Abstract
Purpose
Antimicrobial resistance poses a significant global health challenge, contributing to a lack of effective therapeutic agents, especially against Gram-negative bacteria. Resistance dissemination is accelerated by horizontal gene transfer (HGT) mechanisms. The extended-spectrum beta lactamases CTX-M confer resistance to several beta-lactams, are usually embedded into plasmids and thought to be mainly disseminated by conjugation. However, an increasing number of isolates carry these enzyme-encoding genes in the chromosome, suggesting that they can spread by other means of HGT. In this study, we aimed to test the involvement of natural transformation in the chromosomal acquisition of a blaCTX−M gene.
Methods
Natural transformation assays were performed during motility on wet surfaces. Acquisition of foreign DNA by transformants was screened by antimicrobial susceptibility testing, polymerase-chain reaction (PCR) and whole genome sequencing (WGS).
Results
Acinetobacter baumannii A118, a naturally competent clinical strain, was transformed with naked DNA from Salmonella enterica serovar Typhimurium Sal25, which was isolated from swine meat. The transformation occurred at low frequency (2.7 × 10− 8 ± 2.04 × 10− 8 transformants per recipient) and blaCTX−M was acquired in one transformant, which was named ACI. WGS of the transformant revealed the acquisition of the blaCTX−M−32 as part of a ca. 36 Kb DNA fragment through an ISEc9-mediated transposition event; various mobile genetic elements and other resistance genes were co-transferred. The blaCTX−M−32 gene was subsequently transferred within A. baumannii at a higher frequency (1.8 × 10− 6 ± 2.49 × 10− 6 transformants per recipient).
Conclusion
Our results highlight the importance of natural transformation events in the dissemination of antimicrobial resistance genes and mobile genetic elements between and within species.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-025-05113-9.
Keywords: Antimicrobial resistance, CTX-M, ISEc9, Natural transformation, Transposition
Introduction
Antimicrobial resistance represents one of the major threats to global public health, undermining the effectiveness of treatments and leading to higher mortality rates and healthcare costs associated with multidrug resistant infections [1]. The recently updated World Health Organization (WHO) list of pathogens that urgently need new antibiotics mainly includes Gram-negative bacteria, with Acinetobacter baumannii at the top of the critical group [2], due to the limited therapeutic options and the high rates of multidrug resistance. A. baumannii is also part of the ESKAPE pathogens [3]. Generally considered a nosocomial pathogen [4], A. baumannii can also be found in activated sludge, animals, food, sewage, soil and water [5–8], where it can cohabit with different bacterial species, including Salmonella enterica [9–11].
Amongst the resistance determinants, beta-lactamases are particularly concerning, especially in Gram-negative bacteria, as they confer resistance to the most widely used family of antibiotics, beta-lactams, including those considered as last resort therapeutic options. CTX-M enzymes are plasmid-encoded extended spectrum beta-lactamases (ESBL) commonly found worldwide [12], in isolates of human [13], animal [14] and environmental [15] origin. CTX-M are highly disseminated among Enterobacterales and dissemination by conjugation is well-established [16], but the prevalence in non-fermentative bacteria is less common [17]. CTX-M found in A. baumannii are usually chromosomally-encoded [17, 18] and the horizontal gene transfer (HGT) mechanisms involved in the acquisition are usually unknown; presence of these enzymes in A. baumannii reduces even more the therapeutic options against this multidrug-resistant pathogen. A. baumannii is able to undergo all HGT mechanisms [19–22], and this trait may be of major importance in the acquisition of its multidrug resistance profile. Although conjugation is considered a key driver of resistance genes spread among bacteria [23], natural transformation has been suggested as the main HGT mechanism involved in antibiotic resistance dissemination in A. baumannii [24]. Nonetheless few studies explore this intercellular mechanism [25], as well as the intracellular mobilization of the resistance genes remains scarcely known. Interspecies dissemination of blaCTX−M−2 from Proteus mirabilis to A. baumannii has been previously suggested [26], and the ability of A. baumannii to acquire blaCTX-M-115 from three A. baumannii clinical isolates by natural transformation followed by intracellular homologous recombination has been experimentally demonstrated [18]. Stable acquisition of a plasmid-encoding blaCTX−M−2 gene by A. baumannii A118 after DNA uptake by natural transformation has also been experimentally demonstrated [27].
The main aims of this study were to evaluate the involvement of natural transformation in interspecies blaCTX−M genes dissemination, as well as to understand the intracellular mechanisms associated with their stable acquisition.
Materials and methods
Bacterial isolates
The naturally competent A. baumannii A118, a clinical antimicrobial susceptible isolate [28], A. baylyi BD413, a soil bacterium [29], A. nosocomialis 013 and Acinetobacter sp. 065, clinical multidrug resistant isolates [22], were used as recipient in natural transformation experiments. S. enterica serovar Typhimurium Sal25, isolated from swine meat and carrying a class 1 integron with the aadA1 gene cassette, the mcr-1 gene and a blaCTX−M−1 group gene in an IncHI2 plasmid [30, 31], was the source of the donor DNA. A. baumannii ACI, a transformant that acquired the blaCTX−M−1 group gene, was also used as donor DNA in subsequent transformation assays.
DNA extraction
Genomic DNA for transformation assays was extracted from bacterial cultures using anion exchange columns (QIAGEN, Germany) according to the manufacturers protocol and resuspended in EB buffer, pH 8.5 (QIAGEN, Germany). The DNA concentration was measured with Nanodrop ND-1000 (Nanodrop Technologies, USA).
Natural transformation assays
Natural transformation assays were performed during motility on wet surfaces, as previously described [32]. Briefly, a single colony of the recipient cell was suspended in 20 µl of sterile phosphate-buffered saline (PBS) and mixed with 20 µl of donor DNA (4 µg in water). This mixture was introduced into the semisolid Motility Medium (MM; 0.5% agar (Liofilchem), 5 g/l tryptone (Difco) and 2.5 g/l sodium chloride (Scharlau)) by stabbing the medium seven times with 2 µl of the transformation mixture each time. The plate was sealed with Parafilm to prevent dryness of the medium and incubated at 37°C for 24 h; bacteria were recovered from the medium surface and suspended in 1 ml of PBS, followed by selection on Luria-Bertani (LB; Fluka Analytica) agar with 30 µg/ml of cefotaxime (Sigma). Positive and negative controls were performed with A. baumannii homologous DNA [22] and water instead of donor DNA, respectively. Three independent transformation assays, each repeated in triplicate, were performed.
The transformation frequency (TF) was calculated as the ratio between the number of transformants and the number of viable recipient cells.
Antimicrobial susceptibility testing
Antimicrobial susceptibility of recipient, donor and transformant cells was determined in Mueller-Hinton agar (Sigma-Aldrich) by disk diffusion with antibiotic disks (Oxoid) of amoxicillin (30 µg), amoxicillin-clavulanic acid (30 µg), aztreonam (30 µg), ceftazidime (30 µg), cefotaxime (30 µg), cefepime (30 µg), spectinomycin (10 µg), streptomycin (10 µg). The minimum inhibitory concentration (MIC) of cefotaxime and colistin was determined by broth microdilution in Mueller-Hinton and cation-adjusted Mueller-Hinton broth (CAMHB), respectively, according to EUCAST and CLSI guidelines [33, 34].
Polymerase chain reaction (PCR)-based detection of antimicrobial resistance genes
DreamTaq Green PCR Master Mix (Thermo Fisher Scientific) was used in end-point PCRs; recipient and donor cells were used as negative and positive control, respectively. The acquisition of the blaCTX−M gene by transformant ACI was screened with primers CTX-M/F’ + CTX-M/R’ [35]; co-acquisition of the mcr-1 gene and the class 1 integron was checked with CLR5-F + CLR5-R [36] and 5’-CS + 3’-CS [37], respectively.
Whole genome sequence
Whole genome sequencing (WGS) of A. baumannii A118, S. enterica Sal25 and transformant A. baumannii ACI were obtained with long reads sequencing technology (Oxford Nanopore Technologie, ONT). Genomic DNA was extracted with the DNeasy Blood and Tissue kit (Qiagen), following the manufacturer instructions. Libraries of DNA samples were prepared using the rapid barcoding kit 24 v14 (SQK-RBK114.24, ONT), following the manufacturer instructions. Libraries were sequenced using R10.4.1 flow cells on a MinION instrument. Data were acquired for 72 h, as advised by the manufacturer. Live basecalling was performed by the MinKNOW software using the superaccuracy model. A118 produced 76k reads totalling 183 Mb (46x coverage). ACI produced 92k reads for a total of 183 Mb and Sal25 generated 83k reads for a total of 239 Mb (47x coverage). A118 and ACI genomes were assembled with the ONT long reads using the Hybracter pipeline [38]. The Sal25 genome was obtained through the hybrid assembly of ONT reads and publicly available Illumina reads (Biosample: SAMEA3476857, European Nucleotide Archive) using Hybracter. Genomes were annotated with Bakta (version 1.8.2). Genomes were aligned and compared with the Mauve software (The Darling lab at the University of Technology Sydney) and Easyfig [39]. All genomes are available at NCBI under BioProject PRJNA1209693 with accession numbers CP178253 (A118), CP178251-CP178252 (Sal25) and CP178250 (ACI).
Sal25 plasmid was analysed with Phastest, to search for phage elements [40].
Results
Interspecies transfer and intracellular mobilization of antimicrobial determinants by natural transformation
We sought to test the possibility that A. baumannii could acquire antimicrobial resistant determinants by natural transformation, even from an unrelated species of different origin. The A. baumannii A118 strain was exposed to genomic DNA extracted from Sal25, a strain of the Enterobacterales S. enterica that was reported to encode resistance determinants to cephalosporins and colistin [30]. Following incubation and cefotaxime selection, A118 successfully acquired S. enterica Sal25 DNA at a frequency of 2.7 × 10− 8 ± 2.04 × 10− 8 transformants per recipient (Online Resource 1), a low frequency event, when compared with intraspecies transformation with genomic DNA from A. baumannii 121-1 [22] (TF = 1.4 × 10− 5 ± 1.98 × 10− 5 transformants per recipient). One tested transformant showed a susceptibility profile consistent with the acquisition of a CTX-M, namely reduced growth inhibition diameters to ceftazidime, cefotaxime, amoxicillin, aztreonam and cefepime (Online Resource 2), and the enlargement of the inhibition zone between cephalosporins and amoxicillin-clavulanic acid. The cefotaxime MIC of the transformant, which was named ACI, was > 512 mg/L, which correspond to a 128-fold increase, and the acquisition of the beta-lactamase was confirmed by PCR amplification of the gene. The susceptibility to the tested aminoglycosides (Online Resource 2) as well as the MIC to colistin did not change, which suggested that the transformant did not acquired the class 1 integron nor the mcr-1 gene from the Salmonella donor, respectively [30, 31]; the non-acquisition of these elements was also confirmed by the absence of PCR amplification of the genes.
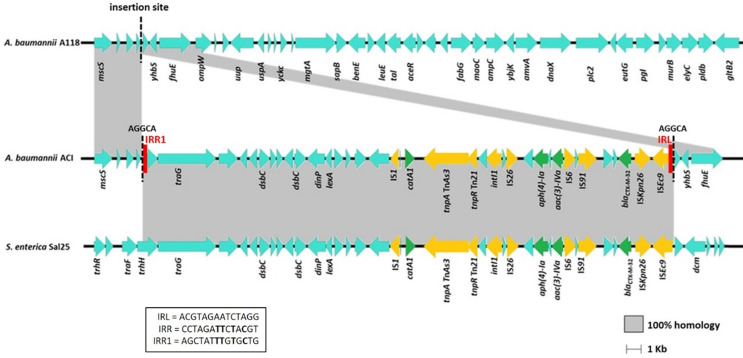
WGS yielded the full genomes of the recipient, donor and transformant cells. A118 and ACI genomes consist of a single circular chromosome while Sal25 showed a chromosome (non-circularized) and a non-circularized 234 kb plasmid. WGS revealed that the A. baumannii ACI transformant acquired a 36,130 bp DNA fragment from the donor S. enterica Sal25, which was inserted into a non-coding region of the A. baumannii A118 chromosome; the only difference between ACI and A118 was the ca. 36 kb acquired fragment. Besides the ESBL-encoding gene, the fragment acquired by ACI included different resistance genes, namely the chloramphenicol O-acetyltransferase catA1 gene and the aminoglycoside transferase genes aph(4)-Ia and aac(3)-IVa, as well as mobile genetic elements, including insertion sequences (IS) from several families and a class 1 integrase intI gene (Fig. 1). The complete sequence of the blaCTX−M−1 group gene identified the gene as the blaCTX−M−32 variant. IS5/ISKpn26 and IS1380/ISEc9 were detected upstream the blaCTX−M−32 gene. The acquired 36 Kb segment originates from a 230 Kb plasmid in Sal25, and with no homologous sequence to A118.
Fig. 1.
Schematic representation of the large DNA fragment acquired by ISEc9-mediated transposition, which includes different resistance genes (coloured in green), and mobile genetic elements (coloured in yellow). Imperfect inverted repeats are coloured in red. The fragment was inserted into a non-coding region of the A. baumannii A118 (marked with dashed line), generating a 5 bp direct repeat (AGGCA). Homologous sequences with the donor and the recipient strains are highlighted in grey
We detected a 5-bp (AGGCA) direct repeat (DR) flanking the acquired fragment, indicating the acquisition was caused by transposition of the DNA segment after uptake by natural transformation into the recipient cytoplasm. One of the 5-bp DR was located immediately adjacent to the left inverted repeat (IRL - ACGTAGAATCTAGG) of ISEc9 (also called ISEcp1). The other DR was located immediately adjacent to the deduced right imperfect inverted repeat (IRR1 - AGCTATTTGTGCTG) of ISEc9. The 14-bp IRR1 sequence detected in transformant A. baumannii ACI was part of the conjugal transfer protein-encoding gene trhH, has 4 base pairs identical to the perfect IRR and has a G residue at the 3’ end (Fig. 1).
Intraspecies transfer of antimicrobial determinants by natural transformation
To test whether the acquired segment could spread by intragenus transfer, the A. baumannii ACI transformant was used in subsequent transformation assays as donor DNA. Among the four recipient isolates belonging to A. baumannii, A. baylyi, A. nosocomialis and Acinetobacter sp., only A. baumannii A118 was successfully transformed by A. baumannii ACI DNA, at a frequency of 1.8 × 10− 6 ± 2.49 × 10− 6 transformants per recipient (Online Resource 1). All tested transformants were resistant to ceftazidime, cefotaxime, amoxicillin and aztreonam (Online Resource 2), and were positive for the blaCTX−M−32 gene acquisition.
Discussion
We have observed the transformation of A. baumannii A118 with naked DNA from S. enterica Sal25 at a frequency of 2.7 × 10− 8 ± 2.04 × 10− 8 transformants per recipient. Interspecies transformation of A. baumannii A118 with DNA from Klebsiella pneumoniae VA360 and Kb18 has been observed at similar frequencies [41]. S. enterica Sal25 was isolated from swine meat and K. pneumoniae VA360 and Kb18 are isolates of human clinical origin, demonstrating that naturally competent A. baumannii is able to acquire DNA from other species and sources.
A. baumannii ACI transformant acquired a long DNA fragment from the donor S. enterica Sal25, which contained several antimicrobial resistance genes and mobile genetic elements, especially insertion sequences. The blaCTX−M−32 gene was detected downstream of IS5/ISKpn26 and IS1380/ISEc9, a genetic environment also previously detected in a E. coli isolated from a healthy bovine in Portugal [42], which may be common among isolates of animal origin.
Integration of the DNA taken up by natural transformation is usually based on homologous recombination. However, no homologous recombination events could be produced by uptake of this plasmid DNA from S. enterica Sal25, as there are no homologous sequences with A118. Neither complete nor partial phage elements were detected in the Sal25 plasmid, ruling out that the plasmid belongs to a phage-plasmid and can be packaged and delivered by a viral particle [43]. It is also unlikely that acquisition was due to a transduction event, as temperate phages have a narrow host range. The detection of a 5-bp DR flanking the 36 Kb DNA fragment and adjacent to the ISEc9 IRL and to an imperfect IRR suggests that the acquisition occurred by transposition mediated by ISEc9. ISEcp1B-mediated transposition with weakly related IRR, comprising 3 to 12 identical base pairs to the perfect IRR, has been observed and a G residue at the 3’ end of the IR was suggested as crucial for the transposition event [44], requirements fulfilled by the identified imperfect IRR. ISEc9 has been associated with the dissemination of blaCTX−M genes and a single copy is able to promote one-ended transposition of downstream genes [45]. Based on WGS analysis, insertion of a blaCTX−M−15 into the chromosome of a clinical isolate of K. pneumoniae via an ISEc9 insertion sequence has been previously suggested [46]. In the same way, in an Escherichia coli isolated from blue mussels, the mobilizable trait of the chromosomal blaCTX−M−14 has been proposed due to the association with ISEc9 [47]. ISEc9 belongs to the IS1380 family, a family that produces a 4–5 bp DR during transposition [48], which is in accordance with the target site duplication we have identified in our transformant. Most of the studies usually employ WGS to understand the mobility potential of the detected antibiotic resistance genes. In this study we experimentally demonstrated the intracellular acquisition of a blaCTX−M−32 by transposition as part of a large DNA fragment taken up by natural transformation. A previous study has also experimentally demonstrated acquisition of blaCTX−M−2 by A. baumannii A118 after uptake of plasmid DNA from Proteus mirabilis Prm9 by natural transformation; in that particular case the blaCTX−M−2-encoding plasmid was maintained as an extrachromosomal element [27]. Although typically involved in the mobilization of fragments no larger than 10 Kb [49–51], ISEc9-mediated mobilization of a 14 Kb and a 41 Kb DNA fragments has been suggested [52]. In conclusion, the results show that A. baumannii could use natural transformation to acquire large DNA from unrelated species, even in the absence of homologous sequence. Instead, integration in the genome was caused by transposition occurring in the course of natural transformation, a low frequency event, likely involving the transient expression of the transposase and resolvase encoded by the donor DNA [53].
In comparison to the frequency of interspecies transformation between A. baumannii A118 and S. enterica Sal25, the intraspecies transfer of the ESBL-encoding gene exhibited a 20-fold increase, underscoring that HGT events that occur at low initial frequencies may subsequently be succeeded by additional occurrences at elevated frequencies, as previously documented in the dissemination of class 1 integrons [54]. The decreased interspecies recombination, as compared with intraspecies events, is called sexual isolation, and several factors may contribute to this barrier, including physical proximity of cells, DNA uptake machineries, and the existence of restriction-modification systems [55]. In fact, a recent study found that A. baumannii A118 has a restriction-modification system that recognizes the RGATCY motif, which limit the acquisition of unmethylated DNA or DNA with different methylation motifs [56]. The intraspecies acquisition of blaCTX−M−32 observed in this study likely occurs by homologous recombination, which is more frequent than transposition [54] and within isolates belonging to the same species [56, 57]. Intracellular acquisition of blaCTX−M genes has also been previously suggested to occur by homologous recombination in A. baumannii [18].
The blaCTX−M genes are usually plasmid-encoded, but there is a growing number of studies that report a chromosomal location in Gram-negative bacteria [18, 58–61]. In conclusion, the combined action of natural transformation and transposition, may explain this observation and lead to the stable incorporation of the acquired DNA into the recipient chromosome. Further studies are needed to clarify the impact of this mechanism in natural contexts, including biological and abiotic settings. Nonetheless, this work demonstrates that natural transformation allows intercellular spread of clinically important resistance genes even between such genetically-distant related bacteria like A. baumannii and S. enterica. The role of natural transformation as a pivotal mechanism facilitating the interspecies transmission of antimicrobial resistance is reinforced, highlighting the possible implications for the control of antimicrobial resistance dissemination within the One Health framework.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financed by the Faculty of Pharmacy of the University of Coimbra and by COMPETE 2020 - Operational Programme for Competitiveness and Internationalisation and Portuguese national funds via FCT – Fundação para a Ciência e a Tecnologia, under projects UIDB/04539/2020, UIDP/04539/2020 and LA/P/0058/2020. Work in the lab of XC was supported by Fondation pour la Recherche Médicale (grant number EQU202303016268).
Author contributions
Conceptualization – S.D., G.J.S.; Investigation – S.D., T.L., C.E., J.P., X.C.; Writing – original draft – S.D.; Writing – review and editing – S.D., T.L., X.C., G.J.S.
Funding
Open access funding provided by FCT|FCCN (b-on).
This work was financed by the Faculty of Pharmacy of the University of Coimbra and by COMPETE 2020 - Operational Programme for Competitiveness and Internationalisation and Portuguese national funds via FCT – Fundação para a Ciência e a Tecnologia, under projects UIDB/04539/2020, UIDP/04539/2020 and LA/P/0058/2020. Work in the lab of XC was supported by Fondation pour la Recherche Médicale (grant number EQU202303016268).
Data availability
Individual genomes are available on GenBank under accession numbers CP178250, CP178251, CP178252 and CP178253.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Antimicrobial Resistance C (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325):629–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO (2024) Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance [DOI] [PubMed]
- 3.Rice LB (2008) Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197(8):1079–1081 [DOI] [PubMed] [Google Scholar]
- 4.Antunes LC, Visca P, Towner KJ (2014) Acinetobacter baumannii: evolution of a global pathogen. Pathogens Disease 71(3):292–301 [DOI] [PubMed] [Google Scholar]
- 5.Eveillard M, Kempf M, Belmonte O, Pailhories H, Joly-Guillou ML (2013) Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Diseases: IJID: Official Publication Int Soc Infect Dis 17(10):e802–e805 [DOI] [PubMed] [Google Scholar]
- 6.Zhang XX, Zhang T (2011) Occurrence, abundance, and diversity of Tetracycline resistance genes in 15 sewage treatment plants across China and other global locations. Environ Sci Technol 45(7):2598–2604 [DOI] [PubMed] [Google Scholar]
- 7.Higgins PG, Hrenovic J, Seifert H, Dekic S (2018) Characterization of Acinetobacter baumannii from water and sludge line of secondary wastewater treatment plant. Water Res 140:261–267 [DOI] [PubMed] [Google Scholar]
- 8.Campos A, Lopes MS, Carvalheira A, Barbosa J, Teixeira P (2019) Survival of clinical and food Acinetobacter spp. Isolates exposed to different stress conditions. Food Microbiol 77:202–207 [DOI] [PubMed] [Google Scholar]
- 9.Ahmed AM, Motoi Y, Sato M, Maruyama A, Watanabe H, Fukumoto Y, Shimamoto T (2007) Zoo animals as reservoirs of Gram-negative bacteria harboring integrons and antimicrobial resistance genes. Appl Environ Microbiol 73(20):6686–6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilharm G, Skiebe E, Higgins PG, Poppel MT, Blaschke U, Leser S, Heider C, Heindorf M, Brauner P, Jackel U, Bohland K, Cuny C, Lopinska A, Kaminski P, Kasprzak M, Bochenski M, Ciebiera O, Tobolka M, Zolnierowicz KM, Siekiera J, Seifert H, Gagne S, Salcedo SP, Kaatz M, Layer F, Bender JK, Fuchs S, Semmler T, Pfeifer Y, Jerzak L (2017) Relatedness of wildlife and livestock avian isolates of the nosocomial pathogen Acinetobacter baumannii to lineages spread in hospitals worldwide. Environ Microbiol 19(10):4349–4364 [DOI] [PubMed] [Google Scholar]
- 11.De Lucia A, Rabie A, Smith RP, Davies R, Ostanello F, Ajayi D, Petrovska L, Martelli F (2018) Role of wild birds and environmental contamination in the epidemiology of Salmonella infection in an outdoor pig farm. Vet Microbiol 227:148–154 [DOI] [PubMed] [Google Scholar]
- 12.Bush K, Bradford PA (2020) Epidemiology of beta-lactamase-producing pathogens. Clin Microbiol Rev 33 (2) [DOI] [PMC free article] [PubMed]
- 13.Yu K, Huang Z, Xiao Y, Bai X, Gao H, Wang D (2024) Epidemiology and molecular characterization of CTX-M-type ESBLs producing Escherichia coli isolated from clinical settings. J Global Antimicrob Resist 36:181–187 [DOI] [PubMed] [Google Scholar]
- 14.Lima T, Fernandes L, Matias M, Mateus A, Silveira E, Domingues S, Pomba C, Da Silva GJ (2022) Longitudinal study detects the co-carriage of ESBL and mcr-1 and – 4 genes in Escherichia coli strains in a Portuguese farrow-to-finish swine herd. Animals: an open access journal from MDPI 12 (17) [DOI] [PMC free article] [PubMed]
- 15.Tacao M, Laco J, Teixeira P, Henriques I (2022) CTX-M-producing bacteria isolated from a highly polluted river system in Portugal. International journal of environmental research and public health 19 (19) [DOI] [PMC free article] [PubMed]
- 16.Poirel L, Bonnin RA, Nordmann P (2012) Genetic support and diversity of acquired extended-spectrum beta-lactamases in Gram-negative rods. Infect Genet Evolution: J Mol Epidemiol Evolutionary Genet Infect Dis 12(5):883–893 [DOI] [PubMed] [Google Scholar]
- 17.Potron A, Munoz-Price LS, Nordmann P, Cleary T, Poirel L (2011) Genetic features of CTX-M-15-producing Acinetobacter baumannii from Haiti. Antimicrob Agents Chemother 55(12):5946–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuillemenot JB, Bour M, Beyrouthy R, Bonnet R, Laaberki MH, Charpentier X, Ruimy R, Plesiat P, Potron A (2022) Genomic analysis of CTX-M-115 and OXA-23/-72 co-producing Acinetobacter baumannii, and their potential to spread resistance genes by natural transformation. J Antimicrob Chemother 77(6):1542–1552 [DOI] [PubMed] [Google Scholar]
- 19.Rumbo C, Fernandez-Moreira E, Merino M, Poza M, Mendez JA, Soares NC, Mosquera A, Chaves F, Bou G (2011) Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother 55(7):3084–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krahn T, Wibberg D, Maus I, Winkler A, Bontron S, Sczyrba A, Nordmann P, Puhler A, Poirel L, Schluter A (2016) Intraspecies transfer of the chromosomal Acinetobacter baumannii blaNDM-1 carbapenemase gene. Antimicrob Agents Chemother 60(5):3032–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leungtongkam U, Thummeepak R, Tasanapak K, Sitthisak S (2018) Acquisition and transfer of antibiotic resistance genes in association with conjugative plasmid or class 1 integrons of Acinetobacter baumannii. PLoS ONE 13(12):e0208468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domingues S, Rosario N, Candido A, Neto D, Nielsen KM, Da Silva GJ (2019) Competence for natural transformation is common among clinical strains of resistant Acinetobacter spp. Microorganisms 7 (2) [DOI] [PMC free article] [PubMed]
- 23.von Wintersdorff CJ, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, Savelkoul PH, Wolffs PF (2016) Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol 7:173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godeux AS, Svedholm E, Barreto S, Potron A, Venner S, Charpentier X, Laaberki MH (2022) Interbacterial transfer of carbapenem resistance and large antibiotic resistance Islands by natural transformation in pathogenic Acinetobacter. mBio 13(1):e0263121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soler-Bistue A (2023) Restriction-methylation systems regulate transformation in Acinetobacter baumannii. Trends Microbiol 31(9):879–881 [DOI] [PubMed] [Google Scholar]
- 26.Nagano N, Nagano Y, Cordevant C, Shibata N, Arakawa Y (2004) Nosocomial transmission of CTX-M-2 beta-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J Clin Microbiol 42(9):3978–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez MS, Merkier AK, Quiroga MP, Centron D (2012) Acinetobacter baumannii is able to gain and maintain a plasmid harbouring In35 found in Enterobacteriaceae isolates from Argentina. Curr Microbiol 64(3):211–213 [DOI] [PubMed] [Google Scholar]
- 28.Ramirez MS, Don M, Merkier AK, Bistue AJ, Zorreguieta A, Centron D, Tolmasky ME (2010) Naturally competent Acinetobacter baumannii clinical isolate as a convenient model for genetic studies. J Clin Microbiol 48(4):1488–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen KM, van Weerelt MD, Berg TN, Bones AM, Hagler AN, van Elsas JD (1997) Natural transformation and availability of transforming DNA to Acinetobacter calcoaceticus in soil microcosms. Appl Environ Microbiol 63(5):1945–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueiredo R, Card RM, Nunez J, Pomba C, Mendonca N, Anjum MF, Da Silva GJ (2016) Detection of an mcr-1-encoding plasmid mediating colistin resistance in Salmonella enterica from retail meat in Portugal. J Antimicrob Chemother 71(8):2338–2340 [DOI] [PubMed] [Google Scholar]
- 31.Figueiredo R, Henriques A, Sereno R, Mendonca N, da Silva GJ (2015) Antimicrobial resistance and extended-spectrum beta-lactamases of Salmonella enterica serotypes isolated from livestock and processed food in Portugal: an update. Foodborne Pathog Dis 12(2):110–117 [DOI] [PubMed] [Google Scholar]
- 32.Wilharm G, Piesker J, Laue M, Skiebe E (2013) DNA uptake by the nosocomial pathogen Acinetobacter baumannii occurs during movement along wet surfaces. J Bacteriol 195(18):4146–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The European Committee on Antimicrobial Susceptibility Testing (2024) Breakpoint tables for interpretation of MICs and zone diameters. Version 14.0. http://wwweucastorg
- 34.Clinical and Laboratory Standards Institute (2023) Performance Standards for Antimicrobial Susceptibility Testing. 33rd ed CLSI supplement M100
- 35.Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L (2003) Prevalence and molecular epidemiology of CTX-M extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob Agents Chemother 47(12):3724–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a Microbiological and molecular biological study. Lancet Infect Dis 16(2):161–168 [DOI] [PubMed] [Google Scholar]
- 37.Levesque C, Piche L, Larose C, Roy PH (1995) PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother 39(1):185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouras G, Houtak G, Wick RR, Mallawaarachchi V, Roach MJ, Papudeshi B, Judd LM, Sheppard AE, Edwards RA, Vreugde S (2024) Hybracter: enabling scalable, automated, complete and accurate bacterial genome assemblies. Microb Genomics 10 (5) [DOI] [PMC free article] [PubMed]
- 39.Sullivan MJ, Petty NK, Beatson SA (2011) Easyfig: a genome comparison visualizer. Bioinformatics 27(7):1009–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wishart DS, Han S, Saha S, Oler E, Peters H, Grant JR, Stothard P, Gautam V (2023) PHASTEST: faster than PHASTER, better than PHAST. Nucleic Acids Res 51(W1):W443–W450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traglia GM, Place K, Dotto C, Fernandez JS, Montana S, Bahiense CDS, Soler-Bistue A, Iriarte A, Perez F, Tolmasky ME, Bonomo RA, Melano RG, Ramirez MS (2019) Interspecies DNA acquisition by a naturally competent Acinetobacter baumannii strain. Int J Antimicrob Agents 53(4):483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leao C, Clemente L, Guerra V, Botelho A, Amaro A (2021) Occurrence of a rare multidrug resistant Escherichia coli coharboring blaCTX-M-32 and blaCTX-M-2 genes in a bovine. Microb Drug Resist 27(8):1155–1157 [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer E, Moura de Sousa JA, Touchon M, Rocha EPC (2021) Bacteria have numerous distinctive groups of phage-plasmids with conserved phage and variable plasmid gene repertoires. Nucleic Acids Res 49(5):2655–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poirel L, Lartigue MF, Decousser JW, Nordmann P (2005) ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob Agents Chemother 49(1):447–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao WH, Hu ZQ (2013) Epidemiology and genetics of CTX-M extended-spectrum beta-lactamases in Gram-negative bacteria. Crit Rev Microbiol 39(1):79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Man TJB, Lutgring JD, Lonsway DR, Anderson KF, Kiehlbauch JA, Chen L, Walters MS, Sjolund-Karlsson M, Rasheed JK, Kallen A, Halpin AL (2018) Genomic analysis of a pan-resistant isolate of Klebsiella pneumoniae, United States 2016. mBio 9 (2) [DOI] [PMC free article] [PubMed]
- 47.Grevskott DH, Salva-Serra F, Moore ERB, Marathe NP (2020) Nanopore sequencing reveals genomic map of CTX-M-type extended-spectrum beta-lactamases carried by Escherichia coli strains isolated from blue mussels (Mytilus edulis) in Norway. BMC Microbiol 20(1):134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siguier P, Gourbeyre E, Varani A, Ton-Hoang B, Chandler M (2015) Everyman’s guide to bacterial insertion sequences. Microbiol Spectr 3(2):MDNA3–0030 [DOI] [PubMed] [Google Scholar]
- 49.Dhanji H, Doumith M, Hope R, Livermore DM, Woodford N (2011) ISEcp1-mediated transposition of linked blaCTX-M-3 and blaTEM-1b from the IncI1 plasmid pEK204 found in clinical isolates of Escherichia coli from Belfast, UK. J Antimicrob Chemother 66(10):2263–2265 [DOI] [PubMed] [Google Scholar]
- 50.Zong Z, Partridge SR, Iredell JR (2010) ISEcp1-mediated transposition and homologous recombination can explain the context of blaCTX-M-62 linked to qnrB2. Antimicrobial agents and chemotherapy 54 (7):3039–3042 [DOI] [PMC free article] [PubMed]
- 51.Zong Z, Ginn AN, Dobiasova H, Iredell JR, Partridge SR (2015) Different IncI1 plasmids from Escherichia coli carry ISEcp1-blaCTX-M-15 associated with different Tn2-derived elements. Plasmid 80:118–126 [DOI] [PubMed] [Google Scholar]
- 52.Shawa M, Furuta Y, Mulenga G, Mubanga M, Mulenga E, Zorigt T, Kaile C, Simbotwe M, Paudel A, Hang’ombe B, Higashi H (2021) Novel chromosomal insertions of ISEcp1-blaCTX-M-15 and diverse antimicrobial resistance genes in Zambian clinical isolates of Enterobacter cloacae and Escherichia coli. Antimicrob Resist Infect Control 10(1):79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kloos J, Johnsen PJ, Harms K (2021) Tn1 transposition in the course of natural transformation enables horizontal antibiotic resistance spread in Acinetobacter baylyi. Microbiology 167 (1) [DOI] [PMC free article] [PubMed]
- 54.Domingues S, Harms K, Fricke WF, Johnsen PJ, da Silva GJ, Nielsen KM (2012) Natural transformation facilitates transfer of transposons, integrons and gene cassettes between bacterial species. PLoS Pathog 8(8):e1002837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Majewski J (2001) Sexual isolation in bacteria. FEMS Microbiol Lett 199(2):161–169 [DOI] [PubMed] [Google Scholar]
- 56.Vesel N, Iseli C, Guex N, Lemopoulos A, Blokesch M (2023) DNA modifications impact natural transformation of Acinetobacter baumannii. Nucleic Acids Res 51(11):5661–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doroghazi JR, Buckley DH (2010) Widespread homologous recombination within and between Streptomyces species. ISME J 4(9):1136–1143 [DOI] [PubMed] [Google Scholar]
- 58.Zhang CZ, Ding XM, Lin XL, Sun RY, Lu YW, Cai RM, Webber MA, Ding HZ, Jiang HX (2019) The emergence of chromosomally located blaCTX-M-55 in Salmonella from foodborne animals in China. Front Microbiol 10:1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leao C, Clemente L, Moura L, Seyfarth AM, Hansen IM, Hendriksen RS, Amaro A (2021) Emergence and clonal spread of CTX-M-65-producing Escherichia coli from retail meat in Portugal. Front Microbiol 12:653595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang M, Liu D, Li X, Xiao C, Mao Y, He J, Feng J, Wang L (2023) Characterizations of blaCTX-M-14 and blaCTX-M-64 in a clinical isolate of Escherichia coli from China. Front Microbiol 14:1158659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon EJ, Gwon B, Liu C, Kim D, Won D, Park SG, Choi JR, Jeong SH (2020) Beneficial chromosomal integration of the genes for CTX-M extended-spectrum beta-lactamase in Klebsiella pneumoniae for stable propagation. mSystems 5 (5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual genomes are available on GenBank under accession numbers CP178250, CP178251, CP178252 and CP178253.