Abstract
The human microbiota comprises a diverse microbial ecosystem that significantly impacts health and disease. Among its components, the gut microbiota plays a crucial role in regulating metabolic, immunologic, and inflammatory responses. Dysbiosis, an imbalance in microbial composition, has been linked to carcinogenesis through mechanisms such as chronic inflammation, metabolic disturbances, epigenetic modifications, and immune system dysregulation. Additionally, dysbiosis influences the efficacy and toxicity of cancer therapies. Given these associations, there is growing interest in leveraging the microbiota as a biomarker for cancer detection and outcome prediction. Notably, distinct microbial signatures have been identified across various cancer types, suggesting their potential as diagnostic markers. Furthermore, modulation of the microbiota presents a promising avenue for improving cancer treatment outcomes through strategies such as antibiotics, prebiotics, probiotics, fecal microbiota transplantation, dietary interventions, small-molecule inhibitors, and phage therapy. To explore these relationships, we conducted a comprehensive literature review using Web of Science, Scopus, PubMed, MEDLINE, Embase, and Google Scholar as our primary online databases, focusing on indexed peer-reviewed articles up to the present year. This review aims to elucidate the role of dysbiosis in cancer development, examine the molecular mechanisms involved, and assess the impact of microbiota on cancer therapies. Additionally, we highlight microbiota-based therapeutic strategies and discuss their potential applications in cancer management. A deeper understanding of the intricate interplay between the microbiota and cancer may pave the way for novel approaches to cancer prevention, early detection, and treatment optimization.
Graphical abstract
Keywords: Microbiota, Cancer, Dysbiosis, Carcinogenesis, Biomarkers, Immune modulation
Introduction
The prized connection between the human microbiota and cancer has become one of the most popular and captivating topics of discussion in the field of biomedical sciences due to its ability to gain deeper insights into disease development, further evolution, and potential treatment strategies [1, 2]. Consisting of numerous microorganisms living mainly in the gastrointestinal tract, the human microbiota plays a critical role in controlling various aspects of human physiology, such as metabolism, immune responses, and inflammation [3]. This mutualistic association, known as eubiosis, highlights the fundamental role of the microbiota in maintaining the well-being and stability of the host [4]. Nevertheless, several diseases, including cancer, correlate with shifts in the microbial structure and its activities, known as dysbiosis [5]. Cancer, a complex human body disease affecting all ages and genders, is one of the worst diseases in the world [6, 7]. Although genetics and environmental conditions remain the primary initiators of cancer and tumour development, current research has delineated the functions of the microbiota in the carcinogenesis process and tiers of therapy [8, 9]. In fact, changes in the balance of harmful microbes in mixed microbial systems can cause or encourage cancer-causing processes like inflammation, changes in DNA methylation, problems with metabolism, and the immune system losing its focus [10]. Furthermore, microbial signalling with the host immune system and tumour microenvironment strongly influences cancer and treatment responses [11]. Consequently, providing an account of the mechanisms that govern microbiota-cancer interactions has emerged as a critical research direction, mainly because it reveals novel approaches for cancer prevention, detection, and therapy. As an application of microbiota, microbial biomarkers—the presence of some microbes in an abnormal way—are now being looked at as a way to find objective signs of when cancer starts or how bad it is [12]. Furthermore, it holds promise in revealing more about the role of microbiota in cancer treatment response and in patients'therapeutic resistance, allowing for the optimal customization of the therapeutic management plan [13]. The intersection of Microbiota research and oncology opens up a new area in the study and treatment of cancer [14]. This includes clinical trials that aim to change the microbiota to find new ways to treat cancer and the use of Microbiota data in the care of cancer patients [15].
Composition of the human microbiota: an overview
The human body hosts a complex and diverse community of microorganisms, including bacteria, viruses, fungi, and archaea, collectively referred to as the human microbiota [16]. This microbiota plays a pivotal role in maintaining physiological homeostasis and modulating various bodily functions, including immune response, metabolism, and protection against pathogens [16]. The composition of the microbiota varies across different locations in the body, with each site harboring a distinct microbial community adapted to its specific environmental conditions [16, 17]. The balance of these microbial communities is essential for health and disease, and disruptions to this balance, known as dysbiosis, have been implicated in various diseases, including cancer. In this context, understanding the normal microbial balance (eubiosis) in different body sites is crucial for understanding the impact of dysbiosis on human health.
Gastrointestinal tract (GIT) microbiota
The gastrointestinal tract is home to the most diverse and dense microbial community in the human body. The composition of the microbiota along the GIT varies depending on the region (stomach, small intestine, and large intestine) and is influenced by dietary habits, age, health status, and antibiotic use [18].
Stomach
Due to its acidic environment (low pH), the microbial load in the stomach is relatively low. However, Helicobacter pylori is a prominent bacterium found in the gastric mucosa, and its presence can lead to gastric diseases such as ulcers and cancer. Despite the acidity, other bacteria such as Lactobacillus and Streptococcus are also found here, though in smaller numbers [19, 20].
Small intestine
The small intestine has a slightly higher pH and a more varied microbial population compared to the stomach. The microbiota here is characterized by the presence of Lactobacilli, Bifidobacteria, and Enterococci, among others. These microorganisms play essential roles in nutrient absorption, digestion, and the synthesis of certain vitamins. However, the bacterial load remains relatively low compared to the large intestine to prevent interference with digestion and absorption [21–23].
Large intestine (colon)
The colon is the most densely populated region of the human microbiota, containing trillions of microorganisms. The microbiota here is dominated by Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, which play critical roles in fermentation of non-digestible carbohydrates, production of short-chain fatty acids (SCFAs) like butyrate, and the regulation of immune function. This complex ecosystem is essential for maintaining gut health, modulating inflammation, and protecting against harmful pathogens [16, 24].
Skin microbiota
The skin is an external barrier and has a unique microbiota that varies by body site, with distinct microbial communities residing in sebaceous (oily), dry, and moist regions. Skin microbiota includes a variety of bacteria, fungi, and viruses that help prevent pathogenic colonization and maintain skin health [16].
Sebaceous areas (e.g., face, scalp)
These areas are rich in lipids, supporting the growth of Propionibacterium acnes and Staphylococcus epidermidis. These microorganisms play roles in preventing infections and modulating immune responses. Disruption of this balance is associated with conditions like acne [25, 26].
Dry areas (e.g., forearms, legs)
The microbiota here is less diverse and dominated by Corynebacteria, Betaproteobacteria, and Firmicutes. These microbes are adapted to low moisture environments and help maintain skin barrier function [16, 27].
Moist areas (e.g., armpits, groin)
Moist areas host a more diverse microbiota, with high populations of Staphylococcus, Corynebacterium, and Malassezia fungi. These areas are more prone to overgrowth of harmful pathogens if the microbiota balance is disturbed, leading to infections such as folliculitis or dermatitis [28, 29].
Vaginal microbiota
The vaginal microbiota is primarily dominated by Lactobacillus species, which produce lactic acid, maintaining an acidic pH that inhibits the growth of pathogens [30]. The diversity and composition of the vaginal microbiota vary during different life stages, such as puberty, menstruation, pregnancy, and menopause, as well as depending on hormonal fluctuations [31].
Healthy vaginal microbiota (Eubiosis)
A healthy vaginal microbiota is primarily composed of Lactobacillus species (e.g., Lactobacillus crispatus, Lactobacillus iners) [32]. These bacteria help maintain an acidic environment, preventing the overgrowth of harmful microorganisms, including Gardnerella vaginalis, Candida albicans, and other pathogens [33].
Dysbiosis in the vaginal microbiota
Dysbiosis in the vaginal microbiota, characterized by a reduction in Lactobacillus and overgrowth of anaerobic bacteria, has been associated with conditions such as bacterial vaginosis, yeast infections, and an increased risk of sexually transmitted infections [34]. Furthermore, dysbiosis in the vaginal microbiota has been linked to preterm birth, pelvic inflammatory disease, and an increased susceptibility to HIV [35].
Respiratory tract microbiota
The respiratory tract, including the nasal cavity, pharynx, and lungs, harbors a diverse community of microbes, although their composition is less dense compared to the gut or skin. The composition of the respiratory microbiota varies with age, environmental exposures, and disease state [36].
Nasal cavity
The nasal microbiota is mainly composed of Staphylococcus, Corynebacterium, Propionibacterium, and Streptococcus. These microorganisms help protect against pathogenic colonization and contribute to the maintenance of respiratory tract immunity [37, 38].
Lower respiratory tract
The lungs, previously thought to be sterile, have been found to host a low-density microbiota, with microbes such as Prevotella, Veillonella, and Fusobacterium species present in healthy individuals [39, 40]. Dysbiosis in the lung microbiota has been associated with respiratory conditions like asthma, chronic obstructive pulmonary disease (COPD), and pneumonia [41, 42].
Urinary tract microbiota
The urinary tract microbiota, particularly in females, plays a significant role in maintaining urinary tract health. The microbiota of the urinary tract is influenced by factors such as age, hygiene, sexual activity, and the use of antibiotics [16, 43].
Healthy urinary tract microbiota
In a healthy state, the urinary microbiota consists mainly of Lactobacillus, Corynebacterium, and Staphylococcus species, which help prevent urinary tract infections (UTIs) by inhibiting the growth of uropathogens [16].
Dysbiosis and UTIs
Disruption of the urinary microbiota, such as a reduction in Lactobacillus species, can lead to the overgrowth of uropathogens like Escherichia coli, resulting in UTIs. This imbalance has also been associated with conditions such as interstitial cystitis and pelvic pain syndrome [44, 45].
The composition of the human microbiota is highly dynamic and varies across different body sites, including the gastrointestinal tract, skin, vaginal, respiratory, and urinary tracts. Eubiosis in these regions ensures proper physiological functioning and protection from pathogens, while dysbiosis can result in adverse health outcomes, including cancer. A better understanding of the role of microbiota in maintaining health and its contribution to cancer pathogenesis could lead to new therapeutic strategies, such as microbiome modulation, for preventing or treating cancer.
This review comprehensively examines the intricate relationship between the gut microbiota and cancer, focusing on its role in carcinogenesis, prognosis, and therapeutic interventions. By analyzing current evidence on microbial dysbiosis, molecular mechanisms, and therapeutic modulation, we aim to elucidate how microbiota influences cancer development, progression, and treatment responses. Additionally, we highlight emerging microbiota-based strategies for cancer prevention, diagnosis, and therapy, offering insights into their potential clinical applications.
Methodology
To achieve the aim of this research, this study adopted a thorough narrative review of articles that explained the involvement of the human gut microbiota in cancer development, prognosis, and therapy. The study encompassed in vitro, animal, and clinical models, as well as in vitro, animal, and clinical examination studies. We identified the study's articles from electronic databases such as PubMed, Medline, Embase, Web of Science, Scopus, and Google Scholar, using keywords like human microbiota, cancer, dysbiosis, carcinogenesis, biomarkers, therapy response, and microbiota-targeted interventions. We included high-quality, relevant, and recent literature. Criteria included publication type, language, date range, study design, relevance, data source, microbiota scope, therapeutic strategies, and exclusion criteria. The review focused on peer-reviewed articles, systematic reviews, meta-analyses, and clinical studies published within the last 10 years. The review excluded non-English articles, non-peer-reviewed sources, older studies, non-relevant focus, limited data, and duplicate publications. We made sure to adhere to the appropriate ethical considerations, including the citation of relevant references.
Overview of the human microbiota and its significance in health
The term “human microbiota” refers to the trillions of microorganisms, including bacteria, viruses, and fungi, that make up the human body [23]. The human body contains these microbes in various areas, with many settling in the digestive system, a collection known as gut flora [46]. The gut microbiota is a set of microorganisms that reside in a person’s digestive tract and is important for most physiological functions that affect an individual’s well-being, such as metabolism, immune response, inflammation, and nutrient absorption [47]. People refer to it as a “vital organ” due to its bidirectional connections with every other organ via neural, endocrine, and immunologic interfaces [48]. According to scientific evidence, a healthy state of the gut microbes, known as eubiosis, is critical for the regulation of microbial diseases as well as human health in general [49]. The composition of the microbiota can be affected by diet, age, geographical location, systemic diseases, and drug use [50]. The entire microbial community, or microbiota, inhabiting the gastrointestinal tract interacts with the host to influence its metabolic homeostasis, immune system, and defence mechanisms [51]. In addition, the composition of the gastro intestinal track (GI) Microbiota plays a significant role starting at birth and continuing through the life cycle, with influences on digestion, and even the nervous system [52]. Dysbiosis refers to an imbalance in the composition of organisms residing in the intestines, and it has been associated with different diseases. Knowledge of the gut microbiota's components and roles is critical for promoting health and preventing diseases caused by microbial community imbalance disruptions [23]. Summarily, microbiota-targeted therapeutic strategies, including probiotics, prebiotics, synbiotics, fecal microbiota transplantation (FMT)., and phage therapy and other related concepts have been summarized in Table 1 below.
Table 1.
Microbiota-targeted therapeutic strategies: mechanisms, applications, and challenges
| Strategy | Definition | Mechanism of action | Therapeutic applications | Applications in cancer patients | Advantages | Limitations/challenges | References |
|---|---|---|---|---|---|---|---|
| Probiotics | Live beneficial microorganisms that confer health benefits when administered in adequate amounts | Compete with pathogenic bacteria, enhance gut barrier function, modulate immune responses, and produce antimicrobial compounds | Used in treating gastrointestinal disorders (IBS, IBD, diarrhea), metabolic diseases, and neurodegenerative conditions | May improve cancer patients'gut microbiota, reduce chemotherapy-induced diarrhea, and enhance immune responses to immunotherapy | Restore gut microbiota balance, reduce inflammation, and enhance gut health | Strain specificity, viability issues during storage and passage through the gut, and risk of infections in immunocompromised individuals | [80, 81] |
| Prebiotics | Non-digestible dietary fibers that selectively stimulate the growth of beneficial gut microbes | Enhance beneficial microbiota growth (e.g., Bifidobacteria, Lactobacilli) by providing substrates for fermentation, producing short-chain fatty acids (SCFAs) | Used in metabolic syndrome, diabetes, cardiovascular diseases, and inflammatory bowel disease (IBD) | Support gut microbiota composition in cancer patients, improve SCFA production, and potentially reduce inflammation linked to tumor progression | Improve gut microbiota composition, promote SCFA production, and support immune function | Potential bloating and gas production, inter-individual variability in response | [81, 82] |
| Synbiotics | A combination of probiotics and prebiotics that work synergistically to enhance gut microbiota composition and function | Enhance the survival of probiotics by providing a specific substrate for their growth, leading to improved colonization and metabolic activity | Applied in gastrointestinal health, obesity management, and immune system modulation | May help in cancer patients by improving gut health, reducing inflammation, and supporting overall well-being during treatment | Synergistic effect, improved colonization and efficacy of probiotics, better gut health benefits | Requires strain-substrate compatibility, production cost, and regulatory challenges | [83, 84] |
| Fecal Microbiota Transplantation (FMT) | Transfer of fecal microbiota from a healthy donor to a recipient to restore gut microbiota composition | Replenishes gut microbiota diversity, restores metabolic and immune functions, and eliminates harmful pathogens | Primarily used for Clostridioides difficile infections (CDI), inflammatory bowel disease (IBD), and metabolic disorders | Investigated for improving gut microbiota diversity in cancer patients undergoing chemotherapy or immunotherapy | Highly effective in refractory CDI, restores a natural microbiota ecosystem | Donor screening challenges, risk of pathogen transmission, ethical and regulatory concerns | [85, 86] |
| Phage Therapy | Use of bacteriophages (viruses that infect bacteria) to selectively target and eliminate pathogenic bacteria | Lyses pathogenic bacteria without affecting beneficial microbiota, reducing antibiotic resistance and promoting gut microbiota balance | Investigated for treating antibiotic-resistant infections, gut dysbiosis, and inflammatory conditions | Potential application in cancer patients to reduce infections, modulate gut microbiota, and decrease inflammation | High specificity, reduced collateral damage to microbiota, potential alternative to antibiotics | Bacterial resistance to phages, regulatory hurdles, stability and delivery challenges | [87–89] |
| Postbiotics | Metabolites or cell components from probiotics that exert beneficial effects without requiring live microorganisms | Modulate immune responses, produce SCFAs, regulate gut barrier function, and exert antimicrobial activity | Potential applications in immune modulation, metabolic disorders, and gut health | May enhance anti-cancer immunity and reduce inflammation in cancer patients | More stable than probiotics, easier to formulate, and free from viability concerns | Research is still emerging, and effectiveness varies by strain-derived metabolites | [90–93] |
| Next-Generation Microbiota-Based Therapies | Includes engineered probiotics, microbial consortia, and Microbiota-modulating drugs for targeted therapeutic effects | Designed to modify specific metabolic pathways, enhance gut-brain axis signaling, and optimize host-microbiota interactions | Potential applications in neurological diseases, metabolic syndromes, and precision medicine approaches | Can be used for precision cancer treatment by modulating gut microbiota to enhance therapy response | Precision-targeted therapies with high efficacy potential | High development cost, regulatory and safety challenges, personalized medicine integration issues | [94–96] |
Mechanisms of microbiota dysbiosis contributing to cancer initiation
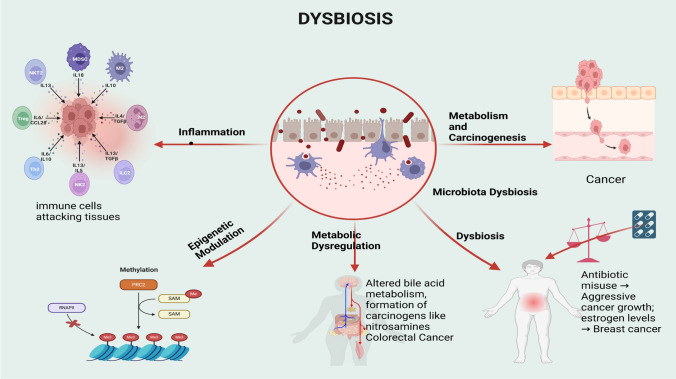
Microbiota dysbiosis disrupts the complexity and functionality of microbial communities within a human body, leading to multiple effects on cancer initiation [53]. Researchers have identified several key mechanisms through which the microbiota can influence cancer as summarized in Fig. 1, and explained below:
Fig. 1.
Mechanisms of microbiota dysbiosis (created in BioRender.com). PRC2—Polycomb Repressive Complex 2, RNAPII—RNA Polymerase II, Me—Methyl group, SAM—S-Adenosylmethionine
1. Inflammation: The microbiota has the ability to influence and induce genome instability, which can lead to cancer, tumour-promoting inflammation, and immunosuppression [54]. It also regulates and controls host immunity, as well as immune cell motion. For instance, researchers have linked certain bacterial species to gut inflammation, thereby increasing the risk of developing colorectal cancer [55]. It also plays an important role in healthy immune system development and regulation, which could potentially aid in cancer detection and immune responses against tumours. Some of the effects that pathogenic bacteria are known to have include disruption of the epithelial layer, which produces inflammation [56]. Dysbiosis can cause an immune reaction, which leads to chronic inflammation. Helicobacter pylori bacteria, for example, can cause an inflammatory state in the stomach's lining tissues and, consequently, are associated with gastric cancer [57]. Zhao et al. [58]reported the immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis.
2. Epigenetic modulation: Several selective organisms within the gut preemptively regulate DNA methylation, DNA repair, and DNA damage, which are critical to cancer development, particularly with regards to the regulation of cell growth and cell signaling [59]. For example, some genotypes of Escherichia coli release colibactin, a genotoxin that causes DNA double-strand breaks [60]. The Epigenetic modulation by gut microbuiota has previously been established by Tian et al. [61], Kim et al. [62], and Pepke et al. [63]. Furthermore, dysbiotic microbiota may increase reactive oxygen and reactive nitrogen species levels, which promote DNA oxidative modification [64].
3. Metabolic dysregulation: Dysbiosis can lead to altered metabolism and carcinogenic conditions. Pathogenic bacteria can alter bile acids into secondary forms that are toxic to the gastrointestinal tract, especially in cases of colorectal cancer as earlier reported [65]. According to a Korean study, cholecystectomy, or the removal of the gall bladder, raises the risk of cancer in the colon, liver, pancreas, biliary system, throat, and oral cavity, among other gastrointestinal sites [66]. Once more, changes in the microbiota result in the formation of toxic compounds such as nitrosamines, which are as dangerous as they are carcinogenic [67].
4. Dysbiosis: Alterations in the environment or the host can trigger these changes, influencing the onset and progression of diseases such as cancer. Abuse of antibiotics can also contribute to the development of cancer and exacerbate its aggressiveness, as they eradicate all bacteria within the body, including those that are beneficial to health, promote the growth of harmful bacteria, and eliminate all bacteria without preserving the harmless ones [68]. Volkova and colleagues established that early-life exposure to the antibiotic penicillin has a negative impact on the expression of amygdala genes, the frontal brain, and the gut flora [69]. Researchers have established a connection between the gut microbiota and cancer in distant organs like the breast, liver, pancreas, and lung [70–73]. Researchers have isolated bacteria not only from the gut, but also from both normal and malignant breast tissues [74]. A microbial imbalance affects hormones that are associated with cancer risk [75]. The enterohepatic circulation involves oestrogens, and reports also suggest a role for bacteria in the gastrointestinal tract. Dysbiosis can also elevate circulating oestrogen levels, for instance, a factor linked to breast and endometrial cancers [76].
5. Metabolism and carcinogenesis: The microbiota plays a role in the breakdown of numerous nutrients and could therefore directly affect the production of carcinogenic metabolites. For instance, certain bacteria have the ability to ferment dietary fibres into short-chain fatty acids (SCFAs,) which are known to possess anti-inflammatory and anti-cancer properties [77]. Other microbial metabolites, such as secondary bile acids and trimethylamine N-oxide (TMAO), are known to promote carcinogenic activity in the gut and other related organisms [78]. Alteration of the microbiota favours the formation of toxic compounds, precursors of nitrosamines that are toxic and carcinogenic [79].
Microbiota as biomarkers in cancer diagnosis
Biomarkers are quantifiable variables used to assess a specific biological state or status [97]. In cancer diagnosis, biomarkers can be substances found in the body, such as those produced by the tumour or changes that occur due to the tumour’s presence [98]. Microbiota as biomarkers for cancer diagnosis is a relatively new approach that aims to gain information about the specific microbial ecosystem in a human body to determine the presence of cancer or even its type [99]. Numerous studies have established a correlation between certain bacterial profiles and various cancer types, including colorectal cancer (CRC), gastric cancer (GC), hepatocellular carcinoma (HCC), pancreatic cancer (PC), and esophageal cancer (EC) [100, 101]. For example, in CRC, colibactin-producing Escherichia coli (CoPEC) adheres strongly to the colonic mucosa, and these bacteria actively contribute to CRC development in preclinical models [99]. As a novel predictive biomarker and therapeutic aim to enhance CRC prognosis and treatment, CoPEC may open a new perspective [102]. Prevotella intermedia, Porphyromonic asaccharolytica, Peptostreptococcus anaerobitis, Parvimonas micra, Dialister pneumosintes, and Anaerococcus vaginalis are some other possible biomarkers for CRC [103]. The identification of the oral Microbiota is another approach that appears to be less invasive and holds potential for diagnosing CRC [104]. Finally, researchers have proposed oral microbiota as biomarkers for CRC diagnosis, but further research is necessary to establish their diagnostic efficiency. Researchers have also attempted to understand the lung microbiota from a lung cancer perspective [105]. Depending on the local environment, researchers have identified some bacterial species as strongly associated with lung cancer, such as the Streptococcus genus in the respiratory tract [106]. Researchers in PC have detected 16S rRNA-known biomarkers in both faecal and saliva samples [107], and the integration of Microbiota data with serum CA19-9 enhances the diagnostic precision [108]. However, the results obtained vary between studies, indicating the need for further validation of the findings. Some studies involving cervical cancer have focused on changes in the composition of the vaginal microbiota. Studies have shown that while Gardnerella vaginalis and Atopobium vaginae, which are normally anaerobic bacteria, are more abundant in cervical cancer and its precursors [109], Lactobacillus species are less prevalent in cervical cancer cases compared to normal women [110]. A study by Wu et al. [111] shows a correlation between changes in reproductive-age women from HPV infection to cervical cancer and the composition of their cervical microbiota, with higher diversity and less Lactobacillus resulting in a more severe pathological state. Variations in the oral microbiota may orchestrate head and neck cancers, according to Benjamin and colleagues [112]. Particularly, studies have highlighted the presence of Porphyromonas gingivalis and Treponema denticola in higher concentrations in any of these cancers [113]. Despite the immense potential of the Microbiota as a cancer biomarker, it is still premature for clinical application. There is a need to engage in larger, prospective, multicentric studies that use uniform sampling and analytical approaches to ascertain the role of the Microbiota as an early cancer marker.
Microbiota and cancer therapy response
Researchers have identified the human Microbiota, most commonly the gut, as having high relevance in regulating the efficacy of cancer treatment. These studies have found different ways that the microbiota can influence cancer therapy outcomes by acting on factors such as immunity response, drug pharmacokinetics, and tumour microenvironment [114]. Some key findings and concepts are as follows and presented in Table 2:
Table 2.
Role of gut microbiota in modulating cancer therapy response: mechanisms and therapeutic implications
| S/no. | Key aspect | Description | Microbial influence | Mechanism of action | Therapeutic impact | Potential interventions | References |
|---|---|---|---|---|---|---|---|
| 1 | Immune Modulation | Gut microbiota affects immune response in cancer therapy | Bifidobacterium, Akkermansia | Stimulates dendritic cell maturation and T-cell infiltration | Enhances immunotherapy response | Probiotic supplementation, microbiota-targeted therapies | [114–117] |
| 2 | Chemotherapy Efficacy | Microbiota influences chemotherapy drug metabolism | Microbial enzymes, metabolites short-chain fatty acids (SCFAs) | Modifies pharmacokinetics and pharmacodynamics of drugs | Can enhance or reduce drug potency | Personalized Microbiota modulation, prebiotics | [118–120] |
| 3 | Drug resistance | Dysbiosis contributes to resistance to cancer therapies | Altered microbiota composition | Affects drug metabolism and immune modulation | Reduces effectiveness of chemotherapy, immune checkpoint inhibitors | Fecal microbiota transplantation (FMT), Microbiota restoration | [121–123] |
| 4 | Tumour microenvironment | Microbial metabolites impact inflammation and tumour progression | SCFAs, butyrate | Regulates immune function and inflammation in tumours | Can promote or inhibit tumour growth | Probiotic and metabolite-based therapies | [124, 125] |
| 5 | Histone modification | Microbiota influences epigenetic regulation in cancer | Butyrate-producing bacteria | Interacts with histone deacetylase, modifies gene expression | Potential role in cancer prevention and therapy | Epigenetic drugs targeting microbiota interactions | [126] |
| 6 | Fecal microbiota transplantation (FMT) | Restoration of healthy microbiota to improve treatment response | Diverse gut microbiota | Balances immune response and improves drug efficacy | Enhances response in patients non-responsive to immunotherapy | Clinical trials, personalized microbiota transplants | [123] |
| 7 | Inflammation control | Gut microbiota regulates inflammation affecting cancer therapy | Anti-inflammatory SCFAs | Reduces pro-inflammatory cytokine levels | Creates a favorable immune environment for therapy | Diet-based microbiota modulation, anti-inflammatory probiotics | [124, 125] |
| 8 | Future directions | Advancing microbiota-based therapies in oncology | Personalized microbiota interventions | Precision medicine approach for cancer treatment | Increased therapy success, reduced resistance | Microbiota sequencing, AI-driven microbiota analysis | [114, 126] |
Immune modulation
The composition of the communities of microbes that dwell in the gut may also promote or inhibit tumour immunity, thereby modulating the effectiveness of immunotherapy, chemotherapy, radiation therapy, and cancer surgery [115]. Some forms of bacteria, such as Bifidobacterium and Akkermansia, have an important role to play in the enhancement of immunotherapy [116]. Some of these commensal bacteria can also increase the body's immune responses by stimulating dendritic cell maturation and increasing T-cell infiltration in tumours [117].
Chemotherapy
The microbiota influences the effectiveness of cancer treatments through the metabolism process. Microbial metabolites, such as short-chain fatty acids, can influence the immune response against cancer cells as well as the efficacy of cancer therapy [118]. Microbial enzymes also influence the pharmacokinetics of chemotherapeutic agents used in cancer treatments [119]. The microbiota has the ability to affect the pharmacokinetics and pharmacodynamics of oral chemotherapy drugs, thus determining their potency [120]. Zhao et al. [121] in their study revealed a link between gut microbiota dysbiosis and resistance to cancer therapies like chemotherapy and immune checkpoint inhibitors. Research shows that restoring a healthy gut microbiota effectively addresses issues of treatment failure [122]. Faecal microbiota transplantation has evidenced a positive potential for enhancing prognoses for patients who are non-responsive to immunotherapy [123].
Inflammation and metabolites
The products of the microbiota, such as metabolites and inflammatory factors, can influence the tumour environment and, thus, the progress and treatment of cancer [124]. For instance, gut bacteria synthesize short-chain fatty acids, which have anti-inflammatory properties and can regulate immune function in the tumour microenvironment [125]. Some pro-biotic microbial metabolites, such as butyrate, interact with histone deacetylase and may control gene expression in a way that helps treat and prevent cancer [126].
Microbiota-targeted therapeutic strategies
The consideration of developing a microbiota-therapeutic approach has hit the limelight because of the profound involvement of the microbiota in physiology and pathophysiology, including cancer development and response to treatment. Such strategies include antibiotic therapy, use of probiotics and prebiotics, foetal microbiota transplantation, diet intervention, small molecule-borne inhibitors, and bacteriophage therapy [127–129]. Antibiotics can neutralise or inactivate dangerous bacteria and also control the immune response. Probiotics introduce friendly bacteria that can replace the undesirable ones and help to strengthen the body’s ability to fight cancer. Lactobacillus and Bifidobacterium are two major types of probiotics that have demonstrated positive results in experimental cancer therapy by inhibiting tumour growth and fortifying immunity [130].
Prebiotics are substances present in food that the human body does not digest, yet they stimulate the growth of beneficial bacteria in the stomach, aiding in coping with chemotherapy side effects and preserving stomach function [131]. Inulin and fructooligosaccharides are popular prebiotics that improve gut bacteria such as Bifidobacteria and Lactobacilli [132]. Faecal microbiota transplantation accelerates the improvement of the disrupted microbiota balance and increases treatment effectiveness [123]. A changing diet can affect the community of microorganisms in the gut and increase the population of beneficial microbes. For instance, high-fibre diets promote the growth of beneficial bacteria in the intestines, which produce short-chain fatty acids (SCFAs), such as butyrate, which play a significant role in promoting anti-inflammatory and anti-cancer effects [133]. Small-molecule inhibitors interfere with dangerous bacterial processes and decrease toxic byproducts. For example, taurine metabolism inhibitors affect bacterial taurine metabolism in order to minimise its immunosuppressive activity during chemotherapy [134]. Phage therapy targets and eliminates only the pathogenic bacteria helping to prevent dysbiosis, while the bacteria aiding digestion remain intact [121]. However, because the human Microbiota is diverse and unique in each patient, these strategies must be patient-specific.
Immunotherapy and radiotherapy influence on microbiota
Recent research suggests that the microbiota plays a crucial role in the efficacy and side effects of cancer treatments. Immunotherapy, including immune checkpoint inhibitors, can be influenced by the gut microbiota, which plays a crucial role in shaping the immune response [135]. Firmicutes and Bacteroidetes, which are specific microbial populations, have been associated with improved outcomes in patients undergoing immune checkpoint inhibitors [136]. The microbiota also influences the development of immune-related adverse events (irAEs), such as colitis or dermatitis [137]. Microbial metabolites, such as short-chain fatty acids (SCFAs), can modulate immune cells and enhance immune responses, improving systemic inflammation or influencing the tumor microenvironment [138].
Radiotherapy can profoundly alter the microbiota, particularly in the gut, due to radiation-induced damage to both normal and cancerous cells [135]. Dysbiosis, particularly in abdominal or pelvic cancer treatments, can lead to gastrointestinal symptoms such as diarrhea, mucositis, and colitis [139]. A healthy microbiota can enhance the anti-tumor effects of radiation by supporting immune responses, while dysbiosis can diminish immune activation, potentially reducing the therapeutic efficacy of radiotherapy. Specific microbes, such as those producing metabolites like butyrate, can counteract radiation-induced immune suppression and improve recovery, potentially alleviating side effects [135, 140].
Limitations of microbiota-based therapies
Microbiota-based therapies, including probiotics, prebiotics, synbiotics, fecal microbiota transplantation (FMT), and Microbiota-targeted drugs, have gained significant attention for their potential in treating various health conditions. However, several challenges and limitations hinder their widespread application. These limitations can be classified into biological, technical, clinical, regulatory, ethical, and economic concerns [121, 135, 141–144].
Biological limitations
The human gut microbiota is extremely personalized, shaped by genetics, nutrition, environment, age, and lifestyle choices. A treatment that is beneficial for one person may not be helpful for another owing to variations in microbial diversity and functioning. Research on Microbiota functionality remains inadequate, and the roles of various bacterial strains and their metabolites in illness prevention or therapy are not well understood. The native gut microbiota has inherent resilience; however, given probiotics may lack long-term persistence, hence limiting their effectiveness. Horizontal gene transfer among bacteria, particularly involving antibiotic resistance genes, may provide health hazards, and the introduction of novel microbial strains might unintentionally exacerbate antibiotic resistance.
Technical limitations
Isolating and characterizing essential microbial strains within gut microbiota is difficult owing to the uncultivable conditions present in laboratory settings. Despite advancements in metagenomic sequencing for identification, culture continues to be a hurdle. Standardization challenges persist, and the heterogeneity among commercial probiotic strains hinders their effectiveness. Challenges related to stability and storage exist, since probiotics and microbiota-derived products may deteriorate with time. The intricacy of microbial relationships complicates result predictions, because modifying one species might adversely affect the total Microbiota equilibrium.
Clinical limitations
The effectiveness of probiotics and fecal microbiota therapy (FMT) is a topic of debate due to several factors. These include inconsistent clinical outcomes, short-term efficacy, difficulty in targeted delivery, adverse effects in vulnerable populations, and limited long-term safety data. Studies often fail to replicate results due to variations in design, dosage, and patient populations. Probiotics often do not permanently colonize the gut, necessitating repeated dosing. Targeted delivery is also challenging, with encapsulation and formulation strategies being explored but still imperfect. Adverse effects in vulnerable populations include infections from live microbial therapies, bacteremia and fungemia in critically ill patients, and limited long-term safety data.
Regulatory limitations
Microbiota-based products face several challenges, including a lack of clear classification, inconsistent approval processes due to different guidelines from regulatory bodies like the FDA (U.S. Food and Drug Administration) and the EMA (European Medicines Agency), and inconsistencies in microbial strain composition and viability due to variability in manufacturing processes. Additionally, many commercial probiotics lack scientific validation and quality assurance. Clinical trial design is complex due to individual Microbiota differences and ethical concerns arise from using fecal microbiota transplantation as a standard treatment without full regulatory approval.
Ethical and safety limitations
Fecal microbiota transplantation (FMT) raises ethical concerns due to rigorous donor screening, potential transmission of pathogens or undesirable traits, and unexplored long-term risks. Dysbiosis, a microbial imbalance, can result from FMT, potentially leading to new health issues and autoimmune or inflammatory diseases. Biocontainment and biosafety issues arise from the potential uncontrolled spread of engineered bacteria or genetically modified microbes in the environment. The impact of microbial interventions on ecosystems and human health is still uncertain. Informed consent challenges arise from patients not fully understanding potential risks and the ethical implications of modifying the gut Microbiota for non-medical purposes.
Economic and logistical limitations
Microbiota-based therapies hold great promise for treating various diseases, but significant challenges remain. High production costs, limited accessibility in low-resource settings, and logistical challenges in storage and transportation pose challenges. The rise of commercially marketed probiotics has led to exaggerated claims and consumer confusion, and many supplements lack clinical evidence. Personalized approaches are needed, as Microbiota profiling may require individualized treatment plans, increasing costs and complexity. Generalized probiotic formulations may not be effective for all patients. Overcoming these limitations will require advanced research, improved clinical trial designs, standardized regulatory guidelines, and more affordable therapeutic approaches. Until then, the integration of microbiota-based therapies into mainstream medicine will remain limited.
Conclusion
The intricate relationship between microbiota and cancer presents promising avenues for both understanding carcinogenesis and developing innovative therapeutic strategies. Beyond summarizing existing knowledge, future research should focus on delineating the precise microbial signatures associated with different cancer types, standardizing microbiota-based biomarkers for early diagnosis, and exploring personalized Microbiota interventions to enhance cancer therapy outcomes. Integrating microbiota-targeted therapies into clinical practice will require rigorous validation through large-scale clinical trials, improved regulatory frameworks, and the development of precise, patient-specific therapeutic approaches. Additionally, understanding how diet, probiotics, prebiotics, and fecal microbiota transplantation can be tailored to improve immunotherapy and chemotherapy responses remains a crucial area for exploration. Advancing this field will not only refine cancer treatment but also pave the way for preventive strategies that leverage the Microbiota’s role in maintaining immune homeostasis and reducing cancer risk.
Acknowledgements
Not applicable.
Author contributions
Authors contributions: Conceptualization: EUA, DEU, OPCU, Writing of original draft,: EUA, BNA, Validation: DEU, FOE, C A, Reviewing and editing: All authors.
Funding
This article did not receive any funding.
Data availability
Data used are within the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All Authors read and approved the manuscript for publication.
Patient consent
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Esther Ugo Alum, Email: esther.alum@kiu.ac.ug, Email: alumesther79@gmail.com.
Daniel Ejim Uti, Email: dan4uti@gmail.com, Email: daniel.ejimuti@kiu.ac.ug.
References
- 1.Khan AA, Nema V, Khan Z. Current status of probiotics for prevention and management of gastrointestinal cancers. Expert Opin Biol Ther. 2021;21:413–22. 10.1080/14712598.2021.1828858. [DOI] [PubMed] [Google Scholar]
- 2.Khan AA, Sirsat AT, Singh H, Cash P. Microbiota and cancer: current understanding and mechanistic implications. Clin Transl Oncol. 2022;24:193–202. 10.1007/s12094-021-02690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ugwu OP-C, Alum EU, Okon MB, Obeagu EI: Mechanisms of microbiota modulation: Implications for health, disease, and therapeutic interventions. Medicine (Baltimore). 2024;103:e38088. 10.1097/MD.0000000000038088 [DOI] [PMC free article] [PubMed]
- 4.Al-Rashidi HE. Gut microbiota and immunity relevance in eubiosis and dysbiosis. Saudi J Biol Sci. 2022;29:1628–43. 10.1016/j.sjbs.2021.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asseri AH, Bakhsh T, Abuzahrah SS, Ali S, Rather IA. The gut dysbiosis-cancer axis: illuminating novel insights and implications for clinical practice. Front Pharmacol. 2023;14:1208044. 10.3389/fphar.2023.1208044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prospect into therapeutic potentials of Moringa oleifera phytocompounds against cancer upsurge: de novo synthesis of test compounds, molecular docking, and ADMET studies. Bulletin of the National Research Centre. 10.1186/s42269-021-00554-6
- 7.Auctores. Beyond conventional therapies: exploring nutritional interventions for cervical cancer patients. https://auctoresonline.org/article/beyond-conventional-therapies-exploring-nutritional-interventions-for-cervical-cancer-patients
- 8.Alum E, Obeagu E, PC U, Orji O, Adepoju A, Oyedeji Amusa M. Exploring natural plant products in breast cancer management: a comprehensive review and future prospects. Int J Innov Appl Res. 2023;11:1–9. 10.58538/IJIAR/2055
- 9.Obeagu EI, Omar DE, Bunu UO, Obeagu GU, Alum EU, Ugwu OP. Leukaemia burden in Africa. Int J Curr Res Biol Med. 2023;1:17–22. 10.22192/ijcrbm.2023.08.01.003
- 10.Garg S, Sharma N, Bharmjeet DA. Unraveling the intricate relationship: influence of microbiome on the host immune system in carcinogenesis. Cancer Rep. 2023;6:e1892. 10.1002/cnr2.1892 [DOI] [PMC free article] [PubMed]
- 11.Ciernikova S, Sevcikova A, Stevurkova V, Mego M. Tumor microbiome—an integral part of the tumor microenvironment. Front Oncol. 2022;12:1063100. 10.3389/fonc.2022.1063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia C, Su J, Liu C, Mai Z, Yin S, Yang C, Fu L. Human microbiomes in cancer development and therapy. MedComm. 2023;4: e221. 10.1002/mco2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Wang Q, He L, Sun X. The critical role of tumor microbiome in cancer immunotherapy. Cancer Biol Ther. 2024;25:2301801. 10.1080/15384047.2024.2301801 [DOI] [PMC free article] [PubMed]
- 14.Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371:eabc4552. 10.1126/science.abc4552 [DOI] [PMC free article] [PubMed]
- 15.Ting NL-N, Lau HC-H, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71:1412–25. 10.1136/gutjnl-2021-326264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekaboruah E, Suryavanshi MV, Chettri D, Verma AK. Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch Microbiol. 2020;202:2147–67. 10.1007/s00203-020-01931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Z, Zuo T, Frey N, Rangrez AY. A systematic framework for understanding the microbiome in human health and disease: from basic principles to clinical translation. Signal Transduct Target Ther. 2024;9:1–36. 10.1038/s41392-024-01946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen BA, Heyndrickx M, Jonkers D, Mackie A, Millet S, Naghibi M, Pærregaard SI, Pot B, Saulnier D, Sina C, Sterkman LG. Small intestine vs. colon ecology and physiology: Why it matters in probiotic administration. Cell Rep Med. 2023;4(9):101190. 10.1016/j.xcrm.2023.101190 [DOI] [PMC free article] [PubMed]
- 19.Zhou C, Bisseling TM, van der Post RS, Boleij A. The influence of Helicobacter pylori, proton pump inhibitor, and obesity on the gastric microbiome in relation to gastric cancer development. Comput Struct Biotechnol J. 2024;23:186–98. 10.1016/j.csbj.2023.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Archambault L, Koshy-Chenthittayil S, Thompson A, Dongari-Bagtzoglou A, Laubenbacher R, Mendes P. Understanding Lactobacillus paracasei and Streptococcus oralis biofilm interactions through agent-based modeling. Msphere. 2021;6(6):e00875–21. 10.1128/mSphere.00875-21 [DOI] [PMC free article] [PubMed]
- 21.Kastl AJ, Terry NA, Wu GD, Albenberg LG. The structure and function of the human small intestinal microbiota: current understanding and future directions. Cell Mol Gastroenterol Hepatol. 2019;9:33. 10.1016/j.jcmgh.2019.07.006 [DOI] [PMC free article] [PubMed]
- 22.Delbaere K, Roegiers I, Bron A, Durif C, Van de Wiele T, Blanquet-Diot S, Marinelli L. The small intestine: dining table of host–microbiota meetings. FEMS Microbiol Rev. 2023;47:fuad022. 10.1093/femsre/fuad022 [DOI] [PMC free article] [PubMed]
- 23.Hou K, Wu Z-X, Chen X-Y, Wang J-Q, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen Z-S. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:1–28. 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu C, Shen H. Microbes in health and disease: human gut microbiota. Appl Sci. 2024;14:11354. 10.3390/app142311354. [Google Scholar]
- 25.Gueniche A, Perin O, Bouslimani A, Landemaine L, Misra N, Cupferman S, Aguilar L, Clavaud C, Chopra T, Khodr A. Advances in microbiome-derived solutions and methodologies are founding a new era in skin health and care. Pathogens. 2022;11:121. 10.3390/pathogens11020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dessinioti C, Katsambas A. The microbiome and acne: perspectives for treatment. Dermatol Ther. 2024;14:31–44. 10.1007/s13555-023-01079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachdeva C, Satyamoorthy K, Murali TS. Microbial interplay in skin and chronic wounds. Curr Clin Microbiol Rep. 2022;9:21–31. 10.1007/s40588-022-00180-4. [Google Scholar]
- 28.Zhang X-E, Zheng P, Ye S-Z, Ma X, Liu E, Pang Y-B, He Q-Y, Zhang Y-X, Li W-Q, Zeng J-H, Guo J. Microbiome: role in inflammatory skin diseases. J Inflamm Res. 2024;17:1057–82. 10.2147/JIR.S441100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hrestak D, Matijašić M, Čipčić Paljetak H, Ledić Drvar D, Ljubojević Hadžavdić S, Perić M. Skin microbiota in atopic dermatitis. Int J Mol Sci. 2022;23:3503. 10.3390/ijms23073503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrientos-Durán A, Fuentes-López A, de Salazar A, Plaza-Díaz J, García F. Reviewing the composition of vaginal microbiota: inclusion of nutrition and probiotic factors in the maintenance of eubiosis. Nutrients. 2020;12:419. 10.3390/nu12020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.France M, Alizadeh M, Brown S, Ma B, Ravel J. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol. 2022;7:367–78. 10.1038/s41564-022-01083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Lu Y, Chen T, Li R. The female vaginal microbiome in health and bacterial vaginosis. Front Cell Infect Microbiol. 2021;11: 631972. 10.3389/fcimb.2021.631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Factories. 2020;19:203. 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Y, Liu Z, Chen T. Role of vaginal microbiota dysbiosis in gynecological diseases and the potential interventions. Front Microbiol. 2021;12: 643422. 10.3389/fmicb.2021.643422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mondal AS, Sharma R, Trivedi N. Bacterial vaginosis: a state of microbial dysbiosis. Med Microecol. 2023;16: 100082. 10.1016/j.medmic.2023.100082. [Google Scholar]
- 36.Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019;17:87. 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Xu C, Zhong C, Lyu Z, Liu J, Chen Z, Dun H, Xin B, Xie Q. Temporal characteristics of the oropharyngeal and nasal microbiota structure in crewmembers stayed 180 days in the controlled ecological life support system. Front Microbiol. 2021;11:617696. 10.3389/fmicb.2020.617696 [DOI] [PMC free article] [PubMed]
- 38.Kang HM, Kang JH. Effects of nasopharyngeal microbiota in respiratory infections and allergies. Clin Exp Pediatr. 2021;64:543–51. 10.3345/cep.2020.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stavropoulou E, Kantartzi K, Tsigalou C, Konstantinidis T, Voidarou C, Konstantinidis T, Bezirtzoglou E. Unraveling the interconnection patterns across lung microbiome, respiratory diseases, and COVID-19. Front Cell Infect Microbiol. 2021;10: 619075. 10.3389/fcimb.2020.619075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utembe W, Kamng’ona AW: Inhalation exposure to chemicals, microbiota dysbiosis and adverse effects on humans. Sci Total Environ. 2024;955:176938. 10.1016/j.scitotenv.2024.176938 [DOI] [PubMed]
- 41.Yuksel N, Gelmez B, Yildiz-Pekoz A. Lung microbiota: its relationship to respiratory system diseases and approaches for lung-targeted probiotic bacteria delivery. Mol Pharm. 2023;20:3320–37. 10.1021/acs.molpharmaceut.3c00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belizário J, Garay-Malpartida M, Faintuch J. Lung microbiome and origins of the respiratory diseases. Curr Res Immunol. 2023;4: 100065. 10.1016/j.crimmu.2023.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawalec A, Zwolińska D. Emerging role of microbiome in the prevention of urinary tract infections in children. Int J Mol Sci. 2022;23:870. 10.3390/ijms23020870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi HW, Lee KW, Kim YH. Microbiome in urological diseases: axis crosstalk and bladder disorders. Investig Clin Urol. 2023;64:126–39. 10.4111/icu.20220357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruța F, Pribac M, Mardale E, Suciu S, Maior R, Bogdan S, Avram C. Associations between gut microbiota dysbiosis and other risk factors in women with a history of urinary tract infections. Nutrients. 2024;16:1753. 10.3390/nu16111753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altveş S, Yildiz HK, Vural HC. Interaction of the microbiota with the human body in health and diseases. Biosci Microbiota Food Health. 202;39:23–32. 10.12938/bmfh.19-023 [DOI] [PMC free article] [PubMed]
- 47.Khaledi M, Poureslamfar B, Alsaab HO, Tafaghodi S, Hjazi A, Singh R, Alawadi AH, Alsaalamy A, Qasim QA, Sameni F. The role of gut microbiota in human metabolism and inflammatory diseases: a focus on elderly individuals. Ann Microbiol. 2024;74:1. 10.1186/s13213-023-01744-5. [Google Scholar]
- 48.Balto H, Al-Hadlaq S, Alhadlaq A, El-Ansary A. Gum-gut axis: the potential role of salivary biomarkers in the diagnosis and monitoring progress of inflammatory bowel diseases. Saudi Dent J. 2023;35:24–30. 10.1016/j.sdentj.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colella M, Charitos IA, Ballini A, Cafiero C, Topi S, Palmirotta R, Santacroce L. Microbiota revolution: how gut microbes regulate our lives. World J Gastroenterol. 2023;29:4368–83. 10.3748/wjg.v29.i28.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7: e7502. 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao T, Hsu R, Rafizadeh DL, Wang L, Bowlus CL, Kumar N, Mishra J, Timilsina S, Ridgway WM, Gershwin ME, Ansari AA, Shuai Z, Leung PSC. The gut ecosystem and immune tolerance. J Autoimmun. 2023;141: 103114. 10.1016/j.jaut.2023.103114. [DOI] [PubMed] [Google Scholar]
- 52.Yao Y, Cai X, Ye Y, Wang F, Chen F, Zheng C. The role of microbiota in infant health: from early life to adulthood. Front Immunol. 2021. 10.3389/fimmu.2021.708472 [DOI] [PMC free article] [PubMed]
- 53.Lopez LR, Bleich RM, Arthur JC. Microbiota effects on carcinogenesis: initiation, promotion and progression. Annu Rev Med. 2021;72:243–61. 10.1146/annurev-med-080719-091604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benešová I, Křížová Ľ, Kverka M. Microbiota as the unifying factor behind the hallmarks of cancer. J Cancer Res Clin Oncol. 2023;149:14429–50. 10.1007/s00432-023-05244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J, Queipo-Ortuño MI. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers. 2020;12:1406. 10.3390/cancers12061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akbar N, Khan NA, Muhammad JS, Siddiqui R. The role of gut microbiome in cancer genesis and cancer prevention. Health Sci Rev. 2022;2: 100010. 10.1016/j.hsr.2021.100010. [Google Scholar]
- 57.Reyes VE. Helicobacter pylori and its role in gastric cancer. Microorganisms. 2023;11:1312. 10.3390/microorganisms11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao M, Chu J, Feng S, Guo C, Xue B, He K, Li L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: a review. Biomed Pharmacother. 2023;164: 114985. 10.1016/j.biopha.2023.114985. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava R, Lodhi N. DNA methylation malleability and dysregulation in cancer progression: understanding the role of PARP1. Biomolecules. 2022;12:417. 10.3390/biom12030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García A, Mannion A, Feng Y, Madden CM, Bakthavatchalu V, Shen Z, Ge Z, Fox JG. Cytotoxic Escherichia coli strains encoding colibactin colonize laboratory mice. Microbes Infect. 2016;18:777–86. 10.1016/j.micinf.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian S, Chen M. Global research progress of gut microbiota and epigenetics: bibliometrics and visualized analysis. Front Immunol. 2024;15:1412640. 10.3389/fimmu.2024.1412640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim B, Song A, Son A, Shin Y. Gut microbiota and epigenetic choreography: Implications for human health: a review. Medicine (Baltimore). 2024;103:e39051. 10.1097/MD.0000000000039051 [DOI] [PMC free article] [PubMed]
- 63.Pepke ML, Hansen SB, Limborg MT. Unraveling host regulation of gut microbiota through the epigenome–microbiome axis. Trends Microbiol. 2024;32:1229–40. 10.1016/j.tim.2024.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Chanin RB, Winter MG, Spiga L, Hughes ER, Zhu W, Taylor SJ, Arenales A, Gillis CC, Büttner L, Jimenez AG, Smoot MP, Santos RL, Winter SE. Epithelial-derived reactive oxygen species enable AppBCX-mediated aerobic respiration of Escherichia coli during intestinal inflammation. Cell Host Microbe. 2020;28:780-788.e5. 10.1016/j.chom.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernstein H, Bernstein C. Bile acids as carcinogens in the colon and at other sites in the gastrointestinal system. Exp Biol Med. 2023;248:79–89. 10.1177/15353702221131858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi YJ, Jin EH, Lim JH, Shin CM, Kim N, Han K, Lee DH. Increased risk of cancer after cholecystectomy: a nationwide cohort study in Korea including 123,295 patients. Gut Liver. 2022;16:465–73. 10.5009/gnl210009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlström M, Moretti CH, Weitzberg E, Lundberg JO. Microbiota, diet and the generation of reactive nitrogen compounds. Free Radic Biol Med. 2020;161:321–5. 10.1016/j.freeradbiomed.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 68.Patangia DV, Anthony Ryan C, Dempsey E, Paul Ross R, Stanton C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen. 2022;11: e1260. 10.1002/mbo3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volkova A, Ruggles K, Schulfer A, Gao Z, Ginsberg SD, Blaser MJ. Effects of early-life penicillin exposure on the gut microbiome and frontal cortex and amygdala gene expression. iScience. 2021;24:102797. 10.1016/j.isci.2021.102797 [DOI] [PMC free article] [PubMed]
- 70.Lv G, Cheng N, Wang H. The gut microbiota, tumorigenesis, and liver diseases. Engineering. 2017;3:110–4. 10.1016/J.ENG.2017.01.017. [Google Scholar]
- 71.Álvarez-Mercado AI, del Valle Cano A, Fernández MF, Fontana L. Gut microbiota and breast cancer: the dual role of microbes. Cancers. 2023;15:443. 10.3390/cancers15020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ivleva EA, Grivennikov SI. Microbiota-driven mechanisms at different stages of cancer development. Neoplasia. 2022;32: 100829. 10.1016/j.neo.2022.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He R, Qi P, Shu L, Ding Y, Zeng P, Wen G, Xiong Y, Deng H. Dysbiosis and extraintestinal cancers. J Exp Clin Cancer Res. 2025;44:44. 10.1186/s13046-025-03313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song X, Wei C, Li X. The relationship between microbial community and breast cancer. Front Cell Infect Microbiol. 2022;12: 849022. 10.3389/fcimb.2022.849022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qasem HH, El-Sayed WM. The bacterial microbiome and cancer: development, diagnosis, treatment, and future directions. Clin Exp Med. 2024;25:12. 10.1007/s10238-024-01523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alizadehmohajer N, Shojaeifar S, Nedaeinia R, Esparvarinha M, Mohammadi F, Ferns GA, Ghayour-Mobarhan M, Manian M, Balouchi A. Association between the microbiota and women’s cancers—cause or consequences? Biomed Pharmacother. 2020;127: 110203. 10.1016/j.biopha.2020.110203. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y, Chen Y-X. Microbiota-associated metabolites and related immunoregulation in colorectal cancer. Cancers. 2021;13:4054. 10.3390/cancers13164054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W, An Y, Qin X, Wu X, Wang X, Hou H, Song X, Liu T, Wang B, Huang X, Cao H. Gut microbiota-derived metabolites in colorectal cancer: the bad and the challenges. Front Oncol. 2021;11: 739648. 10.3389/fonc.2021.739648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz-Saavedra S, García-González H, Arboleya S, Salazar N, Labra-Gayo JE, Díaz I, Gueimonde M, González S, de Los Reyes-Gavilán CG. Intestinal microbiota alterations by dietary exposure to chemicals from food cooking and processing. Application of data science for risk prediction. Comput Struct Biotechnol J. 2021;19:1081–91. 10.1016/j.csbj.2021.01.037 [DOI] [PMC free article] [PubMed]
- 80.Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells. 2023;12:184. 10.3390/cells12010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10:S49–66. 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lau LYJ, Quek SY. Probiotics: health benefits, food application, and colonization in the human gastrointestinal tract. Food Bioeng. 2024;3:41–64. 10.1002/fbe2.12078. [Google Scholar]
- 83.Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al-Habsi N, Al-Khalili M, Haque SA, Elias M, Olqi NA, Al Uraimi T. Health benefits of prebiotics, probiotics, synbiotics, and postbiotics. Nutrients. 2024;16:3955. 10.3390/nu16223955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamamah S, Gheorghita R, Lobiuc A, Sirbu I-O, Covasa M. Fecal microbiota transplantation in non-communicable diseases: recent advances and protocols. Front Med. 2022. 10.3389/fmed.2022.1060581 [DOI] [PMC free article] [PubMed]
- 86.Gholam-Mostafaei FS, Azimirad M, Naseri K, Nabavi-Rad A, Asadzadeh Aghdaei H, Shahrokh S, Ebrahimi Daryani N, Yadegar A, Zali MR. Intestinal microbiota changes pre- and post-fecal microbiota transplantation for treatment of recurrent Clostridioides difficile infection among Iranian patients with concurrent inflammatory bowel disease. Front Microbiol. 2023. 10.3389/fmicb.2023.1147945 [DOI] [PMC free article] [PubMed]
- 87.Olawade DB, Fapohunda O, Egbon E, Ebiesuwa OA, Usman SO, Faronbi AO, Fidelis SC. Phage therapy: a targeted approach to overcoming antibiotic resistance. Microb Pathog. 2024;197: 107088. 10.1016/j.micpath.2024.107088. [DOI] [PubMed] [Google Scholar]
- 88.Bacteriophages and their use in combating antimicrobial resistance. https://www.who.int/europe/news-room/fact-sheets/item/bacteriophages-and-their-use-in-combating-antimicrobial-resistance
- 89.Palma M, Qi B. Advancing phage therapy: a comprehensive review of the safety, efficacy, and future prospects for the targeted treatment of bacterial infections. Infect Dis Rep. 2024;16:1127–81. 10.3390/idr16060092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hijová E. Postbiotics as metabolites and their biotherapeutic potential. Int J Mol Sci. 2024;25:5441. 10.3390/ijms25105441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prajapati N, Patel J, Singh S, Yadav VK, Joshi C, Patani A, Prajapati D, Sahoo DK, Patel A. Postbiotic production: harnessing the power of microbial metabolites for health applications. Front Microbiol. 2023;14:1306192. 10.3389/fmicb.2023.1306192 [DOI] [PMC free article] [PubMed]
- 92.Zhao X, Liu S, Li S, Jiang W, Wang J, Xiao J, Chen T, Ma J, Khan MZ, Wang W, Li M, Li S, Cao Z. Unlocking the power of postbiotics: a revolutionary approach to nutrition for humans and animals. Cell Metab. 2024;36:725–44. 10.1016/j.cmet.2024.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Da M, Sun J, Ma C, Li D, Dong L, Wang LS, Chen F. Postbiotics: enhancing human health with a novel concept. eFood. 2024;5(5):e180.10.1002/efd2.180
- 94.Sahle Z, Engidaye G, Shenkute Gebreyes D, Adenew B, Abebe TA. Fecal microbiota transplantation and next-generation therapies: a review on targeting dysbiosis in metabolic disorders and beyond. SAGE Open Med. 2024;12:20503121241257490. 10.1177/20503121241257486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tiwari A, Krisnawati DI, Susilowati E, Mutalik C, Kuo T-R. Next-generation probiotics and chronic diseases: a review of current research and future directions. J Agric Food Chem. 2024;72:27679. 10.1021/acs.jafc.4c08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yaqub MO, Jain A, Joseph CE, Edison LK. Microbiome-driven therapeutics: from gut health to precision medicine. Gastrointest Disord. 2025;7:7. 10.3390/gidisord7010007. [Google Scholar]
- 97.Bodaghi A, Fattahi N, Ramazani A. Biomarkers: promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon. 2023;9: e13323. 10.1016/j.heliyon.2023.e13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Obeagu EI, Ahmed YA, Obeagu GU, Bunu UO, Ugwu OP, Alum EU. Biomarkers of breast cancer: Overview. Int J Curr Res Biol Med. 2023;1:8–16.10.22192/ijcrbm.2023.08.01.002
- 99.Veziant J, Villéger R, Barnich N, Bonnet M. Gut microbiota as potential biomarker and/or therapeutic target to improve the management of cancer: focus on colibactin-producing escherichia coli in colorectal cancer. Cancers. 2021;13:2215. 10.3390/cancers13092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jardim SR, de Souza LMP, de Souza HSP. The rise of gastrointestinal cancers as a global phenomenon: unhealthy behavior or progress? Int J Environ Res Public Health. 2023;20:3640. 10.3390/ijerph20043640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xuan M, Gu X, Liu Y, Yang L, Li Y, Huang D, Li J, Xue C. Intratumoral microorganisms in tumors of the digestive system. Cell Commun Signal CCS. 2024;22:69. 10.1186/s12964-023-01425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ogunwobi OO, Mahmood F, Akingboye A. Biomarkers in colorectal cancer: current research and future prospects. Int J Mol Sci. 2020;21:5311. 10.3390/ijms21155311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Osman MA, Neoh H, Ab Mutalib N-S, Chin S-F, Mazlan L, Raja Ali RA, Zakaria AD, Ngiu CS, Ang MY, Jamal R. Parvimonas micra, Peptostreptococcus stomatis, Fusobacterium nucleatum and Akkermansia muciniphila as a four-bacteria biomarker panel of colorectal cancer. Sci Rep. 2021;11:2925. 10.1038/s41598-021-82465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Negrut RL, Cote A, Maghiar AM. Exploring the potential of oral microbiome biomarkers for colorectal cancer diagnosis and prognosis: a systematic review. Microorganisms. 2023;11:1586. 10.3390/microorganisms11061586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y, Liu Y, Li S, Peng Z, Liu X, Chen J, Zheng X. Role of lung and gut microbiota on lung cancer pathogenesis. J Cancer Res Clin Oncol. 2021;147:2177–86. 10.1007/s00432-021-03644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng J, Zhou L, Wang H. Symbiotic microbial communities in various locations of the lung cancer respiratory tract along with potential host immunological processes affected. Front Cell Infect Microbiol. 2024;14:1296295. 10.3389/fcimb.2024.1296295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Church DL, Cerutti L, Gürtler A, Griener T, Zelazny A, Emler S. Performance and application of 16S rRNA gene cycle sequencing for routine identification of bacteria in the clinical microbiology laboratory. Clin Microbiol Rev. 2020;33:e00053-e119. 10.1128/CMR.00053-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dai J-H, Tan X-R, Qiao H, Liu N. Emerging clinical relevance of microbiome in cancer: promising biomarkers and therapeutic targets. Protein Cell. 2023;15:239–60. 10.1093/procel/pwad052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fong Amaris WM, de Assumpção PP, Valadares LJ, Moreira FC. Microbiota changes: the unseen players in cervical cancer progression. Front Microbiol. 2024;15:1352778. 10.3389/fmicb.2024.1352778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Auctores. Cervical Cancer Prevention Paradox: Unveiling Screening Barriers and Solutions. https://auctoresonline.org/article/cervical-cancer-prevention-paradox-unveiling-screening-barriers-and-solutions
- 111.Wu S, Ding X, Kong Y, Acharya S, Wu H, Huang C, Liang Y, Nong X, Chen H. The feature of cervical microbiota associated with the progression of cervical cancer among reproductive females. Gynecol Oncol. 2021;163:348–57. 10.1016/j.ygyno.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 112.Benjamin WJ, Wang K, Zarins K, Bellile E, Blostein F, Argirion I, Taylor JMG, D’Silva NJ, Chinn SB, Rifkin S, Sartor MA, Rozek LS. Oral microbiome community composition in head and neck squamous cell carcinoma. Cancers. 2023;15:2549. 10.3390/cancers15092549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou Y, Luo G-H. Porphyromonas gingivalis and digestive system cancers. World J Clin Cases. 2019;7:819–29. 10.12998/wjcc.v7.i7.819 [DOI] [PMC free article] [PubMed]
- 114.Kouidhi S, Zidi O, Belkhiria Z, Rais H, Ayadi A, Ayed FB, Mosbah A, Cherif A, El Gaaied AB. Gut microbiota, an emergent target to shape the efficiency of cancer therapy. Explor Targeted Anti-tumor Therapy. 2023;4(2):240.10.37349/etat.2023.00132 [DOI] [PMC free article] [PubMed]
- 115.Kang X, Lau HC-H, Yu J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep Med. 2024;5: 101478. 10.1016/j.xcrm.2024.101478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao X, Zhao J, Li D, Yang H, Chen C, Qin M, Wen Z, He Z, Xu L. Akkermansia muciniphila: a potential target and pending issues for oncotherapy. Pharmacol Res. 2023;196: 106916. 10.1016/j.phrs.2023.106916. [DOI] [PubMed] [Google Scholar]
- 117.Matson V, Chervin CS, Gajewski TF. Cancer and the microbiome—influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology. 2021;160:600–13. 10.1053/j.gastro.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hersi F, Elgendy SM, Al Shamma SA, Altell RT, Sadiek O, Omar HA. Cancer immunotherapy resistance: the impact of microbiome-derived short-chain fatty acids and other emerging metabolites. Life Sci. 2022;300: 120573. 10.1016/j.lfs.2022.120573. [DOI] [PubMed] [Google Scholar]
- 119.Pathania S, Bhatia R, Baldi A, Singh R, Rawal RK. Drug metabolizing enzymes and their inhibitors’ role in cancer resistance. Biomed Pharmacother. 2018;105:53–65. 10.1016/j.biopha.2018.05.117. [DOI] [PubMed] [Google Scholar]
- 120.Yu H, Xu H, Yang X, Zhang Z, Hu J, Lu J, Fu J, Bu M, Zhang H, Zhai Z, Wang J, Jiang J, Wang Y. Gut microbiota-based pharmacokinetic-pharmacodynamic study and molecular mechanism of specnuezhenide in the treatment of colorectal cancer targeting carboxylesterase. J Pharm Anal. 2023;13:1024–40. 10.1016/j.jpha.2023.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao L-Y, Mei J-X, Yu G, Lei L, Zhang W-H, Liu K, Chen X-L, Kołat D, Yang K, Hu J-K. Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications. Signal Transduct Target Ther. 2023;8:1–27. 10.1038/s41392-023-01406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ji J, Jin W, Liu S, Jiao Z, Li X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm. 2023;4: e420. 10.1002/mco2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang Y-B, Cai Y. Faecal microbiota transplantation enhances efficacy of immune checkpoint inhibitors therapy against cancer. World J Gastroenterol. 2021;27:5362–75. 10.3748/wjg.v27.i32.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luu M, Schütz B, Lauth M, Visekruna A. The impact of gut microbiota-derived metabolites on the tumor immune microenvironment. Cancers. 2023;15:1588. 10.3390/cancers15051588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gomes S, Rodrigues AC, Pazienza V, Preto A. Modulation of the tumor microenvironment by microbiota-derived short-chain fatty acids: impact in colorectal cancer therapy. Int J Mol Sci. 2023;24:5069. 10.3390/ijms24065069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schemczssen-Graeff Z, Pileggi M. Probiotics and live biotherapeutic products aiming at cancer mitigation and patient recover. Front Genet. 2022;13: 921972. 10.3389/fgene.2022.921972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hitch TCA, Hall LJ, Walsh SK, Leventhal GE, Slack E, de Wouters T, Walter J, Clavel T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022;15:1095–113. 10.1038/s41385-022-00564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quaranta G, Guarnaccia A, Fancello G, Agrillo C, Iannarelli F, Sanguinetti M, Masucci L. Fecal microbiota transplantation and other gut microbiota manipulation strategies. Microorganisms. 2022;10:2424. 10.3390/microorganisms10122424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang H, Jiang J, Wang X, Jiang K, Cao H. Exposure to prescribed medication in early life and impacts on gut microbiota and disease development. eClinicalMedicine. 2024;68:102428. 10.1016/j.eclinm.2024.102428 [DOI] [PMC free article] [PubMed]
- 130.Górska A, Przystupski D, Niemczura MJ, Kulbacka J. Probiotic bacteria: a promising tool in cancer prevention and therapy. Curr Microbiol. 2019;76:939–49. 10.1007/s00284-019-01679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang S, Xiao Y, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Rational use of prebiotics for gut microbiota alterations: specific bacterial phylotypes and related mechanisms. J Funct Foods. 2020;66: 103838. 10.1016/j.jff.2020.103838. [Google Scholar]
- 133.Zhang P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int J Mol Sci. 2022;23:9588. 10.3390/ijms23179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu G, Chen T, Zhang X, Ma X, Shi H. Small molecule inhibitors targeting the cancers. MedComm. 2022;3: e181. 10.1002/mco2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Z, Xiong W, Liang Z, Wang J, Zeng Z, Kołat D, Li X, Zhou D, Xu X, Zhao L. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J Hematol Oncol J Hematol Oncol. 2024;17:33. 10.1186/s13045-024-01541-w [DOI] [PMC free article] [PubMed]
- 136.Jiang H, Zhang Q. Gut microbiota influences the efficiency of immune checkpoint inhibitors by modulating the immune system (Review). Oncol Lett. 2024;27:87. 10.3892/ol.2024.14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu M, Lin X, Sun T, Shao X, Huang X, Du W, Guo M, Zhu X, Zhou Y, Tong T, Guo F, Han T, Wu X, Shi Y, Xiao X, Zhang Y, Hong J, Chen H. Gut microbiome for predicting immune checkpoint blockade-associated adverse events. Genome Med. 2024;16:16. 10.1186/s13073-024-01285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu X, Shao J, Liao Y-T, Wang L-N, Jia Y, Dong P, Liu Z, He D, Li C, Zhang X. Regulation of short-chain fatty acids in the immune system. Front Immunol. 2023;14:1186892. 10.3389/fimmu.2023.1186892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akbarali HI, Muchhala KH, Jessup DK, Cheatham S. Chemotherapy induced gastrointestinal toxicities. Adv Cancer Res. 2022;155:131–66. 10.1016/bs.acr.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Z, Zhang Y, Hong W, Wang B, Chen Y, Yang P, Zhou J, Fan J, Zeng Z, Du S. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma Via STING signaling. Gut Microbes. 2022;14:2119055. 10.1080/19490976.2022.2119055 [DOI] [PMC free article] [PubMed]
- 141.Yarahmadi A, Afkhami H. The role of microbiomes in gastrointestinal cancers: new insights. Front Oncol. 2024. 10.3389/fonc.2023.1344328 [DOI] [PMC free article] [PubMed]
- 142.Lan Z, Liu W-J, Cui H, Zou K-L, Chen H, Zhao Y-Y, Yu G-T. The role of oral microbiota in cancer. Front Microbiol. 2023;14:1253025. 10.3389/fmicb.2023.1253025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Z, Guan D, Wang Z, Li X, Dong S, Huang J, Zhou W. Microbiota in cancer: molecular mechanisms and therapeutic interventions. MedComm. 2023;4(6):e417. 10.1002/mco2.417 [DOI] [PMC free article] [PubMed]
- 144.Gulliver EL, Young RB, Chonwerawong M, D’Adamo GL, Thomason T, Widdop JT, Rutten EL, Marcelino VR, Bryant RV, Costello SP, O’Brien CL, Hold GL, Giles EM, Forster SC. Review article: the future of microbiome-based therapeutics. Aliment Pharmacol Ther. 2022;56:192. 10.1111/apt.17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used are within the manuscript.