Abstract
Borrelia species are the causative agents of Lyme disease (LD) and tick-borne relapsing fever (TBRF) in humans and animals. These pathogens are transmitted through hard and soft ticks. The increasing tick population, influenced by climate change, underscores the urgent need for enhanced research on tick-borne diseases.
Iran, situated in southwestern Asia, boasts a diverse climate that supports a wide range of tick species and their vertebrate hosts. While TBRF is endemic to Iran, recent reports suggest the presence of LD in the country as well. Understanding the various Borrelia species, their tick vectors, human cases, affected reservoirs, and geographical distribution is crucial for assessing the epidemiology of TBRF and LD in Iran.
This comprehensive review examines the epidemiological patterns, geographical distribution, detection methods for these pathogens, providing critical insights into their public health significance.
Keywords: Borrelia, Tick, Tick-borne disease, Lyme disease, Relapsing fever, Iran
Graphical abstract

Highlights
-
•
Tick borne relapsing fever (TBRF) is endemic in Iran, and Lyme disease (LD) has recently been reported.
-
•
The distribution of Borrelia in Iran has increased, affecting 20 provinces.
-
•
Mazandaran province shows the highest diversity of Borrelia species, with six types documented.
-
•
Hard ticks (I. ricinus and Rh. annulatus) can also transmit TBRF in Iran, caused by B. miyamotoi and B. theileri.
1. Introduction
Borrelia is classified within the spirochaetia class, comprising organisms that belong to the Spirochaetota phylum within the eubacteria kingdom. The genus Borrelia was recognized over a century after the discovery of Borrelia recurrentis, the pathogen responsible for louse-borne relapsing fever (LBRF) (Saint Girons et al., 1994). Various Borrelia species can cause diseases, including Relapsing Fever (Jakab et al., 2022) and Lyme Disease (LD) (Stanek and Strle, 2003). Relapsing fever is classified as either tick-borne (TBRF) or louse-borne (LBRF) (Felsenfeld,1971). LBRF is an anthroponotic disease caused by B. recurrentis, whereas TBRF is a zoonotic disease resulting from various Borrelia species (Jakab et al., 2022). Borrelia spp. are transmitted through both hard and soft ticks, as well as human body lice (Koutantou et al., 2024). Relapsing fever, encompassing both LBRF and TBRF, is typically observed in the western United States, Africa, Asia, Saudi Arabia, and Spain. These diseases remain epidemic in developing countries due to poverty, war, and famine (Hoch et al., 2015). The TBRF enzootic cycles differ, encompassing various tick species and their hosts, predominantly small rodents. Furthermore, they can infect humans, who act as accidental dead-end hosts (Killilea et al., 2008; Rodino et al., 2020). TBRF has recently been associated with travel-related infections following visits to endemic regions (Rebaudet and Parola, 2006). The global reduction of lice-infested populations has led to LBRF becoming an uncommon disease, while TBRF has become a growing concern in recent years (Jakab et al., 2022) (see Table 1, Table 2).
Table 1.
Summary of published identified and unidentified Borrelia species in hosts across Iran.
| Host | Borrelia species | Location | Clinical complication | Laboratory method/Target gene | Accession Numbers | Treatment | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Identified Borrelia species responsible for TBRF | Accidental dead-end host | Human | B. balthazardi | Ardabil | – | Microscopic examination | – | – | Karimi et al., 1979 |
| Human/7-day-old neonate/Male (Congenital TBRF) | B. persica | Zanjan | Malaise, jaundice, weakness of neonatal reflexes, and sepsis | Microscopic examination | – | Erythromycin | Mahram and Ghavami, 2009 | ||
| Human/5 cases | B. persica | Khorasan-e Razavi | Relapsing episodes of fever, chills, and headache | Microscopic examination | – | – | Shayeghi et al., 2016 | ||
| Two-week-old puppy | B. persica | Tehran | Vomiting, diarrhea, and loss of appetite | Microscopic examination and PCR/IGS | KR816159 | Metoclopramide, Vitamin B12, and Ampicillin | Shirani et al., 2016 | ||
| Reservoir | Meriones persicus | B. duttonii-like spirochetes | East Azerbaijan | – | Microscopic examination, qPCR, and PCR/flaB, glpQ, groEL, p66, rrs, IGS, and 16S rRNA | MW767941-5 for IGS, MW795343-6 for P66, MW79533-5 for flaB, MW737426-9 for rrs, MW795340-2 for groEL, and MW79536-9 for glpQ. | – | Ghasemi et al., 2021 | |
| M. persicus | B. duttonii | East Azerbaijan | – | qPCR/16S rRNA | – | – | Rezaie et al., 2025 | ||
| M. persicus | B. persica | Hamadan | – | qPCR/16S rRNA | – | – | |||
| Identified Borrelia species responsible for LD | Accidental dead-end host | Human/I case | B. burgdorferi | Mazandaran | Erythema migrans | ELISA | – | Doxycycline | Adabi et al., 2004 |
| Dogs | B. burgdorferi | Gilan, Mazandaran, and Golestan | No clinical signs | ELISA and Western blot | – | – | Hanifeh et al., 2012 | ||
| Companion dogs | B. burgdorferi | Ahvaz | No clinical signs | Immunochromatography assay (ICA) | – | – | Mosallanejad et al., 2015 | ||
| Dogs | B. burgdorferi | Ahvaz | – | ELISA | – | – | Rezaei et al., 2016 | ||
| Companion dogs | B. burgdorferi | Fars | No clinical signs | ELISA | – | – | Esmailnejad et al., 2017 | ||
| Human/1 case (neuroborreliosis) | B. burgdorferi | – | Seizures | Chestcomputed tomography (CT) scan, MRI, and serological tests | – | Ceftriaxone | Sayad et al., 2023 | ||
| Guard dogs | B. burgdorferi | Isfahan | No clinical signs | Microscopic examination and PCR/16S rRNA | – | – | Chaleshtory et al., 2023 | ||
| Unidentified Borrelia species | Human/13 cases | Borrelia spp. | Kazeroun | – | Microscopic examination | – | – | Janbakhsh and Ardelan, 1977 | |
| Human/391 cases | Borrelia spp. | Ardabil | Headache, fever, chills, nausea, vomiting, abdominal pain, cough, sweating, hematuria, jaundice, arthralgia, petechiae, photophobia, and eosinophilia | Microscopic examination | – | – | Arshi et al., 2002 | ||
| Human/1 case | Borrelia spp. | Ardabil | Headache, vomiting, fever, positive Kernig's sign, and neck stiffness (Meningitis) | Microscopic examination | – | Doxycycline | Majid-Pour, 2003 | ||
| Borrelia meningitis | |||||||||
| Human/97 cases | Borrelia spp. | Kurdistan | Sweats, nervousness, headache, fever, chills, vomiting, stomach ache, myalgias, nose bleeding, cough, arthralgia, and photophobia | Microscopic examination | – | – | Rafinejad et al., 2012 | ||
| Human/138 cases | Borrelia spp. | Kurdistan | Chills, fever, sweating, headache, abdominal pain, vomiting, myalgia, arthralgia, nervousness, cough, photophobia and nose bleeding | Microscopic examination | – | – | Kassiri et al., 2014b | ||
| Human/148 cases | Borrelia spp. | Kurdistan | Fever, chills and headache | Microscopic examination | – | – | Moemenbellah-Fard et al., 2009 | ||
| Human/11 cases | Borrelia spp. | Kurdistan | Chills, fever, sweating, headache, abdominal pain, vomiting, myalgia, arthralgia, nervousness, cough, photophobia and nose bleeding | Microscopic examination | – | – | Kassiri et al., 2014a | ||
| Human/1 case | Borrelia spp. | Fars | Fever and tachycardia | Microscopic examination | – | Intravenous penicillin G four times a day for 24 h and Oral penicillin for 10 days. | Pouladfar et al., 2008 | ||
| Human/14 cases | Borrelia spp. | Hormozgan | Headache, fever, chills, nausea, vomiting, abdominal pain, cough, sweating, hematuria, jaundice, arthralgia, petechiae, photophobia, eosinophilia, muscle and joint pain | Microscopic examination/PCR | – | Tetracycline | Naddaf et al., 2015 | ||
| One-month-old puppy | Borrelia spp. | Tehran | Icteric mucous membranes, malaise, tenderness upon palpation, pustular lesions, loss of appetite, diarrhea, and fever | Microscopic examination | – | Prednisolone and Doxycycline | Rostami et al., 2011 | ||
| Human/276 cases | Borrelia spp. | Hamedan | – | Microscopic examination | – | – | Nazari and Najafi, 2016 | ||
| Human/2 cases | Borrelia spp. | Jask/Hormozgan | Fever, fatigue, and headache | PCR | Tetracycline | Naddaf et al., 2017 | |||
| Human/1 case | Borrelia spp. | Tehran | Joint pain in the left elbow and left ankle, erythema migrans on foot | Microscopic examination, ELISA, and Western blot | Doxycycline | Siadati, 2006 | |||
| Sheep and goats/1018 cases | Borrelia spp. | West Azerbaijan | – | PCR/glpQ, IGS, and flaB | KX683867 for IGS, KX683864 and KX683865 for glpQ, and KX683866 for flaB | – | Enferadi et al., 2024a | ||
| Cats and dogs | Borrelia spp. | West Azerbaijan | No clinical signs | PCR/5–23 S rRNA | OR770094, OR770195, OR770198, and OR771914 | – | Ownagh et al., 2024 | ||
| Hares and long-eared hedgehogs (Hemiechinus megalopolis) | Borrelia spp. | Sistan and Baluchistan | – | PCR/5–23S rRNA | OR400934 and OR398185 | – | Sarani et al., 2024 | ||
Table 2.
Summary of published identified and unidentified Borrelia species in different tick species in Iran.
| Disease | Tick vector | Tick status in the field (Feeding or questing) | Borrelia species | Province | Laboratory method/Target gene | Accession Numbers | Ref. |
|---|---|---|---|---|---|---|---|
| Identified Borrelia species related to TBRF | O. tholozani | – | B. persica | Markazi | – | - | Moradi-Asl and Jafari, 2020 |
| O. tholozani | Questing and feeding on domestic animals | B. persica | Semnan | Animal inoculation, microscopic examination | – | Nekoui et al., 1999 | |
| O. tartakovskyi | Questing in rodent burrows and bird nests | B. latyschewii | Khorasan-e Razavi | Animal inoculation, microscopic examination | – | Piazak et al., 2000 | |
| O. tholozani | Questing in human dwelling and animal | B. persica | Hamedan | Animal inoculation, microscopic examination | – | Vatandoost et al., 2003 | |
| O. tholozani | Questing in human dwelling, animal shelters | B. persica | Kurdistan | Animal inoculation, microscopic examination | – | Rafinejad et al., 2012 | |
| O. tholozani | Questing in human dwelling and animal | B. persica | Qazvin | Animal inoculation, microscopic examination | – | Aghighi et al., 2007 | |
| O. erraticus | B. microti | – | |||||
| O. tholozani | Questing in human dwelling, animal shelters, and rodent burrows | B. persica | Qazvin | PCR-RFLP/16S rRNA | EU914141 | Barmaki et al., 2010 | |
| O. erraticus | Feeding on rodents | B. microti | Alborz | Animal inoculation, microscopic examination, and PCR/16S rRNA, flaB, glpQ, and IGS | JF803950, JF825472, JF825473, and JQ436580 | Naddaf et al., 2012 | |
| O. tholozani | Questing in human dwelling, animal shelters | B. persica | Khorasan-e Razavi | Animal inoculation, microscopic examination | – | Shayeghi et al., 2016 | |
| A. persicus | Questing in cracks of aviary | B. anserina | Lorestan | PCR/flaB | KY438930 | Chegeni et al., 2017 | |
| I. ricinus | Questing and feeding (camels, goats, donkeys, hedgehogs, sheep, rodents, horses, cattle, and dogs) | B. miyamotoi | Mazandaran | qPCR and PCR/16S rRNA, flaB, and rrs-rrlA | MN958345 to MN958348 | Naddaf et al., 2020 | |
| Rh. annulatus | Questing and feeding (dogs, sheep, cattle, and goats) | B. theileri | Mazandaran | qPCR and PCR/16S rRNA, flaB, and glpQ | OR037296-OR037302 for flaB and OR037292- OR037295 for glpQ | Milani et al., 2024 | |
| Identified Borrelia species related to LD | I. ricinus | Questing and feeding (camels, goats, donkeys, hedgehogs, sheep, rodents, horses, cattle, and dogs) | B. bavariensis, B. valaisiana, B. afzelii | Mazandaran | qPCR and PCR/16S rRNA, flaB, and rrs-rrlA | MN958342, MN958343, MN958344 | Naddaf et al., 2020 |
| I. ricinus | B. garinii | Gilan | MN958341 | ||||
| Unidentified Borrelia species | O. tholozani | Questing in human dwelling, animal shelters, and rodent burrows | Borrelia spp. | Kurdistan | Animal inoculation, microscopic examination | – | Moemenbellah-Fard et al., 2009 |
| O.lahorensis, O.tholozani, and A. persicus | Questing | Borrelia spp. | Ardabil | Animal inoculation, microscopic examination | – | Arshi et al., 2002 | |
| O. lahorensis | Questing in human dwelling, animal shelters, and rodent burrows | Borrelia spp. | Takistan/Qazvin | PCR-RFLP/16S rRNA | – | Barmaki et al., 2010 | |
| O. tholozani | Questing in animal shelters | Q | Zanjan | Animal inoculation, microscopic examination | – | Mohammadi et al., 2013 | |
| O. tholozani/eggs | Questing in animal dwelling | Borrelia spp. | Ardabil | Animal inoculation, microscopic examination and PCR/rrs-rrlA | – | Aghaei et al., 2014 | |
| Rh. turanicus, and Rh. sanguineus | Questing and feeding (camels, goats, donkeys, hedgehogs, sheep, rodents, horses, cattle, and dogs) | Borrelia spp. | Golestan | qPCR and PCR/16S rRNA, flaB, and rrs-rrlA | MN958349 to MN958351 | Naddaf et al., 2020 | |
| Rh. sanguineus, H. asiaticumss, H.aegyptium, H. anatolicum | Feeding on sheep and goats | Borrelia spp. | West Azerbaijan | PCR/16srRNA, 5S-23SrRNA, and ospA | OQ073805.1, OR342388.1, OR352151.1, and OR345451.1. | Enferadi et al., 2024b | |
| Hyalomma aegyptium. and Rh. sanguineus | Feeding on rabbits and hedgehogs. | Borrelia spp. | Sistan and Baluchistan | PCR/5–23S rRNA | – | Sarani et al., 2024 |
LD is recognized as the most rapidly increasing tick-borne zoonotic disease worldwide, with reported cases in more than 60 countries and established endemic regions in North America, Europe, and Asia (Donohoe et al., 2015). It is transmitted through infected hard tick bites, primarily from ticks of the Ixodes genus (Giesen et al., 2024). The reservoir hosts for LD include various mammalian species, particularly rodents, as well as other animals capable of hosting hard ticks (Kazemi-Moghaddam et al., 2019). Between 1982 and 2022, the Centers for Disease Control and Prevention (CDC) documented more than 844,000 cases of LD (Mead et al., 2024). The burden of LD has increased, spreading to previously non-endemic regions (Stone et al., 2017). In this study, we assessed the status of Borrelia spp. in Iran, located in southwestern Asia.
2. Epidemiological pattern of identified Borrelia species
2.1. TBRF history, vectors, and hosts (reservoir and dead-end)
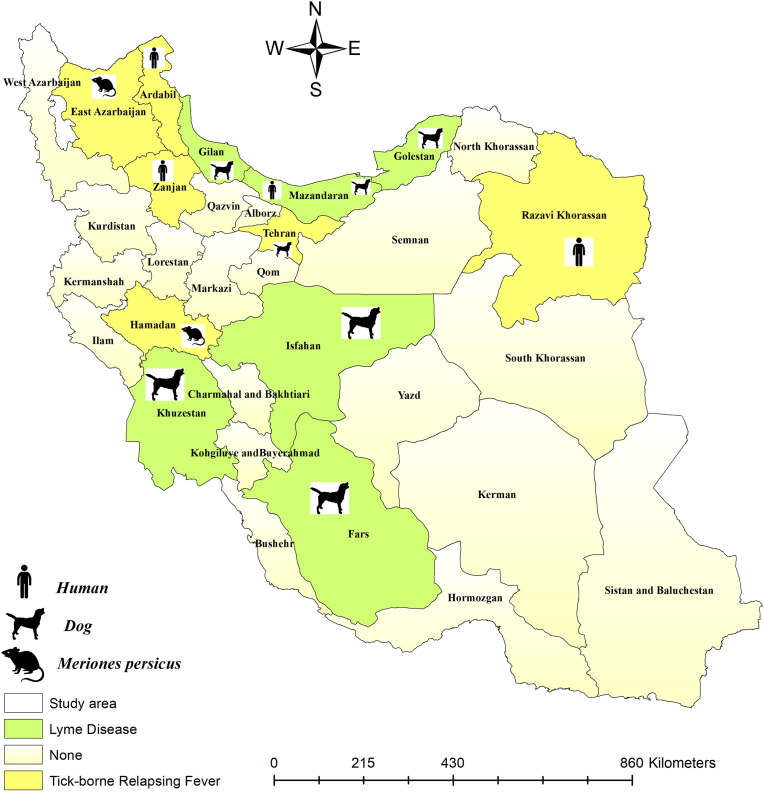
TBRF is recognized as an endemic disease in Iran. Between 1997 and 2006, more than 1400 cases were documented across 19 provinces (Asl et al., 2009). The epidemiology of the disease in various regions is influenced by the interactions between ticks, Borrelia species, and environmental conditions within their respective distribution areas (Vatandoost et al., 2003). Previously, B. persica was identified as a causative agent, with Ornithodoros tholozani reported as the main vector in Iran (Karimi et al., 1979; Naddaf et al., 2015). Additionally, B. baltazardi and B. latyschewii have been recorded in northwestern and northeastern Iran (Karimi et al., 1979). Concerning TBRF within their natural enzootic cycles, both humans and dogs serve as accidental dead-end hosts (Rodino et al., 2020). An exception to this is B. duttonii, for which humans might act as the reservoir (Jakab et al., 2022). B. persica has been documented in human TBRF cases in the provinces of Zanjan and Khorasan-e-Razavi (Mahram and Ghavami, 2009; Shayeghi et al., 2016). B. baltazardi has been detected in the blood serum of patients in Ardabil (Karimi et al., 1979). TBRF can manifest in neonates, including cases of congenital disease. A congenital TBRF case has been documented in Zanjan, where a 7-day-old neonate exhibited malaise, jaundice, diminished neonatal reflexes, and sepsis, but lacked hepatosplenomegaly, fever, and respiratory distress (Mahram and Ghavami, 2009). However, infected human cases in Khorasan-e-Razavi presented with relapsing fever episodes, chills, and headaches (Shayeghi et al., 2016). An instance of B. persica infection has also been documented in a two-week-old puppy in Tehran, which exhibited vomiting, diarrhea, and loss of appetite (Shirani et al., 2016). Wild rodents are generally the primary reservoir hosts (Rebaudet and Parola,2006). The rodent species Meriones persicus, commonly found in Iran, serves as a host for Borrelia species responsible for TBRF. In the provinces of East Azerbaijan and Hamedan, B. persica and B. duttonii, as well as B. duttonii-like spirochetes, have been successfully isolated from M. persicus (Adabi et al., 2004; Ghasemi et al., 2021; Rezaie et al., 2025).
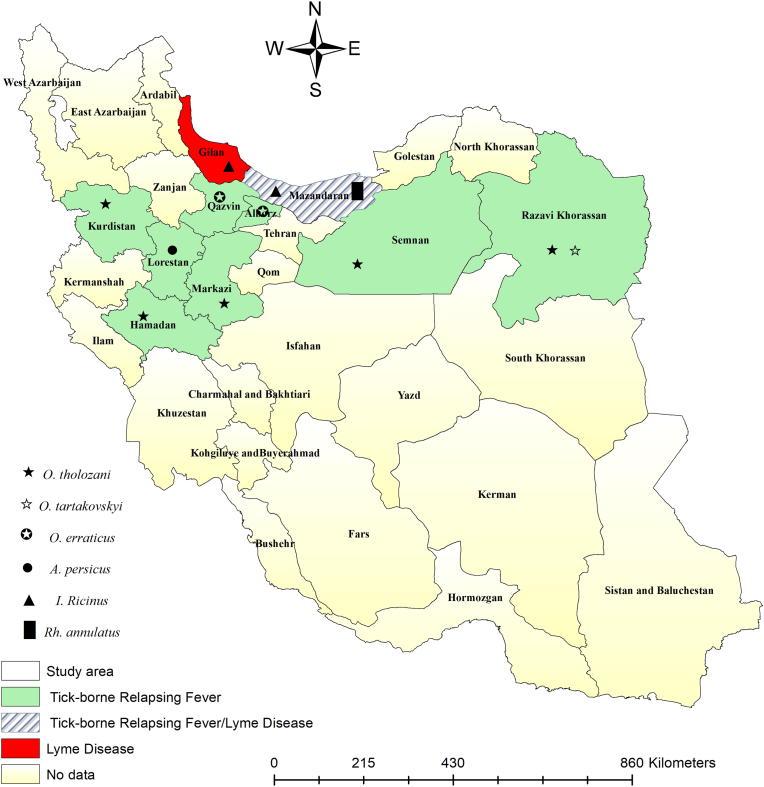
Information on TBRF tick vectors indicates that B. persica has been identified in O. tholozani across the provinces of Markazi, Semnan, Hamedan, Kurdistan, Qazvin, and Khorasan-e- Razavi (Nekoui et al., 1999; Vatandoost et al., 2003; Aghighi et al., 2007; Barmaki et al., 2010; Rafinejad et al., 2012; Shayeghi et al., 2016; Moradi-Asl,2020). B. microti has been reported from O. erraticus in Qazvin and Alborz (Aghighi et al., 2007; Naddaf et al., 2012). Other studies have recorded B. anserine and B. latyschewii from Argas persicus and O. tartakovsky, respectively, in Lorestan and Khorasan-e-Razavi (Piazak et al., 2000; Chegeni et al., 2017).
Recent findings indicate that hard ticks can also transmit TBRF. Ixodes ricinus and Rhipicephalus annulatus have been documented as TBRF hard tick vectors for B. miyamotoi and B. theileri in Mazandaran (Naddaf et al., 2020; Milani et al., 2024).
2.2. LD history, vectors, and hosts (reservoir and dead-end)
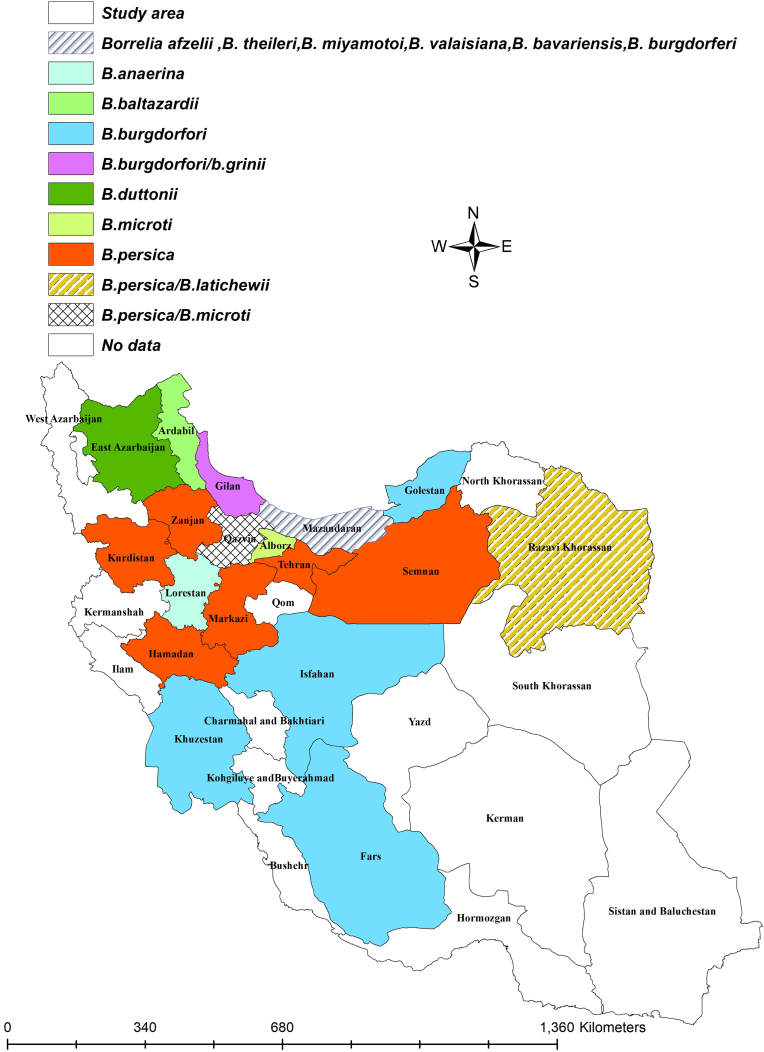
The first report of endemic LD caused by B. burgdorferi in Iran occurred in 1997 (Chams-Davatchi, 1997), serving as an alert for medical professionals to consider LD in their diagnostic evaluations. Following this case report, several LD patients have been documented over time in the country. All identified Borrelia species in Iran are shown in Fig. 1. So far, among the various Borrelia species that cause LD, only B. burgdorferi has been reported from hosts and ticks in Iran. Humans and dogs inadvertently become part of this cycle, acting as dead-end hosts (Humair and Gern, 2000; Wagner and Erb, 2012). Conversely, small wild mammals such as rodents and possibly some migratory birds play a role as reservoirs in maintaining the Lyme borreliosis cycle (Wagner and Erb, 2012). In Iran, there have been two reported cases of human Lyme borreliosis. One case occurred in Mazandaran (Adabi et al., 2004), while the region associated with the Lyme neuroborreliosis case has not been specified (Sayad et al., 2023). In Lyme neuroborreliosis, initially, the patient exhibited low-grade fever, headache, vomiting, and malaise; however, after a week, they were hospitalized due to the onset of seizures (Sayad et al., 2023). This zoonotic disease has also been documented in dogs across various regions of Iran, including Gilan, Mazandaran, Golestan, Khuzestan, Fars, and Isfahan (Hanifeh et al., 2012; Mosallanejad et al., 2015; Rezaei and Gh, 2016; Esmailnejad et al., 2017; Chaleshtory et al., 2023). Notably, B. burgdorferi did not cause any clinical signs in dogs (Hanifeh et al., 2012; Mosallanejad et al., 2015; Esmailnejad et al., 2017; Chaleshtory et al., 2023). Findings regarding tick vectors of LD indicated that B. bavariensis, B. valaisiana, B. afzelii, and B. garinii have been successfully isolated from I. ricinus in Mazandaran and Gilan provinces (Naddaf et al., 2020).
Fig. 1.
Different types of Borrelia species by the counties.
The geographical distribution of identified Borrelia species in hosts is presented in Fig. 2. Additionally, the geographical distribution of identified Borrelia species from different tick species is shown in Fig. 3.
Fig. 2.
Identified Borrelia species in various hosts by the counties. The symbols represent humans, dogs, and rodents, indicating the Borrelia-infected specimens.
Fig. 3.
Identified Borrelia species in various tick species by the counties. The symbols represent different species of ticks infected with Borrelia.
2.3. Unidentified Borrelia species reported in vectors and hosts
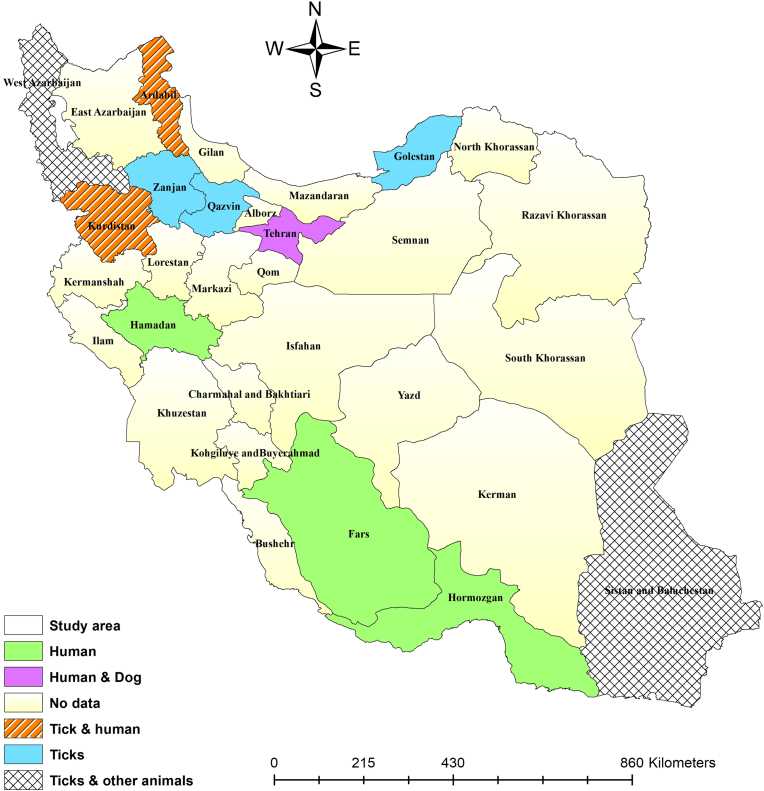
Previous studies have recorded human cases of TBRF symptoms in Fars (Janbakhsh and Ardelan, 1977), Ardabil (Arshi et al., 2002; Majid-Pour, 2003), Kurdistan (Moemenbellah-Fard et al., 2009; Rafinejad et al., 2012; Kassiri et al., 2014; Kassiri et al., 2014), Fars (Pouladfar et al., 2008), Hormozgan (Naddaf et al., 2015, Naddaf et al., 2017), and Hamedan Provinces (Nazari and Najafi, 2016). However, the species of Borrelia involved in these cases have not been identified. A human case with LD clinical symptoms was reported from Tehran, while the type of Borrelia is unknown (Siadati, 2006). A one-month-old puppy was diagnosed with a Borrelia spp. infection in Tehran (Rostami et al., 2011). Blood samples from cats, dogs, sheep, and goats in West Azerbaijan (Enferadi et al., 2024; Ownagh et al., 2024), and hares and long-eared hedgehogs (Hemiechinus megalotis) in Sistan and Baluchistan revealed the presence of Borrelia spp. (Sarani et al., 2024). Unidentified Borrelia species have also been documented in soft and hard tick vectors. The identified soft ticks were infected with Borrelia spp. include O. tholozani from Kurdistan (Moemenbellah-Fard et al., 2009), Ardabil (Arshi et al., 2002; Aghaei et al., 2014), and Zanjan (Mohammadi et al., 2013); O. lahorensis from Ardabil (Arshi et al., 2002) and Qazvin (Barmaki et al., 2010); and A. persicus from Ardabil (Arshi et al., 2002). The identified hard ticks are infected with Borrelia spp. include Rh. turanicus and Rh. sanguineus from Golestan (Naddaf, Mahmoudi et al.,2020); Rh. sanguineus, Hyalomma asiaticum, H. aegyptium, and H. anatolicum from West Azerbaijan (Enferadi et al., 2023); and Hyalomma aegyptium. and Rh. sanguineus from Sistan and Baluchistan (Sarani et al., 2024). The geographical distribution of unidentified Borrelia species in hosts and ticks has been illustrated in Fig. 4.
Fig. 4.
Unidentified Borrelia species in various hosts and tick species by the counties.
3. Laboratory methods for Borrelia detection
3.1. Microscopic examination
Giemsa staining was employed for the identification of Borrelia spp. in the microscopic method. The stained slide demonstrated the presence of spirochetes within the thin blood smear obtained from an infected canine (Rostami et al., 2011; Shirani et al., 2016; Chaleshtory et al., 2023), M. persicus (Ghasemi et al., 2021), and human (Janbakhsh and Ardelan, 1977; Arshi et al., 2002; Majid-Pour, 2003, Siadati, 2006; Pouladfar et al., 2008; Mahram and Ghavami, 2009; Moemenbellah-Fard et al., 2009; Rafinejad et al., 2012; Kassiri et al., 2014; Kassiri et al., 2014; Naddaf et al., 2015; Nazari and Najafi, 2016; Shayeghi et al., 2016).
3.2. Animal inoculation
It is important to understand that the sensitivity of microscopy is defined by specific positivity thresholds, which are estimated to be 105 spirochetes per mL of blood for thin smears and 104 spirochetes per mL for thick smears (Hovette et al., 2001). As a result, more investigations use animal inoculation before direct microscopic examination. Bacteria can be introduced into the animal either by direct injection or by allowing ticks to feed on it. One or two weeks later, blood samples from the inoculated animals were tested for Borrelia using Giemsa staining, microscopic examination, or PCR. In this method, a suspension made from live ticks, including O. lahorensis, O. tholozani, and A. persicus, was injected into the peritoneum of guinea pigs and white mice in Ardabil for Borrelia spp. (Arshi et al., 2002). O. tholozani was inoculated into suckling Syrian hamsters or white mice for Borrelia spp. in Kurdistan (Moemenbellah-Fard et al., 2009). O. tholozani was also intraperitoneally inoculated into white mice with B. persica (Nekoui et al., 1999; Vatandoost et al., 2003; Mohammadi et al., 2013; Shayeghi et al., 2016), and O. erraticus was inoculated into guinea pigs with B. microti (Naddaf et al., 2012). In the tick-feeding method, unfed O. tartakovskyi infected with B. latyschewii, and O. tholozani infected with B. persica were fed on white mice in Khorasan-e-Razavi and Hamedan, respectively (Piazak et al., 2000, Vatandoost et al., 2003). In another study, infected O. tholozani were fed on guinea pigs, while O. erraticus were fed on newborn mice. As a result, B. persica and B. microti were successfully isolated from O. tholozani and O. erraticus, respectively (Aghighi et al., 2007). Unfed O. tholozani infected with Borrelia spp. fed on guinea pigs and mice in Semnan, Zanjan, and Ardabil (Nekoui et al., 1999; Arshi et al., 2002; Mohammadi et al., 2013). In a separate investigation conducted in Kurdistan, O. tholozani was examined for the presence of Borrelia spp. through a three-step process: the ticks were first crushed and inoculated into guinea pigs and white mice, followed by blood-feeding on the guinea pigs, and finally, a subcutaneous injection of the crushed ticks was administered (Rafinejad et al., 2012).
3.3. Molecular technique
The Nested-PCR technique was employed for the identification of Borrelia species after DNA extraction from tissues (tick and animal) or blood samples. This technique uses specific primers to precisely identify the species of spirochete. Multilocus sequence analysis (MLSA) used for Borrelia identification includes the intergenic spacer region (IGS) (Naddaf et al., 2012; Naddaf et al., 2015; Shirani et al., 2016; Naddaf et al., 2017; Ghasemi et al., 2021), 5–23S rRNA gene (Mosallanejad et al., 2015; Enferadi et al., 2023; Enferadi et al., 2024a; Ownagh et al., 2024; Sarani et al., 2024), non-coding rrs-rrlA spacer (Aghaei et al., 2014; Naddaf et al., 2020; Ghasemi et al., 2021), flagellin (flaB) (Chegeni et al., 2017; Naddaf et al., 2017; Naddaf et al., 2020; Ghasemi et al., 2021; Milani et al., 2024), glycerophosphodiester phosphodiesterase (glpQ) (Naddaf et al., 2012; Naddaf et al., 2017; Ghasemi et al., 2021; Milani et al., 2024), groEL (Ghasemi et al., 2021), p66 (Ghasemi et al., 2021), 16S rRNA region (Chaleshtory et al., 2023), and ospA (Enferadi et al., 2023; Enferadi et al., 2024). In several investigations, the qPCR/Real-Time PCR technique was utilized to detect Borrelia spp. using a specific probe targeting the 16S rRNA region (Naddaf et al., 2020; Ghasemi et al., 2021; Milani et al., 2024; Moradkasani et al., 2024; Rezaie et al., 2025). Furthermore, PCR-restriction fragment length polymorphism (RFLP) analysis was employed to identify B. persica in ticks (Barmaki et al., 2010).
3.4. Serological tests
The serological testing for Borrelia species, particularly in LD, generally consists of a two-phase procedure. The initial phase employs an enzyme-linked immunosorbent assay (ELISA) to identify antibodies targeting B. burgdorferi. If the outcome of this first test is either positive or uncertain, a Western blot test is administered to confirm the diagnosis. In several studies, ELISA and/or Western blot, and immunochromatography assay (ICA) techniques were utilized to detect the presence of B. burgdorferi in dogs (Adabi et al., 2004; Siadati, 2006; Hanifeh et al., 2012; Mosallanejad et al., 2015; Rezaei and Gh,2016; Esmailnejad et al., 2017; Sayad et al., 2023).
4. The negative findings regarding Borrelia presence in hosts and ticks
During the period from 2011 to 2012, blood smear examinations of febrile patients in the city of Chabahar revealed no signs of spirochetes associated with Borrelia (Metanat et al., 2014). None of the avian blood samples collected in Lorestan Province showed any signs of infection with B. anserina (Chegeni et al., 2017). In Khorasan Province, PCR testing indicated that there was no evidence of Borrelia spp. infection among 66 patients suffering from morphoea symptoms (Yazdanpanah et al., 2015). Given that small mammals and rodents are considered reservoirs of TBRF and LD disease, it is important to note that, none of the rodents and small mammals in Mazandaran, Gilan, and Golestan Provinces, including Rattus norvegicus, Microtus obscurus, Mus musculus castaneus, Rattus rattus, Apodemus hyrcanicus, Apodemus uralensis, Nesokia indica, Microtus paradoxus, Crocidura caspica, Crocidura suaveolens, and Erinaceus concolor, exhibited evidence of Borrelia spp. presence within their internal organs (Naddaf et al., 2020). In a study involving R. norvegicus gathered from Tehran, antibodies (IgG) against Borrelia spp. were not found (Azimi et al., 2024). Borrelia spp. was not detected in dromedary camels (Camelus dromedarius) assessed in Fars, Kerman, and Sistan and Baluchistan Provinces (Sazmand et al., 2019; Heidari et al., 2023). An investigation was conducted to survey the presence of Borrelia spp. in Iran, focusing on ornamental birds across four provinces: Tehran, East Azerbaijan, Yazd, and Khuzestan. The results indicated that Borrelia was absent in all the birds that were studied (Enferadi et al., 2024). Regarding ticks, no Borrelia was found in these investigations: A. persicus in Qazvin and West Azerbaijan (Barmaki et al., 2010; Enferadi et al., 2024) and I. ricinus in Gilan, Mazandaran, and Golestan (Yousefi-Behzadi et al., 2022).
5. Discussion and conclusion
5.1. Identified Borrelia species regarding TBRF and LD
Our review study found that 20 provinces in Iran are affected by Borrelia species. The geographical distribution map indicates that B. persica and B. burgdorferi are the most prevalent Borrelia species in Iran, respectively. The causative agents of TBRF are chiefly present in the northern regions of Iran. In contrast, B. burgdorferi has been reported in northern provinces, as well as in central, southern, and southwestern regions of Iran. Mazandaran exhibits the highest diversity of Borrelia, where six species of the pathogen have been documented. This notable diversity may be attributed to the province's geographical location and the presence of migratory birds, which have a remarkable ability to host various animal species, especially migratory birds (Jakab et al., 2022), likely contributing to the spread of these pathogens. Moreover, the prolonged attachment and feeding period of hard ticks enhances their ability to migrate over vast distances (Spach et al., 1993; Guberman et al., 1994), facilitating the dissemination of Borrelia species.
5.2. TBRF vectors and hosts
Humans and dogs are reported as dead-end hosts, while M. persicus has been documented as a reservoir in Iran. Considering a documented neonatal case in an endemic region, neonatal borreliosis should be considered in any newborn presenting with symptoms indicative of neonatal sepsis. Additionally, any history of febrile illnesses experienced by the mother during pregnancy, particularly in the final days of gestation, is crucial to consider. For accurate diagnosis, peripheral blood smears from the infant must be obtained at least three times (Mahram and Ghavami, 2009). The transmission of TBRF in Iran is predominantly associated with four species of soft ticks, including O. tholozani, O. erraticus, O. tartokovyskyi, and A. persicus. Surprisingly, hard ticks such as I. ricinus and Rh. annulatus can also transmit TBRF(Naddaf et al., 2020; Milani et al., 2024). Climate change can increase the risk of TBRF by expanding the activity and range of ticks and increasing the number of susceptible hosts (Bouchard et al., 2019).
5.3. LD vectors and hosts
The known dead-end hosts of LD in Iran are humans and dogs. Although B. burgdorferi has been predominantly identified in canine populations, human cases have been reported exclusively in Mazandaran. The incidence of LD in animals has increased over the past several years, specifically from 2009 to 2023. Despite reports of LD cases in six provinces across the country, the investigation of hard tick vectors responsible for the disease have been conducted only in Mazandaran.
5.4. Laboratory methods for Borrelia detection
The diagnosis of TBRF and LD is based on a combination of clinical observations and laboratory tests, none of which alone can definitively confirm infection with Borrelia species (Mead et al., 2024). For TBRF, preparing a thin blood smear followed by microscopic examination is a standard diagnostic method. However, comparative studies have demonstrated that microscopic examination may fail to detect spirochetes in some cases, whereas molecular techniques such as PCR and quantitative PCR (qPCR) can successfully identify their presence (Siadati, 2006). In fact, qPCR has been shown to possess higher sensitivity than traditional PCR, particularly for detecting spirochete DNA at low cycle threshold (Ct) values (Gil et al., 2005; Naddaf et al., 2020; Milani et al., 2024). Therefore, molecular methods could be more reliable techniques for diagnosing TBRF. The use of serological testing for diagnosing TBRF is limited because high seroprevalence does not always indicate an active infection, delayed seroconversion can hinder timely treatment decisions, and assay cross-reactivity complicates distinguishing TBRF from Lyme disease (Magnarelli et al., 1987). In contrast, the diagnosis of LD is primarily depends on serological tests, including ELISA, Western blot, and ICA (Marques,2015).
6. Literature gap and future research
Review studies often highlight gaps in the literature and suggest future research. In this study, we aim to recommend effective field studies to improve understanding and control of tick-borne diseases in Iran. In East Azerbaijan, while M. persicus has been found infected with B. duttonii, ticks in this area have yet to be tested for Borrelia species. Given that infected humans serve as reservoir for B. duttonii, studying local tick populations in this province is crucial. Similarly, an animal case report in West Azerbaijan suggests the presence of Borrelia, but comprehensive investigation into Borrelia species and tick infection are still lacking. In Zanjan Province, the identification of B. persica in O. tholozani remains inconclusive and relies primarily on earlier reports, highlighting the need for updated molecular and phylogenetic studies. Notably, molecular investigations in Hormozgan involving patients with symptoms of TBRF have revealed diverse Borrelia species that cluster into distinct clades. A focused research project on these pathogens has the potential to uncover a novel Borrelia type in Iran. Nanopore-based metagenomic studies have demonstrated higher sensitivity in detecting a wide range of tick-borne viruses and bacteria, enabling the molecular characterization of novel viral and bacterial strains in cases where traditional methods were inadequate or unsuccessful (Ravi et al., 2019; Ergunay et al., 2023; Kipp et al., 2023). Furthermore, in central, southern, and southwestern Iran—regions where LD has been identified in dogs, dedicated studies assessing tick populations are still absent, representing another important gap for future research.
CRediT authorship contribution statement
Parisa Soltan-Alinejad: Writing – review & editing, Writing – original draft, Investigation, Data curation, Conceptualization. Mahmood Nikbakhtzadeh: Writing – review & editing, Visualization, Validation. Eslam Moradi-Asl: Writing – review & editing, Writing – original draft, Software, Methodology.
Ethical approval
The study was conducted by the ethical principles and the national norms and standards for conducting Medical Research in Iran. The study was approved by the Iran National Committee for Ethics in Biomedical Research (Approval ID: IR. ARUMS.REC.1403.483).
Funding
This research was supported by Ardabil University of Medical Sciences (ARUMS), Ardabil, Iran, under Grant No.403000942.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgment
Not applicable.
Contributor Information
Parisa Soltan-Alinejad, Email: parisaalinejad1369@gmail.com.
Mahmood Nikbakhtzadeh, Email: mahmood.nikbakhtzadeh@csusb.edu.
Eslam Moradi-Asl, Email: Moradiasl83@yahoo.com, e.moradiasl@arums.ac.ir.
Data availability
All data generated this study are available upon reasonable request to the corresponding author.
References
- Adabi M., Firoozjahi A., Ghasemi M. Report of a case of Lyme disease in Mazandaran. Iran. j. dermatol. 2004;8(Suppl. l):21–25. [Google Scholar]
- Aghaei A., Ghazinezhad B., Naddaf S.R. Detection of Borrelia DNA in Ornithodoros tholozani ticks and their eggs. JoMMID. 2014;2(3):118–120. [Google Scholar]
- Aghighi Z., Assmar M., Piazak N., Javadian E., Seyedi R.M., Kia E., Rasi Y., Vatandoust H. Distribution of soft ticks and their natural infection with Borrelia in a focus of relapsing fever in Iran. J Arthropod Borne Dis. 2007;1(2):14–18. [Google Scholar]
- Arshi S., Majidpour A., Sadeghi H., Emdadi D., Asmar M., Derakhshan M. Relapsing fever in Ardabil, a northwestern province of Iran. Arch. Iran. Med. 2002;5(3):141–145. [Google Scholar]
- Asl H.M., Goya M., Vatandoost H., Zahraei S., Mafi M., Asmar M., Piazak N., Aghighi Z. The epidemiology of tick-borne relapsing fever in Iran during 1997–2006. Trav. Med. Infect. Dis. 2009;7(3):160–164. doi: 10.1016/j.tmaid.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Azimi T., Nasrollahian S., Sabour S., Hadi N., Azimi L., Fallah F., Pourmand M.R. Detection of Yersinia enterocolitica, Shigella spp. and Salmonella spp. in Rattus norvegicus captured from Tehran, Iran. Future Microbiol. 2024;19(5):377–384. doi: 10.2217/fmb-2023-0138. [DOI] [PubMed] [Google Scholar]
- Barmaki A., Rafinejad J., Vatandoost H., Telmadarraiy Z., Mohtarami F., Leghaei S., Oshaghi M. Study on presence of Borrelia persica in soft ticks in Western Iran. J. Arthropod. Borne Dis. 2010;4(2):19. [PMC free article] [PubMed] [Google Scholar]
- Bouchard C., Dibernardo A., Koffi J., Wood H., Leighton P.A., Lindsay L.R. N increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019;45(4):83–89. doi: 10.14745/ccdr.v45i04a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleshtory M.Z., Keihani P., Momtaz H., Tehrani M.H.A., Hosseini S.R. Prevalence of Borrelia burgdorferi in guard dogs in Isfahan, Iran. J. Vet. Res. 2023;78(3) [Google Scholar]
- Chams-Davatchi C. The first endemic case of lyme borreliosis in Iran. Med. J. Islam. Repub. Iran. 1997;11(3):237–239. [Google Scholar]
- Chegeni A.H., Telmadarraiy Z., Tavakoli M., Faghihi F. Molecular detection of Borrelia anserina in Argas persicus (Acari: argasidae) ticks collected from Lorestan province, west of Iran. Persian J. Acarol. 2017;6(4) [Google Scholar]
- Donohoe H., Pennington-Gray L., Omodior O. Lyme disease: current issues, implications, and recommendations for tourism management. Tour. Manag. 2015;46:408–418. doi: 10.1016/j.tourman.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enferadi A., Ownagh A., Nofouzi K., Khordadmehr M. Molecular and histopathological survey of Francisella spp., Borrelia spp. and Leptospira spp. in ornamental birds of four provinces of Iran. Gene Rep. 2024;35 [Google Scholar]
- Enferadi A., Ownagh A., Tavassoli M. Molecular detection of Borrelia spp. in ticks of sheep and goats by nested PCR method in West Azerbaijan Province, Iran. Vector Borne Zoonotic Dis. 2023;23(12):605–614. doi: 10.1089/vbz.2023.0039. [DOI] [PubMed] [Google Scholar]
- Enferadi A., Ownagh A., Tavassoli M. Veterinary Research Forum. Faculty of Veterinary Medicine, Urmia University; Urmia, Iran: 2024. Molecular detection and phylogenetic analysis of Borrelia spp. from sheep and goats blood samples in West Azerbaijan province, Iran. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergunay K., Dincer E., Justi S.A., Bourke B.P., Nelson S.P., Liao H.-M., Timurkan M.O., Oguz B., Sahindokuyucu I., Gokcecik O.F., Reinbold-Wasson D.D., Jiang L., Achee N.L., Grieco J.P., Linton Y.-M. Impact of nanopore-based metagenome sequencing on tick-borne virus detection. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1177651. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmailnejad A., Tabatabaei M., AbbaszadehHasiri M., Sheikhi F. Serological evidence of Borreliosis among companion dogs in Fars Province, South of IranSerological evidence of Borreliosis among companion dogs in Fars Province, South of Iran. Jundishapur J. Microbiol. 2017;2(1):1–8. [Google Scholar]
- Felsenfeld O. 1971. Borrelia: Strains, Vectors, Human and Animal Borreliosis. [Google Scholar]
- Ghasemi A., Naddaf S.R., Mahmoudi A., Rohani M., Naeimi S., Mordadi A., Cutler S.J., Mostafavi E. Borrelia duttonii-like spirochetes parasitize Meriones persicus in East Azerbaijan Province of Iran. Ticks Tick Borne Dis. 2021;12(6) doi: 10.1016/j.ttbdis.2021.101825. [DOI] [PubMed] [Google Scholar]
- Giesen C., Cifo D., Gomez-Barroso D., Estévez-Reboredo R.M., Figuerola J., Herrador Z. The role of environmental factors in lyme disease transmission in the European Union: a systematic review. Trop. med. infect. 2024;9(5):113. doi: 10.3390/tropicalmed9050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil H., Barral M., Escudero R., García-Pérez A.L., Anda P. Identification of a new Borrelia species among small mammals in areas of northern Spain where Lyme disease is endemic. Appl. Environ. Microbiol. 2005;71(3):1336–1345. doi: 10.1128/AEM.71.3.1336-1345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guberman D., Vardy D.A., Klapholz L., Klaus S.N. Vector-borne infections: a hazard for adventure visitors to Israel. J. Wilderness Med. 1994;5(3):254–262. [Google Scholar]
- Hanifeh M., Malmasi A., Virtala A., Nikbakht-Brujeni G., Salehi T.Z., Rahbari S. Seroprevalence, geographic distribution and risk factor analysis of Borrelia burgdorferi sensu lato in naturally exposed dogs of Iran. Afr. J. Microbiol. Res. 2012;6:5353–5361. [Google Scholar]
- Heidari F., Sharifiyazdi H., Nazifi S., Ghane M., Hosseinzadeh S. Coxiella burnetii and Borrelia spp. in peripheral blood of dromedary camels in Fars, Iran: molecular characterization, hematological parameters, and acute-phase protein alterations. Iran. J. Vet. Res. 2023;24(3):174–181. doi: 10.22099/ijvr.2023.46933.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch M., Wieser A., Löscher T., Margos G., Pürner F., Zühl J., Seilmaier M., Balzer L., Guggemos W., Rack-Hoch A. Louse-borne relapsing fever (Borrelia recurrentis) diagnosed in 15 refugees from northeast Africa: epidemiology and preventive control measures, Bavaria, Germany, July to October 2015. Euro Surveill. 2015;20(42) doi: 10.2807/1560-7917.ES.2015.20.42.30046. [DOI] [PubMed] [Google Scholar]
- Hovette P., Aubron C., Perrier-Gros-Claude J.D., Schieman R., N'Dir M.C., Camara P. [Value of Quantitative Buffy Coat (QBC) in borreliasis-malaria co-infection] Med. Trop. 2001;61(2):196–197. [PubMed] [Google Scholar]
- Humair P.-F., Gern L. The wild hidden face of Lyme borreliosis in Europe. Microb. Infect. 2000;2(8):915–922. doi: 10.1016/S1286-4579(00)00393-2. [DOI] [PubMed] [Google Scholar]
- Jakab Á., Kahlig P., Kuenzli E., Neumayr A. Tick borne relapsing fever-a systematic review and analysis of the literature. PLoS Neglected Trop. Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbakhsh B., Ardelan A. The nature of sporadic cases of relapsing fever in Kazeroun area, southern Iran. Bull. Soc. Pathol. Exot Filiales. 1977;1977 Nov-Dec;70(6):587–589. PMID: 615684. [PubMed] [Google Scholar]
- Karimi Y., Hovind-Hougen K., Birch-Andersen A., Asmar M. Experimental Pathogenicity for Some Animals and Comparison of the Ultrastructure. Annales De Microbiologie. 1979. Borrelia persica and B. baltazardi sp. Nov. [PubMed] [Google Scholar]
- Kassiri H., Kasiri A., Dostifar K., Lotfi M. The epidemiology of tick-borne relapsing fever in Bijar County, North-Western Iran. J. Acute Dis. 2014;3(3):224–227. [Google Scholar]
- Kassiri H., Kasiri A., Karimi M., Kasiri E., Lotfi M. The seven-year longitudinal study on relapsing fever borreliosis in Western Iran. Asian Pac. J. Trop. Dis. 2014;4:S679–S683. doi: 10.1016/S2222-1808(14)60706-4. [DOI] [Google Scholar]
- Kazemi-Moghaddam V., Dehghani R., Hadei M., Dehqan S., Sedaghat M.M., Latifi M., Alavi-Moghaddam S. Rodent-borne and rodent-related diseases in Iran. Comp. Clin. Pathol. 2019;28:893–905. [Google Scholar]
- Killilea M.E., Swei A., Lane R.S., Briggs C.J., Ostfeld R.S. Spatial dynamics of Lyme disease: a review. EcoHealth. 2008;5:167–195. doi: 10.1007/s10393-008-0171-3. [DOI] [PubMed] [Google Scholar]
- Kipp E.J., Lindsey L.L., Khoo B., Faulk C., Oliver J.D., Larsen P.A. Metagenomic surveillance for bacterial tick-borne pathogens using nanopore adaptive sampling. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-37134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutantou M., Drancourt M., Angelakis E. Prevalence of Lyme disease and relapsing fever Borrelia spp. in vectors, animals, and humans within a one health approach in Mediterranean countries. Pathogens. 2024;13(6):512. doi: 10.3390/pathogens13060512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L.A., Anderson J.F., Johnson R.C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. JID (J. Infect. Dis.) 1987;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Mahram M., Ghavami M. EMHJ; 2009. Congenital tick-borne Relapsing Fever: Report of a Case with Transplacental Transmission in the Islamic Republic of Iran. [PubMed] [Google Scholar]
- Majid-Pour A. A case of Borrelia meningitis. Arch. Iran. Med. 2003;6(3):222–223. [Google Scholar]
- Marques A.R. Laboratory diagnosis of Lyme disease: advances and challenges. Infect. Dis. Clin. 2015;29(2):295–307. doi: 10.1016/j.idc.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead P., Hinckley A., Kugeler K. Lyme disease surveillance and epidemiology in the United States: a historical perspective. J. Infect. Dis. 2024;230(Suppl. ment_1):S11–S17. doi: 10.1093/infdis/jiae230. [DOI] [PubMed] [Google Scholar]
- Metanat M., Tabatabaei M., Afsharmanesh A. Prevalence of tick-borne relapsing fever in febrile patients suspicious for malaria admitted in Chabahar. Zahedan J. Res. Med. Sci. 2014;16(4):35–37. [Google Scholar]
- Milani M., Naddaf S.R., Ziapour S.P., Sepahi A.A., Rohani M. Borrelia theileri infections in Rhipicephalus annulatus ticks from the north of Iran. Exp. Appl. Acarol. 2024:1–15. doi: 10.1007/s10493-024-00924-5. [DOI] [PubMed] [Google Scholar]
- Moemenbellah-Fard M., Benafshi O., Rafinejad J., Ashraf H. Tick-borne relapsing fever in a new highland endemic focus of western Iran. Ann. Trop. Med. Parasitol. 2009;103(6):529–537. doi: 10.1179/136485909X451852. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Ghavami M.B., Mohammadi J. Study of phenotypic variability and borrelia infectivity in different populations of Ornithodoros Tholozani in Zanjan Province. J Zanjan Univ Med Sci. 2013;21(87) [Google Scholar]
- Moradi-Asl E. The habitat suitability model for the potential distribution of Ornithodoros tholozani (Laboulbène et Mégnin, 1882) and Ornithodoros lahorensis (Neumann, 1908) (Acari: argasidae):the main vectors of tick-borne relapsing fever in Iran. Ann. Parasitol. 2020;66(3):357–363. doi: 10.17420/ap6603.274. PMID: 33128518. [DOI] [PubMed] [Google Scholar]
- Moradkasani S., Latifian M., Salehi-Vaziri M., Amiri F.B., Mostafavi E., Ghasemi A., Esmaeili S. Molecular investigation of Coxiella burnetii, Brucella spp., Ehrlichia spp., and Borrelia spp. among patients suspected of having Crimean-Congo Hemorrhagic Fever in Iran. J. Infect. Public Health. 2024;17(12) doi: 10.1016/j.jiph.2024.102582. [DOI] [PubMed] [Google Scholar]
- Mosallanejad B., Avizeh R., Jalali M.H.R., Pourmahdi M. A serological survey on Borrelia burgdorferi infection among companion dogs in Ahvaz district, southwestern Iran. Comp. Clin. Pathol. 2015;24:1559–1563. doi: 10.1007/s00580-015-2116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddaf S.R., Ghazinezhad B., Bahramali G., Cutler S.J. Phylogenetic analysis of the spirochete Borrelia microti, a potential agent of relapsing fever in Iran. J. Clin. Microbiol. 2012;50(9):2873–2876. doi: 10.1128/JCM.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddaf S.R., Ghazinezhad B., Kazemirad E., Cutler S.J. Relapsing fever causative agent in Southern Iran is a closely related species to East African borreliae. Ticks Tick Borne Dis. 2017;8(6):882–886. doi: 10.1016/j.ttbdis.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Naddaf S.R., Ghazinezhad B., Sedaghat M.M., Asl H.M., Cutler S.J. Tickborne relapsing fever in southern Iran, 2011–2013. Emerg. Infect. Dis. 2015;21(6):1078. doi: 10.3201/eid2106.141715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddaf S.R., Mahmoudi A., Ghasemi A., Rohani M., Mohammadi A., Ziapour S.P., Nemati A.H., Mostafavi E. Infection of hard ticks in the Caspian Sea littoral of Iran with Lyme borreliosis and relapsing fever borreliae. Ticks Tick Borne Dis. 2020;11(6) doi: 10.1016/j.ttbdis.2020.101500. [DOI] [PubMed] [Google Scholar]
- Nazari M., Najafi A. Epidemiological Study of endemic relapsing fever in Hamadan Province, west of Iran. J Arthropod Borne Dis. 2016;10(4):586–594. [PMC free article] [PubMed] [Google Scholar]
- Nekoui H., Assmar M., Amirkhani A., Pyazak N. Distribution of ticks and their association with Borrelia in Semnan province. J. Public Health. 1999;28(1):103–109. Iran 1-4. [Google Scholar]
- Ownagh A., Rajabi S.A., Enferadi A., Hadian M. Molecular detection and phylogenetic analysis of Borrelia Spp. In blood samples of cats and dogs by the nested-PCR method in West Azerbaijan Province, Iran. Braz. J. Microbiol. 2024;55(3):2915–2922. doi: 10.1007/s42770-024-01401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazak N., Rashti M.S., Assmar M. A survey of prevalence of Ornithodorus tartakovskyi and its infection rate with Borrelia latychevi in Sarakhs county, Khorassan province. J. Public Health. 2000;29(1):103–108. Iran 1-4. [Google Scholar]
- Pouladfar G.R., Alborzi A., Pourabbas B. Tick-borne relapsing fever, a neglected cause of fever in Fars province. Iran. J. Med. Sci. 2008;33 [Google Scholar]
- Rafinejad J., Shemshad K., Banafshi O. Epidemiological study on tick-borne (Acari: argasidae) relapsing fever in Kurdistan Province, Iran, 2000–2004. Fla. Entomol. 2012;95(3):758–763. [Google Scholar]
- Ravi A., Ereqat S., Al-Jawabreh A., Abdeen Z., Abu Shamma O., Hall H., Pallen M.J., Nasereddin A. Metagenomic profiling of ticks: identification of novel rickettsial genomes and detection of tick-borne canine parvovirus. PLoS Neglected Trop. Dis. 2019;13(1) doi: 10.1371/journal.pntd.0006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebaudet S., Parola P. Epidemiology of relapsing fever borreliosis in Europe. FEMS Immunol. Med. Microbiol. 2006;48(1):11–15. doi: 10.1111/j.1574-695X.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- Rezaei A., Gh D. "Seroprevalence of Lyme disease and Q fever in referred dogs to Veterinary Hospital of Ahvaz." Iran. J. Vet. Res. 2016;11(4):34–41. [Google Scholar]
- Rezaie N., Latifian M., Ghasemi A., Mahmoudi A., Baseri N., Omidi A.H., Esmaeili P., Esmaeili S., Mostafavi E. Assessing the diversity of zoonotic bacterial agents in rodents and small mammals in Iran. Eur. J. Publ. Health. 2025;35(Suppl. ment_1):i41–i47. doi: 10.1093/eurpub/ckae132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino K.G., Theel E.S., Pritt B.S. Tick-Borne diseases in the United States. Clin. Chem. 2020;66(4):537–548. doi: 10.1093/clinchem/hvaa040. [DOI] [PubMed] [Google Scholar]
- Rostami A., Zaeimi M., Piazhak N., Mazaheri Nezhad Fard R. A case report of atypical borreliosis in a dog. Iran J Vet Sci Technol. 2011;3(2):49–56. [Google Scholar]
- Saint Girons I., Old I., Davidson B. Molecular biology of the Borrelia, bacteria with linear replicons. Microbiology. 1994;140(8):1803–1816. doi: 10.1099/13500872-140-8-1803. [DOI] [PubMed] [Google Scholar]
- Sarani S., Enferadi A., Hasani S.J., Sarani M.Y., Rahnama M., Sarani F. Identification of zoonotic pathogenic bacteria from blood and ticks obtained from hares and long-eared hedgehogs (Hemiechinus megalofis) in eastern Iran. Comp. Immunol. Microbiol. Infect. 2024;104 doi: 10.1016/j.cimid.2023.102097. [DOI] [PubMed] [Google Scholar]
- Sayad B., Babazadeh A., Barary M., Hosseinzadeh R., Ebrahimpour S., Afshar Z.M. Lyme neuroborreliosis: a case report. Clin. Case Rep. 2023;11(8) doi: 10.1002/ccr3.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazmand A., Harl J., Eigner B., Hodžić A., Beck R., Hekmatimoghaddam S., Mirzaei M., Fuehrer H.-P., Joachim A. Vector-borne bacteria in blood of camels in Iran: new data and literature review. Comp. Immunol. Microbiol. Infect. 2019;65:48–53. doi: 10.1016/j.cimid.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayeghi M., Piazak N., Gollampuor A., Nasirian H., Abolhassani M. Tick-borne relapsing fever in Sabzevar (Khorasan Razavy Province), north-eastern Iran. Bangladesh J. Med. Sci. 2016;15(4) [Google Scholar]
- Shirani D., Rakhshanpoor A., Cutler S.J., Ghazinezhad B., Naddaf S.R. A case of canine borreliosis in Iran caused by Borrelia persica. Ticks Tick Borne Dis. 2016;7(3):424–426. doi: 10.1016/j.ttbdis.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Siadati P.T.A. A case of Lyme disease (Lyme Borreliosis) Acta Med. Iran. 2006:222–224. [Google Scholar]
- Spach D.H., Liles W.C., Campbell G.L., Quick R.E., Anderson Jr D.E., Fritsche T.R. Tick-borne diseases in the United States. N. Engl. J. Med. 1993;329(13):936–947. doi: 10.1056/NEJM199309233291308. [DOI] [PubMed] [Google Scholar]
- Stanek G., Strle F. Lyme borreliosis. Lancet. 2003;362(9396):1639–1647. doi: 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- Stone B.L., Tourand Y., Brissette C.A. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017;17(9):619–629. doi: 10.1089/vbz.2017.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatandoost H., Ghaderi A., Javadian E., Nia A.Z., Rassi Y., Piazak N., Kia E., Shaeghi M., Zelmodarreiy Z., Abolhasani M. "Distribution of soft ticks and their infection with Borrelia in Hamadan province, Iran." Iran. J. Public Health. 2003;32(1):22–24. [Google Scholar]
- Wagner B., Erb H.N. Dogs and horses with antibodies to outer-surface protein C as on-time sentinels for ticks infected with Borrelia burgdorferi in New York State in 2011. Prev. Vet. Med. 2012;107(3):275–279. doi: 10.1016/j.prevetmed.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Yazdanpanah M.J., Sharifi N., Khooei A., Banihashemi M., Khaje-Daluee M., Shamsi A., Ghazvini K. Frequency of Borrelia in Morphea lesion by polymerase chain reaction in Northeast of Iran. Jundishapur J. Microbiol. 2015;8(8) doi: 10.5812/jjm.19730v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi-Behzadi M., Moazzezy N., Rohani M., Naddaf S.R., Mostafavi E., Mohamadi A., Shams-Ghahfarokhi M., Pashootan N., Razzaghi-Abyaneh M. Identification of intestinal fungal microflora and bacterial pathogens in the collected adult ixodes ricinus from the Northern provinces of Iran. J Arthropod Borne Dis. 2022;16(2):97–107. doi: 10.18502/jad.v16i2.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated this study are available upon reasonable request to the corresponding author.