Abstract
TOPLESS (TPL) and TOPLESS-Related (TPR) corepressors are key regulatory proteins that interact with a variety of transcription factors to form specific complexes, thereby modulating a wide range of signaling pathways and metabolic processes. This study explored the function of the rice TPR gene OsTPR1. Transgenic rice lines overexpressing OsTPR1 (OsTPR1-Ox) exhibited reduced lateral root density, whereas OsTPR1 RNA interference lines (OsTPR1-Ri) showed increased lateral root density. To gain further insight, these transgenic lines were crossed with the DR5::GUS auxin reporter line. In 7-day-old seedlings, lateral root formation occurred in the differentiation zone of seminal roots, with GUS staining prominently localized in the lateral root primordia of the DR5::GUS/OsTPR1-Ri line. Similar results were observed in 45-day-old seedlings, where the DR5::GUS/OsTPR1-Ri line exhibited stronger GUS staining and a higher number of lateral roots in the crown root differentiation zones. In contrast, the DR5::GUS/OsTPR1-Ox line showed weaker GUS signals and fewer lateral roots. Additionally, the expression levels of several auxin efflux transporter genes encoding PIN-FORMED (PIN) proteins, including OsPIN1a, OsPIN1b, OsPIN1c, OsPIN2, and OsPIN5a, were increased in the OsTPR1-Ri line but decreased in the OsTPR1-Ox line. These results suggest that OsTPR1 also modulates the expression of multiple OsPIN genes, thereby potentially influencing auxin responses and lateral root development. Beyond root development, OsTPR1 overexpression led to a significant increase in tiller angle and a delay in flowering time, whereas OsTPR1-Ri plants exhibited earlier flowering. These findings indicate that OsTPR1 acts as a negative regulator of the auxin response, with its overexpression leading to reduced auxin sensitivity and altered plant architecture. Our results show that OsTPR1 modulates lateral root development, tiller angle, and flowering time, contributing to the coordinated growth and development in rice.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-10224-6.
Keywords: Rice, TOPLESS-related protein (OsTPR1), Auxin, Lateral root, Tiller angle, Heading date
Subject terms: Biotechnology, Plant sciences
The TOPLESS (TPL) protein and its related family members, the TOPLESS-related proteins (TPRs), were initially identified in Arabidopsis thaliana, where they interact with the homeodomain transcriptional repressor WUSCHEL (WUS)1. WUS suppresses gene transcription through histone deacetylation, thereby negatively regulating apical meristem differentiation2,3. All TPL/TPR homologs contain an N-terminal LisH (Lissencephaly type-1-like homology) domain, followed by a CTLH (C-terminal to LisH) domain, and a C-terminal WD40 repeat domain. Functional analyses in Arabidopsis have demonstrated that TPL/TPR orthologs act as transcriptional corepressors involved in a variety of biological processes, particularly hormone signaling, including auxin4jasmonic acid (JA)5and gibberellic acid (GA)6. Additional functions include regulation of flowering time7leaf and floral development8,9and responses to biotic stress10.
In rice, a TPR gene named ASP1 (ABERRANT SPIKELET AND PANICLE 1) was identified from a TOS17 insertion mutant population11. The asp1 mutant exhibited multiple panicle abnormalities, including reduced inflorescence size, disorganized primary and secondary branching, and malformed spikelets. Aberrant outgrowth of axillary buds and irregular tiller formation were also observed. The FLORAL ORGAN NUMBER2 (fon2-3) mutant, which forms flowers with two or three pistils, displays an enhanced phenotype when combined with the asp1 mutation. Complementation with ASP1 rescued this phenotype, indicating that ASP1 and FON2 function synergistically to regulate floral organ differentiation12. Similarly, asp-lsl (aberrant spikelet-long sterile lemma), a point mutant identified from an EMS-mutagenized population, showed a Gly521Glu substitution that resulted in shorter plants, fewer tillers, reduced grain size, and elongated sterile lemmas13. These findings suggest that ASP1 homologs have pleiotropic roles in rice development.
Mechanistically, TPR proteins act through interaction with transcriptional repressors that contain an EAR (ethylene-responsive element binding factor-associated amphiphilic repression) motif. The CTLH domain of TPRs binds to EAR motifs and recruits histone deacetylases (HDACs), leading to transcriptional repression via reduced histone acetylation14. In rice, the transcriptional repressor NSG1 (NONSTOP GLUMES 1) was shown to interact with all three TPR homologs (OsTPR1, OsTPR2, OsTPR3) and repress the expression of LHS1 (LEAFY HULL STERILE 1) by lowering histone acetylation levels within the first intron of the gene. This repression ultimately affects normal spikelet development15. MYB repressors have also been shown to interact with OsTPRs to regulate spikelet development16,17. The url1 (upward rolled leaf 1) mutant, characterized by adaxially rolled leaves due to reduced bulliform cell number and size, further supports this mechanism. URL1, which contains an EAR motif, functions as a transcriptional repressor by binding OsTPR2 to repress ACL1 (abaxially curled leaf 1), thus modulating leaf architecture18.
The rice root system comprises four root types: seminal roots, crown roots, and large (L-type) and small (S-type) lateral roots19,20. L-type lateral roots are long, branched, and composed of multiple cortical layers, whereas S-type roots are short, unbranched, and contain a single cortical layer21. Lateral roots enhance nutrient and water uptake by increasing root surface area22. Their development, originating from pericycle and endodermal cells, is tightly regulated by auxin19,23,24. In the radicleless 1 (ral1) mutant, which is defective in auxin sensing, both crown and lateral root development are impaired25. The crown rootless 1 (crl1) mutant, isolated from an MNU-treated population, is defective in crown root initiation and encodes an LOB family transcription factor regulated by auxin via ARFs26. These findings underscore the critical role of auxin in root formation.
Auxin also regulates many aspects of plant growth through a signaling pathway involving AUX/IAA repressors and ARF activators27. In the presence of auxin, the F-box protein TIR1 (TRANSPORT INHIBITOR RESPONSE 1) promotes AUX/IAA degradation via the ubiquitin-proteasome pathway. In the absence of auxin, AUX/IAA proteins bind ARFs and recruit TPL corepressors to inhibit transcriptional activation5,28. These results highlight the role of TPL/TPRs in mediating auxin response.
Although many components of auxin signaling have been characterized, the regulatory mechanisms that fine-tune auxin responses across developmental contexts remain incompletely understood. In this study, we show that the transcriptional corepressor OsTPR1 influences auxin response and plays crucial roles in lateral root initiation, tiller architecture, and heading date in rice. Our findings demonstrate that OsTPR1 functions as a negative regulator of auxin sensitivity, contributing to the precise regulation of developmental processes. These results suggest that manipulation of OsTPR1 may provide a potential strategy to improve rice growth and yield.
Results
OsTPR1 regulates plant architecture and flowering time in rice
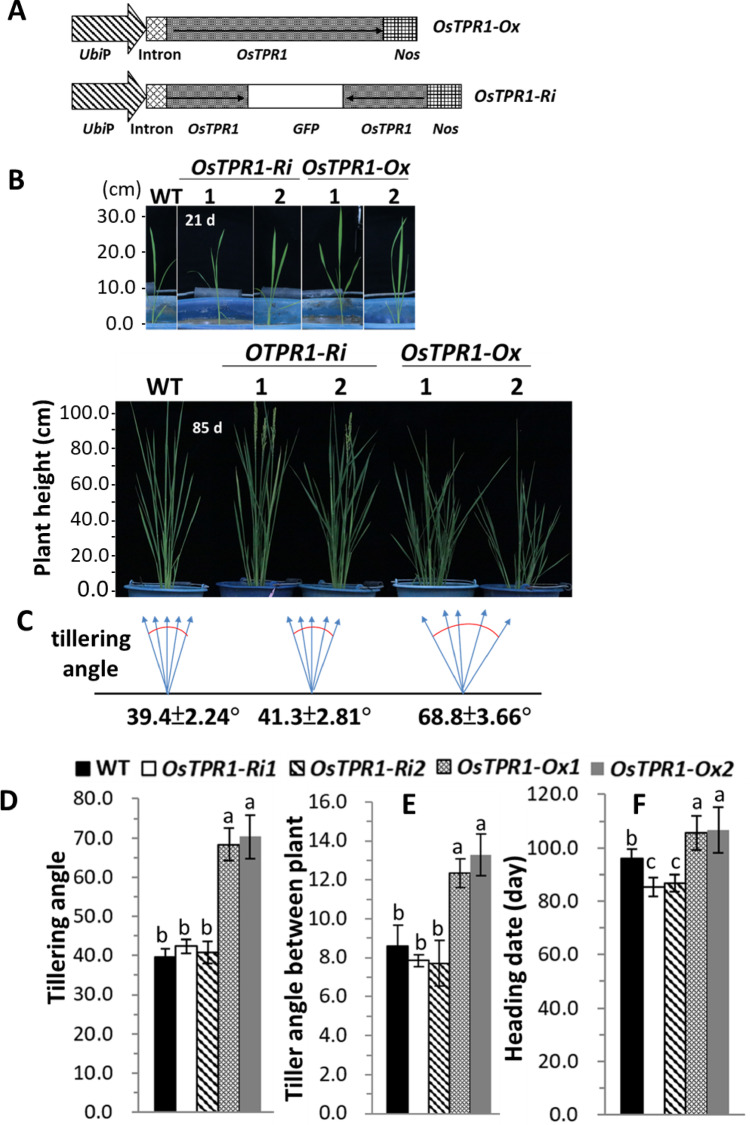
Transcriptional corepressors regulate a variety of physiological processes by interacting with repressor proteins. We previously identified the rice corepressor OsTPR1 (NM_001056122) from a cDNA library generated from sucrose-starved rice suspension cells29. In rice, three OsTPR homologs have been reported11. Amino acid sequence alignment revealed that OsTPR1 shares 68% and 63% identity with OsTPR2 (NM_001067590) and OsTPR3 (NM_001049151), respectively. To investigate the functional role of OsTPR1 in rice development, we generated transgenic rice lines either overexpressing (OsTPR1-Ox) or silencing (OsTPR1-Ri) the gene under the control of the maize ubiquitin promoter30 (Fig. 1A).
Fig. 1.
Comparison of plant height, tiller angle, and heading date among WT, OsTPR1-Ox, and OsTPR1-Ri lines. (A) Schematic representation of the overexpression construct (OsTPR1-Ox) and the RNA interference (RNAi) silencing construct (OsTPR1-Ri). (B) Growth phenotypes of WT and T₃ transgenic rice plants at 21 and 85 days of age. The 21-day-old seedlings were transplanted from culture pots into soil-filled buckets, and phenotypes were analyzed 64 days after transplantation (at 85-day-old). (C) Diagram illustrating how total tiller angle was calculated as the angle between the outermost tillers of each plant. (D) Quantification of total tiller angle among WT, OsTPR1-Ox, and OsTPR1-Ri lines. (E) Quantification of mean inter-plant tiller angles. (F) Comparison of heading date among genotypes. Data represent means ± SD from three independent experiments (n = 3). Statistical significance was determined using one-way ANOVA followed by Tukey’s post-hoc test. Different letters above the bars indicate statistically significant differences (P < 0.01).
The T2 transgenic plants were used for phenotypic analysis. To evaluate whether OsTPR1 affects rice development, 21-day-old WT, OsTPR1-Ox (OsTPR1-Ox-1, OsTPR1-Ox-2), and OsTPR1-Ri (OsTPR1-Ri-1, OsTPR1-Ri-2) seedlings were transplanted into soil (Fig. 1B). By 85 days, OsTPR1-Ox plants exhibited significantly reduced plant height (OsTPR1-Ox-1: 72 ± 2.8 cm; OsTPR1-Ox-2: 70 ± 2.6 cm) compared to WT (90 ± 3.1 cm), whereas OsTPR1-Ri lines (OsTPR1-Ri-1: 92 ± 3.4 cm; OsTPR1-Ri-2: 93 ± 2.7 cm) were slightly taller, with no significant difference from WT.
Heading date analysis revealed that OsTPR1-Ri-1 and OsTPR1-Ri-2 flowered earlier (84 ± 2.5 and 85 ± 2.2 days, respectively) compared to WT (98 ± 2.1 days), while OsTPR1-Ox-1 and OsTPR1-Ox-2 showed delayed flowering (105 ± 3.3 and 103 ± 2.6 days, respectively) (Fig. 1F). Notably, tiller angle was also influenced by OsTPR1. OsTPR1-Ox plants exhibited a significantly wider total tiller angle (~ 68.8°) and inter-plant tiller angle (~ 12.8°), compared to WT and OsTPR1-Ri lines, which had total angles of ~ 39.4° and 41.3° and inter-plant angles of − 8.6° and 7.8°, respectively (Fig. 1C-E). These findings indicate that OsTPR1 promotes increased tiller angle and delays heading date. Given the consistency of these phenotypes, OsTPR1-Ox-1 and OsTPR1-Ri-1 lines were selected for further experiments.
OsTPR1 negatively regulates lateral root development
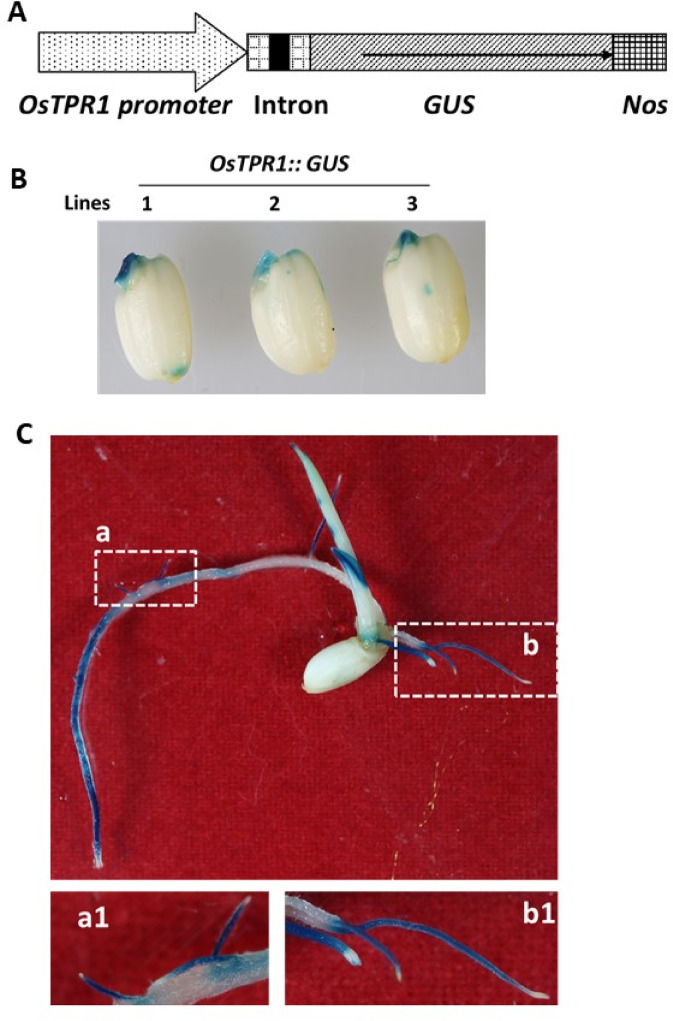
Given the influence of OsTPR1 on shoot architecture, we next examined its role in root development. We constructed an OsTPR1::GUS reporter by fusing the OsTPR1 promoter (including its first intron within the 5′ UTR) to the GUS reporter gene (Fig. 2A). GUS staining was strong in 3-day-old germinating embryos but nearly absent in the endosperm and aleurone layers (Fig. 2B). In 7-day-old seedlings, intense GUS staining was observed in the elongation zones of seminal and crown roots, and in emerging lateral roots, but was weak in the differentiation zone and absent from the root cap (Fig. 2C). These patterns suggest a role for OsTPR1 in root development.
Fig. 2.
Histochemical staining of β-glucuronidase (GUS) activity in transgenic OsTPR1::GUS lines. (A) Diagram of the OsTPR1::GUS construct, including the OsTPR1 promoter and first intron located within the 5′ untranslated region. (B) GUS staining of 3-day-old germinating seeds from three independent lines. (C) GUS staining of 7-day-old seedlings. Dashed boxes indicate local differentiation zones in seminal roots (a), and developing crown roots (b). Corresponding enlarged views are shown in a1 and b1, respectively.
To evaluate its functional role, we quantified lateral root traits in 20-day-old seedlings. OsTPR1-Ri-1 plants exhibited significantly more lateral roots in the crown root differentiation zone (18.3 ± 2.3 cm⁻¹) than WT (12.3 ± 1.8 cm⁻¹), while OsTPR1-Ox-1 plants had fewer lateral roots (6.3 ± 0.9 cm⁻¹) (Fig. 3A, B). Lateral root length also differed significantly: OsTPR1-Ri-1 roots were longest (0.95 ± 0.12 cm), followed by WT (0.45 ± 0.09 cm), with OsTPR1-Ox-1 showing the shortest roots (0.21 ± 0.04 cm) (Fig. 3C). These findings indicate that OsTPR1 negatively regulates lateral root formation and elongation in rice.
Fig. 3.
Comparison of lateral root development between WT and transgenic lines. (A) Representative images of crown root differentiation zones from 20-day-old seedlings. (B) Quantification of lateral root density. (C) Quantification of average lateral root length. Data are presented as means ± SD (n = 3). Statistical significance was evaluated using one-way ANOVA followed by Tukey’s post-hoc test. Different letters above the bars indicate statistically significant differences at P < 0.05 in (B) and P < 0.01 in (C).
OsTPR1 modulates lateral root development through auxin signaling
Given the known role of auxin in root development, and the phenotypic effects observed in OsTPR1 transgenic lines, we hypothesized that OsTPR1 functions through auxin signaling pathways. To test this, OsTPR1-Ox-1 and OsTPR1-Ri-1 lines were crossed with the auxin-responsive DR5::GUS reporter line31,32producing DR5::GUS/OsTPR1-Ox-1 and DR5::GUS/OsTPR1-Ri-1 hybrids. RT-PCR confirmed overexpression and silencing of OsTPR1 in the respective F2 hybrid lines (Fig. 4A, B).
Fig. 4.
Expression of OsTPR1 and GUS staining of roots in hybrid rice lines. (A) RT-PCR analysis of OsTPR1 expression in hybrid rice lines DR5::GUS/OsTPR1-Ox-1 and DR5::GUS/OsTPR1-Ri-1. Total RNA was extracted from 7-day-old embryos and amplified using OsTPR1-specific primers targeting the 3′ untranslated region. (B) Quantification of OsTPR1 transcript levels from panel A. Relative gene expression was normalized to the internal control (OsActin1), with the DR5::GUS value set to 1.0. (C) GUS staining of 7-day-old germinated seedlings. Dashed boxes indicate the differentiation zones of seminal roots in DR5::GUS (a), DR5::GUS/OsTPR1-Ri-1 (b), and DR5::GUS/OsTPR1-Ox-1 (c). Corresponding enlarged views are shown in a1, b1, and c1, respectively. (D) The expression levels of the β-glucuronidase (GUS) gene in the roots of 14-day-old seedlings were analyzed by qRT-PCR. Total RNA was isolated from the differentiation zones (including lateral roots) of the seminal and crown roots of 14-day-old seedlings from the DR5::GUS, DR5::GUS/OsTPR1-Ox, and DR5::GUS/OsTPR1-Ri lines and subjected to qRT-PCR analysis. The relative expression level of the GUS gene was normalized to that of the internal control OsActin. The expression level of the GUS gene in the NAA untreated DR5::GUS line was set as 1.0. (E) GUS staining of 55-day-old roots. Dashed boxes indicate local differentiation zones in seminal roots of DR5::GUS (a), DR5::GUS/OsTPR1-Ri-1 (b), and DR5::GUS/OsTPR1-Ox-1 (c). Corresponding enlarged views are shown in a1, b1, and c1, respectively. (F) Quantification of S-type lateral root density in DR5::GUS, DR5::GUS/OsTPR1-Ri-1, and DR5::GUS/OsTPR1-Ox-1 lines. Data represent means ± SD from three independent experiments (n = 3). Statistical significance was determined using one-way ANOVA followed by Tukey’s post-hoc test. Different letters above the bars indicate statistically significant differences (P < 0.05).
GUS staining of 7-day-old roots revealed strong GUS activity in the elongation zones of all genotypes (DR5::GUS as control, DR5::GUS/OsTPR1-Ox-1, and DR5::GUS/OsTPR1-Ri-1) (Fig. 4C, panels a-c). However, GUS activity in the differentiation zone, particularly in the lateral root primordia, was observed only in DR5::GUS/OsTPR1-Ri-1 roots (Fig. 4C, panel b, b1). No such activity was detected in DR5::GUS or DR5::GUS/OsTPR1-Ox-1 lines (Fig. 4C, panels a and a1, c and c1). These results suggest that OsTPR1 plays a negative role in auxin signaling. To further confirm this finding, we analyzed the expression levels of the GUS gene in these three lines treated with or without 1-naphthaleneacetic acid (NAA). Total RNA was isolated from the differentiation zones (including lateral roots) of the seminal and crown roots of 14-day-old seedlings from the DR5::GUS, DR5::GUS/OsTPR1-Ox, and DR5::GUS/OsTPR1-Ri lines and subjected to qRT-PCR analysis. As shown in Fig. 4D, the GUS gene expression was significantly induced in all three lines following NAA treatment compared to the untreated conditions. Although the differences in fold induction of GUS expression among the three lines were not statistically significant. However, when compared to the DR5::GUS control line, GUS expression was increased in the DR5::GUS/OsTPR1-Ri line and decreased in the DR5::GUS/OsTPR1-Ox line, under both treated and untreated conditions. These results suggest that the negative effect of OsTPR1 on auxin signaling in the roots of rice seedlings.
To assess long-term effects on lateral root development, 10-day-old seedlings were transferred to soil and grown for an additional 45 days. At 55 days, roots were carefully excavated and subjected to GUS staining. DR5::GUS/OsTPR1-Ri-1 roots exhibited stronger GUS activity and more S-type lateral roots in crown root differentiation zones compared to DR5::GUS controls, whereas DR5::GUS/OsTPR1-Ox-1 roots showed weaker GUS staining and fewer lateral roots (Fig. 4E, F). These results support the hypothesis that OsTPR1 negatively regulates lateral root development through modulation of auxin responses.
PIN-FORMED proteins (PINs) are essential for auxin transport, functioning as efflux carriers that mediate the polar movement of auxin from shoot to root tissues33. This directional transport establishes auxin gradients that regulate both shoot growth and root development34. In Fig. 3, analysis of 20-day-old seedlings revealed a significant difference in lateral root number among WT, OsTPR1-Ox, and OsTPR1-Ri lines. To investigate whether OsTPR1 influences the expression of rice OsPIN genes, potentially explaining the observed differences in lateral root development among the three lines. We analyzed the expression of OsPIN genes in the roots of 20-day-old seedlings using qRT-PCR. The results (Fig. 5) show that the expression levels of OsPIN1a, OsPIN1b, OsPIN1c, OsPIN2, and OsPIN5a were upregulated in OsTPR1-Ri lines and downregulated in OsTPR1-Ox lines (Fig. 5B, C, D, F, and G). In contrast, OsPIN10a expression was reduced in OsTPR1-Ri and increased in OsTPR1-Ox plants (Fig. 5H). No significant change was observed in OsPIN1d expression (Fig. 5E). These findings suggest that OsTPR1 affects the expression of multiple OsPIN family members, thereby potentially influencing lateral root development.
Fig. 5.
Expression levels of OsPIN genes in roots of 20-day-old seedlings. Root samples were collected by pooling ten seedlings per line for each biological replicate. Total RNA was isolated from roots of 20-day-old seedling of WT, OsTPR1-Ox-1, and OsTPR1-Ri-1 plants and subjected to qRT-PCR analysis. Relative gene expression was normalized to the internal control (OsActin1), with the WT value set to 1.0. Data represent means ± SD from single biological replicate with three technical repeats (n = 3). Statistical significance was determined using one-way ANOVA followed by Tukey’s post-hoc test. Different letters above the bars indicate statistically significant differences (P < 0.01). Primer sequences and gene accession numbers are provided in Supplementary Table S1.
The SMALL AUXIN UP RNA (SAUR) gene family comprises early auxin-responsive genes that play diverse roles in plant development35,36. To further evaluate whether OsTPR1 influences auxin response and, consequently, affects the expression of OsSAUR genes in roots of rice seedling. We examined the differential expression of OsSAUR genes in WT, OsTPR1-Ri, and OsTPR1-Ox lines. There are 58 OsSAURs in rice35based on expression profiles from the RiceXPro database37 and heatmap analysis36five OsSAUR genes were selected for expression analysis during rice root development, three predicted auxin-induced genes (OsSAUR10, OsSAUR11, and OsSAUR30), and two predicted auxin-repressed genes (OsSAUR9 and OsSAUR55). The results showed that NAA treatment increased OsSAUR10 expression in the roots by 0.28 ± 0.08-fold in WT plants and 0.67 ± 0.06-fold in OsTPR1-Ri plants, but had no effect in OsTPR1-Ox roots (Fig. S1). In contrast, the other four OsSAUR genes examined did not show significant changes in expression in response to NAA treatment. If OsTPR1 influences the auxin response to regulate lateral root development through an OsSAUR-dependent pathway, OsSAUR10 is likely the candidate gene involved.
Discussion
To date, many studies have shown that the TPR family can act as universal corepressors to regulate various biotic and abiotic stresses, plant growth and development, and hormone signaling responses. This regulation is mediated through the TPD domain’s interaction with the EAR motif located in different transcriptional repressors38. Therefore, it is important to understand how TPR corepressors respond to various signals to balance plant growth, development, and environmental stress responses. In this study, we isolated a TPR orthologous gene, OsTPR1, from rice. Transgene overexpression and gene silencing experiments revealed that OsTPR1 increases the tiller angle during the tillering stage and delays the heading date during the flowering stage (Fig. 1). Our results also indicate that OsTPR1 negatively regulates rice lateral root development (Figs. 2, 3 and 4). These findings suggest that OsTPR1 plays multifaceted roles in rice lateral root development, tillering angle, and heading date by influences auxin signaling responses.
Root system is a key agronomic trait that plays an important role in soil conservation, water and nutrient uptake, plant-microbe interactions, and supporting overall plant growth39. There are two main types of root systems in plants, taproot systems in dicot and fibrous root systems in monocot. The fibrous root system consists of adventitious roots (crown roots) growing from the base of the stem, lateral roots, and root hairs40. Among which the lateral roots are a very important part of the plant root system. They can increase the biomass of root hairs, allowing the plant to absorb more water and nutrients, and provide better fixation in the soil41,42. Auxin is required for the initial of lateral root primordium43,44. Auxin transport inhibitors suppress lateral root formation, suggesting that polar auxin transport is required for lateral root initiation45,46. Mutations in the rice cyclophilin gene (OsCYP2) exhibit defective auxin responses and block lateral root initiation47. In this study, we found that OsTPR1 may negatively regulate the sensitivity of auxin signaling, thereby influencing root development in rice seedlings. Results (Fig. 2) show that OsTPR1::GUS is specifically expressed in developing rice roots, indicating that OsTPR1 is functionally related to root development. A synthetic promoter (DR5), composed of nine copies of the auxin response element (TGTCGG), has been shown to exhibit higher affinity for auxin response factor (ARF) binding, resulting in increased sensitivity to the auxin response32. The DR5::GUS construct contains a reporter gene that expresses β-glucuronidase (GUS) in response to auxin, enabling the monitoring of auxin distribution and signaling in different tissues and developmental stages through GUS activity staining48–50. The intensity and location of the GUS staining typically reflect the distribution and concentration of auxin in that region. To investigate the functional correlation between OsTPR1 and auxin, we conducted the DR5::GUS reporter line to hybrid with the OsTPR1-Ox-1 and OsTPR1-Ri-1 lines, respectively. The hybrid line DR5::GUS/OsTPR1-Ri-1 shows stronger GUS activity staining, increased GUS gene expression, and more lateral roots in most differentiation zones of crown roots, while the DR5::GUS/OsTPR1-Ox-1 line exhibits weaker GUS staining, decreased GUS gene expression, and fewer lateral roots (Fig. 4). The results indicate that OsTPR1 plays a functional role in rice lateral root development by negatively regulating auxin signaling responses.
The PIN protein family mediates polar auxin transport and the asymmetric distribution of auxin within plant tissues. The expression of most PIN genes is tissue-specific and induced by auxin33. In rice, there are 12 putative OsPIN genes encoding auxin efflux transporters34,51which perform diverse functions. These include mediating asymmetric auxin distribution (e.g., OsPIN2, OsPIN5a)34,51–53 and regulating seminal, crown, and lateral root development, tillering, and shoot growth (OsPIN1a, OsPIN1b, OsPIN2)34,51,52,54,55. OsPIN2 also plays a role in gravitropic responses51,56. Additionally, OsPIN1c and OsPIN1d are associated with inflorescence development57while OsPIN10a is involved in regulating auxin flux to maintain growth under abiotic stress conditions58. Our results (Fig. 5) show that the expression of OsPIN1a, OsPIN1b, OsPIN1c, OsPIN2, and OsPIN5a is significantly upregulated in the OsTPR1-Ri line and downregulated in the OsTPR1-Ox line. This pattern suggests that in the OsTPR1-Ri line, which exhibits enhanced auxin sensitivity, the increased expression of these five genes may promote lateral root formation (Figs. 3 and 4), as well as increased plant height and an earlier heading date (Fig. 1). In contrast, the expression level of OsPIN10a is increased in the OsTPR1-Ox line but decreased in the OsTPR1-Ri line, while OsPIN1d expression remains unchanged among the lines (Fig. 5E and H). These results suggest that different members of the rice OsPIN gene family exhibit distinct auxin-responsive expression patterns, contributing to the regulation of plant growth in rice.
SAUR proteins act downstream of auxin signaling by promoting cell expansion through the activation of H⁺-ATPases, which acidify the cell wall to facilitate expansion and enhance plant growth59,60. As shown in Fig. S1, among the five OsSAUR genes examined, only OsSAUR10 showed enhanced expression in OsTPR1-Ri roots under NAA treatment. This finding is consistent with previous reports indicating that OsSAUR10 is upregulated by auxin in roots36,61. Given that there are 58 OsSAUR paralogs in rice, it is possible that the other four genes are simply not involved in auxin-responsive expression in roots. However, if OsTPR1 influences root development through a SAUR-dependent pathway, it is likely mediated by OsSAUR10.
The optimal tiller angle constitutes an ideal plant architecture, is crucial for increasing grain yield in rice62. Rice tiller angle is determined as the angle between the middle vertical line and the plants on both sides63. Auxin also regulates tillering and tiller angle in rice. The study found that the lazy1 mutants display defects in the lateral distribution of auxin in the stem in response to gravity, resulting in larger tiller angles in rice64,65. Mutations in the nuclear protein Tiller Angle Control 4 (TAC4) reduce endogenous auxin content, ultimately leading to a larger tiller angle phenotype62. These previous studies confirmed that auxin negatively regulates tiller angle in rice. Consistent with the findings in our study (Fig. 1), the tiller angle in OsTPR1-Ox line was significantly larger than that of the WT and OsTPR1-Ri lines. Combining the results in Figs. 1, 4 and 5, suggests that OsTPR1 reduces the responses of auxin in tiller buds by changing the expression of OsPINs, thereby causing tillering plants to expand outward and produce larger tiller angles.
Heading date is a critical agronomic trait regulated by environmental factors such as photoperiod, temperature, and nutrient availability, as well as genetic factors including hormones and key photoperiod-related genes such as Hd3a (Heading date 3a), RFT1 (RICE FLOWERING LOCUS T1), Ehd1 (Early heading date 1), OsGI (GIGANTEA), Hd1 (Heading date 1), and Ghd7 (Grain number, plant height, and heading date 7)66–68. Among these, Ghd7 can interact with Hd1 to form a strong transcriptional repressor complex that inhibits the expression of Ehd1, a key floral inducer under non-inductive long-day conditions, thereby delaying the heading date69. Further studies have shown that the auxin signaling repressor OsIAA23 downregulates Ghd7 expression, resulting in a delayed heading date under long-day conditions70revealing that auxin also plays a regulatory role in the rice heading stage. Our results demonstrated that the OsTPR1-Ri-1 and OsTPR1-Ox-1 lines exhibited early and delayed heading dates, respectively. Based on the data (Figs. 4 and 5), the corepressor OsTPR1 appears to play a negative regulatory role in auxin signaling. This suggests that OsTPR1-Ri plants may have an enhanced auxin response, leading to earlier flowering, while OsTPR1-Ox plants show a delayed flowering phenotype.
Conclusion
This study demonstrates that OsTPR1 negatively influences lateral root initiation and density in developing rice seedlings. The DR5::GUS/OsTPR1-Ri line exhibited stronger GUS staining and a higher number of lateral roots in the crown root differentiation zones. In contrast, the DR5::GUS/OsTPR1-Ox line showed weaker GUS signals and fewer lateral roots. The expression of several OsPIN genes was increased in the OsTPR1-Ri line and decreased in the OsTPR1-Ox line. Taken together, these results suggest that OsTPR1 attenuates auxin signaling, thereby influencing the expression of specific OsPIN genes, which may contribute to the distinct root development patterns observed between the OsTPR1-Ox and OsTPR1-Ri lines. In addition, OsTPR1 negatively affects tiller number but positively influences tiller angle during the tillering stage. Unexpectedly, OsTPR1 also plays a significant role in regulating heading date, with RNA interference lines (OsTPR1-Ri) exhibiting earlier heading and overexpression lines (OsTPR1-Ox) showing delayed heading. Further investigation of OsTPR1 will enhance our understanding of the molecular mechanisms underlying root development, tillering, and heading date in rice.
Materials and methods
Plant materials
The rice cultivar Oryza sativa L. cv. Tainung 67 was used throughout this study. Among the OsTPR1 overexpression (OsTPR1-Ox) and RNA interference (OsTPR1-Ri) transgenic lines, three independent T2 lines carrying a single-copy transgene were selected to evaluate the functional effects of OsTPR1 on rice development.
Callus induction.
Immature rice seeds, collected 10 days after flowering, were air-dried for 7 days. Thirty seeds were dehulled, surface-sterilized with 2.4% NaOCl for 30 min, and washed thoroughly with sterile water. The sterilized seeds were cultured on callus induction medium composed of N6 salts7110 µM 2,4-D, and 3% sucrose. Cultures were maintained at 28 °C under a 16 h/8 h light/dark photoperiod with 3500 lx light intensity64. After 35 days, calli were transferred to fresh medium and cultured for an additional 7 days before Agrobacterium-mediated transformation.
Primer sequences
The nucleotide sequences of all primers used for PCR, RT-PCR, and qRT-PCR are listed in Supplementary Table S1.
Vector construction
To construct the OsTPR1 overexpression vector (OsTPR1-Ox), a 3357-bp fragment containing the full coding region of OsTPR1 was amplified using primers OsTPR1-F1 and OsTPR1-R1. The PCR product was digested with BamHI and ligated into the BamHI-digested pAHC18 vector under the control of the maize ubiquitin promoter72. The recombinant plasmid was then linearized with HindIII and inserted into the HindIII site of the binary vector pSMY1H73.
To generate the OsTPR1 RNAi construct (OsTPR1-Ri), a 405-bp fragment comprising 65 bp of the 3′ coding region and 340 bp of the 3′ untranslated region of OsTPR1 was amplified with primers OsTPR1-F2 and OsTPR1-R2. The fragment was inserted downstream of the maize ubiquitin promoter in both sense and antisense orientations, separated by a truncated GFP sequence as a spacer74. The construct was linearized with HindIII and inserted into the HindIII site of the pSMY1H binary vector.
To construct the OsTPR1::GUS reporter vector, a 2407 bp promoter fragment, including the 5′ untranslated region (with an 87 bp intron), was amplified using primers OsTPR1-5P and OsTPR1-3B. The PCR product was digested with PstI and BamHI and ligated upstream of the GUS reporter gene in the pBX-2 vector73. The final construct was linearized with PstI and inserted into the PstI site of the pSMY1H binary vector.
Plant transformation
Transgenic rice plants were generated by Agrobacterium-mediated transformation. The recombinant plasmids were introduced into Agrobacterium tumefaciens strain EHA101 via electroporation. Transformed Agrobacterium was co-cultivated with rice calli to deliver the T-DNA, following established protocols64.
Phenotypic analysis
Dehulled rice seeds were surface-sterilized with 2.4% NaOCl for 30 min, rinsed thoroughly with sterile water, and sown on half-strength MS solid medium supplemented with 1.5% sucrose. Cultures were maintained at 28 °C under a 16 h photoperiod with a light intensity of 3500 lx. After 7 days, seedlings were transferred to soil-filled pots and grown for either 14 days (21-day-old plants; Figs. 1B and 3) or 78 days (85-day-old plants; Fig. 1B). Phenotypic traits including plant height, tiller number, tiller angle, root length, and lateral root number were measured. Statistical analyses were performed using one-way ANOVA.
Hydroponic cultivation of the rice seedlings
Ten-day-old seedlings grown in Kimura B hydroponic solution75 were treated with or without 10 µM NAA (1-Naphthaleneacetic acid). After 3 h of treatment (the optimal auxin concentration and induction time for OsSAUR genes are based on previous studies36,61), seminal and crown roots were collected from WT, OsTPR1-overexpressing (OsTPR1-Ox), and RNAi knockdown (OsTPR1-Ri) lines. Quantitative RT-PCR was performed to evaluate the expression levels of selected OsSAUR genes.
Histochemical GUS staining
Sterilized, dehulled rice seeds were germinated on half-strength MS solid medium. Roots from 3-day-old seedlings, 7-day-old roots, and 55-day-old soil-grown plants were collected for GUS staining. Tissues were incubated at 37 °C in the dark for 4 h in staining buffer containing 100 mM sodium phosphate (pH 7.0), 10 mM EDTA, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, 0.1% Triton X-100, and 1 mM 5-bromo-4-chloro-3-indolyl β-D-glucuronide. After staining, samples were stored in 70% ethanol, rinsed with water, and imaged.
Quantitative RT-PCR
Thirty dehulled, sterilized rice seeds were sown on half-strength MS medium with 1.5% sucrose and cultured under a 16 h/8 h photoperiod at 28 °C with a light intensity of 3500 lx. Root samples were collected by pooling ten seedlings per line for each biological replicate. For Figs. 4 and 14-day-old seedlings of DR5::GUS, DR5::GUS/OsTPR1-Ri, and DR5::GUS/OsTPR1-Ox were used. For Figs. 5 and S1, roots from ten seedlings per line of 20-day-old (Fig. 5) or 10-day-old (Fig. S1) WT, OsTPR1-Ri, and OsTPR1-Ox plants were isolated and pooled. Total RNA was extracted using TRIzol reagent (Invitrogen, USA), and genomic DNA was removed with a TURBO DNA-free kit (Ambion, USA). First-strand cDNA synthesis was performed using 5 µg of RNA, M-MuLV reverse transcriptase (New England Biolabs, USA), and oligo(dT) primers. Quantitative RT-PCR was conducted using an Eco Real-Time PCR System (Illumina, USA) following the manufacturer’s protocol. Gene-specific primers (Table S1) targeting the 3′ untranslated region were used. Expression levels were normalized to OsActin1 as an internal control, and expression in WT was set as 1.0. All reactions were performed in three technical replicates.
Statistical analysis
Each experiment was performed with a minimum of three experimental (biological) replicates. Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test. Statistical analyses were conducted using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Significance thresholds were set at P < 0.01 (Figs. 1 and 5) or P < 0.05 (Figs. 3 and 4).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- TPR
TOPLESS-related
- Ox
Overexpression
- Ri
RNA interference
- GUS
β-glucuronidase
- PIN
PIN-FORMED
- SAUR
SMALL AUXIN UP RNA
- qRT-PCR
Quantitative reverse transcription polymerase chain reaction
Author contributions
Conceived and designed the experiments: Shin Lon Ho. Performed the experiments: Yi Hsuan Hou, Chia Ying Kuo, Tin Yau Edwin Lai and Ming Hong Xiao. Analyzed the data and wrote the paper: Shin Lon Ho. All authors reviewed the manuscript.
Funding
This work was supported by grants from the National Science and Technology Council of Taiwan (Grant No. MOST 111-2313-B-415-004- and MOST 108-2313-B-415-014-).
Data availability
The data underpinning the findings of this study are available within the published article and its supplementary materials from the date of publication, and can be obtained from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kieffer, M. et al. Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell18, 560–573. 10.1105/tpc.105.039107 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand, U. et al. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science289, 617–619. 10.1126/science.289.5479.617 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Schoof, H. et al. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell100, 635–644. 10.1016/S0092-8674(00)80700-X (2000). [DOI] [PubMed] [Google Scholar]
- 4.Gallavotti, A. et al. The control of axillary meristem fate in the maize ramosa pathway. Development137, 2849–2856. 10.1242/dev.050872 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels, L. et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature464, 788–791. 10.1038/nature08854 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukazawa, J. et al. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR 1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell26, 2920–2938. 10.1105/tpc.114.126227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goralogia, G. S. et al. CYCLING DOF FACTOR 1 represses transcription through the TOPLESS co-repressor to control photoperiodic flowering in Arabidopsis. Plant J.92, 244–262. 10.1111/tpj.13656 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez, N. et al. A repressor protein complex regulates leaf growth in Arabidopsis. Plant Cell27, 2273–2287. 10.1105/tpc.15.00274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, N. et al. STERILE APETALA modulates the stability of a repressor protein complex to control organ size in Arabidopsis. PLoS Genet.14, e1007218. 10.1371/journal.pgen.1007218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu, Z. et al. Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc. Natl Acad. Sci. USA107, 13960–13965. 10.1073/pnas.1002828107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida, A., Ohmori, Y., Kitano, H., Taguchi-Shiobara, F. & Hirano, H. Y. ABERRANT SPIKELET AND PANICLE1, encoding a TOPLESS-related transcriptional co-repressor, regulates meristem fate in rice. Plant J.70, 327–339. 10.1111/j.1365-313X.2011.04875.x (2012). [DOI] [PubMed] [Google Scholar]
- 12.Suzuki, C., Tanaka, W. & Hirano, H. Y. Transcriptional corepressor ASP1 and CLV-like signaling regulate meristem maintenance in rice. Plant Physiol.180, 1520–1534. 10.1104/pp.19.00178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu, T. et al. A homologous gene of OsREL2/ASP1, ASPLSL, regulates pleiotropic phenotypes including long sterile lemma in rice. BMC Plant Biol.21, 390. 10.1186/s12870-021-03174-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke, J. et al. Structural basis for recognition of diverse transcriptional repressors by the TOPLESS family of corepressors. Sci. Adv.1, e1500107. 10.1126/sciadv.1500107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang, H. et al. NONSTOP GLUMES1 encodes a C2H2 zinc finger protein that regulates spikelet development in rice. Plant Cell32, 392–413. 10.1105/tpc.19.00577 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Y. F. et al. MULTI-FLORET SPIKELET 2, a MYB transcription factor, determines spikelet meristem fate and floral organ identity in rice. Plant Physiol.184, 988–1003. 10.1104/pp.20.00435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren, D. et al. MORE FLORET1 encodes a MYB transcription factor that regulates spikelet development in rice. Plant Physiol.184, 251–265. 10.1104/pp.20.00556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang, J. et al. The URL1-ROC5-TPL2 transcriptional repressor complex represses the ACL1 gene to modulate leaf rolling in rice. Plant Physiol.185, 1722–1744. 10.1093/plphys/kiab004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebouillat, J. et al. Molecular genetics of rice root development. Rice2, 15–34. 10.1007/s12284-008-9016-5 (2009). [Google Scholar]
- 20.Dinh, L. T. et al. Novel QTL for lateral root density and length improve phosphorus uptake in rice (Oryzasativa L.). Rice16, 37. 10.1186/s12284-023-00654-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wissuwa, M., Gonzalez, D. & Watts-Williams, S. J. The contribution of plant traits and soil microbes to phosphorus uptake from low-phosphorus soil in upland rice varieties. Plant Soil448, 523–537. 10.1007/s11104-020-04434-6 (2020). [Google Scholar]
- 22.Banda, J. et al. Lateral root formation in Arabidopsis: A well-ordered LRexit. Trends Plant Sci.24, 9. 10.1016/j.tplants.2019.06.001 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Boerjan, W. et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell7, 1405–1419. 10.1105/tpc.7.9.1405 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okushima, Y. et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell17, 444–463. 10.1105/tpc.104.028316 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarpella, E., Rueb, S. & Meijer, A. H. The RADICLELESS1 gene is required for vascular pattern formation in rice. Development130, 645–658. 10.1242/dev.00292 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Inukai, Y. et al. Characterization of rice mutants deficient in the formation of crown roots. Breed. Sci.51, 123–129. 10.1270/jsbbs.51.123 (2001). [Google Scholar]
- 27.Guilfoyle, T. J. & Hagen, G. Auxin response factors. Curr. Opin. Plant Biol.10, 453–460. 10.1016/j.pbi.2007.08.014 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Vanneste, S. & Friml, J. Auxin: a trigger for change in plant development. Cell136, 1005–1016. 10.1016/j.cell.2009.03.001 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Ho, S. L., Chao, Y. C., Tong, W. F. & Yu, S. M. Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol.125, 877–890. 10.1104/pp.125.2.877 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen, A. H., Sharrock, R. A. & Quail, P. H. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol.18, 675–689. 10.1007/BF00020002 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Yang, J. et al. Dynamic regulation of auxin response during rice development revealed by newly established hormone biosensor markers. Front. Plant Sci.8, 256. 10.3389/fpls.2017.00256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao, C. Y. et al. Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods12, 207–210. 10.1038/nmeth.3279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, J. R. et al. Expression of PIN genes in rice (Oryzasativa L.): Tissue specificity and regulation by hormones. Mol. Plant2, 823–831 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Miyashita, Y., Takasugi, T. & Ito, Y. Identification and expression analysis of PIN genes in rice. Plant Sci.178, 424–428. 10.1016/j.plantsci.2010.02.018 (2010). [Google Scholar]
- 35.Jain, M., Tyagi, A. K. & Khurana, J. P. Genome-wide analysis, evolutionary expansion, and expression of early auxin-responsive SAUR gene family in rice (Oryza sativa). Genomics88, 360–371. 10.1016/j.ygeno.2006.04.008 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Jia, C. et al. Genome-wide identification and expression analysis of SMALL AUXIN UP RNA (SAUR) genes in rice (Oryza sativa). Plant Signal Behav.19, 2391658. 10.1080/15592324.2024.2391658 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato, Y. et al. RiceXPro version 3.0: expanding the informatics resource for rice transcriptome. Nucleic Acids Res.41, D1206–D1213. 10.1093/nar/gks1125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plant, A. R., Larrieu, A. & Causier, B. Repressor for hire! The vital roles of TOPLESS-mediated transcriptional repression in plants. New Phytol.231, 963–973. 10.1111/nph.17428 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Jan, M. et al. Modulating root system architecture: Crosstalk between auxin and phytohormones. Front. Plant Sci.15, 1343928. 10.3389/fpls.2024.1343928 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coudert, Y., Perin, C., Courtois, B., Khong, N. G. & Gantet, P. Genetic control of root development in rice, the model cereal. Trends. Plant Sci.15, 219–226. 10.1016/j.tplants.2010.01.008 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Du, Y. & Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot.69, 155–167. 10.1093/jxb/erx223 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Motte, H., Vanneste, S. & Beeckman, T. Molecular and environmental regulation of root development. Ann. Rev. Plant Biol.70, 465–488. 10.1146/annurev-arplant-050718-100423 (2019). [DOI] [PubMed] [Google Scholar]
- 43.De Smet, I., Vanneste, S., Inzé, D. & Beeckman, T. Lateral root initiation or the birth of a new meristem. Plant Mol. Biol.60, 871–887. 10.1007/s11103-005-2914-3 (2006). [DOI] [PubMed] [Google Scholar]
- 44.De Smet, I. et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development134, 681–690. 10.1242/dev.02753 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Reed, R. C., Brady, S. R. & Muday, G. K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol.118, 1369–1378. 10.1104/pp.118.4.1369 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casimiro, I. et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell13, 843–852. 10.1105/tpc.13.4.843 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang, B. et al. OsCYP2, a chaperone involved in degradation of auxin–responsive proteins, plays crucial roles in rice lateral root initiation. Plant J.74, 86–97. 10.1111/tpj.12108 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell115, 591–602. 10.1016/S0092-8674(03)00924-3 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Petersson, S. V. et al. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell21, 1659–1668. 10.1105/tpc.109.066480 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jedličková, V., Ebrahimi Naghani, S. & Robert, H. S. On the trail of auxin: Reporters and sensors. Plant Cell34, 3200–3213. 10.1093/plcell/koac179 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen, Y., Fan, X., Song, W., Zhang, Y. & Xu, G. Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1. Plant Biotechnol. J.10, 139–149. 10.1111/j.1467-7652.2011.00637.x (2012). [DOI] [PubMed] [Google Scholar]
- 52.Inahashi, H. et al. OsPIN2, encoding an auxin efflux carrier, is involved in root elongation and lateral root formation in rice. Physiol. Plant.164, 216–225. 10.1111/ppl.12707 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Zhou, L. J., Xiao, L. T. & Xue, H. W. Dynamic cytology and transcriptional regulation of rice lamina joint development. Plant Physiol.174, 1728–1746. 10.1104/pp.17.00413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu, M., Zhu, L., Shou, H. & Wu, P. OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol.46, 1674–1681. 10.1093/pcp/pci183 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Li, Y. et al. Functional divergence of PIN1 paralogous genes in rice. Plant Cell Physiol.60, 2720–2732. 10.1093/pcp/pcz159 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Farooq, M., Jan, R. & Kim, K. M. Gravistimulation efects on Oryza sativa amino acid profle, growth pattern and expression of OsPIN genes. Sci. Rep.10, 17303. 10.1038/s41598-020-74531-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu, J., Shi, X., Chang, Z., Ding, Y. & Ding, C. Auxin efflux transporters OsPIN1c and OsPIN1d function redundantly in regulating rice (Oryzasativa L.) panicle development. Plant Cell Physiol.63, 305–316. 10.1093/pcp/pcab172 (2022). [DOI] [PubMed] [Google Scholar]
- 58.Xu, H. et al. Tissue specificity and responses to abiotic stresses and hormones of PIN genes in rice. Biologia77, 1459–1470. 10.1007/s11756-022-01031-9 (2022). [Google Scholar]
- 59.Yin, H. et al. SAUR15 promotes lateral and adventitious root development via activating H1-ATPases and auxin aiosynthesis. Plant Physiol.184, 837–851. 10.1104/pp.19.01250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou, Y. et al. Small auxin-up RNA gene, IbSAUR36, regulates adventitious root development in transgenic sweet potato. Genes15, 760. 10.3390/genes15060760 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang, X., Lu, Z., Zhai, L., Li, N. & Yan, H. The small auxin-Up RNA SAUR10 is involved in the promotion of seedling growth in rice. Plants12, 3880. 10.3390/plants12223880 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li, H. et al. TAC4 controls tiller angle by regulating endogenous auxin content and distribution in rice. Plant Biotechnol. J.19, 64–73. 10.1111/pbi.13442 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, W., Gao, H., Liang, Y., Li, J. & Wang, Y. Molecular basis underlying rice tiller angle: current progress and future perspectives. Mol. Plant15, 125–137. 10.1016/j.molp.2021.11.004 (2022). [DOI] [PubMed] [Google Scholar]
- 64.Zhang, N. et al. A core regulatory pathway controlling rice tiller angle mediated by the LAZY1-dependent asymmetric distribution of auxin. Plant Cell30, 1461–1475. 10.1105/tpc.18.00152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai, Y. et al. LAZY3 interacts with LAZY2 to regulate tiller angle by modulating shoot gravity perception in rice. Plant Biotechnol. J.21, 1217–1228. 10.1111/pbi.13983 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishikawa, R. et al. Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell17, 3326–3336. 10.1105/tpc.105.035451 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zong, W. et al. Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytol.229, 1635–1649. 10.1111/nph.16964 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song, J. et al. Research progress on photoperiod gene regulation of heading date in rice. Curr. Issues Mol. Biol.46, 10299–10311. 10.3390/cimb46060273 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nemoto, Y., Nonoue, Y., Yano, M. & Izawa, T. Hd1, a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J.86, 221–233. 10.1111/tpj.13167 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Zhang, J. et al. OsIAA23 promotes heading by directly downregulating Ghd7 in rice. Rice17, 70. 10.1186/s12284-024-00750-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu, C. C. et al. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sin.5, 659–668 (1975). [Google Scholar]
- 72.Bruce, W. B., Christensen, A. H., Klein, T., Fromm, M. & Quail, P. H. Photoregulation of a phytochrome gene promoter from oat transferred into rice by particle bombardment. Proc. Natl. Acad. Sci. USA86, 9692–9696. 10.1073/pnas.86.24.9692 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho, S. L., Tong, W. F. & Yu, S. M. Multiple mode regulation of a cysteine proteinase gene expression in rice. Plant Physiol.122, 57–66. 10.1104/pp.122.1.57 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho, S. L. et al. Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14–3-3 protein to confer drought tolerance in rice seedlings. Plant Mol. Biol.81, 347–361. 10.1007/s11103-012-9994-7 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Ehara, H., Morita, O., Morimoto, C., Kawashima, M. & Suematsu, M. Effect of nitrogen composition in culture solution on the growth of rice seedlings raised on a cotton mat spread on hydroponic apparatus for vegetables. Jpn J. Crop Sci.70, 359–364. 10.1626/JCS.70.359 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underpinning the findings of this study are available within the published article and its supplementary materials from the date of publication, and can be obtained from the corresponding author upon reasonable request.