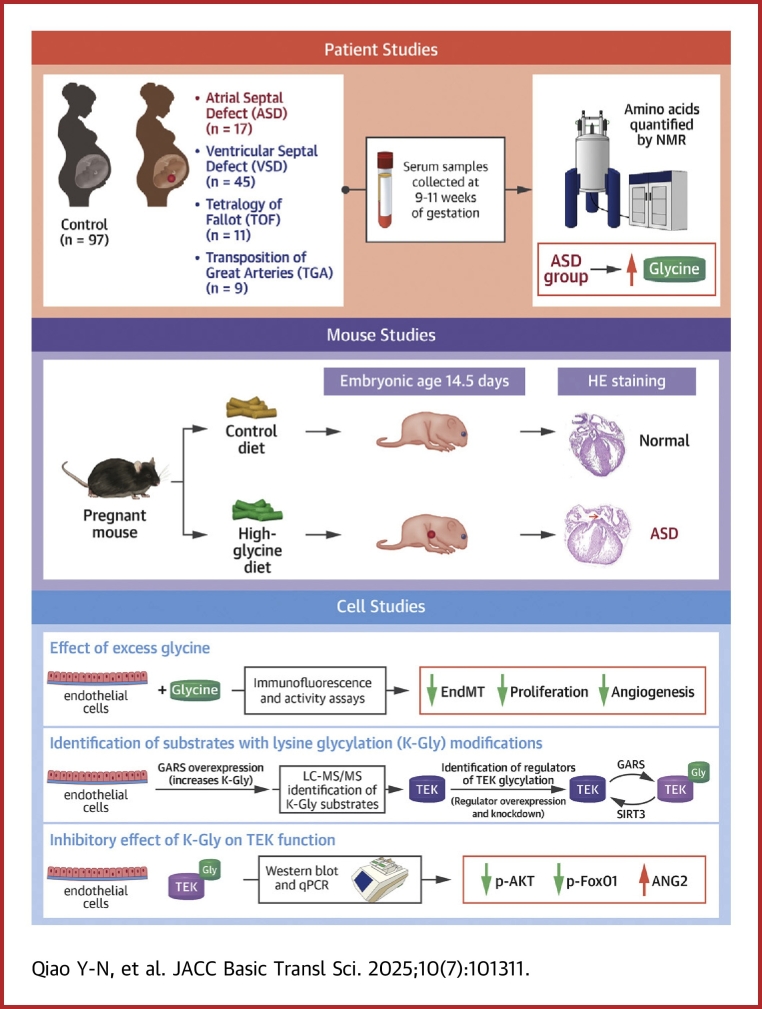
Visual Abstract
Key Words: atrial septal defects, glycine, maternal single-nucleotide variation, pregnancy serum, TEK
Highlights
-
•
Women carrying rs2545801 C/C genotypes exhibit increased glycine levels and increase risk for ASDs in their offspring.
-
•
Feeding pregnant mice with high-glycine-chow increases ASD risk in their offspring.
-
•
High glycine levels increase lysine glycylation of K688 site within TEK receptor tyrosine kinase, suppress TEK membrane localization and PI3K-AKT/FOXO1 signaling in endothelial cells.
-
•
Glycine inhibits proliferation, angiogenesis, and EndMT process of endothelial cells, which may lead to abnormal atrial septum development.
-
•
SIRT3 catalyzes de-glycylation of TEK.
Summary
Amino acid imbalance is linked to increased congenital heart disease risk. Here, we found women carrying rs2545801 C/C genotypes exhibited increased glycine levels and increased risk for atrial septal defects (ASDs) in their offspring. Elevated maternal glycine levels during the first trimester were correlated with a higher ASD risk in the offspring. Additionally, feeding pregnant mice with high-glycine chow increased ASD risk in their offspring. Mechanistically, elevated maternal glycine led to increased lysine-glycylation of lysine-688 within the TEK receptor tyrosine kinase and inhibited TEK-PI3K-AKT/FOXO1 signaling in cardiac endothelial cells. These findings indicate that lysine-glycylation exerts teratogenic effects and may be a target for ASD intervention.
Congenital heart disease (CHD) is the most common type of birth defect and most common cause of infant morbidity and mortality arising from birth defects.1, 2, 3 Normal embryonic development is an intricately choreographed process vulnerable to perturbation by environmental factors such as an imbalance in metabolism or exposure to toxic substances,4,5 and various maternal metabolic imbalances are known to increase CHD risk in the offspring. The relationship between maternal metabolism and embryonic heart development is complex and multifaceted, because maternal metabolism plays a crucial role in providing the necessary nutrients and energy for the developing embryo, including the heart. During pregnancy, the metabolism of the mother undergoes various changes to support fetal growth. The mother's diet and nutrient intake directly affect the availability of key nutrients required for fetal development, including the formation and functioning of the embryonic heart.
Amino acids in the maternal circulatory system provide an important source of nutrition and a developmental environment for the fetus. Abnormalities in amino acid levels during pregnancy can lead to abnormal heart development, such as the accumulation of phenylalanine and decrease in tyrosine caused by phenylketonuria,6,7 as well as maternal folate deficiency induced hyperhomocysteine,8,9 which can increase the risk of congenital heart disease in the offspring. We previously discovered that high leucine levels during pregnancy also increase CHD risk in the offspring, and in both human populations and animal models, the phenotype caused by high leucine levels is primarily a ventricular septal defect.10 In a nonbiased proteomics study assessing pregnant early gestation plasma, we found that a series of changes in amino acid metabolic enzyme expression were associated with CHD risk in the offspring.11 These findings suggest that abnormal maternal amino acid metabolism plays an important role in the development of CHD.
However, the underlying cause of metabolic imbalance leading to CHD remains unclear. Although information about the metabolic status of mothers can be obtained during early pregnancy, tracing this information before conception is challenging because of the uncertainty of pregnancy and variability of maternal metabolism. Nonetheless, nutritional and genetic factors are considered to jointly determine metabolite levels in pregnant women. For example, in addition to nutritional imbalance such as insufficient folate intake leading to low folate and high homocysteine (Hcy) levels, our previous studies suggested that genetic alterations are important factors for changes in folate and Hcy levels in the human body.12, 13, 14, 15 Moreover, the genetic alterations regulating folate and Hcy concentrations are significantly associated with CHD risk in the offspring. Consequently, genetic alterations may possibly serve as markers of metabolic status and can be used to analyze disease risk. In recent years, multiple genome-wide association studies have identified genetic polymorphic loci that are significantly associated with the concentrations of 20 common amino acids in the human body.16, 17, 18 These loci provide a valuable tool for studying the relationship between maternal amino acid metabolism and CHD risk in the offspring.
CHD phenotypes are diverse and encompass several major categories. In our previous clinical sample analysis, we found a strong correlation between metabolic imbalance during pregnancy and the risk of developing mild CHD. Simultaneously, in animal model studies, we observed that, compared with genetic editing-induced severe and complex phenotypes, metabolic imbalances during maternal pregnancy primarily led to mild phenotypes, such as septal defects.19, 20, 21 Taken together, these findings indicate a strong association between nutritional imbalance during pregnancy and moderate-to-mild CHD. Cardiac development is a complex process, and different defect types are closely related to cell lineages and developmental stages.22,23 Although genetic studies have demonstrated distinct genetic mutations in different CHD types,23 the question on whether imbalances in different metabolic types could lead to the appearance of different CHD phenotypes remains to be answered.
In this study, we focused on atrial septal defects (ASDs), which are one of the most common CHD types. We initially examined 50 genetic variants associated with common plasma amino acid concentrations in 545 mothers of children with ASD and 697 mothers of healthy children. We found a positive correlation between the variant rs2545801 of the glycine concentration-related variant, elevated plasma glycine levels, and increased risk of ASD in the offspring. Next, we compared amino acid concentrations in serum samples during early pregnancy between individuals with ASD and healthy offspring and discovered that high levels of glycine were associated with an elevated risk of ASD in the offspring. Our animal model further confirmed the teratogenic effect of glycine on ASD. Finally, our mechanistic studies revealed that high glycine levels inhibit the Tek signaling pathway in the cardiac endothelial cells of the offspring, thereby increasing the risk of ASDs.
Methods
A detailed Methods section, including establishment of high glycine-fed pregnant mouse model, mouse embryo heart isolation and histological analysis, cell culture, plasma construction, immunofluorescence staining, RNA isolation and reverse transcription-quantitative real-time PCR, Western blot, immunoprecipitation, LC-MS/MS analysis, lysine glycylation (K-Gly) modification site identification, nuclear magnetic resonance, and data processing, can be found in the Supplemental Methods. The DNA sequences of all primers and siRNAs used are listed in Supplemental Table 1.
Study participants and ethics
Single-nucleotide variations (SNVs) of mothers were analyzed. The design and conduct of the study was approved and supervised by the Ethics Committee of the Children’s Hospital of Fudan University through Ethic Vote [2021]93, in accordance with the criteria established by the Declaration of Helsinki. Written informed consent was obtained from all human participants. In this group, 545 mothers of children with ASD and 697 mothers of children without ASD were recruited from the Children’s Hospital of Fudan University, from May 2019 to December 2021. The demographic characteristics of mothers having children either with or without ASD, including the use of folic acid, maternal/paternal smoking and drinking status, are shown in Supplemental Table 2.
Maternal serum samples during early pregnancy (10-12 weeks gestation) were analyzed. The design and conduct of the study was approved and supervised by the Ethics Committee of the Obstetrics and Gynecology Hospital of Fudan University through Ethic Vote 2015-17-C1, in accordance with the criteria established by the Declaration of Helsinki, and all experiments conformed to the Department of Health and Human Services Belmont Report. Written informed consent was obtained from all human participants. In this group, 37 pregnant women whose babies were later diagnosed with ASD and 23 control subjects with healthy offspring were recruited from the Obstetrics and Gynecology Hospital of Fudan University, from January 2019 to December 2020. Pregnant women included were in good health when recruited in our study, and the pregnant women in the control group were chosen on the basis of matching the general health status, which was also confirmed by the baseline comparison of their vital signs and physiological indexes. Pregnant women with one of the following situations were excluded: clinical or biochemical signs of infection, multiple gestations, diabetes mellitus, or other severe metabolic disorders. CHD phenotypes were first identified by examining malformations during week 22 of gestation and were confirmed after birth using color echocardiography. All patients with genetic syndromes or known chromosomal abnormalities (eg, Down’s syndrome, Holt–Oram syndrome, Alagille syndrome, DiGeorge syndrome, William syndrome, and Noonan syndrome) or family history of CHD in a first-degree relative (parent, sibling, or child) were excluded. Also, cases combined with other noncardiovascular malformations, tumors, or systematic diseases were not recruited. All participants were unrelated ethnic Han Chinese. The demographic characteristics of pregnant women bearing children either with or without ASD, including the use of folic acid, maternal/paternal smoking and drinking status, and blood biochemical index, are shown in Supplemental Table 2.
Animals and ethics
Female C57BL/6J (8 weeks) mice (17-19 g) were purchased from Beijing Vital River Laboratory Animal Technology Co Ltd (Beijing, China). These mice were housed in cages for 8 weeks under a 12-hour light/dark cycle. Standard laboratory chow and water were provided to the mice ad libitum. All chows were obtained from the Shanghai Feilin Biotechnology Co Ltd (Shanghai, China). All animal procedures were performed in accordance with the Fudan University Institutional Animal Care and Use Committee through Ethic Vote 201902010S, and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All mice were anesthetized with 2% isoflurane gas (spontaneous inhalation) and sacrificed by cervical dislocation. Blood and mouse tissues were collected at specific time points during the experiment.
Quantification and statistical analysis
All data shown represent the results obtained from triplicate independent experiments and are presented as the mean ± SD or mean with SEM as indicated. Two-group comparisons were performed using an unpaired Student's t-test, assuming a normal distribution, and were corrected for multiple hypothesis testing using the Benjamini-Hochberg procedure. Categorical data are presented as count (percentages) and were compared using the chi-square test with Bonferroni adjustment for multiple testing or Hardy-Weinberg equilibrium test in the control participants. Association of SNVs in the case-control studies was assessed using logistic regression adjusted for age and sex and presented as the OR with 95% CI. Statistical analyses were performed using GraphPad Prism version 10.0 (GraphPad Software), and a P value <0.05 was considered statistically significant.
Results
Maternal variation rs2545801 is significantly associated with the risk of ASD in their offspring
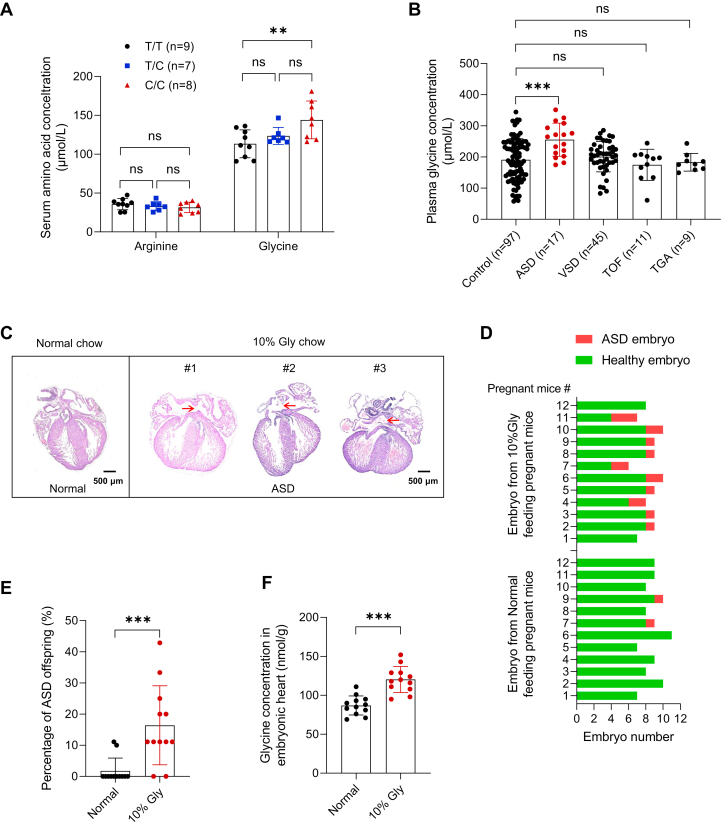
To investigate whether amino acid concentration-related SNVs of mothers were associated with ASD risk in the offspring, we selected 50 SNVs that showed a strong correlation with human blood amino acid levels and genotyped these SNVs in 545 mothers of children with ASD and 697 mothers of healthy children (Table 1, Supplemental Table 2). All SNVs were successfully genotyped in >95% of the samples. The genotype frequencies of all SNVs corresponded to the Hardy-Weinberg equilibrium (P > 0.05) (Supplemental Table 3). Among these SNVs, the genotype distribution of only 1 SNV, rs2545801, was significantly different between mothers with ASD and those with healthy offspring (P < 0.001) (Table 1). Compared with the wild-type T/T genotype, the maternal C/C genotype was associated a 112% increase in the ASD risk in the offspring (OR: 2.12; 95% CI: 1.41-3.18) (Supplemental Table 3). Because the rs2545801 is located near the F12 gene, which is associated with blood arginine and glycine levels,24,25 we verified these conclusions in serum samples from 24 individuals carrying 9 T/T, 7 T/C, and 8 C/C genotypes. Individuals with the C/C genotype exhibited lower arginine levels than those with the T/T genotype, but the decrease was not statistically significant. However, there was a statistically significant increase in glycine levels (Figure 1A). These results suggested that rs2545801-related high glycine levels in mothers may contribute to ASD risk in their offspring.
Table 1.
The SNVs Investigated in the Association Study
| Amino Acid | SNV | Gene | Base Change | Genotype P Value |
Amino Acid | SNV | Gene | Base Change | Genotype P Value |
|---|---|---|---|---|---|---|---|---|---|
| Ala | rs2704797 | FOXP1 | T>C | 0.17 | Gly | rs4646961 | ACADM | G>A | 0.17 |
| rs13232120 | TBL2 | A>T | 0.66 | rs2545801 | T>C | 6 × 10-4 | |||
| rs4237150 | GLIS3 | G>C | 0.70 | rs543159 | SLC22A3 | C>A | 0.33 | ||
| rs7938942 | LDHC | C>T | 0.60 | rs17591030 | GLDC | T>C | 0.57 | ||
| rs4554975 | SLC38A4 | G>A | 0.91 | rs10740134 | REEP3 | C>T | 0.58 | ||
| rs731165 | DLST | T>C | 0.42 | rs9923732 | C16orf46 | G>A | 0.81 | ||
| rs12453290 | SLC16A3 | G>A | 0.44 | rs8078686 | KPNB1 | T>C | 0.09 | ||
| Arg | rs10189479 | VIL1 | C>A | 0.20 | Val | rs964184 | ZPR1 | G>C | 0.56 |
| rs56335308 | SLC7A2 | A>G | 0.79 | rs4801776 | BCAT2 | T>C | 0.12 | ||
| rs7199750 | PRMT7 | C>G | 0.79 | Thr | rs1742425 | MEIOB | A>G | 0.95 | |
| rs11085824 | GCDH | G>A | 0.90 | Tyr | rs917117 | JAZF1 | G>A | 0.82 | |
| Asn | rs1260326 | GCKR | T>C | 0.30 | Ser | rs477992 | PHGDH | A>G | 0.95 |
| rs12587599 | ASPG | C>T | 0.43 | rs715 | CPS1 | T>C | 0.23 | ||
| Asp | rs4690522 | AGA | C>A | 0.44 | Pro | rs10882649 | TCTN3 | A>G | 0.50 |
| rs7964859 | GNPTAB | G>C | 0.51 | Phe | rs710446 | KNG1 | T>C | 0.48 | |
| Glu | rs7979478 | HNF1A | A>G | 0.23 | rs1800787 | FGB | C>T | 0.62 | |
| Gln | rs13281892 | SLC7A2 | G>A | 0.91 | rs4253238 | KLKB1 | C>T | 0.43 | |
| rs3892354 | GLIS3 | G>T | 0.81 | rs2731672 | T>C | 0.063 | |||
| rs7078003 | HOGA1 | C>T | 0.24 | rs1165167 | SLC17A3 | A>G | 0.86 | ||
| rs17602430 | SLC38A4 | C>T | 0.18 | rs2649667 | SLC43A1 | T>G | 0.87 | ||
| His | rs5030062 | KNG1 | A>C | 0.27 | rs869916 | PAH | T>G | 0.32 | |
| rs3733402 | KLKB1 | G>A | 0.40 | Ile | rs493841 | BCAT2 | C>T | 0.13 | |
| Met | rs6891672 | ADGRV1 | C>T | 0.95 | |||||
| Lys | rs2517237 | SLC7A2 | A>G | 0.41 | |||||
| rs7005693 | PPDPFL | A>C | 0.89 | ||||||
| rs2657880 | SPRYD4 | G>C | 0.67 | ||||||
| rs1059263 | SLC25A29 | A>C | 0.75 | ||||||
| rs8056893 | SLC7A6 | C>A | 0.60 | ||||||
The difference in the genotype distributions between the case and control subjects was estimated using the chi-square test with Bonferroni correction for type I error.
SNV = single-nucleotide variation.
Figure 1.
Increased Maternal Glycine Is Associated With the Risk for Developing Atrial Septal Defect in Embryos
(A) Serum arginine and glycine concentrations in individuals with T/T (n = 9), T/C (n = 7), and C/C (n = 8) genotypes; C/C genotype shows higher glycine levels. (B) Plasma glycine concentration in pregnant women at 10 to 12 weeks gestation with normal or offspring with congenital heart disease. n = 97 for control group, n = 17 for the atrial septal defect (ASD) group, n = 45 for the ventricular septal defect (VSD) group, n = 11 for tetralogy of Fallot (TOF) group, n = 9 for transposition of the great arteries (TGA) group. (C) ASD phenotype in embryos from mice with high levels of glycine. Representative histological staining results are shown (scale bar, 500 μm). (D) Number of healthy and embryos with atrial septal defect from pregnant mice fed with normal chow or 10%-glycine chow (n = 12 mice each group). (E) Percentage of offspring with atrial septal defect from pregnant mice fed with normal chow or 10%-glycine chow (n = 12 mice each group). (F) Glycine concentrations in embryonic heart tissues increased in 10%-glycine chow group (n = 12 mice each group). Data are presented as mean ± SD. Statistical analysis among 2 groups was performed using a 2-tailed unpaired Student’s t-test. nsP > 0.05, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Increased levels of circulating glycine during early pregnancy are associated with increased risk for ASD in the offspring
Next, we determined whether glycine levels during early pregnancy were associated with ASD. To further evaluate the correlation between maternal glycine levels and embryonic heart development, we collected fasting serum samples from women (37 bearing newborns with ASD [case group] and 23 bearing healthy newborns [control group]) in their first trimester at weeks 10 to 12 of gestation. Metabolite proofing was performed using nuclear magnetic resonance, as performed in our previous studies.10,26 In total, 23 metabolites were successfully identified and quantified in >95% of the samples. We found that the glycine levels of pregnant women in the case group were significantly increased compared with those of pregnant women in the control group (P < 0.001) (Table 2). We also reanalyzed the data obtained from our previous measurement of amino acid levels in plasma samples of 82 pregnant women bearing newborns with CHD and 101 pregnant women bearing healthy newborns in their first trimester at weeks 10 to 12 of gestation.10 In the case group, 17 individuals had ASD. By reanalyzing the arginine and glycine levels in the case and control groups, we found that increased maternal glycine levels during early pregnancy were associated with ASD in the offspring (Figure 1B). Taken together, these results indicated that increased glycine levels during embryonic heart development increased the risk of developing ASD.
Table 2.
Differences in Metabolites Between Serum in Pregnant Bearing Children With ASD and Healthy Children
| Metabolites (μmol/L) | Control (n = 23) | Case (n = 37) | P Value |
|---|---|---|---|
| Glycine | 197.65 ± 43.30 | 220.40 ± 50.69 | 1.26 × 10−9 |
| Glutamine | 175.44 ± 36.70 | 185.05 ± 67.84 | 0.012 |
| Lysine | 291.62 ± 69.63 | 317.51 ± 152.39 | 0.031 |
| Leucine | 169.92 ± 59.20 | 180.25 ± 48.94 | 0.032 |
| Formate | 5.53 ± 3.33 | 6.02 ± 4.61 | 0.032 |
| Isoleucine | 97.25 ± 36.02 | 104.19 ± 33.07 | 0.032 |
| Acetate | 65.66 ± 11.33 | 71.18 ± 15.79 | 0.038 |
| Asparagine | 34.27 ± 15.00 | 36.88 ± 11.03 | 0.040 |
| Alanine | 305.66 ± 119.55 | 324.15 ± 135.17 | 0.042 |
| Phenylalanine | 47.41 ± 12.02 | 49.36 ± 10.61 | 0.054 |
| Creatine | 87.59 ± 16.91 | 93.48 ± 30.42 | 0.077 |
| Glutamate | 309.00 ± 77.69 | 322.43 ± 100.64 | 0.089 |
| Valine | 173.34 ± 68.94 | 180.62 ± 49.43 | 0.12 |
| Tyrosine | 23.70 ± 9.98 | 25.23 ± 10.82 | 0.18 |
| Citrate | 26.42 ± 10.20 | 28.20 ± 19.43 | 0.20 |
| 3-Hydroxybutytrate | 241.69 ± 240.08 | 263.97 ± 189.15 | 0.21 |
| Aspartate | 30.32 ± 15.95 | 31.85 ± 16.52 | 0.22 |
| Pyruvate | 33.63 ± 16.19 | 35.36 ± 10.41 | 0.23 |
| Choline | 107.39 ± 48.95 | 114.18 ± 58.27 | 0.27 |
| Glycerophosphocholine | 1,842.46 ± 433.64 | 1,935.24 ± 575.59 | 0.28 |
| Dimethylamine | 65.14 ± 41.40 | 69.18 ± 54.55 | 0.39 |
| Tryptophan | 12.19 ± 5.25 | 12.64 ± 6.27 | 0.67 |
| Hypoxanthine | 3.78 ± 4.13 | 3.65 ± 4.24 | 0.68 |
| Inosine | 3.30 ± 2.13 | 3.23 ± 2.19 | 0.83 |
| Lactate | 658.79 ± 353.65 | 652.90 ± 358.87 | 0.86 |
| Histidine | 10.78 ± 10.34 | 10.81 ± 6.64 | 0.86 |
Values are mean ± SD and compared using unpaired Student's t-tests. All values expressed as μmol/L.
Increased glycine levels in pregnant mice induce ASDs in the offspring
We also assessed the teratogenic effects of glycine in a high-amino acid mouse model. Eight-week-old C57BL/6J female mice were fed high-glycine chow, prepared by supplementing normal chow with either 5% or 10% glycine. The ratio of glycine to normal chow was optimized to ensure that the significantly increased circulating glycine levels were comparable to those observed in patients in the clinic (12%-34% increase) (Table 2, Figure 1B). Only chow supplemented with 10% glycine induced an increase (of 16% on average) in circulating glycine levels in mouse serum (Supplemental Figure 1A). Therefore, we used 10% glycine chow to mimic the clinical trends observed in the patients. Pregnant mice were administered the high-amino acid chow and normal chow from embryonic day 0.5 to embryonic day 13.5. The cardiac phenotypes of the embryos at embryonic day 14.5 were examined via histological analysis. Although feeding with high-glycine chow did not change food intake, body weight, blood pressure, pulse, or blood glucose levels in the mice (Supplemental Figures 1B to 1H), the ASD phenotype was observed in the hearts of embryos obtained from high-glycine chow-fed female mice (Figure 1C). No differences were observed in the total number of fetuses or incidence of absorbed fetuses between the groups (Supplemental Figures 2A and 2B). However, the proportion of pregnant mice with ASD offspring was 16.7% (2 of 12) in the control group and 83.3% (10 of 12) in the high-glycine diet group (Figure 1D). The proportion of ASD occurrence among all offspring was 1.9% (2 of 105) and 15.8% (16 of 101) in the control and high-glycine diet groups, respectively (Figure 1E), showing a statistically significant difference (P = 0.001). In contrast, no increase in the incidence of ventricular septal defect was observed in the offsprings (Supplemental Figure 2C). These results indicated that increased maternal glycine levels during early pregnancy were associated with an increased risk of ASD in the offspring. Subsequently, we analyzed glycine levels in the embryonic heart tissues from high-glycine chow-fed and normal chow-fed mice. Glycine levels were notably increased in the heart tissues of embryos from the high-glycine chow-fed group of mice (Figure 1F), thereby supporting the hypothesis that increased glycine levels caused abnormal heart development in our mouse model.
We investigated the effect of elevated glycine levels on ASD development. Our findings revealed that increased levels of glycine, but not other amino acids, contributed to ASD development in the offspring. This result suggested that protein synthesis is not the underlying cause of ASD. Elevated glycine levels also affect the folate cycle, where it serves as a donor for methylation.27 As folate levels showed no alterations between the case and control groups (see Supplemental Table 2), we excluded the possibility that glycine contributes to the onset of ASD by enhancing folate metabolism. Endocardial/endothelial cells play a vital role during embryonic heart development and cushion formation,28 and glycine was reported to be associated with angiogenesis.29,30 Human umbilical vein endothelial cells (HUVECs) share key signaling pathways or transcription regulators (eg, vascular endothelial growth factor, Notch, PI3K-AKT-FOXO1) critical to endothelial functions and were employed as an in vitro surrogate to investigate molecular mechanisms. We treated HUVEC with glycine and observed that glycine exerted an inhibitory effect on HUVECs proliferation (Supplemental Figures 3A and 3B), migration (Supplemental Figure 3C), and angiogenesis (Supplemental Figure 3D), while having no significant impact on apoptosis (Supplemental Figure 3E). These findings suggest a close association between intracellular glycine levels and endothelial cell function.
In our previous studies, we discovered that amino acids such as leucine,10 tyrosine,31 and homocysteine32 exert their pathological effects by modifying proteins. Similarly, glycine modifies protein lysine residues to form K-Gly via the glycyl-tRNA synthetase (GARS).33 As we previously identified, GARS can produce reactive aminoacyl-adenylate (aminoacyl-AMP) compounds, which contain high-energy aminoacyl-phosphate bonds known to modify the ε-amine groups of lysine and potentially catalyze K-Gly formation. To investigate whether GARS-mediated K-Gly is the cause of high glycine-induced teratogenicity, we developed a custom pan–K-Gly antibody as described previously33 and examined whether inhibiting GARS-mediated K-Gly in pregnant mice could reduce the risk of ASD in the offspring. We first validated the specificity of the pan–K-Gly antibody using dot blot analysis (Supplemental Figure 4A). Comparing the K-Gly levels in embryonic heart tissue between normal and high-glycine maternal feeding groups, we found significantly higher K-Gly levels in the latter group (Supplemental Figure 4B). These findings indicated that increased glycine levels determined K-Gly levels in embryonic mouse hearts, and suggested that high levels of glycine induced ASD by inducing K-Gly formation in mouse embryos.
GARS interacts and catalyzes the K-Gly of angiopoietin-1 receptors TEK
We then investigated the role of K-Gly modification in cardiovascular development. Proteins interacting with GARS are more likely to undergo K-Gly modification, and endothelial cell dysfunction plays a crucial role in ASD development. Therefore, we overexpressed GARS in HUVEC and employed tandem affinity purification to identify GARS-interacting proteins (Supplemental Table 4). We also searched for K-Gly modifications in a tryptic peptide library of human liver tissue as previously described33 (Supplemental Table 5). TEK was the only intersection between the 2 sets (Figure 2A), suggesting that TEK is likely regulated by K-Gly in endothelial cells. According to the Protein Atlas database, TEK is primarily expressed in endothelial cells and is linked to the onset of ASD. TEK receptors and angiopoietin ligands regulate angiogenesis and vessel maturation.34 Myocardial Angpt1/endocardial TEK signaling in the atrium promotes the spatiotemporal degradation of cardiac jelly during early cardiac development and is therefore indispensable for atrial chamber morphogenesis.35 Therefore, we screened for K-Gly modified sites on TEK using LC-MS/MS and found that lysine 688 of TEK was glycylated in HUVEC (Figure 2B). Moreover, the MS/MS spectrum of the synthetic TEK peptide with lysine 688 glycylation matched the spectra of tryptic TEK peptide libraries of HUVEC (Figure 2B), confirming that lysine 688 of TEK was glycylated. We also confirmed that ectopically expressed TEK and GARS interacted in HUVEC (Figure 2C). Affinity-purified endogenous GARS was copurified with endogenous TEK in HUVEC (Figure 2D) and human atrial tissue (Figure 2E). These results confirmed the strong interaction between GARS and TEK in endothelial cells. We also verified that lysine 688 of TEK was glycylated in mouse heart tissues (Figure 2F), thereby confirming that the K688 site of TEK was modified by K-Gly.
Figure 2.
TEK Is Glycylated at Lysine 688
(A) Tandem affinity purification assay identified 302 proteins as potential glycyl-tRNA synthetase (GARS)-interacting proteins, and 217 proteins were identified with K-Gly modification in a tryptic peptide library. TEK represents the exclusive intersection of the 2 sets. (B) Lysine 688 site of TEK, identified as being glycylated. MS/MS spectra result of tryptic peptides from human umbilical vein endothelial cells (HUVECs) (top), with the synthetic glycylated peptide bearing the same peptide sequence (bottom). (C) GARS bound to TEK. GARS and TEK interactions are examined by coimmunoprecipitation against exogenous Flag-tagged TEK (left) and Flag-tagged GARS (right). (E) Endogenous GARS interacts with TEK in human atrial tissue. Immunoprecipitation against TEK was performed before Western blot analysis. (F) MS/MS spectra of TEK K688Gly-containing peptides from mice heart tissue.
Using an in vitro assay, we validated that GARS, but not the proven enzyme-inactive mutants, GARSG580R and GARSG472R, glycylated the lysine of a synthetic TEK peptide containing K688 (Figure 3A). We used immunoprecipitation to enrich exogenous TEK in HUVEC and ectopically expressed Flag-TEK in HUVEC to detect the K-Gly modification levels of TEK using Western blot analysis. The levels of K-Gly–modified TEK increased with glycine concentration gradient (Figures 3B and 3C). Furthermore, overexpression of GARS, but not of tRNA-charging defective GARSG580R and GARSH472R mutants, increased the K-Gly levels of both endogenous (Figure 3D) and exogenous TEK (Figure 3E). Conversely, the transient knockdown of GARS using small interfering RNA (siRNA) in HUVEC decreased the K-Gly levels of endogenous TEK protein (Figures 3F and 3G).
Figure 3.
GARS Interacts and Catalyzes Lysine Glycylation of TEK
(A) Purified glycyl-tRNA synthetase (GARS) and the proven enzyme inactive mutants GARSG580R and GARSH472R are assessed for their ability to catalyze lysine glycylation (K-Gly) formation in a synthetic K688 peptide in vitro. (B and C) Glycine increases K-Gly levels of endogenous (B) and exogenously-expressed (C) TEK in a dose-dependent manner in HUVEC. (Right) Quantification of relative intensity of K-Gly blots. n = 3 biological replicates. (D and E) GARS, but not the enzyme inactive mutants GARSG580R and GARSH472R, increases the K-Gly modification of endogenous (D) and exogenously expressed (E) TEK in HUVEC. (Right) Quantification of relative intensity of K-Gly blots. n = 3 biological replicates. (F and G) GARS knockdown deceases K-Gly levels of endogenous (F) and exogenously expressed (G) TEK in HUVEC. (Right) Quantification of relative intensity of K-Gly blots. n = 3 biological replicates. (H) Glycine increases K-Gly levels of wild-type but not K688R mutant TEK. (Right) Quantification of relative intensity of K-Gly blots. n = 3 biological replicates. (I) GARS overexpression increases K-Gly level of wild-type but not K688R mutant TEK. Immunoprecipitation against Flag was performed before Western blot analysis. (Right) Quantification of relative intensity of K-Gly blots. n = 3 biological replicates. Data are presented as mean ± SD. Statistical analysis among 2 groups was performed using a 2-tailed unpaired Student’s t-test. nsP > 0.05, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Abbreviations as in Figure 2.
These results demonstrated that the level of modification was positively correlated with GARS expression levels and glycine concentration. Furthermore, we mutated the lysine 688 site of TEK to arginine (R), an unmodifiable amino acid, and we found that K-Gly levels decreased notably in the TEKK688R mutant, and no change was observed with increasing glycine levels (Figure 3H) or GARS overexpression (Figure 3I). These results collectively confirmed that GARS exhibited lysine glycyltransferase activity to catalyze the K-Gly of TEK, and that K688 was the major modification site of TEK.
K-Gly impedes TEK membrane localization and inactivates TEK
Subsequently, we explored whether the K-Gly modification affects TEK function. First, we found that increasing the glycine concentration in the culture medium did not alter TEK levels at both the mRNA and protein levels in HUVEC (Figures 4A and 4B). These results indicated that the modification had no significant effect on protein expression. Thus, we investigated whether this modification affected the functionality of the protein. We aligned the TEK sequences of different species and found that the TEK K688 site was evolutionarily conserved across species, from Xenopus laevis to Homo sapiens (Figure 4C). According to the structural analysis of PyMOL, K688 is located in the Fn3 domain (Supplemental Figure 5), which mainly mediates homotypic interactions and is required for ligand-induced TEK activation.36 Therefore, we investigated the effect of glycine on the cellular localization of TEK in HUVEC using fluorescence immunostaining. We found that high concentrations of glycine inhibited the translocation of TEK to the cell surface (Figure 4D). The cell surface localization of TEK was confirmed by Western blot after separation of the cell membrane and cytoplasm (Figure 4E). Moreover, we validated that changing lysine 688 to glycine (K688G) in TEK to mimic K-Gly caused the retention of TEK in the cytosol (Figure 4F). This was also confirmed by immunofluorescence staining of TEK and the TEK K688G mutant in HUVEC (Figure 4G), suggesting the dynamic regulation of cell-surface TEK in response to the glycylation level.
Figure 4.
Lysine Glycylation Impedes TEK Membrane Localization and Inactivates TEK
(A and B) Glycine does not affect the protein (A) and mRNA (B) levels of TEK in HUVEC. n = 5 biological replicates. P values are calculated using the 2-tailed Student's t-test. (C) The TEK K688 site is evolutionarily conserved across species. (D and E) TEK membrane localization in cultured cells subjected to various treatments as examined via immunostaining (D) and Western blot (E). Scale bar represents 10 μm. (Right) Quantification of relative intensity of Flag. n = 3 biological replicates. (F and G) Intracellular localization of the TEKK688G mutant, which mimics the glycylation status, examined via Western blot (F) and immunostaining (G). Scale bar represents 10 μm. (Right) Quantification of relative intensity of Flag. n = 3 biological replicates. (H) The phosphorylation levels of S473 of AKT and S256 of FOXO1 and the protein levels of ANG2 in HUVEC subjected to glycine treatment or not. (I) The nuclear localization of FOXO1 in HUVEC subjected to glycine treatment or not. (Right) Quantification of relative intensity of FOXO1. n = 3 biological replicates. (J) The mRNA levels of FOXO1 transcriptional activity targets in HUVEC subjected to different treatments. n = 5 biological replicates. P values are calculated using the 2-tailed Student's t-test. (K and L) Intracellular localization of TEK and TEKK688R mutant, which impeded the lysine glycylation, examined via Western blot (K) and immunostaining (L). Scale bar represents 10 μm. (Right) Quantification of relative intensity of Flag. n = 3 biological replicates. (M) The mRNA levels of FOXO1 transcriptional activity targets in HUVEC subjected to different treatments; n = 5 biological replicates. P values are calculated using the two-tailed Student's t-test. (N) The phosphorylation levels of S473 of AKT and S256 of FOXO1 and the protein levels of ANG2 in HUVEC subjected to different treatments. (Right) Quantification of relative intensity of ANG2. n = 3 biological replicates. Data are presented as mean ± SD. Statistical analysis among 2 groups was performed using a 2-tailed unpaired Student’s t-test. nsP > 0.05, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
As described earlier, Angpt1/TEK signaling plays an important role in early cardiac development, and TEK activation promotes PI3K/AKT signaling. FOXO1 transcription factors are important downstream molecules in TEK-mediated AKT signal transduction, and FOXO1 activation promotes angiopoietin 2 (ANG2) expression in endothelial cells.37, 38, 39 Consequently, we hypothesized that glycylation of TEK may alter the expression level of ANG2 by inhibiting the downstream AKT-FOXO1 signaling pathway. Overexpression of GARS and stimulation with glycine resulted in decreased phosphorylation of S473 of AKT and S256 of FOXO1 and increased expression of ANG2 (Figure 4H). ANG1, an angiogenic factor that signals through the endothelial cell-specific TEK receptor tyrosine kinase, is essential for normal vascular development and atrial chamber morphogenesis.35 ANG2 was identified by homology screening and shown to be a naturally occurring antagonist for ANG1 and suppresses TEK signaling.40 In the case of high glycine, the increase of ANG2 competes with ANG1 to bind TEK, further inhibits TEK signaling and contributes to ASD formation. Furthermore, we verified that the increased nuclear localization of FOXO1 caused by glycine treatment (Figure 4I) led to increased mRNA levels of FOXO1-regulated transcripts such as ANG2, PDK4, OCT4, SOX2, and NOXA in HUVEC (Figure 4J). These genes are all recognized downstream genes of FOXO1, and changes in their transcriptional levels can reflect the activation state of FOXO1. Moreover, these genes are associated with angiogenesis or endothelial-mesenchymal transition (EndMT).41, 42, 43, 44 EndMT is a critical process during the development of embryonic heart and many other tissues, and AKT is a key regulator of EMT.45 Therefore, we examined whether glycine or TEK affects the process of EndMT. HUVECs were treated with TGFβ1 for 7 days to induce EndMT. Glycine treatment significantly decreased SMA-positive cells after TGFβ1 induction (Supplemental Figure 6A), and expression of mesenchymal cell marker N-cadherin (Supplemental Figure 6B). TEK knockdown also exhibited similar effect as glycine to inhibit EndMT process (Supplemental Figures 6C and 6D). These results indicated that K-Gly of TEK inhibited the AKT-FOXO1 pathway, thus restrain the EndMT process of endothelial cells, which may contribute to ASD formation. In addition, we found that glycine-induced nuclear translocation of FOXO1 also inhibit the proliferation of human mesenchymal stem cells (Supplemental Figure 6E). Moreover, lysine 688 was mutated to arginine (K688R) to mimic the nonglycylated form of TEK. The protein level of the TEK K688R mutant on the cell surface was significantly increased in HUVEC and did not respond to glycine concentrations (Figures 4K and 4L). Conversely, transient knockdown of TEK in HUVEC increased the mRNA levels of FOXO1-regulated transcripts such as ANG2 and PDK4 (Figure 4M). Western blot experiments also verified that the protein level of ANG2 increased and phosphorylation of AKT and FOXO1 decreased when TEK was knocked down (Figure 4N). Furthermore, knockdown of TEK in HUVEC diminished the effect of high glycine levels on ANG2 expression and phosphorylation of AKT and FOXO1 (Figures 4M and 4N). A previous study showed that ANG2 competes with ANG1 and acts as a TEK antagonist under baseline conditions.40 Concomitantly, ANG2 overexpression caused embryonic lethality, similarly to the deletion of Ang1 or TEK.46 Taken together, our results suggested that glycylation of the K688 site of TEK prevents its membrane localization, thereby inhibiting the downstream AKT-FOXO1 signaling pathway. This results in an increase in the expression level of ANG2 regulated by FOXO1 and further inhibits the TEK pathway in a positive feedback manner, leading to congenital ASD.
SIRT3 deglycylates and activates TEK
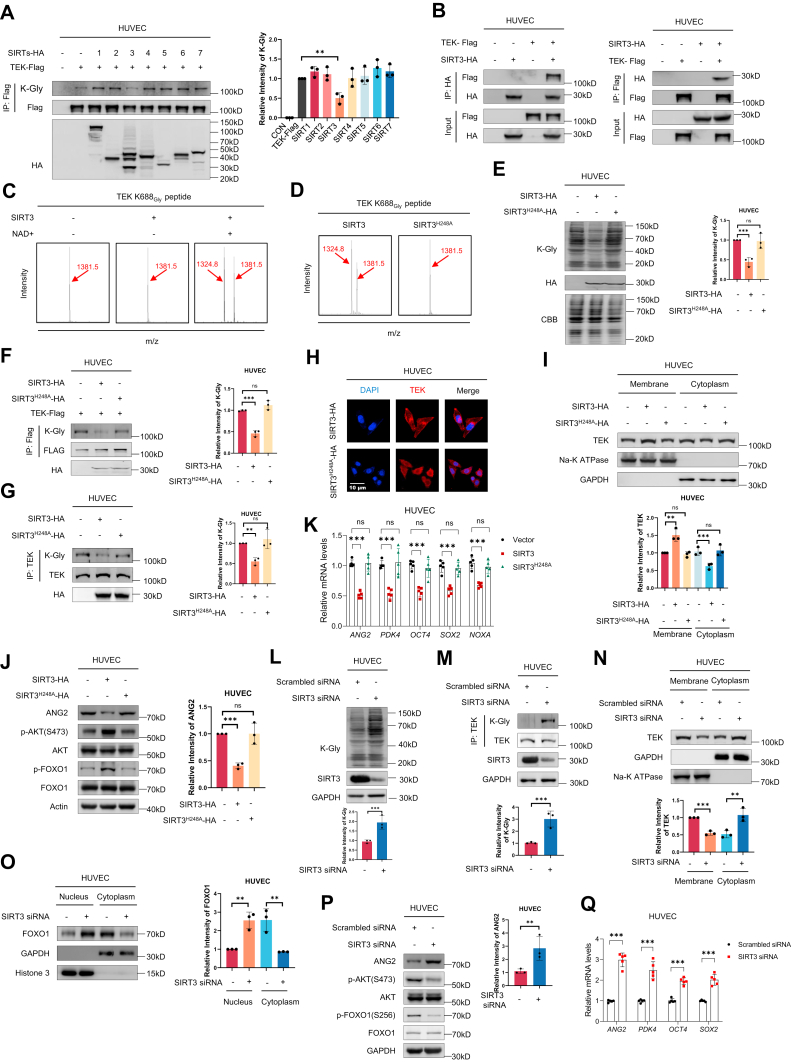
To identify potential human deglycylases, we screened the deaminoacylase activities of the NAD+-dependent sirtuins SIRT1 to SIRT7. Overexpression of SIRT3, but not other SIRTs, in HUVEC led to decreased K-Gly levels in exogenously expressed TEK (Figure 5A). Moreover, coimmunoprecipitation assays in HUVEC demonstrated a direct interaction between TEK and SIRT3 (Figure 5B). We also confirmed that SIRT3 showed NAD+-dependent deglycylase activity toward the synthetic TEK peptide containing K688-Gly (Figure 5C). In contrast, the deaminoacylase-defective mutant SIRT3H248A failed to remove TEK K688-Gly in vitro (Figure 5D). Overexpression of wild-type SIRT3, but not of the catalytically inactive SIRT3H248A mutant, deglycylated total proteins (Figure 5E) and ectopically expressed Flag-TEK (Figure 5F) or endogenous TEK in HUVEC (Figure 5G). Our earlier results showed that glycylation of TEK inhibited its membrane localization. This led us to investigate the effects of SIRT3-mediated removal of TEK glycylation on the cellular localization and function of TEK. Immunofluorescence and Western blot results revealed that wild-type SIRT3, but not the SIRT3H248A mutant, promoted TEK membrane localization in the presence of high glycine levels (Figures 5H and 5I). Consistently, the protein level of ANG2 decreased, and the phosphorylation levels of AKT and FOXO1 were elevated when wild-type SIRT3 was overexpressed in HUVEC (Figure 5J). The mRNA levels of transcripts such as ANG2 and PDK4 were also reduced in the presence of wild-type SIRT3, but not upon SIRT3H248A mutant overexpression (Figure 5K).
Figure 5.
SIRT3 Deglycylates and Activates TEK
(A) SIRT3, but not other sirtuins, decreases K-Gly levels of ectopically-expressed TEK. (Right) Quantification of relative intensity of K-Gly. n = 3 biological replicates. (B) SIRT3 and TEK interactions are examined via coimmunoprecipitation in HUVECs. (C and D) SIRT3 catalyzes deglycylation reactions in vitro (C), in contrast to the deaminoacylase-defective mutant SIRT3H248A (D). (E to G) SIRT3 decreases the K-Gly levels of total protein (E) and exogenous (F) and endogenous TEK (G) in HUVEC. (Right) Quantification of relative intensity of ANG2. n = 3 biological replicates. (H and I) TEK membrane localization in cultured cells overexpressed SIRT3 or SIRT3H248A, examined via immunostaining (H) and Western blot (I); the scale bar represented 10 μm. (Bottom) Quantification of relative intensity of TEK. n = 3 biological replicates. (J) The protein levels of ANG2, the phosphorylation levels of S473 of AKT and S256 of FOXO1 in HUVEC subjected to SIRT3 or SIRT3H248A. (Right) Quantification of relative intensity of ANG2. n = 3 biological replicates. (K) The mRNA levels of FOXO1 transcriptional activity targets in HUVEC subjected to SIRT3 or SIRT3H248A; n = 5 biological replicates. (L and M) SIRT3 knockdown increases K-Gly levels of total protein (L) and TEK (M) in HUVEC. (Bottom) Quantification of relative intensity of K-Gly. n = 3 biological replicates. (N and O) Intracellular localization of the TEK (N) and FOXO1 (O) in HUVEC subjected to different treatments, examined via Western blot. (Bottom) Quantification of relative intensity of TEK (N) and FOXO1 (O). n = 3 biological replicates. (P) SIRT3 knockdown increases the protein levels of ANG2, reduces the phosphorylation levels of S473 of AKT and S256 of FOXO1 in HUVEC. (Right) Quantification of relative intensity of ANG2. n = 3 biological replicates. (Q) The mRNA levels of FOXO1 transcriptional activity targets in HUVEC subjected to SIRT3 knockdown; n = 5 biological replicates. Data are presented as mean ± SD. Statistical analysis among 2 groups was performed using a 2-tailed unpaired Student’s t-test. nsP > 0.05, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Abbreviations as in Figure 2.
To further assess the effect of SIRT3 on deglycylase activity, we transiently knocked down SIRT3 in HUVEC, which increased K-Gly levels of total protein (Figure 5L) and endogenous TEK (Figure 5M). Following SIRT3 knockdown, the membrane localization of TEK was significantly reduced (Figure 5N). We also confirmed that SIRT3 knockdown increased the nuclear localization of FOXO1 (Figure 5O), led to elevated protein levels of ANG2, increased AKT and FOXO1 phosphorylation (Figure 5P), and increased the mRNA levels of FOXO1-regulated transcripts such as ANG2, PDK4, OCT4, SOX2 in HUVEC (Figure 5Q). Collectively, our results suggested that SIRT3 can activate the TEK signaling pathway by removing the glycylation of TEK, thereby ensuring normal development of the heart.
Discussion
In this study, we investigated the association between amino acid concentrations and CHD, particularly ASD, and the underlying mechanisms. We found that excess maternal glycine contributes to the risk of ASD in the offspring. Overall, the interplay between maternal metabolism and embryonic heart development highlights the importance of maintaining maternal metabolic health during pregnancy to ensure optimal cardiovascular development in the growing fetus.
In addition to protein synthesis, glycine plays 2 important physiological roles. First, glycine is used by the body to generate large pools of 1-carbon units. These units activate 1-carbon metabolism, an intracellular bicyclic pathway that couples the methionine and folate cycles via methionine synthase.27 Second, glycine regulates intracellular redox homeostasis, playing a critical role in maintaining cellular redox homeostasis by providing cells with reductive equivalents. One of the main redox-regulating metabolites is glutathione, a tripeptide comprising glycine, cysteine, and glutamate that can scavenge reactive oxygen species. Therefore, proliferating cells require sufficient amounts of glycine via the intracellular serine and glycine synthesis pathways and/or glycine uptake from the cellular environment.47 However, the physiological role of glycine does not explain why increased glycine levels increase the risk of ASD during embryonic organ development.
With the discovery of new amino acid functions and the specific regulation of different cellular signaling pathways by all 20 common amino acids, a functional network of amino acids has been discovered and valued. Aminoacyl-tRNA synthetases (ARS) are a family of enzymes capable of binding and discriminating between amino acids and catalyzing aminoacylation reactions to link each specific amino acid to its cognate tRNA, thus fulfilling this requirement as true amino acid sensors.33 Remarkably, certain ARS and their corresponding amino acids regulate functions other than protein synthesis. For example, both leucine and leucyl-tRNA synthetase activate mTORC1.48, 49, 50 Glutamine and glutamine-bound glutaminyl-tRNA synthetase suppress apoptosis by inhibiting the proapoptotic enzyme apoptosis signal-regulating kinase.33,51,52 Moreover, the binding of amino acids to their corresponding ARS is required for signal transmission by the latter.49,52,53 These results supported the concept that amino acids are sensed by the ARS. During organ development, in addition to substance synthesis and energy supply, amino acids play complex signal regulatory functions. An imbalance in their concentrations can change cell signaling and lead to abnormal development. For example, we previously found that an imbalance of homocysteine9 and leucine10 can lead fetal CHD, and the disease phenotype is mainly concentrated in ventricular septal defects. In this study, we found that high levels of glycine were closely related to the development of the atrial septum. One of the most likely explanations is that ANG1 expression is highly and specifically expressed in atrial myocardium during heart development,35 and ANG1/TEK is indispensable for ADAMTS-mediated atrial chamber morphogenesis. Glycine-increased ANG2 competes with ANG1 to bind with TEK, further suppressing TEK-PI3K-Akt signaling and causes formation of ASD. Moreover, we also found that elevated glycine level inhibited ADAMTS proteinase expression (Supplemental Figure 7) and may also affect the normal development of the atrial septum through this pathway. Importantly, our findings suggest that glycine-induced nuclear translocation of FOXO1 may constitute a central mechanism underlying its biological effects. To validate this hypothesis, pregnant mice fed a high-glycine chow were administered AS1842856, a specific FOXO1 inhibitor, at the same time. AS1842856 treatment significantly attenuated glycine-induced ASD formation (Supplemental Figure 8), indicating the critical role of FOXO1 in mediating glycine's teratogenic effects. Intriguingly, it was also found that FOXO1 is nuclear excluded during early cardiac development (E10.5-E15.5) and myocyte-specific overexpression of FOXO1 resulted in embryonic lethality because of severe myocardial defect,54 demonstrating that precise suppression of FOXO1 activity is essential for normal heart development.
In this study, we found specific genetic alterations that were significantly associated with glycine concentration alone and with the risk of CHD in offspring. These observations not only confirmed the effect of glycine teratogenicity from the perspective of genetic and nutritional interactions but also provided genetic targets for identifying high-risk pregnant women during early pregnancy or even preconception. For example, monitoring glycine levels during pregnancy in women of childbearing age carrying specific genotypes is crucial. The use of genetic markers holds promise for advancing risk prediction for certain conditions, including congenital heart disease. Integrating genetic information with other risk factors and clinical data may enable a more accurate and personalized risk assessment before conception. However, further research is needed to establish the validity and reliability of these genetic markers for predicting specific health outcomes.
Study limitations
The study relies on HUVECs as a surrogate for atrial endocardial cells. Although HUVECs provide valuable insights into conserved endothelial pathways, they differ from embryonic endocardial cells in origin, microenvironmental cues, and tissue-specific functions. Future work using lineage-specific endocardial cell models or in vivo genetic approaches will be essential to validate the relevance of our findings to cardiac development. Numerous substrate proteins are modified by glycine, and in addition to TEK, other proteins may be involved in the regulation of heart development. Further research is needed to elucidate the role of glycine in heart development at the signaling network level. Second, the demodifying enzyme SIRT3 that we screened has multiple demodifying functions, including classic deacetylation and demodification of various amino acids. Therefore, compared with glycine modification, the demodification function of SIRT3 has a broad spectrum of regulatory effects on cellular signaling networks. Notably, in our previous studies on SIRT3 knockout mice, both ventricular and ASDs were observed. We cannot rule out the possibility that there are other, more specific deglycylation enzymes; thus, further exploration is warranted.
Conclusions
We found that at the genetic level, maternal high-glycine-related genetic variants were associated with increased ASD risk in the offspring. At the metabolism level, higher maternal levels of both serum and plasma glycine during early pregnancy led to increased ASD risk in the offspring. In an animal model, increasing glycine levels during early pregnancy in mice led to ASD in the offspring. Finally, we found that glycine overload led to the onset of ASD through inhibition of TEK signaling. Our findings highlight the potential of using genetics and metabolite examination to identify early biomarkers and developing nutritional and pharmaceutical interventions as preventive and therapeutic strategies against ASD.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Amino acid imbalance is linked to increased congenital heart disease risk. We found that maternal abnormal glycine metabolism is linked to ASD risk in offspring. Elevated glycine levels impair TEK-PI3K-AKT/FOXO1 signaling in fetal cardiac endothelial cells by forming K-Gly modification on lysine 688 of TEK, which indicates that lysine-glycylation exerts teratogenic effects and may be a target for ASD intervention.
TRANSLATIONAL OUTLOOK: Using 3 different measurements, we demonstrated that high maternal levels of glycine can increase ASD risk in the offspring by inhibiting embryonic TEK signaling. Our study indicated glycine levels should be monitored in individuals with high-risk alleles before pregnancy to mitigate the teratogenic risks associated with high glycine levels. A sequential combination of glycine concentration-related genetic variant analysis before pregnancy and measurement of circulating glycine levels in early pregnancy could serve as a better predictive biomarker for ASD.
Funding Support and Author Disclosures
This work was supported by the grants from National Natural Science Foundation of China (82330048, 82170236, 81700212, 32000895, 32370824, 82300428), Program of Shanghai Academic Research Leader (21XD1421700), Innovation Program of the Shanghai Municipal Education Commission (2023ZKZD24), and Innovative research team of high-level local universities in Shanghai (SHSMU-ZDCX20211100). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
Contributor Information
Rui Zhao, Email: zr_gre@hotmail.com.
Jian-Yuan Zhao, Email: zhaojianyuan1508@xinhuamed.com.cn.
Appendix
References
- 1.Kalisch-Smith J.I., Ved N., Sparrow D.B. Environmental risk factors for congenital heart disease. Cold Spring Harb Perspect Biol. 2020;12(3) doi: 10.1101/cshperspect.a037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tennant P.W., Pearce M.S., Bythell M., Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375(9715):649–656. doi: 10.1016/S0140-6736(09)61922-X. [DOI] [PubMed] [Google Scholar]
- 3.Liu A., Diller G.P., Moons P., Daniels C.J., Jenkins K.J., Marelli A. Changing epidemiology of congenital heart disease: effect on outcomes and quality of care in adults. Nat Rev Cardiol. 2023;20(2):126–137. doi: 10.1038/s41569-022-00749-y. [DOI] [PubMed] [Google Scholar]
- 4.van der Bom T., Zomer A.C., Zwinderman A.H., Meijboom F.J., Bouma B.J., Mulder B.J. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011;8(1):50–60. doi: 10.1038/nrcardio.2010.166. [DOI] [PubMed] [Google Scholar]
- 5.Bouma B.J., Mulder B.J. Changing landscape of congenital heart disease. Circ Res. 2017;120(6):908–922. doi: 10.1161/CIRCRESAHA.116.309302. [DOI] [PubMed] [Google Scholar]
- 6.Michals-Matalon K., Platt L.D., Acosta P.P., Azen C., Walla C.A. Nutrient intake and congenital heart defects in maternal phenylketonuria. Am J Obstet Gynecol. 2002;187(2):441–444. doi: 10.1067/mob.2002.124276. [DOI] [PubMed] [Google Scholar]
- 7.Rouse B., Matalon R., Koch R., et al. Maternal phenylketonuria syndrome: congenital heart defects, microcephaly, and developmental outcomes. J Pediatr. 2000;136(1):57–61. doi: 10.1016/s0022-3476(00)90050-7. [DOI] [PubMed] [Google Scholar]
- 8.Hobbs C.A., Malik S., Zhao W., James S.J., Melnyk S., Cleves M.A. Maternal homocysteine and congenital heart defects. J Am Coll Cardiol. 2006;47(3):683–685. doi: 10.1016/j.jacc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Mei X., Qi D., Zhang T., et al. Inhibiting MARSs reduces hyperhomocysteinemia-associated neural tube and congenital heart defects. EMBO Mol Med. 2020;12(3) doi: 10.15252/emmm.201809469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Liu L., Chen W.C., et al. Gestational leucylation suppresses embryonic T-box transcription factor 5 signal and causes congenital heart disease. Adv Sci (Weinh) 2022;9(15) doi: 10.1002/advs.202201034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Y.N., Cao L., Wang J., et al. Proteome profiling of early gestational plasma reveals novel biomarkers of congenital heart disease. EMBO Mol Med. 2023;15(12) doi: 10.15252/emmm.202317745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J.Y., Yang X.Y., Gong X.H., et al. Functional variant in methionine synthase reductase intron-1 significantly increases the risk of congenital heart disease in the Han Chinese population. Circulation. 2012;125(3):482–490. doi: 10.1161/CIRCULATIONAHA.111.050245. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J.Y., Yang X.Y., Shi K.H., et al. A functional variant in the cystathionine β-synthase gene promoter significantly reduces congenital heart disease susceptibility in a Han Chinese population. Cell Res. 2013;23(2):242–253. doi: 10.1038/cr.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J.Y., Qiao B., Duan W.Y., et al. Genetic variants reducing MTR gene expression increase the risk of congenital heart disease in Han Chinese populations. Eur Heart J. 2014;35(11):733–742. doi: 10.1093/eurheartj/eht221. [DOI] [PubMed] [Google Scholar]
- 15.Wang D., Wang F., Shi K.H., et al. Lower circulating folate induced by a Fidgetin intronic variant is associated with reduced congenital heart disease susceptibility. Circulation. 2017;135(18):1733–1748. doi: 10.1161/CIRCULATIONAHA.116.025164. [DOI] [PubMed] [Google Scholar]
- 16.Yu B., de Vries P.S., Metcalf G.A., et al. Whole genome sequence analysis of serum amino acid levels. Genome Biol. 2016;17(1):237. doi: 10.1186/s13059-016-1106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhre K., Shin S.Y., Petersen A.K., et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477(7362):54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee E.P., Ho J.E., Chen M.H., et al. A genome-wide association study of the human metabolome in a community-based cohort. Cell Metab. 2013;18(1):130–143. doi: 10.1016/j.cmet.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan X., Liang F., Zhu J., et al. Maternal exposure to PM2.5 and the risk of congenital heart defects in 1.4 million births: a nationwide surveillance-based study. Circulation. 2023;147(7):565–574. doi: 10.1161/CIRCULATIONAHA.122.061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson M., Razaz N., Edstedt Bonamy A.K., Villamor E., Cnattingius S. Maternal overweight and obesity and risk of congenital heart defects. J Am Coll Cardiol. 2019;73(1):44–53. doi: 10.1016/j.jacc.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Auger N., Fraser W.D., Sauve R., Bilodeau-Bertrand M., Kosatsky T. Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environ Health Perspect. 2017;125(1):8–14. doi: 10.1289/EHP171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Soysa T.Y., Ranade S.S., Okawa S., et al. Srivastava D. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature. 2019;572(7767):120–124. doi: 10.1038/s41586-019-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng W., Bais A., He H., et al. Single-cell transcriptomic analysis identifies murine heart molecular features at embryonic and neonatal stages. Nat Commun. 2022;13(1):7960. doi: 10.1038/s41467-022-35691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittemans L.B.L., Lotta L.A., Oliver-Williams C., et al. Assessing the causal association of glycine with risk of cardio-metabolic diseases. Nat Commun. 2019;10(1):1060. doi: 10.1038/s41467-019-08936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., Jernerén F., Lehne B.C., et al. Genome-wide association reveals that common genetic variation in the kallikrein-kinin system is associated with serum L-arginine levels. Thromb Haemost. 2016;116(6):1041–1049. doi: 10.1160/TH16-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z.Y., Wang J.Y., Li Z.X., et al. Branched-chain amino acids deficiency promotes diabetic neuropathic pain through upregulating LAT1 and inhibiting Kv1.2 channel. Adv Sci (Weinh) 2024;11(33) doi: 10.1002/advs.202402086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducker G.S., Rabinowitz J.D. One-carbon metabolism in health and disease. Cell Metab. 2017;25(1):27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piera-Velazquez S., Jimenez S.A. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev. 2019;99(2):1281–1324. doi: 10.1152/physrev.00021.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji-Tamura K., Sato M., Fujita M., Tamura M. Glycine exerts dose-dependent biphasic effects on vascular development of zebrafish embryos. Biochem Biophys Res Commun. 2020;527(2):539–544. doi: 10.1016/j.bbrc.2020.04.098. [DOI] [PubMed] [Google Scholar]
- 30.Verginadis I.I., Avgousti H., Monslow J., et al. A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat Cell Biol. 2022;24(6):940–953. doi: 10.1038/s41556-022-00918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao R., Cai K., Yang J.J., et al. Nuclear ATR lysine-tyrosylation protects against heart failure by activating DNA damage response. Cell Rep. 2023;42(4) doi: 10.1016/j.celrep.2023.112400. [DOI] [PubMed] [Google Scholar]
- 32.Wang D., Zhao R., Qu Y.Y., et al. Colonic lysine homocysteinylation induced by high-fat diet suppresses DNA damage repair. Cell Rep. 2018;25(2):398–412.e6. doi: 10.1016/j.celrep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 33.He X.D., Gong W., Zhang J.N., et al. Sensing and transmitting intracellular amino acid signals through reversible lysine aminoacylations. Cell Metab. 2018;27(1):151–166.e6. doi: 10.1016/j.cmet.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Saharinen P., Eklund L., Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017;16(9):635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 35.Kim K.H., Nakaoka Y., Augustin H.G., Koh G.Y. Myocardial angiopoietin-1 controls atrial chamber morphogenesis by spatiotemporal degradation of cardiac jelly. Cell Rep. 2018;23(8):2455–2466. doi: 10.1016/j.celrep.2018.04.080. [DOI] [PubMed] [Google Scholar]
- 36.Leppänen V.M., Saharinen P., Alitalo K. Structural basis of Tie2 activation and Tie2/Tie1 heterodimerization. Proc Natl Acad Sci U S A. 2017;114(17):4376–4381. doi: 10.1073/pnas.1616166114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly C., Pasnikowski E., Burova E., et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A. 2006;103(42):15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daly C., Wong V., Burova E., et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18(9):1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim I., Kim H.G., So J.N., Kim J.H., Kwak H.J., Koh G.Y. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res. 2000;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 40.Kim M., Allen B., Korhonen E.A., et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J Clin Invest. 2016;126(9):3511–3525. doi: 10.1172/JCI84871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei P., Lin D., Luo C., et al. High glucose promotes benign prostatic hyperplasia by downregulating PDK4 expression. Sci Rep. 2023;13(1) doi: 10.1038/s41598-023-44954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G., Guo X., Hong W., et al. Critical regulation of miR-200/ZEB2 pathway in Oct4/Sox2-induced mesenchymal-to-epithelial transition and induced pluripotent stem cell generation. Proc Natl Acad Sci U S A. 2013;110(8):2858–2863. doi: 10.1073/pnas.1212769110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J., Chen S., Zhuo L., Zhu Y., Zheng H. Regulation of cancer stem cell properties, angiogenesis, and vasculogenic mimicry by miR-450a-5p/SOX2 axis in colorectal cancer. Cell Death Dis. 2020;11(3):173. doi: 10.1038/s41419-020-2361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs Y., Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karimi Roshan M., Soltani A., Soleimani A., Rezaie Kahkhaie K., Afshari A.R., Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–234. doi: 10.1016/j.biochi.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Maisonpierre P.C., Suri C., Jones P.F., et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 47.Jain M., Nilsson R., Sharma S., et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blomstrand E., Eliasson J., Karlsson H.K., Köhnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136(1 Suppl):269S–273S. doi: 10.1093/jn/136.1.269S. [DOI] [PubMed] [Google Scholar]
- 49.Han J.M., Jeong S.J., Park M.C., et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149(2):410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 50.Yoon M.S., Son K., Arauz E., Han J.M., Kim S., Chen J. Leucyl-tRNA synthetase activates Vps34 in amino acid-sensing mTORC1 signaling. Cell Rep. 2016;16(6):1510–1517. doi: 10.1016/j.celrep.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hattori K., Naguro I., Runchel C., Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal. 2009;7:9. doi: 10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko Y.G., Kim E.Y., Kim T., et al. Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276(8):6030–6036. doi: 10.1074/jbc.M006189200. [DOI] [PubMed] [Google Scholar]
- 53.Guo M., Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol. 2013;9(3):145–153. doi: 10.1038/nchembio.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans-Anderson H.J., Alfieri C.M., Yutzey K.E. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102(6):686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.