Abstract
Macrophage pyroptosis has been identified as a critical pathological mechanism in inflammation-related atherosclerosis (AS). In this work, we have demonstrated that Zn2+ features the strongest anti-inflammatory performance by screening 10 representative metal ions, and the MTC1 agonists can trigger lysosomal Zn2+ release and inhibit pyroptosis in macrophages. Based on these findings, we further engineered a mucolipin TRP channel 1 (MTC1)–related therapeutic nanoplatform for endogenously triggering lysosomal zinc release to curb inflammation and block macrophage pyroptosis. This nanoplatform consists of mesoporous silica nanoparticles to deliver MTC1 agonists and carbon nanodots, which could synergistically exert antiatherosclerotic effect by scavenging toxic reactive oxygen species, inhibiting macrophage pyroptosis, modulating macrophage transition, and rebuilding atherosclerotic immune microenvironment. These findings demonstrate that macrophage pyroptosis can be efficiently blocked via leveraging self-lysosomal zinc pool, which provides the paradigm of lysosomal zinc modulation-involved nanotherapeutics for managing other inflammatory diseases.
An antipyroptosis nanoplatform has been developed for efficient management of atherosclerosis.
INTRODUCTION
Atherosclerosis (AS), characterized by oxidative stress and chronic inflammation, remains a substantial cause of morbidity and mortality due to plaque rupture and thrombosis formation (1–2). During atherogenesis, macrophages play a crucial role as integrators of inflammatory signals, substantially influencing the characteristics of arterial plaques and accelerating the progression of AS (3–4). Furthermore, dying macrophages at plaques cause the release of cellular contents and inflammatory cytokines into extracellular space, contributing to exacerbating necrotic core enlargement and destabilizing plaques (5). Recent studies have demonstrated that pyroptosis, an inflammatory form of cell death featured by membrane pore formulation and cell lysis, is highly implicated in accretion of atherosclerotic plaques (6–12). Solid evidence has established that important functional molecules related to macrophage pyroptosis, such as nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing 3 (NLRP3) and caspase-1, are closely related to the emergence of atherosclerotic plaques and plaque instability (7–9, 13). Consequently, macrophage pyroptosis has been recognized as a potential therapeutic target for treatment of AS. Nevertheless, current treatment options for AS are limited and the therapeutic effects are insufficient, underscoring the urgent need for innovative therapies capable of effectively inhibiting inflammation and attenuating cell pyroptosis.
Metal ions are crucial in numerous physiological and pathological processes and are essential for the functionality of approximately half of all enzymes, among which alkali and alkaline earth metals (Ca2+, K+, and Na+) typically serve structural roles, whereas transition metals (Fe2+, Mn2+, and Cu+) are integral to metabolism, catalysis, and structural stabilization (14–15). When responding to sudden changes, metal ions can alter their intracellular concentrations by several orders of magnitude, thus facilitating them naturally fit for signal conductors and functional regulators (16). Consequently, various ion signals, behaving as fundamental vowel phonemes in cellular language, have been studied and explored for treating diseases, such as metalloimmunology for cancer treatment (17). Over recent decades, substantial evidence has verified that zinc could act as both an antioxidant and an anti-inflammatory agent for AS management. Zinc reduces •OH production by competing with Fe2+/Cu2+ ions for binding sites on cell membranes and proteins. In addition, zinc is an inhibitor of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), which catalyzes the generation of •O2− from O2 by using NADPH as electron donor, consequently diminishing the production of reactive oxygen species (ROS) (18–19). Furthermore, zinc participates the activation of nuclear factor κB (NF-κB), a critical transcription factor in immune response, leading to the down-regulation of pro-inflammatory cytokines interleukin1-β (IL-1β) and tumor necrosis factor–α (TNF-α) (20). However, zinc exposure has double-edged sword effect, excessive amounts can disrupt intracellular homeostasis, leading to increased ROS levels, mitochondrial damage, and cell apoptosis (21–22).
Lysosomes are catabolic organelles, which govern a board range of processes, including nutrient signaling, degradation of senescent components, and ion homeostasis (23). As an important cellular compartment, lysosome can remove toxic ions from the cytoplasm and sequester them within their vacuolar membrane in a detoxified form to limit their diffusion (24). Moreover, evidence demonstrated that lysosomes can also trap zinc within their vesicles, serving as a primary source for the free Zn2+ pool in the cytoplasm (25). In addition, mucolipin TRP channel 1 (MTC1), a member of the mucolipin subfamily of transient receptor potential proteins, is a Ca2+/Zn2+/Fe2+ permeable ion channel located on the membranes of late endosomes and lysosomes in all mammalian cell types, participating in the intracellular transport of zinc ions (26–29). Thus, it is more rational to modulate lysosomal function by redistributing Zn2+ between lysosomes and cytoplasm for an anti-inflammatory effect. Nevertheless, the roles of cellular lysosomes in inflammation have yet to be fully elucidated.
MTC1-specific agonists (MLs), which bind directly to MTC1 proteins (29–30), have been used to perform specialized functional roles in various cellular functions, including lipid trafficking, lysosome biogenesis, calcium-related signaling, and autophagy processes (31). Building on our previous explorations of lysosomal function (32), we have uncovered an underappreciated role of lysosomes and demonstrated that activation of MTC1 can facilitate the reutilization of the lysosomal zinc pool to elicit potent anti-inflammatory and antipyroptotic properties. In this study, we constructed biocompatible indocyanine green (ICG) surface-engineered mesoporous silica nanoparticles (MSNs) coloaded with carbon nanodots (C NDs) and MLs (designated as CM@MS NPs) for combined anti-atherosclerotic therapy. MSNs, known for desirable biosafety, were applied for the encapsulation and sustained release of antiatherosclerotic agents, delivering them across endothelial barriers into atherosclerotic lesions (33), where ICG with high photoacoustic (PA) imaging capability, was anchored on the surface of these NPs to visualize the accumulation of the NPs at plaques and monitor their therapeutic process (Fig. 1A). This multifunctional nanoplatform features a MLs component designed to trigger Zn2+ release from lysosomes, thereby inhibiting cytokine release, curbing inflammation, and blocking macrophage pyroptosis. In addition, the C NDs component, with strong ROS scavenging properties, alleviates intravascular oxidative stress (34), synergistically producing a cooperative antiatherosclerotic effect (Fig. 1B). Unlike the sole modulation of exogenous dosage in conventional drugs, the therapeutic efficacy of these drugs depends on the channel activity and the injection dosage of biosafe MTC1 agonists, which could prevent normal tissues and organs from being exposed to highly active therapeutic drugs, thereby reducing severe systemic side effects (table S1). This study presents an efficient antipyroptosis strategy through rational utilization of lysosomal zinc pool and highlights its promise for treating other inflammatory diseases.
Fig. 1. Schematic illustration of synthetic procedure of CM@MS NPs and anti-atherogenic therapy as enabled by CM@MS NPs.
(A) Scheme of synthetic process of CM@MS NPs, including synthesis of MSNs, APTES surface modification, ICG engineering (MS NPs), and C ND and ML coloading (CM@MS NPs). (B) Schematic illustration of the therapeutic procedure of CM@MS NPs for AS management, including ROS elimination by C NDs component and anti-inflammatory property through ML-driven intracellular redistribution of zinc ions.
RESULTS
Pyroptosis is involved in human AS
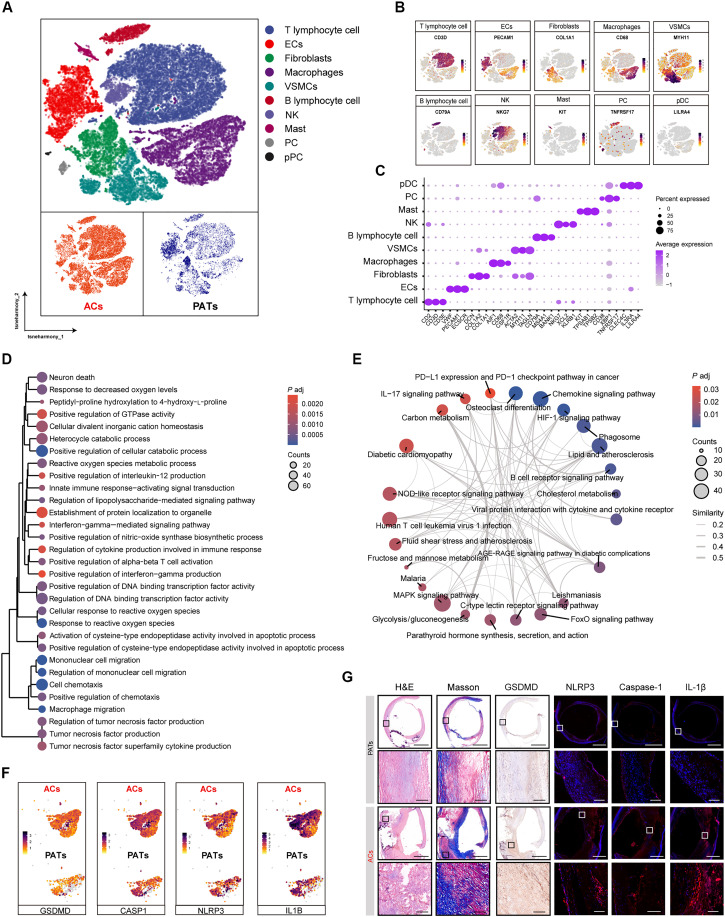
Inflammation is widely acknowledged as a critical factor in the pathogenesis of AS, with macrophage pyroptosis playing a substantial role in the exacerbation of inflammation. A single-cell sequencing (scRNA-seq) analysis of plaque tissues was initially carried out to identify the underlying etiological factors in calcified atherosclerotic core of plaques (ACs) and proximal adjacent tissues (PATs) from patients undergoing carotid endarterectomy based on the scRNA-seq dataset GSE159677, which is available in the Gene Expression Omnibus public database (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE159677). Based on the differential expression of established lineage markers, a t-distributed stochastic neighbor embedding (t-SNE) visualization that categorizes 10 major cell types in atherosclerotic lesions was shown in Fig. 2 (A to C). Subsequently, we compared the macrophage cell line from ACs with that in PATs to elucidate the roles of macrophages in AS genesis. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses indicated that differentially expressed mRNAs (DEMs) in macrophages verified a pronounced focus on processes related cellular response to ROS, macrophage migration, regulation of tumor necrosis factor production, etc. and be involved in inflammatory response, including IL-17 signaling pathway, NOD-like receptor signaling pathway, FoxO signaling pathway (Fig. 2, D and E). Furthermore, pyroptosis-related genes [such as NLRP3 inflammasome, IL-1β, caspase 1, and GSDMD] were observed highly upregulated within ACs as compared with PATs (Fig. 2F). We then collected human plaque tissues to perform histological analysis, where we compared the cells located in the ACs to their PATs. The results indicated that atherosclerotic cores were featured by increased numbers of necrotic cores and high levels of pyroptosis-related molecules (NLRP3 inflammasome, IL-1β, caspase-1, and GSDMD), suggesting that targeting macrophage pyroptosis could be a promising therapeutic strategy for treating AS (Fig. 2G and fig. S1).
Fig. 2. Pyroptosis is involved in human AS.
(A) A t-SNE visualization that identified 10 major cell types exhibited in atherosclerotic lesions from patients undergoing carotid endarterectomy. (B) Feature plots exhibiting the expression profiles of cell type–specific marker genes. (C) Dot plot exhibiting the expression level of key marker genes across the cell types. (D) GO biological process analysis and (E) KEGG pathway enrichment of DEMs in macrophages within atherosclerotic plaques. (F) Dotted lines represented P-adjusted value 0.5 and < −0.5 corresponding to PATs and ACs cells, respectively. t-SNE visualization of the pyroptosis associated differential genes in ACs versus PATs macrophages. (G) Histological analysis of HE, Masson, GSDMD, and immunofluorescence assay of NLRP3, caspase-1, and IL-1β from PATs and ACs in human plaque lesions (n = 3).
MLs triggered lysosomal Zn2+ release and inhibited pyroptosis in macrophages
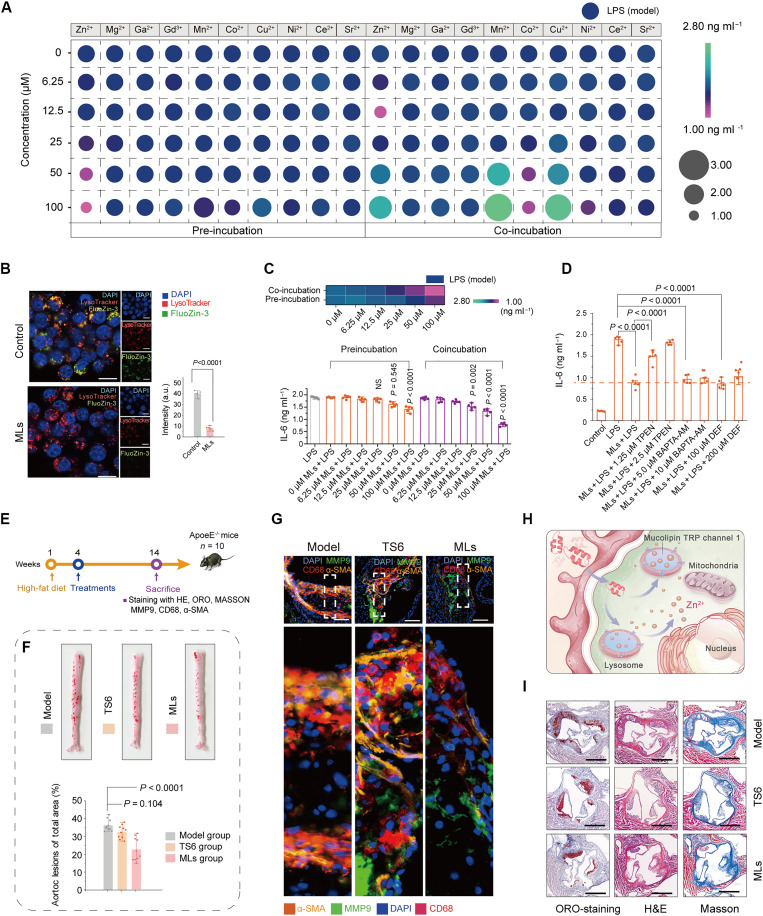
The notable impact of Mn2+ on cancer immunotherapy has sparked growing interest in the concept of “metals in medicine” (35). Previous studies have shown that pretreatment with various metal ions can enhance cellular anti-inflammatory functions (36–37). However, the exposure to these functional ions can also lead to potential side effects in vivo. Therefore, in this study, we conducted an initial screening of 10 different metal ions to assess their potential as therapeutic agents with anti-inflammatory properties, which included nutritional metal ions (such as, Zn2+, Co2+, Cu2+, and Mn2+), commonly used imaging/therapeutic ions (Ga2+ and Gd2+), and ions known to have anti-inflammatory functions (Ni2+, Mg2+, Sr2+, and Ce2+). Two treatment methods, pretreatment and cotreatment, were used to compare the anti-inflammatory properties of these ions and evaluate their potential toxicity in vivo. The results indicated that pretreatment with Ni2+, Co2+, and Zn2+ at concentrations (below 100 μM) exhibited desirable anti-inflammatory effects, with Zn2+ demonstrating the strongest anti-inflammatory properties. However, when cells were co-incubated with Zn2+ at concentrations ranging from 2.5 to 10 μM, a weak anti-inflammatory effect was observed. Notably, a pro-inflammatory effect was induced at Zn2+ concentrations exceeding 25 μM, highlighting the dual nature of zinc ions. These results suggested that while cytosolic Zn2+ is crucial as a trace element in normal cells, excessive Zn2+ levels can inhibit mitochondrial functions, resulting in cellular energy failure, inflammation, and potentially cell death (Fig. 3A and figs. S2 and S3) (38).
Fig. 3. MLs trigger Zn2+ release from lysosome and corresponding therapeutic effect of MLs.
(A) Levels of IL-6 in J774A.1 cells after co-incubated or pre-incubated with various concentrations of metal ions. (B) Fluorescence images of J774A.1 cells stained with FluoZin-3 (–AM) and LysoTracker after different treatments, and corresponding statistical analysis of FluoZin-3 intensities after different treatments. Scale bars, 20 μm. (C) Levels of IL-6 in J774A.1 cells after co-incubated or pre-incubated with MLs. (D) Levels of IL-6 in J774A.1 cells after co-incubated with different formulations. (E) Schematic illustration of the in vivo therapeutic protocols. (F) Representative photographs of ORO-stained aortas of mice following treatment with varied formulations and corresponding statistical analysis after different treatments. (G) Representative immunofluorescent images of aorta sections stained with antibody against CD68, MMP9, and α-SMA. Scale bars, 100 μm. (H) Schematic illustration of the cellular Zn2+ release from lysosomes triggered by MLs. (I) Representative photographs of ORO-stained, and H&E/Masson immunohistochemical images of aortas root sections following treatment with varied formulations. Scale bars, 500 μm. Statistical analysis from [(C), (D), and (F)] was performed by one-way analysis of variance (ANOVA), and statistical analysis from (B) was calculated via the Student’s t test. The data are presented as mean ± standard deviation (SD). DAPI, 4′,6-diamidino-2-phenylindole.
The functional modulation of metal ions is influenced by their subcellular distributions. These distributions vary according to the specific metalloproteins or ion channels within cells, which can cause substantial changes of several orders of magnitude in a short time. Previous studies have identified lysosomes as a primary source of the free Zn2+ pool in the cytoplasm. Activation of MTC1 by MLs could trigger lysosomal Zn2+ release into cytoplasm, prompting us to explore whether modulation of Zn2+ distribution could mitigate cellular inflammatory responses. To this end, FluoZin-3-AM, a cell membrane-permeable Zn2+-sensitive fluorescent dye was applied to monitor cytoplasmic/vesicular Zn2+ levels. The murine macrophages cell line J774A.1 was used as a cell model to study the anti-inflammatory effects mediated by MLs (39). As demonstrated in Fig. 3B, the FluoZin-3 signals were overlapped with LysoTracker-positive red signals within the vesicular compartments of the control group, indicating that lysosomes are the principal intracellular Zn2+ stores in J774A.1 cells. Following treatment with MLs, a pronounced reduction in vesicular FluoZin-3 fluorescence was observed, suggesting that MLs can trigger Zn2+ release from lysosomes. Subsequently, we assessed the anti-inflammatory effects of lysosomal Zn2+ release induced by MLs. Notably, MLs displayed anti-inflammatory performance, as demonstrated by the notable reduction in interleukin-6 (IL-6) expression when cells were co-incubated with 25 μM MLs. MLs had high biosafety and biocompatibility (fig. S4) and could exert anti-inflammatory function even at a high concentration of 100 μM after co-incubation (Fig. 3C and fig. S5). However, lysosomal MTC1 is a nonselective cation channel, which is also permeable to Ca2+ and Fe2+. Giving the established role of Ca2+ and Fe2+ processes related to cell death (40–41), investigated whether these ions could influence the anti-inflammatory effects of lysosomal Zn2+. Therefore, in this study, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM; a membrane-permeable Ca2+ chelator), desferriamine (DEF; a Fe2+ chelator), and N,N,N,N-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN) were applied to identify the roles of these ions in the anti-inflammatory process. Initially, we evaluated whether Ca2+ is required for MLs-involved anti-inflammatory effects. It was noted that the expression of IL-6 was not changed in the BAPTA-AM (a membrane-permeable Ca2+ chelator) + lipopolysaccharide (LPS) + MLs group as compared with that in LPS + MLs group (Fig. 3D). Likewise, similar result was observed when DEF (a Fe2+ chelator) was applied. These results demonstrated that both Ca2+ and Fe2+ did not play a key role in ML-related anti-inflammatory process. In contrast, TPEN, relatively high-affinity Zn2+ chelator, could reverse the MLs-involved anti-inflammatory effects to some extent in a dose-dependent manner, suggesting that MLs-induced Zn2+ release, but not its Ca2+/Fe2+, exert anti-inflammatory efficacy.
Previous studies have shown that zinc can inhibit NLRP3 inflammasome activation, which is a key upstream pathway of canonical pyroptosis (42). After the NLRP3 protein is stimulated, it can recruit and activate pro–caspase-1, and the activated caspase-1 subsequently cleaves gasdermin D (GSDMD) to produce GSDMD-N, thereby triggering pyroptosis (43). To evaluate the anti-pyroptotic potency of MLs, we used LPS plus nigericin to induce pyroptosis in J774A.1 macrophages. The flow cytometry was performed to evaluate the cell survival. J774A.1 cells were incubated with a FAM FLICA caspase-1 assay kit to detect caspase-1 activation. The result suggested that treatment of MLs could reduce the expression level of caspase-1 (a critical executioner of pyroptosis) and effectively inhibit macrophage pyroptosis (fig. S6A). The underlying mechanism was further identified using Western blot analysis, which confirmed a pronounced decrease in GSDMD, GSDMD-N (“killer protein” to execute pyroptosis), and IL-1β levels in ML-treated cells (fig. S6B). These results indicated that treatment with MLs can trigger lysosomal zinc release and subsequently block macrophage pyroptosis.
MLs attenuated diet-induced AS in ApoE−/− mice
Then, we evaluated the therapeutic efficacy of MLs in vivo using a TRAF-STOP6 inhibitor (TS6) as a positive control (44, 45), which reduces the phosphorylation of signaling intermediates in the NF-κB pathway to combat AS. ApoE−/− mice, following 1 month on a high-fat diet to develop early atherosclerotic plaques, were randomly assigned to three groups (n = 10). MLs are poorly soluble in physiological solutions, so we increased their solubility by adding 5% dimethyl sulfoxide (DMSO), enabling their use in in vivo therapy (ML dosage: 0.6 mg kg−1). Before the in vivo experiment, we verified that treatment of 0.6 mg kg−1 exhibited high biosafety in the tested Kunming mice (fig. S7). No appreciable histological abnormalities were observed, and there were no alterations in liver and renal function, as well as hematological indices, in the tested organs following ML treatment. Subsequently, treatment with MLs (5% DMSO, 0.6 mg kg−1) and TS6 (0.6 mg kg−1) continued for 2 months while maintaining the high-fat diet (Fig. 3E). Post-treatment, the entire aorta was stained with Oil Red O (ORO). The result demonstrated that ML treatment could reduce the ORO-positive areas compared with the model group (Fig. 3F and fig. S8, A to E), whereas TS6 treatment showed no significant therapeutic effect at such a low dosage. Immunostaining with anti-CD68 antibody, α-smooth muscle actin (α-SMA) antibody, and matrix metalloproteinase-9 (MMP-9) antibody confirmed that ML treatment decreased the number of macrophages and vascular smooth muscle cells and brought about the largest drops of MMP-9 expression, factors closely associated with plaque vulnerability (Fig. 3G and fig. S8, F to H), indicating that Zn2+ could be released by activation of MTC1 and exert strong anti-atherosclerotic effect. Figure 3I and fig. S8 (I to K) indicated that, relative to the model group, ORO and hematoxylin and eosin (H&E) staining in aortas in the ML-treated group revealed a reduced lipid-rich necrotic core. In addition, Masson staining further suggested that MLs effectively inhibited collagen accumulation.
Single-cell resolution analysis in ApoE−/− mice after MLs treatment
To identify the therapeutic effect by MLs, scRNA-seq analysis after treatment of MLs conducted on ApoE−/− mice treated with MLs using Seurat unsupervised clustering from the R package (Fig. 4, A to C). Focusing on DEMs for each cell type, we found that DEMs after MLs treatment were highly associated with inflammation (Fig. 4, D and E), such as inflammatory response, response to cytokine stimulus, response to TNF, response to TNF-β, extracellular signal–regulated kinase 1 (ERK1)/ERK2 cascade, positive regulation of cytokine production, autophagy-related focal adhesion phosphatidylinositol 3-kinase–AKT–mammalian target of rapamycin signaling pathway, etc. The treatment of MLs could potentially reduce the expression level of pyroptosis-related genes such as caspase 1, caspase 8, IL-1β, and IL-18 (Fig. 4F). These findings suggest that treatment with MLs could exert anti-inflammatory and anti-pyroptotic effects on macrophages by triggering the zinc release from lysosomes.
Fig. 4. Single-cell resolution analysis of gene expression from atherosclerotic plaques of ApoE−/− mice after treatment of MLs.
(A) t-SNE visualization that categorized seven main cell types presented in atherosclerotic plaques from ApoE−/− mice. (B) Key marker genes defining each type of cell cluster in (A) are presented. (C) Heatmap showing cluster-specific genes at plaque regions. (D) Network visualizing the comprehensive inflammatory-related GO terms and pathways across seven cell types. (E) GO analysis of DEMs exhibiting the biological process, cellular component, and molecular function from annotated macrophages. (F) Dotted lines presenting classical pyroptosis-related marker genes for the annotated macrophages. (G) Pseudotime-ordered analysis of CD8+ T cells identified two distinct cell fates from ML-treated group as compared with model group. (H) Bar plot exhibiting the proportions of each subcluster. (I) Two-dimensional plots exhibiting the expression of selected genes along the pseudo-time from model group and ML-treated group. (J) Heatmap displaying the dynamic variation in gene expression along the pseudotime.
The level of immune cell infiltration in the atherosclerotic immune microenvironment plays a crucial role in the progression of AS, primarily depending on the levels of proinflammatory cytokines and immune cell infiltration. Macrophages are considered as the first responders within atherosclerotic microenvironment, presenting antigens to other immune cells and recruiting them into plaque lesions (46). T cells as another notable driver of inflammation, vary in the immune infiltrated cell population at plaques, mainly include T helper (TH) cells, regulatory T cells (Treg cells), CD4+ T cells, CD8+ T cells, and natural killer (NK) T cells. In addition, CD8+ T cells can promote a pro-atherogenic role by enhancing M1 polarization, thus accelerating AS (47). In addition, secretory inflammatory cytokines trigger CD4+ T cells to differentiate into various cell subsets, including TH17 cells and Treg cells (48). Therefore, inhibiting inflammation and pyroptosis could potently reverse M1 macrophage polarization, reduce the antigen presentation to T cells, and modulate the number and categories of T cell subsets. Subsequently, pseudotime analysis ordered CD8+ T cells in a trajectory and identified three key different states based on gene expression, revealing the differentiation path of the plaque immune microenvironment, from naïve CD8+ cells to effector and memory CD8+ cells (Fig. 4G). Notably, the results suggested that treatment with MLs effectively inhibited CD8+ cells infiltration and decreased the proportion of effector CD8+ cells, indicating AS attenuation (Fig. 4H). To identify the inflammation/pyroptosis-related gene expression dynamics along the pseudotime trajectories, we explored DEMs that constantly increased or decreased during AS development, where we classified six grouped gene modules based on their expression changes and found that the inflammation/pyroptosis-related genes (such as Gzma, Gzmb, Pgs1, and Timp1) exhibited a decreasing trend after MLs treatment (Fig. 4, H to J). These findings led us to conclude that MLs could modulate the lysosomal function and exert antiatherosclerotic effect.
Design, synthesis, and characterizations of CM@MS NPs
MLs have low solubility in physiological solutions and limited therapeutic efficacy, which complicates their effective systemic delivery for in vivo therapy. Next, we sought to further explore suitable carriers for MLs in the treatment of AS, optimizing the in vivo therapeutic efficacy. Recently, nanomaterials have been extensively investigated as platforms for delivering therapeutic or imaging contrast agents for AS management (49–51). This is due to the compromised endothelial barrier at plaque lesions, which exhibit substantially decreased junction continuity (52), causing nanomaterials size less than 500 nm can passively target and penetrate the plaque lesions (33). Among various nanosystems, MSNs have emerged as particularly promising for drug delivery (53), which were selected to administer MLs for AS treatment in this study, offering advantages over treatments using free MLs. For monitoring the therapeutic process, ICG molecules, known for their strong light absorption, were chosen as PA agents and attached to the surface of MSNs (54). Furthermore, considering the prevalence of intravascular oxidative stress within the atherosclerotic microenvironment, C NDs with robust ROS scavenging properties were incorporated to enhance the therapeutic efficacy of MLs. The typical transmission electron microscope (TEM) images (fig. S9A) and scanning electron microscopy (SEM) images (fig. S9B) of MSNs show the monodispersed dendrimer-like structure with radial ultralarge mesopores (~28 nm; fig. S9, C and D), which could behave as reservoirs and achieve encapsulation of guest NPs and small molecules. 29Si MAS nuclear magnetic resonance spectra confirmed the successful fabrication of MSNs (fig. S9E). The elemental distribution mappings (fig. S9F) and the x-ray energy dispersive spectroscopy (fig. S9G) in MSNs revealed the presence of Si and O element.
To evaluate the efficacy of nano-sized ML-loaded MSN NPs (ML@MSN NPs), the plaque-bearing ApoE−/− mice were applied. The ApoE−/− mice were randomly separated to five different groups (n = 8) after receiving a high-fat diet for 1 month to develop atherosclerotic plaques, including model, MSN NPs (Si concentration: 1.4 mg kg−1), MLs (0.6 mg kg−1), low-dose ML@MSN (Si concentration: 1.4 mg kg−1; MLs: 0.6 mg kg−1), and high-dose ML@MSN (Si concentration: 1.4 mg kg−1; MLs: 1.2 mg kg−1), followed by twice weekly injections for another 2 months maintaining high-fat feeding. After varied treatments, the entire aorta was isolated and stained using ORO. It was found that large ORO-positive areas were detected in the model group, while the smallest ORO-stained areas were observed in ML@MSN NP-treated group in a dose-dependent manner (figs. S10, A and B and S11, A and B). H&E staining of aortic root sections revealed that the plaque area in the high-dose ML@MSN NP group (11.05 ± 4.04%) was markedly reduced compared to the model group (32.31 ± 2.24%; fig. S11C). Masson’s trichrome staining further demonstrated a pronounced decrease in collagen accumulation within the plaques following ML@MSN NP treatment (fig. S11D). Consistent with these findings, immunohistochemical staining for matrix metalloproteinase–9 (MMP-9) and CD68 antibodies indicated that high-dose ML@MSN NPs treatment notably reduced macrophage infiltration, vascular smooth muscle cell (VSMC) infiltration, and MMP-9 expression in the plaques (fig. S12, A and C to E). Furthermore, it was also noted that ML@MSN NP treatment could reduce the expression of GSDMD level (fig. S12, B and F). These results suggested that ML@MSN NP treatment could block macrophage pyroptosis and inhibit the progression of AS.
Based the above encouraging results, we next explored a ML-based theranostic nanoplatform for AS treatment. First, to enable visualization of AS treatment, the sequential surface modification of 3-aminopropyltriethoxysilane (APTES) and ICG-COOH was performed, and MSN-APTES-ICG NPs (denoted as MS NPs) with the ability of PA imaging guidance were obtained, which were completely inherited the features of counterparts in size and morphology of MSN NPs (fig. S13A). Fourier transform infrared spectroscopy (fig. S13B) and series changes on Zeta potential (fig. S13C) of each modification step of MS NPs substantiated the successful grafting of ICG onto the surface of MSNs, which was further supported by thermogravimetric analysis results (fig. S13D). Subsequently, MLs and ultrasmall C NDs (fig. S13, E to G), which exhibit potent ROS scavenging capacity, were encapsulated into the large mesopores of dendritic MSN NPs (Fig. 5A), forming CM@MS NPs. The particle sizes of CM@MS NPs and the loaded C NDs were ~180 and 5 nm, respectively (fig. S13H). The ultraviolet-visible (UV-vis)–near-infrared (NIR) spectra confirmed the successful MLs loading into CM@MS NPs (Fig. 5B). The dispersity of the developed CM@MS NPs in various solvents [including H2O, simulated body fluid (SBF), saline and Dulbecco’s modified Eagle’s medium (DMEM)] demonstrated high stability, confirming their suitability for both in vitro and in vivo applications (fig. S14). Afterward, x-ray photo-electron spectroscopy (XPS) was conducted to analyze the surface chemical status of CM@MS NPs (Fig. 5, C and D). The C1s XPS results confirmed the presence of various functional groups such as C═C, C═O, C─O, and O─C═O on the surface of C NDs component of the CM@MS NPs. Notably, the content of the C═C bonds was determined to be ~51%, a composition critical for the superoxide dismutase (SOD)–like enzymatic activity of the CM@MS NPs (55). Then, the C ND/ML loading capacity of CM@MS NPs was determined by measuring the change in UV-vis absorbance before and after the incorporation of C NDs and MLs. The results indicated that the approximate mass ratio of MSNs, C NDs, MLs, and ICG within the CM@MS NPs was 15:3.5:4:2.5. After that, the releasing performance of MLs and C NDs was also evaluated. Nearly 25% MLs and 20% C NDs were released from the nanopores of CM@MS NPs during 24 hours of observation (fig. S15). The high light absorption in NIR region endowed them with desirable PA imaging capabilities, which overcome the penetration limitations of optical imaging due to low tissue-attenuation coefficients and enable real-time therapeutic monitoring. Figure 5E showed that the enhanced PA signals were observed as CM@MS NP concentration increased, and the PA signals of CM@MS NPs followed a linear relationship with the CM@MS NPs concentration, demonstrating the desirable PA-imaging ability of CM@MS NPs.
Fig. 5. Characterizations and cellular internalization of CM@MS NPs.
(A) TEM images of CM@MS NPs at different magnifications and schematic illustration of modification process of ICG-engineered CM@MS NPs. Scale bars, (A1) 100 nm, (A2) 50 nm, and (A3) 2 nm. (B) UV-vis–NIR spectra of MSNs, MLs, ICG, C nanodots, and CM@MS NPs. (C) XPS spectrum of CM@MS NPs and (D) corresponding XPS spectrum of C1s region in CM@MS NPs. (E) In vitro concentration-dependent PA signals of CM@MS NPs under 808-nm laser irradiation. (F) Uptake of CM@MS NPs by J774A.1 cells and (G) quantification of uptake based on fluorescence intensity within 8 hours. (H) Colocalized images of CM@MS NPs in J774A.1 cells incubated for different time points. Scale bars, 20 μm. (I) Fluorescence images of J774A.1 cells stained with FluoZin-3 (–AM) and LysoTracker after different treatments and corresponding statistical analysis of FluoZin-3 intensity after different treatments. Scale bars, 20 μm. (J) Schematic illustration of lysosome escape of CM@MS NPs. Statistical analysis was performed by one-way ANOVA. The data are presented as means ± SDs. a.u., arbitrary unit.
ROS-scavenging property of CM@MS NPs
ROS/reactive nitrogen species (RNS) are highly reactive molecules that play a key role in vascular homeostasis. The overproduction of ROS/RNS can induce vascular damage, trigger oxidative modifications of LDL, and promote an inflammation response and thereby contributing to the development of AS. To evaluate the ROS-scavenging capabilities of CM@MS NPs, the commonly 2,20-azinobis (3-ethylbenzthiazoline-6-sulfonate) (ABTS) assay was used to assess the general ROS-scavenging performance of CM@MS NPs. As displayed in fig. S16A, nearly 85% of ABTS•+ radicals were inhibited, indicating high ROS-scavenging ability of CM@MS NPs. To further analyze the catalytic selectivity of CM@MS NPs, and •OH were specifically tested for their ROS-scavenging abilities, while 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) assays were used to evaluate the RNS-scavenging performance. The results indicated that nearly 60% of •OH, 70% of , 75% of DPPH•, and 55% of PTIO• could be eliminated when the Si concentration in CM@MS NPs was 140 parts per million (ppm) (fig. S16, B to E), where the SOD activity was calculated to be about 2.65 U ml−1 (fig. S16C).
In vivo toxicological evaluation and cellular internalization of CM@MS NPs
Macrophages are the key integrators of inflammatory signals, which are vital in the progression of AS (56, 57). Consequently, the ability of CM@MS NPs to enter and function effectively within macrophages is crucial for their therapeutic efficacy in AS treatments, where we used J774A.1 mouse mononuclear macrophage as the cell model (39). Initially, flow cytometry was applied to study the uptake of CM@MS NPs by J774A.1 cells at various time intervals. J774A.1 cells treated with ICG-modified CM@MS NPs exhibited a gradual increase in mean fluorescence intensity over an 8-hour incubation period, indicating efficient cellular internalization (Fig. 5, F and G). To elucidate the intracellular transport pathway of CM@MS NPs, lysosomal colocalization experiments were conducted. During the initial 4 hours of incubation, there was a notable increase in green fluorescence, indicating the internalization of CM@MS NPs. This fluorescence pronouncedly overlapped with the red fluorescence emanating from lysosomal markers, suggesting primary transport through the lysosomal pathway. Over time, the overlap between these fluorescence signals decreased, with clear separation evident at 12 hours, which confirmed the lysosomal escape capabilities of the CM@MS NPs (Fig. 5H). Furthermore, FluoZin-3-AM was also applied to detect the intracellular Zn2+ release in J774A.1 cells triggered by CM@MS NPs. The result displayed that FluoZin-3 fluorescence signals notably decreased after treatment with CM@MS NPs, while other treatments showed no such an effect, suggesting that CM@MS NPs could trigger the lysosomal Zn2+ release (Fig. 5, I and J).
The in vitro biocompatibility of CM@MS NPs confirmed by Cell Counting Kit-8 (CCK-8) test due to both MSNs and C NDs feature the desirable biocompatibility (fig. S17A). Then, the in vivo toxicity of CM@MS NPs was systematically evaluated to ensure their safety for guaranteeing further clinic use. As shown in fig. S17 (B and C), the weight variation of CM@MS NP-treated mice was consistent with that of the control group. No notable difference was observed in all these groups in the levels of the major indexes for the kidney and liver function 30 days post-injection, indicating that CM@MS NPs feature minimal renal and hepatic toxicity. In addition, the routine blood results of mice with injection of CM@MS NPs were not obviously different from the control group (fig. S17D). The H&E staining of major organs showed no notable signs of damage, demonstrating high biocompatibility of CM@MS NPs (fig. S17E). These results confirmed the satisfactory biosafety of CM@MS NPs at the therapeutic dose. Furthermore, the in vivo metabolism was also tested, and the blood circulation half-life was determined to be nearly 90 min (fig. S17F). Accordingly, nearly 7% of total intracellular CM@MS NPs concentration could be accumulated within the plaque lesions after 24-hour treatment (fig. S17G).
In vitro ROS-scavenging and anti-inflammatory properties by CM@MS NPs
Excess ROS induces oxidative stress, causing tissue damage and subsequent inflammation. Therefore, we further investigated whether the CM@MS NPs could mitigate ROS production in J774A.1 cells. As shown in Fig. 6A, the intracellular ROS level of J774A.1 cells increased markedly after treatment with LPS (100 ng ml−1). In contrast, cells treated with CM@MS NPs exhibited markedly reduced fluorescence intensity compared to those treated with other formulations, indicating suppressed ROS production. Further analysis involved separate staining with the •OH probe [3ʹ-(p-aminophenyl)fluorescein, AFP, green signals] and fluorescent probe [dihydroethidium (DHE), red signals]. The results showed that CM@MS NPs treatments could effectively attenuate the generation of •OH and in J774A.1 cells. These findings confirmed that CM@MS NPs could effectively ameliorate the oxidative stress, which further protected the mitochondrial function by reversion of the mitochondrial membrane potential after CM@MS NP treatment (Fig. 6B).
Fig. 6. Evaluation of ROS-scavenging property and anti-inflammatory effect by CM@MS NPs at cellular level.
(A) Confocal microscopy images of total ROS, , and •OH in J774A.1 cells after different treatments. Scale bars, 20 μm. (B) Confocal microscopy images on the changes of mitochondrial membrane potential in J774A.1 cells after different treatments. Scale bars, 20 μm. Levels of (C) IL-6, (D) TNF-α, and (E) IL-1α in LPS-treated J774A.1 cells after varied treatments. (F) Schematic illustration of therapeutic process of CM@MS NPs in cells. (G) Immunofluorescence of IL-6, TNF-α and IL-1α and (H) corresponding fluorescence intensities in LPS-induced J774A.1 cells after different treatments. Scale bars, 50 μm. (I) Confocal fluorescence images showed cellular internalization of Dil-labeled oxidized LDL in J774A.1 cells. For fluorescence observation, nuclei were labeled with DAPI (blue), and the cell membrane was stained with Dil (magenta). (J) Observation of ORO in foam cells derived from J774A.1 cells. Scale bars, 20 μm. (K) Representative flow cytometry plots of M1 subtypes of macrophages percentage after varied treatments. Statistical analysis was performed by one-way ANOVA. The data are presented as means ± SD.
Based on the ROS-scavenging capacities of CM@MS NPs, further evaluation of their ability to inhibit inflammation in J774A.1 cells was conducted. The expression levels of inflammatory cytokines in J774A.1 cells were quantified using enzyme-linked immunosorbent assay (ELISA; Fig. 6, C to E) method and corroborated by immunofluorescence imaging (Fig. 6, G and H). The expression levels of the pro-inflammatory factors—including IL-6, tumor necrosis factor alpha (TNF-α), and IL-1α—were markedly reduced in the CM@MS NP-treated group, noticeably lower than those observed in the C@MS NP-treated group, while LPS-stimulated J774A.1 cells in the model group displayed a notable increase in the secretion of pro-inflammatory cytokines. This result suggested that CM@MS NPs could effectively inhibit inflammation in J774A.1 cells (Fig. 6F). Previously studies demonstrated that foam cells, which form by the imbalance between oxidized low-density lipoprotein (ox-LDL) uptake and cholesterol transport in macrophages, play a key role in the development of AS (58). Our findings demonstrated that treatment with CM@MS NPs effectively inhibited ox-LDL uptake in J774A.1 cells (Fig. 6I) and reduced foam cell formation (Fig. 6J). Furthermore, we explored the effects of CM@MS NPs on macrophage polarization. Notably, after activation with LPS and interferon-γ (IFN-γ), the proportion of M1 macrophages (F4/80+ CD86+ cells) was notably reduced in the CM@MS NP group, indicating a robust anti-inflammatory effect, whereas the C@MS treatment only modestly modulated macrophage transition (Fig. 6K and fig. S18).
In vitro antipyroptosis capacity by CM@MS NPs
To evaluate whether MS NPs, C@MS NPs, and CM@MS NPs inhibit pyroptosis, we added these NPs to LPS plus nigericin primed J774A.1 cells, where nigericin is a K+ ionophore that activates the NLRP3 inflammasome by depletion of cytoplasmic K+. SYTOX Green and cell membrane staining exhibited that extensive cell swelling and giant bubble-like formation were observed in LPS plus nigericin-treated group, while the cells treated with CM@MS NPs showed no notable morphological changes (Fig. 7, A and B), the result of which was further confirmed by bio-TEM observation (fig. S19).
Fig. 7. Evaluation on cell pyroptosis inhibition by CM@MS NPs at cellular level.
(A) SYTOX Green staining of LPS plus nigericin-induced J774A.1 cells after varied treatment. Scale bars, 20 μm. (B) Morphological features of J774A.1 cell membrane observed by CLSM after varied treatments. The cell membrane was stained with PlasMem Bright Red. Scale bars, 20 μm. (C) Circle diagram of enriched biological process terms from GO analysis after treatment with CM@MS NPs. (D) Circle diagram of KEGG enrichment analysis of inflammatory/pyroptosis-involved DEMs. (E) Rank-order plot for RNA-seq of DEMs from J774A.1 cells treated with LPS plus nigericin and LPS plus nigericin plus CM@MS NPs. (F) Friends analysis to identify key pyroptosis-associated DEMs. (G) Quantitative analysis of varied gene expression levels after treatment with CM@MS NPs as determined by PCR. (H) Western blot results of protein expression (including caspase-1, GSDMD, and GSDMD-N) and corresponding quantitative analysis of varied gene expression levels after different treatments. (I) Immunofluorescence images of GSDMD and caspase-1 in LPS plus nigericin-induced J774A.1 cells after different treatments. Scale bars, 20 μm. (J) Pyroptosis of LPS plus nigericin-induced J774A.1 cells after varied treatments. The cells were stained with fluorescein-labeled fluorochrome inhibitor of caspases (FAM-FLICA) caspase-1 assay kit and analyzed by flow cytometry analysis. Statistical analysis was performed by one-way ANOVA.
To shed light on the intrinsic mechanism of CM@MS NP-based nanotherapy in LPS plus nigericin-treated J774A.1 cells, high-throughput transcriptomics analysis was conducted. The heatmap indicated that a total of 1071 mRNAs were differentially expressed after treatment with CM@MS NPs, verifying significant transcriptomics differences between the CM@MS NP group and the model group (fig. S20, A and B). A volcano plot demonstrated that 446 DEMs were up-regulated, while 625 down-regulated in the CM@MS-treated group (fig. S20A). Following the determination of genetic mRNA differences, GO process analysis was conducted to illuminate the biological process, cellular component, and molecular function after treatment of CM@MS NPs (Fig. 7C and fig. S20C), including inflammatory response, cytokine activity, protein kinase activity, and so on. The specific regulatory mRNAs corresponding to the major impact pathways after CM@MS NPs treatment were exhibited in KEGG pathway analysis. The antipyroptosis mechanism in combination with the anti-inflammatory response played key roles in the progression of AS. The result showed that the most significantly regulated mRNAs were ascribed to the TNF signaling pathway, NF-κB signaling pathway, IL-17 signaling pathway, lipid and AS, FoxO signaling pathway, fluid shear stress and AS, NOD-like receptor signaling pathway, etc. (Fig. 7D and fig. S20D). Studies have suggested that zinc could inhibit the NF-κB signaling pathway (20) and subsequently modulate the immune response transcription factors involved in inflammatory-related molecular signaling, such as TNF signaling pathway. Tracing the initial process of pyroptosis, the expression of inflammatory factors/pyroptosis related mRNAs after CM@MS NPs treatment should be monitored as a primary verification task, and the ring numeric heatmap highlighted the expression of major genes involved in inflammation and pyroptosis (fig. S20, E and F), where the appearance of down-regulated signals included IL-1α, Nlrp3, IL-1β, IL-6, Tnf, etc. (Fig. 7, E and F), confirming the anti-inflammatory/antipyroptosis effect of CM@MS NPs. Furthermore, the result of gene set enrichment analysis indicated that gene sets for inflammatory response and pyroptosis were down-regulated in cells after CM@MS NPs treatment, pointing to the anti-inflammatory/antipyroptosis properties of CM@MS NPs (fig. S20, G and H). Quantitative polymerase chain reaction (qPCR) evaluation, Western blot analysis, and immunofluorescence images observation were further conducted to reveal the potential mechanism by analysis of the expression level of pyroptosis-related genes and proteins (Fig. 7, G to I). The result showed that a pronounced decrease of caspase-1 and GSDMD-N level was detected in CM@MS NP-treated cells. Furthermore, pyroptosis inhibition blocked by CM@MS NPs was supported by evaluation of cell survival via both flow cytometry (fig. S21), and the result of which suggested that CM@MS NPs could substantially reduce the expression level of caspase-1 and inhibit the J774A.1 cells pyroptosis (Fig. 7J).
In vivo treatment of AS enabled by CM@MS NPs
Before in vivo treatment, the plaque-bearing ApoE−/− mice were initially used for evaluating the passive accumulation effect of CM@MS NPs by PA imaging. The result showed that, after treatment of CM@MS NPs, the PA signal intensities obviously increased in the CM@MS NP group as compared with the control group, indicating that CM@MS could accumulate to the plaque lesions (fig. S22), mainly attributing to the fact that these NPs could reach the plaque sites via a compromised endothelial barrier.
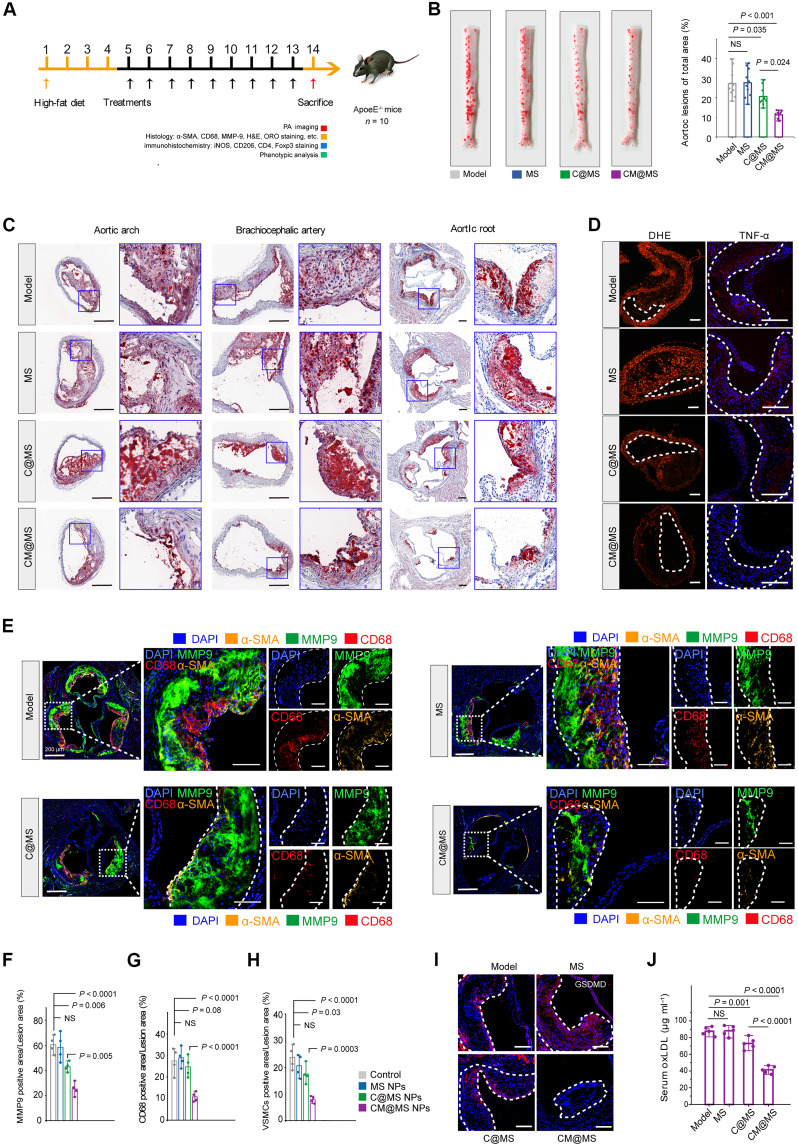
The antioxidant and anti-inflammatory activities of CM@MS NPs were systemically evaluated in vivo. ApoE−/− mice were randomly assigned to four different groups (n = 10) based on the above therapeutic protocol (Fig. 8A). After treatments, the entire aorta was collected, stained with ORO, and characterized. It was noted that both MS and model groups showed large ORO-positive areas and treatment with C@MS NPs afforded limited therapeutic effects, indicating that antioxidant treatment alone exerted compromised antiatherosclerotic efficacy due to intricate cross-talk between ROS signals and inflammatory process. By contrast, considerably low ORO-stained areas were observed in CM@MS NP group. Further quantification showed significant differences among the groups, where the average plaque area was 26.47 ± 6.58, 27.42 ± 7.05, 20.52 ± 4.28, and 14.10 ± 4.66% for mice treated with saline, MS, C@MS NPs, and CM@MS NPs, respectively (Fig. 8B and fig. S23, A and B). We found that plaques in both MS and model groups largely consisted of lipid-rich necrotic cores. Compared to the C@MS group, the necrotic core area was significantly low in the CM@MS NPs (fig. S23, C and D). Staining with the Masson’s trichrome method exhibited a significantly lower content of collagen in CM@MS NP-treated group, indicating CM@MS NP treatment could reduce collagen accumulation and attenuate the AS progression (fig. S23, E and F). Similarly, the ORO-stained sections collected from aortic root, aortic arch, and cephalic brachial arteries also verified that the CM@MS NPs exert the most potent antiatherosclerotic activity (Fig. 8C and fig. S23, G to I).
Fig. 8. Therapeutic effects of CM@MS NPs in ApoE−/− mice.
(A) Schematic illustration of the in vivo therapeutic protocols treated by CM@MS NPs. (B) Representative photographs and quantitative analysis of ORO-stained aortas of mice following treatment with varied formulations. (C) Representative ORO-stained cryosections. Scale bars, 400 μm. (D) Fluorescence images stained with DHE and antibody against TNF-α from ApoE−/− mice after varied treatments. Scale bars, 200 μm. (E) Fluorescence images and (F to H) quantitative analysis of aortic root sections stained with MMP-9, antibody to CD68, and antibody to α-SMA from ApoE−/− mice after varied treatments. Scale bars, 400 μm. (I) Fluorescence images stained with antibody against GSDMD from ApoE−/− mice after varied treatments. Scale bars, 200 μm. (J) Serum levels of ox-LDL. Statistical analysis was performed by one-way ANOVA. NS, not significant.
Previous studies have indicated that lysosomal Ca2+ plays a crucial role in autophagy (59), an intracellular degradation process that facilitates the breakdown of cytoplasmic material and organelles. Autophagy has been found to regulate the efflux of cellular cholesterol from macrophages, contributing to the attenuation of AS (60). Thus, we explored whether CM@MS NP-involved lysosomal Ca2+ release could induce autophagy in J774A.1 cells. First, acridine orange (AO) staining was applied to evaluate the dynamic course of autophagic efflux because it could penetrate into acidic compartments and emit red fluorescence signals when it protonated (fig. S24A). The results demonstrated that week red dots were detected in macrophages after MS NPs treatment, while no obvious fluorescence variation was observed in control group, mainly attributing to the fact that MSN-based NPs themselves could induce autophagy process to some extent (61). After treatment of CM@MS NPs, the fluorescence intensities were comparable with that in MS NPs, suggesting that CM@MS NPs could induce week autophagy. To further explore whether CM@MS NP-involved autophagy could contribute to the remission of AS in vivo, LC3 staining was performed. As shown in fig. S24B, the expression of LC3 in all three experiment groups (MS NPs, C@MS NPs, and C@MS NPs) was a little higher than that in control group. However, the treatment of MS NPs showed no obviously therapeutic effect, suggesting that autophagy did not play a key role in the treatment of AS treated by the designed CM@MS NPs.
To assess whether CM@MS NPs treatment could reduce ROS level and inhibit inflammation, DHE method was carried out to evaluate the ROS scavenging ability of CM@MS NPs. After different treatments, sections of brachiocephalic artery from ApoE−/− mice were observed, where the model group emitted strong fluorescence signal (red), whereas treatment with CM@MS NPs showed the weakest fluorescence intensity, indicating strong ROS-scavenging efficacy of CM@MS NPs, which was further confirmed by reduced expression level of inflammatory cytokines after treatment of CM@MS NPs (Fig. 8D and fig. S23, J and K). Furthermore, anti-CD68 antibody, α-SMA antibody, and MMP-9 antibody staining demonstrated that treatment of CM@MS NPs could efficiently alleviate the macrophage and vascular smooth muscle cells infiltration and reduce the MMP-9 expression at plaques (Fig. 8, E to H). The expression level of pyroptosis-related proteins was also evaluated, and the result indicated that CM@MS NPs treatment could effectively decrease the expression of GSDMD and IL-1β (Fig. 8I and fig. S23, L and M). This was further confirmed by the lowest levels of ox-LDL in serum after treatment with CM@MS NPs (Fig. 8J).
Immune regulation at plaque lesions by CM@MS NPs in ApoE−/− mice
The in vitro ability of CM@MS NPs for inhibiting inflammation and blocking pyroptosis promoted us to explore whether CM@MS NPs could reshape the immune microenvironment at plaque lesions. We analyzed both immune cells from spleen and aortic plaque lesions by flow cytometry. After different treatments, the ApoE−/− mice were euthanized at the 90th day, and single-cell suspensions of plaque lesions were obtained for flow analysis of CD206/CD86 (a marker of the relative proportion of M2 versus M1 cells) to examine whether CM@MS NPs could shift the phenotype of macrophages at plaques. It was noted that CM@MS NP treatment increased M2 macrophages in aortic plaque lesions, while MS NP and C@MS NP treatment showed no such an effect (fig. S25). Recent research has indicated that, in addition to the innate immune system, adaptive immunity also plays a crucial role in the development of AS. T lymphocytes, which are recruited by antigen-presenting cells, are closely associated with the progression of lesion inflammation, whose abundance undergoes the most pronounced changes during this process (62). Studies showed that CD8+ T cells constitute a substantial proportion of the total immune cells in atherosclerotic plaques, which produce a variety of cytokines and promote the development of AS (63). An in-depth flow analysis revealed that the proportions of CD3+ cells, CD4+ T cells, and CD8+ T cells within plaques in the CM@MS NP-treated mice were 0.61, 0.54, and 0.37 times lower, respectively, compared to the model group. Similarly, in the spleen, these proportions were 0.50, 0.77, and 0.48 times lower than those in the model group. In addition, Foxp3+ Treg cells, which could counterbalance pro-inflammatory effector T cells, were further assessed in atherosclerotic plaques and spleen. The number of Foxp3+CD25+ Treg cells significantly increased in the CD4+ T cell populations by 3.80-fold in atherosclerotic plaques and by 3.95-fold in the spleen for the CM@MS NP-treated group compared to the model group. In addition, the frequency of CD4+, CD44+, and CD62L− T cells in the spleen was tested to verify the inhibition of immune system activation enabled by CM@MS NPs (fig. S26). As expected, mice treated by CM@MS NPs exhibited the lowest frequency of CD4+, CD44+, and CD62L− T cells. These results confirmed that CM@MS NPs were capable of regulating macrophages polarization and T cell differentiation (Fig. 9, A to N).
Fig. 9. Immune regulation at plaque lesions by CM@MS NPs in ApoE−/− mice.
(A to F) Representative flow cytometry plots showing CD3+ T cell, CD4+ T cell, CD8+ T cell, and Treg cell ratios in aortas and spleen extracted from mice after different treatments. (G to N) Quantitative analysis of the number of CD3+ T cell and the CD4+ T cell, CD8+ T cell, and Treg cell ratios in aortas and spleen after varied treatments. (O) Immunofluorescence assay showing iNOS (red) and CD206 (green) and (P and Q) Quantitative analysis in the aortic root sections from mice after different treatments. Scale bars, 100 μm. (R) Immunofluorescence assay showing CD4 (red) and Foxp3 (green) and (S and T) quantitative analysis in the aortic root sections from mice after varied treatments. Scale bars, 100 μm. (U) Schematic illustration of CM@MS NPs potentially immunoregulation of mechanism at plaque lesions.
Immunofluorescent staining further confirmed the altered expression of macrophage polarization-associated protein after different treatments. The mice in CM@MS NP-treated group exhibited the increased CD206 expression and decreased expression of inducible nitric oxide synthase (iNOS) within the plaque lesions while comparing with that in the other three groups, indicating that CM@MS NP-treated macrophages were successfully polarized into M2 types from M1. Moreover, greater numbers of FOXP3+CD4+ Treg cells were detected in CM@MS NP-treated group as compared with the model group, which can exert a protective role during AS, while no notable FOXP3+ expression was observed in MS NP group and C@MS NP-treated group (Fig. 9, O to U).
DISCUSSION
AS, characterized by oxidative stress and chronic inflammation, remains a substantial cause of morbidity and mortality. In addition, pyroptosis, characterized by GSDMD-pore formulation and rapid release of inflammatory cytokines, can exacerbate inflammation and induce plaque instability. Inspired by the advances in metals in medicine, we conducted a screening of 10 different metal ions to evaluate their potential as antiatherosclerotic agents, and our results indicated that zinc could exert anti-inflammatory property even at low concentrations (from 2.5 to 10 μM). However, pro-inflammatory effects were also observed when Zn2+ concentrations exceed 25 μM, highlighting the dual nature of zinc ions.
Lysosome can sequester toxic ions within their vacuolar membrane. Furthermore, MTC1, a Ca2+/Zn2+/Fe2+ permeable ion channel located on the membranes of lysosomes, participates in the intracellular transport of zinc ions. This prompted us to investigate whether modulation of MTC1 could exert an anti-inflammatory effect. We found that using MTC1-specific agonists (MLs), which directly bind to MTC1, can trigger lysosomal zinc release and exert desirable anti-inflammatory function even at a high concentration of 100 μM. In addition, chelating Ca2+ and Fe2+ could not alter the anti-inflammatory property by lysosomal zinc. Furthermore, we also confirmed that treatment of MLs could reduce the expression level of caspase-1 and block macrophage pyroptosis. Based on these findings, we propose that the rational modulation of the lysosomal zinc pool to exert anti-inflammatory and antipyroptosis effects may minimize side effects and prevent the direct exposure of normal tissues/organs to exogenous drugs, demonstrating substantial potential for future clinical applications (64–73).
However, MLs have low solubility in physiological solutions and exhibited limited in vivo therapeutic effect. Consequently, we constructed ICG-engineered MSNs as carriers to coload C NDs and MLs within their mesoporous structure (CM@MS NPs), optimizing their in vivo therapeutic efficacy. Both in vitro and in vivo results demonstrated that CM@MS NPs had high biosafety, desirable PA imaging ability, high ROS-scavenging abilities, and strong anti-inflammatory/antipyroptosis performance. Furthermore, we also varied that CM@MS NPs could promote macrophage polarization, reduce immune cell infiltration, and reshape the immune microenvironment at plague lesions.
In summary, we present a multifunctional nanoplatform targeting pyroptosis for PA-guided treatment of AS following intravascular injection, strategically leveraging the self-lysosomal zinc pool. Our findings highlight that CM@MS NPs effectively mitigate AS development and progression, offering an alternative but efficient treatment in scenarios where conventional anti-inflammatory approaches fall short. Collectively, our results underscore the potential of using the self-lysosomal zinc pool as an effective therapeutic strategy for treating AS and other inflammatory diseases.
MATERIALS AND METHODS
Materials
Tetraethyl orthosilicate (TEOS), triethanolamine (TEA), hexadecyl trimethyl ammonium bromide (CTAB), sodium salicylate (NaSal), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC-HCl), N-hydroxysuccinimide (NHS), N,N-dimethylformamide (DMF), and hydrochloric acid (HCl) were obtained from Aladdin Co. Ltd. (Shanghai, China). LPS was obtained by Sigma-Aldrich (Saint Louis, USA). APTES was ordered from Macklin Biochemical Co. Ltd. (Shanghai, China). MLs (C22H22N2O3), nigericin, AFP (C26H17NO5), and DHE were obtained from MedChemexpress Co. Ltd. (Shanghai, China). FluoZin-3, AM, and SYTOX Green were obtained from Thermo Fisher Scientific Co., Ltd. (Boston, USA). ICG-carboxlaic acid (ICG-COOH) was bought from Ruixi Biotechnology Co. Ltd. (Xi’an, China). 2′,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA), annexin V–mCherry/SYTOX Green Apoptosis Detection Kit, JC-1 assay kit, and LysoTracker Red were obtained from Shanghai Beyotime Biotechnology Company (Shanghai, China). Mouse IL-1β, TNF-α, IL-6, and IL-1α ELISA kits were ordered from Multiscience (Hangzhou, China). CCK-8 and PlasMem Bright Red were purchased from Dojindo Molecular Technologies DiI-ox-LDL and ORO were obtained from Jingkehuaxue Company (Shanghai, China).
Characterization
TEM images and corresponding energy dispersive x-ray spectroscopy spectra were obtained on a JEM-2100F electron microscope. SEM images were observed on a field-emission Magellan 400 microscope (FEI Company). XPS spectra were performed on ESCAlab250 (Thermal Scientific). Size and zeta potential measurements were performed on a Zetasizer Nanoseries (Nano ZS90, Malvern Instrument Ltd.). UV-vis–NIR absorption spectra were obtained by a UV-3600 Shimadzu UV-vis–NIR spectrometer. The quantitative analysis of element Si concentration was conducted by inductively coupled plasma–optical emission spectrometry (ICP-OES, Agilent 725, Agilent Technologies, USA). The confocal laser scanning microscopy (CLSM) images were performed on a FV1000 (Olympus Company, Japan).
Synthesis of CM@MS NPs
For synthesis of MSNs, TEA (0.0068 g) was first dissolved into deionized water (25 ml) and placed in a water bath at 80°C with a magnetic stirrer for 1 hour. Then, CTAB (0.38 g) and NaSal (0.168 g) were added to the above solution for another 30 min of stirring, which was followed by the dropwise addition of TEOS (2 ml). After 2 hours of gentle stirring, the product was collected by centrifugation. Afterward, CTAB was removed by using a mixture of ethanol and HCl solution (6%) by extraction at 80°C for 24 hours three times. Last, the MSNs were collected, washed with water and ethanol for several times, and dispersed in ethanol for later use.
For synthesis of MSN-ICG, the modification of the MSNs’ surface with APTES was conducted by suspending MSNs (0.2 g) in anhydrous toluene (10 ml) under an argon atmosphere into a round bottom flask (25 ml), followed by sonicated for 30 min. After that, APTES (0.10 ml) was added dropwise, and the mixture was maintained at 130°C for 24 hours. Then, the products were obtained by centrifugation and washed with ethanol three times. Then, ICG-COOH (1 mg), EDC-HCl (1.44 mg), and NHS (0.8 mg) were dissolved in 1 ml of DMF and stirred at room temperature for 24 hours to acquire carboxyl activated ICG (ICG-COOH). After that, MSN-APTES (5 mg) and carboxyl-activated ICG (ICG-COOH) were dispersed into 2 ml of DMF and stirred at room temperature for 24 hours. Last, the products were collected and washed with DMF three times.
For synthesis of CM@MS NPs, MS NPs (30 mg) were dispersed in 50 ml of ethanol solution, followed by adding 100 mg of C NDs and 100 mg of MLs. After gently stirring for 24 hours, the final products were collected by centrifugation and washed with ethanol and water three times and dispersed in aqueous solution for later use.
ROS-scavenging performance of CM@MS NPs
In ABTS•+-scavenging assay, ABTS solution (7 mM) was initially incubated with potassium persulfate (2.45 mM) for 24 hours to activating ABTS•+ radicals. Then, CM@MS NPs with a serial Si concentration from 0 to 4.375 mM were added into ABTS•+ solutions and measured by a UV-vis–NIR spectrophotometer.
In PTIO•-scavenging activity assay, 3 mg of PTIO was first dissolved in 20 ml of distilled water. Afterward, CM@MS NPs with a serial Si concentration from 0 to 4.375 mM were added into the above solution. Then, the tested reaction mixtures were incubated at 37°C for 2 hours. Last, the samples were tested by a UV-vis–NIR spectrophotometer.
In DPPH-scavenging assay, a mixture of dimethyl sulfoxide containing 50 μM DPPH was plated in a 96-well plate. One hour after incubation, the reaction was induced by the addition of 10 μl of CM@MS NPs solution to 190 μl of DPPH solution for 30 min, which was then measured by a UV-vis–NIR spectrophotometer. The generation of DPPH radicals was conducted by subtracting the absorbance of CM@MS NPs samples mixed with methanol.
In -scavenging assay and SOD-like activity test, CM@MS NPs solutions with varied Si concentrations were set to evaluate the content of residual radicals by a superoxide anion assay kit (Comin, Suzhou, China). As for SOD-like activity, the test samples were performed by a SOD assay kit with WST-8 (Beyotime Biotechnology Company, Shanghai, China). After reacting with CM@MS NP solutions with varied Si concentrations, the SOD-like activity was calculated according to the manufacturer’s instructions.
In •OH-scavenging assay, CM@MS NPs solutions with varied Si concentrations were set to evaluate the content of residual •OH radicals by a hydroxyl radical assay kit according to the manufacturer’s instructions (Comin, Suzhou, China).
Cell uptake study
First, the uptake of CM@MS NPs in J774A.1 cells was studied. Briefly, J774A.1 cells were seeded in 24-well plates (2.5 × 104 cells per well) overnight. CM@MS NPs were added to each well at different time points. Afterward, the cells in each well were washed with phosphate-buffered saline (PBS) for three times and transferred to eppendorf (EP) tubes (1.5 ml) and subsequently centrifuged (1000 rpm) for 3 min. The cells were then resuspended into 200 μl of PBS, and their corresponding fluorescence intensity was measured by flow cytometry (n = 3).
Next, J774A.1 cells were inoculated into confocal dishes overnight to evaluate the intracellular transport pathway of CM@MS NPs. After treatment of CM@MS NPs for different time points, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) and a lysosome tracer and visualized using confocal microscopy.
Cell culture and cell cytotoxicity evaluation
Murine J774A.1 macrophages were planted in the DMEM medium containing 10% fetal calf serum at 37°C humidified incubator with 5% CO2. To evaluate the cell viability of MLs and CM@MS NPs, J774A.1 cells were first cultured into 96-well plates for 24 hours to adhere. Then, the cells were treated with varied concentrations of MLs and CM@MS NPs. After 24 h co-incubation, the CCK-8 assay was performed to evaluate the cell activity. To induce macrophage pyroptosis, J774A.1 cells were primed with LPS (100 ng ml−1) for 4 hours, followed by 10 mΜ nigericin treatment for 1 hour.
FluoZin-3 and LysoTracker imaging
J774A.1 cells were cultured in six-well plates (106 per well) overnight, which was followed by varied formulations, including control, MS (Si concentration of 100 ppm), C@MS (Si concentration of 100 ppm), and CM@MS (Si concentration of 100 ppm). Then, the treated cells were stained with FluoZin-3–AM (3 μM) and LysoTracker red (0.5 μM) to monitor the cellular Zn2+ for 30 min before image collection. Last, fluorescence signals of Zn2+ were detected by CLSM.
ROS-scavenging ability of CM@MS NPs in vitro
Antioxidative stress capacity of CM@MS NPs was assessed by measuring intracellular total ROS, •OH, and , respectively. To monitor the intracellular total ROS, J774A.1 cells were cultured in CLSM dishes (105 cells per dish) overnight. Subsequently, the cells were stimulated with LPS (100 ng ml−1), in combination with varied treatments. The control group was treated with fresh medium alone, while the model group was stimulated with LPS (100 ng ml−1). After 8 hours of incubation, the cells were washed and stained with DCFH-DA (10 μM) and DAPI in free DMEM for 20 min. Last, fluorescence signals were detected by CLSM. Through the similar procedures, the intracellular •OH and was stained with APF (10 μM, 30 min) and DHE (10 μM, 30 min), respectively, which were observed by CLSM.
Enzyme-linked immunosorbent assay
J774A.1 cells were seeded into 24-well plates overnight followed by varied treatments, including control, LPS (100 ng ml−1), MS (Si concentration of 100 ppm) + LPS (100 ng ml−1), C@MS (Si concentration of 100 ppm) + LPS (100 ng ml−1), and CM@MS (Si concentration of 100 ppm) + LPS (100 ng ml−1). The typical inflammatory cytokines were then conducted by ELISA kits.
Evaluation on the changes of mitochondrial membrane potential
J774A.1 cells were seeded on a specific confocal dish (1× 105 cells per dish) overnight followed by varied treatments, including control, LPS (100 ng ml−1), MS (Si concentration of 100 ppm) + LPS (100 ng ml−1), C@MS (Si concentration of 100 ppm) + LPS (100 ng ml−1), and CM@MS (Si concentration of 100 ppm) + LPS (100 ng ml−1). After 12-hour incubation, the treated cell mitochondrial were stained with JC-1 dye and visualized under CLSM.
Inhibition of internalization of ox-LDL enabled by CM@MS NPs
After stimulation with LPS (100 ng ml−1) for 12 hours, J774A.1 cells were treated with varied formulations, including control, MS (Si concentration of 100 ppm), C@MS (Si concentration of 100 ppm), and CM@MS (Si concentration of 100 ppm). The control group was treated with fresh medium alone. After 8 hours, the treated cells were incubated with DiI-oxLDL (40 μg ml−1) for 4 hours. Afterward, the treated cells were washed with (HCl 0.5 M) in 70% ethanol, which were followed by stained with DIO (10 μM) and DAPI, and observed under CLSM.
Effect of CM@MS treatment on foam cell formation
After stimulation with LPS (100 ng ml−1) for 12 hours, J774A.1 cells were treated with varied formulations [PBS, MS (Si concentration of 100 ppm), C@MS (Si concentration of 100 ppm), and CM@MS (Si concentration of 100 ppm)]. The control group was treated with fresh medium alone. After that, the treated cells were incubated with oxLDL at 50 μg ml−1 for 12 hours. After being washed with HCl (0.5 M) in 70% ethanol, these cells were fixed with 4% neutral-buffered formalin, followed by stained with 0.3% ORO and hematoxylin. Last, the treated cells were observed though optical microscopy.
In vitro evaluation of antipyroptosis ability
J774A.1 cells were cultured in six-well plates (106 per well) overnight. Subsequently, cells were incubated with varied formulations, including control, model (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour), MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in MS NPs: 100 ppm), C@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in C@MS NPs: 100 ppm), and CM@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in CM@MS NPs: 100 ppm). After that, cells were collected and stained with annexin V–mCherry/SYTOX Green apoptosis detection kit (Beyotime, China) and analyzed by flow cytometer.
Cell membrane integrity evaluation
J774A.1 cells were seeded in six-well plates overnight followed by varied formulations, including control, model (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour), MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in MS NPs: 100 ppm), C@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in C@MS NPs: 100 ppm), and CM@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in CM@MS NPs: 100 ppm). Afterward, cell membrane integrity was detected by PlasMem Bright Red based on the relevant manufacturers’ instructions. Last, the cells were rinsed by PBS three times and monitored under CLSM.
AO staining
J774A.1 macrophages were cultivated with fresh mediums containing varied formulations including control, MS (Si concentration in MS NPs: 100 ppm), C@MS (Si concentration in C@MS NPs: 100 ppm), and CM@MS (Si concentration in CM@MS NPs: 100 ppm) for 24 hours. Then, the acidic vesicular organelles of cells were observed by CLSM after stained with AO (1 μg ml−1) solution for 20 min.
Western blot experiments
The J774A.1 cells were planted in six-well plates followed by giving varied treatments, including control, model (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour), MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in MS NPs: 100 ppm), C@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in C@MS NPs: 100 ppm), and CM@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in CM@MS NPs: 100 ppm). After that, the treated cells were collected followed by the addition of lysis buffer (Epizyme Biotech, PC101) supplemented with protease inhibitor cocktail (Sigma-Aldrich, 200-664-3, P8340). Then, unwanted lysates were removed, and the collected proteins were separated by electrophoresis. Afterward, the tested samples were transferred to polyvinylidene difluoride B membrane, which was then blocked with 5% skim milk for 1 hour. Subsequently, the samples were incubated with varied primary antibodies against GSDMD (Abcam, ab219800), caspase-1 (Proteintech, catalog no. 22915-1-AP), and β-actin (Servicebio, GB15003-100) overnight. Then, the membranes were washed with Tris-buffered saline with Tween 20 (TBST) for several times followed by incubated with horseradish peroxidase–labeled secondary antibodies for 1 hour. Last, the membranes were added by enhanced chemiluminesence reagent and overserved by infrared scanner.
Immunostaining
Immunofluorescence imaging was performed for evaluating the anti-inflammatory ability of CM@MS NPs. J774A.1 cells were seeded into a 12-well plate overnight to adhere, which were followed by varied treatments, including control, model (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour), MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in MS NPs: 100 ppm), C@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in C@MS NPs: 100 ppm), and CM@MS (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in CM@MS NPs: 100 ppm). Then, the treated cells were washed with PBS three times and fixed with 4% paraformaldehyde (Biosharp, BL539A) for another 30 min. Subsequently, the tested cells were treated by a membrane breaking solution (1% Triton). Thirty minute after treatment, the cells were washed with PBS (Gibco, 10010031) three times and incubated with diluted primary antibody for 12 hours. Then, the cells were washed with PBS three times again, followed by incubation of diluted fluorescent dye-linked secondary antibody for another 1 hour. Last, the treated cells were washed with PBS three times again and sealed with antifluorescence quenching agent and observed by a fluorescence microscope.
RNA sequencing
J774A.1 cells treated with model group (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour) and CM@MS NPs group (LPS: 100 ng ml−1, 4 hours; nigericin: 10 μM, 1 hour; Si concentration in CM@MS NPs: 100 ppm) were collected for RNA extraction using TRIzol regent (Invitrogen, CA, USA) based on the manufacturer’s instructions. RNA quality control was performed by the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The samples with RNA integrity number ≥ 7 were used for the following sequencing. VAHTS Universal V6 RNA-seq Library Prep Kit (Vazyme, Nanjing, China) was used for libraries construction. Afterward, the libraries were sequenced on the Illumina sequencing platform (HiSeqTM 2500) and 125/150–base pair paired-end reads were generated. The RNA libraries were sequenced by OE Biotech Inc., Shanghai, China.
PA imaging performance
For in vitro PA imaging, CM@MS NPs at varied concentrations from 0.125 to 2.0 mg ml−1 dispersed in aqueous solution were performed for PA signals detection, which was obtained using 808-nm excitation light. For imaging parameters, the region of interest was 20 mm, PA gain is 43 dB, 2D gain is 40 dB, wavelength is 808 nm, scanning time is 5 min, pulse repetition frequency is 40 MHz, and safe energy is 250 V.
For in vivo PA imaging, ApoE−/− mice was used as animal model, which were first fed a high-fat diet for 3 months. After plaque lesions built up, these mice were intravenously injected with CM@MS NPs at 5 mg kg−1. PA images of the tested ApoE−/− mice were collected before and after CM@MS treatments. For imaging parameters, the region of interest is 20 mm, PA gain is 43 dB, 2D gain is 40 dB, and wavelength is 808 nm. Both in vitro and in vivo PA imaging experiments were carried out by using a Fujifilm VisualSonics/VEVO LAZR-X PA Imaging System.
Animals
All animal experimental protocols were in agreement with instructions of the Regional Ethics Committee for Animal Experiments and the care regulations approved by Animal Care Ethics Commission of Laboratory Animals of Tongji University (TJHBLAC-2021-012). Female Kunming mice (4 to 5 weeks) and male apolipoprotein E–deficient (ApoE−/−) mice (4 to 5 weeks old) were purchased from the GemPharmatech Co. Ltd. (Nanjing, China). ApoE−/− mice were fed a high-cholesterol diet throughout the entire animal experiment after 7 days of acclimatization.
In vivo biosafe evaluation
Twenty Kunming mice were randomly assigned to four groups (n = 5 mice per group) [control, group and CM@MS NP-treated groups at three elevated doses (7.5, 15, and 30 mg kg−1)], whereas the mice in control group were treated with PBS. Body weight was measured every day for 30 days. Major organ tissues and blood samples were collected at 30 days of injection. Major organ tissues were fixed in 4% paraformaldehyde, followed by embedded in paraffin and sectioned into slices before being stained with H&E.
In vivo pharmacokinetic study of CM@MS NPs
Pharmacokinetic profile of CM@MS NPs was tested in mice after intravenous injection. CM@MS NPs was treated to Kunming mice at 20 mg kg−1, while mice in the control group were administered with the same volume of saline. Blood (15 μl) was collected at different time points for quantitatively analyzing Si content by ICP-MS test. A double-component pharmacokinetic model was conducted to calculate the pharmacokinetics of CM@MS NPs in blood.
In vivo AS treatment by CM@MS NPs
Forty ApoE−/− mice were fed a high-fat diet for 3 months. One month after feeding, they were randomly separated to four groups (n = 10), and treatment with different formulations was performed for an additional 2 months maintaining high-fat feeding. Mice in the model group were administrated with saline, while mice in the other groups were treated with MS NPs, C@MS NPs, and CM@MS NPs at the same Si concentration of 1.4 mg kg−1 (equivalent CM@MS NPs concentration of 5 mg kg−1), where CM@MS NPs contained a dose of MLs and C NDs equivalent to 0.7 and 0.8 mg kg−1, respectively. All formulations were treated twice per week.
ORO staining
After different treatments, the aortic diseased areas were excised and collected followed by stained with ORO. Frozen sections of the aortic root, aortic arch, and brachiocephalic artery were prepared and stained with ORO. The plaque areas were measured using ImageJ software.
Histology and immunohistochemistry
After fixation with 4% paraformaldehyde, aortic sinus sections (4 to 6 μm thick) were stained with H&E and Masson’s trichrome. The brachiocephalic artery tissues were evaluated, followed by fluorescent dye DHE staining to observe the in situ ROS generation. For the immunohistochemical analysis, the aortic tissue sections were incubated with antibodies against THF-α, GSDMD, IL-1β, α-SMA, CD68, and MMP-9 to detect the expression of inflammation/pyroptosis-related proteins and to evaluate the vascular smooth muscle cells, macrophage, and MMP-9 expression, respectively, and iNOS for M1 cells, CD206 for M2 cells, CD4 for CD4+ cells, and Foxp3 for Treg cells. ImageJ software was used for the semiquantitative analysis of images.
Phenotypic analysis via flow cytometry
Cells were collected as described above followed by incubated with allophycocyanin (APC)–Cy7 Rat Anti-Mouse CD45 (BD Pharmingen, catalog no. 557659), FITC Hamster Anti-Mouse CD3e (BD Pharmingen, Cat# 553061), BV650 Rat Anti-Mouse CD4 (BD Pharmingen, catalog no. 563232), BV786 Rat Anti-Mouse CD8a (BD Pharmingen, catalog no. 563332), phycoerythrin (PE) Rat Anti-Mouse CD44 (BD Pharmingen, catalog no. 553134), APC Rat Anti-Mouse CD62L (BD Pharmingen, catalog no. 553152), BV421 Rat Anti-Mouse F4/80 (BD Pharmingen, catalog no. 565411), PE-Cy7 Rat Anti-CD11b (BD Pharmingen, catalog no. 552850), PE Rat Anti-Mouse CD86 (BD Pharmingen, catalog no. 553692), Alexa Fluor 647 Rat Anti-Mouse CD206 (BD Pharmingen, catalog no. 565250), PE Rat Anti-Mouse CD25 (BD Pharmingen, catalog no. 553075), and BV421 Rat Anti-Mouse Foxp3 (BD Pharmingen, catalog no. 562996) using appropriate isotypes at 4°C for 30 min. The stained cells were then analyzed though flow cytometry analysis.
ScRNA-seq and analysis
Initially, the plaque tissues were collected from mice in both model group and MLs group in ice-cold PBS followed by enzymatic digestion to prepare a single-cell suspension. After that, scRNA-seq was carried out by e Chromium Next GEM Single Cell 3′ Library and Gel Beads Kit v3.1 (PN-1000121) according to the manufacturer’s instructions. Then, the Chromium Single Cell Controller was used to obtain a capture of 1 × 105 cells per sample, and cDNA was subsequently synthesized and amplified. Based on the 10X Genomics protocol, libraries were built up, which were further quantified using Qubit and quality assessed by MobiVision software pipeline (version 1.1, MobiDrop). Sequencing was conducted on an Illumina NovaSeq 6000 PE150 System. Then, the sequencing data were performed using the 10X Genomics Cell Ranger software suite for further analysis.
Statistical analysis
All the experimental data in this work were measured at least three times and recorded as means ± SD. Unpaired two-tailed Student’s t tests and one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons were used in this study depending on the data type. The details can be found in the figure legends including test used, sample size (n), and probability (P) value. P < 0.05 was considered to be statistically significant. All the statistical analysis was conducted by GraphPad Prism 7.0 (GraphPad Software, CA, USA). Plots and charts were performed using GraphPad Prism 7.0 and Origin 2021 software (OriginLab, MA, USA).
Acknowledgments
We acknowledge the financial support from the National Key Research and Development Projects (grant no. 2023YFC2306500), the National Natural Science Foundation of China (grant nos. 82371988 and 82171950), the Important Weak Subject Construction Project of Shanghai Pudong New Area Health Commission (grant no. PWZbr2022-12), and the Shanghai Municipal Commission of Health (grant no. 202140250).
Author contributions: Conceptualization: R.H., C.D., B.Z., and Y.C. Methodology: W.F., J.Q., R.H., X.S., C.D., and Y.C. Investigation: W.F., R.H., X.S., H.H., C.D., and Y.C. Visualization: W.F., R.H., C.D., and Y.C. Project administration: R.H., Y.C., C.D., and B.Z. Supervision: R.H., Y.C., C.D., and B.Z. Writing—original draft: R.H., C.D., and Y.C. Writing—review and editing: C.D., Y.C., and B.Z. Resources: R.H., Y.C., H.H., C.D., and B.Z. Data curation: R.H., C.D., and Y.C., Validation: R.H., Y.C., H.H., C.D., and B.Z. Formal analysis: R.H., H.H., C.D., and Y.C. Software: R.H. and X.S. Funding acquisition: C.D., B.Z., and Y.C.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Table S1
Figs. S1 to S26
REFERENCES AND NOTES
- 1.Libby P., The changing landscape of atherosclerosis. Nature 592, 524–533 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Björkegren J. L. M., Lusis A. J., Atherosclerosis: Recent developments. Cell 185, 1630–1645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koelwyn G. J., Corr E. M., Erbay E., Moore K. J., Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 19, 526–537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabas I., Bornfeldt K. E., Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 118, 653–667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinet W., Schrijvers D. M., De Meyer G. R. Y., Necrotic cell death in atherosclerosis. Basic Res. Cardiol. 106, 749–760 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F., Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M., NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 118, 372–385 (2022). [DOI] [PubMed] [Google Scholar]
- 8.He X., Fan X., Bai B., Lu N., Zhang S., Zhang L., Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol. Res. 165, 105447 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Sharma B. R., Kanneganti T. D., NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22, 550–559 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zu Z., Sheng J., Qi J., Miao Y., Zhang Y., Zheng T., Xiang K., Wu H., Lu G., Zhang L., Natural cell patches: melanin nanoparticles for MR imaging-guided antiatherosclerosis therapy via attenuating macrophage pyroptosis. Adv. Funct. Mater. 33, 2212748 (2023). [Google Scholar]
- 11.Xu L., Yang X., Ding S., Qian J., Ge J., Histamine promotes atherosclerotic plaque instability via STAT6-dependent macrophage pyroptosis. Eur. Heart J. 40, 318 (2019). [Google Scholar]
- 12.De Meyer G. R. Y., Zurek M., Puylaert P., Martinet W., Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat. Rev. Cardiol. 21, 312–325 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Xu S., Ge Y., Wang X., Yin W., Zhu X., Wang J., Qiao S., Circ-USP9X interacts with EIF4A3 to promote endothelial cell pyroptosis by regulating GSDMD stability in atherosclerosis. Clin. Exp. Hypertens. 45, 2186319 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Handing K. B., Niedzialkowska E., Shabalin I. G., Kuhn M. L., Zheng H., Minor W., Characterizing metal-binding sites in proteins with X-ray crystallography. Nat. Protoc. 13, 1062–1090 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Que E. L., Domaille D. W., Chang C. J., Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Pradeu T., Vivier E., The discontinuity theory of immunity. Sci. Immunol. 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C., Zhang R., Wei X., Lv M., Jiang Z., Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 145, 187–241 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Prasad A. S., Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Bio. 28, 364–371 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Hughes S., Samman S., The effect of zinc supplementation in humans on plasma lipids, antioxidant status and thrombogenesis. J. Am. Coll. Nutr. 25, 285–291 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M., Weitzmann M. N., Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. 355, 179–186 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Lv C. X., Kang W. Y., Liu S., Yang P. S., Nishina Y., Ge S. H., Bianco A., Ma B. J., Growth of ZIF-8 nanoparticles on graphene oxide nanosheets: A multifunctional nanoplatform for combined ion-interference and photothermal therapy. ACS Nano 16, 11428–11443 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Li J., Liu X., Tan L., Cui Z., Yang X., Liang Y., Li Z., Zhu S., Zheng Y., Yeung K. W. K., Wang X., Wu S., Zinc-doped prussian blue enhances photothermal clearance of staphylococcus aureus and promotes tissue repair in infected wounds. Nat. Commun. 10, 4490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutvei A. P., Nagiec M. J., Blenis J., Balancing lysosome abundance in health and disease. Nat. Cell Biol. 25, 1254–1264 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Berry J. P., Zhang L., Galle P., Ansoborlo E., Henge-Napoli M. H., Donnadieu-Claraz M., Role of alveolar macrophage lysosomes in metal detoxification. Microsc. Res. Tech. 36, 313–323 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Haase H., Beyersmann D., Uptake and intracellular distribution of labile and total Zn(II) in C6 rat glioma cells investigated with fluorescent probes and atomic absorption. Biometals 12, 247–254 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Cuajungco M. P., Basilio L. C., Silva J., Hart T., Tringali J., Chen C.-C., Biel M., Grimm C., Cellular zinc levels are modulated by TRPML1-TMEM163 interaction. Traffic 15, 1247–1265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong X.-P., Cheng X., Mills E., Delling M., Wang F., Kurz T., Xu H., The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, 992–996 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minckley T. F., Zhang C., Fudge D. H., Dischler A. M., LeJeune K. D., Xu H., Qin Y., Sub-nanomolar sensitive GZnP3 reveals TRPML1-mediated neuronal Zn2+ signals. Nat. Commun. 10, 4806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmiege P., Fine M., Blobel G., Li X. C., Human TRPML1 channel structures in open and closed conformations. Nature 550, 366–370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmiege P., Fine M., Li X. C., Atomic insights into ML-SI3 mediated human TRPML1 inhibition. Structure 29, 1295–1302.e3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong D., Wang R., Zhang H., Wang M., Zhang X., Chen H., Induction of lysosomal exocytosis and biogenesis via TRPML1 activation for the treatment of uranium-induced nephrotoxicity. Nat. Commun. 14, 3997 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu R., Dai C., Dong C., Huang H., Ding L., Chen Y., Zhang B., Living macrophage-delivered tetrapod PdH nanoenzyme for targeted atherosclerosis management by ROS scavenging, hydrogen anti-inflammation, and autophagy activation. ACS Nano 16, 15959–15976 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Chan C. K. W., Zhang L., Cheng C. K., Yang H., Huang Y., Tian X. Y., Choi C. H. J., Recent advances in managing atherosclerosis via nanomedicine. Small 14, 1702793 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Cathcart M. K., Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages. Arterioscler. Thromb. Vasc. Biol. 24, 23–28 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Sen S., Won M., Levine M. S., Noh Y., Sedgwick A. C., Kim J. S., Sessler J. L., Arambula J. F., Metal-based anticancer agents as immunogenic cell death inducers: the past, present, and future. Chem. Soc. Rev. 51, 1212–1233 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J., Wang X., Feng Z., Chen Y., Wen D., Liu Z., The protective effect of isoflurane pretreatment on liver IRI by suppressing noncanonical pyroptosis of liver macrophages. Int. Immunopharmacol. 99, 107977 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Zhang C., Yang B., Biazik J. M., Webster R. F., Xie W., Tang J., Allioux F.-M., Abbasi R., Mousavi M., Goldys E. M., Kilian K. A., Chandrawati R., Esrafilzadeh D., Kalantar-Zadeh K., Gallium nanodroplets are anti-inflammatory without interfering with iron homeostasis. ACS Nano 16, 8891–8903 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Sheline C. T., Behrens M. M., Choi D. W., Zinc-induced cortical neuronal death: Contribution of energy failure attributable to loss of NAD+ and inhibition of glycolysis. J. Neurosci. 20, 3139–3146 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao J., Li L., Yao Y., Xing Y., Ma H., Dehydroepiandrosterone exacerbates nigericin-induced abnormal autophagy and pyroptosis via GPER activation in LPS-primed macrophages. Cell Death Dis. 13, 372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong C., Dai X., Wang X., Lu Q., Chen L., Song X., Ding L., Huang H., Feng W., Chen Y., Chang M., A calcium fluoride nanozyme for ultrasound-amplified and Ca2+–overload-enhanced catalytic tumor nanotherapy. Adv. Mater. 34, e2205680 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Lei G., Zhuang L., Gan B. Y., Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Y., Zhang X., Yang L., Wang J., Hu Y., Bian A., Liu J., Ma J., Zinc inhibits high glucose-induced NLRP3 inflammasome activation in human peritoneal mesothelial cells. Mol. Med. Rep. 16, 5195–5202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai Q., Yu S., Zhong Y., Lu Z., Qiu C., Yu Y., Zhang X., Zhang Y., Lei Z., Qiang L., Li B., Pang Y., Qiu X., Wang J., Liu C., A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 378, eabq0132 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Seijkens T. T. P., van Tiel C. M., Kusters P. J. H., Atzler D., Soehnlein O., Zarzycka B., Aarts S. A. B. M., Lameijer M., Gijbels M. J., Beckers L., den Toom M., Slütter B., Kuiper J., Duchene J., Aslani M., Megens R. T. A., van’t Veer C., Kooij G., Schrijver R., Hoeksema M. A., Boon L., Fay F., Tang J., Baxter S., Jongejan A., Moerland P. D., Vriend G., Bleijlevens B., Fisher E. A., Duivenvoorden R., Gerdes N., de Winther M. P. J., Nicolaes G. A., Mulder W. J. M., Weber C., Lutgens E., Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J. Am. Coll. Cardiol. 71, 527–542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bazioti V., Wichapong K., Lacy M., Nitz K., Van Tiel C. M., Weber C., Atzler D., Lutgens E., Atheroprotective CD40-TRAF6 inhibitors: Translational pipeline towards a clinical application. Eur. Heart J. 44, ehad655.3299 (2023). [Google Scholar]
- 46.Ketelhuth D. F. J., Hansson G. K., Adaptive response of T and B cells in atherosclerosis. Circ. Res. 118, 668–678 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Cochain C., Koch M., Chaudhari S. M., Busch M., Pelisek J., Boon L., Zernecke A., CD8+ T cells regulate monopoiesis and circulating Ly6C monocyte levels in atherosclerosis in mice. Circ. Res. 117, 244–253 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Zhou L., Chong M. M. W., Littman D. R., Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009). [DOI] [PubMed] [Google Scholar]
- 49.He Z., Chen W., Hu K., Luo Y., Zeng W., He X., Li T., Ouyang J., Li Y., Xie L., Zhang Y., Xu Q., Yang S., Guo M., Zou W., Li Y., Huang L., Chen L., Zhang X., Saiding Q., Wang R., Zhang M., Kong N., Xie T., Song X., Tao W., Resolvin D1 delivery to lesional macrophages using antioxidative black phosphorus nanosheets for atherosclerosis treatment. Nat. Nanotechnol. 19, 1386–1398 (2024). [DOI] [PubMed] [Google Scholar]
- 50.Chen W., Schilperoort M., Cao Y., Shi J., Tabas I., Tao W., Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 19, 228–249 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Z. M., Chen W., Cao Y., Abdi R., Tao W., Nanomedicine-based strategies for the treatment of vein graft disease. Nat. Rev. Cardiol. 22, 255–272 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beldman T. J., Malinova T. S., Desclos E., Grootemaat A. E., Misiak A. L. S., van der Velden S., van Roomen C. P. A. A., Beckers L., van Veen H. A., Krawczyk P. M., Hoebe R. A., Sluimer J. C., Neele A. E., de Winther M. P. J., van der Wel N. N., Lutgens E., Mulder W. J. M., Huveneers S., Kluza E., Nanoparticle-aided characterization of arterial endothelial architecture during atherosclerosis progression and metabolic therapy. ACS Nano 13, 13759–13774 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu M., Meng Q., Chen Y., Du Y., Zhang L., Li Y., Zhang L., Shi J., Large-pore ultrasmall mesoporous organosilica nanoparticles: Micelle/precursor co-templating assembly and nuclear-targeted gene delivery. Adv. Mater. 27, 215–222 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Lin L., Wang L. V., The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 19, 365–384 (2022). [DOI] [PubMed] [Google Scholar]
- 55.Gao W., He J., Chen L., Meng X., Ma Y., Cheng L., Tu K., Gao X., Liu C., Zhang M., Fan K., Pang D.-W., Yan X., Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 14, 160 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang X. G., Liu C., Kong N., Xiao Y. F., Yurdagul A. Jr., Tabas I., Tao W., Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. 17, 748–780 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao W., Yurdagul A., Kong N., Li W. L., Wang X. B., Doran A., Feng C., Wang J. Q., Islam M. A., Farokhzad O., Tabas I., Shi J. J., siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 12, eaay1063 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Li L., Zhao W., Dou Y., An H., Tao H., Xu X., Jia Y., Lu S., Zhang J., Hu H., Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano 12, 8943–8960 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Huang P., Dong R., Wang P., Xu M., Sun X., Dong X., MCOLN/TRPML channels in the regulation of MTORC1 and autophagy. Autophagy 20, 1203–1204 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laval T., Ouimet M., A role for lipophagy in atherosclerosis. Nat. Rev. Cardiol. 20, 431–432 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nanda S. S., Kim D., Yang H., An S. S. A., Yi D. K., Synergistic effect of SiO2 and Fe3O4 nanoparticles in autophagy modulation. Nanomaterials 14, 1033 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandez D. M., Rahman A. H., Fernandez N. F., Chudnovskiy A., Amir E. D., Amadori L., Khan N. S., Wong C. K., Shamailova R., Hill C. A., Wang Z. C., Remark R., Li J. R., Pina C., Faries C., Awad A. J., Moss N., Bjorkegren J. L. M., Kim-Schulze S., Gnjatic S., Ma’ayan A., Mocco J., Faries P., Merad M., Giannarelli C., Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gewaltig J., Kummer M., Koella C., Cathomas G., Biedermann B. C., Requirements for CD8 T-cell migration into the human arterial wall. Hum. Pathol. 39, 1756–1762 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Ma X., Hao J., Wu J., Li Y., Cai X., Zheng Y., Prussian blue nanozyme as a pyroptosis inhibitor alleviates neurodegeneration. Adv. Mater. 34, e2106723 (2022). [DOI] [PubMed] [Google Scholar]
- 65.Marchetti C., Swartzwelter B., Gamboni F., Neff C. P., Richter K., Azam T., Carta S., Tengesdal I., Nemkov T., D’Alessandro A., Henry C., Jones G. S., Goodrich S. A., Laurent J. P. S., Jones T. M., Scribner C. L., Barrow R. B., Altman R. D., Skouras D. B., Gattorno M., Grau V., Janciauskiene S., Rubartelli A., Joosten L. A. B., Dinarello C. A., OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. U.S.A. 115, E1530–E1539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo X., Weng X., Bao X., Bai X., Lv Y., Zhang S., Chen Y., Zhao C., Zeng M., Huang J., Xu B., Johnson T. W., White S. J., Li J., Jia H., Yu B., A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 57, 102511 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye M., Zhao Y., Wang Y., Xie R., Tong Y., Sauer J.-D., Gong S., NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat. Nanotechnol. 17, 880–890 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Niu X., Xu H., Li Q., Meng L., He M., Zhang J., Zhang Z., Zhang Z., VX-765 attenuates atherosclerosis in ApoE deficient mice by modulating VSMCs pyroptosis. Exp. Cell Res. 389, 111847 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Zhao N., Zhuo X., Lu Y., Dong Y., Ahmed M. E., Tucker D., Scott E. L., Zhang Q., Intranasal delivery of a caspase-1 inhibitor in the treatment of global cerebral ischemia. Mol. Neurobiol. 54, 4936–4952 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu J. J., Liu X., Xia S., Zhang Z., Zhang Y., Zhao J., Ruan J., Luo X., Lou X., Bai Y., Wang J., Hollingsworth L. R., Magupalli V. G., Zhao L., Luo H. R., Kim J., Lieberman J., Wu H., FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rathkey J. K., Zhao J. J., Liu Z. H., Chen Y. H., Yang J., Kondolf H. C., Benson B. L., Chirieleison S. M., Huang A. Y., Dubyak G. R., Xiao T. S., Li X. X., Abbott D. W., Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 3, eaat2738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng Y. Q., Ostermann E., Brune W., A cytomegalovirus inflammasome inhibitor reduces proinflammatory cytokine release and pyroptosis. Nat. Commun. 15, 786 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei Z., Pinfang K., Jing Z., Zhuoya Y., Shaohuan Q., Chao S., Trentini, curcumin improves diabetic cardiomyopathy by inhibiting pyroptosis through AKT/Nrf2/ARE pathway. Mediators Inflamm. 2023, 3906043 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figs. S1 to S26