Abstract
Aging adversely impacts thermoregulatory function, thereby increasing the risk of intraoperative hypothermia. Age-associated alterations-including diminished thermal perception, impaired autonomic responsiveness, reduced thermogenic capacity due to sarcopenia, and decreased cardiovascular adaptability, exacerbate the vulnerability to hypothermia. Concomitant comorbidities and polypharmacy further compromise thermal homeostasis in geriatric patients. Anesthetic agents compound this risk by lowering the thresholds for vasoconstriction and shivering and attenuating the magnitude of thermal responses. Consequently, geriatric populations are predisposed to significant perioperative temperature decline, particularly in cooler operating room (OR) environments. Intraoperative hypothermia is associated with an increased incidence of adverse outcomes, including increased cardiac events, surgical site infections, coagulopathy, protracted pharmacodynamic effects, extended recovery, and hospitalization duration. Although recent investigations suggest a diminished incidence of hypothermia due to minimally invasive surgical techniques and enhanced temperature management protocols, the intrinsic susceptibility of the aged thermoregulatory system persists. Effective temperature management requires precise core temperature monitoring and maintenance of appropriate OR temperatures. Furthermore, the implementation of multimodal warming strategies, including passive insulation, active warming modalities, warming of intravenous fluids, and prewarming before anesthesia induction, is critical. Therefore, a comprehensive and proactive thermal management approach is essential in mitigating hypothermia-related risks and optimizing perioperative outcomes in the geriatric patients.
Keywords: Body temperature, Frail elderly, Geriatric anesthesia, Hypothermia, Perioperative care, Postoperative complications
INTRODUCTION
The global population is increasing overall, with a notable rise in the number of people aged 65 years and over [1]. As the geriatric population grows, addressing the health challenges associated with aging has become increasingly important. When approaching geriatric patients, anesthesiologists must recognize the tremendous heterogeneity or variability in aging, both in the body as a whole and in individual organ systems. Among these challenges, perioperative unintentional hypothermia has become a growing concern for anesthesiologists because of its association with systemic responses to various complications [2,3]. Geriatric populations are especially vulnerable to disturbances in thermoregulation due to the physiological changes that accompany aging and senescence [4,5].
This review examined the factors and management strategies associated with unintentional hypothermia in the geriatric patients. First, we explored age-related changes in the thermoregulatory physiology and other contributing factors. Next, we reviewed the current methods for monitoring and managing hypothermia in this population. Finally, we discussed existing controversies and identified areas that require further investigation.
NORMAL THERMOREGULATION SYSTEM
The normal thermoregulation system is mediated by various of mechanisms (Fig. 1). A normal thermoregulation system comprises afferent thermal sensing, central regulation, and efferent responses [6]. Afferent signals are primarily derived from various tissues, including the skin, deep tissues, and the spinal cord. Cold stimuli are transmitted via Aδ fibers, whereas warm stimuli are conveyed through unmyelinated C fibers [7]. These thermal signals ascend through the spinothalamic tract of the anterior spinal cord to reach the hypothalamus, which is the central integrator of thermoregulatory control [8,9]. Upon integration, effector responses are initiated once the afferent input surpasses specific threshold values, with the response magnitude increasing along a defined slope until the maximal intensity is achieved [10]. Threshold, gain, and maximal response intensities are independently determined for each thermoregulatory effector [11]. The effector systems are categorized into behavioral and autonomic components. Behavioral responses are predominantly regulated by thermal inputs from the skin surface, whereas approximately 80% of autonomic responses are governed by thermal inputs from core structures. Behavioral regulation is the most powerful thermoregulatory effector that allows humans to tolerate extreme environments. In the autonomic cold-defense system, vasoconstriction and thermogenesis play central roles [9]. Vasoconstriction significantly reduces blood flow through arteriovenous shunts on the skin surface, thereby minimizing convective and radiative heat loss and effectively conserving metabolic heat [10]. This response is primarily mediated by local α-adrenergic sympathetic nerve activity. Meanwhile, non-shivering thermogenesis, which occurs in skeletal muscle and brown adipose tissue, enhances metabolic heat production without muscular activity and is regulated by norepinephrine released from adrenergic nerve terminals [12,13]. Shivering thermogenesis, while capable of increasing heat production by 300–500%, contributes less significantly overall. Conversely, autonomic warmth defense mechanisms involve sweating and vasodilation. Sweating, mediated by the postganglionic cholinergic nerves, promotes efficient heat dissipation through evaporative cooling. Vasodilation, the physiological opposite of cold-induced vasoconstriction, is mediated by nitric oxide (NO), which facilitates heat loss by increasing blood flow to the skin. Under extreme heat conditions, blood flow through arteriovenous shunts can reach 6–8 L/min, approximating the entire resting cardiac output [8]. The interthreshold range, which is the temperature interval between the activation thresholds of cold (i.e., vasoconstriction) and warm (i.e., sweating) autonomic responses, defines the zone of normal thermoneutrality wherein autonomic thermoregulatory responses are not triggered. In humans, this range is maintained with high precision, typically within 0.2°C [7]. Moreover, it is reported to be approximately 0.3–0.5°C higher in women compared to men and approximately 0.4°C lower in older individuals [14,15].
Fig. 1.
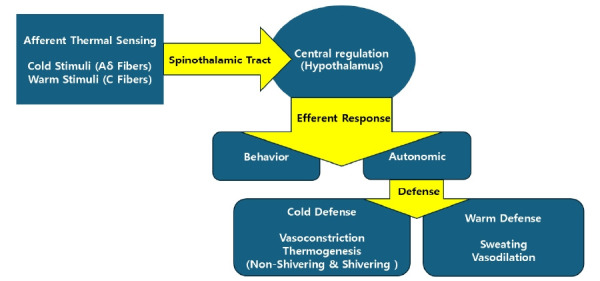
Normal thermoregulation system.
CHANGES IN THERMOREGULATION AMONG GERIATRIC PATIENTS
Various components of the thermoregulatory system are influenced by aging, as well as by associated comorbidities and polypharmacy (Table 1) [15,16]. One of the primary areas affected is the nervous system. The perception of changes in body temperature is significantly blunted in geriatric populations. This impairment is attributed to a decline in the function of thermoreceptors distributed across the skin, with attenuation of warm-signal detection preceding that of cold-signal detection, and with distal regions affected before proximal regions [4,15]. Furthermore, aging diminishes the sensitivity of the central thermoregulatory center, the hypothalamus, to temperature changes. The responses of the autonomic nervous system also become less robust with advancing age [16,17]. The resulting decline in subjective thermal perception, coupled with a reduced awareness of environmental risks, physical limitations such as arthritis, and impaired mobility, collectively contribute to a decreased behavioral thermoregulatory response. Consequently, older adults are more vulnerable to thermal injury [18].
Table 1.
Effect of Aging on the Thermoregulatory System
| Component | Description | Detail |
|---|---|---|
| Nervous system | Blunted perception of temperature changes | Reduced subjective awareness of temperature |
| Reduced function of skin thermoreceptors | Diminished behavioral and autonomic responses | |
| Decreased hypothalamic sensitivity | Increased risk of thermal injury | |
| Impaired autonomic responses | ||
| Cardiovascular | Elevated baseline norepinephrine | Impaired heat conservation (cold exposure) |
| Reduced responsiveness to stimuli | Impaired heat dissipation (hot environments) | |
| Increased ROS, impaired NE synthesis | Higher risk of hypothermia and heat illness | |
| Blunted vasoconstriction/vasodilation | ||
| Decreased cardiac output reserve | ||
| Skin & sweat glands | Thinner dermis | Reduced heat preservation (cold) |
| Decreased vascular density | Impaired heat loss (heat) | |
| Vascular wall hypertrophy (stiffness) | Lower sweating capacity, in lower extremities first | |
| Reduced subcutaneous fat | ||
| Decreased sweating (functional gland decline) | ||
| Skeletal muscle | Sarcopenia (loss of muscle mass) | Reduced shivering thermogenesis |
| Reduced muscle vascularity | Decreased basal/resting metabolic rate | |
| Functional impairment of muscle | Impaired passive heat distribution and insulation | |
| Endocrine system | Decreased hormone levels (epinephrine, thyroxine, cortisol) | Reduced thermogenic capacity |
| Other factors | Reduced caloric intake | Further impaired thermoregulation |
| Lower basal metabolic rate | Increased risk of hypothermia and complications | |
| Reduced body fat mass | ||
| Comorbidities | ||
| Polypharmacy |
ROS: reactive oxygen species, NE: norepinephrine.
In addition to impairing neural components, aging induces significant changes in the cardiovascular system that affect thermoregulation. In geriatric populations, baseline sympathetic norepinephrine levels are elevated, but responsiveness to stimuli is diminished [11,19]. This reduction is attributed to age-related increases in reactive oxygen species (ROS), which impair the synthesis and secretion of norepinephrine. Consequently, the vasoconstrictive response to cold signals is markedly blunted, reducing the ability to conserve heat in cold environments and thereby increasing the risk of hypothermia [17]. Similarly, the vasodilatory response to warm stimuli is attenuated with aging. This decline is primarily due to the increased oxidative degradation of NO resulting from ROS accumulation. As a result, geriatric adults are at greater risk of heat-related illnesses under high-temperature conditions. Age-associated cardiopulmonary senescence, characterized by a limited cardiac output reserve, further exacerbates thermoregulatory challenges during thermal stress.
Aging-associated changes in the skin and sweat glands further contribute to impaired thermoregulation in geriatric populations [20]. With advancing age, the dermis becomes thinner, cutaneous vascular density declines, and the vascular walls undergo hypertrophy, leading to increased stiffness [15,21]. As previously discussed, these vascular changes diminish the thermoregulatory vascular responses to thermal stimuli. Subcutaneous fat decreases with age, thereby reducing the ability of the body to preserve its thermal content in response to cold exposure. Sweating capacity declines, primarily because of the functional deterioration of sweat glands rather than a reduction in their number [15,16]. This decrease begins in the lower extremities and progresses sequentially to the back, anterior chest, upper extremities, and face.
Shivering thermogenesis is attenuated in the geriatric patients [22]. While sarcopenia, the age-related loss of skeletal muscle mass, contributes to this decline, reduced thermogenic capacity has been observed even in geriatric individuals with preserved muscle mass, suggesting that functional impairment plays a predominant role in this decline. Sarcopenia diminishes shivering thermogenesis and impairs passive heat distribution and insulation due to the reduced vascularity of skeletal muscle, ultimately leading to decreased basal and resting metabolic rates, which affect the overall heat production and distribution. Additionally, various hormones, including epinephrine, thyroxine, and cortisol, play key roles in thermogenic mechanisms; thus, aging of the endocrine system further compromises heat production [23]. Cardiopulmonary aging, by limiting oxygen delivery and consumption, exacerbates deficits in thermogenesis. Other factors associated with aging, such as reduced caloric intake, decreased basal metabolic rate, reduced body fat mass, comorbidities, and the effects of polypharmacy, collectively impair thermoregulatory capacity in the geriatric populations [16]. Finally, the diminished physiological reserve associated with aging increases the risk of complications arising from hypothermia.
EFFECTS OF ANESTHESIA ON THERMOREGULATION
The effect of anesthesia on thermoregulation has been well established. During anesthesia, robust behavioral thermoregulatory responses cannot be elicited, and autonomic responses are significantly impaired [2,3,7]. Most anesthetic agents currently in use exert direct vasodilatory effects, particularly by promoting the dilation of thermoregulatory arteriovenous shunts. Consequently, the threshold for the strong cold defense response, characterized by vasoconstriction, decreases proportionally to the anesthetic dose, while the threshold for shivering decreases, maintaining an approximate 1°C difference between the two. The thresholds for warm-defense responses are slightly increased, resulting in a substantial expansion of the inter-threshold range to 2–4°C, roughly 10–20 times wider than the normal range of approximately 0.2°C [7]. Within this expanded inter-threshold range, the core temperature becomes highly labile, resembling that of a poikilothermic state. Anesthesia lowers the thresholds for effector responses and reduces their gain and maximal intensity, resulting in a delayed onset of autonomic responses, with their rate of increase and maximum efficacy diminished [2].
The typically low ambient temperature of operating rooms (ORs) combined with impaired thermoregulation due to anesthesia leads to significant intraoperative heat loss. Approximately 40% of heat loss occurs through radiation, 30% through convection due to air movement, 25% through evaporation, and a minor amount through conduction to the operating table [2,24,25]. However, the rapid initial decrease in core temperature at approximately 1.0–1.5°C within the first hour of anesthesia-cannot be explained by heat loss alone [26]. Instead, this phenomenon is primarily due to the redistribution of body heat from the core to the periphery, facilitated by anesthesia-induced vasodilation, which account for approximately 81% of the initial temperature drop. Subsequently, the core temperature decreases more gradually as a function of the imbalance between heat loss and metabolic heat production, eventually reaching a thermal steady-state after 3–4 h [26].
Neuraxial anesthesia, such as spinal or epidural anesthesia, also impairs thermoregulation by reducing both the vasoconstriction and shivering thresholds by approximately 0.6°C [27]. This impairment is thought to result from the central effects of neuraxial blockade, as well as altered central interpretation of afferent thermal inputs, where the blocked segments are perceived to have elevated temperatures [28]. Additionally, vasodilation in blocked dermatomes increases skin temperature, enhancing patients’ subjective thermal comfort and further blunting behavioral responses, which then creates a dangerous clinical paradox that exacerbates hypothermia [29]. These thermoregulatory impairments are proportional to the number of blocked segments and are characterized by the absence of gain and maximal intensity responses in the affected regions. Unlike general anesthesia, temperature decreases under neuraxial blockade occurs rapidly due to initial redistribution, followed by gradual cooling without a subsequent plateau phase, as ongoing heat loss exceeds metabolic heat production [28,29]. Temperature decreases are more pronounced during combined general and regional anesthesia [30]. In contrast, peripheral nerve blockades such as brachial plexus blocks do not result in clinically significant impairment of thermoregulation even in geriatric patients [2,31]. In a study conducted on geriatric patients undergoing hand surgery under brachial plexus block, the body temperature at the end of surgery remained close to 37°C regardless of prewarming. Moreover, body temperatures measured every 15 min from the start to the end of surgery did not differ from those measured before the brachial plexus block [31].
THERMOREGULATION DURING ANESTHESIA IN GERIATRIC PATIENTS
Thermoregulatory function during anesthesia is further compromised in geriatric patients due to age-associated alterations in the thermoregulatory system, as described earlier [16]. Previous studies have reported that isoflurane anesthesia reduces the vasoconstriction threshold by approximately 1°C, whereas sevoflurane anesthesia decreases it by approximately 0.8°C in geriatric patients [14,32]. Consistent with these findings, intraoperative hypothermia is more pronounced in geriatric patients, with greater decreases in core temperature and prolonged recovery times than in younger individuals. Similar trends have been observed under neuraxial anesthesia [33]. Although earlier studies have indicated an increased susceptibility to intraoperative hypothermia in geriatric patients, some researchers have suggested that the risk may be lower than previously thought or even comparable to that in younger patients [34-36]. This discrepancy remains a subject of active debate and is likely attributable to advances in minimally invasive surgical techniques and the implementation of active warming strategies during surgery. The use of processed electroencephalography, such as the bispectral index, effectively reduces the anesthetic requirements in geriatric patients, thereby mitigating the impact of anesthetics on the thermoregulatory system. Nonetheless, given the well-documented vulnerability of the thermoregulatory system in the geriatric patients, careful interpretation of these newer findings is warranted.
CLINICAL IMPLICATIONS OF PERIOPERATIVE HYPOTHERMIA
Various complications can occur in geriatric patients due to hypothermia (Table 2). Intraoperative hypothermia is defined by previous guidelines and other clinical standards as a decrease in core temperature below 36.0°C during anesthesia [37,38]. Intraoperative hypothermia is associated with several complications. Hypothermia significantly increases the risk of surgical wound infection. Multiple studies have demonstrated that hypothermia impairs bacterial resistance and wound healing, likely through both direct suppression of immune function and indirect effects caused by thermoregulatory arteriovenous vasoconstriction, which reduces local tissue perfusion [39,40]. Moreover, hypothermia impairs platelet function and inhibits the enzymatic activity within the coagulation cascade, leading to coagulopathy, increased blood loss, and increased greater transfusion requirements [41-43]. Neuromuscular blockade is prolonged during hypothermia: the duration of action for agents such as vecuronium and atracurium is extended when core temperature decreases by 2–3°C [44,45]. Furthermore, the pharmacokinetics of anesthetics are altered: plasma concentrations of propofol may increase by approximately 30% with a 3°C drop in core temperature, and the minimum alveolar concentration for volatile anesthetics decreases [45,46]. Collectively, these effects contribute to prolonged anesthetic recovery times and extended hospital stays [47]. Mild hypothermia increases the risk of adverse cardiac complications [48,49]. This is thought to result from sympathetic activation and elevated levels of catecholamine and cortisol, leading to an increased heart rate and a mismatch between myocardial oxygen supply and demand [50]. In addition to these major complications, intraoperative hypothermia can exacerbate minor outcomes, such as thermal discomfort and postoperative shivering [9].
Table 2.
Complications Related to Perioperative Hypothermia
| Surgical wound infection |
| Coagulopathy & bleeding, higher transfusion requirement |
| Prolonged neuromuscular blockade & anesthetic recovery |
| Adverse cardiac complications |
| Thermal discomfort & shivering |
STRATEGY TO MINIMIZE PERIOPERATIVE HYPOTHERMIA IN GERIATRIC PATIENTS
Various strategies are required to maintain perioperative normothermia in geriatric patients (Table 3). Accurate monitoring of core temperature is essential for maintaining intraoperative normothermia. To reliably reflect core body temperature, measurements should be obtained from well-perfused and thermally stable sites. Although pulmonary artery temperature monitoring provides the most accurate measurement, its invasiveness limits its routine use. Therefore, distal esophageal, tympanic membrane (with a contact thermistor or thermocouple), and nasopharyngeal temperature monitoring are considered appropriate and reliable alternatives [51]. Other sites, such as the sublingual area, rectum, skin, axilla, and urinary bladder, are used but are more susceptible to external factors. Thus, caution should be exercised if the temperature is outside the normal range [52]. Numerous recent trials have demonstrated relatively good agreement between zero-heat-flux (ZHF) thermometers and conventional devices for measuring the reference core temperature [53-57]. Although the ZHF thermometer is a skin-surface device, it reflects the temperature of the deep forehead and offers the advantages of being noninvasive, providing an acceptable estimation of the core temperature, and enabling continuous monitoring. Monitoring the distal esophageal temperature is particularly challenging during regional or neuraxial anesthesia, and there are limited options for continuous core temperature measurement in these settings. Therefore, the ZHF thermometer may serve as a valuable tool for reliably estimating the core temperature in geriatric patients undergoing regional or neuraxial anesthesia. Previous guidelines recommend temperature monitoring at least every 30 min during surgery and every 15 min in the recovery room [37,38]. However, the use of a ZHF thermometer enables non-invasive and continuous near-core temperature monitoring, allowing for the implementation of more proactive hypothermia prevention strategies in geriatric patients who are more vulnerable to perioperative hypothermia.
Table 3.
Strategies for Preserving Perioperative Normothermia in Geriatric Patients
| Component | Description | Detail |
|---|---|---|
| Core temperature monitoring | Gold standard: Pulmonary artery (invasive) | Monitor at least every 30 min (intraoperative) and at least every 15 min (at recovery room) |
| Reliable: Distal esophagus, nasopharynx, tympanic membrane (with a direct contact probe) | Zero-heat-flux thermometer is relatively reliable for noninvasive and continuous core temperature measurement | |
| Less reliable: Sublingual, rectum, skin, bladder, axilla | Infrared thermometers (tympanic membrane, temporal artery, forehead) are less reliable | |
| Target temperature | ≥ 36.0°C: Generally safe | No difference in major outcomes between 35.5–37.0°C, but minor outcomes (e.g. shivering) still need attention |
| ≥ 35.5°C: Acceptable | ||
| Operating room temperature | ≥ 21°C: Adults | Ambient temperature is a key factor in preventing heat loss |
| 26°C: Further reduces risk of hypothermia | ||
| Active warming devices | Forced-air warming blankets: Most effective | In all patients with anesthesia for > 30 min |
| Heating mattresses: Less effective, risk of burns | In patients with anesthesia for < 30 min, but are at higher risk of hypothermia (e.g. geriatric patients) | |
| Passive insulation | Blankets and drapes reduce exposed surface area | Key is minimizing exposed surface area |
| Self-heating blanket: Acceptable, easy to use | Coverage is more important than number/type of layers | |
| Supportive measures | Heated/humidified airway gases | Fluid warming prevents hypothermia but does not actively warm patients |
| Warmed intravenous fluids or blood (> 500 ml/h; 37°C) | ||
| Warmed irrigation fluids (38–40°C) | ||
| Preoperative period | Prewarming (At least 10–20 min) reduces redistribution hypothermia | Especially important for geriatric patients |
| Postoperative period | Postwarming improves comfort, reduces shivering | Still recommended for patient comfort |
Effective intraoperative thermal management requires a multimodal approach. The previous guidelines recommended maintaining a patient temperature of at least 36.0°C. However, a recent study suggested that maintaining intraoperative core temperatures above 35.5°C may also be acceptable [58]. This study reported no significant differences in major outcomes such as myocardial injury after non-cardiac surgery, non-fatal cardiac arrest, 30-day mortality, surgical site infection, or transfusion requirements between patients actively warmed to 37.0°C and those maintained at 35.5°C. Subgroup analyses demonstrated no significant differences in outcomes among patients aged ≥ 65 years. Nevertheless, minor outcomes such as postoperative shivering remain a concern, and further attention to these aspects is warranted when determining intraoperative temperature management strategies.
Controlling the ambient temperature of an OR is critical because it is one of the most influential factors for preventing heat loss. An ambient temperature of at least 21°C is recommended, with temperatures above 24°C advised for pediatric surgeries [37,59]. Previous study has demonstrated that maintaining an OR temperature of 26°C significantly reduces the incidence of intraoperative hypothermia and complications [39].
Active warming devices such as heating mattresses and forced air warming systems directly transfer heat to patients [60,61]. Heating mattresses placed beneath the patient are less effective because of the insulation properties of the operating table and carry an increased risk of pressure-related injuries and burns [62,63]. In contrast, forced-air warming blankets combine passive insulation with convective heat transfer at the body surface, offering a more effective method of maintaining body temperature [61,64,65]. Recent meta-analyses support the use of forced-air warming as a highly effective modality [66-68]. Previous guidelines recommend the application of forced-air warming in patients who have anesthesia for more than 30 min or less than 30 min if they are at a higher risk of inadvertent perioperative hypothermia [37,38]. The guideline defined the high risk of hypothermia as American Society of Anesthesiologist grade II to V, preoperative temperature < 36.0°C, combined general and regional anesthesia, major surgery, and risk of cardiac complications. However, geriatric patients are at high risk of developing hypothermia because they are vulnerable to perioperative hypothermia. Passive insulation using blankets or surgical drapes can reduce the exposed surface area and create an insulating air layer between the patient and the covering material [37,69,70]. The type or material of covering did not significantly affect the insulation efficiency, and simply increasing the number of layers did not proportionally enhance the warming effect. Thus, reducing the exposed surface area is key factor. Recently, self-heating blankets have gained popularity as warming devices. According to a recent meta-analysis, self-heating blankets were reported to be as effective as forced-air warmers in preventing hypothermia [71]. Given that they do not require complex equipment for warming, they offer the advantage easy application, particularly for geriatric patients
Other supportive measures include airway gas heating and humidification, as well as warming of intravenous fluids [72,73]. Although warmed fluids cannot actively transfer heat, fluid warming is essential to prevent hypothermia when administering large volumes or during transfusion [2,72,74]. One unit of refrigerated blood or 1 L of unwarmed fluid decreases mean body temperature by approximately 0.25°C and finally increases the risk of hypothermia [75]. Therefore, the guidelines recommend warming intravenous fluids or blood products to 37°C when administering volumes greater than 500 ml/h, and suggest that irrigation fluids should be warmed to 38–40°C [37].
Active warming strategies are effective intraoperatively and during the preoperative and postoperative periods. Preoperative warming (prewarming) aims to increase peripheral heat content and temperature, thereby reducing the core-to periphey temperature gradient and minimizing redistribution hypothermia during the first hour after anesthesia induction [76-78], and even 10–20 min of prewarming was effective in preventing hypothermia [79,80]. Although studies have confirmed the benefits of prewarming in geriatric patients, a previous study suggested that its effectiveness may be relatively limited compared to that in younger patients [81]. This finding indicates that meticulous thermal management is particularly important in geriatric patients. Postoperative warming is less effective than intraoperative warming because vasoconstriction associated with emergence and postoperative pain inhibits peripheral-to-core heat transfer. Nevertheless, postoperative warming remains important for improving the thermal comfort of patients and reducing the incidence of postoperative shivering [37,82].
CONCLUSION
This review has certain limitations. Research focusing exclusively on temperature management in geriatric patients remains limited and no specific guidelines are currently available. Therefore, further studies targeting this population are warranted. With the ongoing development of various temperature monitoring tools, continued evidence of their clinical utility is essential. Given the importance of continuity in temperature management, the relative lack of emphasis on post-warming strategies compared with prewarming and intraoperative measures highlights the need for further investigation in this area. Despite recent advancements in temperature management, geriatric patients remain at increased risk of intraoperative hypothermia owing to their intrinsic physiological vulnerabilities. Comprehensive, multimodal temperature management strategies tailored to this population are essential for improving surgical outcomes and minimizing perioperative hypothermia-related complications.
Footnotes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Jae Hwa Yoo, Tae-Yun Sung, Chung-Sik Oh. Writing - review & editing: Jae Hwa Yoo, Tae-Yun Sung, Chung-Sik Oh. Conceptualization: Chung-Sik Oh.
REFERENCES
- 1.Jensen L, Monnat SM, Green JJ, Hunter LM, Sliwinski MJ. Rural population health and aging: toward a multilevel and multidimensional research agenda for the 2020s. Am J Public Health. 2020;110:1328–31. doi: 10.2105/ajph.2020.305782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387:2655–64. doi: 10.1016/s0140-6736(15)00981-2. [DOI] [PubMed] [Google Scholar]
- 3.Rauch S, Miller C, Bräuer A, Wallner B, Bock M, Paal P. Perioperative hypothermia-a narrative review. Int J Environ Res Public Health. 2021;18:8749. doi: 10.3390/ijerph18168749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grassi G, Seravalle G, Turri C, Bertinieri G, Dell’Oro R, Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation. 2003;108:729–35. doi: 10.1161/01.cir.0000081769.02847.a1. [DOI] [PubMed] [Google Scholar]
- 5.Tan R, Chen Y, Yang D, Long X, Ma H, Yang C. Risk factors for postoperative hypothermia in non-cardiac surgery patients: a systematic review and meta-analysis. BMC Anesthesiol. 2025;25:223. doi: 10.1186/s12871-025-03089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 7.Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–38. doi: 10.1097/aln.0b013e31817f6d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–12. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- 9.De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96:467–84. doi: 10.1097/00000542-200202000-00036. [DOI] [PubMed] [Google Scholar]
- 10.Buggy DJ, Crossley AW. Thermoregulation, mild perioperative hypothermia and postanaesthetic shivering. Br J Anaesth. 2000;84:615–28. doi: 10.1093/bja/84.5.615. [DOI] [PubMed] [Google Scholar]
- 11.Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol (1985) 2010;109:1538–44. doi: 10.1152/japplphysiol.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blondin DP, Haman F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb Clin Neurol. 2018;156:153–73. doi: 10.1016/b978-0-444-63912-7.00010-2. [DOI] [PubMed] [Google Scholar]
- 14.Ozaki M, Sessler DI, Matsukawa T, Ozaki K, Atarashi K, Negishi C, et al. The threshold for thermoregulatory vasoconstriction during nitrous oxide/sevoflurane anesthesia is reduced in the elderly. Anesth Analg. 1997;84:1029–33. doi: 10.1213/00000539-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol (1985) 2003;95:2598–603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- 16.Alvis BD, Hughes CG. Physiology considerations in geriatric patients. Anesthesiol Clin. 2015;33:447–56. doi: 10.1016/j.anclin.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson D, Tyra J, McCray A. Attenuation of the cutaneous vasoconstrictor response to cold in elderly men. J Gerontol. 1992;47:M211–4. doi: 10.1093/geronj/47.6.m211. [DOI] [PubMed] [Google Scholar]
- 18.Kenney WL, Wolf ST, Dillon GA, Berry CW, Alexander LM. Temperature regulation during exercise in the heat: insights for the aging athlete. J Sci Med Sport. 2021;24:739–46. doi: 10.1016/j.jsams.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheitlin MD. Cardiovascular physiology-changes with aging. Am J Geriatr Cardiol. 2003;12:9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- 20.Ezure T, Amano S, Matsuzaki K. Aging-related shift of eccrine sweat glands toward the skin surface due to tangling and rotation of the secretory ducts revealed by digital 3D skin reconstruction. Skin Res Technol. 2021;27:569–75. doi: 10.1111/srt.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CJ, Alexander LM, Kenney WL. Nonuniform, age-related decrements in regional sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol. 2013;305:R877–85. doi: 10.1152/ajpregu.00290.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byard R, Bright F, Winskog C. Reduced body mass index and lethal hypothermia. Aust J Forensic Sci. 2014;46:451–4. doi: 10.1080/00450618.2014.888476. [DOI] [Google Scholar]
- 23.Khan KT, Hemati K, Donovan AL. Geriatric physiology and the frailty syndrome. Anesthesiol Clin. 2019;37:453–74. doi: 10.1016/j.anclin.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Sessler DI. How three linked clinical observations led to an understanding of perioperative heat balance: a personal reflection on the scientific process. J Clin Anesth. 2024;96:111496. doi: 10.1016/j.jclinane.2024.111496. [DOI] [PubMed] [Google Scholar]
- 25.Riley C, Andrzejowski J. Inadvertent perioperative hypothermia. BJA Educ. 2018;18:227–33. doi: 10.1016/j.bjae.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsukawa T, Sessler DI, Sessler AM, Schroeder M, Ozaki M, Kurz A, et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82:662–73. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Kurz A, Sessler DI, Schroeder M, Kurz M. Thermoregulatory response thresholds during spinal anesthesia. Anesth Analg. 1993;77:721–6. doi: 10.1213/00000539-199310000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Leslie K, Sessler DI. Reduction in the shivering threshold is proportional to spinal block height. Anesthesiology. 1996;84:1327–31. doi: 10.1097/00000542-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Ikeda T, Sessler DI, Turakhia M, Jeffrey R. Epidural anesthesia reduces the gain and maximum intensity of shivering. Anesthesiology. 1998;88:851–7. doi: 10.1097/00000542-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Joris J, Ozaki M, Sessler DI, Hardy AF, Lamy M, McGuire J, et al. Epidural anesthesia impairs both central and peripheral thermoregulatory control during general anesthesia. Anesthesiology. 1994;80:268–77. doi: 10.1097/00000542-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Cho SA, Chang M, Lee SJ, Sung TY, Cho CK. Prewarming for prevention of hypothermia in older patients undergoing hand surgery under brachial plexus block. Ann Geriatr Med Res. 2022;26:175–82. doi: 10.4235/agmr.22.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurz A, Plattner O, Sessler DI, Huemer G, Redl G, Lackner F. The threshold for thermoregulatory vasoconstriction during nitrous oxide/isoflurane anesthesia is lower in elderly than in young patients. Anesthesiology. 1993;79:465–9. doi: 10.1097/00000542-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Vassilieff N, Rosencher N, Sessler DI, Conseiller C. Shivering threshold during spinal anesthesia is reduced in elderly patients. Anesthesiology. 1995;83:1162–6. doi: 10.1097/00000542-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Collins S, Budds M, Raines C, Hooper V. Risk factors for perioperative hypothermia: a literature review. J Perianesth Nurs. 2019;34:338–46. doi: 10.1016/j.jopan.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Journeaux M. Peri-operative hypothermia: implications for practice. Nurs Stand. 2013;27:33–8. doi: 10.7748/ns2013.07.27.45.33.e7742. [DOI] [PubMed] [Google Scholar]
- 36.de Brito Poveda V, Galvão CM, dos Santos CB. Factors associated to the development of hypothermia in the intraoperative period. Rev Lat Am Enfermagem. 2009;17:228–33. doi: 10.1590/s0104-11692009000200014. [DOI] [PubMed] [Google Scholar]
- 37. Hypothermia: prevention and management in adults having surgery. National Institute for Health and Care Excellence (NICE) clinical guidelines, No. 65. London, NICE. 2016. [PubMed] [Google Scholar]
- 38. Association for periOperative Registered Nurses (AORN). Guideline for patient temperature management. In: Guidelines for perioperative practice. Edited by Kyle E: Denver, AORN. 2025, pp 677-706. [Google Scholar]
- 39.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–15. doi: 10.1056/nejm199605093341901. [DOI] [PubMed] [Google Scholar]
- 40.Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358:876–80. doi: 10.1016/s0140-6736(01)06071-8. [DOI] [PubMed] [Google Scholar]
- 41.Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71–7. doi: 10.1097/01.anes.0000296719.73450.52. [DOI] [PubMed] [Google Scholar]
- 42.Sun Z, Honar H, Sessler DI, Dalton JE, Yang D, Panjasawatwong K, et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology. 2015;122:276–85. doi: 10.1097/aln.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Poucke S, Stevens K, Marcus AE, Lancé M. Hypothermia: effects on platelet function and hemostasis. Thromb J. 2014;12:31. doi: 10.1186/s12959-014-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heier T, Caldwell JE. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology. 2006;104:1070–80. doi: 10.1097/00000542-200605000-00025. [DOI] [PubMed] [Google Scholar]
- 45.Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80:1007–14. doi: 10.1097/00000539-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 46.Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia. 2013;68:512–22. doi: 10.1111/anae.12168. [DOI] [PubMed] [Google Scholar]
- 47.Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–23. doi: 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Dietrichs ES, Tveita T, Smith G. Hypothermia and cardiac electrophysiology: a systematic review of clinical and experimental data. Cardiovasc Res. 2019;115:501–9. doi: 10.1093/cvr/cvy305. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22:645–57. doi: 10.1016/j.bpa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Greif R, Laciny S, Rajek A, Doufas AG, Sessler DI. Blood pressure response to thermoregulatory vasoconstriction during isoflurane and desflurane anesthesia. Acta Anaesthesiol Scand. 2003;47:847–52. doi: 10.1034/j.1399-6576.2003.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sessler DI. Perioperative temperature monitoring. Anesthesiology. 2021;134:111–8. doi: 10.1097/aln.0000000000003481. [DOI] [PubMed] [Google Scholar]
- 52.Torossian A, Bräuer A, Höcker J, Bein B, Wulf H, Horn EP. Preventing inadvertent perioperative hypothermia. Dtsch Arztebl Int. 2015;112:166–72. doi: 10.3238/arztebl.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Liang H, Tian C, Rong G, Shao X, Ran C. Agreement of zero-heat-flux thermometry compared with infrared tympanic temperature monitoring in adults undergoing major surgery. Eur J Med Res. 2025;30:60. doi: 10.1186/s40001-025-02317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brandes IF, Tirilomis T, Nemeth M, Wieditz J, Bräuer A. Intraoperative zero-heat-flux thermometry overestimates nasopharyngeal temperature by 0.39 °C: an observational study in patients undergoing congenital heart surgery. J Clin Monit Comput. 2025;39:205–15. doi: 10.1007/s10877-024-01204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang H, Wang JY, Liang Y, Shao XF, Ding YL, Jia HQ. Agreement of zero-heat-flux thermometry with the oesophageal and tympanic core temperature measurement in patient receiving major surgery. J Clin Monit Comput. 2024;38:197–203. doi: 10.1007/s10877-023-01078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kollmann Camaiora A, Brogly N, Alsina E, de Celis I, Huercio I, Gilsanz F. Validation of the Zero-Heat-Flux thermometer (SpotOn®) in major gynecological surgery to monitor intraoperative core temperature: a comparative study with esophageal core temperature. Minerva Anestesiol. 2019;85:351–7. doi: 10.23736/S0375-9393.18.12188-2. [DOI] [PubMed] [Google Scholar]
- 57.Boisson M, Alaux A, Kerforne T, Mimoz O, Debaene B, Dahyot-Fizelier C, et al. Intra-operative cutaneous temperature monitoring with zero-heat-flux technique (3M SpotOn) in comparison with oesophageal and arterial temperature: a prospective observational study. Eur J Anaesthesiol. 2018;35:825–30. doi: 10.1097/EJA.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 58.Sessler DI, Pei L, Li K, Cui S, Chan MTV, Huang Y, et al. PROTECT Investigators. Aggressive intraoperative warming versus routine thermal management during non-cardiac surgery (PROTECT): a multicentre, parallel group, superiority trial. Lancet. 2022;399:1799–808. doi: 10.1016/S0140-6736(22)00560-8. [DOI] [PubMed] [Google Scholar]
- 59.Link T. Guidelines in practice: hypothermia prevention. AORN J. 2020;111:653–66. doi: 10.1002/aorn.13038. [DOI] [PubMed] [Google Scholar]
- 60.Shaw CA, Steelman VM, DeBerg J, Schweizer ML. Effectiveness of active and passive warming for the prevention of inadvertent hypothermia in patients receiving neuraxial anesthesia: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth. 2017;38:93–104. doi: 10.1016/j.jclinane.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bräuer A, Quintel M. Forced-air warming: technology, physical background and practical aspects. Curr Opin Anaesthesiol. 2009;22:769–74. doi: 10.1097/aco.0b013e328331d134. [DOI] [PubMed] [Google Scholar]
- 62.Kim HY, Lee KC, Lee MJ, Kim MN, Kim JS, Lee WS, et al. Comparison of the efficacy of a forced-air warming system and circulating-water mattress on core temperature and post-anesthesia shivering in elderly patients undergoing total knee arthroplasty under spinal anesthesia. Korean J Anesthesiol. 2014;66:352–7. doi: 10.4097/kjae.2014.66.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dewar DJ, Fraser JF, Choo KL, Kimble RM. Thermal injuries in three children caused by an electrical warming mattress. Br J Anaesth. 2004;93:586–9. doi: 10.1093/bja/aeh236. [DOI] [PubMed] [Google Scholar]
- 64.Yoo HS, Park SW, Yi JW, Kwon MI, Rhee YG. The effect of forced-air warming during arthroscopic shoulder surgery with general anesthesia. Arthroscopy. 2009;25:510–4. doi: 10.1016/j.arthro.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 65.Tyvold SS. Preventing hypothermia in outpatient plastic surgery by self-warming or forced-air-warming blanket: a randomised controlled trial. Eur J Anaesthesiol. 2019;36:843–50. doi: 10.1097/EJA.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 66.Nieh HC, Su SF. Meta-analysis: effectiveness of forced-air warming for prevention of perioperative hypothermia in surgical patients. J Adv Nurs. 2016;72:2294–314. doi: 10.1111/jan.13010. [DOI] [PubMed] [Google Scholar]
- 67.Chen YC, Cherng YG, Romadlon DS, Chang KM, Huang CJ, Tsai PS, et al. Comparative effects of warming systems applied to different parts of the body on hypothermia in adults undergoing abdominal surgery: a systematic review and network meta-analysis of randomized controlled trials. J Clin Anesth. 2023;89:111190. doi: 10.1016/j.jclinane.2023.111190. [DOI] [PubMed] [Google Scholar]
- 68.Lee Y, Kim K. Optimal application of forced air warming to prevent peri-operative hypothermia during abdominal surgery: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:2517. doi: 10.3390/ijerph18052517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenkilde C, Vamosi M, Lauridsen JT, Hasfeldt D. Efficacy of prewarming with a self-warming blanket for the prevention of unintended perioperative hypothermia in patients undergoing hip or knee arthroplasty. J Perianesth Nurs. 2017;32:419–28. doi: 10.1016/j.jopan.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 70.Torossian A, Van Gerven E, Geertsen K, Horn B, Van de Velde M, Raeder J. Active perioperative patient warming using a self-warming blanket (BARRIER EasyWarm) is superior to passive thermal insulation: a multinational, multicenter, randomized trial. J Clin Anesth. 2016;34:547–54. doi: 10.1016/j.jclinane.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 71.Al-Dardery NM, Abdelwahab OA, El-Samahy M, Seif AM, Mouffokes A, Khaity A. Self-warming blankets versus active warming by forced-air devices for preventing hypothermia: a systematic review and meta-analysis. Medicine (Baltimore) 2023;102:e33579. doi: 10.1097/md.0000000000033579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji N, Wang J, Li X, Shang Y. Strategies for perioperative hypothermia management: advances in warming techniques and clinical implications: a narrative review. BMC Surg. 2024;24:425. doi: 10.1186/s12893-024-02729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munday J, Duff J, Wood FM, Sturgess D, Ralph N, Ramis MA. Perioperative hypothermia prevention: development of simple principles and practice recommendations using a multidisciplinary consensus-based approach. BMJ Open. 2023;13:e077472. doi: 10.1136/bmjopen-2023-077472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andrzejowski JC, Turnbull D, Nandakumar A, Gowthaman S, Eapen G. A randomised single blinded study of the administration of pre-warmed fluid vs active fluid warming on the incidence of peri-operative hypothermia in short surgical procedures. Anaesthesia. 2010;65:942–5. doi: 10.1111/j.1365-2044.2010.06473.x. [DOI] [PubMed] [Google Scholar]
- 75.Bindu B, Bindra A, Rath G. Temperature management under general anesthesia: compulsion or option. J Anaesthesiol Clin Pharmacol. 2017;33:306–16. doi: 10.4103/joacp.joacp_334_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koc BB, Schotanus MGM, Kollenburg JAPAC, Janssen MJA, Tijssen F, Jansen EJP. Effectiveness of early warming with self-warming blankets on postoperative hypothermia in total hip and knee arthroplasty. Orthop Nurs. 2017;36:356–60. doi: 10.1097/nor.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 77.Perl T, Peichl LH, Reyntjens K, Deblaere I, Zaballos JM, Bräuer A. Efficacy of a novel prewarming system in the prevention of perioperative hypothermia. A prospective, randomized, multicenter study. Minerva Anestesiol. 2014;80:436–43. [PubMed] [Google Scholar]
- 78.Horn EP, Bein B, Broch O, Iden T, Böhm R, Latz SK, et al. Warming before and after epidural block before general anaesthesia for major abdominal surgery prevents perioperative hypothermia: a randomised controlled trial. Eur J Anaesthesiol. 2016;33:334–40. doi: 10.1097/EJA.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 79.Becerra Á, Valencia L, Ferrando C, Villar J, Rodríguez-Pérez A. Prospective observational study of the effectiveness of prewarming on perioperative hypothermia in surgical patients submitted to spinal anesthesia. Sci Rep. 2019;9:16477. doi: 10.1038/s41598-019-52960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horn EP, Bein B, Böhm R, Steinfath M, Sahili N, Höcker J. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67:612–7. doi: 10.1111/j.1365-2044.2012.07073.x. [DOI] [PubMed] [Google Scholar]
- 81.Cho SA, Yoon S, Lee SJ, Jee YS, Cho CK, Sung TY. Clinical efficacy of short-term prewarming in elderly and adult patients: a prospective observational study. Int J Med Sci. 2022;19:1548–56. doi: 10.7150/ijms.77578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warttig S, Alderson P, Campbell G, Smith AF. Interventions for treating inadvertent postoperative hypothermia. Cochrane Database Syst Rev. 2014;2014:CD009892. doi: 10.1002/14651858.cd009892.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


