Abstract
Understanding the hydration behavior of amino acids is fundamental to gaining insights into protein solvation mechanisms. Within this framework, examining the solvation properties of amino acids in aqueous media containing protic ionic liquids (PILs) a novel class of environmentally friendly solvents is crucial for characterizing their hydration dynamics. This research focuses on the influence of ammonium-based PILs, specifically mono-, bis-, and tris-(2-hydroxyethyl) ammonium acetate, on the hydration characteristics of DL-alanine. By employing COSMO computational analysis alongside thermophysical property measurements, the study evaluates the hydration energies and σ-profiles of DL-alanine and the PILs. The thermophysical behavior of DL-alanine in aqueous solutions containing varying concentrations of protic ionic liquids (PILs) was systematically analyzed using experimental data and modeling approaches. The density, speed of sound, viscosity, and refractive index were measured for ternary solutions of DL-alanine, water, and PILs across a temperature range of (298.15 to 318.15) K under atmospheric pressure. The standard partial molar volume ( ) measured over the studied temperature range indicates that the solute-solvent interactions between [2-HEA]Ac and DL-alanine in the presence of water is 63.119 cm3·mol-1. Additionally, the hydration layer surrounding DL-alanine is notably influenced by temperature, as higher temperatures lead to the release of more water molecules relative to PIL-containing solutions. This temperature-dependent effect is especially pronounced in the presence of (2-hydroxyethyl) ammonium acetate, underscoring its significant impact on the hydration behavior of DL-alanine.
) measured over the studied temperature range indicates that the solute-solvent interactions between [2-HEA]Ac and DL-alanine in the presence of water is 63.119 cm3·mol-1. Additionally, the hydration layer surrounding DL-alanine is notably influenced by temperature, as higher temperatures lead to the release of more water molecules relative to PIL-containing solutions. This temperature-dependent effect is especially pronounced in the presence of (2-hydroxyethyl) ammonium acetate, underscoring its significant impact on the hydration behavior of DL-alanine.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13065-025-01603-1.
Keywords: DL-alanine, Protic ionic liquids, Volumetric properties, Viscosity, Molar refractionon, COSMO Analysis
Introduction
Water is fundamental to the structure, stability, dynamics, and functionality of biological macromolecules, including proteins and DNA [1]. Water plays an active role in protein folding, molecular recognition, and the assembly of macromolecular structures. The hydrogen bonds involved in these processes, while individually weak, exert significant collective strength, rendering them particularly well-suited for facilitating molecular recognition and structural organization within biological systems [2, 3]. Water functions not merely as a passive solvent but as an integral component of biomolecular systems, influencing their structures while simultaneously being influenced by them [4]. A comprehensive understanding of water’s role is essential for advancing the design and functional understanding of proteins and nucleic acids. The amphoteric nature of amino acids and their hydration characteristics have been extensively analyzed using various experimental techniques [5, 6]. Evidence from NMR spectroscopy and crystallographic studies indicates that amino acids in their cationic form exhibit greater hydration compared to their zwitterion form, with distinct hydrogen bonding patterns identified in crystal structures. Additionally, the hydropathic properties of amino acid side chains in protein environments have been elucidated through investigations of structural changes in water networks, offering valuable insights into local hydrophobicity and hydrophilicity [7, 8].
Protic ionic liquids (PILs) have gained recognition as versatile designer solvents, distinguished by their unique properties derived from extensive hydrogen bonding networks. These networks significantly influence the physicochemical properties of PILs, including density, thermal stability, viscosity, and conductivity [9, 10]. The incorporation of water into PILs further enhances their solvent characteristics, notably by reducing viscosity and increasing conductivity. As research progresses, PILs are anticipated to play a pivotal role in advancing sustainable chemical processes and renewable energy technologies [11]. Protic ionic liquids (PILs) and their interactions with water have attracted considerable interest owing to their distinctive properties and diverse applications. The selection of mono-, bis-, and tris-substituted forms of 2-hydroxyethylammonium acetate allows us to investigate the influence of substitution on solvation properties and hydrogen bonding capabilities. This variation enables a comprehensive analysis of how molecular structure affects the physicochemical behavior of protic ionic liquids. Understanding these differences can provide insights into the design of more effective solvent systems for amino acid interactions [12, 13].
Understanding the molecular interactions between protic ionic liquids (PILs) and biomolecules is crucial for designing effective PIL-based processes. DL-alanine is a non-polar, aliphatic amino acid that plays a crucial role in protein synthesis and serves as a building block for peptides. It exists in two enantiomeric forms, D- and L-alanine, both of which are involved in various metabolic pathways. Due to its small size and simple structure, DL-alanine is often used in studies of solvation dynamics and interactions with solvents. Its unique properties make it a valuable model compound for investigating the effects of different solvent systems, including protic ionic liquids. Although significant progress has been made in exploring protein-PIL interactions, research on peptide-PIL interactions is still in its early stages. Both experimental and computational approaches are being utilized to uncover the underlying mechanisms [14]. Given the complexity of proteins composed of numerous amino acids studying individual amino acids offers a simpler and more focused approach than examining whole proteins. Investigating the thermophysical properties of amino acids in systems containing protic ionic liquids and water provides valuable insights into solute-solvent and solute-solute interactions. In recent years, significant research has focused on the thermodynamic behavior of mixtures of amino acids and first-generation ionic liquids in aqueous media across various temperature ranges [15–17]. The susceptibility of amino acids to denaturation has driven studies on their thermodynamic properties in water, particularly in the presence of protic ionic liquids, highlighting the protective effects these unique solvents can exert against amino acid denaturation [18, 19]. The study of interactions between DL-alanine in polar solvents such as water and protic ionic liquids is crucial for understanding solvation phenomena. Water, as a polar solvent, facilitates significant hydrogen bonding interactions with amino compounds like DL-alanine, enhancing solubility and stability. Protic ionic liquids, due to their ionic nature and ability to form hydrogen bonds, further contribute to these interactions. Factors such as temperature and solvent concentration can influence the equilibrium of these interactions, thereby affecting the physical and chemical properties of the system. The chemical structure of DL-alanine, including its various functional groups, also plays a vital role in determining the nature and strength of these interactions. Spectroscopic techniques, such as NMR and FTIR, provide valuable insights into the interactions and structural changes occurring within the system. Understanding these interactions has practical implications in industrial processes and pharmaceuticals, while computational modeling can offer predictive insights into the behavior of these systems. The specific interactions facilitated by PILs, such as enhanced hydrogen bonding and altered solvation dynamics, can significantly influence amino acid stability and reactivity. By elucidating these effects, we can better understand how PILs modify the properties of amino acids compared to traditional solvents. This insight is crucial for advancing the design of solvent systems in biochemical applications [20, 21].
This study explores the interactions between DL-alanine and specific protic ionic liquids (PILs) namely, 2-hydroxyethylammonium acetate, bis (2-hydroxyethylammonium acetate), and tris (2-hydroxyethylammonium acetate) in aqueous solutions. Experimental measurements were carried out to determine the density, speed of sound, viscosity, and refractive index of aqueous DL-alanine solutions with varying concentrations of these PILs. The experiments were conducted over a temperature range of (288.15 K to 318.15) K under atmospheric pressure. From the experimental data, key thermodynamic and physical parameters, including the partial molar volume ( ), partial molar isentropic compressibility (
), partial molar isentropic compressibility ( ), viscosity B-coefficient (B), and molar refraction (RM), were calculated. Additionally, COSMO computational methods were employed to elucidate the bonding interactions and intrinsic properties of the species, analyzed through σ-profiles and related theoretical results.
), viscosity B-coefficient (B), and molar refraction (RM), were calculated. Additionally, COSMO computational methods were employed to elucidate the bonding interactions and intrinsic properties of the species, analyzed through σ-profiles and related theoretical results.
Experimental
Materials
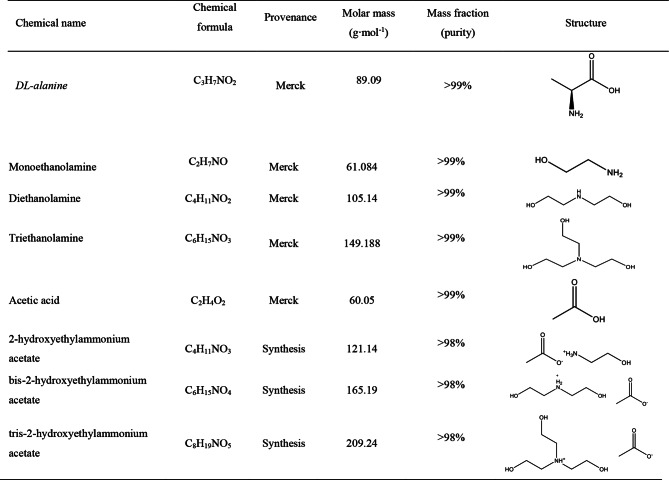
All reagents utilized in this study, including DL-alanine, monoethanolamine, diethanolamine, triethanolamine, and acetic acid, were procured from Merck and used as received, without additional purification. Deionized ultrapure water, with a specific conductance of less than 1 µS·cm− 1, was employed for the preparation of aqueous solutions containing the amino acid and ionic liquids. Detailed information on the reagents and their specifications is provided in Table 1:
Table 1.
Descriptions of the used chemicals
PILs synthesis and purification
In the present study, the synthesis and purification of ionic liquids, specifically 2-hydroxyethylammonium acetate, bis (2-hydroxyethyl) ammonium acetate, and tris (2-hydroxyethyl) ammonium acetate, were conducted. The synthesis process involved the controlled, dropwise equimolar (1:1 molar ) addition of acetic acid to ethanolamine, which was maintained in an ice bath. The reaction mixture was vigorously stirred at room temperature for 24 h using a magnetic stirrer. To ensure high purity, the synthesized ionic liquids were subjected to vacuum drying for 3 h using a D25 vacuum pump (USA). The resulting protic ionic liquids (PILs) were rigorously characterized by proton nuclear magnetic resonance (1H NMR) and Fourier-transform infrared (FT-IR) spectroscopy. Additionally, their water content was precisely determined through Karl-Fischer titration. Detailed characterization results are provided in the supporting information section. As stated thorough the caractrization method (FT-IR, H-NMR, KARL FISHER) the synthesized PILs in this study presented high quality of 98% purity and affirmational synthesized rate gave us 93% yield.
Apparatus and procedure
The solutions were prepared with meticulous precision using a Shimadzu AW-220 analytical balance, ensuring a measurement precision of ± 2 × 10− 4 g. Density and speed of sound were measured with a digital vibrating U-shaped densitometer (Anton Paar DSA5000), providing resolutions of 1 × 10− 6 g·cm− 3 for density and 0.01 m·s− 1 for speed of sound. The associated measurement uncertainties were 4 × 10− 5 g·cm− 3 for density and ± 0.7 m·s− 1 for speed of sound. The reported uncertainty has been calculated thorough the instructions of the national institute of technology (NIST). The apparatus features an integrated thermostat based on Peltier technology, ensuring precise temperature control within ± 0.05 K. The instrument was calibrated using air and distilled water, and speed of sound measurements were conducted at a frequency of 3 MHz.
Viscosity was measured with a digital microviscometer, calibrated at 298.15 K using doubly distilled water. The apparatus features an integrated thermostat based on Peltier technology, ensuring precise temperature control within ± 0.05 K. The refractive index was determined using a digital refractometer (Mettler Toledo) with an accuracy of ± 0.0002 units; calibration was performed twice with doubly distilled water.
For computational analysis, density functional theory (DFT) calculations were performed using the Dmol³ module. The geometries of the protic ionic liquids (PILs) were optimized using the generalized gradient approximation Vosko-Wilk-Nusair-Becke-Perdew (GGA VWN-BP) functional, which combines the BP functional with the VWN framework for local correlation. The COSMO method was employed in a two-step process involving initial geometry optimization followed by energy optimization.
Results and discussion
Molecular fingerprint: σ-Profile
The sigma profile (σ-profile) is a critical concept in COSMO (Conductor-like Screening Model) calculations, widely used to characterize molecular interactions in various chemical environments [22, 23]. In COSMO calculations, the sigma profile represents the distribution of surface charge densities on the molecular surface of a solute. This distribution is derived from quantum chemical simulations, where the molecule is placed in a virtual conductor medium. The conductor environment ensures that the molecular surface is fully polarized, facilitating accurate predictions of interaction energies in non-conducting solvents [24]. The sigma profile is constructed by dividing the molecular surface into segments and calculating the local charge on each segment. The results are then expressed as a histogram, where the sigma-values are plotted against their respective surface area fractions. This histogram provides a quantitative representation of the molecule’s polarity and its capacity to engage in hydrogen bonding, van der Waals interactions, and electrostatic forces.
The GGA VWN-BP functional, implemented within the Dmol³ framework as recommended by its developers, has demonstrated effectiveness in modeling realistic solvent systems [25]. In this study, COSMO calculations were conducted using density functional theory (DFT) within the Dmol³ module of Materials Studio (Biovia, Materials Studio 2023). Molecular geometry optimization was performed using the GGA VWN-BP functional to ensure accurate structural representations. The σ-profiles for the investigated solvents and protic ionic liquids (PILs), are presented in Fig. 1:
Fig. 1.

Sigma profiles of DL-alanine, water, [2-HEA] Ac, [BHEA] Ac, and [THEA] Ac
The calculated cavity volume, cavity surface area, and HOMO and LUMO energy levels of the compounds are summarized in Table 2:
Table 2.
The cavity volume and surface beside hydration energy, and highest occupied molecular orbital numbers and energy, lowest unoccupied molecular orbital of the studied compounds
| Compound | Cavity volume | Cavity surface | Dielectric (hydration) energy | n HOMO | HOMO | n LUMO | LUMO |
|---|---|---|---|---|---|---|---|
| Å3 | Å2 | kcal/mol | eV | eV | |||
| DL -alanine | 105.1877 | 123.0834 | -12.76 | 24 | -5.725eV | 25 | -1.130 eV |
| [2-HEA]Ac | 155.2485 | 188.5375 | -114.85 | 33 | -4.457 eV | 34 | 0.498 eV |
| [BHEA]Ac | 207.5569 | 241.6801 | -111.79 | 45 | -4.439 eV | 46 | 0.226 eV |
| [THEA]Ac | 251.1792 | 271.4756 | -103.36 | 57 | -4.444 eV | 58 | -0.047 eV |
The Generalized Gradient Approximation (GGA) VWN-BP functional, which integrates the Vosko-Wilk-Nusair (VWN) correlation with the Becke-Perdew (BP) exchange, exhibits several limitations that may affect its applicability in computational studies. Notably, it struggles with systems characterized by strong electron correlation, such as transition metal oxides, often resulting in underestimations of binding energies and failing to capture essential physical phenomena. Additionally, the functional inadequately addresses long-range dispersion interactions, which are critical in systems with weak van der Waals forces, leading to inaccuracies in molecular geometries and interaction energies. Furthermore, the presence of self-interaction errors can compromise predictions for systems with unoccupied states, while the treatment of exchange-correlation effects remains limited, particularly in non-uniform electron density distributions. Within the Dmol³ framework, the functional is employed to optimize molecular geometries, calculate electronic properties, and perform DFT-based analyses. Its reliability and precision make it particularly effective for studying solvation behaviors in realistic solvent environments [26].
In this study, water was used as the solvent for all calculations, providing critical insights into the solvation and interaction properties of the systems under investigation. DL-alanine exhibited the smallest cavity volume and surface area among the analyzed molecules, consistent with its compact molecular structure. This compactness was also reflected in its relatively less negative dielectric (hydration) energy, indicating weaker interactions with the solvent compared to the other studied compounds.
In contrast, the mono-, bis-, and tris-(2-hydroxyethyl) ammonium acetate compounds displayed progressively larger cavity volumes and surface areas, reflecting their greater spatial demands and more complex interactions with the solvent. These compounds also exhibited increasingly negative dielectric (hydration) energies, suggesting stronger and more favorable interactions with the solvent environment. Furthermore, all the molecules demonstrated negative HOMO energy levels, while significant variations in their LUMO energy levels were observed. These differences highlight variations in their electronic properties and potential reactivity.
Volumetric properties
The densities of ternary aqueous solutions containing DL-alanine and mono-, bis-, and tris-(2-hydroxyethyl)ammonium acetate were measured at atmospheric pressure over a temperature range of (288.15 to 318.15) K, as shown in Table 3:
Table 3.
The density of ternary solutions containing DL-alanine in aqueous PILs and apparent molar volume of DL-alanine in various concentration of PILs and temperature range of (288.15 to 318.15) K under atmospheric pressure.
| m | d / kg·m− 3 | 106 Vφ / m3·mol− 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| 288.15 K | 298.15 K | 308.15 K | 318.15 K | 288.15 K | 298.15 K | 308.15 K | 318.15 K | |
| [2-HEA]Ac 0.0501 (mol·kg-1) | ||||||||
| 0.0000 | 1000.871 | 998.185 | 995.618 | 993.289 | ||||
| 0.0495 | 1002.276 | 999.556 | 996.970 | 994.627 | 60.596 | 61.373 | 61.847 | 62.215 |
| 0.0999 | 1003.712 | 1000.947 | 998.343 | 995.990 | 60.451 | 61.333 | 61.794 | 62.118 |
| 0.1493 | 1005.120 | 1002.313 | 999.694 | 997.327 | 60.358 | 61.260 | 61.699 | 62.038 |
| 0.1994 | 1006.551 | 1003.689 | 1001.052 | 998.678 | 60.240 | 61.217 | 61.659 | 61.969 |
| 0.2492 | 1007.976 | 1005.065 | 1002.401 | 1000.017 | 60.122 | 61.120 | 61.602 | 61.907 |
| 0.2993 | 1009.397 | 1006.446 | 1003.736 | 1001.374 | 60.065 | 61.047 | 61.618 | 61.811 |
| [2-HEA]Ac 0.1001 (mol·kg-1) | ||||||||
| 0.0000 | 1002.144 | 999.434 | 996.854 | 994.539 | ||||
| 0.0499 | 1003.576 | 1000.826 | 998.227 | 995.886 | 60.261 | 61.149 | 61.619 | 62.225 |
| 0.0996 | 1004.986 | 1002.199 | 999.592 | 997.232 | 60.323 | 61.185 | 61.545 | 62.081 |
| 0.1497 | 1006.408 | 1003.581 | 1000.964 | 998.579 | 60.286 | 61.158 | 61.494 | 62.047 |
| 0.1995 | 1007.806 | 1004.943 | 1002.326 | 999.925 | 60.294 | 61.153 | 61.427 | 61.944 |
| 0.2494 | 1009.179 | 1006.296 | 1003.649 | 1001.274 | 60.390 | 61.177 | 61.536 | 61.860 |
| 0.3005 | 1010.587 | 1007.683 | 1005.050 | 1002.653 | 60.412 | 61.152 | 61.418 | 61.775 |
| [2-HEA]Ac 0.1501 (mol·kg-1) | ||||||||
| 0.0000 | 1003.427 | 1000.690 | 998.098 | 995.779 | ||||
| 0.0496 | 1004.799 | 1002.038 | 999.420 | 997.071 | 61.233 | 61.809 | 62.425 | 63.119 |
| 0.1001 | 1006.202 | 1003.416 | 1000.769 | 998.401 | 61.063 | 61.645 | 62.288 | 62.866 |
| 0.1499 | 1007.602 | 1004.783 | 1002.113 | 999.752 | 60.865 | 61.505 | 62.118 | 62.484 |
| 0.1999 | 1009.010 | 1006.173 | 1003.481 | 1001.093 | 60.706 | 61.299 | 61.892 | 62.323 |
| 0.2493 | 1010.382 | 1007.536 | 1004.834 | 1002.443 | 60.660 | 61.189 | 61.723 | 62.097 |
| 0.2988 | 1011.791 | 1008.924 | 1006.174 | 1003.801 | 60.480 | 61.007 | 61.629 | 61.893 |
| [BHEA]Ac 0.0498 (mol·kg-1) | ||||||||
| 0.0000 | 1001.252 | 998.555 | 995.977 | 993.657 | ||||
| 0.0500 | 1002.686 | 999.953 | 997.359 | 995.034 | 60.115 | 60.927 | 61.337 | 61.516 |
| 0.1004 | 1004.131 | 1001.357 | 998.751 | 996.419 | 60.121 | 60.979 | 61.347 | 61.546 |
| 0.1494 | 1005.561 | 1002.748 | 1000.132 | 997.792 | 60.117 | 60.980 | 61.321 | 61.534 |
| 0.1998 | 1006.943 | 1004.092 | 1001.468 | 999.124 | 60.165 | 61.029 | 61.349 | 61.549 |
| 0.2489 | 1008.372 | 1005.469 | 1002.841 | 1000.476 | 60.133 | 61.051 | 61.340 | 61.602 |
| 0.3000 | 1009.754 | 1006.817 | 1004.165 | 1001.798 | 60.150 | 61.047 | 61.384 | 61.623 |
| [BHEA]Ac 0.0999 (mol·kg-1) | ||||||||
| 0.0000 | 1002.993 | 1000.256 | 997.647 | 995.313 | ||||
| 0.0504 | 1004.445 | 1001.681 | 999.043 | 996.690 | 60.102 | 60.724 | 61.387 | 61.847 |
| 0.0999 | 1005.857 | 1003.071 | 1000.402 | 998.037 | 60.144 | 60.723 | 61.413 | 61.806 |
| 0.1495 | 1007.264 | 1004.454 | 1001.761 | 999.383 | 60.177 | 60.754 | 61.406 | 61.783 |
| 0.1993 | 1008.665 | 1005.823 | 1003.117 | 1000.729 | 60.194 | 60.810 | 61.388 | 61.742 |
| 0.2496 | 1010.075 | 1007.205 | 1004.479 | 1002.082 | 60.196 | 60.819 | 61.380 | 61.716 |
| 0.2994 | 1011.449 | 1008.545 | 1005.812 | 1003.418 | 60.243 | 60.893 | 61.399 | 61.682 |
| [BHEA]Ac 0.1498 (mol·kg-1) | ||||||||
| 0.0000 | 1004.617 | 1001.840 | 999.210 | 996.863 | ||||
| 0.0499 | 1006.042 | 1003.228 | 1000.589 | 998.225 | 60.316 | 61.144 | 61.413 | 61.835 |
| 0.1007 | 1007.476 | 1004.631 | 1001.982 | 999.597 | 60.375 | 61.139 | 61.417 | 61.877 |
| 0.1501 | 1008.869 | 1005.985 | 1003.329 | 1000.929 | 60.369 | 61.172 | 61.434 | 61.871 |
| 0.2004 | 1010.265 | 1007.352 | 1004.691 | 1002.279 | 60.415 | 61.185 | 61.429 | 61.837 |
| 0.2497 | 1011.647 | 1008.691 | 1006.012 | 1003.601 | 60.364 | 61.174 | 61.460 | 61.801 |
| 0.2995 | 1013.015 | 1010.032 | 1007.325 | 1004.912 | 60.393 | 61.175 | 61.523 | 61.827 |
| [THEA]Ac 0.0501 (mol·kg-1) | ||||||||
| 0.0000 | 1002.423 | 999.709 | 997.119 | 994.789 | ||||
| 0.0497 | 1003.861 | 1001.123 | 998.489 | 996.119 | 60.008 | 60.576 | 61.551 | 62.444 |
| 0.0979 | 1005.239 | 1002.465 | 999.801 | 997.385 | 60.072 | 60.772 | 61.620 | 62.590 |
| 0.1498 | 1006.701 | 1003.890 | 1001.199 | 998.737 | 60.209 | 60.945 | 61.712 | 62.687 |
| 0.1994 | 1008.060 | 1005.226 | 1002.522 | 999.998 | 60.412 | 61.105 | 61.770 | 62.840 |
| 0.2501 | 1009.435 | 1006.562 | 1003.838 | 1001.285 | 60.553 | 61.282 | 61.913 | 62.903 |
| 0.3003 | 1010.822 | 1007.891 | 1005.094 | 1002.528 | 60.532 | 61.351 | 62.139 | 63.024 |
| [THEA]Ac 0.0998 (mol·kg-1) | ||||||||
| 0.0000 | 1005.271 | 1002.492 | 999.862 | 997.507 | ||||
| 0.0499 | 1006.682 | 1003.878 | 1001.224 | 998.844 | 60.578 | 61.167 | 61.736 | 62.321 |
| 0.0996 | 1008.082 | 1005.250 | 1002.576 | 1000.177 | 60.543 | 61.163 | 61.695 | 62.221 |
| 0.1501 | 1009.486 | 1006.632 | 1003.940 | 1001.536 | 60.568 | 61.158 | 61.662 | 62.073 |
| 0.1993 | 1010.845 | 1007.983 | 1005.276 | 1002.861 | 60.620 | 61.128 | 61.605 | 61.990 |
| 0.2492 | 1012.213 | 1009.332 | 1006.599 | 1004.189 | 60.641 | 61.142 | 61.647 | 61.952 |
| 0.2994 | 1013.559 | 1010.687 | 1007.972 | 1005.554 | 60.734 | 61.137 | 61.512 | 61.806 |
| [THEA]Ac 0.1502 (mol·kg-1) | ||||||||
| 0.0000 | 1008.077 | 1005.240 | 1002.572 | 1000.192 | ||||
| 0.0499 | 1009.452 | 1006.591 | 1003.909 | 1001.526 | 61.207 | 61.779 | 62.151 | 62.296 |
| 0.0999 | 1010.831 | 1007.949 | 1005.249 | 1002.864 | 61.077 | 61.620 | 62.032 | 62.166 |
| 0.1499 | 1012.233 | 1009.315 | 1006.593 | 1004.201 | 60.854 | 61.485 | 61.937 | 62.101 |
| 0.1948 | 1013.474 | 1010.546 | 1007.801 | 1005.399 | 60.789 | 61.349 | 61.835 | 62.032 |
| 0.2498 | 1015.006 | 1012.072 | 1009.310 | 1006.887 | 60.671 | 61.152 | 61.619 | 61.875 |
| 0.2997 | 1016.448 | 1013.450 | 1010.661 | 1008.224 | 60.393 | 61.022 | 61.517 | 61.790 |
The standard uncertainties for molality, temperature and pressure were u (m) = 0.001 mol·kg− 1, u (T) = 0.2 K, u (P) = 10.5 hPa, respectively with level of confidence 0.68. The standard combined uncertainty for density and apparent molar volume were about, u (d) = 0.06 × 10− 4 kg·m− 3 and u (Vφ) = 5 × 10− 6 m3·mol− 1 (level of confidence 0.68), respectively
The apparent molar volumes of the studied solutions were calculated using the Eq. (1) provided in reference [27], with the resulting values presented in Table 3:
 |
1 |
where, Molar mass of amino acids is shown by M (kg·mol-1), and density of solution and density of solvents (water + PILs) are represented by d and d0 (kg·m-3), respectively. Also m is the molality of the experimental solution (mol·kg-1). The thermodynamic properties of the investigated solutions were analyzed to provide a comprehensive understanding of their behavior. An example depicting the variation of ( ) with the molality of DL-alanine in the coexistence of the PILs, their concentrations, and temperature is presented in Fig. 2:
) with the molality of DL-alanine in the coexistence of the PILs, their concentrations, and temperature is presented in Fig. 2:
Fig. 2.
Variation of apparent molar volumes(  )with, a) temperature, b) concentration of PILs and c) cation size of the PILs
)with, a) temperature, b) concentration of PILs and c) cation size of the PILs
Masson model has been used to calculate the apparent molar properties, as expressed by the Eq. (2):
 |
2 |
The parameters ( ), (
), ( ) presented in Table 4, correspond to the ternary aqueous solutions of PILs. The term (
) presented in Table 4, correspond to the ternary aqueous solutions of PILs. The term ( )represents the intercept of Eq. (2), referred to as the standard partial molar volume. It is postulated that solute molecules are encircled by water molecules, maintaining relatively large distances between them, thereby facilitating polar interactions with the water molecules. The (
)represents the intercept of Eq. (2), referred to as the standard partial molar volume. It is postulated that solute molecules are encircled by water molecules, maintaining relatively large distances between them, thereby facilitating polar interactions with the water molecules. The ( ) values of the studied solutions increase with rising concentrations of PILs and temperature, indicating that the interactions become more pronounced at higher temperatures and elevated concentrations of PILs.
) values of the studied solutions increase with rising concentrations of PILs and temperature, indicating that the interactions become more pronounced at higher temperatures and elevated concentrations of PILs.
Table 4.
Parameters of the Masson equation and standard deviation of DL-alanine in aqeuous PILs for the apparent molar volume at different temperatures. The infinite Dilution apparent molar expansibility  (cm3·mol− 1·k), isobaric thermal expansion coefficient (K− 1) and helper constant values at different temperatures
(cm3·mol− 1·k), isobaric thermal expansion coefficient (K− 1) and helper constant values at different temperatures
| System | T |

|
SV | E |

|
Hc*1000 | σVφ |
|---|---|---|---|---|---|---|---|
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.0501 (mol·kg-1) | 288.15 | 60.874(0.021) | -2.152(0.111) | 0.067 | 11.019 | 0.021 | |
| 298.15 | 61.303(0.016) | -1.325(0.083) | 0.057 | 9.349 | -9.769*10− 4 | 0.016 | |
| 308.15 | 62.134(0.029) | -1.009(0.154) | 0.048 | 7.651 | 0.029 | ||
| 318.15 | 62.345(0.098) | -1.560(0.052) | 0.038 | 6.058 | 0.009 | ||
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1001 (mol·kg-1) | 288.15 | 60.388(0.032) | -0.869(0.170) | 0.034 | 22.687 | 0.032 | |
| 298.15 | 61.094(0.015) | 0.389(0.079) | 0.045 | 17.316 | -1.371*10− 3 | 0.015 | |
| 308.15 | 61.901(0.050) | -1.579(0.262) | 0.063 | 12.347 | 0.049 | ||
| 318.15 | 62.383(0.020) | -0.517(0.107) | 0.083 | 7.448 | 0.020 | ||
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1501 (mol·kg-1) | 288.15 | 61.668(0.040) | -2.945(0.212) | 0.051 | 8.205 | 0.040 | |
| 298.15 | 61.970(0.018) | -3.202(0.094) | 0.045 | 7.182 | -6.093*10− 4 | 0.018 | |
| 308.15 | 62.645(0.032) | -3.386(0.171) | 0.038 | 6.132 | 0.032 | ||
| 318.15 | 62.825(0.022) | -3.784(0.114) | 0.032 | 5.144 | 0.021 | ||
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0498 (mol·kg-1) | 288.15 | 60.089(0.015) | 0.102(0.078) | 0.159 | 26.428 | 0.015 | |
| 298.15 | 60.807(0.015) | 0.601(0.080) | 0.081 | 13.066 | -7.805*10− 3 | 0.015 | |
| 308.15 | 61.444(0.017) | -0.677(0.088) | 0.027 | 1.244 | 0.017 | ||
| 318.15 | 61.601(0.016) | -0.616(0.085) | 0.015 | -12.231 | 0.016 | ||
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0999 (mol·kg-1) | 288.15 | 60.007(0.032) | 0.448(0.170) | 0.084 | 13.916 | 0.032 | |
| 298.15 | 60.843(0.015) | -0.971(0.079) | 0.069 | 11.34 | -1.451*10− 3 | 0.015 | |
| 308.15 | 61.350(0.049) | 0.285(0.262) | 0.054 | 8.881 | 0.049 | ||
| 318.15 | 61.896(0.020) | -0.109(0.107) | 0.040 | 6.458 | 0.020 | ||
| DL-alanine in aqueous solutions of [BHEA]Ac 0.1498 (mol·kg-1) | 288.15 | 60.121(0.040) | 1.171(0.212) | 0.113 | 18.803 | 0.040 | |
| 298.15 | 61.073(0.018) | 0.358(0.094) | 0.073 | 11.895 | -4.04*10− 3 | 0.018 | |
| 308.15 | 61.562(0.032) | -1.020(0.171) | 0.032 | 5.238 | 0.032 | ||
| 318.15 | 61.706(0.021) | 0.918(0.114) | 0.013 | -1.322 | 0.021 | ||
| DL-alanine in aqueous solutions of [THEA]Ac 0.0501 (mol·kg-1) | 288.15 | 59.755(0.056) | 0.662(0.296) | 0.094 | 15.788 | 0.056 | |
| 298.15 | 60.105(0.035) | 1.935(0.187) | 0.092 | 15.262 | -2.644*10− 4 | 0.035 | |
| 308.15 | 61.910(0.054) | -2.835(0.285) | 0.089 | 14.390 | 0.054 | ||
| 318.15 | 62.187(0.022) | 0.919(0.114) | 0.086 | 13.900 | 0.022 | ||
| DL-alanine in aqueous solutions of [THEA]Ac 0.0998 (mol·kg-1) | 288.15 | 60.876(0.032) | -2.082(0.170) | 0.093 | 14.932 | 0.032 | |
| 298.15 | 61.204(0.015) | -0.169(0.079) | 0.068 | 11.026 | 2.535*10− 3 | 0.015 | |
| 308.15 | 61.716(0.049) | 0.324(0.262) | 0.043 | 6.9770 | 0.049 | ||
| 318.15 | 62.551(0.020) | -0.799(0.107) | 0.017 | 2.8500 | 0.020 | ||
| DL-alanine in aqueous solutions of [THEA]Ac 0.1502 (mol·kg-1) | 288.15 | 61.272(0.040) | 0.521(0.212) | 0.062 | 10.168 | 0.040 | |
| 298.15 | 61.905(0.018) | 0.156(0.094) | 0.044 | 7.049 | -1.866*10− 3 | 0.018 | |
| 308.15 | 62.093(0.032) | 1.142(0.171) | 0.025 | 4.023 | 0.032 | ||
| 318.15 | 62.353(0.022) | 0.205(0.114) | 0.012 | 1.013 | 0.022 |
The standard uncertainties for molality, temperature and pressure were u (m) = 0.001 mol·kg-1, u (T) = 0.2 K, u (P) = 10.5 hPa, respectively with level of confidence 0.68
The ( ) value serves as a criterion for evaluating solute-solute interactions, with positive (
) value serves as a criterion for evaluating solute-solute interactions, with positive ( ) values indicating the presence of interactions between DL-alanine molecules. The temperature dependence of the (
) values indicating the presence of interactions between DL-alanine molecules. The temperature dependence of the ( )has been modeled using a second-degree polynomial Eq. (3) [28]:
)has been modeled using a second-degree polynomial Eq. (3) [28]:
 |
3 |
The apparent molar expansibility in infinite dilution at studied pressure was determined by applying the empirical parameters A, B, and C, as described by the Eq. (4) [29].
 |
4 |
The data presented in Table 3 indicate a declining trend in ( ) values by rising the temperature. This behavior implies that as temperature rises, the water molecules are progressively released from the hydration layer. The volumetric expansion of amino acid is notably more pronounced in the presence of mono (2-hydroxyethyl) ammonium acetate compared to other protic ionic liquids (PILs). To provide a deeper understanding of this phenomenon, the apparent isobaric thermal expansion coefficient was calculated using the Eq. (5) [30].
) values by rising the temperature. This behavior implies that as temperature rises, the water molecules are progressively released from the hydration layer. The volumetric expansion of amino acid is notably more pronounced in the presence of mono (2-hydroxyethyl) ammonium acetate compared to other protic ionic liquids (PILs). To provide a deeper understanding of this phenomenon, the apparent isobaric thermal expansion coefficient was calculated using the Eq. (5) [30].
 |
5 |
The calculated values of (α) for the amino acid are shown in Table 4:
The data reveal that (α) decreases with rising temperature, a trend attributed to the disruption of Hydrogen bonds at elevated temperatures. The structural behavior of the solute (DL-alanine) in studied solutions containing water and protic ionic liquids (PILs) can be assessed using the Helper’s constant, which is determined through the Eq. (6) [31]:
 |
6 |
The Helper’s constant values for DL-alanine in (water + PILs) solutions, as presented in Table 4, provide insights into its role as a structure former or structure destructor within the solution. The positive values of the constant indicate that DL-alanine behaves as a structure-forming agent, facilitating the organization of water molecules. The findings indicate that hydrogen bonding interactions between DL-alanine and water are modulated by the coexistence of the studied PILs, resulting in a reorganization of water molecules around both DL-alanine and the PILs.
Ultrasonic and compressibility properties
The isentropic compressibility was determined using the Newton-Laplace equation, as represented by Eq. (7). Additionally, the apparent molar isentropic compressibility of ternary solution (DL-alanine + PILs + water) solutions was calculated applying the corresponding Eq. (8):
 |
7 |
 |
8 |
Here, (u), ( ), (
), ( ) represent the speed of sound, the isentropic compressibility of the solvent, and the isentropic compressibility of the solution, respectively. The isentropic compressibility (
) represent the speed of sound, the isentropic compressibility of the solvent, and the isentropic compressibility of the solution, respectively. The isentropic compressibility ( ) and apparent molar isentropic compressibility (
) and apparent molar isentropic compressibility ( ) data obtained in this study are summarized in Table 5:
) data obtained in this study are summarized in Table 5:
Table 5.
Speed of sound U (m·s− 1), isentropic compressibility κS (pa-1) and apparent molar isentropic compressibility  (m3·mol− 1·pa− 1) of ternary solutions in temperature range (298.15 to 318.15) K and concentration range (0.05 to 0.15) mol·kg− 1
(m3·mol− 1·pa− 1) of ternary solutions in temperature range (298.15 to 318.15) K and concentration range (0.05 to 0.15) mol·kg− 1
| m | U / m s− 1 | κS/pa-1 | m3·mol− 1·pa− 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 288.15 K | 298.15 K | 308.15 K | 318.15 K | 288.15 K | 298.15 K | 308.15 K | 318.15 K | 288.15 K | 298.15 K | 308.15 K | 318.15 K | ||
| DL-alanine 0.0501 +[2HEA]Ac | |||||||||||||
| 0.0000 | 1471.70 | 1501.33 | 1523.71 | 1539.64 | 4.61 | 4.44 | 4.32 | 4.24 | -3.14 | -2.12 | -1.83 | -1.82 | |
| 0.0495 | 1475.29 | 1504.47 | 1526.71 | 1542.52 | 4.58 | 4.42 | 4.30 | 4.22 | -3.04 | -2.26 | -1.97 | -1.75 | |
| 0.0999 | 1478.86 | 1507.79 | 1529.76 | 1545.43 | 4.55 | 4.39 | 4.28 | 4.20 | -2.99 | -2.33 | -1.96 | -1.74 | |
| 0.1493 | 1482.29 | 1510.92 | 1532.78 | 1548.28 | 4.52 | 4.30 | 4.25 | 4.18 | -2.94 | -2.30 | -1.97 | -1.73 | |
| 0.1994 | 1485.66 | 1514.16 | 1535.76 | 1550.85 | 4.50 | 4.34 | 4.23 | 4.16 | -2.89 | -2.31 | -1.95 | -1.64 | |
| 0.2492 | 1489.26 | 1517.49 | 1538.74 | 1553.78 | 4.47 | 4.32 | 4.21 | 4.14 | -2.92 | -2.34 | -1.95 | -1.67 | |
| 0.2993 | 1492.34 | 1520.61 | 1541.53 | 1556.58 | 4.44 | 4.29 | 4.19 | 4.12 | -2.82 | -2.31 | -1.90 | -1.67 | |
| DL-alanine 0.0999 +[2HEA]Ac | |||||||||||||
| 0.0000 | 1476.64 | 1505.84 | 1527.83 | 1543.52 | 4.57 | 4.41 | 4.29 | 4.22 | -3.24 | -2.15 | -2.47 | -2.01 | |
| 0.0499 | 1480.20 | 1509.07 | 1531.01 | 1546.48 | 4.54 | 4.38 | 4.27 | 4.19 | -2.95 | -2.32 | -2.13 | -1.78 | |
| 0.0996 | 1483.58 | 1512.30 | 1533.97 | 1549.30 | 4.52 | 4.36 | 4.25 | 4.17 | -2.83 | -2.32 | -2.01 | -1.72 | |
| 0.1497 | 1487.03 | 1515.65 | 1537.05 | 1552.19 | 4.49 | 4.33 | 4.22 | 4.15 | -2.81 | -2.35 | -2.01 | -1.71 | |
| 0.1995 | 1490.46 | 1518.70 | 1539.92 | 1554.86 | 4.46 | 4.31 | 4.20 | 4.13 | -2.79 | -2.28 | -1.95 | -1.65 | |
| 0.2494 | 1493.82 | 1521.81 | 1542.91 | 1557.73 | 4.44 | 4.29 | 4.18 | 4.11 | -2.75 | -2.2 | -1.93 | -1.66 | |
| 0.3005 | 1497.34 | 1525.05 | 1546.05 | 1560.66 | 4.41 | 4.26 | 4.16 | 4.09 | -2.74 | -2.24 | -1.94 | -1.67 | |
| DL-alanine 0.1501 +[2HEA]Ac | |||||||||||||
| 0.0000 | 1481.70 | 1510.37 | 1532.02 | 1547.43 | 4.53 | 4.38 | 4.26 | 4.19 | -3.06 | -1.92 | -1.95 | -1.68 | |
| 0.0496 | 1485.19 | 1513.51 | 1535.03 | 1550.35 | 4.51 | 4.35 | 4.24 | 4.17 | -2.75 | -2.14 | -1.86 | -1.68 | |
| 0.1000 | 1488.56 | 1516.84 | 1538.01 | 1553.18 | 4.48 | 4.33 | 4.22 | 4.15 | -2.65 | -2.23 | -1.82 | -1.61 | |
| 0.1499 | 1491.96 | 1519.96 | 1541.03 | 1556.15 | 4.45 | 4.30 | 4.20 | 4.13 | -2.65 | -2.20 | -1.84 | -1.66 | |
| 0.1999 | 1495.36 | 1523.29 | 1543.96 | 1559.01 | 4.43 | 4.28 | 4.10 | 4.10 | -2.65 | -2.24 | -1.84 | -1.66 | |
| 0.2493 | 1498.58 | 1526.26 | 1547.22 | 1561.46 | 4.40 | 4.26 | 4.15 | 4.09 | -2.61 | -2.19 | -1.91 | -1.58 | |
| 0.2988 | 1502.08 | 1529.51 | 1549.92 | 1564.31 | 4.38 | 4.23 | 4.13 | 4.07 | -2.65 | -2.22 | -1.86 | -1.60 | |
| DL-alanine 0.0501+[BHEA]Ac | |||||||||||||
| 0.0000 | 1472.64 | 1502.14 | 1524.42 | 1540.25 | 4.60 | 4.43 | 4.32 | 4.24 | -2.72 | -2.25 | -2.09 | -1.61 | |
| 0.0500 | 1476.01 | 1505.29 | 1527.45 | 1543.05 | 4.57 | 4.41 | 4.29 | 4.22 | -2.78 | -2.29 | -2.02 | -1.70 | |
| 0.1004 | 1479.46 | 1508.46 | 1530.39 | 1545.84 | 4.55 | 4.38 | 4.27 | 4.20 | -2.79 | -2.26 | -1.94 | -1.66 | |
| 0.1494 | 1482.88 | 1511.74 | 1533.45 | 1548.77 | 4.52 | 4.36 | 4.25 | 4.17 | -2.78 | -2.30 | -1.96 | -1.71 | |
| 0.1998 | 1486.23 | 1514.86 | 1536.51 | 1551.58 | 4.49 | 4.34 | 4.23 | 4.15 | -2.77 | -2.28 | -1.91 | -1.71 | |
| 0.2489 | 1489.75 | 1518.19 | 1539.37 | 1554.33 | 4.46 | 4.31 | 4.20 | 4.13 | -2.78 | -2.30 | -1.90 | -1.67 | |
| 0.3000 | 1493.03 | 1521.23 | 1542.28 | 1557.08 | 4.44 | 4.29 | 4.18 | 4.11 | -2.75 | -2.26 | -1.90 | -1.66 | |
| DL-alanine 0.0501+[BHEA]Ac | |||||||||||||
| 0.0000 | 1478.03 | 1506.6 | 1529.07 | 1544.47 | 4.56 | 4.40 | 4.28 | 4.21 | -3.63 | -3.14 | -1.62 | -1.64 | |
| 0.0504 | 1481.83 | 1510.29 | 1532.08 | 1547.29 | 4.53 | 4.37 | 4.26 | 4.19 | -3.19 | -2.84 | -1.91 | -1.62 | |
| 0.0999 | 1485.35 | 1513.81 | 1535.03 | 1550.07 | 4.50 | 4.35 | 4.24 | 4.10 | -3.05 | -2.77 | -1.90 | -1.62 | |
| 0.1495 | 1488.78 | 1517.02 | 1538.12 | 1552.89 | 4.47 | 4.32 | 4.21 | 4.14 | -2.95 | -2.61 | -1.94 | -1.63 | |
| 0.1993 | 1491.78 | 1520.23 | 1541.09 | 1555.34 | 4.45 | 4.30 | 4.19 | 4.13 | -2.76 | -2.53 | -1.93 | -1.54 | |
| 0.2496 | 1495.02 | 1523.55 | 1543.67 | 1558.12 | 4.42 | 4.27 | 4.17 | 4.11 | -2.70 | -2.49 | -1.82 | -1.54 | |
| 0.2994 | 1498.28 | 1526.59 | 1546.55 | 1561.01 | 4.40 | 4.25 | 4.15 | 4.09 | -2.66 | -2.41 | -1.81 | -1.57 | |
| DL-alanine 0.1501+[BHEA]Ac | |||||||||||||
| 0.0000 | 1484.24 | 1512.52 | 1533.86 | 1549.24 | 4.51 | 4.36 | 4.25 | 4.18 | -2.42 | -1.99 | -1.82 | -1.63 | |
| 0.0499 | 1487.54 | 1515.66 | 1536.87 | 1552.03 | 4.49 | 4.33 | 4.23 | 4.15 | -2.55 | -2.16 | -1.90 | -1.58 | |
| 0.1007 | 1490.98 | 1518.93 | 1539.86 | 1554.73 | 4.46 | 4.31 | 4.20 | 4.13 | -2.59 | -2.19 | -1.86 | -1.50 | |
| 0.1501 | 1494.35 | 1522.15 | 1542.91 | 1557.62 | 4.43 | 4.29 | 4.18 | 4.11 | -2.60 | -2.21 | -1.89 | -1.57 | |
| 0.2004 | 1497.81 | 1525.25 | 1546.00 | 1560.44 | 4.41 | 4.26 | 4.16 | 4.09 | -2.61 | -2.17 | -1.90 | -1.57 | |
| 0.2497 | 1500.97 | 1528.39 | 1548.73 | 1562.95 | 4.38 | 4.24 | 4.14 | 4.07 | -2.56 | -2.16 | -1.84 | -1.50 | |
| 0.2995 | 1504.60 | 1531.60 | 1551.67 | 1565.83 | 4.36 | 4.22 | 4.12 | 4.05 | -2.61 | -2.16 | -1.82 | -1.53 | |
| DL-alanine 0.0501+[THEA]Ac | |||||||||||||
| 0.0000 | 1475.33 | 1504.63 | 1526.71 | 1542.60 | 4.58 | 4.41 | 4.303 | 4.22 | -3.40 | -2.44 | -2.42 | -1.79 | |
| 0.0497 | 1478.89 | 1507.85 | 1529.91 | 1545.49 | 4.55 | 4.39 | 4.39 | 4.20 | -3.00 | -2.38 | -2.17 | -1.70 | |
| 0.0979 | 1482.15 | 1510.95 | 1532.73 | 1548.16 | 4.53 | 4.36 | 4.36 | 4.18 | -2.86 | -2.34 | -2.00 | -1.61 | |
| 0.1498 | 1485.67 | 1514.23 | 1535.89 | 1551.29 | 4.50 | 4.34 | 4.34 | 4.16 | -2.80 | -2.29 | -1.97 | -1.66 | |
| 0.1994 | 1489.11 | 1517.56 | 1539.19 | 1554.27 | 4.47 | 4.32 | 4.32 | 4.14 | -2.77 | -2.31 | -2.04 | -1.67 | |
| 0.2501 | 1492.51 | 1520.57 | 1541.94 | 1556.82 | 4.44 | 4.29 | 4.29 | 4.12 | -2.73 | -2.23 | -1.93 | -1.57 | |
| 0.3003 | 1495.87 | 1523.84 | 1544.82 | 1560.09 | 4.42 | 4.27 | 4.27 | 4.09 | -2.708 | -2.232 | -1.873 | -1.638 | |
| DL-alanine 0.1001+[THEA]Ac | |||||||||||||
| 0.0000 | 1483.82 | 1512.29 | 1533.75 | 1549.12 | 4.51 | 4.36 | 4.25 | 4.17 | -2.96 | -2.26 | -2.25 | -1.93 | |
| 0.0499 | 1487.39 | 1515.61 | 1536.91 | 1552.05 | 4.49 | 4.33 | 4.22 | 4.15 | -2.85 | -2.35 | -2.04 | -1.69 | |
| 0.0996 | 1490.72 | 1518.98 | 1539.95 | 1554.83 | 4.46 | 4.31 | 4.20 | 4.13 | -2.70 | -2.38 | -1.98 | -1.62 | |
| 0.1500 | 1494.25 | 1522.26 | 1542.99 | 1557.66 | 4.43 | 4.28 | 4.18 | 4.11 | -2.71 | -2.34 | -1.94 | -1.60 | |
| 0.1993 | 1497.68 | 1525.42 | 1545.96 | 1560.60 | 4.41 | 4.26 | 4.16 | 4.09 | -2.70 | -2.30 | -1.92 | -1.64 | |
| 0.2492 | 1501.04 | 1529.01 | 1548.82 | 1563.17 | 4.38 | 4.23 | 4.14 | 4.07 | -2.66 | -2.36 | -1.87 | -1.58 | |
| 0.2994 | 1504.05 | 1531.89 | 1551.94 | 1566.29 | 4.36 | 4.21 | 4.11 | 4.05 | -2.55 | -2.26 | -1.89 | -1.63 | |
| DL-alanine 0.1501+[THEA]Ac | |||||||||||||
| 0.0000 | 1492.41 | 1520.06 | 1540.83 | 1555.69 | 4.45 | 4.30 | 4.20 | 4.13 | -2.96 | -2.70 | -2.16 | -1.78 | |
| 0.0499 | 1496.02 | 1523.38 | 1543.95 | 1558.55 | 4.42 | 4.28 | 4.17 | 4.11 | -2.75 | -2.23 | -1.90 | -1.56 | |
| 0.0999 | 1499.36 | 1526.53 | 1546.91 | 1561.28 | 4.40 | 4.25 | 4.15 | 4.09 | -2.59 | -2.14 | -1.82 | -1.50 | |
| 0.1499 | 1502.73 | 1529.61 | 1549.96 | 1564.13 | 4.37 | 4.23 | 4.13 | 4.00 | -2.56 | -2.09 | -1.82 | -1.52 | |
| 0.1948 | 1505.71 | 1532.51 | 1552.62 | 1566.46 | 4.35 | 4.21 | 4.11 | 4.05 | -2.53 | -2.10 | -1.80 | -1.47 | |
| 0.2498 | 1509.47 | 1536.05 | 1555.89 | 1569.62 | 4.32 | 4.18 | 4.09 | 4.03 | -2.53 | -2.12 | -1.80 | -1.50 | |
| 0.2997 | 1511.98 | 1539.37 | 1558.94 | 1572.40 | 4.30 | 4.16 | 4.07 | 4.01 | -2.37 | -2.15 | -1.81 | -1.50 | |
The standard uncertainties for molality, temperature and pressure were u (m) = 0.002 mol·kg− 1, u (T) = 0.02 K, u (P) = 10 hPa, respectively with level of confidence 0.68. The combined standard uncertainty for speed of sound was, u (u) = 0.6 m·s− 1 with level of confidence 0.68
Analysis of ( ) reveals an inverse relationship with the concentration of DL-alanine. The variation of (
) reveals an inverse relationship with the concentration of DL-alanine. The variation of ( ) with DL-alanine molality, the concentration of the PILs, and temperature is shown in Fig. 3:
) with DL-alanine molality, the concentration of the PILs, and temperature is shown in Fig. 3:
Fig. 3.
Variation of apparent molar isentropic compressibility (  ) with, a) temperature, a) concentration of PILs and c) cation size of the PILs
) with, a) temperature, a) concentration of PILs and c) cation size of the PILs
These findings indicate that the ( ) values decrease with increasing the concentration of DL-alanine. This effect can be attributed to the disruption of the three-dimensional hydrogen bond network among water molecules, which weakens the cohesive forces within the liquid and reduces its overall resistance to flow. The standard partial molar isentropic compressibility of DL-alanine was calculated using the specified Eq. (9) [32];
) values decrease with increasing the concentration of DL-alanine. This effect can be attributed to the disruption of the three-dimensional hydrogen bond network among water molecules, which weakens the cohesive forces within the liquid and reduces its overall resistance to flow. The standard partial molar isentropic compressibility of DL-alanine was calculated using the specified Eq. (9) [32];
 |
9 |
where, ( ) is the partial molar isentropic compressibility and (
) is the partial molar isentropic compressibility and ( ) symbol is the empirical parameters of the Eq. (9). The parameters obtained for the studied solutions are presented in Table 6:
) symbol is the empirical parameters of the Eq. (9). The parameters obtained for the studied solutions are presented in Table 6:
Table 6.
The values of SK (m3·mol− 3/2·kg1/2·pa− 1), BK (m3mol− 2.kg·pa− 1),  (m3·mol− 1·pa− 1) obtained for each mixture and standard deviation at the experimental temperatures from Eq. (7)
(m3·mol− 1·pa− 1) obtained for each mixture and standard deviation at the experimental temperatures from Eq. (7)
| System | T |

|
SK | σ
|
|---|---|---|---|---|
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.0501 (mol·kg-1) | 288.15 | -3.146 | 0.382 | 0.025 |
| 298.15 | -2.121 | -0.920 | 0.023 | |
| 308.15 | -1.833 | -0.904 | 0.017 | |
| 318.15 | -1.825 | 0.293 | 0.025 | |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1001 (mol·kg-1) | 288.15 | -3.244 | 1.653 | 0.029 |
| 298.15 | -2.156 | -1.153 | 0.026 | |
| 308.15 | -2.470 | 1.932 | 0.033 | |
| 318.15 | -2.012 | 1.297 | 0.023 | |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1501 (mol·kg-1) | 288.15 | -3.060 | 1.816 | 0.033 |
| 298.15 | -1.927 | -1.382 | 0.032 | |
| 308.15 | -1.958 | 0.687 | 0.029 | |
| 318.15 | -1.684 | 0.013 | 0.029 | |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0499 (mol·kg-1) | 288.15 | -2.722 | -0.444 | 0.011 |
| 298.15 | -2.250 | -0.236 | 0.016 | |
| 308.15 | -2.091 | 0.395 | 0.027 | |
| 318.15 | -1.614 | -0.512 | 0.021 | |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0999 (mol·kg-1) | 288.15 | -3.630 | 1.922 | 0.042 |
| 298.15 | -3.148 | 1.260 | 0.031 | |
| 308.15 | -1.620 | -1.925 | 0.039 | |
| 318.15 | -1.648 | -1.450 | 0.030 | |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.1498 (mol·kg-1) | 288.15 | -2.423 | -0.795 | 0.022 |
| 298.15 | -1.999 | -1.045 | 0.021 | |
| 308.15 | -1.824 | -0.535 | 0.025 | |
| 318.15 | -1.633 | 0.354 | 0.031 | |
| DL-alanine in aqueous solutions of [THEA]Ac 0.0502 (mol·kg-1) | 288.15 | -3.400 | 2.216 | 0.035 |
| 298.15 | -2.445 | 0.211 | 0.021 | |
| 308.15 | -2.426 | 1.423 | 0.054 | |
| 318.15 | -1.798 | 0.609 | 0.040 | |
| DL-alanine in aqueous solutions of [THEA]Ac 0.0998 (mol·kg-1) | 288.15 | -2.966 | 0.578 | 0.039 |
| 298.15 | -2.267 | -0.710 | 0.033 | |
| 308.15 | -2.253 | 1.122 | 0.021 | |
| 318.15 | -1.937 | 1.502 | 0.033 | |
| DL-alanine in aqueous solutions of [THEA]Ac 0.1502 (mol·kg-1) | 288.15 | -2.969 | 1.094 | 0.046 |
| 298.15 | -2.709 | 2.878 | 0.044 | |
| 308.15 | -2.163 | 1.573 | 0.026 | |
| 318.15 | -1.783 | 1.296 | 0.025 |
The combined standard uncertainty for apparent molar isentropic compressibility were, 10 14 u ( ) = 0.7 m3·mol− 1·pa− 1
) = 0.7 m3·mol− 1·pa− 1
A negative value for this parameter indicates that the amino acid, as the solute, is surrounded by (PILs + water) molecules, which resist compression more effectively compared to the bulk solvent. Furthermore, the ( ) values decrease with increasing temperature, attributed to the intrinsic thermal expansion at elevated temperatures, where pressure-induced volume expansion becomes more pronounced.
) values decrease with increasing temperature, attributed to the intrinsic thermal expansion at elevated temperatures, where pressure-induced volume expansion becomes more pronounced.
Viscosity results
Viscosity is a measure of a fluid’s resistance to flow, influenced by intermolecular interactions and temperature [33]. In solutions, viscosity can vary significantly based on solute concentration and the nature of the solute and solvent. For instance, adding a solute like sugar to water increases viscosity due to enhanced molecular interactions. Understanding viscosity is crucial in fields like pharmaceuticals and food science, where it affects mixing, stability, and flow behavior. The dynamic and kinematic viscosities of DL-alanine in aqueous solutions containing PILs, measured over the temperature range of (288.15 to 318.15) K, are summarized in Table 7.
Table 7.
Dynamic viscosity and kinematic viscosity of DL-alanine in PILs aqueous solutions at different temperatures
| m | η k | η D | ||||||
|---|---|---|---|---|---|---|---|---|
| mol·kg− 1 | mm2·S− 1 | mPa·s | ||||||
| 288.15 K | 298.15 K | 308.15 K | 318.15 K | 288.15 K | 298.15 K | 308.15 K | 318.15 K | |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.0501 (mol·kg-1) | ||||||||
| 0.0000 | 1.1553 | 0.9050 | 0.7392 | 0.6184 | 1.156 | 0.903 | 0.736 | 0.618 |
| 0.0495 | 1.1664 | 0.9187 | 0.7442 | 0.6251 | 1.169 | 0.916 | 0.742 | 0.625 |
| 0.0999 | 1.1791 | 0.9252 | 0.7520 | 0.6301 | 1.183 | 0.926 | 0.751 | 0.631 |
| 0.1493 | 1.1926 | 0.9357 | 0.7622 | 0.6372 | 1.199 | 0.938 | 0.762 | 0.636 |
| 0.1994 | 1.2061 | 0.9462 | 0.7685 | 0.6441 | 1.214 | 0.950 | 0.769 | 0.643 |
| 0.2492 | 1.2258 | 0.9558 | 0.7760 | 0.6526 | 1.231 | 0.961 | 0.778 | 0.650 |
| 0.2993 | 1.2349 | 0.9671 | 0.7841 | 0.6596 | 1.246 | 0.973 | 0.787 | 0.656 |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1001 (mol·kg-1) | ||||||||
| 0.0000 | 1.1703 | 0.9190 | 0.7672 | 0.6425 | 1.173 | 0.918 | 0.766 | 0.639 |
| 0.0499 | 1.1876 | 0.9293 | 0.7726 | 0.6458 | 1.189 | 0.930 | 0.771 | 0.643 |
| 0.0996 | 1.2084 | 0.9442 | 0.7783 | 0.6498 | 1.207 | 0.946 | 0.779 | 0.647 |
| 0.1497 | 1.2228 | 0.9593 | 0.7841 | 0.6557 | 1.231 | 0.962 | 0.785 | 0.653 |
| 0.1995 | 1.2382 | 0.9711 | 0.7918 | 0.6597 | 1.250 | 0.974 | 0.791 | 0.659 |
| 0.2494 | 1.2501 | 0.9801 | 0.7965 | 0.6634 | 1.262 | 0.986 | 0.801 | 0.663 |
| 0.3005 | 1.2664 | 0.9915 | 0.8038 | 0.6686 | 1.284 | 0.998 | 0.805 | 0.668 |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1501 (mol·kg-1) | ||||||||
| 0.0000 | 1.1979 | 0.9401 | 0.7647 | 0.6478 | 1.202 | 0.941 | 0.762 | 0.643 |
| 0.0496 | 1.2116 | 0.9496 | 0.7706 | 0.6526 | 1.217 | 0.952 | 0.770 | 0.650 |
| 0.1003 | 1.2220 | 0.9622 | 0.7809 | 0.6584 | 1.230 | 0.966 | 0.782 | 0.655 |
| 0.1499 | 1.2372 | 0.9705 | 0.7889 | 0.6633 | 1.247 | 0.975 | 0.790 | 0.662 |
| 0.1999 | 1.2541 | 0.9840 | 0.8005 | 0.6695 | 1.268 | 0.987 | 0.801 | 0.669 |
| 0.2493 | 1.2658 | 0.9909 | 0.8087 | 0.6769 | 1.279 | 0.998 | 0.813 | 0.678 |
| 0.2988 | 1.2806 | 1.0019 | 0.8179 | 0.6818 | 1.296 | 1.011 | 0.821 | 0.685 |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0499 (mol·kg-1) | ||||||||
| 0.0000 | 1.1682 | 0.9176 | 0.7459 | 0.6282 | 1.168 | 0.916 | 0.743 | 0.621 |
| 0.0502 | 1.1781 | 0.9256 | 0.7526 | 0.6327 | 1.181 | 0.926 | 0.751 | 0.626 |
| 0.1004 | 1.1923 | 0.9348 | 0.7604 | 0.6418 | 1.196 | 0.936 | 0.759 | 0.635 |
| 0.1494 | 1.2112 | 0.9453 | 0.7706 | 0.6479 | 1.218 | 0.948 | 0.771 | 0.644 |
| 0.1998 | 1.2252 | 0.9582 | 0.7803 | 0.6541 | 1.232 | 0.962 | 0.782 | 0.654 |
| 0.2489 | 1.2348 | 0.9711 | 0.7894 | 0.6626 | 1.245 | 0.976 | 0.792 | 0.663 |
| 0.3001 | 1.2502 | 0.9790 | 0.7947 | 0.6678 | 1.262 | 0.986 | 0.798 | 0.668 |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0999 (mol·kg-1) | ||||||||
| 0.0000 | 1.1829 | 0.9293 | 0.7551 | 0.6302 | 1.186 | 0.931 | 0.753 | 0.627 |
| 0.0504 | 1.1946 | 0.9374 | 0.7612 | 0.6365 | 1.201 | 0.939 | 0.762 | 0.634 |
| 0.0999 | 1.2151 | 0.9539 | 0.7715 | 0.6451 | 1.222 | 0.958 | 0.775 | 0.641 |
| 0.1495 | 1.2266 | 0.9619 | 0.7808 | 0.6520 | 1.236 | 0.966 | 0.782 | 0.652 |
| 0.1993 | 1.2427 | 0.9743 | 0.7911 | 0.6587 | 1.253 | 0.981 | 0.794 | 0.659 |
| 0.2496 | 1.2582 | 0.9849 | 0.7975 | 0.6640 | 1.271 | 0.992 | 0.801 | 0.665 |
| 0.2994 | 1.2802 | 0.9936 | 0.8053 | 0.6708 | 1.295 | 1.002 | 0.812 | 0.672 |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.1498 (mol·kg-1) | ||||||||
| 0.0000 | 1.2269 | 0.9605 | 0.7784 | 0.6519 | 1.229 | 0.962 | 0.776 | 0.651 |
| 0.0499 | 1.2476 | 0.9748 | 0.7915 | 0.6591 | 1.251 | 0.977 | 0.787 | 0.658 |
| 0.1007 | 1.2582 | 0.9836 | 0.7967 | 0.6691 | 1.265 | 0.988 | 0.802 | 0.668 |
| 0.1501 | 1.2768 | 1.0012 | 0.8103 | 0.6766 | 1.287 | 1.002 | 0.812 | 0.677 |
| 0.2004 | 1.2951 | 1.0125 | 0.8195 | 0.6838 | 1.304 | 1.018 | 0.822 | 0.685 |
| 0.2497 | 1.3049 | 1.0208 | 0.8272 | 0.6892 | 1.320 | 1.030 | 0.832 | 0.692 |
| 0.2995 | 1.3214 | 1.0334 | 0.8372 | 0.6973 | 1.339 | 1.044 | 0.843 | 0.702 |
| DL-alanine in aqueous solutions of [THEA]Ac 0.0501 (mol·kg-1) | ||||||||
| 0.0000 | 1.1907 | 0.9357 | 0.7603 | 0.6371 | 1.194 | 0.937 | 0.758 | 0.638 |
| 0.0497 | 1.2018 | 0.9426 | 0.7650 | 0.6415 | 1.206 | 0.944 | 0.764 | 0.643 |
| 0.0979 | 1.2131 | 0.9506 | 0.7718 | 0.6468 | 1.220 | 0.953 | 0.771 | 0.649 |
| 0.1498 | 1.2269 | 0.9614 | 0.7797 | 0.6517 | 1.235 | 0.965 | 0.781 | 0.653 |
| 0.1994 | 1.2397 | 0.9726 | 0.7886 | 0.6586 | 1.251 | 0.978 | 0.789 | 0.659 |
| 0.2501 | 1.2574 | 0.9801 | 0.7952 | 0.6628 | 1.269 | 0.986 | 0.796 | 0.664 |
| 0.3003 | 1.2669 | 0.9926 | 0.8022 | 0.6685 | 1.281 | 1.001 | 0.804 | 0.671 |
| DL-alanine in aqueous solutions of [THEA]Ac 0.0998 (mol·kg-1) | ||||||||
| 0.0000 | 1.2317 | 0.9560 | 0.7801 | 0.6583 | 1.241 | 0.958 | 0.780 | 0.657 |
| 0.0499 | 1.2405 | 0.9692 | 0.7862 | 0.6621 | 1.251 | 0.973 | 0.789 | 0.661 |
| 0.0996 | 1.2539 | 0.9777 | 0.7943 | 0.6671 | 1.268 | 0.983 | 0.798 | 0.668 |
| 0.1501 | 1.2688 | 0.9905 | 0.8052 | 0.6734 | 1.281 | 0.997 | 0.809 | 0.673 |
| 0.1993 | 1.2816 | 1.0047 | 0.8136 | 0.6781 | 1.296 | 1.009 | 0.818 | 0.680 |
| 0.2492 | 1.2944 | 1.0126 | 0.8224 | 0.6818 | 1.310 | 1.022 | 0.827 | 0.685 |
| 0.2994 | 1.3105 | 1.0235 | 0.8285 | 0.6881 | 1.328 | 1.034 | 0.834 | 0.692 |
| DL-alanine in aqueous solutions of [THEA]Ac 0.1502 (mol·kg-1) | ||||||||
| 0.0000 | 1.2686 | 0.9906 | 0.8088 | 0.6757 | 1.279 | 0.996 | 0.811 | 0.679 |
| 0.0499 | 1.2834 | 1.0036 | 0.8145 | 0.6819 | 1.296 | 1.010 | 0.819 | 0.685 |
| 0.0999 | 1.2952 | 1.0133 | 0.8231 | 0.6858 | 1.309 | 1.021 | 0.828 | 0.691 |
| 0.1499 | 1.3076 | 1.0224 | 0.8287 | 0.6921 | 1.327 | 1.032 | 0.836 | 0.696 |
| 0.1948 | 1.3194 | 1.0313 | 0.8365 | 0.6968 | 1.341 | 1.042 | 0.843 | 0.701 |
| 0.2498 | 1.3389 | 1.0437 | 0.8439 | 0.7017 | 1.361 | 1.056 | 0.852 | 0.707 |
| 0.2997 | 1.3505 | 1.0536 | 0.8506 | 0.7068 | 1.378 | 1.062 | 0.860 | 0.713 |
a The standard uncertainties associated with molality, temperature, and pressure were determined as u (m) = 0.001 mol·kg− 1, u (T) = 0.2 K and u (P) = 10.5 hPa, respectively, with a confidence level of 0.68. The standard combined uncertainty for viscosity was approximately u (η) = 0.02 mPa·s corresponding to a confidence level of 0.68
The data indicate that both dynamic and kinematic viscosities increase with higher concentrations of DL-alanine and PILs.
This trend indicates that as the molality of DL-alanine and PILs increases, the frequency of molecular collisions per unit volume rises, which enhances intermolecular interactions, such as hydrogen bonding and ionic interactions. These stronger interactions restrict molecular mobility, leading to an increase in viscosity. Conversely, all studied systems demonstrate a decrease in viscosity with increasing temperature, highlighting reduced resistance to flow at elevated temperatures. This behavior can be attributed to the increased kinetic energy of molecules, which weakens hydrophilic-ionic and hydrophilic-hydrophobic interactions, allowing for greater molecular movement. The dynamic viscosity data were further analyzed using the Jones-Dole equation [34]:
 |
10 |
In this equation, (η) represents the viscosity of the solution, while ( ) refers to the viscosity of the solvent. The parameter A is associated with long-range Coulombic interactions between solute molecules, known as the Falkenhagen coefficient. For non-electrolyte solutes, the values of the A coefficient are typically negligible. Additionally,
) refers to the viscosity of the solvent. The parameter A is associated with long-range Coulombic interactions between solute molecules, known as the Falkenhagen coefficient. For non-electrolyte solutes, the values of the A coefficient are typically negligible. Additionally,  another parameter used to evaluate solute-solute interactions, exhibits a similar trend to the A coefficient and is therefore often disregarded.
another parameter used to evaluate solute-solute interactions, exhibits a similar trend to the A coefficient and is therefore often disregarded.
The viscosity B-coefficient is an empirical parameter used to evaluate solute-solvent interactions. This parameter is influenced by the size, shape, and structural effects arising from solute-solvent interactions. High positive values of the B-coefficient indicate robust solute-solvent interactions, corroborating the findings of Vϕ0 which also serves as an indicator of solute-solvent interactions.
As shown in Table 8, the B-coefficient for all systems exhibits a decreasing trend with increasing temperature, indicating a reduction in solute-solvent interactions as the temperature rises.
Table 8 The viscosity.
B-coefficient (dm3·mol− 1) of DL-alanine in aqueous solution of PILs obtained from Jones-Dole equation.
| T | B |
|---|---|
| K | dm3·mol− 1 |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.0501 (mol·kg-1) | |
| 288.15 | 0.259(0.01) |
| 298.15 | 0.243(0.05) |
| 308.15 | 0.223(0.03) |
| 318.15 | 0.216(0.04) |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1001 (mol·kg-1) | |
| 288.15 | 0.387(0.02) |
| 298.15 | 0.361(0.04) |
| 308.15 | 0.357(0.08) |
| 318.15 | 0.321(0.04) |
| DL-alanine in aqueous solutions of [2-HEA]Ac 0.1501 (mol·kg-1) | |
| 288.15 | 0.453(0.09) |
| 298.15 | 0.430(0.02) |
| 308.15 | 0.422(0.05) |
| 318.15 | 0.415(0.01) |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0499 (mol·kg-1) | |
| 288.15 | 0.320(0.02) |
| 298.15 | 0.305(0.07) |
| 308.15 | 0.293(0.05) |
| 318.15 | 0.290(0.01) |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.0999 (mol·kg-1) | |
| 288.15 | 0.421(0.02) |
| 298.15 | 0.390(0.01) |
| 308.15 | 0.367(0.03) |
| 318.15 | 0.325(0.01) |
| DL-alanine in aqueous solutions of [BHEA]Ac 0.1498 (mol·kg-1) | |
| 288.15 | 0.607(0.02) |
| 298.15 | 0.579(0.07) |
| 308.15 | 0.548(0.04) |
| 318.15 | 0.524(0.09) |
| DL-alanine in aqueous solutions of [THEA]Ac 0.0499 (mol·kg-1) | |
| 288.15 | 0.400(0.08) |
| 298.15 | 0.377(0.05) |
| 308.15 | 0.337(0.01) |
| 318.15 | 0.330(0.02) |
| DL-alanine in aqueous solutions of [THEA]Ac 0.0998( mol·kg-1) | |
| 288.15 | 0.407(0.01) |
| 298.15 | 0.421(0.07) |
| 308.15 | 0.403(0.05) |
| 318.15 | 0.378(0.02) |
| DL-alanine in aqueous solutions of [THEA]Ac 0.1502 (mol·kg-1) | |
| 288.15 | 0.589(0.01) |
| 298.15 | 0.582(0.03) |
| 308.15 | 0.562(0.01) |
| 318.15 | 0.536(0.08) |
The standard uncertainties associated with molality, temperature, and pressure were u (m) = 0.001 mol·kg− 1, u (T) = 0.2 K and u (P) = 10.5 hPa, respectively, corresponding to a confidence level of 0.68. The standard combined uncertainty for viscosity was approximately u (η) = 0.02 mPa·s with a confidence level of 0.68
Additionally, the viscosity B-coefficient values are consistently higher than the A-coefficient values, suggesting that interactions between the solute and solvent are more influential than solute-solute interactions.
Refractive index and molar refraction
The refractive indices of ternary solutions containing DL-alanine, protic ionic liquids (PILs), and water were measured over a temperature range of (288.15 to 318.15) K. The experimentally determined refractive index values for these solutions are presented in Table 9.
Table 9.
Refractive index and the molar refraction of the ternary aqueous solutions of DL-alanine in the presence of PILs at different temperatures
| m | n D | R M | ||||||
|---|---|---|---|---|---|---|---|---|
| mol·kg− 1 | 288.15 K | 298.15 K | 308.15 K | 318.15 K | 288.15 K | 298.15 K | 308.15 K | 318.15 K |
| DL-alanine 0.0501 + [2-HEA]Ac | ||||||||
| 0.0000 | 1.3347 | 1.3338 | 1.3326 | 1.3312 | 3.7228 | 3.7215 | 3.7189 | 3.7134 |
| 0.0497 | 1.3352 | 1.3348 | 1.3336 | 1.3319 | 3.7335 | 3.7396 | 3.7371 | 3.7285 |
| 0.0993 | 1.3358 | 1.3356 | 1.3344 | 1.3329 | 3.7475 | 3.7558 | 3.7534 | 3.7469 |
| 0.1496 | 1.3364 | 1.3366 | 1.3351 | 1.3339 | 3.7613 | 3.7739 | 3.7684 | 3.7651 |
| 0.2001 | 1.3373 | 1.3372 | 1.3358 | 1.3348 | 3.7782 | 3.7880 | 3.7837 | 3.7824 |
| 0.2499 | 1.3383 | 1.3379 | 1.3367 | 1.3355 | 3.7930 | 3.8030 | 3.8008 | 3.7976 |
| 0.2992 | 1.3389 | 1.3386 | 1.3375 | 1.3364 | 3.8100 | 3.8181 | 3.8171 | 3.8148 |
| DL-alanine 0.1001 + [2-HEA]Ac | ||||||||
| 0.0000 | 1.3356 | 1.3345 | 1.3335 | 1.3321 | 3.7249 | 3.7239 | 3.7234 | 3.7182 |
| 0.0494 | 1.3364 | 1.3353 | 1.3343 | 1.3327 | 3.7408 | 3.7401 | 3.7396 | 3.7321 |
| 0.0997 | 1.3371 | 1.3361 | 1.3352 | 1.3334 | 3.7558 | 3.7551 | 3.7547 | 3.7473 |
| 0.1498 | 1.3378 | 1.3367 | 1.3357 | 1.3342 | 3.7707 | 3.7702 | 3.7699 | 3.7636 |
| 0.1993 | 1.3389 | 1.3377 | 1.3365 | 1.3349 | 3.7897 | 3.7883 | 3.7860 | 3.7787 |
| 0.2496 | 1.3397 | 1.3385 | 1.3374 | 1.3359 | 3.8058 | 3.8045 | 3.8033 | 3.7970 |
| 0.2994 | 1.3404 | 1.3391 | 1.3382 | 1.3367 | 3.8211 | 3.8188 | 3.8176 | 3.8134 |
| DL-alanine 0.1501 + [2-HEA]Ac | ||||||||
| 0.0000 | 1.3363 | 1.3352 | 1.3342 | 1.3331 | 3.7272 | 3.7263 | 3.7258 | 3.7234 |
| 0.0501 | 1.3370 | 1.3360 | 1.3349 | 1.3341 | 3.7422 | 3.7424 | 3.7421 | 3.7407 |
| 0.1005 | 1.3379 | 1.3368 | 1.3357 | 1.3348 | 3.7594 | 3.7587 | 3.7575 | 3.7572 |
| 0.1501 | 1.3387 | 1.3374 | 1.3365 | 1.3354 | 3.7754 | 3.7728 | 3.7737 | 3.7714 |
| 0.1993 | 1.3395 | 1.3382 | 1.3375 | 1.3362 | 3.7914 | 3.7889 | 3.7919 | 3.7877 |
| 0.2500 | 1.3402 | 1.3391 | 1.3384 | 1.3371 | 3.8063 | 3.8049 | 3.8090 | 3.8038 |
| 0.3002 | 1.3412 | 1.3399 | 1.3393 | 1.3377 | 3.8242 | 3.8218 | 3.8262 | 3.8188 |
| DL-alanine 0.0501 + [BHEA]Ac | ||||||||
| 0.0000 | 1.3349 | 1.3337 | 1.3324 | 1.3315 | 3.7204 | 3.7191 | 3.7155 | 3.7150 |
| 0.0500 | 1.3359 | 1.3347 | 1.3334 | 1.3323 | 3.7391 | 3.7371 | 3.7336 | 3.7311 |
| 0.1004 | 1.3366 | 1.3356 | 1.3342 | 1.3329 | 3.7540 | 3.7539 | 3.7498 | 3.7454 |
| 0.1494 | 1.3372 | 1.3365 | 1.3351 | 1.3336 | 3.7717 | 3.7711 | 3.7660 | 3.7605 |
| 0.1998 | 1.3381 | 1.3372 | 1.3358 | 1.3342 | 3.7875 | 3.7861 | 3.7821 | 3.7746 |
| 0.2489 | 1.3391 | 1.3383 | 1.3366 | 1.3352 | 3.8059 | 3.8054 | 3.7983 | 3.7909 |
| 0.3000 | 1.3400 | 1.3390 | 1.3374 | 1.3357 | 3.8222 | 3.8205 | 3.8145 | 3.8061 |
| DL-alanine 0.1001 + [BHEA]Ac | ||||||||
| 0.0000 | 1.3356 | 1.3344 | 1.3329 | 1.3320 | 3.7217 | 3.7198 | 3.7144 | 3.7139 |
| 0.0498 | 1.3364 | 1.3351 | 1.3339 | 1.3328 | 3.7377 | 3.7349 | 3.7326 | 3.7302 |
| 0.1001 | 1.3373 | 1.3358 | 1.3346 | 1.3336 | 3.7546 | 3.7498 | 3.7477 | 3.7463 |
| 0.1496 | 1.3382 | 1.3368 | 1.3352 | 1.3345 | 3.7715 | 3.7679 | 3.7617 | 3.7613 |
| 0.1998 | 1.3389 | 1.3377 | 1.3361 | 1.3352 | 3.7865 | 3.7850 | 3.7789 | 3.7787 |
| 0.2494 | 1.3397 | 1.3387 | 1.3368 | 1.3359 | 3.8025 | 3.8024 | 3.7941 | 3.7940 |
| 0.2994 | 1.3405 | 1.3394 | 1.3378 | 1.3367 | 3.8185 | 3.8183 | 3.8124 | 3.8102 |
| DL-alanine 0.1501 + [BHEA]Ac | ||||||||
| 0.0000 | 1.3368 | 1.3353 | 1.3343 | 1.3333 | 3.7278 | 3.7230 | 3.7227 | 3.7213 |
| 0.0498 | 1.3378 | 1.3362 | 1.3352 | 1.3341 | 3.7457 | 3.7401 | 3.7399 | 3.7376 |
| 0.1004 | 1.3387 | 1.3371 | 1.3362 | 1.3351 | 3.7629 | 3.7564 | 3.7562 | 3.7560 |
| 0.1501 | 1.3395 | 1.3382 | 1.3369 | 1.3359 | 3.7788 | 3.7745 | 3.7733 | 3.7722 |
| 0.1997 | 1.3403 | 1.3387 | 1.3376 | 1.3367 | 3.7949 | 3.7897 | 3.7885 | 3.7881 |
| 0.2495 | 1.3410 | 1.3394 | 1.3383 | 1.3374 | 3.8098 | 3.8047 | 3.8036 | 3.8025 |
| 0.2996 | 1.3418 | 1.3403 | 1.3392 | 1.3381 | 3.8258 | 3.8219 | 3.8209 | 3.8185 |
| DL-alanine 0.0498 + [THEA]Ac | ||||||||
| 0.0000 | 1.3354 | 1.3342 | 1.3331 | 1.3322 | 3.7218 | 3.7198 | 3.7184 | 3.7179 |
| 0.0488 | 1.3362 | 1.3350 | 1.3340 | 1.3330 | 3.7377 | 3.7358 | 3.7355 | 3.7342 |
| 0.0987 | 1.3370 | 1.3358 | 1.3347 | 1.3337 | 3.7533 | 3.7516 | 3.7504 | 3.7493 |
| 0.1497 | 1.3378 | 1.3367 | 1.3355 | 1.3345 | 3.7697 | 3.7691 | 3.7670 | 3.7661 |
| 0.1994 | 1.3385 | 1.3374 | 1.3363 | 1.3353 | 3.7847 | 3.7842 | 3.7832 | 3.7825 |
| 0.2472 | 1.3393 | 1.3381 | 1.3369 | 1.3359 | 3.8010 | 3.7996 | 3.7977 | 3.7971 |
| 0.3001 | 1.3401 | 1.3389 | 1.3378 | 1.3368 | 3.8171 | 3.8159 | 3.8153 | 3.8148 |
| DL-alanine 0.0998 + [THEA]Ac | ||||||||
| 0.0000 | 1.3372 | 1.3361 | 1.3350 | 1.3340 | 3.7294 | 3.7286 | 3.7273 | 3.7261 |
| 0.0500 | 1.3380 | 1.3369 | 1.3358 | 1.3347 | 3.7454 | 3.7447 | 3.7436 | 3.7413 |
| 0.1001 | 1.3390 | 1.3378 | 1.3366 | 1.3354 | 3.7633 | 3.7618 | 3.7597 | 3.7566 |
| 0.1499 | 1.3395 | 1.3383 | 1.3372 | 1.3360 | 3.7764 | 3.7750 | 3.7740 | 3.7709 |
| 0.1999 | 1.3403 | 1.3391 | 1.3379 | 1.3369 | 3.7924 | 3.7901 | 3.7891 | 3.7881 |
| 0.2491 | 1.3411 | 1.3397 | 1.3386 | 1.3377 | 3.8085 | 3.8052 | 3.8044 | 3.8043 |
| 0.2993 | 1.3417 | 1.3406 | 1.3395 | 1.3385 | 3.8228 | 3.8224 | 3.8215 | 3.8205 |
| DL-alanine 0.1501 + [THEA]Ac | ||||||||
| 0.0000 | 1.3389 | 1.3378 | 1.3367 | 1.3357 | 3.7360 | 3.7355 | 3.7344 | 3.7332 |
| 0.0498 | 1.3397 | 1.3386 | 1.3375 | 1.3364 | 3.7521 | 3.7517 | 3.7507 | 3.7485 |
| 0.0997 | 1.3405 | 1.3393 | 1.3382 | 1.3372 | 3.7682 | 3.7669 | 3.7659 | 3.7648 |
| 0.1494 | 1.3413 | 1.3401 | 1.3389 | 1.3378 | 3.7843 | 3.7831 | 3.7812 | 3.7791 |
| 0.1999 | 1.3419 | 1.3407 | 1.3396 | 1.3386 | 3.7975 | 3.7964 | 3.7956 | 3.7945 |
| 0.2500 | 1.3429 | 1.3417 | 1.3405 | 1.3393 | 3.8163 | 3.8153 | 3.8136 | 3.8106 |
| 0.3002 | 1.3438 | 1.3425 | 1.3412 | 1.3402 | 3.8331 | 3.8313 | 3.8287 | 3.8278 |
Standard uncertainties (u) for each variable are u (m) = 0.0002 mol·kg− 1, u (T) = 0.01 K; u (nD) = 0.001; u (P) = 1.05 hPa
The temperature dependence of the refractive index follows a trend similar to that of solution density, as outlined in Table 3. According to the data in Table 9, the refractive index decreases with increasing temperature and increases with higher concentrations of DL-alanine.
To calculate the molar refraction, the Lorentz-Lorenz equation was applied [35]:
 |
11 |
In this equation ( ) is mole fraction and (M1), (M2)are molecular weights of each component in the mixture, while (d) denotes the density of the solution. The values of (nD) and molar refraction are provided in Table 9. Molar refraction is an important parameter that reflects solute-solvent interactions and the molecular polarizability within the solution. As shown in Table 9, the molar refraction values increase with higher concentrations of PILs, suggesting enhanced polarizability in the solutions studied and indicating strong interactions between PILs and DL-alanine. Increased molar refraction indicates a substance’s ability to polarize in response to an electric field. This is linked to enhanced polarizability, which refers to the ease with which electron clouds can be distorted. As more electrons are present, or as the molecular structure allows for greater distortion, both molar refraction and polarizability increase. Thus, a direct relationship exists where higher polarizability leads to greater molar refraction. Furthermore, the variations in molar refraction with temperature, PIL concentration, and the cation size of PILs are illustrated in Fig. 4:
) is mole fraction and (M1), (M2)are molecular weights of each component in the mixture, while (d) denotes the density of the solution. The values of (nD) and molar refraction are provided in Table 9. Molar refraction is an important parameter that reflects solute-solvent interactions and the molecular polarizability within the solution. As shown in Table 9, the molar refraction values increase with higher concentrations of PILs, suggesting enhanced polarizability in the solutions studied and indicating strong interactions between PILs and DL-alanine. Increased molar refraction indicates a substance’s ability to polarize in response to an electric field. This is linked to enhanced polarizability, which refers to the ease with which electron clouds can be distorted. As more electrons are present, or as the molecular structure allows for greater distortion, both molar refraction and polarizability increase. Thus, a direct relationship exists where higher polarizability leads to greater molar refraction. Furthermore, the variations in molar refraction with temperature, PIL concentration, and the cation size of PILs are illustrated in Fig. 4:
Fig. 4.
molar refraction variation of ternary solutions with, a) temperature, b) concentration of PILs and c) cation size of the PILs
with, a) temperature, b) concentration of PILs and c) cation size of the PILs
Based on Fig. 4 As the size of cations in protic ionic liquids increases, the molar refraction also tends to increase. Larger cations have more electrons and a more substantial electron cloud, which enhances their polarizability. This increased polarizability leads to a greater ability to distort in an electric field, contributing to higher molar refraction. Consequently, the relationship reflects the interplay between cation size and electron distribution.
Hydration behavior interpretation
This study demonstrates that DL-alanine has the smallest cavity volume among the molecules analyzed, indicating its more compact molecular structure. This compactness suggests that the hydration behavior of DL-alanine may differ from that of the other solutes examined. The smaller cavity volume and surface area imply fewer potential sites for hydrogen bonding (H-bonding) with solvent molecules. Also, DL-alanine exhibits the least negative dielectric energy (hydration energy) among the studied molecules, indicating weaker solute-solvent interactions. The relatively less negative hydration energy further suggests that the hydrogen bonding between DL-alanine and the solvent is weaker compared to the other solutes.
In contrast, the mono-, bis-, and tris (2-hydroxyethyl) ammonium acetate molecules exhibit progressively larger cavity volumes and surface areas, signifying a greater spatial requirement and more complex interactions with the solvent than DL-alanine. Their more negative dielectric energies indicate that these molecules engage in stronger and more favorable interactions with the solvent. The increased cavity volumes and surface areas provide more opportunities for hydrogen bond (H-bond) formation with the solvent, enhancing stronger and more favorable H-bonding interactions.
Additionally, the Helper’s constant is introduced as a useful parameter to evaluate the structure-making or structure-breaking behavior of DL-alanine in the presence of the examined PILs. Positive values of this constant suggest that DL-alanine exhibits structure-making behavior, facilitating the rearrangement of the hydrogen bonding network between the PILs and water molecules. This implies that the presence of the studied PILs may influence the hydrogen bonding interactions between DL-alanine and water molecules, potentially altering the hydration dynamics.
In conclusion, the findings highlight the distinct hydration behavior of DL-alanine compared to the studied PILs. The reduced surface area, smaller cavity volume, and less negative hydration energy of DL-alanine indicate its compact nature and weaker hydrogen bonding interactions with the solvent. In contrast, the protic ionic liquids (PILs), with their larger cavity volumes, expanded surface areas, more negative hydration energies, and higher capacity for hydrogen bonding, demonstrate more intricate interactions with the solvent. The temperature-dependent behavior and the impact of the PILs emphasize the significant role that hydrogen bonding plays in governing the hydration dynamics of DL-alanine.
Conclusions
This investigation reveals that DL-alanine interacts more weakly with water molecules compared to the surrounding protic ionic liquids (PILs), such as mono-, bis-, and tris-(2-hydroxyethyl) ammonium acetate. The weaker interactions observed for DL-alanine are attributed to its compact molecular structure and lower negative dielectric energy. In contrast, the PILs, with their larger molecular size and more complex structures, engage more strongly with water molecules, primarily through hydrogen bonding. Temperature variations significantly affect the hydration layer around DL-alanine, leading to a greater release of water molecules compared to the PIL solutions. The positive value of the Helper constant indicates that DL-alanine facilitates the ordering of water molecules in its surrounding environment. In contrast, the PILs may alter the hydrogen bonding between DL-alanine and water by reorganizing the water molecules and forming their own hydrogen bonds with the solvent.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the support of the grant council of the University of Tabriz.
Author contributions
Conception and design of study: Mohammad Amin Morsali, Hemayat Shekaari, Acquisition of data: Mohammad Amin Morsali analysis and/or interpretation of data: Mohammad Amin Morsali, Drafting the manuscript: Mohammad Amin Morsali Revising the manuscript critically for important intellectual content: Mohammad Amin Morsali, Hemayat Shekaari Approval of the version of the manuscript to be published: Mohammad Amin Morsali, Hemayat Shekaari.
Funding
No founding has been received for this study.
Data availability
The authors confirm that the data supporting the findings of this study are available within the manuscript, figures, tables, and supporting information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biological Water or Rather Water. in Biology?| The Journal of Physical Chemistry Letters. https://pubs.acs.org/doi/10.1021/acs.jpclett.5b01143. Accessed 28 Apr 2025. [DOI] [PubMed]
- 2.Durell SR, Ben-Naim A. Hydrophobic-Hydrophilic forces in protein folding. Biopolymers. 2017;107. 10.1002/bip.23020. [DOI] [PMC free article] [PubMed]
- 3.(2024) Molecular Recognition Mediated by Hydrogen Bonding in Aqueous Media| Request PDF. ResearchGate. 10.1002/anie.202012315
- 4.Water Dynamics in the Hydration Shells of Biomolecules| Chemical Reviews. https://pubs.acs.org/doi/10.1021/acs.chemrev.6b00765. Accessed 28 Apr 2025. [DOI] [PMC free article] [PubMed]
- 5.Water is an. active matrix of life for cell and molecular biology. ResearchGate. 10.1073/pnas.1703781114
- 6.Dargaville BL, Hutmacher DW. Water as the often neglected medium at the interface between materials and biology. Nat Commun. 2022;13:4222. 10.1038/s41467-022-31889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas CG, Tzakos AG, Gerothanassis IP. On the hydration state of amino acids and their derivatives at different ionization states: A comparative multinuclear NMR and crystallographic investigation. J Amino Acids. 2012;2012:565404. 10.1155/2012/565404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abnormal incorporation of. Amino acids into the gas hydrate crystal lattice| request PDF. ResearchGate. 10.1039/c4cp05056h [DOI] [PubMed]
- 9.Protic Ionic Liquids. Properties and Applications| Request PDF. ResearchGate. 10.1021/cr068040u
- 10.Padinhattath SP, Shaibuna M, Gardas RL. Ionic liquids and deep eutectic solvents: A brief prologue and their applications in sustainable extraction and separation processes. J Indian Chem Soc. 2025;102:101638. 10.1016/j.jics.2025.101638. [Google Scholar]
- 11.Understanding the Hydrogen Bonds. in Ionic Liquids and Their Roles in Properties and Reactions| Request PDF. https://www.researchgate.net/publication/298800814_Understanding_the_Hydrogen_Bonds_in_Ionic_Liquids_and_Their_Roles_in_Properties_and_Reactions. Accessed 28 Apr 2025.
- 12.Akbarzadeh Gondoghdi P, Khorsandi M, Mokhtarpour M, et al. Effect of 2-hydroxyethylammonium carboxylate protic ionic liquids on the solubility and cytotoxicity of indomethacin. BMC Chem. 2024;18:109. 10.1186/s13065-024-01212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Protic Ionic Liquids. Evolving Structure-Property Relationships and Expanding Applications| Request PDF. ResearchGate. 10.1021/acs.chemrev.5b00158 [DOI] [PubMed]
- 14.Stream Structure and Nanostructure in Ionic Liquids| Chemical Reviews. https://pubs.acs.org/doi/10.1021/cr500411q. Accessed 29 Apr 2025. [DOI] [PubMed]
- 15.Thermophysical Properties of Amino Acid-. Based Ionic Liquids| Request PDF. ResearchGate. 10.1021/je900660x
- 16.Synthesis and Thermophysical Properties of Biocompatible Cholinium-Based. Amino acid ionic liquids| request PDF. ResearchGate. 10.1021/je301103d
- 17.Synthesis and Characterization of the Choline-. Amino Acid Ionic Liquids [Ch][Ala], [Ch]2[Glut], and [Ch][Threo] and Their Aqueous Binary Solutions| Journal of Chemical & Engineering Data. https://pubs.acs.org/doi/10.1021/acs.jced.5c00014. Accessed 29 Apr 2025.
- 18.Do Ionic Liquids Exhibit the Required. Characteristics to Dissolve, Extract, Stabilize, and Purify Proteins? Past-Present-Future Assessment| Chemical Reviews. https://pubs.acs.org/doi/full/10.1021/acs.chemrev.3c00551. Accessed 29 Apr 2025. [DOI] [PMC free article] [PubMed]
- 19.Toward a Circular Economy of Heteroatom Containing Plastics. A Focus on Heterogeneous Catalysis in Recycling| Langmuir. https://pubs.acs.org/doi/abs/10.1021/acs.langmuir.4c04015. Accessed 29 Apr 2025. [DOI] [PubMed]
- 20.Volumetric and Spectroscopic Analysis of Betaine Hydrochloride–Protic Ionic Liquid Interaction in Aqueous Solution| Journal of Chemical & Engineering Data. https://pubs.acs.org/doi/10.1021/acs.jced.4c00269. Accessed 29 Apr 2025.
- 21.Chen Z, Zhang L, Guo Y, et al. Molecularly engineer protic ionic liquids for simultaneous dissolution of silk fibroin/cellulose and Wet-Spinning composite fibers Preparation through Hydrogen-Bonding-Acceptor-Strengthening strategy. ACS Sustainable Chemistry & Engineering; 2024. 10.1021/acssuschemeng.4c00560.
- 22.(2025) Systematic computational prediction and experimental confirmation of amino acid-based natural deep eutectic solvents for removal of sterically hindered trisulfur. Chem Eng Res Des 216:270–81. 10.1016/j.cherd.2025.03.002
- 23.MLR Data-Driven for the Prediction of Infinite Dilution. Activity Coefficient of Water in Ionic Liquids (ILs) Using QSPR-Based COSMO Descriptors| Journal of Chemical Information and Modeling. https://pubs.acs.org/doi/full/10.1021/acs.jcim.4c02095. Accessed 29 Apr 2025. [DOI] [PMC free article] [PubMed]
- 24.Effects of Conformational Distributions on Sigma Profiles. in COSMO Theories| Request PDF. ResearchGate. 10.1021/jp053859h [DOI] [PubMed]
- 25.Morsali MA, Shekaari H, Golmohammadi B. Hydration behavior of L-proline in the presence of mono, bis, tris-(2-hydroxyethyl) ammonium acetate protic ionic liquids: thermophysical properties. Sci Rep. 2024;14:27229. 10.1038/s41598-024-77341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COSMO-RS σ Profiles - DDBST GmbH. https://www.ddbst.com/ddb-sigma.html. Accessed 29 Apr 2025.
- 27.(2025) Volumetric and viscometric study of caffeine in aqueous D-sorbitol solutions. J Chem Thermodyn 204:107445. 10.1016/j.jct.2024.107445
- 28.Le Donne A, Bodo E. Cholinium amino acid-based ionic liquids. Biophys Rev. 2021;13:147–60. 10.1007/s12551-021-00782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaikh MM, Zodape SP, Patel V, Shaikh VR. Thermodynamic studies of molecular interactions between local anesthetical drug (Procaine Hydrochloride) and amino acid (Glycine) in aqueous solutions at T (= 288.15–318.15 K). J Chem Eng Data. 2025. 10.1021/acs.jced.4c00743. [Google Scholar]
- 30.(2025) Apparent molar volumes of isoprene and limonene in dense-phase CO2. J Supercrit Fluids 221:106544. 10.1016/j.supflu.2025.106544
- 31.Devi S, Chakraborty N, Juglan KC. Ultrasonic, volumetric, and spectroscopic study of 2-Phenoxyethanol with sodium L-Ascorbyl-2-Phosphate/Magnesium ascorbate in aqueous solutions at varying temperatures. Phys Scr. 2025;100:055022. 10.1088/1402-4896/adce44. [Google Scholar]
- 32.Patel V, Jain A, Chandak AS, Zodape SP. Study of molecular interactions between potassium chloride and ionic liquid 1-Methyl-3-propylimidazolium iodide in aqueous solutions at different temperatures: A thermodynamic approach. J Chem Eng Data. 2025. 10.1021/acs.jced.4c00750. [Google Scholar]
- 33.Fluid Viscosity -. an overview| ScienceDirect Topics. https://www.sciencedirect.com/topics/engineering/fluid-viscosity. Accessed 29 Apr 2025.
- 34.The viscosity B. and D coefficient (Jones–Dole equation) studies in aqueous solutions of alkyltrimethylammonium bromides at 298.15 K| request PDF. ResearchGate. 10.1016/j.molliq.2014.11.003
- 35.Impact of Carboxylate Anion Chain Length and Temperature on Physicochemical Properties of Butyrolactam-. Based Protic Ionic Liquids| Journal of Chemical & Engineering Data. https://pubs.acs.org/doi/abs/10.1021/acs.jced.4c00651. Accessed 29 Apr 2025.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the manuscript, figures, tables, and supporting information files.