Abstract
The Aedes aegypti mosquito is a vector for human arboviruses and zoonotic diseases, such as yellow fever, dengue, Zika, and chikungunya, and as such poses a serious threat to public health. Understanding how Ae. aegypti adapts to environmental pressures—such as insecticides—is critical for developing effective mitigation strategies. However, most traditional methods for detecting recent positive selection search for signatures of classic “hard” selective sweeps, and to date no studies have examined soft sweeps in Ae. aegypti. This represents a significant limitation as this is vital information for understanding the pace at which an organism can adapt—populations that are able to immediately respond to new selective pressures are expected to adapt more often via standing variation or recurrent adaptive mutations (both of which may produce soft sweeps) than via de novo mutations (which produces hard sweeps). To this end, we used a machine learning method capable of detecting hard and soft sweeps to investigate positive selection in Ae. aegypti population samples from Africa and the Americas. Our results reveal that soft sweep signatures are significantly more common than hard sweeps in all population samples, including those that have experienced population bottlenecks, which may imply that this species can respond quickly to environmental stressors. This is a particularly concerning finding for vector control methods that aim to eradicate Ae. aegypti through the use of insecticides. We highlight genes under selection that include both well-characterized and putatively novel insecticide resistance genes. These findings underscore the importance of using methods capable of detecting and distinguishing hard and soft sweeps, implicate soft sweeps as a major selective mode in Ae. aegypti, and highlight genes that may aid in the control of Ae. aegypti populations.
INTRODUCTION
A central goal of evolutionary biology is to uncover the genomic underpinnings of adaptation by identifying loci under positive selection. Researchers have made significant progress in this challenging task in a wide array of species. Some well-studied examples include: the hypoxia pathway gene EPAS1 that was implicated in differences in hemoglobin concentrations at high altitude in humans (Huerta-Sánchez et al. 2014) or the Ectodysplasin (EDA) locus in threespine stickleback fish that facilitated their transition from marine to freshwater (Barrett et al. 2008). These discoveries, as well as many others, have been made possible by theoretical and methodological advances which have allowed for the detection and characterization of selective sweep signatures.
In addition to revealing the loci underpinning adaptation, selective sweep signatures can be informative about the rate of adaptation, provided one can identify the type of sweep that occurred. Traditionally, selective sweeps were thought to occur when a de novo beneficial mutation appears and then quickly rises in frequency until it becomes fixed in a population (Smith and Haigh 1974). This type of selective sweep is referred to as a hard sweep and is characterized by a lack of diversity (except that which is introduced by recombination or mutation during the sweep) around the vicinity of the selected site (Smith and Haigh 1974). A hard sweep may also result in increased linkage disequilibrium (LD) on either side of the sweep (Kelly 1997; Kim and Nielsen 2004), or a skew in allele frequency patterns (Fay and Wu 2000; Nielsen et al. 2005). In contrast, soft sweeps act on a mutation that is initially neutral (or weakly deleterious) and evolves under drift until a change in the selective environment causes the mutation to become beneficial and sweep to fixation (Orr and Betancourt 2001; Hermisson and Pennings 2005). They may also occur when a beneficial allele arises via mutation or migration during the selective phase of a sweep (Pennings and Hermisson 2006a; Pennings and Hermisson 2006b). In soft sweeps, the beneficial allele exists on multiple haplotypes that share a common ancestor prior to the onset of the sweep and so the resulting skew in patterns of genetic diversity around the selected region may be both qualitatively different and less pronounced than that of a hard sweep (Prezeworski et al. 2005; Schrider et al. 2015). There is evidence that in at least some populations, adaptation proceeds mainly via soft sweeps (Garud et al. 2015; Schrider and Kern 2017; Xue et al. 2021). This in turn implies that these populations are able to adapt rapidly, as they need not wait for an adaptive mutation to arise (Karasov et al. 2010).
Sweep detection methods, which generally involve calculating single summary and/or test statistics (e.g., (Fay and Wu 2000; Kim and Nielsen 2004; Nielsen et al. 2005; Voight et al. 2006; Ferrer-Admetlla et al. 2014; Garud et al. 2015)), tend to be biased towards detecting hard sweeps because their more prominent genomic footprints are easier to detect (Teshima et al. 2006; Garud et al. 2015; Alachiotis and Pavlidis 2018; Weigand and Leese 2018). Even methods that are capable of detecting soft sweeps often lose potentially valuable information by reducing population genomic diversity to a single statistic (Schrider and Kern 2016). This endeavor is further complicated in populations with complex or unknown demographic histories because certain events, like bottlenecks, can mimic selective sweeps (Simonsen et al. 1995; Jensen et al. 2005; Nielsen et al. 2005). In the last few years, the field has made substantial progress in leveraging powerful machine learning algorithms that have been shown to outperform traditional methods (Flagel et al. 2019; Torada et al. 2019; Adrion et al. 2020; Caldas et al. 2022; Hejase et al. 2022; Mo and Siepel 2023; Whitehouse and Schrider 2023). ML approaches yield impressive discriminatory power as they either use a combination of several summary statistics as input (Pavlidis et al. 2010; Lin et al. 2011; Ronen et al. 2013; Schrider and Kern 2016; Alachiotis and Pavlidis 2018; Kern and Schrider 2018; Mughal et al. 2020; Arnab et al. 2023) or bypass this step entirely and train neural networks to work directly on genome alignments (Chan et al. 2018; Flagel et al. 2019; Torada et al. 2019; Adrion et al. 2020; Sanchez et al. 2021). Central to ML approaches are simulated training datasets which can be generated using estimated population-specific demographic histories. Doing so allows researchers to better approximate the distributions of patterns of polymorphisms produced by selection and neutrality even under more realistic and complex scenarios, thereby improving sweep-detection accuracy (Pybus Oliveras et al. 2015; Schrider and Kern 2016). Finally, some ML approaches have the added capacity to distinguish between hard and soft sweeps (Kern and Schrider 2018; Mughal and DeGiorgio 2019). This functionality is especially important in large, diverse populations where greater levels of standing variation and/or a higher population-scaled mutation rate may mean that adaptation predominantly occurs through soft sweeps (Messer and Petrov 2013).
As a globally distributed vector species with large population sizes and high levels of genetic diversity, the mosquito Aedes aeygpti poses a major threat to public health (Kent et al. 2024). Ae. aegypti transmits yellow and dengue fever, Zika, and chikungunya, making a thorough understanding of natural selection in this species critical for guiding mitigation and vector control strategies. Due to a combination of climate change which is making regions more suitable for Ae. aegypti, increased globalization, and their impressive capacity as an invasive species, Ae. aegypti populations are expanding in parts of Europe, Central America, east Africa, the United States, and Canada (Ryan et al. 2019; Iwamura et al. 2020). In addition to novel environmental stressors experienced during range expansion, Ae. aegypti has also encountered strong selective pressure in the form of insecticides for the better part of the last century (Love et al. 2023; Ware-Gilmore et al. 2023). This, coupled with their population sizes, genetic diversity levels, capacity to develop resistance to insecticides in as little as 5 generations, suggests that signatures of positive selection may be prevalent (Martins et al. 2012; Matthews et al. 2018; Thornton et al. 2020; Kent et al. 2024). However, efforts to detect such signals are complicated by the species’ varied and complex demographic histories.
Originally native to Africa, Ae. aegypti has, over the last four centuries, expanded its geographic range to include most of the world’s tropical belt (Ryan et al. 2019; Rose et al. 2023). Its movement from Africa to the Americas involved multiple introductions, bottlenecks, and expansion events and was likely facilitated by the high ship volume to the Americas during the transatlantic slave trade (Tabachnick 1991; Brown et al. 2014; Powell et al. 2018). Population size estimates and nucleotide diversity in African Ae. aegypti population samples are approximately 105–106 and 0.0370, respectively (Rose et al. 2023; Kent et al. 2024). These estimates are generally larger than those in Drosophila melanogaster—a species known for its large population sizes and high levels of genetic diversity (Terhorst et al. 2017; Kapopoulou et al. 2018). Most of the genetic studies performed on Ae. aegypti has relied on only a small number of genes, mitochondrial DNA, microsatellites, or reduced representation sequencing, and only recently have whole genome-based resequencing and exome-based sequencing been performed (Gonçalves da Silva et al. 2012; Brown et al. 2014; Bennett et al. 2016; Crawford et al. 2017; Matthews et al. 2018; Kelly et al. 2021a; Lozada-Chávez et al. 2025). This is largely due to the size (1.3 Gb) and repetitive nature of its genome which poses a significant challenge (Matthews et al. 2018).
Because whole-genome data for Ae. aegypti have only recently become available (Rose et al. 2020; Kelly et al. 2021b; Love et al. 2023), very little is known about recent positive selection in this species. Currently, sweep scans in Ae. aegpyti based on single-summary statistical approaches have reported positive selection in genomic regions linked to human preference (Rose et al. 2020), increased tolerance to egg desiccation (Venkataraman et al. 2022), and insecticide resistance (Saavedra-Rodriguez et al. 2019; Love et al. 2023; Schmidt et al. 2024; Lozada-Chávez et al. 2025). However, given their large population sizes, high levels of genetic diversity, and growing evidence to suggest that IR-increasing alleles may spread via soft sweeps in several insect species (Garud et al. 2015; Avalos et al. 2017; Xue et al. 2021; Muralidhar and Veller 2022), it is likely that traditional methods may be missing informative signatures of selection. Here, we leverage a robust machine learning approach (Kern and Schrider 2018) to identify genomic targets of positive selection from environmental and anthropogenic stressors in Ae. aegypti. We find that we are able to accurately detect both hard and soft sweeps in four globally distributed population samples with complex population size histories. Importantly, we identify novel insecticide resistance (IR) genes which will improve our understanding of the mechanisms of IR evolution and uncover evidence that soft sweeps may play a central role in adaptation in Ae. aegypti.
RESULTS
Accurate detection of hard and soft selective sweeps in Aedes aegypti
To identify genomic targets of selective sweeps in the yellow-fever mosquito, Aedes aegypti, we used a machine learning approach, diploS/HIC (Kern and Schrider 2018), which seeks to discriminate between hard sweeps, soft sweeps, regions linked to hard or soft sweeps, and purely neutrally evolving regions. We used previously published genomic data from Love et al., 2023 that examined mosquito population samples from Brazil (Santarém), Colombia (Río Claro and Cali), Gabon (Franceville), Senegal (Ngoye), and Kenya (Kaya Bomu). For each population sample, we applied a diploS/HIC classifier that was trained on simulations following the population size trajectory estimates from Kent et al. 2024 that were estimated using SMC++ (Terhorst et al. 2017). Our training simulations also included variation in mutation rates, recombination rates, and selective parameters such as the selection coefficient and the timing of the sweep (see Methods).
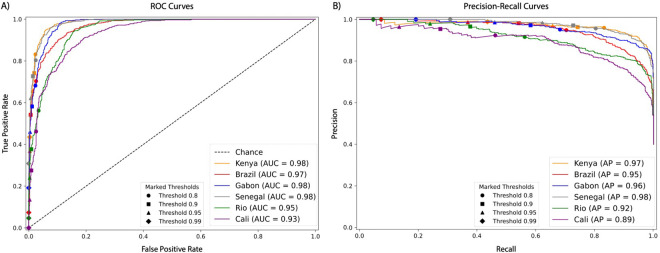
We assessed the performance of our classifiers through Receiver Operating Characteristic curves (ROC), Precision Recall (PR) curves, and confusion matrices (Figure 1). ROC curves measure true positive versus false positive rates across classification thresholds. An ideal classifier has a ROC curve that resembles a step-function with a true positive rate (or recall) as close to 1.0 as possible while achieving a false positive rate as close to 0 as possible. A PR curve measures the precision (or positive predictive value) against recall, with a good classifier being able to maintain high precision even at classification thresholds that yield high rates of recall. Here, our curves evaluate our models’ performance on the binary task of distinguishing selective sweeps (whether hard or soft) from unselected regions (whether sweep-linked or fully neutrally evolving). The excellent quality of our classifiers is clearly discernible by the ROC and PR curves which respectively show an area under the curve (AUC) value greater or equal to 0.97 and an Average Precision (AP) value greater or equal to 0.95 in all population samples except for the two Colombia population samples.
Figure 1.
ROC curves and precision-recall curves summarizing the performance of each population samples’ classifier. A) ROC curves showing the true and false positive rates for the binary classification task of distinguishing between selective sweeps (hard and soft) vs unselected regions (sweep-linked and neutral) with varying threshold cutoffs highlighted with different shapes. B) Precision-recall curves showing the classifiers performance at the same task (sweep vs unselected) with varying threshold cutoffs highlighted with different shapes. The precision is defined by the fraction of regions classified as sweeps that truly were sweeps and the recall is defined by the true positive rate. For the Cali classifier, there were no windows classified as a sweep with probability ≥0.99, so no marker is included on Cali’s precision-recall curve for that threshold.
While ROC and PR curves are useful for summarizing the overall accuracy of a binary classifier, for multi-class inferential models like our trained diploS/HIC neural networks, these curves are limited because they collapse diploS/HIC’s five classes down to two (selected and unselected regions), and thus they are not informative about the precise type of errors made within these two meta-classes. For example, if a hard sweep is correctly detected, how often is it misclassified as soft? Or, when neutrally evolving regions are misclassified as sweeps, are they generally classified as hard sweeps or soft sweeps? We therefore calculated confusion matrices, which show the fraction of test examples from each of the five classes that are correctly assigned to a given class. However, unlike the ROC and PR curves, the confusion matrices only show results from a single class membership probability threshold. We therefore made confusion matrices for a range of thresholds for each population sample (see Methods). Note that the class membership probabilities, which are produced by the softmax activation function from the final layer of the diploS/HIC neural network, may not necessarily be well calibrated such that 80% of examples classified as a hard sweep with 80% probability will be true hard sweeps.
In each population sample our ability to distinguish sweeps from neutral variation was strong and improved even further when imposing sweep probability thresholds (e.g., < 7.5% of neutral windows were misclassified as sweeping in each population sample with no sweep probability cutoff versus < 4.2% in each population sample at the 0.80 cutoff; Supplemental Figures 1–5). Our diploS/HIC classifiers had adequate power to distinguish hard from soft sweeps, albeit with overall higher accuracy in African population samples than the South American population samples (Supplemental Figures 1–5). For example, in Kenya with no sweep probability cutoff, diploS/HIC correctly classified 88% of hard sweeps and 68% of soft sweeps (Supplemental Figure 1); with a 0.80 threshold imposed this increased to 88% and 72%, respectively. This improvement is observed because simulations classified as sweeps but with a combined sweep probability lower than the classification threshold are treated as uncertain and are thus omitted from the calculation, and those high-confidence predictions that remain are more likely to be accurate. When applying cutoff values of 0.90, 0.95, and 0.99 to the Kenya classifier, accuracy values for hard sweeps stayed relatively constant at 88%, 88%, and 90%, respectively, as did soft sweeps at 71%, 69%, 66%. Conversely, for Cali (Supplemental Figure 1) with no cutoff, diploS/HIC correctly classified 80% of hard sweeps and 33% of soft sweeps and increasing the threshold to more stringent values did not dramatically improve these values (e.g., at 0.95, 77% of hard sweeps and 37% of soft sweeps were recovered). At the highest threshold of 0.99, there were no hard or soft sweep simulations for Cali passing the threshold, so the fraction of sweeps correctly predicted could not be calculated. Performance for the other Colombian sample (Río Claro) was similarly poor (Supplemental Figure 1–5), while performance for the Brazil, Gabon, and Senegal classifiers was strong (obtaining similar false positive rates as for Kenya but slightly lower accuracy overall). Given the comparatively poor performance of Colombia’s classifiers, we chose to focus our subsequent analyses on the other four population samples (Brazil, Gabon, Kenya, and Senegal), for which our simulated test data suggested that we could detect and classify sweeps with sufficient accuracy.
Soft sweeps appear to predominate over hard sweeps in Ae. aegypti
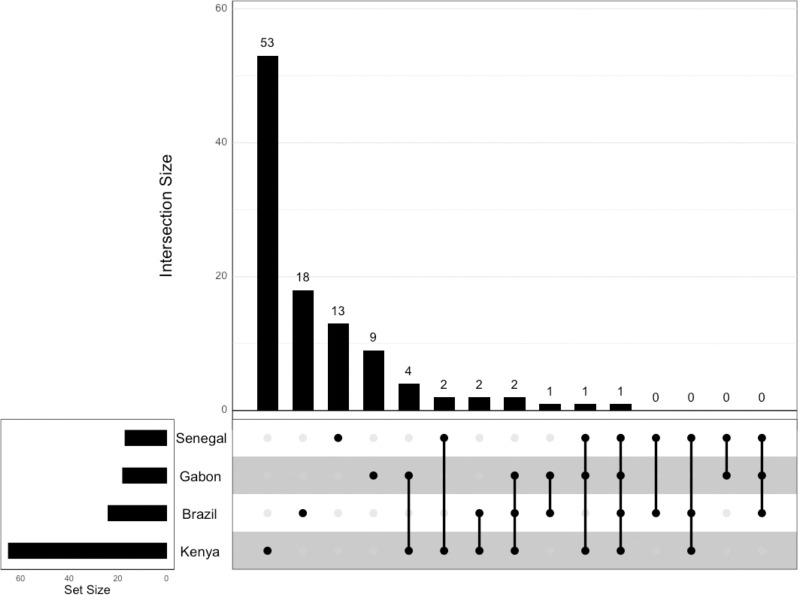
We classified a total of 4598, 4599, 4599, and 4599 windows that passed our data filtering cutoffs in the Brazil, Gabon, Senegal, and Kenya population samples, respectively. Of these windows, 165 were classified as sweeps in Brazil, compared to 150 in Gabon, 79 in Senegal, and 363 in Kenya (Table 1). To construct higher-confidence sets of sweep candidates, we then imposed increasing posterior probability cutoffs on these windows (Table 1). For example, when using a cutoff of 0.95, there were 24 sweep windows in Brazil, 18 in Gabon, 17 in Senegal, and 65 in Kenya. These windows comprise a set of 106 distinct candidate sweeps, some of which were shared by more than one population sample: 1 (0.94%) was shared across all population samples, 1 (0.94%) was shared among the African population samples, and 93 (87.7%) were population-specific sweeps (Figure 2). The remaining 11 (10.4%) sweeps were present in more than one population but did not fit into the categories above.
Table 1.
The number of hard and soft sweeps per population sample at different posterior probability thresholds.
| Pop Sample | No Cutoff | ≥0.80 | ≥ 0.90 | ≥0.95 | ≥0.99 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hard | Soft (%) | Hard | Soft (%) | Hard | Soft (%) | Hard | Soft (%) | Hard | Soft (%) | |
| Brazil | 8 | 157 (95.2%) | 4 | 75 (94.9%) | 1 | 44 (97.8%) | 1 | 23 (95.8%) | 0 | 6 (100%) |
| Gabon | 5 | 145 (96.7%) | 0 | 66 (100%) | 0 | 36 (100%) | 0 | 18 (100%) | 0 | 3 (100%) |
| Senegal | 8 | 71 (89.9%) | 5 | 37 (88.1%) | 4 | 24 (85.7%) | 2 | 15 (88.2%) | 0 | 3 (100%) |
| Kenya | 8 | 355 (97.8%) | 5 | 149 (96.8%) | 4 | 90 (95.7%) | 4 | 61 (93.9%) | 1 | 21 (95.5%) |
Figure 2.
Upset plot showing the intersection of selective sweep locations across the four population samples in this study based on the posterior probability cutoff of a sweep of 0.95.
Across all population samples, the number of soft sweeps was substantially higher than the number of hard sweeps and this pattern did not change with more stringent posterior probability thresholds (Table 1). We further evaluated the robustness of this finding by applying a conservative approach using the error rates in our confusion matrices to estimate the proportion of sweeps that were soft after accounting for hard sweeps that may have been misclassified as soft, and to estimate potential false positive soft sweeps that are truly neutrally evolving—this approach may underestimate the fraction of soft sweeps because we ignore the possibility of soft sweeps being misclassified as hard (see Methods). Even after accounting for these estimated error rates, we found that soft sweeps predominate at every threshold and that the relative proportion of soft sweeps generally increases or remains stable with more stringent threshold cutoffs. Indeed, at every threshold and every population sample, more than 72% of all sweeps are predicted to be soft (Supplemental Table 1). In all population samples other than Senegal, this (conservative) estimate was even higher: > 83.5% across all thresholds.
To further measure the degree of confidence in our sweep calls, we calculated q-values for each sweep window based on the combined posterior probabilities of hard and soft sweeps and our predicted false positive rates obtained from simulations for each population sample (Methods; see Supplemental Table 2 for the sweep candidates and their associated q-values). Notably, for those sweep windows with a combined posterior probability of ≥ 0.95, our q-value estimate could not be distinguished from zero (i.e., our neutral test simulations never achieved a sweep probability above this threshold) in all population samples except for Kenya (where sweeps passing the 0.95 threshold had an estimated q-value of ~0.4).
Insecticide Resistance Genes as Likely Targets of Selection
When examining candidate selective sweeps, we separately considered two sets of regions: shared sweeps (those with a posterior sweep probability of ≥0.95 in more than one population sample) and population-specific sweeps (those where only one population sample had a sweep probability ≥0.95). These two sets contained 13 and 93 sweep windows, respectively. Many of these candidate regions include genes with functions potentially linked to insecticide resistance. To better interpret their relevance, we categorized these genes into two groups: well-characterized IR genes and putative or emerging IR candidates. We assigned genes to these groups based on the nature and extent of evidence for their relevance to IR in the literature, including: (1) functional similarity to known IR genes, (2) differential expression in response to insecticide exposure, and (3) signatures of selection reported in other insect species under insecticide pressure. It is important to note that while we can identify compelling candidate genes within these sweep regions, there are often multiple genes in and around these windows, and we thus cannot conclude with certainty which gene is the target of selection.
Shared Sweep Windows Containing Putative or Emerging IR candidates
Of the 13 windows that were shared between 2 to 4 population samples, 3 windows contain candidate Ae. aegypti insecticide-resistance genes (see Supplemental Table 3 for the complete list). First, we found a high-confidence soft sweep shared in Kenya and Gabon (chr2: 452,250,001–452,500,000) that contains an ankyrin repeat domain-containing protein 29. This same region was predicted to be a soft sweep in Brazil but its sweep probability of 0.94 did not meet our threshold of 0.95. The ankyrin protein domain is a common protein-protein interaction motif that is present in numerous structural proteins and is involved in cytoskeletal anchoring and mechanosensation. Importantly, ankyrin proteins have been shown to directly interact with the well-established target for insecticides, the voltage-gated sodium channel VGSC (Williamson et al. 1996). In a study on pyrethroid resistant Ae. aegypti from Mexico, genes containing the ankyrin-domain were highly associated with pyrethroid resistance (Campbell et al. 2019). Similarly, in pyrethroid-resistant populations of the mosquito Anopheles funestus from Senegal, an ankyrin repeat domain protein was one of the most highly overexpressed genes (Samb et al. 2016). In Ae. aegypti, an ankyrin repeat domain-containing protein was found to be associated with pyrethroid resistance (Cosme et al. 2022), and signatures of selection in two regions containing ankyrin genes (along with other IR candidates) was also reported by Love et al., (2023). Similar patterns have also been observed in other insect species (Kwiatkowska et al. 2013; Gouesbet et al. 2025), further highlighting the role of these gene families in pyrethroid resistance.
The other two shared sweep windows containing putative IR genes were also classified as sweeps in Kenya and Gabon. The first contained a gene that encodes for the RNA binding protein Split ends (spen; chr2: 143,500,001–143,750,000) which provides a protective role against cytotoxicity from the herbicide paraquat in Drosophila (Girard et al. 2020; Bresgen et al. 2023). Although paraquat is an herbicide, it is known to cause oxidative stress in the mosquito An. gambiae (Champion and Xu 2018; Tarimo et al. 2018). The second window contains thioredoxin-2 (chr2: 45,000,001–45,250,000), a key mitochondrial protein that regulates cellular redox and is protective against oxidative stress, a common consequence of insecticide exposure, in Drosophila (J. Svensson and Larsson 2007). Interestingly, the thioredoxin system recycles oxidized glutathione to reduced glutathione, which can then be used by the well-established IR genes glutathione S-transferases (GSTs) for detoxification (Tarimo et al. 2018). Other components of the thioredoxin system, like thioredoxin peroxidase, have been shown to protect insects from insecticide-induced oxidative injury (Zhao et al. 2022; Gao et al. 2025) and thioredoxin is listed as of the candidate IR genes on the Anopheles gambiae 1000 Genomes Project Selection Atlas (Clarkson et al. 2020).
Population-Specific Sweep Windows Containing Well-Characterized IR Genes
We classified 93 windows as a sweep with ≥ 95% probability in a single population sample. Of these 93 windows, six contain genes with well-characterized IR-related functions. We describe three of these genes below (See Supplemental Text for descriptions of the other three genes) and explore their role in insecticide resistance.
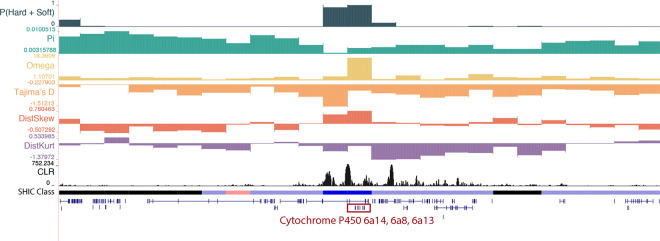
One window classified as a soft sweep in Senegal, located at chr1:271,250,001–271,500,000, contains three cytochrome P450 genes: Cyp 6a8, Cyp 6a13, Cyp 6a14 (Figure 3). Cytochrome P450 monooxygenases (CYPs) are critical for the metabolic detoxification and have been implicated in insecticide resistance in many species, including mosquitoes (Balabanidou et al. 2016; Rahman et al. 2021; Yang et al. 2021). Cyp6a8 specifically has been shown to be upregulated in response to Piper nigrum and DDT in D. melanogaster (Maitra et al. 1996; Jensen et al. 2006). Notably, this sweeping window also contained several SweepFinder Composite Likelihood Ratio (CLR) peaks (Nielsen 2005), with the highest value > 750. We also detected a soft sweep in Kenya in a window that contains cytochrome b5 reductase 4 (chr2:156,500,001–156,750,000). Cytochrome P450’s require cytochrome b5 reductase to function as an electron-transfer intermediate and the latter is therefore likely to be involved in IR (Zhao et al. 2012). Moreover, it has been shown to be upregulated in response to phenobarbital in the cotton bollworm, Helicoverpa armigera (Zhao et al. 2012) and has been linked to cyantraniliprole resistance in the tomato pest Tuta absoluta (Ullah et al. 2025).
Figure 3.
A soft sweep in Senegal at three cytochrome P450 genes: Cyp6a14, Cyp6a8, and Cyp6a13. The diploS/HIC classification track shows the class with the highest posterior probability, with soft sweeps as dark blue, soft sweep-linked regions as light blue, hard sweeps as red, hard sweep-linked as light red, and neutrally evolving regions in black. Above the diploS/HIC classifications are a subset of the summary statistics used by the classifier.
We detected a soft sweep in Brazil in a window containing a glutathione S-transferase (chr2:446,250,001–446,500,000), GSTD7, and that had a corresponding CLR peak with values > 460. Glutathione S-transferase epsilon clusters (GSTe) are another group of metabolic enzymes that are well-known for their role in resistance to several classes of insecticides (Enayati et al. 2005), and other GSTe genes have previously been found to exhibit strong signatures of positive selection (Love et al. 2023; Schmidt et al. 2024). GSTD7, specifically, was found to be under selection in the fall armyworm, Spodoptera frugiperda (Tessnow et al. 2025). Moreover, knockdown of GSTD7 resulted in higher mortality post-imidacloprid exposure in the silver whitefly Bemisia tabaci (He et al. 2018), and GSTD7 is highly expressed in Drosophila suzukii after exposure to malathion (Hamby et al. 2013).
Population-Specific Sweeps Windows with Putative or Emerging IR Candidates
Of these 93 windows that were classified as high-confidence sweeps, 13 contain genes with putative or emerging IR-related functions. We describe seven of these genes below and explore their role in insecticide resistance (see Supplemental Table 4 for the complete list). The remaining six genes and associated role in IR are included in the Supplementary Text.
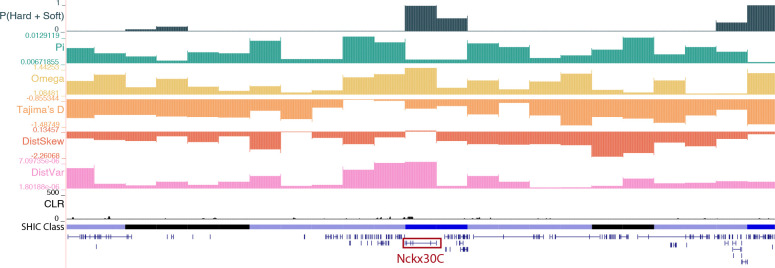
We found three high-confidence windows that were classified as soft sweeps in Kenya which contained arrestin (chr2:246,250,001–246,500,000) and overlapped with the sodium/potassium/calcium exchanger NCKX30C (chr2:288,750,001–289,000,000; Figure 4). In vertebrates, arrestins regulate the signaling and trafficking of G-protein-coupled receptors (Gurevich and Gurevich 2006), and in the mosquito Culex pipiens pallens, arrestin expression was higher in deltamethrin-resistant strains and knockdown via siRNA resulted in decreased viability after deltamethrin treatment (Sun et al. 2012). NCKX30C is likely involved in the maintenance of calcium homeostasis (Haug-Collet et al. 1999; Webel et al. 2002) and was found to be differentially regulated in insecticide resistant strains of An. gambiae (Vontas et al. 2005). The last window classified as a hard sweep in Kenya contains mitochondrial protoheme IX farnesyltransferase (chr2:262,250,001–262,500,000), or COX10. COX10 encodes for an enzyme that catalyzes the farnesylation of heme, which plays a crucial role in cytochrome c oxidase (COX) function (Diaz et al. 2006). COX genes have been linked to insecticide resistance in a number of species (Steele et al. 2018), including D. melanogaster (Song and Scharf 2009), the housefly Musca domestica (Sacktor 1951), the German cockroach Blattella germanica (Pridgeon and Liu 2003), and Ae. aegypti (Pridgeon et al. 2009).
Figure 4.
A soft sweep in Kenya at the sodium/potassium/calcium exchanger NCKX30C. The diploS/HIC classification track shows the class with the highest posterior probability, with soft sweeps as dark blue, soft sweep-linked regions as light blue, hard sweeps as red, hard sweep-linked as light red, and neutrally evolving regions in black. Above the diploS/HIC classifications are a subset of the summary statistics used by the classifier.
In Brazil, we found sweeping windows that contain or overlap the muscle calcium channel subunit alpha-1 (chr2:225,750,001–226,000,000), potassium voltage-gated channel subfamily KQT member 1 (chr2:144,750,001–145,000,000), neuroligin-1 (chr3:351,750,001–352,000,000), and thioredoxin-related transmembrane protein 1 (chr3:12,250,001–12,500,000). Of these four windows, three are classified as soft sweeps while the second window is classified as a hard sweep. Further, the first, third, and fourth window all contain CLR peaks with values greater than 475, 280, and 900, respectively. The second window was also initially classified as a soft sweep in Kenya, although the posterior probability (0.83) did not meet our threshold. Although the role of both the muscle calcium channel subunit alpha-1 and the potassium voltage-channel subfamily KQT member 1 in IR is not well understood, both mechano-susceptible and voltage-gated channels are confirmed targets of various insecticide and pesticide classes. Neuroligin-1 is a synaptic cell-adhesion molecule (Song et al. 1999) that is highly associated with pyrethroid resistance in Ae. aegypti (Campbell et al. 2019), likely a result of insecticides disrupting excitatory synapses. Similar to the thioredoxin-2 sweep window described above, thioredoxin-related transmembrane protein 1 (Trx1) also plays a role in protection against oxidative stress in insects (Zhang et al. 2015) and has been shown to be involved in antioxidant defense in Apis cerana cerana (Yao et al. 2014).
In the Supplementary Text, we discuss additional candidate sweep regions containing the following putative IR genes: acyl-CoA synthetase family member 4, fatty acyl-CoA reductase 1, membrane-associated progesterone receptor component 1, D(2) dopamine receptor A, transcription factor grauzone, and NADH dehydrogenase 1 beta subcomplex subunit 5,
DISCUSSION
Natural selection leaves conspicuous footprints in the genome, and our ability to accurately detect these signatures has significant implications for public health, agriculture, and conservation. While early methods focused heavily on detecting hard selective sweeps, growing evidence supports the widespread occurrence of soft sweeps, particularly in large, diverse populations. Soft sweeps leave a more subtle signature than hard sweeps and are therefore more difficult to detect with traditional approaches. Machine learning has emerged as a powerful tool in this space, well-suited to detecting high-dimensional signals of selection and capable of accounting for complex demographic histories that confound inferences. This is an important advancement for species like Aedes aegypti—a major disease vector with a global impact on public health—which poses a significant challenge to evolutionary analyses due to its high degree of genetic variation and complex demographic history. Here we leverage the machine learning tool diploS/HIC (Kern and Schrider 2018) and recently published demographic history estimates (Kent et al. 2024) to uncover the targets of recent positive selection in Ae. aegypti and provide evidence that soft sweeps may play a central role in adaptive evolution in this important vector species.
This work contributes to a growing body of literature that challenges the traditional view that selective sweeps mainly act on a single-origin de novo mutation. Indeed, our work mirrors the findings of similar studies performed on humans (Schrider and Kern 2017), D. melanogaster (Garud et al. 2015), Anopheles gambiae (Weedall et al. 2020; Xue et al. 2021), and HIV (Feder et al. 2016), that implicate soft selective sweeps as the dominant mode of adaptation (although these studies have proved controversial; see (Harris et al. 2018; Schrider and Kern 2018; Feder et al. 2021; Garud et al. 2021; Johri et al. 2022)). In fact, in all population samples, and at every sweep probability threshold, the percentage of sweeps that were soft was always > 85%. When we applied a conservative approach designed to account for the potential impact of false positives and of soft sweeps being misclassified as hard (while ignoring the possibility of misclassification in the opposite direction), the percentage of soft sweeps was still > 72% in all population samples and thresholds. This suggests that selection in Ae. aegypti can act on standing genetic variation instead of waiting for novel mutations to arise, and Ae. aegypti populations are therefore able to rapidly respond to new selective pressures like insecticides. Soft sweeps even predominated in Brazil—a population sample that has experienced a protracted bottleneck associated with the introduction from Africa to the Americas (Rose et al. 2023; Kent et al. 2024). This suggests that even bottlenecked populations of Ae. aegypti may have sufficient standing variation (or a high enough population-scaled mutation rate; see below) to facilitate rapid adaptation. Out of every population sample, Kenya had substantially more high-confidence soft selective sweeps predicted than any other population sample (61 in Kenya, compared to 23, 18, 15 in Brazil, Gabon and Senegal, respectively, when considering sweeps with a posterior probability ≥ 0.95). This is in line with the findings presented in Kent et al. (2025) where Kenya was inferred to have a larger effective population size than the other population samples and therefore would be expected to experience efficacious natural selection and more soft sweeps.
Our findings are predicated on modeling soft sweeps as selection on standing variation, which is plausible given the high levels of genetic diversity observed. Alternatively, selection on recurrent de novo mutations can also produce sweep signatures similar to that of a soft sweep—albeit typically less pronounced–with multiple haplotypes carrying the beneficial allele (Pennings and Hermisson 2006b). Such sweeps are especially likely in species where the number of new mutations entering the population each generation is large, either due to large population size and/or a high spontaneous mutation rate (Pennings and Hermisson 2006b; Karasov et al. 2010; Garud et al. 2015). As a result, our machine learning models may detect signals of both selection on standing variation and on recurrent mutations, both of which are a sign of a population that need not wait for long periods for a de novo beneficial mutation to arise following a change in the selective environment.
On the other hand, there are several phenomena that may result in signatures of soft sweeps even in the absence of rapid adaptation. First, patterns of genetic diversity in a genomic region flanking a hard sweep can mirror that of a soft sweep (the “soft shoulder” effect; (Schrider et al. 2015)). However, this is an unlikely explanation for our results given that we detected very few hard sweeps in our population samples, and diploS/HIC is designed to account for this shoulder effect by examining polymorphisms across a larger window containing the sweep (Schrider and Kern 2016). Second, allelic gene conversion can “soften” hard selective sweeps by transferring the beneficial mutation onto multiple haplotypes (Jones and Wakeley 2008; Schrider et al. 2015; Schrider 2023). This is more common in large populations where there is more time for gene conversion events to occur (Schrider 2023). Third, the dearth of hard sweeps across all population samples may result from the inherent difficulty in accounting for spatial population structure when detecting selective sweeps. In low-dispersal scenarios (which are common in species with wide geographic ranges) an adaptive mutation cannot rise in frequency as rapidly as it would in a panmictic population. As a result, hard selective sweeps can become enriched in intermediate-frequency variants which can cause them to resemble soft sweeps (Chotai et al. 2024). Thus, the expectation for large populations like those examined here might be that we would detect more soft sweeps than hard sweeps even without selection acting on standing genetic variation or recurrent mutations. Nonetheless, there is direct evidence that Ae. aegypti can adapt rapidly to insecticides (Martins et al. 2012), and Lozada-Chávez et al. (2025) recently reported evidence of selection in out-of-Africa population samples on variants that are segregating in Africa. Thus, true soft sweeps may therefore represent the most parsimonious explanation for the patterns we observe.
While the interpretation of soft sweep signatures is not straightforward, the above arguments suggest that it is essential for selection scans in large/diverse populations to consider soft sweeps in order to detect a larger fraction of the targets of recent positive selection. Previous studies have detected selection on several IR-related genes, including the voltage-gated sodium channel gene (VGSC), glutathione S-transferases (GSTs), ace-1, carboxylesterases, and many cytochrome P450 genes (Love et al. 2023; Schmidt et al. 2024). While these studies have provided insight into how mosquitoes develop resistance to insecticides (e.g., through metabolic detoxification or variation in the insecticide target location (Kliot and Ghanim 2012; Yahouédo et al. 2017)) they did not consider the possibility of soft sweeps, and thus may have missed additional targets of selection. Here we identified both shared and population-specific sweep windows that were almost entirely classified as soft sweeps (96.8% and 93.5%, respectively). Within these windows, we identified 22 genes with either well-characterized or putative roles in IR. To the best of our knowledge, several of our highlighted IR candidates (e.g., ankyrin repeat domain-containing protein 29, split ends, sodium/potassium/calcium exchanger NCKX30C, muscle calcium channel subunit alpha-1, or potassium voltage-gated channel subfamily KQT member 1), have not been reported as IR genes in Ae. aegypti and thus represent novel targets for functional validation. Interestingly, of our 13 shared selective sweep windows, only two of them had corresponding CLR peaks (defined by a CLR value > 200) and in our population-specific dataset, only 22% had corresponding CLR peaks. These results make sense given that CLR is based on a hard selective sweep model and the majority of our selective sweeps were classified as soft, further highlighting the need for methods specifically designed to detect the signatures of soft selective sweeps in order to more fully appreciate the landscape of adaptive evolution.
Although we were able to identify several novel IR candidate regions by searching for selective sweeps, we note that IR is a polygenic trait. One might therefore expect polygenic selection, in which the selected phenotype moves towards its optimum through the combined effect of small shifts in the frequencies of a large number of small-effect alleles (Berg and Coop 2014; Stephan 2016; Höllinger et al. 2019; Hayward and Sella 2022), to be the primary mode of adaptation in Ae. aegypti. Indeed, polygenic adaptation has been implicated in the evolution of complex traits, including insecticide resistance (Chen et al. 2023; Hobbs et al. 2023). However, selective sweeps may still occur during a polygenic shift to a new fitness optimum (Thornton 2019), which may explain our success in finding sweep candidates at both known and potentially novel IR loci, even though diploS/HIC would be underpowered to detect more subtle allele frequency shifts that may also be contributing to adaptation for IR and other traits in Ae. aegypti.
While our examination of candidate sweep regions primarily focuses on genes associated with IR, we note that other traits may also be subject to recent positive selection. Given Ae. aegypti’s broad and expanding geographic distribution, robust invasive capacity, and the impact of ongoing climate change, it is highly likely some of the sweeps reported here are unrelated to IR. This is particularly relevant for the population samples from Brazil and Senegal, which are human specialists unlike the Kenya and Gabon samples which exhibit the ancestral generalist feeding behavior. The Senegal and Brazil samples thus have experienced additional selective pressures associated with domestication (e.g., adapting to urban environments and acquiring a preference for feeding on human blood; (Rose et al. 2020; Lozada-Chávez et al. 2025)). Although we do not analyze non-IR genes in this work, we provide a comprehensive list of all sweep calls with ≥0.95 confidence in any population sample in Supplemental Tables 3 and 4. Our focus on IR-related genes reflects both the urgency of addressing developing IR, as well as the rich body of existing literature on these genes, which helps facilitate the challenging task of interpreting sweep signals.
In summary, in this study we leveraged whole-genome data from multiple Ae. aegypti population samples together with a powerful machine learning tool to enrich our understanding of the genomic targets and mode of positive selection in this important vector species. We discovered several novel insecticide resistance candidate genes, suggesting that current surveillance efforts may be missing variants/loci that contribute substantially to segregating resistance. More broadly, our results suggest that insect vectors, particularly those with large, diverse populations, can rapidly adapt to insecticides and other control strategies, perhaps through selection on standing genetic variation, recurrent originations of adaptive alleles, and/or polygenic selection. This in turn may imply the presence of many additional adaptive variants that have been missed by previous scans for selection in other insect pests.
MATERIALS AND METHODS
Sampling, Sequencing, and Variant Calling
In this study, we used a previously curated whole genome sequencing dataset of 104 individuals from (Love et al. 2023). This dataset includes 18 samples from Santarém, Brazil; 13 from Franceville, Gabon; 19 from Kaya Bomu, Kenya; and 20 from Ngoye, Senegal, all taken from Rose et al. (2020), as well as 10 and 24 samples from Cali and Río Claro, Colombia, respectively, sequenced in Love et al. (2023). Detailed methods for DNA extractions, sequencing, and variant calling and filtering are available in (Love et al. 2023). Briefly, reads from all individuals were aligned to the AaegL5 reference genome (cite; NCBI accession GCF_002204515.2) using bwa-mem2 v. 2.1 (Vasimuddin et al. 2019), indels were removed and SNPs were filtered following GATK’s best practices (Koboldt 2020). Repetitive regions, non-biallelic SNPs, and non-uniquely mappable regions were removed and genotypes with qualities less than 20 were masked.
Using Classifiers to Detect Selective Sweeps
To detect selective sweeps, we used diploS/HIC, a supervised machine learning approach that has been shown to be robust to nonequilibrium demography and has the capacity to distinguish between hard and soft sweeps in unphased data (Schrider and Kern 2016; Kern and Schrider 2018). In short, diploS/HIC classifies individual genomic windows into five categories (hard sweep, soft sweep, hard-linked, soft-linked, or neutral) based on a vector of windowed and transformed summary statistics that are input into a convolutional neural network (CNN). While this classification approach allows for robust inference based on many features jointly, it requires training datasets that consist of examples known to belong to each class. Therefore, we generated the training data using discoal (Kern and Schrider 2016). DiploS/HIC then subdivides each simulated region into a number of equally sized, adjacent subwindows; we used the default value of 11 subwindows. For hard and soft sweeps, we selected the location of selection from a uniform distribution within each of the 11 subwindows. Specifically, we conducted 3,000 simulations where selection occurred uniformly within the leftmost subwindow, another 3,000 for the second subwindow, and so on for all 11 subwindows.
We sought to train each population sample’s classifier on simulations under demographic models that provide a reasonable fit to that population sample’s genomic data. To this end, we used Kent et al (2025)’s population size histories, which were estimated using SMC++ for each population sample examined here. To account for variation in mutation and recombination rates across the genome, we allowed these parameters to vary across our training replicates. We set our mutation rate (μ) to vary uniformly from 8.82×10−10 to 8.82×10−9, giving a mean value of 4.85×10−9 which matches the estimate from Rose et al. (2023). We drew the recombination rate (r) from an exponential distribution with mean 4.85×10−9 (with values greater than 3*r not allowed), choosing this mean value because the average recombination rate in Ae. aegypti appears to be similar to our mean value of μ (Matthews et al. 2018; Chen et al. 2022); note that because this is a truncated exponential distribution the true mean value of r will be somewhat lower than our mean parameter value.
For the simulations involving sweeps, there are several additional parameters whose distributions are unknown, like the strength and timing of selection. For these, we drew from a wide distribution to ensure that the range of parameters seen during training encompasses those likely encountered during downstream inference. Specifically, we drew the selection coefficient from a log-uniform distribution ranging from 0.005 to 0.05 and the time of fixation of the beneficial allele, from U(0, 0.001). For soft and soft-linked sweeps, we simulated selection on a previously neutral standing variant, drawing the frequency of the previously neutral allele at the onset of selection, (f0), from U(0, 0.05). In total, we generated 3,000 simulated replicates for each class for each population. Because our goal was to identify strong selective sweeps that affect a wide stretch of the chromosome, we sought to simulate 2.75 Mb regions, but discoal was unable to simulate such regions due to memory constraints. We therefore simulated 550 kb regions and decreased the selection coefficient to U(0.001, 0.01), resulting in simulated sweeps with the same ratio of s/r as we would have obtained from 2.75 Mb simulations with s~U(0.005, 0.05).
As described above, in our real data we masked genomic positions that had genotype qualities below 20. To incorporate heterogeneity in data quality into our training/test data, for each simulated window, we randomly selected a corresponding window from our empirical dataset and masked the same sites in the simulated window that had been masked in the empirical window. This ensured that our masking procedure affected our simulated data in the same way as our real data. Feature vectors were then calculated for each simulation replicate. These features vectors measure the spatial patterns of a number of population genetic summary statistics, including π (Tajima 1983), (Watterson 1975), Tajima’s D (Tajima 1989), the number of distinct haplotypes, average haplotype homozygosity (also referred to as H1 by Garud et al. 2015), H12 and H2/H1 (Garud et al. 2015), Zns (Kelly 1997), and the maximum value of Kim and Nielsen’s ω (Kim and Nielsen 2004); note that the latter two statistics, which measure linkage disequilibrium, were based on calculations of Rogers and Huff’s estimator of LD for unphased data (Rogers and Huff 2009). In addition to these commonly used statistics, diploS/HIC also includes in its feature vector estimates of the variance, skewness, and kurtosis of the distribution of the number of pairwise differences for all pairs of individuals in the sample; these summaries are useful for detecting hard and soft sweeps and discriminating between them (Kern and Schrider 2018). DiploS/HIC subdivides the simulated region into 11 adjacent windows and calculates each of these statistics in each window, dividing that value by the sum of values for that statistic across all 11 subwindows. If the smallest of these 11 values for a given statistic is less than 0, the value for this subwindow is increased to zero, and the value for each other subwindow is increased by the same amount (i.e., the absolute value of the smallest subwindow is added to each subwindow in such cases).
After computing summary statistics, for each population sample we used diploS/HIC’s makeTrainingSets command to combine our simulations into a training set of 13,500 examples: 2,700 each for the hard sweep class (consisting of simulations where a hard sweep occurred in the central subwindow), the hard-linked class (where a hard sweep occurred in any subwindow but the central one), the soft sweep class (where a soft sweep occurred in the central subwindow), the soft-linked class (where a soft sweep occurred in any other subwindow), and the neutral class (where no sweep occurred). Similarly, we constructed an independent test set of 1,500 examples, 300 of each of the five classes. We then used diploS/HIC to train a CNN classifier for each population, holding out 10% of the training simulations as a validation set used for early stopping during training—if 5 consecutive epochs of training yielded no improvement (defined as a decrease in the categorical cross entropy loss function of at least 0.001), then training terminated and the best-performing set of neural network weights up to that point was used for the final classifier. We then applied each population sample’s classifier to its corresponding test set and evaluated performance using confusion matrices, precision recall curves (PR), and receiver operating characteristic (ROC) curves.
After training our classifiers, we then calculated feature vectors on the genomic data from the corresponding Ae. aegypti population samples, again using 2.75 Mb windows (each subdivided into 11 adjacent 250 kb subwindows). We next applied our classifiers to the genomic data, and we used diploS/HIC’s posterior class membership probability estimates in order to experiment with four different thresholds: 0.80, 0.90, 0.95, and 0.99. For a given threshold, we required the sum of the windows’ hard and soft sweep posterior probabilities to be greater or equal to the threshold before labelling the window as a sweep, and all windows whose highest-probability class was hard or soft but for whom the sweep probability threshold was not met were treated as uncertain and thus were not assigned to a class. We plotted the intersections of selective sweep locations for all populations for a given threshold using the UpsetR package (Conway et al. 2017).
Accounting for the Impact of Classification Errors on the Relative Numbers of Hard and Soft Sweeps
To investigate how false positive rates may impact our ratio of hard sweep to soft sweep calls, we calculated the expected number of false discoveries obtained at a given sweep probability threshold for a specified population sample by using the q-value obtained at that threshold. We then made the conservative assumption that all false sweep discoveries are classified as soft sweeps and reduced our expected number of soft sweeps accordingly. We then examined the confusion matrix for the corresponding probability threshold to obtain the fraction of hard sweeps that are misclassified as soft, further reducing our total number of expected soft sweeps—during this step we conservatively ignored the possibility of soft sweeps being misclassified as hard.
Identifying Novel High-Confidence Sweep Candidates
To generate a list of high-confidence regions that are sweeping in one or more population samples, we imposed sweep probability cutoffs as described above. We also calculated false discovery q-values for each sweep candidate as follows: first, for each sweep in each population sample, we obtained the sweep probability score from diploS/HIC, treating it as our current threshold, and then counted the numbers of both real and simulated sweeps that were equal to or exceeded this threshold. We then obtained the false discovery rate (FDR) by comparing the estimated number of neutral simulations misclassified as a sweep to the total number of predicted sweeps. Once we obtained an FDR for each candidate sweep and its corresponding threshold, q-values were calculated as described in (Storey and Siegmund 2001). This procedure was done separately for each population sample.
Because diploS/HIC is designed to detect the changes in diversity summaries at varying recombination distances away from a sweep, it cannot produce accurate predictions in non-recombining regions of the genome. We therefore calculated weighted average recombination rates in each of our predicted windows (using data from (Matthews et al. 2018)) and removed any regions where the recombination rate was equal to zero. Finally, to exclude regions of low confidence, we removed regions where the fraction of sites that were masked according to the data filtering criteria described above exceeded 0.85. Our set of high-confidence, shared sweep candidates was generated by ensuring that the combined posterior probabilities of a sweep was ≥0.95 in all populations that contained the sweep. Similarly, the set of high-confidence population-specific sweeps consisted of windows where a sweep was predicted with ≥0.95 probability in a single population sample. Visualization of sweep candidates was performed using the UCSC Genome Browser (Kent et al. 2002; Perez et al. 2025), along with custom tracks highlighting several population genetic summary statistics calculated by diploS/HIC and CLR scores (Nielsen 2005; DeGiorgio et al. 2016) generated in Love et al., 2023. When searching for potential targets of selection, we focused on genes that were either contained entirely within or partially overlapped the sweeping window, while noting that it is possible that in some cases selection may be acting on a regulatory region impacting the expression of a gene lying outside of this window.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank members of the Schrider lab for useful discussion. This work was funded by NIH awards R01HG010774 and R35GM138286.
REFERENCES
- Adrion JR, Galloway JG, Kern AD. 2020. Predicting the landscape of recombination using deep learning. Molecular Biology and Evolution 37:1790–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alachiotis N, Pavlidis P. 2018. RAiSD detects positive selection based on multiple signatures of a selective sweep and SNP vectors. Communications Biology 1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnab SP, Amin MR, DeGiorgio M. 2023. Uncovering footprints of natural selection through spectral analysis of genomic summary statistics. Molecular Biology and Evolution 40:msad157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos A, Pan H, Li C, Acevedo-Gonzalez JP, Rendon G, Fields CJ, Brown PJ, Giray T, Robinson GE, Hudson ME. 2017. A soft selective sweep during rapid evolution of gentle behaviour in an Africanized honeybee. Nature Communications 8:1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanidou V, Kampouraki A, MacLean M, Blomquist GJ, Tittiger C, Juárez MP, Mijailovsky SJ, Chalepakis G, Anthousi A, Lynd A. 2016. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proceedings of the National Academy of Sciences 113:9268–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RD, Rogers SM, Schluter D. 2008. Natural selection on a major armor gene in threespine stickleback. Science 322:255–257. [DOI] [PubMed] [Google Scholar]
- Bennett KL, Shija F, Linton Y-M, Misinzo G, Kaddumukasa M, Djouaka R, Anyaele O, Harris A, Irish S, Hlaing T. 2016. Historical environmental change in Africa drives divergence and admixture of Aedes aegypti mosquitoes: a precursor to successful worldwide colonization? Molecular Ecology 25:4337–4354. [DOI] [PubMed] [Google Scholar]
- Berg JJ, Coop G. 2014. A population genetic signal of polygenic adaptation. PLoS Genetics 10:e1004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresgen N, Kovacs M, Lahnsteiner A, Felder TK, Rinnerthaler M. 2023. The janus-faced role of lipid droplets in aging: insights from the cellular perspective. Biomolecules 13:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, Egizi A, Zhao H, Caccone A, Powell JR. 2014. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 68:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas IV, Clark AG, Messer PW. 2022. Inference of selective sweep parameters through supervised learning. bioRxiv:2022.07. 19.500702. [Google Scholar]
- Campbell CL, Saavedra-Rodriguez K, Kubik TD, Lenhart A, Lozano-Fuentes S, Black IV WC. 2019. Vgsc-interacting proteins are genetically associated with pyrethroid resistance in Aedes aegypti. PLoS One 14:e0211497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion CJ, Xu J. 2018. Redox state affects fecundity and insecticide susceptibility in Anopheles gambiae. Scientific Reports 8:13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Perrone V, Spence J, Jenkins P, Mathieson S, Song Y. 2018. A likelihood-free inference framework for population genetic data using exchangeable neural networks. Advances in Neural Information Processing Systems 31. [PMC free article] [PubMed] [Google Scholar]
- Chen C, Compton A, Nikolouli K, Wang A, Aryan A, Sharma A, Qi Y, Dellinger C, Hempel M, Potters M. 2022. Marker-assisted mapping enables forward genetic analysis in Aedes aegypti, an arboviral vector with vast recombination deserts. Genetics 222:iyac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Cohen ZP, Bueno EM, Christensen BM, Schoville SD. 2023. Rapid evolution of insecticide resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Current Opinion in Insect Science 55:101000. [DOI] [PubMed] [Google Scholar]
- Chotai M, Wei X, Messer PW. 2024. Signatures of selective sweeps in continuous-space populations. bioRxiv. [DOI] [PubMed] [Google Scholar]
- Clarkson CS, Miles A, Harding NJ, Lucas ER, Battey CJ, Amaya-Romero JE, Kern AD, Fontaine MC, Donnelly MJ, Lawniczak MK. 2020. Genome variation and population structure among 1142 mosquitoes of the African malaria vector species Anopheles gambiae and Anopheles coluzzii. Genome Research 30:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JR, Lex A, Gehlenborg N. 2017. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33:2938–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme LV, Lima JBP, Powell JR, Martins AJ. 2022. Genome-wide association study reveals new loci associated with pyrethroid resistance in Aedes aegypti. Frontiers in Genetics 13:867231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JE, Alves JM, Palmer WJ, Day JP, Sylla M, Ramasamy R, Surendran SN, Black WC, Pain A, Jiggins FM. 2017. Population genomics reveals that an anthropophilic population of Aedes aegypti mosquitoes in West Africa recently gave rise to American and Asian populations of this major disease vector. BMC Biology 15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio M, Huber CD, Hubisz MJ, Hellmann I, Nielsen R. 2016. SweepFinder2: increased sensitivity, robustness and flexibility. Bioinformatics 32:1895–1897. [DOI] [PubMed] [Google Scholar]
- Diaz F, Fukui H, Garcia S, Moraes CT. 2006. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Molecular and cellular Biology 26:4872–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayati AA, Ranson H, Hemingway J. 2005. Insect glutathione transferases and insecticide resistance. Insect Molecular Biology 14:3–8. [DOI] [PubMed] [Google Scholar]
- Fay JC, Wu C-I. 2000. Hitchhiking under positive Darwinian selection. Genetics 155:1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder AF, Pennings PS, Petrov DA. 2021. The clarifying role of time series data in the population genetics of HIV. PLoS Genetics 17:e1009050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder AF, Rhee S-Y, Holmes SP, Shafer RW, Petrov DA, Pennings PS. 2016. More effective drugs lead to harder selective sweeps in the evolution of drug resistance in HIV-1. Elife 5:e10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Admetlla A, Liang M, Korneliussen T, Nielsen R. 2014. On detecting incomplete soft or hard selective sweeps using haplotype structure. Molecular Biology and Evolution 31:1275–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel L, Brandvain Y, Schrider DR. 2019. The unreasonable effectiveness of convolutional neural networks in population genetic inference. Molecular Biology and Evolution 36:220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Liu D-Y, Deng Q, Feng W-L, Yu J-M, Li M-Y, Liu S. 2025. Characterization of the thioredoxin peroxidase gene and its role in lambda-cyhalothrin tolerance in Agrotis ipsilon (Lepidoptera: Noctuidae). Journal of Asia-Pacific Entomology 28:102403. [Google Scholar]
- Garud NR, Messer PW, Buzbas EO, Petrov DA. 2015. Recent selective sweeps in North American Drosophila melanogaster show signatures of soft sweeps. PLoS Genetics 11:e1005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garud NR, Messer PW, Petrov DA. 2021. Detection of hard and soft selective sweeps from Drosophila melanogaster population genomic data. PLoS Genetics 17:e1009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard V, Goubard V, Querenet M, Seugnet L, Pays L, Nataf S, Dufourd E, Cluet D, Mollereau B, Davoust N. 2020. Spen modulates lipid droplet content in adult Drosophila glial cells and protects against paraquat toxicity. Scientific Reports 10:20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves da Silva A, Cunha IC, Santos WS, Luz SL, Ribolla PE, Abad-Franch F. 2012. Gene flow networks among American Aedes aegypti populations. Evolutionary Applications 5:664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouesbet G, Renault D, Derocles SA, Colinet H. 2025. Strong resistance to β-cyfluthrin in a strain of the beetle Alphitobius diaperinus: a de novo transcriptome analysis. Insect Science 32:209–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. 2006. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacology & Therapeutics 110:465–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby KA, Kwok RS, Zalom FG, Chiu JC. 2013. Integrating circadian activity and gene expression profiles to predict chronotoxicity of Drosophila suzukii response to insecticides. PloS One 8:e68472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB, Sackman A, Jensen JD. 2018. On the unfounded enthusiasm for soft selective sweeps II: examining recent evidence from humans, flies, and viruses. PLoS Genetics 14:e1007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug-Collet K, Pearson B, Webel R, Szerencsei RT, Winkfein RJ, Schnetkamp PPM, Colley NJ. 1999. Cloning and characterization of a potassium-dependent sodium/calcium exchanger in Drosophila. Journal of Cell Biology 147:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LK, Sella G. 2022. Polygenic adaptation after a sudden change in environment. Elife 11:e66697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Xie W, Yang X, Wang S-L, Wu Q-J, Zhang Y-J. 2018. Identification of glutathione S-transferases in Bemisia tabaci (Hemiptera: Aleyrodidae) and evidence that GSTd7 helps explain the difference in insecticide susceptibility between B. tabaci Middle East-Minor Asia 1 and Mediterranean. Insect Molecular Biology 27:22–35. [DOI] [PubMed] [Google Scholar]
- Hejase HA, Mo Z, Campagna L, Siepel A. 2022. A deep-learning approach for inference of selective sweeps from the ancestral recombination graph. Molecular Biology and Evolution 39:msab332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermisson J, Pennings PS. 2005. Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics 169:2335–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs NP, Weetman D, Hastings IM. 2023. Insecticide resistance management strategies for public health control of mosquitoes exhibiting polygenic resistance: A comparison of sequences, rotations, and mixtures. Evolutionary Applications 16:936–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllinger I, Pennings PS, Hermisson J. 2019. Polygenic adaptation: From sweeps to subtle frequency shifts. PLoS Genetics 15:e1008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Sánchez E, Jin X, Asan, Bianba Z, Peter BM, Vinckenbosch N, Liang YU, Yi X, He M, Somel M. 2014. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura T, Guzman-Holst A, Murray KA. 2020. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nature Communications 11:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J. Svensson M, Larsson J. 2007. Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas 144:25–32. [DOI] [PubMed] [Google Scholar]
- Jensen HR, Scott IM, Sims S, Trudeau VL, Arnason JT. 2006. Gene expression profiles of Drosophila melanogaster exposed to an insecticidal extract of Piper nigrum. Journal of Agricultural and Food Chemistry 54:1289–1295. [DOI] [PubMed] [Google Scholar]
- Jensen JD, Kim Y, DuMont VB, Aquadro CF, Bustamante CD. 2005. Distinguishing between selective sweeps and demography using DNA polymorphism data. Genetics 170:1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri P, Stephan W, Jensen JD. 2022. Soft selective sweeps: addressing new definitions, evaluating competing models, and interpreting empirical outliers. PLoS Genetics 18:e1010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Wakeley J. 2008. The influence of gene conversion on linkage disequilibrium around a selective sweep. Genetics 180:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapopoulou A, Pfeifer SP, Jensen JD, Laurent S. 2018. The demographic history of African Drosophila melanogaster. Genome Biology and Evolution 10:2338–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov T, Messer PW, Petrov DA. 2010. Evidence that adaptation in Drosophila is not limited by mutation at single sites. PLoS Genetics 6:e1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ET, Mack LK, Campos M, Grippin C, Chen T-Y, Romero-Weaver AL, Kosinski KJ, Brisco KK, Collier TC, Buckner EA. 2021a. Evidence of local extinction and reintroduction of Aedes aegypti in Exeter, California. Frontiers in Tropical Diseases 2:703873. [Google Scholar]
- Kelly ET, Mack LK, Campos M, Grippin C, Chen T-Y, Romero-Weaver AL, Kosinski KJ, Brisco KK, Collier TC, Buckner EA. 2021b. Evidence of local extinction and reintroduction of Aedes aegypti in Exeter, California. Frontiers in Tropical Diseases 2:703873. [Google Scholar]
- Kelly JK. 1997. A test of neutrality based on interlocus associations. Genetics 146:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent TV, Schrider DR, Matute DR. 2024. Demographic history and the efficacy of selection in the globally invasive mosquito Aedes aegypti. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. 2002. The human genome browser at UCSC. Genome Research 12:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern AD, Schrider DR. 2016. Discoal: flexible coalescent simulations with selection. Bioinformatics 32:3839–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern AD, Schrider DR. 2018. diploS/HIC: an updated approach to classifying selective sweeps. G3: Genes, Genomes, Genetics 8:1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Nielsen R. 2004. Linkage disequilibrium as a signature of selective sweeps. Genetics 167:1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliot A, Ghanim M. 2012. Fitness costs associated with insecticide resistance. Pest Management Science 68:1431–1437. [DOI] [PubMed] [Google Scholar]
- Koboldt DC. 2020. Best practices for variant calling in clinical sequencing. Genome Medicine 12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska RM, Platt N, Poupardin R, Irving H, Dabire RK, Mitchell S, Jones CM, Diabaté A, Ranson H, Wondji CS. 2013. Dissecting the mechanisms responsible for the multiple insecticide resistance phenotype in Anopheles gambiae ss, M form, from Vallee du Kou, Burkina Faso. Gene 519:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Li H, Schlotterer C, Futschik A. 2011. Distinguishing positive selection from neutral evolution: boosting the performance of summary statistics. Genetics 187:229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love RR, Sikder JR, Vivero RJ, Matute DR, Schrider DR. 2023. Strong positive selection in Aedes aegypti and the rapid evolution of insecticide resistance. Molecular Biology and Evolution 40:msad072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada-Chávez AN, Lozada-Chávez I, Alfano N, Palatini U, Sogliani D, Elfekih S, Degefa T, Sharakhova MV, Badolo A, Sriwichai P. 2025. Adaptive genomic signatures of globally invasive populations of the yellow fever mosquito Aedes aegypti. Nature Ecology & Evolution:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S, Dombrowski SM, Waters LC, Ganguly R. 1996. Three second chromosome-linked clustered Cyp6 genes show differential constitutive and barbital-induced expression in DDT-resistant and susceptible strains of Drosophila melanogaster. Gene 180:165–171. [DOI] [PubMed] [Google Scholar]
- Martins AJ, Ribeiro CD e M, Bellinato DF, Peixoto AA, Valle D, Lima JBP. 2012. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PloS One 7:e31889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, Dudchenko O, Kingan SB, Koren S, Antoshechkin I, Crawford JE, Glassford WJ, Herre M, Redmond SN, Rose NH. 2018. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer PW, Petrov DA. 2013. Population genomics of rapid adaptation by soft selective sweeps. Trends in Ecology & Evolution 28:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Siepel A. 2023. Domain-adaptive neural networks improve supervised machine learning based on simulated population genetic data. PLoS Genetics 19:e1011032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal MR, DeGiorgio M. 2019. Localizing and classifying adaptive targets with trend filtered regression. Molecular Biology and Evolution 36:252–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal MR, Koch H, Huang J, Chiaromonte F, DeGiorgio M. 2020. Learning the properties of adaptive regions with functional data analysis. PLoS Genetics 16:e1008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhar P, Veller C. 2022. Dominance shifts increase the likelihood of soft selective sweeps. Evolution 76:966–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. 2005. Molecular signatures of natural selection. Annu. Rev. Genet. 39:197–218. [DOI] [PubMed] [Google Scholar]
- Nielsen R, Williamson S, Kim Y, Hubisz MJ, Clark AG, Bustamante C. 2005. Genomic scans for selective sweeps using SNP data. Genome Research 15:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HA, Betancourt AJ. 2001. Haldane’s sieve and adaptation from the standing genetic variation. Genetics 157:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Jensen JD, Stephan W. 2010. Searching for footprints of positive selection in whole-genome SNP data from nonequilibrium populations. Genetics 185:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings PS, Hermisson J. 2006a. Soft sweeps III: the signature of positive selection from recurrent mutation. PLoS Genetics 2:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings PS, Hermisson J. 2006b. Soft sweeps II—molecular population genetics of adaptation from recurrent mutation or migration. Molecular Biology and Evolution 23:1076–1084. [DOI] [PubMed] [Google Scholar]
- Perez G, Barber GP, Benet-Pages A, Casper J, Clawson H, Diekhans M, Fischer C, Gonzalez JN, Hinrichs AS, Lee CM. 2025. The UCSC Genome Browser database: 2025 update. Nucleic Acids Research 53:D1243–D1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JR, Gloria-Soria A, Kotsakiozi P. 2018. Recent history of Aedes aegypti: Vector genomics and epidemiology records. Bioscience 68:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezeworski M, Coop G, Wall JD. 2005. The signature of positive selection on standing genetic variation. Evolution 59:2312–2323. [PubMed] [Google Scholar]
- Pridgeon JW, Becnel JJ, Clark GG, Linthicum KJ. 2009. Permethrin induces overexpression of cytochrome c oxidase subunit 3 in Aedes aegypti. Journal of Medical Entomology 46:810–819. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Liu N. 2003. Overexpression of the cytochrome c oxidase subunit I gene associated with a pyrethroid resistant strain of German cockroaches, Blattella germanica (L.). Insect Biochemistry and Molecular Biology 33:1043–1048. [DOI] [PubMed] [Google Scholar]
- Pybus Oliveras M, Luisi P, Dall’Olio GM, Uzkudun M, Laayouni H, Bertranpetit J, Engelken J. 2015. Hierarchical boosting: a machine-learning framework to detect and classify hard selective sweeps in human populations. Bioinformatics. 2015 Dec 15; 31 (24): 3946–52. [DOI] [PubMed] [Google Scholar]
- Rahman RU, Souza B, Uddin I, Carrara L, Brito LP, Costa MM, Mahmood MA, Khan S, Lima JBP, Martins AJ. 2021. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Scientific Reports 11:4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AR, Huff C. 2009. Linkage disequilibrium between loci with unknown phase. Genetics 182:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen R, Udpa N, Halperin E, Bafna V. 2013. Learning natural selection from the site frequency spectrum. Genetics 195:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NH, Badolo A, Sylla M, Akorli J, Otoo S, Gloria-Soria A, Powell JR, White BJ, Crawford JE, McBride CS. 2023. Dating the origin and spread of specialization on human hosts in Aedes aegypti mosquitoes. Elife 12:e83524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, Ibe N, Akorli J, Otoo S, Mutebi J-P. 2020. Climate and urbanization drive mosquito preference for humans. Current Biology 30:3570–3579. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. 2019. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Neglected Tropical Diseases 13:e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Campbell CL, Lenhart A, Penilla P, Lozano-Fuentes S, Black IV WC. 2019. Exome-wide association of deltamethrin resistance in Aedes aegypti from Mexico. Insect Molecular Biology 28:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor B. 1951. Some aspects of respiratory metabolism during metamorphosis of normal and DDT-resistant house flies, Musca domestica L. The Biological Bulletin 100:229–243. [DOI] [PubMed] [Google Scholar]
- Samb B, Konate L, Irving H, Riveron JM, Dia I, Faye O, Wondji CS. 2016. Investigating molecular basis of lambda-cyhalothrin resistance in an Anopheles funestus population from Senegal. Parasites & vectors 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Cury J, Charpiat G, Jay F. 2021. Deep learning for population size history inference: design, comparison and combination with approximate Bayesian computation. Molecular Ecology Resources 21:2645–2660. [DOI] [PubMed] [Google Scholar]
- Schmidt TL, Endersby-Harshman NM, van Rooyen AR, Katusele M, Vinit R, Robinson LJ, Laman M, Karl S, Hoffmann AA. 2024. Global, asynchronous partial sweeps at multiple insecticide resistance genes in Aedes mosquitoes. Nature Communications 15:6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrider DR. 2023. Allelic gene conversion softens selective sweeps. bioRxiv. [Google Scholar]
- Schrider DR, Kern AD. 2016. S/HIC: robust identification of soft and hard sweeps using machine learning. PLoS Genetics 12:e1005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrider DR, Kern AD. 2017. Soft sweeps are the dominant mode of adaptation in the human genome. Molecular Biology and Evolution 34:1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrider DR, Kern AD. 2018. On the well-founded enthusiasm for soft sweeps in humans: a reply to Harris, Sackman, and Jensen. Zenodo [Google Scholar]
- Schrider DR, Mendes FK, Hahn MW, Kern AD. 2015. Soft shoulders ahead: spurious signatures of soft and partial selective sweeps result from linked hard sweeps. Genetics 200:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen KL, Churchill GA, Aquadro CF. 1995. Properties of statistical tests of neutrality for DNA polymorphism data. Genetics 141:413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Haigh J. 1974. The hitch-hiking effect of a favourable gene. Genetics Research 23:23–35. [PubMed] [Google Scholar]
- Song C, Scharf ME. 2009. Mitochondrial impacts of insecticidal formate esters in insecticide-resistant and insecticide-susceptible Drosophila melanogaster. Pest Management Science: formerly Pesticide Science 65:697–703. [DOI] [PubMed] [Google Scholar]
- Song J-Y, Ichtchenko K, Südhof TC, Brose N. 1999. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proceedings of the National Academy of Sciences 96:1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele LD, Coates BS, Seong KM, Valero MC, Mittapalli O, Sun W, Clark J, Pittendrigh BR. 2018. Variation in mitochondria-derived transcript levels associated with DDT resistance in the 91-R strain of Drosophila melanogaster (Diptera: Drosophilidae). Journal of Insect Science 18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan W. 2016. Signatures of positive selection: from selective sweeps at individual loci to subtle allele frequency changes in polygenic adaptation. Molecular Ecology 25:79–88. [DOI] [PubMed] [Google Scholar]
- Storey JD, Siegmund D. 2001. Approximate p-values for local sequence alignments: numerical studies. Journal of Computational Biology 8:549–556. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zou P, Yu X-Y, Chen C, Yu J, Shi L-N, Hong S-C, Zhou D, Chang X-L, Wang W-J. 2012. Functional characterization of an arrestin gene on insecticide resistance of Culex pipiens pallens. Parasites & Vectors 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick WJ. 1991. Evolutionary genetics and arthropod-borne disease: the yellow fever mosquito. American Entomologist 37:14–26. [Google Scholar]
- Tajima F. 1983. Evolutionary relationship of DNA sequences in finite populations. Genetics 105:437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarimo BB, Law HCH, Tao D, Pastrana-Mena R, Kanzok SM, Buza JJ, Dinglasan RR. 2018. Paraquat-Mediated Oxidative Stress in Anopheles gambiae Mosquitoes Is Regulated by An Endoplasmic Reticulum (ER) Stress Response. Proteomes 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst J, Kamm JA, Song YS. 2017. Robust and scalable inference of population history from hundreds of unphased whole genomes. Nature Genetics 49:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima KM, Coop G, Przeworski M. 2006. How reliable are empirical genomic scans for selective sweeps? Genome Research 16:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessnow AE, Nagoshi RN, Meagher RL, Gilligan TM, Sadd BM, Carrière Y, Davis HN, Fleischer SJ, Richers K, Palumbo JC. 2025. Genomic patterns of strain-specific genetic structure, linkage, and selection across fall armyworm populations. BMC Genomics 26:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J, Gomes B, Ayres C, Reimer L. 2020. Insecticide resistance selection and reversal in two strains of Aedes aegypti. Wellcome Open Research 5:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton KR. 2019. Polygenic adaptation to an environmental shift: temporal dynamics of variation under Gaussian stabilizing selection and additive effects on a single trait. Genetics 213:1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torada L, Lorenzon L, Beddis A, Isildak U, Pattini L, Mathieson S, Fumagalli M. 2019. ImaGene: a convolutional neural network to quantify natural selection from genomic data. BMC Bioinformatics 20:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah F, Gul H, Panda RM, Murtaza G, Zhang Z, Huang J, Li X, Desneux N, Lu Y. 2025. Nanocarrier-mediated RNAi of CYP9E2 and CYB5R enhance susceptibility of invasive tomato pest, Tuta absoluta to cyantraniliprole. Frontiers in Plant Science 16:1573634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasimuddin M, Misra S, Li H, Aluru S. 2019. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In: 2019 IEEE international parallel and distributed processing symposium (IPDPS). IEEE. p. 314–324. [Google Scholar]
- Venkataraman K, Shai N, Lakhiani P, Zylka S, Zhao J, Herre M, Zeng J, Neal LA, Molina H, Zhao L. 2022. Rapidly evolving genes underlie Aedes aegypti mosquito reproductive resilience during drought. bioRxiv:2022.03. 01.482582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight BF, Kudaravalli S, Wen X, Pritchard JK. 2006. A map of recent positive selection in the human genome. PLoS Biology 4:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontas J, Blass C, Koutsos AC, David J-P, Kafatos FC, Louis C, Hemingway J, Christophides GK, Ranson H. 2005. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Molecular Biology 14:509–521. [DOI] [PubMed] [Google Scholar]
- Ware-Gilmore F, Novelo M, Sgrò CM, Hall MD, McGraw EA. 2023. Assessing the role of family level variation and heat shock gene expression in the thermal stress response of the mosquito Aedes aegypti. Philosophical Transactions of the Royal Society B 378:20220011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson GA. 1975. On the number of segregating sites in genetical models without recombination. Theoretical Population Biology 7:256–276. [DOI] [PubMed] [Google Scholar]
- Webel R, Haug-Collet K, Pearson B, Szerencsei RT, Winkfein RJ, Schnetkamp PPM, Colley NJ. 2002. Potassium-Dependent Sodium-Calcium Exchange through the Eye of the Fly. Annals of the New York Academy of Sciences 976:300–314. [DOI] [PubMed] [Google Scholar]
- Weedall GD, Riveron JM, Hearn J, Irving H, Kamdem C, Fouet C, White BJ, Wondji CS. 2020. An Africa-wide genomic evolution of insecticide resistance in the malaria vector Anopheles funestus involves selective sweeps, copy number variations, gene conversion and transposons. PLoS Genetics 16:e1008822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand H, Leese F. 2018. Detecting signatures of positive selection in non-model species using genomic data. Zoological journal of the Linnean Society 184:528–583. [Google Scholar]
- Whitehouse LS, Schrider DR. 2023. Timesweeper: accurately identifying selective sweeps using population genomic time series. Genetics 224:iyad084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. 1996. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Molecular and General Genetics MGG 252:51–60. [DOI] [PubMed] [Google Scholar]
- Xue AT, Schrider DR, Kern AD. 2021. Discovery of ongoing selective sweeps within Anopheles mosquito populations using deep learning. Molecular Biology and Evolution 38:1168–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahouédo GA, Chandre F, Rossignol M, Ginibre C, Balabanidou V, Mendez NGA, Pigeon O, Vontas J, Cornelie S. 2017. Contributions of cuticle permeability and enzyme detoxification to pyrethroid resistance in the major malaria vector Anopheles gambiae. Scientific Reports 7:11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Li T, Feng X, Li M, Liu S, Liu N. 2021. Multiple cytochrome P450 genes: conferring high levels of permethrin resistance in mosquitoes, Culex quinquefasciatus. Scientific Reports 11:9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P, Chen X, Yan Y, Liu F, Zhang Y, Guo X, Xu B. 2014. Glutaredoxin 1, glutaredoxin 2, thioredoxin 1, and thioredoxin peroxidase 3 play important roles in antioxidant defense in Apis cerana cerana. Free Radical Biology and Medicine 68:335–346. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li Z, Nian X, Wu F, Shen Z, Zhang B, Zhang Q, Liu X. 2015. Sequence analysis, expression profiles and function of thioredoxin 2 and thioredoxin reductase 1 in resistance to nucleopolyhedrovirus in Helicoverpa armigera. Scientific Reports 5:15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Tang T, Liu J, Feng X, Qiu L. 2012. Identification and expression analysis of NADH-cytochrome b5 reductase gene in the cotton bollworm, Helicoverpa armigera. Gene 511:96–102. [DOI] [PubMed] [Google Scholar]
- Zhao L, Cao Y, Wang D-D, Chen N, Li S-G, Liu S, Li M-Y. 2022. A thioredoxin peroxidase protects Pieris rapae from oxidative stress induced by chlorantraniliprole exposure. Archives of Insect Biochemistry and Physiology 111:e21964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.