Abstract
Background
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related mortality worldwide owing to the lack of effective and early diagnostic tools and therapeutic approaches. DNAJC7, a member of the DnaJ heat shock family, is crucial in protein folding and stability; however, its specific functions and mechanisms in HCC remain unclear.
Objective
This study aimed to explore the role of DNAJC7 in HCC progression and evaluate its potential clinical significance as a prognostic marker.
Methods
Public databases (TCGA, ICGC, GEO, and TIMER) were used to assess DNAJC7 expression, correlations with clinical parameters, and related signaling pathways. Proliferation, migration, invasion, and cell cycle assays were performed to evaluate the function of DNAJC7 in HCC. Immune infiltration and associations with checkpoint proteins were analyzed using TIMER, and a Gene Set Enrichment Analysis (GSEA) was used to explore enriched pathways.
Results
DNAJC7 expression was higher in HCC tissues than in adjacent normal tissues and was associated with advanced malignancy and poor prognosis, including a lower overall survival, progression-free survival, and disease-free survival. DNAJC7 knockdown resulted in reduced malignant behavior of HCC cells, leading to S-phase cell cycle arrest. Increased DNAJC7 expression was associated with immune cell infiltration and the presence of immunological checkpoint molecules, including CTLA4 and PD-1. GSEA highlighted the activation of key pathways, including WNT signaling and cell cycle regulation.
Conclusion
DNAJC7 regulates tumor cell proliferation, migration, invasion, and immune evasion by acting as an oncogene in HCC. It can serve as a diagnostic and prognostic biomarker and potential treatment target for HCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-025-06202-0.
Keywords: Hepatocellular carcinoma, DNAJC7, Prognosis, Signaling pathway
Introduction
Hepatocellular carcinoma (HCC) is the third-leading cause of cancer-related mortality worldwide (Bray et al. 2024). This can be attributed to the lack of effective early diagnostic methods and an insufficient understanding of the pathogenesis, limiting the development of effective treatment strategies (Coffman-D’Annibale et al. 2023; Barcena-Varela et al. 2024). Identifying the key molecules associated with HCC will provide insight into its pathogenesis and an important basis for developing precise clinical treatment strategies and determining prognosis.
The pathogenesis of HCC is complex and involves the combined effects of multiple factors, such as genetics, environmental conditions, and immune responses (Yang et al. 2024). This complexity results in high heterogeneity in tumor cell proliferation, migration, and metabolism, facilitating disease progression (Zhang et al. 2024a, b). The pathological course of HCC is accompanied by transcriptional imbalances. Aberrant gene expression is associated with patient prognosis, tumor cell proliferation, apoptosis, resistance to immunotherapy and targeted drugs, and the regulation of the immune microenvironment (Qiu et al. 2024). For example, VEGF overexpression promotes tumor angiogenesis, thereby enhancing the oxygen and nutrient supply to tumor cells and ultimately resulting in a poor prognosis for patients with HCC (Llovet et al. 2021). The abnormal expression of MDR1 and Bcl-2 leads to tumor cell resistance to radiotherapy and chemotherapy (Liu et al. 2021; Xu et al. 2023). Elevated programmed death ligand 1 (PD-L1) expression in HCC contributes to tumor immune escape. PD-L1 can interact with PD-1 receptors on T cells, thereby impairing the ability of the immune system to target tumor cells (Wen et al. 2023). The abnormal expression of these genes holds substantial value for diagnosis and for predicting prognosis in patients with HCC (Wang et al. 2021). Owing to the polygenic nature of HCC, it is necessary to identify novel therapeutic targets and develop multi-targeted combination therapies to improve prognosis.
Due to their rapid proliferation, tumor cells often experience high metabolic demands under intrinsic stress conditions, such as oxidative stress, hypoxia, and nutrient deprivation. These factors can cause protein misfolding, and the accumulation of incorrectly folded or aggregated proteins can impair cellular function (Tilk et al. 2024). Molecular chaperones play a critical role in maintaining cellular protein homeostasis by facilitating the proper folding of proteins or directing the degradation of misfolded proteins. Specifically, molecular chaperones influence tumor cell survival, proliferation, migration, and response to treatment by assisting in the proper folding of newly synthesized proteins, repairing damaged proteins, and regulating cellular stress responses (Roychowdhury et al. 2024). DNAJC7 is a member of the DnaJ heat shock protein family, which is essential for protein folding, preventing aggregation, and ensuring proper protein conformation (Hou et al. 2021). As a molecular chaperone, DNAJC7 is related to the heat shock response, aiding in the maintenance of protein stability under cellular stress conditions, such as elevated temperatures (Dilliott et al. 2022). This suggests that DNAJC7 plays a crucial regulatory role in tumor cell biology. However, research has focused on renal cell carcinoma (Li et al. 2016), highlighting the need for studies of the prognostic value of DNAJC7 in patients with HCC.
In this study, DNAJC7 was identified as a critical prognostic risk factor in HCC. First, we demonstrated that DNAJC7 likely participates in regulating protein folding and tumor cell stability, providing a foundation for further analyses of its role in the pathogenesis of HCC. Second, we systematically explored the mechanisms by which DNAJC7 contributes to HCC progression and evaluate its potential as a prognostic biomarker. The study results deepen our understanding of the molecular basis of HCC and provide novel strategies to optimize therapeutic regimens and prognostic assessments. Finally, our work reveals that DNAJC7 influences patient outcomes via effects on tumor cell proliferation, migration, and therapy resistance.
Materials and methods
Data collection
The Human Protein Atlas (HPA) is a comprehensive public database of human proteins, offering detailed insights into their expression, function, and localization under various physiological and pathological conditions (Colwill et al. 2011). Using data from the HPA, we analyzed DNAJC7 expression at the RNA and protein levels and its distribution across tumor cell lines. Furthermore, the TIMER database is an invaluable tool for investigating tumor immune infiltration, incorporating modules related to gene expression, survival, mutations, and copy number variation (Li et al. 2020). This database was used for a pan-cancer analysis and immune infiltration assessment. The molecular mechanisms underlying tumor occurrence and development cannot be completely captured by genomic data alone. The CPTAC database includes information on molecular processes involved in cancer by identifying proteins linked to changes in the cancer genome. PCAS is a multi-omics analysis platform based on the CPTAC dataset. It integrates proteomics, phosphoproteomics, and transcriptomics data to simplify analyses of multiple types of cancer and the tumor microenvironment (Wang et al. 2024). By leveraging the PCAS platform, we comprehensively analyzed differential DNAJC7 protein expression in tumors to uncover its role in malignant processes.
The Cancer Genome Atlas (TCGA) is a thorough and cooperative resource in cancer genomics. It was founded by the National Cancer Institute and the National Human Genome Project Research Center and covers 33 types of cancer (Li et al. 2024). In this study, we analyzed RNA sequencing data for 374 patients with HCC within the TCGA database. Data for adjacent tumor tissues were only available for 50 of these patients. Consequently, we obtained information for 357 patients through additional examinations. The detailed clinical characteristics of the patients are shown in Table S1. The ICGC database is a comprehensive global cancer resource that encompasses various data types, including transcriptomic, epigenetic, and genomic information (Huang et al. 2024). In this study, we included data for 232 patients with HCC in our follow-up analysis, including clinical characteristics. The GEO database is a vast high-throughput gene expression repository, integrating data from proteomics, genomics, and high-throughput sequencing (Clough et al. 2024). Multiple datasets (GSE54238, GSE36376, GSE25097, GSE54236, and GSE10186) were used for validation in this study.
Cell culture and transfection
Normal hepatocytes (L-O2) and HCC cell lines (SK-HEP-1 and MHCC97H) were sourced from Wuhan Servicebio Technology Co., Ltd. Cells were cultured in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) under standard conditions at 37 °C in a 5% CO2 incubator. The cells were digested, resuspended, uniformly seeded into six-well plates, and cultured at a concentration of 80–90%. Transfection was performed at an approximate cell density of 50%, using Lipofectamine 3000 as the transfection reagent. For the knockdown experiments, we selected three siRNAs: si-1, si-DNAJC7-2, and si-DNAJC7-3. A control group was established using si-NC. RT-PCR was used to identify the most effective siRNA sequence for subsequent analyses. The primer sequences for the reference (18 S rRNA) and target (DNAJC7) genes are presented in Table S2.
RT-qPCR analysis
A specific kit was used to extract total RNA, and a UV spectrophotometer (Cat. # ND-ONEC-W, Thermo Fisher Scientific) was used for quantification. cDNA was synthesized using a reverse transcription kit (Cat. # E047-01B, Novoprotein, China) and amplified using an RNA amplification kit (Cat. #: E096-01 A, Novoprotein). DNAJC7 mRNA expression levels were evaluated quantitatively using StepOnePlus Real-Time PCR (Thermo Fisher Scientific). The 2−ΔΔCt method was used to determine relative expression levels. The RT qPCR system is presented in Table S3.
Cell counting Kit-8 assay
A 500 µL aliquot of the suspension was diluted into 20 mL of culture media and dispensed into a 96-well plate using a multichannel pipette. After 12 h of adhesion, the cells were allowed to adhere for another 12 h before transfection. After transfection for 6–8 h, the culture medium was changed, and CCK-8 reagent (CCK-8 to culture medium) was added at a ratio of 1:10. Absorbance was measured at the 0-hour mark using a microplate reader, with subsequent readings recorded at 24, 48, 72, and 96 h post-transfection. The resulting data were analyzed using GraphPad Prism software.
Colony formation assay
After transfection, the cells were cultured to confluence, digested, and resuspended. Cells in each group were counted separately and diluted to 5,000 cells/mL. After adding 2 mL of culture medium to a 10 L aliquot of the suspension in a six-well plate, the cells were incubated for 14 days. The cells were fixed and stained based on the appearance of colonies formed. The colonies were photographed and examined, and the data were analyzed using GraphPad Prism and ImageJ software.
Cell cycle analysis using flow cytometry
To determine the cell cycle distribution, SK-HEP-1 and MHCC97H cells were analyzed by flow cytometry. After fixation with 70% ethanol, the cells were stained with a solution containing propidium iodide (PI) and ribonuclease A (RNase A) to determine the DNA content, enabling the identification of the G1, S, and G2/M phases. Data were analyzed using ModFit and GraphPad Prism software to ensure correct quantification and the clear display of cell cycle progression, providing critical information regarding cell proliferation.
Wound-healing assay
After transfection for 48 h, the cells were cultured until reaching 90–100% confluence. The medium in the six-well plate was aspirated, and three evenly spaced horizontal lines were drawn on the underside of the plate. Fresh medium was added, and a 100 µL pipette tip was used to create three equally spaced vertical scratches in each well. Next, the wells were rinsed twice with PBS, followed by the addition of fresh medium. The plate was observed under a microscope (10× magnification), and images were taken at 0, 24, 48, and 72 h post-scratch. The migration distances of various cell lines and knockout groups were measured using ImageJ software, and statistical analyses were conducted using GraphPad Prism.
Transwell experiment
Two days after transfection, cells were removed from the mixture, resuspended, and adjusted to a density of 3 × 105 cells/mL. A 100 µL aliquot of the cell suspension from each group was added to the upper chamber of a Transwell plate, and 700 µL of culture medium containing 20% serum was added to the lower chamber and mixed evenly. After 24 h of incubation, the cells were removed, fixed, and stained. Images were captured under a microscope (10× magnification) and analyzed using ImageJ software. Statistical analyses were performed using GraphPad Prism.
Gene set enrichment analysis
Gene Set Enrichment Analysis (GSEA) is a statistical method for exploring molecular signaling pathways associated with gene expression (Candia and Ferrucci 2024). In this study, TCGA and ICGC data were divided into upregulated and downregulated groups according to the median expression level of DNAJC7 in HCC. GSEA (version 4.0.2) software was used to identify the signaling pathways connected to DNAJC7. With respect to key parameter settings, each analysis included 1,000 gene sets ranked by effect size. Pathway significant enrichment was set at p < 0.05, and the false discovery rate (FDR) was < 0.25 to ensure the reliability of the results.
Statistical analysis
Data were analyzed and visualized using R (version 4.2.3) and GraphPad Prism 9. Results were evaluated using unpaired t-tests for two-group comparisons and one-way analysis of variance (ANOVA) for comparisons among multiple groups. The chi-squared test was used to examine the relationship between clinical features and DNAJC7 mRNA expression levels. A survival analysis was performed to evaluate the effect of DNAJC7 expression on patient outcomes, and a Cox regression analysis was used to identify possible risk factors. With statistical significance set at p < 0.05, an ROC curve analysis was used to evaluate the predictive value of DNAJC7.
For the Cox regression analysis, a proportional hazards model was constructed. In this model, the DNAJC7 expression level was considered the primary variable of interest along with potential confounding factors, such as patient age, sex, tumor stage, and other relevant clinical characteristics. These covariates were carefully selected based on prior knowledge and their potential associations with patient survival in HCC. The Cox regression model was used to estimate the hazard ratio (HR) for each variable, which represents the relative risk of an event (such as disease progression or death) occurring in one group compared with another, adjusted for all other variables in the model. A HR greater than 1 indicates an increased risk associated with the variable, while a HR less than 1 suggests a decreased risk. By fitting the data to the Cox regression model, we determined which of the variables, including DNAJC7 expression, was a possible risk factor for patient outcomes in HCC. Statistical significance for all analyses was set at p < 0.05, and the ROC curve analysis was used to evaluate the predictive value of DNAJC7.
Results
DNAJC7 is differentially expressed in various cancers
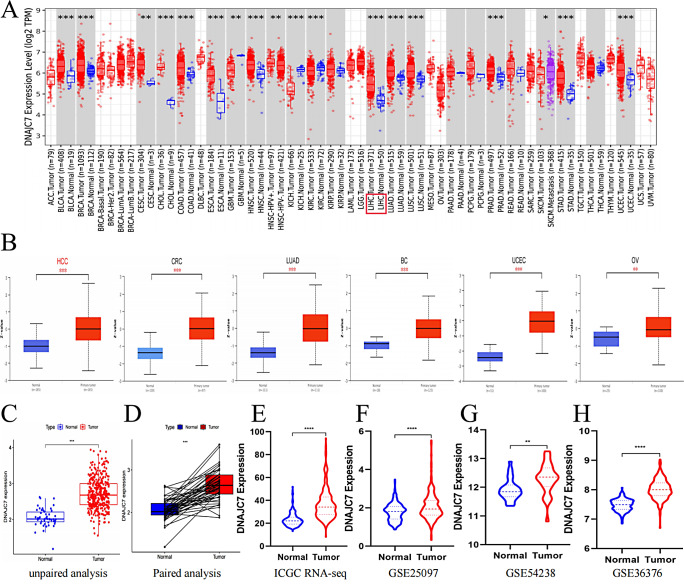
The abnormal expression of numerous genes is crucial in tumor initiation and progression (Zhang et al. 2024a, b). To explore the role of DNAJC7 in tumors, we examined its mRNA and protein expression levels in normal human organs using the HPA database. At the mRNA and protein levels, DNAJC7 was moderately expressed in the liver (Figure S1A, B). Notably, DNAJC7 was highly expressed in various tumor cell lines, including those derived from liver cancer (Figure S1C). We also observed that DNAJC7 was localized in the nucleus and cytoplasm (Figure S1D). Subsequently, DNAJC7 mRNA expression across various tumors was analyzed using the TIMER database. This analysis revealed significant differences in expression between tumor and normal tissues across the 18 tumor types (Fig. 1A). Furthermore, we conducted an in-depth analysis of DNAJC7 protein expression across various tumor types using the PCAS platform. The findings revealed a significant differential expression of DNAJC7 in six cancer types, including HCC (Fig. 1B). These results provide a basis for further research into the molecular mechanism of DNAJC7 in tumorigenesis.
Fig. 1.
Comparison of expression levels of DNAJC7 in tumors and normal tissues. (A) The mRNA expression levels of DNAJC7 in various tumors from the TIMER database. (B) The Protein expression levels of DNAJC7 in different tumor types from the CPTAC database. (C) Based on TCGA RNA-seq analysis, the expression level of DNAJC7 is significantly higher in tumor tissues compared to normal tissues. (D) Paired analysis further confirmed that the expression level of DNAJC7 in tumor tissues is significantly higher than that in normal tissues in the TCGA RNA-seq database. (E-H) Based on the RNA-seq data analysis from ICGC and GEO (GSE25097, GSE54238, and GSE36376), the expression level of DNAJC7 in tumor tissues is significantly higher than that in normal tissues
DNAJC7 is significantly upregulated in HCC
Despite evidence for a correlation between DNAJC7 and tumor development (Li et al. 2016), its correlation with HCC is yet to be documented. Therefore, we analyzed datasets for HCC tumor tissues and matched neighboring tissues from the TCGA-Seq database. DNAJC7 was more highly expressed in HCC tumor tissues than in surrounding tissues (Fig. 1C). Recognizing the potential for bias due to the large number of tumor tissues, we performed a paired analysis, which indicated a statistically significant increase in DNAJC7 expression in tumor tissues (Fig. 1D). To further corroborate these findings, we evaluated RNA-seq data from the ICGC and GEO databases (GSE25097, GSE54238, and GSE36376), yielding consistent results (Fig. 1E-H). This study provides the first evidence that DNAJC7 is highly expressed in HCC, demonstrating its possible association with the pathological course of the disease.
DNAJC7 expression levels are closely associated with malignant clinical features in HCC
Abnormal gene expression often drives malignant tumor characteristics and poor clinical outcomes (Chen et al. 2024). Nonetheless, little is known about the connection between DNAJC7 and the prognosis or malignant clinical characteristics of tumors. Given the elevated expression of DNAJC7 in HCC, we investigated its association with malignant clinical traits and prognosis. First, we analyzed the relationship between high DNAJC7 expression and clinical parameters, such as T stage, tumor stage, and histological grade, in patients with HCC (Fig. 2A-C). We grouped stages 1, 2, 3, and 4 for T stage, tumor stage, and histological grade to improve the accuracy of our analysis and address imbalances in patient numbers throughout the TCGA dataset. High DNAJC7 expression was correlated with more advanced clinical features in patients with HCC (Fig. 2D-F).
Fig. 2.
High expression of DNAJC7 leads to malignant clinical characteristics and poor prognosis in HCC patients. (A-C) Based on TCGA RNA-seq analysis, the high expression of DNAJC7 is significantly associated with T stage, tumor stage, and histological grade in HCC patients. (D-F) The analysis results after data integration indicate that high expression of DNAJC7 leads to more advanced malignant clinical characteristics in HCC patients. (G-I) K-M survival analysis suggests that high expression of DNAJC7 leads to poorer survival in HCC patients. (J-K) The ROC curve indicates that DNAJC7 has significant prognostic diagnostic value in HCC patients
DNAJC7 serves as an independent risk factor in patients with HCC and contributes to adverse clinical outcomes
We conducted univariate and multivariate analyses using data from TCGA and ICGC databases to identify independent prognostic factors affecting survival while controlling for confounding variables. These findings identified DNAJC7 and T stages as independent risk factors for HCC (Figure S2A-D). To evaluate the impact of DNAJC7 on patient prognosis, a Kaplan-Meier survival analysis was performed using clinical data and DNAJC7 expression levels from the TCGA database. High DNAJC7 expression was significantly associated with OS (overall survival), PFS (progression-free survival), and DFS (disease-free survival). This suggests that higher DNAJC7 levels were associated with lower survival rates in patients with HCC (Fig. 2G-I). These results were further validated using a Kaplan-Meier survival analysis based on data from the ICGC and GEO (GSE54236 and GSE10186) databases (Figure S3A-C). Finally, a receiver operating characteristic (ROC) curve analysis was performed to evaluate the prognostic and diagnostic value of DNAJC7 expression in patients with HCC. The results suggested that DNAJC7 may function as an oncogene in HCC, highlighting its clinical significance (Figure S3D, E). In summary, our research indicates that high DNAJC7 expression is a reliable predictor of poor outcomes in patients with HCC and an independent risk factor, highlighting its potential as a novel biomarker and therapeutic target in HCC.
Elevated expression of DNAJC7 promotes proliferation, migration, and invasion in HCC cells
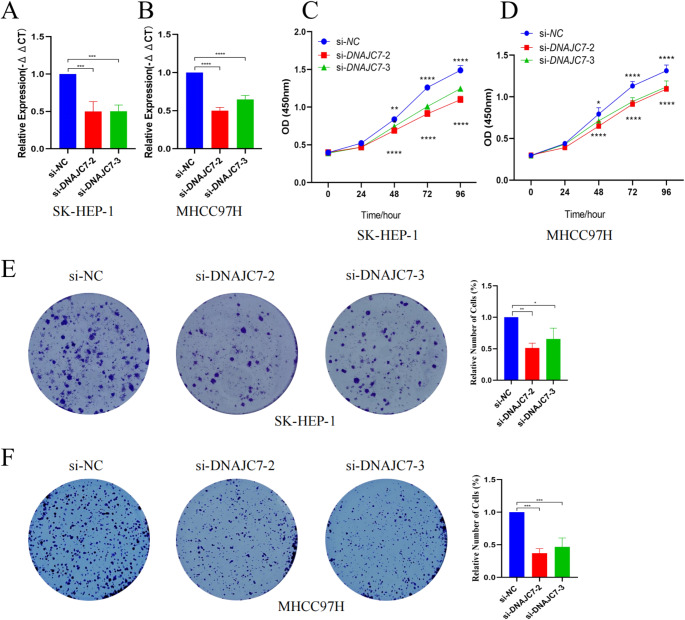
Based on the preliminary data analysis, DNAJC7 may be function as an oncogene in liver cancer and a potential HCC biomarker. To evaluate this hypothesis, we used in vitro assays to assess the effect of DNAJC7 on the biological behavior of HCC cells. Various siRNAs targeting DNAJC7 were used for knockdown in SK-HEP-1 and MHCC97H cells. The siRNA with the highest knockdown efficiency was selected for further experiments (Fig. 3A, B). CCK-8 and colony formation assays were performed to explore the effects of DNAJC7 on cell proliferation. The data showed that DNAJC7 knockdown decreased the growth of HCC cells dramatically, indicating its role in promoting cell growth (Fig. 3C-F). Additionally, Transwell and scratch assays revealed that silencing DNAJC7 markedly reduced the invasive and migratory capacities of these cells (Fig. 4A-C). Flow cytometry also showed that DNAJC7 knockdown in SK-HEP-1 and MHCC97H cells altered the cell cycle distribution (Fig. 5A-D). The percentage of cells in the S phase was significantly decreased in cells with decreased DNAJC7 expression, suggesting S-phase arrest. This disruption in DNA synthesis likely stems from alterations in the regulatory mechanisms controlling DNA replication, which slow progression through the S phase. Shortening of the S phase further affects the overall cell cycle dynamics, ultimately inhibiting cell proliferation and growth. These findings emphasize the pivotal role of DNAJC7 in maintaining cell-cycle integrity and provide novel insights into its molecular function in the pathogenesis of HCC. In summary, our study results confirm that DNAJC7 is an oncogene with a significant role in HCC cells.
Fig. 3.
In vitro experiments indicate that high expression of DNAJC7 promotes the proliferation of HCC cells. (A, B) In vitro assays using SK-HEP-1 and MHCC97H cells with DNAJC7-targeting siRNAs established a knockdown model, selecting the siRNA with the highest efficiency for further experiments. (C, D) CCK-8 assays showed that DNAJC7 knockdown significantly inhibited HCC cell growth. (E, F) Colony formation assays confirmed that DNAJC7 promotes cell proliferation
Fig. 4.
In vitro experiments have shown that high expression of DNAJC7 promotes the invasion and migration ability of HCC cells. (A) Transwell assays showed that silencing DNAJC7 significantly reduced the invasive capacity of the cells. (B, C) Similarly, scratch assays revealed a marked decrease in their migratory ability
Fig. 5.
Flow cytometry analysis shows that high expression of DNAJC7 affects cell cycle distribution by regulating DNA replication. (A) In the SK-HEP-1 cell line, the percentage of cells in the S phase is significantly reduced when DNAJC7 expression is decreased. (B) In the HCC97H cell line, the percentage of cells in the S phase is also significantly reduced when DNAJC7 expression is decreased
Co-expression and GSEA of DNAJC7
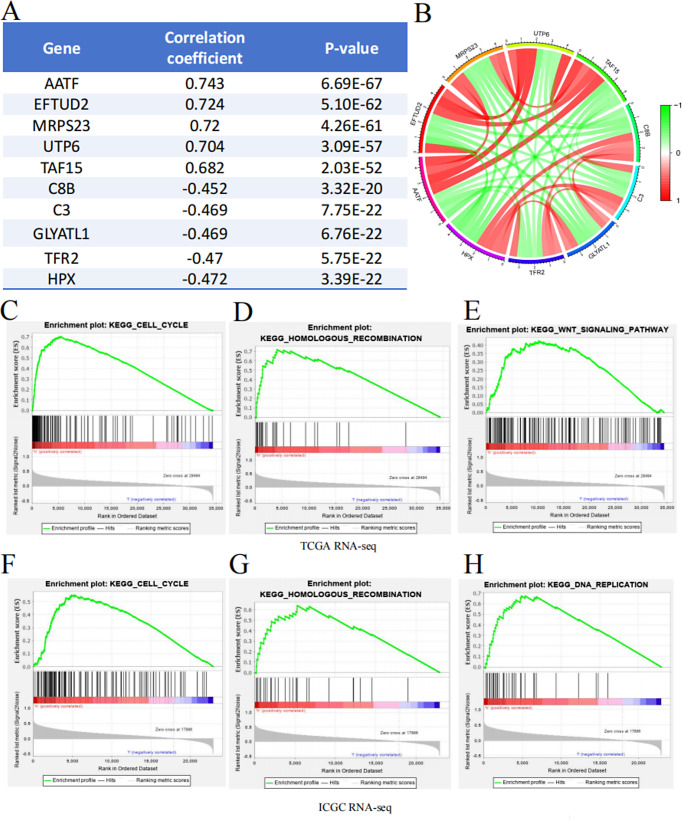
The development and progression of cancer involve the dysregulation of numerous genes, driven by multigenomic processes and the interplay of multiple signaling pathways (Hogg et al. 2020). First, through co-expression analysis, we identified five genes that were most positively correlated with DNAJC7 (AATF, EFTUD2, MRPS23, UTP6, and TAF15) and five most negatively correlated genes (C8B, C3, GLYATL1, TFR2, and HPX). Notably, AATF, EFTUD2, and MRPS23 reportedly facilitate the progression of HCC, supporting our conclusion that DNAJC7 functions as an oncogene in HCC (Fig. 6A, B). Compared with traditional enrichment methods, GSEA provides a comprehensive understanding of the functional roles of specific gene sets in biological processes (Candia and Ferrucci 2024). Using TCGA and ICGC data, we stratified patients into high and low DNAJC7 expression groups and performed GSEA to explore the regulatory mechanisms of DNAJC7 in HCC. High DNAJC7 expression was strongly correlated with the activation of several important signaling pathways, including DNA replication, Cell Cycle, WNT signaling, and Homologous Recombination repair pathways (Fig. 6C-H). In conclusion, analyses using TCGA and ICGC databases consistently showed that DNAJC7 controls the cell cycle signaling pathway to facilitate HCC development. These findings align with our flow cytometry results, which demonstrated the impact of DNAJC7 on cell cycle dynamics, further underscoring its role in cell cycle regulation in HCC.
Fig. 6.
Co-Expression and GSEA Enrichment Analysis of DNAJC7. A summary table lists the top five genes with the strongest synergistic or antagonistic expression relationships with DNAJC7. (B) Visualization of the top five genes showing synergistic or antagonistic expression patterns with DNAJC7. GSEA enrichment analysis results based on TCGA RNA-seq. (C) Cell Cycle; (D) Homologous Recombination; (E) WNT Signaling Pathway. GSEA enrichment analysis results based on ICGC RNA-seq. (F) Cell Cycle; (G) Homologous Recombination; (H) DNA Replication
Association between DNAJC7 and the immune microenvironment in HCC
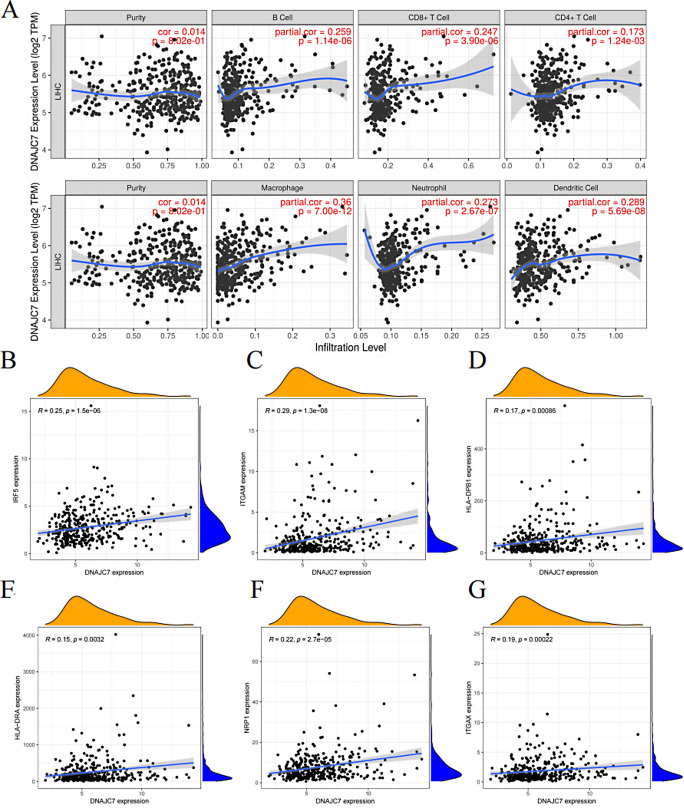
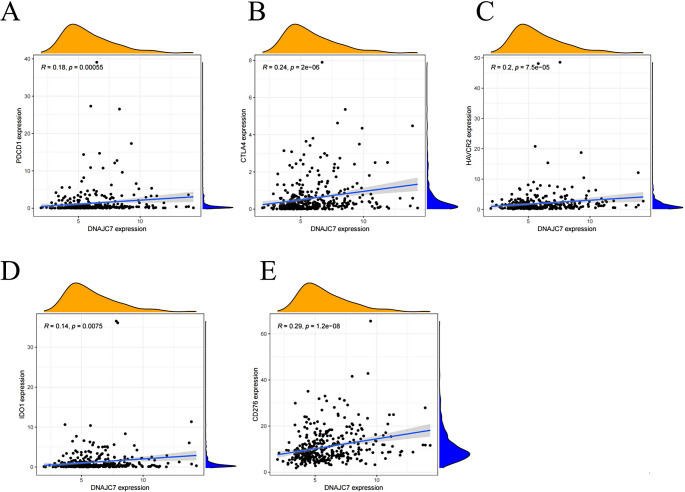
The immunological microenvironment is a key factor in HCC, impacting tumor growth, invasion, and proliferation. Immunotherapy targets often arise from the complex regulatory interactions within the tumor microenvironment (Shen et al. 2024). To explore the relationship between DNAJC7 and immune infiltration, we used the TIMER database. We detected a significant association between the expression of DNAJC7 and the infiltration of different immune cell types, such as B cells, macrophages, CD4+T cells, CD8+T cells, neutrophils, and dendritic cells (Fig. 7A). Previous studies have emphasized the intricate relationship between the immune microenvironment in HCC and immune cell surface markers (Harkus et al. 2022). It is important to better understand the mechanisms involved in immunotherapy prediction and to identify new therapeutic targets. Correlation analyses revealed that DNAJC7 was closely linked to the expression of several immune cell surface markers, particularly those associated with dendritic cells, neutrophils, and macrophages (Fig. 7B-G). We also examined the relationships between immune checkpoint molecules and DNAJC7 expression. Our results showed a positive correlation between DNAJC7 expression and key immune targets, including PDCD1, CTLA4, HAVCR2, IDO1, and CD27 (Fig. 8A-E). In summary, these findings indicated a significant association between DNAJC7 and the immune microenvironment in HCC, suggesting its potential as a promising target for clinical immunotherapy.
Fig. 7.
The regulatory role of DNAJC7 in the immune microenvironment. (A) The expression level of DNAJC7 shows a positive correlation with the infiltration levels of six types of immune cells: B cells, CD8 + T cells, CD4 + T cells, macrophages, neutrophils, and dendritic cells. (B-G) Spearman analysis reveals that the expression level of DNAJC7 is positively correlated with the expression of M1 macrophage marker (IRF5), neutrophil marker (ITGAM), and dendritic cell markers (NRP1, HLA-DPB1, ITGAX, and HLA-DRA)
Fig. 8.
Spearman analysis indicates that the expression level of DNAJC7 is positively correlated with the expression of five immune checkpoints. (A) PDCD1; (B) CTLA4; (C) HAVCR2; (D) IDO1; (E) CD276
Discussion
HCC is among the most lethal cancers globally. This can be attributed to the lack of effective early diagnostic tools, the complexity of its pathogenesis, and inadequate targeted therapies (Lee et al. 2023). Identifying the key genes that drive the high heterogeneity of HCC cells is crucial for understanding the proliferation and migration of cancer cells and progression of HCC (Wang et al. 2023). This study emphasized the importance of transcriptional dysregulation and molecular chaperone activity in the pathogenesis of HCC, providing a basis for improving patient outcomes (Zhou et al. 2024). Our findings clearly demonstrate that DNAJC7 is highly expressed in HCC and drives malignancy and disease progression.
In malignant tumors, abnormal gene expression can play a crucial regulatory role in tumor cell processes (Ng et al. 2022). In this study, DNAJC7 expression levels were examined in HCC tumor tissues and adjacent normal tissues. DNAJC7 expression was significantly elevated in HCC tissues, confirming its involvement in the pathogenesis of HCC. This finding was validated across multiple datasets, including TCGA, ICGC, and GEO. Genes with abnormally high expression often negatively impact cancer prognosis (Deng et al. 2022). A survival analysis also showed that higher DNAJC7 levels were substantially linked to poor OS, PFS, and DFS. These findings suggested that high DNAJC7 expression is associated with unfavorable outcomes and the gene may serve as a prognostic marker for HCC. Furthermore, a Cox regression analysis identified DNAJC7 as an independent risk factor for HCC prognosis, further highlighting its critical role in tumor progression and its potential as a therapeutic target.
Although public database analyses provide valuable insights, potential statistical biases cannot be excluded. Therefore, we conducted in vitro experiments using the SK-HEP-1 and MHCC97H cell lines to validate the role of DNAJC7 in HCC. CCK-8 and colony formation assays demonstrated that DNAJC7 knockdown significantly reduced the proliferative ability of tumor cells. Additionally, flow cytometry revealed a significant delay in S-phase progression, indicating that DNAJC7 plays a critical role in cell cycle regulation. DNAJC7 suppression led to the accumulation of cells in the S phase, potentially disrupting DNA replication and cell division, thereby impairing cell proliferation. These findings support the hypothesis that DNAJC7 drives tumor cell proliferation. Furthermore, the experimental results suggest that DNAJC7 may play a vital role in regulating protein folding and stability in tumor cells, particularly under stress conditions, such as oxidative stress, nutrient deprivation, and hypoxia. As a molecular chaperone, DNAJC7 is essential for maintaining protein homeostasis and tumor cell survival and growth. The malignant growth of HCC is caused by DNAJC7, which promotes tumor cell growth and survival by facilitating improper folding of proteins.
The tumor immune microenvironment in HCC plays pivotal roles in immune evasion and disease progression (Lin et al. 2024). Increased immune cell infiltration within the tumor microenvironment can influence tumor behavior and therapeutic responses significantly (Xie et al. 2024). Analyses using the TIMER database revealed that elevated DNAJC7 expression is associated with increased infiltration of six distinct immune cell types during HCC progression. This suggests that the oncogenic role of DNAJC7 may be mediated by its effect on immune cells in HCC. Notably, DNAJC7 expression in the immune microenvironment of HCC showed a strong positive correlation with immune checkpoint molecules, such as PDCD1, CTLA4, and IDO1, which are essential regulators of tumor immune evasion (Pinter et al. 2023). These findings indicate that DNAJC7 promotes the proliferation and migration of HCC cells. It also plays a key role in immune evasion mechanisms by modulating immune cell infiltration and checkpoint molecules. This emphasizes the potential of DNAJC7 as a novel HCC immunotherapy target.
During HCC progression, oncogenes regulate cellular signaling pathways to exert biological effects (Li et al. 2023). In this study, GSEA revealed that elevated DNAJC7 expression promoted the activation of key signaling pathways, including WNT signaling, cell cycle regulation, DNA replication, and homologous recombination repair. These pathways affect the biological behaviors of HCC cells, including proliferation, migration, stemness, and differentiation. Furthermore, they affect the immune microenvironment, enabling tumor cells to evade and destroy immune cells (Rebouissou and Nault 2020). As a member of the heat shock protein family, DNAJC7 functions as a molecular chaperone and is involved in cell cycle modulation (Hu et al. 2022). Flow cytometry and GSEA results suggest that DNAJC7 promotes HCC progression primarily by modulating the cell cycle pathway. These results emphasize the vital role of DNAJC7 in HCC pathology and highlight its potential as a novel molecular marker for the early diagnosis and prognostic monitoring of HCC.
Conclusion
Our research shows that DNAJC7 is a key factor in the pathogenesis of HCC. It not only promotes the proliferation, migration, and invasion of tumor cells but also promotes immune escape in the tumor microenvironment. High DNAJC7 expression was closely associated with poor prognosis in HCC, highlighting its value as a prognostic marker and the candidate therapeutic target. Given the important role of DNAJC7 in the progression of HCC and its association with multiple key signaling pathways, further exploration of its molecular mechanism of action is expected to bring new breakthroughs in HCC research and in the development of new treatment strategies. However, this study had certain limitations. For example, a detailed exploration of the relevant mechanisms of action was lacking. Furthermore, validation through in vivo studies using animal models and clinical data is needed. Further in-depth research and optimization of research plans are needed to gain a more comprehensive understanding of the mechanism of action of DNAJC7, providing practical guidance for the clinical treatment of HCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our sincere gratitude to Henan Provincial People’s Hospital and Zhengzhou University for their invaluable assistance and support throughout this project.
Author contributions
Jiaxing Chen, Zhizhao Yang: Conducted the experiments, analyzed and interpreted the data, contributed analysis tools or data, and drafted the manuscript.Dongfeng Deng, Zhihao Fu, Yongqiang Cui, and Zhilei Zhao: Performed the experiments and provided reagents, materials, and analysis tools or data.Xiao Zhang: Designed the experiments, analyzed and interpreted the data, and revised the manuscript.
Funding
This study was supported by the Henan Province Youth Health Science and Technology Innovation Talent Training Project (Approval Number: CYQ20220080) and the joint construction project of Henan Province Medical Science and Technology Research and Development Plan (Approval Number: LHGJ20220022, LHGJ20240013, LHGJ20240041).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethical approval
All clinical data in this study were sourced from public databases and do not require an ethical statement.
Competing interests
The authors declare no competing interests.
Footnotes
Chen Jiaxing and Yang Zhizhao are co-first authors
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barcena-Varela M, Monga SP, Lujambio A (2024) Precision models in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 10.1038/s41575-024-01024-w [DOI] [PubMed] [Google Scholar]
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74:229–263. 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- Candia J, Ferrucci L (2024) Assessment of gene set enrichment analysis using curated RNA-seq-based benchmarks. PLoS ONE 19:e0302696. 10.1371/journal.pone.0302696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang C, Xue R, Liu M, Bai J, Bao J, Wang Y, Jiang N, Li Z, Wang W et al (2024) Deep whole-genome analysis of 494 hepatocellular carcinomas. Nature 627:586–593. 10.1038/s41586-024-07054-3 [DOI] [PubMed] [Google Scholar]
- Clough E, Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM et al (2024) NCBI GEO: archive for gene expression and epigenomics data sets: 23-year update. Nucleic Acids Res 52:D138–D144. 10.1093/nar/gkad965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman-D’Annibale K, Xie C, Hrones DM, Ghabra S, Greten TF, Monge C (2023) The current landscape of therapies for hepatocellular carcinoma. Carcinogenesis 44:537–548. 10.1093/carcin/bgad052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill K, Renewable Protein Binder Working Group, Gräslund S (2011) A roadmap to generate renewable protein binders to the human proteome. Nat Methods 8:551–558. 10.1038/nmeth.1607 [DOI] [PubMed] [Google Scholar]
- Deng M, Sun S, Zhao R, Guan R, Zhang Z, Li S, Wei W, Guo R (2022) The pyroptosis-related gene signature predicts prognosis and indicates immune activity in hepatocellular carcinoma. Mol Med (Camb Mass) 28:16. 10.1186/s10020-022-00445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilliott AA, Andary CM, Stoltz M, Petropavlovskiy AA, Farhan SMK, Duennwald ML (2022) DnaJC7 in amyotrophic lateral sclerosis. Int J Mol Sci 23:4076. 10.3390/ijms23084076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkus U, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L (2022) Immune checkpoint inhibitors in HCC: cellular, molecular and systemic data. Semin Cancer Biol 86:799–815. 10.1016/j.semcancer.2022.01.005 [DOI] [PubMed] [Google Scholar]
- Hogg SJ, Beavis PA, Dawson MA, Johnstone RW (2020) Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov 19:776–800. 10.1038/s41573-020-0077-5 [DOI] [PubMed] [Google Scholar]
- Hou Z, Wydorski PM, Perez VA, Mendoza-Oliva A, Ryder BD, Mirbaha H, Kashmer O, Joachimiak LA (2021) DnaJC7 binds natively folded structural elements in Tau to inhibit amyloid formation. Nat Commun 12:5338. 10.1038/s41467-021-25635-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, Mei H, Liu J, Wang W, Liu Q (2022) Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. MedComm 3:e161. 10.1002/mco2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Li Z, Lv X, Tan W (2024) Screening of candidate inflammatory markers of epithelial cells in hepatocellular carcinoma based on integration analysis of TCGA/ICGC databases and single-cell sequencing. Recent Pat Anti-Cancer Drug Discovery. 10.2174/0115748928256530240124093759 [DOI] [PubMed] [Google Scholar]
- Lee Y-T, Fujiwara N, Yang JD, Hoshida Y (2023) Risk stratification and early detection biomarkers for precision HCC screening. Hepatol (Baltim Md) 78:319–362. 10.1002/hep.32779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang J, Hao J, Dong B, Li Y, Zhu X, Ding J, Ren S, Zhao H, Wu S et al (2016) Reduced cytosolic carboxypeptidase 6 (CCP6) level leads to accumulation of serum polyglutamylated DNAJC7 protein: a potential biomarker for renal cell carcinoma early detection. Oncotarget 7:22385–22396. 10.18632/oncotarget.8107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS (2020) TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res 48:W509–W514. 10.1093/nar/gkaa407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Cui X, Li Y, Guo D, He S (2023) Identification of ferroptosis and drug resistance related hub genes to predict the prognosis in hepatocellular carcinoma. Sci Rep 13:8681. 10.1038/s41598-023-35796-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu W, Mojumdar K, Kim H, Zhou Z, Ju Z, Kumar SV, Ng PK-S, Chen H, Davies MA et al (2024) A protein expression atlas on tissue samples and cell lines from cancer patients provides insights into tumor heterogeneity and dependencies. Nat Cancer 5:1579–1595. 10.1038/s43018-024-00817-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Kang K, Chen P, Zeng Z, Li G, Xiong W, Yi M, Xiang B (2024) Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer 23:108. 10.1186/s12943-024-02023-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhuang H, Cao F, Li J, Guo Y, Zhang J, Zhao Q, Liu Y (2021) Shc3 promotes hepatocellular carcinoma stemness and drug resistance by interacting with β-catenin to inhibit its ubiquitin degradation pathway. Cell Death Dis 12:278. 10.1038/s41419-021-03560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7:6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- Ng CKY, Dazert E, Boldanova T, Coto-Llerena M, Nuciforo S, Ercan C, Suslov A, Meier M-A, Bock T, Schmidt A et al (2022) Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat Commun 13:2436. 10.1038/s41467-022-29960-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter M, Scheiner B, Pinato DJ (2023) Immune checkpoint inhibitors in hepatocellular carcinoma: emerging challenges in clinical practice. Lancet Gastroenterol Hepatol 8:760–770. 10.1016/S2468-1253(23)00147-4 [DOI] [PubMed] [Google Scholar]
- Qiu X, Zhou T, Li S, Wu J, Tang J, Ma G, Yang S, Hu J, Wang K, Shen S et al (2024) Spatial single-cell protein landscape reveals vimentinhigh macrophages as immune-suppressive in the microenvironment of hepatocellular carcinoma. Nat Cancer 5:1557–1578. 10.1038/s43018-024-00824-y [DOI] [PubMed] [Google Scholar]
- Rebouissou S, Nault J-C (2020) Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol 72:215–229. 10.1016/j.jhep.2019.08.017 [DOI] [PubMed] [Google Scholar]
- Roychowdhury T, McNutt SW, Pasala C, Nguyen HT, Thornton DT, Sharma S, Botticelli L, Digwal CS, Joshi S, Yang N et al (2024) Phosphorylation-driven epichaperome assembly is a regulator of cellular adaptability and proliferation. Nat Commun 15:8912. 10.1038/s41467-024-53178-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-Y, Zhu Y, Xie S-Z, Qin L-X (2024) Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: current status and prospectives. J Hematol Oncol 17:25. 10.1186/s13045-024-01549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilk S, Frydman J, Curtis C, Petrov DA (2024) Cancers adapt to their mutational load by buffering protein misfolding stress. eLife 12:RP87301. 10.7554/eLife.87301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Lan T, Xiao H, Chen Z-H, Wei C, Chen L-F, Guan J-F, Yuan R-F, Yu X, Hu Z-G et al (2021) The expression profiles and prognostic values of HSP70s in hepatocellular carcinoma. Cancer Cell Int 21:286. 10.1186/s12935-021-01987-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhou L, Ji N, Sun C, Sun L, Sun J, Du Y, Zhang N, Li Y, Liu W et al (2023) Targeting ACYP1-mediated Glycolysis reverses lenvatinib resistance and restricts hepatocellular carcinoma progression. Drug Resist Updates: Rev Comment Antimicrob Anticancer Chemother 69:100976. 10.1016/j.drup.2023.100976 [DOI] [PubMed] [Google Scholar]
- Wang J, Song X, Wei M, Qin L, Zhu Q, Wang S, Liang T, Hu W, Zhu X, Li J (2024) PCAS: an integrated tool for multi-dimensional cancer research utilizing clinical proteomic tumor analysis consortium data. Int J Mol Sci 25:6690. 10.3390/ijms25126690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Zhang Y, Zhang H, Chen Y (2023) Clinical outcomes of PD-1/PD-L1 inhibitors in patients with advanced hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol 149:969–978. 10.1007/s00432-022-04057-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Yu M, Zhang B, Yu Q, Zhao Y, Wu M, Jin L, Yan J, Zhou B, Liu S et al (2024) CRKL dictates anti-PD-1 resistance by mediating tumor-associated neutrophil infiltration in hepatocellular carcinoma. J Hepatol 81:93–107. 10.1016/j.jhep.2024.02.009 [DOI] [PubMed] [Google Scholar]
- Xu J, Dong X, Huang DCS, Xu P, Zhao Q, Chen B (2023) Current advances and future strategies for BCL-2 inhibitors: potent weapons against cancers. Cancers 15:4957. 10.3390/cancers15204957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Cheng J, Zhuang H, Xu H, Wang Y, Zhang T, Yang Y, Qian H, Lu Y, Han F et al (2024) Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell 42:535–551e8. 10.1016/j.ccell.2024.03.004 [DOI] [PubMed] [Google Scholar]
- Zhang X, Xie J, Yang Z, Yu CKW, Hu Y, Qin J (2024a) Tumour heterogeneity and personalized treatment screening based on single-cell transcriptomics. Comput Struct Biotechnol J. 10.1016/j.csbj.2024.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xiao X, Yi Y, Wang X, Zhu L, Shen Y, Lin D, Wu C (2024b) Tumor initiation and early tumorigenesis: molecular mechanisms and interventional targets. Signal Transduct Target Ther 9:149. 10.1038/s41392-024-01848-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hang S, Wang Q, Xu L, Wang P (2024) Decoding the role of O-GlcNAcylation in hepatocellular carcinoma. Biomolecules 14:908. 10.3390/biom14080908 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.