features. Various proteins implicated in RNA processing and splicing have been detected in GBM. RNA-binding proteins are regulators of co- and post-transcriptional mechanisms, including RNA processing (splicing, capping, and polyadenylation), transport, decay, localization, and translation. Many of the proteins had high expression in GBM and glioblastoma stem cell (GSC) samples, and were associated with a poor prognosis. Some of them, i.e., SNRPB, encoding core components of the spliceosome complex SmB/B′, control expression and splicing affecting RNA processing, DNA repair, and chromatin remodeling along with pathways relevant to GBM initiation and development.46,47
In contrast, pathways such as peroxisomal lipid metabolism in CCRCC were unique in their tumor-type-specific association with stemness, consistent with known pathobiological features of these tumors.
Identification of cancer stemness candidate inhibitors using CMap
Drug connectivity analysis, conducted on the set of 100 proteins showing the most positive and negative correlations with stemness in each tumor type, enabled us to identify perturbagens (i.e., small molecules such as drugs or chemical compounds)48 that may repress stemness in the analyzed cancers, thereby providing therapeutic benefit (Figure 6; Table S7B). Among these putative anti-stemness perturbagens shown in Figure 6A, compounds common to several cancer types were identified, such as CGP-60474 for CCRCC, GBM, HNSCC, LUAD, PDA, and UCEC, or compounds targeting only one cancer type, such as prostratin for LSCC or teniposide for UCEC. Several of the identified perturbagens represented drugs that have been studied in preclinical or early-phase clinical trials for various cancers. Of these, bisindolylomaleimide-IX, a pan-protein kinase C (PKC) inhibitor also known as Ro 31–8220,49 showed promise in preclinical studies against BR50,51 and non-small cell lung cancer.52 Vorinostat, a US Food and Drug Administration-approved histone deacetylase (HDAC) inhibitor, identified herein as a stemness perturbagen in CCRCC, GBM, HNSCC, LSCC, and UCEC, has demonstrated an efficacy in cutaneous T cell lymphoma,53 and in a phase 1 clinical trial, it showed some promise in non-small cell lung cancer.54
Figure 6.
Candidate inhibitors targeting stemness-associated pathways
(A) Candidate compounds targeting stemness-associated pathways. Left: identified candidate compounds that may inhibit cancer stemness based on CMap analysis. Right: number of tumor types in which the compounds were identified.
(B) Top drug targets being targeted by at least two candidate compounds inhibiting stemness-associated pathways. Columns show specific drug targets targeted by candidate inhibitors of stemness, which are presented in rows.
(C) Mechanisms of action (MoAs) inhibiting stemness. Left: columns represent identified inhibitors of stemness, and rows show MoAs. Right: MoAs active in tumors.
See also Figure S6.
To provide further support for the clinical relevance of our findings, we searched for stemness-associated candidate targets using the Target LinkedOmics and Drug Gene Interaction Database portals and also found HDAC proteins targeted by vorinostat. Notably, vorinostat has also been shown to enhance the sensitivity of drug-resistant neuroblastoma cells to chemotherapy, indicating its potential in overcoming drug resistance. Vorinostat impaired the ability of these cells to form tumorspheres, a hallmark of stem-like properties, and decreased the expression of several stemness-associated genes, reinforcing its role in repressing key pathways contributing to the maintenance and survival of CSCs.55 Alvocidib, a cyclin-dependent kinase inhibitor, has been evaluated in clinical trials for leukemia,56 and selumetinib, a MEK inhibitor, has been studied in melanoma, glioma, lung cancer, head and neck cancer, and thyroid cancer.57 These compounds represent a subset of investigational agents, each targeting distinct molecular pathways implicated in oncogenesis, and it remains to be seen whether their effects may be mediated by the inhibition of stemness or other biological pathways.
Next, drug connectivity analysis allowed for further identification of specific drug targets for potential inhibitors of cancer stemness. In this analysis, HDACs emerged as the most common targets for these perturbagens (Figures 6B and S6; Table S7B). Connectivity Map (CMap) analysis suggested the mechanisms of action (MoAs) of the identified potential stemness inhibitors (Figure 6C). CGP-60474 acts as an inhibitor of cyclin-dependent kinase; therefore, using this perturbagen could inhibit the stemness phenotype related to cell-cycle-associated pathways in tumors. In contrast, topotecan and teniposide are topoisomerase inhibitors that we identified as specifically inhibiting stemness-associated pathways in UCEC, showing a positive correlation between TOP1, TOP2A, and stemness. Once again, Target LinkedOmics supported our findings by showing topotecan and teniposide as cancer candidate targets. Topoisomerases are primarily involved in maintaining genomic stability during DNA replication and transcription,58 and their inhibition is well known to induce replication stress.59 However, emerging evidence indicates that the MoA for these drugs extends beyond this, suggesting they may also modulate pathways associated with cancer stemness and EMT.60,61 Topotecan (METRO-TOPO) has shown potential as a treatment against invasive prostate cancer, either as a single agent or in combination with docetaxel. Notably, METRO-TOPO was found to reduce stemness by either targeting CSC populations or downregulating EMT-related gene expression collaborating with our findings that TOP1 inhibition plays a role in suppressing cancer stemness.60 Additionally, teniposide, which targets DNA topoisomerase TOP2A, has been shown to be effective in reversing EMT in BR, a process closely linked to stemness.61
Validation of selected stemness-associated proteins by immunohistochemistry confirms their prognostic value
We used immunohistochemistry (IHC) to validate the expression of proteins identified by mass spectrometry (MS) as highly associated with PROTsi. We then interrogated the prognostic association of IHC scores of selected proteins in an independent set of one exemplary tumor type, CCRCC (Figure 7). Based on previous analyses (Figures 2 and S2A; Tables S2 and S3), HEATR1, SNRNP200, and UHRF, proteins positively correlated with stemness, were selected for IHC validation in CCRCC samples. Their increased levels (measured by liquid chromatography-tandem MS) were associated with oncogenic dedifferentiation, increased tumor aggressiveness, and progression. Moreover, SLC27A2, a fatty acid transporter protein 2 involved in lipid metabolism and fatty acid transport,62 was selected as a protein negatively correlated with stemness (Figure S2A).
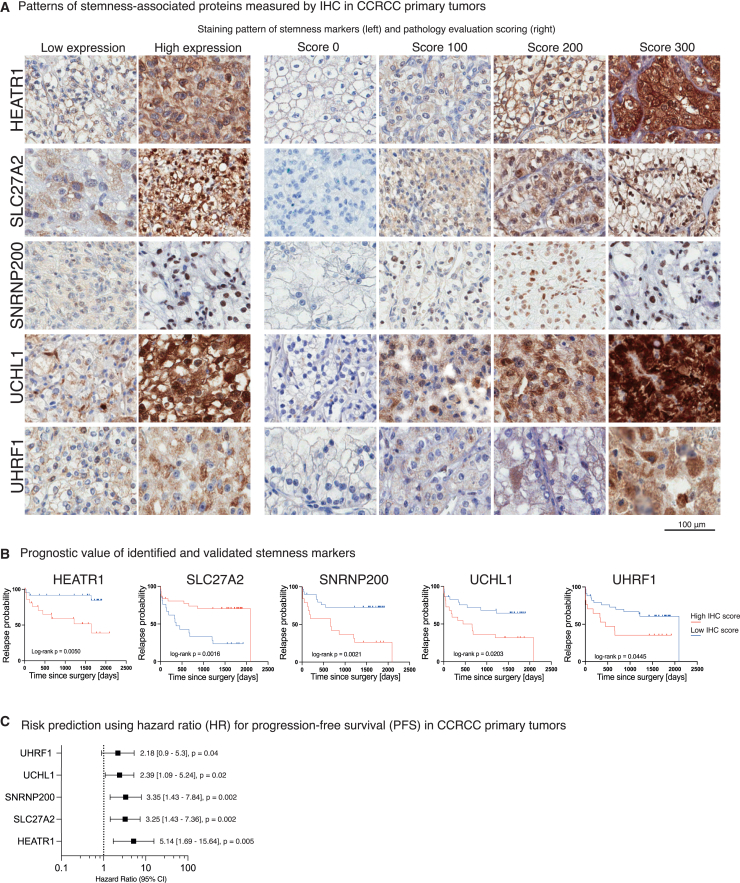
Figure 7.
Selected protein targets identified by PROTsi have prognostic value of cancer progression
(A) Patterns of stemness-associated proteins measured by IHC in CCRCC primary tumors. Representative images show levels (low and high) of selected proteins, along with a four-tier semi-quantitative pathology evaluation scale based on H-score methodology.
(B) Prognostic value of the validated stemness-associated proteins. Kaplan-Meier curves show the correlation between IHC positivity scores and PFS. Log rank p < 0.05 is considered statistically significant.
(C) Risk prediction for the stemness-associated proteins influencing PFS. Hazard ratio and 95% confidence interval were calculated for PFS analysis of validated stemness-related proteins using the log rank test.
In addition, IHC analysis of UCHL1 was performed to validate the prognostic value of UCHL1 in CCRCC, and to confirm key aspects of the stemness concept. UCHL1, participates in ubiquitin processing, protein stability regulation, and influences neuronal development and survival.63 Its role in cancer is debated, with reports suggesting both tumor-suppressive and oncogenic functions. UCHL1 acts as a tumor suppressor in liver,64 breast,65 prostate,66 and esophageal cancer,67 but in certain contexts, it promotes tumor growth, particularly in ER(−) and HER2(+) BR.68 Recent studies indicate that elevated ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) expression serves as a biomarker for high-grade CCRCC with specific genetic alterations.69 In our study, we observed an increased UCHL1 expression in CCRCC tumors with high stemness and confirmed its potential as a predictor of metastasis in CCRCC patients, indicating a possible role in promoting cancer stemness.
Patients with high levels of HEATR1, SNRNP200, UCHL1, and UHRF1 in primary tumors had a higher probability of disease progression (i.e., metastasis) (Figure 7B). In contrast, low SLC27A2 strongly correlated with a shorter time from primary tumor surgery to metastasis appearance. Of these, HEATR1 showed the strongest correlation with poor outcome (hazard ratio [HR] 5.14, p = 0.005; Figure 7C).
Discussion
The implementation of the PROTsi is an effective strategy for assessing the degree of similarity of cancer cells to stem cells. While our previous mRNA-based stemness index contributed to understanding tumor dedifferentiation,4 technological advances now allow robust proteomic data analysis capturing both protein levels and activities. With multiple processes beyond transcript concentration that contribute to establishing the expression level of a protein, there is no linear relationship between the concentration of a transcript and those of the protein. Unlike mRNA levels, proteomics reflects the real-time status of cellular machinery.70 Additionally, PTMs are crucial for short-term adaptations such as phosphorylation at cellular signaling events and contribute to the regulation of protein activities.71,72 By focusing on proteomics, the current stemness score provides a more direct and functional assessment, identifying key proteins, enzymatic events, and critical residues that can be targeted for small-molecule inhibitors, advancing cancer research and personalized medicine.73
Training the OCLR-based predictive model on a proteomic set of stem cells adds credibility to the development of PROTsi. Our model, derived from normal stem cells, captures key aspects of stem cell biology that CSCs often exploit. By coopting normal stem cell pathways, CSCs drive tumor initiation, progression, and therapy resistance, sustaining malignancy.1,74 While proliferative pathways are often the focus, our model also captures plasticity, differentiation potential, and stress resistance, which are key to cancer aggressiveness. This approach allows for a sharper characterization of the stemness state in cancer cells, enabling better prognosis, patient stratification, and therapeutic targets identification.
We demonstrate that PROTsi has advantages over mRNAsi.2018, particularly because proteins more closely relate to cellular phenotype and function. PROTsi provides a potentially more informative measure of cancer cell behavior but can be influenced by factors such as PTMs, protein stability, and degradation, which do not always correlate with mRNA expression. This is particularly relevant in brain tumors,75,76 where distinct molecular processes like neural differentiation and neuroinflammation are pronounced at the protein level.77,78 An adverse association between mRNAsi.2018 and prognosis in adult gliomas was described in the original study.4 Here, the isocitrate dehydrogenase mutant GBM, a subtype known for its more favorable prognosis,79 exhibited the highest mRNAsi.2018 levels, further confirming the unforeseen previous results (Figure S1C). This unexpected stratification of GBM tumors was not observed with PROTsi. The superior stratification of PBT, UCEC, and LUAD samples by PROTsi, which accurately captured tumor aggressiveness unlike gene expression data, reinforces the potential of PROTsi as a tool for predicting clinical outcome. Additionally, we identified stemness-related protein biomarkers with prognostic value independent of gene expression, highlighting PROTsi’s ability to reveal outcome-associated protein patterns undetected by mRNAsi.2018. PROTsi’s ability to capture additional regulatory mechanisms linked to stemness, such as PTMs, further strengthens the rationale for preferring PROTsi over mRNAsi.2018.
Moreover, we reported significant PROTsi associations with clinical outcomes in selected cancer types, suggesting that stemness-associated proteins may serve as valuable prognostic or theranostic biomarkers. Minichromosome maintenance (MCM) proteins stand out in our analysis, which revealed a strong association between the expression of several MCM proteins (MCM2–7) and PROTsi across multiple tumors. Specifically, MCM2, MCM3, and MCM4 expression correlated with worse OS in both the CPTAC PDA and the PDA validation datasets. As key regulators of DNA replication,80 MCM proteins play a critical role in cancer progression and are linked to poor prognosis.81 Recent studies showed that MCM2 regulates CXCL1 secretion in lung and GSCs, influencing key CSC traits, potentially contributing to tumor recurrence and treatment resistance.82 Notably, MCM2 ranks as the top druggable protein among 2,863 candidates in the LinkedOmics Targets portal.23 Targeting MCM proteins could therefore impair CSC survival and improve therapeutic outcomes.
However, not all of these proteins have prognostic value due to the complexity of cancer stemness, the heterogeneity of cancer subtypes, and variations in tumor microenvironments. While some proteins may show a correlation with stemness, their individual contribution to the overall predictive value of PROTsi may be overshadowed by the combined effect of other proteins or factors involved in stemness regulation. For example, in PDA, we found that proteins negatively correlated with PROTsi, like ITGA1 and FBN1, were associated with poor prognosis. This anticorrelation may reflect the high enrichment of stromal cancer-associated fibroblasts in PDA, emphasizing the complexity of PDA biology, where tumor-stromal interactions play as critical a role as stemness in disease progression.
Despite these challenges, PROTsi has led to the identification of therapeutic targets and potential drug inhibitors. By analyzing the correlation between PROTsi and PTMs alongside kinase activity, we identified modified proteins associated with stemness and their activities. These targets may help reduce tumor aggressiveness and therapy resistance. For instance, CHEK1, a member of the CAMK kinase group, showed high activation levels in tumors with elevated stemness, while prexasertib, a CHEK1 inhibitor, is a promising treatment for highly aggressive triple-negative BR and high-grade ovarian carcinoma, both of which exhibit high PROTsi. These findings suggest that inhibiting CHEK1 may also benefit other cancer types characterized by high stemness. Also, glycosylated proteins have been tested as promising targets for chimeric antigen receptor (CAR) T cell therapy.83 Stemness-associated glycosylated proteins may offer unique targets for CAR T therapy as they can be specific to the more resistant undifferentiated cell populations.
PROTsi aids in identifying prognostic proteins and drug targets simultaneously. Drug repositioning, explored through CMap, revealed potential stemness inhibitors. Based on interactions between stemness-related proteins and drug targets involved in the same signaling pathways, potential stemness inhibitors were discovered along with their specific targets in signaling pathways. Insights from CMap are also being supported by studying the kinase activity in relation to stemness. For example, WEE1 kinase, with its strong positive correlation with stemness in all tumors, was found to be a drug target for SA-792987 PKC inhibitor among the top 20 stemness-repressing agents in LSCC. This way we provide a comprehensive compendium of precisely identified drug targets and their promising inhibitors. Further in vitro functional studies could include the administration of selected inhibitors to cancer models such as patient-derived organoids.
Moreover, identification of protein markers may be more directly translated into clinical practice through the use of IHC studies where degree of staining may more readily reflect protein levels detected through MS than mRNA expression. Thus, PROTsi offers potentially greater clinical immediacy than mRNA-based signatures. IHC analysis of stemness-associated proteins like HEATR1, SNRNP200, SLC27A2, UHRF1, and UCHL1 in primary CCRCC tumors verified that PROTsi is a reliable tool for selecting predictive protein markers and further reinforces the role of these proteins in stemness and cancer progression. HEATR1, elevated in various cancers,84,85 was associated with stemness in glioma stem cells and cancer cell proliferation; UHRF1, an epigenetic regulator,86 has oncogenic properties in several cancers,87 including renal cell carcinoma. SLC27A2 negatively correlates with stemness and, although insufficiently explored in renal carcinoma, is associated with tumor progression.88,89 While typically cytoplasmic, recent studies suggest that SLC27A2 subcellular localization can vary under different physiological and pathological conditions.90,91 Although direct evidence of SLC27A2’s nuclear localization is limited,92 our findings suggest its presence in the nucleus of differentiated cancer cells, indicating regulatory functions beyond its cytoplasmic role. This potential nuclear localization broadens our understanding of SLC27A2’s functional diversity, warranting further investigation into its regulatory roles in the nucleus. Significant associations of UCHL1 and SLC27A2 with stemness and clinical outcomes, particularly in metastatic samples, underscore their roles in oncogenesis and cancer progression. Further research is needed to explore SLC27A2’s nuclear functions.
In summary, our study describes the development of PROTsi and provides an updated and enhanced comprehensive framework for understanding cancer stemness by capturing the functional state of proteins, identifying therapeutic targets, and offering prognostic insights. Its ability to correlate protein modifications with stemness enhances the development of personalized treatments, bringing us closer to effective cancer therapies.
Limitations of the study
Our study utilized multiomics data across seven tumor types. While these datasets provided valuable insights, the restricted tumor type coverage may not fully capture the diversity and complexity present across all cancer types, potentially limiting the generalizability of our findings.
Another challenge lies in the limitations of our machine learning approach. While it has successfully identified potential therapeutic targets associated with stemness and uncovered significant insights into the relationship between stemness and PTMs, both unique and shared across tumor types, we acknowledge the need for further validation. Specifically, although we leveraged independent datasets to support our candidate compounds in modulating stemness-associated pathways, rigorous experimental validation will be required to confirm their efficacy, elucidate MoAs, and evaluate clinical utility. These steps, while crucial for translating our discoveries into therapeutic applications, fall beyond the scope of the present study. We anticipate that future studies will build upon our findings to explore these mechanisms in greater detail.
Moreover, due to the lack of in vitro diagnostic antibodies for some of our protein targets, we utilized research antibodies for IHC validation, and their performance characteristics may differ from clinically validated antibodies, potentially affecting the reliability and generalizability of our validation results. Additionally, the relatively small number of CCRCC samples used for IHC validation (n = 52) may reduce the statistical power of our findings.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by Maciej Wiznerowicz (maciej.wiznerowicz@iimo.pl).
Materials availability
The study did not generate new unique reagents.
Data and code availability
Raw and processed proteomics as well as open-access genomic data for the CPTAC Pan-Cancer cohort can be accessed via Proteomic Data Commons at https://pdc.cancer.gov/pdc/cptac-pancancer. Raw genomic and transcriptomic data files can be accessed via the Genomic Data Commons Data Portal at https://portal.gdc.cancer.gov with dbGaP study accession number: phs001287.v16.p6. Processed genomic data with access control can be obtained via the Cancer Data Service at https://dataservice.datacommons.cancer.gov/.
Consortia
The members of the National Cancer Institute Clinical Proteomic Tumor Analysis Consortium for Pan-Cancer are Alexander J. Lazar, Alexey I. Nesvizhski, Alicia Francis, Amanda G. Paulovich, Ana I. Robles, Anna P. Calinawan, Antonio Colaprico, Antonio Iavarone, Arul M. Chinnaiyan, Bing Zhang, Bo Wen, Boris Reva, Bożena Kamińska, Brian J. Druker, Caleb M. Lindgren, Chandan Kumar-Sinha, Chelsea J. Newton, Chen Huang, Chet Birger, Corbin Day, D.R. Mani, Daniel Cui Zhou, Daniel W. Chan, David Fenyö, David I. Heiman, Dmitry Rykunov, Elizabeth G. Demicco, Emerson de Souza Santos, Emily Huntsman, Eric E. Schadt, Eric J. Jaehnig, Erik P. Storrs, Eunkyung An, Felipe da Veiga Leprevost, Fernanda Martins Rodrigues, Francesca Petralia, François Aguet, Gad Getz, Galen Hostetter, Gilbert S. Omenn, Hanbyul Cho, Henry Rodriguez, Houtan Noushmehr, Hui Zhang, Iga Kołodziejczak-Guglas, Jan Lubiński, Jared L. Johnson, Jasmin Bavarva, Jiayi Ji, Jimin Tan, Jonathan T. Lei, Joshua M. Wang, Karen A. Ketchum, Karin D. Rodland, Karl R. Clauser, Karsten Krug, Kelly V. Ruggles, Lewis C. Cantley, Li Ding, Liang-Bo Wang, Lijun Yao, Lizabeth Katsnelson, Maciej Wiznerowicz, Marcin J. Domagalski, Marcin P. Cieslik, Mathangi Thiagarajan, Matthew A. Wyczalkowski, Matthew J. Ellis, Meenakshi Anurag, Mehdi Mesri, Michael A. Gillette, Michael J. Birrer, Michael Schnaubelt, Michele Ceccarelli, Myvizhi Esai Selvan, Nadezhda V. Terekhanova, Nathan Edwards, Nicole Tignor, Özgün Babur, Paweł Kurzawa, Pei Wang, Pietro Pugliese, Qing Zhang, Ratna R. Thangudu, Renan L.S. Simões, Richard D. Smith, Robert Oldroyd, Rossana N. Lazcano Segura, Runyu Hong, Samuel H. Payne, Sara J.C. Gosline, Sara R. Savage, Saravana M. Dhanasekaran, Scott D. Jewell, Shankara Anand, Shankha Satpathy, Shrabanti Chowdhury, Song Cao, Stephan Schürer, Steven A. Carr, Steven M. Foltz, Tania J. Gonzalez Robles, Tao Liu, Tara Hiltke, Tathiane M. Malta, Tobias Schraink, Tomer M. Yaron, Vasileios Stathias, Waldemar Priebe, Weiping Ma, Wen Jiang, Wen-Wei Liang, Wenke Liu, Wilson McKerrow, Xiaoyu Song, Xinpei Yi, Xu Zhang, Yifat Geffen, Yige Wu, Ying Wang, Yingwei Hu, Yize Li, Yizhe Song, Yo Akiyama, Yongchao Dou, Yuxing Liao, Zeynep H. Gümüş, Zhen Zhang, and Zhiao Shi.
Acknowledgments
The CPTAC is supported by the National Cancer Institute of the National Institutes of Health under award numbers U24CA210955, U24CA210985, U24CA210986, U24CA210954, U24CA210967, U24CA210972, U24CA210979, U24CA210993, U01CA214114, U01CA214116, and U01CA214125. Additional funding support was provided by the São Paulo Research Foundation grants 2018/00583-0, 2019/14928-1 (T.M.M.), and 2022/16350-0 (R.L.S.S.).
Author contributions
Study conception and design, T.M.M., L.D., H.N., and M.W.; supervision, T.M.M., M.W., L.D., B.Z., M.M., and A.I.R.; data analysis, I.K.-G., R.L.S.S., E.d.S.S., T.M.M., E.G.D., R.N.L.S., A.J.L., W.M., J.L., P.W., F.P., A.C., P.P., M.C., P.K., and W.P.; data generation, W.M., E.S., A.I.N., F.d.V.L., Y.G., and P.K.; writing, I.K.-G., T.M.M., R.L.S.S., E.d.S.S., E.G.D., and B.K.; visualization, I.K.-G., R.L.S.S., T.M.M., and W.M.; funding acquisition, T.M.M. and M.W. All authors contributed to data interpretation and manuscript editing and revision.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| CPTAC Clinical data and proteomic data | Li et al.19 | https://pdc.cancer.gov/pdc/cptac-pancancer |
| CPTAC genomic, transcriptomic data | Li et al.19 | https://pdc.cancer.gov/pdc/cptac-pancancer |
| https://dataservice.datacommons.cancer.gov/#/ | ||
| Full proteomic data tables | Li et al.19 | https://pdc.cancer.gov/pdc/cptac-pancancer |
| Harmonized acetylation and phosphorylation data | Geffen et al.20 | https://pdc.cancer.gov/pdc/cptac-pancancer |
| HipSci proteomic, genomic data | Kilpinen et al.92 | https://www.hipsci.org/ |
| PROTsi validation dataset - Intrahepatic cholangiocarcinoma (ICC) - 101 tumor samples and 76 normal tissue samples | Cho et al.93 | https://pdc.cancer.gov/pdc/study/PDC000356 |
| PROTsi validation dataset - Lung adenocarcinoma (LUAD) - 89 tumor samples | Chen et al.94 |
https://www.sciencedirect.com/science/article/pii/S0092867420307431?via%3Dihub https://pdc.cancer.gov/pdc/study/PDC000219 |
| PROTsi validation dataset - Acute myeloid leukemia (AML) - 177 tumor samples | Jayavelu et al.95 |
https://www.sciencedirect.com/science/article/pii/S1535610822000587?via%3Dihub#da0010 https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD023201 |
| PROTsi validation dataset - Triple Negative Breast Cancer (TNBC) - 60 tumor samples | Anurag et al.96 |
https://aacrjournals.org/cancerdiscovery/article/12/11/2586/709984/Proteogenomic-Markers-of-Chemotherapy-Resistance https://pdc.cancer.gov/pdc/study/PDC000408 |
| PROTsi validation dataset - Pancreatic ductal adenocarcinoma (PDA) - 153 tumor samples | Hyeon et al.97 | https://proteomic.datacommons.cancer.gov/pdc/study/PDC000248 |
| PROTsi validation dataset - human foreskin fibroblasts (hFF), colon cancer RKO cell line (RKO), embryoid bodies (EB), embryonic stem cells (ESC), induced pluripotent stem cells (iPSC) | Sabatier et al.98 |
https://www.ebi.ac.uk/pride/archive/projects/PXD015874 https://www.ebi.ac.uk/pride/archive/projects/PXD014830 https://www.ebi.ac.uk/pride/archive/projects/PXD018453 |
| Antibodies | ||
| Rabbit polyclonal anti-HEATR1 | Thermo Fisher Scientific | Cat# bs-15438R, RRID:AB_2934056 |
| Rabbit polyclonal anti-UCHL1 | Atlas Antibodies | Cat# HPA005993, RRID:AB_1858560 |
| Rabbit polyclonal anti-UHRF1 | Atlas Antibodies | Cat# HPA049408, RRID:AB_2756317 |
| Rabbit polyclonal anti-SNRNP200 | Atlas Antibodies | Cat# HPA029321, RRID:AB_10604096 |
| Rabbit polyclonal anti-SLC27A2 | Atlas Antibodies | Cat# HPA026089, RRID:AB_1857060 |
| Biological Samples | ||
| Primary tumor and normal tissue samples | See Methods: Experimental Model and Subject Details | N/A |
| Chemicals, Peptides, and Recombinant Proteins | N/A | |
| Critical Commercial Assays | ||
| EnVision FLEX visualizing kit | Agilent Technologies Inc | Cat# K800221-2 |
| Software and Algorithms | ||
| CMap | Lamb et al.99 Subramanian et al.99,100 | https://clue.io |
| Cytoscape | Shannon et al.40 | https://cytoscape.org/ |
| KEA3 | Kuleshov et al.101 | https://appyters.maayanlab.cloud/#/KEA3_Consensus_Kinases |
| STRING | Szklarczyk et al.41 | https://string-db.org |
| Enrichr | Xie et al.102 | https://maayanlab.cloud/Enrichr/ |
| Target LinkedOmics | Savage et al.23 | https://targets.linkedomics.org/ |
Experimental model and subject details
Human subjects
In this pan-cancer study, we used proteogenomic and clinical data obtained from a total of 1325 participants, including 1314 patients with 11 histopathologically-defined cancer types eligible in the Clinical Proteomic Tumor Analysis Consortium (CPTAC). Among these 1314 participants, there were following tumor samples collected for the study: 113 with breast cancer (BR),16 110 with clear cell renal cell carcinoma (CCRCC),103 106 with colon cancer (CO),104 99 with glioblastoma (GBM),105 109 with head and neck squamous cell carcinoma (HNSCC),106 110 with lung squamous cell carcinoma (LSCC),107 112 with lung adenocarcinoma (LUAD),17 92 with ovarian cancer (OV),108 145 with prostate adenocarcinoma (PDA)109 and 100 with uterine corpus endometrial carcinoma (UCEC).110
Institutional review boards at tissue source sites, reviewed protocols and consent documentation adhering to the CPTAC guidelines. We also included 218 samples of pediatric brain tumors (PBT), a group of childhood brain tumors consisting of atypical teratoid rhabdoid tumor (ATRT, n = 12), craniopharyngioma (n = 16), ependymoma (n = 32), ganglioglioma (n = 18), low-grade glioma (n = 93), high-grade glioma (n = 25) and medulloblastoma (n = 22). PBT samples were obtained from the Children’s Brain Tumor Network (CBTN) at the Children’s Hospital of Philadelphia (CHOP).111
All specimens were obtained from patients during routine tumor resections with the informed consent of the patients and in accordance with CPTAC guidelines and standards.
ln total, 2287 samples across 11 tumor types were available, including 1314 primary tumor tissues and a set of 973 non-tumor tissues (Table S1A).
Clinical data annotation
Clinical and molecular data of all CPTAC samples were collected from the tissue source sites and gathered by the Biospecimen Core Resource (BCR, Van Andel Research Institute, Grand Rapids, MI) in the internal CPTAC database called Comprehensive Data Resource (CDR) synchronized with the CPTAC Data Coordinating Center (DCC). Clinical and molecular data can be accessed and downloaded from the NCI’s Proteomic Data Commons (PDC) at https://proteomic.datacommons.cancer.gov. Summary table including clinical and molecular data of all samples used in the study is provided in Table S1A.
Specimen acquisition
The tumor, normal adjacent tissue (NAT), and whole blood samples derived from patients diagnosed with BR, CCRCC, CO, GBM, HNSCC, LSCC, LUAD, OV, PDA and UCEC, used in this study were collected for the CPTAC project.
PBT and whole blood samples were collected at CHOP, according to the process described in Petralia et al. 2020.111
Tumor and NAT material were derived from resection, and all patients had no prior treatment for the disease, including chemo- or radiotherapy. All cases used in the study met the histology acceptance criteria. Detailed information regarding the sample processing can be found in particular flagship publications and CPTAC pan-cancer resource paper.19
Method details
All CPTAC molecular data were downloaded from NCI’s Proteomic Data Commons (PDC) at https://proteomic.datacommons.cancer.gov.
DNA methylation data
A total of 755 PanCancer CPTAC samples across 7 different tumor types were available for DNA methylation, and downloaded from CPTAC DCC and GDC. DNA methylation data was obtained using the robust Illumina Infinium Methylation EPIC BeadChip profiling microarray. The MethylationEPIC array uses an 8-sample version of the Illumina Beadchip capturing >850,000 methylation sites per sample. The EPIC array includes sample plating, bisulfite conversion, and methylation array processing. After scanning, the data was processed through an automated genotype calling pipeline. Data generated consisted of raw idats and a sample sheet as described in Li et al.19
Somatic CNV data
Processing of CNV expression data for each tumor type available has been described in Li et al.19
RNA expression data
Pan-cancer RNA expression data have been processed as described in Li et al.,19 and downloaded from the GDC. We obtained a total of 1612 samples and 34657 transcripts. To apply the stemness model based on pluripotent stem cells (iPSCs) published in 2018, we used 12,265 genes that were common between the gene expression data and the model.
miRNA expression data
Pan-cancer miRNA expression data available for 7 tumor types have been processed as described in Li et al.,19 and downloaded from the GDC. To refine the miRNA expression data across various tumor subtypes, we applied a quantile-based filtering method using the TCGAanalyze_Filtering function. This process retained the top 25% of miRNAs with the highest expression levels, ensuring the focus on highly expressed miRNAs for subsequent analyses. To investigate the correlation between the expression of miRNAs and genes, we considered miRNA and gene interaction provided by miRDB version 6.0 (http://mirdb.org/).112 We considered miRNA-gene pairs where the interaction has been predicted by miRDB database with a score more than 50. For each miRNA, we calculated the Spearman correlation with each target gene. We only considered correlations with p.value < 0.05, and then selected for each gene the miRNA associated with the highest negative correlation, considering an expression inhibition interaction between the selected miRNAs and genes. Although there may be more than a single negatively associated miRNA for each gene, in this analysis we show the one with the highest negative correlation, as being the most promising target for a drug or treatment. These procedures allowed for a systematic analysis of potential interactions between miRNAs and genes, providing a basis for identifying miRNAs that may be associated with the regulation of specific genes.
Protein expression data
Pan-cancer protein expression data have been processed as described in Li et al.,19 and downloaded from the PDC. We obtained a total of 1919 samples and 12930 proteins. A total of 1919 samples across 11 tumor types were available, including primary tumor tissues (1306) and a set of 613 non-tumor tissues (Table S1A).
The iPSCs proteomic data were acquired from the Human Induced Pluripotent Stem Cell Initiative (HipSci) that generated iPSCs from skin biopsies derived from healthy volunteers using a standardized experimental pipeline. One of the unique aspects of the HipSci is its emphasis on obtaining cells from a genetically diverse set of donors. This diversity is crucial for capturing the genetic variations present in the human population, as different individuals may have distinct susceptibilities to diseases or respond differently to treatments.92
For PROTsi validation, we also downloaded the protein expression data from the following studies: Cho et al. 2023 (76 normal and 101 intrahepatic cholangiocarcinoma samples),93 Chen et al. 2020 (89 lung adenocarcinoma samples),94 Jayavelu et al. 2022 (177 acute myeloid leukemia samples),95,96 Anurag et al. 2022 (60 triple negative breast cancer samples),96 Hyeon et al. 2023 (153 pancreatic ductal adenocarcinoma samples)97 and Sabatier et al. 2021 (human foreskin fibroblasts (hFF), colon cancer RKO cell line (RKO), embryoid bodies (EB), embryonic stem cells (ESC), induced pluripotent stem cells (iPSC))98 (Table S1D). The proteomics datasets used for external model validation were first log-transformed, and then normalized by centering each protein’s expression (the mean expression across all samples was calculated for each protein, and individual sample values were adjusted by subtracting the mean). When integrating multiple datasets, batch effects were corrected using the ComBat function from the sva package.
Protein phosphorylation expression data
Harmonized protein phosphorylation expression data for seven CPTAC tumor types—CCRCC, HNSCC, GBM, LSCC, LUAD, PDA, and UCEC—were processed by Geffen et al.20 and accessed through the CPTAC Pan-Cancer Proteomic Data Commons platform (https://pdc.cancer.gov/pdc/cptac-pancancer). The phosphorylation dataset included details on specific phosphoproteins, as well as peptides and phosphosites. This harmonized dataset enabled consistent and cross-tumor analysis of phosphorylation patterns, facilitating insights into phosphorylation dynamics across the selected tumor types.
Protein acetylation data
Protein acetylation data, covering five CPTAC tumor types—BRCA, GBM, LSCC, LUAD, and UCEC—were also harmonized and processed by Geffen et al.,20 with access provided through the CPTAC Pan-Cancer Proteomic Data Commons platform (https://pdc.cancer.gov/pdc/cptac-pancancer). This dataset provided multi-site acetylation data, identifying modified proteins and their lysine acetylation sites. The harmonized acetylation data supported cross-tumor comparisons, enabling a comprehensive examination of acetylation-associated regulatory mechanisms in the selected cancer types.
Protein glycosylation data
Protein glycosylation data have been processed as described in Li et al.,19 and downloaded from the PDC for each CPTAC tumor separately. Glycosylation data were available for 7 CPTAC tumor types: CCRCC, HNSCC, GBM, LSCC, LUAD, PDA and UCEC. The protein glycosylation data consisted of N-glycosylated proteins, their N-glycosylated sites, and the glycans occupying these sites.
Annotation of the identified stemness-associated proteins to their functional families
To identify the functional families of stemness-associated genes and proteins, we utilized the TCGAbiolinks' EAGenes annotation.113,114,115 EAGenes comprises a table containing information about genes, including ID, Gene, Description, Location, and Family. The names of genes encoding a total of 700 stemness-associated proteins across various cancer types, such as CCRCC, HNSCC, GBM, LSCC, LUAD, PDA, and UCEC, were identified. Subsequently, the functional annotations of these proteins were integrated with heatmaps for a comprehensive analysis. The functional classes of stemness-associated proteins included: cytokines, enzymes, G protein-coupled receptors, growth factors, ion channel, kinases, ligand-dependent nuclear receptors, peptidases, phosphatases, transcription regulators, translation regulators, transmembrane receptors and transporters. These annotations can be also found in Table S3A.
In silico validation of stemness-associated biomarkers
To perform thorough validation of stemness-associated protein markers identified using PROTsi, we leveraged external protein expression datasets of pancreatic ductal adenocarcinoma (PDA)97 and lung adenocarcinoma (LUAD).94 PDA dataset also included survival data. First, we obtained the proteomic profiles from PDA and LUAD, and then we applied PROTsi to identify common biomarkers between external PDA and LUAD cohorts and CPTAC PDA and LUAD cohorts. Additionally, using survival data available for external PDA dataset, we assessed the prognostic significance of proteins identified as stemness-associated biomarkers in CPTAC PDA cohort. Survival analysis was performed focusing on proteins showing significant associations with worse OS in both external PDA and CPTAC PDA cohorts. For these proteins hazard ratios (HR) along with 95% confidence intervals (CI) were computed, and statistical significance was determined as p < 0.05. PROTsi values for each sample in external PDA and LUAD cohorts, correlation values for proteins in external PDA and LUAD cohorts, and PDA HR, CI and p values were provided in Table S4.
Verification of stemness-associated proteins in a database of currently existing protein drug targets
The Open Targets platform was used to verify whether and which proteins here identified as stemness-associated belong to currently existing protein drug targets for neoplasms. Open Targets provides a comprehensive tool that enables the systematic identification of therapeutic drug targets.116 Using Open Targets, annotations for the 700 proteins most associated with stemness identified for CCRCC, HNSCC, GBM, LSCC, LUAD, PDA and UCEC were downloaded and a list containing protein-drug target interaction data was retrieved. This way, corresponding annotations were obtained as to whether the stemness-associated proteins analyzed are currently identified as targets for drugs in neoplasms. To corroborate and expand the information on drug targets we also accessed the drug tier compilation published in a study by Savage et al.,23 which integrates information from DrugBank, Guide to Pharmacology (GtoPdb), the Drug Gene Interaction Database, and the in silico human surfaceome. The five target tiers are represented by targets approved for oncology (Tier 1), targets approved for non-cancer treatment (Tier 2), investigational or experimental targets (Tier 3), potentially druggable targets (Tier 4), and cell surface membrane proteins (Tier 5). These annotations were included in the heatmaps generated for the analyzed cancer types and included in Table S3A.
Immunohistochemistry
To validate our findings, we performed immunohistochemistry (IHC) assay using specific antibodies against identified proteins associated with stemness. IHC validation was performed in CCRCC FFPE specimens derived from patients who underwent primary tumor surgery at Józef Struś Multidisciplinary Municipal Hospital in Poznań, Poland. The CCRCC validation cohort consisted of 52 CCRCC cases, including 26 patients who developed metastasis within 5 years from primary tumor surgery, and 26 patients with no relapse. Out of a total 52 cases, 36 cases were enrolled in CPTAC and belonged to CPTAC CCRCC discovery cohort.103 The CCRCC validation cohort clinical data are available in Table S8A.
IHC was performed at a single-site IHC facility using 4-micron FFPE tissue sections. Each IHC run was carried out using a Dako Autostainer Link 48 with the preprogrammed staining protocols and the Dako EnVision visualizing kit. The following antibodies were used in the CCRCC validation cohort: anti-HEATR1 (ThermoFisher, polyclonal, 1:200), anti-SLC27A2 (Atlas Antibodies, polyclonal, 1:500), anti-SNRNP200 (Atlas Antibodies, polyclonal, 1:225), anti-UCHL1 (Atlas Antibodies, polyclonal, 1:3000) and anti-UHRF1 (Atlas Antibodies, polyclonal, 1:2000). Appropriate known positive and negative controls were run for each antibody separately. IHC images were digitally scanned with an automated ScanScope AT Turbo whole slide scanner (Aperio/Leica Microsystems, Vista, CA) using 40X magnification. Digital images were then saved as.svs files recommended for ImageScope IHC slides viewing software (Aperio, Vista, CA) enabling for high quality and expert pathologist review of the IHC images. The digital images were accessed through password-protected Synology Rack Station server (RS18017xs+). IHC slides were manually evaluated by two expert pathologists, and a third pathologist provided tie-breaking independent scoring in case of any discordant noting. The pathologists used well-established H-score methodology for IHC score evaluation, which is based on the value of extent of expression in the tumor cells in percentages (0–100%), and multiplied by the intensity of expression: 0 (negative), 1+ (low), 2+ (moderate) and 3+ (high), resulting in a total score of 0–300.117 The IHC scores for each protein marker analyzed are provided in Table S8B.
Quantification and statistical analyses
Stemness indices derived using OCLR
To calculate a stemness index (si) based on protein expression, we built a predictive model using one-class logistic regression (OCLR)118 algorithm on the human induced pluripotent stem cell samples (iPSCs) from the Human Induced Pluripotent Stem Cell Initiative (HipSci, https://www.hipsci.org/) dataset.92
To ensure compatibility with the CPTAC PanCancer cohort, we considered 7702 proteins with protein expression values obtained in common with the 207 HipSci iPS samples (Table S1C). Then, we applied the OCLR to 207 HipSci iPS cells and the protein-based prediction model was applied to score new samples, generating the protein-based stemness index (PROTsi). We computed Spearman correlations between the model’s weight vector (Table S1B) and the new sample’s expression profile according to Malta et al.4 The mRNA-based stemness index (mRNAsi.2018) was computed as described by Malta et al..4
We also performed an external validation by integrating different available proteomics datasets, as detailed in the key resources table and Table S1D. Among the groups of this validation were samples of normal cells, tumor cells, and stem and tumor cell lines. The results of this external validation align well with previous validation and the biological expectations of the model. The lower indices observed for normal samples compared to tumor samples highlight the model’s ability to distinguish between these states, especially considering that induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) consistently showed high indices. Although we have no evidence of difference between iPSCs and the other groups, likely due to the small sample size available for iPSCs, the indices for iPSCs were the highest among all samples, further supporting the robustness of the model in identifying stemness characteristics.
Having validated the signatures by using cross-validation and external SC data, we then applied the signature to score the CPTAC PanCancer cohort. The indices were subsequently mapped to the [0,1] range by using a linear transformation that subtracted the minimum and divided by the maximum. The mapping was done to assist with interpretation as well as integration with the stemness indices derived from different data platforms. Moreover, we considered stemness indices as continuous values, thus allowing us to identify proteins, multi-omic regulations, and post-translational modifications associated with stemness. Intentionally, we did not define a specific cutoff for low and high stemness across all tumors. Establishing a fixed threshold for stemness classification could hold clinical significance for diagnostic and prognostic purposes, however here we are working in a pan-cancer context, where stemness characteristics vary across different tumor types, making it biologically challenging to set a universal cutoff. Thus, our focus was on developing a tool that can be used to uncover novel molecular aspects of stemness, which can serve in future research for determining clinically relevant cut-off values for stemness classification.
Correlation of protein expression with PROTsi
To perform the Spearman correlation test, only entities (genes) in common in the mRNA, DNA methylation (DNAm) and CNV data with the protein expression data were selected. Spearman correlation was then calculated between all proteins in the protein expression matrix and PROTsi, using the cor.test function from the stats package (Table S2). Afterward, the correlation values were sorted firstly in the decreasing order to select the top 50 positively correlated proteins with PROTsi, and then in increasing order to select the top 50 negatively correlated proteins with PROTsi. Thus, we obtained a list of 100 stemness-associated protein targets for each analyzed CPTAC tumor type (Table S3A). We also provided a list of all proteins significantly associated with PROTsi in Table S2.
Identification of post-translational modifications (PTMs) associated with stemness
Our investigation delved into the intricate correlation between stemness and diverse protein post-translational modifications (PTMs), specifically acetylation, glycosylation, and phosphorylation, across a spectrum CPTAC tumor types, using the available data outlined above. Our analytical approach commenced with the comprehensive identification of shared and unique modification sites for each PTM type across CPTAC tumors (Table S5). To visually elucidate these findings, we employed UpSet plots, providing a clear depiction of the intersecting sets of modified sites that exhibited both positive and negative correlations with stemness. Building upon this foundation, we further focused on the most influential players in this correlation by identifying the top 20 modified proteins associated with acetylation, glycosylation, and phosphorylation. These proteins were carefully scrutinized for their positive and negative correlations with stemness, revealing key molecular entities driving or inhibiting stemness across the tumor types. To enhance the clarity of these associations, chord diagrams were employed, presenting an aesthetically informative visualization of the intricate relationships between stemness and the identified modified proteins. This comprehensive and systematic analysis not only uncovers the nuanced interplay between stemness and PTMs but also highlights specific proteins that play pivotal roles in shaping the molecular landscape of cancer across diverse CPTAC tumor types.
Survival analysis for identified stemness-associated proteins
The proportional hazard model with the PROTsi as a single continuous covariate was used to test whether there was a statistically significant effect on OS or PFS after adjusting for age, sex, and tumor purity (clinical variables that are relevant to the prognosis across all tumors).
In addition, we computed proportional hazard model for each of identified 100 protein targets (Table S3A) and also for all proteins significantly associated with PROTsi (Table S3B) based on protein expression and Overall Survival (OS) and Progression-free Survival (PFS), and reported the HR values and log rank test p-values for OS and PFS. These analyses were next incorporated into heatmaps for each CPTAC tumor (Figure 2 and S2A–S2G). Clinical data along with survival data (OS, PFS) used for the analysis are provided in Table S1A.
Kinase activity score derivation and association with PROTsi
To discover the most activated and deactivated protein kinases associated with stemness in analyzed tumors, we integrated kinase activity score with protein-expression-based stemness index (PROTsi) (Table S6). Kinase activity scores were obtained from phosphoproteomic data available for all tumor samples. Firstly, we derived phosphosite abundance z-scores for all tumor samples per each tumor type. Based on the z-scores, for each sample, we identified the top 500 proteins with the highest phosphosite z-scores, referred to as top 500 phosphoprotein sets for each tumor. Similarly, we identified the bottom 500 proteins with the lowest phosphosite z-scores for each tumor sample. Subsequently, we performed kinase enrichment analysis using KEA3.101 The phosphoprotein sets were inputted into the KEA3 Consensus Kinases Appyter (https://appyters.maayanlab.cloud/#/KEA3_Consensus_Kinases) to derive kinase activity ranks. As a result, we obtained the KEA3 mean rank scores for 519 kinases in each of the KEA3 runs. The lower rank scores indicate higher levels of kinase activity enrichment among the 500 protein sets, consequently suggesting increased kinase activation in the tumor samples. We further normalized kea3 ranks using the same procedure from a previous pan cancer study by Petralia et al..119 Means and standard deviations of each kinase were adjusted by a null distribution estimated from the kea3 ranks of 5000 random 500-protein-lists. Those random protein lists were sampled independently from 8639 proteins represented in the Pan Cancer phosphoproteomics at multi-site level. We acquired reversed z-scores of a kinase after the transformation:
| Normalized Rank Score = – (Observed Rank – μ0) / SD0 |
where μ0 and SD0 are the mean and standard deviation of the sampled null distribution of the same kinase. After the transformation, the higher scores represent higher kinase activities.
To assess the association between kinase activities and PROTsi, we tested the correlation of each kinase with stemness score within each cancer type. Significance of the correlation was determined by the FDR cutoff at 0.05 adjusted across all kinases for a specific cancer type. We further selected significant kinases with consistent pan-cancer correlation for visualization: having the same direction of correlation across 10 tumor types and significant in at least 8 tumor types. Moreover, to each kinase identified we have assigned specific kinase family as follows: AGC family - group of more than 60 kinases related to cAMP-dependent protein kinase 1 (PKA), cGMP-dependent protein kinase (PKG) and protein kinase C (PKC); Atypical family - group of kinases that do not share structural similarity with eukaryotic protein kinases (ePKs) but still exhibit kinase activity; CAMK family - calcium/calmodulin-dependent kinases; CK1 family - casein/cell kinase 1 involved in various cellular processes; CMGC family - group of kinases belonging to main kinase families: cyclin-dependent kinases (CDKs), mitogen-activated protein kinases (MAPKs), glycogen synthase kinases (GSKs) and CDC-like kinases (CLKs); Other family - group of kinases that are ePKs but do not fall into any other defined family; STE family - homologs of the yeast sterile kinases STE7, STE11 and STE20, which are part of MAPK signaling cascades; TK family - tyrosine kinases which phosphorylate tyrosine residues on proteins, playing key role in signaling pathways; TKL family - tyrosine kinase-like kinases which have structural similarities to TKs but typically phosphorylate serine or threonine residues.
Protein-protein interactions among identified stemness-associated proteins
The protein-protein interactions among the identified 100 proteins associated with cancer stemness in analyzed tumors were investigated using Cytoscape, a bioinformatics software for network visualization and analysis.40 To construct the protein-protein interaction network, the STRING database was leveraged, which houses comprehensive and experimentally validated protein interaction data.41 The 100 proteins per tumor type (Table S3A) were inputted into the Cytoscape software, and the STRING app, an integrated plugin within Cytoscape, was employed to access the STRING database and retrieve the relevant protein interaction information. The retrieved data included both physical and functional interactions between the proteins, enabling the creation of an interconnected network representation. To enhance the visualization and interpretation of the network, various layout algorithms and visual styles were applied within Cytoscape, and proteins that did not show any interactions were not shown in the figure.
Biological pathways associated with stemness
We performed pathway analysis on a set of previously identified stemness-associated proteins to provide valuable insights into their functional roles and involvement in various biological processes. For each tumor type, we put 100 stemness-associated protein names into Enrichr web-based analytical tool. Enrichr is a web-based tool that allows to input a list of genes/proteins and perform enrichment analysis against a large collection of gene set libraries, including various pathway databases like KEGG, Reactome, or Gene Ontology (GO). It provides results in an easy-to-interpret format and offers interactive visualizations for further exploration.102 The analysis results from Enrichr provided a list of enriched Reactome120 pathways (Table S7B), highlighting the significant biological processes in which the 100 proteins are involved. Leveraging the results from Enrichr we showed the top 10 enriched Reactome biological pathways as dot charts, and shared Reactome pathways across tumors, along with their corresponding p-values (<0.05) and FDR values.
Drug connectivity analysis for CPTAC tumors
To identify candidate compounds that might repress stemness, as well as their mechanisms of action (MoA) and specific targets of the identified compounds, we performed connectivity analysis using recently updated web-based tool - Connectivity Map (CMap, https://clue.io). CMap is a large-scale collection of about 1.3 million gene expression profiles obtained from cell lines that were treated with bioactive small molecules and genetic modifications (drug perturbagens). The aim of CMap development was to enable data-driven studies on drug repositioning and drug mode-of-action.99,100 To perform drug connectivity analysis, we uploaded into a “query” module of CMap a list of top 50 up- and downregulated proteins previously identified as the most associated with stemness (positively and negatively, respectively) for each CPTAC tumor type separately. The results file contained the compounds with connectivity scores ranging from −100 to 100, representing the functional connection between the uploaded protein signatures and the compounds collected in the CMap. As negative connectivity scores indicate the therapeutic potential of a given drug molecule, we selected a set of candidate compounds with enrichment scores below −97. For each tumor type, we limited the list of candidate compounds to 20 compounds with the lowest enrichment scores, obtaining a selection of compounds that may repress the stemness phenotype in analyzed tumors (Table S7A). All identified compounds were either shared among several tumor types or exclusive for one tumor type. Subsequently, we explored specific targets for each identified drug compound. The targets were also either shared among a few drug compounds or exclusive for a single drug compound. Additionally, based on the CMap we also explored the mechanisms of action (MoA) of the identified potential stemness inhibitors for analyzed tumors.
Predictive value of selected stemness-associated proteins validated by immunohistochemistry (IHC)
To check the association between protein expression level measured by IHC and patients’ Progression-free Survival (PFS) (Table S8A), we performed Kaplan-Meier survival analysis. For each stemness-associated protein validated by IHC, we ranked the H-score values (Table S8B) obtained for all samples in descending order, and divided the samples into three groups. The group with top ⅓ H-score values was considered as the High IHC score group, while the group with bottom ⅓ H-score values was assigned to the Low IHC score group. Additionally, we reported hazard ratio (HR) and 95% confidence interval (95% CI) for each survival analysis considering validated stemness-associated proteins using log -rank method. To ensure consistency in risk assessment across all markers, we define HR > 1 as indicating an increased risk of cancer progression, regardless of whether the at-risk group corresponds to high or low protein expression. This approach aligns with the biological interpretation of stemness-associated proteins while maintaining clarity in statistical comparisons. p < 0.05 was considered as statistically significant.
Published: April 17, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xgen.2025.100851.
Contributor Information
Tathiane M. Malta, Email: tathimalta@usp.br.
Maciej Wiznerowicz, Email: maciej.wiznerowicz@iimo.pl.
Clinical Proteomic Tumor Analysis Consortium:
Alicia Francis, Amanda G. Paulovich, Anna P. Calinawan, Antonio Iavarone, Arul M. Chinnaiyan, Bo Wen, Boris Reva, Brian J. Druker, Caleb M. Lindgren, Chandan Kumar-Sinha, Chelsea J. Newton, Chen Huang, Chet Birger, Corbin Day, D.R. Mani, Daniel Cui Zhou, Daniel W. Chan, David Fenyö, David I. Heiman, Dmitry Rykunov, Emily Huntsman, Eric E. Schadt, Eric J. Jaehnig, Eunkyung An, Fernanda Martins Rodrigues, François Aguet, Gad Getz, Galen Hostetter, Gilbert S. Omenn, Hanbyul Cho, Hui Zhang, Jared L. Johnson, Jasmin Bavarva, Jiayi Ji, Jimin Tan, Jonathan T. Lei, Joshua M. Wang, Karen A. Ketchum, Karin D. Rodland, Karl R. Clauser, Karsten Krug, Kelly V. Ruggles, Lewis C. Cantley, Liang-Bo Wang, Lijun Yao, Lizabeth Katsnelson, Marcin J. Domagalski, Marcin P. Cieslik, Mathangi Thiagarajan, Matthew A. Wyczalkowski, Matthew J. Ellis, Meenakshi Anurag, Michael A. Gillette, Michael J. Birrer, Michael Schnaubelt, Myvizhi Esai Selvan, Nadezhda V. Terekhanova, Nathan Edwards, Nicole Tignor, Özgün Babur, Qing Zhang, Ratna R. Thangudu, Richard D. Smith, Robert Oldroyd, Runyu Hong, Samuel H. Payne, Sara J.C. Gosline, Sara R. Savage, Saravana M. Dhanasekaran, Scott D. Jewell, Shankara Anand, Shankha Satpathy, Shrabanti Chowdhury, Song Cao, Stephan Schürer, Steven A. Carr, Steven M. Foltz, Tania J. Gonzalez Robles, Tao Liu, Tobias Schraink, Tomer M. Yaron, Vasileios Stathias, Wen Jiang, Wen-Wei Liang, Wenke Liu, Wilson McKerrow, Xiaoyu Song, Xinpei Yi, Xu Zhang, Yifat Geffen, Yige Wu, Ying Wang, Yingwei Hu, Yize Li, Yizhe Song, Yo Akiyama, Yongchao Dou, Yuxing Liao, Zeynep H. Gümüş, Zhen Zhang, and Zhiao Shi
Supplemental information
Table S1A: Clinical data of patient samples
Table S1B: Protein weights (prediction model)
Table S1C: List of iPS sample IDs using to train the model
Table S1D: List of sample IDs and PROTsi of the validation dataset
Table S1E: Results of OS and PFS based on the stemness index (Related to Figures S1F and S1G)
Table S3A: Stemness-associated proteins for each tumor type along with related multi-omic and characterization data
Table S3B: Stemness-associated protein biomarkers with significant prognostic value, but whose gene expression is not associated with survival, for each tumor type
References
- 1.Hanahan D. Hallmarks of cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 2.Warrier N.M., Kelkar N., Johnson C.T., Govindarajan T., Prabhu V., Kumar P. Understanding cancer stem cells and plasticity: Towards better therapeutics. Eur. J. Cell Biol. 2023;102 doi: 10.1016/j.ejcb.2023.151321. [DOI] [PubMed] [Google Scholar]
- 3.Shi Z.-D., Pang K., Wu Z.-X., Dong Y., Hao L., Qin J.-X., Wang W., Chen Z.-S., Han C.-H. Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies. Signal Transduct. Target. Ther. 2023;8:113. doi: 10.1038/s41392-023-01383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malta T.M., Sokolov A., Gentles A.J., Burzykowski T., Poisson L., Weinstein J.N., Kamińska B., Huelsken J., Omberg L., Gevaert O., et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell. 2018;173:338–354.e15. doi: 10.1016/j.cell.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng S.W.K., Mitchell A., Kennedy J.A., Chen W.C., McLeod J., Ibrahimova N., Arruda A., Popescu A., Gupta V., Schimmer A.D., et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–437. doi: 10.1038/nature20598. [DOI] [PubMed] [Google Scholar]
- 6.Smith B.A., Balanis N.G., Nanjundiah A., Sheu K.M., Tsai B.L., Zhang Q., Park J.W., Thompson M., Huang J., Witte O.N., Graeber T.G. A Human Adult Stem Cell Signature Marks Aggressive Variants across Epithelial Cancers. Cell Rep. 2018;24:3353–3366.e5. doi: 10.1016/j.celrep.2018.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miranda A., Hamilton P.T., Zhang A.W., Pattnaik S., Becht E., Mezheyeuski A., Bruun J., Micke P., de Reynies A., Nelson B.H. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. USA. 2019;116:9020–9029. doi: 10.1073/pnas.1818210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dezem F.S., Marção M., Ben-Cheikh B., Nikulina N., Omotoso A., Burnett D., Coelho P., Hurley J., Gomez C., Phan-Everson T., et al. A machine learning one-class logistic regression model to predict stemness for single cell transcriptomics and spatial omics. BMC Genom. 2023;24:717. doi: 10.1186/s12864-023-09722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C., Hou J., Tanner J.J., Cheng J. Bioinformatics Methods for Mass Spectrometry-Based Proteomics Data Analysis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meissner F., Geddes-McAlister J., Mann M., Bantscheff M. The emerging role of mass spectrometry-based proteomics in drug discovery. Nat. Rev. Drug Discov. 2022;21:637–654. doi: 10.1038/s41573-022-00409-3. [DOI] [PubMed] [Google Scholar]
- 11.Christopher J.A., Stadler C., Martin C.E., Morgenstern M., Pan Y., Betsinger C.N., Rattray D.G., Mahdessian D., Gingras A.-C., Warscheid B., et al. Subcellular proteomics. Nat. Rev. Methods Primers. 2021;1 doi: 10.1038/s43586-021-00029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller J.B., Geyer P.E., Colaço A.R., Treit P.V., Strauss M.T., Oroshi M., Doll S., Virreira Winter S., Bader J.M., Köhler N., et al. The proteome landscape of the kingdoms of life. Nature. 2020;582:592–596. doi: 10.1038/s41586-020-2402-x. [DOI] [PubMed] [Google Scholar]
- 13.Hanna-Sawires R.G., Schiphuis J.H., Wuhrer M., Vasen H.F.A., van Leerdam M.E., Bonsing B.A., Mesker W.E., van der Burgt Y.E.M., Tollenaar R.A.E.M. Clinical perspective on proteomic and glycomic biomarkers for diagnosis, prognosis, and prediction of pancreatic cancer. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22052655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benns H.J., Wincott C.J., Tate E.W., Child M.A. Activity- and reactivity-based proteomics: Recent technological advances and applications in drug discovery. Curr. Opin. Chem. Biol. 2021;60:20–29. doi: 10.1016/j.cbpa.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y., Sun A., Zhao Y., Ying W., Sun H., Yang X., Xing B., Sun W., Ren L., Hu B., et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257–261. doi: 10.1038/s41586-019-0987-8. [DOI] [PubMed] [Google Scholar]
- 16.Krug K., Jaehnig E.J., Satpathy S., Blumenberg L., Karpova A., Anurag M., Miles G., Mertins P., Geffen Y., Tang L.C., et al. Proteogenomic landscape of breast cancer tumorigenesis and targeted therapy. Cell. 2020;183:1436–1456.e31. doi: 10.1016/j.cell.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillette M.A., Satpathy S., Cao S., Dhanasekaran S.M., Vasaikar S.V., Krug K., Petralia F., Li Y., Liang W.-W., Reva B., et al. Proteogenomic characterization reveals therapeutic vulnerabilities in lung adenocarcinoma. Cell. 2020;182:200–225.e35. doi: 10.1016/j.cell.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi S.K., Nechiporuk T., Bottomly D., Piehowski P.D., Reisz J.A., Pittsenbarger J., Kaempf A., Gosline S.J.C., Wang Y.-T., Hansen J.R., et al. The AML microenvironment catalyzes a stepwise evolution to gilteritinib resistance. Cancer Cell. 2021;39:999–1014.e8. doi: 10.1016/j.ccell.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Dou Y., Da Veiga Leprevost F., Geffen Y., Calinawan A.P., Aguet F., Akiyama Y., Anand S., Birger C., Cao S., et al. Proteogenomic data and resources for pan-cancer analysis. Cancer Cell. 2023;41:1397–1406. doi: 10.1016/j.ccell.2023.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geffen Y., Anand S., Akiyama Y., Yaron T.M., Song Y., Johnson J.L., Govindan A., Babur Ö., Li Y., Huntsman E., et al. Pan-cancer analysis of post-translational modifications reveals shared patterns of protein regulation. Cell. 2023;186:3945–3967.e26. doi: 10.1016/j.cell.2023.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedele M., Cerchia L., Chiappetta G. The Epithelial-to-Mesenchymal Transition in Breast Cancer: Focus on Basal-Like Carcinomas. Cancers. 2017;9 doi: 10.3390/cancers9100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo G., Zhuang J., Zhang K., Zhou Z., Wang Y., Zhang Z. Atypical teratoid/rhabdoid tumor of the central nervous system in children: case reports and literature review. Front. Surg. 2022;9 doi: 10.3389/fsurg.2022.864518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage S.R., Yi X., Lei J.T., Wen B., Zhao H., Liao Y., Jaehnig E.J., Somes L.K., Shafer P.W., Lee T.D., et al. Pan-cancer proteogenomics expands the landscape of therapeutic targets. Cell. 2024;187:4389–4407.e15. doi: 10.1016/j.cell.2024.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan G., Walther D. The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput. Biol. 2015;11 doi: 10.1371/journal.pcbi.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutta H., Jain N. Post-translational modifications and their implications in cancer. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1240115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X., Zhou F., Zhang L. PARP1-DNA co-condensation: the driver of broken DNA repair. Signal Transduct. Target. Ther. 2024;9:135. doi: 10.1038/s41392-024-01832-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poüs C., Klipfel L., Baillet A. Cancer-Related Functions and Subcellular Localizations of Septins. Front. Cell Dev. Biol. 2016;4:126. doi: 10.3389/fcell.2016.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia W., Yao Z., Zhao J., Guan Q., Gao L. New perspectives of physiological and pathological functions of nucleolin (NCL) Life Sci. 2017;186:1–10. doi: 10.1016/j.lfs.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Pecoraro A., Pagano M., Russo G., Russo A. Ribosome biogenesis and cancer: overview on ribosomal proteins. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilmani, D’costa M., D'costa M., Bothe A., Das S., Udhaya Kumar S., Gnanasambandan R., George Priya Doss C. CDK regulators-Cell cycle progression or apoptosis-Scenarios in normal cells and cancerous cells. Adv. Protein Chem. Struct. Biol. 2023;135:125–177. doi: 10.1016/bs.apcsb.2022.11.008. [DOI] [PubMed] [Google Scholar]
- 31.He J., Qiu Z., Fan J., Xie X., Sheng Q., Sui X. Drug tolerant persister cell plasticity in cancer: A revolutionary strategy for more effective anticancer therapies. Signal Transduct. Target. Ther. 2024;9:209. doi: 10.1038/s41392-024-01891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatti-Mays M.E., Karzai F.H., Soltani S.N., Zimmer A., Green J.E., Lee M.-J., Trepel J.B., Yuno A., Lipkowitz S., Nair J., et al. A Phase II Single Arm Pilot Study of the CHK1 Inhibitor Prexasertib (LY2606368) in BRCA Wild-Type, Advanced Triple-Negative Breast Cancer. Oncologist. 2020;25:1013–e1824. doi: 10.1634/theoncologist.2020-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quadri R., Sertic S., Muzi-Falconi M. Roles and regulation of Haspin kinase and its impact on carcinogenesis. Cell. Signal. 2022;93 doi: 10.1016/j.cellsig.2022.110303. [DOI] [PubMed] [Google Scholar]
- 34.Wang P., Hua X., Bryner Y.H., Liu S., Gitter C.B., Dai J. Haspin inhibition delays cell cycle progression through interphase in cancer cells. J. Cell. Physiol. 2020;235:4508–4519. doi: 10.1002/jcp.29328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huertas D., Soler M., Moreto J., Villanueva A., Martinez A., Vidal A., Charlton M., Moffat D., Patel S., McDermott J., et al. Antitumor activity of a small-molecule inhibitor of the histone kinase Haspin. Oncogene. 2012;31:1408–1418. doi: 10.1038/onc.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida-Fukuda H., Tokuhiro K., Ando Y., Matsushita H., Wada M., Tanaka H. Evaluation of the antiproliferative effects of the HASPIN inhibitor CHR-6494 in breast cancer cell lines. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amoussou N.G., Bigot A., Roussakis C., Robert J.-M.H. Haspin: a promising target for the design of inhibitors as potent anticancer drugs. Drug Discov. Today. 2018;23:409–415. doi: 10.1016/j.drudis.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Wang F., Ulyanova N.P., Daum J.R., Patnaik D., Kateneva A.V., Gorbsky G.J., Higgins J.M.G. Haspin inhibitors reveal centromeric functions of Aurora B in chromosome segregation. J. Cell Biol. 2012;199:251–268. doi: 10.1083/jcb.201205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Yang H., Fang Y., Xing Y., Pang X., Li Y., Zhang Y., Liu Y. Function and inhibition of Haspin kinase: targeting multiple cancer therapies by antimitosis. J. Pharm. Pharmacol. 2023;75:445–465. doi: 10.1093/jpp/rgac080. [DOI] [PubMed] [Google Scholar]
- 40.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke C.-Y., Xiao W.-L., Chen C.-M., Lo L.-J., Wong F.-H. IRF6 is the mediator of TGFβ3 during regulation of the epithelial mesenchymal transition and palatal fusion. Sci. Rep. 2015;5 doi: 10.1038/srep12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mardinian K., Adashek J.J., Botta G.P., Kato S., Kurzrock R. SMARCA4: Implications of an Altered Chromatin-Remodeling Gene for Cancer Development and Therapy. Mol. Cancer Ther. 2021;20:2341–2351. doi: 10.1158/1535-7163.MCT-21-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D., Cheng P., Wang J., Qiu X., Zhang X., Xu L., Liu Y., Qin S. IRF6 is directly regulated by ZEB1 and ELF3, and predicts a favorable prognosis in gastric cancer. Front. Oncol. 2019;9:220. doi: 10.3389/fonc.2019.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shain A.H., Pollack J.R. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correa B.R., de Araujo P.R., Qiao M., Burns S.C., Chen C., Schlegel R., Agarwal S., Galante P.A.F., Penalva L.O.F. Functional genomics analyses of RNA-binding proteins reveal the splicing regulator SNRPB as an oncogenic candidate in glioblastoma. Genome Biol. 2016;17:125. doi: 10.1186/s13059-016-0990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song X., Wan X., Huang T., Zeng C., Sastry N., Wu B., James C.D., Horbinski C., Nakano I., Zhang W., et al. SRSF3-Regulated RNA Alternative Splicing Promotes Glioblastoma Tumorigenicity by Affecting Multiple Cellular Processes. Cancer Res. 2019;79:5288–5301. doi: 10.1158/0008-5472.CAN-19-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zi Z., Zhang Y., Zhang P., Ding Q., Chu M., Chen Y., Minna J.D., Yu Y. A proteomic connectivity map for characterizing the tumor adaptive response to small molecule chemical perturbagens. ACS Chem. Biol. 2020;15:140–150. doi: 10.1021/acschembio.9b00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooney L.N., O’Shea K.D., Winfield H.J., Cahill M.M., Pierce L.T., McCarthy F.O. Bisindolyl Maleimides and Indolylmaleimide Derivatives-A Review of Their Synthesis and Bioactivity. Pharmaceuticals. 2023;16 doi: 10.3390/ph16091191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam W.L., Lu H., Buikhuisen J., Soh B.S., Lim E., Reinhardt F., Wu Z.J., Krall J.A., Bierie B., Guo W., et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24:347–364. doi: 10.1016/j.ccr.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey T.A., Luan H., Tom E., Bielecki T.A., Mohapatra B., Ahmad G., George M., Kelly D.L., Natarajan A., Raja S.M., et al. A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J. Biol. Chem. 2014;289:30443–30458. doi: 10.1074/jbc.M114.608992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aggarwal S., Kim S.-W., Ryu S.-H., Chung W.-C., Koo J.S. Growth suppression of lung cancer cells by targeting cyclic AMP response element-binding protein. Cancer Res. 2008;68:981–988. doi: 10.1158/0008-5472.CAN-06-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olsen E.A., Kim Y.H., Kuzel T.M., Pacheco T.R., Foss F.M., Parker S., Frankel S.R., Chen C., Ricker J.L., Arduino J.M., Duvic M. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 54.Ramalingam S.S., Parise R.A., Ramanathan R.K., Lagattuta T.F., Musguire L.A., Stoller R.G., Potter D.M., Argiris A.E., Zwiebel J.A., Egorin M.J., Belani C.P. Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin. Cancer Res. 2007;13:3605–3610. doi: 10.1158/1078-0432.CCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 55.Zheng X., Naiditch J., Czurylo M., Jie C., Lautz T., Clark S., Jafari N., Qiu Y., Chu F., Madonna M.B. Differential effect of long-term drug selection with doxorubicin and vorinostat on neuroblastoma cells with cancer stem cell characteristics. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeidner J.F., Karp J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 2015;39:1312–1318. doi: 10.1016/j.leukres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Markham A., Keam S.J. Selumetinib: First Approval. Drugs. 2020;80:931–937. doi: 10.1007/s40265-020-01331-x. [DOI] [PubMed] [Google Scholar]
- 58.Pommier Y., Sun Y., Huang S.-Y.N., Nitiss J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saxena S., Zou L. Hallmarks of DNA replication stress. Mol. Cell. 2022;82:2298–2314. doi: 10.1016/j.molcel.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitra Ghosh T., Mazumder S., Davis J., Yadav J., Akinpelu A., Alnaim A., Kumar H., Waliagha R., Church Bird A.E., Rais-Bahrami S., et al. Metronomic Administration of Topotecan Alone and in Combination with Docetaxel Inhibits Epithelial-mesenchymal Transition in Aggressive Variant Prostate Cancers. Cancer Res. Commun. 2023;3:1286–1311. doi: 10.1158/2767-9764.CRC-22-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metge B.J., Alsheikh H.A.M., Kammerud S.C., Chen D., Das D., Nebane N.M., Bostwick J.R., Shevde L.A., Samant R.S. Targeting EMT using low-dose Teniposide by downregulating ZEB2-driven activation of RNA polymerase I in breast cancer. Cell Death Dis. 2024;15:322. doi: 10.1038/s41419-024-06694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Black P.N., Ahowesso C., Montefusco D., Saini N., DiRusso C.C. Fatty acid transport proteins: targeting FATP2 as a gatekeeper involved in the transport of exogenous fatty acids. Medchemcomm. 2016;7:612–622. doi: 10.1039/C6MD00043F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham S.H., Liu H. Life and death in the trash heap: The ubiquitin proteasome pathway and UCHL1 in brain aging, neurodegenerative disease and cerebral Ischemia. Ageing Res. Rev. 2017;34:30–38. doi: 10.1016/j.arr.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J., Tao Q., Cheung K.F., Jin H., Poon F.F., Wang X., Li H., Cheng Y.Y., Röcken C., Ebert M.P.A., et al. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology. 2008;48:508–518. doi: 10.1002/hep.22343. [DOI] [PubMed] [Google Scholar]
- 65.Xiang T., Li L., Yin X., Yuan C., Tan C., Su X., Xiong L., Putti T.C., Oberst M., Kelly K., et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ummanni R., Jost E., Braig M., Lohmann F., Mundt F., Barett C., Schlomm T., Sauter G., Senff T., Bokemeyer C., et al. Ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) is a potential tumour suppressor in prostate cancer and is frequently silenced by promoter methylation. Mol. Cancer. 2011;10:129. doi: 10.1186/1476-4598-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan W.-J., Guo M.-Z., Yang Y.-S. [The role of hypermethylation in promoter region of ubiquitin carboxyl-terminal hydrolase L1 in human esophageal cancer] Zhonghua Nei Ke Za Zhi. 2012;51:390–393. [PubMed] [Google Scholar]
- 68.Mondal M., Conole D., Nautiyal J., Tate E.W. UCHL1 as a novel target in breast cancer: emerging insights from cell and chemical biology. Br. J. Cancer. 2022;126:24–33. doi: 10.1038/s41416-021-01516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y., Lih T.-S.M., Dhanasekaran S.M., Mannan R., Chen L., Cieslik M., Wu Y., Lu R.J.-H., Clark D.J., Kołodziejczak I., et al. Histopathologic and proteogenomic heterogeneity reveals features of clear cell renal cell carcinoma aggressiveness. Cancer Cell. 2023;41:139–163.e17. doi: 10.1016/j.ccell.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Molecular Biology of the Cell (Garland Science) 2002. Protein Function. [Google Scholar]
- 71.Liu Y., Beyer A., Aebersold R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. 2016;165:535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 72.McManus J., Cheng Z., Vogel C. Next-generation analysis of gene expression regulation--comparing the roles of synthesis and degradation. Mol. Biosyst. 2015;11:2680–2689. doi: 10.1039/c5mb00310e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hristova V.A., Chan D.W. Cancer biomarker discovery and translation: proteomics and beyond. Expert Rev. Proteomics. 2019;16:93–103. doi: 10.1080/14789450.2019.1559062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wainwright E.N., Scaffidi P. Epigenetics and cancer stem cells: unleashing, hijacking, and restricting cellular plasticity. Trends Cancer. 2017;3:372–386. doi: 10.1016/j.trecan.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Statoulla E., Chalkiadaki K., Karozis D., Gkogkas C.G. Regulation of mRNA translation in stem cells; links to brain disorders. Cell. Signal. 2021;88 doi: 10.1016/j.cellsig.2021.110166. [DOI] [PubMed] [Google Scholar]
- 76.Curry R.N., Glasgow S.M. The role of neurodevelopmental pathways in brain tumors. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.659055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cariaga-Martínez A.E., Gutiérrez K.J., Alelú-Paz R. The Vast Complexity of the Epigenetic Landscape during Neurodevelopment: An Open Frame to Understanding Brain Function. Int. J. Mol. Sci. 2018;19:1333. doi: 10.3390/ijms19051333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ransohoff R.M., Schafer D., Vincent A., Blachère N.E., Bar-Or A. Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics. 2015;12:896–909. doi: 10.1007/s13311-015-0385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parsons D.W., Jones S., Zhang X., Lin J.C.-H., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.-M., Gallia G.L., et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhai Y., Li N., Jiang H., Huang X., Gao N., Tye B.K. Unique Roles of the Non-identical MCM Subunits in DNA Replication Licensing. Mol. Cell. 2017;67:168–179. doi: 10.1016/j.molcel.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 81.Ishimi Y. Regulation of MCM2-7 function. Genes Genet. Syst. 2018;93:125–133. doi: 10.1266/ggs.18-00026. [DOI] [PubMed] [Google Scholar]
- 82.Kahm Y.-J., Kim I.-G., Kim R.-K. Regulation of cancer stem cells by CXCL1, a chemokine whose secretion is controlled by MCM2. BMC Cancer. 2024;24:319. doi: 10.1186/s12885-024-12085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raglow Z., McKenna M.K., Bonifant C.L., Wang W., Pasca di Magliano M., Stadlmann J., Penninger J.M., Cummings R.D., Brenner M.K., Markovitz D.M. Targeting glycans for CAR therapy: The advent of sweet CARs. Mol. Ther. 2022;30:2881–2890. doi: 10.1016/j.ymthe.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bursać S., Jurada D., Volarević S. New insights into HEATR1 functions. Cell Cycle. 2018;17:143–144. doi: 10.1080/15384101.2017.1411325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turi Z., Senkyrikova M., Mistrik M., Bartek J., Moudry P. Perturbation of RNA Polymerase I transcription machinery by ablation of HEATR1 triggers the RPL5/RPL11-MDM2-p53 ribosome biogenesis stress checkpoint pathway in human cells. Cell Cycle. 2018;17:92–101. doi: 10.1080/15384101.2017.1403685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaughan R.M., Dickson B.M., Whelihan M.F., Johnstone A.L., Cornett E.M., Cheek M.A., Ausherman C.A., Cowles M.W., Sun Z.-W., Rothbart S.B. Chromatin structure and its chemical modifications regulate the ubiquitin ligase substrate selectivity of UHRF1. Proc. Natl. Acad. Sci. USA. 2018;115:8775–8780. doi: 10.1073/pnas.1806373115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mancini M., Magnani E., Macchi F., Bonapace I.M. The multi-functionality of UHRF1: epigenome maintenance and preservation of genome integrity. Nucleic Acids Res. 2021;49:6053–6068. doi: 10.1093/nar/gkab293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W., Chakraborty B., Safi R., Kazmin D., Chang C.-Y., McDonnell D.P. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat. Commun. 2021;12:5103. doi: 10.1038/s41467-021-25354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y., Zhao Y., Deng Y., Yang Y., Xu L., Fu J. FATP2 regulates non-small cell lung cancer by mediating lipid metabolism through ACSL1. Tissue Cell. 2023;82 doi: 10.1016/j.tice.2023.102105. [DOI] [PubMed] [Google Scholar]
- 90.Wang X., Li S. Protein mislocalization: mechanisms, functions and clinical applications in cancer. Biochim. Biophys. Acta. 2014;1846:13–25. doi: 10.1016/j.bbcan.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghaemimanesh F. The protein subcellular mislocalization in human cancers. Avicenna J. Med. Biotechnol. (AJMB) 2020;12:1. [PMC free article] [PubMed] [Google Scholar]
- 92.Kilpinen H., Goncalves A., Leha A., Afzal V., Alasoo K., Ashford S., Bala S., Bensaddek D., Casale F.P., Culley O.J., et al. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature. 2017;546:370–375. doi: 10.1038/nature22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho S.Y., Hwang H., Kim Y.-H., Yoo B.C., Han N., Kong S.-Y., Baek M.-J., Kim K.-H., Lee M.R., Park J.G., et al. Refining classification of cholangiocarcinoma subtypes via proteogenomic integration reveals new therapeutic prospects. Gastroenterology. 2023;164:1293–1309. doi: 10.1053/j.gastro.2023.02.045. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y.-J., Roumeliotis T.I., Chang Y.-H., Chen C.-T., Han C.-L., Lin M.-H., Chen H.-W., Chang G.-C., Chang Y.-L., Wu C.-T., et al. Proteogenomics of Non-smoking Lung Cancer in East Asia Delineates Molecular Signatures of Pathogenesis and Progression. Cell. 2020;182:226–244.e17. doi: 10.1016/j.cell.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Jayavelu A.K., Wolf S., Buettner F., Alexe G., Häupl B., Comoglio F., Schneider C., Doebele C., Fuhrmann D.C., Wagner S., et al. The proteogenomic subtypes of acute myeloid leukemia. Cancer Cell. 2022;40:301–317.e12. doi: 10.1016/j.ccell.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 96.Anurag M., Jaehnig E.J., Krug K., Lei J.T., Bergstrom E.J., Kim B.-J., Vashist T.D., Huynh A.M.T., Dou Y., Gou X., et al. Proteogenomic Markers of Chemotherapy Resistance and Response in Triple-Negative Breast Cancer. Cancer Discov. 2022;12:2586–2605. doi: 10.1158/2159-8290.CD-22-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hyeon D.Y., Nam D., Han Y., Kim D.K., Kim G., Kim D., Bae J., Back S., Mun D.-G., Madar I.H., et al. Proteogenomic landscape of human pancreatic ductal adenocarcinoma in an Asian population reveals tumor cell-enriched and immune-rich subtypes. Nat. Cancer. 2023;4:290–307. doi: 10.1038/s43018-022-00479-7. [DOI] [PubMed] [Google Scholar]
- 98.Sabatier P., Beusch C.M., Saei A.A., Aoun M., Moruzzi N., Coelho A., Leijten N., Nordenskjöld M., Micke P., Maltseva D., et al. An integrative proteomics method identifies a regulator of translation during stem cell maintenance and differentiation. Nat. Commun. 2021;12:6558. doi: 10.1038/s41467-021-26879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamb J., Crawford E.D., Peck D., Modell J.W., Blat I.C., Wrobel M.J., Lerner J., Brunet J.-P., Subramanian A., Ross K.N., et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 100.Subramanian A., Narayan R., Corsello S.M., Peck D.D., Natoli T.E., Lu X., Gould J., Davis J.F., Tubelli A.A., Asiedu J.K., et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell. 2017;171:1437–1452.e17. doi: 10.1016/j.cell.2017.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuleshov M.V., Xie Z., London A.B.K., Yang J., Evangelista J.E., Lachmann A., Shu I., Torre D., Ma’ayan A. KEA3: improved kinase enrichment analysis via data integration. Nucleic Acids Res. 2021;49:W304–W316. doi: 10.1093/nar/gkab359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie Z., Bailey A., Kuleshov M.V., Clarke D.J.B., Evangelista J.E., Jenkins S.L., Lachmann A., Wojciechowicz M.L., Kropiwnicki E., Jagodnik K.M., et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021;1 doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark D.J., Dhanasekaran S.M., Petralia F., Pan J., Song X., Hu Y., da Veiga Leprevost F., Reva B., Lih T.-S.M., Chang H.-Y., et al. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell. 2019;179:964–983.e31. doi: 10.1016/j.cell.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vasaikar S., Huang C., Wang X., Petyuk V.A., Savage S.R., Wen B., Dou Y., Zhang Y., Shi Z., Arshad O.A., et al. Proteogenomic analysis of human colon cancer reveals new therapeutic opportunities. Cell. 2019;177:1035–1049.e19. doi: 10.1016/j.cell.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L.-B., Karpova A., Gritsenko M.A., Kyle J.E., Cao S., Li Y., Rykunov D., Colaprico A., Rothstein J.H., Hong R., et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell. 2021;39:509–528.e20. doi: 10.1016/j.ccell.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang C., Chen L., Savage S.R., Eguez R.V., Dou Y., Li Y., da Veiga Leprevost F., Jaehnig E.J., Lei J.T., Wen B., et al. Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell. 2021;39:361–379.e16. doi: 10.1016/j.ccell.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Satpathy S., Krug K., Jean Beltran P.M., Savage S.R., Petralia F., Kumar-Sinha C., Dou Y., Reva B., Kane M.H., Avanessian S.C., et al. A proteogenomic portrait of lung squamous cell carcinoma. Cell. 2021;184:4348–4371.e40. doi: 10.1016/j.cell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang H., Liu T., Zhang Z., Payne S.H., Zhang B., McDermott J.E., Zhou J.-Y., Petyuk V.A., Chen L., Ray D., et al. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell. 2016;166:755–765. doi: 10.1016/j.cell.2016.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao L., Huang C., Cui Zhou D., Hu Y., Lih T.M., Savage S.R., Krug K., Clark D.J., Schnaubelt M., Chen L., et al. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell. 2021;184:5031–5052.e26. doi: 10.1016/j.cell.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dou Y., Kawaler E.A., Cui Zhou D., Gritsenko M.A., Huang C., Blumenberg L., Karpova A., Petyuk V.A., Savage S.R., Satpathy S., et al. Proteogenomic characterization of endometrial carcinoma. Cell. 2020;180:729–748.e26. doi: 10.1016/j.cell.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petralia F., Tignor N., Reva B., Koptyra M., Chowdhury S., Rykunov D., Krek A., Ma W., Zhu Y., Ji J., et al. Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell. 2020;183:1962–1985.e31. doi: 10.1016/j.cell.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y., Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127–D131. doi: 10.1093/nar/gkz757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Colaprico A., Silva T.C., Olsen C., Garofano L., Cava C., Garolini D., Sabedot T.S., Malta T.M., Pagnotta S.M., Castiglioni I., et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44 doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silva T.C., Colaprico A., Olsen C., D’Angelo F., Bontempi G., Ceccarelli M., Noushmehr H. TCGA Workflow: Analyze cancer genomics and epigenomics data using Bioconductor packages. F1000Res. 2016;5:1542. doi: 10.12688/f1000research.8923.2. [version 2; peer review: 1 approved, 2 approved with reservations] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mounir M., Lucchetta M., Silva T.C., Olsen C., Bontempi G., Chen X., Noushmehr H., Colaprico A., Papaleo E. New functionalities in the TCGAbiolinks package for the study and integration of cancer data from GDC and GTEx. PLoS Comput. Biol. 2019;15 doi: 10.1371/journal.pcbi.1006701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ochoa D., Hercules A., Carmona M., Suveges D., Baker J., Malangone C., Lopez I., Miranda A., Cruz-Castillo C., Fumis L., et al. The next-generation Open Targets Platform: reimagined, redesigned, rebuilt. Nucleic Acids Res. 2023;51:D1353–D1359. doi: 10.1093/nar/gkac1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thike A.A., Chng M.J., Fook-Chong S., Tan P.H. Immunohistochemical expression of hormone receptors in invasive breast carcinoma: correlation of results of H-score with pathological parameters. Pathology. 2001;33:21–25. doi: 10.1080/00313020123290. [DOI] [PubMed] [Google Scholar]
- 118.Sokolov A., Paull E.O., Stuart J.M. One-class detection of cell states in tumor subtypes. Pac. Symp. Biocomput. 2016;21:405–416. doi: 10.1142/9789814749411_0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petralia F., Ma W., Yaron T.M., Caruso F.P., Tignor N., Wang J.M., Charytonowicz D., Johnson J.L., Huntsman E.M., Marino G.B., et al. Pan-cancer proteogenomics characterization of tumor immunity. Cell. 2024;187:1255–1277.e27. doi: 10.1016/j.cell.2024.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gillespie M., Jassal B., Stephan R., Milacic M., Rothfels K., Senff-Ribeiro A., Griss J., Sevilla C., Matthews L., Gong C., et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022;50:D687–D692. doi: 10.1093/nar/gkab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1A: Clinical data of patient samples
Table S1B: Protein weights (prediction model)
Table S1C: List of iPS sample IDs using to train the model
Table S1D: List of sample IDs and PROTsi of the validation dataset
Table S1E: Results of OS and PFS based on the stemness index (Related to Figures S1F and S1G)
Table S3A: Stemness-associated proteins for each tumor type along with related multi-omic and characterization data
Table S3B: Stemness-associated protein biomarkers with significant prognostic value, but whose gene expression is not associated with survival, for each tumor type
Data Availability Statement
Raw and processed proteomics as well as open-access genomic data for the CPTAC Pan-Cancer cohort can be accessed via Proteomic Data Commons at https://pdc.cancer.gov/pdc/cptac-pancancer. Raw genomic and transcriptomic data files can be accessed via the Genomic Data Commons Data Portal at https://portal.gdc.cancer.gov with dbGaP study accession number: phs001287.v16.p6. Processed genomic data with access control can be obtained via the Cancer Data Service at https://dataservice.datacommons.cancer.gov/.