Abstract
The discovery of activating mutations in the epidermal growth factor receptor (EGFR) gene has revolutionized the management of lung cancer, enabling the development of targeted tyrosine kinase inhibitors (TKIs). These therapies offer improved survival and reduced side effects compared with conventional treatments. Recent advancements have significantly reshaped the treatment paradigm for EGFR‐mutant non‐small cell lung cancer. TKIs are now incorporated into the management of early stage and locally advanced disease, and phase 3 trials have explored combination strategies in metastatic settings. Although these intensified approaches improve progression‐free survival, they come with increased toxicity and higher costs, underscoring the need for precise patient selection to maximize benefit. Emerging data on biomarkers, such as co‐mutations and circulating tumor DNA, show promise for refining treatment decisions. In addition, significant progress in understanding resistance mechanisms to EGFR TKIs has broadened therapeutic options. This review provides a comprehensive overview of the current landscape of EGFR‐mutant nonsmall cell lung cancer, highlighting recent breakthroughs and discussing strategies to optimize treatment based on the latest evidence.
Keywords: activating mutations, epidermal growth factor receptor (EGFR), non‐small cell lung cancer (NSCLC), tyrosine kinase inhibitors (TKIs)
INTRODUCTION
The discovery of activating mutations in the epidermal growth factor receptor (EGFR) gene introduced the concept of actionable alterations in nonsmall cell lung cancer (NSCLC) and paved the way for precision medicine in lung cancer. Mutations in the tyrosine kinase domain of EGFR represent one of the most common druggable alterations in NSCLC. EGFR kinase domain mutations are found almost exclusively in lung adenocarcinomas (LUADs), with a prevalence ranging from 15% to 50% depending on ethnicity. 1 They occur more commonly in patients with no smoking history, females, and patients of Asian ethnicity. 2 , 3 Among patients with NSCLC of East Asian genomic ancestry—determined by a single‐nucleotide variant approach—the prevalence of EGFR mutations is approximately 50.3% versus 34.2%, 26.8%, 14%, and 13% for patients of South Asian, admixed American, African, and European genomic ancestry, respectively. 4 , 5
EGFR‐mutant NSCLC represents an important health issue worldwide. Although the incidence of smoking‐related lung cancers, such as squamous cell carcinoma or small cell lung cancer, is decreasing, 6 LUAD, which affects both individuals with and without a history of smoking, has become the most common histologic subtype of lung cancer worldwide. LUAD is frequently associated with oncogenic driver alterations such as EGFR mutations, particularly in the absence of a smoking history. Exposure to air pollution has been associated with an increased risk of LUADs, notably EGFR‐mutant NSCLC, 7 and might contribute to their high incidence. Diagnosis of lung cancer in young and never‐smoking patients, often thought to be at low risk of cancer, can be challenging and contributes to delayed diagnosis. Furthermore, despite improvements in survival with tyrosine kinase inhibitors (TKIs) and combination therapies and a better prognosis than that for nononcogene‐addicted NSCLC, EGFR‐mutant NSCLC remains a challenging disease, particularly in the metastatic setting, in which long‐term disease control remains elusive. Recognizing the heterogeneity of EGFR‐mutant NSCLC and the expanding number of treatment options, risk stratification and personalized strategies are relevant clinical questions.
In this review, we aim to provide a comprehensive clinical overview of the management of patients with EGFR‐mutant NSCLC. Unless explicitly stated otherwise, the studies and results discussed in this report apply to the common L858R and exon 19 deletions (ex19del) EGFR mutations. The treatment of insertion mutations in exon 20 (ex20ins) is not covered in this review because ex20ins represents a distinct disease subtype with unique biology, prognosis, and therapy response.
BIOLOGY OF EGFR‐MUTANT NSCLC
Functional consequences of EGFR kinase domain mutations
EGFR is a transmembrane protein localized at the cellular surface and a member of the ERBB family of tyrosine kinase receptors (Figure 1). EGFR is commonly expressed by various epithelial tissues, such as skin, lung, or gastrointestinal mucosa, in which it plays a role in regulating tissue regeneration and wound healing. In normal conditions, the EGFR protein is activated through binding of its ligand, mainly epidermal growth factor and TGF‐α. Ligand binding induces dimerization of the receptor and triggers activation of the intracellular kinase domain, which then activates intracellular pathways implicated in the survival, growth, and proliferation of the cell, such as the MAP kinase and PI3K/AKT/MTOR pathways (Figure 1). 8 EGFR mutations occurring in exons 18 through 21 often lead to constitutive activation of the tyrosine kinase domain.
FIGURE 1.

Structural and functional organization of the EGFR protein. EGFR exists as an inactive monomer embedded in the cell membrane. Upon ligand binding (e.g., epidermal growth factor), the receptor dimerizes, transitioning to an activated state. This activation triggers intracellular signaling cascades, such as MAPK, PI3K‐AKT‐MTOR, which regulate cellular proliferation, survival, and differentiation. Mutations in the kinase domain of EGFR can lead to a constitutive and ligand‐independent activation of the protein and downstream pathways. EGFR indicates epidermal growth factor receptor. Created in BioRender (Maxime Borgeaud, and Timothée Olivier, 2025; https://BioRender.com/s16f601).
EGFR mutation classification
Historically, the L858R point mutation in exon 21 and ex19del, which include the loss of amino acids E746 through A750, have been recognized as the most frequent EGFR mutations, exhibiting high sensitivity to EGFR TKIs. Consequently, most clinical trials primarily include patients with L858R and ex19del mutations. 9 , 10 , 11 Therefore, they are referred to as the common EGFR mutations (Figure 2) and represent around 80% of cases. Ex20ins mutations, which are not the subject of this review, are the most frequent among the uncommon EGFR mutations. In addition to these alterations, many other mutations in the kinase domain are also oncogenic and exhibit diverse sensitivity to EGFR TKIs.
FIGURE 2.
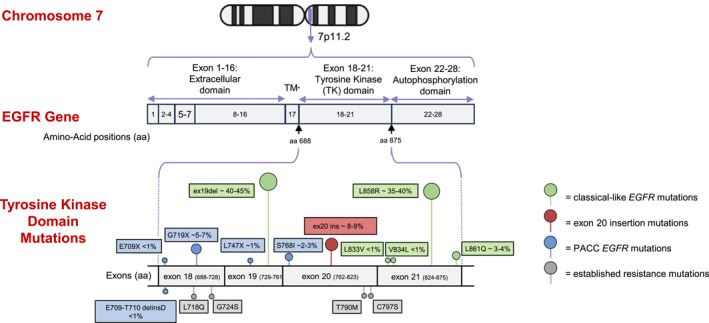
EGFR tyrosine kinase domain mutations. The EGFR gene is located on chromosome 7 (7p11.2). Most frequent mutations within the tyrosine kinase domain in exons 18–21 are shown. Classical‐like mutations include deletions in exon 19, L858R mutations (both are usually referred to as common EGFR mutations), and other rare point mutations. PACC mutations include most other non‐ex20ins and T790M mutations. Insertions in exon 20 represent 8%–9% of EGFR mutations. Most frequent EGFR resistance mutations, acquired after exposure to EGFR TKIs, are shown. T790M represents a resistance mutation to first‐generation or second‐generation TKIs, whereas C797S, G724S, and L718Q represent resistance mutations to third‐generation EGFR TKIs. ex20ins indicates insertion mutation at exon 20; PACC, P‐loop and αC‐helix–compressing; TKIs, tyrosine kinase inhibitors; TM, transmembrane EGFR domain. Created in PowerPoint (Timothée Olivier, 2025).
Various classifications have been proposed regarding EGFR mutations, 12 , 13 often based on sensitivity to EGFR TKIs, which are small molecules designed to inhibit the activated EGFR protein. One of the most comprehensive classifications, The University of Texas MD Anderson Cancer Center classification, 14 clusters different EGFR mutations by their structural impact on the EGFR kinase domain and sensitivity to different generations of EGFR TKIs into four distinct clusters: classical‐like mutations, P‐loop and αC‐helix–compressing (PACC) mutations, ex20ins mutations, and T790M‐like mutations. These four clusters affect differentially on the adenosine triphosphate (ATP) binding pocket of the kinase domain, which constitutes the binding site of EGFR TKIs. Classical‐like mutations represent mutations distant from the ATP binding pocket and do not affect the binding of EGFR TKIs, such as ex19del, L858R, and L861Q. PACC mutations consist of mutations leading to the compression of the region between the P‐loop and the αC‐helix of the EGFR protein, which results in modification of the ATP binding pocket, such as G719X and S768I. The second‐generation TKI afatinib exhibits higher inhibiting properties against PACC mutations than first‐generation or third‐generation TKIs in preclinical models. 14 T790M mutation is a well established gatekeeper resistance mutation that hinders the binding of first‐generation and second‐generation EGFR TKIs to the ATP pocket. This structure‐based classification represents a practical approach that may help in the selection of treatment for rare EGFR mutations based on the prediction of their structural impact.
Genomic landscape of EGFR‐mutant NSCLC
EGFR‐mutant and smoking‐related LUAD represent different diseases from a molecular perspective. EGFR‐mutant cancer cells are addicted to EGFR activation of the MAP kinase pathways and are very sensitive to EGFR inhibition. Activating EGFR mutations are usually mutually exclusive with other oncogenic driver alterations, such as KRAS mutations, at initial diagnosis. Concurrent activating driver mutations were found to exhibit synthetic lethality in preclinical models. 15 Although a rare event, the presence of other oncogenic drivers, such as PIK3CA co‐mutations, seem to be more frequent in patients with a smoking history and are associated with a poorer outcome on EGFR TKIs. 16 Moreover, patients with a higher tumor mutational burden—a feature that is also associated with smoking history and reflects heterogenous disease biology—derive less benefit from EGFR TKIs. 17 Conversely, new oncogenic alterations can arise under the selective pressure of EGFR TKIs as a mechanism of resistance to therapy. Unlike oncogenic driver genes, alterations in tumor‐suppressor genes are rather frequent in EGFR‐mutant NSCLC, with TP53 being the most common, occurring in 40%–50% of cases. 18 TP53 and other tumor‐suppressor genes are thought to favor the acquisition of copy number alterations and genomic instability in cancer cells, which can facilitate resistance to therapy. 19 Indeed, convincing evidence indicates that TP53 mutation and some other concomitant alterations in tumor‐suppressor genes, 20 , 21 such as ARID1A or RB1, seem to confer a further worse prognosis. 19
In addition to somatic EGFR mutations, a T790M germline variant in EGFR was first reported in a European descendant family with several members who developed LUAD. 22 Although T790M is the most described germline EGFR variant, several other germline mutations have been identified, including G724S, T725M, R776H, V769M, V834L/I, and K757R. 23 The prevalence of these germline mutations is probably very low in the general population and among people who have lung cancer, with a frequency of less than 1%. 24 Most patients are female, of European ancestry, without a smoking history, and present with multiple, bilateral, ground‐glass pulmonary nodules. 25 , 26 Oxnard et al. reported that 95% of patients with a germline EGFR mutation have a concomitant somatic EGFR‐activating mutation as a second hit. 25 The risk of developing lung cancer for people carrying a germline EGFR T790M mutation is estimated to exceed 50% before age 60 years, highlighting the need for tailored lung cancer screening in this population. 25
Tumor immune microenvironment in EGFR‐mutant NSCLC
The tumor immune microenvironment (TME) in EGFR‐mutant NSCLC presents distinct characteristics compared with smoking‐related LUAD, with significant implications for immunotherapy strategies. 27 Whereas preclinical models suggest that EGFR mutations could promote programmed death‐ligand 1 (PD‐L1) expression, 28 clinical observations indicate that these mutations are often associated with lower PD‐L1 expression levels. 29 This discrepancy may stem from the unique immunogenic profiles of EGFR‐mutant tumors, typically exhibiting a reduced tumor mutational burden, which is generally correlated with a diminished neoantigen load, leading to a less robust immune response to immune checkpoint inhibitors (ICIs). 30 The TME in EGFR‐mutant NSCLC is generally immunosuppressive, with low CD8‐positive/PD‐L1‐positive tumor‐infiltrating lymphocytes; dysfunctional dendritic cells secondary to the upregulation of IL‐6/STAT3 signaling; higher regulatory T cells, which suppress antitumor immunity; the presence of myeloid‐derived suppressor cells; and predominantly M2 immune‐suppressive tumor‐associated macrophages. 27 Moreover, EGFR‐mutant NSCLC tend to demonstrate an interferon‐γ low immune phenotype, further suggesting an immune‐suppressive TME. 31
MANAGEMENT OF PATIENTS WITH EGFR‐MUTANT NSCLC
The relation between EGFR kinase domain mutations and response to EGFR TKIs was first demonstrated in 2004 in two pivotal studies for patients with metastatic NSCLC. 32 , 33 Three generations of EGFR TKIs are now available in clinical practice for the treatment of patients with EGFR‐mutant NSCLC. The first‐generation TKIs (erlotinib, gefitinib, and icotinib) are reversible EGFR TKIs that compete with ATP binding in the ATP‐binding pocket in the kinase domain. They also have activity on the wild‐type EGFR kinase domain, which is responsible for some of the adverse drug events. Afatinib and dacomitinib represent second‐generation TKIs that bind irreversibly to EGFR and also to some extent inhibit the HER‐2 and HER‐4 kinase domains and the wild‐type EGFR protein. Third‐generation TKIs osimertinib and lazertinib are highly potent, irreversible inhibitors that covalently bind to the C797 residue, 34 , 35 with several advantages over first‐generation and second‐generation drugs, including higher affinity for the mutant EGFR protein and less inhibition of the wild‐type protein, higher intracranial activity, and sustained activity against the T790M gatekeeper resistance mutation. EGFR TKIs have since become the mainstay of treatment of metastatic EGFR‐mutant NSCLC, as discussed below. In the last 5 years, EGFR TKIs have also entered the treatment landscape of nonmetastatic EGFR‐mutant NSCLC.
Management of patients with stage I–III EGFR‐mutant NSCLC
The current management of patients with nonmetastatic NSCLC depends on the resectability of the tumor. For resectable stage I–III tumors, upfront surgery represents the preferred strategy and remains the cornerstone of treatment in otherwise medically fit patients. Definitive chemoradiation is the preferred approach for nonresectable stage III tumors. The following section describes how EGFR TKIs have also become a standard of care in the management of patients with early stage EGFR‐mutant NSCLC as adjuvant therapy after local treatment of the primary tumor and lymph nodes.
Adjuvant tyrosine kinase inhibitors
Gefitinib was the first EGFR TKI evaluated in the adjuvant setting for EGFR‐mutant NSCLC. In the CTONG1104 phase 3 trial (ClinicalTrials.gov identifier NCT01405079), 2 years of adjuvant gefitinib improved median disease‐free survival (DFS) compared with adjuvant cisplatin plus vinorelbine (median DFS, 30.8 vs. 19.8 months; hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.40–0.79). 36 The updated analysis with a follow‐up of 80 months indicates a convergence of the DFS curves, with DFS at 3 and 5 years comparable between the two treatment arms. There was no difference in overall survival (OS) between treatments, likely because most patients in the chemotherapy arm received an EGFR TKI at the time of progression. 37 Thereafter, multiple other randomized trials in the adjuvant setting 38 , 39 , 40 involving first‐generation TKIs demonstrated similar findings that a DFS benefit was lost on longer follow‐up after TKI cessation, and no significant OS benefit was observed.
Osimertinib, a third‐generation TKI, is approved for patients who have stage IB–IIIA NSCLC with classical EGFR mutations after surgery, based on results of the phase 3 ADAURA trial (ClinicalTrials.gov identifier NCT02511106). 41 In this trial, patients with stage IB–IIIA disease according to the American Joint Committee on Cancer's AJCC Cancer Staging Manual, seventh edition (stage IB corresponding to tumor >3 cm without lymph node involvement), were randomly assigned to 3 years of adjuvant osimertinib or placebo, with optional use of adjuvant chemotherapy allowed in both arms. Osimertinib improved DFS in patients who had stage IB–IIIA disease (median DFS, not reached vs. 27.5 months; HR, 0.20; 99% CI, 0.14–0.30) and led to a significant absolute OS benefit of 12% at 5 years for those with stage II–IIIA disease (85% vs. 73%; HR, 0.49; 95% CI, 0.33–0.73). Patients with stage IIIA disease derived the greatest benefit from osimertinib in terms of DFS (HR, 0.12; 95% CI, 0.07–0.20), regardless of adjuvant chemotherapy use. 42 Osimertinib also was associated with a lower rate of central nervous system (CNS) relapse (CNS DFS in stage II–IIIA: HR, 0.24; 95% CI, 0.14–0.42). Notably, however, most CNS relapses in the osimertinib group occurred after the end of the treatment. 43
One of the criticisms of ADAURA has been the suboptimal access to standard‐of‐care therapy at progression in the control group, with only 40% of patients receiving osimertinib at progression, thus potentially affecting OS. Because, in the updated analysis of ADAURA, the benefit of osimertinib seems to be reduced after TKI cessation, 41 a pertinent question is whether adjuvant TKI prevents rather than delays the onset of metastatic disease. However, a counter argument is whether this matters if we improve 5‐year OS rates by 12% with adjuvant osimertinib versus adjuvant chemotherapy, which achieves a less than 5% improvement. 44 Although treatment duration was 3 years in ADAURA, it is currently unknown whether a longer duration would result in a greater benefit. The TARGET phase 2 single‐arm study (ClinicalTrials.gov identifier NCT05526755) is currently evaluating 5 years of adjuvant osimertinib. 45 To that end, improving risk‐stratification strategies to adapt adjuvant treatment strategies is an important research priority. Whether the benefits of adjuvant treatment extend to people with stage IA lung cancer is the subject of the phase 3 ADAURA2 study (ClinicalTrials.gov identifier NCT05120349), which is currently evaluating 3 years of adjuvant osimertinib versus placebo. 46 , 44
In summary, based on these data, we recommend adjuvant osimertinib for 3 years in patients with stage IB (>3 cm) to IIIA resected NSCLC harboring EGFR L858R or ex19del mutations after adjuvant platinum‐based chemotherapy and adjuvant osimertinib for those who are not eligible for chemotherapy. Although criticism regarding the access to osimertinib for people randomly assigned to placebo in the ADAURA trial is valid, in our opinion, it should not prevent the adoption of this recommendation.
Consolidation osimertinib after chemoradiation
For unresectable stage III NSCLC, concurrent chemoradiation represents the current standard of care. Maintenance durvalumab for 1 year has been the standard of care for stage III NSCLC with nonprogressive disease after definitive chemoradiation, based on the PACIFIC trial (ClinicalTrials.gov identifier NCT02125461). 47 However, for the subset of patients with EGFR mutations included in PACIFIC, durvalumab did not appear to improve DFS in a post‐hoc analysis. 48
The phase 3 LAURA trial (ClinicalTrials.gov identifier NCT03521154) evaluated consolidation therapy with osimertinib, which was given until patients developed intolerance or progression, for stage III EGFR‐mutant NSCLC after chemoradiation. 49 The study showed an improvement in progression‐free survival (PFS) compared with placebo (median PFS, 39.1 vs. 5.6 months; HR, 0.16; 95% CI, 0.10–0.24). A trend toward improved OS was also reported in a subsequent follow‐up analysis. 50 In addition, the CNS relapse rate was significantly lower for osimertinib (CNS PFS: HR, 0.17; 95% CI, 0.09–0.32). 51 People on the control arm did poorly, with only 22% of patients alive and without disease progression at 12 months after the initiation of placebo, highlighting that a large proportion of patients with stage III EGFR‐mutant NSCLC harbor micrometastatic disease. In this trial, nearly 50% of patients did not have baseline positron emission tomography/computed tomography staging, 49 raising the possibility that people with occult stage IV disease were inappropriately included. A subgroup analysis was presented at the European Society of Medical Oncology congress subsequently with stratification by whether or not a baseline positron emission tomography/computed tomography scan was performed. In this analysis, there was no difference in PFS with osimertinib versus placebo (HR, 0.23 [95% CI, 0.14–0.40] and 0.24 [95% CI, 0.13–0.38], respectively). 48 , 51 If anything, these data suggest that patients with stage III EGFR‐mutant NSCLC are at a very high risk of relapse. Despite the benefit observed in LAURA, long‐term treatment with osimertinib raises a few issues, such as increased treatment cost and long‐term toxicities, which would be of higher concern if OS were not significantly improved.
In summary, consolidation osimertinib improves PFS in unresectable stage III EGFR‐mutant NSCLC after chemoradiation and is preferred over durvalumab, which is not recommended in this setting. Future trials should explore escalation and de‐escalation treatment strategies.
Role of neoadjuvant EGFR tyrosine kinase inhibitors
The role of neoadjuvant TKI monotherapy for resectable EGFR‐mutant NSCLC has been explored in phase 2 studies. 52 Multiple single‐arm trials demonstrated that a neoadjuvant first‐generation EGFR TKI was generally safe, although modest pathologic responses were achieved. 53 , 54 The phase 2 EMERGING‐CTONG1103 study (ClinicalTrials.gov identifier NCT01407822) was the only randomized trial that enrolled patients with stage IIIA N2 EGFR‐mutant NSCLC and compared perioperative erlotinib (6 weeks and 12 months before and after surgery, respectively) versus perioperative gemcitabine and cisplatin (two cycles before and after surgery). There was no improvement in the objective response rate (ORR) or OS with erlotinib compared with chemotherapy, and the major pathologic response (MPR) rate was low in both groups (9.7% and 0% for erlotinib and chemotherapy, respectively). However, PFS, which was a secondary end point, was significantly improved with erlotinib (median PFS, 21.5 vs. 11.4 months; HR, 0.36; 95% CI, 0.21–0.61). 55 Similarly, several phase 2 trials have evaluated neoadjuvant osimertinib, with rather low MPR and pathologic complete response rates ranging from 10.7% to 24% and from 0% to 3.6%, respectively. 56 , 57 , 58 These findings suggest that EGFR TKIs might have limited capacity for tumor eradication in the neoadjuvant setting.
In the phase 3 NeoADAURA trial (ClinicalTrials.gov identifier NCT04351555), patients with resectable stage II–IIIB NSCLC harboring classical EGFR mutations were randomly assigned either to neoadjuvant osimertinib with or without chemotherapy or to chemotherapy alone. 52 , 59 Of note, adjuvant use of osimertinib was offered to all trial participants and was received by greater than 80% of the patients in each arm. 52 The primary outcomes of MPR was significantly improved with osimertinib monotherapy (MPR, 25%; 95% CI, 17%–34%) and osimertinib chemotherapy (MPR, 26%; 95% CI, 18%–34%) versus chemotherapy alone (MPR, 2%; 95% CI, <1% to 6%). 59 The pathologic complete response rate was less than 10% in both osimertinib groups. The selection of MPR as the primary end point in this trial is a subject of debate, given the uncertainty surrounding its validity as a surrogate for OS and long‐term disease control in the context of targeted therapies. 60 Event‐free survival (EFS) also trended in favor of the osimertinib arms in the NeoADAURA trial; however, the data are still relatively immature to date (15% maturity). Until full maturation of EFS and OS data, the benefit of adding neoadjuvant osimertinib compared with the current standard of care of surgery followed by adjuvant chemotherapy and osimertinib remains uncertain.
Role of immune checkpoint inhibitors in the management of patients with stage I–III disease
There is currently no evidence supporting the use of neoadjuvant, adjuvant or perioperative ICIs in EGFR‐mutant NSCLC. The IMpower010 and Pearls/KEYNOTE‐091 studies (ClinicalTrials.gov identifiers NCT02486718 and NCT02504372, respectively), which evaluated adjuvant atezolizumab and pembrolizumab, respectively, included a limited number of patients with EGFR‐mutant NSCLC. 61 , 62 Within these small subgroups, a positive EFS trend was seen for adjuvant ICIs, with an HR for DFS of 0.44 (95% CI, 0.23–0.84) in the Pearls/KEYNOTE‐091 study 61 and 0.57 (95% CI, 0.26–1.24) for patients who had EGFR‐mutant disease with a PD‐L1 level ≥1% in IMpower010. 62 The phase 3 trials of ICIs in the perioperative setting either excluded 63 , 64 , 65 or included only a very limited number of patients with EGFR‐mutant NSCLC. 66 , 67 In KEYNOTE‐671 (ClinicalTrials.gov identifier NCT03425643), the subgroup with EGFR‐mutant NSCLC seemed to derive significant EFS benefit from perioperative pembrolizumab 66 ; whereas, in AEGEAN (ClinicalTrials.gov identifier NCT03800134), no benefit was seen with perioperative durvalumab. 67 However, these small sample sizes preclude any conclusion; and, in addition to these results, all available data about immunotherapy in patients with EGFR‐mutant NSCLC do not support this idea. An important consideration about ICIs and EGFR‐mutant NSCLC is the increased risk of toxicities for patients receiving osimertinib after ICIs. Several studies have indeed reported an increased risk of pneumonitis and hepatitis. 68 Therefore, we do not recommend the use of neoadjuvant or perioperative ICIs in patients with early stage EGFR‐mutant NSCLC.
Metastatic recurrence during or after adjuvant osimertinib
Recurrence occurring during adjuvant osimertinib will often indicate resistance to osimertinib, whereas recurrence after the end of adjuvant treatment can indicate either resistance or disease flare after cessation of therapy. In all patients, a new biopsy, if feasible, is recommended to evaluate resistance mechanisms to osimertinib. The management of these patients depends on the timing of the recurrence regarding adjuvant treatment, the site and extent of recurrence, and the presence or not of resistance mechanisms. For recurrence occurring after adjuvant osimertinib, without a resistance mechanism on re‐biopsy, we would consider osimertinib rechallenge as a potential option (see Management of patients with metastatic EGFR‐mutant NSCLC, below), although there are no prospective studies supporting this approach. Patients who develop disease recurrence while on adjuvant osimertinib will usually be managed the same as those who have disease progression in the metastatic setting, except in special circumstances, such as oligoprogression amenable to local ablative therapy, in which osimertinib continuation could be considered (see Treatment options at progression after EGFR TKI, below).
Management of patients with metastatic EGFR‐mutant NSCLC
First‐line treatment
EGFR TKI monotherapy
EGFR TKIs have replaced chemotherapy as the first‐line treatment of EGFR‐mutant NSCLC since 2009, when it was demonstrated that gefitinib improved ORR and PFS compared with platinum‐based chemotherapy in the IPASS trial (ClinicalTrials.gov identifier NCT00322452). 10 Similar improvements in ORR and PFS were observed for other first‐generation EGFR TKIs (Figure 3). 69 , 70 The second‐generation TKIs afatinib and dacomitinib have both been shown to improve PFS compared with first‐generation TKIs. 11 , 71 Third generation osimertinib was first approved for the treatment of patients whose disease progressed after a first‐generation TKI treatment, with a T790M EGFR mutation as a resistance mechanism. 72 The safety profile of osimertinib was more favorable than that of first‐generation TKIs, with less frequent skin rash and diarrhea. Osimertinib was then tested against first‐generation TKIs in the first‐line metastatic setting in the FLAURA trial (ClinicalTrials.gov identifier NCT02296125), in which it demonstrated an improvement in PFS (median PFS, 18.9 vs. 10.2 months; HR, 0.46; 95% CI, 0.37–0.57) and OS (median OS, 38.6 vs. 31.8 months; HR, 0.80; 95% CI, 0.64–1.00). 9 Since then, osimertinib has been the standard of care for the first‐line treatment of metastatic EGFR‐mutant NSCLC. Lazertinib demonstrated a comparable PFS benefit compared with first‐generation TKIs in the LASER301 trial (ClinicalTrials.gov identifier NCT04248829). 73 Aumolertinib, furmonertinib, and befotertinib represent other third‐generation TKIs that were developed in China and have also demonstrated prolonged PFS compared with first‐generation TKIs. 74 , 75 , 76
FIGURE 3.

Key clinical data from trials in the first‐line metastatic setting of EGFR‐mutant NSCLC. *The IPASS trial included 10 patients with uncommon EGFR mutations. **The LASER301 trial only reported treatment‐related grade 3 or greater adverse events. AE indicates adverse events; Chemo, chemotherapy; ex20ins, insertion mutation at exon 20; Mo, months; NR, not reached; NSCLC, nonsmall cell lung cancer. Created in PowerPoint (Timothée Olivier, 2025).
In the FLAURA trial, approximately 40% of patients whose cancer progressed on study treatment did not receive further systemic treatment, and 43% of patients whose cancer progressed on a first‐generation TKI received osimertinib at progression. 77 This raises the question of the feasibility of a sequential approach (a first‐generation TKI followed by osimertinib at progression in patients with T790M mutation) and of the advantage of upfront osimertinib. This question was evaluated in the phase 2 APPLE trial (ClinicalTrials.gov identifier NCT02856893). 78 No differences in OS was observed between the two groups, but patients in the sequential approach arm had higher rates of brain progression, and no benefit was observed for the sequential approach in terms of toxicities.
Combination strategies
Despite the high efficacy of EGFR TKIs, treatment resistance invariably develops. Thus several trials have evaluated various combination strategies with TKIs in the first‐line setting (Figure 3).
The NEJ009 trial (Japanese University Hospital Medical Information Network [UMIN] Clinical Trials Registry identifier UMIN000006340) and a study by Noronha et al. demonstrated that the addition of from four to six cycles of chemotherapy to gefitinib improved PFS and PFS2 (defined as the time from randomization to progression on both platinum‐based therapy and gefitinib), at the cost of increased toxicity. 79 , 80 An OS benefit was also seen in both trials at the time of the initial report. However, in an updated analysis of the NEJ009 trial with longer follow‐up, the difference in OS was no longer statistically significant. 81
FLAURA2 (ClinicalTrials.gov identifier NCT04035486) was a global trial that evaluated a similar strategy of combined TKI plus chemotherapy using osimertinib with or without concomitant carboplatin plus pemetrexed for four cycles followed by maintenance pemetrexed plus osimertinib versus osimertinib monotherapy. 82 Compared with osimertinib alone, its combination with chemotherapy led to a statistically significant improvement in PFS (median PFS, 25.5 vs. 16.7 months; HR, 0.62; 95% CI, 0.49–0.79). 82 However, the combination was also associated with more grade 3 or greater adverse events (64% vs. 27%, respectively). The increased toxicities mainly consist of the typical side effects of chemotherapy, such as hematologic adverse events. Although data are immature, a trend toward an OS benefit was observed in the secondary analysis. 77 Interestingly, the combination led to an improvement in CNS PFS compared with osimertinib alone (HR, 0.58; 95% CI, 0.33–1.01). 83 It is worth mentioning, however, that brain imaging was mandated only for patients with baseline brain metastasis and was performed only at the time of progression for patients without a history of brain involvement. In patients with baseline brain metastasis, the PFS was improved with the addition of chemotherapy (median PFS, 24.9 vs. 13.8 months; HR, 0.47; 95% CI, 0.33–0.66). This finding was unanticipated because chemotherapy supposedly has poor intracranial activity compared with third‐generation EGFR TKIs. Interestingly, although the addition of chemotherapy to osimertinib improved PFS by 8 months in the first‐line setting, in the second line, the median PFS observed with chemotherapy alone is only 4.2–4.4 months. 72 , 84
The MARIPOSA trial (ClinicalTrials.gov identifier NCT04487080) evaluated the combination of lazertinib plus amivantamab, an EGFR‐MET–bispecific antibody, in the first‐line metastatic setting for NSCLC with common EGFR mutations. 85 Patients were randomized in a 2:2:1 ratio to amivantamab plus lazertinib, osimertinib monotherapy, and lazertinib monotherapy. Compared with osimertinib monotherapy, amivantamab plus lazertinib improved PFS (median PFS, 23.7 vs. 16.6 months; HR, 0.70; 95% CI, 0.58–0.85). The PFS for lazertinib was similar to that observed for osimertinib.
In the updated analysis, amivantamab plus lazertinib demonstrated a significant survival benefit (median OS, not reached vs. 36.7 months; HR, 0.75; 95% CI, 0.61–0.92). 86 Crossover to amivantamab (e.g., the MARIPOSA‐2 regimen [ClinicalTrials.gov identifier NCT04988295], as discussed below [see Treatment options at progression after EGFR TKI: Agnostic strategies]) at progression was not allowed in the control group. The combination strategy was associated with more toxicities, with grade 3 or greater adverse events in 75% versus 43% of patients treated with osimertinib; paronychia, rash, and infusion reaction were the most common adverse events. Thromboembolic events were also more frequent in the amivantamab plus lazertinib arm (37% vs. 9%). Most thromboembolic events occurred in the first 4 months of treatment, leading to the adoption of prophylactic anticoagulation for the first 4 months in the ongoing trials of the combination, as also stated in the US Food and Drug Administration [FDA] approval of this combination. Interestingly, the combination also seemed to be superior to osimertinib monotherapy in high‐risk subgroups, such as patients with CNS or liver metastasis, TP53 co‐mutation or the persistence of detectable circulating tumor DNA (ctDNA) after two treatment cycles. 87 Of note, serial brain magnetic resonance imaging was mandated in MARIPOSA: every 8 weeks for the first 30 months and 12 weeks thereafter for patients with baseline brain metastases, and every 24 weeks for patients without brain metastasis. This imaging schedule might have led to earlier identification of brain metastasis in MARIPOSA compared with other trials that included less frequent brain monitoring. In the phase 3 PALOMA trial (ClinicalTrials.gov identifier NCT05388669), subcutaneous amivantamab, in combination with lazertinib, was noninferior to intravenous administration in terms of pharmacokinetics and ORR and was associated with lower rates of infusion reactions (13% vs. 66%) and thromboembolic events (9% vs. 14%). 88 Of note, 81% of patients received prophylactic anticoagulation in this trial. Administration time was significantly shorter with the subcutaneous route: approximately 5 minutes versus 5 hours for intravenous amivantamab. Patients' and health care providers' experience was improved overall with subcutaneous administration. The lower rate of infusion reactions and the convenience of administration potentially could allow the delivery of this treatment at home in the future. Interestingly, median duration of response and OS were superior for subcutaneous administration (HR for OS, 0.62; 95% CI, 0.42–0.92), suggesting a potential effect of the route of administration on treatment efficacy. A potential explanation for this difference is the interaction of amivantamab with subcutaneous immune cells. 89
Regarding toxicity of amivantamab plus lazertinib, the phase 2 COCOON study (ClinicalTrials.gov identifier NCT06120140) demonstrated that a prophylactic intensive regimen (consisting of oral doxycycline followed by topical clindamycin, chlorhexidine wash, and skin moisturizers) reduced moderate‐to‐severe dermatologic reactions by almost 50% and increased treatment adherence. 90 The use of dexamethasone premedication 8 mg twice daily for 2 days and 8 mg 1 hour before infusion also was shown to reduce infusion‐related reactions. 91
Several studies have evaluated the role of anti‐angiogenic agents in EGFR‐mutant NSCLC. Two phase 3 studies evaluated the addition of bevacizumab, a humanized monoclonal antibody targeting vascular endothelial growth factor (VEGF), to erlotinib in the first line treatment of advanced or metastatic EGFR‐mutant NSCLC. In both trials, compared with erlotinib alone, the combination of erlotinib and bevacizumab improved PFS (median PFS, 17.9 vs. 11.2 months [HR, 0.55; 95% CI, 0.41–0.73 92 ] and 16.9 vs. 13.3 months [HR, 0.605; 95% CI, 0.417–0.877], respectively). 93 A similar benefit was observed with the combination of erlotinib with ramucirumab, another anti‐VEGF antibody (median PFS, 19.4 vs. 12.4 months for erlotinib monotherapy; HR, 0.59; 95% CI, 0.46–0.76). 94 Importantly, none of these trials demonstrated an OS benefit and also showed increased toxicity. The data regarding the combination of anti‐VEGF with third generation TKI in the first‐line metastatic setting are more limited, with three randomized phase 2 studies and no phase 3 trials. The WJOG9717L and OSIRAM trials (UMIN Clinical Trials Registry identifiers UMIN000030206 and UNIM2080224085) demonstrated no benefit in PFS from the addition of bevacizumab 95 or ramucirumab 96 to osimertinib. Conversely, in the RAMOSE 97 trial (ClinicalTrials.gov identifier NCT03909334), the combination of osimertinib and ramucirumab improved PFS (median PFS, 24.8 vs. 15.6 months; HR, 0.55; 95% CI, 0.32–0.93) with no difference in OS and a higher rate of grade 3 or greater treatment‐related adverse events (53% vs. 41%). 98 Taken together, these data do not support the addition of anti‐VEGF therapies to first‐line osimertinib, especially in light of FLAURA2 and MARIPOSA.
Clinical and molecular factors that may affect first‐line treatment decisions are discussed further below (see Metastatic disease first line—Risk stratification).
Consolidative local therapies
Local ablative treatments of residual disease in patients responding to first‐line EGFR TKIs represent a conceptually interesting strategy because they target potential drug‐persistent cells thus retarding the acquisition of resistance and progression. Moreover, EGFR‐mutant NSCLC frequently progresses at the site of primary disease. 99 Two randomized trials evaluated the addition of radiotherapy to the site of residual disease in the oligometastatic setting. In the phase 2 SINDAS study (ClinicalTrials.gov identifier NCT02893332), patients with oligometastatic disease (five or less locations) were randomized to a first‐generation EGFR TKI with or without stereotactic radiotherapy on all disease sites. 100 The addition of radiotherapy improved PFS (median PFS, 20.2 vs. 12.5 months; HR, 0.22; 95% CI, 0.17–0.46) and OS (median OS, 25.5 vs. 17.6 months; HR, 0.44; 95% CI, 0.28–0.68). This trial, however, has several limitations, such as the presence of mainly bone metastasis, the exclusion of brain metastasis, and a high screen‐failure rate, suggesting that the study involved a very specific and selected population. In another phase 3 study, treatment‐naive patients with oligometastatic disease were randomized to the first‐generation TKI icotinib with or without concurrent thoracic radiotherapy. 101 Radiotherapy to other metastatic sites was allowed at the physician's discretion. The patients receiving thoracic radiotherapy had an improved median PFS of 17.1 versus 10.6 months in the TKI‐alone group (HR, 0.57; p = .004) and improved OS (median OS, 34.4 vs. 26.2 months; HR, 0.62; p = .029). Thoracic radiotherapy was associated with a higher incidence of treatment‐related adverse events of grade 3 or greater (11.9% vs. 5.1%). Conversely, continuation of osimertinib during thoracic radiation therapy has been associated with higher rates of severe pneumonitis. 102 Therefore, although we suggest a temporary interruption of osimertinib during thoracic radiation, we also caution against a prolonged interruption given the risk of tumor flares or progression in other disease sites. Indeed, disease flares have been reported in up to 23% of patients upon TKI cessation, with a median time of 8 days. 103
We suggest that, in oligometastatic disease, radiotherapy to residual sites of disease can be considered in selected patients who respond to first‐line EGFR TKIs. Currently available evidence does not inform the optimal duration of response or depth of response before consolidative therapy can be offered.
Treatment of patients with NSCLC who have uncommon (non‐ex20ins) kinase domain EGFR mutations
Uncommon EGFR mutations, representing activating mutations of the kinase domain other than L858R, ex19del, and ex20ins, represent a challenge. Taken altogether, uncommon mutations generally exhibit lower response rates and PFS with EGFR TKIs. 14 They represent a very heterogenous group, with variable sensitivity to EGFR TKIs. Because of their rarity, uncommon EGFR mutations were excluded from the ADAURA and NeoADAURA (ClinicalTrials.gov identifier NCT04351555) trials, resulting in a lack of prospective studies exploring the role of perioperative TKIs for individuals with NSCLC harboring these mutations. In the metastatic setting, few prospective trials have been conducted for uncommon EGFR mutations. A post‐hoc analysis of the LUX‐Lung trials (ClinicalTrials.gov identifiers NCT00525148, NCT00949650, and NCT01121393) with afatinib reported a response rate of 71.0% (95% CI, 54.1%–84.6%) and a PFS of 10.7 months (95% CI, 5.6–14.7 months) for all uncommon mutations altogether. 12 The ACHILLES study/Thoracic Oncology Research Group study TORG1834 (UMIN Clinical Trials Registry identifier UMIN031180175) is the only randomized trial specifically evaluating a EGFR TKI versus platinum‐pemetrexed chemotherapy in uncommon EGFR mutations. 104 Patients were randomized to receive either afatinib (n = 73) or chemotherapy (n = 36), and the median PFS was 10.6 months with afatinib versus 5.7 months with chemotherapy (HR, 0.422; 95% CI, 0.256–0.694). The overall response rate was also superior with afatinib, at 61.4% versus 47.1% with chemotherapy. At the time of this writing, OS data had not been reported. Several single‐arm studies have evaluated third‐generation TKIs in this setting, with response rates of 50%–55% and a median PFS of 8.2–9.4 months. 105 , 106 The combination of amivantamab plus lazertinib has demonstrated promising activity in uncommon EGFR mutations in the phase 1/1B CHRYSALIS‐2 study (ClinicalTrials.gov identifier NCT04077463), with a 55% response rate (95% CI, 40%–69%) in treatment‐naive patients, with median duration of response not estimable (NE; 95% CI, 9.9 months to NE) and a promising median PFS of 19.5 months (95% CI, 11.0 months to NE). 107
Although the trials described above aggregated all uncommon mutations into a single group, it is now well known that sensitivity to EGFR TKIs depends on the specific mutations. Here, The University of Texas MD Anderson Cancer Center classification described above, which offers a classification of EGFR mutations based on their structural impact and their sensitivity to different generations of TKI, could offer some guidance. PACC mutations are predicted in preclinical models to be more sensitive to second‐generation TKIs, such as afatinib, and less sensitive to third‐generation osimertinib. 14 However, in patients, the activity of osimertinib is not negligible even for PACC mutations, with response rate of approximately 45% in mostly retrospective data. 108 For classical‐like mutations, such as L861Q, in which the structural effect is predicted to be comparable to that of L858R and ex19del mutations, the use of osimertinib seems reasonable. Novel TKIs are also in development for uncommon mutations, such as furmonertinib, a third‐generation EGFR TKI, which led to an ORR of 81.8% (N = 22; 95% CI, 59.7%–94.8%) in patients with NSCLC harboring EGFR PACC mutations in an ongoing phase 1/2 trial. 109
Currently, afatinib is the only guideline‐recommended and FDA‐European Medicines Agency–approved drug for the treatment of patients with uncommon EGFR mutations. For uncommon EGFR mutations, we suggest the use of afatinib for patients harboring uncommon PACC mutations. For classical‐like mutations, such as L861Q, as well as for PACC mutations in some circumstances, such as patients with brain metastasis, we recommend osimertinib if available because of its CNS activity.
Mechanisms of resistance to EGFR TKI therapy
Understanding these mechanisms of resistance is of paramount importance to identify personalized treatment strategies in subsequent lines (Figure 4A). A repeat tumor tissue biopsy at the time of resistance offers broad possibilities of analysis, such as the characterization of additional somatic tumor genomic changes, tumor histology, and the quantification of protein expression levels on the cell surface. It allows for the detection of resistance mechanisms in approximately 50% of patients who progress on osimertinib. 110 Detection of ctDNA by liquid biopsy in plasma allows for the detection of acquired genomic alterations as mechanisms of resistance to targeted treatment. 110 , 111 Liquid biopsy, however, has several limitations, such as nonshedding tumors, inability to detect overexpression of targets like HER2 and MET, and inability to detect histologic transformation. Therefore, the reliability of ctDNA testing should be questioned for cases in which no genomic alteration is retrieved. Modern ctDNA techniques can now determine the proportion of tumor cell DNA in a blood sample (tumor fraction) by using various approaches in combination (the presence of aneuploidy, variant allele frequency, and the presence of canonical alterations). In patients with a high tumor fraction in liquid biopsy, a negative result carries a good negative predictive value, minimizing the need for repeat tissue biopsy to identify an actionable target. 112 Some methods using epigenomic ctDNA profiling might also allow for the detection of small cell transformation from liquid biopsy. 113
FIGURE 4.

(A, B) This figure illustrates key resistance mechanisms to third‐generation TKIs in EGFR‐mutant NSCLC. On‐target resistance occurs through secondary EGFR mutations, such as C797S and G724S, or through EGFR amplification. Cell cycle alterations, including the loss of CDKN2A/B or amplification of CDK4/6 and CCND1, drive uncontrolled proliferation. Resistance can also arise from bypass pathway activation, such as MET or HER2 amplification, or oncogenic mutations in KRAS, BRAF, or PIK3CA. In addition, histologic transformation enables tumor cells to evade therapy by transforming into small cell or squamous cell carcinoma. ctDNA+ indicates circulating tumor DNA‐positive; NSCLC, nonsmall cell lung cancer; TKIs, tyrosine kinase inhibitors. Created in BioRender (Maxime Borgeaud and Timothée Olivier, 2025; https://BioRender.com/s16f601).
The mechanisms of resistance to targeted therapy often consist in the reactivation of specific pathways arising from on‐target (EGFR secondary alterations) or off‐target alterations. Nongenomic acquisition of resistance may occur through transcriptomic changes arising from epigenetic modifications, such as histologic transformation. The paragraphs below describe these mechanisms of resistance.
Acquired on‐target genomic alterations
Secondary mutations of EGFR represent a common mechanism of resistance to EGFR TKIs. The most common mechanisms of resistance to first‐generation and second‐generation TKIs is the acquisition of the T790M mutations that occur in greater than 50% of patients, which increases the affinity for ATP and reduces the binding of TKIs. 114 Resistance to osimertinib can arise secondary to several point mutations, such as C797 (C797S and C797G), G724S, 115 L718 (L718Q and L718V), 116 , 117 and L792 (L792F and L792H). 118 The C797S mutation is the most common because osimertinib forms a covalent bond precisely with the C797 residue. EGFR amplification of the wide‐type allele is also a well known resistance mechanism, leading to increased EGFR signaling, despite the presence of a TKI.
Acquired off‐target genomic alterations
Amplification of MET is the most common mechanism of resistance to osimertinib monotherapy, representing approximately 15% of cases when considering gene amplification 119 and up to 34% of cases when considering MET overexpression by immunohistochemistry (IHC). 120 Various other genomic alterations conferring resistance to osimertinib have been reported with a frequency of less than 5%, including amplifications of HER2, HER3, or KRAS; activating mutations of BRAF (V600E), KRAS, or PIK3CA; and ALK, RET, or BRAF fusions. 110 , 120 In the detection of MET amplification, the gold standard is fluorescence in situ hybridization (FISH) on tissue biopsy. Next‐generation sequencing (NGS) on liquid biopsy can also detect MET amplification, especially in cases of high focal amplification, as illustrated in the INSIGHT‐2 trial (ClinicalTrials.gov identifier NCT03940703), 121 in which 38% of patients who had MET amplification by FISH on tissue biopsy had detectable MET amplification on liquid biopsy NGS. Patients who had MET amplification detected on liquid biopsy NGS had mostly focal MET amplification and high gene copy numbers.
Resistance mechanisms may vary with the use of combination strategies. Based on ctDNA analysis, the acquisition of secondary EGFR mutations and MET amplifications were less frequent with amivantamab plus lazertinib than with osimertinib. 122 In FLAURA‐2, no major difference in the pattern of resistance mechanism was observed between osimertinib and osimertinib plus chemotherapy, but acquired genomic alterations were detected less frequently with disease progression after osimertinib plus chemotherapy. 123
Histologic transformation
Histologic transformation is identified in 10%–15% of patients with EGFR‐mutant NSCLC who progress on EGFR TKIs, mostly among those small cell carcinoma morphology. 110 , 124 The occurrence of small cell transformation has been suspected to be more prevalent with the use of third‐generation TKIs. 124 Evidence suggests that specific genomic alterations, such as RB1 or TP53, could be associated with small cell transformation. 125 , 126 Beyond genomic alterations, multi‐omics analyses indicate that small cell transformation is primarily driven by transcriptional changes through epigenetic reprogramming, 127 , 128 which may be favored in particular genomic contexts, including the loss of the 3p chromosome and possibly TP53 and RB1 silencing. Squamous cell transformation represents another rare histologic transformation and also is associated with significant genomic changes, such as acquired PIK3CA mutations, chromosome 3q amplification, or FGF amplification. 124 Evidence regarding histologic transformation after combination strategies is scarce because the reports on resistance mechanisms in the FLAURA2 and MARIPOSA trials are based on ctDNA analysis. Interestingly, TP53 and RB1 mutations, which sometimes are associated with histologic transformation, were also slightly less frequent after amivantamab plus lazertinib. 122
Drug‐tolerant persister cells
Recent studies have highlighted the presence of drug‐tolerant persister cells in patients with EGFR‐mutant NSCLC, 129 , 130 characterized by reduced cell cycle activity and division and the ability to withstand treatment lethality. These can survive targeted therapies, contributing to disease relapse and progression. 131 , 132 , 133 Evidence suggests that epigenetic mechanisms play a crucial role in the adaptation of these cells, enabling them to withstand therapeutic pressures. 134 , 135 Moreover, it also has been demonstrated that the TME plays a role in the emergence and promotion of drug persister cells, such as tumor‐associated fibroblasts. 130
Understanding the interactions between epigenetic mechanisms and the TME is crucial for developing novel therapeutic strategies.
Treatment options at progression after EGFR TKI
Local therapies for oligoprogression
In patients who present with disease progression isolated to limited metastatic sites (usually from less than three to five sites), local ablative therapy offers a conceptually interesting approach. Local therapy may help to prolong the duration of current therapy and delay the need for a switch in systemic treatment by eradicating the resistant clones. Retrospective data support the use of radiotherapy in this setting, directed to oligo‐progressive sites followed by continuation of a EGFR TKI, with an improvement in PFS. 136 In a single‐arm phase 2 trial, patients who had oligo‐progression on erlotinib were treated with radiotherapy and continuation of erlotinib, with a median PFS of 6 months (95% CI, 2.5–11.6 months) and a median OS of 29 months (95% CI, 21.7–36.3 months). 137
In the setting of oligo‐progression, we suggest local ablative therapy, although no prospective data are available comparing different methods of local ablative therapy.
Biomarker‐selected treatment approaches
Among patients who present with secondary EGFR resistance mutations, treatment strategy differs according to the type of mutation. For patients treated with first‐generation or second‐generation TKIs, the acquisition of a T790M mutation should be treated with osimertinib. 72 Some data suggest that patients who present with secondary G724S mutations or L718Q/V mutations could respond to the second‐generation TKI afatinib, 138 , 139 whereas secondary C797S mutations in trans could be sensitive to first‐generation TKIs such as gefitinib. 140 Fourth‐generation TKIs designed to block the C797S mutation are currently being assessed in early phase trials 141 but are not currently available.
In patients who present with MET amplification as a mechanism of resistance, the combinations osimertinib plus MET inhibitors have been tested in several phase 2 trials. INSIGHT‐2 evaluated the combination of osimertinib plus the MET inhibitor tepotinib, 121 whereas the SAVANNAH and ORCHARD trials (ClinicalTrials.gov identifiers NCT03778229 and NCT03944772) combined osimertinib and savolitinib. In the INSIGHT‐2 trial, among patients who had a MET amplification defined by FISH on tissue (gene copy number five or greater or an MET‐to‐CEP7 ratio ≥2.0) the ORR was 50% (95% CI, 39.7%–60.3%), with a median PFS of 5.6 months (95% CI, 4.2–8.1 months). In SAVANNAH and ORCHARD, the ORRs were 32% (95% CI, 26%–39%) and 41% (80% CI, 25%–59%), respectively. 120 , 142 In the SAVANNAH trial, the response rate correlated with MET amplification or expression, with an ORR of 56% (95% CI, 39%–59%) in patients with high levels of expression (an IHC score of 3+ in ≥90% of tumor cells) or amplification (gene copy numbers ≥10), compared with 9% (95% CI, 4%–18%) in those with lower expression and amplification levels. 120 , 143 Conversely, in INSIGHT‐2, patients who had lower levels of MET amplification experienced only a slightly lower ORR compared with those who had higher levels of amplification (ORR 42.2%; 95% CI, 27.7%–57.8%) versus 56.6% (95% CI, 42.3%–70.2%) for patients with MET gene copy numbers <10 versus ≥10, respectively. Importantly, in INSIGHT‐2 and SAVANNAH, 144 patients receiving tepotinib or savolitinib monotherapy exhibited a much lower ORR (tepotinib: ORR 8.3% [95% CI, 0.2%–38.5%]; savolitinib: ORR, 16% [95% CI, 5%–36%], respectively) highlighting the importance of maintaining EGFR blockade. More recently, the combination of savolitinib plus osimertinib also produced an improvement in PFS compared with chemotherapy among patients with EGFR‐mutant NSCLC who had MET amplification after a previous EGFR TKI, in the phase 3 SACHI trial (ClinicalTrials.gov identifier NCT05015608). 145
Interestingly, the few patients who harbored concurrent bypass mechanisms of resistance in the INSIGHT‐2 trial (BRAF, ALK, RAS) exhibited no response to the combination, highlighting the importance of exhaustive and comprehensive molecular evaluation at the time of progression. The most common mechanisms of resistance to osimertinib plus tepotinib in INSIGHT‐2 consisted of an on‐target alteration of MET or EGFR (loss of MET amplification and secondary resistance mutations of the kinase domain of MET or EGFR C797 mutations). A phase 1 study of combined osimertinib plus telisotuzumab vedotin (a cMet‐targeting antibody–drug conjugate) was recently reported in patients with c‐MET–expressing tumors after progression on prior osimertinib, 146 with an ORR of 58%.
Regarding the concomitant use of inhibitors of bypass resistance mechanisms and osimertinib, the evidence is scarce; therefore, the inclusion in biomarker‐selected clinical trials, 147 , 148 wherever available, should be the priority. Selpercatinib and pralsetinib have been combined with osimertinib in several cases reports and cases series for patients presenting an RET fusion as a mechanism of resistance. 149 , 150 , 151 The combination of osimertinib with alectinib or with BRAF/MEK inhibitors have been reported in the context of acquired ALK fusion or BRAF mutations, respectively. 150 , 152 , 153 , 154
For amplification of the EGFR wild‐type allele, a potential approach could consist of the use of monoclonal EGFR antibodies or an earlier generation of TKIs that also portend activity on the wild‐type EGFR protein. However, because amivantamab also seems to be efficient in the case of EGFR amplification, we would suggest not using another strategy outside of a standard‐of‐care amivantamab plus chemotherapy regimen.
Preclinical data and a few case reports seem to suggest that capivasertib or CDK4/6 inhibitors, in combination with osimertinib, may overcome resistance driven by PI3K pathway or cell cycle gene alterations, respectively. 155 , 156
In HER‐2 amplification as an acquired resistance mechanism, the phase 1/2 TRAEMOS trial (ClinicalTrials.gov identifier NCT03784599) studying osimertinib plus trastuzumab emtansine revealed limited efficacy. 157
Although none of the combination strategies discussed here are FDA approved, up to 45% of patients (Figure 4B) who have disease progression on osimertinib harbor alterations that are potentially actionable with FDA‐approved drugs.
Agnostic strategies
In the second‐line setting, platinum plus pemetrexed chemotherapy has long represented the standard of care. With median a PFS and OS after TKI therapy of 4–5 months 72 , 84 and 14–15 months, respectively, 158 , 159 chemotherapy seems to demonstrate better efficacy in EGFR‐mutant compared versus nononcogene‐addicted NSCLC. The question of pursuing EGFR TKIs beyond progression in addition to chemotherapy—to target both TKI‐resistant and TKI‐sensitive clones and to maintain CNS protection—remains controversial. In the phase 3 IMPRESS trial (ClinicalTrials.gov identifier NCT01544179), continuing gefitinib along with platinum‐based doublet chemotherapy after disease progression on first‐line gefitinib did not improve PFS. 160 The COMPEL trial (ClinicalTrials.gov identifier NCT04765059) is currently evaluating the efficacy of pursuing osimertinib along with chemotherapy in patients with EGFR‐mutant NSCLC who exhibit disease progression after first‐line osimertinib. 161
Several other agnostic strategies have been tested and compared with chemotherapy in the second‐line setting, independent of the mechanisms of resistance. The combination of amivantamab plus chemotherapy with or without lazertinib was evaluated in the MARIPOSA‐2 trial among patients with progressive disease after osimertinib. 84 The primary end point of PFS was improved by the combination of amivantamab plus chemotherapy (median PFS, 6.3 vs. 4.2 months for chemotherapy alone; HR, 0.48; 95% CI, 0.36–0.64) and amivantamab plus chemotherapy plus lazertinib (median PFS, 8.3 vs. 4.2 months for chemotherapy alone; HR, 0.44; 95% CI, 0.35–0.56). The CNS PFS was also improved with amivantamab plus chemotherapy (median CNS PFS, 12.5 vs. 8.3 months for chemotherapy; HR, 0.55; 95% CI, 0.38–0.79) and amivantamab plus chemotherapy plus lazertinib (median CNS PFS, 12.8 vs. 8.3 months for chemotherapy; HR, 0.58; 95% CI, 0.44–0.78). In a biomarker analysis of the CHRYSALIS‐2 trial, which evaluated the efficacy of amivantamab plus lazertinib in the post‐osimertinib and chemotherapy‐naive setting, MET overexpression, defined as an IHC score of 3+ (staining of ≥25% cancer cells), was predictive of a response to the combination, with an ORR of 61% (95% CI, 41%–78%) for MET‐positive tumors versus 12% (95% CI, 5%–25%) for MET‐negative tumors. 162 In this analysis, only one patient exhibited MET amplification on ctDNA and also had MET overexpression on tissue IHC. An analysis of outcomes in MARIPOSA‐2 based on osimertinib‐resistance mechanisms indicated that amivantamab plus chemotherapy improved median PFS regardless of the resistance mechanism. 163
Toxicity was increased by the addition of amivantamab to chemotherapy and increased further by the addition of lazertinib, with grade 3 or greater treatment‐emergent adverse events in 72% of patients who received amivantamab plus chemotherapy, 92% in those who received amivantamab plus lazertinib plus chemotherapy, and 48% in those who received chemotherapy. 84 Discontinuation of one of the study agents because of toxicities occurred in 18% and 34% of patients who received amivantamab plus chemotherapy and amivantamab plus chemotherapy plus lazertinib, respectively, compared with 4% of those who received chemotherapy alone. Hematologic toxicities were predominant and notably increased by the addition of amivantamab and lazertinib. Given the higher toxicity observed with the addition of lazertinib to amivantamab plus chemotherapy and fairly comparable efficacy parameters between amivantamab plus chemotherapy and lazertinib plus amivantamab plus chemotherapy, the FDA approved amivantamab plus chemotherapy for patients who progress after treatment with osimertinib. 164
Numerous trials have evaluated the role of ICIs in combination with chemotherapy 159 , 160 or with chemotherapy and an anti‐VEGF 165 , 166 , 167 for patients with EGFR‐mutant NSCLC who have progressive disease while receiving a TKI. The CheckMate 722 and KEYNOTE‐789 trials (ClinicalTrials.gov identifiers NCT02864251 and NCT03515837, respectively), which evaluated chemotherapy with nivolumab and pembrolizumab, respectively, were negative. 158 , 159 Several trials evaluating the combination of an ICI, chemotherapy, and an anti‐VEGF reported a modest improvement in PFS for ICI plus anti‐VEGF plus chemotherapy combinations and no improvement in OS. 165 , 166 In the IMpower150 phase 3 trial (ClinicalTrials.gov identifier NCT02366143), the combination of platinum‐based chemotherapy, bevacizumab and atezolizumab demonstrated an improvement PFS and OS compared with chemotherapy plus bevacizumab in an exploratory analysis of the small subgroup of patients with EGFR mutations. 167 These results were not reproduced in EGFR‐mutant NSCLC in the phase 3 IMpower151 trial (ClinicalTrials.gov identifier NCT04194203) evaluating the same strategy. 168 Several meta‐analyses confirm a modest gain in PFS of ICI plus chemotherapy and ICI plus anti‐VEGF plus chemotherapy versus chemotherapy alone, with higher toxicity rates and no definitive benefit in terms of OS. 169 , 170
Ivonescimab, a programmed cell death protein 1 (PD‐1)/VEGF–bispecific antibody, was evaluated in combination with chemotherapy in the phase 3 HARMONi‐A trial (ClinicalTrials.gov identifier NCT05184712) for patients with EGFR‐mutant NSCLC who progressed on a previous TKI. 171 Ivonescimab plus chemotherapy improved PFS compared with placebo plus chemotherapy (median PFS, 7.1 vs. 4.8 months; HR, 0.46; 95% CI, 0.34–0.62). OS data are not yet mature.
In the trials that included an anti‐VEGF agent, the relative contribution of the anti–PD‐1/PD‐L1 component in the small PFS gain remains uncertain because all of these trials (except IMpower150) lacked an anti‐VEGF plus chemotherapy arm.
Because several randomized phase 3 trials have reported no benefit in terms of OS and only a modest benefit in terms of PFS, mostly for ICI plus anti‐VEGF plus chemotherapy combinations, at the moment, we do not suggest the use of ICI‐based combinations for EGFR‐mutant NSCLC.
Several antibody–drug conjugates are also being assessed in EGFR‐mutant NSCLC. Datopotamab‐deruxtecan (dato‐DXd), an antitrophoblast cell surface antigen 2 (TROP2) with a topoisomerase I inhibitor payload, was evaluated in the TROPION‐Lung05 phase 2 trial (ClinicalTrials.gov identifier NCT04484142) in 137 previously treated patients with actionable genomic alterations. 172 Dato‐DXd resulted in an ORR of 43.6% (95% CI, 32.4%–55.3%) in 78 patients with EGFR mutations. The median PFS was 5.8 months, and grade 3 or greater adverse events occurred in 47% of patients. Moreover, the phase 3 TROPION‐Lung01 trial (ClinicalTrials.gov identifier NCT04656652), which compared dato‐DXd versus docetaxel in the second line, included a subgroup of patients with actionable genomic alterations. 173 These patients, who harbored mainly EGFR mutations, achieved better PFS on dato‐DXd, with an HR of 0.38 (95% CI, 0.22–0.65). 173 The combination of dato‐DXd plus osimertinib in patients with disease progression on osimertinib showed promising results in the ORCHARD phase 2 trial. 174
Patritumab deruxtecan, an anti‐HER3 with a topoisomerase I inhibitor payload, demonstrated an interesting ORR in an early phase trial 175 but achieved only a modest improvement in PFS compared with chemotherapy in the second‐line setting after an EGFR TKI in the phase 3 HERTHENA‐Lung02 trial (ClinicalTrials.gov identifier NCT04619004; median PFS, 5.8 vs. 5.4 months; HR, 0.77; 95% CI, 0.63–0.94). 176 Other agents under investigation include sacituzumab tirumotecan, an anti‐TROP2 agent with a topoisomerase I inhibitor payload. 177 In the OptiTROP‐Lung03 study (ClinicalTrials.gov identifier NCT05631262), sacituzumab tirumotecan demonstrated an improvement in the ORR and in PFS compared with docetaxel in the third‐line setting after a third‐generation TKI and platinum‐based chemotherapy (ORR, 45.1% vs. 15.6%; median PFS, 6.9 vs. 2.8 months; HR, 0.30; 95% CI, 0.20–0.46). 178
Small cell carcinoma transformation
Small cell carcinoma transformation is associated with a change in disease phenotype, with a more aggressive course and a poor prognosis. 125 , 179 The standard treatment consists of platinum plus etoposide treatment, but the median OS is usually less than 1 year in this situation. 179 Although it is routine to continue EGFR inhibition in cases of other acquired resistance mechanisms, translational studies suggest that, in the context of small cell transformation, even if EGFR mutation is conserved, EGFR expression tends to be downregulated, calling into question the utility of continued EGFR blockade. 180 No prospective trial has specifically tested this strategy in small cell transformation, but some retrospective data suggest that continuing an EGFR TKI during chemotherapy could be associated with better PFS in this setting, potentially treating persisting, EGFR‐driven clones. 181 , 182 Another question is the potential benefit of ICIs in combination chemotherapy, as they lead to a small but significant increment of OS in de‐novo small cell lung cancer 183 , 184 but have limited efficacy in EGFR‐mutant NSCLC, as discussed above. Retrospective studies suggest that an ICI plus chemotherapy combination could improve PFS or OS compared with chemotherapy alone. 181 , 185 Such small retrospective studies carry a high risk of bias, and larger cohorts or prospective trials are needed to validate this strategy. Caution should be taken when ICI therapy is considered concomitantly with osimertinib because severe grade 3–5 pulmonary adverse events have been reported with combining these agents. 186 Therefore, this is not a strategy we recommend for patients who have small cell carcinoma transformation.
Future directions and current challenges in personalizing EGFR‐directed therapies
With the advent of these numerous intensification strategies, which improve PFS or DFS, but at the cost of increased toxicity, it is critical to develop risk‐stratified approaches from early to advanced stages to avoid overtreating patients with a good prognosis while improving outcomes for poor‐risk patients.
Early stage and molecular residual disease
In the adjuvant setting, it is imperative to identify patients at higher risk of relapse who potentially could benefit more from prolonged adjuvant treatment with an EGFR TKI. Several trials have shown promise for ctDNA in NSCLC in the adjuvant setting, 174 including EGFR‐mutant tumors. 187 The half‐life of tumor ctDNA in the blood is relatively short, typically less than 2.5 hours, making it a dynamic biomarker for the monitoring of treatment response. 188 In an analysis of the ADAURA trial, a tumor‐informed ctDNA panel identified molecular residual disease (MRD) with a median lead time of 4.7 months ahead of DFS events in both arms.190 However, in the treatment arm, the majority of MRD events occurred after the end of osimertinib treatment. This underlies the finding that, in the adjuvant setting, efficient targeted therapy can either suppress or decrease ctDNA levels below the detection threshold of current techniques. Although this suggests that the use of MRD detection potentially could be interesting before the introduction of adjuvant targeted treatment, in the study by Herbst et al., only a very small proportion of patients who further relapsed were MRD‐positive at that time. 189 Furthermore, approximately 30% of patients who relapsed were not previously positive for MRD. Better technology with improved sensitivity is needed before MRD‐informed approaches can routinely be used for clinical decision making. 190
Metastatic disease first line—Risk stratification
In the metastatic setting, combination strategies have demonstrated advantages in terms of PFS, and the MARIPOSA regimen, a combination of amivantamab plus lazertinib, led to a statistically significant and clinically meaningful reduction in mortality compared with osimertinib (HR, 0.75; 95% CI, 0.61–0.92). At 3 years, 60% of participants were alive in the experimental arm compared with 51% in the osimertinib arm. 86 After these OS results, the MARIPOSA regimen thus represents a standard first‐line option. However, EGFR‐mutant NSCLC can sometimes follow a long course with prolonged disease control on a single TKI. Given the increased toxicities of combination approaches, we believe that some patients might still benefit from a sequential approach with osimertinib in the first‐line setting. The sequential approach offers the convenience of oral treatment for a prolonged period and minimizes toxicities. The key challenge lies in distinguishing patients who could be offered TKI monotherapy from those who would require a treatment‐intensification approach. Several clinical or molecular poor prognostic factors may help to guide management in this scenario (Figure 5). 87 , 191 , 192 , 193
FIGURE 5.

Clinical and molecular poor prognostic factors. PFS outcomes are shown for different treatments groups from the MARIPOSA and FLAURA2 trials. This figure combines subgroup and exploratory analyses from two separate clinical studies to provide a comprehensive overview; however, caution is warranted when performing cross‐trial comparisons. Ami‐laz indicates amivantamab plus lazertinib; HR, hazard ratio; NE, not estimable; Osi, osimertinib; Osi‐CT, osimertinib plus chemotherapy; PFS, progression‐free survival. Created in BioRender (Maxime Borgeaud, 2025; https://BioRender.com/s16f601).
The type of EGFR mutation is a well known prognostic factor. Patients harboring an L858R mutation in exon 21, for instance, have a worse prognosis on osimertinib monotherapy, with a median PFS of 12.4 months in the FLAURA trial compared with 21.4 months for patients with ex19del. 9 Although patients with L858R mutations also had a poorer prognosis than patients with ex19del in the FLAURA2 and MARIPOSA trials, 82 , 85 both combination regimens improved PFS compared with osimertinib alone in the L858R subgroup. In FLAURA2, osimertinib plus chemotherapy extended PFS to 24.7 months versus 13.9 months for osimertinib alone in patients with L858R mutations. The HR for PFS was comparable between the L858R and ex19del subgroups (ex19del: HR, 0.6; L858R: HR, 0.63). In MARIPOSA, amivantamab plus lazertinib extended PFS to 18.4 months versus 14.8 months for osimertinib in patients with L858R mutations. The PFS benefit appeared greater for ex19del (HR, 0.65) than for L858R (HR, 0.78), and the same trend was observed for OS. 86 Hence, the relative benefit of both combinations did not seem to be higher in patients with L858R mutations versus those with ex19del, even if the absolute PFS was longer with the combination, underpinning a prognostic rather than predictive role for the type of mutation with regard to combination strategies.
Patients with CNS metastasis represent another poor‐prognosis group. Both combination regimes in the FLAURA2 and MARIPOSA trials demonstrated an improvement in CNS PFS in patients with baseline brain metastasis. 83 , 86 The magnitude of benefit from combination therapy seemed greater in patients who had baseline CNS metastases in the FLAURA2 trial, but this trend was not observed in the MARIPOSA trial.
The combination of amivantamab plus lazertinib also improved PFS compared with osimertinib monotherapy in other high‐risk subgroups who are usually associated with a worse prognosis on osimertinib monotherapy. 87 The combination improved PFS for patients harboring TP53 mutations (HR, 0.65; 95% CI, 0.48–0.87), patients without clearance of ctDNA after two cycles (HR, 0.64; 95% CI, 0.48–0.87), and patients with baseline liver metastasis (HR, 0.58; 95% CI, 0.37–0.91).
In an exploratory analysis of FLAURA2, the detection of baseline ctDNA was prognostic and appeared to be predictive of clinical benefit with the combination of osimertinib plus chemotherapy. 193 Among those who had no detectable ctDNA at baseline, compared with single‐agent osimertinib, PFS was prolonged with the combination, although it did not reach statistical significance (median PFS, 33.3 vs. 30.3 months with osimertinib alone; HR, 0.93; 95% CI, 0.51–1.72). For patients who had detectable ctDNA at baseline, PFS was significantly prolonged with the combination (median PFS, 24.8 vs. 13.9 months; HR, 0.60; 95% CI, 0.45–0.80). The clearance of ctDNA at 3 weeks was associated with improved PFS compared with no clearance. However, the relative benefit of the combination versus osimertinib alone was similar regardless of whether there was clearance of ctDNA at 3 weeks, suggesting that ctDNA clearance might be prognostic, but not predictive, in this setting. Thus several trials are evaluating intensification strategies based on ctDNA guidance (ClinicalTrials.gov identifiers NCT04410796 and NCT06020989). For example, the PACE‐LUNG phase 2 trial is currently evaluating osimertinib followed by the addition of chemotherapy in patients who are positive for ctDNA at 3 weeks (EudraCT [European Union Drug Regulating Authorities Clinical Database] registration number 2019‐004757‐88).
These observations in high‐risk subgroups emerge purely from exploratory analyses. Nevertheless, they may inform patient discussions and contribute to shared decision making, particularly when assessing the potential benefits and risks of treatment‐intensification strategies. As technology improves, dynamic ctDNA monitoring may help to individualize treatment decisions in the future. Combinations of third‐generation EGFR TKIs with chemotherapy or with amivantamab are currently approved options.
CONCLUSION
In this review, we have discussed the 2‐decade‐long journey of discovery, identification, and targeting of EGFR mutations in lung cancer. Although these advances have improved survival and quality of life, patients with early stage disease still face a high risk of recurrence, and long‐term disease control remains a challenge in the metastatic setting. Progress is undeniable, but so is the work that remains. We are now entering a new era of precision oncology, driven by improvements in real‐time disease monitoring, ultrasensitive disease‐detection thresholds, and the development of more nuanced treatment strategies based on co‐alterations, tumor burden, and patient characteristics. We predict that novel therapeutics possibly allowing the complete inactivation of EGFR pathways and the ability to bypass mechanisms of resistance will make inroads in the EGFR realm, further improving outcomes in this disease.
Finally, even more for instances in which there are conflicting data or uncertain benefits, we encourage treating clinicians to consider patient comorbidities, financial challenges, preferences, and personal values to facilitate shared decision making and to ensure informed decisions.
CONFLICT OF INTEREST STATEMENT
Maxime Borgeaud reports financial support to attend scientific meetings for registration, travel, and accommodations from AstraZeneca, Bayer, Janssen Pharmaceuticals, Merck Sharp and Dohme (MSD), and PharmaMar outside the submitted work. Jair Bar reports institutional research funding from AbbVie, AstraZeneca/MedImmune, Immunai, MSD Novartis, Oncohost, Pfizer, Roche, and Takeda Pharmaceutical Company; personal/consulting fees from AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb Company, Immunity Bio, Jazz Pharmaceuticals, Merck Serono, MSD Novartis, Regeneron, Rigel Pharmaceuticals, Roche, and Takeda Pharmaceutical Company; and travel support from Rigel Pharmaceuticals outside the submitted work. Stephanie Pei Li Saw reports grants from AstraZeneca; personal/consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo Company, Johnson & Johnson, and Pfizer; honoraria from AstraZeneca, Bristol‐Myers Squibb, Daiichi Sankyo Company, MSD, Pfizer, Roche, and Takeda Oncology;, meeting support from AstraZeneca, MSD, and Pfizer; and advisory board participation for AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo Company, and Pfizer outside the submitted work. Kaushal Parikh reports payment made to his institution from AstraZeneca, Dava Oncology, Guardant Health, Intellisphere, ImmunityBio, Medscape, Regeneron, and Rigel Pharmaceuticals; personal/consulting fees from AstraZeneca, Immunity Bio, Jazz Pharmaceuticals, Regeneron, and Rigel Pharmaceuticals; and travel support from Rigel Pharmaceuticals outside the submitted work. Giuseppe Luigi Banna reports personal/consulting or advisory fees from Accord, Amgen, AstraZeneca, and Merck; and fees for serving on speakers' bureaus from Amgen, Astellas, AstraZeneca, Bayer, Merck, and Pfizer outside the submitted work. Jill Feldman reports personal/consulting fees from AstraZeneca, Blueprint Medicine, Janssen, Johnson & Johnson, and EQRx outside the submitted work. Xiuning Le reports institutional research funding from ArriVent, Boehringer Ingelheim, Dizal, Eli Lilly and Company, EMD Serono, Janssen, Regeneron, Takeda, Teligene, and ThermoFisher; personal/consulting fees from AbbVie, Abion, ArriVent, AstraZeneca, Bayer, BlossomHill, Blueprint Medicines, Boehringer Ingelheim, Daiichi Sankyo Company, Eli Lilly and Company, EMD Serono (Merck KGaA), Hengrui Therapeutics, Janssen Biotech, Novartis, Regeneron Pharmaceuticals, Sensei Biotherapeutics, Spectrum Pharmaceuticals, SystImmune, Taiho, and Teligene; travel support from EMD Serono, Janssen, and Spectrum Pharmaceutics; and owns stock options in BlossomHill outside the submitted work. Alfredo Addeo reports grants from AstraZeneca; personal/consulting or advisory fees from Amgen, Astellas, AstraZeneca, Bristol Myers Squibb Company, Eli Lilly and Company, Merck, MSD, Novartis, Pfizer, and Roche; and fees for serving on speakers' bureaus from Amgen, AstraZeneca, and Eli Lilly and Company outside the submitted work. Timothée Olivier and Claudio De Vito disclosed no conflicts of interest.
Borgeaud M, Olivier T, Bar J, et al. Personalized care for patients with EGFR‐mutant nonsmall cell lung cancer: navigating early to advanced disease management. CA Cancer J Clin. 2025;75(5):387‐409. doi: 10.3322/caac.70024
REFERENCES
- 1. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022;40(6):611‐625. doi: 10.1200/JCO.21.01626 [DOI] [PubMed] [Google Scholar]
- 2. Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non‐small cell lung cancer: a systematic review and meta‐analysis. Oncotarget. 2016;7(48):78985‐78993. doi: 10.18632/oncotarget.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi Y, Au JSK, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154‐162. doi: 10.1097/JTO.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miura K, Shukuya T, Greenstein R, et al. Ancestry‐, sex‐, and age‐based differences of gene alterations in NSCLC: from the real‐world data of cancer genomic profiling tests. J Natl Compr Canc Netw. 2024;22(7):e247021. doi: 10.6004/jnccn.2024.7021 [DOI] [PubMed] [Google Scholar]
- 5. Adib E, Nassar AH, Abou Alaiwi S, et al. Variation in targetable genomic alterations in non‐small cell lung cancer by genetic ancestry, sex, smoking history, and histology. Genome Med. 2022;14(1):39. doi: 10.1186/s13073-022-01041-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10‐45. doi: 10.3322/caac.21871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill W, Lim EL, Weeden CE, et al. Lung adenocarcinoma promotion by air pollutants. Nature. 2023;616(7955):159‐167. doi: 10.1038/s41586-023-05874-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 2017;9(5):52. doi: 10.3390/cancers9050052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382(1):41‐50. doi: 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 10. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947‐957. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 11. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐Lung 7): a phase 2B, open‐label, randomised controlled trial. Lancet Oncol. 2016;17(5):577‐589. doi: 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 12. Yang JCH, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: a combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol. 2015;16(7):830‐838. doi: 10.1016/S1470-2045(15)00026-1 [DOI] [PubMed] [Google Scholar]
- 13. Janning M, Süptitz J, Albers‐Leischner C, et al. Treatment outcome of atypical EGFR mutations in the German National Network Genomic Medicine Lung Cancer (nNGM). Ann Oncol. 2022;33(6):602‐615. doi: 10.1016/j.annonc.2022.02.225 [DOI] [PubMed] [Google Scholar]
- 14. Robichaux JP, Le X, Vijayan RSK, et al. Structure‐based classification predicts drug response in EGFR‐mutant NSCLC. Nature. 2021;597(7878):732‐737. doi: 10.1038/s41586-021-03898-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Unni AM, Lockwood WW, Zejnullahu K, Lee‐Lin SQ, Varmus H. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. Elife. 2015;4:e06907. doi: 10.7554/eLife.06907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eng J, Woo KM, Sima CS, et al. Impact of concurrent PIK3CA mutations on response to EGFR tyrosine kinase inhibition in EGFR‐mutant lung cancers and on prognosis in oncogene‐driven lung adenocarcinomas. J Thorac Oncol. 2015;10(12):1713‐1719. doi: 10.1097/JTO.0000000000000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Offin M, Rizvi H, Tenet M, et al. Tumor mutation burden and efficacy of EGFR‐tyrosine kinase inhibitors in patients with EGFR‐mutant lung cancers. Clin Cancer Res. 2019;25(3):1063‐1069. doi: 10.1158/1078-0432.CCR-18-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tavernari D, Borgeaud M, Liu X, et al. Decoding the clinical and molecular signatures of EGFR common, compound, and uncommon mutations in NSCLC: a brief report. J Thorac Oncol. 2025;20(4):500‐506. doi: 10.1016/j.jtho.2024.12.012 [DOI] [PubMed] [Google Scholar]
- 19. Stockhammer P, Grant M, Wurtz A, et al. Co‐occurring alterations in multiple tumor suppressor genes are associated with worse outcomes in patients with EGFR‐mutant lung cancer. J Thorac Oncol. 2024;19(2):240‐251. doi: 10.1016/j.jtho.2023.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canale M, Petracci E, Delmonte A, et al. Impact of TP53 mutations on outcome in EGFR‐mutated patients treated with first‐line tyrosine kinase inhibitors. Clin Cancer Res. 2017;23(9):2195‐2202. doi: 10.1158/1078-0432.CCR-16-0966 [DOI] [PubMed] [Google Scholar]
- 21. Vokes NI, Chambers E, Nguyen T, et al. Concurrent TP53 mutations facilitate resistance evolution in EGFR‐mutant lung adenocarcinoma. J Thorac Oncol. 2022;17(6):779‐792. doi: 10.1016/j.jtho.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37(12):1315‐1316. doi: 10.1038/ng1671 [DOI] [PubMed] [Google Scholar]
- 23. Sharma P, Mahadevia H, Donepudi S, et al. A novel EGFR germline mutation in lung adenocarcinoma: case report and literature review. Clin Lung Cancer. 2024;25(5):479‐482. doi: 10.1016/j.cllc.2024.04.009 [DOI] [PubMed] [Google Scholar]
- 24. Yap TA, Ashok A, Stoll J, et al. Prevalence of germline findings among tumors from cancer types lacking hereditary testing guidelines. JAMA Netw Open. 2022;5(5):e2213070. doi: 10.1001/jamanetworkopen.2022.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oxnard GR, Chen R, Pharr JC, et al. Germline EGFR mutations and familial lung cancer. J Clin Oncol. 2023;41(34):5274‐5284. doi: 10.1200/JCO.23.01372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan K, Owens J, Elamin Y, et al. Mutational characteristics and clinical outcomes for lung adenocarcinoma with EGFR germline mutations. J Thorac Oncol. 2024;19(10):1438‐1448. doi: 10.1016/j.jtho.2024.06.004 [DOI] [PubMed] [Google Scholar]
- 27. Qiao M, Jiang T, Liu X, et al. Immune checkpoint inhibitors in EGFR‐mutated NSCLC: dusk or dawn? J Thorac Oncol. 2021;16(8):1267‐1288. doi: 10.1016/j.jtho.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 28. Chen N, Fang W, Zhan J, et al. Upregulation of PD‐L1 by EGFR activation mediates the immune escape in EGFR‐driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910‐923. doi: 10.1097/JTO.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 29. To KKW, Fong W, Cho WCS. Immunotherapy in treating EGFR‐mutant lung cancer: current challenges and new strategies. Front Oncol. 2021;11:635007. doi: 10.3389/fonc.2021.635007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vryza P, Fischer T, Mistakidi E, Zaravinos A. Tumor mutation burden in the prognosis and response of lung cancer patients to immune‐checkpoint inhibition therapies. Transl Oncol. 2023;38:101788. doi: 10.1016/j.tranon.2023.101788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Le X, Negrao MV, Reuben A, et al. Abstract 5028: Characterization of the tumor immune microenvironment in treatment‐naive EGFR‐mutant NSCLC uncovers a low IFN‐gamma suppressive immune phenotype. Cancer Res. 2019;79(13 suppl):5028. doi: 10.1158/1538-7445.AM2019-5028 [DOI] [Google Scholar]
- 32. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small‐cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129‐2139. doi: 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 33. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497‐1500. doi: 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 34. Yun J, Hong MH, Kim SY, et al. YH25448, an irreversible EGFR‐TKI with potent intracranial activity in EGFR mutant non–small cell lung cancer. Clin Cancer Res. 2019;25(8):2575‐2587. doi: 10.1158/1078-0432.CCR-18-2906 [DOI] [PubMed] [Google Scholar]
- 35. Cross DAE, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046‐1061. doi: 10.1158/2159-8290.CD-14-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR‐mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open‐label, phase 3 study. Lancet Oncol. 2018;19(1):139‐148. doi: 10.1016/S1470-2045(17)30729-5 [DOI] [PubMed] [Google Scholar]
- 37. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR‐mutant NSCLC: final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021;39(7):713‐722. doi: 10.1200/JCO.20.01820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tada H, Mitsudomi T, Misumi T, et al. Randomized phase III study of gefitinib versus cisplatin plus vinorelbine for patients with resected stage II–IIIA non–small‐cell lung cancer with EGFR mutation (IMPACT). J Clin Oncol. 2022;40(3):231‐241. doi: 10.1200/JCO.21.01729 [DOI] [PubMed] [Google Scholar]
- 39. Ou W, Li N, Wang BX, et al. Adjuvant icotinib versus observation in patients with completely resected EGFR‐mutated stage IB NSCLC (GASTO1003, CORIN): a randomised, open‐label, phase 2 trial. eClinicalMedicine. 2023;57:101839. doi: 10.1016/j.eclinm.2023.101839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He J, Su C, Liang W, et al. Icotinib versus chemotherapy as adjuvant treatment for stage II–IIIA EGFR‐mutant non‐small‐cell lung cancer (EVIDENCE): a randomised, open‐label, phase 3 trial. Lancet Respir Med. 2021;9(9):1021‐1029. doi: 10.1016/S2213-2600(21)00134-X [DOI] [PubMed] [Google Scholar]
- 41. Tsuboi M, Herbst RS, John T, et al. Overall survival with osimertinib in resected EGFR‐mutated NSCLC. N Engl J Med. 2023;389(2):137‐147. doi: 10.1056/NEJMoa2304594 [DOI] [PubMed] [Google Scholar]
- 42. Wu YL, John T, Grohe C, et al. Postoperative chemotherapy use and outcomes from ADAURA: osimertinib as adjuvant therapy for resected EGFR‐mutated NSCLC. J Thorac Oncol. 2022;17(3):423‐433. doi: 10.1016/j.jtho.2021.10.014 [DOI] [PubMed] [Google Scholar]
- 43. Herbst RS, Wu YL, John T, et al. Adjuvant osimertinib for resected EGFR‐mutated stage IB–IIIA non–small‐cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;41(10):1830‐1840. doi: 10.1200/JCO.22.02186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552‐3559. doi: 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 45. Soo RA, de Marinis F, Han JY, et al. TARGET: a phase II, open‐label, single‐arm study of 5‐year adjuvant osimertinib in completely resected EGFR‐mutated stage II to IIIB NSCLC post complete surgical resection. Clin Lung Cancer. 2024;25(1):80‐84. doi: 10.1016/j.cllc.2023.09.005 [DOI] [PubMed] [Google Scholar]
- 46. Tsutani Y, Goldman JW, Dacic S, et al. Adjuvant osimertinib vs. placebo in completely resected stage IA2–IA3 EGFR‐mutated NSCLC: ADAURA2. Clin Lung Cancer. 2023;24(4):376‐380. doi: 10.1016/j.cllc.2023.02.002 [DOI] [PubMed] [Google Scholar]
- 47. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342‐2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 48. Naidoo J, Antonia S, Wu YL, et al. Brief report: durvalumab after chemoradiotherapy in unresectable stage III EGFR‐mutant NSCLC: a post hoc subgroup analysis from PACIFIC. J Thorac Oncol. 2023;18(5):657‐663. doi: 10.1016/j.jtho.2023.02.009 [DOI] [PubMed] [Google Scholar]
- 49. Lu S, Kato T, Dong X, et al. Osimertinib after chemoradiotherapy in stage III EGFR‐mutated NSCLC. N Engl J Med. 2024:585‐597. doi: 10.1056/NEJMoa2402614 [DOI] [PubMed] [Google Scholar]
- 50. Ramalingam SS, Ozguroglu M, Ahn MJ, et al. LBA4: Osimertinib (osi) after definitive chemoradiotherapy (CRT) in patients (pts) with unresectable (UR) stage III EGFR‐mutated (EGFRm) non‐small cell lung cancer (NSCLC): updated overall survival (OS) analysis from the LAURA study [abstract]. J Thorac Oncol. 2025;20(3 suppl 1):S123‐S124. doi: 10.1016/S1556-0864(25)00379-X [DOI] [Google Scholar]
- 51. Lu S, Ahn MJ, Reungwetwattana T, et al. Osimertinib after definitive chemoradiotherapy in unresectable stage III epidermal growth factor receptor‐mutated non‐small‐cell lung cancer: analyses of central nervous system efficacy and distant progression from the phase III LAURA study. Ann Oncol. 2024;35(12):1116‐1125. doi: 10.1016/j.annonc.2024.08.2243 [DOI] [PubMed] [Google Scholar]
- 52. Tsuboi M, Weder W, Escriu C, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR‐mutated resectable non‐small‐cell lung cancer: NeoADAURA. Future Oncol. 2021;17(31):4045‐4055. doi: 10.2217/fon-2021-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tan A, Chua KP, Takano A, et al. P1.17‐07 Neoadjuvant gefitinib in resectable early stage EGFR mutant non‐small cell lung cancer (NSCLC): a window‐of‐opportunity study [abstract]. J Thorac Oncol. 2019;14(10 suppl):S609‐S610. doi: 10.1016/j.jtho.2019.08.1281 [DOI] [Google Scholar]
- 54. Zhang Y, Fu F, Hu H, et al. Gefitinib as neoadjuvant therapy for resectable stage II–IIIA non–small cell lung cancer: a phase II study. J Thorac Cardiovasc Surg. 2021;161(2):434‐442.e2. doi: 10.1016/j.jtcvs.2020.02.131 [DOI] [PubMed] [Google Scholar]
- 55. Zhong WZ, Yan HH, Chen KN, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA‐N2 EGFR‐mutant non‐small‐cell lung cancer: final overall survival analysis of the EMERGING‐CTONG 1103 randomised phase II trial. Signal Transduct Targeted Ther. 2023;8(1):76. doi: 10.1038/s41392-022-01286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lv C, Fang W, Wu N, et al. Osimertinib as neoadjuvant therapy in patients with EGFR‐mutant resectable stage II–IIIB lung adenocarcinoma (NEOS): a multicenter, single‐arm, open‐label phase 2b trial. Lung Cancer. 2023;178:151‐156. doi: 10.1016/j.lungcan.2023.02.011 [DOI] [PubMed] [Google Scholar]
- 57. Blakely CM, Urisman A, Gubens MA, et al. Neoadjuvant osimertinib for the treatment of stage I–IIIA epidermal growth factor receptor–mutated non–small cell lung cancer: a phase II multicenter study. J Clin Oncol. 2024;42(26):3105‐3114. doi: 10.1200/JCO.24.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee JB, Choi SJ, Shim HS, et al. Neoadjuvant and adjuvant osimertinib in stage IA–IIIA, EGFR‐mutant non‐small cell lung cancer (NORA). J Thorac Oncol. 2025;20(5):641‐650. doi: 10.1016/j.jtho.2024.12.023 [DOI] [PubMed] [Google Scholar]
- 59. He J, Tsuboi M, Weder W, et al. Neoadjuvant osimertinib for resectable EGFR‐mutated non‐small‐cell lung cancer. J Clin Oncol. Published online June 2, 2025. doi: 10.1200/JCO-25-00883 [DOI] [PubMed] [Google Scholar]
- 60. Ramalingam SS, Stinchcombe TE. Neoadjuvant osimertinib: a step ahead or just a step? J Clin Oncol. Published online June 2, 2025. doi: 10.1200/JCO-25-01020 [DOI] [PubMed] [Google Scholar]
- 61. O’Brien M, Paz‐Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non‐small‐cell lung cancer (PEARLS/KEYNOTE‐091): an interim analysis of a randomised, triple‐blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274‐1286. doi: 10.1016/S1470-2045(22)00518-6 [DOI] [PubMed] [Google Scholar]
- 62. Felip E, Altorki N, Zhou C, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II–IIIA non‐small cell lung cancer (IMpower010): a randomised, multicentre, open‐label, phase 3 trial. Ann Oncol. 2023;34(10):907‐919. doi: 10.1016/j.annonc.2023.07.001 [DOI] [PubMed] [Google Scholar]
- 63. Cascone T, Awad MM, Spicer JD, et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med. 2024;390(19):1756‐1769. doi: 10.1056/NEJMoa2311926 [DOI] [PubMed] [Google Scholar]
- 64. Provencio M, Nadal E, González‐Larriba JL, et al. Perioperative nivolumab and chemotherapy in stage III non–small‐cell lung cancer. N Engl J Med. 2023;389(6):504‐513. doi: 10.1056/NEJMoa2215530 [DOI] [PubMed] [Google Scholar]
- 65. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973‐1985. doi: 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early‐stage non–small‐cell lung cancer. N Engl J Med. 2023;389(6):491‐503. doi: 10.1056/NEJMoa2302983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non–small‐cell lung cancer. N Engl J Med. 2023;389(18):1672‐1684. doi: 10.1056/NEJMoa2304875 [DOI] [PubMed] [Google Scholar]
- 68. Uchida T, Kaira K, Yamaguchi O, et al. Different incidence of interstitial lung disease according to different kinds of EGFR‐tyrosine kinase inhibitors administered immediately before and/or after anti–PD‐1 antibodies in lung cancer. Thorac Cancer. 2019;10(4):975‐979. doi: 10.1111/1759-7714.13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shi YK, Wang L, Han BH, et al. First‐line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation‐positive lung adenocarcinoma (CONVINCE): a phase 3, open‐label, randomized study. Ann Oncol. 2017;28(10):2443‐2450. doi: 10.1093/annonc/mdx359 [DOI] [PubMed] [Google Scholar]
- 70. Wu YL, Zhou C, Liam CK, et al. First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol. 2015;26(9):1883‐1889. doi: 10.1093/annonc/mdv270 [DOI] [PubMed] [Google Scholar]
- 71. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first‐line treatment for patients with EGFR‐mutation‐positive non‐small‐cell lung cancer (ARCHER 1050): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2017;18(11):1454‐1466. doi: 10.1016/S1470-2045(17)30608-3 [DOI] [PubMed] [Google Scholar]
- 72. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376(7):629‐640. doi: 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cho BC, Ahn MJ, Kang JH, et al. Lazertinib versus gefitinib as first‐line treatment in patients with EGFR‐mutated advanced non–small‐cell lung cancer: results from LASER301. J Clin Oncol. 2023;41(26):4208‐4217. doi: 10.1200/JCO.23.00515 [DOI] [PubMed] [Google Scholar]
- 74. Lu S, Zhou J, Jian H, et al. Befotertinib (D‐0316) versus icotinib as first‐line therapy for patients with EGFR‐mutated locally advanced or metastatic non‐small‐cell lung cancer: a multicentre, open‐label, randomised phase 3 study. Lancet Respir Med. 2023;11(10):905‐915. doi: 10.1016/S2213-2600(23)00183-2 [DOI] [PubMed] [Google Scholar]
- 75. Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first‐line therapy for Chinese patients with locally advanced or metastatic EGFR mutation‐positive non‐small‐cell lung cancer (FURLONG): a multicentre, double‐blind, randomised phase 3 study. Lancet Respir Med. 2022;10(11):1019‐1028. doi: 10.1016/S2213-2600(22)00168-0 [DOI] [PubMed] [Google Scholar]
- 76. Lu S, Dong X, Jian H, et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first‐line therapy for locally advanced or metastatic non–small‐cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40(27):3162‐3171. doi: 10.1200/JCO.21.02641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Planchard D, Boyer MJ, Lee JS, et al. Postprogression outcomes for osimertinib versus standard‐of‐care EGFR‐TKI in patients with previously untreated EGFR‐mutated advanced non–small cell lung cancer. Clin Cancer Res. 2019;25(7):2058‐2063. doi: 10.1158/1078-0432.CCR-18-3325 [DOI] [PubMed] [Google Scholar]
- 78. Remon J, Besse B, Aix SP, et al. Overall survival from the EORTC LCG‐1613 APPLE trial of osimertinib versus gefitinib followed by osimertinib in advanced EGFR‐mutant non–small‐cell lung cancer. J Clin Oncol. 2024;42(12):1350‐1356. doi: 10.1200/JCO.23.01521 [DOI] [PubMed] [Google Scholar]
- 79. Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non–small‐cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38(2):115‐123. doi: 10.1200/JCO.19.01488 [DOI] [PubMed] [Google Scholar]
- 80. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR‐mutated lung cancer. J Clin Oncol. 2020;38(2):124‐136. doi: 10.1200/JCO.19.01154 [DOI] [PubMed] [Google Scholar]
- 81. Miyauchi E, Morita S, Nakamura A, et al. Updated analysis of NEJ009: gefitinib‐alone versus gefitinib plus chemotherapy for non–small‐cell lung cancer with mutated EGFR. J Clin Oncol. 2022;40(31):3587‐3592. doi: 10.1200/JCO.21.02911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without chemotherapy in EGFR‐mutated advanced NSCLC. N Engl J Med. 2023;389(21):1935‐1948. doi: 10.1056/NEJMoa2306434 [DOI] [PubMed] [Google Scholar]
- 83. Jänne PA, Planchard D, Kobayashi K, et al. CNS efficacy of osimertinib with or without chemotherapy in epidermal growth factor receptor‐mutated advanced non‐small‐cell lung cancer. J Clin Oncol. 2024;42(7):808‐820. doi: 10.1200/JCO.23.02219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Passaro A, Wang J, Wang Y, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR‐mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA‐2 study. Ann Oncol. 2024;35(1):77‐90. doi: 10.1016/j.annonc.2023.10.117 [DOI] [PubMed] [Google Scholar]
- 85. Cho BC, Lu S, Felip E, et al. Amivantamab plus lazertinib in previously untreated EGFR‐mutated advanced NSCLC. N Engl J Med. 2024;391(16):1486‐1498. doi: 10.1056/NEJMoa2403614 [DOI] [PubMed] [Google Scholar]
- 86. Yang JCH, Kim YJ, Lee SH, et al. 4O: Amivantamab plus lazertinib vs osimertinib in first‐line (1L) EGFR‐mutant (EGFRm) advanced NSCLC: final overall survival (OS) from the phase III MARIPOSA study [abstract]. J Thorac Oncol. 2025;20(3 suppl 1):S6‐S8. doi: 10.1016/S1556-0864(25)00199-6 [DOI] [Google Scholar]
- 87. Felip E, Cho BC, Gutiérrez V, et al. Amivantamab plus lazertinib versus osimertinib in first‐line EGFR‐mutant advanced non‐small‐cell lung cancer with biomarkers of high‐risk disease: a secondary analysis from MARIPOSA. Ann Oncol. 2024;26(24):805‐816. doi: 10.1016/j.annonc.2024.05.541 [DOI] [PubMed] [Google Scholar]
- 88. Leighl NB, Akamatsu H, Lim SM, et al. Subcutaneous amivantamab vs intravenous amivantamab, both in combination with lazertinib, in refractory EGFR‐mutated, advanced non‐small cell lung cancer (NSCLC): primary results, including overall survival (OS), from the global, phase 3, randomized controlled PALOMA‐3 trial [abstract]. J Clin Oncol. 2024;42(17 suppl):LBA8505. doi: 10.1200/JCO.2024.42.17_suppl.LBA8505 [DOI] [Google Scholar]
- 89. Lim SM, Kang SS, Kim DK, et al. Exploration of immune‐modulatory effects of amivantamab in combination with pembrolizumab in lung and head and neck squamous cell carcinoma. Cancer Res Commun. 2024;4(7):1748‐1764. doi: 10.1158/2767-9764.CRC-24-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Girard N, Li W, Spira A, et al. 10MO: Preventing moderate to severe dermatologic adverse events in first‐line EGFR‐mutant advanced NSCLC treated with amivantamab plus lazertinib: early success of the COCOON trial [abstract]. J Thorac Oncol. 2025;20(3 suppl 1):S14‐S16. doi: 10.1016/S1556-0864(25)00205-9 [DOI] [Google Scholar]
- 91. Spira AI, Paz‐Ares L, Han JY, et al. Preventing infusion‐related reactions with intravenous amivantamab—results from SKIPPirr, a phase 2 study: a brief report. J Thorac Oncol. 2025;20(6):809‐816. doi: 10.1016/j.jtho.2025.01.018 [DOI] [PubMed] [Google Scholar]
- 92. Zhou Q, Xu CR, Cheng Y, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR‐mutated, advanced NSCLC (ARTEMIS‐CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39(9):1279‐1291.e3. doi: 10.1016/j.ccell.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 93. Kawashima Y, Fukuhara T, Saito H, et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR‐mutant non‐small‐cell lung cancer (NEJ026): overall survival analysis of an open‐label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2022;10(1):72‐82. doi: 10.1016/S2213-2600(21)00166-1 [DOI] [PubMed] [Google Scholar]
- 94. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR‐mutated, advanced non‐small‐cell lung cancer (RELAY): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655‐1669. doi: 10.1016/S1470-2045(19)30634-5 [DOI] [PubMed] [Google Scholar]
- 95. Kenmotsu H, Wakuda K, Mori K, et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J Thorac Oncol. 2022;17(9):1098‐1108. doi: 10.1016/j.jtho.2022.05.006 [DOI] [PubMed] [Google Scholar]
- 96. Nakahara Y, Kato T, Isomura R, et al. LBA70 OSIRAM‐1: a multicenter, open label, randomized phase II study of osimertinib plus ramucirumab versus osimertinib alone as initial chemotherapy for EGFR mutation‐positive non‐squamous non‐small cell lung cancer (TORG1833) [abstract]. Ann Oncol. 2023;34(suppl 2):S1313. doi: 10.1016/j.annonc.2023.10.071 [DOI] [Google Scholar]
- 97. Le X, Patel JD, Shum E, et al. A multicenter open‐label randomized phase II study of osimertinib with and without ramucirumab in tyrosine kinase inhibitor–naive EGFR‐mutant metastatic non–small cell lung cancer (RAMOSE trial). J Clin Oncol. 2025;43(4):403‐411. doi: 10.1200/JCO.24.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kenmotsu H. Combination of osimertinib and vascular endothelial growth factor receptor inhibitors for EGFR‐mutated non–small cell lung cancer: old or new regimen? J Clin Oncol. 2025;43(4):369‐372. doi: 10.1200/JCO-24-01921 [DOI] [PubMed] [Google Scholar]
- 99. Al‐Halabi H, Sayegh K, Digamurthy SR, et al. Pattern of failure analysis in metastatic EGFR‐mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol. 2015;10(11):1601‐1607. doi: 10.1097/JTO.0000000000000648 [DOI] [PubMed] [Google Scholar]
- 100. Wang XS, Bai YF, Verma V, et al. Randomized trial of first‐line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR‐mutated non‐small cell lung cancer. J Natl Cancer Inst. 2023;115(6):742‐748. doi: 10.1093/jnci/djac015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sun H, Li M, Huang W, et al. Thoracic radiotherapy improves the survival in patients with EGFR‐mutated oligo‐organ metastatic non–small cell lung cancer treated with epidermal growth factor receptor–tyrosine kinase inhibitors: a multicenter, randomized, controlled, phase III trial. J Clin Oncol. 2025;43(4):412‐421. doi: 10.1200/JCO.23.02075 [DOI] [PubMed] [Google Scholar]
- 102. Banla LI, Tzeng A, Baillieul JP, et al. Pneumonitis in patients receiving thoracic radiotherapy and osimertinib: a multi‐institutional study. JTO Clin Res Rep. 2023;4(10):100559. doi: 10.1016/j.jtocrr.2023.100559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR‐mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011;17(19):6298‐6303. doi: 10.1158/1078-0432.CCR-11-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Miura S, Tanaka H, Misumi T, et al. LBA66 Afatinib versus chemotherapy for treatment‐naive non‐small cell lung cancer with a sensitizing uncommon epidermal growth factor receptor mutation: a phase III study (ACHILLES/TORG1834) [abstract]. Ann Oncol. 2023;34(suppl 2):S1310‐S1311. doi: 10.1016/j.annonc.2023.10.067 [DOI] [Google Scholar]
- 105. Okuma Y, Kubota K, Shimokawa M, et al. First‐line osimertinib for previously untreated patients with NSCLC and uncommon EGFR mutations: the UNICORN phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;22(1):43‐51. doi: 10.1001/jamaoncol.2023.5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with non‐small‐cell lung cancer harboring uncommon EGFR mutations: a multicenter, open‐label, phase II trial (KCSG‐LU15‐09). J Clin Oncol. 2020;38(5):488‐495. doi: 10.1200/jco.19.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cho BC, Wang Y, Felip E, et al. Amivantamab plus lazertinib in atypical EGFR‐mutated advanced non‐small cell lung cancer (NSCLC): results from CHRYSALIS‐2 [abstract]. J Clin Oncol. 2024;42(16 suppl):8516. doi: 10.1200/JCO.2024.42.16_suppl.8516 [DOI] [Google Scholar]
- 108. Borgeaud M, Parikh K, Banna GL, et al. Unveiling the landscape of uncommon EGFR mutations in NSCLC—a systematic review. J Thorac Oncol. 2024;19(7):973‐983. doi: 10.1016/j.jtho.2024.03.016 [DOI] [PubMed] [Google Scholar]
- 109. Le X, Yu Y, Zhao Y, et al. PL04.07 FURTHER: a global, randomized study of firmonertinib at two dose levels in TKI‐naive, advanced NSCLC with EGFR PACC mutations [abstract]. J Thorac Oncol. 2024;19(10 suppl):S5‐S6. doi: 10.1016/j.jtho.2024.09.019 [DOI] [Google Scholar]
- 110. Piotrowska Z, Chen LN, Shum E, et al. Tissue and plasma‐based mechanisms of resistance to first‐line osimertinib in EGFR‐mutant NSCLC: a multi‐institutional cohort [abstract]. J Clin Oncol. 2023;41(16 suppl):9108. doi: 10.1200/JCO.2023.41.16_suppl.9108 [DOI] [Google Scholar]
- 111. Chabon JJ, Simmons AD, Lovejoy AF, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7(1):11815. doi: 10.1038/ncomms11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rolfo CD, Madison RW, Pasquina LW, et al. Measurement of ctDNA tumor fraction identifies informative negative liquid biopsy results and informs value of tissue confirmation. Clin Cancer Res. 2024;30(11):2452‐2460. doi: 10.1158/1078-0432.CCR-23-3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. El Zarif T, Meador CB, Qiu X, et al. Detecting small cell transformation in patients with advanced EGFR mutant lung adenocarcinoma through epigenomic cfDNA profiling. Clin Cancer Res. 2024;30(17):3798‐3811. doi: 10.1158/1078-0432.CCR-24-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res. 2013;19(8):2240‐2247. doi: 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Oztan A, Fischer S, Schrock AB, et al. Emergence of EGFR G724S mutation in EGFR‐mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer. 2017;111:84‐87. doi: 10.1016/j.lungcan.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 116. Bersanelli M, Minari R, Bordi P, et al. L718Q mutation as new mechanism of acquired resistance to AZD9291 in EGFR‐mutated NSCLC. J Thorac Oncol. 2016;11(10):e121‐e123. doi: 10.1016/j.jtho.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 117. Liu Y, Li Y, Ou Q, et al. Acquired EGFR L718V mutation mediates resistance to osimertinib in non‐small cell lung cancer but retains sensitivity to afatinib. Lung Cancer. 2018;118:1‐5. doi: 10.1016/j.lungcan.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 118. Chen K, Zhou F, Shen W, et al. Novel mutations on EGFR Leu792 potentially correlate to acquired resistance to osimertinib in advanced NSCLC. J Thorac Oncol. 2017;12(6):e65‐e68. doi: 10.1016/j.jtho.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 119. Chmielecki J, Gray JE, Cheng Y, et al. Candidate mechanisms of acquired resistance to first‐line osimertinib in EGFR‐mutated advanced non‐small cell lung cancer. Nat Commun. 2023;14(1):1070. doi: 10.1038/s41467-023-35961-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ahn MJ, Kim TM, Bonanno L, et al. 2O: SAVANNAH: savolitinib (savo) + osimertinib (osi) in patients (pts) with EGFRm advanced NSCLC and MET overexpression (OverExp) and/or amplification (Amp) following progressive disease (PD) on osi [abstract]. J Thorac Oncol. 2025;20(3 suppl 1):S4‐S5. doi: 10.1016/S1556-0864(25)00197-2 [DOI] [Google Scholar]
- 121. Wu YL, Guarneri V, Voon PJ, et al. Tepotinib plus osimertinib in patients with EGFR‐mutated non‐small‐cell lung cancer with MET amplification following progression on first‐line osimertinib (INSIGHT 2): a multicentre, open‐label, phase 2 trial. Lancet Oncol. 2024;25(8):989‐1002. doi: 10.1016/S1470-2045(24)00270-5 [DOI] [PubMed] [Google Scholar]
- 122. Besse B, Lee SH, Lu S, et al. LBA55 Mechanisms of acquired resistance to first‐line amivantamab plus lazertinib versus osimertinib in patients with EGFR‐mutant advanced non‐small cell lung cancer: an early analysis from the phase III MARIPOSA study [abstract]. Ann Oncol. 2024;35(suppl 2):S1245‐S1246. doi: 10.1016/j.annonc.2024.08.2297 [DOI] [Google Scholar]
- 123. Lee CK, Robichaux JP, Jänne PA, et al. 514MO Acquired mechanisms of resistance to first‐line (1L) osimertinib with or without platinum‐based chemotherapy (CT) in EGFR‐mutated (EGFRm) advanced NSCLC: preliminary data from FLAURA2 [abstract]. Ann Oncol. 2023;34(suppl 4):S1669‐S1670. doi: 10.1016/j.annonc.2023.10.593 [DOI] [Google Scholar]
- 124. Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor analyses reveal squamous transformation and off‐target alterations as early resistance mechanisms to first‐line osimertinib in EGFR‐mutant lung cancer. Clin Cancer Res. 2020;26(11):2654‐2663. doi: 10.1158/1078-0432.CCR-19-3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Marcoux N, Gettinger SN, O’Kane G, et al. EGFR‐mutant adenocarcinomas that transform to small‐cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. 2019;37(4):278‐285. doi: 10.1200/JCO.18.01585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Offin M, Chan JM, Tenet M, et al. Concurrent RB1 and TP53 alterations define a subset of EGFR‐mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol. 2019;14(10):1784‐1793. doi: 10.1016/j.jtho.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Quintanal‐Villalonga A, Taniguchi H, Zhan YA, et al. Multiomic analysis of lung tumors defines pathways activated in neuroendocrine transformation. Cancer Discov. 2021;11(12):3028‐3047. doi: 10.1158/2159-8290.CD-20-1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang C, Ma S, Zhang J, et al. EHMT2‐mediated transcriptional reprogramming drives neuroendocrine transformation in non‐small cell lung cancer. Proc Natl Acad Sci U S A. 2024;121(23):e2317790121. doi: 10.1073/pnas.2317790121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ebi H. Drug‐tolerant persister cells after EGFR tyrosine kinase inhibitor treatment: their origin and the influences from the tumor microenvironment. J Thorac Oncol. 2023;18(4):399‐401. doi: 10.1016/j.jtho.2022.12.010 [DOI] [PubMed] [Google Scholar]
- 130. Moghal N, Li Q, Stewart EL, et al. Single‐cell analysis reveals transcriptomic features of drug‐tolerant persisters and stromal adaptation in a patient‐derived EGFR‐mutated lung adenocarcinoma xenograft model. J Thorac Oncol. 2023;18(4):499‐515. doi: 10.1016/j.jtho.2022.12.003 [DOI] [PubMed] [Google Scholar]
- 131. Sharma SV, Lee DY, Li B, et al. A chromatin‐mediated reversible drug‐tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69‐80. doi: 10.1016/j.cell.2010.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kobayashi K, Tan AC. Unraveling the impact of intratumoral heterogeneity on EGFR tyrosine kinase inhibitor resistance in EGFR‐mutated NSCLC. Int J Mol Sci. 2023;24(4):4126. doi: 10.3390/ijms24044126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Johnson M, Garassino MC, Mok T, Mitsudomi T. Treatment strategies and outcomes for patients with EGFR‐mutant non‐small cell lung cancer resistant to EGFR tyrosine kinase inhibitors: focus on novel therapies. Lung Cancer. 2022;170:41‐51. doi: 10.1016/j.lungcan.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 134. Cabanos HF, Hata AN. Emerging insights into targeted therapy‐tolerant persister cells in cancer. Cancers (Basel). 2021;13(11):2666. doi: 10.3390/cancers13112666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Russo M, Chen M, Mariella E, et al. Cancer drug‐tolerant persister cells: from biological questions to clinical opportunities. Nat Rev Cancer. 2024;24(10):694‐717. doi: 10.1038/s41568-024-00737-z [DOI] [PubMed] [Google Scholar]
- 136. Fallet V, Matton L, Schernberg A, Canellas A, Cornelis FH, Cadranel J. Local ablative therapy in oncogenic‐driven oligometastatic non‐small cell lung cancer: present and ongoing strategies—a narrative review. Transl Lung Cancer Res. 2021;10(7):3457‐3472. doi: 10.21037/tlcr-20-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Weiss J, Kavanagh B, Deal A, et al. Phase II study of stereotactic radiosurgery for the treatment of patients with oligoprogression on erlotinib. Cancer Treat Res Commun. 2019;19:100126. doi: 10.1016/j.ctarc.2019.100126 [DOI] [PubMed] [Google Scholar]
- 138. Minari R, Leonetti A, Gnetti L, et al. Afatinib therapy in case of EGFR G724S emergence as resistance mechanism to osimertinib. Anticancer Drugs. 2021;32(7):758‐762. doi: 10.1097/CAD.0000000000001064 [DOI] [PubMed] [Google Scholar]
- 139. Sanchis‐Borja M, Guisier F, Swalduz A, et al. Characterization of patients with EGFR mutation‐positive NSCLC following emergence of the osimertinib resistance mutations, L718Q or G724S: a multicenter retrospective observational study in France. Onco Targets Ther. 2024;17:439‐448. doi: 10.2147/OTT.S448909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Enrico D, Tsou F, Catani G, et al. Overcoming resistance to osimertinib by T790M loss and C797S acquisition using gefitinib in a patient with EGFR‐mutant NSCLC: a case report. JTO Clin Res Rep. 2023;4(2):100456. doi: 10.1016/j.jtocrr.2022.100456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lim SM, Fujino T, Kim C, et al. BBT‐176, a novel fourth‐generation tyrosine kinase inhibitor for osimertinib‐resistant EGFR mutations in non‐small cell lung cancer. Clin Cancer Res. 2023;29(16):3004‐3016. doi: 10.1158/1078-0432.CCR-22-3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yu HA, Ambrose H, Baik C, et al. 1239P ORCHARD osimertinib + savolitinib interim analysis: a biomarker‐directed phase II platform study in patients (pts) with advanced non‐small cell lung cancer (NSCLC) whose disease has progressed on first‐line (1L) osimertinib [abstract]. Ann Oncol. 2021;32(5 suppl):S978‐S979. doi: 10.1016/j.annonc.2021.08.1844 [DOI] [Google Scholar]
- 143. Ahn MJ, De Marinis F, Bonanno L, et al. EP08.02‐140 MET biomarker‐based preliminary efficacy analysis in SAVANNAH: savolitinib + osimertinib in EGFRm NSCLC post‐osimertinib [abstract]. J Thorac Oncol. 2022;17(9 suppl):S469‐S470. doi: 10.1016/j.jtho.2022.07.823 [DOI] [Google Scholar]
- 144. Levy BP, de Marinis F, Bonanno L, et al. Efficacy and CNS results from a randomized subset of the phase 2 SAVANNAH study comparing savolitinib (savo) + osimertinib (osi) combination with savo + placebo (PBO) [abstract]. J Clin Oncol. 2025;43(16 suppl):8513. doi: 10.1200/JCO.2025.43.16_suppl.8513 [DOI] [Google Scholar]
- 145. Lu S, Wang J, Yang N, et al. Savolitinib (savo) combined with osimertinib (osi) versus chemotherapy (chemo) in EGFR‐mutant (EGFRm) and MET‐amplification (METamp) advanced NSCLC after disease progression (PD) on EGFR tyrosine kinase inhibitor (TKI): results from a randomized phase 3 SACHI study [abstract]. J Clin Oncol. 2025;43(17 suppl):LBA8505. doi: 10.1200/JCO.2025.43.17_suppl.LBA8505 [DOI] [Google Scholar]
- 146. Horinouchi H, Cho BC, Camidge DR, et al. 515MO Phase Ib study of telisotuzumab vedotin (Teliso‐V) and osimertinib in patients (Pts) with advanced EGFR‐mutated (Mut), c‐Met overexpressing (OE) non‐small cell lung cancer (NSCLC): final efficacy and safety updates [abstract]. Ann Oncol. 2023;34(suppl 4):S1670. doi: 10.1016/j.annonc.2023.10.594 [DOI] [Google Scholar]
- 147. Yu H, Goldberg S, Le X, et al. P2.01‐22 ORCHARD: a phase II platform study in patients with advanced NSCLC who have progressed on first‐line osimertinib therapy [abstract]. J Thorac Oncol. 2019;14(10 suppl):S647. doi: 10.1016/j.jtho.2019.08.1366 [DOI] [Google Scholar]
- 148. Aguilar A, Cobo M, Azkarate A, et al. 79TiP Progress of a phase I trial (TOTEM) of repotrectinib in combination with osimertinib in advanced, metastatic EGFR mutant NSCLC [abstract]. J Thorac Oncol. 2023;18(4 suppl):S85‐S86. doi: 10.1016/S1556-0864(23)00333-7 [DOI] [Google Scholar]
- 149. Rotow J, Patel J, Hanley M, et al. FP14.07 Combination osimertinib plus selpercatinib for EGFR‐mutant non‐small cell lung cancer (NSCLC) with acquired RET fusions [abstract]. J Thorac Oncol. 2021;16(3 suppl):S230. doi: 10.1016/j.jtho.2021.01.150 [DOI] [Google Scholar]
- 150. Offin M, Somwar R, Rekhtman N, et al. Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR‐mutant lung cancers. JCO Precis Oncol. 2018;2:PO.18.00126. doi: 10.1200/PO.18.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR‐mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU‐667 for acquired RET fusion. Cancer Discov. 2018;8(12):1529‐1539. doi: 10.1158/2159-8290.CD-18-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Batra U, Sharma M, Amrith BP, Mehta A, Jain P. EML4‐ALK fusion as a resistance mechanism to osimertinib and its successful management with osimertinib and alectinib: case report and review of the literature. Clin Lung Cancer. 2020;21(6):e597‐e600. doi: 10.1016/j.cllc.2020.05.016 [DOI] [PubMed] [Google Scholar]
- 153. Lu K, Tse V, Altaie G, Husain H. Efficacy and tolerability of osimertinib with dabrafenib and trametinib in BRAF V600E acquired EGFR‐mutant non‐small cell lung cancer: a case series. J Thorac Dis. 2024;16(8):5379‐5387. doi: 10.21037/jtd-23-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Oxnard GR, Yang JCH, Yu H, et al. TATTON: a multi‐arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR‐mutant lung cancer. Ann Oncol. 2020;31(4):507‐516. doi: 10.1016/j.annonc.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 155. Grazini U, Markovets A, Ireland L, et al. Overcoming osimertinib resistance with AKT inhibition in EGFRm‐driven non–small cell lung cancer with PIK3CA/PTEN alterations. Clin Cancer Res. 2024;30(18):4143‐4154. doi: 10.1158/1078-0432.CCR-23-2540 [DOI] [PubMed] [Google Scholar]
- 156. de Jager VD, Stigt JA, Niemantsverdriet M, ter Elst A, van der Wekken AJ. Osimertinib and palbociclib in an EGFR‐mutated NSCLC with primary CDK4 amplification after progression under osimertinib. NPJ Precis Oncol. 2024;8(1):113. doi: 10.1038/s41698-024-00607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Jebbink M, de Langen AJ, Monkhorst K, et al. Trastuzumab‐emtansine and osimertinib combination therapy to target HER2 bypass track resistance in EGFR mutation‐positive NSCLC. JTO Clin Res Rep. 2023;4(4):100481. doi: 10.1016/j.jtocrr.2023.100481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mok T, Nakagawa K, Park K, et al. Nivolumab plus chemotherapy in epidermal growth factor receptor–mutated metastatic non–small‐cell lung cancer after disease progression on epidermal growth factor receptor tyrosine kinase inhibitors: final results of CheckMate 722. J Clin Oncol. 2024;42(11):1252‐1264. doi: 10.1200/JCO.23.01017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yang JCH, Lee DH, Lee JS, et al. Phase III KEYNOTE‐789 study of pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor‒resistant, EGFR–mutant, metastatic nonsquamous non–small cell lung cancer. J Clin Oncol. 2024;42(34):4029‐4039. doi: 10.1200/JCO.23.02747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR‐mutation‐positive non‐small‐cell lung cancer after progression on first‐line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990‐998. doi: 10.1016/S1470-2045(15)00121-7 [DOI] [PubMed] [Google Scholar]
- 161. Sequist LV, Peled N, Tufman A, et al. P47.11 COMPEL: chemotherapy with/without osimertinib in patients with EGFRm advanced NSCLC and progression on first‐line osimertinib [abstract]. J Thorac Oncol. 2021;16(10 suppl):S1101. doi: 10.1016/j.jtho.2021.08.504 [DOI] [Google Scholar]
- 162. Besse B, Baik CS, Marmarelis ME, et al. Predictive biomarkers for treatment with amivantamab plus lazertinib among EGFR‐mutated NSCLC in the post‐osimertinib setting: analysis of tissue IHC and ctDNA NGS [abstract]. J Clin Oncol. 2023;41(16 suppl):9013. doi: 10.1200/JCO.2023.41.16_suppl.9013 [DOI] [Google Scholar]
- 163. Califano R, Passaro A, Tan JL, et al. Amivantamab plus chemotherapy vs chemotherapy in EGFR‐mutant advanced NSCLC after disease progression on osimertinib: outcomes by osimertinib resistance mechanisms in MARIPOSA‐2 [abstract]. J Clin Oncol. 2025;43(16 suppl):8639. doi: 10.1200/JCO.2025.43.16_suppl.8639 [DOI] [Google Scholar]
- 164. Goulart BHL, Larkins E, Beaver JA, Singh H. Continuation of third‐generation tyrosine kinase inhibitors in second‐line trials for EGFR‐mutated non‐small‐cell lung cancer: regulatory considerations. J Clin Oncol. 2023;41(23):3905‐3908. doi: 10.1200/JCO.23.00154 [DOI] [PubMed] [Google Scholar]
- 165. Lu S, Wu L, Jian H, et al. Sintilimab plus chemotherapy for patients with EGFR‐mutated non‐squamous non‐small‐cell lung cancer with disease progression after EGFR tyrosine‐kinase inhibitor therapy (ORIENT‐31): second interim analysis from a double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Respir Med. 2023;11(7):624‐636. doi: 10.1016/S2213-2600(23)00135-2 [DOI] [PubMed] [Google Scholar]
- 166. Park S, Kim TM, Han JY, et al. Phase III, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR‐ or ALK‐rearranged or translocated non–small‐cell lung cancer (ATTLAS, KCSG‐LU19‐04). J Clin Oncol. 2024;42(11):1241‐1251. doi: 10.1200/JCO.23.01891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non‐small‐cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open‐label phase 3 trial. Lancet Respir Med. 2019;7(5):387‐401. doi: 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 168. Zhou C, Dong X, Chen G, et al. OA09.06 IMpower151: phase III study of atezolizumab + bevacizumab + chemotherapy in 1L metastatic nonsquamous NSCLC [abstract]. J Thorac Oncol. 2023;18(11 suppl):S64‐S65. doi: 10.1016/j.jtho.2023.09.059 [DOI] [Google Scholar]
- 169. Zhao Y, He Y, Wang W, et al. Efficacy and safety of immune checkpoint inhibitors for individuals with advanced EGFR‐mutated non‐small‐cell lung cancer who progressed on EGFR tyrosine‐kinase inhibitors: a systematic review, meta‐analysis, and network meta‐analysis. Lancet Oncol. 2024;25(10):1347‐1356. doi: 10.1016/S1470-2045(24)00379-6 [DOI] [PubMed] [Google Scholar]
- 170. Piotrowska Z, Yeap BY, Gainor JF. Chemotherapy and programmed cell death protein 1/programmed death‐ligand 1 inhibitor combinations for tyrosine kinase inhibitor‐resistant, epidermal growth factor receptor‐mutated non‐small‐cell lung cancer: a meta‐analysis. ESMO Open. 2024;9(9):103660. doi: 10.1016/j.esmoop.2024.103660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. HARMONi‐A Study Investigators , Fang W, Zhao Y, et al. Ivonescimab plus chemotherapy in non–small cell lung cancer with EGFR variant: a randomized clinical trial. JAMA. 2024;332(7):561‐570. doi: 10.1001/jama.2024.10613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sands J, Ahn MJ, Lisberg A, et al. Datopotamab deruxtecan in advanced or metastatic non–small cell lung cancer with actionable genomic alterations: results from the phase II TROPION‐Lung05 study. J Clin Oncol. 2025;43(10):1254‐1265. doi: 10.1200/JCO-24-01349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ahn MJ, Lisberg AE, Paz‐Ares L, et al. 509MO Datopotamab deruxtecan (Dato‐DXd) vs docetaxel in previously treated advanced/metastatic (adv/met) non‐small cell lung cancer (NSCLC): results of the randomized phase III study TROPION‐Lung01 [abstract]. Ann Oncol. 2023;34:S1665‐S1666. doi: 10.1016/j.annonc.2023.10.588 [DOI] [Google Scholar]
- 174. Le X, Hendriks L, Morabito A, et al. 1O: Osimertinib (osi) + datopotamab deruxtecan (Dato‐DXd) in patients (pts) with EGFR‐mutated (EGFRm) advanced NSCLC (aNSCLC) whose disease progressed on first‐line (1L) osi: ORCHARD [abstract]. J Thorac Oncol. 2025;20(3 suppl 1):S2‐S4. doi: 10.1016/S1556-0864(25)00196-0 [DOI] [Google Scholar]
- 175. Yu HA, Goto Y, Hayashi H, et al. HERTHENA‐Lung01, a phase II trial of patritumab deruxtecan (HER3‐DXd) in epidermal growth factor receptor–mutated non–small‐cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum‐based chemotherapy. J Clin Oncol. 2023;41(35):5363‐5375. doi: 10.1200/JCO.23.01476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mok TSK, Yu HA, Lim SM, et al. Patritumab deruxtecan (HER3‐DXd) in resistant EGFR‐mutated (EGFRm) advanced non‐small cell lung cancer (NSCLC) after a third‐generation EGFR TKI: the phase 3 HERTHENA‐Lung02 study [abstract]. J Clin Oncol. 2025;43(16 suppl):8506. doi: 10.1200/JCO.2025.43.16_suppl.8506 [DOI] [Google Scholar]
- 177. Fang W, Cheng Y, Chen Z, et al. Abstract CT247: Updated efficacy and safety of anti‐TROP2 ADC SKB264 (MK‐2870) for previously treated advanced NSCLC in phase 2 study. Cancer Res. 2024;84(7 suppl):CT247. doi: 10.1158/1538-7445.AM2024-CT247 [DOI] [Google Scholar]
- 178. Zhang L, Fang W, Li X, et al. Sacituzumab tirumotecan (sac‐TMT) in patients (pts) with previously treated advanced EGFR‐mutated non‐small cell lung cancer (NSCLC): results from the randomized OptiTROP‐Lung03 study [abstract]. J Clin Oncol. 2025;43(16 suppl):8507. doi: 10.1200/JCO.2025.43.16_suppl.8507 [DOI] [Google Scholar]
- 179. Ferrer L, Giaj Levra M, Brevet M, et al. A brief report of transformation from NSCLC to SCLC: molecular and therapeutic characteristics. J Thorac Oncol. 2019;14(1):130‐134. doi: 10.1016/j.jtho.2018.08.2028 [DOI] [PubMed] [Google Scholar]
- 180. Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small‐cell lung cancer. Nat Commun. 2015;6(1):6377. doi: 10.1038/ncomms7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Saalfeld FC, Möller J, Christopoulos P, et al. Small cell transformation in EGFR‐mutated non‐small cell lung cancer: DLL3 expression and efficacy of immune checkpoint inhibitors or tyrosine kinase inhibitors combined with chemotherapy. Eur J Cancer. 2024;213:115065. doi: 10.1016/j.ejca.2024.115065 [DOI] [PubMed] [Google Scholar]
- 182. Wang S, Xie T, Hao X, et al. Comprehensive analysis of treatment modes and clinical outcomes of small cell lung cancer transformed from epidermal growth factor receptor mutant lung adenocarcinoma. Thorac Cancer. 2021;12(19):2585‐2593. doi: 10.1111/1759-7714.14144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Horn L, Mansfield AS, Szczęsna A, et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med. 2018;379(23):2220‐2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 184. Paz‐Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first‐line treatment of extensive‐stage small‐cell lung cancer (CASPIAN): a randomised, controlled, open‐label, phase 3 trial. Lancet. 2019;394(10212):1929‐1939. doi: 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 185. Zhang CY, Sun H, Su JW, et al. A potential treatment option for transformed small‐cell lung cancer on PD‐L1 inhibitor‐based combination therapy improved survival. Lung Cancer. 2023;175:68‐78. doi: 10.1016/j.lungcan.2022.11.016 [DOI] [PubMed] [Google Scholar]
- 186. Ahn MJ, Cho BC, Ou X, et al. Osimertinib plus durvalumab in patients with EGFR‐mutated, advanced NSCLC: a phase 1b, open‐label, multicenter trial. J Thorac Oncol. 2022;17(5):718‐723. doi: 10.1016/j.jtho.2022.01.012 [DOI] [PubMed] [Google Scholar]
- 187. Jung HA, Ku BM, Kim YJ, et al. Longitudinal monitoring of circulating tumor DNA from plasma in patients with curative resected stages I to IIIA EGFR‐mutant non–small cell lung cancer. J Thorac Oncol. 2023;18(9):1199‐1208. doi: 10.1016/j.jtho.2023.05.027 [DOI] [PubMed] [Google Scholar]
- 188. Sánchez‐Herrero E, Serna‐Blasco R, Robado de Lope L, González‐Rumayor V, Romero A, Provencio M. Circulating tumor DNA as a cancer biomarker: an overview of biological features and factors that may impact on ctDNA analysis. Front Oncol. 2022;12:943253. doi: 10.3389/fonc.2022.943253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Herbst RS, John T, Grohé C, et al. Molecular residual disease analysis of adjuvant osimertinib in resected EGFR‐mutated stage IB–IIIA non‐small‐cell lung cancer. Nat Med. 2025;31(6):1958‐1968. doi: 10.1038/s41591-025-03577-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Black JRM, Frankell AM, Veeriah S, et al. LBA55 An ultra‐sensitive and specific ctDNA assay provides novel pre‐operative disease stratification in early stage lung cancer [abstract]. Ann Oncol. 2023;34:S1294. doi: 10.1016/j.annonc.2023.10.049 [DOI] [Google Scholar]
- 191. Valdiviezo N, Gray JE, Jänne PA, et al. MA12.04 FLAURA2: impact of tumor burden on outcomes of 1L osimertinib ± chemotherapy in patients with EGFR‐mutated advanced NSCLC [abstract]. J Thorac Oncol. 2024;19(10 suppl):S102. doi: 10.1016/j.jtho.2024.09.185 [DOI] [Google Scholar]
- 192. Yang JC, Robichaux J, Planchard D, et al. MA12.03 FLAURA2: resistance, and impact of baseline TP53 alterations in patients treated with 1L osimertinib ± platinum‐pemetrexed [abstract]. J Thorac Oncol. 2024;19(10 suppl):S101‐S102. doi: 10.1016/j.jtho.2024.09.184 [DOI] [Google Scholar]
- 193. Jänne PA, Kobayashi K, Robichaux J, et al. Abstract CT017: FLAURA2: exploratory analysis of baseline (BL) and on‐treatment plasma EGFRm dynamics in patients (pts) with EGFRm advanced NSCLC treated with first‐line (1L) osimertinib (osi) ± platinum‐pemetrexed. Cancer Res. 2024;84(7 suppl):CT017. doi: 10.1158/1538-7445.AM2024-CT017 [DOI] [Google Scholar]


