Abstract
The MEGGIC (Magic EGGplant InCanum) population here presented is the first highly inbred eggplant (Solanum melongena) multiparent advanced generation intercross (MAGIC) population developed so far, derived from seven cultivated accessions and one wild Solanum incanum from arid regions. The final 325 S5 lines were high-throughput genotyped using low-coverage whole-genome sequencing (lcWGS) at 3X, yielding 293 783 high-quality SNPs after stringent filtering. Principal component analysis (PCA) and neighbor-joining clustering revealed extensive genetic diversity driven by the unique genetic profile of the wild founder, and lack of genetic structure, suggesting a well-mixed population with a high degree of recombination. The eight founders and a core subset of 212 lines were phenotyped for above- and belowground traits, revealing wide phenotypic diversity. Root morphology traits displayed moderate heritability values, and strong correlation were found between root and aerial traits, suggesting that a well-developed root system supports greater aboveground growth. Genome-wide association studies (GWAS) identified a genomic region on chromosome 6 associated with root biomass (RB), total root length (RL), and root surface area (SA). Within this region, SmLBD13, an LOB-domain protein involved in lateral root development, was identified as a candidate gene. The S. incanum haplotype in this region was linked to reduced lateral root branching density, a trait that may enhance deeper soil exploration and resource uptake. These findings provide key insights into root genetics in eggplant, demonstrating MEGGIC potential for high-resolution trait mapping. Furthermore, they highlight the role of exotic wild germplasm in breeding more resilient cultivars and rootstocks with improved root architecture and enhanced nutrient uptake efficiency.
Introduction
The increasing global population, coupled with the rising demand for food and the negative impact of climate change, are threatening food security [1]. These challenges underscore the urgent need to achieve a more sustainable and productive horticulture. Addressing these intertwined issues requires the development of innovative and efficient strategies to breed more resilient crops capable of thriving under increasingly unpredictable environmental conditions [2, 3]. A deeper understanding of complex traits associated with improved plant performance is crucial, particularly those related to enhanced resource use efficiency, stress tolerance, and overall adaptability to changing climates.
Roots serve as the primary structures anchoring plants to the soil and play an essential role in water and nutrient uptake; therefore, they are key organs affecting plant growth and resilience. Understanding this ‘hidden half’ offers significant potential to optimize crop performance [4]. Thus, breeding for more efficient belowground behavior might drive a ‘second green revolution’ [5, 6]. Despite their relevance, root traits have traditionally been neglected in breeding programs due to their phenotyping challenges. Conventional phenotyping methods, such as root digging and soil boring, are labor-intensive and time-consuming. However, advances in phenomic technologies have enabled the development of automated, non-invasive, and high-throughput methodologies [7]. Integration of root phenotyping with genomics could further accelerate progress in understanding the genetic basis of root development.
Eggplant (Solanum melongena L.) is a major vegetable crop of increasing global significance, ranking fifth in worldwide vegetable production, with an annual output >60.8 million metric tons in 2023 [8]. Despite its substantial economic and agricultural importance, eggplant has lagged considerably behind other Solanaceae crops, such as tomato, in terms of the development of genetic and genomic resources [9, 10]. To address this gap, we developed the first eggplant multiparent advanced generation intercross (MAGIC) population [11]. MAGIC populations have emerged as a powerful tool for crop breeding in genetics, ideal for dissecting complex traits [12]. These populations consist of large sets of recombinant inbred lines, offering genetic mosaics of multiple founders suitable for precise fine mapping [13–15]. MAGIC populations have been developed in several crops and successfully applied to unravel the genetic basis of different traits of interest, including those related to resilience, such as drought and salt tolerance [16–22]. To fully exploit the potential of MAGIC populations, deep marker density is required to capture their extensive genetic variation, given their convoluted crossing design and the large population sizes [23]. Traditionally, two high-throughput genotyping approaches have been predominantly used for MAGIC populations: reduced representation sequencing (RRS)-based methods, such as GBS [20, 24, 25], and commercial single nucleotide polymorphism (SNP) arrays [26–28]. While cost-effective, these methods are limited in genome-wide coverage, as they primarily target predefined genomic regions [29, 30]. To address this limitation, low-coverage whole-genome sequencing (lcWGS) has emerged as a promising alternative. By combining the broad genomic coverage and dense polymorphism detection of whole-genome sequencing (WGS) with the cost-efficiency of RRS and SNP arrays, lcWGS provides a scalable and cost-effective platform for genome-wide analysis, making it ideal for large-scale studies [31, 32]. This approach has been successfully employed in trait-associated loci discovery across various crops, even at ultra-low sequencing coverages as low as 0.02X [33–41].
The eggplant MAGIC population, referred to as MEGGIC (Magic EGGplant InCanum), was derived from an interspecific cross of seven accessions of cultivated eggplant and one accession of its close wild relative Solanum incanum [11]. The seven S. melongena accessions were selected from different geographical origins, including Spain, China, and India, to maximize the phenotypic diversity within the common eggplant, including traits of commercial interest [42]. The S. incanum founder was selected to broaden the genetic diversity of the population by introducing ancestral variation lost during domestication processes. The wild S. incanum accession was originally collected from a desertic region in Israel characterized by significant temperature fluctuations between day and night, being exposed to both heat and cold stresses, as well as severe drought conditions [42, 43]. In such environments, a robust root system is critical for plant survival and performance [44]. A previous study [45] demonstrated that genomic introgressions from the wild S. incanum on chromosome 6 into cultivated eggplant backgrounds positively influence root-related traits, enhancing overall yield under water stress conditions.
Due to root phenotyping difficulties, limited information on genetic control of root development is available for eggplant [46]. In this study, the final MEGGIC lines were grown in expanded clay balls and screened at the seedling stage for different root morphology traits. Lines were genotyped at 3X lcWGS and phenotyped through high-resolution scanning of clean roots to identify potential genomic regions associated with an improved eggplant root architecture through genome-wide association studies (GWAS). Additionally, aboveground traits were evaluated to assess overall plant performance, including some well-known traits in eggplant being used to validate the potential of the MEGGIC population for high-resolution mapping. This study highlights the potential of integrating multiparent populations, cost-effective genomic tools, and high-resolution screening approaches to tackle complex traits such as root architecture, critical for crop adaptation and resilience in the current environmental context. Furthermore, the identification of lines with improved root systems could represent potential elite material for direct release as new cultivars, for inclusion in breeding pipelines as prebreeding resources, or use as new rootstocks. This marks a qualitative leap in eggplant breeding, marking a significant step forward in the development of innovative tools and strategies for genetic improvement.
Results
Polymorphisms among MEGGIC lines
The lcWGS genotyping at 3X coverage of the 325 MEGGIC lines yielded 31 673 278 biallelic SNPs with Freebayes v. 1.3.6 [47]. A final marker set of 293 783 SNPs was selected for subsequent analyses after a rigorous step filtration process (Fig. 1A). The proportion of markers selected from the initial raw set after the comparison with the gold standard (GS), considered as high-confident biallelic SNPs set as they were supported by at least 20 reads in the GS, was 23.66%, totaling 7 492 731 biallelic SNPs (Fig. S1A). The second filtration step adjusted the SNP heterozygosity of each line, with the proportion of heterozygous SNPs ranging from 0.03 to 0.15 (Fig. S1B). While the minimum depth filtering step increased the proportion of missing data (Fig. S1C and S1D), this was subsequently addressed in the preimputation step by applying a maximum missing data threshold (Fig. S1E and S1F) and further corrected during the imputation process. The selection of original sites from the fully genotyped dataset resulted in an overall dosage R-squared (DR2) of 1, indicating high imputation quality. However, sites with a low allele frequency were removed because of being associated with higher allele-specific error rates (Fig. S1G; [48]).
Figure 1.
MEGGIC population genotyping results. (A) SNP filtering pipeline from the 3X lcWGS dataset of 31 673 278 biallelic SNPs to the final subset of 293 783 high-confident SNPs used for the subsequent analysis. The workflow illustrates the impact of each filtering step and the downstream selection of marker subsets. Dashed lines indicate the steps where SNP sets derived from the founders’ 20X resequencing data were used as the gold standard and as the reference panel. (B) Distribution of the final SNP subset along the 12 eggplant chromosomes. Color code indicates the SNP density per Mb.
The distribution of SNPs across the 12 eggplant chromosomes was uneven, with the highest number of SNP loci observed on chromosome 1 (38 659 SNPs) and the lowest on chromosome 9 (9660 SNPs) (Table S1). However, when accounting for physical chromosome length, the SNP density appeared relatively uniform across the genome, with an average of one SNP per 3931 bp (Fig. 1B, Table S1).
Population structure and founder contribution
Principal component analysis (PCA) using the genotypic information was performed to evaluate population structure [49]. The first principal component (PC1; 5.31%), distinctly separated the wild S. incanum from the cultivated S. melongena founders, while the PC2 (4.66%) differentiated the Oriental (A and H) from the Occidental (B, D, E, and G) founders, with founder F of unknown origin clustered in the latter group (Fig. 2A; [42]). Focusing on the main plot where the S. melongena parents and MEGGIC lines were distributed, we found that the MEGGIC lines covered a wide area of the zoomed plot with a shift toward positive PC1 values, possibly due to wild introgressions (Fig. 2B). Very importantly, no population structure was detected, as no distinct groups were observed. Moreover, the first two PCs accounted only for 9.97% of the genetic variance. Given that the PCA revealed weak population structure and a high level of genetic diversity, no further analyses were conducted to assess genetic structure. The neighbor-joining dendrogram yielded consistent conclusions (Fig. 2C). The wild founder (C) displayed a highly divergent genetic profile, while the Occidental founders (B, D, E, and G) clustered together reflecting their closer genetic relationship. Specifically, founders D and E, as well as founders B and G, tightly clustered in the same branch indicating a strong genetic similarity between these pairs.
Figure 2.
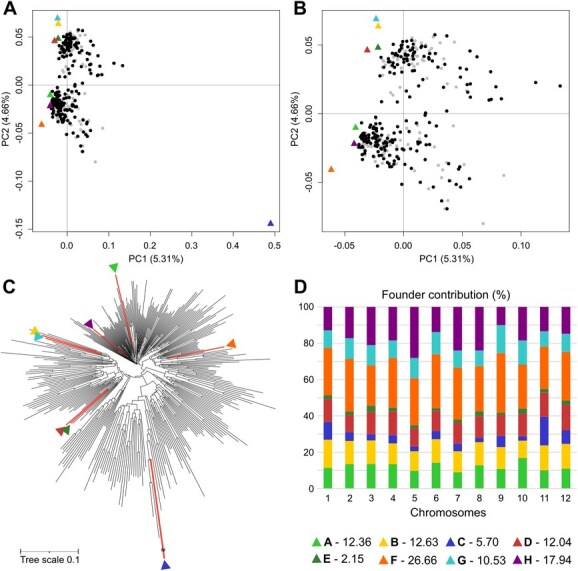
MEGGIC population structure analysis. (A) PCA including the genotyping data of the founders and the MEGGIC population, highlighting the set of lines selected for this study over the entire population. The eight founders A–H are indicated with triangles using a color code. (B) Zoom in on the top left-hand corner of the PCA. (C) Dendrogram indicating founders’ location with colored branches and triangles with the color code. (D) Chromosome-wide and genome-wide allele contribution of parental lines. Numbers in the legend indicate the overall genome-wide contribution of each parental line.
The reconstruction of the genomic mosaics in the MEGGIC lines, based on the eight founder haplotypes, revealed differential haplotype block proportions across different genomic regions for all chromosomes (Fig. S2). The eight-founder crossing design theoretically predicts an equal contribution of ~12.50% from each founder to the genetic diversity of the final population. However, genome-wide and chromosome-wide assessments of parental allelic probabilities revealed deviations from this expectation, with estimated genomic contributions varying across chromosomes. On average, founder contributions align more closely with the expected value than those reported for the S3MEGGIC at an intermediate stage of the population development [11]. However, some founders contributed disproportionately to the estimated genetic background of the population, with founders F and H showing the highest estimated average contributions at 26.66% and 17.94%, respectively. In contrast, founders C and E contributed the least to the estimated contribution to the genetic background, with averages of 5.70% and 2.15%, respectively (Fig. 2D).
Morphological phenotyping
Nine traits, four related to the aerial growth and development (AB, HE, LN, and LA) and five related to root morphology (RB, RL, SA, MD, and MW), were assessed across MEGGIC founders at the seedling stage. Initial evaluation of founder genotypes revealed significant diversity across all measured traits (Fig. 3). Notably, the wild S. incanum founder (C) exhibited a distinctive aerial morphology and root architecture compared to the other founders. In this way, the aerial part of this founder was marked by higher plant height and leaf number although coupled with lower leaf expansion. In contrast, the root system was characterized by minimal lateral root development but significantly deeper roots (Fig. S3). The substantial diversity observed among founders prompted further investigation into plant performance across the entire population to unravel the genetic basis underlying these traits. Consequently, the MEGGIC population was evaluated under the same experimental conditions and following the same methodology. Additionally, PR and AN traits were also collected. A core subset of MEGGIC lines was selected based on seed availability and germination consistency, including lines with data from at least two reliable replicates. After data curation, a core subset comprising 212 lines was retained for further analysis, which was representative of the genetic diversity observed in the entire population (represented as black dots over gray dots in Fig. 2B). These remaining 212 MEGGIC lines displayed a broad range of variation highlighting the wide phenotypic diversity within the population (Table 1). Most traits displayed a continuous distribution slightly skewed to the right (Fig. 4A). Aerial traits exhibited heritability values ranging from 0.21 to 0.31. In contrast, root morphology traits showed higher heritability values ranging from 0.29 to 0.51.
Figure 3.

Boxplots illustrating the diversity among MEGGIC founders (A–H) for different aerial (aerial biomass, AB; plant height, HE; leaf number, LN; and leaf area, LA) and root (root biomass, RB; total root length, RL; surface area, SA; maximum depth, MD; and maximum width, MW) traits.
Table 1.
Mean (±SD), range values, genetic variance ( ), residual variance (
), residual variance (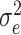 ), and broad-sense heritability (H2) with standard errors (within brackets) of the different traits evaluated in the MEGGIC core subset lines
), and broad-sense heritability (H2) with standard errors (within brackets) of the different traits evaluated in the MEGGIC core subset lines
| Trait | MEGGIC lines (n = 212) | ||||
|---|---|---|---|---|---|
| Mean | Range |

|

|
H2 | |
| Aerial growth | |||||
| AB (g) | 0.73 ± 0.36 | 0.08–2.25 | 0.03 | 0.09 | 0.24 (0.049) |
| HE (cm) | 49.49 ± 11.03 | 24.93–92.82 | 36.54 | 82.26 | 0.31 (0.051) |
| LN | 2.98 ± 0.78 | 1–6 | 0.16 | 0.44 | 0.27 (0.050) |
| LA (cm2) | 40.69 ± 20.72 | 6.13–139.18 | 82.67 | 318.89 | 0.21 (0.047) |
| Root morphology | |||||
| RB (g) | 0.15 ± 0.09 | 0.01–0.60 | 0.002 | 0.006 | 0.29 (0.050) |
| RL (cm) | 171.21 ± 95.60 | 9.96–593.76 | 4862.84 | 4647.16 | 0.51 (0.047) |
| SA (cm2) | 15.66 ± 9.61 | 0.85–65.13 | 40.88 | 51.83 | 0.44 (0.050) |
| MD (cm) | 9.96 ± 3.19 | 2.01–19.48 | 3.13 | 6.86 | 0.31 (0.051) |
| MW (cm) | 4.96 ± 1.50 | 1.36–10.74 | 0.65 | 1.54 | 0.30 (0.051) |
Figure 4.
Statistical analysis of the MEGGIC seedlings phenotyping. (A) Histograms and density plots of the phenotype values among MEGGIC core subset lines for different aerial (aerial biomass, AB; plant height, HE; leaf number, LN; and leaf area, LA) and root (root biomass, RB; total root length, RL; surface area, SA; maximum depth, MD; and maximum width, MW) traits. (B) PCA score plot and (C) loading plot on the first principal components based on all the studied traits for the selected MEGGIC lines. (D) Pairwise phenotypic (lower-left matrix) and genetic (upper-right matrix) correlations among the studied traits. Pearson’s correlation coefficient (r) is shown using a Bonferroni correction at the significance level of 0.05.
The phenotypic PCA performed with the evaluated traits in the MEGGIC lines facilitated the assessment of variation across multiple traits within the population and revealed the relationship among lines. The first two PCs accounted for 66.22% and 10.93% of the total variation, respectively. The projection of the lines in the PCA score plot showed a wide distribution over the graph area, which underscored a substantial phenotypic diversity within the MEGGIC lines (Fig. 4B). Regarding the PCA loading plot, all the traits were placed on the negative values of PC1 (Fig. 4C). Aerial-related traits were mainly located in or close to the negative values of PC2, while root morphology-related traits were plotted close together in the positive values of PC2.
Pearson’s correlation coefficients were calculated to estimate phenotypic correlations both within and across aerial and root traits (Fig. 4D). For the aerial traits, only AB and LA exhibited a high positive correlation (0.92), suggesting that LA is a good indicator of the plant’s overall growth potential. For root morphology traits, strong positive correlations were observed among all traits, with correlations ranging from 0.56 to 0.97 (mean of 0.79). RL and SA showed a high correlation of (0.97), and both also showed a strong correlation with RB (0.93 and 0.95, respectively), all of them contributing to the overall efficiency of the root system. There was a positive correlation between root traits, AB, and LA with values ranging from 0.58 to 0.88 (mean of 0.76), suggesting that a robust root system supports greater aboveground growth, especially in terms of leaf expansion. Genetic correlations were estimated and found to closely align with the phenotypic correlations (Fig. 4D).
By analyzing MEGGIC lines distribution for each trait, several lines were identified that exhibited high vigor in both above- and belowground development (i.e. MEGGIC lines 101 or 148), indicating strong overall growth potential (Fig. S4). Conversely, some lines were observed to display significantly weaker development in both aerial and root systems (i.e. lines 102 or 210). Some lines were found to prioritize LA expansion over LN (i.e. line 114) or prioritize LN production over root development (i.e. line 73).
GWAS analysis and candidate gene identification
To validate the suitability of the MEGGIC population for GWAS, PR and AN traits were initially analyzed, since they are well characterized and associated quantitative trait loci (QTLs) and genes have been proposed. Given the absence of population structure, a general linear model (GLM) model was conducted. The Manhattan plot for PR revealed a strong association peak at the end of chromosome 6 with the highest significantly associated SNP located at 105 615 359 bp, explaining 36.81% of the total phenotypic variance (Fig. 5A). This peak is very close to the SmLOG3 gene (SMEL_006g267050.1, 105 504 136-105 509 693 bp), which has been recently identified as a major gene for prickliness in eggplant [50]. The Manhattan plot for AN displayed a pronounced association peak on chromosome 9, with the top significantly associated SNP positioned at 17 400 739 bp, accounting for 25.61% of the overall phenotypic variance (Fig. 5B). Near this SNP, the SmbHLH69 gene (SMEL_009g326640, 17 862 102-17 872 412 bp), also known as SmTT8, was identified, which promotes anthocyanin biosynthesis in eggplant [51, 52].
Figure 5.
GWAS results for (A) prickles (PR), (B) anthocyanin pigmentation (AN), and (C) different root-related traits, including root biomass (RB), total root length (RL), and surface area (SA). The horizontal lines represent the Bonferroni threshold at P = .05 (LOD = 5.77). The vertical dashed line indicates the associated genomic position. Highlighted dots covered the neighboring positions to the top significantly associated SNP. Genes close to the top significantly associated SNP position are shown, with the proposed candidate gene indicated.
For root-related traits, GWAS analyses were performed to investigate genetic control underlying root development. The Manhattan plot for RL and SA revealed a single peak on chromosome 6, with the SNP over the Bonferroni threshold (limit of detection, LOD = 5.77) located at 1 290 799 bp, explaining 12.33% of the total phenotypic variance (Fig. 5C). Although no SNP exceeded the Bonferroni threshold for RB, there was a clear upward trend at the same position. No trend was observed for MD and MW traits (Fig. S5). Under the peak on chromosome 6, the SmLBD13 gene (SMEL_ 006 g243020.1, 1 386 770-1 388 917 bp), also known as SmTT8, was identified as the best candidate (Table S2). This gene belongs to the lateral organ boundaries (LOB) domain-containing proteins, which are known to play crucial roles in plant development [53]. Variants that predicted high-impact effects on protein function were annotated by SnpEff for founders C, D, and H. Specifically, for founders C and H, an SNP (C/A) leading to a stop gain was identified in two different positions (at 1 388 151 and 1 388 725 bp, respectively); while for founder D a frameshift variant (TTGT/TT) was identified at 1 387 319 bp. These variants were supported by a limited number of reads, which may compromise their reliability (Table S3). For this reason, a comparative analysis of the founder haplotype diversity was performed for the associated genomic region where SmLBD13 is located. As a result, it was observed that MEGGIC lines carrying the founder C haplotype displayed on average reduced lateral root branching density, contributing to smaller RB, RL, and SA mean values (Table 2). In contrast, lines carrying the founder B haplotype exhibited on average a more fibrous root system. No assessed lines were found to carry the founder E haplotype in this genomic region, consistent with its overall low estimated contribution to the population for this region.
Table 2.
Mean (±SD) of the root-related traits evaluated in the MEGGIC core subset lines based on the founders’ haplotype in the associated genomic region on chromosome 6
| MEGGIC lines’ haplotype average | |||
|---|---|---|---|
| Founder | RB (g) | RL (cm) | SA (cm2) |
| A | 0.13 ± 0.08 | 155.72 ± 84.36 | 14.64 ± 8.70 |
| B | 0.22 ± 0.13 | 220.87 ± 138.11 | 22.25 ± 13.48 |
| C | 0.10 ± 0.06 | 112.83 ± 69.43 | 10.45 ± 6.68 |
| D | 0.13 ± 0.09 | 135.93 ± 93.48 | 12.73 ± 8.92 |
| E | |||
| F | 0.14 ± 0.06 | 162.61 ± 69.57 | 14.60 ± 6.70 |
| G | 0.17 ± 0.07 | 207.79 ± 79.90 | 19.00 ± 7.53 |
| H | 0.14 ± 0.06 | 165.56 ± 66.73 | 14.90 ± 6.96 |
Discussion
A comprehensive understanding of traits governing crop adaptation is crucial to cope with increasingly unpredictable environmental conditions. Among these traits, root architecture plays a pivotal role in enhancing the plant ability to adapt to environmental stresses, improving overall plant performance and resource use efficiency [46, 54, 55]. Elucidating the genetic basis of these traits is essential for developing more resilient plants. However, mapping complex quantitative traits such as root architecture using traditional mapping population presents significant challenges. In this context, MAGIC populations have emerged as a cutting-edge resource due to their potential for high-precision association studies, establishing it as a valuable resource for genetic mapping and trait dissection. In this study, we presented the final version of the first eight-way MAGIC population in eggplant (MEGGIC) constituted by 325 highly inbred lines. Among the eight founders, founder C corresponds to an accession of the close wild relative S. incanum [11], whose genetic distinctiveness provides the opportunity to introgress unique traits into a cultivated background [56]. Native to desert areas [42, 43], the S. incanum accession serves as an ideal source for traits related to root development and environmental adaptation.
Coupling the potential of MAGIC populations for genetic mapping with emerging high-throughput genotyping strategies, such as lcWGS, enhances the resolution of trait mapping and accelerates the identification of key genomic regions associated with complex traits. lcWGS provides a cost-effective platform for genome-wide analysis, making it particularly suitable for large-scale studies like those involving MAGIC populations [21, 57, 58]. Here, the 325 MEGGIC lines were high-throughput genotyped at 3X coverage following Baraja-Fonseca et al.’s [59] recommendations, which provided accuracy, sensitivity, and genotypic concordance comparable to 5X coverage while significantly reducing sequencing costs. However, low sequencing coverages inherently pose challenges due to the limited number of reads per site, potentially leading to genotype misclassification and erroneous variant calls [60]. Despite these challenges, the benchmarking study done by Baraja-Fonseca et al. [59] using data from the founders of the MEGGIC population demonstrated that <10% of the SNPs were misclassified as homozygous when they were heterozygous in the high-coverage SNP set, and even fewer SNPs were misclassified as heterozygous when they were homozygous. To address the potential for genotype misclassification in our 3X dataset, we removed sites based on the percentage of heterozygous genotypes and applied a depth of coverage (DP) threshold of 3DP. To minimize noise from sequencing errors, rigorous SNP filtering is critical, with the implementation of a GS being a widely recognized approach for ensuring genotypic reliability [37, 61, 62]. In the context of inbreed populations, GSs are typically constructed using high-coverage resequencing data from the founders [17, 35, 63, 64]. Despite the limited number of individuals included, these GSs capture the full allelic diversity of the population, as no novel alleles are expected beyond those present in the founder genomes. In this study, we implemented a filtering step strategy involving the comparison of the dataset against a GS and the application of a minimum depth coverage threshold [59] to minimize potential false positives and generate a high-confidence set of biallelic SNPs with reliability comparable to variants surveyed at 20X coverage. This genotyping strategy resulted in a significantly higher SNP density (one SNP per 4 kb) and a more evenly distributed set of markers compared to the single-primer enrichment technology (SPET) [29] used for the genotyping of the intermediate-stage S3MEGGIC population, which achieved a density of one SNP per 165 kb [11].
To evaluate the success of the MEGGIC development, genetic diversity and population structure of the final population were thoroughly analyzed using the lcWGS genotyping data. A PCA including the founders’ genetic information reflected the genetic divergence of the wild founder, which was clearly separated from the cultivated founders and the rest of the lines in the population. Genetic similarities were observed within the cultivated founders, as evidenced by their clustering in both the PCA and dendrogram analyses, particularly among those of Occidental origin. The wide distribution of the MEGGIC lines in the PCA graph area, coupled with the low variance explained by the first PCs, highlighted the extensive genetic diversity within the population and the lack of population structure, which is one of the main advantages of this kind of multiparent population [15]. A deeper genotyping characterization of the population enabled a more precise reconstruction of haplotypes, providing more accurate estimations of founder haplotype proportions and better capturing the fine-scale genomic structure of the population [48]. However, genome-wide and chromosome-wide analyses revealed deviations from the expected equal contribution of each founder from an eight-way crossing design, a pattern also observed in other MAGIC populations [65]. Despite an increased marker density, the genetic similarity among some founders may hinder the reliable distinction between them, potentially introducing bias in the estimation of haplotype blocks. The lower contribution of the wild founder could also be attributed to segregation distortion and cryptic selection processes due to reduced fertility, recalcitrant germination, or erratic flowering and fruit set, since they have previously been reported in progenies from crosses between S. incanum and cultivated eggplant [66, 67].
The eight founders and the MEGGIC lines were further evaluated for root system architecture. Seedlings were grown in expanded clay balls, which facilitate straightforward and nondestructive root extraction, enabling high-quality root scanning. This growing system provides an efficient approach for root phenotyping and could be extended to other crops to facilitate studies on root architecture and development. Seedlings were phenotyped for different aerial and root-related traits showing a wide range of variation. Moderate heritability values were obtained for root morphology traits, suggesting that selection for improved root architecture could lead to significant and consistent genetic gains [68, 69]. Since positive correlations were observed among root and aerial traits, selecting for improved root traits could indirectly enhance aboveground growth, promoting overall plant vigor and resilience under stress conditions [70]. Moreover, positive correlations between traits exhibiting differing heritabilities suggested that an indirect selection approach by targeting a genetically correlated trait with higher heritability may represent a more effective strategy for improving traits with lower heritability, thereby optimizing breeding efforts [71]. Some MEGGIC lines exhibited different biological trade-offs between above- and belowground resource allocation, underscoring diverse adaptation strategies for balancing growth and resource use [72].
As proof of concept for testing the potential of the MEGGIC population for the high-precision fine mapping of traits of interest, two extensively studied traits in eggplant, PR and AN, were also phenotyped. GWAS analysis for PR directly pointed to the well-known prickleless (pl) locus on chromosome 6 [73], very close to SmLOG3, recently identified as the responsible gene for prickle losses across the Solanum as well as in distantly related vascular plant lineages [50]. For AN, a differential trend was observed in the genetic regulation across developmental stages of the MEGGIC population. In the S3MEGGIC population, an intermediate stage of population development referred to the third generation of selfing (S3), GWAS for anthocyanin presence in vegetative plant tissues and fruit epidermis identified a major peak on chromosome 10 near to the SmMYB113 gene, a well-known regulatory transcription factor controlling anthocyanin synthesis in eggplant [11]. Additionally, a peak on chromosome 9 was detected close to SmbHLH69 (also commonly referred to as SmTT8), although with a lower LOD score. In contrast, here in the more advanced S5 MEGGIC population, the strongest association signal for anthocyanin presence was the one on chromosome 9. The key difference between the S3MEGGIC and the final MEGGIC phenotyping was the plant development stage (adult plants vs 25-day-old seedlings, respectively). Given that SmTT8 is a basic helix–loop–helix (bHLH) protein that directly binds to SmMYB113 via their amino acid terminus domain to module anthocyanin biosynthesis [52, 74–76], these results suggest a shift in the genetic regulation of anthocyanin accumulation with stage-specific expression patterns. The SmTT8 gene seems to be predominantly expressed during the early stages of plant development, with increased activity in vegetative tissues, while expression may shift toward a higher activation of SmMYB113 gene during reproductive development and in fruit tissues [77]. Further expression analysis at different developmental stages would provide valuable insights into the dynamic interaction between bHLH and MYB genes in anthocyanin regulation.
Different root-related traits, including RB, RL, and SA, were also assessed through GWAS analysis. Given the strong correlation observed among these root traits (r > 0.93), the resulting Manhattan plots were similar. As a result, an associated genomic region was identified at the beginning of chromosome 6. The candidate genomic region colocalized with the SmLBD13 gene (SMEL_ 006 g243020.1), which belongs to LOB domain-containing proteins. These proteins are known to be key regulators of a large number of developmental and metabolic processes in higher plants, particularly in lateral organ formation, including lateral root development and root architecture plasticity [53]. LOB domain proteins are involved in regulating processes like lateral root initiation and formation in other species, such as rice [78], maize [79], or Arabidopsis [80]. Identifying this gene as a key regulator of root morphology in eggplant has important implications for understanding root architecture, which could enhance breeding efforts aimed at improving nutrient uptake efficiency, stress resilience, and overall plant performance. The ‘steep, cheap, and deep’ root ideotype for rapid exploration of deeper soil layers, improving water and nutrient uptake, is crucial for optimizing plant plasticity [81]. One of the key mechanisms contributing to this ideotype is the reduction of lateral root branching density. The presence of the wild haplotype in the associated genomic region on chromosome 6 was found to contribute to a less fibrous root system in the MEGGIC lines. These results were consistent with the S. incanum root morphology shaped by the harsh and resource-scarce environments where it evolved [43]. However, given that dense root hairs are important for the acquisition of immobile soil nutrients, especially phosphorus and potassium [81], lines carrying the S. melongena founder B haplotype, characterized by a more fibrous root system, may also hold agronomic value. The identification of promising lines that combine a more efficient root system provides valuable candidates for further analysis as potential elite breeding materials and might also be useful as rootstocks for eggplant grafting.
Our study marks a significant step forward in eggplant breeding, providing genetic insights that contributes to the development of more resilient crops by improving root architecture, a critical yet often overlooked aspect of crop breeding [1]. The development of a highly inbred MEGGIC population also represents a milestone in eggplant research. By leveraging the power of a MAGIC population, a high-throughput and cost-effective genomic tool, alongside a precise phenotyping strategy, we identified key genomic regions associated with root system traits, highlighting SmLBD13 as a strong candidate gene for lateral root development. These findings provide valuable genetic resources for breeding strategies aimed at enhancing eggplant adaptability to increasingly unpredictable environments.
Materials and methods
MEGGIC population development and genotyping
Plant materials
The MEGGIC (Magic EGGplant InCanum) population was developed by intercrossing seven cultivated common eggplant (S. melongena; A, B, D, E, F, G, and H) and one wild relative (S. incanum; C). Founders were pairwise intercrossed by following a simple ‘funnel’ approach (Fig. S6). After obtaining four simple hybrids (AB, CD, EF, and GH) and two double hybrids (ABCD and EFGH), the latter were intercrossed following a chain pollination scheme obtaining 209 combinations of quadruple hybrids (S0 generation) [11]. Two plants of each S0 progeny were randomly selected and selfed during five generations (S5) by single-seed descent (SSD). Due to the loss of some lines during the SSD process and to some plants failing to set fruit or produce seed, the final MEGGIC population is constituted of 325 highly inbred lines.
Genotyping of the MEGGIC population
The 325 MEGGIC lines were germinated in seedling trays. Young leaf tissue was sampled from each line and genomic DNA was extracted using the silica matrix extraction (SILEX) method [82]. The quality and integrity of the extracted DNA were assessed by agarose gel electrophoresis and NanoDrop spectrophotometer ratios (260/280 and 260/230), while its concentration was estimated using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). All samples were high-throughput genotyped by lcWGS at 3X (3.6 Gb clean data each) using the DNBseq platform at Beijing Genomics Institute (BGI Genomics, Hong Kong, China) following Baraja-Fonseca et al.’s [59] recommendations. Initial raw reads were processed for quality control using fastq-mcf v.1.04.676 [83]. High-quality reads were aligned onto the eggplant reference genome ‘67/3’ v. 3 [74], using the BWA-MEM algorithm v. 0.7.17–r1188 [84] with default parameters. Polymerase chain reaction (PCR) duplicates were removed by using MarkDuplicates software from Picard’s tools v. 1.119 (https://broadinstitute.github.io/picard/).
SNP selection
Variant detection was performed with Freebayes v. 1.3.6 [47], using the standard settings except for a minimum quality threshold of 20 for both mapping and base quality. Biallelic SNPs were selected using BCFtools v. 1.13 [85]. To filter out potential false positives from lcWGS and retain only variants present in the population founders, ensuring that we captured the expected variants and minimized noise from sequencing errors, the dataset was benchmarked against a high-confidence gold standard reference set developed by Baraja-Fonseca et al. [59]. This GS set was developed from 20X resequencing data of the MEGGIC population founders [42]. Following mapping against the eggplant reference genome ‘67/3’ v. 3 [74], SNP calling was performed at the population level using Freebayes, considering data from all eight founders to improve variant detection. To ensure robust genotyping and reliable variant calls, only biallelic SNPs supported by a minimum of 20 reads were retained in the final dataset. No additional filters were applied to avoid removing low-represented alleles, particularly those derived from the wild founder, which may be absent in the other founders but still provide valuable genetic variation within the population. GS reliability was assessed by Baraja-Fonseca et al. [59] through genotypic concordance with independent datasets. Shared positions between the 325 MEGGIC cohort and the GS underwent a rigorous four-step filtration process: (I) elimination of positions with >20% heterozygosity among lines to mitigate the misclassification of SNPs as heterozygous; (II) conversion of genotypes supported by fewer than three reads to missing data to further address misclassification issues; (III) removal of monomorphic sites across lines; and (IV) removal of sites with >50% missing data. For complete genomic representation, imputation was carried out with Beagle v. 22Jul22.46e [86], using the GS as the reference panel, with additional filtering for a minimum depth of 10 using VCFtools v. 0.1.16 (−minDP 10). Only original positions with a minor allele frequency >0.04 was retained, maintaining a minimum distance of 2000 bp between selected positions to ensure data quality and reduce redundancy.
Population structure and founder contribution
A PCA was conducted to study the pattern of genetic variation among the final MEGGIC lines based on the filtered SNPs. Founders’ genetic information was also included. The genetic matrix was calculated using the prcomp function from the stats package in R [87]. The eigenvalues of each principal component (PC) and the proportion of explained variance were used to evaluate the structure of the MEGGIC population. The two first PCs were drawn using R package ggplot2 [88]. A dendrogram was constructed using the neighbor-joining method [89], and the graphical representation was generated and refined using iTOL v.4 software [90]. To estimate the parental contribution to the final population, haplotype blocks, and recombination patterns within the population, the R package HaploBlocker v. 1.7.01 was used [91].
MEGGIC population phenotyping and analysis
Seedling cultivation conditions and experimental design
In an initial exploratory trial, the eight founders were evaluated for trait diversity, followed by assessing the entire population for traits that exhibited significant variation. Seeds of the eight founders and the 325 MEGGIC lines were germinated in Petri dishes, following the Ranil et al. [92] protocol to overcome the seed dormancy commonly observed in wild species and synchronized germination. Subsequently, they were transferred to seedling trays of 0.2 l containing expanded clay balls of 2- to 3-mm diameter (Arlita™, Madrid, Spain) using a completely randomized block design with three replicates. Seedlings (one per replicate) were grown in a climatic chamber under a photoperiod and temperature regime of 16 h light (25°C, 100–112 μmol m−2 s−1) and 8 h dark (18°C) and 70% of relative humidity. They were cultivated for 25 days, and they were fertirrigated three times a week with 50 ml of one-fourth strength Hoagland no. 2 solution [93].
Phenotyping of the MEGGIC seedlings
After 25 days of cultivation, seedlings were cut and partitioned into aerial and root parts for the evaluation of four traits related to the aerial growth and development (aerial biomass, AB; plant height, HE; leaf number, LN; and leaf area, LA) and five traits related to root morphology (root biomass, RB; total root length, RL; surface area, SA; maximum depth, MD; and maximum width, MW) as indicated in Table 3. Additionally, the presence of prickles (PR) and anthocyanin pigmentation (AN) in the leaves and stem were assessed for validating MEGGIC potential for high-precision fine mapping. The aerial part was photographed with a 4 cm2 red calibration area for LA analysis with the Easy Leaf Area software (Fig. S7) [94]. Default parameters were used except for the minimum red-green-blue (RGB) values and G/B ratio, which were set at 0 and 2, respectively. Roots were spread in a transparent tray in a thin layer of water, and they were imaged using a high-resolution scanner. Image acquisition was performed by WinRhizo root scanner (dual lens system STD 4800 root scanner Epson Perfection V700, Regents Instrument Canada Inc.) and analysis was carried out by RhizoVision Explorer (Fig. S7) [96]. Nonroot objects were filtered at a maximum size of two units and root pruning was enabled with a threshold set at five units. MD and MW were measured by Image J software [95].
Table 3.
List of traits used for the MEGGIC characterization with their abbreviations, units, and method of collection
| Abbreviation | Trait | Units | Method of collection |
|---|---|---|---|
| Aerial characteristics | |||
| AB | Aerial biomass | g | Weighed using a precision balance |
| HE | Plant height | cm | Measured from ground level to apical meristem |
| LN | Leaf number | Including mature and young leaves | |
| LA | Leaf area | cm2 | Easy Leaf Area software [94] |
| PR | Prickles | Binary classification for absence (0) or presence [2] | |
| AN | Anthocyanins | Measured from none (0) to very dark pigmentation [95] | |
| Root morphology | |||
| RB | Root biomass | g | Weighed using a precision balance |
| RL | Total root length | cm | RhizoVision Explorer [96] |
| SA | Surface area | cm2 | RhizoVision Explorer [96] |
| MD | Maximum depth | cm | Image J software [95] |
| MW | Maximum width | cm | Image J software [95] |
Statistical analysis
For each trait, the phenotypic mean and range values were calculated for the three replicates. To visualize the distribution of these traits, histograms and density plots were generated using the R package ggplot2 [88]. PCA was performed using the prcomp function to assess the phenotypic variation among the MEGGIC lines, with score and loading plots created using ggplot2. Pearson’s correlation coefficients between traits were calculated and significance was assessed with a Bonferroni correction at a significance level of 0.05 [97, 98], using the R packages psych [99] and corrplot [100]. Additionally, barplots of standardized mean values with error bars were produced using ggplot2 to identify interesting MEGGIC lines.
Genomic heritabilities (H2) were estimated by fitting univariate linear mixed models using the genomic best linear unbiased prediction (GBLUP) framework [101]. Fixed effects only included the intercept and MEGGIC lines were treated as random genetic effects, assumed to be normally distributed with mean 0 and variance equal to genomic relationship matrix (GRM). Residuals were assumed to be independently and normally distributed. The models were fitted using the R package sommer [102]. The genomic relationship matrix was constructed following VanRaden [103] method 1 using R package AGHmatrix [104]. The genomic heritability for each trait was calculated using the formula
 |
where 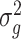 is the genetic variance using marker-based relationships and
is the genetic variance using marker-based relationships and 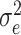 is the residual variance. Standard errors for the heritabilities were obtained using the vpredict function in sommer. Additionally, genetic correlations between traits were estimated by fitting a multivariate linear mixed model using the GBLUP framework within sommer. Fixed effects included only the intercept, and an unstructured variance model between traits was used for both the random genetic effects and residuals to allow correlations across traits to be estimated. Genetic correlations were calculated in R using cor(rho_pheno[lower.tri(rho_pheno)], rho_geno[upper.tri(rho_geno).
is the residual variance. Standard errors for the heritabilities were obtained using the vpredict function in sommer. Additionally, genetic correlations between traits were estimated by fitting a multivariate linear mixed model using the GBLUP framework within sommer. Fixed effects included only the intercept, and an unstructured variance model between traits was used for both the random genetic effects and residuals to allow correlations across traits to be estimated. Genetic correlations were calculated in R using cor(rho_pheno[lower.tri(rho_pheno)], rho_geno[upper.tri(rho_geno).
Genome-wide association study
PR and AN traits were used for validating the potential of the MEGGIC population for GWAS analysis since these traits have been extensively studied in eggplant [11, 50, 73, 105–107]. Several QTLs and candidate genes have been proposed to control these traits, so we aimed to assess whether the results obtained in the MEGGIC population were consistent with the previously reported regions. Additionally, five root-related traits were analyzed as key indicators of root development since they are suggested to be directly related to nutrient acquisition and crop yield [46, 54, 55]. The phenotyping data as the mean value of the three replicates for each trait was used and GWAS analyses were performed using the TASSEL software (ver. 5.0, [108]). GLM analysis was conducted for the association study [109]. The multiple testing was corrected with the Bonferroni method [110] with a significance level of 0.05 [111]. SNPs with an LOD score (calculated as -log10[P-value]) exceeding these specified thresholds or cut-off values in both GWAS models were considered significantly associated with the traits under evaluation. The genes surrounding the highest significant SNPs were retrieved from the ‘67/3’ v. 3 eggplant reference genome [74]. Candidate genes were assessed by SnpEff prediction software v 4.2 [112] based on resequencing data from the eight MEGGIC founders [42] to identify causative mutations associated with phenotypic variation. The Integrative Genomics Viewer (IGV) tool was then used to visually explore the founder genome sequences and validate the SnpEff predictions [113]. Founder haplotypes were estimated for the candidate genomic region and a comparative analysis of founder haplotype diversity across the MEGGIC lines combining genotypic and phenotypic data was performed.
Supplementary Material
Acknowledgements
This work has been funded by grants PID2021-128148OB-I00 funded by MICIU/AEI/10.13039/501100011033/ and by ERDF/EU, PDC2022-133513-I00 funded by MICIU/AEI/10.13039/501100011033/ and European Union Next Generation EU/PRTR, CIPROM/2021/020 from Conselleria d’Educació, Cultura, Universitats i Ocupació (Generalitat Valenciana), and by the Horizon Europe programme, project number 101094738 (Promoting a Plant Genetic Resource Community for Europe; PRO-GRACE). V.B.-F. is grateful to Conselleria d’Educació, Cultura, Universitats i Ocupació (Generalitat Valenciana), for a predoctoral contract (CIACIF/2023/238). A.S. is grateful to MICIU/AEI/10.13039/501100011033/ and FSE+ for a predoctoral grant (PRE2022-102368). P.G. is grateful for the postdoctoral grant RYC2021-031999-I funded by MICIU/AEI/10.13039/501100011033 and the European Union through NextGenerationEU/PRTR.
Contributor Information
Andrea Arrones, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Virginia Baraja-Fonseca, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Andrea Solana, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Mariola Plazas, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Salvador Soler, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Jaime Prohens, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Santiago Vilanova, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Pietro Gramazio, Instituto de Conservación y Mejora de la Agrodiversidad Valenciana, Universitat Politècnica de València, Camino de Vera 14, 46022 Valencia, Spain.
Author contributions
M.P., S.S., J.P., S.V., and P.G. conceived the idea and supervised the manuscript. A.A. and A.S. performed the root phenotyping trials. A.A., V.B.-F., P.G., and S.V. performed the bioinformatic analysis and analyzed the data. A.A. prepared a first draft of the manuscript. All other authors reviewed and edited the manuscript.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA392603 and PRJNA1174391.
Conflict of interests
The authors declare no conflicts of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
References
- 1. Yang Y, Tilman D, Jin Z. et al. Climate change exacerbates the environmental impacts of agriculture. Science. 2024;385:eadn3747. [DOI] [PubMed] [Google Scholar]
- 2. Abbas M, Abid MA, Meng Z. et al. Integrating advancements in root phenotyping and genome-wide association studies to open the root genetics gateway. Physiol Plant. 2022;174:e13787. [DOI] [PubMed] [Google Scholar]
- 3. Qaim M. Role of new plant breeding technologies for food security and sustainable agricultural development. Appl Econ Perspect Policy. 2020;42:129–50 [Google Scholar]
- 4. Maqbool S, Hassan MA, Xia X. et al. Root system architecture in cereals: progress, challenges and perspective. Plant J. 2022;110:23–42 [DOI] [PubMed] [Google Scholar]
- 5. Den Herder G, Van Isterdael G, Beeckman T. et al. The roots of a new green revolution. Trends Plant Sci. 2010;15:600–7 [DOI] [PubMed] [Google Scholar]
- 6. Uga Y. Challenges to design-oriented breeding of root system architecture adapted to climate change. Breed Sci. 2021;71:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teramoto S, Uga Y. Improving the efficiency of plant root system phenotyping through digitization and automation. Breed Sci. 2022;72:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. FAOSTAT . “Food and Agriculture Organization of the United Nations Database of Agricultural Production.” FAO Statistical Databases; 2025. http://www.fao.org/faostat/. Accessed 27 January 2025. [Google Scholar]
- 9. Gramazio P, Alonso D, Arrones A. et al. Conventional and new genetic resources for an eggplant breeding revolution. J Exp Bot. 2023;74:6285–305 [DOI] [PubMed] [Google Scholar]
- 10. Gramazio P, Prohens J, Plazas M. et al. Development and genetic characterization of advanced backcross materials and an introgression line population of Solanum incanum in a S. Melongena background. Front Plant Sci. 2017;8:1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mangino G, Arrones A, Plazas M. et al. Newly developed MAGIC population allows identification of strong associations and candidate genes for anthocyanin pigmentation in eggplant. Front Plant Sci. 2022;13:847789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scott MF, Ladejobi O, Amer S. et al. Multi-parent populations in crops: a toolbox integrating genomics and genetic mapping with breeding. Heredity (Edinb). 2020;125:396–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cavanagh C, Morell M, Mackay I. et al. From mutations to MAGIC: resources for gene discovery, validation and delivery in crop plants. Curr Opin Plant Biol. 2008;11:215–21 [DOI] [PubMed] [Google Scholar]
- 14. Huang BE, Verbyla KL, Verbyla AP. et al. MAGIC populations in crops: current status and future prospects. Theor Appl Genet. 2015;128:999–1017 [DOI] [PubMed] [Google Scholar]
- 15. Mackay I, Powell W. Methods for linkage disequilibrium mapping in crops. Trends Plant Sci. 2007;12:57–63 [DOI] [PubMed] [Google Scholar]
- 16. Abdelraheem A, Thyssen GN, Fang DD. et al. GWAS reveals consistent QTL for drought and salt tolerance in a MAGIC population of 550 lines derived from intermating of 11 upland cotton (Gossypium hirsutum) parents. Mol Gen Genomics. 2021;296:119–29 [DOI] [PubMed] [Google Scholar]
- 17. Diaz S, Ariza-Suarez D, Izquierdo P. et al. Genetic mapping for agronomic traits in a MAGIC population of common bean (Phaseolus vulgaris L.). BMC Genomics. 2020;21:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ravelombola W, Shi A, Huynh BL. Loci discovery, network-guided approach, and genomic prediction for drought tolerance index in a multi-parent advanced generation intercross (MAGIC) cowpea population. Hortic Res. 2021;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravelombola W, Shi A, Huynh BL. et al. Genetic architecture of salt tolerance in a multi-parent advanced generation inter-cross (MAGIC) cowpea population. BMC Genomics. 2022;23:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma V, Mahadevaiah SS, Latha P. et al. Dissecting genomic regions and underlying candidate genes in groundnut MAGIC population for drought tolerance. BMC Plant Biol. 2024;24:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thudi M, Samineni S, Li W. et al. Whole genome resequencing and phenotyping of MAGIC population for high resolution mapping of drought tolerance in chickpea. Plant Genome. 2023;17:e20333. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Ponce KS, Meng L. et al. QTL identification for salt tolerance related traits at the seedling stage in indica rice using a multi-parent advanced generation intercross (MAGIC) population. Plant Growth Regul. 2020;92:365–73 [Google Scholar]
- 23. Arrones A, Vilanova S, Plazas M. et al. The dawn of the age of multi-parent magic populations in plant breeding: novel powerful next-generation resources for genetic analysis and selection of recombinant elite material. Biology (Basel). 2020;9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krishnamurthy SL, Sharma PC, Dewan D. et al. Genome wide association study of MAGIC population reveals a novel QTL for salinity and sodicity tolerance in rice. Physiol Mol Biol Plants. 2022;28:819–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López-Malvar A, Butron A, Malvar RA. et al. Association mapping for maize stover yield and saccharification efficiency using a multiparent advanced generation intercross (MAGIC) population. Sci Rep. 2021;11:3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arrones A, Antar O, Pereira-Dias L. et al. A novel tomato interspecific (Solanum lycopersicum var. cerasiforme and Solanum pimpinellifolium) MAGIC population facilitates trait association and candidate gene discovery in untapped exotic germplasm. Hortic Res. 2024;11:uhae154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fourquet L, Barber T, Campos-Mantello C. et al. An eight-founder wheat MAGIC population allows fine-mapping of flowering time loci and provides novel insights into the genetic control of flowering time. Theor Appl Genet. 2024;137:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan G, Sun K, Yu W. et al. Development of a MAGIC population and high-resolution quantitative trait mapping for nicotine content in tobacco. Front Plant Sci. 2023;13:1086950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barchi L, Acquadro A, Alonso D. et al. Single primer enrichment technology (SPET) for high-throughput genotyping in tomato and eggplant germplasm. Front Plant Sci. 2019a;10:1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Z, Zheng Z, Qi F. et al. Development and evaluation of the utility of GenoBaits Peanut 40K for a peanut MAGIC population. Mol Breed. 2023;43:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar P, Choudhary M, Jat BS. et al. Skim sequencing: an advanced NGS technology for crop improvement. J Genet. 2021;100:38. [PubMed] [Google Scholar]
- 32. Scheben A, Batley J, Edwards D. Genotyping-by-sequencing approaches to characterize crop genomes: choosing the right tool for the right application. Plant Biotechnol J. 2017;15:149–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adhikari L, Shrestha S, Wu S. et al. A high-throughput skim-sequencing approach for genotyping, dosage estimation and identifying translocations. Sci Rep. 2022;12:17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bayer PE, Ruperao P, Mason AS. et al. High-resolution skim genotyping by sequencing reveals the distribution of crossovers and gene conversions in Cicer arietinum and Brassica napus. Theor Appl Genet. 2015;128:1039–47 [DOI] [PubMed] [Google Scholar]
- 35. Clot CR, Wang X, Koopman J. et al. High-density linkage map constructed from a skim sequenced diploid potato population reveals transmission distortion and QTLs for tuber yield and pollen shed. Potato Res. 2024;67:139–63 [Google Scholar]
- 36. Gonda I, Ashrafi H, Lyon DA. et al. Sequencing-based bin map construction of a tomato mapping population, facilitating high-resolution quantitative trait loci detection. Plant Genome. 2019;12:180010. [DOI] [PubMed] [Google Scholar]
- 37. Happ MM, Wang H, Graef GL. et al. Generating high density, low cost genotype data in soybean [Glycine max (L.) Merr.]. G3 (Bethesda). 2019;9:2153–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang X, Feng Q, Qian Q. et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 2009;19:1068–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luo X, Xu L, Wang Y. et al. An ultra-high-density genetic map provides insights into genome synteny, recombination landscape and taproot skin colour in radish (Raphanus sativus L.). Plant Biotechnol J. 2020;18:274–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malmberg MM, Barbulescu DM, Drayton MC. et al. Evaluation and recommendations for routine genotyping using skim whole genome re-sequencing in canola. Front Plant Sci. 2018;9:1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang H, Xu X, Vieira FG. et al. The power of inbreeding: NGS-based GWAS of rice reveals convergent evolution during rice domestication. Mol Plant. 2016;9:975–85 [DOI] [PubMed] [Google Scholar]
- 42. Gramazio P, Yan H, Hasing T. et al. Whole-genome resequencing of seven eggplant (Solanum melongena) and one wild relative (S. incanum) accessions provides new insights and breeding tools for eggplant enhancement. Front Plant Sci. 2019;10:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knapp S, Vorontsova MS, Prohens J. Wild relatives of the eggplant (Solanum melongena L.: Solanaceae): new understanding of species names in a complex group. PLoS One. 2013;8:e57039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Delfin EF, Drobnitch ST, Comas LH. Plant strategies for maximizing growth during water stress and subsequent recovery in Solanum melongena L. (eggplant). PLoS One. 2021;16:e0256342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flores-Saavedra M, Gramazio P, Vilanova S. et al. Introgressed eggplant lines with the wild Solanum incanum evaluated under drought stress conditions. J Integr Agric. 2024;24:2203–16 [Google Scholar]
- 46. Yousefi F, Soltani F, Lalehparvar AR. et al. Genetic diversity of eggplant (Solanum melongena L.) accessions based on morpho-physiological characteristics and root system architecture traits. J Agric Sci Technol. 2024;26:387–401 [Google Scholar]
- 47. Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. Preprint 2012;at arxiv.org/abs/1207.3907
- 48. Pook T, Mayer M, Geibel J. et al. Improving imputation quality in beagle for crop and livestock data. G3 (Bethesda). 2020;10:177–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diouf I, Pascual L. Multiparental population in crops: methods of development and dissection of genetic tratis. Methods Mol Biol. 2021;2264:13–32 [DOI] [PubMed] [Google Scholar]
- 50. Satterlee JW, Alonso D, Gramazio P. et al. Convergent evolution of plant prickles by repeated gene co-option over deep time. Science. 2024;385:eado1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. He Y, Chen H, Zhou L. et al. Comparative transcription analysis of photosensitive and non-photosensitive eggplants to identify genes involved in dark regulated anthocyanin synthesis. BMC Genomics. 2019;20:1–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shi S, Liu Y, He Y. et al. R2R3-MYB transcription factor SmMYB75 promotes anthocyanin biosynthesis in eggplant (Solanum melongena L.). Sci Hortic. 2021;282:110020 [Google Scholar]
- 53. Xu C, Luo F, Hochholdinger F. LOB domain proteins: beyond lateral organ boundaries. Trends Plant Sci. 2016;21:159–67 [DOI] [PubMed] [Google Scholar]
- 54. Katuuramu DN, Wechter WP, Washington ML. et al. Phenotypic diversity for root traits and identification of superior germplasm for root breeding in watermelon. HortScience. 2020;55:1272–9 [Google Scholar]
- 55. Wang H, Wei J, Li P. et al. Integrating GWAS and gene expression analysis identifies candidate genes for root morphology traits in maize at the seedling stage. Genes (Basel). 2019;10:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Flores-Saavedra M, Plazas M, Gramazio P. et al. Growth and antioxidant responses to water stress in eggplant MAGIC population parents, F1 hybrids and a subset of recombinant inbred lines. BMC Plant Biol. 2024b;24:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fang DD, Thyssen GN, Wang M. et al. Genomic confirmation of Gossypium barbadense introgression into G. hirsutum and a subsequent MAGIC population. Mol Gen Genomics. 2023;298:143–52 [DOI] [PubMed] [Google Scholar]
- 58. Han Z, Hu G, Liu H. et al. Bin-based genome-wide association analyses improve power and resolution in QTL mapping and identify favorable alleles from multiple parents in a four-way MAGIC rice population. Theor Appl Genet. 2020;133:59–71 [DOI] [PubMed] [Google Scholar]
- 59. Baraja-fonseca V, Arrones A, Vilanova S. et al. Benchmarking of low coverage sequencing workflows for precision genotyping in eggplant. Preprint 2024;at bioRxiv 2024.10.24.619843
- 60. Meisner J, Albrechtsen A. Inferring population structure and admixture proportions in low-depth NGS data. Genetics. 2018;210:719–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gao Y, Yang Z, Yang W. et al. Plant-ImputeDB: an integrated multiple plant reference panel database for genotype imputation. Nucleic Acids Res. 2021;49:D1480–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zook JM, Hansen NF, Olson ND. et al. A robust benchmark for detection of germline large deletions and insertions. Nat Biotechnol. 2020;38:1347–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saripalli G, Adhikari L, Amos C. et al. Integration of genetic and genomics resources in einkorn wheat enables precision mapping of important traits. Commun Biol. 2023;6:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang M, Qi Z, Thyssen GN. et al. Genomic interrogation of a MAGIC population highlights genetic factors controlling fiber quality traits in cotton. Commun Biol. 2022;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hashemi SM, Perry G, Rajcan I. et al. SoyMAGIC: an unprecedented platform for genetic studies and breeding activities in soybean. Front Plant Sci. 2022;13:945471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barchi L, Lanteri S, Portis E. et al. Segregation distortion and linkage analysis in eggplant (Solanum melongena L.). Genome. 2010;53:805–15 [DOI] [PubMed] [Google Scholar]
- 67. Lefebvre V, Pflieger S, Thabuis A. et al. Towards the saturation of the pepper linkage map by alignment of three intraspecific maps including known-function genes. Genome. 2002;45:839–54 [DOI] [PubMed] [Google Scholar]
- 68. Mathew I, Shimelis H. Genetic analyses of root traits: implications for environmental adaptation and new variety development: a review. Plant Breed. 2022;141:695–718 [Google Scholar]
- 69. Schuster A, Santana AS, Uberti A. et al. Genetic diversity, relationships among traits and selection of tropical maize inbred lines for low-P tolerance based on root and shoot traits at seedling stage. Front Plant Sci. 2024;15:1429901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang Y, Wu X, Wang X. et al. Crop root system architecture in drought response. J Genet Genomics. 2024;52:4–13 [DOI] [PubMed] [Google Scholar]
- 71. Neyhart JL, Lorenz AJ, Smith KP. Multi-trait improvement by predicting genetic correlations in breeding crosses. G3 (Bethesda). 2019;9:3153–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weigelt A, Mommer L, Andraczek K. et al. An integrated framework of plant form and function: the belowground perspective. New Phytol. 2021;232:42–59 [DOI] [PubMed] [Google Scholar]
- 73. Frary A, Frary A, Daunay MC. et al. QTL hotspots in eggplant (Solanum melongena) detected with a high resolution map and CIM analysis. Euphytica. 2014;197:211–28 [Google Scholar]
- 74. Barchi L, Pietrella M, Venturini L. et al. A chromosome-anchored eggplant genome sequence reveals key events in Solanaceae evolution. Sci Rep. 2019b;9:11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moglia A, Francesco EF, Sergio I. et al. Identification of a new R3 MYB type repressor and functional characterization of the members of the MBW transcriptional complex involved in anthocyanin biosynthesis in eggplant (S. melongena L.). PLoS One. 2020;15:e0232986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou L, He Y, Li J. et al. CBFs function in anthocyanin biosynthesis by interacting with MYB113 in eggplant (Solanum melongena L.). Plant Cell Physiol. 2020;61:416–26 [DOI] [PubMed] [Google Scholar]
- 77. Petroni K, Tonelli C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011;181:219–29 [DOI] [PubMed] [Google Scholar]
- 78. Liu H, Wang S, Yu X. et al. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005;43:47–56 [DOI] [PubMed] [Google Scholar]
- 79. Taramino G, Sauer M, Stauffer JL. et al. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 2007;50:649–59 [DOI] [PubMed] [Google Scholar]
- 80. Cho C, Jeon E, Pandey SK. et al. LBD13 positively regulates lateral root formation in Arabidopsis. Planta. 2019;249:1251–8 [DOI] [PubMed] [Google Scholar]
- 81. Lynch JP. Harnessing root architecture to address global challenges. Plant J. 2022;109:415–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vilanova S, Alonso D, Gramazio P. et al. SILEX: a fast and inexpensive high-quality DNA extraction method suitable for multiple sequencing platforms and recalcitrant plant species. Plant Methods. 2020;16:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aronesty E. Comparison of sequencing utility programs. Open Bioinformatics J. 2013;7:1–8 [Google Scholar]
- 84. Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint 2013;at https://arxiv.org/abs/1303.3997v2
- 85. Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Browning BL, Browning SR. Genotype imputation with millions of reference samples. Am J Hum Genet. 2016;98:116–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna: 2023. https://www.R-project.org/ [Google Scholar]
- 88. Wickham H. Getting Started with ggplot2. In: ggplot2 elegant graphics for data analysis. Cham: Springer International Publishing. 2016;11–31 [Google Scholar]
- 89. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25 [DOI] [PubMed] [Google Scholar]
- 90. Letunic I, Bork P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pook T, Schlather M, De Campos G. et al. Haploblocker: creation of subgroup-specific haplotype blocks and libraries. Genetics. 2019;212:1045–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ranil RHG, Niran HML, Plazas M. et al. Improving seed germination of the eggplant rootstock Solanum torvum by testing multiple factors using an orthogonal array design. Sci Hortic. 2015;193:174–81 [Google Scholar]
- 93. Hoagland DR, Arnon DI. Preparing the nutrient solution. The water-culture method for growing plants without soil. In Circ - Calif Agric Exp Stn, Ann Arbor, MI, USA: University of Michigan Library. 1950;347:29–31 [Google Scholar]
- 94. Easlon HM, Bloom AJ. Easy leaf area: automated digital image analysis for rapid and accurate measurement of leaf area. Appl Plant Sci. 2014;2:1400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with image. J Biophotonics Int. 2004;11:36–41 [Google Scholar]
- 96. Seethepalli A, Dhakal K, Griffiths M. et al. RhizoVision explorer: open-source software for root image analysis and measurement standardization. AoB Plants. 2021;13:plab056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–2 [Google Scholar]
- 98. Pearson K. Correlation coefficient. Royal Soc Proc. 1895;58:214 [Google Scholar]
- 99. Revelle WR. Psych: Procedures for Personality and Psychological Research Software. Evanston, IL, USA: Northwestern University, 2017 [Google Scholar]
- 100. Wei T, Simko V, Levy M. et al. Visualization of a correlation matrix. R package “corrplot”. Statistician. 2017;56:316–24 [Google Scholar]
- 101. Clark SA, van der Werf J. Genomic best linear unbiased prediction (gBLUP) for the estimation of genomic breeding values. Methods Mol Biol. 2013;1019:321–30 [DOI] [PubMed] [Google Scholar]
- 102. Covarrubias-Pazaran G. Genome-assisted prediction of quantitative traits using the R package sommer. PLoS One. 2016;11:e0156744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. VanRaden PM. Efficient methods to compute genomic predictions. J Dairy Sci. 2008;91:4414–23 [DOI] [PubMed] [Google Scholar]
- 104. Amadeu RR, Cellon C, Olmstead JW. et al. AGHmatrix: R package to construct relationship matrices for autotetraploid and diploid species: a blueberry example. Plant Genome. 2016;9:plantgenome2016.01.0009. [DOI] [PubMed] [Google Scholar]
- 105. Barchi L, Lanteri S, Portis E. et al. A RAD tag derived marker based eggplant linkage map and the location of QTLs determining anthocyanin pigmentation. PLoS One. 2012;7:e43740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cericola F, Portis E, Lanteri S. et al. Linkage disequilibrium and genome-wide association analysis for anthocyanin pigmentation and fruit color in eggplant. BMC Genomics. 2014;15:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Toppino L, Barchi L, Mercati F. et al. A new intra-specific and high-resolution genetic map of eggplant based on a ril population, and location of QTLS related to plant anthocyanin pigmentation and seed vigour. Genes (Basel). 2020;11:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bradbury PJ, Zhang Z, Kroon DE. et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23:2633–5 [DOI] [PubMed] [Google Scholar]
- 109. Price AL, Patterson NJ, Plenge RM. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9 [DOI] [PubMed] [Google Scholar]
- 110. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70 [Google Scholar]
- 111. Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. J Educ Behav Stat. 2002;27:77–83 [Google Scholar]
- 112. Cingolani P, Platts A, Wang LL. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6:80–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Robinson JT, Thorvaldsdottir H, Turner D. et al. Igv.Js: an embeddable JavaScript implementation of the integrative genomics viewer (IGV). Bioinformatics. 2023;39:btac830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA392603 and PRJNA1174391.





