Abstract
Medicinal plants are effective in treating many infections. Pistacia lentiscus L., whose oil is recognized for its biological properties, is attracting growing interest in medical research. These biological properties are due to their chemical composition (CC), which various conditions, like climate, can affect. P. lentiscus EOs biological properties from December, May, and August were investigated in vitro; gas chromatography-mass spectrometry (GC-MS) helped analyse their CC. Autodock Vina was used for molecular docking and SwissADME was used for ADMET analysis. The Eos of P. lentiscus leaves collected in December showed a remarkable presence of β-caryophyllene and β-myrcene. May Eos, α-pinene dominated, while August Eos, β-pinene, and D-limonene were predominant. The biological activity of Eos from P. lentiscus collected in August was highest compared to other periods. During this time (August), the leave’s Eos showed significant antibacterial, antioxidant, and anti-inflammatory activity. The computational molecular docking and ADMET results align with experimental evidence, lending scientific validation to the traditional medicinal uses of the plants from which these compounds were derived. These results provide valuable information on the seasonal dynamics of the CC of P. lentiscus Eos and underline the importance of taking environmental factors into account in studies of metabolite biosynthesis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-08833-2.
Subject terms: Plant sciences, Environmental sciences
Introduction
Medicinal plants (MP) have been used for hundreds of years in different regions, particularly in Asia, with a notable focus on China and India. These regions have considerably proved their expertise, facilitating knowledge through generations1,2. With the efficiency of MP, many countries have seen a renewal in their interest in using them to prevent and treat infectious diseases3–5.
Because of its efficacy, doctors and health organizations have been convinced and recognized for a long time that medicinal plants are important in treating diseases and their use in the composition of many medicines6.
Today, as modern medicine grapples with the escalating issue of antibiotic resistance, there is a growing interest in medicinal plants and their active compounds, such as essential oils. Antibiotic resistance has emerged as a significant global public health challenge, jeopardizing the effectiveness of existing treatments and posing serious risks to human, animal, and environmental health7. In response to this urgent situation, research is increasingly focusing on natural alternatives, particularly essential oils and extracts from medicinal plants, which show promising potential in combating resistant infections8,9.
These natural compounds are not only recognized for their antimicrobial properties but also exhibit a range of biological activities, including antioxidant and anti-inflammatory effects, influenced by environmental and seasonal factors. Understanding this variability is crucial for optimizing their application in various therapeutic contexts and for identifying effective alternatives to current treatment regimens.
Among the numerous plants investigated for their therapeutic properties, P. lentiscus is one of the medicinal plants whose efficacy has been acknowledged throughout history and is esteemed for its diverse therapeutic applications10,11. Native to Mediterranean regions, P. lentiscus, or lentisk, derives its medicinal potential from its various parts, particularly its leaves and twigs12,13. Its essential oil (Eos), attracting growing interest in medical research, is recognized for its antimicrobial, antioxidant, and anti-inflammatory properties14. Previous scientific research has explored various aspects of the chemical composition (CC) of P. lentiscus Eos in different regions, notably Tunisia and Morocco. A study carried out in Tunisia revealed significant variations in the Eo’s CC according to locality, highlighting three different chemotypes of P. lentiscus Eos11. Moreover, distinct research has investigated the variation in CC, phenolic compound content, and antioxidant activity of different aerial parts of P. lentiscus, considering factors such as sex and harvesting periods15. Additionally, a study conducted in Morocco observed seasonal variations in the CC of P. lentiscus Eos, underscoring the importance of seasonal effects on oil production and composition16,17. However, despite these advances, these studies have yet to exhaustively examine the correlation between its composition and its biological activities in the specific context of Morocco’s Rif region.
We aim to analyse the seasonal variability of this Eos, harvested in the Al-Hoceima region, located in the Rif region (Northern Morocco), characterized by a Mediterranean climate. By exploring this correlation, our study will better understand seasonal variations in P. lentiscus Eos and its impact on its biological activities in specific climatic contexts, thus enriching the scientific knowledge base in this field.
Materials and methods
Eos samples
P. lentiscus was harvested in the early morning hours in Morocco, AL-Hoceima province (commune of Ajdir), (35.2097449°N, 3.9174927°W) during the rainy (December), intermediate (May) and dry (August) seasons. Taxonomic identification of the plant material was performed by Prof. C. Alaoui Jamali, Ecole Normale Supérieure, Tetouan, Morocco. A voucher specimen has been deposited in the Herbarium of the Faculty of Sciences and Techniques of Al Hoceima (FSTH) under the reference number PL022.After harvesting, the leaves were kept at dark for five days to dry. They were then hydrodistilled for 4 h using a Clevenger apparatus to extract the Eos from the plant. The Eos obtained was then stored at -4 °C in a refrigerator until use.
Gas chromatography-mass spectrometry (GC-MS)
The identification of P. lentiscus oil compounds were achieved by comparing their retention times with those of established standards and by analysing their mass spectral fragmentation patterns against the National Institute of Standards and Technology (NIST147) database. A BPX25 capillary column (30 m × 0.25 mm internal diameter × 0.25 μm film thickness) with 5% diphenyl and 95% dimethylpolysiloxane was used in a Shimadzu GC system (Kyoto, Japan) coupled to a QP2010 MS. Pure helium gas (99.99%) was used as the carrier gas at a flow rate of 3 mL/min. The injection, ion source, and interface were set to 250 °C. The column oven temperature program began at 50 °C (maintained for one minute), then the temperature rose to 250 °C at a speed of 10 °C/min and stayed for one min. The P. lentiscus Eos components were ionized using electron ionization (EI) mode at 70 eV and examined within a 40 to 300 m/z mass range18.
Antioxidant capacity in vitro (AC)
The DPPH assay estimated the AC of P. lentiscus Eos. 1 mL of P. lentiscus Eos at concentrations ranging from 600 µg to 2000 µg was mixed with 1 mL of DPPH solution (0.5 mM) and incubated for 20 min in the dark. Under the same conditions, ascorbic acid was used as a positive control. The DPPH inhibition (DI) percentage was calculated using the following formula19,20.
 |
With Ac-absorbance of control, As-Absorbance of samples.
Antibacterial activity
Bacterial strains
Four bacteria strains were used to evaluate the antibacterial activity of P. lentiscus essential oils. The bacterial cells assayed included two Gram-positive: Methicillin-resistant Staphylococcus aureus ATCC 43,300 and Micrococcus luteus ATCC 381, and two Gram-negative bacteria, namely Escherichia coli ATCC 25,922, and Pseudomonas aeruginosa ATCC 27,853.
Antibacterial screening
The antibacterial activity of P. lentiscus Eos was tested with the agar diffusion method and the determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC).
The agar diffusion method was described by Alaoui Jamali et al. (2018). Briefly, bacterial strains (0.1 mL) were suspended on Mueller-Hinton agar (MHA). Next, 10 µL of Pistacia oils was impregnated onto 6 mm filter paper discs. Incubation lasted two hours at 4 °C and then at 37 °C for 24 h. Ciprofloxacin (10 µg/disc) were utilized as reference antibiotic. All tests were performed in triplicate8,21.
Determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC determination for P. lentiscus Eos was carried out by NCCLS guidelines M7-A422. Pistacia lentiscus Eos were dissolved in 4% dimethylsulfoxide (DMSO). Mueller-Hinton Broth (MHB) was used to perform log-phase testing of the microorganism under test, with a cell suspension adjusted to 10⁶ CFU/mL in MHB, and 100 µL was added to each well of 96-well plates, followed by 100 µL of each dilution of the EOs. Negative controls were prepared using the DMSO solvent, and positive controls contained the DMSO and bacteria. The plates were then incubated at 37 °C under normal atmospheric conditions for 24 h.
The MIC is defined as the lower concentration of the Eos at which the microorganism does not show visible growth. The turbidity of the culture indicates microorganism growth23. To determine the MBC, 100 µL of each case in which microbial growth was not observed was spread on Mueller Hinton Agar (MHA) and incubated at 37 °C for 24 h. The MBC was defined as the lower concentration of the Eos at which the incubated microorganism was killed entirely. Each test was performed in triplicate. Ciprofloxacin served as positive antibacterial control8.
Anti-inflammatory activity by inhibition of albumin denaturation
The denaturation method using BSA was employed to study the anti-inflammatory capacity of the Eos of P. lentiscus Samples were prepared at concentrations of 50 µg/mL to 500 µg/mL and mixed with a 0.2% BSA (Bovine Serum Albumin) solution prepared in PBS (Phosphate Buffered Saline) with a pH of 6.4. Each mixture contained a volume of 500 µL. Samples were incubated at 37 °C for 20 min and then in a water bath at 72 °C for five minutes24,25.
Percentage protein denaturation (%I) was estimated according to the following equation:
 |
With C-control, W-white, S-sample.
Statistical analysis
GraphPad Prism version 8 software (GraphPad Inc.) was used to analyse the results of P. lentiscus Eos samples, which were tested in triplicate. The data were analyzed by one-way ANOVA followed by Dunnett’s multiple comparisons test (comparing all test groups to the positive controls), with statistical significance set at p < 0.05. Results are expressed as mean ± SD (n = 3). To analyse variations in the CC of Eos according to tree period, a principal component analysis (PCA) was performed using OriginPro 2024b software.
Molecular docking methodology
In silico molecular docking study was conducted to evaluate the anti-inflammatory, antioxidant and antibacterial potential of bioactive compounds from P. Lentiscus L. Essential Oil. The p38-MAP kinase (PDB ID: 3UVQ)26, cytochrome P450-CYP2C9 (PDB ID: 1OG5)27and DNA gyrase (PDB ID: 6KZV)28 were retrieved from the RCSB Protein Data Bank. The p38-MAPK plays a crucial role in controlling pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6, making it a strategic target for effective anti-inflammatory therapies29. The proteins were prepared for docking analysis through a sequence of optimization steps: water molecules and heteroatoms were removed, missing atoms were added, and Kollman charges were applied using MGL tools 1.5.630. The optimized structures were then saved in PDBQT format. The ChemOffice Suite 2020 was employed to draw 2D and 3D structures of investigated ligands and energetically minimized with the MM2 force field, applying 200 iterations to ensure stability. These minimized structures were converted to PDBQT format via AutoDock tools, facilitating compatibility with docking software. Molecular docking simulations were carried out using AutoDock Vina v.1.2.031. A grid box was configured around respective crystalized ligand within the active binding sites to ensure accurate interaction predictions. For each ligand, up to ten possible conformations were analyzed to identify the optimal docking pose. Visualization of the docking interactions and binding affinities was achieved using Biovia Discovery Studio32,33. Furthermore, SwissADME is employed for evaluating pharmacokinetic properties, including absorption, metabolism, and drug-likeness, to assess compound viability in drug discovery34.
Results and discussion
Chemical composition of P. lentiscus eos
Oils’ CC and yield vary under the influence of multiple factors, such as harvest time, soil composition, plant age, and weather conditions35. These external elements can alter the biosynthesis of volatile compounds, resulting in significant variations in the chemical profile of Eos, which in turn impacts their biological activity35. Despite their importance, research into the effect of seasonality on these oils remains limited.
The results presented in Table 1 show that the extraction of Eos from P. lentiscus in May, which yielded 0.3%, stands out as the highest compared to August (0.22%) and December (0.15%), the latter period having the lowest yield of the three. This significant finding underscores the importance of the flowering period in Eos production. Studies carried out in the province of Taounate revealed a high yield in May (0.32%) and lower values for the December (0.2%) and August (0.24%) periods36.
Table 1.
Chemical composition of essential oils from Pistacia lentiscus L. Leaves.
| Nombre | Compound name | RT | % composition | ||
|---|---|---|---|---|---|
| May | August | December | |||
| 1 | Tricyclene (MH) | 4.951 | 0.7 | - | - |
| 2 | α -Pinene (MH) | 5.135 | 13.65 | 4.13 | 5.97 |
| 3 | Camphene (MH) | 5.388 | 3.27 | 1.44 | - |
| 4 | Sabinene (MH) | 5.774 | 5.25 | 1.42 | 1.44 |
| 5 | β-Pinene (MH) | 5.848 | 3.17 | 18.68 | - |
| 6 | β -Myrcene (MH) | 6.015 | - | - | 18.1 |
| 7 | α-Phellandrene (MH) | 6.277 | 1.48 | 2.79 | - |
| 8 | 4-Carene (MH) | 6.477 | 1.9 | - | - |
| 9 | β-cymene (MH) | 6.61 | 2.16 | - | 1.9 |
| 10 | D-Limonene (MH) | 6.684 | 5.51 | 37.25 | - |
| 11 | γ-Terpinen (MH) | 7.174 | 3.38 | 0.89 | - |
| 12 | 2-Carene (MH) | 7.672 | 2.66 | - | - |
| 13 | 4-Terpineol (MO) | 9.152 | 15.48 | 2 | 4.31 |
| 14 | p-menth-1-en-8-ol (MO) | 9.353 | 7.07 | - | - |
| 15 | Borneol (MO) | 10.801 | 7.18 | 1.75 | - |
| 16 | β -Caryophyllen (SH) | 12.981 | 11.51 | 9.61 | 19.55 |
| 17 | α-Caryophyllene (SH) | 13.425 | 1.72 | 1.61 | 3.05 |
| 18 | γ-Muurolene (SH) | 13.657 | - | 1.62 | - |
| 19 | Copaene (SH) | 13.658 | - | - | 2.11 |
| 20 | Germacrene D (SH) | 13.748 | 8.06 | 8.61 | 8.82 |
| 21 | Germacrene B (SH) | 13.926 | - | 2.8 | - |
| 22 | δ-Cadinene (SH) | 14.172 | - | 2.76 | 4.52 |
| 23 | Caryophyllene oxide (SO) | 14.873 | 1.12 | - | 5.53 |
| 24 | Spathulenol (SO) | 15.242 | - | 6.58 | |
| 25 | τ-Cadinol (SO) | 15.358 | 2.29 | - | 6.8 |
| 26 | τ-Muurolol (SO) | 15.359 | - | 1.51 | - |
| 27 | α-Cadinol (SO) | 15.471 | - | 1.13 | 9.15 |
| 28 | Hexahydropseudoionone (SH) | 16.701 | - | - | 2.17 |
| Hydrocarbon monoterpenes (MH) | 43.13 | 66.6 | 27.41 | ||
| Oxygenated monoterpenes (MO) | 29.73 | 3.75 | 4.31 | ||
| Hydrocarbon sesquiterpenes (SH) | 21.29 | 27.01 | 38.05 | ||
| Oxygenated sesquiterpenes (SO) | 3.41 | 2.64 | 28.06 | ||
| Total identified (%) | 97.56 | 100 | 100 | ||
| Yield (%) | 0.30% | 0.25% | 0.15% | ||
RT: retention time.
Furthermore, research carried out in Algeria during the flowering season indicated a yield of 0.27%, which is close to our result of 0.3% in May37; these findings align with the results of our study but contrast with those from the Fez-Meknes (0.22% in December) and Tunisia (0.009–0.02% in May)11,38. These results underscore the combined importance of phenological stage and geographical factors in Eos production.
The high yield in May can be attributed to the flowering period of P. lentiscus. Indeed, the concentration of volatile compounds responsible for Eos production is often at its highest during flowering, leading to better extraction39.
Significant seasonal variations in compound group percentages are also apparent. Sesquiterpene hydrocarbons show a notable increase in Eos collected in December (38.05%), while monoterpene hydrocarbons are more abundant in May Eos (43.16%) and August Eos (66.6%). This variation could be attributed to the influence of elevated temperatures and summer photoperiods on monoterpene biosynthesis40.
Furthermore, the chromatographic profiles of the Eos, presented in Fig. 1, illustrate the composition of P. lentiscus across the three harvest periods (Fig. 1). The Venn diagram analysis reveals six compounds common to all seasons, including β-caryophyllene, α-pinene, and germacrene D, indicating stable metabolic constituents in P.lentiscus Eos regardless of seasonal variations. Notably, season-specific patterns emerge: December oils contain four exclusive compounds, such as β-myrcene (18.1%), while May oils feature four distinct compounds, including 2-carene and 4-carene. August samples show three unique components (γ-muurolene, germacrene B, and δ-cadinene).These compositional differences highlight the environmental and phenological influences on the production of secondary metabolites (Fig. 2).
Fig. 1.
Chromatogram of E O from Pistacia lentiscus L. Leaves; (a) EO December; (b) Eos May; (c) Eos August.
Fig. 2.
Venn diagram of Eos composition of Pistacia lentiscus L. leaves cross harvest periods :December (green), May (blue), August (red).
Table 1 shows that Eos collected in December, the majority of the compounds identified were β-caryophyllene (19.55%), β-myrcene (18.1%), α-cadinol (9.15%), and germacrene D (8.82%).
Studies in other regions show interesting variations: in eastern Morocco, β-myrcene (39.2%) and limonene (10.3%) dominate41, while in Algeria, leaves show a wide range of concentrations of myrcene (0.1–33.1%), germacrene D (2.7–13.5%), and β-caryophyllene (0.7–2.4%)42. In Sicily, Italy, germacrene D (18.61%) and β-caryophyllene (14.68%) are also present, with a lower concentration of α-cadinol (5.36%) than observed in our study43.
In May EOs, the CC was predominantly α-pinene (13.65%), significantly lower in December (5.97%). Similarly, 4-terpineol (15.48%) shows a much higher concentration than December Eos (4.31%). On the other hand, β-caryophyllene (11.51%) and germacrene D (8.06%) levels were lower in December EOs, and α-cadinol, a major compound during this collection period, was absent in May EOs.
Studies in Italy (Sardinia) and Tunisia (Siliana) show similar concentrations of terpinene-4-ol (14.17% and 23.32%, respectively) and β-caryophyllene (5.03% in Algeria and 22.62% in Tunisia44,45. In Algeria (Bouira), levels of α-pinene (15.47%) and β-pinene (7.31%) were observed, confirming the seasonal variation of these compounds46.
Analysis of the August EOs revealed a dominance of β-pinene (18.68%) and a strong presence of D-limonene (37.25%), which were much less abundant in the essential oils collected in May (5.51%). β-Caryophyllene (9.61%) continued to decrease compared to May Eos, while D-germacrene (8.61%) in August EOs remained at a concentration similar to that observed in May (8.06%).
In Greece (Zakynthos), the primary compounds during summer include α-pinene at 17.1%, limonene at 13.9%, and terpinene-4-ol at 10.6%47. In Morocco (Chaouen), the concentration of β-pinene is notably lower, at 1%, D-limonene (8%), and germacrene D (0.1%), showing significant geographical differences16.
Intersecting areas: Count of shared compounds between seasons.
Non-overlapping areas: Count of season-exclusive compounds.
Central intersection (6): Compounds present in all three seasons (core profile).
December-only (4), May-only (4), August-only (3): Unique seasonal markers.
December-May overlap (3), May-August (6), December-August (2): Pairwise shared.
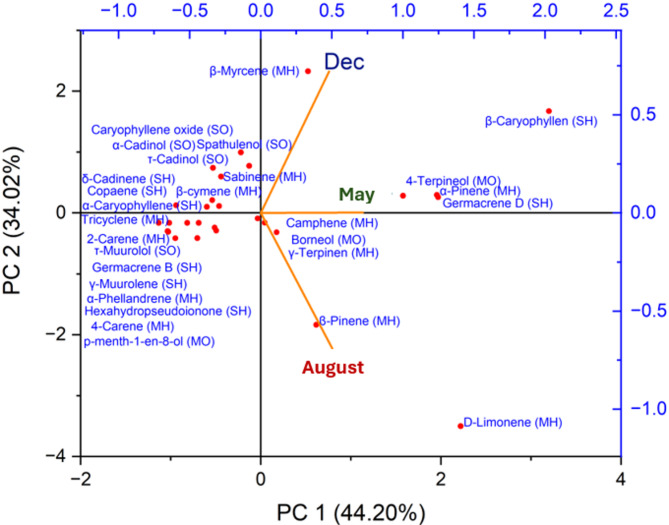
The PCA biplot (Fig. 3), a powerful tool in our research, vividly illustrates the distribution of CC in P. lentiscus Eos over two principal components (PC1 and PC2), which collectively account for a substantial 78.22% of the total variance.
Fig. 3.
Biplot of PCA identified in the Pistacia lentiscus Eos leaves. Dec-December; PCA-principal components analysis.
Axis PC1 (44.20%) represents the principal component explaining the most considerable proportion of the data’s variance. This component strongly influences compounds on the far right of this axis, such as D-limonene. The PC2 axis (34.02%) represents the second principal component, explaining a considerable proportion of the remaining variance. This component influences compounds at the top of this axis, such as β-myrcene.
In December Eos, the dominant compounds are located at the top right of the biplot, including β-myrcene, β-caryophyllene, α-cardinal, and germacrene D. This indicates that these compounds score high on PC2 and are characteristic of P. lentiscus Eos collected in December.
The characteristic May Eos compounds are located near the center, slightly to the right. Notable compounds include α-pinene, 4-terpineol, and germacrene D. This central position suggests that these compounds have intermediate values on PC1 and PC2, indicating a moderate CC compared to other periods.
In August Eos, the dominant compounds are located on the lower right, notably β-pinene (MH) and D-limonene (MH).
The position of D-limonene on the far right indicates a strong influence from PC1, characterizing the essential oils of this period by a high concentration of this compound.
The PCA biplot illustrates the seasonal variation in the CC of P. lentiscus Eos. Each season has a distinct profile influenced by environmental conditions, with specific compounds predominating in winter, spring, and summer.
Anti-inflammatory activity using BSA
The P. lentiscus Eos extracted in August shows high anti-inflammatory activity (IC50 = 0.07 ± 0.01 mg/ml), close to the sodium diclofenac standard (IC50 = 0.035 ± 0.01 mg/ml). This period is characterized by the dominance of β-pinene (18.68%) and D-limonene (37.25%) (Fig. 4). The strong anti-inflammatory activity observed could be attributed to the high concentration of D-limonene, a compound well known for its anti-inflammatory properties. It suppresses key inflammatory mediators, including TNFα (tumor necrosis factor-alpha), IL-1β (interleukin 1 beta), and COX-2 (cyclooxygenase-2), which play an essential role in the inflammatory process, enabling this compound to reduce inflammation and its symptoms48. The significant presence of β-pinene, another compound with anti-inflammatory properties, could also contribute to this high activity49.
Fig. 4.
Effect of Pistacia lentiscus L Eos leaves. on albumin denaturation. The results are presented as mean ± S.D. (n = 3), DF-diclofenac sodium; Dec-December. Values not sharing a common letter (a to c) differ significantly at p < 0.05.
The December Eos of P. lentiscus shows moderate anti-inflammatory activity (IC50 = 0.19 ± 0.11 mg/ml). The moderate anti-inflammatory activity in December Eos is linked to the high presence of β-caryophyllene, known for its anti-inflammatory effects50. However, the absence or low concentration of compounds such as D-limonene could explain the lower activity compared to August Eos.
The May Eos of P. lentiscus shows a slightly different anti-inflammatory activity to December, with an (IC50 = 0.14 ± 0.01 mg/ml). The anti-inflammatory activity in May Eos may be because of 4-terpineol, which suppresses superoxide production and pro-inflammatory cytokines, and to the presence of α-pinene. One study showed that a dose-dependent response (0.50 mL/kg) of alpha-pinene led to a significant decrease in inflammation, indicating its anti-inflammatory activity51,52. Nevertheless, the absence of a highly active compound such as D-limonene, observed in August, could explain why the anti-inflammatory activity is less pronounced.
Antioxidant capacity (AC)
Figure 5 reveals high antioxidant capacity (AC) in August Eos of P. lentiscus (IC₅₀ = 0.72 ± 0.05 mg/ml), which, although significantly higher than the standard ascorbic acid (IC₅₀ = 0.05 ± 0.01 mg/ml), remains the best result among the tested periods. This was followed by the May period (0.83 ± 0.09 mg/ml). The lowest activity was observed in December Eos (1.88 ± 0.36 mg/ml) compared to the other two periods.
Fig. 5.
Antioxidant activity of Pistacia lentiscus Eos leaves by DPPH assay. The results are presented as mean ± S.D (n = 3). Values not sharing a common letter (a to c) differ significantly at p < 0.05.
Studies carried out in Algeria in winter showed IC50 values between 8.8 and 27.48 mg/ml, significantly higher than those observed in our study for December Eos (1.88 mg/ml)53. In Tunisia, at four sites studied in spring, IC50 values ranged from 60 ± 0.01 µg/ml to 110.8 ± 0.004 µg/ml, comparable to those observed in May in our study (0.83 mg/ml)11.
The high AC observed in August Eos of P. lentiscus can be attributed to the elevated concentrations of monoterpene Hydrocarbon compounds (66%) such as D-limonene and β-pinene54. D-limonene is recognized for its antioxidant properties, which can reduce lipid oxidation and promote the functions of antioxidant enzymes like superoxide dismutase and heme oxygenase-155. Studies have also shown that D-limonene reduces mitochondrial oxidation and preserves mitochondrial dehydrogenase activity in brain cells exposed to Aβ1–42 oligomers56. Furthermore, the antioxidant activity of β-pinene and D-limonene is attributed to their ability to inhibit NADPH oxidase subunit activity and increase antioxidant enzyme levels57.
Antibacterial activity
The antibacterial activity of P. lentiscus Eos, collected during three different periods (December, May, and August), was assessed through disk diffusion and the MIC tests against two Gram-positive bacteria (M.R.S.A and M. luteus) and two Gram-negative bacteria (E. coli and P. aeruginosa).
The results obtained from the disk diffusion method indicated that the Eos revealed significant variations in antibacterial activity depending on the collection period (Table 2). Maximum activity was observed with August Eos against different tested bacteria with zones of inhibition greater than 30 mm, followed by May Eos and December Eos which demonstrated moderate antibacterial activity with an inhibition zone ranging from 11.00 to 26.00 mm, and from 10.33 to 24.66 mm, respectively. Ciprofloxacin demonstrates remarkable antibacterial activity that is close to that of P. lentiscus oil in August. This equivalence highlights the potential of P. lentiscus Eos as a natural or complementary alternative to conventional antibiotics.
Table 2.
Antibacterial activity of Eos from leaves of Pistacia lentiscus L. in different seasons of the year using disc diffusion assay.
| Test microorganisms | Inhibition zone diameter (mm) | |||
|---|---|---|---|---|
| P.lentiscus Eos | Antibiotic | |||
| May Eos | August Eos | December Eos | Ciprofloxacin | |
| Gram positive bacteria | ||||
| M.R.S.A | 23.63 ± 0.57 | 25.11 ± 1.05 | 10.33 ± 0.57 | 43.33 ± 1.52 |
| M.luteus | 26.00 ± 0.00 | 30.00 ± 1.00 | 24.66 ± 1.52 | 50.00 ± 0.00 |
| Gram negative bacteria | ||||
| E.coli | 25.82 ± 1.00 | 35.00 ± 1.00 | 12.00 ± 1.00 | 30.66 ± 0.57 |
| P.aeruginosa | 11.00 ± 1.16 | 12.00 ± 1.52 | 11.33 ± 0.57 | 42.66 ± 2.08 |
This result was confirmed by studying the MIC and MBC values using the micro-dilution method (Table 3). The data show that P. lentiscus Eos is active against all tested bacteria depending on the collection period. In August, P. lentiscus oil showed significant antibacterial activity, with an MIC from 0.22 mg/ml to 1.76 mg/ml.
Table 3.
Antibacterial activity of essential oil of Pistacia lentiscus L. leaves in different seasons of the year using the minimum inhibitory concentration method.
| P.lentiscus Eos (mg/ml) | Antibiotic (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| May Eos | August Eos | December Eos | Ciprofloxacin | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Gram positive bacteria | ||||||||
| M.R.S.A | 4.00 | 4.00 | 1.76 | 3.52 | 45.42 | 45.42 | 0.03 | 0.03 |
| M.luteus | 2.00 | 2.00 | 0.88 | 0.88 | 45.42 | 45.42 | 0.01 | 0.01 |
| Gram negative bacteria | ||||||||
| E.coli | 1.00 | 1.00 | 0.22 | 0.22 | 45.42 | 45.42 | 0.07 | 0.07 |
| P.aeruginosa | 4.00 | 8.00 | 1.76 | 3.52 | 45.42 | 45.42 | 1.00 | 1.00 |
The results are presented as mean ± S.D. (n = 3)
MIC-minimum inhibitory concentration; MBC-minimum bactericidal concentration.
In May, the antibacterial activity of P. lentiscus Eos remained remarkable, with a MIC of 1 mg/ml against E. coli and 2 mg/ml against M. luteus. Although the activity is lower than in August, it remains significant. Eos collected in December exhibited reduced activity against all strains, demonstrating a MIC of 45.42 mg/ml against all tested bacteria, indicating a marked decrease in antibacterial efficacy. However, the MIC and MBC differed among the tested microorganisms; in most instances, the MIC was equal to the MBC, suggesting that the Eos exhibited bactericidal properties. A study in the Fez region during May also revealed a significant MIC against various strains. This study also showed a strong zone of inhibition against E. coli (34 mm), which is almost similar to our results58. Furthermore, a recent investigation in the Zinat and Fnideq regions of northern Morocco, conducted during the summer months, confirmed the potent antibacterial properties of P. lentiscus Eos, demonstrating efficacy against clinically relevant strains, including Escherichia coli.59.
The high antibacterial activity of P. lentiscus Eos in August may be justified by the presence of β-pinene at a high concentration (18%), even though the concentration of α-pinene is low (4%). Studies have shown that the antibacterial properties of β-pinene are 2 to 12 times greater than those of α-pinene against Gram-positive and Gram-negative strains. Therefore, this high β-pinene concentration may contribute significantly to the observed activity60. Additionally, the increased presence of compounds such as D-limonene in August Eos may contribute to the high antibacterial activity, particularly against M. luteus. D-limonene is recognized for its efficacy in antibacterial applications61. The high antibacterial activity of P. lentiscus Eos in May can be attributed to the significant presence of both α-pinene and β-pinene. These compounds exhibit strong antibacterial activity against M.R.S.A. Further studies have revealed that combining the two compounds with commercial antibacterial, ciprofloxacin plus α-pinene or β-pinene showed synergistic activity against M.R.S.A62.
The results are presented as mean ± S.D (n = 3). values in the same row not sharing a common letter (a–b) differ significantly at p < 0.05.
Molecular docking
The docking analysis revealed the binding patterns of bioactive compounds targeting anti-inflammatory, antioxidant, and antibacterial proteins. Table 4 outlined detailed results, including binding affinities, hydrogen bonding, hydrophobic interactions, and bond distances (measured in angstroms), for ligands with binding energies (ΔG) exceeding − 5.0 kcal/mol against the target proteins 3UVQ, 1OG5, and 6KZV. The ligands displayed binding energies ranging from − 7.6 to -4.2 kcal/mol. Among them, ligand-01 achieved the most favorable score of -7.3 kcal/mol with 3UVQ, while ligands 03 and 04 demonstrated significant affinities of -6.7 and − 6.8 kcal/mol, respectively, highlighting their anti-inflammatory potential. Key interactions such as hydrophobic contacts with residues such as VAL38, ALA51, and SER154, as well as hydrogen bonding with SER154 were observed. These findings were comparable to the co-crystallized ligand (ΔG = -10.7 kcal/mol). Figure 6 showcased the 2D and 3D interactions of ligand-01 and the co-crystallized ligand (RMSD < 2.0) with 3UVQ.
Table 4.
Molecular Docking score, hydrogen binding, hydrophobic and electrostatic interactions with distances in Angstrom for investigated ligands with 3UVQ, 1OG5, 6KZV.
| Sr. No. | Ligands | Binding Affinity, ΔG (kcal/mol) | H.B.I, Residue (Distance Å) |
Hydrophobic Interaction, Residue (Distance Å) |
|---|---|---|---|---|
| 3UVQ | ||||
| 1 | β-Caryophyllene | -7.3 | - |
VAL38 (5.3), ALA51 (5.4) ALA157 (4.9), LEU167 (5.4) |
| 2 | β-Myrcene | -5.0 | - |
VAL38 (5.0), ALA51 (4.7) LYS53 (3.9), LEU75 (5.1) ILE84 (5.2), ALA157 (3.7) LEU167 (4.3), MET109 (5.3) |
| 3 | α-Cadinol | -6.7 | SER154 (2.5) |
VAL38 (4.8), ALA51 (3.8) ILE84 (5.2), ALA157 (5.1) LEU167 (4.8) |
| 4 | Germacrene D | -6.8 | - |
VAL38 (5.0), ALA51 (3.9) LYS53 (4.0), ALA157 (5.1) |
| 5 | Limonene | -5.6 | - |
VAL38 (4.6), ALA51 (5.0) LYS53 (4.1), LEU75 (4.7) ILE84 (5.4), LEU104 (4.5) |
| 8 | 4-Terpineol | -5.9 |
HIS107 (2.7) MET109 (2.1) |
VAL30 (5.0), ALA51 (4.7) LEU108 (5.2), ALA157 (5.3) |
| C.L | Dibenzosuberone derivative | -10.7 |
GLU71 (2.2) MET109 (2.2) GLY110 (2.4) ASP168 (2.5) |
VAL30 (4.2), VAL38 (4.5) ALA51 (4.9), LYS53 (4.0) LEU74 (5.4), LEU75 (4.4) ILE84 (5.2) |
| 1OG5 | ||||
| 1 | β-Caryophyllene | -7.2 | - | ALA103 (3.7) |
| 2 | β-Myrcene | -5.2 | - |
LEU66 (4.6), ARG97 (4.9) PHE100 (4.2), VAL113 (4.6) PHE114 (3.7), ALA103 (4.4) PRO367 (4.4), PHE476 (4.6) |
| 3 | α-Cadinol | -6.8 | - |
ALA103 (4.5), PHE114 (4.4) LEU208 (5.2), LEU366 (5.2) PRO367 (5.3) |
| 4 | Germacrene D | -7.4 | - |
ARG97 (5.1), ILE99 (3.8) ALA103 (4.5), PHE114 (3.8) PRO367 (5.2) |
| 5 | Limonene | -5.9 | - |
ARG97 (5.1), PHE100 (5.2) ALA103 (4.2), VAL113 (5.1) PHE114 (3.8), LEU366 (5.2) PRO367 (4.2) |
| 6 | α-Pinene | -5.6 | - |
LEU102 (5.1), ALA103 (4.0) ALA106 (3.7), PHE114 (4.7) LEU208 (5.3), LEU233 (4.4) VAL237 (5.4) |
| 7 | β-Pinene | -5.6 | - |
PHE114 (4.7), ALA106 (4.7) LEU208 (5.3), LEU233 (4.4) VAL237 (5.3) |
| 8 | 4-Terpineol | -5.6 | - |
PHE100 (4.3), ALA103 (4.3) PRO367 (4.0), PHE476 (4.9) |
| C.L | S-Warfarin | -8.0 | ASN204 (1.9) |
ALA106 (4.6), LEU208 (5.3) LEU233 (4.6) |
| 6KZV | ||||
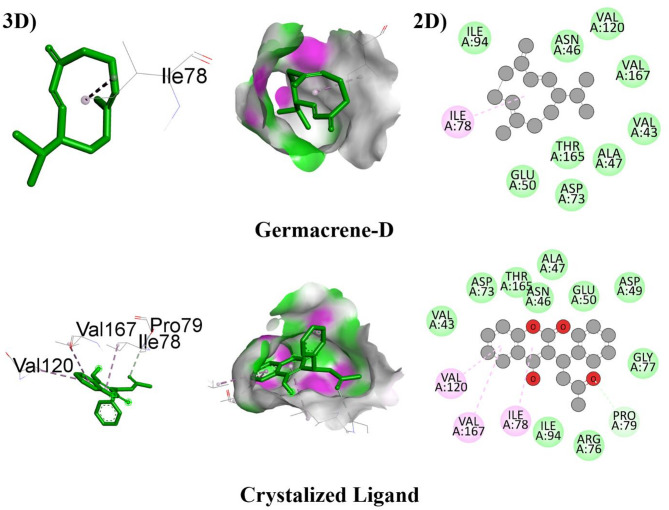
| 1 | β-Caryophyllene | -6.3 | - | ILE78 (4.7) |
| 2 | β-Myrcene | -5.4 | - |
VAL43 (4.3), ALA47 (4.7) VAL71 (4.5), ILE78 (4.5) VAL120 (4.1), VAL167 (4.2) |
| 3 | α-Cadinol | -6.6 | - | ILE78 (4.7) |
| 4 | Germacrene D | -7.6 | - | ILE78 (4.1) |
| 5 | Limonene | -5.8 | - |
VAL43 (4.9), ILE78 (4.9) MET95 (5.4), VAL120 (3.6) VAL167 (4.2) |
| 8 | 4-Terpineol | -5.7 | - |
ILE78 (4.2), VAL120 (3.7) VAL167 (4.8) |
| C.L | 2-Oxo-1,2-dihydroquinoline derivative | -7.9 | PRO79 (3.0) |
ILE78 (4.8), VAL120 (5.0) VAL167 (5.2) |
C.L: Crystalized Ligand H.B.I: Hydrogen Bonding Interaction.
Fig. 6.
The putative 2D and 3D binding mode of investigated compounds with 3UVQ.
For antioxidant activity, ligands 01 and 04 displayed strong affinities, with ΔG values of -7.2 and − 7.4 kcal/mol, respectively, against 1OG5. Residues ARG97, ILE99, ALA103, PHE114, and PRO367 emerged as key interaction points within the binding pocket. Figure 7 provided visualizations of the 2D and 3D interactions for ligands exhibiting binding energies greater than − 7.0 kcal/mol with 1OG5.
Fig. 7.
The putative 2D and 3D binding mode of investigated compounds with 1OG5.
The bioactive compounds also showed notable antibacterial potential, with all ligands achieving ΔG values > -5.0 kcal/mol when interacting with DNA gyrase (6KZV). Ligand-04 displayed the strongest binding affinity (-7.6 kcal/mol), forming hydrophobic interactions with ILE78. Ligands 01 and 03 also exhibited significant binding scores of -6.3 and − 6.6 kcal/mol, respectively. These binding affinities were consistent with the crystallized ligand (ΔG = -7.9 kcal/mol). Figure 8 illustrated the detailed hydrophobic interactions for these compounds.
Fig. 8.
The putative 2D and 3D binding mode of investigated compounds with 6KZV.
Comprehensive 2D and 3D interaction diagrams for ligands with ΔG values greater than − 5.0 kcal/mol against 3UVQ, 1OG5, and 6KZV are provided in the supplementary file (Figure S1 – Figure S16).
ADMET analysis
The investigated ligands exhibited notable physicochemical properties that are crucial for evaluating their potential as drug candidates. They exhibited significant lipid solubility, with XLOGP3 values up to 4.57and moderate to low polarity, reflected by a total polar surface area (TPSA) of approximately 20.23 Ų with most ligands having TPSA values close to zero. Ligands with TPSA values ranged from 60 Ų to 140 Ų are generally well-absorbed following oral administration, indicating low absorption profiles for these compounds. In terms of drug-likeness, the four ligands (02, 03, 05 and 08) adhered to Lipinski’s Rule of Five without any violations. Ligands 03 and 08 displayed a high likelihood of passive gastrointestinal absorption, supported by a bioavailability score of 0.55 and their ablility to cross the blood-brain barrier (BBB). Interestingly, none of the ligands were identified as substrates for P-glycoprotein (P-gp), a protein known to influence drug efflux and reduce intracellular drug concentrations.
The most ligands exhibited interactions with drug-metabolizing enzymes, particularly cytochrome P450-CYP2C9 except ligand 02 and 03. This suggested the potential for these compounds to interact with metabolic pathways, which could influence drug clearance and lead to drug-drug interactions. However minimal interactions were observed with other metabolizing enzymes such as CYP2C19 and CYP2D6. Additionally, these ligands demonstrated high synthetic accessibility with scores below 5, suggesting relatively easy to synthesize. A detailed summary of these interactions is presented in Table 5, emphasizing the pharmacokinetic considerations that must be addressed during further drug development.
Table 5.
The ADMET properties including absorption, distribution, bioavailability, Lipinski violation and synthetic accessibility of investigated ligands.
| Ligand | TPSA | ESOL Log S | Absorption | Metabolism | Lipinski #violations | SA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XLOGP3 | log Kp (cm/s) | GI Absorption |
BBB | P-gp substrate | BA | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | |||||
| 1 | 0 | -3.87 | 4.38 | -4.44 | Low | No | No | 0.55 | Yes | Yes | No | 1 | 4.51 |
| 2 | 0 | -3.05 | 4.17 | -4.17 | Low | Yes | No | 0.55 | No | No | No | 0 | 2.85 |
| 3 | 20.23 | -3.26 | 3.34 | -5.29 | High | Yes | No | 0.55 | No | No | No | 0 | 4.29 |
| 4 | 0 | -4.03 | 4.74 | -4.18 | Low | No | No | 0.55 | No | Yes | No | 1 | 4.55 |
| 5 | 0 | -3.5 | 4.57 | -3.89 | Low | Yes | No | 0.55 | No | Yes | No | 0 | 3.46 |
| 6 | 0 | -3.51 | 4.48 | -3.95 | Low | Yes | No | 0.55 | No | Yes | No | 1 | 4.44 |
| 7 | 0 | -3.31 | 4.16 | -4.18 | Low | Yes | No | 0.55 | No | Yes | No | 1 | 3.73 |
| 8 | 20.23 | -2.78 | 3.26 | -4.93 | High | Yes | No | 0.55 | No | No | No | 0 | 3.28 |
TPSA: Topological polar surface area P-gp: p-glycoprotein BA: Bioavailability F20 BBB: Blood Brain Barrier SA: Synthetic accesscibility.
Conclusion
In summary, our research aims to understand the CC of P. lentiscus Eos and its impact on various biological activities. The results show notable differences between the three periods of the year, with a particularly high level of biological activity observed in August Eos. In summer, P. lentiscus Eos demonstrated significant antibacterial, antioxidant, and anti-inflammatory activity. This increased efficacy can be explained by the oil’s specific CC during this period, characterized by a high concentration of bioactive compounds. These results highlight the promise of P. lentiscus Eos as an effective antibacterial, antioxidant, and anti-inflammatory agent. They underline the importance of seasonal variation in assessing its biological properties. The knowledge gained on this seasonal variation could also guide harvesting practices to maximize the efficacy of P. lentiscus Eos.
Molecular docking simulations serve as a crucial tool in understanding ligand-protein interactions, offering predictive insights into the biological activity of potential therapeutic compounds. Among the studied ligands, ligand-01 (β-Caryophyllene) and ligand-04 (Germacrene D) emerged as promising candidates, demonstrating notable antioxidant and anti-inflammatory activity and anti-bacterial activity. The computational results align with experimental evidence, lending scientific validation to the traditional medicinal uses of the plants from which these compounds were derived. This research not only bridges the gap between traditional medicine and modern drug discovery but also opens avenues for utilizing plant-based compounds to develop safer and more effective treatments for oxidative and inflammatory disorders as well as to combat infections.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to extend their sincere appreciation to the Ongoing Research Funding Program (ORF-2025-437), King Saud University, Riyadh, Saudi Arabia
Author contributions
Conceptualization, original draft writing, reviewing, and editing: Amina Labhar, Yahya El-Mernissi, Karima Ait Alla, Sanae Jahjah, Omar Benamari, Aziz Zouhri. Formal analysis, investigations, funding acquisition, reviewing, and editing: Farah Labhar, Farhan Siddique, Chaima Alaoui Jamali, Elyoussfi Abdellah, Amin Salhi. Resources, data validation, data curation, and supervision: Mhamed Ahari, Hassan Amhamdi, Amira Metouekel, Ahmad Mohammad Salamatullah, Atrsaw Asrat Mengistie.
Funding
This work is financially supported by the Ongoing Research Funding Program (ORF-2025-437), King Saud University, Riyadh, Saudi Arabia
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
IUCN policy statement
The collection of plant material complies with relevant institutional, national, and international guidelines and legislation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amina Labhar, Email: amina.labhar@etu.uae.ac.ma.
Ahmad Mohammad Salamatullah, Email: asalamh@ksu.edu.sa.
Atrsaw Asrat Mengistie, Email: Atrsawresearcher@gmail.com.
References
- 1.Hong, L. et al. Ethnobotanical study on medicinal plants used by Maonan people in China. J. Ethnobiol. Ethnomed.10.1186/s13002-015-0019-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salmerón-Manzano, E., Garrido-Cardenas, J. A. & Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Pub. Health10.3390/ijerph17103376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor, J.L.S., Rabe, T., Mcgaw, L.J., Jäger, A.K. & Van Staden, J. Towards the scientific validation of traditional medicinal plants (2001).
- 4.Abdelkader, H., Nadia, K. & Salima, B. Chemical Composition and Antioxidant Potential of <i>Pistacia lentiscus</i> L. Essential Oil from Oran (Algeria). Adv. Biosci. Biotechnol.07(12), 539–544. 10.4236/abb.2016.712048 (2016). [Google Scholar]
- 5.Jahjah, S. et al. Ethnobotanical investigation of medicinal and aromatic plants used in the traditional treatment of COVID-19 in the rural commune of Bni Hadifa, province of Al Hoceima (Central Rif, Northern Morocco). Multidiscip. Sci. J.6(8), 2024149. 10.31893/multiscience.2024149 (2024). [Google Scholar]
- 6.Marrelli, M. Medicinal Plants. Plants10(7), 1355. 10.3390/plants10071355 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouyahya, A. et al. Résistance aux antibiotiques et mécanismes d’action des huiles essentielles contre les bactéries. Phytothérapie10.1007/s10298-017-1118-z (2017). [Google Scholar]
- 8.Alaoui Jamali, C. et al. Synergistic effects of three Moroccan thyme essential oils with antibiotic cefixime. Phytothérapie10.1007/s10298-017-1107-2 (2017). [Google Scholar]
- 9.Djouahri, A. et al. In vitro synergistic/antagonistic antibacterial and anti-inflammatory effect of various extracts/essential oil from cones of Tetraclinis articulata (Vahl) Masters with antibiotic and anti-inflammatory agents. Ind. Crops Prod.56, 60–66. 10.1016/j.indcrop.2014.02.035 (2014). [Google Scholar]
- 10.Labhar, A. et al. An ethnobotanical investigation of medicinal and aromatic plants used in Al Hoceima National Park. BIO Web Conf. 10.1051/bioconf/202410901001 (2024).
- 11.Bachrouch, O. et al. Variations in composition and antioxidant activity of Tunisian Pistacia lentiscus L. leaf essential oil. Plant Biosyst.149(1), 38–47. 10.1080/11263504.2013.809027 (2015). [Google Scholar]
- 12.Sehaki, C., Jullian, N., Ayati, F., Fernane, F. & Gontier, E. A review of pistacia lentiscus polyphenols: Chemical diversity and pharmacological activities. Plants10.3390/plants12020279 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragović, S. et al. The mastic tree (Pistacia lentiscus L.) leaves as source of BACs: Effect of growing location, phenological stage and extraction solvent on phenolic content. Food Technol. Biotechnol.58(3), 303–313. 10.17113/ftb.58.03.20.6662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadini, A., Azdimousa, A., Khoulati, A., El Bekkaye, K. & Saalaoui, E. Valorization of moroccan pistacia lentiscus L. Leaves: Phytochemical and in vitro antioxidant activity evaluation compared to different altitudes. Sci. World J.10.1155/2022/6367663 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yosr, Z., Imen, B. H. Y., Rym, J., Chokri, M. & Mohamed, B. Sex-related differences in essential oil composition, phenol contents and antioxidant activity of aerial parts in Pistacia lentiscus L. during seasons. Ind. Crops Prod.121, 151–159. 10.1016/j.indcrop.2018.04.067 (2018). [Google Scholar]
- 16.Zrira, S., Elamrani, A. & Benjilali, B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco - A seasonal variation. Flavour Fragr. J.18(6), 475–480. 10.1002/ffj.1221 (2003). [Google Scholar]
- 17.Aouinti, F. et al. New study of the essential oil, mineral composition and antibacterial activity of Pistacia lentiscus L. from Eastern Morocco. Res. Chem. Intermed.40(8), 2873–2886. 10.1007/s11164-013-1134-z (2014). [Google Scholar]
- 18.Loukili, E. L. H. et al. Chemical Composition and Physicochemical Analysis of Opuntia dillenii Extracts Grown in Morocco. J. Chem.2021, 1–11. 10.1155/2021/8858929 (2021). [Google Scholar]
- 19.Alam, Md. N., Bristi, N. J. & Rafiquzzaman, Md. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J.21(2), 143–152. 10.1016/j.jsps.2012.05.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labhar, A. et al. Phytochemical, antioxidant, and anti-inflammatory properties of Tetraclinis articulata (Vahl) masters extracts from Al Hoceima province. E3S Web Conf. 527, 01016. 10.1051/e3sconf/202452701016 (2024).
- 21.Wayne, P. National committee for clinical laboratory standards. In Performance standards for antimicrobial disk susceptibility test 6th edn. Approved standard M2-A6. (1997).
- 22.Wayne, P. National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically 4th edn. Approved Standard M7-A4. (1997).
- 23.Jamali, C. A. et al. Phenological changes to the chemical composition and biological activity of the essential oil from Moroccan endemic thyme (Thymus maroccanus Ball). Ind. Crops Prod.49, 366–372. 10.1016/j.indcrop.2013.05.016 (2013). [Google Scholar]
- 24.Lekouaghet, A. et al. In vitro evaluation of antioxidant and anti-inflammatory activities of the hydroalcoholic extract and its fractions from Leuzea conifera L. roots. S. Afr. J. Bot.132, 103–107. 10.1016/j.sajb.2020.03.042 (2020). [Google Scholar]
- 25.Labhar, A. et al. Phytochemical, Anti-inflammatory and Antioxidant Activities of Pistacia Lentiscus L. Leaves from Ajdir, Al Hoceima Province, Morocco. Ecol. Eng. Environ. Technol.24(7), 172–177. 10.12912/27197050/169935 (2023). [Google Scholar]
- 26.Fischer, S. et al. Dibenzosuberones as p38 mitogen-activated protein kinase inhibitors with low ATP competitiveness and outstanding whole blood activity. J. Med. Chem.56(1), 241–253. 10.1021/JM301539X/SUPPL_FILE/JM301539X_SI_001.PDF (2013). [DOI] [PubMed] [Google Scholar]
- 27.Williams, P. A. et al. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature424(6947), 464–468. 10.1038/nature01862 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Ushiyama, F. et al. Lead Identification of 8-(Methylamino)-2-oxo-1,2-dihydroquinoline Derivatives as DNA Gyrase Inhibitors: Hit-to-Lead Generation Involving Thermodynamic Evaluation. ACS Omega5(17), 10145–10159. 10.1021/ACSOMEGA.0C00865/SUPPL_FILE/AO0C00865_SI_003.XLSX (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Z. et al. <p>p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects</p>. J. Inflamm. Res.15, 723–734. 10.2147/JIR.S348491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen, N. T. et al. Autodock Vina Adopts More Accurate Binding Poses but Autodock4 Forms Better Binding Affinity. J. Chem. Inf. Model60(1), 204–211. 10.1021/ACS.JCIM.9B00778/SUPPL_FILE/CI9B00778_SI_001.PDF (2020). [DOI] [PubMed] [Google Scholar]
- 31.Pham, T. N. H. et al. Improving ligand-ranking of AutoDock Vina by changing the empirical parameters. J. Comput. Chem.43(3), 160–169. 10.1002/JCC.26779 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Jejurikar, B. L. & Rohane, S. H. Drug Designing in Discovery Studio. Asian J. Res. Chem.14(2), 135–138. 10.5958/0974-4150.2021.00025.0 (2021). [Google Scholar]
- 33.Asbabou, A. et al. Phytochemical profile, physicochemical, antioxidant and antimicrobial properties of Juniperus phoenicea and Tetraclinis articulate: in vitro and in silico approaches. Front. Chem.12, 1397961. 10.3389/FCHEM.2024.1397961/BIBTEX (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daina, A., Michielin, O. & Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep.7(1), 1–13. 10.1038/srep42717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labhar, A. et al. Seasonal Variations in the Essential Oil Composition and Biological Activities of Wild Lavandula dentata L.. Nat. Prod. Commun.10.1177/1934578X241230822 (2024). [Google Scholar]
- 36.Farah, A.,Lebrazi, S., Fadil, M.,Haloui, T. & Alaoui, A.B. Effect of harvesting period and drying time on the essential oil yield of Pistacia lentiscus L. leaves. [Online]. Available: http://derpharmachemica.com/archive.html (2015).
- 37.Dlih Boudiaf, N.,Ferchichi, L. & Mizi, A. Chemical composition of the essential and fixed oil of pistacia lentiscus L. Grown in collo east of Algeria. (2021).
- 38.Drioiche, A. et al. Analysis of the Chemical Composition and Evaluation of the Antioxidant, Antimicrobial, Anticoagulant, and Antidiabetic Properties of Pistacia lentiscus from Boulemane as a Natural Nutraceutical Preservative. Biomedicines10.3390/biomedicines11092372 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueiredo, A. C., Barroso, J. G., Pedro, L. G. & Scheffer, J. J. C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J.23(4), 213–226. 10.1002/ffj.1875 (2008). [Google Scholar]
- 40.Tiiva, P., Tang, J., Michelsen, A. & Rinnan, R. Monoterpene emissions in response to long-term night-time warming, elevated CO2 and extended summer drought in a temperate heath ecosystem. Sci. Total Environ.580, 1056–1067. 10.1016/j.scitotenv.2016.12.060 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Amhamdi, H., Aouinti, F., Wathelet, J.-P. & Elbachiri, A. Chemical composition of the essential oil of Pistacia lentiscus L. from Eastern Morocco. Rec. Nat. Prod.3(2), 90–95 (2009). [Google Scholar]
- 42.Mecherara-Idjeri, S., Hassani, A., Castola, V. & Casanova, J. Composition and chemical variability of the essential oil from pistacia lentiscus L. Growing wild in algeria part I: Leaf oil. J. Essent. Oil Res.20(1), 32–38. 10.1080/10412905.2008.9699415 (2008). [Google Scholar]
- 43.Lo Presti, M. et al. Evaluation of the volatile and chiral composition in Pistacia lentiscus L. essential oil. Flavour Fragr. J.23(4), 249–257. 10.1002/ffj.1878 (2008). [Google Scholar]
- 44.Barra, A., Coroneo, V., Dessi, S., Cabras, P. & Angioni, A. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus L. from different origins and its antifungal and antioxidant activity. J. Agric. Food Chem.55(17), 7093–7098. 10.1021/jf071129w (2007). [DOI] [PubMed] [Google Scholar]
- 45.Bachrouch, O. et al. Composition and insecticidal activity of essential oil from Pistacia lentiscus L. against Ectomyelois ceratoniae Zeller and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Stored Prod. Res.46(4), 242–247. 10.1016/j.jspr.2010.07.001 (2010). [Google Scholar]
- 46.Meriem, M. et al. Antimicrobial Activity of the Essential Oil Isolated from Pistacia lentiscus Leaves Against Helicobacter pylori Algerian Clinical Isolates. J. Essent. Oil-Bear. Plants19(2), 466–474. 10.1080/0972060X.2015.1119659 (2016). [Google Scholar]
- 47.Gardeli, C., Vassiliki, P., Athanasios, M., Kibouris, T. & Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem.107(3), 1120–1130. 10.1016/j.foodchem.2007.09.036 (2008). [Google Scholar]
- 48.Maghsoudi, H. & Yazadanpanah, E. Evaluation of the Anti-Inflammatory Properties of D-limonene Compared with Dexamethasone and Ibuprofen in Bovine Synoviocyte. J Bas Res Med Sci. 10(1), 1–14 (2023).
- 49.Santos, E. S. et al. Potential anti-inflammatory, hypoglycemic, and hypolipidemic activities of alpha-pinene in diabetic rats. Process Biochem.126, 80–86. 10.1016/j.procbio.2022.12.023 (2023). [Google Scholar]
- 50.de Morais Oliveira-Tintino, C. D. et al. Anti-inflammatory and anti-edematogenic action of the Croton campestris A. St.-Hil (Euphorbiaceae) essential oil and the compound β-caryophyllene in in vivo models. Phytomedicine41, 82–95. 10.1016/j.phymed.2018.02.004 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Su, C. W., Tighe, S., Sheha, H., Cheng, A. M. S. & Tseng, S. C. G. Safety and efficacy of 4-terpineol against microorganisms associated with blepharitis and common ocular diseases. BMJ Open Ophthalmol.10.1136/bmjophth-2017-000094 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Özbek, H. & Yılmaz, B. S. Anti-inflammatory and hypoglycemic activities of alpha-pinene. Acta Pharm. Sci.55(4), 7–14. 10.23893/1307-2080.APS.05522 (2017). [Google Scholar]
- 53.Gourine, N. et al. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Ind. Crops Prod.31(2), 203–208. 10.1016/j.indcrop.2009.10.003 (2010). [Google Scholar]
- 54.Chafik, T. et al. Toxicity, repellency and chemical composition of essential oils from aerial parts of Pistacia lentiscus (L) against Tribolium casatneum (Coleoptera: Tenebrionidae). J. Essent. Oil Plant Compos.1(3), 176–184. 10.58985/jeopc.2023.v01i03.22 (2023). [Google Scholar]
- 55.Lin, H. et al. D-Limonene: Promising and sustainable natural bioactive compound. Multidisciplinary Digital Publishing Institute (MDPI). 10.3390/app14114605 (2024).
- 56.Piccialli, I. et al. The antioxidant activity of limonene counteracts neurotoxicity triggered byaβ1–42 oligomers in primary cortical neurons. Antioxidants10.3390/antiox10060937 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Omari, N. et al. Expediting Multiple Biological Properties of Limonene and α-Pinene: Main Bioactive Compounds of Pistacia lentiscus L., Essential Oils. Front. Biosci. - Landmark10.1083/j.fbl2809229 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Derwich, E., Manar, A., Benziane, Z. & Boukir, A. GC/MS Analysis and In vitro Antibacterial Activity of the Essential Oil Isolated from Leaf of Pistacia lentiscus Growing in Morocoo. World Appl. Sci. J.8(10), 1267–1276 (2010). [Google Scholar]
- 59.Bouchfara, A. et al. Antibacterial and antioxidant activities of Pistacia lentiscus essential oils: Impact of total phenolic content on antioxidant efficacy. Biocatal. Agric. Biotechnol.10.1016/j.bcab.2025.103532 (2025). [Google Scholar]
- 60.Salehi, B. et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules9(11), 738. 10.3390/biom9110738 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta, A., Jeyakumar, E. & Lawrence, R. Journey of limonene as an antimicrobial agent. J. Pure Appl. Microbiol.15(3), 1094–1110. 10.22207/JPAM.15.3.01 (2021). [Google Scholar]
- 62.da Silva, A. C. R. et al. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules17(6), 6305–6316. 10.3390/molecules17066305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.