Abstract
Background
While the technical aspects of histotripsy have seen significant advancements, research on Magnetic Resonance Imaging (MRI) monitoring remains limited.
Purpose
This preliminary study explored the use of conventional T2‐Weighted (T2‐W) Turbo Spin Echo (TSE) imaging to monitor histotripsy lesions in a pure agar gel, as a potential research tool for MRI‐guided histotripsy (MRgHt).
Methods
Histotripsy experiments were conducted in a 3T MRI scanner using a 2% weight per volume agar solution in water. Pulsed Focused Ultrasound (FUS) was applied at a duty factor of 2%, with a pulse repetition period of 1s, delivering up to 2000 pulses. T2‐W TSE imaging was employed for intra‐procedural monitoring with 10s updates and post‐sonication assessment, with the first prioritizing rapid acquisition and the second focusing on resolution. Supplementary, MR thermometry tracked temperature variations throughout the procedure.
Results
Dynamic T2‐W TSE imaging provided insights into the progress of phantom disruption. Mild thermal effects served as early signs of phantom response, as mechanical effects required more time to manifest. Typical histotripsy lesion characteristics were observed, including a hyperintense core with an elongated shape and a thin hypointense border. The developed temperatures remained well below hyperthermia levels, reaching a maximum of 34°C.
Conclusions
The presented methodology, utilizing a pure agar gel within a simple, versatile setup alongside conventional T2‐W TSE imaging, holds promise for advancing MRgHt research.
Keywords: agar gel, histotripsy, lesion monitoring, MRI, T2‐W
1. INTRODUCTION
Histotripsy, developed about two decades ago, is an innovative technology that employs pulsed focused ultrasound (FUS) to noninvasively fractionate tissue to the subcellular level through controlled acoustic cavitation mechanisms. 1 , 2 Different types of this modality, from intrinsic threshold histotripsy to boiling histotripsy (BH), use short, high‐amplitude FUS pulses at a low duty cycle to drive bubble activity and tissue fractionation, with variations in the pulse duration (µs to ms) and peak acoustic pressure required for the desired bubble dynamics. 3 Tissue fragmentation occurs when FUS pressure exceeds the cavitation threshold inherent to each tissue, creating precise treatment zones with well‐defined boundaries. Ex vivo and in vitro tissue models have been crucial tools for investigating the mechanisms of histotripsy, particularly to understand how different sonication parameters and tissue characteristics impact the cavitation threshold 4 , 5 and volume of tissue liquefaction. 6 , 7 Animal models have been used to explore the potential of histotripsy for treating various conditions, including pancreatic, 8 renal, 9 bone, 10 breast, 11 and prostate 12 tumors. Notably, as studies established the effectiveness of this approach, it received the U.S. Food and Drug Administration (FDA) approval for liver tumor treatment. 13
Although excised tissue and animal models are valuable for simulating tissue responses, they are costly and raise ethical concerns when used repeatedly. As an alternative, tissue‐mimicking phantoms (TMPs) offer a beneficial controlled environment for preliminary testing, prior to using excised tissue or live subjects. 4 , 6 , 7 In this context, agar gels have been employed to examine the relationship between the mechanical properties of the host medium and induced cavitation dynamics, focusing on both the threshold and extent of mechanical fractionation of the treated medium. 4 , 14 , 15
The cost‐effectiveness and widespread adoption of advanced ultrasound imaging modalities for therapy guidance, including contrast‐enhanced ultrasound 16 and shear wave elastography, 17 have also been demonstrated in histotripsy applications. US‐based techniques offer valuable real‐time monitoring capabilities and are more accessible and cost‐effective than Magnetic Resonance Imaging (MRI). However, despite their advantages, US modalities can face limitations in certain contexts, such as reduced image quality due to acoustic interference from cavitation during histotripsy, and challenges in imaging anatomically complex or air‐encased regions (e.g., the brain). MRI, while more expensive, provides superior soft‐tissue contrast, is less susceptible to acoustic artifacts, and enables imaging in these challenging environments.
During histotripsy procedures, B‐mode US imaging is commonly used for real‐time monitoring of cavitational effects, 2 as it helps visualize the cavitational bubbles created, which appear as bright (hyperechoic) areas. 18 , 19 Post‐sonication US imaging assessment shows fractionated tissue as darker (hypoechoic) regions, with their extent correlating to the amount of liquefied tissue. 20 , 21 , 22 However, significant variation in the grayscale appearance of ablation zones across individuals makes it difficult to establish standardized methods for quantifying tissue liquefaction with this modality. 23 , 24 Diagnostic US may also struggle to visualize abdominal tumors, especially deeply situated or obscured ones, due to factors like obesity or bowel gas. 2 It also has limitations in providing quantitative therapy feedback. 23 The integration of Computed Tomography (CT) and MRI into histotripsy has thus become increasingly indispensable over time. 2 , 24 MRI offers a clear advantage as it is non‐ionizing, provides superior soft tissue contrast, and is better at assessing the tumor's location and spread. 25 Thermometry is another key feature of MRI, 26 essential for confirming that temperatures stay below ablative limits in conventional histotripsy or reach desired levels in BH. Accordingly, the role of CT was mostly restricted to enhancing US guidance through image fusion with pre‐procedure scans. 2
A preliminary ex vivo study on MRI‐guided Histotripsy (MRgHt) 27 revealed that by varying the timing of cavitation events relative to the imaging gradients in a motion‐sensitive Gradient Echo (GRE) sequence, images can be selectively tuned for sensitivity or insensitivity to cavitation. In the context of BH, an early study by Hoogenboom et al. 28 in a mouse tumor model reported BH‐induced lesions appearing hyperintense with a surrounding hypointense rim on T2‐weighted Turbo Spin Echo (T2‐W TSE) imaging. T1‐weighted echo planar imaging (T1‐W EPI) thermometry served as a monitoring tool to evaluate and adjust focal heating intraprocedurally. 28 With a high correlation between T2‐W imaging and histological staining outcomes, the study suggested the use of this sequence for intraprocedural and postprocedural assessment. 28
A year later, a follow‐up study 29 expanded on these outcomes, achieving effective mechanical fragmentation in different mouse tumor models. During the same period, Eranki et al. 30 investigated how sonication parameters affect the resulting BH lesion characteristics in ex vivo tissues. The treatment was planned with T2‐W GRE imaging, and a 2D EPI pulse sequence was used to map the temperature in real time, ensuring precise heating. After treatment, additional MRI scans using T2W and T1‐W GRE sequences were conducted to evaluate the damage. 30 Meanwhile, another study investigating intrinsic threshold histotripsy in in vitro porcine tissues 31 suggested that MRI contrast changes from tissue alterations could provide a noninvasive method for tracking treatment effects. T2 sensitivity to histotripsy varied across tissues in both the magnitude and onset of change, with a close correlation to the actual extent of tissue damage.
In the 2020s, Lu et al. 32 demonstrated in vivo MRgHt of pig brains through a human skull, showing consistent tissue ablation despite the lack of pre‐treatment targeting and intraprocedural monitoring. More recently, investigations in transcranial histotripsy 33 , 34 have employed MRI‐based thermometry and acoustic radiation force imaging for precise beam targeting, alongside the development of dedicated FUS systems. 35 , 36 Although MRI‐based monitoring in histotripsy remains scarce, these studies illustrate its emerging role, especially in complex regions like the brain. Generally, the inherent challenges of US propagation through the skull position the brain as a key anatomical target driving the advancement of MRgHt.
Although significant advancements have been made in the technical and preclinical aspects of image‐guided histotripsy, considerable work remains in MRgHt, particularly in terms of intraprocedural monitoring of histotripsy‐induced tissue disruption. To date, no studies in this field have directly monitored lesion formation intraprocedurally. The literature on this topic is limited, with no studies identified in phantoms, despite this being a crucial step in advancing MRgHt applications towards in vivo use. To support progress in this field, the current study explored the use of conventional T2‐W TSE imaging both intra‐ and post‐procedurally to track histotripsy lesions in a pure agar gel, using established ultrasonic parameters. This study builds upon a previous work that introduced this phantom model, 37 , 38 now utilizing it as a tool to investigate MRgHt, with a focus on the feasibility of dynamically monitoring histotripsy effects.
2. MATERIALS AND METHODS
2.1. Histotripsy phantom preparation
A pure agar gel, proven to be susceptible to cavitation‐induced damage, served as the target for all sonications. The gel was prepared with a 2% agar concentration (Merck KGgA, Darmstadt, Germany), dissolved in water, according to the procedure outlined in previous literature. 39 The fabrication process was straightforward, involving a heating and a cooling cycle, with continuous automated stirring to ensure a homogeneous environment for histotripsy experiments. The agar gel was molded in a dedicated plastic container with dimensions of 10 cm (width) × 20 cm (length) × 10 cm (height), featuring handles for attachment to the rest of the setup and allowing for the removal of its bottom surface for bottom‐up sonications. Prior to the experiments, the phantom, as embedded in the mold, was degassed in a vacuum chamber (VC2523AG, Vacuum Chambers, Jodlowa, Poland) using a dual‐stage vacuum pump (VP260, Vacuum Chambers) at a pressure of −1 bar. The density of pure 2% agar gel was measured at 901 ± 3 kg/m3 (n = 10), while its Young's modulus has been reported to be around 40 kPa. 40
2.2. Setup for phantom sonication within MRI scanner
The setup for phantom sonications in the MRI scanner (3T, Magnetom Vida, Siemens Healthineers, Erlangen, Germany) employed a standard water tank arrangement, as shown in Figure 1a. The tank included integrated compartments to securely hold the transducer beneath the phantom holder, which had an open bottom (acoustic window) for ultrasonic delivery. Ruler‐type components with an engraved scale allowed precise 1 mm step adjustments of the transducer's position left‐right and up‐down. Additionally, the phantom could be moved front‐back, thus having full 3‐axis control for accurate adjustment of the focal point within the phantom. An 18‐channel coil (Body_18 Ultraflex Large Coil, Siemens Healthineers) was mounted above the tank to facilitate imaging.
FIGURE 1.
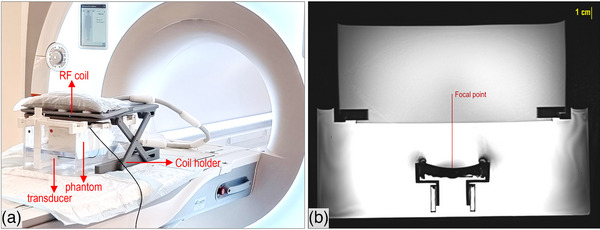
(a) Experimental setup for phantom histotripsy experiments in the MRI setting, and (b) Axial T2‐W TSE image of the setup, showing the location from the transducer to the focal point within the phantom. MRI, Magnetic Resonance Imaging.
2.3. Histotripsy protocol
Sonications were performed using a single‐element concave transducer with a nominal frequency of 2.75 MHz, a 50 mm aperture diameter, a 65 mm focal radius of curvature, and an efficiency of 30%. Pulsed FUS was applied with a duty factor (DF) of 2% and a pulse repetition period (PRP) of 1s, based on prior experimental findings. 41 The employed amplifier (AG1016, AG Series Amplifier, T & C Power Conversion, Inc., New York, USA) was set to deliver 450 W, corresponding to an effective acoustic power of 135 W. This translated to a spatial peak pulse average intensity (SPPA) of 29.4 kW/cm2 and a spatial peak temporal average intensity (SPTA) of 587 W/cm2. A total of 1000 to 2000 pulses were delivered at a focal depth (FD) of 2.5 cm.
2.4. Assessment of histotripsy effects
Histotripsy‐induced structural changes in the phantom were tracked during sonication through the acquisition of axial T2‐W TSE images at 10s intervals with: Repetition time (TR) = 2000 ms, Echo time (TE) = 52 ms, number of averages (NEX) = 1, Flip angle (FA) = 110°, Matrix size = 192 × 192, Slice thickness (ST) = 4 mm, Echo train length (ETL) = 30, Pixel bandwidth (pBW) = 250 Hz/pixel, and Field of view (FOV) = 260 × 260 mm2. The selected sequence parameters represent a balance between image quality, considering both resolution and signal‐to‐noise ratio (SNR), and acquisition time. The chosen acquisition time of 10 s per image, corresponding to 10 FUS pulses, was considered sufficiently short given the inherently slow nature of the process, where this duration is generally insufficient to produce observable outcomes. Proton Resonance Frequency (PRF) shift thermometry enabled monitoring the developed temperatures with a temporal resolution of 2.4s, utilizing Fast Low Angle Shot (FLASH) imaging with the following parameters: TR = 25 ms, TE = 10 ms, NEX = 1, FA = 30°, Matrix size = 96 × 96, ST = 6 mm, ETL = 1, pBW = 250 Hz/pixel, and FOV = 280 × 280 mm2. Thermal maps were generated during sonication and for approximately 250s afterward. Notably, the MR thermometry method and reconstruction software 42 employed in this study have been previously validated by our group in multiple phantom studies related to MR‐guided FUS for thermal ablation. In those studies, MR‐derived temperature maps showed strong agreement with direct thermocouple readings, supporting the reliability of the current approach for precisely capturing temperature changes during sonication. 43
Coronal and axial T2‐W TSE scans were repeated post‐sonication using a modified sequence to enhance SNR and resolution. This was achieved by increasing the TR to 2600 ms and NEX to 2, as well as using a larger matrix size of 256 × 256, while keeping all other parameters unchanged. A representative axial T2‐W TSE scan acquired with this sequence is shown in Figure 1b, which served as a reference for checking/adjusting the focal point location, ensuring proper coupling (i.e., absence of bubbles), and facilitating comparison with post‐sonication images. Quantitative analysis was performed by calculating CNR as , where represents the signal intensity measured in regions of interest (ROIs) within the lesion and phantom and is the standard deviation of the signal measured in an ROI placed in air. Lesion dimensions were also extracted.
Simultaneous or interleaved acquisition of T2W TSE and FLASH sequences was not feasible due to their fundamentally different sequence requirements. Consequently, separate sonications were performed at distinct phantom locations, with either T2W TSE or MR thermometry data collected exclusively in each experiment. Sonication parameters were kept identical across conditions, and the phantom's repeatable response under controlled settings ensured consistent and comparable results.
3. RESULTS
Intraprocedural T2‐W TSE imaging successfully captured histotripsy‐induced phantom changes over time, as shown in the indicative outcomes in Figures 2 and 3. Figure 2 presents the first 10 images acquired following activation of the amplifier ( = 29.4 kW/cm2, PRP = 1 s, DF = 2%, 2000 pulses, FD = 2.5 cm), alongside the reference image. Examination of the first image obtained at 10s revealed that the SNR at the sonicated ROI was nearly identical to that of the surrounding non‐sonicated phantom region (∼170). During this time, only about 10 pulses were delivered, which is typically insufficient to produce visible mechanical changes in the agar. It is also noted that the SNR measured in the reference image was notably higher (∼290), due to noise introduced by activation of the FUS system. The first clear indication of thermally induced hypointensity (onset of mild heating) was observed in the fourth image of the series, around 40s after initiation of the sonication.
FIGURE 2.

Axial T2‐W TSE images showing the first 10 scans acquired following amplifier activation ( = 29.4 kW/cm2, PRP = 1s, DF = 2%, 2000 pulses), alongside the pre‐sonication reference image. DF, duty factor; PRP, pulse repetition period; SPPA, spatial peak pulse average intensity; T2‐W TSE, T2‐weighted turbo echo spin.
FIGURE 3.

Selected axial T2‐W TSE images from a series of scans, spaced at regular intervals throughout the sonication session ( = 29.4 kW/cm2, PRP = 1 s, DF = 2%, 2000 pulses). DF, duty factor; PRP, pulse repetition period; SPPA, spatial peak pulse average intensity; T2‐W TSE, T2‐weighted turbo echo spin.
Histotripsy lesion formation occurred gradually and cumulatively. A selection of axial T2‐W TSE images from the entire series of scans, spaced at regular intervals throughout the sonication session, is shown in Figure 3, demonstrating the progression of the entire process.
Progressive changes in signal intensity are visible across the imaging sequence, reflecting the dynamic response of the phantom and lesion evolution. Note that post‐sonication images (> 2000s) are also shown, clearly delineating the formed lesions, with hypointensity associated with thermal changes being resolved.
Figure 4a presents the changes in CNR observed over the course of the sonication process. Negative CNR values at early time points indicate hypointense regions caused by mild heating. As cavitation effects progress, CNR increases, reflecting lesion formation and hyperintense signal development. The CNR curve continues to increase following the completion of sonication, which is attributed to the resolution of thermal effects.
FIGURE 4.

(a) CNR measured from T2‐W TSE images of the agar phantom during histotripsy sonication. (b) Focal temperature changes during and shortly after sonication ( = 29.4 kW/cm2, PRP = 1s, DF = 2%, 2000 pulses), c) Color‐coded thermal map produced upon completion of sonication, and (c) Corresponding thermal dose map. DF, duty factor; PRP, pulse repetition period; SPPA, spatial peak pulse average intensity; T2‐W TSE, T2‐weighted turbo echo spin.
Thermal mapping data confirmed that the temperatures reached with the highest tested pulse count of 2000 and a PRP of 1s remained consistently below clinically relevant hyperthermia thresholds (< 45°C) throughout the procedure. According to the recorded focal temperature profile, shown in Figure 4b, the temperature initially increased rapidly from 19°C to above 30°C within 3 min, then rose more gradually, almost plateauing at a maximum temperature of 34.5°C (ΔT = 15.5°C). This corresponds to an accumulated thermal dose well below ablative levels (7.64 × 10− 5 CEM43°C), as indicated by the corresponding thermal dose map in Figure 4c.
A higher‐resolution T2‐W TSE image acquired afterward is shown in Figure 5a, with corresponding coronal views at two cross‐sections of the formed lesion shown in Figure 5b. This figure shows the characteristic appearance of histotripsy lesions on the proposed phantom in T2‐W images, with hyperintense signal areas (elongated with a slightly widened tail) surrounded by a hypointense border. Figure 5c shows a photo of the actual lesion's cross‐section at the FD, taken after phantom dissection. The mean lesion diameter from three identical sonications ( = 29.4 kW/cm2, PRP = 1 s, DF = 2%, 2000 pulses, FD = 2.5 cm) was measured as 2.4 mm on MRI scans. The measurement was in close agreement with the actual lesion size of 2.3 mm, measured using a caliper.
FIGURE 5.

(a) High‐resolution T2‐W TSE axial image showing the inflicted histotripsy lesion ( = 29.4 kW/cm2, PRP = 1s, DF = 2%, 2000 pulses) following resolution of thermal effects, (b) Coronal slices at two different cross‐sections (1 and 2) of the lesion, and (c) Photo of the actual lesion after phantom cross‐sectioning at FD. DF, duty factor; FD, focal depth; PRP, pulse repetition period; SPPA, spatial peak pulse average intensity; T2‐W TSE, T2‐weighted turbo echo spin.
Importantly, the employed sequence effectively tracked changes in lesion dynamics in response to variations in ultrasonic parameters, further validating the phantom's ability to reflect these changes. Examples of the effects of pulse number and PRP ( = 29.4 kW/cm2 and DF = 2%) on lesion length and diameter are shown in Figures 6a and 6b, respectively. The T2‐W TSE sequence effectively captured protocol‐dependent differences, revealing visually distinct lesion patterns across the three conditions. Quantitative analysis in Figure 6c confirms these visual differences, demonstrating variation in CNR and lesion area among the protocols. Finally, Figure 6d presents T2‐W TSE images of lesions produced at varying pulse numbers ( = 29.4 kW/cm2 and DF = 2%, PRP = 1) alongside a thermal lesion from continuous FUS exposure at 200 W for 60 s (red arrow). The panel illustrates lesion appearance over 30 min, showing resolution of the thermal lesion while histotripsy‐induced lesions persisted.
FIGURE 6.

(a) High‐resolution T2‐W TSE axial image showing histotripsy lesions created with different protocols: [1] pulse numbers (N) = 2000 and PRP = 1, [2] N = 1000 and PRP = 1, [3] N = 2000 and PRP = 0.5 ( = 29.4 kW/cm2 and DF = 2%), (b) Corresponding coronal slice revealing lesion diameter, with the cross‐section location indicated by the red dotted line in the axial view, (c) Contrast‐to‐noise ratio between lesion and background, and lesion area measured in axial plane. (d) T2‐W TSE images acquired at 5‐, 10‐, 15‐, and 30 min post‐sonication ( = 29.4 kW/cm2 and DF = 2%, PRP = 1) showing lesions formed by histotripsy at pulse counts of [4] N = 1000, [5] N = 750, [6] N = 500, and [7] N = 250, compared to a thermal lesion (200 W, 60 s) indicated by the red arrow. DF, duty factor; PRP, pulse repetition period; SPPA, spatial peak pulse average intensity; T2‐W TSE, T2‐weighted turbo echo spin.
4. DISCUSSION
MRI has been proven effective for analyzing histotripsy‐induced bioeffects, including the success of tissue destruction and lesion characterization, though not employed for monitoring these changes intraprocedurally. In this context, there is a lack of literature reports on MRgHt using hydrogel phantoms, as evidenced by the scarcity of published MRI images of histotripsy lesions in these models. Building on previous studies that established agar gels as tools for MRI‐guided FUS research, 44 , 45 , 46 this study demonstrates the use of conventional MRI to track histotripsy lesion formation in this model.
The use of a pure 2% w/v agar gel provides a straightforward and cost‐effective model, easily manufactured without specialized equipment. It offers a homogeneous environment ensuring consistent sonication results and reproducibility across different trials. Its Young's modulus (∼40 kPa) falls within the upper range of soft tissue stiffness, which spans from approximately 1 kPa for very soft tissues like the brain to 500–1000 kPa for stiffer structures and is quite above that of muscle tissue. 47 Importantly, the phantom was confirmed to be responsive to histotripsy, demonstrating tissue‐like visibility in T2‐W TSE scans. This aligns with its previously measured T2 relaxation time of about 46 ms (at 3T), 37 which is comparable to values seen in muscle, liver, and kidney. 48 Although this simple phantom model does not fully replicate the complexity of biological tissues, it provides a consistent and reproducible testing tool for MRgHt preclinical research.
The employed histotripsy protocol resulted in the formation of a liquefied core within the phantom, which appeared hyperintense on T2‐W images due to an increase in its T2 relaxation time. This corresponds to the area of complete tissue fragmentation seen in biological tissue, where a surrounding hypointense rim has been associated with a transition zone containing apoptotic cells. 28 , 30 Likewise, a thin hypointense border was observed around the phantom lesions, which remained unchanged over time, indicating that it was not related to temporary thermal effects. This visible characteristic suggests a local increase in gel density at the border, likely caused by compaction due to intense mechanical stress.
T2‐W TSE imaging, with carefully selected parameters balancing temporal resolution with data acquisition constraints and sufficient image quality, effectively monitored histotripsy‐induced signal intensity changes in the phantom, demonstrating the progressive formation of lesions (Figure 3). Updates on histotripsy outcomes were provided every 10s, which is considered reasonably high given the slow pace of the histotripsy process. At this point, it is important to clarify that mild thermal effects served as the initial indicators of accurate targeting and the extent of the affected area, while mechanical outcomes required more time to manifest. These thermal effects, observed as hypointense features, served as early signs of phantom response and resolved within a few minutes post‐sonication. The results further confirmed the utility of the employed conventional sequence in monitoring lesion dynamics as a function of varying sonication parameters (Figure 6).
The results demonstrate that histotripsy‐induced lesions remained stable post‐sonication, whereas the thermal lesion produced by continuous high‐power FUS resolved within the first 10 min (Figure 6). Quantitative analysis of the lesion region's dynamic signal changes further supports this distinction (Figure 4). Initially, negative CNR values were observed, attributed to the mild thermal effects causing hypointense regions. Over time, the CNR gradually increased as mechanical disruption from histotripsy led to hyperintense lesion formation. Notably, this increase continued even after sonication ended, reflecting the fading of thermal effects and the clearer emergence of the hyperintense lesion core. These findings, along with thermometry data (Figure 4) collectively support that histotripsy lesions are due to mechanical tissue disruption rather than transient thermal effects.
Previous studies employing conventional or advanced MRI sequences to investigate ultrasound‐mediated microbubble cavitation have primarily focused on bubble dynamics, particularly in contexts such as blood‐brain barrier opening 49 and histotripsy. 50 For example, a recent study demonstrated that MRI sequences with sensitizing gradients could capture individual histotripsy bubble clouds in agar gels, focusing on bubble detection rather than lesion monitoring. 50 In contrast, our work centers on monitoring histotripsy lesion formation in a homogeneous agar phantom and examining the MRI‐visible response to variations in histotripsy protocol. While agar phantoms have been used in previous histotripsy research, MRI has typically been restricted to thermal monitoring through MR thermometry or endpoint evaluation, without capturing the dynamic mechanical disruption process. 33 , 34 This study presents a proof of concept for using conventional T2‐W MRI and simple agar phantoms to enable real‐time monitoring and quality assurance in MRgHt.
When it comes to limitations, ex vivo tissues offer greater biological relevance; however, phantoms remain indispensable tools in the development and standardization of image‐guided ablation techniques. The phantom proposed herein provides immediate visual feedback on the effects of varying pulse parameters, facilitating tuning of treatment protocols. Although the phantom material does not fully replicate the acoustic and mechanical properties of biological tissue; limiting direct extrapolation of lesion size, shape, or treatment thresholds, it is critical for assessing relative parameter sensitivity and establishing parameter–response relationships. Furthermore, the phantom offers a controlled, reproducible, and accessible platform for systematically evaluating MRgFUS system performance, particularly in early‐stage development. It enables quality assurance through repeatable testing of system output, targeting accuracy, spatial fidelity, and temporal control, free from the confounding variables inherent to biological tissues such as heterogeneity. This reproducibility supports cross‐platform comparisons and regulatory approval processes, further enhancing their value for testing and refining MRI monitoring sequences.
The next step will involve performing robotically assisted grid sonications to assess the ability to disrupt a larger area of the phantom under T2‐W TSE imaging monitoring. Another important future direction would be to assess the volumetric evolution of lesions during sonication. Such analysis could provide deeper insights into lesion formation dynamics and improve understanding of treatment effects. Additionally, given the inherent challenges of US propagation through the skull, which make the brain a key target for advancing MRgHt, future work may incorporate ex vivo skull models to investigate transcranial applications.
5. CONCLUSIONS
Overall, the approach demonstrated in this study, utilizing pure agar gel within a compact, versatile MRI‐compatible setup and conventional T2‐W imaging, holds significant potential for advancing preclinical MRgHt research, a field that has lagged behind. The results confirm the feasibility of the employed sequence for tracking histotripsy‐induced effects in the phantom with reasonable temporal and spatial accuracy. The supplementary use of MR thermometry is beneficial for monitoring thermal effects and ensuring proper targeting. The next step will involve performing robotically assisted grid sonications to assess the ability to disrupt a larger area of the phantom under T2‐W TSE imaging monitoring.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The study was funded by the Recovery and Resilience Facility of the NextGenerationEU instrument, through the Research and Innovation Foundation (RIF) of Cyprus, under the project BRAINSONIC (ENTERPRISES/0223/Sub‐Call1/0057).

Authors would also like to express their sincere gratitude to Dr. Samuel Pichardo for providing the MR thermometry code, which was integrated into the MRI‐guided Focused Ultrasound software used in this study.
Antoniou A, Evripidou N, Georgiou L, et al. Magnetic Resonance Imaging monitoring of histotripsy effects in agar phantom. Med Phys. 2025;52:e18054. 10.1002/mp.18054
REFERENCES
- 1. Xu Z, Hall TL, Vlaisavljevich E, Lee FT. Histotripsy: the first noninvasive, non‐ionizing, non‐thermal ablation technique based on ultrasound. Int J Hyperthermia. 2021;38(1):561‐575. doi: 10.1080/02656736.2021.1905189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kisting MA, Jentink MS, Wagner MG, et al. Imaging for targeting, monitoring, and assessment after histotripsy: a non‐invasive, non‐thermal therapy for cancer. EMJ Radiology. doi: 10.33590/emjradiol/10308529 [DOI] [Google Scholar]
- 3. Williams RP, Simon JC, Khokhlova VA, Sapozhnikov OA, Khokhlova TD. The histotripsy spectrum: differences and similarities in techniques and instrumentation. Int J Hyperthermia. 2023;40(1):2233720. doi: 10.1080/02656736.2023.2233720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vlaisavljevich E, Lin KW, Maxwell A, et al. Effects of ultrasound frequency and tissue stiffness on the histotripsy intrinsic threshold for cavitation. Ultrasound Med Biol. 2015;41(6):1651‐1667. doi: 10.1016/j.ultrasmedbio.2015.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vlaisavljevich E, Gerhardson T, Hall T, Xu Z. Effects of f ‐number on the histotripsy intrinsic threshold and cavitation bubble cloud behavior. Phys Med Biol. 2017;62(4):1269‐1290. doi: 10.1088/1361-6560/aa54c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu J, Bigelow TA, Lee H. Effect of pulse repetition frequency and scan step size on the dimensions of the lesions formed in agar by HIFU histotripsy. Ultrasonics. 2013;53(4):889‐896. doi: 10.1016/j.ultras.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 7. Xu J, Bigelow TA. Experimental investigation of the effect of stiffness, exposure time and scan direction on the dimension of ultrasound histotripsy lesions. Ultrasound Med Biol. 2011;37(11):1865‐1873. doi: 10.1016/j.ultrasmedbio.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 8. Hendricks‐Wenger A, Sereno J, Gannon J, et al. Histotripsy ablation alters the tumor microenvironment and promotes immune system activation in a subcutaneous model of pancreatic cancer. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2987‐3000. doi: 10.1109/TUFFC.2021.3078094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knott EA, Swietlik JF, Longo KC, et al. Robotically‐assisted sonic therapy for renal ablation in a live porcine model: initial preclinical results. J Vasc Interv Radiol. 2019;30(8):1293‐1302. doi: 10.1016/j.jvir.2019.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnold L, Hendricks‐Wenger A, Coutermarsh‐Ott S, et al. Histotripsy ablation of bone tumors: feasibility study in excised canine osteosarcoma tumors. Ultrasound Med Biol. 2021;47(12):3435‐3446. doi: 10.1016/j.ultrasmedbio.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hendricks AD, Howell J, Schmieley R, et al. Histotripsy initiates local and systemic immunological response and reduces tumor burden in breast cancer. J Immunol. 2019;202(1):194.30‐194.30. doi: 10.4049/jimmunol.202.Supp.194.30 30455398 [DOI] [Google Scholar]
- 12. Schade GR, Keller J, Ives K, et al. Histotripsy focal ablation of implanted prostate tumor in an ace‐1 canine cancer model. J Urol. 2012;188(5):1957‐1964. doi: 10.1016/j.juro.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu Z, Khokhlova TD, Cho CS, Khokhlova VA. Histotripsy: a method for mechanical tissue ablation with ultrasound. Annu Rev Biomed Eng. 2024;26(1):141‐167. doi: 10.1146/annurev-bioeng-073123-022334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlaisavljevich E, Maxwell A, Warnez M, Johnsen E, Cain CA, Xu Z. Histotripsy‐induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61(2):341‐352. doi: 10.1109/TUFFC.2014.6722618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hendley SA, Bollen V, Anthony GJ, Paul JD, Bader KB. In vitro assessment of stiffness‐dependent histotripsy bubble cloud activity in gel phantoms and blood clots. Phys Med Biol. 2019;64(14):145019. doi: 10.1088/1361-6560/ab25a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trivedi VV, Wallach EL, Bader KB, Shekhar H. Contrast‐Enhanced imaging of histotripsy bubble clouds using chirp‐coded excitation and volterra filtering. IEEE Trans Ultrason Ferroelectr Freq Control. 2023;70(9):989‐998. doi: 10.1109/TUFFC.2023.3289918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang TY, Hall T, Xu Z, Fowlkes J, Cain C. Imaging feedback of histotripsy treatments using ultrasound shear wave elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2012;59(6):1167‐1181. doi: 10.1109/TUFFC.2012.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121(6):742‐749. doi: 10.1161/CIRCULATIONAHA.109.889071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khokhlova TD, Wang YN, Simon JC, et al. Ultrasound‐guided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model. Proc Natl Acad Sci. 2014;111(22):8161‐8166. doi: 10.1073/pnas.1318355111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khokhlova TD, Schade GR, Wang YN, et al. Pilot in vivo studies on transcutaneous boiling histotripsy in porcine liver and kidney. Sci Rep. 2019;9(1):20176. doi: 10.1038/s41598-019-56658-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall T, Fowlkes J, Cain C. A real‐time measure of cavitation induced tissue disruption by ultrasound imaging backscatter reduction. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(3):569‐575. doi: 10.1109/TUFFC.2007.279 [DOI] [PubMed] [Google Scholar]
- 22. Khokhlova VA, Fowlkes JB, Roberts WW, et al. Histotripsy methods in mechanical disintegration of tissue: towards clinical applications. Int J Hyperthermia. 2015;31(2):145‐162. doi: 10.3109/02656736.2015.1007538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang X, Miller RM, Lin KW, et al. Real‐Time feedback of histotripsy thrombolysis using bubble‐induced color doppler. Ultrasound Med Biol. 2015;41(5):1386‐1401. doi: 10.1016/j.ultrasmedbio.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anthony GJ, Bollen V, Hendley S, Antic T, Sammet S, Bader KB. Assessment of histotripsy‐induced liquefaction with diagnostic ultrasound and magnetic resonance imaging in vitro and ex vivo. Phys Med Biol. 2019;64(9):095023. doi: 10.1088/1361-6560/ab143f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shankar L, Montanera W. Computed tomography versus magnetic resonance imaging and three‐dimensional applications. Med Clin N Am. 1991;75(6):1355‐1366. doi: 10.1016/S0025-7125(16)30392-3 [DOI] [PubMed] [Google Scholar]
- 26. Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging. 2008;27(2):376‐390. doi: 10.1002/jmri.21265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allen SP, Hall TL, Cain CA, Hernandez‐Garcia L. Controlling cavitation‐based image contrast in focused ultrasound histotripsy surgery. Magn Reson Med. 2015;73(1):204‐213. doi: 10.1002/mrm.25115 [DOI] [PubMed] [Google Scholar]
- 28. Hoogenboom M, Eikelenboom D, den Brok MH, et al. In vivo MR guided boiling histotripsy in a mouse tumor model evaluated by MRI and histopathology. NMR Biomed. 2016;29(6):721‐731. doi: 10.1002/nbm.3520 [DOI] [PubMed] [Google Scholar]
- 29. Hoogenboom M, Eikelenboom DC, van den Bijgaart RJE, et al. Impact of MR‐guided boiling histotripsy in distinct murine tumor models. Ultrason Sonochem. 2017;38:1‐8. doi: 10.1016/j.ultsonch.2017.02.035 [DOI] [PubMed] [Google Scholar]
- 30. Eranki A, Farr N, Partanen A, et al. Boiling histotripsy lesion characterization on a clinical magnetic resonance imaging‐guided high intensity focused ultrasound system. PLoS One. 2017;12(3):e0173867. doi: 10.1371/journal.pone.0173867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allen SP, Vlaisavljevich E, Shi J, et al. The response of MRI contrast parameters in in vitro tissues and tissue mimicking phantoms to fractionation by histotripsy. Phys Med Biol. 2017;62(17):7167‐7180. doi: 10.1088/1361-6560/aa81ed [DOI] [PubMed] [Google Scholar]
- 32. Lu N, Gupta D, Daou BJ, et al. Transcranial magnetic resonance‐guided histotripsy for brain surgery: pre‐clinical investigation. Ultrasound Med Biol. 2022;48(1):98‐110. doi: 10.1016/j.ultrasmedbio.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta D, Choi D, Lu N, et al. Magnetic resonance thermometry targeting for magnetic resonance–guided histotripsy treatments. Ultrasound Med Biol. 2023;49(5):1102‐1107. doi: 10.1016/j.ultrasmedbio.2022.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta D, Kaovasia TP, Komaiha M, et al. Transcranial MRI‐guided histotripsy targeting using MR‐thermometry and MR‐ARFI. Ultrasound Med Biol. 2025;51(2):330‐335. doi: 10.1016/j.ultrasmedbio.2024.10.010 [DOI] [PubMed] [Google Scholar]
- 35. Verma Y, Perera Molligoda Arachchige AS. Revolutionizing brain interventions: the multifaceted potential of histotripsy. Neurosurg Rev. 2024;47(1):124. doi: 10.1007/s10143-024-02353-9 [DOI] [PubMed] [Google Scholar]
- 36. Lu N, Hall TL, Choi D, et al. Transcranial MR‐Guided histotripsy system. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2917‐2929. doi: 10.1109/TUFFC.2021.3068113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antoniou A, Georgiou L, Christodoulou T, et al. MR relaxation times of agar‐based tissue‐mimicking phantoms. J Appl Clin Med Phys. 2022;23(5):e13533. doi: 10.1002/acm2.13533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menikou G, Damianou C. Acoustic and thermal characterization of agar based phantoms used for evaluating focused ultrasound exposures. J Ther Ultrasound. 2017;5(1):14. doi: 10.1186/s40349-017-0093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drakos T, Giannakou M, Menikou G, Constantinides G, Damianou C. Characterization of a soft tissue‐mimicking agar/wood powder material for MRgFUS applications. Ultrasonics. 2021;113:106357. doi: 10.1016/j.ultras.2021.106357 [DOI] [PubMed] [Google Scholar]
- 40. Zeng T, Chen H, Yoshitomi T, Kawazoe N, Yang Y, Chen G. Effect of hydrogel stiffness on chemoresistance of breast cancer cells in 3d culture. Gels. 2024;10(3):202. doi: 10.3390/gels10030202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou Y, Wang X. Effect of pulse duration and pulse repetition frequency of cavitation histotripsy on erosion at the surface of soft material. Ultrasonics. 2018;84:296‐309. doi: 10.1016/j.ultras.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 42. Filippou A, Georgiou A, Nikolaou A, Evripidou N, Damianou C. Advanced software for MRgFUS treatment planning. Comput Methods Programs Biomed. 2023;240:107726. doi: 10.1016/j.cmpb.2023.107726 [DOI] [PubMed] [Google Scholar]
- 43. Drakos T, Giannakou M, Menikou G, Ioannides C, Damianou C. An improved method to estimate ultrasonic absorption in agar‐based gel phantom using thermocouples and MR thermometry. Ultrasonics. 2020;103:106089. doi: 10.1016/j.ultras.2020.106089 [DOI] [PubMed] [Google Scholar]
- 44. Filippou A, Damianou C. Agar‐based phantom for evaluating targeting of high‐intensity focused ultrasound systems for breast ablation. J Med Phys. 2024;49(3):343‐355. doi: 10.4103/jmp.jmp_52_24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Partanen A, Mougenot C, Vaara T, Ebbini ES. Feasibility of agar‐silica phantoms in quality assurance of MRgHIFU. AIP Conf Proc. 2009:296‐300. doi: 10.1063/1.3131434 [DOI] [Google Scholar]
- 46. Sofokleous P, Damianou C. High‐quality agar and polyacrylamide tumor‐mimicking phantom models for magnetic resonance‐guided focused ultrasound applications. J Med Ultrasound. 2024;32(2):121‐133. doi: 10.4103/jmu.jmu_68_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu J, Zheng H, Poh P, Machens HG, Schilling A. Hydrogels for engineering of perfusable vascular networks. Int J Mol Sci. 2015;16(7):15997‐16016. doi: 10.3390/ijms160715997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bojorquez JZ, Bricq S, Acquitter C, Brunotte F, Walker PM, Lalande A. What are normal relaxation times of tissues at 3 T?. Magn Reson Imaging. 2017;35:69‐80. doi: 10.1016/j.mri.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 49. Mondou P, Mériaux S, Nageotte F, Vappou J, Novell A, Larrat B. State of the art on microbubble cavitation monitoring and feedback control for blood‐brain‐barrier opening using focused ultrasound. Phys Med Biol. 2023;68(18):18TR03. doi: 10.1088/1361-6560/ace23e [DOI] [PubMed] [Google Scholar]
- 50. Allen SP, Hernandez‐Garcia L, Cain CA, Hall TL. MR‐based detection of individual histotripsy bubble clouds formed in tissues and phantoms. Magn Reson Med. 2016;76(5):1486‐1493. doi: 10.1002/mrm.26062 [DOI] [PubMed] [Google Scholar]


